Abstract
In this study a valuable fermented brewer’s spent grain (BSG) was obtained by solid state fermentation (SSF) with Rhizopus sp. and assessed for feed and food applications. SSF conditions were optimized by factorial design and response surface methodology (RSM) to maximize the value of the resulting BSG biomass. Two Rhizopus sp. strains were tested as inoculum (one wild and one mutant strain) and time and temperature were analyzed. Measured response variables included, among others, protein content, soluble protein, degree of hydrolysis, antioxidant activity, total phenolic content and antibacterial activity. Both strains led to the highest protein concentration (31.7 ± 7.6%) and soluble protein (47.4 ± 3.8 mg/g DM) when BSG was fermented at 30 °C for 9 days. The biomass obtained presented a modified amino acid profile resulting in an essential amino acid index (EAAI) of 1.58 compared to FAO human nutrition standard, with antioxidant capacity (59.7 ± 7.7% DPPH reduction) and 11 times higher total polyphenol content (2.7 ± 0.1 mg GAE/g DM). Hereby, results demonstrate that SSF of BSG results in a significant increase of highly appreciated characteristics for feed or food applications, which could lead to a promising valorization alternative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Statement of Novelty
Sustainable livestock development requires novel feed resources, preferably those not competing with human food. Until now, the most used application for beer-industry derived main sub-product (BSG) has been as animal feed. However, commercial value will be much higher if protein and digestibility increase and if amino acid profile and biological activities are improved in a cost-effective way. Fungal SSF is a successful strategy for BSG valorization for production of a valuable, sustainable, non-climate dependent protein source. This study leads previous works presenting the nutritional improvement and availability of amino acids and antioxidant compounds but also its suitability for animal feed compared to other protein sources.
Introduction
Brewer’s spent grain (BSG) is a by-product of the beer brewing industry which corresponds to approximately 85% of total by-products generated during beer production [1]. Its composition can vary depending on the barley variety, harvesting time and malting and mashing conditions [2] but, in general, it is a fiber and protein rich material, around 70 and 20% respectively, followed by lower proportions of lipids and ash, 9–10 and 4–5% respectively [3]. Europe beer production was of 416 million hl in 2016 [4] and for every 100 liters of beer produced, 15–20 kg of BSG was generated [5, 6]. Despite being readily available, it is considered an unstable product and, up to now, no good preservation technologies have been developed [5]. Hence, several strategies have been studied as an alternative to overcome the environmental problems associated with BSG’s disposal and/or combustion. The use of raw BSG has been studied in depth, including use in animal and human nutrition [6, 7], energy production [8], as a brick component [9], cellulose source [10] or adsorbent [11]. In most cases, not modified BSG has a low commercial value due to its high humidity (75–80%) and low digestibility protein [12]. BSG utilization also includes enzymatic recovery of carbohydrate fractions [3, 13] and enzymatic production of antioxidant and antihypertensive compounds [14,15,16]. However, the use of commercial enzymes is costly. In this case, microbial fermentation could be a cost-effective alternative to hydrolyze biomass, and reports include the use of BSG for organic acids production [17] and as substrate for microbial enzyme production [18, 19].
Until now, BSG has been primarily used as animal feed, mainly for ruminants due to its high proportion of fiber [1]. Animal feed producers are interested in high protein, high digestibility and “low-toxin” products, which allow a correct nutritional balance in the recipe. Regular availability, low price and constant characteristics are also appreciated. BSG can fulfill all these requirements, but commercial value would be higher if protein and digestibility increased in a cost-effective process. In addition, some biological activities are important for feed application, including antibacterial and antioxidant activities.
BSG, as other large volumes of food processing by-products, can be transformed from poor-nutrient wastes to animal feeds, food ingredients or enzymes when appropriate technologies are used for their valorization [20]. Industrially interesting compounds can be produced by the use of different fermentation strategies such as solid-state fermentation (SSF) and submerged fermentation (SmF). In recent years, SSF has gained scientific and technological attention because it is considered an efficient process for production of enzymes and other compounds such as phenols, vitamins and flavor compounds, among others [21, 22]. Fungi, the main microorganisms used in SSF, are known to produce enzymes which degrade plant cell walls and could also improve biochemical composition and bioactivity of the employed substrates [23]. Fungal fermentation applied to food by-products improves digestibility and availability of proteins, preventing growth of pathogenic microorganisms and undesirable bacteria through the production of compounds with antibiotic activity [24].
One of the most promising fungi genus for SSF is Rhizopus sp. This genus includes several species used for enzyme production (glucoamylases, cellulases, tannases), organic acids production (lactic acid, fumaric acid) and animal feed preparation [25, 26]. They are also the traditional inoculum for human food production (tempeh, peka, ragi and loog-pang) [27]. Simple nutritional requirements, wide growth temperature interval (from 25 to 45 °C), growth and survival pH range (at least, from 4.5 to 7.5) and board fermentative substrates make Rhizopus a good candidate to ferment agro-industrial by-products in order to obtain products with higher protein and vitamin contents [28]. R. oryzae is a well-known specie of Rhizopus genus, generally recognized as safe (GRAS) by the FDA; it is employed industrially for its capacity to consume a great range of carbon sources [25], lipase and protease production [29, 30] and it has been considered for by-product valorization [31,32,33]. Using Rhizopus oryzae strain during SSF technology, a previous work obtained fruit by-products enriched in fatty acids (FA), amino acid (AA) concentration and with improved AA profile [31]. Most critical parameters for highest biomass production (by-product, surface, Tª, time, and nitrogen) have been previously optimized in model substrate and in some real substrates (fruit and vegetable by-products) [31] but not optimized for feed application.
Along this paper, the optimization of parameters for SSF process of BSG is described, using Rhizopus sp. fermentation as an improving strategy of nutritional composition, compound availability and bioactivity of the final product which could lead to an economically and environmentally feasible valorization strategy. Fermentation conditions were optimized with a rotatory design model for highest protein concentration, optimal degree of hydrolysis (DH), high total phenolic content (TPC) and looking for antioxidant and antimicrobial activity in order to develop a sustainable process for BSG valorization that allows its use as a valuable ingredient for feed or food applications. Resultant biomass is richer in protein and TPC, shows higher antioxidant activity and DH than the unfermented BSG and a modified AA and FA profile. This study leads previous works presenting the nutritional improvement of fermented BSG [24, 34] but also offers insight into the bioavailability of the released compounds during fermentation and discusses fermented BSG suitability for animal feed and human nutrition compared to other protein sources and standards.
Materials and Methods
Microorganism, Mutagens and Culture Media
One food-derived Rhizopus sp. strain isolated and characterized in our laboratory (ROR004) [31] and a strain derived from ROR004 after mutagenesis experiments (ROR004 G) were used as strains.
