Abstract
The expected response of fluvial biofilms to the environment and metal pollution prevailing under different discharge conditions was investigated. The relationship between inter-annual hydrological variability and metal concentration in water and sediments was explored in Mediterranean rivers (Catalonia, NE Spain) affected by low but chronic metal pollution, using monitoring data provided by the Catalan Water Agency (ACA). During the period investigated (2000–2006), metal pollution was characterized by low water concentrations and high concentrations in sediments. The most consistent pattern was observed for sediment cadmium (Cd) concentrations, showing a positive relationship with annual discharge, reaching values of environmental concern (above ecotoxicological benchmarks). A different pattern was observed for Cu, Zn, and As increasing with flow in some sites and decreasing in others. While Cd seems to proceed from diffuse sources being washed by surface runoff, Zn, Pb, and As may proceed from either diffuse or point-sources in the different river sites investigated. The relevance of diffuse metal pollution in the area of study indicates that polluted landfills runoff might be an important source of metals causing repetitive pulses of high metal concentration in the receiving water courses. The experimental results presented demonstrate that metal effects in fluvial biofilms may be accumulative, increasing the toxicity after repetitive pulse exposures. Since draughts and extreme rain events are expected to increase at higher latitudes due to global change, the sources of metal pollution, its final concentration and potential effects on the fluvial ecosystem may also change following the patterns expected for human-impacted Mediterranean rivers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Human activity is one of the major causes of elevated concentrations of metals in fluvial ecosystems causing a great concern over potential toxicity and trophic transfer. Metal concentrations are very variable in time and space depending on the source of pollution (diffuse or point-source), the hydrological regime and the processes affecting their transfer from the water phase to other compartments. Furthermore, it is expected that below average surface flow conditions, will also change the fate and effects of metals at ecosystem scales (Guasch et al., 2009b). Based on recent modeling of the fate and transport of metals in streams (Caruso et al., 2008), higher metal concentrations are predicted during low flow (mostly for Zn). This tendency is common in mining areas where metal inputs come from groundwater. In these cases, metal concentration is lowest at highest discharges due to dilution (Audry et al., 2004; Bambic et al., 2006; Armitage et al., 2007). The influence of flow in sites affected by low but chronic metal pollution (i.e., influenced by urban and agricultural activities) has been poorly investigated (i.e., Brown & Peake, 2006). While the differences in metal concentration between the dry and rainy season described by Fianko et al. (2007) were attributed to dilution, other studies report no linkage between discharge and metal pollution (Benson & Etesin, 2008).
The duration and frequency of increased metal concentration episodes is of great relevance since metal bioaccumulation and toxicity are strongly influenced by the time and frequency of exposure (Meylan et al., 2003). The expected linkages between fluvial hydrology and metal exposure of fluvial biofilm communities were addressed in Guasch et al. (2009b). It was indicated that point-sources of metal pollution might cause chronic and variable metal exposure depending on flow conditions (of lower concentration under high-flow conditions due to dilution). On the other hand, diffuse sources of metal pollution such as the urban runoff would cause intermittent metal exposures directly linked with rainfall episodes. It was also concluded that chronic exposure would lead to community adaptation and a decrease in sensitivity, whereas metal toxicity would be maximum if a non-adapted community was suddenly exposed to peak metal concentrations. Community responses to intermittent metal exposures were not directly addressed in this review article.
Reinert et al. (2002) indicate that the long-term effects on non-target organisms of intermittent pesticide exposure can be a function of the damage sustained during exposure, the capacity of the organisms to recover, and the duration of the recovery period between pulses. Furthermore, sequential exposure can lead to an increased or decreased effect during each subsequent exposure, depending on the mode of action of the toxicant, the resilience of the biotic community, and its adaptation capacity (Macinnis-Ng & Ralph, 2002; Hoang & Klaine, 2008). Results of previous studies (Serra et al., 2009), demonstrated that Cu accumulation kinetics and toxicity differed among fluvial biofilms with different Cu-exposure history. Biofilms that had been continuously exposed to Cu, differed from those unexposed and also from those exposed to short Cu-pulses in their species composition (due to the replacement of sensitive algal classes by tolerant ones) and also on their metal content, several orders of magnitude higher. The non pre-exposed and Cu-pulsed communities were more sensitive to Cu than the chronically exposed community showing a slight decrease in the photosynthetic efficiency after the short exposure to higher Cu concentration (Serra et al., 2009). In this study, photosynthesis inhibition was slightly higher in the Cu-pulsed than in the non pre-exposed community indicating that the pulses may enhance Cu toxicity.
Several authors (Pinto et al., 2003; Torres et al., 2008) have proposed the study of “signals of distress” at molecular level as toxicity biomarkers. Studies performed on algal cultures have already shown the sensibility of several enzymatic biomarkers to the presence metals (Tripathi et al., 2006). Antioxidant enzyme activities (AEA) such as superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), and glutathione S-transferase (GST) activity are of great importance in oxidative stress to cope with free radicals that lead to several disturbances (Geoffroy et al., 2004). Elevated Cu levels induce oxidative stress by generating reactive oxygen species (ROS), such as hydrogen peroxide, superoxide radical, singlet oxygen, and hydroxyl radical, via Haber–Weiss as well as Fenton reactions that can oxidize proteins, lipids and nucleic acids. This often leads to the cell structure being damaged or even cell death (Tripathi & Gaur, 2004; Dewez et al., 2005). AEA characterization may provide early warning systems of detection of toxicity on autotrophic communities at lower exposure time and/or dose than other classical endpoints such as photosynthesis or algal growth (Sabater et al., 2007).
