Abstract
Estimation of the energy capacity of a microbial cell mass on the basis of its lipid content and elemental composition can be used for the comparative evaluation of different microbial sources of biodiesel. Lipomyces starkeyi cell mass concentration reached 94.6 g/L with 37.4 % of lipids in a fed-batch process using xylose and urea as substrates. The fatty acid composition of the yeast oil was quite similar to that of palm oil. L. starkeyi converted more than 80 % of the energy contained in xylose into cell mass energy yield. The approach used in this study makes it possible to determine the energy of a cell mass by its elemental composition. A heat of combustion (Q c) of 25.7 (kJ/g) was obtained for the cell mass after 142 h of fed-batch cultivation, which represents approximately 56 % of the energy content of diesel oil (45.4 kJ/g). The Q c of the triacylglycerols produced was 48.9 (kJ/g), indicating the potential of this oleaginous yeast for biodiesel production. Our work developed here provides a simple and efficient tool for characterization of this cell mass to further our understanding of its use as a feedstock for bioenergy production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The production of microbial oils offers many advantages over that of plant oils, such as short life cycle, lower labor requirements, independence of season and climate, easier scale-up and avoidance of competition with food production [1]. The oleaginous yeasts have the remarkable capability to be cultivated on a plethora of renewable- or waste-type materials in various fermentation configurations (e.g., xylose-based materials, hemi-cellulose hydrolysates, (raw) glycerol, sewage sludge, various types of whey permeate, etc.), while they present a significantly maximum higher specific growth rate compared to molds and algae, therefore yeast lipids can be considered as potential starting materials for the synthesis of this second generation biodiesel [2]. To make microbial lipid-based biofuel and oleochemicals production sustainable, lignocellulosic materials such as agricultural residues and energy crops must be used as inexpensive and abundant carbon sources [3]. Complete hydrolysis of lignocellulosic materials produces a variety of soluble, fermentable sugars that consist mainly of pentoses (e.g., xylose and arabinose) and hexoses (e.g., glucose and mannose) [4]. The highly efficient utilization of lignocellulosic biomass for the production of valuable chemicals depends on obtaining microorganisms that are capable of using pentoses, which are difficult to metabolize by most microorganisms, as a carbon source by screening the natural surroundings or by mutagenesis using traditional or genetic engineering methods. As a result, finding a pentose fermenting strain is critical for any bioconversion that uses lignocellulosic biomass as feedstock [5].
The application of mass and energy balances has not been fully explored in fermentation processing due to the complexity of cell growth and product formation. In some cases, material and energy balances were only roughly estimated in growth processes [6]. Knowing the precise energy characteristics for efficient growth, including cell mass energy yield (η) and heat of combustion (Q c), is very important. In general, the energy contents of microbial cells can be assumed to be constant, although this is not the case with oleaginous yeasts.
To calculate η, a precise elemental composition of the microbial cell dry weight (CDW) is necessary. Combustion heat of CDW indicates the energy content of the microbial cells intuitively and accurately, which is the most essential factor for the selection of promising biodiesel sources [1]. Nevertheless, a bomb calorimeter, which is not available in many laboratories, is needed for analysis of the Q c of the cell mass [7]. Another method to obtain Q c is an equation using the elemental components of cells as mass variables [8, 9], which makes energy analysis of fermentation easier, without the need for laborious and expensive calorimetric measurement of combustion heat.
The present work studied the elemental composition and reductivity of the dry cell mass of Lipomyces starkeyi with the aim of finding possible constancies and interrelations between these characteristics and the lipid content of the cell mass to establish the cell mass energetic yield (η). The relationship between the lipid content and the heat of combustion of the dry cell mass was also studied.
Fundamentals of mass and energy balances
The elemental balance equation of microbial growth can be written as
where C W H x O Y N Z , H g O h N i , CH α O β N δ and fC j H K O l N m denote an elemental composition of substrate, nitrogen, cell mass and product, respectively. For oleaginous yeast, the product (lipids) is inside the cells; therefore CH α O β N δ is related to the cell mass plus lipids and fC j H K O l N m is not used in this study. The carbon balance in Eq. (1) can be written as
where c is the cell mass carbon yield and f is the product carbon yield.
