Abstract
Since periphytic biofilm is an important source of food in lotic ecosystems, it is important to understand how key ecological factors affect the accrual and loss of algal biomass and sediment in the biofilm. We designed a field experiment to evaluate the effects of mesohabitat type (pools and riffles), grazing fish (control and exclusion), and substrate roughness (smooth and rough) on chlorophyll a, ash-free dry mass (AFDM), and total dry mass in a subtropical stream. Mesohabitat type did not influence the effect of grazers on periphyton. However, rough substrates accumulated more total dry mass in pools than in riffles, while smooth substrates accumulated similar amounts of total dry mass in both mesohabitats. The accrual of AFDM and chlorophyll a was greater on rough than on smooth substrates, regardless of mesohabitat. Treatments without fish accrued more total dry mass, AFDM, and chlorophyll a than treatments with fish, showing that fish play a major role in this stream by removing sediment and algal biomass. These results suggest that habitat simplification in the scale of substrate roughness and loss of large grazers may impact the accrual and loss of algal biomass and sediment in lotic ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The periphytic biofilm, composed of algae, heterotrophic microorganisms and organic sediments, is an important source of food in lotic ecosystems (Biggs, 1996). Changes in the quantity and quality of periphyton may affect the entire food web (Lowe & Pan, 1996). Therefore, understanding how key ecological factors, such as biotic interactions and habitat heterogeneity, affect the accrual and loss of algal biomass, organic matter, and sediment is of great importance in view of increasing human perturbations, which are simplifying habitats, altering the intensity of disturbances, and causing the loss and homogenization of assemblages in lotic ecosystems (Rahel, 2000; Cardinale et al., 2002).
Experimental studies have shown that the modification of habitats by grazers is a major mechanism structuring benthic communities in lotic ecosystems. For instance, the effects of grazers on the structure and productivity of streams and rivers have been documented for insects (Moulton et al., 2004; Álvarez & Peckarsky, 2005), immature amphibians (Flecker et al., 1999; Ranvestel et al., 2004), shrimp (Pringle & Blake, 1994; Souza & Moulton, 2005), and fish (Power, 1990; Flecker, 1996; Bertrand & Gido, 2007). Grazers can affect periphyton directly, by ingestion, and indirectly, by removing organic and inorganic sediments (Moulton et al., 2004; Cross et al., 2008). Periphyton responses to grazing range from changes in biomass to changes in the composition, diversity, physiognomy, nutrient content and succession of communities (Feminella & Hawkins, 1995; Steinman, 1996).
Substrate roughness (a type of small-scale habitat heterogeneity; Bergey, 2005) is an important ecological factor that strongly influences benthic algal biomass and sediment accrual in streams, and likely mediates the effect of grazers. Rough substrates support higher algal biomass than smooth substrates (Bergey, 2005; Murdock & Dodds, 2007) and affect sediment retention (Bergey, 1999; Taniguchi & Tokeshi, 2004). Moreover, there is evidence of the efficacy of crevices in rough substrates as refuges for algae (Dudley & D’Antonio, 1991; Bergey & Weaver, 2004; Schneck et al., 2011). If crevices effectively protect algae from grazers, rough substrates should accumulate larger amounts of algal biomass than smooth substrates in the presence of grazers, while similar amounts of algal biomass should be accumulated on rough and smooth substrates in the absence of grazers.
In addition to grazers and substrate roughness, mesohabitat type should also directly and indirectly affect the accrual of algal biomass and organic and inorganic sediment. The different characteristics of pools and riffles, such as current velocity and retention of organic matter and inorganic sediment (Allan & Castillo, 2007), may affect algal growth on rough and smooth substrates. Accordingly, we could expect hard substrates to accumulate more sediment in pools than in riffles, and thus negatively affect algal growth. However, the different environmental conditions of pools and riffles may affect the strength of the interaction between grazers and the periphytic biofilm. For instance, Poff & Ward (1995) found that the interaction strength of a grazing caddisfly varied with current velocity. The caddisfly reduced algal biomass and altered algal assemblage composition at low velocities, but had only weak effects under high-velocity regimes. Similarly, the detritivorous fish Prochilodus mariae Eigenmann strongly influenced sediment accrual in pools, but had no influence in riffles in a tropical Andean stream (Flecker, 1997). However, it should be noted that non-consumptive loss may be greater in habitats where the water current is stronger such as riffles, since the material dislodged by bioturbation would be easily washed downstream. This role of water flow was already suggested as a mechanism related to the removal of periphyton by shrimps (Souza & Moulton, 2005; Moulton et al., 2012).
