Abstract
Trametes versicolor is a white-rot fungus known as a producer of extracellular enzymes such as laccase, manganese-peroxidase, and lignin-peroxidase. The production of these enzymes requires detailed knowledge of the growth characteristics and physiology of the fungus. Submerged cultivations of T. versicolor on glucose, fructose, and sucrose as sole carbon sources were performed in shake flasks. Sucrose hydrolysis catalyzed by the whole cells of T. versicolor was considered as one-step enzymatic reaction described with Michaelis–Menten kinetics. Kinetic parameters of invertase-catalyzed sucrose hydrolysis were estimated (Km = 7.99 g dm−3 and Vm = 0.304 h−1). Monod model was used for description of kinetics of T.versicolor growth on glucose and fructose as sole carbon sources. Growth associated model parameters were estimated from the experimental results obtained by independent experiments \( \left( {\mu_{\max }^{\text{G}} = 0. 1 4 {\text{ h}}^{ - 1} ,\;K_{\text{S}}^{\text{G}} = 8.0 6 {\text{ g dm}}^{ - 3} ,\;\mu_{\max }^{\text{F}} = 0. 3 7 {\text{ h}}^{ - 1} {\text{ and }}K_{\text{S}}^{\text{F}} = 5 4. 8 {\text{ g dm}}^{ - 3} } \right). \) Developed mathematical model is in good agreement with the experimental results.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
White-rot fungi are basidiomycetes commonly found in the nature. They attack wood and cause its degradation because they posses an extracellular enzymatic system mainly composed from laccase, lignin-peroxidase, and manganese-peroxidase, which function together with H2O2-producing oxidases and secondary metabolites [1]. The main characteristic that differentiates white-rot fungi from most other microorganisms is their ability to mineralize all components of lignin to carbon dioxide and water. Possible biotechnological application of white-rot fungi varied from degradation of industrial pollutants and other hazardous materials [2] till production of value-added and efficient ligninolytic enzymes [3].
In order to produce ligninolytic enzymes, white-rot fungi can be cultivated either in solid culture (solid-state fermentation), which is the natural way of fungus growth, or in submerged fermentation. Easier and cheaper methods of enzymes isolation and purification can be reached when submerge fermentation is taking place [4]. If cultivated in submerged fermentation, fungi can form either dispersed mycelial filaments or interwoven mycelial masses called pellets, depending of genetic material of the fungal species, the nature of the inoculum, and chemical and physical cultivation conditions. Obtaining uniforming pellets of the same size and shape is not easy and many factors influence on it [5–8].
The enzyme production by white-rot fungi using different methods of submerged and solid-state cultivation has been intensively studied [3, 9–16]. Although there is an increasing interest in using these fungal cultures, there is little published data of the growth of white-rot fungi [17, 18], mostly due to the difficulty of measuring the biomass concentration [19]. Kinetics of white-rot fungi growth was studied using glucose [17–20] and glycerol [21] as carbon sources only.
Microbial growth kinetics is an indispensable tool in physiology, genetics, ecology, and biotechnology [22]. Modeling of biotransformation processes have a large potential in searching for optimal process conditions, development and process design, control, scale-up, and identifying of the process cost structure leading to significant material and cost savings [23, 24]. For that purpose, black box un-structured cell models are commonly used, while they offer significant simplicity providing general understanding of the process at the same time. Estimation of the kinetic parameters in unstructured model is the most sensitive part of the model development, and it is carried out from the batch, fed batch, and continuous experimental results [25]. However, there are two major disadvantages when unstructured models are used. First of all, hard work is needed to identify large amounts of model parameters, although the physiological significance of these parameters is not completely clear. Second, un-structured models cannot really embrace the dynamic changes due to changes in environmental factors, working conditions and batch fermentation runs, therefore, the general prediction and control performance based on the model is limited [26].
