Abstract
The psychrotolerant yeast Guehomyces pullulans 17-1 grows the best at 15 °C. When the yeast cells grown at 15 °C for 48 h were transferred to new medium and grown at 10, 15, and 25 °C, respectively, trehalose-6-phosphate synthase (Tps1) activity and trehalose content of the yeast cells grown at 25 °C were higher than those of the yeast cells grown at 10 and 15 °C. However, Tps1 activity and trehalose content of the yeast cells grown at 10 °C were lower than those of the yeast cells grown at 15 °C. This may suggest that trehalose synthesized by G. pullulans 17-1 only can play more important role in its adaption to high temperature than in its adaption to low temperature. After the GPTPS1 gene encoding trehalose-6-phosphate synthase was cloned from the psychrotolerant yeast, it was found that the promoter of the gene contained several stress–response elements such as C4T and AG4, indicating that the gene expression might be regulated by heat shock. It was also found that the transcriptional level of the GPTPS1 gene in the yeast cells grown at 25 °C was higher than that of the GPTPS1 gene in the yeast cells grown at 10 and 15 °C. However, the transcriptional level of the GPTPS1 gene in the yeast cells grown at 10 °C was lower than that of the yeast cells grown at 15 °C. This meant that expression of the GPTPS1 gene was constant with the changes in Tps1 activity and trehalose content of the yeast cells.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A psychrophile is defined as an organism that is capable of growth at or below 0 °C, but unable to grow above 20 °C, whereas as a psychrotolerant, while capable of growth at around 0 °C, can grow well above 20 °C (Gerday 2000). It has been reported that 80 % of the biosphere has temperatures below 5 °C. For example, much of the oceans, which cover some 70 % of the Earth’s surface, are at an average temperature of −1 to 5 °C. Polar regions, including Antarctica and those portions of North America and Europe that lie within the Arctic circle, constitute some 20 % of the world’s land surface area (Casanueva et al. 2010). The low temperatures and low liquid water availability within such cold habitats make these regions extremely inhospitable to most forms of life (Casanueva et al. 2010). However, psychrophiles and psychrotolerants can grow well in such cold environments. Therefore, they can play an important role in this world. It has also been well documented that they have potential applications in biotechnology (Hua et al. 2010).
Temperature is the defining factor affecting psychrophiles’ growth and survival. Consequently, it is of considerable significance to know how the psychrophiles survive in their native environments and how they adapt to temperatures exceeding their maximal growth temperature. It has been well documented that induced stress tolerance is related to concomitant synthesis of heat-shock protein, cold-shock protein, antifreeze proteins, ice-binding proteins, glycine, betaine, glycerol, mannitol, sorbitol and unsaturated fatty acids (Deegenaars and Watson 1998; Rossi et al. 2009). The accumulation of these compatible solutes results in a reduction of the freezing point of the cytoplasmic aqueous phase and might also directly stabilize cytoplasmic macromolecules, particularly enzymes. Trehalose is widely present in bacteria, yeasts, and fungi as well as some insects, invertebrates and plants (Schick et al. 1995). Recent results have shown that trehalose does not only primarily function as a reserve carbohydrate, but also as a highly efficient protectant, enhancing the resistance of cellular components against adverse conditions such as high temperature, freezing, low dehydration, high osmotic pressure and high concentration of ethanol (Chi et al. 2003). However, little has been known about the physiological role of trehalose in psychrophiles (Magan 2007) and the exact mechanism by which trehalose protects the cell remains to be elucidated. In our previous studies (Song et al. 2010a), a psychrotolerant yeast Guehomyces pullulans 17-1 was isolated from sea sediment in Antarctica. It was found that it could yield both extracellular and cell-bound β-galactosidase (Song et al. 2010b). In this study, Tps1 activity and trehalose content of the yeast cells grown at different temperatures were examined. After the TPS1 gene trehalose-6-phosphate synthase was cloned from the psychrotolerant yeast, the transcriptional level of the TPS1 gene in the cells grown at different temperatures was also examined. This is the first time to investigate how Tps1 activity, trehalose contents and expression of TPS1 gene in the psychrotolerant yeast grown at different temperatures are changed and such investigation is helpful to understand how the psychrotolerant yeasts adapt to changes in surrounding temperature.