The wild type strain (ROR004) was treated with UV (Atom A/70 Nº557, Barcelona, Spain), 1-methyl-3-nitro-1-nitrosoguanidine (MNNG, Tokyo chemical industry, Zwijndrecht, Belgium) and ethidium bromide (EB, 15585011 Fischer Scientific, Loughborough, UK) at room temperature to induce mutagenesis. For treatment with UV, 10 ml of the spore suspension (4 log cfu/ml) were plate on sterile petri dish (90 mm diameter) and exposed to UV irradiation (254 nm) for 60 and 90 min at 20 cm. During exposition to MNNG, 1 ml of the single spore suspension (4 log cfu/ml) was mixed with 5 ml MNNG (0.1 mg/ml) for 30 and 50 min. Finally, for mutagenesis with EB, 7.4 ml of spore suspension (4 log cfu/ml) were mixed with 600 µl of EB solution (1 mg/ml) for 90 or 100 min. After treatment with MNNG and EB, spores were washed twice by centrifugation (11,330g, 5 min). Supernatant was discarded and pellet was resuspended in 1 ml of sterile water. In all cases, final spore suspension was cultivated on PDA agar plates for 48 h at 20 °C to obtain isolated colonies.
Potato dextrose agar (PDA), Mueller Hilton broth and bacteriological agar were used for bacterial and fungal propagation and count. Buffered peptone water (all from Oxoid, Basingstoke, Hampshire, England) was used for sample dilution when required. All media were prepared as recommended by the producer and sterilized at 121 °C for 15 min. The total microbial and fungal determination was done at 30 °C for 48 h.
Brewer’s Spent Grain (BSG)
BSG was provided by Boga Cooperative (an artisanal brewery located in Mungia, Spain) as by-product after standard barley brewing process. Sample was frozen at − 20 °C until it was used. After defrost (18 h, 4 °C), sample was sterilized at 110 °C for 15 min and cooled down up to room temperature in sterile conditions before inoculation. In all the experiments, substrate density was maintained at 0.09 g/cm2 and no extra nitrogen was added, as previously optimized [31]. Gross composition of BSG is expressed in Table 1.
Analytical Determinations
Gross Composition
Dry matter (DM) was calculated by drying the sample at 60 °C for 24 h, until constant weight. Protein content was determined by Kjeldahl, total lipids by Soxhlet previous acid hydrolysis, and ash gravimetrically [35]. Neutral detergent fiber (NDF) was determined gravimetrically [36]. Mycelia protein content for mutant strain characterization was determined by Biuret method proposed by Satari et al. [37].
Fat extraction for FA profile determination was based on Bligh and Dyer [38]. Biomass (500–1000 mg) was mixed with 3 ml of methanol, 1.5 ml of chloroform (both from Fischer Scientific, Loughborough, UK) and 1.2 ml of water and homogenized for 1 min. In addition, 1.5 ml of chloroform and 1.2 ml of water were added, homogenized again and centrifuged (1450g, 15 min). Lipids were determined in the organic phase. Moisture and impurities were removed by passing through Na2SO4 (Fischer Scientific, Loughborough, UK). The solvent was evaporated under nitrogen flush. Fatty acid profile was determined as methyl esters by adding 5 ml of sodium methylate (0.2%) to previously extracted lipid fraction and boiling for 10 min in a reflux system. Samples were cooled in ice, neutralized by adding HCL (5% v/v) (Fischer Scientific, Loughborough UK) in methanol and again boiled for 10 min, cooled in ice and mixed with 5 ml of n-hexane (Fischer Scientific, UK). Saturated NaCl was added and the organic phase was analyzed by GC-FID. Chromatograph was fitted with a DB-23 column of Agilent Technologies (60 m × 0.25 mm) and programed at 150 °C for 1 min, a 5 ºC/min gradient up to 200 °C, a second gradient of 2 ºC/min up to 230 °C and 20 min at 230 °C. Fatty acid methyl esters were identified by comparing the retention times with standards and were expressed as percentages of total fatty acid methyl esters.
Amino acid profile was determined by liquid chromatography and fluorescence detection after sample derivatization using AccQ Fluor Reagent Kit (WATO52880, Waters, Milford, USA). Samples were neutralized by adding HCL 6 N and left for 24 h at 100 °C. The derivatization was done as specified in the kit. The HPLC- FPLC analysis was fitted with a AccQ.Tag for hydrolysate amino acid analysis column of Agilent Technologies (3.9 × 150, 4 µm, Silica base bonded with C18), fluorescence detector (Ex = 250 nm; Em = 396 nm) and performed in gradient system (60% Acetonitrile-40% Buffer AccQ.Tag (10% v/v) 5 min- 100% Buffer Waters AccQ.tag (10% v/v) for next 9 min. Amino acids were identified by comparing the retention times with standards and are expressed as percentages of total amino acids.
Protein quality was evaluated by the essential amino acid index (EAAI) as described before [39]. EAAI is based on the content of essential amino acids (EAA) compared to a reference protein or specific requirements for human nutrition [40], and it is used as a rapid method to evaluate and optimize the amino acid content of food and feed formulations. EAAI equation is described as follow:
where n is the number of essential amino acids referenced.
Bioactive Compounds Extraction
Bioactive compounds were extracted using water in order to determine the real availability of those compounds. 10 g of sample were mixed with 25 ml of distilled water and the mixture was placed in a rotary shaker (1 h, 180 rpm, 37 °C) in order to homogenize the flask content. Afterwards, the broth was centrifuged (10,000g, 10 min, 4 °C) and the supernatant, containing the water soluble metabolites, was filtered through a 0.45 µm filter. Those extracts were used for soluble protein, DH, antioxidant activity, TPC, antibacterial activity and reducing sugars determination.
Degree of Hydrolysis (DH)
The degree of hydrolysis (DH, %) was determined with the O-phthaldialdehyde (OPA) method [41]. OPA reagent was prepared as follows: 3.7% w/v Na tetraborate decahydrate (B9876, Sigma Aldrich, Steinheim, Germany) and 0.58% w/v Na-dodecyl-sulfate (SDS 99%, 230,421,000 Fischer Scientific, New jersey, USA) dissolved in deionized water. OPA (4% w/v 79,765, Sigma Aldrich, Steinheim, Germany) in methanol was prepared and both solutions were mixed in 51.5:1 proportion. Finally, 0.38% v/v of β-mercaptoethanol (M6250 Sigma Aldrich, Steinheim, Germany) was added to the solution. Nα-Acetyl-L-lysine (250 µM J65083, Alfa Aesar) was prepared in deionized water and subsequent dilutions were used as standard. The sample solution was adjusted to 0.05 g protein/l and 60 µl of sample or standard were added to microplate wells. 180 µl of OPA reagent were added to each well and incubated at ambient temperature for 5 min. The measurement was done at 360 nm excitation wavelength and 460 nm emission wavelength. DH was calculated as described by Nielsen et al. [41], and as recommended for general purpose, htot was used as 0.8 g meq/g protein and α and β were estimated to be 1.00 and 0.40.
