Abstract
The Long-Term Intersite Decomposition Experiment in China (hereafter referred to as LTIDE-China) was established in 2002 to study how substrate quality and macroclimate factors affect leaf litter decomposition. The LTIDE-China includes a wide variety of natural and managed ecosystems, consisting of 12 forest types (eight regional broadleaf forests, three needle-leaf plantations and one broadleaf plantation) at eight locations across China. Samples of mixed leaf litter from the south subtropical evergreen broadleaf forest in Dinghushan (referred to as the DHS sample) were translocated to all 12 forest types. The leaf litter from each of other 11 forest types was placed in its original forest to enable comparison of decomposition rates of DHS and local litters. The experiment lasted for 30 months, involving collection of litterbags from each site every 3 months. Our results show that annual decomposition rate-constants, as represented by regression fitted k-values, ranged from 0.169 to 1.454/year. Climatic factors control the decomposition rate, in which mean annual temperature and annual actual evapotranspiration are dominant and mean annual precipitation is subordinate. Initial C/N and N/P ratios were demonstrated to be important factors of regulating litter decomposition rate. Decomposition process may apparently be divided into two phases controlled by different factors. In our study, 0.75 years is believed to be the dividing line of the two phases. The fact that decomposition rates of DHS litters were slower than those of local litters may have been resulted from the acclimation of local decomposer communities to extraneous substrate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Litter decomposition is an important process that connects many aboveground and belowground processes. The decomposition of dead leaves and roots is one of the major pathways by which carbon (C) fixed during photosynthesis is returned to the atmosphere (Coûteaux et al. 1995) and changed into soil organic matter (SOC). Plant detritus and SOC are the largest carbon pools in terrestrial biosphere (Moore and Braswell 1994). It is estimated that global terrestrial heterotrophic respiration generates 60 PgC y−1, which is 11 times the annual amount of CO2–C released by fossil fuel consumption and industrial activities (Schlesinger 1991). Understanding litter decomposition processes and the factors controlling litter decomposition is important for studying nutrient cycling, developing carbon budgets as well as assessing implications of global climate change. Wardle et al. (2004) concluded that old-growth forest decline was also often associated with reductions in litter decomposition rate, P release from litter, and biomass and activity of decomposer microbes.
Litter decomposition rates are a function of litter quality, biota and microclimate (Seastedt et al. 1983), as well as edaphic properties (Heneghan et al. 1998). In decreasing order of importance, the key factors regulating decomposition are commonly assumed to be climate, litter quality (e.g. N content, C/N ratio, lignin content etc) and decomposer communities (e.g. bacteria, fungi, and soil macro- and micro fauna) (Meentemeyer 1984). Many studies (Coûteaux et al. 1995; Aerts 1997; Moorhead et al. 1999; Gholz et al. 2000; Silver and Miya 2001) have concluded that the combination of climate (e.g. mean annual temperature (MAT), mean annual precipitation (MAP), actual evapotranspiration, etc) and litter quality (N content, C/N ratio, lignin content) are the primary factors controlling litter decomposition. However, the conclusions are highly varied, especially with respect to the contribution of MAP to decomposition rates. Although the litter quality parameters of N content, C/N ratio and lignin contents have been commonly recognized as important variables of litter decomposition rates, Silver and Miya (2001) found that Ca content, was a better predictor of root decomposition rates, which implies that there may be other litter quality variables that have yet to be fully examined that may play important roles in determining litter decomposition rates.
In studying the mechanisms of litter decomposition, three approaches have often been applied, including microcosm tests (Taylor and Parkinson 1988a, b; Taylor et al. 1989), field experiments involving simulated soil warming (Chapin and Shaver 1996; McHale et al. 1998; Shaw and Harte 2001), and long-term intersite decomposition experiments (Vitousek et al. 1994; Gholz et al. 2000).
Long-term intersite decomposition experiments are useful for examining the influence of substrate quality and macroclimate variables on litter decomposition. Because they effectively allow comparisons among many study variables, they have received growing attention in studying litter decomposition. However, due to their significant human and financial resource requirements such experiments have not been commonly used across climatic gradients or ecosystem types.
