Abstract
As the continuation of Fungal Diversity Notes series, the current paper is the 16th contribution to this series. A total of 103 taxa from seven classes in Ascomycota and Basidiomycota are included here. Of these 101 taxa, four new genera, 89 new species, one new combination, one new name and six new records are described in detail along with information of hosts and geographic distributions. The four genera newly introduced are Ascoglobospora, Atheliella, Rufoboletus and Tenuimyces. Newly described species are Akanthomyces xixiuensis, Agaricus agharkarii, A. albostipitatus, Amphisphaeria guttulata, Ascoglobospora marina, Astrothelium peudostraminicolor, Athelia naviculispora, Atheliella conifericola, Athelopsis subglaucina, Aureoboletus minimus, A. nanlingensis, Autophagomyces incertus, Beltrania liliiferae, Beltraniella jiangxiensis, Botryobasidium coniferarum, Calocybella sribuabanensis, Calonarius caesiofulvus, C. nobilis, C. pacificus, C. pulcher, C. subcorrosus, Cortinarius flaureifolius, C. floridaensis, C. subiodes, Crustomyces juniperi, C. scytinostromoides, Cystostereum subsirmaurense, Dimorphomyces seemanii, Fulvoderma microporum, Ginnsia laricicola, Gomphus zamorinorum, Halobyssothecium sichuanense, Hemileccinum duriusculum, Henningsomyces hengduanensis, Hygronarius californicus, Kneiffiella pseudoabdita, K. pseudoalutacea, Laboulbenia bifida, L. tschirnhausii, L. tuberculata, Lambertella dipterocarpacearum, Laxitextum subrubrum, Lyomyces austro-occidentalis, L. crystallina, L. guttulatus, L. niveus, L. tasmanicus, Marasmius centrocinnamomeus, M. ferrugineodiscus, Megasporoporia tamilnaduensis, Meruliopsis crystallina, Metuloidea imbricata, Moniliophthora atlantica, Mystinarius ochrobrunneus, Neomycoleptodiscus alishanense, Nigrograna kunmingensis, Paracremonium aquaticum, Parahelicomyces dictyosporus, Peniophorella sidera, P. subreticulata, Phlegmacium fennicum, P. pallidocaeruleum, Pholiota betulicola, P. subcaespitosa, Pleurotheciella hyalospora, Pleurothecium aseptatum, Resupinatus porrigens, Russula chlorina, R. chrysea, R. cruenta, R. haematina, R. luteocarpa, R. sanguinolenta, Synnemellisia punensis, Tenuimyces bambusicola, Thaxterogaster americanoporphyropus, T. obscurovibratilis, Thermoascus endophyticus, Trechispora alba, T. perminispora, T. subfarinacea, T. tuberculata, Tremella sairandhriana, Tropicoporus natarajaniae, T. subramaniae, Usnea kriegeriana, Wolfiporiella macrospora and Xylodon muchuanensis. Rufoboletus hainanensis is newly transferred from Butyriboletus, while a new name Russula albocarpa is proposed for Russula leucocarpa G.J. Li & Chun Y. Deng an illegitimate later homonym of Russula leucocarpa (T. Lebel) T. Lebel. The new geographic distribution regions are recorded for Agaricus bambusetorum, Bipolaris heliconiae, Crinipellis trichialis, Leucocoprinus cretaceus, Halobyssothecium cangshanense and Parasola setulosa. Corresponding to morphological characters, phylogenetic evidence is also utilized to place the above-mentioned taxa in appropriate taxonomic positions. The current morphological and phylogenetic data is helpful for further clarification of species diversity and exploration of evolutionary relationships in the related fungal groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Table of contents
Ascomycota Caval.-Sm.
Dothideomycetes O.E. Erikss & Winka.
Muyocopronales Mapook, Boonmee & K.D. Hyde.
Muyocopronaceae K.D. Hyde.
1717. Neomycoleptodiscus alishanense Tennakoon, C.H. Kuo & K.D. Hyde, sp. nov. (Contributed by Danushka S. Tennakoon, Chang-Hsin Kuo and Kevin D. Hyde).
Pleosporales Barr.
Lentitheciaceae Y. Zhang ter, C.L. Schoch, J. Fourn., Crous & K.D. Hyde.
1718. Halobyssothecium cangshanense (Z.L. Luo, X.J. Su & K.D. Hyde) M.S. Calabon, K.D. Hyde & E.B.G. Jones, new geographical record (Contributed by Yun Qing and Huang Zhang).
1719. Halobyssothecium sichuanense Y. Qing & H. Zhang, sp. nov. (Contributed by Yun Qing and Huang Zhang).
Nigrogranaceae Jaklitsch & Voglmayr.
1720. Nigrograna kunmingensis T.Y. Du & Tibpromma, sp. nov. (Contributed by Tian-Ye Du and Saowaluck Tibpromma).
1721. Nigrograna heveae R.F. Xu & Tibpromma, new record for Thailand (Contributed by Jing-Yi Zhang and Yong-Zhong Lu).
Pleosporaceae Nitschke.
1722. Bipolaris heliconiae Alcorn, new record for India (Contributed by PN Singh, KS Pawar and SK Singh.)
Trypetheliales Lücking, Aptroot & Sipman.
Trypetheliaceae Eschw.
1723. Astrothelium peudostraminicolor S.H. Jiang, C. Zhang & J.C. Wei, sp. nov. (Contributed by Shu-Hua Jiang and Chao Zhang).
Tubeufiales Boonmee & K.D. Hyde.
Tubeufiaceae M.E. Barr.
1724. Parahelicomyces dictyosporus M.S. Calabon, E.B.G. Jones & K.D. Hyde, sp. nov. (Contributed by Mark S. Calabon, E.B. Gareth Jones, and Kevin D. Hyde).
Eurotiomycetes Tehler ex O.E. Eriksson & K. Winka.
Eurotiales G.W. Martin ex Benny & Kimbr.
Thermoascaceae Apinis.
1725. Thermoascus endophyticus T.M. Silva, C.S. Oliveira & J.D.P. Bezerra, sp. nov. (Contributed by Tatiane M. da Silva, Cristina M. Souza-Motta and Jadson D.P. Bezerra).
Laboulbeniomycetes Engl.
Laboulbeniales Lindau.
Laboulbeniaceae G. Winter.
1726. Autophagomyces incertus W. Rossi & M. Leonardi, sp. nov. (Contributed by Walter Rossi and Marco Leonardi).
1727. Dimorphomyces seemanii W. Rossi & M. Leonardi, sp. nov. (Contributed by Walter Rossi and Marco Leonardi).
1728. Laboulbenia bifida W. Rossi & M. Leonardi, sp. nov. (Contributed by Walter Rossi and Marco Leonardi).
1729. Laboulbenia tschirnhausii W. Rossi & M. Leonardi, sp. nov. (Contributed by Walter Rossi and Marco Leonardi).
1730. Laboulbenia tuberculata W. Rossi & M. Leonardi, sp. nov. (Contributed by Walter Rossi and Marco Leonardi).
Lecanoromycetes O.E. Erikss. & Winka.
Lecanorales Nannf.
Parmeliaceae F. Berchtold & J. Presl.
1731. Usnea kriegeriana A. Gerlach & P. Clerc, sp. nov. (Contributed by Alice Gerlach).
Leotiomycetes O.E. Erikss. & Winka.
Helotiales Nannf.
Rutstroemiaceae Holst-Jensen, Koehn & Schmach.
1732. Lambertella dipterocarpacearum P.N. Singh, S.K. Singh & A.C. Lagashetti, sp. nov. (Contributed by P.N. Singh, S. K. Singh & A. C. Lagashetti).
Sordariomycetes O.E. Erikss. & Winka.
Amphisphaeriales D. Hawksw. & O.E. Erikss.
Amphisphaeriaceae G. Winter.
1733. Amphisphaeria guttulata J.Y. Zhang & Y.Z. Lu, sp. nov. (Contributed by Jing-Yi Zhang and Yong-Zhong Lu).
Beltraniaceae Nann.
1734. Beltrania liliiferae P. Razaghi, M. Raza & L. Cai, sp. nov. (Contributed by Parisa Razaghi, Mubashar Raza and Lei Cai).
1735. Beltraniella jiangxiensis P. Razaghi, M. Raza & L. Cai, sp. nov.
(Contributed by Parisa Razaghi, Mubashar Raza and Lei Cai).
Hypocreales Lindau.
Bionectriaceae Samuels and Rossman.
1736. Synnemellisia punensis K. S. Pawar, P. N. Singh, S. K. Singh, sp. nov.
(Contributed by K. S. Pawar, P. N. Singh & S. K. Singh).
Cordycipitaceae Kreisel ex G. H. Sung, J.M. Sung, Hywel-Jones & Spatafora.
1737. Akanthomyces xixiuensis X. C. Peng & T. C. Wen, sp. nov. (Contributed by Xing-Can Peng and Ting-Chi Wen).
Nectriaceae Tul. & C. Tul.
1738. Paracremonium aquaticum M.S. Calabon, E.B.G. Jones & K.D. Hyde, sp. nov. (Contributed by Mark S. Calabon, E.B. Gareth Jones and K.D. Hyde).
Microascales Luttr. ex Benny & R.K. Benj.
Halosphaeriaceae E. Müll. & Arx ex Kohlm.
1739. Ascoglobospora Abdel-Wahab, gen. nov. (Contributed by Mohamed A. Abdel-Wahab).
1740. Ascoglobospora marina Abdel-Wahab, sp. nov. (Contributed by Mohamed A. Abdel-Wahab).
Pleurotheciales Réblová & Seifert.
Pleurotheciaceae Réblová & Seifert.
1741. Pleurothecium aseptatum J. Ma & Y.Z. Lu, sp. nov. (Contributed by Jian Ma and Yong-Zhong Lu).
Sordariomycetes, genus incertae sedis
1742. Pleurotheciella hyalospora J. Ma & Y.Z. Lu, sp. nov. (Contributed by Jian Ma and Yong-Zhong Lu).
Basidiomycota R.T. Moore.
Agaricomycetes Doweld.
Agaricales Underw.
Agaricaceae Chevall.
1743. Agaricus agharkarii P.N. Singh, S.K. Singh, S. Rana & A.C Lagashetti, sp. nov. (Contributed by P.N. Singh, S.K. Singh, S. Rana and A.C Lagashetti)
1744. Agaricus albostipitatus E. Tarafder, A.K. Dutta & K. Acharya, sp. nov. (Contributed by Entaj Tarafder, Arun Kumar Dutta and Krishnendu Acharya).
1745. Agaricus bambusetorum H. Bashir & Niazi, new host and geographical record (Contributed by Entaj Tarafder, Arun Kumar Dutta and Krishnendu Acharya).
1746. Leucocoprinus cretaceus (Bull.) Locq., new record for Thailand (Contributed by Nakarin Suwannarach, Jaturong Kumla and Saisamorn Lumyong).
Cortinariaceae R. Heim ex Pouzar.
1747. Calonarius caesiofulvus Niskanen, Liimat. & M. E. Sm., sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Matthew E. Smith).
1748. Calonarius nobilis Niskanen & Liimat., sp. nov. (Contributed by Tuula Niskanen and Kare Liimatainen).
1749. Calonarius pacificus Niskanen, Liimat. & Bojantchev, sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Dimitar Bojantchev).
1750. Calonarius pulcher Niskanen, Liimat., & Bojantchev, sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Dimitar Bojantchev).
1751. Calonarius subcorrosus Niskanen & Liimat., sp. nov. (Contributed by Tuula Niskanen and Kare Liimatainen).
1752. Cortinarius flaureifolius Niskanen, Liimat. & M. E. Sm., sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Matthew E. Smith).
1753. Cortinarius floridaensis Niskanen, Liimat. & M. E. Sm., sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Matthew E. Smith).
1754. Cortinarius subiodes Niskanen, Liimat. & M. E. Sm., sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Matthew E. Smith).
1755. Hygronarius californicus Niskanen, Liimat., Bojantchev & Ammirati, sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen, Dimitar Bojantchev and Joe Ammirati).
1756. Mystinarius ochrobrunneus Ammirati, Halling, Niskanen & Liimat., sp. nov. (Contributed by Joe Ammirati, Tuula Niskanen and Kare Liimatainen).
1757. Phlegmacium fennicum Kekki, Kytöv., Niskanen & Liimat., sp. nov. (Contributed by Tapio Kekki, Tuula Niskanen and Kare Liimatainen).
1758. Phlegmacium pallidocaeruleum Niskanen, Liimat. & Bojantchev, sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Dimitar Bojantchev).
1759. Thaxterogaster americanoporphyropus Niskanen, Liimat. & Ammirati, sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Joe Ammirati).
1760. Thaxterogaster obscurovibratilis Niskanen, Liimat. & Ammirati, sp. nov. (Contributed by Tuula Niskanen, Kare Liimatainen and Joe Ammirati).
Cystostereaceae Jülich.
1761. Crustomyces juniperi S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
1762. Crustomyces scytinostromoides S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
1763. Cystostereum subsirmaurense S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
1764. Tenuimyces S.L. Liu & L.W. Zhou, gen. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
1765. Tenuimyces bambusicola S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
Incertae sedis
1766. Henningsomyces hengduanensis S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
Lyophyllaceae Jülich.
1767. Calocybella sribuabanensis N. Suwannarach, J. Kumla & S. Lumyong, sp. nov. (Contributed by Nakarin Suwannarach, Jaturong Kumla and Saisamorn Lumyong).
Marasmiaceae Roze ex Kühner.
1768. Crinipellis trichialis (Lév.) Pat. ex Antonín, Ryoo & H.D. Shin, new record for Thailand (Contributed by Nakarin Suwannarach and Jaturong Kumla).
1769. Marasmius centrocinnamomeus J.S. Kim & Y.W. Lim, sp. nov. (Contributed by Ji Seon Kim and Young Woon Lim).
1770. Marasmius ferrugineodiscus J.S. Kim & Y.W. Lim, sp. nov. (Contributed by Ji Seon Kim and Young Woon Lim).
1771. Moniliophthora atlantica N. A. Ramirez & Niveiro, sp. nov. (Contributed by Natalia Ramirez and Nicolás Niveiro).
Pleurotaceae Kühner.
1772. Resupinatus porrigens J. Z. Xu & Yu Li, sp. nov. (Contributed by Ji Ze Xu and Yu Li).
Psathyrellaceae Vilgalys, Moncalvo & Redhead.
1773. Parasola setulosa (Berk. & Broome) Redhead, Vilgalys & Hopple, new record for Thailand (Contributed by Nopparat Wannathes, Nakarin Suwannarach and Jaturong Kumla).
Strophariaceae Singer & A.H. Sm.
1774. Pholiota betulicola T. Bau & E.J. Tian, sp. nov. (Contributed by Tolgor Bau and Enjing Tian).
1775. Pholiota subcaespitosa E.J. Tian, sp. nov. (Contributed by Enjing Tian).
Atheliales Jülich.
Atheliaceae Jülich.
1776. Athelia naviculispora S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
Byssocorticiaceae Jülich.
1777. Athelopsis subglaucina S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
Atheliales, genus incertae sedis
1778. Atheliella S.L. Liu & L.W. Zhou, gen. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
1779. Atheliella conifericola S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
Boletales E.-J. Gilbert.
Boletaceae Chevall.
1780. Aureoboletus minimus Ming Zhang, C.Q. Wang & T.H. Li, sp. nov. (Contributed by Ming Zhang).
1781. Aureoboletus nanlingensis Ming Zhang, C.Q. Wang & T.H. Li, sp. nov. (Contributed by Ming Zhang).
1782. Hemileccinum duriusculum Mei-Xiang Li, Zhu L. Yang & G. Wu, sp. nov. (Contributed by Mei-Xiang Li, Zhu L. Yang and Gang Wu).
1783. Rufoboletus N.K. Zeng & Zhi Q. Liang, gen. nov. (Contributed by Nian-Kai Zeng and Yun-Xiao Han).
1784. Rufoboletus hainanensis (N.K. Zeng, Zhi Q. Liang & S. Jiang) N.K. Zeng & Zhi Q. Liang, comb. nov. (Contributed by Nian-Kai Zeng and Yun-Xiao Han).
Cantharellales Gäum.
Botryobasidiaceae Jülich.
1785. Botryobasidium coniferarum S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
Gomphales Jülich.
Gomphaceae Donk.
1786. Gomphus zamorinorum Krishnapriya K. & T.K.A. Kumar, sp. nov. (Contributed by K. Krishnapriya and T. K. Arun Kumar).
Hymenochaetales Oberw.
Chaetoporellaceae Jülich.
1787. Kneiffiella pseudoabdita Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1788. Kneiffiella pseudoalutacea Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
Hymenochaetaceae Donk.
1789. Fulvoderma microporum Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1790. Tropicoporus natarajaniae M. Kaliyaperumal, S. Gunaseelan, K. Kezo, Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Malarvizhi Kaliyaperumal, Sugantha Gunaseelan, Kezhocuyi Kezo, Xue-Wei Wang and Li-Wei Zhou).
1791. Tropicoporus subramaniae S. Gunaseelan, M. Kaliyaperumal, K. Kezo, Xue W. Wang & L.W. Zhou., sp. nov. (Contributed by Malarvizhi Kaliyaperumal, Sugantha Gunaseelan, Kezhocuyi Kezo, Xue-Wei Wang and Li-Wei Zhou).
Schizoporaceae Jülich.
1792. Lyomyces austro-occidentalis Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1793. Lyomyces crystallina Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1794. Lyomyces guttulatus Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1795. Lyomyces niveus C.L. Zhao ex L.W. Zhou & Xue W. Wang, sp. nov. (Contributed by Li-Wei Zhou and Xue-Wei Wang).
1796. Lyomyces tasmanicusXue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1797. Xylodon muchuanensis Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
Hymenochaetales, genus incertae sedis
1798. Ginnsia laricicola Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1799. Peniophorella sidera Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
1800. Peniophorella subreticulata Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
Polyporales Gäum.
Irpicaceae Spirin & Zmitr.
1801. Meruliopsis crystallina Xue W. Wang & L.W. Zhou, sp. nov. (Contributed by Xue-Wei Wang and Li-Wei Zhou).
Laetiporaceae Jülich.
1802. Wolfiporiella macrospora X.H. Ji, L.W. Zhou & S.L. Liu, sp. nov. (Contributed by Xiao-Hong Ji, Li-Wei Zhou and Shi-Liang Liu).
Meruliaceae Rea.
1803. Metuloidea imbricata R. Saha, A.K. Dutta & K. Acharya, sp. nov. (Contributed by Rituparna Saha, Arun Kumar Dutta and Krishnendu Acharya).
Polyporaceae Corda.
1804. Megasporoporia tamilnaduensis K. Kezo, M. Kaliyaperumal, S. Gunaseelan, Xue W. Wang & L.W. Zhou., sp. nov. (Contributed by Kezhocuyi Kezo, Malarvizhi Kaliyaperumal, Sugantha Gunaseelan, Xue-Wei Wang and Li-Wei Zhou).
Russulales Kreisel ex P.M. Kirk, P.F. Cannon & J.C. David.
Hericiaceae Donk.
1805. Laxitextum subrubrum R. Saha, A.K. Dutta & K. Acharya, sp. nov. (Contributed by Rituparna Saha, Arun Kumar Dutta and Krishnendu Acharya).
Russulaceae Lotsy.
1806. Russula albocarpa G.J. Li & Chun Y. Deng, nom. nov. (Contributed by Guo-Jie Li and Chun-Ying Deng).
1807. Russula chlorina G.J. Li & Chun Y. Deng, sp. nov. (Contributed by Guo-Jie Li and Chun-Ying Deng).
1808. Russula chrysea G.J. Li & Chun Y. Deng, sp. nov. (Contributed by Guo-Jie Li and Chun-Ying Deng).
1809. Russula cruenta G.J. Li & Chun Y. Deng, sp. nov. (Contributed by Guo-Jie Li and Chun-Ying Deng).
1810. Russula haematina G.J. Li & Chun Y. Deng, sp. nov. (Contributed by Guo-Jie Li and Chun-Ying Deng).
1811. Russula luteocarpa G.J. Li & Chun Y. Deng, sp. nov. (Contributed by Guo-Jie Li and Chun-Ying Deng).
1812. Russula sanguinolenta G.J. Li & Chun Y. Deng, sp. nov. (Contributed by Guo-Jie Li and Chun-Ying Deng).
Trechisporales K.H. Larss.
Hydnodontaceae Jülich.
1813. Trechispora alba S.L. Liu, G. He & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu, Gang He and Li-Wei Zhou).
1814. Trechispora perminispora S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
1815. Trechispora subfarinacea S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
1816. Trechispora tuberculata S.L. Liu & L.W. Zhou, sp. nov. (Contributed by Shi-Liang Liu and Li-Wei Zhou).
Tremellomycetes Doweld.
Tremellales Fr.
Tremellaceae Fr.
1817. Tremella sairandhriana A. Thomas & T.K.A. Kumar, sp. nov. (Contributed by Anjitha Thomas and T. K. Arun Kumar).
Introduction
Fungal taxonomy is a fundamental discipline that aims to recognize all fungi and clarify their kinships, viz. reconstruction of the Fungal Tree of Life (Zhou and May 2023). Since the publication of Species Plantarum in 1753 (Linnaeus 1753), fungi have been considered to be a low-level branch of plants and have been systematically explored for more than 250 years. The known species are no more than 10% of the total number of fungi estimated in various scenarios (Hawksworth 2001; Blackwell 2011; Dai et al. 2015; Hawksworth and Lücking 2017; Wu et al. 2019; Hyde 2020c, 2022). In contrast, however, the reduction of biodiversity is increasing due to habitat loss and climate change in the Anthropocene (Díaz et al. 2019; Wei 2021; Exposito-Alonso et al. 2022). Therefore, it is improtant to record species from all fungal groups for their conservation and utilization.
Traditionally, new fungal taxa are introduced by taxonomists who focus on specific fungal groups among for example, agarics, poroid species, corticioid species, plant pathogens and aquatic fungi. This leads to new being taxa scattered in many papers (one or two new taxa per paper), and thus some of new taxa published in these papers may become buried in the copious publications, some obscure. For example, an order name Xenasmatales Jülich erected in 1981 (Jülich 1981) was published as a new order Xenasmatales K.Y. Luo & C.L. Zhao recently (Luo and Zhao 2022a). The latter is not valid (Note 2 to Art. 6 of the International Code of Nomenclature for algae, fungi, and plants; Turland et al. 2018). Therefore, the Fungal Diversity Notes series provides an outlet to promote newly described taxa at and above the species level (Ariyawansa et al. 2015; Liu et al. 2015a; Hyde et al. 2016; etc.).
In this paper we follow standard taxonomic protocols (Aime et al. 2021) when introducing new taxa. The authors follow the guidelines for describing new species for Basidiomycetes (He et al. 2021) and ascomycetes (Chethana et al. 2021; Maharachchimbura et al. 2021; Pem et al. 2021).
As the continuation of FDN series, this paper introduces, four new genera, 89 new species, one new combination and one new name. In addition, six new records are also included to provide distribution and host information for previously known species. This information is crucial for further preserving and utilizing fungal species (Hyde et al. 2007, 2019). Moreover, along with these new taxa, brief notes on higher taxa are provided for supplementing related taxonomic knowledge.
Materials and methods
The studied fungal materials were collected from Asia, Europe, North America, South America and Oceania, and are preserved in various fungaria or herbaria (see Taxonomy section for acronyms following Index Herbariorum: http://sweetgum.nybg.org/science/ih/). Morphological examination and phylogenetic analyses were performed following standard fungal taxonomic procedures (Aime et al. 2021; Shen et al. 2021, 2023); the detailed procedures are similar to previous FDN series (Boonmee et al. 2021). The new taxa are registered in Index Fungorum (Index Fungorum 2023) and Faces of Fungi databases (Jayasiri et al. 2015).
Taxonomy
Ascomycota Caval.-Sm.
Notes: Ascomycota is not only the largest phylum in the fungal kingdom, but also one of the most diverse and common phylum in eukaryotes (Kirk et al. 2008). The species are widely distributed in a variety of terrestrial and aquatic environments. Cavalier-Smith (1998) officially introduced Ascomycota, which consists of two subphyla (Hemiascomycotina and Euascomycotina), seven classes and nine subclasses. Schoch et al. (2009) provided an outline of Ascomycota including three subphyla (Pezizomycotina, Saccharomycotina and Taphrinomycotina), 16 classes and ten subclasses. Schoch et al. (2009) explored the origin and evolutionary relationship of species within the whole phylum through phylogenetic methods. Recently, Wijayawardene et al. (2018) provide an updated outline of Ascomycota, which includes three subphyla, 19 classes, and approximately 6600 genera.
Dothideomycetes O.E. Erikss. & Winka.
Notes: Dothideomycetes is the largest class in Ascomycota, including two subclasses (Dothideomycetidae and Pleosporomycetidae), 33 orders and 174 families. It also comprises a highly diverse group of freshwater fungi. There are 1022 genera in Dothideomycetes (Wijayawardene et al. 2018). Species in this class are characterized mainly by bitunicate asci (two wall layers, Hyde et al. 2013). In recent years, with the development of molecular technology, more and more scholars used phylogenetic analysis methods to modified Dothideomycetes lineage. Lumbsch and Huhndorf (2010) accepted 41 families consisting of 249 genera in Dothideomycetes. Hyde et al. (2013) introduced ten new families in Dothideomycetes and provided the corresponding descriptions, notes and illustrations based on the phylogenetic tree. Wijayawardene et al. (2014) listed 23 orders, 110 families and 1295 genera of Dothideomycetes following the International Code of Nomenclature for Algae, Fungi, and Plants (ICN; Melbourne Code), which addresses the nomenclature of pleomorphic fungi, became effective from 30 July 2011. They also provided a phylogenetic tree for 23 orders and 75 families. Hongsanan et al. (2020a) further provided an overall phylogenetic tree of families in Dothideomycetes based on combined analysis of LSU, SSU, rpb2 and tef1 sequence data and phylogenetic trees for each order in Dothideomycetidae and Pleosporomycetidae.
Muyocopronales Mapook, Boonmee & K.D. Hyde.
Notes: Muyocopronales was introduced by Mapook et al. (2016) to accommodate the Muyocopronaceae as the type family. This order was related to Acrospermales and Dyfrolomycetales, but morphologically differs in having superficial, flattened, carbonaceous, brittle ascomata, cellular pseudoparaphyses that are longer than the asci and ellipsoid to ovate, unicellular ascospores (Hongsanan et al. 2020a; Mapook et al. 2020). To date, only Muyocopronaceae is accepted in this order and divergence time has been estimated as 171 million years ago (Mya) (Hongsanan et al. 2020a; Wijayawardene et al. 2022).
Muyocopronaceae K.D. Hyde.
Notes: This family was invalidly introduced by Luttrell (1951) and re-established by Hyde et al. (2013) with a single genus Muyocopron. Muyocopronaceae members are commonly found as saprobes on dead leaves, twigs and stems, thus playing important roles in decomposition (Hongsanan et al. 2020a; Tennakoon et al. 2021). Currently, this family comprises nine genera Arxiella, Leptodiscella, Mycoleptodiscus, Muyocopron, Neocochlearomyces, Neomycoleptodiscus, Paramycoleptodiscus, Setoapiospora, and Pseudopalawania (Hongsanan et al. 2020a; Wijayawardene et al. 2022). In this study, we follow the recent treatment of Mapook et al. (2020) for Muyocopronaceae.
Neomycoleptodiscus Hern.-Restr., J.D.P. Bezerra & Crous.
Notes: Neomycoleptodiscus was introduced by Hernández-Restrepo et al. (2019) to accommodate Neomycoleptodiscus venezuelense as the type species. The type species was found on leaf litter of Gyranthera caribensis in Venezuela. Neomycoleptodiscus is distinguished from Mycoleptodiscus by having dark brown conidiogenous cells and conidia which are curved at the apex, and truncate at the base, whereas Mycoleptodiscus has brown conidiogenous cells and conidia with recurved ends (Hernández-Restrepo et al. 2019). Currently, this genus has two species, viz. Neomycoleptodiscus pertusus and N. venezuelense. However, molecular data are available only for the type species. In this study, we introduce Neomycoleptodiscus alishanense as the third species of this genus.
Neomycoleptodiscus alishanense Tennakoon, C.H. Kuo & K.D. Hyde, sp. nov.
Index Fungorum Number: IF559582; Facesoffungi number: FoF10790; Fig. 1
Etymology: The epithet "alishanense" refers to the locality mountain (Ali Shan), from where the taxon was collected.
Holotype: MFLU 19-1732.
Saprobic on senescent leaves of Trachycarpus fortunei. Sexual morph: Undetermined. Asexual morph: Coelomycetous. Conidiomata sporodochial, 120–150 µm high, 130–160 µm in diam. (x̅ = 135 × 145 μm, n = 30), superficial, dark brown to black, circular, dull, undulate, umbonate, rough. Conidiogenous cells evanescent. Conidia 16–19 × 3–4 µm (x̅ = 17.5 × 3.6 μm, n = 30), 1-septate, cylindrical, hyaline, guttulate, straight or slightly curved, with filamentous appendages at both ends, 7–7.6 μm long, 0.4–1 μm wide, smooth-walled.
Culture characteristics: Colonies on PDA reaching 15 mm diameter after 2 weeks at 25 °C, colonies medium dense, circular, convex, surface slightly smooth with entire edge, effuse, velvety to hairy, margin well-defined and curled, colony from above: grey at the margin, brown at the centre; reverse, light brown to grey at the margin, brown to black at the centre; mycelium grey to greenish with tufting; not producing pigments in PDA.
Material examined: China, Taiwan Province, Chiayi, Fanlu Township area, Dahu forest, on dead leaves of Trachycarpus fortune, 6 August 2019, D.S. Tennakoon, GSP014A (MFLU 19-2732, holotype), ex-type living culture, MFLUCC 19-0390; ibid., 11 August 2019, GSP014B (NCYU 19-0006, paratype), NCYUCC 19-0391; ibid., 13 August 2019, GSP014C (NCYU 19-0401), NCYUCC 19-0392.
GenBank numbers: MFLUCC 19-0390: ITS = ON024153, LSU = ON024150; NCYUCC 19-0391: ITS = ON024154, LSU = ON024151; NCYUCC 19-0392: ITS = ON024155, LSU = ON024152.
Notes: The characteristics of our collection (MFLU 19-2732 and NCYU 19-0006) tally with the type of Neomycoleptodiscus in having superficial, dark brown to black, circular, sporodochial conidiomata and 1-septate, cylindrical, hyaline, straight or slightly curved conidia with filamentous appendages at both ends (Hernández-Restrepo et al. 2019). Multi-gene phylogeny (LSU, SSU and ITS) generated herein indicates that our collection forms a strongly supported lineage sister to the clade containing N. venezuelense and Mycoleptodiscus endophyticus (85% ML, 1.00 BYPP, Fig. 2). Our collection can be distinguished from N. venezuelense in having smaller conidia (16–19 × 3–4 µm), whereas N. venezuelense has larger conidia (18–27 × 3–5 µm). In addition, a comparison of the 632 nucleotides across the ITS (+ 5.8S) gene region of our collection and N. venezuelense (CBS 100519) reveals 21 base pair differences (3.32%). Therefore, based on both morphology and phylogenetic evidence, we introduce our collections as a new species, N. alishanense from dead leaves of Trachycarpus fortunei (Arecaceae). Mycoleptodiscus. endophyticus was introduced by Tibpromma et al. (2018) from healthy leaves of Freycinetia sp. (Pandanaceae) as an endophytic species based on mycelial characteristics. However phylogenetically, M. endophyticus has a close relationship with Neomycoleptodiscus species (Hernández-Restrepo et al. 2019). Therefore, further taxonomic work is needed to precisely resolve identification and relationships between M. endophyticus and Neomycoleptodiscus species.
The best scoring RAxML tree with a final likelihood value of − 14,874.014066 for combined dataset of LSU, SSU and ITS sequence data. The topology and clade stability of the combined gene analyses was compared to the single gene analyses. The tree is rooted with Lophium mytilinum (AFTOL-ID 1609) and Mytilinidion rhenanum (CBS 135.45). The matrix had 1024 distinct alignment patterns with 36.09% undetermined characters and gaps. Estimated base frequencies were as follows; A = 0.241588, C = 0.238793, G = 0.293284, T = 0.226335; substitution rates AC = 1.340756, AG = 2.207875, AT = 1.322261, CG = 1.183116, CT = 5.116204, GT = 1.000000; gamma distribution shape parameter α = 0.586892. Ex-type strains are in bold and newly generated sequences are in red. Bootstrap support values for ML equal to or greater than 60% and BYPP equal to or greater than 0.90 are given above the nodes
Pleosporales Luttr. ex M.E. Barr
Notes: Pleosporales (Barr 1987) is an order of the class Dothidiomycetes within the division Ascomycota. It is estimated to contains 91 families and 655 genera (Wijayawardene et al. 2022). The majority of species are saprobes on decaying plant material in freshwater (Shearer et al. 2009), marine (Suetrong et al. 2009) or terrestrial environments, but some species are also associated with living plants as parasites, epiphytes or endophytes (Zhang et al. 2009). The best studied species cause plant diseases on important agricultural crops e.g. Cochliobolus heterostrophus, causing southern corn leaf blight on maize, Phaeosphaeria nodorum (Stagonospora nodorum) causing glume blotch on wheat, sorghum and on various other host plants (Ellis 1971; Sivanesan 1987; Berbee et al. 1999). Some species of this order are coprophilous found on animal dung (Kruys et al. 2006) and a few members occur as lichens (Nelsen et al. 2009).
Lentitheciaceae Y. Zhang ter, C.L. Schoch, J. Fourn., Crous & K.D. Hyde.
Notes: Lentitheciaceae was introduced by Zhang et al. (2009) to accommodate Katumotaa, Keissleriella and Lentithecium. Phylogenetically, the family is highly-supported as monophyletic in Pleosporales (Wanasinghe et al. 2014; Tanaka et al. 2015; Liu et al. 2017a). The species in this family have lenticular ascomata, fusiform to filiform, hyaline to pale yellow, 1-septate ascospores, sometimes becoming 3-septate when mature, rarely multiseptate (Zhang et al. 2009; Hyde et al. 2013). According to the latest report, there are 14 genera in Lentitheciaceae, viz. Darksidea, Halobyssothecium, Katumotoa, Keissleriella, Lentithecium, Murilentithecium, Neoophiosphaerella, Phragmocamarosporium, Pleurophoma, Poaceascoma, Pseudomurilentithecium, Setoseptoria, Tingoldiago and Towyspora (Hongsanan et al. 2020a).
Halobyssothecium Dayar., E.B.G. Jones & K.D. Hyde.
Notes: Halobyssothecium was introduced by Dayarathne et al. (2018) to accommodate H. obiones, which was previously regarded as Byssothecium obiones. The species in Halobyssothecium have subglobose or ellipsoidal, immersed to semi-immersed, ostiolate, carbonaceous, dark brown to black ascomata, septate, branched pseudoparaphyses, 8-spored, clavate to subcylindrical, short pedicellate asci, without an apical apparatus and versicolored, septate ascospores. Dayarathne et al. (2018) suggested that the most distinct character of this genus were the versicolored ascospores with brown central cells and hyaline end cells, which was similar to Byssothecium. Subsequently, Calabon et al. (2021) collected three new Halobyssothecium species and also transferred Lentithecium cangshanense, L. carbonneanum, L. kunmingense, L. unicellulare, and L. voraginesporum to Halobyssothecium based on the result of their phylogeny. Hyde et al. (2021) introduced H. thailandicum from submerged wood in Thailand. Halobyssothecium bambusicola, H. kunmingense, H. phragmitis and H. unicellulare are asexual morphs, while H. cangshanense, H. carbonneanum, H. thailandicum and H. voraginesporum possess brown ascospores with uniform color, which differs from the original description of the genus. Bootstrap support values for Halobyssothecium in the phylogenetic tree of Calabon et al. (2021) had low maximum likelihood and maximum parsimony lower than 50% and Bayesian posterior probabilities less than 0.90, which is in agreement with Hyde et al. (2021) and this study (Fig. 3). Therefore, more studies need to be done to confirm whether Halobyssothecium is monophyletic. Herein we follow Calabon et al. (2021) and Hyde et al. (2021), with eleven species in the genus.
The best scoring Maximum Likelihood (RAxML) tree of family Lentitheciaceae, based on LSU, SSU, ITS and TEF1-α sequence dataset. The tree is rooted with Corynespora smithii (CABI5649b) and Corynespora cassiicola (CBS 100822). Maximum likelihood support values greater than 70% and Bayesian posterior probabilities greater than 0.95 are shown near the nodes. Newly strain in this study is shown in red, and new species is shown in blue. Ex-type strains are shown in bold. The optimal score of RAxML analysis results ln L = − 36,913.321704. Estimated base frequencies are as follows: A = 0.238921, C = 0.248704, G = 0.273797, T = 0.238578; substitution rates AC = 1.113695, AG = 2.495521, AT = 1.345905, CG = 1.136632, CT = 5.923529, GT = 1.000000; gamma distribution shape parameter a = 0.220373
Halobyssothecium cangshanense (Z.L. Luo, X.J. Su & K.D. Hyde) M.S. Calabon, K.D. Hyde & E.B.G. Jones, Mycol. Progr. 20: 715 (2021).
Index Fungorum number: IF 558092; Facesoffungi number: FoF 09434; Fig. 4
Saprobic on decaying bamboo in freshwater habitats. Sexual morph: Ascomata 170–270 µm high, 150–250 µm in diam., scattered or clustered, semi-immersed to partially erumpent, conical or subglobose, black, papillate in the center, ostiolate. Peridium 20–30 µm wide, composed of several layers of pseudoparenchymatous cells, arranged in a textura angularis, hyaline to subhyaline at the inner part, pigmented at the outer part. Pseudoparaphyses 2.5 − 3.5 μm wide, numerous, septate, embedded in a mucilaginous matrix. Asci 65–114 × 9–12 µm (x̅ = 87.8 × 10.8 μm, n = 15), 8-spored, bitunicate, clavate, pedicellate, with a rounded apex and an ocular chamber. Ascospores 15.5–19.5 × 3.5–5.5 µm (x̅ = 17.6 × 4.5 μm, n = 20), 1–2-seriate, broadly fusiform, straight or slightly curved, yellowish brown, 1-septate in the central, slightly constricted at the septum, upper cell wider, guttulate when young, smooth-walled. Asexual morph: Undetermined.
Culture characteristics: On PDA, colony circular on the whole, germinating within 24 h, reaching 40 mm in 1 month at room temperature (25 °C), dark brown from above and below, surface rough and dry, umbonate, with dense brown mycelium in the middle, sparse mycelium in the regular edge.
Material examined: China, Sichuan Province, Yibin, Changning river, on submerged bamboo, 29 March 2021, Y. Qing, CN16 (IFRD, new record for China).
GenBank numbers: LSU = KU991149, SSU = KU991150.
Notes: Halobyssothecium cangshanense was originally introduced as Lentithecium cangshanense by Su et al. (2016) from Yunnan Province, China, and subsequently transferred to Halobyssothecium by Calabon et al. (2021) based on phylogenetic analysis. Phylogenetically, our isolate clusters with H. cangshanense with strong support. Morphologically, our isolate fits the characters of H. cangshanense except for the slightly narrower conidia (3.5–5.5 µm vs. 6–7 μm). This is the first time to report it from Sichuan Province, China.
Halobyssothecium sichuanense Y. Qing & H. Zhang, sp. nov.
Index Fungorum number: IF 559791; Facesoffungi number: FoF 12672; Fig. 5
Etymology: Referring to this species collected from Sichuan.
Holotype: CN12 (IFRD).
Saprobic on decaying wood in freshwater habitats. Sexual morph: Undetermined. Asexual morph: Conidiomata 90–180 µm high, 180–310 µm in diam, pycnidial, scattered, semi-immersed to superficial, ellipsoidal, dark brown to black, unilocular, sometimes white at the top, ostiolate. Conidiomatal wall 19–35 μm, composed of thick-walled, dark brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–7 × 2–4 μm, holoblastic, determinate, cylindrical to subcylindrical, aseptate, hyaline, smooth-walled. Conidia 8–15 × 6–8 µm (x̅ = 12.4 × 6.7 μm, n = 20), ellipsoidal to ovoidal, aseptate, rarely 1-septate, hyaline, thin-walled, smooth, with one large guttule or several smaller ones in each cell.
Culture characteristics: On PDA, colony circular, conidia germinated within 24 h, reaching 23 mm in 1 month at room temperature (25 °C), grey to brown from above, dark brown from below, surface rough and dry, umbonate, with dense white mycelium in the middle, regular edge.
Material examined: China, Sichuan Province, Yibin, Changning River, on submerged wood, 29 March 2021, Y. Qing, CN12 (IFRD, holotype).
GenBank numbers: ITS = ON124829, LSU = ON124829.
Notes: Halobyssothecium sichuanense is a distinct species and close to the sexual species H. thailandica (Fig. 3). Halobyssothecium bambusicola, H. kunmingense, H. phragmitis and H. unicellulare are also known from their asexual morphs. Halobyssothecium sichuanense morphologically differs from H. bambusicola and H. phragmitis in conidial shape (ellipsoidal to ovoidal in H. sichuanense vs. globose to obovate in H. bambusicola vs. ovoid to fusoid-ellipsoidal in H. phragmitis, Calabon et al. 2021); from H. kunmingense in having smaller conidiomata (210–250 μm high vs. 90–180 μm high, Dong et al. 2020); and from H. unicellulare in having larger conidia (8–15 × 6–8 μm vs. 6–9 × 4–5 μm) and cylindrical to subcylindrical conidiogenous cells as compared to globose, subglobose to pear-shaped ones in H. unicellulare (Hyde et al. 2016). All of the four known asexual species are phylogenetically distinguished from our new isolate. Therefore, it is introduced as a new species H. sichuanense.
Nigrogranaceae Jaklitsch & Voglmayr.
Notes: Nigrogranaceae was established by Jaklitsch and Voglmayr (2016) to accommodate Nigrograna based on its unique morphology. Nigrograna is the monotypic genus of Nigrogranaceae and it is considered to be a geographically and ecologically diverse group (Kolařík et al. 2017; Kolařík 2018). The divergence time estimates for this family are given as crown age of 72 Mya (44–124 Mya) and stem age of 131 Mya (86–180 Mya) during the Cretaceous period (Liu et al. 2017a; Mapook et al. 2020).
Nigrograna Gruyter, Verkley & Crous.
Notes: Nigrograna was introduced by de Gruyter et al. (2013) with N. mackinnonii as the type species. Based on the related molecular data of N. mackinnonii and the type species of Biatriospora (B. marina), Ahmed et al. (2014) synonymized Nigrograna under Biatriospora, and N. mackinnonii was transferred to Biatriospora. Later, Jaklitsch and Voglmayr (2016) found three new collections clustered with the above two Biatriospora species in the molecular study, but the new collections were different morphologically and ecologically from B. marina; therefore, they established the family Nigrogranaceae to accommodate Nigrograna, and N. mackinnonii was designated as the type species (Zhang et al. 2020). Subsequently, Kolařík et al. (2018) synonymized four endophytic species of Biatriospora, viz. B. antibiotica, B. carollii, B. peruviensis and B. yasuniana under Nigrograna. Members of this genus have been recorded from a wide range of hosts, i.e. marine and terrestrial habitats as saprobes, endophytes, and pathogens (Hyde et al. 2017; Tibpromma et al. 2017; Kolařík 2018; Zhao et al. 2018; Dayarathne et al. 2020; Zhang et al. 2020; Boonmee et al. 2021). Nigrograna is characterized by black ascomata, clavate, short pedicellate asci and pale to chocolate brown, asymmetric, fusoid to narrowly ellipsoid septate ascospores (Zhang et al. 2020; Boonmee et al. 2021). Currently, the genus includes 20 species epithets in Index Fungorum (2022). All of these species were confirmed with molecular data.
Nigrograna kunmingensis T.Y. Du & Tibpromma, sp. nov.
Index Fungorum number: IF559865; Facesoffungi number: FoF 12956; Fig. 6
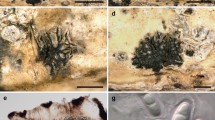
Nigrograna kunmingensis (ZHKU 22-0141, holotype). a, b Appearance of ascomata on the substrate. c A section through ascoma. d–f Asci. g Hairs of an ascoma. h Peridium. i–n Ascospores. o Pseudoparaphyses. p Germinating ascospore. q, r Colony on PDA from above and below. Scale bars: c = 200 µm, d–f = 50 µm, g = 20 µm, h = 50 µm, i–p = 10 µm
Etymology: named after the city Kunming from which the holotype was collected.
Holotype: ZHKU 22-0141.
Saprobic on dead stems of Gleditsia sinensis. Sexual morph: Ascomata 300–500 µm high × 390–450 µm diam. (x̅ = 356 × 413 µm, n = 10), aggregated in a disc, immersed in substrate, appearing as black irregular protrusions and cracks, subglobose to globose, brown to dark brown, hairs of ascomata 2–3.5 wide, brown, septate, branched. Ostiole inconspicuous, without papilla. Peridium 20–60 µm wide, comprising several layers, thick-walled cells, comprising brown to dark brown cells of textura angularis. Hamathecium comprising 1.5–2.5 µm wide, filiform, hyaline, septate, branched pseudoparaphyses. Asci (56–)65–80 × 10–12.5(–14) µm (x̅ = 70.3 × 11.4 µm, n = 20), bitunicate, fissitunicate, 8-spored, cylindrical to clavate, straight or slightly curved, with a short pedicel, apically rounded. Ascospores 13–16 × 5–6(–7) µm (x̅ = 14 × 5.4 µm, n = 30), uniseriate, brown to dark brown, broadly fusiform or inequilateral, with slightly obtuse ends, upper part or second cell slightly wider, 3-septate when mature, slightly constricted at the septum, straight or slightly curved, guttulate, without appendages. Asexual morph: Undetermined.
Culture characteristics: Ascospores germinated on PDA within 24 h at 28 ℃ and germ tubes were produced from several cells. Mycelium raised, entire, white aerial hyphae; yellow to brown in reverse.
Material examined: China, Yunnan Province, Kunming City, Kunming Institute of Botany, on dead stems of Gleditsia sinensis, 24 March 2021, S.C. Karunarathna, KMD8 (ZHKU 22-0141, holotype); ex-type living culture: ZHKUCC 22-0242, ZHKUCC 22-0243.
GenBank numbers: ZHKUCC 22-0242: LSU = OP456379, ITS = OP456214, SSU = OP456382, TEF1-α = OP471608. ZHKUCC 22-0243: LSU = OP456380, ITS = OP484334, SSU = OP456383, TEF1-α = OP471609.
Notes: In the present phylogenetic analyses, Nigrograna kunmingensis clusters with the sister taxon N. magnoliae (MFLUCC 20-0020, MFLUCC 20-0021) with 100% ML and 1.00 PP statistical support (Fig. 7). Nigrograna kunmingensis and N. magnoliae are similar in the shape and size of asci and ascospores. However, N. kunmingensis differs from N. magnoliae in having ascomata with hairs, aggregated in a disc, immersed in the substrate, septate and branched pseudoparaphyses, and uniseriate, brown to dark brown, broadly fusiform or inequilateral ascospores, while N. magnoliae has immersed to erumpent ascomata, septate pseudoparaphyses, and uni to bi-seriate, yellowish-brown to brown, ellipsoid ascospores (Wanasinghe et al. 2020). Therefore, based on both phylogenetic analyses and morphological comparison, N. kunmingensis associated with Gleditsia sinensis is introduced as a new species from China.
Phylogram generated from maximum likelihood analysis based on a combined dataset of LSU, ITS, SSU, TEF1 and RPB2 sequence data of Nigrograna. Forty-one strains are included in the combined sequence analysis, which comprises 3961 characters with gaps. Tree topology of the ML analysis was similar to the BYPP. The best scoring RAxML tree with a final likelihood value of − 15,644.521340 is presented. The matrix had 949 distinct alignment patterns, with 30.90% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.249098, C = 0.246692, G = 0.264559, T = 0.239652; substitution rates: AC = 1.741432, AG = 5.232798, AT = 1.725788, CG = 1.315174, CT = 13.366562, GT = 1.000000; gamma distribution shape parameter α = 0.546634. Bootstrap support values for ML equal to or greater than 50% and BYPP equal to or greater than 0.90 are given above the nodes. The ex-types are in black bold; while the new isolates are in blue. The tree is rooted with Seriascoma didymospora (MFLUCC 11-0179) and S. didymospora (MFLUCC 11-0194)
Nigrograna heveae R.F. Xu & Tibpromma, Mycosphere 14: 663–744 (2023).
Index Fungorum number: IF559985; Facesoffungi number: IF559985; Fig. 8
Holotype: ZHKU 22-0152.
Saprobic on dead branch. Sexual morph: undetermined. Asexual morph: Coelomycetous. Conidiomata immersed in natural host, visible as black spots, solitary, scattered or aggregated, globose to depressed globose, papillate. Conidiophores arising from the wall, up to 28 µm long, and mainly 3 µm wide, in palisadic arrangement, filiform, septate, 1 to 3-celled, hyaline, unbranched or sparsely branched. Conidiogenous cells 4–12.5 × 1–3 µm (\(\overline{x }\) = 7.5 × 2.5 µm, n = 10), terminal phialide, cylindrical, hyaline. Conidia 3–4 × 1.5–2 μm (\(\overline{x }\) = 3.5 × 1.8 µm, n = 20), oblong to cylindrical, unicellular, with 1–2 small guttules, hyaline, smooth-walled.
Culture characteristics: Conidia germinating on PDA within 12 h and germ tube produced from conidia. Colonies growing on PDA, reaching 20 mm diam. in 10 days at 25 °C, circular, flat, entire edge with a protuberance in the center, brown to yellowish towards the edge in reverse and not producing pigment in culture.
Material examined: Thailand, Chiangrai Province, Mae Fah Luang University, on dead branch in a forest, 07 January 2019, J.Y. Zhang, TF01-2 (MFLU 19-1393), living culture, ex-type living culture, MFLUCC 22-0051 (new record for Thailand).
GenBank numbers: LSU = OQ101584, ITS = OQ101581, TEF1α = OQ054804.
Notes: Morphologically, our collection (MFLUCC 22-0051) matches with generic concept of Nigrograna and is similar to N. fuscidula in the shape of conidiophores, conidiogenous cells and conidia (Jaklitsch & Voglmayr 2016). However, our collection differs from N. fuscidula in having shorter conidiophores (up to 28 μm vs. up to 55 μm, Jaklitsch & Voglmayr 2016). In the phylogenetic tree, our isolate (MFLUCC 22-0051) clustered as sister taxon to the strains of Nigrograna heveae (ZHKUCC 22-0284 and ZHKUCC 22-0285) with support (100% ML/1.00 PP, Fig. 9). The comparison of LSU, ITS, and TEF1α sequences between our isolate and type strain (ZHKUCC 22-0285) showed 99.77% (850/852 bp), 99.34% (907/913 bp) and 99.79% (939/941 bp) sequence similarity, respectively. Based on the molecular evidence and phylogenetic result, we recognize they are the same species. Nigrograna heveae was introduced with its ascomycetous sexual morph on the stem of Hevea brasiliensis (Euphorbiaceae) in China (Hyde et al. 2023). In this study, we identify our collection as N. heveae with coelomycetous asexual morph and report a new record of the species in Thailand.
Phylogram generated from maximum likelihood analysis based on combined ITS and TEF1α sequence data. 40 taxa were included in the combined analyses, which comprised 1382 characters (ITS: 493 bp, TEF1α: 889 bp) after alignment. The best scoring RAxML tree with a final likelihood value of -5723.168531 is presented. The matrix had 441 distinct alignment patterns, with 15.59% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.224465, C = 0.293279, G = 0.250321, T = 0.231935; substitution rates: AC = 2.046193, AG = 3.037116, AT = 2.343899, CG = 0.810670, CT = 9.890958, GT = 1.000000; gamma distribution shape parameter α = 0.176114. Bootstrap support values for ML equal to or greater than 50% and BYPP equal to or greater than 0.95 are given above the nodes. The tree is rooted with Occultibambusa bambusae MFLUCC 13-0855 and O. pustula MFLUCC 11-0502. The newly-generated strain is shown in blue. Ex-type strains are indicated by black and bold
Pleosporaceae Nitschke.
Notes: Pleosporaceae (Nitschke 1869), includes 36 genera and 769 species (Kirk et al. 2008), and the species are cosmopolitan. They are parasites or saprobes on wood and dead herbaceous stems or leaves (Sivanesan 1984). This includes the genera like Alternaria, Bipolaris, Cochliobolus, Curvularia, Drechslera, Helminthosporium, Exserohilum and Pleospora.
Bipolaris Shoemaker.
Notes: Bipolaris was established by Shoemaker (1959) and comprises 69 species according to Wijayawardene et al. (2022). Bipolaris species cause leaf spots, leaf blights, root rots, foot rots and other disease symptoms in the family Poaceae, including rice, maize, wheat and sorghum and on some other host plants (Ellis 1971; Sivanesan 1987; Berbee et al. 1999). We follow the recent treatment of Bhunjun et al. (2020) and Wijayawardene et al. (2022).
Bipolaris heliconiae Alcorn, Aust. Syst. Bot. 9(5): 814 (1996).
Mycobank number: MB 436839; Facesoffungi number: FoF11035; Fig. 10
Bipolaris heliconiae NFCCI 5165 (AMH10416, holotype). a Substratum (leaf of Dypsis lutescens). b Stereomicroscopic surface view of infected leaf. c Colony morphology on PDA (front view on 7th days). d Branched conidiophores. e An unbranched cylindrical conidiophore. f–m Different types of conidia. n–o SEM images of conidia. p SEM images of branched conidiophores with numerous conidia. Scale bars d–o = 10 μm, p = 20 μm
Holotype: AMH10416.
Colour code follows: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
On leaf of Dypsis lutescens in terrestrial habitats. Infection spots amphiphilous, fructifications amphigenous, small, circular, dark brown, latter collapse to each other. Sexual morph: Undetermined. Asexual morph: stromatal cells 2–5 in groups, light to olivaceous brown (7D8). Setae and hyphopodia absent. Conidiophores superficial, straight to curved, solitary or in groups, unbranched to rarely dichotomously branched, basal cell of conidiophores slightly bulbous, geniculate in fertile region, scars slightly thickened, smooth walled, light brown (7D8), 2–12 septate, 103.30–335.50 × 7.0–16.5 μm. Conidia acropleurogenous, cylindrical, clavate to obclavate, smooth walled, light brown (7D8), transversely septate, 1–11 septate, hilum slightly thickened, non-protuberant, base truncate, tip obtuse, 43.0–192.0 × 7.5–17.0 μm.
In-vitro culture, vegetative hyphae, unbranched to branched, septate, thin and thick, pigmented, subhyaline to very light olivaceous brown(7D8), 3.0–9.0 μm wide. Chlamydospores absent. Setae and hyphopodia absent. Conidiophores arising from superficial hyphae, macronematous, mononematous, unbranched to dichotomously branched, straight to flexuous, multi-septate, smooth walled, scars slightly thickened, light olivaceous brown (7D8), up to 612.0 × 9.5 μm. Conidia acropleurogenous, straight to slightly curved, clavate to obclavate, non-protuberant, hilum slightly thickened, olivaceous to light olivaceous brown (7D8), transversely septate, smooth walled, base truncate, tip obtuse, pseudoseptate, 3–7 septate, inner cells discoid, 72.5–10.0 × 15.5–21.5 μm.
Culture characteristics: On semi-synthetic agar medium PDA (Potato Dextrose Agar) greyish white (5A1), greyish brown (5A2) reaching 3 cm diam in 8 days at 25 °C, with irregular margin, puffy, surface filamentous.
Material examined: India, Maharashtra, Pune District, on infected leaves of Dypsis lutescens (Arecaceae), 5 September 2021, P.N. Singh, AMH10416 (holotype); ex-type living culture, NFCCI5165 (National Fungal Culture Collection of India- WDCM 932, new record for India).
GenBank numbers: ITS = ON028647, LSU = ON032299, TEF1-α = ON148454.
Notes: The present taxon is distinct from type species Bipolaris heliconiae (BRIP 17186) (Alcorn 1996) in having smaller conidia (72.65–99.75 × 15.72–21.55 μm with 3–7 septa vs. 65–150 × 15–19 μm with 7–10 septa). In addition to this, the conidia of NFCCI 5165 are clavate to obclavate, whereas conidia of BRIP 17186 are fusoid to clavate fusoid. Bipolaris heliconiae (NFCCI 5165) clusters with Bipolaris heliconiae (BRIP 17189) and B. heliconiae (BRIP 17186) (Alcorn 1996) with 97% bootstrap support. On megablast analysis, our ITS sequence is showing 99% similarity (397/400) with 1 gap (0%) with both Bipolaris heliconiae (BRIP 17189) and Bipolaris heliconiae (BRIP 17186) (Fig. 11).
Phylogenetic tree of Bipolaris heliconiae (NFCCI 5165) constructed based on combined sequence data of ITS, LSU and TEF by Maximum-Likelihood method. Curvularia tuberculata CBS 146.63 was used as out-group. The analysis involved 51 nucleotide sequences. Evolutionary analyses were conducted in IQ–TREE multicore version 1.6.11 (Nguyen et al. 2015) by the Maximum–Likelihood method using the best suitable model (TITNe + R2 model). Newly generated sequence is in blue. One–thousand bootstrap replicates were analyzed to get ultrafast bootstrap values, and the values above 50% were represented on nodes in the tree. Ex-type strains are in bold and new isolate is in blue
However, based on similarity of morphological characteristics, and phylogenetic analysis, the present collection is confirmed as Bipolaris heliconiae (Alcorn 1996). This is the first report of Bipolaris heliconiae from India.
Trypetheliales Lücking, Aptroot & Sipman.
Notes: The order Trypetheliales was established by Aptroot et al. (2008) to accommodate the lichen-forming family Trypetheliaceae (Hyde et al. 2013), and recently another family, Polycoccaceae, including lichenicolous fungi, was included in this order (Ertz et al. 2015). The order is characterized by perithecioid ascomata solitary or aggregated in the pseudostromata, branched and anastomosing paraphyses forming a network, and hyaline or rarely brown ascospores, transversely septate to muriform often with diamond-shaped lumina (Aptroot et al. 2008; Hyde et al. 2013; Ertz et al. 2015).
Trypetheliaceae Eschw.
Notes: Trypetheliaceae now includes 18 genera and more than 400 species (Hongsanan et al. 2020b). Most members in this family were found in tropical lowland to lower montane, rain forest, dry forest, and savanna habitats, but a few species extended into temperate regions.
Astrothelium Eschw.
Notes: The genus Astrothelium includes pyrenocarpous lichen-forming fungi within Trypetheliaceae (Harris 1984, 1995). It is originally restricted to species with lateral, fused ostioles and transversely septate ascospores. In its revised delimitation, the genus comprises the majority of species in the Trypetheliaceae (Aptroot and Lücking 2016), with variable ascoma arrangement and ascospore septation (Hongsanan et al. 2020b). In both its traditional and its current circumscription, the genus has a pantropical distribution (Harris 1984; Awasthi 1991; Aptroot et al. 2008; Hyde et al. 2013).
Astrothelium peudostraminicolor S.H. Jiang, C. Zhang & J.C. Wei, sp. nov.
Mycobank number: MB 841113; Facesoffungi number: FoF 13377; Figs. 12, 13
Phylogenetic tree showing the internal phylogeny of Astrothelium, constructed through Bayesian analysis based on ITS with an alignment length of 455 bp. Bayesian inference posterior probabilities above 95% (left) and Maximum likelihood bootstrap support above 70% (right) are shown at nodes (B–PP / ML–BP)
Etymology: The epithet “peudostraminicolor” refers to the similarity with Astrothelium straminicolor.
Holotype: HMAS − L 151080.
Thallus crustose, corticate, olive-green to yellowish, shiny, uneven to bullate, continuous, cortex distinct, prothallus not observed, 0.2‒0.4 mm thick, covering areas up to 8 cm in diam., not inducing gall formation of the host bark. Algae trentepohlioid. Ascomata perithecia, conical or pyriform, black, 0.3‒0.7 mm in diam., erumpent, covered by thallus except for dark ostiolar area surrounded by whitish rim, solitary or aggregated in pseudostromata. Pseudostromata rounded to irregular, erumpent to prominent, covered by a thallus layer, with flattened top. Ostiole appearing as blackish dot, eccentric, fused, 30‒100 μm in diam. Ascomata Wall carbonized, black, 20‒90 μm thick. Hamathecium composed of densely anastomosing, net-like paraphyses, inspersed with oil drops. Asci cylindrical to clavate, 120–140 × 16–21 μm. Ascopores 8 per ascus, biseriate to irregular, hyaline, transversely 3-septate, 17‒30 × 6‒10 μm, fusiform, ends rounded, lumina diamond-shaped, surrounded by a smooth gelatinous sheath, 2‒8 μm wide. Pycnidia not seen.
Chemistry: Thallus UV-. Pseudostromata UV-. No substance detected by TLC.
Habitat and distribution: The new species grows on the bark of tropical and subtropical regions, and is currently only found in China.
Material examined: China, Guangxi Province, Xing’an county, Maoershan National Nature Reserve, 25° 52′ 10′′ N, 110° 24′ 47′′ E, 2025 m alt., on bark, 21 August 2017, X.L. Wei, R.D. Liu, X. Qian, Y.B. Zuo, X.M. Cheng 20191091 (HMAS−L 151080, holotype); ibid., XA2017068 (HMAS−L 0139996).
GenBank numbers: HMAS−L 151080: ITS = OM001629; HMAS−L 0139996: ITS = OM001628.
Notes: This species keys out in the recent world key (Aptroot 2021) in key K at couplet 6. It is similar to Astrothelium straminicolor, but the latter often has prominent pseudostromata, laterally covered by a thallus, with one to several groups of fused ascomata forming broad, flat, dark ostiolar areas often fused in a lobate pattern (Aptroot and Lücking 2016). Another similar species is A. pyrenastrosulphureum, but its pseudostromata are often prominent, covered by a thallus, with one to several groups of fused ascomata with dark, papilliform, always separate ostiolar areas, but without a whitish rim (Aptroot and Lücking 2016).
Tubeufiales Boonmee & K.D. Hyde.
Notes: Boonmee et al. (2014) established Tubeufiales based on multi-locus phylogeny and morphology. Tubeufiales comprises three families: Bezerromycetaceae (3 genera), Tubeufiaceae (47 genera) and Wiesneriomycetaceae (6 genera, Wijayawardene et al. 2022). The latest treatments and updated accounts of Tubeufiales by Hongsanan et al. (2020b) was followed in this paper.
Tubeufiaceae M.E. Barr.
Notes: Tubeufiaceae, typified by Tubeufia, was introduced by Barr (1979), who accepted an additional five genera: Letendrae, Melioliphila, Podonectria, Rebentischia and Thaxteriella. Various authors later introduced and accepted other genera in Tubeufiaceae (Rossman 1987; Kirk et al. 2001; Lumbsch and Huhndorf 2010; Boonmee et al. 2014; Brahmanage et al. 2017; Chaiwan et al. 2017; Liu et al. 2018; Lu et al. 2018). Forty-seven genera are presently included in Tubeufiaceae with Acanthostigma (60 species) and Tubeufia (ca. 60 species) being the most speciose genera (Dong et al. 2020). For the latest treatments and updated accounts of Tubeufiaceae see Hongsanan et al. (2020b).
Parahelicomyces Goh.
Notes: Lu et al. (2018) introduced Pseudohelicomyces with P. talbotii as type species and included four others, P. aquaticus, P. hyalosporus, P. paludosus and P. indicus. Phookamsak et al. (2019) and Jayasiri et al. (2019) introduced two more species, P. menglunicus and P. quercus, respectively. The former was observed from unidentified seed in China, while the latter from fruit pericarp of Quercus in Thailand. Later, Hsieh et al. (2021) collected Pseudohelicomyces talbotii from decaying culm of Miscanthus floridulus in Taiwan, China and an unidentified wood submerged in a stream. Since Pseudohelicomyces under Tubeufiaceae was a homonym of Pseudohelicomyces belonging to Hymenogastraceae (Agaricales, Agaricomycetes, Valenzuela and Garnica 2000). Hsieh et al. (2021) renamed the genus as Parahelicomyces, and transferred Pseudohelicomyces talbotii and the other six illegitimate Pseudohelicomyces species. Currently, Parahelicomyces species are accepted, and all have molecular sequence data. Parahelicomyces thrives in terrestrial and freshwater habitats (Jayasiri et al. 2019; Calabon et al. 2022). Four species were reported in the latter: Parahelicomyces aquaticus (Lu et al. 2018), P. hyalosporus (Luo et al. 2017) and P. talbotii (Lu et al. 2018; Hsieh et al. 2021). In this paper, we introduce one novel species of Parahelicomyces with unique morphology from Spartina sp. in salt marsh habitat in Thailand.
Parahelicomyces dictyosporus M.S. Calabon, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF 559841; Facesofungi number: FoF 12738; Fig. 14
Parahelicomyces dictyosporus (MFLU 22-0119, holotype). a Colonies in natural substrates. b–f Dictyochlamydospores. g Germinated conidium. h, i Colonies on MEA from surface and in reverse. j Dictyochlamydospores in culture. k–q Development of dictyochlamydospores. r–u Dictyochlamydospores Scale bars: a, j = 100 µm, b–g, k–u = 20 µm
Etymology: “dictyosporus” referring to dictyospores of this fungus.
Holotype: MFLU 22-0119.
Saprobic on submerged decaying wood in a freshwater stream. Sexual morph: Undetermined. Asexual morph: Hyphomycetous, dictyosporous. Conidiophores lacking. Conidiogenous cells holoblastic, monoblastic, integrated, cylindric, apical, hyaline to pale brown. Dictyospores 40–70 × 25–70 μm (x̄ = 52.3 × 43.1 μm, n = 20) acrogenous, carbonaceous, friable, solitary, mostly globose, subglobose to ovoid indistinctly dictyoseptate, verrucose, brown when young, dark brown to black when matured.
Culture characteristics: Conidia germinating on malt extract agar (MEA) and producing germ tubes within 24 h. Colonies growing on MEA, circular, with flat surface, edge entire to filiform, reaching 30–35 mm in 4 weeks at 25 °C, from above brown to dark brown, from below dark brown. Mycelia superficial and partially immersed, branched, septate, hyaline to pale brown. Sporulation in culture. Conidiophores lacking. Conidiogenous cells holoblastic, monoblastic, integrated, cylindric, apical, hyaline to pale brown. Dictyospores 35–70 × 25–70 μm (x̄ = 53.1 × 45.2 μm, n = 50) acrogenous, carbonaceous, friable, solitary, variable in shape, broadly oval to ellipsoidal when young, mostly globose when mature, indistinctly dictyoseptate, verrucose, hyaline to pale brown when young, dark brown to black when matured.
Habitat and distribution: Parahelicomyces dictyosporus was observed from submerged decaying culms of Spartina sp. in tropical saltmarsh area, and is currently only found in Thailand.
Material examined: Thailand, Prachuap Khiri Khan, Pran Buri, 12°23′43.0"N 99°58′28.3"E, on submerged decaying culms of Spartina sp. (Poaceae), 20 July 2019, M.S. Calabon, MCFWBB (MFLU 22-0119, holotype), ex-type living culture, MFLUCC 22-0080.
GenBank numbers: ITS = OP216409, LSU = OP216404, TEF1-α = OP251194, RPB2 = OP251198.
Notes: The isolate MFLU 22-0119 is morphologically similar with Tubeufia dictyospora (MFLU 17–1173) with globose to subglobose dictyospores but the latter has a larger dictyospores [60–100 × 60–70(–80) μm vs. 40–70 × 25–70 μm] (Lu et al. 2018). NCBI BLASTn of the sequences of isolate MFLUCC 22-0119 showed the this is closely related to Parahelicomyces: LSU (P. hyalosporus CBS 283.51, 99.51%), ITS (P. aquaticus MFLUCC 16-0234, 92.20%), TEF1-α (P. aquaticus MFLUCC 16-0234, 98.46%; P. quercus MFUCC 17-0895, 98.02%), and RPB2 (P. hyalosporus AFTOL-ID, 94.30%). The multi-locus phylogenetic analysis placed the isolate within Parahelicomyces in a subclade with a terrestrial species P. chiangmaiensis and the freshwater species P. aquaticus (Fig. 15). In pairwise nucleotide comparisons of P. dictyosporus with the sister taxon P. chiangmaiensis (MFUCC 21-0159), there is a nucleotide differences of 3.36% (13 bp) in ITS (of 387 nucleotides altogether), 1.86% (16 bp) in TEF1-α (of 861 nucleotides altogether), and 3.26% (26 bp) in RPB2 (of 797 nucleotides altogether). Parahelicomyces dictyosporus is a dictyosporous species and this morphological character is unique compared to other Parahelicomyces species with cylindrical, branched conidiophores, and acropleurogenous, helicoid conidia. Parahelicomyces dictyosporus is the first species of Parahelicomyces reported from marine habitats, and the third species recorded from aquatic environments in Thailand, wherein Para. aquaticus and Para. talbotii were earlier recorded by Lu et al. (2018).
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS, TEF1-α, and RPB2 sequence data representing Tubeufiaceae (Tubeufiales). One hundred strains are included in the combined analyses which comprised 3726 characters (806 characters for LSU, 967 characters for ITS, 910 characters for TEF1-α, and 1043 characters for RPB2) after alignment. Bezerromyces pernambucoensis (URM7412) and Bezerromyces pseudobrasiliensis (URM7414) in Tubeufiaceae (Tubeufiales) were used as the outgroup taxa. The best scoring RAxML tree with a final likelihood value of − 44,231.079169 is presented. The matrix had 1741 distinct alignment patterns, with 33.38% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.242684, C = 0.256704, G = 0.264190, T = 0.236422; substitution rates: AC = 1.008141, AG = 3.810958, AT = 1.774984, CG = 0.705605, CT = 6.978420, GT = 1.000000; gamma distribution shape parameter α = 0.240917. Bootstrap support values for ML equal to or greater than 70% are given above the nodes (left side). Bayesian posterior probabilities (BYPP) equal to or greater than 0.95 are given above the nodes (right side). Ex-type strains are in bold and newly generated sequences are in blue
Eurotiomycetes Tehler ex O.E. Eriksson & K. Winka.
Notes: For the latest treatments and updated accounts of Eurotiomycetes, see Wijayawardene et al. (2022).
Eurotiales G.W. Martin ex Benny & Kimbr.
Notes: The order Eurotiales has many species with economically important uses and negative impacts on human activities (Houbraken et al. 2020); currently, it has five accepted families harbouring about 28 genera (Wijayawardene et al. 2022).
Thermoascaceae Apinis.
Notes: The family Thermoascaceae was introduced by Apinis (1967), and it has been recently revisited based on morphology and phylogeny; currently, this family has two accepted genera, Paecilomyces and Thermoascus (Houbraken et al. 2020; Wijayawardene et al. 2022).
Thermoascus Miehe.
Notes: The genus Thermoascus has 12 records in Index Fungorum and MycoBank databases (3 July 2022), and about seven species are accepted in this genus (Houbraken et al. 2020; Wijayawardene et al. 2022). Species in this genus are thermophilic and morphologically characterised by the “production of orange-yellow, brown or red-brown, soft cleistothecia formed in a more or less continuous crust-like layer with a pseudoparenchymatous wall”, and the anamorph can be absent or differ significantly (Houbraken et al. 2020).
Thermoascus endophyticus T.M. Silva, C.S. Oliveira & J.D.P. Bezerra, sp. nov.
Mycobank number: MB 846409; Facesoffungi number: FoF 13376; Fig. 16
Thermoascus endophyticus (UFG 34289, holotype). a Colonies (verse and reverse) on MEA, PDA, DG18 and CZ at 25 °C after 1 week in the dark and PDA after 30 days. b Cleistothecial ascomata and ascospores. c‒e Asci and ascospores. f‒j Conidiophores and conidia. k Conidia. Scale bars: b = 50 µm, c‒k = 10 µm
Etymology: The epithet "endophyticus" refers to the fungus’s lifestyle, which was found to occur endophytically in Brosimum gaudichaudii.
Holotype: UFG 34289.
Cleistothecial ascomata, globose to subglobose, light brown to brown, abundantly present with colony age (178‒)418(‒465) × (108‒)372(‒465) µm. Asci globose to subglobose, 8-spored, 13.5‒16.5 × 11‒13.5 µm. Ascospores ellipsoids, ornamented, light brown to brown with age (5.5‒)8 × (4‒)5.5 µm. Conidiophores straight to flexuous, septate, branched, hyaline, smooth-walled, abundantly present at the initial colony growth stage (40.5‒)108‒148(‒216) × (4‒)5.5 µm. Phialides ampulliform with a cylindrical basal portion and tapering to a thin neck, hyaline, smooth-walled, in groups of two to three (occasionally one) on short metulae, single phialides are occasionally born directly on the hyphae (13.5‒)16‒19(‒21.5) × (3‒)5.5 µm. Conidia cylindrical, occasionally subglobose, aseptate, hyaline to light brown with age, smooth walled, produced in chains (5.5‒)8‒11 × 3(‒5.5) µm. Chlamydospores not observed.
Culture characteristics: Colonies on PDA, MEA and DG18 growing fast and attaining a diameter of 90 mm at 25 °C after 1 week in the dark and growing up to 70 mm on CZ. On PDA, colonies are plane, floccose, whitish, yellowish to orange and orange with age, exudate yellowish; reverse yellowish to orange. On MEA, colonies are plane, slightly cottonose, whitish, salmon to orange with age, concentric circles, exudate salmon to orange; reverse brownish to orange. On DG18, colonies are slightly cottonose, whitish to light orange with age, exudate not observed; reverse white and yellowish to orange. On CZ, colonies are plane, floccose, yellowish to orange, exudate not observed; reverse light brown to orange. At 36 °C after 1 week in the dark, colonies on PDA, MEA and DG18 growing fast and attaining a diameter of 90 mm and morphologically similar as described at 25 °C.
Habitat and distribution: The new species occur endophytically in branches of Brosimum gaudichaudii Trécul (Moraceae) and is currently only found in Brazil’s Cerrado biome.
Material examined: Brazil, Goiás state, Goiânia municipally, Escola de Agronomia of the Universidade Federal de Goiás, 16º 35′ 58.5″ S, 49º 16′ 45.8″ W, isolated as an endophyte from branches of Brosimum gaudichaudii (Moraceae), 20 October 2020, T. M. Silva & J.D.P. Bezerra (UFG 34289, holotype), ex-type living culture, FCCUFG 19 = URM 8565, ibid., FCCUFG 20 and FCCUFG 21.
GenBank numbers: FCCUFG 19 = URM 8565: ITS = OP325230, CAL = OP351562, TUB2 = OP351559, RPB2 = OP351565; FCCUFG 20: ITS = OP325231, CAL = OP351563, TUB2 = OP351560, RPB2 = OP351566; FCCUFG 21: ITS = OP325232, CAL = OP351564, TUB2 = OP351561, RPB2 = OP351567.
Notes: The new species is phylogenetically placed in a well-supported clade (ML-BS = 100% and BYPP = 1), having Thermoascus aegyptiacus and Thermoascus crustaceus as related species (Fig. 17). Morphologically, T. endophyticus differs from T. aegyptiacus by the size of cleistothecia (250‒550 µm), asci (14‒18 × 11‒15 µm) and ascospores (6.0‒8.5 × 4.0‒5.5 µm) in the teleomorph; and in the anamorph by the size of conidiophores (50‒300 × 5‒7 µm), phialides (12‒30 × 3‒6 µm) and conidia (4.5‒11 × 3‒4 µm) (Salar and Aneja 2007). The new species also differs from T. crustaceus by the size of orange cleistothecia (300‒900 µm diameter), asci (16‒20 × 13‒15 µm) and ascospores (6.5‒8 × 5‒6.5 µm) in the teleomorph; in the anamorph by the size of conidiophores (up to 1000 µm long, 7‒12 µm wide at the base and 4‒5 µm at the apex), phialides (15‒30 µm long) and conidia (6‒10 × 3‒6 µm, Stolk 1965).
Phylogram generated from maximum likelihood analysis based on combined ITS, TUB2, CAL and RPB2 sequence data representing Thermoascus in Thermoascaceae, Eurotiales. Eleven strains are included in the combined analyses which comprised 2815 characters (610 characters for ITS, 533 characters for TUB2, 625 characters for CAL and 1047 characters for RPB2) after alignment. Paecilomyces niveus (CBS 100.11) and Paecilomyces variotii (CBS 102.74) in Thermoascaceae (Eurotiales) were used as the outgroup taxa. The best scoring RAxML tree with a final likelihood value of -9050.155654 is presented. The matrix had 699 distinct alignment patterns, with 20.42% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.212703, C = 0.286868, G = 0.279343, T = 0.221086; substitution rates: AC = 0.719132, AG = 1.931043, AT = 0.828377, CG = 0.434883, CT = 3.720518, GT = 1.000000; gamma distribution shape parameter α = 0.268628. Bootstrap support values for ML equal to or greater than 70% and Bayesian posterior probabilities (BYPP) equal to or greater than 0.95 are given near nodes. Ex-type strains are in bold and newly generated sequences (Thermoascus endophyticus) are in blue
Laboulbeniomycetes Engl.
Laboulbeniales Lindau.
Notes: This order includes more than 2100 species described as obligate ectosymbionts on Arthropods. Wijayawardena et al. (2022) accepted three families viz. Ceratomycetaceae, Euceratomycetaceae and Laboulbeniaceae in this order.
Laboulbeniaceae G. Winter.
Notes: Goldmann and Weir (2018) based on SSU rDNA sequence data identified this family to consist of mostly terrestrial and sexually reproducing taxa with simple or compound endogenous antheridia. Santamaria & Pedersen (2021) accepted 147 genera in this family.
Autophagomyces Thaxt.
Notes: After the revision by Benjamin (2001), the concept of this genus was narrowed to 12 species, nine of which occurr on Coleoptera Anthicidae and the others on Phalacridae and Scaphiidae. The seven characteristics listed by Benjamin for Autophagomyces in the limited concept are: (1) cell III very close or in contact with cell I; (2) cell I and II separated by a transverse cross wall; (3) 1–3 free, slender appendages borne from cell III; (4) absence of the remain of the original spore apex; (5) trichogyne consisting of 2 or rarely 3 cells; (6) trichogynic remnant not visible; and (7) perithecium with five tiers of outer wall cells. No DNA sequence is available for any species in Autophagomyces.
Autophagomyces incertus W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: 559783; Facesoffungi number: FoF 12769; Fig. 18
Etymology: From Latin, meaning uncertain, because of the uncertain taxonomic position of the new species.
