Abstract
To characterize the partitioning of metals in a stream ecosystem, concentrations of trace metals including As, Cd, Cu, Pb, and Zn were measured in water, colloids, sediment, biofilm (also referred to as aufwuchs), macroinvertebrates, and fish collected from the Boulder River watershed, Montana. Median concentrations of Cd, Cu, and Zn in water throughout the watershed exceeded the U.S. EPA acute and chronic criteria for protection of aquatic life. Concentrations of As, Cd, Cu, Pb, and Zn in sediment were sufficient in the tributaries to cause invertebrate toxicity. The concentrations of As, Cu, Cd, Pb, and Zn in invertebrates from lower Cataract Creek (63, 339, 59, 34, and 2,410 μg/g dry wt, respectively) were greater than the concentrations in invertebrates from the Clark Fork River watershed, Montana (19, 174, 2.3, 15, and 648 μg/g, respectively), that were associated with reduced survival, growth, and health of cutthroat trout fed diets composed of those invertebrates. Colloids and biofilm seem to play a critical role in the pathway of metals into the food chain and concentrations of As, Cu, Pb, and Zn in these two components are significantly correlated. We suggest that transfer of metals associated with Fe colloids to biological components of biofilm is an important pathway where metals associated with abiotic components are first available to biotic components. The significant correlations suggest that Cd, Cu, and Zn may move independently to biota (biofilm, invertebrates, or fish tissues) from water and sediment. The possibility exists that Cd, Cu, and Zn concentrations increase in fish tissues as a result of direct contact with water and sediment and indirect exposure through the food chain. However, uptake through the food chain to fish may be more important for As. Although As concentrations in colloids and biofilm were significantly correlated with As water concentrations, As concentrations in fish tissues were not correlated with water. The pathway for Pb into biological components seems to begin with sediment because concentrations of Pb in water were not significantly correlated with any other component and because concentrations of Pb in the water were often below detection limits.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Watersheds throughout the West are affected by drainage from historical mines, mine wastes, and mill tailings, all of which can be sources of metals to sediment and water in streams and for uptake by biofilm, macroinvertebrates, and fish. Although many ecological studies (Blus et al. 1991; Kiffney and Clements 1993; Woodward et al. 1995) have expanded our understanding of the extent of metals contamination and apparent biological effects in mining-affected watersheds, fundamental questions about metal pathways generally have not been addressed. Few studies have had sufficient detail to help develop an understanding of the mechanisms and processes responsible for the transfer of metals from abiotic to biotic components and between biological components within aquatic ecosystems. One such study (Farag et al. 1998) determined the pathway of metals from contaminated sources to organisms in the Coeur d’Alene River watershed by characterizing the partitioning of metals in water and sediment (abiotic components) and the relative concentration of metals in biofilm, invertebrates, and fish (biotic community) at many sites throughout the watershed.
Colloids (particles 0.001 to 1 μm in size) are one component of stream ecosystems that are important for metal transport (Kimball et al. 1995; Schemel et al. 2000) and in the accumulation of metals in sediment (Church et al. 1997) and likely in biofilm (Besser et al. 2001) in streams draining mine sites. However, the role of colloids in the transfer of metals to biota has not been well studied.
This study was designed to better understand the pathways of metals in streams affected by historical mining and the role colloids play in these pathways. The first objective of this study was to characterize the partitioning of metals in a stream ecosystem. Components of interest included water, colloids, sediment, biofilm (also referred to as aufwuchs), macroinvertebrates, and fish. The second objective of this study was to discern and describe how metals in water, colloids, and sediment are associated with uptake in biofilm, macroinvertebrates, and fish throughout a watershed. Once the partitioning of metals among various components is defined (objective number 1), the interrelationship and pathways of metals uptake in these components may be identified (objective number 2). For example, metals dissolved in stream water can be transferred to abiotic or biotic components. Metals transported downstream from mine sites also can play an important role in the accumulation of metals in sediment and biofilm. Biofilm is a critical link in the movement of metals from abiotic to biotic components in a stream because it is composed of algae, bacteria, and fine detrital material that adhere to substrates in water bodies (Ruttner 1968). The interrelationships among the combined metal data for these ecological components in streams could facilitate construction of models to quantitatively predict the movement of metals associated with water, colloids, and sediment to aquatic life. For instance, concentrations of some metals in sediment and water might be correlated with uptake of these metals in invertebrates and specific fish tissues. Similarly, concentrations of other metals in colloids might be correlated with uptake in biofilm or other fish tissues. With this kind of information, we can define the importance of water and colloid/sediment pathways in the bioaccumulation of metals from a complex mixture.
The study described here supplements the Coeur d’Alene study (Farag et al. 1998) because the mix of metals in the Boulder River watershed is different than in the Coeur d’Alene watershed. The current study advances our knowledge base because it includes water-column colloids, which play an important role in metal transport and transfer (Kimball et al. 1995).
Our study was conducted in the Boulder River watershed in southwest Montana (Figure 1). Several studies conducted in this watershed reported reduced fitness of aquatic life and concluded that these biological effects were related to metals. In an ecological study of the effects of metals contamination on aquatic life, Martin (1992) documented elevated concentrations of cadmium (Cd), copper (Cu), and zinc (Zn) in water, sediment, aquatic invertebrates, and fish and attributed these high concentrations to sources of metals in the Cataract Creek drainage. More recently, Farag et al. (2003) documented that metals affect fish populations and fish health in the Boulder River watershed. Although effects from metals have been reported in the Boulder River watershed, little is known about the partitioning and pathways of metals in the aquatic environment. Without this information, it is not possible to fully understand the fate of metals in the watershed or to determine what reasonable remediation objectives might be. Furthermore, a comprehensive understanding of metal pathways in the Boulder River watershed should provide data that is transferable to metal pathways in other watersheds.
