Abstract
Dynamic magnetic resonance imaging (MRI) allows real-time characterization of upper airway collapse in sleeping subjects with obstructive sleep apnea (OSA). The aim of our study was to use sleep MRI to compare differences in upper airway collapse sites between BMI-matched subjects with mild OSA and severe OSA. This is a prospective, nested case–control study using dynamic sleep MRI to compare 15 severe OSA subjects (AHI >40) and 15 mild OSA (AHI <10) subjects, who were matched for BMI. Upper airway imaging was performed on sleeping subjects in a 3.0 T MRI scanner. Sleep MRI movies were used by blinded reviewers to identify retropalatal (RP), retroglossal (RG), and lateral pharyngeal wall (LPW) airway collapse. Mean AHI in the severe OSA group was 70.3 ± 23 events/h, and in the mild group was 7.8 ± 1 events/h (p < 0.001). All mild and severe OSA subjects demonstrated retropalatal airway collapse. Eighty percent in the mild group showed single-level RP collapse (p < 0.001). All subjects in the severe group showed multi-level collapse: RP + LPW (n = 9), RP + RG + LPW (n = 6). All severe OSA subjects showed LPW collapse, as compared with three subjects in the mild group (p < 0.001). LPW collapse was positively associated with AHI in simple regression analysis (β = 51.8, p < 0.001). In conclusion, severe OSA patients present with more lateral pharyngeal wall collapse as compared to BMI-matched mild OSA patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Obstructive sleep apnea (OSA) is characterized by recurrent upper airway (UA) obstruction associated with oxygen desaturation during sleep. Polysomnography (PSG) is the gold standard diagnostic tool for OSA, and provides a comprehensive array of physiologic data. It does not, however, provide anatomic information about the region of UA collapse that leads to apnea and desaturation. As more medical and surgical treatments become available for targeted therapy, precise phenotyping the OSA patient for individualized therapy is pertinent and timely.
From static imaging to awake and asleep endoscopic examinations, clinicians have attempted numerous ways to characterize the dynamic airway of a sleeping patient. Much work has been done via static and awake imaging, and surrogate measures that correlate with severity of OSA have been published [1–5]. There is need for examination of upper airway collapse during sleep.
Dynamic magnetic resonance imaging (MRI) has been used to investigate the sites of airway obstruction in OSA patients who are either awake, under sedation, or during natural sleep. To date, sleep MRI studies have focused on observations using sagittal views of the UA, which only allows for the identification of retropalatal and retroglossal airway collapse in an anterior to posterior direction [6–9].
The aim of our study was to use sleep MRI to compare differences in upper airway collapse sites between BMI-matched subjects with mild and severe OSA.
Materials and methods
Study design
This was a nested case–control study, where subjects were selected from 64 consecutive participants who successfully completed in the dynamic sleep MRI study from July 2012 to June 2014. Inclusion criteria for the study subjects included complaint of excessive daytime somnolence, presence of snoring, and OSA diagnosed by attended PSG. Subjects with nasal obstruction or who had nasal or oropharyngeal surgery for OSA were excluded. Subjects with image sequences obscured by swallowing or mandibular movements were also excluded. Of the 64 subjects, 15 subjects with severe OSA (AHI >40) and 15 BMI-matched subjects with mild OSA (AHI <10) were selected. The study was approved by the institutional review boards of Cathay General Hospital and the National Taiwan University Hospital, with informed written consent obtained from all enrolled subjects.
Data collection
Polysomnography
All subjects underwent standard overnight in-lab PSG with at least 6 h of sleep data recording. Alcohol or other sedatives were not allowed the day before the study. Electro-physiological parameters, including central electro-encephalogram (EEG), electro-oculogram, electro-myogram, electrocardiogram, nasal/oral airflow, thoracic and abdominal efforts, oxygen (O2) saturation, and snoring sound, were recorded. AHI was defined as the sum of total apnea and hypopnea episodes per hour of sleep. Apnea was defined by a decreasing of airflow by 90 % lasting longer than 10 s, and hypopnea defined as oxygen desaturation of 3 % or more with EEG confirmed arousal.