Holotype: CAMB WR2471.
Thallus uniformly straw-yellow. Basal cell of the receptacle (cell I) tapered downward to hyaline and aciculate tip of foot. Cell II quadrangular, slightly longer than broad, its outer margin slightly concave. Cell III relatively large, wedge-shaped, united on the inside to cell II, reaching the base of cell VI and the top of cell I. Appendage simple, free, consisting of 5 superposed cells, the lower two of which are slightly longer than broad, the third subquadrate, the fourth much smaller and broader than long, bearing an antheridium on the inner side, while the fifth, slightly larger than the forth, bears two antheridia. Antheridia with a very narrow and slightly curved efferent tube, the terminal one bearing a tiny and hardly visible spine. Cell VI short, about as long as maximum width, abruptly constricted at the base. Cell VII and basal cells relatively large and elongate, forming together a stipe slightly exceeding the ascigerous part of the perithecial body. Perithecium slightly asymmetrical, with the ventral side more convex, broadest below the middle, than tapering upwards, becoming abruptly narrower just above the median tier of the outer wall cells, than nearly uniform in width, the tip rather abruptly and symmetrically tapered, the apex rounded. Length from foot to perithecial apex 285–330 µm; length from foot to tip of uppermost antheridium 160–165 µm; perithecium, including basal cells 200–225 × 70–75 µm.
Material examined: Australia, New South Wales, Headwaters Lookout Barrington Tops, in leaf litter, 19 January 1992, leg. V. Lorimer, on the dorsal side of the abdomen of Palimbolus sp. (Coleoptera, Staphylinidae, Pselaphinae) CAMB WR2471 (holotype).
Notes: Utilizing the key given by Tavares (1985), the inclusion of this fungus within the genus Autophagomyces is out of question. The new species also displays at least five of the seven characteristics listed by Benjamin (2001) for Autophagomyces in the limited concept. A sixth remains uncertain, because the structure of the trichogyne is not visible in the available thalli. The only feature in contrast with Benjamin’s concept of the genus is the presence of a spinous process on the appendage, which is not, in our opinion, a conclusive feature. The fact that the spine, which is actually the remain of the original spore apex, is sometimes minute and evanescent, and has been observed in only one of almost 700 recognized species of Laboulbenia (Dima et al. 2021) and in only one of the 91 species of Rhachomyces (Santamaria et al. 2020; Buyck et al. 2021) would seem to support our opinion.
Dimorphomyces Thaxt.
Notes: Dioecious genus consisting of 29 recognized species, of which the most recently described is D. carolinae (Rossi 2010). The female thallus consists of a row of small cells bordered below by an extension of the basal cell; these cells produce from their upper side either perithecia or secondary appendages. The male thallus is much smaller, being composed of a few celled receptacle, compound flask-shaped antheridia and a simple appendage. The majority of the hosts of Dimorphomyces are rove beetles (Coleoptera, Staphylinidae), but a few species are found on other insect families (viz. Carabidae and Tenebrionidae), one on ants and two on mites. No DNA sequence is available for any of the species in Dimorphomyces.
Dimorphomyces seemanii W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: 559784; Facesoffungi number: FoF 12924; Fig. 19
Etymology: Named after the Australian acarologist Owen D. Seeman, who supplied us with the materials utilized for the description of this new species.
Holotype: CAMB WR2471.
Female thallus broadly fan-shaped, tinged with amber yellow. Lower portion of the basal cell (Cell I) forming a well-defined stalk. Cell II distinctly longer than broad, regularly enlarging from below upwards. Primary appendage consisting of three cells, the lower of which is quadrangular in outline, slightly to distinctly longer than broad, the following smaller, pale gray and barrel-shaped, delimited above and below by thick, dark septa, the uppermost cell hyaline rounded or slightly longer than broad; the uppermost cell is usually missing in mature thalli. Secondary receptacle usually curved, forming an almost right angle with the primary receptacle, consisting of a row of 7–10 gradually smaller cells bearing one or two perithecia or three celled appendages similar to the primary appendage. Perithecia paler than the receptacle, subfusiform, with a truncate apex. Length from foot to perithecial apex 120–175 µm; perithecium, including stalk cell 110 × 25 µm.
Male thallus translucent, tinged with very pale yellow. Basal cell of the receptacle slender and elongate, separated from the shorter and stockier suprabasal cell by a very oblique septum. The latter cell gives rise distally to the appendage and bears laterally the short antheridial stalk-cell and a small rounded cell which protrudes outwards under the antheridium. Appendage similar to those of the female thalli. Antheridium flask-shaped, with the venter almost rounded, rather abruptly distinguished from the strongly tapering and slightly outcurved neck. Length from foot to antheridial apex 67 µm.
Material examined: Australia, SE Queensland, Lamington N. P., rainforest, 1142 m, 28.259°S, 153.162°E, 27 January 2008, leg. C. Burwell, on Micromegistus sp. (Acarina, Parantennulidae) WR3782 (holotype). NE New South Wales, Gibraltar Range, pitfall trap, 450 m, 29° 32′ S, 152° 22′ E, 1980–1981, leg. G.B. Monteith, on Micromegistus sp., WR3783.
Notes: The new species is very different from Dimorphomyces clavulifer and D. triangularis, the other two species occurring on mites. Among other differences, both the latter fungi have much longer appendages and much shorter perithecia. Dimorphomyces seemanii is easily distinguishable from all other species in the same genus for the spherical distal cell of the appendages and the rounded cell protruding under the antheridium.
An undescribed species of Dimorphomyces on an undescribed species Micromegistus associated with the ground beetle Trichosternus subvirens was reported for the first time by Seeman and Nahrung (2000), who also provided a drawing of the new fungus in Fig. 1a on page 5.
Laboulbenia Mont. & C.P. Robin.
Notes: With about 700 species, Laboulbenia is by far the largest genus among the Laboulbeniales, representing almost one third of all species in the order. The species of Laboulbenia occur on various families of beetles (Coleoptera), but are also found on flies (Diptera), true bugs (Hemiptera), ants (Hymenoptera), termites (Isoptera), cockroaches (Blattodea), crickets (Orthoptera), and mites (Acarina) (Kaishian et al. 2020; Kong et al. 2020; Santamaria and Pedersen 2021). Very few published sequences of species in this large genus are available to date, with most of the taxa, including the type species, lacking this molecular information.
Laboulbenia bifida W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: 559786; Facesoffungi number: FoF 12925; Fig. 20
Etymology: From Latin bifidus = bifid, divided in two parts, referring to the outer appendage consisting of two subequal branches.
Holotype: FI WR3571.
Basal cell (cell I) grayish yellow, relatively small, about twice longer than maximum width, tapering below. Suprabasal cell (II) paler and much larger than the former, dirty yellow, almost twice longer than broad, slightly enlarging upwards. Cell III much smaller than cell II, bicolored, with the lower portion concolorous with the underlying cell and the upper grayish yellow, distinctly darker. Cell IV as long as cell III but slightly wider, dark gray, becoming paler on the inner side. Cell V small, shield-shaped and yellowish. Insertion cell black and thick. Outer appendage consisting of a dirty yellow, relatively large, broader than long and irregularly quadrangular basal cell, bearing distally two simple and elongate branches. The latter are similar except in color, the outer one being darker in the lower portion, with the two lowermost cells dark gray, the third light gray, the others dirty yellow. Cell VI quadrangular, distinctly shorter than the flanking cell III. Perithecium more than half free, about two and a half times longer than broad, irregularly yellowish gray, darker above its basal cells, not abruptly tapering to the tip, ending in a hyaline and subtruncate apex pointing outwards, subtended by a blackish area more extended on the ventral side and darker on the dorsal. Length from foot to perithecial apex 325–425 µm; longest appendage 275 µm; perithecium 175–195 × 65–75 µm.
Material examined: New Zealand, South Island, mouth of Taieri River, under driftwood, sandy beach, January 2007, J. Nunn, on various parts of the body of Otagonia chathamensis Bordoni (Coleoptera, Staphylinidae), FI WR3571 (holotype).
Notes: Although Laboulbenia, with almost 700 species, is by far the largest genus among the Laboulbeniales, the species of this genus occurring on Staphylinidae are just over thirty. None of these fungi bear an outer appendage consisting of two subequal branches arising from the basal cell, which makes Laboulbenia. bifida distinguishable at first sight. The new species might be compared only with L. micrandra, with which it shares a bifurcate outer appendage, an inner appendage lacking sterile branches, and a septum between cells IV and V not reaching cell III. However, the latter species, parasitic on Lobrathium sp. from Ecuador, is distinctly smaller and slenderer, with the outer appendage dividing above the second cell (not above the first, as in Laboulbenia bifida), and with very different perithecial tip and apex (Rossi 2011).
Laboulbenia tschirnhausii W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: 559788; Facesoffungi number: FoF 12927; Fig. 21
Etymology: Named after the German dipterologist Michael von Tschirnhaus, who supplied us with the flies bearing the new parasites.
Holotype: CAMB WR3382.
Basal cell (cell I) large brownish yellow, short, trapezoidal, slightly broader than long. Suprabasal cell (cell II) concolorous with the former but much longer, slightly and evenly enlarging from below upwards. Stalk cell of the appendage (cell III + IV + V) darker, slightly longer than broad, with the outer margin distinctly convex and the inner united to the stalk- and basal cells of the perithecium. Insertion cell small, dark brown, nearly isodiametric, adherent to the base of the perithecium on the inner side, bearing apically four cells, of which the two outer ones are larger and more elongate. These cells give rise to short and irregularly ramified branches ending in elongate antheridia or slender, hyaline and curled branchlets. Stalk cell of the perithecium (cell VI) almost flattened. Perithecium, with its basal cells, chestnut brown, becoming paler above and below, flask-shaped, the inflated venter evenly tapering to the long neck, ending with two large, short, cylindrical, truncate and symmetrically diverging outgrowths, which are subtended by a rounded prominence on the ventral side. Length from insect surface to perithecial apex 360–430 µm; length from insect surface to tip of longest appendage 290 µm; perithecium 215–270 × 85–105 µm.
Material examined: Australia, Queensland, Carnarvon National Park, Mt. Moffatt Camp Site along small creek, 10 October 2002, M. von Tschirnhaus, on the head of an undescribed species of Apotropina (Diptera, Chloropidae), CAMB WR3382 (holotype), FI WR3383.
Notes: Laboulbenia tschirnhausii clearly belongs to a small group of species occurring on flies characterized by rhizoids penetrating the body of the host insects and by an undivided stalk-cell of the appendage. This group includes L. dahlii, L. curtonoti, L. penetrans and L. perforans (Thaxter 1901; Rossi and Kirk-Spriggs 2011; Rossi and Leonardi 2018; Rossi et al. 2019). Laboulbenia tschirnhausii differs from all the latter parasites for its stockier habitus, the nearly isodiametric stalk-cell of the appendage, the more inflated perithecial venter, and especially the peculiar perithecial outgrowths.
The thalli of Laboulbenia tschirnhausii were all found projecting forward from the head of the host insects, which means that the transfer of the sticky spores from a parasitized insect to other members of the same species did not happen during sexual contacts. Actually, although sexual contacts represent the more frequent method by which Laboulbeniales pass from one host to another, these fungi take advantage also of other contacts resulting from social behaviors not directly related with mating. The contacts by means of the antennae, which are frequent among insects, seems to be the way utilized by Laboulbenia tschirnhausii. Several other Laboulbeniales benefit from this behavior. For example, among the species occurring on flies can be cited Stigmatomyces hydrelliae and the above mentioned Laboulbenia dahlii (Thaxter 1901; Weir and Rossi 1995).
The description of a new species bearing haustoria penetrating the host insect gives us the opportunity to briefly comment on a recent paper that deals with this topic (Haelewaters et al. 2022). In this paper the authors suggest that species with haustoria are older than the species without haustoria and that the latter are derived from the former. However, phylogenetic analyses based on DNA sequence data have shown that the genera bearing haustoria are scattered through the phylogenetic tree and do not occupy a basal position relative to genera without (evident) haustoria (Goldmann and Weir 2018). This pattern clearly suggests that the character ‘haustorium’ evolved independently in different clades of Labulbeniomycetes. Moreover, under the term ‘haustoria’ are grouped very different structures. For example, the genera Herpomyces and Hesperomyces, have inconspicuous haustoria originating from the foot, while the haustoria of Laboulbenia and Rhizomyces, when present, are in comparison very large, sometimes very long and ramified, originating from the basal cell [these four genera are present in the tree of Goldmann and Weir (2018)]. Therefore, it is possible that these different types of haustoria do not represent homologous structures. While the evolutionary origin of haustoria certainly needs further research, the hypothesis by Haelewaters et al. (2022) is clearly not supported by available data.
Laboulbenia tuberculata W. Rossi & M. Leonardi, sp. nov.
Index Fungorum number: 559787; Facesoffungi number: FoF 12926; Fig. 22
Etymology: From Latin tuberculatus, which means “covered with wart-like projections”.
Holotype: FI WR4035.
Basal cell (cell I) of the receptacle slender and elongate, with almost parallel margins, hyaline above the foot, then gradually darkening from below upwards becoming brown in the upper portion, its surface distinctly warty. Suprabasal cell (cell II) with a wavy surface, broadly pentagonal, much shorter and paler than the basal, from which is separated by a thick, blackish septum looking like a prominent ring. Similar septa, but shorter and symmetrically oblique, divide cell II from cell III and cell VI. Cell III about as long as cell II but distinctly narrower, dark brown, its surface covered with large, dark warts, the warty surface extending to cell VI and to the lower, outer portion of cell IV. The latter as long as cell III but slightly broader and somewhat paler. Cell V very small and lens-like. Insertion cell thick, subtended by a paler line. Outer appendage consisting of a basal cell almost twice longer than broad followed by a linear series of hyaline cells gradually longer and slenderer. Inner appendage consisting of a small and flattened basal cell giving rise to a short cell oriented inwards and bearing distally a pair of falcate antheridia. Cell VI rhombic, shorter than the adjacent cell III. Perithecium colored pale brown, darker in the lower portion, adnate to the receptacle for 3/5 of its length, oblong, slightly inflated, its surface slightly undulate, the darker tip abruptly distinguished, ending in rounded and subhyaline lips, one of which is larger than the others. Length from foot to perithecial apex 160–180 µm; length from foot to tip of longest appendage 420 µm; perithecium 60–63 × 23–25 µm.
Material examined: Papua New Guinea, Western Province, Yaromdeng tem, Cave near Finim tel, 9 November 1975, leg. Ph. Chapman, on Altagonum sphodrum Darlington (Coleoptera, Carabidae), FI WR4035 (holotype), FI WR4036.
Notes: The large, irregularly arranged dark warts that cover most of the surface of the receptacle make it possible to distinguish at first sight Laboulbenia tuberculata from any other described species. Smaller warts on the surface of the receptacle are found in L. tuberculifera and L. rugosa, but in the latter species the warts are found only on cells III, IV and V, while in the former these are restricted to cell II (Thaxter 1908; Rossi and Leonardi 2020). The surface of the receptacle of Laboulbens scabra is also completely covered with warts, but in this case the warts are small, uniformly distributed and concolorous with the remaining parts of the thallus (Kong et al. 2020).
Lecanoromycetes O.E. Erikss. & Winka.
Notes: Lecanoromycetes is the largest and most varied class of lichenized fungi with 15,131 species and 701 genera. It includes four subclasses (Acarosporomycetidae, Candelariomycetidae, Lecanoromycetidae, Ostropomycetidae), 17 orders and 75 families. This class includes orders predominantly or exclusively lichenized, with the exception of Ostropales (Lücking et al. 2017). Species in this class are characterized mainly by their ascohymenial ascomatal ontogeny, with a predominance of apothecial fruiting bodies, although of diverse construction and shape. In most lineages, asci have a multilayered ascal wall of which two layers are thick enough to be visible with light microscopy and display different types of dehiscence (Miadlikowska et al. 2006). Members of Lecanoromycetes form bipartite symbiotic associations with a broad range of photobionts, representing chlorococcalean algae, filamentous algae and cyanobacteria. Molecular studies have substantially challenged phenotypically based groupings applied to previous classifications, as well as resolved placement of many sterile taxa, and taxa with uncertain taxonomic affiliation. Kraichak et al. (2018) proposed a revised classification, based on temporal banding approach, of orders and families in the two major subclasses of Lecanoromycetes with seven orders in Lecanoromycetidae and eight in Ostropomycetidae.
Lecanorales Nannf.
Notes: Lecanorales was introduced by Nannfeldt (1932) to accommodate the Lecanoraceae as the type family. It is the largest order of lichen fungi with 6231 species and 234 genera distributed into 19 families (Lücking et al. 2017). The main ascus dehiscence, structural differences, and staining reactions of the tholus induced by Lugol’s iodine was used to name ascus type according to genera (e.g. Lecanora-type, Bacidia-type, Acarospora-type) although with little agreement on naming conventions. A Lecanora-type ascus may have been ancestral in the Lecanorales. (Ekman et al. 2008).
Parmeliaceae F. Berchtold & J. Presl.
Notes: Parmeliaceae is the largest family of lichenized fungi, with 2765 species and 77 genera (Lücking et al. 2016). The family belongs to the core of the Lecanorales closely related to other large families like the Lecanoraceae and Cladoniaceae (Crespo et al. 2007). Parmeliaceae includes morphologically very diverse lichens, including crustose (e.g., Protoparmelia), peltate (e.g., Omphalodiella), subcrustose (e.g., Karoowia), foliose (e.g., Parmelia), umbilicate (e.g., Xanthomaculina), fruticose (e.g., Usnea) or subfruticose (e.g., Almbornia) species and even lichenicolous fungi devoid of any own photosynthetic partner, such as Phacopsis and Nesolechia (Crespo et al. 2007) tested hypotheses of phylogenetic relationships in Parmeliaceae based on morphology and molecular biology (three ribosomal markers and the nuclear gene RPB1). In this study, they point out that the species of Usnea are strongly supported as a monophyletic group and that six more monophyletic clades were found in Parmeliaceae: alectorioide, cetrarioid, hypogymnioid, letharioid, parmelioid and psiloparmelioid. A review of the family Parmeliaceae is provided by Thell et al. (2012).
Usnea Dill. ex Adans.
Notes: The genus Usnea forms a strong supported clade (named “usneoid”) within the phylogeny of Parmeliaceae (Crespo et al. 2007) sister to Cornicularia normoerica (Divakar et al. 2015). Usnea is a fruticose lichen genus easily recognized by its thallus branches with radial symetry, the presence of a central cartilaginous axis and the production of usnic acid in the cortex. The first major work about the genus Usnea was published by Motyka (1936), who published more than 750 names in his world monograph, many of them are now considered synonyms of well-known species (Clerc 1998). Usnea is among the ten richest genus among the lichenized fungi in number of species with 350 species (Lücking et al. 2016), but actually molecular phylogeny indicated that the number could be twice (Lücking et al. 2020). Modern revisions of the genus were done in Africa (e.g., Temu et al. 2019), Australia (e.g., Stevens 2004), Europe (e.g., Clerc and Otte 2018), India (e.g., Shukla et al. 2014), Japan, China and Russia (e.g., Ohmura 2001; Ohmura et al. 2017), New Zealand (e.g., Galloway 2007), North America (e.g. Herrera-Campos 2016), South America (e.g. Gerlach et al. 2020), and Polar regions (e.g. Wirtz et al. 2012).
Usnea kriegeriana A. Gerlach & P. Clerc, sp. nov.
Index Fungorum number: IF 900342; Facesoffungi number: FoF 14476; Fig. 23
Usnea kriegeriana. a basal part with annular cracks (white arrows) ((2016/P19, holotype). b lateral branches slightly constricted at ramification point, with annular cracks (arrows) (Krieger 13474). c section through thallus with matt cortex (holotype). d large and confluent soralia (Krieger 13474). Scales bars: a, b = 2 mm, c = 500 μm, d = 2 mm
Etymology: In honor of the Brazilian Priest Leopoldo Krieger, retired botany teacher of the Federal University of Juiz de Fora (Brazil), who collected many Usnea specimens chiefly in the south of Minas Gerais and in the Paraná State. The oldest specimen of this new species was collected by him in 1975.
Holotype: 2016/P19 (ICN).
Diagnosis: Thallus shrubby, basal part with annular cracks, lateral branches not constricted at ramification point, main branches covered with eroded tubercles and convex soralia, cortex mat, thin to ± thick (6.5–)7.5–9%–10.5(–11.5) and medulla with fumarprotocetraric acids as main medullar secondary metabolite.
Lichenized Ascomycota fungi corticolous on bark of Araucaria angustifolia, on exotical Pinus spp, or lignicolous on fences. Thallus shrubby, up to 10 cm long; ramifications mainly isotomic-dichotomous; basal part up to 0.5 cm long, concolorous to the main branches, sometimes paler or with a faint orange tinge, regularly with annular cracks; main branches up to 1.8 mm diameter (n = 16), cylindrical to slightly irregular; branches segments cylindrical, sometimes slightly swollen; lateral branches not (main branches) to sometimes slightly constricted (secondary branches) at ramification point; annular cracks present, thin, often with medullar extrusions, sparse to frequent on the whole thallus, 1–2 annular cracks/0.5 cm; foveolae, depressions, maculae and pseudocyphellae absent; papillae absent or sparse; tubercles present, often numerous, verrucous, eroded at top; fibrils slender, unevenly distributed; fibercles sparse; soralia punctiform to large, convex, often circular or building elliptical to irregular masses of soralia, usually numerous on main branches, well delimited to confluent towards the apices of the branches, arising from the top of tubercles, with granular soredia; isidiomorphs usually numerous; isidiofibrils rare; apothecia and pycnidia not seen; cortex moderately thin to ± thick [(6.5–)7.5–9%–10.5(–11.5)], mat (n = 16); medulla ± thin to thick [19–25.5%–32(–48)], dense; axis sometimes with an orange tinge, thin to ± thin [(19–)24–31.5%–38.5(–40)]; A/M = 0.6–1–2. Photobiont trebouxioid. Medulla K + yellow turning red. Secondary chemistry detected by thin layer chromatography: fumarprotocetraric acid, ± protocetraric acid, ± an unknown substance orange after charring Rf classes A/B/C: 1–2/2–3/1–2 (US2) (n = 20).
Material examined: Brazil, Santa Catarina: Alfredo Wagner, Reserva Particular do Patrimônio Natural Rio das Furnas, ca. 27° 40′ 28.3″ S, 49° 10′ 37.9″ W, ca. 900 m, on twigs, 13 May 2016, Gerlach et al., 2016/P19 (ICN, holotype), Bosque das Araucarias, sobre Araucaria angustifolia, ca. 900 m, 2016, Gerlach et al. P37 (G); Joinville, estrada das Laranjeiras, sobre mourão, 5 October 2013, Gerlach & Beilke 1133a (ICN); Urubici, Parque Nacional de São Joaquim, arredores do alojamento, ca. 1300 m, 03 February 2014, Gerlach & Alves 1327 (ICN); São Bento do Sul, APA Rio Vermelho, on Araucaria angustifolia, 12 March 2013, Gumboski 4269 (ICN). Espírito Santo, Domingos Martins, in Weide am Morro do Cruzeiro, 1200 m, 20° 26′ S, 41° 00′ W, 11 October 1988, Schäfer-Verwimp & Verwimp (G). Minas Gerais, Serra de Ibitipoca, 19 May 1975, Krieger 13474 (JPB). Paraná: Curitiba, en allant vers Vila Velha, 25°21'S, 49°34'W, 4 March 1989, Grundlehner (G). Rio Grande do Sul: Cambará do Sul, Parque Nacional dos Aparados da Serra, Cânion do Itaimbezinho, 30 April 1989, Fleig 3577 (ICN); ibid., mata nebular próximo ao centro de visitantes, 14 March 2014, Gerlach & Akkerman 1403 (ICN). São Paulo, Zwischen Guapira und Apiaí, in einem Kleinen Pinus-Forest, an Pinus spec., 800 m, 23 August 1980, Kalb (G); Serra da Mantiqueira, Campos do Jordão, etwa 150 km nordöstlich von São Paulo, an freistehenden Pinus spec. 1700 m, 26 May 1978, Kalb & Plöbst (G).
GenBank number: ITS = MF669873.
Notes: Usnea is a ultradiverse genus with more than 450 species worldwide (Lücking et al. 2020) mainly corticolous species in Africa (e.g. Swinscow and Krog 1979; Temu et al. 2019), Europe (e.g. Clerc 1987, 2011; Halonen et al. 1998; Clerc and Otte 2018), Asia (e.g. Ohmura 2001, 2012; Stevens 2004; Ohmura and Clerc 2019), South America (e.g. Rodriguez et al. 2011; Truong et al. 2011, 2013; Truong and Clerc 2012, 2013, 2016; Gerlach et al. 2017, 2019a, b, 2020) and North America (e.g. Clerc 2007; Herrera-Campos 2016). The genus is characterized by the fruticose thallus, branches holding a central axis and the presence of usnic acid in the cortex (Clerc 1998). Usnea kriegeriana is characterized by the annulate and cracked basal part, the lateral branches that are not constricted at attachment point, the numerous tubercles eroded at the top, the large soralia, the mat cortex, the thin to thick medulla (22.5–25%–35) and the presence of fumarprotocetraric acid as main secondary substance in the medulla. The density of the annular cracks with medullar extrusion and isidiomorphs is variable in this species. The lateral branches are most of the time not constricted; only rarely slightly constricted at attachment point. The shape of the segments varies from slightly swollen to cylindrical. Soralia are convex and often well delimited on main branches. Well-developed specimens have confluent soralia that become larger up to the half of the branches diameter towards the apices. Usnea kriegeriana produces fumarprotocetraric often accompanied by protocetraric acid and an unknown substance (refereed as US2; only one specimen without protocetraric and another one without US2 were found) in the medulla.
Usnea kriegeriana is morphologically similar with U. flammea, which, however, differs mainly by the shape of the soralia, that are even with the cortex surface, and by the absence of tubercles (Clerc 2006). Usnea subflammea is similar by the presence of numerous tubercles, but it differs by the thicker cortex [12–16%; instead 7.5–9%–10.5(–11.5) in U. kriegeriana] and the soralia that do not enlarge (Clerc 2006). Moreover, U. flammea and U. subflammea have stictic acid in the medulla whereas U. kriegeriana has fumarprotocetraric acid. Moreover, Usnea kriegeriana builds a strongly supported clade, unrelated to Usnea flammea and U. subflammea (Fig. 24; Fig. 1 as Usnea sp. 5 in Gerlach et al. 2019a).
Maximum likelihood (ML) tree reconstruction, on RAxML Black Box on the CIPRES server. Outlined on yellow gradient is the outgroup. The new species are in blue. Specimens of Usnea used in the study including voucher information, chemotype and GenBank accession numbers for the nuclear ribosomal internal transcribed spacer region (ITS) are available on supplementary information
Until our knowledge there are relatively few species with fumarprotocetraric acid as secondary metabolity recorded for South America: Usnea aurantiaco-atra belong to the Neuropogon group, differing by the saxicolous and by the fertile thallus. U. bornmuelleri is also saxicolous. U. brasiliensis differs from U. kriegeriana mainly by the CMA and by the absence of tubercles. U. grandispora is a fertile species without soralia. Usnea pallida is a erect-shrubby species belonging to the Usnea articulata group; Usnea rubropallens belonging to the Usnea cornuta aggregate and differ by the presence of a faint red pigment subcortical (see Gerlach et al. 2020 for more details); U. subrubicunda differs from U. kriegeriana by the vitreous cortex and the presence of a red pigment cortical; U. vitrea is a fertile species with apothecia and does not present vegetative propagules.
Usnea kriegeriana is relatively frequent in the mountainous areas above 900 m in Araucaria Forest. So far known only in Brazil (Southern and Southeast Region): Espírito Santo, Minas Gerais, Paraná, Rio Grande do Sul, Santa Catarina and São Paulo states.
Leotiomycetes O.E. Erikss. & Winka.
Notes: Eriksson and Winka (1997) introduced the class Leotiomycetes (Ascomycota) comprising of 4 orders, 19 families and 641 genera (Kirk et al. 2008). Leotiomycetes contains numerous species with anamorphs placed within the fungi imperfecti (deuteromycota), that have only recently found their place in the phylogenetic system. Earlier, Leotiomycetes was placed into Discomycetes clade (inoperculate Discomycetes). Molecular studies have recently shed lights to the still ambiguous systematics. Most researchers consider Leotiomycetes a sister group to Sordariomycetes in the phylogenetic tree of Pezizomycotina. Its division into sub-classes have received strong support by the molecular data, but the overall monophyly of Leotiomycetes is uncertain. The order Lichinodiales and family Lichinodiaceae, newly circumscribed by Prieto et al. (2019) to contain the cyanolichen genus Lichinodium, which is the first known group of lichenized fungi in the Leotiomycetes. Most Leotiomycetes form apothecia containing asci (seldom cleistothecia). The asci are found cylindrical and have no operculum. Hyaline spores are of various shapes, disperse through a circular apical pore (Prieto et al. 2019). Most of the genera in this class are saprophytic growing on a wide variety of substrates like dead plant materials, on dung as well as endophytes. Many of the genera belonging to Leotiomycetes, like Botrytis, Erysiphe, Uncinula, Blumeria, Podosphaera, Phyllactinia, Diplocarpon, Scytalidium etc. are well known to cause serious plant diseases on a variety of plants.
Helotiales Nannf.
Notes: Helotiales (Nannf 1932) is an order of the class Leotiomycetes within the division Ascomycota (Lumbsch and Hundorf 2007). According to the dictionary of fungi estimate, the order contains 10 families, 501 genera, and 3881 species (Kirk et al. 2008). This is the largest order of inoperculate discomycetes. The order is distinguished by its disc or cup-shaped apothecia. Most fungal genera of this order live as saprobes on dead logs, soil humus, manure and other organic matter. This order includes most fungi associated with ericoid mycorrhiza. Including Rhizoscyphus ericae (Zhang and Zhuang 2004), Meliniomyces bicolor (Hambleton and Sigler 2005) and Cairneyella variabilis (Midgley et al. 2016). Many of the fungi of this order are deadly severe plant pathogens such as Sclerotinia sclerotiorumcausing lettuce drop and other diseases (de Bary 1884), Monilinia fructicola causing brown rot on stone fruits (Honey 1928), Diplocarpon rosae causing black spot of roses (Wolf 1912) and Sclerotium cepivorum causing soft rot of onions (Berkeley 1841).
Rutstroemiaceae Holst-Jensen, Koehn & Schmach.
Notes: The species in this family (Holst-Jensen et al. 1997) have a cosmopolitan distribution, especially in temperate areas (Cannon and Kirk 2007). This family includes the genera like Dicephalospora, Lambertella, Lanzia, Poculum, Rutstroemia, Scleromitrula etc.
Lambertella Höhn.
Notes: The genus Lambertella was established by von Höhnel (1918). The genus is characterized by the presence of a substratal stroma with epidermoid cells and an ectal excipulum composed of thin-walled prismatic cells. Pigmented ascospores are also one of the distinguishing characters of the genus Lambertella (Zhao et al. 2016a). It is one of the largest genera in the family Rutstroemiaceae consists of 78 species according to Index Fungorum records (http://www.indexfungorum.org/names/Names.asp). Studies have already shown the polyphyly in the genus Lambertella based on the phylogenetic analysis of ITS, LSU, and RPB2 regions suggesting its phylogenetic heterogeneity. However, a highly supported clade called Lambertella sensu stricto has been confirmed consists of species characterized by brown ascospores before discharged from asci (Zhao et al. 2016a).
Lambertella dipterocarpacearum P.N. Singh, S.K. Singh & A.C. Lagashetti, sp. nov.
Index Fungorum number: IF900321; Facesoffungi number: FoF0773601; Figs. 25, 26
Lambertella dipterocarpacearum (AMH10226, holotype). a Substratum (leaf of Shorea). b Colony morphology on PDA (front view on 5th days). c Stereomicroscopic surface view of colony showing dark orange shiny gleosporic mass of conidial heads. d–g Coiled and anastomosed hyphae. h, i Hyphae bearing developing phialides with conidia. j, k branched conidiophores with sterile arm. l Conidiophore with solitary phialide. Scale bars: d–f = 10 μm, g = 20 μm, h = 20 μm, i = 5 μm, j, k = 10 μm, l = 20 μm
Etymology: specific epithet ‘dipterocarpacearum’ refers to the host family.
Holotype: AMH 10226.
Colour code follows: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
On leaf phylloplane of Shorea robusta in terrestrial habitats. Asexual morph: vegetative hyphae smooth walled, branched, septate, anastomoses, sometimes coiled, subhyaline to light olivaceous up to 10.15 µm wide. Stroma none. Chlamydospores absent. Setae and hyphopodia absent. Conidiophores macronematous, mononematous, unbranched to dichotomously densely branched, straight, short, septate, smooth walled, subhyaline to light olivaceous, 4.5–138.90 × 4–6.35 μm, sometimes reduced into conidiogenous cells. Occasionally, a sterile arm is produced near axel of conidiophore. Conidiogenous cells holoblastic, terminal to lateral, determinate, polyblastic, ampulliform, persistent, aseptate, hyaline, 2.88–12.75 × 1.61–7.20 μm (x̅ = 6.30 × 2.70 μm, n = 30). Phialides solitary or aggregated, densely produced, ampulliform to lageniform, aseptate, sometimes single to multi- septate, smooth walled, hyaline, 2.59–22.92 × 1.67–4 μm (x̅ = 6.31 × 2.73 μm, n = 30). Conidia mostly aggregated in slimy mass heads at the apex of phialides, sometimes produced in chains, globose to sub-globose, smooth walled, sub-hyaline to light olivaceous, 1.84–4.22 × 1.81–2.80 μm (x̅ = 2.38 × 2 μm, n = 30). Sexual morph: Undetermined.
Culture characteristics: on semi-synthetic agar medium PDA (Potato Dextrose Agar) white (1A1), reaching 6.5 cm diam. in 5 days at 25 °C, with irregular margin, surface filamentous, reverse light yellow (4A4). After aging light yellow (4A3), reverse light yellow (4A3). Hyphae septate, unbranched to branched, sometimes constricted near septa, smooth and thin walled, subhyaline to light olivaceous, 2–10.15 μm wide.
Materials examined: India, Uttar Pradesh, Gorakhpur District, on Shorea robusta (leaf infested with black mold), 5 May 2016, P.N. Singh, AMH10226 (holotype); ex type living culture, NFCCI 4826 (National Fungal Culture Collection of India-WDCM 932).
GenBank Numbers: AMH10226: ITS = MT227126, LSU = MT227018, RPB2 = OM313014.
Notes: The present taxon is morphologically distinct from allied species of Lambertella. All the species of Lambertella have been reported to be found in apothecial forms rather producing conidial stage. The sexual stage in the present taxon was not found. Therefore, it was not compared with the allied taxons of the genus Lambertella. The genus Lambertella is morphologically most closely related to anamorphs of Cadophora, Phialocephala, Mollerodiscus and Longia. The present taxon is compared with Cadophora fastigiata (Lagerberg et al. 1927), which is the anamorph of the genus Lambertella. The L. dipterocarpacearum was found in anamorphic state in having subhyaline to light olivaceous globose to subglobose conidia (1.84–4.22 × 1.81–2.80 μm) produced on tips of phialides in compact dark orangish gleosporic mass. In addition, densely produced phialides, conidiogenous cells, presence of coiled/anastomoses hyphae and sterile arms in axel of conidiophores are the distinct features of this taxon. These features are lacking in Cadophora fastigiata. Flask-shaped, light brown phialides that are produced laterally from hyphae and having funnel-shaped collarette conidiogenous cells in C. fastigiata. Conidia are generally oval to button shaped (3.0–5.5 × 1.5–2.5 µm). Thus, based on these morphological distinction, L. dipterocarpacearum, is described here as species new to science. Phylogenetic analysis of ITS, LSU and RPB2 sequence data indicates that L. dipterocarpacearum (NFCCI 4826), is a new species in Rutstroemiaceae, which is different from other known species of Lambertella forming a sister clade to Lambertella sensu stricto (Zhao et al. 2016a) with strong ultrafast bootstrap value of 98 (Fig. 27). On megablast analysis, ITS sequence of L. dipterocarpacearum showed 92% (485/525) identity and 13 gaps (2%) with L. corni-maris CBS 184.93 and L. corni-maris CBS 774.95, 92% (483/523) identity and 14 gaps (2%) with L. himalayensis CBS 230.77, 92% (471/512) identity and 14 gaps (2%) with L. pruni CBS 199.47, 92% (472/511) identity and 12 gaps (2%) with L. hicoriae, and 91% (472/519) identity and 12 gaps (2%) with L. pyrolae. To our knowledge, this was isolated for the first time from leaf of Shorea collected from Kusumi Forest, District Gorakhapur, Uttar Pradesh region in India. Moreover, this species is being reported for the first time from India.
Phylogenetic tree of Lambertella dipterocarpacearum (NFCCI 4826) by Maximum-Likelihood method based on combined sequence data of ITS, LSU and RPB2. Rutstroemia firma CBS 115.86 and Rutstroemia firma CBS 341.62 were used as outgroup. The analysis involved 20 nucleotide sequences. Evolutionary analyses were conducted in IQ–TREE multicore version 1.6.11 (Nguyen et al. 2015) by the Maximum–Likelihood method using the best suitable model (TIM2e + G4 model). Ex-type strains are in bold and newly generated sequence is in blue. One–thousand bootstrap replicates were analyzed to get ultrafast bootstrap values, and the values above 50% were represented on nodes in the tree
Sordariomycetes O.E. Erikss. & Winka.
Notes: The class Sordariomycetes was established by (Eriksson and Winka 1997). This is the second largest class of Ascomycota (Kirk et al. 2008; Maharachchikumbura et al. 2016). According to Kirk et al. (2008), 15 orders, 64 families, 1,119 genera and 10,564 species belong to class Sordariomycetes; however, as per the recent classification, the Sordariomycetes now comprises six subclasses, 28 orders, 90 families and 1,344 genera (Maharachchikumbura et al. 2015). Fungi of this class have worldwide distribution containing mostly terrestrial taxa including some pathogens causing disease to plants, arthropods and mammals along with some aquatic fungi (Maharachchikumbura et al. 2016). Main characteristic of large number of Sordariomycetes species is presence of flask-shaped hollow fruiting body containing inoperculate unitunicate asci (Zhang et al. 2006).
Amphisphaeriales D. Hawksw. & O.E. Erikss.
Notes: Based on divergence time estimations provided by Samarakoon et al. (2016) and Hongsanan et al. (2017), Amphisphaeriales was supported as a distinct order in Xylariomycetidae and 17 families were accepted.
Amphisphaeriaceae G. Winter.
Notes: The family Amphisphaeriaceae was established by Winter (1885) as ‘Amphisphaerieae’ and later established as Amphisphaeriaceae to accommodate the type genus Amphisphaeria and its allies (Winter 1887). Members of Amphisphaeriaceae are saprobes on decaying wood in terrestrial, freshwater and marine habitats (Wang et al. 2004; Liu et al. 2015a; Senanayake et al. 2015; Samarakoon et al. 2019, 2020; Dissanayake et al. 2020; Hyde et al. 2020b). According to the latest update of Amphisphaeriaceae by Samarakoon et al. (2020), Lepteutypa was synonymized under Amphisphaeria based on holomorphic morphology and multigene phylogeny, and Amphisphaeria was accepted as the only genus in Amphisphaeriaceae.
Amphisphaeria Ces. & De Not.
Notes: Amphisphaeria, the type genus of Amphisphaeriaceae, was introduced by Cesati and De Notaris (1863), and is typified by A. umbrina with asexual morph. Samarakoon et al. (2020) accepted 27 species among 287 epithets listed under Amphisphaeria. It should be noted that several taxa were introduced as new to science within the genus Amphisphaeria with a weak bootstrap values support, which implied more fresh collections and isolations are expected in future studies (Samarakoon et al. 2019, 2020; Dissanayake et al. 2020).
Amphisphaeria guttulata J.Y. Zhang & Y.Z. Lu, sp. nov.
Index Fungorum number: IF 900043; Facesoffungi number: FoF 13259; Fig. 28
Amphisphaeria guttulata (MFLU 22-0078, holotype). a, b Ascomata on the substrate. c, d Vertical section of ascoma. e ostiole. f paraphyses. g peridium. h–k asci. l–r ascospores. s Germinating ascospore. t, u Colony on PDA from above and below. Scale bars: a, b = 1000 μm, c–e = 50 μm, g–k = 20 μm, f, l–s = 10 μm
Etymology: refers to the ascospores with guttules.
Holotype: MFLU 22-0078.
Saprobic on dead branch. Sexual morph: Ascomata 193–268 μm high × 104–208 μm in diam. (\(\overline{x }\) = 225 × 161 μm, n = 8), immersed, visible as black spots, surrounded by a grey halo area, solitary, scattered or aggregated, globose to subglobose, dark reddish brown, centric ostioles with periphyses. Hamathecium composed of paraphyses, septate, unbranched, 2.5–5 μm wide, not anastomosing, hyaline. Perridium comprising 4–7 layers of dark brown cells of textura angularis, 12.5–24 μm wide, thick-walled. Asci 77–88 × 8.5–12.5 μm (\(\overline{x }\) = 81 × 10.5 μm, n = 15), 8-spored, unitunicate, cylindrical, sessile, thin-walled, apically round. Ascospores 12–16 × 4.5–5.5 μm (\(\overline{x }\) = 14 × 5 μm, n = 20), uniseriate, partly overlapping, oblong or narrowly fusiform, 1-septate, 2-celled, slightly constricted at the septum, guttulate, pale brown, smooth-walled. Asexual morph: undetermined.
Culture characteristics: Ascospores germinated on WA within 12 h at 25–28 °C. The hyaline germ tube germinates from a point of one cell of the ascospores. Colonies growing on PDA, reaching 19 mm in diam. after two weeks at 25 °C, flat, section to fan shape at the surface, fimbriate margin with a smooth surface, yellowish white from above; cream yellow mycelium in the middle and brown yellow mycelium in the outer ring in reverse, and not producing pigment in the culture.
Material examined: Thailand, Loei Province, Chang Wat, Phu Kradueng, Tambon Huai Som (16° 55′ 9″ N, 101° 55′ 21″ E), on dead branch in a forest, 27 February 2020, J.Y. Zhang, Y196 (MFLU 22-0078, holotype); ex-type living culture, MFLUCC 22-0052.
GenBank numbers: MFLU 22-0078: LSU = OQ101583, ITS = OQ101582.
Notes: The morphological characteristics of the new isolate match well with the generic concept of Amphisphaeria and is most similar to Amphisphaeria micheliae in the shape of asci and ascospores (Samarakoon et al. 2020). However, it is recognized from A. camelliae by its shorter asci (77–88 × 8.5–12.5 μm vs. 92–135 × 7–10.5 μm) and smaller ascospores (12–16 × 4.5–5.5 μm vs. 15.5–21 × 6–7.5 µm, Samarakoon et al. 2020). The phylogenetic tree showed that the new collection formed a separate clade within the genus Amphisphaeria, and is sister to A. yunnanensis (Fig. 29). Therefore, we introduce the new collection as Amphisphaeria guttulata sp. nov.
Phylogram generated from maximum likelihood analysis based on combined LSU and ITS sequence data. 26 taxa were included in the combined analyses, which comprised 1782 characters (LSU: 1160 bp, ITS: 622 bp) after alignment. The best scoring RAxML tree with a final likelihood value of -7242.965669 is presented. The matrix had 614 distinct alignment patterns, with 17.29% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.257803, C = 0.216953, G = 0.269172, T = 0.256073; substitution rates: AC = 0.829912, AG = 2.560479, AT = 1.266564, CG = 0.816478, CT = 4.838135, GT = 1.000000; gamma distribution shape parameter α = 0.264935. Bootstrap support values for ML equal to or greater than 50% and BYPP equal to or greater than 0.90 are given above the nodes. The tree is rooted with Bartalinia pondoensis CBS 125525 and B. pini CBS 143891. The newly-generated strain is shown in blue and bold. Ex-type strains are indicated by black and bold
Beltraniaceae Nann.
Notes: Beltraniaceae was introduced by Nannizzi (1934) to accommodate the genus Beltrania and allied genera including Beltrania, Beltraniella, Beltraniopsis, Hemibeltrania, Parapleurotheciopsis, Porobeltraniella, Pseudobeltrania, Pseudosubramaniomyces and Subsessila (Wijayawardene et al. 2022).
Beltrania Penz.
Notes: Species of Beltrania are characterized by setae with radially lobed basal cells, conidiophores with separating cells, and biconic conidia with a hyaline transverse band and apical tubular appendage. There are 26 epithets for Beltrania listed in Index Fungorum (2022).
Beltrania liliiferae P. Razaghi, M. Raza & L. Cai, sp. nov.
Index Fungorum number: IF 900337; Facesoffungi number: FoF 12673; Fig. 30
Beltrania liliiferae (HMAS 352088, holotype). a–d Upper and reverse views of cultures on PDA and SNA after 1 week of inoculation, respectively. e Exudate droplets on PDA. f Conidiophores and setae on pine needle on SNA. g, h Setae, conidiophores, conidiogenous cells and conidia. i–l Conidiophores and radial lobed basal cells of conidiophores. m Separating cells. n Conidia. Scale bars: g, h = 20 µm, i–n = 5 µm
Etymology: The name refers to the host “Magnolia liliifera”.
Holotype: HMAS 352088.
Setae numerous, erect, arising from radially lobed basal cells, straight to flexuous, unbranched, 2–6-septate, thick-walled, verrucose, dark brown to black, 155–207 µm long, 4.5–7 µm wide, tapering to a pointed apex. Conidiophores macronematous, mononematous, single or in small groups, erect, unbranched, hyaline to pale brown, smooth, straight to flexuous, mostly geniculate at the apical region, 2–12(–23) septate, 37–91(–380) × 4–7.5 µm, arising from basal cells of setae or from separate dark brown, swollen, radially lobed cells, 11–19 μm (av. = 13.47 ± 2.32 μm) diam. Conidiogenous cells polyblastic, integrated, terminal, sympodial, with several flat-tipped denticles, cylindrical, clavate, subhyaline to pale brown, smooth (14.5–)18–24.5 × 4.5–8.5 μm (av. = 20.71 ± 4.37 × 6.11 ± 1.35 μm). Separating cells hyaline, finely roughened, aseptate, ellipsoidal, obovoid, 9.5–13 × 5–7 μm (av. = 10.81 ± 0.97 × 5.68 ± 0.47 μm), with several apical, flat-tipped denticles. Conidia arise directly from conidiogenous cells or from separating cells, acrogenous, biconic, rhomboidal, aseptate, solitary, smooth, pale brown to pale smoky-brown with a hyaline to subhyaline equatorial transverse band, with distinct granules, rounded or 1-denticulate at base, 20–25 × 8.5–10 μm (av. = 22.66 ± 1.39 × 9.36 ± 0.51 μm), apical appendage 7–12 μm (av. = 8.94 ± 1.29 μm) long, tapering to an acutely rounded tip. Sexual morph: Undetermined.
Culture characteristics: Colonies on PDA raised, with undulate edge, smooth, grayish green from above, reverse centric circles in grayish green and cream, velutinous, producing olivaceous exudate droplets on aerial mycelia, reaching 71–77 mm in diam after 7 d at 25 °C; on SNA flat, with undulate edge, colorless, sparse aerial mycelia, reaching 36–42 mm diam after 7 d at 25 °C.
Material examined: Thailand, on dead leaves of Magnolia liliifera, 2010, Anonymous (HMAS 352088, holotype), ex-holotype living culture, CGMCC 3.23464 = LC0063. Thailand, on dead leaves of Magnolia liliifera, 2010, Anonymous, living culture, LC15869.
GenBank numbers: CGMCC 3.23464: ITS = OP022176, LSU = OP022172; LC15869: ITS = OP022177, LSU = OP022173.
Notes: Based on phylogenetic analysis of combined LSU and ITS sequence data, two isolates of Beltrania liliiferae grouped together and formed a distinct clade closely related to B. rhombica (Fig. 31). However, it differs from the latter by having longer setae (155–207 µm vs. 103–167 µm), larger conidiophores [37–91(–380) × 4–7.5 µm vs. 22.5–46 × 2–4.5] μm and wider conidia (8.5–10 vs. 6–8.5 μm, Lin et al. 2017). Beltrania rhombica lacks type material; therefore, to ensure better designation of the species, recollecting on Citrus limon in Italy is required.
Phylogenetic tree generated from Maximum likelihood analysis (RAxML) based on combined ITS and LSU sequence data of Beltraniaceae. Fifty-three strains are included in the combined analyses which comprised 1373 characters (547 characters for ITS and 820 characters for LSU) after alignment. Anthostomella leucospermi (CBS 110126) was used as the outgroup taxon. The best scoring RAxML tree with a final likelihood value of -4929.358117 is presented. The matrix had 335 distinct alignment patterns, with 11.74% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.2705, C = 0.2648, G = 0.2173, T = 0.2474; substitution rates: AC = 0.6116, AG = 3.9808, AT = 1.0000, CG = 0.6116, CT = 1.9749, GT = 1.0000; gamma distribution shape parameter α = 0.4680. Bootstrap support values for ML equal to or greater than 50% are given above the nodes (left side). Bayesian posterior probabilities (BYPP) equal to or greater than 0.80 are given above the nodes (right side). Ex-type strains are in bold and newly generated sequences are in blue
Beltraniella Subram.
Notes: Beltraniella was established with B. odinae as the type species, found on dead leaves of Odina wodier in India. The genus is characterized by setiform conidiophores and polyblastic, sympodial, denticulate conidiogenous cells, acropleurogenous, turbinate or biconic conidia with a distinct hyaline transverse band at the equatorial zone (Subramanian 1952). There are 31 epithets listed in Index Fungorum (2022).
Beltraniella jiangxiensis P. Razaghi, M. Raza & L. Cai, sp. nov.
Index Fungorum number: IF 900338; Facesoffungi number: FoF 12674; Fig. 32
Beltraniella jiangxiensis (HMAS 352097, holotype). a–d Upper and reverse views of cultures on PDA and SNA after 1 week of inoculation, respectively. e Conidiophores and setae on pine needle on SNA. f–j Setae, conidiophores and conidiogenous cells. k–m Conidiophores and conidiogenous cells. n Separating cells. o Conidia. Scale bars: 5 µm
Etymology: The name refers to the location it was collected.
Holotype: HMAS 352097.
Setae numerous, erect, arising from radially lobed basal cells, straight or flexuous, unbranched, single or in small groups, thick-walled, coarsely verrucose, olivaceous brown, paler towards apex, 81–304 μm long, 5–8.5 μm wide, tapering to a pointed apex, up to 16-septate, arising from a dark brown, swollen, radially lobed basal cell, 11– 22 μm in diameter. Conidiophores macronematous, short, simple or branched at apical regions, 3–6 septate, reduced to conidiogenous cells, verrucose, thin-walled, swollen at the base, subhyaline to pale olivaceous, arising from basal cells of setae or from separate, 36–65 × 4–7 µm. Conidiogenous cells polyblastic, integrated, determinate, terminal, geniculate, denticulate, cylindrical, oblong, hyaline to subhyaline, smooth, 9–19 × 4–6 μm (av. = 12.62 ± 3.27 × 4.68 ± 0.59 μm). Separating cells obclavate, thin-walled, smooth, hyaline to subhyaline, 1-denticulate at each end, 8–11 × 3.5–5 μm (av. = 9.91 ± 0.89 × 3.95 ± 0.47 μm). Conidia arise directly from conidiogenous cells or from separating cells, aggregated, acrogenous, simple, dry, straight, sometimes verrucose, thin-walled turbinate to pyriform, rostrate to pointed at proximal end, truncate at distal end, hyaline to subhyaline without a hyaline transverse band, 20–24 × 5.5–7.5 μm (av. = 22.12 ± 1.25 × 6.55 ± 0.64 μm). Sexual morph: Undetermined.
Culture characteristics: Colonies on PDA raised with concave edge, with undulate edge, dense, colony cream from above and reverse, velutinous, sterile, reaching 55–57 mm diam after 7 d at 25 °C; on SNA dome shaped, with rhizoid edge, white, reaching 53–60 mm diam after 7 d at 25 °C.
Material examined: China, Jiangxi Province, Ganzhou, Fengshan, on Camellia sinensis, August 2013, Y. Zhang (HMAS 352097, holotype), ex-holotype living culture, CGMCC 3.23486 = LC3449. China, Jiangxi Province, Ganzhou, on leaves of Camellia sinensis, August 2013, Y. Zhang, living culture, LC15868.
GeneBank numbers: CGMCC 3.23486: ITS = OP022178, LSU = OP022174; LC15868: ITS = OP022179, LSU = OP022175.
Notes: Two isolates of Beltraniella jiangxiensis formed a well-supported and distinct clade on the ML tree generated from ITS and LSU sequences (Fig. 31), closely related to B. pandanicola, B. portoricensis and B. fertilis. Morphologically, our novel species is distinguished from the most closely related species, B. pandanicola isolated from Pandanus sp. in Thailand (Tibpromma et al. 2018) by producing more septate (16 μm × 2–4 μm) and relatively longer setae (81–304 μm vs. 114–200 μm), longer conidiophores (36–65 μm vs. 20–42 µm) and longer conidiogenous cells (9–19 μm vs. 6–10 μm).
Hypocreales Lindau.
Notes: The order Hypocreales was established by Lindau (1897) and is the monophyletic order under the subclass Hypocreomycetidae. Hypocreales contains a wide range of economical important fungi from plant pathogens to bio-control agents (Rossman 1996). Most of the members of this order are found in temperate or tropical and subtropical regions (Põldmaa 2011). This order accommodates ten families, Bionectriaceae, Clavicipitaceae, Cordycipitaceae, Flammocladiaceae, Hypocreaceae, Nectriaceae, Niessliaceae, Ophiocordycipitaceae, Stachybotriaceae and Tilachlidiaceae.
Bionectriaceae Samuels & Rossman.
Notes: Bionectriaceae is the monophyletic family within order Hypocreales introduced by Rossman et al. (1999) to accommodate 26 genera. The genera of Bionectriaceae are perpetuating in soil, dead wood and as endophytes in tropical and subtropical areas (Huanraluek et al. 2020). As per recent classification, this family contains 39 genera with Bionectria as the type genus (Rossman et al. 2001; Maharachchikumbura et al. 2015). This family is characterized by white, yellow, orange to tan colored uniloculate, perithecial or sometimes cleistothecial ascomata (Rossman et al. 1999).
Synnemellisia N.K. Rao, Manohar. & Goos.
Notes: Synnemellisia was proposed by Rao et al. (1989). The genus is characterized by synnamatous conidiomata with conidiogenous cells at their tips. The conidia are holoblastic, usually single, hyaline, lanceolate and aseptate. This is one of the smallest genera of Bionectriaceae family and contains only four species according to Index Fungorum records (http://indexfungorum.org/Names/Names.asp).
Synnemellisia punensis K. S. Pawar, P. N. Singh, S. K. Singh, sp. nov.
Index Fungorum number: IF900322; Facesoffungi number: FoF10810; Figs. 33, 34
Synnemellisia punensis (NFCCI 5166, holotype). a, b Colony morphology on PDA (front view). c colony morphology on grass leaf media. d Stereomicroscopic surface view of colony grown on PDA with hyphal bundles forming synnemata. e Stereomicroscopic surface view of colony grown on PCA with pale orange exudate. f Stereomicroscopic surface view of colony grown on grass leaf media with watery exudate. g, h Whitish puffy mass of sporodochia. i Anastomoses with phialides bearing long chains of conidia. j parallel hyphal bundles of hyphae forming synnemata. k anastomosed hyphae. l bipolar germination in spores (showing arrow). m Germinating conidia directly producing phialide with conidia (showing arrow). Scale bars: i–m = 10 μm
Synnemellisia punensis (NFCCI 5166, holotype). a, b Conidiophores with phialides bearing conidia. c Conidiophores with phialides bearing conidia (in higher magnification). d Conidiophores with phialides bearing chevron/zipper like arrangement of conidia (showing arrow). e Conidiophores, phialides with chains of conidia. f Numerous conidia. g SEM images of branched conidiophores, phialides and conidia. h Conidiophore with primary and secondary metulae bearing phialides and conidia. i Chain of conidia. Scale bars: a–f = 10 μm, g, h = 2 μm, i = 1 μm
Etymology: specific epithet ‘punensis’ refers to name Pune (a district of Maharashtra state).
Holotype: NFCCI 5166.
Colour code follows: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Isolated in vitro from the leaf litter. Asexual morph: Synnemata produced in the form of aggregated mass of parallel bundles hyphae, hyaline. Vegetative hyphae aggregated in loose parallel bundles, rope like, branched to unbranched, anastomoses, smooth walled, thin and thick, hyaline, up to 3 μm wide. Setae and hyphopodia absent. Chlamydospores absent. Conidiophores arising from superficial hyphae, forming floccose sporodochia, unbranched to branched, branching unilateral to bilateral verticillate, hyaline, smooth walled, 9.68–127.7 × 1.97–2.77 μm. Conidiogenous cells polyblastic and polyphialidic, smooth walled, hyaline, 4.71–35.91 × 1.35–3.46 μm. Phialides solitary or in group of 2–4, sometimes directly produced from superficial hyphae, cylindrical, attenuated, smooth walled. Metulae 1–2, primary metulae solitary, spatulate, variable in size (8.59–10.27 × 1.05–1.83 μm), secondary metulae paired (6.98 × 2.12 μm), 8.29–25.36 × 1.35–3.58 μm. Conidia produced in long chain from polyblastic and polyphialidic conidiogenous cell, sometimes conidia accumulated in the form of chevron or zipper like, produced in loose mass on the tip of phialides, oval to broadly ovoid, allantoid to fusoid, base broader, sometimes base rarely tapered, base truncate, tip obtuse, aseptate, smooth walled, 1–2 gutulate, smooth walled, hilum unthickened, hyaline, 2.22–6.43 × 1.2–4 μm (\(\overline{x }\) = 3.84 × 2.17 μm, n = 30). Sexual morph: Undetermined.
Culture characteristics: on semi-synthetic agar medium PDA white (6A1) to orange white (6A2), reaching 3.0 cm diam. in 5 days at 25 °C, forming synnemata, margin irregular, reverse wrinkled, pale orange (5A3); on PCA (Potato Carrot Agar) white (6A1), reverse yellowish white (4A2), reaching 3.7 cm diam. in 5 days at 25 °C, rarely forming dome like synnemata with pale orange (5A3) exudate. No sporulation on PDA and PCA but abundantly sporulating on grass leaf agar.
Material examined: India, Maharashtra, Pune District, from leaf litter, 1 October 2020, K.S. Pawar, NFCCI 5166 (holotype), ex-type living culture, National Fungal Culture Collection of India, WDCM 932.
GenBank numbers: ITS = ON059361, LSU = ON059433.
Notes: Synnemellisia punenesis is morphologically distinct from allied species of Synnemellisia. The conidia of S. hyalospora are solitary, slightly curved, 40–52 μm long, 4.2–5 μm wide at the middle, and 1–1.4 μm wide at the base (Rao et al. 1989). The conidia of type species S. aurantia (COAD 2070) are aggregated on a cushion-like head, navicular to fusiform, 23–30 long and 7–9 μm wide, apex subacute, base obtuse to subtruncate, aseptate, guttulate, subhyaline and smooth. (Crous et al. 2016). In present taxon S. punensis (NFCCI 5166), the conidia are produced from polyblastic, polyphialidic conidiogenous cells, ovoid, aseptate 2.22–6.43 μm long and 1.2–4 μm wide. Thus, S. punenesisis morphologically different in having much smaller shape and dimension of conidia as compared to the conidial dimension of S. hyalospora and S. aurantia. In addition, the conidia in new taxon produced in long chains and in loose aggregated mass, also forming zipper or chevron like pattern. Tan et al. (2021) described both the taxa based only on sequencing and phylogenetic analysis. Hence, morphological features of S. urenae (BRIP 71675) and S. acacia (BRIP 71652) are missing and hence cannot be compared.
Phylogenetic analysis of ITS and LSU sequence data specifies that S. punensis (NFCCI 5166), is a new species in Bionectriaceae, which is dissimilar from other known species of Synnemellisia (Fig. 35). Based on megablast analysis, ITS sequence of Synnemellisia punensis (NFCCI 5166) showed 94% (541/574) identity and 13 gaps (2%) with ex-type strain of Synnemellisia aurantia (COAD 2070), 96% (544/569) identity and 6 gaps (1%) with ex-type strain Synnemellisia urenae (BRIP 71675), and 95% (543/572) identity and 10 gaps (1%) with Synnemellisia acacia (BRIP 71652). Sequence data of Synnemellisia hyalospora is not available in the literature. Based on morphological and phylogenetic analysis, Synnemellisia punensis (NFCCI 5166) is proposed as novel species with strong ultrafast bootstrap (96%).
Phylogenetic tree of Synnemellisia punensis (NFCCI 5166) inferred from Maximum-Likelihood analysis based on combined sequence data of ITS, LSU. Verrucostoma freycinetiae MAFF 240100 was used as outgroup. The analysis involved 32 nucleotide sequences. Evolutionary analyses were conducted in IQ–TREE multicore version 1.6.11 (Nguyen et al. 2015) by the Maximum–Likelihood method using the best suitable model (TNe + I + G4 model). Ex-type strains are in bold and newly generated sequence is in blue. One–thousand bootstrap replicates were analyzed to get ultrafast bootstrap values, and the values above 50% were represented on nodes in the tree
Cordycipitaceae Kreisel ex G.H. Sung, J.M. Sung, Hywel-Jones & Spatafora.
Notes: Species in the Cordycipitaceae have two phenotypes; teleomorph and anamorph. Among them, the main characteristics of the teleomorph are brightly colored, fleshy stromata and filiform ascospores (Sung et al. 2007). Asexual forms are more diverse, e.g. the insect host was covered in powdery conidia, with synnemate emerging from specific parts of the host (Mongkolsamrit et al. 2018). Currently, Cordycipitaceae is composed of 16 genera, with Cordyceps as the type genus (Sung et al. 2007; Kepler et al. 2017; Mongkolsamrit et al. 2018; Thanakitpipattana et al. 2020; Wang et al. 2020a; Chen et al. 2021a). Furthermore, species in the Cordycipitaceae have important roles in food (Cordyceps militaris, Wen et al. 2014, 2016), medicine (Cordyceps cicadae, Zha et al. 2018, 2019) and biocontrol (Beauveria bassiana, Biryola et al. 2022; Beauveria pseudobassiana, Villamizar et al. 2020).
Akanthomyces Lebert.
Notes: Akanthomyces was introduced by Lebert (1858) based on the type species Akanthomyces aculeatus. Akanthomyces is usually parasitic in spiders and moth (Chen et al. 2019a; Aini et al. 2020), but also attacks Hemiptera, Coleoptera and Orthoptera (Hodge et al. 2003; Kepler et al. 2017; Mongkolsamrit et al. 2018; Chen et al. 2020a; Nishi et al. 2022). Based on morphological and phylogenetic analyses, Insecticola, Lecanicillium and Torrubiella, are all basionyms of Akanthomyces (Samson and Evans 1974; Kepler et al. 2017). Now, this genus has more than 31 species (Index Fungorum, http://www.indexfungorum.org/Names/Names.asp, 19th September 2022).
Akanthomyces xixiuensis X. C. Peng & T. C. Wen, sp. nov.
Index Fungorum number: IF900124; Facesofungi number: FoF 13898; Figs. 36, 37
Phylogram generated from maximum likelihood analysis based on combined ITS, SSU, LSU, TEF, RPB1 and RPB2 sequence data representing Cordycipitaceae in Hypocreales. One hundred and four strains are included in the combined analyses which comprised 4633 characters (519 for ITS, 947 for SSU, 774 for LSU, 877 for TEF, 678 for RPB1 and 840 for RPB2) after alignment. Purpureocillium lilacinum CBS 431.87 and Purpureocillium lilacinum CBS 284.36 in Ophiocordycipitaceae were used as the outgroup taxa. The best scoring RAxML tree with a final likelihood value of − 44,136.925268 is presented. Estimated base frequencies were as follows: A = 0.244444, C = 0.269605, G = 0.267391, T = 0.218561; substitution rates: AC = 1.445523, AG = 3.809695, AT = 0.889234, CG = 0.984428, CT = 8.185559, GT = 1.000000; gamma distribution shape parameter α = 0.543314. Bootstrap support values for ML equal to or greater than 60 are given above the nodes (left side). Bayesian posterior probabilities (BYPP) equal to or greater than 0.7 are given above the nodes (right side). Bold black dots mean support for the two analyses were 100 and 1. Ex-type strains are in bold and newly generated sequences are in blue
Etymology: xixiuensis (Lat.) referring to the collecting site “Xixiu District”.
Holotype: HKAS 125851.
Asexual morph: The host is ca. 14 mm long, moth (Lepidoptera). Several synnemata arising from adult of insect host, cylindrical, yellowish, 2‒6.5 mm long and 0.2‒0.7 mm wide. Phialides 6‒27 × 2‒4 µm (x̅ = 12.4 × 3.0 μm, n = 15), cylindrical to ellipsoidal with papillate end. Conidia smooth-walled, fusoid to ovoid, 3.5‒5.6 × 2.2‒3.2 µm (x̅ = 4.3 × 2.6 μm, n = 15).
Culture characteristics: Colonies on PDA, attaining a diameter of 17‒23 mm within 14 d at 20 ℃, dense, cottony, yellowish to white, reverse pale yellow. Mycelium smooth, branched, hyaline, 0.7‒4.8 μm diam. Conidia and reproductive structures not observed.
Habitat and distribution: The new species grow on the pine needle litter of mixed forest, and is currently only found in China.
Material examined: China, Guizhou Province, Xixiu District, 26°09′13.38′′ N, 106°05′13.19′′ E, 1293 m alt., on moth (Lepidoptera), 17 August 2021, X. C. Peng, XX21081764 (HKAS 125851, holotype); ex-type living culture XX21081764).
GenBank numbers: HKAS 125851: ITS = OP693461; SSU = OP693479; LSU = OP693481; TEF = OP838888; RPB1 = OP838890; RPB2 = OP838892. XX21081764: ITS = OP693460; SSU = OP693478; LSU = OP693480; TEF = OP838887; RPB1 = OP838889; RPB2 = OP838891.
Notes: Akanthomyces xixiuensis is characterized by gregarious synnemate with tapering gradually toward the apex, unbranched, obclavate, and yellowish. Two samples of Akanthomyces xixiuensis group together with strong statistical support (100/1), and form a separate clade at the basal portion of the Akanthomyces lineage. Akanthomyces xixiuensis is morphological similar to A. aculeatus, A. pyralidarum, and A. tortricidarum, and the host is Lepidoptera. However, the new species differs from Akanthomyces aculeatus in having shorter and wider synnemate, and fusoid conidia (Mains 1950), Akanthomyces tortricidarum has two kinds of structures of the synnemate, phialides, and conidia (Aini et al. 2020), while the asexual state of the Akanthomyces pyralidarum is undetermined (Aini et al. 2020).
Nectriaceae Tul. & C. Tul.
Notes: Nectriaceae, typified by Nectria, was introduced by Tulasne and Tulasne (1865). Wijayawardene et al. (2022) accepted 70 genera under Nectriaceae wherein members have worldwide distribution with various lifestyles as saprobes, endophytes, or pathogens of wide-ranging host species (Rossman et al. 1999; Lombard et al. 2015). Sexual morphs of Nectriaceae are characterized by uniloculate, white, yellow, orange-red or purple ascomata that change colour in KOH while asexual morphs are mainly hyphomycetous with phialidic conidiogenous cells producing amerosporous to phragmosporous conidia (Lombard et al. 2015; Hyde et al. 2020b). The latest treatment and updated accounts of Nectriaceae by Perera et al. (2023) is followed in this paper.
Paracremonium L. Lombard & Crous.
Notes: Lombard et al. (2015) introduced Paracremonium based on different strains previously treated as Acremonium recifei with formation of sterile coils from which conidiophores radiate with inconspicuously swollen septa in the hyphae. Ten species are currently accepted under Paracremonium [P. apiculatum, P. bendijkiorum, P. binnewijzendii, P. contagium, P. ellipsoideum, P. inflatum (type species), P. lepidopterorum, P. moubasheri, P. pembeum and P. variiforme] isolated from various sources (i.e., soil, water, sewage and Homo sapiens) (Lombard et al. 2015; Lynch et al. 2016; Crous et al. 2017, 2021a; Zhang et al. 2017a, 2021a; Al-Bedak 2019; Ming et al. 2021). Zhang et al. (2021a) noted that the sterile coil as a distinguishing feature of Paracremonium is no longer significant in characterizing this genus to other acremonium-like genera.
Paracremonium aquaticum M.S. Calabon, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF 559840; Facesoffungi number: FoF 05445; Fig. 38
Paracremonium aquaticum (MFLU 22-0120, holotype). a, c Conidiophores and conidia. d Conidia. e Germinated conidium. f Colonies on MEA from surface and in reverse. g–ab Sporulation on MEA. g–t Various moniliform hyphal elements. m–t Chlamydospores. w–aa Conidiophores, conidiogenous cells, and conidia. ab Conidia Scale bars: a–c = 50 µm, d, e, i–k, m–t = 20 µm, g, h, l = 100 µm, w–ab = 10 µm
Etymology: “aquaticum” in reference to the aquatic habitat.
Holotype: MFLU 22-0120.
Sexual morph: Undetermined. Asexual morph: Hyphomycetous. Saprobic on submerged decaying wood from a freshwater habitat. Colonies on natural substrate effuse, greyish white, velvety. Mycelium immersed, composed of hyaline to pale brown, branched, septate hyphae. Conidiophores 18–30 × 1.4–2.4 μm (x̄ = 23.8 × 1.8 μm, n = 15), erect, subcylindrical, unbranched, hyaline, smooth. Conidiogenous cells terminal, elongate-ampulliform, tapering towards apex, with prominent periclinal thickening and inconspicuous collarette, hyaline, smooth. Conidia 4.5–8.5 × 2.0–3.0 μm (x̄ = 6.3 × 2.5 μm, n = 50), formed in heads at apex of conidiogenous cells, aseptate, ellipsoidal to fusiform, acute at both ends, smooth, slightly to strongly curved, with two large guttules. Chlamydospores not observed.
Culture characteristics: Conidia germinating on malt extract agar (MEA) and producing germ tubes at both ends within 24 h. Colonies growing on MEA, circular, with flat surface, margin entire, reaching 40–45 mm in 4 weeks at 25 °C, powdery, from above pale yellow to white from edge to center, from white to pale yellow from edge to center. Hyphae 2–5.5 μm wide, septate, hyaline, mostly smooth, thick-walled, moniliform, abundant. Conidiophores erect, simple or mostly branched, septate, bearing whorls of 2–4 conidiogenous cells. Conidiogenous cells 8–15 × 1.5–6.0 μm (x̄ = 11.2 × 3.6 μm, n = 20), terminal or lateral, straight, flask-shaped, tapering towards apex, hyaline, with prominent periclinal thickening and inconspicuous collarette, 1.5–2.0 µm in diam. Conidia 2.5–8.5 × 1.5–3 μm (x̄ = 5.8 × 2.4 μm, n = 50), unicellular, hyaline, ellipsoidal to fusiform, smooth-walled, with slightly apiculate base, sightly curved, guttulate. Chlamydospores hyaline, formed intercalary in chains or solitary, smooth, thin-walled, ellipsoidal to mostly cylindrical, guttulate.
Habitat and distribution: Paracremonium aquaticum was observed from submerged decaying wood in stream, and is currently only found in Thailand.
Material examined: Thailand, Chiang Mai Province, Mushroom Research Center, on submerged wood in an artificial lake, 13 September 2019, M.S. Calabon, MRC67 (MFLU 22-0120, holotype), ex-type living culture, MFLUCC 22-0077.
GenBank numbers: ITS = OP216410, LSU = OP216405, RPB2 = OP251199, TEF1-α = OP251195, TUB2 = OP251200.
Notes: Multi-locus phylogenetic analysis showed that Paracremonium aquaticum MFLUCC22-0077 shared the same subclade with the type species P. inflatum CBS 485.77 (Fig. 39). Paracremonium aquaticum MFLUCC 22-0077 differs from P. inflatum in having flask-shaped conidiogenous cells with larger conidia (2.5–8.5 × 1.5–3 μm vs. 5–6 × 1–2 μm). In pairwise nucleotide comparisons of P. aquaticum with the type strain of P. inflatum CBS 124513, there is a nucleotide difference of 2.51% (14/558 bp) in ITS, 4.70% (25/532 bp) in TEF1-α, 8.37% (72/860 bp) in RPB2 and 3.15% (11/349 bp) in TUB2 genes. Another strain of P. inflatum CBS 482.78, is a sister taxon to P. aquaticum (Fig. 39), but pairwise nucleotide comparison showed 0.89% (5 bp) in ITS (of 564 nucleotides altogether), 1.16% (10 bp) in RPB2 (of 862 nucleotides altogether) and 0.37% (2 bp) in TEF1-α (of 533 nucleotides altogether). Morphological comparison is not possible between P. inflatum CBS 482.78 and P. aquaticum because the former does not have morphological data. As the strain P. inflatum CBS 482.78 forms a sister taxon to P. aquaticum, we rename this strain as P. aquaticum CBS 482.78. Paracremonium contagium also clustered with strains of P. pembeum but with inclusions of three more additional loci (calmodulin, histone H3-like and actin) in a separate phylogenetic analysis of Paracremonium species (data not shown), P. contagium has a distinct lineage basal to P. pembeum strains. Phylogenetic analysis also shows that two strains of P. lepidopterorum DY10351 and DY10352, a species isolated from an insect pupa in China (Ming et al. 2021), did not cluster within the Paracremonium clade, but forms a strongly supported clade with members of Cordycipitaceae (Fig. 39). A wider taxon sampling of taxa under Cordycipitaceae will help establish the phylogenetic placement of P. lepidopterorum within this family. Paracremonium aquaticum from freshwater habitats in Thailand is the third freshwater species of Paracremonium, with P. binnewijzendii recorded from submerged wood and P. variiforme from cave water, both from China (Zhang et al. 2017a; Luo et al. 2019).
Phylogram generated from maximum likelihood analysis based on combined ITS, LSU, RPB2, TEF1-α, and TUB2 sequence data representing Nectriaceae (Hypocreales) and closely related families. Seventy-eight strains are included in the combined analyses which comprised 3663 characters (ITS: 499, LSU: 817, RPB2: 1037, TEF1-α: 714, TUB2: 596) after alignment. Seven Phaeoisaria species (P. annesophiea e MFLU 19-0531; P. aquatica MFLUCC 16–1298; P. clematidis MFLUCC 18–1017; P. fasciculata CBS 127885, DAOM 230055; P. pseudoclematidis MFLUCC 11-0393; P. sedimenticola CGMCC 3.14949) in Pleurotheciaceae (Pleurotheciales) were used as the outgroup taxa. The best scoring RAxML tree with a final likelihood value of − 30,486.223016 is presented. The matrix had 1831 distinct alignment patterns, with 38.42% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.230840, C = 0.279253, G = 0.276292, T = 0.213616; substitution rates: AC = 1.237490, AG = 2.611546, AT = 1.138497, CG = 0.936411, CT = 6.064431, GT = 1.000000; gamma distribution shape parameter α = 0.282744. Bootstrap support values for ML equal to or greater than 75% are given above the nodes (left side). Bayesian posterior probabilities (BYPP) equal to or greater than 0.95 are given above the nodes (right side). Ex-type strains are in bold and newly generated sequences are in blue
Microascales Luttr. ex Benny & R.K. Benj.
Notes: Benny and Kimbrough (1980) established the order Microascales to accommodate three families: Chadefaudiellaceae, Microascaceae and Pithoascaceae. Later, it was expanded to include another four families: Ceratocystidaceae, Gondwanamycetaceae, Halosphaeriaceae and Graphiaceae (Réblová et al. 2011; Maharachchikumbura et al. 2016). The order currently has seven families and 110 genera (Hyde et al. 2020b).
Halosphaeriaceae E. Müll. & Arx ex Kohlm.
Notes: Halosphaeriaceae is the largest family of the order Microascales introduced by Müller and von Arx (Kohlmeyer 1972) with Halosphaeria appendiculata as the type species. The family currently comprises 167 species in 64 genera (Jones et al. 2017, 2019; Nourel-Din et al. 2022). Halosphaeriaceous genera are aquatic with most of them described from marine habitats and usually possess a pseudoparenchymatous centrum that break up into catenophyses, mostly with deliquescing asci and appendaged ascospores.
Ascoglobospora Abdel-Wahab, gen. nov.
Index Fungorum number: IF 559818; Facesoffungi number: FoF 12719;
Etymology: Named after the globose-shaped asci of the fungus.
Saprobic on decaying driftwood. Sexual morph: Ascomata globose to subglobose, erumpent to superficial, membranous, hyaline to light-brown, ostiolate, papillate, surrounded by septate hyphae. Neck hyaline to light-brown in color, cylindrical, periphysate. Peridium one-layered, hyaline, forming textura-angularis, cell layers consists of elongated, thick-walled, polygonal hyaline cells. Catenophyses present. Asci eight-spored, subglobose, thin-walled, deliquesce early, without an apical apparatus. Ascospores ellipsoidal, with rounded ends, multiseriate, one-septate, not constricted at the septum, hyaline, thick-walled, with bipolar, hamate, apical appendages. Appendages extend beyond the median septa, uncoil in water to form long filaments. Asexual morph: unknown.
Type species: Ascoglobospora marina Abdel-Wahab.
Notes: Undescribed marine ascomycete with early deliquescing subglobose asci with uncoiling polar appendages to the ascospores was recorded from driftwood collected from a rocky beach at Umikaze Park, Yokohama, Japan and listed as Aniptodera sp. (MF966) in Abdel-Wahab (2011). Molecular phylogenetic analyses of the combined SSU and LSU rDNA placed the new taxon with Aniptosporopsis lignatilis in all the analyses performed with high statistical support (100 ML/100 MP/100 BYPP, Fig. 40). Other taxa with polar uncoiling appendages in the clade include: Aniptodera chesapeakensis, A. aquibella and Ascosacculus heteroguttulatus (Fig. 40). Ascoglobospora marina cannot be placed in the genus Aniptosporopsis because it has an early deliquescing asci that are subglobose in shape, thin-walled and without an apical apparats. Aniptosporopsis lignatilis has clavate asci with a flattened thickened tip, with apical pore, plasmalemma retraction, pedunculate, persistent, with active spore release (Hyde 1992; Jones et al. 2017). A comparison of the 572 nucleotides of the D1/D2 region of the LSU rDNA of Ascoglobospora marina and Aniptosporopsis lignatilis shows 30 base pair differences (6%), which justifies the erection of a new genus. The pairwise distance of the partial 28S rDNA of genera in the Halosphaeriaceae generally ranges between 3 and 9% (Jones et al. 2017).