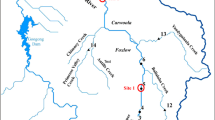
Map of the Boulder River watershed with designated sites where samples for water, colloid, sediment, and biology were collected in the Boulder River watershed, Montana. LBR = Little Boulder River, BMT = Bullion Mine Tributary, JC = Jack Creek, USG = Uncle Sam Gulch, MCC = middle Cataract Creek, LCC = lower Cataract Creek, LHO = lower High Ore Creek, UHO = upper High Ore Creek, UBR = upper Boulder River, LBC = lower Basin Creek, BRRC = Boulder River below Red Rock Creek, BRBC = Boulder River below Basin Creek, BRCC = Boulder River below Cataract Creek, BRGG = Boulder River below Galena Gulch
The Boulder River watershed is an excellent area to conduct a comprehensive study of metal partitioning and pathways because a continuum of metals and aquatic life exist in the watershed. Fish populations are absent from some reaches directly below historical mines, but are found in reaches further downstream. Fish species in the watershed include brook trout (Salvelinus fontinalis), rainbow trout (Oncorhynchus mykiss), and westslope cutthroat trout (Oncorhynchus clarki lewisi) (Bob Wintergerst, U.S. Forest Service, Missoula, Montana, personal communication). Similarly, metal concentrations in water and streambed sediment range over several orders of magnitude throughout the watershed. Some fishless reaches in Jack Creek, Uncle Sam Gulch, and High Ore Creek coincide with dissolved metal concentrations of greater than 50 μg Cd/L and 5,000 μg Zn/L in the ambient water at low flow (Nimick and Cleasby, 2000). The streams of concern in the Boulder River watershed include the three tributaries (Basin, Cataract, and High Ore Creeks) affected by historical mining and the reach of the Boulder River affected by these tributaries (Figure 1). Drainage from the Bullion mine enters Jack Creek, which flows into Basin Creek. Basin Creek then flows into the Boulder River below Rock Creek. The Crystal mine in Uncle Sam Gulch provides most of the metal contamination in Cataract Creek (Cleasby et al. 2000), which flows into the Boulder River downstream of Basin Creek. The Comet mine is the major source of metals in High Ore Creek (Church et al. 2004; Nimick and Cleasby 2004), which flows into the Boulder River upstream of Galena Gulch.
The objectives of this study were to (1) characterize the pathway and partitioning of metals in water, sediment, biofilm, macroinvertebrates, and fish; and (2) to help define the influence of water, colloids, and sediment in the transfer of metals to aquatic life.
Materials and Methods
Water, sediment, colloids, biofilm, and macroinvertebrates were collected from 12 sites in the Boulder River watershed. Five sites were on the mainstem Boulder River (Boulder River below Galena Gulch, BRGG; Boulder River below Cataract Creek, BRCC; Boulder River below Basin Creek, BRBC; Boulder River below Red Rock Creek, BRRC; Upper Boulder River, UBR), and seven sites were on tributaries (Little Boulder River, LBR; lower High Ore Creek, LHO; lower Cataract Creek, LCC; middle Cataract Creek, MCC; upper Cataract Creek, UCC; lower Basin Creek, LBC; Jack Creek, JC) (Figure 1). BRRC, UBR, and LBR were upstream of areas of significant historical mining and were designated reference sites. Fish were collected at five sites (UBR, BRRC, BRGG, LBC, LCC), with UBR and BRRC being reference sites for tributaries and mainstem, respectively.
Water Sampling Methods
Water samples were collected periodically during 1996–1999 to characterize total recoverable (unfiltered) and filtered metal concentrations during low-flow and high-flow conditions. Samples were composited from multiple verticals across the stream using depth- and width-integration methods described by Edwards and Glysson (1988). These methods provide a vertically and laterally discharge-weighted sample that is representative of the entire flow through the cross-section of a stream. Instantaneous streamflow at the time of water sampling was determined by direct measurement (Rantz 1982). Sample processing, including 0.45-μm filtration and preservation with ultrapure nitric acid, was performed according to procedures described by Horowitz et al. (1994) and Ward and Harr (1990).
Colloid Sampling Methods
Some water samples were processed further to determine metal concentrations in the dissolved and colloidal phases. Dissolved concentrations were determined in ultrafiltered aliquots. Ultrafiltration was conducted using a 10,000-Da molecular weight membrane. Concentrations of metals in the colloidal fraction were determined indirectly by subtracting the ultrafiltered metal concentration from the total recoverable (unfiltered) metal concentration. Colloid data also are presented for three additional sites located between one of the main sampling sites and an upstream inactive mine to demonstrate the large amount of colloidal material that drains from abandoned mine lands in the watershed. These additional sites are Bullion Mine tributary (BMT) below the Bullion mine, Uncle Sam Gulch (USG) below the Crystal mine, and upper High Ore Creek (UHO) below the Comet mine.
Many of the Cu concentrations for ultrafiltrates (see Table 2) were greater than the filtered concentration. In general, this disparity only occurred when total-recoverable concentrations were less than about 30 μg/L, indicating a source of low-level Cu contamination during filtration with the 10,000-Da filter. The highest concentrations of Cu near mining sources, however, were much greater than the level of contamination, and, therefore, the Cu contamination has little effect on the results of this study.
Sediment Sampling Methods
A composite sediment sample from the streambed surface was collected at each site over a 50-m reach using a plastic scoop. The sample was wet sieved at the site using stream water. In the laboratory, the sample was dry sieved to <80 mesh (<0.15 mm) and then digested using a warm 2M HCl-1% H2O2 leach extraction for 3 hours with continuous agitation (Fey et al. 1999).
Biofilm Sampling Methods
Biofilm was collected from surfaces of rocks that were removed from near-shore areas of the stream and gently scraped with plastic, acid-washed utensils. The biofilm was placed directly into acid-washed, plastic vials. The rocks were left out of the water after sampling to ensure that they were not scraped again. Four separate samples were collected from different, randomly selected, riffles at each site.