Magnetic resonance imaging
Subjects were scanned after 11 pm with continuous monitoring by a radiologist and an otolaryngologist. A 3 T MRI (Verio 3 T Syngo MR B17, Siemens Healthcare, Munich, Germany) was used with a head and neck surface coil. Subjects were in supine position and orbito-auricular plane at 90° angle to the horizontal plane. Airtight earplugs were inserted into the external auditory canal to minimize noise disturbance. Sleep MRI images were obtained with rapid MRI 2D multi-slice fast low-angle shot (FLASH) sequence, first performed with the subjects awake. Once sleep was determined with our study definitions, patients were monitored for 30 min. The technical parameters were: echo time 1.73 ms, repetition time 2.74 ms; flip angle 6°; matrix 1.0 × 1.0.8.0 mm; and section thickness 8 mm. The imaging time per slice for this sequence was 0.5 s. 100 consecutive images were obtained as a single section, with total imaging time of 50 s, where each image represents 0.5 s.
Determination of sleep
Pulse rate with oxygen saturation and snoring sound were continuously recorded during scan. State of sleep was determined by simultaneous recordings of irregular snoring heard through the optical-fiber microphone.
Snoring recording and synchronizing
Snoring was recorded using an MRI-compatible microphone (FOMRI-III, dual channel optical-fiber microphone, Optoacoustics, Yehuda, Israel) and a universal serial bus sound card sampling at 44.1 kHz with 24-bit resolution (Sound Blaster X-Fi, Creative, Singapore City, Singapore). The microphone was installed inside the MRI head coil for snore sound recording. To ensure the quality of sound recording, the distance between the mouth and microphone was consistent at 5–20 mm in supine position, depending on the subject’s head size. The details of MRI and snoring recording/synchronizing procedures were validated in our previous studies [10, 11].
To eliminate MRI noise from the recording, we identified the segments (slices) of each FLASH sequence in the recording and applied principle component analysis method to the matrix of segments. The snoring sound recorded during MRI imaging was reconstructed by subtracting the templates from different FLASH sequences of the original data. The audio data and the slices of each FLASH sequence were aligned segment by segment to synchronize MRI imaging and snoring sound (Video 1).
Data analysis
Starting with the mid-sagittal slice, UA was divided into retropalatal and retroglossal spaces. Retropalatal (RP) space was defined between levels of the hard palate to inferior border of the uvula. The retroglossal space (RG) was defined from the inferior border of the uvula to the base of the epiglottis (Fig. 1). RP and RG collapses were determined by dynamic sagittal and axial sequences. Rating of lateral pharyngeal wall collapse was based on coronal slices from sleep MRI.
Rating of airway collapse was binary, where collapse (airway obstruction) was defined as a narrowing greater than 75 % of the airway. Rating was performed by two blinded investigators (SYCL and LKH).
Statistical analysis
Intra-rater consistency and inter-rater reliability were checked with Cronbach’s and Kappa correlations, respectively. Simple regression analyses were used to measure the association between sites of UA collapse and severity of OSA. Fisher exact and Mann–Whitney U tests were used for group comparisons. To determine the sample size, we set confidence level at 80 % with significance level p < 0.01.
Results
Demographics
All subjects were male, and of Han Chinese ethnicity. There were no statistically significant differences in age, neck circumference, and BMI between the 15 subjects with severe OSA and 15 subjects with mild OSA (Table 1). AHI was 70.3 ± 22.9 events per hour in the severe group and 7.8 ± 1.4 events per hour in the matched, mild OSA group (p < 0.001).
Dynamic UA collapse
Distribution of sites of UA collapse for both groups is provided in Table 2. All subjects with severe OSA had multi-level collapse (RP + LPW, or RP + RG + LPW). In comparison, 80 % of the mild OSA subjects showed single level collapse at RP only. Only 20 % of the mild OSA subjects showed multi-level collapse with 13.3 % (RP + RG) and 6.7 % (RP + RG + LPW).
When compared by sites of collapse, all subjects (mild and severe OSA) showed RP collapse. All subjects with severe OSA had LPW collapse, as compared with 6.7 % in the mild OSA group (p < 0.0001) (Table 3). Internal consistency for intra-rater Cronbach’s alpha coefficient ranged from 0.95 to 1.00, and inter-rater reliability Kappa coefficient was 0.90–1.00.
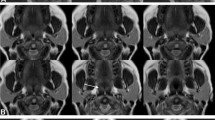
We demonstrate the coronal view of a dynamic sleep MRI movie of a 46-year-old subject with AHI of 71 events/h (Video 2) is compared with an age and BMI-matched subject with AHI of 7 events/h (Video 3). This is the view used to rate lateral pharyngeal wall collapse, which is 1 in the severe OSA subject, and 0 in the mild OSA subject. Figure 2 shows axial views of lateral pharyngeal wall collapse sequences, and Fig. 3 showed dynamic coronal view of lateral pharyngeal wall collapse sequences.
