Abstract
To investigate the responses of the cut rose ‘Charming Black’ to drought stress at different growth stages, we defined five stages of flowering and shoot development defined from initiation of the axillary bud to full complement of the floral parts. Drought stress was applied from earliest bud break (stage 1) to floral bud appearance (stage 5). After the stage of treatment, irrigation was restored to normal levels. Results showed that rose plant growth was not only influenced by the environment but also by different stages of floral bud appearance. The stem length as well as the time it took to reach different stages of rose development was influenced by the applied irrigation and supplemental lighting conditions. Drought stress did not have negative effects on the quality of the flower at stage 1. Drought stress reduced the vegetative growth phase and promoted flowering in the early stages (2–3), and significantly decreased shoot length, shoot weight and leaf area. However, at the stage prior to flower appearance (stage 5) drought stress was more severe. The most damage was accompanied by malformed floral buds that had shorter petal length and distorted petals. Furthermore, photosynthesis was negatively affected by drought stress at stage 5, even after re-irrigation, for which this negative effect could not be compensated.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Roses are the most important cut flower. The plants are self-inductive for flower initiation and exhibit recurrent flowering year-round (Zieslin and Moe 1985). The production system of cut roses indicates changes in biomass production during each flowering cycle; the plant biomass of cut roses may change at different stages of flower development during each flowering cycle and the pattern of biomass change is cyclically repeated during the harvesting periods (Kim and Lieth 2012; Zeislin and Mor 1990).
In water deficit conditions, water is supplied at critical growth stages; thus, it is important to define the critical growth stages for this crop. There are differences in the growth rate of cowpea during its vegetative stage (Watanabe et al. 1997), with the flowering and pod-filling stages being the most sensitive drought stress (Turk et al. 1980). In Panicum Miliaceum L., drought stress can decrease the yield and water use efficiency (WUE) during the ear emergence stage, as well as promote floret death and reduced seed size (Seghatoleslami et al. 2008). In roses, drought stress during the flowering stage of development (floral initiation until stamen initiation is complete) has detrimental effects on the quantity and quality of rose production. Drought stress prior to petal initiation affected the quality of the floral buds and reduced the number of well-formed petals and the height of the floral buds (Chimonidou-Pavlidou 1996, 1999).
A previous study showed drought stress and supplemental lighting altered plant growth and photosynthesis (Shi and Kim 2014, 2015). Sigmoid function analysis indicated a water deficit signal during vegetative growth, and a significant difference could be observed in shoot growth rate due to drought stress when vegetative growth began to switch to flowering and retarded shoot elongation. Thus, it is important to investigate the effect of drought stress at different developmental stages in rose plants, and to identify the critical growth stages.
2 Materials and methods
2.1 Plant materials and treatments
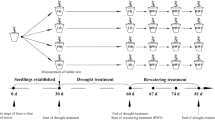
Rosa hybrida ‘Charming Black’ was planted in Rockwool slabs that were 100 cm long, 15 cm wide, and 7.5 cm deep (UR Rockwool, Pocheon, Korea) at a plant density of 5 plants m−2 in an experimental glass-covered greenhouse, located at the University of Seoul. The greenhouse was controlled the temperature at 23–26°C during the day and 17 °C during the night, a relative humidity of 61.3% with a range of 40.2–71.1%, and a daily light cycle maintained at 200 µmol m−2 s−1 between 10:00–11:00 and 14:00–18:00; 500 µmol m−2 s−1 between 11:00–14:00; and 90 µmol m−2 s−1 at night. A high-pressure sodium lamp (GEO-NH 400 W-L/P, Daekwang, Yeosu, Korea) was used as the light source. Supplemental lighting periods were 16:00–24:00 and 02:00–10:00 h with a 2 h night break. The Rosa hybrida ‘Charming Black’ plants were grown using the bending technique (Kool and Lenssen 1997), which consisted of bending over the stems that were not considered useful for flower production. Irrigation with nutrient solution of electrical conductivity (EC) 1.0 ds m−1, pH 6.0 ± 0.2 was provided every day. The supplemental nutrient solution was composed of 1841 g m−3 Ca(NO3)2·4H2O, 2323 g m−3 KNO3, 64.5 g m−3 EDTA-Fe, 204.8 g m−3 Mg(NO3)2·6H2O, 575 g m−3 (NH4)2PO4, 12.05 g m−3 MnSO4·5H2O, 8.63 g m−3 ZnSO4·7H2O, 9.27 g m−3 H3BO3, 1.25 g m−3 CuSO4·5H2O, and 0.88 g m−3 (NH4)6MO7O24·4H2O, H2SO4 provided 281 Ml. The water content was measured using the FDR method (Coco-100, Mirae Sensor, Seoul, Korea). The changes in growth stages were noted so that drought stress would be applied at exactly the right time.
