Abstract
Brewer’s spent grain (BSG) represents 85 % of brewing industry by-products. Currently, BSG is underutilised as low-value animal fodder. The current study aims to expose additional nutritional and economic benefits of BSG as a food ingredient in wheat breads. The raw material properties were studied revealing that BSG by-product contains (on a w/w) 22.13 % protein (including exceptionally high levels of essential amino acids), 1.13 % minerals, 131.0 mg/L polyphenols, 28.22 % total fibre and 3.6 % essential fatty acids. Additionally, BSG was fermented (BSG SD), using the lactic acid bacteria, Lactobacillus plantarum FST 1.7, to elucidate the benefits of traditional sourdough for processing crude BSG. Fermentation resulted in softer breads with increased springiness. Farinograph results revealed that wheat flour incorporating BSG had increased water absorption. Rheological measurements showed a positively correlated increase in dough resistance in line with BSG or BSG SD incorporation. Supplemented breads had sensory acceptability up to levels of 10 % BSG or BSG SD, resulting in breads comparing favourably with wholemeal breads from a nutritional, technological and textural perspective. Using BSG as a main stream food ingredient would increase the market value of this by-product, thus enhancing its economic potential, a factor that is also discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brewer’s spent grain (BSG) is the primary bulky by-product of the brewing industry representing 85 % of the total by-products generated [1]. Traditionally, this is viewed negatively by the industry from waste removal or treatment cost and sustainability perspectives. Nowadays, there is an increasing pressure to reduce by-products from industrial processes by reusing them in secondary processes or revaluing them as co-products instead. The economic opportunities for brewhouse by-products, which are currently associated with fiscal loss and are an environmental burden, can be realised through expanding their functionality. Thus, there is a renewed emphasis placed on increasing the nutritive value of suitable industrial by-/co-products for use as food ingredients [2] like in this study, or by extracting value-added products from industrial materials for use as nutraceutical ingredients [3, 4], protein-rich fractions [5], xylooligosaccharides [6], xylitol [7], antioxidant phenolic extracts [8], etc.
In this research, BSG and fermented BSG are evaluated as ingredients for bread production. BSG is acquired from one of the initial brewing steps called mashing. During this elevated temperature process, all soluble matter is extracted into the mash from the barley malt which, after lautering (or mash filtration), is separated into wort (liquid) and spent grain (solid) components. The BSG is generally incorporated into animal feeds currently making them a low-value product, maximally marketed between €1 and 6/tonne, or alternatively BSG is composted, dried and incinerated, dumped or anaerobically fermented [9]. The ‘Environmental, Health, and Safety Guidelines for Breweries’ as set out by the International Finance Corporation (IFC, World Bank Group) suggests that reduction of brewery by-products is the most efficient way of increasing profitability, thus illustrating the significant financial burden associated with brewhouse by-products [10].
Paradoxically, scientific research has revealed that BSG has highly desirable nutritional characteristics from a human dietary standpoint. Typical BSG compositions vary but always include high levels of dietary fibre, protein and particularly essential amino acids, as well as appreciable levels of minerals, polyphenols and lipids [1, 7, 11, 12]. These quality characteristics, in addition to its low cost and high levels of availability, make BSG suitable as a food ingredient. However, some previous attempts to incorporate BSG as a cereal food ingredient have resulted in low-quality sensory/flavour, textural and coloured breads, cookies, baked snacks, etc. As such, we prevented this by further processing the raw material by milling and lactic acid fermentation before using it in a wheat bread formulation.
Lactobacillus plantarum FST 1.7, which was originally isolated from malted barley [13], was used to make a BSG sourdough (BSG SD) during this research. Lactic acid fermentation is known to contribute a number of beneficial characteristics to cereal-baked products, such as increased shelf-life, flavour, aroma, nutritive value and amelioration of the dough handling properties [13–16].
The aim of this research project was to convert an economically low-value universal brewery by-product into a high-nutrition value-added bakery ingredient. Specifically, BSG has high levels of quality essential amino acids, in particular lysine, which counteracts the low availabilities of such nutrients typically associated with baked cereal goods. We aim to incorporate either or both BSG and BSG SD ingredients into a simple bread formulation to ascertain their influence on technological properties of the bread in addition to taste and quality. Additionally, we intend to produce a product that was comparable, but nutritionally superior, to wholemeal wheat bread without compromising on technological or sensory aspects of the bread.
Materials and methods
Materials and strains
Barley (Hordeum vulgare var. Sebastian) was harvested in Ireland in 2008, was purchased from Cork Malting Company, Ireland, and was used to produce the brewer’s spent grain (BSG) in UCC. Odlum’s bakers wheat flour (Odlum Group, Dublin, Ireland), coarse wholemeal flour (Odlum Group), salt (Salt Union, Cheshire, UK) and dried yeast (Mauripan, Burns Philip Food Ltd., UK) were also used in the dough and bread-making recipes.
Media used for lactic acid bacteria (LAB) fermentation was a modified version of MRS (de Man, Rogosa, Sharpe) broth, it contained; 10 g casein peptone, 5 g meat extract, 5 g yeast extract, 1 mL tween 80, 2 g K2HPO4, 5 g NaCH3COO·3H2O, 3 g NH4Cl, 0.1 g MgSO4·7H2O, 0.05 g MnSO4·H2O, 4 g KH2PO4, 0.5 g cysteine-HCl dissolved in distilled H2O, combined with; 5 g glucose, 10 g maltose and 5 g fructose after autoclaving at 121 °C for 15 min to make a 1-L broth supplemented with 1-mL filter sterilised vitamin mixture (0.2 g/L cobalamin, 0.2 g/L folic acid, 0.2 g/L nicotinic acid, 0.2 g/L pantothenic acid, 0.2 g/L pyridoxal phosphate and 0.2 g/L thiamine, dissolved in distilled H2O). The agar was prepared similarly except 0.05 g bromocresol blue, and 15 g agar were also added before autoclaving.