The main objective of this article is to present the expected response of the biota (fluvial biofilms) to the metal pollution prevailing under different discharge conditions. In order to reach this objective the following specific objectives were addressed:
-
1.
To explore the relationship between inter-annual hydrological variability and metal concentration in water and sediments in Mediterranean watersheds (Catalonia, NE Spain) affected by low but chronic metal pollution.
-
2.
To present a specific example dealing with the response of fluvial biofilms to repetitive Cu-pulses using AEA as early warning systems of toxicity detection.
-
3.
To provide a conceptual framework about the influence of temporal dynamics of metal exposure on metal accumulation and toxicity in fluvial biofilms.
In order to reach the first specific objective, monitoring data provided by the Catalan Water Agency (ACA) were selected in order to cover a large environmental gradient and temporal scale (from 2000 to 2006).
In an experimental study, changes in the defense capacity of biofilms were used to evaluate the effects of several pulses of Cu. This complemented a series of investigations dealing with the significance of the time of exposure on the fate and effects of Cu on fluvial biofilms (Serra & Guasch, 2009; Serra et al., 2009).
Materials and methods
Monitoring
Nine sites located in seven watersheds were included in this study (Table 1). The physical and chemical characterization including nutrient content, water metal concentration, sediment metal concentration, and flow was performed at each site. Furthermore, the Catalan River basins were characterized according to their typology and their impact degree. Since sediment metal concentration was analyzed once a year, average or integrated annual values of the rest of variable were included for comparison. Temporal variability was based on the inter-annual variability over 7 years (2000–2006). Conductivity was determinate by electrical conductivity (ISO 7888:1985), pH by continuous instrument, dissolved oxygen by electrochemical probe method (ISO 5814-1990). Ammonium by flow analysis (continuous flow and flow injection analysis) and spectrometric detection (ISO 11732:2005) and phosphate was determinate by ammonium molybdate spectrometric method (ISO 6878:2004). Metal concentration in water was analyzed by inductively coupled plasma mass spectrometry (ICP-MS) and metal concentration in sediments was determinate by inductively coupled plasma atomic emission spectroscopy (ICP-AS). Flow was obtained from the Catalan network of gauging stations.
Statistical analysis
Non parametric correlation analysis (Spearman’s rho) was carried out between the relative flow recorded at each sampling station and the metal concentration in water and sediment. The environmental variables (physical, chemical and heavy metal concentration in water and sediments) along the study period were obtained from the Catalan Water Agency (ACA). Some of the variables available were selected on the basis of their relevance for the distribution and abundance of the biofilm community. The variables used were copper, lead, cadmium, arsenic, mercury, chromium, nickel, and zinc concentration in the sediment, and zinc in the water. As metal content and dynamics were likely to be substantially affected by time, the year in which the data were collected was included as independent variables in partial correlation analyses to describe the linear relationship between the variables while controlling for the effects of temporal variations in the metal content in the streams.
Significant differences in metal concentration in streams between flow conditions were examined using one way ANOVA (Winer, 1971). Two possible flow conditions were considered as factors in ANOVA, either high or low flow. Each site was classified in either of both classes according to their deviation from the standard condition. Deviations were calculated as anomalies by subtracting the mean from each observation, then dividing by the standard deviation. The contribution of spatial and temporal random effects to the variance of the sediment metal concentration was assessed by variance components procedure. Maximum likelihood (ML) method was used in the Variance Components procedure. The maximum likelihood method accounts for the temporal and spatial dependence of the observations that characterize our dataset. Using the Variance Components procedure, the year effect’s contribution to the random variation in a given variable can be estimated. Multiple comparisons between means were analyzed in all the cases with a Tukey HSD (Honest significant difference) test (Winer, 1971). Statistical analyses were performed using SPSS for Windows (version 13.0; SPSS Inc., Illinois).
Influence of Cu pulses on the structure and function of biofilms
Fluvial biofilms were cultivated and exposed to copper under controlled conditions. The experiments were carried out in an indoor channel system consisting of six Perspex channels (each 170 cm long and 9 cm wide) as described in Serra et al. (2009). Briefly, water was supplied from 10 l carboys located at the end of each channel and was recirculated at a rate of 1 l min−1 through centrifuge pumps. Light was provided by halogen lamps (80–100 μmol m −2 s−1) with a 12 h light/12 h dark cycle and the temperature was kept between 19 and 20°C using a cooling bath. Two consecutive colonization experiments were performed: one with no copper added (No-Cu); and the other with several Cu pulses (referred to as Cu-pulsed). In each colonization experiment, biofilms were allowed to colonize the surface of etched glass substrata (8.5 * 12 cm) placed at the bottom of each channel. In the No-Cu colonization treatment, no copper was added during the whole colonization period (5 weeks). In the Cu-pulsed colonization treatment, biofilms were exposed to three pulses of 20 μg/l Cu (nominal concentration) on the 5th week of colonization. Each pulse lasted for 2.5 h. After each pulse, water from the system was replaced by water without Cu.