Five stages of rose development from the initiation of the axillary bud to stage of full complement of floral parts were defined and modified based on Chimondifou-Pavlidou (2000):
- Stage 1:
-
Earliest bud break, where the bud separated before elongation had occurred
- Stage 2:
-
First 3-leaflet leaf clearly separated
- Stage 3:
-
First 5-leaflet leaf clearly separated
- Stage 4:
-
Second 5-leaflet leaf clearly separated
- Stage 5:
-
Floral bud appearance.
The treatments were applied as follows:
- T1:
-
Drought stress was applied at stage 1 and then irrigation was restored to 80% (84.5 ± 1.1) water content in slab since field capacity is reported to contain 80% solution until the stage when the flowering shoot reached the market stage
- T2:
-
Drought stress was applied at stage 2 and then irrigation was restored
- T3:
-
Drought stress was applied at stage 3 and then irrigation was restored
- T4:
-
Drought stress was applied at stage 4 and then irrigation was restored
- T5:
-
Drought stress was applied at stage 5 and then irrigation was restored
- Control:
-
Irrigation was applied throughout the stages of bud development and until the flower shoot reached the market stage.
In a preliminary experiment, critical water content for the ‘Charming Black’ in the greenhouse was 46.6%; when the water content in the slab was below the critical water content drought stress occurred. Therefore, in all the treatments, drought stress was applied for a 3–4 d period, according to the water content in the slab (30–40%). The rate of shoot growth was measured every day until the floral buds appeared (stage 5). The quality of cut flowers was evaluated by the length and weight of the cut flower, the length of the petals, the number of petals, and the number of days to flowering.
2.2 Photosynthesis parameter measurements
The photosynthetic parameters were measured when the youngest compound leaf had fully expanded. Photosynthetic rate was measured using a portable photosynthesis system Li-6400 (Li-Cor, Lincoln, NE, USA). Photosynthetic photon flux density (PPFD) was gradually decreased from 2000 to 0 (2000; 1500; 1000; 700; 300; 100; 80; 50; and 0 μmol m−2 s−1) in order to avoid limiting photosynthesis in high light conditions due to insufficient stomatal opening that is caused by the initial low light intensities (Singsaas et al. 2001).
Chlorophyll fluorescence in rose leaves was performed using FluorCam (FluorCam 800MF, Photon System Inc., Brno, Czech). The second leaflet was dark-adapted for 30 min and then observed using quenching kinetics analysis method. The actinic light (Act1), shutter, and intensity of light were set at 50%, 20 μs, and 80%, respectively.
To determine the leaf chlorophyll content, 0.1 g crushed leaf samples were extracted from the second leaflet, to which 10 mL 100% methanol was added. Samples were then stored in a dark place for 24 h to ensure complete extraction. Chlorophyll content was assessed by the absorbance measured at 470 nm, 652 nm, and 655 nm with a spectrophotometer (UV-2450, Shimadzu, Kyoto, Japan). Chlorophyll a, b, and a + b, and carotenoid content was measured according to the method described by Lichtenthaler (1987).
Statistical analysis included analysis of variance (ANOVA) and was calculated using statistical analysis software (version 9.3, SAS Institute Inc., Cary, NC, USA). Parameter values in the model equations were estimated with SAS protocols. Sigmoid function was performed with Sigma Plot software (Systat Software, Inc., Chicago, IL, USA).
