Abstract
Temperature and freeze-thaw events are two key factors controlling litter decomposition in cold biomes. Predicted global warming and changes in freeze-thaw cycles therefore may directly or indirectly impact litter decomposition in those ecosystems. Here, we conducted a 2-year-long litter decomposition experiment along an elevational gradient from 3000 to 3600 m to determine the potential effects of litter quality, climate warming and freeze-thaw on the mass losses of three litter types [dragon spruce (Picea asperata Mast.), red birch (Betula albosinensis Burk.), and minjiang fir (Abies faxoniana Rehd. et Wild)]. Marked differences in mass loss were observed among the litter types and sampling dates. Decay constant (k) values of red birch were significantly higher than those of the needle litters. However, mass losses between elevations did not differ significantly for any litter type. During the winter, lost mass contributed 18.3–28.8 % of the net loss rates of the first year. Statistical analysis showed that the relationships between mass loss and litter chemistry or their ratios varied with decomposition periods. Our results indicated that short-term field incubations could overestimate the k value of litter decomposition. Considerable mass was lost from subalpine forest litters during the wintertime. Potential future warming may not affect the litter decomposition in the subalpine forest ecosystems of eastern Tibetan Plateau.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Temperature and litter quality are two key factors controlling litter decomposition (Aerts 1997, 2006). In high-altitude and high-latitude ecosystems, seasonal snow cover and associated freeze-thaw events play an important role in litter decomposition. The CO2 emissions from decaying litter accounts for a considerable part of the total CO2 efflux during the winter (Uchida et al. 2005). Global air temperatures are predicted to increase 1.8–4.0 °C over this century, with greater warming occurring in cold biomes (IPCC 2007). This warming may affect seasonal snow cover and freeze-thaw cycles in these ecosystems (Sjursen et al. 2005). Thus, projected global warming can directly affect litter decay rates via accelerating biochemical reaction rates (Aerts 2006). Moreover, future warming can also indirectly influence litter decomposition rates by altering soil moisture, litter quality and freeze-thaw cycles (Uchida et al. 2005; Aerts 2006; Cornelissen et al. 2007). Decomposition of plant litter is an important component of the global carbon budget (Robinson 2002). Thus, any changes in factors that control litter decomposition rates may have important repercussions for the global carbon budget (Cornelissen et al. 2007).
For better insight into the mechanisms underlying litter responses, artificial warming experiments have been done (e.g., Aerts 2006; Luo et al. 2009; Su et al. 2010), but they have not shown any consistent warming effects on litter decay; positive, negative, and neutral effects have all been reported. Moreover, responses of litter decay to experimental warming varied among plant species, ecosystems and sites (Aerts 2006; Cornelissen et al. 2007), and in snowy regions, climate change can covary with factors such as freeze-thaw cycle (Sjursen et al. 2005). It is apparent that litter decomposition could be complicated by extreme soil temperature and freeze-thaw cycles in alpine regions.
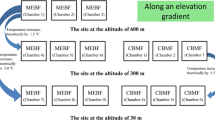
The subalpine/alpine forests located at the transition zone from the Tibetan Plateau to the Sichuan basin could be very sensitive to climate change with important consequences for the global carbon balance (Xu et al. 2010a, b). Red birch (Betula albosinensis Burk.), dragon spruce (Picea asperata Mast.) and minjiang fir (Abies faxoniana Rehd. et Wild) are the three dominant tree species in this area (Liu and Lin 2009). Altitudinal gradients can be considered as natural, long-term analogues for climate change (Aerts 2006), and freeze-thaw cycle patterns vary with elevations in the Tibetan Plateau region (Tan et al. 2011). In this study, we conducted a 2-year litter decomposition experiment along an elevational gradient (3000, 3300 and 3600 m) to facilitate our understanding of the interactions of climate warming and freeze-thaw events on litter decomposition. The objectives of this work were to (i) determine how litter mass losses are controlled by microclimatic conditions and litter quality and (ii) quantify the mass loss of litters under the snow during the winter.
