Abstract
Microbial oils can be used for biodiesel production and fumaric acid (FA) is widely used in the food and chemical industries. In this study, the production of lipids and FA by Aureobasidium pullulans var. aubasidani DH177 was investigated. A high initial carbon/nitrogen ratio in the medium promoted the accumulation of lipids and FA. When the medium contained 12.0% glucose and 0.2% NH4NO3, the yeast strain DH177 accumulated 64.7% (w/w) oil in its cells, 22.4 g/l cell biomass and 32.3 g/l FA in a 5-L batch fermentation. The maximum yields of oil and FA were 0.12 g/g and 0.27 g/g of consumed sugar, respectively. The compositions of the produced fatty acids were C14:0 (0.6%), C16:0 (24.9%), C16:1 (4.4%), C18:0 (2.1%), C18:1 (57.6%), and C18:2 (10.2%). Biodiesel obtained from the extracted oil burned well. This study provides the pioneering utilization of the yeast strain DH177 for the integrated production of oil and FA.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Single cell oils (SCOs) extracted from oleaginous yeasts, similar to plant lipids, can be used as raw materials for biodiesel production [1,2,3]. Having the advantages of being renewable, easily biodegradable, and free of sulfur components, biodiesel has received considerable interest from researchers [4, 5]. Biodiesel is typically produced by chemically reacting lipids with short-chain alcohols to produce fatty acid esters. The composition of fatty acids significantly affects the quality of biodiesel. Fatty acid compositions in SCOs produced by oily yeasts generally satisfies the criteria required for biodiesel production, e.g., a particular chain length and saturation degree [1]. Oleaginous microorganisms can accumulate more than 20% of their biomass in intracellular lipids [6]. Oleaginous yeasts typically belong to multiple genera, including Candida, Cryptococcus, Lipomyces, Rhodosporidium, Rhodotorula and Yarrowia [7]. However, only a minor proportion (3–10%) of the 1600 known yeast species have been proven to be oleaginous microorganisms [1]. Screening novel oily yeast species for biodiesel production is, therefore, very important.
In this study, a novel oleaginous yeast strain, DH177, of Aureobasidium pullulans var. aubasidani was obtained. Notably, the yeast strain DH177 also produced large amounts of fumaric acid (FA) in the culture. FA or trans-butenedioic acid is a naturally occurring organic acid involved in the tricarboxylic acid (TCA) cycle [8]. With its unique structure (C4, dicarboxylic acid groups and a carbon–carbon double bond), FA is considered a promising building-block chemical in many applications such as those found in the food, pharmaceutical, and chemical industries [9, 10]. FA is produced petrochemically from maleic anhydride or by microbial fermentation [8, 11]. With the advantages of environment-friendly and sustainable development process, an increasing number of researchers have paid close attention to FA production by fermentation. High levels of FA have been produced by Rhizopus oryzae [12,13,14], Rhizopus arrhizus [15], and Rhizopus delemar [16]. Although many studies focus on exploiting the production of FA or SCOs by microbial fermentation, the integrated production of lipids and FA is rarely studied.
SCO tends to accumulate in microbial cells under such stress conditions as high concentrations of carbon sources and nitrogen deprivation [17,18,19,20], which are also typical triggers of C4-dicarboxylic acid (FA and malate) accumulation [9, 21,22,23]. The induction of SCOs and C4-dicarboxylic acids by identical conditions provides substantial advantages for the simultaneous production of SCOs and FA in the yeast strain DH177.
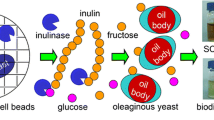
The main objective of this study was, therefore, to investigate the simultaneous accumulation of SCOs and FA by the yeast strain DH177 (Fig. 1). Our results revealed that the yeast strain DH177 simultaneously could produce large amounts of SCOs and FA from glucose when nitrogen was limited in the medium. This study provides valuable insights into the utilization of A. pullulans var. aubasidani DH177 for the integrated production of SCOs and FA.
Materials and methods
Yeast strain and cultivation media
A. pullulans var. aubasidani DH177 isolated from the leaves of Weigela florida at Ya-Shan national forest park in Shandong Province of China was used in this study and was maintained at 4 °C on YPD slants composed of (%, w/v) yeast extract 1.0, peptone 2.0, and dextrose 2.0 or on PDA slants composed of (%, w/v) potato 20, dextrose 2.0 and agar 2.0. The medium for the evaluation of lipid-accumulating ability (EL medium) contained (%, w/v) glucose 4.0, KH2PO4 0.7, Na2HPO4 0.25, MgSO4·7H2O 0.15, yeast extract 0.05, (NH4)2SO4 0.1, FeCl3·7H2O 0.015, CaCl2 0.015, and ZnSO4·7H2O 0.002, pH 6.0 [18]. The medium for both SCO and FA production (SF medium) contained (%, w/v) glucose 12.0, NH4NO3 0.2, KH2PO4 0.01, MgSO4·7H2O 0.01, and KCl 0.05 and CaCO3, Na2CO3 or NaOH was used as a pH buffer.
