Abstract
Brewer’s spent grain (BSG) is a major by-product in the beer-brewing process which contributes to 85% of the entire generated by-product in the brewing process. BSG is rich in proteins, and most of the malt proteins (74–78%) remain insoluble in BSG after the mashing process. Solid-state fermentation (SSF) is a promising bioprocess that enables microorganisms to survive in environments with minimal water and has shown to enhance the nutritional composition of BSG. In this review, the potential application of protein, amino acids (proline, threonine, and serine), phenolic contents, and soluble sugars (glucose, fructose, xylose, arabinose, and cellobiose) extracted from BSG by various microorganisms using SSF is explored. Incorporation of BSG into animal feed, human diets, and as a substrate for microorganisms are the prospects that could be implemented in the industrial scale. This review also discussed various advances to improve the fermentation yield such as symbiotic fermentation, the addition of nitrogen supplements, and an optimal mixture of the agro-industrial waste substrate. Future perspectives on SSF are also addressed to provide important ideas for immediate and future studies. However, challenges include optimizing SSF conditions and design of bioreactors, and operational costs must be addressed in the future to overcome current obstacles. Overall, this mini review highlights the potential benefits of BSG utilization and SSF in a sustainable way.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brewer’s spent grain (BSG) is a major by-product in the beer-brewing process when the barley and cereal grains are solubilized to enable proper wort extraction. BSG contributes 85% of the entire generated by-product in the brewing process. It consists of 31% original malt weight, and the beer produced is 20 kg per 100 L [1]. The barley malt was partially liquefied during the brewing process, and the wort was filtered from the raw material, leaving BSG as a residual product. Generally, the BSG is disposed to landfill/incineration as waste or utilized as livestock feed due to its high composition of protein and sugar level, while the wort is subsequently brewed into beer [2]. BSG is indeed the most common by-product of the brewery industry, with a yearly global production of roughly 39 million tonnes, 10% of which is generated in Europe alone [3]. Given its high moisture content of around 80% and its richness in polysaccharides and proteins, BSG is highly prone to microbial contamination, leading to a short lifespan ranging from 7 to 10 days [4]. These characteristics exert various impacts across different levels. At an environmental level, BSG poses a significant risk when disposed of without appropriate treatment. Specifically, every ton of BSG discarded in landfills results in the release of roughly 514 kg of CO2 greenhouse gases equivalent. Therefore, BSG contributes to substantial waste generation and adverse environmental effects [5]. At an industrial level, the described characteristics contribute to the high cost of transporting BSG, create difficulties in storage, and restrict their stability and usability [6]. For instance, the drying process necessary to prevent contamination can be costly, ultimately hindering the commercial value of BSG [7]. To address issues associated with BSG aggregation and minimize waste and pollution stemming from the brewing industry, there is a growing interest in pursuing alternative valorization routes through biotechnological processing. In addition to their nutritional value, the year-round availability of spent grains at an exceptionally low cost, around 40.23 USD per ton of BSG, makes them an attractive raw material for microbial fermentation [8, 9].
BSG can be viewed as a heterogeneous material due to the different brewing conditions that might affect the BSG’s compositions. BSG is majorly constituted of 30–50% fiber (20–25 hemicellulose, 12–35% cellulose), 19–30% proteins, 12–28% lignin, and others such as 10% lipid and 2–5% ash [10]. BSG is rich in proteins and most of the malt proteins remain insoluble in BSG after the mashing process. In BSG, roughly 30% of the total protein composition is made up of essential amino acids [11]. The essential amino acids found in BSG are specifically tryptophan, lysine, phenylalanine, histidine, and methionine, while the non-essential amino acids are proline, alanine, serine, and glycine. Significant valuable amounts of phenolic compounds, primarily ferulic acid and p-coumaric acid, are also present in BSG [12].
The brewing process of beer consists of six major steps which include malting, milling, mashing, brewing, chilling, and fermentation, whereas BSG is produced after the mashing steps which is shown in Fig. 1. Firstly, malting is the process of steeping, germinating, and kilning barley grains in order to cleanse and dry it. In the mashing process, starch is converted into fermentable and non-fermentable sugars through the use of alpha and beta amylases, while proteins are partly hydrolyzed into short peptide chains and amino acids. Then, the wort is produced after the lautering step. The insoluble BSG is obtained after the filtration. The wort is boiled with hops for sterilization to enhance the bitterness and fragrance. Lastly, prior to fermentation, the wort must be cooled, and the yeasts were added [13,14,15].
BSG is a lignocellulosic material and made up of complex natural plant cell wall components which include lignin, cellulose, and hemicellulose that integrate together to form a refractory structure. Pre-treatment is necessary to break up the cellular rigid of lignocellulosic and enable their segregation before they can be used in the biorefinery processes [16]. Pre-treatments can be categorized into two major methods, which are chemical and physical pre-treatments. Chemical pre-treatment method including alkaline pre-treatment, acid pre-treatment, and ionic liquids increases the biodegradability of cellulose. Moreover, they have less of an effect on depolymerization and cellulose complex crystallinity. The drawback of chemical pre-treatment is the potential of hazardous by-products to emerge which could negatively impact anaerobic digestion and the cost expenses of the chemicals. Physical pre-treatments including hydrothermal, ultrasonic, mechanical, and microwave radiation pre-treatments disrupt the cellulosic structure and increase the accessibility of enzymes. With the assistance of enzymes in the treatment, cellulose and hemicellulose in BSG were hydrolyzed which resulted in a higher percentage of monosaccharides yield [17, 18].
The combination of both chemical and physical pre-treatment is called physiochemical pre-treatment methods. Alterations to the physical parameters, chemical bonds, and intermolecular interactions of lignocellulosic materials can be achieved through the use of physicochemical pre-treatment [19]. Examples of physicochemical pre-treatments include ammonia fiber explosion [20], steam explosion [21], liquid hot water [22], and carbon dioxide explosion pre-treatment [23]. The limitations of physicochemical pre-treatment are significant, despite their effectiveness. These include a lack of selectivity, damage biopolymer basic units, create unwanted and potentially harmful compounds, require high energy consumption, and produce effluents and residues with negative environmental impacts [24]. During the pre-treatment process, the internal structure of biomass is altered by the breakdown of intra and inter hydrogen bonds, as well as glycosidic bonds. This breakdown paves the way for the degradation of biomass into sugars [25]. In pre-treatment lignocellulosic hydrolysates, fermentation inhibitors like acetic acid, furfural, and 5-hydroxymethyl furfural (HMF) are commonly present [26]. The production of acetic acid is attributed to the degradation of acetyl and ester bonds in hemicellulose, while furfural and HMF are released during the degradation of pentose and hexose carbohydrates. Furfural emerges as the most abundant and potent inhibitor among all inhibitors. The dehydration of the acetyl group in hemicellulose is responsible for the production of acetic acid in hydrolysates obtained from all lignocellulosic biomass, without exception. Acetic acid exists in its undissociated form under acidic conditions and exhibits liposolubility, leading to the accumulation of anions inside cells. Subsequently, acetic acid dissociates and infiltrates the cell membrane, triggering a decrease in internal pH that eventually results in cell death [27, 28]. The presence of inhibitors undoubtedly creates unfavorable environments for fermentative microorganisms, leading to reduced yield by prolonging the lag phase, diminishing cell density, and slowing down the growth rates of the fermenting microbes [25].
Other than chemical-physical pre-treatment methods, biological pre-treatment methods offer a new future alternative for the processing of lignocellulosic biomass. The primary approach of biological pre-treatment methods is the use of fungi and bacteria, along with their enzymes, which involves multiple variables such as strain types, enzymes produced, culture conditions, culture time, and degradation mechanisms [29]. Compared to other methods, biological pre-treatment has several benefits, including easy operation, the absence of chemical recovery after pre-treatment, low energy consumption, and low cost of downstream treatment. Nevertheless, the low hydrolysis rates and extended consumption time are major obstacles to the industrial application of biological methods [30]. Turet et al. [31] demonstrated the used of biological pre-treatment method by using Aspergillus niger and Thermoascus aurantiacus fungal strains on BSG to produce lignocellulolytic enzymes. The self-produced enzymes hydrolyzed the same BSG to obtain sugar-rich hydrolysates that serve as an alternative carbon source for polyhydroxyalkanoates (PHA) production during the second round of fermentation.
Solid wastes, including agricultural, domestic, human, and animal waste, are being produced as a result of urbanization, economic growth, and rapid population growth [32]. To minimize landfill and environmental problems, several efforts have been focused on converting the waste into value-added products [33]. The biotechnology sector has been focusing on the manufacture of high value-added bio-products employing microorganisms, primarily bacteria and fungi. Especially, BSG is used as a substrate for biotechnological applications because of its high nutritional content [34]. The method of valorizing BSG involves two different types of fermentation which are submerged fermentation (SmF) and solid-state fermentation (SSF). SmF is a method involving the growing of microorganisms in a liquid medium that consist of required nutrients. The amount of water available for bacterial growth has an impact on the mechanisms which affects the mass transfer, metabolism, and biomass development [35]. SmF is ideal for the synthesis of secondary metabolites that must be used in their liquid form since they are usually secreted in the fermentation broth and for microorganisms that require high moisture content [36]. This method has a higher product yield than other varieties since it is less likely to have substrate inhibition [37]. SmF can be used on an industrial scale as a result of the well-developed scale-up techniques and bioreactors for this kind of fermentation [38]. However, it has several disadvantages such as time-consuming process, prone to fungal and bacterial contamination, requires energy for sterilization, and high operational cost as the nutrients have to be supplemented constantly [39]. To ensure a successful SmF, key factors such as carbon and nitrogen sources, temperature, pH, agitation, and aeration must be monitored. Angel et al. [40] examined how different carbon sources, including inulin, sucrose, xylose, fructose, and glucose, affected inulinase production from Bacillus sp., Pseudomonas sp., Lactobacillus sp., and Achromobacter sp. Among these sources, inulin was found to be the most favorable carbon source for inulinase production across all bacterial strains under SmF. Nitrogen is an integral element of amino acids, nucleic acids, and various co-enzymes, and any changes made to its concentration in the media can lead to significant alterations in cellular biosynthesis [41]. The optimal temperature and pH required for growth and development can vary across different species. Therefore, it is crucial to optimize the culture conditions to ensure maximum growth and productivity [42]. Proper agitation is essential to maintain heat, oxygen, and mass transfer rates while promoting surface absorption. In addition, the physical morphology of an organism can significantly impact the oxygen transfer rate. For instance, maintaining oxygen supply through agitation is easier in bacterial cultures than in fungal cultures with long hyphal threads. This is mainly because the coalescence of bubbles in rigid filamentous fungal broth reduces the transfer area between the gas and liquid phases, thereby altering turbulence and liquid-film conditions [43]. When it comes to the extraction and purification stages of the downstream process, several obstacles must be overcome, including the need to achieve high product quality and purity, ensure stability, and determine the optimal formulation for the final product. The substances in need of purification belong to a heterogeneous group, making their characterization a challenging task. Furthermore, there is a notable demand for a remarkably high level of purity. To ensure the degree of purity, multiple-step downstream processes are usually necessary, resulting in lower product yields due to sample loss throughout the process [44].