We experimentally excluded large grazers (fish) from smooth and rough artificial substrates used for periphyton colonization in pools and riffles. We evaluated the independent and interacting effects of mesohabitat type, large grazers, and substrate roughness on periphyton (total organic and inorganic mass, organic matter, and algal biomass). We hypothesized that the interaction strength between grazers and periphyton would be mediated by mesohabitat types and by substrate roughness. Additionally, we hypothesized that the effect of substrate roughness would be mediated by mesohabitat types. Specifically, we predicted that grazers would have a higher grazing impact in pools than in riffles, and that in the absence of large grazers, pools would retain more biomass and total dry mass than riffles. We also predicted that, in the presence of large grazers, crevices on rough substrates will act as refuges and protect algae, and thus rough substrates would accumulate more algal biomass than smooth substrates, while smooth and rough substrates would accumulate similar amounts of algal biomass in the absence of grazers in the same mesohabitat type. For the last hypothesis, we predicted that rough substrates would accumulate more organic matter and sediment in pools than in riffles, but the accrual on smooth substrates would not differ between mesohabitat types.
Materials and methods
Study area
We conducted the experiment in Marco stream (28°36′S; 49°54′W), São José dos Ausentes, state of Rio Grande do Sul, southern Brazil. The vegetation in the catchment is Campos grassland with patches of Araucaria Forest, and the climate is high-altitude subtropical (Cfb), with uniform precipitation throughout the year (Behling, 2002). The study site is a fourth-order stream at approximately 1,100 m asl that drains a catchment with low human impact and oligotrophic waters (Buckup et al., 2007). Stream width ranges from 6 to 10 m, and the depth ranges from 0.1 to 0.4 m in the reaches studied. The stream bed is composed mostly of rough basaltic stones, boulders, and bedrock. During the study period, the water had high dissolved oxygen concentrations (mean of 8.6 mg l−1), low electrical conductivity (21 μS cm−1), and slight acidity pH (6.6). In pools, the current velocity ranged from 0 to 7 cm s−1, with a mean of 3.7 cm s−1, while in riffles the velocity ranged from 18 to 35 cm s−1, with a mean of 25.6 cm s−1. Velocity was measured close to the substrates in all pools and riffles used in this study (see below).
The fish fauna of Marco stream is composed of 11 species, mostly small characiforms and siluriforms (Winckler-Sosinski et al., 2009). Common grazers on algae and detritus in Marco stream include the armored catfishes (Loricariidae) Rineloricaria sp. and Pareiorhaphis hystrix Pereira & Reis, and the curimatid Steindachnerina biornata Braga & Azpelicueta (Bowen, 1983; Winckler-Sosinski et al., 2009; Dias & Fialho, 2011). The poeciliid Cnesterodon brevirostratus Rosa & Costa probably feeds mostly on detritus, as described for other species of the genus (e.g., Quintans et al., 2009). Eurycheilichthys pantherinus Reis & Schaefer is a common armored catfish in the stream, but differently from most Loricariidae, it was described as an insectivore in Marco stream (Dias & Fialho, 2011). The benthic algal assemblage is composed mostly of diatoms and some filamentous green algae (Schneck et al., 2011).