Materials and methods
Microorganisms and media
The psychrotolerant yeast G. pullulans 17-1 (the culture collection number is 2E00825 at the Marine Culture Collection of China) used in this study was isolated from sea sediment in Antarctica (ZS1: S69◦22′22′′, salt 3.0 % and temperature 1.5 °C; the samples were collected on 9 March 2007) (Song et al. 2010a). The yeast strain grew the best and produced high levels of both extracellular and cell-bound β-galactosidase at 15 °C (Song et al. 2010a). The yeast strain was cultivated and maintained on YPD medium which contained 2.0 % (w/v) glucose, 2.0 % (w/v) polypeptone, 1.0 % (w/v) yeast extract, and 2.0 % (w/v) agar. The Escherichia coli strain used in this study was DH5a [F − endA1 hsdR17(rK − /mK +) supE44 thi − 1k − recA1 gyr 96Δ lacU169(u80lac-ZΔM15)] kept in this laboratory and was grown in 5.0 mL of Luria broth (LB) at 37 °C overnight. The E. coli transformants were grown in 5.0 mL of LB medium with 100 μg/mL of ampicillin at 37 °C overnight.
The cell cultivation at different temperatures
The yeast cells of G. pullulans 17-1 were grown in 5.0 mL of YPD medium at 15 °C and 180 rpm for 24 h. When cell density of the yeast culture reached OD600nm = 4.0, 1.0 mL of the culture was transferred to 50.0 mL of new YPD medium and the new culture was grown at 15 °C and 180 rpm for 48 h. 5.0 mL of the yeast culture was harvested and washed by centrifugation at 4 °C and 5,000×g for 10 min. 5.0 mL of the washed yeast cells was transferred to 50.0 mL of new YPD medium and the mixture was cultivated at 10, 15, and 25 °C and 180 rpm for 48 h. During the cultivation, the yeast cells were collected and washed at the interval of 6 or 12 h for determination of trehalose contents and Tps1 activity as described below. At the same time, cell density (OD600nm) of the yeast cultures was also measured using spectrophotometer.
Trehalose extraction and quantitative determination
Trehalose in the yeast cells was extracted with 0.5 M trichloroacetic acid as described by Chi et al. (2003) and trehalose content in the extract was assayed by the Anthrone Method (Stewart 1982).
Measurement of cell dry weight
The yeast cells from 5.0 mL of culture were harvested and washed three times with distilled water by centrifugation at 5,000×g for 10 min. Then, the cells in the tube were dried at 100 °C until the cell dry weight was constant.
Preparation of intracellular enzymes of the yeast
The washed cells were resuspended in 10.0 mL of disruption buffer (0.06 M Na2HPO4·7H2O, 0.04 M NaH2PO4·H2O, 0.01 M KCl, 0.001 M MgSO4·7H2O, pH 7.0). The cell suspension was mixed with 19 mL of glass beads (0.25–0.30 mm diameter). The mixture was shaken in a Merckenschlager cell homogenizer (Braun-Melsungen, Germany) with CO2 cooling for 7 min. The disrupted cells were centrifuged at 4 °C and 12,000×g for 20 min and the supernatants obtained were used as the intracellular crude enzymes. Total protein quantity in the supernatant was determined using Coomassie brilliant blue assay (Bradford 1976).
Measurement of Tps1 activity
Tps1 activity in the supernatant obtained above was measured as described by Hottiger et al. (1987). 0.24 mL of the supernatant and 0.16 mL of the mixture containing 0.05 mM Hepes–KOH (pH 7.0), 5.0 mM UDP-glucose, 10.0 mM 6-phosphate-glucose and 12.5 mM MgCl2 were incubated at 22 °C for 20 min. The reaction was stopped at 100 °C for 5.0 min. After cooling, the heated mixture was mixed with 0.6 mL of HCl solution (final HCl concentration was 100.0 mM) and the mixture was heated again at 100 °C for 10 min. After cooling, the mixture was mixed with 0.6 mL of NaOH solution (final NaOH concentration was 150.0 mM) and heated at 100 °C for 10 min. The above treatment will destroy all the sugars in the mixture except 6-phosphate-trehalose formed during the reaction. 6-phosphate-trehalose formed was quantitatively determined using the Anthrone Method (Stewart 1982). One unit of Tps1 activity was defined as the amount of Tps1 producing 1.0 μM 6-phosphate-trehalose per min under the conditions used in this study. The specific Tps1 activity was expressed as the units per mg of protein.