Materials and methods
Site description
This study was conducted at the Bipenggou Nature Reserve of Lixian County in the eastern Tibetan Plateau of China (31°14′–31°19′N, 102°53′–102°57′E, 2458–4619 m a.s.l.). The mean annual temperature ranges from 2 to 4 °C with a maximum of 23 °C and minimum of −18 °C. Annual precipitation is about 850 mm; the monthly variation in precipitation during the study period (from November 2008 to October 2010) is shown in Fig. 1. The growing season generally ranges from May to October, with a maximum snow depth of ca. 20–60 cm in the winter, depending on the elevation and slope direction. The freeze-thaw season generally starts in early December, and soil remains frozen about 4 months. However, there are obvious freeze-thaw cycles before the soil completely freezes or thaws (Tan et al. 2011). The forest soils are classified as Cambisols and Primosols (Gong et al. 2007). Forests are mainly dominated by minjiang fir, dragon spruce and red birch (Liu and Lin 2009). The understory is dominated by Fargesia spathacea, Rhododendron delavayi, Berberis sargentiana, Hippophae rhamnoides, Carex capilliformis and Anemone rivularis.
Experimental design
We established a litter decomposition experiment at three sites with similar environmental factors such as slope, aspect and canopy density but at an elevational gradient: 3000, 3300 and 3600 m. Soil temperature was recorded every 2 h using a DS1923G Thermochron iButton data logger (DS1921-F5#, Maxim/Dallas Semiconductor Inc., USA) that was placed on the forest floor close to the litterbags. A freeze-thaw cycle was identified whenever the temperature dropped below 0 °C for at least 3 h, followed by a rise above 0 °C for at least 3 h during the winter (November to April) (Konestabo et al. 2007).
Litter collection and litterbag construction
Late October 2008, naturally fallen leaves of dragon spruce, red birch and minjiang fir were collected randomly from the selected forests. Litter samples were air dried to a constant mass. Subsamples of the leaves from each species were oven dried at 70 °C for 48 h to calculate a moisture correction factor and were then analyzed for initial nutrient concentrations.
The litterbag technique was used to quantify the leaf litter decomposition rate (Bocock and Gilbert 1957). We constructed 20 × 20 cm litter bags using 0.5 × 0.5 mm mesh nylon cloth on the bottom and 1 × 1 mm mesh nylon cloth on the top. Air-dried litter samples were placed into litterbags. Duplicate sets of litterbags were deployed on the soil surface in the three sites (3000, 3300 and 3600 m) in early November 2008. Litterbags of each litter type were retrieved randomly with five replicates every 2 months. In the laboratory, extraneous matter such as other plant materials, rocks and soil animals were handpicked from the decomposed litters, and the clean samples were then oven-dried at 70 °C to a constant mass. Mass loss was calculated as the difference between the initial dry mass and the actual dry mass of leaves at each sampling date.
Chemical analyses
For each sampling date, litters of the same plant species were pooled for chemical analyses after determination of dry mass. Carbon and nutrients in samples were determined as described by Lu (1999). Carbon content was measured with the dichromate oxidation–sulphate ferrous titration method. Subsamples of 0.2500 g were acid digested with an 8 mL H2SO4 and 3 mL H2O2 solution at 190 °C for 10 min. The digested solution was then transferred to a 100 mL volumetric flask, rationed, and stored for N, P, K, Ca and Mg quantification. N and P were determined by semi-micro Kjeldahl and phosphorus molybdenum-blue colorimetry. K, Ca and Mg were measured by atomic absorption spectroscopy (Bao 1999). Lignin and cellulose were measured using the acid detergent lignin method (Vanderbilt et al. 2008).
Statistical analyses
All statistical tests were performed using the software Statistical Package for the Social Sciences (SPSS) version 11.0. Decomposition rates were calculated from dry mass remaining using a single negative exponential decay model x t /x 0 = e−kt, where x t /x 0 is the fraction mass remaining at time t, t the time elapsed in years and k the annual decay constant (Olson 1963). Repeated-measures analysis of variation with Fisher’s LSD test was performed to examine the effects of elevation, litter type, sampling date and their interactions on litter mass loss. One-way ANOVA was used to examine significant differences among litter types in decay constant (k) and initial leaf litter chemistry.
