Abstract
Dental sleep appliances achieve a 50% response in around 65% of patients with obstructive sleep apnea and a complete response in 35–40%. This means that all practitioners will need to augment the effect of a dental sleep appliance at some stage. There are many ways in which adjunctive therapies can be used to augment both the objective and subjective outcomes of DSA therapy. This chapter discusses the use of multiple adjunct therapies including positional therapies, positive airway pressure therapies, therapies aimed at stabilizing or improving compromised anatomy in the upper airway, and therapies aimed at improving the subjective outcomes of sleep.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Dental sleep appliance
- Obstructive sleep apnea
- Positional obstructive sleep apnea
- Cognitive behavioral therapy for insomnia
- Circadian rhythm disorders
- Bright light therapy
- Oral EPAP
- Nasal EPAP
1 Introduction
Dental sleep appliances (DSA) are preferred to continuous positive airway pressure (CPAP) by a majority of patients with obstructive sleep apnea (OSA) [1]. As patient choice is an important aspect of successful OSA therapy, this will sometimes place clinicians in a situation where patients who would be better treated with CPAP may instead choose to use a DSA. Clinicians may need to incorporate adjunct therapies to maximize DSA clinical outcomes.
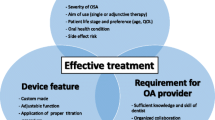
Adjunct therapies are added to DSA therapy to improve DSA outcomes. These outcomes may be the reduction of OSA severity, or they may involve quality-of-life issues that improve the tolerance to the DSA appliance (Fig. 12.1). The decision to start adjunct therapy can be made at multiple places during OSA treatment, both before and after the provision of a DSA based on the initial case assessment or close monitoring of polysomnographic or qualitative improvements in sleep. We will discuss adjunct therapies based on their ability to:
-
1.
Improve the severity of anatomical compromise
-
Single and multilevel surgical techniques.
-
Weight loss.
-
-
2.
Improve sleep phenotypes
-
Supine and REM OSA.
-
Positioners for supine OSA.
-
-
Airway collapsibility.
-
Continuous positive airway pressure.
-
Expiratory positive airway pressure.
-
-
-
3.
Improve quality-of-life issues for
-
Insomnia.
-
Circadian rhythm disorders.
-
2 Positional Therapies
2.1 Positional Obstructive Sleep Apnea: Prevalence and Definitions
The prevalence of positional obstructive sleep apnea (POSA) is around 50% in the general population and as much as 75% among patients with OSA [2]. Of the 75% of patients with POSA, 33–35% have exclusive POSA [2]. There are multiple definitions of POSA which vary around the required ratio of the supine: non-supine apnea-hypopnea index (AHI) [3,4,5,6,7,8,9]. The prevalence of POSA varies based on the different scoring criteria used. A composite-criteria incorporating the percentage of time spent in the best and worst sleep positions and the AHI in the best position had a superior specificity and positive predictive value compared to the classic definition of a 50% difference in the supine/non-supine AHI [3, 4]. The classic definition of POSA was not significantly affected by changes to scoring rules for OSA from the 2007 to 2012 criteria [10].
Positional therapy (PT) prevents or minimizes sleep in the supine position [11]. To achieve this, many devices use a design based on the traditional “tennis ball technique,” in which an external device is worn as a mechanical barrier to prevent rolling to the supine position. PT devices mimicking the concept of the tennis ball techniques include the “dorsal fin” or wedge-like devices, usually worn with a waistband, upper torso strap, shoulder holster, or vest to stabilize them [12]. Other PT options available include positional auditory alarms, vibrational alarms, vests, and positional pillows [13].
2.2 Mechanisms of Action of Positional Therapies
Positional apneics have an increased number and duration of apneas while in the supine position [14]. The supine position has detrimental effects on airway stability [15], lung volumes [16], and airway dimensions [17]. These effects may be mediated by lateral versus supine head and body positions [18]. The effect of the supine position on the severity of apneas is maintained across both REM and NREM sleep [11] and is consistent over a 6-year time span in 70.4% of positional apneics [19]. Positional therapies act by minimizing the time spent in the supine position [6, 20]. It is important to note that PT has no impact on non-supine respiratory events or events that are sleep stage dependent such as REM apnea. Lateral snoring and the lateral AHI will likely remain unchanged by PT. This emphasizes the importance of a careful pretreatment assessment of the baseline PSG for lateral versus supine snoring and apneas (Fig. 12.2).
2.3 Efficacy of Positional Therapy
There are no official guidelines about the use of PT as a standalone therapy either in patients with mixed POSA, or specifically in the 33% with supine-isolated POSA.
This subgroup of patients could be considered target candidates who would potentially benefit from PT as a standalone therapy; however, PT has not become widely adopted. Patients with exclusive POSA may be younger, less obese, and have a lower AHI [14, 21], lower sleepiness, higher snoring [22], and lower CPAP compliance [21, 23] than other apneic groups. CPAP is considered the gold standard therapy for OSA [24] and has generally been found to be superior to PT for treatment of POSA [25,26,27]. Recent studies have found that PT may be non-inferior to auto-adjusting positive airway pressure (APAP) in mild-moderate POSA [28] and may be efficacious in the treatment of some obese subjects [29].
Compliance with CPAP therapy is often poor [30, 31] which compromises the long-term health benefits of treatment. When CPAP compliance was <50% of the night in severe apneics (n = 292), greater reductions in the AHI were achieved using PT compared to CPAP [14]. Reducing the time spent, supine significantly reduced the number and severity of respiratory events [12] and may have reduced the number and magnitude of the swings in air pressure needed to maintain airway patency during POSA [32]. It is possible that joint use of PT and CPAP may improve CPAP compliance by lowering pressure requirements during supine sleep [12, 33, 34]. There may also be a subpopulation of patients with POSA and a large time spent supine in which PT may be an efficacious standalone therapy [12, 34]. However, the therapeutic utility of PT is also dependent on long-term compliance with the use of the devices.
2.4 Compliance with Positional Therapy
Reasonable compliance of around 7 h/night has been demonstrated for PT for up to 3 months [34,35,36,37]. Although the device retains its efficacy for preventing supine position, loss of long-term compliance is often related to discomfort, with around 40% of study populations discontinuing use after 6 months [33, 35,36,37]. The lack of long-term compliance highlights both the need for improvements in PT design and its lack of reliability as a standalone long-term therapy for POSA except for patients who refuse all other treatment options [38].
2.5 New Generation Design Modifications
New designs of PT have been introduced to try and improve efficacy and compliance. The next generation of positional devices includes built-in position sensors and data storage for the collection of objective compliance data [35]. Small “active” PT devices have been developed which deliver additional auditory or vibrational stimuli while the patient is in the supine position. These devices are usually worn around the neck or at the chest level (Fig. 12.3). The streamlined design improves tolerability and compliance [37].
Microphones and accelerometers have been incorporated for the detection of snoring, movement, and sleep position. Results have been validated by comparison with video footage and data from attended type 1 PSGs. These devices can track compliance for up to 12 months, with reports available for the last 6 nights of use [39].
2.6 Combinations of Positional and Dental Sleep Appliance Therapy
Different authors have found supine OSA to be predictive of both increased [40, 41] and decreased treatment success using oral appliances. In addition, persistent supine-dependent OSA is common during DSA therapy [42]. Up to 37.5% of patients convert from non-supine to supine-dependent OSA during therapy, and 34% of patients have persistent supine-dependent OSA with DSA in place [43, 44]. Therefore, a need exists for adjunct PT modalities for these patients in order to optimize treatment outcomes and reduce the time spent sleeping in the supine position.
2.7 Efficacy of Combination Dental Sleep Appliance and Positional Therapy
There is an ongoing debate about how the efficacy of treatment for OSA should be measured. The AHI does not capture all of the clinically relevant features of OSA [45, 46]. Oxygen desaturation indices have clinical relevance in several OSA-related outcomes including vigilance [47, 48], small vessel disease [49], and excessive sleepiness [50]. Levels of oxygen desaturation have been investigated in studies combining DSA + PT. Improvements in oxygen desaturation have been found in line with improvements in respiratory statistics in studies investigating combinations of DSA only, PT only, and DSA + PT [51].
2.7.1 Active Devices
Studies combining active PT with DSA are few and use multiple definitions of supine-dependent apnea and different sleep scoring criteria. They often involve small numbers, with inadequate follow-up, so care must be taken in interpreting their results. Although the primary outcome is generally change to the AHI or oxygen desaturation, secondary outcomes have included patient satisfaction, compliance, sleepiness, and adverse outcomes.
While the outcomes of research investigating the outcomes of combinations of DSA + PT have had promising results [51, 52], the research base is small, and the study populations have been small. In general, DSA and PT have equivalent outcomes for AHI reduction [29]. The combination of DSA + PT leads to greater improvements in AHI metrics [51, 52]. Compliance remains the greatest problem for PT. Although high compliance and lack of habituation have been reported [53], most studies report that discomfort or habituation to the active alarm may result in discontinuation of treatment [29]. In the future, it may be possible to vary the frequency or amplitude of the alarm to prevent habituation [54].
In addition to compliance monitoring, some devices offer type 3 monitoring of nocturnal sleep. These are used in conjunction with pulse oximetry, plethysmography, and sound analysis to improve clinical understanding of nocturnal changes in OSA metrics (Fig. 12.4).
2.7.2 Passive Devices
Passive devices for POSA do not use alarms to maintain a non-supine posture during sleep and include the tennis ball and fin and wedge devices (Fig. 12.5). Passive devices are also successful at reducing time spent spine during sleep [55, 56]. There has been recent interest in the contribution of head position during sleep to POSA. Two studies investigated the use of positioning pillows and found improvements in respiratory and oxygen desaturation indices and quality-of-life outcomes over 2–6 months of use [57, 58]. In one study, outcomes were improved by the use of DSA + pillow [57].
2.8 Modifying the Dental Sleep Appliance to Counteract Positional Apnea
Although it is not within the scope of this chapter to give a detailed discussion of DSA appliance design (see Chap. 10), specific design features can be modified to provide a personalized approach to DSA therapy and improve DSA outcomes in POSA. These modifications include customizing the thickness of the construction material to improve tongue space and the addition of an expiratory positive airway pressure (EPAP) valve [59, 60]. Minimizing the vertical dimension of the appliance may maximize the available mandibular protrusion [61, 62] and improve patient compliance [63,64,65]. The addition of hooks to a DSA allows the use of vertical elastics and reduces increases in vertical dimension due to mouth opening during sleep [64, 65]. The use of elastics in DSA therapy is analogous to other DSA design elements which limit vertical opening during sleep (Fig. 12.6). These design features include the monobloc (one piece) appliance [40] and anterior coupling (upper and lower DSA components attached in the incisor region) [66] and two-part appliances [67].
The use of vertical elastics in conjunction with DSA therapy can be considered a form of PT, as it controls the mandibular position during POSA. Milano et al. [64] investigated the use of elastics to manage POSA in a retrospective study of 230 patients, 188 males (51.1 ± 11.8 years) and 42 females (55.2 ± 8.9 years), diagnosed with POSA using a two-part DSA. The use of 85–170 g elastics, 3/8–3/16″ by 92 subjects in conjunction with DSA therapy at 5 mm vertical dimension was superior to DSA therapy alone (n = 188) after 4–8 weeks of device titration. The reductions in the AHI, supine AHI, and non-supine AHI were significantly greater in the DSA + elastics group. The use of elastics resulted in 3.8 times greater odds of achieving treatment success (defined as a 75% reduction in the AHI). Female gender, lower BMI, lower baseline AHI, and younger age all contributed to improved chances for treatment success.
Further insights into the effect of vertical opening and DSA design on OSA are provided by retrospective studies on DSA outcomes [41, 67]. Reviewing 471 patients, Marklund et al. reported six times greater odds of successful therapy (defined as lateral and supine AHI < 10) in males with supine OSA (defined as a lateral AHI < 10 and supine AHI > 10) using monobloc appliances and vertical dimensions of 1.3–13 mm in inverse relation to the AHI. Each additional millimeter of mandibular advancement increased the odds for success 1.3 times. In contrast in 425 patients, Sutherland et al. [67] reported a reduced rate of treatment success in supine apneics using a two-piece appliance. Supine-isolated and predominant apneics had response rates of 20–22% (AHI < 5) and 42–4.9% (AHI <10 and >50% reduction), respectively, compared with the response rates of non-positional apneics of 44 and 59%, respectively. The variation in the results is difficult to interpret due to differences in multiple factors including definition of success, baseline population characteristics, and AHI scoring criteria, as well as appliance design.
2.8.1 Practice Points
-
Patients with POSA may have a lower presenting AHI.
-
If there is no supine sleep during the PSG, the contribution of POSA to the patient’s condition is unknown.
-
During DSA therapy more than 30% of patients will convert to supine sleep.
-
In type 3 and 4 studies, the persistence of clusters of respiratory and oxygen desaturation events may indicate the presence of POSA.
-
The use of PT is limited by the patient’s ability to sleep in the lateral position.
3 Positive Airway Pressure as an Adjunct Therapy for Dental Sleep Appliances
Not all DSA therapy failures are due to the presence of POSA [44]. Other forms of adjunct support may be needed which can target intermittent or generalized severe OSA. Positive airway pressure (PAP) therapies include CPAP and expiratory EPAP. Both are able to maintain airway patency in the presence of severe closing pressures.
3.1 Continuous Positive Airway Pressure
CPAP is the gold standard treatment for OSA [68], but CPAP intolerance is a common reason for patients to seek DSA therapy. CPAP works by providing a pneumatic splint that maintains airway patency [32]. Many patients are unable to cope with the high pressures required, especially during multilevel obstructions. Combination CPAP-DSA therapy may provide a compromise between excessive CPAP pressures, excessive mandibular protrusion, and the requirement for normalization of polysomnographic measures of OSA severity. This has the potential to improve patient tolerance, compliance, and long-term health outcomes. To date, there is not a large research base investigating this aspect of adjunct therapy. For CPAP-intolerant patients with an incomplete response to DSA therapy, combined DSA-CPAP therapy may eliminate residual respiratory events and improve oxygen desaturation values [69] and the level of excessive daytime sleepiness [70].
Using CPAP-DSA therapy may reduce required CPAP pressures by as much as 29–45% [59, 69] in a dose-dependent manner [71]. This pressure reduction may potentially lead to improvements in comfort, air leaks, and compliance [72]. The major mechanisms behind the expected increase of comfort involves improvements in epiglottic pressure swings [73], a reduction in oropharyngeal resistance [74], and improved velopharyngeal airway patency [75]. Before final conclusions can be made about the efficacy of this form of combination therapy, larger randomized controlled trials are required over longer time periods to determine its long-term feasibility.
3.2 Expiratory Positive Airway Pressure
3.2.1 Definition
EPAP devices contain a mechanical valve that provides very low inspiratory resistance but high expiratory resistance. Two options exist: One option is a small central valve, commonly 0.25 in. in diameter that is designed to sit just inside each nostril, with adhesive surrounding it to provide a seal (Fig. 12.7). They are single-use devices that are commercially available, and manufacturers have developed a range of resistance settings to accommodate for individual patient needs ranging from 5 to 20 cm of water [76].
More recently a second EPAP delivery option has become available with the development of a DSA with a built-in oral airway. The oral EPAP (oEPAP) valve consists of a nylon body containing an internal silicone flapper with holes to serve as a one-way valve. It is inserted into the oral airway on the DSA. The size and the number of holes enable this device to offer a range of different fixed expiratory resistance valves. This allows the practitioner to replace the valve in order to titrate the EPAP resistance to best suit the patient’s needs.
3.2.2 The Rationale for EPAP Therapy
Imaging studies have shown that DSA therapy primarily increases airway volume at the lateral walls of the velopharynx in treatment responders [77]. The amount of forward movement of the tongue that is produced with DSA wear is inversely correlated to the severity of the AHI [78]. As the severity of the AHI increases, the percentage of multisite collapse that occurs during sleep increases [79,80,81], and the effectiveness of DSA therapy decreases. A retrospective report on 425 patients found that REM and supine apnea, which often have the most severe OSA [82, 83], respond least well to DSA therapy [67]. This means that adjunctive therapy that reduces airway collapsibility in patients with severe REM and supine apnea may improve DSA outcomes.
Expiratory resistance devices have been developed that can be applied to either the nasal [76, 84] or oral airway [59, 85] to generate EPAP and reduce pharyngeal collapsibility. Utilizing a combination of an EPAP device with a DSA may improve the efficacy of OSA treatment for the subset of patients who experience multilevel airway collapse.
3.2.3 Mechanisms of Action of Expiratory Positive Pressure Devices
Both inspiratory and expiratory closing forces contribute to airway collapse during sleep [87]. Independent adjustment of PAP pressure during inspiration and expiration has found that the required pressure to control OSA is lower during expiration than during inspiration [88], opening opportunities for alternatives to CPAP. An automatic mask-delivered EPAP has been shown to deliver equivalent results to CPAP for treating OSA [89]. Mahadevia et al. reported that EPAP is an effective treatment for OSA at a pressure of 10 cm H20 [90]. This level of EPAP has been found to increase the functional residual capacity (FRC) (Fig. 12.8) by 13.3 ml and to reduce the AHI [91].
