Abstract
Alpine ecosystems are harsh environments where low temperatures are generally a limiting factor. Predicted global warming is thus expected to have a profound impact on alpine ecosystems in the future. This study was conducted to compare the effect of experimental warming on soils in two contrasting forest ecosystems (a dragon spruce plantation and a natural forest) using the open top chamber (OTC) method in the Eastern Tibetan Plateau of China. The OTC enhanced average daily mean soil temperatures by 0.61°C (plantation) and 0.55°C (natural forest), respectively, throughout the growing season. Conversely, soil volumetric moisture declined by 4.10% in the plantation and by 2.55% in the natural forest. Across all measuring dates, warming increased average soil CO2 efflux by 10.6% in the plantation and by 15.4% in the natural forest. However, elevated temperatures did not affect the respiration quotient in either forest. Two-stage sulfuric acid hydrolysis was used to quantify labile and recalcitrant C and N fractions in the two contrasting soils. Warming significantly reduced labile C and N fractions in both ecosystems but did not influence the total, recalcitrant and microbial biomass C and N pools. Labile C, N and microbial biomass C showed significant interactions in warming × forest type × season. Irrespective of warming treatments, all measured pools were significantly larger in the natural forest compared to the plantation. Taken together, our results indicate that the lowered soil labile C and N pools may be induced by the increased soil CO2 efflux. The responses of the natural forest soil were more sensitive to experimental warming than those of the plantation. We conclude that reforestation dramatically lowers soil C and N pools, further affecting the responses of forest soils to future global warming.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The world’s soils contain about 1,500 Gt carbon (C) and 300 Gt nitrogen (N) in the top 1 m (Jobbagy and Jackson 2000). It is believed that soil, especially forest soil, acts as a vital pool, source and sink of C and N, and plays an important role in the cycling of C and N in terrestrial ecosystems. Global air temperatures are predicted to increase by 1.8–4.0°C over the course of this century, with a greater warming occurring in higher latitude and higher altitude ecosystems (IPCC 2007). Temperature is a key factor that regulates almost all biogeochemical processes of terrestrial ecosystems, such as soil respiration and decomposition of soil organic matter (SOM; Rustad et al. 2001). Warmer air temperatures would likely result in warmer soil temperatures, which could, in turn, affect the quality and quantity of SOM (Kirschbaum 2004). Therefore, the sensitivity of SOM to climate warming could have great potential to influence the interactions between climatic change and C cycling.
Soil respiration, as an indicator of soil biological activity, is influenced strongly by temperature and soil moisture (e.g., Xu and Qi 2001; Emmett et al. 2004). Global warming is therefore expected to increase the efflux of CO2 from soils. Experimental ecosystem warming generally increases CO2 efflux from soils in various biomes—this effect being more significant in forested than in low-tundra and grassland ecosystems (Rustad et al. 2001). Moreover, a particular ecosystem or vegetation type may have further specific impacts on soil biological activity, mostly through altering the quality and quantity of SOM, and by its characteristic heat and soil moisture regimes (Emmett et al. 2004; Lellei-Kovács et al. 2008). The magnitude of warming on the Tibetan Plateau is projected to be large relative to many other regions, and soils on the Tibetan Plateau contain large amounts of SOM (Xu et al. 2003; Jiang et al. 2009). Thus, respiratory C loss from the alpine forest soils in this region could be more pronounced than in other ecosystems.
The impacts of climate change on the C and N cycling of terrestrial ecosystems depend largely on changes in the quantity and quality of soil C and N pools. SOM consists of a variety of components with different chemical characteristics and physical accessibility that influence their susceptibility to biodegradation. Differentiation of the total SOM into labile fractions and recalcitrant fractions could provide more understanding of the cycling of C and N under global warming (Sun et al. 2004; Belay-Tedla et al. 2009). Microbial biomass C (MBC) and N (MBN) pools are also thought to be important labile soil C and N pools, with a turnover time from days to weeks (Parton et al. 1987). Microbes play a critical role in C and nutrient transformation in forest soils. Any slight changes in microbial biomass or community structure could affect the cycling of C and N, and N availability to plants (Saffigna et al. 1989). Therefore, soil microbial properties are considered to be potential indicators of the impacts of global warming on soils (Zhang et al. 2005; Rudrappa et al. 2006). In recent years, a few studies have mentioned the effects of experimental warming on microbial properties. However, these studies have not shown consistent warming effects, with positive, negative, and neutral effects all being reported (Rillig et al. 2002; Staddon et al. 2003; Zhang et al. 2005; Biasi et al. 2008).