Yeast isolation
The leaves, fruits, and flowers from 23 species of plants were collected from Ya-Shan national forest park (longitude 120°83′, latitude 37°28′) in Shandong Province of China. Five grams of the leaves, fruits, and flowers form different plants were suspended in YPD medium (40.0 ml) supplemented with 0.04% chloramphenicol in 250-ml shaking flasks, followed by cultivation at 28 °C and 180 rpm for 3 days. The cell cultures were then diluted with sterilized water, and the diluted mixture was plated onto YPD plates and incubated at 28 °C for 3 days. Yeast colonies from the plates were maintained at 4 °C.
Lipid determination and observation of lipid particles in the yeast cells
Lipid accumulations by the yeast strains isolated above were assessed in flasks containing EL medium (50 ml) after inoculating with 2.5 milliliters of the seed culture (2.0 × 108 cells/ml). The culture was incubated at 28 °C in a rotary shaker at 180 rpm for 3 days. The cells were then harvested and washed three times by centrifugation at 5000g and 4 °C with distilled water. The cell biomass was calculated gravimetrically after drying at 80 °C. Total intracellular lipids were extracted from the cells [24], and oil content was calculated as the number of grams of oil per 100 g of dry cell weight [19]. Nile Red dye (0.5 mg/l in DMSO) was employed to stain intracellular lipid particles [2, 18]. Lipid particles were then observed using a fluorescence microscope (Olympus BX51, Japan).
Yeast identification and phylogenetic analysis
The yeast strain DH177 was identified at the molecular level using the D1/D2 26S rDNA region as a marker. For DNA extraction, the cell wall of the yeast strain DH177 was first digested using lyticase (Sigma). The resulting yeast protoplast pellet was disrupted, and the genomic DNA was isolated using a Yeast Genomic DNA Kit (Tiangen, China). The DNA obtained above was used as a template for the amplification of D1/D2 26S rDNA region using the primers NL1 (forward 5′-GCATATCAATAAGCGGAGGAAAAG-3′) and NL4 (reverse 5′-GGTCCGTGTTTCAAGACGG-3′). The generated sequence was analyzed using the BLASTN (http://www.ncbi.nlm.nih.gov/BLAST.cgi). ClustalX software was used for multiple alignments, and a dendrogram was constructed using MEGA 5.0.
Cloning and sequencing of the gene encoding fumarase
The genomic DNA of the yeast strain DH177 was isolated as described above. The primers for cloning of the fumarase gene in the yeast strain DH177 were designed according to the fumarase genes from R. oryzae (accession no. HM130701), Aureobasidium subglaciale (accession no. XM_013484505), Aureobasidium namibiae (accession no. XM_013571509), and Saccharomyces cerevisiae (accession no. J02802.1). The forward primer was 5′-ATGTTSAGAASCGGATCGGCAGCYGT-3′ and the reverse primer was 5′- TTAMTYCTTSTCCTTGGGRGCAAGC-3′. The parameters for the PCR amplification were as follows: an initial denaturation at 95 °C for 15 min, followed by 32 cycles of denaturation at 95 °C for 50 s, annealing at 55 °C for 40 s, extension at 72 °C for 1.5 min, and a final extension at 72 °C for 10 min. The amplified fragments by PCR were cloned into pMD18-T Simple Vector and sequenced. The amino acid sequence of the gene cloned above was deduced. The protein sequences obtained were aligned using software DNAMAN 6.0.
Determination of the intracellular fumarase activity
The yeast cells grown on EL medium for 48 h were suspended in 10.0 ml of sodium phosphate buffer (0.05 M, pH 7.3) and were disrupted as described [25]. The cells debrises were removed by centrifugation at 12,000g and 4 °C for 20 min. The supernatant obtained was used as the intracellular crude enzyme. The reaction mixture containing 0.1 ml of the crude enzyme and 0.9 ml of sodium phosphate buffer (0.05 M, pH 7.3) containing 0.05 M L-malate was incubated at 28 °C for 10 min [26]. The reaction was inactivated immediately at 100 °C for 10 min. The amount of fumarate in the reaction mixture was determined by high-performance liquid chromatography (HPLC) as described below. One fumarase unit was defined as the amount of enzyme that produced one micromole of fumarate per minute under the assay conditions used in this study. Total protein concentration in the supernatant was measured using Coomassie brilliant blue assay [27].
