Abstract
This is a continuity of a series of taxonomic papers where materials are examined, described and novel combinations are proposed where necessary to improve our traditional species concepts and provide updates on their classification. In addition to extensive morphological descriptions and appropriate asexual and sexual connections, DNA sequence data are also analysed from concatenated datasets (rDNA, TEF-α, RBP2 and β-Tubulin) to infer phylogenetic relationships and substantiate systematic position of taxa within appropriate ranks. Wherever new species or combinations are being proposed, we apply an integrative approach (morphological and molecular data as well as ecological features wherever applicable). Notes on 125 fungal taxa are compiled in this paper, including eight new genera, 101 new species, two new combinations, one neotype, four reference specimens, new host or distribution records for eight species and one alternative morphs. The new genera introduced in this paper are Alloarthopyrenia, Arundellina, Camarosporioides, Neomassaria, Neomassarina, Neotruncatella, Paracapsulospora and Pseudophaeosphaeria. The new species are Alfaria spartii, Alloarthopyrenia italica, Anthostomella ravenna, An. thailandica, Arthrinium paraphaeospermum, Arundellina typhae, Aspergillus koreanus, Asterina cynometrae, Bertiella ellipsoidea, Blastophorum aquaticum, Cainia globosa, Camarosporioides phragmitis, Ceramothyrium menglunense, Chaetosphaeronema achilleae, Chlamydotubeufia helicospora, Ciliochorella phanericola, Clavulinopsis aurantiaca, Colletotrichum insertae, Comoclathris italica, Coronophora myricoides, Cortinarius fulvescentoideus, Co. nymphatus, Co. pseudobulliardioides, Co. tenuifulvescens, Cunninghamella gigacellularis, Cyathus pyristriatus, Cytospora cotini, Dematiopleospora alliariae, De. cirsii, Diaporthe aseana, Di. garethjonesii, Distoseptispora multiseptata, Dis. tectonae, Dis. tectonigena, Dothiora buxi, Emericellopsis persica, Gloniopsis calami, Helicoma guttulatum, Helvella floriforma, H. oblongispora, Hermatomyces subiculosa, Juncaceicola italica, Lactarius dirkii, Lentithecium unicellulare, Le. voraginesporum, Leptosphaeria cirsii, Leptosphaeria irregularis, Leptospora galii, Le. thailandica, Lindgomyces pseudomadisonensis, Lophiotrema bambusae, Lo. fallopiae, Meliola citri-maximae, Minimelanolocus submersus, Montagnula cirsii, Mortierella fluviae, Muriphaeosphaeria ambrosiae, Neodidymelliopsis ranunculi, Neomassaria fabacearum, Neomassarina thailandica, Neomicrosphaeropsis cytisi, Neo. cytisinus, Neo. minima, Neopestalotiopsis cocoës, Neopestalotiopsis musae, Neoroussoella lenispora, Neotorula submersa, Neotruncatella endophytica, Nodulosphaeria italica, Occultibambusa aquatica, Oc. chiangraiensis, Ophiocordyceps hemisphaerica, Op. lacrimoidis, Paracapsulospora metroxyli, Pestalotiopsis sequoiae, Peziza fruticosa, Pleurotrema thailandica, Poaceicola arundinis, Polyporus mangshanensis, Pseudocoleophoma typhicola, Pseudodictyosporium thailandica, Pseudophaeosphaeria rubi, Purpureocillium sodanum, Ramariopsis atlantica, Rhodocybe griseoaurantia, Rh. indica, Rh. luteobrunnea, Russula indoalba, Ru. pseudoamoenicolor, Sporidesmium aquaticivaginatum, Sp. olivaceoconidium, Sp. pyriformatum, Stagonospora forlicesenensis, Stagonosporopsis centaureae, Terriera thailandica, Tremateia arundicola, Tr. guiyangensis, Trichomerium bambusae, Tubeufia hyalospora, Tu. roseohelicospora and Wojnowicia italica. New combinations are given for Hermatomyces mirum and Pallidocercospora thailandica. A neotype is proposed for Cortinarius fulvescens. Reference specimens are given for Aquaphila albicans, Leptospora rubella, Platychora ulmi and Meliola pseudosasae, while new host or distribution records are provided for Diaporthe eres, Di. siamensis, Di. foeniculina, Dothiorella iranica, Do. sarmentorum, Do. vidmadera, Helvella tinta and Vaginatispora fuckelii, with full taxonomic details. An asexual state is also reported for the first time in Neoacanthostigma septoconstrictum. This paper contributes to a more comprehensive update and improved identification of many ascomycetes and basiodiomycetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Table of contents
Ascomycota
Dothideomycetes
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
Asterinaceae Hansf.
367. Asterina cynometrae Hongsanan & K.D. Hyde, in Fungal Diversity 81: 12 (2016), new species
Botryosphaeriales C.L. Schoch et al.
Botryosphaeriaceae Theiss. & Syd.
368. Dothiorella iranica Abdollahz. et al., in Abdollahzadeh et al., Persoonia, Mol. Phyl. Evol. Fungi 32: 4 (2014), new host record, p 15
369. Dothiorella sarmentorum (Fr.) A.J.L. Phillips et al., Mycologia 97: 522. 2005, new host record, p 17
370. Dothiorella vidmadera W.M. Pitt et al., Fungal Diversity 61 (1): 216 (2013), new host record, p 17
Capnodiales Woron.
Mycosphaerellaceae Lindau
371. Pallidocercospora thailandica (Crous et al.) Phookamsak, Wulandari & K.D. Hyde, in Fungal Diversity 81: 21 (2016), new combination
Dothideales Lindau
Dothideaceae Chevall.
372. Dothiora buxi Jayasiri, Camporesi & K.D. Hyde, in Fungal Diversity 81: 30 (2016), new species
Hysteriales Lindau
Hysteriaceae Chevall.
373. Gloniopsis calami Konta. & K.D. Hyde, in Fungal Diversity 81: 34 (2016), new species
Dictyosporiaceae Boonmee & K.D. Hyde
374. Pseudocoleophoma typhicola E.B.G. Jones, Kamolhan, Boonmee & K.D. Hyde, in Fungal Diversity 81: 34 (2016), new species
375. Pseudodictyosporium thailandica C.G. Lin, Yong Wang bis & K.D. Hyde, in Fungal Diversity 81: 37 (2016), new species
Pleosporales Luttr. ex M.E. Barr
Didymellaceae Gruyter et al.
376. Neomicrosphaeropsis cytisi W.J. Li, Camporesi & K.D. Hyde, in Fungal Diversity 81: 38 (2016), new species
377. Neomicrosphaeropsis cytisinus Tennakoon, Camporesi & K.D. Hyde, in Fungal Diversity 81: 39 (2016), new species
378. Neomicrosphaeropsis minima W.J. Li, Camporesi & K.D. Hyde, in Fungal Diversity 81: 39 (2016), new species
379. Neodidymelliopsis ranunculi W.J. Li, Camporesi & K.D. Hyde, in Fungal Diversity 81: 41 (2016), new species
380. Platychora ulmi (J. Schröt.) Petr., Annls mycol. 23(1/2): 103 (1925), reference specimen, p 41
381. Stagonosporopsis centaureae Tennakoon, Camporesi & K.D. Hyde, in Fungal Diversity 81: 43 (2016), new species
Didymosphaeriaceae Munk
382. Montagnula cirsii Qing Tian, Camporesi & K.D. Hyde, in Fungal Diversity 81: 43 (2016), new species
383. Tremateia arundicola Wanasinghe, E.B.G. Jones & K.D. Hyde, in Fungal Diversity 81: 45 (2016), new species
384. Tremateia guiyangensis J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu in Fungal Diversity 81: 48 (2016), new species
Lentitheciaceae Y. Zhang et al.
385. Lentithecium unicellulare Abdel-Aziz, in Fungal Diversity 81: 53 (2016), new species
386. Lentithecium voraginesporum Abdel-Wahab, Bahkali & E.B.G. Jones, in Fungal Diversity 81: 53 (2016), new species
Leptosphaeriaceae M.E. Barr
387. Leptosphaeria cirsii Jayasiri, Camporesi & K.D. Hyde, in Fungal Diversity 81: 55 (2016), new species
388. Leptosphaeria irregularis R.H. Perera, E.B.G. Jones & K.D. Hyde, in Fungal Diversity 81: 59 (2016), new species
Lindgomycetaceae K. Hiray. et al.
389. Arundellina Wanasinghe, E.B.G. Jones & K.D. Hyde, in Fungal Diversity 81: 59 (2016), new genus
390. Arundellina typhae Wanasinghe, E.B.G. Jones & K.D. Hyde, in Fungal Diversity 81: 61 (2016), new species
391. Lindgomyces pseudomadisonensis Tak. Takah. & Kaz. Tanaka, in Fungal Diversity 81: 61 (2016), new species
Lophiostomataceae Sacc.
392. Vaginatispora fuckelii (Sacc.) Thambugala, Wanasinghe, Kaz. Tanaka & K.D. Hyde, Fungal Diversity 74: 242. 2015, new host record, p 62
Lophiotremataceae K. Hiray. & Kaz. Tanaka
393. Hermatomyces mirum (Starbäck) C.G. Lin, Yong Wang bis & K.D. Hyde, in Fungal Diversity 81: 69 (2016), new combination
394. Hermatomyces subiculosa C.G. Lin, Yong Wang bis & K.D. Hyde, in Fungal Diversity 81: 73 (2016), new species
395. Lophiotrema bambusae Phookamsak, S.C. Karunarathana & K.D. Hyde, in Fungal Diversity 81: 73 (2016), new species
396. Lophiotrema fallopiae A. Hashim. & Kaz. Tanaka, in Fungal Diversity 81: 74 (2016), new species
Massariaceae Nitschke
397. Neomassaria Mapook, Camporesi & K.D. Hyde, in Fungal Diversity 81: 77 (2016), new genus
398. Neomassaria fabacearum Mapook, Camporesi & K.D. Hyde, in Fungal Diversity 81: 77 (2016), new species
Massarinaceae Munk
399. Stagonospora forlicesenensis Phukhamsakda, Camporesi & K.D. Hyde, in Fungal Diversity 81: 77 (2016), new species
Melanommataceae G. Winter
400. Bertiella ellipsoidea Ekanayaka, Q. Zhao & K.D. Hyde, in Fungal Diversity 81: 79 (2016), new species
Occultabambusaceae Dai et al.
401. Occultibambusa aquatica Huang Zhang & K.D. Hyde, in Fungal Diversity 81: 81 (2016), new species
402. Occultibambusa chiangraiensis Phukhamsakda & K.D. Hyde, in Fungal Diversity 81: 81 (2016), new species
Phaeosphaeriaceae M.E. Barr
403. Camarosporioides W.J. Li & K.D. Hyde, in Fungal Diversity 81: 83 (2016), new genus
404. Camarosporioides phragmitis W.J. Li & K.D. Hyde, in Fungal Diversity 81: 85 (2016), new species
405. Chaetosphaeronema achilleae S.K. Huang & K.D. Hyde, in Fungal Diversity 81: 85 (2016), new species
406. Dematiopleospora alliariae Thambugala, Camporesi & K.D. Hyde, in Fungal Diversity 81: 89 (2016), new species
407. Dematiopleospora cirsii Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, in Fungal Diversity 81: 89 (2016), new species
408. Juncaceicola italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 81: 93 (2016), new species
409. Leptospora rubella (Pers.) Rabenh., Klotzschii Herb. Viv. Mycol., Edn 2: no. 532 (1857), reference specimen, p 93
410. Leptospora galii de Silva & K.D. Hyde, in Fungal Diversity 81: 96 (2016), new species
411. Leptospora thailandica Phukhamsakda & K.D. Hyde, in Fungal Diversity 81: 100 (2016), new species
412. Muriphaeosphaeria ambrosiae S.K. Huang & K.D. Hyde, in Fungal Diversity 81: 104 (2016), new species
413. Nodulosphaeria italica Phookamsak, Camporesi & K.D. Hyde, in Fungal Diversity 81: 106 (2016), new species
414. Poaceicola arundinis W.J. Li, Camporesi, D.J. Bhat & K.D. Hyde, in Fungal Diversity 81: 111 (2016), new species
415. Pseudophaeosphaeria Jayasiri, Camporesi & K.D. Hyde, in Fungal Diversity 81: 111 (2016), new genus
416. Pseudophaeosphaeria rubi Jayasiri, Camporesi & K.D. Hyde, in Fungal Diversity 81: 112 (2016), new species
417. Wojnowicia italica Qing Tian, Camporesi & K.D. Hyde, in Fungal Diversity 81: 112 (2016), new species
Pleosporaceae Nitschke
418. Comoclathris italica Tibpromma, Camporesi & K.D. Hyde, in Fungal Diversity 81: 117 (2016), new species
Roussoellaceae J.K. Liu et al.
419. Neoroussoella lenispora J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu, in Fungal Diversity 81: 119 (2016), new species
Torulaceae Corda
420. Neotorula submersa Z.L. Luo, H.Y. Su & K.D. Hyde, in Fungal Diversity 81: 121 (2016), new species
Tubeufiales Boonmee & K.D. Hyde
Tubeufiaceae M.E. Barr
421. Aquaphila albicans Goh, K.D. Hyde & W.H. Ho, Mycol. Res. 102(5): 588 (1998), reference specimen, p 121
422. Chlamydotubeufia helicospora Boonmee, Y. Z. Lu & K.D. Hyde, in Fungal Diversity 81: 123 (2016), new species
423. Helicoma guttulatum Y.Z. Lu, Boonmee & K.D. Hyde, in Fungal Diversity 81: 125 (2016), new species
424. Neoacanthostigma septoconstrictum (Promp. & A.N. Mill.) S. Boonmee & K.D. Hyde, Fungal Diversity 68(1): 279 (2014), reference specimen, 125
425. Tubeufia hyalospora Y.Z. Lu, Boonmee & K.D. Hyde, in Fungal Diversity 81: 126 (2016), new species
426. Tubeufia roseohelicospora Y.Z. Lu, Boonmee & K.D. Hyde, in Fungal Diversity 81: 128 (2016), new species
Dothideomycetes family, incertae sedis
Pleurotremataceae K.D. Hyde et al.
427. Pleurotrema thailandica Dayarathne, Jones E.B.G. & K.D. Hyde, in Fungal Diversity 81: 131 (2016), new species
Trypetheliaceae Eschw.
428. Alloarthopyrenia Phukhamsakda, Lücking & K.D. Hyde, in Fungal Diversity 81: 131 (2016), new genus
429. Alloarthopyrenia italica Phukhamsakda, Camporesi, Ariyawansa & K.D. Hyde, in Fungal Diversity 81: 135 (2016), new species
Pleosporales genera incetae sedis
430. Neomassarina Phookamsak & K.D. Hyde, in Fungal Diversity 81: 136 (2016), new genus
431. Neomassarina thailandica Phookamsak & K.D. Hyde, in Fungal Diversity 81: 138 (2016), new species
Eurotiomycetes
Eurotiales G.W. Martin ex Benny & Kimbr.
432. Aspergillus koreanus Hyang B. Lee, T.T. Duong & T.T.T. Nguyen, in Fungal Diversity 81: 142 (2016), new species
Chaetothyriales M.E. Barr
Chaetothyriaceae Hansf. ex M.E. Barr
433. Ceramothyrium menglunense Mapook, J.F. Li & K.D. Hyde, in Fungal Diversity 81: 142 (2016), new species
Herpotrichiellaceae
434. Minimelanolocus submersus Z.L. Luo, H.Y. Su & K.D. Hyde, in Fungal Diversity 81: 143 (2016), new species
Trichomeriaceae Chomnunti & K.D. Hyde
435. Trichomerium bambusae Hongsanan & K.D. Hyde, in Fungal Diversity 81: 145 (2016), new species
Leotiomycetes
Rhytismatales M.E. Barr ex Minter
Rhytismataceae Chevall.
436. Terriera thailandica Jayasiri & K.D. Hyde, in Fungal Diversity 81: 146 (2016), new species
Pezizomycetes
Pezialaes J. Schrot.
Helvelaceae Fr.
437. Helvella tinta Q. Zhao, B. Feng & K.D. Hyde, in Fungal Diversity 81: 149 (2016), new species
438. Helvella floriforma Q. Zhao & K.D. Hyde, in Fungal Diversity 81: 154 (2016), new species
439. Helvella oblongispora Harmaja, Karstenia 18(2): 57 (1978), new distribution record, p 157
Pezizaceae Dumort.
440. Peziza fruticosa Lantieri, Medardi & Vizzini, in Fungal Diversity 81: 157 (2016), new species
Sordariomycetes
Coronophorales Nannf.
Coronophoraceae Höhn.
441. Coronophora myricoides H.X. Wu & K.D. Hyde, in Fungal Diversity 81: 164 (2016), new species
Diaporthales Nannf.
Diaporthaceae Höhn. ex Wehm.
Diaporthe Nitschke
442. Diaporthe aseana Dissanayake, Tangthirasunun & K.D. Hyde, in Fungal Diversity 81: 167 (2016), new species
443. Diaporthe eres Nitschke, Pyrenomycetes Germanici 2: 245 (1870), new host record, 167
444. Diaporthe foeniculina Niessl, in von Thümen, Contr. Ad. Fl. Myc. Lusit. 2: 30. 1880. new record, p 169
445. Diaporthe garethjonesii Dissanayake, Tangthirasunun & K.D. Hyde, in Fungal Diversity 81: 171 (2016), new species
446. Diaporthe siamensis Udayanga et al., Cryptogamie Mycologie 33: 298 (2012), new host record, p 171
Valsaceae Tul. & C. Tul.
447. Cytospora cotini Norphanphoun, Bulgakov & K.D. Hyde, in Fungal Diversity 81: 176 (2016), new species
Glomerellales Chadef. ex Réblová et al.
Glomerellaceae Locq.
448. Colletotrichum insertae Jayawardena, Bulgakov & K.D. Hyde, in Fungal Diversity 81: 176 (2016), new species
Reticulascaceae Réblová & W. Gams
449. Blastophorum aquaticum Z.L. Luo, Bhat, H.Y. Su & K.D. Hyde, in Fungal Diversity 81: 177 (2016), new species
Hypocreales Lindau
Ophiocordycipitaceae G.H. Sung et al.
450. Ophiocordyceps hemisphaerica Mafalda-Freire, Reck & Drechsler-Santos, in Fungal Diversity 81: 181 (2016), new species
451. Ophiocordyceps lacrimoidis Mafalda-Freire, Reck & Drechsler-Santos, in Fungal Diversity 81: 186 (2016), new species
452. Purpureocillium sodanum Papizadeh, Soudi, Wijayaw., Shahz.-Faz. & K.D. Hyde, in Fungal Diversity 81: 186 (2016), new species
Hypocreales genus incertae sedis
453. Alfaria spartii Senan., Camporesi & K.D. Hyde, in Fungal Diversity 81: 187 (2016), new species
Bionectriaceae Samuels & Rossman
454. Emericellopsis persica Papizadeh, Wijayaw, Soudi & K.D. Hyde, in Fungal Diversity 81: 191 (2016), new species
Meliolales Gäum. ex D. Hawksw. & O.E. Erikss.
Meliolaceae G.W. Martin ex Hansf.
455. Meliola citri-maximae X.Y. Zeng, K.D. Hyde & T.C. Wen, in Fungal Diversity 81: 192 (2016), new species
456. Meliola pseudosasae I. Hino, Bull. Faculty of Agriculture, Yamaguchi University 9: 882 (1958), reference specimen, p 196
Xylariales Nannf.
Apiosporaceae K.D. Hyde et al.
457. Arthrinium paraphaeospermum Senan. & K.D. Hyde, in Fungal Diversity 81: 198 (2016), new species
Bartaliniaceae Wijayaw. et al.
458. Neotruncatella Hyang B. Lee & T.T.T. Nguyen, in Fungal Diversity 81: 198 (2016), new genus
459. Neotruncatella endophytica Hyang B. Lee, P.M. Kirk, K.D. Hyde, S.S.N. Maharachch, & T.T.T. Nguyen, in Fungal Diversity 81: 198 (2016), new species
Cainiaceae J.C. Krug
460. Cainia globosa Senan., Camporesi & K.D. Hyde, in Fungal Diversity 81: 201 (2016), new species
Pestalotiopsidaceae Maharachch. & K.D. Hyde
461. Ciliochorella phanericola Norphanphoun, T.C. Wen & K.D. Hyde, in Fungal Diversity 81: 207 (2016), new species
462. Neopestalotiopsis cocoës Norphanphoun, T.C. Wen & K.D. Hyde, in Fungal Diversity 81: 207 (2016), new species
463. Neopestalotiopsis musae Norphanphoun, T.C. Wen & K.D. Hyde, in Fungal Diversity 81: 209 (2016)
464. Pestalotiopsis sequoiae W.J. Li, Camporesi & K.D. Hyde, in Fungal Diversity 81: 210 (2016), new species
Xylariaceae Tul. & C. Tul.
465. Anthostomella ravennica Daranagama, Camporesi & K. D. Hyde, in Fungal Diversity 81: 210 (2016), new species
466. Anthostomella thailandica Daranagama & K.D. Hyde, in Fungal Diversity 81: 213 (2016)
Sordariomycetes incertae sedis
Sporidesmiaceae Fr.
467. Sporidesmium pyriformatum J. Yang & K.D. Hyde, in Fungal Diversity 81: 215 (2016), new species
468. Sporidesmium aquaticivaginatum J. Yang & K.D. Hyde, in Fungal Diversity 81: 217 (2016), new species
469. Sporidesmium olivaceoconidium J. Yang & K.D. Hyde, in Fungal Diversity 81: 220 (2016), new species
Distoseptisporaceae Su et al.
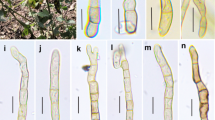
470. Distoseptispora multiseptata J. Yang & K.D. Hyde, in Fungal Diversity 81: 220 (2016), new species
471. Distoseptispora tectonae Doilom & K.D. Hyde, in Fungal Diversity 81: 222 (2016), new species
472. Distoseptispora tectonigena Doilom & K.D. Hyde, in Fungal Diversity 81: 222 (2016), new species
Sordariomycetidae, Incertae sedis
473. Paracapsulospora Konta & K.D. Hyde, in Fungal Diversity 81: 223 (2016), new genus
474. Paracapsulospora metroxyli Konta & K.D. Hyde, in Fungal Diversity 81: 223 (2016), new species
Basidiomycota
Agaricomycetes
Agaricales Underw.
Clavariaceae Chevall.
475. Clavulinopsis aurantiaca Araujo-Neta, Silva & Gibertoni, in Fungal Diversity 81: 225 (2016), new species
476. Ramariopsis atlantica Araujo-Neta, Silva & Gibertoni, in Fungal Diversity 81: 226 (2016), new species
Cortinariaceae R. Heim ex Pouzar
477. Cortinarius fulvescens Fr., Epicr. syst. mycol. (Upsaliae): 311 (1838), neotype, 227
478. Cortinarius fulvescentoideus Kytöv., Niskanen & Liimat., in Fungal Diversity 81: 230 (2016), new species
479. Cortinarius nymphatus Kytöv., Niskanen, Liimat. & Bojantchev, in Fungal Diversity 81: 230 (2016), new species
480. Cortinarius pseudobulliardioides Kytöv., Niskanen, Liimat. & Ammirati, in Fungal Diversity 81: 232 (2016), new species
481. Cortinarius tenuifulvescens Kytöv., Niskanen & Liimat., in Fungal Diversity 81: 232 (2016), new species
Entolomataceae Kotl. & Pouzar
482. Rhodocybe indica K.N.A. Raj & Manim., in Fungal Diversity 81: 236 (2016), new species
483. Rhodocybe luteobrunnea K.N.A. Raj & Manim., in Fungal Diversity 81: 241 (2016), new species
484. Rhodocybe griseoaurantia K.N.A. Raj & Manim., in Fungal Diversity 81: 242 (2016), new species
Agaricaceae Chevall.
485. Cyathus pyristriatus B. Thongbai, C. Richt. & M. Stadler, in Fungal Diversity 81: 244 (2016), new species
Polyporales Gäum.
Polyporaceae Fr. ex Corda
486. Polyporus mangshanensis B.K. Cui, J.L. Zhou & Y.C. Dai, in Fungal Diversity 81: 249 (2016), new species
Russulales Kreisel ex P.M. Kirk et al.
Russulaceae Lotsy
487. Russula indoalba A. Ghosh, Buyck, A. Baghela, K. Das & R.P. Bhatt, in Fungal Diversity 81: 250 (2016), new species
488. Russula pseudoamoenicolor A. Ghosh, Buyck, K. Das, A. Baghela & R.P. Bhatt, in Fungal Diversity 81: 251 (2016)
489. Lactarius dirkii Uniyal, K. Das, A. Baghela & R.P. Bhatt, in Fungal Diversity 81: 252 (2016), new species
Mortierellomycotina Kerst. Hoffm. et al.
Mortierellales Caval.-Sm.
Mortierellaceae A. Fisch.
Mortierella fluviae Hyang B. Lee, K. Voigt & T.T.T. Nguyen, in Fungal Diversity 81: 254 (2016), new species
Mucorales Dumort.
Cunninghamellaceae Naumov ex R.K. Benj.
490. Cunninghamella gigacellularis A.L. Santiago, C.L. Lima & C.A. de Souza, in Fungal Diversity 81: 255 (2016), new species
Materials and methods
Sampling, isolation and identification
Specimens from a number of plants examined in this study were collected from at least 15 countries around the world, including Brazil, China, Egypt, Germany, Iran, India, Italy, Japan, Philippines, Republic of Korea, Russia, Saudi Arabia, Thailand and the UK. Soil samples collected from the Atlantic rainforest in Recife, Brazil to isolate Cunninghamella gigacellularis followed methods outlined by Benny (2008). The color designation of C. gigacellularis colonies was established according to Maerz and Paul (1950). Samples were observed under the microscope; macro-morphological (e.g. from basidiomycetes) and micro-morphological characters (e.g. ascomata sections, peridium structures, asci and ascospores) were examined, described and photographed. Most measurements (e.g. from basidiospores and ascospores) were taken from at least 20 representatives and both the mean and the standard deviation for both the length and the width, together with the range of spore quotient (Q, the length/width ratio) and its mean value (Qm) are given. Axenic cultures (following single spore isolations) and specimens with duplicates have been deposited in Culture Collections and Herbaria where appropriate and accession numbers are provided in the taxonomic descriptions. Faces of Fungi and Index Fungorum numbers are given as outlined by Jayasiri et al. (2015) and Index Fungorum (2016). Wherever possible, appropriate techniques were employed to induce the formation of the asexual morph in culture using sterilized pieces of plant materials. Colony characters were observed and recorded. We have tried to maintain consistency in terminology, however this may not always be possible as different authors prefer to use different terms. For example the use of ascostromata is variable and often difficult to interpretate and is left to each author’s discretion.
DNA extraction, PCR amplification and sequencing
For most fungal samples (especially ascomycetes), total genomic DNA was extracted from fresh fungal mycelium grown on appropriate media agar at room temperature with appropriate Genomic DNA Extraction Kit purchased from countries where samples were collected following manufacturer’s instructions. Under circumstances where fungi fail to grow in culture, DNA was extracted directly from fruiting bodies using aseptic techniques. The procedure described by Izumitsu et al. (2012) was employed for extracting genomic DNA from dried Rhodocybe specimens. For Cunninghamella isolates, genomic DNA extraction was carried out with macerated material according to Góes-Neto et al. (2005).
DNA amplification for most samples was performed by polymerase chain reaction (PCR) using universally standard primers such as LROR and LR5 (Vilgalys and Hester 1990) for the partial large subunit nuclear rDNA (28S, LSU); NS1 and NS4 (White et al. 1990) for the small subunit nuclear rDNA (18S, SSU); ITS4 and ITS5 (White et al. 1990) for the internal transcribed spacers (5.8S, ITS); EF1-983F and EF1-2218R (Rehner 2001) for the translation elongation factor 1-alpha gene (TEF1α); and fRPB2-5F and fRPB2-7cR (Liu et al. 1999) for the partial RNA polymerase second largest subunit (RPB2). Primer pair LR1/LSU2 were used to amplify LSU region of Cunninghamella isolates (van Tuinen et al. 1998) while LROR/LR7 for (Vilgalys and Hester 1990) and b6Fb7.1R (Matheny 2005) for RPB2 region were used for Rhodocybe specimens. Thermal cycle program for ITS, LSU and TEF1α amplification were as follows: initial 94 °C for 3 mins, followed by 35 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 50 s, elongation at 72 °C for 1 mins, and final extension at 72 °C for 10 mins. For ascomycetes, the thermal cycle program for RPB2 was as follows: initial 95 °C for 5 mins, followed by 40 cycles of denaturation at 95 °C for 1 mins, annealing at 52 °C for 2 mins, elongation at 72 °C for 90 s, and final extension at 72 °C for 10 mins. Thermal cycle program for Cunninghamella isolates were follows: 95 °C for 5 mins, followed by 39 cycles of denaturation at 94 °C for 45 s, annealing at 60 °C for 1 min, elongation at 72 °C for 1 min, and final extension at 72 °C for 7 mins. For Rhodocybe specimens, thermal profiling and amplification reactions of ITS, nLSU and RPB2 regions were performed following Latha et al. (2015). Prior to sequencing, quality of PCR amplicons were checked and purified with appropriate purification kits as per manufacturer’s guidelines before being subjected to automated DNA sequencing using the same primers used for PCR.
Sequence alignment and phylogenetic analyses
A careful verification of all sequences obtained were done especially with appropriate reference sequences following a Blast search in GenBank to ensure that no erroneous sequences are used in further analyses and then submitted to GenBank. Following sequence verification and Blast search, DNA sequences from appropriate taxonomic ranks were downloaded to construct datasets for phylogenetic analyses. BioEdit sequence alignment editor (Hall 1999), AliView v.1.17 (Larsson 2014), CLUSTALX (Larkin et al. 2007), Mega 6.0.5 (Tamura et al. 2013) and MAFFT: multiple sequence alignment software version 7.215 (Katoh et al. 2002) were used for alignment purposes. Under most circumstances, concatenated DNA datasets are analyzed to generate gene trees but in cases of limited availability of DNA sequences from respective gene regions, phylogenies are inferred from single or two genes datasets and either a consensus of these gene phylogenies or one of the most parsimonious phylogeny is used to infer phylogenetic relationships across taxa sampled. Selection of outgroup (s) for rooting purposes was based on knowledge of potential common ancestor to our in-group as well as taxon sampling from previously published studies. Phylogenetic analyses were performed by maximum parsimony (MP), maximum likelihood (RAxML) and Bayesian inference (BI) analyses.
A maximum parsimony (MP) analysis was performed with stepwise additions of sequences by using PAUP v. 4.0b10 (Swofford 2002). The heuristic search option with 1000 random sequences addition and tree-bisection reconnection (TBR) of branch-swapping algorithm were performed. Maxtrees were setup at 5000, branches of zero length were collapsed and gaps were treated as missing data. Tree length (TL), consistency index (CI), retention index (RI), rescaled consistency index (RC) and homoplasy index (HI) were calculated for trees generated under different optimality criteria. The robustness of the most parsimonious trees was evaluated by 1000 bootstrap replications resulting from maximum parsimony analysis with each 100 replicates of random stepwise addition of taxa (Liu et al. 2011, 2012).
Maximum likelihood (ML) analysis was performed using RAxML v.8.0.26 (Stamatakis 2014) with 1000 rapid ML bootstrap replicates. The available substitution models comprised a generalized time reversible (GTR) for nucleotides was applied with a discrete gamma distribution (Silvestro and Michalak 2012). A discrete GAMMA (Yang 1994) was complemented for each substitution model. Rapid bootstrap analysis (Stamatakis et al. 2008) and search for a best-scoring ML tree were applied (Silvestro and Michalak 2012). The parameters were followed; run mode = ML + rapid bootstrap, replicates = 1000, BS brL = selected and Model = GTRGAMMA.
Bayesian analysis was performed by MrBayes v. 3.0b4 (Ronquist and Huelsenbeck 2003) with the best-fit model of sequence evolution estimated with MrModeltest 2.2 (Nylander et al. 2008). Markov Chain Monte Carlo sampling (BMCMC) was used to determine the posterior probabilities (PP) (Rannala and Yang 1996; Zhaxybayeva and Gogarten 2002) in MrBayes v. 3.0b4 (Huelsenbeck and Ronquist 2001). Six simultaneous Markov chains were run for 1,000,000 generations sampling one tree every 100th generations of trees (resulting 10,001 total trees). The burn-in (first 2000 trees) which represented the phase of the analysis were discarded and the remaining 8000 trees were used to build a majority rule consensus tree (Liu et al. 2011, 2012) with posterior probabilities (PP).
Phylograms were visualized in Treeview (Page 2001) or FigTree 1.4.2 (Rambaut 2014) with bootstrap values above or below the nodes. All the sequences generated in this study have been deposited in GenBank and accession numbers provided where appropriate.
Dothideomycetes
We follow Hyde et al. (2013) and Wijayawardene et al. (2014a) for the latest arrangement of this class.
Asterinales M.E. Barr ex D. Hawksw. & O.E. Erikss.
The order was monographed by Hongsanan et al. (2014). Guatimosim et al. (2015) provided sequence data for Asterina and Parmularia directly from ascomata, but did not include other sequence data used in Hofmann (2009). Ertz and Diederich (2015) provided sequence data for taxa of Melaspileaceae and placed them in Asterinales based on their phylogenetic data. However, Ertz et al. (2016) indicated that Asterina species are segregated in two unrelated clades. Asterotexis cucurbitacearum and Inocyclus angularia (Parmulariaceae) clustered with strains of Asterina provided by Hofmann (2009) and Hongsanan et al. (2014), and formed a sister group to Melaspileaceae (Ertz et al. 2016). Thus, these Asterinales strains were considered to represent the order Asterotexiales based on the type species of Asterotexis cucurbitacearum (Ertz et al. 2016). In the present study we treat the main Asterinales clade, which includes Asterotexis cucurbitacearum, as Asterinales sensu stricto because most of the Asterinales strains, from both Asterina and Lembosia cluster in this clade and also because the large clade supporting Asterinales as circumscribed by Ertz et al. (2016) does not have any phylogenetic support. We therefore synonymize the younger Asterotexiales (in December, 2015) under Asterinales (in 1986). It is questionable that the other clade, which contains the putatively named, type species of Asterina is actually Asterinales as most strains in this order cluster in Asterinales sensu stricto. It may be that DNA was amplified from other taxa in the black mildew colonies. Since Parmularia represents a distinct monophyletic clade with high support outside Asterinales sensu stricto, we reinstate Parmulariaceae to accommodate this clade (Fig. 1).
Phylogram generated from maximum likelihood and Bayesian analyses based on LSU sequence data from species of Asterinales and Asterotexales. The first set of numbers above the nodes are RAxML bootstrap value expressed from 1000 repetitions with values above 50 % shown. The second set of numbers above the nodes are Bayesian posterior probabilities, with values above 0.85 shown. The new isolates are in blue bold and other ex-type strains are in bold. The tree is rooted with Capronia munkii Unter
Asterinaceae Hansf.
The family Asterinaceae was established in Myriangiales by Hansford (1946). Several studies placed Asterinaceae in an uncertain position in the Dothideomycetes incertae sedis (Cannon and Kirk 2007; Kirk et al. 2008). Phylogenies of Hongsanan et al. (2014) place Asterinaceae within Asterinales in Dothideomycetes. They also accepted 17 genera in the family based mainly on morphology. In this paper, we introduce a new species, Asterina cynometrae with morphological details and molecular data (Figs. 1, 2).
Asterina cynometrae (holotype). a Appearance of thyriothecia on leaf. b, c Thyriothecia with star-like opening when viewed in squash mounts. d Superficial hyphae with hyphopodia. e Upper wall of thyriothecium, f Ascus in Melzer’s reagent. g Ascus at maturity. h, i Ascospores. Scale bars b, c = 100 μm, d, e = 20 μm, f–i = 10 μm
Asterina Lév.
The genus Asterina is the type genus of the family Asterinaceae which was introduced by Léveillé (1845). Asterina is the largest genus in Asterinaceae, and has a worldwide distribution in tropical and subtropical regions (Hongsanan et al. 2014). According to Ertz et al. (2016) Asterina species cluster in two well-supported distinct clades, which are placed in the order Asterinales and in the incertae sedis clade in this study (Fig. 1).
Asterina cynometrae Hongsanan & K.D. Hyde, sp. nov.
Index Fungorum number: IF552216; Facesoffungi number: FoF02430, Fig. 2
Holotype: MFLU 13-0373.
Epiphytes on the upper surface of leaves. Superficial hyphae branched, septate, darker at the septum, brown, with hyphopodia. Hyphopodia 11–13 μm high × 4–8 μm wide (\( \bar{x} \) = 12 × 6 μm, n = 20) capitate, alternate, rarely opposite on hyphae, near to hyphal septum, 1-celled, with 2 branches at the apex, brown. Sexual morph Thyriothecia 150–200 μm diam. (\( \bar{x} \) = 160 μm, n = 10), superficial on the surface of host, solitary to gregarious, circular, flattened, with star-like opening, sometimes variously shaped. Upper wall comprising parallel arrangement of cells radiating from the center, base poorly developed. Hamathecium not observed. Asci up to 40 μm diam., vertically arranged within thyriothecium cavity, 6-spored, bitunicate, fissitunicate, subglobose to globose, short pedicellate, without an ocular chamber, with evanescent wall. Ascospores 18–22 μm × 10–12 μm (\( \bar{x} \) = 20 × 11 μm, n = 15), 2–3-seriate, oblong, hyaline to dark brown, uniseptate at the middle, constricted and with dark band at the septum, smooth-walled, ends rounded. Asexual morph Undetermined.
Material examined: PHILIPPINES, Luzon, Laguna Province, Mount Makiling, on living leaves of Cynometra sp. (Fabaceae), February 2012, Pamela Alva (MFLU 13-0373, holotype).
Notes: Asterina cynometrae was collected on living leaves of Cynometra sp. (Fabaceae) and is most similar to A. trachycarpa Syd. & P. Syd. in the shape and size of ascospores, the latter species being found on Derris atro-violacea Elmer (Fabaceae) in the Philippines. However, it differs from A. trachycarpa in having a thick and dark band at the septum of ascospores, with 2–3 branching hyphopodia. We therefore introduce A. cynometrae as a new species based on host and morphology (Fig. 2). Furthermore, we place our new species within Asterinales based on the phylogeny of LSU sequence data (Fig. 1). As we were unable to isolate the new taxon because of its obligate parasitic habitat, sequence data was prepared directly from thyriothecia and ascospores.
Botryosphaeriales C.L. Schoch et al.
The order Botryosphaeriales has undergone significant taxonomic changes during the past decade by addition of several new families. Currently, there are seven families, Aplosporellaceae, Botryosphaeriaceae, Melanopsaceae, Planistromellaceae, Phyllostictaceae, Saccharataceae and Septorioideaceae (Schoch et al. 2006; Minnis et al. 2012; Wikee et al. 2013; Slippers et al. 2013; Wyka and Broders 2016) and several new genera (Liu et al. 2012; Crous et al. 2015b). Botryosphaeriales is a diverse order with a worldwide distribution, comprising species that vary from endophytes to pathogens (Slippers and Wingfield 2007) and occurring on a wide range of monocotyledonous, dicotyledonous, and gymnosperm hosts (Liu et al. 2012; Crous et al. 2015b) and lichens (Barr 1987; von Arx 1987). Many are considered as pathogens that cause disease on a wide range of economically and ecologically significant plants (Slippers et al. 2013).
Dothiorella Sacc.
The species in the genus Dothiorella (Botryosphaeriaceae, Botryosphaeriales, Dothideomycetes) is characterized based on conidia that become pigmented and 1-septate while they are still attached to the conidiogenous cells (Phillips et al. 2013). Due to wide host ranges and morphological plasticity, identification of species in this genus is almost impossible without the support of molecular data (Slippers et al. 2013). We provide an updated tree for the genus (Fig. 3).
One of 309 most parsimonious trees obtained from combined ITS and EF-1α sequence data, for all ex-types from species in Dothiorella. Isolate numbers of new host records are in blue. Maximum parsimony bootstrap values (>70 %) and Bayesian inference values (>0.9) are given on the nodes. The tree is rooted with Spencermartinsia viticola
Dothiorella iranica Abdollahz. et al., in Abdollahzadeh et al., Persoonia, Mol. Phyl. Evol. Fungi 32: 4 (2014)
Facesoffungi number: FoF02202, Fig. 4
Saprobic on Paliurus bark. Asexual morph Conidiomata 280–305 μm high, 275–310 μm diam. (\( \bar{x} \) = 290 × 295 μm, n = 5), acervular, solitary to gregarious, superficial to immersed, unilocular, globose to subglobose, dark brown to black. Conidiomata wall 30–40 μm wide, composed of brown, thin or thick-walled cells of textura angularis, apex and base thicker than middle, with setae. Conidiophores 7–9 × 2–3 μm (\( \bar{x} \) = 8 × 2.5 μm, n = 10), cylindrical, filiform, septate, branched, hyaline. Conidiogenous cells 9–17 × 4–6 μm, (\( \bar{x} \) = 12 × 5 μm, n = 20), holoblastic, annellidic, integrated or discrete, hyaline, determinate. Conidia 20–25 × 8–11 μm (\( \bar{x} \) = 22 × 9 μm, n = 30), cylindrical, oval or ellipsoid, 1-septate, hyaline when immature, brown to dark brown when mature. Sexual morph Undetermined.
Culture characteristics: Ascospores germinating on MEA within 36 h. Colonies growing on MEA attaining 2 cm diam. in 1 week at 28 °C. Mycelium superficial, felted, gummy, dark brown to black. Asexual structures not formed in culture.
Material examined: ITALY, Province of Forlì-Cesena, Monte Pallareto—Meldola, on Paliurus bark (Rhamnaceae), 1 January 2012, Erio Camporesi IT 962 (MFLU 15-1402, KUN, new host record), living culture, MFLUCC 15-0656, KUNCC
Notes: Phylogenetically this species resides in a distinct subclade in Dothiorella with high support and closely related to D. ulmacea (Fig. 3). The conidia of D. iranica are longer than those of all other Dothiorella species except D. casuarini J. de Wet et al. (27 × 11 μm). Our isolate is morphologically and phylogenetically similar to Dothiorella iranica (strain IRAN 1587C), but associated with a different host. Dothiorella iranica (type) was recorded on Olea europaea L. (Oleaceae) and our collection was found on Paliurus (Rhamnaceae) bark. Host distribution is poorly known in this genus, but according to Dissanayake et al. (2016), Dothiorella species may be host specific, although Dothiorella iberica A.J.L. Phillips et al., D. sarmentorum (Fr.) A.J.L. Phillips et al. and D. symphoricarposicola W.J. Li occur on many host families and orders.
Dothiorella sarmentorum (Fr.) A.J.L. Phillips, J. Luque & A. Alves, Mycologia 97: 522. 2005
≡ Sphaeria sarmentorum Fr., K. svenska Vetensk-Acad. Handl. 39: 107. 1818.
≡ Diplodia sarmentorum (Fr.) Fr., Summ. veg. Scand. (Stockholm) 2: 417. 1849.
= Botryosphaeria sarmentorum A.J.L. Phillips, J. Luque & A. Alves, Mycologia 97: 522. 2005.
Facesoffungi number: FoF02148, Fig. 5
Saprobic on Celtis occidentalis L. Sexual morph Undetermined. Asexual morph Conidiomata 140–240 μm high × 175–300 μm diam. (\( \bar{x} \) = 230 × 180 μm, n = 10), stromatic, solitary or scattered in small groups, immersed, uni or biloculate, black, globose to subglobose, ostiolate. Conidiomatal wall 25–45 μm (\( \bar{x} \) = 35 μm, n = 15), comprising several layers; outer layers comprising thick-walled, dark brown, somewhat flattened cells of textura angularis and inner layers of larger, thin-walled, lightly pigmented or hyaline cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 6–12 × 2–4 μm (\( \bar{x} \) = 8.6 × 3.3 μm, n = 15), lining the conidiomatal cavity, holoblastic, hyaline, subcylindrical, proliferating at the same level giving rise to periclinal thickenings. Conidia 17.8–22.4 × 8.3–11 μm (\( \bar{x} \) = 20.2 × 9.9 μm, n = 30), ovoid, with a broadly rounded apex and truncate base, initially hyaline to lightly pigmented and aseptate, becoming dark brown and 1-septate, slightly constricted at the septum, smooth-walled.
Culture characteristics: Conidia germinating on PDA within 18 h and germ tubes produced from one end or both cells. Colonies on PDA at 25 °C, covering 9 cm Petri-dishes in few days, circular, flat, dense, surface initially white, becoming grey with reverse black, smooth surface with entire to slightly undulate edge.
Material examined: RUSSIA, Rostov Region, Shakhty City, former Shakhty forestry, on Celtis occidentalis L. (Cannabaceae), 26 February 2014, Timur Bulgakov T 35 (MFLU 16-1008, new host record), living culture MFLUCC 15-0443. RUSSIA, Rostov region, Shakhty city, Central Park (47.7055886°E, 40.2059913°N), on Morus alba (Moraceae), 12 March 2014, Timur S. Bulgakov T 07 (MFLU 16-1274, new host record), living culture, MFLUCC 14-0889, MUCL.
Notes: Dothiorella sarmentorum was introduced by Phillips et al. (2005) based on the asexual morph of Botryosphaeria sarmentorum A.J.L. Phillips et al. This species has been recorded from 34 different host species (Phillips et al. 2005, 2013). The sexual morph of D. sarmentorum is characterized by partially erumpent ascomata with papillate ostiole, 4–6(–8)-spored asci and oblong to ovate, (0–)1-septate, finely verruculose ascospores, which are widest in the middle part (Phillips et al. 2013). As the morphological information does not provide any clear difference, we would like to report our collections as new host records of D. sarmentorum. This is the first report of Dothiorella sarmentorum on Celtis occidentalis and Morus alba from Russia. ITS and EF-1α based phylogenies also depict that all D. sarmentorum isolates cluster together and is phylogenetically related to D. americana (Fig. 3)
Dothiorella vidmadera W.M. Pitt et al., Fungal Diversity 61: 216 (2013)
Facesoffungi number: FoF02206, Fig. 6
Dothiorella vidmadera (MFLU 16-1273). a Robinia pseudoacacia with fungus. b Conidiomata on host substrate. c Vertical section through a conidioma. d Peridium of conidioma. e Conidium attached to conidiogenous cell. f–i Mature and immature conidia. Scale bars b = 500 μm, c = 100 μm, d = 20 μm, e–i = 10 μm
Saprobic or weak pathogenic on twigs of Robinia pseudoacacia L. Sexual morph Undetermined. Asexual morph Conidiomata 240–280 μm high × 280–320 μm diam. (\( \bar{x} \) = 263 × 298 μm, n = 10), pycnidial, stromatic, mostly solitary, semi-immersed to immersed in the host, globose, dark brown to black, ostiolate, apapillate. Peridium 20–30 μm wide at the base, 25–35 μm wide at the side, comprising 5–6 layers, heavily pigmented, thick-walled, blackish to dark brown, angular cells, becoming flattened towards the outer layers. Conidiogenous cells 5–9 μm high × 2–4 μm wide, holoblastic, cylindrical to subcylindrical, hyaline, the first conidium produced holoblastically and subsequent conidia enteroblastically forming typical phialides with periclinal thickenings, swollen at the base, discrete, producing a single conidium at the apex. Conidia 18–22 × 8–10 μm (\( \bar{x} \) = 19.9 × 8.6 μm, n = 30), initially hyaline, unicellular, becoming dark brown and 1-septate while still attached to conidiogenous cells; detached conidia, hyaline, sepia or blackish-brown, unicellular or 1-septate, moderately thick-walled, wall externally smooth, roughened on the inner surface, oval to ovoid, widest in the center, apex obtuse, base truncate or rounded, guttulate when young.
Culture characteristics: Colonies on MEA reaching 5 cm diam. after 30 days at 16 °C, circular, smooth margin, greyish-green to blackish-green after 28 days flat on the surface, without aerial mycelium, reverse greyish-brown to black. Hyphae septate branched, hyaline, thin, smooth-walled.
Material examined: RUSSIA, Rostov region, Rostov-na-Donu city, Botanical garden of Southern Federal University, Higher Park, underwood (47.2389405°E, 39.6484137°N), on Robinia pseudoacacia (Fabaceae), 26 March 2014, Timur S. Bulgakov T 06 (MFLU 16-1273, new host record), living culture, MFLUCC 14-0888, MUCL.
Notes: In the combined phylogenetic analysis (ITS and EF1-α), MFLUCC 14-0888 strain is phylogenetically most closely related to D. vidmadera (Fig. 3). Our isolate resembles D. vidmadera in the shape and size of the conidia but our collection (MFLU 16-1273) has darker conidia than DAR78992 of Dothiorella vidmadera (Pitt et al. 2013). As the morphological information does not provide any clear divergence, we report our collection as a new host record of D. vidmadera. This species has been previously recorded only from Vitis vinifera and Fraxinus ornus. This is the first record of Dothiorella vidmadera on Robinia species.
Capnodiales Woron.
The order Capnodiales comprises human pathogens, plant saprotrophs and rock-inhabiting species. This order was reviewed by Chomnunti et al. (2011) and in this paper we follow the recent publication of Hyde et al. (2013).
Mycosphaerellaceae Lindau
The family Mycosphaerellaceae was introduced by Lindau (1897) and typified by Mycosphaerella, with M. punctiformis (Pers.) Starbäck as the type species. The family was designated to accommodate Dothideomycete species having small ascomata and often ascostromata forming on various hosts, mostly parasitic, but also saprobic on dead plants (von Arx and Müller 1975; Hyde et al. 2013). Species in Mycosphaeriallaceae lack pseudoparaphyses and ascospores are often 2-celled, oblong to clavate, or ellipsoidal (Hyde et al. 2013). Based on these characters, the family was initially placed in the order Dothideales (von Arx and Müller 1975; Hawksworth et al. 1995; Hyde et al. 2013; Liu et al. 2015a). Kirk et al. (2001) treated the family in a separate order—Mycosphaerellales. Schoch et al. (2006) assigned Mycosphaerellaceae to Capnodiales based on phylogenetic support and this was followed by various mycologists (Crous et al. 2007, 2009; Kirk et al. 2008; Hyde et al. 2013; Wijayawardene et al. 2014a; Liu et al. 2015a). Recently, more than 50 sexual and asexual genera have been accommodated in Mycosphaerellaceae (Wijayawardene et al. 2014a).
Pallidocercospora Crous et al.
Pallidocercospora was introduced by Crous et al. (2013) to accommodate cercospora-like species, but not congeneric with Cercospora and is typified by P. heimii (Crous) Crous. Crous et al. (2013) designated the genus based on its pale brown cercosporoid conidia, which are generally referred to as the Mycosphaerella heimii complex (Crous et al. 2004a, 2013). Seven species were initially included in the genus based on multi-gene phylogenetic analyses (Crous et al. 2013). However, they did not synonymize Pseudocercospora colombiensis and P. thailandica under Pallidocercospora, even though these two species clustered with other Pallidocercospora species (Crous et al. 2013). Subsequently, these two species have been stated as Pallidocercospora colombiensis and P. thailandica (Crous et al. 2013; Pérez et al. 2013; Quaedvlieg et al. 2014), although, the species combinations have not been formally established. Therefore the sexual morph, Mycosphaerella thailandica is transferred to Pallidocercospora in this study and this is congruent with our rDNA based phylogenies (Fig. 7).
Phylogram generated from maximum likelihood analysis (RAxML) based on combined ITS and LSU sequence data of respective genera in Mycosphaerellaceae. Bootstrap support values for maximum likelihood (ML, left) and maximum parsimony (MP, right) equal to or greater than 50 % are given above the nodes. The values of the Bayesian posterior probabilities from MCMC analyses (BYPP) equal or higher than 95 % are given below the nodes. The tree is rooted with Lecanosticta acicola (CBS 871.95). Ex-type and ex-epitype strains are in bold. The generated sequences in this study are indicated in blue
Pallidocercospora thailandica (Crous et al.) Phookamsak, Wulandari & K.D. Hyde, comb. nov.
= Mycosphaerella thailandica Crous et al., in Crous et al., Stud. Mycol. 50(2): 465 (2004)
≡ Pseudocercospora thailandica Crous et al., in Crous et al., Stud. Mycol. 50(2): 465 (2004)
Index Fungorum number: IF552204; Facesoffungi number: FoF02258, Fig. 8
Pallidocercospora thailandica (MFLU 11-0170, MFLU 11-0177). a Herbarium material with leaf spots. b Appearance of ascomata on the host surface (a = from MFLU 11-0177, b = MFLU-110170). c Close up of ascomata on the host (MFLU 11-0170). d Section through the ascomata (MFLU 11-0170). e Section through peridium (MFLU 11-0170). f–i Asci (MFLU 11-0170). j–n Ascospores (MFLU 11-0170). o–p Culture characteristics (MFLU 11-0170; o = from above, p = from below). Scale bars d = 20 μm, e = 10 μm, f–i = 5 μm, j–n = 2 μm
Biotrophic, hemibiotrophic, or saprotrophic on various hosts, leaf spots on the margins of leaves, causing tip blight, or lesions or lesions initially start from the tip of leaves, irregular in shape, dried, pale brown to brown at the middle, and reddish-brown to dark brown at margin of the lesions. Sexual morph Ascomata 45–70 μm high, 45–80 μm diam., as small black dots on the host surface, scattered, sometimes clustered, gregarious, immersed to semi-immersed, with protruding papilla, globose to subglobose, glabrous, ostiole central, with minute papilla. Peridium 5–10 μm wide, thin-walled, composed of 2–3 cell layers of brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium lacking pseudoparaphyses. Asci (23–)25–35(–37.5) × 7–9 μm (\( \bar{x} \) = 29 × 7.9 μm, n = 30), 8-spored, bitunicate, fissitunicate, obclavate, rarely ovoid, subsessile, apically rounded, with well-developed ocular chamber. Ascospores (8–)9–12 × 2.5–3.5 μm (\( \bar{x} \) = 10.8 × 3.1 μm, n = 30), overlapping uni- to tri-seriate, clavate, hyaline to subhyaline, 1-septate, not contricted at the septum, smooth-walled, upper cell wider and shorter than lower cell. Asexual morph Hyphomycetous, pseudocercospora-like (see notes).
Culture characteristics: Colonies on PDA reaching 23.5–30 mm diam. after 4 weeks at 25–30 °C; colony from above, dark greenish at the margin, paler greenish hair-like at the center; from below, dark greenish to black; dense, irregular, flattened to raised, with undulate edge, with entire margin, surface smooth, velvety to cottony; not producing pigmentation in agar.
Material examined: THAILAND, Phrae, Rongkwang District, Maejo University Phrae campus grounds, on dead leaves of Dracaena loureiri Gagnep (Ruscaceae), 20 August 2010, R. Phookamsak, RP0050 (MFLU 11-0170), living culture, MFLUCC 11-0134, KUMCC; ibid. Chiang Rai, Muang District, Mae Fah Luang University campus grounds, on living leaves of Rhapis sp. (Arecaceae), 4 August 2010, N.F. Wulandari, RP0057 (MFLU 11-0177), living culture, MFLUCC 11-0141, KUMCC.
Notes: Pallidocercospora thailandica was introduced as Mycosphaerella thailandica by Crous et al. (2004b) who noted its asexual morph as Pseudocercospora thailandica Crous et al. (2004b). The asexual morph was described with “mycelium composed of medium brown, branched, septate, and smooth hyphae; conidiophores dense, pale brown, subcylindrical, unbranched, 0–2-septate, straight to curved, smooth-walled, arising from the upper cells of the stroma; conidiogenous cells terminal, pale brown, subcylindrical, tapering to flat tipped apical loci, proliferating sympodially; conidia solitary, pale brown, narrowly obclavate to subcylindrical, subobtuse at the apex, with long obconically subtruncate at the base, 3–6-septate, smooth-walled, and guttulate” (Crous et al. 2004b).
Based on phylogenetic analysis, Mycosphaerella thailandica clustered with Pallidocercospora species (Crous et al. 2013; Quaedvlieg et al. 2014). However, Crous et al. (2013) mentioned that Pseudocercospora colombiensis and Ps. thailandica were typical members of Pseudocercospora sensu stricto based on its morphological features. Therefore, these taxa were not synonymized under the genus Pallidocercospora when Crous et al. (2013) introduced this genus. However, the name “Pallidocercospora thailandica” has been used instead of “Pseudocercospora thailandica” (Crous et al. 2013; Quaedvlieg et al. 2014), but the name “Pallidocercospora thailandica” was not formally synonymized and thus, this name was invalid.
In this study, two isolates were collected from dead leaves of Dracaena loureiri (Ruscaceae) and living leaves of Rhapis sp (Arecaceae). Combined ITS and LSU phylogenetic analyses show that these isolates grouped with Pseudocercospora colombiensis and Ps. thailandica, and clustered with Pallidocercospora species, with high bootstrap support (97 % ML, 100 % MP, 1.00 PP, Fig. 7). Furthermore, Pseudocercospora colombiensis and Ps. thailandica form a distinct clade with Pseudocercospora sensu stricto which is congruent to Crous et al. (2013) and Quaedvlieg et al. (2014). Therefore, we transfer the species Mycosphaerella thailandica to the genus Pallidocercospora.
Pallidocercospora thailandica is morphologically distinct from P. colombiensis, but it is difficult to distinguish these two species based on phylogenetic analyses (Quaedvlieg et al. 2014). However, Quaedvlieg et al. (2014) applied the pairwise homoplasy index (PHI) test with the GCPSR and CSC concepts and proposed that these two species are different taxa.
Pallidocercospora thailandica has been collected from various hosts in Australia, Laos, Thailand and West Indies (Acacia mangium Willd., Eucalyptus camaldulensis Dehnh., Musa sp.) and is mostly found as a pathogen on the hosts (Crous et al. 2004b, 2013; Arzanlou et al. 2008; Cheewangkoon et al. 2008). In this study, this species was associated with leaf spots on Rhapis sp., and as a saprobe on Dracaena loureiri; both are new hosts for P. thailandica.
Dothideales Lindau
For Dothideales, we follow Li et al. (2016).
Dothideaceae Chevall.
The family Dothideaceae was introduced by Chevallier (1826) as ‘Dothideae’, and later Fuckel (1869) established this family with Dothidea as the type genus and D. gibberulosa as the type species. Thambugala et al. (2014) treated the family Dothideaceae with 15 genera. Dothideaceae is characterized by ‘immersed to erumpent or superficial, uni or multi-loculate ascostromata, 8- or polyspored, bitunicate asci and hyaline or brown, transversely septate, sometimes muriform ascospores’ (Thambugala et al. 2014). We provide an updated phylogeny in Fig. 9.
Dothiora buxi Jayasiri, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552173; Facesoffungi number: FoF02223, Fig. 10
Etymology: The specific epithet buxi is based on the host genus from which the taxon was collected.
Holotype: MFLU 15-3404.
Saprobic on Buxus sempervirens L. Sexual morph Ascostromata 500–1000 μm long × 220–250 μm high, 320–340 μm diam., erumpent through the epidermis, solitary or clustered, globose, brown to black, with single locules, with a central longitudinal slit-like opening. Peridium 32–83 μm wide, two-layered, outer layer composed of dark brown or brown, thick-walled cells of textura angularis, inner layer composed of hyaline, thin-walled cells of textura angularis. Hamathecium lacking pseudoparaphyses. Asci 100–115 × 14–21 μm (\( \bar{x} \) = 102 × 17 μm, n = 20), 32-spored, bitunicate, fissitunicate, cylindro-clavate, short pedicellate, apically rounded, with a small ocular chamber. Ascospores 11–15 × 5.4–7 μm (\( \bar{x} \) = 13 × 6 μm, n = 30), bi-seriate to multi-seriate, hyaline to very pale brown, aseptate, fusoid to ovoid, one end narrower than other, smooth-walled with granular contents, with a thin mucilaginous sheath.
Material examined: ITALY, Province of Forlì-Cesena [FC]), near Passo delle Forche—Galeata on dead branch of Buxus sempervirens (Buxaceae), 17 November 2014, E. Camporesi, IT 2284 (MFLU 15-3404, holotype, KUN, isotype).
Notes: Dothiora was introduced by Fries (1849) with D. pyrenophora (Fr.) Fr. as the type species. Our isolate shares common characters with the genus Dothiora. DNA was extracted from fruiting bodies and multi-gene phylogenetic analysis placed Dothiora buxi as a sister taxon to Dothiora elliptica (Fig. 9). The latter is similar in having epidermal erumpent, hysteriiforme ascostromata and hyaline ascospores but differs from Dothiora buxi in having 32-spored asci and aseptate, fusoid to ovoid, ascospores narrowed at one end (Saccardo 1889). Therefore, we introduce Dothiora buxi as a new species.
Hysteriales Lindau
For Hysteriales, we follow Hyde et al. (2013).
Hysteriaceae Chevall.
Chevallier (1826) introduced the family Hysteriaceae as ‘Hysterineae’ and this family has been treated with different genera by various authors (Zogg 1962; von Arx and Müller 1975; Kirk et al. 2001; Lumbsch and Huhndorf 2010). Recent multi-gene phylogenetic studies placed Hysteriaceae in Hysteriales, Pleosporomycetidae (Boehm et al. 2009a, b; Hyde et al. 2013; Wijayawardene et al. 2014a, Thambugala et al. 2016b). Hyde et al. (2013) and Wijayawardene et al. (2014a) accepted 13 genera, while de Almeida et al. (2014) introduced a new genus Hysterodifractum in this family. The family now contains 14 genera. A phylogenetic tree for the family is presented in Thambugala et al. (2016b) (Fig. 11).
Gloniopsis De Not.
The genus Gloniopsis is typified by Gloniopsis praelonga (Schwein.) Underw. & Earle [as ‘praelongum’]. Based on morphology and molecular phylogenetic analyses Gloniopsis is placed in Hysteriaceae (Boehm et al. 2009b; Wijayawardene et al. 2014a; Thambugala et al. 2016b). The genus now contains 65 epithets listed in Index Fungorum (2016). The genus Gloniopsis is characterized by hyaline to yellow dictyospores, often inequilateral, curved, multi-septate, with one or more longitudinal septa, constricted at the first-formed septum, sometimes constricted at additional septa, and usually surrounded by a gelatinous sheath which may dissipate with age (Zogg 1962).
Gloniopsis calami Konta & K.D. Hyde. sp. nov.
Index Fungorum number: IF552234; Facesoffungi number: FoF02366, Fig. 12
Etymology: Name reflects the host genus Calamus.
Holotype: MFLU 15-1470.
Saprobic on dead Calamus sp. Sexual morph Hysterothecia 195–215 μm high × 160–170 μm wide, erumpent to superficial, solitary to gregarious, scattered, dark, straight to flexuous. Peridium 37–47 μm wide, carbonaceous, thick-walled, not having distinct layers, relatively smooth on the outer surface. Hamathecium 1.2–2 μm wide, composed of dense, branched, hyaline, septate, pseudoparaphyses. Asci 60–80 × 15–21 μm (\( \bar{x} \) = 71 × 17 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, with knob-like pedicel, apically rounded, with a well-developed ocular chamber, external layer easily broken. Ascospores 17–20 × 6–8 μm (\( \bar{x} \) = 19 × 7 μm, n = 10), dictyosporous, overlapping 1–2-seriate, fusiform, slightly curved to straight, 4–6-trans-septate and with 2–4 vertical septa, reddish-brown to brown, constricted at the septa, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Ascospores germinated on MEA within 24 h and germ tubes produced from all cells. Colonies on MEA 7–7.5 cm diam. after 2 weeks at 25 °C, grey to dark green, outwardly with strongly radiating colony. After 1 month of incubation, colonies irregular, convex, spongy, medium dense, margin undulate.
Material examined: THAILAND, Phang-Nga Province, on dead Calamus sp. (Arecaceae), 6 December 2014, S. Konta, DNH07i (MFLU 15-1470, holotype, isotype HKAS 95030); ex-type living culture, MFLUCC 15-0739.
Notes: Molecular analyses indicate that the new species belongs to the genus Gloniopsis in the Hysteriaceae clade and is nested in between G. praelonga (type) and G. subrugosa and appear to be phylogenetically distinct (Fig. 11). The ascospores of G. calami are similar in shape, but smaller in size and have a different septation to G. praelonga (type) and G. subrugosa. However, G. calami is distinct from G. arciformis in its ascospores being constricted at the septa, while in G. arciformis they are not constricted.
Pleosporales Luttr. ex M.E. Barr
We follow Tanaka et al. (2015).
Dictyosporiaceae Boonmee & K.D. Hyde
The family Dictyosporiaceae was introduced by Boonmee et al. (2016) to accommodate Aquaticheirospora, Cheirosporium, Dictyocheirospora, Dictyopalmispora, Dictyosporium, Digitodesmium, Pseudocoleophoma and Pseudodictyosporium. The asexual morphs of the family Dictyosporiaceae are hyphomycetous with brown, multi-septate, cheirosporous conidia (Boonmee et al. 2016). In this study we provide an updated backbone tree for Dictyosporiaceae (Fig. 13) and introduce the new species Pseudodictyosporium thailandica and Pseudocoleophoma typhicola.
Phylogenetic tree generated from maximum parsimony (MP) analysis based on combined ITS and LSU sequence data of genera of the family Dictyosporiaceae. Bootstrap support values for maximum parsimony (MP) and maximum likelihood (ML) greater than 50 % and Bayesian posterior probabilities greater than 0.80 are indicated above or below the nodes as MPBS/MLBS/PP. The ex-type strains are in bold and the new isolates are in red bold. The tree is rooted with Letendraea helminthicola
Pseudocoleophoma typhicola E.B.G. Jones, Kamolhan, Boonmee & K.D. Hyde, sp. nov.
Index Fungorum number: IF552326; Facesoffungi number: FoF02444, Figs. 14 and 15
Pseudocoleophoma typhicola (holotype). a Appearance of conidiomata on host substrate. b Close up of conidioma. c Vertical section through conidiomata .d Conidia attached to conidiogenous cells. e–l Immature to mature conidia. m Germinated conidium. Scale bars a = 1 mm b = 100 μm, c = 50 μm, d–l = 5 μm, m = 10 μm
Etymology: Referring to the host plant Typha latifolia.
Holotype: MFLU 16-0966.
Saprobic on submerged stems in freshwater. Sexual morph Undetermined. Asexual morph Conidiomata forming as dark spots on the host surface, 140–150 μm high × 60–100 μm diam. (\( \bar{x} \) = 143 × 80 μm, n = 15), semi-erumpent in the host tissue, uniloculate solitary to scattered, subglobose, brown to black. Peridium 40–45 μm at base, 40–45 μm at sides, comprising 4–5 layers, hyaline to dark brown, thick-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 2–5 × 2–5 μm (\( \bar{x} \) = 2 × 3 μm, n = 15), enteroblastic, smooth-walled, hyaline. Conidia 9–11 × 2–3 μm (\( \bar{x} \) = 10 × 3 μm, n = 20), hyaline, oblong to cylindrical, with rounded or obtuse ends, 1-euseptate, smooth, thin-walled, guttulate.
Culture characteristics: Colonies on PDA 3 cm diam. after 4 weeks at 16 °C, dirty white to pale brown at the margin, pale brown to creamy at the center; reverse iron, thin, curled, flat.
Material examined: UK, Hampshire, Swanick Lakes, on submerged stems of Typha latifolia (Typhaceae) in freshwater, 28 August 2015, E.B.G. Jones, GJ190 (MFLU 16–0966, holotype, HKAS94520 isotype), ex-type living culture, MFLUCC 16–0123, KUMCC 16-0007.
Notes: Pseudocoleophoma typhicola is different from P. polygonicola and P. calamagrostidis in having conidiomata without neck and with a wide peridium (40–45 μm at base, 40–45 μm at sides), enteroblastic, 3-septate conidia while P. polygonicola and P. calamagrostidis have conidiomata with a long neck, phialidic, aseptate conidia. Phylogenetic analyses indicate that Pseudocoleophoma typhicola is related to P. polygonicola and P. calamagrostidis with high support, but it can be recognized as a new species as it stands on its own with relatively good support (Fig. 13).
Pseudodictyosporium Matsush.
The genus Pseudodictyosporium was established by Kobayasi (1971) and is typified by P. wauense Matsush. Three species are accepted in this genus, P. wauense, P. elegans and P. indicum (Boonmee et al. 2016). Molecular phylogenetic analyses place Pseudodictyosporium within the family Dictyosporiaceae (Pleosporales) (Tanaka et al. 2015; Boonmee et al. 2016). In this study we introduce P. thailandica as a new species.
Pseudodictyosporium thailandica C.G. Lin, Yong Wang bis & K.D. Hyde, sp. nov.
Index Fungorum number IF552165; Facesoffungi number: FoF02227, Fig. 16
Etymology: Referring to the country where the fungus was first collected.
Holotype: MFLU 16-1301.
Saprobic on decaying bamboo stem. Sexual morph Undetermined. Asexual morph Conidiophores macronematous, mononematous, scattered or caespitose, erect, flexuous, irregularly branched, smooth, septate, slightly constricted at septa, hyaline to brown, often geniculate, 23–305 μm long (\( \bar{x} \) = 77 μm, n = 27), 2.5–7.9 μm wide (\( \bar{x} \) = 5 μm, n = 96). Conidiogenous cells holoblastic, polyblastic, discrete, determinate or sympodial, terminal and intercalary. Conidia solitary, acropleurogenous, dry, cheiroid, ellipsoidal, ovoid, smooth, multi-septate, subhyaline to grey-brown, 14–31 μm long (\( \bar{x} \) = 20 μm, n = 70), 12–26.5 μm wide (\( \bar{x} \) = 17 μm, n = 70) at the widest point.
Culture characteristics: Conidia germinating on PDA within 36 h. Colonies on MEA reaching 20–35 mm diam. after 4 months at room temperature (25 °C), effuse, hairy, grey-white from above, brown at the center, yellowish-white at margin from below.
Material examined: THAILAND, Phetchaburi, Cha-am District, on decaying bamboo stem, 28 July 2015, Chuan-Gen Lin, KNP 5-3 (MFLU 16-1301, holotype; HKAS 95053, isotype), ex-type living culture, MFLUCC 16-0029.
Notes: Phylogenetic analysis of combined ITS, LSU, SSU and TEF sequence data indicate that our species belongs in the genus Pseudodictyosporium (Fig. 13) with 100 % MP bootstrap support, 100 % ML bootstrap support and 100 % Bayesian posterior probabilities, and forms a separate clade within Pseudodictyosporium.
The conidiophores of P. thailandica are longer than those of P. wauense (up to 100 μm) and P. elegans (10–56 μm), but shorter than those of P. indicum (284–630 μm). In addition, the conidia of our species (12–26.5 μm) are wider than earlier described species (P. elegans 9–16.5 μm, P. indicum 12.5–16 μm and P. wauense 12–19 μm) (Rao and Subhedar 1976; Tzean and Chen 1990; Kirschner et al. 2013).
Didymellaceae Gruyter et al.
The family Didymellaceae was introduced by De Gruyter et al. (2009), with type species Didymella exigua (Niessl) Sacc., to accommodate most species in Phoma sensu lato and allied genera. The family contains numerous plants pathogenic, saprobic and endophytic species associated with a wide range of hosts (Aveskamp et al. 2010; Chen et al. 2015a). Aveskamp et al. (2010) revised the taxonomy of Didymellaceae based on multi-gene analyses and included eleven genera in the family, i.e. Ascochyta, Boeremia, Chaetasbolisia, Didymella, Epicoccum, Leptosphaerulina, Macroventuria, Microsphaeropsis, Peyronellaea, Phoma and Stagonosporopsis. Subsequently, more genera and information were added (Wijayawardene et al. 2012; Zhang et al. 2012a; Hyde et al. 2013; Ariyawansa et al. 2015a). Chen et al. (2015a) utilized the RPB2 gene combined with ITS, LSU as well as tub2 to distinguish Phoma and related genera and accepted 17 genera in Didymellaceae. However Microsphaeropsis was excluded from the Didymellaceae, and a new family Microsphaeropsidaceae was proposed to accommodate these Microsphaeropsis species. Thambugala et al. (2016a) included an additional genus Neomicrosphaeropsis due to its morphological similarity with Microsphaeropsis species, but this is phylogenetically closely related to Didymellaceae. In this study, three new species are added in Neomicrosphaeropsis based on both morphology and phylogeny. Moreover, a new collection of Platychora ulmi (J. Schröt.) Petr. MFLUCC 14-1189 together with another strain CBS 361.52 from GenBank clustered with the Phoma group (Fig. 17), but LSU gene data are only available for these two strains. LSU and SSU sequence data does not provide sufficient phylogenetic information to distinguish closely related genera or species (Aveskamp et al. 2009, 2010; Chen et al. 2015a). Thus, the genus Platychora should be re-evaluated based on additional genes. Didymellocamarasporium was introduced in Didymellaceae based on LSU and SSU sequence data by Wijayawardene et al. (2016), but they cannot be well separated from other genera in present study (data not shown). Hence, this genus also needs additional genes to confirm its placement in Didymellaceae. To date, 21 genera are included in Didymellaceae.
