Abstract
Pesticide pollution is one of the main current threats on water quality. This paper presents the potential and functioning principles of a “Wet” forest buffer zone for reducing concentrations and loads of glyphosate, isoproturon, metazachlor, azoxystrobin, epoxiconazole, and cyproconazole. A tracer injection experiment was conducted in the field in a forest buffer zone at Bray (France). A fine time-scale sampling enabled to illustrate that interactions between pesticides and forest buffer substrates (soil and organic-rich litter layer), had a retarding effect on molecule transfer. Low concentrations were observed for all pesticides at the forest buffer outlet thus demonstrating the efficiency of “Wet” forest buffer zone for pesticide dissipation. Pesticide masses injected in the forest buffer inlet directly determined concentration peaks observed at the outlet. Rapid and partially reversible adsorption was likely the major process affecting pesticide transfer for short retention times (a few hours to a few days). Remobilization of metazachlor, isoproturon, desmethylisoproturon, and AMPA was observed when non-contaminated water flows passed through the forest buffer. Our data suggest that pesticide sorption properties alone could not explain the complex reaction mechanisms that affected pesticide transfer in the forest buffer. Nevertheless, the thick layer of organic matter litter on the top of the forest soil was a key parameter, which enhanced partially reversible sorption of pesticide, thus retarded their transfer, decreased concentration peaks, and likely increased degradation of the pesticides. Consequently, to limit pesticide pollution transported by surface water, the use of already existing forest areas as buffer zones should be equally considered as the most commonly implemented grass buffer strips.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Point and non-point source pesticide pollution is of increasing concern and regulated through the Water Framework Directive (2000/60/EC) in Europe and the Clean Water Act (USEPA 1972) in the USA. To limit a transfer of pesticides from agricultural fields to surface waters, buffer zones can be implemented as complementary measures to those related to reduction of the pesticide application.
Buffer zones are areas that do not receive any pesticide application, and are located between agricultural fields, where pesticides are applied, and aquatic ecosystems that need to be protected against pesticide pollution. Buffer zones work by intercepting and reducing pesticide transfer to downstream aquatic ecosystems. They can affect pollution transported either by air (crop-dusting drift) or water via surface runoff or shallow hypodermic flows (i.e., subsurface tile-drains or agricultural ditches) (Reichenberger et al. 2007). On pesticide drift, buffer zones act simply by keeping aquatic ecosystems away from areas where pesticides are applied. For their impact on water pollution, buffer zones will be classified here into two main types depending on their hydrologic regime. “Dry” buffer zones are systems that enhance water infiltration, and include the well-known “grass buffer zones”, as well as landscape features like prairies, hedges, and woods. “Wet” buffer zones like constructed wetlands are those exhibiting free surface water and are characterized by distinct vegetation. The efficiency of buffer zones is closely related to their ability to intercept water flows and support pesticide degradation by micro-organisms (Carluer et al. 2011; CORPEN 2007; Lacas et al. 2005). Degradation is the ultimate goal of buffer zones as it results in breakdown of pesticide parent molecules, contrary to non-degradative processes like adsorption–desorption that only delay pesticide transfer. Biodegradation occurs through different processes depending on the type of buffer zone. In “Dry” buffer zones, the initial key step before degradation relies on pesticide retention via partially reversible sorption in buffer zone soil and organic substrates present in top of buffer zone surfaces (e.g., grass, dead leaves) (Benoit et al. 2008; Margoum et al. 2006; Passeport et al. 2011). During contaminated flow infiltration, adsorption onto buffer zone soil particles is the most efficient process for limiting downstream pesticide pollution (Muscutt et al. 1993). Conversely, in “Wet” buffer zones, long water retention times are needed to promote abiotic and microbial degradation of pesticides or their uptake by plants. In “Dry” buffer zones where most incoming flows are infiltrated, variations in temperature of the soil pore water are dampened by soil temperature buffer effect. Temperature is therefore likely to affect microbial processes to a lower extent in “Dry” than “Wet” buffer zones.
An intermediate case between “Dry” and “Wet” buffer zones is the subject of the present paper. Indeed, if “Dry” buffer zones’ infiltration capacity is drastically limited due to soil compaction or large soil clay content, surface runoff can temporarily be produced on the top of the soil thus hindering an efficient functioning of this type of buffer zones (Souiller et al. 2002). In a forest buffer zone, this constraint is suspected to have a smaller effect on pesticide reduction than in the more commonly known grass buffer strips. Indeed, when a forest buffer soil is saturated, surface runoff flows through a thick mat of dead leaves in decay (i.e., the litter layer), which is rich in organic matter. The role of organic matter in enhancing pesticide sorption has been highlighted in the literature. Particulate organic matter in a forest soil was shown to exhibit high sorption capacities for diflufenican, epoxiconazole, metazachlor, and isoproturon, although isoproturon and metazachlor were easily desorbed from the soil (Benoit et al. 2008; Passeport et al. 2011). Similarly, leaves in decay, as found in vegetated ditches or forest buffers, had higher sorption capacity than bottom sediments for isoproturon, metazachlor, diflufenican, and diuron (Margoum et al. 2006; Passeport et al. 2011).