Results
Soil temperature along the elevations
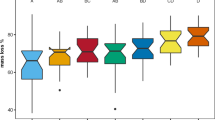
The annual average values over 2 years were 4.9, 4.2 and 4.0 °C for soil temperature at 3000, 3300 and 3600 m, respectively (Fig. 2). There was no significant difference in soil temperature between 3300 and 3600 m, but the temperature of 3000 m was significantly higher than at 3600 m. The patterns in soil temperature among elevations differed between growing season and non-growing seasons. For example, over the growing season, soil temperatures were 9.6, 9.0, and 8.6 °C at 3000, 3300 and 3600 m, respectively, whereas during the non-growing season they were 0.8, 1.2 and 0.1 °C, respectively.
Soils were frozen longer at higher elevations (Fig. 2). During the winter of 2008/2009, the total amount of time soils remained frozen was 118, 125 and 155 days, respectively, at 3000, 3300 and 3600 m. Moreover, there were 7, 15 and 32 freeze-thaw cycles, respectively, at 3000, 3300 and 3600 m during the winter of 2008/2009, whereas the frequency of freeze-thaw cycles was 44, 22 and 24 over the winter of 2009/2010.
Initial litter chemistry
There were significant differences in initial litter chemistry among litter types (Table 1). Total C and lignin contents were significantly higher in the minjiang fir litter than in the dragon spruce and red birch litter, yet for cellulose the opposite was true (Table 1). Initial N, P and Ca concentrations were highest in red birch litter, followed by the minjiang fir and dragon spruce litter (Table 1). Initial K concentration did not differ among litter types. Conversely, the order of initial Mg concentration was minjiang fir > red birch > dragon spruce (Table 1). Red birch litter had the lowest C:N, C:P and lignin:N ratios relative to the two needles (Table 1). However, initial N:P ratio was lowest in the dragon spruce litter compared with the other litter types (Table 1).
Mass losses of the three litter types among the elevations
Marked differences in mass loss were observed among the litter types and sampling dates (Fig. 3; Table 2). In general, red birch litter decayed more rapidly relative to the two needle litters over the sampling dates, (Fig. 3; Table 2). After 2 years of decomposition, the remaining litter mass was ~55 % of the initial in the two needles types, but <50 % in red birch at each elevation (Fig. 3).
Additionally, effects of litter quality on mass losses depended on seasons (P < 0.05). There were no significant differences in mass losses between elevations in any litter type (Fig. 3; Table 2). However, repeated ANOVA showed that the interactive effects of elevation and date were significant on mass losses (P < 0.05). Litter decay process were well described by exponential model, x t /x 0 = e−kt (values of r 2 ranged between 0.88 and 0.97, and P < 0.01; Table 2). In general, the values of decay constant, k, of red birch were significantly higher than those of needle litters (Table 2).
The litters lost considerable mass during the winter period (Fig. 3). Over the winter of 2008/2009, 8.3–12.5 % of mass was lost from the studied forest litters (Fig. 3). The lost mass contributed 18.3–28.8 % of mass to the net loss rates of the first year for three litter types (Fig. 3). Compared with the first winter, only slight mass losses were observed during the winter of 2009/2010 (3.6–3.8 %, Fig. 3). Additionally, over the 2-year incubation, approximately 90 % of the mass was lost from the studied litters during the first year (Fig. 3).