Dynamic UA collapse and disease severity
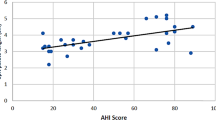
On simple regression analysis, lateral pharyngeal wall collapse showed positive correlation with AHI (β = 51.8, p < 0.001) (Table 4).
Discussion
Methods for predicting sites of UA obstruction during sleep in OSA patients include cephalometry, computed tomography (CT), MRI, nasopharyngoscopy, fluoroscopy, and drug-induced sedation endoscopy (DISE). They are all limited by providing information about the airway during static, awake, or pharmacologically induced sleep states [1, 2, 12–15]. Dynamic sleep MRI provides excellent temporal resolution to changes of the upper airway during sleep. Previous sleep MRI studies have demonstrated retropalatal and retroglossal airway collapse in subjects with OSA [6]. In this study, we found that retropalatal airway collapse is common in all OSA subjects, which is compatible with previous observations [6–9, 15]. We did not, however, find any isolated retroglossal airway collapse in subjects with severe OSA as described in previous imaging studies [7, 9]. All of our severe OSA subjects showed retroglossal or retropalatal airway collapse in combination with lateral pharyngeal wall collapse. The most important finding from our dynamic sleep MRI study is patients with severe OSA had a more lateral pharyngeal wall collapse compared with BMI-matched mild OSA patients.
Pathogenesis of OSA remains complex. Two prevailing mechanisms for upper airway collapse during sleep include: (1) anatomic theory: fat deposits around the pharynx and thickening of soft tissue of pharyngeal wall; and (2) neural hypothesis: decreased neural output to pharyngeal dilator muscles during sleep, leading to muscle hypotonia [16].
For the first theory, obesity is a predisposing factor in the development and progression of OSA [17, 18]. Fat pads in the pharyngeal wall are increased in obese patients with OSA, contributing to narrowing of the pharyngeal lumen [19–21]. Larger neck circumference has been associated with greater severity of OSA [22]. However, this does not apply to OSA patients who are of normal BMI [2, 23]. Also, studies by Akan et al. [24] and Schwab et al. [25] did not ascribe importance to the parapharyngeal fat pads in airway obstruction during sleep.
Our findings in the setting of BMI-matched non-obese subjects illustrate the neural hypothesis as it relates to multi-level collapse. Our results highlight the importance of increased lateral pharyngeal wall collapsibility as a major determinant of airway obstruction in severity of OSA. Increased collapsibility of the lateral pharyngeal wall may reflect inadequate neuromechanical response to airway obstruction in severe OSA subjects, while in the matched mild OSA subjects, coordinated airway dilation mechanism during sleep is preserved.
The association of lateral pharyngeal wall collapse with severity of OSA has been reported by observation during nasopharyngoscopy and DISE. There is a strong association between lateral pharyngeal wall collapse and severity of oxygen desaturation during DISE [26]. Soares et al. also reported the presence of severe lateral pharyngeal wall on preoperative DISE is associated with sleep surgical failure [27]. The most effective surgical treatment for OSA besides a tracheostomy is maxillomandibular advancement (MMA), and both Li et al. and Liu et al. reported that reduction of lateral pharyngeal wall collapse is associated with MMA surgical success [28, 29].
Our findings need to be interpreted with the following limitations. Our comparison groups are subjects with mild OSA and severe OSA. There is not a true control group who do not have OSA. While the strength in our study is the BMI-matching of both groups, all subjects are of Han Chinese ethnicity, which may limit the generalizability of our results. There is also a selection bias, since we are only able to assess those patients who fell asleep in the scanner. Sleeping in MRI in this study is not representing a true natural sleep due to the short scanning time (30 min after subjects fall asleep). Without EEG in our MRI scanners, we do not have information about the patients’ sleep staging. It has been suggested that patients who could fall asleep in the scanner remain mostly in stages I and II [7]. Finally, an increase in number of subjects would allow multivariate analyses examining other anatomic or physiologic contributors to lateral pharyngeal wall collapse.