As mentioned above, the sub-alpine and alpine forest ecosystems in the Eastern Tibetan Plateau located in the transition zone from Qinghai-Tibet plateau to Sichuan basin could be very sensitive to global climate change, with important consequences for the global C and N balance (Wang et al. 2003). In the last century, natural coniferous forests in southwestern China were deforested and reforested with dragon spruce (Picea asperata Mast.). Currently, there are over 1 million ha dragon spruce plantation in southwestern China, accounting for approximately 50% of the forest area in this region. To date, artificial warming studies in this region have focused more on aboveground components, with less focus on soils and belowground ecosystem processes (Yin et al. 2008; Zhang et al. 2008; Zhao and Liu 2009; Han et al. 2009; Xu et al. 2009).
Ecosystem response could depend strongly on the initial conditions of the ecosystem, such as stocks and initial turnover rates of SOM, the relative size of the plant and soil C pools, and the chemical composition and turnover rates of plant residues (Shaver et al. 2000). Therefore, it is very important to synchronously investigate different soil C and N components under different land-uses in a warming experiment. Additionally, soil responses to warming are likely to be complicated by land-use change (Luo et al. 2001). Comparing responses of soils to warming under different land-uses could more accurately predict the impacts of global warming on terrestrial ecosystems. Hence, in this study, we conducted an experiment in two contrasting ecosystems (dragon spruce plantation versus spruce-fir dominated natural forest) to assess the effects of experimental warming on soil CO2 efflux, labile and recalcitrant C and N pools. Specifically, we hypothesized that (1) an increase in temperature will stimulate soil surface CO2 fluxes in both forest ecosystems; (2) soil warming will reduce soil labile C, N pools in two sites; and (3) there will be responsive differences to warming between two contrasting forest soils.
Materials and methods
Experimental site
The study was conducted on two sites within approximately 300 m of each other. One site was in a dragon spruce plantation (65 years old) and the other was in a spruce-fir-dominated natural forest (200 years old). There are some mosses and grasses (e.g., Carex capilliformis, Deyeuxia arundinacea, Festuca ovina) in the plantation. Conversely, the understory is dominated by mosses, woody trees (e.g., Betula albo-sinensis, Acer mono, Lonicera spp.) and grasses (e.g., Carex capilliformis, Anemone rivularis) in the natural forest. Both experimental sites were located at the Miyaluo Experimental Forest of Lixian County, eastern Tibetan Plateau (31°35′ N; 102°35′ E; 3,150 m a.s.l). Soils at two sites are classified as belonging to the mountain brown soil series (Chinese taxonomy). The basic soil properties as determined in July 2008 are as follows: organic C 44.82 ± 3.25 g kg−1, total N 2.95 ± 0.34 g kg−1, total P 0.65 ± 0.03 g kg−1, total K 14.16 ± 0.39 g kg−1 and pH 6.19 ± 0.47 for the plantation; organic C 145.02 ± 14.87 g kg−1, total N 9.56 ± 1.19 g kg−1, total P 0.67 ± 0.07 g kg−1, total K 11.31 ± 0.51 g kg−1 and pH 5.85 ± 0.65 for the natural forest. For basic climate conditions in the study region see Yang et al. (2007).
In order to increase soil temperature, six open top chambers (OTCs) were set up in each of the two contrasting forests with similar canopy. One control plot was established randomly in the vicinity of each OTC. The OTCs used in this study were hexagonal and 80 cm high, made of solar transmitting material, with 2.45 m2 as the ground area tapering to 1.64 m2 at the open-top area. It was expected that all of the selected plots were similar in microhabitat characteristics. The OTC installations were completed in late September 2008 and observations were initiated from late April 2009.
Microclimate monitoring
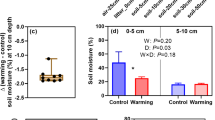
To quantify the environmental factors affected by the OTCs, two automatic recording systems were set up. Air and soil temperatures at 30 cm above and 5 cm below the soil surface, respectively, were measured in the centre of each plot by alternating among sensors connected to a datalogger (Avalon Campbell AR5, Campbell Scientific, Logan, UT). Temperature monitoring was carried out in three OTCs and three control plots. Data were taken at 60 -min intervals from early May to late October in 2009. Soil volumetric moisture content at 10 cm below the soil surface was measured with a hand-held probe (IMKO, Ettlingen, Germany) at approximately weekly intervals. Measurements of soil moisture were carried out in the area of the OTC without rainfall interception so as to avoid any edge effects of the OTC.
