Abstract
Purpose
To assess the direction and strength of climate and leaf litter trait effects on decomposition dynamics throughout the litter decomposition process.
Methods
We performed a three-year-long litterbag translocation experiment along an elevational gradient in a transitional mixed forest in China. We explored temporal shifts in the relative contribution of microclimate and litter traits of seven dominant species to mass loss throughout the decomposition process.
Results
Air temperature and soil moisture imparted no significant effects on mass loss in the initial decomposition stage (0–6 months) but exerted positive effects after 6 months’ incubation (p < 0.05). Initial specific leaf area (SLA) was positively associated with mass loss only during the early decomposition stages (0–12 months). Litter P concentration was positively while N concentration was negatively associated with mass loss for almost all decomposition stages. Both litter Mg and Ca concentrations were negatively associated with mass loss throughout the whole decomposition process (p < 0.05). The relative contribution of microclimate was weaker than that of litter traits during the early stages but increased in the late decomposition stage (after 36 months). SLA and Mg were the most important traits during the early stages (0-12 months), whereas N and Mg were relatively stronger during the later stages (30–36 months).
Conclusion
Our results have highlighted that microclimate (particularly temperature) exerted dominant control over later-stage litter decomposition. Often-overlooked litter traits such as Mg, are key potential drivers of litter decomposition dynamics and should be more explicitly incorporated into current biogeochemical models to better understand litter-driven nutrient and carbon cycling.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Leaf litter decomposition represents a fundamental ecological process that influences nutrient cycling and soil organic carbon formation in ecosystems. This decomposition process is a key determinant of carbon fluxes between forests and the atmosphere, as it releases a large amount of CO2 into the atmosphere along with nutrients such as nitrogen (N) and phosphorus (P) into the soils, which can be effectively utilized by plants for growth (Cotrufo et al. 2015; García-Palacios et al. 2016; Zanne et al. 2015). Therefore, there is a growing need to better understand the factors controlling the decomposition process to accurately predict how climate change may affect carbon and nutrient cycles via biotic feedback mechanisms. Numerous studies have demonstrated that prevailing climatic conditions along with leaf litter traits of species are among the main drivers of litter decomposition across diverse ecosystems (Berg 2014; Cornwell et al. 2008; García-Palacios et al. 2016; Suseela and Tharayil 2018). Still, our understanding of the drivers of litter decomposition remains relatively limited across decomposition stages and climates.
First, while leaf litter decomposition rate is tightly linked to the stage of decomposition, temporal dynamics of litter decomposition need to be further studied (Adair et al. 2010; Canessa et al. 2021; García-Palacios et al. 2016). The majority of past studies have explored leaf litter decomposition with a small number of harvests of litterbags (Berg and McClaugherty 2020; Cornwell and Weedon 2014). This approach strongly facilitates the robust evaluation of how this short-term decomposition might be influenced by various factors (García-Palacios et al. 2016; Moore et al. 2017). The problem with such an approach is that it provides a poor representation of the long-term dynamics of decomposition for various species in many ecosystems (Canessa et al. 2021; Moore et al. 2017). Therefore, this approach could yield inaccurate estimates across different decomposition stages (Cornwell and Weedon 2014; Manzoni et al. 2012). A more straightforward method is that litterbags are retrieved with several harvests over a longer duration.
Second, variation in the relative importance of climate versus leaf litter traits of tree species during the course of litter decomposition has not been fully elucidated (Canessa et al. 2021; Kou et al. 2020; Moore et al. 2017; Oberle et al. 2020). Though there is little reason to expect the shifting influence of climatic controls through the decomposition process, several empirical studies have suggested that the role of climate and litter traits could vary among different decomposition stages (Canessa et al. 2021; Currie et al. 2010; García-Palacios et al. 2016; Moore et al. 2017). Specifically, studies have highlighted a temporal shift in the control of decomposition by climatic factors between high- and low-quality litter (García-Palacios et al. 2016). However, these analyses only focused on the arbitrary classification of litters (rather than quantitative litter traits), which could lead to an inaccurate estimation of the role of litter traits of species (García-Palacios et al. 2013). Furthermore, a study conducted by Zanne et al. (2015) in temperate forests inferred that litter traits could be more relevant in the early stages of decomposition, while climate may be more important at later stages. However, other studies have indicated that litter decomposition was strongly regulated by the climate in early stages and by litter traits in late stages (Berg et al. 2015; Hobbie 2015; Waring 2012). These contradictory findings provide further justification for more empirical studies to accurately parse the relative roles of climate and leaf litter traits throughout the decomposition process (García-Palacios et al. 2016; Santonja et al. 2019).
Finally, while previous studies have demonstrated that the control of leaf litter decomposition by climate and leaf litter traits of species is forest-specific (Aerts 1997; Fujii et al. 2018; Hättenschwiler et al. 2011; Keller and Phillips 2019), field observations of litter decomposition are strongly biased toward tropical and temperate forests. Furthermore, our understanding of litter decomposition in transitional forests located between tropical and temperate regions is lacking, which seriously limits our understanding of litter decomposition across diverse forests. Litter decomposition in tropical regions with year-round high temperatures and seasonal precipitation is mostly moisture- and P-controlled, whereas the process is primarily temperature- and N-controlled in temperate regions with seasonally variable temperatures (Aerts 1997; Jiang et al. 2021; Marklein et al. 2016; Powers and Salute 2011). Since these ecosystems have different climate conditions and species composition compared to transitional forests, we expect that previously made generalizations and conclusions about decomposition drivers may not be correct and thus lead to uncertainty. More specifically, to develop the findings from temperate and tropical forests, new insights into the decomposition process in this under-represented forest are urgently needed.
To address these knowledge gaps, we have identified and quantified the relative contribution of specific microclimate and leaf litter traits for the decomposition process for different decomposition stages in a transitional mixed forest located between tropical and temperate forests. Specifically, we conducted a three-year-long leaf litter translocation decomposition experiment using the litterbag technique along an elevational climatic gradient in Central China. We measured leaf litter mass loss and initial traits of seven dominant tree species as well as local-scale microclimate (air temperature and soil moisture) during the decomposition trajectory. Based on the above-mentioned evidence from other studies, we predicted that (a) the relative importance of leaf litter traits and microclimate would vary temporally during the decomposition process, and (b) the decomposition dynamics of our targeted forest would show some common and unique features relative to tropical and temperate forests.