Phylogenetic relationship of Ascoglobospora marina with related genera in Halosphaeriaceae based on the nucleotide sequences of the combined SSU and LSU rDNA. The maximum likelihood (ML) tree was constructed in RAxMLGUI v. 2.0.8 (Silvestro & Michalak 2012) using the GTR + GAMMA substitution model with 1000 bootstrap replicates. The best RAxML tree with a final likelihood value of − 12,686.5456 is presented. The maximum parsimonious data set of the combined genes consisted of 51 taxa with 3 representatives of Xylariales used as outgroup. The combined dataset includes 1662 total characters, of which 1004 were constant, 280 parsimony-uninformative and 378 parsimony-informative. The parsimony analyses of the data matrix yielded 4 equally most parsimonious trees with a tree length of 2082 steps (CI = 0.4539, RI = 0.5734, RC = 0.2602). Phylogenetic trees obtained from ML, maximum parsimony (MP) and Bayesian inference posterior probabilities (BYPP) were similar in topology. Bootstrap support on the nodes represents ML and MP ≥ 70%. Branches with a BYPP of ≥ 95% are in bold. The new taxon, Ascoglobospora marina in blue bold and type strains are in bold
Ascoglobospora marina Abdel-Wahab, sp. nov.
Index Fungorum number: IF 559819; Facesoffungi number: FoF 12718; Fig. 41
Etymology: Named after the marine habitat of the fungus.
Holotype: CBS H-23855.
Saprobic on decaying driftwood. Sexual morph: Hyphae 2–3 μm in diam, light-brown, septate. Ascomata 110–180 μm in diam., globose to subglobose, erumpent to superficial, membranous, hyaline to light-brown, ostiolate, papillate, surrounded by septate hyphae. Neck 22–45 μm long, 18–21 μm wide hyaline to light-brown in color, cylindrical, periphysate. Peridium 15–24 μm thick, one-layered, hyaline, forming textura-angularis, consists of 9–12 cell layers of elongated, thick-walled, polygonal hyaline cells, the first outer layer is hyaline to light-brown in color. Catenophyses present. Asci 42–48 × 32–37 μm, eight-spored, subglobose, thin-walled, deliquesce early, without an apical apparatus. Ascospores 22–30 × 8–9 μm (x̅ = 26.3 × 8.2 μm, n = 50), ellipsoidal, with rounded ends, multiseriate, one-septate, not constricted at the septum, hyaline, thick-walled (0.5–0.7 μm), with bipolar, hamate, apical appendages. Appendages 15–20 μm long extend beyond the median septa, uncoil in water to form long filaments. Asexual morph: unknown.
Culture characteristics: Single spore isolates of Ascoglobospora marina growing on PDA are light brown with tufts of aerial mycelium, reaching a 20–25 mm radius after one month at 25 ºC. No sporulation structure was observed.
Material examined: Japan, Yokohama, Umikaze Park, 35° 16′ 36″ N, 139° 41′ 02″ E, on decaying driftwood, 12 October 2007, M.A. Abdel-Wahab, CBS-H-23855 (holotype), ex-type living culture, NBRC 105278.
GenBank numbers: CBS-H-23855: ITS = OP150939, SSU = OP151088.
Notes: Ascoglobospora marina differs from Aniptosporopsis lignatilis by having globose, early deliquescing, thin-walled asci without apical apparatus, smaller ascospore dimensions that are ellipsoidal with rounded ends. The latter species has clavate, persistent asci, with a thickened plate, plasmalemma retraction, apical pore and active spore dispersal. Ascospores in A. lignatilis are fusiform with acute apices and larger than ascospores of A. marina (Hyde 1992; Jones et al. 2017).
Pleurotheciales Réblová & Seifert.
Notes: Pleurotheciales was established by Réblová et al. (2016) based on the type family Pleurotheciaceae. Hongsanan et al. (2017) reported that Pleurotheciales clustered with Conioscyphales, Fuscosporellales and Savoryellales in a monophyletic clade within Sordariomycetes. Hence, they transferred Pleurotheciales to a newly introduced sub-class Savoryellomycetidae based on molecular evidence and divergence time (Hongsanan et al. 2017). This treatment was confirmed and acknowledged by Dayarathne et al. (2019) and Hyde et al. (2020a).
Pleurotheciaceae Réblová & Seifert.
Notes: The family Pleurotheciaceae was established by Réblová et al. (2016) with Pleurothecium as the type genus. Currently, there are 13 genera in this family, viz. Adelosphaeria, Anapleurothecium, Coleodictyospora, Dematipyriforma, Helicoascotaiwania, Melanotrigonum, Neomonodictys, Phaeoisaria, Phragmocephala, Pleurotheciella, Pleurothecium, Saprodesmium and Sterigmatobotrys (Bao et al. 2022). It should be noted that some species of Pleurotheciaceae were identified as opportunistic human pathogens (Réblová et al. 2012, 2016; Luo et al. 2019; Hyde et al. 2020a).
Pleurothecium Höhn.
Notes: Pleurothecium was introduced by Höhnel (1919) with P. recurvatum (≡ Acrothecium recurvatum) as the type species. Pleurothecium is a holomorphic genus, and there are currently 12 species, of which only two species, viz. P. recurvatum and P. semifecundum, have sexual morphs (Réblová et al. 2012). The asexual morph of Pleurothecium is characterized by having distinct brown conidiophores, polyblastic, sympodially denticulate conidiogenous cells, and solitary, unicellular or septate, cylindrical, ellipsoidal, fusiform or clavate conidia (Réblová et al. 2012; Luo et al. 2019). In addition, most species of Pleurothecium are reported as saprobes from freshwater or terrestrial habitats in Australia, Brunei, China and Seychelles (Tsui et al. 2001; Luo et al. 2019; Shen et al. 2022).
Pleurothecium aseptatum J. Ma & Y.Z. Lu, sp. nov.
Index Fungorum number: IF 900172, Facesoffungi number: FoF 13907; Fig. 42
Etymology: referring to the aseptate conidia.
Holotype: GZAAS 22–2019.
Saprobic on submerged decaying wood. Sexual morph: undetermined. Asexual morph: Colonies on the substratum superficial, effuse, gregarious, white. Mycelium composed of partly immersed, partly superficial, hyaline, septate, branched hyphae, with a little of glistening conidia. Conidiophores macronematous, mononematous, erect, cylindrical, mostly unbranched, a few branched, 46–59 μm long (\(\overline{x }\) = 53 μm, n = 15), 1.5–2.5 μm wide (\(\overline{x }\) = 2 μm, n = 15), tapering to 0.8–1.3 μm wide near apex, 0–1-septate, sometimes reduced to conidiogenous cells, hyaline, smooth-walled. Conidiogenous cells polyblastic, integrated, terminal, sympodial, cylindrical, 41–53 μm, denticulate, hyaline; denticles cylindrical, long, narrow, 3–5 × 0.6–0.8 μm (\(\overline{x }\) = 4 × 0.7 μm, n = 20). Conidia solitary, acrogenous, subcylindrical, slightly curved, 8.5–10 μm × 2–3 μm (\(\overline{x }\) = 9.5 × 2.5 μm, n = 30), rounded at the apex, obtuse and tapering towards base, aseptate, with 2–4 large guttules, hyaline, smooth-walled.
Culture characteristics: Conidia germinating on water agar and germ tubes produced from conidia within 8 h. Colonies growing on PDA, circular, with flat surface, edge entire, reaching 45 mm in 30 days at 25 °C, pale brown to brown.
Material examined: China, Guizhou Province, Qiandongnan Miao and Dong Autonomous Prefecture, Tianzhu County, on decaying wood in a freshwater stream, 16 January 2021, Jian Ma, TZX8 (GZAAS 22–2019, holotype), ex-type living culture, GZCC 22–2019.
GenBank numbers: LSU = OQ002372, ITS = OQ002375.
Notes: Phylogenetically, Pleurothecium aseptatum is clustered within Pleurothecium and formed a basal clade to P. recurvatum and P. semifecundum (Fig. 43). Morphologically, P. aseptatum differs from P. recurvatum and P. Semifecundum by having hyaline conidiophores and aseptate conidia, while P. recurvatum and P. semifecundum having 3-septate conidia and brown conidiopheres (Réblová et al. 2012; Luo et al. 2019). Furthermore, the denticles of conidiogenous cells in P. aseptatum are rather long, while P. recurvatum and P. semifecundum having tiny denticles (Réblová et al. 2012; Luo et al. 2019).
Phylogram generated from maximum likelihood analysis based on combined ITS, LSU, SSU and rpb2 sequence data representing the species of pleurotheciaceae. Thirty-nine taxa were included in the combined analyses, which comprised 3393 characters (ITS: 621, LSU: 870, SSU = 986, rpb2 = 916) after alignment. Bootstrap support values for ML equal to or greater than 50% and BYPP equal to or greater than 0.95 are given above the nodes. Conioscypha minutispora (CBS 137253) and C. tenebrosa (GZCC 19-0217) were used as the outgroup taxa. The newly-generated strain is shown in blue and bold. Ex-type strains are indicated by black and bold
Sordariomycetes, genus incertae sedis
Pleurotheciella Réblová, Seifert & J. Fourn.
Notes: Pleurotheciella was introduced by Réblová et al. (2012) with P. rivularia as the type species. The asexual morph of Pleurotheciella is characterized by having hyaline or brown, mononematous or synnematous conidiophores, integrated, polyblastic, sympodially denticulate conidiogenous cells, and hyaline, unicellular or septate, ellipsoidal, obovoid, clavate, lunate or suballantoid conidia (Réblová et al. 2012; Luo et al. 2019). Currently, 15 species are included in the genus, with 14 species from freshwater habitats and only one species from dead branches of Malus (Luo et al. 2019; Shi et al. 2021).
Pleurotheciella hyalospora J. Ma & Y.Z. Lu, sp. nov.
Index Fungorum number: IF 900171; Facesoffungi number: FoF 13906; Fig. 44
Etymology: “hyalospora” referring to the hyaline conidia.
Holotype: GZAAS 22–2018.
Saprobic on decaying wood. Sexual morph: undetermined. Asexual morph: Colonies on the substratum superficial, effuse, gregarious, white. Mycelium composed of partly immersed, partly superficial, hyaline to pale brown, septate, branched hyphae, with a little of glistening conidia. Conidiophores macronematous, mononematous, unbranched, straight or flexous, cylindrical, 73–100 × 3.5–5 μm (\(\overline{x }\) = 88 × 4 μm, n = 20), dark brown at the base, hyaline to pale brown toward the apex, smooth-walled. Conidiogenous cells polyblastic, terminal, cylindrical, 17–30 × 3–4 μm (\(\overline{x }\) = 25 × 3.5 μm, n = 20), with denticles, pale brown near base, hyaline towards apex, smooth-walled. Conidia solitary, acropleurogenous, fusoid or clavate, slightly curved, rounded at the apex, obtuse and tapering towards base, 11–20 × 3–5.5 μm (\(\overline{x }\) = 16 × 4.5 μm, n = 30), hyaline, 1-septate, often with 1–3 guttules in each cell, smooth-walled.
Culture characteristics: Conidia germinating on water agar and germ tubes produced from conidia within 8 h. Colonies growing on PDA, circular, with flat surface and filiform margin, reaching 35 mm in 30 days at 25 °C, brown in center, pale brown at the entire margin.
Material examined: China, Guizhou Province, Guiyang City, Xiaochehe Park, on decaying wood in a forest, 29 March 2020, Jian Ma, XCH20 (GZAAS 22–2018, holotype), ex-type living culture, GZCC 22–2018; Guizhou Province, Longli County, on decaying wood submerged in a freshwater stream, 3 December 2020, Jian Ma, LLSZ2 (GZAAS 22–2023), living culture, GZCC 22–2023.
GenBank numbers: GZCC 22–2018: LSU = OQ002371, ITS = OQ002374, SSU = OQ002377, RPB2 = OP999221; GZCC 22–2023: LSU = OQ002370, ITS = OQ002373, SSU = OQ002376, RPB2 = OP999220.
Notes: The proposed new species viz. Pleurotheciella hyalospora is morphologically similar to P. uniseptata in conidiophores and conidia, but it can be recognized from P. uniseptata by having smaller conidiophores (73–100 × 3.5–5 μm vs. 100–150 × 4.5–5 μm). Besides, the conidia of P. hyalospora are slightly curved, while P. uniseptata is characterized by straight conidia (Réblová et al. 2016). Phylogenetically, P. hyalospora forms a sister clade to P. uniseptata and the phylogentic tree showed that they are distinct species (Fig. 43).
Basidiomycota R.T. Moore.
Notes: For the latest updated account of Basidiomycota see Zhao et al. (2017).
Agaricomycetes Doweld.
Notes: For the latest treatments and updated accounts of Agaricomycetes see Hibbett et al. (2014) and Zhao et al. (2017).
Agaricales Underw.
Notes: Agaricales is the largest order of Agaricomycetes with 17,291 species in 508 genera (He et al. 2019). There is no morphological synapomorphy that unites this order. Although they are dominated by pileate-stipitate forms with lamellate hymenophores, this order also includes many other forms such as false truffles, puffballs, bird-nest fungi, resupinate, coralloid, cyphelloid, and pileate with poroid hymenophores. On the other hand, the pileate-stipitate traditional morphology is not exclusive to Agaricales (Hibbett et al. 2014).
Agaricaceae Chevall.
Notes: The family Agaricaceae was established by Chevallier (1826). It is named after the type genus Agaricus, originally confined by Linnaeus (1753). For the latest treatments and updated accounts of Agaricaceae see Zhao et al. (2016b), Zhou et al. (2016a) and Hyde et al. (2017).
Agaricus L.
Notes: The genus Agaricus was established by Linnaeus (1753). Agaricus is characterized by having a fleshy cap or pileus, from the underside of which grow a number of radiating plates or gills, on which are produced the naked chocolate-brown spores. Members of Agaricus have a stipe, which elevates it above the object on which the mushroom grows, or substrate, and a partial veil, which protects the developing gills and later forms a ring or annulus on the stalk. The latest treatments and updated accounts of Agaricus follow Karunarathna et al. (2016), Zhao et al. (2016b) and Zhou et al. (2016a).
Agaricus agharkarii P.N. Singh, S.K. Singh, S. Rana & A.C Lagashetti, sp. nov.
Index Fungorum number: IF 559727; Facesoffungi number: FoF11794; Figs. 45, 46, 47
Agaricus agharkarii (AMH10341, holotype). a A basidium with attached basidiospores on sterigmata. b Basidia with basidiospores and cheilocystidia on lateral side. c A basidium with sterigmata. d A clavate cheilocystidium. e Numerous basidiospores arranged in tetrad manner (photographs taken from surface of gill). f, g Numerous basidiospores (arrow showing slightly tapered basal end). h An elongated basidiospore with thickened wall (showing arrow). i–k Pileocystidia (pileal tissues). l–m Stipitipellis (stipe tissues arranged in parallel manner). Scale bars: a–m = 10 µm
Etymology: species named ‘agharkarii’ in the surname of Professor Shankar Puroshattam Agharkar, founder Director of Agharkar Research Institute.
Holotype: AMH 10341.
Colour code follows: Methuen Handbook of Colour (Kornerup and Wanscher 1978).
Basidiomes solitary or in groups of 2–4, variable in size, agaricoid, stipitate. Pileus 80–110 mm in diam, broadly umbonate, broadly ovate when young, 8 mm thick at the disk, fleshy, convex to plano-convex, when mature the pileus becomes upwards, surface dry, squamulose, color rufescent to greyish brown (8F3) when young, paraboloid when young, surface dry with greyish brown spots (8F3) when mature, margin entire, nonappendiculate. Lamellae free, regular, become dark brown with age (6F8); lamellulae present in 1–3 tiers, 8–10 mm broad, wavy, greyish brown (8F3). Stipe 70–96 × 20–22 mm, compact, attenuated towards length, base slightly bulbus with white (1A1) mycelial threads, fragile, greyish brown (8F3), surface dry, after cutting or touching with finger becomes dark brown (7F5). Volva absent. Annulus present, drooping or skirt like, membranous covering around the stipe, with double edged margin, white (1A1) to light brown (5D8). Volva absent. Spore print dark brown (7F5). Odour mushroomy. Taste not recorded. Context thick and fleshy, spongy, whitish (1A1) in pileus and pithy in stipe. Macrochemical reactions; negative in Schäffer’s reaction, no reaction with Ammonia and Potassium hydroxide.
Basidia 21.80–36.10 × 3.5–9.55 µm, clavate to narrowly cylindrical, subhyaline to light olivaceous, pigmented, smooth walled, variable in size, frequently tetrasporic. Sterigmata straight to curved sometimes resembles like an incisor canine, hyaline smooth walled, up to 3.52 × 1.90 µm. Basidiospores 5.15–9.19 × 3.92–5.67 µm (\(\overline{x }\) = 6.55 × 4.70 μm, n = 30), Qm = 1.43, Q = 1.11–1.94, oval, ellipsoid to oblong, sometimes slightly tapered towards base with one or two guttulate, dark olivaceous brown, smooth walled, wall thickened and darkened (up to 0.85 µm thick). Hilum protuberant. Lamellar surface fertile, made-up of basidia, cystidia, and marginal cell. Cheilocystidia pedicillate, ampulliform to narrowly clavate, apex rounded, non-fertile, base narrow, subhyaline to light olivaceous, smooth walled, wall thickened and darkened, 19.75–42.47 × 7–8.12 µm. Pileocystidia hyphoid, fusiod, unbranched to branched, smooth walled, hyaline, up to 45.5–230 × 13–35 µm. Subhymenium 22.5–25 µm thick, composed of globose to oval cells, 6.9–18.65 × 6.55–15.25 µm, light olivaceous, Hymenial trama hyphoid, upto 113.35 μm wide, composed of parallel to interwoven thick and thin hyphae, subhyaline to light olivaceous, with distant septation in hyphae, branched, variable in dimensions. Pileipellis (pileus trama) dark brown, interwoven, hyphae septate, up to 34.80 μm in diam, branched, pigmented, light olivaceous. Clamp connections absent. Stipitipellis hyphal, arranged in parallel bundles of hyphae, irregularly septate, subhyaline to light olivaceous, cells irregular, variable in dimensions, 20.38–26.75 µm wide.
Materials examined: INDIA, Maharashtra, Pune District, on garden soil, 9 July 2021, P.N. Singh, AMH10341 (holotype).
Host and habitat: Solitary or in groups, free living in botanical garden under Albizia tree.
GenBank numbers: ITS = MZ198899, LSU = MZ198900.
Notes: In the present taxon, the basidiomes are medium to large sized, squmulose with off-white linings with greyish brown pileus that have negative reaction with Schäffer’s test. There is no reaction with KOH and Ammonia on the flesh of fruiting body. The stipe base is white with swollen base and mild mushroomy odour. The pileipellis hyphae are hyphoid and variable in shape and size (10.5–34.80 μm in diam). The presence of cheilocystidia in the present taxon is a key feature of the A. sect. Flocculenti under the A. subg. Pseudochitonia (Zhao et al. 2011; He et al. 2018). Morphologically, new taxon, Agaricus agharkarii is different from its related taxa in having a larger convex pileus with broader lamellae as compared to A. erectosquamosus and A. pallidobrunneus (Zhao et al. 2016b). The length of cheilocystidia in A. agharkarii is comparatively larger than in A. erectosquamosus and A. pallidobrunneus. In addition to this, the dimension of basidiomes and basidiospores is apparently larger compared to A. erectosquamosus and A. pallidobrunneus (Table 1).
ITS and LSU sequence comparison has revealed that the sequences of the new species Agaricus agharkarii differ at 33 positions in ITS and 10 positions in LSU. Beside this, one deletion and five insertions (four of 1 bp and one of 3 bp) were observed in ITS sequence of the present taxon. The position of the present taxon within Agaricus sect. Flocculenti was further confirmed by molecular phylogeny based on combined ITS and LSU rDNA data. Phylogenetic analysis based on combined ITS and LSU sequence data indicates that Agaricus agharkarii (AMH 10341) is a new species of A. sec. Flocculenti, which is different from the other known species of A. sec. Flocculenti. The present taxon forms a distinct clade from other species of the A. sec. Flocculenti (A. erectosquamosus and A. pallidobrunneus) with a moderately supported ultrafast bootstrap value of 85% (Fig. 48).
Phylogenetic tree of Agaricus agharkarii (AMH 10341) by Maximum-Likelihood method based on combined sequence data of ITS and LSU. Agaricus biberi LAPAG687 was used as an outgroup. The analysis involved 17 nucleotide sequences. Evolutionary analyses were conducted in IQ–TREE multicore version 1.6.11 (Nguyen et al. 2015) by the Maximum–Likelihood method using the best suitable model (TPM3u + F + R2 model). One–thousand bootstrap replicates were analysed to get ultrafast bootstrap values, and the values above 50% were represented on nodes in the tree
On megablast analysis, ITS sequence of Agaricus agharkarii showed 95% (654/691) identity and 8 gaps (1%) with Agaricus erectosquamosus SDBR-NK0080 and Agaricus erectosquamosus SDBR-CJ0032, 95% (653/690) identity and 8 gaps (1%) with Agaricus pallidobrunneus SDBR-NK0368, 94% (642/684) identity and 6 gaps (0%) with Agaricus sparsisquamosus PU320, 94% (641/684) identity and 6 gaps (0%) with Agaricus sparsisquamosus PU256, 93% (640/685) identity and 8 gaps (1%) with Agaricus sparsisquamosus PU320, and 94% (641/684) identity and 9 gaps (1%) with Agaricus sparsisquamosus PU320.
The distinct morphology compared to other species of A. sec. Flocculenti (A. erectosquamosus and A. pallidobrunneus), as well as the phylogenetic analysis, clearly establishes Agaricus agharkarii as a novel species.
Agaricus albostipitatus E. Tarafder, A.K. Dutta & K. Acharya, sp. nov.
MycoBank number: MB 843678; Facesoffungi number: FoF 10832; Figs. 49, 50
Agaricus albostipitatus (CAL 1871, holotype). a Immature stage of the basidiomes showing stipe context and lamellae colouration. b Basidiomes showing pileus surface feature. c Basidiomes showing developing stages and gradual maturity of the lamellae. d Mature basidiomes showing lamellae colouration and annulus features. Scale bars: a–d = 20 mm
Etymology: The epithet “albostipitatus” refers to an entirely white coloured stipe.
Holotype: CAL 1871.
Pileus 12–60 mm in diam., convex when young, becoming broadly convex to plano-convex on maturity, overall white (1A1), rarely with slight light brown (6D5-6) patches especially at the center, smooth, margin incurved, associated with traces of partial veilar remnants, often rimose; context ca. 4 mm thick at the centre, gradually thinner towards margin (up to 1 mm), white (1A1). Lamellae 4–6 mm broad, free, crowded with two series of lamellulae, light pinkish (14A2) when young, becoming brown (7F4) to dark chocolate brown (6F4) on maturity, edge even, concolorous. Stipe 25–70 × 5–7 mm, central, cylindrical with slightly broader at base (up to 10 mm), rarely tapered toward base, slightly curved, smooth, sometimes slightly fibrillose at the apex, entirely white, basal portion turning brownish orange (6C4–5) on bruising; context narrowly hollow, white. Annulus supramedian, entire, simple, persistent, rarely fragile and remains at traces on the stipe surface, upper side brown, smooth, lower side white, fibrillose. Odour mushroomy.
Basidiospores (6.5–)7.4–8.5(–9.7) × (4.4–)5.3–5.9(–6.5) μm (Xmr = 7.9–8.2 × 5.4–5.8 μm, Xmm = 8.1 ± 0.9 × 5.6 ± 0.5 μm, Qmr = 1.41–1.45, Qmm = 1.43 ± 0.1, n = 60 spores, s = 2 specimens), ellipsoid, brown to dark brown, smooth, thick-walled. Basidia 22–32 × 8–10 μm, clavate, hyaline, thin-walled, 4-spored; sterigmata 1–3 μm long, hyaline. Basidioles 14–25 × 5–7.5 µm, clavate, hyaline, thin-walled. Cheilocystidia 11–21 × 6–10 μm, pyriform to ovoid, smooth, thin-walled. Pleurocystidia absent. Pileipellis a cutis, composed of hyphae measuring 5–7 μm broad, cylindrical, smooth constricted at the septa, branched, hyaline, thin-walled. Stipitipellis hyphae 5–7 μm broad, unbranched, cylindrical, hyaline, thin-walled. Annulus hyphae 5–7 μm broad, hyaline, thin-walled.
Chemistry: KOH reaction negative on pileus and stipe surface. Schäffer’s reaction negative on pileus surface.
Habitat and distribution: The new species grows on humus mixed soil in a grassy field, and is currently only found in India.
Material examined: India, West Bengal, Nadia district, Village Chandirampur, 22°59′54.48" N, 88°31′43.3" E, 9.0 m alt., on humus mixed soil, 1 May 2020, E. Tarafder, ENTAJ 01/2020 (CAL 1871, holotype); West Bengal, Village Chandirampur, 22°59′54.5"N, 88°31′42.92"E, 9.0 m alt., on humus mixed soil, 9 May 2020, E. Tarafder, CUH AM688.
GenBank numbers: CAL 1871: ITS = OM654932, LSU = OM677382; CUH AM688: ITS = OM654933, LSU = OM677383.
Notes: Agaricus albostipitatus is characterized by its entirely white, smooth pileus, crowded lamellae with two series of lamellulae, a cylindrical stipe with slightly broader base coloured white all over that turning brownish orange on bruising, a supramedian annulus with smooth, brown upper side and fibrillose, white lower side, ellipsoid basidiospores measuring 7.9–8.2 × 5.4–5.8 μm, and pyriform to ovoid cheilocystidia (11–21 × 6–10 μm).
Considering the overall morphological features and phylogenetic analyses (Figs. 51 and 52), Agaricus albostipitatus appears related to A. argyropotamicus and A. inilleasper. However, A. argyropotamicus differs from A. albostipitatus by its smaller basidiospores (5.5–7.5 × 4.3–5.4 µm) and almost indistinguishable cheilocystidia (Kerrigan 2016; Liu et al. 2020). Agaricus inilleasper has fused pileus margin with the stipe, and ellipsoid to subglobose, somewhat differently sized basidiospores measuring 6.0–8.0 × 5.0–7.0 μm (Lebel and Syme 2012) compared to A. albostipitatus.
Phylogram generated from maximum likelihood analysis based on ITS sequence data. Seventy-four vouchers are included in the ITS analyses which comprised 703 characters after alignment. Heinemanomyces sp. ZRL185 was used as the outgroup taxa. The best scoring RAxML tree with a final likelihood value of − 5901.668865 is presented. The matrix had 364 distinct alignment patterns, with 7.57% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.227109, C = 0.202541, G = 0.237172, T = 0.333179; substitution rates: AC = 0.869861, AG = 4.823902, AT = 0.890497, CG = 0.397316, CT = 5.110861, GT = 1.000000; gamma distribution shape parameter α = 0.764408. Bootstrap support values for ML equal to or greater than 70% are given above the nodes (left side of ‘/’). Bayesian posterior probabilities (BYPP) equal to or greater than 0.90 are given above the nodes (right side of ‘/’). Ex-type specimens are in black bold and the sequences of the newly described taxa for the present study are placed in blue font to highlight its phylogenetic position in the tree. Voucher numbers for all of the sequences are indicated in the tree
Phylogram generated from maximum likelihood analysis based on LSU sequence data. Fifty vouchers are included in the LSU analyses which comprised 598 characters after alignment. Heinemanomyces sp. ZRL185 was used as the outgroup taxa. The best scoring RAxML tree with a final likelihood value of -1645.607832 is presented. The matrix had 96 distinct alignment patterns, with 0.84% of undetermined characters or gaps. Estimated base frequencies were as follows: A = 0.257065, C = 0.195979, G = 0.302519, T = 0.244438; substitution rates: AC = 0.614943, AG = 6.869678, AT = 2.033440, CG = 0.301621, CT = 17.053330, GT = 1.000000; gamma distribution shape parameter α = 0.748655. Bootstrap support values for ML equal to or greater than 70% are given above the nodes (left side of ‘/’). Bayesian posterior probabilities (BYPP) equal to or greater than 0.90 are given above the nodes (right side of ‘/’). Ex-type specimens are in black bold and the sequences of the newly described taxa for the present study are placed in blue font to highlight its phylogenetic position in the tree. Voucher numbers for all of the sequences are indicated in the tree
In the phylogenetic analyses (Figs. 51 and 52), the newly described taxon, Agaricus albostipitatus, clustered together with well-represented members of the sect. Agaricus of subg. Agaricus confirming its position within that section and subgenus. In the ITS sequence based phylogenetic tree (Fig. 51), two newly generated sequences of A. albostipitatus (CUH AM688 and CAL 1871) cluster together with five unnamed sequences of Agaricus species (ZRL2010103, LD201247, F2272, ZRL2010102 and ZRL2010010) with strong statistical support values (99% BS, 1.00 PP), suggesting all of them to be the morphotype of the same taxon. However, other than the sequence data, no other morphological features were associated with those sequences in any published literature for comparison of those specimens with the newly described species.
Among the five unnamed Agaricus species (ZRL2010103, LD201247, F2272, ZRL2010102 and ZRL2010010), the LSU sequence data of only Agaricus sp. ZRL2010010 was available in the NCBI GenBank nucleotide database. However, in the phylogenetic tree constructed using the LSU sequences (Fig. 52), the sequence deposited after the name Agaricus sp. ZRL2010010 cluster in a different clade with the members of Agaricus subg. Flavoagaricus, which suggests that the sequence might have been mistakenly named after Agaricus sp. ZRL2010010.
Agaricus bambusetorum H. Bashir & Niazi, in Bashir, Chen, Jabeen, Ullah, Khan, Rehman Khan Niazi, Zhang, Khalid, Parra & Callac, Scientific Reports 11(no. 12905): 9 (2021).
Index Fungorum number: IF 828314; Facesoffungi number: FoF 10833; Figs. 53, 54
Pileus 44–95 mm in diam., convex when young, becoming broadly convex to applanate with a slight central depression, surface smooth, white (1A1), reddish brown (8D8, 9D-E8) on bruising, margin split radially; context white (1A1), gradually discoloring to light-yellow when cut. Lamellae 5–6 mm broad, free, crowded with up to two series of lamellulae, pinkish to pinkish-brown when young, dark brown (6F6) on maturity, edge entire, concolorous. Stipe 32–96 × 6–12 mm, cylindrical with slightly bulbous base (up to 15 mm), curved at the base, surface smooth, white, immediately turning brown on brushing. Annulus superous, simple, pendant, membranous, thin, smooth in both sides, fragile, white (1A1). Odour pleasant, mushroomy.
Basidiospores (5.9–)7.4–8.5(–8.8) × (3.5–)4.4–5.6(–5.9) μm (Xmr = 6.9–8.3 × 4.5–5.3 μm, Xmm = 7.8 ± 0.9 × 5.0 ± 0.6 μm, Qmr = 1.5–1.6, Qmm = 1.56 ± 0.1, n = 41 spores, s = 3 specimens), ellipsoid, brown to dark brown when viewed with KOH, smooth, with a prominent apiculus measuring 0.8–1.5 µm long. Basidia 15–22 × 5.5–7.5 µm, clavate to broadly clavate, hyaline, 4-spored, sterigmata 1.5–2.5 µm long. Basidioles 12–17 × 4–6 µm, clavate, hyaline, thin-walled. Cheilocystidia 13–37 × 5–17 µm, pyriform to ovoid or broadly clavate, thin-walled, simple septate at the base, basal cells 10–15 × 3.5–5 µm, cylindrical. Pleurocystidia absent. Pileipellis a cutis, composed of hyphae measuring 4.5–7 µm broad, branched, frequently septate, wider at middle, constricted at the septa, elements 5–12 µm broad with round apices. Stipitipellis hyphae 5–8.5 µm broad, cylindrical, parallel, hyaline, smooth, branched, thin-walled. Annulus hyphae 4–7 µm broad, cylindrical, short branched, hyaline, smooth, thin-walled.
Chemistry: KOH reaction positive, pileus surface pastel yellow (1A4) to light yellow (1A5) when fresh, greyish yellow (4C5) on dry specimen; greenish yellow (1A6-7, 1B6-7) on stipe surface; and light yellow (1A5) on lamellae surface. Schäffer’s reaction negative on dry specimen.
Habitat and distribution: The species grows solitary to scattered on leaf litter mixed soil in the roadside vegetation covered by Phoenix dactylifera and is currently found in Pakistan and India.
Material examined: India, West Bengal, South 24-parganas district, Mathurapur, 22°7′7.24" N, 88°23′34.43" E, alt. 8.0 m asl., on leaf litter mixed soil, 4 August 2019, E. Tarafder, CAL 1872 (new record for India); Mathurapur, 22°7′10.25" N, 88°23′28.78" E, alt. 10.0 m asl., on leaf litter mixed soil, 7 August 2019, E. Tarafder, CUH AM749 (new record); East Midnapur District, Narayangarh, Datan, Jenkapur, 21°53′50.01" N, 87°22′56.45" E, alt. 17.0 m asl., on leaf litter mixed soil, 11 August 2019, A. K. Dutta, CUH AM750 (new record for India).
GenBank numbers: CAL 1872: ITS = OM278635, LSU = ON171824; CUH AM749: ITS = OM278636, LSU = ON171824; CUH AM750: ITS = OM278638, LSU = OM677380.
Notes: Agaricus bambusetorum was originally described from Pakistan (Bashir et al. 2021) in habitat dominated by bamboo forests. However, in case of the present collection, the specimen was collected growing on leaf litter mixed soil in the roadside vegetation covered by Phoenix dactylifera. Besides, the cheilocystidia of the present collection shows slightly longer in size compared to the Pakistani collection. In the phylogenetic analyses (Figs. 51 and 52), three newly generated sequences from the Indian collections clustered together with sequences deposited from Pakistan, suggesting all of them to be the morphotypes of the same taxon.
Among morphologically related taxa, Agaricus biannulatus has a pileus coloured dull to brownish-ochre with ochraceous to brownish-pink scales on its surface, and globose or pyriform to sphaeropedunculate cheilocystidia (Parra et al. 2011). Agaricus hondensis differs from A. bambusetorum by its larger pileus, phenolic odor, smaller basidiospores (3.4–5.7 × 3.0–3.5 µm), and considerably smaller (10–15 × 10–15 µm), subglobose cheilocystidia (Kerrigan 2016).
Leucocoprinus Pat.
Notes: The genus Leucocoprinus has been accepted by Singer (1986) with L. cepistipes as the type species. This genus is characterized by a pluteoid habit, convex to umbonate pileus with floccose pulverulent squamules, a striate-sulcate margin, a very thin context, free lamellae, and the absence of colour changes with ammonia vapour, a central stipe with bulbous-clavate base and annulus, and a white to pale pink spore-print (Singer 1986; Vellinga 2001; Wartchow et al. 2008). The Leucocoprinus species is widely distributed in tropical, subtropical and temperate areas throughout the world as saprobes (Kirk 2008). According to Singer (1986), this genus occupies a position intermediate between Macrolepiota and Leucoagaricus. The generally small-sized basidiomes and the absence of clamp-connections differentiate Leucocoprinus from Macrolepiota, while it can be distinguished from Leucoagaricus by the fragile coprinoid basidiomes, the plicate-sulcate-striate pileal margin, large cheilocystidia, and the presence of pseudoparaphyses (pavement cells) in the hymenium (Singer 1986). Currently, there are 100 accepted species of Leucocoprinus in the Index Fungorum (2022).
Leucocoprinus cretaceus (Bull.) Locq., Bull. mens. Soc. linn. Soc. Bot. Lyon 14: 93 (1945).
Index Fungorum number: IF287726; Facesoffungi number: FoF3137; Fig. 55
Pileus 30–90 mm in diameter, at first ovoid then convex to broadly conical, umbonate in maturity, white (1A1), covered by easily removed, granular floccose, white (1A1) to yellowish white (1A2) squamules, conical to pyramidal on the umbo; margin entire, sulcate. Lamellae distantly free, rather broad, thin; white, edge slightly fimbriate, white (1A1). Stipe 50–80 × 4 mm, broad at apex, gradually enlarged downward to a broadly clavate to somewhat fusiform 6–14 mm broad base; sometimes coarsely farinose to slightly flocculose-farinose below annulus, subfarinose above; white (1A1) to yellowish white (1A2). Annulus white, very soft, rather flaring, median to superior. Spore print white.
Basidiospores 7–11 × 5–8 μm, Q = 1.31–1.47 (n = 50), dextrinoid, ellipsoid to amygdaliform, thick-walled, with an apical germ pore covered with a hyaline lens. Basidia 16–24 × 8–12 μm, pyriform to clavate with a bulbous base, hyaline, thin-walled, bearing four sterigmata, 1.0–3.0 μm long, surrounded by 4 pseudoparaphyses. Pseudoparaphyses 10–13 × 7–10 μm, sphaeropedunculate to broadly clavate to pyriform, very broad point of attachment. Pleurocystidia absent. Cheilocystidia 25–65 × 6.5–18 μm, thin-walled, hyaline, subcylindrical to narrowly fusiform to slightly narrowly lageniform, mucronate, rarely obtuse, moderately pedicellate. Pileipellis made up of hyaline hyphae with smooth walls and cylindric terminal elements with excrescences, branched in different shapes (H, T and Y), 24–45 × 7.5–15 μm. Stipitipellis a cutis of narrowly cylindrical, 2–7 μm broad hyphae. Clamp connections absent.
Habit and distribution: Solitary to gregarious, on decomposed wood. Known from Argentina, Brazil, Europe, North America, Sri Lanka and Thailand (Candusso and Lanzoni 1990; Birkebak 2010; Ferreira and Cortez 2012; Niveiro et al. 2012; Tipbromma 2017; this study).
Material examined: Thailand, Chiang Mai Province, Muang District, Chiang Mai University, 18°48′14″N 98°57′14″E, elevation 331 m, solitary to gregarious on decomposed wood, 27 July 2019, J. Kumla and N. Suwannarach, SDBR-CMUNK0559 (new record for Thailand); Sukhothai Province, Si Satchanalai National Park, Natural trail; 17°55′12″N 99°48′44″E, elevation 277 m, solitary to gregarious on decomposed wood, 23 August 2020, N. Wannathes, J. Kumla, N. Suwannarch and S. Khuna, SDBR-CMUNW1461 (new record for Thailand).
GenBank numbers: SDBR-CMUNK0559: ITS = OP503474; SDBR-CMUNW1461: ITS = OP503476.
Notes: Leucocoprinus cretaceus is easily recognized by its fleshy white basidiomes covered by floccose squamules that are easily removed when touched and by a fusoid stipe inflated toward the base. Phylogenetic analysis indicated that L. cretaceus forms monophyletic clade and clearly separated from other species of Leucocoprinus (Fig. 56). Morphologically, this species is related to L. squamulosus and L. cepistipes. Leucocoprinus cretaceus differs from L. squamulosus by its which lacks an annulus and an inflated stipe base (Dennis 1952; Wartchow et al. 2008). Leucocoprinus cepistipes separate from L. cretaceus based on the ochraceus to pale brown squamules covering the pileus surface (Candusso and Lanzoni 1990; Wartchow et al. 2008; Rother and Silveira 2009).
Phylogenetic tree derived from maximum likelihood analysis of ITS gene of 19 sequences and the aligned dataset was comprised of 787 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00563. A best scoring RAxML tree was established with a final ML optimization likelihood value of -3057.9735. The matrix had 296 distinct alignment patterns with 13.97% undetermined characters or gaps. Estimated base frequencies were found to be: A = 0.2280, C = 0.2242, G = 0.2429, T = 0.3047; substitution rates AC = 0.4870, AG = 4.0084, AT = 1.4356, CG = 0.2018, CT = 3.7781, GT = 1.0000. Leucoagaricus laoensis HNL501802 and Leucoagaricus umbonatus LAHSHL1 were used as outgroup. Numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap values ≥ 70% and PP ≥ 0.90 are shown at each branch. The bar represents 0.1 substitutions per nucleotide position. Type strains are in bold. The newly generated sequences are indicated in blue
Cortinariaceae R. Heim ex Pouzar.
Notes: Cortinariaceae typified by Cortinarius violaceus belongs to Agaricales. It is a large family with a worldwide distribution (Liimatainen et al. 2022). The basidiomes range from small to large size and from agaricoid to sequestrate form. Species of the group are characterized by a cobweb-like partial veil (cortina), ornamented basidiospores and a cinnamon brown spore print. They are important ectomycorrhizal fungi forming associations with different trees and shrubs: Fagaceae, Salicaceae, Caesalpiniaceae, Cistaceae, Dipterocarpaceae, Myrtaceae, Rhamnaceae, Rosaceae and Pinaceae, as well as some herbaceous plants in the Cyperaceae and Polygonaceae. The classification of the group was recently revised by Liimatainen et al. (2022) and the family was divided into ten genera: Aureonarius, Austrocortinarius, Calonarius, Cortinarius, Cystinarius, Hygronarius, Mystinarius, Phlegmacium, Thaxterogaster and Volvanarius.
Calonarius Niskanen & Liimat.
Notes: This species-rich genus with an estimated around 200 species is only known from the Northern Hemisphere. The species are predominantly calcicolous or calciphilous, many are rare with narrow ecological preferences and thus are included in national red lists in several countries. Typical for the members of this genus are medium- to large-sized, pileocarpic, often brightly coloured basidiomes with a more or less, usually distinctly marginated bulb at the base of the stipe. The pileus is viscid to glutinous, and the stipe is dry. The basidiospores are amygdaloid to citriform and coarsely verrucose, and the pileipellis is simplex. Part of the species have a positive KOH-reaction. The genus includes three subgenera: Calonarius, Calochroi and Fulvi (Liimatainen et al. 2022).
Calonarius caesiofulvus Niskanen, Liimat. & M. E. Sm., sp. nov.
Index Fungorum number: IF 900300; Facesoffungi number: FoF 14857; Figs. 57a, 58a, 59
Basidiomes of the species of the genus Calonarius. a Calonarius caesiofulvus (K-M 001434088, holotype). b Calonarius nobilis (K-M 001434089, holotype). c Calonarius pacificus (K-M 001434090, holotype). d Calonarius pulcher (K-M 001434092, holotype). e Calonarius subcorrosus (K-M 001434093, holotype). Photographs: a Matthew E. Smith, b–e Kare Liimatainen
Basidiospores of the species of the genus Calonarius. a Calonarius caesiofulvus (K-M 001434088, holotype). b Calonarius nobilis (K-M 001434089, holotype). c Calonarius pacificus (K-M 001434090, holotype). d Calonarius pulcher (K-M 001434092, holotype). e Calonarius subcorrosus (K-M 001434093, holotype). Drawings: Tuula Niskanen. Scale bars: 10 µm
The best scoring RAxML tree of the genera Calonarius and Mystinarius based on the ITS region. Related sequences were retrieved from GenBank. Twenty-five specimens were included in the analysis of the ITS region which comprises 663 characters after alignment. The tree is rooted with Mystinarius. Estimated base frequencies were: A = 0.246760, C = 0.199165, G = 0.201141, T = 0.352933; substitution rates AC = 0.954938, AG = 3.189949, AT = 1.426518, CG = 0.990207, CT = 5.977045, GT = 1.000000, gamma distribution shape parameter α = 0.312392. Maximum likelihood bootstrap values higher than 50% are given at the nodes. The holotype specimens retrieved from the GenBank are in bold and black. The new species are in bold and blue. The subgenus of the species is indicated after the collection/GenBank no. of each specimen
Etymology: The name refers to the colours of the basidiomes.
Holotype: K-M 001434088.
Pileus 4–10 cm in diam., at first hemispherical, then low convex to almost plane with a long-incurved margin, innately fibrillose, at first pale greyish purple, becoming yellow on the margin and brown in centre, viscid. Lamellae crowded, yellow. Stipe 4–7 cm long, 1–2 cm thick at the apex, marginate bulbous, pale yellow, covered up to halfway by a bluish purple universal veil. Context in pileus white to very pale yellow, in stipe, and especially at the base of the stipe, yellow. Universal veil bluish purple. Mycelium white to yellow. Odour not recorded. Basidiospores 9.5–11 × 5–5.5 µm, narrowly amygdaloid-citriform, moderately to strongly coarsely verrucose. Some basidia with brownish purplish-red granulose contents in 5% KOH. ITS sequence (GenBank ON843392, ex holotype) distinct from other members of Calonarius and with 97% similarity to the closest known species C. alcalinophilus.
Habitat and distribution: In deciduous forests with Fagaceae and so far known from Florida, USA.
Material examined: USA. Florida, Gainesville, University of Florida campus, under oak, 12 January 2015, coll. Richard Kneal, T. Niskanen 14–167, K-M 001434088 (holotype in K-M; isotype in H); Gainesville, Rock Creek Neighborhood, under oaks and pines, 10 January 2017, coll. Matthew E. Smith, MES-2020, FLAS-F-60313 (FLAS).
GenBank numbers: TN14-167: ITS = ON843392; FLAS-F-60313: ITS = MF074799.
Notes: The species can be recognized by the combination of yellow lamellae, pale yellow stipe, initially greyish purple pileus and narrowly amygdaloid-citriform, 9.5–11 × 5–5.5 µm basidiospores. It belongs to Calonarius subgenus Fulvi section Fulvi.
Calonarius nobilis Niskanen & Liimat., sp. nov.
Index Fungorum number: IF900301; Facesoffungi number: FoF 14858; Figs. 57b, 58b, 59
Etymology: The species is rare and included in the red list e.g. in Sweden, thus the name nobilis meaning nobel, distinct.
Holotype: K-M 001434089.
Pileus 3–8 cm in diam, at first hemispherical, then low convex to almost plane, at first greenish yellow, soon greenish yellow brown, viscid. Lamellae crowded, greenish yellow. Stipe 4–7 cm long, 1–2 cm thick at the apex, with a marginate bulb, greenish yellow with some purplish tint. Context in most parts with white with a purplish tint, except in the bulb and cortex bright greenish yellow. Universal veil greenish yellow, on the margin of the bulb. Mycelium white to yellow. Odour slightly yeast-like. KOH-reaction on pileus, on context of the base of the stipe, and mycelium red. Basidiospores (11.5–)12–13.5(–14) × 8–9 µm, broadly citriform, moderately coarsely verrucose. Some basidia with dark brownish purplish-red contents. ITS sequence (GenBank ON843393, ex holotype) distinct from other members of Calonarius and with 96% similarity to the closest known species.
Habitat and distribution: In coniferous forests on calcareous ground and so far known from Europe: Estonia, Finland, France, Germany and Spain.
Material examined: Estonia, Hiiumaa, Pÿhalepa, NE of Suuremõisa, Vahtrepa, Kallaste pank, dryish to damp grass-herb Picea forest with Pinus, Corylus, Betula, Populus tremula and deciduous bushes under the precipice, 17 Sepetember 2001, T. Niskanen, I. Kytövuori 01-0051 (H). FINLAND, Ahvenanmaa, Jomala, Önningby, N of Södervik, mesic (to moist) spruce (Picea abies) forest with some Pinus sylvestris and Betula on calcareous ground, 27 October 2006, K. Liimatainen, T. Niskanen 06–312, 001434089 (holotype in K-M; isotype in H).
GenBank numbers: IK01-0051: ITS = ON843432; TN06-312: ITS = ON843393.
Notes: Typical characters for Calonarius nobilis are the greenish yellow colours in pileus, lamellae, stipe and universal veil combined with a purplish tint on stipe and context of the pileus and stipe, at least when young. The spores are broadly citriform and large. The species belongs to C. subgenus Calonarius and has previously been called C. cedretorum in the Nordic countries.
Calonarius pacificus Niskanen, Liimat. & Bojantchev, sp. nov.
Index Fungorum number: IF 900302; Facesoffungi number: FoF 14859; Figs. 57c, 58c, 59
Etymology: The species was first found in California, a state in the Western United States by the Pacific Ocean.
Holotype: K-M 001434090.
Pileus 5–9 cm in diam, at first hemispherical, then low convex to almost plane, can be quite purplish when young but becoming more and more ochre brown with age, with small, dark yellowish brown patches in the centre, glutinous. Lamellae crowded, pale brownish purple when young, becoming somewhat darker brown and losing the purple tint when old. Stipe 6–10 cm long, 1–2 cm thick at the apex, up to 4.5 cm at the base, with a marginate bulb, completely purple or upper part of the stipe purple when young, later very pale ochre brown. Context in pileus at first purple/white marbled hygrophanous, becoming pale yellow with age and on exposure, in the stipe or apex of the stipe purple/white, at the base of the stipe brownish ochre/white marbled hygrophanous. Mycelium white with yellowish brown patches. Universal veil not recorded. Odour indistinct. Basidiospores 12.5–14.5 × 6.5–7.5 µm, amygdaloid-citriform to citriform, moderately, coarsely verrucose. Part of the basidia with pale yellowish contents. ITS sequence (GenBank ON843394, ex holotype) distinct from other members of Calonarius and with 97% similarity to the closest known species C. suaveolens.
Habitat and distribution: In oak forests and so far known from California, USA.
Material examined: USA, California, Humboldt Co., Willow Creek, camping site, live oak forest, 30 November 2012, K. Liimatainen, J. Olsson, T. Niskanen 12–172, 001434090 (holotype in K-M; isotype in H); Santa Cruz Co., Scotts Valley, NAMA Foray site 12, 15 December 2012, K. Liimatainen, T. Niskanen 12–332, 001434091 (K-M).
GenBank number: ITS = ON843394.
Notes: Characteristic for Calonarius pacificus are the purple colours in all parts of basidiomes, at least when young, large basidiospores and habitat with oaks in Western North America. The morphology supports its placement in the C. subgenus Calochroi as a sister species to European C. suaveolens, although the placement of C. suaveolens in the C. subgenus Calochroi was not always been well supported by the previous phylogenetic studies (Frøslev et al. 2007; Liimatainen et al. 2022).
Calonarius pulcher Niskanen, Liimat., & Bojantchev, sp. nov.
Index Fungorum number: IF 900303; Facesoffungi number: FoF 14860; Figs. 57d, 58d, 59
Etymology: The epiteth pulcher means beautiful, nobel.
Holotype: K-M 001434092.
Pileus 8–12 cm in diam, at first hemispherical, then low convex, reddish brown, edge purple when young, yellow when old. Lamellae crowded, at first pale brownish grey, later pale brown. Stipe 6–8 cm long, 2–3 cm thick at the apex, up to 5 cm at the base, with a marginate bulb, yellowish white, sometimes with a purple tint when young, base becoming reddish brown when handled. Context in pileus and lower part of the stipe yellow, at the upper part of stipe white, with a purplish tint at least when young. Universal veil yellow, at the margin of the bulb. Mycelium white (to yellow). Odour indistinct or very faintly aniseed. Basidiospores 10.5–12 × 6.5–7.5 µm, broadly citriform, moderately to strongly, coarsely verrucose. Some basidia with brownish purplish-red granulose contents in 5% KOH. ITS sequence (GenBank ON843396, ex holotype) distinct from other members of Calonarius and with 97% similarity to the closest known species C. saxamontanus.
Habitat and distribution: In coniferous forests on calcareous ground and so far known from Alaska, USA.
Material examined: USA, Alaska, Fairbanks, Ballaine Lake trails, NE of University campus, Picea dominated forest with some Betula, Populus, Salix and Alnus, on rich ground, 14 August 2011, K. Liimatainen, T. Niskanen 11-060, 001434092 (holotype in K-M; isotype in H).
GenBank number: ITS = ON843396.
Notes: Calonarius pulcher has pale brownish grey lamellae when young, purple tints occur in the pileus and stipe at least when young, and the basidiospores are broadly citriform and rather large. The pileus is reddish brown with a purple edge when young and yellow when old. The species belongs to Calonarius subgenus Calonarius.
Calonarius subcorrosus Niskanen & Liimat., sp. nov.
Index Fungorum number: IF 900304; Facesoffungi number: FoF 14861; Figs. 57e, 58e, 59
Etymology: The species is close to C. calojanthinus (C. corrosus ss auct.).
Holotype: K-M 001434093.
Pileus 3–6 cm in diam, hemispherical, then low convex to almost plane, ochre brown from the centre with small brownish spots, paler and more yellowish towards the margin, margin bright citrus yellow, somewhat viscid. Lamellae crowded, very pale brownish grey when young, later brown. Stipe 4–6 cm long, 1.3–1.7 cm thick at the apex, up to 2.5 cm at the base, with a marginate bulb, white with a very faint, yellow tint. Context white. Universal veil not recorded. Mycelium white. Odour indistinct. Basidiospores 10.5–12 × 6–7 µm, amygdaloid-citriform to citriform, moderately to strongly, coarsely verrucose. Basidia hyaline, with hyaline oil drops. ITS sequence (GenBank ON843397, ex holotype) distinct from other members of Calonarius and with 98% similarity to the closest known species, C. calojanthinus (C. corrosus ss auct.).
Habitat and distribution: In coniferous forests on calcareous ground and and so far known from China and North America, Alaska, Arizona and Mexico.
Material examined: Mexico, Veracruz, Cofre de Perote, coniferous forest, 1965, H. L. Evans no. 4 (K(M)). USA, Alaska, Fairbanks, trails at the NW side of the campus, at the end of Yukon Road (trail starting from parking place), Picea dominated forest with some Betula, Populus and Alnus, on rich ground, 15 August 2011, K. Liimatainen, T. Niskanen 11-083, 001434093 (holotype in K-M; isotype in H).
GenBank numbers: HLE 4: ITS = ON843431; TN11-083: ITS = ON843397.
Notes: Typical characters for the species are the lack of purplish tints in the basidiomes, ochre brown pileus with a bright yellow margin, white stipe with a very faint yellow tint, and white context. The species belongs to the C. subgenus Calochroi. The North American-European sister species C. calojanthinus (C. corrosus ss auct.) has pale ochraceous to cream-coloured pileus.
Cortinarius (Pers.) Gray.
Notes: This is the most species-rich genus of the family with an estimated over 2000 species and a worldwide distribution. The species are characterized by mainly stipitocarpic development and a pileipellis duplex with a more or less developed hypoderm. The basidiomes range from very small to large size and from dry to glutinous contexture. Majority of species have brown colours in their basidiomes, but some species also have purple, red, yellow, orange, green or black colours. Eleven subgenera are recognized: Cortinarius, Camphorati, Dermocybe, Illumini, Infracti, Iodolentes, Leprocybe, Myxacium, Orellani, Paramyxacium and Telamonia (Liimatainen et al. 2020, 2022).
Cortinarius flaureifolius Niskanen, Liimat. & M. E. Sm., sp. nov.
Index Fungorum number: IF 900305; Facesoffungi number: FoF 14862; Figs. 60a, 61a, 62
Basidiomes of the species of the genera Cortinarius, Hygronarius and Mystinarius. a Cortinarius flaureifolius (K-M 001434094, holotype). b Cortinarius floridaensis (K-M 001434095, holotype). c Cortinarius subiodes (K-M 001434108, holotype). d Hygronarius californicus (K-M 001434114, holotype). e Mystinarius ochrobrunneus (CR 4064459, holotype). Photographs: a Matthew E. Smith, b–d Kare Liimatainen, e Joseph F Ammirati
Basidiospores of the species of the genera Cortinarius, Hygronarius and Mystinarius. a Cortinarius flaureifolius (K-M 001434094, holotype). b Cortinarius floridaensis ((K-M 001434095, holotype). c Cortinarius subiodes (K-M 001434108, holotype). d Hygronarius californicus (K-M 001434114, holotype). e Mystinarius ochrobrunneus (CR 4064459, holotype). Drawings: Tuula Niskanen. Scale bars: 10 µm
The best scoring RAxML tree of the genera Cortinarius and Hygronarius based on the ITS region. Related sequences were retrieved from GenBank. Forty-nine specimens were included in the analysis of the ITS region which comprises 732 characters after alignment. The tree is rooted with Hygronarius. Estimated base frequencies were: A = 0.242037, C = 0.200516, G = 0.214242, T = 0.343206; substitution rates AC = 1.323688, AG = 3.480113, AT = 1.611311, CG = 0.756641, CT = 4.952595, GT = 1.000000, gamma distribution shape parameter α = 0.362036. Maximum likelihood bootstrap values higher than 50% are given at the nodes. The holotype specimens retrieved from the GenBank are in bold and black. The new species are in bold and blue. The section of the species is indicated after the collection/GenBank no. of each specimen
Etymology: Derived from the name of the sister species Cortinarius aureifolius and the location Florida.
Holotype: K-M 001434094.
Pileus 1.5–3.5 cm in diam, convex to plane, tomentose fibrillose, brown. Lamellae medium spaced, at first yellow, later brown. Stipe 2–4 cm long, up to 0.4 cm thick at the apex, cylindrical, at first pale greyish white silky fibrillose, later pale yellowish to ochre brown. Context not recorded. Universal veil ochre (brown), quite sparse. Mycelium not recorded. Odour indistinct. Basidiospores 11–13 × 3.5–4 µm, boletoid, almost smooth. Basidia hyaline. ITS sequence (GenBank ON843398, ex holotype) distinct from other members of C. subgen. Dermocybe and with 92% similarity to the closest known species C. aureifolius.
Habitat and distribution: With Pinus on sandy soil and so far only known from Florida, USA.
Material examined: USA, Florida, Melrose, Ordway-Swisher Reserve, in sandy soil with mostly pines but some oaks, 1 December 2014, M. E. Smith, MES811, 001434094 (holotype in K-M; isotype in FLAS).
GenBank number: ITS = ON843398.
Notes: In the field the species looks like a representative of Cortinarius sect. Dermocybe or an Inocybe. The species is easy to recognize by the narrow, boletoid basidiospores. The sister species C. aureifolius has yellow orange to ochraceus orange lamellae when young. For an unambiguous identification ITS sequence is recommended.
Cortinarius floridaensis Niskanen, Liimat. & M. E. Sm., sp. nov.
Index Fungorum number: IF 900306; Facesoffungi number: FoF 14863; Figs. 60b, 61b, 62
Etymology: The species was first found from Florida, USA.
Holotype: K-M 001434095.
Pileus 1.5–5.5 cm in diam, at first hemispherical, then low convex to almost plane, often with a quite low and broad umbo, distinctly innately fibrillose, greyish brown when young, later more olive yellow brown, hygrophanous. Lamellae medium spaced, brown to dark brown. Stipe 2.5–5.5 cm long, 0.5–0.8 cm thick at the apex, cylindrical to clavate, blue at the apex, greyish white fibrillose lower down, becoming pale yellowish brown with age. Context in pileus brown, in stipe apex pale greyish brown, in the rest of the stipe brown. Universal veil ochraceous yellow to ochraceous brown, forming a thin sock or some bands on the lower half of the stipe. Mycelium white. Odour raphanoid. Basidiospores 8–9 × 5.5–6 µm, broadly obovoidly ellipsoid, rather strongly verrucose. Basidia hyaline, some with pale brownish-yellow contents in 5% KOH. ITS sequence (GenBank ON843399, ex holotype) distinct from other members of C. subgen. Telamonia and with 90% similarity to the closest known species C. alces.
Habitat and distribution: In mixed forests with Fagaceae and so far known from Eastern USA: Florida, Massachusetts, Michigan and Tennessee.
Material examined: USA, Florida, Wakulla Co., Crawfordville, 306 Wakulla Beach Road, under Pinus, mixed deciduous and Pinus forest with some Quercus virginiana, Magnolia grandiflora and sweetgum, sandy soil, on calcareous bedrock, 24 December 2014, K. Liimatainen, T. Niskanen 14-060, 001434096 (K-M). loc. cit. 27 December 2014, K. Liimatainen, T. Niskanen 14-096, 001434097 (K-M). loc. cit. 30 December 2014, K. Liimatainen, T. Niskanen 14–112, 001434098 (K-M). Tallahassee Co., Lake Talquin State Forest, by the road 267, Bear Creek Educational forest, Fagus, Carpinus, Castanea, Pinus, laurel oak, live oak, white oak, on sandy soil, 25 December 2014, K. Liimatainen, T. Niskanen 14-068, 001434099 (K-M). loc. cit. mostly evergreen oaks, 29 December 2014, K. Liimatainen, T. Niskanen 14–107, 001434100 (K-M). Gainesville, Sweetwater, Eastern trail, Xeric oak forest with some pines, 9 January 2015, K. Liimatainen, T. Niskanen 14–135, 001434101 (K-M). High Springs, O’Leno State Park, Xeric oak forest with pine hammock, 11 January 2015, K. Liimatainen, T. Niskanen 14–139, 001434102 (K-M). loc. cit. 11 January 2015, K. Liimatainen, T. Niskanen 14–144, 001434103 (K-M). loc. cit. 11 January 2015, K. Liimatainen, T. Niskanen 14–150, 001434104 (K-M). loc. cit. 11 January 2015, K. Liimatainen, T. Niskanen 14–152, 001434105 (K-M). loc. cit. 11 January 2015, K. Liimatainen, T. Niskanen 14–163, 001434106 (K-M). Columbia Co., River Rise State Park, laurel oak, live oak, Pinus, Magnolia, sweetgum, 13 January 2015, K. Liimatainen, T. Niskanen 14–174, 001434095 (holotype in K-M; isotype in H). Paynes Prairie Preserve State Park, parking place in east side of the road at Puggy Road junction, Park-like oak spp. dominated forest, 14 January 2015, K. Liimatainen, T. Niskanen 14–244, 001434107 (K-M).
GenBank numbers: TN14-174: ITS = ON843399; TN14-060: ITS = ON843400; TN14-096: ITS = ON843401; TN14-112: ITS = ON843402; TN14-068: ITS = ON843403; TN14-107: ITS = ON843404; TN14-135: ITS = ON843405; TN14-139: ITS = ON843406; TN14-144: ITS = ON843407; TN14-150: ITS = ON843408; TN14-152: ITS = ON843409; TN14-163: ITS = ON843410; TN14-224: ITS = ON843411.
Notes: Although a brown species of C. subgenus Telamonia, C. floridaensis has a combination of characters that make it rather easy to identify. It is a medium-sized species with an innately fibrillose, olive yellow brown pileus, the apex of the stipe is blue, the universal veil is ochraceous yellow to ochraceous brown, and the smell is raphanoid. The systematic position of the species in the subgenus was not resolved, but the species is most reminiscent of those in sections Brunneotincti, Valgi and Bovini.
Cortinarius subiodes Niskanen, Liimat. & M. E. Sm., sp. nov.
Index Fungorum number: IF 900307; Facesoffungi number: FoF 14864; Figs. 60c, 61c, 62
Holotype: K-M 001434108.
Etymology: The name refers to affinity with Cortinarius iodes.
Pileus 3–7.5 cm in diam, at first hemispherical, then low convex to almost plane, greyish blue to purple, and becoming pale yellow from the centre with age, innately fibrillose, somewhat slimy. Lamellae subcrowded to medium spaced, greyish blue when young, later greyish brown. Stipe 2–4.5 cm long, 0.7–1.5 cm thick at the apex, more or less marginate bulbous, white fibrillose. Context faint greyish or yellowish white with bluish tints. Universal veil glutinous, blue, forming a sock-like sheet or some belts at the bottom of stipe. Mycelium white. Odour in lamellae sweet. Basidiospores 9.5–10.5 × 6–6.5 µm, ellipsoid, moderately verrucose. Basidia hyaline. ITS sequence (GenBank ON843412, ex holotype) distinct from other members of Cortinarius and with 98% similarity to the closest known species C. iodes.
Habitat and distribution: In mixed forest (oaks and Pinus) and so far known from Florida, USA.
Material examined: USA, Florida, Franklin Co., by the bridge, when entering to Franklin Co., boat ramp place, young oak-dominated forest with some Pinus, 26 December 2014, K. Liimatainen, T. Niskanen 14-075, 001434110 (K-M). Wakulla Co., Crawfordville, 306 Wakulla Beach Road, under Pinus, mixed deciduous and Pinus forest with some Quercus virginiana, Magnolia grandiflora and sweetgum, sandy soil, on calcareous bedrock, 27 December 2014, K. Liimatainen, T. Niskanen 14-078, 001434111 (K-M). Otter Lake Road, young evergreen oak and Pinus forest, on sandy soil, 28 December 2014, K. Liimatainen, T. Niskanen 14-099, 001434112 (K-M). Tallahassee Co., Lake Talquin State Forest, by the road 267, Bear Creek Educational forest, mostly evergreen oaks, 29 December 2014, K. Liimatainen, T. Niskanen 14–106, 001434113 (K-M). Alachua Co., Gainesville, Sweetwater Preserve, Eastern trail, Xeric oak forest with some pines, 9 January 2015, K. Liimatainen, T. Niskanen 14–132, 001434109 (K-M). loc. cit. 15 January 2015, K. Liimatainen, T. Niskanen 14–286, 001434108 (holotype in K-M; isotype in H).
GenBank numbers: TN14-286: ITS = ON843412; TN14-132: ITS = ON843413; TN14-075: ITS = ON843414; TN14-078: ITS = ON843415; TN14-099: ITS = ON843416; TN14-106: ITS = ON843417.
Notes: Cortinarius subiodes is easy to spot in the field due to its bright greyish blue to purple colours. The pileus and universal veil are slimy and the basidiospores are ellipsoid. The species belongs to C. cf. subgenus Camphorati, section Delibuti. The sister species C. iodes can be distinguished by relatively longer, cylindrical to slightly clavate stipe.
Hygronarius Niskanen & Liimat.
Notes: This small genus with bihemispheric distribution has an estimated 10 species including small- to medium-sized, stipitocarpic, agaricoid (telamonioid) species with brownish colours. The stipe is dry and the pileus is dry or viscid and hygrophanous. The basidiospores are subglobose or ellipsoid and the pileipellis is duplex with a more or less developed hypoderm. Two subgenera, Hygronarius and Visincisi are recognized (Liimatainen et al. 2022).
Hygronarius californicus Niskanen, Liimat., Bojantchev & Ammirati, sp. nov.
Index Fungorum number: IF 900308; Facesoffungi number: FoF 14865; Figs. 60d, 61d, 62
Etymology: The species was first found from California, USA.
Holotype: K-M 001434114.
Pileus 1.5–3.5 cm in diam, at first hemispherical, then low convex to almost plane, sometimes with a broad and low umbo, red brown, strongly hygrophanous. Lamellae medium spaced, pale brown. Stipe 4–6 cm long, 0.5–1.0 cm thick at the apex, cylindrical, yellowish brown to ochre brown fibrillose. Context yellow brown to red brown. Universal veil and cortina absent. Mycelium white. Odour indistinct. Basidiospores 6.5–7.5 × 5–5.5(–5.7) µm, somewhat obovoid-subglobose to very broadly obovoid-ellipsoid, moderately to almost strongly verrucose. ITS sequence (GenBank ON843418, ex holotype) distinct from other members of Hygronarius and with 98% similarity to the closest known species H. renidens.
Habitat and distribution: In mixed coniferous forests and so far known from North America: Alberta, British Columbia, California, Oregon and Washington.
Material examined: Canada, Alberta, Hinton, 05 September 2011, T. Niskanen 11–381, 001434115 (K-M). USA, California, Mendocino Co, Jackson Demonstration State Forest, crossing of the roads 408 and 409, mixed conifer dominated forest (Tsuga), 17 November 2012, T. Niskanen 12-080, 001434114 (holotype in K-M; isotype in H). Oregon, Detroit, Breitenbush, surroundings of the Breitenbush Hot Springs Retreat & Conference centre, mixed coniferous forest of Tsuga heterophylla and Pseudotsuga mentziesi with some Castanopsis chrysophylla, 12 October 2007, K. Liimatainen, T. Niskanen 07–482, 001434116 (K-M). Washington, Mount Baker, Easy Pass trailhead, coniferous forest (Tsuga heterophylla, Abies and some Picea engelmannii), 28 September 2009, K. Liimatainen, T. Niskanen 09-017, 001434117 (K-M).
GenBank numbers: TN12-080: ITS = ON843418; TN11-381: ITS = ON843419; TN07-482: ITS = ON843420; TN09-017: ITS = ON843421.
Notes: Hygronarius californicus is characterised by the red brown pileus, absence of universal veil, and small, obovoid-subglobose to very broadly obovoid-ellipsoid basidiospores. The spores are slightly longer than those in the North American-European sister species H. renidens (6–7 × 5–5.5 µm), but for an unambiguous identification an ITS sequence is needed. Hygronarius californicus belongs to the H. subgen. Hygronarius.
Mystinarius Niskanen & Liimat.
Notes: The species of this small (< 5 species), bihemispheric genus have medium-sized, stipitocarpic, agaricoid (myxacioid/phlegmacioid) basidiomes with a yellow to reddish brown, somewhat viscid to almost dry pileus, and a white to yellow, dry or very slightly viscid stipe. The basidiospores are medium-sized and the pileipellis is duplex (Liimatainen et al. 2022).
Mystinarius ochrobrunneus Ammirati, Halling, Niskanen & Liimat., sp. nov.
Index Fungorum number: IF 900309; Facesoffungi number: FoF 14866; Figs. 60e, 61e
Etymology: Named for the general coloration of the basidiomes.
Holotype: CR 4064459.
Pileus 2.5–4 cm in diam, campanulate to obtuse-umbonate, rarely plano-umbonate, margin often irregular, 1/3–1/2 pellucid striate, orange yellow brown to orange brown or reddish brown, margin ochraceous brown to light ochraceous tawny with darker brown striations or more orange ochraceous brown, edge sometimes very pale, viscid.
Lamellae crowded to subcrowded, adnexed, bright ochraceous brown, pallid to whitish in places. Stipe 7–9 cm long, 0.4–0.6 cm thick at the apex, up to 1.3 cm at the base, clavate-bulbous, at first white then yellowish, colour often watery, viscid at mid-stipe when young. Context to 3–4 mm on disc, very thin over lamellae, watery and ± concolorous (watery brown), in stipe hollow, cortex white to watery white, in hollow ochraceous to pale watery yellowish. Universal veil white, forming thin patches on the stipe. Mycelium white. Odour fungoid. Taste of gluten and context bitter. Basidiospores 8–9 × 5–5.5 µm, amygdaloid to somewhat ellipsoid, finely verrucose. ITS sequence (GenBank ON843422, ex holotype) distinct from M. lustrabilis with 98% similarity.
Habitat and distribution: In Quercus spp. forests and so far known from Costa Rica.
Material examined: Costa Rica, San José, Pérez Zeledón, Paramo, R.F. Los Santos, San Gerardo de Dota, Albergue de Montaña Savegre, 2200–2500 m, Quercus spp. gregarious in deep leaf litter, 7 June 2004, J. F. Ammirati, 4064459 (holotype in CR; isotypes in NY & K).
GenBank number: ITS = ON843422.
Notes: Mystinarius ochrobrunneus is a rather small and fragile species with bitter taste, viscid orange brown pileus, white, somewhat viscid stipe when young, and amygdaloid to somewhat ellipsoid basidiospores. The sister species M. lustrabilis occurs in Europe. Morphologically the species also resembles those of Thaxterogaster sect. Vibratiles.
Phlegmacium (Fr.) Wünsche.
Notes: This predominantly Northern Hemispheric genus is estimated to include around 200 species. Basidiomes are medium- to large-sized, rarely small. Typical for the species are a dry stipe and viscid to glutinous pileus, or if dry, then the KOH reaction in the context of the pileus is usually yellow. Most species have a pileipellis duplex with a more or less developed hypoderm but the species of the subgenus Cyanicium and some lineages of P. subgen. Phlegmacium have a simplex pileipellis. The genus includes four subgenera: Phlegmacium, Bulbopodium, Carbonella and Cyanicium (Liimatainen et al. 2022).
Phlegmacium fennicum Kekki, Kytöv., Niskanen & Liimat., sp. nov.
Index Fungorum number: IF 900310; Facesoffungi number: FoF 14867; Figs. 63a, 64a, 65
Basidiomes of the species of the genera Phlegmacium and Thaxterogaster. a Phlegmacium fennicum (K-M 001434119, holotype). b Phlegmacium pallidocaeruleum (K-M 001434120, holotype). c Thaxterogaster americanoporphyropus (K-M 001434121, holotype). d Thaxterogaster obscurovibratilis (K-M 001434124, holotype). Photographs: a Tapio Kekki, b–d Kare Liimatainen
Basidiospores of the species of the genera Phlegmacium and Thaxterogaster. a Phlegmacium fennicum (K-M 001434119, holotype). b Phlegmacium pallidocaeruleum (K-M 001434120, holotype). c Thaxterogaster americanoporphyropus (K-M 001434121, holotype). d Thaxterogaster obscurovibratilis (K-M 001434124, holotype). Drawings: Tuula Niskanen. Scale bars: 10 µm
The best scoring RAxML tree of the genera Phlegmacium and Thaxterogaster based on the ITS region. Related sequences were retrieved from GenBank. Twenty-six specimens were included in the analysis of the ITS region which comprises 671 characters after alignment. The tree is rooted with Thaxterogaster. Estimated base frequencies were: A = 0.246332, C = 0.202234, G = 0.200934, T = 0.350500; substitution rates AC = 1.534815, AG = 3.904253, AT = 1.485681, CG = 0.452034, CT = 6.187457, GT = 1.000000, gamma distribution shape parameter α = 0.257017. Maximum likelihood bootstrap values higher than 50% are given at the nodes. The holotype specimens retrieved from the GenBank are in bold and black. The new species are in bold and blue. The section of the species is indicated after the collection/GenBank no. of each specimen
Etymology: The species has thus far only been found from Finland.
Holotype: K-M 001434119.
Pileus 4.5–7 cm in diam, at first hemispherical, then low convex to almost plane, yellowish brown with brown fibrils, with hygrophanous streaks. Lamellae subcrowded, pale brown to pale greyish brown when young, brown when old. Stipe 6–8 cm long, 1–1.5 cm thick at the apex, up to 3 cm at base, bulbous with a somewhat marginate to more rarely rounded bulb, at first very pale yellowish brown, later pale yellowish brown, fibrillose. Context pale yellowish brown. Universal veil pale brown, at the margin of the bulb. Mycelium white. Odour indistinct. Taste somewhat bitter. KOH reaction in pileus, in the context of bulb and in bulbipellis negative. Basidiospores (8.5–)9–10(–10.5) × 5–6(–6.5) µm, amygdaloid-citriform to citriform, coarsely verrucose. ITS sequence (GenBank ON843423, ex holotype) distinct from other members of Phlegmacium and with 92% similarity to the closest known species.
Habitat and distribution: In Picea abies forest on calcareous ground and so far known only from Finland.
Material examined: Finland, Perä-Pohjanmaa, Keminmaa, Kallinkangas, W part, herb-rich Picea abies forest on calcareous ground, 28 June 2015, T. Kekki 1774, 215553 (TUR). loc. cit., 25 June 2019, T. Kekki 3657, 001434119 (holotype in K-M; isotype in OULU).
GenBank numbers: TK1774: ITS = ON843434; TK3657: ITS = ON843423.
Notes: Phlegmacium fennicum is a precocious species producing basidiomes already in June. It can be recognized by the combination of yellowish brown basidiomes without bluish tints, amygdaloid-citriform to citriform, coarsely verrucose basidiospores and habitat with Picea on calcareous ground. It belongs to the P. subgenus Bulbopodium sect. Arcifolia. The two other known species of the section, P. arcifolium and P. subhygrophanum, occur with Fagus in Europe.
Phlegmacium pallidocaeruleum Niskanen Liimat. & Bojantchev, sp. nov.
Index Fungorum number: IF 900311; Facesoffungi number: FoF 14868; Figs. 63b, 64b, 65
Etymology: Named for the general coloration of the basidiomes.
Holotype: K-M 001434120.
Pileus 4.5–7.5 cm in diam, at first hemispherical, then low convex to almost plane, innately fibrillose, ochraceous in the middle, silvery bluish grey towards margin, ochraceous part enlarging with age, viscid, somewhat hygrophanous striate. Lamellae subcrowded to crowded, bluish grey, adnexed to adnate. Stipe 5–9 cm long, 1–2 cm thick at the apex, up to 3 cm at the base, with a rounded bulb, at first pale greyish blue, later more whitish, becoming brownish ochraceous when handled or gaining brownish ochraceous spots with age. Context when young bluish white marbled hygrophanous in the pileus and upper part of the stipe, white at the lower part of the stipe, when old white or yellowish white in the pileus and the base of stipe, greyish blue at the apex of stipe. Universal veil white at the very base of the stipe/bulb. Mycelium white, often forming strands. Odour indistinct. Basidiospores 8.5–10 × 5–6 µm, obovoidly ellipsoid, moderately verrucose, somewhat more strongly at the apex. Basidia hyaline in 5% KOH. ITS sequence (GenBank ON843424, ex holotype) distinct from other members of Phlegmacium and with 98% similarity to the closest known species P. aurescens.
Habitat and distribution: In mixed coniferous forests and so far known from California, USA.
Material examined: USA, California, Mendocino Co., Caspar, Caspar cemetery, mixed conifer dominated forest with Tsuga, Abies and Picea, 22 November 2012, K. Liimatainen, T. Niskanen 12-098, 001434120 (holotype in K-M; isotype in H).
GenBank number: ITS = ON843424.
Notes: Phlegmacium pallidocaeruleum is a typical member of the P. sect. Caerulea. It has pallid blue colours, the pileus is innately fibrillose and becoming more ochraceous from the centre with age, and the basidiospores are ellipsoid. The sister species P. aurescens, currently known from Washington, USA, has less bluish basidiomes and longer (10–11 × 5.5–6.5 µm) basidiospores.
Thaxterogaster Singer.
Notes: The centre of the diversity of this genus is in the Southern Hemisphere and it is estimated to include around 200 species. The size of the basidiomes ranges from small to large and varies in coloration from white, ochraceous, greenish, brown to purple. Typical characters for all agaricoid species, however, are a pileipellis duplex and a negative or, more rarely, red KOH reaction. Several lineages of this genus have a honey-like or sweet smell in the context, otherwise known only in Cortinarius subgen. Myxacium. The development of basidiomes ranges from stipitocarpic to pileocarpic type. Six subgenera are currently recognized: Thaxterogaster, Cretaces, Multiformes, Riederorum, Scauri and Variegati (Liimatainen et al. 2022).
Thaxterogaster americanoporphyropus Niskanen, Liimat. & Ammirati, sp. nov.
Index Fungorum number: IF 900312; Facesoffungi number: FoF 14869; Figs. 63c, 64c, 65
Etymology: The species is known from North America and is reminiscent of T. porphyropus.
