Abstract
Aims
Warming has the potential to alter plant litter mass loss and nutrient release during decomposition. However, a great deal of uncertainty remains concerning how other factors such as litter species or substrate quality might modify the effects of increased temperature on decomposition. Meanwhile, the temperature sensitivity of plant litter decay in tropical and subtropical forest ecosystems remains poorly resolved.
Methods
This study was designed to assess the effects of experimental warming on litter decomposition and nutrient release of two contrasting tree species (Schima superba and Machilus breviflora) by translocating model forest ecosystems from the high-elevation sites to the lower-elevation sites in subtropical China. Translocating model mountain evergreen broad-leaved forest (MEBF) to the altitude of 300 m and 30 m increased the average monthly soil temperature at 5 cm depth by 0.88 and 1.84 °C, respectively during the experimental period. Translocating model coniferous and broad-leaved mixed forest (CBMF) to the altitude of 30 m increased the average monthly soil temperature at 5 cm depth by 0.85 °C.
Results
We found that experimental warming accelerated litter decomposition in both model forest types, and the promoting efficiency was greater when the temperature increased. The litter with high quality (Schima superba) had stronger response to warming than low quality litter (Machilus breviflora). Warming accelerated Na, K, Mg, P, N and Ca release from Schima superba litter, but only simulated Ca release from Machilus breviflora litter. Overall, litter decomposition was controlled by the order: soil temperature > litter quality > soil moisture > litter incubation forest type under experimental warming in the subtropical China.
Conclusion
We conclude that leaf litter decomposition was facilitated by experimental warming in subtropical China. Litter species might modify the effects of increased temperature on litter decomposition; however, forest type has no effect on litter decomposition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Over the past 100 years, the global average temperature has increased by 0.74 ± 0.18 °C (IPCC 2014). Previous studies showed that global warming had strong effects on terrestrial ecosystem process. Global warming can directly and indirectly affect litter decomposition (Berg and McClaugherty 2008) via the changes of soil temperature, humidity and litter quality. Meanwhile, litter decomposition acts as an important process of the ecosystem carbon (C) and nutrient cycle could strongly feedback to global warming (Shaver et al. 2000; Rustad et al. 2001). Therefore, understanding the response mechanisms of litter decomposition to warming under the background of global warming can provide basic data for studying soil C and nutrient cycle in ecosystems, and provide reference for ecosystem management and species selection.
Litter decomposition rate was jointly affected by abiotic factors (e.g., temperature, humidity, and litter quality) (Silver and Miya 2001; Hobbie 2008; Hättenschwiler and Jørgensen 2010) and biotic factors (e.g., soil detritivores and microbes) (Hobbie et al. 2006). Many studies showed that elevated soil temperature would accelerate litter decomposition rates (Dang et al. 2009; Aerts et al. 2012; Zhang et al. 2017). However, other studies reveal opposite results. Christiansen et al. (2017) found that enhanced summer warming significantly restricted litter mass loss by 32% at the dry site and 17% at the wet site. Warming may stimulate litter decomposition only as long as the associated increases in evapotranspiration do not lead to moisture limitation of microbial activity (Bardgett et al. 2008; Bokhorst et al. 2010). In addition, Xu et al. (2012) demonstrated that litter decomposition rate was different between divergent species under warming as litter quality often varied. Previous other studies also reported that the magnitude and direction of responses of litter decomposition to warming varied with species (Wang et al. 2000; Schindlbacher et al. 2009; Hagedorn et al. 2010). High quality litters are often characterized by higher N concentrations and lower C:N and lignin:N ratios, and can decompose faster compared to low quality litters (Sanchez 2001). Further, different vegetation types may also generate different microclimate effects on litter decomposition (Cornelissen et al. 2007). Therefore, investigating the direct effects of warming on litter decomposition, together with its interaction with litter quality and vegetation type, is essential to understanding how litter mass and nutrient loss will be altered under global warming.