Measurements of soil surface CO2 efflux
We measured soil CO2 efflux with a Li-Cor 6400 portable CO2 infrared gas analyzer (Li-Cor, Lincoln, NE) to monitor the change in CO2 concentration over time in the flux chambers. Three chambers (10 cm in diameter, 5 cm in height) were installed in each plot in early May 2009. Living plants inside the soil collars were clipped at the soil surface to avoid leaf respiration. Surface vegetation (mosses) was left in the chambers. Fluxes were measured every month during the 2009 growing season. Soil CO2 efflux in each chamber was measured continuously for three cycles, and the three measurements were averaged to produce a mean soil flux. Soil CO2 efflux measurements were also taken in the center of the plot to avoid edge effects. To examine the seasonal temperature sensitivity of soil respiration, we conducted nonlinear regression models using R s=aebT, where R s is soil respiration, T is soil temperature, coefficient a is the intercept of soil respiration when temperature is zero, and coefficient b represents the temperature sensitivity of soil respiration. In this analysis, we used the soil temperatures (5 cm depth) measured at the same time as the soil CO2 efflux. On the other hand, Q 10 was used to assess actual warming effects on soil CO2 effluxes at each time the respiration rates were measured. Q 10 is calculated as R p^(10/W), where R p is proportional respiration rate; W is the magnitude of the warming.
Soil sampling
Soil samples were collected from the topsoil (0–15 cm) in early May, mid-July and late September of 2009. Five cores (3 cm diameter, 15 cm deep) were taken randomly from each plot and mixed to get one composite sample and delivered immediately to the laboratory. Each composite sample was passed through a sieve (4 mm diameter), and any visible living plant material was removed manually from the sieved soil. The sieved soil was kept in the refrigerator at 4°C prior to the analysis of microbial properties. A subsample of each soil was air-dried and ground (<250 µm) prior to chemical analysis.
Soil analysis
A two-step acid hydrolysis procedure with H2SO4 as the extractant was used to determine labile and recalcitrant C and N Pools (Rovira and Vallejo 2002). Briefly, 20 ml 2.5 M H2SO4 was added to 500 mg soil, and the sample was hydrolyzed for 30 min at 105°C in sealed Pyrex tubes, after which the hydrolysate was recovered by centrifugation and decantation. The residue was washed with 20 ml de-ionized water and the washing added to the hydrolysate. This hydrolysate was taken as labile pool I (LPI) and analyzed for labile pool I carbon (LPI-C) and nitrogen (LPI-N). The remaining residue was hydrolyzed with 2 mL 13 M H2SO4 overnight at room temperature under continuous shaking. The concentration of the acid was then brought down to 1 M by dilution with de-ionized water and the sample was hydrolyzed for 3 h at 105°C with occasional shaking. This second hydrolysate was taken as labile pool II (LPII) and analyzed for labile pool II carbon (LPII-C) and nitrogen (LPII-N).
Total soil organic C (TOC) was analyzed with a TOC analyzer (Multi N/C 2100, Analytik, Jena, Germany) and soil total N (TN) was determined using a UDK152 apparatus (Velp Scientifica, Ulpiate, Milan, Italy). Recalcitrant C (RP-C) and N (RP-N) pools were calculated as the difference between the total concentration of the elements (TOC or TN) and the labile pools (LPI and LPII summed together; Belay-Tedla et al. 2009).
Soil microbial biomass C (MBC) and N (MBN) were determined using the fumigation-extraction method (Vance et al. 1987). Soil extractable organic C and total N in the K2SO4 extracts before and after the fumigation were quantified using a total C/N analyzer (Multi N/C 2100, Analytik Jena). The released C and N were converted to MBC and MBN, respectively, using K ec−0.45 and K en−0.45 (Vance et al. 1987).
Statistical analysis
Three-way analysis of variance (ANOVA) was used to examine the effects of warming, forest type, sampling date, and their interactions for labile C and N pools I and II (LPI-C, LPI-N, LPII-C, LPII-N), recalcitrant C and N (RP-C and RP-N), total C, N (TC and TN), and soil microbial biomass C and N (MBC and MBN) and their ratio (MBC/MBN). Before analysis, all data were tested for the assumptions of ANOVA. If data were heterogeneous, they were ln-transformed before analysis. For specific sampling dates, Student t-tests were used to compare the effect of the experimental warming. The statistical tests were considered significant at the P < 0.05 level. All statistical tests were performed using SPSS version 11.0.