At the end of each colonization period, three glass substrata were removed at random from three different channels to characterize the chlorophyll fluorescence of the different algal classes composing the biofilm communities by means of a Phyto-PAM chlorophyll fluorometer (Heinz Walz, Effeltrich, Germany).
These mature communities were thereafter exposed to higher Cu concentration to assess the influence of Cu pre-exposure on Cu sensitivity. Three channels were exposed for 24 h to 100 μg/l Cu (nominal concentration) and the other three channels were used as controls (maintained without Cu in the water) following the procedures described in Serra et al. (2009). In order to follow accumulative effects, biofilms were collected at time 0 and after 6 and 24 h of exposure to study antioxidant enzyme activities. Cu accumulation, the algal biomass (Fo), and quantum yield (effective and optimal quantum yield) were also analyzed following the procedures described in Serra et al. (2009). All fluorescence measurements were performed using the Phyto-PAM. It employs an array of light-emitting diodes (LED) to excite chlorophyll fluorescence at different measuring lights (470, 520, 645, and 665 nm), and to illuminate samples with actinic light and saturation pulses. The deconvolution of the overall fluorescence signal into the contributions of three algal groups is based on the internal ‘reference excitation spectra’ of a pure culture (Schmitt-Jansen & Altenburger, 2008). The differences in pigment composition of the antenna complexes of photosystem II can be determined because the shapes of the excitation spectra depend on the spectra of three algal groups (Ruser et al., 1999). Reference spectra which have previously been validated for periphyton communities were used (Schmitt-Jansen & Altenburger, 2008). The fluorescence linked to cyanobacteria, referred to as F(Bl), the fluorescence linked to green algae, referred to as F(Gr) and the fluorescence linked to diatoms, referred to as F(Br), were used for evaluating the relative contribution (in percentage) of each algal class to the whole community. The measurements of in vivo chlorophyll fluorescence of PSII were used to estimate F, which corresponds to the steady-state fluorescence in the given actinic irradiance, and F’m, which refers to the maximum fluorescence yield of an actinic-adapted sample. These two parameters were used to calculate the effective and optimal quantum yield (Y) according to Genty et al. (1989). In our study, Y was based on the fluorescence obtained with 665 nm light-emitting diode (F4). Y measurements were used to follow changes in the photosynthetic efficiency of the communities after Cu exposure. The measurements were performed at room temperature (20°C). Saturation pulses were applied in the same actinic light conditions as the ones used for periphyton colonization.
Effective quantum yield (eff QY) is taken as a measure of the photosynthetic efficiency of the community. Yield inhibition indicates that the toxicant is reducing electron flow in the PSII. The optimal quantum yield (opt QY) provides information about the maximum electron flow. It is a potential estimate and is expected to change after longer exposures if the treatment produces alterations in the photosynthetic apparatus (i.e., shade-adapted chloroplasts). Basal fluorescence (Fo) may increase if the toxicant induces fluorescence production (i.e., in the case of herbicides blocking electron flow). In most cases, however, Fo is used to estimate algal biomass since chlorophyll fluorescence is proportional to total chlorophyll content. Therefore, it is expected that Fo will decrease if the treatment causes a reduction in the number of cells due to cell death (structural damage).
Antioxidant enzyme activities
Biofilms were removed from the glass substrata with a cell scraper and centrifuged at 2,300g for 5 min (+10°C) to remove the excess water. The pellets were frozen immediately in liquid nitrogen and stored at −80°C until the enzyme assays. Samples were homogenized for 3 min on ice by adding 2.5 ml of homogenization buffer, containing 100 mM potassium phosphate buffer (pH 7.4), 100 mM KCl, 1 mM EDTA and 10% (w/v) PPVP (Polyvinylpolypyrrolidone), to the pellet. Homogenates were then centrifuged at 10,000g for 30 min at +4°C and the supernatants were used as the enzyme source. The protein contents of the supernatants were determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Catalase activity was measured spectrophotometrically at 240 nm according to Aebi (1984). 750 μl of reaction mixture was contained in a final concentration of 80 mM potassium phosphate buffer (pH 7.0), 20 mM H2O2 and enzyme extract (~60 μg protein). The optimum substrate concentration and protein content of enzyme extract were determined by using 5.0, 10, 15, 20, and 25 mM H2O2 concentrations and approximately 10, 30, 35, and 60 μg protein in the test media, respectively. The decomposition of CAT was determined by measuring the decrease in absorbance at 25°C for 1–5 min during optimization procedures. Enzymatic activity was measured after monitoring for 1 min, at the end of which linearity was shown. CAT activity was calculated as μmol H2O2/mg protein/min.
Ascorbate peroxidase activity was assessed by monitoring the decrease in absorbance at 290 nm at 25°C for 2 min due to ascorbate oxidation according to Nakano & Asada (1981). Test medium was contained in a final concentration of 80 mM phosphate buffer (pH 7.0), 0.3 mM Na-Ascorbate, 0.5 mM H2O2 and enzyme extract (~65 μg protein) in a final volume of 1 ml. The optimum Na-Ascorbate, H2O2 concentration and protein content of enzyme extract were determined by using 0.1, 0.2, 0.3, 0.5 and 0.1, 0.2, 0.3, 0.5 and approximately 10, 25, 30, 45 μg protein in the test media, respectively. APX activity was calculated as μmol Ascorbate/mg protein/min.