Contrary to grass buffer zones whose efficiency is well documented (CORPEN 2007; Muscutt et al. 1993; USDA-NRCS 2000), very few studies were conducted at the field scale to demonstrate the efficiency of forest buffer zones for pesticide pollution mitigation (Gay et al. 2006; Lowrance et al. 1997; Pinho et al. 2007; Vellidis et al. 2002). Three of the above-mentioned papers were conducted on the same mixed buffer zone including grass and forest areas, and all four studies were carried out in Georgia, USA. In this buffer zone, atrazine and alachlor influent concentrations, i.e. 12.7–34.1 (atrazine) and 1.3–9.1 (alachlor) μg/L, were reduced to less than 1 μg/L for both a 38-m (Vellidis et al. 2002) and a 50-m long buffers (Lowrance et al. 1997). Infiltration and degradation of atrazine was also observed by Gay et al. (2006) who reported more than 84 % removal for atrazine and its metabolites. Combining laboratory and field studies, Pinho et al. (2007) found that 47 and 28 % mass removal rates for atrazine and picloram, respectively, were mainly attributed to forest infiltration capacity. In addition, they concluded that adsorption processes only played a role for reducing concentrations of atrazine, but not for those of picloram.
Most of these studies either involved comparing pesticide concentrations and loads between forest buffer zones’ inlet and outlet, or were conducted under laboratory controlled conditions. The present paper describes an experiment conducted at an intermediate scale, in the field, under real climatic conditions, but under partially controlled hydrologic conditions. An injection experiment was conducted, involving a high sampling frequency at the outlet of a forest buffer zone to monitor the transfer dynamics of pesticides through the system. The objective of this experiment was to demonstrate at the field scale the potential of forest buffers to reduce the concentrations and loads of pesticides with different physico-chemical properties. The design of this experiment provided crucial information on removal processes of pesticides under real dynamic conditions.
Site description
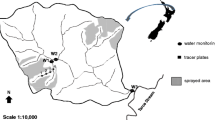
The forest buffer zone (0.5 ha) is located at the outlet of a tile-drained agricultural watershed (46 ha) at Bray (France), and described in details in Passeport et al. (2010). The forest main components were oak trees (Quercus robur, approximately 60 years old). To intercept watershed outlet flows, distribute them through the forest buffer, and collect them before they return to the stream, two ditches were constructed in the forest inlet and outlet (Fig. 1). At the inlet, a flat ditch with no outlet first filled in with a portion of watershed outlet water volumes (Fig. 1). Once full, water overflowed along the ditch length and ran off through the experimental forest buffer as a sheet flow. At the forest buffer outlet, water runoff was collected in another ditch before it reached a natural creek (Fig. 1). The forest buffer soil is a silty clay loam considered as a hydromorphic soil with the clay and organic carbon contents of 28.4 and 6.91 %, respectively.
Diagram of the “Wet” forest buffer zone including the experimental plot (hatchings), the locations of the injection solution (I), flowrate measurements (I), and outlet sampling (S). The gray rectangles represent constructed ditches for inlet overflow distribution (bottom ditch), and outlet flow collection (top ditch). The red cross on the bottom ditch (i.e., the inlet ditch) indicates that this ditch has no outlet at this point, as water exits the ditch by overflowing along its length. The black arrows indicate flow direction
Material and methods
Chemicals
An injection solution (20.5 L) was prepared with six pesticides and potassium bromide as a conservative tracer. Pesticides were provided by farmers and diluted in deionized water before injection. For information only, the commercial solutions that were used are indicated into parentheses: three herbicides, glyphosate (Glyphogan), isoproturon (Isoproturon), and metazachlor (Novall), and three fungicides, azoxystrobin (Priori Xtra), cyproconazole (Amistar Xtra), and epoxiconazole (Opus) were selected for their contrasting properties and wide use in agriculture (Table 1). Table 2 presents pesticide concentrations, masses, and expanded uncertainties in the injection solution. Injection concentrations were selected to represent peak concentrations that can punctually reach agricultural tile-drained watershed outlets (Passeport et al. 2013).
Tracer experiment
The forest buffer tracer experiment took place for a period of 14 days, from 19 February 2009, 10:50 to 5 March 2009, 13:20 in a reduced portion of the forest buffer, using watershed outlet flows as incoming flows into the forest buffer. The experimental plot was delimited with soil border levees leading to a 54-m2 surface area (36 m × 1.5 m). Only one significant rainfall event occurred on 308.5 h after the start of the experiment, with a cumulative rainfall depth of 9.94 mm, measured with the on-site tipping bucket rain gauge (R01 3030A Danae, Précis Mécanique, Bezons, France). Water temperature was 5.9 ± 3.7 °C during the course of the experiment, and was close to or greater than monthly averages. The inlet flow rate was 0.32 ± 0.08 L/s. At the outlet, a flow restriction helped manually measuring flow rates by frequently timing the filling of a container with a known volume. These gaugings were compared to the corresponding inlet flow rates resulting in a 0.61 ± 0.28 ratio on average between outlet and inlet flow rates. Outlet flow rates were estimated applying this ratio to continuously measured inlet flow rates for further calculation purposes. Water from the watershed was allowed to flow through the forest buffer experimental plot on 18 February 2009 at 15:50, in order to saturate the soil and ensure a permanent flow rate for the next day injection. Two peristaltic pumps (Eijkelkamp 12 V SDEC Reignac-sur-Indre, France) were used to ensure a 0.30 L/s injection flow rate during 78 s. Grab water samples or samples collected by means of a time-dependent automated sampler (ISCO 3700 Neotek, Trappes, France) were taken at the outlet of the experimental plot. The sampling frequency was modified along the course of the experiment: every 15 min for the first 7 h, every 30 min until 28.5 h after the start of the experiment, then every 3 h until 94 h since injection, and every 10 h from days 4 to 10 following the start of the experiment. Finally, five grab water samples were taken at forest buffer inlet to control pesticides’ background concentrations coming from the artificially drained watershed.