Phylogenetic tree inferred from a maximum Likelihood analysis based on a concatenated alignment of LSU, ITS, RPB2, TUB2 sequence data representing Didymellaceae and allied families. The RAxML bootstrap support values (MLBS) greater than 50 % and Bayesian posterior probabilities (BPP) greater than 0.95 are given at the nodes (MLBS/BPP). The ex-type strains are in bold and the new isolates are in blue. The tree is rooted with Leptosphaeria doliolum
Neomicrosphaeropsis Thambugala et al.
Thambugala et al. (2016a) included Neomicrosphaeropsis in Didymellaceae. In this study, three new species are added in Neomicrosphaeropsis based on both morphology and phylogeny.
Neomicrosphaeropsis cytisi W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552212; Facesoffungi number: FoF02347, Fig. 18
Neomicrosphaeropsis cytisi (holotype). a Herbarium specimen. b, c Appearance of brown coniodiomata on the host. d Wall of conidiomata. e Vertical section of conidiomata. f–h Conidiophores, conidiogenous cells and developing conidia. j Germinating spore. i, k, l Conidia. m Culture. Scale bars b = 500 μm, c = 200 μm, d, j = 10 μm, e = 20 μm, f–h, i, k–n = 5 μm, n = 25 mm
Etymology: Named after the host genus Cytisus.
Holotype: MFLU 16-1871
Saprobic on dead stem of Cytisus sp. (Fabaceae), forming numerous, conspicuous, oval, dark brown, conidiomata. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 75–155 μm diam. × 75–130 μm high, dark brown, solitary to gregarious or confluent, pycnidial, globose to subglobose, immersed, unilocular, thick-walled, smooth, ostiolate. Ostiole single, short, with acute apex, centrally located. Wall of conidiomata 10–24 μm wide, composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 2–4 μm long × 3.5–6 μm wide, hyaline, enteroblastic, phialidic, doliiform to ampulliform, determinate, discrete, glabrous. Conidia 4.5–7 × 3–5 μm (\( \bar{x} \) = 5.7 × 3.9, n = 30), initially hyaline, becoming pale brown to dark brown at maturity, globose to obovate, ellipsoidal to subcylindrical, rounded at both ends, unicellular, thick-walled, smooth.
Culture characteristics: Colonies on PDA attaining 30–40 mm diam. after 4 weeks at 20–25 °C, with circular margin, dark jacinth to orange red to dark olivaceous, flattened, dense, aerial mycelium on the surface, reverse similar in colour.
Material examined: ITALY, Province of Arezzo [AR], Bagno di Cetica, on dead stem of Cytisus sp. (Fabaceae), 7 October 2012, Erio Camporesi, IT-784 (MFLU 16-1871, holotype); ex-type living culture, MFLUCC 13-0396, ICMP; ibid. IT-784B (HKAS 93585, isotype); living culture, KUMCC 16-0026.
Notes: Neomicrosphaeropsis cytisi differs from N. cytisinus in the form of the conidiomata. Neomicrosphaeropsis cytisi has immersed, ostiolate conidiomata that are smaller than those of N. cytisinus which are semi-immersed when immature, and become erumpent at maturity (190–220 μm high × 210–250 μm diam.)
Neomicrosphaeropsis cytisinus Tennakoon, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552262; Facesoffungi number: FoF02396, Fig. 19
Neomicrosphaeropsis cytisinus (holotype). a Appearance of conidiomata on host. b Close-up of conidiomata. c Section of conidioma. d Section of peridium. e Conidiogenous cells. f–i Conidia. j. Germinated conidia. l Colony from above. m Colony from below. Scale bars c = 50 μm, d = 20 μm, e = 5 μm, f–i = 2 μm, j = 10 μm
Etymology: Name reflects the host genus Centaurea, from which the holotype was collected.
Holotype: MFLU 16-1364.
Saprobic on Cytisus sp. Sexual morph Undetermined. Asexual morph Coelomycetous Conidiomata 190–220 μm high × 210–250 μm diam. (\( \bar{x} \) = 232 × 204, n = 10), stromatic, solitary, immersed to semi-immersed when immature, becoming erumpent at maturity, globose to subglobose, uniloculate, black, dehiscing by an irregular split of the host epidermis. Peridium 20–25 μm wide, composed of 4–5 layers of light brown cells of textura angularis to textura prismatica. Conidiogenous cells 1–2 μm wide, phialidic, hyaline, thin-walled, smooth, integrated, producing a single conidium at the apex. Conidia 5–7 × 3–5 μm (\( \bar{x} \) = 67 × 4.2, n = 30), initially hyaline, becoming light brown, moderately thick-walled, smooth, aseptate, ovoid, obtuse at apex, truncate or rounded at base.
Culture characteristics: Colonies on PDA reaching 25–30 mm diam. after 8 days at 20–25 °C, medium sparse, circular, flat, slightly rough at surface with entire edge, with a well-defined margin, cottony to fairly fluffy with sparse mycelium; from above: white to cream at the margin, white to yellowish at the centre; from below, light yellow to light brown at the margin, yellowish at the centre; mycelium white to cream with tufting; not producing pigments in PDA medium.
Material examined: ITALY, Province of Arezzo [AR], near Croce di Pratomagno, on dead stem of branch of Cytisus scoparius L. (Fabaceae), 24 June 2012, E. Camporesi, IT 472 (MFLU 16-1364, holotype; HKAS 93703, isotype), ex-type living cultures, MFLUCC 16-0790, KUMCC 15-0557.
Notes: Neomicrosphaeropsis cytisinus resembles N. cytisi in sharing the size range of conidiophores and conidiogenous cells, but differs in the size of conidiomata (75–130 × 74–157 μm) and thickness of peridium (10–24 μm). Neomicrosphaeropsis cytisinus differs from Neomicrosphaeropsis minima in the size of conidiomata (3–5.5 × 2–4 μm), conidiophores and host. Phylogenetic analyses show that they are distinct with high bootstrap support (98 % ML, 1.00 BYPP, Fig. 17).
Neomicrosphaeropsis minima W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552213; Facesoffungi number: FoF02348, Fig. 20
Neomicrosphaeropsis minima (holotype). a Herbarium specimen. b, c Appearance of black coniodiomata on the host. d, e Vertical section of conidiomata. f Wall of conidiomata. g–k Conidiophores, conidiogenous cells and developing conidia. l Germinating spore. m, n Conidia. o Culture. Scale bars b, c = 200 μm, d, e = 50 μm, f = 15 μm, g–n = 5 μm, o = 50 mm
Etymology: Named for the small conidiomata.
Holotype: MFLU 16-1490
Saprobic on dead stems of Verbascum sp. (Scrophulariaceae), forming numerous, conspicuous, rounded, black conidiomata. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 60–80 μm diam., 60–95 μm high, black, solitary to gregarious or confluent, pycnidial, globose to subglobose, immersed or semi-immersed, unilocular, thick-walled, smooth, ostiolate. Ostiole single, short, centrally located. Wall of conidiomata 8–15 μm wide, composed of thick-walled, brown to hyaline cells of textura angularis. Conidiophores 2.7–5.5 μm long × 3.2–5.5 μm wide, occasionally present, hyaline, doliiform to ampulliform, arising from inner layers of the pycnidial wall. Conidiogenous cells 2.6–5.5 μm long × 2–3.5 μm wide, hyaline, enteroblastic, phialidic, doliiform or cylindrical to ampulliform, with a periclinal wall thickening at the tip, smooth. Conidia 2.8–5.4 × 2–3.6 μm (\( \bar{x} \) = 4.1 × 2.8, n = 30), hyaline when young, becoming brown at maturity, oval, rounded at both ends, unicellular, thick-walled, smooth, guttulate.
Culture characteristics: Colonies on PDA attaining 15–20 mm diam. after 7d at 20–25 °C with circular margins, white to dark brown, flattened, with felt-like, dense, aerial mycelium on the surface, reverse with dark brown in the central zone, white on the edge, sporulating.
Material examined: ITALY, Province of Arezzo [AR], near Montemignaio, on dead stems of of Verbascum sp. (Scrophulariaceae), 1 October 2012, Erio Camporesi, IT-765 (MFLU 16-1490, holotype); ex-type living culture, MFLUCC 13-0394; ibid. IT-765B (HKAS 95027, isotype); living culture, KUMCC 16-0024.
Notes: Phylogenetic tree based on multi-gene (LSU, ITS, RPB2 and β-tubulin) shows that the three collections (MFLUCC 13-0394, MFLUCC 13-0396, KUMCC 15-0557) cluster with newly introduced genus Neomicrosphaeropsis (Thambugala et al. 2016a). However, these strains formed a separate branch, basal to N. rossica Thambugala, Bulgakov & K.D. Hyde. Morphologically, they share similar conidia characters with the type species N. italica. Neomicrosphaeropsis minima is closely related to N. cytisi in our phylogenetic tree (Fig. 17), but can be easily distinguished by the dimensions of the conidiomata. Neomicrosphaeropsis minima has immersed to semi-immersed conidiomata smaller than these of N. cytisi which has conidiomata 75–155 μm diam. × 75–130 μm high. In addition, N. minima has conidiogenous cell walls, thickened at the apex, while this character is not observed in N. cytisi.
Neodidymelliopsis Q. Chen & L. Cai
Neodidymelliopsis was introduced to accommodate Neodidymelliopsis cannabis (G. Winter) Q. Chen & L. Cai. as the type species, N. polemonii (Cooke) Q. Chen & L. Cai, N. xanthine (Sacc.) Q. Chen & L. Cai, and two unidentified species (Chen et al. 2015b). The conidia of this genus are variable in shape: ovoid to ellipsoidal, cylindrical, allantoid, hyaline to pale brown or pale yellowish, 0–1-septate.
Neodidymelliopsis ranunculi W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552214; Facesoffungi number: FoF02349, Fig. 21
Neodidymelliopsis ranunculi (holotype). a Herbarium specimen. b, c Appearance of black coniodiomata on the host. d Vertical section of conidioma. e, f Vertical section of peridium. g–j Conidiogenous cells and developing conidia. k Germinating conidium. l–n Conidia. o Culture on PDA. Scale bars b = 200 μm, c–d = 100 μm, e = 50 μm, f = 20 μm, g–i = 10 μm. k–p = 5 μm, q, r = 20 mm
Etymology: Named after host genus Ranunculus.
Holotype: MFLU 16-1870
Saprobic on dead stem of Ranunculus sp. (Ranunculaceae), Sexual morph undetermined. Asexual morph Coelomycetes. Conidiomata 100–120 μm high, 95–110 μm diam., pycnidial, solitary, globose to subglobose, black, immersed to semi-immersed, unilocular, ostiolate, wall 14–32 μm wide, composed of dark brown to light brown, thick-walled cells of textura angularis. Ostiole single, short, eccentric. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3–5 μm long × 4.5–10 μm wide, hyaline, enteroblastic, phialidic, determinate, discrete, doliiform, with a small collarette, smooth, arising from inner layers of conidiomata. Conidia 3–5 × 7.5–10 μm (\( \bar{x} \) = 4.3 × 8.7 μm, n = 30), initially hyaline, becoming pale brown at maturity, ellipsoidal, rounded at both ends or 1-septate, straight or slightly curved, guttulate, smooth-walled.
Culture characteristics: Colonies on PDA attaining 40–50 mm diam. after 7d at 20–25 °C, margins circular, floccose, white, pale grey to olivaceous near the center; reverse with white margin, dark brown at central zone, sporulating after 4 weeks.
Material examined: ITALY, Province of Forlì-Cesena [FC], Castrocaro Terme e Terra del Sole, near Converselle, on dead stem of Ranunculus sp. (Ranunculaceae), 2 December 2012, Erio Camporesi, IT-936 (MFLU 16-1870, holotype); ex-type living culture, MFLUCC 13–0490, ICMP; ibid. (KUN, HKAS 95028, isotype), living culture, KUMCC 16-0025.
Notes: Neodidymelliopsis ranunculi fits well within the morphological concept for Neodidymelliopsis. Based on both morphology and phylogeny, N. ranunculi is introduced as a new species in the genus.
Platychora Petr.
The genus Platychora is typified by P. ulmi (J. Schröt.) Petr. and comprises the type species and P. alni (Peck) Petr. (Index Fungorum 2016). This genus is characterized by multi-loculate stromatic tissues and it produces apiospores.
Platychora ulmi (J. Schröt.) Petr., Annls mycol. 23(1/2): 103 (1925)
= Sphaeria ulmi Schleich. ex Fr., Observ. mycol. (Havniae): 173 (1815)
Index Fungorum number: IF280985; Facesoffungi number: FoF02431, Fig. 22
Platychora ulmi (MFLU 16-1972). a, b Ascostromata on substrate. c, d Cross section of ascostromata. e Peridium f, i. Asci j–l. Ascospores. m Conidiomata on MEA. o Cross section of conidiomata. n, p, q Conidia attached to possible conidiogenous cell. r Conidia. Scale bars a = 500 μm, b, c, m, o = 100 μm, d = 50 μm, e = 20 μm, f–i = 10 μm, j–l, n, p–r = 5 μm
Reference specimen: MFLU 16-1972
Saprobic on dead leaves of Ulmus sp. Sexual morph Stromata up to 1 mm broad, sub-epidermal, solitary to aggregated, superficial, cushion-shaped, multi-loculate, black. Locules 100–130 μm high × 120–170 μm diam. (\( \bar{x} \) = 216 × 250 μm, n = 10), immersed in stromatic tissues, globose to subglobose, coriaceous, papillate, ostiolate. Peridium 13–16 μm wide (\( \bar{x} \) = 15 μm, n = 10), comprising thick-walled, dark brown cells of textura angularis. Hamathecium comprising 3.5–5.5 μm wide (\( \bar{x} \) = 5 μm, n = 10), filiform, hyaline, branched pseudoparaphyses. Asci 50–100 × 8.5–10.5 μm (\( \bar{x} \) = 76 × 10 μm, n = 10), 8-spored, bitunicate, cylindrical, short pedicellate. Ascospores 10–15 × 3–6 μm (\( \bar{x} \) = 13 × 5 μm, n = 10), obliquely uni-seriate, apiosporous, hyaline, ovoid, with a transverse septum near the lower end, not constricted at the septum. Asexual morph Conidiomata 130–140 μm high × 190–210 μm diam. (\( \bar{x} \) = 138 × 200 μm, n = 10), subcuticular, acervular, brown to black, applanate to pulvinate. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 10–15 μm high × 2–4 μm wide (\( \bar{x} \) = 138 × 200 μm, n = 10), solitary, septate at the base, olivaceous, smooth, cylindrical, straight, holoblastic, annellidic. Conidia 3–5.5 × 2.5–3 μm (\( \bar{x} \) = 5 × 2.7 μm, n = 10), oval, pale brown, aseptate, thin-walled, smooth, ornamented.
Culture characteristics: Colonies growing on MEA, becoming 1 cm within 7 days at 18 °C, circular, flat, irregular margin, with sparse aerial mycelium, white.
Material examined: ITALY, Province of Forlì-Cesena [FC], Isola di Santa Sofia, on dead leaves of Ulmus sp. (Ulmaceae), 20 January 2014, Erio Camporesi, IT 1670 (MFLU 16-1972, reference specimen designated here), living culture, MFLUCC 14-1186.
Notes: The phylogenetic placement of Platychora ulmi is not clear. Most phylogenetic studies placed this genus in Pleosporales without assignment to any family (Winton et al. 2007). However Hyde et al. (2013) included Platychora within Didymellaceae and combined ITS, LSU, RPB2 and Beta tubulin gene analysis in this study (Fig. 17) support the taxonomic placement of Platychora ulmi in Didymellaceae. We also illustrate the asexual morph of Platychora ulmi from culture.
Stagonosporopsis Died.
Stagonosporopsis was introduced by Diedicke (1912). This genus is characterised by having conidia with one, two or occasionally three septa. Jaczewski (1917) treated Stagonosporopsis as a subgenus of Ascochyta, whereas Petrak (1925) reduced this genus to synonymy with Ascochyta.
Stagonosporopsis centaureae Tennakoon, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552261; Facesoffungi number: FoF02397, Fig. 23
Stagonosporopsis centaureae (holotype). a Appearance of ascomata on host. b Close-up of ascoma. c Section of ascoma. d Section of peridium. e Pseudoparaphyses. f–i Asci. j–l Ascospores. m Germinated ascospore. n Colony from above. o Colony from below. Scale bars c = 20 μm, d, e = 10 μm, f–i = 20 μm, j–m = 5 μm
Etymology: Name reflects the host genus Centaurea, from which the species was collected.
Holotype: MFLU 16-1365
Saprobic on dead stem of Centaurea cyanus. Sexual morph Ascomata 110–120 μm high 120–130 μm diam., solitary, scattered, partly immersed to superficial, raised, dark brown to black, globose to subglobose, glabrous, uni-loculate, with centrally located ostiole with minute papilla. Peridium 22–27 μm wide, thin-walled, with unequal thickness, composed of 4–5 layers of dark brown to black pseudoparenchymatous cells, arranged in textura angularis to textura prismatica. Hamathecium composed of numerous, 2–2.5 μm wide, filamentous, distinctly septate, cellular pseudoparaphyses, non-constricted at the septum, anastomosing at the apex, embedded in a hyaline gelatinous matrix. Asci 35–48 × 7.2–8.2 μm (\( \bar{x} \) = 42.5 × 8.1 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical or clavate, short pedicellate, with furcate pedicel, apically rounded with an indistinct ocular chamber. Ascospores 13–15 × 3–5 μm (\( \bar{x} \) = 14.3 × 4.3 μm, n = 30), overlapping, uni- to bi-seriate, hyaline, ellipsoidal to clavate or fusiform with rounded ends, 1-septate, slightly constricted at the septum, echinulate, straight to slightly curved. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 25–30 mm diam. after 8 days at 20–25 °C, colonies medium sparse, circular, flat, slightly rough surface with entire edge, well defined margin, cottony to fairly fluffy with sparse mycelium; from above: dark brown to yellowish at the margin, dark brown to black at the centre; from below: dark brown to yellowish-brown at the margin, dark brown at the centre; mycelium light brown to yellowish with tufting; not producing pigments in PDA medium.
Material examined: ITALY, Province of Arezzo [AR], near Quota, on dead stem of Centaurea cyanus (Asteraceae), 24 June 2015, E. Camporesi, IT 2548 (MFLU 16-1365 holotype; HKAS 93705 isotype), ex-type living cultures, MFLUCC 16-0787, KUMCC 15-0559.
Notes: Stagonosporopsis centaureae clusters with Stagonosporopsis trachelii with high support (93 % ML, 0.99 BYPP, Fig. 17). It can be easily distinguished from S. trachelii which has smaller ascomata (60–80 μm diam.) and smaller ascospores (4–6 × 1.5–2.5 μm).
Didymosphaeriaceae Munk
Details of this family can be seen in Ariyawansa et al. (2014a), Li et al. (2016) and Wanasinghe et al. (2016).
Montagnula Berl.
The genus Montagnula was introduced by Berlese (1896) to accommodate M. infernalis (Niessl) Berl. and M. gigantea (Mont.) Berl. Ariyawansa et al. (2014c) placed Montagnula in the family Didymosphaeriaceae including some phragmosporous and didymosporous species, based on morphology and available phylogenetic analysis. Montagnula was accepted as a distinct genus in the family Didymosphaeriaceae in several recent studies (Ariyawansa et al. 2014c; Hongsanan et al. 2015a; Li et al. 2016). Presently, there are 28 species in this genus including two recently described species, M. bellevaliae Wanasinghe et al. and M. scabiosae Wanasinghe et al. (Hongsanan et al. 2015a).
Montagnula cirsii Qing Tian, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552184; Facesoffungi number: FoF02255, Fig. 25
Etymology: In reference to the host genus Cirsium, from which this holotype was collected.
Holotype: MFLU 14-0730.
Saprobic under periderm or semi-immersed in woody plant substrates. Sexual morph Ascomata 385–415 μm diam. × 510–525 μm high (\( \bar{x} \) = 400 × 515.5 μm, n = 10), semi-immersed to erumpent, solitary, scattered, or sometimes gregarious, globose, black, smooth-walled, neck long, with a small, flattened, ellipsoid ostiole at the apex. Peridium 41–58.5 μm (\( \bar{x} \) = 43.3 μm, n = 6), 2-layered, the outer layer composed of irregular, thick-walled, brown cells of textura angularis; the inner layer composed of hyaline, smaller cells of textura angularis. Hamathecium comprising 1–2 μm broad, septate, long, colourless, branched or simple pseudoparaphyses, surrounding the asci. Asci 84.5–119.5 × 10.5–13.5 μm (\( \bar{x} \) = 101 × 12 μm, n = 10), 8-spored, bitunicate, clavate, long pedicellate, apically rounded with an ocular chamber. Ascospores 18–23.5 × 6.5–9.5 μm (\( \bar{x} \) = 21.5 × 8 μm, n = 10), overlapping uni-seriate or uni-seriate, 3-septate, ellipsoid to fusiform, curved, yellow to brown, constricted at the septa, broader at the middle two cells, tapering or obtuse at both ends, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Ascospore germinating on PDA within 12 h. Colonies on PDA, reaching 8 mm diam. in 7 days at 25 °C. Mycelium superficial, hyaline, hairy, with entire edge, floccose at the center, drift white from above and light brown at the center from below.
Material examined: ITALY, Province of Forlì-Cesena [FC], Balze-Verghereto, on dead stem of Cirsium sp. (Compositae), 21 May 2013, Erio Camporesi (MFLU 14-0730, holotype); ibid., (HKAS 94523, isotype); ex-type living culture, MFLUCC 13-0680, KUMCC 16-0018.
Notes: Montagnula cirsii is morphologically similar to M. bellevaliae and M. scabiosae (Hongsanan et al. 2015a). However, they can be distinguished by various characters, such as host, shape of ascomata and different sizes of asci. Montagnula bellevaliae occurs on dead stems of Bellevalia romana, M. scabiosae on dead stems of Scabiosa sp. and M. cirsii on dead stems of Cirsium sp. The orientation of ascomata is also different. Montagnula bellevaliae has an eccentric papilla, while the other two species have regular papilla. The size of asci is also quite different; Montagnula cirsii is smaller than M. scabiosae (84.5–119.5 × 10.5–13.5 μm vs. 110–130 × 14–20 μm) but larger than M. bellevaliae which is 70–100 μm in length, 9–12 μm in width.). Based on phylogenetic analysis (Fig. 24), our strain clusters with M. scabiosae in Montagnula with relatively high support (99 % MP /1.00 PP). We therefore introduce a new species based both on morphology and phylogeny.
RAxML tree based on a combined dataset of LSU, SSU, ITS and TEF partial sequences of Didymosphaeriaceae. Bootstrap support values for maximum likelihood (ML) higher than 60 % and Bayesian posterior probabilities (BYPP) greater than 0.90 are defined as above the nodes respectively. The tree is rooted to Pleospora herbarum. The ex-type strains are in bold; the new isolates are in blue
Montagnula cirsii (holotype). a Herbarium material. b, c Ascomata semi-immersed in the stem. d Vertical hand section of ascoma. e Ostiole. f Vertical hand section of peridium. g Immature asci. h, i, k Asci with ascospores. Note the bitunicate asci. j Pseudoparaphyses. n Germinating ascospore. o–s Ascospores. l Colony on PDA from above. m Colony on PDA from below. Scale bars b = 500 μm, c = 100 μm, d = 50 μm, e–f, n = 20 μm, g–j = 10 μm, k, o–s = 5 μm
Tremateia Kohlm.
Facesoffungi number: FoF00223
Tremateia was introduced as a facultative marine genus, characterized by ‘depressed globose, immersed ascomata, numerous cellular pseudoparaphyses, fissitunicate and clavate asci, ellipsoid muriform ascospores, and a Phoma-like asexual morph’ (Kohlmeyer et al. 1995; Ariyawansa et al. 2014a). Earlier the genus was identified as similar to Lewia and Diademosa and placed in Pleosporaceae (Kohlmeyer et al. 1995). DNA sequences based studies have revealed that T. halophila groups in Didymosphaeriaceae (\( \bar{x} \) = Montagnulaceae), sister to Bimuria novae-zelandiae (Schoch et al. 2009; Suetrong et al. 2009; Ariyawansa et al. 2014a, 2015a, b, c; Liu et al. 2015a; this study). Ariyawansa et al. (2014a) confirmed the familial status of Tremateia in Didymosphaeriaceae.
In this study the novel isolates of Tremateia arundicola and T. guiyangensis grouped with T. halophila sister to Bimuria novae-zelandiae. However these Tremateia members are separated from Bimuria novae-zelandiae with high statistical support in combined (LSU, SSU, ITS and TEF) gene analyses (98 % ML and 1.00 BYPP, Fig. 24)
Tremateia arundicola Wanasinghe, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF552134; Facesoffungi number: FoF02210, Fig. 26
Etymology: Name reflects the place Arun River, from which the species was collected.
Holotype: MFLU 16-1275
Saprobic on dead herbaceous stems. Sexual morph Ascomata 200–300 μm high 250–350 μm diam. (\( \bar{x} \) = 259.3 × 295.1 μm, n = 5) immersed to semi-erumpent, solitary, scattered, broadly oblong to sub globose and flattened, dark brown to black, coriaceous, ostiolate. Ostiole 40–60 μm high 50–70 μm diam. papillate, black, smooth, filled with hyaline cells. Peridium 10–20 μm wide at the base, 15–25 μm wide in sides, thick, with 4–5 layers, outer layer heavily pigmented, thick-walled, comprising reddish to dark brown cells of textura angularis, inner layer composed of hyaline thin-walled cells of textura angularis. Hamathecium comprising numerous, 3–4 μm wide, filamentous, branched, septate, pseudoparaphyses. Asci 170–200 × 15–20 μm (\( \bar{x} \) = 183.3 × 18.4 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, pedicellate, thick-walled at the apex, with minute ocular chamber. Ascospores 20–30 × 6–9 μm (\( \bar{x} \) = 25.9 × 6.6 μm, n = 50), overlapping uni-seriate, muriform, ellipsoidal to subfusiform, slightly curved, upper part wider than the lower part, 3–6 transversely septate, with 1 vertical septum, constricted at the septa, initially hyaline, becoming pale-brown at maturity, ends remaining cone-shaped, with pointed upper ends and rounded lower ends, lacking a mucilaginous sheath or disappear with maturity. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 2 cm diam. after 30 days at 18 °C, circular, smooth margin white at first, dirty white after 4 weeks flat on the surface, without aerial mycelium, reverse greenish-grey. Hyphae septate branched, hyaline, thin, smooth-walled.
Material examined: UK, England, Arun River, 6 April 2015, on herbaceous stem, E.B.G. Jones, GJ126 (MFLU 16-1275, holotype); (isotype in BBH)
Notes: Tremateia arundicola resembles T. guiyangensis and T. halophila in having globose to subglobose ascomata with a thin peridium comprising cells of textura angularis and brown muriform ascospores. Both T. guiyangensis and T. halophila have comparatively shorter (\( \bar{x} \) = 150 μm) asci. They also have ascospores with more than 6 transverse septa and 2 vertical septa, while T. arundicola ascospores have less than 6 transverse septa and only one vertical septum.
Tremateia guiyangensis J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF552160; Facesoffungi number: FoF02235, Fig. 27
Etymology: Name reflects the place Guiyang, where the holotype was collected.
Holotype: MFLU 16-1299
Saprobic on dead herbaceous stems. Sexual morph Ascomata 130–280 μm high, 190–400 μm diam., scattered to clustered, subglobose to ovoid, with flattened base, coriaceous, immersed to semi-immersed or breaking through the host epidermis, dark brown to black, ostiolate, minutely papillate. Peridium up to 9–16 μm, composed of several layers of light to brown, thick-walled cells of textura angularis, becoming thin-walled and hyaline towards the centrum. Hamathecium comprising numerous, 2.3–5.1 μm wide, hypha-like, septate pseudoparaphyses, tapering towards the terminal cells, and intermingled among asci, embedded in a gelatinous matrix. Asci 152–160 × 21–27 μm (\( \bar{x} \) = 149 × 24 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical-clavate, with a furcate pedicel, apically rounded, with a minute ocular chamber. Ascospores 20–28 × 9–12 μm (\( \bar{x} \) = 25 × 11 μm, n = 30), muriform, normally overlapping 1–2-seriate, ellipsoid to broad fusiform, both parts of the spore ±equal in size, with 3–5 transverse septa, and 1 longitudinal septum in each row, obviously constricted in the central septum and slightly constricted at other septa, initially hyaline, becoming golden-brownish to brown when mature, rounded at both ends and lacking any gelatinous sheath or appendages. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 3.5 cm diam. after 12 days at 25 °C, circular, raised at center, margin smooth, white at first, palely pigmented after 10 days at the center, reverse yellowish to reddish. Hyphae septate, branched, hyaline, thin, smooth-walled.
Material examined: CHINA, Guizhou, Guiyang, around Guizhou Academy of Agriculture Sciences, on dead herbaceous stems, 17 August 2015, J.F. Zhang, GZ-01 (MFLU 16-1299, holotype), ex-type living culture, MFLUCC.
Notes: Tremateia guiyangensis is phylogenetically close to T. arundicola and T. halophila, and also morphologically similar in having globose to subglobose or flattened, immersed to semi-immersed ascomata, with clavate asci and muriform ascospores. However, T. guiyangensis was collected from terrestrial habitat, while the other two species were collected from aquatic habitats. In addition, the asci of T. guiyangensis are shorter than T. arundicola (\( \bar{x} \) = 149 vs. 185 μm) and the ascospores are smaller than T. halophila (\( \bar{x} \) = 26 × 6.5 μm vs. 30 × 16 μm). There are more transverse and vertical septa in T. guiyangensis than in T. arundicola, and the ascospores of T. arundicola are paler than the other two species.
Lentitheciaceae Yin. Zhang et al.
Zhang et al. (2009b) established Lentitheciaceae to accommodate the saprobic genera Katumotoa, Keissleriella, Lentithecium and Tingoldiago described from aquatic and terrestrial habitats. The type genus of the family is the aquatic genus Lentithecium. Several new genera and species from different habitats have since been introduced in Lentitheciaceae based on molecular data. Quaedvlieg et al. (2013) established Setoseptoria to accommodate saprobic coelomycetes that are Septoria-like but with setose conidiomata and belong to Lentitheciaceae. Knapp et al. (2015) described the endophytic genus, Darksidea from semiarid sandy grasslands and multi-genes phylogenetic analyses placed Darksidea in Lentitheciaceae. Phookamsak et al. (2015) introduced Poaceascoma to accommodate a species with setose ascomata and filiform ascospores. Wijayawardene et al. (2015) introduced Phragmocamarosporium Wijayawardene et al. to accommodate two coelomycetous species with brown phragmospores and phialidic conidiogenesis. The family currently includes 12 genera (Tanaka et al. 2015). In this paper we introduce two new species of Lentithecium and two new species of Poascoma and provide a new phylogenetic tree for Lentitheciaceae (Fig. 28).
Phylogram generated from maximum Likelihood analysis (MEGA6) based on combined dataset of SSU and LSU rDNA sequences of the two Lentithecium strains and related taxa in the family Lentitheciaceae. The tree is rooted to Dothidea sambuci. Maximum Likelihood bootstrap support values greater than 50 % are indicated. The new species are in blue and ex-types in bold
Lentithecium K.D. Hyde et al.
The genus Lentithecium was established to accommodate Massarina arundinacea (Sowerby) Leuchtm., M. fluviatilis Aptroot & Van Ryck. and Keissleriella linearis E. Müll. ex Dennis. The genus currently contains seven species that were described from aquatic (Suetrong et al. 2009; Tanaka et al. 2015), or L. arundinaceum (Sowerby) K.D. Hyde et al. and L. rarum (Kohlm. et al.) Suetrong et al. from marine habitats. Lentithecium species have been described from decayed submerged parts of Phragmites (three species), Juncus (one species), and submerged wood (three species) (Zhang et al. 2009b; Suetrong et al. 2009; Tanaka et al. 2015).
Lentithecium unicellulare Abdel-Aziz, sp. nov.
Index Fungorum number: IF552267; Facesoffungi number: FoF02433, Fig. 29
Etymology: In reference to the unicellular conidia.
Holotype: CBS H-22674.
Saprobic on decayed wood in freshwater habitats. Sexual morph Undetermined. Asexual morph Conidiomata 115–235 μm high × 140–235 wide μm (\( \bar{x} \) = 176.6 × 252.5 μm, n = 6), pycnidial, solitary or aggregated, uniloculate, immersed, erumpent to superficial, globose, subglobose, ovate, elongated, papillate, ostiolate, dark brown to black, surrounded by brown, septate, thick-walled hyphae, 3–4 μm wide. Pycnidial wall 18–25 μm thick, composed of 6–8 layers of yellow-brown to black brown cells of textura angularis, hyaline inner layer lining bearing conidiogenous cells. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 9–14 × 8–12 μm (\( \bar{x} \) = 11.8 × 10.3 μm, n = 12), holoblastic, determinate, smooth-walled, hyaline, globose, subglobose to pear-shaped with rounded or truncate base, sometimes proliferating once and each conidiogenous cell producing one to two conidia. Conidia 6–9 × 4–5 μm (\( \bar{x} \) = 8.1 × 4.3 μm, n = 50), subglobose, ovate, clavate, ellipsoid, allantoid, rectangular or irregular, with or without truncate base, hyaline, unicellular, smooth-walled.
Culture characteristics: Colonies on PDA 45–50 mm diam. after 10 days at 23 °C, dark grey with irregular margins, reverse black, dense growth with aerial and submerged mycelia without diffusible pigments. Pycnidia were produced in pure cultures after 4 weeks. Dimensions of pycnidia and conidia were similar to those recorded from natural substrates.
Material examined: EGYPT, Sohag City, on decayed wood submerged in the River Nile, 14 August 2014, F. A. Abdel-Aziz (CBS H-22674, holotype), ex-type living culture MD 6004.
Notes: Asexual genera reported so far under Lentitheciaceae are coelomycetous with variable morphology and these include: Phragmocamarosporium, Pleurophoma, Setoseptoria and Stagonospora macropycnidia (Tanaka et al. 2015). Lentithecium unicellulare differs from Stagonospora species in having globose, unicellular, holoblastic, determinate conidia that are smaller. Species of Stagonospora have cylindrical, multi-septate conidia, while conidiogenesis is holoblastic, occasionally annellidic, with single proliferation, discrete and indeterminate (Sutton 1980). The genus Stagonospora is polyphyletic and considered as the asexual morph of Phaeosphaeria (Leuchtmann 1984). Pleurophoma species differ from L. unicellulare in having long, filiform, septate, branched conidiophores and enteroblastic, phialidic, integrated, determinate conidiogenous cells (Sutton 1980). Setoseptoria species differ from L. unicellulare in having subcylindrical, transversely euseptate conidia, becoming constricted at septa and disarticulating into phragmospores when old. Conidiogenesis in Setoseptoria rarely has percurrent proliferations (Quaedvlieg et al. 2013).
Lentithecium voraginesporum Abdel-Wahab, Bahkali & E.B.G. Jones, sp. nov.
Index Fungorum number: IF 552266; Facesoffungi number: FoF02432, Fig. 30
Etymology: After the Latin word “Voraginem”, meaning “gulf”, where the fungus was recorded.
Holotype: CBS H-22560.
Saprobic on submerged wood in mangroves. Sexual morph Ascomata 125–215 μm diam., globose to subglobose, immersed to erumpent, ostiolate, papillate, coriaceous. Peridium 25–30 μm thick at the upper part around the ascomatal venter, two-layered, outer layer with yellow-brown cells forming a textura-angularis, inner-layer comprising thin-walled, hyaline, flattened cells; 11–17 μm thick at the basal part of the ascomata. Asci 38–50 × 8–10 μm (\( \bar{x} \) = 44 × 9.3 μm, n = 15), 8-spored, bitunicate, clavate, short pedicellate, developing at the base of venter. Ascospores 15–21 × 5–6 μm (\( \bar{x} \) = 18.8 × 5.3 μm, n = 50), bi-seriate, yellow-brown to reddish-brown, ellipsoidal with rounded ends, 1-septate, septum sub-median, upper cell is longer and wider, with roughened surface. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA 20–25 mm diam. after 10 days at 23 °C, brown to dark-brown aerial and immersed mycelia with dark-brown reverse with circular margins, without diffusible pigments.
Material examined: SAUDI ARABIA, Arabian Gulf, Tarut mangroves, on submerged, decayed Phragmites australis (Cav.) Trin. ex Steud. (Poaceae), stem inside the mangrove stand, 28 March 2013, M.A. Abdel-Wahab (CBS H-22560, holotype), ex-type living culture MD 1342.
Notes: Lentithecium voraginesporum is the third marine species in the genus and differs from the other seven described Lentithecium species in having small ascomata, asci and ascospores. Ascospores of the new species are characterized by their brown colour, roughened surface and the absence of a gelatinous sheath. The phylogenetic analyses of both SSU and LSU sequence data place L. voraginesporum within the family Lentitheciaceae in a well-supported clade with the recently described species, L. cangshanense Z.L. Luo et al. and L. unicellulare Abdel-Aziz. Both species were described from freshwater habitats (Su et al. 2016b; this article). Lentithecium voraginesporum differs from L. cangshanense in having smaller ascomata (125–215 μm vs. 210–320 μm) and asci (38–50 × 8–10 μm vs. 65–78 × 11–13 μm), longer and narrower ascospores with a rough surface (15–21 × 5–6 μm vs. 16.5–17.5 × 6–7 μm) and its marine habitat. Lentithecium unicellulare produces dark brown to black pycnidia and unicellular, hyaline conidia and its sexual morph is unknown. Molecular data delimit L. voraginesporum from L. cangshanense and L. unicellulare (Fig. 28). Lentithecium fluviatile differs from L. voraginesporum in having hyaline, longer and wider ascospores (24–31 × 7–10 μm vs. 15–21 × 5–6 μm) that are 2–3-septate and surrounded by a wide expanding mucilaginous sheath (van Ryckegem and Aptroot 2001). Lentithecium clioninum and L. pseudocloninum have larger asci and ascospores than those reported for L. voraginesporum. Ascospores of the latter two species are hyaline and surrounded by gelatinous sheath (Tanaka et al. 2015).
Leptosphaeriaceae M.E. Barr
The family Leptosphaeriaceae was established by Barr (1987) in the order Pleosporales and is typified by Leptosphaeria. Species of Leptosphaeriaceae can be saprobic, hemibiotrophic or parasitic on stems and leaves of herbaceous or woody plants in terrestrial habitats (Hyde et al. 2013). Members of this family are characterized by single, papillate, immersed or erumpent, perithecial ascomata, with relatively thick peridia, bitunicate cylindrical asci and hyaline to brown, transversely septate ascospores (Hyde et al. 2013). The asexual morphs of the family Leptosphaeriaceae can be coelomycetous or hyphomycetous (Alves et al. 2013; De Gruyter et al. 2013; Hyde et al. 2013; Zhang et al. 2012b). In recent classifications Alternariaster, Heterospora, Leptosphaeria, Neophaeosphaeria, Paraleptosphaeria, Plenodomus, and Subplenodomus were included in the family (Ariyawansa et al. 2015b). In this study we introduce two new species within the genus Leptosphaeria.
Leptosphaeria Ces. & De Not.
For classification of Leptosphaeriaceae we follow Ariyawansa et al. (2015b) and provide an updated tree (Fig. 31).
RAxML maximum likelihood phylogenetic tree based on a LSU and ITS sequence data from species of Leptosphaeriaceae. Maximum likelihood bootstrap support values greater than 50 % are shown above the nodes. The ex-type strains are in bold and the new isolates are in red. The tree is rooted with Alternariaster helianthi
Leptosphaeria cirsii Jayasiri, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552155; Facesoffungi number: FoF02220, Fig. 32
Leptosphaeria cirsii (holotype). a, b Ascomata on host. c Section through the ascoma. d Peridium. e Pseudoparaphyses. f Mature ascus. g Immature ascus. h, i Ascospores. j Germinated ascospore. k Ascospores stained in Indian ink. l, m Culture from above (l) and below (m). n Fruiting body in culture. o Vertical section of conidioma. p Conidioma wall. q, r Conidiogenous cells and conidia s Conidia. Scale bars a = 500 μm, b = 200 μm, c = 100 μm, d, e–g, o, p = 30 μm, h–k = 10 μm, l–m = 4 cm, q–s = 5 μm
Etymology: The specific epithet circii is based on the host genus from which the taxon was collected.
Holotype: MFLU 15-1072
Saprobic on dead stem of Cirsium sp. Sexual morph Ascomata 285–315 × 335–360 μm (\( \bar{x} \) = 304 × 347 μm, n = 10), solitary or scattered, superficial, globose to subglobose, broadly or narrowly conical, coriaceous, smooth-walled, ostiolate. Ostiole usually papillate, darkened at the base. Peridium 22–27 μm (\( \bar{x} \) = 24 μm, n = 20) wide, comprising two types of cells, outer cells of 1–2 layers of heavily pigmented cells of textura angularis, inner layer composed of small, light brown to hyaline cells of textura angularis. Hamathecium of septate, long, hyaline, cellular pseudoparaphyses, between and above the asci. Asci 75–110 × 10–12 μm (\( \bar{x} \) = 95 × 11 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindro-clavate, with a short, broad pedicel, thickened and rounded at the apex, with a distinct ocular. Ascospores 19–22 × 4–4.5 μm (\( \bar{x} \) = 20 × 4.2 μm, n = 40), overlapping uni-seriate, hyaline to light brown when immature, becoming brown to chestnut brown when mature, ellipsoidal with narrowly rounded ends, 3-septate, smooth-walled with mucilaginous sheath. Asexual morph Coelomycetous on MEA. Conidiomata 150–200 × 195–220 μm (\( \bar{x} \) = 190 × 205 μm, n = 10), pycnidial, superficial, immersed in media, globose to subglobose, black, without an ostiole. Conidiomata wall 15–25 μm wide, composed cells of textura angularis in multiple layers, pale yellowish-brown, remaining hyaline in inner layer. Conidiogenous cells 2–5 × 2–4 μm (\( \bar{x} \) = 3.5 × 2.5 μm, n = 30), enteroblastic, phialidic, determinate integrated, subglobose to short conical. Conidia 3–6 × 1–3 μm (\( \bar{x} \) = 3.8 × 1.5 μm, n = 50), hyaline, aseptate, oblong to cylindrical, thin-walled, smooth, guttulate, rounded at both ends.
Culture characteristics: Colonies on MEA 60 mm diam. after 4 weeks at 18 °C, white on the top and reverse, reverse yellow to brown near the middle, some area dense, circular, regular margin, without diffusible pigments.
Material examined: ITALY, Province of Trento [TN], near Vermiglio—Val di Sole, on dead stem of Cirsium sp. (Asteraceae), 9 August 2014, E. Camporesi IT 2044, (MFLU 15-1072, holotype); (KUN, isotype); ex-type living culture, MFLUCC 14-1170, KUNCC
Notes: Leptosphaeria cirsii is introduced here based on both morphology and phylogeny. Leptosphaeria cirsii is typical of Leptosphaeria in having a peridium of plectenchymatous cells and reddish to yellowish-brown, fusoid, 3-septate ascospores. Phylogenetically, L. cirsii clusters with L. cichorium with relatively moderate support (61 % ML) and also shares affinities to L. italica. Morphologically L. cirsii is similar to L. cichorium in having similar asci, ascospores, cellular pseudoparaphyses and asexual state morphology, but differs in having, superficial, globose to subglobose, broadly or narrowly conical ascomata and ascospores with a mucilaginous sheath (Ariyawansa et al. 2015b). Leptosphaeria cirsii resembles L. italica but differs as L. cirsii has long and narrow ascospores and lacks an ostiolar canal (Dayarathne et al. 2015).
Leptosphaeria irregularis R.H. Perera, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF552172, Facesoffungi number: FoF02234, Fig. 33
Leptosphaeria irregularis (holotype). a, b Appearance of ascomata on host substrate. c Vertical section through ascoma. d Close up of the peridium. e Close up of the ostiole. f Pseudoparaphyses. g, h Asci. i–k Immature and mature ascospores. l Germinating ascospore. m, n Colonies on MEA. Scale bars b = 1 mm, c = 200 μm, d–h = 50 μm, i–i = 20 μm
Etymology: Refers to the irregular shape of the fruiting body.
Holotype: MFLU 16-1091
Saprobic on unidentified plant stem. Sexual morph Ascomata 245–370 μm high, 180–368 μm diam. (\( \bar{x} \) = 285 × 255 μm, n = 10), superficial, appearing as black raised spots on the host, solitary or arranged in groups of 2–5, globose to irregularly globose, uniloculate, black, ostiolate. Ostiole aperiphysate. Peridium 30–67 μm wide, composed of 8–16 rows of scleroplectenchymatous cells, outer layer of amorphous black cells, inner layers composed of hyaline to pale brown cells of textura angularis. Hamathecium comprising numerous, 1.5–2.2 μm (n = 25) wide, filamentous, branched, septate, pseudoparaphyses. Asci 110–147 × 6–8.5 μm (\( \bar{x} \) = 130 × 8 μm, n = 30), 8-spored, bitunicate, long-cylindrical, with a short furcate pedicel, rounded at the apex. Ascospores 23.5–29 × 5–7 μm (\( \bar{x} \) = 27 × 6 μm, n = 40), uni-seriate to overlapping uni-seriate, fusiform, 3-septate, constricted at the septa, initially hyaline, becoming reddish to yellowish-brown at maturity, conical at the ends, without a mucilaginous sheath. Asexual morph Undetermined.
Culture characters: Colonies growing on MEA, reaching 4 cm diam. in 21 days at 16 °C, white, dense, moderate aerial mycelium on the surface, underneath pale yellow, margins even.
Material examined: UK, Hampshire, Swanick Wood, on unidentified plant stem, 28 September 2015, E.B. Gareth Jones, GJ 199 (MFLU 16-1091, holotype), (isotype in HKAS); ex-type living cultures MFLUCC 15-1118, GAAS).
Notes: Leptosphaeria irregularis is typical of Leptosphaeria in having a peridium of scleroplectenchymatous cells and 3-septate, reddish to yellowish-brown, fusiform ascospores. Phylogenies herein show that L. irregularis is a sister taxon to L. slovacica and can be considered distinct to other known Leptosphaeria species (Fig. 31). Morphologically L. irregularis is similar to L. slovacica, but differs in its larger (23.5–29 μm vs. 18–22 μm) ascospores.
Lindgomycetaceae K. Hirayama et al.
The family Lindgomycetaceae was introduced by Hirayama et al. (2010) for the freshwater genus Lindgomyces (L. breviappendiculatus, L. cinctosporae, L. ingoldianus and L. rotundatus) and its sister taxon, Massariosphaeria typhicola. Molecular phylogenetic studies using ribosomal sequence data from different lineages of Dothideomycetes also showed that Lindgomycetaceae is a unique lineage among the Pleosporales (Zhang et al. 2012b; Hyde et al. 2013; Wijayawardene et al. 2014b; Ariyawansa et al. 2015c; Wanasinghe et al. 2015). Currently there are 12 species included the genus Lindgomyces including the novel taxon introduced below. Multi-gene phylogenetic analyses also placed Arundellina typhae, Clohesyomyces aquaticus, Hongkongmyces pedis, Lolia aquatica, Massariosphaeria typhicola, Phyllosticta flevolandica and Trematosphaeria hydrela in Lindgomycetaceae (Abdel-Aziz and Abdel-Wahab 2010; Zhang et al. 2012b; Tsang et al. 2014; this study). Most of the Lindgomycetaceae members have been recorded from freshwater habitats, but Hongkongmyces is associated with IgG4-related sclerosing disease of humans (Tsang et al. 2014).
Arundellina Wanasinghe, E.B.G. Jones & K.D. Hyde, gen. nov.
Index Fungorum number: IF552132; Facesoffungi number: FoF02208.
Etymology: Name reflects the Arun River, and town Arundel, from where the holotype was collected.
Saprobic on dead stem, sheaths and leaves. Sexual morph Ascomata immersed, solitary, scattered, globose, dark brown to black, coriaceous, ostiolate. Ostiole papillate, black, smooth, filled with hyaline to pale brown cells. Peridium thin, with 4–5 layers, outer layer heavily pigmented, thick-walled, comprising reddish to dark brown cells of textura angularis, inner layer composed of hyaline thin-walled cells of textura angularis. Hamathecium comprising numerous, filamentous, branched, septate pseudoparaphyses. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, pedicellate, thick-walled at the apex, with an ocular chamber. Ascospores overlapping 1–2-seriate, fusiform, slightly curved, widest in the center, 3–4 transversely septate, without vertical septa, constricted at the septa, initially hyaline, becoming golden-pale brown at maturity, ends remaining cone-shaped, with pointed ends, without a mucilaginous sheath. Asexual morph Undetermined.
Type species: Arundellina typhae Wanasinghe, E.B.G. Jones & K.D. Hyde
Notes: The genus Arundellina is characterized by immersed, globose, ascomata, papillate ostiole, a thin peridium composed of cells of textura angularis, cylindrical to cylindric-clavate asci with a short pedicel and thick-walled apex and fusiform, golden-pale brown ascospores with 3–4 transverse septa and cone-shape pointed ends. The globose ascomata, cylindrical to cylindric-clavate asci and fusiform ascospores with 3–4 transverse septa of Arundellina resemble those of Equiseticola, Galiicola, Loratospora, Paraleptosphaeria and Phaeosphaeria in Phaeosphaeriaceae, Neolophiostoma in Halotthiaceae and Mytilinidion in Mytilinidiaceae. LSU and SSU combined gene phylogenetic analyses indicate that Arundellina belongs in Lindgomycetaceae, but is distinct from other genera in the family (Fig. 34).
Phylogram generated from maximum likelihood analysis based on combined LSU and SSU sequence data for species of Lindgomycetaceae. Maximum likelihood, Maximum parsimony bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.90 are near the nodes. The new isolates are in blue bold, and other ex-type strains are in black bold. The scale bar indicates 0.006 changes. The tree is rooted with Aigialus mangrovis and A. grandis
Arundellina typhae Wanasinghe, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF552133; Facesoffungi number: FoF02209, Fig. 35
Etymology: Name reflects the host genus Typha.
Holotype: MFLU 16-1276
Saprobic on submerged stem and sheaths of Typhaceae sp. Sexual morph Ascomata 250–300 μm high × 200–250 μm diam. (\( \bar{x} \) = 272.1 × 229.5 μm, n = 5), immersed, solitary, scattered, globose, dark brown to black, coriaceous, ostiolate. Ostiole 70–90 μm high, 40–60 μm diam. (\( \bar{x} \) = 80.2 × 51.4 μm, n = 5), papillate, black, smooth, filled with hyaline to pale brown cells. Peridium 18–22 μm wide at the base, 20–30 μm wide in sides, with 4–5 layers, outer layer heavily pigmented, thick-walled, comprising reddish to dark brown cells of textura angularis, inner layer composed of hyaline, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2.5–3.5 μm wide, filamentous, branched, septate, pseudoparaphyses. Asci 110–115 × 15–20 μm (\( \bar{x} \) = 112.4 × 17.5 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, pedicellate, thick-walled at the apex, with an ocular chamber. Ascospores 30–40 × 5–7 μm (\( \bar{x} \) = 34.2 × 6.1 μm, n = 30), overlapping 1–2-seriate, fusiform, slightly curved, widest in the center, 3–4 transversely septate, without vertical septa, constricted at the septa, initially hyaline, becoming golden-pale brown at maturity, ends remaining cone-shaped, with pointed ends, without a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 2 cm diam. after 30 days at 16 °C, circular, margin smooth, dirty white at first, greenish dark brown after 4 weeks flat on the surface, without aerial mycelium, reverse blackish-brown. Hyphae septate branched, hyaline, thin, smooth-walled.
Material examined: UK, England, Arun River, on dead submerged stem of Typha sp (Typhaceae), 6 April 2015, E.B.G. Jones, GJ122 (MFLU 16-1276, holotype); (isotype in BBH), ex-type culture, MFLUCC 16-0310, MUCL. UK, Dorset, West Lulworth, Lulworth Cove, 24 February 2015, on Typha sp., E.B.G. Jones, GJ096 (MFLU 16-1277, paratype); ex-paratype culture, MFLUCC 16-0309, MUCL.
Lindgomyces pseudomadisonensis Tak. Takah. & Kaz. Tanaka, sp. nov.
MycoBank number: MB817598; Facesoffungi number: FoF02434, Fig. 36
Lindgomyces pseudomadisonensis (holotype). a, b Appearance of ascomata on substrate. c Ascoma in longitudinal section. d Peridium in longitudinal section. e Asci. f Pseudoparaphyses. g–j Ascospores. k Germinating ascospore. Scale bars a = 400 μm, b = 200 μm, c = 50 μm, d = 25 μm, e = 10 μm, f–k = 5 μm
Etymology: Referring to its resemblance to Lindgomyces madisonensis.
Holotype: HHUF 30513
Saprobic on submerged wood. Sexual morph Ascomata 190–240 μm high, 230–360 μm diam., globose to subglobose, black, scattered to grouped, immersed to erumpent. Ostiolar neck 90–100 μm long, 40–55 μm wide, papillate, central. Peridium 50–55 μm thick, composed of an inner layer of polygonal to subglobose, hyaline to pale brown cells and an outer layer of brown cells. Pseudoparaphyses cellular, numerous, 1.5–2.5 μm wide, anastomosed, branched. Asci 92.5–130 × 13.5–17.5 μm (\( \bar{x} \) = 108.3 × 15.6 μm, n = 8), fissitunicate, clavate, rounded at the apex, with an apical chamber, with eight overlapping bi-seriate ascospores. Ascospores 28–41(–45.5) × (5.5–)7–10 μm (\( \bar{x} \) = 36.3 × 7.9 μm, n = 63), l/w 3.7–6.1 (\( \bar{x} \) = 4.6, n = 63), fusiform with acute ends, straight or slightly curved, with the primary septum almost supramedian (0.40–)0.42–0.51 (\( \bar{x} \) = 0.47, n = 62), slightly constricted at the primary septum, with a broad upper cell, hyaline, smooth, becoming 3-septate and pale brown with age, surrounded by an entire gelatinous sheath ca. 2 μm wide. Asexual morph Undetermined.
Material examined: JAPAN, Aomori, Nishimeya, Seisyu trail, Ooshirosawa River, on submerged dead twigs of woody plant, 28 August 2010, K. Tanaka et al., KT 2742 (HHUF 30513, holotype); ex-type living culture, MAFF 245610.
Notes: Lindgomyces pseudomadisonensis is morphologically similar to L. madisonensis and phylogeny also support a close association between them (Fig. 34). However, the former has shorter ascospores (\( \bar{x} \) = 39 ± 2 × 7 ± 1 μm). In addition, the ascospores of L. pseudomadisonensis have an entire gelatinous sheath. The identities of ITS sequences between L. pseudomadisonensis and L. madisonensis are rather low [GenBank KT207819; Identities = 541/564 (95.9 %), Gaps = 9/564 (1.5 %)].
Lophiostomataceae Sacc.
The family Lophiostomataceae was introduced by Nitschke (1869) with Lophiostoma macrostomum (Tode) Ces. & De Not. as the type species (Eriksson 1981; Mugambi and Huhndorf 2009; Thambugala et al. 2015b). Most species in this family are widely distributed on twigs, stems, or bark of various woody plants and herbaceous plants in terrestrial and aquatic environments (Ellis and Ellis 1985; Mugambi and Huhndorf 2009; Zhang et al. 2009b; Hirayama and Tanaka 2011; Hyde et al. 2013; Thambugala et al. 2015b).
Vaginatispora fuckelii (Sacc.) Thambugala, Wanasinghe, Kaz. Tanaka & K.D. Hyde, Fungal Diversity 74: 242. 2015.
Index Fungorum Number: IF551535; Facesoffungi number: FoF00829
Basionym: Lophiostoma fuckelii Sacc., Michelia 1(no. 3): 336 (1878)
= Lophiostoma pulveraceum Sacc., Michelia 1: 336, 1878
= Didymosphaeria lophospora Sacc. & Speg., Michelia 1: 376, 1878
= Lophiosphaera mendax Rehm, Ann. Myc. 5: 544, 1907
Saprobic on dead branch of Rosa sp. Sexual morph Ascomata 145–205 μm high, 140–258 μm diam., solitary, scattered to clustered, immersed to semi-immersed or erumpent, globose to subglobose, glabrous, uniloculate, rarely bi-loculate, papillate, visible as raised, black spots on host surface. Peridium 18–26 μm wide, thin-walled, unevenly thickened, two layered; inner layer comprising 3–5 cell layers of flattened, hyaline cells of textura prismatica; outer layer comprising several layers of dark brown to black cells of textura angularis. Hamathecium composed of dense, 1.7–2.5 μm wide, filamentous, indistinctly septate, cellular pseudoparaphyses, anastomosing at the apex, embedded in a hyaline gelatinous matrix. Asci 43–72 × 4.6–6.8 μm (\( \bar{x} \) = 57 × 5.6 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicel with furcate to obtuse ends, apically rounded with an indistinct ocular chamber. Ascospores 15–23 × 3–4.7 μm (\( \bar{x} \) = 19.7 × 3.8 μm, n = 30), overlapping bi-seriate, hyaline, subfusoid, with rounded or obtuse ends, initially aseptate, becoming 1-septate at maturity, strongly constricted at the septum, enlarged near the septum at the upper cell, smooth-walled, guttulate, surrounded by thin distinctive sheath when immature, invisible at maturity, bearing appendages at both ends. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA, 33–35 mm diam. after 3 weeks, colonies medium dense, irregular, flat, slightly raised, surface smooth with crenate edge, fluffy to velvety with smooth aspects, zonate with different sector yellowish-grey to yellowish-brown at the margin whitish-grey to brownish-grey at the centre; reverse whitish-grey at margin, yellowish-brown with fimbriate aspect at the midlle, black at the centre, no pigmentation produced in media.
Material examined: CHINA, Yunnan Province, Kunming, on dead branch of Rosa sp. (Rosaceae), 9 June 2015, Wen Jing Li, NI005 (HKAS 92495); living culture KUMCC 15-0523.
Notes: This is the first record of Vaginatispora fuckelii on dead branch of Rosa species (Rosaceae) in China. The current maximum likelihood analysis shows that the new strain (KUMCC 15-0523) clusters with other Vaginatispora fuckelii strains with low bootstrap support (Fig. 37). The new strain, however, differs from the others in asci and ascospores size. Vaginatispora fuckelii has 60–90 × 9–12 μm asci and 15–17 × 4–5 μm ascospores (Thambugala et al. 2015b), whereas the new strain has 43–72 × 4.6–6.8 μm asci and 15–23 × 3–4.7 μm ascospores. Vaginatispora fuckelii has been recorded from various dead herbaceous twigs including Vitis coignetiae (Thambugala et al. 2015b) and also occurs on leaves of Mangifera indica (Wang and Lin 2004). The known distribution of this species is Sweden, UK, Germany, Switzerland, Taiwan and Japan (Wang and Lin 2004, Thambugala et al., 2015b). Vaginatispora fuckelii is distinct from other Vaginatispora species in having 1-septate, guttulate, ascospores with a sheath and appendages at both ends (Fig. 38).
RAxML tree based on analysis of a combined dataset of ITS, LSU, SSU and TEF partial sequences of Lophiostomataceae. Bootstrap support values for maximum likelihood (ML, black) higher than 50 % and Bayesian posterior probabilities (BYPP, red) greater than 0.95 are defined as above the nodes. The tree is rooted to Melanomma pulvis-pyrius. All type strains are in bold. New strains are given in blue
Vaginatispora fuckelii (HKAS 92495). a Herbarium material. b Appearance of ascomata on host. c Vertical section through ascoma. d Peridium. e–g Asci. h Pseudoparaphyses. i–k Ascospores. l Ascospore stained in Indian ink. m Germinating ascospore. n Upper view of culture on PDA. o Lower view of culture on PDA. Scale bars c = 50 μm, d, e–g, m = 20 μm i–l = 10 μm
Lophiotremataceae K. Hiray. & Kaz. Tanaka
The family Lophiotremataceae was introduced by Hirayama and Tanaka (2011) to accommodate a single genus Lophiotrema, typified by L. nucula (Fr.) Sacc. Lophiotrema shares morphological characters with Lophiostoma due to its carbonaceous ascomata with compressed, crest-like apex and was previously regarded as a synonym of Lophiostoma (Chesters and Bell 1970; Zhang et al. 2009b, 2012b; Hirayama and Tanaka 2011; Hyde et al. 2013). However, these two genera can be distinguished by their peridial structure and shape of ascus (Zhang et al. 2009a, b; Hirayama and Tanaka 2011, Hyde et al. 2013). The peridial structure of Lophiotrema is usually thin, of equal thickness, composed of cells of textura angularis to textura globulosa and asci are often oblong to cylindrical with hyaline ascospores (Zhang et al. 2009a, b; Hirayama and Tanaka 2011; Hyde et al. 2013). In Lophiostoma the peridium is of unequal thickness and is usually broader near the base, while asci are mostly clavate (Zhang et al. 2009b; Hirayama and Tanaka 2011). Doilom et al. (2016) accepted two other genera, Hermatomyces and Aquasubmersa in the Lophiotremataceae as in the phylogenetic analyses these two genera are related to Lophiotrema.
Species in Lophiotrema are not well-studied and lack modern taxonomic treatments and molecular data. There are 159 epithets recorded in Index Fungorum (2016), but only 96 sequences from six species in GenBank. In this study, we introduce two new taxa, Lophiotrema bambusae and L. fallopiae. We also provide a new combination for Scyphostroma mirum under Hermatomyces mirum and introduce H. subiculosa sp. nov.
Hermatomyces mirum (Starbäck) C.G. Lin, D.J. Bhat, Yong Wang bis & K.D. Hyde, comb. nov.
Basionym: Scyphostroma mirum Starbäck, Bih. K. svenska Vetensk Akad. Handl., Afd. 3 25(no. 1): 23 (1899)
= Subicularium reticulatum M.L. Farr & Goos, Mem. N. Y. bot. Gdn 49: 66 (1989)
Index Fungorum number: IF552284; Facesoffungi number: FoF02435
Notes: Farr and Goos (1989) introduced a monotypic genus, Subicularium, with S. reticulatum M.L. Farr & Goos as type, and provisionally placed the genus in the order Agonomycetales of Deuteromycotina. The Dictionary of Fungi (Kirk et al. 2008) recorded S. reticulatum under an older name Scyphostroma mirum Starbäck (Starbäck 1899), apparently based on their similar morphology, while Seifert et al. (2011) treated them differently. Incidentally, the genus Scyphostroma Starbäck, typified by Sc. mirum Starbäck, was found to be wrongly described by the original author (Starbäck 1899). The description ran as follows: “Subiculum dark-brown, with reticulately branched hyphae, forming a cup-like nest with a distinct, thick margin. Perithecia minute, sphaerical, with distinct stalk, perched in the dense stromatic cup”. Starbäck (1899) mistook the conidia for perithecia. The species description indicated that the cup-like conidiomata measured 0.5–4 mm diam. and conidia (wrongly referred as ‘perithecia’) measured 40–50 μm diam. It is clear that Hermatomyces is the same as Scyphostroma.
Although Scyphostroma is the first name for the genus, we preferred to use Hermatomyces because the latter name is presently in use and the earliest name was wrongly described in the literature and has never been referred to any taxonomic discourses of the genus complex. Accordingly, in this study, we propose the synonymy of Scyphostroma mirum and Subicularium reticulatum, under the new combination Hermatomyces mirum, based on their morphological similarities and phylogenetic analysis (Fig. 39).
Phylogram generated from maximum parsimony analysis based on combined LSU, SSU and TEF1α sequence data of Lophiotremataceae. Maximum likelihood (left)/ parsimony (right) bootstrap support values greater than 50 % are shown above the nodes. The ex-type strains are in bold and the new species are indicated in blue. The tree is rooted to Dothidotthia symphoricarpi and D. aspera
Hermatomyces subiculosa C.G. Lin, D.J. Bhat, Yong Wang bis & K.D. Hyde, sp. nov.
Index Fungorum number: IF552285, Facesoffungi number: FoF02436, Fig. 40
Hermatomyces subiculosa (holotype). a Host (decaying wood). b, c Subiculum, conidiophores and conidia on the host surface, (1) subiculum (2) conidia. d Infertile hyphae. e Conidiophores. f Conidiophores, conidiogenous cells and conidia, (3) parts of the conidiogenous cells, (4) conidiophores. f–h Conidia. l, m 20-day old colonies on MEA, l from above, m from below. Scale bars b = 500 μm, c = 200 μm, d–f = 20 μm, g–k = 10 μm
Etymology: Referring to the conspicuous subiculum on the host surface.
Holotype: MFLU 16-1300.
Saprobic on decaying wood. Sexual morph Undetermined. Asexual morph Colonies subiculate on natural substrate, superficial, effuse, floccose, greyish to dark brown. Mycelium superficial, composed of septate, flexuous, repeatedly branched, pale to brown, 2–4 μm wide infertile hyphae. Conidiophores micronematous or semi-macronematous, mononematous, brown, smooth, confined to the center and surrounded by infertile hyphae, 3–5.5 μm wide. Conidiogenous cells holoblastic, subhyaline, 3–5.5 μm wide. Conidia solitary, dry, acrogenous, muriform, deeply constricted at septa, globose, oblong, ellipsoidal, pyriform, verruculose, subhyaline to pale brown when young, dark brown when mature, 15–35 μm (\( \bar{x} \) = 26.23 μm, n = 39) long, 18–30 μm (\( \bar{x} \) = 24.28 μm, n = 39) wide at the broadest part, sometimes with part of conidiogenous cell attached.
Culture characteristics: Colonies on MEA attaining a diam. of 2.5–3.5 cm at room temperature (25 °C) in 3 weeks, effuse, hairy, grey above, pale brown at the margins, dark brown at the center from below.
Material examined: THAILAND, Chiang Rai, Mae Sai District, Ang Kep Nam Wat Tham Khao Hin Phayanak (Wat Tham Sao Hin Payanak), 20°19′16.58″–20°19′30.12″N, 99°51′40.72″–99°51′54.50″E, on decaying wood, 19 June 2015, Chuan-Gen Lin, WTSP 1-1 (MFLU 16-1300, holotype; HKAS 95052, isotype), ex-type living culture MFLUCC 15-0843.
Notes: We found that our new species forms a separate well-supported clade within the genus Hermatomyces, sister to H. thailandica (MFLUCC 14-1143, MFLUCC 14-1145 and MFLUCC 14-1144) and H. tectonae (MFLUCC 14-1140 and MFLUCC 14-1141) in the family Lophiotremataceae. The new species is morphologically similar to H. mirum in having a conspicuous subiculum and the conidia of these two species are hyaline to pale brown when young, and dark brown when mature. However, the conidia in our new species (18–30 μm) are smaller than H. mirum (40–50 μm) verruculose and, in addition, the subiculum of H. mirum are more or less disc-shaped, with individual discs 500–4000 μm in diam., while the subiculum in the new species are effuse on the natural substrate. Therefore, based on differences in morphology and molecular data, we introduce the new species, Hermatomyces subiculosa.
Lophiotrema bambusae Phookamsak, S.C. Karunarathana & K.D. Hyde, sp. nov.
Index Fungorum number: IF552203; Facesoffungi number: FoF02257, Fig. 41
Lophiotrema bambusae (MFLU 11-0150, holotype). a Appearance of ascostromata on the host surface. b Section through the ascostroma. c Section through peridium. d Pseudoparaphyses stained in cotton blue. e–h Asci. i Ocular chamber stained in Melzer’s reagent. j–l Ascospores. m Ascospores stained in Indian ink. n, o Culture characteristics (n = from above, o = from below). Scale bars b = 100 μm, c–h = 20 μm, i = 10 μm, j–m = 5 μm
Etymology: The specific epithet “bambusae” refers to the host.
Holotype: MFLU 11-0150.
Saprobic on bamboo. Sexual morph Ascostromata 140–270 μm high, 250–370 μm diam., dark brown to black, scattered, gregarious, immersed to semi-immersed in host cortex, raised, conical to quadrilateral, or irregular in shape, uni-loculate, glabrous, coriaceous, ostioles central or lateral, with rim-like opening. Peridium 20–50 μm wide, thin- to thick-walled, of unequal thickness, slightly thick at the sides towards apex, composed of several layers of small, dark brown to black, pseudoparenchymatous cells, with host cells plus fungal tissue, arranged in a textura angularis to textura epidermoidea. Hamathecium composed of dense, 2–3 μm wide, cellular pseudoparaphyses, distinctly septate, anastomosing among the asci, embedded in a hyaline gelatinous matrix. Asci 65–100 × 8–10 μm (\( \bar{x} \) = 83.3 × 8.7 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical, short pedicellate, apically rounded with an ocular chamber. Ascospores 18–25 × 4–5 μm (\( \bar{x} \) = 21.3 × 4.4 μm, n = 25), overlapping bi-seriate, hyaline, fusiform, with rounded ends, 1-septate, rarely 3–5-septate, upper cell larger that lower cell, constricted at the central septum, smooth-walled, with guttules, surrounded by a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA reaching 39–45 mm diam. after 4 weeks at 25–30 °C; colony from above, dark green, with black concentric ring at the margin, black in the centre, with white turfs; from below, dark green, with black concentric ring at the margin, dark green to black at the centre, slightly radiating; medium dense, irregular, flattened, slightly raised at the middle, edge undulate, with entire margin, surface smooth, slightly rough, with white tufts, or black granules, woolly; not producing pigmentation in agar.
Material examined: THAILAND, Chiang Rai, Mae Fah Luang District, Doi Tung, on dead stem of bamboo, 28 April 2010, S.C. Karunarathana, RP0030 (MFLU 11-0150, holotype), ex-type living cultures, MFLUCC 10-0558, BBC.
Notes: Lophiotrema bambusae shares a size range of asci and ascospores, including number of ascospore septa with many Lophiotrema species, such as L. alpinum (Ellis & Everh.) M.E. Barr, L. arundinariae Rehm, L. culmifragum Speg., L. incisum Ellis & Everh., L. paspalicola Speg. and L. radicans (Ellis & Everh.) Sacc. However, the concept of Lophiotrema was unclear, when many of these species were introduced or transferred to the genus and they need restudying. The ascospore septation of L. bambusae is typically 1-septate (rarely 3–5-septate), similar to L. alpinum, L. culmifragum and L. incisum, while, L. arundinariae, L. paspalicola and L. radicans usually have 3-septate ascospores. Nevertheless, L. bambusae differs from L. alpinum and L. incisum based on host, with L. bambusae collected from bamboo, and the others collected from conifer and Ribes. Lophiotrema bambusae is most similar to L. culmifragum given its host and habitat. However, L. bambusae has slightly larger asci and mature ascospores have more septa. Phylogenetic analyses show that L. bambusae is related to L. lignicola and distinct from other taxa in Lophiotrema (Fig. 39). Therefore, the new species is established.
Lophiotrema fallopiae A. Hashim. & Kaz. Tanaka, sp. nov.
MycoBank number: MB817597; Facesoffungi number: FoF02475, Fig. 42
Lophiotrema fallopiae. a, b Appearance of ascomata on substrate. c Ascoma in longitudinal section. d Peridium of ascoma. e, f Ascus. g Ascus apex. h Pseudoparaphyses. i–k Ascospores. l Ascospore with gelatinous sheath (in Indian ink). m Germinating ascospore. n, o Conidiomata in culture. p Conidioma in longitudinal section. q Peridium of conidioma. r, s Conidiogenous cells. t, u Conidia. v Germinating conidium. a–m From HHUF 30506 (holotype); n–v from MAFF 245612 (ex-type culture). Scale bars a = 1 mm, b, n, o = 250 μm, c, p = 20 μm, d–f, q = 10 μm, g–m, r–v = 5 μm
Etymology: Referring to the generic name of host plant.
Holotype: HHUF 30506.
