Abstract
Background
In 1984, Cartwright suggested that physicians should differentiate between patients with either positional obstructive sleep apnoea (POSA) or non-positional OSA. Treatment of POSA has advanced dramatically recently with the introduction of a new generation of positional therapy (PT), a small device attached to either the neck or chest which corrects the patient from adopting the supine position through a vibrating stimulus. Encouraging data have been published suggesting that this simple therapy successfully prevents patients with POSA from adopting the supine position without negatively influencing sleep efficiency, as well as allowing for good adherence. Unfortunately, evaluating the efficacy of PT and comparing results are hindered by the fact that there are no universally used POSA criteria. In 1984, Cartwright introduced the arbitrary cut-off point of a difference of 50 % or more in apnoea index between supine and non-supine positions.
Introduction
The aim of this project was to introduce a new classification system, which ideally should identify suitable candidates for PT: patients that will benefit from a clinically significant improvement of their OSA with PT. The shared use of this classification can facilitate collection of data across multiple centres and comparison of results across studies. We report on the development and process that resulted in the Amsterdam Positional OSA Classification (APOC).
Method
A panel of three field experts were instructed to independently assign the diagnosis POSA to 100 randomly selected patients they considered likely to benefit from a clinically significant improvement of their OSA with PT. In a group setting, the completed lists were compared. Discrepancies were discussed until consensus was met. This resulted in the consensus standard used to calibrate the new classification. Using the nominal group technique, the APOC was developed.
Results
The APOC criteria evolve around the percentage of total sleep time spent in either the worst sleeping position (WSP) or the best sleeping position (BSP) and the apnoea–hypopnoea index (AHI) in BSP. On applying APOC, one discriminates between the true positional patient, the non-positional patient and the multifactorial patient, whose OSA severity is influenced in part by sleep position. APOC has an increased sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) compared to previously applied POSA criteria in identifying patients that will benefit from positional therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increasing amount of literature is being published on the role of sleep position in obstructive sleep apnoea (OSA) and methods to avoid the worst sleeping position (WSP) [1]. It has become apparent that in the majority of patients with OSA, the frequency and duration of apnoeas are influenced by body position as well, the so-called position-dependent OSA (POSA) [2].
To treat patients with POSA, positional therapy (PT) can be considered, aimed at preventing patients from sleeping in the WSP. Various techniques have been described such as positional alarms or verbal instructions for example. The majority of studies on PT apply the so-called tennis ball technique (TBT): a bulky mass strapped to the patient’s back [1]. Even though TBT is simple and cheap, as well as effective in reducing the apnoea–hypopnoea index (AHI), results are unsatisfactory [2]. Ineffectiveness, backache, discomfort and no improvement in sleep quality or daytime alertness have been responsible for poor compliance and the subsequent disappointing long-term results of PT [3]. Compliance rates reported in the literature range from 40 % short term to 10 % long term [3–5].
Three recent studies have seen the introduction of a new generation of PT, a small device attached to either the neck or chest which corrects the patient from adopting the supine position through a vibrating stimulus [6–8]. The studies present encouraging data suggesting that this simple therapy successfully prevents patients with POSA from adopting the supine position without negatively influencing sleep efficiency as well as allowing for good adherence. It is to be expected that PT will gain momentum in the scope of OSA treatment, but evaluating the efficacy of new-generation PT and comparison of results are hindered by the fact that there are no universally used POSA criteria [9].
In 1984, Cartwright suggested that physicians should differentiate between patients with either positional or non-positional OSA. She described the arbitrary cut-off point of a difference of 50 % or more in apnoea index between supine and non-supine positions [10]. Despite being the most common classification system and definition used to date, various modified versions of Cartwright’s criteria have been applied in literature. In 1998, Marklund et al. defined supine-dependent sleep apnoea as follows: a supine AHI ≥ 10, together with a lateral AHI < 10 [11]. Both Mador’s and Permut’s groups defined POSA as follows: an AHI of fewer than five events per hour whilst in the non-supine position as well as a >50 % decrease in the AHI between the supine and non-supine postures [12,13]. In the study of Bignold et al., patients who met the following criteria were deemed position-dependent: overall AHI ≥ 15/h, supine AHI ≥ twice the non-supine AHI, ≥20 min of sleep in supine and non-supine postures and non-supine AHI < 15 [9].
