Abstract
Proteases are ubiquitous in prokaryotes and eukaryotes. Plant proteases are key regulators of various physiological processes, including protein homeostasis, organelle development, senescence, seed germination, protein processing, environmental stress response, and programmed cell death. Proteases are involved in the breakdown of peptide bonds resulting in irreversible posttranslational modification of the protein. Proteases act as signaling molecules that specifically regulate cellular function by cleaving and triggering receptor molecules. Peptides derived from proteolysis regulate ROS signaling under oxidative stress in the plant. It degrades misfolded and abnormal proteins into amino acids to repair the cell damage and regulates the biological process in response to environmental stress. Proteases modulate the biogenesis of phytohormones which control plant growth, development, and environmental stresses. Protein homeostasis, the overall balance between protein synthesis and proteolysis, is required for plant growth and development. Abiotic and biotic stresses are major factors that negatively impact cellular survivability, biomass production, and reduced crop yield potentials. Therefore, the identification of various stress-responsive proteases and their molecular functions may elucidate valuable information for the development of stress-resilient crops with higher yield potentials. However, the understanding of molecular mechanisms of plant protease remains unexplored. This review provides an overview of proteases related to development, signaling, and growth regulation to acclimatize environmental stress in plants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Proteases play a crucial role in plant growth, reproduction, development, photosynthesis, programmed cell death (PCD), immune response, and defense against unwanted stress. They also play an essential role in regulated cell death (RCD) and respond to environmental stimuli (Zamyatnin 2015). Protease regulates the protein quality and homeostasis in different plant organelles (Schuhmann and Adamska 2012) by degrading photodamaged proteins. Protein homeostasis is maintained by controlling the rate of protein synthesis and protein degradation by the plant’s regulatory mechanism (Li et al. 2017). Plant protease is involved in development and nutrient recycling by degrading misfolded proteins (García-Lorenzo 2007). Protease degrades photoinhibition proteins and plays a crucial role in repairing plant cells (Bailey et al. 2002), and controls the protein quality by removing damaged proteins (Komenda 2006).
Plant proteases are ubiquitous enzymes that function in various processes. They play an essential role in nutrient remobilization (Van der Hoorn 2008) and controls protein localization in cells, and acts as a signaling molecule (Turk 2006). Proteases are substrate-specific, they are present in different subcellular compartments, and their activity is highly regulated temporally and spatially (Martins et al. 2019). Proteases cleave misfolded or damaged proteins at the N-terminal (aminopeptidases), C-terminal (carboxypeptidases), or internal peptide bonds. Different plants have different numbers of proteases. For example, Arabidopsis thaliana contains over 800 proteases in 60 families, and rice (Oryza sativa) contains 600 proteases (Van der Hoorn 2008). Cysteine protease plays an essential role in signaling, PCD, protein maturation, nutrient remobilization, hormone synthesis, and degradation of misfolded proteins (Rocha et al. 2017).
The MEROPS database provides information on plant proteases. Proteases have been divided into five classes as serine, aspartate, cysteine, threonine, and metalloproteases based on their structural properties and activities (Rawlings et al. 2018). Serine, aspartic, cysteine, and metalloproteases are the major catalytic classes of plant protease (Schaller 2004).
Plants face environmental stress such as heat, ultraviolet light, drought, cold, pesticides, pathogens, and salinity, which are the major factors that reduce crop yield potential and negatively impact their survival (Costa and Farrant 2019). Climate change decreases the productivity of crops, such as maize, rice, and wheat (Tigchelaar 2018). Plants have various strategies to adapt and acclimate to the changing environmental conditions by activating different protective mechanisms that trigger physiological, morphological, and biochemical changes (Bernstein 2019; Hirayama and Shinozaki 2010). Environmental stresses enhance the production of reactive oxygen species (ROS) such as O2– (superoxide), O2 (singlet oxygen), and H2O2 (hydrogen peroxide) in different cellular compartments like mitochondria, chloroplasts, and peroxisomes. Production of ROS is very common in the plant during all types of stress (Jaspers and Kangasjärvi 2010). ROS triggers programmed cell death by creating adverse conditions in the organelles (Petrov et al. 2015). ROS leads to oxidative stress in different cellular compartments, which damage the proteins, lipids, metabolites, and nucleic acids, affecting multiple biological processes (Hasanuzzaman et al. 2012). Molecular chaperon refolds proteins, while protease rapidly degrades the misfolded and aggregated proteins by proteolysis. Proteolysis is necessary to remove misfolded or unwanted proteins and regulate signaling molecules.
This review demonstrates the role of plant protease as a growth regulator and signaling molecule in response to environmental stress in crops. It also provides a comprehensive assessment of plant protease to identify various proteases in crop plants for a better understanding of the biological processes for improving crop yield and upcoming applications.
Relationship of proteases with reactive oxygen species
ROS is produced in response to abiotic and biotic stresses. ROS triggers oxidative stress involving various biological processes, such as autophagy, apoptosis, and necrosis. Under environmental stress conditions, plants generate ROS, which affects the different biochemical and physiological reactions. ROS is an important signal molecule of a plant for controlling growth and development (Cappetta et al. 2020). Several studies have been demonstrated the relations between ROS and protease in the plant. The peptide molecule is involved in the ROS signaling process. The Peptides derived from proteolysis regulate ROS signaling under oxidative stress in the plant (Møller and Sweetlove 2010). Photosystem II (PSII) of chloroplast produces ROS, which accelerates the damage of the D1 protein of PSII. The FtsH protease has been involved in removing the damaged D1 protein in the plant (Nixon et al. 2010). In addition, Deg protease, an ATP- dependent serine protease, has been linked in the degradation of D1 protein with the cooperative action of FtsH protease (Kato and Sakamoto 2018). MtbHLH2 and MtCP77 in Medicago truncatula showed a crucial role in ROS accumulation, PCD, and nodule senescence (Deng et al. 2019). OsNAC2 induces the expression of caspase‐like protease OsAP37 and leads to PCD producing ROS (Mao et al. 2018). The cysteine protease HopN1 reduces the production of ROS in the chloroplast (Rodríguez‐Herva et al. 2012). Therefore, protease has an essential role in the regulation of ROS in the plant.
Proteases in response to abiotic and biotic stresses
Plants encounter various stress during their growth phase. Environmental stress includes drought, salinity, cold, temperature, UV- B radiation, pathogen, pesticides, and metal toxicity, which delays plant growth and yield potentials. Plants acclimatize the environmental stress at physiological, molecular, and biochemical levels, primarily via proteolysis machinery (Table 1).
Drought
Drought is often considered a major environmental factor that reduces crop yield and negatively impacts plant survival. The effects have particularly been accentuated with global warming, especially in crop plants such as maize, rice, and wheat (Furlan et al. 2016). Nevertheless, plants have developed various strategies to minimize the damage due to drought (Shah et al. 2017). Plants commonly respond to drought stress by producing ROS, which causes oxidative stress in different cellular compartments. Oxidative stress damages proteins, nucleic acids, metabolites, and lipids. Dehydration stress increases the number of misfolded, aggregated, and post-translationally modified proteins, which, in turn, activate the chaperone system. Chaperones refold the proteins damaged by oxidation, and proteases degrade the misfolded and aggregated proteins (Pulido et al. 2017). Plant protease significantly contributes to a drought stress response. Protein homeostasis and nutrient remobilization are essential for plant responses to drought stress. Protease plays an essential role in many events, including removal of damaged, denatured, and aggregated proteins, remobilization of amino acids, and signal transduction (Van der Hoorn 2008).
Drought induces aspartic protease activity, mainly in beans (Phaseolus vulgaris and Vigna unguiculata) (de Carvalho et al. 2001). ASPG1 (Aspartic protease in guard cell 1) plays an essential role in drought avoidance in A. thaliana (Yao et al. 2012). Overexpression of APA1 in A. thaliana ensures drought tolerance compared to the Wild-type (WT) plant. Overexpressing APA1 causes lower stomatal density and smaller stomatal aperture compared to that in WT plants (Sebastián et al. 2020). Lon protease is an ATP-dependent, nuclear-encoded, mitochondrial protease that belongs to the serine protease family (Pinti et al. 2016). Lon protease is necessary for cellular homeostasis and many developmental changes that have been induced by stress (Pinti et al. 2016). Lon protease has been involved in mitochondrial homeostasis. Lon protease (Atlon4) in modified A. thaliana makes the plant more tolerant to drought stress than the wild-type plant (Li et al. 2010). SUMO (small ubiquitin-like modifier) protease belongs to the cysteine protease family, found in plants, eukaryotes, and yeast, and is crucial for stress tolerance. SUMO protease influences drought tolerance in wheat (T. aestivum) (Le Roux et al. 2019) by SUMOylation, which is also crucial for plant development. The ERD1 (Early response to dehydration 1) of A. thaliana induces the expression of the ClpA and ClpB protein levels during dehydration stress (Kiyosue et al. 1993). In addition, it has been reported that ASPG1 protease plays an essentials role in drought avoidance in A. thaliana (Yao et al. 2012).