Saprobic on dead stem of Fallopia japonica. Sexual morph Ascomata 200–300 μm high, 180–250 μm diam., scattered, immersed, erumpent at the neck, subglobose in section. Ostiolar neck up to 40 μm high, compressed, composed of carbonaceous, black, thick-walled cells, without clypeus. Peridium 17.5–25.5 μm thick, 5–8 layers, composed of polygonal to elongate, thin-walled, 8–13 × 2.5–3 μm cells. Hamathecium comprises branched pseudoparaphyses numerous, trabeculate, 1–1.5 μm wide, septate. Asci (77–)93–125 × (5.5–)7–9 μm (\( \bar{x} \) = 105.8 × 7.6 μm, n = 10), 8-spored, numerous, bitunicate, fissitunicate, cylindrical, with a short stipe (5–11 μm long, \( \bar{x} \) = 7.4 μm, n = 10), apically rounded with an ocular chamber. Ascospores 19–24 × 4.5–6 μm (\( \bar{x} \) = 21.7 × 5.2 μm, n = 50), l/w 3.7–5.3 (\( \bar{x} \) = 4.2, n = 50), fusiform with rounded ends, straight, 1-septate, constricted, with a primary septum nearly median [0.45–0.55, \( \bar{x} \) = 0.51, n = 50], hyaline, smooth, guttulate when young, with an entire gelatinous sheath (2.5–6 μm wide at sides). Asexual morph Coelomycetes. Conidiomata pycnidial, globose to subglobose, up to 200 μm high in section, 120–180 μm diam., scattered, semi-immersed, solitary, black. Peridium 10–14 μm wide; outer layers composed of 7.5–10.5 × 4–7.5 μm, subglobose, brown cells; inner layers composed of 5–7.5 × 0.5–1.5 μm, “porrecta”, hyaline cells. Conidiophores reduced. Conidiogenous cells enteroblastic, phialidic, 7–9 × 2–4 μm, lageniform, hyaline, smooth. Conidia ellipsoidal with rounded ends, 2–3.5 × 1–2 μm (\( \bar{x} \) = 2.7 × 1.5 μm, n = 60), l/w 1.2–2.5 (\( \bar{x} \) = 1.8, n = 60), hyaline, aseptate, smooth.
Culture characteristics: Colonies on PDA attaining 12–16 mm diam. within 21 days at 20 °C in the dark.
Material examined: JAPAN, Aomori, Hirakawa, Kuzukawa, near Aseishi River, on dead stem of Fallopia japonica, 5 September 2010, K. Tanaka, KT 2748 (HHUF 30506, holotype); ex-holotype living culture, MAFF 245612.
Notes: The characters of L. fallopiae fit into the generic concept of Lophiotrema in having compressed ostiolar necks, a peridium composed of textura angularis, and cylindrical asci with short stipes. This species is similar to L. nucula, the type species of Lophiotrema, but L. nucula has shorter ascospores [17–21(–25) × (4–)5–6.5 μm; Zhang et al. 2009a]. Lophiotrema fallopiae is phylogenetically close to L. vagabundum (Fig. 39), but L. vagabundum has slightly narrower ascospores (20–26 × 4–5.5 μm, l/w 4.3–5.9; Tanaka and Harada 2003).
Massariaceae Nitschke
The family Massariaceae was introduced by Nitschke (1869) to accommodate the genus Massaria with M. inquinans (Tode) De Not. as the type species. The family is characterized by immersed globose, subglobose to pyriform, ascomata, with a thin-walled peridium comprising cells of textura angularis, oblong to cylindrical asci with a wide ocular chamber and refractive ring, and large, oblong to ellipsoidal ascospores, surrounded by a gelatinous sheath (Hyde et al. 2013). Seven new species were introduce in Massaria with molecular analyses of combined LSU, SSU, RPB2 and TEF1 sequence data. In this study, the new genus Neomassaria is introduced based on molecular and morphological comparison with descriptions and illustrations. The phylogenetic tree, based on combined LSU, SSU and TEF1 sequence data for the new taxon is presented in Fig. 43.
Phylogram generated from RAxML analysis of combined LSU, SSU and TEF sequence data of Massariaceae. Maximum Likelihood (ML) bootstrap support values greater than 50 % and Bayesian posterior probabilities (PP) greater than 0.95 are shown above and below branches. The ex-type strains are in bold and the new isolate is in blue. The tree is rooted with Acrospermum gramineum and A. compressum
Neomassaria Mapook, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF552273; Facesoffungi number: FoF02437
Etymology: The generic epithet refers to a new genus in the family Massariaceae.
Saprobic on dead branches. Sexual morph Ascomata immersed, solitary or scattered, coriaceous, globose to subglobose, brown to dark brown. Ostiole central. Peridium comprising light brown cells of textura angularis. Hamathecium comprising cylindrical to filiform, septate, branched, pseudoparaphyses. Asci 8-spored, bitunicate, oblong to cylindrical, short pedicellate, with wide ocular chamber. Ascospores overlapping 1–2-seriate, hyaline, ellipsoid to broadly fusiform, 1-septate, constricted at the septa, surrounded by gelatinous sheath. Asexual morph Undetermined.
Type species: Neomassaria fabacearum Mapook, Camporesi & K.D. Hyde, sp. nov.
Notes: Neomassaria fabacearum was collected from a dead branch of Hippocrepis emerus (L.) Lassen. Molecular data places Neomassaria in the family Massariaceae with high bootstrap support (91, ML with 0.99, PP) along with other species of Massaria. However, Neomassaria is distinct in morphology of ascomata, asci and ascospores. Phylogenies also reveal that Neomassaria can be considered as distinct genus given that it did not cluster with any Massaria species. Instead it is basal to them with high support (Fig. 43).
Neomassaria fabacearum Mapook, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552274; Facesoffungi number: FoF02438, Fig. 44
Neomassaria fabacearum (holotype). a, b Appearance of ascomata on substrate. c Section through ascoma. d Ostiole. e Peridium. f Pseudoparaphyses. g, h Asci. i–k Ascospores. l Ascospores surrounded by hyaline gelatinous sheath in Indian ink. Scale bars a = 500 μm, b = 200 μm, c, g–h = 50 μm, d = 20 μm, e, f, i–l = 10 μm
Etymology: Name reflects the host family Fabaceae, from which this holotype was collected.
Holotype: MFLU 16-1875.
Saprobic on a dead branch of Hippocrepis emerus. Sexual morph Ascomata (150–)200–220 μm high × (100–)130–150 μm diam. (\( \bar{x} \) = 190 × 130 μm, n = 5), immersed, solitary or scattered, coriaceous, globose to subglobose, brown to dark brown, Ostiole central. Peridium 10–20 μm wide, comprising light brown cells of textura angularis. Hamathecium comprising 1–2 μm wide, cylindrical to filiform, septate, branched, pseudoparaphyses. Asci 65–75(–85) × 10–15 μm (\( \bar{x} \) = 75 × 12 μm, n = 5), 8-spored, bitunicate, oblong to cylindrical, short pedicellate, with wide ocular chamber. Ascospores 18–20 × (4–)5–6 μm (\( \bar{x} \) = 19 × 5 μm, n = 15), overlapping 1–2-seriate, hyaline, ellipsoid to broadly fusiform, 1-septate, constricted at the septum, surrounded by hyaline gelatinous sheath observed clearly when mounted in Indian ink. Asexual morph Undetermined.
Material examined: ITALY, Forlì-Cesena, Cusercoli-Civitella di Romagna, on dead branch of Hippocrepis emerus (L.) Lassen (Fabaceae), 11 September 2014, E. Camporesi (MFLU 16-1875, holotype), ex-type culture MFLUCC 14-1117, (isotype as HKAS 95078 in HKAS).
Massarinaceae Munk
The family Massarinaceae was introduced by Munk (1956) and is typified by Massarina with M. eburnea (Tul. & C. Tul.) Sacc. as the type species. Currently Byssothecium, Corynespora, Helminthosporium, Massarina, Pseudodidymosphaeria, Pseudosplanchnonema, Stagonospora, and Suttonomyces are included in Massarinaceae (Chethana et al. 2015; Tanaka et al. 2015; Thambugala et al. 2015a; Fig. 45). Corynespora leucadendri Quaedvl. et al. and C. olivacea (Wallr.) M.B. Ellis cluster within Massarinaceae, however the generic type of Corynespora, C. cassiicola (Berk. & M.A. Curtis) C.T. Wei clusters in Corynesporaceae, which is a distinct family. Therefore, these two Corynespora species should be renamed. In this study we introduce a new species of Stagonospora.
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS, SSU, and TEF1-α sequence data from species of Massarinaceae. Maximum likelihood bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.90 are shown above the nodes. Ex-type strains are in bold and the new isolate is in blue. The tree is rooted with Periconia digitata
Stagonospora (Sacc.) Sacc.
Stagonospora is typified by S. paludosa (Sacc. & Speg.) Sacc., a species from Carex pseudocyparus. Quaedvlieg et al. (2013), reassembled septoria-like genera and introduced Stagonospora sensu stricto in Massarinaceae because of its pycnidial conidiomata, immersed, globose, ostiolate, conidiophores reduced to conidiogenous cells, with holoblastic, percurrent proliferations, and doliiform, cylindrical to ellipsoid, hyaline, guttulate conidia. Tanaka et al. (2015) revised Massarinaceae and included twelve species in Stagonospora.
Stagonospora forlicesenensis Phukhamsakda, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552238; Facesoffungi number: FoF02384, Fig. 46
Stagonospora forlicesenensis (holotype). a Appearance of conidiomata on host surface. b Conidioma on host surface. c Vertical section of conidioma. d Basal mycelium. e Setae at side of conidioma. f Pycnidial walls. g–i Developing stages of conidia. j, l Conidia with campanulate appendage. k Conidia stained with cotton blue reagent. m, n Culture characters on PDA. Scale bars b = 200 μm, c = 100 μm, d, e, h, i, j = 20 μm, f = 50 μm, g, j, k = 10 μm
Etymology: In reference to the location where the fungus was collected.
Holotype: MFLU 16-1337
Saprobic on dead branches of Phragmites australis (Cav.) Trin. ex Steud. Sexual morph Undetermined. Asexual morph Conidiomata 70–210 μm high × 70–160 μm diam. (\( \bar{x} \) = 141 × 117 μm, n = 10), pycnidial, solitary, uniloculate, scattered, immersed in the host, dark brown to black, globose, containing basal mycelium, ostiole central. Ostiole 22–72 μm high × 27–62 μm diam. (\( \bar{x} \) = 53 × 46 μm, n = 5), papillate, dark brown. Setae 48 × 3 μm, on the side of the pycnidial walls, acicular, curved, constricted at the septate, light brown to brown. Pycnidial wall 8–28 μm (–31 μm at apex), composed of 4–5 layers of brown-walled cells of textura angularis, two hyaline inner layers, lining bearing conidiogenous cells. Conidiophores reduced to conidiogenous cells with one supporting cell. Conidiogenous cells 4–10 × 2–6 μm, (\( \bar{x} \) = 7 × 4 μm, n = 30), holoblastic, determinate, discrete, ampulliform to clavate, hyaline, smooth, formed from the inner cells of the pycnidial wall. Conidia 25–34 × 6–11 μm (\( \bar{x} \) = 30 × 8 μm, n = 50), broad fusiform to oblong, obtuse at both ends, thick-walled, with minute and large guttules in each cell, 3 transverse eusepta, rarely 2-septate, sometimes slightly constricted at the septa, hyaline; apical appendage, infundibuliform to campanulate-like, gelatinous, 7–8 μm wide.
Culture characteristics: Colonies on PDA, reaching 90 mm diam. after 14 days at 16 °C, colonies covering surface, sparse, cream, with abundant aerial mycelium, margins lobate; reverse white at the edges, cream at the center, radiating, circular, flattened, margin rough, not pigmented.
Material examined: ITALY, Province of Forlì-Cesena, Pian di Spino—Meldola, on dead and stem of Phragmites australis (Poaceae), 22 December 2014, E. Camporesi, IT 2306 (MFLU 16-1337, holotype), isotype in HKAS 94613, ex-type living culture, MFLUCC 15-0054, KUMCC 16-0028.
Notes: Based on a morphological comparison, Stagonospora forlicesenensis is similar to Stagonospora (Neottiosporina) paspali G.F. Atk. B. Sutton & Alcorn (CBS 331.37), a species introduced from Paspalum laeve Michaux (Poaceae). In our study, sequence data also reveals a close phylogenetic affinity of Stagonospora forlicesenensis to Stagonospora (Neottiosporina) paspali (Fig. 45). Therefore we compare the morphology with Neottiosporina. Sutton (1980) illustrated seven species of Neottiosporina. Neottiosporina australiensis B. Sutton & Alcorn and N. clavata B. Sutton are also reported from Phragmites australis (Poaceae) (Sutton and Alcorn 1974; Sutton 1980). They are phenotypically similar in having thin pycnidial walls, central ostioles, and holoblastic, determinate, 3-septate, hyaline conidia with infundibuliform apical appendage (Sutton and Alcorn 1974; Sutton 1980). Stagonospora forlicesenensis differs from N. australiensis and N. clavata in having immersed and smaller conidiomata, with brown setae on the pycnidial walls, and conidia which are obtuse at both ends. Therefore Tanaka et al. (2015) suggested these species should be treated under Stagonospora based on morphology and phylogenetic analysis. However, sequence data for the type species are not available and therefore the position remains unconfirmed.
Melanommataceae G. Winter
Tian et al. (2015) accepted 20 genera in this family based on morphology and phylogenetic analyses. In this paper, we introduce a new species of Bertiella, and an updated phylogenetic tree for the family (Fig. 47).
Phylogenetic tree generated by maximum likelihood (RAxML) analysis of combined LSU, SSU, RPB2 and EF-1α sequence data from species of Melanommataceae. Bootstrap support values for maximum likelihood equal or greater than 50 % are given above the nodes. Bayesian posterior probabilities equal or greater than 0.90 are given below the nodes. Ex-type strains, reference strains and new isolates are in bold. Newly generated sequences are in blue. The tree is rooted with Hysterium angustatum CBS 123334 and H. angustatum
Bertiella (Sacc.) Sacc. & P. Syd.
The genus Bertiella is typified by B. macrospora (Sacc.) Sacc. & Traverso. Previously this genus was assigned to Massarina (as M. macrospora (Sacc.) O.E. Erikss. & J.Z. Yue) (Lumbsch and Huhndorf 2010). However, recent phylogenetic studies support its placement within Melanommataceae close to Byssosphaeria (Tian et al. 2015). Taxa are mainly saprobes on woody hosts and characterized by black, superficial, subglobose ascomata with carbonaceous peridium, composed of few layers of pigmented cells (Tian et al. 2015).
Bertiella ellipsoidea Ekanayaka, Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF552201; Facesoffungi number: FoF02229, Fig. 48
Bertiella ellipsoidea (holotype). a Herbarium material. b Ascomata on wood. c Ascoma on wood. d, e Cross section of an ascoma. f Vertical section of the ascoma at margin. g Septate hairs. h Aseptate, branched pseudoparaphyses. i–l Cylindrical asci. m Apical apex. n–q Ovoid ascospores. Scale bars b = 500 μm, c, h = 100 μm, d = 400 μm, e = 200 μm, f = 70 μm, g = 50 μm, i–l = 40 μm, m = 25 μm, n–q = 10 μm
Etymology: The specific epithet ellipsoidea is refers to the shape of the ascospores.
Holotype: MFLU 16-0583
Saprobic on dead stems. Sexual morph Ascomata 170–280 × 200–250 μm (\( \bar{x} \) = 255 × 236 μm, n = 10), arising in small groups, sessile, erumpent from the substrate, subglobose with a flattened base, black, carbonaceous, ostiole area black, ascomata covered with outwardly projecting, long hairs. Peridium 50–60 μm (\( \bar{x} \) = 56.6 μm, n = 10) wide, outer part composed of thick-walled, strongly melanized cells of textura angularis and inner part filled with elongate, hyaline cells of textura angularis. Hamathecium comprising numerous, 1.3–1.8 μm wide (\( \bar{x} \) = 1.5 μm, n = 20), long, filiform, aseptate, trabeculate pseudoparaphyses, branching and anastomosing between and above the asci. Asci 112–160 × 10–12 μm (\( \bar{x} \) = 131 × 11.4 μm, n = 30), 8-spored, bitunicate, cylindric–clavate, short pedicellate, rounded at the apex, with an ocular chamber. Ascospores 14–18 × 6–7 μm (\( \bar{x} \) = 16.3 × 6.7 μm, n = 40), 1–2 seriate, ellipsoid, greenish, 1-septate, constricted at septum, with 1–2 globules, acute at the apex, smooth, thick-walled. Asexual morph Undetermined.
Material examined: THAILAND, Chiang Rai, Mae Fah Luang University, on dead stems, 17 October 2015, A.H. Ekanayaka (MFLU 16-0583, holotype).
Notes: Bertiella ellipsoidea is well distinguished by ascomata arising in small groups, sessile, erumpent from the substrate, covered with outwardly projecting, long hairs, trabeculate pseudoparaphyses, branching and anastomosing between and above the asci, bitunicate, 8-spored, cylindric-clavate, asci with an ocular chamber and (14–18 × 6–7 μm) ellipsoid, 1–2 seriate, greenish, 1-septate ascospores.
Sequence data obtained directly from the type material clustered within the family Melanommataceae, close to the type species B. macrospora (Sacc.) Sacc. & Traverso (Fig. 47). Bertiella ellipsoidea has ellipsoid ascospores, while in other species in the genus they are fusiform. Furthermore, B. ellipsoidea has greenish, 1-septate ascospores, while in B. macrospora ascospores are brownish and 3-septate. Byssosphaeria villosa (Samuels & E. Müll.) Boise is similar to Bertiella ellipsoidea but differs in having ascospores with a gelatinous sheath (Mugambi and Huhndorf 2009).
Occultabambusaceae Dai et al.
Dai et al. (2016) introduced Occultabambusaceae to accommodate two pleomorphic genera Occultibambusa and Seriascoma, a coelomycetous genus Versicolorisporium and Neooccultibambusa. In their study, the morphology of sexual morphs and asexual morphs were discussed. In this paper, we introduce two new species, Occultibambusa aquatica and O. chiangraiensis and provide an updated tree (Fig. 49).
RAxML Maximum lkelihood phylogenetic tree based on a LSU and SSU sequence data from species of order Pleosporales. Maximum likelihood bootstrap support values greater than 50 % are shown on near the nodes. Some branches were shortened to fit the page—these are indicated by two diagonal lines with the number of times a branch was shortened indicated next to the lines. The new isolates are in red. The tree is rooted with Hysterium angustatum
Occultibambusa aquatica Huang Zhang & K.D. Hyde, sp. nov.
Index Fungorum number: IF552366; Facesoffungi number: FoF02439, Fig. 50
Occultibambusa aquatica (holotype). a Appearance of ascomata on host surface. b Vertical section through ascoma. c Peridium. d–f Asci. g, h Pseudoparaphyses. i, j Ascospores. k Ascospore stained with Indian ink. l Germinating ascospore. m, n Culture characters on PDA (m = from above, n = from below). Scale bars b = 100 μm, c = 40 μm, d, e, l = 20 μm, f = 30 μm, g, h, i–k = 10 μm
Etymology: Refers to its aquatic habitat.
Holotype: MFLU 11-1141.
Saprobic on submerged wood in freshwater stream. Sexual morph Ascomata perithecioid, 100–250 μm high, 180–280 μm diam., scattered or in small groups, immersed to semi-immersed, subglobose, with a flattened base, brown to dark brown, with minute ostiolate, central papilla, with rounded slot. Papilla up to 40 μm long, 50 μm diam., dark brown. Peridium carbonaceous and fragile, unequal in thickness, 35–45 μm thick at the sides, 25–35 μm thick at the base and near ostiole, composed of 5–7 × 1.5–2.5 μm, rectangular to polygonal celadon cells. Hamathecium comprises 2–3 μm wide, septate, hypha-like, numerous, branched, anastomosing, pseudoparaphyses embedded in mucilage. Asci 73–86 × 9–13 μm (\( \bar{x} \) = 78.2 × 11.1 μm, n = 10), 8-spored, bitunicate, fissitunicate, clavate, with a short furcate pedicel (5–8 μm), which elongates after discharge (up to 55 μm), apically rounded with a small ocular chamber (1–3 × 4–4.5 μm). Ascospores 19–25 × 3.5–6.5 μm (\( \bar{x} \) = 22.2 × 5 μm, n = 20), L/W 3.8–4.8 (mostly 4.5), mostly 2-seriate, narrowly fusiform with acute ends, 1-septate, not constricted at the septum, septum mostly median, upper cell slightly broader than lower cell, slightly swollen near the septum, straight to curved, brownish, with one large guttule in each cell, smooth-walled, thin-walled, surrounded by a 5–10 μm thick sheath. Germ tube mainly formed from both end cells. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on WA within 12–24 h. Colonies on PDA, dense, dark grey, reaching up to 1 cm diameter at after 13 days at 25–28 °C, raised, central embossing, velvety, aerial mycelium and entire edge smooth, clear.
Material examined: THAILAND, Chiang Rai, Hui Kang Pla Waterfall, on submerged bamboo, 16 November 2010, Huang Zhang a50 (MFLU 11-1141, holotype), ex-type living culture, MFLUCC 11-0006.
Notes: Occultibambusa aquatica is similar to O. bambusae in having clavate asci and 1-septate, fusiform, brown ascospores with a mucilaginous sheath. However, O. bambusae has larger ascomata (400–550 μm diam.) and larger ascospores (23.5–27.5 × 4.5–7 μm). A close phylogenetic relatedness is noted between O. aquatica and O. pustula in a monophyletic subclade basal to other Occultibambusa species (Fig. 49) (99 % MLBP, PP greater than 0.90). Occultibambusa aquatica has no statistical support to O. pustula and is similar in having fusiform, 1-septate ascospores, but the latter has raised and darker ascomata and hyaline to pale brown ascospores. Based on morphological characters and multi-gene phylogenetic analyses, we introduce a novel species in the genus Occultibambusa.
Occultibambusa chiangraiensis Phukhamsakda & K.D. Hyde, sp. nov.
Index Fungorum number: IF552240; Facesoffungi number: FoF02382, Fig. 51
Occultibambusa chiangraiensis (holotype). a Appearance of ascostromata on host substrate. b, c Close up of ascostromata on host. d Vertical section of ascostroma. e Section through peridium. f Cellular pseudoparaphyses. g–i Developmental stages of asci. j–o Developmental stages of ascospores. p Germinated ascospore. q, r Culture characters. Scale bars b, c = 500 μm, d = 100 μm, e = 50 μm, f–i = 20 μm, j–p = 10 μm
Etymology: The epithet “chiangraiensis” refers to Chiang Rai Province where the holotype was collected.
Holotype: MFLU 16-1334
Saprobic on dead stem of Bambusoideae. Sexual morph Ascostromata 195–295 μm high × 352–520 μm diam. (\( \bar{x} \) = 264 × 460 μm, n = 5), erumpent, solitary, scattered, depressed globose to subglobose, flattened at the base, brown to light brown. Ostioles central, with slit-like opening. Peridium (8–)12–34 μm wide, of unequal thickness, thickened at sides, with an outer layer of 7–9 layers of brown to dark brown, heavily pigment cells, arranged in a textura angularis and textura prismatica, and polygonal at the base. Hamathecium composed of dense, 1.6–4(–4.7 in enlarged cells) μm wide (n = 30), transversely septate, branched, cellular pseudoparaphyses. Asci 47–92 × 12–16 μm (\( \bar{x} \) = 69 × 14 μm, n = 20), 8-spored, bitunicate, clavate-oblong, with a short pedicel, apically obuse, with an ocular chamber, clearly visible when immature. Ascospores 16–24 × 5–7 μm (\( \bar{x} \) = 22 × 6 μm, n = 30), overlapping bi-seriate, hyaline when immature, pale brown to red-brown at maturity, fusiform, tapering towards the ends, guttulate in each cell, (1–)3-septate, strongly contricted at the median septum, sometimes the cells above median septum wider, smooth-walled, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA, reaching 50 mm diam. after 4 weeks at 25 °C, dark brown to black at the edge, surface covered with grey mycelium, rarely with aerial mycelium, margins lobate, reverse black radiating, dense, umbonate, margin uneven, producing reddish pigment in agar.
Material examined: Thailand, Chiang Rai Province, on dead stem of Bambusoideae sp. (Poaceae), 16 April 2015, C. Phukhamsakda, CP012 (MFLU 16-1334, holotype), ex-type living culture, MFLUCC 16-0380, KUMCC 16-0027; ibid. (KUN; HKAS 94617, isotype).
Notes: Occultibambusa chiangraiensis is closely related to O. bambusae Dai & K.D. Hyde, the type species of Occultibambusa, based on maximum likelihood analysis (87 % ML). However, in O. chiangraiensis the ascostromata are erumpent, solitary, with a mixing of peridium cell types, while in O. bambusae the ascostromata and peridium cells are only textura angularis. The asci of O. bambusae are oblong-clavate and ascospores 3-septate without a sheath (Dai et al. 2016). Occultibambusa chiangraiensis also shares similarity with O. fusispora Phookamsak et al., but the latter has smaller, solitary ascostromata (135–185 μm high × 240–275 diam.), a thick-walled peridium (up to 60 μm wide), and narrowly fusiform ascospores, acutely tapering at the ends, and not constricted at the septa (Dai et al. 2016). Doilom et al. (2016) introduced Neooccultibambusa chiangraiensis from Tectona grandis. Even though ascospores are similar, N. chiangraiensis has immersed ascomata, with obvious ostioles, larger asci (128 × 20 μm diam.), and cylindrical to subcylindrical, larger ascospores (37 × 10 μm diam.) surrounded by a mucilaginous sheath.
Phaeosphaeriaceae M.E. Barr
The family Phaeosphaeriaceae was introduced by Barr (1979) to accommodate Dothideomycete species, mostly on monocotyledons and some dicotyledons (Shoemaker and Babcock 1989; Schoch et al. 2006, 2009; Zhang et al. 2009a, 2012a; de Gruyter et al. 2010; Hyde et al. 2013; Phookamsak et al. 2014; Wijayawardene et al. 2014b; Ariyawansa et al. 2015c). Species in Phaeosphaeriaceae vary in morphological characters and comprise more than 35 sexual and asexual genera (Phookamsak et al. 2014; Wijayawardene et al. 2014a; Ariyawansa et al. 2015c).
Species in Phaeosphaeriaceae have often been confused with taxa in Leptosphaeriaceae (Zhang et al. 2012a; Hyde et al. 2013; Phookamsak et al. 2014; Ariyawansa et al. 2015b; Tennakoon et al. 2016). Based on phylogenetic analyses, Phaeosphaeriaceae is a heterogeneous group of taxa with shared similar morphology, but is phylogenetically distinct from species of Leptosphaeriaceae and Phaeosphaeria sensu lato (Zhang et al. 2012b; Hyde et al. 2013; Phookamsak et al. 2014; Ariyawansa et al. 2015b; Liu et al. 2015a; Tennakoon et al. 2016). Therefore, several genera have been introduced to accommodate ambiguous phaeosphaeriaceous taxa (Ariyawansa et al. 2015b; Li et al. 2015; Liu et al. 2015a; Phukhamsakda et al. 2015; Tibpromma et al. 2015; Tennakoon et al. 2016). In this study we introduce the new genera Camarosporioides with a single species, Ca. phragmitis, and Pseudophaeosphaeria, with Ps. Rubi. We also introduce the new species, Chaetosphaeronema achilleae, Dematiopleospora alliariae, De. cirsii, Juncaceicola italica, Leptospora galii, Le. aquatica, Le. thailandica, Muriphaeosphaeria ambrosiae, Neodidymelliopsis ranunculi, Nodulosphaeria italica, Poaceicola arundinis and Wojnowicia italica and provide an updated tree (Fig. 52).
RAxML tree based on analysis of a combined dataset of ITS, LSU and SSU partial sequences. Bootstrap support values for maximum likelihood (ML, black) higher than 50 % and Bayesian posterior probabilities (BYPP, Red) greater than 0.95 are defined as above the nodes. The tree is rooted to Didymella exigua. All type strains are in bold. New stains are given in blue
Camarosporioides W.J. Li & K.D. Hyde, gen. nov.
Index Fungorum number: IF552209; Facesoffungi number: FoF02350
Etymology: Morphologically resembling the genus Camarosporium, but phylogenetically distinct.
Saprobic on dead stems of Poaceae. Sexual morph Undetermined. Asexual morph Conidiomata yellowish to brown, separate or aggregated, pycnidial, obpyriform, immersed, unilocular, thick-walled, smooth, ostiolate. Ostiole single, circular, with hyaline periphyses, centrally located, papillate. Periphyses hyaline, hyphae-like, smooth, subcylindrical, with obtuse apex, unbranched, septate. Wall of conidiomata composed of thick-walled, brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells hyaline to pale brown, holoblastic, long lageniform, swollen at the base, discrete, determinate, formed from the cells lining the inner wall of the conidioma. Conidia pale brown to brown, finely roughened, ellipsoidal to oval, obtuse at the apex, slightly truncate at base, with primary transverse septa, with a longitudinal septum, constricted at septa, verruculose, thick-walled.
Type species: Camarosporioides phragmitis W.J. Li & K.D. Hyde, sp. nov.
Notes: Combined rDNA gene sequence data reveals that our new genus belongs to the Phaeosphaeriaceae and in particular is close to Stagnospora neglecta and S. foliicola (Fig. 52). Morphologically, Camarosporioides shares similar conidial morphology with Phragmocamarosporium (Massarinaceae), but Phragmocamarosporium lack periphyses. In addition, both genera can be distinguished by the form of conidiomata. Camarosporioides species have yellowish to brown, obpyriform, immersed conidiomata, while Phragmocamarosporium species have black, globose to subglobose conidiomata (Wijayawardene et al. 2016). In combining both morphology and phylogeny, we introduce Camarosporioides as a new genus.
Camarosporioides phragmitis W.J. Li & K.D. Hyde, sp. nov.
Index Fungorum number: IF552210; Facesoffungi number: FoF02351, Fig. 53
Camarosporioides phragmitis (holotype). a Herbarium specimen. b Appearance of black coniodiomata on the host. c Vertical section of conidioma. d Ostiole. e, f Section of peridium. g–j Conidiophores, conidiogenous cells and developing conidia. k, l Germinated conidia. m–p Conidia. q Culture on PDA. Scale bars c = 100 μm, d–e = 50 μm, f, k–1 = 20 μm, g–j, m–p = 5 μm, q = 25 mm
Etymology: Named after the host genus Phragmites.
Holotype: MFLU 16-1488.
Saprobic on dead stem of Phragmites australis (Cav.) Trin. ex Steud. (Poaceae). Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 143–170 μm diam., 160–177 μm high, yellowish to brown, separate or aggregated, pycnidial, obpyriform, immersed, unilocular, thick-walled, smooth, ostiolate. Ostiole 30–70 × 33–70 μm, single, circular, with hyaline periphyses, centrally located, papillate. Periphyses hyaline, hyphae-like, smooth, subcylindrical, with obtuse apex, septate, unbranched. Wall of conidiomata composed of thick-walled, brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 3.5–10 μm long × 3–7 μm wide, hyaline to pale brown, holoblastic, long lageniform, swollen at the base, discrete, determinate, formed from the cells lining the inner wall of the pycnidium. Conidia 11–18 × 5–8 μm (\( \bar{x} \) = 14 × 6 μm, n = 30), pale brown to brown, finely roughened, ellipsoidal to oval, obtuse at the apex, slightly truncate at base, with 1–3 primary transverse septa, and occasionally 1–2 longitudinal septum, constricted at septa, verruculose, thick-walled.
Culture characteristics: Colony on PDA, reaching 40–50 mm diam. in 7 days, with circular margin, white and fluffy, dense, aerial mycelium on the surface, reverse similar in colour.
Material examined: GERMANY, wet meadow, on dead stem of Phragmites australis (Poaceae), 6 April 2012, René K. Schumacher, G3 (MFLU 16-1488, holotype); ex-type living culture, MFLUCC 13-0365 (HKAS 95025, isotype); living culture, KUMCC 15-0599.
Chaetosphaeronema Moesz
The genus Chaetosphaeronema was introduced by Moesz (1915) and is represented by C. hispidulum (Corda) Moesz (type) and C. herbarum (Hollós) Moesz. In this genus the conidiomata are pycnidial and globose and have setae. De Gruyter et al. (2009, 2010) used 18S rDNA and 28S rDNA data to show that Chaetosphaeronema was related to Phaeosphaeriaceae and Pleosporaceae and treated Chaetosphaeronema sensu stricto in Phaeosphaeriaceae. Petrak (1944) and Zhang et al. (2009b) suspected that Chaetosphaeronema may be asexual morph of Ophiobolus. Phookamsak et al. (2014) confirmed the taxonomic position of Chaetosphaeronema in Phaeosphaeriaceae.
Chaetosphaeronema achilleae S.K. Huang & K.D. Hyde, sp. nov.
Index Fungorum number: IF552176; Facesoffungi number: FoF02240, Fig. 54
Chaetosphaeronema achilleae (holotype). a Herbarium specimen. b Conidiomata on host. c Immersed conidioma. d Conidioma in vertical section. e Wall of conidioma. f, g Conidiophores and conidia. h–j Conidia. k, l Culture characters on PDA. Notes g stained in lactophenol cotton blue. Scale bars b = 500 μm, c = 200 μm, d = 100 μm, e = 50 μm, f–g = 10 μm, h–j = 2 μm
Etymology: Named after the host genus Achillea.
Holotype: MFU 15-1922.
Saprobic on dead cone of Achillea nobilis L. Sexual morph Undetermined. Asexual morph Conidiomata 200–290 μm high × 180–245 wide μm (\( \bar{x} \) = 240 × 200 μm, n = 5) diam., pycnidial, solitary, uniloculate, immersed, subglobose, brown to dark brown, with minute papilla. Ostiole central, short, lined with periphyses. Wall of conidiomata 25–35 μm, membranaceous, composed of dark brown, or brown to hyaline cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 7–12 × 1–2 μm (\( \bar{x} \) = 10 × 1.5 μm, n = 20), enteroblastic, phialidic, cylindrical to subcylindrical, smooth-walled, hyaline, arising from the inner layers of conidioma. Conidia 9–16 × 1.5–3 μm (\( \bar{x} \) = 12 × 2 μm, n = 50), cylindrical to subcylindrical, slightly curved, hyaline, aseptate to 1-septate, smooth-walled.
Culture characteristics: Colonies on PDA reaching 10 mm diam. after 2 weeks at 16 °C, white at the margin, dark brown to green at the center; reverse cream to white at the margins, dark brown to black at the center, curled, circular, umbonate, without diffusible pigments.
Material examined: RUSSIA, Rostov Region, Oktyabrsky District, southern outskirts of Persianovky settlement, Khoruli gully (rus. balka Khoruli), on dead stems of Achillea nobilis (Asteraceae), 28 April 2015, T.S. Bulgakov (MFLU 15-1922, holotype, GZU 16022408, isotype), ex-type living culture, MFLUCC 16-0476, GZUCC 16022408.
Notes: Chaetosphaeronema achilleae is morphologically similar to C. hispidulum (type species of Chaetosphaeronema) in having globose pycnidial, cylindrical conidiogenous cells, and cylindrical to subcylindrical, slightly curved, and hyaline conidia. However, C. hispidulum is distinct from C. achilleae in having comparatively large conidiomata (up to 450 μm diam.) with numerous setae, and a pycnidial wall comprising cells of textura prismatica, while C. achilleae has smaller conidiomata, lacking setae and the pycnidial wall comprises cells of textura angularis (Sutton 1980). Phylogenetic data also indicate C. achilleae is closely to C. hispidulum, but morphological differences stated above support our establishment of a new species (Fig. 52).
Dematiopleospora Wanasinghe et al.
Wanasinghe et al. (2014) introduced Dematiopleospora as a monotypic genus in the family Phaeosphaeriaceae to accommodate D. mariae Wanasinghe et al. Subsequently, a second species D. luzulae Wanasinghe et al., was added by Ariyawansa et al. (2015a). The genus is characterized by thick, brown, periphyses in the ostiole, immersed to superficial ascomata and yellowish-brown to brown, muriform ascospores with light end cells. Two new additions are proposed in the genus.
Dematiopleospora alliariae Thambugala, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum Number: IF552265; Facesoffungi number: FoF02440, Fig. 55
Etymology: The specific epithet alliariae refers to the host genus on which the fungus occurs.
Holotype: MFLU 15-0545.
Saprobic on Alliaria petiolata (M. Bieb.) Cavara & Grande. Sexual morph Ascomata 210–350 μm high × 175–300 μm diam. (\( \bar{x} \) = 270 × 224 μm, n = 6), solitary to gregarious, scattered, immersed, slightly erumpent, coriaceous, dark brown to black, subglobose to pyriform, thickened at the apex, ostiolate. Ostiole central, papillate, with a pore-like opening. Peridium up to 60 μm wide, thin at the sides, broad at the apex, comprising two strata, outer stratum composed of small, brown to dark brown, somewhat flattened, thick-walled cells of textura angularis, fusing and indistinguishable from the host tissues, inner stratum composed of few layers of lightly pigmented to hyaline cells of textura angularis. Hamathecium comprising 1–2 μm wide, septate, cellular pseudoparaphyses, embedded in a gelatinous matrix. Asci 100–125 × 10–12 μm (\( \bar{x} \) = 110 × 10.9 μm, n = 15), 8-spored, bitunicate, fissitunicate, cylindrical, pedicellate, rounded at the apex, with a distinct ocular chamber. Ascospores 14–17.5 × 6.4–8.4 μm (\( \bar{x} \) = 15.5 × 7.5 μm, n = 35), uniseriate, partially overlapping, initially hyaline, becoming yellowish-brown at maturity, ellipsoidal to fusiform, muriform, transversely 3-septate, with 0–3(–4) vertical septa, slightly curved, deeply constricted at the central septum, rounded at the ends, smooth-walled, lacking a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on PDA within 24 h and producing germ tubes from several cells. Colonies on PDA, reaching 10–12 mm diam. after 7 days at 25 °C, surface dirty white to buff, spreading with moderate aerial mycelium, and even, smooth and entire margins.
Material examined: ITALY, Forlì-Cesena [FC] Province, Ridracoli—Bagno di Romagna, on dead stem of Alliaria petiolata (M. Bieb.) Cavara & Grande (Brassicaceae), 18 November 2012, Erio Camporesi IT 915 (MFLU 15-0545, holotype); ex-type living culture, MFLUCC 13-0070, ICMP.
Notes: Dematiopleospora alliariae is introduced as the third species of Dematiopleospora. Dematiopleospora alliariae mainly differs from the other two species in having ascomata with a crest-like ostiole and uni-seriate ascospores with 3(–4) transverse septa and 2–4 vertical septa. There is also support herein from our molecular data analysis that D. alliariae warrants new species status as it did not cluster with other Dematiopleospora species (Fig. 52).
Dematiopleospora cirsii Wanasinghe, Camporesi, E.B.G. Jones & K.D. Hyde, sp. nov.
Index Fungorum number: IF552135; Facesoffungi number: FoF02205, Fig. 56
Dematiopleospora cirsii (holotype). a Appearance of ascomata immersed beneath host substrate. b, c Sections of ascomata. d Close up of ostiole with short, light brown, setose hyphae. e Peridium. f Pseudoparaphyses. g–j Asci. k–n Ascospores. o Germinated ascospore. p, q Culture on PDA (note q reverse). Scale bars a = 500 μm, b = 100 μm, c, d = 50 μm, e, k–o = 10 μm, f = 5 μm, g–j = 20 μm
Etymology: Name reflects the host genus Cirsium, from which the species was collected.
Holotype: MFLU 16-0145.
Saprobic on dead and hanging branches of Cirsium sp. Sexual morph Ascomata 250–300 μm high 250–350 μm diam. (\( \bar{x} \) = 271.1 × 299.5 μm, n = 5), immersed to semi-erumpent, solitary, scattered, broadly oblong and flattened, dark brown to black, coriaceous, cupulate when dry, ostiolate. Ostiole 50–65 μm high 40–50 μm diam. (\( \bar{x} \) = 58.2 × 43.4 μm, n = 5), papillate, black, smooth, with short and light brown setae. Peridium 10–20 μm wide at the base, 15–30 μm wide in sides, thick, with 4–5 layers, outer layer heavily pigmented, thick-walled, comprising reddish to dark brown cells of textura angularis, inner layer composed of hyaline thin-walled cells of textura angularis. Hamathecium comprising numerous, 1.5–2 μm wide, filamentous, branched, septate, pseudoparaphyses. Asci 80–120 × 10–14 μm (\( \bar{x} \) = 100.9 × 12.3 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, pedicellate, thick-walled at the apex, with minute ocular chamber. Ascospores 20–30 × 6–9 μm (\( \bar{x} \) = 25.9 × 6.6 μm, n = 50), overlapping 1–2-seriate, muriform, ellipsoidal to subfusiform, slightly curved, upper part wider than the lower part, 6–7 transversely septate, with 1 vertical septum, deeply constricted at the central septum, initially hyaline, becoming golden-brown at maturity, ends remaining cone-shaped, with rounded ends, without a mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA, reaching 1.5 cm diam. after 30 days at 18 °C, circular, smooth margin white at first, greenish-grey after 4 weeks, flat on the surface, without aerial mycelium, reverse blackish-green. Hyphae septate, branched, hyaline, thin, smooth-walled.
Material examined: ITALY, Arezzo [AR] Province, Papiano, dead herbaceous branches of Cirsium sp. (Asteraceae), 13 June 2013, E. Camporesi (MFLU 16-0145, holotype); (isotype in BBH), ex-type living culture, MFLUCC 13-0615
Notes: In this study, our strain MFLUCC 13-0615 groups with Dematiopleospora mariae (type strain of Dematiopleospora) with very high bootstrap and Bayesian support (Fig. 52). Dematiopleospora cirsii resembles D. mariae in having brown periphyses in the ostiole, a peridium comprising brown to dark brown cells of textura angularis and multi-septate ascospores, but differs in having immersed ascomata, ascospores with 7 transverse septa and much longer lower part than upper part, while D. mariae has superficial ascomata, ascospores with 9–10 transverse septa, and upper and lower parts of equal length.
Juncaceicola Tennakoon et al.
The genus Juncaceicola (Phaeosphaeriaceae) was introduced by Tennakoon et al. (2016), with J. luzulae as the type species.
Juncaceicola italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number IF552246; Facesoffungi number: FoF02388, Fig. 57
Etymology: Refers to the name of the country where the holotype was collected
Holotype: MFLU 14-0685.
Saprobic on dead stem of Dactylis glomerata L. Sexual morph Ascomata 115–130 μm high × 130–140 μm diam. (\( \bar{x} \) = 125 × 134 μm, n = 4), superficial, solitary, scattered, globose to subglobose, with a flattened base, easy to remove from the host, shiny, ostiole central, black, smooth-walled. Peridium 5–8 μm, yellow-brown, a single stratum comprised of 2–3 cell layers of textura prismatica. Hamathecium comprising numerous 1.3–3 μm wide, filamentous, guttulate, branched anastomosing, septate pseudoparaphyses. Asci 62–88 × 11–20 μm (\( \bar{x} \) = 77 × 17 μm, n = 10), 6–8-spored, bitunicate, clavate to cylindrical, thin-walled, short pedicellate, apex rounded, ocular chamber not well-developed. Ascospores 18–21 × 6–8 μm (\( \bar{x} \) = 18 × 7 μm, n = 10), fusiform with broadly to narrowly rounded ends, 3-septate, constricted at septa, enlarged at the second cell, green-yellow, guttulate, surrounded by mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: on PDA reaching 2 cm diam. after 2 weeks at 16 °C, later with dense mycelium, with circular, rough margin, flattened; upper surface white at first, dark brown after 4 weeks, producing pigments in PDA media agar; hyphae septate, branched, thick-walled.
Material examined: ITALY, Forlì-Cesena Province, Monte Falco, on dead stem of Dactylis glomerata (Poaceae), 15 July 2013, Erio Camporesi, IT1378 (MFLU 14-0685, holotype); ex-type living culture, MFLUCC 13-0750; ibid. (HKAS94558 bis, paratypes).
Notes: Phylogenetic results show Juncaceicola italica to be related to J. dactylidis; however, we found our new taxon is well-differentiated from J. dactylidis based on morphological differences. Our new taxon is characterized by superficial ascomata, a peridium of textura prismatica, 6–8-spored asci and 3-septate ascospores, while J. dactylidis has immersed or erumpent ascomata, a peridium of textura angularis, 8-spored asci and 4-septate ascospores (Tennakoon et al. 2016).
Leptospora Rabenh.
Leptospora is typified by L. rubella (Pers.) Rabenh. Persoon (1801) originally assigned the genus to Sphaeria rubella Pers., however, Rabenhorst (1858) transferred it to Dothideomycetes under Leptospora. Leptospora frequently stains the surface of host tissues red to purple and the red colour is also present at the apical part of ostiolar canal. The taxa has multi-septate ascospores. Leptospora is similar to Ophiobolus Riess base on their ascospore morphology (Shoemaker 1976; Crous et al. 2006). Therefore, in this study we reconstruct the phylogenetic analysis (Fig. 52) for Phaeosphaeriaceae and introduce three new species, L. aquatica, L. galii, L. thailandica with provide a reference specimen for L. rubella (96 %ML, 1.00 PP).
Leptospora rubella (Pers.) Rabenh., Klotzschii Herb. Viv. Mycol., Edn 2: no. 532 (1857)
Index Fungorum number: IF552325; Facesoffungi number: FoF02442, Fig. 58
Leptospora rubella (reference specimen). a Appearance of ascomata on host. b Close up of an ascoma. c, d Vertical section of ascoma. e Pseudoparaphyses. f, g Asci. h Ascospores. i Germinated ascospore. j Mycelium. k, l Culture on PDA (note l reverse). Scale bars a = 1 mm b = 200 μm, c = 100 μm, d = 50 μm, e = 5 μm, f–j = 20 μm
Saprobic on decaying wood submerged in freshwater. Sexual morph Ascomata 250–260 μm high, 185–190 μm diam., solitary or aggregated, immersed to semi-immersed, erumpent, globose to subglobose, coriaceous, black, apapillate, ostiolate. Ostiole central, ostiolar canal filled with hyaline to brown cells. Peridium 8–9 μm at base, 20–24 μm at sides comprising 3–4 layers of pale brown to brown, inwardly lighter, thin-walled cells of textura angularis. Hamathecium comprising numerous, 2–3 μm wide, branched, cellular, hyaline pseudoparaphyses. Asci 100–150 × 5–9 μm (\( \bar{x} \) = 107 × 6 μm, n = 10), 8-spored, bitunicate, long-cylindrical, short pedicellate, apex rounded, with minute ocular chamber. Ascospores 90–125 × 2–3 μm (\( \bar{x} \) = 115 × 2.5 μm, n = 10), arranged spirally in the ascus, initially hyaline becoming pale brown at maturity, filiform, ends narrowly rounded, straight or curved, thick-walled, smooth-walled, ascospores are sometimes longer than asci. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA 4 cm diam. after 4 weeks at 16 °C, dirty white to creamy at the margins, grey to pale-brown at the center; reverse grey to pale brown and cream at the center, gauzy, curled, flat.
Material examined: UK, Hampshire, Swanick Lakes, on decaying wood submerged in freshwater, 28 August 2015, E.B.G. Jones, GJ187 (MFLU 16-0965, reference specimen designated here, HKAS94519), living culture, MFLUCC 16-0122, KUMCC 16-0006.
Notes: The strain MFLU 16-0965 has morphologically identical to Leptospora rubella, although, it differs from the type of L. rubella as the ostiole lacks a distinct papilla. In the phylogenetic analysis (Fig. 52), our collection grouped with the Leptospora rubella (CPC 11006), thus they are not phylogenetically distinct (Fig. 52).
Leptospora galii de Silva & K.D. Hyde, sp. nov.
Index Fungorum number: IF552317; Facesoffungi number: FoF02441, Fig. 59
Etymology: Name reflects the host genus Galium.
Holotype: HKAS 92493a.
Saprobic on dead branch of Galium sp. Sexual morph Ascomata 105–226 μm high, 109–188 μm diam., solitary, scattered to clustered, immersed to semi-immersed to erumpent, globose to subglobose, glabrous, visible as raised, black spots on host surface. Peridium 18–25 μm wide, unevenly thickened, two-layered; inner layer comprising 3–4 cell layers of hyaline cells of textura angularis or 3–4 cell layers of flattened, hyaline cells of textura prismatica; outer layer comprising several layers of brown to dark brown cells of textura angularis. Hamathecium composed of dense, 1.5–2.5 μm wide, filamentous, septate, cellular pseudoparaphyses, anastomosing at the apex. Asci 55–87 × 3.7–6.9 μm (\( \bar{x} \) = 70 × 5.2 μm, n = 30), 8-spored, bitunicate, cylindric-clavate, short pedicellate, with an obtuse to slightly furcate end, apically rounded, with ocular chamber when immature and indistinct at maturity. Ascospores 17–32 × 2.1–3.3 μm (\( \bar{x} \) = 23.2 × 2.7 μm, n = 30), overlapping bi-seriate, hyaline, elongate fusiform with obtuse ends, and sometimes curved at ends, 1-septate at maturity, guttulate. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA 32–35 mm diam. after 3 weeks, colonies sparse, circular, flat, surface smooth with entire edge, velvety with smooth aspects, zonate with different sector light brown to brown at the margin whitish-grey at the centre; reverse light brown at margin, brown at the middle, dark brown at the centre.
Material examined: ITALY, Forlì-Cesena Province, Monte Mirabello-Predappio, on dead branch of Galium sp. (Rubiaceae), 11 May 2015, E. Camporesi, IT2478 (HKAS 92493, holotype), (MFLU 15-1230, isotype); ex-type living culture KUMCC 15-0521, MFLUCC.
Notes: Our new species clearly fits into Leptospora where all species constitute a monophyletic clade with high support (Fig. 52). In addition, phylogenies also reveal that L. galli is phylogenetically distinct as it branches basal to other species with high support. Leptospora galii differs from other Leptospora species by having hyaline, elongate, fusiform, rarely light yellow, mature, 1-septate ascospores, 17–32 μm long, while other Leptospora species in the same clade have brown, filiform, multi-septate ascospores more than 60 μm long.
Leptospora thailandica Phukhamsakda & K.D. Hyde, sp. nov.
Index Fungorum number: IF552239; Facesoffungi number: FoF02381, Fig. 60
Leptospora thailandica (holotype). a Specimens. b Appearance of ascomata on host surface. c Vertical section of ascoma. d Ostiolar canal. e Section through peridium. f Cellular pseudoparaphyses. g–i Developing state of asci. j–l Developing stages of ascospores. m Ascospore stained with Indian ink to show septa. Scale bars c = 100 μm, d, g–m = 50 μm, e, f = 10 μm
Etymology: The name reflects the country, where the holotype was collected.
Holotype: MFLU 16-1335
Saprobic on dead branches of Duranta sp. Sexual morph Ascomata 188–207 μm high × 112–170 μm wide diam. (\( \bar{x} \) = 197 × 141 μm, n = 10), immersed to erumpent through host tissue with papilla, solitary, scattered, globose, smooth, brown to dark brown, ostiole central. Ostioles 66–115 μm high × 53–102 μm diam. (\( \bar{x} \) = 79 × 83 μm, n = 10), papillate, dark brown to light brown, heavily pigmented at outer layer, smooth, filled with periphyses, orange around pore. Peridium (5–)10–24(–27) μm wide, up to 30 μm wide at the apex, thick-walled, brown to dark brown, pseudoparenchymatous cells, composed of 5–7 layers of textura angularis, in hyaline gelatinous layers, thin. Hamathecium comprising numerous, filamentous, 1.7–4 μm (n = 50) wide, broad, branched, transversely septate, cellular pseudoparaphyses. Asci 68–114 × 7–13 μm (\( \bar{x} \) = 94 × 10 μm, n = 35), 8-spored, bitunicate, cylindrical to cylindrical-clavate, with short furcate pedicel, apically rounded, ocular chamber visible when immature. Ascospores 63–89 × 1.8–3.8 μm (\( \bar{x} \) = 76 × 3 μm, n = 40), fasciculate, scolecosporous, filiform, tapering towards the ends, minute guttules in each cell, hyaline when immature, pale brown at maturity, (14–)20–22-septate, contricted at the septa. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA, reaching 20 mm diam. after 4 weeks at 16 °C, surface cream, dense, circular, convex with moderate aerial mycelium, downy, slightly irregular at margins; reverse cream at the edges, brown at the center, dense, margin rough, not pigmented.
Material examined: THAILAND, Chiang Rai, on dead branches of Duranta sp. (Verbenaceae), 12 June 2015, C. Phukhamsakda, CP014 (MFLU 16-1335, holotype), isotype in HKAS 94616, ex-type living culture, MFLUCC 16-0385, KUMCC 16-0030.
Notes: Leptospora thailandica forms a sister clade (99 % ML, 100 %PP) with L. aquatica, L. galii (this study), and L. rubella (Pers.) Rabenh., the type species of Leptospora. Leptospora thailandica is similar to Leptospora in staining the host substrate pinkish-red and having remarkable reddish colour at the apical part of ostiole canal, with pale brown and cylindrical ascospores (Crous et al. 2006).
Muriphaeosphaeria C. Phukhamsakda et al.
Muriphaeosphaeria was introduced by Phukhamsakda et al. (2015) as a monotypic genus with M. galatellae Phukhamsakda et al. as the type species. The genus is characterized by superficial ascomata, muriform ascospores and conidiomata with cylindrical to subclavate, 1–3-septate and brown conidia. This genus was accepted in Phaeosphaeriaceae based on combined ITS, 18S and 28S sequence data (Fig. 52).
Muriphaeosphaeria ambrosiae S.K. Huang & K.D. Hyde, sp. nov.
Index Fungorum number: IF552177; Facesoffungi number: FoF02241, Fig. 61
Muriphaeosphaeria ambrosiae (holotype). a Herbarium packet. b Herbarium material. c Conidiomata on host. d Close up of conidiomata, superficial on host. e Conidioma in vertical section. f Wall of conidioma. g–i Conidiophores and conidia. j–m Conidia. Notes h–i, m stained in lactophenol cotton blue, g stained in Indian ink. Scale bars c = 1 mm, d = 200 μm, e = 100 μm, f–i = 10 μm, j–m = 5 μm
Etymology: The specific epithet ambrosiae is based on the host genus from which the holotype was collected.
Holotype: MFU 15-1971
Saprobic on dead branch of Ambrosia artemisiifolia L. Sexual morph Undetermined. Asexual morph Conidiomata 228–328 μm high × 224–268 wide μm (\( \bar{x} \) = 281 × 249 μm, n = 5), globose, cleistothecial, solitary, uniloculate, black, superficial, globose to subglobose. Wall of conidiomata 10–14 μm diam., membranaceous, composed of dark brown, or hyaline to brown cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 7–12 × 1.5–3 μm (\( \bar{x} \) = 9 × 2 μm, n = 20), enteroblastic, phialidic, oblong to cylindrical, smooth-walled, hyaline. Conidia 9.5–11.5 × 3–4 μm (\( \bar{x} \) = 10 × 3.5 μm, n = 50), oval, fusiform to oblong, 1-septate, initially hyaline, pale brown at maturity, smooth-walled, with three inconspicuous and filiform, apical appendages at the apex.
Material examined: RUSSIA, Rostov Region, Shakhty City, Cotton Fabric Urban Microdistrict, Grushevka steppe slopes, near Grushevsky Pond, on dead stems of Ambrosia artemisiifolia (Asteraceae), 12 May 2015, T.S. Bulgakov (MFLU 15-1971, holotype, GZU 2016022413, isotype).
Notes: We could not obtain a culture from single conidia. Therefore fungal DNA was extracted directly from the conidiomata. Muriphaeosphaeria ambrosiae is a sister to M. galatellae with moderate support (Fig. 52). Both these species are characterized by globose to subglobose, uniloculate conidiomata, and pycnidial wall comprising cells of textura angularis. They were found on Asteraceae. The asexual morph of M. galatellae is distinct from M. ambrosiae in having cylindrical to subclavate, 1–3-septate conidia, while M. ambrosiae has 1-septate, oval to oblong conidia with three inconspicuous appendages at the apex (Phukhamsakda et al. 2015).
Nodulosphaeria Rabenh.
Nodulosphaeria is a relatively poorly studied genus lacking a modern taxonomic treatment and phylogenetic analyses (Hyde et al. 2013; Phookamsak et al. 2014). Since 2014, mycologists have attempted to resolve its natural placement in Phaeosphaeriaceae by recollecting samples and investigated their phylogeny (Ariyawansa et al. 2015c; Li et al. 2015; Liu et al. 2015a). Presently, there are 28 sequences from seven species available in GenBank, with 64 epithets in Index Fungorum (2016). The most recent treatment of Nodulosphaeria which includes molecular data is that of Mapook et al. (2016) and this is followed here.
Nodulosphaeria has relatively large ascomata, and phragmospores or scolecospores, with an enlarged cell in the upper part of the ascospores. Nodulosphaeria differs from Ophiobolus in having brown, setae-like periphyses in the ostiole, and ascospores do not separate into part spores (Phookamsak et al. 2014; Mapook et al. 2016). Many Nodulosphaeria species were transferred to Ophiobolus by Shoemaker (1976, 1984). Subsequent authors introduced new species and designated reference specimens to resolve the placement of Nodulosphaeria (Phookamsak et al. 2014; Ariyawansa et al. 2015a; Li et al. 2015; Liu et al. 2015a; Mapook et al. 2016). However, many species still lack modern taxonomic treatments or molecular data to clarify their placement.
Nodulosphaeria italica Phookamsak, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552202; Facesoffungi number: FoF02261, Fig. 62
Etymology: The specific epithet “italica” refers to the country, where the holotype was collected.
Holotype: MFLU 16-1359
Saprobic on Cirsium sp. Sexual morph Ascomata 240–330 μm high (excluding necks), 280–330 μm diam., dark brown to black, scattered, gregarious, immersed to erumpent through the host cortex, globose to subglobose, uni-loculate, setose, covered by dark brown, septate, vegetative hyphae. Papilla 190–330 μm high, 120–140 μm diam., truncate to cylindrical, composed of several layers of thick, brown to dark brown, pseudoparenchymatous cells, arranged in a textura angularis, ostiole central, pore-like opening, with brown, 2–3-septate, setae-like periphyses. Peridium 16–34 μm wide, thin-walled, of equal thickness, composed of two types of brown to dark brown, pseudoparenchymatous cells, inner layers comprising 2–3 layers, of flattened, dark brown cells, arranged in textura prismatica to textura angularis, outer layers comprising 1–2 layers, of thickened, subhyaline to black cells, arranged in textura angularis to textura globulosa. Hamathecium composed of dense, 2–4 μm wide, cellular pseudoparaphyses, distinctly septate, anastomosing at the apex, embedded in a hyaline gelatinous matrix. Asci (157–)170–200(–206) × (11–)12–14(–15) μm (\( \bar{x} \) = 183.5 × 13.2 μm, n = 30), 8-spored, bitunicate, fissitunicate, cylindrical, short pedicellate, with obtuse to furcate pedicel, apically rounded, with well-developed ocular chamber. Ascospores (114–)120–150(–158) × (2–)3–4(–5) μm (\( \bar{x} \) = 141.5 × 3.8 μm, n = 30), tri- to tetra-seriate in parallel, not fasciculate, yellowish-brown to brown, filiform, with rounded ends, tapered towards lower cell, enlarged at the 8th cell, multi-septate (17–20 septa), constricted at the central septum, smooth-walled, with guttules. Asexual morph Undetermined.
Material examined: ITALY, Province of Trento[TN], Mezzana, Marilleva 900, on dead stem of thistle (Cirsium sp.), 3 June 2014, E. Camporesi, IT 549 (MFLU 16-1359, holotype; MFLU 16-1360, isotype); ibid. 9 June 2014, E. Camporesi (MFLU 16-1361); 16 July 2012, E. Camporesi (MFLU 15-0449).
Notes: Nodulosphaeria italica is similar to N. cirsii (P. Karst.) L. Holm in having brown, scolecosporous ascospores with multi-septa. However, they can be distinguished by the size of asci and ascospores, as N. italica has smaller asci and ascospores than N. cirsii. Based on phylogenetic analyses, N. italica sits with N. senecionis in a robust clade (92 % ML, 0.95 PP), while N. scabiosae is basal to both species (Fig. 52).
Poaceicola W.J. Li et al.
The genus Poaceicola was introduced by Li et al. (2015) to accommodate taxa from Poaceae. The genus includes Po. arundinis Li et al. (type species), Po. bromi Wijayawardene et al. and Po. elongata (Wehm.) Shoemaker & C.E. Babc.) Li et al. Poaceicola is characterized by immersed to semi-immersed ascomata, subepidermal, globose, papillate, usually reddish-brown and fusiform ascospores (Li et al. 2015). Specimens in the genera produce brown conidia and are coelomycetous. Poaceicola elongata has a sexual morph which is phaeosphaeria-like (Ariyawansa et al. 2014b). Li et al. (2015) introduced the asexual morph into Phaeosphaeriaceae based on combined LSU and ITS sequence data. From the analysis they found Poaceicola is closely related to P. elongata (96 % ML), and therefore they synonymized Phaeosphaeria elongata under Poaceicola elongata. In this study, we reconstruct the phylogeny of genera of Phaeosphaeriaceae and our strain (MFLUCC 14-1060) clusters together with Po. arundinis (MFLUCC 15-0702) with high support. We therefore describe the sexual morph for Po. arundinis.
Poaceicola arundinis W.J. Li, Camporesi, D.J. Bhat & K.D. Hyde, Mycosphere 6 (6): 681 (2015)
Facesoffungi number: FoF02383, Fig. 63
Poaceicola arundinis (holotype). a Appearance of ascoma on host surface. b Vertical section of ascoma. c Ostiole. d Section through peridium. e Cellular pseudoparaphyses. f Immature ascus with ocular chamber. g Mature ascus. h–k Developing state of ascospores. l Ascospore stained with Indian ink to show sheath. m Germinated ascospore. n, o Culture character on PDA from surface and reverse. Scale bars a, b = 100 μm, c, d = 50 μm, e–g, m = 20 μm, h–l = 10 μm
Saprobic on dead stem of Dactylis sp. Sexual morph Ascomata 164–230 μm high × 210–290 μm wide diam. (\( \bar{x} \) = 200 × 235 μm, n = 10), superficial, solitary, scattered, or sometimes gregarious, globose, black to dark brown, ostiole central. Ostioles 70–85 μm high × 87–110 μm diam. (\( \bar{x} \) = 80 × 100 μm, n = 5), papillate, dark brown, smooth, without periphyses. Peridium 21–35 μm wide., up to 45 μm wide at the apex, composed of 6–7 layers of textura angularis, outer region heavily pigment, cells 4–8 μm wide, inner layer composed of hyaline gelatinous cells, thin, merging with pseudoparaphyses. Hamathecium comprising numerous, long, 2–3 μm (n = 40) wide, transversely septate, branched, cellular pseudoparaphyses. Asci 65–105 × 8–19 μm (\( \bar{x} \) = 87 × 13 μm, n = 20), 7–8-spored, bitunicate, cylindric-clavate, with short bulbose pedicel, apically rounded, with an ocular chamber up to 1–2 μm wide × 1–2 μm high. Ascospores 23–34 × 4.5–8 μm (\( \bar{x} \) = 29 × 6 μm, n = 30), bi-seriate or overlapping, hyaline when immature, pale brown to yellowish at maturity, fusiform, tapering towards the ends, (5–)8–9-distoseptate, slightly contricted at third septa, third cell from apex enlarged, smooth-walled, surrounded by thick, distinct mucilaginous sheath. Asexual morph Coelomycetous. Conidiomata 100–150 μm high, 100–200 μm diam., pycnidioid, dark brown, solitary or aggregated, semi-immersed, unilocular, globose, papillate. Pycnidial wall comprised of cells of textura angularis, gradually merging with the outer, surrounding layers of brown, textura oblita. Ostiole central. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, phialidic, hyaline, smooth-walled, discrete. Conidia 30–40 × 6.5–10 μm, pale brown, cylindrical, flexuous, up to 8-euseptate, slightly curved, smooth-walled, with middle cells wider than end cells, guttulate, with an acute apex, truncate at the base (Li et al. 2015).
Culture characteristics: Colonies on PDA reaching 43 mm diam. after 14 days at 16 °C, surface white, with moderate aerial mycelium, fluffy, fimbriate margins; reverse white at the edges, dark green at the center, radiating light green outwardly, dense, circular, flattened, margin rough, not pigmented.
Material examined: ITALY, Trento [TN], Forte Strino—Vermiglio, on dead stem of Dactylis sp. (Poaceae), 12 September 2014, E. Camporesi, IT 541 (MFLU 16-1336); KUN HKAS 94614, living culture, MFLUCC 14-1060, KUMCC 16-0029.
Notes: Based on the combined gene analysis, Poaceicola arundinis (MFLUCC 14-1060) has a close relationship with P. arundinis (MFLUCC 15-0702), reported from Arundo plinii (98 % ML, 100 % PP support). Poaceicola arundinis (MFLUCC 14-1060) is similar to P. elongata, but relatively smaller than the epitype (Ariyawansa et al. 2014b). Li et al. (2015) introduced P. arundinis from the asexual morph and the phylogeny support them to be the same strain as our sexual morph. We therefore introduce the sexual morph to accommodate P. arundinis.
Pseudophaeosphaeria Jayasiri, Camporesi & K.D. Hyde, gen. nov.
Index Fungorum number: IF552207; Facesoffungi number: FoF02345
Etymology: Named because of its morphological similarity to the genus Phaeosphaeria.
Saprobic on Rubus idaeus L. Sexual morph Ascomata scattered, immersed to slightly erumpent through host tissue, visible as small irregular black spots on the host surface, uniloculate, globose to subglobose, glabrous, dark brown, ostiole central, with a minute papilla. Peridium wide, thin-walled, of equal thickness, composed of 3–5 layers of flattened cells, outer layer dark brown, inner layer of pseudoparenchymatous cells, arranged in textura angularis. Hamathecium composed of numerous, wide, filiform, distinctly septate, frequently anastomosing, narrow, cellular pseudoparaphyses, embedded in mucilaginous matrix between and above asci. Asci 8-spored, bitunicate, fissitunicate, broadly cylindrical, subsessile with minute knob-like pedicel, apically rounded, with a well-developed ocular chamber. Ascospores overlapping 1–3-seriate, hyaline, phragmosporous, narrowly fusiform, mostly 3-septate, indistinctly constricted at the septa, slightly curved, smooth-walled. Asexual morph Undetermined.
Type species: Pseudophaeosphaeria rubi Jayasiri, Camporesi & K.D. Hyde
Notes: Phylogenetic analyses of combined LSU, SSU and ITS sequence data indicate that Pseudophaeosphaeria is a distinct genus in Phaeosphaeriaceae, which forms a clade sister to Paraphoma, Setomelanomma and Xenoseptoria clades. Pseudophaeosphaeria, differs from Setomelanomma in having irregular ascomata and narrowly fusiform ascospores, while Setomelanomma has regular ascomata and ellipsoidal to broadly fusiform ascospores. Paraphoma and Xenoseptoria are asexual genera of this family. Pseudophaeosphaeria is morphologically most closely related to Phaeosphaeria sensu stricto, which has immersed to slightly erumpent, irregular, black ascomata, broadly cylindrical asci with small subsessile knob-like pedicels, and narrowly fusiform, 3-septate ascospores. However, this relationship is not supported by sequence data, as Pseudophaeosphaeria is phylogenetic distinct from Phaeosphaeria sensu stricto (Fig. 52). Pseudophaeosphaeria rubi stands phylogenetically apart other genera with relatively high support (Fig. 52).
Pseudophaeosphaeria rubi Jayasiri, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum Number: IF552174; Facesoffungi number: FoF02221, Fig. 64
Pseudophaeosphaeria rubi (holotype). a, b Appearance of ascomata on host surface. c, d Sections through the ascomata. e Papilla. f Peridium. g Pseudoparaphyses. h Asci with pseudoparaphyses in gelatinous matrix. i–h Asci. m–p Ascospores. Scale bars c, d = 50 μm, e = 20 μm, f, g–l = 20 μm, m–p = 5 μm
Etymology: The specific epithet rubi is based on the host genus from which the taxon was collected.
Holotype: MFLU 15-1400
Saprobic on Rubus idaeus L. Sexual morph Ascomata 105–145 μm high, 125–135 μm diam., scattered, immersed to slightly erumpent through host tissue, visible as small irregular black spots on the host surface, uniloculate, globose to subglobose, glabrous, dark brown, ostiole central, with a minute papilla. Peridium 12–18 μm wide, thin-walled, of equal thickness, composed of 3–5 layers of flattened cells, outer layer dark brown, inner layer pseudoparenchymatous cells, arranged in textura angularis. Hamathecium composed of numerous, 1.8–2.4 μm wide, filiform, distinctly septate, frequently anastomosing, narrow cellular pseudoparaphyses, embedded in mucilaginous matrix between and above asci. Asci 59–75 × 7–9 μm (\( \bar{x} \) = 66.5 × 8.2 μm, n = 20), 8-spored, bitunicate, fissitunicate, broadly cylindrical, subsessile with minute knob-like pedicel, apically rounded, with a well-developed ocular chamber. Ascospores 17–21 × 2.3–3.9 μm (\( \bar{x} \) = 18.6 × 2.8 μm, n = 30), overlapping 1–3-seriate, hyaline, phragmosporous, narrowly fusiform, mostly 3-septate, slightly constricted at the septa, slightly curved, smooth-walled. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating on MEA within 36 h. Colonies on MEA, reaching 2.2 cm diam. in 1 week at 28 °C. Mycelium superficial, felty, gummy, pale grey to black. No asexual morph formed in culture.
Material examined: ITALY, Forlì-Cesena, Passo la Calla—Santa Sofia, on dead branch of Rubus idaeus (Rosaceae), 28 January 2014, Erio Camporesi IT 1680 (MFLU 15-1400, holotype), (isotype in KUN), ex-type culture MFLUCC 14-0259, KUMCC.
Wojnowicia Sacc.
The genus Wojnowicia was introduced by Saccardo (1892) to accommodate W. hirta. The sexual morph of Wojnowicia has not been reported. Presently, there are 14 species epithets in Wojnowicia (Index Fungorum 2016) which are coelomycetous fungi. However, W. graminis (McAlpine) Sacc. & D. Sacc. and W. tenella Pat. have been synonymized under W. hirta (Sutton 1980) and W. buxi Bertault & Malençon under W. ephedrae Hollós (Farr and Bills 1995). Wojnowicia bryophila Racov., W. exilis (Corda) Sacc. & Traverso, W. viburni Wijayaw. et al. and W. lophostoma (Höhn.) Sacc. were excluded from Wojnowicia (Sutton 1980; Farr and Bills 1995; Wijayawardene et al. 2013; Crous et al. 2015a). Li et al. (2016) introduced and illustrated W. spartii based on morphological characters and phylogenetic analysis. In the present study, the sexual morph of Wojnowicia is described and eight species are accepted in the genus.
Wojnowicia italica Qing Tian, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552183; Facesoffungi number: FoF02254, Fig. 65
Wojnowicia italica (holotype). a Herbarium material. b, c Appearance of ascomata semi-immersed in the host. d–f Hand sections of ascomata showing peridia. g–j Asci with ascospores. k–o Ascospores. p Pseudoparaphyses. q Germination of spore. r Colony on PDA from above. s Colony on PDA from below. Scale bars b–c = 200 μm, d–e = 50 μm, f = 20 μm, g–j, p–q = 10 μm, k–o = 5 μm
Etymology: In reference to the occurrence of this species in Italy.
Holotype: MFLU 14-0732.
Colonies growing on a dead branch of Spartium junceum L. Sexual morph Ascomata 230–260 × 130–165 μm (\( \bar{x} \) = 240 × 150 μm, n = 7), immersed or semi-immersed and erumpent in the host, solitary, scattered, sometimes gregarious, subglobose to globose, black, up to 200 μm high. Ostiole inconspicuous. Peridium thick at the apex 30–60.5 μm (\( \bar{x} \) = 42 μm, n = 7), thinner at the base and sides 17–30 μm (\( \bar{x} \) = 24 μm, n = 7), one-layered, composed of brown to black, thin-walled, cells of textura angularis. Asci 60–93 × 15–20 μm (\( \bar{x} \) = 77 × 16 μm, n = 10), 8-spored, bitunicate, clavate, short-pedicellate, apically rounded, with an inconspicuous ocular chamber. Hamathecium consisting of 2–3 μm broad long, colourless, branched, pseudoparaphyses, with transverse sept. Ascospores 18–27 × 5–8 μm (\( \bar{x} \) = 22 × 8 μm, n = 10), overlapping uni-seriate or bi-seriate, light brown to brown, ellipsoid to fusiform, slighty curved, muriform, with 4–7 transverse septa, and one longitudinal septum in some central cells, constricted at the septa, end cells conical, smooth-walled, lacking a mucilaginous sheath. Asexual morphs Coelomycetous. Conidiomata pycnidial, dark brown, scattered, immersed to semi-immersed, globose to subglobose, glabrous, ostiolate. Ostiole centrally located, papillate. Wall of conidiomata composed of 4–5-cell layers. Conidiogenous cells phialidic, hyaline, integrated, flask-shaped, thick-walled. Conidia dark brown, fusiform or cylindrical, 7–12-septate.