Static lung volumes. (Figure adapted from [86])
Maximal expiration is associated with the largest airway caliber up until the transition from expiration to inspiration. At this point in the respiratory cycle, the airway pressure has generally dropped to zero, and upper airway dilator muscle activity is at its lowest. The period of end-expiration on the breath preceding an apnea corresponds with the narrowest cross-sectional area of the retropalatal airway [92]. This narrowing of the pharyngeal airway is greatest immediately prior to an apnea. The upper airway is then at risk of either complete or partial collapse during the subsequent inspiration [87, 92]. EPAP devices aim to stabilize this activity and prevent expiratory-related airway collapse.
The aim of the therapy is to stabilize airway caliber during the critical end-expiratory period. There are a number of proposed mechanisms by which EPAP, and specifically nasal EPAP (nEPAP) and oEPAP, may work. To date our understanding of the probable mechanisms comes from studies examining the controlled “in-lab” use of inspiratory or EPAP [87, 88, 90,91,92] and not from the modified devices that have been produced as nEPAP or oEPAP. Changes in the FRC due to the expiratory resistance generated by EPAP increases expiratory and total respiratory time and reduces the minute ventilation. The increased FRC is associated with traction on the trachea which is thought to decrease airway collapsibility. These changes are linked to an increase in the levels of end tidal carbon dioxide which may stabilize ventilatory control by increasing respiratory drive and increasing respiratory muscle responsiveness [84, 87, 88, 90,91,92,93]. As the magnitude of the response varies between individuals, it is crucial that the outcomes of any nEPAP or oEPAP therapy are objectively verified to confirm its efficacy [94].
It is reasonable to enquire whether nEPAP or oEPAP can produce equivalent effects to controlled “in-lab” EPAP investigations. While valve pressures for nEPAP of 50 and 110 cmH2O L/s may be given as experimental resistance values, there have generally been no controls accounting for time spent breathing orally [76, 95,96,97]. Patel et al. [98] used a pneumotachograph to monitor intranasal pressure during nEPAP treatment as an analogue for nasal airflow. They also measured CPAP pressure, arterial carbon dioxide, airway closing pressures, and awake lung volumes. They found that successful reduction of the AHI was associated with sustained elevated end expiratory pressure but that the level of expiratory pressure varied from 5–23 cm H2O across individual patients by sleep stage and sleep position. They were unable to discern any mechanisms for this. More controlled work needs to be done to determine the effect of potential confounders such as pretreatment nasal resistance, mouth breathing, and sleep posture on primary outcomes in nEPAP therapy. There has only been one clinical trial investigating oEPAP, which did not investigate mechanisms of action of the device [59].
3.2.4 Efficacy and Compliance with nEPAP Therapy
A number of studies have shown efficacy for a nEPAP device used as a standalone therapy. These studies have found that there are statistically significant reductions in the level of oxygen desaturation [99, 100]; the full night, REM, NREM, and supine AHI [76, 96,97,98,99,100,101]; excessive daytime sleepiness [76, 97, 98, 101], and sleep quality [96]. A recent meta-analysis of nEPAP results [102] found that across 345 patients there was a decrease in the AHI from 27.3 ± 22.7 to 12.8 ± 16.9, an improvement in the oxygen desaturation index (ODI) for 247 patients from 21.2 ± 19.3 to 12.4 ± 14.1, and a change in excessive sleepiness from 9.9 ± 5.3 to 7.4 ± 5.0 in 359 patients. Similar to DSA therapy, the efficacy of nEPAP was better in patients with less severe OSA [96, 98] and in patients without nasal obstruction [101]. All studies found interindividual variation in the results, with no overall discernable reasons for that variation [102]. One small trial evaluated the use of nEPAP in children (n = 14: 8–16 years of age) and concluded that nEPAP should only be used in children under direct polysomnographic supervision due to the interindividual variability of the results [103].
Most studies on nEPAP were conducted over time spans of up to 1 month, but one group reviewed patients at 3 months [76] and again at 12 months [97] and found no loss of efficacy of the device. Compliance has been a major issue. Although many studies report that the device is worn for more than 80% of nights, the compliance dropped from 82 to 66.7% from 3 to 12 months of use [76, 101]. Side effects across different studies were minor, but common, and included difficulty in breathing, dry mouth, nasal symptoms, insomnia, and headache [102].
3.2.5 Efficacy and Compliance of oEPAP Therapy
Only one group so far has studied the combination of DSA with EPAP devices (Fig. 12.9). In a study of 22 patients with an incomplete or non-response to initial DSA therapy, DSA + oEPAP or DSA + oEPAP + nEPAP was tested in a randomized order across one night, monitored using type1 PSG [59]. They found statistically significant incremental improvements in the NREM AHI, ODI, and minimum oxygen saturation going from baseline to DSA only to DSA + oEPAP and then DSA + oEPAP + nEPAP. There was no significant change in sleep efficiency from DSA only to DSA + oEPAP, but sleep efficiency significantly decreased from DSA + oEPAP to DSA + oEPAP + nEPAP from 88 ± 10% to 78 ± 19%. The number of patients with an AHI < 5 increased to 9% with the DSA + oEPAP device and to 41% with DSA + oEPAP + nEPAP. There is no information on long-term efficacy as yet for this treatment. Further investigation of the use of DSA + EPAP valves is required to determine the efficacy of its long-term use as an adjunct therapy for patients who are CPAP noncompliant or who are DSA non- or partial responders. It is also possible that the use of EPAP valves in conjunction with DSA therapy may make it possible to reduce the extent of mandibular protrusion.
In general, the use of EPAP valves as an adjunctive therapy to improve DSA outcomes seems promising. Further work is needed to determine and then optimize protocols to maximize treatment outcomes. In particular, further research is required to determine the long-term efficacy and compliance, the reasons for the variability in treatment response, and the effects of better control of mode of breathing during treatment.
3.2.5.1 Practice Points
-
nEPAP reduces the AHI and improves indices of oxygen desaturation.
-
The results are variable and the mechanisms are not fully understood.
-
DSA + EPAP valve may have superior outcomes.
-
More research is required in this field.
4 Adjunct Therapy for Anatomical Compromise in Dental Sleep Appliance Therapy
It is currently believed that most patients with OSA have some form of anatomical compromise of the upper airways [104, 105]. As DSA therapy has a limited ability to correct upper airway anatomy during sleep [77], it may be necessary to assess multilevel anatomical compromise at multiple time points during DSA therapy. The purpose of this section is to introduce the concept of improvement of anatomical compromise as an adjunctive treatment during DSA therapy. Therapies for anatomical compromise can be behavioral or surgical, but for a more comprehensive review of surgical procedures for OSA, see Chap. 11.
4.1 Surgery as an Adjunct Therapy
Surgical correction of the upper airway anatomy can be an adjunct therapy for OSA [106]. A lack of level I evidence with long-term outcome measures, together with the wide variety of surgical procedures, and a lack of standardization of techniques make the extrapolation of surgical results difficult [107]. Surgical procedures rarely provide definitive therapy for OSA, but as surgical compliance is 100%, it has been suggested that the efficacy/compliance/effectiveness ratio for surgical treatment of OSA is similar to CPAP [108]. A multidisciplinary team is required to determine the role and timing of surgical treatment in the effective management of OSA [109] and to assess and manage other disorders which can affect both surgical and DSA outcomes, such as the existence of the medical, mood, and sleep disorders, and the severity of the sleep-disordered breathing [110]. Commonly multidisciplinary care for DSA therapy may involve co-treatment with a sleep physician, general practitioner, dentist, and otolaryngologist.
Surgical interventions have multiple functions and forms in DSA therapy for OSA including the following:
Assessment of the severity of collapse during OSA.
Prediction of DSA outcomes.
Improvement of compromised nasal anatomy.
Correction of oropharyngeal anatomy including:
-
Soft palate.
-
Tongue base.
-
Hyoid position.
Hypoglossal nerve stimulation for mandibular advancement.
Multilevel upper airway approaches.
Maxillo-mandibular advancement.
Bariatric surgery.
4.2 Drug-Induced Sleep Endoscopy for Assessment of Airway Collapse and Prediction of Treatment Outcomes in Dental Sleep Appliance Therapy
Both drug-induced sleep endoscopy (DISE) and awake nasendoscopy were developed to assess the severity and site of airway collapse during sleep (Fig. 12.10), to enable selection of the appropriate surgical procedure, and to assist in the planning of multidisciplinary OSA management [112]. The addition of the bispectral index as a monitoring tool may solve the issue of the effects of incorrect depth of sedation on airway properties [113, 114]. Patients who display retrognathia or tongue base collapse that improves with jaw lift during DISE may benefit from DSA therapy [115, 116], and both DISE [117, 118] and awake nasendoscopy [119, 120] have been used with varying levels of success to predict DSA therapy outcomes. For further information on these techniques, please see Chap. 11.
Drug-induced sleep endoscopy of positional changes in the location of upper airway collapse. Arrows point to the airway. (1) Soft palate collapse in supine and lateral positions during sleep. (2) Improvement in the base of the tongue collapse from the supine to the lateral position. (Adapted from [111])
4.3 Improving Compromised Nasal Airway Anatomy
Nasal resistance accounts for 50% of the total resistance of the upper airway during awake nasal breathing [121]. Nasal resistance increases when body position changes from the upright to the supine position [122]. Increased nasal resistance may be a side effect of CPAP therapy [123] and may also contribute to CPAP noncompliance [124] and the requirement for DSA therapy as an alternative treatment. Increased nasal resistance has been associated with a worse response to DSA therapy [41, 124, 125], so treatment aimed at improving nasal airway volume may be an important adjunct measure that should be assessed and discussed with the patient prior to the construction of a DSA. Initial assessment of the nasal airway prior to DSA therapy includes assessment of hard and soft tissue features including the presence of external asymmetry, functional nasal valve collapse, the position of the nasal septum, the presence of polyposis, enlarged adenoids and inflamed turbinates (Fig. 12.11) or enlargement of the nasal turbinates, the presence of rhinitis, and the effect that this is having on the sleep disorder [126].
Treatment for nasal obstruction may take several forms including:
-
Behavioral modification to limit exposure to environmental allergens
-
Use of intranasal medications to improve nasal symptoms
-
Use of nasal stents to limits nasal valve collapse during respiratory events
-
Nasal surgery
4.3.1 Behavioral Modifications
Contact of the nasal mucosa with an allergen elicits an immune response including sneezing, itching, nasal obstruction, and production of nasal secretions in sensitized individuals [127]. Although there are many different allergens, the most common may include house dust mites, pollens, mold, animal dander, and nicotine smoke. The presence of allergy is associated with increased snoring and risk for OSA, especially in the pediatric population [128]. While nicotine and pollens should be avoided, dust mites require exposure to dry heat at 60 °C or freezer temperatures of −17 °C (0 °F) in order to kill the mites and their eggs [129]. Nasal rinsing with saline or steroid solutions can be used to relieve intranasal soft tissue inflammation [130].
Other environmental issues related to increased nasal resistance include supine posture, cold air, nonallergic forms of rhinitis, aspirin, and alcohol [131]. Elevation of the head of the bed by as much as 15 cm [132, 133] results in small reductions in the AHI, which may be related to reduced nasal resistance. These interventions may make slight improvements in sleep quality and may improve DSA tolerance.
4.3.2 Intranasal Medications
Multiple topical nasal steroid medications have been used to improve nasal airflow in both allergic and nonallergic individuals with greater success in those who suffer from allergy. In pediatric populations, intranasal topical steroid preparations have been found to significantly reduce the AHI and the size of the nasal adenoids [134, 135]. A recent meta-analysis found that the use of topical intranasal medications provides small improvements in both objective and subjective OSA metrics, including the RDI and the minimum oxygen saturation [136]. Nasal sprays are considered an adjunctive therapy [106, 137], and their most common use is for treatment of rhinitis associated with CPAP therapy [138], indicating a possible use in patients with increased nasal resistance during DSA therapy.
4.3.3 Nasal Dilators
Nasal dilators provide support for the anterior nasal valve to prevent collapse during respiratory events in sleep for patients with OSA (Fig. 12.12). They stabilize and enlarge the cross-sectional area of the nasal anatomy directly in contact with the dilator, increasing the duration, maximum flow, peak flow, and volume of air [139]. There are four classes of mechanical dilators, which include external strips, nasal stents, nasal clips, and septal stimulators [140]. The use of nasal dilators includes indices of subjective sleep quality [141, 142] but has minimal and nonsignificant effects on objective indices of OSA [141, 144,145,145] and on the levels of CPAP pressure needed to prevent airway obstruction [146, 147]. Internal stents may have a greater effect on sleep indices including oxygen desaturation and the AHI than external devices [146, 148, 149], and the effect may be related to the length and position of the area of stabilization. A study investigating the effect of a nasal stent with a length of 120–145 mm worn in one nostril found significant improvements to the AHI, oxygen desaturation, RDI, and snore volume, but 30% of patients were unable to tolerate the device [149]. A recent meta-analysis concluded that although the internal dilators had a slightly larger effect on the AHI, the dilators as a group did not improve objective sleep indices [150]. It is likely that at this stage the major indication for nasal dilators as an adjunctive DSA therapy is to improve subjective sleep quality in patients with repetitive nasal congestion.
Mechanism of action of nasal strips and dilators. (1) Arrows indicate the direction of stabilizing force applied to the nasal airway by nasal strips. (2) Green rings indicate passive placement of nasal dilators to stabilize the internal volume of the nasal valve (blue). (Figure adapted from [151])
4.3.4 Nasal Surgery
Nasal surgery has been extensively studied. As a standalone therapy, nasal surgery improves subjective [153,154,155,156,156] but not objective indices of OSA [152, 154, 157,158,159,160,160], except in the group of patients with mild-to-moderate OSA and a BMI <30 kg/m2 in which isolated nasal surgery has shown efficacy at reducing objective indices [155, 161, 162]. As well as BMI, the ability to re-establish a nasal mode of breathing post-surgery may also affect treatment outcomes [158]. Despite the lack of efficacy as a standalone therapy, nasal surgery improves CPAP compliance [163], so it may be a useful treatment to include in the DSA armamentarium to improve sleep quality and acceptance of a DSA appliance [106]. It may also be useful as a component of a multilevel surgical plan aimed at improving anatomical compromise in multiple airway locations [106, 164, 165]. (See Chap. 11 for more detailed information on surgical techniques for OSA.)
5 Improving the Compromised Oropharyngeal Anatomy
Compromised anatomy of other oropharyngeal tissues can also have detrimental effects on DSA therapy outcomes and may require adjunctive modification. The tissues commonly affected include the following:
-
Soft palate.
-
Tongue.
-
Obesity-related fat deposits.
5.1 Soft Palate
Repetitive snoring and upper airway obstruction lead to vibrationally mediated inflammation, edema [166, 167], and nerve lesions [168, 169] in the soft palate of patients with sleep-disordered breathing (Fig. 12.13). This inflammation may be exacerbated by the presence of gastroesophageal reflux [170] and may ultimately contribute to the severity of the disorder. Nasal CPAP has been shown to reverse the edema in the oropharynx [171], but DSA therapy may take longer to implement due to the need for titration and may produce an incomplete response, leading to a need to stabilize or reduce the bulk and level of collapsibility of this tissue.
Adjunctive treatment for vibrationally mediated edema may include oral-myofunctional therapy (Chap. 14) [172] and multiple surgical procedures (Chap. 11) [173, 174]. The inflammation and edema are clinically apparent prior to treatment and can be monitored during therapy, but surgical interventions to improve soft palate anatomy may lead to pain and swelling [175], which may further compromise DSA therapy during healing, so a decision to include soft-palate surgical adjunctive procedures is usually best made prior to the start of treatment.
5.2 Tongue
An enlarged tongue is common in patients with OSA [176]. Patients with OSA have a larger tongue volume [177] and larger deposits of sublingual fat than healthy controls [178]. In addition, patients with severe OSA tend to have less forward movement of the tongue base with a DSA in place than those with less severe OSA [78]. As indices of oxygen desaturation may be correlated to tongue size [179], there is a rationale for provision of surgically mediated tongue reduction to improve DSA therapy outcomes.
Despite these relationships, there can be multiple other reasons for an enlarged tongue, which need to be assessed at the time of examination. These conditions include congenital disorders associated with macroglossia [180], amyloidosis [181], hypothyroidism, and acromegaly [182]. See Chapters 4 and 5 for more details. There are multiple surgical techniques for the reduction of tongue size, most of which have been associated with significant improvements in the AHI. Transoral robotic surgery (n = 353 patients) was associated with a 68.4% postoperative surgical success rate (defined as a reduction in AHI of 50% and with a residual AHI < 20), which dropped to 23.8% postoperatively if AHI < 5 was used as a measurement of success [183]. The glossectomy procedure (n = 522) had a surgical success rate of 59.6%, a surgical cure rate of 22.5%, and an acute complication rate of 16.4% [184].
6 Obesity
Obesity is a key risk factor for OSA [185, 186], and weight loss is an essential component of OSA management. Therapies for OSA are used over many years. A gain of 3.6 kg over 5 years of DSA use has been shown to be sufficient to worsen subjective sleep quality and oxygen saturation indices [187]. It is also important for adjunctive DSA therapy planning to note that weight loss has been shown to improve OSA more during non-supine than supine sleep [188] .