Experimental design
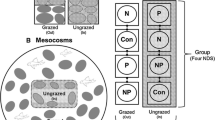
We designed a 3-factor split-split-plot field experiment with the following hierarchical treatments: (i) mesohabitat, with two levels, pools and riffles (5 replications); (ii) grazer occurrence, with the presence or absence of fish (10 replications); and (iii) substrate roughness, with smooth and rough substrates (20 replications). We randomly selected 5 pools and 5 riffles along approximately 200 m in the stream reach. In each of these 10 locations, we placed two flat paving stones (50 × 50 × 8 cm) that constituted the grazer factor, i.e., one paving stone was randomly assigned to receive the electrical exclusion treatment (see below), and the other to be the control. Each paving stone had smooth and rough acrylic substrates (5 × 5 cm) glued on it. The rough substrates had nine longitudinal grooves, each groove (crevice) 1 mm wide and 1 mm deep. The experiment consisted of 40 experimental units (2 substrate types × 2 grazer treatments × 2 mesohabitats × 5 locations) and each experimental unit consisted of two sampling units (acrylic substrates).
We excluded grazers by using electrified fences that were constructed similarly to the description by Landeiro et al. (2008). We used commercial fence electrifiers (Shock 8 Lite; JFL Equipamentos Eletrônicos Ind. Com. Ltda, Santa Rita do Sapucaí, Minas Gerais, Brazil) labeled as having an electric pulse of 8,000 V per second, 1.8 J, and maximum current of 1.6 A. Each of the 10 electrifiers (one for each location) was connected to a 12-V car battery. We fixed two copper wires on each 50 × 50 × 8 cm flat paving stone. We fixed one of the wires on three sides of the paving stone (40 cm of wire on each side) and connected its ends to the exit and return connectors of the electrifier. The two parallel sides were stripped and acted as the negative electrodes. A second wire was also connected to the electrifier, and its final 40-cm-long stripped part was fixed between and parallel to the two stripped sides of the first wire. This second central wire acted as the positive electrode.
Since the electrifiers emit low-intensity pulses, they are able to exclude only large organisms. Landeiro et al. (2008) used an electrifier very similar to the one we used and were able to exclude organisms larger than 1 cm. We tested the efficacy of electrical exclusion during preliminary experiments and monitored the exclusions during the two first days and the two last days that the experiment was running and observed that organisms larger than 1 cm were indeed excluded. The electric shocks caused reactions in small fish, which immediately left the electrified area. We also observed armored catfishes on the control treatments, but never on the exclusion treatments. Macroinvertebrates were not excluded, and species of Trichoptera, Ephemeroptera, and Chironomidae were present on both the electrified and the control substrates (F. Schneck, personal observation). Based on these observations, we considered that our exclusion treatment was effective against fish species, including grazers such as the small armored catfishes and poeciliids present in the stream.
We constructed the wire fences on all paving stones used for the experiment (i.e., in the exclusion and control treatments), and placed the pair of paving stones approximately 50 cm apart. We placed the paving stones with the fences and artificial substrates in the stream at the end of September 2010 for algal colonization. We monitored the precipitation in the studied region during the period of colonization of the substrates (45 days) and there were no spates for approximately 20 days before we set up the electrified fences. The exclusion experiment started on 1 November 2010 when we randomly assigned one paving stone of the pair to be connected to the electrifier at each of the 10 locations. Sampling was carried out on 8 November. Under visual inspection, the biomass of periphyton and inorganic sediment accrual appeared to be higher in the exclusion treatments than in the control treatments after 7 d. We planned the duration of the experiment based on several short-term studies on the effects of grazers/bioturbators on periphyton. For instance, an increase in sediment accrual (dry mass and ash-free dry mass) was observed 6 days after the exclusion of ephemeropterans (Moulton et al., 2004) and 7 days after the exclusion of a detritivorous fish (Flecker, 1996) in relation to non-exclusion treatments. Further, Opsahl et al. (2003) found greater amounts of chlorophyll a in electrified than in non-electrified treatments, but did not find a significant trend over time for chlorophyll a after the exclusion of invertebrate grazers during 7 weeks.
Sampling and laboratory analyses
We removed two sampling units (25 cm², each) of each substrate type per paving stone to form a composite sample of 50 cm². We used a toothbrush to scrub the upper surfaces of the substrates to remove the periphytic material, adjusted the samples to a defined volume (100 ml), and preserved them on ice in the dark until laboratory processing (24–30 h after collection). In the laboratory, each sample (experimental unit) was divided into two subsamples for analyses of chlorophyll a (50 ml) and periphyton dry mass (50 ml). We determined chlorophyll a by extracting pigments with ethanol after filtration through a Whatman GF/C filter, followed by spectrophotometrically measuring chlorophyll a according to standard procedures (Biggs & Kilroy, 2000).