Results
Microclimates
The OTCs had parallel heating effects in the experimental sites. In contrast to the control plot, soil and air temperatures in the OTCs increased on average by 0.61°C and by 1.24°C, respectively, in the plantation throughout the growing season (Fig. 1a,c) and by 0.56°C and by 1.15°C, respectively, in the natural forest (Fig. 1b,d). Conversely, soil volumetric moisture content declined by 4.10% in the plantation and by 2.55% in the natural forest (Fig. 2a,b). Moreover, there was no obvious seasonality in soil volumetric moisture content in the two forest ecosystems regardless of treatment (Fig. 2a,b).
Soil surface CO2 efflux
Warming tended to increase soil CO2 effluxes over the growing season in both sites (Fig. 3a,b). Across all measuring dates, warming increased the average soil respiration rate by 10.6% in the plantation and by 15.4% in the natural forest. Statistical analyses showed that warming and measuring date had significant effects on soil respiration rates in both forests but there was no significant interaction between the two factors (warming × date). Q 10 values at each measuring time in both forests varied among seasons (Fig. 3a,b). The exponential relationships between soil temperature and soil CO2 flux were highly significant for all treatments (Table 1). Soil warming did not affect the respiration quotient in either forest ecosystem. Nevertheless, irrespective of the warming regimes, respiration quotient values were significantly larger in the natural forest than in the plantation (Table 1).
Soil CO2 fluxes (grey and open bars) based on chamber measurements in warmed OTC and control plots during the growing season in natural and plantation forest ecosystems. Bars Mean and SD on each date. * P < 0.05, ns P > 0.05. Soil temperatures (solid and open circles) recorded at the same time as efflux measurements are also shown. Numerals shown on the bars are Q 10 values at each measuring period
Soil labile and recalcitrant C pools
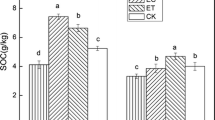
Warming significantly reduced LPI-C and LPII-C pools in both forest soils (Table 2). On average, over the sampling dates, warming declined LPI-C and LPII-C by 12.7% and 9.2%, respectively, in the plantation (Fig. 4a,b). Likewise, elevated soil temperature decreased LPI-C and LPII-C by 15.9% and 11.3%, respectively, in the natural forest (Fig. 4d,e). When LPI-C and LPII-C pools were summed together, warming decreased labile C by 10.4% in the plantation and by 12.2% in the natural forest. Irrespective of the warming regimes, both LPI-C and LPII-C pools in both sites varied significantly between seasons (Table 2). Soil LPI-C and LPII-C pools were dramatically larger in the natural forest than in the plantation (Table 3). The effect of warming on LPII-C pool depended on forest type, which showed a marginal significance for warming × forest type (Table 2). In addition, there were interactive effects of warming, forest type and date on LPI-C, but not on LPII-C (Table 2).
The recalcitrant C pools contributed to a large amount of total C compared to labile C fractions (Table 3). Warming did not affect the recalcitrant and total C contents in either site (Fig. 4c,f; Table 2). There were significant differences in RP-C and total C contents between two contrasting forest ecosystems (Table 2). No other main or interactive effects on soil RP-C and total C pools were detected (Table 2). Additionally, warming did not affect labile and recalcitrant C pools significantly when expressed as a percentage of total C (Table 3). However, the percentages for most measured pools clearly differed between forest types (Table 3).
Soil labile and recalcitrant N pools
Warming significantly lowered LPI-N pool but not LPII-N pool (Table 2). Over three sampling dates, warming averagely declined LPI-N by 9.6% in the plantation and by 10.9% in the natural forest, respectively (Fig. 5a,d; Table 2). Similar to labile C pools, soil labile N pools in both forests also showed a clear seasonality, and were greater in the natural forest than in the plantation (Table 2, Fig. 5). There were interactive effects of forest type and date on LPI-N and LPII-N (Table 2), whereas three-way interactions of warming, date and forest type were significant only in LPI-N (Table 2).
Warming did not affect the recalcitrant and total N contents in both sites (Fig. 5c, f; Table 2). There were significant differences in soil RP-N and total N contents between two contrasting forest ecosystems (Table 2). Additionally, warming did not affect labile and recalcitrant N pools when expressed as a percentage of total N (Table 4). However, the percentages for most measured pools clearly differed between the two forest types (Table 4).
Soil microbial biomass C and N
There were no significant warming effects on MBC and MBN in the plantation among the sampling dates (Fig. 6a,b; Table 2). Likewise, warming did not cause significant differences in the ratio of MBC to MBN in the plantation (Fig. 6c, Table 2). In contrast to the plantation, warming increased MBC and MBN in the natural forest only in late September, but did not affect the ratios of MBC to MBN over the sampling dates (Fig. 6f). MBC and MBN showed different seasonal dynamics regardless of the treatments in both forest ecosystems (Fig. 6). MBC, MBN and MBC/MBN all were significantly different over three seasons. There were interactive effects of warming, forest type and date on MBC (Table 2).