Materials and methods
Study site
The study site was located on the southern slope of Shennongjia Mountain in Central China. This region constitutes a geographical transition zone between the (sub-) tropical and temperate climates and is well known as an important biodiversity conservation hotspot both in China and globally (Xie and Shen 2021). The mean annual temperature is 10.6 °C and annual precipitation ranges between 1306–1722 mm. The dominant soil classes are mountain yellow–brown soil and mountain brown soil. Further details about the site have been previously reported (Ge et al. 2013) and are available in Appendix S1 and S2.
The mixed evergreen and deciduous broad-leaved forest, the zonal vegetation in this region, is unique among forests at similar latitudes (ca. 31oN) which are typically dominated by Fagaceae species in the Northern Hemisphere (Ge et al. 2013; Xie and Shen 2021). The mixed forest forms a critical climate and geographical transitional zone in Central China between year-round warm (sub-) tropical evergreen broad-leaved forest and seasonally temperate broad-leaved forest (See Appendix S1 for more details on this transitional forest). This forest harbors one of the highest levels of biodiversity in the world, and is particularly sensitive to climate change (Ge et al. 2019; Ge and Xie 2017; Wu 1980). Therefore, this kind of transitional forest provides a powerful model to comprehensively address how climate, along with the shift in litter traits of tree species will affect leaf litter decomposition dynamics.
Experimental design and setup
We performed a three-year-long (2011–2014) leaf litter translocation decomposition experiment along an elevational gradient using the litterbag method to measure litter mass loss over time. In November 2011, we established five experimental sites along an elevational gradient at 800, 1000, 1300, 1600, and 1800 m a.s.l. The selection of these sites allowed us to address a range of contrasting microclimates (in particular temperature), which could increase our current understanding of the effects of climate and leaf litter traits (Ge et al. 2013; Salinas et al. 2011; Sundqvist et al. 2013). We performed the same experimental layout for the seven tree species used. We collected leaf litter samples produced by seven dominant tree species of the transitional mixed forest in 2011, specifically Cyclobalanopsis multinervis (CM, the synonym of Quercus hypargyrea), Cyclobalanopsis oxyodon (CO, the synonym of Quercus oxyodon), Quercus engleriana (QE), Fagus engleriana (FE), Fagus pashanica (FP), Quercus serrata var. brevipetiolata (QS) and Quercus aliena var. acuteserrata (QA). The nomenclature of plant species here follows the Flora of China (The Editorial Board of Flora of China 2004). Samples from each species were collected independently and oven-dried at 65℃ for two days to reach a constant mass before being homogenized. For each species, 10.00 g of leaf litter was sealed in 15 cm × 10 cm litterbags constructed from 1.5-mm mesh nylon netting. Eighteen bags (for 18 samplings across three years) for each species were placed in five clusters (replicates) at each site. Samples were harvested ca. every two months with one litterbag from each of the five replicated clusters at each one of five sites. Therefore, a total of 3150 litterbags (5 replicates × 18 sample litterbags × 5 sites × 7 species) were installed along this elevational gradient (See details in Appendix S2). After collection, litterbags were immediately transported to the laboratory where the remaining litter was cleaned and brushed carefully to remove roots, fauna, and soil particles adhered to the litterbags. Finally, the litterbags were oven-dried to a constant mass at 65℃ and weighed.
Leaf litters of these selected dominant tree species, constituting the major components of litterfall in this kind of forest (Ge et al. 2017; Gilliam 2016; Shen et al. 2019), are characterized by a wide range of initial litter traits which are expected to influence litter decomposition in this transitional forest (Canessa et al. 2021; Cornwell et al. 2008; Pérez-Harguindeguy et al. 2013). Here, we measured and calculated the initial mean specific leaf area (SLA) of leaf litter for each tree species using a leaf area meter (LI-3000, Li-Cor, USA). Furthermore, we determined initial concentrations of nitrogen (N), phosphorus (P), calcium (Ca), and magnesium (Mg) of leaf litter samples for each tree species. The laboratory analyses and corresponding results are available in Appendix S2.
Given the recent indication that local site conditions in which decomposition takes place may be poorly represented by macroclimatic parameters (Bradford et al. 2014; Bütikofer et al. 2020; Canessa et al. 2021), we used HOBO Onset microclimatic recorders (Onset Computer Corporation, USA) to measure actual microclimatic variables including air temperature (AT) and soil moisture (SM) at one-minute intervals for each site. See Figure S2 for temporal dynamics of AT and SM conditions for the five study sites along the elevational gradient during these incubation periods.
Calculations and statistical analysis
Because our studied forests experience strong climate seasonality (Ge et al. 2019), the initial decomposition rate will be influenced by the season in which the incubations were started. This issue could be partially remedied by reporting mass loss estimates across different decomposition stages. Thus, we chose mass loss as a proxy of decomposition and we analyzed these data using different litter harvests (6-month intervals). We calculated relative mass loss (%) for each sampling period by dividing the mass loss at any harvest by the initial mass (× 100) (Canessa et al. 2021; Santonja et al. 2019).
We analyzed these data by decomposition stage. First, we evaluated the effects of species identity and elevation on litter mass loss over time using a factorial ANOVA. Elevation, species, and incubation time were fixed effects in the model, while the cluster was a random effect. To interpret significant interaction terms, we used a simple main effects test. The Tukey’s HSD test was used for post hoc comparisons of factors with more than two levels. Second, to further evaluate the underlying temporal drivers of mass loss across different tree species and multiple sites, we conducted commonly used multiple linear regression models and variation partition analysis for each decomposition stage. Here, grouped litterbag data were selected and defined at six decomposition stages, specifically 6, 12, 18, 24, 30, and 36 months after litterbag installation. We selected 6-month intervals since this time frame demonstrated a much greater ability to characterize the temporal patterns of different controls of litter decomposition as compared to 2- or 4-month intervals in our preliminary data exploration, although similar conclusions were achieved with the above-mentioned different time frames. See more details in Appendix S3.