Culture characteristics: Ascospore germinating on PDA within 12 h. Colonies on PDA reaching 10 mm diam. in 7 days at 16 °C. Mycelium superficial, initially white, later becoming light brown or grey, hairy, with entire edge.
Material examined: ITALY, Forlì-Cesena [FC] Province, Teodorano-Meldola, on dead stem of Spartium junceum (Fabaceae), 15 December 2012, Erio Camporesi (MFLU 14-0732, holotype), (HKAS 94522, isotype); ex-type living cultures, MFLUCC 13-0447, KUMCC 16-0017.
Notes: The sexual morph of Wojnowicia had not been previously reported. However, we found the sexual morph of W. italica. Its placement in Wojnowicia is supported by phylogenetic analyses of combined ITS, LSU and SSU sequence data.
Pleosporaceae Nitschke
The family Pleosporaceae was introduced by Nitschke (1869) and Pleospora is the type genus of this family. Pleosporaceae is the largest family in the Pleosporales and is representative of the order (Zhang et al. 2012b; Hyde et al. 2013; Wijayawardene et al. 2014b). A backbone tree for Pleosporaceae was provided by Ariyawansa et al. (2015c) and this is updated in Fig. 66. Taxa of this family are pathogenic or saprobic on wood and dead herbaceous stems or leaves (Ariyawansa et al. 2015c).
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS and SSU sequence data of Pleosporaceae. Maximum likelihood bootstrap support values greater than 60 % are shown above nodes. The ex-type strains are in bold and the new isolate is in blue. The tree is rooted with Leptosphaeria doliolum
Comoclathris italica Tibpromma, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number IF552245; Facesoffungi number: FoF02387, Fig. 67
Etymology: refers to the name of the country where the holotype was collected.
Holotype: MFLU 15-1491.
Saprobic on dead stem of Thalictrum sp. Sexual morph Ascomata 195–215 μm high × 225–250 μm diam. (\( \bar{x} \) = 203 × 230 μm, n = 5), globose to subglobose, solitary, dark brown to black, immersed, black, smooth, ostiolar canal filled with sparse periphyses. Peridium 6–27 μm wide, comprising 3–4 layers of brown to reddish-brown cells of textura angularis. Hamathecium comprising 1.8–3.7 μm wide, dense, septate, hyaline, pseudoparaphyses. Asci 75–115 × 22–29 μm (\( \bar{x} \) = 94 × 25 μm, n = 10), 8-spored, bitunicate, fissitunicate, cylindric-clavate, short pedicellate, thick-walled at the apex, with a minute ocular chamber. Ascospores 20–29 × 9–13 μm (\( \bar{x} \) = 24 × 11 μm, n = 20), overlapping 1–2-seriate, hyaline when young, becoming golden yellow at maturity, muriform, 5–7 transverse septate with 2–3 longitudinal septa in all cells and rarely in end cells, slightly constricted in the middle, conical and narrowly rounded at the ends, surrounded by large mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA 60 mm diam. after 4 weeks at 16 °C, circular colony with entire edge, smooth, raised on surface, white-grey.
Material examined: ITALY, near Mezzana, province of Trento [TN], dead stem of Thalictrum sp. Tourn. ex L. (Ranunculaceae), 16 July 2014, Erio Camporesi, IT1998B (MFLU 15-1491, holotype); ex-type living culture, MFLUCC 15-0073); ibid. (HKAS 94599 bis, paratypes).
Notes: Comoclathris lanata is the type species of Comoclathris which was introduced by Clements (1909). The genus is characterized by ascomata with circular lid-like openings and applanate, reddish-brown to dark reddish-brown, muriform ascospores, with a single longitudinal septum (Shoemaker and Babcock 1992). The phylogenetic classification in this study shows that Comoclathris italica clusters with C. spartii (Fig. 66), although it is differentiated from C. spartii based on molecular phylogeny and morphological support. Our new taxon is characterized by ascomata with ostioles and golden yellow ascospores with conical and narrowly rounded ends, while C. spartii has yellow to pale brown ascospores with broadly fusiform, obtuse ends, constricted at primary septum (Crous et al. 2014).
Roussoellaceae J.K. Liu et al.
Roussoellaceae was introduced by Liu et al. (2014) and is typified with Roussoella. This family is characterized by immersed, clypeate, gregarious ascostromata, cylindrical, bitunicate asci, and brown, 2-celled ascospores, and is phylogenetically close with Biatriosporaceae and Pleomassariaceae (Liu et al. 2014; Wijayawardene et al. 2014a). Appendispora, Cytoplea, Elongatopedicellata, Neoroussoella, Roussoella and Roussoellopsis are placed in this family (Ariyawansa et al. 2015a), and members mostly occur on bamboo and palms in terrestrial habitats. In this study we introduce a new species of Neoroussoella with support from molecular data (Fig. 68).
Maximum Parsimony tree obtained from a heuristic search with 1000 random taxon additions of combined LSU and ITS sequence data using PAUP v. 4.0b10. Bootstrap support values of more than 50 % are indicated above the nodes, and Bayesian posterior probabilities more than 0.9 are indicated in bold. The isolate from this study is shown in blue background, and Biatriospora marina as the out group taxon, and the scale bar shows 10 changes. The ex-type strains are in bold
Neoroussoella lenispora J.F. Zhang, J.K. Liu, K.D. Hyde & Z.Y. Liu, sp. nov.
Index Fungorum number: IF552324; Facesoffungi number: FoF02443, Fig. 69
Neoroussoella lenispora (holotype). a, b Appearance of ascostromata on the host surface. c Pseudoparaphyses. d Vertical section through ascostroma. e Section of peridium. f–i Long cylindrical asci with ascospores. j–m Ascospores. Scale bars a = 500 μm, b = 100 μm, c = 30 μm, d = 50 μm, e = 10 μm, f–i = 20 μm, j–m = 5 μm
Etymology: Name reflects the ascospores being smooth-walled, from the Latin lenis referring to smooth and spora.
Holotype: GZAAS 16-0011.
Saprobic on decaying branch. Sexual morph Ascostromata semi-immersed to erumpent, clypeate, globose to subglobose, solitary to gregarious, black, coriaceous, centrally ostiolate, visible as black, dome-shaped spots. Peridium up to 22–45 μm, composed of an outer stratum of dark brown, thick-walled cells fusing with host tissue, and an inner stratum of pale brown, thin-walled cells arranged in a textura prismatica. Hamathecium comprising 1.4–2.8 μm wide, anastomosing pseudoparaphyses, branched at apex, and embedded in a gelatinous matrix. Asci (74–)89–113(–127) × (5.9–)6.5–7(–9) μm (\( \bar{x} \) = 101 × 6.8 μm, n = 20), 8-spored, bitunicate, cylindrical, long pedicellate, with a furcate pedicel, apically rounded with a minute ocular chamber. Ascospores (10–)12–14.5(–16) × (3.5–)4.5–5 μm (\( \bar{x} \) = 13 × 4.5 μm, n = 30), uni- to bi-seriate or overlapping, ellipsoidal to fusiform, 2-celled, constricted at the septum, pale brown to brown, guttulate when young, smooth-walled, without any mucilaginous sheath and appendages. Asexual morph Undetermined.
Culture characteristics: Ascospore geminating on WA within 12 h and colonies on PDA reaching 24 mm diam. in 10 days at 25 °C; circular, irregular, aerial hypha dense, raised, white to pale brown in forward and rufous in reverse. Mycelium superficial to immersed in/on media, with branched, smooth-walled hyphae.
Material examined: CHINA, Guizhou, Libo County, Maolan National Natural Preserve, on decaying branch of unidentified host, 8 July 2015, J.F. Zhang, MLC-13 (GZAAS 16-0011, holotype), ex-type living culture, GZCC16-0020.
Notes: The new taxon is typical of species in the family Roussoellaceae, and the sequence data also shows that it is most phylogenetically close to Neoroussoella bambusae (Fig. 68). However, it differs in the morphology of ascostromata. In N. lenispora ascostroma are globose or subglobose, while in N. bambusae, they are flattened at the base. The asci and ascospores also differ (asci: 101 × 6.8 μm vs. 72.2 × 5.6 μm; ascospores: 13 × 4.5 μm vs. 9 × 3 μm). The ascospores of N. bambusae are ornamented with longitudinal ribs and surrounded by a mucilaginous sheath, whereas those of N. lenispora lack a sheath and are smooth-walled when mature.
Torulaceae Corda
= Dendryphiaceae Corda, Icon. fung. (Prague) 4: 32 (1840)
Corda introduced the family Torulaceae (Sturm 1829). Crous et al. (2015a) revisited this family, provided molecular data for four species of Torula; accepted two genera (Dendryphion and Torula) and placed Torulaceae in the order Pleosporales. Su et al. (2016a) introduced a new genus Neotorula in this family and provided molecular data for three species of Dendryphion including two new species. Presently, there are three genera accepted in the family Torulaceae (Su et al. 2016a). Torulaceae is known only by asexual taxa characterized by erect, micro- or macronematous conidiophores, with or without apical branches, and doliiform to ellipsoid or clavate, brown, smooth to verruculose, mono- to polyblastic, often cupulate, conidiogenous cells and subcylindrical, phragmosporous, acrogenous, brown, dry, smooth to verrucose conidia, characteristically produced in branched chains (Crous et al. 2015a; Su et al. 2016a). A phylogenetic tree for the family is presented in this paper (Fig. 70).
Phylogram generated from maximum likelihood analysis (RAxML) based on combined ITS and LSU sequence data. Bootstrap support values for maximum likelihood (ML, red) and maximum parsimony (MP, green) equal to or greater than 50 % are given above the nodes. The values of the Bayesian posterior probabilities from MCMC analyses (BYPP, blue) equal or higher than 95 % are given below the nodes. The tree was rooted to Melanomma pulvis-pyrius. Newly generated sequences are indicated in red, and other ex-type strains are in bold
Neotorula Ariyawansa et al.
Neotorula was established by Su et al. (2016a) to accommodate a Torula-like species collected from freshwater habitats in China, based on both morphology and phylogeny. Neotorula shares similarities with Torula in having clavate to subcylindrical, brown conidia, with rounded ends that are borne in branched chains. The young conidia of N. aquatica are pale green, and are pale yellow in N. submersa. The conidiogenesis in Neotorula is polytretic, whereas Torula has polyblastic cupulate and dark-coloured conidiogenous cells. Neotorula has distinct conidiophores which comprise a few cells, while comprise a single or few cells in Torula. Our new species have polytretic, macronematous conidiophores. In this study, we introduce a new species in Neotorula based on morphological characters. The phylogenetic analysis also supports our taxon as a new species (Fig. 70).
Neotorula submersa Z.L. Luo, H.Y. Su & K.D. Hyde, sp. nov.
Index Fungorum number: IF552138; Facesoffungi number: FoF02217, Fig. 71
Etymology: With reference to the submerged habitat.
Holotype: HKAS 92660.
Saprobic on submerged decaying wood. Sexual morph Undetermined. Asexual morph Colonies on the substrate superficial, effuse, gregarious, hairy, dark brown. Mycelium immersed, composed of septate, branched, thin-walled, smooth, pale brown hyphae. Conidiophores mononematous, macronematous, branched or unbranched, septate, erect, straight or flexuous, smooth, pale brown, cylindrical, 2–5-septate, 18–48 μm (\( \bar{x} \) = 33 μm, SD = 15, n = 10) long, 4–5 μm (\( \bar{x} \) = 4.5 μm, SD = 0.5, n = 10) wide. Conidiogenous cells mono- to polytretic, integrated or discrete, terminal, pale brown, doliiform or lageniform. Conidia acrogenous, in short chains, dry, clavate to subcylindrical, rounded at the apex, 2–4-septate, dark bands at the septa, verruculose, pale yellow when young, tawny to brown when mature, 16–22 μm (\( \bar{x} \) = 19 μm, SD = 3, n = 25) long, 5–6 μm (\( \bar{x} \) = 5.5 μm, SD = 0.5, n = 25) wide. Conidial secession schizolytic.
Material examined: CHINA, Yunnan Province, saprobic on decaying wood submerged in Dulong River, May 2015, X.C. Tao, HD 1–10–7 (HKAS 92660, holotype), ex-type culture, KUMCC 15-0280, MFLUCC.
Notes: Neotorula submersa was collected from north-western Yunnan Province. It differs from N. aquatica in having thinner conidiophores (4–5 μm vs. 6–7 μm) and longer conidia (16–22 μm vs. 11–16 μm). The conidia of N. aquatica are pale green when young and brown when mature, in N. submersa they are pale yellow when young and tawny to brown when mature. Phylogenies also reveal a close relationship between these two taxa (Fig. 70).
Tubeufiales Boonmee & K.D. Hyde
For Tubeufiales, we follow Boonmee et al. (2014)
Tubeufiaceae M.E. Barr
The most recent treatments of the family and order are Boonmee et al. (2014) and their findings are followed here. In this study we provide and updated backbone tree for Tubeufiaceae (Fig. 72) and introduce Chlamydotubeufia helicospora, Helicoma guttulatum, Neoacanthostigma septoconstrictum, Tubeufia hyalospora and T. roseoelicospora. A reference specimen is also designated for Aquaphila albicans.
RAxML phylogenetic tree generated from combined LSU and ITS sequence data from species of Tubeufiaceae. The final ML optimization likelihood is −16263.541876. Bootstrap support values greater than 50 % are shown at the nodes and Bayesian posterior probabilities greater than 0.95 are marked with an asterisk (*). The tree is rooted with Hysteropatella elliptica (Patellariaceae). Type species are in bold and marked (T) and reference specimens are marked (R)
Aquaphila Goh et al.
Aquaphila is a genus known only from freshwater habitats and is typified by A. albicans which is connected with the sexual morph Tubeufia asiana Sivichai & K.M. Tsui (Tsui et al. 2007). Boonmee et al. (2014) synonymized T. asiana under Aquaphila and named Aquaphila asiana based on phylogenetic evidence.
Aquaphila albicans Goh, K.D. Hyde & W.H. Ho, Mycol. Res. 102(5): 588 (1998)
Index Fungorum number: IF443558; Facesoffungi number: FoF02356, Fig. 73
Reference specimen: MFLU 16-1136
Saprobic on decaying wood in flowing freshwater stream. Colonies on natural substrate effuse, translucent, chalky white or pale yellowish when dry. Mycelium partly immersed in woody substrate and partly superficial, consisting of septate, branched, hyaline, smooth, thin-walled hyphae. Sexual morph Undetermined. Asexual morph hyphomycetous; phragmosporous. Conidiophores arising singly as lateral branches from procumbent hyphae, up to 33 μm long, 4–6 μm wide, simple or branched, thin-walled and smooth, indistinctly septate, flexuous, hyaline. Conidiogenous cells monoblastic or polyblastic, sympodial with cylindrical denticles, hyaline, with numerous integrated tiny pegs. Conidia 70–86 × 8–10 μm, holoblastic, acrogenously borne on denticles, solitary, obclavate, predominantly fusoid to sickle-shaped, sometimes sigmoid, slightly curved and acute at both ends, basal cell obconical, up to 14-euseptate, slightly constricted at the septa, hyaline to pale yellowish, densely guttulate, smooth-walled.
Culture characteristics: Conidia germinating on water agar (WA) within 12 h and germ tubes produced at both ends. Colonies on malt extract agar (MEA), reaching 10 mm in 4 weeks at 28 °C, brown to dark brown in MEA media. Mycelium superficial and partially immersed, branched, septate, hyaline to pale brown, smooth.
Material examined: THAILAND, Prachuap Khiri Khan, Bang Sapan, Ron Thai, N11°14′47.028″ E99°19′59.682″, elev. ca. 171 msl., on decaying wood in flowing freshwater stream, 30 July 2015, K.D. Hyde TF04 (MFLU 16-1136, reference specimen designated here), MFLU 16-1138, BBH 41052,; ex-type living culture, MFLUCC 16-0010, TBRC.
Sequence data: LSU = KX454166 and KX454168, ITS = KX454165 and KX454167.
Notes: Two new collections of Aquaphila albicans (MFLU 16-1136 and MFLU 16-1138) were found on submerged wood in southern Thailand. Their morphology is identical to the type species A. albicans (HKU (M) 2856) collected in Queensland, Australia (Goh et al. 1998). Additionally, phylogenetic analysis placed the two new specimens in the same clade with other A. albicans strains and its sexual morph A. asiana (strain BCC3463) with high support (Fig. 72). We therefore designate MFLU 16-1136 as a reference specimen (sensu Ariyawansa et al. 2014a, b, c) for Aquaphila albicans. We do not designate an epitype as this was not collected from Australia.
Chlamydotubeufia Boonmee & K.D. Hyde
Chlamydotubeufia is a genus recognized by black pigmented with multi-septate dictyochlamydosporous conidia and a typical sexual morph (Boonmee et al. 2011). Chlamydotubeufia a small genus with only four species presently recorded and typified by Ch. huaikangplaensis Boonmee & K.D. Hyde (Index Fungorum 2016).
Chlamydotubeufia helicospora Boonmee, Y.Z. Lu & K.D. Hyde, sp. nov.
Index Fungorum number: IF552217; Facesoffungi number: FoF02357, Fig. 74
Chlamydotubeufia helicospora (MFLU 16–1338, holotype). a Conidia arise directly from hyphal cells on natural substrate. b Conidiophores with attached conidia. c Conidiogenous cells. d Germinating conidium. e–h Conidia. i–j Colonies on MEA from above and below. Scale bars a = 500 μm, b, e–h = 50 μm, c = 20 μm, d = 100 μm, i–j = 20 mm
Holotype: MFLU 16-1338.
Etymology: ‘helicospora’ referring to asexual helicospores.
Saprobic on woody substrate. Mycelium partly immersed, partly superficial, white, septate, sparsely branched hyphae, with masses of crowded conidia. Sexual morph Undetermined. Asexual morph Conidiophores 15–25 μm long × 4–6 μm wide (\( \bar{x} \) = 18 × 5 μm, n = 10), pale brown, macronematous, erect, short, smooth-walled. Conidiogenous cells 11–33 μm long × 4–5 μm wide (\( \bar{x} \) = 18 × 4.5 μm, n = 10), monoblastic, integrated, each with single conidium. Conidia 63–119 μm diam. and conidial filament 5–7 μm wide (\( \bar{x} \) = 80 × 6 μm, n = 20), 405–546 μm long, loosely coiled 1–2.5 times, rounded at apical end, up to 68-septate, slightly constricted at septa, hyaline, smooth-walled.
Culture characteristics: Conidia germinating on water agar (WA) within 8 h and germ tubes produced from conidia. Colonies on malt extract agar (MEA) reaching 4 mm in 1 week at 28 °C, brown to dark brown. Mycelium superficial and partially immersed, branched, septate, hyaline to pale brown, smooth.
Material examined: THAILAND, Uttaradit, Laplae, Mae Phun, Ban Ton Klua, on decaying wood in flowing freshwater stream, 24 October 2015, Saranyaphat Boonmee, UTD15–1 (MFLU 16-1338, holotype, BBH 41053, isotype); ex-type living culture, MFLUCC 16-0213, TBRC.
Notes: According to phylogenetic analysis, Chlamydotubeufia helicospora clusters in the Chlamydotubeufia clade with high support. The molecular analyses (Fig. 72) confirms C. helicospora as a new taxon that is basal to C. khunkornensis, while C. chlamydospora and C. huaikangplaensis constitutes a different sublineage with very high statistical support. Hence, we assign our collection as a new species based on morphology and phylogeny (Fig. 72). Morphologically, C. helicospora is characterized by slender hyphae, moderately long conidiophores and helicosporous conidia. The conidia are different from other species in Chlamydotubeufia (Boonmee et al. 2011).
Helicoma Corda
The genus Helicoma was established by Corda with the type species H. muelleri Corda. The genus is distinguished by its relatively short, erect, thick, dark brown, smooth conidiophores, holoblastic conidiogenous cells and helicoid, hyaline, thick-walled, brown to dark brown conidia forming from terminal, denticulate conidiophores.
Helicoma guttulatum Y.Z. Lu, Boonmee & K.D. Hyde, sp. nov.
Index Fungorum number: IF552218; Facesoffungi number: FoF02358, Fig. 75
Helicoma guttulatum (holotype). a Conidiophores with attached apical conidium on natural substrate. b, c Conidiophores with conidia at the apex. d Conidiogenous cell with conidiophores. e Germinating conidium. f–h Conidia. i–j Colonies on MEA from above and below. Scale bars a = 200 μm, b, c, e–h = 20 μm, d = 10 μm, i–j = 20 mm
Holotype: MFLU 16-1339
Etymology: ‘guttulatum’ referring to conidia containing numerous guttules.
Saprobic on woody substrates. Mycelium composed of partly immersed and partly superficial, pale brown, septate hyphae. Sexual morph Undetermined. Asexual morph Conidiophores 74–182(197) μm long, 4–6 μm wide (\( \bar{x} \) = 120 × 5 μm, n = 20), macronematous, crowded, erect, subhyaline to yellowish, brown towards the base, septate, unbranched, smooth-walled. Conidiogenous cells monoblastic to polyblastic, subhyaline to pale brown, smooth-walled. Conidia 18–23 μm diam., and conidial filament 6–8 μm wide (\( \bar{x} \) = 20 × 7 μm, n = 20), tightly coiled 1–1½ times, hyaline to pale brown, tapering toward flat end, 8–9-septate, rounded at the apex, conico-truncate at the base, smooth-walled.
Culture characteristics: Conidia germinating on water agar (WA) within 12 h. Colonies on malt extract agar (MEA) reaching 5 mm in 1 week at 28 °C, pale brown to brown. Mycelium superficial and partially immersed, branched, septate, hyaline to pale brown, smooth.
Material examined: THAILAND, Chiang Rai, Muang, Ban Nang Lae Nai, on decaying wood in flowing freshwater stream, 28 November 2015, Yong-Zhong Lu and Saranyaphat Boonmee, TUB02–1 (MFLU 16-1339, holotype, BBH 41054, isotype); ex-type living culture, MFLUCC 16-0022, TBRC.
Notes: Helicoma guttulatum was collected from northern Thailand. Phylogenetic results recognize H. guttulatum as belonging to the genus Helicoma as well as its recognition as a new species. Helicoma guttulatum segregates from H. dennisii and H. inthanonense with full support in the phylogenetic analysis (Fig. 72). Helicoma guttulatum forms an asexual morph on the natural substrate, which is morphologically similar to H. muelleri (Boonmee et al. 2014). However, its conidiophores (74–182 μm) are longer and narrower (4–6 μm) than H. muelleri (80–154 μm) and (7–10 μm). The conidial diameter (18–23 μm) is also greater than H. moelleri (16–19 μm). Therefore, H. guttulatum is introduced here as a novel species.
Neoacanthostigma Boonmee et al.
The genus Neoacanthostigma was introduced to accommodate three sexual taxa, characterized by dark pigmented ascomata, surrounded by reddish-brown to dark brown setae, bitunicate asci, fasciculate cylindrical and hyaline ascospores, and having a helicosporous asexual state (Boonmee et al. 2014). Here we collected Acanthostigma septoconstrictum Promp. & A.N. Mill. and transfer it to Neoacanthostigma.
Neoacanthostigma septoconstrictum (Promp. & A.N. Mill.) S. Boonmee & K.D. Hyde, Fungal Diversity 68(1): 279 (2014)
≡ Acanthostigma septoconstrictum Promp. & A.N. Mill., Mycologia 102(3): 579 (2010)
Index Fungorum number: IF550683; Facesoffungi number: FoF02360, Fig. 76
Reference specimen: MFLU 16-1134
Saprobic on decaying wood in flowing freshwater stream. Mycelium partly superficial, partly immersed, composed of brown, septate, sparsely branched hyphae, with masses of crowded conidia. Sexual morph Undetermined. Asexual morph hyphomycetous; helicosporous. Conidiophores brown, macronematous, erect, short, smooth-walled. Conidiogenous cells monoblastic, integrated, each with single conidium. Conidia 115–155 μm diam., filament 9–11 μm wide (\( \bar{x} \) = 139 × 10 μm, n = 20), 775–920 μm long, loosely coiled 1½–2½ times, rounded at apical end, up to 71-septate, not constricted at septa, pale brown to brown, smooth-walled.
Culture characteristics: Conidia germinating on water agar (WA) within 12 h and germ tubes produced from conidia. Colonies growing on malt extract agar (MEA), reaching 25 mm in 4 weeks at 28 °C, slightly convex, with an undulate edge, pale yellow to pale brown, brown in MEA media. Mycelium superficial and partially immersed, branched, septate, hyaline to pale brown, smooth.
Material examined: THAILAND, Prachuap Khiri Khan, Bang Sapan, Ron Thai, on decaying wood in flowing freshwater stream, 30 July 2015, K.D. Hyde, KH02 (MFLU 16-1134, BBH 41051); living culture, MFLUCC 15–1248, TBRC.
Notes: Neoacanthostigma was introduced by Boonmee et al. (2014) with N. fusiforme as the type species. An asexual morph has not been previously reported in this genus. In this study, N. septoconstrictum was found in its asexual form (Fig. 72) on decaying wood in a flowing freshwater stream and is characterized by pale brown to brown, large filament, multi-septate conidia. Phylogenetic analysis placed our collection in a cluster with the sexual morph N. septoconstrictum (strain ANM 536.1) with high support (Fig. 72). Therefore, we describe this fungus under the sexual name N. septoconstrictum.
Tubeufia Penz. & Sacc.
The genus Tubeufia is the type genus of the family Tubeufiaceae. It is characterized by white, cream-pink to brownish, vertically oblong to ovoid ascomata and cylindrical, fusiform to vermiform, multi-septate ascospores (Barr 1980).
Tubeufia hyalospora Y.Z. Lu, Boonmee & K.D. Hyde, sp. nov.
Index Fungorum number: IF552220; Facesoffungi number: FoF02361, Fig. 77
Holotype: MFLU 16-1135
Etymology: ‘hyalospora’ referring to hyaline helicospores of asexual morph.
Saprobic on decaying woody in flowing freshwater stream. Mycelium partly immersed, partly superficial, composed of pale brown, septate, sparsely branched hyphae, with masses of crowded conidia. Sexual morph Undetermined. Asexual morph hyphomycetous; helicosporous. Conidiophores pale brown, macronematous, erect, short, smooth-walled. Conidiogenous cells monoblastic, integrated, each with single conidium. Conidia 16–33 μm diam., conidial filament 3–5 μm wide (\( \bar{x} \) = 22 × 4 μm, n = 20), 110–225 μm long, coiled 2½–3½ times, rounded at apical end, multi-septate, slightly constricted at septa, hyaline, smooth-walled.
Culture characteristics: Conidia germinating on water agar (WA) within 12 h and germ tubes produced from conidia. Colonies growing on malt extract agar (MEA), reaching 26 mm in 3 weeks at 28 °C, with an undulate edge, pale brown to brown, dark brown, white at margin. Mycelium superficial and partially immersed, branched, septate, hyaline to pale brown, smooth.
Material examined: THAILAND, Prachuap Khiri Khan, Bang Sapan, Ron Thai, on decaying wood in flowing freshwater stream, 30 July 2015, K.D. Hyde, TF03 (MFLU 16-1135, holotype); ex-type living culture, MFLUCC 15-1250, TBRC.
Notes: Tubeufia hyalospora grouped within the Tubeufia clade, but did not cluster with any known species (Fig. 72). Tubeufia hyalospora forms an asexual morph on the natural substrate, which is morphologically similar to T. tectonae (Doilom et al. 2016). Its conidia (diam. 16–33 μm) are narrower than those of T. tectonae (32–55 μm) and are tightly coiled, but in T. tectonae most are loosely coiled or uncoiled.
Tubeufia roseohelicospora Y.Z. Lu, Boonmee & K.D. Hyde, sp. nov.
Index Fungorum number: IF02362; Facesoffungi number: FoF02362, Fig. 78
Etymology: ‘roseohelicospora’ referring to light pink helicospores of asexual morph.
Holotype: MFLU 16-1133.
Saprobic on decaying woody in flowing freshwater stream. Colonies on natural substrate, superficial with partly immersed mycelium and bright, septate, sparsely branched hyphae, with masses of crowded conidia. Sexual morph Undetermined. Asexual morph Hyphomycetes, helicosporous. Conidiophores macronematous, erect, short, pale brown, smooth-walled. Conidiogenous cells monoblastic, integrated, each with single conidium. Conidia 36–48 μm diam., filaments 5–7 μm wide (\( \bar{x} \) = 41 × 6 μm, n = 20), 210–283 μm long, coiled 2½–3½ times, tight to loose, rounded at apical end, 29–35-septate, slightly constricted at septa, light pink on substrate, hyaline when seen under light microscope, smooth-walled.
Culture characteristics: Conidia germinating on water agar (WA) within 12 h and germ tubes produced from conidia. Colonies growing on malt extract agar (MEA) reaching 10 mm in 2 weeks at 28 °C, with an undulate edge, pale brown to brown, dark brown. Mycelium superficial and partially immersed, branched, septate, hyaline to pale brown, smooth-walled.
Material examined: THAILAND, Prachuap Khiri Khan, Bang Sapan, Ron Thai, on decaying wood in flowing freshwater stream, 30 July 2015, K.D. Hyde KH01 (MFLU 16-1133, holotype; BBH 41050, isotype); ex-type living culture, MFLUCC 15-1247, TBRC.
Notes: Tubeufia roseoelicospora clusters in Tubeufia sister to T. tectonae based on the phylogenetic analysis of combined LSU and ITS sequence data (Fig. 72). The conidiophores of T. roseoelicospora are tightly coiled while those of T. tectonae are mostly loosely coiled or uncoiled; its conidia filament is 5–7 μm wide, while that of T. tectonae is 2–5 μm wide. On the natural substrate colonies of T. roseohelicospora are light pink, while T. tectonae colonies are hyaline to white. Therefore, T. hyalospora is introduced here as a novel species based on its morphological and phylogenetic differences from other Tubeufia species.
Dothideomycetes family, incertae sedis
Pleurotremataceae Walt. Watson
Currently Pleurotremataceae comprises the genus Pleurotrema and Dyfrolomyces species may need transferring to this genus (Maharachchikumbura et al. 2016). Whether the freshwater species of Saccardoella belong to this family will require further studies at the molecular level.
Pleurotrema Müll. Arg.
Watson (1929) introduced the family Pleurotremataceae based on Pleurotrema polysemum (Nyl.) Mull. Arg. Eriksson and Hawksworth (1993) suspected Pleurotremataceae to be synonym of Pyrenulaceae. Pleurotrema was synonymized under Lithothelium as a member of Pyrenulaceae by Aptroot (1991). Later, Harris re-examined the isotype of P. polysemum, and showed its similarity to Melomastia and Saccardoella (Barr 1994). Based on its cylindrical asci, with a non amyloid apical ring and hyaline, distoseptate ascospores Barr (1994) transferred Pleurotremataceae to Xylariales. Five genera, Phomatospora, Melomastia, Pleurotrema, Saccardoella, Daruvedia, were included in Pleurotremaceae on account of their non-fissitunicate asci (Barr 1994). Hawksworth et al. (1995) disagreed with this justification and retained Pleurotrema in the order Pyrenulales. Saccardoella and Melomastia were placed in Ascomycota genera incertae sedis (Kirk et al. 2001; Lumbsch and Huhndorf 2010), but there is no phylogenetic evidence to support this. Pleurotremataceae was accepted as monotypic via Pleurotrema and placed under Chaetosphaeriales without giving a reason by Maharachchikumbura et al. (2015). However, Maharachchikumbura et al. (2016) excluded this family from class Sordariomycetes and this is an earlier name for Dyfrolomycetaceae (Dothideomycetes).
Pleurotrema thailandica Dayarathne, Jones E.B.G. & K.D. Hyde, sp. nov.
Index Fungorum number: IF552244; Facesoffungi number: FoF02445, Fig. 80
Etymology: Name reflects the country Thailand, from where the holotype was collected.
Holotype: MFLU 16-1173.
Saprobic on pneumatophores of Avicennia marina in mangrove vegetation. Sexual morph Ascomata 430–350 μm diam. × 242–280 μm high (\( \bar{x} \) = 428 × 264 μm, n = 10), solitary, semi-immersed, clypeate, globose to subglobose, dark brown to black, ostiolate, ostiolar canal filled with dark cells, apapillate. Peridium 26–35 μm wide, comprising an outer layer of dark brown cells of textura angularis, becoming lighter inwardly. Hamathecium comprising numerous, 2.1–3.6 μm wide, septate pseudoparaphyses embedded in a gelatinous matrix. Asci 146–158 × 7–9 μm (\( \bar{x} \) = 152 × 8.2 μm, n = 10), 8-spored, bitunicate, cylindrical, short-pedicellate, with a thickened apex. Ascospores 24–32 × 6–8 μm (\( \bar{x} \) = 28 × 6.5 μm, n = 20), slightly overlapping uni-seriate, hyaline, ellipsoidal, mostly 3–5-septate, slightly constricted at septa, with several small guttules, surrounded by a gelatinous sheath, 1.2–2.4 μm thick. Asexual morph Undetermined.
Culture characteristics: Colonies on PDA, reaching 3 cm in 14 days at 25–28 °C, white at first, becoming yellowish when mature, undulate and reverse yellowish-white.
Material examined: THAILAND, Phetchaburi Province, Hat Chao Samran, 47°72506′E, 40°25038′N, 0 m asl., on pneumatophores of Avicennia marina, 28 July 2015, Monika Dayarathne, CHAM 006 (MFLU 16-1173, holotype); ex-type living culture, MFLUCC 15-0945, ICMP.
Notes: Pleurotrema thailandica is similar to other species of Pleurotrema, but can be differentiated based on ascospore characteristics, in particular ascospore septation. Ascospores of P. thailandica are 3–5-septate, while those of P. tiomanensis, P. mangrovei, P. marinospora and P. rhizophorae are 20–24, 7–9, 3 and 4–6 septate, respectively. According to our maximum likelihood analysis based on combined LSU and SSU sequence data, D. thailandica forms a distinct lineage, sister to P. rhizophorae with 99 % bootstrap support (Fig. 79).
Trypetheliaceae Eschweiler
Trypetheliaceae is mainly a lichen-forming family distributed mostly in tropical to subtropical areas (Harris 1984; Aptroot et al. 2008; Nelsen et al. 2014). The family was introduced by Eschweiler (1824) and is typified by T. eluteriae Sprengel. It is characterized by a corticolous thallus, perithecioid ascomata often organized in pseudostromata, richly branched and anastomosing, net-like pseudoparaphyses, bitunicate asci, ascospores often with angular wall thickenings and diamond-shaped lumina, and a trentepohlioid photobiont in lichenized taxa (Harris 1984; Aptroot 1998, 2008; Hyde et al. 2013; Nelsen et al. 2009, 2014).
Pleurotrema thailandica (holotype). a Appearance of ascomata on host surface. b Close up of clypeus of ascomata. c, d Vertical sections through ascomata. e Peridium. f–i Asci. j Apical thickening. k Pseudoparaphyses. l–o Ascospores with sheath. p Germinating ascospore. Scale bars b = 1 mm, c, d, f–i = 100 μm, e, l–p = 20 μm, j = 10 μm
Based on phylogenetic analyses, the family forms a monophyletic clade in Trypetheliales, sister to Polycoccaceae (Ertz et al. 2015). In this paper we introduce a new genus, Alloarthopyrenia, in Trypetheliaceae to accommodate a unique, new, non-lichenized lineage resembling Arthopyrenia (Fig. 81).
Phylogram generated from maximum likelihood analysis based on combined LSU and SSU sequence data of selected taxa. Maximum likelihood bootstrap support values greater than 60 % are given above the nodes. The type strains of each genera are in bold and the new isolates are in blue bold. The tree is rooted with Arthoniomycetes taxa
Alloarthopyrenia Phukhamsakda, Lücking & K.D. Hyde, gen. nov.
Index Fungorum number: IF552236, Facesoffungi number: FoF02379
Etymology: The generic name Alloarthopyrenia refers to its morphological resemblance with Arthopyrenia.
Saprobic on living tree branches. Thallus absent but area around the ascomata in part whitish. Sexual morph Ascostromata covered with a blackened pseudoclypeus, semi-immersed, with only ostioles visible, coriaceous, solitary, scattered or gregarious, depressed globose to obpyriform, wall rough, black to dark brown, ostiolate. Ostiole centrally located, filled with periphyses. Peridium dark brown to light brown, cells of textura angularis and textura epidermoidea, easy to break, at base indistinguishable from host tissue. Hamathecium composed of branched, anastomosing, trabeculate pseudoparaphyses, embedded in a gelatinous matrix. Asci 8-spored, bitunicate, obviod to suboblong, short pedicellate, thick-walled, faintly bluish in IKI + , apically rounded, with ocular chamber, lightly IKI + bluish. Ascospores partially overlapping or bi-seriate, hyaline, oviod or ellipsoid, septate, constricted at the septa, wall rough, indentations present when mature, surrounded by a mucilaginous sheath. Asexual morph Undetermined.
Type species: Alloarthopyrenia italica Phukhamsakda, Camporesi, Ariyawansa & K.D. Hyde.
Notes: Based on phylogenetic analysis, Alloarthopyrenia is introduced as a monospecific genus to accommodate a species forming a distinct lineage in Trypetheliaceae (Fig. 81). The genus Arthopyrenia sensu lato is polyphyletic with tropical, lichenized species clustering in Trypetheliaceae and a single, non-lichenized taxon, Arthopyrenia salicis, which falls in Pleosporales: there, A. salicis clusters with Roussoella and Roussoellopsis, and Liu et al. (2014) introduced Roussoellaceae to accommodate these taxa. Arthopyreniaceae was kept as a separate family in Pleosporales, based on its morphology and anatomy based on the type species, A. cerasi (Schrad.) A. Massal. which has not yet been sequenced. The new genus, Alloarthopyrenia is similar to other non-lichenized, temperate species formerly placed in Arthopyrenia sensu lato, including A. cerasi, in having a carbonaceous pseudoclypeus, branched and anastomosing pseudoparaphyses, bitunicate asci with shortpedicels, and hyaline ascospores (Coppins 1988; Hyde et al. 2013). Upreti and Pant (1993) illustrated species of Arthopyrenia from India. Alloarthopyrenia italica differs from Arthopyrenia cerasi in having depressed-globose to obpyriform ascostromata, wider hamathecium filaments (1.4 vs. 0.5–0.7 μm), obovoid to suboblong asci, and 1-septate, ovoid to ellipsoid ascospores; in contrast, A. cerasi has K + greenish, larger and hemisphaerical ascostromata (300–500 μm), less branched and narrower pseudoparaphyses, cylindrical-clavate asci, and 3-septate, oblong ascospores (Coppins 1988; Nelsen et al. 2009; Hyde et al. 2013). It is therefore highly unlikely that the two species are congeneric and that A. cerasi also falls within Trypetheliaceae.
Although the family Trypetheliaceae mainly comprises lichenized fungi, Nelsen et al. (2014) found some weakly or non-lichenized taxa in the family, forming basal lineages in the tree. This particularly applies to a non-lichenized, temperate species of Julella, and hence, the placement of a further, non-lichenized lineage basally in the family is not surprising. The internal anatomy of Alloarthopyrenia is otherwise typical of Trypetheliaceae, with the hamathecium forming a network of anastomosing hyphae embedded in a gelatinous matrix, bitunicate asci, transversely septate, and hyaline ascospores (Harris 1984; Prado et al. 2006; Nelsen et al. 2009). In the present study we introduce a new genus for this species in Trypetheliaceae, based on data from analysis of combined LSU and SSU sequence data.
Alloarthopyrenia italica Phukhamsakda, Camporesi, Ariyawansa & K.D. Hyde, sp. nov.
Index Fungorum number: IF552237; Facesoffungi number: FoF02380, Fig. 82
Alloarthopyrenia italica (holotype). a Herbarium material. b, c Ascostromata on host surface. d Section of ascostroma. e Ostiole. f Section of peridium. g Pseudoparaphyses. h–i Ocular chamber (i ocular chamber staining in Melzer’s reagent). j Immature asci. k–l Mature asci. m Ascus staining in Melzer’s reagent. n–r Ascospores. Scale bar b = 200 μm, c = 100 μm, d = 20 μm, e–m = 50 μm, h–i = 5 μm, n–r = 10 μm
Etymology: Name reflects the country “Italy”, where the holotype was collected.
Holotype: MFLU 15-0399.
Saprobic on living tree branches of Fraxinus ornus L. Thallus absent, algae not visible. Sexual morph Ascostromata 110–185 μm high × 100–270 μm diam. (\( \bar{x} \) = 139 × 173 μm, n = 5), covered with a blackened pseudoclypeus, semi-immersed, with only ostioles visible, coriaceous, solitary, scattered or gregarious, depressed globose to obpyriform, wall rough, black to dark brown, flattened, ostiolate. Ostiole centrally located, oblong, filled with periphyses. Peridium 12–23(–21 at apex) μm wide, composed of 5–6 layers of dark brown to light brown, cells of textura angularis and textura epidermoidea, easy to break, at base indistinguishable from host tissue. Hamathecium of numerous, dense, 1–1.8 μm wide (\( \bar{x} \) = 1.4 μm, n = 60), narrow, transversely septate, branched, anastomosing, trabeculate pseudoparaphyses, embedded in a gelatinous matrix. Asci 45–99 × 9–20 μm (\( \bar{x} \) = 66 × 15 μm, n = 30), 8-spored, bitunicate, fisitunicate, obviod to suboblong, short pedicellate, thick-walled, lightly faintly bluish in IKI + , apically rounded, with ocular chamber up to 1–2 μm high. Ascospores 15–20 × 4–9 μm (\( \bar{x} \) = 18 × 7 μm, n = 50), partially overlapping or bi-seriate, hyaline, oviod or ellipsoid, slightly narrow at the apex when young, rounded at the apex when mature, 1-septate, strongly constricted at the septum, lower end tapering, wall rough, not uniform, smooth, indentations present when mature, surrounded by 2.5–4 μm wide, mucilaginous sheath. Asexual morph Undetermined.
Material examined: ITALY, Forlì-Cesena Province, Monte Mirabello—Predappio, on dead branch of Fraxinus ornus L. (Oleaceae), 15 September 2014, E. Camporesi, IT 122 (MFLU 15-0399, holotype, isotype in HKAS 94615).
Notes: Alloarthopyrenia italica was collected on living branches of Fraxinus ornus (Oleaceae) in Italy; it is non-lichenized and probably saprobic. Due to the taxon having similarities with Arthopyrenia, we compared its morphology with Arthopyrenia species reported from Fraxinus. Five species have been recorded from Fraxinus in Europe, including A. cerasi (Schrader) A. Massal (type species of Arthopyrenia, see above), A. cinereopruinosa (Schaerer) A. Massal., A. carneobrunneola B.J. Coppins, A. fraxini A. Massal., and A. ranunculospora B.J. Coppins & P. James (Coppins 1988; Index Fungorum 2016). Arthopyrenia carneobrunneola and A. ranunculospora differ in being lichenized (Coppins 1988). Arthopyrenia cinereopruinosa has been reported from Italy, but it is weakly lichenized and has larger ascostromata [(200–)300–440 μm] with 3-septate ascospores (Coppins 1988; Aptroot et al. 2008). Arthopyrenia fraxini differs in having larger ascostromata (250–500 μm), longer, clavate to cylindrical asci, and thick-walled, larger ascospores (20–30 × 4–9 μm), with blunt ends and lacking indentations (Coppins 1988; Upreti and Pant 1993). Our species is also similar to A. grisea (Schleich. ex Schaer.) Körb. but the latter has a yellow thallus, clavate to cylindrical uni-seriate asci, and 3-septate ascospores (Upreti and Pant 1993). As most Arthopyrenia species lack sequence data, their placement is uncertain.
Pleosporales genera incetae sedis
The following tree (Fig. 83) represents taxa which do not cluster in the main families of Dothideomycetes.
RAxML maximum likelihood phylogenetic tree based on a LSU and SSU sequenced data from species of order Pleosporales. Maximum likelihood bootstrap support values greater than 50 % are shown in near the nodes. Some branches were shortened to fit the page—these are indicated by two diagonal lines with the number of times a branch was shortened indicated next to the lines. The new isolates are in red. The tree is rooted with Hysterium angustatum
Neomassarina Phookamsak, Jayasiri & K.D. Hyde, gen. nov.
Index Fungorum number: IF552225; Facesoffungi number: FoF02259
Etymology: The generic epithet “Neomassarina” refers to the resemblance to Massarina
Saprobic on Agave angustifolia. Sexual morph Ascostromata black, solitary, scattered to clustered, immersed, erumpent through host surface, slightly raised, globose to subglobose, uni- to bi-loculate, glabrous, ostiole central, with a minute papilla. Peridium thin-walled, of unequal thickness, composed of 3–5 layers of dark pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium composed of dense, 0.5–1 μm wide, cellular pseudoparaphyses, anastomosing among the asci, embedded in a hyaline gelatinous matrix. Asci 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate. Ascospores overlapping uni- to bi-seriate, hyaline, pale brown at maturity, fusiform, 1-septate, constricted at the septum, smooth-walled with guttules, surrounded by a distinct mucilaginous sheath. Asexual morph Undetermined.
Type species: Neomassarina thailandica Phookamsak, Jayasiri & K.D. Hyde
Notes: Neomassarina is introduced as a monotypic genus to accommodate the massaina-like species, but not congeneric with Massarina. Neomassarina is similar to Massarina in having globose to subglobose ascostromata, with a thin-walled peridium and typically hyaline, fusiform ascospores, with broadly cellular pseudoparaphyses. However, the phylogeny indicates that they are distinct. Based on multi-gene phylogenetic analyses, Neomassarina form a single clade basal to Floricolaceae (Fig. 83). Therefore, the new genus is introduced in this study.
Neomassarina thailandica Phookamsak, Jayasiri & K.D. Hyde, sp. nov.
Index Fungorum number: IF552226; Facesoffungi number: FoF02260, Fig. 84
Neomassarina thailandica (holotype). a Appearance of ascostromata on the host surface. b Section through the ascostroma. c Section through peridium. d Pseudoparaphyses stained in Melzer’s reagent. e–h Asci. i–k Ascospores. l Ascospores becoming pale brown at maturity. m, n Culture characteristics (m = from above, n = from below). Scale bars b = 100 μm, c = 20 μm, d–h, l = 10 μm, i–k = 5 μm
Etymology: The specific epithet “thailandica” refers to the country, from which the holotype was collected.
Holotype: MFLU11-0144.
Saprobic on dead bract-like leaves from flower stalk of Agave angustifolia Haw. Sexual morph Ascostromata 130–180 μm high, 100–200 μm diam., black, solitary, scattered to clustered, immersed, erumpent through host surface, slightly raised, with dark area around ostioles, globose to subglobose, uni- to bi-loculate, glabrous, ostiole central, with minute papilla. Peridium 7–16 μm wide, thin-walled, of unequal thickness, slightly thick at the apex, composed of 3–5 layers of flattened, dark brown to black, pseudoparenchymatous cells, arranged in a textura angularis. Hamathecium composed of dense, 0.5–1 μm wide, cellular pseudoparaphyses, distinctly septate, anastomosing among the asci, embedded in a hyaline gelatinous matrix. Asci (70–)75–90(–93) × 7–8(–8.5) μm (\( \bar{x} \) = 81.8 × 7.6 μm, n = 20), 8-spored, bitunicate, fissitunicate, cylindrical to cylindric-clavate, short pedicellate, apically rounded, with an obtuse, ocular chamber. Ascospores (17.5–)18–20 × 3–4(–5) μm (\( \bar{x} \) = 18.6 × 4 μm, n = 25), overlapping uni- to bi-seriate, hyaline, pale brown at maturity, fusiform, 1-septate, constricted at the septum, smooth-walled, guttulate, surrounded by a distinct mucilaginous sheath. Asexual morph Undetemined.
Culture characteristics: Colonies on PDA reaching 20–25 mm diam. after 3 weeks at 25–30 °C; colony from above, white to cream; from below, white to cream at the margin, pale yellowish at the centre; medium dense, irregular, flattened, with undulate edge, surface slightly rough, cottony; not producing pigmentation in agar.
Material examined: THAILAND, Chiang Mai, Muang District, Medicinal Plant Garden in Doi Suthep-Pui, on dead bract-like leaves from flower stalks of Agave angustifolia (Asparagaceae), 23 November 2009, R. Phookamsak, RP0015 (MFLU 11-0144, holotype), ex-type living cultures, MFLUCC 10-0552, BCC.
Eurotiomycetes
Eurotiales G.W. Martin ex Benny & Kimbr.
Section Cremei
Aspergillus P. Micheli ex Haller
The genus Aspergillus (Trichocomaceae, Eurotiales) was established by Micheli (1729). The species belonging to this genus are characterized by their conidiophore patterns and the production of conidial heads (Samson et al. 2014). Species of Aspergillus are ubiquitous and are particularly abundant in the soil and in contaminated foods; about 339 species (Samson et al. 2014) are divided into four subgenera with 20 sections (Houbraken et al. 2014). About 60 species are known to be medically relevant pathogens, while many species are common contaminants as mycotoxins producers on various foods, others, such as A. oryzae, are important in commercial microbial fermentations as Koji mould. Currently, aspergillum is known as a name of an asexual spore-forming structure which is common to all species of Aspergillus (Dyer and O’Gorman 2011); about one-third of species are known to have a sexual morph. Among the sections of Aspergillus, section Cremei (known as A. cremeus group) was first described by Raper and Fennell (1965) for five species. Colonies of species belonging to this section are characterized by shades of yellowish-brown to brown or grey-green, with bi-seriate conidial heads and long conidiophores (Samson et al. 2014). Their conidia are pale grey-green to yellow-brown (Peterson 1995). Among species assigned to this section, A. wentii is known as a source of enzymes (Raper and Fennell 1965; Lowe 1992) and A. inflatus is reported to produce sterigmatocystin—a precursor to the even more potent compounds, the aflatoxins (Rank et al. 2011). During the investigation of the fungi from rhizosphere soil of pine trees in forests of Gunsan, Korea in August 2015, an interesting species of Aspergillus was discovered. It proved to be sufficiently different from previously described species (Stolk and Malla 1971; Samson et al. 2014) to warrant description as a new species, forming a distinct lineage. Previously only one species, A. cibarius from traditional Meju, has been reported as a new species in Korea (Hong et al. 2012).
Aspergillus koreanus Hyang B. Lee, T.T. Duong & T.T.T. Nguyen, sp. nov.
MycoBank number: MB816938; Facesoffungi number: FoF02476, Fig. 88
Etymology: koreanus, referring to the country from which the species was first isolated (Korea)
Holotype: EML-GSNP1-1
Colonies on MEA reaching 41–44 mm diam. at 25 °C in 7 days, initially white to white-cotton, turning greyish-brown in the center with age and the abundant sporulation; reverse white to pale yellowish. Conidiophores hyaline, smooth, septate, varying greatly in length, 4.5–6 μm diam. Vesicles subglobose to globose, 7.5–12 μm diam. Conidial heads bi-seriate, consisting of many metulae bearing several phialides. Phialides ampulliform, 6–9 × 1.5–2.5 μm, extending from metulae. Conidia grey-green, slightly roughened, borne in short or long chains, subglobose to globose, 2.5–3.5 μm diam. Cleistothecia not observed.
Notes: Aspergillus koreanus is distinct from A. inflatus, growing rapidly when cultivated on MEA producing subglobose to globose vesicles successively and bearing metulae which develop simultaneously. The metulae consist of several (commonly 3) phialides. The conidial colour is consistently grey-green with a thin band in the middle to connect conidia into a chain, while that of A. inflatus is brownish, with mostly two roughly parallel very thin bands. A part from phylogenetic characterization; phylogenetic comparisons from single and multi-gene sequence analyses clearly indicate that A. koreanus strains are related to A. inflatus and represent a new taxon (Figs. 85, 86, 87).
Phylogenetic tree of Aspergillus koreanus and related species based on maximum parsimony analysis of a combined dataset from RNA polymerase II (RPB2), ITS and 28S rDNA sequence data. Sequences data from Talaromyces bacillisporus was used as the outgroup taxon. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replicates. The bar indicates the number of substitutions per position. New taxa are in blue
Phylogenetic tree of Aspergillus koreanus and related species within the sect. Cremei based on maximum parsimony analysis of ITS sequence data. Sequence data for Aspergillus tamarii was used as the outgroup taxon. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replicates. The bar indicates the number of substitutions per position. New taxa are in blue
Phylogenetic tree of Aspergillus koreanus and related species within the sect. Cremei based on maximum parsimony analysis of combined dataset for beta tubulin (BenA), calmodulin (CaM), ITS rDNA, 28S rDNA and RPB2 sequence data. Sequence data for Aspergillus tamarii is used as the outgroup taxon. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replicates. The bar indicates the number of substitutions per position. New taxa are in blue
Material examined: REPUBLIC OF KOREA, Jeonnam Province, Gunsan City, Sinsido Island (35.82°N, 126.45°E), from a rhizosphere soil of pine tree in forest, 7 August 2015; EML-GSNP1-1 holotype, (ex-type) at Culture Collection of National Institute of Biological Resources (NIBR), Incheon, and preserved as glycerol stock at −80 °C in the Chonnam National University Fungal Collection (CNUFC); living culture (ex-type) deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:012334).
The isolate was observed to grow over a wide range of temperatures, with varying growth rates The average growth rates on MEA, PDA, YES, OA, CYA, and CREA were 6, 5.5, 4.5, 4, 3, and 0.2 mm per day, respectively at 25 °C. Optimal growth was observed at 25 °C, slow growth was observed at below 20 °C and no growth at 35 °C (Fig. 88).
Aspergillus koreanus (holotype). a, d Colonies on malt extract agar (MEA). b, e Colonies on yeast extract sucrose agar (YES). e, f Colonies on Czapek yeast autolysate agar (CYA) (a–c from above, d–f from below). g–i Developing bi-seriate conidial heads on stalks. j Phialides finely differentiated on metulae covering the entire surface of vesicle. k Conidiophore and phialides (green arrow). l Detail of a metula bearing 3 phialides (red arrow). m Initial young conidia (yellow arrow) developed at the tips of phialides and scars remaining after conidial detachment (white arrow). n Mature conidia with slightly roughened scale-like surface. Scale bars g–j, k, l = 20 μm, m, n = 5 μm
Chaetothyriales M.E. Barr
Chaetothyriales comprises Chaetothyriaceae, Cyphellophoraceae and Herpotrichiellaceae based on molecular analysis (Réblová et al. 2013), and includes the sooty mould families Chaetothyriaceae, Coccodiniaceae and Trichomeriaceae (Chomnunti et al. 2012b, 2014).
Chaetothyriaceae Hansf. ex M.E. Barr
For the family Chaetothyriaceae, we follow Chomnunti et al. (2014).
Ceramothyrium menglunense Mapook, J.F. Li & K.D. Hyde, sp. nov.
Index Fungorum number: IF552275, Facesoffungi number: FoF02477, Fig. 90
Etymology: Named for the town, Menglun, where the holotype was collected.
Holotype: MFLU 16-1874
Saprobic on a dead leaves of Syzygium sp. Sexual morph Ascomata (205–)245–255(–370) μm high × 140–165 μm diam. (\( \bar{x} \) = 270 × 150 μm, n = 5), superficial, solitary or scattered, coriaceous, globose to subglobose, dark brown to black with scattered brown setae, Ostiole central, minutely papillate. Peridium (10–)20–60 μm wide, 2-layered, outer layer comprising dark brown to black cells of textura globulosa, inner layer comprising light brown to hyaline cells of textura angularis. Hamathecium comprising 2–3 μm wide, cylindrical to filiform, septate, branched, pseudoparaphyses. Asci 50–75 × 24–30(–37) μm (\( \bar{x} \) = 65 × 28 μm, n = 10), 8-spored, bitunicate, clavate to pyriform, short pedicellate, with an ocular chamber. Ascospores 25–35 × 10–12 μm (\( \bar{x} \) = 28 × 11 μm, n = 10), overlapping, hyaline, ellipsoid to obovoid, muriform, with 4–7 transverse septa, and 1–5 vertical septa when mature, constricted at the septa, smooth-walled, without mucilaginous sheath. Asexual morph Undetermined.
Material examined: CHINA, Yunnan Province, Xishuangbanna, Menglun, on dead leaves, 13 February 2015, J.F. Li (MFLU 16-1874, holotype), ex-type culture MFLUCC 14-1120, (isotype in HKAS, under the code of HKAS 95077).
Notes: Maximum likelihood (RAxML) and Bayesian analyses of combined LSU and ITS sequences data showed that our collection is closely related to Ceramothyrium thailandicum. However, our collection is morphologically distinct in having larger ascomata with brown setae, shorter and narrow asci and wider ascospores with vertical septa. Thus, based on morphology and phylogeny support, C. menglunense is a new species (Figs. 89, 90).
Phylogenetic tree generated from maximum parsimony analysis (RAxML) of a combined dataset of LSU and ITS sequence data. Bootstrap support values for maximum likelihood (ML, left), equal to or greater than 60 %, are indicated above or below branches. Bayesian posterior probabilities (PP, right) equal to or greater than 0.95 are indicated on the branches. New isolates are in blue, and other ex-type strains are in bold. The tree is rooted with Catapyrenium daedaleum
Ceramothyrium menglunense (holotype). a, Herbarium material. b Superficial ascoma on substrate. c, d Squash of ascomata wall. e Section through ascoma. f Setae. g Minutely papillate ostiole. h Pseudoparaphyses. i–j Asci. k–n Ascospores. Scale bars c, e = 100 μm, i, j = 50 μm, d, f, g, h, k–n = 10 μm
Herpotrichiellaceae Munk
The family Herpotrichiellaceae was established by Munk and encompasses loculoascomycetes with small, superficial, inconspicuous, setose ascomata, bitunicate asci with a thickened endotunica and greenish-grey to brown, septate ascospores (Munk 1953). Barr (1976) included the family Herpotrichiellaceae in the order Chaetothyriales. Phylogenetic analysis also supported the family Herpotrichiellaceae as belonging to the order Chaetothyriales (Liu et al. 2015b).
Minimelanolocus R.F. Castañeda & Heredia
Castañeda-Ruiz et al. (2001) introduced the genus Minimelanolocus with M. navicularis (R.F. Castañeda) R.F. Castaneda as the type species. They transferred ten species from the genera Pseudospiropes, Helminthosporium, and Belemnospora into Minimelanolocus, and described one new species, M. curvisporus. Several species of Minimelanolocus have since been described from a wide range of hosts worldwide based on morphology (Zhang et al. 2010; Ma et al. 2011a, b; Hernández-Restrepo et al. 2013; Xia et al. 2014; Liu et al. 2015b). Liu et al. (2015b) listed 28 species of Minimelanolocus and provided the morphological characters of these taxa. A phylogenetic tree of Chaetothyriales, based on combined LSU and ITS sequence data, is provided in this paper (Fig. 89).
Minimelanolocus submersus Z.L. Luo, H.Y. Su & K.D. Hyde, sp. nov.
Index Fungorum number: IF552154, Facesoffungi number: FoF02219, Fig. 91
Minimelanolocus submersus (holotype). a, b Appearance of the fungus on wood. c–g Conidiophores and conidia. h–m Conidia. n Germinating conidium. o Surface view of culture on PDA. p Reverse view of culture on PDA. Scale bars c = 20 μm, d–e = 25 μm, f = 40 μm, g = 25 μm, h–i = 10 μm, j–m = 20 μm, n = 25 μm
Etymology: With reference to the submerged habitat.
Holotype: HKAS 92593.
Saprobic on submerged decaying wood. Sexual morph Undetermined. Asexual morph Colonies on the substrate superficial, effuse, hairy, scattered, brown. Mycelium mostly immersed, composed of septate, dark brown, smooth hyphae. Conidiophores mononematous, macronematous, unbranched, erect, straight or slightly flexuous, smooth, cylindrical, septate, dark brown, gradually paler towards the apex, 110–180 μm (\( \bar{x} \) = 145.5 μm, SD = 35.5 μm, n = 10) long, 5–6 μm (\( \bar{x} \) = 5.5 μm, SD = 0.5 μm, n = 10) wide. Conidiogenous cells holoblastic, integrated, sympodially proliferating, terminal, pale brown or subhyaline. Conidia acrogenous, clavate to fusiform, solitary, immature conidia 1-septate, mostly 2–5-septate at maturity, dry, subhyaline to pale brown, 19–33 μm (\( \bar{x} \) = 26 μm, SD = 7 μm, n = 20) long, 3.5–4.5 μm (\( \bar{x} \) = 4 μm, SD = 0.5 μm, n = 20) wide. Conidial secession schizolytic.
Culture characteristics: Colonies on PDA 25 mm diam. after 3 weeks at room temperature, pale brown at the margins, dark brown at the center; reverse steel grey, medium dense, circular, fairly tight.
Material examined: CHINA, Yunnan Province, Dali, Erhai Lake, saprobic on submerged decaying wood, June 2015, X.Y. Liu, S-323 (HKAS 92593, holotype), ex-type culture KUMCC 15-0206, MFLUCC.
Notes: In the phylogenetic analysis, Minimelanolocus submersus clustered with species of Minimelanolocus; and formed a sister group to M. obscurus (Matsush.) R.F. Castañeda & Heredia. M. submersus closely resembles M. aquaticus H.Y. Su etal. and M. asiaticus H.Y. Su et al. but differs from M. aquaticus in having 2–5-septate, narrower conidia (3.5–4.5 μm vs. 4–8 μm), and differs from M. asiaticus in having longer and wider conidiophores (110–181 × 5–6 μm vs. 112–162 × 3.5–4.5 μm) (Liu et al. 2015b).
Trichomeriaceae Chomnunti & K.D. Hyde
The family was introduced and placed in Chaetothyriales by Chomnunti et al. (2012a), with the generic type Trichomerium. Trichomeriaceae comprises several species of sooty moulds. The family presently comprises three genera, Bradymyces, Knufia, and Trichomerium (Hubka et al. 2014). Trichomeriaceae includes strains that were isolated from the surface of rocks as well as fungi associated with ants (Hubka et al. 2014). In this study, we introduce a new species isolated from a plant, based on morphology and phylogenetic analysis. We provide a tree for Trichomeriaceae below (Fig. 92).
Phylogram generated from maximum likelihood and Bayesian analyses based on ITS sequence data from species of Trichomeriaceae. The numbers above the nodes are RAxML bootstrap values expressed from 1000 repetitions with values above 50 % shown. The ex-type strains are in bold and the new isolates is in blue.The tree is rooted with Phaeococcomyces catenatus
Trichomerium Lév.
Trichomerium species differ from Capnodiaceae species in having loose mycelium beneath the ascomata, the appearance of ascomatal setae, and fusoid ascospores, with three transverse septa or sometimes with longitudinal septa. Phylogenetic analyses from previous studies indicate that Trichomeriaceae species belong to Eurotiomycetes, and Capnodiaceae belongs to Dothideomycetes.
Trichomerium bambusae Hongsanan & K.D. Hyde, sp. nov.
Index Fungorum number: IF552365; Facesoffungi number: FoF02446, Fig. 93
Trichomerium bambusae (holotype). a Appearance of ascomata on leaf. b, c Section through ascoma. d Peridium at the base of ascoma. e Mycelium. f Ascomatal setae. g Apical ring. h Ascus. i Ascus in Melzer’s reagent. k, l Ascospores. j Ascospore in Melzer’s reagent. Scale bars b = 100 μm, c, f = 50 μm, e, h, i = 20 μm d, g, k–l = 10 μm, f–i = 10 μm
Etymology: Named after the host bamboo.
Holotype: MFLU 16-2286
Epiphytes, saprobic on the surface of culm of bamboo. Superficial hyphae 5–6 μm wide, branched, septate, constricted at the septa, brown. Sexual morph Ascomata 140–180 high × 105–130 wide μm (\( \bar{x} \) = 154 × 118 μm, n = 7), superficial, solitary to gregarious, mainly globose to subglobose, with long ostiole at the center, held to the leaf surface by basal mycelium, brown to greyish, with apical setae. Setae 50–80 × 5–8 μm (\( \bar{x} \) = 65 × 7.5 μm, n = 10), surrounding the ascomata, usually at the upper part of ascomata, dark brown to black, slightly narrower and paler at the apex, straight, dark brown to black. Peridium 13–17 μm (\( \bar{x} \) = 14 μm, n = 10), comprising two layers, outer layer composed of pigmented, thick-walled cells of textura angularis, inner layer composed of pale, flattened cells of textura angularis. Hamathecium not observed. Asci 53–62 × 19–24 μm (\( \bar{x} \) = 57 × 22 μm, n = 10), 8-spored, bitunicate, broadly cylindrical or oblong, short pedicellate, ocular chamber when immature. Ascospores 15–18 × 5–7 μm (\( \bar{x} \) = 17 × 6 μm, n = 15), 2–3-seriate, oblong, hyaline, 3-septate, usually not constricted at the septa, but sometimes slightly constricted at the lowest septum, with thin mucilaginous sheath at the middle, smooth-walled, end cells narrow and smaller than central cell. Asexual morph Undetermined.
Material examined: THAILAND, Chiang Rai, Bandoo, on dead of culm of bamboo (Poaceae), December 2012, SC Karunarathna HSA59 (MFLU 16-2286, holotype; isotype in KIB); ex-type living culture, MFLUCC 13-0097.
Notes: Trichomerium bambusae differs from other species within Trichomerium in having a long central ostiole, and 2–3-septate ascospores, with very thin mucilaginous sheath. The new species is most similar to T. gloeosporum Chomnunti & K.D. Hyde based on the shape of ascomata, with raised ostioles, however, T. bambusae has a longer ostioles than T. gloeosporum. The ascospores of T. gloeosporum have a thick mucilaginous sheath, while its mucilaginous sheath is poorly developed in T. bambusae. In the phylogenetic analysis T. bambusae clusters with strains of Chaetothyriales isolated from carton fungi (fungi associated on ant carton nests) and other Trichomerium species. Ant fungi are a polyphyletic group which also present in Capnodiales (Voglmayr et al. 2010).
Leotiomycetes
We follow Wang et al. (2006) for the classification of this order.
Rhytismatales M.E. Barr ex Minter
Rhytismatales is an order of endophytic, parasitic or saprotrophic fungi in the class Leotiomycetes (Ascomycota), the inoperculate discomycetes. Four families are currently recognized in the order.
Rhytismataceae Chevall.
Rhytismataceae is largest family (Johnston 2001) and includes 44 genera (Lumbsch and Huhndorf 2010), Lophodermium is the largest genus with more than 100 species currently accepted (Lantz et al. 2011).
Terriera B. Erikss.
The genus Terriera is a member of Rhytismataceae (Kirk et al. 2008). The type species is T. cladophila (Lév. in Moug. & Nestl.) B. Erikss (syn. Lophodermium cladophilum (Lév. In Moug. & Nestl.) Rehm) (Eriksson 1970). Johnston (1988, 1989) enumerated some important features for delimitation of this genus, such as oblong to sublinear ascomata, the lack of lip cells, covering stroma forming a platform in vertical section, and the triangular space in section between the covering stroma and basal stroma filled with vertically oriented cells.
Terriera thailandica Jayasiri & K.D. Hyde, sp. nov.
Index Fungorum number: IF552171; Facesoffungi number: FoF02222, Fig. 95
Etymology: thailandica is based on country where species was found.
Holotype: MFLU 16-0945.
Saprobic on decaying woody branches in terrestrial habitats, associated with bleached, pale brown areas. Sexual morph Ascomata hysterothecial, 1100–1900(–900) long × 222–250 high, 210–268 μm diam., in surface view, matt, elliptical, ends rounded to subacute, margins diffuse, the central part of the ascomata strongly raising the surface of the substrate at maturity, opening by a longitudinal split that extends almost the whole length of the ascoma. Peridium 17–44 μm, carbonaceous, brittle, of heavily pigmented, basal cells of textura angularis and globulosa, covering stroma consisting of an outer layer of host cuticle. Hamathecium comprising 1–2 μm wide, hyaline, aseptate, excipulum moderately developed, borne in a gel matrix, closely adhering to the covering stroma and the extension, arising from the marginal paraphyses. Asci 80–105 × 3.4–6.6 μm (\( \bar{x} \) = 90 × 5 μm, n = 15), 8-spored, crowded to somewhat parallel, cylindrical, but cylindrical-clavate when immature, apex obtuse to truncate, thin-walled, without a circumapical thickening, long stalked J-, discharging spores through a small apical pore. Ascospores 38–60 × 1–1.5 μm (\( \bar{x} \) = 45 × 1.2 μm, n = 25), arranged in a fascicle, hyaline, filiform, tapering slightly towards both ends, aseptate, pluriguttulate, cell wall not clear, without a gelatinous sheath or appendages. Asexual morph Undetermined.
Culture characters: Colonies on MEA 25 mm diam. after 7 days at 25 °C, raised, with lobate margin; colony two layered, outer layer off white and bound to media, inner layer white and finely floccose to woolly aerial mycelia. Reverse white with black colour patches, prominent in middle.
Material examined: THAILAND. Chiang Rai, Doi Pui: dead branch of undetermined tree, 15 June 2014, Subashini C. Jayasiri. (MFLU 16-0945, holotype), (isotype in KUN), ex-type culture, MFLUCC 14-0818, KUNCC.
Notes: Terriera thailandica is introduced here based on both morphology and phylogeny. Terriera was segregated from Lophodermium based mainly on its swollen paraphyses at the apex to form an epithecium and the lack of lip cells (Eriksson 1970). Terriera thailandica shares these characters with other species of the genus. Terriera thailandica clusters with T. camelliicola in a clade with high ML support and distinct from T. thailandica and T. minor (Fig. 94). Terriera thailandica shares similar morphological characters with T. camelliicola, but the latter species has hysterothecia which are more or less curved, lacks an epithecium, and has observable conidiomata near the hysterothecia (Zhang et al. 2015).
Pezizomycetes
Pezizales J. Schrot.
The order Pezizales has long attracted the attention of mycologists from different parts of the world. Currently, there are 16 families, namely, Ascobolaceae, Ascodesmidaceae, Caloscyphaceae, Carbomycetaceae, Chorioactidaceae, Discinaceae, Glaziellaceae, Helvellaceae, Karstenellaceae, Morchellaceae, Pezizaceae, Pyronemataceae, Rhizinaceae, Sarcoscyphaceae, Sarcosomataceae and Tuberaceae in the order (Hibbett et al. 2007; Kirk et al. 2008). This order is distinguished by asci generally open by rupturing to form a terminal or eccentric lid or operculum (Hansen and Pfister 2006).
Helvellaceae Fr.
This family was introduced by Fries (1822) and is widely distributed in temperate to arctic-alpine areas (e.g. Abbott and Currah 1997; Dissing 1966; Ying and Zang 1994; Zhuang 2004), with a few taxa known from the tropics (Dissing 1979). To date, the family includes four genera, viz. Balsamia., Barssia, Helvella and Wynnella, that comprise approximately 100 species (Kirk et al. 2008; Nguyen et al. 2013; Zhao et al. 2015b, 2016a, b; Wang et al. 2016).
Helvella L.
Helvella is the type genus of Helvellaceae, is characterized by its epigeous, stipitate, cupulate, saddle-shaped to irregularly-lobed ascomata, with whitish, cream, greyish, brown to black hymenium, glabrous, pubescent to villose receptacle surface, and terete or externally sulcate, solid, hollow or lacunose stipe (Dissing 1966; Abbott and Currah 1997).
This genus has received much attention in China, Europe and North America, in the last decade (Zhuang 2004; Zhuang and Yang 2008; Ariyawansa et al. 2015a; Hwang et al. 2015; Zhao et al. 2015b, 2016a, b; Wang et al. 2016). To date, 20 species have been described from China (Wang et al. 2016). In this study we introduced two new species based on molecular support (Fig. 96) and a new record for Helvella oblongispora.
Helvella tinta Q. Zhao, B. Feng & K.D. Hyde, sp. nov.
Index Fungorum number: IF552355, Facesoffungi number: FoF02447, Fig. 97
Etymology: Named because of its tints hymenium and receptacle surface.
Holotype: HKAS 82560.
