Abstract
The presence of heavy metal concentrations was examined in natural sediments from four sites along the Jajrood river in northeast of Tehran, the capital of Iran. Besides determination of elemental concentrations (Pb, Cu, Zn, Cd, Ni and Cr), X-ray fluorescence and X-ray diffraction tests were carried out to determine other chemical components in these adsorbents. Also the ability of sediments to adsorb these heavy metal ions from aqueous solutions was investigated. Results show that the extent of adsorption increases with increase in adsorbent concentration. The amount of adsorbed Pb, Cu and Zn in sediments was much greater than that of the other metals, and Cr was adsorbed much less than others. The adsorbabilities of sediments to heavy metals increased in the order of Pb > Cu > Zn > Cd > Ni > Cr. Based on the adsorption data, equilibrium isotherms were determined at selected areas to characterize the adsorption process. The adsorption data followed Freundlich and Langmuir isotherms in most cases. Correlation and cluster analysis was performed on heavy metals adsorption and sediment components at each site to evaluate main adsorbing compounds in sediments for each metal. Results demonstrated that heavy metals sorption is mostly related to load of organic matter in the Jajrood river sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The adsorption of metals onto sediments is an important process that can potentially reduce the concentrations of toxic metals in natural aquatic environments (Comber et al. 1996; Jannasch et al. 1988). Sediments also have the main role in transportation of metals in such systems (Larsen and Jensen 1989) since they act as sink of contaminants (Izquierdo et al. 1997; Larsen and Gaudette 1995; Tam and Wong 1995; Cortesao and Vale 1995; Dassenakis et al. 1997; Bruces Williamson et al. 1996; Balls et al. 1997). Release or sorption of metals from/to river sediments has had a significant influence on the quality of the river waters (Förstner and Müller 1973; Förstner and Wittman 1979; Xianghua and Herbert 1999). The study of sediment composition can reveal its role in removal of pollutants especially heavy metals from aqueous phase and its lasting effects on water pollution. The concentration and mobility of heavy metals in sediments has been widely studied in the last decades (Alloway 1995; Hooda and Alloway 1998; Baptista et al. 2000; Hatje et al. 2003; Jain et al. 2004; Saeedi et al. 2004; Fan et al. 2007).
Zhou and Kot (1995), in a study on sediments of Hanjiang River in China, investigated the effective parameters in the process of adsorption. According to their findings, exobiological factors such as temperature, ion number, sediment type, and size of sediment particles, pH, and the simultaneous existence of several heavy metals affect the amount of heavy metals adsorption on the sediments. Some studies on the heavy metals pollution and their behavior in Iranian rivers have been conducted recently (Saeedi et al. 2003, 2004; Saeedi and Karbassi 2008; Karbassi and Nadjafpour 1996; Karbassi et al. 2007, 2008), but the adsorption process of metals onto the sediments is rarely studied.
The Jajrood River flows into the Latian dam reservoir, which is one of the main water resources of Tehran, capital of Iran. It is continuously exposed to industrial, urban and agricultural wastes including some heavy metals. Due to heavy metals, pollution remains a serious environmental and public problem. Taking into account that the river water is consumed as the source of Tehran (capital of Iran) drinking water, dissolved metals and their concentration in aqueous phase is important from the health risks point of view. This issue becomes more important knowing that the drinking water treatment plants of Tehran are conventional and are not equipped with advanced treatment units to remove heavy metals. In recent years, an increasing effort has been put into improvement of the water quality of this river. Research has shown that river bed sediments play an important role as sink of metals in aquatic systems. Hence, adsorption of metals on sediments as a sink process can reduce the hazard of the dissolved metallic ions in Jajrood River.
In the present study, the ability of sediments to adsorb dissolved heavy metal ions from Jajrood River has been investigated at different concentrations of the adsorbents. To better understanding of the process, chemical composition as well as mineralogy of the sediments has been studied and cluster analysis based on correlation between concentrations of components has conducted. Moreover, the adsorption equilibrium was modeled using the Langmuir and Freundlich isotherm models to understand the adsorption mechanism and evaluate the adsorption capacity of these natural adsorbents.
Methodology
Study area
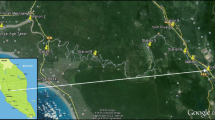
The Jajrood River flows in the great basin area about 710 km2 and lies between latitude 35°48′N to 36°03′N and longitude 51°25′E to 51°42′E (Fig. 1). The climate in this region is moderate. The average annual rainfall in the area is about 210 mm, a major part of which is received during the monsoon period. There is no effective forest cover and there are many metal works and plating workshops as well as recreational centers within the river watershed without environmental controls and a heavily traffic road along side the river as point and non-point sources of pollution. Annual average water quality of this river is presented in Table 1. The sediments which are stored in Latian Dam reservoir remain as potential source of metal pollution of the river water particularly in accidental or rapid change of pH or other physical–chemical properties.