Antibacterial Activity
The antibacterial activity of the extracts was determined by agar diffusion method based on Bougherra et al. [42]. Two bacterial species were used as test microorganisms, Salmonella enterica (CECT 4156) and Escherichia coli (CECT 516), and sub-cultured in Mueller–Hinton agar at 37 °C for 24–48 h.
Sensible strains were inoculated in 10 ml of Mueller-Hilton broth until optical density reached 0.50 (approx. 108 cfu/ml) and incubated at 30 °C for 1 h. Then, 100 µl of bacterial suspension was added to 4 ml of Mueller Hilton soft agar (0.7% bacteriological agar w/v) maintained at 48 °C, vortexed and quickly cast in a Mueller Hilton petri plate. After solidification, the wells (5 mm diameter Whatman filter no 1) and the extracts were added (10 µl per each well) and incubated at 30 °C 24–48 h. Antibacterial activity was measured as the diameter of the clear zone of pathogen growth compared to a positive antibacterial-control, tetracycline (> 98% Sigma–Aldrich, Steinheim, Germany), (1 g/l) and a negative control (sodium phosphate buffer, 0.01 M, pH 7.5).
Antioxidant Activity (DPPH Radical Scavenging Activity)
Antioxidant activity of samples was measured using the DPPH radical scavenging activity (DRSA) method based on Brand-Williams et al. [43] with slight modifications. DPPH (2,2-Diphenyl-1-picrylhydrazyl, D9132 Sigma Aldrich, Steinheim, Germany) in methanol (25 ppm) was prepared and 280 µl of this solution were added to 20 µl of sample solution. The mixture was incubated at room temperature in the dark for 30 min. Absorbance was measured at 515 nm. The standard comprised of water–methanol (50% v/v) and different concentrations of Trolox (218940050, Acros Organics, New Jersey, USA). The antioxidant capacity was expressed as Trolox equivalent antioxidant capacity (TEAC), µg Trolox equivalents/g DM using the calibration curve. The percentage of reduction of DPPH compared to the blank also was calculated:
Total Phenolic Content (TPC)
TPC was measured using the Folin–Ciocalteu method [44] with modifications. Initially, 30 µl of Folin–Ciocalteu (J/4100/08, Fischer Scientific, Loughborough, UK) solution were added to 140 µl sample, blank or standard and 140 µl of Na2CO3 7% (w/v) (Sigma Aldrich, Steinheim, Germany). The mixture was incubated at room temperature in the dark for 1 h and the absorbance was measured at 750 nm. Gallic acid (G7384, Sigma Aldrich, Steinheim, Germany) was used as standard at a concentration range of 1.4–20 ppm and results were expressed as mg gallic acid equivalent (GAE) per g of DM sample.
Reducing Sugars
Reducing sugars were determined by Dinitrosalicylic (DNS) acid reagent method [45] adjusted to microplate assay procedure. Briefly, 25 µl of DNS reagent were mixed with 25 µl of sample, blank or standard (0–2.5 g/l of D-glucose) and incubated for 10 min at 100 °C. The microplate was cooled in an ice bath and 250 µl of distilled water were added to each well. Absorbance was read at 540 nm.
Soluble Protein
Soluble protein was determined by BCA (Bicinchoninic Acid) method (Pierce™ BCA Protein Assay Kit) adjusted to microplate assay procedure. Bovine serum albumin (BSA 0-2.5 mg/ml) was used as standard. Absorbance was read at 550 nm.
Solid State Fermentation (SSF)
All SSF experiments were carried out on 135 mm diameter sterile plate (surface area 143.1 cm2) at 30 °C. SSF process was done using ROR004 strain and all variables were monitored from time 0 to 192 h (n = 4 in all cases except for time 0 h, where n = 2).
Factorial design methodology was used for optimal conditions determination. Factorial design consisted on two continuous factors (time and temperature) and one categorical factor (fungal strain). Considered runs included 23 factorial design (eight runs) and central points (four runs) to determine the best combination between incubation time and temperature and the effect of used strain (category factor) on several response parameters (protein, soluble protein, DH, TEAC, DPPH reduction and TPC). Overview of the complete design is described in Table 2.
In all cases, substrate density was 0.09 g of dry BSG/cm2 and 10% of spore suspension (107 cfu/ml) was added to the fermentation substrates. Spore suspension was prepared as described before [31].
Submerged Fermentation (SmF)
During mutant selection, SmF was used to control changes in biomass production. Growth medium includes (g/l): 20 glucose (G/0450/65, Fischer Scientific, Loughborough, UK), 0.75 nitrogen (Protease Peptone, Oxoid LP0085, Basingstoke, Hampshire, England), 3.0 KH2PO4 (Panreac Química SA, Barcelona, Spain), 0.5 MgSO4 * 7H2O, 0.5 FeSO4 (both from Sigma Aldrich, Steinheim, Germany) and 0.5 KCl (Merck, Darmstadt, Germany). SmF was carried out on sterile 150 ml Erlenmeyer flasks (100 ml medium) at 170 rpm, incubated at 30 °C for 72 h in dark conditions. Fungal spore suspensions were prepared as described before [30], adjusted to 107 cfu/ml just before inoculation (1% v/v).
Statistical Analysis
Experimental factors were considered significant when their probability (p-value) was less than 0.05 and were analyzed with one-way ANOVA (analysis of variance) using SPSS (IBM Corp. V 24.0, New York, USA). Differences between fermented and unfermented BSG composition, AA and FA profile were determined by T-student statistics. Normal data distribution was verified using Shapiro Wilk test and Levene test to assess the equality of variances. When equal variances were not assumed, Welch statistic was used to compare samples.
For factorial design analysis (software package Statgraphics Centurion XVI), the F test was used to analyze the statistical significance of proposed equations for each interesting parameter and the analysis of variance (ANOVA) for the response surface model. Factors were considered significant when their probability (p-value) was lower than 0.05.
Results and Discussion
Wild Strain Modification
Rhizopus ROR004 is a wild strain obtained from spoiled fruit samples. This strain presents good technological properties and has been described before [31], but looking for an increase in the growth rate and final biomass production, several procedures for genetical modifications were considered in this work. Treatment with UV result in 98.8 ± 0.4% of spore’s mortality and similar ratio is obtained with EB and MNNG, 98.7 ± 0.5 and 88.4 ± 15.6% respectively.