Isolation of DNA, restriction digestions, and transformation
Yeast genomic DNA for amplification of the gene encoding Tps1 was isolated with TIANamp Yeast Genomic DNA Kits (Tiangen Biotech (Beijing) Co., Ltd.). Restriction endonuclease digestions and DNA ligations were performed according to the manufacturer’s recommendations. E. coli was transformed with plasmid DNA according to Sambrook et al. (1989). E. coli transformants were plated onto LB medium containing 100 μg/mL of ampicillin.
Cloning of the full-length TPS1 gene
The conserved amino acid sequences of the Tps1 from different species of eukaryotic microorganisms were downloaded from GenBank (http://www.ncbi.nlm.nih.gov/) and were used to design the degenerate primers to clone these homologs. Codon usage database of G. pullulans was also taken into account for designing the degenerate primers to clone these homologs. The degenerate sense primer and antisense primer were reDs and reDa (Table 1), respectively. The DNA fragment encoding the conserved amino acid sequence of the Tps1 was PCR amplified from the genomic DNA of G. pullulans 17-1 using the primers reDs and reDa and sequenced. Then, inverse PCR was performed to clone the gene encoding Tps1 according to the methods described by Sambrook et al. (1989). The primers Ose and Oan for inverse PCR were designed according to the partial gene encoding the Tps1 in G. pullulans 17-1 (Table 1). The genomic DNA of G. pullulans 17-1 was digested with HindIII. The DNA fragments were purified with TIANquick Mini Purification Kits [Tiangen Biotech (Beijing) Co., Ltd.]. The DNA fragments were circulated by T4 DNA ligase (MBI) at 22 °C for 4 h. The circulated DNA was purified again with TIANquick Mini Purification Kits. Inverse PCR system was composed of 5.0 μL 10× La Taq buffer (TaKaRa, Japan), 8.0 μL 2.5 mM dNTP, 1.0 μL 20.0 μM of each primer, 1.0 μL the circulated DNA, 0.5 μL La Taq DNA polymerase, and 33.5 μL double-distilled water. Touchdown PCR conditions were 94 °C 8 min; 94 °C 30 s, 66.5–0.5 °C 40 s, 72 °C 2 min 30 s, 20 cycles; 94 °C 30 s, 56.5 °C 40 s, 72 °C 2 min 30 s, 15 cycles, final extension at 72 °C for 15 min. After the PCR products were checked and separated by agarose gel electrophoresis and sequenced, the TPS1 gene in G. pullulans 17-1 was obtained. In order to obtain the full-length of the TPS1 gene including 3′-noncoding and 5′-noncoding regions, genome walking techniques were applied using three forward primers (Z1, Z2, and Z3) and three reverse primers (F1, F2, and F3) (Table 1). After the sequence of the PCR products was analyzed with NCBI ORF finder program and aligned with the known sequences of the genes encoding Tps1 from different fungi by NCBI BLASTn, the full-length of the TPS1 gene in G. pullulans 17-1 was obtained and named GPTPS1 gene (accession number: JX046041).
The cells of G. pullulans 17-1 for RNA extraction were prepared and total RNA in the collected cells was extracted using RNAiso Reagent (TaKaRa) and the contaminating DNA was removed by DNAase I. RNA content in the sample was measured at 260 nm and the RNA amount was adjusted to 1.0 μg per 50 μL. ORF of the GPTPS1 gene was obtained by PCR using the primers rcs and rca (Table 1) and the PCR product was sequenced.