Holotype: K-M 001434121.
Pileus 4–8 cm in diam, at first hemispherical, then low convex, pale silvery grey to ochraceous, viscid- sticky to glutinous. Lamellae medium spaced, at first purplish grey, pale brown with age, becoming strongly purple when touched. Stipe 5–8 cm long, 0.8–1.3 cm thick at the apex, up to 2 cm at the base, clavate, whitish grey, with more or less purplish tints, especially when young, becoming strongly purple when touched. Context completely purple or only in parts of the pileus, cortex and at the base of the stipe, and then other parts white. Universal veil white, sparse. Mycelium white. Odour at the base of the stipe honey-like. Basidiospores 8.5–9.5 × 5–6 µm, amygdaloid to obovoidly amygdaloid-ellipsoid, strongly verrucose. Basidia hyaline in 5% KOH. ITS sequence (GenBank ON843425, ex holotype) distinct from other members of T. subgen. Scauri sect. Purpurascentes and with 98.5% similarity to the closest known species, T. porphyropus and T. occidentalis.
Habitat and distribution: In mixed coniferous forests and so far known from North America: Alberta, British Columbia, Quebec and Washington.
Material examined: Canada, Alberta, Hinton, 5 September 2011, K. Liimatainen, T. Niskanen 11–392, 001434123 (K-M). USA, Washington, Mount Baker, Easy Pass trailhead, coniferous forest with Tsuga heterophylla, Abies and some Picea engelmannii, 28 September 2009, K. Liimatainen, T. Niskanen 09-020, 001434122 (K-M). Snohomish Co., Barlow past, Mount Baker-Snoqualmie national forest, mixed coniferous forest with mainly Tsuga heterophylla and some Pseudotsuga mentziesi and Abies amabilis, 3 October 2009, K. Liimatainen, T. Niskanen 09-042, 001434121 (holotype in K-M; isotype in H).
GenBank numbers: TN09-042: ITS = ON843425; TN09-020: ITS = ON843426; TN11-392: ITS = ON843427.
Notes: In the field the species looks like T. porphyropus and also their basidiospores are similar. Thus far, T. porphyropus is not known from the Western side of the Rocky Mountains and there the default identification would be T. americanoporphyropus. In the east side an ITS sequence is needed for unambiguous identification. The closely related C. occidentalis differs by having purple basidiomes and somewhat smaller (7–9 × 4.5–5.5 µm), slightly to moderately verrucose basidiospores.
Thaxterogaster obscurovibratilis Niskanen, Liimat. & Ammirati, sp. nov.
Index Fungorum number: IF 900313; Facesoffungi number: FoF 14870; Figs. 63d, 64d, 65
Etymology: The species belongs to Thaxterogaster sect. Vibratiles and has a darker pileus than other species of the section.
Holotype: K-M 001434124.
Pileus 2.5–5 cm in diam, at first hemispherical, later low convex to almost plain, sometimes with an umbo, dark reddish brown to pale yellowish brown, viscid to glutinous. Lamellae crowded, very pale greyish brown. Stipe 4–6 cm long, 0.4–0.8 cm thick at the apex, up tp 1.5 cm at the base, clavate to rarely almost bulbous with a tapered base, almost dry, whitish silky fibrillose. Context dark brown to brown in the pileus, white at the apex of the stipe, pale yellow towards the base. Universal veil white, sparse. Mycelium white. Taste very bitter on the pileus. Odour in lamellae and context more or less raphanoid. Basidiospores 6.5–7.5 × 4.5–5 µm, ellipsoid to amygdaloid-ellipsoid, almost smooth to very finely verrucose. Basidia hyaline, with hyaline oil drops. Lamellar trama hyphae hyaline with oil drops in 5% KOH. ITS sequence (GenBank ON843428, ex holotype) distinct from other members of Thaxterogaster and with 93% similarity to the closest known species T. vibratilis.
Habitat and distribution: In mixed forests and so far known from USA: California and Washington.
Material examined: USA, California, Humboldt Co., Big Lagoon, trail starting from the school, spruce forest with moss cover, 3 December 2012, J. Olsson & K. Liimatainen, T. Niskanen 12–225, 001434127 (K-M); Mendocino Co., Jackson State Forest, S of the crossing of roads 408 and 409 by road 720, mixed forest (Lithocarpus and Pseudotsuga), 26 November 2012, K. Liimatainen, T. Niskanen 12–151, 001434126 (K-M). Close to 408 road, Pseudotsuga and Lithocarpus, 8 December 2012, K. Liimatainen, T. Niskanen 12–276, 001434125 (K-M). loc. cit., K. Liimatainen, T. Niskanen 12–281, 001434124 (holotype in K-M; isotype in H). Washington, Gray’s Habor County, Ocean Shores, caespitose to gregarious under Tsuga heterophylla, 17 December 1979, M. McCaw, J. F. Ammirati 8464 (WTU).
GenBank numbers: TN12-151: ITS = ON843430; TN12-276: ITS = ON843429; TN12-281: ITS = ON843428; JFA8464: ITS = ON843433.
Notes: A unique looking species in the Thaxterogaster sect. Vibratiles due to the dark colour of the pileus and thus easy to distinguish from the other members of the section. Otherwise, the species has the typical characters of the section: basidiomes are rather small, pileus is viscid to glutinous, stipe is white and somewhat viscid to almost dry, and the spores are small, amygdaloid-ellipsoid and finely verrucose.
Cystostereaceae Jülich.
Notes: Cystostereaceae was established by Jülich (1981) with Cystostereum as the type genus. Additional six genera, viz. Crustomyces, Cystidiodontia, Parvobasidium, Parvodontia and Rigidotubus are also accepted in this family (Larsson 2007a; Song et al. 2018c). Moreover, one new genus is described below as Tenuimyces in Cystostereaceae.
Crustomyces Jülich.
Notes: Crustomyces was erected by Jülich (1978) with C. subabruptus as the type species. Five species were described worldwide (Jülich 1978; Hjortstam 1987a, 1987b, 1995). Crustomyces is morphologically circumscribed by smooth to odontioid hymenophore, a dimitic hyphal system, and the presence of gloeocystidia (Jülich 1978; Bernicchia and Gorjón 2010).
Crustomyces juniperi S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901033; Facesoffungi number: FoF 14803; Figs. 66, 67
Etymology: juniperi (Lat.) referring to inhabiting Juniperus przewalskii.
Holotype: LWZ 20180905–5 (HMAS).
Diagnosis: Differing from Crustomyces heteromorphus by its thin-walled gloeocystidia with an apical rounded bulb and relatively larger basidiospores (Hallenberg 1980).
Basidiomes resupinate, effused, adnate, crustaceous, up to 8 cm long, 4 cm wide, 200 µm thick. Hymenophore smooth with scattered tubercles, olivaceous buff when fresh, concolorous when dry, cracked. Margin white, thinning out as byssoid, 2 mm wide.
Hyphal system dimitic; generative hyphae with clamp connections, hyaline, thin-walled, moderately branched in subiculum, 2–3 µm in diam; skeletal hyphae hyaline, unbranched or occasionally unbranched, with a narrow to wide lumen, 1.5–2 µm in diam; tissues becoming a clay-buff colour in KOH. Gloeocystidia numerous, with an empty content, thin-walled, clavate, with an apical rounded bulb, 20–30 × 5–7 µm. Dendrohyphidia absent. Basidia subclavate, 4-sterigmate, basal clamp present, 12–18 × 4.5–6 μm. Basidiospores ellipsoid to subcylindrical, hyaline, smooth, thin-walled, IKI–, CB–, (4–)4.5–5.2(–5.5) × 2.2–3(–3.3) µm, L = 4.9 µm, W = 2.7 µm, Q = 1.8–1.9 (n = 90/3).
Material examined: China, Qinghai Province, Menyuan Hui Autonomous County, Qilian Mountains National Nature Reserve, Xianmi Forest Farm, Sigou, on base of living Juniperus przewalskii, 9 September 2018, L.W. Zhou, LWZ 20180905–5 (HMAS, Holotype), LWZ 20180905–3 (HMAS), LWZ 20180905–4 (HMAS).
GenBank numbers: LWZ 20180905–5: ITS = OR557241, LSU = OR527267; LWZ 20180905–3: ITS = OR557242, LSU = OR527268; LWZ 20180905–3: ITS = OR557243, LSU = OR527269.
Notes: Crustomyces juniperi is nested within the Crustomyces clade from a phylogenetic perspective (Fig. 68). Morphologically, C. juniperi resembles C. indecorus by olivaceous buff hymenophore and similar basidiospores; however, the latter species differs in lack of apical schizopapilla on gloeocystidia and presence of dendrohyphidia (Hjortstam 1987b).
Phylogeny generated by the maximum likelihood algorithm based on combined ITS and nLSU regions is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Holotypes are in bold and the newly generated sequences are in blue. Coniophora olivacea FP-104386 and C. puteana FP-105438 were selected as the outgroup taxa
Crustomyces scytinostromoides S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901046; Facesoffungi number: FoF 14804; Figs. 69, 70
Etymology: scytinostromoides (Lat.) referring to the similarity to the genus Scytinostroma.
Holotype: LWZ 20190808-14b (HMAS).
Diagnosis: Differing from C. subabruptus by the dominance of skeletal hyphae in subhymenium and subiculum (Eriksson and Ryvarden 1975; Jülich 1979).
Basidiomes resupinate, effused, adnate, at first ceraceous, with age crustaceous, up to 15 cm long, 5 cm wide, 200 µm thick. Hymenophore smooth with scattered tubercles, whitish to cream when fresh, cream to buff when dry, not cracked. Margin white, thinning out as byssoid, 1 mm wide.
Hyphal system dimitic; generative hyphae with clamp connections, hyaline, thin-walled, 2–3 µm in diam; skeletal hyphae dominant in subhymenium and subiculum, hyaline to yellowish, unbranched, 1.5–2.5 µm in diam; tissues becoming a clay-buff colour in KOH. Gloeocystidia numerous, thin-walled, clavate, sometimes with an apical rounded bulb, 20–30 × 5–7 µm. Dendrohyphidia numerous, hyaline. Basidia, basidioles and basidiospores not seen.
Material examined: China, Sichuan Province, Ganzi Tibetan Autonomous Prefecture, Jiulong County, Wuxuhai Scenic Spot, on fallen branch of angiosperm, 8 August 2019, L.W. Zhou, LWZ 20190808-14b (HMAS, Holotype), LWZ 20190807-15b (HMAS).
GenBank numbers: LWZ 20190808-14b: ITS = OR557244, LSU = OR527270; LWZ 20190807-15b: ITS = OR557245, LSU = OR527271.
Notes: Crustomyces scytinostromoides has a close phylogenetic relationship with other species of Crustomyces (Fig. 68). Morphologically, C. scytinostromoides has smooth hymenophore, a dimitic hyphal system, and the presence of gloeocystidia, which makes it fit with the concept of Crustomyces. However, the tubercles scattered on smooth hymenophore and the presence of skeletal hyphae in both subhymenium and subiculum make C. scytinostromoides distinct from other species in this genus. Although both specimens of C. scytinostromoides are sterile, the above-mentioned unique morphological characters are acceptable for describing a new species, like previous publications (Tchoumi et al. 2020; Yu et al. 2021).
Cystostereum Pouzar.
Notes: Cystostereum, a genus of corticioid fungi in the family Cystostereaceae, contains nine species. Species in the Cystosrereum have a dimitic hyphal system with very scarce lightcouriered skeletal hyphae, numerous vesicular gloeocystidia, and inamyloid basidiospores (Pouzar 1959; Bernicchia and Gorjón 2010; Kaur et al. 2019).
Cystostereum subsirmaurense S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901050; Facesoffungi number: FoF 14805; Figs. 71, 72
Etymology: subsirmaurense (Lat.) referring to the similarity to Cystostereum sirmaurense.
Holotype: LWZ 20170618–10 (HMAS).
Diagnosis: Differing from C. sirmaurense in thick-walled, wider cystidia (Kaur et al. 2019).
Basidiomes annual, resupinate, effused, adnate, corky to hard and brittle, up to 20 cm long, 4 cm wide, 200 µm thick. Hymenophore smooth, with minute scattered tubercles, white when fresh, becoming yellowish white to orange white on drying. Margin thinning or indeterminate, concolorous or paler.
Hyphal system dimitic; generative hyphae with clamp connections, hyaline, thin-walled, 2–3 µm in diam; skeletal hyphae subhyaline, unbranched, 1–1.5 µm in diam. Cystidia subglobose to clavate, generally with an obtuse apex, thick-walled, with a basal clamp connection, embedded in hymenium and subhymenium, 30–50 × 7–15 μm. Basidia subclavate, 4-sterigmate, with a basal clamp connection, 15–20 × 5–6 μm. Basidiospores ellipsoid to subcylindrical, hyaline, smooth, thin-walled, IKI–, CB–, 3.5–4 × 2.6–3 µm, L = 3.7 µm, W = 2.8 µm, Q = 1.3 (n = 30/1).
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Jinghong, Mengla County, Xishuangbanna National Park of Tropical Rainforests, on fallen branch of angiosperm, 18 June 2017, L.W. Zhou, LWZ 20170618–10 (HMAS, Holotype).
GenBank numbers: ITS = OR557246, LSU = OR527272.
Notes: Cystostereum subsirmaurense is nested within the Cystostereum clade from a phylogenetic perspective (Fig. 68). Morphologically, this species is recognized by smooth hymenophore with minute scattered tubercles, which makes it similar to C. sirmaurense. However, C. sirmaurense differs in slightly thick-walled, narrower cystidia (7–8.5 µm in width, Kaur et al. 2019).
Tenuimyces S.L. Liu & L.W. Zhou, gen. nov.
Index Fungorum number: IF 901051; Facesoffungi number: FoF 14801.
Etymology: Tenuimyces (Lat.) referring to thin basidiomes.
Diagnosis: Distinguished from other genera in Cystostereaceae by extremely thin basidiomes, a monomitic hyphal system, the presence of cystidioles and hyphidia, and aculeate, slightly thick-walled basidiospores.
Basidiomes annual, resupinate, effused, thin, up to 50 μm thick in section. Hymenophore smooth, white to cream. Margin thinning or indeterminate, concolorous or paler. Hyphal system monomitic; generative hyphae with clamp connections, hyaline, thin-walled. Cystidioles and hyphidia present. Basidia subclavate, 4-sterigmate. Basidiospores broadly ellipsoid, hyaline, aculeate, slightly thick-walled, IKI–, CB–.
Type species: Tenuimyces bambusicola S.L. Liu & L.W. Zhou.
Notes: Three corticioid specimens from Southwest China form a distinct lineage with strong support within Cystostereaceae in phylogenetic analysis (Fig. 68). Morphologically, these three specimens have smooth hymenophore, a monomitic hyphal system, aculeate, slightly thick-walled basidiospores that are distinguished from known genera in Cystostereaceae (Song et al. 2018c). Therefore, a new genus Tenuimyces is described on the basis of these three specimens.
Tenuimyces bambusicola S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901052; Facesoffungi number: FoF 14806; Figs. 73, 74
Etymology: bambusicola (Lat.) referring to the growth on bamboo.
Holotype: LWZ 20200816-4a (HMAS).
Diagnosis: Characterized by cream, extremely thin basidiomes, and aculeate basidiospores.
Basidiomes annual, resupinate, effused, thin, up to 50 μm thick in section. Hymenophore smooth, white to cream when fresh, concolorous when dry, cracked. Margin thinning or indeterminate, concolorous or paler.
Hyphal system monomitic; generative hyphae with clamps, hyaline, thin-walled, 1.5–2 µm in diam; tissues darkening in KOH. Cystidioles subfusiform, rare in the hymenium, thin-walled, with a basal clamp connection, 30–50 × 7–15 μm. Hyphidia rare, branched or unbranched. Basidia subclavate, 4-sterigmate, with a basal clamp connection, 15–20 × 5–6 μm. Basidiospores broadly ellipsoid, hyaline, aculeate, slightly thick-walled, IKI–, CB–, 3.5–4 × 2.6–3 µm, L = 3.7 µm, W = 2.8 µm, Q = 1.3 (n = 90/3).
Material examined: China, Sichuan Province, Leshan, Mabian Yi Autonomous County, Dafengding National Nature Reserve, on dead bamboo, 16 August 2020, L.W. Zhou, LWZ 20200816-4a (HMAS, Holotype), LWZ 20200816-5a (HMAS), LWZ 20200816-2b (HMAS).
GenBank numbers: LWZ 20200816-4a: ITS = OR557247, LSU = OR527273; LWZ 20200816-5a: ITS = OR557249, LSU = OR527274; LWZ 20200816-2b: ITS = OR557248, LSU = OR527275.
Notes: Tenuimyces bambusicola is characterized by white to cream basidiomes with smooth hymenophore, subfusiform cystidioles, slightly thick-walled, aculeate basidiospores, and the growth on bamboo.
Incertae sedis
Henningsomyces Kuntze.
Henningsomyces, typified by H. candidus, is characterized by annual hymenophores consisting of sparse or gregarious tubes, a monomitic hyphae system usually bearing both clamp connections and simple septa, the absence of cystidia, and globose to subglobose basidiospores (Wei and Qin 2009).
Henningsomyces hengduanensis S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901053; Facesoffungi number: FoF 14807; Figs. 75, 76
Etymology: hengduanensis (Lat.) referring to the type locality Hengduan Mountains.
Holotype: LWZ 20190807-11b (HMAS).
Diagnosis: Differing from Henningsomyces minimus in smaller tubes and smaller basidiospores (Wei and Qin 2009).
Basidiomes annual, soft when fresh, becoming a little chalky when drying, without odour or taste, white when fresh, turned cream when bruised or drying, consisting of tiny tubes. Tubes densely aggregated, each 0.2–0.3 mm long, 0.07–0.1 mm in diam. Subiculum absent.
Hyphal system monomitic; generative hyphae bearing both clamp connections and simple septa; tramal hyphae in tubes hyaline, smooth, thin-walled, often branched, winding, more or less tightly parallel along the tubes, 1.5–2 μm in diam; hyphae at tube margin finely branched. Cystidia absent. Basidia broadly clavate, with four sterigmata and a basal clamp connection, 15–20 × 6–8 μm. Basidiospores subglobose, hyaline, thin-walled, smooth, IKI–, CB–, 5–6 × (4–)4.5–5 µm, L = 5.5 µm, W = 4.7 µm, Q = 1.2 (n = 60/2).
Material examined: China, Sichuan Province, Ganzi Tibetan Autonomous Prefecture, Jiulong County, Wahuishan Nature Reserve, on fallen trunk of Abies, 7 August 2019, L.W. Zhou, LWZ 20190807-11b (HMAS, Holotype); ibid., on fallen trunk of Abies, 7 August 2019, L.W. Zhou, LWZ 20190807-22b (HMAS).
GenBank numbers: LWZ 20190807-11b: ITS = OR557250, LSU = OR527276; LWZ 20190807-22b: ITS = OR557251, LSU = OR527277.
Notes: Henningsomyces hengduanensis has typical morphological characters of this genus, and falls within the clade of Henningsomyces as a distinct lineage (Fig. 77). Morphologically, H. hengduanensis resembles H. candidus, H. leptus and H. minimus by the absence of subiculum, but differs mainly in its smaller tubes (0.5–1 mm in length and about 0.2 mm in diam in H. candidus, 1.2–1.8 mm in length and 0.1–0.15 mm in diam in H. leptus, and up to 0.5 mm in length and 0.1 mm in diam in H. minimus; Wei and Qin 2009).
Phylogeny generated by the maximum likelihood algorithm based on combined ITS and nLSU regions is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Holotypes are in bold and the newly generated sequences are in blue. Radulomyces copelandii Dai 15061 and Pterula echo AFTOL-ID 711 were selected as the outgroup taxa
Lyophyllaceae Jülich.
Notes: Lyophyllaceae was circumscribed by Jülich (1981) and Lyophyllum is the generic type. Currently, Lyophyllaceae contains 19 genera based on molecular phylogenetic analyses (He et al. 2019; Wijayawardena et al. 2022).
Calocybella Vizzini, Consiglio & Setti.
Notes: The genus Calocybella (Lyophyllaceae, Agaricales) was circumscribed by Vizzini et al. (2015) with C. pudica as the type species. Calocybella is saprotrophic and distributed in Europe, Dominican Republic and India (Vizzini et al. 2015, 2017; Latha et al. 2016, 2020; Corriol et al. 2017). Species of Calocybella are characterized by collybioid or slenderly tricholomatoid basidiomes, a cutis- or a trichoderm-type pileipellis, and clamped hyphae (Vizzini et al. 2015, 2017; Latha et al. 2016). Currently, there are eight accepted species of Calocybella in the Index Fungorum (2022).
Calocybella sribuabanensis N. Suwannarach, J. Kumla and S. Lumyong, sp. nov.
Index Fungorum number: IF559933; Facesoffungi number: FoF 12909; Fig. 78
Etymology: “sribuabanensis” referring to Sri Bua Ban Subdistrict where holotype was found.
Holotype: SDBR-CMUNK0910.
Basidiomes small, collybioid. Pileus 10–25 mm broad, convex or hemispherical when young, becoming campanulate and finally plano-convex with a small indistinct umbo; surface initially reddish brown (7E7) to brown (7E8) except at the margin which is grayish orange (5B6), not striate, somewhat granulose all over; margin initially incurved, becoming decurved to somewhat straight with age, slightly wavy or somewhat lobate. Lamellae emarginate with a small decurrent tooth, moderately crowded, initially grayish yellow (4B6), becoming light yellow (4A5) at maturity, up to 3 mm wide, with lamellulae of 3–6 lengths; edge finely torn, concolorous with the sides. Stipe 20–32 × 3–4 mm, central, terete or slightly compressed, equal or slightly tapering towards the base, stuffed; light yellow (4A4) to grayish yellow (4B5), appressed-fibrillose all over; base slightly enlarged, whitish. Context up to 2 mm thick at the center of the pileus, light yellow (4A4). Odor not distinctive.
Basidiospores 5–8 × 3–5 μm, Q = 1.33–2.00, Qm = 1.65, ellipsoid to oblong-ellipsoid, with a fine verrucose ornamentation, hyaline, thin- to slightly thick-walled, acyanophilous, inamyloid. Basidia 22–25 × 7–8 μm, clavate or rarely pedicellate-clavate, hyaline, thin-walled, 4-spored, rarely 2-spored; sterigmata up to 6 μm long. Lamella-edge fertile. Pleurocystidia and cheilocystidia absent. Lamellar trama subregular to almost regular; hyphae 3–6 μm wide, hyaline, thin-walled, not discoloring in KOH, inamyloid. Pileus trama interwoven; hyphae 4–10 μm wide, with a pale yellowish brown wall pigment which is darker towards the pileipellis, thin-walled, not discoloring in KOH, inamyloid. Pileipellis a cutis; hyphae 3–5 μm wide, with a pale yellow wall pigment, thin-walled, slightly gelatinized. Stipitipellis a cutis composed of narrow, interwoven hyphae coated with a resinous material; hyphae 3–7 μm wide, with a pale yellow wall pigment which is paler towards the stipe base, thin-walled. Caulocystidia absent. Clamp connections observed on all hyphae.
Habitat and distribution: Solitary to gregarious on soil. Known only from Thailand.
Material examined: Thailand, Lamphun Province, Sri Bua Ban Subdistrict, Chiang Mai University Haripunchai Campus, 18°32′18″N 99°7′2″E, elevation 368 m, solitary to gregarious on soil, 16 August 2020, J. Kumla and N. Suwannarach, SDBR-CMUNK0910 (holotype); 18°32′19″N 99°7′4″E, elevation 371 m, solitary to gregarious on soil, 19 August 2022, J. Kumla and N. Suwannarach, SDBR-CMUNK1783.
GenBank numbers: SDBR-CMUNK0910: ITS = OP503445; SDBR-CMUNK1783: OP503940.
Notes: Phylogenetic analysis of the ITS sequence confirmed that C. sribuabanensis stands within the genus Calocybella and clearly separate from the other Calocybella species (Fig. 79). This is the first record of the genus Calocybella from Thailand. Morphologically, C. sribuabanensis is similar to C. goethei; however, C. goethei has a longer stipe (30–45 × 3–5 mm) than C. sribuabanensis (20–32 × 3–4 mm, Crous et al. 2021b). A phylogenetic tree showed that C. sribuabanensis and C. goethei formed distinct species. Calocybella sribuabanensis forms a sister taxon to C. dicholamellata known from India with high support value (100% BS and 1.00 PP, Latha et al. 2020). However, the latter species is distinct by its brownish yellow pileus and narrower size of basidia (20–25 × 5–7 μm, Latha et al. 2020). An ITS sequence of C. sribuabanensis exhibit 97.68% similarity with both sequences of the C. dicholamellata CAL1242 (holotype) and DKP522.
Phylogenetic tree derived from maximum likelihood analysis of ITS gene of 30 sequences and the aligned dataset was comprised of 804 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00893. A best scoring RAxML tree was established with a final ML optimization likelihood value of -6247.2241. The matrix had 511 distinct alignment patterns with 18.35% undetermined characters or gaps. Estimated base frequencies were found to be: A = 0.2542, C = 0.2004, G = 0.2186, T = 0.3266; substitution rates AC = 1.5589, AG = 4.1460, AT = 1.7968, CG = 0.7747, CT = 5.1818, GT = 1.0000. Entoloma hainaense GDGN 27990 and E. tiliae LE < RUS > 254179were used as outgroup. Numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap values ≥ 70% and PP ≥ 0.90 are shown at each branch. The bar represents 0.1 substitutions per nucleotide position. Type strains are in bold. The newly generated sequences are indicated in blue
Marasmiaceae Roze ex Kühner.
Notes: Kühner (1980) established Marasmiaceae with the type genus Marasmius and with a combination of three tribes viz. Marasmieae, Collybieae and Myceneae of Tricholomataceae in the classification of Singer (1986). Molecular phylogeny has revealed that Marasmiaceae is monophyletic, and comprises 10 genera (Amyloflagellula, Brunneocorticium, Campanella, Chaetocalathus, Crinipellis, Hymenogloea, Marasmius, Moniliophthora, Neocampanella and Tetrapyrgos) with more than 750 species (Matheny et al. 2006; He et al. 2019; Wijayawardene et al. 2020). Marasmiaceae is a lineage mainly integrated by white-spored agaricoid fungi with small, membranous and toughness pileus, and saprophytes on wood and leaf-litter or occasionally biotrophs (Moniliophthora).
Crinipellis Pat.
Notes: The genus Crinipellis (Marasmiaceae, Agaricales) was established by Patouillard (1900) with C. scabella (syn. Agaricus stipitarius and C. stipitaria) as the type species (Singer 1943). This genus is characterized by a presence of thick-walled, dextrinoid, hair-like terminal cells in the pileipellis (Kerekes and Desjardin 2009). The Crinipellis species has been recognized both saprotrophic and parasitic species (Singer 1943; Aime and Phillips-Mora 2005; Kerekes and Desjardin 2009). This genus comprises over 150 species and is considered to have a worldwide distribution (Kirk et al. 2008; Antonín et al. 2009; Kerekes and Desjardin 2009; Antonín and Noodeloos 2010; Antonín 2012).
Crinipellis trichialis (Lév.) Pat. ex Antonín, Ryoo & H.D. Shin, Mycotaxon 108: 432 (2009).
Index Fungorum number: IF 543142; Facesoffungi number: FoF 12910; Fig. 80
Pileus 5–18 mm in diameter, hemispherical to convex-cylindrical when young, becoming convex to plano-convex or applanate with age, disc with a tuft of scales forming a small papilla, with concentric zones of erect fibrils or scales around the disc, margin fibrillose; papilla and central zone dark brown (6F6–7F8), middle zone brown (6F6–7F8), margin brownish yellow (5C5–5C8). Context < 1 mm thick, white. Lamellae adnexed to free, close to crowded with 2–4 series of lamellulae, white to pale yellowish white (3A1–3A2). Stipe 5–25 × 0.5–1.5 mm, central, cylindrical, equal with an enlarged base, tough, pliant, fibrillose to hairy overall, insititious, brown to dark brown. Rhizomorphs absent.
Basidiospores 6–11 × 5–8 μm, Q = 1.0–2.0 (n = 50), ellipsoid, smooth, hyaline, inamyloid, thin-walled. Basidia 18–35(–40) × 6–8 μm, clavate, 4-spored. Pleurocystidia 30–60 × 6–10 μm, clavate, hyaline, inamyloid, thick-walled. Cheilocystidia 20–32 × 5–10 μm, clavate, simple or a majority with 1–4 apical appendages, hyaline, inamyloid, thin-walled. Pileipellis a cutis, 5–6 μm in diam, hyaline, inamyloid, with spiral encrustations, giving rise to terminal hairs with basal clamp connections. Hairs 50–460 × 2–5 μm diam, cylindrical, apex rounded to acute, with secondary septations, thick-walled, dextrinoid, yellowish-brown to greenish brown in KOH. Stipitipellis composed of repent cortical hyphae and terminal hairs; cortical hyphae 1–3 μm in diam, cylindrical, dextrinoid, light yellow brown to green in KOH; medullary hyphae 3–5 μm in diam, cylindrical, strongly dextrinoid, hyaline; terminal hairs 100–325 × 7.5–10 μm, cylindrical, apices acute, with secondary septations, dextrinoid, yellowish brown to greenish brown in KOH. Clamp connections present.
Habitat and distribution: Solitary to gregarious, on decomposing wood and sticks (mostly found in bamboo). Known from Brazil, Indonesia, Malaysia, Thailand and Venezuela (Kerekes and Desjardin 2009; This study).
Material examined: Thailand, Lamphun Province, Sri Bua Ban subdistrict, Chiang Mai University Haripunchai Campus, 18°31′59″N 99°7′46″E, elevation 401 m, solitary to gregarious on decaying wood, 16 August 2020, J. Kumla and N. Suwannarach, SDBR-CMUNK0900 (new record for Thailand).
GenBank number: ITS = OP503450 (SDBR-CMUNK0900).
Notes: Phylogenetic analysis indicated that Crinipellis trichialis separated from sister taxon C. scabella and C. rhizomaticola (Fig. 81). Crinipellis scabella differs from C. trichialis by negative KOH reaction (Singer 1943). The narrower size of basidiospores in C. rhizomaticola (8.5–10 × 4–5.25 μm, Antonín et al. 2009) clearly distinguishes it from C. trichialis (6–11 × 5–8 μm).
Phylogenetic tree derived from maximum likelihood analysis of ITS gene of 20 sequences and the aligned dataset was comprised of 782 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00637. A best scoring RAxML tree was established with a final ML optimization likelihood value of -3314.6448. The matrix had 347 distinct alignment patterns with 18.53% undetermined characters or gaps. Estimated base frequencies were found to be: A = 0.2579, C = 0.1949, G = 0.2212, T = 0.3258; substitution rates AC = 1.0835, AG = 3.5775, AT = 1.5611, CG = 0.3860, CT = 4.5691, GT = 1.0000. Marasmius iras KLU M 73 and Marasmius ochropoides KLU M 89 were used as outgroup. Numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap values ≥ 70% and PP ≥ 0.90 are shown at each branch. The bar represents 0.1 substitutions per nucleotide position. Type strains are in bold. The newly generated sequences are indicated in blue
Marasmius Fr.
Notes: Marasmius is a saprotrophic fungus that decomposes leaves or wood debris and distributes throughout the world. Marasmius contains more than 1000 species and is known as polyphyletic genus (Owings and Desjardin 1997; Wannathes et al. 2009). This genus was first divided into 12 sections by Singer, but some of them have been reclassified into several genera with the introduction of molecular analysis. Marasmius is restricted to include only seven sections: Globules, Hygrometrici, Scotophysini, Leveilleani, Marasmius, Neosessiles and Sicci. Among them, Marasmius section Marasmius, sect. Sicci, and sect. Globulares formed a strongly supported lineage, and this clade was designated as Marasmius sensu stricto (Owings and Desjardin 1997; Wilson and Desjardin 2005).
Marasmius centrocinnamomeus J.S. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF900042; Facesoffungi number: FoF 13254; Fig. 82
Basidiomes and microscopic drawings of Marasmius centrocinnamomeus (SFC201807014-02, holotype). A Field pictures of the basidiomes, B Drawings of the microscopic features. Abbreviations: s Basidiospores. b Basidia. ch Cheilocystidia. p pileipellis cells. ca Caulocystidia. Scale bars: A = 1 cm, B = 20 μm
Etymology: ‘centrocinnamomeus’ refers to the cinnamon (bright brown) colored center of pileus.
Holotype: SFC20180704-02.
Pileus 12–67 mm in diam, convex to campanulate when young, becoming plano-convex to uplifted when old, sometimes slightly depressed in or near the center, rugulose, glabrous, hygrophanous, greyish orange (5B5) to brown (6E6) when moist, yellowish white (3A2) to greyish yellow (4B6), margin white to yellowish white (3A2 to 4A2). Lamellae adnexed, distant (L = 12–22), with 1–3 series of lamellulae, white to yellowish white (3A2 to 4A2), concolorous with margin. Stipe 30–110 × 11–50 mm, central, cylindrical, greyish yellow (4B4) to orange white (5A2) toward apex, brownish orange (5C4) to light brown (6D5) towards the base, non-insititious, with whitish basal mycelium.
Basidiospores (5.8–)6.1–8(–8.7) × 3.4–4.6(–5.1) μm (x̅ = 7.1 × 4 μm, n/s = 30), Q = 1.3–2.1 (x̅ = 1.78), asymetrical, ellipsoid to oblong, hyaline, inamyloid, thin-walled, sometimes with oil drops. Basidia (29–)31.2–38.8(–42) × 5.4–7.3 μm, cylindrical, clavate. Basidioles 20–37 × 3.3–7 μm, cylindrical, fusoid, clavate, hyaline, thin-walled. Pleurocystidia absent. Cheilocystidia (18–)20–32.5(–37) × (5.5–)6–9.9(–11) μm, (broadly) clavate, obovoid, hyaline, thin- to slightly thick-walled. Tramal hyphae formed by cylindrical or sometimes inflated, thin walled, hyaline, up to 12 μm wide. Pileipellis a hymeniderm composed of cells 13.5–26 × 7–17 μm, broadly clavate to sphaeropedunculate, vesiculate, thin- to slightly thick-walled, smooth, yellowish white (1A2) in KOH. Stipe tramal hyphae 3–7(–7.6) μm broad, cylindrical, hyaline, moderately dextrinoid, slightly thick-walled. Caulocystidia (13–)14.5–38(–41) × (4.5–)5.2–8.5(–9.3) μm, abundant, cylindrical to clavate, fusoid, branched, hyaline, inamyloid, thin-walled. Clamp connections present in all tissues.
Material examined: Republic of Korea, Jeollanam-do, Wando-gun, cedar Recreation Forest, 34°20′47.4"N 126°40′14.4"E, on leaf litter dominated with pine needles, 4 July 2018, Young Woon Lim, SFC20180704-02 (holotype); Jeju island, Jeolmul Natural Recreation Forest, 33°26′22.7"N 126°37′34.7"E, on leaf litter, 3 July 2014, Jae Young Park (SFC20140703-03); Incheon, Ganghwa-gun, Manisan, 37°36′41.8"N 126°26′05.4"E, on leaf litter in a mixed forest dominated with Carpinus laxiflora, 16 July 2015, Jae Young Park, SFC20150716-07; Incheon, Ongjin-gun, Jangbongdo, 37°32′20.7"N 126°20′12.0"E, on leaf litter of broadleaf tree, 26 July 2016, Nam Kyu Kim, SFC20160726-33.
Habitat: On litter in a mixed forest of conifer and broadleaf trees.
GenBank numbers: SFC20180704-02: ITS = OP730947; SFC20140703-03: ITS = OP730948; SFC20150716-07: ITS = OP730949; SFC20160726-33: ITS = OP730946.
Notes: The diagnostic characteristics of Marasmius centrocinnamomeus are convex to plano-convex and brownish pileus, adnexed and distant lamellae, central, cylindrical, greyish yellow to light brown colored, non-insititious stipe that becomes brownish toward the base, ellipsoid to oblong basidiospores, (broadly) clavate to obovoid cheilocystidia, and a hymeniderm pileipellis composed of broadly clavate to sphaeropenduculate cells. Marasmius maximus is morphologically similar species and phylogenetically close species to M. centrocinnamomeus (Fig. 83). However, Marasmius maximus differs from M. centrocinnamomeus by shorter stipe with a darker brownish base, larger basidiospores, and bigger caulocystidia (Hongo 1962; Antonín et al. 2010).
Phylogenetic tree conducted from maximum likelihood analysis based on ITS sequence data of Marasmius species with Bayesian Information Criterion values. Related sequences are mostly taken from Oliveira et al. (2020). A total of 49 Marasmius and two Crinipellis species as outgroup taxa are included in the phylogenetic analysis. Crinipellis malesiana and Crinipellis brunneipurpurea are used as the outgroup taxa. Support values at the nodes consist of BS over 70 and PP over 0.95. The nodes with 100 BS are represented as thicker stems. The sequences obtained in this work are indicated in blue
Marasmius ferrugineodiscus J.S. Kim & Y.W. Lim, sp. nov.
Index Fungorum number: IF900041; Facesoffungi number: FoF 13255; Fig. 84
Basidiomes and microscopic drawings of Marasmius ferrugineodiscus (SFC20160714-57, holotype). A Field pictures of the basidiomes, B Drawings of the microscopic features. Abbreviations: s Basidiospores. b Basidia. ch Cheilocystidia. p pileipellis cells. ca Caulocystidia. Scale bars: A = 1 cm, B = 20 μm
Etymology: ‘ferrugineodiscus’ refers to the light brown color of the center of the pileus.
Holotype: SFC20160714-57.
Pileus 15–30 mm in diam, convex when young, becoming plano-convex to undulate when old, sometimes slightly depressed in center, wrinkled, glabrous, orange grey (5B2), greyish orange (5B5) to light brown (6D4), margin yellowish grey (4B2) to greyish orange (5B3), hygrophanous. Lamellae adnexed to sinuate, subdistant (L = 20–32), with 2–4 series of lamellulae, white with concolorous edge. Stipe 23–68 × 1.3–3 mm, central, cylindrical, hollow, brownish orange (5B4), grayish brown (5D3) to brownish orange (6C3) towards base, yellowish grey (4A2) to orange grey (5B2) towards apex, non-insititious, with whitish basal mycelium.
Basidiospores 5.4–7.8 (–8.4) × 2.7–4(–4.5) μm (x̅ = 6.7 × 3.5 μm, n/s = 30), Q = 1.6–2.6 (x̅ = 1.98), oblong to amygdaliform, hyaline, inamyloid, thin-walled, with oil drops. Basidia 26.3–30.8 × 3.5–6 μm, cylindrical, (narrowly) clavate. Basidioles 19.2–29.4 × 2.8–5.6(–6) μm, cylindrical, fusoid to narrowly clavate, hyaline, thin-walled. Pleurocystidia absent. Cheilocystidia 16–27 × (5.4–)5.7–12.6 μm, variable in shape, (broadly) clavate, pyriform, obovoid, branched, hyaline. Tramal hyphae formed by cylindrical, slightly thick-walled, hyaline, up to 12 μm wide cells. Pileipellis a hymeniderm composed of cells 15.6–22.3 × 8.7–17.5 μm, ellipsoid, (broadly) clavate, obovoid, vesiculate, thin-walled, smooth, yellowish white (1A2) in KOH. Stipe tramal hyphae 3.5–9.2(–11) μm broad, cylindrical to fusoid, hyaline, strongly dextrinoid, slightly thick-walled. Caulocystidia 20–42 × 5.5–12 μm, sometimes clustered, cylindrical to clavate, hyaline, inamyloid, slightly thin-walled. Clamp connections present in all tissues.
Material examined: Republic of Korea, Gyeongsangbuk-do, Ulleung-gun, Ulleung-eup, 37°30′44.4"N 130°54′31.1"E, on the decomposed branch, 14 July 2016, Jae Young Park, SFC20160714-57 (holotype); Seoul, Gwanak-gu, Seoul National University, 37°27′51.8"N 126°57′08.0"E, on leaf litter, 21 August 2014, Jae Young Park, SFC20140821-08; Incheon, Ongjin-gun, Yeongheung-myeon, 37°15′25.0"N 126°27′37.1"E, on leaf litter, 19 July 2016, Nam Kyu Kim, SFC20160719-13.
GenBank numbers: SFC20160714-57: ITS = OP730950; SFC20140821-08: ITS = OP730951; SFC20160719-13: ITS = OP730952.
Habitat: On litter of leaves and branches in a mixed forest of conifer and broadleaf trees.
Notes: Marasmius ferrugineodiscus is characterized by a convex to undulate pileus which is orange grey to light brown colored center but gets whiter as it goes to margin, subdistant and adnexed lamellae, central and cylindrical stipe, oblong to amygdaliform basidiospores, (broadly) clavate to pyriform cheilocystidia, and a hymeniderm formed pileipellis composed of clavate, obovoid, vesiculate cells. These characteristics place this species under sect. Globulares. The macromorphological characteristics of this species are similar to Marasmius wynneae. Marasmius wynneae has more whitish and greyish pileus when comparing with M. ferrugineodiscus. Marasmius ferrugineodiscus is phylogenetically close to Marasmius albimyceliosus (Fig. 83). However, M. albimyceliosus can be distinguished from M. ferrugineodiscus by pale cream pileus, fewer lamellae, and the lack of caulocystidia (Wannathes et al. 2009).
Moniliophthora H.C. Evans, Stalpers, Samson & Benny.
Notes: Moniliophthora was described by Evans et al. (1978) as an incertae sedis, monotypic genus of basidiomycetes, with M. roreri, a parasitic fungus of T. cacao, as the type (Niveiro et al. 2020). Later, based on phylogenetic studies, Aime and Phillips-Mora (2005) placed M. roreri within Marasmiaceae (Agaricales), and included M. perniciosa (= M. Crinipellis) in Moniliophthora. The authors speculated that other Crinipellis species, especially those currently placed in section Iopodinae, would be found to belong to Moniliophthora (Aime and Phillips-Mora 2005). The primary morphological diagnostic characters that separate Crinipellis and Moniliophthora are pliant vs. stiff (Crinipellis) stipes, and a tendency toward production of pink to orange pigments in the basidiome that do not change to green or olive when treated with KOH or NaOH (Moniliophthora). Additionally, many Moniliophthora species appear to have a biotrophic habit, including important pathogens of tropical crops such as cocoa (Theobroma cacao), while those of Crinipellis are primarily saprotrophic (Niveiro et al. 2020). Ten species of Moniliophthora are currently known (Evans et al. 1978; Aime and Phillips-Mora 2005; Arruda et al. 2005; Kerekes and Desjardin 2009; Kropp and Albee-Scott 2012; Antonín et al. 2014; Lisboa et al. 2020; Niveiro et al. 2020; Izhar et al. 2022).
Moniliophthora atlantica N. A. Ramirez & Niveiro, sp. nov.
Index Fungorum number: IF555944; Facesoffungi number: FoF 10838; Figs. 85, 86
Etymology: referring to the ecoregion where the species was collected, the Atlantic Forest.
Holotype: OS17-2–7 (CTES 0568284).
Pileus 3–20 mm in diam, circular, convex to broadly convex when mature, some subumbonated, slightly depressed at center, surface pale red (7A3-8A3), pastel red (7A4-8A4), dull red (8B4-8C4) to greyish red (8B5-8C5), usually darker near the center, reddish brown (8D7-8E6-7); surface fibrillose to pubescent, dry, opaque, not hygrophanous, sulcate-striate; margin incurved to plane. Context thin (< 1 mm), membranaceous, yellowish white (1A2), odor and taste not tested. Lamellae free to slightly adnexed, subdistant, regular, sometimes furcate towards the margin and with intervenose, lamellulae in 2–3 series, yellowish white (1A2) to pale yellow (1A3), smooth margin, concolorous with sides. Stipe 2–12 × 0.8–2 mm, central, sometimes slightly lateral, cylindrical, equal or slightly thinner towards the middle, hollow, insititious, apex concolorous with lamellae, yellowish white (4A2), turning pale orange (6A4-6B4) towards the middle of the stipe, darkening towards the base, reddish brown (9E6-7) to dark brown (9F6-8), surface fibrillose, pubescent under lens, more distinctive toward the base. Spore-print not observed, presumably yellowish white (1A2). KOH and NaOH reactions on pileus surface negative. Basidiospores (7–)8.2–11.1 × 4.5–6.2(–7.2) µm, x̅ = 9.4 ± 0.77 × 5.6 ± 0.45 µm, Q = 1.4–2.1, Qx = 1.7 ± 0.12, n = 25, N = 2, ellipsoidal to oblong, amygdaliform in lateral view, with a slight suprahilar depression, thin-walled, smooth, hyaline, inamyloid, without germ-pore. Basidia 34.7–50 × 7.5–9 μm, clavate to narrowly clavate, 4-spored, thin-walled. Basidioles 22.2–34 × 4–7.7 μm, clavate, thin-walled, abundant. Pleurocystidia absent. Cheilocystidia 18–33.2 × 2.5–6.4 μm, subcylindrical to narrowly clavate, sometimes with a finger-like apical protuberance, rounded apex, thin-walled, hyaline. Hymenophoral trama subregular, composed of cylindrical interwoven hyphae, up to 7 μm in diam, thin-walled, hyaline. Pileipellis a cutis of repent, more or less interwoven cylindrical hyphae, up to 8 μm broad, thin-walled, smooth, hyaline, inamyloid, covered by clusters of dextrinoid hairs. Hairs of the pileus surface 50–315 × 4–8.5 μm, setiform, acicular, thick-walled, up to 3 μm broad, dextrinoid, sometimes with secondary septa, rounded apex, KOH– (do not turn olive green). Stipitipellis a cutis of repent hyphae, up to 8 µm in diam, parallel, elongated, dextrinoid. Hairs of the stipe surface 20.1–44.1 × 4.4–8.3 μm, setiform, similar to hairs of the pileus surface, thick-walled, up to 2 μm broad, dextrinoid, more abundant in young specimens. Clamp connections presents.
Habitat and distribution: Marasmioid, growing on small freshly fallen branches of unidentified dicots and stems of living lianas. In Misiones Province, Argentina.
Material examined: Argentina, Misiones, San Ignacio, Osununú Private Reserve, 27°17′09.3" S, 055°34′03.6" O, 206 m asl, on recently fallen branches, 30 September 2018, on recently fallen branches leg. Ramirez N. et al., OS17-2–7 (CTES 0568284, holotype), OS18-2–5 (CTES); ibid., Teyú Cuaré Provincial Park, 27°17′05.5" S, 055°35′21.1" O, 177 m asl, on living liana, 1 October 2018, leg. Ramirez N. et al., TC19-2–1 (CTES); Iguazú, Iguazú National Park, 25°43′29.2" S, 054°28′08.6" O, 239 m asl, 2 October 2018, leg. Ramirez N. et al., IG16-3–11 (CTES); ibid., Palmital, 25°41′19.7" S, 054°28′26.3" O, 186 m asl, 13 October 2017, leg. Ramirez N. et al., IG10-1–1 (CTES).
GenBank numbers: OS17-2–7: ITS = ON180675; IG16-3–11: ITS = ON180677.
Notes: The matrix included 35 sequences belonging to 20 taxa of Moniliophthora and related genera, such as Crinipellis, Chaetocalathus and Marasmius. A Tetrapyrgos nigripes sequence was used to root the tree (Kropp and Albee-Scott 2012). The alignment resulted in a total of 602 characters, of which 367 were conserved sites, 235 variables and 150 parsimony-informative. The best substitution models were estimated as TPM2uf + G, K80, and SYM + G for ITS1, 5.8S, and ITS2 respectively.
Since the Maximum Likelihood and Bayesian Inference analysis yielded trees with similar topology, only the latter is shown in which the support values of PP/BS for the compatible nodes between them (Fig. 87). In the phylogeny, two main clades are observed, although without statistical support: Chaetocalathus, which groups all species of this genera included, and the clade Moniliphthora/Crinipellis. Within the latter, M. atlantica, the new species proposed here, forms a well-supported clade (0.99/100) as sister group to M. perniciosa (1/98).
Bayesian Inference Analysis based on ITS sequences of Moniliophthora and related genera. The sequences obtained in this work are indicated in blue, type material in bold. Support values, when present, consist of the posterior probability (PP) followed by the bootstrap (BS), separated by a /. Only nodes with support above 50% were scored, and support values greater than 0.90/70 are showed (- indicates a lower value)
Moniliophthora atlantica is characterized by its small to medium sized basidiomes, reddish to violet in color, covered by hairs that preserve their color in contact with KOH. The most closely related species, both morphologically and phylogenetically (Fig. 87), is M. perniciosa. Both species share the general appearance and coloration of the basidiomes, but M. perniciosa differs in the lageniform and more elongated cheilocystidia (35–50 × 9–14 μm), the shorter basidia (31–32 μm), and shorter hairs of the pileus surface (80–150 μm, Singer 1976). Another very similar species in terms of coloration is M. marginata known from Malaysia, but it is differentiated by its marginate lamellae with a reddish-brown edges (10D6), basidiospores smaller on average (x̅ = 7.0 ± 1.0 × 4.0 ± 0.6 μm), shorter basidia (35–36 μm), and longer stipe hairs (60–150 μm, Kerekes and Desjardin 2009).
Moniliophthora perniciosa is a widely studied species since it is the cause of the main disease of cocoa (Theobroma cacao) called “witches’ broom”, and has been reported in numerous hosts of different families, such as Malvaceae, Malpighiaceae, Solanaceae, Bixaceae, Sapindaceae, Asteraceae, and lianas possibly belonging to the Bignoniaceae (Lisboa et al. 2020). These authors observed in the hosts the presence of symptoms caused by the fungus, concluding that there are non-pathogenic life forms (on lianas and Allophylus edulis) and pathogenic forms (in the other hosts studied). Considering the absence of symptoms or visible anomalies in the hosts of M. atlantica at the time of collection, this species can be classified as non-pathogenic. However, it should be highlighted that a more extensive sampling, with a more detailed study of its hosts and studies including other regions of the genome is considered necessary to define this issue.
Pleurotaceae Kühner.
Notes: Pleurotaceae is erected by Kühner (1980) with the type genus Pleurotus. The fungi in this family feed on dead wood, nematodes and bacteria, so they can often be seen growing on dead trees and logs. Most of them are directly attached to wood. They often grow in loose or dense clusters (Thorn 2000).
Resupinatus Nees ex Gray.
Notes: Resupinatus was established by Gray (1821). Singer (1986) proposed Hohenbuehelia and Resupinatus belong to the tribe Resupinateae, family Tricholomataceae. Thorn and Barron (1986) judged Hohenbuehelia and Resupinatus as different taxonomic levels, arguing that the two genera are fundamentally different: Hohenbuehelia possesses nematophagous anamorph and Resupinatus is non-nematophagous and lacks a conidial anamorph (Thorn and Barron 1986). Until 2000, further studies by Thorn et al. (2000) based on ribosomal DNA sequences showed that other members of the Resupinateae, Asterotus and Resupinatus are a monophyletic group within family Tricholomataceae, distinct from family Pleurotaceae, but Resupinatus is now a part of the family Pleurotaceae. There are 83 records in the Index, of which 55 are valid records, including 7 variants. There is no clear opinion on the division of its subordinates.
Resupinatus porrigens J.Z. Xu & Yu Li, sp. nov.
Index Fungorum number: IF 901101; Fungal names number: FN 571222; Fig. 88
Etymology: “porrigens” refers to the macroscopic morphological features resembling a conch.
Holotype: HMJU 261.
Basidiomes small, sessile, mostly lateral or dorsal, fleshy. Pileus up to 7 mm in diam, surface beige (7F3), red-brown (8E4) toward the margin, convex, orbicular when young, becoming conchate, flabelliform, discoid when mature, smooth; margin incurved, entire to variously cracked or wavy. Hymenophore lamella-like with radiating folds, separated by 4–6 series of lamellulae interconnected, reddish brown (8E5). Basidiospores (3.8–)4.1–4.6(–4.9) × (3.0–)3.3–4.0(–4.3) μm (\(\overline{x }\) = 4.3 × 3.6 μm, n = 20), Q = 1.04–1.40, Qm = 1.19, globose to subglobose, with a small hilar appendage, faint bump, multiguttulate, thin-walled, hyaline. Basidia (17.2–)19.0–25.1(–27.4) × 5.0–6.9 μm (\(\overline{x }\) = 22 × 5.9 μm, n = 20), narrowly clavate, tetrasporic, sterigmata up to 3.2 μm long, with numerous oil drops. Cheilocystidia 2.1–4.2 μm wide, clavate to subcoralloid, with 1 or 2 branches, hyaline. Divergent hymenophoral trama. Pileipellis intricately interwoven layer of hyphae 1.6–4.4 μm in diam, thin-walled, compact. Clamp connections are present in all hyphae.
Material examined: China, Qinghai Province, Halihatu National Forest Park, on the rotting coniferous trunk, 7 August 2018, Jize Xu, HMJU 261 (holotype); Qinghai Province, Halihatu National Forest Park, 22 July 2021, HMJU 3836.
GenBank numbers: HMJU 261: ITS = OP727724, LSU = OP727807; HMJU 3836: ITS = OP729420, LSU = OP727819.
Notes: The main characteristic of Resupinatus porrigens is the lateral or dorsal, beige pileus with conchate, flabelliform, discoid, entire to variously cracked or wavy form, and reddish brown hymenium, lamella-like with radiating folds, separated by 4–6 series of lamellulae interconnected. The pileus is beige similar to Resupinatus merulioides, but R. merulioides is differentiated by taupe and alveolate hymenium (Redhead and Nagasawa 1987). Resupinatus odoratus resemblance R. porrigens due to cracked edge, but R. odoratus has a sticky and striate pileus with a beige and poroid hymenium when young (Bijeesh 2020). Resupinatus vinosolividus is distinguished from R. porrigens by lamellulae, but R. vinosolividus having the livid vinaceus and floccose pileus with alveolate and livid hymenium (Cooper 2012). Resupinatus porrigens, R. applicatus and R. trichotis are lamella-like of hymenium distributed in China, but R. porrigens has thickened lamella, similar to Cantharellus, and the relationships were not closed in the phylogenetic analysis tree (Fig. 89).
Bayesian and ML phylogenetic trees of Resupinatus based on ITS and LSU sequences, nodes were annotated if supported by > 0.95 Bayesian posterior probability (PP) (left) or > 80% ML bootstrap proportions (BP) (right) values. Newly generated sequences are shown in bold black. The ex-types are in bold; the new isolates are in blue
Resupinatus porrigens also has many microscopic similarities with other species of the genus Resupinatus. Resupinatus merulioides showes similarities with R. porrigens in the appearance of globose basidiospores; however, R. merulioides is differentiated by smooth basidiospores, longer basidia, and clavate to cylindrical cheilocystidia (Redhead 1987). Resupinatus porosus also has globose basidiospores, but R. porosus has clavate-acanthophysoid or coralloid cheilocystidia, often bearing granular to crystalline encrustation (Thorn et al. 2005). Resupinatus stictoideus shows similarities with R. porrigens in the appearance of basidia; however, R. stictoideus is differentiated by ellipsoid basidiospores and coralloid-diverticulate pileipellis (Nakasone 2008). In phylogenetic analysis, R. porrigens has the highest similarity with R. striatulus, but it is a clade of its own (Fig. 89).
Psathyrellaceae Vilgalys, Moncalvo & Redhead.
Notes: Psathyrellaceae was classified from other coprinoid taxa based on molecular phylogenetic data, which circumscribed by Psathyrella and Lacrymaria, together with related species in the polyphyletic genus Coprinus sensu lato that were transferred to the genera Coprinellus, Coprinopsis or Parasola (Redhead et al. 2001). Afterwards, several other genera were included in Psathyrellaceae currently comprising 21 accepted genera (Wijayawardena et al. 2022).
Parasola Redhead, Vilgalys & Hopple.
Notes: Parasola is a genus of coprinoid mushroom that forms a small, umbrella-like, non-deliquescent pilei lacking of veils (Redhead et al. 2001; Nagy et al. 2009). Parasola plicatilis is the specific type species (Redhead et al. 2001). The species are common decomposers of leaf-litter, wood and herbivore dung (Schafer 2014), and are distributed worldwide. Species of Parasola are divided into section Auricomi (the presence of thick-walled, brown sclerocystidia) and section Parasola (the absence of thick-walled, brown sclerocystidia, Schafer 2010). There are 48 epithets listed in the Index Fungorum (2022). However, the genus currently has 24 species based on recent taxonomic treatments dealing with morphological and molecular studies (Nagy et al. 2010; Schafer 2014; Hussain et al. 2016, 2017, 2018; Szarkándi et al. 2017; Schafer et al. 2022).
Parasola setulosa (Berk. & Broome) Redhead, Vilgalys & Hopple, in Redhead, Vilgalys, Moncalvo, Johnson & Hopple, Taxon 50: 236 (2001).
Index Fungorum number: IF474661; Facesoffungi number: FoF 12911; Fig. 90
Pileus 20–30 mm in diam, very thin, conical when young, expanding to broadly conical in age; dull, dry, plicato-striate with minute, erect, brown setules; brownish orange (7C6, 6C4) at center, light brown (7D4) to greyish brown (7D3) fading to orange white (5A2), orange grey (5B2) or brownish grey (5C2) at margin. Context very thin, orange grey (5B2). Lamellae free to adnate, moderately crowded with 1–2 series of lamellulae, narrow, orange grey (5B2) finally black, not deliquescent. Stipe 65–90 × 1.5–2 mm, cylindrical, hollow, central, glabrous, dull, orange grey (5B2).
Basidiospores (8–)9–11 × 7–9 × (6–)7–8 μm, Q = 1.13–1.43, q = 1.25 ± 0.10 (n = 25), lentiform, broadly ovoid to subglobose in frontal view, ellipsoid to broadly ellipsoid in lateral view, smooth, dark brown, moderately thick-walled, germ-pore central, 1.5–2 μm wide. Basidia 17–22 × 9–11 μm, clavate to broadly clavate, with 4 sterigmata, thin-walled, inamyloid. Lamella-edge heteromorphous with cheilocystidia. Cheilocystidia common, 31–45 × 16–21 µm, clavate to broadly clavate, hyaline, inamyloid, thin-walled. Pleurocystidia numerous, 26–35 × 13–25 µm, pyriform to turbinate, hyaline, inamyloid, thin-walled. Pileipellis a non-stratified epithelium of hyaline elements and numerous sclerocystidia, elements 19–40 × 15–23 µm, clavate to pyriform, hyaline, inamyloid, thin-walled; sclerocystidia setoid 56–190 × 14–19 µm, lageniform, cylindrical, brown, thick-walled (up to 4 µm).
Habitat and distribution: Solitary on dead wood or the ground, and known from Sri Lanka, Pakistan and Thailand (Redhead et al. 2001; Szarkándi et al. 2017; This study).
Material examined: Thailand, Nakhon Ratchasima Province, Khao Yai National Park, trail to Kong Kaew waterfall, 14°26′22′′ N, 101°22′19′′ E, elevation 744 m, 24 September 2018, N. Wannathes, N. Suwannarach, J. Kumla, S. Khuna, P. Lumyong and S. Lumyong, SDBR-CMUNW1161 (new record for Thailand).
GenBank number: ITS = OP503478.
Notes: Parasola setulosa was classified into Parasola section Auricomi based on morphological and phylogenetic analysis (Fig. 91). This species is characterized by a small to medium-sized coprinoid basidiomes with light brown to greyish brown, radial plicato-striate pileus, moderately crowded lamella, cylindrical, orange grey stipe, lentiform basidiospores with broadly ovoid to subglobose in frontal view with the size of 8–11 × 7–9 × 6–8 µm, and central germ pore. The pileipellis is composed of hyaline sclerocystidia with brown thick wall, and presence of cheilo- and pleurocystidia. The Thai specimen matches nicely with the description of type study reported by Nagy et al. (2010) and is in concordance with the note of Pegler (1986). Parasola setulosa is morphologically similar to P. malakandensis, but differs by forming larger basidiospores (13–18 × 12.5–16 × 10–13 µm, Hussain et al. 2016).
Phylogenetic tree derived from maximum likelihood analysis of ITS gene of 23 sequences and the aligned dataset was comprised of 667 characters including gap. The average standard deviation of the split frequencies of the BI analysis was 0.00826. A best scoring RAxML tree was established with a final ML optimization likelihood value of -2987.9897. The matrix had 268 distinct alignment patterns with 9.05% undetermined characters or gaps. Estimated base frequencies were found to be: A = 0.2290, C = 0.2428, G = 0.2255, T = 0.3024; substitution rates AC = 1.3958, AG = 2.6467, AT = 2.2120, CG = 0.2505, CT = 4.1800, GT = 1.0000. Coprinopsis pseudomarcescibilis AH33711 HNL501802 and Coprinopsis villosa NL1758 were used as outgroup. Numbers above branches are the bootstrap statistics percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap values ≥ 70% and PP ≥ 0.90 are shown at each branch. The bar represents 0.1 substitutions per nucleotide position. Type strains are in bold. The newly generated sequences are indicated in blue
Strophariaceae Singer & A.H. Sm.
Notes: The family Strophariaceae was established by Overeem in 1927. It is an important group in Basidiomycota. This family accommodates 18 genera and about 1300 species, distributing worldwide (Kirk et al. 2008). There are many edible and medicinal properties in this family, such as Pholiota nameko, P. adiposa, Stropharia rugosoannulata and so on (Dai et al. 2008, 2010).
Pholiota (Fr.) P. Kumm.
Notes: Pholiota is a widespread agaric genus, containing about 150–155 species worldwide, with most species being found in the north temperate zone (Smith and Hesler 1968; Jacobsson 1990; Holec 2001; Kirk et al. 2008; Noordeloos 2011; Holec et al. 2014). The genus Pholiota is mainly characterized by a glabrous or fibrillose to scaly pileus often with yellow and brown tint, a partial veil forming an annulus on the stipe, rusty brown to yellow–brown spore deposit, smooth basidiospores frequently with a germ pore, and often typical chrysocystidia or prominent lageniform pleurocystidia (Smith and Hesler 1968; Jacobsson 1990).
Pholiota betulicola T. Bau & E.J. Tian, sp. nov.
Index Fungorum number: IF559944; Facesoffungi number: FoF 12906; Figs. 92, 93
Etymology: The epithet betulicola refers to the tree which this species grows on.
Holotype: HMJAU37328.
Diagnosis: Differs from other Pholiota species by yellowish pileus with light brown minute fibrillose spots, distinct spicy-aromatic content, whitish stipe with brownish longitudinal stripes and basal yellowish tomenta, plerocystidia buried in hymenium, sometimes sub-rectangle basidiospores, as well as growing on living birch.
Pileus 11 cm in diam, convex; surface slightly viscid, with appressed light brown (6D6) minute fibrillose spots; brownish yellow (5C8) at center, becoming yellowish white (1A2) to white towards the margin; the margin irregularly undulate. Context thick, firm, yellowish white (2A2), odor distinct spicy-aromatic. Lamellae adnexed, broad, close, L = 60–65 mm, I = 3–7 mm, dark blonde (5D4), the edges even. Stipe 5 cm long, 1.9 cm thick, central or eccentric, slightly enlarged at the base, hard, solid, smooth and white above the annulus, ground color whitish and with brownish longitudinal stripes and brown fibrillose squamules below the annulus, with yellowish white (1A2) tomenta near the base. Partial veil leaving an annular zone. Spore print cinnamon brown (6D6).
Basidiospores (6.5–)7.5–10 × (3.7–)4.5–5 μm, Q = (1.25–)1.5–2, in face view elliptic, oblong to sub-rectangle, rarely broadly elliptic, in side view elliptic, somewhat inequilateral to slightly reniform, wall smooth and thick, germ pore minute, pale rusty, yellow-brownish to pale brown in KOH, slightly paler in Melzer’s reagent. Basidia 22–28 × 5–7.5 μm, 4-spored, clavate, hyaline in KOH. Pleurocystidia 15–27 × 4.5–5 μm, buried in the hymenium, clavate to subfusiform, sometimes with refractive and amorphous-granular content, wall thin, smooth, pale tawny to dark yellow brown in KOH. Cheilocystidia 17–25 × 5–8 μm, clavate, clavate-irregular to utriform or lageniform to versiform, rarely branched, thin-walled, smooth, content homogeneous, hyaline to pale yellow brown in KOH. Caulocystidia not observed. Gill trama of parallel hyphae hyaline to yellowish in KOH, walls smooth, thin to thick, 3.5–10 μm in diam. Pileus cutis of yellowish brown to cinnamon hyphae 2.5–5 μm in diam, thin-walled, smooth. Content hyphae hyaline to yellowish in KOH, cells inflated, smooth, thin-walled, interwoven. Clamp connections present in all tissues.
Habitat: Solitary at the base of living birch in mixed broadleaf-conifer forest in late summer.
Materials examined: China, Jilin Province, Jiaohe, Hongye Valley, elev. 520 m, solitary at the base of living birch in mixed broadleaf-conifer forest, 27 August 2016, Tolgor Bau, HMJAU37328 (holotype); Jiaohe, Shansongling, 1 September 2020, Yuhou Zhai, HMJAU37369.
Genbank numbers: HMJAU37328: ITS = OP244886, LSU = MN251156, RPB2 = MN329729, TEF1-α = MN311972; HMJAU37369: ITS = OP244887, LSU = OP223414.
Notes: This species is readily recognized due to its yellowish pileus with light brown minute fibrillose spots, thick content with distinct spicy-aromatic odor reminding of Tricholoma matsutake, whitish stipe with brownish longitudinal stripes and yellowish tomenta at the base, as well as numerous plerocystidia buried in hymenium and sometimes sub-rectangle basidiospores.
Growing on living trees and the large and robust basidiomes of this species remind one of Pholiota populnea. However, the pileus of the latter has no yellow tone and covers with conspicuous floccose or patches scales, and the lack of the pleurocystidia and cylindric-capitate cheilocystidia also makes it different from P betulicola.
In the phylogenetic analysis (Fig. 94), the samples of Pholiota betulicola occupied an isolated phylogenetic position apart from the other species of Pholiota. Furthermore, in the multiple alignment, this species has a unique insertion of 181 bp.
Pholiota subcaespitosa E.J. Tian, sp. nov.
Index Fungorum number: IF559945; Facesoffungi number: FoF 12907; Figs. 95, 96
Etymology: Based on the similarity to Pholiota caespitosa.
Holotype: HMJAU37330.
Diagnosis: Differs from other Pholiota species by the pileus with umbonate or depressed disc, slim stipe, brownish to brown fibrillose scales on the surface of pileus and stipe, small basidiospores, pleurocystidia as chrysocystidia, cheilocystidia mainly with two shapes: fusoid-ventricose and cylindric with subcapitate, the former small and rare with short cells chained at base, the latter often flexuous and at times with branches.
Pileus 18–35 mm broad, convex, becoming plane, with obtuse umbo or slightly depressed on the disc in age, with a waved margin at maturity, yellowish white (4A2), fading to near whitish toward margin, light brown (5D4) on the disc, slightly viscid when wet, decorated with brownish fibrillose scales appressed and often not apparent in age. Lamellae adnate to sinuate-uncinate or with a decurrent tooth, light brown to brown, narrow, close, edges even. Stipe 40–70 mm long, 2–5 mm thick, equal, solid becoming stuffed to hollow, dingy pallid, becoming light brownish below, naked above, elsewhere with dingy brown fibrils or scales. Veil fibrillose, leaving an evanescent ring zone.
Spore deposit brown. Basidiospores 5–7 × 3.2–4 μm (Q = 1.5–2), in face view elliptic, oblong to subovate, in side view slightly inequilateral, wall smooth and thick, germ pore distinct, sometimes making the spore apex somewhat truncate, rust brown (6E8) to light brown (6D8) in KOH, slightly paler in Melzer’s reagent. Basidia 20–24 × 5.5–6.5 μm, 4-spored, clavate, hyaline in KOH. Pleurocystidia as chrysocystidia, 30–42 × 8–9.5 μm, subfusoid, fusoid-ventricose to clavate-mucronate, with thin and smooth wall, hyaline to yellow brown in KOH, content showing a refractive amorphous inclusion as revived in KOH. Cheilocystidia 20–67 × 5–8 μm, cylindric with subcapitate apex, often flexuous, at times with branches, or fusoid-ventricose, some ventricose with 2–3 short cells chained at base, wall thin, rare slightly thick, hyaline in KOH, rarely yellowish, content homogeneous. Gill trama parallel, hyphae 3–24 μm diam, thin-walled, hyaline to yellowish brown in KOH. Pileus hyphae 2–8.6 μm in diam, yellowish to yellowish brown in KOH, wall thin, with spiral incrustations. Context hyphae 3–10 μm in diam, thin-walled, hyaline in KOH. Clamps regularly present.
Habitat: Caespitose on hardwood stumps in autumn.
Materials examined: China, Jilin Province, Changchun, Saman huanle Valley, Jingyuetan National Forest Park, 10 September 2016, Guangcheng Cao, HMJAU37330 (holotype); ibid., 1 September 2012, Enjing Tian, HMJAU37368.
GenBank numbers: HMJAU37330: ITS = OP244888, LSU = OP223415; HMJAU37368: ITS = OP244889, LSU = OP223416.
Notes: Pholiota subcaespitosa is similar to P. caespitosa in shape, germ pore and size of basidiospores, the pleurocystidia, and caespitose growth (Smith and Hesler 1968). However, the latter lack cylindric cheilocystidia. In the phylogram (Fig. 94), P. subcaespitosa clustered together with P. caespitosa and P. gummosa. Pholiota gummosa has the similar cylindric cheilocystidia with P. subcaespitosa, but the former can be easily distinguished by its absence of pleurocystidia (Smith and Hesler 1968).
Atheliales Jülich.
Notes: Sulistyo et al. (2021) recently emended the taxonomic frame of Atheliales. For now, five families are accepted in this order, viz. Atheliaceae, Byssocorticiaceae, Lobuliciaceae, Pilodermataceae and Tylosporaceae (Sulistyo et al. 2021).
Atheliaceae Jülich.
Notes: Atheliaceae, typified by Athelia, is a family dominated by saprotrophic taxa (but with one lichenicolous species Athelia arachnoidea; Sulistyo et al. 2021). Two genera, viz. Athelia and Fibulomyces are included in this family (Sulistyo et al. 2021).
Athelia Pers.
Notes: Athelia was originally erected by Persoon (1818), and more than one hundred years later Athelia epiphylla was designated as the type species of this genus (Donk 1957). Athelia is the largest genera in Atheliales, with 117 names are recorded in Index Fungorum (18 November 2022). Morphologically, Athelia is characterized by thin and pellicular basidiomes, loosely intertwined subicular hyphae and inamyloid basidiospores (Bernicchia and Gorjó 2010; Maekawa et al. 2020).
Athelia naviculispora S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901054; Facesoffungi number: FoF 14808; Figs. 97, 98
Etymology: naviculispora (Lat.) referring to the navicular basidiospores.
Holotype: LWZ 20200921-21a (HMAS).
Diagnosis: Differing from other species of Athelia in the absence of cystidia and the presence of navicular to broadly subfusiform basidiospores.
Basidiomes resupinate, effused, thin, loosely adnate, athelioid, fragile, up to 10 cm long, 2 cm wide, 100 µm thick. Hymenophore smooth, reticulate or continuous, grayish white when fresh, pale mouse-grey to olivaceous buff when dry, not cracked. Margin thinning out as byssoid, finely fibrillose, white, 0.8 mm wide.
Hyphal system monomitic; generative hyphae without clamp connections, hyaline, thick-walled, frequently branched at right angles, 6–8 µm in diam. Cystidia absent. Basidia clavate, 4-sterigmata, simple-septate at the base, 18–22 × 9–13 µm. Basidiospores navicular to broadly subfusiform, hyaline, thin to slightly thick-walled, smooth, IKI–, CB–, 9–11 × 5–6 µm, L = 10 µm, W = 5.5 µm, Q = 1.8 (n = 60/2).
Material examined: China, Sichuan Province, Liangshan Yi Autonomous Prefecture, Leibo County, Mamize Provincial Nature Reserve, on fallen trunk of angiosperm, 21 September 2020, L.W. Zhou, LWZ 20200921-21a (HMAS, holotype); Anhui Province, Jinzhai County, Tianma National Nature Reserve, on fallen trunk of Pinus, 12 October 2020, L.W. Zhou, LWZ 20201012–13 (HMAS).
GenBank numbers: LWZ 20200921-21a: ITS = OR557252, LSU = OR527278; LWZ 20201012–13: ITS = OR557253.
Notes: Phylogenetically, Athelia naviculispora is nested within the core clade of Athelia including the generic type A. epiphylla (Fig. 99). Athelia naviculispora may be morphologically confused with A. epiphylla by grayish hymenophore and absence of cystidia; however, A. epiphylla differs in its cylindrical to ellipsoid basidiospores (Eriksson and Ryvarden 1973; Bernicchia and Gorjón 2010).
Phylogeny generated by the maximum likelihood algorithm based on combined ITS and nLSU regions is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Coniophora marmorata MUCL 31667 and Pseudomerulius aureus FP-103859 were selected as the outgroup taxa. Holotypes are in bold and the newly generated sequences are in blue
Byssocorticiaceae Jülich.
Notes: Byssocorticiaceae is originally erected in Atheliales by Jülich (1981). Recently, Sulistyo et al. (2021) emended the circumscription of Byssocorticiaceae and accepted Athelopsis, Byssocorticium and Leptosporomyces in this family.
Athelopsis Oberw. ex Parmasto.
Notes: Athelopsis, typed by A. glaucina, is characterized by the pellicular basidiomes, usually with yellowish or green tints and ellipsoid, allantoid to cylindrical basidiospores (Bernicchia and Gorjón 2010; Gorjón et al. 2011). Athelopsis is similar to Athelia in micromorphology, but differs in its basidia arranged in a compacted layer (Bernicchia and Gorjón 2010; Gorjón et al. 2011).
Athelopsis subglaucina S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901055; Facesoffungi number: FoF 1480; Figs. 100, 101
Etymology: subglaucina (Lat.) referring to the similarity to A. glaucina.
Holotype: LWZ 20180512–13 (HMAS).
Diagnosis: Differing from A. glaucina in whitish to ash-gray basidiomes and wider basidiospores (Eriksson and Ryvarden 1973; Bernicchia and Gorjón 2010).
Basidiomes resupinate, effused, thin, loosely adnate, up to 12 cm long, 3 cm wide, 100 µm thick. Hymenophore smooth, continuous, whitish to ash-gray when fresh, grayish when dry, not cracked. Margin thinning out, concolorous.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline, thin-walled, frequently branched, 2–3 µm. Cystidia absent. Basidia clavate, 14–16 × 6–7 µm, with four sterigmata and a basal clamp connection. Basidiospores cylindrical, some narrowed toward the apex, hyaline, thin walled, smooth, IKI–, CB–, 8.5–10 × 2.5–3 µm, L = 9 µm, W = 2.6 µm, Q = 3.5 (n = 30/1).
Material examined: Australia, Victoria, Yarra Ranges National Park, Dandenong Ranges Botanic Garden, on fallen branch of Eucalyptus, 12 May 2018, L.W. Zhou, LWZ 20180512–13 (HMAS, holotype).
GenBank numbers: ITS = OR557255, LSU = OR527279.
Notes: Athelopsis subglaucina is morphologically similar to A. glaucina and these two species have a close phylogenetic relationship (Fig. 99). However, A. glaucina differs in yellowish basidiomes and narrower basidiospores (2–2.5 µm in width; Eriksson and Ryvarden 1973; Bernicchia and Gorjón 2010).
Atheliales, genus incertae sedis
Atheliella S.L. Liu & L.W. Zhou, gen. nov.
Index Fungorum number: IF 901056; Facesoffungi number: FoF 14802;
Etymology: Atheliella (Lat.) referring to the close relationship with Athelia.
Diagnosis: Characterized by the pellicular basidiomes with cracked and peeped out, brownish subiculum, loosely intertwined subicular hyphae, the absence of cystidia, and ellipsoid basidiospores.
Basidiomes resupinate, effused, thin, loosely adnate, soft, membranous. Hymenophore smooth, continuous, whitish. Subiculum cracked, peeped out brownish. Hyphal system monomitic; generative hyphae with clamp connections. Cystidia absent. Basidia clavate, with four sterigmata. Basidiospores ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–. On wood.
Type species: Atheliella conifericola S.L. Liu & L.W. Zhou.
Notes: Atheliella falls into the Atheliales, but is separated from any known families and genera (Fig. 99). The family position of this genus needs to be further clarified. Atheliella seems to be morphologically related to members of Atheliaceae; however, Atheliella is distinct by the combination of brownish subiculum and ellipsoid basidiospores (Eriksson and Ryvarden 1973; Bernicchia and Gorjón 2010).
Atheliella conifericola S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901057; Facesoffungi number: FoF 14810; Figs. 102, 103
Etymology: conifericola (Lat.) referring to occurrence on conifers.
Holotype: LWZ 20191104–36 (HMAS).
Diagnosis: Characterized by the pellicular basidiomes with cracked and peeped out, brownish subiculum, loosely intertwined subicular hyphae, the absence of cystidia, and ellipsoid basidiospores.
Basidiomes resupinate, effused, thin, loosely adnate, soft, membranous, up to 10 cm long, 5 cm wide, 200 µm thick. Hymenophore smooth, continuous, whitish, pinkish buff when dry. Subiculum cracked, peeped out brownish. Margin thinning out, brownish.
Hyphal system monomitic; generative hyphae with clamp connections; subicular hyphae yellowish, thick-walled, moderately branched at right angles, 3 µm in diam; subhymenia hyphae hyaline, thin-walled, frequently branched. Cystidia absent. Basidia clavate, with four sterigmata and a basal clamp connection, 14–18 × 3.5–5 µm. Basidiospores ellipsoid, hyaline, thin-walled, smooth, IKI–, CB–, 3.8–4.2 × 2–2.3 µm, L = 4 µm, W = 2.2 µm, Q = 1.8 (n = 60/2).
Material examined: China, Yunnan Province, Dali Bai Autonomous Prefecture, Cangshanerhai National Nature Reserve, Cangshan Mountain, on stump of Pinus, 4 November 2019, L.W. Zhou, LWZ 20191104–36 (HMAS, holotype); ibid., on fallen trunk of Pinus, 4 November 2019, L.W. Zhou, LWZ 20191104–37 (HMAS).
GenBank numbers: LWZ 20191104–36: LSU = OR527280; LWZ 20191104–37: ITS = OR557254, LSU = OR527281.