The success of our modifications was determined considering the growth rate of the obtained strains in PDA and comparing them with the wild strain. During initial selection round, strains with similar (or higher) growth rate than the wild strain were selected and isolated. These results were confirmed in successive re-growth steps in PDA. Finally, 4 strains presented a consistently higher growth rate than ROR004 and were selected for further selection.
Due to the difficulty of fungal growth estimation in SSF, possible mutants (4 strains) were grown in SmF at optimal conditions and results were compared to wild type ROR004. After 72 h, growth, biomass, protein, and glucose consumption were analyzed. ROR004 G was significantly the strain with the highest biomass production (g/l) and biomass yield (g DM/g consumed glucose) (Table 3), and, therefore, was used together with ROR004 during SSF optimization.
BSG Fermentation with Rhizopus sp.
In order to determine the variables that change along the SSF, initial experiments were performed and several characteristics, like protein concentration, biological activities and technological properties, were analyzed.
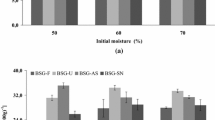
The evolution of determined parameters in fermented BSG is very dependent on the considered incubation periods (up to 192 h) (Figs. 1, 2, 3). Clearly, SSF with Rhizopus sp. changes BSG composition and its TEAC, TPC and DH. Protein content significantly increases up to 50% of initial value, until a final concentration of 31.5 ± 3.7% after 192 h of incubation (Fig. 1). Other authors have required urea supplementation to obtain similar protein (32.9 ± 0.6% after 168 h) on fermented BSG using R. oligosporus [34] but our ROR004 seems to have lower nitrogen requirements (3.3% in our samples compared to 3.9% in the supplemented one, DM basis). In other nitrogen rich substrates, fermentation with Rhizopus sp. resulted in similar protein increase, such as rice bran (2.4% nitrogen DM basis) [21, 46] and nitrogen supplemented fruit wastes (1.6% nitrogen DM basis) [31]. Vegetable mixture (3.5% nitrogen DM basis) also showed similar protein increase using Aspergillus niger as fermentation agent [47]. When low nitrogen substrates are used, otherwise, protein increase is not quite significant [31].
Soluble protein is 6.5 times higher in fermented BSG compared to non-fermented one, probably due to high fungal protease activity (Fig. 1). Other authors have reported lower increase (four times) compared to the non-fermented BSG although total soluble protein content was higher [34], which depends on the extraction system used. Highest soluble protein value is obtained after 192 h (29.5 ± 6.2 mg/g DM), while the highest increase is observed in the initial 96 h.
The DH (%) is significantly higher after 96 h (59.0 ± 5.4%) compared to other fermentation periods (Fig. 2). This could be related to maximal peptide release happens at that moment. As fermentation continues, those peptides and AA are used for fungal protein formation. In any case, fermentation increases dramatically the free amino acid content of the product, and this could improve the protein availability in feed formulations.
TEAC and DPPH reduction are also significantly higher in the final extracts. The highest values are obtained at longest fermentation time, although there are no significant differences between 168 and 192 h, with a DPPH reduction of 52.0 ± 4.7% and TEAC of 965 ± 84 µg/g DM (Fig. 3). TPC also increases together with fermentation time until a final value of 2043 ± 114 µg GAE/g DM (Fig. 2). Results show a significant lineal relation between TPC and TEAC (p < 0.05), with a regression coefficient of 0.984 and R2 of 96.8. Buenrostro-Figueroa et al. [48] also found a positive correlation between antioxidant activity and TPC release during SSF of fig by-products. Our values show nine times higher TPC in the final extracts compared to unfermented BSG from 224 ± 30 to 2043 ± 114 µg GAE/g DM, higher than the values reported previously. This release could be related to the carbohydrate-cleaving enzymes (β-glucosidase) of the fungi, which hydrolyze β-glycosidic linkages [49] and release free aglycones with potential high antioxidant activity. DPPH reduction, otherwise, is lower compared to those studies. TPC and antioxidant activity are closely related; it has been reported that the use of high polyphenol diets in ruminants have promising effects in animal well-being and could be a future strategy for producing meat and milk with antioxidant properties [50,51,52].
No antibacterial activity is detected in the extracts obtained from fermented BSG, while positive inhibition is obtained with tetracycline (1 mg/ml) for Salmonella enterica (1 mm) and for Escherichia coli (2 mm). Therefore, antibacterial activity is not taken into account for the optimization process. These results agree with those obtained by Almeida et al. [14], who reported a negative antibacterial activity of BSG extracts. A similar situation is observed in reducing sugars concentration (result not shown), and it is discarded for further studies.
Finally, the visual inspection of the obtained product (Online resource 1, with plates photographed during fermentation evolution) corroborates that BSG is an ideal substrate for SSF with filamentous fungi, due to its composition and its particle size [12]. Small particles with rigid internal structure allow a complete fungal growth in a short period of time and a complete use of the inter-particle space [53]. This results in a homogenous fermented material and it avoids the presence of under-fermented areas (usually the inner parts of the solid substrates).
Optimization of Fermentation Conditions
The statistical analysis of the results obtained shows that proposed models are significant for the response factors considered (Table 4). As an overview, equations derived from proposed variables can predict positively protein proportion, soluble protein concentration, DH, TEAC, DPPH reduction and TPC. In all cases, p-values are lower than 0.05 (Table 4). The percentage of variation (R2-value) that can be attributed to the independent variables indicates that those significant variables can explain in around 80–90% of the final value (65.5 for total protein, 96.8 for soluble protein, 77.7 for DH, 78.4 for TEAC, 78.6 for DPPH reduction and 92.6 for TPC).
Protein concentration (% DM) depends on the temperature and on the interaction of time and temperature. When fermentation time is shorter there are no differences between samples; otherwise, when fermentation time increases, higher temperature increases the protein content (Fig. 4a). Fermented BSG is composed of 31.7 ± 7.6% of protein at 30 °C and 9 days of fermentation (Table 1).
Soluble protein concentration significantly depends on time, temperature and the interaction between those two (Fig. 4b). High temperature and long fermentation have a positive significant effect in protein content. Our model describes the maximal soluble protein (47.4 ± 3.8 mg/g DM) after 9 days of fermentation (Table 5). Other authors [34] have reported a maximum soluble protein after 6 days of fermentation.