Phylogenetic analysis
After the amino acid sequences of the Tps1s of Saccharomyces cerevisiae (accession number: CAA85083), Zygosaccharomyces rouxii (accession number: CAAK69413), Yarrowia lipolytica (accession number: CAA09463), Aspergillus niger (accession number: Q00075), Schizosaccharomyces pombe (accession number: CAB95998), Cryptococcus neoformans var. grubii (accession number: AAT40476.1), and G. pullulans 17-1 (accession number: JX04601) were downloaded from NCBI, a phylogenetic tree of the Tps1s was constructed by MEGA 4.0. The outgroup we used was Emericella nidulans (accession number: CAAO72737).
Fluorescent RT-PCR
The cultures cultivated at different temperatures for 24 h were centrifuged at 5,000×g and 4 °C for 10 min and the pellets obtained were used as the samples for total RNA isolation. Total RNA was purified by a RNAprep pure Tissue Kit (TIANGEN, China). Reverse transcription was performed using PrimeScript RT reagent Kit (TaKaRa, Japan) according to the manufacturer’s protocol. The fluorescent real-time RT-PCR assay was carried out in 8-strip tubes (Aikb, China) in a 20.0-μL reaction volume per well containing 9 μL of SYBR Green PCR Master Mix (Applied Biosystems, USA), 0.5 μL of 1:20 diluted cDNA, and 50 mM of each forward and reverse primer. The primers Rts and Rta used for fluorescent real-time PCR were designed according to the GPTPS1 gene sequence (accession number: JX046041) in G. pullulans 17-1 (Table 1). The primers 26Sf and 26Sr were designed according to the 26S rDNA gene sequence in G. pullulans 17-1 (GenBank accession no.: EU596445) (Table 1). The fluorescent real-time PCR was performed on a 7500 Real-Time PCR System (Applied Biosystems, USA). The thermal profile was one initial cycle of 2 min at 95 °C, followed by 40 cycles of 20 s at 95 °C, 20 s at 60 °C, and 20 s at 72 °C. The fluorescent real-time RT-PCR data obtained were analyzed with 7500 System SDS Software v1.4.0 (Applied Biosystems). The comparative Ct method (Livak and Schmittgen 2001) was used to analyze the relative expression level of the gene in the cultures grown at different temperatures. The Ct for the target amplified the GPTPS1 gene and the Ct for the internal control 26S rDNA was determined for each sample. The Ct values for each set of three reactions were averaged for all the subsequent calculations. Each datum represents the average of three experiments. The relative expression quantity was calculated using the formula RATE = 2−ΔΔCt. The sample data obtained from the real-time PCR analysis were subjected to one-way analysis of variance (Wu and Hamada 2000). P values were calculated by Student’s t test (n = 3). P values less than 0.05 were considered as statistically significant. Statistical analysis was performed using SPSS 19 for Windows (SPSS Inc., Chicago, IL, USA).
Results and discussion
The changes in cell growth and trehalose contents in the cells cultivated at different temperatures
In our previous studies (Song et al. 2010a), it was found that the cells of G. pullulans 17-1 isolated from sea sediment in Antarctica grew the best at 15 °C. As shown in Fig. 1, it also could grow at 25 and 10 °C. It has been reported that trehalose can play an important role in stress response in microorganisms (Chi et al. 2003). However, little has been known about the role of trehalose in adaption to stress in the psychrotolerant yeast, such as G. pullulans 17-1 used in this study. Therefore, trehalose contents in G. pullulans 17-1 cultivated at different temperatures were examined. The results in Fig. 2 showed that when it grew at 25 °C, trehalose contents of the yeast cells were higher than those of the yeast cells grown at 15 °C. In contrast, when it grew at 10 °C, trehalose contents of the yeast cells were lower than those of the yeast cells grown at 15 °C (Fig. 2). These results may reveal that trehalose plays an important role in thermotolerance of G. pullulans 17-1, but plays less important role in cold tolerance of the yeast. This may also suggest that the psychrotolerant yeast is more sensitive to high temperature (above 20 °C) than to low temperature (below 10 °C) (Fig. 2), so that more trehalose needs to be synthesized to protect the cells grown at high temperature.