In this study, T. versicolor growth on glucose, fructose, and sucrose in shake flasks was studied. Mathematical model of the process composed of sucrose hydrolysis catalyzed by intracellular enzyme (probably invertase) and microbial growth on glucose and fructose was developed. Kinetic parameters for invertase-catalyzed reaction and for biomass growth on glucose and fructose were estimated using experimental results obtained from single substrate experiments. Mathematical model was verified and sensitivity analysis of estimated parameters was performed. To the best of our knowledge, this is the first attempt to be modeled white-rot fungi growth on fructose and sucrose as substrates.
Mathematical model
Trametes versicolor growth (X) was studied in shake flasks during its submerged cultivation on glucose (G), fructose (F), and sucrose (S) as sole carbon sources. When sucrose is used as carbon source, reaction scheme given by Eq. 1–3 was used for model development. First, sucrose is enzymatically hydrolyzed to glucose and fructose followed by biomass growth simultaneously using glucose and fructose.
For simplification, invertase-catalyzed sucrose degradation is considered as one-step enzymatic reaction. Viscosity of reaction media remains constant during cultivation. Taken these basic assumptions into accounts, mathematical model composed of T. versicolor growth on glucose and fructose and enzymatic hydrolysis of sucrose by intracellular invertase was developed. The Monod type model was used for the description of biomass growth on glucose (Eq. 4) and fructose (Eq. 5), while Michaelis–Menten type kinetics was applied for the description of invertase-catalyzed sucrose degradation (Eq. 6)
where μ is biomass growth rate, μmax is maximal biomass growth rate, γ is concentration, K S is Monod constant, r is rate of enzymatic hydrolysis, V m is maximal reaction rate, and K m is Michaelis–Menten constant. Overall biomass growth rate is given as sum of biomass growth rate on glucose and on fructose (Eq. 7). Glucose and fructose are the only limiting substrates. All other nutrients, such as nitrogen, phosphate, and other growth factors are not limiting.
The mass balances of biomass (Eq. 8), glucose (Eq. 9), fructose (Eq. 10), and sucrose (Eq. 11) in batch reactor are represented by the following differential equations:
where Y is yield biomass–substrate and t is reaction time.
Materials and methods
Materials
Microorganism
Trametes versicolor MZKI G-99 was kindly donated by the Microbial culture collection of the National Institute of Chemistry, Ljubljana, Slovenia. The culture was cultivated at 30 °C, stored at 4 °C on medium containing 2% malt-extract agar and 2% agar–agar, and transferred at one-month intervals.
Chemicals
Fructose was purchased from Sigma-Aldrich (Steinheim, Germany); sucrose was purchased from Kandit (Osijek, Croatia); glucose, Na2HPO4, KH2HPO4, agar–agar, and citric acid were purchased from Kemika (Zagreb, Croatia); MgSO4·7H2O and CaCl2·2H2O were purchased from Merck (Darmstadt, Germany); peptone, malt-extract agar, and yeast extract were purchased from Difco–Becton, Dickinson and Co (New Jersey, USA).
Methods
Analytics
The biomass concentration was determined by dry weight method. The culture medium was filtered through 0.45 μm filter paper (5 H/N, Munktell & Filtrak GmbH, Barenstein, Germany). Retained biomass was washed with distilled water and dried at a temperature of 80 °C for 24 h.
The concentration of glucose, fructose, and sucrose were determined by high-pressure liquid chromatography (Shimadzu, Kyoto, Japan). The HPLC was equipped with a C18 column (Carbohydrate Ca2+, 300 × 6.5 mm, CS-Chromatographie service GmbH, Germany) and an index refraction detector. The analysis was performed at 80 °C with the flow of mobile phase (redistilled water) of 0.5 cm3 min−1. Retention times for sucrose, glucose, and fructose were 7.6, 9.1, and 10.6 min, respectively.