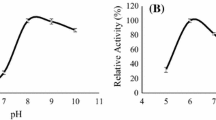
Time and temperature–time interaction are significant parameters for DH (Fig. 4c). The DH is higher at middle fermentation time (72 h). When time is shorter, higher temperature increases the DH, but when fermentation time is longer, lower temperature leads to higher DH. As mentioned above, fungal metabolism is higher at 30 °C and as fermentation time increases, this activity would result in amino acid assimilation for biomass production and would lead to a decrease in the DH of soluble proteins. However, DH is higher at any fermentation time compared to the unfermented BSG, showing that even short fermentation process would improve the amino acid availability of the BSG protein.
TEAC and DPPH reduction depend on time and temperature (Fig. 4d, e). Higher fermentation time and temperature provide the highest antioxidant activity, 723 ± 21 (strain ROR004) and 867 ± 15 (strain ROR004 G) µg Trolox equivalent/g DM, and DPPH reduction, 54.2 ± 1.2 (strain ROR004) and 65.1 ± 1.1% (strain ROR004 G) (Table 5). There is no significant interaction between time and temperature. TPC also depends on time and temperature (Fig. 4f). Long fermentation time and high temperature is the best combination for the highest TPC (2747 ± 112 µg GAE/g DM). Temperature and time interaction is also a significant factor, while at lower fermentation periods temperature effect is lower, at higher fermentation time this effect increases. Other authors have also reported a release of phenolic compounds and an increase of antioxidant activity during rice bran and plum fruit fermentation using R. oryzae and R. oligosporus as fermentation agents [21, 54, 55]; nonetheless, to the best of our knowledge there are no studies related to BSG.
During the SSF processes, the production of enzymes (amylases, pectinases, xylanases, proteases, β-glucosidase, tannase, and ellagitannase) and their synergic activity would increase the TPC release, enhancing the TEAC of the obtained extracts.
Strain effect is not significant for any of the studied variables, that could be because mutagens did not have any significant effect on the protein synthesis metabolism, TPC release and other studied variables.
Optimal conditions for all variables, except for DH, are high temperature and long fermentation time (30 °C, 216 h, Table 6). However, for DH, optimal conditions are high temperature but short fermentation time (Table 6). Although there are no statistical differences between strains, protein concentration, DH and DPPH reduction are maximal using strain 2 (ROR004) and the highest soluble protein, TEAC and TPC are obtained using strain 1 (ROR004 G). As an overall strategy for BSG valorization, the attempt is to optimize all responses at a time; in that case, the optimal conditions are 30 °C, 216 h of fermentation time. These conditions would lead to a fermented BSG with 31.1% protein, 48.6 mg soluble protein/g DM, 29.9% DH, 746 µg Trolox equivalent/g DM, 55.9% DPPH reduction and 2736 µg GAE g/DM.
Fermented BSG Composition and Amino Acid and Fatty Acid Profile
Fermented BSG gross composition changes during fermentation (day 9, 30 °C) (Table 1). Total lipid content goes from 11.1 ± 0.1% in the not fermented sample to 3.8 ± 0.4% after complete fungal growth (Table 1). Other authors have reported also a decrease in total lipids of fermented rice bran [56] due to fungal lipase activity [23]. Protein and NDF, however, increase. NDF is composed by cellulose, hemicellulose, lignin and cutin, and it is also known as fibrous carbohydrate [57]. Its main objective is to maintain ruminal function avoiding the appearance of digestive disorders [58] and therefore, minimum quantities are stablished depending on the specie and production objective. Unfermented BSG is already used by ruminants due to its high proportion of fiber compounds and like proportion is slightly higher in the fermented product, it should continue using as ruminant animal feed [1]. However, there are studies that reported the use of unfermented BSG as fish feed (30% w/w) with improvement of body weight gain [59]. The increase in protein proportion is one of the most interesting effects of SSF in the fermented biomass, which could convert it into a high value alternative of plant proteins. In any case, important factors such as, metabolizable, digestible and absorbable protein should be determined before production of ruminant feed [58].
The AA profile of the fermented BSG is dominated by Glu, Asp, Ala, Leu and Lys (Table 7), with high proportion of essential amino acids (EAA up to 43.1%). Fermented BSG AA profile differs from the unfermented sample in some of the AA: an increase in Met, Gly and Ala proportion and a decrease of Glu, Arg, Pro, Leu and Phe percentage. However, the total EAA percentage does not differ significantly (p < 0.05) within the samples.
Although the general profile does not differ significantly, the total amount of almost all AA significantly increases in the fermented BSG (Table 7). Compared to unfermented BSG, the concentration of Asp, Ser, Glu, His, Thr, Ala, Cys, Val, Met, Lys, Ile, Leu, Tyr, Arg and Phe (mg AA/g BSG) is significantly higher in the fermented BSG. In consequence, overall EAA content in fermented BSG is 1.5 times higher than in the unfermented one. AA composition of fermented BSG was evaluated for the essential amino acid index (EAAI). Higher EAAI indicates the presence of higher concentration of EAA. EAAI for fermented BSG is 1.58 compared to human nutrition FAO standard [40], what means that AA fulfills human requirements.
The essential AA profile of fermented BSG is comparable to standardized feed (fish meal and soybean meal) [60, 61] except for Met. However, each EAA content (mg/g product) of fermented BSG is lower than fish meal due to its high protein proportion, 64–70%, and more similar to soybean meal [62]. Lysine is a critical component in protein source for aquatic feed and feed ingredients. Lysine percentage in fermented BSG is similar (6.8 ± 0.4%) compared to fish meal and soybean meal (7.5 and 6.08% respectively). The essential amino acid composition of fermented BSG makes it an interesting alternative protein source and could contribute greatly towards promoting a sustainable protein source for feed and food applications.
Fermentation modifies the fat fraction of the BSG. For raw or fermented BSG, predominant FA include palmitic (C16:0), oleic (C18:1n-9) and linoleic (C18:2n-6), but fermentation changes FA profile (Table 8). Fungal growth result in a significant increase of C16:0, C18:0, C18:1n-9, C20:0, C20:1n-9, C22:0 and C24:0, leading to a significant increase of the saturated fatty acids (SFA) and monounsaturated fatty acids (MUFA). Linoleic acid (C18:2n-6) decreases significantly in the fermented BSG leading to an overall significant decrease of polyunsaturated fatty acid (PUFA) proportion. Gamma linolenic acid (GLA C18:3n-6), a characteristic FA of Rhizopus sp. biomass [56, 63] and related to anti-inflammatory effects [64], is only detected in fermented BSG as expected. Several medium and long-FA (14:1, 15:0, 15:1, 17:0, 17:1, 20:5n-3, 22:1, 22:5n-3 and 22:6n-3) are not detected in any of the samples.