Notes: Morphologically, Atheliella conifericola resembles Athelia bombacina by thin and pellicular basidiomes, loosely intertwined subicular hyphae with encrusted crystals, clamp-connected hyphae, the absence of cystidia, and ellipsoid basidiospores (Eriksson and Ryvarden 1973; Bernicchia and Gorjón 2010). However, Athelia bombacina differs in its whitish basidiomes and larger basidiospores (4.5–6 × 2.5–3 µm, Eriksson and Ryvarden 1973; 4.5–5.5 × 2.5–3 µm, Bernicchia and Gorjón 2010).
Boletales E.-J. Gilbert.
Notes: Boletales was first formally proposed by Gilbert (1931). It is a diverse order that includes stipitate-pileate forms, puffball-like forms, resupinate or crust-like fungi, and polypore-like species (Binder and Hibbett 2006). This order contains five suborders (Boletineae, Sclerodermatineae, Suillineae, Coniophorineae and Tapinellineae), 16 families, 141 genera, and more than 2000 species (Kirk et al. 2001; Binder and Hibbett 2006; He et al. 2019). Phylogenomic analysis estimates that the Boletales lineage arose in the early Cretaceous period approximately 113 Mya (Watkinson and Eastwood 2012).
Boletaceae Chevall.
Notes: Boletaceae was introduced by Chevallier (1826) as a family separated from the Agaricaceae to accommodate Boletus. Species of Boletaceae are mainly characterized by stipitate-pileate forms with fleshy context and a tubulate, sometimes lamellate or loculate hymenophore, while few species in this family are puffball-like fungi (Binder and Hibbett 2006; Kirk et al. 2008; Wu et al. 2014, 2016a). The family contains approximately 92 genera, which are interesting and important in forestry for their mycorrhizal properties and the edibility of many species (Zeng et al. 2013, 2014; Chai et al. 2019; He et al. 2019).
Aureoboletus Pouzar.
Notes: Aureoboletus (Boletaceae, Basidiomycota) is a relatively small but cosmopolitan genus; species in this genus can be found in tropical, subtropical and temperate regions of different continents, especially rich in Asia and North America (Klofac 2010; Wu et al. 2016a; Zhang et al. 2019). This group of fungi can form ectomycorrhizal symbiosis with an array of ectotrophic plants of the families Fagaceae, Myrtaceae and Pinaceae (Pouzar 1957; Yang et al. 2003; Klofac 2010; Wu et al. 2016a; Zhang et al. 2019). Phylogenetic analyses demonstrated that Aureoboletus is a monophyletic group, and about 50 species have been reported worldwide (Klofac 2010; Wu et al. 2016a; Zhang et al. 2019; Wang et al. 2020b).
Aureoboletus minimus Ming Zhang, C.Q. Wang & T.H. Li, sp. nov.
Index Fungorum number: IF 900314; Facesoffungi number: FoF 14038; Fig. 104
Etymology: “minimus” refers to the small basidiomes.
Holotype: GDGM44400.
Macroscopic characters: Basidiomes small-sized. Pileus 0.8–2 cm wide, obtuse to convex at first, becoming broadly convex to plane in age, fleshy, surface dry or slightly viscid when wet, covered with fibrillose to tomentose squamules, light orange (6A6), pastel red (7A6–9A6) to grayish red (7B5–9B5, 8C5–9C5), usually cracking when mature; margin somewhat involute at young, nearly flat when mature, without veil remnants at margin. Context 1–2.5 mm thick at stipe, firm and tough in youth, soft when matured, white on the whole, greyish red (10B5–11B5) beneath pileipellis, slightly becoming greyish-pinkish or greyish red (10B5–11B5) when exposed. Tubes 2–3 mm deep, distinctly depressed around stipe, yellowish white, yellowish gray to pale yellow (2A2–4A3, 2A3–4A3, 2B2–3B2), often with an olive tint, unchanging when bruised. Pores 2–3 per mm, irregular angular, slightly elongated around stipe at maturity, concolorous with tubes, unchanging when bruised. Stipe 15–40 × 3–6 mm, central, cylindrical or narrowly clavate, solid, slightly enlarged toward the base, concolourous with pileus, covered with white to yellowish brown fibril or tomentum, with white basal mycelium. Odour not distinct. Taste mild.
Microscopic characters: Basidiospores 13–16 × 5–6 µm, (\(\overline{x }\) = 14.5 × 5.4 μm, n = 20), subfusiform, inequilateral in side view, smooth, yellowish to yellowish brown in 5% KOH, thin-walled. Basidia 25–30 × 7–10 µm, clavate, 4-spored, sterigmata 2–4.5 µm long, yellowish white to hyaline in 5% KOH, without basal clamps. Pleurocystidia 32–50 × 10–13 μm, fusiform, thin-walled. Cheilocystidia frequent, similar to pleurocystidia in shape and size. Hymenophoral trama subparallel, yellowish white to hyaline in 5% KOH, with 4–10 μm broad. Pileipellis a trichodermium of erect hyphae 12–22 μm in diam, yellowish white to hyaline in 5% KOH; terminal cells 37–70 × 12–22 µm, cylindrical, clavate or nearly fusoid. Stipitipellis a layer of repent to suberect branching hyphae 4–8 μm in diam, hyaline in 5% KOH. Clamp connections absent in all tissues.
Habit, habitat and distribution: Solitary to scattered on soil or decayed wood in broadleaf forest dominated by Fagaceae trees. Currently only known from central Vietnam.
Material examined: Vietnam, Lam Dong Province, Da Lat, Bi Doup Nui Ba National Park, alt. 1500 m, 15 October 2017, Ming Zhan, GDGM44400 (holotype), Tai-Hui Li, GDGM44401.
GenBank numbers: GDGM44400: LSU = OP901640, TEF1-α = OP918149, RPB1 = OP918155, RPB2 = OP918152; GDGM44401: LSU = OP901641, TEF1-α = OP918150, RPB1 = OP918156, RPB2 = OP918153.
Notes: Aureoboletus minimus is characterized by its small basidiomes with a dry and usually cracked pileus densely covered with fibrillose to tomentose squamules, white context slightly changing greyish-pinkish or greyish red when expose, yellowish gray to pale yellow hymenophore unchanging when bruised, subfusiform and relatively larger basidiospores. The above combination of features makes it easily distinguished from other species in Aureoboletus.
Morphologically, A. minimus is similar to A. glutinosus and A. tenuis. However, A. glutinosus differs by its strongly glutinous or mucilaginous basidiomes, reddish-brown to greyish-ruby pileus with irregular reticulation and gelatinous veil remnants at margin, and smaller basidiospores 10–13.5 × 4.5–5 µm (Zhang et al. 2019). Aureoboletus tenuis differs in having relatively larger baisidomata, glutinous and wrinkled pileus, and smaller basidiospores 11–12 × 4–5 μm (Zhang et al. 2014).
Phylogenetic analyses based on the combined sequence of LSU, TEF1-α, RPB1, RPB2 shown that two specimens of A. minimus formed a distinct lineage in Aureoboletus, and showed close relationships with A. auriflammeus, A. garciae and A. miniatoaurantiacus but with low support value and larger genetic distance (Fig. 105). In morphology, A. auriflammeus and A. miniatoaurantiacus can be easily distinguished by their pileus minutely covered with orange yellow, orange to reddish-orange tomentum or powder (Zhang et al. 2019). Aureoboletus garciae, recently reported from Mexico, differs in having vivid blue to light blue pileus with some reddish tones, bright yellow hymenophore, and smaller basidiospores 9–14 × 4–5(–6) µm (Haelewaters et al. 2020).
Phylogram generated from maximum likelihood analysis based on combined LSU, tef1, rpb1 and rpb2 sequence data of Aureoboletus. Bootstrap support values for maximum likelihood greater than 50% are indicated above or below the nodes, and branches with Bayesian posterior probabilities greater than 0.95 are given in bold. The new species are in blue. The tree is rooted with Phylloporus imbricatus HKAS68642 and Xerocomus subtomentosus HKAS58865
Aureoboletus nanlingensis Ming Zhang, C.Q. Wang & T.H. Li, sp. nov.
Index Fungorum number: IF 900315; Facesoffungi number: FoF 14039; Fig. 106
Etymology: “nanling” refers to the type locality Nanling National Forest Park.
Holotype: GDGM71707.
Macroscopic characters: Basidiomes small-sized. Pileus 2–4.5 cm wide, hemispheric when young, becoming convex to nearly plane in age, fleshy, dry, subtomentose, grayish yellow to brownish orange (4C5–7C5), slightly paler toward pileus margin; margin thin, slightly incurved when young, becoming nearly straight in age, without veil remnants. Context 4–7 mm thick at center, firm and tough in youth, becoming soft, white, unchanging when exposed. Tubes 3–6 mm deep, pastel yellow, light yellow, greenish yellow to greyish yellow (1A4–1A2, 2A4–2A5, 1B4–2B4), unchanging when bruised. Pores small, 2–3 per mm, somewhat larger around the stipe, circular to angular, concolourous with tubes, unchanging when bruised. Stipe 40–60 × 8–10 mm, central, cylindrical or clavate, equal to slightly enlarged downwards, surface dry, yellowish white to pale yellow (1A2–2A2, 1A3–2A3), with obscure fibrous stripes. Stipe context white, unchanging or slightly changing greyish red when exposed, especially in the lower part. Basal mycelium white. Odour none. Taste mild.
Microscopic characters: Basidiospores 7–8.5 × (4–)4.5–5 µm, (\(\overline{x }\) = 7.7 × 4.7 μm, n = 20), ellipsoidal to broadly ellipsoidal, oblong in ventral view, smooth, yellowish to yellowish brown in 5% KOH, thin-walled. Basidia 25–30 × 8–10 µm, clavate, 4-spored, sterigmata 3–5 µm long, yellowish white to hyaline in 5% KOH, without basal clamps. Pleurocystidia 35–60 × 10–13 μm, fusiform, thin-walled. Cheilocystidia frequent, similar to pleurocystidia in shape and size. Hymenophoral trama subparallel, yellowish white to hyaline in 5% KOH, with 4–10 μm broad. Pileipellis a trichodermium of suberect hyphae 8–15 μm in diam, branched, yellowish white to hyaline in 5% KOH; terminal cells 40–70 × 8–15 µm, cylindrical, clavate or nearly fusoid. Stipitipellis a layer of repent hyphae 4–8 μm in diam, hyaline in 5% KOH. Clamp connections absent in all tissues.
Habit, habitat and distribution: Solitary to scattered on soil in broadleaf forest dominated by Fagaceae trees. Currently only known from southern China.
Material examined: China, Guangdong Province, Shaoguan, Nanxiong Town, Qingzhangshan-Xiaoliukeng Provincial Natural Reserve, alt. 500 m, 16 May 2018, Ming Zhang, GDGM71707 (holotype); ibid., alt. 580 m, 17 May 2018, Ming Zhang, GDGM44729.
GenBank numbers: GDGM71707: LSU = MN204556, RPB1 = MN473174, RPB2 = MN549710; GDGM44729: LSU = OP901643, TEF1-α = OP918151, RPB1 = OP918157, RPB2 = OP918154.
Notes: Aureoboletus nanlingensis is characterized by its dry, subtomentose and grayish yellow to brownish orange pileus, white context unchanging when exposed, light yellow to greyish yellow hymenophore unchanging when bruised, and ellipsoidal to broadly ellipsoidal basidiospores. The combination of above features corresponds to the define of “Clade II” of Aureoboletus in Zhang et al. (2019).
Morphologically, A. nanlingensis resembles A. raphanaceus and A. solus. However, A. raphanaceus differs by its yellowish-white to pinkish-white pileus covered with fibrillose to tomentose squamules, radish smell and broader basidiospores 7.5–9 × 5–6 μm (Zhang et al. 2019); A. solus differs by its brownish-yellow to greyish-red pileus, glabrous stipe and larger basidiospores (7–)8–10.5(–11) × (4–)4.5–5 μm (Zhang et al. 2019).
Phylogenetically, A. nanlingensis nested well into the “Clade II” of genus Aureoboletus, which defined by Zhang et al. (2019), and closely related to A. velutipes. However, the latter differs by its brown orange to reddish-brown pileus, light yellow to pastel yellow stipe covered with obviously fibrillose to tomentose squamules, and larger basidiospores 10–13 × 4–6.5 μm (Zhang et al. 2019).
Hemileccinum Šutara.
Notes: The genus Hemileccinum was erected with type species H. impolitum (Šutara 2008). Species in this genus can be distinguished by their rugose pileus often covered with inconspicuous subtomentose or pulverulent squamules, unchanging context and hymenophore when bruised, often yellowish punctate squamules on the stipe surface, fusoid basidiospores ornamented with irregular warts, and hyphoepithelium to subepithelium pileipellis. Currently Hemileccinum comprises 15 species (Šutara 2008; Halling et al. 2015; Wu et al. 2016a; Kuo and Ortiz-Santana 2020; Li et al. 2021). Among them, seven species were recorded from China including the new species described below.
Hemileccinum duriusculum Mei-Xiang Li, Zhu L. Yang & G. Wu, sp. nov.
Index Fungorum number: IF 901102; Facesoffungi number: FoF 14841; Figs. 107, 108, 109
Etymology: The epithet ‘duriusculum’ refers to the wrinkled pileus.
Holotype: HKAS 125907.
Basidiomes stipitate-pileate, medium-sized to large. Pileus up to 9 cm in diam, convex to plano-convex, surface glabrous, but some areas often covered with inconspicuous subtomentose or pulverulent squamules, often strong rugose, light brown to brown (5D5, 6D5–6E5), sometimes much paler with light red tinge, margin and surface ridges lighter in color, dry; context yellowish white to pale yellow (1A2–1A3), unchanging when bruised, ca. 10–15 mm thick. Hymenophoral tubes concolorous with pores, pale yellow (3A3) to grayish yellow (4B4), unchanging when bruised; pores polygonal to roundish, 1.5–2 per mm, tubes ca. 13 mm long, unchanging when bruised. Stipe 10–13 × 1.2–2.1 cm, subcylindrical, clavate, slightly bulbous at the base, upper part light yellow (4A5), lower part reddish to grayish reddish (5C4–8C5); surface covered with finely granular yellowish to brownish yellowish scales; context of stipe cream to pale yellow, unchanging when bruised; basal mycelium cream.
Basidia 24–35 × 8–12 µm, clavate, thin-walled, 4-spored; sterigmata 4–6 µm long, colorless in KOH. Basidiospores [40/2/2] 12–15 × 4–6 µm [Q = (2.5–) 2.6–2.8 (–3), Qm = 2.74 ± 0.17], subfusiform in side view with slight suprahilar depression, subfusoid in ventral view, yellowish to brownish in KOH, smooth under light microscopy, but ornamented with irregularly warts under SEM. Hymenophoral trama nearly phylloporoid type with hyphae of the lateral strata touching or almost touching each other with hyphae diverging from the central strand to the subhymenium; hyphae subcylindrical to cylindrical, 5–13 µm wide. Cheilocystidia 25–49 × 10–14 µm, lanceolate to clavate or ventricose, thin-walled, colorless in KOH. Pleurocystidia 33–43 × 8–10 µm, ventricose-subfusiform, with long beak, thin-walled. Pileipellis a subepithelium 110–130 µm thick, composed of moniliform hyphal segments 5–30 µm wide, thin-walled, colorless in KOH, with narrowly cylindrical to shortly cystidioid terminal cells 6–23 × 4–20 µm. Pileal trama composed of interwoven hyphae 4–24 µm wide. Stipitipellis ca. 130 µm thick, hymeniform, with narrowly or broadly clavate terminal cells 11–25 × 7–12 µm. Caulobasidia abundant, 16–32 × 9–12 µm, thin-walled. Stipe trama composed of parallel hyphae 4–10 µm wide. Clamp connections absent in all tissues.
Habitat and distribution: Scattered on soil in subtropical forests dominated by Fagaceae (Quercus spp., Castanopsis spp. and Lithocarpus spp.). Currently known from eastern, central and northeastern China.
Material examined: China, Zhejiang Province, Huzhou, Anji County, 1400 m elev., 21 September 2021, Yan-Jia Hao 2527 (HKAS 125907, holotype; EFHAAU2843, isotype). Henan Province, Nanyang, Neixiang County, 600 m elev., 31 July 2010, Xiao-Fei Shi 416 (HKAS76664). Liaoning Province, Anshan, 1980 m elev., 14 August 2015, Jing Li 251 (HKAS91279).
GenBank numbers: HKAS 125907: LSU = OQ600185, TEF1-α = OQ718485, RPB1 = OQ750573; HKAS76664: LSU = OQ600186, TEF1-α = OQ718484, RPB1 = OQ750572, RPB2 = OQ718482; HKAS91279: LSU = OQ600187, TEF1-α = OQ718483, RPB1 = OQ750573, RPB2 = OQ718481.
Notes: Hemileccinum duriusculum is characterized by the yellowish brown to brown wrinkled pileus, context and hymenophore unchanging in color when cut, scaly stipe surface and a subepitheliod pileipellis. Phylogenetically, Hemileccinum rugosum is closely related to H. duriusculum (Fig. 110). However, H. rugosum differs from H. duriusculum in its narrower basidiospores (10–12 × 4–5 µm) and the lighter color (light orange to reddish orange) of the pileus (Wu et al. 2016a). Morphologically, Hemileccinum hortonii originally described from Illinois, USA is somewhat similar to H. duriusculum, but differs from the latter by its narrower basidiospores (12–15 × 3.5–4.5 µm, Smith and Thiers 1971).
Rufoboletus N.K. Zeng & Zhi Q. Liang, gen. nov.
Index Fungorum number: IF559490; Facesoffungi number: FoF 14856.
Etymology: Lain “rufo-”, meaning the hymenophore and context of the new genus turning red when injured.
Basidiomes stipitate-pileate with tubular hymenophore. Pileus hemispherical, convex to applanate, surface tomentose; context thick, white, changing blue quickly, then turning red and finally black when injured. Hymenophore thin, yellow, changing blue quickly, then turning red and finally black when injured. Stipe central, subcylindrical; surface without reticulations; context white, changing blue quickly, then turning red and finally black when injured. Basal mycelium white. Basidiospores subfusiform to ellipsoid, smooth. Pleuro- and cheilocystidia present. Pileipellis a trichoderm. Clamp connections absent.
Type species: Rufoboletus hainanensis (N.K. Zeng, Zhi Q. Liang & S. Jiang) N.K. Zeng & Zhi Q. Liang.
Notes: Rufoboletus is characterized by a large basidiome, a pileus with thick context and thin hymenophore, a blue-red–black color change of hymenophore and context when injured, a stipe without reticulations, and smooth basidiospores. In previous studies, species of Rufoboletus was placed into the genus Butyriboletus (Liang et al. 2016; Chai et al. 2019). However, species of Butyriboletus has a blue (without red) color change of hymenophore and context when injured, and a stipe with obvious reticulations. The thick context and thin hymenophore characterized by Rufoboletus are also reminiscent of the genus Baorangia; however, species of Baorangia has a blue (without red) color change of hymenophore and context when injured, and a stipe usually with reticulations (Wu et al. 2016b; Zhang et al. 2021b).
Phylogenetically, the type species of Rufoboletus, viz. B. hainanensis and Boletus sp. (KUN-HKAS 52525) formed an independent generic clade (Fig. 111), which was labeled as “hainanensis clade” by Biketova et al. (2022) and also supported by the phylogenetic analyses of Gelardi et al. (2015), Liang et al. (2016), Fu et al. (2022) and Wang et al. (2022a, b).
Rufoboletus hainanensis (N.K. Zeng, Zhi Q. Liang & S. Jiang) N.K. Zeng & Zhi Q. Liang, comb. nov.
≡ Butyriboletus hainanensis N.K. Zeng, Zhi Q. Liang & S. Jiang, in Liang, An, Jiang, Su & Zeng, Phytotaxa 267(4): 257 (2016).
Index Fungorum number: IF559491; Facesoffungi number: FoF 14871; Figs. 112, 113
Basidia 32–38 × 8–9 μm clavate, colorless to yellowish in KOH, thin-walled, four-spored; sterigmata 4–6 μm long. Basidiospores [66/3/2] (6.5–)7–9 × 4–5 μm, Q = (1.4–)1.5–2.13, Qm = 1.81 ± 0.18, subfusiform to ellipsoid, slightly thick-walled (up to 0.8 μm), olive brown to yellowish brown in KOH, smooth. Hymenophoral trama boletoid. Cheilocystidia 32–52 × 5–8 μm, abundant, subfusiform or fusiform, thin- to slightly thick-walled (up to 0.5 μm), sometimes with yellowish brown contents, without encrustations. Pleurocystidia 38–70 × 8–12 μm, abundant, fusiform or subfusiform, thin- to slightly thick-walled (up to 0.5 μm), sometimes with brown or golden brown contents, without encrustations. Pileipellis an intricate trichodermal type, 110–160 μm thick, composed of colorless, pale yellowish brown to yellowish brown in KOH, thin- to slightly thick-walled (up to 0.5 μm) hyphae 3–9 μm broad; terminal cells 28–44 × 4–11 μm, narrowly clavate or subcylindrical, with obtuse apex. Pileus trama composed of thin- to slightly thick-walled (up to 0.5 μm) hyphae 3–9 μm broad. Stipitipellis a trichoderm-like structure 90–160 μm thick, composed of thin- to slightly thick-walled (up to 0.5 μm) hyphae with narrowly or broadly clavate, subfusiform or fusiform terminal cells (14–30 × 6–10 μm). Stipe trama composed of cylindrical, thin to slightly thick-walled (up to 0.5 μm), parallel hyphae 4–10 μm broad. Clamp connections absent in all tissues.
Materials examined: China, Hainan Province, Yinggeling of Hainan Tropical Rainforest National Park, elev. 850 m, 26 July 2009, N.K. Zeng334 (KUN-HKAS 59816, holotype), N.K. Zeng332 (KUN-HKAS 59814, paratype), N.K. Zeng333 (KUN-HKAS 59815), 16 June 2013, N.K. Zeng1197 (FHMU2410), 31 July 2015, N.K. Zeng2418 (FHMU2437).
GenBank numbers: KUN-HKAS 59814: ITS = KU317762, LSU = KF112336, TEF1-α = KF112199; FHMU2410: ITS = KU961653, LSU = KU961651, RPB2 = KU961658; FHMU2437: ITS = KU961654, LSU = KU961652, TEF1-α = KU961656, RPB2 = KX453856.
Notes: Rufoboletus hainanensis was firstly described from Hainan, tropical China. The description of macrostructures has been provided by Liang et al. (2016); the microstructures have been reexamined in the present study.
Cantharellales Gäum.
Notes: Cantharellales with Cantharellaceae as the type family is a morphologically diverse fungal order in Agaricomycetes. Although less than 300 species are known in this order (Kirk et al. 2008), these species may have corticioid, stipitate or coralloid basidiomes with smooth, hydnoid, poroid or veined hymenophores (Hibbett et al. 2014). The most striking morphological character of Cantharellales is the dominant presence of two, five, six or eight sterigmata in basidia, instead of four sterigmata present in other orders of Agaricomycetes (Hibbett et al. 2014). Moncalvo et al. (2006) performed the first comprehensive phylogenetic analyses of Cantharellales (the cantharelloid clade) with the help of multiloci. Later, Veldre et al. (2013) recognized four families, viz. Botryobasidiaceae, Ceratobasidiaceae, Hydnaceae (synonym of Cantharellaceae) and Tulasnellaceae in Cantharellales, while later Botryobasidiaceae, Clavulinaceae and Hydnaceae accepted in this order by Zhao et al. (2017). Nevertheless, the taxonomic frame of Cantharellales still needs to be further explored with more samples.
Botryobasidiaceae Jülich.
Notes: Botryobasidiaceae was originally erected to accommodate Botryobasidium (Jülich 1981). Phylogenetically, besides Botryobasidium, Botryohypochnus is also included in the well-supported Botryobasidiaceae clade, which is a sister to Ceratobasidiaceae, Hydnaceae and Tulasnellaceae (Larsson 2007a; Cao et al. 2021).
Botryobasidium Donk.
Notes: Botryobasidium, typified by B. subcoronatum, is a saprobic genus in Cantharellales, and characterized by discontinuous, arachnoid to porulose basidiomes and short, subcylindrical basidia usually with 6–8 sterigmata (Bernicchia and Gorjón 2010; Bernicchia et al. 2010). Botryobasidium was protected over Acladium, Allescheriella, Alysidium, Haplotrichum, Physospora, and Sporocephalium (Stalpers et al. 2021).
Botryobasidium coniferarum S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901058; Facesoffungi number: FoF 14811; Figs. 114, 115
Etymology: coniferarum (Lat.) referring to occurrence on conifers.
Holotype: LWZ 20210928–3 (HMAS).
Diagnosis: Differing from Botryobasidium conspersum in thick-walled hyphae in subiculum (Bernicchia and Gorjón 2010).
Basidiomes resupinate, thin, separable, up to 15 cm long, 6 cm wide, 150 µm thick. Hymenophore smooth, arachnoid, whitish to cream when fresh, honey-yellow to olivaceous buff when dry, not cracked. Margin white, thinning out as byssoid, finely fibrillose, 0.5 mm wide.
Hyphal system monomitic; generative hyphae without clamp connections; subhymenial hyphae hyaline, thin-walled, 6–8 µm in diam; basal hyphae hyaline to yellowish, thick-walled, frequently branched at right angles, 6–10 µm in diam. Cystidia absent. Basidia subcylindrical, 16–20 × 7–9 µm, 6-sterigmata, simple-septate at the base. Basidiospores navicular, hyaline, thin-walled, smooth, IKI–, CB–, 7–8 × 3–3.5 µm, L = 7.5 µm, W = 3.3 µm, Q = 2.3 (n = 60/2).
Anamorph not found.
Material examined: China, Guizhou Province, Jiulong County, Wuxuhai Scenic Spot, on fallen branch of Pinus, 28 September 2019, L.W. Zhou, LWZ 20210928–3 (HMAS, holotype); ibid., on fallen branch of Pinus, 28 September 2019, L.W. Zhou, LWZ 20210928–4 (HMAS); Sichuan Province, Xichang, Luojishan Scenic Spot, on fallen branch of gymnosperm, 11 August 2019, L.W. Zhou, LWZ 20190811-36a (HMAS). VIETNAM, Da Lat, Bidoup Nui Ba National Park, on fallen branch of Pinus, 16 October 2017, L.W. Zhou, LWZ 20171016–5 (HMAS), LWZ 20171016–15 (HMAS).
GenBank numbers: LWZ20171016-5: ITS = OR557261; LSU = OR527285; LWZ20171016-15: ITS = OR557262; LSU = OR527286; LWZ20190811-36a: LSU = OR527284; LWZ 20210928-3: ITS = OR557259; LSU = OR527282; LWZ LWZ 20210928-3: ITS = OR557260; LSU = OR527283.
Notes: Besides differing from the most phylogenetic close (Fig. 116) and morphological similar species Botryobasidium conspersum in thick-walled subicular hyphae (Bernicchia and Gorjón 2010), B. coniferarum also resemble Botryobasidium chilense and B. yutajense. However, B. chilense, originally described from Chile and New Zealand, is separated by broadly rounded basidiospores with a lateral and prominent basal apiculus at the base (Holubová-Jechová 1980) and B. yutajense differs in its shorter basidiospores (6–7 µm in length, Ryvarden et al. 2005).
Phylogeny generated by the maximum likelihood algorithm based on combined ITS and nLSU regions is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Holotype is in bold and the newly generated sequences are in blue. Dacrymyces stillatus UPS:F-939814 was selected as the outgroup taxon
Gomphales Jülich.
Notes: The order Gomphales consists 633 described species in three families and 18 genera (González-Ávila et al. 2017; He et al. 2022). They are abundantly reported from the temperate zones of Northern Hemisphere (Kirk et al. 2008) with a very sparse reports from tropical and subtropical zones (Corner 1966; Petersen 1971; Kirk et al. 2008; González-Ávila et al. 2017). The gomphoid fungi shows great variations in basidiomal morphologies, from hypogeous or epigeous, stalked ramarioid or clavarioid to cantharelloid-gomphoid, resupinate, odontoid, to sequestrate form (Giachini et al. 2010; González-Ávila et al. 2020). Here, we follow the phylogenetic treatment of Gomphales by Giachini et al. (2010).
Gomphaceae Donk.
Notes: The family Gomphaceae was proposed by Donk (1961) in the order Aphyllophorales to incorporate macroscopically heterogenous fungi, which differs in their hymenial constrictions (Giachini 2004). He included the resupinate-odontoid genera Kavinia and Ramaricium, the stalked clavarioid genera Lentaria and Ramaria, the stalked hydnoid genus Beenakia, the stipitate agaricoid genus Gloeocantharellus, and the pileate genera Chloroneuron and Gomphus in his familial classification (Giachini 2010). Later, members of the family Gomphaceae were divided into several smaller families and placed in a new order Gomphales (Jülich 1981). Giachini (2004) reviewed the generic concepts in the family Gomphaceae and recombined the species of Gomphus sensu lato into Gloeocantharellus, Gomphus sensu stricto, Phaeoclavulina and Turbinellus (Giachini et al. 2010). Currently the family Gomphaceae consists 14 genera. They are Araeocoryne, Ceratellopsis, Delentaria, Destuntzia, Gautieria, GloeocantharelIus, Gomphus, Phaeoclavulina, Protogautieria, Pseudogomphus, Ramaria, Ramaricium, Terenodon and Turbinellus (Kirk et al. 2008; Index Fungorum 2022).
Gomphus Pers.
Notes: The genus Gomphus sensu lato was proposed by Persoon (1796) to include the species with wrinkled hymenia and verrucose spore in the cantharelloid-gomphoid clade (Giachini and Castellano 2011; Giachini 2004). The genus currently comprises 16 described species (Index Fungorum 2022). No species was assigned in the genus Gomphus, when it was introduced to generic level. Later, it was Gray (1821) who described Gomphus clavatus as the type species for the genus Gomphus (Giachini and Castellano 2011). Lacks of distinctive morphological characters make it difficult in the taxonomic identification of the genus Gomphus. According to the latest classification by Giachini and Castellano (2011) for Gomphus sensu lato (Gomphus sensu stricto, Gloeocantharellus, Phaeoclavulina and Turbinellus), Gomphus sensu stricto is the only genus with strictly violet, lavender-brown, or milky-coffee colored hymenia.
Gomphus zamorinorum Krishnapriya K. & T.K.A. Kumar, sp. nov.
Mycobank number: MB 844405; Facesoffungi number: FoF 11792; Fig. 117
Etymology: In honour of the Zamorins, the hereditary monarchs (1124 CE – 1806 CE) of the kingdom of Kozhikode that was an important trading port on the south-western coast of India (South Malabar).
Holotype: ZGCKP203A.
Basidiomes gregarious, growing as a cluster, two to three basidiomes in a cluster, 30 to 40 mm long, 6 to 10 mm wide at the apex, not differentiated into stipe and pileus. Young basidiomes cylindrical, arising from a thick rhizomorph like structure, branched (2–3) towards the apex, branches 5 to 6 mm thick, branching irregular, apex pyxidate, round or obtuse, not acute, glabrous to fibrillose. Mature basidiomes becoming partially lobed downwards, branched upwards, round, with broad hymenial folds or wrinkles, ellipsoid in cross section, solid, fleshy, fragile, bright violet fruitbody, with a purplish tint towards the apex, no color change on drying, odour pleasant. Positive reaction in FeCl3.
Basidiospores 6–7 × 4–5 µm (Q = 1.2–1.7 µm; Qm = 1.4 µm), phaseoliform in side view, oblong in front view, with guttulate contents (uniguttulate), verrucose, thin to thick-walled, hyaline, apiculus prominent (up to 1 µm long), in some hilar appendage protruded up to 6–7 µm long, inamyloid, cyanophilic in cotton blue. Basidia 25–50 × 4–7 µm, uniguttulate to agguttulate, cylindrical to clavate, not smooth, incrustations present on the basidia, sterigmata 2–4 (up to 4–7 µm long), cyanophilic. Hymenial cystidia 21–60 × 5–6 µm, cylindrical to flexuose, projecting from the hymenium, thin-walled, inamyloid, pileocystidia absent. Hymenium 80–110 µm wide. Subhymenium not distinguishable. Context composed of interwoven, irregularly arranged, encrusted, and agglutinated hypahe, hyphal constrictions present, septate, 4–8 µm wide, blackish in group, gleophorous hyphae present, in some bulged at the septal portion (up to 11 µm), thin walled, cyanophilic, inamyloid. Hyphal clamp-connections absent.
Habitat: In gregarious clusters among leaf litters on soil.
Material examined: India, Kerala State, Wayanad District, Banasura sagar dam site area (11.6942° N, 75.9081° E), 29 September 2019, Krishnapriya K., ZGCKP203A (holotype), KP203B (isotype).
GenBank numbers: ZGCKP203A: ITS = ON732852; KP203B: ITS = ON732853.
Notes: In ITS based phylogenetic analysis, Gomphus zamorinorum settled in the Gomphus clade, sister to Gomphus clavatus (Fig. 118). Gomphus zamorinorum is characterised by its deep violet basidiomes with wrinkled hymenophore, branched, comparatively smaller basidiospores and basidia, presence of hymenial cystidia, presence of gleophorous hypahe, generative hyphae with ampulliform swelling and absence of clamp connections. Gomphus zamorinorum is similar to G. clavatus macroscopically in having the wrinkled hymenophore with deep violet colour (Giachini et al. 2012). However, G. zamorinorum differs from the latter morphologically, with its branched basidiomes, smaller basidiospore and basidia, absence of pileocystidia, presence of gloeophorous hyphae and absence of clamp-connections. Besides this, hyphal encrustations are observed in G. zamorinorum, which is absent in the latter.
Phylogram generated from maximum likelihood analysis based on ITS sequence data showing the placement of Gomphus zamorinorum. This analysis involved 36 nucleotide sequences. There was a total of 837 positions in the final dataset. Calocera cornea was taken as the out-group. The tree with the highest log likelihood (− 17,846.00) is shown. The percentage of trees in which the associated taxa clustered together is shown next to the branches. Type strains are in bold and newly generated sequences are in blue
Hymenochaetales Oberw.
Notes: Hymenochaetales is one of the largest orders of the class Agaricomycetes. Oberwinkler (1977) included the characters of xanthochoroid polypores in Série des Igniaires (Patouillard 1900), the concept of subfamily Hymenochaetoidae (Donk 1948) of Hymenochaetaceae and raised to the order Hymenochaetales. Micromorphological features of Hymenochaetales varies widely from resupinate, effused-reflexed, imbricate, pileate, stipitate, coral-like to spathulate pilei with smooth, poroid, hydnoid or lamellate hymenophore. Microscopically, mono, mono-di, di or trimitic hyphal system, absence of clamp connections, and globose to cylindrical, smooth to finely ornamented basidiospores, with or without sterile elements such as cystidioles and setae are the distinct characteristic features.
Chaetoporellaceae Jülich.
Notes: Chaetoporellaceae was introduced with Chaetoporellus as the type genus (Jülich 1981). However, due to the conservation of Hyphodontia against its synonyms Chaetoporellus and some other genera (Eriksson et al. 1982; Gams 1993; Langer et al. 1996), the family name Chaetoporellaceae has long been in oblivion. Recently, Wang et al. (2021a) revealed that Chaetoporellus is a later synonym of Kneiffiella and both genera occupy a distinct lineage in Hymenochaetales; therefore, Chaetoporellaceae was reinstated as a monotypic family.
Kneiffiella P. Karst.
Notes: Kneiffiella is a prior synonym over Chaetoporellus and thus represents the single accepted genus in Chaetoporellaceae (Wang et al. 2021a). See Wang et al. (2021a) for the latest comprehensive summarization of Kneiffiella.
Kneiffiella pseudoabdita Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900289; Facesoffungi number: FoF 14052; Figs. 119, 120
Etymology: pseudoabdita (Lat.) referring to the similarity to Kneiffiella abdita.
Holotype: LWZ 20210624-6b (HMAS).
Diagnosis: Characterized by annual, resupinate, adnate basidiomes, poroid hymenophore, the presence of two types of generative hyphae, subcylindrical to subclavate cystidia, clavate basidia, and ellipsoid, thick-walled basidiospores.
Basidiomes annual, resupinate, adnate, cracked and brittle when dry, without odour. Hymenophore poroid with angular and variable pores, buff to buff-yellow. Margin thinning out, paler than hymenial surface, indeterminate, sometimes pruinose.
Hyphal system monomitic; two types of generative hyphae: 1) with clamp connections, hyaline, dichotomous branching, interwoven, slightly thick-walled, 2.5–3.5 μm in diam; 2) simple septate, brown, slightly thick-walled with a wide lumen, usually dichotomous branching, interwoven, 3–4 μm in diam. Cystidia subcylindrical to subclavate, 25–45 × 4.5–5.5 μm. Basidia clavate, 16.5–20 × 4.5–5.5 μm, with four sterigmata and a basal clamp; basidioles similar in shape to basidia, but smaller. Basidiospores narrowly to broadly ellipsoid, with a small oily drop, hyaline, smooth, thick-walled, IKI–, CB–, (5.2–)5.3–6.4(–6.5) × (3.1–)3.2–4.3(–4.4) μm, L = 5.76 μm, W = 3.81 μm, Q = 1.51 (30/1).
Material examined: China, Jiangxi Province, Jiujiang, Lushan Mountain, on fallen trunk of Pinus, 24 June 2021, L.W. Zhou, LWZ 20210624-6b (HMAS, holotype).
GenBank numbers: ITS = OQ540894; LSU = OQ540852.
Notes: Kneiffiella pseudoabdita is distinct in this genus by the presence of two types of generative hyphae. Phylogenetically, Kneiffiella pseudoabdita groups with K. abdita (Fig. 121). These two species share similar poroid hymenophore, tubular cystidia and clavate basidia; however, K. abdita differs in its narrowly allantoid basidiospores (3–4 × 0.5–1 μm, Wang et al. 2021a). Kneiffiella pilaecystidiata resembles K. pseudoabdita by sharing ellipsoid basidiospores, but differs in having odontioid hymenophore and capitate cystidia (Eriksson and Ryvarden 1976). Moreover, K. pilaecystidiata grows on decayed wood of Salix and Populus (Eriksson and Ryvarden 1976), while K. dimitica was found on Pinus.
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Kneiffiella pseudoalutacea Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900290; Facesoffungi number: FoF 14053; Figs. 122, 123
Etymology: pseudoalutacea (Lat.) referring to the similarity to Kneiffiella alutacea.
Holotype: LWZ 20210625-5b (HMAS).
Diagnosis: Characterized by annual, resupinate, adnate basidiomes, smooth to slightly tuberculate, lime to cream hymenophore, a monomitic hyphal system, subcylindrical cystidia, subclavate basidia and narrowly allantoid basidiospores.
Basidiomes annual, resupinate, adnate, membranous, cracked and brittle when dry, without odour. Hymenophore smooth to slightly tuberculate, lime to cream. Margin concolorous with subiculum, thinning out, indeterminate.
Hyphal system monomitic; generative hyphae in trama with clamp connections, hyaline, slightly thick-walled with a wide lumen, parallel, 3.5–4.5 μm in diam; generative hyphae in subhymenium with clamp connections, hyaline, thin-walled to slightly thick-walled with a wide lumen, usually dichotomous branching, 3–4 μm in diam. Cystidia subcylindrical, smooth, thin-walled, 35–50 × 5.5–6 μm, projecting up to 10 µm beyond the hymenial surface. Basidia subclavate with a median constriction, 12–15 × 4–4.7 μm, with a clamp connection at the base; basidioles similar in shape to basidia, but smaller. Basidiospores narrowly allantoid, hyaline, smooth, thin-walled, IKI–, CB–, (6–)6.1–6.8(–7) × 1.3–2.2(–2.3) μm, L = 6.38 μm, W = 1.78 μm, Q = 3.58 (30/1).
Material examined: China, Jiangxi Province, Jiujiang, Lushan Mountains, on fallen branch of gymnosperm, 25 June 2021, L.W. Zhou, LWZ 20210625-5b (HMAS, holotype).
GenBank numbers: ITS = OQ540895; LSU = OQ540853.
Notes: Kneiffiella pseudoalutacea is phylogenetically close to K. alutacea (Fig. 121). Morphologically, these two species share a monomitic hyphal system, thin-walled cylindrical to subcylindrical cystidia, subclavate basidia and allantoid basidiospores; however, K. alutacea differs in the presence of creamish white to ochraceous, odontioid to coralloid hymenophore and longer cystidia (50–75 µm in length, Eriksson and Ryvarden 1976).
Hymenochaetaceae Donk.
Notes: Hymenochaetaceae proposed by Donk (1948), are white rot saprophytes or parasites causing wood decaying in Angiosperms and Gymnosperms. They are characterized by annual to perennial, yellow to brownish basidiomes with a xanthochroic reaction to KOH, smooth, poroid or corticioid hymenophore with microscopic features such as monomitic, mono-dimitic or dimitic hyphal system, clampless generative hyphae, presence or absence of cystidioles and setae with thin to thick walled, hyaline to brown, globose to cylindrical basidiospores (Ryvarden 1991; Dai 2010).
Fiasson and Niemela (1984), studied the morphological, cultural, chemical, and nuclear behavioural of Phellinus and Inonotus complex and raised Fomitiporia, Fulvifomes, Fuscoporia, Ochroporus, Phellinidium, Phellinus s.s. and Porodaedalea from Phellinus s.l.; Inonotus s.s., Inocutis and Inonotopsis from Inonotus s.l. Góes-Neto et al. (2000) proposed 12 genera namely, Aurificaria, Clavariachaete, Coltricia, Coltriciella, Cyclomyces, Hydnochaete, Hymenochaeteae, Inonotus, Phellinus, Phylloporia, Pyrrhoderma and Stiptochaete under this family. Dai (2010) studied 170 Hymenochaetoid fungi and grouped into 23 genera. Subsequently, several new genera, like Coniferiporia, Cylindrosporus, Fulvoderma, Sanghuangporus and Tropicoporus (Zhou 2015; Zhou et al. 2016b, c, 2018) were introducedin this family. Recently, Wu et al. (2022a) revised the systematics of 672 poroid hymenochaetoid fungi, and classified them under 34 genera.
Fulvoderma L.W. Zhou & Y.C. Dai.
Notes: Fulvoderma typified by F. australe was segregated from Pyrrhoderma to accommodate the generic type and another species F. scaurum (Zhou et al. 2018). Fulvoderma is characterized by the combination of sessile to stipitate basidiomes, the absence of any setal materials, and hyaline, thin-walled basidiospores within Hymenochaetaceae (Zhou et al. 2018).
Fulvoderma microporum Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900291; Facesoffungi number: FoF 14054; Figs. 124, 125
Etymology: microporum (Lat.) referring to the small pores.
Holotype: LWZ 20210626-12b (HMAS).
Diagnosis: Characterized by fulvous to snuff brown pileal surface, umber pore surface, small, circular pores, the absence of cystidia and cystidioles, and small, broadly ellipsoid to globose basidiospores.
Basidiomes annual, laterally stipitate, solitary or imbricate, without odor or taste when fresh, hard corky. Pilei projecting up to 7 cm long, 8 cm wide and 1 cm thick at base. Pileal surface fulvous to snuff brown, distinctly concentrically zonate, tuberculate and warty; margin acute, colour consistent with pileal surface, wavy. Pore surface umber, glancing; sterile margin cinnamon-buff, up to 5 mm wide; pores mostly circular, 7–8 per mm; dissepiments thin, entire. Context honey-yellow, hard corky, up to 7 mm thick. Tubes clay-buff, hard corky, up to 3 mm long. Stipe up to 3 cm long and 2.5 cm in diam.
Hyphal system monomitic; generative hyphae simple septate; tissue xanthochroic. Contextual hyphae pale yellowish to yellowish, thick-walled with a wide lumen, rarely branched, simple septate, interwoven, 5–6 µm in diam. Tramal hyphae hyaline, pale yellowish to yellowish, thick-walled with a narrow lumen, unbranched, simple septate, interwoven, 3–4 µm in diam. Hymenial setae dark brown, thick-walled with a narrow lumen, ventricose and apex sharp, 20–35 × 7–7.5 µm. Cystidia and cystidioles absent. Basidia barrel-shaped to subclavate, simple septate with four sterigmata, 10 × 5–6 µm; basidioles in shape similar to basidia but slightly smaller. Basidiospores broadly ellipsoid to globose, with a big guttule, hyaline, thin-walled, IKI–, CB–, 4–4.5(–5) × 3–3.5 µm, L = 4.25 µm, W = 3.25 µm, Q = 1.29–1.31 (n = 60/2).
Material examined: China, Jiangxi Province, Jiujiang, Bailudong Academy, ground under gymnosperm, 26 June 2021, L.W. Zhou, LWZ 20210626-12b (HMAS, holotype); ibid., LWZ 20210626-24b (HMAS).
GenBank numbers: LWZ 20210626-12b: ITS = OQ540896; LSU = OQ540854; LWZ 20210626-24b: ITS = OQ540897; LSU = OQ540855.
Notes: Fulvoderma microporum resembles the other two species of Fulvoderma, F. australe and F. scaurum, for their annual, sessile to laterally stipitate, solitary or imbricate basidiomes and yellowish brown pileal surface. However, F. australe has larger angular pores (5–6 per mm) and basidiospores (4.5–5.5 × 4–4.5 μm), and F. scaurum is also distinct by larger pores (4–5 per mm) and basidiospores (5–6 × 4–4.6 μm, Zhou et al. 2018). Moreover, F. microporum occupies a distinct lineage from F. australe and F. scaurum within Fulvoderma (Fig. 126).
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Tropicoporus L.W. Zhou, Y.C. Dai & Sheng H. Wu.
Notes: Based on molecular systematics and microscopical illustrations, Zhou et al. (2016c) proposed Tropicoporus as a new genus along with seven new combinations from the Inonotus linteus complex. Tropicoporus accommodates species with annual to perennial, resupinate, effused-reflexed to pileate brown, dark greyish to black pilei, velutinate, hispid, tomentose with glabrous to radially (un)cracked pilear surface. Context vary from homogeneous to duplex with or without black line. Microscopically, a dimitic or mono-dimitic hyphal system with simple septate generative hyphae, presence or absence of cystidioles, presence of hymenial setae with smooth, fairly thick walled to thick walled, yellowish, subglobose to ellipsoid basidiospores are characteristic features (Zhou et al. 2016c; Wu et al. 2022a).
Later, T. boehmeriae (Wu et al. 2015), T. stratificans (Coelho et al. 2016), T. drechsleri (Salvador-Montoya et al. 2018), T. texanus (Brown et al. 2020), T. flabellatus and T. nullisetus (Lima et al. 2022) were added to the genus Tropicoporus. Wu et al. (2022a, b) described seven new species viz., T. angustisulcatus, T. hainanicus, T. lineatus, T. minus, T. ravidus, T. substratificans and T. tenuis with 24 new combinations. Of known species of Tropicoporus, T. nullisetus was reported without setae (Lima et al. 2022).
Tropicoporus natarajaniae M. Kaliyaperumal, S. Gunaseelan, K. Kezo, Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF559816; Facesoffungi number: FoF 12715; Figs. 127, 128
Morphological characters of Tropicoporus natarajaniae. a Basidiomes (Holotype). b Pore surface. c Cross-section of basidiome with obtuse margin. d Indistinctly stratified tube layer. e Contextual hyphae. f Tramal hyphae. g–h Hymenial setae. i Cystidioles. j Basidia. k Basidioles. l–p Basidiospores: l Basidiospore. m Basidiopore in water. n Basidiopore in KOH. o Basidiopore in cotton blue. p Basidiopore in Melzer’s reagent. Scale bars: e–p = 5 μm
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Etymology: The species epithet “natarajaniae” referring to the Indian mycologist, Krishnamoorthy Natarajan, for his contributions to the Indian mycology especially on taxonomic studies in Agaricomycetes.
Holotype: MUBL4020.
Diagnosis: Tropicoporus natarajaniae is characterized by velutinate to abundantly tuberculae or warted pilear, uncracked pilear surface, on maturity becoming greyish brown with few cracks only near attachment, obtuse, velutinate margin and stratified tube with mono-dimitic hyphal system, presence of cystidioles and setae with broadly ellipsoid to subglobose basidiospores.
Basidiomes perennial, solitary, pileate, sessile, light in weight, hard when dry. Pileus dimidiate, applanate, with no distinct crust, projecting up to 5.4 cm, 6.6 cm wide and 3.6 cm thick near the attachment. Pilear surface on first pubescent to velutinate, smooth with few tuberculae or warts in young basidiomes, uncracked, azonate, yellowish brown (5E5, 5F8) to brown (6E8), on maturation pilei becoming velutinate with abundant tuberculate or warts on the surface, becoming greyish brown (6F3) with few cracks near attachment. Margin entire, obtuse, yellowish brown (5F8). Pore surface brown (6E6) to dark brown (6F6). Pores round to angular, regular, 5–7 per mm. Dissepiments entire, thick. Context duplex with no blackline, up to 1.5 cm, light brown (6D8) to brown (6E7). Tubes 0.7 cm in length, indistinctly stratified, each stratum up to 3 mm, brown (6E8).
Hyphal system monomitic in the context and dimitic in the tubes, tissue darkening with KOH without swelling. Context generative hyphae, thin to thick walled, hyaline to golden yellow, simple septate, rarely branched, 2–5 μm in diam. Tramal generative hyphae dominant, thin to thick walled, hyaline to pale yellow, septate, occasionally branched, 2–4.8 μm in diam. Skeletal hyphae thick-walled with narrow to wide lumen, yellowish brown, aseptate, unbranched, 2–4.5 μm in diam. Hymenial setae dark brown, thick-walled, ventricose to subulate with a sharp to blunt tips, 6–18.7 × 4.3–5.3 μm. Cystidia absent. Cystidioles hyaline, thin-walled, ventricose to fusoid with elongated tapering apical portion, 4.8–27 × 3.8–6 μm. Basidia clavate to subclavate, 6–18 × 3.8–6.2 μm, with four sterigmata and a simple septum at the base. Basidioles clavate, 4.8–16 × 3.5–6 μm. Basidiospores broadly ellipsoid to subglobose, pale yellow in water, turning golden yellow to brown in KOH, fairly thick walled to thick–walled, smooth, inamyloid, nondextrinoid, acyanophilous, (4.6–) 5–6 (–6.5) × (4.1–) 4.6–4.9 (–5.2) μm (n = 50/1), Q = 1.1 (Q range 1.05–1.3).
Material examined: India, Tamil Nadu, Thiruvannamalai district, Sathanur, Pennaiyar river, 12º 08′00.34″N, 78º 56′48.63″E, on living angiosperm tree (Albizia amara), 03 Febraury 2018, Sugantha Gunaseelan, MUBL4020 (holotype).
GenBank number: ITS = OP003881.
Notes: Tropicoporus natarajaniae is characterized by pileate basidiomes with a mono-dimitic hyphal system, while other Tropicoporus species such as T. boehmeriae, T. hainanicus, T. minus, T. ravidus, T. stratificans, T. tenuis and T. texanus are reported to be resupinate with a dimitic hyphal system (Wu et al. 2015, 2022a, b; Coelho et al. 2016; Brown et al. 2020). Absence of setae makes T. nullisetus (Lima et al. 2022) distinct from other reported Tropicoporus species.
Tropicoporus angustisulcatus, T. excentrodendri, T. lineatus and T. substratificans (Wu et al. 2022a, b) have a dimitic hyphal system and larger pores (Zhou et al. 2016c; Wu et al. 2022a, b), when compared with T. natarajaniae that has a mono-dimitic hyphal system and smaller pores (5–7 per mm).
Tropicoporus guanacastensis (Zhou et al. 2016c) is similar to T. natarajaniae in sharing a mono-dimitic hyphal system, stratified tube layer and presence of ventricose setae but differs from later in radially cracked, concentrically zonate and sulcate basidiomes, small pores (7–8 per mm), absence of cystidioles and ellipsoidal spores. While our Indian collection has pilei with abundant warts, large pores (5–7 per mm), presence of cystidioles and broadly ellipsoid to subglobose spores.
Tropicoporus flabellatus (Lima et al. 2022) and T. natarajaniae shows significant variations in basidiomes characters and microscopical features, the former have radially folded pilear surface with projections, velutinate to black and brown concentric zones, acute margin with smaller pores (7–9 per mm), absence of cystidioles and ellipsoidal basidiospores, whereas T. natarajaniae have abundant warts without any black or brown zones, obtuse margin with large pores (5–7 per mm), presence of cystidioles and broadly ellipsoid to subglobose basidiospores.
Tropicoporus drechsleri (Salvador˗Montoya et al. 2018), similar with T. natarajaniae in a mono˗dimitic hyphal system, presences of setae and cystidioles, yet T. drechsleri differs widely with pilear and basidiospores characters such as concentrically sulcate, radially deeply cracked pilei with broadly ellipsoid to ellipsoid basidiospores, while T. natarajaniae has frequently warted pilear surface, with few cracks near attachment and broadly ellipsoid to subglobose basidiospores.
Tropicoporus subramaniae S. Gunaseelan, M. Kaliyaperumal, K. Kezo, Xue W. Wang & L.W. Zhou., sp. nov.
Index Fungorum: IF559817; Facesoffungi number: FoF 12714; Figs. 129
Microscopic structures of Tropicoporus subramaniae. a Basidiomes (Holotype). b Pore surface. c Cross-section of basidiome. d Stratified tube layer. e branched generative hyphae from context. f Contextual hyphae. g Tramal hyphae. h–j Hymenial setae. k Basidioles. l Basidia. m–q Basidiospores: m Basidiopore in water. n Basidiopore in KOH. o Basidiopore in cotton blue. p Basidiopore in Melzer’s reagent. Scale bars: e–p = 5 μm
Etymology: The species epithet “subramaniae” referring to the Indian mycologist C. V. Subramanian, for his contributions to the fungal taxonomic studies in India.
Holotype: MUBL4021.
Diagnosis: Tropicoporus subramaniae is characterized by sulcate, deeply rimose, irregularly cracked pilear surface, acute margin, homogenous context and stratified tube with monomitic context and dimitic hyphal system in trama, absence of cystidioles and presence of setae and broadly ellipsoid to subglobose basidiospores.
Basidiomes perennial, solitary, pileate, sessile, woody hard, light in weight when dry. Pileus dimidiate, applanate, ungulate, projecting up to 9.3 cm, 13.7 cm wide and 6.4 cm thick near the attachment. Pilear surface glabrous to sulcate, deeply rimose with irregular cracks, with crust, brownish grey (6F2), greyish brown (6E3) to almost black. Margin entire, acute, golden brown (5D7) to brownish grey (6F2). Pore surface brown (6E7). Pores round to angular, regular, 4–6 per mm. Dissepiments entire, thick. Context homogenous, up to 0.5 cm, light brown (6D8) to brown (6E8). Tube layer up to 3.7 cm, stratified, each strata up to 0.4 cm thick.
Hyphal system monomitic in the context and dimitic in the tubes, tissue darkening with KOH without swelling; Context generative hyphae, thin- to thick-walled, hyaline to golden yellow, simple septate, rarely branched, 2–5.2 μm in diam. Trama generative hyphae, dominant, thin to thick-walled, hyaline to pale yellow, septate, occasionally branched, 2–4.2 μm in diam. Skeletal hyphae, thick-walled with narrow to wide lumen, yellowish brown, aseptate, unbranched, 2–4 μm in diam. Hymenial setae dark brown, thick-walled, ventricose to subulate with a sharp to blunt tips, 12–28 × 4.5–8 μm. Cystidia and cystidioles absent. Basidia clavate to sub clavate, 8–18.2 × 3.8–6.2 μm, with four sterigmata and a simple septum at the base. Basidioles clavate, 5–16.5 × 3–5.8 μm. Basidiospores broadly ellipsoid to subglobose, pale yellow to golden yellow in water, turning brown in KOH, thick-walled, smooth, inamyloid, nondextrinoid, acyanophilous, (5–)5.3–6.3(–6.7) × (4.5–)4.8–5(–5.2) μm (n = 50/1), Q = 1.2 (Q range 1.1–1.5).
Material examined: India, Tamil Nadu, Thiruvannamalai district, Sathanur, Pennaiyar river, 12º 08′00.34″N, 78º 56′48.63″E, on living angiosperm tree (Albizia amara), 3 Febraury 2018, Sugantha Gunaseelan, MUBL4021 (holotype).
GenBank number: ITS = OP003882.
Notes: Tropicoporus subramaniae, has a mono˗dimitic hyphal system, and deeply and irregularly cracked basidiomes; however, T. angustisulcatus, T. excentrodendri, T. lineatus and T. substratificans (Zhou et al. 2016c; Wu et al. 2022a, b), differs from our Indian collection in having a dimitic hyphal system and uncracked basidiomes.
Despite sharing a mono˗dimitc hyphal system and absence of cystidioles, Tropicoporus flabellatus differs from T. subramaniae by having velutinate to black and brown zones in pilear surface, uncracked basidiomes with smaller pores (7–9 per mm), duplex context with black line and ellipsoidal spores (4.5–5 × 3.5–4 μm), whereas T. subramaniae has sulcate, deeply cracked pilei with larger pores (4–6 per mm), homogenous context and broadly ellipsoid to subglobose basidiospores (5–6.7 × 4.5–5 μm).
Tropicoporus guanacastensis and T. subramaniae are similar in having sulcate, cracked basidiomes, stratified tube, a mono˗dimitic hyphal system, absence of cystidioles and presence of ventricose setae, but the former differs in smaller pores (7–8 per mm) and ellipsoidal spores (Zhou et al. 2016c), while our Indian species have comparatively larger pores (4–6 per mm) and broadly ellipsoid to subglobose spores.
Tropicoporus subramaniae and T. drechsleri (Salvador˗Montoya et al. 2018), shares concentrically sulcate deeply cracked pilei with a mono˗dimitc hyphal system, larger pores (< 6 per mm), but the South American species varies with indistinctly stratified tube layer, presence of cystidioles and broadly ellipsoid to ellipsoid basidiospores (4–5.5 × 3–4.5 μm), while T. subramaniae have stratified tramal layer without cystidioles and broadly ellipsoid to subglobose basidiospores (5–6.7 × 4.5–5 μm).
Tropicoporus boehmeriae, T. hainanicus, T. minus, T. ravidus, T. stratificans, T. tenuis and T. texanus are having resupinate basidiomes with a dimitic hyphal system (Coelho et al. 2016; Brown et al. 2020; Wu et al. 2022a, b), whereas T. subramaniae could be easily distinguished by its pileate basidiomes and a mono˗dimtic hyphal system.
Schizoporaceae Jülich.
Notes: Schizoporaceae with Schizopora as the type genus was introduced by Jülich (1981). Similar to Chaetoporellaceae, due to Schizopora being against by the conservation of Hyphodontia, the family name Schizoporaceae has long been ignored, until that Wang et al. (2021a) reinstated Schizoporaceae to accommodate Fasciodontia, Lyomyces and Xylodon (a prior synonym over Schizopora).
Lyomyces P. Karst.
Notes: Lyomyces typified by L. serus is a well supported monophyletic genus. See Wang et al. (2021a) for the latest comprehensive summarization of Lyomyces.
Lyomyces austro-occidentalis Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900292; Facesoffungi number: FoF 14055; Figs. 130, 131
Etymology: austro-occidentalis (Lat.) referring to the distribution of this species in Southwest China.
Holotype: LWZ 20190816-40a (HMAS).
Diagnosis: Characterized by annual, resupinate, adnate basidiomes, smooth, white to cream hymenophore, a monomitic hyphae system with encrusted subhymenial hyphae, capitulate cystidia, clavate basidia, and ellipsoid basidiospores.
Basidiomes annual, resupinate, adnate, cracked and brittle when dry, without odour. Hymenophore smooth, white to cream colored. Margin concolorous with or slightly paler than subiculum, abrupt.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline, dichotomous branching, interwoven, thin-walled, 2–3.5 μm in diam, subhymenial hyphae usually with encrustation. Cystidia capitate, ca. 20 × 3 μm. Basidia clavate, 15–20 × 5–5.5 μm, with four sterigmata ca. 3–4 μm long and a clamp connection at the base; basidioles similar in shape to basidia, but smaller. Basidiospores ellipsoid, with a large oily drop, hyaline, smooth, thin-walled, IKI–, slightly CB + , 4.2–5.2 × 3.1–3.5 μm, L = 4.63 μm, W = 3.27 μm, Q = 1.39–1.44 (60/2).
Material examined: China, Sichuan Province, Leshan, Ebian Yi Autonomous County, Heizhugou National Forest Park, on fallen branch of angiosperm, 16 August 2019, L.W. Zhou, LWZ 20190816-40a (HMAS, holotype), LWZ 20190816-8a (HMAS).
GenBank numbers: LWZ 20190816-8a: LSU = OQ540856; LWZ 20190816-40a: LSU = OQ540857.
Notes: Phylogenetically, Lyomyces austro-occidentalis and L. ochraceoalbus (CLZhao 9819) are nested within a single clade (Fig. 132). Morphologically, L. austro-occidentalis is highly similar to the original description of L. ochraceoalbus in Luo et al. (2021). Therefore, these two names indeed represent the same species. Unfortunately, Luo et al. (2021) did not effectively published the name L. ochraceoalbus according to Art. 40.7 of the International Code of Nomenclature for algae, fungi, and plants (Turland et al. 2018). Here, we redescribed this species as L. austro-occidentalis.
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Lyomyces crustosus and L. austro-occidentalis have a close phylogenetic relationship (Fig. 132), and share white to cream colored hymenophoral surface; however, L. crustosus differs in grandinioid to odontioid hymenophore, narrowly ellipsoid to cylindrical, sometimes slightly allantoid, longer basidiospores (5–7 μm in length, Langer 1994). Lyomyces juniperi resembles L. austro-occidentalis by smooth, white to cream hymenophore and ellipsoid basidiospores, but L. juniperi is differentiated by its subulate cystidia and suburniform basidia (Langer 1994).
Lyomyces crystallina Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900296; Facesoffungi number: FoF 14059; Figs. 133, 134
Etymology: crystallina (Lat.) referring to crystalline materials covering generative hyphae.
Holotype: LWZ 20190810-6b (HMAS).
Diagnosis: Characterized by annual, resupinate, adnate basidiomes, smooth to slightly grandinioid, cream to pale buff hymenophore, fusoid cystidia with subulate apex, subcylindrical basidia, and narrowly ellipsoid to oblong basidiospores.
Basidiomes annual, resupinate, adnate, cracked and brittle when dry, without odour. Hymenophore smooth to slightly grandinioid, cream to pale buff. Margin concolorous with subiculum, abrupt.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline, dichotomous branching, interwoven, thin-walled, 2.5–3.5 μm in diam, encrusted with coarse crystalline materials throughout the hymenium. Cystidia fusoid with subulate apex, 20–25 × 3.5–4 μm. Basidia subcylindrical and constricted, with four sterigmata and a clamp connection at the base, 30–35 × 5–5.5 μm; basidioles subclavate, 13–20 × 4.5–5.5 μm. Basidiospores narrowly ellipsoid to oblong, usually with an oily drop, hyaline, smooth, thin-walled, IKI–, CB–, (5–)5.1–5.6(–5.7) × 3.1–3.4(–3.5) μm, L = 5.32 μm, W = 3.26 μm, Q = 1.63 (30/1).
Materials examined: China, Sichuan Province, Ganzi Tibetan Autonomous Prefecture, Jiulong County, on fallen branch of angiosperm, 10 August 2019, L.W. Zhou, LWZ 20190810-6b (HMAS, holotype).
GenBank number: ITS = OQ540901.
Notes: Lyomyces juniperi resembles L. crystallina for sharing annual, resupinate, adnate basidiomes, slightly grandinioid hymenophore, fusoid cystidia with subulate apex, and ellipsoid basidiospores; however, L. juniperi differs in its larger basidiospores (5–6.5 × 3.5–4 μm, Langer 1994).
Lyomyces guttulatus Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900294; Facesoffungi number: FoF 14057; Figs. 135, 136
Etymology: guttulatus (Lat.) referring to the numerous oil-like globules on basidia.
Holotype: LWZ 20200921-29a (HMAS).
Diagnosis: Characterized by annual, resupinate, adnate basidiomes, smooth to slightly grandinioid hymenophore, crystalline hyphae, tapering cystidia, subclavate to cylindrical basidia with numerous oil-like globules and broadly ellipsoid basidiospores.
Basidiomes annual, resupinate, adnate, cracked and brittle when dry, without odour. Hymenophore smooth to slightly grandinioid, lime to cream colored. Margin concolorous with subiculum, abrupt.
Hyphal system monomitic; generative hyphae with numerous clamp connections, hyaline, dichotomous branching, interwoven, thin-walled. Subhymenial hyphae frequently encrusted by crystals, 2.5–4.5 μm in diam. Tapering cystidia colourless, smooth, thin-walled, ca. 20 × 3 μm. Basidia subclavate to cylindrical, with numerous oil-like globules, ca. 20 × 4 μm, with four sterigmata and a basal clamp connection; basidioles abundant, similar in shape to basidia, but slightly smaller. Basidiospores broadly ellipsoid, with a large oily drop, hyaline, smooth, thin-walled, IKI–, slightly CB + , 4–6 × 3–4 μm, L = 4.98 μm, W = 3.47 μm, Q = 1.43–1.44 (60/2).
Material examined: China, Sichuan Province, Liangshan Yi Autonomous Prefecture, Leibo County, Mamize Nature Reserve, on fallen trunk of Picea, 21 September 2020, L.W. Zhou, LWZ 20200921-29a (HMAS, holotype). China, Sichuan Province, Yaan, Shimian County, Hongba Nature Reserve, on fallen branch of angiosperm, 10 August 2019, L.W. Zhou, LWZ 20190810-20b (HMAS).
GenBank numbers: LWZ 20190810-20b: ITS = OQ540898; LSU = OQ540858; LWZ 20200921-29a: ITS = OQ540899; LSU = OQ540859.
Notes: Lyomyces niveus and L. sambuci resembles L. guttulatus by having resupinate basidiomes, light colored hymenophore and crystalline hyphae. However, L. niveus differs from L. guttulatus in having capitate, fusiform cystidia and barreled basidia (Luo et al. 2021), while L. sambuci differs in its capitate cystidia (Langer 1994).
Lyomyces niveus C.L. Zhao ex L.W. Zhou & Xue W. Wang, sp. nov.
Index Fungorum number: IF900293; Facesoffungi number: FoF 14056.
Based on Lyomyces niveus C.L. Zhao, in Luo, Chen & Zhao, Nordic Jl Bot.(e03414): 7 (2021), invalid name, not effectively published.
Holotype: CLZhao 6496 (SWFC).
Basidiomes annual, resupinate, subcoriaceous when fresh, becoming pruinose upon drying, up to 20 cm long and 3.5 cm wide, 50–130 μm thick. Hymenial surface smooth, white when fresh, turning white to pale buff upon drying. Margin indistinct, white.
Hyphal system monomitic; generative hyphae with clamp connections, colorless, thin-walled, frequently branched; subhymenium with moderately encrusted crystals, 1.5–4.0 μm in diameter; IKI–, CB–; tissues unchanged in KOH.
Cystidia of two types: 1) capitate cystidia colorless, thin-walled, smooth, 12.5–20.5 × 4.5–5.0 μm; 2) fusiform cystidia colorless, thin-walled, smooth, 12.5–22.0 × 3.0–5.0 μm; cystidioles absent. Basidia barreled, with 4 sterigmata and a basal clamp connection, 9.5–15.0 × 3.5–5.5 μm; basidioles abundant, in shape similar to basidia, but slightly smaller.
Basidiospores broadly ellipsoid, colorless, thin-walled, smooth, IKI–, cyanophilous, with a single oil-like globule, (3.0–)3.5–5.0(–6.5) × (2.5–)3.0–4.0(–5.0) μm, L = 4.45 μm, W = 3.31 μm, Q = 1.28–1.46 (n = 150/5).
Material examined: China, Yunnan Province, Yuxi, Xinping County, Mopanshan National Forestry Park, 101°57′E, 23°57′N, 2185 m a.s.l., on the trunk of Pinus armandii, leg. 19 January 2018, C.L. Zhao, CLZhao 6496 (SWFC, holotype).
GenBank numbers: CLZhao 6496: ITS = MZ262545, LSU = MZ262530.
Notes: Lyomyces niveus was published with a detailed description and phylogenetic analyses inferred from combined ITS and nLSU regions (Luo et al. 2021). However, it was indicated that the holotype (CLZhao 6496) was deposited at two separated herbaria (SWFC and HKAS, Luo et al. 2021), which is contrary to Art. 40.7 of the International Code of Nomenclature for algae, fungi, and plants (Turland et al. 2018). The current phylogeny (Fig. 132) confirms the phylogenetic conclusion of Luo et al. (2021), and thus we validate L. niveus here by reproducing the original description and selecting the piece of CLZhao 6496 at SWFC as the holotype. Then, the other piece of CLZhao 6496 at HKAS should be the isotype.
Lyomyces tasmanicus Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900295; Facesoffungi number: FoF 14058; Figs. 137, 138
Etymology: tasmanicus (Lat.) referring to the type locality Tasmania.
Holotype: LWZ 20180515–17 (HMAS).
Diagnosis: Characterized by annual, resupinate, adnate basidiomes, smooth, white to cream hymenophore, fusoid cystidia with subulate apex, subcylindrical basidia, and broadly ellipsoid basidiospores.
Basidiomes annual, resupinate, adnate, cracked and brittle when dry, without odour. Hymenophore smooth, white to cream. Margin concolorous with subiculum, abrupt.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline, dichotomous branching, interwoven, thin-walled, 2–3.5 μm in diam, without encrustation. Cystidia fusoid with tapering apex, 25–30 × 3.5–4 μm. Basidia subcylindrical and constricted, with four sterigmata and a clamp connection at the base, 32–35 × 4.5–6 μm; basidioles subclavate to suburniform, 13–20 × 4.5–5.5 μm. Basidiospores broadly ellipsoid, usually with an oily drop, hyaline, smooth, thin-walled, IKI–, CB–, 5–5.4(–5.5) × 4–4.7(–4.8) μm, L = 5.25 μm, W = 4.36 μm, Q = 1.20 (30/1).
Material examined: Australia, Tasmania, Tahune Adventures, The look-in look-out, on fallen twig of angiosperm, 15 May 2018, L.W. Zhou, LWZ 20180515–17 (HMAS, holotype).
GenBank number: ITS = OQ540900, LSU = OQ540860.
Notes: Lyomyces tasmanicus has a close relationship with L. crystallina from a phylogenetic perspective (Fig. 132). These two new species also have similar morphological characters, except that L. crystallina has encrusted generative hyphae and narrower basidiospores (3.1–3.4 µm). Lyomyces organensis resembles L. tasmanicus for sharing annual, resupinate, adnate basidiomes and fusoid cystidia. However, L. organensis differs in its crystalline hyphal system, and ellipsoid to narrowly ellipsoid, thin- to slightly thick-walled, narrower basidiospores (2.5‒3.5 µm in width, Yurchenko et al. 2017). Lyomyces bambusinus is also similar to L. tasmanicus by its annual, resupinate, adnate basidiomes, white to cream hymenophore, and fusoid cystidia, but it differs in the presence of capitate cystidia, thick-walled basidiospores and the growth on bamboo (Chen and Zhao 2020).
Xylodon (Pers.) Gray.
Notes: Xylodon typified by X. quercinus is a well supported monophyletic genus and has a priority over Schizopora the type genus of Schizoporaceae. See Wang et al. (2021a) for the latest comprehensive summarization of Xylodon.
Xylodon muchuanensis Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900297; Facesoffungi number: FoF 14060; Figs. 139, 140
Etymology: muchuanensis (Lat.) referring to the type locality Muchuan County, Sichuan Province, China.
Holotype: LWZ 20200819-2b (HMAS).
Diagnosis: Characterized by annual, resupinate, adnate basidiomes, poroid, cream to buff hymenophore, a monomitic hyphal system, the presence of capitate cystidia, clavate to subcylindrical basidia, and broadly ellipsoid basidiospores.
Basidiomes annual, resupinate, adnate, cracked and brittle when dry. Hymenophore poroid, cream to buff at young prat and cinnamon-buff at old part. Margin concolorous or slightly paler than subiculum, abrupt.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline, dichotomous branching, interwoven, slightly thick-walled, 3.5–4.5 μm in diam, occasionally with encrustation. Capitate cystidia embedded in hymenium, ca. 30 × 3.5 μm, with a round swollen top, about 7.5–8.5 μm in diam. Basidia clavate to subcylindrical, 15–20 × 4–5 μm, with four sterigmata and a clamp connection at the base; basidioles similar in shape to basidia but smaller. Basidiospores broadly ellipsoid, with an oily drop, hyaline, smooth, thin-walled, IKI–, slightly CB + , (4.1–)4.2–5.2(–5.3) × 3.1–3.6 μm, L = 4.46 μm, W = 3.32 μm, Q = 1.33–1.36 (60/2).
Material examined: China, Sichuan Province, Leshan, Muchuan County, on fallen branch of angiosperm, 19 August 2020, L.W. Zhou, LWZ 20200819-2b (HMAS, holotype); ibid., on fallen trunk of angiosperm, 19 August 2020, L.W. Zhou, LWZ 20200819-3a (HMAS).
GenBank numbers: LWZ 20200819-2b: ITS = OQ540902; LSU = OQ540861; LWZ 20200819-3a: ITS = OQ540903; LSU = OQ540862.
Notes: Phylogenetically, Xylodon muchuanensis groups with several other poroid species of Xylodon (Fig. 141). Of these species, X. muchuanensis is most similar to X. taiwanianus by sharing poroid hymenophore and ellipsoid basidiospores; however, Xylodon taiwanianus differs from X. muchuanensis by its suburniform basidia and the presence of resinous matter on the top of capitate cystidia (Wu 2001).
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Hymenochaetales, genus incertae sedis
Ginnsia Sheng H. Wu & Hallenb.
Notes: Ginnsia typified by G. viticola is a monotypic genus segregated from Phanerochaete (Wu et al. 2010a). The placement of G. viticola in Hymenochaetales was supported by phylogenetic analyses (Wu et al. 2010a; Ghobad-Nejhad et al. 2015).
Ginnsia laricicola Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900285; Facesoffungi number: FoF 14049; Figs. 142, 143
Etymology: laricicola (Lat.) referring to the host genus Larix.
Holotype: LWZ 20180830–9 (HMAS).
Diagnosis: Characterized by annual resupinate basidiomes, byssoid to fibrillose subiculum, cinnamon to yellowish brown hymenophore, a monomitic hyphal system with densely encrusted subicular hyphae, cylindrical cystidia, clavate basidia, and ellipsoid to broadly ellipsoid basidiospores.
Basidiomes resupinate, annual, flaking easily off subiculum when dry. Subiculum byssoid to fibrillose. Hymenophore smooth, cinnamon to yellowish brown; margin abrupt, concolorous with subiculum.
Hyphal system monomitic; generative hyphae hyaline, thin-walled, simple septate. Subicular hyphae hyaline, thin-walled, frequently branched, simple septate, interwoven, densely encrusted, 3.5–4 µm in diam. Subhymenium thin, poorly delimited from context. Cystidia cylindrical with obtuse apex, thin- to slightly thick-walled, 60–90 × 9–10 µm. Basidia clavate with stalked bases, thin-walled, simple septate with four sterigmata, guttulate, 20–30 × 8.5–9.5 µm; basidioles in shape similar to basidia but slightly smaller. Hyphidia hyaline, thin-walled, smooth, usually branched, 2.5–3.5 µm in diam. Basidiospores ellipsoid to broadly ellipsoid, with blunt apex, hyaline, thin-walled, guttulate, IKI − , CB − , (8.5–)9–9.5 × 5–6 µm, L = 9.29 µm, W = 5.52 µm, Q = 1.66–1.68 (n = 90/3).
Material examined: China, Shaanxi Province, Baoji, Mei County, Taibai Mountain, on stump of Larix, 30 August 2018, L.W. Zhou, LWZ 20180830–9 (HMAS, holotype); ibid., LWZ 20180830–5 (HMAS), LWZ 20180830–6 (HMAS).
GenBank numbers: LWZ 20180830–5: ITS = OQ540881; LSU = OQ540842; LWZ 20180830–6: ITS = OQ540882; LSU = OQ540843; LWZ 20180830–9: ITS = OQ540883; LSU = OQ540844.
Notes: Ginnsia laricicola resembles the generic type G. viticola; however, G. viticola differs in its slightly longer cystidia (60–125 µm) and basidia (25–50 µm, Burdsall 1985). Moreover, G. laricicola was only found on Larix, while G. viticola has a wider host range. Phylogenetically, two vouchers labeled as G. viticola are separated from each other (Fig. 144), which indicates that the concept of G. viticola may be a species complex and needs to be further clarified.
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Peniophorella P. Karst.
Notes: Peniophorella typified by P. pubera was introduced as a monotypic genus (Karsten 1889). After a long time of oblivion, Larsson (2007b) reinstated the independency of Peniophorella from Hyphoderma in Hymenochaetales. Since then, the known species number of Peniophorella has been improved to about 30 (Yurchenko et al. 2020). Although several papers placed Peniophorella in Rickenellaceae (He et al. 2019; Olariaga et al. 2020), its taxonomic position at the family level in Hymenochaetales needs to be further clarified with more comprehensive phylogenetic analyses, which is beyond the the current scope of phylogenetic analysis (Fig. 145).
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Peniophorella sidera Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900287; Facesoffungi number: FoF 14051; Figs. 146, 147
Etymology: sidera (Lat.) referring to the star-like crystals covering subhymenial hyphae.
Holotype: LWZ 20180921–15 (HMAS).
Diagnosis: Characterized by annual, resupinate basidiomes, grandinioid, white to cream hymenophore, a monomitic hyphal system, star-like crystals, fusoid to subcylindrical cystidia, urniform basidia, and ellipsoid to subcylindrical basidiospores.
Basidiomes annual, resupinate, adnate, ceraceous, without odour or taste when fresh, becoming cracked upon drying. Hymenophore grandinioid, white to cream, margin abrupt.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline. Subicular hyphae thin- to slightly thick-walled, smooth, 4.5–5 μm in diam. Subhymenial hyphae thin- to slightly thick-walled, strongly encrusted with star-like crystals, moderately branched, 4–5.5 μm in diam. Cystidia scattered, embedded in hymenium, fusoid to subcylindrical, somewhat constricted, smooth, thin-walled, 30–35 × 9–12 μm. Basidia urniform, thin-walled, smooth, with four sterigmata and a basal clamp connection, 17–18 × 7.5–8.5 μm; basidioles in shape similar to basidia but slightly smaller. Basidiospores ellipsoid to subcylindrical, with flat or slightly concave adaxial side, thin-walled, IKI–, CB–, (7.5–)8.2–10 (–10.2) × (4–)4.1–5.1(–5.3) μm, L = 8.95 μm, W = 4.55 μm, Q = 1.95–1.98 (n = 150/5).