Since the usage of SmF has been fully developed in the industrial-scale, this review will be focusing solely on SSF. SSF is a bioprocessing technique with a rich history in the food and fermentation sectors, which finds extensive use in Asia for the production of various foods like bread, cheese, koji, miso, soy sauce, tempeh, and natto. Over the past century and in recent years, SSF has been instrumental in generating significant biochemical compounds and valuable products, including amino acids, enzymes, organic acids, pharmaceutical antibiotics, textiles, and biofuels [45]. SSF is a well-established bioprocess that potentially generates microbial secondary metabolites from industrial by-products and agricultural waste. Large amounts of biomass waste such as corn residue, seeds, husks, bran, whole pomace, peels, BSG, and other waste products are generated but either underutilized or ended up in landfills. The use of the BSG has received a lot of attention recently since they are easily accessible, inexpensive renewable substrates that can be converted into a variety of essential compounds [46]. SSF has been proposed as a viable approach for recycling wastes by employing solid wastes as substrates for the cultivation of microorganisms to enhance the nutritional composition within the solid wastes. SSF enables microorganisms to thrive in the environments with minimum or no free water. SSF replicates the natural habitat of the majority of microorganisms, primarily fungi and molds. It offers enhanced enzyme effectiveness for several enzymes and is less prone to bacterial contamination [47]. This method claims to have various advantages compared to the submerged fermentation, including less water waste contamination, low production cost as the process does not involve anti-foam chemicals, low maintenance fee in terms of downstream process, environmentally friendly, and the absence of catabolite repression [48]. In the study conducted by Sim and Oh [49], BSG was first employed as a substrate for cellulase production using SSF. As time went on, SSF techniques were implemented to elevate the nutritional value of BSG especially in terms of protein [50,51,52]. Additionally, efforts were made to improve other nutritional aspects like phenolic compounds [53, 54] and soluble sugars [55, 56], and the study evaluated the potential use of BSG in both animal and human feed [57,58,59].
The fundamental of SSF could also possibly generate nutritional added value to the substrate when given a compatible substrate with the proper microorganism. For instance, bioactive compounds such as flavonoids, phenolic acids, and saponins that are initially produced by vegetables may also be present in endophytic fungi colonies, which are emerging as a promising source of antioxidants [60]. In addition to its impact on adding nutritional value to the substrate, SSF can also affect by increasing the extractability of the nutritional compounds within the substrate. This extractability is depending on various physicochemical properties and molecular interactions, including the potential compartmentalization of bioactive compounds within cellular structures [61]. During SSF, microorganisms may initially struggle to access certain plant cell wall components due to their chemical connections to lignin. However, microorganisms are naturally equipped with various enzymes that allow for the degradation of lignin, such as lignin peroxidase, enabling them to access energy-rich polysaccharides for growth and metabolism, while releasing insoluble-bound compounds from the lignocellulosic structure [62].
SSF faces a major obstacle in scaling up, as it is largely confined to flask scale. Scaling up SSF is a process-specific challenge, and there is a dearth of information on bioreactor design and optimal conditions for microorganism biomass production. At the onset of fermentation development, little is known about the specifics of a particular process. Despite the longstanding challenge of scale-up, it is predicted that the majority of SSF’s issues can be addressed by designing appropriate bioreactors. Heat accumulation and mass transfer are two major challenges associated with bioreactors. Temperature management poses challenges in the SSF process due to the heat generated by microbial activity, which accumulates in the system [63]. To ensure optimal microbial growth and product formation, it is necessary to remove excess heat from the system and prevent overheating [64]. The fermentation process experiences low yield due to the hindered mass transfer and increased heat accumulation caused by the high concentration of BSG substrate [65]. One way to overcome this challenge is by introducing air into the system to expel the heat generated, typically through a gas outlet [66]. The diffusion of oxygen in the solid matrix is a challenging aspect of SSF. The growth of microorganisms in the solid substrate, along with culture biomass, leads to a reduction in the permeability of oxygen through the matrix. Adequate oxygen levels are crucial for the proper growth of microorganisms [67]. The use of a bioreactor with improved oxygen diffusion rate in the substrate enhances fermentation efficiency by facilitating longer air and liquid contact time [68]. Zhao et al. [69] demonstrated that implementing the microbubbles technique enhances mass transfer in BSG and results in a twofold increase in fermentable sugar production.
An excellent bioreactor design should fulfill several essential criteria. These include the use of inexpensive, inert, corrosion-resistant, and abrasion-resistant materials, preferably creating a microbiologically contamination-free system to prevent potential hazards associated with biological pollution. Furthermore, the bioreactor design should facilitate efficient control and regulation of operational parameters, ensure biomass uniformity, and simplify maintenance, loading and unloading, and product recovery [70].
Countless of research has been done in the past few years about the exploitations of nutritional value in BSG via SSF. However, limited review papers were published to compare the effectiveness of fermentation methods and approaches that could improve the commercial value of BSG. Hence, the aim of this review is to describe the nutritional added value to the BSG via SSF bioprocess as the microorganism feeds on the lignocellulosic content in BSG and improve the nutritional components of it. A specific focus is placed on multiple value-added compounds which include proteins, phenolic compounds, and soluble sugars in BSG. Therefore, SSF and its effect on the formation of value-added products by this process were reviewed and discussed.
Nutritional Value in BSG
Protein
The microorganism that feeds on the substrate during the SSF produces enzymes that hydrolyzed directly on the cellular structure of the substrate, subsequently releasing the phytochemical substances attached to the solid matrix and improve their extractability. Therefore, SSF can increase the substrate’s bioactivity and nutritional value through the biosynthesis process [71].
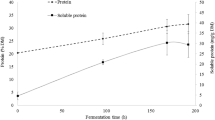
Many researches have proven that SSF is able to improve the protein content of BSG under various optimal fermentation conditions. Canedo et al. [72] evaluated the up-value of the protein content of BSG under different parameters that include initial moisture levels and types of nitrogen supplement using Rhizopus oligosporus. It can be expected that supplementing with nitrogen source would lead to the better proliferation of fungi, primarily due to the increased nitrogen content and the resulting lower C:N ratio, which provides optimal conditions for fungal growth [73]. Results showed that an initial moisture level of 70% with the supplementation of nitrogen source (ammonium sulfate, urea, and sodium nitrate) for the fermented BSG has outperformed the initial moisture level of 50% and 60%. The average protein content of BSG fermented by R. oligosporus with an initial moisture level of 70% is nearly 1.7 times higher than unfermented BSG, at 30.6 mg/g on average. In another study by Ibarruri et al. [74], BSG incubated under SSF for 192 h significantly increased the protein content by up to 50% compared to the unfermented BSG. Throughout the fermentation process, Rhizopus sp. continuously produced protease, resulting in 6.5 times higher soluble protein, up to 47.4 mg/g. Astoundingly, the nitrogen requirements were lower, 3.3% compared to another research conducted by FazeliNejad et al. [75] which used 3.9% to get similar results. Rhizopus genus has been broadly used in the food industry especially for the tempeh production and rice wine production. R. oligosporus secretes various enzymes such as xylanases, pectinases, amylases, and cellulases, enabling it to effectively infiltrate the lignocellulosic structure. These enzymes play a crucial role in substrate colonization, leading to increased fungal biomass protein and improved substrate protein concentration during SSF [76].
Sousa et al. [77] conducted a study to find out the effect of SSF on BSG, exhausted grape marc, and exhausted olive pomace using three different fungi A. niger, A. ibericus, and A. uvarum. Although BSG has the highest initial protein content among the other agro-food waste, the increase in protein content was the lowest after the fermentation. BSG fermented with A. ibericus has the greatest increase in protein content, at 38.5%, which resulted in 277 mg/g. A study conducted by Adewale Ogunjobi et al. [78] used SSF for a fermentation of 35 days with A. oryzae which was the fungal strain isolated from the BSG itself. The results indicated that the protein content has reached its highest concentration of 284 mg/g, from the initial value of 183.3 mg/g. Contrarily, the crude fiber content decreased throughout the fermentation process. The ability of A. oryzae to digest crude fiber to build up their biomass could be responsible for these changes in the nutritional composition, which resulted in an increase in the concentration of protein content [79]. In order to disrupt the lignocellulosic rigid structure of BSG, Zeng et al. [55] implemented the aid of ultrasonic pre-treatment prior to the SSF Bacillus velezensis. The results demonstrated that the pre-treated BSG has a higher protein value of 315.9 mg/g compared with untreated BSG of 296.6 mg/g after a 6 days fermentation. Ultrasonic pre-treatment applies high pressure and intense shear forces, utilizing the cavitation effect to generate a multitude of pores on the surface of BSG. This pore formation significantly enhances the porosity and expands the surface area of BSG, allowing for improved contact and utilization by microorganisms. Furthermore, ultrasonic pre-treatment holds the potential to reduce cellulase secretion. This can be showed from Zeng et al. [55], the degradation of cellulose and hemicellulose were reduced, while a higher number of polysaccharides were produced. The implosion of air bubbles within the substrate due to ultrasound irradiation assists in defracting lignocelluloses and lysing cell walls and membranes. Consequently, the reduced requirement for hydrolyzing the lignocellulosic structure leads to a decline in cellulase secretion [80]. These findings suggest that nutrient release in pre-treated BSG was beneficial for microorganism uptake and utilization.