Tropical and subtropical forests can provide crucial ecosystem services to natural systems and human beings (i.e., carbon sequestration, biodiversity conservation, climatic regulation). However, the effects of warming on ecosystem process in these areas have not been well studied. Cavaleri et al. (2015) pointed out that the tropics are indeed “a high priority region” for future warming research. Up to date, no field experiment has been conducted in tropical or subtropical forests of China, which are experiencing a significant increase in surface temperature (Zhou et al. 2011). In this study, we conducted a translocation experiment from the high-elevation sites to the low-elevation sites, to study the effects of altitudinal transplant-induced soil temperature increase on the leaf-litter decomposition as well as nutrient loss from the decomposing litter. Two vascular plant litters of different qualities were subjected to decay in two different model forest types. We measured litter mass and nutrient loss to test the following hypotheses: (1) Warming would increase litter decomposition, and hence lead to more nutrient release; (2) The effects of warming on leaf-litter decomposition would differ between different litter species; and (3) Forest type difference would also affect responses of litter decomposition to warming as substrate soil is different between different forest types.
Materials and methods
Study site
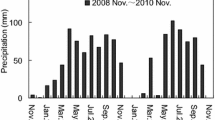
This study was conducted at the Dinghushan Biosphere Reserve (DBR), with an elevation ranging from 10 to 1000 m (a.s.l.), located in the central Guangdong Province in southern China (112°10′E, 23°10′N). The climate is typical subtropical monsoon. Mean annual temperature is ca. 21 °C, ranging from the mean coldest in January (12.6 °C) to the hottest in July (28.0 °C). Mean annual precipitation is ca. 1700 mm, and nearly 80% of the rain falls in the wet season (April–September) and 20% in the dry season (October–March). There are three major forest types at DBR: the monsoon evergreen broad-leaved forest, the coniferous and broad-leaved mixed forest (CBMF) and the mountain evergreen broad-leaved forest (MEBF) located at 30 m, 300 m and 600 m (a.s.l.), respectively. Soils are oxisols (lateritic red earths) formed from sandstone approximately 30 to 70 cm in depth.
Translocation experiment design
We initiated warming treatments by translocating model forest ecosystems from the high-elevation sites to the lower-elevation sites so that temperature is the main altered environmental factor. Model MEBF was translocated from an altitude of 600 m (as the control) to the altitudes of 300 m and 30 m, respectively. Temperature would be increased theoretically by 1.5 °C and 3.0 °C, respectively. Model CBMF was translocated from an altitude of 300 m (as the control) to an altitude of 30 m. Temperature would increase theoretically by 1.5 °C (Fig. 1).
Three 3 × 3 m growth chambers were located in an open area at the altitude of 600 m site. Six 3 × 3 m growth chambers were located in the open areas at the altitude of 300 m and 30 m sites, respectively. The 0.8-m deep below-ground section in each growth chamber was surrounded by concrete brick wall bonding with ceramic tile to prevent the lateral or vertical movement of water or element from the surrounding soils. There was one hole at the top and the bottom of the wall. The holes (inner diameter 2 cm), which were capped by a 2 mm net to prevent losses other than those of leachates, were connected to as stainless steel water collection drum (Li et al. 2016; Fang et al. 2016).
In April 2012, soil and individual seedlings that were 1-year-old were collected from a MEBF near the control site (at the altitude of 600 m). Three different layers of soils (0–20, 20–40 and 40–70 cm) were homogenized separately. Seedlings of six species were stored in shade in containers with soil from the collection sites. In May 2012, three different layers of soils were transferred into the growth chambers correspondingly at the three sites (the altitudes of 600, 300 and 30 m, respectively). Meanwhile, soil and individual seedlings that were 1-year-old were collected from a CBMF near the control site (at the altitude of 300 m). Three different layers of soils were homogenized separately. In May 2012, three different layers of soils were transferred into the growth chambers correspondingly at the two sites (the altitudes of 300 and 30 m, respectively). The seedlings were transplanted into each growth chambers in all sites in a randomized block design (n = 6 replicates per species). Model forest ecosystems of MEBF and CBMF were then established in each site.
The six species in model MEBF were specifically selected due to their common occurrence and distribution range (exist in almost all regions along the altitudinal gradient from 600 m to 30 m altitudes). They included Schima superba, Syzygium rehderianum, Machilus breviflora, Itea chinensis, Myrsine seguinii, and Ardisia lindleyana. Accordingly, the six species of seedlings in model CBMF were specifically selected for this study due to their common occurrence and distribution range in the CBMF. They included Schima superba, Syzygium rehderianum, Machilus breviflora, Pinus massoniana, Castanopsis hystrix and Ardisia lindleyana (Li et al. 2016; Fang et al. 2016). The trees grew very well and fast. One tree per species was harvested at the end of 2014 and 2015, to avoid crowding in the chambers.