Optimization of the medium for lipid and FA production
The effects of different glucose concentrations and neutralizing agents (CaCO3, Na2CO3 and NaOH) on cell growth and lipid and FA production by the yeast strain DH177 were assessed by incubating the culture in SF medium supplemented with different concentrations of glucose and different kinds of neutralizing agents. A total of 2.5 ml of the seed described above was transferred to 50 ml of the medium. The flasks were then incubated at 28 °C in a rotary shaker at 180 rpm for 5 days. The biomass, oil production, oil content and FA production of the cultures were analyzed.
Batch fermentation in a bioreactor
A total of 175 ml of the seed culture described above was transferred to 3500 ml of SF medium supplemented with 5.0 g/100 ml calcium carbonate in a 5-L fermenter (5BG-7000A-Baoxing Bio-Equipment Co., Ltd., China). Fermentation was performed at 28 °C, 250 rpm, and 480 l/h aeration for 8 days. A subset of 50 ml of the culture was collected at regular intervals for the analysis of cell growth and oil, FA, and residual sugar content. The amount of residual sugar in the culture was assayed with the Nelson-Somogyi method [28].
Fatty acid compositions determination, biodiesel preparation and estimation of biodiesel properties
To determine fatty acid composition, the extracted lipids were first transmethylated, and gas chromatography (GC) (Agilent 5890-II, USA) was then to used to analyze the fatty acid methyl esters according to a previously described procedure [29]. Lipids extracted from the yeast strain DH177 were transformed into biodiesel by a previously described method [18, 29]. In brief, 40.0 ml of extracted lipids was mixed with 500 ml of H2SO4 solution (0.1 M in methanol). The mixture was then incubated at 75 °C on a magnetic stirrer for 30 min to complete transmethylation. After being separated by centrifugation, the resulting lower organic phase was heated at 90 °C for 1.5 h. The remaining organic compounds obtained above were then used as biodiesel. Six parameters, iodine value, cetane number, higher heating value (HHV), viscosity, cloud point and specific gravity, were calculated in previously described manners to estimate the biodiesel properties [19, 30, 31].
Determination of FA content by HPLC
FA content was estimated by HPLC (Agilent 1100, USA). Briefly, the culture broth that had been obtained above was diluted with distilled water to dissolve potential calcium fumarate precipitate, and the excess carbonate in the culture broth was then removed by the addition of HCl (1 M) [16]. The processed fermentation broth was centrifuged at 5000g and 4 °C for 10 min. The exopolysaccharide (EPS) in 10 ml of the supernatant was first precipitated with 5 ml of methanol (supernatant-to-methanol ratio (v/v), 1:0.5) [32]. After the sample was centrifuged at 4 °C for 20 min, the EPS material was discarded. The resulting supernatant was separated on a ZORBAX SB-AQ C18 column (4.6 × 250 mm, 5 µm, Agilent, USA) by elution with 0.01 M (NH4)2HPO4 (pH 2.7) in 10.0% methanol at a flow rate of 0.5 ml/min, column temperature of 30 °C, sample volume of 20.0 µl and detection wavelength of 210 nm.
Results and discussion
Screening of oleaginous yeast strains for their ability to produce high levels of lipids and FA
More than 100 yeast strains were isolated at the first stage and purified from a variety of samples, including leaves, fruits, and flowers (data not shown). After lipid production by the different yeast strains was determined, a candidate labeled “DH177” was proven to produce the most oil. As shown in Fig. 2a, the yeast strain DH177 could produce 11.9 g/l dry cell mass and accumulate 61.1% (w/w) intracellular oil in its cells. The FA production by the yeast strain DH177 was also investigated. As shown in Fig. 2a, 7.7 g/l of FA was obtained when the yeast strain DH177 was grown in the EL medium for 3 days.
The intracellular lipid bodies produced by the yeast strain DH177 were stained with Nile Red and observed according to the methods described above. The results showed that more than ten lipid bodies were produced in each cell of the yeast strain DH177 and that even whole cells of the yeast strain DH177 were filled with oil bodies (Fig. 2b, c), demonstrating that the yeast strain DH177 produced high level of lipid. Triacylglycerols (TAGs) and steryl esters (SEs) stored in the hydrophobic core of oil bodies in oleaginous yeast cells can be dyed by the lipophilic Nile Red [33]. A phospholipid monolayer typically surrounds the neutral lipid cores of oil particles [34]. The average diameter of lipid particles in S. cerevisiae was between 0.3 and 0.4 µm [35], while the average diameter of lipid particles formed by the yeast strain DH177 was between 2.0 and 3.0 µm (Fig. 2b, c). The number of lipid particles in each oleaginous yeast cell, e.g., Pichia guilliermondii [18], Papiliotrema laurentii [19] and Yarrowia lipolytica [2, 36] cells, ranged from one to three. However, the reason why the yeast strain DH177 accumulated many more lipid particles than P. guilliermondii, Pa. laurentii and Y. lipolytica is unknown.