As the protein content increased, the number of amino acids in fermented BSG was found to be increased as well. This could be attributed to the enzyme hydrolyzation produced by the microorganism such as peptidases which converts long peptide chain in BSG into simple amino acids [81]. According to Tan et al. [81], the amount of amino acid after the SSF of BSG using Bacillus subtilis WX-17 has significantly increased. Proline was observed to have the most significant increase of 3.5-fold, among the amino acids. This resulted from the starch being broken down into glucose by microorganisms during the tricarboxylic acid (TCA) cycle, which was then converted into energy. Glucogenic amino acids are created all along the cycle. In this case, proline, which was derived from glutamate, was being produced the most. After 2 days of fermentation, the amount of total amino acids increased from 0.9 mg/g in unfermented BSG to 1.9 mg/g in fermented BSG.
Ibarruri et al. [74] composed an amino acid profile for the fermented BSG. The results showed that the amount of practically all amino acids increases significantly in the fermented BSG, even though the overall profile does not greatly differ from each other. Likewise, the total essential amino acid content of the fermented BSG is 1.5 times greater than that of the unfermented BSG, from 83.9 to 135.8 mg/g. Similarly, Cooray and Chen [34] were able to observe an increase in amino acid content in BSG after 3 days of fermentation, the total amino acid concentrations in the fermented BSG increased from 3.8 mg/g in the unfermented BSG to 7.8 mg/g. The greatest changes were seen in threonine (sixfold) and serine (3.6-fold). During the TCA cycle, serine is produced from a 3-step pathway initiating with 3-phosphoglycerate, converting into 3-phosphohydroxypyruvate, then phosphoserine. As the process is reversible, the synthesis of threonine was aided as well [82].
Apparently, symbiotic fermentation was one of the methods to further improve the fermentation yield. Zeng et al. [52] used B. velezensis K8 and Levilactobacillus brevis LZB2 fungal strain as the microorganism for the SSF of BSG. Among the total protein content in BSG, glutamic acid represents the second highest proportion and can be converted into γ-aminobutyric acid to enhance its nutritional value [83]. L. brevis plays a crucial role in this conversion process by exhibiting significant glutamate decarboxylase activity, which catalyzes the synthesis of γ-aminobutyric acid from glutamate [84]. However, L. brevis has limited ability to efficiently utilize polysaccharide biomass and proteins [85]. Therefore, B. velezensis K8 was utilized, as previous research by Zeng et al. [52] demonstrated its capacity to enhance protein and soluble sugar content. The total amino acid content increased significantly, with the maximum values reaching 55.6 mg/g (ratio of 5:5) and 51.2 mg/g (ratio of 5:5.5), an increase of 52.2% and 43.5%, respectively. Table 1 summarized the enhancement of protein and amino acid yield from BSG at different SSF conditions with various types of microorganism.
The degree of hydrolysis (DH) is a critical parameter to consider in SSF, and it is influenced by time and temperature. Higher temperatures result in higher DH for shorter fermentation times, while lower temperatures lead to higher DH for longer fermentation times. Even a brief fermentation process improves amino acid availability in BSG protein compared to unfermented BSG [74].
Nitrogen supplements are crucial for regulating microorganism growth during fermentation. Inadequate control or substrate depletion can cause sporulation, reduced growth, or cell death [86]. Lareo et al. [87] and Suhet and Fioreze [88] observed sporulation when glucose metabolism declines after glucose depletion. Maintaining optimal nitrogen supplement concentration preserves soluble sugars. Ammonium sulfate is suitable for enhancing protein content in BSG [72].
Symbiotic fermentation faces challenges due to operational heterogeneity. B. velezensis fermentation effectively enhance the soluble sugar and protein content in BSG. However, Zeng et al. [52] demonstrated a slight decline in soluble protein content during symbiotic fermentation, attributed to increased presence of L. brevis consuming proteins and amino acids. In contrast, Tsai et al. [89] demonstrated that incorporating B. velezensis compensated for lower enzyme production by L. brevis during soybean meal fermentation, resulting in enhanced enzyme activities and elevated soluble peptide levels. Optimizing parameters in symbiotic fermentation is crucial for desired outcomes.
SSF can greatly enhance the protein and amino acid content in BSG, allowing it to be utilized more effectively. It has been reported that fermented agro-industrial waste can serve as a partial or complete substitute for animal feed. The primary challenge associated with conventional animal feed lies in its expensive production cost. As a result, industries are increasingly turning to non-traditional plant protein sources as viable alternatives. However, the elevated fiber content and insufficient protein levels impose limitations on the utilization of unconventional dietary feed ingredients. The utilization of fermentation as a means to improve the quality of these feedstuffs is a viable strategy. Through SSF, feed utilization can be enhanced by reducing fiber content, increasing crude protein and lipid levels, improving vitamin availability, optimizing protein solubility and amino acid profiles, and enhancing the palatability of the feedstuffs [90,91,92]. SSF typically involves the utilization of beneficial bacteria, yeast, and fungi. Probiotics offer a range of advantages, including enhanced growth performance, increased feed value, enzymatic assistance in digestion, inhibition of adherence, and colonization by pathogenic microorganisms in the gastrointestinal tract, promotion of hematological parameters, and reinforcement of immune response [93,94,95]. For instance, the introduction of Bacillus sp. for substrate fermentation and subsequent inclusion in carp diets has shown significant enhancements in growth performance, survival rate, and feed conversion ratio [96]. Nonetheless, while SSF improved the protein content in BSG, the microorganisms involved in the process produce proteases that can break down long peptide chains, potentially resulting in the formation of bioactive peptides. Bioactive peptides are short amino acid chains with low molecular weights, typically composed of 2 to 20 residues. Numerous studies have indicated that bioactive peptides exhibit therapeutic and regulatory activities in organisms, including antioxidant, antihypertensive, antitumor, antimicrobial, antithrombotic, antidiabetic, and atherosclerosis prevention effects [97]. Consequently, the application of BSG after SSF is not limited solely to the food industry but also holds potential benefits in the nutraceutical industry.
Phenolic Contents
The two main components of BSG are lignocellulose and hemicellulose with the former component predominantly made up of arabinoxylan, which is a combination of xylose residues that have been -(1,4)-linked with arabinose residues and cross-linked with ferulic or diferulic acids [10]. It may be important to break down BSG components to effectively utilize value-added components such as bound phenolic compounds but it involves numerous enzymes [98]. The synthesis of microbial hydrolytic enzymes during SSF could increase the phenolic compound in BSG. More precisely, the matrix structure of BSG is softened, cell walls are broken down, and bound bioactive chemicals are released with the aid of enzymes like α-amylase and β-glucosidase [71]. There are various cases when SSF has successfully increased the number of bioactive components in BSG, specifically the concentration of polyphenols and antioxidant properties [53, 54, 56, 58, 74, 99, 100].
For instance, BSG from different breweries fermented with five different filamentous fungi strains R. oryzae, A. terreus, A. niger, A. awamori, and A. oryzae showed an increase in terms of total phenolic content (TPC) and the antioxidant activity against DPPH radicals [53]. Among the fungal strains, the outstanding readings were obtained from A. oryzae and A. terreus. Both microorganisms depicted results that are 7 times higher for TPC, 8.2 mg gallic acid equivalents (GAE)/g and 5 times higher of antioxidant value, 15.1 mg Trolox equivalents (TE)/100 g as compared to unfermented BSG. Similar results were shown in the study by Tišma et al. [56]. After 14 days of fermentation using Trametes versicolor, the TPC has increased by 3.4-fold from 2.5 to 8.7 mg GAE/g. The total organic carbon (TOC) was evaluated as an indication of microbial growth. As expected, the total mass loss of BSG decreased from 35 to 10.5%. Likewise, results from Ibarruri et al. [74] showed that the TPC and antioxidant activity of BSG after fermentation is 9 times higher than the unfermented BSG from 0.2 to 2.0 mg GAE/g. The increment of TPC could be attributed to the release of β-glucosidase in the fungi which hydrolyzes β-glycosidic bonds and generate free aglycones. A study by Goh and Ken [58] used 8 different filamentous fungal strains for the fermentation of BSG. All the fermented BSG has shown significant improvement in the TPC. Among the strains, A. oryzae M-1 increased from 1.4 mg GAE/g in unfermented BSG to 9.5 mg GAE/g.
Compared with other agro-industrial wastes, BSG has a lower concentration of phenolic compounds due to the fact that most of them are attached to the hemicellulose fraction and lignin [99]. Studies by Ong and Ken [100] and Leite et al. [101] showed that after the SSF, the increment of TPC value in BSG was significantly lower compared with other agro-industrial wastes such as okara, exhausted mixed white and red grape marc, vine shoots trimming, grape stalks, crude olive pomace, and exhausted olive pomace. The low increment of TPC in treated BSG was relatively lesser due to the lack of nutrient accessibility for microorganisms as most of the phenolic compounds are linked to the polysaccharides. However, the SSF has proven to be effective by increasing the TPC for 2 to 13-folds, maximum extraction of 2.7 mg GAE/g and 11 mg GAE/g, respectively. In order to compensate for the minimal amount of phenolic compound, an aqueous extraction was conducted on a mixture of agro-industrial wastes which includes BSG prior to the SSF with A. niger. This method resulted in a 1.4-fold increase in TPC and a 1.5-fold increase in antioxidant activity, leading to values of 13.3 mg GAE/g and 1.5 mg TE/100 g, respectively [54].