Symbiotic in coniferous forests of Picea euphratica Oliv. Sexual morph Pileus cupulate to irregularly cupulate, 2–3 cm high, 1.5–3.5 cm broad, margin slightly flattened; hymenium glabrous, even, brown to dark brown, usually mottled with patches of paler pigmentation, greyish to dark brown when fresh, blackish-brown when dried; receptacle surface pubescent, concolourous with the hymenium, ribs absent or extending onto basal quarter only. Stipe 2.5–4 cm long, 0.7–1.5 cm broad, glabrous, flaring and merging with apothecium, white to smoky grey, becoming cream and tough when dried, with 6–8 ribs, with rounded-edge, few anastomosis between ribs, basal mycelium white. Medullary excipulum 370–500 μm broad, of textura intricata, hyaline, composed of 4–6 μm broad hyphae, J−. Ectal excipulum 70–180 μm broad, of textura angularis, outermost cells catenuliform in long fascicled tufts, 14–38 × 8–18 μm, brown, evenly blue in cotton blue, J+. Asci 270–350 × 14–20 μm, pleurorhynchous base, 8-spored, uni-seriate, subcylindrical to clavate. Paraphyses filiform, 3–5 μm broad, slightly exceeding the asci, light brown, with a slightly yellow refractive content in Melzer’s reagent, blue in cotton blue, apex slightly enlarged, 4–5.5 μm broad. Ascospores [30/2/2, in H2O] (15–)16–19(–20) × 10–13(–14) μm [Q = 1.36–1.77, Q = 1.59 ± 0.09)], ellipsoid, smooth-walled. All tissues J−. Asexual morph Undetermined.
Habitat and distribution: Scattered or gregarious on the moss, under Picea euphratica Oliv., forests. Known only from southwestern China.
Material examined: CHINA, Sichuan Province, Hongyuan County, alt. 3300 m, 12 August 2013, Bang Feng 1454 (HKAS 82560, holotype).
Notes: Helvella tinta is well characterized by its cupulate to irregularly cupulate pileus with a consistently mottled, greyish to dark brown hymenium, and a lacunose stipe possessing ribs extending onto 1/4 of the receptacle surface. Paraphyses light brown, 3–5 μm. The brown pigments of the receptacle surface are visible in cotton blue or Melzer’s reagent.
Morphologically, H. tinta is similar to H. maculata N.S. Weber, a species originally described from North America, but the latter has a saddle-shaped pileus with a drab, buffy brown to snuff brown hymenium, margin curved to receptacle surface, a white to cream stipe and large ascospores (Weber 1975). Helevlla floriforma also shares some features with H. tinta, but it has a peculiar apothecial shape and colour.
Phylogenetically, H. tinta clusters with H. griseoalba N.S. Weber (66 % bootstrap support). However, H. griseoalba differs from H. tinta in having grey to cinnamon hymenium and receptacle surface and hyaline paraphyses (Weber 1972; Landeros et al. 2012).
Helvella floriforma Q. Zhao & K.D. Hyde, sp. nov.
Index Fungorum number: IF552356, Facesoffungi number: FoF02448, Fig. 98
Etymology: the epithet refers to the flower-shaped apothecia.
Holotype: HKAS 90224.
Symbiotic in the coniferous forests of Platycladus orientalis (L.) Franco. Sexual morph Pileus slightly infundibuliform to central strongly depressed but not hollow when mature, 2–3 cm high, 2–6 cm broad, margin rolled to receptacle surface, wavy; hymenium glabrous, drab, buffy brown to snuff brown when fresh, yellowish-brown when dried; receptacle surface pubescent, cream, pale, apricot greyish when fresh, becoming yellowish when dried, branching and anastomosing ribs extending to marginal region. Stipe 2–4 cm long, 1.5–3 cm broad, cream, becoming yellowish when dried, glabrous, sulcate, branched and anastomosed ribs extending to the receptacle surface margin. Medullary excipulum 290–450 μm broad, of textura intricata, hyaline, composed of 3–5 μm broad hyphae, with a red refractive content in Melzer’s reagent, blue in cotton blue, J−. Ectal excipulum 100–130 μm broad, of textura angularis, outermost cells catenuliform in long fascicled tufts, hyaline, evenly blue in cotton blue, cylindrical end cells 13–25 × 7–13 μm, J+. Stipitipellis 50–70 μm, hyaline, composed of textura angularis, terminal cells 13–27 × 7–10 μm, clavate, with a yellow refractive content in Melzer’s reagent, blue in cotton blue, J−. Asci 260–310 × 16–22.5 μm, pleurorhynchous base, 8-spored, uni-seriate, subcylindrical to clavate. Paraphyses filiform, 3–4 μm broad, slightly exceeding the asci, with a slightly yellow refractive content in Melzer’s reagent, blue in cotton blue, apex 4–5 μm broad, J−. Ascospores [40/2/1, in H2O] (14)14.5–20 × 10.5–16 μm [Q = 1.16–1.59, Q = 1.34 ± 0.11)], subglobose to subellipsoid, smooth-walled. Asexual morph Undetermined.
Habitat and known distribution: Scattered or gregarious on the ground, under Platycladus orientalis. Currently known only in southwestern China.
Material examined: CHINA, Yunnan Province, Gucheng County, alt. 2500 m, 19 August 2013, Qi Zhao 2000 (HKAS 90224, holotype).
Notes: Helvella floriforma is characterized by its slightly infundibuliform to strongly depressed pileus with a wavy, curved to receptacle surface margin, a glabrous, drab, buffy brown to snuff brown hymenium, and a pubescent, cream, pale, apricot greyish, ribs branching and anastomosing extending to marginal region receptacle surface. Stipe cream, glabrous, sulcate, rounded-edge, branched and anastomosed ribs extending to the receptacle surface margin. Stipe inner and ectal excipulum J+.
In our phylogenetic analysis, H. floriforma is sister to H. robusta S.P. Abbott with relatively high statistical support (91 %) (Fig. 96). However, the latter species is irregularly cupulate with a large central depression to irregularly bi-lobed pileus, free margin, a subpubescent to pubescent, ribbed receptacle surface and a subpubescent to pubescent, lacunose stipe with sharp ribs; medullary excipulum and stipe inner and stipitipellis J+; ectal excipulum J− (Landeros et al. 2012).
Helvella tinta and H. maculata also have mottled hymenium surface and lacunose stipe. However, H. tinta has a cupulate to irregularly cupulate pileus, with a greyish to dark brown hymenium, a mottled receptacle surface only near the stipe possessing ribs, all tissues J−. Helvella maculata has a saddle-shaped pileus, margin rolled or straight toward the hymenium and a white to cream stipe, all tissues J− (Weber 1975; Abbott and Currah 1997; Landeros et al. 2012).
Helvella oblongispora Harmaja, Karstenia 18(2): 57 (1978)
Facesoffungi number: FoF02449, Fig. 99
Symbiotic in the coniferous forests of Picea euphratica Oliv. Sexual morph Pileus cupulate to irregularly cupulate, 0.5–2.5 cm high, 0.5–2.5 cm broad, margin slightly splitting; hymenium glabrous, even to slightly undulate at center, greyish-brown to brown when fresh, blackish-brown when dried; receptacle surface finely pubescent, greyish to brown at the margin, cream to white below, white near stipe, ribs absent or extending onto basal quarter only. Stipe 0.5–1.5 cm long, 0.3–0.5 cm broad, flaring and merging with apothecium, white, becoming cream and tough when dried, glabrous to finely pubescent, ribs prominent, rounded, few anastomosis between ribs, basal mycelium white. Medullary excipulum 220–315 μm broad, of textura intricata, hyaline, composed of 3–4 μm broad hyphae. Ectal excipulum 60–115 μm broad, of textura angularis, hyaline, evenly blue in cotton blue, outermost cells 17–45 × 7–27 μm, clavate to subglobose. Stipitipellis 100–160 μm, hyaline, composed of textura angularis, terminal cells 14–59 × 8–21 μm, clavate. Asci 260–330 × 15–21 μm, aporhynchous base, 8-spored, uni-seriate, subcylindrical to clavate. Paraphyses filiform, slightly exceeding the asci, with a yellow refractive content in Melzer’s reagent, 3–5 μm broad. Ascospores [80/4/4, in H2O] (17–)18–20(–21) × 11–13 μm [Q = 1.36–1.77, Q = 1.59 ± 0.09)], subellipsoid, smooth-walled under the light microscope. All tissues J−. Asexual morph Undetermined.
Known distribution: Scattered or gregarious on moss under Picea euphratica, P. retroflexa Mast. and P. purpurea Mast. Picea spp. forests. Known in Europe (Harmaja 1978) and Asia (Liu et al. 1985).
Material examined: CHINA, Sichuan Province, Jiuzhaigou County, on forest ground with Picea euphratica Oliv., alt. 3200 m, 19 Jun 2014, Qi Zhao 2046 (HKAS 87726), Jiuzhaigou County, alt. 3300 m, 22 June 2014, Qi Zhao 2064 (HKAS 87744), same location, 23 June 2014, Qi Zhao T24352, T24387, T24470, T24477, respectively (HKAS 87693; HKAS 87694; HKAS 87698; HKAS 87700), Xinjiang Autonomous Region,Zhaosu County, alt. 2330 m, 9 July 2015, Qi Zhao 2380 (HKAS 90254).
Notes: Helvella oblongispora is characterized by deeply cupulate pileus, slightly serrate margin, brown, even to slightly undulate hymenium, white to pale brownish receptacle surface, and prominent, rounded ribs, white stipe without few crossed veins or pockets; aporhynchous asci, paraphyses apex 3–5 μm broad and subellipsoid, 18–20 × 11–13 μm ascospores. The species was re-examined by Landeros et al. (2012).
Helvella oblongispora is very similar to H. leucomelaena in gross morphology and anatomical structure. However, H. leucomelaena has dark grey to blackish apothecia, deeply cupuliform, generally subsessile or with a very short stipe and less or more visible ribs and 20–23 × 11–13 μm ascospores (Dissing 1966; Abbott and Currah 1997).
Pezizaceae Dumort.
Pezizaceae, known as the cup-fungi, is recognized by the fleshy, soft, brittle, cupulate ascomata, with amyloid asci. This family formed a strongly supported monophyletic with Ascobolaceae as its sister group (Hansen and Pfister 2006). To date, GBIF lists 54 genera, approximately 1214 species (http://www.gbif.org/species/8399, 27 July 2016) and, of these, 31 genera, 230 species were accepted (Kirk et al. 2008).
Peziza Dill. ex Fr.
Peziza is the type genus of the family Pezizaceae, estimated to comprise 104 species (Kirk et al. 2008). Molecular phylogenetic studies have shown that the genus Peziza is not monophyletic, composed of at least 14 distinct lineages (Hansen and Pfister 2006) (Figs. 100, 101).
Phylogeny of Peziza based on a Bayesian and maximum likelihood analysis of ITS dataset. Bayesian posterior probability (BPP) values (in bold) ≥0.7 and maximum likelihood bootstrap (MLB) values ≥50 % are shown on the branches. Thickened branches indicate BPP ≥ 0.95 and MLB support ≥70 %. Peziza subcitrina was chosen as outgroup taxon
Phylogeny of Peziza based on a Bayesian and maximum likelihood analysis of RPB2 dataset. Bayesian posterior probability (BPP) values (in bold) ≥0.7 and maximum likelihood bootstrap (MLB) values ≥50 % are shown on the branches. Thickened branches indicate BPP ≥ 0.95 and MLB support ≥70 %. Newly sequence collections are in bold. Peziza polaripapulata and P. obtuspiculata are chosen as the outgroup taxa
Peziza fruticosa Lantieri, Medardi & Vizzini, sp. nov.
Index Fungorum number: IF552367; Facesoffungi number: FoF02450, Figs. 102 and 103
Peziza fruticosa (holotype). a, b Ascospores in Lactic blue. c–e Ascospores in Melzer’s reagent. f Hymenium in water. g Ascus and paraphyses in Melzer’s reagent. h Asci and paraphyses in water. i Apothecia in situ. Scale bars a = 8 μm, b = 4 μm, c = 3 μm, d, e = 2.5 μm, f = 30 μm, g, h = 15 μm, i = 2 mm (Photo G. Medardi)
Diagrammatic representation of the microscopic characters of Peziza fruticosa (holotype). a Vertical section of one apothecium. a1 Hymenium. a2 Subhymenium. a3 Upper medullar excipulum. a4 Median medullar excipulum. a5 Lower medullar excipulum. a6 Ectal excipulum. b Ascospores. c Asci and ascospores. d Paraphyses. Scale bars a = 100 μm, b = 10 μm, c, d = 25 μm (drawing by Del. G. Medardi)
Etymology: from Latin “fruticosa”, clustered, for its way of fruiting.
Holotype: AMB 17135.
Saprobic on the sand of the high dunes. Sexual morph Apothecium cup shaped, sessile, irregularly rounded or compressed-lobed because of mutual contact; on average 15 mm diam. Hymenium smooth, often wavy, dull to dark brown, with reddish reflections, darker on the bottom. Receptacle surface smooth, concolorous with hymenium; margin whole (rarely cracked), even to lobed, wavy, curved. Flesh fragile, waxy, up to 1.5 mm thick, pale brown. Medullar excipulum up to 950 μm thick, 3-layered, upper layer of textura globulosa-angularis, cells 30–80 (100) μm diam.; intermediate layer of textura intricate; hyphae 4–6 μm diam., with presence of a few rounded cells, 10–15 μm diam.; lower layer identical to the upper one. Ectal excipulum up to 250 μm thick, textura globulosa or globulosa-angularis, cells 15–20 μm diam. Subhymenium up to 100 μm thick, textura globulosa-angularis (cells 5–10 μm diam.), mixed with intricate hyphae. Asci 190–225 × 12–15 μm, cylindrical, amyloid, 8-spored, amyloidity visible only on the top, lower part manifestly destrinoid. Paraphyses clavate to irregularly swollen, several bent in the upper part, a few with oil drops, septate, simple or forked, with some moniliform cells, 2.5–4 μm in the lower part, up to 10 μm at the apex. Ascospores 15–17 × 7.5–9.5 μm, elliptical, distinctly and very delicately warted, ornamentation as very fine warts, punctiform or slightly elongated (0.4–0.6 μm), hyaline, without oil drops, uniseriate in the ascus. All tissues hyaline to brownish, except the ectal excipulum that is pale reddish-brown. Asexual morph Undetermined.
Habitat and known distribution: on the sand in dune zones and on high dunes, near degraded remnants of Ammophila arenaria and Eryngium maritimum; so far known only from Italy and Spain. Autumn-spring.
Material examined: ITALY, Calambrone, Pisa, Leg. G. Medardi, 26 April 1996, AMB 17136, Rosolina Mare, Rovigo, Leg. A. Lantieri and G. Medardi, 7 December 2014, AMB 17135 (holotype). SPAIN, Playa de Xagò, Asturias, Leg. C. Lopez-Alvarez, 15 February 2006, K(M) 140524 (sub ammophila).
Notes: In the year 1996, on the dunes near Calambrone (Pisa, Italy), we collected one portion of a strange fungal structure, made up by a sort of hemisphaerical accumulation of small, brown apothecia, quite overlapping; due to the particularly rainy period and the advanced age, the specimen was not in good conditions to be photographed, but we preserved it however (AMB 17136) because of its interesting microscopic characters. In 2006 one Spanish colleague collected one identical formation on sand in Asturias, which revealed the same microscopic features, still referable to any known species; we placed this collection in herbarium under a provisional name [K(M) 140524], waiting for other more detailed studies. In December 2014 near Rosolina Mare (Rovigo, Italy) we newly found this fungus (AMB 17135). The microscopic study showed the same characteristics of the two previous collections, and the following molecular analyses confirmed it as a new species.
According to current information, Peziza fruticosa seems to be a rare species, however its easily identifiable by well-defined macro- and microscopic features, such as the clustered-overlapping apothecia, amyloid asci only at the apex, and the manifest destrinoidity. Phylogenetically, P. fruticosa is closely related to P. domiciliana, P. perdicina P. ampliata and P. ninguis (Fig. 100). However, P. domiciliana Cooke differs by growing gregarious or occasionally cespitose on cellars, damp walls, plaster, mortar, mushroom-caves, and smaller ascospores, 14.5–15.5 × 7–8 μm, with two oil drops (Seaver 1917; Le Gal 1941; Maas Geesteranus 1967; Rifai 1968; Hansen et al. 2002). Peziza perdicina (Velen.) Svrček has ascospores 15–16 × 6–7 μm, and occurs on dung (Svrček 1976; Donadini 1981; Häffner 1985; Cacialli et al. 1997; Doveri 2004; Medardi 2006). Peziza ampliata Pers. has ascospores 18–20.5 × 9.5–11.5 μm, smooth and grows on hardwood trunks, rich soil mixed with wood-chips, chalk (Svrček 1970; Dennis 1981; Hansen et al. 2002; Medardi 2006).
Concerning P. ninguis Donadini & Trimbach, we had the possibility to examine the collection ref. JF908536, consisting of one specimen kept in MCVE (ref. 11883), and we noticed it fully matches the characters of P. varia (Hedw. : Fr.) Alb. & Schwein; the name under which its sequence was filed in GenBank is therefore incorrect. P. ninguis is one of the synonyms of P. nivalis (R. Heim & L. Rémy) M.M. Moser, that has divergent microscopic characters: ascospores remarkably larger and differently ornamented, 18–22.5 × 9–10 μm vs. 14–16 (17.5) × 9–11 (12) μm for P. varia (Medardi et al. 2012), with a very feeble and inconstant punctuation instead of the typical superficial crimp, and growth in nival environment only in solstitial period, often immersed in water from melting snow fields. The fungi of MCVE 11883 were collected on 04/05/1996 in the municipality of Trasaghis (Udine, Italy), one town located only a few hundred meters above sea level and therefore not in the expected typical glacial environment, and moreover in an inappropriate period. The sample was previously identified as “P. ninguis var. fortoulii”, but also in this case the characters are not coincident, because the ascospores of P. fortoulii Donadini & Neville are larger [16–18 (19) × 10.5–12.5 μm] and smooth (our personal observations).
Among the morphologically closest species, P. proteana (Boud.) Seaver f. sparassoides (Boud.) Korf shares similar habits with P. fruticosa, such as several apothecia often coalesced into a large, irregular compound cauliflower-like mass, but differs in smaller ascospores 10–13 × 5–7 μm with a strongly warty ornamentation, and by growing on burnt soil (Seaver 1917, 1928; Durand 1919; Korf 1956, 1973; Dennis 1981; Donadini 1981; Geesink 1984; Hohmeyer 1986; Van Vooren 2003; Medardi 2006; Barseghyan and Wasser 2007).
The sand-associated P. ammophila Durieu & Lév. shows semi-hypogean, funnel-shaped not coalesced apothecia, at first sphaerical and then opened and splitting into a stellate shape, long stalked, and ascospores 16–18 × 9.5–11 μm, elliptical and smooth (Donadini 1981; Hansen et al. 2002).
Sordariomycetes
For Sordariomycetes for follow Maharachchikumbura et al. (2016).
Coronophorales Nannf.
Member of the order Coronophorales are wood inhabiting fungi and comprise the families Bertiaceae, Chaetosphaerellaceae, Coronophoraceae, Nitschkiaceae and Scortechiniaceae (Maharachchikumbura et al. 2015, 2016). The taxa in the order are characterized by mostly superficial ascomata, sometimes with an extensive hyphal subiculum or well-developed basal stroma, that often becomes cupulate or collapsed, and in some cases the ostiolar opening is either indistinct or lacking (Mugambi and Huhndorf 2010; Maharachchikumbura et al. 2016).
Coronophoraceae Höhn.
The order and family were treated by Maharachchikumbura et al. (2015, 2016) and is followed here.
Coronophora Fuckel
The genus Coronophora was introduced by Fuckel (1864) and Mugambi and Huhndorf (2010) reported a single collection of Coronophora gregaria Fuckel from USA. It is characterized by immersed, erumpent or superficial ascomata, lack of ostiole, thin-walled asci with long stipe and large ascospore numbers. Maharachchikumbura et al. (2015, 2016) showed the family Coronophoraceae is monophyletic, however it was only represented by the type species C. gregaria.
Coronophora myricoides H.X. Wu & K.D. Hyde, sp. nov.
Index Fungorum number: IF552180; Facesoffungi number: FoF02451, Fig. 104
Etymology: Referring to the resemblance of the ascomata (under the stereomicroscope) to the berries of Myrica rubra (waxberry).
Holotype: IFRD 9201.
Saprobic on the surface of dead wood. Asexual morph Undetermined. Sexual morph Ascomata 373–395 μm high × 282–340 μm diam. (\( \bar{x} \) = 383 × 308 μm, n = 10), perithecial, superficial or erumpent through bark of host, clustered in small to large groups, subglobose, surface tuberculate, dark brown to black, lacking ostioles. Peridium 27–135 μm (27–56 μm at the apex, 93–135 μm at the base), composed of five layers of dark brown-walled cells of textura globosa, inner layer of hyaline pseudoparenchymatic cells, slightly melanized on the outer surface. Paraphyses not seen. Asci 102–147 × 11–27 μm (\( \bar{x} \) = 119 × 22 μm, n = 15), numerous, polysporous, unitunicate, cylindrical to clavate, pedicel 11–38 × 4–7 μm. Ascospores 6–8 × 1–2 μm (\( \bar{x} \) = 7 × 1.7 μm, n = 30), numerous, hyaline, 1-celled, cylindrical or ellipsoidal, narrowly rounded at both ends, smooth-walled.
Material examined: CHINA, Yunnan Province, Jing Dong, on dead wood of unidentified plant, 13 September 2013, Wu Hai Xia (IFRD 9201, holotype).
Notes: The genus Coronophora is typified by Coronophora gregaria and there are 28 epithets in Index Fungorum (2016). Kirk et al. (2008) estimated that there are five species in Coronophora from wood. There are two sequences for Coronophora gregaria in GenBank. In this paper, we introduce a new species of Coronophora; C. myricoides which differs from C. gregaria in the shape of the ascomata and also the shape of ascospores. The ascospores of Coronophora myricoides (6–8 × 1–2 μm) are smaller the type species (8–12 × 2–3 μm). We directly extracted DNA using ascomata and sequencing. We add the molecular data to better understand the generic and familial circumscriptions.
Diaporthales Nannf.
Members of Diaporthales are pathogens, parasites, and endophytes of plants, human-animal pathogens, saprobes and soil inhabitants (Rossman et al. 2007). The order Diaporthales is characterized by perithecia with an elongate beak, often forming within stromatic tissues (Rossman et al. 2007). Asci generally deliquesce at the base when mature and have a characteristic refractive apical annulus. The order Diaporthales comprises 12 families: Cryphonectriaceae, Diaporthaceae, Gnomoniaceae, Harknessiaceae, Macrohilaceae, Melanconidaceae, Pseudoplagiostomataceae, Pseudovalsaceae, Schizoparmaceae, Stilbosporaceae, Sydowiellaceae and Valsaceae (Maharachchikumbura et al. 2016).
Diaporthaceae Höhn. ex Wehm.
Von Höhnel (1917) established the family Diaporthaceae and accommodated it in the order Diaporthales. The family has undergone various taxonomic revisions during the past century (Wehmeyer 1975; Barr 1978; Castlebury et al. 2002; Dai et al. 2014). Maharachchikumbura et al. (2015) accepted eleven genera in Diaporthaceae: Allantoporthe, Apioporthella, Clypeoporthella, Diaporthe, Diaporthella, Diaporthopsis, Leucodiaporthe, Mazzantia, Mazzantiella, Ophiodiaporthe and Pustulomyces.
Diaporthe Nitschke
Diaporthe has a worldwide distribution as endophytes, pathogens and saprobes (Udayanga et al. 2011). The asexual morph was previously known as Phomopsis. These genera were linked in Wijayawardene et al. (2014a) and Maharachchikumbura et al. (2015). Rossman et al. (2015) proposed to conserve Diaporthe over Phomopsis, to resolve nomenclatural problems. An update of the phylogeny of Diaporthales species with new species and records is presented herein (Fig. 105).
One of 255 most parsimonious trees obtained from analyses of combined ITS, EF-1α, β-tubulin and CAL sequence data for all ex-types from species in Diaporthe. Isolate numbers of new species and new host records are in blue. Maximum parsimony bootstrap values (>70 %) and Bayesian inference values (>0.8) are given on the nodes. The tree is rooted to Diaporthe corylina
Diaporthe aseana Dissanayake, Tangthirasunun & K.D. Hyde, sp. nov.
Index Fungorum number: IF551402; Facesoffungi Number: FoF00925, Fig. 106
Diaporthe aseana (MFLU 13-0256, holotype). a Specimen on dead leaf. b Conidiomata on the host surface. c Longitudinal section of a conidioma. d, e Longitudinal section of a conidioma wall. f, g Conidiogenous cells with developing conidia. h–k Conidia. l Germinating conidium. m, n Colonies on PDA, m from above n from below. Scale bars c = 100 μm, d = 50 μm, e–l = 10 μm
Etymology: In reference to the ASEAN, the ‘Association of Southeast Asian nations’ where the fungus was collected in Thailand, one of the member countries.
Holotype: MFLU 13-0256.
Saprobic on dead leaves. Sexual morph Undetermined. Asexual morph Conidiomata 140–200 μm diam. × 220–300 μm high (\( \bar{x} \) = 185 × 260 μm, n = 10), associated with necrotic leaf tissue; pycnidial, globose, unilocular, black, erumpent, ostiolate, walls comprising 5–6 layers of dark brown cells of textura angularis. Conidiophores 8–15 × 2–3 μm, hyaline, smooth, densely aggregated, cylindrical, straight to sinuous. Conidiogenous cells 6–10 × 2–3 μm, phialidic, cylindrical, terminal and lateral, slightly tapering towards the apex. Paraphyses not observed. Alpha conidia 6–9 × 2–3 μm, aseptate, hyaline, smooth, guttulate, fusoid to ellipsoid, tapering towards both ends, straight, apex subobtuse. Beta conidia not observed.
Culture characteristics: Colonies covering 9 cm Petri dish after 2 weeks in the dark at 28 °C. On PDA surface with fluffy white aerial mycelium with patches of saffron, in reverse patches of luteous to olivaceous-grey.
Material examined: THAILAND, Phayao, Jam Pa Thong Waterfall, on dead leaf, 12 March 2012, N. Tangthirasunun (MFLU 13-0256, holotype); ex-type living cultures MFLUCC 12-0299a, KUMCC 15-0112.
Notes: Diaporthe aseana did not produce a sexual morph in culture or on the host. Multi-gene phylogenies reveal a close relationship of D. aseana to D. hongkongensis, D. lithocarpus, D. eucalyptorum, but support our new taxa as not being conspecific. Our phylogenetic analyses position D. aseana basal to the above species (Fig. 105). Based on a Blast search of NCBI’s GenBank nucleotide database, the closest matches for the ITS sequence of D. aseana are an undescribed endophytic Diaporthe sp. InaCC F-238 from Cinchona calisaya, Indonesia (AB899784; Identities = 544/549 (99 %), Gaps = 2/549 (0 %), and an endophytic Diaporthe sp. M23-2 from Abies beshanzuensis from Zhejiang Province, China (HM595506; Identities = 538/549 (98 %)).
Diaporthe eres Nitschke, Pyrenomycetes Germanici 2: 245 (1870)
Facesoffungi number: FoF02182, Fig. 107
Diaporthe eres (MFLUCC 12-0351). a Specimen on dead cone of Picea excelsa. b Conidiomata on the host surface. c Longitudinal section of a conidiomata. d–g Longitudinal section of peridium. h–m Conidiogenous cells with developing conidia. n–s Conidiogenous cells with developing conidia stained with lactophenol cotton blue. t Conidia. u Conidia stained with lactophenol cotton blue. v Germinating conidium. w–x Colonies on PDA, w from top, x from reverse. Scale bars c = 100 μm; d–g = 50 μm; h–u = 5 μm; v = 10 μm
Saprobic on dead cone of Picea excelsa found on land. Sexual morph Undetermined. Asexual morph Conidiomata 225–400 μm diam. × 235–280 μm high (\( \bar{x} \) = 330 × 255 μm, n = 10), pycnidial, sub-globose to globose, unilocular, black, immersed to sub-immersed, ostiolate, peridium consisting of 3–5 layers of dark brown cells of textura angularis. Conidiophores 5–9 × 1–1.5 μm, hyaline, smooth, cylindrical, straight to sinuous. Conidiogenous cells 0.5–1 μm diam, phialidic, cylindrical, terminal and lateral, with slight taper towards apex. Paraphyses not observed. Alpha conidia 5–6 × 2–3 μm aseptate, hyaline, smooth, guttulate, fusoid to ellipsoid, tapering towards both ends, straight, apex subobtuse. Beta conidia not observed.
Culture characteristics: Colonies white and pale brown on surface, reverse pale brown to black. Aerial mycelium white and brown, feathery, with concentric zonation, margin fimbriate, with visible conidiomata at maturity.
Material examined: ITALY, on dead cone of Picea excelsa (Pinaceae), 12 April 2012, Erio Camporesi (MFLU 13-0334), living cultures MFLUCC 12-0351.
Note: Diaporthe eres is an important plant pathogen (Gomes et al. 2013; Udayanga et al. 2014a, b; Dissanayake et al. 2015) and has been reported on various woody hosts (from 121 host genera from 61 host families; https://nt.ars-grin.gov/fungaldatabases). This is the first record of D. eres on Picea excelsa. In our phylogenetic analysis of combined ITS, EF-1α, β-tubulin and CAL sequence data of Diaporthe species (Fig. 105), strain MFLUCC 12-0351 grouped together with ex-type strains of D. eres (ex-epitype culture AR5193).
Diaporthe foeniculina Niessl, in von Thümen, Contr. Ad. Fl. Myc. Lusit. 2: 30. 1880.
Facesoffungi number: FoF02183, Fig. 108
Diaporthe foeniculina (MFLUCC 12-0668). a Specimen on dead branch of Laburnum sp. b Conidiomata on the host surface. c, d Longitudinal section of a conidiomata. e, f Longitudinal section of peridium. g–l developing conidia. m–p Developing conidia stained with lactophenol cotton blue. q Alpha conidia. r Alpha conidia stained with lactophenol cotton blue. s Beta conidia. t Beta conidia stained with lactophenol cotton blue. u Germinating conidium. v, w Colonies on PDA, v from top, w from reverse. Scale bars c, d = 100 μm; e, f = 50 μm; g–t = 5 μm; u = 10 μm
Saprobic on dead branch of Laburnum. Sexual morph Undetermined. Asexual morph Conidiomata 215–390 μm diam. × 160–235 μm high (\( \bar{x} \) = 360 × 185 μm, n = 10), pycnidial, eustromatic, unilocular, semi-immersed, dark brown, scattered or aggregated, ostiolate, peridium consisting of brown thick-walled cells of textura angularis, conidial mass globose to conical and exuding in cirrhi, yellow to reddish brown. Conidiophores 20–28 × 2–3 (\( \bar{x} \) = 24 × 2 μm, n = 10) μm hyaline, subcylindrical or cylindrical, filiform, tapering towards the apex. Alpha conidia 6–9 × 2–3 μm (\( \bar{x} \) = 7 × 3 μm, n = 10), hyaline, oblong to ellipsoidal, apex bluntly rounded, base obtuse to sub-truncate, bi- to multi-guttulate. Beta conidia 26–34 × 1–2 μm (\( \bar{x} \) = 28 × 1 μm, n = 10), hyaline, smooth, slightly curved.
Culture characteristics: Colony is entirely white both on surface and reverse. Aerial mycelium cottony and feathery, colonies reaching 60 mm diam. after 5 days in 28 °C.
Material examined: ITALY, on dead branch of Laburnum (Fabaceae), 21 August 2012, Erio Camporesi (MFLU 13-0320), living cultures MFLUCC 12-0668.
Note: Diaporthe foeniculina has been reported on various woody hosts (from 20 host genera from 16 host families; https://nt.ars-grin.gov/fungaldatabases). This is the first record of D. foeniculina on Laburnum sp. In our phylogenetic analysis of combined ITS, EF-1α, β-tubulin and CAL sequence data of Diaporthe species (Fig. 105), strain MFLUCC 12-0668 grouped together with ex-type strains of D. foeniculina with very high support (ex-epitype culture CBS 111553).
Diaporthe garethjonesii Dissanayake, Tangthirasunun & K.D. Hyde, sp. nov.
Index Fungorum number: IF551403; Facesoffungi Number: FoF00926, Fig. 109
Diaporthe garethjonesii (MFLU 13-0261, holotype). a Diseased leaves. b Conidiomata on the host surface. c Longitudinal section of conidiomata. d Conidiomata on host. e–g Conidiogenous cells with developing conidia. h–m Conidiogenous cells with developing conidia stained with lactophenol cotton blue. n Alpha conidia. o Beta conidia. p Germinating conidium. q, r Colonies on PDA, q from above, r from below. Scale bars c, = 200 μm, e–p = 10 μm
Etymology: In reference to the significant contribution of E.B. Professor Gareth Jones made to Thai mycology.
Holotype: MFLU 13-0261.
Saprobic on dead leaves. Sexual morph Undetermined. Asexual morph Conidiomata 85–125 μm diam. × 80–100 μm high (\( \bar{x} \) = 115 × 85 μm, n = 10), associated with necrotic leaf tissue; pycnidial, globose, unilocular, black, immersed to sub-immersed, ostiolate, walls consisting of 5–6 layers of dark brown cells of textura angularis. Conidiophores 5–12 × 1–1.5 μm, hyaline, smooth, cylindrical, straight to sinuous. Conidiogenous cells 0.5–1 μm diam., phialidic, cylindrical, terminal and lateral, with slight taper towards apex. Paraphyses not observed. Alpha conidia 5–6 × 2–3 μm, aseptate, hyaline, smooth, guttulate, fusoid to ellipsoid, tapering towards both ends, straight, apex subobtuse. Beta conidia 40–50 × 3–4 μm hyaline, smooth, less common than alpha conidia, straight, curved or hamate.
Culture characteristics: Colonies with sparse aerial mycelium covering the dish after 2 weeks in the dark at 28 °C. On PDA buff, honey to isabelline, reverse smoke-grey.
Material examined: THAILAND, Kanjanaburi, on dead leaf, 5 May 2012, Jayarama Bhat (MFLU 13-0261, holotype), ex-type living cultures MFLUCC 12-0542a, KUMCC15-0117.
Notes: Diaporthe garethjonesii forms a well-supported clade in our phylogenetic analysis with high bootstrap and Bayesian values (Fig. 105). The BLAST comparison of the ITS sequence of D. garethjonesii showed a 99 % match to a fungal endophyte from Hong Kong (DQ485955) (Identities = 543/548 (99 %), Gaps = 4/548 (0 %)), a 98 % match to the ITS sequence of USA isolate NY8658c (HQ108026) (Identities 537/550 (98 %), Gaps 4/550 (0 %)). The distinct clade that D. garethjonesii forms in the phylogenetic analysis, represents a separate species.
Diaporthe siamensis Udayanga et al., Cryptogamie Mycologie 33: 298 (2012)
Facesoffungi number: FoF02398, Fig. 110
Diaporthe siamensis (MFLUCC 12-0300, NTCL056-1). a Herbarium specimen. b Appearance of conidiomata on the host surface. c Vertical section of conidioma. d Peridium. e Ostiole. f, g Conidiogenous cells with developing conidia. h Alpha conidia. i Beta conidium. j Germinating alpha conidium. k–l Culture on PDA, k top, l reverse. Scale bars b = 500 μm, c, d = 100 μm, e = 50 μm, f–j = 10 μm
Saprobic on dead leaves of woody plants. Sexual morph Undetermined. Asexual morph Conidiomata pycnidial, 185–200 μm high, 150–200 μm diam. (\( \bar{x} \) = 190 × 170 μm, n = 10), ampulliform, scattered, immersed, ostiolate, with elongate black neck. Pycnidial wall, comprising 2–3 layers, with heavily pigmented outer layer, thick-walled, comprising blackish to dark brown cells of textura angularis, with lighter cells towards the inside, with inner layer composed of 1–2 layers, hyaline, thin-walled cells of textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic with percurrent annellations, integrated, solitary, hyaline, smooth-walled and formed from the inner layer of pycnidium wall. Paraphyses extending above conidiophores and hyaline, 20–35 μm long. Alpha conidia 3–5 × 2–3 μm (\( \bar{x} \) = 3.5 × 2.4 μm, n = 30), hyaline, smooth-walled, mono- or bi-guttulate. Beta conidia15–20 × 1.5–2 μm (\( \bar{x} \) = 18.5 × 1.8 μm, n = 30), aseptate, hyaline, hamate or curved, apex acutely rounded.
Culture characteristics: Colonies on PDA 30 mm diam. after 4 weeks at 25 °C, cream to white mycelium, cottony and lobate at the margins, white at the center; reverse yellowish.
Material examined: THAILAND, Chiang Rai Province, Thasud, Muang District, Mae Fah Luang University, on dead leaves of Castanopsis sp. (Fagaceae), 21 March 2012, N. Tangthirasunun (MFLU 13-0257, NTCL056-1), living culture, MFLUCC 12-0300.
Notes: Diaporthe siamensis was introduced by Udayanga et al. (2012). This species was described on leaves of Dasymaschalon sp. (Annonaceae) from Chiang Rai, Thailand. Since then this species has not been reported from any locality worldwide. Here we have collected D. siamensis for the first time on Castanopsis sp. (Fagaceae) from a close locality in Thailand. The morphological characters are similar to the type specimen. Based on the phylogenetic analysis of combined ITS, EF1-α, CAL sequence data, shows high support to its ex-type strain MFLUCC 10-0573a, clusters with high support with D. siamensis MFLUCC 12-0300 (Fig. 105). According to Udayanga et al. (2012) this species was found in different habitats of Dasymaschalon sp. whereas, in this study we isolated it as a saprobic fungus. Identification of D. siamensis, within the same locality on two different plant groups, reveals the potential of this species to occur on multiple hosts in different habitats.
Valsaceae Tul. & C. Tul.
The family Valsaceae was introduced by Tulasne and Tulasne (1861) and placed in Diaporthales by Barr (1978). Most of Valsaceae species are plant pathogens causing canker and dieback disease, with damage to several economic crops worldwide (Adams et al. 2005; Fan et al. 2014, 2015a, b; Ariyawansa et al. 2015a, Li et al. 2016, Maharachchikumbura et al. 2015, 2016). Currently this family comprises 13 genera: Amphicytostroma, Chadefaudiomyces, Cryptascoma, Cytospora, Ditopellina, Durispora, Harpostroma, Hypospilina, Kapooria, Leptosillia, Maculatipalma, Pachytrype and Paravalsa (Maharachchikumbura et al. 2015, 2016). Valsaceae was restricted to Cytospora as asexual morph and four sexual morph genera viz. Valsa, Leucostoma, Valsella, and Valseutypella (Fries 1838; Saccardo 1884; Gvritishvili 1982; Spielman 1985; Adams et al. 2002, 2005; Castlebury et al. 2002; Bulgakov 2010; Yang et al. 2015; Li et al. 2016; Maharachchikumbura et al. 2015, 2016). Therefore, Adams et al. (2005) synonymized all sexual genera under Valsa as a subgenus or species without additional infrageneric rank, according to the International Code of Nomenclature for Algae, Fungi, and Plants (ICN) in 2011 for placement on the list of protected fungi (Adams et al. 2005; Fotouhifar et al. 2010, Fan et al. 2015a; Wingfield et al. 2012; Crous et al. 2015a; McNeill et al. 2012; Rossman et al. 2015; Ariyawansa et al. 2015a, b, c; Li et al. 2016; Maharachchikumbura et al. 2015, 2016). Cytospora species have been reported as the asexual morph of most taxa in Valsaceae, and they are characterized by single or labyrinthine locules, filamentous conidiophores and allantoid, hyaline conidia. In moist conditions, conidia emerge from the fruiting bodies as yellow masses, which later become orange to red (Spielman 1983, 1985; Adams et al. 2005, 2006; Li et al. 2016; Maharachchikumbura et al. 2015, 2016). There are 579 epithets for Cytospora in Index Fungorum (2016). Ex-type sequence data are available for only a few species and it is, thus, difficult to identify isolates to the species level (Liu et al. 2015a; Ariyawansa et al. 2015a, b, c; Li et al. 2016; Maharachchikumbura et al. 2015, 2016). Further studies and recommended on Cytospora to clarify cryptic species with updated gene trees (Adams et al. 2002; Fotouhifar et al. 2010; Hyde et al. 2010, 2014; Fan et al. 2015a, b; Liu et al. 2015a; Ariyawansa et al. 2015a, b, c; Yang et al. 2015; Li et al. 2016).
Cytospora Ehrenb.
Cytospora was introduced by Ehrenberg (1818), and it contains species that are one of the most important pathogens, causing canker disease on branches leading to large areas of dieback on a wide range of plants (Adams et al. 2005, 2006). The genus Cytospora has sexual morphs in Valsa, Leucostoma, Valsella, and Valseutypella (Adams et al. 2005). All sexual genera were synonymized with Valsa either as a subgenus or species. Cytospora (1818) is an older name than Valsa (1849) and the Cytospora morph is more common in nature. Therefore, Valsa species are treated as synonyms of Cytospora. Cytospora species identification has generally been established according to host affiliation, while morphological descriptions generally lacked detail. However, a single species of Cytospora often occurs on taxonomically unrelated host plants (Adams et al. 2005; Wang et al. 2011, Fan et al. 2015a, b; Ariyawansa et al. 2015a, b, c; Liu et al. 2015a; Maharachchikumbura et al. 2015, 2016). There are 579 epithets for Cytospora in Index Fungorum (2016) with an estimated 110 species in Kirk et al. (2008). In this study with introduce two new species with support from molecular data (Figs. 111, 112).
Maximum parsimony (MP) majority rule consensus tree of Cytospora isolates based on a combined dataset of ITS, LSU, RPB2 and ACT sequence data. Values above the branches indicate maximum parsimony and maximum likelihood bootstrap ≥70 %, (MPBS/MLBS). Values at the third positions, respectively, above or below the branches represent posterior probabilities (BI PP ≥ 0.90) from Bayesian inference analysis. The tree is rooted with Phomopsis vaccinii (ATCC 18451). The strain numbers are mentioned after the species names. The species obtained in this study are in blue bold and ex-type strains are in black bold
Maximum parsimony (MP) majority rule consensus tree of Cytospora isolates based on ITS sequence data. Values above the branches indicate maximum parsimony and maximum likelihood support ≥ 70 %, (MPBS/MLBS). Values at the third positions, respectively, above or below the branches represent posterior probabilities (BI PP ≥ 0.90) from Bayesian inference analysis. The tree is rooted to Phomopsis vaccinii. New strains are in blue bold and ex-type strains are in black bold
Cytospora cotini Norphanphoun, Bulgakov & K.D. Hyde, sp. nov.
Index Fungorum number: IF552231; Facesoffungi Number: FoF02365, Fig. 113
Cytospora cotini (holotype). a Appearance of fruiting bodies in wood. b Fruiting bodies on substrate. c Close up of fruiting body. d Cross section of the conidioma. e Peridium. f Conidiophores with conidia. g Conidia. h Germinating spore. i Culture characters on MEA. Scale bars a = 2 mm, b = 1 mm, c = 400 μm, d = 300 μm, e = 20 μm, f, g = 10 μm, h = 40 μm
Etymology: from generic name of host plant, Cotinus coggygria.
Holotype: MFLU 14-0783.
Necrotrophic on dying branches of Cotinus coggygria Scop. Sexual morph Undetermined. Asexual morph Conidiomata 800–1000 μm diam. pycnidial, solitary, immersed in host tissue, multi-locule, dark brown, ostiolate. Ostiole 250–350 μm diam. at the same level as the disc surface. Peridium comprising a few to several layers of cells of textura angularis, with inner most layer thin, hyaline, outer layer brown to dark brown. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, phialidic, formed from the innermost layer of pycnidial wall, hyaline, smooth. Conidia (4.9–)5.6–6.5 × 0.8–1.4(–1.7) μm (\( \bar{x} \) = 5.9 × 1.2 μm, n = 30), unicellular, allantoid to subcylindrical, hyaline, smooth-walled.
Culture characteristics: Colonies on MEA, reaching 1.7 cm diam. after 7 days at 25 °C, producing dense mycelium, lobate circular, with white rough margin, after 5 days, flat or effuse on the surface, without aerial mycelium.
Material examined: RUSSIA, Rostov region, Shakhty city, near Grushevsky pond, shelterbelt artificial forest, on dead branches of Cotinus coggygria Scop. (Anacardiaceae), 18 May 2014, Timur S. Bulgakov (MFLU 14-0783, holotype; KUM, isotype); ex-type living cultures, MFLUCC 14-1050, KUMCC.
Notes: Cytospora cotini is a weak pathogen on Cotinus coggygria Scop., and is often associated with Pseudocamarosporium cotinae Norphanphoun et al. The present study using morphology and phylogenetic analyses, places Cytospora cotini in Valsaceae. The new species has immersed, multi-locular conidiomata, with a single ostiole and shares common walls with the host tissue. Phylogenetic analyses, using ITS sequence data (Fig. 112), indicate that C. cotini can be distinguished from other species within the genus Cytospora. The analyses based on combined ITS, LSU, RPB2 and ACT sequence data also demonstrate that C. cotini separates from other sequenced species, and is close to Cytospora tanaitica Norphanphoun et al. (Figs. 111, 112). However, C. tanaitica differs in having a single locule with smaller conidia than our species.
Glomerellales Chadef. ex Réblová et al.
Order “Glomerellales” was proposed by Chadefaud (1960), without a Latin diagnosis which made it invalid. Glomerellales was validly published by Réblová et al. (2011) and comprises of four families Australiascaceae, Glomerellaceae, Plectosphaerellaceae and Reticulascaceae (Maharachchikumbura et al. 2016).
Glomerellaceae Locq. ex Seifert & W. Gams
The family Glomerellaceae was invalidly published by Locquin (1984) and was validated in Zhang et al. (2006). This family was accepted as one of the three families of Glomerellales in Réblová et al. (2011). Glomerellaceae is a monotypic family, mainly comprised pathogens, and characterized by the Colletotrichum asexual morph and the Glomerella sexual morph, which was synonymized under Colletotrichum (Hyde et al. 2014; Maharachchikumbura et al. 2015, 2016).
Colletotrichum Corda
This genus was introduced by Corda (1831), for C. lineola Corda (Damm et al. 2009). Colletotrichum comprises mainly pathogens, as well as endophytes and saprobes (Cannon et al. 2012; Hyde et al. 2014). Kirk et al. (2001, 2008) and Réblová et al. (2011) placed Colletotrichum in the family Glomerellaceae and Maharachchikumbura et al. (2015) further confirmed the placement of this genus. In the latter study the use of the name Colletotrichum over its sexual name Glomerella was followed.
Colletotrichum insertae Jayawardena, Bulgakov & K.D. Hyde, sp. nov.
Index Fungorum number: IF552260; Facesoffungi number: FoF02399, Fig. 115
Etymology: Based on the host species.
Hoolotype: MFLU 15-1895.
Saprobic on dying twigs and leaf stalks of Parthenocissus inserta (A. Kern.) Fritsch. Sexual morph Undetermined. Asexual morph Conidiomata 250–620 μm (\( \bar{x} \) = 420 μm, n = 10) diam., black, acervulus, oval, solitory, gregarious. Setae straight or ±bent, abundant, dark brown, becoming paler towards the apex, opaque, smooth-walled, septate, 1–5-septate, 55–150 μm long, base cylindrical, 6.7–8.9 μm diam., apex rounded, Conidiophores simple, to 20 μm long, hyaline to pale brown, smooth-walled. Conidiogenous cells 11–24 × 2.8–4.9 μm (\( \bar{x} \) = 8.5 × 2.5 μm, n = 20), hyaline, smooth-walled, cyllindrical to slighty inflated, opening 1–2 μm wide, collarette or periclinal thickening not observed. Conidia 17.3–23.4 × 2.6–5.2 μm (\( \bar{x} \) = 19.3 × 3.8 μm, n = 40), L/W ratio 5, hyaline, smooth or verruculose, aseptate, curved, gradually tapering towards the round to slightly acute apex and truncate base, guttulate. Appressoria not observed.
Material examined: RUSSIA, Rostov Region, Rostov-on-Don City, Botanical garden of Southern Federal University, High Park, dying twigs and leafstalks, on Parthenocissus inserta (L.) Planch. (Vitaceae), 15 April 2015, T.S. Bulgakov (T191), (MFLU 15-1895, holotype).
Notes: No culture is available for this species; hence direct DNA extraction from conidiomata was conducted. The Colletotrichum dematium species complex is mainly characterized by curved conidia (Damm et al. 2009). Multi-gene analyses reveal a close association between C. insertae and C. dematium with high support (75 % BT/ 1.0 PP, Fig. 114). This species differs from C. dematium in having abundant setae per acervulus, which becomes hyaline towards the rounded apex and 1–5 septa, as well as smaller conidia (C. dematium conida L/W = 6). This species differs from C. lineola in having shorter setae with 1–5 septa which can be observed along the seta, conidiogenous cells not having a distinct collarette, as well as having smaller conidia (C. lineola conidia L/W = 6.6) (Fig. 115).
Phylogram generated from maximum parsimony analysis based on combined ITS, GADPH, CHS, ACT and TUB2 sequence data from species of dematium species complex. Maximum parsimony bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.70 are shown above the branches. The new isolate is in blue. The tree is rooted with Colletotrichum nigrum
Reticulascaceae Réblová & W. Gams
The family Reticulascaceae was introduced to accommodate two holomorphic genera Reticulascus and Porosphaerellopsis Samuels & E. Müll., supported by analysis of combined ITS, LSU, SSU and RPB2 sequence data. Even though the characters and ontogeny of these genera differ, the centrum and interthecial tissues are quite similar (Réblová et al. 2011). Presently, three genera are accepted in Reticulascaceae (Maharachchikumbura et al. 2015, 2016). In this study, we place the genus Blastophorum in family Reticulascaceae based on phylogenetic analysis (Fig. 116) and morphological characters.
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS sequence data from species of Glomerellales. Maximum likelihood bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.90 are shown in above and below and branches with maximum parsimony bootstrap support values greater than 50 % are in bold. The new isolates is in red and other ex-type strains are in bold. The tree is rooted with Microascus longirostris
Blastophorum Matsush
Matsushima (1971) introduced the genus Blastophorum with B. truncatum as the type species. Index fungorum (2016) lists three additional species in this genus, namely B. fusarioides K. Matsush. & Matsush., B. pini Minter & Hol.-Jech., and B. uniseptatum Matsush. There is no sequence data in GenBank, and fresh collections are needed to provide a natural classification of the genus.
Blastophorum aquaticum Z.L. Luo, Bhat & K.D. Hyde, sp. nov.
Index Fungorum number: IF552241; Facesoffungi number: FoF02218, Fig. 117
Etymology: In reference to the aquatic habitat of this fungus.
Holotype: DLU 084.
Saprobic on submerged decaying wood. Sexual morph Undetermined. Asexual morph Colonies on the substrate superficial, effuse, velvety, brownish-grey to greyish-brown. Conidiophores macronematous, mononematous, unbranched, percurrently proliferating, smooth, septate, dark brown, 190–250 μm (\( \bar{x} \) = 220 μm, SD = 26.5 μm, n = 10) long, 6–8 μm (\( \bar{x} \) = 7 μm, SD = 1 μm, n = 10) wide. Conidiogenous cells terminal, integrated, initially enteroblastic, subsequently polyblastic and sympodial, subhyaline at the base and hyaline above, with inconspicuous flattened denticles at conidiogenous loci. Conidia 1–3-septate, elongated, cuneiform, rounded at the tip, narrowed towards the flattened base, smooth, slimy, 19–24 μm (\( \bar{x} \) = 21.5 μm, SD = 2.5 μm, n = 20) long, 6.5–7.5 μm (\( \bar{x} \) = 7 μm, SD = 0.5 μm, n = 20) wide. Conidial secession schizolytic.
Culture characteristics: Colonies on MEA 70 mm diam. after 6 weeks at room temperature, dark brown at the margins, cream to white at the center; reverse yellowish to dark brown and orangish at the center, medium dense, circular, umbonate.
Material examined: CHINA, Yunnan Province, Dali, Cangshan Mountain, Heilong stream, saprobic on submerged decaying wood, March 2014, X.Y Liu, S-084 (DLU084, holotype), ex-type living culture, MFLUCC 15-0264.
Notes: Blastophorum resembles Kylindria in having terminal, integrated, initially enteroblastic, subsequently polyblastic and sympodial conidiogenous cells, but Blastophorum differs in having cuneiform and larger conidia. The phylogeny analysis (Fig. 116) also showed Blastophorum separates from Kylindria. Blastophorum aquaticum differs from B. pini in having elongated, cuneiform and wider conidia (6.5–7.5 μm vs. 1.5–2.5 μm) (Minter and Holuboá-Jechová 1981). It differs from B. truncatum in having longer conidiophores (190–250 μm vs. 18.3–132.6 μm), elongated, cuneiform, 1–3-septate and larger conidia (19–24 × 6.5–7.5 μm vs. 8.7–17 × 2.6–4.3 μm) (Chen and Tezan 2008).
Hypocreales Lindau
Hypocreales is an order within the class Sordariomycetes, represented by Bionectriaceae, Clavicipitaceae, Cordycipitaceae, Flammocladiaceae, Hypocreaceae, Nectriaceae, Niessliaceae, Ophiocordycipitaceae, Stachybotriaceae and Tilachlidiaceae. Maharachchikumbura et al. (2016)
Ophiocordycipitaceae G.H. Sung et al.
Ophiocordycipitaceae was proposed in Sung et al. (2007), based mainly on phylogenetic analyses. According to most recent studies on the family, it contains six genera, Drechmeria, Harposporium, Ophiocordyceps, Polycephalomyces, Purpureocillium and Tolypocladium (Quandt et al. 2014). Ophiocordyceps was first described to accommodate species possessing ascospores that usually do not break into part-spores at maturity, asci with thin apical caps (Petch 1931a, 1932), and often producing a dark pigmented, tough to pliant stromata, commonly with perithecial apices (Sung et al. 2007). This genus is associated with several asexual morphologies, Sorosporella, Hirsutella, Hymenostilbe, Stilbella, Syngliocladium, and Paraisaria (Quandt et al. 2014). Hymenostilbe was proposed by Petch (1931b) and contains species that produce conidia from multiple denticles on conidiogenous cells forming a palisade-like layer along the surface of synnemata (Mains 1950). There is evidence to restrict the use of Hymenostilbe to the “O. sphecocephala clade” within the genus Ophiocordyceps (Sung et al. 2007). The phylogenetic tree, based on ITS, composed by species of this clade, including two new species, is presented in Fig. 118.
Phylogram generated from Bayesian analysis based on ITS sequence data of Ophiocordyceps. Metacordyceps taii is used as outgroup taxon. Maximum likelihood bootstrap values greater than 50 % and Bayesian posterior probabilities over 0.90 are indicated above and below the nodes (BBP/BP), respectively. The new species are indicated in blue, holotypes in bold
Ophiocordyceps hemisphaerica Mafalda-Freire, Reck & Drechsler-Santos, sp. nov.
Index Fungorum number: IF552122; Faces of fungi number: FoF02193, Fig. 119
Ophiocordyceps hemisphaerica. a Overview of the stromata and the host (FURB 45181). b Fertile head (FLOR 59525, holotype). c Cross section showing the complete immersed perithecia. d Ascoma entirely isolated from the fertile head. e Conidia. f Asci. g Apical cap. h–i Asci with part-spores. Scale bars a = 2 cm, b–c = 1 mm, d = 1000 μm, e = 10 μm, h–i = 10 μm
Etymology: Referring to the hemisphaeric form of the fertile head of the stroma.
Holotype: FLOR 59525
Specimens found on twigs of living plants, parasitic in true flies of Muscidae. Sexual morph capitated. Stromata 12–20 × 0.8–1 mm, rarely branched, stipitate, usually 2, up to 4, arising from the host thorax between the wings, tough, with morphology unaffected when dried. Stipe 11–19 mm long, 0.8–1 mm wide, cylindrical, with a fertile apex, flexible when fresh, hard and tough when dried, smooth, brown to greyish-brown, epidermal layer compact, medullar region white to cream, not compacted. Fertile head 1–1.2 mm long, 2–4 mm diam., hemisphaerical, truncate, applanate at the base, pale to dark yellowish, ostioles inconspicuous on the upper surface, smooth-like. Ascomata obpyriform, slightly curved, 780–860 × 220–290 μm (\( \bar{x} \) = 820 × 255 μm, n = 40), completely immersed, yellow, thick-walled, perithecia easily detachable from the fertile region. Asci narrow cylindrical, 500–640 × 5–6 μm (\( \bar{x} \) = 570 × 5.5 μm, n = 40), apex thickened, 8-spored, hyaline. Apical cap 5–6 μm (\( \bar{x} \) = 5.5 μm, n = 40) diam. Ascospores filiform, smooth, almost as long as asci, hyaline, more than 52 septa, easily breaking into part-spores. Part-spores cylindrical to unusually fusoid, 7–10 × 1–1.5 μm (\( \bar{x} \) = 8.5 × 1.25 μm, n = 40), hyaline, smooth. Asexual morph Hymenostilbe-like. Synnemata cylindrical, 6–12 × 0.5–1 mm, simple or branched, 1 to 3, smooth, brown to greyish-brown, arising from the thorax and abdomen of the host, unusually arising from aborted stromata. Conidiogenous cells phialidic, clavate, surface slightly rugose, hyaline, compactly arranged. Conidia obovoid, 6.2–8.3 × 2.5–3.5 μm (\( \bar{x} \) = 7.25 × 3 μm, n = 40), hyaline, slightly verrucous, with persistent appendix.
Material examined: BRAZIL. Santa Catarina, Ituporanga, Rio do Norte, 27°24′00″S, 49°36′00″W, on dead flies of Muscidae, 19 October 2014, Bittencourt FB 246 (FURB 45181); Florianópolis, 27°35′33″S, 48°28′42″W, 2010, Mafalda-Freire FMF 33 (FLOR 59524); Joaçaba, Parque Natural Municipal Rio do Peixe, 27º10′22″S, 51º30′23″W, 10 March 2010, Mafalda-Freire FMF 104 (FLOR 59525, holotype); 24 January 2014, Mafalda-Freire FMF 331 (FLOR 59526); Mafalda-Freire FMF 332 (FLOR 59527); Mafalda-Freire FMF 333 (FLOR 59528); 27 September 2014, Mafalda-Freire FMF 295 (FLOR 59529); Mafalda-Freire FMF 305 (FLOR 59530); 27 September 2014, Mafalda-Freire FMF 296 (FLOR 59531); Mafalda-Freire FMF 297 (FLOR 59532); Mafalda-Freire FMF 298 (FLOR 59533); Mafalda-Freire FMF 309 (FLOR 59534); Mafalda-Freire FMF 310 (FLOR 59535); Mafalda-Freire FMF 311 (FLOR 59536); Mafalda-Freire FMF 312 (FLOR 59537); Mafalda-Freire FMF 313 (FLOR 59538); Mafalda-Freire FMF 314 (FLOR 59539); Mafalda-Freire FMF 315 (FLOR 59540); Mafalda-Freire FMF 316 (FLOR 59541); Mafalda-Freire FMF 317 (FLOR 59542); Mafalda-Freire FMF 318 (FLOR 59543); Mafalda-Freire FMF 319 (FLOR 59544); Mafalda-Freire FMF 320 (FLOR 59545); Mafalda-Freire FMF 321 (FLOR 59546); Mafalda-Freire FMF 322 (FLOR 59547); Mafalda-Freire FMF 323 (FLOR 59548); Mafalda-Freire FMF 324 (FLOR 59549); 28 September 2014, Mafalda-Freire FMF 328 (FLOR 59553); Águas Mornas, Sítio Portal, 27°41′47″S, 48°49′29″W, 26 October 2013 Mafalda-Freire FMF 141 (FLOR 59550); Blumenau, Parque das Nascentes, 26°57′41″S, 49°04′11″W, 22 February 2012, Drechsler-Santos DS 785 (FLOR 59551).
Additional material examined (as Ophiocordyceps dipterigena): JAPAN, Ibaraki: Gozenyama, 29 July 2001, F. Ihara 1021 (personal collection of F. Ihara); 26 July 2002, F. Ihara 02024 (personal collection of F. Ihara); F. Ihara 2025 (personal collection of F. Ihara); 7 August 2002, F. Ihara 04063 (personal collection of F. Ihara); 23 July 2007, F. Ihara 7044 (personal collection of F. Ihara); F. Ihara 07045 (personal collection of F. Ihara); Kyushu, July 1950, (MICH 274571); USA, North Carolina, Cranberry, August 1887, R. Thaxter, Reliquiae Farlowianae 612 (FH 5086); R. Thaxter, Reliquiae Farlowianae 612 (FH 5077); R. Thaxter, Reliquiae Farlowianae 612 (MICH 274593).
Notes: Ophiocordyceps hemisphaerica is characterized by the tough stipitate stromata, with hemisphaerical and pale to dark yellowish fertile-head, with applanate base. The tough stromata are resistant and the morphology is unaffected even when dried. The primordium stromata has conidiogenous cells and conidia during the development. Additionally, the species parasitizes true big flies of Muscidae, which are not covered by mycelium. All parasitized flies were found above branches of living trees and always in advanced stage of decomposition. ITS based phylogenies group all O. hemisphaerica isolates in a strongly supported monophyletic clade and sharing a sister relationship to O. dipterigena species (Fig. 118). Ophiocordyceps hemisphaerica is morphologically similar to O. dipterigena (Berk. & Broome) G.H. Sung et al. However, it can be differentiated by its larger stromata (12–20 × 0.8–1 mm vs. 5–10 × 1 mm) and hemisphaerical fertile region, which are not observed in O. dipterigena (Petch 1932; Kobayasi and Shimizu 1978, Luangsa-ard et al. 2008). Additionally, O. dipterigena was described originally by Berkeley and Broome (1874) from material collected in Sri Lanka (Ceylon). Cordyceps muscicola Möller, a heterotypic synonymy of O. dipterigena, was described from material collected in Blumenau, Santa Catarina, Brazil. In a taxonomical perspective O. dipterigena corresponds to a species-complex, O. hemisphaerica differs from C. muscicola by its slightly smaller perithecia and asci (780–860 × 220–290 μm and 500–640 × 5–6 μm vs. 850–920 × 230–300 μm and 550–700 × 5 μm, respectively). Furthermore, C. muscicola presents six stromata, smaller (9–13 mm) than those observed for O. hemisphaerica (12–20 mm); the host of C. muscicola was described as covered by mycelium found adhered to the abaxial region of the leaves (Möller 1901).
Ophiocordyceps lacrimoidis Mafalda-Freire, Reck & Drechsler-Santos, sp. nov.
Index Fungorum number: IF552123, Facesoffungi number: FoF02194, Fig. 120
Ophiocordyceps lacrimoidis. a Holotype, overview of the stromata and the host attached by mycelium on a twig. b Fertile region. c Fertile head in a cross section showing the complete immersed perithecia. d Vertical section showing the superficial perithecia. e Asci. f Layer of conidiogenous cells with a conidium attached. g Conidia. Scale bars a = 5 mm, b–d = 1 mm, e = 20 μm, f = 20 μm, g = 25 μm
Etymology: Referring to the lacrimoid form of the conidia.
Holotype: FLOR 59552.
Specimen found attached by mycelium on a twig, parasitic in true flies of Muscidae (host very damaged). Sexual morph capitated. Stromata 4–5 × 1 mm, simple, stipitate, 2 stromata arising from the thorax. Stipe 3–4 mm long, 1 mm wide, cylindrical, with a fertile apex, fleshy, robust, smooth, epidermal layer brown, compact, medullar region white to cream, soft, not compacted. Fertile head 1.2 mm long, 1.8–2.2 mm diam., discoid, pale to dark yellowish, ostioles inconspicuous on the upper surface. Ascomata obpyriform, slightly curved, 650–700 × 200–250 μm (\( \bar{x} \) = 675 × 225 μm, n = 40), completely immersed, yellow, thick-walled, perithecia easily detachable from the fertile region. Asci narrow cylindrical, 350–450 × 5 μm (\( \bar{x} \) = 400 × 5 μm, n = 40), apex thickened, 8-spored, hyaline. Apical cap 3–4 × 5–6 μm (\( \bar{x} \) = 3.5 × 5.5 μm, n = 40). Ascospores filiform, smooth, almost as long as asci, hyaline, more than 56 septa, easily breaking into part-spores. Part-spores cylindrical, 8–14 × 2 μm (\( \bar{x} \) = 11 × 2 μm, n = 40), hyaline, smooth. Asexual morph Hymenostilbe-like. Synnemata cylindrical, 3 × 0.3 mm, simple, unique, smooth, orange brown, arising from the abdomen of the host. Conidiogenous cells phialidic, clavate, surface roughened, hyaline, pseudoparenchymatous, compactly arranged. Conidia lacrimoid, 4–5 × 3–5 μm (\( \bar{x} \) = 4.5 × 4 μm, n = 40), hyaline.
Material examined: BRAZIL. Amazonas, Manaus, Reserva Florestal Adolfo Ducke, 3°00′16″S 59°55′07″W, on a dead fly attached on a twig, 2012, Mafalda-Freire FMF148 (FLOR 59552, holotype).
Additional material examined (as Ophiocordyceps discoideicapitata): JAPAN, Yamagata, 8 July 1982 (TNS-F. 181545, NMNS); Iwate, Morioka, 18 July 2007, F. Ihara 07031 (personal collection of F. Ihara); F. Ihara 07032 (personal collection of F. Ihara); 11 August 2007, F. Ihara 07116 (personal collection of F. Ihara); F. Ihara 07117 (personal collection of F. Ihara); F. Ihara 07118 (personal collection of F. Ihara).
Notes: Ophiocordyceps lacrimoidis is characterized by a robust short stipe, discoid fertile region, cylindrical synnemata and lacrimoid conidia. Additionally, the species parasitizes true flies of Muscidae, which are not covered by mycelium. According to phylogenetic analysis (Fig. 118), O. lacrimoidis is in a distinct lineage in the “O. sphecocephala clade”, as sister of O. australis (Speg.) G.H. Sung et al. Morphologically, O. lacrimoidis is closely related to O. discoideicapitata (Kobayasi & Shimizu) G.H. Sung et al., but it can be differentiated by its darker fertile region with inconspicuous ostioles. Ophiocordyceps lacrimoidis slightly smaller perithecia than those of the O. discoideicapitata specimen studied by us (TNS-F. 181545: 700–820 × 270–280 μm) and similar to those described by Kobayasi and Shimizu (1982, 620–700 × 250–300 μm). The presence of synnemata and lacrimoid conidia was not observed for O. discoideicapitata. Furthermore, O. discoideicapitata was originally described from Japan, while the new species is described from Brazilian Amazon. Ophiocordyceps dipterigena and O. hemisphaerica also parasize flies, however the size of the stromata, and the morphology of fertile region and part-spores are very different (Table 1).
Purpureocillium Luangsa-ard et al.
The genus Purpureocillium was introduced by Luangsa-ard et al. (2011) with P. lilacinum (Thom) Luangsa-ard et al. as the type species, and it was reported as important pathogens in humans. Luangsa-ard et al. (2011) also showed that it belongs to Ophiocordycipitaceae, Hypocreales. Besides the type species, Purpureocillium comprises three species viz. P. atypicola (Yasuda) Spatafora et al., P. lavendulum Perdomo et al. and P. takamizusanense (Kobayasi) S. Ban, Azuma & Hirok. Sato (Index Fungorum 2016).
Purpureocillium sodanum Papizadeh, Soudi, Wijayaw., Shahz.-Faz. & K.D. Hyde, sp. nov.
Faces of Fungi number: IF552178; Facesoffungi Number: FoF02247, Fig. 122
Etymology: Named after isolation of this species from salt crystals.
Holotype: IBRC-H 2020
Saprobic on salt crystals. Sexual morph Undetermined. Asexual morph Hyphae hyaline, smooth-walled, 1–2 μm wide. Conidiophores growing from the superficial mycelium very long, consisting of a stipe, bearing 6–8 μm long monoverticillate branches with terminal whorls of 2–3 phialides; stipe erect, septate, hyaline to pale purple-brown, rough-walled, profusely verrucose and up to 5 μm wide. Conidiophores growing from the aerial mycelium 25 to 40 nm long, irregularly branched, with 3–4 phialides per branch, septate, hyaline and smooth-walled. Phialides 5–13 × 2–3 mm, with a cylindrical basal portion tapering into a distinct neck up to 3 μm long. Conidia were in long dry chains, unicellular, subglobose with apiculate base or limoniform, 3.5–5.5 × 3–4.5 μm, smooth-walled, mostly subhyaline, pinkish-purple in mass. Acremonium-like conidiophores were rarely observed, and when present, up to 50 μm long, simple, usually reduced to a cylindrical phialides, 9–22 × 1.5–2 mm. Chlamydospores and sexual morph were not observed.
Culture characteristics: Colonies at 25 °C on MEA attained 28–30 mm diam. after 7 days incubation, consisting of a dense basal felt of numerous conidiophores giving a powdery texture, pale vinaceous (9B2), with sparse aerial mycelium, and producing a very faint yellow diffusible pigment. Colony features on CYA, CMA and PDA were similar to those observed on MEA, except that on PDA and CYA they were radially folded toward the periphery. Such a folding pattern was observed on MEA exclusively at 25 °C. Colonies on CYA were floccose and pale vinaceous (9A2). The optimum growth temperature was 30 °C (on MEA, 33–35 mm diam. after 7 days incubation) and the minimum was 20 °C. Colonies at 35 °C on MEA attained 23–25 mm diam. after 7 days incubation. Growth was insignificant at 37 °C and absent at 40 °C.
Material examined: IRAN, Markazi Province, Mighan (Miqan) saline Lake, salt crystals, 2013, M. Papizadeh (IBRC-H 2020, holotype), ex-type culture IBRC-M 30175.
Notes: In our phylogenetic analyses, the new strain clusters as a distinct clade from other species (Fig. 121). This clade is sister to the type species, but is separated from high bootstrap value (86 %). Hence, we introduce our strain as a new species based on DNA sequence analyses. P. sodanum is distinguished from other Purpureocillium species based on its growth at 30 up to 37 °C.
Hypocreales incertae sedis
Alfaria Crous et al.
The monotypic genus Alfaria was introduced and typified by A. cyperi-esculenti Crous et al. The genus is placed in Hypocreales incertae sedis based on molecular data (Fig. 123).
Phylogram generated from maximum likelihood analysis based on combined LSU, ITS and RPB2 sequence data for taxa of Hypocreales. Maximum likelihood bootstrap support values greater than 50 % are shown near the nodes. The new isolates are in blue and ex-type sequences in bold. The tree is rooted with Gelasinospora tetrasperma and Neurospora crassa
Alfaria spartii Senan., Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552303; Facesoffungi number: FoF02455, Fig. 124
Etymology: In reference to the host genus Spartium.
Holotype: MFLU 16-1969.
Saprobic on Spartium junceum L. Sexual morph Ascomata 220–250 μm high × 190–275 μm diam. (\( \bar{x} \) = 216 × 250 μm, n = 10), scattered, solitary, immersed, black, globose to subglobose, coriaceous, ostiolate. Ostiole lined with hyaline, filiform periphyses. Hamathecium comprises few, septate, hyphae-like, hyaline paraphyses. Peridium comprising thick-walled, brown cells of textura angularis. Asci 140–180 × 30–40 μm (\( \bar{x} \) = 155 × 37 μm, n = 20), 8-spored, unitunicate, oblong to fusiform, base sessile, apex oblong, with J-apical ring. Ascospores 30–35 × 10–12 μm (\( \bar{x} \) = 32 × 11 μm, n = 20), overlapping bi-seriate, hyaline, oval to ellipsoid, unicellular, thick-walled, guttulate, guttules concentrated at the ends. Asexual morph Undetermined.
Culture characteristics: Colonies on MEA reaching 2 cm after 14 days at 18 °C, circular, umbonate, filiform, white, dense colonies, tightly attached to the media.
Material examined: ITALY, Santa Sofia, Province of Forlì-Cesena [FC], Camposonaldo, on dead branch of Spartium junceum (Fabaceae), 13 September 2013, Erio Camporesi, IT 1452 (MFLU 16-1969, holotype; MFLU 16-1978, paratype), ex-type living culture, MFLUCC 13-0799.
Notes: Alfaria spartii is the second species of genus Alfaria. In combined analyses of LSU and ITS sequence data, A. spartii clustered with A. cyperi-esculenti with high support (Fig. 123). Alfaria spartii differs from A. cyperi-esculenti in having large, oval ascospores with distinctly periphysate ostioles.