Inoculum preparation
Stock culture was inoculated into 500 cm3 shake-flask containing 50 cm3 medium (pH 6.13) with 10 g dm−3 glucose, 0.2 g dm−3 peptone, 0.3 g dm−3 yeast extract, 0.8 g dm−3 KH2HPO4, 0.2 g dm−3 Na2HPO4, and 0.5 g dm−3 MgSO4·7H2O. The cotton-stopped flasks were incubated in an orbital shaker (Innova 4330, New Brunswick Scientific, Edison, NJ, USA) at 140 rpm and 27 °C. After 72 h of cultivation, cultures were harvested by filtration (5 H/N, Munktell & Filtrak GmbH, Barenstein, Germany). Image analysis technique (Motic Images Plus 2.0 ML, Motic China Group Co. Ltd., 1999–2004) was used for determination of size and shape of produced pellets. Only pellets of the same size (diameter of 3 ± 0.5 mm) and shape (spherical) were used for all further experiments (Fig. 1).
Kinetic measurements
Kinetic measurements were carried out in 250 cm3 glass bioreactor containing 50 cm3 of reaction media. Reaction media was composed of 10 g dm−3 or 20 g dm−3 of sucrose, 0.8 g dm−3 of peptone, 2 g dm−3 of yeast extract, 0.8 g dm−3 of KH2PO4, 0.2 g dm−3 of Na2HPO4, 0.5 g dm−3 of MgSO4·7H2O, 0.05 g dm−3 CaCl2·2H2O, and 0.15 g dm−3 citric acid. Reaction was started with addition of 4.3 g dm−3 of mycelial pellets (dry weight). Reaction mixture was stirred on magnetic stirrer at 200 rpm and thermostated at 27 °C. Samples were taken every 15 min for 2 h, centrifuged at 9,000 rpm for 10 min (Centric 150, Tehtnica, Železniki, Slovenia), filtered (0.25 μm, Macherey–Nagel GmbH & Co. KG, Düren, Germany) and analyzed on HPLC for determination of glucose, fructose, and sucrose concentrations.
Batch cultivation
Experiments were carried out in 500 cm3 Erlenmayer flasks on orbital shaker (Innova 4330, New Brunswick Scientific, Edison, NJ, USA) at T = 27 °C and 140 rpm. Reaction mixture contained sole carbon source (glucose or fructose or sucrose), 0.8 g dm−3 of peptone, 2 g dm−3 of yeast extract, 0.8 g dm−3 of KH2PO4, 0.2 g dm−3 of Na2HPO4, 0.5 g dm−3 of MgSO4·7H2O, 0.05 g dm−3 CaCl2·2H2O, and 0.15 g dm−3 of citric acid. The initial concentration of glucose, fructose, sucrose, and biomass (dry weight) for different batch experiments are given in Table 1. Reaction was started by addition of mycelial pellets.
Each experiment was conducted simultaneously in 14 flasks. At irregular intervals, a sample from each flask was taken, filtered, and analyzed on HPLC for the concentration of sugar. When significant change of sugar concentration was experimentally observed, content of two flasks was quantitatively transferred on filter paper, and biomass was quantified as described above. The seven cycles for determination of biomass concentration were carried out during one experiment.
Data handling
Model parameters were estimated by nonlinear regression analysis using Simplex and Least Squares method implemented in the SCIENTIST software (MicroMath®, Salt Lake City, USA). They were evaluated by fitting the model to the experimental data for sucrose hydrolysis or from batch cultivation experiments for biomass growth. The calculated data were compared with the experimental data, recalculated in the optimization routine, and fed again to the integration step until a minimal error between experimental and integrated values was achieved. The residual was defined as the sum of the squares of the differences between the experimental and the calculated data. “Episode” algorithm for the stiff system of differential equations, implemented in the SCIENTIST software was used for simulations of batch reactor mathematical models.
Results and discussion
Kinetic of invertase-catalyzed sucrose degradation
Invertase-catalyzed sucrose degradation was studied in batch reactor. It is considered as one-step enzymatic reaction with glucose and fructose as products (Eq. 1). Experiments were carried out at high initial concentration of mycelial pellets (4.3 g dm−3) at two different initial concentration of sucrose (10 g dm−3—Fig. 2a, and 20 g dm−3—Fig. 2b). The high initial concentration of mycelial pellets was used to decrease the influence of the biomass growth on produced glucose and fructose on total biomass concentration. During the reaction time of 2 h, the consumption of glucose and fructose are not supposed to be seen.