The LAB used in these trials was Lactobacillus plantarum FST 1.7 (School of Food and Nutritional Science, UCC, Cork, Ireland culture stock) and was originally isolated from malted barley [13]. It was routinely maintained on modified MRS agar anaerobically for short periods and stored in glycerol at −80 °C for longer periods.
BSG preparation and analyses
BSG is the main by-product from the brewing process and represents the solid residue present after wort mashing [17]. After the wort was lautered; filtered through the spent grain bed in the lauter tun traditionally and in this research (or using a mash filter in modern brewhouses), the BSG was removed and dried. The drying process was under air at 40 °C, for 3 days. Subsequently, the dried BSG was passed through a mill feeder (Model 3170, Perten Instruments, Segeltorp, Sweden) at a rate of 300 g/min as recommended by the manufacturers (setting II). Immediately, the milled BSG was sealed in polyethylene bags and stored at room temperature.
Standard methods from the Analytica European Brewery Convention (A-EBC), Deutsche Gesellschaft für Fettwissenschaft (DGF), American Association of Cereal Chemists (AACC), or Methodensammlung der Mitteleuropäischen Brautechnischen Analysenkommission (MEBAK) were used for most analyses of barley, malt and BSG. Total nitrogen was quantified by Kjeldhal, A-EBC 4.3.1 [18] and using the conversion factor 6.25; the total protein contents were obtained. Amino acids were analysed by HPLC using ion-exchange chromatography (IEC) (AACC Method 07-01, 11, 20). Carbohydrate content was analysed by IEC and included total fermentable sugars (HPLC AACC Method 80-04), glucose, fructose, saccharose, maltose and maltotriose, in cold extracts of barley, malt and BSG [19]. Total polyphenol contents were calculated according to the Folin–Ciocalteau (Sigma Aldrich) method and reported as gallic acid equivalents [20]. Hop bitter acids were analysed by HPLC using MEBAK Method 4.3.2.1. β-glucan contents were measured according to AACC standard method 32-23 using the K-BGLU enzyme kit (Magazyme Int., Bray, Ireland). Crude fat contents were quantified using MEBAK method 2.5.6 (3.5), and the fatty acid compositions were analysed using the method DGF C-VI 11e, which uses derivatisation with TMSH (Trimethylsulfonium hydroxide). Fibre contents were ascertained according to the method AACC 32-07 using the K-TDFR enzyme kit (Megazyme). Mineral contents were quantified according to the method DIN EN ISO 11885 (E22) Deutsches Institut für Normung [21].
BSG sourdough preparation and analyses
A certain proportion of the milled BSG-flour was fermented by Lactobacillus plantarum FST 1.7. The lactic acid bacteria (LAB) were incubated anaerobically on modified MRS agar for 48 h at 30 °C. Subsequently, a single colony was inoculated into 10 mL of MRS broth and incubated anaerobically for 24 h at 30 °C. This culture was then inoculated at a level of 1 % into MRS broth and incubated for 12–18 h at 30 °C. After this time, the broth was centrifuged, and the pellets were washed twice with Ringer’s solution and suspended in 20 mL of sterile tap water (~5 × 109 CFU/mL) for use in fermentations. The fermentation inoculum was added to BSG to make a sourdough. The fermentation consisted of 350 g BSG, 930 mL of sterile tap water and 40 mL of the washed LAB suspension, which was mixed for 1 min at speed I in a food mixer (Kenwood Classic KM 800, Kenwood Ltd. Hampshire, UK), the mixer bowl sides were scraped down and mixing continued for a further 1 min. The BSG mixture was fermented at 30 °C for 48 h, and the sourdough was incorporated into the bread dough at levels of 5, 10, 15 and 20 % (Table 1B).
During sourdough fermentation, samples were taken at 0, 6, 24 and 48 h for analyses. pH and TTA (total titratable acidity) analyses were done for the sourdough samples (Table 5). For this, 10 g rootlet sourdough, 95 mL distilled water and 5 mL acetone were mixed together using a magnetic stirrer, and the pH was measured subsequently. The TTA was determined by titrating the mixture against 0.1 M NaOH and calculating the volume (mL) needed to reach pH 8.5 [22].
Dough recipe preparation
In advance of recipe formulation, levels of water incorporation needed to be ascertained. The wholemeal flour and the four BSG-supplemented Baker’s wheat flours (5, 10, 15 and 20 % supplementation), which were mixed for 3 min. at speed I in a food mixer (Kenwood Classic), were analysed for moisture by AACC Method 44-15A at 130 °C. Subsequently, water absorbance of the flour blends was ascertained with a Brabender farinograph (Brabender OHG, Duisberg, Germany) using AACC Method 54-21. Based on the moisture and water absorption values, the recipe formulations for wheat, wholemeal wheat, BSG-wheat and fermented BSG-wheat breads were adjusted to reach 500 Brabender units (BU) (Table 1A, B).