Excess adipose tissue surrounding the upper airway contributes to airway collapse and compromised lung volumes via reductions in the FRC during sleep [189]. See Chap. 9 on CPAP therapy. In addition, patients with OSA experience different patterns of fat deposition around the upper airway in comparison with weight-matched healthy controls. Patients with OSA accumulate more fat in the soft palate and tongue areas [190] and adjacent to the pharyngeal airway [191]. Reductions in fat deposits in the tongue are correlated to improvements in AHI during weight loss [192].
6.1 Dietary Interventions
A 10% weight loss predicts a 26% reduction in the AHI [186], making it a key therapeutic target in OSA, but there are doubts about the long-term efficacy of weight loss programs. Recently Kuna et al. published the 10-year follow-up of the Sleep AHEAD Study (n = 134), in which obese patients with OSA and type 2 diabetes were randomized to receive intensive lifestyle interventions aimed at weight loss or diabetes education [193]. At the 10-year follow-up, remission from OSA was 34.4% in the diet/lifestyle intervention group and 22.2% in the diabetes education group. Previous work has shown that a 5-year weight loss maintenance is associated with continued AHI reduction benefits [194].
6.2 Bariatric Surgery
The lessons learned from dietary interventions are also applicable to bariatric surgery. Bariatric surgery improves both subjective and objective indices of OSA [196,197,197]. While initial weight loss is higher than for dietary and lifestyle interventions [198], a residual AHI remains [199, 200], and some weight may be regained over the subsequent years [201, 202].
6.2.1 Practice Points
-
Initial assessment prior to DSA therapy should include examination for anatomical compromise in the oropharyngeal and nasal airways.
-
Adjunctive therapy to improve anatomical compromise may involve multidisciplinary staged treatment.
-
Careful consideration must be given to the timing of this treatment.
-
Most anatomically based adjunctive therapies improve objective and subjective indices of OSA but are not suitable as standalone therapies.
7 Adjunctive Therapies for Sleep Quality During Dental Sleep Appliance Therapy
While the primary outcome of DSA therapy is the reduction of the severity of OSA-related sleep metrics, subjective changes in the quality of life (QOL) of the patient must also be addressed during DSA therapy. Failure to improve these issues may lead to treatment failure due to lack of compliance with appliance wear. While it is not within the scope of this chapter to give a comprehensive view of QOL-directed adjunct therapies (for a more comprehensive coverage of other sleep disorders, see Chap. 3), it is still essential that the clinician has an understanding of various adjunct therapies that can help to improve QOL-related factors during DSA therapy for OSA. For the purposes of this section, we will limit our discussion to adjunct therapies available for insomnia and circadian rhythm disorders which are both commonly comorbid with OSA and may impact DSA acceptance [203].
7.1 Insomnia
The prevalence of insomnia is increasing globally. In America from 2007 to 2008, 37.6% of participants responding to the National Health and Nutrition Survey reported sleeping ≤7 h/work night, and 19.2% reported poor sleep quality [204]. The International Classification of Sleep Disorders (ICSD-3) [205] defines insomnia as difficulty with sleep initiation, consolidation, duration, or quality despite sufficient sleep opportunity. The ICSD-3 currently recognizes six forms of insomnia:
-
1.
Chronic insomnia disorder
-
2.
Short-term insomnia disorder
-
3.
Other insomnia disorder
-
4.
Isolated symptoms and normal variants
-
5.
Excessive time in bed
-
6.
Short sleeper
(For further information please see Chap. 3.)
7.1.1 Comorbidity of Insomnia and Obstructive Sleep Apnea
Insomnia has a high rate of comorbidity with OSA which can reach 50–84% in a sleep center population [207,208,209,209]. The presence of comorbid insomnia and OSA is more common than either disorder occurring singly [207, 210]. Approximately 30–35% of these patients may report difficulty initiating, maintaining sleep, or having early morning awakenings [211]. A telephone review of 188 patients previously treated with DSA therapy found that the presence of insomnia was the most important factor in the self-impression of the lack of improvement after DSA therapy [212], which is consistent with findings for poor CPAP outcomes as well [213, 214]. Clearly the clinician must be prepared to treat both OSA and insomnia for any treatment strategy to maintain long-term success. When the treatment strategy is DSA therapy, this will mean that there will be some treatment options that the dental practitioner may feel equipped to handle, and other times the involvement of a multidisciplinary team will be required.
Therapy for insomnia can be divided broadly into behavioral and pharmacologic strategies. Although taking patient preference into consideration is an important feature of any co-therapy, it is considered wiser to formulate a staged treatment plan using behavioral therapies and then transitioning to pharmacologic therapies if required. The first element of any treatment strategy is to assess the patient’s sleep using an interview detailing the sleep environment, sleep habits, sleep versus work patterns and any circadian concerns [215]. This may involve the use of sleep questionnaires, sleep diaries, and a comprehensive evaluation of psychosocial and pain-related factors. (For resources see: https://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/DC-TMDAssessment/Diagnosis-IADR.com.)
The structured patient interview should include questions about any factors which may precipitate, perpetuate, or predispose for insomnia (Fig. 12.14). These may include a history of psychoactive substances such as caffeine, alcohol, nicotine, recreational drugs, and other polypharmacy [215].
The behavioral model of insomnia. Predisposing, precipitating, and perpetuating factors that can affect DSA therapy success. (Figure adapted from [216])
There are multiple behavioral therapies for insomnia, which are used either as standalone treatments or in combination. Below is a brief review of some of the most commonly used behavioral modalities which can be used as adjuncts to DSA therapy.
7.1.1.1 Cognitive Behavioral Therapy for Insomnia
Cognitive behavioral therapy for insomnia (CBT-I) is the first-line therapy for insomnia [215, 217]. CBT-I is both efficacious and cost-effective when compared to pharmacological therapies for insomnia [218]. It can be delivered individually, as a group, and online [219]. It employs a combination of multiple techniques including stimulus control therapy, sleep restriction therapy, sleep hygiene/education, relaxation training, and psychological strategies to reduce sleep-related stress [215, 220]. Exercise has also recently been included as an element of CBT-I [220]. As a combined behavioral intervention, CBT-I has a large effect on sleep quality, self-report of insomnia severity and sleep quality, sleep onset latency, and wake-after-sleep onset. It has a medium effect on objective sleep quality and a small effect on total sleep time (TST) and the number of awakenings after sleep [221]. As each component of CBT-I can be used as a standalone therapy, they will be discussed separately.
Summary: CBT-I is the recommended first-line therapy for insomnia.
It is a mix of multiple behavioral therapies.
It has the best risk-benefit ratio of any insomnia therapy.
7.1.1.2 Sleep Restriction Therapy
Sleep restriction therapy (SRT) aims to improve sleep efficiency by first limiting the amount of time spent in bed, followed by a gradual increase as sleep efficiency is restored [217]. The effects of SRT are similar to those produced by CBT-I but with smaller effect sizes. There are improvements in total sleep time and in the remission rates from insomnia [222, 223]. There have also been reports of improvements in the symptoms of depression, pre-sleep arousal, and maladaptive beliefs about sleep [223]. Evidence to date suggests that the risk of developing excessive sleepiness after both SRT and CBT-I is either transient [224, 225] or negligible [226].
7.1.1.3 Stimulus Control Therapy
Stimulus control therapy (SCT) works to strengthen the association between bed and sleep and to improve sleep patterns [217]. Moderate-effect sizes have been reported for SCT for the treatment of insomnia [227]. Instructions for SCT may include to only go to bed when sleepy, do not conduct activities in bed that are not associated with sleeping (e.g., reading or watching TV), to get out of bed if you wake and cannot go back to sleep, and to get out of bed at the same time every day [227].
7.1.1.4 Sleep Hygiene
Sleep hygiene is an integral component of sleep education [228]. The content frequently changes and covers behavioral aspects aimed at normalizing sleep timing [228, 229]. There is doubt as to its efficacy in improving sleep [230]. Sleep hygiene is less effective than CBT-I for insomnia [223, 231]. This may be because sleep hygiene practices focus on patient education and not initiating behavioral change [232]. Nevertheless, a sleep hygiene program personalized to the needs of the individual patient with their active participation has the potential to improve sleep habits on a long-term basis (Fig. 12.15). This was shown in a prospective cohort study (n = 3000 ages 20–60) which found that late evening use of nicotine, light and noise disturbance, and an irregular sleep schedule were significantly related to the presence of current and future insomnia at 1-year follow-up. Importantly both pain and a psychiatric/mood disorders were also related to the presence of insomnia at 1 year [233].
Summary: Light and noise disturbance.
Late evening nicotine use.
Irregular sleep schedules are all related to current and future insomnia
7.1.1.5 Psychoactive Substances
7.1.1.5.1 Caffeine
Caffeine use is common in an OSA population [234] and may be associated with the onset of insomnia [235], smaller sleep duration, and non-restorative sleep [236]. The effects of caffeine on sleep and insomnia increase with advancing age [237]. While caffeine is used to improve subjective sleepiness, the objective effects of caffeine on reaction time are less clear [239,240,241,241] and may be dose dependent. Smaller doses may cause higher levels of brain activation than larger doses [238]. An intake of 400 mg of caffeine within 6 h of usual sleep time has been associated with PSG-measured changes to sleep architecture. These changes include a reduction in TST and an increase in sleep onset latency, increased sleep fragmentation and sleep arousals, and reductions in the time spent in all sleep stages except for REM [242]. Drinking eight cups of coffee a day is associated with an odds ratio of 1.5 for the presence of sleep bruxism [243].
Summary: Caffeine intake should be moderate.
Caffeine intake should stop at least 6 h prior to habitual sleep.
7.1.1.5.2 Alcohol
Higher levels of alcohol consumption are associated with a 25% increased risk for OSA [244]. Alcohol intake causes muscle hypotonia and a decreased arousal response to apneas, resulting in longer apneas [245] and worsened respiratory and oxygen desaturation data [246]. Decreasing alcohol consumption should be a primary treatment aim in DSA therapy when indicated (Fig. 12.16). Alcohol consumption is also associated with insomnia and poor sleep quality. The effects of alcohol on insomnia vary depending on whether alcohol use is acute or chronic. In late adolescence acute alcohol use acts as a sedative, decreasing sleep onset latency, increasing slow wave sleep (SWS) in the first half of the night and decreasing REM in the second half [247, 248]. Chronic alcohol use is associated with a decrease in SWS and an increase in Stage 1 and REM sleep [247].
Possible interactions of untreated insomnia and dental sleep appliance therapy (adapted from [249])
Summary: Alcohol increases the severity of OSA. Acute alcohol use is a sedative and decreases sleep onset latency.
Acute alcohol use increases the amount of SWS in the first half of the night.
Acute and chronic alcohol are related to fragmented sleep and decreased sleep efficiency.
Chronic alcohol use is associated with increased REM sleep and decreased SWS.
7.1.1.5.3 Nicotine
The use of nicotine has a bidirectional [250] dose-dependent effect on sleep quality with large inter-individual differences [251]. One study found that 31% of the high-dose group had worsened sleep, but 16% had improved self-reports of sleep quality [251]. When examined using PSG, both acute and chronic nicotine use has similar effects to caffeine on sleep, with decreased TST [252], REM [253], and SWS sleep and increased sleep onset and REM latency [252]. The effects of nicotine on sleep are similar for conventional and electronic cigarettes [254] and may be increased when the nicotine is taken in the late evening [255]. During withdrawal from nicotine, there is REM rebound [256], decreased sleep onset and REM latency, and increased TST with increased time spent awake after sleep [253]. The effects of nicotine withdrawal on sleep parameters may be felt for up to 3 weeks [253] and are not improved by the use of nicotine withdrawal therapy [257]. There is a circular relationship between the presence of insomnia and the use of psychoactive substances such as nicotine [258]. Insomnia itself can be a major cause of failure to cease smoking [259].
Summary: There is a bidirectional relationship between nicotine and insomnia.
Chronic and acute nicotine increases sleep onset and REM latency and decreases TST, SWS, REM, and sleep efficiency.
Nicotine withdrawal also affects sleep for up to 3 weeks.
7.1.1.6 Relaxation Therapy
Like CBT-I, relaxation therapy comprises several techniques aimed at reducing stress to improve sleep behavior [260]. These may include progressive muscle relaxation, meditation, and control of intrusive thoughts at bedtime [215]. Although relaxation techniques such as mindfulness-based stress reduction provide significant improvements in sleep quality [262,263,263] and are readily available on electronic media, to date they have not proven to be superior to CBT-I [261].
7.1.1.7 Exercise
Exercise has been recommended as an adjunct therapy for DSA treatment [203] and is considered an adjunct therapy for OSA in general [264, 265]. Exercise can improve excessive sleepiness [267,268,268] and sleep apnea metrics [266, 269,270,270] (see Chap. 15 for a review of oropharyngeal exercise for OSA). Recent reviews found that exercise improved self-reported sleep quality [271, 272] as well as some PSG-based measurements such as sleep onset latency and sleep efficiency [273]. The effects of exercise may be dose- and time-of-day-dependent. Morning exercise may improve sleep efficiency and sleep fragmentation [274, 275], while exercise 3 days/week was needed to improve insomnia in middle-aged women [276]. Exercise improves sleep quality in smokers [277] and morning exercise may be related to improvements in SWS, sleep onset, and sleep maintenance during nicotine withdrawal [257]. The type of exercise may modulate how well it treats insomnia. Aerobic exercise [272, 278, 279] may be more effective than low-impact stretching or yoga [272, 279] and has a long-term positive impact on sleep quality.
Summary: Exercise can make small improvements to the AHI and excessive sleepiness.
Exercise improves subjective and objective sleep quality.
The effect of exercise is dose- and time-of-day-dependent.
7.1.1.8 Pharmacological Agents
Pharmacologic agents are effective in the treatment of insomnia but carry a larger risk: benefit ratio than CBT-I. Side effects of medication used to treat insomnia include cognitive issues, falls, and slowing of reaction times [219]. The major classes of medications approved to treat insomnia in the United States include benzodiazepine receptor agonists (benzodiazepines and non-benzodiazepine “Z-drugs”), a selective melatonin receptor agonist (ramelteon), a selective histamine receptor antagonist (doxepin), and a dual orexin receptor antagonist (suvorexant) [280]. The general recommendation is that pharmaceutical agents should only be used in the treatment of insomnia after failure of CBT-I or if CBT-I is either unavailable or unsuitable for the patient [219]. Evidence for pharmaceuticals in the treatment of insomnia is for short-term use, with concerns for severe side effects with longer use [215, 219].
Summary: Pharmacologic agents should only be used for insomnia if CBT-I is not efficacious or suitable.
Pharmacologic agents are only recommended for short-term use.
8 Circadian Rhythm Disorders
Circadian rhythm disorders (CRD) occur when there is a mismatch between the homeostatic process, which controls sleep need, and the circadian system, which controls timing [281]. There are seven CRDs included in the ICSD-3 diagnostic criteria [205]:
-
1.
Delayed sleep-wake phase disorder.
-
2.
Advanced sleep-wake phase disorder.
-
3.
Irregular sleep-wake rhythm disorder.
-
4.
Non-24 h sleep-wake rhythm disorder.
-
5.
Shift work sleep disorder.
-
6.
Jet lag disorder.
-
7.
Circadian sleep-wake disorder not otherwise specified.
There are multiple interactions between the CRDs and other sleep disorders including insomnia [282] and OSA. This makes it difficult to independently investigate the circadian aspects nested within other disorders or to estimate the prevalence of CRD, which may be 0.1–10% of the population [284,285,285]. The major treatment modalities of CRDs are chronotherapy, bright light therapy (BLT), and melatonin. In general combinations of all three therapeutic options are commonly used.
8.1 Chronotherapy
Chronotherapy is generally used in conjunction with sleep hygiene to reset sleeping and waking times by 3 hours/2 days until the required sleep-wake schedule is reached [286]. The process is slow and must be rigidly followed for the best results [283].
8.2 Bright Light Therapy
BLT uses timed exposure to light to either advance or delay sleep times. Exposure to bright light in the morning advances the sleep phase (earlier sleep onset), while exposure to light in the evening delays sleep onset [283]. Two weeks of exposure to 1–3 h of 2500 lux light or broad-spectrum (2000–10,000 lux) light in the morning combined with dull light in the evening advances sleep onset times [287]. The efficacy of BLT in shift work disorder is less clear. There is uncertainty about the long-term stability of improvements of BLT in adolescents with delayed sleep phase disorder [288], but short-term changes of up to 2 h over 3 days have been reported for jet lag disorder [289]. A shorter exposure of 30 min to morning bright light may produce a phase shift equivalent to 75% of that gained by the 2-h exposure [290]. Blue light exposure at around 460–470 nm may have the highest phase shifting outcomes [291], and avoiding or blocking blue light in the evening by limiting exposure to electronic media [292] or using tinted glasses [293] can also lead to an advancement of sleep onset and improvement of sleep quality.
Summary: Bright light can either advance or delay sleep times.
Blue light is the most efficacious color for phase shifting sleep.
