Abstract
Yarrowia lipolytica ACA-DC 50109 is an oleaginous yeast. In order to know the function of pyruvate carboxylase (PYC) in lipid biosynthesis, the PYC gene cloned from Pichia guilliermondii Pcla22 was overexpressed in the oleaginous yeast. The lipid contents in the wild-type strain ACA-DC 50109 and the transformants P4, P7, and P103 were 30.2 % (w/w) 36.5 % (w/w), 38.2 % (w/w), and 37.9 % (w/w). However, the amount of the secreted citric acids by strains ACA-DC 50109, P4, P77, and P103 were 0.5, 10.1, 11.5, and 9.4 g/L. In order to reduce the amount of the secreted citric acid, the PYC gene and endogenous ACL1 gene encoding ATP citrate lyase (ACL1) were simultaneously overexpressed in the oleaginous yeast. The lipid contents of the transformants PA19, PA56, PA124 were 44.4 % (w/w), 45.3 % (w/w), and 43.7 % (w/w). At the same time, the amount of the secreted citric acid by the transformants PA19, PA56, and PA124 was reduced to 5.4, 6.2, and 6.3 g/L. The PYC and ACL1 activities and their gene transcriptional levels in all the transformants were greatly enhanced compared to those in their wild-type strain ACA-DC 50109. During 10-L fermentation, lipid content in the transformant PA56 was 49.6 % (w/w) and the amount of secreted citric acid was 2.9 g/L. This meant that PYC and ACL1 can play an important role in accumulation of intracellular lipid of the oleaginous yeast Y. lipolytica ACA-DC 50109.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
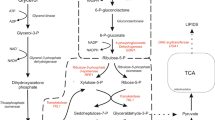
It has been well known that microbial oils, especially yeast oils, can be used for production of biodiesel and specific lipid derivatives such as lubricants, adhesives, and plastics (Beopoulos et al. 2011). Therefore, it is very important to enhance oil biosynthesis by the oleaginous yeasts which contain more than 20 % of oils in their cells. Many strategies have been developed for enhancement of lipid biosynthesis in yeast cells. A high C/N ratio (an excess of carbon source and limitation of nitrogen) is a most effective way to promote lipid biosynthesis (Li et al. 2010). According to the pathway of lipid biosynthesis (Fig. 1) (Ratledge 2004), overexpression of malic enzyme (ME) in order to regenerate the NADPH+ cofactor supply for fatty acid synthesis; ATP citrate lyase (ACL) to increase the acetyl-CoA, the main precursor of fatty acid synthesis; and acetyl-CoA carboxylase (ACC) to catalyze the first and rate-limiting step in de novo lipid synthesis have been found to be able to enhance lipid biosynthesis. Deletion of the MIG1 encoding the main effector of glucose repression, the glycerol-3-phosphate dehydrogenase gene (GUT2) and the six POX genes encoding acyl-CoA oxidases, abolishing β-oxidation results in a significant increase in lipid accumulation (Beopoulos et al. 2011; Wang et al. 2013).
The pathway for fatty acids and triglycerides (TAGs) biosynthesis in oleaginous yeasts. PYC pyruvate carboxylase, ME malic enzyme, MD malate dehydrogenase, ACC acetyl-CoA carboxylase, FAS fatty acid synthetase, FAD fatty acid dehydrogenase, ACL ATP citrate lyase, CS citrate synthetas, ICDH iso-citrate dehydrogenase, ACO aconitase, GPD glycerol-3-phosphate dehydrogenase, GUT1 glycerol kinase, GUT2 glycerol-3-phosphate dehydrogenase, POX acyl-CoA oxidase, PA phosphatidic acid, DAG diacylglycerol, TAGs triacylglycerols, FFA free fatty acids, PEP phosphoenolpyruvate, OAA oxaloacetic acid
Pyruvate carboxylase (PYC) which is a key enzyme in the cytosolic reductive TCA pathway is a biotin-dependent tetrameric enzyme that catalyzes the carboxylation of pyruvic acid to oxaloacetic acid (Fig. 1). All the microbial PYC enzymes have been found to be biotin dependent, existing either as an α4 or an α4β4 multimeric complex. The α4 PYC enzymes are allosteric, requiring acetyl-CoA for their activation, and are inhibited by aspartate, while α4β4 PYC enzymes are not subjected to allosteric control (Lietzan and Maurice 2013). When yeasts are grown on acetate, PYC-catalyzed oxaloacetate formation is repressed. So far, it has been well known that PYC can play an important role in biosynthesis of malic acid, polymalate, succinic acid, fumaric acid, citric acid, α-ketoglutaric acid and all the kinds of amino acids among the carboxylic acids produced by microorganisms (Brown et al. 2013; Ma et al. 2013; Yin et al. 2012; Xu et al. 2012; Gokarn et al. 1998; Guo et al. 2013). Because it catalyzes the carboxylation of pyruvic acid to oxaloacetic acid, a precursor for citrate and fatty acids biosynthesis (Fig. 1), it may also play an important role in biosynthesis of fatty acid and triglycerides. In order confirm this, the PYC gene cloned from Pichia guilliermondii Pcla22 which can produce malic acid and the endogenous ACL1 gene from Yarrowia lipolytica were simultaneously overexpressed in the oleaginous yeast Y. lipolytica ACA-DC 50109. Then, lipid production in the genetically engineered yeasts was examined.