Analytical method
Water sample analysis
Subsamples (4 mL) were taken from water samples, filtered (0.20 μm, PET 20/15 MS Macherey-Nagel, VWR) and analyzed for bromide with ion chromatography (DX-120, Dionex, Sunnyvale, CA, U.S.A.) and an IonPac AS9-HC column. The limit of quantification (LQ) was 1 mg/L. Remaining water samples were frozen until further pesticide analysis was conducted by a subcontracted laboratory (Institut Pasteur de Lille, France). Metazachlor, cyproconazole, epoxiconazole, azoxystrobin, isoproturon and two of its metabolites, desmethylisoproturon and 1-(4-isopropylphenyl)urea, were extracted by solid-phase extraction on pre-filtered samples (syringe filters Macherey-Nagel PET-20/15 MS), and then analyzed by high-performance liquid chromatography (Agilent 1200) coupled with triple quadrupole mass spectrometry (LC-MS-MS, Micro Mass Ultima or API 4000 Sciex). Limits of quantification were 0.02 μg/L for these seven pesticides and metabolites. Glyphosate and its main metabolite, AMPA, were first derivatized with 9-fluorenylmethyl chloroformate (FMOC) before LC-MS-MS analysis (LQ = 0.1 μg/L for both glyphosate and AMPA).
Litter and soil sampling and analysis
Litter (i.e., dead leaves in decay forming a mat on top of the forest soil), and soil grab samples were taken in the forest experimental plot at the end of the tracer experiment. Another litter and soil samples were collected outside the experimental plot to compare with those collected inside the experimental plot. All samples were frozen before pesticide analysis. Glyphosate and AMPA were extracted by ultrasonic waves in water, then derivatized with FMOC and analyzed by LC-MS-MS, whereas extraction for the other molecules from soil samples was carried out with ultrasonic waves in acetone. Extracts were analyzed by LC-MS-MS. Litter samples were treated with an internal procedure developed by the laboratory (Institut Pasteur de Lille). Limits of quantification were 0.01 mg/kg dry weight for each compound.
Data analysis
The hydraulic retention time was calculated based on the bromide conservative tracer using the moment theory on residence time distribution (see Passeport et al. (2010), Kadlec and Wallace (2008)). Loads were calculated as follows:
where L out(t i ) is the outlet cumulated mass of a pesticide at time t i after injection, C out is the concentration of a pesticide at the outlet (in microgram per liter). Concentrations lower than the limits of quantification were set to the LQ divided by 2. No difference was provided by the laboratory for detection and quantification thresholds. Dimensionless concentrations were calculated by dividing outlet concentrations (C) by maximal concentration (C max) thus helping graph comparisons among pesticides and bromide tracer.
Uncertainty analyses
Uncertainties on pesticide loads were calculated from uncertainties on concentration and flow rates.
Uncertainties (u) on each concentration C j , noted u(C j ), were calculated in regard to two main components. First, from the uncertainty (P, %, corresponding to twice the coefficient of variation) due to the analytical procedure provided by the laboratory, we calculated the analytical uncertainties, noted u an(C j ) by Eq. (2) below.
Second, as explained above, data below the LQ were replaced with LQ/2 for mass calculation purposes, although such non-quantifiable concentrations could take any value between zero (min) and LQ (max). In such cases, the best estimate of the uncertainty generated by this arbitrary choice (herein noted u LQ(Cj)) is given by Eq. (3) (EURACHEM/CITAC 2000).
Consequently, unless concentrations were above the LQ, in which case u LQ(Cj) was zero, combined uncertainties on concentrations were determined as follows (Eq. (4)):
Uncertainties on loads (u(L j )) were obtained from Eq. (5) using the first-order Taylor series expansion. The variables were assumed to be independent, thus allowing to neglect the covariance term (Eq. (5)).
Uncertainties on forest outlet flow rate estimations (u(Q out)) were the result of two sources that were combined together according to a similar equation as Eq. (5) for uncertainties on loads. First, a constant uncertainty was calculated for the inlet flow rate (u(Q in) = 0.0059 L/s) from data given by the manufacturer. Second, the uncertainty due to estimation of the forest outlet flowrate (Q out) by 0.61 × Q in was calculated as u(Q out) = 0.61/√3 (EURACHEM/CITAC 2000). The uncertainty on the time period (u(δt)) was assumed to be null.
Concentration and load data were presented with their corresponding expanded uncertainties, U(C j ) and U(L j ), respectively, determined using a coverage factor of 2 for a 95 % level of confidence.
Statistical analyses
Pearson correlation coefficients were determined with the R software (R Development Core Team 2005) to detect possible correlations among pesticide concentrations, injected masses, and pesticide physico-chemical properties.
Results
Hydrology
Water ran off through the forest buffer experimental plot as a shallow sheet flow with an average outlet flow rate of 0.18 ± 0.11 L/s (average ± expanded uncertainty for 95 % confidence interval). Bromide started to be detected 1 h after injection and reached a concentration peak 1.8 h after injection (Fig. 2). Bromide recovery rate and hydraulic residence time were 74 % and 6.3 h, respectively.