Comparing the response to heat stress in six yeast species which include Mrakia stokesii, Mrakia frigida, Mrakia gelida, Leucosporidium antarcticum, Leucosporidium fellii, and Leucosporidium scottii isolated from Antarctica, relatively high endogenous levels of trehalose, also elevated upon a heat shock, were exhibited by all the species at 25 °C and the greatest increase was observed in L. scottii with a 3-fold increase in trehalose after a heat shock at 25 °C for 3 h (Deegenaars and Watson 1998). Unfortunately, the response to cold stress in the six yeast species is still unknown. However, comparisons of Hebeloma spp. from Arctic and temperate regions have indicated that substantial accumulation of trehalose occurred in the Arctic species when grown at low temperature (Tibbet et al. 1998). Similarly, Humicola marvini and Mortierella elongate, psychrophiles isolated from Signy Island, Antarctica and grown at 5 and 15 °C, accumulated trehalose intracellularly to a significantly greater extent (75 % more for the latter species) at 5 than 15 °C (Weinstein et al. 2000). Studies by Weinstein et al. (1997) also suggested that sugar alcohols such as mannitol as well as trehalose were increased in isolates of H. marvini, compared with the non-psychrophilic species H. fuscoatra. It has been well known that trehalose functions as a thermoprotectant by stabilizing cell membranes and accumulates markedly in cells exposed to a non-lethal heat shock (Walker and Dijck 2006). The differences may be due to different genetic background and regulation modes of different yeast species. This meant that the response to heat shock in G. pullulans 17-1 was similar to that in M. stokesii, M. frigida, M. gelida, L. antarcticum, L. fellii, and L. scottii isolated from Antarctica, but was different from that in H. marvini and M. elongate.
The changes in Tps1 activity in the cells cultivated at different temperatures
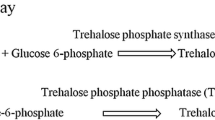
In general, in yeasts, trehalose is synthesized in a two-step process. First, trehalose-6-phosphate (T-6-P) is formed from uridine diphosphate glucose (UDPG) and α-glucose-6-phosphate by T-6-P synthase (Tps1). Then, T-6-P is dephosphorylated to trehalose by T-6-P phosphatase (Tps2) (Chi et al. 2009). Therefore, Tps1 activity of G. pullulans 17-1 grown at different temperatures was tested. The results in Fig. 3 showed that when G. pullulans 17-1 grew at 25 °C, Tps1 activity of the yeast cells was higher than that of the yeast cells grown at 15 °C. In contrast, when G. pullulans 17-1 grew at 10 °C, Tps1 activity of the yeast cells was lower than that of the yeast cells grown at 15 °C (Fig. 3). These results were in agreement with those in Fig. 2. However, the most dramatic temperature downshift response in the fungi isolated from fellfield soil in the maritime Antarctic is the induction of the trehalose-synthesizing enzymes Tps1 and Tps2 in the near-freezing temperature and the resulting production of large amounts of trehalose (Weinstein et al. 2000). This meant that trehalose accumulation and Tps1 activity in G. pullulans 17-1 grown at lower temperature were different from those in the fungi isolated from fellfield soil in the maritime Antarctic.