Macroinvertebrate Sampling Method
Macroinvertebrates were sampled with a 3-mm mesh net attached to a 1.2- × 0.6-m frame. The substrate in approximately 6 m2 of riffle was overturned with hooked tools, and the dislodged macroinvertebrates were collected in a downstream net. Macroinvertebrates were removed from the net with plastic acid-washed forceps and placed into acid-washed plastic vials. Four samples were collected from different, randomly selected, riffles at each site.
Fish-Tissue Sampling Method
Sampling methods as well as analytical data for fish tissues were reported by Farag et al. (2003). Metal-concentration data for fish tissues are presented in this study to define accumulation of metals in fish tissues compared to accumulations of metals in water, colloids, sediment, biofilm, and invertebrates.
Metal Analyses
Metals in water samples were analyzed by inductively coupled plasma-mass spectrometry (ICP-MS) by the USGS National Water Quality Laboratory in Denver, Colorado. Some of the ultrafiltrate samples were analyzed by inductively coupled plasma-atomic emission spectrometry (ICP-AES) by a USGS laboratory in Boulder, Colorado, or by ICP-MS by the USGS Geologic Division laboratory in Denver, Colorado. Metals in digests of streambed sediment were analyzed by ICP-AES by the USGS Geologic Division laboratory in Denver, Colorado.
Biofilm and macroinvertebrate samples were processed and analyzed for As, Cd, Cu, Pb, and Zn by ICP-MS at the USGS Columbia Environmental Research Center, Columbia, Missouri. All biology samples were lyophilized to a constant weight and briefly homogenized by pulverizing with a glass rod. Subsamples (0.5 g dry weight) were digested with microwave heating in sealed CEM Teflon vessels after the addition of 5 ml 16N HNO3 and 1 ml 30% H2O2. An aliquot of each liquid sample digestate was quantitatively transferred and diluted 5× in an acid-cleaned 15-ml polyethylene centrifuge tube. Further dilution during analysis was performed as necessary with a CETAC ASX500-ADX100 autodiluter and ranged from 10× to 100×, depending on sample and analyte. Internal standards were metered into the sample solutions during analysis with a peristaltic pump. The effective concentrations for internal standards were as follows: 50 ng/ml Ge (for Cu, Zn, and As), 10 ng/ml Rh (for Cd), and 10 ng/ml Bi (for Pb). Calibration standards for analysis ranged from 0 to 40 ng/ml for Cd and Pb, 0 to 100 ng/ml for As and Cu, and 0 to 500 ng/ml for Zn. Masses monitored included Cu-63 and Cu-65, Zn-66 and Zn-68, As-75, Cd-111 and Cd-114, and Pb-206, 207, and 208 (all three masses summed).
Quality control was maintained for all chemical analyses. Instrument calibration was verified by analyzing certified calibration solutions during each instrumental run. These external reference standards were generally within 80% to 120% of the nominal concentrations. For colloidal samples, certified internal samples were run through the analysis schedule to allow for corrections due to any instrument drift. Precision for metal analyses used in calculation of the colloidal concentrations was generally less than 10%. All sample spikes for sediment, biofilm, and macroinvertebrates were within 80% to 120% recovery. The percent recovery of sample spikes for the fish matrix ranged from 94% to 121%. Measured concentrations were within 15% of the certified limits for three reference samples (copepod, mussel, and oyster tissues) analyzed with biofilm and macroinvertebrates. Sample preparation blanks were analyzed to document that the samples were not contaminated with metals during the digestion procedure. Metal concentrations in the preparation blanks generally were below the detection limit.
Analysis of Data
Concentrations of metals measured in water, colloids, sediment, biofilm, macroinvertebrates, gill, liver, and whole fish collected from the experimental sites were compared to concentrations in samples collected from the reference sites. Data from two reference tributary sites (LBR and UBR) were combined into one group and referred to as the pooled reference (REF 1) for colloid, sediment, biofilm, and invertebrate metal concentrations. No pooling was performed for water because concentrations of water are presented as ranges. UBR was the only reference tributary site sampled for gill, liver, and whole fish and was designated as REF 1 for fish tissue collected from tributary sites. BRRC was the only reference used for sites on the mainstem of the Boulder River. Data for all sites were tested for homogeneity of variance and transformed when necessary. For biological samples, an ANOVA was performed using the assumption of equal variances to test for differences among means. If data failed to meet the homogeneity or normality assumptions, they were log transformed before the analysis. If the ANOVA detected a difference, a Tukey test (Zar 1984) was performed to make comparisons among means for each metal. Significant correlations of metal accumulation among abiotic and biotic components were also defined. Because fish tissues were collected from five, rather than 12 sites, all correlations performed with fish tissues utilized a data set that contained only samples collected from the five fish sampling sites. Statistical significance was defined at p≤ 0.05.
Results
Water
In stream water, concentrations of As and Pb were greatest at LHO (Table 1, total recoverable reported as μg/L in this text; 64 and 54, respectively) during high-flow conditions. Copper concentrations also were greater at most sites during high flow, although the highest total recoverable concentrations at any site occurred during low flow at JC (140 μg/L). Unlike As and Pb, the concentrations of Cd and Zn were greater during low rather than high flow. Zinc concentrations were greatest at LHO during low flow (1,970 μg/L). The greatest concentrations of Cd were measured at JC (12 μg /L) and MCC (9.4 μg /L) during low flow. The pH ranged from 7.0 to 8.5 at all sites regardless of flow, and these data are not presented.