Conclusion
In conclusion, patients with severe OSA present with more multi-level and lateral pharyngeal wall collapse as compared to BMI-matched mild OSA patients under sleep MRI. When examining patients with suspected sleep-disordered breathing, identification of lateral pharyngeal wall collapse with nasopharyngoscopy or drug-induced sleep endoscopy may correlate with OSA severity.
References
Abramson Z, Susarla S, August M, Troulis M, Kaban L (2010) Three-dimensional computed tomographic analysis of airway anatomy in patients with obstructive sleep apnea. J Oral Maxillofac Surg 68(2):354–362. doi:10.1016/j.joms.2009.09.087
Battagel JM, Johal A, Kotecha B (2000) A cephalometric comparison of subjects with snoring and obstructive sleep apnoea. Eur J Orthod 22(4):353–365
Susarla SM, Abramson ZR, Dodson TB, Kaban LB (2010) Cephalometric measurement of upper airway length correlates with the presence and severity of obstructive sleep apnea. J Oral Maxillofac Surg 68(11):2846–2855. doi:10.1016/j.joms.2010.06.196
Terris DJ, Hanasono MM, Liu YC (2000) Reliability of the Muller maneuver and its association with sleep-disordered breathing. Laryngoscope 110(11):1819–1823. doi:10.1097/00005537-200011000-00010
Guilleminault C, Riley R, Powell N (1984) Obstructive sleep apnea and cephalometric roentgenograms. Am Rev Respir Dis 130(1):145–146
Barrera JE (2011) Sleep magnetic resonance imaging: dynamic characteristics of the airway during sleep in obstructive sleep apnea syndrome. Laryngoscope 121(6):1327–1335. doi:10.1002/lary.21810
Kavcic P, Koren A, Koritnik B, Fajdiga I, Dolenc Groselj L (2014) Sleep magnetic resonance imaging with electroencephalogram in obstructive sleep apnea syndrome. Laryngoscope. doi:10.1002/lary.25085
Ikeda K, Ogura M, Oshima T, Suzuki H, Higano S, Takahashi S, Kurosawa H, Hida W, Matsuoka H, Takasaka T (2001) Quantitative assessment of the pharyngeal airway by dynamic magnetic resonance imaging in obstructive sleep apnea syndrome. Ann Otol Rhinol Laryngol 110(2):183–189
Moriwaki H, Inoue Y, Namba K, Suto Y, Chiba S, Moriyama H (2009) Clinical significance of upper airway obstruction pattern during apneic episodes on ultrafast dynamic magnetic resonance imaging. Auris Nasus Larynx 36(2):187–191. doi:10.1016/j.anl.2008.04.004
Chang YC, Huon LK, Pham VT, Chen YJ, Jiang SF, Shih TT, Tran TT, Wang YH, Lin C, Tsao J, Lo MT, Wang PC (2014) Synchronized imaging and acoustic analysis of the upper airway in patients with sleep-disordered breathing. Physiol Meas 35(12):2501–2512. doi:10.1088/0967-3334/35/12/2501
Chen YJ, Shih TT, Chang YC, Hsu YC, Huon LK, Lo MT, Pham VT, Lin C, Wang PC (2015) Acoustic-integrated dynamic MR imaging for a patient with obstructive sleep apnea. Magn Reson Imaging. doi:10.1016/j.mri.2015.08.004
Shepard JW Jr, Gefter WB, Guilleminault C, Hoffman EA, Hoffstein V, Hudgel DW, Suratt PM, White DP (1991) Evaluation of the upper airway in patients with obstructive sleep apnea. Sleep 14(4):361–371
Borowiecki B, Pollak CP, Weitzman ED, Rakoff S, Imperato J (1978) Fibro-optic study of pharyngeal airway during sleep in patients with hypersomnia obstructive sleep-apnea syndrome. Laryngoscope 88(8 Pt 1):1310–1313. doi:10.1288/00005537-197808000-00012
Guilleminault C, Hill MH, Simmons FB, Powell N, Riley R, Stoohs R (1997) Passive constriction of the upper airway during central apneas: fiberoptic and EMG investigations. Respir Physiol 108(1):11–22
Chuang LP, Chen NH, Li HY, Lin SW, Chou YT, Wang CJ, Liao YF, Tsai YH (2009) Dynamic upper airway changes during sleep in patients with obstructive sleep apnea syndrome. Acta Otolaryngol 129(12):1474–1479. doi:10.3109/00016480902780242