Time and temperature have a significant impact on the increase in phenolic content in BSG. Longer fermentation time and higher temperatures result in higher phenolic contents. Microorganisms produce various enzymes during extended fermentation, which work together to enhance phenolic content [74]. β-Glucosidase plays a key role in increasing free phenolic compounds by hydrolyzing β-glucosidic bonds, releasing phenolics [102, 103]. Fungal enzymes also enhance antioxidant activity by releasing hydroxycinnamic acids from hemicellulose and lignin fractions [104].
Contrarily, Goh and Ken [58] suggest minimizing fermentation time to reduce undesirable microbial activity. Phenolic compounds may polymerize due to oxidative enzymes activated in response to stress caused by nutrient depletion from microbial contamination [105]. Autoclaving sterilization can be used to overcome this challenge. Leite et al. [54] found similar release of phenolic compounds in both sterilized and unsterilized substrates during SSF. However, unsterilized substrate experienced a significant decrease in enzyme production. Sterilization serves as a pre-treatment to facilitate nutrient access for desired microorganisms in solid substrates. The enhancement of phenolic contents from BSG at different SSF conditions with various types of microorganism is summarized in Table 2.
Soluble Sugars
As mentioned above, BSG is basically a by-product from the beer brewing process, whereby most of the fermentable sugars are extracted out. BSG primarily comprises the husk of the initial barley grain, obtained during wort preparation. Since the barley husk is composed of lignocellulosic material, BSG likely contains sugars that are polymerized into cellulose (glucose) and hemicellulose (mainly xylose and arabinose), which can be released through a hydrolysis process [106]. Lignocellulolytic enzymes produced by microorganisms during fermentation are required to convert the polysaccharides into monosaccharides which leads to the production of soluble sugars. Results from Tišma et al. [56] showed that during the fermentation of BSG with T. versicolor, a gradually decreased sucrose concentration with time was observed. As the sucrose was broken down by the enzymes, the fructose concentration increased 30-fold from 0.1 to 2.4 mg/g. However, the glucose concentration did not increase due to the metabolism of the fungi. A positive outcome could be expected when symbiotic fermentation is used. B. velezensis and L. brevis with a ratio of 5:5 significantly increased the total soluble sugar content in BSG after the fermentation with an improvement of 69.1%, 73.1 mg/g [52].
Among the available pre-treatment methods, dilute acid hydrolysis is widely recognized as an effective approach for solubilizing hemicellulose sugars. This method offers notable advantages, including high sugar yield, low production cost, and minimal acid concentration requirements [6]. Moreover, this method comes with high conventional heating methods which require extensive amounts of energy and are not environmentally friendly. Furthermore, it leads to the formation of numerous inhibitory compounds, such as furaldehydes, formaldehyde, aliphatic acids, vanillic acid, uronic acid, 4-hydroxybenzoic acid, cinnamaldehyde, and phenol which could potentially interfere with the growth of fermentative microorganisms during the fermentation process. Therefore, emerging pre-treatment methods such as non-ionizing radiation, ionizing radiation, pulsed-electric field, high pressure, and ultrasound are more encouraging [107]. Ultrasonic pre-treatment is one of the alternative methods to decrease the crystallinity of cellulose, increase the number of pores on the surface of BSG, and promote the interaction between enzymes and substrates in order to enhance accessibility [80]. A study conducted by Zeng et al. [55] utilized ultrasonic pre-treatment on BSG prior to the fermentation with B. velezensis. The contents of xylose, glucose, arabinose, cellobiose, and fructose were substantially increased after 4 days of fermentation, while the amounts of mannose were marginally increased as well. The total soluble sugar content has also increased by 172.9%, from 180.7 to 312.4 mg/g. These findings suggested that lignocellulose might be efficiently degraded by B. velezensis fermentation and converted into a range of monosaccharides or oligosaccharides. The most notable increase in fructose concentration may be attributable to microorganisms’ fructofuranosidase function, which encourages fructose conversion and release in BSG. According to this finding, ultrasonic treatment exposes the lignocellulosic rigid structure of BSG, which facilitates attachment and destruction by lignocellulolytic enzymes produced by microorganisms. Similar results were able to observe by Fernandes et al. [59], as the enzymatic pre-treated BSG was incorporated into the plant-based diets for European seabass. Due to the high xylanase and cellulase activity secreted by A. ibericus to disrupt the lignocellulosic structure of BSG, the amount of glucose, xylose, and arabinose in BSG has increased by an average of 197%, resulting in 18.9 mg/g. Pre-treatment steps and enzymatic hydrolysis have become a trend for researchers to address the difficulties of extracting nutritional-value content embedded beneath the rigid cellulosic structure of BSG.
Prolonged cultivation time does not necessarily increase soluble sugar content in BSG. When T. versicolor was grown on BSG, sucrose concentration decreased while fructose concentration significantly increased after 14 days [56]. T. versicolor utilized the soluble sugars, leading to sucrose hydrolysis and subsequent fructose utilization [108]. Similarly, Zeng et al. [55] reported a decrease in soluble sugar content after reaching its peak on the fourth day of fermentation.
Symbiotic fermentation is an effective approach to enhance soluble sugar release, but the synergistic activity between the two microorganisms could result in a negative outcome [52]. Hence, clearly defining research objectives and selecting appropriate microorganisms are crucial for desired outcomes. Pre-treatment techniques are also effective in increasing soluble sugar release in BSG. Feeding fish diets with pre-treated BSG has shown lower plasma glucose levels compared to untreated BSG diets [59]. Establishing definitive criteria for assessing BSG’s nutritional value is important for its certification as a beneficial dietary supplement. Soluble sugar from BSG at different SSF conditions with various types of microorganism is summarized in Table 3.
Applications of Nutritional Values from BSG
Proteins
Even without the enhancement of SSF, unfermented BSG is already used by aquaculture industry to improve the body weight of the fish [109]. By increasing the protein and essential amino acids in BSG, the incorporation of BSG in animal feeds or even human diets could improve the absorbability and digestibility in order to achieve weight gain in a shorter period of time. In a recent study conducted by Nazzaro et al. [110] examined the impact of partially incorporating brewery spent grain (BSG) into the diets of gilthead sea bream and rainbow trout. The study found that after a 30-day feeding trial and fecal collection, the digestibility of these fish ranged from 71 to 88%, similar to commercial fish diets. Furthermore, the incorporation of 20–30% BSG resulted in good protein, lipid, and amino acid digestibility, indicating its potential as a substitute for commercial aquaculture diets.
BSG contains a high amount of hordein, which is the most prevalent protein component trapped within its matrix. This protein is rich in essential (E), proline (P), leucine (L), valine (V), phenylalanine (F), and tyrosine (Y) residues, which are known to possess antioxidant properties. Food peptides’ specific bioactivity depends on their amino acid chain length, hydrophobicity, and molecular weight. Researchers are increasingly interested in using bioactive peptides derived from food proteins to combat chronic diseases and maintain good health by inhibiting enzymes such as angiotensin-converting enzyme (ACE) and dipeptidyl peptidase IV (DPP IV) [35]. In spontaneously hypertensive rats, the ingestion of a semi-pilot scale BSG protein hydrolysate led to notable hypotensive effects 6 h later. Peptides containing the amino acid isoleucine demonstrated greater in vitro bioactivity than those containing leucine. As a result, BSG peptides have been identified as potential naturally derived ingredients for managing type 2 diabetes and cardiovascular disease [111].
Phenolic Contents
Phenolic compounds that remain in BSG provides several benefits on human health such as antioxidant, anti-inflammatory, and anti-cancer properties [112,113,114]. According to reports, feeding ruminant diets high in phenolic compounds has positive effects on the welfare of the animals and may be a new method to produce milk and meat that have antioxidant characteristics [115, 116]. In addition to being used as a feed additive in animal nutrition, BSG can also be incorporated into human food products. The phenolic contents and antioxidants capacity in cookies and cereal-based snacks which were incorporated with BSG were tested [117, 118]. Incorporating 20% of BSG into cookies resulted in a significant increase in ferulic acid content, up to 3.5-fold higher compared to control cookies. Ferulic acid has potential health benefits, including preventing lipid hydroperoxide propagation into the gastrointestinal tract and mediating prebiotic modulation in gut microbiota. Additionally, by incorporating BSG into cereal-based snacks, there was a remarkable sevenfold increase observed in the phenolic content. The antioxidant effect of phenolic contents in BSG were investigated by McCarthy et al. [112] as well. A variety of oxidants including H2O2, 3 morpholinosydnonimine hydrochloride, 4-nitroquinoline oxide, and tert-butylhydroperoxide were used to induce oxidative DNA damage in the U937 cell line. Pre-treating U937 cells with BSG extracts containing ferulic acid significantly protected them against H2O2-induced DNA damage, indicating that BSG may be capable of offering protection against oxidant-induced DNA damage through Fe chelation.