Microclimate monitoring
Rainfall was recorded using Campbell Scientific (TB4MM, Logan, USA) in each elevation gradient. Soil profile temperatures (at 5 cm) were recorded in each chamber using Campbell 109 constantan-copper thermocouples. Volumetric water content was measured from the soil surface to a depth of 5 cm using Campbell CS616 water content reflectometer probes. Data were recorded every hour using Campbell Scientific (Logan, UT, USA) CR1000 data loggers since May 2013 (Fang et al. 2016).
Leaf-litter decomposition experiment
Naturally senesced leaf-litters of Schima superba and Machilus breviflora were collected in litter fall traps in a natural forest of CBMF at the site of 300 m altitude. The initial quality of these two litters was shown in Table 1. A total of 15 g (ratios of oven dried mass were determined by the proportion of air-dried mass of the leaf-litter after drying for 48 h at 70 °C) of leaf litter of Schima superba or Machilus breviflora was placed in 15 × 20 cm litterbags. The mesh bags had a 1 mm mesh nylon top and a 0.2 mm mesh Dacron cloth bottom to reduce fragmented litter losses, and to allow microorganisms and small soil animal access. For each leaf-litter of Schima superba or Machilus breviflora, there were totally 75 litterbags (n = 5 replicates per chambers). Litterbags were placed on the soil surface in each growth chamber at all three sites. The chemical properties of the substrate soil (0–10 cm) were shown in Table 2. Decomposition was followed for about 450 days from July 2014 to October 2015. The total rainfall amounts during the whole experimental period were 3464, 3929 and 3734 mm at the altitude of 600, 300 and 30 m, respectively. One litterbag of Schima superba and one litterbag of Machilus breviflora were retrieved in each chamber every three months. At each removal, the litter samples were sorted to remove foreign material, weighed for mass loss calculation after drying for 48 h at 70 °C, and then finely ground for element concentration analysis. Mass loss was calculated as the difference between the initial dry mass and the remaining dry mass of leaves at each sampling date.
Leaf-litter chemical composition
Nutrient loss via the leaf-litter composition, nutrient concentration in the initial leaf litter and the residual litter were determined. Carbon concentration was determined following the Walkley-Black’s wet digestion method (Nelson and Sommers 1982). Phosphorus concentration was measured photometrically after samples were digested with H2SO4-H2O2. N concentration was measured using the Kjeldahl method (Bremner and Mulvaney 1982). The concentrations of K, Ca and Mg were measured by inductively coupled plasma atomic emission spectroscopy (ICP-AES; Optima-2000 DV, PerkinElmer, USA) after acid digestion. Acid-insoluble C fraction (AIF) was determined using the method of Xiong et al. (2013). AIF is considered to be comprised of lignin and other recalcitrant structures like condensed tannins, cutin and suberin, and was difficult for microbes to decompose (Ryan et al. 1990).
The chemical properties in the substrate soil at the beginning of experiment were analyzed. Soil pH was determined with a glass electrode in the supernatant after shaking for 2 h and sedimentation in a beaker for 24 h in 0.1 M KCl. The soil to extractant ratio was 1:2.5. Total P concentration was analyzed colorimetrically (Anderson and Ingram 1989), organic matter was determined following Walkley Black’s wet digestion method (Nelson and Sommers 1982), and total N was measured using the micro Kjeldahl method (Jackson 1964). After soil was extracted using a 1 M KCl solution, the extracted NO3-N and NH4-N were determined by an ultraviolet spectrophotometer. Soil available P was extracted with a solution containing 25 mM HCl and 30 mM NH4F (the soil to extractant ratio was 1:7) and then measured using an ultraviolet spectrophotometer.
Data analysis
Data were transformed to meet the assumptions of normality and homogeneity of variances when necessary. A repeated-measures general linear model was used to evaluate the effects of the downward translocation (warming treatment) on soil temperature and moisture, litter mass remaining, element remaining in the leaf litter residue in both model forest types. It was also used to examine the effects of warming treatment, litter species, sampling time, incubation forest and their interactions on litter mass loss and element (C, N, P, K, Ca and Mg) release from decomposition of leaf litter. One-way ANOVA with the Fisher’s LSD test was used to identify significant differences between litter species and chambers at different altitudes in the initial chemical properties. Correlation of decomposition constant K value with annual soil mean temperature and soil moisture was tested by Pearson correlation coefficient. All statistical analyses were performed by the SAS software (Statistical Analysis System, version 9.2, SAS Institute, Inc.) and statistical significance was determined at P < 0.05.