Discussion
On a smaller spatial scale, litter quality is considered as the most important factor influencing decomposition rate (Aerts and Caluwe 1997). High quality litters are often characterized by higher N concentrations and lower C:N and lignin:N ratios and can decompose faster in comparison with low quality litters (Sanchez 2001). In this study, the leaf litters of red birch had higher N concentrations and lower C:N, C:P and lignin:N ratios relative to the two coniferous needles. As a consequence, red birch leaf litter decayed faster than dragon spruce and minjiang fir. The values of decay constant, k, of red birch were significantly higher than those of both needles in any elevation. These tendencies are similar to those found in previous studies of the decomposability of plants in this region (Deng et al. 2009). The significant differences in the rate of litter decomposition between litter types can, therefore, mainly be attributed to differences in litter quality. Additionally, red birch has a more fragile structure, while dragon spruce and minjiang fir are more fibrous and their physically resistant cuticular boundary of the needles may be more difficult for microbes to break down (Rustad and Fernandez 1998). Therefore, the differences in physical structures between red birch litter and two needles may also partly contribute to the differences in mass losses.
Litter decomposition is a complex ecological process that is strongly influenced by environmental factors (Aerts 1997, 2006; Zhang et al. 2008). Temperature is a key factor that governs litter decomposition (Ruark 1993; Aerts 1997). To get a better insight into mechanisms underlying litter responses, some direct techniques (e.g., open-top chambers, heating cables and infrared heaters) have been used to manipulate temperature experimentally (Aerts 2006; Luo et al. 2009; Su et al. 2010). In addition to this approach, patterns along present-day natural gradients can be included. Altitudinal gradients can be considered as natural, long-term analogues for climate change. At present, warming effects on litter decomposition have widely been reported in various terrestrial ecosystems (Aerts 2006), but no consistent patterns have been observed. For example, increases in litter mass loss in response to warming were reported in arctic dwarf shrub, subalpine meadow and boreal forest (Robinson et al. 1995; Rustad and Fernandez 1998; Verburg et al. 1999; Shaw and Harte 2001). Nevertheless, in a forest–tundra ecotone, experimental warming did not affect or sometimes even decreased litter decay rates (Sjögersten and Wookey 2004). Aerts (2006) found that litter decay is hierarchically controlled by the triad climate > litter quality > soil organisms, and warming effects on litter mass loss were strongly dependent on the method used. Open top chambers (OTCs) declined mass losses (Sjögersten and Wookey 2004), whereas heating cables increased mass loss (e.g., Robinson et al. 1995; Verburg et al. 1999).
In the present case, contrary to our initial hypothesis that relatively higher temperature at lower elevations would be correlated with increased rates of litter decomposition, we did not observe significant differences between elevations in mass losses in any of the litter types. Along an elevational gradient, climate factors can covary with edaphic factors (Murphy et al. 1998; Scowcroft et al. 2000). The low responsiveness noted in some studies was mainly due to moisture-limited mass losses in warming treatments, especially at mesic and xeric sites (Aerts 2006). At the regional scale, litter mass loss as mediated by temperature and moisture has been corroborated by latitudinal gradient studies (Berg et al. 1993; Zhou et al. 2008). Additionally, previous altitudinal gradient studies have found that the importance of temperature was overridden by soil moisture or other site factors (i.e., soil fertility) (Murphy et al. 1998; Scowcroft et al. 2000). In an alpine meadow, an elevational gradient from 3200 to 3800 m was accompanied by a 3.0 °C temperature difference, which resulted in significant differences in litter decomposition rates between elevations (Luo et al. 2009; Su et al. 2010). However, in our study, there was only approximately 0.9 °C difference from 3000 to 3600 m, and forest litters were less decomposable compared with the alpine grassland. In addition, in another study, short-term warming (3.2 °C increment in soil temperature by heating cable) accelerated the mass loss of red birch litter but did not affect the mass loss of dragon spruce needle (Xu et al. 2012). Thus, low temperature sensitivity of litter decomposition might, to some extent, be attributable to a small increase in soil temperature and low quality of forest litters. However, during the winter, seasonal snow cover associated with freeze-thaw cycles may promote mass loss by fragmenting litter and causing release of soluble compounds that are either respired or leached (Taylor and Jones 1990; Hobbie and Chapin 1996). The snow cover was deeper and snow cover lasted longer at upper elevations than at lower elevations. In addition, the frequency of freeze-thaw cycles increased with elevation. Therefore, the patterns of freeze-thaw cycles during the wintertime may partly mask the effect of temperature induced by elevation.