8.3 Melatonin
Melatonin can advance or delay the sleep phase. Avoiding evening blue light results in an advance in the timing of nighttime melatonin secretion [295]. The production of endogenous melatonin in the pineal gland is regulated by the suprachiasmatic nucleus [296]. Exogenous melatonin shortens sleep latency [297] and decreases core body temperature (pooled effect size 0.22°C at the tympanic membrane) [298]. The greatest phase advance occurs when melatonin is taken 5 h prior to the dim light melatonin onset (around 7.30–9.30 pm in adults) [299, 300], and delays occur if it is taken 6–15 h after this (Fig. 12.17) [302,303,303]. The reduction of the body temperature is associated with the onset of sleepiness [304], and the peak plasma level of melatonin corresponds to the nadir of the sleep core body temperature [305]. The amount of phase shifting produced by melatonin is related to the change in body temperature [306]. A meta-analysis of 5 trials of 91 adults and 4 trials of 226 children found that exogenous melatonin advanced the mean endogenous melatonin onset by 1.18 h and time spent asleep by 0.62 h and decreased sleep onset latency by 23.27 min [296]. More research is needed to determine the best way to use circadian therapies to provide long-term phase shifts. The long-term side effects of melatonin are not well understood, but fatigue and mood changes may be ameliorated by adjusting the timing and the dose of melatonin to integrate the dose with natural circadian rhythms [307].
Relationship between melatonin core body temperature and sleep phase. Figure adapted from [294].  = endogenous melatonin levels.
= endogenous melatonin levels.  = minimum core body temperature
= minimum core body temperature
Summary: Melatonin can advance or delay the timing of sleep.
Its effects are tied to light and the circadian timing of body temperature.
The effect of melatonin on sleep onset latency is small at 23.27 min.
8.3.1 Practice Points
-
Sleep quality is an important component of DSA therapy.
-
Poor sleep quality can be related to incomplete treatment response to DSA therapy or to the presence of other sleep or medical disorders.
-
Behavioral strategies are the first choice for poor sleep quality.
-
Pharmacologic strategies are effective but are only approved for short-term therapy.
Change history
17 November 2022
In chapter 12, an author’s name has been changed from Charolte de Coursey to Charlotte de Courcey-Bayley.
Abbreviations
- AHI:
-
Apnea-hypopnea index
- APAP:
-
Auto-adjusting positive airway pressure
- BLT:
-
Bright light therapy
- CBT-I:
-
Cognitive behavioral therapy for insomnia
- CPAP:
-
Continuous positive airway pressure
- CRD:
-
Circadian rhythm disorders
- DISE:
-
Drug-induced sleep endoscopy
- DSA:
-
Dental sleep appliance
- EPAP:
-
Expiratory positive airway pressure
- FRC:
-
Functional residual capacity
- nEPAP:
-
Nasal EPAP
- ODI:
-
Oxygen desaturation index
- oEPAP:
-
Oral EPAP
- OSA:
-
Obstructive sleep apnea
- PAP:
-
Positive airway pressure
- POSA:
-
Positional obstructive sleep apnea
- PT:
-
Positional therapy
- QOL:
-
Quality of life
- SCT:
-
Stimulus control therapy
- SRT:
-
Sleep restriction therapy
- SWS:
-
Slow wave sleep
- TST:
-
Total sleep time
References
Dieltjens M, Braem MJ, Vroegop AVMT, Wouters K, Verbraecken JA, De Backer WA, Van de Heyning PH, Vanderveken OM. Objectively measured vs self-reported compliance during oral appliance therapy for sleep-disordered breathing. Chest. 2013;144:1495–502. https://doi.org/10.1378/chest.13-0613.
Heinzer R, Petitpierre NJ, Marti-Soler H, Haba-Rubio J. Prevalence and characteristics of positional sleep apnea in the HypnoLaus population-based cohort. Sleep Med. 2018;48:157–62. https://doi.org/10.1016/j.sleep.2018.02.011.
Cartwright RD. Effect of sleep position on sleep apnea severity. Sleep. 1984;7:110–4. https://doi.org/10.1093/sleep/7.2.110.
Frank MH, Ravesloot M, van Maanen JP, Verhagen E, de Lange J, de Vries N. Positional OSA part 1: towards a clinical classification system for position-dependent obstructive sleep apnoea. Sleep Breath. 2015;19:473–80. https://doi.org/10.1007/s11325-014-1022-9.
Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ. Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest. 2005;128:2130–7. https://doi.org/10.1378/chest.128.4.2130.
Oksenberg A, Silverberg D, Offenbach D, Arons E. Positional therapy for obstructive sleep apnea patients: a 6-month follow-up study. Laryngoscope. 2006;116:1995–2000. https://doi.org/10.1097/01.mlg.0000237674.66716.a7.
Oksenberg A, Arons E, Radwan H, Silverberg DS. Positional vs. nonpositional obstructive sleep apnea patients: anthropomorphic, nocturnal polysomnographic and multiple sleep latency test data. Chest. 1997;112:629–39. https://doi.org/10.1378/chest.112.3.629.
Richard W, Kox D, den Herder C, Laman M, van Tinteren H, de Vries N. The role of sleep position in obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2006;263:946–50. https://doi.org/10.1007/s00405-006-0090-2.
Pevernagie DA, Shepard JW Jr. Relations between sleep stage, posture and effective nasal CPAP levels in OSA. Sleep. 1992;15:162–7. https://doi.org/10.1093/sleep/15.2.162.
Levendowski DJ, Oksenberg A, Vicini C, Penzel T, Levi M, Westbrook PR. A systematic comparison of factors that could impact treatment recommendations for patients with positional obstructive sleep apnea (POSA). Sleep Med. 2018;50:145–51. https://doi.org/10.1016/j.sleep.2018.05.012.
Cartwright R, Ristanovic R, Diaz F, Caldarelli D, Alder G. A comparative study of treatments for positional sleep apnea. Sleep. 1991;14:546–52. https://doi.org/10.1093/sleep/14.6.546.
Permut I, Diaz-Abad M, Chatila W, Crocetti J, Gaughan JP, D’Alonzo GE, Krachman SL. Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med. 2010;6:238–43. https://doi.org/10.5664/jcsm.27820.
Zuberi NA, Rekab K, Nguyen HV. Sleep apnea avoidance pillow effects on obstructive sleep apnea syndrome and snoring. Sleep Breath. 2004;8:201–7. https://doi.org/10.1055/s-2004-860897.
Oksenberg A, Gadoth N, Töyräs J, Leppänen T. Prevalence and characteristics of positional obstructive sleep apnea (POSA) in patients with severe OSA. Sleep Breath. 2019;24:1–9. https://doi.org/10.1007/s11325-019-01897-1.
Neill AM, Angus SM, Sajkov D, McEvoy RD. Effects of sleep posture on upper airway stability in patients with obstructive sleep apnea. Am J Respir Crit Care Med. 1997;155:199–204. https://doi.org/10.1164/ajrccm.155.1.9001312.
Series F, Cormier Y, La Forge J. Role of lung volumes in sleep apnoea-related oxygen desaturation. Eur Respir J. 1989;2:26–30. https://doi.org/10.1136/thx.44.1.52.
Yildirim N, Fitzpatrick MF, Whyte KF, Jalleh R, Wightman AJ, Douglas NJ. The effect of posture on upper airway dimensions in normal subjects and in patients with the sleep apnea/hypopnea syndrome. Am Rev Respir Dis. 1991;144:845–7. https://doi.org/10.1164/ajrccm/144.4.845.
Zhu K, Bradley TD, Patel M, Alshaer H. Influence of head position on obstructive sleep apnea severity. Sleep Breath. 2017;21:821–8. https://doi.org/10.1007/s11325-017-1525-2.
Oksenberg A, Goizman V, Eitan E, Nasser K, Gadoth N, Leppänen T. Obstructive sleep apnea: do positional patients become nonpositional patients with time? Laryngoscope. 2020;130:2263–8. https://doi.org/10.1002/lary.28387.
Ravesloot M, Van Maanen JP, Dun L, De Vries N. The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea—a review of the literature. Sleep Breath. 2013;17:39–49. https://doi.org/10.1007/s11325-012-0683-5.
Sabil A, Blanchard M, Trzepizur W, Goupil F, Meslier N, Paris A, Pigeanne T, Priou P, Le Vaillant M, Gagnadoux F. Positional obstructive sleep apnea within a large multicenter French cohort: prevalence, characteristics, and treatment outcomes. J Clin Sleep Med. 2020;16(12):2037–46. https://doi.org/10.5664/jcsm.8752.
Joosten SA, O'Driscoll DM, Berger PJ, Hamilton GS. Supine position related obstructive sleep apnea in adults: pathogenesis and treatment. Sleep Med Rev. 2014;18:7–17. https://doi.org/10.1016/j.smrv.2013.01.005.
Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15:343356. https://doi.org/10.1016/j.smrv.2011.01.003.
Epstein LJ, Kristo D, Strollo PJ Jr, Friedman N, Malhotra A, Patil SP, Ramar K, Rogers R, Schwab RJ, Weaver EM, Weinstein MD, Adult Obstructive Sleep Apnea Task Force of the American Academy of Sleep, Medicine. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. https://doi.org/10.5664/jcsm.27497.
Mok Y, Tan A, Hsu PP, Seow A, Chan YH, Wong HS, Poh Y, Wong KKH. Comparing treatment effects of a convenient vibratory positional device to CPAP in positional OSA: a crossover randomised controlled trial. Thorax. 2020;75:331–7. https://doi.org/10.1136/thoraxjnl-2019-213547.
Barnes H, Edwards BA, Joosten SA, Naughton MT, Hamilton GS, Dabscheck E. Positional modification techniques for supine obstructive sleep apnea: a systematic review and meta-analysis. Sleep Med Rev. 2017;36:107–15. https://doi.org/10.1016/j.smrv.2016.11.004.
Ha SC, Hirai HW, Tsoi KK. Comparison of positional therapy versus continuous positive airway pressure in patients with positional obstructive sleep apnea: a meta-analysis of randomized trials. Sleep Med Rev. 2014;18:19–24. https://doi.org/10.1016/j.smrv.2013.05.003.
Berry RB, Uhles ML, Abaluck BK, Winslow DH, Schweitzer PK, Gaskins RA Jr, Doekel RC Jr, Emsellem HA. NightBalance sleep position treatment device versus auto-adjusting positive airway pressure for treatment of positional obstructive sleep apnea. J Clin Sleep Med. 2019;15:947–56. https://doi.org/10.5664/jcsm.7868.
Beyers J, Vanderveken OM, Kastoer C, Boudewyns A, De Volder I, Van Gastel A, Verbraecken JA, De Backer WA, Braem MJ, De Heyning V, Paul H. Treatment of sleep-disordered breathing with positional therapy: long-term results. Sleep Breath. 2019;23:1141–9. https://doi.org/10.1007/s11325-019-01792-9.
Grote L, Hedner J, Grunstein R, Kraiczi H. Therapy with nCPAP: incomplete elimination of sleep related breathing disorder. Eur Respir J. 2000;16:921927. https://doi.org/10.1183/09031936.00.16592100.
Kribbs NB, Pack AI, Kline LR, Smith PL, Schwartz AR, Schubert NM, Redline S, Henry JN, Getsy JE, Dinges DF. Objective measurement of patterns of nasal CPAP use by patients with obstructive sleep apnea. Am Rev Respir Dis. 2012;147:2405–34. https://doi.org/10.1164/ajrccm/147.4.887
Sullivan C, Berthon-Jones M, Issa F, Eves L. Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet. 1981;317:862–5. https://doi.org/10.1016/s0140-6736(81)92140-1.
Bignold JJ, Deans-Costi G, Goldsworthy MR, Robertson CA, McEvoy D, Catcheside PG, Mercer JD. Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med. 2009;5:428–30. https://doi.org/10.5664/jcsm.27597.
Heinzer RC, Pellaton C, Rey V, Rossetti AO, Lecciso G, Haba-Rubio J, Tafti M, Lavigne G. Positional therapy for obstructive sleep apnea: an objective measurement of patients’ usage and efficacy at home. Sleep Med. 2012;13:425–8. https://doi.org/10.1016/j.sleep.2011.11.004.
Ravesloot MJL, White D, Heinzer R, Oksenberg A, Pepin JL. Efficacy of the new generation of devices for positional therapy for patients with positional obstructive sleep apnea: a systematic review of the literature and meta-analysis. J Clin Sleep Med. 2017;13:813–24. https://doi.org/10.5664/jcsm.6622.
de Vries GE, Hoekema A, Doff MH, Kerstjens HA, Meijer PM, van der Hoeven JH, Wijkstra PJ. Usage of positional therapy in adults with obstructive sleep apnea. J Clin Sleep Med. 2015;11:131–7. https://doi.org/10.5664/jcsm.4458.
van Maanen JP, de Vries N. Long-term effectiveness and compliance of positional therapy with the sleep position trainer in the treatment of positional obstructive sleep apnea syndrome. Sleep. 2014;37:1209–15. https://doi.org/10.5665/sleep.3840.
Calik MW. Treatments for obstructive sleep apnea. J Clin Outcomes Manag. 2016;23:181–92. PMID: 27134515
Levendowski DJ, Seagraves S, Popovic D, Westbrook PR. Assessment of a neck-based treatment and monitoring device for positional obstructive sleep apnea. J Clin Sleep Med. 2014;10:863–71. https://doi.org/10.5664/jcsm.3956.
Marklund M, Persson M, Franklin KA. Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest. 1998;114:1630–5. https://doi.org/10.1378/chest.114.6.1630.
Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–8. https://doi.org/10.1378/chest.125.4.1270.
Sutherland K, Chan A, Ngiam J, Dalci O, Darendeliler A, Cistulli PA. Multimodal phenotyping for prediction of Oral appliance treatment outcome in obstructive sleep apnea. In: A98. Does this mean i have to wear that dsak? non pap therapies for SDB. New York, New York: American Thoracic Society; 2016. p. A2635. https://doi.org/10.5664/jcsm.7484.
Ten Berge DM, Braem MJ, Altenburg A, Dieltjens M, Van de Heyning PH, Vanhaecht K, Vanderveken OM. Evaluation of the impact of a clinical pathway on the organization of a multidisciplinary dental sleep clinic. Sleep Breath. 2014;18:325–34. https://doi.org/10.1007/s11325-013-0888-2.
Dieltjens M, Braem MJ, Van de Heyning PH, Wouters K, Vanderveken OM. Prevalence and clinical significance of supine-dependent obstructive sleep apnea in patients using oral appliance therapy. J Clin Sleep Med. 2014;10:959–64. https://doi.org/10.5664/jcsm.4024.
Pevernagie DA, Gnidovec-Strazisar B, Grote L, Heinzer R, McNicholas WT, Penzel T, Randerath W, Schiza S, Verbraecken J, Arnardottir ES. On the rise and fall of the apnea− hypopnea index: a historical review and critical appraisal. J Sleep Res. 2020;29:e13066. https://doi.org/10.1111/jsr.13066.
Cielo CM, Tapia IE. Diving deeper: rethinking AHI as the primary measure of OSA severity. J Clin Sleep Med. 2019;15:1075–6. https://doi.org/10.5664/jcsm.7856.
Kainulainen S, Duce B, Korkalainen H, Oksenberg A, Leino A, Arnardottir ES, Kulkas A, Myllymaa S, Toyras J, Leppanen T. Severe desaturations increase psychomotor vigilance task-based median reaction time and number of lapses in obstructive sleep apnoea patients. Eur Respir J. 2020;55:1901849. https://doi.org/10.1183/13993003.01849-2019.
McCloy K, Duce B, Swarnkar V, Hukins C, Abeyratne U. Polysomnographic risk factors for vigilance-related cognitive decline and obstructive sleep apnea. Sleep Breath. 2020:1–9. https://doi.org/10.1007/s11325-020-02050-z.
Zirak P, Gregori-Pla C, Blanco I, Fortuna A, Cotta G, Bramon P, Serra I, Mola A, Solà-Soler J, Giraldo-Giraldo BF. Characterization of the microvascular cerebral blood flow response to obstructive apneic events during night sleep. Neurophotonics. 2018;5:045003. https://doi.org/10.1117/1.nph.5.4.045003.
Kainulainen S, Töyräs J, Oksenberg A, Korkalainen H, Sefa S, Kulkas A, Leppänen T. Severity of desaturations reflects OSA-related daytime sleepiness better than AHI. J Clin Sleep Med. 2019;15:1135–42. https://doi.org/10.5664/jcsm.7806.
Dieltjens M, Vroegop AV, Verbruggen AE, Wouters K, Willemen M, De Backer WA, Verbraecken JA, de Heyning V, Paul H, Braem MJ, de Vries N. A promising concept of combination therapy for positional obstructive sleep apnea. Sleep Breath. 2015;19:637–44. https://doi.org/10.1007/s11325-014-1068-8.
To KW, Chan TO, Ng S, Ngai J, Hui DS. Role of nasal positive end expiratory pressure valve as an alternative treatment for obstructive sleep apnoea in Chinese patients. Respirology. 2016;21:541–5. https://doi.org/10.1111/resp.12703.
Levendowski D, Cunnington D, Swieca J, Westbrook P. User compliance and behavioral adaptation associated with supine avoidance therapy. Behav Sleep Med. 2018;16:27–37. https://doi.org/10.1080/15402002.2016.1163704.