The balance of both sides of Eq. (1) with respect to the available electrons is given by
where
The degree of reductance (γ) is the number of equivalent available electrons/g carbon atom based on carbon (4), hydrogen (1), oxygen (−2) and nitrogen (−3) atoms. A value of −3 is used for nitrogen since the γ of nitrogen in cell mass and ammonia is −3.
The elemental composition of freeze-dried cells was determined to estimate the η. η is the term used to represent the energetic yield for cell mass formation [10] and is the ratio of the heat produced by oxidation of the cell mass to that of the substrate used, where oxidation results in the production of CO2, H2O and NH3. According to Erickson et al. [11], η may be correlated with cell mass yield, Y X/S, as follows:
where σ S and σ B are the weight fractions of carbon in the organic substrate and cell mass, respectively.
Heijnen and Roels [12] reported that the average values of γ B and σ B calculated from the data available in the literature were 4.17 and 0.489, respectively, and the coefficient of variation is less than 5 % for both γ B, and σ B. Therefore, γ B and σ B can be assumed to be constant for nonoleaginous microorganisms, whereas this assumption is not valid for oleaginous microorganisms, since the thermodynamic energy content of oleaginous microorganisms increases with accumulation of intracellular lipids [13]. For most microorganisms, Eq. (7) becomes
In accordance with the thermodynamic analysis of microbial growth [14], phosphorous was not considered in this work. γ of the organic substance with the chemical formula C5H10O5 (xylose) was calculated based on Eq. (3).
Heat of combustion (kJ/g) of cell mass can be determined not only by bomb calorimetry, but also by calculation based on the elemental composition of the cells according to Eq. (9) which is based on Dulong’s formula and usually gives higher theoretical heating values within a 5 % margin of error [15]:
where C, H, O and N are the weights of the corresponding elements per g cell mass.
The relationship between lipid content and Q c of the cells can also be simulated. This approach is applied here to determine the energy of the cell mass by its elemental composition without the laborious and expensive calorimetric measurement of combustion heats.
Materials and methods
Organism, media and chemicals
The oleaginous yeast L. starkeyi DSM 70296 was used throughout this study. Agar slants (YPD medium) were stored in a refrigerator and propagated monthly or stored through cryopreservation at −80 °C with 10 % (v/v) glycerol. The composition of the inoculum and cultivation medium was (per liter): 60 g of xylose, 1.03 g of urea, 1 g of Na2HPO4, 1 g of KH2PO4, 0.4 g of Mg2SO .4 7H2O, 0.04 g of CaCl .2 2H2O, 0.08 g of ZnSO .4 7H2O, 0.001 g of CuSO .4 5H2O, 0.001 g of CoCL .2 6H2O and 0.005 g of (NH4)2Mo2O7 (pH 5.5). The carbon-to-nitrogen (C/N) ratio was fixed at 50. To avoid darkening of the medium due to the reaction of the sugars (caramelization and Maillard reactions), the xylose solution was sterilized in a separate flask. The inoculum was prepared through two successive cell propagations in liquid media at 28 °C and 150 rpm in an orbital shaker. In the first, the cells were incubated for 48 h and in the second, for 30 h, where a CDW of 10 g/L (equivalent to 1 × 108 cells/mL) was obtained.
The cultivation was carried out in a 3.0 L bioreactor (BioFlo/CelliGen 115, New Brunswick Scientific, USA) with an initial working volume of 1.2 L, an aeration rate of 1 vvm, a temperature of 28 °C and an agitation speed of 400 rpm. The pH was maintained at 5.5 through the automatic addition of 2 M NaOH. Aliquots were collected at various intervals and stored at −20 °C until their analysis. Concentrated xylose solution (600 g/L) was used for the fed-batch mode and the concentration of the nutrient solution (urea plus salts) was ten times higher than that of the culture medium. The fed-batch culture was carried out with three feeding pulses, using a peristaltic pump to increase the xylose concentration to 60 g/L immediately after the residual sugar concentration dropped to 0–5 g/L. The other nutrients (urea and salts) were fed in at the same time to achieve a C/N ratio of 50 at each feeding pulse.
