Abstract
To test the impact of nasal dilator strips (NDSs) on insomnia severity, sleep-disordered breathing (SDB) symptoms, sleep quality, and quality of life. Randomized, controlled trial of 4 weeks' duration. Community sample of nonobese, adults with a primary sleep complaint of chronic sleep-maintenance insomnia and mild to moderate SDB symptoms (treatment, n=42; control, n=38). Primary outcomes were four validated scales: Insomnia Severity Index (ISI), Pittsburgh Sleep Quality Index (PSQI), Functional Outcomes of Sleep Questionnaire (FOSQ), and Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ). Secondary outcomes were sleep indices, nonrestorative sleep ratings, and SDB symptoms, assessed retrospectively and prospectively. Both groups received nonspecific education about sleep disorders. Treatment group also received a brief SDB education and nasal strip instructions. At 4 weeks' follow-up, the treatment group demonstrated significant (p=.0001), large improvements in ISI and PSQI (mean Cohen's d=1.18) and significant (p<.02), medium-sized improvements in FOSQ and QLESQ (mean d=0.51) compared to small, nonsignificant changes in control group (Cohen's d range=0.36–0.09). Treatment group change scores among all four primary variables were significantly correlated (mean r=0.50, p=0.01). Secondary prospective and retrospective outcomes showed medium to large improvements in treatment compared to controls for sleep indices (mean d=0.52 vs 0.28), nonrestorative sleep ratings (mean d=0.69 vs 0.11), and sleep breathing symptoms (mean d=0.47 vs 0.09). Significance was obtained for prospective sleep indices (p=0.01), retrospective, and prospective nonrestorative sleep ratings (p=0.003, <0.05), and retrospective sleep breathing symptoms (p=0.03). SDB education and NDSs demonstrated therapeutic efficacy in a select sample of insomnia patients with SDB symptoms. Replication of results requires placebo controls and objectively confirmed SDB cases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
More than 30 years ago, Guilleminault et al. (1973) described three cases of sleep-maintenance insomnia caused by sleep-disordered breathing [1]. More recent studies demonstrated strong associations between these two common sleep disorders [2–4], and several studies showed higher-than-expected prevalence of obstructive sleep apnea (OSA) or upper airway resistance syndrome (UARS) among chronic insomnia patients in select populations [5–10]. One study measured flow limitation events, consistent with upper airway resistance, at three times the rate of apneas and hypopneas [5].
A sleep fragmentation mechanism was proposed through which sleep-disordered breathing (SDB) causes or aggravates insomnia, and UARS might be the predominant or earliest form of SDB among affected insomnia patients [11]. Upper airway resistance is associated with craniofacial abnormalities, including deviated septum, arched or narrow hard palates, and excessive soft palate tissue [12] as well as somatic conditions, including allergic rhinitis and nasal congestion [13, 14]. The nasal resistance observed in these conditions has been associated with insomnia [13, 15, 16].
Rappai et al. (2003) reviewed salient relationships between nasal resistance and SDB, concluding that “technologies and treatments aimed at facilitation of nasal breathing should be explored further in the context of SDB [17].” Treatment of nasal resistance in SDB among insomnia patients has been studied rarely. Guilleminault et al. (2002) conducted the only prospective, randomized controlled trial (RCT) to test a specific nasal resistance treatment (turbinectomy) on insomnia parameters [15]. Of 30 postmenopausal women receiving continuous positive airway pressure (CPAP) or radiofrequency turbinectomy, the latter group achieved more consistent improvements in fatigue, sleep quality, and objective polysomnograph (PSG) sleep parameters; however, overall results were mixed, and no validated insomnia scales were measured [15]. In a case series, three chronic insomnia patients with suspected SDB reported substantial improvements in sleep quality and insomnia with nasal dilator strip (NDS) therapy [18]. In 11 other NDS studies, improvements were exhibited in respiratory, sleep, and arousal indices as well as daytime sleepiness [19–29]. However, only three studies were RCTs [25, 27, 29], and their significant findings seemed dependent on patient types, tending to show modest benefits for those with less severe Apnea/Hypopnea Index (AHI) and/or minimal obesity [25, 27, 29].
The current pilot study was an RCT, testing NDS therapy on a select sample of nonobese, sleep-maintenance insomnia patients, with mild to moderate SDB symptoms. We hypothesized that NDS therapy would produce small to moderate improvements in insomnia, sleep quality, SDB symptoms, and quality of life.