Biomass composition is a critical aspect for using fungi biomass protein (FBP) for animal feeding or human consumption purposes. Using filamentous fungi as fermentation of BSG leads to a protein rich substrate with higher EAA content and DH. SSF also increases the antioxidant activity of the fermented BSG related to the release of phenolic compounds, what makes SSF a promising alternative to revalorize this agro-industrial by-product as high valuable ingredient for feed and food applications. Furthermore, BSG is also known for containing peptides related to antihypertensive activity [15], for its beneficial nutritional composition (high fiber, protein and β-glucans content) [5] and for specific phenolic compounds (ferulic, caffeic and p-coumaric acid) related to anti-cancer, anti-atherogenic and anti-inflammatory effects [6]. Further research is needed to evaluate the effect of SSF by Rhizopus sp. in the liberation of those specific peptides and phenolic compounds.
Conclusion
Hereby, fungal SSF is a successful strategy for BSG valorization in order to produce a valuable, sustainable, non-climate dependent protein source for animal feed or human food formulations. Compared to the original BSG, the fermented product has a higher protein content, DH and total soluble protein. Furthermore, the TPC of the final product is 11 times higher leading to an increase of its TEAC and is richer in EAA. In any case, specific feed formulations, in vitro digestibility analysis and feeding trials are vital to determine the effect of using fermented BSG as animal feed and fermentation optimization at higher scale is required to validate the BSG fermentation as a cost-effective industrial process.
Abbreviations
- AA:
-
Amino acids
- EAA:
-
Essential amino acids
- EAAI:
-
Essential amino acid index
- FA:
-
Fatty acids
- DH:
-
Degree of hydrolysis
- TEAC:
-
Trolox equivalent antioxidant capacity
- DPPH:
-
2,2-Diphenyl-1-picrylhydrazyl
- TPC:
-
Total phenolic content
References
Mussatto, S.I., Dragone, G., Roberto, I.C.: Brewers’ spent grain: generation, characteristics and potential applications. J. Cereal Sci. 43(1), 1–14 (2006). https://doi.org/10.1016/j.jcs.2005.06.001
Santos, M., Jiménez, J.J., Bartolomé, B., Gómez-Cordovés, C., del Nozal, M.J.: Variability of brewer’s spent grain within a brewery. Food Chem. 80(1), 17–21 (2003). https://doi.org/10.1016/S0308-8146(02)00229-7
Steiner, J., Procopio, S., Becker, T.: Brewer’s spent grain: source of value-added polysaccharides for the food industry in reference to the health claims. Eur. Food Res. Technol. 241(3), 303–315 (2015). https://doi.org/10.1007/s00217-015-2461-7
The Brewers of Europe.: Beer statistics 2017 edition. The Brewers of Europe, Bruxelles (2017)
Ikram, S., Huang, L.Y., Zhang, H.J., Wang, J., Yin, M.: Composition and nutrient value proposition of brewers spent grain. J. Food Sci. 82(10), 2232–2242 (2017). https://doi.org/10.1111/1750-3841.13794
McCarthy, A.L., O’Callaghan, Y.C., Piggott, C.O., FitzGerald, R.J., O’Brien, N.M.: Brewers’ spent grain; bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: a review. Proc. Nutr. Soc. 72(1), 117–125 (2013). https://doi.org/10.1017/S0029665112002820
Ozturk, S., Ozboy, O., Cavidoglu, I., Koksel, H.: Effects of brewer’s spent grain on the quality and dietary fibre content of cookies. J. Inst. Brew. 108(1), 23–27 (2002)
Weger, A., Jung, R., Stenzel, F., Hornung, A.: Optimized energetic usage of brewers’ spent grains. Chem. Eng. Technol. 40(2), 306–312 (2017). https://doi.org/10.1002/ceat.201600186
Russ, W., Mörtel, H., Meyer-Pittroff, R.: Application of spent grains to increase porosity in bricks. Constr. Build. Mater. 19(2), 117–126 (2005). https://doi.org/10.1016/j.conbuildmat.2004.05.014
Mishra, P.K., Gregor, T., Wimmer, R.: Utilising brewer’s spent grain as a source of cellulose nanofibres following separation of protein-based biomass. Bioresources. 12(1), 107–116 (2017). https://doi.org/10.15376/biores.12.1.107-116
Chiang, P.C., Chang, P., You, J.H.: Innovative technology fr controlling voc emissions. J. Hazard. Mater. 31(1), 19–28 (1992). https://doi.org/10.1016/0304-3894(92)87036-f
Xiros, C., Christakopoulos, P.: Biotechnological potential of brewers spent grain and its recent applications. Waste Biomass Valoriz. 3(2), 213–232 (2012). https://doi.org/10.1007/s12649-012-9108-8
Carvalheiro, F., Esteves, M.P., Parajó, J.C., Pereira, H., Gírio, F.M.: Production of oligosaccharides by autohydrolysis of brewery’s spent grain. Bioresour. Technol. 91(1), 93–100 (2004). https://doi.org/10.1016/S0960-8524(03)00148-2
Almeida, A.D., Geraldo, M.R.F., Ribeiro, L.F., Silva, M.V., Maciel, M., Haminiuk, C.W.I.: Bioactive compounds from brewer’s spent grain: phenolic compounds, fatty acids and in vitro antioxidant capacity. Acta Sci.-Technol. 39(3), 269–277 (2017). https://doi.org/10.4025/actascitechnol.v39i3.28435
Connolly, A., O’Keeffe, M.B., Piggott, C.O., Nongonierma, A.B., FitzGerald, R.J.: Generation and identification of angiotensin converting enzyme (ACE) inhibitory peptides from a brewers’ spent grain protein isolate. Food Chem. 176, 64–71 (2015). https://doi.org/10.1016/j.foodchem.2014.12.027
Vieira, E., Teixeira, J., Ferreira, I.: Valorization of brewers’ spent grain and spent yeast through protein hydrolysates with antioxidant properties. Eur. Food Res. Technol. 242(11), 1975–1984 (2016). https://doi.org/10.1007/s00217-016-2696-y
Radosavljevic, M., Pejin, J., Kocic-Tanackov, S., Mladenovic, D., Djukic-Vukovic, A., Mojovic, L.: Brewers’ spent grain and thin stillage as raw materials in l-(+)-lactic acid fermentation. J. Inst. Brew. 124(1), 23–30 (2018). https://doi.org/10.1002/jib.462