Bionectriaceae Samuels & Rossman
Bionectriaceae Samuels & Rossman was introduced by Rossman et al. (1999) to accommodate Bionectria and other allied genera. Members of this family are characterized by uniloculate hyaline to brightly coloured, soft perithecia with or without a well-developed stroma and unitunicate asci (Maharachchikumbura et al. 2016). Maharachchikumbura et al. (2016) accepted 39 genera and confirmed the familial placement in Hypocreales, Sordariomycetes in their phylogenetic analyses.
Emericellopsis Beyma
The genus Emericellopsis was introduced by van Beyma (1939−1940) with Emericellopsis terricola Beyma as the type species. Grum-Grzhimaylo et al. (2013) placed this genus in Bionectriaceae based on sequence analyses, but Maharachchikumbura et al. (2016) treated it as Hypocreales, genera incertae sedis. Based on habitats, species of Emericellopsis have been detected as saprobes. Moreover, Grum-Grzhimaylo et al. (2013) showed that Emericellopsis species can be classified into three categories based on their origins; marine, soda lakes, and terrestrial. Our new collection, Emericellopsis persica, falls into the terrestrial clade of this genus although detected in saline soils.
Emericellopsis persica Papizadeh, Wijayaw, Soudi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552358; Facesoffungi Number: FoF 02517, Fig. 126
Etymology: Epithet taken from its isolation from Iran (formerly known as Persia).
Holotype: IBRC-H 2022.
Isolated from soil from lake. Sexual morph Ascomata dark brown, superficial on the substrate, globose, 280–320 μm diam., non-ostiolate. Peridium 19–25 μm thick, multi-layered, pseudoparenchymatous, composed of 3–5 layers of compressed cells. Asci saccate, 15–18 μm diam., with thin deliquescent wall, soon dissolving, unitunicate, scattered irregularly in the ascocarp. Ascospores ellipsoid, pale brown, with uneven surfaces, 8–11 × 4–5 μm (\( \bar{x} \) = 9.5 × 4.5), surrounded by 3 longitudinal, hyaline, sharp appendages, width up to 1 μm. Asexual morph hyphomycetous, acremonium-like. Conidiophores mostly simple orthotropic. Conidiogenous cells 30–40 μm long, phialidic, tapering from 2–2.5 μm at the base to 0.5–0.7 μm at the apex. Conidia narrowly ellipsoid, smooth-surfaced, 4.5–6.5 × 2 μm (\( \bar{x} \) = 5.5 × 2), hyaline, adhering in slimy heads. Chlamydospores mostly intercalary, 10–22 × 7–17 μm, hyaline.
Culture characteristics: Colonies on CYA agar fast-growing, reaching 21–23 mm diam. in 7 days at 25 °C. On MEA (pH 6.5) growing slower, reaching 17–18 mm diam. in 7 days. Colonies orange-salmon-pink, later darkening in centre due to the formation of ascomata with tufted aerial mycelium. Reverse colourless. Exudate absent. Decumbent vegetative hyphae thin-walled, hyaline, 0.5–1.5 μm wide. Mycelium consisting of hyaline, smooth-walled, septate hyphae, 1–2 μm wide, often fasciculate.
Material examined: IRAN, Lake Urmia, soil, 2011, M. Papizadeh, M.R. Soudi (IBRC-H 2022, holotype), ex-type culture IBRC-M 30046.
Sequence: ITS sequence GenBank KX668543. The aligned dataset used in the analysis has been submitted with the TreeBase under the submission ID 19688 for the combined set of ITS and β-tub.
Notes: In our phylogenetic analyses, Emericellopsis persica clusters as a distinct clade from other species. Emericellopsis donezkii and E. humicola are the closest species, but separated with high bootstrap support (100 %). Hence, we introduce our strain as a new species based on DNA sequence analyses (Figs. 125, 126).
Meliolales Gäum. ex D. Hawksw. & O.E. Erikss.
Hongsanan et al. (2015b) provided a monograph and a backbone tree for Meliolales. Meliolales is in Sordariomycetes, which also comprises the family Armatellaceae. A new tree incorporating Meliola citri-maximae and M. pseudosasae (Fig. 127) is provided below.
Phylogram generated from Bayesian analysis based on LSU sequence data from species of Meliolaceae. Maximum parsimony/likelihood bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.9 are shown in the first and second set, respectively. Ex-type strains are in bold and the new sequence are in blue bold
Meliolaceae G.W. Martin ex Hansf.
The family Meliolaceae was introduced by Martin (1941) without a Latin diagnosis and validated by Hansford (1946). It is typified by Meliola with M. nidulans (Schwein.) Cooke as the type species. At various times, this family has been placed in the orders Dothideales, Erysiphales, Meliolales, Myriangiales and Hypocreales (Martin 1941; Luttrell 1951, 1989; Roger 1953; Ainsworth et al. 1971; Müller and von Arx 1973; Yarwood 1973; Barr 1976; Eriksson 1981; Hawksworth et al. 1983). Kirk et al. (2001) introduced a new subclass, Meliolomycetidae (Sordariomycetes) for members of Meliolaceae, but without a description or diagnosis. The placement of Meliolomycetidae in Sordariomycetes was confirmed by Justavino et al. (2015) based on their phylogenetic analyses and validated in Maharachchikumbura et al. (2015).
Ainsworth et al. (1971) and Eriksson and Hawksworth (1993) listed 50 genera in the family, but this was reduced to 25 genera (Hawksworth et al. 1995) and later 22 genera (Kirk et al. 2008). Lumbsch and Huhndorf (2010) placed Meliolaceae in the class Sordariomycetes with 26 genera. Hongsanan et al. (2015b) accepted only seven genera Amazonia, Appendiculella, Asteridiella, Cryptomeliola, Endomeliola, Irenopsis and Meliola in this family.
Meliola Fr.
The genus Meliola is typified by M. nidulans (Schwein.) Cooke. Meliola is the largest genus of Meliolaceae. Several studies have provided sequence data from Meliola members (Gregory and John 1999, Justavino et al. 2015, Pinho et al. 2012, 2014, Hongsanan et al. 2015b), and phylogenetic placement of Meliola was clarified. However, there is still little sequence data for the entire subclass available in GenBank.
Meliola citri-maximae X.Y. Zeng, K.D. Hyde & T.C. Wen, sp. nov.
Index Fungorum number IF552271; Facesoffungi number: FoF02253, Fig. 128
Meliola citri-maximae (MFLU14-0288, holotype). a Host leaves. b Colony on surface of leaf. c Ascoma on host substrate. d Hyphae with hyphopodia and phialides. e Hyphae with hyphopodia. f Hyphae with phialides. g Outer layer of peridium. h Inner layer of peridium. i Hamathecium. j Hyphal setae. k–q Asci from young state to mature state. r–t Ascospore from young state to mature state. Scale bars c, d = 200 μm, j = 100 μm, e–i, k–t = 20 μm
Etymology: Referring to the host Citrus maxima (Burm. f.) Merr.
Holotype: MFLU 14-0288.
Epiphytes on the surface of living leaves. Colonies hypophyllous, scattered to dense, sometimes confluent. Hyphae superficial, brown, substraight, radiating outwardly, branched, septate, darker at septa, reticulate, with hyphal setae. Hyphal setae up to 320 μm long, dark brown, denticulate at the apex. Hyphopodia 20–22 × 8–10 μm (\( \bar{x} \) = 21 × 9 μm, n = 20), 2-celled, brown, spathulate, form one or two near the septa, alternate to unilateral, sometimes opposite antrorse. Sexual morph Perithecia up to 250 μm, superficial, subdense, globose to subglobose, thick-walled, with ostiole, without perithecial setae. Peridium two strata, comprising hyaline inner cells and dark brown outer wall of textura angularis. Hamathecium with evanescent paraphyses. Asci unitunicate, 2–3-spored, ovoid to ellipsoid when young, with short pedicel, asci wall attenuated or broken when mature, evanescent. Ascospores 42–48 × 16–21 μm (\( \bar{x} \) = 44 × 19 μm, n = 20), 2–3-seriate, cylindrical, hyaline at young state, becoming dark brown when mature, 3–4-septate, constricted and darker at the septa, middle cell a little larger, rounded at both ends, smooth-walled. Asexual morph Phialides 15–22(–25) × 7–9 μm (\( \bar{x} \) = 20 × 8 μm, n = 10), ampulliform, form one at top of hyphal cell, alternate or unilateral, sometimes mixed with capitate hyphopodia. Conidia undetermined.
Material examined: THAILAND, Chiang Rai, Horticulture Research Centre, on living leaves of Citrus maxima (Rutaceae), 12 March. 2014, Xiang-Yu Zeng (MFLU 14-0288, holotype)
Notes: Species of Meliolaceae found on Citrus include Amazonia butleri, Meliola amyridis, M. camelliae and M. citricola. Morphologically, the new collection is typical to the genus Meliola in having hyphal setae, but differs from M. camelliae and M. citricola as it has 2–3-spored asci instead of 8-spored asci in M. camelliae and shorter setae, larger perithecia and larger ascospores than M. citricola. It is most similar to M. amyridis in having hypophyllous and dense colonies, alternate hyphopodia, and ascospore size, but differs in having shorter setae, larger perithecia and hyphopodia. Phylogenetically, our new collection is related to M. brachyodonta and M. trichostroma. The sequence data of M. brachyodonta and M. trichostroma, which are unpublished, were provided by Pinho et al. (2013). However, these two species were found on Croton curranii (Euphorbiaceae) and Psidium guajava (Myrtaceae) respectively, which are on the different host family from this new collection.
Although LSU phylogenies did not fully resolve the affinities of M. citri-maximae with other known Meliola species, we suggest it is a new taxon. Further analyses should target a more variable region such as ITS or β-tubulin, as the LSU gene is too conserved to differentiate species (Jeewon et al. 2002, 2003).
Meliola pseudosasae I. Hino, Bull. Faculty of Agriculture, Yamaguchi University 9: 882 (1958)
Facesoffungi number: FoF02458, Fig. 129
Epiphytes on the upper surface of leaves of Sasa borealis (Hack.) Makino & Shibata. Superficial hyphae branched, septate, darker at the septa, brown, with hyphopodia, hyphal setae present. Hyphopodia 10–11 μm diam. (\( \bar{x} \) = 10.5 μm, n = 20), head cell capitate, 22–25 μm long (\( \bar{x} \) = 23 μm, n = 20), alternate or opposite on hyphae, near to hyphal septum, 2-celled, upper cell cylindrical and curly, brown. Hyphal setae 170–210 μm high × 9–12 wide μm (\( \bar{x} \) = 180 × 10 μm, n = 10), arising from hyphae, comprising 2–3 arms, with 2–4-branches in each arm, acute and hyaline at the apex, septate, brown to dark brown, smooth-walled. Sexual morph Ascomata 97–135 μm high × 105–160 μm diam. (\( \bar{x} \) = 120 × 140 μm, n = 10), superficial on the surface of hosts, solitary to gregarious on superficial hyphae, globose to subglobose, thick-walled, ascomatal setae and appendages absent, surface of ascomata verrucose. Peridium 17–20 μm comprising dark brown cells of textura angularis when viewed in squash mounts, with two strata, outer stratum thick-walled, dark brown cells of irregular textura angularis, and inner stratum of flattened, hyaline cells. Hamathecium not observed. Asci not observed. Ascospores 45–52 μm high × 18–23 wide μm (\( \bar{x} \) = 50 × 22 μm, n = 10), 2-seriate, oblong to broadly cylindrical, hyaline to dark brown, 4-septate, constricted and darker at the septa, smooth-walled, ends rounded. Asexual morph Undetermined.
Material examined: CHINA, Xishuangbanna, on living leaves of Sasa borealis (Pinaceae), 22 November 2015, R Phookamsak Xb002 (MFLU 16-2136, in KIB, reference specimen designated here).
Notes: Our fresh specimen is similar to Meliola pseudosasae both in host (Sasa borealis) and morphology. It has hyphal setae with 2–3 arms and with 2–4-branches at the apex, superficial hyphae with hyphopodia and 4-septate, brown ascospores as in the protologue. We were unable to isolate our specimen in culture, thus, DNA was extracted from ascomata and ascospores directly. Phylogenetic analysis (Fig. 127) indicate that our collection clusters with members of Meliolaceae within the subclass Meliolomycetidae, Sordariomycetes. Meliola pseudosasae is related to M. clerodendricola Henn. strain, which was published as a reference specimen by Hongsanan et al. (2015b). Asteridiella, Appendiculella and Endomeliola species clustered with Meliola species. Thus, further molecular data are needed to clarify if they are polyphyletic. We designate our collection as a reference specimen (sensu Ariyawansa et al. 2014b).
Xylariales Nannf.
For the order Xylariales, we follow Daranagama et al. (2016).
Apiosporaceae K.D. Hyde et al.
The monophyletic family Apiosporaceae was introduced by Hyde et al. (1998), and is typified by the genus Apiospora. Apiospora typified by A. montagnei Sacc. is characterized by apiospores and a basauxic, Arthrinium-like conidiogenesis (Samuels et al. 1981; Hyde et al. 1998; Bahl 2006, Senanayake et al. 2015).
Arthrinium Kunze
Crous and Groenewald (2013) provided a monograph on this genus and Index Fungorum (2016) listed 60 species. The older name Arthrinium was conserved as it is a more commonly encountered name and more frequently used in literature (Crous and Groenewald 2013). We provide an updated tree here (Fig. 130).
Consensus tree resulting from a Bayesian analysis of the ITS sequence alignment of Arthrinium species. RAxML bootstrap support values (MLB above 50 %) and Bayesian posterior probabilities (PP above 50 %) are given at the nodes (MLB/PP). The newly introduced sequences are in blue bold. The scale bar represents the expected number of changes per site. The tree is rooted to Xylaria hypoxylon
Arthrinium paraphaeospermum Senan., & K.D. Hyde, sp. nov.
Index Fungorum number: IF552137, Facesoffungi number: FoF02459, Fig. 131
Etymology: In reference to the large conidia and conidiogenous cells and morphological similarity to the A. phaeospermum.
Holotype: MFLU 16-1974
Saprobic on bamboo. Sexual morph Undetermined. Asexual morph Mycelium consisting of smooth, hyaline, branched, septate, 3–5 μm diam. hyphae. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 25–30 × 4–6 μm (\( \bar{x} \) = 27 × 4.7 μm), basauxic, aggregated in clusters on hyphae, hyaline, smooth, elongated, conical to ampulliform. Conidia 10–19 μm diam., (\( \bar{x} \) = 13 μm) brown, smooth, somewhat granular, globose to ellipsoid in surface view, with a median scar, lenticular in side view, with pale equatorial slit. Sterile cells 20–30 × 9–13 μm (\( \bar{x} \) = 24 × 11 μm), forming on solitary loci on hyphae, brown, finely roughened, ellipsoid to clavate.
Culture characteristics: Colonies on MEA flat, circular, smooth, spreading, with fluffy aerial mycelium, mycelia not tightly attached to the surface, dirty white, with patches of iron-grey to black due to sporulation.
Material examined: THAILAND, Chiang Mai Prov., Doi Inthanon, Hwy 1009 at 25 km marker, N18°32.54′, E98°33.51′, alt. 1076 m, on dead clumps of Bambusa sp. L. (Poaceae), 4 November 2012, I. C. Senanayake, CHUNI 33, (MFLU 16-1974, holotype), ex-type living culture, MFLUCC 13-0644.
Notes: Arthrinium paraphaeospermum is phylogenetically closely related to A. rasikravindrii and A. hydei (Fig. 130). However A. paraphaeospermum is phylogenetically distinct with high support (97/0.9, Fig. 130) and morphologically differs in having ellipsoid to clavate, brown, sterile tissues, elongated, conical conidiogenous cells and conidia with a median scar.
Bartaliniaceae Wijayaw. et al.
The family Bartaliniaceae was introduced by Senanayake et al. (2015) based on analysis of ITS and 28S rDNA sequence data, and includes Bartalinia, Broomella, Dyrithiopsis, Hyalotiella, Truncatella and Zetiasplozna. The polyphyletic genera were placed in this family based on similarities in morphology and their phylogeny (Senanayake et al. 2015; Maharachchikumbura et al. 2016). Members of Bartaliniaceae including Truncatella species produce secondary metabolites with potential use in biotechnological applications including control of plant diseases (Flores-Bustamante et al. 2010; Zhao et al. 2015c).
Truncatella was established by Steyaert (1949). The genus was previously placed in Amphisphaeriaceae, Xylariales, a family including 41 genera, although some genera are uncertain position (Lumbsch and Huhndorf 2010). The primary reason for introducing a separate genus is to accommodate a species which is distinct in having 3-septate conidia (Steyaert 1949; Maharachchikumbura et al. 2012). Molecular data has been used to evaluate pestalotiod fungi, including species of Truncatella (Jeewon et al. 2002; Lee et al. 2006; Maharachchikumbura et al. 2012). During a study on the Amphisphaeriales from an Abies firma leaf in Korea, a new genus that differed morphologically and phylogenetically (Fig. 132) from related species was isolated and is described as new.
Phylogenetic tree of Neotruncatella endophytica EML-AS5-1 and EML-AS5-2 and related species based on maximum likelihood analysis of ITS rDNA sequences. Sequence of Pestalotiopsis malayana was used as outgroup. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replications. The bar indicates the number of substitutions per position. New taxa are in blue
Neotruncatella Hyang B. Lee & T.T.T. Nguyen, gen. nov.
MycoBank number: MB 817422; Facesoffungi number: FoF02460
Etymology: The genus name “Neotruncatella” refers to the similarity with Truncatella
Endophyte of Abies firma. Sexual morph Undetermined. Asexual morph Conidiomata globose to subglobose, black. Conidiogenous cells hyaline, ampulliform to subcylindrical, formed from the inner cells of the peridial wall. Conidia fusiform with rounded ends, yellow to yellowish-brown at maturity, mostly 3-septate; apical appendages short.
Type species: Neotruncatella endophytica Hyang B. Lee, P.M. Kirk, K.D. Hyde, S.S.N. Maharachch., & T.T.T. Nguyen
Neotruncatella endophytica Hyang B. Lee, P.M. Kirk, K.D. Hyde, S.S.N. Maharachch., & T.T.T. Nguyen, sp. nov.
MycoBank number: MB 816958; Facesoffungi number: FoF02478, Fig. 133
Neotruncatella endophytica (holotype). a, d Colonies in potato dextrose agar (PDA). b, e Colonies in oatmeal agar (OA). c, f Colonies in malt extract agar (MEA) (a–c obverse view, d–f reverse view). g, h Conidioma and peridium. i–k Conidia attached to conidiogenous cells. l 3-septate conidia with apical and basal appendages. Scale bars g = 50 μm, h–l = 20 μm
Etymology: endophytica, referring to the endophytic habitat
Holotype: EML-AS5-1
Endophyte of plants. Sexual morph Undetermined. Asexual morph Conidiomata globose to subglobose, black, 75–190 × 75–215 μm diam. Conidiogenous cells hyaline, phialidic, ampulliform to subcylindrical, formed from the inner cells of the peridial wall. Conidia fusiform with rounded ends, yellow to yellowish-brown at maturity, mostly 3-septate, 25–34 × 3–5 μm; apical cell slightly yellow, thin-walled, bearing a single apical appendage, 3–5 (av. 4) μm long; apical appendages short, 4.5–6.5 μm long; two median cells yellow to yellowish-brown; second cell from the base 7.5–11.5 μm long, third cell 6.5–10.5 μm long; basal cell conic or with a truncate base, slightly yellowish-white, 5–7 μm long, basal appendages present or absent, if appendages present, very shortly at base, 1–3.5 μm long.
Culture characteristics: Colonies on PDA reaching 82–85 mm diam. at 25 °C after 14 days, initially greenish-glaucous, but finally whitish with a white margin; reverse yellowish with irregular margin; on OA powdery white. The isolate was observed to grow over a wide range of temperatures with average growth rates of EML-AS5-1 on PDA, MEA, and OA of 6.8 mm, 5.8 mm, and 6.5 mm per 24 h, respectively. Optimal growth was observed around 23 °C–27 °C, slow growth was observed at below 15 °C, and no growth at 35 °C (Fig. 132). Sporulation is excellent on oatmeal agar and malt extract agar.
Notes: Neotruncatella is morphologically distinct from Truncatella and Bartalinia by having narrower conidia with only a shorter apical appendage. In the phylogenetic trees, the ITS and 28S rDNA sequences (KX216520 and KX216518, respectively) of the strain formed a separate branch distinct with them, showing it represents a new species. In addition, ITS based phylogenies resulted in a clearly defined monophyletic subclade for our novel genus basal to Bartalinia (Fig. 132). Morphological comparison also reveals that Neotruncatella is characterized by a centric basal appendages as compared to Bartalinia.
Material examined: REPUBLIC OF KOREA, Jeonnam Province, garden of the Chonnam National University located in Gwangju (35°10′N 126°55′E), from a Abies firma leaf, 3 August 2015 (EML-AS5-1, holotype); EML-AS5-1 (ex-type) at Culture Collection of National Institute of Biological Resources (NIBR), Incheon, Chonnam National University Fungal Collection (CNUFC-EML-AS5-1) and Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:012333).
Cainiaceae J.C. Krug
The family Cainiaceae was introduced to accommodate species with a characteristic apical ring comprising a series of rings (Krug 1978). This family comprises Amphibambusa, Atrotorquata, Arecophila, Cainia, Ceriophora, Reticulosphaeria, Seynesia and Ommatomyces (Kohlmeyer and Volkmann-Kohlmeyer 1993; Hyde 1996; Senanayake et al. 2015, Maharachchikumbura et al. 2016). Rhabdospora-like species have been reported as the asexual morph of this family (Müller and Corbaz 1956).
Cainia Arx & E. Müll.
Cainia is typified by C. graminis (Niessl) Arx & E. Müll., and comprises eight species (Index Fungorum 2016). Members of Cainia occur on grasses and are characterized by immersed ascomata, cylindrical asci with a characteristic J+ apical ring with series of rings, brown, two-celled, ascospores with longitudinal striations, and ascospores surrounded by a mucilaginous sheath (Krug 1978; Senanayake et al. 2015). An updated tree for Cainia is provided (Fig. 134).
Consensus tree resulting from a maximum likelihood analysis of combined LSU and ITS sequence alignment of taxa in Xylariales. RAxML bootstrap support values (MLB above 50 %) and Bayesian posterior probabilities (PP above 50 %) are given at the nodes (MLB/PP). The newly introduced sequences are in blue bold. The scale bar represents the expected number of changes per site. The tree was rooted to Vialaea mangiferae MFLUCC 12-0808
Cainia globosa Senan., Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552136, Facesoffungi number: FoF02461, Fig. 135
Cainia globosa (MFLU 14-0738, holotype). a Appearance of ascomata on host substrate as minute clypeate ostiolar dots. b Section through ascoma. The blackened clypeal area is narrow. c Peridium. d Papilla. e Pseudoparaphyses. f Ascus in Melzer’s reagent showing lightly bluing apical apparatus. g–j Immature and mature unitunicate ascospores in asci. k–o Ascospores. p Mucilaginous sheath. q Conidiomata on MEA. r Cross section of conidiomata. s, t Conidiogenous cells with conidia. u Conidia. Scale bars a, q, r = 200 μm, b = 100 μm, c, d, g–j, s–u = 50 μm, e, f, k–p = 20 μm
Etymology: In reference to the globose ascospores.
Holotype: MFLU 14-0738.
Saprobic on standing culms of Anthoxanthum odoratum L. Sexual morph Ascomata 250–360 μm high × 210–315 μm diam. (\( \bar{x} \) = 326 × 264 μm, n = 10), solitary to scattered, immersed beneath clypeus, dark brown to black, globose to subglobose, uniloculate, coriaceous, with a central ostiole. Peridium 20–25 μm wide, composed of inner, thick-walled, compressed, hyaline cells of textura angularis and outer, dark brown to black cells of textura angularis. Hamathecium comprising 3–4 μm wide, septate, guttulate, sometimes branched, hyaline paraphyses. Asci 175–190 × 40–50 μm (\( \bar{x} \) = 188 × 46 μm, n = 20), 8-spored, unitunicate, cylindric-clavate, straight, short pedicellate, with a layered, apical ring, bluing lightly in Melzer’s reagent. Ascospores 30–35 × 14–17 μm (\( \bar{x} \) = 33 × 16 μm, n = 20), uni-seriate, ellipsoid to oval, rounded at both ends, hyaline when immature, becoming pale brown to dark brown when mature, medianly 1-septate, not constricted at the septum, guttulate when immature, striate, surrounded by a mucilaginous sheath. Asexual morph Conidiomata pycnidia, superficial, solitary, formed at the colony margins. Conidiophores reduced to conidiogenesis cells. Conidiogenous cells 4–7 × 1.5–3 μm, (\( \bar{x} \) = 5.5 × 2.4 μm), holoblastic, cylindrical, hyaline, unbranched, septate. Conidia 7–10 × 3.5–5 μm (\( \bar{x} \) = 8 × 4.4 μm, n = 20), globose to oval, 0–1-septate, brown, striate.
Culture characteristics: Colonies on MEA reaching 2 cm after 14 days at 18 °C, circular, dense, white, velvety, radiating towards the slightly undulate edge, not pigmented, after 4 weeks, small superficial, pycnidia formed mainly at margin of the colonies.
Material examined: ITALY, Province of Forlì-Cesena, Santa Sofia, near San Paolo in Alps, on dead culms of Anthoxanthum odoratum (Poaceae), 2 May 2013, E. Camporesi, IT 1217 (MFLU 14-0738, holotype), ex-type living culture, MFLUCC 13-0663.
Notes: Cainia globosa is morphologically distinct from other species in having ascospores with small guttules in each cell, a very lightly bluing bi-lobed apical ring and 1-septate, striate conidia. Cainia globosa clustered together with C. graminis and C. anthoxanthis. However C. globosa separates from these species with high support (85/0.9).The asexual morph of Cainia observed from a pure culture of C. desmazieresii was reported by Müller and Corbaz (1956) as Rhabdospora-like. However, Krug (1978) was unable to obtain the asexual morph from C. desmazieresii in culture (Kang et al. 1999). Here we observed a coelomycetous asexual morph from the culture (Fig. 135).
Pestalotiopsidaceae Maharachch. & K.D. Hyde
Pestalotiopsidaceae was introduced by Maharachchikumbura and Hyde (2015), which possesses pestalotiopsis-like asexual morphs (Senanayake et al. 2015, Maharachchikumbura et al. 2016). Six genera belong to Pestalotiopsidaceae; Ciliochorella, Monochaetia, Neopestalotiopsis, Pestalotiopsis, Pseudopestalotiopsis and Seiridium (Senanayake et al. 2015, Maharachchikumbura et al. 2016). Most taxa in Pestalotiopsidaceae are phytopathogens that cause a variety of diseases in plants and some are often isolated as saprobes or endophytes and are widely distributed throughout tropical and temperate regions (Guba 1961; Barr 1975; Nag Raj 1993; Maharachchikumbura et al. 2014a, b, 2016).
Ciliochorella Syd.
Ciliochorella was introduced by Sydow & Mitter (1935) with C. mangiferae Syd. as the type species. Seven species of Ciliochorella (C. bambusarum Shanor, C. buxifoliae Allegr., Eliades & Aramb., C. castaneae Munjal, C. eucalypti T.S. Viswan, C. indica Kalani, C. mangiferae Syd., C. splendida Nag Raj & R.F. Castañeda) are recorded in Index Fungorum (2016). Only two strains of Ciliochorella have sequence data (LSU) in GenBank (Tangthirasunun et al. 2015). Tangthirasunun et al. (2015) provided sequence data for the reference specimen of C. mangiferae found on dead leaves in Thailand.
One of the 16 equally most parsimonious trees obtained from combined analyses set of LSU, ITS and β-tubulin sequence data (CI = 0.627, RI = 0.821, RC = 0.514, HI = 0.373). MP values (>50 %) resulting from 1000 bootstrap replicates and Bayesian posterior probabilities above 0.95 are given at the nodes. The tree is rooted to Pseudopestalotiopsis theae (MFLUCC 12-0055). Ex-type strains are in bold and newly introduced species is in blue
Ciliochorella phanericola C. Norphanphoun, T.C. Wen & K.D. Hyde, sp. nov.
Index Fungorum number: IF552264; Facesoffungi Number: FoF02462, Fig. 137
Etymology: In reference to the host Phanera, and Latin cola meaning loving.
Holotype: MFLU 14-0776.
Pathogen or saprobic on leaf. Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata semi-immersed, circular areas, carbonaceous, black, mostly aggregated, sometimes solitary, in cross section 1000–1200 μm diam., 170–200 μm high. Peridium comprising a few to several layers of cell of textura angularis, with inner most layer thin, pale-brown, outer layer dark brown to black. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic phialidic, formed from the inner most layer of wall, hyaline to pale brown, smooth. Conidia (12.5–)13–15 × 2.8–3.5(–4) μm (\( \bar{x} \) = 15 × 3.7 μm, n = 30), allantoid to subcylindrical, 2-septate, hyaline to pale brown, smooth-walled; apical cell with 2 tubular apical appendages, 15–23 μm (\( \bar{x} \) = 20 μm), unequal; basal appendage 9–11.5 μm (\( \bar{x} \) = 11 μm). Sexual morph Undetermined.
Culture characteristics: Colonies on MEA, reaching 2.5 cm diam. after 7 days at 25 °C, producing dense mycelium, circular, margin rough, white, without aerial mycelium.
Material examined: THAILAND, Chiang Rai, Mae Fah Luang University, on dead leaves of Phanera purpurea (L.) Benth. (Leguminosae), August 2014, Ausana Mapook (MFLU 14-0776, holotype; KUN, isotype); ex-type-living cultures, MFLUCC 14-0984, KUMCC.
Notes: Phylogenetic analyses based on maximum parsimony and Bayesian analyses of LSU, ITS and TUB sequence data, provide good evidence that our strain belongs in Ciliochorella. Phylogenetic analysis confirms C. phanericola is a new species with high support (Fig. 136). However, C. castaneae differs mainly by 0–2 septate, branched conidiophores occasionally reduced to conidiogenous cells (Allegrucci et al. 2011). Ciliochorella phanericola, has conidia of a similar size (12–17 × 2–4 μm) as C. mangiferae, the latter has fusiform, 3-septate conidia, while those of C. phanericola are allantoid to subcylindrical, 2-septate with longer basal and apical appendages.
Neopestalotiopsis Maharachch. et al.
Neopestalotiopsis species are important plant pathogens, causing post-harvest diseases, fruit rots, and leaf blights in plants worldwide. Pestalotiopsis was divided into three genera by Maharachchikumbura et al. (2014b), viz Neopestalotiopsis, Pestalotiopsis, and Pseudopestalotiopsis with Neopestalotiopsis being distinguished from Pseudopestalotiopsis and Pestalotiopsis by its versicolorous median cells. Conidiophores in Neopestalotiopsis are indistinct and often reduced to conidiogenous cells (Xu et al. 1999; Das et al. 2014, b; Maharachchikumbura et al. 2013, 2014b; Hyde et al. 2014; Jayawardena et al. 2015).
Neopestalotiopsis cocoës Norphanphoun, T.C. Wen & K.D. Hyde, sp. nov.
Index Fungorum number: IF552232; Facesoffungi Number: FoF02364, Fig. 139
Etymology: The specific epithet cocoes refers to the host.
Holotype: MFLU 15-0220.
Pathogenic on Cocos nucifera L. Sexual morph Undetermined. Asexual morph Conidiomata 170–260 × 140–190 μm (\( \bar{x} \) = 250 × 180 μm, n = 10), acervuli, with basal stroma and lateral wall of 1–2 layers, thin, wall cells pale brown, textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, discrete, simple, short, filiform. Conidia 19–22.5 × 7.5–9.5 μm (\( \bar{x} \) = 21 × 9.3 μm, n = 30), fusiform to ellipsoid, straight to slightly curved, 4-septate; basal cell conic, hyaline, thin and smooth-walled, 3.4–4.6 μm long (\( \bar{x} \) = 4.5 μm); three median cells 13.4–14.5 μm long (\( \bar{x} \) = 14 μm), brown to pale brown, versicolored, verruculose; second cell from base pale brown to olivaceous, 3.8–5.5 μm (\( \bar{x} \) = 5.2 μm); third cell darker brown to olivaceous, 4.2–5.5 μm (\( \bar{x} \) = 5.5 μm); fourth cell darker brown, 3.6–4.9 μm (\( \bar{x} \) = 4–3 μm); apical cell 2.1–3.2 μm long (\( \bar{x} \) = 2.3 μm), hyaline, cylindric to subcylindric; apical appendages 14.9–21 μm long (\( \bar{x} \) = 19.6 μm), 2–3 (mostly 3), basal appendage present (1 or rarely absent), filiform 3.2–8.1 μm (\( \bar{x} \) = 6 μm).
Culture characteristics: Colonies on MEA, reaching 4 cm diam. after 7 days at 25 °C, producing dense mycelium, circular, rough margin white.
Material examined: THAILAND, Chiang Rai, Mushroom Research Foundation, on leaf of Cocos nucifera L. (Arecaceae), 4 February 2015, K.D. Hyde (MFLU 15-0220, holotype; KUN, isotype); ex-type-living cultures, MFLUCC 15-0152, KUMCC.
Notes: Neopestalotiopsis cocoës was associated with leaf blight disease of Cocos nucifera. In the phylogenetic analyses Neopestalotiopsis egyptiaca Ismail et al., is sister to our strain (Fig. 138). However, this species differs from N. cocoës in having larger conidia (22.5–28 μm). The conidia of N. cocoës are also wider (7.5–9.5 μm) than those of N. egyptiaca (6–7.5 μm) (Jayawardena et al. 2016) (Fig. 139).
Maximum parsimony (MP) majority rule consensus tree for the analyzed Neopestalotiopsis isolates based on a combined dataset of ITS, LSU, TUB and TEF sequence data. Values above or below the branches indicate maximum parsimony bootstrap equal or greater than 60 %. The tree is rooted with Pestalotiopsis trachicarpicola (OP068). The strain numbers are mentioned after the species names. The species obtained in this study are shown in blue bold and ex-type strains are in black bold
Neopestalotiopsis cocoës (holotype). a Leaf bright disease on coconut. b, c Conidiomata on host substrate. d Cross section of conidioma. e, f Conidiogenous cells with developing conidia. g Immature conidia. h–l Mature conidia. m Colony on PDA. Scale bars a = 2 mm, b = 500 μm, c = 250 μm, d = 100 μm, e, f = 20 μm, g–l = 15 μm
Neopestalotiopsis musae Norphanphoun, T.C. Wen & K.D. Hyde, sp. nov.
Index Fungorum number: IF552233, Facesoffungi Number: FoF02363, Fig. 140
Neopestalotiopsis musae (holotype). a Leaf bright disease on banana. b, c Conidiomata on host substrate. d Cross section of conidioma. e Peridium. f Apex. g, h Conidiogenous cells with developing conidia. i–j Mature conidia. k Germinating spore. l Colony on PDA. Scale bars d, e = 50 μm, f–h = 20 μm, i–j = 15 μm, k = 25 μm
Etymology: Named after the host genus on which the fungus occurs.
Holotype: MFLU 16-1279.
Pathogenic on Musa sp. Sexual morph Undetermined. Asexual morph Conidiomata 175–180 × 110–160 μm (\( \bar{x} \) = 175 × 140 μm), acervuli, with basal stroma and lateral wall 1–3 cells thick; the wall cells hyaline, textura angularis. Conidiophores reduced to conidiogenous cells. Conidiogenous cells enteroblastic, discrete, simple, short, filiform. Conidia 18.6–25 × 4.1–5 μm (\( \bar{x} \) = 20.5 × 4.5 μm), fusiform to ellipsoid, straight to slightly curved, 4-septate; basal cell conic, hyaline, thin and smooth-walled, 3.2–5.2 μm long (\( \bar{x} \) = 3.5 μm); three median cells 12.1–16 μm long (\( \bar{x} \) = 15 μm), hyaline, versicolored, verruculose; second cell from base pale brown to olivaceous, 4.1–6.1 μm (\( \bar{x} \) = 5 μm); third cell pale brown to olivaceous, 3.2–6.1 μm (\( \bar{x} \) = 5.5 μm); fourth cell pale brown to olivaceous, 3.3–6 μm (\( \bar{x} \) = 4.5 μm); apical cell 3.6–5.2 μm long (\( \bar{x} \) = 3.7 μm), hyaline, cylindric to subcylindric; apical appendages 16.3–25 μm long (\( \bar{x} \) = 16 μm), 2–3 (mostly 3), basal appendage filiform, 4.6–10.3 μm long (\( \bar{x} \) = 5 μm).
Culture characteristics: Colonies on PDA, reaching 5 cm diam. after 10 days at 25 °C, producing dense mycelium, circular, rough margin white, after 2 weeks, flat or effuse on the surface, without aerial mycelium.
Material examined: THAILAND, Chiang Rai, Pha Chang, on leaf of Musa sp. (Musaceae), 4 February 2015, Chada Norphanphoun (MFLU 16-1279, holotype; KUN, isotype); ex-type-living cultures, MFLUCC 15-0776, KUMCC.
Notes: Molecular analysis provides good evidence that our strain belongs to Neopestalotiopsis and is closest to N. honoluluana Maharachch. et al. and N. zimbabwana Maharachch. et al. (Fig. 138). However, N. honoluluana and N. zimbabwana has longer and wider conidia (\( \bar{x} \) = 28 × 8.3 μm, = 25.3 × 7.7 μm, respectively) with N. honoluluana having three dark median cells, unlike N. musae which has smaller conidia (\( \bar{x} \) = 20.5 × 4.5 μm), and pale-brown median cell of conidia (Maharachchikumbura et al. 2014b).
Pestalotiopsis sequoiae W.J. Li, Camporesi & K.D. Hyde, sp. nov.
Index Fungorum number: IF552215; Facesoffungi number: FoF02352, Fig. 141
Etymology: Named after the host genus Sequoia.
Holotype: 16-1489.
Saprobic on dead stem of Sequoia sempervirens (Lamb. ex D. Don) Endl. (Cupressaceae). Sexual morph Undetermined. Asexual morph Coelomycetous. Conidiomata 160–305 μm diam. × 162–326 μm high, black, solitary to aggregated or confluent, subperidermal in origin, erumpent at maturity, pycnidioid, globose to subglobose, unilocular, glabrous, straw-coloured with dark center; wall 10–20 μm wide, composed of thin-walled, hyaline cells of textura angularis to textura globulosa. Conidiophores reduced to conidiogenous cells. Conidiogenous cells 4–24 μm long × 2–4 μm wide, hyaline, enteroblastic, lageniform to subcylindrical, arising from the cells lining the inner wall of the pycnidium. Conidia 21–30 × 7.5–10 μm ( = 25 × 8.5 μm, n = 30 μm), fusiform, 4-septate, straight or slightly curved, bearing tubular, unbranched, flexuous appendage at both ends, basal cell 2.9–5.7 μm (\( \bar{x} \) = 4.3 μm) long, hyaline, obconic with a truncate base, 3 median cells, together 14.7–20 μm long (second cell from base 4.1–7 (\( \bar{x} \) = 5.6 μm, third cell 5.4–6.9 μm (\( \bar{x} \) = 6.2 μm), fourth cell 4.6–6.7 μm (\( \bar{x} \) = 5.6 μm), cylindrical, pale brown to brown and concolourous, septa and periclinal wall darker, apical cell 2.9–4.8 μm (\( \bar{x} \) = 4 μm) long, hyaline, conical, with mostly 4 apical appendages, 3–17 μm (\( \bar{x} \) = 11 μm) long, with independent loci of origin along upper half of the apical cell, often forming aggregated crest, basal appendage 4–11 μm (\( \bar{x} \) = 6 μm) long, single, unbranched.
Culture characteristics: Colony on PDA reaching 40–50 mm diam. in 7 days, with circular margin, white and fluffy, dense, aerial mycelium on the surface, reverse similar in colour.
Material examined: ITALY. Province of Forlì-Cesena [FC]), Castrocaro Terme, on dead stem of Sequoia sempervirens (Cupressaceae), 20 October 2012, Erio Camporesi, IT-832 (MFLU 16-1489, holotype); ex-type living culture, MFLUCC 13-0399, ICMP; IT-832B (HKAS 95026, isotype); living culture, KUMCC 15-0642.
Notes: Phylogenetically, based on megablast search of NCBIs GenBank nucleotide database, the closest hit using ITS sequence is Pestalotiopsis hollandica (GenBank KM199328; Identities = 548/548(100 %); Gaps = 0/548(0 %)), Followed by uncultured fungus clone (GenBank = KP889337; Identities = 549/550(99 %); Gaps = 0/550(0 %)), and P. monochaeta (GenBank = KM199327; Identities = 547/547(100 %); Gaps = 0/547(0 %)). Closest hits using the TEF sequence yielded highest similarity to P. hollandica (GenBank = KM199481; Identities = 237/238(99 %); Gaps = 1/238(0 %)), Pestalotiopsis sp. (GenBank = KP781881; Identities = 228/230(99 %); Gaps = 2/230(0 %)), P. verruculosa (GenBank = JX399061; Identities = 233/238(98 %); Gaps = 1/238(0 %)).
Morphologically, the conidia of Pestalotiopsis sequoiae is smaller than those of P. hollandica [(25–)25.5–33(–34) × 8.5–10(–10.5) μm], P. monochaeta [(25–)27–40(–42) × 7–11(–11.5) μm] and P. verruculosa [(28–35 × 9–11 μm ( = 30:6 × 10:3 μm)]. Based on morphology and phylogeny (Fig. 138), Pestalotiopsis sequoiae is introduced as novel species in Pestalotiopsis.
Xylariaceae Tul. & C. Tul.
The family Xylariaceae currently comprises 87 genera (Maharachchikumbura et al. 2016). Xylariaceous members are well-known for their secondary metabolite production (Stadler 2011). Xylariaceae has been traditionally classified into two subfamilies (Chesters and Greenhalgh 1964; Ju and Rogers 1996; Bitzer et al. 2008). In this study we introduce new species to genus Anthostomella.
Anthostomella ravennica Daranagama, Camporesi & K. D. Hyde, sp. nov.
Index Fungorum number: IF552286; Facesoffungi number: FoF02418, Fig. 143
Etymology: “ravennica” refers to the province from where it was first collected
Holotype: MFLU 16-0972
Saprobic on dead stem of Ammophila arenaria (L.) Link. Sexual morph Ascomata 170–190 × 180–200 μm (\( \bar{x} \) = 175 μm × 192 μm, n = 10), immersed, visible as black, irregular to dome-shaped areas, coriaceous, solitary, scattered, in cross section globose, with inconspicuous ostiole. Ostiole black, papillate. Peridium 30–50 μm wide (\( \bar{x} \) = 35 μm, n = 10), with two cell layers, outwardly comprising thick-walled, compressed, dark brown cells of textura angularis and inwardly comprising a few layers thin-walled, hyaline cells of textura angularis. Paraphyses 3.3–3.8 μm wide at base (\( \bar{x} \) = 3.5 μm, n = 30), shorter than the asci, numerous, filamentous, septate. Asci 116–127 × 11–13.6 μm (\( \bar{x} \) = 124 × 12.8 μm, n = 20), 8-spored, unitunicate, cylindrical, short pedicellate, with conspicuous, J+, discoid, apical ring, 0.7–1.5 × 0.2–0.8 μm (\( \bar{x} \) = 1.2 × 0.5 μm, n = 20). Ascospores 14–17 × 7–8 μm (\( \bar{x} \) = 16.2 × 7.6 μm, n = 20), uni-seriate, inequilaterally ellipsoidal, with one convex surface, pointed ends, light brown-dark brown, smooth-walled, without germ slit. Asexual morph Undetermined.
Culture characteristics: Colonies on OA 9 cm diam. after 4 weeks at 27 °C, white at the margins, pale yellowish at the center; reverse yellowish to cream, colony azonate, cottony appearance, dense.
Material examined: ITALY, Ravenna Province, Lido di Dante, on dead stems of Ammophila arenaria (L.) Link, 28 January 2015, E. Camporesi, IT 2358 (MFLU 16-0972, holotype), ibid. HKAS, isotype; ex-type living culture, MFLUCC 15-0012, KUMCC.
Notes: Anthostomella ravennica is morphologically reminiscent of A. zongluensis K.D. Hyde. However, the new species can be distinguished as the latter has larger ascomata with a central ostiolar canal, a thin peridium, while A. ravennica has irregular or sometimes dome-shaped ascomata, with an inconspicuous ostiole. Ascospores in A. zongluensis have conspicuous mucilaginous sheaths and asci have a distinct, J+, wedged-shaped apical ring, while ascospores in A. ravennica lack mucilaginous sheaths and asci have a J+, discoid apical ring which is inconspicuous. Due to these morphological differences we designate our collection as a new species. Molecular data also support A. ravennica to be phylogenetically distinct (Figs. 142, 143)
Consensus tree resulting from a Bayesian analysis of the LSU and ITS sequence of taxa in Sordariomycetes. RAxML bootstrap support values (MLB above 50) and Bayesian posterior probabilities (PP above 90 %) are given at the nodes (MLB/PP). The newly introduced sequences are in blue bold. The scale bar represents the expected number of changes per site. The tree was rooted to Sordaria fimicola (HKUCC3414)
Anthostomella thailandica Daranagama & K.D. Hyde, sp. nov.
Index Fungorum number: IF552287; Facesoffungi number: FoF02419, Fig. 144
Etymology: “thailandica” refers to the country it was first collected.
Holotype: MFLU 16-0971
Saprobic on leaves of grasses. Sexual morph Ascomata 150–200 × 140–155 μm (\( \bar{x} \) = 167 × 148 μm, n = 10), immersed, visible as black, irregular areas, coriaceous, solitary, sometimes clustered in groups of two, scattered, in cross section subglobose. Ostiole inconspicuous, without conspicuous papilla. Peridium 14–22 μm wide (\( \bar{x} \) = 17 μm, n = 10), comprising two cell layers, outwardly comprising thin-walled, loosely arranged, light brown cells of textura globosa and inwardly comprising a few layers of thin-walled, hyaline cells of textura angularis. Paraphyses 4.2–6.5 μm wide at base (\( \bar{x} \) = 4.9 μm, n = 30), as long as asci, few, filamentous, septate. Asci 87–118 × 14.1–17.8 μm (\( \bar{x} \) = 92.6 × 15.2 μm, n = 20), 8-spored, unitunicate, cylindrical-clavate, apedicellate, with conspicuous, J+, discoid apical ring, 3.1–3.8 × 1.1–1.9 μm (\( \bar{x} \) = 3.5 × 1.5 μm, n = 20). Ascospores 12–17 × 5.5–9 μm (\( \bar{x} \) = 15.4 × 7.2 μm, n = 20), uni-seriate, equilateral ellipsoidal, with broad ends, olivaceous, smooth-walled, with one large, central guttule, without germ slit. Asexual morph Undetermined.
Culture characteristics: Colonies on OA 9 cm diam. after 4 weeks at 27 °C, white at the margins, pale yellowish at the center; reverse yellowish to cream, colony azonate, cottony appearance, dense.
Material examined: THAILAND, Chiang Rai Province, Mae Fah Luang University garden, on dead leaves of a grass, 12 August 2015, D.A. Daranagama, KM24 (MFLU 16-0971, holotype), ibid. HKAS, isotype; ex-type living culture, MFLUCC 15-0017, KUMCC.
Notes: Our collection is reminiscent of both A. zongluensis K.D. Hyde and A. consanguinea (Ces.) Sacc. However, A. zongluensis has ascospores with more parallel sides, minutely verruculose walls and ascomata with periphysate ostiolar canals (Lu and Hyde 2000). Ascospores of A. thailandica are similar in size to those of A. consanguinea (Ces.) Sacc. However, ascospores of A. consanguinea have verruculose walls and a short, straight germ slit, while those in A. thailandica have a smooth wall and lack germ slits. In the phylogenetic analysis A. thailandica clusters in a separate clade with A. formosa Kirschst., A. conorum (Fuckel) Sacc., A. rubicola Speg. ex Sacc. & Trotter and A. obesa Daranagama, E. Camporesi & K.D. Hyde.
Sordariomycetes incertae sedis
Sporidesmiaceae Fr.
The family Sporidesmiaceae was introduced by Fries (1849), but the taxon has not been widely used in modern classification. The family is typified by Sporidesmium Link with S. atrum Link as the type species. Su et al. (2016a) treated Ellisembia as a synonym of Sporidesmium based on molecular data and placed in the presently monotypic Sporidesmiaceae. Based on molecular data, Sporidesmiaceae shows a sister relationship to Papulosaceae and Trichosphaeriaceae (Fig. 145).
Phylogram generated from Bayesian analysis based on combined LSU and ITS sequence data from species of Sordariomycetes. Maximum parsimony/likelihood bootstrap support values greater than 50 % and Bayesian posterior probabilities greater than 0.90 are shown in above and below. The new isolates are in red and other ex-type strains are in bold. The tree is rooted with Sordaria fimicola
Sporidesmium Link
The genus Sporidesmium was typified by S. atrum. Ellis (1958) considered S. ehrenbergii be the same as the type. Sporidesmium is characterized by unbranched or sparingly branched conidiophores; monoblastic or percurrently proliferating conidiogenous cells with broad scar; euseptate, rostrate conidia, sometimes with a mucilaginous apex (Shenoy et al. 2006; Seifertk et al. 2011). Sporidesmium is morphologically similar to the distoseptate genus Ellisembia. However, Su et al. (2016a) considered the euseptate/distoseptate difference between Sporidesmium and Ellisembia to have little taxonomic significant and that did not appear to be supported by any molecular data. Sporidesmium is shown to be polyphyletic based on phylogenetic studies (Shenoy et al. 2006; Su et al. 2016a).
Sporidesmium pyriformatum J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF552227; Facesoffungi number: FoF02245, Fig. 146
Etymology: Referring to the pyriform conidia.
Holotype: MFLU 15-1155
Saprobic on submerged wood in a stream. Colonies on substrate superficial, effuse, dark brown, hairy. Mycelium mostly immersed, composed of branched, septate, hyaline to pale brown hyphae. Sexual morph Undetermined. Asexual morph Conidiophores macronematous, mononematous, solitary or caespitose, cylindrical, straight or slightly flexuous, smooth, 2–5-septate, brown, paler towards the apex, 65–190 × 3–5.5 μm (\( \bar{x} \) = 118.5 × 4 μm, n = 20). Conidiogenous cells holoblastic, integrated, lageniform, terminal, brown to olivaceous, 9–24.5 × 4–7 μm, darkened and truncate at the apex, with up to three lageniform percurrently proliferating. Conidia acrogenous, solitary, pyriform or obclavate, 3–4-euseptate, the lower 3–4 cells olivaceous to brown, with pale brown to subhyaline apical cell, darkened at the septa, 18.5–32.5 × 8–15.5 μm (\( \bar{x} \) = 25.5 × 12 μm, n = 20).
Culture characteristics: Conidia germinating on PDA within 24 h. Germ tubes produced from the apex. Colonies on MEA reaching 10–15 mm diam. after 2 weeks at 25 °C, white in the middle, pale yellow at edge, with dense white mycelium on surface, sparser to the edge; in reverse with a yellow middle with yellowish irregular margin.
Material examined: THAILAND, Prachuap Khiri Khan Province, Hua Hin, stream flowing outside Kaeng Krachan National Park, on submerged wood, 25 December 2014, Jaap van Strien Site4-26-3 (MFLU 15-1155, holotype); ex-type living culture, MFLUCC15-0620; ibid. (MFLU 15-1162, paratype).
Note: Phylogenetic analysis of combined LSU and ITS sequence data, supports the placement of our taxon within Sporidesmium and reveals a close relationship (Fig. 145) to S. parvum (S. Hughes) M.B. Ellis and S. fluminicola H.Y. Su & K.D. Hyde. We therefore introduced this as a new species, Sporidesmium pyriformatum. The conidial shape of S. pyriformatum resembles those of S. penzigii Cooke & Ellis, S. jasminicola M.B. Ellis, S. bambusinum N.D. Sharma, S. fraxini-paxianae Jian Ma & X.G. Zhang, S. submersum H.Y. Su & K.D. Hyde, S. parvum and S. fluminicola. However, the conidia of the new taxon are larger than those of S. parvum (18–24 × 8–9 μm), but smaller than those of the other taxa that listed above. Sporidesmium pyriformatum is distinguished from the newly introduced taxa S. submersum and S. fluminicola in having much longer conidiophores and fewer septa (Su et al. 2016a).
Sporidesmium aquaticivaginatum J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF552229; Facesoffungi number: FoF02369, Fig. 147
Sporidesmium aquaticivaginatum (MFLU 15-1159, holotype). a Colonies on wood. b, c Conidiophores with conidia. d Conidium developing on the apex of conidiophore. e Conidiophores. f–j Conidia. k Germinated conidium on PDA medium. l, m Culture, l from above, m from below. Scale bars a = 100 μm, b–k = 30 μm
Etymology: Referring to the aquatic habitat and the mucilaginous sheath.
Holotype: MFLU 15-1159
Saprobic on decaying plant twigs. Sexual morph Undetermined. Asexual morph Colonies effuse, olivaceous, hairy. Mycelium partly immersed, partly superficial on the substrate. Conidiophores macronematous, mononematous, solitary or sometimes caespitose, cylindrical, straight or slightly flexuous, smooth, dark brown, triangular or globose at the base, 1–3-septate, 60–125 × 4–6 μm (\( \bar{x} \) = 82.5 × 5 μm, n = 15). Conidiogenous cells holoblastic, monoblastic, integrated, terminal, determinate or with one percurrent proliferation, cylindrical, smooth, mid to dark brown. Conidia acrogenous, solitary, dry, olivaceous to pale brown, paler at the apex, obclavate, tapering gradually towards the apex, smooth, straight or curved, 6–10-distoseptate, sometimes with a mucilaginous sheath, 49.5–80.5 × 10.5–14 μm (\( \bar{x} \) = 66.5 × 12 μm, n = 20), rounded and 2.3–3.5 μm at the apex, with a thick, flat, colourless, sheath extending from the tip to half way down, truncate and 3.5 × 5 μm at the base with conspicuous darkened scars.
Culture characteristics: Colonies on PDA reaching 10–15 mm diam. after 4 weeks at 25–30 °C, colony from above, dark greenish in the middle becoming greyish-green at the umbonate margin; from below, greyish-green in the centre and the third layer, dark greenish to black at the second layer and the edge; medium raised to umbonate in the middle.
Material examined: THAILAND, Prachuap Khiri Khan Province, Hua Hin, stream flowing outside Kaeng Krachan National Park, on submerged wood, 25 December 2014, Jaap van Strien, Site4-44-2 (MFLU 15-1159, holotype); ex-type living culture, MFLUCC15-0624; ibid. (HKAS95046, isotype).
Note: The combined LSU and ITS phylogenetic analyses indicate Sporidesmium aquaticivaginatum represents a sister taxon to S. olivaceoconidium within the Sporidesmiaceae (Fig. 145). In conidial shape and sheath, S. aquaticivaginatum resembles S. bambusae M.B. Ellis, S. minigelatinosa Matush., S. novozymium W.P. Wu. and S. palmicola W.P. Wu. Conidia in these species are characterized by a globose to subclavate, mucilaginous sheath at the apex. The conidiophores of S. aquaticivaginatum (60–125 × 4–6 μm) are obviously longer than those of S. bambusae (30–70 × 4–5 μm), S. minigelatinosa (40–100 × 4–5 μm) and S. palmicola (20–35 × 4–6 μm), but shorter than S. novozymium (150–200 × 6–8 μm). The conidia of S. hainanense (40–50 × 10–13 μm) and S. minigelatinosa (45–52 × 8–12 μm) are smaller than the conidia of S. aquaticivaginatum (49.5–80.5 × 10.5–14 μm). Moreover, among all these species, S. hainanense and S. palmicola are described as having euseptate conidia while in others the conidia are euseptate and distoseptate. The olivaceous conidia with a basal scar of S. aquaticivaginatum is clearly distinguishable from the brown or dark brown conidia of S. bambusae, S. minigelatinosa, S. novozymium and S. palmicola.
Sporidesmium olivaceoconidium J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF552230; Facesoffungi number: FoF02368, Fig. 148
Sporidesmium olivaceoconidium (MFLU 15-1175). a Substrate. b Colonies on wood. c, d Conidiophores. e Conidiogenous cell. f Fruiting body. g–k Conidia. l Germinated conidium on PDA medium. m, n Culture, m from above, n from below. Scale bars b = 50 μm, c–e, h–k = 15 μm, f = 20 μm, g = 30 μm, l = 40 μm
Etymology: Referring to the olivaceous conidia.
Holotype: MFLU 15-1175.
Saprobic on decaying plant twigs. Sexual morph Undetermined. Asexual morph Colonies effuse, olivaceous, hairy. Mycelium partly immersed, partly superficial on the substrate. Conidiohores macronematous, mononematous, solitary or sometimes caespitose, cylindrical, straight or slightly flexuous, smooth, dark brown, slightly swollen at the base, 1–3-septate, 25–55 × 3–4 μm (\( \bar{x} \) = 40 × 3.5 μm, n = 20). Conidiogenous cells holoblastic, monoblastic, integrated, terminal, determinate, cylindrical, lageniform, smooth, mid to dark brown, 11–15 × 3–5 μm (\( \bar{x} \) = 13 × 4 μm, n = 20). Conidia acrogenous, solitary, dry, olivaceous to pale brown, paler towards the tip, obclavate, smooth, straight or slightly curved, 6–10-septate, sometimes with a rounded mucilaginous sheath at the tip, 25–50 × 6–10 μm (\( \bar{x} \) = 40 × 8 μm, n = 20), rounded at the apex, truncate at the base.
Culture characteristics: Conidia germinating on PDA within 24 h and germ tube produced from the apex. Colonies on MEA slow-growing, reaching 5–10 mm diam. in 14 days, with dense white mycelium in the center, becoming sparse at the edge, folded in the middle; in reverse, brown in the middle and paler, smooth in the margin.
Material examined: THAILAND, Chiang Rai Province, stream flowing in Tham Luang Nang Non Cave, on submerged wood, 25 November 2014, Jing Yang YJ-14 (MFLU 15-1175, holotype), ex-type living culture, MFLUCC 15-0380, GZCC 16-0008.
Notes: The placement of Sporidesmium olivaceoconidium is revealed by molecular data within Sporidesmiaceae as sister taxon to S. aquaticivaginatum (Fig. 145). However, the phylogenetic tree contains a single long branch of the taxa S. olivaceoconidium and S. aquaticivaginatum with all other branches being short. It requires more collections for the future study of these two taxa. Sporidesmium olivaceoconidium is morphologically similar to S. aquaticivaginatum. It differs from S. aquaticivaginatum by the shorter conidiophores and smaller conidia.
Distoseptisporaceae K.D. Hyde & McKenzie
The family was introduced by Su et al. (2016a) and we follow this here. We introduce a new species of Distoseptispora and provide an updated tree (Fig. 145).
Distoseptispora K.D. Hyde et al.
We follow Su et al. (2016a)
Distoseptispora multiseptata J. Yang & K.D. Hyde, sp. nov.
Index Fungorum number: IF552206; Facesoffungi number: FoF02244, Fig. 149
Distoseptispora multiseptata (MFLU 15-1144, holotype). a Colonies on wood. b–e Conidiophores and conidia. f–j Conidia. k Conidiophore. l Germinated conidium on PDA medium. m, n Culture on MEA medium, m from above, n from below. Scale bars a = 200 μm, b–g = 50 μm, h, i = 40 μm, j, k = 20 μm, l = 100 μm
Etymology: Referring to the multi-septate conidia.
Holotype: MFLU 15-1144.
Saprobic on wood submerged in stream. Colonies effuse, dark olive-green, hairy or velvety. Mycelium mostly immersed, comprised of branched, septate, smooth, hyaline to pale brown hyphae. Sexual morph Undetermined. Asexual morph Conidiophores macronematous, mononematous, solitary, brown, 2–3-septate, straight or slightly flexuous, erect, slightly tapering distally, truncate at the apex, olive-green to mid brown, 23–65 × 4.5–8.5 μm (\( \bar{x} \) = 41.5 × 6 μm, n = 20). Conidiogenous cells holoblastic, monoblastic, integrated, terminal, brown, determinate, cylindrical. Conidia acrogenous, solitary, obclavate, rostrate, multi-distoseptate, tapering towards the apex, dark olivaceous green, 95–290 μm (\( \bar{x} \) = 160 μm, n = 30) long, 11–20 μm (\( \bar{x} \) = 16 μm, n = 30) wide at the broadest part, rounded and 5.5–9 μm (\( \bar{x} \) = 7.5 μm, n = 30) wide at the apex, basal cell subcylindrical, truncate and 3–5.5 μm (\( \bar{x} \) = 4 μm, n = 30) wide at the base. Conidial secession schizolytic.
Culture characteristics: Conidia germinating on PDA within 24 h. Germ tubes produced from both ends. Colonies on MEA reaching 10–15 mm diam. after 2 weeks at 25 °C, greyish-green on the surface, with dense, fluffy mycelium folded in the middle, dark green in reverse, with smooth margin.
Material examined: THAILAND, Prachuap Khiri Khan Province, Hua Hin, a stream flowing outside Kaeng Krachan National Park, on decaying submerged wood, 25 December 2014, Jaap van Strien, Site4-4-2 (MFLU 15-1144, holotype), ex-type culture, MFLUCC 15-0609, GZCC; ibid. (HKAS 95045, isotype).
Notes: Distoseptispora multiseptata is placed within the recently established genus Distoseptispora and it is well-supported by both morphology and molecular data. Distoseptispora multiseptata clusters with D. fluminicola, D. tectonae and D. tectonigena. Distoseptispora multiseptata however, is distinguished by its dark olivaceous green conidia, while the conidia are yellowish to dark reddish brown in D. fluminicola, D. tectonae and D. tectonigena. The new species also differs from D. fluminicola and D. tectonae in having larger conidiophores and conidia, but smaller than those of D. tectonigena (Su et al. 2016a). Distoseptispora tectonigena is the only species in the genus having percurrently proliferating conidiogenous cells at the apex of conidiophores. Distoseptispora multiseptata resembles D. aquatica in conidia shape and colour, but differs by its larger conidia (Su et al. 2016a).
Distoseptispora tectonae Doilom & K.D. Hyde, sp. nov.
Index Fungorum number: IF552223; Facesoffungi number: FoF01877, Fig. 150
Etymology: Name refers to the host genus Tectona on which the fungus was collected.
Holotype: MFLU 15-3417.
Saprobic on dead twig and branch of Tectona grandis L.f. Sexual morph Undetermined. Asexual morph Colonies on natural substrate, superficial, numerous, hairy, dark brown, scattered, single or in groups. Conidiophores up to 40 μm long, 4–6 μm wide, macronematous, mononematous, simple, erect to slightly curved, unbranched, 2–4-septate, slightly constricted at the septa, pale brown to dark brown, cylindrical. Conidiogenous cells 7.5–9.5 × 3.5–5 μm, monoblastic, integrated, terminal, cylindrical. Conidia (90–)130–140(–170) × (11–)13–14(–16) μm (\( \bar{x} \) = 128 × 14 μm, n = 20), 3.5–5.5 μm wide at the protruding truncate base (rostrum), 20–28-distoseptate, flexuous, cylindric-obclavate, elongate, straight or slightly curved, rounded at the apex, obconically truncate at the base, verruculose, dark reddish brown, slightly paler towards the apex, thick-walled, smooth, secession schizolytic.
Culture characteristics: Conidia germinating on PDA within 24 h. Germ tubes produced at the apex and septa of conidia. Colonies on MEA reaching 8–10 mm diam. after 7 days in the dark at 25 °C (\( \bar{x} \) = 8.9 mm, n = 5), undulate, irregular shape, flat or effuse at the edge, velvety, brownish-grey (4E2) from above, olive brown (4F3) from below.
Material examined: THAILAND, Utaradit Province, on dead twig of Tectona grandis (Lamiaceae), 29 December 2012, M. Doilom (MFLU 15-3417, holotype), ex-type living culture MFLUCC 12-0291, MKT 030, ICMP 21156; Phrae Province, Song District, on dead branch of T. grandis, 30 October 2011, M. Doilom, (MFLU 15-3416), living culture MFLUCC 12-0288, MKT 025, ICMP 21154.
Notes: Distoseptispora tectonae is introduced here as a novel species based on its morphological and phylogenetic differences from known Distoseptispora species. Phylogenetically, D. tectonae lies in a subclade together with D. fluminicola McKenzie et al. 2016 and D. tectonigena sp. nov. (this study), but in a distinct lineage with support 67 %MPBS, 55 % MLBS and 1.00 PP (Fig. 145). The conidia of D. tectonae ((90–)130–140(–170) × (11–)13–14(–16)) are shorter and slightly narrower than those of D. fluminicola (125–250 × 13–15), D. adscendens ((80)350–500 × 15–18), and shorter and wider than D. tectonigena ((83–)148–225(–360) × (10–)11–12(–13)), but longer than those of D. leonensis ((38–)50–75(–85) × 11–15). The conidia of D. tectonae also have more septa than those of D. leonensis, but fewer than those of D. adscendens, D. fluminicola and D. tectonigena. In addition, the conidiophores of D. tectonae are shorter than those of D. adscendens, D. leonensis and D. tectonigena, but longer than D. fluminicola (Table 2).
Distoseptispora tectonigena Doilom & K.D. Hyde, sp. nov.
Index Fungorum number: IF552224; Facesoffungi number: FoF01878, Fig. 151
Etymology: Name refers to the host genus Tectona on which the fungus was collected,
gena are used for a second species from the same host.
Holotype: MFLU 15-3418.
Saprobic on dead twig of Tectona grandis. Sexual morph Undetermined. Asexual morph Colonies on natural substrate, superficial, numerous, hairy, dark brown, scattered, single or in groups. Conidiophores up to 110 μm long, 5–11 μm wide, macronematous, mononematous, simple, erect, straight to slightly curved, septate, slightly constricted at the septa, pale brown to dark brown, 2–6-septate, cylindrical. Conidiogenous cells 4–10 × 2–5.5 μm, monoblastic, integrated, terminal, lageniform or cylindrical, brown, smooth, with lageniform or doliiform. Conidia (83–)148–225(–360) (rostrum included) × (10–)11–12(–13) μm thick (\( \bar{x} \) = 170 × 11 μm, n = 30), 2.5–6 μm wide at the truncate base (rostrum), older conidia 20–46-distoseptate, flexuous, cylindric-obclavate, elongated, straight or slightly curved, verruculose, rounded at apex, sometimes percurrently proliferating 5–10 times at apex, obconically truncate at base, dark reddish-brown and slightly paler towards the apex, thick-walled, smooth, secession schizolytic.
Culture characteristics: Conidia germinating on PDA within 24 h. Germ tubes produced at the apex and other cells of conidia. Colonies on MEA reaching 6–13 mm diam. after 7 days in the dark at 25 °C (\( \bar{x} \) = 9.9 mm, n = 5), edge entire to undulate, flat or effuse, velvety, aerial, medium sparse, grey (7F1) from both above and below.
Material examined: THAILAND, Chiang Rai Province, Mae Lao District, on dead twig of Tectona grandis (Lamiaceae), 12 March 2012, M. Doilom (MFLU 15-3418, holotype), ex-type living cultures MFLUCC 12-0292, MKT 033, ICMP 21157.
Notes: Although phylogenies reveal a close affinity between D. fluminicola, D. tectonae and D. tectonigena (Fig. 145). Distoseptispora tectonigena differs from D. fluminicola and D. tectonae by its conidiophores and conidial dimensions, conidial septation and apex of conidia as well as by phylogenetic analyses. Conidiophores of D. tectonigena are longer than those of D. tectonae and D. fluminicola. Its conidia are longer and narrower than D. tectonae, shorter and narrower than D. fluminicola. The conidia have more septa than those of D. tectonae and D. fluminicola (Table 2). The conidial apex of D. tectonigena is sometimes rounded like that of D. tectonae, but sometimes D. tectonigena produces an annellidic apex (Table 2).
Sordariomycetidae, genera incertae sedis
Paracapsulospora Konta. & K. D. Hyde, gen. nov.
Saprobic on dead Metroxylon sagu (Arecaceae). Sexual morph Ascomata solitary, immersed, uniloculate, globose to subglobose, surrounded by a dark brown layer of peridium cells. Peridium composed of outer layers of black, thick-walled cells of textura angularis inner layers hyaline and thin-walled. Asci, 8-spored, unitunicate, cylindrical, long pedicellate. Ascospores uni-seriate, oblong to cylindrical, unicellular, hyaline, with two guttules, thin-walled, smooth-walled, have appendages with a distinct mucilaginous sheath. Asexual morph Undetermined.
Note: Paracapsulospora is introduced to accommodate a single species P. metroxyli which was collected from Metroxylon in Thailand. The genus is morphologically similar to Capsulospora and Xylochrysis in having cylindrical asci with uni-seriate and oblong to cylindrical, aseptate ascospores. Paracapsulospora is most similar to Capsulospora in having immersed ascomata, with uni-seriate, hyaline, oblong to cylindrical, aseptate ascospores. Paracapsulospora differs from Xylochrysis in forming immersed ascomata, with hyaline ascospores, while Xylochrysis forms ascostromata erumpent through host tissue by long neck and forms paraphyses, with pale brown ascospores However, these two genera can be distinguished as Paracapsulospora has appendages at both ends of ascospores and does not produce paraphyses at the centrum, whereas Capsulospora forms paraphyses buts lacks appendages. Phylogenetic analyses show that Paracapsulospora is close to the orders Magnaporthales and Amplistromatales but cannot be confidently assigned to these order due to weak support (Fig. 152). Based on the limited sequences data in this group, we could not clarify the natural placement of our genus. Thus, we treat the new genus in the Sordariomycetes genera incertae sedis.
Maximum likelihood phylogenetic tree generated by RAxML (GTR+G model) based on LSU sequence data. ML/MP values (>50 %) resulting from 1000 bootstrap replicates are given at the nodes and branches with Bayesian posterior probabilities greater than 0.90 are in bold. The original isolate or specimen numbers are noted after the species names. The tree is rooted to Colletotrichum asianum. Newly generated sequence is in red and other ex-type strains are in bold
Type species: Paracapsulospora metroxyli Konta. & K. D. Hyde
Paracapsulospora metroxyli Konta & K. D. Hyde. sp. nov.
Index Fungorum number: IF552257; Facesoffungi number: FoF02367, Fig. 153
Paracapsulospora metroxyli (holotype). a Appearance of ascomata on host substrate. b Close up of ascomata. c Peridium. d Section of ascoma. e–j Asci. k–q Ascospores. r Germinated ascospore. s Culture characters on MEA. Scale bars a = 1 mm, b = 200 μm, c = 10 μm, d = 100 μm, e = 50 μm, f–j = 20 μm, k–r = 5 μm
Etymology: The specific epithet “metroxyli” refers to host of which the taxon was collected.
Holotype: MFLU 15-0704
Saprobic on dead Metroxylon sagu (Arecaceae). Sexual morph Ascomata 87–95 μm high × 158–167 μm wide, solitary, immersed, uniloculate, globose to subglobose, surrounded by a dark brown layer of peridium cells. Peridium 9–17 μm wide, composed of outer layers of black, thick-walled cells of textura angularis, inner layers hyaline and thin-walled. Asci 93–100 × 3–4 μm (\( \bar{x} \) = 97 × 4 μm, n = 10), 8-spored, unitunicate, cylindrical, long pedicellate. Ascospores 9–11 × 1.7–2.6 μm (\( \bar{x} \) = 12.2 × 2.2 μm, n = 10), uni-seriate, oblong to cylindrical, unicellular, hyaline, with two guttules, thin-walled, smooth-walled, with a distinct mucilaginous sheath. Asexual morph Undetermined.
Culture characteristics: Ascospores germinating after 24 h on MEA. Colony on MEA reaching 1.5–2.3 cm diam. after 13 weeks at 25 °C. Center of the colony grey, slightly wrinkled, margin uneven; reddish-brown pigment diffusing into the agar.
Material examined: THAILAND, Krabi Province, on dead Metroxylon sagu Rottb. (Arecaceae), 8 December 2014, S. Konta (MFLU 15-0704, holotype, HKAS 95031, isotype), ex-type living culture, MFLUCC 15-0250.
Basidiomycota
Agaricales Underw.
Clavariaceae Chevall.