3 Results
The effect of drought stress on rose shoot growth over time is plotted in Fig. 1. Drought stress significantly affected the growth of ‘Charming Black’ plants. Drought stress applied at the beginning of stage 1 and stage 2 (T1 and T2) did not affect plant growth. Treatments with water stress during stage 3 caused a reduction in growth by about 24.6% at the bloom stage compared to the control treatment. Moreover, drought stress applied at stage 4 and stage 5 (T4 and T5) caused growth inhibition. Three weeks after irrigation was stopped at stage 3, the shoot growth rate was inhibited and reduced by 54.1% at bloom stage. Similarly, shoot growth impairment was observed in T5, where shoot growth rate was reduced by 31.9% at the bloom stage.
When drought stress was applied at different developmental stages, it had different negative effects on the shoot growth and flower quality (Table 1). Water deficiencies during stages 3, 4, and 5 decreased the plant height and reduced leaf area but significantly promoted the transition from vegetative to flowering stages; flowering time occurred sooner by 2, 6, and 5 d in T3, T4, and T5, respectively. Plant height in ‘Charming Black’ roses subjected to water regimes in T3, T4, and T5 treatments was less than the control by 14.1, 22.2, and 7.7, and plant weight subjected to water regimes in T3, T4, and T5 treatments was less than the control by 20.8 g, 23.4 g, and 20.3 g, respectively. When drought stress was applied at stages 3 and 4, stem diameter decreased by 0.3–0.4 cm, and leaf area decreased by 243.9–280.4 cm2 compared to the control, respectively. Application of drought stress during stages 3, 4, and 5 “significantly damaged” the floral bud; petal length under treatments T3, T4, and T5 was reduced by 1.3, 1.2 and 2.3, respectively, in relation to the control. Moreover, T4 and T5 treatments caused a decrease in the average number of petals by 9 and 22.8, respectively. Therefore, malformed flowers with irregular petals occurred in T4 (40%) and malformed flowers (71.4%) appeared when drought stress was applied at stage 5. A significant reduction in floral bud diameter and petal length was observed (Table 1) .
Photosynthesis was measured after irrigation resumed in all treatment groups (Fig. 2). No significant difference was recorded among control, T1 and T2 treatments. However, net CO2 assimilation rates (An) in response to drought stress applied in stages 3, 4, and 5 were significantly reduced. The An values were 7.23 µmol CO2 m−2 s−1, 9.38 µmol CO2 m−2 s−1, and 5.66 µmol CO2 m−2 s−1 at 1000 µmol m−2 s−1 PPFD for these stages, respectively. Compared to the control treatment, we observed a reduction of 3.67 µmol CO2 m−2 s−1, 1.52 µmol CO2 m−2 s−1, 5.24 µmol CO2 m−2 s−1 in plants treated with drought stress at stages 3–5.
Leaf stomatal conductance (gs) was 0.25 mol H2O m−2 s−1 and 0.05 mol H2O m−2 s−1 in T4 and T5 at 1000 µmol m−2 s−1 PPFD, which was then reduced by 1.00 mol H2O m−2 s−1 and 1.20 mol H2O m−2 s−1, respectively. A significant decrease of 2.46 H2O m−2 s−1 at 1000 µmol m−2 s−1 PPFD was observed when the drought stress was applied at stage 5. However, T5 caused an increase in stomatal limitation (LS) by 0.18, and 6.61 in water use efficiency (E) at 1000 µmol m−2 s−1 PPFD (Fig. 3).
Chlorophyll was measured at stages 3 and 5, and at the harvest stage. Results demonstrated that the highest value occurred in T2 when chlorophyll was measured at stage 3. In relation to control, 30.6% increase could be found. When chlorophyll was measured at stage 3, the highest value was 23.91 mg.g−1 FW, but dropped by about 6.12 and 7.16 in T4 and T5. However, at harvest stage, all the stressed treatments showed a reduction compared with control. A decrease of 9.8%, 14%, 11.4%, 15.3% and 31.7% in T1, T2, T3, T4, and T5 could be observed, respectively (Fig. 4).