Four sites were selected for sampling of sediments along the river by investigating topographical and geological maps, access ways to river bank, pollution sources (like existing workshops and factories in the basin area) and by considering the natural conditions such as tributaries of the river. Figure 1 shows the location of the sampling sites (Ahar, Ushan, Fasham, Roodak).
Sampling of sediments
Five surface sediment samples were collected from the river at sampling sites with a small dredge, taking portions from the center of the dredge with a polyethylene spoon to avoid contamination by metallic parts. Equal weights of samples from each station were mixed to get a composite sample and transferred to the laboratory in plastic bags under 4°C. The sediments were wetly filtered through mesh 230 with 0.63-μm pore diameters. The filtered sediments were set in flat plastic dishes and dried (<40°C) to a constant weight. The dried sediments were stored at 4°C in polypropylene containers until analysis.
Experimental methods
In order to characterize the sediment samples, X-ray fluorescence (XRF) and diffraction analyses were carried out. The chemical composition of dried samples was determined by XRF (PHILIPS PW 2404). Mineralogical composition and clay mineralogy of samples was determined by X-ray diffractometer (PHILIPS Expert-Pro XRD).
For adsorption isotherm studies, dosages of 1, 2, 3 and 5 g/l of adsorbents were added to five beakers containing matrix of 2 mg/l of stock metals (Cd, Cr, Pb, Zn, Cu, and Ni) solution. The fifth beaker as blank was treated as others without adding dissolved metals. Initial and final pH and temperature were measured and recorded during the experiments. After adding the adsorbent, the solutions were mixed by a mechanical stirrer for 3 h. Then it was left aside constantly for 30 min to settle out the particles. Supernatants were sampled and filtered through 0.45-μm Whatman paper filter before analyzing for metals concentration. Decreasing pH to less than 2 using HNO3, elemental concentrations (Cd, Cr, Pb, Cu, Zn and Ni) were determined using atomic absorption spectrometry (Buck Scientific 210 VP model) according to U.S.EPA 7000s series methods. Blank samples of spiked river water containing no adsorbents were also run and analyzed during all experiments to check the recovery and interferences such as colloidal particles effects which showed negligible effects.
To determine the adsorption capacity of sediments, 20 ml solutions containing 1, 2.5, 5 and 10 mg/l of metals were mixed with 1 g of adsorbents for 24 h according to ASTM D 4646 method (ASTM 1990). A mixture without adding the metals also prepared as blank. All the batch-mixing experiments were conducted in duplicates to ensure quality control of the results, which showed less than 5% error in experiment results. Among the existing clustering techniques (Lance and Williams 1966; Anderson 1971; Davis 1973), the weighted-pair group method (Davis 1973) was used in this study to evaluate the correlation of chemical composition of the sediments with adsorption capacity of metals. This method employs the linear correlation coefficient as a similarity measure. The highest similarities are clustered/linked first, and variables connected only if they are highly correlated. After two variables are clustered, their correlations with all the other variables are averaged.
Results and discussion
Characteristics of sediments
Chemical composition of the sediment samples of each sampling site is presented in Table 2. Results show that SiO2, Al2O3, CaO and organic matter (OM) are found in large quantities in sediments. Analysis of total OM in the sediments was performed using the “loss-on-ignition” method (Bighman and Bartels 1996). Iron and aluminum oxides and OM are present in the sediments in considerable amounts, which may play important roles in adsorbing heavy metals. Although the chemical composition of sediments of four sites are relatively similar, there are some differences particularly in OM and Iron oxide contents of Roodak in comparison with other stations. These differences are important when interpreting the adsorption isotherms. Figure 2 displays XRD spectrum of the sediments at four different stations. It reveals that Quartz, calcite, feldspar, hematite, dolomite and some clayey minerals exist in the sediments. Clay minerals such as Chlorite, Illite and Montmorillonite are present in sediments of the study area while the presence of large quantities of quartz (SiO2), albite, dolomite and clinochlore can clearly be distinguished from X-ray patterns.
Bhattacharyya and Gupta (2008) demonstrated that clays with these characteristics have been good adsorbents because of the existence of several types of active sites on the surface- and ion-exchange sites. Because of same mineralogy for sediment samples at different sites (according to XRD and XRF results), it is concluded that there is no significant change in geology of study area. This subject is confirmed by general geology of region under study.