Over the last few decades, considerable research has focused on quantifying litter decomposition during the growing season; comparatively little effort has focused on litter decomposition under snow-covered or frozen soils (Uchida et al. 2005). This lack is partly because forest ecosystems at high altitudes or latitudes were once thought to be dormant during the long winter, but a growing number of studies have convincingly demonstrated that winter soil respiration is a pronounced component of the annual carbon budget in these ecosystems (Monson et al. 2002; Suzuki et al. 2006; Wang et al. 2010). However, the sources of this CO2 efflux in alpine forests during the winter remain unclear. A large percentage of the annual leaf litter is supplied to the forest floor during autumn months. Therefore, microbial activity and physical processes (e.g., fragmentation or leaching) in the litter layer may contribute significantly to the CO2 efflux in winter. Recent studies have also found that significant CO2 effluxes were emitted from the snow-cover soils and that litter decomposition contributed significantly to winter soil respiration in the subalpine coniferous forests (Zhou et al. 2009; Xiong et al. 2015). This study found that mass losses from the three species over the winter constitute 18.3–28.8 % of total first-year mass losses. This result was consistent with the estimated values (26 %) in a cool-temperate broad-leaved deciduous forest ecosystem (Uchida et al. 2005). Nevertheless, nearly all mass loss of tundra litter occurred during the winter when soils were mostly frozen (Hobbie and Chapin 1996) because snow cover in arctic and subarctic tundra ecosystems is significantly deeper and lasts longer than in temperate alpine forest ecosystems.
In our study region, red birch is a fast-growing, early-successional species, while dragon spruce and minjiang fir are two slow-growing, late-successional species. Correspondingly, red birch, dragon spruce and minjiang fir forest are three dominant forest types. Several models predict that future warming may dramatically enhance vegetation productivity (Cramer 2001; Saleska 2002). Increased productivity will probably increase litter production, leaves being the predominant source of annual aboveground litter production (Cornelissen et al. 2007). Additionally, experimental warming could, to some degree, reduce the litter quality of the subalpine coniferous forests of eastern Tibetan Plateau (Liu and Lin 2009). In the present study, a small increase in soil temperature did not affect the mass losses of the three litters, and the temperature sensitivity of litter decomposition may not be as high as expected in the subalpine forests. If this is true, future warming, at least at an early stage, may not influence the amount of CO2 released to the atmosphere from litters in this area. On the other hand, considerable litter decayed in the winter in the subalpine forests although the underlying mechanisms were unknown. In general, climate models predict the greatest increases in temperatures will occur during the autumn and winter months (IPCC 2007), which will likely lead to associated decreases in the duration of snow cover in winter and increases in the frequency of freeze-thaw events (Sjursen et al. 2005). Thus, predictions of the impact of climate change on litter decomposition in montane ecosystems and other cold biomes should take such factors into account.