Materials and methods
Strains, media, and plasmids
The oleaginous yeast used in this study was Y. lipolytica ACA-DC 50109 (wild-type strain) (collection number 2E00680 at the Marine Microorganisms Culture Collection of China) which was kindly offered by Dr. Seraphim Papanikolaou from Laboratory of Food Microbiology and Biotechnology, Department of Food Science and Technology, Greece (Papanikolaou and Aggelis 2003). A uracil mutant (collection number 2E00681 at MCCC) was isolated from Y. lipolytica ACA-DC 50109 using 5′-fluororotic acid (5′-FOA) (Wang et al. 2009) and was grown in the medium containing 1.7 g/L of YNB (yeast nitrogen base without amino acids and ammonium sulfate), 5.0 g/L of (NH4)2SO4, 10.0 g/L of glucose, 25.0 g/L of agar and 0.01 g/L of uracil. P. guilliermondii Pcla22 (collection number 2E00691 at MCCC) is an oleaginous yeast and a producer of malate (Wang et al. 2012). Yeast strains were grown in yeast peptone dextrose (YPD medium) (10.0 g/L yeast extract, 20.0 g/L bacto peptone, 20.0 g/L glucose). The yeast transformants were selected on YNB-N5000 medium (1.7 g/L YNB, 10.0 g/L glucose, 5.0 g/L ammonium sulfate). The Escherichia coli strain used in this study for plasmid recovery and cloning experiments was DH5α [F − endA1 hsdR17 (rK_/mK +) supE44 thi − λ − recA1gyr96DlacU169 (j80lacZDM15)] and was grown in Luria–Bertani broth (LB). The E. coli transformants were grown in LB medium with 100.0 μg/mL of ampicillin. The compositions of the medium for lipid production were 40.0 g/L glucose, 20.0 g/L CaCO3, 7.0 g/L KH2PO4, 2.5 g/L Na2HPO4, 1.5 g/L MgSO4·7H2O, 0.15 g/L CaCl2, 0.15 g/L FeCl3·6H2O, 0.02 g/L ZnSO4·7H2O, 0.06 g/L MnSO4·H2O, and 0.5 g/L yeast extract (Papanikolaou et al. 2002).
Plasmids
The expression vector piNA1312-GY was constructed in this laboratory and pMD19-T simple for amplification of the target genes in E. coli was purchased from TaKaRa (Japan).
Isolation of DNA, restriction digestions, and transformation
The genomic DNAs were isolated from the yeasts P. guilliermondii Pcla22 and Y. lipolytica ACA-DC 50109 and DNA manipulations were carried out using standard methods (Sambrook et al. 1989). Bacterial plasmid DNA was purified using TIANprep Mini Plasmid Kit (TIANGEN). Restriction endonuclease digestions and DNA ligations were performed according to the manufacturer’s recommendations. E. coli was transformed with plasmid DNA according to Sambrook et al. (1989). Y. lipolytica was transformed according to the methods described by Xuan et al. (1988).
Construction of the vectors for expression of the PYC gene and ACL1 gene in Y. lipolytica
To express the PYC gene from P. guilliermondii Pcla22 in Y. lipolytica ACA-DC 50109, the primers for amplification of the PYC gene were designed according to the sequence of the gene (accession no. XM_001484326.1). The forward primer was P1 and the reverse primer was P2 (Table S1 in the supplementary file and Fig. 2). The genomic DNA of P. guilliermondii Pcla22 was used as the template for PCR. Meanwhile, in order to increase the copy numbers of the endogenous ACL1 gene in Y. lipolytica ACA-DC 50109, the primers (A1 and A2) for amplification of the ACL1 gene were designed according to the sequence of the gene (accession no. XM_504787.1). The forward primer was A1 and the reverse primer was A2 (Table S1 and Fig. 2). The genomic DNA of Y. lipolytica ACA-DC 50109 was used as the template for PCR. The PCR reaction system was 50.0 μL containing 1.0 μL La Taq, 5.0 μL 10× La PCR buffer II (Mg2+ Plus), 8.0 μL 2.5 mM dNTPs, 1.0 μL 20.0 μM each primer, 1.0 μL of 10 ng/mL the genomic DNA, sterile deionized water up to 50.0 μL. The conditions for the PCR amplification were initial denaturation at 94 °C for 10 min, denaturation at 94 °C for 1 min, annealing temperature at 55 °C for 1 min, extension at 72 °C for 4 min, final extension at 72 °C for 10 min. PCR was run for 30 cycles. The PCR products (3.6 kb) were separated by agarose gel electrophoresis and ligated into the plasmid pMD19-T simple vector. The recombinant vector was transformed into E. coli DH5α. The recombinant vectors carrying the PCR products were extracted from the E. coli transformants and purified. The purified recombinant vectors carrying the PYC gene or the ACL1 gene were digested with SacII and SpeI, and the digests were ligated into pNA1312-GY digested with the same enzymes, respectively (data not shown). The resulting plasmid carrying the PYC gene was designated as pINA1312-GY-PYC and the resulting plasmid carrying the ACL1 gene was designated as pINA1312-GY-ACL1 (data not shown).