Results
Soil temperature and moisture
During the litter decomposition experimental period (about 15 months), the average monthly soil temperatures at 5 cm depth in the chambers of MEBF were 20.84, 21.72 and 22.68 °C at the altitudes of 600, 300 and 30 m, respectively. Translocating MEBF to the altitudes of 300 and 30 m increased the average monthly soil temperature by 0.88 and 1.84 °C, respectively. During the same period, soil moisture was also significantly affected by translocation treatments (Fig. 2; P < 0.05). The average monthly soil moisture was 17.61%, 19.77% and 16.11% in the chambers at the altitudes of 600, 300 and 30 m, respectively.
The average monthly soil temperatures at 5 cm depth in the chambers of CBMF were 21.80 and 22.65 °C at the altitudes of 300 and 30 m, respectively. Translocating CBMF to the altitude of 30 m increased the average monthly soil temperature at 5 cm depth by 0.85 °C during the litter decomposition experimental period. During the same period, soil moisture was also significantly affected by translocation treatments (Fig. 3; P < 0.05). The average monthly soil moistures were 22.41% and 16.36% in the chambers at the altitudes of 300 and 30 m, respectively.
Effects of warming on leaf-litter mass loss
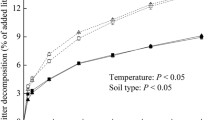
Mass loss was significantly affected by downward translocation for both litter species in MEBF (Table 3, Fig. 4). At the end of the experiment, the average litter remaining for S. superba litter was 42.2%, 41.26% and 32.86% in the chambers of MEBF at the altitudes of 600, 300 and 30 m, respectively. As for M. breviflora litter, the average litter remaining was 60.8%, 57.55% and 54.56% in the chambers of MEBF at the altitude of 600, 300 and 30 m, respectively. Greater litter decomposition rate was found in the warmed chambers than the control chambers. S. superba litter decomposed faster than M. breviflora litter (Fig. 4). In the chambers of CBMF, the average mass remaining for S. superba litter at the end of experiment was 37.49% and 31.69% at the altitudes of 300 and 30 m, respectively. However, mass remaining of M. breviflora litter was 54.04% and 51.33% in the chambers of CBMF at the altitude of 300 and 30 m, respectively. There was no difference between the mass remaining of M. breviflora litter in the chambers at the altitude of 300 and 30 m. Forest type did not affect mass loss during litter decomposition process for both species (Table 3) although higher soil quality was shown in the CBMF than in the MEBF (Table 2).
Litter mass remaining (%) in the decomposition litter under control and warming treatments in the model mountain evergreen broad-leaved forests (a and b) and model coniferous and broad-leaved forests (c and d). p < 0.05 represents the significance of warming treatments on litter decomposition for each species. S. superba, Schima superba; M. breviflora, Machilus breviflora
The decay rate constant (k) of S. superba litter decomposition was 0.611, 0.710 and 0.865 for the chambers of MEBF at the altitude of 600, 300 and 30 m, respectively, with the significantly higher values for the warmed chambers than for the control chambers. The k of M. breviflora litter decomposition was 0.355, 0.377 and 0.435 for the chambers of MEBF at the altitude of 600, 300 and 30 m, respectively. Downward translocation also induced higher k of M. breviflora litter. However, in the chambers of CBMF, downward translocation only increased k of S. superba litter. No obvious difference existed between the k of M. breviflora litter in the chambers at the altitudes of 300 and 30 m.
Effects of warming on nutrient loss during leaf-litter decomposition
Warming, litter type, incubation time and forest type all affected nutrient release (Table 3). Nutrient loss was faster in the decomposing litter of S. superba than M. breviflora in the chambers of both forest types. The K, Na, C and Mg were released faster in the decomposing litter than the other elements. Especially for K and Na, more than half of the original weight was released in the three-month litter incubation (Figs. S1, S2, S3 and S4). In the chambers of MEBF, the losses of C, N, P, K, Ca and Mg in the decomposing litter of S.superba were all increased by the warming treatments. However, the warming treatments only increased the losses of P in the decomposing litter of M. breviflora. Translocating CBMF from the altitude of 300 m to 30 m only induced greater losses of C, N, P, K, Ca and Mg in the decomposing litter of S. superba. Warming treatment did not affect nutrient loss in the decomposing litter of M. breviflora in the CBMF. Overall, the warming-induced effects on nutrient loss were substantially larger than the litter quality and forest types (Table 3).