Obviously, the fungus T. versicolor has intracellular invertase as its constituent, while sucrose biotransformation to glucose and fructose was experimentally proved. The proposed kinetic model for the intracellular invertase-catalyzed sucrose degradation (Eq. 6) was fitted to experimental data obtained at lower initial sucrose concentration γ S,0 = 10 g dm−3 (Fig. 2a). Kinetic parameters K m = 7.99 g dm−3 and V m = 0.304 h−1 were estimated (Table 2). This was first attempt to estimate K m and V m for sucrose degradation catalyzed by whole cells of T. versicolor as a source of intracellular invertase. Estimated K m for intracellular invertase is in accordance with reported values for free invertase from baker’s yeast (K m = 8.21 g dm−3; [27]) and significantly lower in comparison with immobilized invertase from Cladosporium cladosporioides (K m = 153 g dm−3; [28]). Estimated parameters were validated for the higher initial sucrose concentration γ S,0 = 20 g dm−3 at same process conditions (Fig. 2b). Developed kinetic model describes experimental data quite well even for higher initial sucrose concentration. Glucose and fructose consumption was experimentally not observed at reaction period of 2 h.
Biomass growth on glucose
Kinetics of biomass growth on glucose as a sole carbon source was investigated in batch experiments. Experiments were performed at different initial concentrations of glucose and different initial concentrations of mycelial pellets (Table 1). Kinetic parameters of the Monod model (Eq. 4) were estimated from the experiment performed at γ G,0 = 11.0 g dm−3 and γ X,0 = 0.083 g dm−3. Model simulation results and experimental data are shown in Fig. 3, while estimated model parameters are given in Table 2. Yield coefficient biomass–glucose was calculated from experimental results for each initial concentration of glucose and biomass.
Estimated maximal specific growth rate on glucose, \( \mu_{\max }^{\text{G}} = \, 0. 1 4 {\text{ h}}^{ - 1} \) is significantly higher then values reported for T. versicolor growth on glucose as a sole carbon source (0.034–0.036 h−1 for growth in shake flasks and 0.032–0.052 h−1 for growth in stirred tank reactor). On the other hand, estimated Monod constant \( K_{\text{S}}^{\text{G}} = 8.0 6 {\text{ g dm}}^{ - 3} , \) and calculated yield coefficient Y X/G = 0.24 g g−1 are similar to the literature data [17, 19]. Assumed kinetic model (Eq. 4) for T. versicolor growth on glucose as a sole carbon substrate was validated using experimental results obtained in shake flasks for different initial glucose concentrations and initial biomass concentrations. Experimental data and model simulation results for specified initial process conditions (Table 1, Experiments 2, 3 and 4) are presented in Fig. 4.
Model simulation and experimental results for biomass growth on glucose in (a) Experiment 2 (γ G,0 = 24.0 g dm−3, γ X,0 = 0.078 g dm−3, and Y X/G = 0.18 g g−1), (b) Experiment 3 (γ G,0 = 5.7 g dm−3, γ X,0 = 0.113 g dm−3, and Y X/G = 0.20 g g−1), (c) Experiment 4 (γ G,0 = 12.1 g dm−3, γ X,0 = 0.667 g dm−3, and Y X/G = 0.27 g g−1), and (d) Experiment 5 (γ G,0 = 36.0 g dm−3, γ X,0 = 0.074 g dm−3, and Y X/G = 0.17 g g−1) (experimental data shown as average value of two data sets with confidence interval calculated using significance level of 0.05)
Proposed mathematical model is able to describe process behavior for a wide range of different initial concentration of glucose (5.7–24 g dm−3) and initial concentrations of biomass (0.078–0.667 g dm−3; Fig. 4a–c). For higher initial concentration of glucose (36 g dm−3), model cannot quantitatively describe dynamic of glucose consumption (Fig. 4d). This is probably due to inhibition by glucose or unknown product. On the other hand, biomass concentration change obtained experimentally was in good correlation with model prediction results. Experimentally obtained yield coefficients biomass–glucose did not change significantly for different initial process conditions concerning glucose and biomass (Y X/G = 0.17–0.27 g g−1).