Before performing multiple linear regression analysis, we checked all potential explanatory variables including microclimate (AT and SM) and the above-mentioned initial leaf litter traits (SLA, N, P, K, Ca, and Mg) for normality, and then log-transformed these variables when necessary. Microclimatic data were trimmed to the corresponding decomposition stages for statistical analyses following previous studies (Canessa et al. 2021; García-Palacios et al. 2016). Each explanatory variable was additionally standardized by subtracting the mean and dividing by the standard deviation before analysis to enable direct comparison of estimated regression coefficients for each decomposition stage (Bradford et al. 2016; García-Palacios et al. 2018; Ge et al.2022; Zuur et al. 2009). We also evaluated collinearity among the explanatory variables by evaluating the variance inflation factor (VIF) for each decomposition stage model (Dormann et al. 2013). Separate models were run for each decomposition stage. Model selection was performed using both forward and backward selection. At each step, we quantified the variance inflation of the added explanatory variable to restrict multicollinearity in the model. In this study, no multicollinearity issues were found in the most parsimonious models for any decomposition stage. We then selected the best-fit models by calculating Akaike’s information criterion (AIC) following the methods of similar studies (Canessa et al. 2021; García-Palacios et al. 2018). The model with the lowest AIC value within a given set of models for each decomposition stage was considered to have the greatest support (Ge et al. 2019; Zuur et al. 2009).
For the selected regression model for each decomposition stage, we further parsed the relative contribution of each explanatory predictor under consideration as drivers of mass loss. To do so, we calculated the relative effect of the parameter estimates for each of the predictors compared with the effect of all parameter estimates in the model (García-Palacios et al. 2018; Gross et al. 2017). This method is similar to variance decomposition analysis since we have standardized all explanatory predictors before analysis. We also examined the overall relative contribution of climatic and leaf litter traits following the same method for each decomposition stage.
The statistical analyses carried out in this study were implemented in R 3.6.0 (R Core Team, 2013) using the basic statistical package, “car” package (Fox and Weisberg, 2019), and “MuMin” package (Bartoń, 2020). See Appendix S3 and S4 for additional information on data processing and statistical analyses.
Results
Leaf litter mass loss across species along the elevational gradient
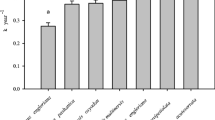
At the end of the experiment (after 36 months), mean leaf litter mass loss was 71.14% across all sites and tree species. The maximum leaf litter mass loss recorded for a sample was 95.7% for Quercus aliena var. acuteserrata. Mean mass loss for the seven individual tree species varied from 62.40% for Fagus engleriana to 78.76% for Quercus serrata var. brevipetiolata, which were significantly different from one another (p < 0.05) (Fig. 1). Mean mass loss across all species among the sites ranged from 61.58% at 1800 m to 77.19% at 1000 m (Fig. 2). Leaf litter mass loss continuously increased with incubation time, and temporal patterns of leaf litter mass loss depended on the studied species and elevation, as indicated by the significant two-way interactions (Table 1 and Appendix S5).
Boxplot of leaf litter mass loss for seven tree species of the studied mixed evergreen and deciduous broad-leaved forest after three three-year of field incubation. Different capital letters at the top represent significant differences (p < 0.05) among the seven tree species (Tukey’s post-hoc test). The abbreviations are used: FE = Fagus engleriana, CO = Cyclobalanopsis oxyodon, CM = Cyclobalanopsis multinervis, FP = Fagus pashanica, QE = Quercus engleriana, QA = Quercus aliena var. acuteserrata, and QS = Quercus serrata var. brevipetiolata. The nomenclature of plant species here follows the Flora of China (The Editorial Board of Flora of China 2004)
Microclimate and leaf litter trait effects on mass loss at various decomposition stages
We found that different factors controlled litter mass loss across decomposition stages (Fig. 3 and Appendix S5). Microclimatic variables (AT and SM) did not display any significant effects on mass loss in the early decomposition stage (0–6 months). After 6 months, both AT and SM exerted consistent, significantly positive effects on mass loss (p < 0.05). The effects of individual leaf litter traits changed throughout the decomposition process Fig. 3). Initial leaf litter SLA was positively associated with mass loss in the period from 0 to 12 months (p < 0.05), but this positive relationship disappeared after 12 months (p > 0.05). Leaf litter P concentration correlated positively with mass loss, while leaf litter N displayed a consistently negative association with mass loss at almost all decomposition stages (p < 0.05). Leaf litter Mg and Ca remained negatively associated with mass loss throughout the whole decomposition process (p < 0.05) (Fig. 3).
Standardized coefficients for microclimate and individual initial leaf litter traits included in the final regression model for each decomposition stage. Coefficients represent relative percent change in mass loss for one standard deviation increase in the variable. Blue and red circles indicate negative and positive effects on mass loss, respectively. Circle size indicates the magnitude of effects. The number in the circles represents the effect size on mass loss. Note that the following microclimatic predictors were included with abbreviations: AT = air temperature, SM = soil moisture
Temporal shifts in the relative contribution of microclimate and leaf litter traits
We found that the relative importance of microclimate and litter traits changed with the progression of litter decay (Fig.4). Overall, the relative contribution of microclimate was smaller than that of litter traits, but increased with advanced decomposition and exceeded the overall contribution of litter traits until 36 months of field incubation (Appendix S6). Specifically, the relative role of air temperature (20.50% averaged across stages) was higher than soil moisture (14.54% averaged across stages) over the whole decomposition period. The relative contribution of single litter traits varied among the decomposition stages (Appendix S5). For instance, SLA and Mg ranked highest in importance among these litter traits in the period of 0 to 12 months, while N and Mg were relatively stronger at advanced stages (i.e. until 36 months) (Fig. 3 and 4). The roles of litter P and Ca were intermediate compared with other litter traits. The total explanatory power of both microclimate and leaf litter traits decreased from 70.96% to 49.82% as decomposition advanced (Appendix S6).