Colloids
The presence of colloids in the water column was best indicated by the high concentration of colloidal Fe (Table 2) and, to a lesser extent, Al (data not presented) at most sites including the reference sites (Table 2). The general pattern of partitioning of metals between dissolved and colloidal fractions in the water column depended primarily on the metal and, to a lesser extent, on the proximity of the sampling site to sources of metals. Iron and Pb almost always occurred in the colloidal fraction whereas Cd and Zn were primarily dissolved. Large amounts of As and Cu also partitioned to the colloidal phase, but unlike Pb, As and Cu also existed in the dissolved phase.
The spatial pattern of colloidal metal concentrations generally was the same as metal concentrations in water and sediment in that the greatest colloidal metal concentrations were directly downstream from inactive mines. Metal-rich acidic water draining from these mines is neutralized in mixing zones in streams, producing substantial loads of metal-rich colloids that then are transported long distances downstream. Substantial colloid loads occurred in the Bullion Mine tributary (BMT) and Jack Creek (JC) downstream from the Bullion mine, in Uncle Sam Gulch (USG) and Cataract Creek (MCC) downstream from the Crystal mine, and in High Ore Creek (UHO and LHO) downstream from the Comet mine (Table 2). For the 12 main sampling sites, the highest concentrations of colloidal metals were at LHO (0.5 μg/L Cd, 8.2 μg/L Pb, and 322 μg/L Zn) and JC (24 μg/L As and 74 μg/L Cu). Concentrations at sites nearer inactive mines (BMT, USG, and UHO) were even higher, with 13 μg/L Pb at BMT, 256 μg/L Cu at USG, and 102 μg/L As, 44 μg/L Pb, and 710 μg/L Zn at UHO (Table 2). Colloidal concentrations of As, Cu, Pb, and Zn increased in the Boulder River in a stepwise downstream pattern as each of the three mining-affected tributaries entered the mainstem.
Sediment
In sediment, the concentrations of all metals except Cu were highest at LHO (all measurements reported as μg/g dry wt; 740 As, 14 Cd, 1,100 Pb, and 3,400 Zn) (Table 3). The concentrations of As, Cd, and Zn in Cataract and Basin Creeks were 2× to 5× less than concentrations in LHO but were still larger than the concentrations at the pooled reference site (REF 1). Cd concentrations were similar in Cataract Creek (9.3 at MCC and 11 at LCC) compared to LHO. The greatest Cu concentrations were in Cataract Creek (450 at MCC, 440 at LCC) followed by Jack Creek (180 at JC) and High Ore Creek (140 at LHO).
In the Boulder River, metal concentrations in sediment generally were less than in the tributaries. However, concentrations of all metals, except Cd at BRBC, were greater at the three downstream mainstem sites compared to the reference site (BRRC). For instance, As concentrations were 99 μg/g at BRGG, far higher than the 8.3 μg/g measured at BRRC.
Biofilm
Metal concentrations in biofilm decreased at sites furthest downstream from historical mining areas (Table 4). The greatest concentrations of Cd, Cu, and Pb were measured in Jack Creek (all data presented as μg/g ± standard error of the mean (SEM); 70 ± 4; 4,620 ± 860; 885 ± 84, respectively at JC) followed by Cataract Creek (60 ± 22; 1,940 ± 850; 660 ± 150, respectively at MCC), then High Ore (41 ± 3; 321 ± 9; 1,100 ± 30, respectively, at LHO). The concentrations of these metals decreased substantially downstream in Basin Creek (LBC), but were still 18× to 23× greater than the pooled reference used for the tributary sites. The concentrations of Cd and Pb, but not Cu, were significantly elevated in biofilm from LHO compared to the pooled reference.
Biofilm from High Ore Creek had the greatest concentrations of As and Zn (3,300 ± 400 and 18,100 ± 1,700, respectively, at LHO) followed by Jack Creek (2,600 ± 170 and 6,210 ± 170, respectively, at JC) and Cataract Creek (1,700 ± 700 and 6,000 ± 2,240, respectively, at MCC). Concentrations of As and Zn decreased further downstream at LCC and LBC but were still >26× the concentrations measured at the pooled reference site.
Elevated concentrations of metals persisted in the mainstem of the Boulder River: 262 μg As/g was measured in biofilm at BRGG. The Zn concentrations at BRGG were similar to the concentrations measured at LCC (3,200 ± 250 vs. 3,870 ± 420), although the concentrations of As in biofilm from the mainstem below Cataract Creek (BRBC) were less than LCC (38 ± 4 vs. 731 ± 67). Metal concentrations in biofilm from the mainstem below Basin Creek (BRBC) and Cataract Creek (BRCC) were also elevated above the reference (BRRC).
Benthic Macroinvertebrates
The concentrations of metals were generally less in benthic macroinvertebrates compared to biofilm, but invertebrates from many of the sites had concentrations greater than at the reference site (Table 5). This trend was apparent at sites throughout the watershed although not all such differences were statistically significant. Furthermore, some of the greatest concentrations of Cd, Cu, and Pb were observed in lower Cataract Creek (59 ± 5; 340 ± 130; 34 ± 16, respectively, at LCC). In general, though concentrations of metals persisted in invertebrates collected from sites on the Boulder River, they were less than concentrations of invertebrates from the tributaries. One exception to this observation was Pb; invertebrates from BRGG had the greatest mean concentration of Pb measured in the watershed. These results demonstrate that metals are being transported downstream where they can be transferred to the food chain.
Fish Tissues
Farag et al. (2003) described the metal concentrations in fish tissues in detail. In summary, mean concentrations of metals, especially As, Cd, and Cu, were greatest in gill, liver, and whole fish from LCC (Table 6). Cd concentrations in gill, liver, and whole fish from LCC were many times greater than those for the reference, UBR (90× for gill, 47× for liver, 33× for whole fish). Likewise, Cu concentrations in fish from LCC ranged from 4× to 8× the concentrations in fish from the reference. Although metal concentrations were less in fish from the mainstem, elevated As, Cd, and Zn concentrations were measured in fish as far downstream as BRGG.