Clavariaceae is a family of the order Agaricales and is characterised by erect, simple or branched, white, pale or brightly coloured basidiomata. The hyphal system is mono- or dimitic, with inflated hyphae or not, clamped or not. The cystidia are rare and the basidiospores are variable in shape, usually globose to ellipsoid, with smooth to ornamented walls, hyaline to pale yellow, and non-amyloid (Donk 1964). The family has seven genera and about 120 species (Kirk et al. 2008), widely distributed in the world (Corner 1950, 1970). They are reported as saprobes, ectomycorrhizal, and possibly biotrophs (Birkebak et al. 2013).
Clavulinopsis Overeem
A genus among the clavarioid fungi that comprises 33 species distributed worldwide (Kirk et al. 2008). The species are usually terrestrial, rarely lignicolous, solitary or gregarious, and characterized by white, yellow, orange or red, simple or branched basidiomata; hyaline or slightly yellow, globose or ellipsoid basidiospores, usually smooth, but echinulate in some species, and tramal hyphae and basidia with clamp connections (Corner 1950, 1970; Petersen 1978). However, the circumscriptions of Clavulinopsis sensu Corner (1950, 1970) and Petersen (1978) were not supported by Birkebak et al. (2013). The phylogenetic tree is presented in Fig. 154.
Phylogenetic tree of the Clavulinopsis and Ramariopsis obtained by analyses from rDNA sequences. Taxa with two accession numbers were analyzed by concatenated ITS and partial LSU rDNA; only the partial LSU rDNA was used to analyze the other individuals. Support values (from top) are maximum likelihood (ML) and Bayesian analyses. Sequences obtained in this study are in boldface. Only support values of at least 50 % are shown. The tree was rooted with Clavaria acuta
Clavulinopsis aurantiaca Araujo-Neta, Silva & Gibertoni, sp. nov.
MycoBank MB 816939; Facesoffungi number: FoF02463, Fig. 155
Etymology: aurantiaca (Latin) = orange-coloured, referring to the colour of the basidiomata.
Holotype: URM 84216.
Basidiomata simple, 1.5–3 cm, solitary or in small groups, slender, with sharp tips, acute, robust, orange (48), with whitish base (78 W) when fresh.
Basidiospores globose, rarely subglobose, 5–6 × 5–6 μm, Q = 1.01, hyaline, guttulate or not, thin-walled, with short apiculus (0.2–0.5 μm), IKI-. Hyphal system monomitic, tramal hyphae parallel, hyaline, abundantly clamped, 2–3 μm wide, inflated up to 10 μm, also clamped, subhymenium tightly interwoven. Cystidia of any kind absent. Basidia elongate-clavate, 35–37 × 5–9 μm, hyaline, guttulate, with 4 (rarely 5) stout sterigmata, 5–6 μm long, clamped at the base.
Material examined: BRAZIL, Pernambuco: Tamandaré, Reserva Biologica de Saltinho, August 2011, L.S. Araujo-Neta (URM 84216, holotypus; isotype in O); BRAZIL, Pernambuco, Tamandaré, Reserva Biológica de Saltinho, February 2011, L.S. Araujo-Neta (URM 84212), July 2011, L.S. Araujo-Neta (URM 84212), March 2012, L.S. Araujo-Neta (URM 84272), June 2013, L.S. Araujo-Neta & V.R. Coimbra 44NA (URM 85691), L.S. Araujo-Neta & V.R. Coimbra 45NA (URM 85692), L.S. Araujo-Neta & V.R. Coimbra 46NA (URM 85693).
Notes: This species was found as several solitary basidiomata scattered on soil and is characterized by the slender, flexible, and orange basidiomata when fresh, brittle when dry, and hyaline, mostly globose basidiospores.
The specimens of C. aurantiaca were placed in a separated, well supported clade (100/1.0) more closely related, but with little support (63/0.58), to Ramariopsis aurantio-olivacea R.H. Petersen, C. fusiformis (Sowerby) Corner, C. helvola (Pers.) Corner and C. laeticolor (Berk. & M.A. Curtis) R.H. Petersen (Fig. 154). These species have simple, orange basidiomata, but R. aurantio-olivacea has narrower, subglobose to ovate basidiospores (5.4–6.5 × 3.6–5 μm) and so far is restricted to New Zealand (Petersen 1988). Clavulinopsis fusiformis, C. helvola and C. laeticolor have been reported in the Neotropics (Petersen 1968; Corner 1950, 1970; Furtado et al. 2016), but C. helvola differs from C. aurantiaca by the angular basidiospores. Clavulinopsis fusiformis (Sowerby) Corner has, on average, larger, globose, subglobose to broadly ovate basidiospores [4.8–7.5(–9.2) × 4.5–7.2(–9.2) μm], while C. laeticolor (Berk. & M.A. Curtis) R.H. Petersen has broadly ellipsoid to subglobose basidiospores (4.3–7.5 × 3.5–6 μm).
Other species of Clavulinopsis with yellow to orange, simple basidiomata have been reported from the Neotropics. Clavulinopsis aurantiocinnabarina (Schwein.) Corner, however, has hyaline to pale yellow, subglobose basidiospores (5–7.1 × 4–7.1 μm), while C. calocera (G.W. Martin) Corner has longer, cylindrical to suballantoid basidiospores (8.5–10 × 4–5 μm). Clavulinopsis amoena (Zoll. & Moritzi) Corner presents pale yellow basidiomata and subglobose basidiospores (4–7 × 4–6.5 μm) (Petersen 1968; Corner 1950, 1970).
Ramariopsis (Donk) Corner
A genus among the clavarioid fungi that comprises 44 species distributed worldwide. The species have variously coloured, branched or rarely simple basidiomata; hyaline, ellipsoid or subglobose, finely verruculose or echinulate basidiospores, and tramal hyphae and basidia with clamp connections (Corner 1950, 1970). The species are terrestrial, rarely lignicolous, solitary or gregarious (Corner 1950, 1970; Petersen 1978). García-Sandoval et al. (2005) considered Ramariopsis sensu Corner (1950, 1970) as a monophyletic group, limiting their species to those with branched basidiomata, echinulate basidiospores and cyanophilous spore ornamentation derived from the tunica. According to Birkebak et al. (2013), the circumscriptions of Ramariopsis sensu Corner (1950, 1970) and Petersen (1978) were not supported. A phylogenetic tree is presented in Fig. 154.
Ramariopsis atlantica Araujo-Neta, Silva & Gibertoni, sp. nov.
MycoBank MB816940; Facesoffungi number: FoF02464, Fig. 156
Etymology: referring to the Atlantic Rain Forest.
Holotype: URM 84210.
Basidiomata branched, 5–3.2 cm, with dichotomous, erect and cylindrical branches, solitary to gregarious, white (2B) when fresh, beige to straw when dry (52B, 50S). Basidiospores broadly ellipsoid, 4 × 3 μm, Q = 1.33, hyaline to pale yellow, guttulate or not, echinulate, spines 0.2–0.5 μm, IKI-. Hyphal system monomitic, tramal hyphae hyaline to yellow in mass, 2–3 μm, conspicuously clamped, some inflated up to 10 μm, hardly clamped; subhymenium loose. Cystidia of any kind absent, but hyphal ends resembling gloeocystidia occasionally present. Hymenium collapsed, basidia not seen.
Material examined: BRAZIL, Pernambuco, Tamandaré, Reserva Biológica de Saltinho, July 2011, L.S. Araujo-Neta (URM 84210 holotype, isotype in O). BRAZIL, Pernambuco, Tamandaré, Reserva Biologica de Saltinho, July 2011, L.S. Araujo-Neta (URM 84213). A culture was obtained from URM 84213 and deposited in the URM Culture Collection (URM 6985).
Notes: Ramariopsis atlantica was collected as several basidiomata scattered on soil and is characterized by branched basidiomata, with dichotomous, cylindrical branches, inflated hyphae and echinulate, ellipsoid to subglobose basidiospores. Ramariopsis kunzei (Fr.) Corner has similar basidiomata (branched, whitish), but smaller basidiospores (3–4.5 × 2.5–3 μm, minutely echinulate, verruculose or asperulate).
Ramariopsis atlantica was placed in a separated, well supported subclade (100/1.0), closely related with good support (81/0.97) to R. aff. kunzei and R. tenuiramosa Corner (Fig. 154). The new species can be distinguished from R. tenuiramosa by the larger basidiospores (3.5–4.5 × 3–3.5 μm in R. tenuiramosa) (Corner 1950, 1970). Additionally, R. atlantica was distantly related to all specimens identified as R. kunzei from the USA and the UK.
Cortinariaceae R. Heim ex Pouzar
The limits of the family Cortinariaceae remain unknown. Currently, this family harbors about 12 genera, 2104 species (Kirk et al. 2008). The majority of the species in this family are in the genus Cortinarius. Many genera formerly placed in the Cortinariaceae, e.g., Phaeocollybia, Hebeloma, and Galerina, and some others have been moved to other families in Agaricales. On the other hand, the sequestrate genera, Thaxterogaster, Quadrispora, Protoglossum and Hymenogaster as well as Cuphocybe, Rapacea and species of Rozites, once thought to be genera within the Cortinariaceae, are currently included in the genus Cortinarius (Peintner et al. 2001, 2002). The basidiocarps range from agaricoid to sequestrate, and many have poorly to well-developed veils. The basidiospores are typically ornamented and cinnamon brown in deposit.
Cortinarius (Pers.) Gray
Cortinarius is the largest genus of Agaricales with a cosmopolitan distribution and over 2000 described species (Kirk et al. 2008). The species are important ectomycorrhizal fungi and are associated with trees and shrubs, belonging to the families Fagaceae, Salicaceae, Caesalpiniaceae, Cistaceae, Dipterocarpaceae, Myrtaceae, Rhamnaceae, Rosacea and Pinaceae, as well as some herbaceous plants in the Cyperaceae and Polygonaceae. Revealing the true diversity of species using only morphological and ecological characteristics has proven to be a difficult if not an impossible task. The use of DNA sequence data has made it possible to elucidate phylogenetic relationships within the genus, to show patterns of speciation, and to help define new, convergent and cryptic species (Fig. 157).
Phylogram resulting from the RAxML (Stamatakis 2014) analysis of ITS sequence data. Bootstrap values greater than 50 % are indicated above branches. The names in blue represent the species of Cortinarius presented in this paper and the specimens in boldface are type specimens of the species. The tree is rooted with Obtusi species
Cortinarius sect. Fulvescentes Melot and Cortinarius sect. Laeti Melot were initially included in subgenus Telamonia (Brandrud et al. 1998), but recent phylogenetic analyses suggest that they form a distinct lineage in genus Cortinarius (Peintner et al. 2004; Harrower et al. 2011; Garnica et al. 2016). These two sections were traditionally distinguished from one another by the colour of the universal veil; the species of the section Fulvescentes have pinkish to vinaceous universal veil and species of the section Laeti yellow to ochraceous veil, but sometimes the veil is very indistinct and difficult to observe. The phylogenetic analysis of Harrower et al. (2011) and Garnica et al. (2016), however, suggested that the division based on the veil colour is at least partly unnatural, and it seems a likely hypothesis based on our analysis too. Additional studies are needed to reveal the natural divisions within the clade Fulvescentes/Laeti. Characteristics of the species in these two sections are mat (dull and flat without a shine), hygrophanous pileus, silky-shiny fibrillose stipe, uniformly yellowish-brown context in the stipe, and indistinct smell. Below we propose a neotype for C. fulvescens Fr. and describe four new species belonging to this clade.
Cortinarius fulvescens Fr., Epicr. syst. mycol. (Upsaliae): 311 (1838)
Facesoffungi number: FoF02465, Fig. 158
a, f Cortinarius fulvescens 04-935 (neotype, H). b, g C. fulvescentoideus 03-1675 (H). c, i C. pseudobulliardioides 11-452 (holotype, H). d, j C. tenuifulvescens 04-572 (holotype, H). e, h C. nymphatus DBB21430 (UC). Scale bar 10 mm. (a–d Photographs by K. Liimatainen, e by D. Bojantchev. f–j Drawings by T. Niskanen and I. Kytövuori.)
Neotype: T. Niskanen 04-935 (H), designated here.
Pileus 25–50 mm diam., conical to hemisphaerical, later low convex with an umbo, narrowly pellucid-striate, surface mat, red brown to vinaceous red brown, hygrophanous. Lamellae adnexed, subdistant, moderately broad, moderately thick, at first light medium brown, becoming cinnamon brown, edges remaining pale for some time, then concolorous. Stipe 50–120 mm long, apex 3–9 mm thick, equal, whitish silky-fibrillose. Universal veil pale pink, very sparse, forming incomplete girdles on the stipe. Basal mycelium white. Context in pileus dark red brown, in stipe medium yellow brown. Odor indistinct. Basidia 4-spored, 8–9 × 27–34 μm, clavate. Basidiospores 7.9–9.5 × 4.5–5.2 μm, Q = 1.64–1.90, narrowly amygdaloid to narrowly amygdaloid-ellipsoid, moderately, often somewhat sharply verrucose, slightly to moderately dextrinoid. Lamella trama hyphae moderately to fairly strongly encrusted in MLZ. Pileipellis duplex (in MLZ): Epicutis hyphae 4–13 μm wide, smooth to encrusted. Hypoderm distinct, elements up to 30 × 65 μm. Trama hyphae 4–13 μm wide, encrusted. ITS sequence (holotype) distinct from the other known members of the clade Fulvescentes/Laeti, and differs from them in the ITS region by more than 14 substitutions and indel positions.
Ecology and distribution: In mesic to dry coniferous forests with Picea and/or Pinus and Tsuga. Known from eastern North America and Europe.
Material examined: CANADA, Québec, Montebello, mixed forest, under Tsuga canadensis, 27 September 2010, leg. K. Liimatainen & T. Niskanen 10-153, 10-169 (H). FINLAND, Varsinais-Suomi, Lohja, dry Pinus sylvestris heath forest on sandy soil with lime dust effect, 19 September 2004, leg. I. Kytövuori & T. Niskanen 04-866/H6030077 (H). Vihti, Sipilänmäki, Lintumäki, mesic Picea-dominated forest, 7 October 2001, leg. H. Tuovila & I. Kytövuori (H). Uusimaa, Kirkkonummi, submesic Picea abies dominated forest with some Betula and Populus tremula, in places herb-rich in others more Pinus dominated, 23 September 2004, leg. T. Niskanen 04-935, H6031281 (neotype, H; isoneotype, K). Uusimaa, Sipoo, Paippinen, dryish to mesic, mossy coniferous forest (Picea abies, Pinus sylvestris) with some Betula, 14 September 2004, leg. K. Liimatainen & T. Niskanen 04-792, H6029527 (H). Etelä-Häme, Orivesi, Päilahti, Ojanperä, mesic Picea abies forest with some Pinus sylvestris and deciduous bushes, 26 September 1995, leg. I. Kytövuori 95-1547 (H).
Notes: Cortinarius fulvescens is a medium-sized species and a typical member of traditional section Fulvescentes with mat, red-brown to vinaceous red-brown pileus, whitish silky-fibrillose stipe, pale pink universal veil, and uniformly yellow brown context in the stipe. It can be distinguished from most other species with pinkish to vinaceous veil by somewhat robuster appearance and the narrowly amygdaloid, somewhat more strongly verrusoce spores. The species was described by Fries (1838) and the short description fits well to our species, except the pale pinkish universal veil is not mentioned. This characteristics, however, is easy to miss since the veil is very sparse and sometimes invisible as e.g. in the basidiomata in the middle in Fig. 158. Fries also has two unpublished plates of C. fulvescens (S0313 and S0314, available at the Krypto-S database in the Swedish Museum of Natural History, http://andor.nrm.se/fmi/xsl/kryptos/kbo/publFindspecies.xsl?-view&-db=kbo_svampregister&-token.languagecode=en-GB), painted after the description of the species. They both represent typical species of traditional section Fulvescentes: the stature is slender, the colour of the pileus is brown to reddish-brown, flesh is brownish-yellow and they do not have any strong veil bands in the stipe. However, the two plates may represent two different species. The basidiomata presented in S0313 illustrate a species common to coniferous heath forests of Scandinavia. It also fits with the current interpretation of the species (Niskanen and Kytövuori 2012). We therefore propose specimen 04-935 as a neotype of the species.
Cortinarius fulvescentoideus Kytöv., Niskanen & Liimat., sp. nov.
Index Fungorum number: IF552368; Facesoffungi number: FoF02466, Fig. 158
Etymology: The name refers to affinity to C. fulvescens.
Holotype: K. Liimatainen & T. Niskanen 03-1634 (H).
Pileus 10–25 mm diam., conical to somewhat hemisphaerical, umbonate, later low convex to plane with an umbo or not, up to 1/2 pellucid-striate, surface mat, warm red-brown, hygrophanous. Lamellae adnexed, subdistant, moderately broad to broad, moderately thick, at first light medium brown, becoming rich brown, edges remaining pale for some time, then concolorous. Stipe 40–100 mm long, apex 3–5 mm thick, equal, whitish silky-fibrillose. Universal veil pale pink, very sparse, forming incomplete girdles on the stipe. Basal mycelium white. Context in pileus dark red-brown, in stipe medium yellow-brown. Odour indistinct. Basidia 4-spored, 8–9 × 30–35 μm, clavate. Basidiospores 8.2–9.5 × (4.5–)5–5.4 μm, Q = 1.64–1.76, ellipsoid to narrowly ellipsoid, finely to moderately verrucose, slightly to somewhat moderately dextrinoid. Lamella trama hyphae moderately to strongly encrusted in MLZ. Pileipellis duplex (in MLZ): Epicutis hyphae 5–12 μm wide, smooth to encrusted. Hypoderm distinct, elements up to 28 × 60 μm. Trama hyphae 5–13 μm wide, encrusted. ITS sequence (holotype) differs from the sister species C. fulvescens and C. tenuifulvescens more than 7 substitutions and indel positions.
Ecology and distribution: In mesic to moist coniferous forests with Picea and Tsuga, often in Sphagnum. Widely distributed and found from Japan, western North America and Europe.
Material examined: FINLAND, Etelä-Häme, Orivesi, Päilahti, mesic to moist Picea abies dominated forest with some Pinus sylvestris and hardwood bushes, 26 September 1995, leg. I. Kytövuori 95-1550 (H). SLOVAKIA, Liptovská kotlina basin, Važec, Važecké lúky, Picea abies forest, 29 September 2003, leg. K. Liimatainen & T. Niskanen 03-1634, H7018165 (holotype, H; isotype, K), 30 September 2003 leg. K. Liimatainen & T. Niskanen 03-1675, H7018157 (H).
Additional specimens: CANADA, British Columbia, cloneSWUBC478, root tip, GenBank no. DQ481834. JAPAN, Yamanashi, Mt. Fuji, Tsuga diversifolia, June 2011, spYM454 root tip, GenBank no. AB848431. U.S.A., Alaska, Bonanza Creek LTER, Site TKN0109, Picea mariana, 2004, clone3199N24,
Notes: Cortinarius fulvescentoideus looks like a slender C. fulvescens and forms a well-supported clade with C. fulvescens and C. tenuifulvescens but strains from two species are partitioned in two highly supported monophyletic subclades that support their delineation (Fig. 157). It is morphologically very similar to C. tenuifulvescens and the two species can easily be misidentified. Our data also indicates that they may have somewhat different ecologies; the collections of C. fulvescentoideus are made from mesic to moist coniferous forests, whereas C. tenuifulvescens prefers sandy, well-drained soils, at least in northern Europe. From the other members of the section Fulvescentes C. fulvescentoideus can most easily be distinguished by the spores. The spores of C. fulvescens are narrowly amygdaloid whereas the spores of C. bulliardioides, C. pseudobulliardioides and C. subfloccopus are broader, >5.5 μm wide. The spores of C. badiovinaceus are small, ovoid-subglobose.
Cortinarius nymphatus Kytöv., Niskanen, Liimat. & Bojantchev, sp. nov.
Index Fungorum number: IF551705; Facesoffungi number: FoF02467, Fig. 158
Etymology: Name based on a Greek word nymphe, meaning fairy, since the species is small and elegant
Holotype: I. Kytövuori 95-1549 (H)
Pileus 10–30 mm diam., at first conical to hemisphaerical, later low convex to almost plane with an umbo, surface mat, brown to dark red-brown, paler towards the margin, hygrophanous. Lamellae adnexed, subdistant, moderately broad, moderately thick, at first light brown, becoming medium brown, edges remaining pale for some time, then concolorous. Stipe 40–60 mm long, apex 2–4 mm thick, equal, whitish silky-fibrillose. Universal veil yellow to ochraceous, forming incomplete girdles on the stipe. Basal mycelium white. Context in pileus dark red-brown, in stipe medium yellow-brown. Odour indistinct. Basidia 4-spored, 6.5–8 × 25–30 μm, clavate. Basidiospores 6.8–8.2 × 4.3–4.8 μm, Q = 1.56–1.76, amygdaloid, finely to moderately, sharply verrucose, slightly to moderately dextrinoid. Lamella trama hyphae moderately encrusted in MLZ. Pileipellis duplex (in MLZ): Epicutis hyphae 4.5–10 μm wide, encrusted. Hypoderm distinct, elements up to 30 × 60 μm. Trama hyphae 5–13 μm wide, somewhat encrusted. ITS sequence (holotype) distinct from the other known members of the clade Fulvescentes/Laeti, and differs from them in the ITS region by more than 7 substitutions and indel positions.
Ecology and distribution: In dry to mesic coniferous forests (Pinus, Picea). Known from Europe, Canada, British Columbia.
Material examined: CANADA, British Columbia, near Squamish, temperate coniferous rainforest (Picea sitchensis, Pseudotsuga menziesii), 19 October 2009, leg. D. Bojantchev DBB21430 (UC). FINLAND, Etelä-Häme, Orivesi, Päilahti, mesic to moist Picea abies forest with some Pinus sylvestris and hardwood bushes, 26 September 1995, leg. I. Kytövuori 95-1549 (holotype, H; isotype, K). Kainuu, Suomussalmi, W of Hossa, dry Pinus sylvestris heath forest on sandy soil, 16 September 2002, leg. I. Kytövuori, K. Liimatainen & T. Niskanen 02-565 (H).
Notes: Cortinarius nymphatus looks like a small Fulvescentes species, but with a yellow-ochraceous veil. Also based on our phylogenetic analysis it seems to belong to section Laeti s. str. with the yellow-veiled type species, C. laetus, of section Laeti. With the combination of small fruiting bodies and small spores it can be distinguished from the other yellow veiled species of the clade Fulvescentes/Laeti.
Cortinarius pseudobulliardioides Kytöv., Niskanen, Liimat. & Ammirati, sp. nov.
Index Fungorum number: IF551704; Facesoffungi number: FoF02468, Fig. 158
Etymology: The name refers to affinity and similar appearance to C. bulliardioides.
Holotype: J.F. Ammirati & K. Liimatainen 11-452 (H).
Pileus 25–45 mm diam., at first conical, later low conical to almost plane with an umbo surface mat, dark reddish-brown, somewhat paler towards the margin, hygrophanous. Lamellae adnexed-emarginate, subdistant, moderately broad, moderately thick, at first light medium brown, becoming medium brown, edges remaining pale for some time, then concolor. Stipe 40–90 mm long, apex 4–7 mm thick, equal, whitish silky-fibrillose. Universal veil pink, forming broad, incomplete girdles on the stipe. Basal mycelium white. Context in pileus dark red brown, in stipe medium to light yellow brown. Odor indistinct. Basidia 4-spored, 9–11 × 35–40 μm, clavate. Basidiospores 8.4–10 × 5.5–6.6 μm, Q = 1.38–1.54, ellipsoid, finely verrucose, moderately dextrinoid. Lamella trama hyphae moderately to strongly encrusted in MLZ. Pileipellis duplex (in MLZ): Epicutis hyphae 6–14 μm wide, encrusted. Hypoderm distinct, elements up to 25 × 40 μm. Trama hyphae 5–12 μm wide, encrusted. ITS sequence (holotype) distinct from the other known members of the clade Fulvescentes/Laeti, and differs from them in the ITS region by more than 7 substitutions and indel positions.
Ecology and distribution: In coniferous forests (Abies, Picea, Pinus, Tsuga). Widely distributed and known from western North America and Europe.
Material examined: FINLAND, Koillismaa, Kuusamo, Oulanka National Park, forests close to Kiutaköngäs, dry Pinus sylvestris heath forest with some Picea and Betula on sandy and calcareous soil, 18 September 2002, leg. I. Kytövuori, K. Liimatainen & T. Niskanen 02-689 (H). U.S.A, Washington, Kittitas County, Table Mountain, coniferous forest (Abies, Picea, Pinus, Larix), 9 October 2011, leg. J.F. Ammirati & K. Liimatainen 11-452 (holotype, H; isotype, K).
Additional specimens: CANADA, British Columbia, Tsuga heterophylla, cloneSLUBC12.
Notes: Cortinarius pseudobulliardioides is a rather large species in clade Fulvescentes/Laeti, in appearance similar to sister species C. subfloccopus and somewhat more distantly related C. bulliardioides. Cortinarius subfloccopus, however, usually has vinaceous red universal veil and C. bulliardioides has broader spores, >6 μm wide.
Cortinarius tenuifulvescens Kytöv., Niskanen & Liimat., sp. nov.
Index Fungorum number: IF551703; Facesoffungi number: FoF02469, Fig. 158
Etymology: The species looks like a slender C. fulvescens.
Holotypus: K. Liimatainen & T. Niskanen 04-572, H6031278 (H)
Pileus 10–30 mm diam., conical to somewhat hemisphaerical, umbonate, later low convex to plane with an umbo or not, up to 1/3 pellucid-striate, surface mat, warm red-brown, hygrophanous. Lamellae adnexed, subdistant, moderately broad, moderately thick, at first light brown, becoming medium brown, edges remaining pale for some time, then concolorous. Stipe 40–110 mm long, apex 2–4 mm thick, equal, whitish silky-fibrillose. Universal veil pale pink, very sparse, forming incomplete girdles on the stipe. Basal mycelium white. Context in pileus dark red-brown, in stipe medium yellow-brown. Odour indistinct. Basidia 4-spored, 8–9 × 27–35 μm, clavate. Basidiospores 7.5–9.3 × 4.8–5.4 μm, Q = 1.48–1.70, ellipsoid to narrowly ellipsoid, finely to fairly finely, densely, evenly verrucose, slightly to somewhat moderately dextrinoid. Lamella trama hyphae moderately to strongly encrusted in MLZ. Pileipellis duplex (in MLZ): Epicutis hyphae 4.5–13 μm wide, smooth to encrusted. Hypoderm distinct, elements up to 30 × 70 μm. Trama hyphae 5–13 μm wide, encrusted. ITS sequence (holotype) distinct from the other known members of the clade Fulvescentes/Laeti, and differs from them in the ITS region by more than 7 substitutions and indel positions.
Ecology and distribution: In dry to mesic coniferous forests, in northern Europe often on sandy soil, one collection from spruce-hardwood swamp. Widely distributed and found from western and eastern North America and Europe.
Material examined: CANADA, Newfoundland, Avalon Peninsula, Butter Pot Provincial Park, mesic to damp Picea dominated forest with some Abies, Larix and Betula, 25 September 2007, leg. K. Liimatainen & T. Niskanen 07-292, H7000956 (H). FINLAND, Perä-Pohjanmaa, Rovaniemi, Pisavaara nature reserve area, 31 August 2004, leg. K. Liimatainen & T. Niskanen 04-572, H6031278 (holotype, H; isotype, K). SWEDEN, Jämtland, Berg, dry Pinus sylvestris heath forest with some Picea and Betula on sandy soil, leg. I. Kytövuori, K. Liimatainen & T. Niskanen 03-907, H7018168 (H). Ångermanland, Strömsund, Bodum, at a small sand pit, Picea-hardwood swamp, leg. I. Kytövuori 97-500b (H).
Additional specimens: CANADA, British Columbia, Mt. Washington, Trail to Rossiter Lake, in mossy detritus, 13 Sep 2001, leg. O. Ceska OC43, F17115 (UBC)
Notes: See discussion under C. fulvescentoideus.
Entolomataceae Kotl. & Pouzar
Entolomataceae was introduced by Kotlaba and Pouzar (1972) and typified by Entoloma, is highly variable in terms of sporocarp morphology and micromorphology (Noordeloos 2004). To date, this family harbors four genera, 1071 species (Kirk et al. 2008).
Rhodocybe Maire
The genus Rhodocybe is characterised by pink, vinaceous white or grey spore print and inamyloid, cyanophilic basidiospores that are angular in polar view and with an undulate-pustulate ornamentation (Baroni 1981; Singer 1986). Based on a molecular phylogenetic study, Kluting et al. (2014) split Rhodocybe sensu lato into four genera, namely Rhodocybe, Clitopilopsis, Rhodophana and Clitocella. The amended Rhodocybe now incorporates only clampless species (Kluting et al. 2014). In the course of our studies on the Entolomataceae of Kerala State, India, we came across three species of Rhodocybe, which were found to be new to science. They are described here based on both morphology and molecular phylogeny. Maximum likelihood analysis placed the three new species in Rhodocybe clade with significant (76 %) ML bootstrap support (Fig. 159)
RPB2-based phylogram generated from maximum likelihood analysis (RAxML) depicting the placement of Rhodocybe indica, R. luteobrunnea and R. griseoaurantia within the genus Rhodocybe. Values at nodes indicate bootstrap support. BS values ≥50 % are indicated above or below the nodes, new species are in blue bold. The tree is rooted with Tricholoma flavovirens (KC816997) and Catathelasma imperiale (KC816994)
Rhodocybe indica K.N.A. Raj & Manim., sp. nov.
MycoBank MB 816841; Facesoffungi number: FoF02179, Fig. 160
Etymology: The specific epithet refers to India, the country where this species was first observed.
Holotype: CAL 1323.
Basidiocarp small, mycenoid. Pileus 15 mm diam., broadly convex with a small umbo; surface brown (6E7/OAC599) on and around the umbo, brownish-yellow (5C7/OAC790) towards the margin, weakly hygrophanous and becoming slightly paler, faintly pellucid-striate towards the margin, glabrous, somewhat tacky; margin slightly incurved and somewhat wavy. Lamellae narrowly adnate to adnate, subventricose, close, greyish-orange (6B3/OAC695), up to 3 mm wide, with lamellulae of 3–7 lengths; edge crenulate, concolourous with the sides. Stipe 30 × 2 mm, central, equal, slightly flexuous, cartilaginous, solid; surface brownish-yellow (5C7/OAC790), glabrous to the naked eye, finely pruinose all over under a lens; base with white mycelial cords. Odour and taste not distinctive.
Basidiospores 6.5–8 × 5.5–7 (7.27 ± 0.49 × 6.1 ± 0.38) μm, (Q = 1–1.3, Qm = 1.19), subglobose or lacrymoid, undulate-pustulate, with or without a suprahilar depression in profile view, 6 angled in polar view, hyaline, thin-walled. Basidia 18–33 × 7–8 μm, narrowly clavate to clavate, pale yellow, thin-walled, 4-spored; sterigmata up to 5 μm long. Lamella-edge heterogeneous. Cheilocystidia and pleurocystidia present as pseudocystidia. Pseudocystidia 24–40 × 4–9 μm, scattered, fusiform, lanceolate or ventricose-rostrate, with glittering, yellow, granular contents, thin-walled. Lamellar trama subregular; hyphae 4–7 μm wide, hyaline or pale yellow, thin-walled. Subhymenium inconspicuous. Pileus trama subregular; hyphae 5–14 μm wide, pale yellow, thin-walled. Pileipellis an undifferentiated cutis, made up of closely septate and compactly arranged hyphae with widely scattered pileocystidia; hyphae 5–13 μm wide, slightly gelatinised, with a pale brownish-yellow wall pigment and fine hyaline encrustations, thin- to slightly thick-walled. Pileocystidia 21–42 × 3–11 μm, scattered, versiform: nettle hair-shaped, narrowly fusiform or flexuous, hyaline or pale yellow, thin-walled. Stipitipellis a cutis, rarely disrupted by flaring out hyphae; hyphae 3–9 μm wide, with a pale brownish-yellow wall pigment, thin-walled. Caulocystidia absent. Clamp connections not observed on any hyphae.
Habitat: on a decaying twig, solitary.
Specimens examined: INDIA, Kerala, Kollam District, Thenmala, Thenmala Forest, 17 August 2013, K. P. Deepna Latha DKP130 (CAL 1323, holotype).
Notes: Rhodocybe indica is well-characterized by its small basidiocarps with a brownish-yellow, umbonate pileus; subglobose or lacrymoid basidiospores; smaller, fusiform, lanceolate or ventricose-rostrate pseudocystidia; a pileipellis with pileocystidia and a stipitipellis lacking encrusted hyphae. Characters such as the centrally stipitate basidiocarps and the presence of pseudocystidia indicate the section Rhodocybe (Baroni 1981). Rhodocybe pruinosistipitata T.J. Baroni et al., a species reported from Pakaraima Mountains of Guyana (Henkel et al. 2010), is somewhat comparable to the present species because of a similar looking pileus, a pruinose stipe, adnate lamellae, somewhat similar sized basidiospores, abundant pseudocystidia and a similar habitat. However, R. pruinosistipitata has a longer stipe, pip-shaped basidiospores, larger, broadly ventricose to lageniform pseudocystidia, a pileipellis lacking pileocystidia and a stipitipellis with encrusted hyphae (Pegler 1977, 1986). Rhodocybe retroflexa (Berk. & Broome) Pegler reported from Sri Lanka (Pegler 1977, 1986) has somewhat similar sized basidiocarps with similarly coloured pileus, almost similarly attached lamellae, almost similar sized basidiospores of similar shape, pseudocystidia with granular contents, and an undifferentiated cutis-type pileipellis. However, that species has a depressed pileus, a pseudoparenchymatous subhymenium, and a pileipellis devoid of encrusted hyphae and pileocystidia.
Comparison of the RPB2 (687 bp), ITS (412 bp) and nLSU (884 bp) sequence data derived from Rhodocybe indica with the nucleotide sequences of taxa available in GenBank suggests that the sequences of the present species are different. In a megablast search of the GenBank nucleotide database using the RPB2 and ITS sequences, no close hits with zero e-values were obtained. While using the nLSU sequence, the closest hit was Rhodocybe fallax (GenBank AF261283; Identities = 853/884 (96 %)). Rhodocybe fallax (Quél.) Singer), belonging to the section Decurrentes (Baroni 1981), and differs from the present species in almost all macro- and micromorphological characters.
In the resulting phylogenetic tree after the ML analysis (Fig. 159), Rhodocybe indica, R. collybioides and R. caelata formed a distinct clade with significant support (73 % BS). Within this clade, R. indica is related to R. collybioides with weak support (67 % BS).
Rhodocybe luteobrunnea K.N.A. Raj & Manim., sp. nov.
MycoBank MB 816842; Facesoffungi number: FoF02180, Figs. 161 and 162
Etymology: The specific epithet refers to the yellowish-brown pileus.
Holotype: CAL 1322.
Basidiocarps small, mycenoid. Pileus 5–14 mm diam., initially somewhat conico-convex, becoming convex or broadly convex with a small umbo; surface yellowish-brown (5E8/OAC734) on umbo and striations, brownish-yellow (5D5, 5D6/OAC743) elsewhere, rather hygrophanous and becoming paler, finely pellucid-striate, glabrous; margin slightly incurved when young, becoming deflexed to almost straight with age, finely wavy. Lamellae sinuate or emarginate with a small decurrent tooth, close, greyish-orange (5B3/OAC574), up to 3 mm wide, with lamellulae of 3 lengths; edge entire to the naked eye, finely torn under a lens, concolourous with the sides. Stipe 7–26 × 1–2 mm, central or slightly eccentric, terete, equal, straight or somewhat flexuous, cartilaginous, solid; surface brownish-orange (5C3/OAC730), glabrous to the naked eye, finely appressed fibrillose all over under a lens, finely pruinose towards the apex; base with white mycelial cords. Odour and taste not distinctive.
Basidiospores 5–7 × 3–4.5 (5.67 ± 0.54 × 3.97 ± 0.49) μm, Q = 1.2–1.7, Qm = 1.44, lacrymoid or pip-shaped, with or without a suprahilar depression in profile view, up to 8, weak angular facets in polar view, finely undulate-pustulate all over, thin-walled. Basidia 16–21 × 5.5–6.5 μm, clavate, pale yellow, thin-walled, 4-spored; sterigmata up to 4 μm long. Lamella-edge heterogeneous. Cheilocystidia and pleurocystidia present as pseudocystidia. Pseudocystidia 16–30 × 4–6 μm, narrowly fusiform, narrowly utriform or cylindrical with an acute apex, with golden yellow or reddish-yellow contents, thin-walled. Lamellar trama subregular; hyphae 4–9 μm wide, hyaline or pale yellow, thin-walled. Subhymenium inconspicuous. Pileus trama subregular; hyphae 4–7 μm wide, with a pale yellow wall pigment and fine brown spiral encrustations, thin- to slightly thick-walled. Pileipellis an undifferentiated cutis; hyphae 4–7 μm wide, with a pale brownish-yellow wall pigment and fine brown encrustations, thin- to slightly thick-walled. Stipitipellis a cutis often disrupted by bunches of flaring out hyphae; hyphae 3–7 μm wide, with a pale brownish-yellow wall pigment and fine brown encrustations, thin- to slightly thick-walled. Caulocystidia 10–14 × 2–4 μm, versiform: lageniform, cylindrical with a short rostrate apex or flexuous, with a pale brownish-yellow wall pigment, thin-walled. Clamp connections not observed on any hyphae.
Habitat: in small groups, on forest floor, among decaying litter.
Material examined: INDIA, Kerala State, Thrissur District, Peechi, Peechi Forest, 26 July 2010, K. N. Anil Raj AR180 (CAL 1322, holotype).
Notes: Rhodocybe luteobrunnea is distinguished by its yellowish-brown basidiocarps with a finely pellucid-striate pileus; sinuate or emarginate lamellae; lacrymoid or pip-shaped basidiospores; cylindrical to fusiform pseudocystidia and encrusted pileipellis. A combination of characters such as the centrally stipitate basidiocarps and the presence of pseudocystidia with coloured contents (in KOH) lead the present species to the section Rhodocybe. Rhodocybe perplexa T.J. Baroni & Watling from Malaysia (Baroni and Watling 1999) resembles R. luteobrunnea in having a pileus of similar size and shape, somewhat similar sized basidiospores, presence of pseudocystidia, pileipellis with encrusted hyphae and clampless hyphae (Baroni and Watling 1999). However, R. perplexa has differently coloured basidiocarps, adnate lamellae, shorter stipe, subglobose to ellipsoid basidiospores and larger pseudocystidia. Rhodocybe naucoria Singer, an Argentinean species has similar shaped pileus, similar-sized and somewhat similar-shaped basidiospores and presence of pseudocystidia (Baroni 1981). However, that species differs from the present one in having smaller sized and differently-coloured basidiocarps, differently attached lamellae, larger pseudocystidia and presence of birefringent crystals in the hymenial hyphae.
Comparison of RPB2 (648 bp), ITS (675 bp) and nLSU (830 bp) sequence data derived from the present Rhodocybe species with the nucleotide sequences of taxa available in GenBank suggests that the sequences are different. In a BLASTn search using the ITS sequence derived from the present species, no close hits with a zero e-value were obtained. Rhodocybe aureicystidiata is the closest hit while conducting a BLASTn search using both nLSU (GenBank AY380407; Identities = 840/852 (99 %)) and RPB2 (GenBank AY337412; Identities = 604/648 (93 %)) sequences.
Rhodocybe aureicystidiata Lennox ex T.J. Baroni, a species belonging to the section Rhodocybe (Baroni 1981), shows some similarity to the present species in having rather similar-coloured basidiocarps, encrusted hyphae in the pileipellis and presence of pseudocystidia. However, that species has larger basidiocarps, a depressed pileus which becomes dark red on bruising, inrolled margin, larger and amygdaliform basidiospores, larger and differently shaped pseudocystidia and a different geographical location (Baroni 1981).
In the phylogram (Fig. 159) generated from the ML analysis, Rhodocybe luteobrunnea and R. aureicystidiata, R. pruinosostipitata, R. mellea and Rhodocybe sp. formed a distinct clade with significant support (99 % BS). Within this, R. luteobrunnea and R. aureicystidiata formed another subclade also with significant support (90 % BS).
Rhodocybe griseoaurantia K.N.A. Raj & Manim., sp. nov.
MycoBank MB 816843; Facesoffungi number: FoF02181, Figs. 163 and 164
Etymology: The specific epithet refers to the greyish-orange colour of the pileus.
Holotype: CAL 1324.
Basidiocarp small. Pileus 14 mm diam., convex with a slightly raised centre; surface greyish-orange (6B6/OAC694) at the centre and pale orange (6A3/OAC695) toward the margin, hygrophanous and soon becoming orange-white (6A2/OAC696) after collection, not striate, finely appressed squamulose on and around the centre and minutely pubescent towards the margins; margin slightly incurved, rather wavy. Lamellae adnate to short decurrent, close, pale orange (6A3/ OAC695), up to 3.5 mm wide, occasionally furcate, with lamellulae of 1–4 lengths; edge entire or finely torn, concolourous with the sides. Stipe 31 × 4 mm, central, terete, slightly tapering towards the apex, solid; surface orange-white (6A2/OAC696), finely appressed-fibrillose all over, finely pruinose towards the apex; base slightly swollen, with whitish mycelial cords. Odour and taste not distinctive.
Basidiospores 5–7 × 3.5–4.5 (5.85 ± 0.67 × 3.82 ± 0.33) μm, Q = 1.25–2, Qm = 1.53; with 6–7 facets in polar view, ellipsoid in profile with or without a suprahilar depression, weakly undulate-pustulate all over, hyaline, thin-walled. Basidia 19–25 × 5–7 μm, clavate, hyaline, thin-walled, 4-spored; sterigmata up to 3 μm long. Lamella-edge often fertile or occasionally heterogeneous. Cheilocystidia 14–29 × 3–9 μm, infrequent, scattered, often filiform or cylindrical, sometimes flexuous or narrowly fusiform. Pleurocystidia none. Lamellar trama subregular; hyphae 4–5.5 μm wide, hyaline, thin-walled. Subhymenium inconspicuous. Pileus trama subregular; hyphae 3.5–8 μm wide, pale yellow, thin-walled. Pileipellis a cutis often disrupted by scattered or rarely clustered ascending hyphae; hyphae 2.5–6 μm wide, with a pale yellow wall pigment and occasionally with faint, hyaline encrustations, thin- to slightly thick-walled. Stipitipellis a cutis occasionally disrupted by scattered or clustered flaring-out hyphae; hyphae 3.5–6 μm wide, hyaline or pale yellow, thin-walled. Caulocystidia absent. Clamp connections not observed on any hyphae.
Habitat: on soil, among moss, solitary.
Material examined: INDIA, Kerala State, Wayanad District, Muthanga, Muthanga Wildlife Sanctuary, 6 September 2011, K. N. Anil Raj AR865 (CAL 1324, holotype).
Notes: Rhodocybe griseoaurantia is well characterized by its small basidiocarps with a greyish-orange pileus; adnate to short decurrent lamellae; ellipsoid basidiospores and filiform or cylindrical cheilocystidia are the diagnostic features of R. griseoaurantia. Owing to the centrally stipitate basidiocarp, presence of cheilocystidia and the absence of both pseudocystidia and clamp connections, R. griseoaurantia can be placed in the section Rufrobrunnea (Baroni 1981). Rhodocybe alutacea Singer, a North American species (Baroni 1981; Baroni and Horak 1994), is similar to the present species in having similar coloured basidiocarps, a similar looking pileus, narrow lamellae with similar type of attachment, a solid stipe, almost similar-sized basidiospores of similar shape, infrequent, scattered cheilocystidia, pileipellis with encrusted hyphae and non-encrusted hyphae of stipitipellis. However, R. alutacea has larger basidiocarps, an umbilicate pileus, basidiospores with more facets (7–9) in profile view, larger, septate cheilocystidia, presence of caulocystidia and a different habitat and geographical location.
Comparison of the RPB2 (681 bp), ITS (733 bp) and nLSU (908 bp) sequence data derived from R. griseoaurantia with the nucleotide sequences of taxa available in GenBank suggests that the present species is different. In the BLASTn search using the RPB2 sequence, the closest hit was Rhodocybe truncata (GenBank EF421019; Identities = 616/659 (93 %)). For ITS sequence, the closest hit was an unidentified Rhodocybe species (Rhodocybe sp. 1 GMB-2014) (GenBank KP012803; Identities = 719/737 (98 %)) from Australia, followed by R. truncata (GenBank EF421110; Identities = 556/644 (86 %)). In the BLASTn search with the nLSU sequence, the closest hits was Rhodocybe gemina (GenBank DQ071715; Identities = 894/908 (98 %)). Rhodocybe truncata (Schaeff.) Singer, a European species, differs from the present one in almost all macro- and microscopic characters, although it has cheilocystidia and rather similar-sized (5–6.5 × 4–5 μm) basidiospores (Baroni 1981). Rhodocybe gemina (Paulet) Kuyper & Noordel., another European species belonging to the section Rufrobrunnea, differs from the present species in having larger and differently-coloured basidiocarps, crowded lamellae, a perfumed odour, unpleasant taste, slightly larger and differently shaped basidiospores (5–7 × 4–5.5 μm), and a cutis with transition to a trichoderm type of pileipellis (Noordeloos 1988).
In the phylogram (Fig. 159), R. griseoaurantia, R. truncata and R. gemina formed a strongly support clade. This clade received significant support (98 % BS) and within it R. griseoaurantia was found to be phylogenetically distinct from R. truncata and R. gemina.
Agaricaceae Chevall.
We follow Li et al. (2016)
Cyathus Haller
The genus Cyathus belongs in the Agaricaceae, despite the unusual form of its basidiomata (also known as peridia), which resemble a bird’s nest and grow on wood or dung. These peridia are typically vase-, trumpet- or urn-shaped with dimensions of 4–8 mm wide and 7–18 mm high. The type species, Cyathus striatus (Huds.) Willd. was described from Europe, but the genus has a cosmopolitan distribution and comprises ca. 45 species (Das et al. 2016). Besides the studies of Zhao et al. (Zhao et al. 2006, 2007), the tropical species are less known, and we here describe a new taxon from Thailand that was recently identified as a producer of novel terpene alkaloid antibiotics. A phylogenetic tree in support of the new species is provided in Fig. 165.
Phylogenetic tree generated with RAxML (GRT GAMMA) based on ITS sequence data aligned with MAFFT. Bootstrap values higher than 50 % are shown. The sequence of the holotype strain is in bold. The tree is rooted with Nidula niveotomentosa SWFC3000. Species names are followed by strain numbers. Ex-type strains are highlighted in bold and new isolates are in blue
Cyathus pyristriatus B. Thongbai, C. Richt. & M. Stadler, sp. nov.
MycoBank number: MB817167; Facesoffungi number: FoF02385, Fig. 166
Cyathus pyristriatus (holotype). a Basidiomata. b Basidiomata in top view showing peridioles. c Peridioles and plications on the inner surface of peridium by longitudinal section. d, e Basidiomata in side view showing fluffy hairs. f Basidiospores. g Purse hyphae showing clamp-connections. Scale bars a = 3 mm; b–d = 1 μm; f, g = 10 μm
Etymology: Named for its production of pyristriatins A and B.
Holotype: MFLU15-1416.
Saprobic on rotten wood in forest with Fagaceae. Basidiomata clavate to broadly obconic, without stipe, 5.5–7 mm high and 4–6 mm diam., wide at the top, external peridium covered with shaggy or fluffy yellowish-brown or buff hairs with age, surface of inner peridium grey, darkening with age, distinctly plicate. Peridioles 3–3.5 mm in diam. wide, greyish-brown to dark grey covered with minute, greyish to greyish-brown hairs. Basidiospores 14–17 × 8–10 μm (av. = 15.18 × 8.12, Q = 1.5–1.91, Qm = 1.70, n = 40), ellipsoid to broadly ellipsoid, some ovoid, rarely subglobose, hyaline, smooth, thin-walled, 1.5–3 μm thick. Basidia not observed. Clamp connections present in all tissues including the mycelial culture.
Material examined: THAILAND, Chiang Mai Province, Mae-Taeng District, near the Mushroom Research Centre (http://www.mushroomresearchcentre.com/), N19°07.200′ E98°44.044′, 12 August 2014, Thongbai, Richter & Stadler M68 (MFLU 15-1416, holotype), ex-type culture MFLUCC14-0770.
Notes: Cyathus pyristriatus closely resembles C. striatus (Huds.) Willd. 1787 in the account of shaggy or fluffy yellowish-brown or buff hairs of peridium, distinctly plicate inner peridium, while peridioles are greyish-brown to dark grey. Based on morphological comparison between C. striatus and C. pyristriatus the latter has smaller basidioma and also smaller ellipsoid basidiospores. However, peridioles are covered with a distinctive minutely greyish to greyish-brown hairs, while peridioles of C. striatus are smooth. Remarkably, C. striatus was reported producing pale to dark pigments from basidiomata, which vary in size (Kuo 2014; Wood and Stevens 2015).
An initial BLAST search of the ITS nucleotide sequence from NCBI database (http://www.ncbi.nlm.nih.gov/) gave the closest hit to a Cyathus striatus strain (EU784194) with a maximum similarity of 94.5 % (Brock et al. 2009). The BLAST search of the LSU nucleotide sequence of C. pyristriatus resulted in a high degree similarity of 98.8 % (DQ071742) with C. striatus (Garnica et al. 2007). Figure 165 illustrates the phylogenetic relationships of C. pyristriatus, C. striatus, and other Cyathus species. The ITS based phylogenies clearly depict that our new taxon belongs to the genus Cyathus. A close affinity between C. pyristriatus and C. stercoreus collected from China, is also noted with high support. Cyathus stercoreus, however, differs in having a smooth surface of inner peridium and larger globose to oval basidiospores (Zhao et al. 2008).
The ex-type culture of the new species also produces novel terpene alkaloid antibiotics named pyristriatins, which have never been found in Cyathus, despite the fact that this genus has been evaluated intensively for secondary metabolites for several decades (Richter et al. 2016).
Polyporales Gäum.
Polyporaceae Fr. ex Corda
We follow Zhou et al. (2016).
Polyporus P. Micheli ex Adans.
Polyporus, the type genus of Polyporaceae, is characterized by annual stipitate basidiocarps, a dimitic hyphal system with generative hyphae and skeleto-binding hyphae, hyaline and thin-walled cylindrical basidiospores, and are the cause of white rot (Gilbertson and Ryvarden 1987). Phylogenetic analysis reports Polyporus as polyphyletic (Ko and Jung 2002; Krüger et al. 2006; Sotome et al. 2008, 2011). Recently, based on a multi-locus phylogeny, species of Polyporus, Favolus and Neofavolus were divided into six highly supported clades, i.e. Favolus clade, Melanopus clade, Neofavolus clade, Polyporellus clade, Polyporus clade and Squamosus clade (Zhou et al. 2016). A phylogenetic tree in support of the new species is provided in Fig. 167.
Phylogeny of Polyporus and related genera generated by maximum likelihood based on combined ITS + LSU sequence data. Branches are labeled with maximum likelihood bootstrap proportions (the former values) higher than 50 %, maximum parsimony bootstrap proportions (the middle values) higher than 50 % and Bayesian posterior probabilities (the latter values) more than 0.95
Polyporus mangshanensis B.K. Cui, J.L. Zhou & Y.C. Dai, sp. nov.
Index Fungorum number: IF552159; Facesoffungi number: FoF02471, Figs. 168 and 169
Polyporus mangshanensis (holotype). a Basidiospores. b Basidia and basidioles. c Hyphae from context. c1 Generative hyphae, c2 Skeleto-binding hyphae. d Hyphae from cuticle. d1 Contextual cuticle hyphae. d2 Stipe cuticle hyphae. e Hyphae from stipe. e1 Generative hyphae. e2 Skeleto-binding hyphae. f Hyphae from trama. f1 Generative hyphae. f2 Skeleto-binding hyphae. Scale bars a–f = 10 μm
Etymology: mangshanensis, referring to the locality of the type specimen.
Holotype: BJFC 018267.
Basidiocarps annual, eccentrically to almost laterally stipitate, solitary, corky when dry; pileus reniform, projecting up to 5.8 cm long, 10.5 cm wide and 2.5 cm thick; pileal surface beige, saffron yellow to yellowish-orange when dry, azonate, with slightly radial stripes, smooth and glabrous; margin sharp and involute upon drying; pore surface brown beige to olive brown when dry; pores angular, 3–5 per mm, occasionally elongated to 1 mm long and 0.5 mm wide; dissepiments thin, entire to lacerate; context buff when dry, up to 1.2 mm thick; tubes concolorous with the pore surface, slightly decurrent on the stipe, up to 1.5 mm long; stipe cylindrical, context of stipe buff when dry, bearing dark brown cuticle, becoming tan towards the tuber layer, glabrous, curvy and wrinkled upon drying, up to 2 cm long and 7 mm diam. Hyphal system dimitic; generative hyphae bearing both clamp connections and simple septa; skeleto-binding hyphae IKI–, CB–; tissues unchanged in KOH. Contextual generative hyphae frequent, colourless, thin-walled, bearing clamp connections, frequently branched, 2.5–8 μm diam., usually inflating at the branching areas or clamping areas, up to 14 μm in diam.; contextual skeleto-binding hyphae dominant, colourless, thick-walled with a narrow to wide lumen, frequently branched, flexuous, interwoven, 2.5–6.5 μm diam., occasionally inflated up to 11 μm diam.; contextual cuticle hyphae simple septate, thin-walled, frequently branched, interwoven, 1.5–4.5 μm diam. Tramal generative hyphae frequent, colourless, thin-walled, bearing clamp connections and simple septa, infrequently branched, 2.5–4 μm diam.; tramal skeleto-binding hyphae dominant, colourless, thick-walled with a narrow to wide lumen, frequently branched, flexuous, interwoven, 1.3–4 μm diam. Stipe generative hyphae frequent, colourless, thin-walled, bearing clamp connections, occasionally branched, 1.5–6 μm diam.; stipe skeleto-binding hyphae dominant, colourless, thick-walled, subsolid or with a narrow to wide lumen, moderately branched, flexuous, interwoven, 2.5–6 μm diam.; stipe cuticle generative hyphae dominant, with buff inclusion inside, thick-walled with a wide lumen, bearing clamp connections; stipe cuticle skeleto-binding hyphae frequent, with light brown to orange brown inclusion inside, thick-walled with a narrow lumen, occasionally branched, 2.4–4.5 μm diam. Cystidia and cystidioles absent; basidia clavate, with a basal clamp and four sterigmata, 16.5–24 × 7–10 μm; basidioles in shape similar to basidia, but smaller and without sterigmata. Basidiospores cylindrical, few, oblong, colourless, thin-walled, smooth, with guttules, IKI–, CB–, (6.5–)7.5–10.5(–11) × 3.5–4.5(–5) μm, L = 8.7 μm, W = 4.07 μm, Q = 1.84–2.53, Qm = 2.14 (n = 60/1).
Type of rot: White rot.
Specimens examined: CHINA, Hunan Province, Yizhang County, Mangshan Nature Reserve, on fallen angiosperm branch, 17 August 2014, Dai 15151 (BJFC 018267, holotype).
Notes: Recently, taxonomic and phylogenetic studies of wood-rotting fungi in subtropical China have been carried out, and many new species have been described based on both morphological characters and molecular data (Chen and Cui 2014, 2016; Chen et al. 2015c, 2016; Han et al. 2016; Li and Cui 2013; Li et al. 2014; Song et al. 2014, 2016; Zhao et al. 2013, 2015a). In the current study, Polyporus mangshanensis is an additional new species described from subtropical China on the basis of morphological characters and phylogenetic analysis. Morphologically, P. mangshanensis is characterized by its beige to yellowish-orange pileal surface with slightly radial stripes, brown beige to olive brown pore surface, dark brown stipe, generative hyphae bearing both clamp connections and simple septa, and oblong to cylindrical basidiospores (7.5–10.5 × 3.5–4.5 μm).
Phylogenetically, P. mangshanensis clustered with P. leprieurii Mont. and P. guianensis Mont.; morphologically, they produce similar pileal surface, pore surface and dark stipe, however, P. mangshanensis differs from the latter two species by producing both clamped and simple-septate generative hyphae, contextual generative hyphae and skeleto-binding hyphae usually inflated over 10 μm in diam. Moreover, pores and basidiospores of P. mangshanensis are smaller than P. guianensis (pores 1–2 per mm, basidiospores 8–12 × 2.5–4 μm), while larger than P. leprieurii (pores 5–8 per mm, basidiospores 4.5–7 × 2–2.5 μm; Núñez and Ryvarden 1995).
Polyporus subvarius C.J. Yu & Y.C. Dai has a similar pileal and pore surface to P. mangshanensis, but the former has larger pores (1–2 per mm) and basidiospores (9.2–12.6 × 3.9–4.9 μm, L = 10.7 μm, W = 4.48 μm), only clamped generative hyphae, and grows on living trees of Salix (Dai et al. 2007).
Polyporus admirabilis Peck resembles P. mangshanensis in having a laterally dark stipe, a tan pileal surface and similar pore size, but it differs in its subulate cystidioles, only clamped generative hyphae, and slender basidiospores (7.8–9 × 3–3.5 μm, L = 8.29 μm, W = 3.12 μm, Qm = 2.66; Dai 1999).
Both Polyporus badius (Pers.) A.B. De and P. submelanopus H.J. Xue & L.W. Zhou have simple-septate generative hyphae, dark stipe and cylindrical basidiospores, which are similar to P. mangshanensis. Polyporus badius has darker pileal surface and smaller pores (5–6 per mm), only simple-septate generative hyphae (Dai 1999); while P. submelanopus has larger pores (2–3 per mm) and basidia (24–33 × 5–8 μm), and a terrestrial habit (Xue and Zhou 2012).
Russulales Kreisel ex P.M. Kirk et al.
Russulaceae Lotsy
The family Russulaceae is one of the dominant and morphologically diverse (Miller et al. 2006) ectomycorrhizal mushroom families in the Himalayas. Apart from its three corticoid genera, i.e. Boidinia, Gloeopeniophorella and Pseudoxenasma (Larsson and Larsson 2003; Miller et al. 2006), this family has four predominantly agaricoid genera: Lactifluus, Lactarius, Multifurca and Russula (Buyck et al. 2008, 2010), some of which may also contain secotioid-hypogeous or pleurotoid species.
Russula Pers.
The monophyletic agaricoid genus Russula (Buyck et al. 2008, 2010) has an enormous diversity in Indian Himalaya (Rawla 2001; Das and Sharma 2005; Das et al. 2006, 2014, 2013, 2010) showing its wide range of distribution from tropical to subalpine areas and associations with broadleaf to coniferous trees. Apart from morphology-based six subgenera (Sarnari 1998; R. subg. Compactae, R. subg. Heterophyllidia, R. subg. Ingratula, R. subg. Amoenula, R. subg. Incrustatula and R. subg. Russula), three additional subgenera have been recently described (Hongsanan et al. 2015a; R. subg. Archaea Buyck & V. Hofstetter, R. subg. Brevipes Buyck & V. Hofstetter and R. subg. Malodora Buyck & V. Hofstetter). Here, two novel species (belonging to R. subg. Heterophyllidia and R. subg. Amoenula respectively), collected from the northwestern part of Indian Himalaya are introduced together with their morphology and phylogenetic placement (Figs. 170, 173).
Phylogram generated from maximum likelihood method based on ITS-rDNA sequences from MEGA6 under Kimura 2-parameter model (Kimura 1980). The tree with the highest log likelihood (−1342.1525) is shown. One-thousand bootstrap replicates were analyzed to obtain the nodal support values. The novel species having GenBank Accession Number KX234820 (ITS-rDNA) is shown in blue. The R. brevipes, R. chloroides, R. emetica are considered as the out groups
Russula indoalba A. Ghosh, Buyck, A. Baghela, K. Das & R.P. Bhatt, sp. nov.
Index Fungorum number: IF552159; Facesoffungi number: FoF02471, Figs. 171 and 172
Etymology: referring to white basidiomata growing in India
Holotype: CAL 1328.
Basidiomata 70–100 mm. in height. Pileus 30–95 mm. in diam., broadly convex when young, gradually planoconvex to applanate with depressed center, finally uplifted at maturity; margin decurved to plane, entire, tuberculately striate; surface dry, viscid when moist, cracked-areolate when mature, white (1A1–2A1) with greyish-yellow (4B4–4B6) to pale yellow (3A3) spots at the center; lemon-yellow or pale yellow to pastel yellow (1A3–1A4) with KOH; cap context white (1A1–2A1), lemon-yellow or pale yellow to pastel yellow (1A3–1A4) with KOH. Lamellae adnexed to almost free with age, equal, subdistant to close (7–10/cm), forked near the stipe apex, white (1A1–1A2), with entire, concolourous gill edges. Stipe 20–65 × 10–23 mm., subclavate, central, dry, smooth, white (1A1–2A1), lemon-yellow or pale yellow to pastel yellow (1A3–1A4) with KOH; context stuffed, becoming hollow with age, white (1A1–2A1), greyish-orange (5B4–5B5) with FeSO4, unchanging with guaiacol and NH4OH. Taste mild. Odour indistinctive. Spore print yellowish-cream. Basidiospores 5.5–7.42–9.5 × 5.5–6.4–8 μm (n = 50, Q = 1–1.16–1.36), subglobose to broadly ellipsoid, rarely ellipsoid; ornamentation amyloid, composed of short (0.3–0.5 μm) and long (0.7–1 μm) conical to cylindric warts, mostly connected to form incomplete reticulum, few isolated, apiculi up to 2 μm high; suprahilar spot not amyloid. Basidia 45–60 × 9–13 μm, 4-spored, cylindrical to subclavate, sterigmata up to 5 μm long. Pleurocystidia 47–90 × 8–16 μm, subclavate to clavate with capitate, moniliform, appendiculate and rounded apex, emergent up to 38 μm beyond the basidiole tips. Cheilocystidia 55–68 × 6–8 μm, cylindrical to subclavate with appendiculate apex. Subhymenium layer up to 26 μm thick, pseudoparenchymatous. Lamellar trama consists predominantly of sphaerocytes. Gill edges fertile. Pileipellis up to 120 μm thick, distinctly divided into suprapellis and supellis; subprapellis composed of erect to suberect chains of 3–6 rows of cells; subterminal cells 8–20 × 6–18 μm, mostly rounded (inflated), ellipsoid; terminal cells mostly, cylindrical to subulate; subpellis composed of. horizontal, interwoven hyphae. Pileocystidia rare, one-celled, slender, thin-walled, up to 7 μm wide, dispersed as terminal cells in the suprapellis.
Habitat and distribution: Under Quercus sp. in mixed forests dominated by Quercus, Rhododendron, Abies and Cupressus.
Material examined: INDIA, Uttarakhand, Rudraprayag district, Baniyakund, alt. 2630 m, N30°28.914′ E79°10.854′, 1 September 2014, A. Ghosh, AG 15-541 (GUH); ibid., 14 July 2015, A. Ghosh, AG 15-628 (CAL 1328, holotype); ibid., A. Ghosh, 16 July 2015, AG 15-661 (GUH); ibid., 1 August 2015, A. Ghosh, AG 15-797 (GUH).
Notes: Russula indoalba which is characterized by white pileus with greyish-yellow to pale yellow spots at center, tuberculately striate and wavy to interrupted pilear margin, equal gills, yellowish-cream spore print and mild taste is a typical member of Russula subg. Heterophyllidia sect. Virescentinae.
In the field Russula kanadii A.K. Dutta & K. Acharya (also reported from India) appears to be quite similar to the present taxon. However, R kanadii has a white spore print (1A1), smaller basidiospores [(4.5)–5.5–5.7–6.5(–7) × (4.5)–5.3–5.5(–6) μm] and grows at very low altitudes (56 m) being ectomycorrhizal with dipterocarps, viz. Shorea robusta (Dutta et al. 2015). Russula alboareolata Hongo, described from Japan in association with Castanopsis (Fagaceae), but also reported from dipterocarps in Taiwan (Watling and Lee 1998) and Thailand is also very similar to R. indoalba but has smaller basidiospores 6.5–8.5 × 5.5–7 μm (Hongo 1979). Our phylogeny (Fig. 170) shows that R. alboareolata is genetically closer to R. kanadii than to our new species or to the other Virescentinae (Fig. 170). Furthermore, the phylogeny also suggests, although without support, that R. indoalba is basal to the core group of Virescentinae (R. virescens and allies) suggesting a migration from India via Asia (Fig. 170, sequence UDB014226) to both Europe and North America. All species in this core-group differ from other Virescentinae such as those belonging to the R. crustosa-R. mustelina lineage and also R. parvovirescens Buyck et al. (Buyck et al. 2006) in the absence of dermatocystidia in the lower subpellis (Buyck, http://www2.muse.it/russulales-news/id_virescentinae.asp).
Phylogram generated from maximum likelihood method based on ITS-rDNA sequences: The evolutionary history was inferred by using the maximum Likelihood method based on the Kimura 2-parameter model (Kimura 1980). The tree with the highest log likelihood (−2215.6038) is shown. One-thousand bootstrap replicates were analyzed to obtain the nodal support values. The novel species having GenBank Accession Number KX234819 (ITS-r-DNA) is shown in blue. The R. emetica and R. nana were considered as the out group taxa. Evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013)
Russula pseudoamoenicolor A. Ghosh, Buyck, K. Das, A. Baghela & R.P. Bhatt, sp. nov.
MycoBank No.: MB 817101; Facesoffungi number: FoF02522, Figs. 174 and 175
Russula pseudoamoenicolor (holotype). a, b Fresh basidiomata. c–e Transverse section through lamellae showing pleuromacrocystidia. f Radial section through pileipellis. g–j Elements of pileipellis. k Basidiospores. l SEM micrograph of basidiospores. Scale bars a = 100 mm; c, d = 50 μm; e, g–k = 10 μm; f = 100 μm; l = 2 μm
Etymology: referring to the lookalike of Russula amoenicolor, an European species.
Holotype: CAL 1330.
Basidiomata up to 100 mm in height. Pileus 50–100 mm in diam., globose, plano-convex to applanate with broadly depressed center, becoming uplifted when mature; margin decurved, entire, tuberculately striate, torn when mature, surface dry, viscid when moist, subvelvety, purplish-red-violet-red (14B5–14B8) or light lilac, light violet to pastel violet (16A4–16A5) with purplish-white (14A2) to violet-white (16A2) towards margin, rarely dark violet (15A6–15A8) towards the depression; cuticle peeling 3/4th of the radius; cap context white (1A1–2A1), unchanging when bruised. Lamellae adnexed to subdecurrent, close to rather crowded, white (1A1–2A1), forked near the stipe; edges marginate near the cap margin, lamellulae absent. Stipe 45–70 × 10–14 mm, equal, slightly tapered towards the base, dry, smooth, brittle, central, reddish-white to pink-rose or pink-red (12A3–12A5), context stuffed, white (1A1–2A1), pink-rose (12A3–12A5) with guaiacol. Taste mild. Spore print not obtained.
Basidiospores 6–7.30–9.5 × 5–6.33–8 μm (n = 25, Q = 1.03–1.16–1.33), subglobose to broadly ellipsoid, rarely ellipsoid, ornamentation amyloid, composed mostly of ridges and warts (up to 1 μm high) aligned or connected to form an incomplete reticulum, with few isolated warts, apiculi up to 2 μm high. Basidia 35–55 × 9–13 μm, cylindrical, subclavate to clavate, 4-spored, sterigmata up to 6 μm high. Subhymenium layer up to 30 μm thick, pseudoparenchymatous. Hymenophoral trama mainly consisting of sphaerocytes measuring 17–40 × 16–34 μm. Pleurocystidia 90–117 × 10–21 μm, ventricose, subfusiform to fusiform with blunt apex, thick walled (1 μm thick), emergent up to 60 μm; content blank or insignificant. Gill edges fertile, with basidia and cystidia. Cheilocystidia 30–85 × 7–10 μm, same as pleurocystidia. Pileipellis up to 90 μm thick, composed of clustered erect to suberect elements composed of chains of 4–7 cells; terminal cells ellipsoid, conical, subfusoid to occasionally subulate (11–65 × 4–10 μm wide); subterminal cells mostly cylindrical to rectangular, few ellipsoid or rounded (inflated), measuring up to 14 μm wide.
Habitat and distribution: grows in close association with Quercus sp. with undergrowth of Rhododendron sp. in moist deciduous and mixed (broadleaf and coniferous) forest.
Material examined: INDIA, Uttarakhand, Pauri Garhwal, along the road side of khirsu, alt. 1835 m., N30°10.155′ E78°52.134′, 24 July 2015, A. Ghosh, AG 15-739 (CAL 1330, holotype)
Notes: The combination of characters in Russula pseudoamoenicolor comprising a purplish-red to violet-red or lilac subvelvety pileus with darker center, reddish-violet to pink-rose stipe, occasional occurrence of typically subulate terminal cells of pileipellis, absence of dermatocystidia and inamyloid suprahilar spot place it in R. subg. Amoenula Sarnari. In the field, the European species, R. amoenicolor Romagn. appears to be quite similar to the present taxon but the former (Sarnari 1998) has purple to green or variegated pileus, smaller basidiospores (6.7–8.4 × 5.6–7.4 μm) and mostly subulate terminal cells in pileipellis (terminal cells mainly ellipsoid, conical, subfusoid or occasionally subulate in R. pseudoamoenicolor) and its ITS nucleotide sequence are dissimilar (93 % identity with R. pseudoamoenicolor for 100 % query coverage using BLAST) from the present species. From a phylogenetic standpoint, R. pseudoamoenicolor is closely related to R. violeipes and cluster together in a subclade with high bootstrap support (Fig. 173).
The European species, R. violeipes Quél. was recently reported from South Korea (Park et al. 2013), yet these collections differs significantly from European material (GenBank acc. no. AY061726). These Korean collections (GenBank accession nos. KF361797 and KF361783) are here, however, recovered as identical to our material from the Indian Himalaya.
Interestingly, two Australian species, R. variispora T. Lebel and R. rostraticystidia T. Lebel, previously of undetermined position within R. subg. Heterophyllidia (Lebel and Tonkin 2007) are here for the first time recovered with significant support (93 % BS) as very closely related to or likely members of R. subsect. Amoeninae. Russula variispora and R. rostraticystidia both are sequestrate species and hence, have an altogether different morphology from other known Amoeninae, or even from all other known species in R. subg. Heterophyllidia as secotioid-gasteroid taxa have never been reported from other subsections in this subgenus (Kong et al. 2015). The rostrate pleurocystidia of R. rostraticystidia and the similarly shaped terminal cells in the less developed pileipellis of these Australian secotioid species (Lebel and Tonkin 2007) support their placement in Amoeninae.
Lactarius Pers.
The well-known milkcaps are split into two genera: Lactarius and Lactifluus (Buyck et al. 2008, 2010). The genus Lactarius comprises three subgenera, L. subg. Piperites (Fr. ex J. Kickx f.) Kauffman, L. subg. Russularia (Fr. ex Burl.) Kauffman and L. subg. Plinthogali (Burl.) Hesler & A.H. Sm. and is one of the common ectomycorrhizal associates in Indian Himalaya (Rawla 2002; Das and Sharma 2005; Das and Verbeken 2011, 2012; Das and Chakraborty 2014; Das et al. 2015). One undescribed species of L. subg. Plinthogali collected from northwest part of Indian Himalaya is introduced here with morphological details and phylogenetic evidence. A phylogenetic tree is presented in Fig. 176.
Phylogram generated from maximum likelihood method based on ITS-rDNA sequences: the evolutionary history was inferred by using the maximum likelihood method based on the Kimura 2-parameter model (Kimura 1980). The tree with the highest log likelihood (−1884.3936) is shown. One-thousand bootstrap replicates were analysed to obtain the nodal support values. The novel species having GenBank Accession Number KX254611 (ITS-r-DNA) is shown in blue. The Lactifluus vellereus was considered as the out group. Evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013)
Lactarius dirkii Uniyal, K. Das, A. Baghela & R.P. Bhatt, sp. nov.
MycoBank: MB 817126; Facesoffungi number FoF02521, Figs. 177 and 178
Lactarius dirkii (holotype). a Fresh basidiomata. b White latex exuding from cut lamellae. c Transverse section through lamellae edge showing cheiloleptocystidia. d, e Radial section through pileipellis showing pileopseudocystidia (arrows). f Radial section through pileipellis. g Terminal elements of pileipellis. h, i SEM micrograph of basidiospores. Scale bars a = 100 mm; c, f = 50 μm; d, e, g = 10 μm; h = 20 μm; i = 2 μm
Etymology: commemorating Dr. Dirk Stubbe for his contribution to Lactarius subg. Plinthogali.
Holotype: CAL 1332.