Mainland China situated between 18°12′ N and 53°03′ N, also has a broad range of climatic zones including (moving south to north): tropical, south subtropical, mid subtropical, north subtropical, warm temperate and cold temperate climates. Correspondingly, regional vegetation includes tropical rainforest, monsoon evergreen broadleaved forest, subtropical evergreen broadleaved forest, temperate mixed broadleaved and coniferous forest. The great diversity in climate and vegetation provides a unique opportunity to conduct a long-term intersite decomposition experiment in China.
The Long-Term Intersite Decomposition Experiment in China (hereafter referred to as LTIDE-China) was launched in 2002. It is the first intersite litter decomposition experiment in China. In this study, we analyzed mass loss and decomposition rates of leaf litter during 0–2.5 years of decomposition in the field and related these changes to interregional climate and substrate quality factors to test the impacts of climate and substrate quality on decomposition rates.
Materials and methods
Description of the LTIDE-China and related sites
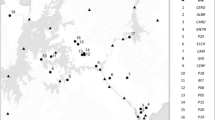
The LTIDE-China project was a litterbag study involving the translocation of leaf litter from 12 forests in eight locations across China (Fig. 1). All eight study locations are part of the Chinese National Field Ecosystem Research Station Network (Table 1). Leaf litter from the south subtropical evergreen broadleaf forest in Dinghushan (DHS) was sent to all 12 forest types in eight locations, and leaf litter from the other 11 forest types was kept in their respective original forest types.
Litter collection, preparation and experiment processing
There were 12 types of leaf samples, each of which was from one of the 12 forest types as described in Table 1. The samples were consisted of freshly fallen leaves from mature trees collected on mesh screens during days without rain. For regional forests, the experimental samples were obtained by mixing the leaf litter of each tree canopy according to their litterfall percentages.
All samples were air dried and sent to the Dinghushan Forest Ecosystem Research Station (DHS) for preparation and then distributed to the corresponding sites and forests. Ten gram of leaf litter was sealed in mesh bags 20 × 15 cm2 in size. The mesh bags had a 1-mm mesh nylon top and a 0.2-mm mesh Dacron cloth bottom to reduce fragmentation losses. Initial moisture contents, oven-dry weights and chemistries were obtained from sub-samples.
For each of the above forest types, two types of litterbags (DHS and local litters) were placed on the corresponding forest floor during 2002 to 2003 at five plots as replicates. We believed that the five replicates would represent the variation of microenvironment within the same forest type. For each plot, there were 20 litterbags (ten for DHS and ten for local leaf litter). Thus, there were 100 (20 × 5) litterbags in total in each forest type. Litterbags were placed flat on the top of the existing litter layer. All of the bags were then left undisturbed until they were collected for analyses.
Every 3 months, two litterbags were collected from each site. Therefore, ten litterbags (five for DHS and five for local samples) were removed in every forest type at each sampling interval. Theoretically, it would take ten sampling intervals (i.e., 30 months) to complete the experiment. However, in the tropical sites (Jianfengling and Xishuangbanna), it was necessary to collect four litterbags after the sixth sampling interval to make sufficient sample quantity for chemical analyses due to their rapid decomposition. Thus, there were only eight sampling intervals (24 months) for Jianfengling and Xishuangbanna.
Laboratory analyses
Biomass
Retrieved litterbags were examined at time of sampling on site and any extraneous materials (e.g., sand, stone, etc) obviously not from the initial litter source were carefully removed. Samples were then wrapped up for analysis. Fresh weight of the litter was recorded before the each sample was dried to constant weight. Dry mass weight was obtained after it dried for at least 48 h at 55°C to constant weight.
Caloric values
Caloric values of initial litter and retrieved decomposing litter samples were determined with an oxygen bomb calorimeter with automatic temperature control system (Parr 1261, USA). The results of duplicated tests were within 0.5% of the mean value.
Litter quality analysis
C and N concentrations of litter samples were analyzed using a Total Carbon Analyzer (TOC-VCPH SHIMADZU, Japan) and the Kjeldahl method, respectively. Phosphorus content was measured using Molybdenum Blue method, and concentrations of potassium, sodium and magnesium were measured using ICP (Optima 2000DV Perkin Elmer, USA). Based on nutrient concentration and biomass, nutrient contents for all analyzed nutrients for each litter sample were calculated.
Climate data
Monthly climate data were provided by the Chinese National Field Ecosystem Research Station Network.
Annual actual evapotranspiration (AAET) of each forest type was calculated using the systematic method developed by Zhou et al. (2008).