Transformation and selection
The plasmids pINA1312-GY-PYC and pINA1312-GY-ACL1 obtained above were digested with the enzyme NotI. The linear fragments carrying the PYC gene or ACL1 gene were separated in agarose gel and recovered using TaKaRa Agarose Gel DNA Purification Kit Ver.3.0. The recovered linear fragments (about 9 kb) carrying the PYC gene or ACL1 gene were transformed into the cells of the uracil mutant of Y. lipolytica ACA-DC 50109 mentioned above by lithium acetate methods (Xuan et al. 1988). The transformants obtained were spread on YNB-N5000 plates without uracil. The positive transformants were grown in the lipid production medium at 28 °C for 96 h and lipid contents in the cells of the different positive transformants were determined as described below, respectively, and Y. lipolytica ACA-DC 50109 was used as a control. After determination of lipid contents in the cells of over 200 positive transformants, it was found that lipid contents in the cells of the transformants P4, P77, and P103 among them only carrying the PYC gene were the highest while lipid contents in the cells of the transformants PA19, PA56, and PA124 among them carrying both the PYC gene and ACL1 gene were the highest. Therefore, the transformants P4, P77, P103, PA19, PA56, and PA124 were used as the lipid producers, subsequently.
Confirmation of the integrated PYC gene and ACL1 gene
The genomic DNAs in the transformants mentioned above and Y. lipolytica ACA-DC 50109 were extracted as described above and used as the templates for PCR. The DNA fragments (the PYC gene) were PCR amplified using the primers P1 and P2 and the DNA fragments (the ACL1 gene) were PCR amplified using the primers A1 and A2 (Table S1). The sizes of the PCR products were estimated using the Automated Documentation and Analysis System (Gene-Genius, USA). The PCR products were sequenced by Nanjing Genscript Company (Zhao et al. 2010). The integrated PYC gene and ACL1 gene were also confirmed by Southern blotting as described by Watanabe et al. (2008).
Single cell oil production at flask level
The cells of the transformants mentioned above and its wild-type strain ACA-DC 50109 were transferred to 50.0 mL of the medium (YPD medium) for the seed culture and cultivated at the shaking speed of 180 rpm and 28 °C for 24 h, respectively. Two milliliters of the culture (2.5 × 108 cells/mL) were transferred to 50.0 mL of the lipid production medium and the yeast cells were cultivated at the shaking speed of 180 rpm and 28 °C for 72 h. The measurements of the cell mass, lipid contents, and secreted citric acid concentration were performed as described below.
Determination of lipid contents
The total lipids in the cells (1.0 g) were extracted according to Folch et al. (1957) and Zhao et al. (2010). The extracted lipids were weighed and oil content per 100 g of cell dry weight was calculated.
Preparation of cell-free extracts
The cell-free extracts of the transformants mentioned above and Y. lipolytica ACA-DC 50109 were prepared as described by Zhang et al. (2013). The disrupted cells were centrifuged at 12,000×g for 20 min at 4 °C and the supernatants obtained were used for determination of PYC and ACL1 activities. Total protein quantity in the supernatants was determined using Coomassie brilliant blue assay (Bradford 1976). The crude intracellular enzymes heated at 100 °C for 5 min were used as the inactivated intracellular enzymes.
Measurement of pyruvate carboxylase and ATP citrate lyase activities
Pyruvate carboxylase (PYC) activity was measured according to the methods described by Chávez-Cabrera et al. (2010). A unit of PYC activity was defined as the amount of enzyme that catalyzed the oxidization of one micromole of NADH per minute. ATP citrate lyase activity (ACL1) was measured using the hydroxamate assay (Lipmann and Tuttle 1945).