Material examined: China, Yunnan Province, Baoshan, Gaoligong Mountains National Nature Reserve, Baihua Ridge, on fallen branch of angiosperm, 21 September 2018, L.W. Zhou, LWZ 20180921–15 (HMAS, holotype); ibid., on fallen twig of Pinus, 22 September 2018, L.W. Zhou, LWZ 20180922–62 (HMAS); Hainan Province, Bawangling National Nature Reserve, on fallen branch of angiosperm, 12 June 2017, L.W. Zhou, LWZ 20170612–41 (HMAS); ibid., on fallen twig of angiosperm, 12 June 2017, L.W. Zhou, LWZ 20170612–43 (HMAS); Hunan Province, Yizhang County, Mangshan National Nature Reserve, on fallen branch of angiosperm, 28 July 2016, L.W. Zhou, LWZ 20160728–16 (HMAS).
GenBank numbers: LWZ 20180921–15: ITS = OQ540892; LSU = OQ540850; LWZ 20180922–62: ITS = OQ540893; LSU = OQ540851; LWZ 20170612–41: ITS = OQ540890; LSU = OQ540848; LWZ 20170612–43: ITS = OQ540891; LSU = OQ540849; LWZ 20160728–16: ITS = OQ540889.
Notes: Peniophorella sidera is distinct from other species of Peniophorella by the subicular hyphae encrusted by star-like. Peniophorella reticulata is similar to P. sidera by the presence of peg-like hyphal structures, smooth, immersed cystidia, and the absence of stephanocysts and echinocysts; however, P. reticulata differs in its wider basidiospores (5–6 μm in width, Yurchenko et al. 2020). Peniophorella rude resembles P. sidera by similar hymenophoral configuration, but differs in the presence of stephanocysts and wider basidiospores (6 μm in width, Larsson 2007b).
Peniophorella subreticulata Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900288; Facesoffungi number: FoF 14050; Figs. 148, 149
Etymology: subreticulata (Lat.) referring to the similarity to P. reticulata.
Holotype: LWZ 20200921-49b (HMAS).
Diagnosis: Characterized by annual, resupinate, basidiomes, grandinioid to odontioid, white to cream hymenophore, a monomitic hyphal system, embedded, fusoid to subcylindrical leptocystidia and subcylindrical asterocystidia, the presence of stephanocysts, and ellipsoid to oblong basidiospores.
Basidiomes annual, resupinate, adnate, ceraceous, without odour or taste when fresh, becoming cracked upon drying. Hymenophore grandinioid to odontioid, white to cream when fresh, turn to cream upon drying, margin abrupt.
Hyphal system monomitic; generative hyphae with clamp connections, hyaline. Subicular hyphae thin-walled, 4–5 μm in diam. Subhymenial hyphae thin- to slightly thick-walled, occasionally encrusted with granules, moderately branched, 4.5–5.5 μm in diam. Stephanocysts present in subhymenium. Cystidia two types: 1) leptocystidia scattered, embedded in subhymenium and hymenium, fusoid to subcylindrical, thin-walled, often with granules on the surface, 45–50 × 9–10 μm; 2) asterocystidia subcylindrical, thick-walled, 15–35 × 4–5.5 μm. Large solitary of aggregated crystals occurring in lower subhymenium and between hyphae in peg-like projections. Basidia clavate, thin-walled, smooth, with four sterigmata and a basal clamp connection, 15–20 × 7.5–8 μm; basidioles in shape similar to basidia but slightly smaller. Basidiospores narrowly ellipsoid to oblong with flat or slightly concave adaxial side, thin-walled, IKI–, CB–, (7.5–)7.8–9.3(–10.3) × 3.8–4.7(–5.1) μm, L = 8.51 μm, W = 4.33 μm, Q = 1.95–1.99 (n = 90/3).
Material examined: China, Sichuan Province, Liangshan Yi Autonomous Prefecture, Leibo County, Mamize Nature Reserve, on fallen branch of gymnosperm, 21 September 2020, L.W. Zhou, LWZ 20200921-49b (HMAS, holotype); ibid., on fallen trunk of Picea, 21 September 2020, L.W. Zhou, LWZ 20200921-61a (HMAS); Liangshan Yi autonomous prefecture, Meigu County, Dafengding National Nature Reserve, on fallen branch of Cryptomeria fortunei, 18 August 2019, L.W. Zhou, LWZ 20190818-35b (HMAS); Ganzi Tibetan Autonomous Prefecture, Jiulong County, Wuxuhai Scenic Spot, on fallen trunk of Picea, 13 September 2019, L.W. Zhou, LWZ 20190913–21 (HMAS); ibid., on fallen branch of Picea, 12 August 2020, L.W. Zhou, LWZ 20200812-37a (HMAS).
GenBank numbers: LWZ 20200921-49b: ITS = OQ540884; LSU = OQ540845; LWZ 20200921-61a: ITS = OQ540885; 20190818-35b: ITS = OQ540887; LWZ 20190913–21: ITS = OQ540888; LSU = OQ540847; LWZ 20200812-37a: ITS = OQ540886; LSU = OQ540846.
Notes: Peniophorella reticulata phylogenetically groups with P. subreticulata. Morphologically, P. reticulata differs in wider basidiospores (5–6 μm in width, Q = 1.5–1.7), and the absence of stephanocysts and asterocystidia (Yurchenko et al. 2020).
Polyporales Gäum.
Notes: Polyporales is a large order of poroid to irpicoid to dentate, sometimes lamellate macro-fungi having roughly 1800 species under 216 genera and 13 families described worldwide (Kirk et al. 2008). Binder et al. (2005, 2013) divided Polyporales into four major clades based on molecular phylogenetic studies such as core polyporoid clade, phlebioid clade, Antrodia clade and residual polyporoid clade. Some genera have uncertain positions in this order at the family level. Most of the genera are polyphyletic. Therefore, it is necessary to construct a phylogenetic tree to systematically show the position of the taxa of Polyporales. The latest updated accounts of Polyporales in Phookamsak et al. (2019) and Wijayawardene et al. (2020).
Irpicaceae Spirin & Zmitr.
Notes: Irpicaceae introduced by Spirin (2003) with Irpex as the type genus is one of the three families of the phlebiod clade accommodating wood-inhabiting fungi in Polyporales (Justo et al. 2017). Chen et al. (2021b) updated the taxonomic circumscription of Irpicaceae from both morphological and phylogenetic perspective, and accepted 13 genera in this family. Species diversity in Irpicaceae was further explored recently by Li et al. (2022).
Meruliopsis Bondartsev.
Notes: Meruliopsis typified by M. taxicola has long been considered to be a synonym of Gloeoporus (Ryvarden and Gilbertson 1993), but molecular phylogeny supported Meruliopsis as an independent genus (Justo et al. 2017). Chen et al. (2020b) redelimited the taxonomic concept of Meruliopsis according to phylogenetic analyses. Meruliopsis is characterized by resupinate to effused-reflexed basidiomes with merulioid to poroid hymenophore, a monomitic hyphal system with simple septate generative hyphae, and ellipsoid to cylindrical, or cylindrical to allantoid basidiospores (Chen et al. 2020b).
Meruliopsis crystallina Xue W. Wang & L.W. Zhou, sp. nov.
Index Fungorum number: IF900298; Facesoffungi number: FoF 14061; Figs. 150, 151
Etymology: crystallina (Lat.) referring to the rhombic crystals in subicular hyphae.
Holotype: LWZ 20190726–28 (HMAS).
Diagnosis: Characterized by white to cream basidiomes, a monomitic hyphal system with strongly rhombic crystallized subicular hyphae, presence of leptocystidia, cylindrical to clavate basidia with numerous oily drops, and ellipsoid basidiospores.
Basidiomes annual, resupinate, effuse, adnate, membranaceous. Pore surface white to cream when dry; pores 4–5 per mm, round; tubes up to 65 μm deep, concolorous with pore surface; dissepiments up to 80 μm thick, entire, with continuous hymenium; margin slightly paler than pore surface, thinning out.
Hyphal system monomitic; generative hyphae simple septate. Subicular hyphae hyaline, thick-walled, strongly dichotomous branched, interwoven, strongly encrusted with rhombic crystals, 3.5–6.5 μm in diam. Tramal hyphae hyaline, slightly thick- to thick-walled, strongly dichotomous branched, interwoven, 3.5–5.5 μm in diam. Leptocystidia cylindrical, hyaline, projecting from hymenium, 25–30 × 4.5–5.5 μm. Basidia cylindrical to clavate, 4-sterigmata, with numerous oily drops, without clamp connection, 15–20 × 4–5 μm; basidioles in shape similar to basidia but slightly smaller. Basidiospores ellipsoid, hyaline, thin-walled, smooth, IKI–, CB + , usually with one or two oily drops, (3.7–)3.8–4.5 × (1.9–)2.0–2.3(–2.5) μm, L = 4.15 μm, W = 2.17 μm, Q = 1.90–1.92 (n = 120/4).
Material examined: China, Beijing, Yanqing County, Songshan National Forest Park, on fallen trunk of Pinus, 26 July 2019, L.W. Zhou, LWZ 20190726–28 (HMAS, holotype), LWZ 20190726–15 (HMAS), LWZ 20190726–27 (HMAS); ibid., on fallen branch of angiosperm, 26 July 2019, L.W. Zhou, LWZ 20190726–34 (HMAS).
GenBank numbers: LWZ 20190726–15: ITS = OQ540904, LSU = OQ540863; LWZ 20190726–27: ITS = OQ540905, LSU = OQ540864; LWZ 20190726–28: ITS = OQ540906, LSU = OQ540865; LWZ 20190726–34: ITS = OQ540907, LSU = OQ540866.
Notes: Phylogenetically, Meruliopsis crystallina is closely related to M. leptocystidiata (Fig. 152). These two species share white to cream pore surface, strongly encrusted subicular hyphae and the presence of leptocystidia. However, M. leptocystidiata differs by smaller basidiospores (3–4 × 1.5–2 μm) and the absence of oily drops in basidia (Chen et al. 2020b).
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Laetiporaceae Jülich.
Notes: Laetiporaceae was established by Jülich (1981) with Laetiporus as the type genus. Laetiporaceae belongs to the antrodia clade within the Polyporales, and accommodates species that cause a brown rot (Liu et al. 2022a). Five genera, viz. Kusaghiporia, Laetiporus, Macrohyporia, Wolfiporiella and Wolfiporiopsis are included in Laetiporaceae (Liu et al. 2022a). Laetiporaceae is closely related to Phaeolaceae (Liu et al. 2022a), and the later family includes some important pathogens on conifer trees (Yuan et al. 2022).
Wolfiporiella B.K. Cui & Shun Liu.
Notes: Wolfiporiella with Wolfiporia dilatohypha as type species was separated from Wolfiporia because of its smaller pores (Liu et al. 2022a).
Wolfiporiella macrospora X.H. Ji, L.W. Zhou & S.L. Liu, sp. nov.
Index Fungorum number: IF 901059; Facesoffungi number: FoF 14812; Figs. 153, 154
Etymology: macrospora (Lat.) referring to the large basidiospores.
Holotype: LWZ 20170821–7 (HMAS).
Diagnosis: Differing from other species of Wolfiporiella by having large basidiospores.
Basidiomes annual, resupinate, soft to juicy when fresh, without odour or taste, becoming fragile, light in weight when dry, up to 15 cm long, 12 cm wide and 5 mm thick. Pore surface buff yellow when fresh, white when bruised, buff to pinkish buff when dry; sterile margin distinct, buff, up to 5 mm wide; pores round to angular, 4–6 per mm; dissepiments thin, lacerate. Context vinaceous buff, corky, very thin, up to 2 mm thick. Tubes concolourous with the pore surface, fragile, up to 2 mm long.
Hyphal system dimitic in trama, monomitic in context; generative hyphae simple septate; skeletal hyphae dominant; IKI–, CB–. Generative hyphae in context hyaline, fairly thick-walled with a large lumen, frequently branched, simple septate, agglutinated, 6–11 µm in diam. Generative hyphae in tube hyaline, thin- to slightly thick-walled, frequently branched, simple septate, 3.5–5 μm in diam; skeletal hyphae frequent, pale yellow, thick-walled with a narrow lumen, unbranched, aseptate, interwoven, 4–6 μm in diam. Basidia broadly clavate to barrel-shaped with four sterigmata and a basal simple septum, 13–15 × 5–7 µm; basidioles similar to basidia in shape, but smaller. Basidiospores ellipsoid, hyaline, thin-walled, smooth, IKI–, CB + , 5–6.1(–7) × (3.4–)3.6–4.1 µm, L = 5.7 µm, W = 3.8 µm, Q = 1.5 (n = 60/2).
Material examined: China, Hubei Province, Danjiangkou County, Wudangshan Scenic Area, on base of dead angiosperm, 21 August 2017, L.W. Zhou, LWZ 20170821–7 (HMAS, holotype); ibid., on stump of angiosperm, 21 August 2017, L.W. Zhou, LWZ 20170821–8 (HMAS).
GenBank numbers: LWZ 20170821–7: ITS = OR557256, LSU = OR527287; LWZ 20170821–8: ITS = OR557257, LSU = OR527288.
Notes: Phylogenetically, Wolfiporiella macrospora falls within the clade of Wolfiporiella and has a close relationship with Wolfiporiella cartilaginea and W. dilatohypha (Fig. 155). Morphologically, the latter two species differ by smaller basidiospores (3.2–4.5 × 2.2–3 µm in W. cartilaginea, Ryvarden et al. 1986; 3.8–4.7 × 2.9–3.1 µm in W. dilatohypha, Dai et al. 2011).
Phylogeny generated by the maximum likelihood algorithm based on combined ITS and nLSU regions is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Pycnoporellus fulgens CA-20 and Sparassis crispa AFTOL ID 703 were selected as the outgroup taxa. Holotypes are in bold and the newly generated sequences are in blue
Meruliaceae Rea.
Notes: Species belonging to the family Meruliaceae, generally possess waxy appearance when dry; a monomitic type of hyphal system, rarely dimitic and tightly interwoven; hyphae with or without clamp-connection; thin walled, hyaline, smooth basidiospores; mostly presence or sometimes absence of cystidia; and causing white-rot symptoms (Justo et al. 2017).
Metuloidea G. Cunn.
Notes: The genus Metuloidea belonging to the family Meruliaceae having distinctive characteristic features like pileate basidiomes, brownish coloured, corky to somewhat waxy with a sweet smell when fresh, and entire margins; poroid or hydnoid hymenophore; a dimitic type of hyphal system, generative hyphae clamped, strongly branched yellowish skeletal hyphae; variable shaped cystidia, thin- to thick-walled, usually with lumen and apically encrusted with crystals, fusoid-ventricose to clavate; thin-walled septocystidia (M. reniforme) and ellipsoid to cylindrical shaped basidiospores (Westphalen et al. 2021). Steccherinum, the largest genus in the family Steccherinaceae, is microscopically characterized by the presence of thick-walled skeletocystidia, a dimitic type of hyphal system and clamped generative hyphae. Mainly, Steccherinum included only hydnoid species, but the phylogenetic studies showed that different types of hymenophore present in this genus, and it also includes poroid species (Miettinen et al. 2012; Miettinen and Ryvarden 2016; Westphalen et al. 2018). With the addition of molecular data, some authors used broad treatments of the Steccherinaceae and consequently some genera were transferred to the Meruliaceae (Larsson 2007a; Zmitrovich 2018). Some species, such as Steccherinum murashkinskyi previously belonged to the genus Steccherinum; however, phylogenetic studies showed that these species do not belong to Steccherinum, and they have been transferred to the genus, Metuloidea. About six species of Metuloidea are known till date (Miettinen and Ryvarden 2016; Westphalen et al. 2021). Of them, the type species is Metuloidea tawa (Westphalen et al. 2021).
Metuloidea imbricata R. Saha, A.K. Dutta & K. Acharya, sp. nov.
Index Fungorum number: IF559643; Facesoffungi number: FoF 11346; Figs. 156, 157
Etymology: The specific epithet ‘imbricata’ means overlapping or closely put together, referring to the growth habits of the basidiomes.
Holotype: CUH AM338.
Basidiomes annual, sessile, imbricate, pileate. Pileus 30–50 × 23–40 mm in diam, 5–13 mm thick at base, coriaceous, glabrous, concentrically zonate, even, reddish white (8A2) when fresh, becoming greyish orange (5B3) on drying. Margin distinct, even to slightly wavy, 0.7–2 mm thick, white (1A1) when fresh, greyish orange (5B3) on drying. Context fibrillose, greyish orange (5B3) on drying, 0.5–2 mm thick at base. Hymenophore hydnoid; spine 1–5 mm long, conical, dense, reddish grey (8B2) when fresh, brownish orange (7C3) to brown (7E4) on drying, 4–5 per mm. Odour sweet. Taste unknown.
Hyphal system dimitic. Context generative hyphae coralloid, solid, sometimes skelerified, 2.9–5.3 µm broad, clamped, thin- to thick-walled; skeletal hyphae 3.9‒6.7 µm broad, hyaline, unbranched, thick-walled. Spine hyphae dimitic, parallel; skeletal hyphae 3.3–6.7 µm broad, thick-walled with narrow lumen, unbranched, hyaline, (–) ve in cotton blue; generative hyphae 1.3–3.3 µm broad, thin-walled, hyaline, branched. Skeletocystidia 3.9–6.7 µm broad, subventricose, hyaline, epically encrusted, deeply rooted, thick-walled. Septocystidia 3.5–6 µm broad, apex clavate to subclavate, thick-walled with adventitious septa, hyaline, smooth. Basidia 9.9–16.7 × 2.9–3.8 µm in diam, clavate, hyaline, thin-walled, 4-sterigmatic with basal clamps, CB − . Basidiospores thin-walled, ellipsoid to elongate, hyaline, (2.9–)3.37–4.0 × (1.3–)1.88–3.4 μm, Q = 1.1–2.5, Qm = 1.90, inamyloid, non-dextrinoid, CB − .
Habitat and distribution: The new species grows on the dead wooden logs of unknown angiosperm and is currently only found in India.
Material examined: India, West Bengal, Jalpaiguri District, Apalchand forest, 26.8648°N, 88.7421°E, elevation 151 m alt., on dead wooden logs of unknown angiosperm, 13 October 2017, R. Saha, K. Acharya, CUH AM338 (holotype), on dead wooden logs of unknown angiosperm, 14 October 2017, R. Saha, K. Acharya, CUH AM773.
GenBank numbers: CUH AM338: ITS = ON227062, LSU = ON227059; CUH AM773: ITS = ON227063, LSU = ON227064.
Notes: Characteristic features of Metuloidea imbricata include pileate, imbricate, sessile basidiomes smelled sweetish, reddish grey coloured, hydnoid hymenophore consisting of conical spines; presence of a dimitic hyphal system with clamped generative hyphae, and thick-walled, unbranched skeletal hyphae reacting (–) ve in cotton blue; epically encrusted, deeply rooted, thick-walled, subventricose skeletocystidia; often with septocystidia and ellipsoid to elongate basidiospores measuring 2.9–4.0 × 1.3–3.4 μm.
Among similar taxa, Metuloidea cinnamomea differs from Metuloidea imbricata by the presence of poroid hymenophore; sub-cylindrical basidiospores, less than 2 µm wide and lack of any smell (Miettinen and Ryvarden 2016). Metuloidea tawa differs by having solitary, conchate, applanate, or effused-reflexed, occasionally resupinate basidiomes, pileus upper surface azonate, coarsely strigose, hairs adpressed to radiately arranged, hymenophore poroid, pores angular or round, hyphal system trimitic and basidiospores elliptical, up to 2 µm wide (Cunningham 1965). Metuloidea rhinocephala differs by having a strong smell in herbarium sample; basidiomes solitary, effused-reflexed, pileus upper surface hairy; hymenophore poroid; generative hyphae simple septate and basidiospores globose to obovate, 4–4.5 µm in diam (Miettinen and Ryvarden 2016). Metuloidea fragrans differs by having annual, pileate, sessile, more rarely effused reflexed, poroid basidiomes with a strong scent of coumarin, trimitic type of hyphal system and ovoid basidiospores measuring 3–4 × 2–3 µm (David and Tortic 1986). Metuloidea reniformis differs by having deep brown coloured basidiomes with pleasant, coumarin-like smell and yellowish skeletal hyphae (Westphalen et al. 2021).
In the phylogenetic tree, Metuloidea imbricata appears to be close to Steccherinum cf. murashkinskyi (96% MLBS, 0.99 BYPP; Figs. 158 and 159). However, details of Steccherinum cf. murashkinskyi cannot be verified because of lacking any morphological details in the form of published literature. Metuloidea murashkinskyi differs from Metuloidea imbricata by having pileate to decurrent to resupinate basidiomes, cap surface tomentose, cinnamon brown; hymenophore hydnoid, sometimes irpicoid or poroid; spicy odour; context hyphae monomitic with thick walled sklerified generative hyphae and spine hyphae dimitic; skeletal hyphae of spine ( +) ve in cotton blue and basidiospores short-cylindrical (Spirin et al. 2007). Steccherinum confragosum differs by the presence of clavate to lageniform cystidia, and absence of septocystidia (Geesteranus and Lanquetin 1975).
Phylogenetic analyses were based on data set of nrDNA ITS sequences. Reference sequences were selected from relevant literature (Westphalen et al. 2021; Wu et al. 2021), BLAST searches (Altschul et al. 1997) and data retrieved from GenBank (Clark et al. 2016). Two sequences of the genus Byssomerulius and Irpex, were chosen as the outgroup for rooting purpose (Wu et al. 2021). ClustalX2 (Thompson et al. 1997) with the use of default settings, and finally the alignment was upgraded wherever needed using MEGA v. 7.0 (Kumar et al. 2016) for manual adjustments. For maximum likelihood (ML) analyses, the statistically best fit models of nucleotide substitution using Cyber Infrastructure for Phylogenetic Research (CIPRES) web portal (https://www.phylo.org/portal2/) were determined by jModeltest 2.1.6 (Darriba et al. 2012) on XSEDE (Miller et al. 2010). Based on the Bayesian information criterion (BIC), the GTR + I + G (13,859.931977) model chosen as the most suitable model for the alignment. Maximum Likelihood analysis was performed using RAxML-HPC2 ver. 8.2.12 (Stamatakis 2006) with bootstrap statistics worked out from 1000 rapid bootstrap replicates on the CIPRES NSF XSEDE resource. Bayesian inference (BI) of the phylogeny was worked out with the application of MrBayes v.3.2.2 (Ronquist et al. 2012) using the selected model operating Markov chain Monte Carlo (MCMC) analyses (Geyer 1991). Bayesian analyses extended to a standard deviation of split frequencies of 0.003 after 106 generations. After 25% preliminary burn in (Hall 2004), MrBayes was used to figure out the remaining trees’ 50% majority rule consensus phylogram to enumerate the PPs (posterior probabilities) of the groups. MLBS and PP values over 50% and 0.50 are displayed in the resulting phylogenetic tree. The newly described species are represented in blue bold font to highlight its position in the phylogenetic tree
Phylogenetic analyses were based on data set of nrDNA LSU sequences. Reference sequences were selected from relevant literature (Westphalen et al. 2021; Wu et al. 2021), BLAST searches (Altschul et al. 1997) and data retrieved from GenBank (Clark et al. 2016). Two sequences of the genus Byssomerulius and Irpex, were chosen as the outgroup for rooting purpose (Wu et al. 2021). ClustalX2 (Thompson et al. 1997) with the use of default settings, and finally the alignment was upgraded wherever needed using MEGA v. 7.0 (Kumar et al. 2016) for manual adjustments. For maximum likelihood (ML) analyses, the statistically best fit models of nucleotide substitution using Cyber Infrastructure for Phylogenetic Research (CIPRES) web portal (https://www.phylo.org/portal2/) were determined by jModeltest 2.1.6 (Darriba et al. 2012) on XSEDE (Miller et al. 2010). Based on the Bayesian information criterion (BIC), the GTR + I + G (5329.291785) model chosen as the most suitable model for the alignment. Maximum Likelihood analysis was performed using RAxML-HPC2 ver. 8.2.12 (Stamatakis 2006) with bootstrap statistics worked out from 1000 rapid bootstrap replicates on the CIPRES NSF XSEDE resource. Bayesian inference (BI) of the phylogeny was worked out with the application of MrBayes v.3.2.2 (Ronquist et al. 2012) using the selected model operating Markov chain Monte Carlo (MCMC) analyses (Geyer 1991). Bayesian analyses extended to a standard deviation of split frequencies of 0.006 after 106 generations. After 25% preliminary burn in (Hall 2004), MrBayes was used to figure out the remaining trees’ 50% majority rule consensus phylogram to enumerate the PPs (posterior probabilities) of the groups. MLBS and PP values over 50% and 0.50 are displayed in the resulting phylogenetic tree. The newly described species are exhibited in blue bold font to highlight its position in the phylogenetic tree
Polyporaceae Fr. ex Corda.
Notes: Corda (1839) proposed the family Polyporaceae with characters such as smooth, hyaline, thin-walled, typically cylindrical, non-amyloid, non-dextrinoid spores and usually lack of true hymenial cystidia, and since then these characters have been widely used in the identification and taxonomy of polyporoid fungi. Ryvarden (1991) classified the family Polyporaceae into 11 groups, using hyphal types as the main criterion. He grouped 16 genera of fungi mostly with a trimitic hyphal system and white rotting habit, viz. Cerrena, Coriolopsis, Cryptoporus, Daedaleopsis, Datronia, Earliella, Elmerina, Fomitella, Hexagonia, Lenzites, Megasporoporia, Microporus, Mollicarpus, Pycnoporus, Trametes and Trichaptum into the Trametes group. A recent monograph of the family on the Chinese species was published and 217 speices are described by Cui et al. (2019), and more new species are described from China very recently (Zhou et al. 2021; Mao et al. 2023). At present, MycoBank has recorded 187 genera as of 6 August, 2022.
Megasporoporia Ryvarden & J.E. Wright.
Notes: Megasporoporia was established by Ryvarden et al. (1982) based on Poria setulosa. The genus is characterized by resupinate basidiomes, large basidiospores, a dimitic to trimitic hyphal structure with clamped generative hyphae and dextrinoid skeletal hyphae, presence of rhomboid or bipyramidic crystals, dendrohyphidia, hyphal pegs, presence or absence of cystidioles/cystidia, and causing a white rot mostly on fallen angiosperm branches or twigs (Ryvarden et al. 1982; Dai and Li 2002; Dai and Wu 2004; Zhou and Dai 2008; Du and Cui 2009; Wang et al. 2022c). At present MycoBank has recorded 16 species under this genus as of 23 July, 2022. However, in the recent years, some of the species listed under Megasporoporia has been grouped under different genera such as Megasporia cavernulosa, Jorgewrightia cystidiolophora, Jorgewrightia ellipsoidea, Megasporia hexagonoides, Jorgewrightia major, Jorgewrightia violacea, Megasporia mexicana, Grammothele quercina, Mariorajchenbergia rhododendri and Mariorajchenbergia subcavernulosa (Li and Cui 2013; Yuan et al. 2017; Lira et al 2021).
Megasporoporia tamilnaduensis K. Kezo, M. Kaliyaperumal, S. Gunaseelan, Xue W. Wang & L.W. Zhou., sp. nov.
Index Fungorum: IF559790; Facesoffungi number: FoF 12713; Figs. 160, 161
Microscopic structures of Megasporoporia tamilnaduensis (MUBL4022, holotype). a Basidiomes. b1 Duplex Context. b2 Tube layer. c Pore surface. d Cross section of hymenium. e Crystals. f Dendrohyphidia. g Basidioles. h Basidia. i Basidiospores. j Phloxine. k Water. l Cotton blue. m Melzer. Scale bars: b, c = 1 mm, d–m = 10 μm
Phylogram generated by the maximum likelihood algorithm based on combined nLSU and ITS sequence data is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Type specimens are in bold and the isolates of new species characterized are in blue
Etymology: “tamilnaduensis” refers to the place of collection.
Holotype: MUBL4022.
Diagnosis: Basidiomes annual, resupinate with white to brownish grey pore surface and duplex context, a dimitic hyphal system with clamped generative hyphae, presence of dendrohyphidia and tetrahedric to polyhedric crystals in the hymenium, absence of cystidia and cystidioles and cylindrical, hyaline, thin-walled basidiospores.
Basidiomes annual, resupinate, without odor or flavor when fresh, becoming corky upon drying, up to 9 cm long, 2.5 cm wide and 1.5 mm thick at the centre. Margin white (5A1) when fresh becoming yellowish white (4A2) on drying, up to 0.5 mm wide. Pore surface reddish white (7A2) when fresh to brownish grey (6C3) on drying; pores round to angular, 1–2 per mm; dissepiments thin. Context duplex without black line, yellowish white (4A2) towards substrate, white (5A1) towards the tube, up to 0.5 mm thick. Tubes concolorous with the pore surface, up to 1 mm long.
Hyphal system dimitic, skeletal hyphae dominant in context, while in trama generative hyphae. Context generative hyphae thin- to thick-walled, hyaline, clamped septate, branched, 2–3 μm in diam, CB + , IKI–; skeletal hyphae thick-walled with narrow lumen, unbranched, aseptate, 2.5–6.2 μm in diam, CB + , IKI–. Trama generative hyphae thin-walled, hyaline, clamped septate, branched, 1.8–2.5 μm in diam, CB + , IKI–; skeletal hyphae thick-walled with narrow lumen, aseptate, unbranched, 2.5–6 μm in diam, CB + , IKI–. Abundant tetrahedric to polyhedric crystals present in the hymenium. Cystidia and Cystidioles absent. Dendrohyphidia present, 1.8–2.7 μm in diam. Basidioles clavate, 14–20 × 7–7.8 μm. Basidia clavate, with four sterigmata, 16–24.2 × 7–8.2 μm. Basidiospores cylindrical, hyaline, thin-walled, smooth, IKI–, CB–, (9.5–)9.7–11.5(–12) × (3.3–)3.5–4(–4.2) μm, Q = 2.85 (n = 30/2).
Material examined: India, Tamil Nadu, Thiruvannamalai district, 12°29′15.4"N, 78°55′01.8"E, on dead wood, 14 November, 2019, Kezhocuyi Kezo, MUBL4022 (holotype). INDIA, Tamil Nadu, Thiruvannamalai district, Jawadhu hills, Nallapathur Reserve Forest, 12°32′49.9"N, 78°54′05.9"E, on dead wood, 14 January 2020, Kezhocuyi Kezo, KSM-NP7.
GenBank numbers: ITS = ON249127, LSU = ON254196.
Notes: Megasporoporia tamilnaduensis (MUBL4022) shares similarities with M. bannaensis (Li and Cui 2013) by annual, resupinate basidiomes and pores size (1–2 per mm). However, the former differs by the presence of dendrohyphidia and smaller basidiospore size (9.5–12 × 3.3–4.2 μm). The spore size of M. tamilnaduensis and M. inflate are similar; however, the latter has smaller pore size (2–3 per mm) and lacks dendrohyphidia (Wang et al. 2021b). Our Indian species shares similar pore size with M. setulosa but the latter varies by having larger basidiospore (10–14 × 4.2–5.7 μm) and absence of dendrohyphidia (Lira et al. 2021). Megasporoporia tamilnaduensis share similar basidiospore and pore size with M. neosetula, but the latter varies in the absence of dendrohyphidia and homogenous context (Lira et al. 2021). Megasporoporia minuta varies from M. tamilnaduensis by small pores (6–8 per mm), narrowly ovoid basidiospores, and lack of hyphal pegs, dendrohyphidia and tetrahedric or polyhedric crystals (Zhou and Dai 2008).
Russulales Kreisel ex P.M. Kirk, P.F. Cannon & J.C. David.
Notes: Russulales is well-supported clade in Agaricomycetes. About 10 families, 99 genera and 4436 species had been reported in this order according to the latest overview of Basidiomycota (He et al. 2019; Wu et al. 2020). A large number of studies were focused on the taxonomy and phylogeny of Russulales taxa in last ten years. A total of 13 new genera have been proposed in the order in this peroid (Audet 2010; Wu et al. 2010b; Zhou and Dai 2013; Larsson 2014; Ryvarden and Tutka 2014; Chen et al. 2016; Liu et al. 2017b). The genus/family-level classification of Russulales are continously provided (Miller et al. 2006; Hibbett et al. 2007; Zhao et al. 2017; He et al. 2019). In addition, a certain number of edible and medicinal and pathogenic species are the memebrs of Russulales (Dai and Yang 2008; Li et al. 2015d; Yuan et al. 2021).
Hericiaceae Donk.
Notes: Species belonging to the family Hericiaceae generally having resupinate, effused-reflexed to pileate, membranous to fleshy basidiomes; smooth, odontoid to toothed hymenophore; white or pallid, fleshy to membranous context; a monomitic type of hyphal system with thin- to thick-walled, clamped hyphae; presence of gloeocystidia; clavate, 4-sterigmate basidia; and globose, ovoid to ellipsoid, smooth to echinulate, amyloid basidiospores (Sharma 2012).
Laxitextum Lentz.
Notes: The genus Laxitextum includes three species (retrieved from www.mycobank.org, 20 April 2022) till date having distinctive characteristic features like resupinate to subpileate basidiomes; brown, tomentose, zonate pileus upper surface; white when fresh, smooth hymenial surface; a monomitic type of hyphal system with thin-walled, clamped generative hyphae; numerous, subulate gloeocystidia; and subglobose to ellipsoid, echinulate and amyloid basidiospores (Lee and Jung 2006). The type species of Laxitextum is L. bicolor (Lee and Jung 2006).
Laxitextum subrubrum R. Saha, A.K. Dutta & K. Acharya, sp. nov.
Index Fungorum number: IF559644; Facesoffungi Number: FoF 11347; Figs. 162, 163
Etymology: Named after sub- (Latin) = under, almost, and rubrum (Latin) = red, referring to the reddish or dull red colouration of the pileus.
Holotype: CUH AM774.
Basidiomes pileate, sessile, soft-spongy when fresh and brittle on drying, 10–30 mm long, 5–20 mm wide and up to 1–2 mm thick. Pileus upper surface uneven, slightly zonate, velutinous, dull red (9C4). Margin thin, wavy, brownish orange (7C3). Hymenophore smooth, white (1A1), cracked on drying. Context soft, thin, dull red (9C3).
Hyphal system monomitic, generative hyphae 2.9–5.9 µm wide, hyaline, thin- to thick-walled, clamped. Oleiferous hyphae 3.5–6.5 µm wide. Gloeocystidia 102–156 × 8.5–15 µm, lanceolate, sometimes apex moniliform, pale orange (5A3), filled with oily substance. Basidia 23.5–38 × 5.5–9 µm, narrowly clavate, hyaline, thin-walled, 4-sterigmate, with basal clamp. Basidiospores (3.5–)4.74–5.9 × (2.9–)3.06–3.5 µm, Q = 1.2–2, Qm = 1.54, thin-walled, hyaline, ellipsoid, apiculate, finely echinulate, strongly amyloid in Melzer’s reagent.
Habitat and distribution: The new species grows on dead wood of unknown angiosperm, and is currently only found in India.
Material examined: India, West Bengal, Jalpaiguri District, Moraghat range, Khuttimari, 26.4728°N, 88.5957°E, elevation 144 m alt., on dead wooden logs of unknown angiosperm, 7 October 2018, R. Saha & K. Acharya, CUH AM774 (holotype), 8 October 2018, R. Saha, K. Acharya, CUH AM775.
GenBank numbers: CUH AM774: ITS = ON227060, LSU = ON226874; CUH AM775: ITS = ON227061, LSU = ON227065.
Notes: Characteristic features of the present specimens include pileate, sessile basidiomes with slightly zonate, velutinous, dull red upper surface; brownish orange margin, smooth, white hymenophore; dull red context; presence of a monomitic hyphal system with clamped generative hyphae; lanceolate, pale orange gloeocystidia, apex sometimes moniliform; narrowly clavate, hyaline, thin-walled, 4-sterigmate basidia; and thin-walled, hyaline, ellipsoid, apiculate, finely echinulate, strongly amyloid in Melzer’s reagen basidiospores measuring 3.5–5.9 × 2.9–3.5 µm.
Among similar taxa, Laxitextum bicolor differs by having resupinate to effused-reflexed basidiomes; brown coloured and finely tomentose pileus upper surface with appressed hyphal hairs (Lentz 1955). Laxitextum incrustatum differs by the presence of resupinate and widely effused basidiomes; white to pallid, cottony to matted tomentose margin; tubular gloeocystidia, sometimes with a bulbous swelling near base and hyphidia (Hjortstam and Ryvarden 1981). Laxitextum lutescens differs by having resupinate to distinctly reflexed basidiomes; deep yellow to pale brown velutinous, azonate pileus upper surface; cream-yellowish to straw coloured hymenium with a light purple-brown tint (Hjortstam and Ryvarden 1981).
In the phylogenetic tree, Laxitextum subrubrum appears to be close to Laxitextum cf. bicolor (83% MLBS, 0.65 BYPP; Figs. 164 and 165). However, details of Laxitextum cf. bicolor cannot be verified because of lacking any morphological details in the form of published literature.
Phylogenetic analyses were based on data set of nrDNA ITS sequences. Reference sequences were selected from relevant literature (He and Zhao 2022; Hofstetter et al. 2019), BLAST searches (Altschul et al. 1997) and data retrieved from GenBank (Clark et al. 2016). Two sequences of the genus Gloeophyllum, was chosen as the outgroup for rooting purpose (He and Zhao 2022). ClustalX2 (Thompson et al. 1997) with the use of default settings, and finally the alignment was upgraded wherever needed using MEGA v. 7.0 (Kumar et al. 2016) for manual adjustments. For maximum likelihood (ML) analyses, the statistically best fit models of nucleotide substitution using Cyber Infrastructure for Phylogenetic Research (CIPRES) web portal (https://www.phylo.org/portal2/) were determined by jModeltest 2.1.6 (Darriba et al. 2012) on XSEDE (Miller et al. 2010). Based on the Bayesian information criterion (BIC), the GTR + I + G (10,768.817815) model chosen as the most suitable model for the alignment. Maximum Likelihood analysis was performed using RAxML-HPC2 ver. 8.2.12 (Stamatakis 2006) with bootstrap statistics worked out from 1000 rapid bootstrap replicates on the CIPRES NSF XSEDE resource. Bayesian inference (BI) of the phylogeny was worked out with the application of MrBayes v.3.2.2 (Ronquist et al. 2012) using the selected model operating Markov chain Monte Carlo (MCMC) analyses (Geyer 1991). Bayesian analyses extended to a standard deviation of split frequencies of 0.002 after 106 generations. After 25% preliminary burn in (Hall 2004), MrBayes was used to figure out the remaining trees’ 50% majority rule consensus phylogram to enumerate the PPs (posterior probabilities) of the groups. MLBS and PP values over 50% and 0.50 are displayed in the resulting phylogenetic tree. The newly described species are exhibited in black bold font to highlight its position in the phylogenetic tree
Phylogenetic analyses were based on data set of nrDNA LSU sequences. Reference sequences were selected from relevant literature (He and Zhao 2022; Hofstetter et al. 2019), BLAST searches (Altschul et al. 1997) and data retrieved from GenBank (Clark et al. 2016). Two sequences of the genus Gloeophyllum, was chosen as the outgroup for rooting purpose (He and Zhao 2022). ClustalX2 (Thompson et al. 1997) with the use of default settings, and finally the alignment was upgraded wherever needed using MEGA v. 7.0 (Kumar et al. 2016) for manual adjustments. For maximum likelihood (ML) analyses, the statistically best fit models of nucleotide substitution using Cyber Infrastructure for Phylogenetic Research (CIPRES) web portal (https://www.phylo.org/portal2/) were determined by jModeltest 2.1.6 (Darriba et al. 2012) on XSEDE (Miller et al. 2010). Based on the Bayesian information criterion (BIC), the GTR + I + G (5221.325866) model chosen as the most suitable model for the alignment. Maximum Likelihood analysis was performed using RAxML-HPC2 ver. 8.2.12 (Stamatakis 2006) with bootstrap statistics worked out from 1000 rapid bootstrap replicates on the CIPRES NSF XSEDE resource. Bayesian inference (BI) of the phylogeny was worked out with the application of MrBayes v.3.2.2 (Ronquist et al. 2012) using the selected model operating Markov chain Monte Carlo (MCMC) analyses (Geyer 1991). Bayesian analyses extended to a standard deviation of split frequencies of 0.002 after 106 generations. After 25% preliminary burn in (Hall 2004), MrBayes was used to figure out the remaining trees’ 50% majority rule consensus phylogram to enumerate the PPs (posterior probabilities) of the groups. MLBS and PP values over 50% and 0.50 are displayed in the resulting phylogenetic tree. The newly described species are exhibited in black bold font to highlight its position in the phylogenetic tree
Russulaceae Lotsy.
Notes: The family was typified by Russula (Persoon 1796), and its concept was originally described to accommodate the genera that have granular flesh, thick gills, spiny spores with amyloid ornamentations, and sphaerocytes in context (Lotsy 1907). Subsequently, the members of Russulaceae have been revised for several times, and this family has been reclassified as the development of molecular phylogeny (Hibbett and Thorn 2001; Larsson and Larsson 2003). Recently, systematic studies on the taxonomy and phylogeny of some sequestrate genera of Russulaceae have been reported. The phylogenetic results showed that the russuloid genera nested with the non-sequestrate Russulaceae members (Calonge and Martín 2000; Lebel and Tonkin 2007; Buyck et al. 2010). Although Buyck et al. (2018) presented systematic analyses of Russulaceae at subgenus level based on sequences from LSU, TEF1-α, mtSSU, RPB1 and RPB2 genes, geographically comprehensive studies on this family are still lacking. Further analyses of Russulaceae are still needed in the future.
Russula Pers.
Notes: Russula is a large, world-wide distributed genus growing in many types of forest ecosystem from tropical broad-leaved forest to subalpine coniferous forest (Li 2014; Li et al. 2015a, d; Looney et al. 2016). The genus is characterized by absence of veils or rings on stipes, brittle lamellae and context caused by the presence of sphaerocysts, and lacks of latex secretion when basidiomes are injured (Romagnesi 1985; Sarnari 1998). China has become one of the main sources of new species of Russula in recent ten years (Li et al. 2011, 2012, 2013a, b, 2015b, c, 2016, 2018a, b, 2020; Ariyawansa et al. 2015; Zhao et al. 2015; Sang et al. 2016; Das et al. 2017; Jiang et al. 2017; Tibpromma et al. 2017; Zhang et al. 2017b; Li and Deng 2018; Song et al. 2018a, b, 2019; Caboň et al. 2019; Chen et al. 2019b; Wang et al. 2019a; Yuan et al. 2019; Buyck et al. 2020; Rossi et al. 2020; Wu et al. 2022b). Specific delimitation, DNA barcode selection and population genetics of Russula species have also been analysed (Li et al. 2010, 2019; Cao et al. 2013; Kleine et al. 2013; Looney 2015; Wang et al. 2015).
Russula albocarpa G.J. Li & Chun Y. Deng, nom. nov.
Fungal Names Number: FN 570688; Facesoffungi number: FoF 14524.
= Russula leucocarpa G.J. Li & Chun Y. Deng, Mycosystema 39(4): 624, 2020. nom. illeg.
Etymology: Referring to the white color of basidiomes.
Holotype: HGAS-MF 009910.
Notes: The name Russula leucocarpa G.J. Li & Chun Y. Deng (Li et al. 2020) is an illegitimate later homonym of Russula leucocarpa (T. Lebel) T. Lebel (Lebel 2017). Thus a new name Russula albocarpa is proposed herein as a replacement of R. leucocarpa G.J. Li & Chun Y. Deng.
Russula chlorina G.J. Li & Chun Y. Deng, sp. nov.
Fungal Names Number: FN 570737; Facesoffungi number: FoF 14523; Figs. 166a, b, 167, 168a
Etymology: Referring to the pale yellowish green pileus.
Holotype: HBAU 15024.
Basidiomes small to medium sized. Pileus 29–37 mm in diam, first hemispheric, then convex, plane when mature, not depressed at center, a tinge of pale green centrally intermixed with yellow, light green (DDE5D9, E3E8DC), to pale yellowish green (B5CF61, BFD833), sometimes faded to a paler yellowish tinge (F1F2F1, EFEFDF), sometimes intermixed with yellowish ocher tinge (91672C, D8AC59), smooth, dull, slightly viscid when wet; margin not striate, not cracked, peeling 1/4–1/3 from the edge, sometimes entirely faded to pale yellow (F1E5BE, EFEFDF). Lamellae adnate, 2–3 mm in height, 17–20 pieces per centimeter at edge, often forked near the stipes, interveined, white (FFFFFF), unchanging when injured, lamellulae absent. Stipes 26–38 × 8–12 mm, central, cylindrical, rugulose longitudinally, white (FFFFFF), turning pale ocher (F1E5BE, F4EAE0) when bruised, smooth, dull, not viscid when wet, slightly tapered downward the base, first stuffed, hollow when old, annulus absent. Context 2 mm thick at pileus center, white (FFFFFF), unchanging, taste mild, smell indistinct. Spore print white to pale cream (Romagnesi Ib–IIa).
Basidiospores [150/3/3] (5.3–)5.6–6.9(–7.3) × (4.6–)4.9–5.9 μm, Q = 1.07–1.28 (1.31), Q = 1.19 ± 0.09), hyaline, subglobose to broad ellipsoid, rarely globose and ellipsoid, ornamentations amyloid, up to 0.7 μm in height, composed of warts and short ridges interconnected as incomplete network, certain amount of isolated warts and short crests also exist, suprahilar area plage indistinct and inamyloid. Basidia 33–44 × 6–10 μm, subcylindrical, subclavate to clavate, rarely cylindrical, four-spored, projecting 5–20 μm beyond hymenium, hyaline, sterigmata 5–7 μm long. Hymenial cystidia infrequent, 58–77 × 8–14 μm, fusiform to clavate, sometimes ventricose towards the apex, contents crystal, unevenly distributed, blackish grey in sulfovanillin (SV), apex obtuse, rarely subacute. Pileipellis two layered, vaguely delimited from the spherocytes in context; epipellis a trichoderm, ca. 50–100 μm deep, hyphae erect, oblique to repent, hyaline, mostly 2–4 μm wide, rarely 5–7 μm; terminal cells fusiform, subulate to bayonet-shaped, attenuate towards the apex; pileocystidia abundant, 5–8 μm wide, rarely septate, cylindrical, narrowing towards the apex, contents crystal, unevenly distributed, blackish in SV; subpellis composed of somewhat gelatinized, densely interweaved, branched and septate, hyaline hyphae 2–5 μm wide, rarely intermixed with spherocytes 15–25 μm.
Habitat: Single or scattered in coniferous and broad-leaved intermixed forest.
Material examined: China, Guizhou Province, Yinjiang County, Chanxi Township, Fengxiangping Nature Reserve, in broad-leaved forest, 31 July 2019, Lu-Yao Shi, Guo-Jie Li 20190156 (HBAU 15024, holotype).
GenBank number: ITS = MT505888.
Notes: The new species Russula chlorina clusters with R. grisea, R. ionochlora, R. subalpinogrisea and an undescribed Russula speciemen from Papua New Guinea (TU 110491) in phylogenetic analysis (Fig. 169). The morphological differences between R. chlorina and the other three known species are as follows: R. grisea differs by its violet, bluish, rarely greenish pileus, lilac shade on stipes, cream spore print (IIc), larger basidiospores (6.4–8.5 × 5.4–6.5 μm), and longer hymenial cystidia (6.4–8.5 × 5.4–6.5 μm) with lower warts up to 0.5 μm; R. ionochlora can be distinguished by its larger basidiospores (6.4–8 × 4.7–6 μm), inflated subapical cells, and baynet shaped terminal cells in pileipellis (Sarnari 1998); R. subalpinogrisea is separated by its strongly viscous pileus, arger basidiospores (6.16–9.45 × 5.85–8.38 μm), longer basidia 47–59 × 10.5–13.5 μm, narrower pileocystidia 4.8–5.2 μm wide, and a habitat of subalpine mixed forest (Das et al. 2018).
Maximum likelihood tree illustrating the phylogeny of Russula chlorina and R. luteocarpa (holotype) with related species in R. sect. Heterophyllae based on ITS sequences. Branches are labeled with maximum likelihood bootstrap higher than 50%, and Bayesian posterior probabilities more than 0.9 respectively. Sequences of R. sect. Substriatinae were used as outgroup to root trees. The new isolates are in bold
Russula chrysea G.J. Li & Chun Y. Deng, sp. nov.
Fungal Names Number: FN 570739; Facesoffungi number: FoF 14521; Figs. 168b, 170a, b, 171
Etymology: Referring to the pale yellowish tinge of pileus.
Holotype: HBAU 15023.
Basidiomes small to medium sized. Pileus 26–35 mm in diam, hemisphere to plano-convex, rarely applanate, a tinge of pale greenish yellow (E2E3A7, E3ECA6), sometimes faded to slight yellow tinge (EFEFDF, F5EED3), smooth, glabrous, not viscid when wet; margin not striate, not cracked, peeling 1/5–1/4 from the edge. Lamellae adnate, 2–3 mm in height, 16–20 pieces per centimeter at edge, sometimes forked near the stipes, interveined, white (FFFFFF), pale cream (F4EAE0) when mature, unchanging when bruised, lamellulae not observed. Stipes 25–33 × 5–11 mm, central to subcentral, cylindrical, slightly ventricose downward the base, rugulose longitudinally, white (FFFFFF), unchanging or slightly turning pale ocher (B78B5F, B6855A) when injured, smooth, dull, not viscid when wet, stuffed when young, hollow after mature, annulus absent. Context 1–3 mm thick at pileus center, white (FFFFFF), unchanging or turning pale yellow (F0E1BF, ECD6A3) when bruised, taste mild, smell indistinct. Spore print cream to pale ocher (Romagnesi IIc–IId).
Basidiospores [150/3/3] 5.6–7.2 (–7.6) × (4.3–) 4.6–6 μm, Q = 1.05–1.27 (1.36), Q = 1.18 ± 0.08), hyaline, subglobose to broad ellipsoid, rarely ellipsoid, ornamentations amyloid, up to 1 μm in height, composed of long ridges interconnected as complete reticulum, mixed up with a few isolated warts and short crests, suprahilar area plage distinct and amyloid. Basidia 34–52 × 9–12 μm, clavate to subclavate, rarely subcylindrical, four-spored, projecting 10–30 μm beyond hymenium, hyaline, sterigmata 4–7 μm long. Hymenial cystidia infrequent, 56–71 × 8–9 μm, fusiform to subclavate, sometimes clavate, contents granulate, sparse, unchanging or slightly grey in SV, apex obtuse, rarely subacute. Pileipellis two layered, not clearly distinguished from the context; epipellis a trichoderm, ca. 30–70 μm deep; terminal cells hyaline, cylindrical, rarely branched, often 3–5 μm wide, seldom up to 8 μm; primordial hyphae abundant, septate, 5–8 μm wide, surface with acido-resistant incrustations; pileocystidia absent; subpellis composed of somewhat gelatinized, densely interlaced, rarely branched and septate, hyaline hyphae 3–6 μm wide.
Habitat: Single or scattered in coniferous and broad-leaved intermixed forest.
Material examined: China, Guizhou Province, Yinjiang County, Chanxi Township, Fengxiangping Nature Reserve, in broad-leaved forest, 31 July 2019, 16 June 2014, Guo-Jie Li, Lu-Yao Shi 20190155 (HBAU 15023, holotype).
GenBank number: ITS = MT505890.
Notes: Russula chrysea forms an independent clade with two unidentified samples from Japan in phylogenetic analyses (Fig. 172). This clade has a close relationship with members of Russula section Amethystinae. The yellowish-capped species of this section, R. helios, R. ochracea, R. postiana and R. risigallina are different from R. chrysea in follwing morphological characters: R. helios has larger basidiospores (8–9.5 × 6.3–7.8 μm) and narrower primordial hyphae 3–4 μm in diam; R. ochracea can be distinguished its thick and flashy pileus and larger basidiospores (7–9.2 × 5.3–7.2 μm) with isolated warts; R. postiana is differeciated in having greenish yellow tinged pileus, larger basidiospres (8–11 × 8.7 µm), and longer and wider hymenial cystidia (70–85 × 10–12 μm); R. risigallina differs in often reddish tinged pileus, context smell of old fruit, larger basidiospores (7–9 × 5.8–6.7 μm) with ornamentation composed of mostly isolated warts (Romagnesi 1985; Sarnari 2005).
Maximum likelihood tree illustrating the phylogeny of Russula chrysea and R. cruenta (holotypes) with related species in “crown clades” as illustrated in Looney et al. (2016) based on ITS sequences. Branches are labeled with maximum likelihood bootstrap higher than 50%, and Bayesian posterior probabilities more than 0.9 respectively. Sequences of R. sect. Auratinae were used as outgroup to root trees. The new isolates are in bold
Russula cruenta G.J. Li & Chun Y. Deng, sp. nov.
Fungal Names Number: FN 570740; Facesoffungi number: FoF 14522; Figs. 168c, 170c, d, 173
Etymology: Referring to the reddish tinge of pileus.
Holotype: HGAS-MF 013964.
Basidiomes small to medium sized. Pileus 29–38 mm in diam, hemispheric when young, then convex, expanded when old, a tinge of brightly red (D66467, CB3145), often faded to a paler orange reddish tinge (F4512C, E9693D) towards the margin, smooth, glabrous, slightly viscid when wet; margin not striate, rarely cracked, exfoliated in small patches, peeling 1/3–1/2 from the edge. Lamellae adnate, 3–4 mm in height, 13–16 pieces per centimeter at edge, not forked, interveined, white (FFFFFF), pale cream (F4EAE0) when mature, unchanging when bruised, lamellulae not observed. Stipes 29–48 × 10–13 mm, central to subcentral, cylindrical, rugulose longitudinally, white (FFFFFF), partly pale pink (F1ACB0, EEB0AE), unchanging when bruised, smooth, not viscid when wet, slightly ventricose downward the base, first stuffed, hollow when mature, annulus absent. Context 2 mm thick at pileus center, white (FFFFFF), unchanging when injured, taste mild to slightly acrid, smell indistinct to fruity. Spore print cream to pale ocher (Romagnesi IIc–IId).
Basidiospores [150/3/3] (6.6–) 7–5.9 (–10.3) × (5.4–) 5.9–8.5 (–9.1) μm, Q = 1.00–1.34 (1.42), Q = 1.17 ± 0.07), hyaline, subglobose, broad ellipsoid to ellipsoid, rarely globose, ornamentations amyloid, up to 1.2 μm in height, composed of isolated warts rarely connected as short crests, not forming reticulum, suprahilar area plage distinct but inamyloid. Basidia 34–53 × 10–12 (–15) μm, cylindrical, subcylindrical to subclavate, rarely clavate four-spored, projecting 10–20 μm beyond hymenium, hyaline, sterigmata 4–6 μm long. Hymenial cystidia scarce, 60–70 × 8–10 μm, fusiform to subclavate, sometimes subfusiform or clavate, contents sparsely distributed, granulate, greyish in SV, apex obtuse to subacute. Pileipellis two layered, not clearly delimited from the spherocytes in context; epipellis trichoderm, ca. 30–60 μm deep, erect to ascending, rarely horizontal; terminal cells hyaline, sometimes inflated, 5–10 μm wide; primordial hyphae abundant, 5–8 μm wide, septate, surface with acido-resistant incrustations; pileocystidia absent; subpellis composed of somewhat gelatinized, densely interlaced, rarely branched and septate, hyaline hyphae 3–6 μm wide. Stipitipellis a cutis, composed of parallel hyaline hyphae 3–5 μm wide.
Habitat: Single or scattered in coniferous and broad-leaved intermixed forest.
Material examined: China, Guizhou Province, Songtao County, in coniferous and broad-leaved intermixed forest, 20 October 2017, HGAS-MF 013964 (holotype).
GenBank number: ITS = MT505893.
Notes: The new species Russula cruenta clusters with an unknown Russula sample from Japan (Fig. 172). The Cruenta clade loosely groups with another clade which is composed of two North American species R. ballouii and R. burlinghamiae (Fig. 172). These two species can be distinguished from R. cruenta by their yellowish tinged pileus with fractured pigment patches. Russula ballouii also differs in basidiospore ornametations forming incomplete reticulum (Peck 1913; Buyck et al. 2003). Russula cruenta is also morpholoically similar to two reddish-capped species: one is the newly described Asian R. clavatohyphata from India, and the other is the North American species R. luteobasis. Russula clavatohyphata differs in having rare lamellulae at pileus margin, pink to pale red stipe, and appendages up to 30 µm long at the hymenial cystidium apex (Wang et al. 2019b). Russula luteobasis can be differentiated in its yellowish turning pileus, low basidiospore ornamentations 0.1–0.3 μm without isolated warts, and hymenial cystidium apex mainly with 4–10 μm long appendage (Adamčík et al. 2018).
Russula haematina G.J. Li & Chun Y. Deng, sp. nov.
Fungal Names Number: FN 570741; Facesoffungi number: FoF 14527; Fig. 174
Etymology: Referring to the brightly red pileus.
Holotype: HGAS-MF 013965.
Basidiomes medium sized. Pileus 35–48 mm in diam, first plano-hemisphere, then convex, flat when mature, a tinge of brightly red (B82928, C52242), often intermixed with pinkish red tinge (F87B90, F58FA3), sometimes faded to a pinkish yellow tinge (FCC1A2, FED1B0) at center, smooth, dull, slightly viscid when wet; margin not striate, rarely cracked, peeling about 1/3 from the edge. Lamellae adnate, 2–4 mm in height, 12–16 pieces per centimeter at edge, not forked, interveined, white (FFFFFF), unchanging when bruised, lamellulae not observed. Stipes 31–43 × 10–14 mm, central to subcentral, cylindrical, rugulose longitudinally, pale pinkish red (E08087, F496A1), partly white (FFFFFF), turning pale ocher (B49B57, 9A7C37) when injured and old, smooth, dull, not viscid when wet, gradually attenuate downward the base, first stuffed, hollow when old, annulus absent. Context 3–4 mm thick at pileus center, white (FFFFFF), turning pale ocherous yellow (BC8B1F, DBB960) when bruised and old, taste mild, smell indistinct. Spore print pale cream (Romagnesi IIa–IIb).
Basidiospores [150/3/3] (6.3–) 6.6–8.4 (–8.8) × (5.3–) 5.7–7.7 (–8.2) μm, Q = 1.01–1.27, Q = 1.13 ± 0.06), hyaline, subglobose to broad ellipsoid, rarely globose, ornamentations amyloid, up to 0.7 μm in height, composed of long ridges interconnected as complete reticulum, isolated warts and short crests absent, suprahilar area plage distinct and amyloid. Basidia 31–42 × 10–12 μm, subcylindrical to subclavate, rarely clavate, four-spored, projecting 10–20 μm beyond hymenium, hyaline, sterigmata 5–7 μm long. Hymenial cystidia rare, 65–90 × 12–14 μm, fusiform, unchanging or slightly greyish in SV, apex constricted, mostly forming an elongate appendage. Pileipellis two layered, not clearly delimited from the spherocytes in context; epipellis a trichoderm, ca. 30–70 μm thick, hyphae erect to oblique, often branched, hyaline; terminal cells cylindrical to flexuous, 5–10 μm wide, often with a capitate or mucronate apex, rarely containing granulate contents slightly greyish in SV, subapical cells sometimes protruding, irregular, 7–14 μm wide; primordial abundant, hyphae 3–6 μm wide, cylindrical, surface with acido-resistant incrustations; subpellis composed of somewhat gelatinized, loosely interweaved, rarely branched and septate, hyaline hyphae 3–7 μm wide, rarely intermixed with spherocytes 10–25 μm in diam.
Habitat: Single or scattered in coniferous and broad-leaved intermixed forest.
Material examined: China, Guizhou Province, Guiyang, Yunyan District, Qianlingshan Park, in coniferous and broad-leaved forest, 1 September 2019, Chun-Ying Deng 2018–48 (HGAS-MF 013965, holotype).
GenBank number: ITS = MT505891.
Notes: Phylogentically, in Russula sect. Roseinae (Fig. 175), the new species Russula haematina forms a clade with speciemens of Rosa clade. The other members of the clade can be distinguished from R. haematina as follows: R. amarissima has violaceous purple to cigar brown pileus, bitter taste of context, narrower basidia 8–10 μm wide, and longer hymenial cystidia up to 115 μm long; R. rosea has longer hymenial cystidia with obtuse apex and narrower epipellis hyphae 3.2–4.5 μm wide (Romagnesi 1985; Sarnari 2005). There are two species, R. guangxiensis and R. hakkae of R. sect. Roseinae which were described from South China. These two species also have brightly red pileus. Russula guangxiensis can be differentiated from R. haematina by its white stipe, mild tasted context, smaller basidiospores (5.9–6.9 × 4.9–6.1 μm), and shorter and narrower hymenial cystidia (55–63 × 7–12 μm); R. hakkae differs in the ornamentations of basidiospores composed of dense and isolated warts (0.9–1.2 μm in height), longer and wider basidia, and round to subacute, often shortly appendiculate to subcapitate apex of hymenial cystidia (40–50 × 10–15 μm, Ariyawansa et al. 2015).
Maximum Parsimony strict consensus tree illustrating the phylogeny of Russula haematina (holotype) with related species in R. sect. Roseinae based on ITS sequences. Branches are labeled with maximum likelihood bootstrap higher than 50%, and Bayesian posterior probabilities more than 0.9 respectively. Sequences of R. sect. Lilaceinae were used as outgroup to root trees. The new isolates are in bold
Russula luteocarpa G.J. Li & Chun Y. Deng, sp. nov.
Fungal Names Number: FN570738; Facesoffungi number: FoF 14526; Figs. 166c, d, 168d, 176
Etymology: Referring to the yellowish pileus of basidiomes.
Holotype: HGAS-MF 013966.
Basidiomes medium sized. Pileus 37–48 mm in diam, first plano-hemisphere to convex, turning applanate to slightly depressed at center when mature, ocherous tinged, brownish yellow (947563, B0876D) to dark yellow (BC9C69, D1A14A), faded to a pale yellow tinge (F1DE9C, F9E397) towards the margin, smooth, glabrous, somewhat sticky when wet; margin not striate to slightly striate, seldom cracked, peeling 1/3–1/2 from the edge. Lamellae adnate, 3–4 mm in height, 14–18 pieces per centimeter at edge, often forked near the stipes, interveined, white (FFFFFF) when young, cream (F1DDCF, F0D8C3) when mature, unchanging when bruised, lamellulae not observed. Stipes 42–56 × 10–14 mm, central to subcentral, cylindrical, rugulose longitudinally, white (FFFFFF), slowly turning pale ocher (B88E4D, B48834) when bruised, smooth, dull, not viscid when wet, slightly attenuate downward the base, first stuffed, hollow when old, annulus absent. Context 2–3 mm thick at pileus center, slightly turning white (FFFFFF), pale ocher (BD7B27, D5A372) when injured, taste mild, smell indistinct. Spore print cream (Romagnesi IIc–IId).
Basidiospores [150/3/3] (4.8–)5.1–7.1 (–7.4) × (4.1–) 4.4–6.6 (–7.1) μm, Q = 1.02–1.31 (1.36), Q = 1.18 ± 0.07), hyaline, subglobose to broad ellipsoid, rarely globose and ellipsoid, ornamentations amyloid, up to 0.5 μm in height, composed of long ridges interconnected as nearly complete reticulum, often intermixed with rare isolated warts, suprahilar area plage indistinct and inamyloid. Basidia 32–44 × 7–10 μm, cylindrical, subcylindrical to subclavate, rarely clavate four-spored, projecting 10–20 μm beyond hymenium, hyaline, sterigmata 4–6 μm long. Hymenial cystidia 61–76 × 11–14 μm, fusiform to cylindrical, sometimes subclavate, contents granulate, sparse, weakly greyish in SV, apex lanceolate, sometimes subacute. Pileipellis two layered, indistinctly delimited from the spherocytes in context; epipellis a trichoderm, ca. 100–150 μm thick, hyphae erect to ascending, rarely branched, cylindrical, hyaline, 2–4 μm wide, rarely septate, some hyphae inflated to 5–7 μm, multi-septate; terminal cells attenuated towards the apex; pileocystidia rare, 6–8 μm wide, cylindrical to clavate, often with an obtuse apex, contents dense, granulate, blackish in SV; subpellis composed of somewhat gelatinized, densely interlaced, rarely branched and septate, hyaline hyphae 3–6 μm wide.
Habitat: Single or scattered in coniferous and broad-leaved intermixed forest.
Material examined: China, Guizhou Province, Weining County, Caohai Nature Reserve, in coniferous and broad-leaved intermixed forest, 4 August 2016, Chun-Ying Deng CH2016080404 (HGAS-MF 013966, holotype).
GenBank number: ITS = MT505889.
Notes: Topologically, Russula luteocarpa lies in the clade of Russula subsect. Heterophyllae, but the phylogenetic position of this new species in this subsection is still not clear (Fig. 168). Future multi-gene phylogenetic analyses are needed to clarify the concrete relationships between R. luteocarpa and the other species of this subsection. The viscid yellowish, not furfuraceous or areolate pileus of R. luteocarpa is unique in R. subsect. Heterophyllae, but is reminiscent of R. section Ingratae. However, members of that section are different from R. luteocarpa, because they often have strongly striate pileus margin and acrid context taste.
Russula sanguinolenta G.J. Li & Chun Y. Deng, sp. nov.
Fungal Names Number: FN 570742; Facesoffungi number: FoF 14525; Fig. 177
Etymology: Referring to the brightly reddish tinge of pileus.
Holotype: HGAS-MF 013963.
Basidiomes small to medium sized. Pileus 29–57 mm in diam., first hemispheric, then convex, applanate when mature, a tinge of brightly red (9B2335, BC243C), often partly faded to a paler pinkish red tinge (EC5D73, EC5D73) at center, rarely turning pale orange pink (EEB0AE, FEA188) to white (FFFFFF) smooth, glabrous, slightly viscid when wet; margin slightly striate, rarely cracked, peeling 1/4–1/2 from the edge. Lamellae adnate, 2–4 mm in height, 11–18 pieces per centimeter at edge, rarely forked near the stipes, interveined, white (FFFFFF) first, pale cream (F4EAE0) when mature, unchanging when bruised, lamellulae not observed. Stipes 29–48 × 10–13 mm, central to subcentral, cylindrical, rugulose longitudinally, pale pink (E08087, FABCB1), rarely faded to white (FFFFFF), a darker pale pinkish red tinge (ED5656, FA7268) near the base, unchanging when injured, smooth, dull, not viscid when wet, slightly tapered downward the base, first stuffed, hollow when mature, annulus absent. Context 3–4 mm thick at pileus center, white (FFFFFF), unchanging, taste acrid, smell indistinct. Spore print cream to pale ocher (Romagnesi IIc–IId).
Basidiospores [150/3/3] (5.6–) 6.1–8 (–8.5) × (4.7–) 5–6.8 (–7.1) μm, Q = (1.00–)1.05–1.37(–1.41), Q = 1.21 ± 0.09, hyaline, subglobose to broad ellipsoid, rarely globose and ellipsoid, ornamentations amyloid, up to 1 μm in height, composed of short crests interconnected as incomplete network, intermixed with a few isolated warts, suprahilar area plage indistinct but amyloid. Basidia 34–43 × 9–13 μm, clavate to subclavate, rarely cylindrical, four-spored, projecting 15–25 μm beyond hymenium, hyaline, sterigmata 5–6 μm long. Hymenial cystidia abundant, 57–99 × 10–16 μm, fusiform, sometimes clavate, ventricose towards the apex, contents crystal, densely distributed, blackish in SV, apex contracted. Pileipellis two layered, distinctly delimited from the spherocytes in context; epipellis an ixotrichoderm, ca. 100–150 μm thick, hyphae hyaline, ascending to erect, rarely septate, 2–4 μm wide; terminal cells cylindrical, tapering towards the apex; pileocystidia abundant, cylindrical to subclavate, 6–9 μm wide, contents granulate, dense, blackish in SV, apex obtuse, sometimes tapering; subpellis a cutis, hyphae repent, slightly gelatinized, densely interweaved, rarely branched, septate, hyaline, 3–6 μm wide.
Habitat: Scattered in coniferous forest.
Material examined: China, Guizhou Province, Guiyang, Huaxi District, Fengbaoyun Village, in coniferous forest, 1 July 2017, Chun-Ying Deng 2017–131 (HGAS-MF 013963, holotype).
GenBank number: ITS = MT505892.
Notes: A combination of brightly red pileus, pinkish tinged stipe, cream to ocher spore print, and a habitat of coniferous forest indicates this new specie close to Russula sanguinea. This affinity is also supported in phylogenetic analyses (Fig. 178). The R. sanguinolenta group includes R. sanguinea and R. sanguinaria. Russula sanguinea differs from R. sanguinolenta by its subdecurrent lamellae, larger basidiospores (7.2–9.6 × 6.3–7.4 μm) with lower ornamentations up to 0.8 μm, longer basidia (40–54 × 9–12 μm), and wider hymenial cystidia up to 16 μm (Sarnari 1998). Russula sanguinaria is distinguished from R. sanguinolenta by its yellowish turing pileus and larger basidiospores (7.5–9 × 6.5–7.5 μm, Rauschert 1989). The other two closely related species are R. helodes and R. renidens. Russula helodes is distinguished by distinctly greyish context, basidiospores up to 10.5 μm in diam with ornamentations of a complete network, and a habitat of Sphagnum environment. Russula renidens differs in its shiny to glazer pileus, abundant lamellulae in pileus margin, larger basidiospores (7.5–9 × 6.5–7.5 μm), and narrower hymenial cystidia up to 11 μm wide (Sarnari 1998).
Maximum likelihood tree illustrating the phylogeny of Russula sanguinolenta (holotype) with related species in R. sect. Persicinae and R. sect. Sardoninae based on ITS sequences. Branches are labeled with maximum likelihood bootstrap higher than 50%, and Bayesian posterior probabilities more than 0.9 respectively. Sequences of R. sect. Emeticinae were used as outgroup to root trees. The new isolates are in bold
Trechisporales K.H. Larss.
Notes: Trechisporales was erected by Hibbett et al. (2007) to replace its equivalent order namer Hydnodontales, due to that the type genus Trechispora has a priority over the synonym Hydnodon and is the speciose genus in this order. Recently, Liu et al. (2022b) reduced the circumscription of Trechisporales by segregating Sertulicium and Sistotremastrum to a new order. Phylogenetically, Trechisporales is a well suppported order, accommodating a single family Hydnodontaceae.
Hydnodontaceae Jülich.
Notes: Hydnodontaceae was erected by Jülich (1981) with Hydnodon as the type genus that is a later synonym of Trechispora (Ryvarden 2002). For now, 12 genera are accepted in this family (Liu et al. 2022b).
Trechispora P. Karst.
Notes: The genus Trechispora was erected by Karsten (1980) with T. onusta as the type species. The main morphological character that defines the genus is remarkable ampullate hyphal septa, and in addition, most species in this genus have ellipsoid, ornamented basidiospores (Liu et al. 2022b). A total of 90 species have been previously recorded all over the world (Liu et al. 2022b; Luo and Zhao 2022b). Here, four additional new lineages revealed from the phylogenetic analysis (Fig. 179) are described as new species.
Phylogeny generated by the maximum likelihood algorithm based on combined ITS and nLSU regions is presented along with the bootstrap values and the Bayesian posterior probabilities above 50% and 0.8, respectively, at the nodes. Dextrinocystis calamicola He 5693, Porpomyces mucidus Dai 12692 and Tubulicium raphidisporum He 3191 were selected as the outgroup taxa. Holotypes are in bold and the newly generated sequences are in blue
Trechispora alba S.L. Liu, G. He & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901060; Facesoffungi number: FoF 14813; Figs. 180, 181, 182
Etymology: alba (Lat.) referring to the white colour of basidiomes.
Holotype: HG 19350 (HMAS).
Diagnosis: Differing from Trechispora pallescens in slightly wider basidiospores (de Meiras-Ottoni et al. 2021).
Basidiomes annual, clavarioid, solitary or in small groups, densely branched, moderately open, fleshy consistency, white when fresh, cream to curry-yellow when dry, 2–8 cm high. Branches polychotomous, axils V-shaped, somewhat palmately branched, slightly flattened, 2–5 mm wide, occasionally flattened to 10 mm, tips subacute to blunt. Stipe white to cream, 5–15 × 2–3 mm.
Hyphal system monomitic; generative hyphae with clamp connections. Subicular hyphae hyaline, thick-walled, cylindrical, moderately branched and septate, subparallel, 2–3.5 μm in diam; ampullate septa usually present in the hyphae at the base of the stipe, up to 6–7 μm wide. Subhymenial hyphae short-celled and wide, 2–3.5 µm in diam, much branched. Basidia suburniform to subclavate, hyaline, thin-walled, with four sterigmata and a basal clamp connection, agglutinated, 20–26 × 5–8 µm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores ellipsoid, hyaline, thin-walled, aculeate, IKI–, CB–, 5–7(–7.5) × (3.3–)3.5–5(–5.5) µm, L = 5.7 µm, W = 4.1 µm, Q = 1.4 (n = 60/2).
Material examined: China, Yunnan Province, Xishuangbanna Dai Autonomous Prefecture, Mengla County, on ground, 30 July 2019, G. He and Y. Chen, HG 19350 (HMAS, holotype); Yunnan Province, Honghe Hani and Yi Autonomous Prefecture, Honghe County, Dayangjie Township, on ground, 22 August 2021, J.W. Guo, CH 21384 (HMAS).
GenBank numbers: HG 19350: ITS = OM523516; CH 21384: ITS = OR557258.
Notes: Trechispora alba is similar to T. caulocystidiatus and T. gelatinosa by white to yellowish white basidiomes when fresh and the presence of cystidial structures. However, T. caulocystidiatus differs from T. alba in the presence of reddish-brown apices on basidiomes, caulocystidial hairs in stipitipellis and smaller basidiospores (3.5–4.5 × 3–3.5 µm, Furtado et al. 2021); T. gelatinosa differs in its dull yellow to brownish orange basidiomes when dried and smaller basidiospores (3.2–4.5 × 2.5–3.5 µm, de Meiras-Ottoni et al. 2021).
Trechispora perminispora S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901061; Facesoffungi number: FoF 14814; Figs. 183, 184, 185
Etymology: perminispora (Lat.) referring to very small basidiospores.
Holotype: LWZ 20190816-39a (HMAS).
Diagnosis: Differing from other species of Trechispora in the smallest basidiospores.
Basidiomes annual, resupinate, effused, thin, soft and fragile, easily separated from substrates, up to 15 cm long, 3 cm wide. Subiculum too thin to be seen. Hymenophore grandinioid with numerous small aculei, white to cream when fresh, cream to buff when dry. Margin white, slightly fimbriate, up to 0.5 mm wide.
Hyphal system monomitic; generative hyphae with clamp connections. Tramal hyphae distinct, hyaline, thin-walled, frequently branched, smooth, interwoven, 3–3.5 μm in diam. Cystidia absent. Crystals aggregated, rhomboidal flakes. Basidia cylindrical with a slight median constriction, hyaline, thin-walled, with four sterigmata and a basal clamp connection, 9–12 × 4–4.5 µm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores ellipsoid, hyaline, slightly thick-walled, verrucose, IKI–, CB–, (2.5–)2.6–3(–3.2) × 1.8–2.1(–2.3) µm, L = 2.8 µm, W = 2 µm, Q = 1.4 (n = 30/1).
Material examined: China, Sichuan Province, Ebian Yi Autonomous County, Heizhugou National Nature Reserve, on fallen branch of angiosperm, 16 August 2019, L.W. Zhou, LWZ 20190816-39a (HMAS, holotype).
GenBank numbers: ITS = OM523525, LSU = OM339329.
Notes: In Trechispora, basidiospores are mostly more than 3.5 µm in length and 2.5 µm in width. Two species, viz. Trechispora microspora and T. minispora, have smaller basidiospores than these sizes (Larsson 1996; de Meiras-Ottoni 2021). However, even in comparison with these two species, T. perminispora has smaller basidiospores, which make it distinguished in this genus.
Trechispora subfarinacea S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901062; Facesoffungi number: FoF 14815; Figs. 186, 187, 188
Etymology: subfarinacea (Lat.) referring to the morphological similarity and phylogenetic kinship to Trechispora farinacea.
Holotype: LWZ 20200921-33a (HMAS).
Diagnosis: Differing from T. farinacea in smooth hymenophore and smaller basidiospores (Larsson 1995).
Basidiomes annual, resupinate, effused, thin, soft, easily separated from substrates, up to 20 cm long, 2 cm wide, 50–80 µm thick. Hymenophore smooth, arachnoid, white when fresh, ash-grey to pale mouse-gray when dry, not cracked. Margin white, thinning out as byssoid, 0.2 mm wide.
Hyphal system monomitic; generative hyphae with clamp connections. Subicular hyphae long-celled, hyaline, thin-walled, frequently branched and septate, interwoven, 3–4 µm in diam, ampullate septa up to 5 μm wide. Subhymenium composed of indistinct generative hyphae, much branched. Cystidia absent. Crystals rare, aggregated, rhomboidal flakes. Basidia cylindrical with a slight median constriction, hyaline, thin-walled, with four sterigmata and a basal clamp connection, 10–15 × 5–6 µm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores subglobose, hyaline, thick-walled, verrucose, IKI–, CB–, 3–3.5(–3.7) × 2.8–3(–3.1) µm, L = 3.2 µm, W = 2.9 µm, Q = 1.1 (n = 30/1).
Material examined: China, Sichuan Province, Liangshan Yi Autonomous Prefecture, Leibo County, Mamize Nature Reserve, on fallen branch of Picea, 21 September 2020, L.W. Zhou, LWZ 20200921-33a (HMAS, holotype).
GenBank numbers: ITS = OM523528, LSU = OM339331.
Notes: Trechispora gracilis is quite similar to T. subfarinacea by thin basidiomes and smooth hymenophore, but differs in cream to light ash-grey hymenophore when fresh and slightly smaller basidiospores (2.8–3.2 × 2.3–2.8 µm, Liu et al. 2022b).
Trechispora tuberculata S.L. Liu & L.W. Zhou, sp. nov.
Index Fungorum number: IF 901063; Facesoffungi number: FoF 14816; Figs. 189, 190, 191
Etymology: tuberculata (Lat.) referring to tubercules on spines of basidiospores.
Holotype: BJFC024964.