Vrijland van Beest EC. 10 problems and solutions for positional therapy: technical aspects of the sleep position trainer. In: Positional Therapy in Obstructive Sleep Apnea. New York: Springer; 2015. p. 279–87. https://doi.org/10.1007/978-3-319-09626-1_25.
Braver HM, Block AJ. Effect of nasal spray, positional therapy, and the combination thereof in the asymptomatic snorer. Sleep. 1994;17:516–21. https://doi.org/10.1093/sleep/17.6.516.
Jokic R, KliDSAzewski A, Crossley M, Sridhar G, Fitzpatrick MF. Positional treatment vs continuous positive airway pressure in patients with positional obstructive sleep apnea syndrome. Chest. 1999;115:771–81. https://doi.org/10.1378/chest.115.3.771.
Newell J, Mairesse O, Neu D. Can positional therapy be simple, effective and well tolerated all together? A prospective study on treatment response and compliance in positional sleep apnea with a positioning pillow. Sleep Breath. 2018;22:1143–51. https://doi.org/10.1007/s11325-018-1650-6.
Newell J, Mairesse O, Smith P, Neu D. Preliminary data of a prospective study on the effectiveness and compliance of a mandibular advancement device alone versus a mandibular advancement device combined with a sleep positioning pillow in the treatment of mild to moderate sleep apnea. Sleep Med. 2017;40:e240. https://doi.org/10.1016/j.sleep.2017.11.700.
Lai V, Tong BK, Tran C, Ricciardiello A, Donegan M, Murray NP, Carberry JC, Eckert DJ. Combination therapy with mandibular advancement and expiratory positive airway pressure valves reduces obstructive sleep apnea severity. Sleep. 2019;42(zsz119) https://doi.org/10.1093/sleep/zsz119.
Lai V, Tong B, Tran C, Ricciardiello A, Donegan M, Murray N, Carberry J, Eckert D. Combination therapy with mandibular advancement and expiratory positive airway pressure valves reduces OSA severity. J Sleep Res. 2018;27 https://doi.org/10.1093/sleep/zsz119.
Mayoral P, Lagravère MO, Míguez-Contreras M, Garcia M. Antero-posterior mandibular position at different vertical levels for mandibular advancing device design. BMC Oral Health. 2019;19:85. https://doi.org/10.1186/s12903-019-0783-8.
Barbero M, Flores-Mir C, Blanco JC, Nuño VC, Casellas JB, Calvo Girado JL, Amezaga JA, De Carlos F. Tridimensional upper airway assessment in male patients with OSA using oral advancement devices modifying their vertical dimension. J Clin Sleep Med. 2020;16(10):1721–9. https://doi.org/10.5664/jcsm.8666.
Pitsis AJ, Darendeliler MA, Gotsopoulos H, Petocz P, Cistulli PA. Effect of vertical dimension on efficacy of oral appliance therapy in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:860–4. https://doi.org/10.1164/rccm.200204-342oc.
Milano F, Mutinelli S, Sutherland K, Milioli G, Scaramuzzino G, Cortesi A, Siciliani G, Lombardo L, Cistulli P. Influence of vertical mouth opening on oral appliance treatment outcome in positional obstructive sleep apnea. J Dent Sleep Med. 2018;5:17–23. https://doi.org/10.15331/jdsm.6918.
Norrhem N, Marklund M. An oral appliance with or without elastic bands to control mouth opening during sleep—a randomized pilot study. Sleep Breath. 2016;20:929–38. https://doi.org/10.1007/s11325-016-1312-5.
Chung JW, Enciso R, Levendowski DJ, Morgan TD, Westbrook PR, Clark GT. Treatment outcomes of mandibular advancement devices in positional and nonpositional OSA patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;109:724–31. https://doi.org/10.1016/j.tripleo.2009.11.031.
Sutherland K, Takaya H, Qian J, Petocz P, Ng AT, Cistulli PA. Oral appliance treatment response and polysomnographic phenotypes of obstructive sleep apnea. J Clin Sleep Med. 2015;11(8):861–8. https://doi.org/10.5664/jcsm.4934.
Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of sleep medicine systematic review, meta-analysis, and GRADE assessment. J Clin Sleep Med. 2019;15:301–34. https://doi.org/10.5664/jcsm.7640.
El-Solh AA, Moitheennazima B, Akinnusi ME, Churder PM, Lafornara AM. Combined oral appliance and positive airway pressure therapy for obstructive sleep apnea: a pilot study. Sleep Breath. 2011;15:203–8. https://doi.org/10.1007/s11325-010-0437-1.
Liu H, Chen Y, Lai Y, Huang C, Huang Y, Lin M, Han S, Chen C, Yu C, Lee P. Combining MAD and CPAP as an effective strategy for treating patients with severe sleep apnea intolerant to high-pressure PAP and unresponsive to MAD. PLoS One. 2017;12(10):e0187032. https://doi.org/10.1371/journal.pone.0187032.
Bamagoos AA, Eckert DJ, Sutherland K, Ngiam J, Cistulli PA. Dose-dependent effects of mandibular advancement on optimal positive airway pressure requirements in obstructive sleep apnoea. Sleep Breath. 2020;24(3):961–9. https://doi.org/10.1007/s11325-019-01930-3.
De Vries GE, Doff M, Hoekema A, Kerstjens HA, Wijkstra PJ. Continuous positive airway pressure and oral appliance hybrid therapy in obstructive sleep apnea: patient comfort, compliance, and preference: a pilot study. Journal of dental. J Dent Sleep Med. 2016;3(1):5–10. https://doi.org/10.15331/jdsm.5362.
Tong BK, Tran C, Ricciardiello A, Donegan M, Chiang AK, Szollosi I, Amatoury J, Carberry JC, Eckert DJ. CPAP combined with oral appliance therapy reduces CPAP requirements and pharyngeal pressure swings in obstructive sleep apnea. J Appl Physiol. 2020;129(5):1085–91. https://doi.org/10.1152/japplphysiol.00393.2020.
Borel J, Gakwaya S, DSAse J, Melo-Silva CA, Sériès F. Impact of CPAP interface and mandibular advancement device on upper airway mechanical properties assessed with phrenic nerve stimulation in sleep apnea patients. Respir Physiol Neurobiol. 2012;183(2):170–6. https://doi.org/10.1016/j.resp.2012.06.018.
El-Solh AA, Moitheennazima B, Akinnusi ME, Churder PM, Lafornara AM. Combined oral appliance and positive airway pressure therapy for obstructive sleep apnea: a pilot study. Sleep Breat. 2011;15(2):203–8. https://doi.org/10.1007/s11325-010-0437-1.
Berry RB, Kryger MH, DSAsie CA. A novel nasal expiratory positive airway pressure (EPAP) device for the treatment of obstructive sleep apnea: a randomized controlled trial. Sleep. 2011;34(4):479–85. https://doi.org/10.1093/sleep/34.4.479.
Chan AS, Sutherland K, Schwab RJ, Zeng B, Petocz P, Lee RW, Darendeliler MA, Cistulli PA. The effect of mandibular advancement on upper airway structure in obstructive sleep apnoea. Thorax. 2010;65:726–32. https://doi.org/10.1136/thx.2009.131094.
Brown EC, Cheng S, McKenzie DK, Butler JE, Gandevia SC, Bilston LE. Tongue and lateral upper airway movement with mandibular advancement. Sleep. 2013;36:397–404. https://doi.org/10.5665/sleep.2458.
Huon L, Liu SY, Shih TT, Chen Y, Lo M, Wang P. Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Otorhinolaryngol. 2016;273(11):4021–6. https://doi.org/10.1007/s00405-016-4131-1.
Vanderveken OM, Vroegop AV, de Heyning V, Paul H, Braem MJ. Drug-induced sleep endoscopy completed with a simulation bite approach for the prediction of the outcome of treatment of obstructive sleep apnea with mandibular repositioning appliances. Oper Tech Otolaryngol Head Neck Surg. 2011;22:175–82. https://doi.org/10.1016/j.otot.2011.05.001.
Marques M, Genta P, Sands SA, Taranto Montemurro L, Azarbarzin A, De Melo C, White DP, Wellman A. Characterizing site and severity of upper airway collapse To guide patient selection for Oral appliance therapy for obstructive sleep apnea. In: A80-a. NOVEL THERAPIES FOR OSA. Am J Respir Crit Care Med. 2017;195:A2584. https://doi.org/10.1093/sleep/zsx005.
Oksenberg A, Arons E, Nasser K, Vander T, Radwan H. REM-related obstructive sleep apnea: the effect of body position. J Clin Sleep Med. 2010;6(4):343–8. https://doi.org/10.5664/jcsm.27875.
Younes M. Contributions of upper airway mechanics and control mechanisms to severity of obstructive apnea. Am J Respir Crit Care Med. 2003;168:645–58. https://doi.org/10.1164/rccm.200302-201oc.
Braga CW, Chen Q, Burschtin OE, Rapoport DM, Ayappa I. Changes in lung volume and upper airway using MRI during application of nasal expiratory positive airway pressure in patients with sleep-disordered breathing. J Appl Physiol. 2011;111(5):1400–9. https://doi.org/10.1152/japplphysiol.00218.2011.
Tong BK, Tran C, Ricciardiello A, Chiang A, Donegan M, Murray N, Szollosi I, Amatoury J, Carberry JC, Eckert DJ. Efficacy of a novel oral appliance and the role of posture on nasal resistance in obstructive sleep apnea. J Clin Sleep Med. 2020;16:483–92. https://doi.org/10.5664/jcsm.8244.
Biggs DA. Spirometry. In: Data Interpretation in Anesthesia. New York: Springer; 2017. p. 431–4. https://doi.org/10.1007/978-3-319-55862-2.
Sanders MH, Moore SE. Inspiratory and expiratory partitioning of airway resistance during sleep in patients with sleep apnea. Am Rev Respir Dis. 1983;127(5):554–8. https://doi.org/10.1164/arrd.1983.127.5.554.
Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal DSAk: physiologic and clinical implications. Chest. 1990;98(2):317–24. https://doi.org/10.1378/chest.98.2.317.
Liu Y, Ying Y, Pandu JS, Wang Y, Dou S, Li Y, Ma D. Efficacy and safety assessment of expiratory positive airway pressure (EPAP) mask for OSAHS therapy. Auris Nasus Larynx. 2019;46(2):238–45. https://doi.org/10.1016/j.anl.2018.08.013.
Mahadevia AK, Önal E, Lopata M. Effects of expiratory positive airway pressure on sleep-induced respiratory abnormalities in patients with hypersomnia-sleep apnea syndrome. Am Rev Respir Dis. 1983;128(4):708–11. https://doi.org/10.1164/arrd.1983.127.5.554.
Heinzer RC, Stanchina ML, Malhotra A, Jordan AS, Patel SR, Lo Y, Wellman A, Schory K, Dover L, White DP. Effect of increased lung volume on sleep disordered breathing in patients with sleep apnoea. Thorax. 2006;61(5):435–9. https://doi.org/10.1164/arrd.1983.127.5.554.
Morrell MJ, Arabi Y, Zahn B, Badr MS. Progressive retropalatal narrowing preceding obstructive apnea. Am J Respir Crit Care Med. 1998;158(6):1974–81. https://doi.org/10.1164/ajrccm.158.6.9712107.
Deegan PC, Nolan P, Carey M, McNicholas WT. Effects of positive airway pressure on upper airway dilator muscle activity and ventilatory timing. J Appl Physiol. 1996;81(1):470–9. https://doi.org/10.1152/jappl.1996.81.1.470.
Schiza SE, Mermigkis C, Bouloukaki I. Expiratory positive airway pressure (EPAP) nasal device therapy: a welcome addition to obstructive sleep apnea syndrome therapy. Sleep and Breathing. 2015;19(3):775–6. https://doi.org/10.1007/s11325-014-1069-7.
White DP. Auto-PEEP to treat obstructive sleep apnea. J Clin Sleep Med. 2009;5(6):538–9. https://doi.org/10.5664/jcsm.27654.
Rosenthal L, Massie CA, Dolan DC, Loomas B, Kram J, Hart RW. A multicenter, prospective study of a novel nasal EPAP device in the treatment of obstructive sleep apnea: efficacy and 30-day adherence. J Clin Sleep Med. 2009;5(6):532–7. https://doi.org/10.5664/jcsm.27653.
Kryger MH, Berry RB, Massie CA. Long-term use of a nasal expiratory positive airway pressure (EPAP) device as a treatment for obstructive sleep apnea (OSA). J Clin Sleep Med. 2011;7(5):449–53B. https://doi.org/10.5664/jcsm.1304.
Patel AV, Hwang D, Masdeu MJ, Chen G, Rapoport DM, Ayappa I. Predictors of response to a nasal expiratory resistor device and its potential mechanisms of action for treatment of obstructive sleep apnea. J Clin Sleep Med. 2011;7(1):13–22. https://doi.org/10.5664/jcsm.28036.
Friedman M, Hwang MS, Yalamanchali S, Pott T, Sidhu M, Joseph NJ. Provent therapy for obstructive sleep apnea: impact of nasal obstruction. Laryngoscope. 2016;126(1):254–9. https://doi.org/10.1002/lary.25312.
Colrain IM, Brooks S, Black J. A pilot evaluation of a nasal expiratory resistance device for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2008;4(5):426–33. https://doi.org/10.5664/jcsm.27277.
Walsh JK, Griffin KS, Forst EH, Ahmed HH, Eisenstein RD, Curry DT, Hall-Porter JM, Schweitzer PK. A convenient expiratory positive airway pressure nasal device for the treatment of sleep apnea in patients non-adherent with continuous positive airway pressure. Sleep Med. 2011;12(2):147–52. https://doi.org/10.1016/j.sleep.2010.06.011.
Riaz M, Certal V, Nigam G, Abdullatif J, Zaghi S, Kushida CA, Camacho M. Nasal expiratory positive airway pressure devices (Provent) for OSA: a systematic review and meta-analysis. Sleep Disord. 2015;2015:734798. https://doi.org/10.1155/2015/734798.
Kureshi SA, Gallagher PR, McDonough JM, Cornaglia MA, Maggs J, Samuel J, Traylor J, Marcus CL. Pilot study of nasal expiratory positive airway pressure devices for the treatment of childhood obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(6):663–9. https://doi.org/10.5664/jcsm.3796.
Carberry JC, Amatoury J, Eckert DJ. Personalized management approach for obstructive sleep apnea. Chest. 2017;153(3):744–55. https://doi.org/10.1016/j.chest.2017.06.011.
Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am J Respir Crit Care Med. 2013;188:996–1004. https://doi.org/10.1164/rccm.201303-0448oc.
MacKay SG, Lewis R, McEvoy D, Joosten S, Holt NR. Surgical management of obstructive sleep apnoea: a position statement of the Australasian Sleep Association. Respirology. 2020;25(12):1292–308. https://doi.org/10.1111/resp.13967.
Certal V, Nishino N, Camacho M, Capasso R. Reviewing the systematic reviews in OSA surgery. Otolaryngol Head Neck Surg. 2013;149(6):817–29. https://doi.org/10.1177/0194599813509959.
Ravesloot M, De Vries N. Reliable calculation of the efficacy of non-surgical and surgical treatment of obstructive sleep apnea revisited. Sleep. 2011;34(1):105–10. https://doi.org/10.1093/sleep/34.1.105.
Mantero M, Carioli D, Romano M, Borsa N, Marra M, Tobaldini E. Multidisciplinary evaluation can find effective alternative treatment to CPAP in OSA patients. Eur Respir J. 2017;50:PA2295. https://doi.org/10.1183/1393003.congress-2017.pa2295.
Camacho M, Riley RW, Capasso R, O'Connor P, Chang ET, Reckley LK, Guilleminault C. Sleep surgery tool: a medical checklist to review prior to operating. J Cranio-Maxillofac Surg. 2017;45(3):381–6. https://doi.org/10.1016/j.jcms.2017.01.001.
Won T, Lee CH, Rhee C. Changes in site of obstruction in obstructive sleep apnea patients according to sleep position. In: Positional Therapy in Obstructive Sleep Apnea. New York: Springer; 2015. p. 119–28. https://doi.org/10.1007/978-3-319-09626-1_10.
Croft CB, Pringle M. Sleep nasendoscopy: a technique of assessment in snoring and obstructive sleep apnoea. Clin Otolaryngol Allied Sci. 1991;16(5):504–9. https://doi.org/10.1111/j.1365-2273.1991.tb02103.x.
De Vito A, Llatas MC, Vanni A, Bosi M, Braghiroli A, Campanini A, de Vries N, Hamans E, Hohenhorst W, Kotecha BT. European position paper on drug-induced sedation endoscopy (DISE). Sleep Breath. 2014;18(3):453–65. https://doi.org/10.1007/s11325-014-0989-6.
Lechner M, Wilkins D, Kotecha B. A review on drug-induced sedation endoscopy–technique, grading systems and controversies. Sleep Med Rev. 2018;41:141–8. https://doi.org/10.1016/j.smrv.2018.02.001.
Battagel JM, Johal A, Kotecha BT. Sleep nasendoscopy as a predictor of treatment success in snorers using dental sleep appliances. J Laryngol Otol. 2005;119:106–11. https://doi.org/10.1258/0022215053419916.