Dough analyses: rheofermentometer
The Chopin rheofermentometer (Villeneuvela-Garenne, France) was used to measure dough development. Dough was prepared as for the baking studies (Table 1A, B). Dough heights were measured using 300 g quantities of dough. Displacement of a 1,500 g weight by the rising dough was measured over a 3-h period and is directly related to the volume of gas produced.
Dough analyses: rheology
All rheological measurements were performed at 30 °C using an Anton Paar Physica MCR 301 (Anton Paar Gmbh, Ostfildern, Germany) with sealed 50-mm serrated parallel plate geometry and a 1-mm gap for the compressed sample. The rheometer utilises a temperature controlled water bath in combination with a Pelletier heating system. In addition, excess sample was removed and sealed with mineral oil (Sigma) to ensure negligible water loss from the sample during measurement.
The samples, which were prepared as for bread-making trials below, excepting the exclusion of yeast, included BSG dough (at 5, 10, 15 and 20 % BSG incorporation), BSG sourdough (at 5, 10, 15 and 20 % BSG sourdough incorporation), wheat dough (0 % BSG incorporation) and wholemeal wheat dough (0 % BSG incorporation). Due to the small size of the dough samples, a modified glutomatic was used to mix the ingredients for 4 min before analyses.
During pre-trials, an optimum complex modulus (a measure of the resistance to deformation), G*, was determined to allow accurate comparison of all sample measurements over the various temperatures and times evaluated. An amplitude sweep test was performed to determine the linear viscoelastic region (LVR) for all samples. The amplitude used was at an increasing deformation of 0.001–100 % at an angular frequency of 10 Hz. Once the LVR was determined, a target strain was utilised (0.01 %) which ensured that all samples were measured uniformly in a non-destructive manner. All experimental measurements were performed within the non-destructive LVR for each sample over the test period, and a 5-min rest was incorporated into the rheology cycles before measurement commenced to allow the sample to rest.
A single-frequency test was performed, thus measuring the initial complex modulus (G*) or ability to resist an applied force of 10 Hz and a target strain of 0.01 %, as predetermined during the amplitude sweep. Measurements were taken at 15 min intervals over a 48-h period. BSG-water pastes, mixed in the same way as for fermentation, were used as controls with incorporation of erythromycin (200 mg/kg of dough) and chloramphenicol (60 mg/kg of dough) to inhibit any growth of bacteria that may have been naturally present in the BSG.
Finally, to determine the effect of various levels of addition of BSG and BSG sourdough incorporation into the dough, a single-frequency sweep was performed over 15 min at an angular frequency range of 50–1 s−1 where the target strain was 0.01 %.
Bread making
The control (wheat and wholemeal flour) and BSG dough were made using the recipe described above (Table 1A, B). On a 100 % flour, BSG-flour or BSG sourdough-flour basis, the recipe contained water (as calculated from farinograph analyses) and 2 % each of salt and yeast. The BSG-flour or fermented BSG-flour was wheat flour substituted by 5, 10, 15 or 20 % dry milled BSG or fermented BSG sourdough, respectively.
The dough was prepared by weighing out dry ingredients, with fermented BSG where applicable excluding the yeast, which was incorporated into the water and then added to the other ingredients. Everything was combined in a mixer (Kenwood Chef Classic KM336) at speed 1 for 60 s followed by scraping down the sides of the bowl and subsequently at speed II for 90 s. After mixing, the dough was rested in a proofer (Koma, Koeltechnische, The Netherlands) at 30 °C and 85 % relative humidity for 15 min before it was divided into 75 g portions and placed in non-stick baking tins (35 mm × 9 mm × 5 mm, Sasa UK, Middex, UK). The dough was then proofed for 60 min under the same conditions and baked immediately in a pre-heated deck oven (MIWE condo oven, MIWE Michael Wenz GmbH, Arnstein, Germany) at 220 °C top and bottom heat for 22 min. The oven was pre-steamed (1,000 mL) before and again after putting the bread in. For staling experiments, loaves were subjected to modified atmosphere packaging (MP100, Gustav Muller and Co KG, Bad Homburg, Germany) 120 min after baking, when adequately cooled, with 60 % N2/40 % CO2 and were stored at room temperature for 2 and 5 days.
Bread analyses
After cooling, bake loss was determined (subtracting loaf weight from pre-baked dough weight) and calculated as a percentage. The rapeseed displacement method (Kontec, Japan) was used to determine loaf volume, and the specific volume was hence calculated [loaf volume (mL) divided by bake loss weight (g)]. A Chromameter (Minolta CR-300, Osaka, Japan) was used to measure crust colour (CIE Lab* values). CIE L* values (brightness), a* values (red-green) and b* values (yellow-blue) were measured. Five readings on the top crust of three loaves in three independent batches (i.e. average of 45 readings for each result reported) were recorded.
Texture analysis was performed 120 min (day 1), 2 days and 5 days after baking using texture profile analysis (TPA) tests with a TA-XT2i texture analyser (Stable Micro Systems, Surrey, UK) equipped with a 25-kg load cell and a 20-mm aluminium cylindrical probe. The settings used were a test speed of 2.0 mm/s with a trigger force of 20 g to compress the middle of the breadcrumb to 40 % of its original height. Results were analysed using Texture Expert 1.17 software (Stable Micro Systems). Values for hardness, springiness, chewiness and staling were calculated using the software. Additionally, the Aqua lab CX-2 (Decagon Devices Inc, Washington, USA) was used to determine the water activity (a w) of the loaf crumbs.