Soluble Sugars
Microorganisms used in SSF converts the starch and cellulosic materials presents in BSG into soluble sugars. The soluble sugars can then serve as the primary substrate for a further round of fermentation in which ethanol-producing microbes will convert them into ethanol and PHA [35, 119]. Microorganisms metabolize D-xylose through an initial pathway that involves its conversion to D-xylulose in the ethanol production. Xylose isomerase is typically utilized by bacteria, while yeasts and mycelial fungi employ a two-step oxidation–reduction pathway. The resulting D-xylulose is then transformed into D-xylulose-5-phosphate and enters the pentose phosphate pathway. Within the pathway, ribulosephosphate-3-epimerase, transaldolase, and transketolase facilitate non-oxidative rearrangements of xylulose-5-phosphate, leading to the formation of glyceraldehyde-3-phosphate and fructose-6-phosphate. These compounds can undergo fermentation reactions in the Embden–Meyerhoff–Parnas pathway, ultimately resulting in the production of ethanol [120]. The synthesis of PHA involves three pathways, each associated with the production of precursors in different metabolic pathways. The first pathway is mediated by β-ketothiolase (encoded by phaA), which catalyzes the condensation of two acetyl-CoA molecules, resulting in the formation of acetoacetyl-CoA. The second step involves the reduction of acetoacetyl-CoA by NADPH-dependent acetoacetyl-CoA dehydrogenase. Finally, poly [3-hydroxybutyrate] [P(3HB)] synthase polymerizes (R)-3-hydroxybutyryl-CoA monomers to generate P(3HB) [121]. The second pathway is the β-oxidation of fatty acids. Fatty acids are degraded through β-oxidation, a process that involves the sequential removal of 2 carbon atoms in the form of acetyl-CoA. During each cycle of this degradation, the acyl-CoA molecule generated is oxidized to form 3-keto-acyl-CoA, utilizing (S)-3-hydroxyacyl-CoA as an intermediate. As a result, the fatty acid molecule is shortened by n-2 carbon atoms, providing the option for it to renter the degradation cycle or enter alternative pathways. However, the intermediate (S)-3-hydroxyacyl-CoA cannot be directly used for PHA biosynthesis. To overcome this, the enzyme (R)-enoyl-CoA hydratase is required to convert (S)-3-hydroxyacyl-CoA into (R)-3-hydroxyacyl-CoA thioester, which acts as a substrate for PHA polymerase. The third pathway is the de novo synthesis of fatty acids. In the initial step, acetyl-CoA is carboxylated to form malonyl-CoA, which is then transferred to the acyl carrier protein to generate malonyl-ACP. Subsequently, the required residues are added to complete the synthesis of the final fatty acid molecule. The enzyme (R)-3-hydroxyacyl-ACP-CoA transferase (PhaG) plays a key role in connecting the de novo synthesis of fatty acids with the synthesis of medium-chain-length compounds. PhaG facilitates the conversion of (R)-3-hydroxyacyl-ACP to (R)-3-hydroxyacyl-CoA, leading to the synthesis of PHA [122]. For instance, a combination of sequential organosolv process and enzymatic saccharification was used to generate BSG hydrolysate containing approximately 72 g/L of monomeric sugars. Rhodosporidium toruloides was then cultivated using this hydrolysate, resulting in a maximum dry weight biomass of 18.44 ± 0.96 g/L with a lipid content of 56.45 ± 0.76% [123]. In another study, BSG was utilized as a substrate for the cultivation of three fungal strains (A. niger, T. aurantiacus, and Trichoderma ressei) using SSF, which yielded high xylanase activities and cellulase side activity. The feasibility of PHA production from BSG was also demonstrated, with Cupriavidus necator producing a maximum of 9.0 ± 0.44 mg PHA per gram of BSG [31]. In Rojas-Chamorro et al. [6] study, both acid and enzymatic hydrolysis techniques were employed to recover 92% of the total sugars found in BSG. The resulting sugar hydrolysate was then fermented by Escherichia coli SL100, producing an ethanol yield of 17.9 g per 100 g of raw BSG.
Applications of BSG in the Industrial Scale
Among the various applications of brewer’s spent grain (BSG) that have achieved commercial success on a large scale, its incorporation into human diets and animal feed stands out prominently. A company known as “Saving Grains” has capitalized on BSG by transforming it into all-purpose grain flour renowned for its excellent protein and fiber content. This flour serves as a key ingredient for developing value-added products, including granola, biscuits, cookies, pasta, and chapati [124]. Additionally, a pet food company based in Portland leverages the locally available BSG from breweries to produce nourishing dog biscuits [125]. Apart from its applications in food, BSG can serve as a substrate for cultivating microorganisms. “Eclo,” a mushroom production company, utilizes BSG as a growth medium for mycelium. They annually utilize 360 tons of BSG to cultivate exotic mushrooms such as shiitake, eryngii, nameko, maitake, and pompom [126]. Furthermore, in 2019, a DB Export campaign awarded a $40,000 prototype fund to an innovative concept utilizing BSG to produce PHA, a material that can be transformed into bioplastic pellets [127]. Since this idea is relatively new to the industry, it may take a few more years to fully develop and scale up this business.
Future Perspectives
There has been a growing research focus on BSG in recent years, driven by its potential applications in the field of biotechnology. Numerous articles have been published concerning how BSG can be used for industrial purposes and how it can mitigate environmental issues. BSG incorporates various nutrients providing ideal conditions for microorganism proliferation. As the microorganisms have the capability to utilize BSG, the approach of using the traditional fermentation method, SSF has been proven to be reliable in upscaling the extraction yield of nutritional value from BSG. Significant improvement in terms of protein content can be observed in BSG as the concept of symbiotic fermentation and the addition of nitrogen supplements during the fermentation. Moreover, BSG alone has a lower concentration of phenolic compounds since most of them are attached to the lignocellulosic structure. Therefore, an optimum mixture of the agro-industrial waste substrate is recommended to be used in SSF to enhance the extraction of the phenolic compounds. In fact, SSF reduces operational and production expenses while also improving solid waste management and reducing environmental pollution as it valorises agro-industrial waste. The fundamental concern of SSF is that the efficiency of hydrolyzation of lignocellulosic components is too slow, making it impossible to use this method as a potential process at the industrial level.
Lignocellulose is a complex biopolymer composed of cellulose, hemicellulose, and lignin. However, the recalcitrance of lignocellulosic components to hydrolysis and the complexity of the BSG matrix make it difficult to effectively utilize the substrate. The use of non-chemical pre-treatment methods on BSG, such as enzymatic hydrolysis and ultrasonic-assisted procedures, should be emphasized in future studies in order to hasten the process and improve SSF performance. Combination approaches could be developed and established for SSF to progress from the laboratory scale to the industrial scale. The type of reactor used can significantly affect the performance of SSF, particularly the rate of substrate utilization, the yield of the desired product, and the quality of the final product. The optimization of the bioreactor design is critical to ensure that the process is efficient in good mass and heat transfer, cost-effective, and scalable to an industrial level [48, 128] . The utilization of BSG offers the potential for generating a range of high-value products. It is crucial for industrial stakeholders to embrace this prospect as part of their comprehensive strategy to tackle waste challenges in the food and agro-industry. By embracing environmentally friendly practices and harnessing BSG to produce enzymes, secondary metabolites, chemicals, and biofuels, they can successfully address the environmental concerns associated with discarding BSG into landfills.
Conclusion
The utilization of BSG as a substrate in SSF bioprocess offers a promising approach to enhance the nutritional value of BSG for industrial applications. This review presents a comprehensive overview of recent progress and applications concerning various value-added compounds, including proteins, phenolic compounds, and soluble sugars found in BSG. It examines the efficacy of strategies such as nitrogen supplementation, symbiotic fermentation, agro-industrial waste mixture, and pre-treatments in enhancing the performance of SSF and achieving higher nutritional value in BSG. Nevertheless, there are still considerable challenges to overcome in order to enhance the value of BSG and increase the commercial competitiveness of the SSF bioprocess. These challenges include the need to optimize bioreactor design specifically for the utilization of BSG in SSF and to implement BSG at an industrial scale. In recent years, the rising number of discussions and studies addressing the improvement of nutritional added value in BSG via SSF indicates the commercial value of BSG and its potential for growth.
Abbreviations
- BSG:
-
Brewer’s spent grain
- HMF:
-
5-Hydroxymethyl furfural
- SmF:
-
Submerged fermentation
- SSF:
-
Solid-state fermentation
- TCA:
-
Tricarboxylic acid
- DH:
-
Degree of hydrolysis
- TPC:
-
Total phenolic content
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- TOC:
-
Total organic carbon
- GAE:
-
Gallic acid equivalents
- TE:
-
Trolox equivalents
References
Guido, L., & Moreira, M. (2017). Techniques for extraction of brewer’s spent grain polyphenols: A review. Food and Bioprocess Technology, 10(7), 1192–1209.
Nasir, N. (2019). Hydrothermal liquefaction of lignocellulosic biomass. PhD thesis, University of Sheffield.
Verni, M, Pontonio, E, Krona, A, Jacob, S, Pinto, D, Rinaldi, F, Verardo, V, Díaz-de-Cerio, E, Coda, R & Rizzello, C. (2020). Bioprocessing of brewers’ spent grain enhances its antioxidant activity: characterization of phenolic compounds and bioactive peptides. Frontiers in Microbiology, 11.
Kavalopoulos, M., Stoumpou, V., Christofi, A., Mai, S., Barampouti, E. M., Moustakas, K., Malamis, D., & Loizidou, M. (2021). Sustainable valorisation pathways mitigating environmental pollution from brewers’ spent grains. Environmental Pollution, 270, 116069.
Martín, D. S., Orive, M., Iñarra, B., Castelo, J., Estévez, A., Nazzaro, J., Iloro, I., Elortza, F., & Zufía, J. (2020). Brewers’ spent yeast and grain protein hydrolysates as second-generation feedstuff for Aquaculture feed. Waste and Biomass Valorization, 11(10), 5307–5320.
Rojas-Chamorro, J. A., Cara, C., Romero, I., Castro, E., Romero-García, J. M., & Mussatto, S. I. (2018). Ethanol production from brewers’ spent grain pretreated by dilute phosphoric acid. Energy & Fuels, 32(4), 5226–5233.
Aboltins, A., & Palabinskis, J. (2015). Research in brewer’s spent grain drying process. Engineering for Rural Development, 5, 230–235.
Teixeira, M. R., Guarda, E. C., Freitas, E. F., Galinha, C. F., Duque, A. F., & Reis, M. A. (2020). Valorization of raw brewers’ spent grain through the production of volatile fatty acids. New Biotechnology, 57, 4–10.
Buffington, J. (2014). The economic potential of brewer’s spent grain (BSG) as a biomass feedstock. Advances in Chemical Engineering and Science, 4(3), 308–318.