Superoxide dismutase activity was measured by the indirect method involving the inhibition of cytochrome c reduction, which SOD competes with for superoxide radicals, generated by the hypoxanthine/xanthine oxidase system at 550 nm for 1 min (McCord & Fridovich, 1969). The reaction buffer contained 50 mM potassium phosphate buffer (pH 7.8), 0.1 mM EDTA, 10 mM cytochrome c, 0.05 mM hypoxanthine, and enzyme extract (~25 μg protein). The reaction was started by adding 1.87 mU/ml xanthine oxidase in a final volume of 1 ml, which gives a 0.02 absorbance increase at 550 nm. A unit of SOD activity was defined as the amount of enzyme that causes 50% inhibition of cytochrome c reduction and was given as unit/mg protein.
Glutathione S-transferase activity was evaluated by the absorbance increase at 340 nm due to conjugation of reduced glutathione (GSH) and CDNB (1-chloro-2,4-dinitrobenzene) (Habig et al., 1974). The reaction buffer contained 100 mM potassium phosphate buffer (pH 7.4), 1 mM GSH, 1 mM CDNB, and enzyme extract (~250 μg protein) in a final volume of 1 ml. GST activity was calculated as nmol/min/mg protein.
Catalase activity was measured in biofilms for the No-Cu experiment and CAT, APX, SOD, and GST for the Cu-pulsed experiment.
Statistical analysis
One way ANOVA test was used to examine differences in the community structure between the no-Cu and the Cu-pulsed treatments and also to compare photosynthesis, and AEA between controls, and Cu exposed communities during acute exposure (100 μg/l Cu). Statistical analyses were performed using SPSS for Windows (version 13.0; SPSS Inc., Illinois).
Results
Monitoring
The nine selected sites include karsts fed rivers, lowland Mediterranean rivers, and large watercourses from intermediate to high degree of impact (Table 1). Average water discharge values were between 1 and 10 m3/s, ranging from 0 to 65 m3/s (Table 2). Total Organic Carbon (TOC) ranged from low (1 mg/l) to high (20 mg/l) concentration. Dissolved metal concentration was in general low and below detection limits in most cases (data not shown) except for Zn concentration ranging between 13 and 201 μg/l. Sediment metal concentrations (in μg/g) were well above background concentrations and above ecotoxicological benchmarks in many cases (Table 2).
Comparing flow categories, the most consistent pattern was shown by Cd in sediments increasing at higher flow (Table 3). Comparing sites, the most consistent pattern between metals was found in Besós-b (Montornès del Vallès) and Francolí (20–86% and 22–42% increase with water flow, respectively). On the other hand, a negative relationship between flow and metal contents (with the exception of Cd) was found in Besós-a (Montcada i Reixac), 21–39% higher in the lower flow category.
Overall, Cd in sediment varied significantly (P < 0.001) across the river flow condition classes. The low flow condition class shows an average Cd concentration in sediment of 0.98 μg/g ranging from 0.41 to 2.31 μg/g while sites within the high flow condition class have Cd concentrations between 0.51 and 4.13 μg/g with mean = 1.57. A significant correlation between Cd sediment values and flow was obtained when temporal variation among sample collection was controlled for (i.e., variables were treated as covariables in partial correlation analyses). The contribution of the different components evaluated to total random variance was 0.128 due to temporal variation (years), 0.003 explained by spatial variation (basin) and 0.007 due to error.
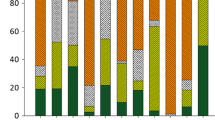
The comparison of Cd concentrations over time shows that Cd values in sediment peaked during 2003 in most of the rivers (Fig. 1a) although no clear correspondence appears to exist between water discharge and Cd sediment concentratrions (Fig. 1b). Another simple way of looking at the relationship between flow and heavy metal concentrations is by grouping water discharge into different classes (Fig. 1c). After grouping water discharge into different classes it can, however, be observed that Cd content in sediment is higher in high flow conditions (Fig. 1c).
Average annual Cd sediment concentrations in the different study rivers (a); average annual relative water discharge for all study rivers (b); and average annual values for all study rivers grouped in discharge classes (c). The water discharge classes were calculated by determining an “upper anchor” and “lower anchor” (the upper and lower 10th percentile) for the average relative annual flow and in doing so the width of the high low water discharge band. The width of the remaining classes was calculated by dividing the interval between the upper and lower anchors equally
Influence of Cu pulses on the structure and function of biofilms
The exposure of mature biofilms to 20 μg/L of Cu for 2.5 h did not alter the effective quantum yield (Table 4). Furthermore, at the end of the three consecutive pulses (Cu-pulsed community), algal biomass (based on basal fluorescence, Fo), and the community composition (in terms of the percentage of fluorescence of different algal groups) was similar to the non pre-exposed community (no-Cu) (Table 5).
The effects caused by the acute exposure (100 μg/l Cu for 24 h) differed between the no-Cu and the Cu-pulsed communities (Table 6). Effects on photosynthesis (in terms of effective quantum yield) and photosynthetic capacity (in terms of optimal quantum yield) were similar, but the AEA showed a different pattern. While CAT showed a slight increase in the non pre-exposed community (no-Cu), it showed a marked reduction in the Cu-pulsed one (Table 6). SOD and GST were also inhibited after 24 h of exposure (Fig. 2).