Sensory evaluation
Breads substituted with BSG and fermented BSG were evaluated for sensory attributes as previously described [23, 24]. The attributes analysed included dryness, aroma, saltiness, sweetness and acidulous aroma and flavour. These attributes were rated on a scale from 0 to 10, where 10 was the highest score. Breads for the sensory evaluation were baked as described above, and the 8 types of breads that were tasted at the sensory evaluation were wheat bread, wholemeal bread, 5, 10 and 15 % BSG-substituted breads and 5, 10 and 15 % BSG sourdough substituted bread. Each bread was blind tasted three times by a sensory panel (n = 10). Three slices were cut from each loaf using a 20-mm slice regulator. Numbered paper plates were used with four bread slices on each plate covered using cling film. This made a total of 6 plates per person with bread slices on plates that were randomly labelled. Each person was given a sheet to rate the bread sensory attributes and a glass of water. Each panellist tasted 8 bread samples at a time, had a 15–20-min break and then tasted the next two plates of bread.
Statistical analyses
Data mean values and standard deviations were calculated from at least three replicates in all analyses cases, unless otherwise stated. A Q-test was used to exclude any raw data that were below a 95 % confidence level. JMP statistical analysis software [Tukey–Kramer Honestly Significant Difference (HSD)] was used to compare and contrast wheat control, wholemeal control, BSG-supplemented and BSG sourdough supplemented dough and breads, thus differentiating results that are statistically different at p < 0.05.
Results and discussion
BSG and fermented BSG nutrient analyses
The quantity of BSG protein and, in particular, the presence of a high proportion of essential amino acids make it a high nutrition potential food ingredient. The total protein levels of the BSG used in this research were 22.13 % (w/w), which is at least double that of wheat flour, wholemeal flour, barley or malt (Table 2). These values are in keeping with previously reported studies [1]. In addition, BSG protein is of good quality with essential amino acids representing approximately 30 % of the total protein content (Table 2). In particular, lysine, which is known to be the limiting amino acid in cereal foods for human nutrition [26], accounts for 14.3 % of the total BSG protein content making it an ideal candidate for plant-based food fortification such as bread, a staple baked product in much of the world.
Typical BSG composition is approximately 17 % cellulose, 28 % each non-cellulosic polysaccharides and lignin [1, 7]. In general, it is expected that carbohydrate levels in barley malt are drastically reduced during malting, being hydrolysed to oligosaccharides and simple sugars, thus leaving BSG poor in such nutrients. However, in this study, total BSG carbohydrates amounted to 64.9 % (w/w) which is only 20 % lower than the malt content (Table 3). However, the starch levels present were negligible due to extensive amylolysis during mashing [17], when compared to barley and malt that had levels of 0.8, 61.5 and 63.4 % (w/w), respectively. Therefore, starch was not expected to heavily influence the yeast activity or shelf-life during dough development or storage, respectively. Surprisingly, BSG also has a high proportion of simple sugars (approximately 15 % w/w), relative to barley or malt (less than 3 and 6 %, respectively) (Table 3). Additionally, the levels of dietary (total) fibre are very high (48.2 % w/w), more than double that of barley and malt (Table 3), and particularly the insoluble fraction (48.3 % total fibre). This has hugely important implications in human nutrition due to its bulking effect, delayed intestinal transit time, gastric emptying and slower transit through the small bowel, resulting in a reduced rate of nutrient absorption and binding of bile acids, carcinogens or mutagens [27]. There are additional prebiotic effects as well as a variety of local and possibly also systemic effects, associated with dietary fibre [28], especially oat and barley β-glucan intake. Additionally, barley β-glucans are known to constructively contribute to the glycaemic, insulin and cholesterol responses to foods [29].
Another important BSG macro-nutrient includes lipids and fatty acids. The total fat content of BSG is 7.1 % (w/w) which in excess of the levels present in the wheat flour, wholemeal flour, barley or malt (Table 4), with essential fatty acids accounting for over half of total BSG lipids. This is lower than, but comparable to, barley and malt that have a greater proportion of essential fatty acids than BSG, at 58.6 and 62.1 % (w/w), respectively. The lipid content of the BSG used in these trials was in excess of those reported in a previous study (10.6 % w/w) [7]. Generally, the importance of fat in wheat breads is its contribution to volume, cell wall uniformity, softness, improved crumb and overall quality of baked goods [30]. Additionally, fat is important in dough behavioural characteristics including machinability, pliability and general handling [30].
Micronutrients including minerals and polyphenols are also important components when considering the nutritional characteristics of a potential food ingredient. Total BSG mineral levels are 1.13 % (w/w) which is in excess of proportions available in either barley or malt that have levels of 0.81 and 0.71 % (w/w), respectively (Table 2). Additionally, the distribution of individual BSG minerals is similar to that of the barley and malt. Conversely, BSG is relatively high in calcium, magnesium and phosphorous, whilst being lower in potassium when compared to barley, malt or either flour. Given the ongoing debate regarding calcium fortification of cereal goods, particularly in the USA, increased amounts of this mineral may contribute to reducing the risks of osteoporosis and colon cancer [31]. Additionally, the estimated average requirement for magnesium in adults is 255 mg/day [32]. This suggests that a diet supplemented with increased magnesium levels may offer a protective effect against diseases such as Type 2 diabetes mellitus [33] and obesity [34]. Phosphorous levels were also elevated in BSG when compared to barley or malt, which is important due to the anti-nutrition effect of phytate on this mineral. Interestingly, LAB fermentation leads to an increase of minerals such as magnesium and phosphorous by decreasing phytic acid and other anti-nutrient levels [35]. Thus, BSG, and particularly BSG SD supplementation of staple wheat bread, can offer a viable mineral fortification tool. Total polyphenol levels are also higher in the BSG sample (131 mg/L) than barley (90 mg/L), malt (112 mg/L), wheat flour (1.3 mg/L) or wholemeal flour (8.2 mg/L) (Table 2). Poignantly, polyphenols are inclusive of antioxidants, and BSG has previously been exploited for this reason [8]. Specifically, these have been reported to have DNA-protective effects by acting as powerful antioxidants. The authors of this study concluded that BSG is a suitable target for development as a health-promoting food supplement [8].