Lynch, K., Steffen, E., & Arendt, E. (2016). Brewers’ spent grain: A review with an emphasis on food and health. Journal of the Institute of Brewing, 122(4), 553–568.
Farcas, A., Socaci, S., Mudura, E., Dulf, F., Vodnar, D. C., Tofana, M., & Salanță, L. C. (2017). Exploitation of Brewing Industry Wastes to Produce Functional Ingredients. InTech EBooks. https://doi.org/10.5772/intechopen.69231
Cooray, S, Lee, J & Chen, W. (2017). Evaluation of brewers’ spent grain as a novel media for yeast growth. AMB Express, 7(1). https://doi.org/10.1186/s13568-017-0414-1
Xiros, C., & Christakopoulos, P. (2012). Biotechnological potential of brewers spent grain and its recent applications. Waste and Biomass Valorization, 3(2), 213–232.
Coronado, M., Montero, G., Montes, D., Valdez-Salas, B., Ayala, J., García, C., Carrillo, M., León, J., & Moreno, A. (2020). Physicochemical characterization and SEM-EDX analysis of brewer’s spent grain from the craft brewery industry. Sustainability, 12(18), 7744.
Jackowski, M., Niedźwiecki, Ł, Jagiełło, K., Uchańska, O., & Trusek, A. (2020). Brewer’s spent grains—valuable beer industry by-product. Biomolecules, 10(12), 1669.
Su, Y., et al. (2015). Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: Characterization of its major components. Food and Bioproducts Processing, 94, 322–330.
Marta, K., et al. (2020). Evaluation of ultrasound pretreatment for enhanced anaerobic digestion of Sida hermaphrodita. BioEnergy Research, 13(3), 824–832.
Beldman, G., Hennekam, J., & Voragen, A. G. (1987). Enzymatic hydrolysis of beer brewers’ spent grain and the influence of pretreatments. Biotechnology and Bioengineering, 30(5), 668–671.
Taylor, M. S., Alabdrabalameer, H. A., & Skoulou, V. (2019). Choosing physical, physicochemical and chemical methods of pre-treating lignocellulosic wastes to repurpose into solid fuels. Sustainability, 11(13), 3604.
Menon, V., & Rao, M. (2012). Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Progress in Energy and Combustion Science, 38(4), 522–550.
Zhengdao, Y., Bailiang, Z., Yu, F., Xu, G., & Song, A. (2012). A real explosion: The requirement of steam explosion pretreatment. Bioresource Technology, 121, 335–341.
Zhuang, X., Wang, W., Yu, Q., Qi, W., Wang, Q., Tan, X., Zhou, G., & Yuan, Z. (2016). Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresource Technology, 199, 68–75.
Zheng, Y., Lin, H., & Tsao, G. T. (1998). Pretreatment for cellulose hydrolysis by carbon dioxide explosion. Biotechnology Progress, 14(6), 890–896.
Chen, S., Zhang, X., Singh, D., Yu, H., & Yang, X. (2010). Biological pretreatment of lignocellulosics: Potential, progress and challenges. Biofuels, 1(1), 177–199.
Kumar, V., Yadav, S., Kumar, J., & Ahluwalia, V. (2020). A critical review on current strategies and trends employed for removal of inhibitors and toxic materials generated during biomass pretreatment. Bioresource Technology, 299, 122633.
Mussatto, S. (2004). Alternatives for detoxification of diluted-acid lignocellulosic hydrolyzates for use in fermentative processes: A review. Bioresource Technology, 93(1), 1–10.
Harmsen, P., Huijgen, W. J. J., Bermudez, L., & Bakker, R. (2010). Literature review of physical and chemical pretreatment processes for lignocellulosic biomass. Wageningen UR-Food & Biobased Research. No. 1184. https://edepot.wur.nl/150289
Zabed, H. M., Akter, S., Yun, J., Zhang, G., Awad, F. N., Qi, X., & Sahu, J. N. (2019). Recent advances in biological pretreatment of microalgae and lignocellulosic biomass for biofuel production. Renewable & Sustainable Energy Reviews, 105, 105–128.
Masran, R., Zanirun, Z., Shirosaki, Y., Ibrahim, M. N. M., Yee, P. L., & Abd-Aziz, S. (2016). Harnessing the potential of ligninolytic enzymes for lignocellulosic biomass pretreatment. Applied Microbiology and Biotechnology, 100(12), 5231–5246.
Rezania, S., Oryani, B., Park, J., Talaiekhozani, A., Sabbagh, F., Hashemi, B., Rupani, P. F., & Mohammadi, A. (2020). Different pretreatment technologies of lignocellulosic biomass for bioethanol production: An overview. Energy, 199, 117457.
Turet, J. L., Martínez-Avila, O., Serrano, E. S., Corchado-Lopo, C., Llenas, L., Salas, S. F. R., & Gea, T. (2020). Brewer’s spent grain biotransformation to produce lignocellulolytic enzymes and polyhydroxyalkanoates in a two-stage valorization scheme. Biomass Conversion and Biorefinery, 12(9), 3921–3932.
Guo, H., Wu, S., Tian, Y., Zhang, J., & Liu, H. (2021). Application of machine learning methods for the prediction of organic solid waste treatment and recycling processes: A review. Bioresource Technology, 319, 124114.
Chaitanya Reddy, C., Khilji, I., Gupta, A., Bhuyar, P., Mahmood, S., Saeed AL-Japairai, K., & Chua, G. (2021). Valorization of keratin waste biomass and its potential applications. Journal of Water Process Engineering, 40, 101707.
Cooray, S., & Chen, W. (2018). Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value. Journal of Functional Foods, 42, 85–94.
Chetrariu, A., & Dabija, A. (2020). Brewer’s spent grains: Possibilities of valorization, a review. Applied Sciences, 10(16), 5619.
Subramaniyam, R. S., & Vimala, R. (2012). Solid state and submerged fermentation for the production of bioactive substances: a comparative study. International Journal of Science and Nature, 3(3), 480–486.
Soccol, C. R., et al. (2017). Recent developments and innovations in solid state fermentation. Biotechnology Research and Innovation, 1(1), 52–71.
Doriya, K. et al. (2016). Solid-state fermentation vs submerged fermentation for the production of L-asparaginase. Marine Enzymes Biotechnology: Production and Industrial Applications, Part I - Production of Enzymes, 115–135.
Hashemi, M., et al. (2011). The potential of Brewer’s spent grain to improve the production of α-amylase by bacillus sp. KR-8104 in submerged fermentation system. New Biotechnology, 28(2), 165–172.
Angel, S. J., Kavitha, C., Vidyadharani, G., Roy, P., & Dhandapani, R. (2012). Isolation of inulinase producing bacteria from sugarcane soil. International Journal of Applied Biology and Pharmaceutical Technology.
Zherebtsov, N. A., Shelamova, S. A., & Abramova, I. N. (2002). Biosynthesis of inulinases by Bacillus bacteria. Applied Biochemistry and Microbiology, 38, 544–548.
Singh, R. B., Chauhan, K., & Kennedy, J. F. (2017). A panorama of bacterial inulinases: Production, purification, characterization and industrial applications. International Journal of Biological Macromolecules, 96, 312–322.
Brierley, M. R., & Steel, R. (1959). Agitation-aeration in submerged fermentation. Applied Microbiology, 7(1), 57–61.
Weuster-Botz, D., Hekmat, D., Puskeiler, R., & Franco-Lara, E. (2006). Enabling technologies: fermentation and downstream processing. In Springer eBooks, 205–247.
Olukomaiya, O. O., Fernando, C., Mereddy, R., Li, X., & Sultanbawa, Y. (2019). Solid-state fermented plant protein sources in the diets of broiler chickens: A review. Animal Nutrition, 5(4), 319–330.
Mitri, S., Salameh, S., Khelfa, A., Leonard, E., Maroun, R., Louka, N., & Koubaa, M. (2022). Valorization of brewers’ spent grains: Pretreatments and fermentation, a review. Fermentation, 8(2), 50.
Šelo, G., Planinić, M., Tišma, M., Tomas, S., Koceva Komlenić, D., & Bucić-Kojić, A. (2021). A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods, 10(5), 927.
Webb, C. (2017). Design aspects of solid-state fermentation as applied to microbial bioprocessing. Journal of Applied Biotechnology & Bioengineering, 4(1).
Sim, T., & Oh, J. C. S. (1990). Spent brewery grains as substrate for the production of cellulases by Trichoderma reesei QM9414. Journal of Industrial Microbiology, 5(2–3), 153–158.
Onyimba, I. A., Ogbonna, C. I. C., Akueshi, C. O., & Chukwu, C. O. (2009). Changes in the nutrient composition of brewery spent grain subjected to solid state natural fermentation. Nigerian Journal of Biotechnology, 20(1), 55–60.
Iyayi, E. A., & Aderolu, Z. A. (2003). Enhancement of the feeding value of some agro-industrial by-products for laying hens after their solid state fermentation with Trichoderma viride. African Journal of Biotechnology, 3(3), 182–185.
Zeng, J., Sheng, F., Hu, X., Huang, Z., Tian, X., & Wu, Z. (2022). Nutrition promotion of brewer’s spent grain by symbiotic fermentation adding Bacillus velezensis and Levilactobacillus brevis. Food Bioscience, 49, 101941.
da Costa Maia, I., dos Thomaz D’Almeida, S. C., Guimarães Freire, D., d’Avila Costa Cavalcanti, E., Cameron, L., Furtado Dias, J., & Simões Larraz Ferreira, M. (2020). Effect of solid-state fermentation over the release of phenolic compounds from brewer’s spent grain revealed by UPLC-MSE. LWT, 133, 110136.
Leite, P., Belo, I., & Salgado, J. (2021). Co-management of agro-industrial wastes by solid-state fermentation for the production of bioactive compounds. Industrial Crops and Products, 172, 113990.