The application of various classifications hinders the comparison of the studies on PT. Furthermore, it can be questioned which classification is best suited to identify ideal candidates for new-generation PT. For example, Mador et al. felt that their definition was more clinically relevant, given that avoidance of the supine sleeping position by patients who fit their description would result in normalization of the AHI and subsequent relief of symptoms of OSA [10]. Bignold et al. were the first to include a minimum sleeping time per position [9].
Bearing this in mind, the aim of this project was to introduce a new classification system, which ideally should identify suitable candidates for PT: patients that will benefit from a clinically significant improvement of their OSA with PT. The shared use of this classification can facilitate collection of data across multiple centres and comparison of results across studies.
The objective of this article was to report on the development and results of the process that resulted in the Amsterdam Positional OSA Classification (APOC).
Material and methods
As Cartwright’s criteria are most commonly used in medical literature on POSA, we sought to evaluate Cartwright’s criteria and identify their weaknesses. Using the nominal group technique, a new classification system was developed. To test the accuracy of a new classification, comparison against a gold standard benchmark is necessary. Even though Cartwright’s classification is most commonly applied, there is no gold standard test to identify patients with POSA. Therefore, we constructed a ‘consensus standard’.
Patients
To perform the consensus method, 100 OSA patients were randomly selected from our institutional database, consisting of patients who underwent a polysomnography (PSG, see paragraph below) from April 2010 until October 2010. Patients were excluded from analysis if aged <18 years, sleep efficiency below 80 %, failure of the position sensor and therefore unknown sleeping position, or in case of an AHI < 5. Weight, length and date of birth were registered. The body mass index (BMI) was calculated, and the following BMI grading system was implemented: obese (BMI 30–34.9), severely obese (BMI 35–39.9), morbidly obese (BMI 40–49.9) and super obese (BMI > 50) [10, 14]. From all patients, an informed consent was obtained. The data described were entered in an encoded study database.
Polysomnography
PSG recordings were carried out using a digital polygraph system (Embla A10, Broomfield, USA). This records the electroencephalogram (FP2-C4/C4-O2), electrooculogram, EKG and submental and anterior tibial electromyogram. Nasal airflow was measured by a pressure sensor and arterial oxygen saturation by finger pulse oximetry. Thoraco-abdominal motion was recorded by straps containing piezoelectric transducers. Snoring was recorded through a piezo snoring sensor. Body position was determined by a position sensor (SleepSense, St. Charles, USA), which was attached to the midline of the upper abdominal wall. This sensor differentiated between the upright, left side, right side, prone and supine positions. All signals were recorded with digital sampling, digital filtering and digital storage (DDD) recording technology and a sample rate up to 200 Hz. Storage was done on a PCMCIA flashcard. The following day, data were downloaded to the computer and analysed by dedicated sleep software (Somnologica, Broomfield, USA). The data were manually reviewed for analysis by an experienced sleep investigator.
Obstructive respiratory events were analysed according to the 2007 AASM criteria [11]. Obstructive apnoeas were defined as decrease of airflow of more than 90 % for at least 10 s, in the presence of respiratory efforts. Central apnoeas were defined as a decrease of airflow of more than 90 % for at least 10 s and no respiratory effort of the thorax or abdomen. Hypopnoeas were defined as a decrease of airflow of 30–90 % for at least 10 s, with a continuation of respiratory effort and leading to a decrease in haemoglobin saturation of at least 3 %. The AHI was calculated as the sum of total events (apnoeas and hypopnoeas) per hour of sleep. An AHI of 5–15/h is mild OSA, an AHI of 15–30/h is moderate OSA and an AHI >30/h is severe OSA, as assessed by PSG.
Consensus standard
We created a consensus standard as to be able to calibrate the new classification. A moderator and three panellists were appointed. Field experts with a minimum of 2 years of field experience treating OSA patients within a multidisciplinary unit, with special focus on treatment of patients with POSA, were appointed as panellists.