Dough analyses
BSG and BSG SD containing bread-making formulations were optimised (Table 1) based on preliminary flour and dough analyses. In general, flour water absorption increased in line with increasing levels of BSG and BSG SD addition, as measured by the farinograph (results not shown). This dictated the water and BSG SD levels used in various dough formulations (Table 1A, B) ensuring that each dough was 500 Brabender Units (BU) on the farinograph. Overall, BSG or BSG SD substitution into wheat bread formulations was found to have a negative correlation with moisture levels of the dough and bread, thus suggesting that these high fibre ingredients have an increased ability to hold onto moisture than flour, which was expected [36–38].
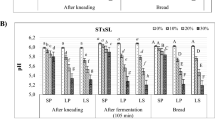
The L. plantarum FST 1.7 fermented BSG SD reduced the BSG pH from 5.59 to 2.67 after a 48-h fermentation, with TTA increasing from 2.67 to 14.92 mL over the same period (Table 5), indicating heterofermentative LAB acid production. BSG and BSG SD were incorporated into wheat dough at levels of 5–20 %, in both instances, resulting in increased flour water absorption (farinograph). Additionally, decreased dough development was observed over the wheat dough control, but still compared favourably with the wholemeal dough up to 10 % incorporation levels (rheofermentometer, Table 6). This is supported by the lower dough elasticity observed during rheological measurements (Figs. 1, 2).
The complex modulus [G*] of brewer’s spent grain over a 48-h L. plantarum FST 1.7 fermentation with rheological measurements at a single-frequency sweep of 10 Hz and strain of 0.01 %. Control is represented by the line and Lactobacillus plantarum FST 1.7 is represented by the broken line. The fermented BSG results (406000 Pa at 0 h) are fitted to the control for ease of comparison
Decreasing frequency sweep (50–1 [1/s]) of the dough at a continuous target strain (0.01 %), showing decreasing complex moduli (G*). Samples included two control dough; wheat (dark solid line) and wholemeal (light dashed line), as well as fermented BSG-substituted dough; 5 % (dark dotted line), 10 % (dark dashed line), 15 % (light solid line) and 20 % (light dotted line)
The rheofermentometre was used to measure dough development during a 3-h fermentation. The development curve of the two controls, BSG and BSG SD containing dough, was compared. As expected, the maximum dough development was observed in the control wheat dough, with 5 % incorporation of BSG or BSG SD not making a significant difference. An addition of 10 % BSG decreased dough height, but to a lesser extent than fermented BSG (Table 6), likely due to decreased elasticity and a weakening of the gluten matrix through gluten–fibre interactions and a gluten dilution effect. However, BSG and BSG SD incorporation up to 15 % resulted in dough with a similar or higher development curve than the wholemeal wheat control.
The rheological behaviour of BSG during a 48-h fermentation with L. plantarum FST 1.7 was examined at a single-frequency sweep of 10 Hz and a target strain of 0.01 %. Rheological measurements showed no significant (p < 0.05) change in resistance to deformation [G*] of the SD supplemented dough when compared to the unfermented control until the latter stages of the test where a slight increase of G* is observed in the BSG SD dough (Fig. 1). This can be attributed to L. plantarum FST 1.7 acid production and pH decrease during the fermentation process, which has been shown to increase the G* of cereal formulations [39] and fermented rootlet substituted dough (Waters et al. submitted for publication). Generally, all dough had a resistance to deformation which was lower than for wholemeal flour dough, except for 20 % fermented rootlet substituted wheat flour dough. At substitution levels of 5 and 10 %, the dough behaved similarly to the wheat flour control.
Overall, the rheology results obtained indicate that at levels of 5 and 10 %, the fermented BSG dough behave in a similar way to the wheat control. Additionally, the viscoelastic behaviour and resistance to deformation increased as BSG SD substitution into the dough increased.
Bread analyses
In general, consumers associate particular physical attributes, including colour, size and shape, with certain products and deviation for this norm can result in rejection of a food before taste, aroma or texture are experienced. Also, aberrations result in an overall decreased perception of flavour and acceptability.
Increasing the substitution levels of BSG and BSG SD into the wheat dough resulted in a reduced specific volume in the final breads (Table 7), when compared to the wheat bread control. However, dough with up to 10 % substitution levels were statistically larger than or the same size as the wholemeal breads. Decrease of volume in brewery by-product substituted breads, relative to wheat bread, is expected due to a disruption of the gluten/protein viscoelastic network as well as the conferred gluten dilution effect [40].
The crust colour is not affected by any level of BSG substitution when compared to the wheat bread control. However, the L* crust colour had a negative correlation with increasing levels of fermented BSG additions, relative to wholemeal bread crust colour (Table 7). The lightening tendency of crust colour due to BSG SD addition is likely due to a number of factors. This effect has previously been observed after fermentation where LAB induced a reduction of polyphenols and fatty acids [41]. This may prevent the polymerisation of endogenous phenolic compounds, as well as polyphenol oxidase-mediated discolouration. Additionally, the fermentative activity of the L. plantarum could also metabolise free sugars leading to a potential reduction in the Maillard reaction colour products.