Analytical methods
The optical density of the cell was measured at 600 nm with a nanophotometer (Implen GmbH, Germany). The cell dry weight (CDW) was determined after lyophilization of the harvested cells. The cellular concentration was determined using a Neubauer-improved chamber, and the number of nonviable cells (dead cells) was obtained through measurement with methylene blue. Xylose was measured by ion chromatography (Metrohm, Switzerland) according to Tapia et al. [16]; however, during the fermentations, the total reducing sugars were assayed as described by Somogyi-Nelson [17, 18]. Urea was measured using an enzymatic colorimetric method. In short, urea in the sample was hydrolyzed enzymatically into ammonia (NH4 +) and carbon dioxide (CO2). The ammonia ions formed reacted with salicylate and hypochloride (NaClO) in the presence of nitroprusside to form a green indophenol. The absorbance of the samples and that of the reference at 580 nm were compared to a blank. For lipid analysis, the cells were treated with hydrochloric acid (2 M), incubated at 80 °C for 1 h and centrifuged at 6000g and 4 °C for 15 min, and the lipids were then extracted and quantified using Bligh–Dyer’s method. The crude lipid was initially converted into fatty acid methyl ester (FAME) [19] and then analyzed using a GC/MS gas chromatography (Shimadzu, Japan) system according to Tapia et al. [16]. Kinetic parameters of cell mass yield (Y X/S, gCDW/gsugar), lipid yield (Y L/S, glipid/gsugar), maximum specific growth rate (μ max), volumetric substrate consumption (Q s), specific substrate consumption rate (q s), specific lipid accumulation rate (q p) and maintenance coefficient (m s) were calculated according to Stephanopoulos et al. [20]. All of the analyses were performed in triplicate and error bars denote the standard deviation.
Results
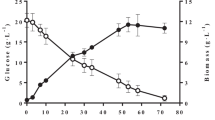
Fed-batch cultivation by L. starkeyi
In our early work, we optimized the lipid cultivation conditions and achieved high lipid content and cell density with a mixture of glucose:xylose (30:70, w/w) in a fed-batch process [21]. In the present study, we wanted to evaluate the η obtained from a fed-batch cultivation using xylose as substrate. The results for xylose and urea consumption, cell growth and lipid accumulation are shown in Fig. 1. After the first feeding pulse, an increase in cell mass concentration of 58.3 % is observed. After the third feeding pulse, a stationary growth phase is observed with an increase in cell mass concentration of greater than 18 %, reaching 94.6 g/L with 37.4 % lipids (35.4 g/L) at the end of cultivation (148 h). The lipids produced mainly include palmitic acid (42.3 %) and oleic acid (38.6 %). Maximum cell mass yield (Y X/S) and lipid yield (Y L/S) were achieved at 70 h of cultivation (0.771 and 0.173 g/g, respectively), highlighting the potential for lipid production by L. starkeyi (Table 2). Cell mass and lipid productivities reached 0.638 and 0.227 g/L h, respectively, at 148 h.
A maximum specific growth rate of 0.077 was observed in batch phase, where ms of 0.03 g/gCDW h was also obtained. For intervals between the feeding pulses, the estimated m s was zero. The volumetric xylose consumption rate (Q s) was higher at the first and second feeding pulse intervals. The highest specific lipid accumulation (q p) of 0.018 g/gCDW h was obtained after the second feeding pulse of the fed-batch cultivation.