Biomass growth on fructose
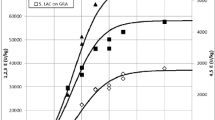
In order to evaluate kinetic parameters for biomass growth on fructose as a sole carbon source, batch experiments in shake flasks were performed. Experiments were carried out for two different initial concentrations of mycelial pellets (Table 1, Experiment 6 and 7). The kinetic model (Eq. 5) was fitted to experimental data obtained from the experiment performed at γ F,0 = 10.5 g dm−3 and γ X,0 = 0.408 g dm−3. The kinetic parameters for biomass growth on fructose are presented in Table 2, while the comparison of the model with the experimental data for different initial concentration of biomass is presented in Fig. 5. Yield coefficient biomass–fructose was calculated from experimental results for both experiments.
Model simulation and experimental results for biomass growth on fructose in (a) Experiment 6 (γ F,0 = 10.5 g dm−3, γ X,0 = 0.408 g dm−3, and Y X/F = 0.41 g g−1), (b) Experiment 7 (γ F,0 = 10.5 g dm−3, γ X,0 = 0.113 g dm−3, and Y X/F = 0.59 g g−1) (experimental data shown as average value of two data sets with confidence interval calculated using significance level of 0.05)
Kinetic parameters \( \mu_{\max }^{\text{F}} = \, 0. 3 7 {\text{ h}}^{ - 1} \) and \( K_{\text{S}}^{\text{F}} = 54. 8 {\text{ g dm}}^{ - 3} \) for T. versicolor growth on fructose as a sole carbon source are estimated for the first time to our best knowledge. Obviously, T. versicolor shows higher affinity toward glucose as a growth substrate, which is indicated by lower estimated K S value (Table 2). Estimated kinetic parameters were validated using experimental results obtained for lower initial biomass concentration (Fig. 5b). Assumed Monod model (Eq. 5) describes experimental data quite well. Experimentally obtained yield coefficients of biomass–fructose was significantly influenced by different initial concentration of biomass (Y X/F = 0.41 g g−1 in Experiment 6 and Y X/F = 0.59 g g−1 in Experiment 7), which was not the case with experiments that were performed with glucose as sole carbon source.
Mass-transfer resistance during pellet’s growth
In batch experiments of T. versicolor cultivation on glucose and fructose as a sole carbon source, before measuring biomass concentration, pellets were photographed and analyzed using an image analysis technique as described in “Inoculum preparation”. In order to investigate the influence of mass-transfer resistance during pellet’s growth by calculating Thiele module, two representative experiments with glucose and fructose as sole carbon source were chosen (Experiment 3 and Experiment 7, Table 1). Changes in the average radius of all the pellets and in the radius of the largest pellets during fungi cultivation were calculated according to the results of area and the parameters obtained by an image analysis technique. Thiele module (Φ) was calculated according to the equation \( \Phi = {\frac{{V_{\text{P}} }}{{A_{\text{P}} }}} \cdot \sqrt {{\frac{{(\mu_{ \max } \cdot c_{\text{S}} )/(Y_{\text{X/G}} \cdot K_{\text{S}} )}}{{D_{\text{S}} }}}} , \) for both, average and maximal values of radius using previously estimated kinetic parameters (Table 2) and diffusion coefficient D s = 0.57 × 10−5 cm2 s−1 [29]. The results are presented in Table 3.