Materials and methods
Patient sample and study design
The study was approved by the institutional review boards of Sandia National Laboratories and the University of New Mexico Health Sciences Center. All participants provided informed consent after a complete description of the study. Financial compensation was not provided. Three waves of recruitment (June 2002 thru December 2003) attracted volunteers from community sources, including working individuals in an occupational health setting, elderly adults through advertisements on a classical music radio station, and the general population through advertisements on a talk-radio station, electronic bulletins, and flyers. To attract patients with sleep-maintenance insomnia and SDB symptoms, advertising included two sets of symptoms: for sleep-maintenance insomnia (middle-of-the-night awakenings and difficulty returning to sleep) and for SDB (breathing symptoms, nocturia, awakening with a dry mouth, and morning headaches).
The study was an RCT of NDSs involving two arms. Both the control and treatment groups received a nonspecific educational review of their current sleep complaints (described below), and the treatment group also received a brief SDB education and NDS instruction.
Screening and eligibility
A screening questionnaire queried demographics, insomnia type, severity, and chronicity, current and past insomnia treatments, medical and psychiatric history, and medications for sleep and mental health. All screened patients responded to five categorical questions about well-described physiological symptoms of SDB: snoring [30], gasping or choking during sleep [30], dry mouth upon awakening [30], nocturia [31], and morning headache [32]. These symptoms, along with information from the subsequent intake measures, were used to confirm sleep-maintenance insomnia and to presumptively distinguish between individuals with severe SDB symptoms (exclusion criteria) and those with mild to moderate SDB symptoms (inclusion criteria).
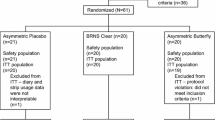
Of 251 screened cases, 229 reported a primary sleep complaint of chronic sleep-maintenance insomnia defined as moderate difficulties sustaining sleep at night or wake time after sleep onset of 30 min or greater as well as related impairment [33]. Of these 229 patients, 223 reported one or more screening SDB symptoms (mean=2.75 symptoms). To reduce our sample to patients with mild to moderate SDB symptoms without complicating factors, 80 patients were excluded due to obesity [body mass index (BMI)>30], signs, symptoms, or previous PSG diagnosis of severe OSA, other comorbid sleep disturbances (e.g., restless legs, leg jerks, chronic nightmares, circadian rhythm disturbances), previously diagnosed severe or unstable breathing disorders [e.g., chronic obstructive pulmonary disease (COPD)], current psychiatric disorder or psychotropic medication usage, including sedatives or other sleep aids, untreated or uncontrolled allergic rhinitis, or other severe or unstable medical conditions (Fig. 1). Patients were not excluded if they had briefly used nasal strips for allergies or colds at a distant point in the past. If patients reported allergic rhinitis, they were included only if their symptoms were essentially eliminated with assorted treatments prior to completing the baseline intake and actual protocol.
Intake and prerandomization sleep log
Of 143 eligible participants, 118 completed a full individual intake, which was then reviewed to reconfirm SDB symptoms, based on American Academy of Sleep Medicine self-report research criteria (1999) [34]. PSG must be performed to confirm an SDB diagnosis, but for this pilot study, objective testing was not conducted due to funding restrictions. After reviewing the intakes and completing physical exams (see below), another ten patients who reported very severe or frequent breathing symptom reports such as regular episodes of witnessed apneas, appeared more obese than in their screening reports, or showed signs of uncontrolled allergies were excluded. One hundred-eight remaining participants completed sleep logs for 7 consecutive days, submitted electronically each morning. The log phase prospectively confirmed sleep-maintenance insomnia, familiarized participants with recording procedures, and clarified participants' capacity to complete the program. Another 17 patients withdrew because they deemed logs as too burdensome. Ninety-one individuals were eligible for randomization.
Randomization and blinding procedures
After completing the 1-week, prerandomization sleep log, an individual interview was conducted in which all participants, prior to randomization, received nonspecific educational reviews of their sleep logs and intake questionnaires. The responses on the logs and the intake questionnaires were reviewed with the patient, who was permitted to raise questions about sleep problems. However, no therapeutic recommendations were offered. For example, two patients said, “It seems I spend a lot of time in bed not sleeping,” and the interviewer replied by rephrasing the statement without interpreting the point or recommending an action, thereby avoiding cognitive-behavioral instructions [35]. Prior to randomization, all patients—whose group status was unknown to the patient and therapist at the time—received this nonspecific, educational review.