Cell mass energetic yield of fed-batch lipid cultivation
Results of the cell mass elemental analysis of the fed-batch culture are summarized in Table 1. The cell mass elemental composition changed upon lipid accumulation. N and O contents declined continuously, while C and H contents increased during the fed-batch cultivation. At 31 h, C and N contents were 45.4 and 4.4 %, respectively. Based on the elemental composition data, chemical formulas were obtained for L. starkeyi cell mass at different times during the fed-batch cultivation. The γ of the cell mass was calculated using Eq. 5 and more reduced cell mass was produced during the cultivation. The increase in the weight fraction of carbon in the cell mass (from 0.45 to 0.55) as a function of lipid content is shown in Fig. 2. η was estimated according to Eq. (7) and is shown in Table 1. Equation (7) is very useful; however, it is important to calculate σ B γ B, and thus to acquire the η value. Energy content (σ B γ B), which requires the elemental composition data, can be estimated by lipid content. When σ B γ B is plotted with the lipid content data from fed-batch cultivation, a straight line is obtained. In Fig. 2, the energy content (σ B γ B) of our oleaginous yeast is shown to increase with the accumulation of intracellular lipids. Least-squares analysis gave a linear correlation between lipid content, L (%), and σ B γ B as follows:
According to the linear relationship in Fig. 2 and Eq. 7, η could also be written as Eq. 11. The relative error between η obtained from Eq. 7 or that obtained from Eq. 11 was less than 6 %. Thus, energy efficiency could be obtained based on lipid experimental data, while elemental composition of cell mass was unnecessary. According to Eq. 1, the overall energy efficiency of the lipid production shown in Fig. 1 was 0.53, similar to that based on Eq. 2.
Nevertheless, when Eq. 11, established by our experimental data, was utilized to estimate the η of L. starkeyi, the values obtained were similar to those calculated based on elemental composition with relative errors less than 5.5 %.
Combustion heat of cell mass
Using elemental data, the Q c of yeast cells was estimated according to Eq. 9. A maximum Q c value of 25.7 (kJ/g) was obtained after 142 h of cultivation, up to 56 % of the energy content of diesel oil (45.4 kJ/g). Estimating the Q c using Eq. 9 for the triacylglycerols (TAGs) produced, mainly composed of palmitic acid, oleic acid and linoleic acid, gave a value of 48.9 (kJ/g), indicating the potential of this oleaginous yeast for biodiesel production (Table 1). When Q c was plotted with lipid content, Eq. 12 was developed to estimate Q c of cell mass based on lipid content. The Q c calculated by Eq. 12 (Table 1) fit the experimental data quite well according to Fig. 3.
The Q c values estimated by Eq. 9 and those by Eq. 12 were similar with a maximum relative error of 5.1 %. Thus, Q c could be obtained based on the experimental data on lipids
Discussion
One of the main challenges associated with the production of second-generation biodiesel is increasing the microbial conversion of low-cost substrates, such as agricultural residues, lignocellulosic materials and wastes, into lipids. Lipid accumulation in oleaginous yeasts is triggered by a nutrient imbalance between the excess carbon source and the limited concentration of nitrogen source in the culture medium. High cell mass (94.6 g/L) and lipid (37.4 %) concentrations were achieved in the present fed-batch culture using xylose and urea as substrates. Ratledge and Cohen [22] suggested that an oil content of 40 % is probably the optimum amount for maximizing the overall cell mass yield, considering that substrate is used to produce lipids besides the other components of the yeast cell. To achieve higher oil contents, it is therefore not a matter of making more oil but making less oil-free cell mass. High microbial oil concentration and productivity can only be attained if a high yeast cell density is achieved at high productivity during the growth phase [23].
Fed-batch cultivation modes have been widely applied in microbial lipid production. Tapia et al. [16] performed fed-batch cultures by L. starkeyi mutant strains using xylose plus glucose as carbon sources and obtained 86.6 g/L of cells and 58.9 % of lipids (Table 2). Anschau et al. [21] obtained similar results of cell mass and lipid concentrations by L. starkeyi using xylose plus glucose at repeated fed batch. Continuous culture, with dilution rate of 0.03 h−1 presented the highest overall cell mass (0.443 g/g) and lipid yields (0.236 g/g).
Wiebe et al. [24] reported constant and intermittent fed-batch cultivation by Rhodosporidium toruloides using glucose, xylose and arabinose as carbon sources. Lipid production was most efficient with glucose (48–75 % lipids at up to 0.21 g/L h) as the sole carbon source, but high lipid concentrations were also produced by L. starkeyi using xylose (32 %). Ykema et al. [25] studied lipid production using Candida curvatus. A cell concentration of 91.4 g/L and a lipid content of 33 % were observed using a partial recycling method, while both nitrogen and carbon sources were supplied.