Johal A, Battagel JM, Kotecha BT. Sleep nasendoscopy: a diagnostic tool for predicting treatment success with dental sleep appliances in obstructive sleep apnoea. Eur J Orthod. 2005;27:607–14. https://doi.org/10.1093/ejo/cji063.
Huntley C, Cooper J, Stiles M, Grewal R, Boon M. Predicting success of oral appliance therapy in treating obstructive sleep apnea using drug-induced sleep endoscopy. J Clin Sleep Med. 2018;14(8):1333–7. https://doi.org/10.5664/jcsm.7266.
Vroegop AV, Vanderveken OM, Dieltjens M, Wouters K, Saldien V, Braem MJ. Sleep endoscopy with simulation bite for prediction of oral appliance treatment outcome. J Sleep Res. 2013;22:348–55. https://doi.org/10.1111/jsr.12008.
Sutherland K, Chan AS, Ngiam J, Darendeliler MA, Cistulli PA. Qualitative assessment of awake nasopharyngoscopy for prediction of oral appliance treatment response in obstructive sleep apnoea. Sleep Breath. 2018;22(4):1029–36. https://doi.org/10.1007/s11325-018-1624-8.
Okuno K, Sasao Y, Nohara K, Sakai T, Pliska BT, Lowe AA, Ryan CF, Almeida FR. Endoscopy evaluation to predict oral appliance outcomes in obstructive sleep apnoea. Eur Respir J. 2016;47:1410–9. https://doi.org/10.1183/13993003.01088-2015.
Ferris BG Jr, Mead J, Opie LH. Partitioning of respiratory flow resistance in man. J Appl Physiol. 1964;19:653–8. https://doi.org/10.1152/jappl.1964.19.4.653.
Miljeteig H, Cole P, Haight JS. Nasal resistance in recumbency and sleep. Rhinology. 1995;33(2):82–3. PMID: 7569657
Franklin K, Rehnqvist N, Axelsson S (2007) Obstructive Sleep Apnoea Syndrome:: a Systematic Literature Review. Swedish Council on Technology Assessment in Health Care. PMID: 28876733.
Sugiura T, Noda A, Nakata S, Yasuda Y, Soga T, Miyata S, Nakai S, Koike Y. Influence of nasal resistance on initial acceptance of continuous positive airway pressure in treatment for obstructive sleep apnea syndrome. Respiration. 2007;74(1):56–60. https://doi.org/10.1159/000089836.
Zeng B, Ng AT, Qian J, Petocz P, Darendeliler MA, Cistulli PA. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep. 2008;31(4):543–7. https://doi.org/10.1093/sleep/31.4.543.
Avidan AY, Kryger M. Physical examination in sleep medicine in thePrinciples and Practice of Sleep Medicine (Sixth Edition) Elsevier 2017:587–606e3. https://doi.org/10.1016/b978-0-323-24288-2.00059-3.
Augé J, Vent J, Agache I, Airaksinen L, Campo Mozo P, Chaker A, Cingi C, Durham S, Fokkens W, Gevaert P. EAACI position paper on the standardization of nasal allergen challenges. Allergy. 2018;73(8):1597–608. https://doi.org/10.1111/all.13416.
McColley SA, Carroll JL, Curtis S, Loughlin GM, Sampson HA. High prevalence of allergic sensitization in children with habitual snoring and obstructive sleep apnea. Chest. 1997;111(1):170–3. https://doi.org/10.1378/chest.111.1.170.
Miller JD. The role of dust mites in allergy. Clin Rev Allergy Immunol. 2019;57(3):312–29. https://doi.org/10.1007/s12016-018-8693-0.
Kanjanawasee D, Seresirikachorn K, Chitsuthipakorn W, Snidvongs K. Hypertonic saline versus isotonic saline nasal irrigation: systematic review and meta-analysis. Am J Rhinol Allergy. 2018;32(4):269–79. https://doi.org/10.1177/1945892418773566.
Sahin E, Çakır B, Vogt K. Clinical assessment of nasal airway obstruction. In: All around the nose. New York: Springer; 2020. p. 93–100. https://doi.org/10.1007/978-3-030-21217-9_11.
de Barros Souza FJF, Souza B, Fabrício FJ, Genta PR, de Souza Filho AJ, José A, Wellman A, Lorenzi-Filho G. The influence of head-of-bed elevation in patients with obstructive sleep apnea. Sleep Breath. 2017;21:815–20. https://doi.org/10.1007/s11325-017-1524-3.
Skinner MA, Kingshott RN, Jones DR, Homan SD, Taylor DR. Elevated posture for the management of obstructive sleep apnea. Sleep Breath. 2004;8(4):193–200. https://doi.org/10.1055/s-2004-860896.
Jung YG, Kim HY, Min J, Dhong H, Chung S. Role of intranasal topical steroid in pediatric sleep disordered breathing and influence of allergy, sinusitis, and obesity on treatment outcome. Clin Exp Otorhinolaryngol. 2011;4(1):27–32. https://doi.org/10.3342/ceo.2011.4.1.27.
Kheirandish-Gozal L, Gozal D. Intranasal budesonide treatment for children with mild obstructive sleep apnea syndrome. Pediatrics. 2008;122:149. https://doi.org/10.1542/peds.2007-3398.
Nguyen D, Liang J, Durr M. Topical nasal treatment efficacy on adult obstructive sleep apnea severity: a systematic review and meta-analysis. Int Forum Allergy Rhinol. 2020;11(2):153–61. https://doi.org/10.1002/alr.22658.
Morgenthaler TI, Kapen S, Lee-Chiong T, Alessi C, Boehlecke B, Brown T, Coleman J, Friedman L, Kapur V, Owens J, Pancer J, Swick T. Standards of Practice Committee; American Academy of Sleep Medic Practice parameters for the medical therapy of obstructive sleep apnea. Sleep. 2006;29(8):1031–5. https://doi.org/10.1093/sleep/29.8.1031.
Charakorn N, Hirunwiwatkul P, Chirakalwasan N, Chaitusaney B, Prakassajjatham M. The effects of topical nasal steroids on continuous positive airway pressure compliance in patients with obstructive sleep apnea: a systematic review and meta-analysis. Sleep Breath. 2017;21(1):3–8. https://doi.org/10.1007/s11325-016-1375-3.
Hornung DE, Smith DJ, Kurtz DB, White T, Leopold DA. Effect of nasal dilators on nasal structures, sniffing strategies, and olfactory ability. Rhinology. 2001;39(2):84–7.
Kiyohara N, Badger C, Tjoa T, Wong B. A comparison of over-the-counter mechanical nasal dilators: a systematic review. JAMA Facial Plast Surg. 2016;18(5):385–9. https://doi.org/10.1001/jamafacial.2016.0291.
Krakow B, Melendrez D, Sisley B, Warner TD, Krakow J, Leahigh L, Lee S. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: a randomized controlled trial. Sleep Breath. 2006;10(1):16–28. https://doi.org/10.1007/s11325-005-0037-7.
Scharf MB, McDannold M. A subjective evaluation of a nasal dilator on sleep & snoring. Ear Nose Throat J. 1994;73(6):395–401. https://doi.org/10.1177/014556139407300609.
Scho B, Franklin KA, Bru H, Wehde H, Ko D. Effect of nasal-valve dilation on obstructive sleep apnea. Chest. 2000;118(3):587–90. https://doi.org/10.1378/chest.118.3.587.
Yagihara F, Lorenzi-Filho G, Santos-Silva R. Nasal dilator strip is an effective placebo intervention for severe obstructive sleep apnea. J Clin Sleep Med. 2017;13(2):215–21. https://doi.org/10.5664/jcsm.6450.
Metes A, Cole P, Hoffstein V, Miljeteig H. Nasal airway dilation and obstructed breathing in sleep. Laryngoscope. 1992;102(9):1053–5. https://doi.org/10.1288/00005537-199209000-00017.
Matteo G, Pierluigi I, Giuseppe P, Vitaliano NQ, Onofrio R, Nicola Q, Giorgio C. Internal nasal dilator in patients with obstructive sleep apnea syndrome and treated with continuous positive airway pressure. Acta Biomed. 2019;90(2-S):24–7.
Schönhofer B, Kerl J, Suchi S, Köhler D, Franklin KA. Effect of nasal valve dilation on effective CPAP level in obstructive sleep apnea. Respir Med. 2003;97(9):1001–5. https://doi.org/10.1016/s0954-6111(03)00125-2.
Matteo G, Giuseppe P, Brigida S, Nicola Q, Giorgio C, on Snoring, Italian Study Group. Internal and external nasal dilatator in patients who snore: a comparison in clinical practice. Acta Biomed. 2019;90(2-S):10–4.
Ohtsuka K, Baba R, YaDSAawa W, Shirahama R, Hattori Y, Senoura H, Betsuyaku T, Fukunaga K. The effectiveness of nasal airway stent therapy for the treatment of mild-to-moderate obstructive sleep apnea syndrome. Respiration. 2020;100(3):193–200. https://doi.org/10.1159/000512319.
Camacho M, Malu OO, Kram YA, Nigam G, Riaz M, Song SA, Tolisano AM, Kushida CA. Nasal dilators (breathe right strips and NoZovent) for snoring and OSA: a systematic review and meta-analysis. Pulm Med. 2016;2016:4841310. https://doi.org/10.1155/2016/4841310.
Gruber RP, Lin AY, Richards T. Nasal strips for evaluating and classifying valvular nasal obstruction. Aesthet Plast Surg. 2011;35(2):211–5. https://doi.org/10.1007/s00266-010-9589-4.
Verse T, Maurer JT, Pirsig W. Effect of nasal surgery on sleep-related breathing disorders. Laryngoscope. 2002;112(1):64–8. https://doi.org/10.1097/00005537-200201000-00012.
Ishii L, Roxbury C, Godoy A, Ishman S, Ishii M. Does nasal surgery improve OSA in patients with nasal obstruction and OSA? A meta-analysis. Otolaryngol Head Neck Surg. 2015;153(3):326–33. https://doi.org/10.1177/0194599815594374.
Friedman M, Tanyeri H, Lim JW, Landsberg R, Vaidyanathan K, Caldarelli D. Effect of improved nasal breathing on obstructive sleep apnea. Otolaryngol Head Neck Surg. 2000;122(1):71–4. https://doi.org/10.1016/s0194-5998(00)70147-1.
Nakata S, Noda A, Yasuma F, Morinaga M, Sugiura M, Katayama N, Sawaki M, Teranishi M, Nakashima T. Effects of nasal surgery on sleep quality in obstructive sleep apnea syndrome with nasal obstruction. Am J Rhinol. 2008;22(1):59–63. https://doi.org/10.2500/ajr.2008.22.3120.
Choi JH, Kim EJ, Kim YS, Kim TH, Choi J, Kwon SY, Lee HM, Lee SH, Lee SH. Effectiveness of nasal surgery alone on sleep quality, architecture, position, and sleep-disordered breathing in obstructive sleep apnea syndrome with nasal obstruction. Am J Rhinol Allergy. 2011;25(5):338–41. https://doi.org/10.2500/ajra.2011.25.3654.
Yalamanchali S, Cipta S, Waxman J, Pott T, Joseph N, Friedman M. Effects of endoscopic sinus surgery and nasal surgery in patients with obstructive sleep apnea. Otolaryngol Head Neck Surg. 2014;151(1):171–5. https://doi.org/10.1177/0194599814528296.
Koutsourelakis I, Georgoulopoulos G, Perraki E, Vagiakis E, Roussos C, Zakynthinos SG. Randomised trial of nasal surgery for fixed nasal obstruction in obstructive sleep apnoea. Eur Respir J. 2008;31(1):110–7. https://doi.org/10.1183/09031936.00087607.
McLean HA, Urton AM, Driver HS, Tan A, Day AG, Munt PW, Fitzpatrick MF. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur Respir J. 2005;25(3):521–7. https://doi.org/10.1183/09031936.05.00045004.
Li H, Wang P, Chen Y, Lee L, Fang T, Lin H. Critical appraisal and meta-analysis of nasal surgery for obstructive sleep apnea. Am J Rhinol Allergy. 2011;25(1):45–9. https://doi.org/10.2500/ajra.2011.25.3558.
Park CY, Hong JH, Lee JH, Lee KE, Cho HS, Lim SJ, Kwak JW, Kim KS, Kim HJ. Clinical effect of surgical correction for nasal pathology on the treatment of obstructive sleep apnea syndrome. PLoS One. 2014;9(6):e98765. https://doi.org/10.1371/journal.pone.0098765.
Kamal I. Objective assessment of nasal obstruction in snoring and obstructive sleep apnea patients: experience of a police authority hospital. Ann Saudi Med. 2002;22(3-4):158–62. https://doi.org/10.5144/0256-4947.2002.158.
Camacho M, Riaz M, Capasso R, Ruoff CM, Guilleminault C, Kushida CA, Certal V. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: a systematic review and meta-analysis. Sleep. 2015;38(2):279–86. https://doi.org/10.5665/sleep.4414.
El-Anwar MW, Amer HS, Askar SM, Elsobki A, Awad A. Could nasal surgery affect multilevel surgery results for obstructive sleep apnea? J Craniofac Surg. 2018;29(7):1897–9. https://doi.org/10.1097/scs.0000000000004883.
Sieśkiewicz A, Walenczak I, Olszewska E, Luczaj J, Rogowski M. The assessment of nasal surgery and uvulopalatopharyngoplasty (UPPP) in the treatment of patients with mild and moderate obstructive sleep apnea (OSA). Pol Merkur Lekarski. 2007;22(128):130–3.
Woodson BT, Toohill RJ, Garancis JC. Histopathologic changes in snoring and obstructive sleep apnea syndrome. Laryngoscope. 1991;101(12 Pt 1):1318–22. https://doi.org/10.1002/lary.5541011211.
Sekosan M, Zakkar M, Wenig BL, Olopade CO, Rubinstein I. Inflammation in the uvula mucosa of patients with obstructive sleep apnea. Laryngoscope. 1996;106(8):1018–20. https://doi.org/10.1097/00005537-199608000-00021.
Friberg D, Ansved T, Borg K, Carlsson-Nordlander B, Larsson H, Svanborg E. Histological indications of a progressive snorers disease in an upper airway muscle. Am J Respir Crit Care Med. 1998;157:586–93. https://doi.org/10.1164/ajrccm.157.2.96-06049.
Hagander L, Harlid R, Svanborg E. Quantitative sensory testing in the oropharynx: a means of showing nervous lesions in patients with obstructive sleep apnea and snoring. Chest J. 2009;136:481–9. https://doi.org/10.1378/chest.08-2747.
Gouveia CJ, Yalamanchili A, Ghadersohi S, Price CP, Bove M, Attarian HP, Tan BK. Are chronic cough and laryngopharyngeal reflux more common in obstructive sleep apnea patients? Laryngoscope. 2019;129(5):1244–9. https://doi.org/10.1002/lary.27557.
Ryan CF, Lowe AA, Li D, Fleetham JA. Magnetic resonance imaging of the upper airway in obstructive sleep apnea before and after chronic nasal continuous positive airway pressure therapy. Am Rev Respir Dis. 1991;144(4):939–44. https://doi.org/10.1164/ajrccm/144.4.939.
Kayamori F, Bianchini EMG. Effects of orofacial myofunctional therapy on the symptoms and physiological parameters of sleep breathing disorders in adults: a systematic review. Revista CEFAC. 2017;19(6):868–78. https://doi.org/10.1590/1982-0216201719613317.
Browaldh N, Nerfeldt P, Lysdahl M, Bring J, Friberg D. SKUP3 randomised controlled trial: polysomnographic results after uvulopalatopharyngoplasty in selected patients with obstructive sleep apnoea. Thorax. 2013;68(9):846–53. https://doi.org/10.1136/thoraxjnl-2012-202610.
Pang KP, Plaza G, Reina CO, Chan YH, Pang KA, Pang EB, Wang CMZ, Rotenberg B. Palate surgery for obstructive sleep apnea: a 17-year meta-analysis. Eur Arch Otorhinolaryngol. 2018;275(7):1697–707. https://doi.org/10.1007/s00405-018-5015-3.
Ryan CF, Love LL. Unpredictable results of laser assisted uvulopalatoplasty in the treatment of obstructive sleep apnoea. Thorax. 2000;55(5):399–404. https://doi.org/10.1136/thorax.55.5.399.
Lowe AA, Gionhaku N, Takeuchi K, Fleetham JA. Three-dimensional CT reconstructions of tongue and airway in adult subjects with obstructive sleep apnea. Am J Orthod Dentofac Orthop. 1986;90(5):364–74. https://doi.org/10.1016/0889-5406(86)90002-8.
Iida-Kondo C, Yoshino N, Kurabayashi T, Mataki S, Hasegawa M, Kurosaki N. Comparison of tongue volume/oral cavity volume ratio between obstructive sleep apnea syndrome patients and normal adults using magnetic resonance imaging. J Med Dent Sci. 2006;53(2):119–26. https://doi.org/10.11480/jmds.530205.
Kim AM, Keenan BT, Jackson N, Chan EL, Staley B, Poptani H, Torigian DA, Pack AI, Schwab RJ. Tongue fat and its relationship to obstructive sleep apnea. Sleep. 2014;37(10):1639–48. https://doi.org/10.5665/sleep.4072.