The bake loss measured for the supplemented breads was between both control bread values and thus is within the acceptable range (Table 7). As such, BSG or BSG SD supplemented breads can be considered as viable high fibre replacements for wholemeal breads.
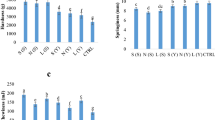
Texture profile analyses revealed hardness, springiness, chewiness and staling values; after 2 and 5 days of storage, for the wheat and wholemeal control breads as well as SBG and SBG SD-substituted breads. Fermented BSG substituted at the lower level resulted in softer bread than the wheat bread control, whereas up to 15 % substitution levels resulted in breads that were softer than (5–10 %) or compared to (15 %) the wholemeal control bread. To produce acceptable bread, levels of 15 % BSG or BSG SD supplementation should not be exceeded. The increased hardness associated with BSG incorporation is likely related to the arabinoxylan, glucan and xylooligosaccharides present in the formulation. These potentially interfere with the viscoelastic dough network as previously reported for insoluble arabinoxylans in wheat bread [42], providing a hurdle to dough development. Additionally, rheological studies showed that dough G* increased with the incorporation of BSG due to the additional mechanical stress necessary to manipulate the dough formulation. This, in turn, restricts dough expansion and thus final bread volume. Conversely, fermented BSG incorporation into dough resulted in a softer dough at low levels (5 %) but a harder dough at increased levels (10–20 %), compared to the wheat bread control. The former effect is likely due to LAB fermentation modifying the proteins and starch, which may reduce elasticity and network connectivity [39]. However, at higher levels of incorporation, the decreased pH has a hardening effect on the gluten network and thus, the resultant bread [39]. Additionally, acid production causing a decreased pH, in combination with an increased TTA, provides a less favourable environment for yeast activity, thus for gas production and dough cell expansion [43]. Staling was notably retarded, particularly in BSG SD-substituted breads compared to the wheat bread control. This anti-staling effect was previously noted after L. plantarum FST 1.7 fermentation and is related to amylolytic activity which can modify the starch resulting in retardation of retrogradation [13]. Conversely, the higher fibre content of the unfermented BSG increases the water absorption of the dough whilst reducing availability of water, thus increasing the pace of starch retrogradation [44].
Sensory evaluation of the breads was ascertained with a ten person panel and was used for sensory analyses of the control breads and the BSG or fermented BSG SD-substituted breads (Table 8). The breads were accepted from a sensory perspective up to a 10 % level of either BSG or BSG SD substitution.
Perceived sweetness of the bread was decreased upon increasing addition of BSG, and this effect was more pronounced upon addition of fermented BSG (Table 8). BSG high polyphenol content has previously been associated with a decreased sweetness and increased bitterness when incorporated into baked cereal goods. Additionally, the acidulous lactic acid fermentation of the BSG leads to a further reduction of sweetness in BSG SD incorporated breads, as expected. It is well known that the acidity increases associated with LAB fermentation lead to a partial lowering of sweetness and increased acidulous aroma/flavours. Generally, saltiness and dryness of the baked breads were between the levels found for the wheat and wholemeal controls for all substituted breads, or not statistically different. Overall aroma of the breads were also in line with the control breads, and thus, the BSG and BSG SD-substituted breads represent a viable alternative to wholemeal breads.
Conclusions
Overall, wheat bread formulations with up to 10 % incorporation of BSG or BSG SD resulted in dough with improved handling properties compared to the wholemeal control, based on rheological analyses. Additionally, the 10 % substituted breads had similar technological properties to wholemeal bread with BSG or BSG SD breads having improved softness and staling. The main sensory differences between control and test breads were from an acidulous aroma and flavour perspective, with both increasing in line with increasing levels of BSF or fermented BSG addition. Sweetness decreased as substitution levels increased and was lower for breads substituted with fermented BSG. Acidulous aroma, flavour and sweetness negative effects associated with BSG or BSG SD addition were more pronounced for the fermented rootlet breads. However, up to a level of 10 % addition of BSG or SBG SD, the breads were completely accepted by the sensory panel.
The acceptability of the 10 % substituted breads, in combination with the increased nutritional properties of BSG, particularly protein, dietary fibre and mineral levels, makes this raw material a suitable fortifying food ingredient imparting textural and dough handling technological benefits to the bread formulation, particularly after fermentation with L. plantarum FST 1.7. Furthermore, we should consider the dietary fibre daily adult intake recommendations from the EFSA (European Food Safety Authority) and USDA/FDA (United States Department of Agriculture/Food and Drug Administration) [45], which are at levels of 25 and 21–38 g/day (state-dependant), respectively [46]. For example, the suggested daily intake of bread is 6 servings per day (25–35 g/slice is one portion as calculated from EFSA and WHO country-dependant recommendations in Western Europe) [46, 47]. Thus, incorporating 10 % BSG into wheat bread could add an additional 13.9 g/day of dietary fibre through consumption of this BSG-fortified staple food, which would contribute to approximately 50 % of the recommended daily intake.
Additionally, using the BSG industrial by-product, which has a low monetary value of between €1-6/tonne, as high-nutrient functional ingredient will enhance the economic potential of the brewhouse as well as improving the dietary attributes of food formulations. To conclude, the resultant BSG and BSG SD-substituted breads are a viable potential replacement for wholemeal breads from economical, nutritional and environmental by-/co-product revalorisation perspectives.