High cell mass and lipid yields were obtained using xylose and urea as carbon and energy sources in the present study. Urea is the organic nitrogen source that has received much attention due to its low cost and growth promotion ability [26]. The maximum theoretical stoichiometry for the conversion of glucose to triacylglycerol is 33 % (w/w) without production of any other cell material. In practice, the best conversion of sugar into oil that has been achieved is 0.224 g/g [22]. Our results represent 80 % of the maximum practical value estimated by Ratledge and Cohen [22].
Production cost is the major factor limiting the broader use of microbial lipids, and it can be significantly reduced by improvements in productivity [27]. Our study achieved higher cell mass and lipid productivities than those obtained by other authors using glucose as the carbon source [27, 28]. Taking into account that the initial carbon source concentration in the medium (60 g/L) was the same, our cultivation achieved a higher cell productivity than those of Tapia et al. [16] and Zhang et al. [29], who used xylose:glucose and glucose, respectively (Table 2). Our study presented also higher Y L/S than fed-batch studies which used mixed glucose and xylose as carbon sources [16, 21, 24].
A potential high value-added end use of microbial oil is a substitute for vegetable oils and animal fats in the oleochemical production of cosmetics, pharmaceuticals, paints, lubricants and polymer additives, among other products [23]. Analysis of fatty acid composition revealed that the major constituent fatty acids were oleic acid, palmitic acid and linoleic acid. The fatty acid composition profile obtained was quite similar to that for palm oil [30], indicating that the lipids produced by L. starkeyi have great potential as a feedstock for biodiesel production and other oleochemical industry applications.
The m s indicates the amount of substrate needed per unit cell mass per hour to maintain cells in an active and proper physiological condition. The reason for a zero m s after the batch phase of the cultivation can be attributed to the high cell activity and proliferation, which requires more energy for motility, active transport of molecules and synthesis of macromolecules such as lipids. A kinetic model study by Jahic et al. [31] describes that the lower the maintenance, the higher is the maximum cell density achievable in a constant feed fed-batch process. Shen et al. [32] obtained a maximum m s of 5.7 gglucose/gCDW h at continuous cultivation by R. toruloides using glucose as sole carbon source. Meeuwse, Tramper and Rinzema [33] obtained q p values ranging from 0.023 to 0.069 Cmol/Cmol h for oleaginous yeasts.
Tsigie et al. [34] studied the effect of urea and peptone on cell mass concentration and lipid production by Yarrowia lipolytica. The addition of urea did not significantly affect cell mass production, but resulted in lower cellular lipid accumulation than using peptone. Gao et al. [3] cultivated Mortierella isabellina on xylose. Different organic and inorganic nitrogen sources were used as single nitrogen sources, including yeast extract, urea, peptone, glycine, KNO3, NH4NO3 and (NH4)2SO4. For organic nitrogen sources, the highest cell mass and lipid concentrations were achieved when yeast extract was used as sole nitrogen source. Urea and (NH4)2SO4 yielded comparable amounts of cell biomass, but (NH4)2SO4 yielded less lipid. Other researchers have also reported urea to be an excellent nitrogen source for lipid production in yeasts such as Rhodotorula glutinis [35] and Trichosporon fermentans [36]. Evans and Ratledge [37] investigated the effects of different nitrogen sources on lipid accumulation by yeast and suggested that different metabolisms of nitrogen sources resulted in different concentrations of metabolites such as NH4 +, 2-oxoglutarate or glutamate, which induced different levels of lipid accumulation.