Statistical analysis
The decomposition constants (k) for initial and decomposing litter were derived from the negative exponential decomposition model proposed by Olson (1963) and Gholz et al. (2000).
where y is the fraction of mass remaining at time t (years).
Analyses of variance (ANOVA) were performed to test the difference between DHS and local litters for a specific site, and the difference between sites.
The relationship between climatic factors (i.e., MAT, MAP and AAET) and decomposition constant (k) were analyzed using nonlinear regression model fitting. Mean standard error (MSE), Pseudo-R 2 (Helland 1987; Motulsky and Christopoulous 2003), and the 95% confidence interval of the model parameters were used to determine goodness-of-fit. A p-value <0.05 was used to reject the null hypothesis that the difference is not significant. Apparent Q 10 values were estimated from the nonlinear regression model between k value and MAT.
Results
C and N contents and C/N ratio
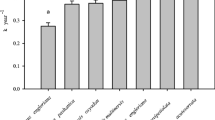
Initial C concentrations of the litter samples ranged from 47.8 ± 0.4% to 54.6 ± 0.2%. The average initial C concentrations for DHS, BLL (local broadleaved litter) and NLL (local needle leaf litter) were 51.4 ± 0.04%, 50.6 ± 1.44% and 54.1 ± 0.4%, respectively. Initial N concentrations varied from 0.67 ± 0.02% to 1.57 ± 0.02%. Mean initial N concentrations for DHS, BLL and NLL was 1.09 ± 0.004%, 1.09 ± 0.21% and 0.78 ± 0.09%, respectively. The difference in both initial C and N concentrations between DHS/or BLL and NLL was statistically significant (p < 0.05). Due to variances in concentration of C and N between broadleaved and needle leaf litters, the initial C/N ratios varied greatly among species and forest types, ranging from 31.7 ± 0.2% to 80.8 ± 0.7%. The average initial C/N ratios (70.5 ± 0.8) of NLL litters (i.e., HT2, BJ2 and GGS2) were significantly higher than those of DHS (47.3 ± 0.14) and BLL (48.2 ± 0.7) litters (p < 0.0001). Thus, our experiment materials have different substrate qualities with a wide range in C/N ratio (Fig. 2).
During the decomposition process, C content gradually declined in the first 0.75 years, and then remained stable (for DHS and BLL litters) or increased (for NLL litters). N content increased during the first 0.75 years and then declined. C/N ratio changed over the course of decomposition experiment for all litter types, decreasing uniformly for the first 0.75 years and then increasing thereafter (p < 0.0001). However, the unexpected increase in C/N ratio of NLL litter is probably due to addition of external materials to the bags and so that can not be taken as a rule. There was no statistical difference (p > 0.05) in C/N ratios of DHS litters among different sites during decomposition. This result indicates that the environment variables do not affect the C/N ratio of a decomposing substrate (Fig. 3).
Elements
Initial P concentrations ranged from 0.04 ± 0.00% to 0.06 ± 0.01% for all litter samples. The average initial P concentrations for DHS, NLL and BLL was 0.04 ± 0.00%, 0.05 ± 0.00% and 0.052 ± 0.01%, respectively, with significant difference (p < 0.05) between DHS and NLL/BLL litters. The initial concentrations of other elements were all highly variable among litter types, ranging from 0.06 ± 0.01% to 0.27 ± 0.01% for Mg, 0.1 ± 0.01% to 0.48 ± 0.03% for K and 0.36 ± 0.01% to 1.99 ± 0.02% for Ca (Fig. 2).
Statistical tests showed that averages of K contents in decomposing stages were significantly lower (p < 0.0001) than the initial values. K content during decomposition process experienced a sharp drop during 0–0.25 years of decomposing, and then gradually remained stable. Ca content continuously increased through all the first 1 year of decomposition stages, which reflected that the percentage of structural matter was enhancing as decomposition proceeded. No consistent pattern was found for the P and Mg contents during the decomposition process. However, P and Mg contents in the first 1 year of decomposition were significantly higher (p < 0.0001) than those of initial values for all litters (Fig. 3).