Afterwards, a sealed envelope was opened to reveal randomization status to the control group, whose members only received the encounter just described, or to the treatment group, whose members also received individual education about SDB and NDS instructions. Data were coded and entered by a technician who was masked to group status as well as to the nature of the experimental manipulation.
Patient encounters and treatment delivery
All sessions were completed by the first, second or third author, and all were supervised by the principal investigator (PI; first author). Participants randomized to treatment received SDB education, which included a picture of the upper airway to demonstrate how SDB manifests through obstruction or resistance at various sites, provoking an increase in brain activity to correct disrupted breathing, which then stimulates arousals and awakenings, which then induces sleep fragmentation and subsequent insomnia. A single-teaching PSG epoch with an SDB-induced arousal was reviewed with the participant. No cognitive-behavioral therapy directed at psychophysiological conditioning, sleep hygiene, or other elements of insomnia was provided.
After discussing the SDB paradigm, NDS education involved proper sizing, positioning, application, and removal. Breathe Right® Nasal Strips (CNS Inc., Minneapolis, MN) are small adhesive strips containing a flexible tine, which is placed below the bridge of the nose, above or over the nasal flanges of the nostrils. When correctly placed, the tine causes expansion of the nasal valve region of the nose, presumably by stabilizing the lateral vestibular walls of the nose, which in turn increases its minimum cross-sectional area.
Measurements
Three widely used sleep scales, previously validated with PSG, were chosen as primary outcomes and were completed at intake and 4-week follow-up to test the three main sleep hypotheses: Insomnia Severity Index (ISI) [36], which assesses insomnia severity; Pittsburgh Sleep Quality Index (PSQI) [37], which assesses global sleep quality; and Functional Outcomes of Sleep Questionnaire (FOSQ) [38], which assesses sleepiness-related impairment likely due to suspected SDB. Because of the critical importance in demonstrating change in quality of life following insomnia treatment [35], the Quality of Life Enjoyment and Satisfaction Questionnaire [39] summary sheet (QLSESQ) was also used as the fourth primary outcome.
Secondary measures provided additional tests of insomnia severity, sleep quality, and SDB symptoms in three sets of related variables, respectively, composed of:
-
1.
Five standard sleep indices [33], including sleep-onset latency (SOL), total sleep time (TST), calculated sleep efficiency (SE%), estimated awakenings at night (WAKES), and time awake after sleep onset (WASO), measured retrospectively at baseline and follow-up on a sleep medicine history form as well as monitored prospectively in a daily sleep log;
-
2.
Five standard ratings of nonrestorative sleep [33] (including refreshing sleep, sleep quality, depth of sleep, ability to sustain sleep, and ability to return to sleep if awakened) were measured on the sleep history, and the first three items were monitored on the sleep log;
-
3.
Seven standard outcome measures for SDB [30–34] (nocturia, morning headache, dry mouth upon awakening, impaired memory, impaired concentration, sleepiness, and tiredness) were measured on the sleep history, and the first three items were measured on the sleep log.
Retrospective and prospective items were measured with visual analog scales from 0 to 10 (e.g., sleepiness), rating scales from 0=no difficulty to 4=severe difficulty (e.g., impaired concentration), or by actual count (e.g., episodes of nocturia). Typical SDB tools such as the Epworth Sleepiness Scale were not used because, anecdotally, we find that many insomnia patients underestimate their sleepiness when asked to link this condition to behavioral circumstances. The sleep log was the same one used during prerandomization and was completed for 28 days during the randomized controlled period and submitted daily by fax or email.
Physical examination and acoustic rhinometry
All randomized participants underwent a brief physical examination (conducted or supervised by the PI) of the face, nose, and upper airway to assess structural abnormalities suggestive of SDB [40]. Airway crowding was determined using Mallampati classification [41]. At baseline, control and treatment patients underwent acoustic rhinometry (Hood Laboratories, Pembroke, MA; Eccovision Software) in an upright, seated position [42]. Treatment patients also underwent acoustic rhinometry following instructional NDS placement to measure changes in minimum cross-sectional area of the nasal valve region.