Salinity
Soil salinity is a critical environmental stress factor that reduces the quality and yield of crops worldwide. Hypersalinity has multiple impacts on plants, such as genotoxicity, nutrition deficiency, oxidative stress, osmotic stress, and ionic imbalance (Shah et al. 2017). Consequently, this decreases photosynthesis rate, generates an excessive amount of ROS, and affects the electron transport chain. Nevertheless, plants adopt various strategies, like exclusion and salt compartmentalization (Wang et al. 2016). In plant cells, both enzymatic and non-enzymatic systems operate to minimize salinity stress. Many proteins and genes are involved in these resistance mechanisms for increasing tolerance to salinity, which includes H + -pyrophosphate, OsMYB3R-2, and AtSOS1 (Dai et al. 2007). Plant protease activity significantly increases in response to high salinity stress. Salt stress reduces the synthesis of proteins, carbohydrates, lipids, DNA, and RNA and increases the degradation of damaged or harmful proteins by proteases (Parida et al. 2004). Salt stress affects various physiological and metabolic processes such as ion transport, synthesis and accumulation of osmotic solute, protein turnover, osmotic adjustment, nitrogen metabolism, and compartmentation.
Plant protease significantly contributes to the salt stress response. Cysteine protease, also called thiol protease, is an essential family of plant protease. Plant Protease plays a vital role in plant growth and development by degradation of endogenous proteins through proteolysis (Van Wyk et al. 2014). Salt stress enhances the level of ROS and oxidized proteins in different cell compartments. Degradation of oxidized proteins is necessary for recycling, which ensures plant growth and development under salt stress. Cysteine protease often plays a crucial role in the degradation of oxidized proteins and regulates ROS levels (Van der Hoorn 2008). RD21a and RD19a are cysteine proteases that belong to the papain-like family, and these two genes are induced by dehydration and salinity stress (Koizumi et al. 1993). The Cyp15a gene in peas (Pisum sativum) is a cysteine protease that induces and modulates mRNA levels in plants in response to high salt stress (Jones and Mullet 1995). The SPCP2 gene demonstrated tolerance during salinity and drought stress (Chen et al. 2010). Many of the cysteine protease genes are involved in altering physiological responses during salinity stress. Therefore, cysteine protease plays an essential role in the signaling pathway and the coping mechanism against salt stress. The serine protease gene HtrA in Halothece sp. PCC7418 is highly upregulated in response to salt stress (Patipong et al. 2020). The ClpD1 protein expression in A. thaliana has been increased in response to high salinity stress compared to the wild-type (Mishra and Grover 2016). Cyp15a gene of pea (Pisum sativum) is a cysteine protease induced and modulates mRNA levels in the plant in response to high salt stress (Jones and Mullet 1995).
Cold
One-third of the global land area is used for agriculture, and 42% of the land temperature is less than –20 °C (Miura and Furumoto 2013). There are two types of cold stress like chilling (0 to –15 °C) and freezing (< 0 °C). Plants in cold areas are constantly exposed to chilling and freezing temperatures. So, they require specialized mechanisms to survive in such low temperatures. Cold stress restricts crop production, development, and growth. The harmful impact of cold stress on photosynthesis and metabolic function further affects the production, metabolism, and growth of the plants. Phenotypic symptoms in cold stress response include wilting, chlorosis, necrosis, stunted seedlings, poor germination, and reduced leaf expansion. Therefore, the development of cold stress tolerance in crops is vital to increase crop production. Light is converted to chemical energy by photosynthesis to form carbohydrates, and this process is sensitive to cold stress. The PSII efficiency and photosynthetic rate are altered at low temperatures. RuBisCo is the most crucial protein for carbon assimilation during photosynthesis.
The production of the large subunit of RuBisCo is reduced during cold treatment. It indicates that cold stress reduces the photosynthetic efficiency mainly by degrading the large subunit of RuBisCo. Several novel proteins such as GroEL, FtsH-like protein, the 26S proteosome unit, and legumin play a crucial role in response to cold stress (Susin et al. 2006). Transcription factor ZmFtsH2B has been upregulated in leaves, whereas ZmFtsH2A has been constitutively expressed in the roots and leaves under cold stress (Yue et al. 2010). FtsH, Clp, and DegP are the prominent protease families located in the chloroplast. These proteases display constitutive gene expression and are involved in protein quality control and maintenance of homeostasis, rather than degrading proteins (van Wijk 2015). Under low temperature, Clp protease expression has been increased in rice (Oryza Sativa) (Cui et al. 2005).
Temperature
The ambient temperature has a significant impact on growth, development, and plant productivity. Consequently, plants can acclimate rapidly and evolve various mechanisms to detect their environment and respond with physiological, developmental, and cellular changes for efficient reproduction and optimal growth. High temperatures affect the physiological, metabolic, molecular, and biochemical changes in plants. In addition, high temperature induces cellular homeostasis and alters multiple genes of plants.
The Clp protease is constitutively expressed in plant tissues, primarily in the chloroplasts of green leaves. Many proteases function as molecular chaperones, such as the ClpB, ClpC, and ClpD protein subunits. The ClpB gene in A. thaliana has been constitutively expressed in chloroplasts and mitochondria during high-temperature stress response (Lee et al. 2007). In addition, the ClpB gene in O. sativa has been upregulated in response to heat stress (Singh et al., 2010). The ClpB gene in lima beans (Phaseolus lunatus) has been constitutively expressed in chloroplasts during high-temperature stress (Keeler et al. 2000).
The metalloprotease FtsH (Filamentous temperature-sensitive H protease) is essential for photosynthesis at high ambient temperatures. Mutant FtsH causes a filamentous temperature-sensitive phenotype in E. coli (Begg et al. 1992). FtsH is crucial for plant survival during high-temperature stress (Langer 2000). The mutant FtsH11 gene reduces the photosynthetic efficiency of PSI and PSII at high temperatures. FtsH11 in A. thaliana plays a vital role in thermotolerance (Chen et al. 2018). The matrix metalloproteinase (MMP) Gm1-MMP has been found in the plasma membrane and is highly expressed in mature leaves, mature seeds, and old leaves associated with seed development. Gm1-MMP is essential for high-temperature tolerance and has been overexpressed during the development of leaves and roots in A. thaliana at high temperatures (Liu et al. 2017). Hence, it is well established that protease is essential for thermotolerance in many organisms.
Pathogen
Pathogen infection, which accounts for many harmful diseases, reduces crop yield and affects food security globally. Pathogens affect plant physiology, including respiration, pathogenesis, cell membrane permeability, transcription and translation, and translocation of water and nutrients. Plants have developed several conserved and sophisticated strategies to protect from pathogens. Plants create many antimicrobial compounds and physical barriers to deal with most of the pathogens. When plants perceive PAMPs (pathogen-associated molecular patterns), such as chitin, by membrane-localized PRRs, multiple immune responses are triggered within a cell. The success of the defense response depends on how soon the plant detects the invasion signals, which leads to the initiation of the defense response.
Protease is an integral part of the immune system of plants. These processes are influenced by proteases in different cellular compartments. Apoplastic serine protease SBT3.3 (Subtilase 3.3) in A. thaliana regulates the defense response. The mutant protease sbt3.3 gene in A. thaliana is hyper susceptible to bacterial pathogen Hyaloperonospora arabidopsidis and Pseudomonas syringae ( Ramírez et al. 2013).
Cathepsin-B is a papain-like cysteine protease that generates a hypersensitive response (HR). CathB is necessary for the hypersensitive response activated by co-expression of the potato gene R3a and the Phytophthora infestans gene Avr3a (Gilroy et al. 2007). Aspartic protease OsCDR1 (Oryza sativa) expressed in A. thaliana enhanced resistance against fungal and bacterial pathogens (Prasad et al. 2009). The papain-like proteases Rcr3 and Pip1 are both inhibited by Avr2 secreted by the fungal pathogen Cladosporium fulvum (Shabab et al. 2008). In plants, deletion of Pip1 causes hypersusceptibility to Pseudomonas syringae, Cladosporium fulvum, and Phytophthora infestans (Ilyas et al. 2015). AtMC1 and AtMC2 are cytosolic metacaspase protease in A. thaliana, regulating hypersensitive response in an antagonistic manner. AtMC1 acts as a positive regulator of the HR during cell death caused by Pseudomonas syringae, and AtMC2 acts as a negative regulator (Coll et al. 2010).