Relationships Among Components
The concentrations of all metals, except Pb, measured in low flow total, filtered, or dissolved water were almost always significantly correlated (p < 0.05) with one or more of the biological components (Table 7). Significant correlations were more frequent between water and biology for Cd, Cu, and Zn than for As. Copper and Zn in total, filtered, and dissolved water were significantly correlated with biofilm and benthic macroinvertebrates. However, for As, the correlations with biofilm were significant only for total and filtered water, and all correlations between water and invertebrates were not significant (p > 0.05). The strength of the significant relationships between water and biofilm or invertebrates generally were less for As (r ≤ 0.884) than they were for Cd, Cu, and Zn (r = 0.656–0.994). There were no significant correlations between As in water and fish, whereas Cd, Cu, and Zn in water generally were significantly correlated to fish tissues. For example, Cu in water was significantly correlated with gill, liver, and whole fish; Cd in water was significantly correlated with gill and liver; and Zn in water was significantly correlated with liver. In summary, Cd, Cu, and Zn appear to have accumulated in various levels of the food chain and, based on the correlations between water and fish, may have accumulated in fish directly from the water column.
There were some significant correlations (p < 0.05) between metals in water and sediment, but r values (range = 0.537–0.877) generally were less than those between water and biological components. Some of the strongest correlations between metals in water and sediment were for dissolved Zn and Cd (r = 0.877 and 0.857, respectively).
Metal concentrations in sediment frequently correlated significantly (p < 0.05) with the metal concentrations in the biological components. Metals in sediment almost always correlated with biofilm and macroinvertebrates for all metals (r = 0.667–0.952). The strongest correlations were for Zn in biofilm (r = 0.952) and macroinvertebrates (r = 0.899). Additionally, Cd and Cu in sediment were significantly correlated to gill, liver, and whole fish (r ≥ 0.922). Arsenic in sediment was significantly correlated to liver and whole fish (r = 0.978 and r = 0.882, respectively); and Zn in sediment was significantly correlated to liver (r = 0.953).
Concentrations of metals in colloids were significantly (p < 0.01) correlated with concentrations in biofilm for all metals except Cd (r ≥ 0.819). Concentrations of As, Cu, and Zn in colloids were significantly correlated (p < 0.05) with macroinvertebrates (r > 0.647). Finally, metals in colloids were significantly correlated (p < 0.05) with liver and whole fish for Cu and whole fish for Pb.
Arsenic, Cu, Pb, and Zn in biofilm and macroinvertebrates were significantly correlated (p < 0.05) with one another. However, the correlations (r ≤ 0.828) were not as strong as in other comparisons. Biofilm and invertebrates were correlated with gill, liver, and whole fish for Cd, and Cu, liver, and whole fish for As and Zn (except no significant correlation between Zn in macroinvertebrates and whole fish), and gill for Pb. Therefore, all metals apparently accumulate in fish, at least in part, through the food chain.
Discussion
The first objective of this study was to characterize the pathway and partitioning of metals into water, sediment, biofilm (aufwuchs), macroinvertebrates, and fish. Metals were elevated in the three tributaries (Basin, Cataract, and High Ore Creeks). In addition, metals accumulated in all components and accumulated to the greatest extent at sites nearest inactive mines. Furthermore, the release of metals from abandoned mines and adits has resulted in concentrations of metals in water and sediment that may adversely affect the aquatic health in the Boulder River watershed.
Median concentrations of Cd, Cu, and Zn found in the water column at most sites exceeded the U.S. EPA acute and chronic criteria for protection of aquatic life (USEPA 1999, 2001). Because hardness often alleviates the toxic effects of metals, the criteria vary with changes in hardness where a greater hardness (mg/L as CaCO3) translates into a higher concentration for the criteria. In the Boulder River watershed, hardness was generally less than 50 mg CaCO3 /L for all sites except LHO. At 50 mg/L hardness, a conservative estimate for the Boulder watershed, the criteria are acute/chronic: 1.0/0.15 μg Cd/L, 7.0/5.0 μg Cu/L, and 66/65 μg Zn/L. These values were exceeded at some of the tributary sites by greater than 10×, suggesting that the water quality of most tributary sites posed an increased risk of detrimental effects to aquatic life. At some of the tributary sites, Farag et al. (2003) directly observed detrimental effects including acute mortality of experimental fish as well as reduced growth and biomass of wild fish compared to those at reference sites. Even at some of the mainstem sites (Cd and Zn at BRCC and BRGG), concentrations in water exceeded criteria, and metals concentrations in fish tissues from those locations were elevated.
Cadmium, Cu, and Zn were elevated in sediment throughout the Boulder River watershed. Additionally, As and Pb persisted in the sediment although these metals generally were not elevated in filtered water. Based upon large datasets of co-occurring sediment chemical concentrations and biological effects, sediment quality guidelines have been developed, which predict that at concentrations greater than about 33 μg As/g, 5 μg Cd/g, 149 μg Cu/g, 128 μg Pb/g, and 459 μg Zn/g, adverse effects to benthic communities were probable (Ingersoll et al. 2001; MacDonald et al. 2000). In the Boulder River watershed, sediment from all tributary sites exceeded these probable effects concentrations (PECs) for As and Pb, and the PEC for As was exceeded on the mainstem as far downstream as BRGG. All tributary sites with the exception of MCC exceeded the PEC for Zn. And several of the tributary sites exceeded the PEC for Cd and Cu. Therefore, this study suggests that the exposure to metals in sediment in the Boulder River watershed would lead to invertebrate toxicity in the watershed. This finding of probable invertebrate toxicity in the Boulder River watershed is supported by Boyle and Gustina (2000). These researchers defined the invertebrate community structure at some of the same sites investigated during this study and observed a depleted number of Ephemeroptera-Plecoptera-Tricoptera (EPT) taxa in High Ore Creek, lower Cataract Creek, and Jack Creek.