For dry mass determination, we transferred the subsamples to pre-combusted and pre-weighed porcelain crucibles. We dried the samples at 70°C until constant weight (total dry mass) and then burned the organic content in a muffle furnace at 500°C for 3 h to estimate ash-free dry mass (AFDM) (Schwarzbold, 1990). We calculated the Autotrophic Index (AFDM/chlorophyll a) to determine the trophic status of the periphyton. The index usually ranges from 50 to 200 for autotrophic periphyton, while higher ratios indicate heterotrophic periphyton (Steinman et al., 2007). We also calculated the indices proposed by Lakatos (1989), based on total dry mass (low mass: < 2 mg cm−2; medium: 2–4; or high: > 4), on the proportions of organic and inorganic matter (inorganic periphyton: > 75% ash in relation to total dry mass; inorganic–organic: 50–75%; organic–inorganic: 25–50%; or organic: < 25%), and on the proportions of autotrophic and heterotrophic components (autotrophic periphyton: > 0.60% chlorophyll a in relation to total dry mass; auto-heterotrophic: 0.25–0.60%; hetero-autotrophic: 0.10–0.25%; or heterotrophic: < 0.10%). It should be noted that a high proportion of inorganic mass (ash) in relation to organic mass and high chlorophyll a content may be related to the occurrence of a diatom-dominated community, since the siliceous valves greatly contribute to the weight of ashes in the periphyton.
Data analysis
We used 3-factor split-split-plot Analyses of Variance (ANOVAs) to test for the effects of treatments on total dry mass, AFDM, and chlorophyll a. Data were log-transformed to meet the assumptions of homogeneity of variance and normality of residuals. The 10 locations (5 riffles, 5 pools) were considered the main-plot (blocks), the grazer factor was the sub-plot (paving stones), and the substrate factor was the sub–sub-plot (acrylic substrates). Mesohabitat was included in the model as a between-plot factor. Analyses were carried out in the R statistical environment using the function aov (R Development Core Team, 2010).
Results
The periphyton was mainly heterotrophic, since the Autotrophic Index ranged from 305 to 13,000, with an average of 3,812. According to the classification of Lakatos (1989), the periphytic biofilm had mostly low to medium amounts of biomass (range: 0.13–16.08 mg cm−2; median: 1.15 mg cm−2), was inorganic–organic to inorganic (range: 18–94% ash; median: 71%), and heterotrophic (range: 0.001–0.14% chlorophyll a; median: 0.01%).
Total dry mass accrual was dependent on the interaction between mesohabitat and substrates (P = 0.037; Table 1). Total dry mass on smooth substrates was similar between pools and riffles, while on rough substrates the accrual of total dry mass was 2.6 times greater in pools than in riffles (Fig. 1a). On the other hand, the interactions between mesohabitat and grazers and between grazers and substrates were not significant (Table 1). The amount of total dry mass increased 1.8 times in the exclusion treatment in relation to the control, independently of mesohabitat and substrate type (P = 0.001; Table 1; Fig. 1a).
No interaction term was important for AFDM (Table 1). Exclusion treatments accrued 2.2 times more AFDM than control treatments (P = 0.023; Table 1; Fig. 1b), and rough substrates accumulated 3.1 times more AFDM than smooth substrates (P < 0.001; Table 1; Fig. 1b).
There were no significant interactions in the determination of the amounts of chlorophyll a between treatments (Table 1). Chlorophyll a was 1.6 times higher in the exclusion treatment than in the control (P = 0.005; Table 1), and 1.4 times higher on rough than on smooth substrates (P = 0.006; Table 1) (Fig. 1c). However, although not significant (P = 0.104), there was a tendency toward an interaction between the presence/absence of fish and substrate types (Table 1; Fig. 1c). While rough substrates accumulated on average only 1.2 times more chlorophyll a than smooth substrates in the exclusion treatments, in the control treatments, the difference between rough and smooth substrates averaged 1.9 times (Fig. 1c).