Fluorescent real-time PCR
Fluorescent real-time PCR was performed according to the methods described by Liu et al. (2011). All the primers used for fluorescent real-time PCR were designed according to the corresponding gene sequences of P. guilliermondii Pcla22 and Y. lipolytica ACA-DC 50109 (Table S1). The primers P3 and P4 were designed according to the PYC gene sequence (GenBank accession no. XM_001484326.1) in P. guilliermondii, the primers A3, A4, A5, and A6 were designed according to the ACL1 gene sequence (GenBank accession no. accession no. XM_504787.1) and the ACL2 gene sequence (GenBank accession no. XM_503231.1), respectively, in Y. lipolytica ACA-DC 50109 and the primers 26s1 and 26s2 were designed according to 26S rRNA gene sequence (GenBank accession no. JQ690257.1) in Y. lipolytica ACA-DC 50109 (Table). The transcriptional levels of the PYC gene, ACL1 gene, and ACL2 gene in Y. lipolytica ACA-DC 50109 were regarded as 100 %.
Lipid production by batch fermentation
Lipid production by batch fermentation was carried out in a 10-L fermentor [BIOQ-6005-6010B, Huihetang Bio-Engineering Equipment (Shanghai) Co., Ltd]. The seed culture of the transformant PA56 grown in YPD medium at the shaking speed of 180 rpm and 28 °C for 24 h was prepared as described above. A volume of 700.0 mL of the seed culture (OD600nm = 18.0) was transferred to 7.0 L of the lipid production medium with initial 40.0 g/L glucose. The fermentation was performed under the conditions of an agitation speed of 200 rpm, an aeration rate of 7 L/min, a temperature of 28 °C and a fermentation period of 288 h. Only 10.0 mL of the culture was collected in the interval of 12 h and was centrifuged at 5000×g and 4 °C for 5 min. The lipid contents, cell mass, reducing sugar, and citric acid in the supernatant obtained were determined as described above and below. The cell dry weight in 5.0 mL of the culture during the 10-L fermentation was also measured.
Determination of citric acid
Citric acid (CA) was estimated by the methods described by Camp and Farmer (1967).
Determination of reducing sugar in the fermented media
Reducing sugar in the fermented media was determined by the Nelson–Somogyi method (Spiro 1966).
Measurement of cell dry weight
Cell dry weight was measured according to the methods described by Chi et al. (2001).
Results
Effects of the PYC gene expression on cell growth, oil contents, and citric acid production
Y. lipolytica ACA-DC 50109 has been reported to be an oleaginous yeast and significant quantities of lipids were accumulated inside the yeast cells (Papanikolaou et al. 2002). It has been reported that P. guilliermondii Pcla22 is an oleaginous yeast and a producer of malate whose biosynthesis is associated high pyruvate carboxylase activity (Brown et al. 2013; Wang et al. 2012). In order to know if pyruvate carboxylase can play a role in lipid biosynthesis and further enhance lipid production in this oleaginous yeast. The PYC gene from P. guilliermondii Pcla22 was integrated into the genomic DNA of the uracil mutant of Y. lipolytica ACA-DC 50109 as described in the “Materials and methods” section. After determination of cell mass, oil contents and the secreted citric acid by the different PYC transformants and their wild-type strain ACA-DC 50109, the results in Fig. 2 indicated that the lipid contents of the wild-type strain ACA-DC 50109 and the transformants P4, P7, and P103 were 30.2 % (w/w) 36.5 % (w/w), 38.2 % (w/w) and 37.9 % (w/w) while the amount of the secreted citric acids by wild-type strain ACA-DC 50109 and the transformants P4, P77, and P103 were 0.5, 10.1, 11.5, and 9.4 g/L (Fig. 2). However, their cell growth was not significantly affected (Fig. 2). The data clearly showed that after expression of the heterologous PYC gene, lipid biosynthesis and citric acid biosynthesis by the transformants were indeed greatly enhanced.
Effects of simultaneous expression of the PYC gene and ACL1 gene on cell growth, oil contents, and citric acid production
As shown in Fig. 2, after expression of the heterologous PYC gene, lipid contents of the transformants P4, P7, and P103 were increased. However, the secreted citrate was also greatly increased. It can be seen from the pathway of fatty acid biosynthesis in Fig. 1 that after citric acid is transformed into acetyl-CoA and oxaloacetic acid by ACL1, the acetyl-CoA formed is the precursor for biosynthesis of fatty acids and the oxaloacetic acid formed can be further metabolized to offer NADPH2 for fatty acid biosynthesis. This meant that it was very important to overexpress the ACL1 gene in the transformants in order to reduce the formed citric acid. Therefore, the PYC gene and ACL1 gene were simultaneously expressed in Y. lipolytica ACA-DC 50109 as described in the “Materials and methods” section. It can be clearly observed from the data in Fig. 3 that compared to lipid contents (30.2 % w/w) in the cells of the wild-type strain ACA-DC 50109, those in the transformants PA19, PA56, and PA124 were 44.4 % (w/w), 45.3 % (w/w) and 43.7 % (w/w). At the same time, compared to the amount of the secreted citric acid in Fig. 2, the amount of the secreted citric acid by the transformants PA19, PA56, and PA124 was reduced to 5.4, 6.2, and 6.3 g/L, respectively (Fig. 3). However, their cell growth of was not significantly affected (Fig. 3). This demonstrated that after expression of the ACL1 gene, lipid contents in the transformants were further increased due to the decrease in citric acid.