Flowrate at the forest outlet (gray triangle, bottom panel, in liter per second), and dimensionless (C/C max) concentration pattern during the first 24 h (left panels) and the next 350 h (right panels) after injection, for molecules that exhibited the clearest transfer pattern: metazachlor (white triangles), azoxystrobin (white diamonds), cyproconazole (white circles), and bromide (black squares). The double slash bars (//) indicate a change in time step. C concentration at time t; C max peak concentration measured 2 h (metazachlor, azoxystrobin, and cyproconazole) and 1.8 h (bromide) after injection. No rainfall event occurred during the first 24 h; rain beyond 24 h (bottom-most right-hand side panel) is plotted on the right-hand vertical axis, in reverse order. Error bars correspond to dimensionless expanded uncertainties, i.e., expanded uncertainties on concentrations (U, coverage factor = 2), divided by C max
Inlet water quality
During the experiment, watershed tile-drain flows continuously entered the experimental plot at a controlled flow rate of 0.3 L/s. To determine if some of the studied pesticides entered also the experimental plot via watershed flows during the course of the experiment, five grab water samples were taken at the forest inlet during the course of the experiment. Non-negligible concentrations of isoproturon, desmethylisoproturon, glyphosate, AMPA and metazachlor were measured (Table 3). Epoxiconazole was detected once (6.8 h after injection) but with a concentration at the limit of quantification. The most recent applications of glyphosate and metazachlor on the Bray watershed were approximately 16 months before the start of the experiment. The relatively high concentrations of isoproturon (higher than 1.2 μg/L, Table 3) measured at the watershed outlet may be explained by its application only 2 months before the experiment.
Pesticide dynamics description
Table 4 presents the main characteristics for pesticide concentration peaks, dynamics and mass balances. Apart from isoproturon, concentrations were lower than 0.50 μg/L for AMPA and metazachlor, and did not exceed 0.15 μg/L for the other pesticides (glyphosate, azoxystrobin, epoxiconazole, cyproconazole, desmethylisoproturon, and 1-(4-isopropylphenyl)urea).
Only injections of metazachlor, azoxystrobin and cyproconazole resulted in a clear transfer pattern at the forest plot outlet (Fig. 2). Two hours after injection, these pesticides exhibited concentration peaks of 0.48 ± 0.10, 0.08 ± 0.02, and 0.07 ± 0.02 μg/L for metazachlor, azoxystrobin, and cyproconazole, respectively. These concentration peaks were observed closely after that of the conservative tracer, which was recorded 1.8 h after injection (Table 4). Azoxystrobin and cyproconazole concentrations were consistently at or below the LQ 10.0 and 13.5 h after injection, respectively, and remained at these levels until the end of the monitoring period. Metazachlor concentrations also significantly decreased during the next 6 h following concentration peak time and stabilized between 0.17 ± 0.04 and 0.28 ± 0.06 μg/L until 82.5 h after injection. A second concentration decrease with values at or below the LQ was then observed for metazachlor until the occurrence of a rainfall event, 308.5 h after injection, which rapidly generated a new concentration peak at 0.44 ± 0.09 μg/L.
For glyphosate, AMPA, epoxiconazole, and 1-(4-isopropylphenyl)urea, concentrations at the forest plot outlet were so low that only a qualitative assessment of the data can reasonably be performed. In addition, high background concentration levels of isoproturon and desmethylisoproturon hindered an accurate quantitative analysis of the data for these two molecules.
In all water samples, glyphosate concentrations were below the LQ and those for AMPA never exceeded 0.30 ± 0.08 μg/L. No temporal variation was observed for these molecules, besides two small AMPA concentration rises, one after injection (between 1.8 and 3.8 h) and a second one after the rainfall event (between 318.5 and 328.5 h).
Maximum concentrations for isoproturon, desmethylisoproturon, and 1-(4-isopropylphenyl)urea were 1.70 ± 0.41, 0.14 ± 0.03, and 0.02 ± 0.01 μg/L, respectively, before the rainfall event at 308.5 h after injection. Isoproturon and desmethylisoproturon concentrations were always higher than the LQ, whereas 1-(4-isopropylphenyl)urea were at or below the LQ. Isoproturon concentrations seemed to exhibit a peak 2.5 h after injection, followed by a decreasing trend down to a fairly steady concentration level between 0.80 ± 0.19 to 1.10 ± 0.26 μg/L. However, this pattern is not clear due to high background level responsible for this steady level in isoproturon concentration (Table 3). Desmethylisoproturon concentrations exhibited a fairly steady trend with no significant variation over time. Sharp (for isoproturon) and moderate (for desmethylisoproturon) concentration decreases started 52 h following injection before reaching down to 0.31 ± 0.07 and 0.06 ± 0.01 μg/L, respectively. Afterwards, the rainfall event occurring at 308.5 h generated a drastic increase in the concentration of both molecules, leading to peak concentration values of 6.50 ± 1.56 (isoproturon) and 0.37 ± 0.09 μg/L (desmethylisoproturon).
Epoxiconazole concentrations at the forest plot outlet were all very low. However, a concentration rise up to 0.03 ± 0.01 μg/L (one value at 0.04 ± 0.01 μg/L at 2.8 h) was observed between 1.5 and 7.0 h following injection. Epoxiconazole concentrations then slowly decreased to an average concentration of 0.02 ± 0.01 μg/L from 7.5 to 52.5 h and stabilized at or below the LQ afterwards. As similarly observed for glyphosate, azoxystrobin, and cyproconazole, and due to low concentrations at the inlet, the rainfall event occurring at 308.5 h did not trigger an observable increase in epoxiconazole concentrations.
Concentration peaks for the injected molecules were significantly correlated (p value = 1.75 × 10−5) with background concentrations, highlighting the strong influence that this artifact exerted on the results. The second strongest correlation (despite not significant at a α = 5 % significance level) was between pesticide concentration peaks and injected masses. Because of the strong influence of these two factors on concentration peaks, no other strong correlation was found between peak concentrations and pesticide physico-chemical properties listed in Table 1. The ratios between concentration peaks and injected mass were calculated. Small ratios indicate low concentration peaks compared to injected masses and thus possible higher retention in the forest plot. It is interesting to note that these ratios decreased (i.e., retention increased) while increasing pesticide sorption coefficients. Sorption is therefore suspected to play an important role in such buffer zones. However, with this small dataset, no statistically significant correlations were found between the ratios and the pesticide sorption properties.