Plots of AEA: catalase (CAT); ascorbate peroxidase (APX); superoxide dismutase (SOD); and gutation-S-transferase (GST) versus Cu contents in biofilms after acute Cu exposure on biofilms non pre-exposed (No-Cu) and biofilms pre-exposed to three consecutive short pulses of 20 μg/l Cu (Cu-pulsed). The regression curves (linear for CAT and SOD and exponential decay for GST) and the corresponding regression coefficients and probabilities are also indicated
Discussion
Monitoring
Dissolved metal concentration was low and below detection limits in many cases but sediment metal concentration (in μg/g) was in general high. Sediment metal concentrations were within the values obtained in river sediments which range 0.073–9.5 (Cd); 19.7–72.5 (Cr); 12–131 (Cu); 15–150 (Pb); and 37–303 (Zn) (Huang & Lin, 2003; Jain, 2004; Adamo et al. 2005; Demirak et al., 2006). Cd, Pb, Zn, and Cu values were characteristic of human-impacted rivers (Peplow & Edmonds, 2005; Demirak et al., 2006) well above background concentrations. Previous study has shown that the relationship between metal loads and diatom species composition can be very poor probably due to the fact that metal concentration in the water was a bad indicator of metal availability (Guasch et al., 2009a). Metal contents in the water phase represented 9.8% of variation in diatom species composition, including only the most polluted sites: lower part of the Besós and Francolí rivers, both affected by urban, industrial, and agricultural activities. In the current study, sediment metal pollution was remarkable in these sites, but also in the lower part of the Llobregat and Tordera rivers where Cd, Cu, Pb, Ni, and Zn concentrations was also high, above ecotoxicological benchmarks in many occasions.
Focusing on temporal variability, the year effect appeared to account for the majority of the variation of the sediment content of cadmium. In fact, temporal variation explained 93% of total random variance, while spatial distribution of the sampling sites and error explained 2 and 5%, respectively. The comparison of Cd concentrations over time shows that Cd values in sediment peaked during 2003 in most of the rivers followed by a subsequent decrease over the next few years (Fig. 1). Remarkably, the river Tordera was the most polluted river in 2003. The average relative water discharge at river Tordera has increased since 2000 showing a sharp decrease since peak values in 2003 (Fig. 1). Although most of the river basins studied are characterized by an increase in sediment Cd concentration coincident with high discharge during 2003, Cd values in sediment do not behave in a predictive manner according to flow in all of them. Precisely, sediment Cd values did not differ under different water discharge conditions within a same basin. For example, sediment Cd concentrations at Francolí were high in 2003 when mean discharge was also above average while Cd values drop to a minimum in 2005 coinciding with the highest discharge for the study period (Fig. 1). However, overall, Cd content in sediment increases as water discharge rises (Fig. 1c). A different pattern was observed for Cu, Zn, and As increasing with flow in some sites and decreasing in others. The differences observed in the total measured heavy metal concentrations can be a consequence of the relative contribution of the different pathways of metal pollution. The relationship between concentration of a substance and discharge exhibits a non-linear dependency. Under this framework the low flow conditions reflect the dominance of point sources from base flow. At higher flow conditions it seems that the input from diffuse sources dominates the transport. While Cd seems to proceed from diffuse sources being washed by surface runoff, Zn, Pb, and As may proceed from either diffuse or point-sources in the different river sites investigated. Although sediment sampling frequency was not optimal (only once a year), sediment characteristics are far more conservative than water metal concentrations and allow an insight within inter-annual variability. Temporal patterns occurring at shorter time scales may also take place. A more frequent sampling is required for a better characterization of metal pollution patterns.
Influence of Cu pulses on the structure and function of biofilms
Cu pulses were not toxic but caused a progressive Cu accumulation increasing the sensitivity of the Cu-pulsed community in comparison with the non pre-exposed one. Effects on photosynthesis (in terms of effective quantum yield) were similar, but the AEA showed a different pattern. While CAT was activated in the non pre-exposed community (no-Cu), it was clearly inhibited in the Cu-pulsed one. In this later case, SOD and GST were also inhibited indicating that toxicity exceeded the antioxidant cell defenses. The response variability of the antioxidant enzymes may be related to several factors, such as ROS production due to toxic effects of Cu, which can be dependent on the specific toxicity at different cellular sites, organelle localizations of these enzymes, metal concentration and exposure duration (Sauser et al., 1997; Pinto et al., 2003; Li et al., 2006). Decreased CAT and GST activities may be related to the direct binding of Cu on the sulfhydryl groups of the enzyme or elevated levels of ROS leading to deleterious effects on cell structure. In addition, Cu bioaccumulation may cause GSH depletion due to the high affinity of Cu to GSH, which leads to a decrease in GST activity. GST activity in Fucus sp. was found to be higher in less contaminated regions than in more contaminated regions of the Portuguese Atlantic coast, which is impacted by complex discharges of contaminants such as petroleum derived products as well as industrial and urban effluents (Cairrao et al., 2004). In the microalgae Scenedesmus sp., Cu caused an increase in CAT and APX activity at lower Cu concentrations (up to 5 μM), while at higher concentrations (up to 40 μM) the activities decreased. This was associated with metals binding to sulfhydryl groups on the enzyme or by displacement of an essential element (Tripathi & Gaur, 2004). In conclusion, decreased CAT, SOD, and GST activities appear to be related to high levels of oxidative stress caused by Cu, which is also in agreement with other studies (Sauser et al., 1997; Tripathi & Gaur, 2004; Dewez et al., 2005). It is emphasized that antioxidant responses to toxicants can also depend on the magnitude of stress. Increased antioxidant enzyme activities due to the metal-induced disruption of the oxidative balance can be seen under low dose exposures. However, toxic effects of pollutants may exceed the antioxidant defenses and cell death or damage of cellular mechanisms may follow under acute conditions (Andrade et al., 2006). This is also supported in our data.