Threonine protease PBA1 is an essential protease that is triggered by Pseudomonas syringae (Hatsugai et al. 2009). Vacuolar processing enzymes (VPEs) have caspase-1-like activity and are crucial for virus-induced HR upon infection by TMV (Tobacco mosaic virus) in Nicotiana benthamiana ( Hatsugai et al. 2004). C14 and RD21 are both orthologous papain-like cysteine proteases found in Arabidopsis thaliana and Solanum lycopersicum, respectively (Thomas and Van der Hoorn 2018).
Light
Light plays a vital role in the growth and development of plants. It is required for photosynthesis, whereby plants convert energy from sunlight to chemical energy. Two proteins, D1 and D2, are found at the core of the PSII subunit (Hankamer et al. 2001). Deg2 is a serine protease that primarily cleaves photodamaged D1 proteins of PSII at high light intensity (Haußühl et al. 2001). The metalloprotease FtsH1 is responsible for the degradation of the D1 protein (Andersson and Aro 2001; Adam et al. 2005).
Nevertheless, it is not yet clear how FtsH functions in regulation. Prohibitin-like proteins form a large complex with FtsH homohexamers and modulate its proteolytic activity in E. coli (Kihara et al. 1996). Prohibitin-like proteins probably form a 2MD complex with the mitochondrial FtsH in A. thaliana (Piechota et al. 2010). Besides, the RC47 complex interacts with the FtsH protease, and this interaction is essential for the degradation of the D1 protein by the FtsH protease (Komenda et al. 2006; Kato et al. 2009; Krynická et al. 2015). EngA is a unique family of GTPase protease (Verstraeten et al. 2011) located on the thylakoid membrane. EngA interacts with the ATPase domain of the FtsH protease and affects its turnover. This interaction is essential for the degradation of the D1 protein. FtsH turnover is accelerated under high light stress in Chlamydomonas reinhardtii (Wang et al. 2017). It is well established that Deg and FtsH proteases are essential for tolerance to light-induced stress.
Protease in signaling and phytohormone mediated regulations during stress
The Arabidopsis mitochondrial FtsH3, FtsH4, and FtsH10 are involved in the biogenesis of oxidative phosphorylation (Marta et al. 2007). Loss of AtFtsH4 gene showed morphological and physiological abnormality of Arabidopsis during short-day conditions (Gibala et al. 2009). It has been found that FtsH protease acts through the production of ROS to change the leaf morphology (Kato et al. 2009). Protease rapidly degrades misfolded and aggregated proteins by proteolysis under stress. ROS acts as a signaling molecule to manage numerous biological processes, including PCD, pathogen defense, and stomatal development; thus, controlling the growth and development of the plant in response to environmental stress (Apel and Hirt 2004). Under stress, a close association occurs between ROS and plant hormones, such ABA, salicylic acid, jasmonic acid, GA, and ethylene (Bright et al. 2006; Gudesblat et al. 2007). The homeostasis and signaling of auxin have been induced by the apoplastic reactive oxygen species (Blomster et al. 2011). ROS negatively affects the auxin response in Arabidopsis thaliana. The expression of the auxin-inducible genes has been downregulated due to the mutation of mekk1 and mpk4 (Nakagami et al. 2006). The morphology of the plant aggravates due to the production of ROS and its impact on auxin signaling (Potters et al. 2007). ROS mediates the interaction between auxin and ABA signaling (He et al. 2012). Auxin homeostasis alters by hydrogen peroxide and promotes change in the gene expression of PINOID, which disturbs the polar transport of auxin (Pasternak et al. 2005). It has been suggested through various morphological studies that ROS interacts with auxin to control growth and development in the plant. Therefore, comprehensive studies are required to elucidate the interaction between ROS and auxin to dissect the molecular mechanism.
FtsH4 is an ATP-dependent AAA-protease having proteolytic activity. FtsH4 is associated with plant dwarfism, and mutation of FtsH4–4 shows numerous axillary branches due to a decrease of IAA concentration. It has been hypothesized that the FtSH4 is involved in auxin synthesis to regulate plant growth and development (Zhang et al. 2014a, b). FtsH4-dependent assembly of various proteins inhibits hydrogen peroxide (H2O2) production, which affects the regulation of Indole-3-acetic acid (IAA) metabolism, signaling, and transport of auxin.
Aspartic protease plays a crucial role in response to stress (Guo et al. 2013). It is a large family of proteolytic enzyme involved in programmed cell death, senescence, and reproduction. Overexpression of aspartic protease induces the expression of various genes, which promote ABA synthesis and signaling (Fig. 1). Additionally, the overexpression of APA1 in A. thaliana confers drought tolerance (Sebastian et al. 2020).
There is a close relation between ROS, signaling molecule, and phytohormones such as abscisic acid (ABA), salicylic acid (SA), gibberellic acid (GA), jasmonic acid (JA), nitric oxide, and ethylene (Bright et al. 2006; Desikan et al. 2008; Galvez-Valdivieso et al. 2009; Gudesblat et al. 2007). Mitochondrial FtsH4 in Arabidopsis thaliana regulates the expression of WRKY gene through modifying the ROS level, and WRKY genes control the salicylic acid synthesis and signaling (Zhang et al. 2017).
Therefore, the identification of various stress-responsive protease and their phytohormone function may elucidate valuable information for the preparation of crops with higher stress tolerance and increase yield potentials (Table 2).
Protease in plant development
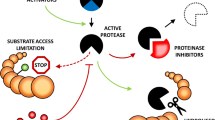
Protease, Proteolytic enzyme, Proteinase, and Peptidase are the enzymes that hydrolyze the peptide bond of stress-damaged proteins (Mótyán et al. 2013). Protease is a crucial component of plants, distributed in the different subcellular compartments, such as vacuole, chloroplast, endoplasmic reticulum, cytoplasm, nucleus, mitochondria. Protease plays a significant role in stress responses, pathogen recognition, systematic defense response, stomata development, and plastid development (Fig. 2). Protease participates in the various cellular process, including the signaling pathway, which moderates multiple biological functions such as PCD (Thomas and Van der Hoorn 2018; Moschou et al. 2016).
Plant protease in promoting growth, development, and degradation of misfolded protein in the different organelle. Misfolded protein is mainly cleaved by proteases and repairs cell damage during stress. Proteolysis plays a crucial role in removing toxic proteins and provides amino acids for the synthesis of new protein
Papain-like cysteine proteases (PLCP) are involved in proteolysis and various physiological process. The HvPap-1, a GA-induced PLCP, is involved in protein remobilization during barley seed germination (Cambra et al. 2012). Other PLCPs proteolytic enzymes like CEP1 play an essential role in tapetal programmed cell death and pollen development in Arabidopsis thaliana (Zhang et al. 2014a, b). In addition, PLCPs play a crucial role in protein degradation via proteolysis during leaf senescence, such as SAG12, AtRD19A, RD21A (Gepstein, et al. 2003; Lohman et al. 1994; Yamada et al. 2001). PLCPs such as ATRD21A and ATRD19A are two essential protein-marker that plays a vital role in the adaption of dehydration, salt, and drought stresses (Koizumi et al. 1993). The malfunctioning of protease leads to various pleiotropic effects in the plant, such as reduced plant growth, impaired chloroplast development, leaf variegation, and photosynthesis (Sjögren et al. 2006).
Conclusion and future perspectives
Protease plays a crucial role in all living organisms. Protein turnover requires for the growth and differentiation facilitated by the specific proteolytic enzymes. Protease plays other essential functions, including amino acids recycling by the degradation of non-functional proteins and regulation of regulatory proteins and essential enzymes. Most of the previous studies have been accomplished in the model plant. However, the role of the protease of crop plants is mostly unexplored. Therefore, exploration of the underlying molecular mechanism of crop plant protease is very crucial to adapt the environmental stress. Plant productivity decreases severely with increasing environmental stresses, which must be a significant concern in the present scenario. With the increasing world population, crop production needs to be increased several folds in the near future. Therefore, the identification of novel protease would contribute a significant role in plant science. The plant protease would facilitate the development of stress tolerance crop plants for improving growth and yield potentials during environmental stress conditions. Understanding plant protease in response to stress will provide a vast field to develop climate-resilient crop plants.