Step 1
As a first step, each panellist was sent a list, containing data (overall AHI, AHI and total sleep time (TST) in supine position, AHI and TST in non-supine position) of 100 patients randomly selected by SPSS from the above-mentioned institutional database. The panellists were instructed to independently assign the diagnosis POSA to the subjects who were likely to benefit from a clinically significant improvement of their OSA with PT. The panellists were not allowed to consult each other nor use any aids such as a calculator. The panellists distinguished between non-positional and positional OSA based on their clinical experience and the provided data. We attributed the terminologies WSP (in the majority of cases the supine position) and best sleeping position (BSP) (in the majority of cases non-supine).
Step 2
During a group meeting, facilitated by the moderator, the three completed lists were compared. Subject data were discussed if panellists had attributed a different rank to data. The panellist who was a ‘minority’ was prompted to discuss and clarify his or her motives followed by a group discussion. In most cases, consensus was met; if not, the ‘majority’ decided which rank would be attributed. The results from step 2, the diagnosis attributed to the 100 randomly selected patients, were considered the consensus standard and used as a surrogate gold standard to validate the new classification system.
Step 3
The nominal group technique, a structured meeting that attempts to provide an orderly procedure for obtaining qualitative information from target groups who are most closely associated with a problem area, was applied to gain consensus and to build a new classification [15–18].
During group discussions, the panellists were invited to make proposals to resolve the weaknesses of Cartwright’s classification, which had been identified during step 2. This led to the construction of a new classification: the APOC.
Step 4
After precise formulation of the APOC criteria, another group meeting was convened during which the criteria for the APOC were discussed and checked. After approval of these criteria by all experts, the proposed APOC criteria were compared to the consensus standard. Finally, a handout for the easy application of APOC in clinical practice was designed.
Statistical analysis
All statistical analyses were conducted in SPSS (version 18, SPSS Inc., Chicago, USA). Descriptive statistics were calculated for baseline characteristics. Results of continuous data are reported with means (SDs) and categorical data as number and percentage. To assess interrater agreement, the intercorrelation coefficient was calculated. As we appointed three panellists, instead of two, the intercorrelation coefficient (ICC) was used instead of Cohen’s kappa. Sensitivity, specificity and positive and negative predictive values were calculated to test the performance of the various classification systems in comparison to the consensus standard.
Results
Of the 343 patients who underwent a PSG during the 7-month study period, 100 OSA patients were randomly selected for the consensus list, of whom 66 were male and 34 female. Patient characteristics are summarized in Table 1. Based on the PSG results, 45 % of the patients had mild OSA, 32 % moderate OSA and 23 % severe OSA.
Consensus standard
The ICCs of the selected panellists were calculated: before the group meeting, the ICC was 0.817 (p < 0.00).
Evaluation of Cartwright’s classification
When applying Cartwright’s criteria, 64 % of the patients were diagnosed with POSA. When comparing Cartwright’s classification to the consensus standard, a sensitivity of 96 %, a specificity of 68 %, a positive predictive value (PPV) of 75 % and a negative predictive value (NPV) of 94 % were measured (see Table 2). All false-positive and false-negative cases were examined during the group meeting.
Discussion topics
During the consensus group meeting, the following topics were discussed:
-
1.
Insufficient distribution of the various sleeping positions: A few cases, albeit rare, were identified in which there was insufficient distribution of the various sleeping positions. For example, one patient had an overall AHI of 25.3/h. The patient spent 99.5 % of the TST in supine position. The AHI in supine position was 25.2/h, whilst in non-supine position, 0/h. According to Cartwright’s criteria, this patient has POSA. The following discussion arose: How long must a patient sleep in a certain position for the AHI measured to be valid and representative. So far, only Bignold et al. mention a specific cut-off value of 20 min that a patient should sleep in the WSP [9]. The panel felt that a patient should sleep more than 10 % of the TST in the WSP before PT should be considered.
-
2.