Confirmation of the integrated PYC and ACL1 genes in the transformants
In order to know if the PYC gene and the ACL1 gene have been integrated into the genomic DNA in the transformants, the PCR products from the genomic DNAs of the transformants P77 and PA56 and their wild-type strain ACA-DC 50109 were amplified. The results in Fig. 4a revealed that the PCR products (around 3.6 kb) carrying the whole sequence of the PYC gene were PCR amplified from the genomic DNAs of both the transformants P77 and PA56 using the primers P1 and P2 (Table S1). However, no such PCR products were PCR amplified from the genomic DNAs of their wild-type strain ACA-DC 50109. At the same time, the results in Fig. 4b indicated that the PCR products (around 2.06 kb) carrying the sequence of the ACL1 gene were PCR amplified from only the genomic DNA of the transformant PA56 using the primers A1 and C1 (Table S1). However, no such PCR products were PCR amplified from the genomic DNAs of the transformant P77 and their wild-type strain ACA-DC 50109 using the same primers. The correct integrations of the replacement constructs were also verified by PCR and Southern blot analysis (data not shown). The results demonstrated that both the PYC gene and the ACL1 gene were indeed integrated into the genomic DNAs of both the transformant PA56 and the PYC gene was indeed only integrated into the genomic DNA of the transformant PA56 because both the expression vectors piNA1312-GY-PYC and piNA1312-GY-ACL1 contain the zeta-elements (Madzak et al. 2004).
Pyruvate carboxylase and ATP citrate lyase activities and transcriptional levels of their genes
After determination of pyruvate carboxylase activity and its gene transcriptional levels in the transformants P77, PA56, and their wild-type strain ACA-DC 50109, the results in Fig. 5a showed that PYC activities of the wild-type strain ACA-DC 50109 and the transformants (P77 and PA56) were 0.32, 0.83, and 0.84 U/mg and the results in Fig. 5b showed that the transcriptional levels of the PYC gene in the transformants P77 and PA56 were much higher (more than three-fold) than those of the PYC gene in their wild-type strain ACA-DC 50109. These results confirmed that the PYC gene was indeed overexpressed and pyruvate carboxylase activity was greatly enhanced in the transformants P77 and PA56. Meanwhile, the results in Fig. 5c showed that ACL activity of the transformant PA56 was 0.36 U/mg while ACL activities of the transformant P77 and the wild-type strain ACA-DC 50109 were 0.23 and 0.25 U/mg. The results in Fig. 5d indicated that the transcriptional levels of the ACL1 gene in the transformant PA56 were much higher than those of the ACL1 gene in the transformant P77 and their wild-type strain ACA-DC 50109. It was interesting to note that the transcription of the ACL2 gene could be enhanced by the overexpression of ACL1 in the yeast of Y. lipolytica and the relative transcriptional levels of the gene ACL2 in the strain ACA-DC 50109, P77, and PA56 were 100, 94.3, and 180.5 %, respectively (Fig. 5e). These results also evidenced that the ACL1 gene was indeed overexpressed and ATP citrate lyase activity was greatly enhanced in the transformant PA56.
Lipid production by the transformant PA56 during the batch fermentation
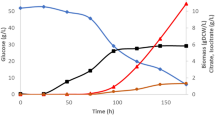
During the time where transformant PA56 was grown in the 10-L fermenter, oil content, cell growth, glucose concentration, and citric acid concentration were monitored. It can be seen from the data in Fig. 6 that oil content in the cells of the transformant PA56 and cell growth reached the highest (49.6 % w/w and 12.2 g/L) within 72 h of the fermentation, while the amount of the secreted citric acid reached the highest (2.9 g/L) within 36 h of the fermentation. Therefore, the titer of lipid was 6.21 g/L, the productivity of lipids was 0.084 g/L/h and the yield of the lipid was 0.16 g/g of glucose. The results in Fig. 6 also indicated that only 1.7 g/L of reducing sugar was left in the fermented medium, suggesting that 95.75 % of the added glucose was transformed into cells, lipid, and citric acid. After the data in Figs. 3 and 6 were compared, we can see that lipid biosynthesis in the transformant PA56 was increased from 45.3 (w/w) to 49.6 % (w/w) and the amount of secreted citric acid was decreased from 6.2 to 2.9 g/L when the cell cultivation at flask level was changed to that in fermenter. This may be due to the fact that the cultivation conditions in the fermentor were better than those in the flask, leading to more citric acid which was transformed into lipid in the cells.