Characterization of the yeast strain DH177
To identify the yeast strain DH177, the morphological characterizations and the phylogenetic relationships were investigated. The results showed that arachnoid mycelia in the marginal areas of colonies were observed (Fig. 3a, b). After 10 days of incubation, pigmentation was formed by the yeast strain DH177 grown in PDA plates (Fig. 3b). These culture characteristics were similar to those of A. pullulans var. aubasidani [37]. The sequence of D1/D2 26S rDNA from the yeast strain DH177 was investigated and deposited in GenBank (accession no. MG209839). Many phylogenetically related yeast species were found when this sequence was compared against the NCBI database using the BLASTN program. A dendrogram was constructed to study the phylogenetic relationship between these yeast strains. Consistent with the morphological characteristics (Fig. 3a, b), the yeast strain DH177 was clustered in one branch with A. pullulans var. aubasidani (Fig. 3c). In recent years, oleaginous yeasts such as Rhodosporidium toruloides [5], Rhodotorula glutinis [38, 39], Sporidiobolus pararoseus [20], Lipomyces starkeyi [40] and Y. lipolytica [41], have been deeply investigated to use the lipids that they produce as a source of biodiesel. However, only approximately 30 of 1600 known yeast species can accumulate over 25% (w/w) SCOs in the cells [7]. The identification of the yeast strain DH177 as A. pullulans var. aubasidani is, therefore, concordant with the lipid-accumulating ability of this strain.
Cloning of the fumarase gene and intracellular fumarase activity in the yeast strain DH177
As shown in Fig. 2a, the yeast strain DH177 could produce a large amount of FA in the culture. In order to know whether the yeast strain DH177 genome contains the gene encoding fumarase, the fumarase gene was cloned as described above. After the fumarase gene was sequenced, the results indicated that the fumarase gene from the yeast strain DH177 was found to have 1597 bp and an intron (accession no. MH568696, Supplementary Fig. S1). The amino acids from the fumarase gene obtained were deduced and a multiple alignments of the deduced amino acid sequence with fumarases from other fungi was performed. It was found that the fumarase gene from the yeast strain DH177 encoded 509 amino acid residues, which exhibited high similarity with other fumarases (Fig. 4). This confirmed that the yeast strain DH177 genome indeed had the gene encoding fumarase. After determination fumarase activity in the yeast strain DH177, the results showed that the fumarase activity was 1.85 µmol min−1 (mg protein)−1 when the yeast strain DH177 was grown in the EL medium for 48 h (data not shown). A previous study showed that 1.44–3.00 µmol min−1 (mg protein)−1 of fumarase activities were detected in R. oryzae NRRL 1526 that could produce large amounts of fumaric acid [42]. Therefore, high fumarase activity may enhance FA production in this oleaginous yeast.
Simultaneous production of SCOs and FA by the yeast strain DH177 in flasks
Nitrogen starvation (a high initial carbon/nitrogen ratio in the medium) is required for C4-dicarboxylic acid biosynthesis [21, 43] and is also a typical trigger of oil accumulation in oleaginous yeasts [17, 20]. Based on these observations, we hypothesized that increasing the carbon/nitrogen ratio would simultaneously boost FA and lipid production. The effects of different glucose concentrations (from 6 to 14%) in the SF medium using Na2CO3 as a neutralizing agent were investigated. The results showed that when the nitrogen level remained constant, increasing the amount of glucose in the medium could significantly increase the production of oil and FA (Fig. 5). Large amounts of lipids (51.1–63.4%, w/w) were accumulated in the yeast strain DH177 cells when the initial glucose concentration exceeded 60 g per liter (Fig. 5). As shown in Fig. 5, the biosynthesis of FA by the yeast strain DH177 required a higher carbon nitrogen ratio than SCO accumulation. The results showed that FA production peaked at 27.8 g/l when the medium contained 120.0 g/l glucose (Fig. 5).