Discussion
As a passive warming system, OTCs are considered an appropriate tool for studying the responses of ecosystems to artificial warming (Marion et al. 1997; Aronson and McNulty 2009). In this study, the OTC increased daily mean soil temperature by approximately 0.6°C on average over the growing season in both forest ecosystems. However, when we focused on daily maximum soil temperature, soil temperature inside the OTC increased above 1°C, even up to 2°C during the growing season at both sites. The magnitude of soil warming in our study is similar to that seen in other studies (Allison and Treseder 2008; Biasi et al. 2008; Thomas et al. 2008).
Considerable evidence is now available showing that soil moisture inside warming treatments is generally lower compared to the control soil (Rustad et al. 2001; Allison and Treseder 2008). For example, a 2.0°C increment decreased soil moisture by 6.4% in a tallgrass prairie (Luo et al. 2001). Our result is not an exception to this rule, in that warming averagely reduced soil volumetric moisture content by 4.10% in the plantation and by 2.55% in the natural forest throughout the growing season. However, this warming-induced decline in soil moisture may not have had a major influence on belowground ecological processes because soil moisture in the warmed plot still remained at a relatively high level (~30%) over the growing season due to frequent rainfall.
Soil temperature and moisture are the most important factors controlling soil CO2 fluxes (Rustad et al. 2000). In line with our initial hypothesis, we found that warming significantly stimulated soil surface CO2 efflux in both sites, particularly in the middle of the growing season, with warming increasing average soil CO2 efflux by 10.6% in the plantation and by 15.4% in the natural forest. This positive response in soil respiration is consistent with many other studies (Rustad et al. 2001). Both seasonal respiration quotient and Q 10 values at each measuring time indicates that soil CO2 fluxes in both forest ecosystems are significantly sensitive to temperature. Previous studies have demonstrated that temperature sensitivity of soil respiration (Q 10) was affected negatively by soil temperature (Xu and Qi 2001; Wang et al. 2008). Our results partly support this conclusion because Q 10 values were relatively small in summer compared with spring and autumn. However, in the meta-analysis of Rustad et al. (2001), the size of the response varied among the ecosystems studied, mainly because of variability in warming magnitude and the quality and quantity of SOM between biomes. Recently, some studies found that warming did not increase, or even decreased, soil CO2 fluxes in temperate or boreal forest ecosystems (Lellei-Kovács et al. 2008; Allison and Treseder 2008). Warming-induced soil drying can explain the decline in soil respiration in these studies.
The respiration quotient reflects the sensitivity of soil CO2 flux to temperature, and varies among ecosystems and across temperature ranges (Peng et al. 2008). In this study, artificial warming did not affect the temperature sensitivity of soil respiration in both forest ecosystems. This result is different from the findings of other studies, which demonstrate that short-term experimental warming lowers the temperature sensitivity of soil respiration (Luo et al. 2001; Allison and Treseder 2008). Warming-induced soil drying is one important underlying mechanism. As stated above, soil drying was not a factor in our experiment. Additionally, the temperature sensitivity of soil respiration may be less in soils with low carbon content (Luo et al. 2001). However, in our experimental sites, soil carbon contents were relatively high in comparison to other experiments at 4.5% in the plantation and 14.8% in the natural forest. The respiration quotient was significantly greater in the natural forest than in the plantation, probably because the soil C pool, particularly labile C pool, was dramatically larger in the natural forest as compared to the plantation.