Gregori, A., Švagelj, M., Pahor, B., Berovič, M., Pohleven, F.: The use of spent brewery grains for Pleurotus ostreatus cultivation and enzyme production. N Biotechnol. 25(2), 157–161 (2008). https://doi.org/10.1016/j.nbt.2008.08.003
Sandhya, C., Sumantha, A., Szakacs, G., Pandey, A.: Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process Biochem. 40(8), 2689–2694 (2005). https://doi.org/10.1016/j.procbio.2004.12.001
Nigam, P.S., Pandey, A.: Biotechnology for agro-industrial residues utilization. Springer, Dordrecht (2009)
Kupski, L., Cipolatti, E., da Rocha, M., Oliveira, M.D., Souza-Soares, L.D., Badiale-Furlong, E.: Solid-state fermentation for the enrichment and extraction of proteins and antioxidant compounds in rice bran by Rhizopus oryzae. Brazil. Arch. Biol. Technol. 55(6), 937–942 (2012). https://doi.org/10.1590/S1516-89132012000600018
Lizardi-Jimenez, M.A., Hernandez-Martinez, R.: Solid state fermentation (SSF): diversity of applications to valorize waste and biomass. 3 Biotech. 7(1), 44 (2017). https://doi.org/10.1007/s13205-017-0692-y
Abd Razak, D.L., Abd Rashid, N.Y., Jamaluddin, A., Sharifudin, S.A., Abd Kahar, A., Long, K.: Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 16(2), 127–134 (2017). https://doi.org/10.1016/j.jssas.2015.04.001
Cooray, S.T., Chen, W.N.: Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value. J. Funct. Foods. 42, 85–94 (2018). https://doi.org/10.1016/j.jff.2017.12.027
Ghosh, B., Ray, R.R.: Current commercial perspective of Rhizopus oryzae: a review. J. Appl. Sci. 11(14), 2470–2486 (2011). https://doi.org/10.3923/jas.2011.2470.2486
Meussen, B.J., de Graaff, L.H., Sanders, J.P., Weusthuis, R.A.: Metabolic engineering of Rhizopus oryzae for the production of platform chemicals. Appl. Microbiol. Biotechnol. 94(4), 875–886 (2012). https://doi.org/10.1007/s00253-012-4033-0
Cantabrana, I., Perise, R., Hernández, I.: Uses of Rhizopus oryzae in the kitchen. Int. J. Gastron. Food Sci. 2(2), 103–111 (2015). https://doi.org/10.1016/j.ijgfs.2015.01.001
Villas-Boas, S.G., Esposito, E., Mitchell, D.A.: Microbial conversion of lignocellulosic residues for production of animal feeds. Anim. Feed Sci. Technol. 98(1–2), 1–12 (2002). https://doi.org/10.1016/s0377-8401(02)00017-2
Lopez, E., Deive, F.J., Longo, M.A., Sanroman, M.A.: Strategies for utilisation of food-processing wastes to produce lipases in solid-state cultures of Rhizopus oryzae. Bioprocess. Biosyst. Eng. 33(8), 929–935 (2010). https://doi.org/10.1007/s00449-010-0416-8
Hsiao, N.-W., Chen, Y., Kuan, Y.-C., Lee, Y.-C., Lee, S.-K., Chan, H.-H., Kao, C.-H.: Purification and characterization of an aspartic protease from the Rhizopus oryzae protease extract. Peptidase R. Electron. J. Biotechnol. 17(2), 89–94 (2014). https://doi.org/10.1016/j.ejbt.2014.02.002
Ibarruri, J., Hernández, I.: Rhizopus oryzae as fermentation agent in food derived sub-products. Waste Biomass Valoriz. 9(11), 2107–2115 (2018). https://doi.org/10.1007/s12649-017-0017-8
Ferreira, J.A., Lennartsson, P.R., Niklasson, C., Lundin, M., Edebo, L., Taherzadeh, M.J.: Spent sulphite liquor for cultivation of an edible Rhizopus sp. Bioresources 7(1), 173–188 (2012)
FazeliNejad, S., Ferreira, J.A., Brandberg, T., Lennartsson, P.R., Taherzadeh, M.J.: Fungal protein and ethanol from lignocelluloses using Rhizopus pellets under simultaneous saccharification, filtration and fermentation (SSFF). Biofuel Res. J. 3(1), 372–378 (2016). https://doi.org/10.18331/brj2016.3.1.7
Canedo, M.S., de Paula, F.G., da Silva, F.A., Vendruscolo, F.: Protein enrichment of brewery spent grain from Rhizopus oligosporus by solid-state fermentation. Bioprocess Biosyst. Eng. 39(7), 1105–1113 (2016). https://doi.org/10.1007/s00449-016-1587-8
Centro de Investigación y Control de la Calidad: Análisis de alimentos: métodos oficiales y recomendados por el Centro de Investigación y Control de la Calidad. Ministerio de Sanidad y Consumo, Madrid (1985)
UNE EN ISO.: Animal feeding stuffs—determination of amylase-treated neutral detergent fibre content (aNDF). (2006)
Satari, B., Karimi, K., Taherzadeh, M.J., Zamani, A.: Co-production of fungal biomass derived constituents and ethanol from citrus wastes free sugars without auxiliary nutrients in airlift bioreactor. Int. J. Mol. Sci. 17(3), 302 (2016). https://doi.org/10.3390/ijms17030302
Bligh, E.G., Dyer, W.J.: A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37(8), 911–917 (1959). https://doi.org/10.1139/o59-099
Waghmare, A.G., Salve, M.K., LeBlanc, J.G., Arya, S.S.: Concentration and characterization of microalgae proteins from Chlorella pyrenoidosa. Bioresour. Bioprocess. 3(1), 1 (2016). https://doi.org/10.1186/s40643-016-0094-8
FAO/WHO/UNU Expert Consultation. Protein and amino acid requirements in human nutrition, vol. 935. WHO Technical Report Series, Geneva (2007)
Nielsen, P.M., Petersen, D., Dambmann, C.: Improved method for determining food protein degree of hydrolysis. J. Food Sci. 66(5), 642–646 (2001). https://doi.org/10.1111/j.1365-2621.2001.tb04614.x
Bougherra, F., Dilmi-Bouras, A., Balti, R., Przybylski, R., Adoui, F., Elhameur, H., Chevalier, M., Flahaut, C., Dhulster, P., Naima, N.: Antibacterial activity of new peptide from bovine casein hydrolyzed by a serine metalloprotease of Lactococcus lactis sub lattice BR16. J. Funct. Foods. 32(Supplement C), 112–122 (2017). https://doi.org/10.1016/j.jff.2017.02.026
Brand-Williams, W., Cuvelier, M.E., Berset, C.: Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 28(1), 25–30 (1995). https://doi.org/10.1016/S0023-6438(95)80008-5
Singleton, V.L., Rossi, J.A.: Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 16(3), 144 (1965)
Miller, G.L.: Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 31(3), 426–428 (1959). https://doi.org/10.1021/ac60147a030