Self-correction of the WSP: Cases were identified in which patients had a high AHI in the WSP and had spent the majority of the night in BSP. For example, a patient had an overall AHI of 12.2/h and spent 12.1 % of the TST in supine position and 87.9 % in non-supine position. The AHI in supine position was 45.9/h, whilst in non-supine position, 7.5/h. Despite a high supine AHI, the patient avoided the WSP. The panel hypothesized that such patients ‘self-correct’, and although they are diagnosed with POSA (Cartwright’s classification), the treatment effect of PT would be very limited.
-
3.
Clinical relevance of elimination of the WSP: In some rare cases, the group concluded that despite a ≤50 % difference between the AHI in the WSP and BSP, elimination of the WSP would still be clinically relevant; PT might make a clinically relevant difference. For example, a patient had an overall AHI of 64.4/h and spent 50.4 % of the TST in supine position and 49.6 % in non-supine position. The AHI in supine position was 85.7/h, whilst in non-supine position, 43.1/h. It was assumed that the maximum achievable result of PT in this patient would be to reduce the overall AHI from 64.4/h to 43.1/h. Although the patient would remain in the same OSA severity category and therefore would still have severe OSA and would still be continuous positive airway pressure (CPAP) dependent, the patient would need significantly lower CPAP pressure, which, in many cases, may lead to better compliance. In case the patient does not tolerate (or refuses) CPAP or oral appliances, PT as salvage therapy could lower AHI and consequently increase the patient’s quality of life and reduce cardiovascular risk.
Proposal of an improved classification
These insights led to new criteria that will henceforth be referred to as the APOC.
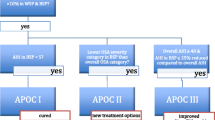
In clinical practice, patients meeting the following criteria can be diagnosed with POSA, according to the APOC criteria (see Fig. 1 flow chart):
-
1.
Diagnosed with OSA according to the American Academy of Sleep Medicine (AASM) criteria [19]
-
2.
Greater than 10 % of the TST in both BSP and WSP
-
3.
Have a BSP AHI of less than 5
-
4.
Have a BSP AHI in a lower OSA severity category
-
5.
Have an overall AHI of at least 40 and at least a 25 % lower BSP AHI.
When adopting the APOC classification system, one discriminates between the true positional patient, the non-positional patient and the multifactorial patient, whose OSA severity is influenced in part by sleep position. The patients with true POSA could be cured by PT alone and are categorized as APOC I, whilst patients classified as APOC II or III can benefit from PT but not cured. Patients may benefit from a combination of therapies. By lowering the OSA severity category, patients could be eligible for less aggressive primary therapy (for example, lower CPAP pressure, less invasive surgery), especially since PT is simple, cheap, well tolerated and reversible. In patients who do not tolerate (or refuse) CPAP or oral appliances, PT can be considered as salvage therapy.
In Table 3, we describe the best possible outcome per category of the APOC.
Validation of the APOC
When applying APOC, 55 % of the patients were diagnosed with POSA. In comparison to the results described earlier when comparing Cartwright’s classification to the consensus standard, when applying the APOC criteria, these values increased to a sensitivity of 98 %, a specificity of 88 %, a PPV of 89 % and a NPV of 98 % (see Table 2). Characteristics of patients diagnosed with POSA when applying the various POSA criteria and APOC are summarized in Table 4. The independent samples T test did not provide any significant differences between the POSA group defined by APOC or Cartwright although the DI has a significance level of 0.051. In Table 5, the distribution of OSA severity is specified.
Discussion
In this paper, we introduce the APOC criteria, a new clinically relevant positional OSA classification system, aimed at accurately identifying candidates who will benefit from a clinically significant improvement of their OSA with PT. We advocate that more patients can benefit from positional therapy than only true positional patients, especially since PT is simple, cheap, well tolerated and reversible.
In contrast to previous classification systems, the APOC discriminates between the true positional patient, the non-positional patient and the multifactorial patient, whose OSA severity is influenced in part by sleep position. The patients with true POSA could be cured by PT alone and are categorized as APOC I.
Patients classified as APOC II or III can benefit from PT, by going down in OSA class or a decrease in AHI, resulting in less aggressive primary treatment. For example, as the AHI drops, so does the CPAP pressure needed, potentially improving tolerance and compliance.