The lack of change in the optimal quantum yield (Table 5) indicates that the photosynthetic apparatus was not damaged and that Cu exposure was probably too low to cause cell death in fluvial biofilms after 24 h of exposure. In addition, the effects on the effective quantum yield were slight. In agreement with our results, catalase activity was found to be more sensitive than the photosynthetic activity in detecting the effects of the herbicide flumioxazin (Geoffroy et al., 2004).
Time-varying or repeated exposures can have a variety of consequences. The first pulse may select more tolerant species, causing an apparent lessening in toxic response (Allin & Wilson, 2000). Induced community tolerance was observed by Tlili et al. (2008) as a result of the exposure of fluvial biofilms to consecutive diuron pulses. A clearance interval between consecutive pulses may allow time for the community to recover from exposure by depurating or detoxifying the contaminant. However, if the contaminant is not completely depurated or detoxified between transient pollution events, thus the compound could accumulate and result in a cumulative toxic effect over several doses. In a study of the effects of multiple pulses of Cu and Irgarol on the photosynthesis performance of Zostera, Macinnis-Ng & Ralph (2002) reported a clear recovery after the first pulse, but a clear damage in the photosynthetic apparatus after the second one, indicating cumulative toxic effects.
Organisms experience toxicity when toxicant accumulation exceeds their tolerance. Fluvial biofilms were not apparently damaged during the short exposure durations in our study. However, marked effects on their AEA did occur after the fourth exposure to higher dose and longer duration. This suggests that Cu accumulation exceeded the tolerance of organisms inhabiting the biofilm after repeated exposures. In contrast with the unexposed biofilm, total Cu content in the Cu-pulsed community was above 30 μg Cu/g DW (36.5 on average) after the three short-pulses suggesting that the biofilm was already exposed and that residual biofilm Cu contents increased the sensitivity of the community. This suggestion is supported by Guan & Wang (2004), who reported that more than about 30% of the accumulated trace metals (Cd, Zn, and Se) remained in Daphnia magna after 8 days holding in a clean environment causing retarded lethal effects.
Conceptual model linking temporal variability of metal exposure with fluvial biofilm community responses
The results obtained in our Cu-pulsed experiment contribute to our understanding of the response of fluvial biofilms to metal pulses. Temporal dynamics of total metal concentration in water, the corresponding pattern of variation of biofilm metal concentration and the expected physiological and structural changes are theoretically represented in Fig. 3.
Conceptual figure showing the expected effects of metal exposure episodes of different duration on fluvial biofilms. Changes in total metal concentration in water and the corresponding pattern of variation of biofilm metal concentration (upper panel); expected physiological responses: photosynthesis and antioxidant enzyme activities, AEA (middle pannel) and structural changes: biomass and percentage of sensitive species in the community (lower pannel)
Input to fluvial systems by metals may occur in pulses and by continuous exposure. Biofilm communities exposed to sublethal levels for short periods may lead to progressive internal dosage (bioaccumulation). Once entering the cell, the heavy metal ions may either be detoxified or adversely affect cell processes. Acute exposure may lead to transitory physiological effects. If the community is not adapted, metal exposure is expected to cause oxidative stress, increased activity of the enzymes that may cope with this stress and finally photosynthesis inhibition and toxicity that may damage the cell defense capacity (Fig. 3b). On the other hand, frequent episodes of exposure or chronic exposure may induce an increase in community tolerance driven by the substitution of sensitive species by tolerant ones becoming more resistant to higher concentrations. It is also expected that metal adaptation will be linked to photosynthesis recovery, increase of antioxidant enzyme activities, slight reduction in biomass, and a higher metal accumulation capacity (Fig. 3c).
Conclusions
Diffuse metal pollution is expected to be high in human-impacted areas, such as the densely populated Mediterranean region. In these areas, pollutants may be accumulated in the terrestrial systems due to low rain, and pollution may be linked to rain events that create urban runoff or industrial spill. The experimental results presented demonstrate that metal effects in fluvial biofilms may be accumulative increasing the toxic effects after repetitive pulse exposures. Longer exposure may allow the community to adapt by the replacement of sensitive species by tolerant ones. Community adaptation is also linked with higher bioaccumulation capacity, in agreement with the results obtained in sediment field samples (monitoring data).
Below average surface flow conditions as well as floods are expected to increase at higher latitudes due to global change. Consequently, the sources of metal pollution, its final concentration and potential effects on the fluvial ecosystem may also change following the patterns expected for human-impacted Mediterranean rivers.