Follow-up
Of 91 randomized participants, 11 did not complete the trial, including 4 with intercurrent illness, 5 who withdrew due to time constraints, and 2 who developed adverse effects, which may have been due to nasal strips. Eighty participants, including 42 in treatment and 38 in control groups, completed the 4-week RCT (Fig. 1).
All patients monitored results and adverse effects by completing daily logs for 28 days, and this log supplied the prospective data for the study, with the initial week of data prior to randomization serving as the baseline. Participants informed the clinician of any adverse effect such as skin irritation or increased nasal congestion from using nasal strips. Missing information on sleep logs, although rare, was retrieved by phone. The ISI and QLESQ were completed at the halfway mark in the protocol. All patients completed a follow-up appointment at the end of 4 weeks, during which the four primary outcome scales and other retrospective measurements were reassessed. After the study, all participants were provided the opportunity to continue or start NDS therapy for several weeks, and these crossover and longitudinal data will be the subject of a separate report.
Treatment receipt, adherence, and fidelity
To ensure participants understood the information provided in the SDB treatment session, a 10-question quiz was given, and incorrect answers were discussed with participants. Treatment adherence was measured through participants' completion of questions on the daily log about NDS use, indicating number of nights used and number of hours used each night, the latter equivalent to time in bed. To insure treatment fidelity, the PI completed mock treatments (control and treatment arms) with the second and third authors, and then he completed sessions for the initial four randomized patients with the second and third authors observing. Afterwards, the second author, a registered PSG technologist with 6 years of experience in working with sleep patients, completed 27 sessions, and the third author, a sleep research coordinator with 4 years of experience in working with sleep patients, completed the remaining 49 sessions. The PI supervised the clinicians and, at the conclusion of each session, reviewed lingering patient questions or concerns about NDS therapy (average time <5 min).
Data analysis
To reduce the possibility of Type I errors due to the use of more than 30 dependent variables, repeated-measure multivariate analysis of variance (MANOVA) was conducted on seven related sets of variables. All tests used a Treatment×Group design, predicting that the interaction would demonstrate significantly greater changes in the treatment group compared to the control group. The primary MANOVA tested the four validated scales (ISI, PSQI, FOSQ, and QLESQ). In the treatment group, change scores were correlated for these four primary outcomes.
The remaining secondary MANOVAs tested retrospective and prospective sleep indices, nonrestorative sleep ratings, and SDB physiological symptoms. Prospective data were collected daily and aggregated into five weekly time points. Testing of the repeated measures using the five weekly time points revealed no systematic differences compared to testing baseline and end point prospective values; therefore, the two-point analysis (baseline vs endpoint) was used for both retrospective (Sleep History) and prospective (Sleep Log) items in the MANOVAs. Repeated categorical measurements were analyzed using a Z test for difference in change proportions between groups, and treatment patients' self-assessments of NDS therapy were analyzed with one-sample t tests, which compared participant ratings to the measurement scale midpoint labeled “no change.”
For continuous measures, within-subjects' effect sizes are reported separately for treatment and control groups as Cohen's d, the standardized mean difference, based on mean group change scores divided by the pooled standard deviation. The difference in treatment vs control d reflects the net treatment effect, a measure that controls for spontaneous improvements in the control group and preexisting random group differences at baseline. By convention, Cohen's d effect sizes are described as small (0.20), medium (0.50), or large (>0.80) [43]. For categorical measurements, effect sizes were reported as the difference in proportions between baseline and end point, with the net treatment effect indicated by the difference in the change proportions between groups. An alpha of p=0.05 was used.
Results
Sample characteristics and treatment credibility
Baseline characteristics of the two groups were not significantly different for all primary and secondary variables, sociodemographics, mental health history as well as for chronicity, prior assessments, and treatments of sleep problems. Features of airway crowding were omnipresent, with 100% showing at least one site of nasal or oral airway obstruction. For possible sites of nasal obstruction, 86% had at least one, and 32% exhibited at least two or more (Table 1). Acoustic rhinometry at baseline was equivalent for both groups. For the treatment group, the mean change in minimum cross-sectional area of the nasal valve region, pre- and post-NDS, was 0.52 cm2 (F 1,41=91.29, p<0.0001; d=1.39). Actual NDS use was consistent with a mean of 27.05 days of the 28-day period and a nightly average of 7.53 h, indicative of time in bed and not hours of sleep. Only five patients missed more than 2 days of use (range 3–9 days).