The majority of previous POSA classification systems do not take the TST spent in different positions into account but use the differential advantage of the non-supine AHI over the supine AHI calculated as a ratio. In the APOC classification, the TST is taken into consideration by requesting that a patient should sleep more than 10 % of the TST in the WSP before PT should be considered and by means of the expected decrease of the overall AHI. Since the overall AHI is directly related to the TST, the APOC gives a more thorough evaluation of the potential role of PT.
In this current study, the APOC criteria were found to be more effective in identifying patients, shown by an increase in sensitivity and specificity and predictive value, that will (not) benefit from PT, thus resulting in a more cost-efficient treatment. Furthermore, the shared use of this classification can facilitate collection of data across multiple centres and comparison of results across studies.
Considerations
Our study is not without flaws. Even though Cartwright’s classification is most commonly applied to discern whether a patient is positional, this is not a gold standard test. We constructed a consensus standard but recognize that a consensus standard is not equal to a gold standard diagnostic test. Another limitation of the study is that all panellists came from the same multidisciplinary treatment group in Amsterdam. This of course may have led to a selection bias when constructing the consensus standard. International participation in the consensus standard would have increased its validity but was logistically not possible within the current time frame.
Even though new-generation PT can treat any WSP (such as the prone position) as to simplify the consensus meeting, the data sent to the panellists did not include the distribution of the AHI and TST in the various non-supine positions. Therefore, when applying APOC to the consensus list, we were only able to take the AHI and TST in supine position and in non-supine position into consideration. It can be expected that the sensitivity, specificity and positive and negative predictive values would have been higher if APOC had been applied whilst taking the AHI and TST of each specific sleeping position. In clinical practice, if available, one should consider each sleeping position individually.
Assumptions were made to validate the current classification system. We considered PT to be effective in preventing patients from adopting the WSP, 100 % efficacy in combination with absolute compliance. It remains to be studied whether elimination of the WSP reduces the overall AHI to the BSP AHI (the mean AHI in all positions except the WSP). One can question whether it might be necessary to add a certain correction percentage for residual supine sleep, but as reports show that the median TST in WSP was between 0 and 5 % with new-generation PT, we considered this point negligible [1,7–9].
Consequently, it can be questioned whether all patients with a pretreatment BSP AHI < 5 will have a posttreatment overall AHI < 5. As with other treatments, it is advisable to repeat PSG after PT as to check for residual disease and measure the efficacy of the treatment.
Furthermore, it is important to stress that even though new-generation PT devices are not internationally available as yet, APOC was developed with new-generation PT devices in mind rather than conventional PT (TBT). New-generation PT can be defined as follows: a well-tolerated device which prevents a patient from adopting the supine position without negatively influencing sleep efficiency, as objectified by a full-night PSG. It is to be expected that PT will gain momentum in the scope of OSA treatment.
Conclusion
With the increase of compliance in PT, the importance of an easy, clinically applicable positional classification system increases. This classification system must not only be able to identify patients that will benefit but also identify which patients will not benefit from PT. This new classification system (APOC) has an increased sensitivity, specificity, PPV and NPV compared to Cartwright’s classification in identifying patients that will benefit from positional therapy. The APOC criteria for POSA are more effective in identifying patients that will benefit from PT, thus resulting in a more cost-efficient treatment for patients with POSA.
Conflict of interest
Nico de Vries is a medical advisor of MSD, ReVENT and NightBalance; is an investigator for Inspire; is a consultant for Philips; and has stock options in ReVENT. None of the other authors have any conflict of interest regarding the material discussed in this article.