Diagnosis: Differing from other species of Trechispora by having numerous tubercules on spines of basidiospores.
Basidiomes annual, resupinate, effused, thin, soft, easily separated from substrates, up to 4 cm long, 3 cm wide. Hymenophore hydnoid with numerous, small aculei, straw-yellow to bluish grey when dry. Margin undifferentiated.
Hyphal system monomitic; generative hyphae with clamp connections. Subicular hyphae hyaline, thick-walled, moderately branched and septate, subparallel, 3–5 µm in diam. Tramal hyphae distinct, hyaline, thin or thick-walled, moderately branched, smooth, subparallel, 2–3.5 μm in diam. Cystidia absent. Crystals usually present, bipyramidic, aggregated. Basidia cylindrical with a slight median constriction, hyaline, thin-walled, with 4 sterigmata and a basal clamp connection, 15–20 × 5–6 µm; basidioles in shape similar to basidia, but slightly smaller. Basidiospores abundant, ellipsoid, hyaline, thin to slightly thick-walled, aculeate, with numerous tubercules on spines, IKI–, CB–, (3–)3.3–4(–4.3) × 2.7–3.2 µm, L = 3.7 µm, W = 3 µm, Q = 1.2 (n = 30/1).
Material examined: Brazil, Pernambuco State, Recife, Recife Botanical Garden, on fallen angiosperm trunk, 16 May 2017, Yu-Cheng Dai, Dai 17433 (BJFC024964, holotype; isotype in HMAS).
GenBank numbers: ITS = OM523507, LSU = OM339314.
Notes: Trechispora tuberculata resembles T. constricta by hydnoid hymenophore; however, T. constricta differs in the presence of thick-walled generative hyphae and aculeate basidiospores with a slight constriction in the middle-upper part of spines (Liu et al. 2022b).
Tremellomycetes Doweld.
Notes: For the latest updated account of Tremellomycetes see Liu et al. (2020).
Tremellales Fr.
Notes: For the latest updated account of Tremellales see Liu et al. (2020).
Tremellaceae Fr.
Notes: For the latest updated account of Tremellaceae see Fan et al. (2021).
Tremella Pers.
Notes: For the latest updated account of Tremella see Liu et al. (2015b), Fan et al. (2021).
Tremella sairandhriana A. Thomas & T.K.A. Kumar, sp. nov.
Mycobank number: MB 844398; Facesoffungi number: FoF11779; Fig. 192
Tremella sairandhriana (ZGCAT89, holotype). A Basidiomes. B Cross section in water. C–E Diferent septations in mature basidia. F Vesicle. G Haustoria. H Basidiospores, budding, and germination via germ tube. I Hyphidia with basidia of different developmental stages. J Collapsed basidia (Black Arrow) and conidia from its sterigmata (White Arrow). K Septate sterigmata (Black Arrow). L Bifurcating sterigmata bearing basidiospores. Scale bars: A = 10 mm, B = 30 µm, C–L = 10 µm
Etymology: refers to the collection locality.
Holotype: ZGCAT89.
Diagnosis: The species is characterised by whitish, resupinate, cerebriform basidiomes, and clamped hyphidia, and conidial formation from sterigmata. This species differs from the closely related T. resupinata by their larger basidiomes, presence of hyphidia and conidia borne on sterigmata.
Basidiomes medium sized, 10–45 × 1.5–3.5 mm, soft gelatinous, resupinate, cerebriform, sessile, broadly attached, easy to separate from the substratum. Margin sometimes wavy, white to pale yellowish white when fresh and pale yellowish brown when dry. Spore print whitish.
Hymenium pale brown in water. Hyphidia 3–7 µm, branched, septate, thick-walled, with clamp connections. Basidia 27–35 × 26–27.5 µm, globose to subglobose, two to four celled, longitudinally, transversely (rare), or obliquely septate, thin-walled, guttulate, hyaline, with basal clamp connection. Basidioles clavate to obovoid. Sterigma 59–96 × 6.5–9 µm, sometimes with swollen tip (up to 11 µm), septate, branched, occasionally producing conidia. Conidia from sterigma 3–7 × 3–7 µm, globose, hyaline, smooth, thin-walled. Basidiospores 16–19 × 17–20 µm (Q = 0.84–1 µm, Qm = 0.96 µm), globose to subglobose, hyaline, thin-walled, smooth, guttulate, apiculate. Secondary spore production, and yeast cell formation from basidiospores observed. Yeast cells 3–7 × 3–7 µm, globose, subglobose, broadly ellipsoid. Vesicles 10.4–12.7 × 5.9–7.8 µm, ellipsoid, oval, thick-walled. Swollen cells absent. Tramal hyphae 2–5.5 µm wide, slightly thick- to thick-walled, branched, smooth, hyaline, frequently anastomosing, with clamp connections. Haustoria abundant on hyphidia and subhymenium, rarely branching, with basal clamp connection, sometimes with forked tip.
Habitat and distribution: On unidentified fungal host growing on dead and decaying wood log.
Material examined: India, Kerala State, Palakkad District, Silent Valley National Park, 4 July 2019, A. Thomas, ZGCAT89 (holotype).
GenBank numbers: ITS = ON668075, LSU = ON668076.
Notes: Tremella sairandhriana is characterised by whitish, resupinate basidiomes, presence of hyphidia, globose to subglobose basidia, formation of condia from sterigmata, and rarely branching haustoria. Among the whitish Tremella members, T. sairandhriana show close similarity to T. resupinata in the resupinate nature of basidiomes and larger size of basidia and basidiospores. However, T. sairandhriana differs from T. resupinata in the following characters: relatively large basidiomes, branched, septate, thick-walled, clamped hyphidia, and conidial formation from sterigmata.
Tremella cheejenii has similarity with T. sairandhriana in its whitish cerebriform basidiomes, and in the formation of conidia from sterigmata. However, T. cheejenii has smaller basidiospores (5–10 µm) when compared to T. sairandhriana. Tremella latispora also have whitish basidiomes and are similar in morphology with T. sairandhriana, but T. latispora differs by their smaller basidia (17.2–24.0 µm) and basidiospores (10.1–11.8 µm, Fan et al. 2021).
In the molecular phylogenetic analysis, T. sairandhriana clusters with T. resupinata (Fig. 193), and is a sister clade to the T. fibulifera complex of Fan et al. (2021). Members of T. fibulifera complex are morphologically characterised by cerebriform whitish basidiome and abundant clamp connections (Fan et al. 2021). The resupinate nature of basidiome, and the larger sized basidia and basidiospores of T. sairandhriana and T. resupinata segregate them from the T. fibulifera complex.
Phylogram generated from maximum likelihood analysis based on nITS and nLSU sequences. The dataset contained 92 representative accessions with 1583 characters including gaps. Cryptococcus depauperatus were used as outgroup (Malysheva et al. 2015). Type strains are in bold and newly generated sequence is in blue
References
Adamčík S, Jančovičová S, Buyck B (2018) The Russulas described by Charles Horton Peck. Cryptogam Mycol 39:3–108
Ahmed SA, van de Sande WWJ, Stevens DA, Fahal A, van Diepeningen AD, Menken SBJ, de Hoog GS (2014) Revision of agents of black-grain eumycetoma in the order of Pleosporales. Persoonia 33:141–154
Aime MC, Phillips-Mora W (2005) The causal agents of witches’ broom and frosty pod rot of cacao (chocolate, Theobroma cacao) form a new lineage of Marasmiaceae. Mycologia 97:1012–1022
Aime MC, Miller AN, Aoki T, Bensch K, Cai L, Crous PW, Hawksworth DL, Hyde KD, Kirk PM, Lücking R, May TW, Malosso E, Redhead SA, Rossman AY, Stadler M, Thines M, Yurkov AM, Zhang N, Schoch CL (2021) How to publish a new fungal species, or name, version 3.0. IMA Fungus 12:11
Al-Bedak O (2019) Paracremonium moubasheri, a new species from an alkaline sediment of Lake Hamra in Wadi-El-Natron, Egypt with a key to the accepted species. Stud Fungi 4:216–222
Alcorn JL (1996) Cochliobolus heliconiae sp. nov. (Ascomycota). Aust Syst Bot 9:813–817
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Aini AN, Mongkolsamrit S, Wijanarka W, Thanakitpipattana D, Luangsa-Ard JJ, Budiharjo A (2020) Diversity of Akanthomyces on moths (Lepidoptera) in Thailand. MycoKeys 71:1–22
Antonín V (2012) Chaetocalathus and Crinipellis (Basidiomycota, Marasmiaceae) from tropical Africa: taxonomic novelties. Cryp Mycol 33:395–410
Antonín V, Noodeloos ME (2010) A monograph of marasmioid and collybioid fungi in Europe. IHW-Verlag, Eching
Antonín V, Ryoo R, Shin HD (2009) Marasmioid and gymnopoid fungi of the Republic of Korea. 1. Three interesting species of Crinipellis (Basidiomycota, Marasmiaceae). Mycotaxon 108:429–440
Antonín V, Ryoo R, Shin HD (2010) Marasmioid and gymnopoid fungi of the Republic of Korea. 2. Marasmius Sect Globulares Persoonia 24:49–59
Antonín V, Ryoo R, Ka HK, Sou HD (2014) Three new species of Crinipellis and one new variety of Moniliophthora (Basidiomycota, Marasmiaceae) described from the Republic of Korea. Phytotaxa 170:86–102
Apinis AE (1967) Dactylomyces and Thermoascus. Trans Br Mycol Soc 50:573–582
Aptroot A (2021) World key to the species of Pyrenulaceae and Trypetheliaceae. Arch Lichenol 29:1–91
Aptroot A, Lücking R (2016) A revisionary synopsis of the Trypetheliaceae (Ascomycota: Trypetheliales). Lichenologist 48:763–982
Aptroot A, Lücking R, Sipman HJM, Umaña L, Chaves JL (2008) Pyrenocarpous lichens with bitunicate asci: a frst assessment of the lichen biodiversity inventory in Costa Rica. Bibl Lichenologica 97:1–162
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal-Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Thorsten Lumbsch H, Matsumura M, Moncada B, Nuankaew S, Parnmen S, Santiago ALCMDA, Sommai S, Song Y, de Souza CAF, de Souza- Motta CM, Su HY, Suetrong S, Wang Y, FongWS Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH, Miettinen O, Spirin V, Hernawati (2015) Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–274
Arruda MCC, Sepuvelda GF, Miller RNG, Ferreira MASV, Santiago DVR, Resende MLV, Dianese JC, Felipe MSS (2005) Crinipellis brasiliensis, a new species based on morphological and molecular data. Mycologia 97:1378–1361
Audet S (2010) Essai de découpage systématique du genre Scutiger (Basidiomycota): Albatrellopsis, Albatrellus, Polyporoletus, Scutiger et description de six nouveaux genres. Mycotaxon 111:431–464
Awasthi DD (1991) A key to the microlichens of India, Nepal and Sri Lanka. Bibl Lichenologica 40:1–340
Bao DF, Bhat DJ, Boonmee S, Hyde KD, Luo ZL, Nalumpang S (2022) Lignicolous freshwater ascomycetes from Thailand: Introducing Dematipyriforma muriformis sp. nov., one new combination and two new records in Pleurotheciaceae. MycoKeys 93:57–79
Barr ME (1979) A classification of Loculoascomycetes. Mycologia 71:935–957
Barr ME (1987) Prodromus to class Loculoascomycetes. University of Massachusetts, Amherst
Bary A (1884) Vergleichende Morphologie und Biologie der Pilze, Mycetozoen und Bacterien. Wilhelm Engelmann, Leipzig
Bashir H, Chen J, Jabeen S, Ullah S, Khan J, Niazi AR, Zhang M, Khalid AN, Parra LA, Callac P (2021) An overview of Agaricus section Hondenses and Agaricus section Xanthodermatei with description of eight new species from Pakistan. Sci Rep 11:1–35
Benjamin RK (2001) Autophagomyces, Bordea, and a new genus, Rossiomyces, (Laboulbeniales). Aliso 19:99–136
Berbee ML, Pirsyedi M, Hubbard SC (1999) Cochliobolous phylogenetics and the origin of known, highly virulent pathogens, inferred from ITS and glceraldehyde-3 phosphate-dehydrogenase gene sequences. Mycologia 91:964–977
Berkeley MJ (1841) Notices of British fungi [138–207]. Annals and Magazine of Natural History Ser 1(6):359
Bernicchia A, Gorjón SP (2010) Fungi Europaei 12. Corticiaceae s.l. Edizioni Candusso, Alassio
Bernicchia A, Langer G, Gorjón SP (2010) Botryobasidium sassofratinoense sp. nov. (Cantharellales, Basidiomycota) from Italy. Mycotaxon 111:403–409
Bhunjun CS, Dong Y, Jayawardena RS, Jeewon R, Phukhamsakda C, Bundhun D, Hyde KD, Sheng J (2020) A polyphasic approach to delineate species in Bipolaris. Fungal Divers 102:225–256
Bijeesh C, Kumar AM, Pradeep CK (2020) A new species of Resupinatus (Agaricomycetes) with merulioid hymenophore from India. Phytotaxa 464:167–174
Biketova AY, Gelardi M, Smith ME, Simonini G, Healy RA, Taneyama Y, Vasquez G, Kovács Á, Nagy L, Wasser S, Peintner U, Nevo E, Bunyard B, Vizzini A (2022) Reappraisal of the genus Exsudoporus (Boletaceae) worldwide based on multi-gene phylogeny, morphology and biogeography, and insights on Amoenoboletus. J Fungi 8:101
Binder M, Hibbett DS (2006) Molecular systematics and biological diversification of Boletales. Mycologia 98:971–981
Binder M, Hibbett DS, Larsson KH, Larsson E, Langer E, Langer G (2005) The phylogenetic distribution of resupinate forms across the major clades of mushroom-forming fungi (homobasidiomycetes). Syst Biodivers 3:113–157
Binder M, Justo A, Riley R, Salamov A, Lopez-Giraldez F, Sjökvist E, Copeland A, Foster B, Sun H, Larsson E, Larsson KH, Townsend J, Grigoriev IV, Hibbett DS (2013) Phylogenetic and Phylogenomic overview of the Polyporales. Mycologia 105:1350–1373
Birkebak J (2010) The genus Leucocoprinus in western Washington. Mycotaxon 112:83–102
Biryola S, Demirbağa Z, Erdoğanb P, Demir I (2022) Development of Beauveria bassiana (Ascomycota: Hypocreales) as a mycoinsecticide to control green peach aphid, Myzus persicae (Homoptera: Aphididae) and investigation of its biocontrol potential. J Asia-Pac Entomol 25(2):101878
Blackwell M (2011) The fungi: 1, 2, 3 … 5.1 million species? Am J Bot 98:426–438
Boonmee S, Rossman AY, Liu JK, Li WJ, Dai DQ, Bhat JD, Jones EBG, McKenzie EHC, Xu JC, Hyde KD (2014) Tubeufiales, ord. nov., integrating sexual and asexual generic names. Fungal Divers 68:239–298
Boonmee S, Wanasinghe DN, Calabon MS, Huanraluek N, Chandrasiri SKU, Jones GEB, Rossi W, Leonardi M, Singh SK, Rana S, Singh PN, Maurya DK, Lagashetti AC, Choudhary D, Dai YC, Zhao CL, Mu YH, Yuan HS, He SH, Phookamsak R, Jiang HB, Martín MP, Dueñas M, Telleria MT, Kałucka IL, Jagodziński AM, Liimatainen K, Pereira DS, Phillips AJL, Suwannarach N, Kumla J, Khuna S, Lumyong S, Potter TB, Shivas RG, Sparks AH, Vaghefi N, Abdel-Wahab MA, Abdel-Aziz FA, Li GJ, Lin WF, Singh U, Bhatt RP, Lee HB, Nguyen TTT, Kirk PM, Dutta AK, Acharya K, Sarma VV, Niranjan M, Rajeshkumar KC, Ashtekar N, Lad S, Wijayawardene NN, Bhat DJ, Xu RJ, Wijesinghe SN, Shen HW, Luo ZL, Zhang JY, Sysouphanthong P, Thongklang N, Bao DF, Aluthmuhandiram JVS, Abdollahzadeh J, Javadi A, Dovana F, Usman M, Khalid AN, Dissanayake AJ, Telagathoti A, Probst M, Peintner U, Garrido-Benavent I, Bóna L, Merényi Z, Boros L, Zoltán B, Stielow JB, Jiang N, Tian CM, Shams E, Dehghanizadeh F, Pordel A, Javan-Nikkhah M, Denchev TT, Denchev CM, Kemler M, Begerow D, Deng CY, Harrower E, Bozorov T, Kholmuradova T, Gafforov Y, Abdurazakov A, Xu JC, Mortimer PE, Ren GC, Jeewon R, Maharachchikumbura SSN, Phukhamsakda C, Mapook A, Hyde KD (2021) Fungal diversity notes 1387–1511: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 111:1–335
Brahmanage RS, Lu YZ, Bhat DJ, Wanasinghe DN, Yan JY, Hyde KD, Boonmee S (2017) Phylogenetic investigations on freshwater fungi in Tubeufiaceae (Tubeufiales) reveals the new genus Dictyospora and new species Chlamydotubeufia aquatica and Helicosporium flavum. Mycosphere 8:917–933
Brown AA, Lawrence DP, Baumgartner K (2020) Role of basidiomycete fungi in the grapevine trunk disease esca. Plant Pathol 69:205–220
Burdsall HH Jr (1985) A contribution to the taxonomy of the genus Phanerochaete. Mycol Mem 10:1–165
Buyck B, Hallling R, Mueller GM (2003) The inventory of Russula in Costa Rica: discovery of two very rare North American species in montane oak forest. Bolletino del Gruppo Micologico “G. Bresadola” (Nuova Serie) 46:57–74
Buyck B, Hofstetter V, Verbeken A, Walleyn R (2010) Proposal to conserve Lactarius nom. cons. (Basidiomycota) with conserved type. Taxon 59:447–453
Buyck B, Zoller S, Hofstetter V (2018) Walking the thin line… ten years later: the dilemma of above- versus below-ground features to support phylogenies in the Russulaceae (Basidiomycota). Fungal Divers 89:267–292
Buyck B, Wang XH, Adamčíková K, Caboň M, Jančovičová S, Hofstetter V, Adamčík S (2020) One step closer to unravelling the origin of Russula: subgenus Glutinosae subg. nov. Mycosphere 11:285–304
Buyck B, Eyssartier G, Dima B, Consiglio G, Noordeloos ME, Papp V, Bera I, Ghosh A, Rossi W, Leonardi M, Das K (2021) Fungal Biodiversity Profiles 101–110. Cryptogam Mycol 42:63–89
Caboň M, Li GJ, Saba M, Kolařík M, Jančovičová S, Khalid AN, Moreau PA, Wen HA, Pfister DH, Adamčík S (2019) Phylogenetic study documents different speciation mechanisms within the Russula globispora lineage in boreal and arctic environments of the Northern Hemisphere. IMA Fungus 10:5
Calabon MS, Jones EB, Hyde KD, Boonmee S, Tibell S, Tibell L, Pang KL, Phookamsak R (2021) Phylogenetic assessment and taxonomic revision of Halobyssothecium and Lentithecium (Lentitheciaceae, Pleosporales). Mycol Prog 20:701–720
Calabon MS, Hyde KD, Jones EBG, Luo ZL, Dong W, Hurdeal VG, Gentekaki E, Rossi W, Leonardi M, Thiyagaraja V, Lestari AS, Shen HW, Bao DF, Boonyuen N, Zeng M (2022) Freshwater Fungal Numbers Fungal Divers 114:3–235
Calonge FD, Martín MP (2000) Morphological and molecular data on the taxonomy of Gymnomyces, Martellia and Zelleromyces (Elasmomycetaceae, Russulales). Mycotaxon 76:9–15
Candusso M, Lanzoni G (1990) Lepiota s.l. Fungi Europei 4. Saronno, Giovanna Biella
Cannon PF, Kirk PM (2007) Fungal families of the world. CABI, Wallingford
Cao Y, Zhang Y, Yu ZF, Mi F, Liu CL, Tang XZ, Long YX, He XX, Wang PF, Xu JP (2013) Structure, gene flow, and recombination among geographic populations of a Russula virescens ally from southwestern China. PLoS ONE 8:e73174
Cao T, Hu YP, Yu JR, Wei TZ, Yuan HS (2021) A phylogenetic overview of the Hydnaceae (Cantharellales, Basidiomycota) with new taxa from China. Stud Mycol 99:100121
Cavalier-Smith T (1998) A revised six-kingdom system of Life. Biol Rev 73:203–266
Cesati V, De Notaris G (1863) Schema di classificazione degli sferiacei italici aschigeri: più o meno appartenenti al genere Sphaeria nell'antico significato attribuitogli da Persoon. Tip. del RI de'Sordo-muti
Chai H, Liang ZQ, Xue R, Jiang S, Luo SH, Wang Y, Wu LL, Tang LP, Chen Y, Hong D, Zeng NK (2019) New and noteworthy boletes from subtropical and tropical China. MycoKeys 46:55–96
Chaiwan N, Lu YZ, Tibpromma S, Bhat DJ, Hyde KD, Boonmee S (2017) Neotubeufia gen. nov. and Tubeufia guangxiensis sp. nov. (Tubeufiaceae) from freshwater habitats. Mycosphere 8:1443–1456
Chen JZ, Zhao CL (2020) Morphological and molecular identification of four new resupinate species of Lyomyces (Hymenochaetales) from southern China. MycoKeys 65:101–118
Chen JJ, Cui BK, Dai YC (2016) Global diversity and molecular systematics of Wrightoporia s.l. (Russulales, Basidiomycota). Persoonia 37:21–36
Chen WH, Liu C, Han YF, Liang JD, Tian WY, Liang ZQ (2019a) Akanthomyces araneicola, a new araneogenous species from Southwest China. Phytotaxa 409(4):227–232
Chen B, Jiang XM, Song J, Liang JF, Wang SK, Lu JK (2019b) Morphological and phylogenetic analyses reveal the new species Russula pallidula from China. Sydowia 71:1–10
Chen WH, Han YF, Liang JD, Liang ZQ (2020a) Akanthomyces neocoleopterorum, a new verticillium-like species. Phytotaxa 432(2):119–124
Chen CC, Chen CY, Lim YW, Wu SH (2020b) Phylogeny and taxonomy of Ceriporia and other related taxa and description of three new species. Mycologia 112:64–82
Chen WH, Han YF, Liang JD, Tian WY, Liang ZQ (2021a) Multi-gene phylogenetic evidence indicates that Pleurodesmospora belongs in Cordycipitaceae (Hypocreales, Hypocreomycetidae) and Pleurodesmospora lepidopterorum sp. nov. on pupa from China. MycoKeys 80:45–55
Chen CC, Chen CY, Wu SH (2021b) Species diversity, taxonomy and multi-gene phylogeny of phlebioid clade (Phanerochaetaceae, Irpicaceae, Meruliaceae) of Polyporales. Fungal Divers 111:337–442
Chethana KWT, Manawasinghe IS, Hurdeal VG, Bhunjun CS, Appadoo MA, Gentekaki E, Raspé O, Promputtha I, Hyde KD (2021) What are fungal species and how to delineate them? Fungal Divers 109:1–25
Chevallier FF (1826) Flore Générale des Environs de Paris. Chez Ferra Jeune, Paris
Clark K, Karsch-Mizrachi I, Lipman DJ, Ostell J, Sayers EW (2016) GenBank. Nucleic Acids Res 44:D67–D72
Clerc P (1987) Systematics of the Usnea fragilescens aggregate and its distribution in Scandinavia. Nord J Bot 7:479–495
Clerc P (1998) Species concepts in the genus Usnea (lichenized Ascomycetes). Lichenologist 30:321–340
Clerc P (2006) Synopsis of Usnea (lichenized Ascomycetes) from the Azores with additional information on the species in Macaronesia. Lichenologist 38:191–212
Clerc P (2007) Usnea. In Lichen Flora of the Greater Sonoran Desert Region (T. H. Nash III, C. Gries & F. Bungartz, eds): 302–335. Tempe, Arizona: Lichens Unlimited, Arizona State University
Clerc P (2011) Usnea. In: Nordic Lichen Flora Vol. 4 (A. Thell & R. Moberg, eds.):107–127. Uddevalla: Nordic Lichen Society
Clerc P, Otte V (2018) Usnea viktoriana (Ascomycota, Parmeliaceae), a new European taxon of the Usnea barbata-dasopoga group, with a key to the shrubby-subpendulous sorediate Usnea species in Europe. Lichenologist 50:513–527
Coelho G, Silveira AO, Antonelli ZI, Yurchenko E (2016) Tropicoporus stratificans sp. nov. (Hymenochaetales, Basidiomycota) from southern Brazil. Phytotaxa 245:144–152
Cooper JA (2012) Nomenclatural Novelties Index Fungorum 3:1–1
Corda ACJ (1839) Icones fungorum hucusque cognitorum 3. J.G. Calve, Prague
Corner EJH (1966) A monograph of Canthareilloid fungi. Annals of Botany Memorandum 2:1–255
Corriol G, Moreau PA, Bellanger JM (2017) Calocybe hymenoderma sp. nov., Calocybella juncicola comb. nov. et les contours taxinomiques de la section Rugosomyces. Errotari 14:35–46
Crespo A, Lumbsch HT, Mattsson JE, Blanco O, Divakar PK, Articus K, Wiklund E, Bawingan PA, Wedin M (2007) Testing morphology-based hypotheses of phylogenetic relationships in Parmeliaceae (Ascomycota) using three ribosomal markers and the nuclear RPB1 gene. Mol Phylogenet Evol 44:812–824
Crous PW, Wingfield MJ, Burgess TI, Hardy GSJ, Crane C, Barrett S, Cano-Lira JF, Le Roux JJ, Thangavel R, Guarro J, Stchigel AM, Martín MP, Alfredo DS, Barber PA, Barreto RW, Baseia IG, Cano-Canals J, Cheewangkoon R, Ferreira RJ, Gené J, Lechat C, Moreno G, Roets F, Shivas RG, Sousa JO, Tan YP, Wiederhold NP, Abell SE, Accioly T, Albizu JL, Alves JL, Antoniolli ZI, Aplin N, Araújo J, Arzanlou M, Bezerra JDP, Bouchara J-P, Carlavilla JR, Castillo A, Castroagudín VL, Ceresini PC, Claridge GF, Coelho G, Coimbra VRM, Costa LA, da Cunha KC, da Silva SS, Daniel R, de Beer ZW, Dueñas M, Edwards J, Enwistle P, Fiuza PO, Fournier J, García D, Gibertoni TB, Giraud S, Guevara-Suárez M, Gusmão LFP, Haituk S, Heykoop M, Hirooka Y, Hofmann TA, Houbraken J, Hughes DP, Kautmanová I, Koppel O, Koukol O, Larsson E, Latha KPD, Lee DH, Lisboa DO, Lisboa WS, López-Villalba Á, Maciel JLN, Manimohan P, Manjón JL, Marincowitz S, Marney TS, Meijer M, Miller AN, Olariaga I, Paiva LM, Piepenbring M, Poveda-Molero JC, Raj KNA, Raja HA, Rougeron A, Salcedo I, Samadi R, Santos TAB, Scarlett K, Seifert KA, Shuttleworth LA, Silva GA, Silva M, Siqueira JPZ, Souza-Motta CM, Stephenson SL, Sutton DA, Tamakeaw N, Telleria MT, Valenzuela-Lopez N, Viljoen A, Visagie CM, Vizzini A, Wartchow F, Wingfield BD, Yurchenko E, Zamora JC, Groenewald JZ (2016) Fungal planet description sheets: 469–557. Persoonia 37:218–403
Crous PW, Wingfield MJ, Burgess TI, Carnegie AJ, Hardy GESJ, Smith D, Summerell BA, Cano-Lira JF, Guarro J, Houbraken J, Lombard L, Martín MP, Sandoval-Denis M, Alexandrova AV, Barnes CW, Baseia IG, Bezerra JDP, Guarnaccia V, May TW, Hernández-Restrepo M, Stchige AM, Miller AN, Ordoñez ME, Abreu VP, Accioly T, Agnello C, Agustin Colmán A, Albuquerque CC, Alfredo DS, Alvarado P, Araújo-Magalhães GR, Arauzo S, Atkinson T, Barili A, Barreto RW, Bezerra JL, Cabral TS, Camello Rodríguez FC, Cruz RHSF, Daniëls PP, Da Silva BDB, De Almeida DAC, De Carvalho AA, Decock CA, Delgat L, Denman S, Dimitrov RA, Edwards J, Fedosova AG, Ferreira RJ, Firmino AL, Flores JA, García D, Gené J, Giraldo A, Góis JS, Gomes AAM, Gonçalves CM, Gouliamova DE, Groenewald M, Guéorguiev BV, Guevara-Suarez M, Gusmão LFP, Hosaka K, Hubka V, Huhndorf SM, Jadan M, Jurjević KB, Kučera V, Kumar TKA, Kušan I, Lacerda SR, Lamlertthon S, Lisboa WS, Loizides M, Luangsa-Ard JJ, Lysková P, Mac Cormack WP, Macedo DM, Machado AR, Malysheva EF, Marinho P, Matočec N, Meijer M, Mešić A, Mongkolsamrit S, Moreira KA, Morozova OV, Nair KU, Nakamura N, Noisripoom W, Olariaga I, Oliveira RJV, Paiva LM, Pawar P, Pereira OL, Peterson SW, Prieto M, Rodríguez-Andrade E, Rojo De Blas C, Roy M, Santos ES, Sharma R, Silva GA, Souza-Motta CM, Takeuchi-Kaneko Y, Tanaka C, Thakur A, Smith MT, Tkalčec Z, Valenzuela-Lopez N, Van Der Kleij P, Verbeken A, Viana MG, Wang XW, Groenewald JZ (2017) Fungal planet description sheets: 625–715. Persoonia 39:270–467
Crous PW, Hernández-Restrepo M, Schumacher RK, Cowan DA, Maggs-Kölling G, Marais E, Wingfield MJ, Yilmaz N, Adan OCG, Akulov A, Álvarez Duarte E, Berraf-Tebbal A, Bulgakov TS, Carnegie AJ, De Beer ZW, Decock C, Dijksterhuis J, Duong TA, Eichmeier A, Hien LT, Houbraken JAMP, Khanh TN, Liem NV, Lombard L, Lutzoni FM, Miadlikowska JM, Nel WJ, Pascoe IG, Roets F, Roux J, Samson RA, Shen M, Spetik M, Thangavel R, Thanh HM, Thao LD, Van Nieuwenhuijzen EJ, Zhang JQ, Zhang Y, Zhao LL, Groenewald JZ (2021a) New and interesting Fungi. 4. Fungal Syst Evol 7:255–343
Crous PW, Osieck ER, Jurjevi Ž, Boers J, Van Iperen AL, Starink-Willemse M, Dima B, Balashov S, Bulgakov TS, Johnston PR, Morozova OV, Pinruan U, Sommai S, Alvarado P, Decock CA, Lebel T, McMullan-Fisher S, Moreno G, Shivas RG, Zhao L, Abdollahzadeh J, Abrinbana M, Ageev DV, Akhmetova G, Alexandrova AV, Altés A, Amaral AG, Angelini C, Antonín V, Arenas F, Asselman P, Badali F, Baghela A, Bañares A, Barreto RW, Baseia IG, Bellanger J, Berraf-Tebbal A, Biketova AY, Bukharova NV, Burgess TI, Cabero J, Câmara MP, Cano-Lira JF, Ceryngier P, Chávez R, Cowan DA, de Lima AF, Oliveira RL, Denman S, Dang QN, Dovana F, Duarte IG, Eichmeier A, Erhard A, Esteve-Raventós F, Fellin A, Ferreira FG, RJ, Ferrer A, Finy P, Gaya E, Geering AD, Gil-Durán C, Glässnerová K, Glushakova AM, Gramaje D, Guard FE, Guarnizo AL, Haelewaters D, Halling RE, Hill R, Hirooka Y, Hubka V, Iliushin VA, Ivanova DD, Ivanushkina NE, Jangsantear P, Justo A, Kachalkin AV, Kato S, Khamsuntorn P, Kirtsideli IY, Knapp DG, Kochkina GA, Koukol O, Kovács GM, Kruse J, Kumar TK, Kušan I, Læssøe T, Larsson E, Lebeuf R, Levicán G, Loizides M, Marinho P, Luangsa-ard JJ, Lukina EG, Magaña-Dueñas V, Maggs-Kölling G, Malysheva EF, Malysheva VF, Martín B, Martín MP, Matočec N, McTaggart AR, Mehrabi-Koushki M, Mešić A, Miller AN, Mironova P, Moreau P, Morte A, Müller K, Nagy LG, Nanu S, Navarro-Ródenas A, Nel WJ, Nguyen TH, Nóbrega TF, Noordeloos ME, Olariaga I, Overton BE, Ozerskaya SM, Palani P, Pancorbo F, Papp V, Pawłowska J, Pham TQ, Phosri C, Popov ES, Portugal A, Pošta A, Reschke K, Reul M, Ricci GM, Rodríguez A, Romanowski J, Ruchikachorn N, Saar I, Safi A, Sakolrak B, Salzmann F, Sandoval-Denis M, Sangwichein E, Sanhueza L, Sato T, Sastoque A, Senn-Irlet B, Shibata A, Siepe K, Somrithipol S, Spetik M, Sridhar P, Stchigel AM, Stuskova K, Suwannasai N, Tan YP, Thangavel R, Tiago I, Tiwari S, Tkalčec Z, Tomashevskaya MA, Tonegawa C, Tran HX, Tran NT, Trovão J, Trubitsyn VE, Van Wyk J, Vieira WA, Vila J, Visagie CM, Vizzini A, Volobuev SV, Vu DT, Wangsawat N, Yaguchi T, Ercole E, Ferreira BW, de Souza AP, Vieira BS, Groenewald JZ (2021b) Fungal planet description sheets: 1284–1382. Persoonia 47:178–374
Cui BK, Li HJ, Ji X, Zhou JL, Song J, Si J, Yang ZL, Dai YC (2019) Species diversity, taxonomy and phylogeny of Polyporaceae (Basidiomycota) in China. Fungal Divers 97:137–392
Cunningham GH (1965) Polyporaceae of New Zealand. Bull New Zealand Dept Sci Ind Res 164:1–304
Dai YC (2010) Hymenochaetaceae (Basidiomycota) in China. Fungal Divers 45:131–343
Dai YC, Li TH (2002) Megasporoporia major (Basidiomycota), a new combination. Mycosystema 21:519–521
Dai YC, Wu SH (2004) Megasporoporia (Aphyllophorales, Basidiomycota) in China. Mycotaxon 89:379–388
Dai YC, Yang ZL (2008) A revised checklist of medicinal fungi in China. Mycosystema 27:801–824
Dai YC, Zhou LW, Yang ZL (2010) A revised checklist of edible fungi in China. Mycosystema 29:1–21
Dai YC, Zhou LW, Steffen K (2011) Wood-decaying fungi in eastern Himalayas. 1. Polypores from Zixishan Nature Reserve. Yunnan Province Mycosystema 30:674–679
Dai YC, Cui BK, Si J, He SH, Hyde KD, Yuan HS, Liu XY, Zhou LW (2015) Dynamics of the worldwide number of fungi with emphasis on fungal diversity in China. Mycol Prog 14:62
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Das K, Ghosh A, Chakraborty D, Li JW, Qiu LH, Baghela A, Halama M, Hembrom ME, Mehmood T, Parihar A, Pencakowski B, Bielecka M, Reczynska K, Sasiela D, Singh U, Song Y, Swierkosz K, Szczesniak K, Uniyal P, Zhang JB, Buyck B (2017) Fungal biodiversity profiles 31–40. Cryptogam Mycol 38:353–406
Das K, Rossi W, Leonardi M, Ghosh A, Bera I, Hembrom ME, Bajpai R, Nayaka JS, Upreti DK, Wang XH, Hofstetter V, Buyck B (2018) Fungal biodiversity profiles 61–70. Cryptogam Mycol 39:381–418
David A, Tortic M (1986) Contribution à l’étude de quatre polypores européens peu connus. Cryptogam Mycol 7:1–13
Dayarathne MC, Wanasinghe DN, Jones EB, Chomnunti P, Hyde KD (2018) A novel marine genus, Halobyssothecium (Lentitheciaceae) and epitypification of Halobyssothecium obiones comb. nov. Mycol Prog 17:1161–1171
Dayarathne MC, Maharachchikumbura SSN, Jones EBG, Dong W, Devadatha B, Yang J, Ekanayaka AH, De Silva W, Sarma VV, Al-Sadi AM, Khongphinitbunjong K, Hyde KD, Zhao RL (2019) Phylogenetic revision of Savoryellaceae and evidence for its ranking as a subclass. Front Microbiol 10:840
Dayarathne MC, Jones EBG, Maharachchikumbura SSN, Devadatha B, Sarma VV, Khongphinitbunjong K, Chomnunti P, Hyde KD (2020) Morpho-molecular characterization of microfungi associated with marine based habitats. Mycosphere 11:1–188
de Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2013) Rediposition of phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
de Lima VX, de Oliveira VRT, de Lima-Junior NC, Oliveira-Filho JRC, Santos C, Lima N, Gibertoni TB (2022) Taxonomy and phylogenetic analysis reveal one new genus and three new species in Inonotus s.l. (Hymenochaetaceae) from Brazil. Cryptogam, Mycol 43:1–21
de Meiras-Ottoni A, Larsson KH, Gibertoni TB (2021) Additions to Trechispora and the status of Scytinopogon (Trechisporales, Basidiomycota). Mycol Prog 20:203–222
Dennis RWG (1952) Lepiota and allied genera in Trinidad, British West Indies. Kew Bull 7:459–499
Díaz S, Settele J, Brondízio ES, Ngo HT, Agard J, Arneth A, Balvanera P, Brauman KA, Butchart SHM, Chan KMA, Garibaldi LA, Ichii K, Liu J, Subramanian SM, Midgley GF, Miloslavich P, Molnár Z, Obura D, Pfaff A, Polasky S, Purvis A, Razzaque J, Reyers B, Chowdhury RR, Shin YJ, Visseren-Hamakers I, Willis KJ, Zayas CN (2019) Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366:eaax3100
Dima B, Brandrud TE, Corriol G, Jansen GM, Jordal JB, Khalid AN, Larsson E, Lorås J, Morozova OV, Naseer A, Noordeloos ME, Rossi W, Santamaria S, Sarwar S, Sesli E, Usman M, Afshan NS, Ahmad I, Banerjee A, Banerjee1 K, Bendiksen E, Rodrigues da Silva Colombo D, De Kesel A, Dovana F, Feresin G, Hussain S, Islam S, Jesus AL, Kaygusuz O, Krisai-Greilhuber I, Mahammad S, Mishra DK, Nath PS, de Oliveira da Paixão SC, Panja B, Papp V, Pires-Zottarelli CLA, Radnóti A, Rana D, Saha R, Türkekul I, Haelewaters D (2021) Fungal systematics and evolution: FUSE7. Sydowia 73:271–234
Dissanayake LS, Samarakoon MC, Mortimer PE, Lu YZ, Li QR, Hyde KD, Kang JC (2020) Morpho-molecular characterization of two novel amphisphaeriaceous species from Yunnan, China. Phytotaxa 446:144–158
Divakar PK, Crespo A, Wedin M, Leavitt SD, Hawksworth DL, Myllys L, McCune B, Randlane T, Bjerke JW, Ohmura Y, Schmitt I, Boluda CG, Alors D, Roca-Valiente B, Del-Prado R, Ruibal C, Buaruang K, Núñez-Zapata J, de Paz GA, Rico VJ, Molina MC, Elix JA, Esslinger TL, Tronstad IKK, Lindgren H, Ertz D, Gueidan C, Saag L, Mark K, Singh G, Grande FD, Parnmen S, Beck A, Benatti MN, Blanchon D, Candan M, Clerc P, Goward T, Grube M, Hodkinson BP, Hur JS, Kantvilas G, Kirika PM, Lendemer J, Mattsson JE, Messuti MI, Miadlikowska J, Nelsen M, Ohlson JI, Pérez-Ortega S, Saag A, Sipman HJM, Sohrabi M, Thell A, Thor G, Truong C, Yahr R, Upreti DK, Cubas P, Lumbsch HT (2015) Evolution of complex symbiotic relationships in a morphologically derived family of lichen-forming fungi. New Phytol 208:1217–1226
Dong W, Wang B, Hyde KD, McKenzie EHC, Raja HA, Tanaka K, Abdel-Wahab MA, Abdel-Aziz FA, Doilom M, Phookamsak R, Hongsanan S, Wanasinghe DN, Yu XD, Wang GN, Yang H, Yang J, Thambugala KM, Tian Q, Luo ZL, Yang JB, Miller AN, Fournier J, Boonmee S, Hu DM, Nalumpang S, Zhang H (2020) Freshwater dothideomycetes. Fungal Divers 105:319–575
Donk MA (1948) Notes on Malesian fungi. I. Bulletin Du Jardin Botanique De Buitenzorg 17:473–482
Donk MA (1957) The generic names proposed for Hymenomycetes. VII: “Thelephoraceae.” Taxon 6:17–28
Donk MA (1961) Four new families of Hymenomycetes. Persoonia 1:405–407
Du P, Cui BK (2009) Two new species of Megasporoporia (Polyporales, Basidiomycota) from tropical China. Mycotaxon 110:131–138
Ekman S, Andersen HL, Wedin M (2008) The limitations of ancestral state reconstruction and the evolution of the ascus in the Lecanorales (lichenized Ascomycota). Syst Biol 57:141–156
Ellis MB (1971) Dematiaceous hyphomycetes. Commonwealth Mycological Institute, Kew
Eriksson J, Ryvarden L (1973) The Corticiaceae of North Europe. 2. Fungiflora, Oslo
Eriksson J, Ryvarden L (1975) The Corticiaceae of North Europe vol. 3: Coronicium–Hyphoderma. Fungiflora, Oslo
Eriksson J, Ryvarden L (1976) The Corticiaceae of North Europe. 4. Fungiflora, Oslo
Eriksson OE, Winka K (1997) Supraordinal taxa of Ascomycota. Myconet 1:1–16
Eriksson J, Hjortstam K, Parmasto E, Ryvarden L (1982) (674) Proposal to conserve Hyphodontia Eriksson, 1958 (Fungi, Corticiaceae) over Kneiffiella Karsten, 1889. Taxon 31:744–746
Ertz D, Diederich P, Lawrey JD, Berger F, Freebury CE, Coppins B, Gardiennet A, Hafellner J (2015) Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyetic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with phoma-like anamorphs resurrected and segregated form Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Divers 74:53–89
Evans HC, Stalpers JA, Samson RA, Benny GL (1978) On the taxonomy of Monilia roreri, an important pathogen of Theobroma cacao in South America. Can J Bot 56:2528–2532
Exposito-Alonso M, Booker TR, Czech L, Gillespie L, Hateley S, Kyriazis CC, Lang PLM, Leventhal L, Nogues-Bravo D, Pagowski V, Ruffley M, Spence JP, Arana SET, Weiß CL, Zess E (2022) Genetic diversity loss in the Anthropocene. Science 377:1431–1435
Fan LF, Alvarenga RLM, Gibertoni TB, Wu F, Dai YC (2021) Four new species in the Tremella fibulifera complex (Tremellales, Basidiomycota). MycoKeys 82:33
Ferreira AJ, Cortez VG (2012) Lepiotoid Agaricaceae (Basidiomycota) from São Camilo State Park, Paraná State, Brazil. Mycosphere 3:962–976
Fiasson JL, Niemela T (1984) The Hymenochaetales: a revision of the European poroid taxa. Karstenia 24:14–28
Frøslev TG, Jeppesen TS, Læssoe T, Kjøller R (2007) Molecular phylogenetics and delimitation of species in Cortinarius section Calochroi (Basidiomycota, Agaricales) in Europe. Mol Phylogenet Evol 44:217–227
Fu HY, Li T, Li F (2022) Two new species of Butyriboletus from China. Phytotaxa 544:207–219
Furtado ANM, Daniëls PP, Reck MA, Neves MA (2021) Scytinopogon caulocystidiatus and S. foetidus spp. nov., and five other species recorded from Brazil. Mycotaxon 136:107–130
Galloway D (2007) Flora of New Zealand: Lichens, including lichen-forming and lichenicolous Fungi (Revised 2nd).
Gams W (1993) Report of the Committee for Fungi and Lichens: 3. Taxon 42:112–118
Geesteranus RAM, Lanquetin P (1975) Observations sur quelques champignons hydnoides de l’Afrique. Persoonia 8:145–165
Gelardi M, Simonini G, Ercole E, Davoli P, Vizzini A (2015) Cupreoboletus (Boletaceae, Boletineae), a new monotypic genus segregated from Boletus sect. Luridi to reassign the Mediterranean species B. poikilochromus. Mycologia 107:1254–1269
Gerlach ACL, Clerc P, Silveira RMB (2017) Taxonomy of the corticolous, shrubby, esorediate, neotropical species of Usnea Adans. (Parmeliaceae) with an emphasis on southern Brazil. Lichenologist 49:199–238
Gerlach ACL, Toprak Z, Naciri Y, Caviró EA, Borges da Silveira RM, Clerc P (2019a) New insights into the Usnea cornuta aggregate (Parmeliaceae, lichenized Ascomycota): molecular analysis reveals a high genetic diversity correlated with chemistry. Mol Phylogenet Evol 131:125–137
Gerlach ACL, Silveira RMB, Clerc P (2019b) Usnea oreophila (Parmeliaceae), a new saxicolous species from the mountains of Brazil. Bryologist 122:122–129
Gerlach ACL, Borges da Silveira RM, Rojas C, Clerc P (2020) Naming and describing the diversity in the Usnea cornuta aggregate (Parmeliaceae) occurring in Brazil. Plant Fungal Syst 65:272–302
Geyer CJ (1991) Markov chain Monte Carlo maximum likelihood. In: Keramidas EM (ed.) Computing Science and Statistics: Proceedings of the 23rd Symposium on the Interface, Seattle, Washington, April 21–24, Interface Foundation of North America, Fairfax Station, pp 156–163
Ghobad-Nejhad M, Liu SH, Langer E, Dai YC (2015) Molecular and morphological evidence reveal a new non-cystidiate species belonging to the core Phanerochaete (Polyporales). Mycol Prog 14:68
Giachini AJ (2004) Systematics of the Gomphales: the genus Gomphus Pers. sensu lato. PhD Dissertation, Department of Forest Science, Oregon State University, Corvallis
Giachini AJ, Castellano MA (2011) A new taxonomic classification for species in Gomphus sensu lato. Mycotaxon 115:183–201
Giachini AJ, Hosaka K, Nouhra E, Spatafora J, Trappe JM (2010) Phylogenetic relationships of the Gomphales based on nuc-25S-rDNA, mit-12S-rDNA, and mit-atp6-DNA combined sequences. Fungal Biol 114:224–234
Giachini AJ, Camelini CM, Rossi MJ, Soares CR, Trappe JM (2012) Systematics of the Gomphales: the genus Gomphus sensu stricto. Mycotaxon 120:385–400
Gilbert EJ (1931) Les Livres du Mycologue Tome I-IV, Tom. III: Les Bolets:1–254
Góes-Neto A, Loguercio-Leite C, Guerrero RT (2000) Taxonomy and qualitative ecological aspects of poroid Hymenochaetales in a Brazilian seasonal tropical forest. Mycotaxon 76:197–211
Goldmann L, Weir A (2018) Molecular phylogeny of the Laboulbeniomycetes (Ascomycota). Fungal Biol 122:87–100
González-Ávila A, Contreras-Medina R, Espinosa D, Luna-Vega I (2017) Track analysis of the order Gomphales (Fungi: Basidiomycota) in Mexico. Phytotaxa 316:22–38
González-Ávila A, Martínez-González CR, Alvarado-Sizzo H, Valenzuela R, Luna-Vega I (2020) Three new combinations in Gloeocantharellus (Gomphales, Agaricomycetes) from Mexico based on molecular evidence. Phytotaxa 447:42–50
Gorjón SP, Greslebin AG, Rajchenberg M (2011) The genus Athelopsis (Atheliales, Basidiomycota) in the Patagonian Andes. Sydowia 64:29–37
Gray SF (1821) Natural arrangement of British plants. vol. 1. Baldwin, Craddock and Joy, London
Haelewaters D, Dima B, Abdel-Hafiz AII, Abdel-Wahab MA, Abul-Ezz SR, Acar I, Aguirre-Acosta E, Aime MC, Aldemir S, Ali M, Ayala-Vásquez O, Bakhit MS, Bashir H, Battistin E, Bendiksen E, Castro-Rivera R, Faruk Çolak Ö, De Kesel A, De la Fuente JI, Dizkırıcı A, Hussain S, Jansen GM, Kaygusuz O, Khalid AN, Khan J, Kiyashko AA, Larsson E, Martínez-González CR, Morozova OV, Niazi AR, Noordeloos ME, Pham THG, Popov ES, Psurtseva NV, Schoutteten N, Sher H, Türkekul I, Verbeken A, Ahmad H, Afshan NS, Christe P, Fiaz M, Glaizot O, Liu JY, Majeed J, Markotter W, Nagy A, Nawaz H, Papp V, Péter Á, Pfliegler WP, Qasim T, Riaz M, Sándor AD, Szentiványi T, Voglmayr H, Yousaf N, Krisai-Greilhuber I (2020) Fungal systematics and evolution 6. Sydowia 72:231–356
Haelewaters D, Lubbers M, De Kesel A (2022) The haustorium as a driving force for speciation in thallus-forming Laboulbeniomycetes. IMA Fungus 13:1
Hall BG (2004) Phylogenetic trees made easy: a how-to manual, 2nd edn. Sinauer Associates, Sunderland
Hallenberg N (1980) New taxa of Corticiaceae from N. Iran (Basidiomycetes). Mycotaxon 11(2):447–475
Halling RE, Fechner N, Nuhn M, Osmundson T, Soytong K, Arora D, Binder M, Hibbett D (2015) Evolutionary relationships of Heimioporus and Boletellus (Boletales) with an emphasis on Australian taxa including new species and new combinations in Aureoboletus, Hemileccinum, and Xerocomus. Aust Syst Bot 28:1–22
Halonen P, Clerc P, Goward T, Brodo IM, Wulff K (1998) Synopsis of the genus Usnea (lichenized Ascomycetes) in British Columbia, Canada. Bryologist 101:36–60
Hambleton S, Sigler L (2005) Meliniomyces, a new anamorph genus for root–associated fungi with phylogenetic affinities to Rhizoscyphus ericae (= Hymenoscyphus ericae), Leotiomycetes. Stud Mycol 53:1–27
Harris RC (1984) The family Trypetheliaceae (Loculoascomycetes: lichenized Melanommatales) in Amazonian Brazil. Supplement Acta Amazonica 14:55–80
Harris RC (1995) More Florida Lichens. Including the 10¢ Tour of the Pyrenolichens. Published by the author, Bronx
Hawksworth DL (2001) The magnitude of fungal diversity: the 1.5 million species estimate revisited. Mycol Res 105:1422–1432
Hawksworth DL, Lücking R (2017) Fungal diversity revisited: 2.2 to 3.8 million species. Microbiol Spectrum 5:FUNK–0052–2016
He X, Zhao CL (2022) Diversity of Wood-Decaying Fungi in Wuliangshan Area, Yunnan Province, P.R. China. Diversity 14:131
He MQ, Chuankid B, Hyde KD, Cheewangkoon R, Zhao RL (2018) A new section and species of Agaricus subgenus Pseudochitonia from Thailand. MycoKeys 40:53–67
He MQ, Zhao RL, Hyde KD, Begerow D, Kemler M, Yurkov A, McKenzie EHC, Raspé O, Kakishima M, Sánchez-Ramírez S, Vellinga EC, Halling R, Papp V, Zmitrovich IV, Buyck B, Ertz D, Wijayawardene NN, Cui BK, Schoutteten N, Liu XZ, Li TH, Yao YJ, Zhu XY, Liu AQ, Li GJ, Zhang MZ, Ling ZL, Cao B, Antonín V, Boekhout T, da Silva BDB, De Crop E, Decock C, Dima B, Dutta AK, Fell JW, Geml J, Ghobad-Nejhad M, Giachini AJ, Gibertoni TB, Gorjón SP, Haelewaters D, He SH, Hodkinson BP, Horak E, Hoshino T, Justo A, Lim YW, Menolli N Jr, Mešić A, Moncalvo JM, Mueller GM, Nagy LG, Nilsson RH, Noordeloos M, Nuytinck J, Orihara T, Ratchadawan C, Rajchenberg M, Silva-Filho AGS, Sulzbacher MA, Tkalčec Z, Valenzuela R, Verbeken A, Vizzini A, Wartchow F, Wei TZ, Weiß M, Zhao CL, Kirk PM (2019) Notes, outline and divergence times of Basidiomycota. Fungal Divers 99:105–367
He MQ, Zhao RL, Liu DM, Denchev TT, Begerow D, Yurkov A, Kemler M, Millanes AM, Wedin M, McTaggart AR, Shivas RG, Buyck B, Chen J, Vizzini A, Papp V, Zmitrovich IV, Davoodian N, Hyde KD (2022) Species Diversity of Basidiomycota Fungal Divers 114:281–325
Hernández-Restrepo M, Bezerra JDP, Tan YP, Wiederhold N, Crous PW, Guarro J, Gené J (2019) Re-evaluation of Mycoleptodiscus species and morphologically similar fungi. Persoonia 42:205–227
Herrera-Campos MA (2016) Usnea in Mexico Bibliotheca Lichenologica 110:505–620
Hibbett DH, Thorn RG (2001) Basidiomycota: Homobasidiomycetes. In: McLaughlin DJ, McLaughlin EG, Lemke PA (eds) The Mycota. Springer-Verlag, Berlin, VIIB. Systematics and Evolution, pp 121–168
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lucking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Day Y-C, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside IE, Koljalg U, Kurtzman CP, Larsson K-H, Lichtwardt R, Longcore J, Miądlikowska J, Miller A, Moncalvo J-M, Mozley-Standtridge S, Oberwinkler F, Parmasto E, Reeb W, Rogers J-D, Roux C, Ryvarden L, Sampaio JP, Schussler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao Y-J, Zhang N (2007) A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547
Hibbett DS, Bauer R, Binder M, Giachini AJ, Hosaka K, Justo A, Larsson E, Larsson KH, Lawrey JD, Miettinen O, Nagy LG, Nilsson RH, Weiss M, Thorn RG (2014) Agaricomycetes. In: McLaughlin DJ, Spatafora JW (eds) Systematics and evolution. The Mycota (A comprehensive treatise on fungi as experimental systems for basic and applied research), vol 7A. Springer-Verlag, Berlin Heidelberg, pp 373–429
Hjortstam K (1987a) A check-list to genera and species of corticioid fungi (Hymenomycetes). Windahlia 17:55–85
Hjortstam K (1987b) Studies in tropical Corticiaceae (Basidiomycetes) VII. Specimens from East Africa collected by L. Ryvarden. II. Mycotaxon 28(1):19–37
Hjortstam K (1995) Two new genera and some new combinations of corticioid fungi (Basidiomycotina, Aphyllophorales) from tropical and subtropical areas. Mycotaxon 54:183–193
Hjortstam K, Ryvarden L (1981) Studies in tropical Corticiaceae (Basidiomycetes) III. Two New Species of Laxitextum Mycotaxon 13:35–40
Hodge KT (2003) Clavicipitaceous anamorphs. Mycol Ser 19:75–124
Hofstetter V, Buyck B, Eyssartier G, Schnee S, Gindro K (2019) The unbearable lightness of sequenced-based identification. Fungal Divers 96:243–284
Holec J (2001) The genus Pholiota in central and western Europe. Libri Botanici 20:1–220
Holec J, Kolařík M, Bizio E (2014) Pholiota chocenensis—a new European species of section Spumosae (Basidiomycota, Strophariaceae). Mycol Prog 13(2):399–406
Holst-Jensen A, Kohn LM, Schumacher T (1997) Nuclear rDNA phylogeny of the Sclerotiniaceae. Mycologia 89:885–899
Holubová-Jechová V (1980) Botryobasidium chilense sp. nov., a teleomorph of Haplotrichum chilense. Mycotaxon 12(1):117–121
Honey EE (1928) The monilioid species of Sclerotinia. Mycologia 20:127–157
Hongo T (1962) Notulae mycologicae. Memoirs of the Faculty of Liberal Arts of the Shiga University 12:39–43
Hongsanan S, Maharachchikumbura SSN, Hyde KD, Samarakoon MC, Jeewon R, Zhao Q, Al-Sadi AM, Bahkali AH (2017) An updated phylogeny of Sordariomycetes based on phylogenetic and molecular clock evidence. Fungal Divers 84:25–41
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Boonmee S, Lücking R, Pem D, Bhat JD, Liu N, Tennakoon DS, Karunarathna A, Jiang SH, Jones EBG, Phillips AJL, Manawasinghe I, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Dissanayake AJ, Zeng XY, Luo Z, Tian Q, Phukhamsakda C, Thambugala KM, Dai D, Chethana TKW, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Konta S, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, de Silva NI, Thiyagaraja V, Zhang H, Bezerra JDP, Miranda-Gonzáles R, Aptroot A, Kashiwadani H, Harishchandra D, Aluthmuhandiram JVS, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Chomnunti P, Samarakoon MC, Suetrong S, Chaiwan N, Dayarathne MC, Jing Y, Rathnayaka AR, Bhunjun CS, Xu J, Zheng J, Liu G, Feng Y, Xie N (2020a) Refined families of dothideomycetes: dothideomycetidae and pleosporomycetidae. Mycosphere 11:1553–2107
Hongsanan S, Hyde KD, Phookamsak R, Wanasinghe DN, McKenzie EHC, Sarma VV, Lücking R, Boonmee S, Bhat JD, Liu NG, Tennakoon DS, Pem D, Karunarathna A, Jiang SH, Jones GEB, Phillips AJL, Manawasinghe I, Tibpromma S, Jayasiri SC, Sandamali D, Jayawardena RS, Wijayawardene NN, Ekanayaka AH, Jeewon R, Lu YZ, Phukhamsakda C, Dissanayake AJ, Zeng XY, Luo ZL, Tian Q, Thambugala KM, Dai DQ, Samarakoon MC, Chethana KWT, Ertz D, Doilom M, Liu JK, Pérez-Ortega S, Suija A, Senwanna C, Wijesinghe SN, Niranjan M, Zhang SN, Ariyawansa HA, Jiang HB, Zhang JF, Norphanphoun C, de Silva NI, Aptroot A, Kashiwadani H, Harishchandra D, Sérusiaux E, Abeywickrama PD, Bao DF, Devadatha B, Wu HX, Moon KH, Gueidan C, Schumm F, Bundhun D, Mapook A, Monkai J, Bhunjun CS, Chomnunti P, Suetrong S, Chaiwan N, Dayarathne MC, Yang J, Rathnayaka AR, Xu JC, Zheng JS, Liu G, Feng Y, Xie N (2020b) Refined families of Dothideomycetes: orders and families incertae sedis in Dothideomycetes. Fungal Divers 105:17–318
Houbraken J, Kocsubé S, Visagie CM, Yilmaz N, Wang XC, Meijer M, Kraak B, Hubka V, Bensch K, Samson RA, Frisvad JC (2020) Classification of Aspergillus, Penicillium, Talaromyces and related genera (Eurotiales): an overview of families, genera, subgenera, sections, series and species. Stud Mycol 95:5–169
Hsieh SY, Goh TK, Kuo CH (2021) New species and records of Helicosporium sensu lato from Taiwan, with a reflection on current generic circumscription. Mycol Prog 20:169–190
Huanraluek N, Jayawardena RS, Aluthmuhandiram JVS, Chethana KWT, Hyde KD (2020) Bionectria pseudochroleuca, a new host record on Prunus sp. in northern Thailand. Stud Fungi 5:358–367
Hussain S, Afshan NS, Ahmad H (2016) First record of Parasola lilatincta from Pakistan. Mycotaxon 131:317–323
Hussain S, Afshan NS, Ahmad H, Khalid AN, Niazi AR (2017) Parasola malakandensis sp. nov. (Psathyrellaceae; Basidiomycota) from Malakand. Pakistan Mycoscience 58:69–76
Hussain S, Ahmad H, Ullah S, Afshan NU, Pfister DH, Sher H, Ali H, Khalid AN (2018) The genus Parasola in Pakistan with the description of two new species. MycoKeys 30:41–60
Hyde KD (1992) Tropical Australian freshwater fungi. I Some Ascomycetes Aust Syst Bot 5:109–116
Hyde KD (2022) The Numbers of Fungi Fungal Divers 114:1
Hyde KD, Bussaban B, Paulus B, Crous PW, S. Lee S, McKenzie EHC, Photita W, Lumyong S (2007) Diversity of saprobic microfungi. Biodivers Conserv 16:7–35
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake I, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yan JY, Yacharoen S, Zhang M (2013) Families of Dothideomycetes Fungal Divers 63:1–313
Hyde KD, Hongsanan S, Jeewon R, Bhat DJ, McKenzie EHC, Jones EBG, Phookamsak R, Ariyawansa HA, Boonmee S, Zhao Q, Abdel-Aziz FA, Abdel-Wahab MA, Banmai S, Chomnunti P, Cui BK, Daranagama DA, Das K, Dayarathne MC, de Silva NI, Dissanayake AJ, Doilom M, Ehanayaka AH, Gibertoni TB, GoesNeto A, Huang SK, Jayasiri SC, Jayawardena RS, Konta S, Lee HB, Li WJ, Lin CG, Liu JK, Lu YZ, Luo ZL, Manawasinghe IS, Manimohan P, Mapook A, Niskanen T, Norphanphoun C, Papizadeh M, Perera RH, Phukhamsakda C, Richter C, de Santiago ALCM, Drechsler-Santos ER, Senanayake IC, Tanaka K, Tennakoon TMDS, Thambugala KM, Tian Q, Tibpromma S, Thongbai B, Vizzini A, Wanasinghe DN, Wijayawardene NN, Wu HX, Yang J, Zeng XY, Zhang H, Zhang JF, Bulgakov TS, Camporesi E, Bahkali AH, Amoozegar MA, Araujo-Neta LS, Ammirati JF, Baghela A, Bhatt RP, Bojantchev D, Buyck B, de Silva GA, de Lima CLF, de Oliveira RJV, de Souza CAF, Dai YC, Dima B, Duong TT, Ercole E, Mafalda-Freire F, Ghosh A, Hashimoto A, Kamolhan S, Kang JC, Karunarathna SC, Kirk PM, Kytovuori I, Lantieri A, Liimatainen K, Liu ZY, Liu XZ, Lucking R, Medardi G, Mortimer PE, Nguyen TTT, Promputtha I, Raj KNA, Reck MA, Lumyong S, Shahzadeh-Fazeli SA, Stadler M, Soudi MR, Su HY, Takahashi T, Tangthirasunun N, Uniyal P, Wang Y, Wen TC, Xu JC, Zhang ZK, Zhao YC, Zhou JL, Zhu L (2016) Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 80:1–270
Hyde KD, Norphanphoun C, Abreu VP, Bazzicalupo A, Chethana KWT, Clericuzio M, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, He MQ, Hongsanan S, Huang SK, Jayasiri SC, Jayawardena RS, Karunarathna A, Konta S, Kušan I, Lee H, Li J, Lin CG, Liu NG, Lu YZ, Luo ZL, Manawasinghe IS, Mapook A, Perera RH, Phookamsak R, Phukhamsakda C, Siedlecki I, Soares AM, Tennakoon DS, Tian Q, Tibpromma S, Wanasinghe DN, Xiao YP, Yang J, Zeng XY, Abdel-Aziz FA, Li WJ, Senanayake IC, Shang QJ, Daranagama DA, De Silva NI, Thambugala KM, Abdel-Wahab MA, Bahkali AH, Berbee ML, Boonmee S, Bhat DJ, Bulgakov TS, Buyck B, Camporesi E, Castañeda-Ruiz RF, Chomnunti P, Doilom M, Dovana F, Gibertoni TB, Jadan M, Jeewon R, Jones GEB, Kang JC, Karunarathna SC, Lim YW, Liu JK, Liu ZY, Plautz HL Jr, Lumyong S, Maharachchikumbura SSN, Matočec N, Mckenzie EHC, Mešić A, Miller D, Pawłowska J, Pereira OL, Promputtha I, Romero AI, Ryvarden L, Su HY, Suetrong S, Tkalčec Z, Vizzini A, Wen TC, Wisitrassameewong K, Wrzosek M, Xu JC, Zhao Q, Zhao RL, Mortimer PE (2017) Fungal diversity notes 603–708: taxonomic and phylogenetic notes on genera and species. Fungal Divers 87:1–235
Hyde KD, Xu J, Rapior S, Jeewon R, Lumyong S, Niego AGT, Abeywickrama PD, Aluthmuhandiram JVS, Brahamanage RS, Brooks S, Chaiyasen A, Chethana KWT, Chomnunti P, Chepkirui C, Chuankid B, de Silva NI, Doilom M, Faulds C, Gentekaki E, Gopalan V, Kakumyan P, Harishchandra D, Hemachandran H, Hongsanan S, Karunarathna A, Karunarathna SC, Khan S, Kumla J, Jayawardena RS, Liu J-K, Liu N, Luangharn T, Macabeo APG, Marasinghe DS, Meeks D, Mortimer PE, Mueller P, Nadir S, Nataraja KN, Nontachaiyapoom S, O’Brien M, Penkhrue W, Phukhamsakda C, Ramanan US, Rathnayaka AR, Sadaba RB, Sandargo B, Samarakoon BC, Tennakoon DS, Siva R, Sriprom W, Suryanarayanan TS, Sujarit K, Suwannarach N, Suwunwong T, Thongbai B, Thongklang N, Wei D, Wijesinghe SN, Winiski J, Yan J, Yasanthika E, Stadler M (2019) The amazing potential of fungi, 50 ways we can exploit fungi industrially. Fungal Diversity 97:1–136
Hyde KD, Jeewon R, Chen YJ, Bhunjun CS, Calabon MS, Jiang HB, Lin CG, Norphanphoun C, Sysouphanthong P, Pem D, Tibpromma S, Zhang Q, Doilom M, Jayawardena RS, Liu JK, Maharachchikumbura SSN, Phukhamsakda C, Phookamsak R, Al-Sadi AM, Naritsada Thongklang N, Wang Y, Gafforov Y, Jones EBG, Lumyong S (2020a) The numbers of fungi: is the descriptive curve flattening? Fungal Divers 103:219–271
Hyde KD, Dong Y, Phookamsak R, Jeewon R, Bhat DJ, Jones EBG, Liu NG, Abeywickrama PD, Mapook A, Wei DP, Perera RH, Manawasinghe IS, Pem D, Bundhun D, Karunarathna A, Ekanayaka AH, Bao DF, Li JF, Samarakoon MC, Chaiwan N, Lin CG, Phutthacharoen K, Zhang SN, Senanayake IC, Goonasekara ID, Thambugala KM, Phukhamsakda C, Tennakoon DS, Jiang HB, Yang J, Zeng M, Huanraluek N, Liu JK, Wijesinghe SN, Tian Q, Tibpromma S, Brahmanage RS, Boonmee S, Huang SK, Thiyagaraja V, Lu YZ, Jayawardena RS, Dong W, Yang EF, Singh SK, Singh SM, Rana S, Lad SS, Anand G, Devadatha B, Niranjan M, Sarma VV, Liimatainen K, Aguirre-Hudson B, Niskanen T, Overall A, Alvarenga RLM, Gibertoni TB, Pfliegler WP, Horváth E, Imre A, Alves AL, da Silva Santos AC, Tiago PV, Bulgakov TS, Wanasinghe DN, Bahkali AH, Doilom M, Elgorban AM, Maharachchikumbura SSN, Rajeshkumar KC, Haelewaters D, Mortimer PE, Zhao Q, Lumyong S, Xu JC, Sheng J (2020b) Fungal diversity notes 1151–1276: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Divers 100:5–277
Hyde KD, Norphanphoun C, Maharachchikumbura SSN, Bhat DJ, Jones EBG, Bundhun D, Chen YJ, Bao DF, Boonmee S, Calabon MS, Chaiwan N, Chethana KWT, Dai DQ, Dayarathne MC, Devadatha B, Dissanayake AJ, Dissanayake LS, Doilom M, Dong W, Fan XL, Goonasekara ID, Hongsanan S, Huang SK, Jayawardena RS, Jeewon R, Karunarathna A, Konta S, Kumar V, Lin CG, Liu JK, Liu NG, Luangsa-ard J, Lumyong S, Luo ZL, Marasinghe DS, McKenzie EHC, Niego AGT, Niranjan M, Perera RH, Phukhamsakda C, Rathnayaka AR, Samarakoon MC, Samarakoon SMBC, Sarma VV, Senanayake IC, Shang QJ, Stadler M, Tibpromma S, Wanasinghe DN, Wei DP, Wijayawardene NN, Xiao YP, Yang J, Zeng XY, Zhang SN, Xiang MM (2020c) Refined Families of Sordariomycetes Mycosphere 11:305–1059
Hyde KD, Suwannarach N, Jayawardena RS, Manawasinghe IS, Liao CF, Doilom M, Cai L, Zhao P, Buyck B, Phukhamsakda C, Su WX, Fu YP, Li Y, Zhao RL, He MQ, Li JX, Tibpromma S, Lu L, Tang X, Kang JC, Ren GC, Gui H, Hofstetter V, Ryoo R, Antonín V, Hurdeal VG, Gentekaki E, Zhang JY, Lu YZ, Senanayake IC, Yu FM, Zhao Q, Bao DF (2021) Mycosphere notes 325–344 – Novel species and records of fungal taxa from around the world. Mycosphere 12:1101–1156
Hyde KD, Norphanphoun C, Ma J, Yang HD, Zhang JY, Du TY, Gao Y, Gomes de Farias AR, Gui H, He SC, He YK, Li CJY, Liu XF, Lu L, Su HL, Tang X, Tian XG, Wang SY, Wei DP, Xu RF, Xu RJ, Yang Q, Yang YY, Zhang F, Zhang Q, Bahkali AH, Boonmee S, Chethana KWT, Jayawardena RS, Lu YZ, Karunarathna SC, Tibpromma S, Wang Y, Zhao Q (2023) Mycosphere notes 387–412 – novel species of fungal taxa from around the world. Mycosphere 14(1):663–744
Index Fungorum (2023) http://www.indexfungorum.org/
Izhar A, Asif M, Niazi A, Khalid N (2022) A new crinipelloid species (Marasmiaceae, Agaricales) from Pakistan. Phytotaxa 538:197–212
Jacobsson S (1990) Pholiota in Northern Europe Windahlia 19:1–86
Jaklitsch WM, Voglmayr H (2016) Hidden diversity in Thyridaria and a new cicumscription of the Thyridariaceae. Stud Mycol 85:35–64
Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai Y-C, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu J-K, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo J-M, Ghobad-Nejhad M, Nilsson H, Pang K-L, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen T-C, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li W-J, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao R-L, Zhao Q, Kang J-C, Promputtha I (2015) The Faces of Fungi database: fungal names linked with morphology, phylogeny and human impacts. Fungal Divers 74:3–18
Jayasiri SC, Hyde KD, Jones EBG, McKenzie EHC, Jeewon R, Phillips AJL, Bhat JD, Wanasinghe DN, Liu JK, Lu YZ, Kang JC, Xu J, Karunarathna SC (2019) Diversity, morphology and molecular phylogeny of Dothideomycetes on decaying wild seed pods and fruits. Mycosphere 10:1–186
Jiang XM, Li YK, Liang JF, Wu JR (2017) Russula brunneovinacea sp. nov. from northeastern China. Mycotaxon 132:789–797
Jones EBG, Ju WT, Lu CL, Guo SY, Pang KL (2017) The Halosphaeriaceae revisted. Bot Mar 60:453–468
Jones EBG, Pang KL, Abdel-Wahab MA, Scholz B, Hyde KD, Boekhout T, Ebel R, Rateb ME, Henderson L, Sakayaroj J, Suetrong S, Dayarathne MC, Kumar V, Raghukumar S, Sridhar KR, Bahkali AH, Gleason F, Norphanphoun C (2019) An online resource for marine fungi. Fungal Divers 96:347–433
Jülich W (1978) Studies in resupinate Basidiomycetes - V. Some New Genera and Species Persoonia 10(1):137–140
Jülich W (1981) Higher Taxa of Basidiomycetes Bibl Mycol 85:1–485
Justo A, Miettinen O, Floudas D, Ortiz-Santana B, Sjökvist E, Lindner D, Nakasone K, Niemelä T, Larsson KH, Ryvarden L, Hibbett DS (2017) A revised family-level classification of the Polyporales (Basidiomycota). Fungal Biol 121:798–824
Kaishian P, Rossi W, Weir A (2020) New species of Laboulbenia (Laboulbeniales, Ascomycota) on Gerridae (Hemiptera, Insecta), a new host family. Mycologia 112:570–576
Karsten PA (1889) Kritisk öfversigt af Finlands Basidsvampar (Basidiomycetes; Gastero- & Hymenomycetes). Bidrag till Kännedom Om Finlands Natur Och Folk 48:1–470
Karsten PA (1980) Fragmenta Mycologica XXIX Hedwigia 29:147–149
Karunarathna SC, Chen J, Mortimer PE, Xu JC, Zhao RL, Callac P, Hyde KD (2016) Mycosphere Essay 8: a review of genus Agaricus in tropical and humid subtropical regions of Asia. Mycosphere 7:417–439
Kaur R, Singh AP, Dhingra GS (2019) Cystostereum sirmaurense sp. nov. from India. Mycotaxon 134(3):577–580
Kepler RM, Luangsa-Ard JJ, Hywel-Jones NL, Quandt CA, Sung GH, Rehner SA, Aime MC, Henkel TW, Sanjuan T, Zare R, Chen MJ, Li ZZ, Rossman AY, Spatafora JW, Shrestha B (2017) A phylogenetically-based nomenclature for Cordycipitaceae (Hypocreales). IMA Fungus 8(2):335–353
Kerekes JF, Desjardin DE (2009) A monograph of the genera Crinipellis and Moniliophthora from Southeast Asia including molecular phylogeny of the nrITS region. Fungal Divers 37:101–152
Kerrigan RW (2016) Agaricus of North America. Mem N Y Bot Gard 114:1–574
Kirk PM, Cannon PF, David JC, Minter DW, Stalpers JA (2008) Dictionary of the fungi, 10th edn. CABI, Wallingford
Kleine CS, McClean T, Miller SL (2013) Genetic divergence among disjunct populations of three Russula spp. from Africa and Madagascar. Mycologia 105:80–89
Klofac W (2010) The genus Aureoboletus, a world-wide survey. A contribution to a monographic treatment. Österreichische Zeitschrift Für Pilzkunde 19:133–174
Kohlmeyer J (1972) A revision of Halosphaeriaceae. Can J Bot 50:1951–1963
Kolařík M (2018) New taxonomic combinations in endophytic representatives of the genus Nigrograna. Czech Mycol 70:123–126
Kolařík M, Spakowicz DJ, Gazis R, Shaw J, Kubátová A, Nováková A, Chudíčková M (2017) Biatriospora (Ascomycota: Pleosporales) is an ecologically diverse genus including facultative marine fungi and endophytes with biotechnological potential. Plant Syst Evol 303:35–50
Kong V, Yorn T, Rossi W (2020) New species and new records of Laboulbenia (Ascomycota, Laboulbeniales) from Cambodia. Phytotaxa 474:119–131
Kornerup A, Wanscher JH (1978) Methuen handbook of color, 3rd edn. Eyre Methuen Ltd., London
Kraichak E, Huang JP, Nelsen M, Leavitt SD, Lumbsch HT (2018) A revised classification of orders and families in the two major subclasses of Lecanoromycetes (Ascomycota) based on a temporal approach. Bot J Linn Soc 188:233–249
Kropp BR, Albee-Scott S (2012) Moniliophthora aurantiaca sp. nov., a Polynesian species occurring in litoral forest. Mycotaxon 120:493–503
Kruys Å, Eriksson OE, Wedin M (2006) Phylogenetic relationships of coprophilous Pleosporales (Dothideomycetes, Ascomycota), and the classification of some bitunicate taxa of unknown position. Mycol Res 110:527–536
Kühner R (1980) Les Hyménomycètes agaricoïdes (Agaricales, Tricholomatales, Pluteales, Russulales). Etude générale et classification. Bull Soc Linn Lyon 50:1–1927
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis ver. 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874
Kuo M, Ortiz-Santana B (2020) Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia 112:197–211
Lagerberg T, Lundberg G, Melin E (1927) Biological and practical researches into Blueing in Pine and Spruce. Svenska Skogsvårdsföreningens Tidskrift 2:145–272
Langer E (1994) Die Gattung Hyphodontia John Eriksson. Bibl Mycol 154:1–298
Langer E, Hallenberg N, Knudsen H, Kõljalg U, Langer G, Larsson KH, Oberwinkler F, Parmasto E, Ryvarden L, Vesterhol J (1996) 1255) Proposal to reject the names Xylodon and Schizopora in favour of Hyphodontia, nom. cons. (Fungi, Corticiaceae. Taxon 45:685–686
Larsson KH (1995) Taxonomy of Trechispora farinacea and proposed synonyms I. Species with a grandinioid or hydnoid hymenophore. Symb Bot Ups 30:101–118
Larsson KH (1996) Taxonomy of Trechispora farinacea and proposed synonyms II. Species with a smooth hymenophore. Nord J Bot 16:73–82
Larsson KH (2007a) Re-thinking the classification of corticioid fungi. Mycol Res 111:1040–1063
Larsson KH (2007b) Molecular phylogeny of Hyphoderma and the reinstatement of Peniophorella. Mycol Res 111:186–195
Larsson KH (2014) Index Fungorum no. 131. www.indexfungorum.org/IndexFungorum/Publications/
Larsson E, Larsson KH (2003) Phylogenetic relationships of russuloid basidiomycetes with emphasis on aphyllophoralean taxa. Mycologia 95:1037–1065
Latha KPD, Raj KNA, Thushara C, Sharafudheen SA, Manimohan P (2016) Three new species of Calocybella from India based on morphology and molecular phylogeny. Phytotaxa 255:133–143
Latha KPD, Raj KNA, Manimohan P (2020) Diversity of the genus Calocybella Vizzini, Consiglio & Setti in Kerala State, India. Cryptogam Mycol 41:147–156
Lebel T (2017) Nomenclatural changes and corrections for some previously described Australasian truffle-like fungi (Basidiomycetes). Muelleria 36:8–14
Lebel T, Syme A (2012) Sequestrate species of Agaricus and Macrolepiota from Australia: new species and combinations and their position in a calibrated phylogeny. Mycologia 104:496–520
Lebel T, Tonkin JE (2007) Australasian species of Macowanites are sequestrate species of Russula. Aust Syst Bot 20:355–381
Lebert H (1858) Ueber einige neue oder unvollkommen gekannte Krankheiten der Insekten, welche durch Entwicklung niederer Pflanzen im lebenden Körper enstehen. Zeitschrift Für Wissenschaftliche Zoologie 9:439–453
Lee JS, Jung HS (2006) Taxonomic study on Korean Aphyllophorales (5) - on some unrecorded genera and species. Mycobiology 34:166–175
Lentz PL (1955) Stereum and allied genera of fungi in the upper Mississippi valley. Agric Monogr 24:1–74
Li GJ (2014) Taxonomy of Russula from China. PhD dissertation. Institute of Microbiology, Chinese Academy of Sciences & University of Chinese Academy of Sciences, Beijing
Li HJ, Cui BK (2013) Taxonomy and phylogeny of the genus Megasporoporia and its related genera. Mycologia 105:368–383
Li F, Deng QL (2018) Three new species of Russula from South China. Mycol Prog 17:1305–1321
Li MC, Liang JF, Li YC, Feng B, Yang ZL, James TY, Xu JP (2010) Genetic diversity of dahongjun, the commercially important “big red mushroom” from southwestern China. PLoS ONE 5:1–11
Li GJ, Li SF, Wen HA (2011) Russula zhejiangensis sp. nov. from east China. Cryptogam Mycol 32:127–133