Effects of warming on the changes in acid-insoluble C fraction during leaf-litter decomposition
The concentrations of acid-insoluble C fraction (AIF) for both litter types increased at the beginning of experiment, and then decreased after the 9-month decomposing process. M. breviflora litter showed higher concentration of AIF than S. superba litter in the chambers of both forest types. In the chambers of MEBF, warming treatments increased the concentration of AIF in the decomposing litter of M. breviflora, however, only S. superba litter showed greater release amount of AIF induced by the warming treatment (p = 0.001, Fig. 5). In the chambers of CBMF, warming treatments did not affect the concentration and the total release amount of AIF in both litters (Fig. 6). Litter incubation forest type did not affect the release amount of recalcitrant fractions (Table 3).
Changes in concentration of acid-insoluble C fraction (AIF) and amounts of AIF remaining (as % of initial amount) in the leaf litter residue during decomposition process under the control and warming treatments in the model mountain evergreen broad-leaved forests. p < 0.05 represents the significance of warming treatments on litter decomposition for each species. S. superba, Schima superba; M. breviflora, Machilus breviflora
Changes in concentration of acid-insoluble C fraction (AIF) and amounts of AIF remaining (as % of initial amount) in the leaf litter residue during decomposition process under the control and warming treatments in the model coniferous and broad-leaved forests. p < 0.05 represents the significance of warming treatments on litter decomposition for each species. S. superba, Schima superba; M. breviflora, Machilus breviflora
Discussion
Our first hypothesis that warming would increase litter decomposition is partly supported. Except for M. breviflora litter in the CBMF, mass loss of litter at the lower-elevation site was significantly higher (p < 0.05) than those at higher-elevation site by the end of this study. Increases in litter mass loss in response to warming were also reported in arctic dwarf shrub, subalpine meadow and boreal forest (Rustad and Fernandez 1998; Verburg et al. 1999; Shaw and Harte 2001). Since nutrient release was tightly related to mass loss, the release of C, N, P, K, Ca and Mg in the S. superba litter was significantly higher (p < 0.05) at the lower-elevation site. The contents of P, Ca, Mg and K in the litter were also found to be positively related to litter decomposition rates in tropical ecosystems (Mo et al. 2006; Zhang et al. 2008; Waring 2012). This is also consistent with the result of Bothwell et al. (2014). They found that the percentage of leaf litter N remaining after six months declined linearly with increasing mean annual temperature from ∼ 88% of initial N at the coolest site to ∼74% at the warmest site. Consistent with the above results, the decay rate constant (k) of litter at the lower-elevation site was significantly higher (p < 0.05) than those at the higher-elevation site except for M. breviflora in CBMF. Translocating model forests from 600 m to 30 m led to higher litter decomposition rates than translocating model forests from 300 m to 30 m, suggesting the higher temperature increased, the faster litter decomposed. In our experiment, soil moisture was also decreased by the downward translocation (from the altitude 300 m to 30 m). However, litter decomposition rates of both litter species had not been inhibited in both model forest types. We did the regression analysis of decomposition constant k with soil temperature and moisture at top 0–5 cm soil depth (Fig. 7). We found that although both soil temperature and moisture were controlling factors on litter decomposition, the responses of litter decomposition to soil temperature was substantially larger than to soil moisture, which made soil moisture a minor factor in controlling leaf decomposition in our experiment.
Our second hypothesis that effects of warming on leaf-litter decomposition would differ between different litter species was supported. Temperature sensitivity of litter decay could strongly depend on the initial litter quality, such as nutrient concentration, carbon (C) fractions (labile and recalcitrant) and C:N (nitrogen) ratio (Loranger et al. 2002; Arunachalam and Singh 2004; Fierer et al. 2005; Wang et al. 2016). In our experiment, higher contents of N, P, K and Mg were detected in the S. superba litter than in the M. breviflora litter (Table 1), which led to greater decomposition rates in the S. superba litter in all chambers. Numerous studies have well documented that litter N is one of the most common factors limiting litter decomposition as it determines the growth and turnover of microbial biomass mineralizing the organic C (Taylor et al. 1989). Our study did confirm that litter decomposition rates were positively correlated with litter N and other nutrients. Further, our results showed that the release rate of acid-insoluble C (AIF) was lower than the rate of mass loss, so AIF concentration started to decrease from the 9 month. AIF would undoubtedly contain all recalcitrant C fraction but mainly lignin (Effland 1977). Higher contents of acid-insoluble C in the M. breviflora litter than in the S. superba litter further inhibited the litter decomposition of M. breviflora. Xu et al. (2012) studied warming effects on the early decomposition of red birch and dargon spruce, and they also found that there were marked differences between the two species in the decomposition rates and nutrient remaining percentages as the two species had quite different litter quality.