Discussion
The distinct pattern of higher algal biomass, organic matter, and total dry mass in the exclusion than in the control treatment indicates that grazing fishes play a major role in this stream through both bioturbation and consumption of algae and detritus (Cross et al., 2008). This strong interaction has been reported in streams around the world, in which different fish species, such as a detritivorous fish in Venezuela (Flecker, 1996), armored catfishes in Panama (Power, 1990), grazing minnows in North America (Power et al., 1985; Bertrand & Gido, 2007), and the ayu fish in Japan (Abe et al., 2007), act as keystone species or ecosystem engineers (sensu Jones et al., 1994). Our observations add important information on processes affecting the functioning of high-altitude subtropical streams.
The second result found in this study was the greater accrual of algal biomass and AFDM on rough than on smooth substrates, a pattern usually attributed to the greater availability of refuges on rough than on smooth substrates (e.g., Bergey & Weaver, 2004; Bergey, 2005). However, we found that this result was statistically not dependent on the presence or absence of grazing fish, contradicting the occurrence of a refuge effect of crevices against grazing fish. On the other hand, although not statistically significant, the larger difference in algal biomass between rough and smooth substrates in the presence (1.9 times more chlorophyll a on rough substrates) than in the absence (1.2 times more chlorophyll a on rough substrates) of fish suggests that crevices may act as refuges and protect part of the algal biomass from grazing fish. This refuge effect is consistent with the previous studies on habitat heterogeneity and complexity in a wide range of ecosystems and scales of observation (reviewed in Kovalenko et al., 2012). For instance, structural heterogeneity generated by macrophytes reduced the predation of invertebrates by a Neotropical fish (Padial et al., 2009) and the protection of algal biomass on substrates exposed to Campostoma anomalum Rafinesque was positively related to decreasing crevice size (Bergey & Weaver, 2004). Moreover, previous studies conducted in the same system and using the same types of substrates as the present study, found greater algal richness and assemblage persistence on rough than on smooth substrates and suggested that the most plausible mechanism to explain these results is the presence of refuges (Schneck et al., 2011, Schneck & Melo, 2013).
It is worth noting that our experiment excluded only large organisms, such as fish that prey on invertebrates, but did not exclude grazing insects. Grazing insects can contribute to the removal of large amounts of algal biomass. For instance, Barbee (2005) excluded only insects and found that insects removed 30% more algal biomass in the control than in the exclusion treatments, while common grazing-fish species did not contribute to differences between treatments. Also, invertebrate grazers could have benefited from the exclusion of predatory fish, thereby removing a larger amount of biomass and decreasing the difference between the exclusion and control treatments in our study. Similar trophic cascades have been suggested to occur in some Neotropical streams, with shrimp inhibiting the activity of grazing baetid mayflies (Moulton et al., 2004), and fish negatively affecting shrimp and grazing baetid mayflies (Moulton et al., 2010). Unfortunately, we lack quantitative data on invertebrates to test this hypothesis, but it was evident during the experiment that larvae of caddisflies and midges were more abundant in the exclusion than in the control treatments (F. Schneck, personal observation).
The only significant effect observed for mesohabitat was its positive interaction with substrate roughness in the accrual of total dry mass, reflecting the synergistic effect of higher rates of sediment deposition in pools (Allan & Castillo, 2007) and the capacity of rough substrates to retain greater amounts of organic matter and sediment than smooth substrates (e.g., Taniguchi & Tokeshi, 2004). Most of this mass accumulated on rough substrates in pools was inorganic sediment (the difference between total dry mass and AFDM), which could have imposed negative impacts on algal biomass. However, mesohabitat type had no effect on the accrual of algal biomass, as shown by the results for AFDM and chlorophyll a.