References
Adamo, P., M. Arienzo, M. Imperato, D. Naimo, G. Nardi & D. Stanzione, 2005. Distribution and partition of heavy metals in surface and sub-surface sediments of Naples city port. Chemosphere 61: 800–809.
Aebi, H., 1984. Catalase in vitro. Methods in Enzymology 105: 121–176.
Allin, C. J. & R. W. Wilson, 2000. Effects of pre-acclimation to aluminum on the physiology and swimming behavior of juvenile rainbow trout (Oncorhynchus mykiss) during pulsed exposure. Aquatic Toxicology 51: 213–224.
Andrade, S., L. Contreras, J. W. Moffet & J. A. Correa, 2006. Kinetics of copper accumulation in Lissonia nigrescens (Phaeophyceae) under conditions of environmental stress. Aquatic Toxicology 78: 398–401.
Armitage, P. D., M. J. Bowes & H. M. Vincet, 2007. Long-term changes in macroinvertebrate communities of a heavily metal polluted stream: the river Went (Cumbria, U.K.) after 28 years. River Research Applications 23: 997–1015.
Audry, S., G. Blanc & J. Schäfer, 2004. Cadmium transport in the Lot-Garonne River system (France) – temporal variability and a model for flux estimation. The Science of the Total Environment 319: 197–213.
Bambic, D. G., C. N. Alpers, P. G. Green, E. Fanellid & W. K. Silo, 2006. Seasonal and spatial patterns of metals at a restored copper mine site. I. Stream copper and zinc. Environmental Pollution 144: 774–782.
Benson, N. U. & U. M. Etesin, 2008. Metal contamination of surface water, sediment and Tympanotonus fuscatus var. radula of Iko River and environmental impact due to Utapete gas flare station, Nigeria. Environmentalist 28: 195–202.
Brown, J. N. & B. M. Peake, 2006. Sources of heavy metals and polycyclic aromatic hydrocarbons in urban stormwater runoff. Science of the Total Environment 359: 145–155.
Cairrao, E., M. Couderchet, A. M. V. M. Soares & L. Guilhermino, 2004. Glutathione-S-transferase activity of Fucus sp. as a biomarker of environmental contamination. Aquatic Toxicology 70: 277–286.
Caruso, B. S., L. T. J. Cox, R. L. Runkel, M. L. Velleux, K. E. Bencala, D. K. Nordstrom, P. Y. Julien, B. A. Butler, C. N. Alpers, A. Marion & K. S. Smith, 2008. Metals fate and transport modelling in streams and watersheds: state of the science and SEPA workshop review. Hydrological Processes 22: 4011–4021.
Demirak, A., F. Yilmaz, A. L. Tuna & N. Ozdemir, 2006. Heavy metals in water, sediment and tissues of Leuciscus cephalus from a stream in southwestern Turkey. Chemosphere 63: 1451–1458.
Dewez, D., L. Geoffroy, G. Vernet & R. Popovic, 2005. Determination of photosynthetic and enzymatic biomarkers sensitivity used to evaluate toxic effects of copper and fludioxonil in alga Scenedesmus obliquus. Aquatic Toxicology 74: 150–159.
Fianko, J. R., S. Osae, D. Adomako, D. K. Adotey & Y. Serfor-Armah, 2007. Assessment of heavy metal pollution of the Iture Estuary in the central region of Ghana. Environmental Monitoring Assessment 131: 467–473.
Genty, B., J. M. Briantais & N. R. Baker, 1989. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochimica et Biophysica Acta 990: 87–92.
Geoffroy, L., C. Frankart & P. Eullaffroy, 2004. Comparison of different physiological parameter responses in Lemna minor and Scenedesmus obliquus exposed to herbicide flumioxazin. Environmental Pollution 131: 233–241.
Guan, R. & W. X. Wang, 2004. Cd and Zn uptake kinetics in Daphnia magna in relation to exposure history. Environmental Science & Technology 38: 6051–6058.
Guasch, H., M. Leira, B. Montuelle, A. Geiszinger, J. L. Roulier, E. Tornés & A. Serra, 2009a. Use of multivariate analyses to investigate the contribution of metal pollution to diatom species composition. Hydrobiologia 627:143–158.
Guasch, H., A. Serra, N. Corcoll, B. Bonet & M. Leira, 2009b. Metal ecotoxicology in fluvial biofilms: potential influence of water scarcity. In: The Handbook of Environmental Chemistry review series. Springer.
Habig, W. H., M. J. Pabst & W. B. Jakoby, 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. Biological Chemistry 249: 7130–7139.
Hoang, T. C. & S. J. Klaine, 2008. Characterizing the toxicity of pulsed selenium exposure to Daphnia magna. Chemosphere 71: 429–438.
Huang, K.-M. & S. Lin, 2003. Consequences and implication of heavy metal spatial variations in sediments of the Keelung river drainage basin, Taiwan. Chemosphere 53: 1113–1121.
Jain, C. K., 2004. Metal fractionation study on bed sediments of river Yamuna, India. Water Research 38: 569–578.
Li, M., C. Hu, Q. Zhu, L. Chen, Z. Kong & Z. Liu, 2006. Copper and zinc induction of lipid peroxidation and effects on antioxidant enzyme activities in the microalga Pavlova viridis (Prymnesiophyceae). Chemosphere 62: 565–572.