Cloning and characterization of the TPS1 gene
It has been reported that trehalose-6-phosphate synthase (Tps1) is a subunit of trehalose synthase complex in fungi and it plays a key role in the biosynthesis of trehalose (Chi et al. 2009). In order to know how the TPS1 gene is expressed in G. pullulans 17-1 grown at different temperatures, the GPTPS1 gene including 3′-nonconding region and 5′-noncoding region in G. pullulans 17-1 was cloned as described in “Materials and methods”. The results in Fig. 4 indicated that open reading frame of the GPTPS1 gene contained 1950 bp encoding a polypeptide of 530 amino acids with a predicted molecular mass of 59.8 kDa and the gene was disrupted by 7 introns. After analysis of its promoter, it was found that the promoter contained one TATA box, one STRE (C4T) and two heat-shock elements (AG4) (Fig. 4; Table 2). The ZrTPS1 gene cloned from Z. rouxii consists of 1572 nucleotides, containing an ORF of 1476 bp, which encodes a polypeptide of 492 amino acids with a molecular mass of 56.0 kDa (Kwon et al. 2003). The MaTPS1 cDNA amplified from filamentous fungi Metarhizium anisopliae is composed of 1836 nucleotides encoding a protein of 517 amino acids with a molecular mass of 58.0 kDa and the conserved predicted amino acid sequence of the MaTps1 aligned with those of homologous ORFs from several other filamentous fungi showed that it belonged to glycosyltransferase family 20 (GT-20) of carbohydrate active enzymes (Cai et al. 2009). CCCCT (C4T) is regarded as an essential component of this stress–response sequence and a possible Migl protein binding site in the promoter of the stress-inducible gene in S. cerevisiae (Bell et al. 1992) and AG4 is also thought to be a heat-shock element in S. cerevisiae. C4T, AG4 and possible Migl protein binding site also occurs in the promoter of the TPS1 gene cloned from Candida albicans (Zaragoza et al. 1998) and other fungi (Table 2). However, such elements are not present in the promoter of the TPS1 gene cloned from Saccharomycopsis fibulegira A11 (Table 2). It was true that neither activation of Tps1 activity nor changes in trehalose content or the expression of the TPS1 gene was observed under the stress exposure of S. fibuligera A11 cells (Chi et al. 2009).
The deduced amino acids of the GPTps1 contained one N-glycosylation site (Fig. 4) and its amino acid sequence was closely related to that of Tps1 produced by C. neoformans var. grubii, but was far related to that produced by S. cerevisiae, Z. rouxii, and Y. lipolytica (Fig. 5).
Transcript level of the GPTPS1 gene in the cells cultivated at different temperatures
It has been well documented that trehalose accumulation and trehalose-synthesizing gene expression in S. cerevisiae cells are greatly enhanced when they are stressed by high temperature, low temperature, high ethanol concentration and high oxidative conditions (Herdeiro et al. 2006; Benaroudj et al. 2001). The stress-responsive element which contains CCCCT sequence in the promoter of the gene encoding Tps1 in S. cerevisiae is responsible for the stress response (Chi et al. 2006; Kobayashi and McEntee 1993). Therefore, the transcript levels of the GPTPS1 gene were analyzed using real-time PCR as described in “Materials and methods”. It was found that the expression level (832.8 % compared to the expression level of 26S rDNA) of the GPTPS1 gene in the yeast cells grown at 25 °C was much higher than that (342.2 % compared to the expression level of 26S rDNA) of the GPTPS1 gene in the yeast cells grown at 15 °C (Fig. 6). However, the expression level (100.0 % compared to the expression level of 26S rDNA) of the GPTPS1 gene in the yeast cells grown at 10 °C was much lower than that (342.2 % compared to the expression level of 26S rDNA) of the GPTPS1 gene in the yeast cells grown at 15 °C (Fig. 6). All the results were consistent with those shown in Figs. 2 and 3. Indeed, as shown in Fig. 6 and Table 2, one copy of STRE and two copies of heat-shock element were present in its 5′-upstream sequence of the GPTPS1 gene. This result also confirmed that the expression of the GPTPS1 gene was regulated by temperature at transcriptional level.
In S. cerevisiae, the TPS1 gene is expressed at very low levels, and increases dramatically with heat shock. In S. cerevisiae, upon temperature downshift to 10, 4 or 0 °C, the transcript levels of the TPS1 gene also increase dramatically so that at 0 °C there is a 20-fold increase in the levels of the TPS1 mRNA 15–20 h after temperature shift (Bell et al. 1992). This suggests that biosynthesis and accumulation of trehalose might be necessary for cold tolerance and energy preservation in S. cerevisiae (Murata et al. 2006). The transcription of tpsB gene in A. niger is enhanced strongly upon heat shock, which agrees with the presence of several copies of a C4T stress-responsive element in its 5′-upstream sequences (Wolschek and Kubicek 1997). But in Z. rouxii (Kwon et al. 2003), the ZrTPS1 gene is highly and constitutively expressed, and fluctuates slightly after heat shock, but salt stress reduced the expression of the ZrTPS1 gene. This may imply that regulation of trehalose biosynthesis and function of trehalose in the psychrotolerant yeast G. pullulans 17-1 are significantly different from those in any other fungi.