Mass balances
Uncertainties on forest outlet flow rates and concentrations resulted in large uncertainties on calculated loads. Treating the “<LQ” data as concentrations equal to LQ/2 had significant impacts on loads calculated on pesticides whose concentrations were frequently below the LQs (e.g., glyphosate, AMPA, 1-(4-isopropylphenyl)urea, epoxiconazole). Figure SM-1 (Supplementary Material, SM) shows comparisons between 24-h percent recoveries calculated under different initial assumptions on how to treat “<LQ” data, whether “<LQ” were treated as 0, as the LQ value, or as LQ/2. For example, as glyphosate was almost never quantified above the LQ, a 40 % difference in recovery was noted between the two extreme cases (“<LQ” = 0 and “<LQ” = LQ). Conversely, calculated recovery rates for isoproturon were independent on initial assumption on “<LQ” data because isoproturon concentrations were never lower than the LQ. In addition, background concentrations coming from the watershed for isoproturon, desmethylisoproturon, and metazachlor, also strongly affected mass balances and hindered drawing reasonable conclusions.
In the forest buffer soil and dead leaves, isoproturon and epoxiconazole were quantified at the limit of quantification level (Table SM-1). Quantification occurred mainly in the inlet and central portions of the experimental zone. In addition, both pesticides were quantified in the litter layer; whereas, only isoproturon was also quantified in the soil. None of the other pesticides and metabolites was detected in the soil or litter.
Discussion of the results for these pesticides will be mostly based on their transfer dynamics. Consequently, loads are only presented here for azoxystrobin and cyproconazole. The cumulated loads for these two molecules at the forest outlet were integrated over a period between the injection and the time when the outlet concentration equalled that of the inlet, namely, 10.0 h for azoxystrobin and 13.5 h for cyproconazole (Table 4). At the forest outlet, 22 and 45 % of the injected masses of azoxystrobin and cyproconazole, respectively, were recovered, corresponding to 247 ± 60 μg (azoxystrobin, total mass ± expanded uncertainty) and 257 ± 55 μg (cyproconazole).
Discussion
Hydrology
The ratio between outlet and inlet flow rates (0.61), and the bromide recovery rate (74 %) are suggestive of some water losses outside the experimental plot, via infiltration, possibly due to poor soil levee compaction, earthworm burrows, and tree roots. Indeed, tree root system probably supported some of the downward water losses, especially because this oak forest has been established for more than 60 years, and was therefore associated with wide and numerous roots. In addition, the presence of the forest outlet ditch helped drain any potential shallow water table that could have limited infiltration. For soils affected by an impervious layer close to the soil surface, a shallow water table could form and move upward to show on the surface. In such a case, a different behavior of water and pesticide transfer would certainly be observed. The particularity of this forest buffer site is the high soil clay content (28.4 %) which is likely to be the main factor limiting water losses via infiltration. This is different from most “Dry” buffer zones’ functioning where infiltration is crucial for pesticide removal. This is also the reason why this forest buffer is classified as a “Wet” buffer zone, which exhibits a shallow water table for a longer period of time than in classic “Dry” buffer zones. Sideward water losses through the forest experimental plot delimiting soil levees might also explain part of the water losses. Finally, because this experiment was conducted in the winter, evaporation and water uptake by vegetation were suspected to be negligible. Overall, for an on-site study conducted under real climatic conditions, a 74 % bromide recovery can be considered satisfactory. It should also be noted that, for the purpose of this experiment, a narrow experimental plot was constructed by raising soil levees thus resulting in a one-dimensional plug flow-like water transfer. A wider experimental plot would provide more opportunities for mixing and sideward flows, which could result in a longer residence time.
Delayed transport of pesticides
As expected, bromide and pesticide dynamics differed in two points. First, pesticide concentration peaks were measured later than that of the bromide conservative tracer. Second, the plots of pesticide concentration over time exhibited wider bell-shaped curves than for bromide (Fig. 2). These data indicate that a lag is present that delays the transport of pesticides compared to that of bromide.
Forest buffer efficiency for pesticide removal
Mass balances (recovery rates) were only calculated for azoxystrobin and cyproconazole. The lower recovery rate of azoxystrobin (22 %) indicates that it was better removed than cyproconazole (45 % recovered). Injected pesticide masses directly affected pesticide concentration peaks. As observed for grass buffer zones (Lacas et al. 2005), vegetated ditches (Bennett et al. 2005; Moore et al. 2001) or constructed wetlands (Moore et al. 2006), buffer zone length is critical in controlling pesticide removal. It is reasonable to assume that a smaller effect would be observed if a longer or wider experimental plot was studied. However, to date, more research is needed to help site managers in designing the efficient forest buffer zones. A key conclusion of our study relies on the fact that, for most pesticides, very low concentrations were measured at the forest outlet, thus demonstrating the efficiency of such buffer zones for pesticide removal. Because of the fine sampling frequency used in this study, which is very rarely done at the field scale, a few hypotheses can be raised to explain the observed transfer pattern.