Ahn SH, Kim J, Min HJ, Chung HJ, Hong JM, Lee J, Kim C, Cho H. Tongue volume influences lowest oxygen saturation but not apnea-hypopnea index in obstructive sleep apnea. PLoS One. 2015;10(8):e0135796. https://doi.org/10.1371/journal.pone.0135796.
Simmonds JC, Patel AK, Mildenhall NR, Mader NS, Scott AR. Neonatal macroglossia: demographics, cost of care, and associated comorbidities. Cleft Palate Craniofac J. 2018;55(8):1122–9. https://doi.org/10.1177/1055665618760898.
Srivastava A, Pandey A, Srivastava S. Amyloidosis is a rare disease but still a frequent cause of macroglossia. Indian J Sci Res. 2017:GALE|A520586709.
Wittmann A. Macroglossia in acromegaly and hypothyroidism. Virchows Arch A Pathol Anat Histol. 1977;373(4):353–60. https://doi.org/10.1007/bf00432533.
Miller SC, Nguyen SA, Ong AA, Gillespie MB. Transoral robotic base of tongue reduction for obstructive sleep apnea: a systematic review and meta-analysis. Laryngoscope. 2017;127(1):258–65. https://doi.org/10.1002/lary.26060.
Murphey AW, Kandl JA, Nguyen SA, Weber AC, Gillespie MB. The effect of glossectomy for obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;153(3):334–42. https://doi.org/10.1177/0194599815594347.
Young T, Peppard PE, Taheri S. Excess weight and sleep-disordered breathing. J Appl Physiol. 2005;99:1592–9. https://doi.org/10.1152/japplphysiol.00587.2005.
Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000;284:3015–21. https://doi.org/10.1001/jama.284.23.3015.
Vuorjoki-Ranta T, Aarab G, Lobbezoo F, Tuomilehto H, Ahlberg J. Weight gain may affect mandibular advancement device therapy in patients with obstructive sleep apnea: a retrospective study. Sleep Breath. 2019;23(2):531–4. https://doi.org/10.1007/s11325-018-1728-1.
Kulkas A, Leppänen T, Sahlman J, Tiihonen P, Mervaala E, Kokkarinen J, Randell J, Seppä J, Töyräs J, Tuomilehto H. Weight loss alters severity of individual nocturnal respiratory events depending on sleeping position. Physiol Meas. 2014;35(10):2037–52. https://doi.org/10.1088/0967-3334/35/10/2037.
Stadler DL, McEvoy RD, Bradley J, Paul D, Catcheside PG. Changes in lung volume and diaphragm muscle activity at sleep onset in obese obstructive sleep apnea patients vs. healthy-weight controls. J Appl Physiol. 2010;109(4):1027–36. https://doi.org/10.1152/japplphysiol.01397.2009.
Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burman ED, Longmore DB, Guz A. Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J. 1989;2(7):613–22. https://erj.ersjournals.com/content/2/7/613
Li Y, Lin N, Ye J, Chang Q, Han D, Sperry A. Upper airway fat tissue distribution in subjects with obstructive sleep apnea and its effect on retropalatal mechanical loads. Respir Care. 2012;57(7):1098–105. https://doi.org/10.4187/respcare.00929.
Wang SH, Keenan BT, Wiemken A, Zang Y, Staley B, Sarwer DB, Torigian DA, Williams N, Pack AI, Schwab RJ. Effect of weight loss on upper airway anatomy and the apnea–hypopnea index. The Importance of Tongue Fat. Am J Respir Crit Care Med. 2020;201(6):718–27. https://doi.org/10.1164/rccm.201903-0692oc.
Kuna ST, Reboussin DM, Strotmeyer ES, Millman RP, Zammit G, Walkup MP, Wadden TA, Wing RR, Pi-Sunyer FX, Spira AP. Effects of weight loss on obstructive sleep apnea severity: 10-year results of the sleep AHEAD study. Am J Respir Crit Care Med. 2020;203(2):221–9. https://doi.org/10.1164/rccm.201912-2511oc.
Tuomilehto H, Seppä J, Uusitupa M, Peltonen M, Martikainen T, Sahlman J, Kokkarinen J, Randell J, Pukkila M, Vanninen E. The impact of weight reduction in the prevention of the progression of obstructive sleep apnea: an explanatory analysis of a 5-year observational follow-up trial. Sleep Med. 2014;15(3):329–35. https://doi.org/10.1016/j.sleep.2013.11.786.
Greenburg DL, Lettieri CJ, Eliasson AH. Effects of surgical weight loss on measures of obstructive sleep apnea: a meta-analysis. Am J Med. 2009;122:535–42. https://doi.org/10.1016/j.amjmed.2008.10.037.
Sillo TO, Lloyd-Owen S, White E, Abolghasemi-Malekabadi K, Lock-Pullan P, Ali M, Perry A, Robinson SJ, Wadley MS. The impact of bariatric surgery on the resolution of obstructive sleep apnoea. BMC Res Notes. 2018;11(1):385. https://doi.org/10.1186/s13104-018-3484-5.
Zhang Y, Wang W, Yang C, Shen J, Shi M, Wang B. Improvement in nocturnal hypoxemia in obese patients with obstructive sleep apnea after bariatric surgery: a meta-analysis. Obes Surg. 2019;29(2):601–8. https://doi.org/10.1007/s11695-018-3573-5.
Dixon JB, Schachter LM, O'Brien PE, Jones K, Grima M, Lambert G, Brown W, Bailey M, Naughton MT. Surgical vs conventional therapy for weight loss treatment of obstructive sleep apnea: a randomized controlled trial. JAMA. 2012;308:1142–9. https://doi.org/10.1001/2012.jama.11580.
Elbahrawy A, Bougie A, Loiselle S, Demyttenaere S, Court O, Andalib A. Medium to long-term outcomes of bariatric surgery in older adults with super obesity. Surg Obes Relat Dis. 2018;14(4):470–6. https://doi.org/10.1016/j.soard.2017.11.008.
Lettieri CJ, Eliasson AH, Greenburg DL. Persistence of obstructive sleep apnea after surgical weight loss. J Clin Sleep Med. 2008;4:333–8. https://doi.org/10.5664/jcsm.27233.
Karmali S, Brar B, Shi X, Sharma AM, de Gara C, Birch DW. Weight recidivism post-bariatric surgery: a systematic review. Obes Surg. 2013;23(11):1922–33. https://doi.org/10.1007/s11695-013-1070-4.
Lynch A. When the honeymoon is over, the real work begins: Gastric bypass patients’ weight loss trajectories and dietary change experiences. Soc Sci Med. 2016;151:241–9. https://doi.org/10.1016/j.socscimed.2015.12.024.
Alghothani L, Iftikhar I. Comparative efficacy of treatments for restless legs syndrome: a network meta-analysis. Chest J. 2016;150:1267A. https://doi.org/10.1093/ndt/gfz097.
Yong LC, Li J, Calvert GM. Sleep-related problems in the US working population: prevalence and association with shiftwork status. Occup Environ Med. 2017;74(2):93–104. https://doi.org/10.1136/oemed-2016-103638.
American Academy of Sleep Medicine (2014) International classification of sleep disorders—third edition (ICSD-3). Darien, IL American Academy of Sleep Medicine https://doi.org/10.1378/chest.14-0970.
Krakow B, Romero E, Ulibarri VA, Kikta S. Prospective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: a pilot study of subjective and objective causes. Sleep. 2012;35:1685–92. https://doi.org/10.5665/sleep.2244.
Luyster FS, Buysse DJ, Strollo PJ Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6:196–204. https://doi.org/10.5664/jcsm.27772.
Lichstein KL, ThoDSA SJ, Woosley JA, Geyer JD. Co-occurring insomnia and obstructive sleep apnea. Sleep Med. 2013;14(9):824–9. https://doi.org/10.1016/j.sleep.2013.02.0087.
Subramanian S, Guntupalli B, Murugan T, Bopparaju S, Chanamolu S, Casturi L, Surani S. Gender and ethnic differences in prevalence of self-reported insomnia among patients with obstructive sleep apnea. Sleep Breath. 2011;15(4):711–5. https://doi.org/10.1007/s11325-010-0426-4.
Wickwire EM, Collop NA. Insomnia and sleep-related breathing disorders. Chest. 2010;137(6):1449–63. https://doi.org/10.1378/chest.09-1485.
Krell SB, Kapur VK. Insomnia complaints in patients evaluated for obstructive sleep apnea. Sleep Breath. 2005;9(3):104–10. https://doi.org/10.1007/s11325-005-0026-x.
Machado MAC, de Carvalho LBC, Juliano ML, Taga M, do Prado LBF, do Prado GF. Clinical co-morbidities in obstructive sleep apnea syndrome treated with mandibular repositioning appliance. Respir Med. 2006;100(6):988–95. https://doi.org/10.1016/j.rmed.2005.10.002.
Pieh C, Bach M, Popp R, Jara C, Crönlein T, Hajak G, Geisler P. Insomnia symptoms influence CPAP compliance. Sleep Breath. 2013;17(1):99–104. https://doi.org/10.1007/s11325-012-0655-9.
Wickwire EM, Smith MT, Birnbaum S, Collop NA. Sleep maintenance insomnia complaints predict poor CPAP adherence: a clinical case series. Sleep Med. 2010;11(8):772–6. https://doi.org/10.1016/j.sleep.2010.03.012.
Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, Espie CA, Garcia-Borreguero D, Gjerstad M, Gonçalves M. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. https://doi.org/10.1111/jsr.12594.
Perlis ML, Smith MT, Jungquist C, Nowakowski S, Orff H, Soeffing J. Cognitive-behavioral therapy for insomnia. In: Clinical handbook of insomnia. New York: Springer; 2010. p. 281–96. https://doi.org/10.1007/978-1-60327-042-7_22.
Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–33. https://doi.org/10.7326/M15-2175.
Natsky AN, Vakulin A, Chai-Coetzer CL, Lack L, McEvoy RD, Lovato N, Sweetman A, Gordon CJ, Adams RJ, Kaambwa B. Economic evaluation of cognitive behavioural therapy for insomnia (CBT-I) for improving health outcomes in adult populations: a systematic review. Sleep Med Rev. 2020;54:101351. https://doi.org/10.1016/j.smrv.2020.101351.
Wilson S, Anderson K, Baldwin D, Dijk D, Espie A, Espie C, Gringras P, Krystal A, Nutt D, Selsick H. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: an update. J Psychopharmacol. 2019;33(8):923–47. https://doi.org/10.1177/0269881110379307.
Anderson KN. Insomnia and cognitive behavioural therapy—how to assess your patient and why it should be a standard part of care. J Thorac Dis. 2018;10(Suppl 1):S94–S102. https://doi.org/10.21037/jtd.2018.01.35.
van Straten A, Geraedts A, Verdonck-de Leeuw I, Andersson G, Cuijpers P. Psychological treatment of depressive symptoms in patients with medical disorders: a meta-analysis. J Psychosom Res. 2010;69(1):23–32. https://doi.org/10.1016/j.jpsychores.2010.01.019.
Drake CL, Kalmbach DA, Arnedt JT, Cheng P, Tonnu CV, Cuamatzi-Castelan A, Fellman-Couture C. Treating chronic insomnia in postmenopausal women: a randomized clinical trial comparing cognitive-behavioral therapy for insomnia, sleep restriction therapy, and sleep hygiene education. Sleep. 2019;42(2):zsy217. https://doi.org/10.1093/sleep/zsy217.
Kalmbach DA, Cheng P, Arnedt JT, Anderson JR, Roth T, Fellman-Couture C, Williams RA, Drake CL. Treating insomnia improves depression, maladaptive thinking, and hyperarousal in postmenopausal women: comparing cognitive-behavioral therapy for insomnia (CBTI), sleep restriction therapy, and sleep hygiene education. Sleep Med. 2019;55:124–34. https://doi.org/10.1016/j.sleep.2018.11.019.
Kyle SD, Miller CB, Rogers Z, Siriwardena AN, MacMahon KM, Espie CA. Sleep restriction therapy for insomnia is associated with reduced objective total sleep time, increased daytime somnolence, and objectively impaired vigilance: implications for the clinical management of insomnia disorder. Sleep. 2014;37(2):229–37. https://doi.org/10.5665/sleep.3386.
Kyle SD, Morgan K, Spiegelhalder K, Espie CA. No pain, no gain: an exploratory within-subjects mixed-methods evaluation of the patient experience of sleep restriction therapy (SRT) for insomnia. Sleep Med. 2011;12(8):735–47. https://doi.org/10.1016/j.sleep.2011.03.016.
Cheng P, Kalmbach D, Fellman-Couture C, Arnedt JT, Cuamatzi-Castelan A, Drake CL. Risk of excessive sleepiness in sleep restriction therapy and cognitive behavioral therapy for insomnia: a randomized controlled trial. J Clin Sleep Med. 2020;16(2):193–8. https://doi.org/10.5664/jcsm.8164. Epub 2020 Jan 13
Sidani S, Epstein DR, Fox M, Collins L. Comparing the effects of single-and multiple-component therapies for insomnia on sleep outcomes. Worldviews Evid-Based Nurs. 2019;16(3):195–203. https://doi.org/10.1111/wvn.12367.
Hauri PJ. Sleep hygiene, relaxation therapy, and cognitive interventions. In: Case studies in insomnia. New York: Springer; 1991. p. 65–84. https://doi.org/10.1007/978-1-4757-9586-8_5.
Stepanski EJ, Wyatt JK. Use of sleep hygiene in the treatment of insomnia. Sleep Med Rev. 2003;7:215–25. https://doi.org/10.1053/smrv.2001.0246.
Irish LA, Kline CE, Gunn HE, Buysse DJ, Hall MH. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23–36. https://doi.org/10.1016/j.smrv.2014.10.001.
Chung K, Lee C, Yeung W, Chan M, Chung EW, Lin W. Sleep hygiene education as a treatment of insomnia: a systematic review and meta-analysis. Fam Pract. 2018;35(4):365–75. https://doi.org/10.1093/fampra/cmx122.
Mead MP, Irish LA. Application of health behaviour theory to sleep health improvement. J Sleep Res. 2020;29(5):e12950. https://doi.org/10.1111/jsr.12950.
Jansson-Fröjmark M, Evander J, Alfonsson S. Are sleep hygiene practices related to the incidence, persistence and remission of insomnia? Findings from a prospective community study. J Behav Med. 2019;42(1):128–38. https://doi.org/10.1007/s10865-018-9949-0.
Ogeil RP, Prasad S, O'Driscoll DM, Li WY, Lubman DI, Young AC. Psychoactive drug and medication use among patients referred to a tertiary sleep laboratory population. Psychiatry Res. 2020;294:113545. https://doi.org/10.1016/j.psychres.2020.113545.
Jin M, Yoon C, Ko H, Kim H, Kim A, Moon H, Jung S. The relationship of caffeine intake with depression, anxiety, stress, and sleep in Korean adolescents. Korean J Fam Med. 2016;37(2):111–6. https://doi.org/10.4082/kjfm.2016.37.2.111.
Chaudhary NS, Grandner MA, Jackson NJ, Chakravorty S. Caffeine consumption, insomnia, and sleep duration: results from a nationally representative sample. Nutrition. 2016;32(11-12):1193–9. https://doi.org/10.1016/j.nut.2016.04.005.
Frozi J, de Carvalho HW, Ottoni GL, Cunha RA, Lara DR. Distinct sensitivity to caffeine-induced insomnia related to age. J Psychopharmacol. 2018;32(1):89–95. https://doi.org/10.1177/0269881117722997.
Zhang B, Liu Y, Wang X, Deng Y, Zheng X. Cognition and brain activation in response to various doses of caffeine: a near-infrared spectroscopy study. Front Psychol. 2020;11:1393. https://doi.org/10.3389/fpsyg.2020.01393.
Pasman WJ, Boessen R, Donner Y, Clabbers N, Boorsma A. Effect of caffeine on attention and alertness measured in a home-setting, using web-based cognition tests. JMIR Res Protoc. 2017;6(9):e169. https://doi.org/10.2196/resprot.6727.
Wang C, Zhu Y, Dong C, Zhou Z, Zheng X. Effects of various doses of caffeine ingestion on intermittent exercise performance and cognition. Brain Sci. 2020;10(9):595. https://doi.org/10.3390/brainsci10090595.
Cornelis MC, Weintraub S, Morris MC. Recent caffeine drinking associates with cognitive function in the UK biobank. Nutrients. 2020;10(9):595. https://doi.org/10.3390/nu12071969.
Drake C, Roehrs T, Shambroom J, Roth T. Caffeine effects on sleep taken 0, 3, or 6 hours before going to bed. J Clin Sleep Med. 2013;9:1195–200. https://doi.org/10.5664/jcsm.3170.
Bertazzo-Silveira E, Kruger CM, De Toledo IP, Porporatti AL, Dick B, Flores-Mir C, Canto GDL. Association between sleep bruxism and alcohol, caffeine, tobacco, and drug abuse: a systematic review. J Am Dent Assoc. 2016;147(11):859–866.e4. https://doi.org/10.1016/j.adaj.2016.06.014.
Simou E, Britton J, Leonardi-Bee J. Alcohol and the risk of sleep apnoea: a systematic review and meta-analysis. Sleep Med. 2018;42:38–46. https://doi.org/10.1016/j.sleep.2017.12.005.