Considering similar mineralogy of samples of four sites, probable differences in adsorption capacity and behavior of different sediment samples are expected to be related to chemical composition of sediments especially the presence of OM, calcium carbonates and iron oxides.
Adsorbabilities of heavy metals
Adsorption capability experiment results are presented in Table 3. As expected, an increase in the adsorbent concentration generally leads to a decrease in the final metal concentration. However, the rate at which the final metal concentration decreases with increasing adsorbent dosage is not linear, suggesting that the adsorption capacity approaches a maximum value. Similar findings were observed by Saeedi et al. (2004). Also, Jain et al. (2004) and Jain and Ram (1997) reported that the extent of adsorption of the metal ions on sediments increases with increasing adsorbent doses and decreases with adsorbent particle size. Although the final concentration of metals in solution decreases with increasing in adsorbent dose, removal efficiency of dissolved metals per unit mass of adsorbents decreases. It should be pointed out that there might be uncertainties related to the metals carry over through the filtration of samples after experiments and desorption of them before analyzing, which assumed to be insignificant. Considering the difference between the initial and final concentration of the dissolved metals, removal of dissolved metals by sediments at different stations shows that the order of adsorption of metals are as Pb > Cu > Zn > Cd > Ni > Cr, which is different than what has been found in other studies (Coquery and Welbourn 1995). The reason for this disagreement probably relates to differences in the surface water quality and sediment characteristics in the regions under study. Table 4 shows removal percentages for the dissolved metals in solution. An overall assessment of the results reveals that Cr and to some extent Ni have a relatively conservative behavior during riverine mixing, while Pb and Cu show nearly complete non-conservative behavior. Thus, discharges containing dissolved Cr and Ni may cause more environmental risks than those of other dissolved metals (Pb, Cu, Zn and to some extent Cd).
The Freundlich and Langmuir isotherm models for the experiments
The Langmuir and Freundlich models are the most frequently employed models to describe experimental data of adsorption isotherms. In this work, both models were used to describe the relationship between the amount of heavy metal adsorbed and its equilibrium concentration in solutions. The Langmuir isotherm model is based on the assumption that binding sites are homogeneously distributed over the adsorbent surface. These binding sites have the same affinity for adsorption as a single molecular layer. This model assumes that there is no interaction between adsorbed molecules. The linear form of the Langmuir isotherm model can be represented as follows:
where q e is the amount adsorbed at equilibrium (mg/g), C e is the equilibrium concentration of the adsorbate (mg/l), and a (mg/g) and b (l/mg) are the Langmuir constants related to the maximum adsorption capacity and the energy of adsorption, respectively. These constants can be evaluated from the intercept and the slope of the linear plot of experimental data of 1/q e versus 1/C e. The Freundlich model also considers monomolecular layer coverage of solute by the sorbent. However, it assumes that the sorbent has a heterogeneous valance distribution and then has deferent affinity for adsorption. The linear form of the Freundlich isotherm model is given by the following equation:
where k F and 1/n are Freundlich constants related to adsorption capacity and adsorption intensity, respectively, of the sorbent. The values of k F and 1/n can be obtained from the intercept and slope, respectively, of the linear plot of experimental data of ln q e versus ln C e.
Figure 3 shows plot of 1/q e versus 1/C e in four sampling stations for heavy metals adsorption on sediments, which has produced a straight line that indicates the applicability of the Langmuir isotherm for the system under consideration. Also, when ln q e is plotted against ln C e, a straight line with slope 1/n and intercept ln k F is obtained (Fig. 4). The corresponding Langmuir and Freundlich parameters along with correlation coefficients are given in Table 5, respectively. A detailed analysis of the regression coefficients showed that Langmuir and Freundlich models adequately described the adsorption data, as can be seen in Figs. 3 and 4.
Correlation matrix and cluster analysis
Correlation analysis was performed on the amount of heavy metals adsorption per unit mass of sediment samples of different sites, from solution with concentration of 10 mg/l of metals, and chemical composition of bed sediment at each site. This analysis was performed to determine the governing adsorbing agents in sediments for different metals. The correlation matrix (Table 6) shows correlation with both positive and negative values among different pairs of variables. According to data summarized in this table, Cd presents high levels of correlation with Cu and Ni (0.85 and 0.96, respectively). Pb was significantly correlated with Cr (0.99) and Fe2O3 (0.95). There is a good correlation between the concentration of Zn with Ni and MnO (0.8 and 0.87, respectively). The dendrogram in Fig. 5 shows the cluster analysis of the studied parameters (metals adsorption and sediment composition) in the sediment samples at four stations along Jajrood River based on correlation matrix. The first cluster, on the left side of the dendrogram, shows a first level of association with the pair Cd–Ni, as the nearest variables. Then Cu, very close to the previous elements, is added, and finally, CaO is added, forming a cluster on the left side of the dendrogram.