Our hypothesis that different environmental conditions of pools and riffles would mediate the strength of the interaction between grazers and periphyton was not supported, as shown by the non-significant interaction terms between mesohabitat type and grazers for all response variables. This result was unexpected, since pools and riffles have different environmental conditions that would be expected to influence the performance of grazers, and consequently regulate ecological processes such as sediment and biomass accrual (e.g., Flecker, 1997). However, a plausible explanation for this result is that different species of grazing fish may have preference for pool or riffle habitats, so that there are grazing fish occurring at both pools and riffles. The occurrence of habitat specialists among grazing fish was already shown by Buck & Sazima (1995) who found that different species of armored catfishes occupied from shallow and slow-current to deep and fast-current habitats in a stream in southeastern Brazil. The absence of grazer-induced difference in periphyton mass between mesohabitat types may thus be reflecting similar grazing pressure between habitats.
Our results showed that substrate roughness and grazing fish are key ecological factors in this subtropical stream, determining the accrual and loss of sediment, organic material, and algal biomass. Mesohabitat plays a minor role, affecting sediment accrual, but not other periphyton metrics. The independent and interacting effects of these three factors are not simple, and even subtle changes in biotic interactions and in habitat heterogeneity (here measured at the mesohabitat and substrate roughness scales) may have strong impacts on ecosystem properties. For instance, the loss of large grazers may lead to the accumulation of organic and inorganic mass, which may affect the structure of habitats. On the other hand, the simplification of habitats, even at the small scale of substrate roughness, may decrease the availability of food resources by slowing the rate of algal biomass accrual and retention of organic matter. Therefore, understanding the effects of these key factors may have important implications for management and conservation efforts.
References
Abe, S., K. Uchida, T. Nagumo & J. Tanaka, 2007. Alterations in the biomass-specific productivity of periphyton assemblages mediated by fish grazing. Freshwater Biology 52: 1486–1493.
Allan, J. D. & M. M. Castillo, 2007. Stream Ecology, 2nd ed. Springer, Dordrecht.
Álvarez, M. & B. L. Peckarsky, 2005. How do grazers affect periphyton heterogeneity in streams? Oecologia 142: 576–587.
Barbee, N., 2005. Grazing insects reduce algal biomass in a neotropical stream. Hydrobiologia 532: 153–165.
Behling, H., 2002. South and southeast Brazilian grasslands during Late Quaternary times: a synthesis. Palaeogeography, Palaeoclimatology, Palaeoecology 177: 19–27.
Bergey, E. A., 1999. Crevices as refugia for stream diatoms: effect of crevice size on abraded substrates. Limnology and Oceanography 44: 1522–1529.
Bergey, E. A., 2005. How protective are refuges? Quantifying algal protection in rock crevices. Freshwater Biology 50: 1163–1177.
Bergey, E. A. & J. E. Weaver, 2004. The influence of crevice size on the protection of epilithic algae from grazers. Freshwater Biology 49: 1014–1025.
Bertrand, K. N. & K. B. Gido, 2007. Effects of the herbivorous minnow, southern redbelly dace (Phoxinus erythrogaster), on stream productivity and ecosystem structure. Oecologia 151: 69–81.
Biggs, B. J. F., 1996. Patterns in benthic algae of streams. In Stevenson, J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Elsevier, San Diego: 31–56.
Biggs, B. J. F. & C. Kilroy, 2000. Stream Periphyton Monitoring Manual. National Institute of Water and Atmospheric Research, Christchurch.
Bowen, S. H., 1983. Detritivory in Neotropical fish communities. Environmental Biology of Fishes 9: 137–144.
Buck, S. & I. Sazima, 1995. An assemblage of mailed catfishes (Loricariidae) in southeastern Brazil: distribution, activity, and feeding. Ichthyological Exploration of Freshwaters 6: 325–332.
Buckup, L., A. A. P. Bueno, G. Bond-Buckup, M. Casagrande & F. Majolo, 2007. The benthic macroinvertebrate fauna of highland streams in southern Brazil: composition, diversity and structure. Revista Brasileira de Zoologia 24: 294–301.
Cardinale, B. J., M. A. Palmer, C. M. Swan, S. Brooks & N. L. Poff, 2002. The influence of substrate heterogeneity on biofilm metabolism in a stream ecosystem. Ecology 83: 412–422.