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Aerts R (2006) The freezer defrosting: global warming and litter decomposition rates in cold biomes. J Ecol 94:713–724
Aerts R, Caluwe HD (1997) Nutritional and plant mediated control son leaf litter decomposition. Ecology 78:244–260
Bao SHD (1999) Agriculture soil chemical analysis. Science Press, Beijing, pp 263–271
Berg B, Berg MP, Bottner P, Box E, Breymeyer A, CadeAnta R, Couteaux M, Escudero A, Gallardo A, Kratz W, Madeira M, Mälkönen E, McClaugherty C, Meentemeyer V, Munoz F, Piussi P, Remacle J, Vide Santo A (1993) Litter mass loss in pine forests of Europe and Eastern United States: some relationships with climate and litter quality. Biogeochemistry 20:127–159
Bocock KL, Gilbert OJW (1957) The disappearance of leaf litter under different woodland conditions. Plant Soil 9:179–185
Cornelissen JHC, Van Bodegom PM, Aerts R, Callaghan TV, Van Logtestijn RSP, Alatalo J, Chapin FS, Gerdol R, Gudmundsson J, Gwynnjones D, Hartley AE, Hik DS, Hofgaard A, Jónsdóttir JS, Karlsson S, Klein JA, Laundra J, Magnusson B, Michelsen A, Molau U, Onipchenko VG, Quested HM, Sandvik SM, Schmidt IK, Shaver GR, Solheim B, Soudzilovskaia NA, Stenström A, Tolvanen A, Totland Ø, Wada N, Welker JM, Zhao XQ, Team MOL (2007) Global negative vegetation feedback to climate warming responses of litter decomposition rates in cold biomes. Ecol Lett 10:619–627
Cramer WA (2001) Global response of terrestrial ecosystem structure and function to CO2 and climate change: results from six dynamic global vegetation models. Glob Change Biol 7:357–373
Deng RJ, Yang WQ, Feng RF, Hu JL, Qin JL, Xiong XJ (2009) Mass loss and element release of litter in the subalpine forest over one freeze-thaw season. Acta Ecol Sin 29:5730–5735
Gong Z, Zhang G, Chen Z (2007) Pedogenesis and soil taxonomy. Science Press, Beijing (In Chinese)
Hobbie S, Chapin IIIFS (1996) Winter regulation of tundra litter carbon and nitrogen dynamics. Biogeochemistry 35:327–338
IPCC (2007) Climate change 2007: the physical science basis. Working Group I contribution to the IPCC fourth assessment report
Konestabo HS, Michelsen A, Holmstrup M (2007) Responses of springtail and mite populations to prolonged periods of soil freeze-thaw cycles in a sub-arctic ecosystem. Appl Soil Ecol 36:136–146
Liu Q, Lin B (2009) Effects of global climate change on subalpine coniferous forest trees. Science Press, Beijing (In Chinese)
Lu RK (1999) Soil and agro-chemical analytical methods. China Agricultural Science and Technology Press, Beijing (In Chinese)
Luo C, Xu G, Chao Z, Wang S, Lin X, Hu Y, Zhang Z, Duan J, Chang X, Su A, Li Y, Zhao X, Du M, Tang Y, Kimball B (2009) Effect of warming and grazing on litter mass loss and temperature sensitivity of litter and dung mass loss on the Tibetan plateau. Glob Change Biol 6:1–12
Monson RK, Turnipseed AA, Sparks JP (2002) Carbon sequestration in a high-elevation, subalpine forest. Glob Change Biol 8:459–478
Murphy KL, Klopatek JM, Klopatek CC (1998) The effects of litter quality and climate on decomposition along an elevational gradient. Ecol Appl 8:1061–1071
Olson JS (1963) Energy storage and the balance of produces and decomposers in ecological systems. Ecology 44:322–332
Robinson CH (2002) Controls on decomposition and soil nitrogen availability at high latitudes. Plant Soil 242:65–81
Robinson CH, Wookey PA, Parsons AN (1995) Responses of plant litter decomposition and nitrogen mineralization to simulated environmental change in a high arctic polar semi-desert and a subarctic dwarf shrub heath. Oikos 74:503–512
Ruark GA (1993) Modeling soil temperature effects on in situ decomposition rates for fine roots of loblolly pine. For Sci 9:118–129
Rustad LE, Fernandez IJ (1998) Soil warming: consequences for foliar litter decay in a spruce-fir forest in Maine, USA. Soil Sci Soc Am J 62:1072–1080
Saleska SR (2002) Plant community composition mediates both large transient decline and predicted long-term recovery of soil carbon under climate warming. Glob Biogeochem Cycles. doi:10.1029/2001GB001573
Sanchez FG (2001) Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. For Ecol Manag 152:85–96