Abbreviations
- ROS:
-
Reactive oxygen species
- ETC:
-
Electron transport chain
- PCD:
-
Programmed cell death
- RCD:
-
Regulated cell death
- ASPG1:
-
Aspartic protease in guard cell 1
- SUMO:
-
Small ubiquitin-like modifier
- WT:
-
Wilf type
- ERD1:
-
Early response to dehydration 1
- FtsH:
-
Filamentous temperature sensitive H protease
- HR:
-
Hypersensitive response
- IAA:
-
Indoleacetic acid
References
Adam Z, Zaltsman A, Sinvany-Villalobo G, Sakamoto W (2005) FtsH proteases in chloroplasts and cyanobacteria. Physiol Plant 123:386–390. https://doi.org/10.1111/j.1399-3054.2004.00436.x
Ahn JW, Kim M, Lim JH, Kim GT, Pai HS (2004) Phytocalpain controls the proliferation and differentiation fates of cells in plant organ development. Plant J 38:969–981. https://doi.org/10.1111/j.1365-313X.2004.02102.x
Andersson B, Aro E-M (2001) Photodamage and D1 protein turnover in photosystem II. In: Regulation of photosynthesis. Springer, pp 377–393. https://doi.org/10.1007/0-306-48148-0_22
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399. https://doi.org/10.1146/annurev.arplant.55.031903.141701
Bailey S, Thompson E, Nixon PJ, Horton P, Mullineaux CW, Robinson C, Mann NH (2002) A critical role for the Var2 FtsH homologue of Arabidopsis thaliana in the photosystem II repair cycle in vivo. J Biol Chem 277:2006–2011. https://doi.org/10.1074/jbc.M105878200
Barry CS, Aldridge GM, Herzog G, Ma Q, McQuinn RP, Hirschberg J, Giovannoni JJ (2012) Altered chloroplast development and delayed fruit ripening caused by mutations in a zinc metalloprotease at the lutescent2 locus of tomato. Plant Physiol 159:1086–1098. https://doi.org/10.1104/pp.112.197483
Bartel B, Fink GR (1995) ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science 268:1745–1748. https://doi.org/10.1126/science.7792599
Begg K, Tomoyasu T, Donachie W, Khattar M, Niki H, Yamanaka K, Hiraga S, Ogura T (1992) Escherichia coli mutant Y16 is a double mutant carrying thermosensitive ftsH and ftsI mutations. J Bacteriol 174:2416–2417. https://doi.org/10.1128/jb.174.7.2416-2417.1992
Belanger FC, Brodl MR, Ho T-H (1986) Heat shock causes destabilization of specific mRNAs and destruction of endoplasmic reticulum in barley aleurone cells. Proc Natl Acad Sci 83:1354–1358. https://doi.org/10.1073/pnas.83.5.1354
Bernstein N (2019) Plants and salt: Plant response and adaptations to salinity. In: Model Ecosystems in Extreme Environments. Elsevier, pp 101–112. https://doi.org/10.1016/B978-0-12-812742-1.00005-2
Blomster T, Salojärvi J, Sipari N, Brosché M, Ahlfors R, Keinänen M, Overmyer K, Kangasjärvi J (2011) Apoplastic reactive oxygen species transiently decrease auxin signaling and cause stress-induced morphogenic response in Arabidopsis. Plant Physiol 157:1866–1883. https://doi.org/10.1104/pp.111.181883
Bölter B, Nada A, Fulgosi H, Soll J (2006) A chloroplastic inner envelope membrane protease is essential for plant development. FEBS Lett 580:789–794. https://doi.org/10.1016/j.febslet.2005.12.098
Bozhkov PV, Suarez MF, Filonova LH, Daniel G, Zamyatnin AA, Rodriguez-Nieto S, Zhivotovsky B, Smertenko A (2005) Cysteine protease mcII-Pa executes programmed cell death during plant embryogenesis. Proc Natl Acad Sci 102:14463–14468. https://doi.org/10.1073/pnas.0506948102
Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45:113–122. https://doi.org/10.1111/j.1365-313X.2005.02615.x
Cambra I, Martinez M, Dáder B, González-Melendi P, Gandullo J, Santamaría ME, Diaz I (2012) A cathepsin F-like peptidase involved in barley grain protein mobilization, HvPap-1, is modulated by its own propeptide and by cystatins. J Exp Bot 63:4615–4629. https://doi.org/10.1093/jxb/ers137
Cappetta E, Andolfo G, Di Matteo A, Ercolano M (2020) Empowering crop resilience to environmental multiple stress through the modulation of key response components. J Plant Physiol 246:153134. https://doi.org/10.1016/j.jplph.2020.153134
Castro PH, Couto D, Freitas S, Verde N, Macho AP, Huguet S, Botella MA, Ruiz-Albert J, Tavares RM, Bejarano ER (2016) SUMO proteases ULP1c and ULP1d are required for development and osmotic stress responses in Arabidopsis thaliana. Plant Mol Biol 92:143–159. https://doi.org/10.1007/s11103-016-0500-9
Chen H-J, Su C-T, Lin C-H, Huang G-J, Lin Y-H (2010) Expression of sweet potato cysteine protease SPCP2 altered developmental characteristics and stress responses in transgenic Arabidopsis plants. J Plant Physiol 167:838–847. https://doi.org/10.1016/j.jplph.2010.01.005
Chen G, Law K, Ho P, Zhang X, Li N (2012) EGY2, a chloroplast membrane metalloprotease, plays a role in hypocotyl elongation in Arabidopsis. Mol Biol Rep 39:2147–2155. https://doi.org/10.1007/s11033-011-0962-4
Chen J, Burke JJ, Xin Z (2018) Chlorophyll fluorescence analysis revealed essential roles of FtsH11 protease in regulation of the adaptive responses of photosynthetic systems to high temperature. BMC Plant Biol 18:1–13. https://doi.org/10.1186/s12870-018-1228-2
Coll NS, Vercammen D, Smidler A, Clover C, Van Breusegem F, Dangl JL, Epple P (2010) Arabidopsis type I metacaspases control cell death. Science 330:1393–1397. https://doi.org/10.1126/science.1194980
Coll N, Smidler A, Puigvert M, Popa C, Valls M, Dangl J (2014) The plant metacaspase AtMC1 in pathogen-triggered programmed cell death and aging: functional linkage with autophagy. Cell Death Differ 21:1399–1408. https://doi.org/10.1038/cdd.2014.50
Costa M-CD, Farrant JM (2019) Plant Resistance to Abiotic Stresses. In. Multidisciplinary Digital Publishing Institute
Cui S, Huang F, Wang J, Ma X, Cheng Y, Liu J (2005) A proteomic analysis of cold stress responses in rice seedlings. Proteomics 5:3162–3172. https://doi.org/10.1002/pmic.200401148
Dai X, Xu Y, Ma Q, Xu W, Wang T, Xue Y, Chong K (2007) Overexpression of an R1R2R3 MYB gene, OsMYB3R-2, increases tolerance to freezing, drought, and salt stress in transgenic Arabidopsis. Plant Physiol 143:1739–1751. https://doi.org/10.1104/pp.106.094532
de Carvalho MHC, d’Arcy-Lameta A, Roy-Macauley H, Gareil M, El Maarouf H, Pham-Thi A-T, Zuily-Fodil Y (2001) Aspartic protease in leaves of common bean (Phaseolus vulgaris L.) and cowpea (Vigna unguiculata L. Walp): enzymatic activity, gene expression and relation to drought susceptibility. FEBS Lett 492:242–246. https://doi.org/10.1016/S0014-5793(01)02259-1
Delorme VG, McCabe PF, Kim D-J, Leaver CJ (2000) A matrix metalloproteinase gene is expressed at the boundary of senescence and programmed cell death in cucumber. Plant Physiol 123:917–928. https://doi.org/10.1104/pp.123.3.917
Deng J, Zhu F, Liu J, Zhao Y, Wen J, Wang T, Dong J (2019) Transcription factor bHLH2 represses CYSTEINE PROTEASE77 to negatively regulate nodule senescence. Plant Physiol 181:1683–1703. https://doi.org/10.1104/pp.19.00574
Desikan R, Horák J, Chaban C, Mira-Rodado V, Witthöft J, Elgass K, Grefen C, Cheung M-K, Meixner AJ, Hooley R (2008) The histidine kinase AHK5 integrates endogenous and environmental signals in Arabidopsis guard cells. PLoS ONE 3:e2491. https://doi.org/10.1371/journal.pone.0002491
Dixon MS, Golstein C, Thomas CM, Van der Biezen EA, Jones JD (2000) Genetic complexity of pathogen perception by plants: the example of Rcr3, a tomato gene required specifically by Cf-2. Proc Natl Acad Sci 97:8807–8814. https://doi.org/10.1073/pnas.97.16.8807
Doelling JH, Phillips AR, Soyler-Ogretim G, Wise J, Chandler J, Callis J, Otegui MS, Vierstra RD (2007) The ubiquitin-specific protease subfamily UBP3/UBP4 is essential for pollen development and transmission in Arabidopsis. Plant Physiol 145:801–813. https://doi.org/10.1104/pp.106.095323
Fourquin C, Beauzamy L, Chamot S, Creff A, Goodrich J, Boudaoud A, Ingram G (2016) Mechanical stress mediated by both endosperm softening and embryo growth underlies endosperm elimination in Arabidopsis seeds. Development 143:3300–3305. https://doi.org/10.1242/dev.137224
Furlan AL, Bianucci E, Castro S (2016) Signaling role of ROS in modulating drought stress tolerance. In: Drought Stress Tolerance in Plants, Vol 1. Springer, pp 309–330. https://doi.org/10.1007/978-3-319-28899-4_13
Galvez-Valdivieso G, Fryer MJ, Lawson T, Slattery K, Truman W, Smirnoff N, Asami T, Davies WJ, Jones AM, Baker NR (2009) The high light response in Arabidopsis involves ABA signaling between vascular and bundle sheath cells. Plant Cell 21:2143–2162. https://doi.org/10.1105/tpc.108.061507
García-Lorenzo, M., 2007. The role of proteases in plant development (Doctoral dissertation, Kemi).