In both experiments, when using the average pellet’s radius, mass resistance was not significant, and the calculated Thiele module was below the value of the three. According to the criteria for assessing the magnitude of mass-transfer effects on overall kinetics when Thiele module is higher than three, diffusion is limiting rate process [29]. Only when the maximum radius of pellets was taken into account (Experiment 3 with glucose as the sole carbon source), the maximum value of Thiele module from 3.31 to 3.52 during the exponential phase of growth were calculated, indicating the influence of mass-transfer resistance to the growth of biomass.
Sensitivity analysis of the process model
In order to qualify the sensitivity of model predictions with respect to kinetic parameters of T. versicolor growth on sucrose as substrate, sensitivity analysis has been performed. For this purpose, the mathematical model parameters \( \left( {K_{\text{m}} , \, V_{\text{m}} , \, \mu_{\max }^{\text{G}} ,K_{\text{S}}^{\text{G}} ,\mu_{\max }^{\text{F}} ,K_{\text{S}}^{\text{F}} } \right) \) were varied in the range of ±50%, and their effect on the final biomass concentration was determined (Fig. 6). All simulations were performed for a period of 80 h.
The process is significantly influenced by kinetics of invertase-catalyzed hydrolysis of sucrose, while sucrose degradation participates in the biomass growth. Change the parameters related to the growth of T. versicolor on glucose has less impact on the final biomass concentration. On the other hand, kinetic parameters have great influence on the growth of T. versicolor on fructose. This is because the yield coefficient biomass-substrate is 2.5 times higher for fructose.
Batch experiment with sucrose
Developed mathematical model of the process (Eq. 4–11), which is composed of sucrose hydrolysis catalyzed by the intracellular invertase from T. versicolor, and its growth on glucose and fructose has been validated in batch experiment using sucrose as carbon source. Experiments were performed in duplicate with the following initial conditions of sucrose and biomass: γ S,0 = 21.1 g dm−3 and γ X,0 = 0.076 g dm−3, respectively. Kinetic parameters (Table 2) and yield coefficients, which were estimated in independent experiments with glucose (Experiment 2) and fructose (Experiment 6) as the sole carbon sources were used for model simulations. Experimental results and model simulation results are presented in Fig. 7a.
Model simulation and experimental results in batch experiment with sucrose as carbon source (a) using kinetic parameters from Table 2 and for γ S,0 = 21.1 g dm−3, γ X,0 = 0.076 g dm−3, Y X/G = 0.24 g g−1, and Y X/F = 0.41 g g−1, (b) using kinetic parameters from Table 3 and for γ S,0 = 21.1 g dm−3, γ X,0 = 0.076 g dm−3
The simulated results, in Fig. 7a, are not in qualitative agreement with the experimental observations. Deviation of the predicted and measured values was probably the results of the use of kinetic parameters estimated from the independently performed experiments with glucose and fructose as sole carbon sources for model simulation. However, developed model seems to have a good predictive ability for the simulation. Model sensitivity analysis showed that V m and \( \mu_{\max }^{\text{F}} \) have a significant influence on the process. Therefore, these parameters were estimated directly from the experimental data of batch experiment performed on sucrose. Additionally, when cultivated on sucrose, biomass growth occurs simultaneously on two substrates, glucose and fructose, respectively. Therefore, yield coefficients Y X/G and Y X/F were also estimated from the data of the same batch experiment. Re-estimate model parameters are given in Table 4, while the experimental results and model simulation results, which were calculated using parameters given in Table 3, are shown in Fig. 7b.