Neutralizing agents in FA and lipid production
The pH must be neutralized when FA is produced by fermentation [14, 44]. Two neutralizing agents, CaCO3 and Na2CO3, have been most commonly used in FA fermentation [8]. The effects of different neutralizing agents on FA fermentation by the yeast strain DH177 are shown in Table 1. The highest FA concentration (31.4 g/l) was measured when CaCO3 was used as a neutralizer, while FA production only reached 17.5 g/l when NaOH was used as a neutralizer (Table 1). The highest oil yield (0.12 g/g of consumed glucose) was also obtained when calcium carbonate was used as a neutralizer (Table 1). The yields of FA were 0.27 g/g, 0.23 g/g, and 0.15 g/g of consumed glucose when CaCO3, Na2CO3, and NaOH were used as neutralizers (Table 1), respectively.
It has been well documented that carbonate can be used as a source of carbon dioxide when pyruvate carboxylase catalyzes pyruvic carboxylation in FA fermentation [8]. The resulting oxaloacetic acid (OAA) is catalytically reduced to malic acid by NAD-malate dehydrogenase [45]. Finally, fumarase catalyzes the dehydration of malate to fumarate. In oleaginous yeast cells, the formation of OAA from citrate cleavage by ATP-citrate lyase (ACL) is important for lipid synthesis. Nitrogen limitation results in rapid reduction of AMP, which is catalyzed by AMP deaminase. The resulting ammonia ions constitute a supplemental nitrogen source for cell metabolism [18]. The activity of isocitrate dehydrogenase (ICDH) is then lost, resulting in the accumulation of isocitric and citric acids in the mitochondria. Finally, non-metabolized citrate is transported to the cytoplasm and then cleaved into OAA and acetyl-CoA by ACL [19]. The resulting acetyl-CoA is the main precursor for fatty acid biosynthesis [17]. In addition, the resulting OAA is a feedstock for FA synthesis. In our previous study, carboxylation of pyruvate also played an important role in lipid accumulation, and the carbonate-buffered medium increased oil biosynthesis by an engineered Y. lipolytica strain [41]. The yeast strain DH177 could, therefore, simultaneously produce high amounts of oils and FA in media with high initial carbon/nitrogen ratios and the presence of carbonate.
SCO and FA production by 5-L fermentation
Five-liter fermentations were performed to scale up SCO and FA production. As shown in Fig. 6, cell mass and lipid and FA production steadily increased with the consumption of glucose. The dry cell mass peaked at 22.4 g/l after 132 h of fermentation (Fig. 6). Lipid accumulation and the biosynthesis of FA lagged behind biomass growth (Fig. 6). The results in Fig. 6 indicated that 64.7% (w/w) of oil was accumulated in its cell after 156 h of fermentation and that FA production in the fermented medium peaked at 32.3 g/l after 180 h of fermentation. At the end of the fermentation, 0.1% (w/v) of glucose was detected in the medium (Fig. 6), indicating that most of the glucose that had been added was transformed into FA, microbial lipids and cell biomass. The yields of biomass, lipids and FA were calculated. The maximum yields of biomass, lipids and FA were 0.19 g/g, 0.12 g/g, and 0.27 g/g of consumed glucose, respectively (data not shown). In a 7-L stirred-tank bioreactor, 35.42 g FA was produced per liter of culture by R. delemar NRRL1526 over 3 days of fermentation [16]. Culturing R. oryzae ATCC20344 for 36 h in the CaCO3-buffered medium resulted in the maximum FA concentration of 37.2 g/l [14]. In another study, adding citrate to the culture medium could increase FA production by R. oryzae ATCC20344 and the final FA titer was 21.9 g/l after 96 h of fermentation [13]. In our previous study, P. guilliermondii Pcla22 accumulated 60.6% (w/w) intracellular oil, and 20.4 g/l biomass in a 2-L fermentation [18]. The biomass and oil content of L. starkeyi DSM 70296 in a fed-batch cultivation were 94.5 g/l and 37.4% (w/w), respectively [3]. The fermentation of glucose by A. pullulans var. melanogenum P10 resulted in 66.3% (w/w) of cell dry weight in lipid content [29]. The maximum oil production of 51 g/l and the intracellular oil content of 60% were obtained by the strain S. pararoseus CCTCCM 2010326 [20]. Culturing R. toruloides AS 2.1389 for 196 h in sugarcane bagasse hydrolysate medium resulted in a cell mass and lipid content of 23.5 g/l and 52.5%, respectively [5]. In another study, the yeast strain BCRC 21418 of R. glutinis could accumulate 11.7 g/l of biomass and 55 ± 4% of oil content from crude glycerol in an airlift bioreactor [39]. Pa. laurentii AM113 produced 54.6% (w/w) intracellular lipid and 18.2 g/l biomass within 108 h [19]. The yeast strain DH177 used in this study can clearly produce much more FA and SCOs than most other yeast strains.