Colloids and biofilm appear to play a critical role in the pathway of metals to the food chain. Colloidal Fe oxyhydroxides are formed immediately downstream of mine drainage mixing zones and are involved in the downstream transport of Cu, Pb, and Zn (Schemel et al. 2000). The association of colloids and metals is dynamic, and the metals may sorb or desorb frequently as pH changes occur in mixing zones during transport. Therefore, the formation of Fe colloids may play an important role in the transport of metals downstream and in the transfer of metals to biofilm. Furthermore, Verbost et al. (1995) documented the enhanced toxicity of mixing zones to brown trout. Brown trout experienced necrosis and apoptosis of cells in their gills after a short (60 min) exposure to the mixing zone followed by a recovery period downstream of the mixing zone. The possible role of colloids in this toxicity is not defined and freshly formed colloids may provide a direct route of metal to fish gills in the water column.
During transport downstream, colloids are commonly trapped by biofilm on rock surfaces. Newman and McIntosh (1989) questioned the bioavailability of metals associated with Fe oxyhydroxides. However, we suggest that the transfer of metals associated with Fe colloids to biological components of biofilm is an important pathway where metals associated with abiotic components are first presented to biotic components. Significant accumulations may occur if only small portions of these metals are bioavailable. The significant correlations we observed between concentrations of As, Cu, Pb, and Zn in colloids and biofilm support the theory that colloids transport metals, at least in part, to biofilm. And we have documented that As, in addition to Cu, Pb, and Zn as documented by Schemel et al. (2000), likely is transported downstream by colloids. Furthermore, metals in biofilm are associated with both the abiotic and biotic components present in biofilm (Newman et al. 1983, 1985). This association suggests that biofilm is a critical link in the movement of metals directly into the food chain.
Biofilm may also be accumulating metals by means other than the exposure received from colloids. This metal accumulation seems especially evident for Cd and Zn. The concentrations of these metals were greater in filtered water in comparison to the concentrations in colloids. We observed strong correlations between water and biofilm for Cd, but not between colloids and biofilm. The metal concentrations and the strong correlation suggest that water is the primary source of the Cd in biofilm. Dissolved Zn concentrations were greater than Zn concentrations in colloids, and the correlations were strong among water, colloids, sediment, and biofilm for Zn. These correlations (Table 7) suggest that biofilm may receive Zn from water, colloids, and sediment.
Concentrations of Zn in biofilm collected from the Boulder River watershed may be sufficient to affect growth of benthic invertebrates in the watershed. Courtney and Clements (2002) observed reduced growth of Baetis tricaudatus that were fed biofilm collected from the Animas River, Colorado. B. tricaudatus contained >1,100 μg Zn/g after being fed biofilm that contained > 2,300 μg Zn/g for 7 days. These same invertebrates exhibited reduced growth compared to reference invertebrates. The concentrations of metals in biofilm and invertebrates from most of the tributaries sampled in the Boulder River watershed exceeded the thresholds reported for the Animas River, Colorado. Courtney and Clements (2002) also noted lesser amounts of chlorophyll a in the biofilm with Zn concentrations sufficient to cause reduced growth. Therefore, the authors were not able to define the indirect effects of food quality on their results. Chlorophyll a was not measured during the current study.
The concentrations of metals in the invertebrates collected from the Boulder River may be sufficient to affect the health of fish feeding upon them. The concentrations of As, Cu, Cd, Pb, and Zn in invertebrates from lower Cataract Creek (63, 340, 59, 34, and 2,410 μg/g, respectively) were greater than the concentrations in invertebrates from the Clark Fork River watershed, Montana (19, 174, 2.3, 15, and 648 μg/g, respectively) that were associated with reduced survival, growth, and health of cutthroat trout fed diets composed of these invertebrates from the Clark Fork River watershed, Montana (Farag et al. 1994; Woodward et al. 1995).
Concentrations of metals are elevated in many watersheds with historical mining activity throughout the intermountain western United States. Although As, Cu, Cd, Pb, and Zn may be generally elevated in these watersheds, the metals with the greatest concentrations compared to reference sites appear to vary among watersheds. For example, concentrations of As and Zn in invertebrates from the Boulder River watershed are greater when compared to the Clark Fork River, Montana, and the Couer d’Alène River, Idaho (Farag et al. 1998). But concentrations of Cu are greatest at some sites in the Clark Fork River, Montana, and concentrations of Pb are greatest in the Coeur d’Alène River, Idaho.
The pattern of accumulation in the components measured during this study differed from patterns previously observed. Farag et al. (1998) found that the pattern of metal concentrations in the Coeur d’Alene River watershed was (from greatest to least): biofilm and sediment > macroinvertebrates > whole fish. However, in the Boulder River watershed, the pattern was biofilm > macroinvertebrates ≥ sediment > fish tissues > water and colloids. In fact, the concentrations of metals in biofilm in the Boulder River watershed were often greater than the concentrations in sediment. This predominance of metals in biofilm over sediment may result from the entrapment of colloid-bound metals by the biofilm, which tends to integrate the water-column concentrations over time. Additionally, the accumulation of metals in the biological component of biofilm could contribute to greater concentrations of metals in biofilm than sediment. Researchers have documented that algae and microbes, which are contained in biofilm, absorb and retain metals for longer periods of time than do metals associated with Fe oxides, as would be found in sediment (Calmano et al. 1988). Therefore, metals from colloidal and biological components in biofilm may influence the accumulation of metals in biofilm in the Boulder River watershed.