Sorption as part of a complex set of removal processes
As water ran off as a shallow sheet flow, adequate surface contact between forest substrates (soil and litter) and pesticides was possible, which is essential to enhance pesticide retention (Margoum et al. 2003). Azoxystrobin and cyproconazole dissipation rates and sorption are known to increase with organic matter content, despite they were likely slowed down due to the low temperatures measured in this study (Gardner et al. 2000; Ghosh and Singh 2009; Singh and Singh 2010). Sorption properties cannot explain pesticide removal efficiency alone. Indeed, both pesticides have similar sorption coefficients, but differ in their solubilities and hydrophobicities (log K ow, see Table 1): cyproconazole is more soluble in water than azoxystrobin; however, it is also more hydrophobic than azoxystrobin which significantly complicates the interpretation of these results. It is interesting to note that azoxystrobin injected mass was roughly twice that of cyproconazole, but the former was better eliminated than the latter through the forest buffer experimental plot. Potential for removal of these pesticides by forest buffer exists, but more research is needed to elucidate the removal processes.
The high sorption coefficients of glyphosate, AMPA (K f/oc = 8,027 mg1−nf kg−1 Lnf (n f = 0.8), FOOTPRINT (2012)), and epoxiconazole may partly explain their low concentrations measured at the forest outlet. Contrary to glyphosate and AMPA, epoxiconazole was detected on dead leaves at the forest plot inlet and middle zones 14 days after injection even after large rainfall events. This supports a possible strong adsorption of epoxiconazole onto the forest litter. Because epoxiconazole was not detected in the soil below the litter layer, it is likely that the litter layer acted as a key sorption material that prevents strongly sorbing pesticides from leaching to deep soil horizons. Conversely, the detection of isoproturon in both the litter and soil components might be due to the lower sorption coefficient of this herbicide that could therefore reach deeper horizons. Previous laboratory results reported a high sorption of epoxiconazole on hydrophobic humic substances (Roy et al. 2000). In addition, a laboratory adsorption and desorption experiment conducted with the forest soil and litter from the herein studied site concluded on a large sorption potential of both of these substrates for isoproturon, metazachlor and epoxiconazole (Passeport et al. 2011). It was found that the sorption of epoxiconazole in forest soil and litter was much larger than that of isoproturon and metazachlor. Moreover, the sorption of the latter two pesticides was similar in the forest soil; however, the forest litter sorbed better metazachlor than isoproturon (Passeport et al. 2011). Margoum et al. (2001) studied isoproturon sorption on dead leaves and soil from an oak wood and found that increasing contact time enhanced isoproturon sorption, particularly on leaves. This “ageing” effect was shown to enhance pesticide sorption with soil components over time (Gevao et al. 2000; Mamy and Barriuso 2007). Benoit et al. (2008) investigated the sorption and desorption of two herbicides with different physico-chemical properties (isoproturon and diflufenican). They showed that enhanced sorption was found in high organic matter hydrophobicity (larger for forest soil than grass buffer zone soil), and low organic matter particle size (which determines the number of sorptive sites). The results presented here are a field-scale demonstration that forest soil and litter delay the transfer of pesticides, which is likely controlled by sorption–desorption processes. It is likely that sorption played a more significant role than degradation in pesticide removal, due to low retention time recorded in the forest experimental plot. However, as mentioned above, sorption coefficients (K f/oc) were probably not the unique parameters, which might explain these results; solubility and hydrophobicity characteristics are highly suspected to play an essential role in explaining pesticide removal. However, it seems that for pesticides presenting high K f/oc values (>1,000 mg1−nf kg−1 Lnf), it is likely that sorption is the main process, which reduced the concentrations of pesticides at the outlet. Increasing water retention time in forested buffer zones could more strongly retain some pesticides. Nevertheless, no statistical correlations between the peak concentration to injected mass ratios and the sorption coefficients were found. A complex set of factors are therefore likely to affect pesticide concentrations in forest buffers. The three fungicides and the three herbicides had both overlapping and distinct physico-chemical properties which prevented from exhibiting a clear difference in the behavior of these two pesticide groups. Molecular mass, structure, aromaticity, solubility, hydrophobicity, number of halogenated groups, sorption coefficients, half-lives, are among those characteristics that could potentially affect pesticide removal. More investigation is needed to identify the most influent factors and understand more thoroughly their interactions with pesticides. A meta-analysis conducted by Stehle et al. (2011) on constructed wetland (“Wet” buffer zones) potential for pesticide removal also showed the absence of a statistically significant relationship between the pesticide sorption coefficients and the efficiencies of the constructed wetland.
Degradation and remobilization of pesticides
Field half-lives for azoxystrobin and cyproconazole are much longer than the duration of this study; in addition, the experiment was conducted during the coldest month of the year. Therefore, degradation as one of the possible removal processes in the forest buffer zone is likely less important for both pesticides. However, degradation of azoxystrobin was found to occur through cometabolism processes, which might take place in the forest buffer due to high availability of organic substrates (Bending et al. 2006). In addition, azoxystrobin half-life is lower in flooded than non-flooded soils (Singh and Singh 2010), which suggests high potential for Bray “Wet” forest buffer zone for azoxystrobin degradation. Due to the moderately long half-lives of their parent molecules, glyphosate and isoproturon, the detection of AMPA and desmethylisoproturon at the beginning of the experiment can hardly be attributed to the injected parent molecules. It should be noted that AMPA, isoproturon, and desmethylisoproturon were detected at the forest plot inlet indicating that these molecules were also transferred to the experimental plot from the tile-drain watershed. Glyphosate and isoproturon were applied previously on the agricultural watershed and may have been partially degraded in the catchment and forest buffer soils thus generating these metabolites. Madrigal et al. (2007) found that isoproturon biodegradation in forest soil top horizon was greater than in deeper horizons, the former presenting larger carbon and biomass content than the latter. However, at the time-scale of this experiment (approx. 350 h), it is unlikely that degradation was a dominant process explaining pesticide losses.