Results of C and N elemental analysis of cell mass from the fed-batch culture were similar to those of conventional microbial cell mass [9]. In general, N and O contents declined continuously, while C and H contents increased upon lipid accumulation [6]. This was in accordance with the profile for cellular lipid content (Table 1). Unlike Y X/S, η helped to distinguish between microbial growth on substrates with different energy contents by considering the energy content of the substrates in the calculations [6]. Our data showed (Table 1) that at 70 h the η value of L. starkeyi was higher (0.85) than the maximum value of 0.7 proposed for heterotrophic microbial growth [11]. This probably resulted from the additional energy produced and stored as lipid. Pan and Rhee [38] collected data from 21 sources and showed that η was mostly from 0.6 to 0.8. Eroshin and Krylova [13] reported η values from 0.15 to 0.31 for L. Starkeyi strains grown on ethanol. Zhou et al. [6] obtained the highest cell mass energetic yield of 0.643 when adding glucose to a culture growing R. toruloides in bioethanol wastewater. It is evident that higher energetic yields are dependent on the conditions of lipid accumulation. Through the above analysis, it was observed that L. starkeyi is highly efficient in converting energy from xylose into biomass.
Cell mass yields of the oleaginous yeasts were consistently lower than those of nonoleaginous microorganisms, whereas their energetic yields were higher [6]. Higher energetic yields are related to many industrially important parameters, such as a lower requirement for oxygen, a lower generation of metabolic heat, a lower maintenance energy demand and a lower production of carbon dioxide [39]. Zhou et al. [6] obtained a similar linear relationship for η by R. toruloides with glucose as sole carbon source. Using the equation σ B γ B = 0.0278L + 1.5655, Eroshin and Krylova [13] demonstrated that there was a good linear relationship between σ B γ B and cellular lipid content for oleaginous yeast growth on ethanol. Pan and Rhee [40] achieved a linear relationship (σ B γ B = 0.0308L + 1.6) valid for glucose-grown oleaginous yeasts, although it was derived from ethanol-grown oleaginous yeasts. When the energy content (σ B γ B) of substrates utilized by oleaginous microorganisms is determined, η of the biomass can also be estimated, which helps in choosing the optimum cultural medium for the production of lipid [6]. Thus, oleaginous yeasts that have higher energetic yields should be favorable candidates for high cell density culture because in bioreactors, mass and heat transfers are primary physical constraints.
Akdeniz and Gündoğdu [8] experimentally obtained a Q c of 17.89 kJ/g and calculated a Q c of 17.09 kJ/g using Eq. 9 with a difference of 4.47 % between these two values. At the end of the fed-batch cultivation, a Q c of 25.7 kJ/g was obtained with Eq. 9 (using data on elemental composition of cell mass). Our results for Q c were lower than those obtained by Minkevich et al. [1] for L. starkeyi. These authors achieved 39 kJ/g using ethanol as sole carbon source. The present data provide a better understanding of growth and lipid accumulation by L. starkeyi using xylose and urea as substrates and should be very helpful in developing and designing a more efficient bioprocess for microbial lipid production. Equations 11 and 12 can be applied to investigate the efficiency of energy conversion of other microbial growth processes in media with different energy contents for lipid production.
We described a fed-batch process for lipid production by L. starkeyi with high cell mass and lipid concentrations using xylose and urea as carbon and energy sources. This process will be useful in the further engineering of cost-effective microbial lipid production processes. The preliminary study provided an analysis of cell mass yields and energetic yields by L. starkeyi, examining the relationship of η and heat of combustion to lipid content. Mass and energy balances could be applied to predict the cell mass yield from xylose in fed-batch cultivation. The work developed here offers simple and efficient tools for characterization of cell mass, which could be useful for furthering our understanding of the process of lipid production by oleaginous yeast.