Cross, W. F., A. Ramírez, A. Santana & L. Silvestrini-Santiago, 2008. Toward quantifying the relative importance of invertebrate consumption and bioturbation in Puerto Rican streams. Biotropica 40: 477–484.
Dias, T. S. & C. B. Fialho, 2011. Comparative dietary analysis of Eurycheilichthys pantherinus and Pareiorhaphis hystrix: two Loricariidae species (Ostariophysi, Siluriformes) from Campos Sulinos biome, southern Brazil. Iheringia, Serie Zoologia 101: 49–55.
Dudley, T. L. & C. M. D’Antonio, 1991. The effects of substrate texture, grazing, and disturbance on macroalgal establishment in streams. Ecology 72: 297–309.
Feminella, J. W. & C. P. Hawkins, 1995. Interactions between stream herbivores and periphyton: a quantitative analysis of past experiments. Journal of the North American Benthological Society 14: 465–509.
Flecker, A. S., 1996. Ecosystem engineering by a dominant detritivore in a diverse tropical stream. Ecology 77: 1845–1854.
Flecker, A. S., 1997. Habitat modification by tropical fishes: environmental heterogeneity and the variability of interaction strength. Journal of the North American Benthological Society 16: 286–295.
Flecker, A. S., B. P. Feifarek & B. W. Taylor, 1999. Ecosystem engineering by a tropical tadpole: density-dependent effects on habitat structure and larval growth rates. Copeia 1999: 495–500.
Jones, C. G., J. H. Lawton & M. Shachak, 1994. Organisms as ecosystem engineers. Oikos 69: 373–386.
Kovalenko, K. E., S. M. Thomaz & D. M. Warfe, 2012. Habitat complexity: approaches and future directions. Hydrobiologia 685: 1–17.
Lakatos, G., 1989. Composition of reed periphyton (biotecton) in the Hungarian part of Lake Fertö. BFB-Bericht 71: 125–134.
Landeiro, V. L., N. Hamada & A. S. Melo, 2008. Responses of aquatic invertebrate assemblages and leaf breakdown to macroconsumer exclusion in Amazonian “terra firme” streams. Fundamental and Applied Limnology 172: 49–58.
Lowe, R. L. & Y. Pan, 1996. Benthic algal communities as biological monitors. In Stevenson, J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Elsevier, San Diego: 705–739.
Moulton, T. P., M. L. Souza, R. M. L. Silveira & F. A. M. Krsulovic, 2004. Effects of ephemeropterans and shrimps on periphyton and sediments in a coastal stream (Atlantic forest, Rio de Janeiro, Brazil). Journal of the North American Benthological Society 23: 868–881.
Moulton, T. P., M. L. Souza, R. M. L. Silveira, F. A. M. Krsulovic, M. P. Silveira, J. C. F. Assis & C. N. Francischetti, 2010. Patterns of periphyton are determined by cascading trophic relationships in two neotropical streams. Marine and Freshwater Research 61: 57–64.
Moulton, T. P., M. L. Souza, E. F. Brito, M. R. A. Braga & S. E. Bunn, 2012. Strong interactions of Paratya australiensis (Decapoda: Atyidae) on periphyton. Marine and Freshwater Research 63: 834–844.
Murdock, J. N. & W. K. Dodds, 2007. Linking benthic algal biomass to stream substratum topography. Journal of Phycology 43: 449–460.
Opsahl, R. W., T. Wellnitz & N. L. Poff, 2003. Current velocity and invertebrate grazing regulate stream algae: results of an in situ electrical exclusion. Hydrobiologia 499: 135–145.
Padial, A. A., S. M. Thomaz & A. A. Agostinho, 2009. Effects of structural heterogeneity provided by the floating macrophyte Eichhornia azurea on the predation efficiency and habitat use of the small Neotropical fish Moenkhausia sanctaefilomenae. Hydrobiologia 624: 161–170.
Poff, N. L. & J. V. Ward, 1995. Herbivory under different flow regimes: a field experiment and test of a model with a benthic stream insect. Oikos 71: 179–188.