Zeng, J., Huang, W., Tian, X., Hu, X., & Wu, Z. (2021). Brewer’s spent grain fermentation improves its soluble sugar and protein as well as enzymatic activities using Bacillus velezensis. Process Biochemistry, 111, 12–20.
Tišma, M., Jurić, A., Bucić-Kojić, A., Panjičko, M., & Planinić, M. (2018). Biovalorization of brewers’ spent grain for the production of laccase and polyphenols. Journal of the Institute of Brewing, 124(2), 182–186.
Wang, X., Xu, Y., Teo, S. Q., Heng, C. W., Lee, D. P. S., Gan, A. X., & Kim, J. E. (2023). Impact of solid-state fermented Brewer’s spent grains incorporation in biscuits on nutritional, physical and sensorial properties. LWT, 182, 114840.
Goh, L. L., & Ken, C. L. L. (2021). Biovalorisation of brewer’s spent grain (BSG) and sensory evaluation of BSG bread. Agriculture and Food Chemistry. https://doi.org/10.26434/chemrxiv.14491842.v1
Fernandes, H., Castro, C., Salgado, J., Filipe, D., Moyano, F., Ferreira, P., Oliva-Teles, A., Belo, I., & Peres, H. (2022). Application of fermented brewer’s spent grain extract in plant-based diets for European seabass juveniles. Aquaculture, 552, 738013.
Verduzco-Oliva, R., & Gutiérrez-Uribe, J. A. (2020). Beyond enzyme production: Solid state fermentation (SSF) as an alternative approach to produce antioxidant polysaccharides. Sustainability, 12(2), 495.
Dey, T. B., Chakraborty, S., Jain, K. K., Sharma, A., & Kuhad, R. C. (2016). Antioxidant phenolics and their microbial production by submerged and solid-state fermentation process: A review. Trends in Food Science and Technology, 53, 60–74.
Gupta, V. K., Kubicek, C. P., Berrin, J., Wilson, D., Couturier, M., Berlin, A., Filho, E. X. F., & Ezeji, T. C. (2016). Fungal enzymes for bio-products from sustainable and waste biomass. Trends in Biochemical Sciences, 41(7), 633–645.
Nigam, P. S. N., & Pandey, A. (2009). Solid-state fermentation technology for bioconversion of biomass and agricultural residues. In Springer eBooks, 197–221.
Pandey, A. (1991). Aspects of fermenter design for solid-state fermentations. Process Biochemistry, 26(6), 355–361.
Alves, L., Coelho, E. A., Romaní, A., & Domingues, L. (2019). Intensifying ethanol production from brewer’s spent grain waste: Use of whole slurry at high solid loadings. New Biotechnology, 53, 1–8.
De Castro, A. M., Castilho, L. R., & Freire, D. M. G. (2015). Performance of a fixed-bed solid-state fermentation bioreactor with forced aeration for the production of hydrolases by Aspergillus awamori. Biochemical Engineering Journal, 93, 303–308.
Ashok, A., Doriya, K., Rao, D. V., & Kumar, D. S. (2017). Design of solid state bioreactor for industrial applications: An overview to conventional bioreactors. Biocatalysis and Agricultural Biotechnology, 9, 11–18.
Moteshafi, H., Mousavi, S. M., & Hashemi, M. (2019). Aeration challenge in high BSG suspended fermentation: Impact of stirred-tank bioreactor scale. Biomass & Bioenergy, 130, 105386.
Zhao, L., Sun, G., Jiang, J., Chen, L., & Huang, J. (2021). Role of microbubbles coupling fibrous-bed bioreactor in butyric acid production by Clostridium tyrobutyricum using Brewer’s spent grain as feedstock. Biochemical Engineering Journal, 172, 108051.
Raghavarao, K., Ranganathan, T. V., & Karanth, N. G. (2003). Some engineering aspects of solid-state fermentation. Biochemical Engineering Journal, 13(2–3), 127–135.
Canoy Postigo, L., Jacobo-Velázquez, D., Guajardo-Flores, D., Garcia Amezquita, L., & García-Cayuela, T. (2021). Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: recent advances and its industrial feasibility. Food Bioscience, 41, 100926.
Canedo, M., de Paula, F., da Silva, F., & Vendruscolo, F. (2016). Protein enrichment of brewery spent grain from Rhizopus oligosporus by solid-state fermentation. Bioprocess and Biosystems Engineering, 39(7), 1105–1113.
Bisaria, R., Madan, M., & Vasudevan, P. (1997). Utilisation of agro-residues as animal feed through bioconversion. Bioresource Technology, 59(1), 5–8.
Ibarruri, J., Cebrián, M., & Hernández, I. (2019). Solid state fermentation of brewer’s spent grain using Rhizopus sp to enhance Nutritional value. Waste and Biomass Valorization, 10(12), 3687–3700.
FazeliNejad, S., Ferreira, J., Brandberg, T., Lennartsson, P., & Taherzadeh, M. (2016). Fungal protein and ethanol from lignocelluloses using Rhizopus pellets under simultaneous saccharification, filtration and fermentation (SSFF). Biofuel Research Journal, 3(1), 372–378.
Ibarruri, J., & Hernández, I. (2018). Rhizopus oryzae as fermentation agent in food derived sub-products. Waste and Biomass Valorization, 9(11), 2107–2115.
Sousa, D., Venâncio, A., Belo, I., & Salgado, J. (2018). Mediterranean agro-industrial wastes as valuable substrates for lignocellulolytic enzymes and protein production by solid-state fermentation. Journal of the Science of Food and Agriculture, 98(14), 5248–5256.
Ogunjobi, A. A., Mejeha, O. K., & Fagade, O. E. (2011). Protein enrichment of brewery spent grains using Aspergillus oryzae. AU Journal of Technology, 15(1), 53–56.
Duarte, L., Carvalheiro, F., Lopes, S., Neves, I., & Gírio, F. (2007). Yeast biomass production in brewery’s spent grains hemicellulosic hydrolyzate. Applied Biochemistry and Biotechnology, 148(1–3), 119–129.
Bundhoo, Z., & Mohee, R. (2018). Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrasonics Sonochemistry, 40, 298–313.
Tan, Y., Mok, W., Lee, J., Kim, J., & Chen, W. (2019). Solid state fermentation of brewers’ spent grains for improved nutritional profile using Bacillus subtilis WX-17. Fermentation, 5(3), 52.
Newsholme, P., Stenson, L., Sulvucci, M., Sumayao, R., & Krause, M. (2011). Amino acid metabolism. Comprehensive Biotechnology, 3–14. https://doi.org/10.1016/b978-0-444-64046-8.00002-1
Waters, D. L., Jacob, F., Titze, J., Arendt, E. K., & Zannini, E. (2012). Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. European Food Research and Technology, 235(5), 767–778.
Kim, J. K., Lee, M., Ji, G. E., Lee, Y., & Hwang, K. C. (2009). Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. International Journal of Food Microbiology, 130(1), 12–16.
Hasegawa, M., Yamane, D., Funato, K., Yoshida, A., & Sambongi, Y. (2018). Gamma-aminobutyric acid fermentation with date residue by a lactic acid bacterium, Lactobacillus brevis. Journal of Bioscience and Bioengineering, 125(3), 316–319.
De Lima, L. J. L. F. (2009). Modelo de inferência para a estimação da umidade do leito de um biorreator de fermentação no estado sólido. https://hdl.handle.net/1884/24023
Lareo, C., Sposito, A. P. B., Bossio, A. L., & Volpe, D. C. (2006). Characterization of growth and sporulation of Mucor bacilliformis in solid state fermentation on an inert support. Enzyme and Microbial Technology, 38(3–4), 391–399.
Suhet, M. I., & Fioreze, R. (2011). Produção de proteína unicelular a partir do resíduo da industrialização do abacaxi utilizando fermentação em estado semi-sólido. Revista Brasileira De Tecnologia Agroindustrial, 5(2).
Tsai, C., Lin, L. J., Wang, C., Tsai, C., Chang, S., & Lee, T. (2021). Assessment of intestinal immunity and permeability of broilers on partial replacement diets of two-stage fermented soybean meal by Bacillus velezensis and Lactobacillus brevis ATCC 367. Animals, 11(8), 2336.
Del Carmen Flores-Miranda, M., Luna-González, A., Cortés-Espinosa, D. V., Álvarez-Ruiz, P., Cortés-Jacinto, E., Valdez-González, F. J., & González-Ocampo, H. A. (2015). Effects of diets with fermented duckweed (Lemna sp.) on growth performance and gene expression in the Pacific white shrimp. Litopenaeus vannamei. Aquaculture International, 23(2), 547–561.
Zhang, D., Zhang, Y., Liu, B., Jiang, Y., Zhou, Q., Wang, J. J., Wang, H., Xie, J., & Kuang, Q. (2017). Effect of replacing fish meal with fermented mushroom bran hydrolysate on the growth, digestive enzyme activity, and antioxidant capacity of allogynogenetic crucian carp (Carassius auratus gibelio). Turkish Journal of Fisheries and Aquatic Sciences, 17(5), 1039–1048.
Jannathulla, R., Dayal, J. S., Vasanthakumar, D., Ambasankar, K., & Muralidhar, M. (2017). Effect of fermentation methods on amino acids, fiber fractions and anti nutritional factors in different plant protein sources and essential amino acid index for Penaeus (Litopenaeus) vannamei. Indian Journal of Fisheries, 64(2).
Van Doan, H., Hoseinifar, S. H., Dawood, M. A., Chitmanat, C., & Tayyamath, K. (2017). Effects of Cordyceps militaris spent mushroom substrate and Lactobacillus plantarum on mucosal, serum immunology and growth performance of Nile tilapia (Oreochromis niloticus). Fish & Shellfish Immunology, 70, 87–94.
Elsabagh, M., Mohamed, R. A., Moustafa, E. M., Hamza, A. M., Farrag, F., Decamp, O., Dawood, M. A., & Eltholth, M. (2018). Assessing the impact of Bacillus strains mixture probiotic on water quality, growth performance, blood profile and intestinal morphology of Nile tilapia. Oreochromis niloticus. Aquaculture Nutrition, 24(6), 1613–1622.