Sforza E, Bacon W, Weiss T, Thibault A, Petiau C, Krieger J (2000) Upper airway collapsibility and cephalometric variables in patients with obstructive sleep apnea. Am J Respir Crit Care Med 161(2 Pt 1):347–352. doi:10.1164/ajrccm.161.2.9810091
Vgontzas AN, Papanicolaou DA, Bixler EO, Hopper K, Lotsikas A, Lin HM, Kales A, Chrousos GP (2000) Sleep apnea and daytime sleepiness and fatigue: relation to visceral obesity, insulin resistance, and hypercytokinemia. J Clin Endocrinol Metab 85(3):1151–1158. doi:10.1210/jcem.85.3.6484
Young T, Shahar E, Nieto FJ, Redline S, Newman AB, Gottlieb DJ, Walsleben JA, Finn L, Enright P, Samet JM, Sleep Heart Health Study Research Group (2002) Predictors of sleep-disordered breathing in community-dwelling adults: the Sleep Heart Health Study. Arch Intern Med 162(8):893–900
Shelton KE, Woodson H, Gay S, Suratt PM (1993) Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis 148(2):462–466. doi:10.1164/ajrccm/148.2.462
Pahkala R, Seppa J, Ikonen A, Smirnov G, Tuomilehto H (2014) The impact of pharyngeal fat tissue on the pathogenesis of obstructive sleep apnea. Sleep Breath 18(2):275–282. doi:10.1007/s11325-013-0878-4
Horner RL, Mohiaddin RH, Lowell DG, Shea SA, Burman ED, Longmore DB, Guz A (1989) Sites and sizes of fat deposits around the pharynx in obese patients with obstructive sleep apnoea and weight matched controls. Eur Respir J 2(7):613–622
Hoffstein V, Mateika S (1992) Differences in abdominal and neck circumferences in patients with and without obstructive sleep apnoea. Eur Respir J 5(4):377–381
Johns FR, Strollo PJ Jr, Buckley M, Constantino J (1998) The influence of craniofacial structure on obstructive sleep apnea in young adults. J Oral Maxillofac Surg 56(5):596–602 (discussion 602–603)
Akan H, Aksoz T, Belet U, Sesen T (2004) Dynamic upper airway soft-tissue and caliber changes in healthy subjects and snoring patients. AJNR Am J Neuroradiol 25(10):1846–1850
Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI (1995) Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med 152(5 Pt 1):1673–1689. doi:10.1164/ajrccm.152.5.7582313
Lan MC, Liu SY, Lan MY, Modi R, Capasso R (2015) Lateral pharyngeal wall collapse associated with hypoxemia in obstructive sleep apnea. Laryngoscope. doi:10.1002/lary.25126
Soares D, Sinawe H, Folbe AJ, Yoo G, Badr S, Rowley JA, Lin HS (2012) Lateral oropharyngeal wall and supraglottic airway collapse associated with failure in sleep apnea surgery. Laryngoscope 122(2):473–479. doi:10.1002/lary.22474
Liu SY, Huon LK, Powell NB, Riley R, Cho HG, Torre C, Capasso R (2015) Lateral pharyngeal wall tension after maxillomandibular advancement for obstructive sleep apnea is a marker for surgical success: observations from drug-induced sleep endoscopy. J Oral Maxillofac Surg. doi:10.1016/j.joms.2015.01.028
Li KK, Guilleminault C, Riley RW, Powell NB (2002) Obstructive sleep apnea and maxillomandibular advancement: an assessment of airway changes using radiographic and nasopharyngoscopic examinations. J Oral Maxillofac Surg 60(5):526–530 (discussion 531)
Acknowledgments
This study was sponsored by the National Taiwan University Hospital, Cathay General Hospital Joint Research Fund (No. 102-CGN08) and the National Central University, Cathay General Hospital Joint Research Fund (Nos. 101CGH-NCUA4, 103CGH-NCU-A1 and 104CGH-NCU-A1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MP4 13166 kb)
Supplementary material 2 (MP4 9635 kb)
Supplementary material 3 (MP4 10679 kb)
Rights and permissions
About this article
Cite this article
Huon, LK., Liu, S.YC., Shih, T.TF. et al. Dynamic upper airway collapse observed from sleep MRI: BMI-matched severe and mild OSA patients. Eur Arch Otorhinolaryngol 273, 4021–4026 (2016). https://doi.org/10.1007/s00405-016-4131-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-4131-1