The other level of aggregation is established with the pair Pb–Fe2O3 and Cr which, at the same time, forms a cluster with the pair Zn–OM. These two pairs are associated with each other in the next stage and later with MnO. Thus, the variables are grouped in two main clusters: Cluster 1 Cd–Ni–Cu–CaO and Cluster 2 Pb–Fe2O3–Cr–Zn–OM–MnO. The association between the metals included in these two clusters is also supported by the results of correlation analysis shown in Table 6. Alternately, low correlations were noted between some metals, suggesting that they have different affinities for adsorption by the various sorbents present within the sediments. Based on the dendrogram shown in Fig. 5, calcium oxides and probably calcium carbonates appear to be responsible for adsorbing most of the Cd, Ni, and Cu. On the other hand, Fe/Mn oxides and OM may be the main adsorbing agents of Pb, Cr and Zn. Based on the results, one can conclude that most of Zn is adsorbed and consequently transported by OM in the river while most of Pb and Cr are adsorbed and transported in particulate form with iron oxides in addition to OM.
The relationship between organic matter and heavy metals
Lin and Chen (1998) reported that the organic content in the sediments can be used as a simple index for assessing the degree of pollution of the sediment and that the adsorbabilities of heavy metals of sediments increase with increasing OM content. In this study, with the exception of Cu, which presents a negligible affinity with OM, there is a significant positive correlation between OM and other components. Correlation coefficients were high for Cd, Pb, Zn, Ni and Cr (0.54, 0.92, 0.75 and 0.85, respectively). Therefore, it appears that the OM content is a good indicator of how effective river sediments will be in removing metals from solution through adsorption.
Conclusions
The results of this study clearly show the potential that river sediments have to adsorb dissolved heavy metals from water. Adsorption increases with increasing metal concentration and can be modeled with either Langmuir or non-linear Freundlich isotherms. The decreasing adsorption efficiency with increasing metal concentration occurs as colloids approach monolayer saturation with adsorbed metals.
Jajrood River sediments showed a consistent preference, in terms of percent adsorbed, for Pb > Cu > Zn > Cd > Ni > Cr. All of these metals (except Cu) showed a high correlation with sediment OM content, suggesting that this is the dominant factor influencing the partitioning of metals between the dissolved and particulate phase.
The two metals exhibiting the least amount of adsorption in lab experiments were Cr (generally <26%) and Ni (between 44 and 53%). Adsorption percentages for the other four metals ranged between 73 and 96%. Therefore, it appears that the release of Cr and Ni poses the greatest bio-ecological risk. Experimental data also revealed that the potential for adsorption to occur diminishes as metal concentrations increase. These findings suggest that Iran’s environmental protection organization should continue its efforts to control the levels of heavy metals that are discharged to rivers through industrial effluents and wastewater disposal.
References
Alloway BJ (1995) Heavy metals in soils. Wiley International, Glasgow
American Society for Testing and Materials (ASTM) (1990) Batch-type measurement of contaminant sorption by soils and sediments. ASTM-D4646
Anderson AJB (1971) Numerical examination of multivariate of soil samples. Math Geol 3:1–14
Balls PW, Hull S, Miller BS, Pirie JM, Proctor W (1997) Trace metal in Scottish estuarine and coastal sediments. Mar Pollut Bull 34:42–50
Baptista JA, Smith BJ, McAllister JJ (2000) Heavy metal concentrations in surface sediments in a nearshore environment, Jurujuba Sound, southeast Brasil. Environ Pollut 109:1–9
Bhattacharyya KG, Gupta SS (2008) Adsorption of a few heavy metals on natural and modified kaolinite and montmorillonite: a review. Adv Colloid Interface Sci 140:114–131
Bighman J, Bartels J (1996) Methods of oil analysis, Part 3, chemical methods. Soil Science Society of America, Book Series 5, Madison