Primary outcome analyses
All four primary variables intercorrelated (mean r=0.41) at baseline among all participants. For the four validated scales, the Treatment×Group interaction was highly significant (F 4,75=5.197, p<0.001), with large sized improvement in insomnia severity (ISI) and sleep quality (PSQI) in the treatment group compared to small- to moderate-sized changes in the control group (Fig. 2a and b). Sleepiness impairment scores (FOSQ) and quality of life (QLESQ) summary scores demonstrated medium-sized improvements in the treatment group but only very small-sized changes in the control group (Fig. 2c and d). Among 42 participants in treatment, change scores were highly correlated for all four primary variables (Table 2).
a–d Four primary outcomes for treated (N=42) and control (N=38) participants at baseline and end point. The dashed line represents the clinical cutoff. The Insomnia Severity Index (ISI) quantifies perceived insomnia severity using seven items on 5-point scales (0–4) with a total range of 0–28. Scores greater than or equal to 11 reflect clinically meaningful insomnia. The Pittsburgh Sleep Quality Index (PSQI) assesses global sleep quality and disturbances on 18 items on 4-point scales (0–3), with a total score range from 0 to 21. Scores greater than or equal to 5 represent poor sleep quality. Functional Outcomes of Sleep Questionnaire (FOSQ) assesses the impact of daytime fatigue and sleepiness on daily functioning on 30 items on 5-point scales, with a global score range of 5–20. Scores less than 17.4 reflect clinically meaningful daytime impairment. The Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q) summary sheet assesses the degree of enjoyment and overall satisfaction levels in various areas of daily functioning using 16 questions scored on a 5-point scale (1–5) to provide an average item score. A Q-LES-Q score of 3 equals “fair,” and a score of 4 equals “good.” An inverse scoring model exists for the FOSQ and the Q-LES-Q; the lower the score, the greater the impairment. All participants completed a 2-week ISI and Q-LES-Q; therefore, 2-week time points are displayed in the graphs for these instruments. Cohen's d represents the actual standardized unit change from baseline to 4-week, that is, the effect size. P values represent the Treatment×Group interaction from a repeated-measures ANOVA
Secondary outcome analyses
Testing insomnia severity on sleep indices (SOL, TST, SE%, WAKES, and WASO) with retrospective data showed no differences (F 5,74=0.936, p=0.46), but prospective data revealed a significant Treatment×Group interaction (F 5,74=3.122, p=0.01). The treatment group showed consistently larger effect sizes for both retrospective and prospective (mean d=0.52) sleep indices compared to small effect sizes in the control group (mean d=0.27) (Table 3).
Testing for sleep quality with retrospective nonrestorative sleep ratings (refreshing sleep, sleep quality, depth of sleep, ability to sustain sleep, and ability to return to sleep) was significant (F 5,74=3.999, p=0.003), as were prospective ratings (refreshing sleep, sleep quality, and depth of sleep) (F 3,76=3.012, p<0.05). The treatment group showed consistently larger changes retrospectively and prospectively (mean d=0.69) compared to very small improvements in the control group (mean d=0.11) (Table 4).
Testing SDB symptom reports on retrospective data (dry mouth upon awakening, morning headache, nocturia, impaired memory, impaired concentration, daytime sleepiness, and daytime tiredness) were significant (F 7,72=2.366, p=0.03), but prospective data (dry mouth upon awakening, morning headache, and nocturia) were not significant (F 3,76=1.695, p=0.18). The treatment group demonstrated consistently larger improvements retrospectively and prospectively (mean d=0.47) compared to negligible changes in the control group (mean d=0.09) (Table 5).
Moderating variables
Moderating variables, including baseline characteristics and Table 1 items, were tested for interactions with treatment- and group-independent variables, but no systematic differences were observed. For example, when comparing 45 participants who reported infrequent to occasional classic breathing symptoms (loud snoring, gasping or choking for breath, and rare witnessed apnea) to 35 participants who did not report breathing symptoms, no systematic differences were observed. No effects were observed for the two groups based on past history of allergic rhinitis. In addition, surprisingly, no effects were dependent upon potential sites of nasal or oral airway obstruction. Last, no significant differences in outcomes were demonstrated, based on the three clinicians conducting sessions or on the three sources of recruitment.