Increases in the concentrations of metazachlor, isoproturon, desmethylisoproturon, and AMPA were observed after the rainfall event occurring 308.5 h after injection. This phenomenon was likely due to remobilization of previously retained pesticides, either adsorbed or temporarily stored in water puddles. Isoproturon and metazachlor desorption from forest soil and litter was also observed in laboratory experiments (Benoit et al. 2008; Passeport et al. 2011). Similarly, the initial increases in AMPA and desmethylisoproturon concentrations between 1.8 and 3.8 h after injection could be due to remobilization of these metabolites.
“Dry” vs. “Wet” buffer zone
In this study, the “Wet” forest buffer soil had a high clay content thus limiting downward infiltration. Even if water losses via infiltration might occur, it could not explain alone the observed pesticide removal. It is a fundamental difference with “Dry” buffer zones like grass areas, where infiltration plays a crucial role. The second major difference between grass and forest buffer zones lies in the presence of thick litter layer rich in organic matter in the latter. The litter provides many sorption sites for pesticides and is biologically active, thereby biodegrading retained pesticides. Consequently, when buffer zone soil is saturated, pesticide sorption and degradation should more easily occur in forested areas than in grass areas, provided that the contaminated water runs off through the litter layer as a shallow and slow water flow.
Conclusions
The objective of this experiment was to demonstrate at the field scale the potential of forest buffer zones to reduce the concentrations and loads of pesticides presenting a wide range of physico-chemical properties. Very low concentrations were measured at the forest outlet thus suggesting a potential of the forest buffer to effectively reduce the pollution with pesticides. Understanding processes, which govern the removal of pesticides through the forest buffer was beyond the scope of this study. However, the fine sampling frequency used in this study helped to provide some explanations about the observed dynamics of pesticide transfer through the forest buffer zone. At short time-scales (lower than a month), retention processes are suspected to dominate. Our results highlighted the dual role of organic matter. On the one hand, organic substrates enabled rapid adsorption of pesticides transported in highly contaminated flows. On the other hand, when fresher (i.e., less contaminated) flows crossed the forest buffer, previously adsorbed pesticides were shown to desorb thus being released back to the water column. Organic matter also plays an indirect role in this process as it supports growth of microbial populations. Any forested area adequately located in the landscape could be used as an efficient buffer zone for reducing pesticide pollution. Indeed, even old wood that were not necessarily well maintained could be good candidates for buffering pesticide contaminated flows provided a thick litter layer has had time to accumulate over time. At a short time scale (here approx. 350 h), highly organic material would therefore mainly act as a retarding factor that temporarily affect pesticide dynamics. For extended periods of water retention, degradation reactions leading to metabolites are likely to occur, however, more research is needed to confirm the extent of pesticide degradation that could be achieved. The results of this study are suggestive of a high potential of “Wet” forest buffer zone for the reduction of downstream pesticide concentrations and loads. Further research should investigate the efficiency of forest buffers for pesticide removal (1) under various climatic conditions, and for a wide range of forest buffer (2) sizes and shapes, and (3) locations in the watershed (headstream vs. downstream). Such results are needed to better understand pesticide fate and the role of the litter layer, and to establish guidelines to design forest buffer zones and incorporate them in land management strategies.
References
Bending GD, Lincoln SD, Edmondson RN (2006) Spatial variation in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties. Environ Pollut 139:279–287
Bennett ER, Moore MT, Cooper CM, Smith S, Shields FD, Drouillard KG, Schulz R (2005) Vegetated agricultural drainage ditches for the mitigation of pyrethroid-associated runoff. Environ Toxicol Chem 24:2121–2127
Benoit P, Madrigal I, Preston CM, Chenu C, Barriuso E (2008) Sorption and desorption of non-ionic herbicides onto particulate organic matter from surface soils under different land uses. Eur J Soil Sci 59:178–189
Carluer N, Tournebize J, Gouy V, Margoum C, Vincent B, Gril JJ (2011) Role of buffer zones in controlling pesticides fluxes to surface waters. Proc Environ Sci 9:21–26
CORPEN GZT (2007) Les fonctions environnementales des zones tampons, Les bases scientifiques et techniques des fonctions de protection des eaux. Ministère de l'écologie dlé, du développement durable et de l'aménagement du territoire et Ministère de l'agriculture et de la pêche (Hrsg.), Paris, France
EURACHEM/CITAC (2000) Guide CG 4 Quantifying uncertainty in analytical measurement - Second Edition. http://www.measurementuncertainty.org/mu/QUAM2000-1.pdf Verified 17 March 2013.