Transcriptional regulation and activation of the Tps1 gene in S. cerevisiae at 0 °C is dependent on the Msn2, 4 pathway, whereby Msn2 and Msn4 are transcription factors which bind STREs and up-regulate transcription (Al-Fageeh and Smales 2006).
All the results mentioned above showed that expression of the GPTPS1 gene was in agreement with the changes in Tps1 activity and trehalose content in the psychrotolerant yeast G. pullulans 17-1 used in this study. However, it is still completely unknown how transcription of the GPTPS1 gene in G. pullulans 17-1 is regulated at different temperatures. This further work is being done in this laboratory.
References
Al-Fageeh MB, Smales CM (2006) Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochem J 397:247–259
Bell W, Klaassen P, Ohnacker M, Boller T, Herweijer M, Schoppink P, Vanderzee P, Wiemken A (1992) Characterization of the 56-kDa subunit of yeast trehalose-6-phosphate synthase and cloning of its gene reveal its identity with the product of CIF1, a regulator of carbon catabolite inactivation. Eur J Biochem 209:951–959
Benaroudj N, Lee DH, Goldberg AL (2001) Trehalose accumulation during cellular stress protects cells and cellular proteins from damage by oxygen radicals. J Biol Chem 276:24261–24267
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–253
Cai Z, Peng G, Cao Y, Liu Y, Jin K, Xia Y (2009) Trehalose-6-phosphate synthase 1 from Metarhizium anisopliae: clone, expression and properties of the recombinant. J Biosci Bioeng 107:499–505
Casanueva A, Tuffin M, Cary C, Cowan DA (2010) Molecular adaptations to psychrophily: the impact of ‘omic’ technologies. Trend Microbiol 18:374–381
Chi ZM, Liu J, Ji J, Meng Z (2003) Enhanced conversion of soluble starch to trehalose by a mutant of Saccharomycopsis fibuligera sdu. J Biotechnol 102:135–141
Chi ZM, Liang LK, Zhu KL, Zhang FL (2006) Advances in metabolism and regulation of trehalose in yeasts. J Ocean Univ Chin 26:209–219
Chi ZM, Zhe Chi Z, Liu GL, Wang F, Ju L, Zhang T (2009) Saccharomycopsis fibuligera and its applications in biotechnology. Biotechnol Adv 27:423–431
Deegenaars ML, Watson K (1998) Heat shock response in psychrophilic and psycrotrophic yeast from Antarctica. Extremophiles 2:41–49
Gerday C (2000) Cold-adapted enzymes: from fundamentals to biotechnology. TBTECH 18:103–107
Herdeiro RS, Pereira MD, Panek AD, Eleutherio ECA (2006) Trehalose protects Saccharomyces cerevisiae from lipid peroxidation during oxidative stress. Biochim Biophys Acta 1760:340–346
Hottiger T, Schmutz P, Wiemken A (1987) Heat-induced accumulation and futile cycling of trehalose in Saccharomyces cerevisiae. J Bacteriol 169:5518–5522
Hua MX, Chi Z, Liu GL, Buzdar MA, Chi ZM (2010) Production of a novel and cold-active killer toxin by Mrakia frigida 2E00797 isolated from sea sediment in Antarctica. Extremophiles 14:515–521
Kobayashi N, McEntee K (1993) Identification of cis and trans components of a novel heat shock stress regulatory pathway in Saccharomyces cerevisiae. Mol Cell Biol 13:248–256
Kwon HB, Yeo ET, Hahn SE, Bae SC, Kim DY, Byun MO (2003) Cloning and characterization of genes encoding trehalose-6-phosphate synthase (TPS1) and trehalose-6-phosphate phosphatase (TPS2) from Zygosaccharomyces rouxii. FEMS Yeast Res 3:433–440
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using realtime quantitative PCR and the 2−ΔΔCt method. Methods 25:402–408
Magan M (2007) Fungi in extreme environments. In: The Mycota IV Kubicek CP and Druzhinina IS (eds) Environmental and microbial relationships, 2nd edn. Springer, Berlin, pp 85–103