Lowry, O. H., N. J. Rosebrough, N. J. Farr & R. J. Randall, 1951. Protein measurements with the Folin phenol reagent. Journal Biological Chemistry 193: 265–275.
Macinnis-Ng, C. M. O. & P. J. Ralph, 2002. Towards a more ecologically relevant assessment of the impact of heavy metals on the photosynthesis of the seagrass, Zostera capricorni. Marine Pollution Bulletin 45: 100–106.
McCord, J. M. & I. Fridovich, 1969. Superoxide dismutase: an enzymatic function for erythrocuprein (hemocuprein). Journal Biological Chemistry 244: 6049–6055.
Meylan, S., R. Behra & L. Sigg, 2003. Accumulation of Cu and Zn in periphyton in response to dynamic variations of metal speciation in freshwater. Environmental Science & Technology 37: 5204–5212.
Nakano, Y. & K. Asada, 1981. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant & Cell Physiology 22: 867–880.
Peplow, D. & R. Edmonds, 2005. The effects of mine waste contamination at multiple levels of biological organization. Ecological Engineering 24: 101–119.
Pinto, E., T. C. S. Sigaud-Kutner, M. A. S. Leitao, O. K. Okamoto, D. Morse & P. Colepicolo, 2003. Heavy metal-induced oxidative stress in algae. Journal of Phycology 39: 1008–1018.
Reinert, K. H., J. M. Giddings & L. Judd, 2002. Effects analysis of time-varying or repeated exposures in aquatic ecological risk assessment of agrochemicals. Environmental Toxicology and Chemistry 21(9): 1977–1992.
Ruser, A., P. Popp, J. Kolbowski, M. Reckermann, P. Feuerpfeil, B. Egge, C. Reineke & K. H. Vanselow, 1999. Comparison of chlorophyll-fluorescence-based measuring systems for the detection of algal groups and the determination of chlorophyll-a concenrations. Berichte Forsch.- u. Technologiezentr. Westküste d. Univ. Kiel. 19:27–38.
Sabater, S., H. Guasch, M. Ricart, A. M. Romaní, G. Vidal, C. Klünder & M. Schmitt-Jansen, 2007. Monitoring the effect of chemicals on biological communities. The biofilm as an interface. Analytical Bioanalytical Chemistry 387: 1425–1434.
Sauser, K. R., J. K. Liu & T. Y. Wong, 1997. Identification of a copper-sensitive ascorbate peroxidase in the unicellular green alga Selenastrum capricornutum. BioMetals 10: 163–168.
Schmitt-Jansen, M. & R. Altenburger, 2008. Community-level microalgal toxicity assessment by multiwavelength-excitation PAM fluorometry. Aquatic Toxicolology 86: 49–58.
Serra, A. & H. Guasch, 2009. Effects of chronic copper exposure on fluvial systems: linking structural and physiological changes of fluvial biofilms with the in-stream copper retention. Science of the Total Environment 407: 5274–5282.
Serra, A., H. Guasch & N. Corcoll, 2009. Copper accumulation and toxicity in fluvial periphyton: the influence of exposure history. Chemosphere 74(5): 633–641.
Tlili, A., U. Dorigo, B. Montuelle, C. Margoum, N. Carluer, V. Gouy, A. Bouchez & A. Bérard, 2008. Responses of chronically contaminated biofilms to short pulses of diuron. An experimental study simulating flooding events in a small river. Aquatic Toxicology 87: 252–263.
Torres, M. A., M. P. Barros, S. C. G. Campos, E. Pinto, S. Rajamani, R. T. Sayre & P. Colepicolo, 2008. Biochemical biomarkers in algae and marine pollution. Ecotoxicology and Environmental Safety 71: 1–15.
Tripathi, B. N. & J. P. Gaur, 2004. Relationship between copper- and zinc-induced oxidative stress and proline accumulation in Scenedesmus sp. Planta 219: 397–404.
Tripathi, B. N., S. K. Mehta, A. Amar & J.-P. Gaur, 2006. Oxidative stress in Scenedesmus sp. during short- and long-term exposure to Cu2+ and Zn2+. Chemosphere 62: 538–544.
Winer, B. J., 1971. Statistical Principles in Experimental Design. McGraw Hill, New York: 907.
Acknowledgments
The “Serveis Científics i Tècnics” at the University of Girona provided its facilities and technical help for ICP-MS metal analysis. The research was funded by the Spanish Ministry Science and Education (FLUVIALFITOMARC CGL2006-12785 and FLUVIALMULTISTRESS CTM2009-14111-CO2-01-745), and the EC Sixth Framework Program (MODELKEY 511237-2 GOCE and KEYBIOEFFECTS MRTN-CT-2006-035695). Alexandra Serra and Berta Bonet benefit from a FPI grant of the Spanish Ministry Science and Education. Güluzar Atli benefit from an Erasmus grant between the University of Çukurova (Turkey) and the University of Girona (Spain).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest editors: R. J. Stevenson, S. Sabater / Global Change and River Ecosystems – Implications for Structure, Function and Ecosystem Services
Rights and permissions
About this article
Cite this article
Guasch, H., Atli, G., Bonet, B. et al. Discharge and the response of biofilms to metal exposure in Mediterranean rivers. Hydrobiologia 657, 143–157 (2010). https://doi.org/10.1007/s10750-010-0116-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-010-0116-z