Gepstein S, Sabehi G, Carp MJ, Hajouj T, Nesher MFO, Yariv I, Dor C, Bassani M (2003) Large-scale identification of leaf senescence-associated genes. Plant J 36:629–642. https://doi.org/10.1046/j.1365-313X.2003.01908.x
Gibala M, Kicia M, Sakamoto W, Gola EM, Kubrakiewicz J, Smakowska E, Janska H (2009) The lack of mitochondrial AtFtsH4 protease alters Arabidopsis leaf morphology at the late stage of rosette development under short-day photoperiod. Plant J 59:685–699. https://doi.org/10.1111/j.1365-313X.2009.03907.x
Gilroy EM, Hein I, Van Der Hoorn R, Boevink PC, Venter E, McLellan H, Kaffarnik F, Hrubikova K, Shaw J, Holeva M (2007) Involvement of cathepsin B in the plant disease resistance hypersensitive response. Plant J 52:1–13. https://doi.org/10.1111/j.1365-313X.2007.03226.x
Golldack D, Popova OV, Dietz K-J (2002) Mutation of the matrix metalloproteinase At2-MMP inhibits growth and causes late flowering and early senescence in Arabidopsis. J Biol Chem 277:5541–5547. https://doi.org/10.1074/jbc.M106197200
Goodman RN, Király Z, Wood KR (1986) The biochemistry and physiology of plant disease. University of Missouri Press. https://doi.org/10.1086/415445
Gudesblat GE, Iusem ND, Morris PC (2007) Guard cell-specific inhibition of Arabidopsis MPK3 expression causes abnormal stomatal responses to abscisic acid and hydrogen peroxide. New Phytol 173:713–721. https://doi.org/10.1111/j.1469-8137.2006.01953.x
Guo R, Xu X, Carole B, Li X, Gao M, Zheng Y, Wang X (2013) Genome-wide identification, evolutionary and expression analysis of the aspartic protease gene superfamily in grape. BMC Genomics 14:1–18. https://doi.org/10.1186/1471-2164-14-554
Han S, Green L, Schnell DJ (2009) The signal peptide peptidase is required for pollen function in Arabidopsis. Plant Physiol 149:1289–1301. https://doi.org/10.1104/pp.108.130252
Hankamer B, Morris E, Nield J, Carne A, Barber J (2001) Subunit positioning and transmembrane helix organisation in the core dimer of photosystem II. FEBS Lett 504:142–151. https://doi.org/10.1016/S0014-5793(01)02766-1
Hasanuzzaman M, Hossain MA, da Silva JAT, Fujita M (2012) Plant response and tolerance to abiotic oxidative stress: antioxidant defense is a key factor. In: Crop stress and its management: perspectives and strategies. Springer, pp 261–315 https://doi.org/10.1007/978-94-007-2220-0_8
Hatsugai N, Kuroyanagi M, Yamada K, Meshi T, Tsuda S, Kondo M, Nishimura M, Hara-Nishimura I (2004) A plant vacuolar protease, VPE, mediates virus-induced hypersensitive cell death. Science 305:855–858. https://doi.org/10.1126/science.1099859
Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I (2006) A cellular suicide strategy of plants: vacuole-mediated cell death. Apoptosis 11:905–911. https://doi.org/10.1007/s10495-006-6601-1
Hatsugai N, Iwasaki S, Tamura K, Kondo M, Fuji K, Ogasawara K, Nishimura M, Hara-Nishimura I (2009) A novel membrane fusion-mediated plant immunity against bacterial pathogens. Genes Development 23:2496–2506. https://doi.org/10.1101/gad.1825209
Haußühl K, Andersson B, Adamska I (2001) A chloroplast DegP2 protease performs the primary cleavage of the photodamaged D1 protein in plant photosystem II. EMBO J 20:713–722. https://doi.org/10.1093/emboj/20.4.713
He J, Duan Y, Hua D, Fan G, Wang L, Liu Y, Chen Z, Han L, Qu L-J, Gong Z (2012) DEXH box RNA helicase–mediated mitochondrial reactive oxygen species production in Arabidopsis mediates crosstalk between abscisic acid and auxin signaling. Plant Cell 24:1815–1833
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J 61:1041–1052. https://doi.org/10.1111/j.1365-313X.2010.04124.x
Hollósy F (2002) Effects of ultraviolet radiation on plant cells. Micron 33:179–197. https://doi.org/10.1016/S0968-4328(01)00011-7
Hunt L, Gray JE (2009) The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development. Curr Biol 19:864–869. https://doi.org/10.1016/j.cub.2009.03.069
Ilyas M, Hörger AC, Bozkurt TO, Van Den Burg HA, Kaschani F, Kaiser M, Belhaj K, Smoker M, Joosten MH, Kamoun S, Van Der Hoorn RA (2015) Functional divergence of two secreted immune proteases of tomato. Current Biology 25: 2300–2306.https://doi.org/10.1016/j.cub.2015.07.030
Jaspers P, Kangasjärvi J (2010) Reactive oxygen species in abiotic stress signaling. Physiol Plant 138:405–413. https://doi.org/10.1111/j.1399-3054.2009.01321.x
Johnson KL, Faulkner C, Jeffree CE, Ingram GC (2008) The phytocalpain defective kernel 1 is a novel Arabidopsis growth regulator whose activity is regulated by proteolytic processing. Plant Cell 20:2619–2630. https://doi.org/10.1105/tpc.108.059964
Jones JT, Mullet JE (1995) A salt-and dehydration-inducible pea gene, Cyp15a, encodes a cell-wall protein with sequence similarity to cysteine proteases. Plant Mol Biol 28:1055–1065. https://doi.org/10.1007/BF00032666
Kanaoka MM, Urban S, Freeman M, Okada K (2005) An Arabidopsis Rhomboid homolog is an intramembrane protease in plants. FEBS Lett 579:5723–5728. https://doi.org/10.1016/j.febslet.2005.09.049
Kashiwagi J, Krishnamurthy L, Upadhyaya HD, Krishna H, Chandra S, Vadez V, Serraj R (2005) Genetic variability of drought-avoidance root traits in the mini-core germplasm collection of chickpea (Cicer arietinum L.). Euphytica 146:213–222. https://doi.org/10.1007/s10681-005-9007-1
Kato Y, Sakamoto W (2018) FtsH protease in the thylakoid membrane: physiological functions and the regulation of protease activity. Front Plant Sci 9:855. https://doi.org/10.3389/fpls.2018.00855
Kato Y, Miura E, Ido K, Ifuku K, Sakamoto W (2009) The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen species. Plant Physiol 151:1790–1801. https://doi.org/10.1104/pp.109.146589
Keeler SJ, Boettger CM, Haynes JG, Kuches KA, Johnson MM, Thureen DL, Keeler CL, Kitto SL (2000) Acquired thermotolerance and expression of the HSP100/ClpB genes of lima bean. Plant Physiol 123:1121–1132. https://doi.org/10.1104/pp.123.3.1121
Kihara A, Akiyama Y, Ito K (1996) A protease complex in the Escherichia coli plasma membrane: HflKC (HflA) forms a complex with FtsH (HflB), regulating its proteolytic activity against SecY. EMBO J 15:6122–6131. https://doi.org/10.1002/j.1460-2075.1996.tb01000.x
Kiyosue T, Yamaguchishinozaki K, Shinozaki K (1993) Characterization of cDNA for a dehydration-inducible gene that encodes a CLP A, B-like protein in Arabidopsis thaliana L. Biochem Biophys Res Commun 196:1214–1220. https://doi.org/10.1006/bbrc.1993.2381
Kmiec-Wisniewska B, Krumpe K, Urantowka A, Sakamoto W, Pratje E, Janska H (2008) Plant mitochondrial rhomboid, AtRBL12, has different substrate specificity from its yeast counterpart. Plant Mol Biol 68:159–171. https://doi.org/10.1007/s11103-008-9359-8
Koizumi M, Yamaguchi-Shinozaki K, Tsuji H, Shinozaki K (1993) Structure and expression of two genes that encode distinct drought-inducible cysteine proteinases in Arabidopsis thaliana. Gene 129:175–182. https://doi.org/10.1016/0378-1119(93)90266-6
Komenda J, Barker M, Kuviková S, de Vries R, Mullineaux CW, Tichý M, Nixon PJ (2006) The FtsH protease slr0228 is important for quality control of photosystem II in the thylakoid membrane of Synechocystis sp. PCC 6803. J Biol Chem 281:1145–1151. https://doi.org/10.1074/jbc.M503852200