Dawood, M. A., Koshio, S., Ishikawa, M., Yokoyama, S., Dawood, M. A., Hossain, M. S., Nhu, T. H., Moss, A. S., Dossou, S., & Wei, H. (2017). Dietary supplementation of β-glucan improves growth performance, the innate immune response and stress resistance of red sea bream. Pagrus major. Aquaculture Nutrition, 23(1), 148–159.
Yanbo, W., & Zirong, X. (2006). Effect of probiotics for common carp (Cyprinus carpio) based on growth performance and digestive enzyme activities. Animal Feed Science and Technology, 127(3–4), 283–292.
Manfredini, P. C., Cavanhi, V. A. F., Costa, J. A. V., & Colla, L. M. (2021). Bioactive peptides and proteases: characteristics, applications and the simultaneous production in solid-state fermentation. Biocatalysis and Biotransformation, 39(5), 360–377.
Aliyu, S., & Bala, M. (2011). Brewer’s spent grain: A review of its potentials and applications. African Journal of Biotechnology, 10(3), 324–331.
Niemi, P., Faulds, C., Sibakov, J., Holopainen, U., Poutanen, K., & Buchert, J. (2012). Effect of a milling pre-treatment on the enzymatic hydrolysis of carbohydrates in brewer’s spent grain. Bioresource Technology, 116, 155–160.
Ong, A., & Ken, C. L. L. (2021). Synergistic Effect of a Mixed Culture in Solid-state Fermentation. Agriculture and Food Chemistry. https://doi.org/10.26434/chemrxiv.14483262.v1
Leite, P., Silva, C., Salgado, J., & Belo, I. (2019). Simultaneous production of lignocellulolytic enzymes and extraction of antioxidant compounds by solid-state fermentation of agro-industrial wastes. Industrial Crops and Products, 137, 315–322.
Georgetti, S. R., Vicentini, F. T. M. C., Yokoyama, C. Y., De Fátima Borin, M., Spadaro, A. C. C., & Fonseca, M. J. V. (2009). Enhanced in vitro and in vivo antioxidant activity and mobilization of free phenolic compounds of soybean flour fermented with different β-glucosidase-producing fungi. Journal of Applied Microbiology, 106(2), 459–466.
Rashid, N., Jamaluddin, A., Ghani, A., Razak, D., Jonit, J., Mansor, A., & Manan, M. (2018). Quantification of phenolic compounds changes by Aspergillus oryzae on rice bran fermentation. Food Research, 3(2), 133–137.
McCarthy, A. J., O’Callaghan, Y. C., Neugart, S., Piggott, C. O., Connolly, A., Jansen, M. A. K., Krumbein, A., Schreiner, M., FitzGerald, R. J., & O’Brien, N. M. (2013). The hydroxycinnamic acid content of barley and brewers’ spent grain (BSG) and the potential to incorporate phenolic extracts of BSG as antioxidants into fruit beverages. Food Chemistry, 141(3), 2567–2574.
Vattem, D. A., Deng, S., Labbe, R. G., & Shetty, K. J. (2004). Phenolic antioxidant mobilization in cranberry pomace by solid-state bioprocessing using food grade fungus Lentinus edodes and effect on antimicrobial activity against select food borne pathogens. Innovative Food Science and Emerging Technologies, 5(1), 81–91.
Mussatto, S. I., & Roberto, I. C. (2006). Chemical characterization and liberation of pentose sugars from brewer’s spent grain. Journal of Chemical Technology & Biotechnology, 81(3), 268–274.
Hassan, S. S., Williams, G. A., & Jaiswal, A. K. (2018). Emerging technologies for the pretreatment of lignocellulosic biomass. Bioresource Technology, 262, 310–318.
Tišma, M., Sudar, M., Vasić-Rački, Đ, & Zelić, B. (2009). Mathematical model for Trametes versicolor growth in submerged cultivation. Bioprocess and Biosystems Engineering, 33(6), 749–758.
Kaur, V. (2004). Incorporation of brewery waste in supplementary feed and its impact on growth in some carps. Bioresource Technology, 91(1), 101–104.
Nazzaro, J., Martin, D. S., Perez-Vendrell, A., Padrell, L., Iñarra, B., Orive, M., & Estévez, A. (2021). Apparent digestibility coefficients of brewer’s by-products used in feeds for rainbow trout (Oncorhynchus mykiss) and gilthead seabream (Sparus aurata). Aquaculture, 530, 735796.
Cermeño, M., Connolly, A., O’Keeffe, M. B., Flynn, C., Laohakunjit, N., Aluko, R. E., & FitzGerald, R. J. (2019). Identification of bioactive peptides from brewers’ spent grain and contribution of Leu/Ile to bioactive potency. Journal of Functional Foods, 60, 103455.
McCarthy, A. J., O’Callaghan, Y. C., Connolly, A., Piggott, C. O., FitzGerald, R. J., & O’Brien, N. M. (2012). Phenolic extracts of brewers’ spent grain (BSG) as functional ingredients – assessment of their DNA protective effect against oxidant-induced DNA single strand breaks in U937 cells. Food Chemistry, 134(2), 641–646.
He, K., Pauli, G. F., Zheng, B., Wang, H., Bai, N., Peng, T., Roller, M., & Zheng, Q. (2006). Cimicifuga species identification by high performance liquid chromatography–photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. Journal of Chromatography A, 1112(1–2), 241–254.
Hole, A. S., Grimmer, S., Jensen, M. S., & Sahlstrøm, S. (2012). Synergistic and suppressive effects of dietary phenolic acids and other phytochemicals from cereal extracts on nuclear factor kappa B activity. Food Chemistry, 133(3), 969–977.
Paraskevakis, N. (2015). Effects of dietary dried Greek oregano (Origanum vulgare ssp. hirtum) supplementation on blood and milk enzymatic antioxidant indices, on milk total antioxidant capacity and on productivity in goats. Animal Feed Science and Technology, 209, 90–97.
Castillo, C., et al. (2013). Effect of supplementation with antioxidants on the quality of bovine milk and meat production. The Scientific World Journal, 2013, 1–8.
Heredia-Sandoval, N. G., Del Carmen Granados-Nevárez, M., De La Barca, A. M. C., Vásquez-Lara, F., Malunga, L. N., Apea-Bah, F. B., Beta, T., & Islas-Rubio, A. R. (2020). Phenolic acids, antioxidant capacity, and estimated glycemic index of cookies added with brewer’s spent grain. Plant Foods for Human Nutrition, 75(1), 41–47.
Reis, S., & Abu-Ghannam, N. (2014). Antioxidant capacity, arabinoxylans content and in vitro glycaemic index of cereal-based snacks incorporated with brewer’s spent grain. Lebensmittel-Wissenschaft & Technologie, 55(1), 269–277.
Simpson, T. J., Scheel, R. A., Nomura, C. T., Ramarao, B. V., & Kumar, D. (2021). Production of polyhydroxybutyrate and polyhydroxybutyrate-co-MCL copolymers from brewer’s spent grains by recombinant Escherichia coli LSBJ. Biomass Conversion and Biorefinery.
Kuhad, R. C., Gupta, R., Khasa, Y. P., Singh, A. K., & Zhang, Y. P. (2011). Bioethanol production from pentose sugars: Current status and future prospects. Renewable & Sustainable Energy Reviews, 15(9), 4950–4962.
Chavan, S., Yadav, B., Tyagi, R. D., & Drogui, P. (2021). A review on production of polyhydroxyalkanoate (PHA) biopolyesters by thermophilic microbes using waste feedstocks. Bioresource Technology, 341, 125900.
Prados, E., & Maicas, S. (2016). Bacterial production of hydroxyalkanoates (PHA). Universal Journal of Microbiology Research, 4(1), 23–30.
Matsakas, L., Mikes, F., & Bühler, S. (2018). Valorization of brewers’ spent grain for the production of lipids by oleaginous yeast. Molecules, 23(12), 3052.
Zachariah, P. (2022). Would you eat Diwali laddoos made with beer waste? Mintlounge. Website: https://lifestyle.livemint.com/food/discover/would-you-eat-diwali-laddoos-made-with-beer-waste-111666324853997.html
McCabe, C. (2022). Portland Pet Food Company turns brew waste into dog treats. KGW8. Website: https://www.kgw.com/article/life/pets/portland-pet-food/283-e4dd786d-8c18-42a2-92ee-7d4225c00aca
Quentin. (2022). Eclo raises €4.7M to create a high-tech circular organic mushroom substrate factory. Eclo | Mushrooms & Microgreens Grown in Brussels. Website: https://eclo.farm/2022/07/eclo-raises-e4-7m-to-create-a-high-tech-circular-organic-mushroom-substrate-factory/
O’Connell, T. (2019). Nelson fisherman making a difference with beer-related plastic alternative. Stuff. Website: https://www.stuff.co.nz/nelson-mail/news/110338378/nelson-fisherman-making-a-difference-with-beerrelated-plastic-alternative
Krishania, M., Sindhu, R., Binod, P., Ahluwalia, V., Kumar, V., Sangwan, R. S., & Pandey, A. (2018). Design of bioreactors in solid-state fermentation. In Current Developments in Biotechnology and Bioengineering.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. TJL performed the literature search and wrote this article. ZWL and SHM reviewed and edited the manuscript. All the authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to Participate
This article does not contain any studies with human participants or animals performed by any of the authors. Therefore there is no consent to participate needed.
Consent for Publication
This article does not contain any studies with human participants or animals performed by any of the authors. Therefore, there is no consent to publish needed.
Competing Interests
All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lock, T.J., Mah, S.H. & Lai, Z.W. Versatile Applications of Brewer’s Spent Grain: Solid-State Fermentation and Nutritional Added Value. Appl Biochem Biotechnol 196, 5508–5532 (2024). https://doi.org/10.1007/s12010-023-04769-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-023-04769-3