Fatty acid composition
The fatty acid profile of the oils extracted from the yeast strain DH177 was investigated by GC. The results showed that the fatty acid profile in the yeast strain DH177 consisted of 0.6% myristic (C14:0), 24.9% palmitic (C16:0), 4.4% palmitoleic (C16:1), 2.1% stearic (C18:0), 57.6% oleic (C18:1) and 10.2% linoleic (C18:2) acids (data not shown). Most fatty acids from oleaginous yeasts are C16–C18 fatty acids [7]. The fatty acid profile of the yeast strain DH177 is very similar to that of oils from plants, e.g., corn, canola, palm and rapeseed [31, 46]. Biodiesel making requires long chain fatty acids such as C16–C18 fatty acids. The lipids extracted from the yeast strain DH177 are, therefore, ideal feedstocks for making biodiesel.
Biodiesel production and the estimation of biodiesel properties
Oils extracted from the yeast strain DH177 were transformed into biodiesel as described above. In total, 80.9% of the oils were converted into biodiesel under the conditions used in this study, and the biodiesel that was prepared burned well (data not shown). Biodiesel properties were estimated according to predictive equations [30, 31]. The results showed that the viscosity, cetane value and specific gravity for all biodiesel derived from yeasts and plants met the US biodiesel ASTM-D6751 and EU biodiesel EN-14214 standards (Table 2). The high content of unsaturated components in oil can lead to lower iodine values and a higher cloud point [46]. HHVs are related to the energy content of the fuel, and high HHVs indicate better energy performance [19]. As shown in Table 2, HHVs varied within the narrow range of 39.84–43.1 MJ/kg for all 5 biodiesel types. “First generation” biodiesel is generally derived from vegetable oils, whereas “second generation” biodiesel is derived from microbial oils [17]. The results of this study reinforce the notion that SCOs accumulated in the cells of A. pullulans var. aubasidani DH177 may serve as raw materials for biodiesel production.
FA analysis by HPLC
After the fermentation broth was centrifuged, HPLC was employed to analyze the products in the supernatant. As shown in Fig. 7a, the major component in the supernatant was FA. A. pullulans can also produce poly(β-malic acid) (PMLA) [21, 32]. A strong acid (1 M sulfuric acid at 85–90 °C) can break the ester bonds in PMLA, releasing much malic acid [32, 47]. However, after the supernatants of cultures were mixed with an equal volume of 2 M sulfuric acid and incubated at 90 °C for 10 h, only a small amount of malic acid was detected (Fig. 7b), indicating that the yeast strain DH177 produced little PMLA. These observations further confirmed that A. pullulans var. aubasidani DH177 could serve as a potential FA producer.
Conclusions
A. pullulans var. aubasidani DH177 was capable of accumulating large amounts of intracellular lipids and FA simultaneously. When the yeast strain DH177 was grown in a 5-L stirred-tank bioreactor in a medium containing 120.0 g/l glucose for 180 h, its biomass reached 22.4 g/l, the intracellular oil reached 64.7% (w/w) and the FA titer peaked at 32.3 g/l. Most of the fatty acids isolated from the yeast strain DH177 were C16–C18 fatty acids. This newly isolated yeast strain DH177 may serve as a promising candidate for FA and lipid production.