The second objective of this study was to define the influence of water, colloids, and sediment in the transfer of metals to aquatic life. A clear interdependency among the components measured during this study exists, and it seems that all components are important in the movement of metals up the food chain. The numerous significant correlations of metal concentrations among the various components support this interpretation. However, the primary pathway varies with the metal. For example, Cu, Cd, and Zn may move independently to biota (biofilm, invertebrates, and fish tissues) from water and sediment. In fact, not only do metal concentrations in biofilm increase similarly to concentrations in water and sediment, but concentrations of metals in fish tissues increase directly with the water and sediment concentrations. Therefore, the possibility exists that these metal concentrations increase in fish tissues as a result of direct contact with water and sediment and indirect exposure through the food chain. The pathway of As may be slightly different because, although As in colloids and biofilm was significantly correlated with water, As in water was not significantly correlated with fish tissues. Therefore for fish, the food chain pathway may be more predominant for As. Recent observations by Hansen et al. (2004) that growth inhibition in rainbow trout was correlated with food-chain arsenic exposure emphasize the potential importance of this pathway for aquatic exposure of As. The movement of Pb into biological components seems to be the result of a pathway that begins with sediment because concentrations of Pb in water and the other components were not correlated.
Correlations between water and sediment were weaker than expected. There are two possible explanations for this observation. First, colloids present in the water and on sediment particles may affect the concentrations and diminish the strength of correlations. Iron colloids are also likely to be present in biofilm, and concentrations of metals in the biofilm for this study were correlated with water and/or sediment for most metals. If colloids make up a significant portion of the biofilm, these correlations of water and sediment with biofilm may be a result, in part, of the significant colloid presence. A second possibility is the spatial aspect of the process by which metals are transferred from the water column to the sediment. The process occurs as colloids form in mixing zones and then metals react with the colloids; this metal-colloid interaction occurs during transport downstream. Thus, a sample at a single site may not reflect the whole process and correlations may not be good. Where a sequence of samples was collected in Uncle Sam Gulch during a metal loading study (Kimball et al. 2004), the process was evident, but any single sample would not have indicated the process.
In summary, the pathway of metals to fish in the Boulder River watershed, Montana, clearly includes water, sediment, biofilm, and benthic macroinvertebrates. Metals have accumulated in all of these components and to the greatest extent at sites nearest historical mining activities. The concentrations of metals in water and sediment routinely are such that aquatic life will be affected in several locations of the watershed. Finally, the interrelationship of the metals accumulating in the components measured suggests that fish are exposed to metals both directly from water and sediment and indirectly through the food chain, especially for Cu, Cd, and Zn. The food chain pathway may be more important for As uptake in fish, and sediment concentrations rather than water may play an important role for Pb transfer to biological components.
References
Besser JM, Brumbaugh WG, May TW, Church SE, Kimball BA (2001) Bioavailability of metals in stream food webs and hazards to brook trout (Salvelinus fontinalis) in the Upper Animas River watershed, Colorado. Arch Environ Contam Toxicol 40:48–59
Boyle TP, Gustina GW (2000) A strategy for use of multivariate methods of analysis of benthic macroinvertebrate communities to assess mine-related ecological stress. In Proceedings of the Fifth International Conference on Acid Rock Drainage, Denver, Colorado, May 21–24, 2000. Littleton, Colorado, Society for Mining, Metallurgy, and Exploration, pp 1425–1432
Blus LJ, Henny CJ, Hoffman DJ, Grove RA (1991) Lead toxicosis in tundra swans near a mining and smelting complex in northern Idaho. Arch Environ Contam Toxicol 21:549–555
Calmano W, Ahlf W, Forstner U (1988) Study of metal sorption desorption processes on competing sediment componenets with a multichamber device. Environ Geol Wat Sci 11:77–84
Church SE, Kimball BA, Fey DL, Ferderer DA, Yager TJ, Vaughn RB (1997) Source, transport, and partitioning of metals between water, colloids, and bed sediments of the Animas River, Colorado. U.S. Geological Survey Open-File Report 97–151, 136 pp
Church SE, Unruh DM, Fey DL, Sole, TC (2004) Trace elements and lead isotopes in streambed sediment in streams affected by historical mining. In: Nimick DA, Church SE, Finger SE (eds), Integrated investigation of environmental effects of historical mining in the Basin and Boulder mining districts, Boulder River watershed, Jefferson County, Montana. U.S. Geological Survey Professional Paper 1652. Reston, Virginia, pp 279–335
Cleasby, TE, Nimick DA, Kimball BA (2000) Quantification of metal loads by tracer-injection and synoptic-sampling methods in Cataract Creek, Jefferson County, Montana, August 1997. U.S. Geological Survey Water Resources Investigations Report 00-4237, 39pp
Courtney LA, Clements WH (2002) Assessing the influence of water and substratum quality on benthic macroinvertebrate communities in a metal-polluted stream: an experimental approach. Freshwater Biol. 47:1766–1778
Edwards TK, Glysson GD (1988) Field methods for measurement of fluvial sediment. U.S. Geological Survey Open-File Report 86-531, 118 pp
Farag AM, Boese CJ, Woodward DF, Bergman HL (1994) Physiological changes and tissue metal accumulation in rainbow trout exposed to foodborne and waterborne metals. Environ Toxicol Chem 13:2021–2029