Quantitative and qualitative changes in SOM largely determine the carbon balance in terrestrial ecosystems under a warmer world (Cardon 1996; Davidson and Janssens 2006). Temperature is a key factor controlling the decomposition processes of SOM. Differentiating total SOM into labile and recalcitrant fractions is very important in understanding the underlying mechanisms modulating the overall cycling of C and N pools under future global warming (Sun et al. 2004; Belay-Tedla et al. 2009). In this study, based on a two-step sulfuric acid hydrolysis, experimental warming significantly lowered labile C and N concentrations in both sites. This was consistent with other reports (Rustad et al. 2001; Niinistö et al. 2004; Hartley et al. 2007; Bradford et al. 2008) and our initial hypothesis that the size of the labile SOM pool may decrease under warming as a result of the increased soil CO2 efflux and the enhanced oxidation of the most labile SOM compound. In the present case, the increased soil CO2 efflux mentioned above may be linked directly to the loss of labile C. Additionally, some studies have reported that warming has reduced the root biomass in boreal soils (Bergner et al. 2004; Bronson et al. 2008). This could also induce a decline in labile pools of SOM. Conversely, an experiment conducted in a tallgrass prairie soil has recently found that artificial warming increased the labile pools of SOM (Belay-Tedla et al. 2009). This was mainly because warming induced greater above- and below-ground biomass production as compared to the increased soil respiration. On the other hand, in this study, the warming-induced decrease in the labile pools of SOM was relatively large in the natural forest as compared to the plantation. One possible reason for this is that the size of the most labile pools (easily oxidized) was substantially greater in the natural forest than in the plantation (Figs. 4, 5). All in all, responses of labile pools of SOM to experimental warming may depend strongly on ecosystem type or forest management practice.
Microbial biomass C (MBC) and N (MBN) pools are also thought to be important labile soil C, N pools with a turnover time from days to weeks (Parton et al. 1987). Moreover, soil microbial properties have been proposed to be the potential indicators of impacts of global warming and forest management on soils (Rudrappa et al. 2006). In our experiment, MBC and MBN did not differ significantly between the warmed and control plot in the plantation. Likewise, in the natural forest, warming produced statistically higher MBC and MBN only late in the growing season. The lack of a clear effect on microbial biomass noted in our study was consistent with the studies conducted in arctic tundra and tallgrass prairie (Zhang et al. 2005; Biasi et al. 2008). In our experimental sites, experimental warming significantly increased the availability of nutrients (unpublished data) but did not affect soil microbial biomasses, indicating that microbes themselves did not profit directly from the higher availability of nutrients in the warmed plots.
The ecosystem response could depend strongly on initial conditions of ecosystems, such as stocks and initial turnover rates of soil organic matter, the relative size of the plant and soil C pools and the chemical composition and turnover rates of plant residues (Shaver et al. 2000). Additionally, interactions between the C cycle and climatic change are likely to be complicated by land-use change (Striegl and Wichland 1998). Reforestation, as an important forest practice, often has profound impacts on the soil properties of forest ecosystems. As stated in this study, almost all measured pools (labile, recalcitrant and total C, N contents) were much larger in the natural forest than in the plantation. Additionally, there were some response differences under warming between the two contrasting ecosystems. Obviously, forest management practice (deforestation or reforestation) can dramatically influence the soil C and N fractions, and further affect the responses of soil C and N cycling to global warming. However, it is important to note that soil C and N pools in the plantation have not restored back to their original status more than 60 years after reforestation. Soil C and N losses due to deforestation/reforestation could be much greater compared to the decreases caused by warming. Apparently, protecting current natural forests is very important to mitigate global climate change.
Conclusions
This present study demonstrated that experimental warming significantly increased soil surface CO2 efflux, reduced labile pools of SOM, but did not influence the total and recalcitrant C, N pools and microbial biomass in either natural or plantation forest ecosystems in the 1st year of the warming experiment. The warming-induced decline in labile C and N pools may be linked to the enhanced soil surface CO2 efflux. Such changes imply that alpine coniferous forests on the eastern Tibetan Plateau could provide a positive feedback to global climate change, at least in the early stage. As many other studies have also demonstrated that labile components of SOM are sensitive to elevated temperature, it is very important to monitor the labile components of SOM in terrestrial ecosystems.
Measured pools (labile, recalcitrant and total C, N) were greater in the natural forest than in the plantation, and the responses of the labile C and N pools to experimental warming depended, to some degree, on forest type and season, with the natural forest being more sensitive than the plantation. Thus, forest management practice (deforestation and reforestation) can dramatically influence the soil C and N fractions, and further affect the responses of soil C, N cycling to global warming.