Discussion
Our current work has identified whether and how the relative importance of microclimate and leaf litter traits on litter decomposition shifted across different decomposition stages by tracking a longer incubation period than that of previous studies (Moore et al. 2017; Oberle et al. 2020; Waring 2012; Zhang et al. 2008). We found that microclimate displayed a relatively smaller influence than initial litter traits at early stages of decomposition, but had a larger influence at later stages. The overall effect of initial litter traits faded with the progression of decomposition, and the relative contribution of specific litter traits depended not only on the decomposition stage but also on the trait studied. These findings provide complementary evidence for reconciling the seemingly debated paradigms regarding the relative contributions of climate vs. litter traits for decomposition (Bradford et al. 2016; Cornwell et al. 2008; Hoeber et al. 2020) and emphasize the urgent need to capture a more complete picture of temporal-dependent key drivers of litter decomposition dynamics.
Microclimate predominantly drove later-stage litter decomposition
Several previous studies have indicated that climate has a negligible influence at the initial but is stronger at later stages of decomposition (Canessa et al. 2021; García-Palacios et al. 2016), while the opposite trend was detected in others (Berg et al. 2015; Hobbie 2015; Waring 2012). Here, we have simultaneously taken temporal dynamics of local concurrent microclimate and mass loss into account and minimized any error that may arise from a temporal-scale mismatch between mass loss data and long-term climate averages (Bradford et al. 2017; Currie et al. 2010; García-Palacios et al. 2016). Our results indicate that microclimate, and in particular air temperature, took precedence over leaf litter traits as the main predictor of later-stage decomposition (after 36 months of field incubation) in our transitional mixed forest (Figs. 3 and 4), a contrasting pattern compared to previous findings (Berg et al. 2015; Waring 2012).
Here, we assumed that this delay in the appearance of microclimatic effects on decomposition may be linked to the relative availability of labile versus recalcitrant compounds of leaf litters. During the early stages of decomposition, microbes preferentially target labile compounds such as cellulose and hemicellulose (Berg 2014; Suseela et al. 2013). As the majority of labile compounds could be utilized by a wide range of microbial communities, representing various climatic tolerances, the potential of climate to influence the utilization of these labile compounds by microbes could be largely restricted (Berg 2014; Suseela et al. 2013; Zanne et al. 2015). Therefore, the decomposition process at the early stage is insensitive to climate. As litter decomposition progresses into later phases, most of these labile substrates have been metabolized and exhausted by the microbial decomposers and recalcitrant complexes of leaf litters have been resided and formed (Averill and Waring 2018; Berg and McClaugherty 2020; Hobbie 2015). At this advanced stage, the ability of microbes to degrade more recalcitrant compounds requires favorable climatic conditions and therefore a higher activation energy (García-Palacios et al. 2016; Keiser and Bradford 2017).
Distinct litter traits related to distinct stages of decomposition
Although initial litter traits are known to be key drivers of litter decomposition across tropical and temperate forests, their specific temporal role throughout the decomposition process is not well documented (Li et al. 2020; Parsons et al. 2014; Suseela and Tharayil 2018; Zanne et al. 2015). Here, we have selected commonly used litter traits including SLA, leaf litter N, and P, along with non-conventional litter traits such as Ca and Mg (Berg et al. 2021; Cornwell et al. 2008), to identify their specific roles during the course of decomposition. We found that these litter traits were adequate predictors of litter decomposition in this transitional forest, but their effects varied among different decomposition stages. Specifically, initial litter traits prevailed over microclimatic drivers of litter decomposition during the first 30 months of field incubation (Appendix S6). This result is in contradiction with the findings of Trofymow et al. (2002) and Santonja et al. (2019), who observed that leaf litter traits increased in predictive importance throughout the course of decomposition but were in qualitative agreement with some studies which stated that litter traits were relatively more important than the climate in controlling initial decomposition, and their relative importance declined in subsequent decomposition stages (Canessa et al. 2021; Currie et al. 2010; García-Palacios et al. 2016). The results among various studies may be related to the study duration of field decomposition incubation and the definition of decomposition stage (mass loss classes vs. specific period) (Canessa et al. 2021; García-Palacios et al. 2016) along with different targeted ecosystem types (forests vs. shrubland) that may respond differently to changes in climatic conditions (Cusack et al. 2009; Moore et al. 2017; Santonja et al. 2019).
Leaf litter SLA emerged as the main driver of initial-stage decomposition
Interestingly, SLA has played a positive role in the initial decomposition stage, but this stimulatory effect disappeared after 12 months of incubation. The vast majority of litter decomposition studies have also reported that SLA was positively correlated with litter decomposition in various forests globally (Cornwell et al. 2008; Santiago 2007; Sun et al. 2018). For example, Cornwell and others (2008) found that global-scale litter decomposition increased significantly with leaf litter SLA. However, these studies have considered relatively short experimental duration, mainly encompassing the early stages of decomposition alone (Cornwell and Weedon 2014; Currie et al. 2010; Moore et al. 2017), which may not hold for later decomposition stages (Coûteaux et al. 1995; Moore et al. 2017; Oberle et al. 2020).
Here, two possible but not mutually exclusive explanations could account for the positive role of SLA observed only during the early stages of litter decomposition. First, initial litter SLA is tightly linked to leaf palatability and concentration of soluble substances, which is likely due to the relative differences in carbon investments in protective compounds (Chomel et al. 2016; Hättenschwiler and Jørgensen, 2010). Higher SLA renders leaf litter more immediately available and accessible to microbes and macro-decomposers such as soil animals during the initial physical transformation process of litter materials (Fujii et al. 2018; Li et al. 2020; Manzoni et al. 2012). Second, the positive effect of SLA on leaf litter decomposition may be indirect, as this trait is tightly associated with other structural characteristics such as leaf litter physical toughness and litter water saturation, both of which improve micro-environmental conditions (Makkonen et al. 2012; Santonja et al. 2019). Following initial transformations on litter decomposition by soil fauna, initial species differences of litter SLA become less distinct. Thus the strong positive effect of SLA disappeared and was progressively replaced by other traits as decomposition progressed (Fig. 4). This finding further emphasizes the need to take caution when applying SLA as an adequate descriptor of litter physical traits to explore the effects of litter traits in the late-stage decomposition.