References
Sitepu IR, Garay LA, Sestric R, Levin D, Block DE, German JB, Boundymills K (2014) Oleaginous yeasts for biodiesel: current and future trends in biology and production. Biotechnol Adv 32:1336–1360
Wang G, Guo L, Liang W, Chi Z, Liu L (2017) Systematic analysis of the lysine acetylome reveals diverse functions of lysine acetylation in the oleaginous yeast Yarrowia lipolytica. AMB Express 7:94
Anschau A, Franco TT (2015) Cell mass energetic yields of fed-batch culture by Lipomyces starkeyi. Bioprocess Biosyst Eng 38:1517–1525
Helwani Z, Othman MR, Aziz N, Fernando WJN, Kim J (2009) Technologies for production of biodiesel focusing on green catalytic techniques: a review. Fuel Process Technol 90:1502–1514
Zhao X, Peng F, Du W, Liu C, Liu D (2012) Effects of some inhibitors on the growth and lipid accumulation of oleaginous yeast Rhodosporidium toruloides and preparation of biodiesel by enzymatic transesterification of the lipid. Bioprocess Biosyst Eng 35:993–1004
Ratledge C, Wynn JP (2002) The biochemistry and molecular biology of lipid accumulation in oleaginous microorganisms. Adv Appl Microbiol 51:1–51
Ageitos JM, Vallejo JA, Veiga-Crespo P, Villa TG (2011) Oily yeasts as oleaginous cell factories. Appl Microbiol Biotechnol 90:1219–1227
Engel CAR, Straathof AJJ, Zijlmans TW, Van Gulik WM, Der Wielen LAMV (2008) Fumaric acid production by fermentation. Appl Microbiol Biotechnol 78:379–389
Chen X, Wu J, Song W, Zhang L, Wang H, Liu L (2015) Fumaric acid production by Torulopsis glabrata: engineering the urea cycle and the purine nucleotide cycle. Biotechnol Bioeng 112:156–167
Xu Q, Liu Y, Li S, Jiang L, Huang H, Wen J (2016) Transcriptome analysis of Rhizopus oryzae in response to xylose during fumaric acid production. Bioprocess Biosyst Eng 39:1267–1280
Xu Q, Li S, Huang H, Wen J (2012) Key technologies for the industrial production of fumaric acid by fermentation. Biotechnol Adv 30:1685–1696
Cao N, Du J, Gong CS, Tsao GT (1996) Simultaneous production and recovery of fumaric acid from immobilized Rhizopus oryzae with a rotary biofilm contactor and an adsorption column. Appl Environ Microbiol 62:2926–2931
Liu Y, Lv C, Xu Q, Li S, Huang H, Ouyang P (2015) Enhanced acid tolerance of Rhizopus oryzae during fumaric acid production. Bioprocess Biosyst Eng 38:323–328
Zhou Y, Du J, Tsao G (2002) Comparison of fumaric acid production by Rhizopus oryzae using different neutralizing agents. Bioprocess Biosyst Eng 25:179–181
Kenealy WR, Zaady E, Du Preez JC, Stieglitz B, Goldberg I (1986) Biochemical aspects of fumaric acid accumulation by Rhizopus arrhizus. Appl Environ Microbiol 52:128–133
Zhou Z, Du G, Hua Z, Zhou J, Chen J (2011) Optimization of fumaric acid production by Rhizopus delemar based on the morphology formation. Bioresour Technol 102:9345–9349
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part I: biochemistry of single cell oil production. Eur J Lipid Sci Technol 113:1031–1051
Wang G, Chi Z, Song B, Wang Z, Chi Z (2012) High level lipid production by a novel inulinase-producing yeast Pichia guilliermondii Pcla22. Bioresour Technol 124:77–82
Wang G, Lin L, Liang W (2018) Single cell oil production from hydrolysates of inulin by a newly isolated yeast Papiliotrema laurentii AM113 for biodiesel making. Appl Biochem Biotechnol 184:168–181
Han M, Xu ZY, Du C, Qian H, Zhang WG (2016) Effects of nitrogen on the lipid and carotenoid accumulation of oleaginous yeast Sporidiobolus pararoseus. Bioprocess Biosyst Eng 39:1425–1433
Chi Z, Liu G, Liu C, Chi Z (2016) Poly(β-l-malic acid) (PMLA) from Aureobasidium spp. and its current proceedings. Appl Microbiol Biotechnol 100:3841–3851
Zelle RM, de Hulster E, Kloezen W, Pronk JT, van Maris AJ (2010) Key process conditions for production of C(4) dicarboxylic acids in bioreactor batch cultures of an engineered Saccharomyces cerevisiae strain. Appl Environ Microbiol 76:744–750
Romano AH, Bright MM, Scott WE (1967) Mechanism of fumaric acid accumulation in Rhizopus nigricans. J Bacteriol 93:600–604
Folch J, Lees M, Sloane Stanley GH (1957) A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem 226:497–509
Zhang F, Wang ZP, Chi Z, Raoufi Z, Abdollahi S, Chi ZM (2013) The changes in Tps1 activity, trehalose content and expression of TPS1 gene in the psychrotolerant yeast Guehomyces pullulans 17–1 grown at different temperatures. Extremophiles 17:241–249
Kanarek L, Hill RL (1964) The preparation and characterization of fumarase from swine heart muscle. J Biol Chem 239:4202–4206