Chlorophyll fluorescence measurements were also applied at stage 3, stage 5 and harvest stage, respectively (Fig. 5). No significant difference could be found at stage 3 and stage 5. However, at harvest stage, FV/FM ratio exhibited a decline in T1, T2, T3, T4, and T5 by 43.0%, 71.9%, 45.9%, 61.5% and 55.6%, respectively. NPQ is considered as an important stress index, T4 and T5 showed lower NPQ value at stage 3 (53.0% and 63.5% to the control), and then increased significantly at stage 5 (139.5% and 141.4% to the control). At the harvest stage, non-photochemical quenching (NPQ) increased with the development stage by 248%, 186%, 495%, 757% and 690% in T1, T2, T3, T4, and T5, respectively (Fig. 5).
4 Discussion
This study characterized plant growth and physiology in response to drought stress at different growth stages in the cut rose ‘Charming Black’. Photosynthesis and growth (biomass production) are the primary processes affected by drought stress (Chaves and Oliveira 2004; Sapeta et al. 2013). Drought stress was applied from stage 3 to stage 5 of plant development. Water deficit not only restricted shoot elongation (Fig. 1), but also shortened the vegetative phase and promoted flowering for about 5 d (Table 1). A previous study also reported that shoot under drought stress conditions, floral buds appeared earlier and on shorter stems compared to plants that received less severe stress treatment (Chimonidou-Pavlidou 2001).
Early induction of floral buds and shortened branches under drought stress has been reported (Sharp et al. 2009). Drought stress affected the quality of Syringa meyeri ‘Palibin’ by altering plant growth. Under drought stress conditions, restricted growth is a morphological adaptation of the plant to reduce to water loss by minimizing the transpiration area (Koniarski and Matysiak 2013). The shoot length in T3 and T4 treatment groups was reduced by 27.0% and 42.4%, and leaf area in T3 and T4 reduced by 46.9% and 54.0%, respectively. Reduction in leaf area during a drought period not only reduced water loss but also reduced plant carbon assimilation, which consequently restricted growth (Banon et al. 2006). However, when drought stress was applied at stages 4 and 5, malformed flowers developed. Previous work on malformation in roses under water deficit conditions has been reported at the flower initiation stage; the flowering shoots produced under drought stress showed less well-formed petals and short floral buds (Chimonidou-Pavlidou 2004). Varying resistance to the transportation of water inside the plant has been found in different parts of cut roses. There were two regions of low conductance that can be observed in rose plant: the first region is the distal peduncle and second is the abscission zone, which is at the junction of the reproductive and vegetative organs of the rose, also known as the safety zone. The abscission zone between the rose stem and peduncle had a significant resistance to water flow and minimized water loss to limit drought stress effects (Darlington and Dixon 1991). These two regions worked in conjunction to control rose plant growth and development. To protect the main axis of the plant from drought stress, many plants sacrifice peripheral organs (Zimmermann 1978; Milburn 1979). The principal site of water loss in rose plants is the flower; if water loss from floral buds is extensive, the abscission zone will restrict water flow to the flower (Darlington and Dixon 1991). Under drought stress, the flower part is removed to protect the shoot, resulting in plants with reduced or malformed flowers. Due to competition between flower shoots and floral buds, the shorter shoot in water stress conditions can be explained.
Photosynthetic parameters were measured after irrigation was restored in all treatments. Plants in T1 and T2 treatment groups recovered their photosynthetic ability and there was no significant difference in the photosynthetic parameters of the experimental groups compared to the control treatment. However, a reduction in the net CO2 assimilation rate was observed when drought stress was applied in stages 3, 4, and 5. If the stress was applied when floral buds appeared, the lowest An value was measured, which correlated with a significant decrease in stomatal conductance. It has been reported that response to drought stress in plants is a reduced stomatal conductivity, which also decreases gas exchange and transpiration. It is considered to be the main mechanism that regulates carbon assimilation and water relations in crops (Hetherington and Woodward 2003). Excessive water loss was limited by reduced stomatal conductance, although it also prevented the infiltration of carbon dioxide into the assimilation parenchyma (Chaves et al. 2003). Decreased water supply in leaves, as a result of drought stress, induced stomatal closure and caused a reduction in available CO2 to the mesophyll cells, which consequently led to a decrease in photosynthesis (Lu and Zhang 1999).