Discussion
In order to know the function of pyruvate carboxylase in lipid biosynthesis in Y. lipolytica and offer the precursor oxaloacetic acid for citric acid and lipid biosynthesis according to the pathway for fatty acids and triglycerides (TAGs) biosynthesis in oleaginous yeasts (Fig. 1), the heterologous PYC gene was overexpressed in the oleaginous yeast Y. lipolytica ACA-DC 50109 (data not shown). It was found that lipid contents in the genetically engineered yeast strains were enhanced (Fig. 2), However, the amount of the secreted citric acid was also increased (Fig. 2). Yin et al. (2012) showed that after overexpression of heterologous pyruvate carboxylase genes in Y. lipolytica WSH-Z06, α-ketoglutaric acid (KGA) production (62.5 g/L) by the genetically engineered Y. lipolytica-RoPYC2 was enhanced. It also has been reported that concentrations of the by-products fumarate (FA), malate (MA), succinic acid (SA), and pyruvic acid (PA) decreased significantly by overproduction of fumarase (FUM) and increased by overproduction of PYC and also of FUM and PYC simultaneously in the recombinant Y. lipolytica strains (Yin et al. 2012).
It has been confirmed that there is a strong correlation between the presence of ACL activity and the ability to accumulate lipid in yeasts (Ratledge 2004). It also has been known that ACL in Y. lipolytica, unlike some other organism, is encoded by two genes, ACL1 (accession no. XM_504787 in NCBI) and ACL2 (accession no. XM_503231 in NCBI) (Beopoulos et al. 2011; Liu et al. 2013). In order to transform the produced citric acid into acetyl-CoA and oxaloacetic acid for lipid biosynthesis according to the pathway for fatty acids and TAGs biosynthesis in oleaginous yeasts (Fig. 1), both the PYC and ACL1 gene were simultaneously overexpressed in the oleaginous yeast Y. lipolytica ACA-DC 50109 (data not shown). In this case, the amount of the produced citric acid was lyzed to produce acetyl-CoA for malonyl-CoA and lipid biosynthesis according to the pathway for fatty acids and TAGs biosynthesis in oleaginous yeasts (Fig. 1). Thus, lipid contents were further increased in the genetically engineered yeast strains due to high PYC and ACL activities (Figs. 3 and 5). For example, lipid contents in the transformant PA56 was 45.3 % (w/w) and the amount of the secreted citric acid by the transformants PA56 was reduced to 6.2 g/L (Fig. 3). The results in Fig. 5e showed that after the expression of the ACL1 gene, expression of the ACL2 gene was also enhanced. It was possible that the enhanced ACL2 gene expression in Y. lipolytica ACA-DC 50109 was due to coordinated regulation after the expression of the ACL1 gene. This phenomenon will be elucidated in this laboratory. In contrast, in our previous studies (Liu et al. 2013), we found that after removal of some of the ACL1 gene in citric acid-producing yeast Y. lipolytica SWJ-1b, lipid contents were decreased and the secreted citric acid was increased. Overexpression of the ACL gene in Y. lipolytica WSH-Z06 enhanced KGA production (increased from 36.3 to 46.7 g/L) (Zhou et al. 2012). After the ACL gene from Mus musculus was expressed in Y. lipolytica WSH-Z06, the ACL activity in the transformants was 11.6-fold increased and the amount of the secreted KGA was increased from 36.3 to 46.7 g/L (Zhou et al. 2012). After the simultaneous coexpression of ACC1 gene encoding acetyl-CoA carboxylase and DGA1 gene encoding diacylglycerol acyltransferase in the wild-type Y. lipolytica W29 strain (ATCC20460), lipid content in the ACC1+DGA1 transformant reached to 41.4 % (w/w), demonstrating synergistic effects of ACC1+DGA1 coexpression (Tai and Stephanopoulos 2013). Furthermore, after lipogenesis capability in Y. lipolytica was drastically increased by multiplexing genomic engineering, the saturated cells containing upwards of 90 % lipid content and titers exceeding 25 g/L lipids were attained (Blazeck et al. 2014). The oleaginous yeast Y. lipolytica was also genetically engineered to produce eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) (Xue et al. 2013).