Krüger J, Thomas CM, Golstein C, Dixon MS, Smoker M, Tang S, Mulder L, Jones JD (2002) A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 296:744–747. https://doi.org/10.1126/science.1069288
Krynická V, Shao S, Nixon PJ, Komenda J (2015) Accessibility controls selective degradation of photosystem II subunits by FtsH protease. Nature Plants 1:1–6. https://doi.org/10.1038/nplants.2015.168
Kuroda H, Maliga P (2003) The plastid clpP1 protease gene is essential for plant development. Nature 425:86–89. https://doi.org/10.1038/nature01909
Langer T (2000) AAA proteases: cellular machines for degrading membrane proteins. Trends Biochem Sci 25:247–251. https://doi.org/10.1016/S0968-0004(99)01541-8
Lee U, Rioflorido I, Hong SW, Larkindale J, Waters ER, Vierling E (2007) The Arabidopsis ClpB/Hsp100 family of proteins: chaperones for stress and chloroplast development. Plant J 49:115–127. https://doi.org/10.1111/j.1365-313X.2006.02940.x
Lehfeldt C, Shirley AM, Meyer K, Ruegger MO, Cusumano JC, Viitanen PV, Strack D, Chapple C (2000) Cloning of the SNG1 gene of Arabidopsis reveals a role for a serine carboxypeptidase-like protein as an acyltransferase in secondary metabolism. Plant Cell 12:1295–1306. https://doi.org/10.1105/tpc.12.8.1295
Le Roux ML, Kunert KJ, Van der Vyver C, Cullis CA, Botha A-M (2019) Expression of a small ubiquitin-like modifier protease increases drought tolerance in wheat (Triticum aestivum L.). Frontiers in plant science 10:266. https://doi.org/10.3389/fpls.2019.00266
Li J, Lease KA, Tax FE, Walker JC (2001) BRS1, a serine carboxypeptidase, regulates BRI1 signaling in Arabidopsis thaliana. Proc Natl Acad Sci 98:5916–5921. https://doi.org/10.1073/pnas.091065998
Li X, Mu Y, Sun X, Zhang L (2010) Increased sensitivity to drought stress in atlon4 Arabidopsis mutant. Chin Sci Bull 55:3668–3672. https://doi.org/10.1007/s11434-010-4166-4
Li L, Nelson C, Fenske R, Trösch J, Pružinská A, Millar AH, Huang S (2017) Changes in specific protein degradation rates in Arabidopsis thaliana reveal multiple roles of Lon1 in mitochondrial protein homeostasis. Plant J 89:458–471. https://doi.org/10.1111/tpj.13392
Liu Y, Wang F, Zhang H, He H, Ma L, Deng XW (2008) Functional characterization of the Arabidopsis ubiquitin-specific protease gene family reveals specific role and redundancy of individual members in development. Plant J 55:844–856. https://doi.org/10.1111/j.1365-313X.2008.03557.x
Liu Y, Qin L, Han L, Xiang Y, Zhao D (2015) Overexpression of maize SDD1 (ZmSDD1) improves drought resistance in Zea mays L. by reducing stomatal density. Plant Cell. Tissue and Organ Culture (PCTOC) 122:147–159. https://doi.org/10.1007/s11240-015-0757-8
Liu S, Liu Y, Jia Y, Wei J, Wang S, Liu X, Zhou Y, Zhu Y, Gu W, Ma H (2017) Gm1-MMP is involved in growth and development of leaf and seed, and enhances tolerance to high temperature and humidity stress in transgenic Arabidopsis. Plant Sci 259:48–61. https://doi.org/10.1016/j.plantsci.2017.03.005
Lohman KN, Gan S, John MC, Amasino RM (1994) Molecular analysis of natural leaf senescence in Arabidopsis thaliana. Physiol Plant 92:322–328. https://doi.org/10.1111/j.1399-3054.1994.tb05343.x
Lopez L, Camas A, Shivaji R, Ankala A, Williams P, Luthe D (2007) Mir1-CP, a novel defense cysteine protease accumulates in maize vascular tissues in response to herbivory. Planta 226:517–527. https://doi.org/10.1007/s00425-007-0501-7
Lu W, Deng M, Guo F, Wang M, Zeng Z, Han N, Yang Y, Zhu M, Bian H (2016) Suppression of OsVPE3 enhances salt tolerance by attenuating vacuole rupture during programmed cell death and affects stomata development in rice. Rice 9:1–13. https://doi.org/10.1186/s12284-016-0138-x
Mao C, Ding J, Zhang B, Xi D, Ming F (2018) Os NAC 2 positively affects salt-induced cell death and binds to the Os AP 37 and Os COX 11 promoters. Plant J 94:454–468. https://doi.org/10.1111/tpj.13867
Marta K, Marta G, Adam U, Hanna J (2007) Significance of Arabidopsis AAA proteases for activity and assembly/stability of mitochondrial OXPHOS complexes. Physiologia plantarum 129: 135–142 .https://doi.org/10.1111/j.1399-3054.2006.00835.x
Martin MN, Saladores PH, Lambert E, Hudson AO, Leustek T (2007) Localization of members of the γ-glutamyl transpeptidase family identifies sites of glutathione and glutathione S-conjugate hydrolysis. Plant Physiol 144:1715–1732. https://doi.org/10.1104/pp.106.094409
Martins A, Ring A, Omnus DJ, Heessen S, Pfirrmann T, Ljungdahl PO (2019) Spatial and temporal regulation of the endoproteolytic activity of the SPS-sensor–controlled Ssy5 signaling protease. Mol Biol Cell 30:2709–2720. https://doi.org/10.1091/mbc.E19-02-0096
McKinnon LJ, Fukushima J, Inoue K, Theg SM (2019) Lipid Chaperoning of a Thylakoid Protease Whose Stability is Modified by the Protonmotive Force. bioRxiv 788471. https://doi.org/10.1101/788471
McLellan H, Gilroy EM, Yun BW, Birch PR, Loake GJ (2009) Functional redundancy in the Arabidopsis Cathepsin B gene family contributes to basal defence, the hypersensitive response and senescence. New Phytol 183:408–418. https://doi.org/10.1111/j.1469-8137.2009.02865.x
Menzel C, Simpson D (1988) Effect of temperature on growth and flowering of litchi (Litchi chinensis Sonn.) cultivars. Journal of Horticultural Science 63:349–360. https://doi.org/10.1080/14620316.1988.11515869
Mishra RC, Grover A (2016) Constitutive over-expression of rice ClpD1 protein enhances tolerance to salt and desiccation stresses in transgenic Arabidopsis plants. Plant Sci 250:69–78. https://doi.org/10.1016/j.plantsci.2016.06.004
Mithöfer A, Schulze B, Boland W (2004) Biotic and heavy metal stress response in plants: evidence for common signals. FEBS Lett 566:1–5. https://doi.org/10.1016/j.febslet.2004.04.011
Miura K, Furumoto T (2013) Cold signaling and cold response in plants. Int J Mol Sci 14:5312–5337. https://doi.org/10.3390/ijms14035312
Møller IM, Sweetlove LJ (2010) ROS signalling–specificity is required. Trends Plant Sci 15:370–374. https://doi.org/10.1016/j.tplants.2010.04.008
Moschou PN, Gutierrez-Beltran E, Bozhkov PV, Smertenko A (2016) Separase promotes microtubule polymerization by activating CENP-E-related kinesin Kin7. Dev Cell 37:350–361. https://doi.org/10.1016/j.devcel.2016.04.015
Mótyán JA, Tóth F, Tőzsér J (2013) Research applications of proteolytic enzymes in molecular biology. Biomolecules 3:923–942. https://doi.org/10.3390/biom3040923
Munns R, Tester M (2008) Mechanisms of salinity tolerance. Annu Rev Plant Biol 59:651–681. https://doi.org/10.1146/annurev.arplant.59.032607.092911
Nakagami H, Soukupová H, Schikora A, Zárský V, Hirt H (2006) A mitogen-activated protein kinase kinase kinase mediates reactive oxygen species homeostasis in Arabidopsis. J Biol Chem 281:38697–38704. https://doi.org/10.1074/jbc.M605293200
Nayyar H, Kaur S, Smita SK, Dhir K, Bains T (2005) Water stress-induced injury to reproductive phase in chickpea: evaluation of stress sensitivity in wild and cultivated species in relation to abscisic acid and polyamines. J Agron Crop Sci 191:450–457. https://doi.org/10.1111/j.1439-037X.2005.00184.x
Nixon PJ, Michoux F, Yu J, Boehm M, Komenda J (2010) Recent advances in understanding the assembly and repair of photosystem II. Ann Bot 106:1–16. https://doi.org/10.1093/aob/mcq059