N/P ratio
Generally, initial N/P ratios of leaf litter samples decreased with increase in latitudes of forest communities (p < 0.05, Fig. 2). N/P ratios of leaf litter samples both from low latitudes and regional forest communities were higher than 18. N/P ratios of needle leaf litters were lower (p < 0.05) than that of broadleaved leaf litter at same latitude. The reason that the N/P ratio in JFL was not as high as expected is partly due to its less maturity than typical tropical montane rainforest and low N deposition in the remote area of Hainan Island. There was an increase trend in N/P ratio during decomposition processes (Fig. 3), similar to N/C ratio.
Caloric value
The initial caloric values for DHS, BLL and NLL litters were 18.88 ± 0.10, 18.64 ± 0.07 and 20.52 ± 0.07 MJ/kg, respectively, with significant difference (p < 0.05) between NLL and DHS/BLL litters (Fig. 3). Zhang et al. (1998) reported that the fat concentrations for broadleaved and needle leaf litters were 2.4–2.7% and 3.9–4.1% of dry weight, respectively, which are similar to our results. Changes of caloric values of the three types of leaf litter during decomposition processes are shown in Fig. 3. The greatest change occurred in NLL litters and the least in DHS litters. The difference may be a result of the difference in caloric values of needle and broadleaved leaves. The curve of NLL can be divided into three phases. In phase 1, the caloric value in the remaining mass increased, in phase 2, the caloric value in the remaining mass decreased and in phase 3, the caloric value increased again.
Fraction of mass remaining (FMR) and k-values
Figure 4 shows FMR and fitted y, k-values during decomposition process. Mass remaining percentages were significantly and negatively exponential correlated with decomposition time (the time since the litter bags were placed), in spite of different sources and qualities in litter samples (Fig. 4). The k-values derived from fitted exponential models (with all R 2 ≥ 0.93, p < 0.0001 and n = 35) ranged from 0.169 to 1.454 per year. The result suggests that there are macroclimate variables, such as MAT and MAP, controlling differences in decomposition across these ecosystems.
There was no significant difference (p < 0.05) in FMR between DHS and local needle leaf litter (NLL) at HT2, BJ2 and GGS2 sites during the period studied. The averaged FMRs of DHS and NLL samples were 84.2 ± 7.9% and 83.5 ± 9.6% after 0.5 year of decomposition, respectively, and 56.7 ± 12.4% and 55.9 ± 13.7% after 1.75 years of decomposition.
Decomposition rates of DHS litters from the sites colder than Dinghushan (i.e., HT, BJ, MES, ALS, and GGS1) were significantly lower (p = 0.0172) than those of local broadleaved litters (BLL). In contrast, decomposition rates of DHS litters from the sites being warmer than Dinghushan (JFL and XBN) were not significantly different to those of local litters (p > 0.05). The result implied that decomposition rates of the DHS litters were sensitive to temperature decrease.
Analysis on climatic factors influencing k-values
Impacts of interregional climatic factors of MAT, MAP and AAET on k-values are shown in Fig. 5.
Relationship between k-values and MAT, MAP and AAET. Q 10 values were from fitted curves based on quadratic least-squares regression (p < 0.0001). The fitted equations of k-values and MAT for local and DHS litters are y = 0.283e 0.0598x (R 2 = 0.57, p < 0.0001) and y = 0.179e 0.0727x (R 2 = 0.84, p < 0.0001). The fitted equations of k-values and MAP for local and DHS litters are y = 0.347 ln(x) − 1.775 (R 2 = 0.26, p = 0.02) and y = 0.391 ln(x) − 2.259 (R 2 = 0.44, p = 0.006). The fitted equations of k-values and AAET for local and DHS litters are y = 0.216e 0.0017x (R 2 = 0.53, p < 0.001) and y = 0.119e 0.0022x (R 2 = 0.86, p < 0.0001)
The results showed that air temperature has a significant impact (p < 0.0001) on k-values for the two types of litters (DHS and local). The apparent Q 10 values were 2.07 and 1.82 for DHS and local litters, respectively. The R 2 between k-values of DHS litters and MAT was 0.84. Without difference in substrate quality, the k-values of DHS litters at various sites were mainly controlled by MAT and other related environmental factors, and thus, the statistical relationship was much stronger. Compared with DHS litters, the 12 types of local litter had greater difference in substrate quality, the statistical relationship between k-values of local samples and MAT was weaker, with R 2 = 0.57.