During 10-L fermentation, oil content in the cells of the transformant PA56 and cell growth reached the highest (49.6 % w/w and 12.2 g/L) within 72 h of the fermentation while the amount of the secreted citric acid reached the highest (2.9 g/L) within 36 h of the fermentation (Fig. 6). After the MIG1 gene in the same oleaginous yeast Y. lipolytica ACA-DC 50109 was disrupted and the disruptant M25 obtained had more lipid bodies than its parent yeast strain and the disruptant M25 contained 48.7 % (w/w) of oil based on its cell weight while the parent yeast strain only contained 36.0 % (w/w) of oil (Wang et al. 2013). In a Y. lipolytica Po1d genetic background, GPD1 (encoding the catabolic dehydrogenase) overexpression, GUT2 (encoding the anabolic dehydrogenase) inactivation or both mutations together result in 1.5-, 2.9-, and 5.6-fold respective increases in the level of glycerol-3-phosphate (G3P), leading to an increase of TAG accumulation (Dulermo and Jean-Marc 2011). At the same time, deletion of POX1-6 or MFE1 genes (encoding β-oxidation pathway of fatty acids) increased TAG and free fatty acids content (Dulermo and Jean-Marc 2011). It has been thought that in order to increase the acyl-CoA support to regenerate the NADPH+ cofactor supply and enhance malonyl-CoA offer, ATP citrate lyase (ACL1), malic enzyme (ME) and acyl-CoA carboxylase (ACC1/2) overexpression may increases lipid accumulation in oleaginous yeasts (Beopoulos et al. 2011). As mentioned above, the ACC1+DGA1 transformant grown in a 2-L bioreactor fermentation achieved 61.7 % lipid content after 120 h. The overall yield and productivity were 0.195 g/g and 0.143 g/L/h, respectively, during the lipid accumulation phase of the fermentation (Tai and Stephanopoulos 2013). Therefore, this is the first time to report that overexpression of both the PYC gene and ACL1 gene in the oleaginous yeast Y. lipolytica ACA-DC 50109 can enhance lipid biosynthesis.
References
Beopoulos A, Nicaud JM, Gaillardin C (2011) An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl Microbiol Biotechnol 90:1193–1206
Blazeck J, Hill A, Liu L, Knight R, Miller J, Pan A, Otoupal P, Alper HS (2014) Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat Commun 5:3131
Bradford MM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal Biochem 72:248–253
Brown SH, Bashkirova L, Berka R, Chandler T, Doty T, McCall K, McCulloch M, McFarland S, Thompson S, Yaver D, Berry A (2013) Metabolic engineering of Aspergillus oryzae NRRL 3488 for increased production of l-malic acid. Appl Microbiol Biotechnol 97:8903–8912
Camp BJ, Farmer L (1967) A rapid spectrophotometric method for the determination of citric acid in blood. Clin Chem 13:501–505
Chávez-Cabrera C, Flores-Bustamante ZR, Marsch R, Montes MC, Sánchez S, Cancino-Díaz JC, Flores-Cotera LB (2010) ATP-citrate lyase activity and carotenoid production in batch cultures of Phaffia rhodozyma under nitrogen-limited and nonlimited conditions. Appl Microbiol Biotechnol 85:1953–1960
Chi ZM, Liu J, Zhang W (2001) Trehalose accumulation from soluble starch by Saccharomycopsis fibuligera sdu. Enzym Microb Technol 28:240–245
Dulermo T, Jean-Marc N (2011) Involvement of the G3P shuttle and β-oxidation pathway in the control of TAG synthesis and lipid accumulation in Yarrowia lipolytica. Metab Eng 13:482–491
Folch J, Lees M, Slane-Stanley JA (1957) A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem 226:497–509
Gokarn RR, Eiteman MA, Altman E (1998) Expression of pyruvate carboxylase enhances succinate production in Escherichia coli without affecting glucose uptake. Biotechnol Lett 20:795–798
Guo X, Wang J, Xie X, Xu Q, Zhang C, Chen N (2013) Enhancing the supply of oxaloacetate for l-glutamate production by pyc overexpression in different Corynebacterium glutamicum. Biotechnol Lett 35:943–950
Li M, Liu GL, Chi Z, Chi ZM (2010) Single cell oil production from hydrolysate of cassava starch by marine-derived yeast Rhodotorula mucilaginosa TJY15a. Biomass Bioenergy 4:101–107
Lietzan AD, Maurice MS (2013) Insights into the carboxyltransferase reaction of pyruvate carboxylase from the structures of bound product and intermediate analogs. Biochem Biophys Res Commun 441:377–382
Lipmann F, Tuttle LC (1945) A specific micromethod for the determination of acyl phosphates. J Biol Chem 159:21–28