Abbreviations
- AHI:
-
Apnoea–hypopnoea index
- APOC:
-
Amsterdam Positional OSA Classification
- BMI:
-
Body mass index
- BSP:
-
Best sleeping position
- CPAP:
-
Continuous positive airway pressure
- DI:
-
Desaturation index
- ICC:
-
Intercorrelation coefficient
- NPV:
-
Negative predictive value
- OSA:
-
Obstructive sleep apnoea
- POSA:
-
Position-dependent obstructive sleep apnoea
- PPV:
-
Positive predictive value
- PSG:
-
Polysomnography
- PT:
-
Positional therapy
- SaO2 :
-
Saturation oxygen
- TBT:
-
Tennis ball technique
- TST:
-
Total sleep time
- WSP:
-
Worst sleeping position
References
Ravesloot MJ, van Maanen JP, Dun L, de VN (2013) The undervalued potential of positional therapy in position-dependent snoring and obstructive sleep apnea-a review of the literature. Sleep Breath 17:39–49
Oksenberg A, Gadoth N (2013) Are we missing a simple treatment for most adults sleep apnea patients? The avoidance of the supine sleep position. J Sleep Res 23:204–210. doi:10.1111/jsr.12097
Bignold JJ, Deans-Costi G, Goldsworthy MR, Robertson CA, McEvoy D, Catcheside PG, Mercer JD (2009) Poor long-term patient compliance with the tennis ball technique for treating positional obstructive sleep apnea. J Clin Sleep Med 5:428–430
Wenzel S, Smith E, Leiacker R, Fischer Y (2007) Efficacy and longterm compliance of the vest preventing the supine position in patients with obstructive sleep apnea. Laryngorhinootologie 86:579–583
Oksenberg A, Silverberg D, Offenbach D, Arons E (2005) Positional therapy for obstructive sleep apnea patients: a 6-month follow-up study. Laryngoscope 116:1995–2000
Marklund M, Verbraecken J, Randerath W (2012) Non-CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J 39:1241–1247
van Maanen JP, Meester KA, Dun LN et al (2013) The sleep position trainer: a new treatment for positional obstructive sleep apnoea. Sleep Breath 17:771–779
van Maanen JP, Richard W, Van Kesteren ER et al (2012) Evaluation of a new simple treatment for positional sleep apnoea patients. J Sleep Res 21:322–329
Bignold JJ, Mercer JD, Antic NA, McEvoy RD, Catcheside PG (2011) Accurate position monitoring and improved supine-dependent obstructive sleep apnea with a new position recording and supine avoidance device. J Clin Sleep Med 7:376–383
Cartwright RD (1984) Effect of sleep position on sleep apnea severity. Sleep 7:110–114
Marklund M, Persson M, Franklin KA (1998) Treatment success with a mandibular advancement device is related to supine-dependent sleep apnea. Chest 114:1630–1635
Mador MJ, Kufel TJ, Magalang UJ, Rajesh SK, Watwe V, Grant BJ (2005) Prevalence of positional sleep apnea in patients undergoing polysomnography. Chest 128:2130–2137
Permut I, Diaz-Abad M, Chatila W et al (2010) Comparison of positional therapy to CPAP in patients with positional obstructive sleep apnea. J Clin Sleep Med 6:238–243
IFSO (2014) https://www.ifso.com. Ref Type: Internet Communication. IFSO, Naples
Allen J, Dyas J, Jones M (2004) Building consensus in health care: a guide to using the nominal group technique. Br J Community Nurs 9:110–114
Cartwright RD, Diaz F, Lloyd S (1991) The effects of sleep posture and sleep stage on apnea frequency. Sleep 14:351–353
Fink A, Kosecoff J, Chassin M, Brook RH (1984) Consensus methods: characteristics and guidelines for use. Am J Public Health 74:979–983
Van de Ven AH, Delbecq AL (1972) The nominal group as a research instrument for exploratory health studies. Am J Public Health 62:337–342
Iber C, Ancoli-Israel S, Chesson AL Jr, Quan SF, for the American Academy of Sleep Medicine (2007) The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications, 1st edn. American Academy of Sleep Medicine, Westchester, IL
Author information
Authors and Affiliations
Corresponding author
Additional information
M.H. Frank and M.J.L. Ravesloot equally contributed to this article.
Rights and permissions
About this article
Cite this article
Frank, M.H., Ravesloot, M.J.L., van Maanen, J.P. et al. Positional OSA part 1: towards a clinical classification system for position-dependent obstructive sleep apnoea. Sleep Breath 19, 473–480 (2015). https://doi.org/10.1007/s11325-014-1022-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11325-014-1022-9