Leaf litter N slowed while P stimulated decomposition throughout the decomposition process
While both initial leaf litter N and P generally affect decomposition, their specific influences on decomposition are rather complex (Berg 2014; Canessa et al. 2021; Hobbie 2015). We found that initial litter N showed a strong, consistently negative effect while initial litter P was positively correlated with mass loss and the overall contribution of litter N was much more important than that of P across the stages of decomposition. This finding contrasts the widely held notion that litter N typically stimulates decomposition in temperate short-term studies and global-scale analyses (Berg et al. 2015; Cornwell et al. 2008; Cusack et al. 2009), but broadly agrees with previous findings in tropical forests (Hättenschwiler et al. 2011; Waring 2012; Wieder et al. 2009).
The contrasting roles of litter N and P in driving litter decomposition may be related to the nature and relative availability of these nutrients for microbes over time (Berg and McClaugherty 2020; Marklein et al. 2016). The negative influence of N on decomposition is related to the different forms and localizations of organic N in leaf litters (Berg 2014; Hobbie 2015; Suseela et al. 2013). For instance, high N concentrations in initial litters could also reflect high concentrations of N-based defense secondary compounds such as alkaloids, or the formation of compounds via covalent bonding to macromolecules that resist microbial attack and in turn inhibit decomposition (Berg and McClaugherty 2020; Hobbie 2015; Zanne et al. 2015). Another possibility is that N is preferentially immobilized into litter while P is preferentially released from the litter during decomposition, leading to a potential mismatch between nutrient availability of decomposing litters and decomposer nutrient demand (Keiser and Bradford 2017; Marklein et al. 2016). We caution, however, that our inferences regarding both litter N and P effects are based on the rather limited size of observational samples. Therefore, we suggest that site-based multi-species field studies that focus on nutrient dynamics of decomposing litters across decomposition stages are urgently needed to further clarify the specific role of these elements during the litter decomposition process.
The suppressed roles of litter Mg and Ca with progressive decomposition
Whereas initial litter micronutrients such as Ca and Mg may be key predictors of litter decomposition, the nature, and extent of their specific effects throughout the decomposition process have been often overlooked in comparison with those of litter N and P, especially for transitional forests such as ours (Makkonen et al. 2012; Waring 2012; Zhou et al. 2020). Here, our findings expand on previous studies in underscoring the key inhibitory roles of Mg and Ca during the decomposition process (García-Palacios et al. 2016; Moore et al. 2017; Zanne et al. 2015). We found that litter Ca and Mg explained a larger amount of variation in the decomposition process compared to the role of N or P litter traits. The strength of the negative effect of Mg was overwhelmingly greater in the early stage of decomposition. These results contrast with some findings in temperate and tropical forests which concluded that both litter Ca and Mg were positively correlated with mass loss (Makkonen et al. 2012) but supported other observations for leaf and root litter decomposition in temperate forests (Goebel et al. 2011; Jiang et al. 2021).
How do we reconcile our results with those of previous studies that observe different litter Mg- and Ca- decomposition relationships? While both litter Mg and Ca favor decomposition since they are vital for the general function and are also components of decomposition enzymes in decomposers (Makkonen et al. 2012; Zhou et al. 2020), prior laboratory and field studies have demonstrated a suppressing role for Mg and Ca via chelation or cross-linking with decomposers (e.g. cation bridges) (Jiang et al. 2021; Powers and Salute 2011). Magnesium and calcium oxalate are common stable components of fungal cell walls (Powers and Salute 2011), where they provide a hydrophobic coating that in turn prevents hyphae from becoming hydrated and thus reduces microbial attachment. Fungal populations can therefore easily colonize Mg- and Ca-rich leaf litters and impede their decomposition by forming the Mg- and Ca-rich fungal hyphae (Powers and Salute 2011; Wiesmeier et al. 2019). Alternatively, it may be the case that Mg- and Ca-rich decomposing litters easily form secondary compounds, such as antibiotics and antifungals, which may inhibit the activity of microbes and in turn impede litter decomposition (Jiang et al. 2021; Powers and Salute 2011; Rowley et al. 2018). While it is not possible to distinguish among these specific mechanisms with our current analyses, our results indeed highlight that future work should consider both stimulatory and inhibitory effects of a wider range of elements that are traditionally studied in driving leaf litter decomposition and biogeochemical cycling across various forests.
Main implications of current findings
In comparison to previous decomposition studies that focus on tropical and temperate forests, our transitional forest study has some potential implications. Here, we found that litter decomposition in our targeted forest displayed unique features compared to tropical and temperate forests (Fig. 5). For example, air temperature rather than soil moisture strongly controls leaf litter decomposition in this transitional forest, similar to temperate but not tropical forests. Additionally, litter decomposition seems to be controlled both by N and P, a transitional feature between temperate and tropical forests (Cusack et al. 2016; Marklein et al. 2016; Waring 2012). The observed negative effect of litter Mg concentration on leaf litter decomposition, in contrast to that of temperate and tropical regions (Makkonen et al. 2012; Zhou et al. 2020), has highlighted the urgent necessity to incorporate these decomposition drivers into our current understanding of broad-leaved forest latitudinal transitions. We also highlight the need for more direct evidence with additional manipulative experiments and observational studies to provide more powerful information that can generalize their temporal roles of climate and particularly traits over the decomposition process throughout diverse forests, but particularly in under-studied ecosystems. Additionally, future studies should incorporate these potential dynamic shifts in the major drivers of decomposition to better predict temporal dynamics of carbon and nutrient cycling via leaf litter decomposition pathways (Cornwell and Weedon 2014; Ge et al. 2017; Oberle et al. 2020).