References
Allison SD, Treseder KK (2008) Warming and drying suppress microbial activity and carbon cycling in boreal forest soils. Glob Change Biol 14:2898–2909
Aronson El, McNulty SG (2009) Appropriate experimental ecosystem warming methods by ecosystem, objective, and practically. Agric For Meteorol 149:1791–1799
Belay-Tedla A, Zhou X, Su B, Wan S, Luo Y (2009) Labile, recalcitrant and microbial carbon and nitrogen pools of a tallgrass prairie soil in the US Great Plains subjected to experimental warming and clipping. Soil Biol Biochem 41:110–116
Bergner B, Johnstone J, Treseder KK (2004) Experimental warming and burn severity alter soil CO2 flux and soil functional groups in a recently burned boreal forest. Glob Change Biol 10:1996–2004
Biasi C, Meyer H, Rusalimova O, Hämmerle R, Kaise C, Baranyi C, Daims H, Lashchinsky N, Barsukov P, Richter A (2008) Initial effects of experimental warming carbon exchange rates, plant growth and microbial dynamics of a lichen-rich dwarf shrub tundra in Siberia. Plant Soil 307:191–205
Bradford MA, Davies CA, Frey SD, Maddox TR, Melillo JM, Mohan JE, Reynolds JF, Tressder KK, Wallenstein MD (2008) Thermal adaptation of soil microbial respiration to elevated temperature. Ecol Lett 11:1316–1327
Bronson DR, Gower ST, Tanner M, Linder S, Van Herk I (2008) Responses of soil surface CO2 flux in a boreal forest to ecosystem warming. Glob Change Biol 14:856–867
Cardon ZG (1996) influence of rhizodepositions under elevated CO2 on plant nutrition and soil organic matter. Plant Soil 187:277–288
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Emmett B, Beier C, Estiarte M, Tietema A, Kristensen HL, Williams D, Peñuelas J, Schmidt I, Sowerby A (2004) The response of soil processes to climate change: Results from manipulation studies of shrub lands across an environmental gradient. Ecosystems 7:625–637
Han C, Liu Q, Yang Y (2009) Short-term effects of experimental warming and enhanced ultraviolet-B radiation on photosynthesis and antioxidant defense of Picea asperata seedlings. Plant Growth Regul 58:153–162
Hartley IP, Heinemeyer A, Ineson P (2007) Effects of three years of soil warming and shading on the rate of soil respiration: substrate availability and not thermal acclimation mediates observed response. Glob Change Biol 13:1761–1770
IPCC (2007) Climate change 2007: the physical science basis. Working Group I contribution to the IPCC Fourth Assessment Report. http://www.ipcc.ch/
Jiang F, Sun H, Lin B, Liu Q (2009) Dynamic changes of topsoil organic carbon in subalpine spruce plantation at different succession stages in western Sichuan Province (in Chinese). Chin J Appl Ecol 20:2581–2587
Jobbagy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436
Kirschbaum MF (2004) Soil respiration under prolonged soil warming: are rate reductions caused by acclimation or substrate loss? Glob Change Biol 10:1870–1877
Lellei-Kovács E, Kovács-Láng E, Kalapos T, Botta-Dukát Z, Barabás S, Beier C (2008) Experimental warming does not enhance soil respiration in a semiarid temperate forest-steppe ecosystem. Commun Ecol 9:29–37
Luo YQ, Wan SQ, Hui DF, Wallace LL (2001) Acclimatization of soil respiration to warming in a tall grass prairie. Nature 413:622–625
Marion GM, Henry GHR, Freckman DW, Johnstone J, Jones G, Jones MH, Lévesque E, Molau U, Mølgaard P, Parsons AN, Svoboda J, Virginia RA (1997) Open-top designs for manipulating field temperature in high-latitude ecosystems. Glob Change Boil 3:20–32
Niinistö SM, Silvola J, Kellomäki S (2004) Soil CO2 efflux in a boreal pine forest under atmospheric CO2 enrichment and air warming. Glob Change Boil 10:1363–1376
Parton WJ, Schimel DS, Cole CV, Ojima D (1987) Analysis of factors controlling soil organic matter levels in the Great Plains grasslands. Soil Sci Soc Am J 51:1173–1179
Peng SS, Piao SL, Wang T, Sun JY, Shen ZH (2008) Temperature sensitivity of soil respiration in different ecosystems in China. Soil Biol Biochem 41:1008–1014
Rillig MC, Wright SF, Shaw MR, Field CB (2002) Artificial climate warming positively affects arbuscular mycorrhizae but decreases soil aggregation. Oikos 97:52–58
Rovira P, Vallejo VR (2002) Labile and recalcitrant pools of carbon and nitrogen in organic matter decomposing at different depths in soil: an acid hydrolysis approach. Geoderma 107:109–141
Rudrappa L, Purakayastha TJ, Singh D, Bhadraray S (2006) Long-term maturing and fertilization effects on soil organic carbon pools in a Typic Haplustept of semi-arid sub-tropical India. Soil Tillage Res 88:180–192