Issa FG, Sullivan CE. Alcohol, snoring and sleep apnea. J Neurol Neurosurg Psychiatry. 1982;45:353–9. https://doi.org/10.1016/j.sleep.2017.12.005.
Kolla BP, Foroughi M, Saeidifard F, Chakravorty S, Wang Z, Mansukhani MP. The impact of alcohol on breathing parameters during sleep: a systematic review and meta-analysis. Sleep Med Rev. 2018;42:59–67. https://doi.org/10.1136/jnnp.45.4.353.
Irwin MR, Bjurstrom MF, Olmstead R. Polysomnographic measures of sleep in cocaine dependence and alcohol dependence: implications for age-related loss of slow wave, stage 3 sleep. Addiction. 2016;111(6):1084–92. https://doi.org/10.1111/add.13300.
Chan JK, Trinder J, Andrewes HE, Colrain IM, Nicholas CL. The acute effects of alcohol on sleep architecture in late adolescence. Alcohol Clin Exp Res. 2013;37(10):1720–8. https://doi.org/10.1111/acer.12141.
O’Malley MB, O’Malley EB. Psychophysiological insomnia. In: Clinical Handbook of Insomnia. New York: Springer; 2010. p. 155–65. https://doi.org/10.1007/978-1-60327-042-7_11.
Pasman JA, Smit DJ, Kingma L, Vink JM, Treur JL, Verweij KJ. Causal relationships between substance use and insomnia. Drug Alcohol Depend. 2020;214:108151. https://doi.org/10.1101/2020.04.06.027003.
AlRyalat SA, Kussad S, El Khatib O, Hamad I, Ahmad A, Alshnneikat M, AbuMahfouz B. Assessing the effect of nicotine dose in cigarette smoking on sleep quality. Sleep Breath. 2020;25(3):1319–24. https://doi.org/10.1007/s11325-020-02238-3.
Teofilo L. Medications and their effects on sleep. Sleep Med. 2008; https://doi.org/10.1016/s1556-407x(18)30019-5.
Jaehne A, Loessl B, Bárkai Z, Riemann D, Hornyak M. Effects of nicotine on sleep during consumption, withdrawal and replacement therapy. Sleep Med Rev. 2009;13(5):363–77. https://doi.org/10.1016/j.smrv.2008.12.003.
Brett EI, Miller MB, Leavens EL, Lopez SV, Wagener TL, Leffingwell TR. Electronic cigarette use and sleep health in young adults. J Sleep Res. 2020;29(3):e12902. https://doi.org/10.1111/jsr.12902.
Spadola CE, Guo N, Johnson DA, Sofer T, Bertisch SM, Jackson CL, Rueschman M, Mittleman MA, Wilson JG, Redline S. Evening intake of alcohol, caffeine, and nicotine: night-to-night associations with sleep duration and continuity among African Americans in the Jackson heart sleep study. Sleep. 2019;42(11):zsz136. https://doi.org/10.1093/sleep/zsz136.
Bola KI, Lesage SR, Gamaldo CE, Neubauer DN, Funderburk FR, Cadet JL, David PM, Verdejo-Garcia A, Benbrook AR. Sleep disturbance in heavy marijuana users. Sleep. 2008;31(6):901–8. https://doi.org/10.1093/sleep/31.6.901.
Soreca I, Conklin CA, Vella EJ, Salkeld RP, Joyce CJ, Mumma JM, Jakicic JM, Kupfer DJ. Can exercise alleviate sleep disturbances during acute nicotine withdrawal in cigarette smokers? Exp Clin Psychopharmacol. 2020;30(1):82–92. https://doi.org/10.1037/pha0000390.
Patterson F, Ashare R. Improved sleep as an adjunctive treatment for smoking cessation. In: Sleep and Health. Amsterdam: Elsevier; 2019. p. 283–301. https://doi.org/10.1016/b978-0-12-815373-4.00022-8.
Short NA, Mathes BM, Gibby B, Oglesby ME, Zvolensky MJ, Schmidt NB. Insomnia symptoms as a risk factor for cessation failure following smoking cessation treatment. Addict Res Theory. 2017;25(1):17–23. https://doi.org/10.1080/16066359.2016.1190342.
Marques M, Pereira AT, Azevedo J, Xavier S, Bento E, Soares MJ, Freitas V, Macedo A. Validation of the insomnia assessment scale-adapted in a community sample of Portuguese pregnant women. Eur Psychiatry. 2016;33:S269. https://doi.org/10.1016/j.eurpsy.2016.01.705.
Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32(5):449–57. https://doi.org/10.1200/jco.2012.47.7265.
Chen C, Pei Y, Chen N, Huang L, Chou S, Wu KP, Ko P, Wong AMK, Wu C. Sedative music facilitates deep sleep in Young adults. J Altern Complement Med. 2014;20(4):312–7. https://doi.org/10.1089/acm.2012.0050.
Wang Y, Wang F, Zheng W, Zhang L, Ng CH, Ungvari GS, Xiang Y. Mindfulness-based interventions for insomnia: a meta-analysis of randomized controlled trials. Behav Sleep Med. 2020;18(1):1–9. https://doi.org/10.1080/15402002.2018.1518228.
Aiello KD, Caughey WG, Nelluri B, Sharma A, Mookadam F, Mookadam M. Effect of exercise training on sleep apnea: a systematic review and meta-analysis. Respir Med. 2016;116:85–92. https://doi.org/10.1016/j.rmed.2016.05.015.
Mendelson M, Lyons OD, Yadollahi A, Inami T, Oh P, Bradley TD. Effects of exercise training on sleep apnoea in patients with coronary artery disease: a randomised trial. Eur Respir J. 2016;48:142–50. https://doi.org/10.1183/13993003.01897-2015.
Norman JF, Von Essen SG, Fuchs RH, McElligott M. Exercise training effect on obstructive sleep apnea syndrome. Sleep Res Online. 2000;3(3):121–9. https://doi.org/10.1183/13993003.01897-2015.
Schutz TCB, Cunha TCA, Moura-Guimaraes T, Luz GP, Ackel-D'Elia C, Alves ES, Pantiga Junior G, Mello MT, Tufik S, Bittencourt L. Comparison of the effects of continuous positive airway pressure, oral appliance and exercise training in obstructive sleep apnea syndrome. Clinics. 2013;68(8):1168–74. https://doi.org/10.6061/clinics/2013(08)17.
Drager LF, Brunoni AR, Jenner R, Lorenzi-Filho G, Bensenor IM, Lotufo PA. Effects of CPAP on body weight in patients with obstructive sleep apnoea: a meta-analysis of randomised trials. Thorax. 2015;70:258–64. https://doi.org/10.1136/thoraxjnl-2014-205361.
Kline CE, Crowley EP, Ewing GB, Burch JB, Blair SN, Durstine JL, Davis JM, Youngstedt SD. The effect of exercise training on obstructive sleep apnea and sleep quality: a randomized controlled trial. Sleep. 2011;34(12):1631–40. https://doi.org/10.5665/sleep.1422.
Guimaraes KC, Drager LF, Genta PR, Marcondes BF, Lorenzi-Filho G. Effects of oropharyngeal exercises on patients with moderate obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2009;179:962–6. https://doi.org/10.1164/rccm.200806-981oc.
Banno M, Harada Y, Taniguchi M, et al. Exercise can improve sleep quality: a systematic review and meta-analysis. PeerJ. 2018;6:e5172. https://doi.org/10.7717/peerj.5172.
Rubio-Arias JÁ, Marín-Cascales E, Ramos-Campo DJ, Hernandez AV, Pérez-López FR. Effect of exercise on sleep quality and insomnia in middle-aged women: a systematic review and meta-analysis of randomized controlled trials. Maturitas. 2017;100:49–56. https://doi.org/10.1016/j.maturitas.2017.04.003.
Lowe H, Haddock G, Mulligan LD, Gregg L, Fuzellier-Hart A, Carter L, Kyle SD. Does exercise improve sleep for adults with insomnia? A systematic review with quality appraisal. Clin Psychol Rev. 2019;68:1–12. https://doi.org/10.1016/j.maturitas.2017.04.003.
Morita Y, Sasai-Sakuma T, Inoue Y. Effects of acute morning and evening exercise on subjective and objective sleep quality in older individuals with insomnia. Sleep Med. 2017;34:200–8. https://doi.org/10.1016/j.sleep.2017.03.014.
Ramos-Campo DJ, Ávila-Gandía V, Luque AJ, Rubio-Arias JÁ. Effects of hour of training and exercise intensity on nocturnal autonomic modulation and sleep quality of amateur ultra-endurance runners. Physiol Behav. 2019;198:134–9. https://doi.org/10.1016/j.physbeh.2018.10.020.
Morse CD, Klingman KJ, Jacob BL, Kodali L. Exercise and insomnia risk in middle-aged women. J Nurse Pract. 2019;15:236–240.e2. https://doi.org/10.1016/j.nurpra.2018.10.020.
Purani H, Friedrichsen S, Allen AM. Sleep quality in cigarette smokers: associations with smoking-related outcomes and exercise. Addict Behav. 2019;90:71–6. https://doi.org/10.1016/j.addbeh.2018.10.023.
Bonardi JM, Lima LG, Campos GO, Bertani RF, Moriguti JC, Ferriolli E, Lima NK. Effect of different types of exercise on sleep quality of elderly subjects. Sleep Med. 2016;25:122–9. https://doi.org/10.1016/j.sleep.2016.06.025.
Ebrahimi M, Guilan-Nejad TN, Pordanjani AF. Effect of yoga and aerobics exercise on sleep quality in women with type 2 diabetes: a randomized controlled trial. Sleep Sci. 2017;10(2):68–72. https://doi.org/10.5935/1984-0063.20170012.
Neubauer DN, Pandi-Perumal SR, Spence DW, Buttoo K, Monti JM. Pharmacotherapy of insomnia. J Cent Nerv Syst Dis. 2018;10:1179573518770672. https://doi.org/10.1111/j.1469-7580.2009.01106.x.
Kim JH, Duffy JF. Circadian rhythm sleep-wake disorders in older adults. Sleep Med Clin. 2018;17(2):241–52. https://doi.org/10.1016/j.jsmc.2017.09.004.
Kandeger A, Selvi Y, Tanyer DK. The effects of individual circadian rhythm differences on insomnia, impulsivity, and food addiction. Eat Weight Disord. 2019;24(1):47–55. https://doi.org/10.1007/s40519-018-0518-x.
Barion A, Zee PC. A clinical approach to circadian rhythm sleep disorders. Sleep Med. 2007;8(6):566–77. https://doi.org/10.1016/j.sleep.2006.11.017.
Yazaki M, Shirakawa S, Okawa M, Takahashi K. Demography of sleep disturbances associated with circadian rhythm disorders in Japan. Psychiatry Clin Neurosci. 1999;53(2):267–8. https://doi.org/10.1046/j.1440-1819.1999.00533.x.
Schrader H, Bovim G, Sand T. The prevalence of delayed and advanced sleep phase syndromes. J Sleep Res. 1993;2(1):51–5. https://doi.org/10.1111/j.1365-2869.1993.tb00061.x.
Weitzman ED, Czeisler CA, Coleman RM, Spielman AJ, Zimmerman JC, Dement W, Pollak CP. Delayed sleep phase syndrome: a chronobiological disorder with sleep-onset insomnia. Arch Gen Psychiatry. 1981;38(7):737–46. https://doi.org/10.1001/archpsyc.1981.01780320017001.
Chesson AL, Littner M, Davila D, Anderson WM, Grigg-Damberger M, Hartse K, Johnson S, Wise M. Practice parameters for the use of light therapy in the treatment of sleep disorders. Standards of Practice Committee, American Academy of Sleep Medicine. Sleep. 1999;22(5):641–60. https://doi.org/10.1093/sleep/22.5.641.
Richardson C, Cain N, Bartel K, Micic G, Maddock B, Gradisar M. A randomised controlled trial of bright light therapy and morning activity for adolescents and young adults with delayed sleep-wake phase disorder. Sleep Med. 2018;45:114–23. https://doi.org/10.1016/j.sleep.2018.02.001.
Burgess HJ, Crowley SJ, Gazda CJ, Fogg LF, Eastman CI. Preflight adjustment to eastward travel: 3 days of advancing sleep with and without morning bright light. J Biol Rhythm. 2003;18:318–28. https://doi.org/10.1177/0748730403253585.
Crowley SJ, Eastman CI. Phase advancing human circadian rhythms with morning bright light, afternoon melatonin, and gradually shifted sleep: can we reduce morning bright-light duration? Sleep Med. 2015;16(2):288–97. https://doi.org/10.1016/j.sleep.2014.12.004.
Warman VL, Dijk D, Warman GR, Arendt J, Skene DJ. Phase advancing human circadian rhythms with short wavelength light. Neurosci Lett. 2003;342(1-2):37–40. https://doi.org/10.1016/s0304-3940(03)00223-4.
Exelmans L, Van den Bulck J. Bedtime, shuteye time and electronic media: sleep displacement is a two-step process. J Sleep Res. 2017;26(3):364–70. https://doi.org/10.1111/jsr.12510.
Esaki Y, Kitajima T, Ito Y, Koike S, Nakao Y, Tsuchiya A, Hirose M, Iwata N. Wearing blue light-blocking glasses in the evening advances circadian rhythms in the patients with delayed sleep phase disorder: an open-label trial. Chronobiol Int. 2016;33(8):1037–44. https://doi.org/10.1080/07420528.2016.1194289.
Lack LC, Wright HR. Circadian rhythms and insomnia. In: Clinical handbook of insomnia. New York: Springer; 2010. p. 243–53. https://doi.org/10.1007/978-1-60327-042-7_18.
Zerbini G, Kantermann T, Merrow M. Strategies to decrease social jetlag: reducing evening blue light advances sleep and melatonin. Eur J Neurosci. 2018;51(12):2355–66. https://doi.org/10.1111/ejn.14293.
van Geijlswijk IM, Korzilius HP, Smits MG. The use of exogenous melatonin in delayed sleep phase disorder: a meta-analysis. Sleep. 2010;33(12):1605–14. https://doi.org/10.1093/sleep/33.12.1605.
Hughes RJ, Badia P. Sleep-promoting and hypothermic effects of daytime melatonin administration in humans. Sleep. 1997;20(2):124–31. https://doi.org/10.1093/sleep/20.2.124.
Marrin K, Drust B, Gregson W, Atkinson G. A meta-analytic approach to quantify the dose–response relationship between melatonin and core temperature. Eur J Appl Physiol. 2013;113(9):2323–9. https://doi.org/10.1007/s00421-013-2668-x.
Van der Heijden KB, Smits MG, Van Someren EJ, Boudewijn Gunning W. Prediction of melatonin efficacy by pretreatment dim light melatonin onset in children with idiopathic chronic sleep onset insomnia. J Sleep Res. 2005;14(2):187–94. https://doi.org/10.1111/j.1365-2869.2005.00451.x.
Burgess HJ, Revell VL, Eastman CI. A three pulse phase response curve to three milligrams of melatonin in humans. J Physiol Lond. 2008;586:639–47. https://doi.org/10.1113/jphysiol.2007.143180.
Lewy AJ, Emens JS, Bernert RA, Lefler BJ. Eventual entrainment of the human circadian pacemaker by melatonin is independent of the circadian phase of treatment initiation: clinical implications. J Biol Rhythm. 2004;19(1):68–75. https://doi.org/10.1177/0748730403259670.
Lewy AJ, Ahmed S, Jackson JML, Sack RL. Melatonin shifts human orcadian rhythms according to a phase-response curve. Chronobiol Int. 1992;9:380–92. https://doi.org/10.3109/07420529209064550.
Cardinali DP, Furio AM, Reyes MP, Brusco LI. The use of chronobiotics in the resynchronization of the sleep–wake cycle. Cancer Causes Control. 2006;17(4):601–9. https://doi.org/10.1007/s10552-005-9009-2.
Skene DJ. Optimization of light and melatonin to phase-shift human circadian rhythms. J Neuroendocrinol. 2003;15(4):438–41. https://doi.org/10.1046/j.1365-2826.2003.01006.x.
Luboshizsky R, Lavie P. Sleep-inducing effects of exogenous melatonin administration. Sleep Med Rev. 1998;2(3):191–202. https://doi.org/10.1016/s1087-0792(98)90021-1.
Deacon S, Arendt J. Melatonin-induced temperature suppression and its acute phase-shifting effects correlate in a dose-dependent manner in humans. Brain Res. 1995;688:77–85. https://doi.org/10.1016/0006-8993(95)96872-i.
Foley HM, Steel AE. Adverse events associated with oral administration of melatonin: a critical systematic review of clinical evidence. Complement Ther Med. 2019;42:65–81. https://doi.org/10.1016/j.ctim.2018.11.003.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
de Courcey-Bayley, C., McCloy, K. (2022). Adjunctive Therapies for Dental Sleep Appliances. In: Demerjian, G.G., Patel, M., Chiappelli, F., Barkhordarian, A. (eds) Dental Sleep Medicine. Springer, Cham. https://doi.org/10.1007/978-3-031-10646-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-031-10646-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-10645-3
Online ISBN: 978-3-031-10646-0
eBook Packages: MedicineMedicine (R0)