MAP also impacted k-values significantly (p < 0.05), with R 2 between k-values of DHS litters or local litters and MAT equaling to 0.44 and 0.26, respectively. MAP can explain 44% variation of k-values for DHS litters, while it can only explain 26% variation of k-values for local litters. The difference in R 2 likely resulted from the same causes as in MAT.
AAET influenced k-values with similar statistical significance (p < 0.0001). The R 2 between k-values of DHS litters or local litters and MAT equaled to 0.86 and 0.53, respectively.
The selected climatic variables are correlated (Table 2). MAT and AAET are highly correlated (r = 0.95, p < 0.0001), while the correlation between MAP and AAET is 0.77 (p = 0.0035), and 0.56 (p = 0.059) between MAT and MAP.
Discussion
Dynamics of substrate quality in decomposition processes
Figure 3 showed that there is an initial phase (0–0.75 years) of N immobilization in the processes of litter decomposition, which is consistent with the result reported by Parton et al. (2007). Our results also support a finding of some researchers as Aber et al. (1990) that the decomposition process may apparently be divided into two phases (N immobilization and N releasing) controlled by different factors. In our study, 0.75 years is believed to be the dividing line of the two phases.
Comparison with other LIDET studies
Gholz et al. (2000) reported a systematic decomposition experiment across North and Central America, from Artic tundra to tropical rainforest with latitude ranging from 9°10′ N to 64°45′ N. Our experiment spanned from 18°46′ N to 45°23′ N. Comparison of the k-values obtained from these two experiments is shown in Fig. 6.
Values of parameter k along latitude series for the two experiments. In Gholz et al. (2000) experiment, the decomposition material was Drypetes glauca leaf litter; DHS and local leaf litters referred to our experiment
Without considering variation of altitudes and longitudes, the k-values along the latitude gradient in our study were highly comparable with that of Gholz et al. (2000). The k values in our study (local and DHS litters) had less variation along the latitude gradient as well as within the same latitude zone. Although the decomposing substrates were totally different, the k-values were comparable. This implies, over broad regions, that environmental factors have key control of litter decomposition and the influence of litter quality is secondary.
However, our results showed that the k values were very significantly affected by both AAET and MAT, different from that of Gholz et al. (2000) in which more complex variables such as AAET and DEFAC explained more than MAT or MAP for the change mechanism of k values. The difference is due to a much larger range of MAP in LIDET than that in our LTIDE. The MAP ranges from 209 to 3,914 mm in LIDET (Gholz et al. 2000), bigger than the 589 to 2,651 mm in our study (Table 1). Moreover, the sites in LTIDE are fairly wet with no water stress, which explains why temperature is such a good indicator of decomposition rate.
Difference in FMR between DHS and local litters
Decomposition rates of DHS litters in terms of FMR or k-values were not as rapid as those of local litters when decomposition experiments were done in same sites. The phenomena have been shown in Figs. 4 and 5. From the results in the two figures, Fig. 7 is calculated and shows the difference in FMR between DHS and local litters with MAT and decomposition time.
Under various MAT and decomposition durations, the differences in FMR or k-values between local and DHS litters varied nonlinearly with MAT and decomposition time, could be as much as 16% of the original mass and decreased with increasing MAT. There was a maximum difference for every MAT. The decomposition time needed to reach the maximum difference was shorter as MAT increased, from 4 years in site of MAT = 1°C to 1 year in site of MAT = 25°C.
We hypothesize the above phenomenon resulted mainly from the acclimation of decomposer communities to substrate. Compared to DHS site, the colder the site is, the larger the differences in environmental conditions and decomposer community will be, and the more difficult the local decomposer community acclimates to extraneous substrate. This may lead to lower decomposition rates of DHS litters. However, if this hypothesis is correct, the importance of intersite decomposition experiment will be reduced.
Our experiment may have partly addressed the impacts of substrate quality on FMR or k-values. The comparable FMR and k-values (p > 0.05) of DHS and local NLL litters in the sites HT2, GGS2 and BJ2 (Fig. 4) may have resulted from the combination effects of difference in substrate qualities and the acclimation of decomposer communities to substrate. The substrate qualities of DHS litter should have favored decomposition more than those of local NLL litters, which should have caused a significant increase in the decomposition rates of DHS litters compared with the local NLL litters. However, this may have been counteracted by the slower acclimation of local decomposer community to DHS litters and their decomposition rates were therefore similar to the NLL litters.