Liu GL, Wang DS, Wang LF, Zhao SF, Chi ZM (2011) Mig1 is involved in mycelial formation and expression of the genes encoding extracellular enzymes in Saccharomycopsis fibuligera A11. Fungal Genet Biol 48:904–913
Liu XY, Chi Z, Liu GL, Madzak C, Chi ZM (2013) Both decrease in ACL1 gene expression and increase in ICL1 gene expression in marine-derived yeast Yarrowia lipolytica expressing INU1 gene enhance citric acid production from inulin. Mar Biotechnol 15:26–36
Ma Y, Wang GY, Liu GL, Wang ZP, Chi ZM (2013) Overproduction of poly(β-malic acid) (PMA) from glucose by a novel Aureobasidium sp. P6 strain isolated from mangrove system. Appl Microbiol Biotechnol 97:8931–8939
Madzak C, Gaillardin C, Beckerich JM (2004) Heterologous protein expression and secretion in the non-conventional yeast Yarrowia lipolytica: a review. J Biotechnol 109:63–81
Papanikolaou S, Aggelis G (2003) Selective uptake of fatty acids by the yeast Yarrowia lipolytica. Eur J Lipid Sci Technol 105:651–655
Papanikolaou S, Muniglia L, Chevalot I, Aggelis G, Marc I (2002) Yarrowia lipolytica as a potential producer of citric acid from raw glycerol. J Appl Microbiol 92:737–744
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimica 86:807–815
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Beijing, pp 367–370, Chinese translating ed
Spiro RG (1966) Analysis of sugars found in glycoproteins. Methods Enzymol 8:3–26
Tai M, Stephanopoulos G (2013) Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab Eng 15:1–9
Wang F, Yue LX, Wang L, Madzak C, Li J, Wang XH, Chi ZM (2009) Genetic modification of the marine-derived yeast Yarrowia lipolytica with high-protein content using a GPI-anchor-fusion expression system. Biotechnol Prog 25:1297–1303
Wang GY, Chi Z, Song B, Wang ZP, Chi ZM (2012) High level lipid production by a novel inulinase-producing yeast Pichia guilliermondii Pcla22. Bioresour Technol 124:77–82
Wang ZP, Xu HM, Wang GY, Chi Z, Chi ZM (2013) Disruption of the MIG1 gene enhances lipid biosynthesis in the oleaginous yeast Yarrowia lipolytica ACA-DC 50109. Biochim Biophys Acta 1831:675–682
Watanabe H, Hatakeyama N, Sakurai H, Uchimiya H, Sato T (2008) Isolation of industrial strains of Aspergillus oryzae lacking ferrichrysin by disruption of the dffA gene. J Biosci Bioeng 106:488–492
Xu G, Liu L, Chen J (2012) Reconstruction of cytosolic fumaric acid biosynthetic pathways in Saccharomyces cerevisiae. Microb Cell Factories 11:24–28
Xuan JM, Fournier P, Gaillardin C (1988) Cloning of the LYS5 gene encoding saccharopine dehydrogenase. Curr Genet 14:15–21
Xue Z, Sharpe PL, Hong SP, Yadav NS, Xie D, Short DR, Damude HG, Rupert RA, Seip JE, Wang J, Pollak DW, Bostick MW, Bosak MD, Macool DJ, Hollerbach DH, Zhang H, Arcilla DM, Bledsoe SA, Croker K, McCord EF, Tyreus BD, Jackson EN, Zhu Q (2013) Production of omega-3 eicosapentaenoic acid by metabolic engineering of Yarrowia lipolytica. Nat Biotechnol 31:734–741
Yin X, Madzak C, Du G, Zhou J, Chen J (2012) Enhanced alpha-ketoglutaric acid production in Yarrowia lipolytica WSH-Z06 by regulation of the pyruvate carboxylation pathway. Appl Microbiol Biotechnol 96:1527–1537
Zhang F, Wang ZP, Chi Z, Raoufi Z, Abdollahi S, Chi ZM (2013) The changes in Tps1 activity, trehalose content and expression of TPS1 gene in the psychrotolerant yeast Guehomyces pullulans 17–1 grown at different temperatures. Extremophiles 17:241–249
Zhao CH, Cui W, Liu XY, Chi ZM, Madzak C (2010) Expression of inulinase gene in the oleaginous yeast Yarrowia lipolytica and single cell oil production from inulin-containing materials. Metab Eng 12:510–551
Zhou J, Yin X, Madzak C, Du G, Chen J (2012) Enhanced α-ketoglutarate production in Yarrowia lipolytica WSH-Z06 by alteration of the acetyl-CoA metabolism. J Biotechnol 161:257–264
Acknowledgments
This research was supported by Hi-Tech Research and Development Program of China (Grant No. 2013BAB01B05).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
(PDF 75 kb)
Rights and permissions
About this article
Cite this article
Wang, GY., Zhang, Y., Chi, Z. et al. Role of pyruvate carboxylase in accumulation of intracellular lipid of the oleaginous yeast Yarrowia lipolytica ACA-DC 50109. Appl Microbiol Biotechnol 99, 1637–1645 (2015). https://doi.org/10.1007/s00253-014-6236-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-6236-z