References
Mussatto SI, Dragone G, Roberto IC (2006) Brewers’ spent grain: generation, characteristics and potential applications. J Cer Sci 43(1):1–14. doi:10.1016/j.jcs.2005.06.001
Baik B-K, Ullrich SE (2008) Barley for food: characteristics, improvement, and renewed interest. J Cer Sci 48(2):233–242. doi:10.1016/j.jcs.2008.02.002
Kanaucki O, Mitsuyama K, Araki Y (2001) Development of a functional germinated barley foodstuff from brewer’s spent grain for the treatment of ulcerative colitis. J Am Soc Brew Chem 59(2):59–62
Ishiwaki N, Murayama H, Awayama H, Kanauchi O, Sato T (2000) Development of high value uses of spent grain by fractionation technology. Tech Quart Master Brewer Assoc Am 37(2):261–265
Celus I, Brijs K, Delcour JA (2007) Enzymatic hydrolysis of brewers’ spent grain proteins and techno functional properties of the resulting hydrolysates. J Agric Food Chem 55(21):8703–8710. doi:10.1021/jf071793c
Carvalheiro F, Esteves MP, Parajó JC, Pereira H, Gírio FM (2004) Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour Technol 91(1):93–100
Mussatto SI, Roberto IC (2005) Acid hydrolysis and fermentation of brewer’s spent grain to produce xylitol. J Sci Food Agric 85(14):2453–2460. doi:10.1002/jsfa.2276
McCarthy AL, O’Callaghan YC, Connolly A, Piggott CO, FitzGerald RJ, O’Brien NM (2012) Phenolic extracts of brewers’ spent grain (BSG) as functional ingredients—assessment of their DNA protective effect against oxidant-induced DNA single strand breaks in U937 cells. Food Chem 134(2):641–646. doi:10.1016/j.foodchem.2012.02.133
Fillaudeau L, Blanpain-Avet P, Daufin G (2006) Water, wastewater and waste management in brewing industries. J Clean Prod 14(5):463–471
IFC IFC (2007) Environmental, health, and safety guidelines for breweries. Environmental, health, and safety guidelines. World Bank Group, Washington
Kanauchi O, Mitsuyama K, Araki Y (2001) Development of a functional germinated barley foodstuff from brewer’s spent grain for the treatment of ulcerative colitis, vol 59, vol 2. American Society of Brewing Chemists, St. Paul (ETATS-UNIS)
Vietor RJ, Voragen AGJ, Angelino SAGF (1993) Composition of non-starch polysaccharides in wort and spent grain from brewing trials with malt from a good malting quality barley and a feed barley, vol 99, vol 3. Institute of Brewing, London (ROYAUME-UNI)
Dal Bello F, Clarke CI, Ryan LAM, Ulmer H, Schober TJ, Ström K, Sjögren J, van Sinderen D, Schnürer J, Arendt EK (2007) Improvement of the quality and shelf life of wheat bread by fermentation with the antifungal strain Lactobacillus plantarum FST 1.7. J Cereal Sci 45(3):309–318. doi:10.1016/j.jcs.2006.09.004
Barber B, Ortolá C, Barber S, Fernández F (1992) Storage of packaged white bread. Z Lebensm Unters For A 194(5):442–449. doi:10.1007/bf01197726
Hammes WP, Gänzle MG (1997) Sourdough breads and related products. In: Wood BJB (ed) Microbiology of fermented foods, vol 1, 2nd edn. Blackie Academic and Professional, London, pp 199–216
Clarke CI, Arendt EK (2005) A Review of the application of sourdough technology to wheat breads. Adv Food Nutr Res 49:137–161. doi:10.1016/s1043-4526(05)49004-x
Bamforth CW (2003) Beer tap into the art and science of brewing, 2nd edn. Oxford University Press, New York
Mitteleuropaische Brautechnische Analysenkommission (2011) Method 4.3.1 Total nitrogen of malt: Kjeldahl method. In: Jacob F (ed) MEBAK Raw Materials: Barley, Adjuncts, Malt, Hops and Hop Products
Keßler M, Kreisz S, Zarnkow M, Back W (2005) Investigations about the relative Hartong Extract at 45 °C. Monatsh Brauwiss 58(4):56–62
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Method Enzymol 299:152–178
Deutsches Institut für Normung (1998) Method: DIN EN ISO 11885 E22
Arbeitsgemeinschaft Getreideforschung A (1994) Uberarbeitete und erweiterte Auflage. In: Standard-Methoden für Getreide, Mehl und Brot (Standard methods for cereals, flour and bread) 7 edn. Verlag Moritz Schäfer, Detmold, Germany
Haglund Å, Johansson L, Dahlstedt L (1998) Sensory evaluation of wholemeal bread from ecologically and conventionally grown wheat. J Cereal Sci 27(2):199–207. doi:10.1006/jcrs.1997.0155
Rizzello C, Nionelli L, Coda R, Di Cagno R, Gobbetti M (2010) Use of sourdough fermented wheat germ for enhancing the nutritional, texture and sensory characteristics of the white bread. Eur Food Res Technol 230(4):645–654. doi:10.1007/s00217-009-1204-z
Hager A-S, Wolter A, Jacob F, Zannini E, Arendt EK (in press) Nutritional properties and ultra-structure of commercial gluten free flours from different botanical sources compared to wheat flours. J Cer Sci
Young V, Pellett P (1994) Plant proteins in relation to human protein and amino acid nutrition. Am J Clin Nutr 59(5):1203S–1212S