References
Minkevich IG, Dedyukhina EG, Chistyakova TI (2010) The effect of lipid content on the elemental composition and energy capacity of yeast biomass. Appl Microbiol Biotechnol 88(3):799–806
Papanikolaou S, Aggelis G (2011) Lipids of oleaginous yeasts. Part II: technology and potential applications. Eur J Lipid Sci Tech 113(8):1052–1073
Gao D, Zeng J, Zheng Y, Yu X, Chen S (2013) Microbial lipid production from xylose by Mortierella isabellina. Bioresour Technol 133:315–321
Coradini ALV, Anschau A, Vidotti ADS, Reis EM, Xavier MCA, Coelho RS, Franco TT (2015) Microorganism for bioconversion of sugar hydrolysates into lipids. In: Kamm B (ed) Microorganisms in Biorefineries, vol 26., vol Microbiology MonographsSpringer-Verlag, Berlin Heidelberg, pp 51–78
Huang C, Chen XF, Xiong L, Chen XD, Ma LL, Chen Y (2013) Single cell oil production from low-cost substrates: the possibility and potential of its industrialization. Biotechnol Adv 31(2):129–139
Zhou W, Li Y, Zhang Y, Zhao Z (2012) Energy efficiency evaluation of lipid production by oleaginous yeast Rhodosporidium toruloides. J Therm Anal Calorim 108(1):119–126
Di Y-Y, Tan Z-C, Sun X-H, Wang M-H, Xu F, Liu Y-F, Sun L-X, Zhang H-T (2004) Low-temperature heat capacity and standard molar enthalpy of formation of 9-fluorenemethanol (C14H12O). J Chem Thermodyn 36(2):79–86
Akdeniz F, Gundogdu M (2007) Direct and alkali medium liquefaction of Laurocerasus officinalis Roem. Energy Convers Manage 48(1):189–192
Cordier JL, Butsch BM, Birou B, Vonstockar U (1987) The relationship between elemental composition and heat of combustion of microbial biomass. Appl Microbiol Biotechnol 25(4):305–312
Minkevich IG, Eroshin VK (1973) Productivity and heat generation of fermentation under oxygen limitation. Folia Microbiol 18(5):376–385
Erickson LE, Minkevich IG, Eroshin VK (1978) Application of mass and energy balance regularities in fermentation. Biotechnol Bioeng 20(10):1595–1621
Heijnen JJ, Roels JA (1981) A macroscopic model describing yield and maintenance relationships in aerobic fermentation processes. Biotechnol and Bioeng 23(4):739–763
Eroshin VK, Krylova NI (1983) Efficiency of lipid synthesis by yeasts. Biotechnol Bioeng 25(7):1693–1700
Patel SA, Erickson LE (1981) Estimation of heats of combustion of biomass from elemental analysis using available electron concepts. Biotechnol Bioeng 23(9):2051–2067
Meier D, Larimer DR, Faix O (1986) Direct liquefaction of different lignocellulosics and their constituents.1. Fractionation, elemental composition. Fuel 65(7):910–915
Tapia EV, Anschau A, Coradini AL, Franco TT, Deckmann AC (2012) Optimization of lipid production by the oleaginous yeast Lipomyces starkeyi by random mutagenesis coupled to cerulenin screening. AMB Express 2(1):64–71
Somogyi M (1945) A new reagent for the determination of sugars. J Biol Chem 160(1):61–68
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153(2):375–380
Lewis T, Nichols PD, McMeekin TA (2000) Evaluation of extraction methods for recovery of fatty acids from lipid producing microheterotrophs. J Microbiol Meth 43(2):107–116
Stephanopoulos GN, Aristidou AA, Nielsen J (1998) Metabolic Engineering Principles and Methodologies. Academic Press, San Diego
Anschau A, Xavier MCA, Hernalsteens S, Franco TT (2014) Effect of feeding strategies on lipid production by Lipomyces starkeyi. Bioresour Technol 157:214–222
Ratledge C, Cohen Z (2008) Microbial and algal oils: do they have a future for biodiesel or as commodity oils? Lipid Technol 20(7):155–160
Koutinas AA, Chatzifragkou A, Kopsahelis N, Papanikolaou S, Kookos IK (2014) Design and techno-economic evaluation of microbial oil production as a renewable resource for biodiesel and oleochemical production. Fuel 116:566–577