Self-assessment and side effects
On average, 42 treatment patients indicated they perceived a moderate, systematic effect of nasal strips in improving breathing and sleep quality as well as decreasing insomnia symptoms (Fig. 3), and no other intercurrent factors were reported by the patients as affecting their results. Two patients withdrew because of perceived side effects from the nasal strips, described as itchiness around the nose and increased nasal congestion.
Treated participants’ (N=42) self-assessment of nasal dilator strip therapy. At 4 weeks follow-up, NDS-treated participants assessed the overall impact of NDS therapy on their ease of breathing, insomnia, sleep quality, and SDB symptoms. Ease of breathing refers to the sensation of increased airflow through the nasal passages while awake. Using one-sample t tests, all improvements were statistically significant. Error bars represent the 95% confidence interval of the difference
Discussion
In a pilot investigation of NDS therapy, this over-the-counter, nasal breathing aide proved unexpectedly efficacious in the treatment of a select sample of insomnia patients with SDB symptoms. Consistent improvements were demonstrated on validated scales for insomnia severity, sleep quality, sleepiness-related impairment, and quality of life, albeit retrospectively assessed and prospectively monitored sleep variables were less consistent in attaining reliable differences between groups. Although effect sizes were consistently larger for primary and secondary variables in the NDS group compared to controls, these results require cautious interpretation and replication with PSG testing of participants.
For this select sample of patients with insomnia and SDB symptoms, the findings support the theory that SDB-induced awakenings and sleep fragmentation contribute to sleep-maintenance insomnia [1, 7, 11, 44]. This pathophysiological mechanism might occur in the following way: If nasal strips decreased nasal resistance, which improved sleep breathing, then sleep fragmentation and related arousals would decrease, resulting in greater consolidation of sleep and improved sleep quality. This consolidation would decrease insomnia at night and decrease fatigue and sleepiness during the daytime, which, in turn, would be expected to improve quality of life. Consistent with this mechanism, change scores in the treatment group for the four primary outcomes (insomnia severity, sleep quality, sleepiness impairment, and quality of life) were moderately to strongly correlated. However, because of the complex nature of these hypothesized relationships, this proposed mechanism remains speculative and must be tested with PSG and evidence-based SDB treatments.
Limitations include the lack of a placebo device compared to NDS, the delivery of an unblinded therapy, and the absence of PSG corroboration of SDB diagnoses and NDS effects. In our view, nasal strip placebos are inadequate. Innovative protocols might include a three-way comparison among NDS, sedatives, and a placebo pill, or nasal strips could be tested in two groups of insomniacs, one with and one without SDB. A blinded SDB therapy requires the use of other devices such as CPAP-placebo [45], which should be attempted to measure CPAP efficacy in the treatment of insomnia in those with comorbid SDB. However, because the relationship between SDB and insomnia is not intuitive, protocols must incorporate SDB education to attain reasonable perceptions of treatment credibility, which, in turn, could create bias and demand characteristics as might have occurred in our protocol. Without PSG corroboration of SDB diagnoses and NDS effects on breathing, the interpretation of these unexpected findings must be properly tempered. Moreover, the absence of testing in this protocol should not be construed as an indication that PSG is not required for diagnosing SDB. In fact, all patients in the study were strongly encouraged to follow-up at local sleep centers to undergo PSG and seek evidence-based therapies for SDB. To date, among 17 patients tested, all SDB diagnoses were confirmed. Last, the results do not establish NDS as a proven SDB therapy.
Caution is also advised because other factors may have influenced outcomes. For example, our treatment patients did not monitor turning from the supine to the lateral sleeping position, which is known to affect SDB events [27]. In addition, our sample was predominantly non-Hispanic white, whereas the nasal contour of individuals of another ethnicity may limit NDS therapy [46, 47]. Overall, our sample represents a select group of patients; therefore, this study lacks generalizability to a substantial number of insomnia patients who may or may not suffer from SDB.
Conclusion
The current work highlights pertinent clinical relationships between insomnia and SDB. First, certain insomnia patients may be suffering from resistance in the upper airway. Second, treatment of this sleep-related respiratory disorder in relevant insomnia cases likely requires a much wider range of therapeutic options such as CPAP [48], oral appliances [49, 50], and site-specific surgery [40]. Finally, treatment of nasal resistance may prove highly informative in the investigation of sleep breathing among chronic insomnia patients.