Power, M. E., 1990. Resource enhancement by indirect effects of grazers: armored catfish, algae, and sediment. Ecology 71: 897–904.
Power, M. E., W. J. Matthews & A. J. Stewart, 1985. Grazing minnows, piscivorous bass, and stream algae: dynamics of a strong interaction. Ecology 66: 1448–1456.
Pringle, C. M. & G. A. Blake, 1994. Quantitative effects of atyid shrimp (Decapoda: Atyidae) on the depositional environment in a tropical stream: use of electricity for experimental exclusion. Canadian Journal of Fisheries and Aquatic Sciences 51: 1443–1450.
Quintans, F., F. Scasso, M. Loureiro & A. Yafe, 2009. Diet of Cnesterodon decemmaculatus (Poeciliidae) and Jenynsia multidentata (anablepidae) in a hypertrophic shallow lake of Uruguay. Iheringia, Serie Zoologia 99: 99–105.
Rahel, F. J., 2000. Homogenization of fish faunas across the United States. Science 288: 854–856.
Ranvestel, A. W., K. R. Lips, C. M. Pringle, M. R. Whiles & R. J. Bixby, 2004. Neotropical tadpoles influence stream benthos: evidence for the ecological consequences of decline in amphibian populations. Freshwater Biology 49: 274–285.
Schneck, F. & A. S. Melo, 2013. High assemblage persistence in heterogeneous habitats: an experimental test with stream benthic algae. Freshwater Biology 58: 365–371.
Schneck, F., A. Schwarzbold & A. S. Melo, 2011. Substrate roughness affects stream benthic algal diversity, assemblage composition, and nestedness. Journal of the North American Benthological Society 30: 1049–1056.
Schwarzbold, A., 1990. Métodos ecológicos aplicados ao estudo do perifíton. Acta Limnologica Brasiliensia 3: 545–592.
Souza, M. L. & T. P. Moulton, 2005. The effect of shrimp on benthic material in a Brazilian island stream. Freshwater Biology 50: 592–602.
Steinman, A. D., 1996. Effects of grazers on freshwater benthic algae. In Stevenson, R. J., M. L. Bothwell & R. L. Lowe (eds), Algal Ecology: Freshwater Benthic Ecosystems. Elsevier, San Diego: 341–373.
Steinman, A. D., G. A. Lamberti & P. R. Leavitt, 2007. Biomass and pigments of benthic algae. In Hauer, R. & G. A. Lamberti (eds), Methods in Stream Ecology, 2nd ed. Elsevier, San Diego: 357–379.
Taniguchi, H. & M. Tokeshi, 2004. Effects of habitat complexity on benthic assemblages in a variable environment. Freshwater Biology 49: 1164–1178.
R Development Core Team, 2010. R. A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. ISBN 3-900051-07-0. URL http://www.R-project.org/.
Winckler-Sosinski, L. T., A. Schwarzbold & U. H. Schultz, 2009. Fish assemblage structure in altitude rivers under the effect of exotic species introduction, northeast of Rio Grande do Sul, Brazil. Acta Limnologica Brasiliensia 21: 473–482.
Acknowledgments
We are grateful to Ana Pressi, Flávia Montagner, Geraldo Schneck, Marcelo Saraiva, Silvia Milesi, and Marlon Vasconcelos for invaluable field assistance, and to Victor Landeiro for suggestions regarding the construction of electrical fences. Sidinei M. Thomaz, Leandro Duarte, Luciane Crossetti, and two anonymous referees provided useful comments on the manuscript. FS received a student fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). ASM received research grants (476304/2007-5; 474560/2009-0) and a research fellowship (302482/2008-3) from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Katya E. Kovalenko
Rights and permissions
About this article
Cite this article
Schneck, F., Schwarzbold, A. & Melo, A.S. Substrate roughness, fish grazers, and mesohabitat type interact to determine algal biomass and sediment accrual in a high-altitude subtropical stream. Hydrobiologia 711, 165–173 (2013). https://doi.org/10.1007/s10750-013-1477-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-013-1477-x