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Spiro RG (1966) Analysis of sugars found in glycoproteins. Methods Enzymol 8:3–26
Wang CL, Yang L, Xin FH, Liu YY, Chi ZM (2014) Evaluation of single cell oil from Aureobasidium pullulans var. melanogenum P10 isolated from mangrove ecosystems for biodiesel production. Process Biochem 49:725–731
Tanimura A, Takashima M, Sugita T, Endoh R, Kikukawa M, Yamaguchi S, Sakuradani E, Ogawa J, Shima J (2014) Selection of oleaginous yeasts with high lipid productivity for practical biodiesel production. Bioresour Technol 153:230–235
Sajjadi B, Raman AAA, Arandiyan H (2016) A comprehensive review on properties of edible and non-edible vegetable oil-based biodiesel: Composition, specifications and prediction models. Renew Sust Energ Rev 63:62–92
Wang YK, Chi Z, Zhou HX, Liu GL, Chi ZM (2015) Enhanced production of Ca2+-polymalate (PMA) with high molecular mass by Aureobasidium pullulans var. pullulans MCW. Microb Cell Fact 14:115
Sitepu IR, Ignatia L, Franz AK, Wong DM, Faulina SA, Tsui M, Kanti A, Boundymills K (2012) An improved high-throughput Nile red fluorescence assay for estimating intracellular lipids in a variety of yeast species. J Microbiol Methods 91:321–328
Zweytick D, Athenstaedt K, Daum G (2000) Intracellular lipid particles of eukaryotic cells. Biochim Biophys Acta 1469:101–120
Leber R, Zinser E, Paltauf F, Daum G, Zellnig G (1994) Characterization of lipid particles of the yeast, Saccharomyces cerevisiae. Yeast 10:1421–1428
Wang G, Li D, Miao Z, Zhang S, Liang W, Liu L (2018) Comparative transcriptome analysis reveals multiple functions for Mhy1p in lipid biosynthesis in the oleaginous yeast Yarrowia lipolytica. Biochim Biophys Acta 1863:81–90
Zalar P, Gostincar C, De Hoog GS, Ursic V, Sudhadham M, Gundecimerman N (2008) Redefinition of Aureobasidium pullulans and its varieties. Stud Mycol 61:21–38
Taskin M, Ortucu S, Aydogan MN, Arslan NP (2016) Lipid production from sugar beet molasses under non-aseptic culture conditions using the oleaginous yeast Rhodotorula glutinis TR29. Renew Energ 99:198–204
Yen HW, Liao YT, Liu YX (2015) The growth of oleaginous Rhodotorula glutinis in an airlift bioreactor on crude glycerol through a non-sterile fermentation process. Bioprocess Biosyst Eng 38:1541–1546
Zhao X, Kong X, Hua Y, Feng B, Zhao ZK (2008) Medium optimization for lipid production through co-fermentation of glucose and xylose by the oleaginous yeast Lipomyces starkeyi. Eur J Lipid Sci Technol 110:405–412
Wang GY, Zhang Y, Chi Z, Liu GL, Wang ZP, Chi ZM (2015) Role of pyruvate carboxylase in accumulation of intracellular lipid of the oleaginous yeast Yarrowia lipolytica ACA-DC 50109. Appl Microbiol Biotechnol 99:1637–1645
Peleg Y, Battat E, Scrutton MC, Goldberg I (1989) Isoenzyme pattern and subcellular localisation of enzymes involved in fumaric acid accumulation by Rhizopus oryzae. Appl Microbiol Biotechnol 32:334–339
Chi Z, Wang Z, Wang G, Khan I, Chi Z (2016) Microbial biosynthesis and secretion of l-malic acid and its applications. Crit Rev Biotechnol 36:99–107
Straathof AJJ, Gulik WMV (2012) Production of fumaric acid by fermentation. In: Wang X, Chen J, Quinn P (eds) Reprogramming microbial metabolic pathways. Springer Netherlands, Dordrecht, pp 225–240
Tan MJ, Chen X, Wang YK, Liu GL, Chi ZM (2016) Enhanced citric acid production by a yeast Yarrowia lipolytica over-expressing a pyruvate carboxylase gene. Bioprocess Biosyst Eng 39:1289–1296
Hoekman SK, Broch A, Robbins C, Ceniceros E, Natarajan M (2012) Review of biodiesel composition, properties, and specifications. Renew Sust Energ Rev 16:143–169
Zou X, Zhou Y, Yang S (2013) Production of polymalic acid and malic acid by Aureobasidium pullulans fermentation and acid hydrolysis. Biotechnol Bioeng 110:2105–2113
Acknowledgements
This research was supported by the Research Foundation for Advanced Talents of Qingdao Agricultural University (Grant No. 6631114335) and Taishan Scholar Construction Foundation of Shandong Province (Grant No. 6631114314).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, G., Bai, T., Miao, Z. et al. Simultaneous production of single cell oil and fumaric acid by a newly isolated yeast Aureobasidium pullulans var. aubasidani DH177. Bioprocess Biosyst Eng 41, 1707–1716 (2018). https://doi.org/10.1007/s00449-018-1994-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-018-1994-0