As one of the major chloroplast components of photosynthesis, chlorophyll content is highly correlated with photosynthetic rate (Guo and Li 1996). In the rose cultivar ‘Charming Black’, young leaves are initially red and then turn green. The highest value of chlorophyll content at stage 3 was observed in the T2 treatment, potentially because the drought stress at this early stage accelerated floral bud appearance and reduced vegetative growth. However, at stage 5 and the harvest stage, chlorophyll content significantly reduced in the T4 and T5 treatments, which implied that drought stress at later developmental stages has a negative effect on chlorophyll content. It has been reported that drought stress always causes a reduction in leaf greenness in C3 plants due to chlorophyll degradation (Flexas and Medrano 2002).
The variation in FV/FM was between 0.75 and 0.85, which indicated that the photosynthetic apparatus was intact. When the value was below 0.75, the stressful condition could be observed (Santos et al. 2013). At the harvest stage, FV/FM demonstrated the highest value with the lowest NPQ value. In later developmental stages, the NPQ increased significantly. Therefore, plant tolerance to drought stress can be effectively assessed by measuring the decline in the quantum efficiency of photosystem II (FV/FM) (Resco et al. 2008). Under drought stress conditions, photosynthesis was limited due to excess energy. If the energy cannot be dissipated safely, over-excitation of the photosystem II reaction centers can occur and cause increased ROS production in the chloroplasts (Carvalho 2008). Chlorophyll fluorescence imaging is a useful and intuitive technique to investigate plant photosynthetic performance, and under drought stress it can be a useful detection technique in greenhouse rose cultivation (Calatayud 2006).
In conclusion, drought stress has different effects on plant growth and physiology in cut rose cultivar ‘Charming Black’. At developmental stage 1, there was no significant negative effect observed after drought stress treatment; however, when drought stress was applied on stages 2, 3, and 4, the water deficit impaired shoot growth. In particular, at stages 3 and 4 the leaf area was significantly reduced to restrict water loss in the plant. Moreover, malformed flowers with distorted petal were observed when the stress was applied at stage 5. At early developmental stages, a shortened vegetative growth period and accelerated floral bud appearance were effects of drought stress, resulting in shorter shoots. However, when the stress appeared at later developmental stages, the floral buds were more affected and resulted in reduced floral bud diameter and length, and well-formed flower petals. When irrigation was restored to the control conditions, photosynthetic ability was recovered in all treatments except T5. In addition, chlorophyll fluorescence imaging was a useful tool for detecting drought stress in the greenhouse-grown cut rose cultivar ‘Charming Black’.
References
Banon S, Ochoa J, Franco JA, Alarcon JJ, Sanchez-Blanco MJ (2006) Hardening of oleander seedlings by deficit irrigation and low air humidity. Environ Exp Bot 56:36–43
Calatayud A, Roca D, Martinez PF (2006) Spatial-temporal variations in rose leaves under water stress conditions studied by chlorophyll fluorescence imaging. Plant Physiol Biochem 44:564–573
Carvalho MHC (2008) Drought stress and reactive oxygen species. Plant Signal Behav 3:156–165
Chaves MM, Oliveira MM (2004) Mechanisms underlying plant resilience to water deficits: prospects for water-saving agriculture. J Exp Bot 55:2365–2384
Chaves MM, Maroco JP, Pereira JS (2003) Understanding plant responses to drought from genes to the whole plant. Funct Plant Biol 30:239–264
Chimonidou-Pavlidou D (1996) Effect of drought stress at different stages of rose development. Acta Hortic 424:45–51
Chimonidou-Pavlidou D (1999) Irrigation and sensitive stages of development. Acta Hortic 481:393–401
Chimonidou-Pavlidou D (2000) Correlation between internal and external stages of rose development. Acta Hortic 515:233–244