Wiebe MG, Koivuranta K, Penttila M, Ruohonen L (2012) Lipid production in batch and fed-batch cultures of Rhodosporidium toruloides from 5 and 6 carbon carbohydrates. BMC Biotechnol 12(1):26
Ykema A, Verbree EC, Kater MM, Smit H (1988) Optimization of lipid production in the oleaginous yeast Apiotrichum curvatum in whey permeate. Appl Microbiol Biot 29(2–3):211–218
Hsieh CH, Wu WT (2009) Cultivation of microalgae for oil production with a cultivation strategy of urea limitation. Bioresource Technol 100(17):3921–3926
Zhao X, Hu C, Wu S, Shen H, Zhao ZK (2011) Lipid production by Rhodosporidium toruloides Y4 using different substrate feeding strategies. J Ind Microbiol Biotechnol 38(5):627–632
Wild R, Patil S, Popovic M, Zappi M, Dufreche S, Bajpai R (2010) Lipids from Lipomyces starkeyi. Food Technol Biotechnol 48(3):329–335
Zhang J, Fang XL, Zhu X, Li Y, Xu H, Zhao B, Chen L, Zhang X (2011) Microbial lipid production by the oleaginous yeast Cryptococcus curvatus O3 grown in fed-batch culture. Biomass Bioenerg 35(5):1906–1911
Liu YF, Meng Z, Zhang FQ, Shan L, Wang XG (2010) Influence of lipid composition, crystallization behavior and microstructure on hardness of palm oil-based margarines. Eur Food Res Technol 230(5):759–767
Jahic M, Rotticci-Mulder J, Martinelle M, Hult K, Enfors S-O (2002) Modeling of growth and energy metabolism of Pichia pastoris producing a fusion protein. Bioprocess Biosyst Eng 24(6):385–393
Shen H, Gong Z, Yang X, Jin G, Bai F, Zhao ZK (2013) Kinetics of continuous cultivation of the oleaginous yeast Rhodosporidium toruloides. J Biotechnol 168(1):85–89
Meeuwse P, Tramper J, Rinzema A (2011) Modeling lipid accumulation in oleaginous fungi in chemostat cultures. II: validation of the chemostat model using yeast culture data from literature. Bioprocess Biosyst Eng 34(8):951–961
Tsigie YA, Wang CY, Kasim NS, Diem QD, Huynh LH, Ho QP, Truong CT, Ju YH (2012) Oil production from Yarrowia lipolytica Po1 g using rice bran hydrolysate. J Biomed Biotechnol 2012:378384
Saenge C, Cheirsilp B, Suksaroge TT, Bourtoom T (2011) Efficient concomitant production of lipids and carotenoids by oleaginous red yeast Rhodotorula glutinis cultured in palm oil mill effluent and application of lipids for biodiesel production. Biotechnol Bioprocess Eng 16(1):23–33
Zhu LY, Zong MH, Wu H (2008) Efficient lipid production with Trichosporon fermentans and its use for biodiesel preparation. Bioresource Technol 99(16):7881–7885
Evans CT, Ratledge C (1984) Influence of nitrogen metabolism on lipid accumulation by Rhodosporidium toruloides CBS-14. J Gen Microbiol 130:1705–1710
Pan JG, Rhee JS (1986) Biomass yields and energetic yields of oleaginous yeasts in batch culture. Biotechnol Bioeng 28(1):112–114
Pan JG, Rhee JS (1986) Kinetic and energetic analyses of lipid accumulation in batch culture of Rhodotorula glutinis. J Ferment Technol 64(6):557–560
Pan JG, Rhee JS (1985) Mass and energy balance for analysis of oleaginous yeast growth. Korean J Chem Eng 2(1):81–85
Li YH, Zhao ZB, Bai FW (2007) High-density cultivation of oleaginous yeast Rhodosporidium toruloides Y4 in fed-batch culture. Enzyme Microb Technol 41(3):312–317
Acknowledgments
The authors would like to acknowledge the financial support received from Shell Oil Company and the Brazilian scientific agencies: Federal Agency for Support and Evaluation of Graduate Education (CAPES), National Council for Scientific and Technological Development (CNPq) and São Paulo Research Foundation (FAPESP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anschau, A., Franco, T.T. Cell mass energetic yields of fed-batch culture by Lipomyces starkeyi . Bioprocess Biosyst Eng 38, 1517–1525 (2015). https://doi.org/10.1007/s00449-015-1394-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-015-1394-7