In addition to soil temperature, moisture and initial litter quality, forest type was also reported to be an important variable to influence litter quality (Smith and Bradford 2003; Wang et al. 2016). Cornelissen et al. (2007) reported that litter responses to warming were likely to be influenced by vegetation change. Against our third hypothesis, although significant difference of soil chemical properties was shown between MEBF and CBMF (Table 2) in our study, litter decomposition rates were not affected by the forest type difference. It suggests that soil properties’ difference (via forest type difference) is not a key control factor on litter decomposition in subtropical area. Recently, Gregorich et al. (2017) also demonstrated that litter decay was controlled by temperature other than soil properties in a large agricultural region (ten sites spanning a 3500-km transect) of Canada.
Temperature is expected to increase rapidly across all tropical land surfaces, resulting in temperature regimes that do not exist in the tropics today (Christensen et al. 2007). However, the potential responses of the tropical forest to this imminent climatic change are still uncertain (Cavaleri et al. 2015). There is urgent need for conducting warming experiments in tropical forests (Cavaleri et al. 2015). Using altitudinal gradients in the tropics offers great opportunities for observational studies of temperature effects on plant and ecosystem functioning (Malhi et al. 2010). In our study, we initiated the warming treatments by translocating model forest ecosystems from the high-elevation sites to the lower-elevation sites. Temperature was significantly increased in both model forest types. Although high temperature has existed in tropical area, simulated warming still stimulated litter decomposition in our study. Marked differences between the two litter species in the decomposition rates and nutrient release were detected. However, litter incubation forest type did not affect litter decomposition for both litter types. Overall, litter decomposition was controlled by soil temperature > litter quality >soil moisture > forest type in the subtropical model forest under the experimental warming in our study.
The S. superba and M. breviflora are common and widely distributed species in subtropical China. M. breviflora is also a heat-resistant plant. As litter mass loss and the warming response of M. breviflora were relatively small compared with that of S. superba litter, more C would be stored in the local ecosystems when we would plant more M. breviflora than S. superba under the future global warming. In that case, M. breviflora litter had slower decomposition and greater nutrient retention than S. superba, thus, the amount of C released to the atmosphere from litter could decline in this area. As experimental plots were set up only two years before the decomposition experiment it is likely that not the complete decomposer community has been established in our study. Hence, constant litter decomposition experiments should be conducted in order to achieve the improved understanding of warming effects on the litter decomposition in subtropical China.
Conclusions
We investigated how translocation-induced warming affected litter decomposition and nutrient release of two contrasting tree species in the subtropical model forests. Although relatively high temperature already exists in subtropical area, we found that increased temperature still increased litter decomposition rate and nutrient loss for both litter species. The stimulation effect for the litter decay with high quality was stronger than the litter with low quality. However, forest type difference did not affect litter decomposition although soil substrate quality in these two forests was different. In short, litter decomposition was controlled by the order: soil temperature > litter quality > soil moisture > litter incubation forest type under the experimental warming in the subtropical China.