Conclusion
Developed mathematical model accurately correlates sucrose hydrolysis catalyzed by intracellular invertase and microbial growth of T. versicolor on glucose and fructose in submerged fermentation performed in shake flasks. Hydrolysis of sucrose into glucose and fructose, which was assumed to exist, was experimentally confirmed and verified with the model. Kinetic parameters K m = 7.99 g dm−3 and V m = 0.304 h−1 for invertase-catalyzed hydrolysis of sucrose were estimated and verified, respectively. The proposed assumption of simultaneous growth of T. versicolor on glucose and fructose was also experimentally confirmed. Monod type kinetics of biomass growth on glucose and on fructose was assumed, and kinetic parameters were estimated \( \left( {\mu_{\max }^{\text{G}} = \, 0. 1 4 {\text{ h}}^{ - 1} ,K_{\text{S}}^{\text{G}} = 8.0 6 {\text{ g dm}}^{ - 3} ,\mu_{\max }^{\text{F}} = \, 0. 3 7 {\text{ h}}^{ - 1} \;{\text{and }}K_{\text{S}}^{\text{F}} = 54. 8 {\text{ g dm}}^{ - 3} } \right). \) The highest biomass yield (Y X/F = 0.59 g g−1) was obtained in experiment performed with fructose as sole carbon source. Additionally, glucose uptake ends before consumption of fructose, which is in accordance with higher affinity of T. versicolor to glucose as a growth substrate.
Therefore, based on experiments and the model developed in this study, we could assume that T. versicolor has an enzyme for the degradation of sucrose, even before it starts to grow on glucose and fructose.
Abbreviations
- D s :
-
Diffusion coefficient (cm2 s−1)
- K m :
-
Michaelis–Menten constant (g dm−3)
- K S :
-
Monod constant (g dm−3)
- r :
-
Radius (mm)
- r :
-
Rate of enzymatic hydrolysis (g dm−3 h−1)
- T :
-
Temperature (°C)
- t :
-
Time (h)
- V m :
-
Maximal reaction rate (h−1)
- Y :
-
Yield coefficient biomass–substrate (g g−1)
- γ :
-
Concentration (g dm−3)
- μ:
-
Biomass growth rate (h−1)
- μmax :
-
Maximal biomass growth rate (h−1)
- Φ :
-
Thiele module (–)
- 0:
-
Initial conditions
- F:
-
Fructose
- G:
-
Glucose
- S:
-
Sucrose
- X:
-
Biomass
References
Teerapatsakul C, Parra R, Bucke C, Chitradon L (2007) Improvement of laccase production from Ganoderma sp. KU-Alk4 by medium engineering. World J Microbiol Biotechnol 23:1519–1527
Asgher M, Bhatti HN, Ashraf M, Legge RL (2008) Recent developments in biodegradation of industrial pollutants by white rot fungi and their enzyme system. Biodegradation 19:771–783
Schlosser D, Grey R, Fritsche W (1997) Patents of lignolitic enzymes in Trametes versicolor. Distribution of extra- and intracellular enzyme activities during cultivation on glucose wheat straw and beech wood. Appl Microbiol Biotechnol 47:412–418
Holker U, Hofer M, Lenz J (2004) Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl Microb Bitechnol 64:175–186
Papagianni M (2004) Fungal morphology and metabolite production in submerged mycelial processes. Biotechnol Adv 22:189–259
Cui YQ, Okkerse WY, van der Lans RGJM, Luyben KChAM (1998) Modeling and measurements of fungal growth and morphology in submerged fermentations. Biotechnol Bioeng 60:216–229
Znidarsic P, Komel R, Pavko A (1998) Studies of pelleted growth form of Rhizopus nigricans as a biocatalyst for progesterone 11α-hydroxylation. J Biotechnol 60:207–216
Moreira MT, Feijoo G, Lema JM (2003) Fungal bioreactors: application to white-rot fungi. Rev Environ Sci Biotechnol 2:247–259
Arrora DS, Gill PK (2000) Laccase production by some white rot fungi under different nutritional conditions. Biores Technol 73:283–285
Kahraman SS, Gurdal IH (2002) Effect of synthetic and natural culture media on laccase production by white rot fungi. Biores Technol 82:215–217