Blackwood A, Salter J, Dettmar P, Chaplin M (2000) Dietary fibre, physicochemical properties and their relationship to health. J R Soc Promot Health 120(4):242–247
Champ M, Langkilde AM, Brouns F, Kettlitz B, Collet YB (2003) Advances in dietary fibre characterisation. 1. Definition of dietary fibre, physiological relevance, health benefits and analytical aspects. Nutr Res Rev 16(1):71–82
Brennan CS, Cleary LJ (2005) The potential use of cereal (1 → 3, 1 → 4)-β-d-glucans as functional food ingredients. J Cereal Sci 42(1):1–13. doi:10.1016/j.jcs.2005.01.002
O’Brien C, Mueller A, Scannell A, Arendt E (2003) Evaluation of the effects of fat replacers on the quality of wheat bread. J Food Eng 56(2–3):265–267. doi:10.1016/S0260-8774(02)00266-2
Newmark HL, Heaney RP, Lachance PA (2004) Should calcium and vitamin D be added to the current enrichment program for cereal-grain products? Am J Clin Nutr 80(2):264–270
Medicine Io (1999) Magnesium, dietary reference intakes for calcium, phosphorous, magnesium, vitamin D, and fluoride. National Academy Press, Washington
Venn BJ, Mann JI (2004) Cereal grains, legumes and diabetes. Cereal Grain Legum Diabetes 58(11):1443–1461
Melanson KJ, Angelopoulos TJ, Nguyen VT, Martini M, Zukley L, Lowndes J, Dube TJ, Fiutem JJ, Yount BW, Rippe JM (2006) Consumption of whole-grain cereals during weight loss: effects on dietary quality, dietary fiber, magnesium, vitamin B-6, and obesity. J Am Diet Assoc 106(9):1380–1388
Lopez HW, Krespine V, Guy C, Messager A, Demigne C, Remesy C (2001) Prolonged fermentation of whole wheat sourdough reduces phytate level and increases soluble magnesium. J Agric Food Chem 49(5):2657–2662. doi:10.1021/jf001255z
Helmy IMF, Nadir AS, Abdel-Hamid AA (2003) Pan bread quality as affected by supplementation of brewing by-products. Egypt J Food Sci 31:1–27
Salama AA, El-Sahn MA, Mesallam AS, Shehata AME (1997) Evaluation of the quality of bread, biscuit and butcher’s sausage supplemented with rootlets of malt sprouts. Food/Nahrung 41(4):228–231. doi:10.1002/food.19970410409
Salama A-RA, El-Sahn MA, Mesallam AS, Shehata AME-T (1997) The chemical composition, the nutritive value and the functional properties of malt sprout and its components (acrospires, rootlets and husks). J Sci Food Agric 75(1):50–56. doi:10.1002/(sici)1097-0010(199709)75:1<50:aid-jsfa833>3.0.co;2-0
Moroni AV, Bello FD, Zannini E, Arendt EK (2011) Impact of sourdough on buckwheat flour, batter and bread: biochemical, rheological and textural insights. J Cereal Sci 54(2):195–202. doi:10.1016/j.jcs.2011.04.008
Sullivan P, O’Flaherty J, Brunton N, Arendt E, Gallagher E (2011) The utilisation of barley middlings to add value and health benefits to white breads. J Food Eng 105(3):493–502. doi:10.1016/j.jfoodeng.2011.03.011
Corsetti A, Settanni L (2007) Lactobacilli in sourdough fermentation. Food Res Int 40(5):539–558. doi:10.1016/j.foodres.2006.11.001
Courtin CM, Roelants A, Delcour JA (1999) Fractionation—reconstitution experiments provide insight into the role of endoxylanases in bread-making. J Agric Food Chem 47(5):1870–1877. doi:10.1021/jf981178w
Di Cagno R, De Angelis M, Corsetti A, Lavermicocca P, Arnault P, Tossut P, Gallo G, Gobbetti M (2003) Interactions between sourdough lactic acid bacteria and exogenous enzymes: effects on the microbial kinetics of acidification and dough textural properties. Food Microbiol 20(1):67–75
Stojceska V, Ainsworth P, Plunkett A, İbanogˇlu S (2008) The recycling of brewer’s processing by-product into ready-to-eat snacks using extrusion technology. J Cer Sci 47(3):469–479. doi:10.1016/j.jcs.2007.05.016
Garza, C (ed) (2005) Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. The National Academies Press, Washington
EFSA Panel on Dietetic Products NaA (2012) Scientific opinion on dietary reference values for carbohydrates and dietary fibre. EFSA J 8(3):1462–1538. doi:10.2903/j.efsa.2010.1462
World Health Organisation W (2003) Food based dietary guidelines in the WHO European region. WHO Regional Office for Europe, Copenhagen
Acknowledgments
Funding for this research was provided by FIRM Department of Agriculture, Food and Forestry (DAFF) from October 2008 to September 2012. FIRM DAFF was not involved in study design; in acquisition, analyses or interpretation of data; or in writing and submitting this article for publication. The authors wish to acknowledge the contribution of Sascha Wunderlich and Andrea Lötterle who aided in data compilation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waters, D.M., Jacob, F., Titze, J. et al. Fibre, protein and mineral fortification of wheat bread through milled and fermented brewer’s spent grain enrichment. Eur Food Res Technol 235, 767–778 (2012). https://doi.org/10.1007/s00217-012-1805-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-012-1805-9