References
Aerts R, Callaghan TV, Dorrepaal E, van Logtestijn RSP, Cornelissen JHC (2012) Seasonal climate manipulations have only minor effects on litter decomposition rates and N dynamics but strong effects on litter P dynamics of sub-arctic bog species. Oecologia 170(3):809–819
Anderson JM, Ingram J (1989) Tropical soil biology and fertility. CAB international, Wallingford
Arunachalam A, Singh ND (2004) Decomposition of Mesua ferrea litter in humid tropics of Arunachal Pradesh, India. J Trop For Sci 16:151–159
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2(8):805–814
Berg B, McClaugherty C (2008) Plant litter. Decomposition, humus formation, carbon sequestration. 2nd Ed, Springer
Bokhorst S, Bjerke JW, Melillo J, Callaghan TV, Phoenix GK (2010) Impacts of extreme winter warming events on litter decomposition in a sub-Arctic heathland. Soil Biol Biochem 42:611–617
Bothwell LD, Selmants PC, Giardina CP, Litton CM (2014) Leaf litter decomposition rates increase with rising mean annual temperature in Hawaiian tropical montane wet forests. PeerJ 2:e685. https://doi.org/10.7717/peerj.685
Bremner J, Mulvaney C (1982) Nitrogen-total methods of soil analysis, part 2, chemical and microbiological properties. (2ndEdn). American Society of Agronomy. Inc., Publisher, Madison
Cavaleri MA, Reed SC, Smith WK, Wood TE (2015) Urgent need for warming experiments in tropical forests. Glob Chang Biol 21:2111–2121
Christensen JH, Hewitson B, Busuioc A, Chen A, Gao X, Held R, Jones R, Kolli RK, Kwon W, Laprise R (2007) Regional climate projections. Climate change, 2007: the physical science basis. Contribution of Working group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, University Press, Cambridge, Chapter 11, pp 847–940
Christiansen CT, Haugwitz MS, Priemé A, Nielsen CS, Elberling B, Michelsen A, Grogan P, Blok D (2017) Enhanced summer warming reduces fungal decomposer diversity and litter mass loss more strongly in dry than in wet tundra. Glob Chang Biol 23:406–420
Cornelissen JHC, van Bodegom PM, Aerts R, Callaghan TV, van Logtestijn RSP, Alatalo J, Stuart Chapin F, Gerdol R, Gudmundsson J, Gwynn-Jones D, Hartley AE, Hik DS, Hofgaard A, Jónsdóttir IS, Karlsson S, Klein JA, Laundre J, Magnusson B, Michelsen A, Molau U, Onipchenko VG, Quested HM, Sandvik SM, Schmidt IK, Shaver GR, Solheim B, Soudzilovskaia NA, Stenström A, Tolvanen A, Totland Ø, Wada N, Welker JM, Zhao X (2007) Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10(7):619–627
Dang CK, Schindler M, Chauvet E, Gessner MO (2009) Temperature oscillation coupled with fungal community shifts can modulate warming effects on litter decomposition. Ecology 90:122–131
Effland MJ (1977) Modified procedure to determine acid insoluble lignin in wood and pulp. Tappi J 60:143–144
Fang X, Zhou GY, Li YL, Liu SZ, Chu GW, Xu ZH, Liu JX (2016) Warming effects on biomass and composition of microbial communities and enzyme activities within soil aggregates in subtropical forest. Biol Fertil Soils 52:353–365
Fierer N, Craine JM, McLauchlan K, Schimel JP (2005) Litter quality and the temperature sensitivity of decomposition. Ecology 86:320–326
Gregorich EG, Janzen H, Ellert BH, Helgason BL, Qian B, Zebarth BJ, Angers DA, Beyaert RP, Drury CF, Duguid SD (2017) Litter decay controlled by temperature, not soil properties, affecting future soil carbon. Glob Chang Biol 23:1725–1734
Hagedorn F, Martin M, Rixen C, Rusch S, Bebi P, Zürcher A, Siegwolf RT, Wipf S, Escape C, Roy J (2010) Short-term responses of ecosystem carbon fluxes to experimental soil warming at the Swiss alpine treeline. Biogeochemistry 97:7–19
Hättenschwiler S, Jørgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98(4):754–763
Hobbie SE (2008) Nitrogen effects on decomposition: a five-year experiment in eight temperate sites. Ecology 89:2633–2644
Hobbie SE, Reich PB, Oleksyn J, Ogdahl M, Zytkowiak R, Hale C, Karolewski P (2006) Tree species effects on decomposition and forest floor dynamics in a common garden. Ecology 87(9):2288–2297
IPCC (2014) Synthesis report. Contribution of three working groups to the fifth assessment report of the Intergovernmental Panel on Climate Change
Jackson ML (1964) Chemical composition of soils. Chemistry of the Soil 2:71–141