Scowcroft PG, Turner DR, Vitousek PM (2000) Decomposition of Metrosideros polymorpha leaf litter along elevational gradients in Hawaii. Glob Change Biol 6:73–85
Shaw MR, Harte J (2001) Control of litter decomposition in a subalpine meadow sagebrush ecotone under climate change. Ecol Appl 11:1206–1223
Sjögersten S, Wookey PA (2004) Decomposition of mountain birch leaf litter at the forest-tundra ecotone in the Fennoscandian mountains in relation to climate and soil conditions. Plant Soil 262:215–227
Sjursen H, Michelsen A, Holmstrup M (2005) Effects of freeze-thaw cycles on microarthropods and nutrient availability in a sub-Arctic soil. Appl Soil Ecol 28:79–93
Su A, Lin Q, Li Y, Du M (2010) Effects of litter quality and climate change along an elevation gradient on litter mass loss in an alpine meadow ecosystem on the Tibetan plateau. Plant Ecol 209:257–268
Suzuki S, Ishizuka S, Kitamura K (2006) Continuous estimation of winter carbon dioxide efflux from the snow surface in a deciduous broadleaf forest. J Geophys Res. doi:10.1029/2005JD006595
Tan B, Wu FZ, Yang WQ (2011) Soil hydrolase characteristics in late soil-thawing period in subalpine/alpine forests of west Sichuan. Chin J Appl Ecol 22:1162–1168
Taylor BR, Jones HG (1990) Litter decomposition under snow in a balsam fir forest. Can J Bot 68:112–120
Uchida M, Mo W, Nakatsubo T (2005) Microbial activity and litter decomposition under snow cover in a cool-temperate broad-leaved deciduous forest. Agric For Meteorol 134:102–109
Vanderbilt KL, White CS, Hopkins O (2008) Aboveground decomposition in arid environments: results of a long-term study in central New Mexico. J Arid Environ 72:696–709
Verburg PSJ, Van Loon WKP, Lükewille A (1999) The CLIMEX soil-heating experiment: soil response after 2 years of treatment. Biol Fertil Soils 28:271–276
Wang W, Peng S, Wang T (2010) Winter soil CO2 efflux and its contribution to annual soil respiration in different ecosystems of a forest-steppe ecotone, north China. Soil Biol Biochem 42:451–458
Xiong L, Xu Z, Yang W, Yin R, Tang S, Xu L, Chang C (2015) Aboveground litter contribution to soil respiration in a subalpine dragon spruce plantation of western Sichuan. Acta Ecol Sin. doi:10.5846/stxb201312042891
Xu Z, Wan C, Xiong P, Tang Z, Hu R, Cao G, Liu Q (2010a) Initial responses of soil CO2 efflux and C, N pools to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China. Plant Soil 336:183–195
Xu ZF, Tang Z, Wan C, Xiong P, Cao G, Liu Q (2010b) Effects of experimental warming on soil enzyme activities in two coniferous forests, Western Sichuan. Chin J Appl Ecol 21:2727–2733
Xu Z, Pu X, Yin H, Zhao C, Liu Q, Wu F (2012) Warming effects on the early decomposition of three litter types, Eastern Tibetan Plateau, China. Eur J Soil Sci 63:360–367
Zhang DQ, Hui DF, Luo YQ (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 6:85–93
Zhou G, Guan L, Wei X, Tang X, Liu S, Liu J, Zhang D, Yan J (2008) Factors influencing leaf litter decomposition: an intersite decomposition experiment across China. Plant Soil 311:61–72
Zhou FF, Lin B, Liu Q (2009) Soil respiration of subalpine coniferous forest in winter in the east of the Qinghai–Tibet Plateau, China. Chin J Appl Environ Biol 15:761–767
Author information
Authors and Affiliations
Corresponding author
Additional information
Project funding: This work was supported by the National Natural Science Foundation of China (31570445, 31570601, 31500509 and 31570605), Postdoctoral Science Foundation of China (2013M540714 and 2014T70880) and Collaborative Innovation Center of Ecological Security in the Upper Reaches of Yangze River.
The online version is available at http://www.springerlink.com
Corresponding editor: Hu Yanbo
Rights and permissions
About this article
Cite this article
Xu, Z., Zhu, J., Wu, F. et al. Effects of litter quality and climate change along an elevational gradient on litter decomposition of subalpine forests, Eastern Tibetan Plateau, China. J. For. Res. 27, 505–511 (2016). https://doi.org/10.1007/s11676-015-0180-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11676-015-0180-3