Substrate quality on k-values
Although the impacts of single substrate quality index such as initial N content, N/P ratio and C/N ratio on k-values are hard to separate from other factors and their interactions, we can get some hints in impacts of substrate quality on k-values from considering the difference in FMR between DHS and local litters.
The values of k increased positively with initial N concentration (k = 1.208N% − 0.539, R 2 = 0.60, p < 0.0001) and initial N/P ratio (k = 0.0451 N/P − 0.2363, R 2 = 0.58, p < 0.0001), negatively with initial C/N ratio (k = −0.0186 C/N + 1.685, R 2 = 0.47, p < 0.0001) for the local litters at all sites. These results are consistent with the conclusions from other studies (Heneghan et al. 1998; Taylor et al. 1989; Vitousek et al. 1994; Moorhead et al. 1999; Silver and Miya 2001). However, the R 2 in our experiment is much smaller than that in Heneghan et al. (1998), probably owing to the fact that our result is from the intersite experiment in which the interregional environmental factors such as MAT and MAP made a more important contribution to the k-values. The relationships between N content or C/N ratio and k-values can be improved if the relationships were developed from one single site (when the impacts of interregional environmental factors are excluded). The following results were based on the BJ site k = 0.881N% − 0.503 (R 2 = 0.998, p < 0.05) and k = −0.0027 C/N + 0.3185 (R 2 = 0.880, p < 0.05). Because of similarity in MAT, MAP, AAET and decomposer community in the three forest types of BJ site, the substrate quality became a significant variable influencing litter decomposition. This clearly indicates that within a narrow range of climates that substrate quality can explain a great deal of the variation in litter decomposition rate-constants.
Lignin or ratio of Lignin to N has been extensively used as a litter quality indicator in studying litter decomposition process and nutrient cycling (Melillo et al. 1982; Prescott 2005). Lignin contents or ration of Lignin to N were not measured in this study. However, we used published lignin data as well as our nitrogen data for litters of the broadleaf species in this study. We found that lignin contents in leaf litters for main species in the 8 studied regional broadleaf forests were similar, ranging from 24.5% to 26.5% (Zhang et al. 1998; Wang and Huang 2001; Li 2007; Li et al. 2007; Xu et al. 2007). The ratios of lignin to N are from 16.4 to 28.6, which are consistent with the data from Melillo et al. (1982). This may imply that the results of the study on broadleaf forest litters may not be influenced by litter quality variable. A more solid conclusion must await further examination on the effects of lignin or Lignin/N ratio on litter decomposition in the all studied forest litter types in the future.
Conclusions
FMR had a high, negative exponential correlation with decomposition time for all litters regardless of substrate quality. The fitted k-values ranged from 0.169 to 1.454/year. Climatic factors control the decomposition rate, in which MAT and AAET are dominant and MAP is subordinate. Decomposition process may apparently be divided into two phases controlled by different factors. In our study, 0.75 years is believed to be the dividing line of the two phases. There is significant difference in initial C and N content, N/P and C/N ratios between broadleaved and needle leaf litters. Initial N/P ratios decreased with increasing site latitude. In addition to initial C/N ratio, we suggest that initial N/P ratio should also be evaluated to determine if it is an important factor for litter decomposition. That the decomposition rates of DHS litters were slower than those of local litters probably resulted from the acclimation of local decomposer communities to extraneous substrate. Decrease in temperature would result in a bigger decrease in decomposition rates of DHS litters.