Chimonidou-Pavlidou D (2001) Effect of irrigation and shading at the stage of flower bud appearance. Acta Hortic 547:245–251
Chimonidou-Pavlidou D (2004) Malformation of roses due to drought stress. Sci Hort 99:79–87
Darlington AB, Dixon MA (1991) The hydraulic architecture of roses (Rosa hybrida). Can J Bot 69:702–710
Flexas J, Medrano H (2002) Drought-inhibition of photosynthesis in C3 plants: stomatal and non-stomatal limitations revisited. Ann Bot 89:183–189
Guo P, Li M (1996) Studies on photosynthetic characteristics in rice hybrid progenies and their parents I. Chlorophyll content, chlorophyll-protein complex and chlorophyll fluorescence kinetics. J Trop Subtrop Bot 4:60–65
Hetherington AM, Woodward FI (2003) The role of stomata in sensing and driving environmental change. Nature 424:901–908
Kim WS, Lieth JH (2012) Simulation of year-round plant growth and nutrient uptake in Rosa hybrida over flowering cycles. Hortic Environ Biotechnol 53:193–203
Koniarski M, Matysiak B (2013) Effect of regulated deficit irrigation on growth, flowering and physiological responses of potted Syringa meyeri ‘Palibin’. Acta Agrobotanica 66:73–80
Kool MTN, Lenssen EFA (1997) Basal-shoot formation in young rose plants: effects of bending practices and plant density. J Hortic Sci 72:635–644
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148:350–382
Lu CM, Zhang JH (1999) Effects of salt stress on PSII function and photoinhibition in the cyanobacterium Spirulina platensis. J Plant Physiol 155:740–745
Milburn JA (1979) Water flow in plants. Longman, London, p 225
Resco V, Ignace DD, Sun W, Huxman TE, Weltzin JF, Williams DG (2008) Chlorophyll fluorescence, predawn water potential and photosynthesis in precipitation pulse-driven ecosystems-implications for ecological studies. Funct Ecol 22:479–483
Santos SAP, Santos C, Silva S, Pinto G, Torres LM, Nogueira AJA (2013) The effect of sooty mold on fluorescence and gas exchange properties of olive tree. Turk J Biol 37:620–628
Sapeta H, Miguel Costa J, Lourenço T, Maroco J, Linde PVD, Margarida Oliveira M (2013) Drought stress response in Jatropha curcas: growth and physiology. Environ Expt Bot 85:76–84
Seghatoleslami MJ, Kafi M, Majidi E (2008) Effect of drought stress at different growth stages on yield and water use efficiency of five Proso Millet (Panicum Miliaceum L.) genotypes. Pak J Bot 40:1427–1432
Sharp RG, Else MA, Cameron RW, Davies WJ (2009) Water deficits promote flowering in Rhododendron via regulation of pre and post initiation development. Sci Hortic 120:511–517
Shi L, Kim WS (2014) Shoot growth and physiological disorder of cut rose ‘Charming Black’ as affected by drought stress during nocturnal supplemental lighting. Hortic Environ Biotechnol 55:91–96
Shi L, Kim WS (2015) Effect of drought stress during supplemental lighting on diurnal photosynthesis of cut rose ‘Charming Black’. Hortic Environ Biotechnol 56:582–587
Singsaas EL, Ort DR, DeLucia EH (2001) Variation in measured values of photosynthetic quantum yield in ecophysiological studies. Oecologia 128:15–23
Turk KJ, Hall AE, Asbell CW (1980) Drought adaptation of cowpea 1. Influence of drought on seed yield. Agron J 72:413–420
Watanabe S, Hakoyama S, Terao T, Singh BB (1997) Evaluation methods for drought tolerance of cowpea. In: Singh BB et al (eds) Advances in cowpea research. IITA/JIRCAS, IITA, Ibadan, pp 87–98
Zieslin N, Moe R (1985) Rosa. In: Halevy AH (ed) Handbook of flowering, vol 4. CRC Press, Boca Raton, pp 214–225
Zieslin N, Mor Y (1990) Light on roses: a review. Sci Hortic 43:1–14
Zimmermann MH (1978) Hydraulic architecture of some diffuse porous trees. Can J Bot 56:2286–2295
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shi, L., Wang, Z. & Kim, W.S. Effect of drought stress on shoot growth and physiological response in the cut rose ‘charming black’ at different developmental stages. Hortic. Environ. Biotechnol. 60, 1–8 (2019). https://doi.org/10.1007/s13580-018-0098-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13580-018-0098-8