References
Guilleminault C, Eldridge FL, Dement WC (1973) Insomnia with sleep apnea: a new syndrome. Science 181(102):856–858
Krakow B, Melendrez D, Ferreira E et al (2001) Prevalence of insomnia symptoms in patients with sleep-disordered breathing. Chest 120(6):1923–1929
Smith S, Sullivan K, Hopkins W et al (2004) Frequency of insomnia report in patients with obstructive sleep apnoea hypopnea syndrome (OSAHS). Sleep Med 5(5):449–456
Krakow B (2004) An emerging interdisciplinary sleep medicine perspective on the high prevalence of co-morbid sleep-disordered breathing and insomnia. Sleep Med 5(5):431–433
Krakow B, Melendrez D, Pedersen B et al (2001) Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry 49(11):948–953
Krakow B, Melendrez D, Johnston L et al (2002) Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis 190(7):442–452
Guilleminault C, Palombini L, Poyares D et al (2002) Chronic insomnia, postmenopausal women, and sleep disordered breathing: part 1. Frequency of sleep disordered breathing in a cohort. J Psychosom Res 53(1):611–615
Gold AR, Dipalo F, Gold MS et al (2003) The symptoms and signs of upper airway resistance syndrome: a link to the functional somatic syndromes. Chest 123(1):87–95
Krakow B, Haynes PL, Warner TD et al (2004) Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress 17:257–268
Gold AR, Dipalo F, Gold MS et al (2004) Inspiratory airflow dynamics during sleep in women with fibromyalgia. Sleep 27:459–466
Chung KF, Krakow B, Melendrez DC et al (2003) Relationships between insomnia and sleep-disordered breathing. Chest 123:310–313
Guilleminault C, Stoohs R, Kim YD et al (1995) Upper airway sleep-disordered breathing in women. Ann Intern Med 122(7):493–501
Lavie P, Gertner R, Zomer J et al (1981) Breathing disorders in sleep associated with “microarousals” in patients with allergic rhinitis. Acta Otolaryngol 92(5–6):529–533
Staevska MT, Mandajieva MA, Dimitrov VD (2004) Rhinitis and sleep apnea. Curr Allergy Asthma Rep 4(3):193–199
Guilleminault C, Palombini L, Poyares D et al (2002) Chronic insomnia, premenopausal women and sleep disordered breathing: part 2. Comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of chronic insomnia. J Psychosom Res 53(1):617–623
Ferguson BJ (2004) Influences of allergic rhinitis on sleep. Otolaryngol Head Neck Surg 130:617–629
Rappai M, Collop N, Kemp S et al (2003) The nose and sleep-disordered breathing: what we know and what we do not know. Chest 124(6):2309–2323
Krakow B, Melendrez D, Sisley B et al (2004) Nasal dilator strip therapy for chronic sleep maintenance insomnia: a case series. Sleep Breath 8(3):133–140
Scharf MB, Brannen DE, McDannold M (1994) A subjective evaluation of a nasal dilator on sleep and snoring. Ear Nose Throat J 73(6):395–401
Turnbull GL, Rundell OH, Rayburn WF et al (1996) Managing pregnancy-related nocturnal nasal congestion. The external nasal dilator. J Reprod Med 41(12):897–902
Scharf MB, Berkowitz DV, McDannold MD et al (1996) Effects of an external nasal dilator on sleep and breathing patterns in newborn infants with and without congestion. J Pediatr 129(6):804–808
Ulfberg J, Fenton G (1997) Effect of breathe right nasal strip on snoring. Rhinology 35(2):50–52
Liistro G, Rombaux P, Dury M et al (1998) Effects of breathe right on snoring: a polysomnographic study. Respir Med 92(8):1076–1078
Todorova A, Schellenberg R, Hofmann HC et al (1998) Effect of the external nasal dilator breathe right on snoring. Eur J Med Res 3(8):367–379
Redline S, Adams N, Strauss ME et al (1998) Improvement of mild sleep-disordered breathing with CPAP compared with conservative therapy. Am J Respir Crit Care Med 157(3 Pt 1):858–865
Di Somma EM, West SN, Wheatley JR et al (1999) Nasal dilator strips increase maximum inspiratory flow via nasal wall stabilization. Laryngoscope 109:780–784
Bahammam AS, Tate R, Manfreda J et al (1999) Upper airway resistance syndrome: effect of nasal dilation, sleep stage, and sleep position. Sleep 22(5):592–598