Concluding remarks
Collectively, this study has quantified temporal shifts in local microclimate and leaf litter traits control of litter decomposition at varying decomposition stages in a transitional forest between temperate and tropical regions over a longer decomposition period than the average of earlier studies (Dale et al. 2015; Moore et al. 2017; See et al. 2019; Waring 2012). This expands our current knowledge of litter decomposition beyond these relatively well-studied tropical and temperate forests (García-Palacios et al. 2016; Wieder et al. 2009; Zanne et al. 2015). We have underscored that microclimate and in particular, temperature exerts a relatively smaller influence than initial litter traits at early but larger at later stages of litter decomposition. We have further provided observational evidence that the decomposition process in our targeted forest displays some unique characteristics. Specifically, often overlooked litter traits such as Mg, as key potential drivers of temporal dynamics of litter decomposition found here, should be more explicitly incorporated into a coherent framework with which to better understand and predict litter-driven nutrient and carbon cycling in current biogeochemical models (García-Palacios et al. 2016; Jiang et al. 2021; Makkonen et al. 2012).
Data availability
The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
Code availability
The code analyzed during the current study are available from the corresponding author upon reasonable request.
References
Adair EC, Hobbie SE, Hobbie RK (2010) Single-pool exponential decomposition models: potential pitfalls in their use in ecological studies. Ecology 91:1225–1236
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Averill C, Waring B (2018) Nitrogen limitation of decomposition and decay: How can it occur? Global Change Biol 24:1417–1427. https://doi.org/10.1111/gcb.13980
Bartoń K (2020). MuMIn: Multi-Model Inference. R package version 1.43.17. Available via https://CRAN.R-project.org/package=MuMIn
Berg B (2014) Decomposition patterns for foliar litter–A theory for influencing factors. Soil Biol Biochem 78:222–232
Berg B, Kjønaas OJ, Johansson MB, Erhagen B, Åkerblom S (2015) Late stage pine litter decomposition: Relationship to litter N, Mn, and acid unhydrolyzable residue (AUR) concentrations and climatic factors. Forest Ecol Manag 358:41–47
Berg B, McClaugherty C (2020) Plant litter-decomposition, humus formation and carbon sequestration. Springer Nature, Switzerland
Berg B, Sun T, Johansson M-B, Sanborn P, Ni X, Åkerblom S, Lönn M (2021) Magnesium dynamics in decomposing foliar litter-A synthesis. Geoderma 382:114756. https://doi.org/10.1016/j.geoderma.2020.114756
Bradford MA, Berg B, Maynard DS, Wieder WR, Wood SA (2016) Understanding the dominant controls on litter decomposition. J Ecol 104:229–238. https://doi.org/10.1111/1365-2745.12507
Bradford MA, Veen GF, Bonis A, Bradford EM, Classen AT, Cornelissen JHC, Crowther TW, De Long JR, Freschet GT, Kardol P, Manrubia-Freixa M, Maynard DS, Newman GS, Logtestijn RSP, Viketoft M, Wardle DA, Wieder WR, Wood SA, van der Putten WH (2017) A test of the hierarchical model of litter decomposition. Nature Ecology & Evolution 1:1836–1845. https://doi.org/10.1038/s41559-017-0367-4
Bradford MA, Warren Ii RJ, Baldrian P, Crowther TW, Maynard DS, Oldfield EE, Wieder WR, Wood SA, King JR (2014) Climate fails to predict wood decomposition at regional scales. Nat Clim Change 4:625–630. https://doi.org/10.1038/nclimate2251
Bütikofer L, Anderson K, Bebber DP, Bennie JJ, Early RI, Maclean IMD (2020) The problem of scale in predicting biological responses to climate. Global Change Biol 26:6657–6666. https://doi.org/10.1111/gcb.15358
Canessa R, van den Brink L, Saldaña A, Rios RS, Hättenschwiler S, Mueller CW, Prater I, Tielbörger K, Bader MY (2021) Relative effects of climate and litter traits on decomposition change with time, climate and trait variability. J Ecol 109:447–458. https://doi.org/10.1111/1365-2745.13516
Chomel M, Guittonny-Larchevêque M, Fernandez C, Gallet C, DesRochers A, Paré D, Jackson BG, Baldy V (2016) Plant secondary metabolites: a key driver of litter decomposition and soil nutrient cycling. J Ecol 104:1527–1541. https://doi.org/10.1111/1365-2745.12644
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Díaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. https://doi.org/10.1111/j.1461-0248.2008.01219.x
Cornwell WK, Weedon JT (2014) Decomposition trajectories of diverse litter types: a model selection analysis. Methods Ecol Evol 5:173–182
Cotrufo MF, Soong JL, Horton AJ, Campbell EE, Haddix ML, Wall DH, Parton WJ (2015) Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat Geosci 8:776–779. https://doi.org/10.1038/ngeo2520
Coûteaux M-M, Bottner P, Berg B (1995) Litter decomposition, climate and liter quality. Trends Ecol Evol 10:63–66. https://doi.org/10.1016/S0169-5347(00)88978-8
Currie W, Harmon M, Burke I, Hart S, Parton W, Silver W (2010) Cross-biome transplants of plant litter show decomposition models extend to a broader climatic range but lose predictability at the decadal time scale. Global Change Biol 16:1744–1761
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Global Change Biol 15:1339–1355
Cusack DF, Karpman J, Ashdown D, Cao Q, Ciochina M, Halterman S, Lydon S, Neupane A (2016) Global change effects on humid tropical forests: Evidence for biogeochemical and biodiversity shifts at an ecosystem scale. Rev Geophys 54:523–610
Dale S, Turner B, Bardgett R (2015) Isolating the effects of precipitation, soil conditions, and litter quality on leaf litter decomposition in lowland tropical forests. Plant Soil 394:225–238. https://doi.org/10.1007/s11104-015-2511-8
Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G, Marquéz JRG, Gruber B, Lafourcade B, Leitão PJ, Münkemüller T, McClean C, Osborne PE, Reineking B, Schröder B, Skidmore AK, Zurell D, Lautenbach S (2013) Collinearity: a review of methods to deal with it and a simulation study evaluating their performance. Ecography 36:27–46. https://doi.org/10.1111/j.1600-0587.2012.07348.x
Fox J, Weisberg S (2019) An R Companion to Applied Regression, Third Edition. Sage: Thousand Oaks. URL: https://socialsciences.mcmaster.ca/jfox/Books/Companion/
Fujii S, Cornelissen JH, Berg MP, Mori AS (2018) Tree leaf and root traits mediate soil faunal contribution to litter decomposition across an elevational gradient. Funct Ecol 32:840–852
García-Palacios P, Gross N, Gaitán J, Maestre FT (2018) Climate mediates the biodiversity–ecosystem stability relationship globally. Proc Natl Acad Sci 115:8400–8405. https://doi.org/10.1073/pnas.1800425115
García-Palacios P, Maestre FT, Kattge J, Wall DH (2013) Climate and litter quality differently modulate the effects of soil fauna on litter decomposition across biomes. Ecol Lett 16:1045–1053. https://doi.org/10.1111/ele.12137
García-Palacios P, Shaw EA, Wall DH, Hättenschwiler S (2016) Temporal dynamics of biotic and abiotic drivers of litter decomposition. Ecol Lett 19:554–563
Ge J, Berg B, Xie Z (2019) Climatic seasonality is linked to the occurrence of the mixed evergreen and deciduous broad-leaved forests in China. Ecosphere 10:e02862. https://doi.org/10.1002/ecs2.2862
Ge J, Wang Y, Xu W, Xie Z (2017) Latitudinal patterns and climatic drivers of leaf litter multiple nutrients in Chinese broad-leaved tree species: Does leaf habit matter? Ecosystems 20:1124–1136. https://doi.org/10.1007/s10021-016-0098-4
Ge J, Xie Z (2017) Geographical and climatic gradients of evergreen versus deciduous broadleaved tree species in subtropical China: Implications for the definition of the mixed forest. Ecol Evol 7:3636–3644. https://doi.org/10.1002/ece3.2967
Ge J, Xiong G, Zhao C, Shen G, Xie Z (2013) Short-term dynamic shifts in woody plants in a montane mixed evergreen and deciduous broadleaved forest in central China. Forest Ecol Manag 310:740–746. https://doi.org/10.1016/j.foreco.2013.09.019
Ge J, Xu W, Xiong G, Zhao C, Li J, Liu Q, Tang Z, Xie Z (2022) Depth-dependent controls over soil organic carbon stock across Chinese shrublands. Ecosystems. https://doi.org/10.1007/s10021-022-00757-6
Gilliam FS (2016) Forest ecosystems of temperate climatic regions: from ancient use to climate change. New Phytol 212:871–887. https://doi.org/10.1111/nph.14255
Goebel M, Hobbie SE, Bulaj B, Zadworny M, Archibald DD, Oleksyn J, Reich PB, Eissenstat DM (2011) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monogr 81:89–102
Gross N, Bagousse-Pinguet YL, Liancourt P, Berdugo M, Gotelli NJ, Maestre FT (2017) Functional trait diversity maximizes ecosystem multifunctionality. Nature Ecology & Evolution 1:0132. https://doi.org/10.1038/s41559-017-0132
Hättenschwiler S, Coq S, Barantal S, Handa IT (2011) Leaf traits and decomposition in tropical rainforests: revisiting some commonly held views and towards a new hypothesis. New Phytol 189:950–965. https://doi.org/10.1111/j.1469-8137.2010.03483.x
Hättenschwiler S, Jørgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763. https://doi.org/10.1111/j.1365-2745.2010.01671.x
Hobbie SE (2015) Plant species effects on nutrient cycling: revisiting litter feedbacks. Trends Ecol Evol 30:357–363. https://doi.org/10.1016/j.tree.2015.03.015
Hoeber S, Fransson P, Weih M, Manzoni S (2020) Leaf litter quality coupled to Salix variety drives litter decomposition more than stand diversity or climate. Plant Soil 453:313–328. https://doi.org/10.1007/s11104-020-04606-0
Jiang L, Wang H, Li S, Fu X, Dai X, Yan H, Kou L (2021) Mycorrhizal and environmental controls over root trait–decomposition linkage of woody trees. New Phytol 229:284–295. https://doi.org/10.1111/nph.16844
Keiser AD, Bradford MA (2017) Climate masks decomposer influence in a cross-site litter decomposition study. Soil Biol Biochem 107:180–187. https://doi.org/10.1016/j.soilbio.2016.12.022
Keller AB, Phillips RP (2019) Leaf litter decay rates differ between mycorrhizal groups in temperate, but not tropical, forests. New Phytol 222:556–564
Kou L, Jiang L, Hättenschwiler S, Zhang M, Niu S, Fu X, Dai X, Yan H, Li S, Wang H (2020) Diversity-Decomposition Relationships in Forests Worldwide Elife 9:e55813. https://doi.org/10.7554/eLife.55813
Li Q, Zhang M, Geng Q, Jin C, Zhu J, Ruan H, Xu X (2020) The roles of initial litter traits in regulating litter decomposition: a “common plot” experiment in a subtropical evergreen broadleaf forest. Plant Soil 452:207–216. https://doi.org/10.1007/s11104-020-04563-8
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041. https://doi.org/10.1111/j.1461-0248.2012.01826.x
Manzoni S, Piñeiro G, Jackson RB, Jobbágy EG, Kim JH, Porporato A (2012) Analytical models of soil and litter decomposition: solutions for mass loss and time-dependent decay rates. Soil Biol Biochem 50:66–76
Marklein AR, Winbourne JB, Enders SK, Gonzalez DJX, van Huysen TL, Izquierdo JE, Light DR, Liptzin D, Miller KE, Morford SL, Norton RA, Houlton BZ (2016) Mineralization ratios of nitrogen and phosphorus from decomposing litter in temperate versus tropical forests. Global Ecol Biogeogr 25:335–346. https://doi.org/10.1111/geb.12414
Moore TR, Trofymow JA, Prescott CE, Titus BD (2017) Can short-term litter-bag measurements predict long-term decomposition in northern forests? Plant Soil 416:419–426. https://doi.org/10.1007/s11104-017-3228-7
Oberle B, Lee MR, Myers JA, Osazuwa-Peters OL, Spasojevic MJ, Walton ML, Young DF, Zanne AE (2020) Accurate forest projections require long-term wood decay experiments because plant trait effects change through time. Global Change Biol 26:864–875. https://doi.org/10.1111/gcb.14873
Parsons SA, Congdon RA, Lawler IR (2014) Determinants of the pathways of litter chemical decomposition in a tropical region. New Phytol 203:873–882. https://doi.org/10.1111/nph.12852