Farag AM, Woodward DF, Goldstein JN, Brumbaugh WG, Meyer JS (1998) Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d’Alene River Basin, Idaho. Arch Environ Contam Toxicol 34:119–127
Farag AM, Skaar D, Nimick DA, MacConnell E, Hogstrand C (2003) Characterizing aquatic health using fish mortality, physiology, and population estimates in the Boulder River watershed, Montana. Trans Am Fish Soc 132:450–467
Fey DL, Unruh DM, Church SE (1999) Chemical data and lead isotopic compositions in stream-sediment samples from the Boulder River watershed, Jefferson County, Montana. U.S. Geological Survey Open-File Report 99-575, 147 pp
Hansen JA, Lipton J, Welsh PG, Cacela D, MacConnell B (2004) Reduced growth of rainbow trout (Oncorhynchus mykiss) fed a live invertebrate diet pre-exposed to metal-contaminated sediments. Environ Toxicol Chem 23:1902–1911
Horowitz AJ, Demas CR, Fitzgerald KK, Miller TL, Rickert DA (1994) U.S. Geological Survey protocol for the collection and processing of surface-water samples for the subsequent determination of inorganic constituents in filtered water. U.S. Geological Survey Open-File Report 94-539, 57 pp
Ingersoll CG, MacDonald DD, Wang N, Crane JL, Field LJ, Haverland PS, Kemble NE, Lindskoog RA, Severn C, Smorong DE (2001) Predictions of sediment toxicity using consensus-based freshwater sediment quality guidelines. Arch Environ Contam Toxicol 41:8–21
Kiffney PM, Clements WH (1993) Bioaccumulation of heavy metals by benthic invertebrates at the Arkansas River, Colorado. Environ Toxicol Chem 12:1507–1517
Kimball BA, Callender E, Axtmann EV (1995) Effects of colloids on metal transport in a river receiving acid mine drainage, upper Arkansas River, Colorado, U.S.A. Applied Geochem 10:285–306
Kimball BA, Runkel RL, Cleasby TE, Nimick DA (2004) Quantification of metal loading by tracer-injection and synoptic-sampling studies, 1997–98, Chapter D6 in Nimick DA, Church SE, Finger SE (eds), Integrated investigation of environmental effects of historical mining in the Basin and Boulder mining districts, Boulder River watershed, Jefferson County, Montana. U.S. Geological Survey Professional Paper 1652. Reston, Virginia, pp 191–263
MacDonald DD, Ingersoll CG, Berger T (2000) Development and evaluation of consensus-based sediment quality guidelines for freshwater ecosystems. Arch Environ Contam Toxicol 39:20–31
Martin DS (1992) Acid mine/rock drainage effects on water quality, sediments, invertebrates and fish located in Uncle Sam Gulch, Cataract Creek and the Boulder River, nothern Jeffereson County, Montana. M.S. thesis. Montana College of Mineral Science and Technology, Butte, Montana. 117 pp
Newman MC, McIntosh AW (1989) Appropriateness of aufwuchs as a monitor of bioaccumulation. Environ Pollut 60:83–100
Newman MC, McIntosh AW, Greenhut VA (1983) Geochemical factors complicating the use of aufwuchs as a biomonitor for lead levels in two New Jersey reservoirs. Wat Res 17:625–630
Newman MC, Alberts JJ, Greenhut VA (1985) Geochemical factors complicating the use of auwfuchs to monitor bioaccumulation of arsenic, cadmium, chromium, copper and zinc. Wat Res 19:1157–1165
Nimick DA, Cleasby TE (2000) Water-quality data for streams in the Boulder River watershed, Jefferson County, Montana. U.S. Geological Survey Open-File Report 00-99, 70 pp
Nimick DA, Cleasby TE (2004) Trace elements in water in streams affected by historical mining. In: Nimick DA, Church SE, Finger SE (eds), Integrated investigation of environmental effects of historical mining in the Basin and Boulder mining districts, Boulder River watershed, Jefferson County, Montana. U.S. Geological Survey Professional Paper 1652. Reston, Virginia, pp 155–190
Rantz SE (1982) Measurement and computation of streamflow, v.1, Measurement of stage and discharge. U.S. Geological Survey Water Supply Paper 2175, pp 1–284
Ruttner F (1968) Fundamentals of Limnology. University of Toronto Press, Toronto, Canada
Schemel LE, Kimball BA, Bencala KE (2000) Colloid formation and metal transport through two mixing zones affected by acid mine drainage near Silverton, Colorado. Appl Geochem 15:1003–1018
U.S. Environmental Protection Agency (1999) National recommended water quality criteria, Correction Washington, D.C., Office of Water, EPA 822-Z-99-001, 25 pp
U.S. Environmental Protection Agency (2001) 2001 update of ambient water quality criteria for cadmium. Washington, D.C., Office of Water, EPA 822-R-01-001, 35 pp
Verbost PM, Berntsen MHG, Kroglund F, Lydersen E, Witters HE, Rosseland BO, Salbu B, Wenderlaar Bonga SE (1995) The toxic mixing zone of neutral and acidic river water: acute aluminum toxicity in brown trout (Salmo trutta L.). Wat Air Soil Pollut 85:341–346
Ward JR, Harr CA (1990) Methods for collection and processing of surface-water and bed-material samples for physical and chemical analyses. U.S. Geological Survey Open-File Report 90-140. Reston, Virginia 71 pp
Woodward DF, Farag AM, Bergman HL, DeLonay AJ, Little EE, Smith CE, Barrows FT (1995) Metals-contaminated benthic invertebrates in the Clark Fork River, Montana: effects on age-0 brown trout and rainbow trout. Can J Fish Aquat Sci 52:1994–2004
Zar JH (1984) Biostatistical analysis. Prentice-Hall, Englewood Cliffs, NJ
Acknowledgments
We are grateful to Tom Cleasby, Jack Goldstein, Brad Mueller, Darren Rhea, and other USGS staff for excellent technical assistance and to two anonymous reviewers. This project was funded in part by the US Geological Survey Abandoned Mine Land Initiative and the USDA Forest Service, Ray TeSoro, project manager.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Farag, A.M., Nimick, D.A., Kimball, B.A. et al. Concentrations of Metals in Water, Sediment, Biofilm, Benthic Macroinvertebrates, and Fish in the Boulder River Watershed, Montana, and the Role of Colloids in Metal Uptake. Arch Environ Contam Toxicol 52, 397–409 (2007). https://doi.org/10.1007/s00244-005-0021-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-005-0021-z