References
Aber JD, Melillo JM, McClaugherty CA (1990) Predicting long-term patterns of mass loss, nitrogen dynamics, and soil organic matter formation from initial fine litter chemistry in temperate forest ecosystems. Can J Bot 68:2201–2208
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Chapin FS III, Shaver GR (1996) Physiological and growth responses of arctic plants to a field experimental simulating climatic change. Ecology 77:822–840
Coûteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Trends Ecol Evol 10:63–66
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Glob Chang Biol 6:751–765
Helland IS (1987) On the interpretation and use of R2 in regression analysis. Biometrics 43:61–69
Heneghan L, Coleman DC, Zou X, Crossley DA Jr, Haines BL (1998) Soil microarthropod community structure and litter decomposition dynamics: a study of tropical and temperate sites. Appl Soil Ecol 9:33–38
Li KX (2007) Effects of nitrogen deposition on litter decomposition of two main coniferous tree species in Changbai mountain. J Northeast For Univ 35(2):17–19
Li XF, Han SJ, Zhang Y (2007) Foliar decomposition in a broadleaf-mixed Korean pine (Pinus koraiensis Sieb. Et Zucc) plantation forest: the impact of initial litter quality and the decomposition of three kinds of organic matter fraction on mass loss and nutrient release rates. Plant Soil 295:151–167
McHale PJ, Mitchell MJ, Bowles FP (1998) Soil warming in northern hardwood forest: trace gas fluxes and leaf litter decomposition. Can J For Res 28:1365–1372
Melillo JM, Aber JD, Muratore JF (1982) Nitrogen and lignin control of hardwood leaf litter decomposition dynamics. Ecology 63:621–626
Meentemeyer V (1984) The geography of organic decomposition rates. Ann Assoc Am Geogr 74:551–560
Moore B, Braswell III (1994) Planetary metabolism: understanding the carbon cycle. Ambio 23:4–12
Moorhead DL, Currie WS, Rasttetter EB, Parton WJ, Harmon ME (1999) Climate and litter quality controls on decomposition: an analysis of modeling approaches. Glob Biogeochem Cycles 13:575–589
Motulsky HJ, Christopoulos A (2003) Fitting models to biological data using linear and nonlinear regression: a practical guide to curve fitting. GraphPad Software Inc., San Diego, pp 34–35
Olson JS (1963) Energy stores and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS et al (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Prescott CE (2005) Do rates of litter decomposition tell us anything we really need to know? For Ecol Manag 220:66–74
Schlesinger WH (1991) Biogeochemistry: an analysis of global change. Academic, New York
Seastedt TR, Crossley DA, Meentemeyer V Jr, Waide JB (1983) A two-year study of leaf litter decomposition as related to macroclimatic factors and microarthropod abundance in the southern Appalachians. Holarct Ecol 6:11–16
Shaw MR, Harte J (2001) Control of litter decomposition in a subalpine meadow-sagebrush steppe ecotone under climate change. Ecol Appl 11(4):1206–1223
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality. Oecologia 129:407–419
Taylor BR, Parkinson D (1988a) A new microcosm approach to litter decomposition studies. Can J Bot 66:1933–1939
Taylor BR, Parkinson D (1988b) Respiration and mass loss rates of aspen and pine leaf litter decomposing in laboratory microcosms. Can J Bot 66:1948–1959
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decomposition rates: a microcosm test. Ecology 70(1):97–104
Vitousek PM, Turner DR, Parton WJ, Sanford RL (1994) Litter decomposition on the Mauna Loa environmental matrix, Hawaii: patterns, mechanisms, and models. Ecology 75(2):418–429
Wang J, Huang JH (2001) Comparison of major nutrient release patterns in leaf litter decomposition in warm temperate zone of China. Acta Phytoecologica Sinica 25(3):375–380
Wardle DA, Walker LR, Bardgett RD (2004) Ecosystem properties and forest decline in contrasting long-term chronosequences. Science 305:509–513
Xu XJ, Zhang K, Liu B, Lan CC, Xu XN (2007) Review on litter decomposition in forest ecosystems. Sci Soil Water Conserv 5(4):108–114
Zhang DQ, Yu QF, Kong GH, Zhang YC (1998) Chemical properties of forest floor litter in Dinghushan monsoon evergreen broadleaved forest. Acta Ecol Sin 18(1):96–100
Zhou GY, Sun G, Wang X, Zhou CY, McNulty SG, Vose JM et al (2008) Estimating forest ecosystem evapotranspiration at multiple temporal scales with a dimension analysis approach. J Am Water Resour Assoc 44(1):208–221
Acknowledgements
The LTIDE study was funded by NSFC projects 30725006 and 40730102 and CERN. We thank Drs. Li Yide, Wang Silong, Tang Jianwei, Sang Weiguo, Guo Qingxi, Liu Yuhong, and Cheng Genwei for their assistance in field data collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Rights and permissions
About this article
Cite this article
Zhou, G., Guan, L., Wei, X. et al. Factors influencing leaf litter decomposition: an intersite decomposition experiment across China. Plant Soil 311, 61–72 (2008). https://doi.org/10.1007/s11104-008-9658-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-008-9658-5