Li GJ, Li SF, Liu XZ, Wen HA (2012) Russula jilinensis sp. nov. (Russulaceae) from northeast China. Mycotaxon 120:49–58
Li GJ, Zhao D, Li SF, Yang HJ, Wen HA, Liu XZ (2013a) Russula changbaiensis sp. nov. from northeast China. Mycotaxon 124:269–278
Li GJ, Zhao Q, Zhao D, Yue SF, Li SF, Wen HA, Liu XZ (2013b) Russula atroaeruginea and R. sichuanensis spp. nov. from southwest China. Mycotaxon 124:173–188
Li GJ, Li SF, Zhao D, Wen HA (2015a) Recent research progress of Russula (Russulales, Agaricomycetes): a review. Mycosystema 34:821–848
Li GJ, Zhao D, Li SF, Wen HA (2015b) Russula chiui and R. pseudopectinatoides, two new species from southwestern China supported by morphological and molecular evidence. Mycol Prog 14:33
Li YK, Zhang X, Yuan Y, Cao Z, Liang JF (2015c) Morphological and molecular evidence for a new species of Russula (Russulaceae) from southern China. Phytotaxa 202:94–102
Li Y, Li TH, Yang ZL, Bau T, Dai YC (2015d) Altas of Chinese macrofungal resources. Central Plain Farmer Press, Zhengzhou
Li GJ, Hyde KD, Zhao RL, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, Crop ED, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Goes-Neto A, Gorczak M, Haitjema GH, Hapuar-achchi KK, Hashimoto A, He MQ, Henske JK, Hirayama K, Iribarren MJ, Jayasiri SC, Jayawardena RS, Jeon SJ, Jerônimo GH, Jesus AL, Jones EBG, Kang JC, Karunarathna SC, Kirk PM, Konta S, Kuhnert E, Langer E, Lee HS, Lee HB, Li WJ, Li XH, Liimatainen K, Lima DX, Lin CG, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Lücking R, Lumbsch HT, Lumyong S, Leaño EM, Marano AV, Matsumura M, McKenzie EHC, Mongkol-samrit S, Mortimer PE, Nguyen TTT, Niskanen T, Norphan-phoun C, O’Malley MA, Parnmen S, Pawłowska J, Perera RH, Phookamsak R, Phukhamsakda C, Pires-Zottarelli CLA, Raspé O, Reck MA, Rocha SCO, de Santiago ALCMA, Senanayake IC, Setti L, Shang QJ, Singh SK, Sir EB, Solomon KV, Song J, Srikitikulchai P, Stadler M, Suetrong S, Takahashi H, Takahashi T, Tanaka K, Tang LP, Thambugala KM, Thanakitpipattana D, Theodorou MK, Thongbai B, Thummarukcharoen T, Tian Q, Tibpromma S, Verbeken A, Vizzini A, Vlasák J, Voigt K, Wanasinghe DN, Wang Y, Weerakoon G, Wen HA, Wen TC, Wijayawardene NN, Wongkanoun S, Wrzosek M, Xiao YP, Xu JC, Yan JY, Yang J, Yang SD, Hu Y, Zhang JF, Zhao J, Zhou LW, Persoh D, Phillips AJL, Maharachchikumbura SSN (2016) Fungal diversity notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 78:1–237
Li GJ, Zhang CL, Lin FC, Zhao RL (2018a) Hypogeous gasteroid Lactarius sulphosmus sp. nov. and agaricoid Russula vinosobrunneola sp. nov. (Russulaceae) from China. Mycosphere 9:838–858
Li GJ, Zhang CL, Zhao RL, Lin FC (2018b) Two new species of Russula from Northeast China. Mycosphere 9:431–443
Li GJ, Zhao RL, Zhang CL, Lin FC (2019) A preliminary DNA barcode selection for the genus Russula (Russulales, Basidiomycota). Mycology 10:61–74
Li GJ, Deng CY, Shi LY, Wang J, Meng QF, Li SM (2020) Three new species of Russula subsect. Lactarioideae from China Mycosystema 39:1–19
Li MX, Wu G, Yang ZL (2021) Four new species of Hemileccinum (Xerocomoideae, Boletaceae) from Southwestern China. J Fungi 7:823
Li Y, He SH, Chen CC, Nakasone KK, Ma HX (2022) Global taxonomy and phylogeny of Irpicaceae (Polyporales, Basidiomycota) with descriptions of seven new species and proposals of two new combinations. Front Microbiol 13:911978
Liang ZQ, An DY, Juang S, Su MS, Zeng NK (2016) Butyriboletus hainanensis (Boletaceae, Boletales), a new species from tropical China. Phytotaxa 267:256–262
Liimatainen K, Niskanen T, Dima B, Ammirati JF, Kirk P, Kytövuori I (2020) Mission impossible completed: unlocking the nomenclature of the largest and most complicated subgenus of Cortinarius, Telamonia. Fungal Divers 104:291–331
Liimatainen K, Kim JT, Pokorny L, Kirk PM, Dentinger B, Niskanen T (2022) Taming the beast: a revised classification of Cortinariaceae based on genomic data. Fungal Divers 112:89–170
Lin CG, Hyde KD, Lumyong S, Mckenzie EHC (2017) Beltrania-like Taxa from Thailand Cryptogam Mycol 38:301–319
Lindau G (1897) Hypocreales. In: Englier HA, Prantl KAE (eds) Die Naturlichen Pflanzenfamilien 1(1). Verlag W, Engelman, Leipzig, pp 343–372
Linnaeus C (1753) Species plantarum. Impensis Laurentii Salvii, Stockholm
Lira CRS, Alvarenga RLM, Soares AMS, Ryvarden L, Gibertoni TB (2021) Phylogeny of Megasporoporia s.lat. and related genera of Poyporaceae: New genera, new species and new combinations. Mycosphere 12:1262–1289
Lisboa DO, Evans HC, Araújo JPM, Elias SG, Barreto RW (2020) Moniliophthora perniciosa, the mushroom causing witches’ broom disease of cacao: Insights into its taxonomy, ecology and host range in Brazil. Fungal Biol 124:983–1003
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, D’souza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe D, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, SriIndrasutdhi V, Tian CM, Verbeken A, Brackel WV, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015a) Fungal diversity notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Liu XZ, Wang QM, Göker M, Groenewald M, Kachalkin AV, Lumbsch HT, Millanes AM, Wedin M, Yurkov AM, Boekhout T, Bai FY (2015b) Towards an integrated phylogenetic classification of the Tremellomycetes. Stud Mycol 81:85–147
Liu JK, Hyde KD, Jeewon R, Phillips AJL, Maharachchikumbura SSN, Ryberg M, Liu ZY, Zhao Q (2017a) Ranking higher taxa using divergence times: a case study in Dothideomycetes. Fungal Divers 84:75–99
Liu SL, Zhao Y, Dai YC, Nakasone KK, He SH (2017b) Phylogeny and taxonomy of Echinodontium and related genera. Mycologia 109:568–577
Liu JK, Lu YZ, Cheewangkoon R, To-Anun C (2018) Phylogeny and morphology of Helicotubeufia gen. nov., with three new species in Tubeufiaceae from aquatic habitats. Mycosphere 9:495–509
Liu AQ, Dai RC, Zhang MZ, Cao B, Xi YL, Wei SL, Zhao RL (2020) Species of Agaricus section Agaricus from China. Phytotaxa 452:1–18
Liu S, Chen YY, Sun YF, He XL, Song CG, Si J, Liu DM, Gates G, Cui BK (2022a) Systematic classification and phylogenetic relationships of the brown-rot fungi within the Polyporales. Fungal Divers. https://doi.org/10.1007/s13225-022-00511-2
Liu SL, He SH, Wang XW, May TW, He G, Chen SL, Zhou LW (2022b) Trechisporales emended with a segregation of Sistotremastrales ord. nov. (Basidiomycota). Mycosphere 13:862–954
Lombard L, van der Merwe NA, Groenewald JZ, Crous PW (2015) Generic concepts in Nectriaceae. Stud Mycol 80:189–245
Looney BP (2015) Molecular annotation of type specimens of Russula species described by WA Murrill from the southeast United States. Mycotaxon 129:255–268
Looney BP, Ryberg M, Hampe F, Sánchez-García M, Matheny PB (2016) Into and out of the tropics: global diversification patterns in a hyperdiverse clade of ectomycorrhizal fungi. Mol Ecol 25:630–647
Lotsy JP (1907) Vorträge über botanische Stammesgeschichte. Gustav Fischer, Jena
Lu YZ, Liu JK, Hyde KD, Jeewon R, Kang JC, Fan C, Boonmee S, Bhat DJ, Luo ZL, Lin CG, Eungwanichayapant PD (2018) A taxonomic reassessment of Tubeufiales based on multi-locus phylogeny and morphology. Fungal Divers 92:131–344
Lücking R, Hodkinson BP, Leavitt SD (2017) The 2016 classification of lichenized fungi in the Ascomycota and Basidiomycota – approaching one thousand genera. Bryologist 119:361–416
Lücking R, Nadel MRA, Araujo E, Gerlach A (2020) Two decades of DNA barcoding in the genus Usnea (Parmeliaceae): how useful and reliable is the ITS? Plant Fungal Syst 65:303–357
Lumbsch HT, Huhndorf SM (2007) Outline of Ascomycota. Myconet. Chicago, USA: The Field Museum, Department of Botany 13:1–58
Lumbsch HT, Huhndorf SM (2010) Myconet Volume 14. Part One. Outline of Ascomycota—2009. Fieldiana Life Earth Sci 1:1–922
Luo ZL, Bhat DJ, Jeewon R, Boonmee S, Bao DF, Zhao YC, Chai HM, Su HY, Su XJ, Hyde KD (2017) Molecular phylogeny and morphological characterization of asexual fungi (Tubeufiaceae) from freshwater habitats in Yunnan, China. Cryptogam Mycol 38:27–53
Luo ZL, Hyde KD, Liu JK, Maharachchikumbura SSN, Jeewon R, Bao DF, Bhat DJ, Lin CG, Li WL, Yang J, Liu NG, Lu YZ, Jayawardena RS, Li JF, Su HY (2019) Freshwater Sordariomycetes Fungal Divers 99:451–660
Luo X, Chen YH, Zhao CL (2021) Morphological and phylogenetic characterization of fungi within Hymenochaetales: introducing two new species from southern China. Nord J Bot 39:e03414
Luo KY, Zhao CL (2022a) Morphology and multigene phylogeny reveal a new order and a new species of wood-inhabiting basidiomycete fungi (Agaricomycetes). Front Microbiol 13:970731
Luo KY, Zhao CL (2022b) A molecular systematics and taxonomy research on Trechispora (Hydnodontaceae, Trechisporales): concentrating on three new Trechispora species from East Asia. J Fungi 8:1020
Luttrell ES (1951) Taxonomy of Pyrenomycetes Univ Missouri Stud 24:1–120
Lynch SC, Twizeyimana M, Mayorquin JS, Wang DH, Na F, Kayim M, Kasson MT, Thu PQ, Bateman C, Rugman-Jones P, Hulcr J, Stouthamer R, Eskalen A (2016) Identification, pathogenicity and abundance of Paracremonium pembeum sp. nov. and Graphium euwallaceae sp. nov.-two newly discovered mycangial associates of the polyphagous shot hole borer (Euwallacea sp.) in California. Mycologia 108:313–329
Maekawa N, Yokoi H, Sotome K, Matsuura K, Tanaka C, Endo N, Nakagiri A, Ushijima S (2020) Athelia termitophila sp. nov. is the teleomorph of the termite ball fungus Fibularhizoctonia sp. Mycoscience 61:323–330
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang SK, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu JK, Liu ZY, Norphanphoun C, Pang KL, Perera RH, Senanayake IC, Shang QJ, Shenoy BD, Xiao YP, Bahkali AH, Kang JC, Somrothipol S, Suetrong S, Wen TC, Xu JC (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat DJ, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ, Xiao YP, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang JC, Li QR, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen TC, Wijayawardene NN (2016) Families of Sordariomycetes Fungal Divers 79:1–317
Maharachchikumbura SSN, Chen Y, Ariyawansa HA, Hyde KD, Haelewaters D, Perera RH, Samarakoon MC, Wanasinghe DN, Bustamante DE, Liu J, Lawrence DP, Cheewangkoon R, Stadler M (2021) Integrative approaches for species delimitation in Ascomycota. Fungal Diversity 109:155–179
Mains EB (1950) Entomogenous species of Akanthomyces, Hymenostilbe and Insecticola in north America. Mycologia 42:566–589
Malysheva VF, Malysheva EF, Bulakh EM (2015) The genus Tremella (Tremellales, Basidiomycota) in Russia with description of two new species and proposal of one nomenclatural combination. Phytotaxa 238:40–70
Mapook A, Hyde KD, Dai DQ, Li J, Jones EG, Bahkali AH, Boonmee S (2016) Muyocopronales, ord. nov., (Dothideomycetes, Ascomycota) and a reappraisal of Muyocopron species from northern Thailand. Phytotaxa 265:225–237
Mao WL, Wu YD, Liu HG, Yuan Y, Dai YC (2023) A contribution to Porogramme (Polyporaceae, Agaricomycetes) and related genera. IMA Fungus 14:5
Mapook A, Hyde KD, McKenzie EH, Jones EG, Bhat DJ, Jeewon R, Stadler M, Samarakoon MC, Malaithong M, Tanunchai B, Buscot F, Wubet T, Purahong W (2020) Taxonomic and phylogenetic contributions to fungi associated with the invasive weed Chromolaena odorata (Siam weed). Fungal Divers 101:1–175
Matheny PB, Curtis JM, Hofstetter V, Aime MC, Moncalvo JM, Ge ZW, Yang ZL, Slot JC, Ammirati JF, Baroni TJ, Bougher NL, Hughes KW, Lodge DJ, Kerrigan RW, Seidl MT, Aanen DK, DeNitis M, Daniele GM, Desjardin DE, Kropp BR, Norvell LL, Parker A, Vellinga EC, Vilgalys R, Hibbett DS (2006) Major clades of Agaricales: a multilocus phylogenetic overview. Mycologia 98:982–995
Miadlikowska J, Kauff F, Hofstetter V, Fraker E, Grube M, Hafellner J, Reeb V, Hodkinson BP, Kukwa M, Lucking R, Hestmark G, Otalora MG, Rauhut A, Buedel B, Scheidegger C, Timdal E, Stenroos S, Brodo I, Perlmutter GB, Ertz D, Diederich P, Lendemer JC, May P, Schoch CL, Arnold AE, Gueidan C, Tripp E, Yahr R, Robertson C, Lutzoni F (2006) New insights into classification and evolution of the Lecanoromycetes (Pezizomycotina, Ascomycota) from phylogenetic analyses of three ribosomal RNA- and two protein-coding genes. Mycologia 98:1088–1103
Midgley DJ, Rosewarne CP, Greenfield P, Li D, Vockler CJ, Hitchcock CJ, Sawyer NA, Brett R, Edwards J, Pitt JI, Tran-Dinh N (2016) Genomic insights into the carbohydrate catabolism of Cairneyella variabilis gen. nov. sp. nov., the first reports from a genome of an ericoid mycorrhizal fungus from the southern hemisphere. Mycorrhiza 26:345–352
Miettinen O, Ryvarden L (2016) Polypore genera Antella, Austeria, Butyrea, Citripora, Metuloidea and Trulla (Steccherinaceae, Polyporales). Ann Bot Fenn 53:157–172
Miettinen O, Larsson E, Sjökvist E, Larsson KH (2012) Comprehensive taxon sampling reveals unaccounted diversity and morphological plasticity in a group of dimitic polypores (Basidiomycota, Polyporales). Cladistics 28:251–270
Miller SL, Larsson E, Larsson KH, Verbeken A, Nuytinck J (2006) Perspectives in the new Russulales. Mycologia 98:960–970
Miller MA, Pfeiffer W, Schwartz T (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In: Proceedings of the gateway computing environments workshop (GCE). IEEE, New Orleans, pp 1–8
Ming DQ, Luo LY, He XX, Wang MS, Fang WX, Chen SF, Chen WH, Han YF, Liang ZQ (2021) Paracremonium lepidopterorum, a new insect-associated fungus. Phytotaxa 524:85–91
Moncalvo JM, Nilsson RH, Koster B, Dunham SM, Bernauer T, Matheny PB, Porter TM, Margaritescu S, Weiß M, Garnica S, Danell E, Langer G, Langer E, Larsson E, Larsson KH, Vilgalys R (2006) The cantharelloid clade: dealing with incongruent gene trees and phylogenetic reconstruction methods. Mycologia 98:937–948
Mongkolsamrit S, Noisripoom W, Thanakitpipattana D, Wutikhun T, Spatafora JW, Luangsa-Ard J (2018) Disentangling cryptic species with isaria-like morphs in Cordycipitaceae. Mycologia 110:230–257
Motyka J (1936) Lichenum generis Usnea: studium monographicum. Pars systematica. Privately printed, Leopoldi
Nagy LG, Kocsubé S, Papp T, Vágvölgyi C (2009) Phylogeny and character evolution of the coprinoid mushroom genus Parasola as inferred from LSU and ITS nrDNA sequence data. Persoonia 22:28–37
Nagy LG, Vágvölgyi C, Papp T (2010) Type studies and nomenclatural revisions in Parasola (Psathyrellaceae) and related taxa. Mycotaxon 112:103–141
Nakasone KK (2008) Type studies of corticioid Hymenomycetes described by Bresadola. Cryptogam Mycol 29:231–257
Nannfeldt JA (1932) Studien uber die Mophologie und Systematik der nicht–lichenisierten inoperculaten discomyceten. Nova Acta Regiæ Societatis Scientiarum Upsaliensis 8:5–368
Nelsen MP, Lücking R, Grube M, Mbatchou JS, Muggia L, Rivas Plata E, Lumbsch HT (2009) Unravelling the phylogenetic relationships of lichenised fungi in Dothideomyceta. Stud Mycol 64:135–144
Nguyen LT, Schmidt HA, Von Haeseler A, Minh BQ (2015) IQ–TREE: a fast and effective stochastic algorithm for estimating maximum–likelihood phylogenies. Mol Biol Evol 32:268–274
Nishi O, Sushida H, Higashi Y, Iida Y (2022) Entomopathogenic fungus Akanthomyces muscarius (Hypocreales: Cordycipitaceae) strain IMI 268317 colonises on tomato leaf surface through conidial adhesion and general and microcycle conidiation. Mycology 13(2):133–142
Nitschke TRJ (1869) Grundlage eines Systems der Pyrenomyceten. Verhandlungen Des Naturhistorischen Vereins Der Preussischen Rheinlande, Westfalens Und Des Regierungsbezirks Osnabrück 26:70–77
Niveiro N, Popoff O, Albertó E (2012) Presence of Leucocoprinus cretaceus and L. fragilissimus in Argentina. Mycotaxon 121:265–273
Niveiro N, Ramírez NA, Michlig A, Lodge DJ, Aime MC (2020) Studies of Neotropical tree pathogens in Moniliophthora: a new species, M. mayarum, and new combinations for Crinipellis ticoi and C. brasiliensis. MycoKeys 66:39–54
Noordeloos ME (2011) Strophariaceae s. l. Fungi Europaei, vol. 13. Edizioni Candusso, Alassio
Nourel-Din AAH, Abdel-Aziz FA, Abdel-Wahab MA (2022) Qarounispora grandiappendiculata gen. et sp. nov. (Halosphaeriaceae, Microascales) from Qaroun Lake. Egypt Phytotaxa 530:086–094
Oberwinkler F (1977) Das neue System der Basidiomyceten. In: Frey W, Hurka H, Oberwinkler F (eds) Beiträge zur Biologie der niederen Pflanzen. Gustav Fischer Verlag, Stuttgart, pp 59–104
Ohmura Y (2001) Taxonomic study of the genus Usnea (lichenized Ascomycetes) in Japan and Taiwan. J Hattori Bot Lab 90:1–96
Ohmura Y (2012) A synopsis of the lichen genus Usnea (Parmeliaceae, Ascomycota) in Taiwan. Memoirs of the National Museum of Nature and Science 48:91–137
Ohmura Y, Clerc P (2019) Lectotypification of Usnea confusa (Parmeliaceae, Ascomycota). Bulletin of the National Museum of Nature and Science, Series B, Botany 45:63–70
Ohmura Y, Skirina I, Skirin F (2017) Contribution to the knowledge of the genus Usnea (Parmeliaceae, Ascomycota) in southern far East Russia. Bulletin of the National Museum of Nature and Science, Series B 43:1–10
Olariaga I, Huhtinen S, Læssøe T, Petersen JH, Hansen K (2020) Phylogenetic origins and family classification of typhuloid fungi, with emphasis on Ceratellopsis, Macrotyphula and Typhula (Basidiomycota). Stud Mycol 96:155–184
Oliveira JJS, Moncalvo JM, Margaritescu S, Capelari M (2020) A morphological and phylogenetic evaluation of Marasmius sect. Globulares (Globulares-Sicci complex) with nine new taxa from the Neotropical Atlantic Forest. Persoonia 44:240–277
Owings P, Desjardin DE (1997) A molecular phylogeny of Marasmius and selected segregate genera. Inoculum 48:29
Parra LA, Mua A, Cappelli A, Callac P (2011) Agaricus biannulatus sp. nov., a new species of the section Xanthodermatei collected in Sardinia and Sicily. Micol Veget Medit 26:3–20
Patouillard N (1900) Essai taxonomique sur les familles et les genres des Hyménomycètes. Lucien Declume, Lons-le-Saunier
Peck CH (1913) Species not before reported. Bulletin of the New York State Museum 167:23–33
Pegler DN (1986) Agaric flora of Sri Lanka. Kew Bull Addit Ser 12:370–371
Pem D, Jeewon R, Chethana KWT, Hongsanan S, Doilom M, Suwannarach N, Hyde KD (2021) Species concepts of Dothideomycetes: classification, phylogenetic inconsistencies and taxonomic standardization. Fungal Divers 109:283–319
Perera RH, Hyde KD, Jones EBG, Maharachchikumbura SSN, Bundhun D, Camporesi E, Akulov A, Liu JK, Liu ZY (2023) Profile of Bionectriaceae, Calcarisporiaceae, Hypocreaceae, Nectriaceae, Tilachlidiaceae, Ijuhyaceae fam. nov., Stromatonectriaceae fam. nov. and Xanthonectriaceae fam. nov. Fungal Divers online
Persoon CH (1796) Descriptiones tam novorum quan notabilium fungorum. Observations Mycologicae 1:1–221
Persoon CH (1818) Traité sur les Champignons Comestibles. Belin-Leprieur, Paris
Petersen RH (1971) The genera Gomphus and Gloeocantharellus in North America. Nova Hedwigia 21:1–118
Phookamsak R, Hyde KD, Jeewon R, Bhat DJ, Jones EBG, Maharachchikumbura SSN, Raspé O, Karunarathna SC, Wanasinghe DN, Hongsanan S, Doilom M, Tennakoon DS, Machado AR, Firmino AL, Ghosh A, Karunarathna A, Mešić A, Dutta AK, Thongbai B, Devadatha B, Norphanphoun C, Senwanna C, Wei D, Pem D, Ackah FK, Wang GN, Jiang HB, Madrid H, Lee HB, Goonasekara ID, Manawasinghe IS, Kušan I, Josep Cano J, Gené J, Li JF, Das K, Acharya K, Raj KNA, Latha KPD, Thilini Chethana KWT, He MQ, Dueñas M, Jadan M, Martín MP, Samarakoon MC, Dayarathne MC, Raza M, Park MS, Telleria MT, Chaiwan N, Matočec N, de Silva NI, Pereira OL, Singh PN, Manimohan P, Uniyal P, Shang QJ, Bhatt RP, Perera RH, Alvarenga RLM, Nogal-Prata S, Singh SK, Vadthanarat S, Oh SY, Huang SK, Rana S, Konta S, Paloi S, Jayasiri SC, Jeon SJ, Mehmood T, Gibertoni TB, Nguyen TTT, Singh U, Thiyagaraja V, Sarma VV, Dong W, Yu XD, Lu YZ, Lim YW, Chen Y, Tkalčec Z, Zhang ZF, Luo ZL, Daranagama DA, Thambugala KM, Tibpromma S, Camporesi E, Bulgakov TS, Dissanayake AJ, Senanayake IC, Dai DQ, Tang LZ, Khan S, Zhang H, Promputtha I, Cai L, Chomnunti P, Zhao RL, Lumyong S, Boonmee S, Wen TC, Mortimer PE, Xu JC (2019) Fungal diversity notes 929–1035: taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers 95:1–273
Põldmaa K (2011) Tropical species of Cladobotryum and Hypomyces producing red pigments. Stud Mycol 68:1–34
Pouzar Z (1957) Nova Genera Macromycetum i Ceská Mykologie 11:48–50
Pouzar Z (1959) New genera of higher fungi III. Ceská Mykologie 13:10–19
Prieto M, Schultz M, Olariaga I, Wedin M (2019) Lichinodium is a new lichenized lineage in the Leotiomycetes. Fungal Divers 94:23–39
Rao NK, Manoharachary C, Goos RD (1989) Forest litter hyphomycetes from Andhra Pradesh, India. IV. A new genus of synnematous hyphomycetes. Mycologia 81:790–793
Rauschert S (1989) Nomenklatorische Studien bei höheren Pilzen I. Russulales (Täublinge und Milchlinge). Ceská Mykologie 43:193–209
Réblová M, Gams W, Seifert KA (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud Mycol 68:163–191
Réblová M, Seifert KA, Fournier J, Štěpánek V (2012) Phylogenetic classification of Pleurothecium and Pleurotheciella gen. nov. and its dactylaria-like anamorph (sordariomycetes) based on nuclear ribosomal and protein-coding genes. Mycologia 104:1299–1314
Réblová M, Seifert KA, Fournier J, Štěpánek V (2016) Newly recognised lineages of perithecial ascomycetes: the new orders Conioscyphales and Pleurotheciales. Persoonia 37:57–81
Redhead SA, Nagasawa E (1987) Resinomycena japonica and Resupinatus merulioides, new species of Agaricales from Japan. Can J Bot 65:972–976
Redhead SA, Vilgalys R, Moncalvo J-M, Johnson J, Hopple JS Jr (2001) Coprinus Pers. and the disposition of Coprinus species sensu lato. Taxon 50:203–241
Rodriguez JM, Estrabou C, Truong C, Clerc P (2011) The saxicolous species of the genus Usnea subgenus Usnea (Parmeliaceae) in Argentina and Uruguay. Bryologist 114(3):504–525
Romagnesi H (1985) Les Russules d’Europe et d’Afrique du Nord. Reprint with supplement. J. Cramer, Lehre
Ronquist F, Teslenko M, Van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP (2012) MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Syst Biol 61:539–542
Rossi W (2010) New Laboulbeniales (Ascomycota) parasitic on Staphylinidae from Ecuador. Mycol Prog 9:407–415
Rossi W (2011) New species of Laboulbenia from Ecuador, with evidence for host switch in the Laboulbeniales. Mycologia 103:184–194
Rossi W, Kirk-Spriggs AH (2011) A new species of Laboulbenia (Ascomycota) parasitic on an African fly (Diptera: Curtonotidae), with a brief review of Diptera-associated species of the genus. Afr Invert 52:211–216
Rossi W, Leonardi M (2018) New species and new records of Laboulbeniales (Ascomycota) from Sierra Leone. Phytotaxa 358:91–116
Rossi W, Leonardi M (2020) New Laboulbeniales from China (Ascomycota). Cryptogam Mycol 41:1–7
Rossi W, Vávra JC, Barták M (2019) New species and new records of Laboulbeniales (Ascomycota) from the Czech Republic and Slovakia. Nova Hedwigia 109:149–159
Rossi W, Das K, Hembrom ME, Santamaria S, Parihar A, Ghosh A, Henkel TW, Hofstetter V, Randrianohany E, Vizzini A, Wang XH, Buyck B (2020) Fungal biodiversity profiles 91–100. Cryptogam Mycol 41:69–107
Rossman AY (1987) The Tubeufiaceae and similar Loculoascomycetes. Mycol Pap 157:1–71
Rossman AY (1996) Morphological and molecular perspectives on systematics of the Hypocreales. Mycologia 88:1–19
Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999) Genera of Bionectriaceae, Hypocreaceae and Nectriaceae (Hypocreales, Ascomycetes). Stud Mycol 42:1–248
Rossman AY, McKemy JM, Pardo-Schultheiss RA, Schroers HJ (2001) Molecular studies of the Bionectriaceae using large subunit rDNA sequences. Mycologia 93:100–110
Rother MS, Borges da Silveira RM (2009) Leucocoprinus Pat. (Agaricaceae, Basidiomycota) no Parque Estadual de Itapuã, Viamão, RS. Brasil Acta Bot Brasil 23:720–728
Ryvarden L (1991) Genera of polypores: nomenclature and taxonomy. Fungiflora, Oslo
Ryvarden L (2002) A note on the genus Hydnodon Banker. Synopsis Fungorum 15:31–33
Ryvarden L, Gilbertson RL (1993) European polypores, Part 1: Abortiporus-Lindtneria. Synopsis Fungorum 6:1–387
Ryvarden L, Tutka S (2014) Perplexostereum Ryvarden & Tutka nov. gen. Synopsis Fungorum 32:72–75
Ryvarden L, Wright JE, Rajchenberg M (1982) Megasporoporia, a new genus of resupinate polypores. Mycotaxon 16:172–182
Ryvarden L, Xu LW, Zhao JD (1986) A note of the Polyporaceae in the Chang Bai Shan Forest Reserve in northeastern China. Acta Mycol Sin 5:226–234
Ryvarden L, Hjortstam K, Iturriaga T (2005) Studies in corticioid fungi from Venezuela II (Basidiomycotina, Aphyllophorales). Synopsis Fungorum 20:42–78
Salar RK, Aneja KR (2007) Thermophilic fungi: Taxonomy and biogeography. J Agric Technol 3:77–107
Salvador-Montoya CA, Popoff OF, Reck M, Drechsler-Santos ER (2018) Taxonomic delimitation of Fulvifomes robiniae (Hymenochaetales, Basidiomycota) and related species in America: F. squamosus sp. nov. Plant Syst Evol 304:445–459
Samarakoon MC, Hyde KD, Promputtha I, Hongsanan S, Ariyawansa HA, Maharachchikumbura SSN, Daranagama DA, Stadler M, Mapook A (2016) Evolution of Xylariomycetidae (Ascomycota: Sordariomycetes). Mycosphere 7:1746–1761
Samarakoon MC, Liu JK, Hyde KD, Promputtha I (2019) Two new species of Amphisphaeria (Amphisphaeriaceae) from northern Thailand. Phytotaxa 391:207–217
Samarakoon MC, Maharachchikumbura SS, Liu JK, Hyde KD, Promputtha I, Stadler M (2020) Molecular phylogeny and morphology of Amphisphaeria (= Lepteutypa) (Amphisphaeriaceae). J Fungi 6:174
Samson RA, Evans HC (1974) Notes on entomogenous fungi from Ghana: II. The Genus Akanthomyces Acta Botanica Neerlandica 23(1):28–35
Sang XY, Li XD, Wang YW, Fan L (2016) Four new sequestrate species of Russulaceae found in China. Phytotaxa 289:101–117
Santamaria S, Pedersen J (2021) Laboulbeniomycetes (Fungi, Ascomycota) of Denmark. Eur J Taxon 781:1–425
Santamaria S, Cuesta-Segura AD, Guardia L (2020) New and remarkable species of Laboulbeniales (Ascomycota) from Spain. Nova Hedwigia 110:347–367
Sarnari M (1998) Monografia illustrate de genere Russula in Europa. Tomo Primo. AMB, Centro Studi Micologici, Trento
Sarnari M (2005) Monografia illustrate de genere Russula in Europa. Tomo Secondo. AMB, Centro Studi Micologici, Trento
Seeman OD, Nahrung HF (2000) Mites as fungal vectors? The ectoparasitic fungi of mites and their arthropod associates in Queensland. Aust Mycol 19:3–9
Senanayake IC, Maharachchikumbura SS, Hyde KD, Bhat JD, Jones E, McKenzie EH, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73:73–144
Sharma JR (2012) Aphylloporales of Himalaya. Botanical Survey of India, Kolkata
Shearer CA, Raja HA, Miller AN, Nelson P, Tanaka K, Hirayama K, Marvanová L, Hyde KD, Zhang Z (2009) The molecular phylogeny of freshwater Dothideomycetes. Stud Mycol 64:145–153
Shen S, Liu SL, Jiang JH, Zhou LW (2021) Addressing widespread misidentifications of traditional medicinal mushrooms in Sanghuangporus (Basidiomycota) through ITS barcoding and designation of reference sequences. IMA Fungus 12:10
Shen HW, Bao DF, Bhat DJ, Su HY, Luo ZL (2022) Lignicolous freshwater fungi in Yunnan Province, China: an overview. Mycology 13(2):119–132
Shen S, Liu SL, Zhou LW (2023) Taxonomy of Hyphodermella: a case study to show that simple phylogenies cannot always accurately place species in appropriate genera. IMA Fungus 14:11
Shi L, Yang H, Hyde KD, Wijayawardene NN, Wang GN, Yu XD, Zhang H (2021) Freshwater Sordariomycetes: new species and new records in Pleurotheciaceae, Pleurotheciales. Phytotaxa 518:143–166
Shoemaker RA (1959) Nomenclature of Drechslera and Bipolaris, grass parasites segregated from ‘Helminthosporium’. Can J Bot 37:879–887
Shukla P, Upreti DK, Tewari LM (2014) Lichen genus Usnea (Parmeliaceae, Ascomycota) in Uttarakhand, India. Curr Res Environ Appl Mycol J Fungal Biol 4:188–201
Schafer DJ (2010) Keys to sections of Parasola, Coprinellus, Coprinopsis and Coprinus in Britain. Field Mycol 11:44–51
Schafer DJ (2014) The genus Parasola in Britain including Parasola cuniculorum sp. nov. Field Mycol 15:77–99
Schafer D, Alvarado P, Smith L, Liimatainen K, Loizides M (2022) Coprinoid Psathyrellaceae species from Cyprus: three new sabulicolous taxa from sand dunes and a four-spored form of the fimicolous species Parasola cuniculorum. Mycol Prog 21:52
Schoch CL, Sung GH, López-Giráldez F, Townsend JP, Miadlikowska J, Hofstetter V, Robbertse B, Matheny PB, Kauff F, Wang Z (2009) The Ascomycota tree of life: a phylum-wide phylogeny clarifies the origin and evolution of fundamental reproductive and ecological traits. Syst Biol 58:224–239
Silvestro D, Michalak I (2012) raxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Singer R (1943) A monographic study of the genera Crinipellis and Chaetocalathus. Lilloa 8:441–534
Singer R (1976) Marasmieae (Basidiomycetes-Tricholomataceae). Flora Neotrop 17:1–347
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Koenigstein
Sivanesan A (1984) The Bitunicate Ascomycetes and their Anamorphs. J. Cramer, Vaduz
Sivanesan A (1987) Graminicolous species of Bipolaris, Curvularia, Drechslera, Exserohilum and their teleomorphs. Mycol Pap 158:1–261
Smith AH, Hesler LR (1968) The North American Species of Pholiota. Hafner Publishing Company, New York
Smith AH, Thiers HD (1971) The boletes of Michigan. University of Michigan Press, Ann Arbor
Song J, Xing JH, Ji X, Sun YF, Cui BK, Dai YC (2018a) Rigidotubus tephroleucus gen. et sp. nov. (Cystostereaceae, Agaricales) evidenced by morphological characters and phylogenetic analyses. Phytotaxa 333(2):259–266
Song Y, Buyck B, Li JW, Yuan F, Zhang ZW, Qiu LH (2018b) Two novel and a forgotten Russula species in sect. Ingratae (Russulales) from Dinghushan iosphere Reserve in southern China. Cryptogam Mycol 39:1–17
Song Y, Li JW, Buyck B, Zheng JF, Qiu LH (2018c) Russula verrucospora sp. nov. and R. xanthovirens sp. nov., two novel species of Russula (Russulaceae) from southern China. Cryptogam Mycol 39:129–142
Song J, Liang JF, Mehrabi-Koushki M, Krisai-Greilhuber I, Ali B, Bhatt VK, Cerna-Mendoza A, Chen B, Chen ZX, Chu HL, Corazon-Guivin MA, da Silva GA, De Kesel A, Dima B, Dovana F, Farokhinejad R, Ferisin G, Guerrero-Abad JC, Guo T, Han LH, Ilyas S, Justo A, Khalid AN, Khodadadi-Pourarpanahi S, Li TH, Liu C, Lorenzini M, Lu JK, Mumtaz AS, Oehl F, Pan XY, Papp V, Qian W, Razaq A, Semwal KC, Tang LZ, Tian XL, Vallejos-Tapullima A, van der Merwe NA, Wang SK, Wang CQ, Yang RH, Yu F, Zapparoli G, Zhang M, Antonín V, Aptroot A, Aslan A, Banerjee A, Chatterjee S, Dirks AC, Ebrahimi L, Fotouhifar K-B, Ghosta Y, Kalinina LB, Karahan D, Maiti M, Mookherjee A, Nath PS, Panja B, Saha J, Ševčíková H, Voglmayr H, Yazıcı K, Haelewaters D (2019) Fungal systematics and evolution 5. Sydowia 71:141–245
Spirin W (2003) Antrodiella romellii (Irpicaceae, Basidiomycetes) in Russia. Mycena 3:48–52
Spirin W, Zmitrovich I, Malysheve V (2007) Steccherinum tenuispinum (Polyporales, Basidiomycota), a new species from Russia and notes on three other species. Ann Bot Fenn 44:298–302
Stalpers JA, Redhead SA, May TW, Rossman AY, Crouch JA, Cubeta MA, Dai YC, Kirschner R, Langer GJ, Larsson KH, Mack J, Norvell LL, Oberwinkler F, Papp V, Roberts P, Rajchenberg M, Seifert KA, Thorn RG (2021) Competing sexual-asexual generic names in Agaricomycotina (Basidiomycota) with recommendations for use. IMA Fungus 12:22
Stamatakis A (2006) RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22:2688–2690
Stevens GN (2004). Usneaceae. In: Flora of Australia Vol. 56A, Lichens 4 (P. M. McCarthy & K. Mallett, eds.). ABRS/CSIRO, Melbourne, pp 78–98 & 107–115.
Stolk AC (1965) Thermophilic species of Talaromyces Benjamin and Thermoascus Miehe. Anton Leeuw 31:262–276
Su HY, Luo ZL, Liu XY, Su XJ, Hu DM, Zhou DQ, Bahkali AH, Hyde KD (2016) Lentithecium cangshanense sp. nov. (Lentitheciaceae) from freshwater habitats in Yunnan Province. China Phytotaxa 267:61–69
Subramanian C V (1952) Fungi imperfecti from Madras—III. In: Proceedings of the Indian Academy of Sciences-Section B. Springer, India, pp 223–228
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Sulistyo BP, Larsson K-H, Haelewaters D, Ryberg M (2021) Multigene phylogeny and taxonomic revision of Atheliales s.l.: Reinstatement of three families and one new family. Lobuliciaceae Fam Nov Fungal Biol 125:239–255
Sung GH, Hywel-Jones NL, Sung JM, Luangsa-Ard JJ, Shrestha B, Spatafora JW (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59
Šutara J (2008) Xerocomus s.l. in the light of the present state of knowledge. Czech Mycol 60:29–62
Swinscow TDV, Krog H (1979) The fruticose species of Usnea subgenus Usnea in East Africa. Lichenologist 11:207–252
Szarkándi JG, Schmidt-Stohn G, Dima B, Hussain S, Kocsubé S, Papp T, VágvÖlgyi C, Nagy LG (2017) The genus Parasola: phylogeny of the genus and the description of three new species. Mycologia 109:620–629
Tan YP, Bishop-Hurley MAR, Shivas RG (2021) Nomenclatural novelties. Index Fungorum 503:1
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y (2015) Revision of the massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136
Tavares II (1985) Laboulbeniales (Fungi, Ascomycetes). Mycol Mem 9:1–627
Tchoumi JMT, Coetzee MPA, Rajchenberg M, Roux J (2020) Poroid Hymenochaetaceae associated with trees showing wood-rot symptoms in the garden route National Park of South Africa. Mycologia 112:722–741
Temu SG, Clerc P, Tibell L, Tibuhwa DD, Tibell S (2019) Phylogeny of the subgenus Eumitria in Tanzania. Mycology 10:250–260
Tennakoon DS, Kuo CH, Maharachchikumbura SS, Thambugala KM, Gentekaki E, Phillips AJ, Bhat DJ, Wanasinghe DN, de Silva NI, Promputtha I, Hyde KD (2021) Taxonomic and phylogenetic contributions to Celtis formosana, Ficus ampelas, F. septica, Macaranga tanarius and Morus australis leaf litter inhabiting microfungi. Fungal Divers 108:1–215
Thanakitpipattana D, Tasanathai K, Mongkolsamrit S, Khonsanit A, Lamlertthon S, Luangsa-Ard JJ (2020) Fungal pathogens occurring on Orthopterida in Thailand. Persoonia 44(1):140–160
Thaxter R (1901) Preliminary diagnoses of new species of Laboulbeniaceae. III Proc Amer Acad Arts Sci 36:397–414
Thaxter R (1908) Contribution toward a monograph of the Laboulbeniaceae. Part II. Mem Am Acad Arts Sci 13:217–269
Thell A, Crespo A, Divakar PK, Kärnefelt I, Leavitt SD, Lumbsch HT, Seaward MRD (2012) A review of the lichen family Parmeliaceae–history phylogeny and current taxonomy. Nord J Bot 30:641–664
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Thorn RG, Barron GL (1986) Nematoctonus and the tribe Resupinateae in Ontario, Canada. Mycotaxon 25:321–453
Thorn RG, Moncalvo JM, Reddy CA, Vilgalys R (2000) Phylogenetic analyses and the distribution of nematophagy support a monophyletic Pleurotaceae within the polyphyletic pleurotoid-lentinoid fungi. Mycologia 92:241–252
Thorn RG, Moncalvo JM, Redhead SA, Lodge DJ, Martin MP (2005) A new poroid species of Resupinatus from Puerto Rico, with a reassessment of the cyphelloid genus Stigmatolemma. Mycologia 97:1140–1151
Tibpromma S, Hyde KD, Jeewon R, Maharachchikumbura SSN, Liu JK, Bhat DJ, Jones EBJ, McKenzie EHC, Camporesi E, Bulgakov TS, Doilom M, de Azevedo SALCM, Das K, Manimohan P, Gibertoni TB, Lim YW, Ekanayaka AH, THONGBAI B, Lee HB, Yang JB, Kirk PW, Sysouphanthong P, Singh SK, Boonmee S, Dong W, Raj KNA, Latha KPD, Phookamsak R, Phukhamsakda C, Konta S, Jayasiri SC, Norphanphoun C, Tennakoon DS, Li JF, Dayarathne MC, Perera RH, Xiao YP, Wanasinghe DN, Senanayake IC, Goonasekara ID, de Silva NI, Mapook A, Jayawardena RS, Dissanayake AJ, Manawasinghe IS, Chethana KWT, Luo ZL, Hapuarachchi KK, BAGHELA A, Soares AM, Vizzini A, MeirasOttoni A, Mešić A, Dutta AK, de Souza CAF, Richter C, Lin CG, Chakrabarty D, Daranagama DA, Lima DX, Chakraborty D, Ercole E, Wu F, Simonini G, Vasquez G, da Silva GA, PlautzJr HL, Ariyawansa HA, Lee H, Kušan I, Song J, Sun JZ, Karmakar J, Hu KF, Semwal KC, Thambugala KM, Voigt K, Acharya K, Rajeshkumar KC, Ryvarden L, Jadan M, Hosen MI, Mikšk M, Samarakoon MC, Wijayawardene NN, Kim NK, Matočec N, Singh PN, Tian Q, Bhatt RP, de Oliveira RJV, Tulloss RE, Aamir S, Kaewchai S, Marathe SD, Khan S, Hongsanan S, Adhikari S, Mehmood T, Bandyopadhyay TK, Svetasheva TY, Nguyen TTT, Antonn V, Li WJ, Wang Y, Indoliya Y, Tkalčec Z, Elgorban AM, Bahkali AH, Tang AMC, Su HY, Zhang H, Promputtha I, LuangsaArd J, Xu JC, Yan JY, Kang JC, Stadler M, Mortimer PE, Chomnunti P, Zhao Q, Phillips AJL, Nontachaiyapoom S, Wen TC, Karunarathna SC (2017) Fungal diversity notes 491–602: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 83:1–261
Tibpromma S, Hyde KD, McKenzie EHC, Bhat DJ, Phillips AJL, Wanasinghe DN, Samarakoon MC, Jayawardena RS, Dissanayake AJ, Tennakoon DS, Doilom M, Phookamsak R, Tang AMC, Xu JC, Mortimer PE, Promputtha I, Maharachchikumbura SSN, Khan S, Karunarathna SC (2018) Fungal diversity notes 840–928: micro-fungi associated with Pandanaceae. Fungal Divers 93:1–160
Truong C, Clerc P (2012) The lichen genus Usnea (Parmeliaceae) in tropical South America: species with a pigmented medulla, reacting C+ yellow. Lichenologist 44:625–637
Truong C, Clerc P (2013) Eumitrioid Usnea species (Parmeliaceae, lichenized Ascomycota) in tropical South America and the Galapagos. Lichenologist 45:383–395
Truong C, Clerc P (2016) New species and new records in the genus Usnea (Parmeliaceae, lichenized Ascomycota) from tropical South America. Lichenologist 48(71):93
Truong C, Bungartz F, Clerc P (2011) The lichen genus Usnea (Parmeliaceae) in the tropical Andes and the Galapagos: species with a red–orange cortical or subcortical pigmentation. Bryologist 114:477–503
Truong C, Rodriguez JM, Clerc P (2013) Pendulous Usnea species (Parmeliaceae, lichenized Ascomycota) in tropical South America and the Galapagos. Lichenologist 45:505–543
Tsui CKM, Hyde KD, Hodgkiss IJ (2001) Longitudinal and temporal distribution of freshwater ascomycetes and dematiaceous hyphomycetes on submerged wood in the Lam Tsuen River, Hong Kong. J North Am Benthol Soc 20:533–549
Tulasne LR, Tulasne C (1865) Selecta fungorum carpologia 3. Jusseau, Paris
Turland NJ, Wiersema JH, Barrie FR, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Kusber WH, Li DZ, Marhold K, May TW, McNeill J, Monro AM, Prado J, Price MJ, Smith GF (2018) International code of nomenclature for algae, fungi, and plants (Shenzhen code) adopted by the nineteenth international botanical congress Shenzhen, China, July 2017. Koeltz Botanical Books, Glashütten
Valenzuela E, Garnica S (2000) Pseudohelicomyces, a new anamorph of Psilocybe. Mycol Res 104:738–741
Veldre V, Abarenkov K, Bahram M, Martos F, Selosse MA, Tamm H, Kõljalg U, Tedersoo L (2013) Evolution of nutritional modes of Ceratobasidiaceae (Cantharellales, Basidiomycota) as revealed from publicly available ITS sequences. Fungal Ecol 6:256–268
Vellinga EC (2001) Leucocoprinus. In: Noordeloos ME et al (eds) Flora Agaricina Neerlandica 5. A.A. Balkema Publishers, Lisse, pp 76–84
Villamizar LF, Barrera G, Marshall SDG, Richena M, Harland D, Jackson TA (2020) Three-dimensional cellular aggregates formed by Beauveria pseudobassiana in liquid culture with potential for use as a biocontrol agent of the African black beetle (Heteronychus arator). Mycology 12(2):105–111
Vizzini A, Consiglio G, Setti L, Ercole E (2015) Calocybella, a new genus for Rugosomyces pudicus (Agaricales, Lyophyllaceae) and emendation of the genus Gerhardtia. IMA Fungus 6:1–11
Vizzini A, Angelini C, Ercole E (2017) Is the species diversity in the lyophylloid genera Calocybella and Gerhardtia (Agaricales, Basidiomycota) underestimated? Two new species from the Dominican Republic. Phytotaxa 291:241–252
von Höhnel F (1918) Fragmente zur Mykologie. (XXI. Mitteilung, Nr. 1058 bis 1091). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Math.-naturw. Klasse Abt i 127:329–393
von Höhnel F (1919) Fünfte vorläufige Mitteilung mycologische Ergebnisse (Nr. 399–500). Berichte Der Deutschen Botanischen Gesellschaft 37:153–161
Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Ariyawansa HA, Wijayawardene NN, Mortimer PE, Xu J, Yang JB, Hyde KD (2014) An exciting novel member of Lentitheciaceae in Italy from Clematis vitalba. Cryptogam Mycol 35:323–337
Wanasinghe DN, Wijayawardene NN, Xu JC, Cheewangkoon R, Mortimer PE (2020) Taxonomic novelties in Magnolia-associated pleosporalean fungi in the Kunming Botanical Gardens (Yunnan, China). PLoS ONE 15(7):e0235855)
Wang YZ, Aptroot A, Hyde KD (2004) Revision of the genus Amphisphaeria. Hong Kong SAR. China Fungal Divers Res Ser 13:1–168
Wang PF, Zhang Y, Mi F, Tang XZ, He XX, Cao Y, Liu CL, Yang D, Dong JY, Zhang KQ, Xu JP (2015) Recent advances in population genetics of ectomycorrhizal mushrooms Russula spp. Mycology 6:110–120
Wang J, Buyck B, Wang XH, Tolgor B (2019a) Visiting Russula (Russulaceae, Russulales) with samples from southwestern China finds one new subsection of R. subg. Heterophyllidia with two new species. Mycol Prog 18:771–784
Wang XH, Das K, Bera I, Chen YH, Bhatt RP, Ghosh A, Hembrom ME, Hofstetter V, Parihar A, Vizzini A, Xu TM, Zhao CL, Buyck B (2019b) Fungal biodiversity profiles 81–90. Cryptogam Mycol 40:57–95
Wang YB, Wang Y, Fan Q, Duan DE, Zhang GD, Dai RQ, Dai YD, Zeng WB, Chen ZH, Li DD, Tang DX, Xu ZH, Sun TT, Nguyen T, Tran NL, Dao VM, Zhang CM, Huang LD, Liu YJ, Zhang XM, Yang DR, Sanjuan T, Liu XZ, Yang ZL, Yu H (2020a) Multigene phylogeny of the family Cordycipitaceae (Hypocreales): new taxa and the new systematic position of the Chinese cordycipitoid fungus Paecilomyces hepiali. Fungal Divers 103(1):1–46
Wang Y, Su MS, Jiang S, Xue R, Wu LL, Xie HJ, Zhang YZ, Liang ZQ, Zeng NK (2020b) The genus Hourangia in China and a description of Aureoboletus erythraeus sp. nov. Phytotaxa 472:87–106
Wang XW, May TW, Liu SL, Zhou LW (2021a) Towards a natural classification of Hyphodontia sensu lato and the trait evolution of basidiomes within Hymenochaetales (Basidiomycota). J Fungi 7:478
Wang YR, Wu YD, Vlasák J, Yuan Y, Dai YC (2021b) Phylogenetic analysis demonstrating four new species in Megasporoporia sensu lato (Polyporales, Basidiomycota). Mycosphere 12:1012–1037
Wang Y, Tuo YL, Wu DM, Gao N, Zhang ZH, Rao G, Wang XM, Wang J, Dai D, Li Y, Zhang B (2022a) Exploring the relationships between four new species of boletoid fungi from Northern China and their related species. J Fungi 8:218
Wang YR, Dai YC, Liu HG, Vlasák J, Buchanan P, Yuan Y, Wu YD (2022b) A new contribution to Megasporoporia sensu lato: Six new species and three new combinations. Front Microbiol 13:1046777
Wannathes N, Desjardin DE, Hyde KD, Perry BA, Lumyong S (2009) A monograph of Marasmius (Basidiomycota) from northern Thailand based on morphological and molecular ITS sequences data. Fungal Divers 37:209–306
Wartchow F, Putzke J, Cavalcanti MAQ (2008) Agaricaceae Fr. (Agaricales, Basidiomycota) from areas of Atlantic Forest in Pernambuco. Brazil Acta Bot Brasil 22:287–299
Watkinson S, Eastwood D (2012) Serpula lacrymans, wood and buildings. Adv Appl Microbiol 78:121–149
Wei FW (2021) Toward post-2020 global biodiversity conservation: Footprint and direction in China. Innovation 2:100175
Wei YL, Qin WM (2009) Notes on cyphelloid homobasidiomycetes in China 1. Henningsomyces (Basidiomycota, Agaricales). Mycosystema 28(5):672–674
Weir A, Rossi W (1995) Laboulbeniales parasitic on British Diptera. Mycol Res 99:841–849
Wen TC, Kang JC, Hyde KD, Li GR, Chen X (2014) Phenotypic marking of Cordyceps militaris fruiting-bodies and their cordycepin production. Chiang Mai J Sci 41(4):846–857
Wen TC, Kang C, Meng ZB, Qi YB, Hyde KD, Kang JC (2016) Enhanced Production of Cordycepin by Solid State Fermentation of Cordyceps militaris using Additives. Chiang Mai J Sci 43(5):972–984
Westphalen MC, Rajchenberg M, Tomšovský M, Gugliotta AM (2018) A re-evaluation of Neotropical Junghuhnia s.l. (Polyporales, Basidiomycota) based on morphological and multigene analyses. Persoonia 41:130–141
Westphalen MC, Motato-Vásquez V, Tomšovský M, Gugliotta AM (2021) Additions to the knowledge of hydnoid Steccherinaceae: Cabalodontia, Etheirodon, Metuloidea, and Steccherinum. Mycologia 113:791–806
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, D’souza MJ, Diederich P, Dissanayake A (2014) Naming and outline of Dothideomycetes–2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55
Wijayawardene NN, Hyde KD, Lumbsch HT, Liu JK, Maharachchikumbura SSN, Ekanayaka AH, Tian Q, Phookamsak R (2018) Outline of Ascomycota: 2017. Fungal Divers 88:167–263
Wijayawardene NN, Hyde KD, Al-Ani LKT, Tedersoo L, Haelewaters D, Rajeshkumar KC, Zhao RL, Aptroot A, Leontyev DV, Saxena RK, Tokarev YS, Dai DQ, Letcher PM, Stephenson SL, Ertz D, Lumbsch HT, Kukwa M, Issi IV, Madrid H, Phillips AJL, Selbmann L, Pfliegler WP, Horváth E, Bensch K, Kirk PM, Kolaříková K, Raja HA, Radek R, Papp V, Dima V, Ma J, Malosso E, Takamatsu S, Rambold G, Gannibal PB, Triebel D, Gautam AK, Avasthi S, Suetrong S, Timdal E, Fryar SC, Delgado G, Réblová M, Doilom M, Dolatabadi S, Pawłowska J, Humber RA, Kodsueb R, Sánchez-Castro I, Goto BT, Silva DKA, de Souza FA, Oehl F, da Silva GA, Silva IR, Błaszkowski J, Jobim K, Maia LC, Barbosa FR, Fiuza PO, Divakar PK, Shenoy BD, Castañeda-Ruiz RF, Somrithipol S, Lateef AA, Karunarathna SC, Tibpromma S, Mortimer PE, Wanasinghe DN, Phookamsak R, Xu J, Wang Y, Tian F, Alvarado P, Li DW, Kušan I, Matočec N, Maharachchikumbura SSN, Papizadeh M, Heredia G, Wartchow F, Bakhshi M, Boehm E, Youssef N, Hustad VP, Lawrey JD, Santiago ALCMA, Bezerra JDP, Souza-Motta CM, Firmino AL, Tian Q, Houbraken J, Hongsanan S, Tanaka K, Dissanayake AJ, Monteiro JS, Grossart HP, Suija A, Weerakoon G, Etayo J, Tsurykau A, Vázquez V, Mungai P, Damm U, Li QR, Zhang H, Boonmee S, Lu YZ, Becerra AG, Kendrick B, Brearley FQ, Motiejūnaitė J, Sharma B, Khare R, Gaikwad S, Wijesundara DSA, Tang LZ, He MQ, Flakus A, Rodriguez-Flakus P, Zhurbenko MP, McKenzie EHC, Stadler M, Bhat DJ, Liu JK, Raza M, Jeewon R, Nassonova ES, Prieto M, Jayalal RGU, Erdoğdu M, Yurkov A, Schnittler M, Shchepin ON, Novozhilov YK, Silva-Filho AGS, Liu P, Cavender JC, Kang Y, Mohammad S, Zhang LF, Xu RF, Li YM, Dayarathne MC, Ekanayaka AH, Wen TC, Deng CY, Pereira OL, Navathe S, Hawksworth DL, Fan XL, Dissanayake LS, Kuhnert E, Grossart HP, Thines M (2020) Outline of Fungi and fungus-like taxa. Mycosphere 11:1060–1456
Wijayawardene NN, Hyde KD, Dai DQ, Sánchez-García M, Goto BT, Saxena RK, Erdoğdu M, Selçuk F, Rajeshkumar KC, Aptroot A, Błaszkowski J, Boonyuen N, da Silva GA, de Souza FA, Dong W, Ertz D, Haelewaters D, Jones EBG, Karunarathna SC, Kirk PM, Kukwa M, Leontyev KJ, DV, Lumbsch HT, Maharachchikumbura SSN, Marguno F, Martínez-Rodríguez P, Mešić A, Monteiro JS, Oehl F, Pawłowska J, Pem D, Pfliegler WP, Phillips AJL, Pošta A, He MQ, Li JX, Raza M, Sruthi OP, Suetrong S, Suwannarach N, Tedersoo L, Thiyagaraja V, Tibpromma S, Tkalčec Z, Tokarev YS, Wanasinghe DN, Wijesundara DSA, Wimalaseana SDMK, Madrid H, Zhang GQ, Gao Y, Sánchez-Castro I, Tang LZ, Stadler M, Yurkov A, Thines M (2022) Outline of Fungi and fungus-like taxa – 2021. Mycosphere 13:53–453
Wilson AW, Desjardin DE (2005) Phylogenetic relationships in the gymnopoid and marasmioid fungi (Basidiomycetes, euagarics clade). Mycologia 97:667–679
Winter G (1885) Ascomyceten: Gymnoasceen und Pyrenomyceten. Rabenhorst’s Kryptogamen-Flora Von Deutschland, Oesterreich Und Der Schweiz 21:1–928
Winter G (1887) Pilze, Ascomyceten. Rabenhorst’s Kryptogamen-Flora Von Deutschland, Oesterreich Und Der Schweiz 1:1–928
Wirtz N, Printzen C, Lumbsch HT (2012) Using haplotype networks, estimation of gene flow and phenotypic characters to understand species delimitation in fungi of a predominantly Antarctic Usnea group (Ascomycota, Parmeliaceae). Org Divers Evol 12:17–37
Wolf FA (1912) The perfect stage of Actinonema rosae. Bot Gaz 54:218–234
Wu SH (2001) Three new species of Hyphodontia with poroid hymenial surface. Mycologia 93:1019–1025
Wu SH, Nilsson HR, Chen CT, Yu SY, Hallenberg N (2010a) The white-rotting genus Phanerochaete is polyphyletic and distributed throughout the phleboid clade of the Polyporales (Basidiomycota). Fungal Divers 42:107–118
Wu SH, Wang DM, Yu SY (2010b) Neoaleurodiscus fujii, a new genus and new species found at the timberline in Japan. Mycologia 102:217–223
Wu G, Feng B, Xu JP, Zhu XT, Li YC, Zeng NK, Hosen MI, Yang ZL (2014) Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers 69:93–115
Wu F, Qin WM, Euatrakool O, Zhou LW (2015) Tropicoporus boehmeriae sp. nov. (Hymenochaetaceae, Basidiomycota) from Thailand, a new member of the Inonotus linteus complex. Phytotaxa 231:73–80
Wu G, Li YC, Zhu XT, Zhao K, Han LH, Cui YY, Li F, Xu JP, Yang ZL (2016a) One hundred noteworthy boletes from China. Fungal Divers 81:25–188
Wu G, Zhao K, Li YC, Zeng NK, Feng B, Halling RE, Yang ZL (2016b) Four new genera of the fungal family Boletaceae. Fungal Divers 81:1–24
Wu B, Hussain M, Zhang W, Stadler M, Liu X, Xiang M (2019) Current insights into fungal species diversity and perspective on naming the environmental DNA sequences of fungi. Mycology 10:127–140
Wu F, Yuan Y, Chen JJ, Cui BK, Zhou M, Dai YC (2020) Terrestriporiaceae fam. nov., a new family of Russulales (Basidiomycota). Mycosphere 11:2755–3276
Wu YX, Wu JR, Zhao CL (2021) Steccherinum tenuissimum and S. xanthum spp. nov. (Polyporales, Basidiomycota): New species from China. PLoS ONE 16:e0244520
Wu F, Zhou LW, Vlasák J, Dai YC (2022a) Global diversity and systematics of Hymenochaetaceae with poroid hymenophore. Fungal Divers 113:1–192
Wu F, Wang XH, Qin WQ, Hao YJ, Guo T, Zhang P, Chen ZH (2022b) Five new species and one new record of Lactarius (Russulaceae, Russulales) from tropical and subtropical regions of China. Mycosystema 41:1234–1253
Yang ZL, Wang XH, Binder M (2003) A study of the type and additional materials of Boletus thibetanus. Mycotaxon 86:283–290
Yu J, Wang XW, Liu SL, Shen S, Zhou LW (2021) Taxonomy and phylogeny of Resinicium sensu lato from Asia-Pacific revealing a new genus and five new species (Hymenochaetales, Basidiomycota). IMA Fungus 12:19
Yuan Y, Ji XH, Chen JJ, Dai YC (2017) Three new species of Megasporia (Polyporales, Basidiomycota) from China. MycoKeys 20:37–50
Yuan F, Song Y, Buyck B, Li JW, Qiu LH (2019) Russula viridicinnamomea F. Yuan & Y. Song, sp. nov. and R. pseudocatillus F. Yuan & Y. Song, sp. nov., two new species from southern China. Cryptogam Mycol 40:45–56
Yuan Y, Chen JJ, Korhonen K, Martin F, Dai YC (2021) An updated global species diversity and phylogeny in the forest pathogenic genus Heterobasidion (Basidiomycota, Russulales). Front Microbiol 11:596393
Yuan Y, Wu YD, Wang YR, Zhou M, Qiu JZ, Li DW, Vlasák J, Liu HG, Dai YC (2022) Two new forest pathogens in Phaeolus (Polyporales, Basidiomycota) on Chinese coniferous trees were confirmed by molecular phylogeny. Front Microbiol 13:942603
Yurchenko E, Riebesehl J, Langer E (2017) Clarification of Lyomyces sambuci complex with the descriptions of four new species. Mycol Prog 16:865–876
Yurchenko E, Wu SH, Maekawa N (2020) Three new species of Peniophorella (Basidiomycota) from East Asia. Nova Hedwigia 111:473–495
Zeng NK, Tang LP, Li YC, Tolgor B, Zhu XT, Zhao Q, Yang ZL (2013) The genus Phylloporus (Boletaceae, Boletales) from China: morphological and multilocus DNA sequence analyses. Fungal Divers 58:73–101
Zeng NK, Wu G, Li YC, Liang ZQ, Yang ZL (2014) Crocinoboletus, a new genus of Boletaceae (Boletales) with unusual boletocrocin polyene pigments. Phytotaxa 175:133–140
Zha LS, Huang SK, Xiao YP, Boonmee S, Eungwanichayapant PD, McKenzie EHC, Kryukov V, Wu XL, Hyde KD, Wen TC (2018) An evaluation of common Cordyceps (Ascomycetes) species found in Chinese markets. Int J Med Mushrooms 20(12):1149–1162
Zha LS, Xiao YP, Jeewon R, Zou X, Wang X, Boonmee S, Eungwanichayapant PD, McKenzie EHC, Kevin DH, Wen TC (2019) Notes on the medicinal mushroom Chanhua (Cordyceps cicadae (Miq.) Massee). Chiang Mai J Sci 46(6):1023–1035
Zhang YH, Zhuang WY (2004) Phylogenetic relationship of some members in the genus Hymenoscyphus (Ascomycetes, Helotiales). Nova Hedwigia 78:475–484
Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH (2006) An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia 98:1076–1087
Zhang Y, Schoch CL, Fournier J, Crous PW, De GJ, Woudenberg JHC, Hirayama K, Tanaka K, Pointing SB, Spatafora JW, Hyde KD (2009) Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang M, Li TH, Song B (2014) A new slender species of Aureoboletus from southern China. Mycotaxon 128:195–202
Zhang ZF, Liu F, Zhou X, Liu XZ, Liu SJ, Cai L (2017a) Culturable mycobiota from Karst caves in China, with descriptions of 20 new species. Persoonia 39:1–31
Zhang JB, Li JW, Li F, Qiu LH (2017b) Russula dinghuensis sp. nov. and R. subpallidirosea sp. nov., two new species from southern China supported by morphological and molecular evidence. Cryptogam Mycol 38:191–203
Zhang M, Li TH, Wang CQ, Zeng NK, Deng WQ (2019) Phylogenetic overview of Aureoboletus (Boletaceae, Boletales), with descriptions of six new species from China. MycoKeys 61:111–145
Zhang JF, Liu JK, Thambugala KM, Yang J, Meng ZH, Liu ZY (2020) Two new species and a new record of Nigrograna (Nigrogranaceae, Pleosporales) from China and Thailand. Mycol Prog 19:1365–1375
Zhang ZF, Zhou SY, Eurwilaichitr L, Ingsriswang S, Raza M, Chen Q, Zhao P, Liu F, Cai L (2021a) Culturable mycobiota from Karst caves in China II, with descriptions of 33 new species. Fungal Divers 106:29–136
Zhang X, Liang ZQ, Jiang S, Xu C, Fu XH, Zeng NK (2021b) Baorangia duplicatopora (Boletaceae, Boletales), a new bolete from tropical China. Phytotaxa 508:49–58
Zhao RL, Karunarathna SC, Raspé O, Parra LA, Guinberteau J, Moinard M, De Kesel A, Barroso G, Courtecuisse R, Hyde KD, Guelly AK, Desjardin ED, Callac P (2011) Major clades in tropical Agaricus. Fungal Divers 51:279–296
Zhao Q, Li YK, Zhu XT, Zhao YC, Liang JF (2015) Russula nigrovirens sp. nov. (Russulaceae) from southwestern China. Phytotaxa 236:249–256
Zhao YJ, Hosaka K, Hosoya T (2016a) Taxonomic re-evaluation of the genus Lambertella (Rutstroemiaceae, Helotiales) and allied stroma-forming fungi. Mycol Prog 15:1215–1228
Zhao RL, Zhou JL, Chen J, Margaritescu S, Sánchez-Ramírez S, Hyde KD, Callac P, Parra LA, Li GJ, Moncalvo JM (2016b) Towards standardizing taxonomic ranks using divergence times - a case study for reconstruction of the Agaricus taxonomic system. Fungal Divers 78:239–292
Zhao RL, Li GJ, Sánchez-Ramírez S, Stata M, Yang ZL, Wu G, Dai YC, He SH, Cui BK, Zhou JL, Wu F, He MQ, Moncalvo JM, Hyde KD (2017) A six-gene phylogenetic overview of Basidiomycota and allied phyla with estimated divergence times of higher taxa and a phyloproteomics perspective. Fungal Divers 84:43–74
Zhao YZ, Zhang ZF, Cai L, Peng WJ, Liu F (2018) Four new filamentous fungal species from newly-collected and hive-stored bee pollen. Mycosphere 9:1089–1116
Zhou XS, Dai YC (2008) A new species of Megasporoporia (Polyporales, Basidiomycota) from China. Mycol Prog 7:253–255
Zhou LW, Dai YC (2013) Taxonomy and phylogeny of wood-inhabiting hydnoid species in Russulales: two new genera, three new species and two new combinations. Mycologia 105:636–649
Zhou LW, May TW (2023) Fungal taxonomy: current status and research agendas for the interdisciplinary and globalisation era. Mycology 14:52–59
Zhou JL, Su SY, Su HY, Wang B, Callac P, Guinberteau J, Hyde KD, Zhao RL (2016a) A description of eleven new species of Agaricus sections Xanthodermatei and Hondenses collected from Tibet and the surrounding areas. Phytotaxa 257(2):99–121
Zhou LW, Vlasák J, Dai YC (2016b) Taxonomy and phylogeny of Phellinidium (Hymenochaetales, Basidiomycota): a redefinition and the segregation of Coniferiporia gen. nov. for forest pathogens. Fungal Biol 120:988–1001
Zhou LW, Vlasák J, Decock C, Assefa A, Stenlid J, Abate D, Wu SH, Dai YC (2016c) Global diversity and taxonomy of the Inonotus linteus complex (Hymenochaetales, Basidiomycota): Sanghuangporus gen. nov., Tropicoporus excentrodendri and T. guanacastensis gen. et spp. nov., and 17 new combinations. Fungal Divers 77:335–347
Zhou LW, Ji XH, Vlasák J, Dai YC (2018) Taxonomy and phylogeny of Pyrrhoderma: a redefinition, the segregation of Fulvoderma gen. nov. and four new species. Mycologia 110:872–889
Zhou M, Dai YC, Vlasák J, Yuan Y (2021) Molecular phylogeny and global diversity of the genus Haploporus (Polyporales, Basidiomycota). J Fungi 7:96
Zmitrovich IV (2018) Conspectus systematis Polyporacearum v. 1.0. Folia Cryptogamica Petropolitana 6:3–145
Acknowledgements
Li-Wei Zhou, Shi-Liang Liu and Xue-Wei Wang are supported by the National Key Research and Development Program of China (No. 2022YFC2601200), the National Natural Science Foundation of China (Nos. 31970012, 32100004, 32111530245 & 31770008), and the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment, China (No. 2019HJ2096001006). Guo-Jie Li and Chun-Ying Deng is supported by the Science and technology support project of Guizhou Province [(2019)2451-2], the Biodiversity Survey and Assessment Project of the Ministry of Ecology and Environment of China (2019HJ2096001006), the Science and technology project of Guizhou Province [(2019)4007], the National Natural Science Foundation of China (31500013, 30770013), and Talent Introduction Scientific Research Special Project of Hebei Agricultural University (YJ201849), the Earmarked Fund for Hebei Edible Fungi Innovation Team of Modern Agro-industry Technology Research System (Project ID: HBCT2018050205), and Special Foundation for Key Agricultural Generic Technology Development (16227303D). Water Rossi and Marco Leonardi wish to thank the entomologists who supplied them with arthropods bearing the new species of Laboulbeniales and/or identified the hosts: Petar Beron, Arnaldo Bordoni, Owen D. Seeman, Michael von Tschirnhaus; they also wish to thank S. Santamaria for the photographs of the fungi. Joe Ammirati is grateful to Museo Nacional de Costa Rica Costa Rica. San José and the New York Botanical Garden U.S.A. New York. Bronx. Mohamed A. Abdel-Wahab is grateful to JSPS for an award of a postdoctoral fellowship and the research grants No. 185701000001 and No. 18-06620. Matthew E. Smith was supported by the US National Science foundation grant DEB-2106130 and NIFA-USDA award FLA-PLP-005289. Ming Zhang acknowledges the financial support received from the Science and Technology Planning Project of Guangdong Forestry Bureau (LC-2021124). Enjing Tian was supported by National Natural Science Foundation of China (No. 31300017). Arun Kumar Dutta acknowledges support from the Department of Science & Technology (DST), New Delhi, India (DST/INSPIRE/04/2018/001906, dated 24 July, 2018). Ji Seon Kim and Young Woon Lim were supported by National Institute of Biological Resources (NIBR202203112). Alice Gerlach acknowledges the financial support received from CNPq (Brazil) and Swiss Government Excellence Scholarship that supported her PhD study and her yearlong stay in Switzerland (2015-2017). Parisa Razaghi and Mubashar Raza thank CAS President’s International Fellowship Initiative (PIFI) for funding his postdoctoral research (grant numbers. 2019PB0060 and 2020PB0115). Mark S. Calabon is grateful to the Mushroom Research Foundation and Department of Science and Technology-Science Education Institute (Philippines). E.B. Gareth Jones is supported under the Distinguished Scientist Fellowship Program (DSFP), King Saud University, Kingdom of Saudi Arabia. Rituparna Saha would like to express special gratitude to the University Grant Commission (UGC) for providing financial support. T. K. Arun Kumar, K. Krishnapriya and Anjitha Thomas thank the Chief Conservator of Forests & Chief Wildlife Warden, Kerala, for permission for fieldwork in the forest areas of Kerala. T. K. Arun Kumar, K. Krishnapriya and Anjitha Thomas thank the Chief Conservator of Forests & Chief Wildlife Warden, Kerala, for permission for fieldwork in the forest areas of Kerala. Malarvizhi Kaliyaperumal and Sugantha Gunaseelan would like to thank DST, SERB-EMR (EMR/2016/003078) for the financial assistance. Malarvizhi Kaliyaperumal and Kezhocuyi Kezo thank RUSA 2.0 (Theme-1 Group-1/2021/49) for providing grant. Malarvizhi Kaliyaperumal, Sugantha Gunaseelan and Kezhocuyi Kezo are grateful to PCCF of Tamil Nadu Forest Department for providing permission (E2/20458/2017), assistance and support during field visit in Eastern Ghats. S.K. Singh, P.N. Singh, A.C. Lagashetti, K.S. Pawar, S. Rana thank Director, MACS- Agharkar Research Institute, Pune for providing necessary facilities to carry out the research work. A.C. Lagashetti and K.S. Pawar thanks SPPU, Pune for providing admission to Ph.D. degree. CSIR, New Delhi and UGC, New Delhi are acknowledged for providing fellowships to ACL (SRF) and KSP (JRF), respectively. Shuhua Jiang and Chao Zhang are indebted to Q.X. Yang in the Fungarium-Lichenum of the Institute of Microbiology, Chinese Academy of Sciences (HMAS–L) for the loan of specimens, to X.L. Wei, R.D. Liu, X. Qian, Y.B. Zuo, and X.M. Cheng (Beijing, China) for help during field collections. Shuhua Jiang and Chao Zhang was funded by the National Natural Science Foundation of China (Project Nos. 31800010, 31750001). Huang Zhang and Yun Qing were mainly supported by National Natural Science Foundation of China (Project ID: NSF 31500017 to Huang Zhang). Ting-Chi Wen and Xing-Can Peng are supported by the National Natural Science Foundation of China (No. 32060012). Natalia A. Ramirez and Nicolás Niveiro acknowledge Secretaría General de Ciencia y Técnica of the Universidad Nacional del Nordeste (SGCyTUNNE- PI19P001), Agencia de Promoción Científica y Tecnológica (FONCYT-PICT2016-2529) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Natalia A. Ramirez and Nicolás Niveiro thank the Administración de Parques Nacionales and Ministerio de Ecología of the Misiones Province for the collection permits granted. Also, Natalia A. Ramirez and Nicolás Niveiro are grateful to the International Barcode of Life (IBOL) and the Canadian Centre for DNA Barcoding (CCDB) for the processing and sequencing of the samples. Gang Wu acknowledges the financial support received from Yunnan Young & Elite Talents Project (YNWR-QNBJ-2018-266). Entaj Tarafder and Krishnendu Acharya acknowledge the Department of Environment, Government of West Bengal, India for providing financial assistance. Danushka S. Tennakoon would like to thank the Department of Plant Medicine, National Chiayi University (NCYU), China for providing facilities for DNA molecular analysis and financial support. Jadson D.P. Bezerra and colleagues are grateful to the Conselho Nacional de Desenvolvimento Científico e Tecnológico (processes numbers CNPq 404989/2021-7, 159163/2021-8, 311187/2022-6, and 303939/2022-2), and to the Fundação de Amparo à Pesquisa do Estado de Goiás (process number FAPEG CAP2022061000159). Gang He was supported by the National Natural Science Foundation of China (Project No. 31860007 and 31900018), and the fund of National Science and Technology Basic Work Priorities Program of China (2013FY110400). Xiao-Hong Ji was supported by the National Natural Science Foundation of China (Project No. 32000014). Nian-Kai Zeng was supported by the Integrated Survey and Monitoring of Resources in Hainan Tropical Rainforest National Park (No. 1074688). Nakarin Suwannarach and Jaturong Kumla thank the Plant Genetic Conservation Project under the Royal initiative of Her Royal Highness Princess Maha Chakri Sirindhorn, Chiang Mai University, Thailand. Saisamorn Lumyong also thanks Chiang Mai University, Thailand for financial and facility support. Nopparat Wannathes is grateful to Pibulsongkram Rajabhat University, Thailand for facility support. K.D. Hyde thanks the National Research Council of Thailand (NRCT) grant “Total fungal diversity in a given forest area with implications towards species numbers, chemical diversity and biotechnology” (grant no. N42A650547).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no confict of interest.
Additional information
Handling Editor: Ruilin Zhao.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, SL., Wang, XW., Li, GJ. et al. Fungal diversity notes 1717–1817: taxonomic and phylogenetic contributions on genera and species of fungal taxa. Fungal Diversity 124, 1–216 (2024). https://doi.org/10.1007/s13225-023-00529-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-023-00529-0