Gosepath J, Amedee RG, Romantschuck S et al (1999) Breathe right nasal strips and the respiratory disturbance index in sleep related breathing disorders. Am J Rhinol 13(5):385–389
Djupesland PG, Skatvedt O, Borgersen AK (2001) Dichotomous physiological effects of nocturnal external nasal dilation in heavy snorers: the answer to a rhinologic controversy? Am J Rhinol 15(2):95–103
American Academy of Sleep Medicine (1997) The international classification of sleep disorders. Allen, Lawrence, pp 52–58
Umlauf MG, Chasens ER (2003) Sleep disordered breathing and nocturnal polyuria: nocturia and enuresis. Sleep Med Rev 7(5):403–411
Loh NK, Dinner DS, Foldvary N et al (1999) Do patients with obstructive sleep apnea wake up with headaches? Arch Intern Med 159(15):1765–1768
Sateia MJ, Doghramji K, Hauri PJ et al (2000) Evaluation of chronic insomnia. An American Academy of Sleep Medicine review. Sleep 23(2):243–308
American Academy of Sleep Medicine (1999) Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The report of an American Academy of Sleep Medicine Task Force. Sleep 22(5):667–689
Harvey AG, Tang NK (2003) Cognitive behaviour therapy for primary insomnia: can we rest yet? Sleep Med Rev 7(3):237–262
Bastien CH, Vallieres A, Morin CM (2001) Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med 2(4):297–307
Buysse DJ, Reynolds CF III, Monk TH et al (1989) The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28(2):193–213
Weaver TE, Laizner AM, Evans LK et al (1997) An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep 20(10):835–843
Endicott J, Nee J, Harrison W et al (1993) Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull 29(2):321–326
Goldberg AN, Schwab RJ (1998) Identifying the patient with sleep apnea: upper airway assessment and physical examination. Otolaryngol Clin North Am 31(6):919–930
Liistro G, Rombaux P, Belge C et al (2003) High Mallampati score and nasal obstruction are associated risk factors for obstructive sleep apnoea. Eur Respir J 21(2):248–252
Viviano JS (2002) Acoustic reflection: review and clinical applications for sleep-disordered breathing. Sleep Breath 6(3):129–149
Cohen J (2005) Statistical power analysis for the behavioral sciences. Erlbaum, Hillsdale
Krakow B, Melendrez D, Lee SA et al (2004) Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath 8(1):15–29
Henke KG, Grady JJ, Kuna ST (2001) Effect of nasal continuous positive airway pressure on neuropsychological function in sleep apnea–hypopnea syndrome. A randomized, placebo-controlled trial. Am J Respir Crit Care Med 163(4):911–917
Portugal LG, Mehta RH, Smith BE et al (1997) Objective assessment of the breathe-right device during exercise in adult males. Am J Rhinol 11(5):393–397
Ho WK, Wei WI, Yuen AP et al (2000) Effect of the external nasal dilator on nasal minimal cross-sectional area in orientals as assessed by acoustic rhinometry. J Otolaryngol 29(6):367–370
Sullivan CE, Issa FG, Berthon-Jones M et al (1981) Reversal of obstructive sleep apnoea by continuous positive airway pressure applied through the nares. Lancet 1(8225):862–865
Schmidt-Nowara W (1999) Recent developments in oral appliance therapy of sleep disordered breathing. Sleep Breath 3(3):103–106
Gotsopoulos H, Chen C, Qian J et al (2002) Oral appliance therapy improves symptoms in obstructive sleep apnea: a randomized, controlled trial. Am J Respir Crit Care Med 166(5):743–748
Acknowledgement
Support for this study was provided by an unrestricted grant from CNS., Inc. (Minneapolis, MN), the manufacturer of Breathe Right® nasal strips.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Krakow, B., Melendrez, D., Sisley, B. et al. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: a randomized controlled trial. Sleep Breath 10, 16–28 (2006). https://doi.org/10.1007/s11325-005-0037-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-005-0037-7