Parida AK, Das AB, Mittra B, Mohanty P (2004) Salt-stress induced alterations in protein profile and protease activity in the mangrove Bruguiera parviflora. Zeitschrift Für Naturforschung C 59:408–414. https://doi.org/10.1515/znc-2004-5-622
Pasternak T, Potters G, Caubergs R, Jansen MA (2005) Complementary interactions between oxidative stress and auxins control plant growth responses at plant, organ, and cellular level. J Exp Bot 56:1991–2001. https://doi.org/10.1093/jxb/eri196
Patipong T, Hibino T, Kageyama H, Waditee-Sirisattha R (2020) The evolutionarily conserved HtrA is associated with stress tolerance and protein homeostasis in the halotolerant cyanobacterium Halothece sp. PCC7418. Extremophiles 1–13. https://doi.org/10.1007/s00792-020-01162-4
Perroud PF, Meyberg R, Demko V, Quatrano RS, Olsen OA, Rensing SA (2020) DEK1 displays a strong subcellular polarity during Physcomitrella patens 3D growth. New Phytol 226:1029–1041. https://doi.org/10.1111/nph.16417
Petrov V, Hille J, Mueller-Roeber B, Gechev TS (2015) ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci 6:69. https://doi.org/10.3389/fpls.2015.00069
Piechota J, Kolodziejczak M, Juszczak I, Sakamoto W, Janska H (2010) Identification and characterization of high molecular weight complexes formed by matrix AAA proteases and prohibitins in mitochondria of Arabidopsis thaliana. J Biol Chem 285:12512–12521. https://doi.org/10.1074/jbc.M109.063644
Pingping WU, Chubin WU, Biyan ZHOU (2017) Drought stress induces flowering and enhances carbohydrate accumulation in Averrhoa Carambola. Horticultural Plant Journal 3:60–66. https://doi.org/10.1016/j.hpj.2017.07.008
Pinti M, Gibellini L, Nasi M, De Biasi S, Bortolotti CA, Iannone A, Cossarizza A (2016) Emerging role of Lon protease as a master regulator of mitochondrial functions. Biochim Biophys Acta 1857:1300–1306. https://doi.org/10.1016/j.bbabio.2016.03.025
Popp HW, Brown F (1933) A review of recent work on the effect of ultraviolet radiation upon seed plants. Bull Torrey Bot Club. https://doi.org/10.2307/2480419
Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MA (2007) Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci 12:98–105. https://doi.org/10.1016/j.tplants.2007.01.004
Prasad BD, Creissen G, Lamb C, Chattoo BB (2009) Overexpression of rice (Oryza sativa L.) OsCDR1 leads to constitutive activation of defense responses in rice and Arabidopsis. Mol Plant Microbe Interact 22:1635–1644. https://doi.org/10.1094/MPMI-22-12-1635
Pulido P, Llamas E, Rodriguez-Concepcion M (2017) Both Hsp70 chaperone and Clp protease plastidial systems are required for protection against oxidative stress. Plant Signal Behav 12:e1290039. https://doi.org/10.1080/15592324.2017.1290039
Ramírez V, López A, Mauch-Mani B, Gil MJ, Vera P (2013) An extracellular subtilase switch for immune priming in Arabidopsis. PLoS Pathog 9:e1003445. https://doi.org/10.1371/journal.ppat.1003445
Rawlings ND, Barrett AJ, Thomas PD, Huang X, Bateman A, Finn RD (2018) The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res 46:D624–D632. https://doi.org/10.1093/nar/gkx1134
Rocha GL, Fernandez JH, Oliveira AEA, Fernandes KVS (2017) Programmed cell death-related proteases in plants. Enzyme Inhibitors and Activators. https://doi.org/10.5772/65938
Rodriguez P, Dell’Amico J, Morales D, Sanchez Blanco MdJ, Alarcón J (1997) Effect of salinity on growth, shoot water relations and root hydraulic conductivity in tomato plants. J Agric Sci 128:439–444. https://doi.org/10.1017/s0021859697004309
Rodríguez-Herva JJ, González-Melendi P, Cuartas-Lanza R, Antúnez-Lamas M, Río-Alvarez I, Li Z, López-Torrejón G, Díaz I, Del Pozo JC, Chakravarthy S (2012) A bacterial cysteine protease effector protein interferes with photosynthesis to suppress plant innate immune responses. Cell Microbiol 14:669–681. https://doi.org/10.1111/j.1462-5822.2012.01749.x
Sakamoto W, Tamura T, Hanba-Tomita Y, Sodmergen MM (2002) The VAR1 locus of Arabidopsis encodes a chloroplastic FtsH and is responsible for leaf variegation in the mutant alleles. Genes Cells 7:769–780. https://doi.org/10.1046/j.1365-2443.2002.00558.x
Sánchez-Morán E, Jones GH, Franklin FCH, Santos JL (2004) A puromycin-sensitive aminopeptidase is essential for meiosis in Arabidopsis thaliana. Plant Cell 16:2895–2909. https://doi.org/10.1105/tpc.104.024992
Schaller A (2004) A cut above the rest: the regulatory function of plant proteases. Planta 220:183–197. https://doi.org/10.1007/s00425-004-1407-2
Schuhmann H, Adamska I (2012) Deg proteases and their role in protein quality control and processing in different subcellular compartments of the plant cell. Physiol Plant 145:224–234. https://doi.org/10.1111/j.1399-3054.2011.01533.x
Sebastián DI, Fernando FD, Gabriela RDG, GM, (2020) Overexpression of Arabidopsis aspartic protease APA1 gene confers drought tolerance. Plant Sci. https://doi.org/10.1016/j.plantsci.2020.110406
Shabab M, Shindo T, Gu C, Kaschani F, Pansuriya T, Chintha R, Harzen A, Colby T, Kamoun S, van der Hoorn RA (2008) Fungal effector protein AVR2 targets diversifying defense-related cys proteases of tomato. Plant Cell 20:1169–1183. https://doi.org/10.1105/tpc.107.056325
Shah ZH, Rehman HM, Akhtar T, Daur I, Nawaz MA, Ahmad MQ, Rana IA, Atif RM, Yang SH, Chung G (2017) Redox and ionic homeostasis regulations against oxidative, salinity and drought stress in wheat (a systems biology approach). Front Genet 8:141. https://doi.org/10.3389/fgene.2017.00141
Shen G, Yan J, Pasapula V, Luo J, He C, Clarke AK, Zhang H (2007) The chloroplast protease subunit ClpP4 is a substrate of the E3 ligase AtCHIP and plays an important role in chloroplast function. Plant J 49:228–237. https://doi.org/10.1111/j.1365-313X.2006.02963.x
Shi L, Wang Z, Kim WS (2019) Effect of drought stress on shoot growth and physiological response in the cut rose ‘charming black’at different developmental stages. Hortic Environ Biotechnol 60:1–8. https://doi.org/10.1007/s13580-018-0098-8
Shimada T, Sugano SS, Hara-Nishimura I (2011) Positive and negative peptide signals control stomatal density. Cell Mol Life Sci 68:2081–2088. https://doi.org/10.1007/s00018-011-0685-7
Shirley AM, McMichael CM, Chapple C (2001) The sng2 mutant of Arabidopsis is defective in the gene encoding the serine carboxypeptidase-like protein sinapoylglucose: choline sinapoyltransferase. Plant J 28:83–94. https://doi.org/10.1046/j.1365-313X.2001.01123.x
Singh A, Singh U, Mittal D, Grover A (2010) Genome-wide analysis of rice ClpB/HSP100, ClpC and ClpD genes. BMC Genomics 11:1–18. https://doi.org/10.1186/1471-2164-11-95
Sjögren LL, Stanne TM, Zheng B, Sutinen S, Clarke AK (2006) Structural and functional insights into the chloroplast ATP-dependent Clp protease in Arabidopsis. Plant Cell 18(10):2635–2649. https://doi.org/10.1105/tpc.106.044594
Soares AFM (2017) RLR1 and RLR2, two novel Arabidopsis thaliana atypical aspartic proteases involved in primary root development and lateral root formation. Universidade de Coimbra http://hdl.handle.net/10316/31662
Sultana N, Ikeda T, Itoh R (1999) Effect of NaCl salinity on photosynthesis and dry matter accumulation in developing rice grains. Environ Exp Bot 42:211–220. https://doi.org/10.1016/S0098-8472(99)00035-0