Pérez-Harguindeguy N, Díaz S, Garnier E, Lavorel S, Poorter H, Jaureguiberry P, Bret-Harte MS, Cornwell WK, Craine JM, Gurvich DE, Urcelay C, Veneklaas EJ, Reich PB, Poorter L, Wright IJ, Ray P, Enrico L, Pausas JG, de Vos AC, Buchmann N, Funes G, Quétier F, Hodgson JG, Thompson K, Morgan HD, ter Steege H, van der Heijden MGA, Sack L, Blonder B, Poschlod P, Vaieretti MV, Conti G, Staver AC, Aquino S, Cornelissen JHC (2013) New handbook for standardised measurement of plant functional traits worldwide. Aust J Bot 61:167–234. https://doi.org/10.1071/BT12225
Powers JS, Salute S (2011) Macro-and micronutrient effects on decomposition of leaf litter from two tropical tree species: inferences from a short-term laboratory incubation. Plant Soil 346:245–257
R Core Team (2013) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available via http://www.R-project.org/
Rowley MC, Grand S, Verrecchia ÉP (2018) Calcium-mediated stabilisation of soil organic carbon. Biogeochemistry 137:27–49
Salinas N, Malhi Y, Meir P, Silman M, Roman Cuesta R, Huaman J, Salinas D, Huaman V, Gibaja A, Mamani M (2011) The sensitivity of tropical leaf litter decomposition to temperature: results from a large-scale leaf translocation experiment along an elevation gradient in Peruvian forests. New Phytol 189:967–977
Santiago LS (2007) Extending the leaf economics spectrum to decomposition: evidence from a tropical forest. Ecology 88:1126–1131
Santonja M, Milcu A, Fromin N, Rancon A, Shihan A, Fernandez C, Baldy V, Hättenschwiler S (2019) Temporal shifts in plant diversity effects on carbon and nitrogen dynamics during litter decomposition in a Mediterranean shrubland exposed to reduced precipitation. Ecosystems 22:939–954. https://doi.org/10.1007/s10021-018-0315-4
See CR, Luke McCormack M, Hobbie SE, Flores-Moreno H, Silver WL, Kennedy PG (2019) Global patterns in fine root decomposition: climate, chemistry, mycorrhizal association and woodiness. Ecol Lett 22:946–953. https://doi.org/10.1111/ele.13248
Shen G, Chen D, Wu Y, Liu L, Liu C (2019) Spatial patterns and estimates of global forest litterfall. Ecosphere 10:e02587. https://doi.org/10.1002/ecs2.2587
Sun T, Hobbie SE, Berg B, Zhang H, Wang Q, Wang Z, Hättenschwiler S (2018) Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc Natl Acad Sci 115:10392–10397. https://doi.org/10.1073/pnas.1716595115
Sundqvist MK, Sanders NJ, Wardle DA (2013) Community and ecosystem responses to evational gradients: processes, mechanisms, and insights for global change. Annu Rev Ecol Evol Syst 44:261–280
Suseela V, Tharayil N (2018) Decoupling the direct and indirect effects of climate on plant litter decomposition: Accounting for stress-induced modifications in plant chemistry. Global Change Biol 24:1428–1451. https://doi.org/10.1111/gcb.13923
Suseela V, Tharayil N, Xing B, Dukes JS (2013) Labile compounds in plant litter reduce the sensitivity of decomposition to warming and altered precipitation. New Phytol 200:122–133
The Editorial Board of Flora of China (2004) Flora of China. Science Press, Beijing
Waring BG (2012) A meta-analysis of climatic and chemical controls on leaf litter decay rates in tropical forests. Ecosystems 15:999–1009
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90:3333–3341
Wiesmeier M, Urbanski L, Hobley E, Lang B, von Lützow M, Marin-Spiotta E, van Wesemael B, Rabot E, Ließ M, Garcia-Franco N (2019) Soil organic carbon storage as a key function of soils-A review of drivers and indicators at various scales. Geoderma 333:149–162
Wu Z (1980) Vegetation of China. Science Press, Beijing
Xie Z, Shen G (2021) The outstanding universal value and conservation of Hubei Shennongjia: The World Natural Heritage Site. Springer Nature.
Zanne AE, Oberle B, Dunham KM, Milo AM, Walton ML, Young DF (2015) A deteriorating state of affairs: How endogenous and exogenous factors determine plant decay rates. J Ecol 103:1421–1431
Zhou S, Butenschoen O, Barantal S, Handa IT, Makkonen M, Vos V, Aerts R, Berg MP, McKie B, Van Ruijven J, Hättenschwiler S, Scheu S (2020) Decomposition of leaf litter mixtures across biomes: The role of litter identity, diversity and soil fauna. J Ecol 108:2283–2297. https://doi.org/10.1111/1365-2745.13452
Zuur A, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Science & Business Media.
Acknowledgements
We wish to acknowledge all participants for their contributions to the fieldwork and laboratory analysis in this study. We would like to thank Professor Björn Berg at the University of Helsinki for his insightful comments on this manuscript. We also would like to thank Dr. Morgan Furze at Yale University and Dr. Shannon Elliot at Michigan State University for their assistance with the English language and grammatical editing. The National Key Research and Development Program of China (Grant No. 2019YFD1100403), The National Natural Science Foundation of China (Grant No.31600360), and The State Key Laboratory of Vegetation and Environmental Change of China (Grant No. Y7206F1016) financed this study.
Funding
Both the National Key Research and Development Program of China (Grant No. 2019YFD1100403) and the National Natural Science Foundation of China (Grant No.31600360) financed this study.
Author information
Authors and Affiliations
Contributions
J.G. and Z.X. conceived of and designed study, performed research, and wrote the manuscript. J.G., B.M., B.B., W.X., and C.Z analyzed data and revised this manuscript. All the authors approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Responsible Editor: Alfonso Escudero.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ge, J., Ma, B., Xu, W. et al. Temporal shifts in the relative importance of climate and leaf litter traits in driving litter decomposition dynamics in a Chinese transitional mixed forest. Plant Soil 477, 679–692 (2022). https://doi.org/10.1007/s11104-022-05425-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05425-1