Li YY, Zhou GY, Huang WJ, Liu JX, Fang X (2016) Potential effects of warming on soil respiration and carbon sequestration in a subtropical forest. Plant Soil 409:247–257
Loranger G, Ponge JF, Imbert D, Lavelle P (2002) Leaf decomposition in two semi-evergreen tropical forests: influence of litter quality. Biol Fertil Soils 35:247–252
Malhi Y, Silman M, Salinas N, Bush M, Meir P, Saatchi S (2010) Introduction: elevation gradients in the tropics: laboratories for ecosystem ecology and global change research. Glob Chang Biol 16:3171–3175
Mo JM, Brown S, Xue J, Fang Y, Li Z (2006) Response of litter decomposition to simulated N deposition in disturbed, rehabilitated and mature forests in subtropical China. Plant Soil 282:135–151
Nelson DW, Sommers LE (1982) Carbon and organic matter. In: Page AL, Mille RH, Keeney DR (eds) Methods of soil analysis—Part 2: chemical and microbiological properties. American Society of Agronomy, Madison, pp 561–579
Rustad LE, Fernandez IJ (1998) Soil warming: consequences for foliar litter decay in a spruce-fir forest in Maine, USA. Soil Sci Soc Am J 62:1072–1080
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J, Gcte-News (2001) A meta-analysis of the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Ryan MG, Melillo JM, Ricca A (1990) A comparison of methods for determining proximate carbon fractions of Forest litter. Can J For Res 20:166–171
Sanchez FG (2001) Loblolly pine needle decomposition and nutrient dynamics as affected by irrigation, fertilization, and substrate quality. For Ecol Manag 152:85–96
Schindlbacher A, Zechmeister-Boltenstern S, Jandl R (2009) Carbon losses due to soil warming: do autotrophic and heterotrophic soil respiration respond equally? Glob Chang Biol 15:901–913
Shaver GR, Canadell J, Chapin FS, Gurevitch J, Harte J, Henry G, Ineson P, Jonasson S, Melillo J, Pitelka L, Rustad L (2000) Global warming and terrestrial ecosystems: a conceptual framework for analysis. Bioscience 50:871–882
Shaw MR, Harte J (2001) Control of litter decomposition in a subalpine meadow-sagebrush steppe ecotone under climate change. Ecol Appl 11:1206–1223
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Smith VC, Bradford MA (2003) Do non-additive effects on decomposition in litter-mix experiments result from differences in resource quality between litters? Oikos 102:235–242
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104
Verburg PSJ, Van Loon WKP, Lukewille A (1999) The CLIMEX soil-heating experiment: soil response after 2 years of treatment. Biol Fertil Soils 28:271–276
Wang Q, Li L, Bai Y, Xing X (2000) Effects of simulated climate change on the decomposition of mixed litter in three steppe communities. Acta Phytoecologica Sin 24:674–679
Wang J, You YM, Tang ZX, Sun XL, Sun OJ (2016) A comparison of decomposition dynamics among green tree leaves, partially decomposed tree leaf litter and their mixture in a warm temperate forest ecosystem. J Forestry Res 27:1037–1045
Waring BG (2012) A meta-analysis of climatic and chemical controls on leaf litter decay rates in tropical forests. Ecosystems 15:999–1009
Xiong YM, Fan PP, Fu SL, Zeng H, Guo DL (2013) Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 363:19–31
Xu ZF, Pu XZ, Yin HJ, Zhao CZ, Liu Q, Wu FZ (2012) Warming effects on the early decomposition of three litter types, eastern Tibetan plateau, China. Eur J Soil Sci 63:360–367
Zhang DQ, Hui DF, Luo YQ, Zhou GY (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93
Zhang XH, Sun XX, Mao R (2017) Effects of litter evenness, nitrogen enrichment and temperature on short-term litter decomposition in freshwater marshes of Northeast China. Wetlands 37:145–152
Zhou G, Wei X, Wu Y, Liu S, Huang Y, Yan J, Zhang D, Zhang Q, Liu J, Meng Z, Wang C, Chu G, Liu S, Tang X, Liu X (2011) Quantifying the hydrological responses to climate change in an intact forested small watershed in Southern China. Glob Chang Biol 17(12):3736–3746
Acknowledgments
This study was jointly funded by the National Natural Science Foundation of China (Grant Nos. 31570482, 31370530 and 41430529).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, J., Liu, S., Li, Y. et al. Warming effects on the decomposition of two litter species in model subtropical forests. Plant Soil 420, 277–287 (2017). https://doi.org/10.1007/s11104-017-3392-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-017-3392-9