Bruces Williamson R, Van Dam LF, Bell RG, Green MO, Kim JP (1996) Heavy metal and suspended sediment fluxes from a contaminated intertidal inlet (Manukau Harbour, New Zealand). Mar Pollut Bull 32:812–822
Comber SDW, Gardner MJ, Gunn AM, Whalley C (1996) Kinetics of trace metal sorption to estuarine suspended particle matter. Chemosphere 33:1027–1040
Coquery M, Welbourn PW (1995) The relationship between metal concentration in the aquatic macrophyte Eriocaulon septangular. Water Res 29:2094–2102
Cortesao C, Vale C (1995) Metals in sediments of the Sado Estuary, Portugal. Mar Pollut Bull 30:34–37
Dassenakis M, Scoullos M, Gaitis A (1997) Trace metals transport and behavior in the mediterranean estuary of Acheloos river. Mar Pollut Bull 34:103–111
Davis JC (1973) Statistics and data analysis in geology. Wiley International, London
Fan Q, He J, Xue H, Ch LU, Saruli YL, Sun Y, Shen L (2007) Competitive adsorption, release and speciation of heavy metals in the Yellow River sediments, China. Environ Geol 53:239–251
Förstner U, Müller G (1973) Heavy metal accumulation in river sediments. Geoforum 14:53–61
Förstner U, Wittman GTW (1979) Metal pollution in the aquatic environment. Springer, Berlin, pp 532
Hatje V, Payne TE, Hill DM, McOrist G, Birch GF, Szymcza R (2003) Kinetics of race metal uptake and release by particles in estuarine waters: effects of pH, salinity, and particle loading. Environ Int 29:619–629
Hooda PS, Alloway BJ (1998) Cadmium and lead sorption behavior of selected English and Indian soils. Geoderma 84:121–134
Izquierdo C, Usero J, Gracia I (1997) Speciation of heavy metals in sediments from salt marshes on the Southern Atlantic Coast of Spain. Mar Pollut Bull 34:123–128
Jain CK, Ram D (1997) Adsorption of metal ions on bed sediments. Hydrol Sci J 42:13–723
Jain CK, Singhal DC, Sharma MK (2004) Adsorption of zinc on bed sediment of River Hindon: adsorption models and kinetics. J Hazard Mater 114:231–239
Jannasch HW, Honeyman BD, Balisrieri LS, Murray JW (1988) Kinetics of trace element uptake by marine particles. Geochim Cosmochim Acta 52:567–577
Karbassi AR, Nadjafpour Sh (1996) Flocculation of dissolved Pb, Cu, Zn and Mn during estuarine mixing of river water with the Caspian Sea. Environ Pollut 93:257–260
Karbassi AR, Nouri J, Ayaz GO (2007) Flocculation of trace metals during mixing of Talar River water with Caspian Sea water. Int J Environ Res 1:66–73
Karbassi AR, Nouri J, Mehrdadi N, Ayaz GO (2008) Flocculation of heavy metals during mixing of freshwater with Caspian Sea water. Environ Geol 53:1811–1816
Lance GN, Williams WT (1966) A generalized sorting for computer classifications. Nature 212:218
Larsen PF, Gaudette H (1995) Spatial and temporal aspect of sedimentary trace metal concentrations in mid-coast Maine. Mar Pollut Bull 30:437–444
Larsen B, Jensen A (1989) Evaluation of the sensitivity of sediment stations in pollution monitoring. Mar Pollut Bull 20:556–560
Lin JG, Chen SY (1998) The relationship between adsorption of heavy metal and organic matter in river sediment. Environ Int 24:345–352
Saeedi M, Karbassi AR (2008) Estuarine capacity in removal of trace metals from contaminated river water-southern Caspian Sea. Water Environ J 22:193–198
Saeedi M, Karbassi AR, Mehrdadi N (2003) Flocculation of dissolved Mn, Zn, Ni and Cu during the mixing of Tadjan river water with Caspian Sea water. Int J Environ Stud 60:575–580
Saeedi M, Daneshvar Sh, Karbassi AR (2004) Role of riverine sediment and particulate matter in adsorption of heavy metals. Int J Environ Sci Technol 1:143–148
Tam NFY, Wong YS (1995) Spatial and temporal variations of heavy metals contamination in sediments of a mangrove swamp in Hong Kong. Mar Pollut Bull 31:254–261
Xianghua W, Herbert EA (1999) Mobilization of heavy metals from Le An River Sediment. Sci Total Environ 227:101–108
Zhou XD, Kot SC (1995) Heavy metals ion adsorption on sediments of the Weiho and Hanjiang rivers-China. J Environ Hydrol 3:125–132
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saeedi, M., Hosseinzadeh, M. & Rajabzadeh, M. Competitive heavy metals adsorption on natural bed sediments of Jajrood River, Iran. Environ Earth Sci 62, 519–527 (2011). https://doi.org/10.1007/s12665-010-0544-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-010-0544-0