Sun X, Wang L, Zhang L (2007) Involvement of DEG5 and DEG8 proteases in the turnover of the photosystem II reaction center D1 protein under heat stress in Arabidopsis thaliana. Chin Sci Bull 52:1742–1745. https://doi.org/10.1007/s11434-007-0275-0
Susin MF, Baldini RL, Gueiros-Filho F, Gomes SL (2006) GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. J Bacteriol 188:8044–8053. https://doi.org/10.1128/JB.00824-06
Suzuki K, Nagasuga K, Okada M (2008) The chilling injury induced by high root temperature in the leaves of rice seedlings. Plant Cell Physiol 49:433–442. https://doi.org/10.1093/pcp/pcn020
Taffouo V, Wamba O, Youmbi E, Nono G, Akoa A (2010) Growth, yield, water status and ionic distribution response of three bambara groundnut (Vigna subterranea (L.) Verdc.) landraces grown under saline conditions. Int J Bot 6:53–58. https://doi.org/10.3923/ijb.2010.53.5810.3923/ijb.2010.53.5810.3923/ijb.2010.53.58
Tan BC, Schwartz SH, Zeevaart JA, McCarty DR (1997) Genetic control of abscisic acid biosynthesis in maize. Proc Natl Acad Sci 94:12235–12240. https://doi.org/10.1073/pnas.94.22.12235
Thomas EL, Van der Hoorn RA (2018) Ten prominent host proteases in plant-pathogen interactions. Int J Mol Sci 19:639. https://doi.org/10.3390/ijms19020639
Thompson EP, Llewellyn Smith SG, Glover BJ (2012) An Arabidopsis rhomboid protease has roles in the chloroplast and in flower development. J Exp Bot 63:3559–3570. https://doi.org/10.1093/jxb/ers012
Tigchelaar M, Battisti DS, Naylor RL, Ray DK (2018) Future warming increases probability of globally synchronized maize production shocks. Proc Natl Acad Sci 115:6644–6649. https://doi.org/10.1073/pnas.1718031115
Tran D, Galletti R, Neumann ED, Dubois A, Sharif-Naeini R, Geitmann A, Frachisse J-M, Hamant O, Ingram GC (2017) A mechanosensitive Ca 2+ channel activity is dependent on the developmental regulator DEK1. Nat Commun 8:1–8. https://doi.org/10.1038/s41467-017-00878-w
Tsitsekian D, Daras G, Alatzas A, Templalexis D, Hatzopoulos P, Rigas S (2019) Comprehensive analysis of Lon proteases in plants highlights independent gene duplication events. J Exp Bot 70:2185–2197. https://doi.org/10.1093/jxb/ery440
Turk B (2006) Targeting proteases: successes, failures and future prospects. Nat Rev Drug Discovery 5:785–799. https://doi.org/10.1038/nrd2092
Turk H, Erdal S, Dumlupinar R (2020) Carnitine-induced physio-biochemical and molecular alterations in maize seedlings in response to cold stress. Archives of Agronomy and Soil Science 66:925–941. https://doi.org/10.1080/03650340.2019.1647336
Van der Hoorn RA (2008) Plant proteases: from phenotypes to molecular mechanisms. Annu Rev Plant Biol 59:191–223. https://doi.org/10.1146/annurev.arplant.59.032607.092835
van Wijk KJ (2015) Protein maturation and proteolysis in plant plastids, mitochondria, and peroxisomes. Annu Rev Plant Biol 66:75–111. https://doi.org/10.1146/annurev-arplant-043014-115547
Van Wyk SG, Du Plessis M, Cullis CA, Kunert KJ, Vorster BJ (2014) Cysteine protease and cystatin expression and activity during soybean nodule development and senescence. BMC Plant Biol 14:1–13. https://doi.org/10.1186/s12870-014-0294-3
Verstraeten N, Fauvart M, Versées W, Michiels J (2011) The universally conserved prokaryotic GTPases. Microbiol Mol Biol Rev 75:507–542. https://doi.org/10.1128/MMBR.00009-11
Wagner R, Aigner H, Funk C (2012) FtsH proteases located in the plant chloroplast. Physiol Plant 145:203–214. https://doi.org/10.1111/j.1399-3054.2011.01548.x
Wang W-H, He E-M, Guo Y, Tong Q-X, Zheng H-L (2016) Chloroplast calcium and ROS signaling networks potentially facilitate the primed state for stomatal closure under multiple stresses. Environ Exp Bot 122:85–93. https://doi.org/10.1016/j.envexpbot.2015.09.008
Wang Q, Guo Q, Guo Y, Yang J, Wang M, Duan X, Niu J, Liu S, Zhang J, Lu Y (2018) Arabidopsis subtilase SASP is involved in the regulation of ABA signaling and drought tolerance by interacting with OPEN STOMATA 1. J Exp Bot 69:4403–4417. https://doi.org/10.1093/jxb/ery205
Went F (1953) The effect of temperature on plant growth. Annu Rev Plant Physiol 4:347–362. https://doi.org/10.1146/annurev.pp.04.060153.002023
Yamada K, Matsushima R, Nishimura M, Hara-Nishimura I (2001) A slow maturation of a cysteine protease with a granulin domain in the vacuoles of senescing Arabidopsis leaves. Plant Physiol 127:1626–1634. https://doi.org/10.1104/pp.010551
Yan N, Doelling JH, Falbel TG, Durski AM, Vierstra RD (2000) The ubiquitin-specific protease family from Arabidopsis. AtUBP1 and 2 are required for the resistance to the amino acid analog canavanine. Plant Physiol 124:1828–1843. https://doi.org/10.1104/pp.124.4.1828
Yang P, Smalle J, Lee S, Yan N, Emborg TJ, Vierstra RD (2007) Ubiquitin C-terminal hydrolases 1 and 2 affect shoot architecture in Arabidopsis. Plant J 51:441–457. https://doi.org/10.1111/j.1365-313X.2007.03154.x
Yao X, Xiong W, Ye T, Wu Y (2012) Overexpression of the aspartic protease ASPG1 gene confers drought avoidance in Arabidopsis. J Exp Bot 63:2579–2593. https://doi.org/10.1093/jxb/err433
Yilmaz H, Kina A (2008) The influence of NaCl salinity on some vegetative and chemical changes of strawberries (Fragaria x ananssa L.). Afr J Biotechnol. https://doi.org/10.5897/AJB08.574
Yue G, Hu X, He Y, Yang A, Zhang J (2010) Identification and characterization of two members of the FtsH gene family in maize (Zea mays L.). Mol Biol Rep 37(2):855–863. https://doi.org/10.1007/s11033-009-9691-3
Zamyatnin A (2015) Plant proteases involved in regulated cell death. Biochem Mosc 80:1701–1715. https://doi.org/10.1134/S0006297915130064
Zhang D, Liu D, Lv X, Wang Y, Xun Z, Liu Z, Li F, Lu H (2014a) The cysteine protease CEP1, a key executor involved in tapetal programmed cell death, regulates pollen development in Arabidopsis. Plant Cell 26:2939–2961. https://doi.org/10.1105/tpc.114.127282
Zhang S, Wu J, Yuan D, Zhang D, Huang Z, Xiao L, Yang C (2014b) Perturbation of auxin homeostasis caused by mitochondrial FtSH4 gene-mediated peroxidase accumulation regulates Arabidopsis architecture. Mol Plant 7:856–873. https://doi.org/10.1093/mp/ssu006
Zhang S, Li C, Wang R, Chen Y, Shu S, Huang R, Zhang D, Li J, Xiao S, Yao N (2017) The Arabidopsis mitochondrial protease FtSH4 is involved in leaf senescence via regulation of WRKY-dependent salicylic acid accumulation and signaling. Plant Physiol 173:2294–2307. https://doi.org/10.1104/pp.16.00008
Acknowledgements
DG is gratefully acknowledging SERB-DST grant (SRG/2020/001024) and Central University of Rajasthan research support. PS is gratefully acknowledging Central University of Rajasthan for financial support.
Author information
Authors and Affiliations
Contributions
DG conceived the idea and designed the concept. The manuscript was prepared with the potential contribution of PS and DG. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by Aryadeep Roychoudhury.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sharma, P., Gayen, D. Plant protease as regulator and signaling molecule for enhancing environmental stress-tolerance. Plant Cell Rep 40, 2081–2095 (2021). https://doi.org/10.1007/s00299-021-02739-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-021-02739-9