FOOTPRINT (2012) Pesticide Properties Database (PPDB) developed by the Agriculture & Environment Research Unit (AERU), University of Hertfordshire, funded by UK national sources and the EU-funded FOOTPRINT project (FP6-SSP-022704). Verified 17 March 2013
Gardner DS, Branham BE, Lickfeldt DW (2000) Effect of turfgrass on soil mobility and dissipation of cyproconazole. Crop Sci 40:1333–1339
Gay P, Vellidis G, Delfino JJ (2006) The attenuation of atrazine and its major degradation products in a restored riparian buffer. T ASABE 49:1323–1339
Gevao B, Semple KT, Jones KC (2000) Bound pesticide residues in soils: a review. Environ Pollut 108:3–14
Ghosh RK, Singh N (2009) Effect of organic manure on sorption and degradation of azoxystrobin in soil. J Agr Food Chem 57:632–636
Kadlec RH, Wallace SD (2008) Treatment wetlands second edition. CRC, Boca Raton, FL
Lacas JG, Voltz M, Gouy V, Carluer N, Gril JJ (2005) Using grassed strips to limit pesticide transfer to surface water: a review. Agron Sustain Dev 25:253–266
Lowrance R, Vellidis G, Wauchope RD, Gay P, Bosch DD (1997) Herbicide transport in a managed riparian forest buffer system. T ASAE 40:1047–1057
Madrigal I, Benoit P, Barriuso E, Real B, Dutertre A, Moquet M, Trejo M, Ortiz L (2007) Pesticide degradation in vegetative buffer strips: grassed and tree barriers: case of isoproturon. Agrociencia 41:205–217
Mamy L, Barriuso E (2007) Desorption and time-dependent sorption of herbicides in soils. Eur J Soil Sci 58:174–187
Margoum C, Gouy V, Madrigal I, Benoit P, Smith J, Johnson AC, Williams RJ (2001) Sorption properties of isoproturon and diflufenican on ditch bed sediments and organic matter rich materials from ditches, grassed strip and forest soils. BCPC Symp Ser 78:183–188
Margoum C, Gouy V, Laillet B, Dramais G (2003) Retention of pesticides by farm ditches. Revue des sciences de l'eau 16:389–405
Margoum C, Malessard C, Gouy V (2006) Investigation of various physicochemical and environmental parameter influence on pesticide sorption to ditch bed substratum by means of experimental design. Chemosphere 63:1835–1841
Moore MT, Bennett ER, Cooper CM, Smith S, Shields FD, Milam CD, Farris JL (2001) Transport and fate of atrazine and lambda-cyhalothrin in an agricultural drainage ditch in the Mississippi Delta, USA. Agr Ecosyst Environ 87:309–314
Moore MT, Bennett ER, Cooper CM, Smith S, Farris JL, Drouillard KG, Schulz R (2006) Influence of vegetation in mitigation of methyl parathion runoff. Environ Pollut 142:288–294
Muscutt AD, Harris GL, Bailey SW, Davies DB (1993) Buffer zones to improve water quality: a review of their potential use in UK agriculture. Agr Ecosyst Environ 45:59–77
Passeport E, Tournebize J, Jankowfsky S, Proemse B, Chaumont C, Coquet Y, Lange J (2010) Artificial wetland and forest buffer zone: hydraulic and tracer characterization. Vadose Zone J 9:73–84
Passeport E, Benoit P, Bergheaud V, Coquet Y, Tournebize J (2011) Selected pesticides adsorption and desorption in substrates from artificial wetland and forest buffer. Environ Toxicol Chem 30:1669–1676
Passeport E, Tournebize J, Chaumont C, Guenne A, Coquet Y (2013) Pesticide contamination interception strategy and removal efficiency in forest buffer and artificial wetland in a tile-drained agricultural watershed. Chemosphere 91:1289–1296
Pinho AP, Matos AT, Morris LA, Costa LM (2007) Atrazine and picloram adsorption in organic horizon forest samples under laboratory conditions. Planta Daninha 25:125–131
R Development Core Team (2005) R: a language and environment for statistical computing, R Foundation, Vienna, Austria
Reichenberger S, Bach M, Skitschak A, Frede H-G (2007) Mitigation strategies to reduce pesticide inputs into ground- and surface water and their effectiveness: a review. Sci Total Environ 384:1–35
Roy C, Gaillardon P, Montfort F (2000) The effect of soil moisture content on the sorption of five sterol biosynthesis inhibiting fungicides as a function of their physicochemical properties. Pest Manag Sci 56:795–803
Singh N, Singh SB (2010) Effect of moisture and compost on fate of azoxystrobin in soils. J Environ Sci Heal B 45:676–681
Souiller C, Coquet Y, Pot V, Benoit P, Réal B, Margoum C, Laillet B, Labat C, Vachier P, Dutertre A (2002) Capacités de stockage et d'épuration des sols de dispositifs enherbés vis-à-vis des produits phytosanitaires Première partie: Dissipation des produits phytosanitaires à travers un dispositif enherbé; mise en évidence des processus mis en jeu par simulation de ruissellement et infiltrométrie. Etude et Gestion des Sols 9:269–285
Stehle S, Elsaesser D, Gregoire C, Imfeld G, Niehaus E, Passeport E, Payraudeau S, Schaefer RB, Tournebize J, Schulz R (2011) Pesticide risk mitigation by vegetated treatment systems: a meta-analysis. J Environ Qual 40:1068–1080
USDA-NRCS (2000) Conservation buffers to reduce pesticide losses. USEPA, Washington D.C., USA
USEPA (1972) Clean Water Act (CWA), National Pollutant Discharge Elimination System (NPDES). USEPA, Washington D.C., USA http://cfpub.epa.gov/npdes/cwa.cfm?program_id=45, Verified 17 March 2013
Vellidis G, Lowrance R, Gay P, Wauchope RD (2002) Herbicide transport in a restored riparian forest buffer system. T ASAE 45:89–98
Acknowledgments
This research was financially supported by the French National Research Institute of Science and Technology for Environment and Agriculture (Irstea), the French Ministère de l’Alimentation, de l’Agriculture et de la Pêche and the Direction Générale des Politiques Agricole, Agroalimentaire, et des Territoires, as well as the European LIFE project ArtWET (06/ENV/F/000133). The authors would like to thank Prof Yves Coquet for his helpful comments on earlier versions of this paper and Sylvain Moreau for his help in data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Hongwen Sun
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 166 kb)
Rights and permissions
About this article
Cite this article
Passeport, E., Richard, B., Chaumont, C. et al. Dynamics and mitigation of six pesticides in a “Wet” forest buffer zone. Environ Sci Pollut Res 21, 4883–4894 (2014). https://doi.org/10.1007/s11356-013-1724-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-013-1724-8