Rustad LE, Huntington TG, Boone RD (2000) Controls on soil respiration: implications for climate change. Biogeochemistry 48:1–6
Rustad LE, Campbell JL, Marion GM, Norby RJ, Mitchell MJ, Hartley AE, Cornelissen JHC, Gurevitch J (2001) A meta-analysisof the response of soil respiration, net nitrogen mineralization, and aboveground plant growth to experimental ecosystem warming. Oecologia 126:543–562
Saffigna PG, Powlson DS, Brookes PC, Thomas GA (1989) Influence of sorghum residues and tillage on soil organic matter and soil microbial biomass in an Australian Vertisol. Soil Biol Biochem 21:759–765
Shaver GR, Canadell J, Chapin FS III, Gurevitch J, Harte J, Henry G, Ineson I, Jonasson S, Melillo J, Piteka L, Rustad L (2000) Global warming and terrestrial ecosystems: a conceptual framework for analysis. Bioscience 50:871–882
Staddon PL, Thompson K, Jakobsen I, Grime JP, Askew AP, Fitter AH (2003) Mycorrhizal fungal abundance is affected by long-term climatic manipulations in the field. Glob Change Biol 9:1365–2486
Striegl RG, Wichland KP (1998) Effects of a clear-cut harvest on soil respiration in a jack-lichen woodland. Can J For Res 28:534–539
Sun OJ, Campbell J, Law BE, Wolf V (2004) Dynamics of carbon stocks in soils and detritus across chronosequences of different forest types in the Pacific Northwest, USA. Glob Change Biol 10:1470–1481
Thomas K, Anton F, Markus BR, Clemens A (2008) Short-term effects of temperature enhancement on growth and reproduction of alpine grassland species. Basic Appl Ecol 9:263–274
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wang K, Kellomäki S, Zha T (2003) Modifications in photosynthetic pigments and chlorophyll fluorescence in 20-year-old pine trees after a four-year exposure to carbon dioxide and temperature elevation. Photosynthetica 41:167–175
Wang X, Zhua B, Gao M, Wang Y, Zheng X (2008) Seasonal variations in soil respiration and temperature sensitivity under three land-use types in hilly areas of the Sichuan Basin. Aust J Soil Res 46:727–734
Xu M, Qi Y (2001) Spatial and seasonal variations of Q10 determined by soil respiration measurements at a Sierra Nevadan forest. Global Biogeochem Cycles 15:687–696
Xu Y, Ding Y, Li D (2003) Climatic change over Qinghai and Xizang in the 21st century (in Chinese). Plateau Meteorol 22:451–457
Xu Z, Hu T, Wang K, Zhang Y, Xian J (2009) Short-term responses of phenology, leaf traits and shoot growth of four alpine shrubs in timberline ecotone to simulated global warming, Eastern Tibetan Plateau, China. Plant Species Biol 24:27–34
Yang Y, Liu Q, Han C, Yao X, Yin H (2007) Influence of water stress and low irradiance of Picea asperata seedlings. Photosynthetica 45:613–619
Yin H, Liu Q, Lai T (2008) Warming effects on growth and physiology in the seedlings of the two conifers Picea asperataand and Abies faxoniana under two contrasting light conditions. Ecol Res 23:459–469
Zhang W, Parker KM, Luo Y, Wan S, Wallace L, Hu S (2005) Soil microbial responses to experimental warming and clipping in a tallgrass prairie. Glob Change Biol 11:266–277
Zhang Y, Duan B, Qiao Y, Wang K, Korpelainen H, Li C (2008) Leaf photosynthesis of Betula albosinensis seedlings as affected by elevated CO2 and planting density. Forest Ecol Manag 255:1937–1944
Zhao C, Liu Q (2009) Growth and photosynthetic responses to two coniferous species experimental warming and nitrogen fertilization. Can J For Res 39:1–11
Acknowledgments
We are grateful to anonymous reviewers and the section editor for their valuable comments and suggestions. We thank the staff in the Forestry Bureau of Western Sichuan for their kind help with field investigations. This study was supported jointly by the Key Program of the National Natural Science Foundation of China (30530630 and 30800165), Western Light Foundation of 491 the CAS (2008) and the Talent Plan of CIB, the Chinese Academy of Sciences 492 (O8B2031), Knowledge Innovation Engineering of the Chinese Academy of Sciences (Y0B2021100) and (KSCX2-YW-Z1023).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Liz Shaw.
Rights and permissions
About this article
Cite this article
Xu, Z., Wan, C., Xiong, P. et al. Initial responses of soil CO2 efflux and C, N pools to experimental warming in two contrasting forest ecosystems, Eastern Tibetan Plateau, China. Plant Soil 336, 183–195 (2010). https://doi.org/10.1007/s11104-010-0461-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-010-0461-8