Murata Y, Homma T, Kitagawa E, Momose Y, Sato MS, Odani M, Shimizu H, Hasegawa-Mizusawa M, Matsumoto R, Mizukami S, Fujita K, Parveen M, Komatsu Y, Iwahashi H (2006) Genome-wide expression analysis of yeast response during exposure to 4 °C. Extremophiles 10:117–128
Rossi M, Buzzini P, Cordisco L, Amaretti A, Sala M, Raimondi S, Ponzoni C, Pagnoni UM, Matteuzzi D (2009) Growth, lipid accumulation, and fatty acid composition in obligate psychrophilic, facultative psychrophilic, and mesophilic yeasts. FEMS Microbiol Ecol 69:363–372
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Beijing, pp 367–370 (Chinese translated edn)
Schick I, Haltrich D, Kulbe KD (1995) Trehalose phosphorylase from Pichia fermentans and its role in the metabolism of trehalose. Appl Microbiol Biotechnol 43:1088–1095
Song CL, Chi ZM, Li J, Wang XH (2010a) β-Galactosidase production by the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica and lactose hydrolysis. Bioprocess Biosyst Eng 33:1025–1031
Song CL, Liu GL, Xu JL, Zhen-Ming Chi ZM (2010b) Purification and characterization of extracellular β-galactosidase from the psychrotolerant yeast Guehomyces pullulans 17-1 isolated from sea sediment in Antarctica. Proc Biochem 45:954–960
Stewart PR (1982) In: Prescott DM (ed) Methods in cell biology, vol 12. Academic Press, London and New York, pp 111–147
Tibbet M, Sanders FE, Cairney JWG (1998) The effect of temperature and inorganic phosphorous supply on growth and acid phosphatase production in arctic and temperate strains of ectomycorrhizal Hebeloma spp. Mycol Res 102:129–135
Walker GM, Dijck PV (2006) Physiological and molecular responses of yeasts to the environment. In: Graham H. Fleet (eds) The Yeast Handbook Amparo Querol, Yeasts in food and beverages © Springer, Berlin Heidelberg, pp 111–152
Weinstein RN, Palm ME, Johnstone K, Wynn-Williams DD (1997) Ecological and physiological characterization of Humicola marvinii, a new psychrophilic fungus from fellfields in the maritime Antarctic. Mycologia 89:706–711
Weinstein RN, Montiel PO, Johnstone K (2000) Influence of growth temperature on lipid and soluble carbohydrate synthesis by fungi isolated from fellfield soil in the maritime Antarctic. Mycologia 92:222–229
Wolschek MF, Kubicek CP (1997) The filamentous fungus Aspergillus niger contains two “differentially regulated” trehalose-6-phosphate synthase-encoding genes, tpsA and tpsB. J Biol Chem 272:2729–2735
Wu JCF, Hamada M (2000) Experiments planning analysis and parameter design optimization. Wiley, New York, pp 23–34
Zaragoza O, Blazquez MA, Gancedo C (1998) Disruption of the Candida albicans TPS1 Gene encoding trehalose-6-phosphate synthase impairs formation of hyphae and decreases infectivity. J Bacteriol 180:3809–3815
Acknowledgments
The authors would like to thank the State Oceanic Administration People’s Republic of China for providing financial support to carry out this work. The grant number is 201005032.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Driessen.
Rights and permissions
About this article
Cite this article
Zhang, F., Wang, ZP., Chi, Z. et al. The changes in Tps1 activity, trehalose content and expression of TPS1 gene in the psychrotolerant yeast Guehomyces pullulans 17-1 grown at different temperatures. Extremophiles 17, 241–249 (2013). https://doi.org/10.1007/s00792-013-0511-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00792-013-0511-2