Oliveira, M.d.S., Feddern, V., Kupski, L., Cipolatti, E.P., Badiale-Furlong, E., de Souza-Soares, L.A.: Physico-chemical characterization of fermented rice bran biomass. Caracterización fisico-química de la biomasa del salvado de arroz fermentado. CyTA—J. Food. 8(3), 229–236 (2010). https://doi.org/10.1080/19476330903450274
Rajesh, N., Imelda, J., Raj, R.P.: Value addition of vegetable wastes by solid-state fermentation using Aspergillus niger for use in aquafeed industry. Waste Manag. 30(11), 2223–2227 (2010). https://doi.org/10.1016/j.wasman.2009.12.017
Buenrostro-Figueroa, J.J., Velázquez, M., Flores-Ortega, O., Ascacio-Valdés, J.A., Huerta-Ochoa, S., Aguilar, C.N., Prado-Barragán, L.A.: Solid state fermentation of fig (Ficus carica L.) by-products using fungi to obtain phenolic compounds with antioxidant activity and qualitative evaluation of phenolics obtained. Process Biochem. 62, 16–23 (2017). https://doi.org/10.1016/j.procbio.2017.07.016
Ajila, C.M., Gassara, F., Brar, S.K., Verma, M., Tyagi, R.D., Valéro, J.R.: Polyphenolic antioxidant mobilization in apple pomace by different methods of solid-state fermentation and evaluation of its antioxidant activity. Food Bioprocess Technol. 5(7), 2697–2707 (2012). https://doi.org/10.1007/s11947-011-0582-y
Fruet, A.P.B., Stefanello, F.S., Rosado Júnior, A.G., Souza, A.N.M.d., Tonetto, C.J., Nörnberg, J.L.: Whole grains in the finishing of culled ewes in pasture or feedlot: performance, carcass characteristics and meat quality. Meat Sci. 113, 97–103 (2016). https://doi.org/10.1016/j.meatsci.2015.11.018
Paraskevakis, N.: Effects of dietary dried Greek Oregano (Origanum vulgare ssp. hirtum) supplementation on blood and milk enzymatic antioxidant indices, on milk total antioxidant capacity and on productivity in goats. Anim. Feed Sci. Technol. 209, 90–97 (2015). https://doi.org/10.1016/j.anifeedsci.2015.09.001
Castillo, C., Pereira, V., Abuelo, A., Hernandez, J.: Effect of supplementation with antioxidants on the quality of bovine milk and meat production. Sci. World J. (2013) https://doi.org/10.1155/2013/616098
Wang, D., Sakoda, A., Suzuki, M.: Biological efficiency and nutritional value of Pleurotus ostreatus cultivated on spent beer grain. Bioresour. Technol. 78(3), 293–300 (2001). https://doi.org/10.1016/S0960-8524(01)00002-5
Dulf, F.V., Vodnar, D.C., Socaciu, C.: Effects of solid-state fermentation with two filamentous fungi on the total phenolic contents, flavonoids, antioxidant activities and lipid fractions of plum fruit (Prunus domestica L.) by-products. Food Chem. 209, 27–36 (2016). https://doi.org/10.1016/j.foodchem.2016.04.016
Correia, R.T.P., McCue, P., Magalhães, M.M.A., Macêdo, G.R., Shetty, K.: Production of phenolic antioxidants by the solid-state bioconversion of pineapple waste mixed with soy flour using Rhizopus oligosporus. Process Biochem. 39(12), 2167–2172 (2004). https://doi.org/10.1016/j.procbio.2003.11.034
Oliveira, M.D., Feddern, V., Kupski, L., Cipolatti, E.P., Badiale-Furlong, E., de Souza-Soares, L.A.: Changes in lipid, fatty acids and phospholipids composition of whole rice bran after solid-state fungal fermentation. Bioresour. Technol. 102(17), 8335–8338 (2011). https://doi.org/10.1016/j.biortech.2011.06.025
FEDNA. Fibra neutro detergente, Ácido detergente Y Lignina (FND,FAD,LAD secuenciales). http://fundacionfedna.org/tecnicas_de_analisis/fibra-neutro-detergente-%C3%A1cido-detergente-y-lignina-fndfadlad-secuenciales. Accessed 30 Sep, 2018
Ferret, A., Calsamiglia, S., Bach, A., Devant, M., Fernández, C., García-Rebollar, P.: Necesidades nutricionales para rumiantes de cebo. In: FEDNA (Fundación Española para el Desarrollo de la Nutrición Animal) (2008)
Kaur, V.: Incorporation of brewery waste in supplementary feed and its impact on growth in some carps. Bioresour. Technol. 91(1), 101–104 (2004). https://doi.org/10.1016/s0960-8524(03)00073-7
Miles, R.D., Chapman, F.A.: The benefits of fish meal in aquaculture diets. Institute of Food and Agricultural Sciences, University of Florida, Florida (2006)
Asadollahzadeh, M., Ghasemian, A., Saraeian, A., Resalati, H., Taherzadeh, M.: Production of fungal biomass protein by filamentous fungi cultivation on liquid waste streams from pulping process. BioResources. 13(1), 5013–5031 (2018). https://doi.org/10.15376/biores.13.3.5013-5031
Nitayavardhana, S., Issarapayup, K., Pavasant, P., Khanal, S.K.: Production of protein-rich fungal biomass in an airlift bioreactor using vinasse as substrate. Bioresour. Technol. 133, 301–306 (2013). https://doi.org/10.1016/j.biortech.2013.01.073
Wei, D., Li, M., Zhang, X., Ren, Y., Xing, L.: Identification and characterization of a novel delta12-fatty acid desaturase gene from Rhizopus arrhizus. FEBS Lett. 573(1–3), 45–50 (2004). https://doi.org/10.1016/j.febslet.2004.06.100
Innes, J.K., Calder, P.C.: Prostaglandins: Omega-6 fatty acids and inflammation. Leukot. Essent. Fatty Acids. 132, 41–48 (2018). https://doi.org/10.1016/j.plefa.2018.03.004
Acknowledgements
Authors thank to Boga Cooperative for providing the BSG. This work was funded by the Basque Government (Department of Economic and Infrastructure Development, Agriculture, Fisheries and Food policy). This paper is Contribution No. 901 from AZTI (Food Research).
Funding
Funding was provided by Ekonomiaren Garapen eta Lehiakortasun Saila, Eusko Jaurlaritza.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ibarruri, J., Cebrián, M. & Hernández, I. Solid State Fermentation of Brewer’s Spent Grain Using Rhizopus sp. to Enhance Nutritional Value. Waste Biomass Valor 10, 3687–3700 (2019). https://doi.org/10.1007/s12649-019-00654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12649-019-00654-5