Tsujiyama S, Minami M (2005) Production of phenol oxidizing enzymes in the interaction between white-rot fungi. Mycoscience 46:268–271
Songulashvili G, Elisashvili V, Wasser SP, Nevo E, Hadar Y (2007) Basidiomycetes laccase and manganese peroxidase activity in submerged fermentation of food industry waste. Enzyme Microb Technol 41:57–61
Revankar MS, Lele SS (2006) Increased production of extracellular laccase by the white rot fungus Coriolus versicolor MTCC 138. World J Microbiol Biotechnol 22:921–926
Winquist E, Moilanen U, Mettala A, Leisola M, Hatakka A (2008) Production of lignin modifying enzymes on industrial waste material by solid-state cultivation of fungi. Biochem Eng J 42:128–132
Elisashvili V, Kachlishvili E, Penninckx M (2008) Effect of growth substrates, method of fermentation, and nitrogen source on lignocellulose-degrading enzymes production by white-rot basidiomycetes. J Ind Microbiol Biotechnol 35:1531–1538
Mazumder S, Basu SK, Mukherjee M (2009) Laccase production in solid state and submerged fermentation by Pleurotus ostreatus. Eng Life Sci 9:45–52
Thiruchelvam AT, Ramsay AJ (2007) Growth and laccase production kinetics of Trametes versicolor in a stirred tank reactor. Appl Microbiol Biotechol 74:547–554
Jang MY, Ryu WR, Cho MH (2002) Laccase production from repeated batch cultures using free mycelia of Trametes sp. Enzyme Microb Technol 30:741–746
Tavares APM, Coelho MAZ, Coutinho JAP, Xavier AMRB (2005) Laccase improvement in submerged cultivation: induced production and kinetic modeling. J Chem Technol Biotechnol 80:669–676
Ryan DR, Leukes WD, Burton SG (2005) Fungal bioremediation of phenolic wastewaters in an air lift reactor. Biotechnol Prog 21:1068–1074
Barclay CD, Legge RL, Farquhar GF (1993) Modeling the growth kinetics of Phanerochaete chrysosporium in submerged static cultures. Appl Environ Microbiol 59:1887–1892
Kovarova-Kovar K, Egli T (1998) Growth kinetics of suspended microbial cells: from single substrate-controlled growth to mixed-substrate kinetics. Microbiol Mol Biol Rev 62:646–666
Vrsalovic Presecki A, Findrik Z, Zelic B (2006) Modeling of the biotransformation processes. Chem Biochem Eng Q 20:227–241
Vasic-Racki D, Kragl U, Liese A (2003) Benefits of enzyme kinetics modelling. Chem Biochem Eng Q 17:7–18
Zelic B, Vasic Racki D, Wandrey C, Takors R (2004) Modeling of the pyruvate production with Escherichia coli in a fed-batch bioreactor. Bioprocess Biosyst Eng 26:249–258
Zhang C, Shi Z, Gao P, Duan Z, Mao Z (2005) On-line prediction of products concentrations in glutamate fermentation using metabolic network model and linear programming. Biochem Eng J 25:99–108
de Almeida ACS, de Araujo LC, Costa AM, de Abreu CAM, de Andrade Lima MAG, de Los Angeles Perez Fernandez Palha M (2005) Sucrose hydrolysis catalyzed by auto-immobilized invertase into intact cells of Cladosporium cladosporioides. Electron J Biotehnol 8:54–62
Akgol S, Kacar Y, Denizli A, Arica MY (2001) Hydrolysis of sucrose by invertase immobilized onto novel magnetic polyvinylalcohol microspheres. Food Chem 74:281–288
Bailey JE, Ollis DF (1977) Biochemical engineering fundamentals. McGraw-Hill, New York
Acknowledgments
This study was supported by the Croatian Ministry of Science, Education and Sports (Contract Grant Number 125-1252086-2793), and by The National Foundation for Science, Higher Education and Technological Development of the Republic of Croatia (Program NZZ Installation Grant).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tišma, M., Sudar, M., Vasić-Rački, Đ. et al. Mathematical model for Trametes versicolor growth in submerged cultivation. Bioprocess Biosyst Eng 33, 749–758 (2010). https://doi.org/10.1007/s00449-009-0398-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-009-0398-6