Pileus 20–86 mm. in diam.; convex when young, becoming planoconvex with shallowly depressed center with maturity; margin decurved, entire to irregularly undulating, often interrupted; surface dry, slightly wrinkled towards margin when mature; white to orange-white (6A2) with a pinkish-white (7A2) or paler tinge, spotted with reddish-grey (7B2) scrobicules, changing to light yellow (2A5) with 10 % KOH. Lamellae subdecurrent, close to rather crowded (13–14/10 mm), some forked near the stipe apex, white to orange-white (5A2) to pale orange (5A3), becoming pinkish to pale red (7A2) to pastel red (7A3/4) when bruised or cut, lamellulae numerous. Stipe 30–58 × 5–8 mm., cylindrical, tapering toward base, central, dry, concolourous with pileus, white at base with minute hairs; Context whitish to yellowish-white (4A2), turning light orange on cutting, hollow in stipe; Latex abundant, white, unchanging when isolated, but drying pinkish on cut lamellae. Taste bitter to acrid. Odour insignificant. Spore print light yellow (4A5). Basidiospores 6.5–7.7–8.5 × 6–6.9–7.5 μm, (n = 70, Q = 1.06–1.11–1.21), subglobose to broadly ellipsoid, rarely broadly ellipsoid, winged; ornamentations amyloid, composed mostly of broad wings and few small ridges but never forming a reticulum, up to 2 μm high, edges smooth, isolated warts also present between ridges, plage amyloid. Basidia 44–62 × 11–14 μm, subclavate, 4-spored, sterigmata 4–7 μm long. Hymenophoral trama composed of lactifers and rosettes of sphaerocytes. Pleurocystidia absent. Pseudocystidia abundant, emergent, cylindrical, sometimes tortuous, branched, up to 5.5 μm wide. Lamellar edge sterile. Cheiloleptocystidia 24–35 × 4–5.5 μm, mostly cylindrical, rarely subfusiform, thin walled. Subhymenium up to 20 μm thick, pseudoparenchymatous. Lactifers in hymenophoral trama up to 7 μm wide. Pileipellis trichopalisade, 55–121 μm thick, suprapellis formed of cylindrical to subfusiform cells and septate hyphal elements 10–57 × 3.5–6.5 μm; subpellis composed of cells with irregular shapes, cells up to 11 μm wide; Lactifers in pilear trama up to 8 μm wide, sometimes becoming gradually thin toward suprapellis and projecting in form of pileopseudocystidia, wide up to 4.5 μm. Stipitipellis trichoderm, up to 100 μm thick, hyphae up to 5 μm wide.
Habitat & distribution: Under Quercus sp., Rhododendron arboreum in temperate mixed forest dominated by Abies, Cupressus, Quercus and Rhododendron.
Material examined: INDIA, Uttarakhand, Rudraprayag district, Baniyakund, 2630 m, N30°28.914′ E79°10.854′, 29 August 2015, P. Uniyal, PU 15-1004 (CAL 1332, holotype); ibid., Baniyakund, 2630 m, N30°28.914′ E79°10.854′, 9th Aug 2014, P. Uniyal, PU 15-360 (GUH); ibid., 25 August 2014, P. Uniyal, PU 15-451 (GUH); ibid., 1 August 2015, P. Uniyal, PU 15-803 (GUH).
Notes: The combination of morphological features such as whitish dry pileus, concolourous (to pileus) stipe, light yellow spore print, winged basidiospores, trichopalisade pattern of pileipellis and absence of hymenial macrocystidia undoubtedly place Lactarius dirkii under L. subg. Plinthogali (Burl.) Hesler & A.H. Sm. (Basso 1999; Das and Sharma 2004; Stubbe et al. 2008). Among this subgenus, the new species is fairly easy to recognize in the field by its white to orange-white basidiomata with pinkish tinge and darker scrobicules on cap surface, yellow reaction with KOH, close to rather crowded lamellae turning pinkish to orange on bruising, hollow stipe and abundant white latex that remains unchanged when isolated but becoming pinkish on cut lamellae. Micromorphologically, occurrence of subglobose to broadly ellipsoid basidiospores with up to 2 μm high wing like ornamentations and warts that never form a reticulum, abundant emergent pseudocystidia and sterile lamellar edges containing cylindrical cheiloleptocystidia are also worth mentioning.
In the field, L. dirkii can be mistaken for L. oomsisiensis Verbeken & Halling (probably morphologically closest species, which was reported from Papua New Guinea and Thailand and labeled with GenBank accession numbers EF560680 and EF560679, respectively in Fig. 176). Both have whitish basidiomata but the latter can be distinguished macromorphologically by distant orange-brown lamellae, white latex drying cream on gills and mild taste (Le et al. 2007) and micromorphologically, by comparatively narrow pileipellis (40–80 μm thick) with brown intracellular pigmentation in upper layer and absence of pileopseudocystidia in suprapellis. Lactarius dirkii is also closely related to another Asian taxon, L. friabilis H.T. Le & Stubbe (reported from Thailand as well and labelled with GenBank accession numbers EF560663 and EF560664 but, the latter differs from L. dirkii in having distant (to subdistant) lamellae, larger spores (7.8–7.9–8.4–9.1 × 7.1–7.5–7.8–8.7 μm) with incompletely reticulated ornamentation of warts and ridges with mostly crenulate edges (Le et al. 2007). Phylogenetically, both L. oomsisiensis (95 % identity with L. dirkii for 95 % query coverage using BLAST) and L. friabilis (94 % identity with L. dirkii for 98 % query coverage using BLAST) are also distinct.
In L. subg. Plinthogali, molecular phylogeny already confirmed the existence of eight European species (Stubbe and Verbeken 2012) of which, seven species: L. acris (Bolton: Fr.) Gray (GenBank acc. nos. JQ446084 and JQ446083), L. azonites (Bull.) Fr. (GenBank acc. nos. JQ446094 and JQ446095), L. fuliginosus (Fr.: Fr.) Fr. (GenBank acc. nos. JQ446111 and JQ446110), L. picinus Fr. (GenBank acc. nos. JQ446129 and JQ446130), L. pterosporous Romagn. (GenBank acc. nos. JQ446138 and JQ446136), L. romagnesii Bon (GenBank acc. nos. JQ446143 and EF560662) and L. ruginosus Romagn. (GenBank acc. nos. JQ446106 and EF560660) are found to be closely related (93–95 % identity with L. dirkii for 95–98 % query coverage using BLAST) to L. dirkii (showing significant support with the Asian taxa of L. subg. Plinthogali in Fig. 176). However, mostly these species are dark coloured in contrast to the white basidiome of L. dirkii, making it easily distinguishable from them in the field. Moreover, L. acris shows a completely different pattern of pileipellis i.e., ixooedotrichoderm to ixotrichopalisade with capitate terminal elements (Heilmann-Clausen et al. 1998); L. fuliginosus and L. picinus (also reported from India) have dark coloured cap, scarce latex, trichoepithelium pattern of pileipellis and lower (1 μm) spore ornamentations (Heilmann-Clausen et al. 1998) and strictly conifer association in later species (Stubbe and Verbeken 2012). Among other close European taxa, L. romagnesii and L. ruginosus have fuscous colour of pileus, irregularly crenate to grooved margin, distant gills and rather sparse latex and spores with high ornamentations that are reticulate in L. romagnesii but zebroid in L. ruginosus (Stubbe and Verbeken 2012), while L. azonites can be separated from the present taxon by distant gills, larger spores (7.3–9.3 × 6.8–8.3 μm), lower ornamentations (up to 1 μm) and hyphoepithelium to trichoepithelium type of pileipellis (Heilmann-Clausen et al. 1998). Lactarius pterosporus shares similar pileipellis structure (a trichopalisade of 80–120 μm thick), but differs in having dark greyish-buff coloured cap, sparse latex and higher (up to 2.5 μm) spore ornamentations (Heilmann-Clausen et al. 1998).
Molecular data also indicates genetic closeness (95 % identity with L. dirkii for 95 % query coverage using BLAST) of L. dirkii with L. fumosibrunneus A.H. Sm. & Hesler (reported from North American continent and labelled with GenBank acc. nos. JQ797634 and JQ797633 in Fig. 176), which is different from the former in brownish, rugose cap, negative reaction of KOH on surface and reddening of tissue by latex. Micromorphologically, hymenoepithelium nature of pileipellis and presence of abundant cheilocystidia in L. fumosibrunneus are also quite distinct (Bandala and Montoya 2010).
Few Indian species of L. subg. Plinthogali namely, L. crenulatus K. Das & Verbeken, L. montoyae K. Das & J.R. Sharma, L. croceigalus K. Das & Verbeken and L. vesterholtii K. Das & D. Chakr. partly resemble micromorphologically (nature of pileipellis, absence of macrocystidia, pinkish discoloration of flesh and high spore ornamentations). But, L. crenulatus has distinctly smaller papillate dark-coloured pileus and slender stipe (Das and Verbeken 2012). Lactarius montoyae (also reported from Thailand) shows subdistant to distant lamellae, brown coloured basidiomata, white unchanging latex which does not stain tissues (Le et al. 2007; Das and Sharma 2005). Lactarius croceigalus differs from L. dirkii on the basis of larger, papillate and dark colored pileus, distant lamellae and larger spores (9–9.2–10.1 × 7.8–8.7–9.5 μm) as mentioned by Das and Verbeken (2012). Finally, Lactarius vesterholtii, which was recently discovered from Himalayan India under L. subg. Plinthogali, can be separated morphologically from the present species by crowded lamellae, typically orange discolouration of latex on exposed gills, brown to greyish-brown pileus, lack of pileopseudocystidia and showing palisade to lampropalisade nature of stipitipellis (Das and Chakraborty 2014).
Mortierellomycotina Kerst. Hoffm. et al.
Mortierellales Caval.-Sm.
Mortierellaceae A. Fisch.
We follow Li et al. (2016).
Mortierella Coem.
The genus Mortierella (Mortierellaceae, Mortierellales) was described by Coemans (1863) with type species M. polycephala Coem. To date, nearly 100 species of Mortierella have been described (Wagner et al. 2013). The species belonging to this genus are characterized by the production of a mainly coenocytic but becoming irregularly septate mycelium. Sporangiophores are simple or variously branched terminating with sporangia and occasionally with a swelling at the base. Sporangia are globose, multi-, few- or uni-spored. Species of Mortierella typically exhibit rapid growth at temperatures ranging from 15 °C to 25 °C. They are frequently isolated from soil and dead or dying plant tissue or from animal dung (Gams 1977; Benny 2006, 2008). Many of them show potential as producers of polyunsaturated fatty acids (Shinmen et al. 1989; Ogawa et al. 2012). In addition, several species of Mortierella have been used as pesticide degrading agent, suggesting that they might have potential for the bioremediation of sites contaminated with organochlorine pesticides (Kataoka et al. 2010).
Based on morphological characters, Gams (1977) divided Mortierella into nine sections: Actinomortierella, Alpina, Haplosporangium, Hygrophila, Mortierella, Schmuckeri, Simplex, Spinosa and Stylospora. Recently, molecular data have been used to evaluate the genus Mortierella (Petkovits et al. 2011; Wagner et al. 2013; Ariyawansa et al. 2015a, b, c).
While examining the diversity of fungi of the order Mortierellales isolated from freshwater sample of Yeongsan River in Gwangju, Korea, a new species was isolated and is described here based on morphological characteristics and phylogenetic analyses.
Mortierella fluviae Hyang B. Lee, K. Voigt & T.T.T. Nguyen, sp. nov.
MycoBank number: MB 817071; Facesoffungi number: FoF02474, Fig. 181
Etymology: referring to the freshwater which from the species was first isolated
Holotype: EML-YR25716-1.
Colonies reaching 45–48 mm diam. at 20 °C after 7 days incubation on PDA, cotton in the center with a white margin, the reverse white and regularly zonate: on OA, aerial hyphae dispersed on agar surface. Sporangiophores 115–350 μm long, 3.5–7.5 μm wide at the tip, 8.5–17 μm wide at the base, arising from the aerial stolons with 1–4 branches (av. 2–3). Sporangia 19–51 × 23–52 μm, globose, multi-spored, with deliquescent wall, sometimes with a bell-shaped apophysis. Columellae 3.5–8 × 5–10 μm, hemisphaerical or subglobose, with small projection on the apex. Collarette appearing after sporangium maturation. Spores globose to ellipsoidal or pyriform, 6.5–11.5 × 5.5–8.5 μm. Chlamydospores absent in aerial mycelia. Zygospores not observed.
The isolate was observed to grow over a wide range of temperatures with varying growth rates on PDA, OA (oatmeal agar), and WA (water agar). The average growth rates on PDA, OA, and WA were 6.5 mm, 6 mm, and 5 mm per 24 h, respectively. Optimal growth was observed around 15–25 °C, maximum around 30 °C, and no growth at 35 °C (Fig. 181).
Notes: Mortierella fluviae is similar to M. gamsii but differs by shorter sporangiospores and having a bell-shaped apophysis. Spores are variable in shape. In the phylogenetic trees the ITS and 28S rDNA sequences of the strain formed a branch separate from other species of Mortierella, showing it represents a new species (Figs. 179, 180, respectively).
Phylogenetic tree of Mortierella fluviae EML-YR25716-1 and EML-YR25716-2 and related species before based on maximum likelihood analysis of ITS rDNA sequences. Sequence of Umbelopsis isabellina was used as outgroup. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replications. The bar indicates the number of substitutions per position. New taxa are in blue
Phylogenetic tree of Mortierella fluviae EML-YR25716-1 and EML-YR25716-2 and related species based on maximum likelihood analysis of 28S rDNA sequences. Sequence of Umbelopsis isabellina was used as outgroup. Numbers at the nodes indicate the bootstrap values (>50 %) from 1000 replications. The bar indicates the number of substitutions per position. New taxa are in blue
Material examined: REPUBLIC OF KOREA, Jeonnam Province, Yeongsan River located in Gwangju (35°10′N 126°55′E), from a freshwater sample, 15 February 2016; holotype, ex-type living culture EML-YR25716-1 at Culture Collection of Nakdonggang National Institute of Biological Resources (NNIBR), Sangju, Gyeongbuk Province, and preserved as glycerol stock at −80 °C in the Chonnam National University Fungal Collection (CNUFC) under deposition number (CNUFC-EML-YR25716-1) and living culture (ex-type) deposited at Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:012332).
Mortierella fluviae (holotype). a, d Colony in potato dextrose agar. b, e Colony in oatmeal agar. c, f Colony in water agar (a–c above view, d–f reverse view) (g–r light microscope; s–u SEM). g–j Young and mature sporangia on sporangiophores and apophysis (i, s) (white arrow). k–m Single and branched sporangiophores. n–r Different shapes of columellae (r, t) (purple arrow) with collarette (green arrow) and sporangial septum (yellow arrow). u Spores. Scale bars g–j, n, p–r = 20 μm, k–m, o = 50 μm, s = 30 μm, t = 10 μm, u = 5 μm
Mucoromycotina Benny
Mucorales Fr.
Cunninghamellaceae Naumov ex R.K. Benj.
Cunninghamella Matr.
Cunninghamella includes saprobes species commonly isolated from soil, decaying fruit or rotten wood, and some species have been responsible for disseminating infections in humans, especially immunocompromised patients (Yu et al. 2015). Species of Cunninghamella have traditionally been distinguished based on their morphology, especially in their sporangial stages (Baijal and Mehrotra 1980; Zheng and Chen 2001; Yu et al. 2015). Since 1991, other characteristics, such as maximum growth temperature, the color and texture of colonies, mating compatibility, zygospore formation as well as molecular tools have been used to delimitate the species (Zhou and Huang 1991; Zheng and Chen 1994, 1998; Liu et al. 2001; Gherbawy and Voigt 2010). Zheng and Chen (2001) monographed the genus based on morphological characteristics, maximum growth temperature and mating experiments, as well as the entire length of the ITS region, describing twelve species and three varieties: C. bertholletiae Stadel, C. blakesleeana Lendn., C. binarie R.Y. Zheng, C. clavata R.Y. Zheng & G.Q. Chen, C. echinulata var. antarctica (Caretta & Piont.) R.Y. Zheng & G.Q. Chen, C. echinulata var. echinulata (Thaxt.) Thaxt. ex Blakeslee, C. echinulata var. nodosa R.Y. Zheng, C. echinulata var. verticillata (F.S. Paine) R.Y. Zheng & G.Q. Chen, C. elegans Lendn., C. homothallica Komin. & Tubaki, C. intermedia K.B. Deshp. & Mantri, C. multiverticillata R.Y. Zheng & G.Q. Chen, C. phaeospora Boedijn and C. septata R.Y. Zheng. Liu et al. (2011) and Yu et al. (2015) amplified the ITS rDNA region and TEF-1α of all the species described by Zheng and Chen (2001) and found their results to be consistent with morphological studies performed by previous authors.
Cunninghamella gigacellularis A.L. Santiago, C.L. Lima & C.A. de Souza, sp. nov.
Index Fungorum number: IF552131; Facesoffungi number: FoF02204, Fig. 184
Etymology: gigacellularis. A reference to the giant cells produced in the mycelium
Holotype: URM 7400.
Colonies white, floccose, reaching 9 cm diam. and touching the plate lid after 4 days on BDA at 25 °C. Reverse light yellow. Rhizoids frequent, long or short, simple or weakly branched. Stolons present, coenocytic, some with septa near the site of origin of the sporophore. Sporophores erect, straight or recumbent, smooth-walled, arising from stolons or from aerial hyphae; main axes of the sporophores usually equal in width throughout, (5–)6.5–10(–12.5) μm diam., predominantly terminated with a vesicle, but with some dividing in branches at the apices; branches monopodial, verticillate and pseudoverticillate; short and long branches (up to 500 × 7.5 μm) in the same sporophore are common, repeatedly branching 1–6 times, terminating with a vesicle. Some branches arise from a bulbous base. Septa in sporophores may be present at its base, near to the branches of the sporophore or below the vesicles. Vesicles of the main sporophores light grey, smooth-walled, globose and subglobose, some lightly depressed, but rarely obovoid or piriform, (14–)20–35 μm diam. Lateral vesicles light grey, smooth-walled, globose and subglobose (10–)12.5–20(–25) μm diam. Sporangioles hyaline, subsmooth to very shortly echinulate, mostly globose 5–7.5 μm diam., some subglobose and ellipsoid (12.5–)7.5–9 × 6–8(–10) μm, rarely with a pointed end. The larger sporangioles are light grey to pale brown and more echinulate than the smaller ones. Giant cells subglobose, some hypha-like and irregularly swollen, simple or branched. Zygosporangia not observed.
Material examined: BRAZIL, Itambé: Instituto Agronômico de Pernambuco (7°97′60″S, 35°47′88″W), in soil samples. Soil, 11.X.2013, leg. C. Lima (URM 7400, holotype) and deposited in the Jena Microbial Resource Collection (University of Jena and Leibniz Institute for Natural Product Research and Infection Biology, Jena, Germany) (JMRC:SF:012269).
Media and temperature tests: On BDA. At 15 °C—very limited growth (2.6 cm in diam. in 120 h); total lack of reproductive structures. At 20 °C—slow growth (5 cm in 120 h); good sporulation. At 25 °C—good growth (8 cm in 120 h); good sporulation. At 30 and 35 °C—excellent growth (9 cm in 96 h); excellent sporulation. At 40 °C—6.8 cm in 96 h; rare sporophores formed, very poor sporulation. At 42 °C—total lack of growth and sporulation. The growth of C. gigacellularis on MEA was slightly slower than on PDA at all tested temperatures, except at 25 °C. Abundant sterile mycelium was observed on PDA and MEA cultures at 30, 35 and 40 °C.
Notes: Cunninghamella gigacellularis is distinguished from other species of the genus as it produces giant cells that have not so far been reported for this genus. The new species also simultaneously presents a white colony and produces monopodially, verticillately and pseudoverticillately branched sporophores, as well as producing long and short branches in the same repeatedly branched sporophore. At first, C. gigacellularis could be confused with C. bertholletiae Stadel because of the branching pattern of the sporophores. However, only the former produces giant cells. Colonies of C. gigacellularis are persistently white, in contrast to the drab to mouse grey colonies of C. bertholletiae (Zheng and Chen 2001), and sporophores of C. gigacellularis seem to be more repeatedly branched than those of C. bertholletiae. Additionally, the sporangiospores of C. bertholletiae are 4.5–9(–11) or 5–14.5(–16) × 3.5–9(–11) μm (Zheng and Chen 2001), larger than those of C. gigacellularis. Our molecular analysis (ITS and LSU rDNA regions, Figs. 182, 183, respectively) placed C. gigacellularis in a separate clade in ITS and LSU trees, confirming that it is genetically different from other species of the genus.
Phylogenetic tree of Cunninghamella constructed using the ITS rDNA sequences. Absidia anomala and Halteromyces radiatus were used as outgroup taxa. Sequences are labeled with their database accession numbers. Support values are from Bayesian inference. Sequences obtained in this study are clones annotated in blue
Phylogenetic tree of Cunninghamellaceae constructed using the large subunit (LSU) rDNA sequence data. Mortierella parvispora species was used as outgroup. Sequences are labeled with their database accession numbers. Support values are from Bayesian inference. The sequences obtained in this study are clones annotated in blue
References
Abbott SP, Currah RS (1997) The Hevellaceae: systematic revision and occurrence in northern and northwestern North America. Mycotaxon 62:1–125
Abdel-Aziz FA, Abdel-Wahab MA (2010) Lolia aquatica gen. et sp. nov. (Lindgomycetaceae, Pleosporales), a new coelomycete from freshwater habitats in Egypt. Mycotaxon 114:33–42
Adams GC, Surve-Iyer RS, Iezzoni AF (2002) Ribosomal DNA sequence divergence and group I introns within the Leucostoma species L. cinctum, L. persoonii, and L. parapersoonii sp. nov., ascomycetes that cause Cytospora canker of fruit trees. Mycologia 94:947–967
Adams GC, Wingfield MJ, Common R, Roux J (2005) Phylogenetic relationships and morphology of Cytospora species and related teleomorphs (Ascomycota, Diaporthales, Valsaceae) from Eucalyptus. Stud Mycol 52:1–144
Adams GC, Roux J, Wingfield MJ (2006) Cytospora species (Ascomycota, Diaporthales, Valsaceae): introduced and native pathogens of trees in South Africa. Australas Plant Pathol 35:521–548
Ainsworth GC, James PW, Hawksworth DL (1971) Ainsworth & Bisby‘s dictionary of the fungi, 6th edn. CAB, Kew
Allegrucci N, Elíades L, Cabello M, Arambarri A (2011) New species Koorchaloma and Ciliochorella from xeric forests in Argentina. Mycotaxon 115:175–181
Alves JL, Woudenberg JHC, Duarte LL, Crous PW, Barreto RW (2013) Reappraisal of the genus Alternariaster (dothideomycetes). Persoonia 31:77–85
Aptroot A (1991) A monograph of the Pyrenulaceae (excluding Anthracothecium and Pyrenula) and the Requienellaceae, with notes on the Pleomassariaceae, the Trypetheliaceae, and Mycomicrothelia (lichenized and nvoglon-lichenized ascomycetes). Bibl Lichenol 44:1–178
Aptroot A (1998) The integration of the taxonomy of lichenized and non-lichenized pyrenocarpous ascomycetes. Lichenologist 30:501–514
Aptroot A, Lücking R, Sipman HJM, Umaña L, Chaves JL (2008) A first assessment of the lichen biodiversity inventory in Costa Rica: Pyrenocarpous lichens with bitunicate asci. Bibl Lichenol 97:1–162
Ariyawansa HA, Camporesi E, Thambugala KM, Mapook A, Kang JC, Alias SA, Chukeatirote E, Thines M, Mckenzie EHC, Hyde KD (2014a) Confusion surrounding Didymosphaeria, phylogenetic and morphological evidence suggest Didymosphaeriaceae is not a distinct family. Phytotaxa 176:102–119
Ariyawansa HA, Hawksworth DL, Hyde KD, Jones EBG, Maharachchikumbura SSN, Manamgoda DS, Thambugala KM, Udayanga D, Camporesi E, Daranagama A, Jayawardena R, Liu JK, McKenzie EHC, Phookamsak R, Senanayake IC, Shivas RG, Tian Q, Xu JC (2014b) Epitypification and neotypification: guidelines with appropriate and inappropriate examples. Fungal Divers 69:57–91
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014c) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (=Montagnulaceae). Fungal Divers 68:69–104
Ariyawansa HA, Hyde KD, Jayasiri SC, Buyck B, Chethana KWT, Dai DQ, Dai YC, Daranagama DA, Jayawardena RS, Lücking R, Ghobad-Nejhad M, Niskanen T, Thambugala KM, Voigt K, Zhao RL, Li GJ, Doilom M, Boonmee S, Yang ZL, Cai Q, Cui YY, Bahkali AH, Chen J, Cui BK, Chen JJ, Dayarathne MC, Dissanayake AJ, Ekanayaka AH, Hashimoto A, Hongsanan S, Jones EBG, Larsson E, Li WJ, Li QR, Liu JK, Luo ZL, Maharachchikumbura SSN, Mapook A, McKenzie EHC, Norphanphoun C, Konta S, Pang KL, Perera RH, Phookamsak R, Phukhamsakda C, Pinruan U, Randrianjohany E, Singtripop C, Tanaka K, Tian CM, Tibpromma S, Abdel-Wahab MA, Wanasinghe DN, Wijayawardene NN, Zhang JF, Zhang H, Abdel-Aziz FA, Wedin M, Westberg M, Ammirati JF, Bulgakov TS, Lima DX, Callaghan TM, Callac P, Chang CH, Coca LF, Dal-Forno M, Dollhofer V, Fliegerová K, Greiner K, Griffith GW, Ho HM, Hofstetter V, Jeewon R, Kang JC, Wen TC, Kirk PM, Kytövuori I, Lawrey JD, Xing J, Li H, Liu ZY, Liu XZ, Liimatainen K, Thorsten Lumbsch H, Matsumura M, Moncada B, Nuankaew S, Parnmen S, Santiago ALCMDA, Sommai S, Song Y, de Souza CAF, de Souza- Motta CM, Su HY, Suetrong S, Wang Y, FongWS Yuan HS, Zhou LW, Réblová M, Fournier J, Camporesi E, Luangsa-ard JJ, Tasanathai K, Khonsanit A, Thanakitpipattana D, Somrithipol S, Diederich P, Millanes AM, Common RS, Stadler M, Yan JY, Li XH, Lee HW, Nguyen TTT, Lee HB, Battistin E, Marsico O, Vizzini A, Vila J, Ercole E, Eberhardt U, Simonini G, Wen HA, Chen XH, Miettinen O, Spirin V, Hernawati (2015a) Fungal diversity notes 111–252—taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 75:27–274
Ariyawansa HA, Phukhamsakda C, Thambugala KM, Bulgakov TS, Wanasinghe DN, Perera RH, Mapook A, Camporesi E, Kang JC, Jones EBG, Bahkali AH, Jayasiri SC, Hyde KD, Liu ZY, Bhat JD (2015b) Revision and phylogeny of Leptosphaeriaceae. Fungal Divers 74:19–51
Ariyawansa HA, Thambugala KM, Manamgoda DS, Jayawardena R, Camporesi E, Boonmee S, Wanasinghe DN, Phookamsak R, Hongsanan S, Singtripop C, Chukeatirote E (2015c) Towards a natural classification and backbone tree for Pleosporaceae. Fungal Divers 71(1):85–139
Arzanlou M, Groenewald JZ, Fullerton RA, Abeln ECA, Carlier J, Zapater MF, Buddenhagen IW, Viljoen A, Crous PW (2008) Multiple gene genealogies and phenotypic characters differentiate several novel species of Mycosphaerella and related anamorphs on banana. Persoonia 20:19–37
Aveskamp MM, Verkley GJM, de Gruyter J, Murace MA, Perelló A, Woudenberg JHC, Groenewald JZ, Crous PW (2009) DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 101:359–378
Aveskamp MM, de Gruyter J, Woudenberg JHC, Verkley GJM, Crou PW (2010) Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud Mycol 65:1–60
Bahl J (2006) Molecular evolution of three morphologically similar families in the Xylariomycetidae (Apiosporaceae, Clypeosphaeriaceae, Hyponectriaceae). Degree of Doctor of Philosophy at The University of Hong Kong in December 2006
Baijal U, Mehrotra BS (1980) The genus Cunninghamella—a reassessment. Sydowia 33:1–13
Bandala VM, Montoya L (2010) Lactarius fumosibrunneus in a relict Fagus grandifolia var. mexicana population in a Mexican montane cloud forest. Mycotaxon 114:333–342
Baroni TJ (1981) A revision of the genus Rhodocybe Maire (Agaricales). Nova Hedwig Beih 67:1–194
Baroni TJ, Horak E (1994) Entolomataceae in North America III: new taxa, new combinations and notes on species of Rhodocybe. Mycologia 86:138–145
Baroni TJ, Watling R (1999) Taxonomic and mycogeographic notes on some Malaysian fungi IV. Notes on Clitopilus and Rhodocybe. Mycotaxon 72(3):57–72
Barr ME (1975) Pestalosphaeria, a new genus in the Amphisphaeriaceae. Mycologia 67:187–194
Barr ME (1976) Perspectives in the Ascomycotina. Mem N Y Bot Gard 28:1–8
Barr ME (1978) The Diaporthales in North America with emphasis on Gnomonia and its segregates. Mycol Mem 7:1–232
Barr ME (1979) A classification of Loculoascomycetes. Mycologia 71:935–957
Barr ME (1980) On the family Tubeufiaceae (Pleosporales). Mycotaxon 12:137–167
Barr ME (1987) Prodromus to class Loculoascomycetes. Published by the author, Amherst
Barr ME (1994) Notes on the Amphisphaeriaceae and related families. Mycotaxon 51:191–224
Barseghyan GS, Wasser SP (2007) Peziza proteana f. sparassoides—a rare taxon for Asian mycobiota from Israel. Mycol Balc 4:161–164
Basso MT (1999) Lactarius Pers. Fungi Europaei 7. Mykoflora, Alassio
Benny GL (2006) Zygomycota. Published on the internet at www.zygomycetes.org
Benny GL (2008) Methods used by Dr. R.K. Benjamin, and other mycologists, to isolate zygomycetes. Aliso 26:37–61
Berkeley MJ, Broome CE (1874) Enumeration of the fungi of Ceylon. Part II. Bot J Linn Soc 14:29−141
Berkeley MJ, Broome CE (1875) The Rev. M.J. Berkeley and Mr. C.E. Broome on the fungi of Ceylon. Journal of the Linnean Society of London 2:110–111
Berlese AN (1896) Icones Fungorum omnium hucusque cognitorum ad usum Sylloges Saccardianae adcommodatae. Avellino E Pergola 2:29−84
Birkebak JM, Mayor JR, Ryberg KM, Matheny PB (2013) A systematic, morphological and ecological overview of the Clavariaceae (Agaricales). Mycologia 105:896–911
Bitzer J, Læssøe T, Fournier J, Kummer V, Decock C, Tichy HV, Piepenbring M, Peršoh D, Stadler M (2008) Affinities of Phylacia and the daldinoid Xylariaceae, inferred from chemotypes of cultures and ribosomal DNA sequences. Mycol Res 112:251–270
Boehm EW, Schoch CL, Spatafora JW (2009a) On the evolution of the Hysteriaceae and Mytilinidiaceae (Pleosporomycetidae, Dothideomycetes, Ascomycota) using four nuclear genes. Mycol Res 113:461–479
Boehm EWA, Mugambi G, Miller AN, Huhndorf S, Marincowitz S, Schoch CL, Spatafora JW (2009b) A molecular phylogenetic reappraisal of the Hysteriaceae, Mytilinidiaceae and Gloniaceae (Pleosporomycetidae, Dothideomycetes) with keysto world species. Stud Mycol 64:49–83
Boonmee S, Zhang Y, Chomnunti P, Chukeatirote E, Tsui CKM, Bahkali AH, Hyde KD (2011) Revision of lignicolous Tubeufiaceae based on morphological reexamination and phylogenetic analysis. Fungal Divers 51:63–102
Boonmee S, Rossman AY, Lui JK, Li WJ, Dai DQ, Bhat JD, Jones EBG, McKenzie EHC, Xu JC, Hyde KD (2014) Tubeufiales, ord. nov., integrating sexual and asexual generic names. Fungal Divers 68:239–298
Boonmee S, D’souza MJ, Luo Z, Pinruan U, Tanaka K, Su H, Bhat DJ, McKenzie EHC, Jones EBG, Taylor JE, Phillips AJL, Hirayama K, Eungwanichayapant PD, Hyde KD (2016) Dictyosporiaceae fam. nov. Fungal Divers. doi:10.1007/s13225-016-0363-z
Brandrud TE, Lindström H, Marklund H, Melot J, Muskos S (1998) Cortinarius Flora photographica IV. Matfors, Cortinarius
Brock PM, Döring H, Bidartondo MI (2009) How to know unknown fungi: the role of a herbarium. New Phytol 181:719–724
Bulgakov TS (2010) Microfungi of Leucostoma and Valsa genera and their Cytospora anamorphs on arboreal plants in the steppe zone of Southern Russia. Actual problems of ecology: Mater. IV All-Russian science conference in Vladikavkaz, North Ossetian State University, pp 40–45 (in Russian)
Buyck B, Mitchell D, Parrent J (2006) Russula parvovirescens sp. nov., a common but ignored species in the eastern United States. Mycologia 98:612–615
Buyck B, Hofstetter V, Eberhardt U, Verbeken A, Kauff F (2008) Walking the thin line between Lactarius and Russula: the dilemma of Russula sect. Ochricompactae. Fungal Divers 28:15–40
Buyck B, Hofstetter V, Verbeken A, Walleyn R (2010) Proposal 1919: To conserve Lactarius nom. cons. (Basidiomycota) with a conserved type. Mycotaxon 111:504–508
Cacialli G, Caroti V, Doveri F (1997) Peziza perdicina e Peziza moravecii, una sola entità? Funghi e Ambiente 74–75:39–40
Cannon PF, Kirk PM (2007) Fungal families of the world. CABI Bioscience, Wallingford
Cannon PF, Damm U, Johnston PR, Weir BS (2012) Colletotrichum-current status and future directions. Study Mycol 73:181–213
Castañeda-Ruiz RFC, Heredia G, Reyes M, Arias RM, Decock C (2001) A revision of the genus Pseudospiropes and some new taxa. Cryptogam Mycol 22:3–18
Castlebury LA, Rossman AY, Jaklitsch WJ, Vasilyeva LN (2002) A preliminary overview of the Diaporthales based on large subunit nuclear ribosomal DNA sequences. Mycologia 94:1017–1031
Chadefaud M (1960) Les végétaux non vasculaires (Cryptogamie). In: Chadefaud M, Emberger L (eds) Traité de botanique systématique. Tome I. Masson et Cie, Paris, pp 613–616
Cheewangkoon R, Crous PW, Hyde KD, Groenewald JZ, To-Anan C (2008) Species of Mycosphaerella and related anamorphs on Eucalyptus leaves from Thailand. Persoonia 21(1):77–91
Chen JJ, Cui BK (2014) Phlebiporia bubalina gen. et sp. nov. (Meruliaceae, Polyporales) from Southwest China with a preliminary phylogeny based on rDNA sequences. Mycol Prog 13:563–573
Chen YY, Cui BK (2016) Phylogenetic analysis and taxonomy of the Antrodia heteromorpha complex in China. Mycoscience 57:1–10
Chen JL, Tezan SS (2008) Hyphomycetes—Beltrania and allied species from Taiwan. Taiwania 53:301–307
Chen Q, Jiang JR, Zhang GZ, Cai L, Crous PW (2015a) Resolving the Phoma enigma. Mycol Res 82:137–217
Chen Q, Zhang K, Zhang GZ, Cai L (2015b) A polyphasic approach to characterise two novel species of Phoma (Didymellaceae) from China. Phytotaxa 197:267–281
Chen YY, Li HJ, Cui BK (2015c) Molecular phylogeny and taxonomy of Fibroporia (Basidiomycota) in China. Phytotaxa 203:47–54
Chen JJ, Cui BK, Dai YC (2016) Global diversity and molecular systematics of Wrightoporia s. l. (Russulales, Basidiomycota). Persoonia 37:21–36
Chesters CGC, Bell A (1970) Studies in the Lophiostomataceae. Myc Pap 120:1–51
Chesters CGC, Greenhalgh GN (1964) Geniculosporium serpens gen. et sp. nov., the imperfect morph of Hypoxylon serpens. Trans Br Mycol Soc 47:393–401
Chethana T, Liu M, Ariyawansa HA, Konta S, Wanasinghe DN, Zhou Y, Yan J, Camporesi E, Bulgakov TS, Chukeatirote E, Hyde KD, Bahkali AH, Liu J, Li X (2015) Splanchnonema-like species in Pleosporales: introducing Pseudosplanchnonema gen. nov. in Massarinaceae. Phytotaxa 231:133–144
Chevallier FF (1826) Flore Générale des Environs de Paris [General Flora of the Area Around Paris], vol 1. Ferra Jeune, France, pp 1–674
Chomnunti P, Schoch CL, Aguirre-Hudson B, KoKo TW, Hongsanan S, Jones EBG, Kodsub R, Chukeatirote E, Bahkali AH, Hyde KD (2011) Capnodiaceae. Fungal Divers 51:103–134
Chomnunti P, Bhat DJ, Jones EBG, Chukeatirote E, Bahkali AH, Hyde KD (2012a) Trichomeriaceae, a new sooty mould family of Chaetothyriales. Fungal Divers 56:63–76
Chomnunti P, KoKo TW, Chukeatirote E, Cai L, Jones EBG, Kodsueb R, Chen H, Hassan BA, Hyde KD (2012b) Phylogeny of Chaetothyriaceae in northern Thailand including three new species. Mycologia 104:382–395
Chomnunti P, Hongsanan S, Hudson BA, Tian Q, Peršoh D, Dhami MK, Alias AS, Xu J, Liu X, Stadler M, Hyde KD (2014) The sooty moulds. Fungal Divers 66:1–36
Clements FE (1909) The genera of fungi, 1st edn. H.W. Wilson, Minneapolis, p 114
Coemans E (1863) Quelques Hyphomycètes nouveaux. 1. notice: Mortierella polycephala et Martinella pectinata. Bull Cl Sci Acad R Belg 15:536–540
Coppins BJ (1988) Notes on the genus Arthopyrenia in the British Isles. Lichenologist 20:305–325
Corda ACI (1831) Die Pilze Deutschlands. In: Sturm J (ed) Deutschlands Flora in Abbildungen nach der Natur mit Beschreibungen, vol 12 (3). Sturm, Nürnberg, pp 33–64
Corner EJH (1950) A Monograph of Clavaria and Allied Genera. Oxford University Press, Oxford
Corner EJH (1970) Supplement to a monograph of Clavaria and allied genera. Beih Nova Hedwig 33:1–299
Crous PW, Groenewald JZ (2013) A phylogenetic re-evaluation of Arthrinium. IMA Fungus 4:133–154
Crous PW, Groenewald JZ, Mansilla JP, Hunter GC, Wingfield MJ (2004a) Phylogenetic reassessment of Mycosphaerella spp. and their anamorphs occurring on Eucalyptus. Stud Mycol 50:195–214
Crous PW, Groenewald JZ, Pongpanich K, Himaman W, Arzanlou M, Wingfield MJ (2004b) Cryptic speciation and host specificity among Mycosphaerella spp. occurring on Australian Acacia species grown as exotics in the tropics. Stud Mycol 50:457–469
Crous PW, Verkley GJ, Groenewald JZ (2006) Eucalyptus microfungi known from culture. 1. Cladoriella and Fulvoflamma genera nova, with notes on some other poorly known taxa. Stud Mycol 31:53–63
Crous PW, Braun U, Groenewald JZ (2007) Mycosphaerella is polyphyletic. Stud Mycol 58:1–3
Crous PW, Schoch CL, Hyde KD, Wood AR, Gueidan C, de Hoog GS, Groenewald JZ (2009) Phylogenetic lineages in the Capnodiales. Stud Mycol 64:17–47
Crous PW, Braun U, Hunter GC, Wingfield MJ, Verkley GJ, Shin HD, Nakashima C, Groenewald JZ (2013) Phylogenetic lineages in Pseudocercospora. Stud Mycol 75:37–114
Crous PW, Wingfield MJ, Schumacher RK, Summerell BA, Giraldo A, Gené J, Guarro J, Wanasinghe DN, Hyde KD, Camporesi E, Gareth Jones EB, Thambugala KM, Malysheva EF, Malysheva VF, Acharya K, Álvarez J, Alvarado P, Assefa A, Barnes CW, Bartlett JS, Blanchette RA, Burgess TI, Carlavilla JR, Coetzee MP, Damm U, Decock CA, den Breeÿen A, de Vries B, Dutta AK, Holdom DG, Rooney-Latham S, Manjón JL, Marincowitz S, Mirabolfathy M, Moreno G, Nakashima C, Papizadeh M, Shahzadeh Fazeli SA, Amoozegar MA, Romberg MK, Shivas RG, Stalpers JA, Stielow B, Stukely MJ, Swart WJ, Tan YP, van der Bank M, Wood AR, Zhang Y, Groenewald JZ (2014) Fungal planet description sheets: 281–319. Persoonia 33:212–289
Crous PW, Carris LM, Giraldo A, Groenewald JZ, Hawksworth DL, Hernández-Restrepo M, Jaklitsch WM, Lebrun MH, Schumacher RK, Stielow JB, van der Linde EJ, Vilcāne J, Voglmayr H, Wood AR (2015a) The Genera of Fungi - fixing the application of the type species of generic names—G2: Allantophomopsis, Latorua, Macrodiplodiopsis, Macrohilum, Milospium, Protostegia, Pyricularia, Robillarda, Rotula, Septoriella, Torula, and Wojnowicia. IMA Fungus 6(1):163–198
Crous PW, Müller MM, Sánchez RM, Giordano L, Bianchinotti MV, Anderson FE, Groenewald JC (2015b) Resolving Tiarosporella spp. allied to Botryosphaeriaceae and Phacidiaceae. Phytotaxa 202:073–093
Dai YC (1999) Changbai wood-rotting fungi 11. Species of Polyporus sensu stricto. Fungal Sci 14:67–77
Dai YC, Yu CJ, Wang HC (2007) Polypores from eastern Xizang (Tibet), western China. Ann Bot Fennici 44:135–145
Dai DQ, Wijayawardene NN, Bhat DJ, Chukeatirote E, Bahkali AH, Zhao RL, Xu JC, Hyde KD (2014) Pustulomyces gen. nov. accommodated in Diaporthaceae, Diaporthales, as Revealed by Morphology and Molecular Analyses. Cryptogamie Mycol 35:63–72
Dai DQ, Phookamsak R, Wijayawardene NN, Li WJ, Bhat DJ, Xu JC, Taylor JE, Hyde KD, Chukeatirote E (2016) Bambusicolous fungi. Fungal Divers (in press)
Damm U, Woudenberg JHC, Cannon PF, Crous PW (2009) Colletotrichum species with curved conidia from herbaceous hosts. Fungal Divers 39:45–87
Das K, Chakraborty D (2014) Lactarius vesterholtii, a new species from India. Mycotaxon 129:477–484
Das K, Sharma JR (2004) Lactarius in Kumaon Himalaya 2: new and interesting species of subgenus Plinthogali. Mycotaxon 89:289–296
Das K, Sharma JR (2005) Russulaceae of Kumaon Himalaya. Botanical Survey of India, Kolkata
Das K, Verbeken A (2011) Three new species of Lactarius from Sikkim, India. Cryptogam Mycol 32:365–381. doi:10.7872/crym.v32.iss4.2011.365
Das K, Verbeken A (2012) New species of Lactarius subg. Plinthogalus and new records of Lactifluus subg. Gerardii (Russulaceae) from Sikkim, India. Taiwania 57:37–48
Das K, Sharma JR, Atri NS (2006) Russula in Himalaya 3: a new species of subgenus Ingratula. Mycotaxon 95:271–275
Das K, Van de Putte K, Buyck B (2010) New or interesting Russula from Sikkim Himalaya (India). Cryptogam Mycol 31:373–387
Das K, Atri NS, Buyck B (2013) Three new species of Russula (Russulales) from India. Mycosphere 4:707–717
Das K, Dowie N, Li GJ, Miller SL (2014) Two new species of Russula (Russulales) from India. Mycosphere 5:612–622
Das K, Verbeken A, Nuytinck J (2015) Morphology and phylogeny of four new Lactarius species from Himalayan India. Mycotaxon 130:105–130
Das K, Hembrom ME, Parihar A, Zhao RL (2016) A new species of Cyathus (Agaricaceae) from India. Turk J Bot 40:97–103
Dayarathne MC, Phookamsak R, Ariyawansa HA, Jones EBG, Camporesi E, Hyde KD (2015) Phylogenetic and morphological appraisal of Leptosphaeria italica sp. nov. (Leptosphaeriaceae, Pleosporales) from Italy. Mycosphere 6:634–642
de Almeida DAC, Gusmão LFP, Miller AN (2014) Brazilian semi-arid ascomycetes I: new and interesting records of hysteriaceous ascomycetes. Mycosphere 5:379–391
De Gruyter J, Aveskamp MM, Woudenberg JHC, Verkley GJM, Groenewald JZ, Crous PW (2009) Molecular phylogeny of Phoma and allied anamorph genera: towards a reclassification of the Phoma complex. Mycol Res 113:508–519
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2010) Systematic reappraisal of species in Phoma section Paraphoma, Pyrenochaeta and Pleurophoma. Mycologia 102:1066–1081
De Gruyter J, Woudenberg JHC, Aveskamp MM, Verkley GJM, Groenewald JZ, Crous PW (2013) Redisposition of Phoma-like anamorphs in Pleosporales. Stud Mycol 75:1–36
Dennis RWG (1981) British ascomycetes. Addenda and corrigenda. J Cramer, Vaduz, 40 pp
Diedicke H (1912) Die Abteilung Hyalodidymae der Sphaerioideen. Ann Mycol 10:135–152
Dissanayake AJ, Liu M, Zhang W, Chen Z, Udayanga D, Chukeatirote E, Li XH, Yan JY, Hyde KD (2015) Morphological and molecular characterization of Diaporthe species associated with grapevine trunk disease in China. Fungal Biology 119:283–294
Dissanayake AJ, Camporesi E, Hyde KD, Phillips AJL, Fu CY, Yan JY, Li XH (2016) Dothiorella species associated with woody hosts in Italy. Mycosphere 7:51–63
Dissing H (1966) The genus Helvella in Europe, with special emphasis on the species found in Norden. Dansk Botanisk Arkiv 25:1–172
Dissing H (1979) Helvella papuensis, a new species from Papua New Guinea. Sydowia Annales Mycologici Beihefte 8:156–161
Doilom M, Dissanayake AJ, Wanasinghe DN, Boonmee S, Liu JK, Bhat DJ, Taylor JE, Bahkali AH, McKenzie EHC, Hyde KD (2016) Microfungi on Tectona grandis (teak) in Northern Thailand. Fungal Divers. doi:10.1007/s13225-016-0368-7
Donadini JC (1981) Le genre Peziza dans le Sud-Est de la France. Thèse. Lab. de chimie gén., univ. de Provence, Marseille
Donk MA (1964) A conspectus of the families of Aphyllophorales. Persoonia 3:199–324
Doveri F (2004) Fungi Fimicoli Italici. Associazione Micologica Bresadola, Trento, p 1104
Durand EJ (1919) Peziza proteana var. sparassoides in America. Mycologia 11:1–3
Dutta AK, Paloi S, Pradhan P, Acharya K (2015) A new species of Russula (Russulaceae) from India based on morphological and molecular (ITS sequence) data. Turk J Bot 39:850–856
Dyer PS, O’Gorman CM (2011) A fungal sexual revolution: Aspergillus and Penicillium show the way. Curr Opin Microbiol 14:649–654
Ehrenberg CG (1818) Sylvae mycologicae Berolinenses. Bruschke, Berlin, pp 1–32
Ellis MB (1958) Clasterosporium and some allied Dematiaceae Phragmosporae, I. Mycol Pap 70:1–89
Ellis MB, Ellis JP (1985) Microfungi on land plants. An identification handbook. Macmillan, London
Eriksson B (1970) On ascomycetes on Diapensales and Ericales in Fennoscandia. 1. Discomycetes. Symb Bot Upsal 19:1–71
Eriksson OE (1981) The families of bitunicate ascomycetes. Nord J Bot 1:800
Eriksson OE, Hawksworth DL (1993) Outline of the ascomycetes-1993. Syst Ascomycetum 12:51–257
Ertz D, Diederich P (2015) Dismantling Melaspileaceae: a first phylogenetic study of Buelliella, Hemigrapha, Karschia, Labrocarpon and Melaspilea. Fungal Divers 71:141–164
Ertz D, Diederich P, Lawrey JD, Berger F, Freebury CE, Coppins B, Gardiennet A, Hafellner J (2015) Phylogenetic insights resolve Dacampiaceae (Pleosporales) as polyphyletic: Didymocyrtis (Pleosporales, Phaeosphaeriaceae) with Phoma-like anamorphs resurrected and segregated from Polycoccum (Trypetheliales, Polycoccaceae fam. nov.). Fungal Divers 74:53–89
Ertz D, Heuchert B, Braun U, Freebury CE, Common RS, Diederich P (2016) Contribution to the phylogeny and taxonomy of the genus Taeniolella, with a focus on lichenicolous taxa. Fungal Biol. doi:10.1016/j.funbio.2016.05.008
Eschweiler FG (1824) Systema Lichenum. Nürnberg
Fan XL, Liang YM, Ma R, Tian CM (2014) Morphological and phylogenetic studies of Cytospora (Valsaceae, Diaporthales) isolates from Chinese scholar tree, with description of a new species. Mycoscience 55:252–259
Fan XL, Hyde KD, Liu M, Liang YM, Tian CM (2015a) Cytospora species associated with walnut canker disease in China, with description of a new species C. gigalocus. Fungal Biol 119:310–319
Fan XL, Hyde KD, Yang Q, Liang YM, Ma R, Tian CM (2015b) Cytospora species associated with canker disease of three anti desertification plants in northwestern China. Phytotaxa 197:227–244
Farr DF, Bills GF (1995) Wojnowicia colluvium sp. nov. isolated from conifer litter. Mycologia 87:518–524
Farr ML, Goos RD (1989) Subicularium reticulatum gen. et sp. nov., an unusual fungus from Venezuela. Mem N Y Bot Gard 49:66–69
Flores-Bustamante ZR, Rivera-Orduña FN, Martínez-Cárdenas A, Flores-Cotera LB (2010) Microbial paclitaxel: advances and perspectives. J Antibiot 63:460–467
Fotouhifar KB, Hedjaroude GA, Leuchtmann A (2010) ITS rDNA phylogeny of Iranian strains of Cytospora and associated teleomorphs. Mycologia 102:1369–1382
Fries EM (1822) Morchella, Helvella. Systema Mycologicum 2:1–275
Fries EM (1838) Epicrisis systematis mycologici, seu synopsis Hymenomycetum, vol i−xii. Typographia Academica, Uppsala, pp 1–612
Fries EM (1849) Summa vegetabilium scandinaviae. Part 2. Bonnier, Leipzig
Fuckel KWGL (1864) Fungi Rhenani exsiccati, Fasc. IX-X, no 801-1000. Hedwigia 3:161–165
Fuckel KWGL (1869) Sytnbolae mycologicae. J Niedner, Wiesbaden, pp 1–459
Furtado ANM, Daniëls PP, Neves MA (2016) New species and new records of Clavariaceae (Agaricales) from Brazil. Phytotaxa 253:1–26
Gams W (1977) A key to the species of Mortierella. Persoonia 9:381–391
García-Sandoval R, Cifuentes J, De Luna E, Estrada-Torres A, Villegas M (2005) A phylogeny of Ramariopsis and allied taxa. Mycotaxon 94:265–292
Garnica S, Weiss M, Walther G, Oberwinkler F (2007) Reconstructing the evolution of agarics from nuclear gene sequences and basidiospore ultrastructure. Mycol Res 111:1019–1029
Garnica S, Schön ME, Abarenkov K, Riess K, Liimatainen K, Niskanen T, Dima B, Soop K, Frøslev TG, Jeppesen TS, Peintner U, Kuhnert-Finkernagel R, Brandrud TE, Saar G, Oertel B, Ammirati JF (2016) Determining threshold values for barcoding fungi: lessons from Cortinarius (Basidiomycota), a highly diverse and widespread ectomycorrhizal genus. FEMS Microbiol Ecol. doi:10.1093/femsec/fiw045
Geesink J (1984) Peziza proteana var. sparassoides new record for the Netherlands. Coolia 27(2):33–35
Gherbawy Y, Voigt K (2010) Molecular identification of fungi. Springer, Berlin
Gilbertson RL, Ryvarden L (1987) North American polypores. Fungiflora, Oslo
Góes-Neto A, Loguercio-Leite C, Guerrero RT (2005) DNA extraction from frozen field collected and dehydrated herbarium fungal basidiomata: performance of SDS and CTAB-based methods. Biotemas 18:19–32
Goh TK, Hyde KD, Ho WH (1998) Aquaphila albicans gen et sp. nov., a hyphomycete from submerged wood in the tropics. Mycol Res 102:587–592
Gomes RR, Glienke C, Videira SIR, Lombard L, Groenewald JZ, Crous PW (2013) Diaporthe: a genus of endophytic, saprobic and plant pathogenic fungi. Persoonia 31:1–41
Gregory SS, John WT (1999) Phylogenetic relationships of Meliola and Meliolina inferred from nuclear small subunit rRNA sequences. Mycol Res 103:1049–1056
Grum-Grzhimaylo AA, Georgieva ML, Debets AJM, Bilanenko EN (2013) Are alkalitolerant fungi of the Emericellopsis lineage (Bionectriaceae) of marine origin? IMA Fungus 4:213–228
Guatimosim E, Firmino AL, Bezerra JL, Pereira OL, Barreto RW, Crous PW (2015) Towards a phylogenetic reappraisal of Parmulariaceae and Asterinaceae (Dothideomycetes). Persoonia 35:230–241
Guba EF (ed) (1961) Monograph of Monochaetia and Pestalotia. Harvard University Press, Cambridge
Gvritishvili MN (1982) The fungal genus Cytospora in the USSR. Izdatelstve Sabchota Sakarstvelo, Tbilisi
Häffner J (1985) Peziza perdicina (Velen.) Svrček ein wenig bekannter Becherling auch in der Bundesrepublik Deutschland gefunden. Natur Gesell Nürnb e V 40:21–23
Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acid Symp Ser 41:95–98
Han ML, Chen YY, Shen LL, Song J, Vlasák J, Dai YC, Cui BK (2016) Taxonomy and phylogeny of the brown-rot fungi: Fomitopsis and its related genera. Fungal Divers. doi:10.1007/s13225-016-0364-y
Hansen K, Pfister DH (2006) Systematics of the Pezizomycetes—the operculate discomycetes. Mycologia 98:1029–1040
Hansen K, Læssøe T, Pfister DH (2002) Phylogenetic diversity in the core group of Peziza inferred from ITS sequences and morphology. Mycol Res 106:879–902
Hansford CG (1946) The foliicolous Ascomycetes, their parasites and associated fungi. Mycol Pap 15:1–240
Harmaja H (1978) New species and combinations in Helvella and Gyromitra. Karstenia 18:57
Harris RC (1984) The family Trypetheliaceae (Loculoascomycetes: lichenized Melanommatales) in Amazonian Brazil. Acta Amazonica 14:55–80
Harrower E, Ammirati JF, Cappuccino AA, Ceska O, Kranabetter JM, Kroeger P, Lim S, Taylor T, Berbee ML (2011) Cortinarius species diversity in British Columbia and molecular phylogenetic comparison with European specimen sequences. Botany 89:799–810
Hawksworth DL, Sutton BC, Ainsworth GC (1983) Ainsworth and Bisby’s dictionary of the fungi, 7th edn. Commonwealth Mycological Institute, Kew
Hawksworth DL, Kirk PM, Sutton BC, Pegler DN (1995) Ainsworth & Bisby’s dictionary of the fungi, 8th edn. CAB International, Wallingford
Heilmann-Clausen J, Verbeken A, Vesterholt J (1998) The genus Lactarius. The Danish Mycological Society, Odense
Henkel TW, Aime MC, Largent DL, Baroni TJ (2010) The Entolomataceae of the Pakaraima Mountains of Guyana III: New species of Rhodocybe. Mycoscience 51:23–27
Hernández-Restrepo M, Castañeda-Ruiz RF, Gené J, Guarro J, Minter DW, Stadler M (2013) Microfungi from Portugal: Minimelanolocus manifestus sp. nov. and Vermiculariopsiella pediculata comb. nov. Mycotaxon 122:135–143
Hibbett DS, Binder M, Bischoff JF, Blackwell M, Cannon PF, Eriksson OE, Huhndorf S, James T, Kirk PM, Lücking R, Thorsten Lumbsch H, Lutzoni F, Matheny PB, McLaughlin DJ, Powell MJ, Redhead S, Schoch CL, Spatafora JW, Stalpers JA, Vilgalys R, Aime MC, Aptroot A, Bauer R, Begerow D, Benny GL, Castlebury LA, Crous PW, Dai YC, Gams W, Geiser DM, Griffith GW, Gueidan C, Hawksworth DL, Hestmark G, Hosaka K, Humber RA, Hyde KD, Ironside JE, Kõljalg U, Kurtzman CP, Larsson KH, Lichtwardt R, Longcore J, Miadlikowska J, Miller A, Moncalvo JM, Mozley-Standridge S, Oberwinkler F, Parmasto E, Reeb V, Rogers JD, Roux C, Ryvarden L, Sampaio JP, Schüssler A, Sugiyama J, Thorn RG, Tibell L, Untereiner WA, Walker C, Wang Z, Weir A, Weiss M, White MM, Winka K, Yao YJ, Zhang N (2007) A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547
Hirayama K, Tanaka K (2011) Taxonomic revision of Lophiostoma and Lophiotrema based on reevaluation of morphological characters and molecular analyses. Mycoscience 52:401–412
Hirayama K, Tanaka K, Raja HA, Miller AN, Shearer CA (2010) A molecular phylogenetic assessment of Massarina ingoldiana sensu lato. Mycologia 102:729–746
Hofmann TA (2009) Plant parasitic Asterinaceae and Microthyriaceae from the Neotropics (Panama). PhD thesis, The faculty of biological sciences at the J.W.Goethe-University, Frankfurt am Main
Hohmeyer H (1986) Ein Schlüssel zu den europaeischen Arten der Gattung Peziza L. Z Mykol 52:161–188
Hong SB, Lee M, Kim DH, Meijer M, Majoor E, VanKuyk PA, Samson RA (2012) Aspergillus cibarius sp. nov., from traditional Meju in Korea. J Microbiol 50:712–714
Hongo T (1979) Notulae mycologicae (16). Mem Shiga Univ 29:99–104
Hongsanan S, Li YM, Liu JK, Hofmann T, Piepenbring M, Bhat JD, Boonmee S, Doilom M, Singtripop C, Tian Q, Mapook A, Zeng XY, Bahkali AH, Xu JC, Mortimer PE, Wu XH, Yang JB, Hyde KD (2014) Revision of genera in Asterinales. Fungal Divers 68:1–68
Hongsanan S, Hyde KD, Bahkali AH, Campores E, Chomnunti P, Ekanayaka H, Gomes AAM, Hofstetter V, Jones EBG, Pinho DB, Pereira OL, Tian Q, Wanasinghe DN, Xu J-C, Buyck B (2015a) Fungal biodiversity profiles 11–20. Cryptogam Mycol 36:355–380
Hongsanan S, Qing T, Peršoh D, Zeng XY, Hyde KD, Chomnunti P, Boonmee S, Bahkali AH, Wen TC (2015b) Meliolales. Fungal Divers 74:91–141
Houbraken J, de Vries RP, Samson RA (2014) Modern taxonomy of biotechnologically important Aspergillus and Penicillium species. Adv Appl Microbiol 86:199–249
Hubka V, Reblova M, Rehulka J, Selbmann L, Isola D, de Hoog SG, Kolarik M (2014) Bradymyces gen. nov (Chaetothyriales, Trichomeriaceae), a new ascomycete genus accommodating poorly differentiated melanized fungi. Antonie Van Leeuwenhoek 106:979–992
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Hwang J, Zhao Q, Yang ZL, Wang Z, Townsend JP (2015) Solving the ecological puzzle of mycorrhizal associations using data from annotated collections and environmental samples—an example of saddle fungi. Environ Microbiol Rep. doi:10.1111/1758-2229.12303
Hyde KD (1996) Fungi from palms. XXIX. Arecophila gen. nov. (Amphisphaeriaceae, Ascomycota), with five new species and two new combinations. Nova Hedwig 63:81–100
Hyde KD, Fröhlich J, Taylor JE (1998) Fungi from palms. XXXVI. Reflections on unitunicate ascomycetes with apiospores. Sydowia 50:21–80
Hyde KD, Abd-Elsalam K, Cai L (2010) Morphology: still essential in a molecular world. Mycotaxon 114:439–451
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake IC, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yacharoen S, Yan JY, Zhang M (2013) Families of dothideomycetes. Fungal Divers 63:1–313
Hyde KD, Nilsson RH, Alias SA, Ariyawansa HA, Blair JE, Cai L, de Cock AWAM, Dissanayake AJ, Glockling SL, Goonasekara ID, Gorczak M, Hahn M, Jayawardena RS, van Kan JAL, Laurence MH, Lévesque CA, Li XH, Liu JK, Maharachchikumbura SSN, Manamgoda DS, Martin FN, McKenzie EHC, McTaggart AR, Mortimer PE, Nair PVR, Pawłowska J, Rintoul TL, Shivas RG, Spies CFJ, Summerell BA, Taylor PWJ, Terhem RB, Udayanga D, Vaghefi N, Walther G, Wilk M, Wrzosek M, Xu JC, Yan JY, Zhou N (2014) One stop shop:backbones trees for important phytopathogenic genera: I. Fungal Divers 67:21–125
Index Fungorum (2016) http://www.indexfungorum.org/Names/Names.asp
Izumitsu K, Hatoh K, Sumita T, Kitade Y, Morita A, Gafur A, Ohta A, Kawai M, Yamanaka T, Neda H, Ota Y, Tanaka C (2012) Rapid and simple preparation of mushroom DNA directly from colonies and fruiting bodies for PCR. Mycoscience 53:396–401
Jaczewski AA (1917) Opredelitel’ gribov. T. 2. Nesovershennye griby, Petrograd, p 803
Jayasiri SC, Hyde KD, Abd-Elsalam KA, Abdel-Wahab MA, Ariyawansa HA, Bhat J, Buyck B, Dai YC, Ertz D, Hidayat I, Jeewon R, Jones EBG, Karunarathna SC, Kirk P, Lei C, Liu JK, Maharachchikumbura SSN, McKenzie E, Ghobad-Nejhad M, Nilsson H, Pang KL, Phookamsak R, Rollins AW, Romero AI, Stephenson S, Suetrong S, Tsui CKM, Vizzini A, Wen TC, De Silva NI, Promputtha I, Kang JC (2015) The faces of fungi database: fungal names linked with morphology, molecular and human attributes. Fungal Divers 74:18–357
Jayawardena RS, Zhang W, Liu M, Maharachchikumbura SSN, Zhou Y, Huang JB, Nilthong S, Wang ZY, Li XH, Yan JY, Hyde KD (2015) Identification and characterization of Pestalotiopsis-like fungi related to grapevine diseases in China. Fungal Biol 119:348–361
Jayawardena RS, Liu M, Maharachchikumbura SSN, Zhang W, Xing Q, Hyde KD, Nilthong S, Li X, Yan J (2016) Neopestalotiopsis vitis sp. nov. causing grapevine leaf spot in China. Phytotaxa 258:63–074
Jeewon R, Liew ECY, Hyde KD (2002) Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Mol Phylogenet Evol 25:378–392
Jeewon R, Cai L, Liew ECY, Zhang KQ, Hyde KD (2003) Dyrithiopsis lakefuxianensis gen. et sp. nov. from Fuxian lake, Yunnan, China, and notes on the taxonomic confusion surrounding Dyrithium. Mycologia 95:911–920
Johnston PR (1988) A new species of Meloderma (Rhytismataceae), with notes on Meloderma and related genera. Mycotaxon 33:423–436
Johnston PR (1989) Rhytismataceae in New Zealand 2. The genus Lophodermium on indigenous plants. N Z J Bot 27:243–274
Johnston PR (2001) Monograph of the monocotyledon-inhabiting species of Lophodermium. Mycol Pap 176:1–239
Ju YM, Rogers JD (1996) A revision of the genus Hypoxylon. Mycologia memoir no. 20. APS Press, St. Paul, p. 365
Justavino DR, Kirschner R, Piepenbring M (2015) New species and new records of Meliolaceae from Panama. Fungal Divers 70:73–84
Kang JC, Hyde KD, Kong RYC (1999) Studies on the Amphisphaeriaceae. The Cainiaceae. Mycol Res 103:1621–1627
Kataoka R, Takagi K, Sakakibara F (2010) A new endosulfan-degrading fungus, Mortierella species, isolated from a soil contaminated with organochlorine pesticides. J Pest Sci 35:326–332
Katoh K, Misawa K, Kuma K, Miyata T (2002) MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res 30:3059–3066
Kimura M (1980) A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences. J Mol Evol 16:111–120
Kirk PM, Cannon PF, David JC, Stalpers JA (2001) Ainsworth and Bisby’s dictionary of the fungi, 8th edn. CABI Publishing, London
Kirk PM, Cannon PF, Minter DW, Stalpers JA (2008) Ainsworth & Bisby’s dictionary of the fungi, 10th edn. CABI, Wallingford
Kirschner R, Pang KL, Jones EBG (2013) Two cheirosporous hyphomycetes reassessed based on morphological and molecular examination. Mycol Prog 12:29–36
Kluting KL, Baroni TJ, Bergemann SE (2014) Toward a stable classification of genera within the Entolomataceae: a phylogenetic re-evaluation of the Rhodocybe-Clitopilus clade. Mycologia 106:1127–1142
Knapp DG, Kovács GM, Zajta E, Groenewald JZ, Crous PW (2015) Dark septate endophytic pleosporalean genera from semiarid areas. Persoonia 35:87–100
Ko KS, Jung HS (2002) Phylogenetic evaluation of Polyporus s. str. based on molecular sequences. Mycotaxon 82:315–322
Kobayasi Y (1971) Mycological reports from New Guinea and the Solomon Islands. 1–11. Nat Sci Mus Bull 14:367–551
Kobayasi Y, Shimizu D (1978) Cordyceps species from Japan. Bull Natl Mus 4:43–63
Kobayasi Y, Shimizu D (1982) Cordyceps species from Japan 4. Bull Natl Mus 8:79–91
Kohlmeyer J, Volkmann-Kohlmeyer B (1993) Atrotorquata and Loratospora: new ascomycete genera on Juncus roemerianus. Systema Ascomycetum 12:7–22
Kohlmeyer J, Volkmann-Kohlmeyer B, Eriksson OE (1995) Fungi on Juncus roemerianus. 2. New dictyosporous ascomycetes. Bot Mar 38:165–174
Kong A, Cifuentes J, Estrada-Torres A, Guzmán-Dávalos L, Garibay-Orijel R, Buyck B (2015) Russulaceae associated with mycoheterotroph Monotropa uniflora (Ericaceae) in Tlaxcala, Mexico: a phylogenetic approach. Cryptogam Mycol 36:479–512
Korf RP (1956) Daleomyces, Durandiomyces and other sparassoid forms of operculate Discomycetes. Mycologia 48:711–718
Korf RP (1973) Sparassoid ascocarps in Pezizales and Tuberales. Rep Tottori Mycol Inst (Japan) 10:389–403
Kotlaba F, Pouzar Z (1972) Taxonomic and nomenclatural notes on some Macromycetes. Česká Mykologie 26:217–222
Krug JC (1978) The genus Cainia and a new family, Cainiaceae. Sydowia 30:122–133
Krüger D, Petersen RH, Hughes KW (2006) Molecular phylogenies and mating study data in Polyporus with special emphasis on group ‘‘Melanopus’’ (Basidiomycota). Mycol Prog 5:185–206
Kuo M (2014) http://www.mushroomexpert.com/cyathus_striatus.html
Landeros F, Iturriaga T, Guzmán-Dávalos L (2012) Type studies in Helvella (Pezizales) 1. Mycotaxon 119:35–63
Lantz H, Johnston PR, Park D, Minter DW (2011) Molecular phylogeny reveals a core clade of Rhytismatales. Mycologia 103:57–74
Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948
Larsson A (2014) AliView: a fast and lightweight alignment viewer and editor for large datasets. Bioinformatics 30:3276–3278
Larsson E, Larsson KH (2003) Phylogenetic relationships of russuloid basidiomycetes with emphasis on aphyllophoralean taxa. Mycologia 95:1037–1065
Latha KPD, Raj KNA, Sharafudheen SA, Manimohan P (2015) Clitocybula sulcatada new species from India. Phytotaxa 208:63–69
Le Gal M (1941) Les Aleuria et les Galactinia. Revue de Mycologie, Supplément 6:56–82
Le HT, Stubbe D, Verbeken A, Nuytinck J, Lumyong S, Desjardin DE (2007) Lactarius in Northern Thailand: 2. Lactarius subgenus Plinthogali. Fungal Divers 27:61–94
Lebel T, Tonkin JE (2007) Australasian species of Macowanites are sequestrate species of Russula (Russula, Basidiomycota). Aust Syst Bot 20:355–381
Lee S, Crous PW, Wingfield MJ (2006) Pestalotioid fungi from Pestiona in the Cape Floral Kingdom. Stud Mycol 55:175–187
Leuchtmann A (1984) Über Phaeosphaeria Miyake und andere bitunicate Ascomyceten mit mehrfach querseptierten Ascosporen. Sydowia 37:75–194
Léveillé JH (1845) Champignons exotiques. Ann Sci Nat Bot 3:38–71
Li HJ, Cui BK (2013) Taxonomy and phylogeny of the genus Megasporoporia and its related genera. Mycologia 105:368–383
Li HJ, Cui BK, Dai YC (2014) Taxonomy and multi-gene phylogeny of Datronia (Polyporales, Basidiomycota). Persoonia 32:170–182
Li WJ, Bhat DJ, Camporesi E, Tian Q, Wijayawardene NN, Dai DQ, Phookamsak R, Chomnunti P, Bahkali AH, Hyde KD (2015) New asexual morph taxa in Phaeosphaeria. Mycosphere 6:681–708
Li GJ, Hyde KD, Zhao RN, Hongsanan S, Abdel-Aziz FA, Abdel-Wahab MA, Alvarado P, Alves-Silva G, Ammirati JF, Ariyawansa HA, Baghela A, Bahkali AH, Beug M, Bhat DJ, Bojantchev D, Boonpratuang T, Bulgakov TS, Camporesi E, Boro MC, Ceska O, Chakraborty D, Chen JJ, Chethana KWT, Chomnunti P, Consiglio G, Cui BK, Dai DQ, Dai YC, Daranagama DA, Das K, Dayarathne MC, Crop ED, De Oliveira RJV, de Souza CAF, de Souza JI, Dentinger BTM, Dissanayake AJ, Doilom M, Drechsler-Santos ER, Ghobad-Nejhad M, Gilmore SP, Góes-Neto A, Gorczak M, Haitjema GH, Hapuarachchi KK, Hashimoto A, He MQ, Henske JK, Hirayama K, Iribarren MJ, Jayasiri SC, Jayawardena RS, Jeon SJ, Jerônimo GH, Jesus AL, Jones EBG, Kang JC, Karunarathna SC, Kirk PM, Konta S, Kuhnert E, Langer E, Lee HS, Lee HB, Li WJ, Li XH, Liimatainen K, Lima DX, Lin CG, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Lücking R, Lumbsch HT, Lumyong S, Leaño EM, Marano AV, Matsumura M, McKenzie EHC, Mongkolsamrit S, Mortimer PE, Nguyen TTT, Niskanen T, Norphanphoun C, O’Malley MA, Parnmen S, Pawłowska J, Perera RH, Phookamsak R, Phukhamsakda C, Pires-Zottarelli CLA, Raspé O, Reck MA, Rocha SCO, de Santiago ALCMA, Senanayake IC, Setti L, Shang QJ, Singh SK, Sir EB, Solomon KV, Song J, Srikitikulchai P, Stadler M, Suetrong S, Takahashi H, Takahashi T, Tanaka K, Tang LP, Thambugala KM, Thanakitpipattana D, Theodorou MK, Thongbai B, Thummarukcharoen T, Tian Q, Tibpromma S, Verbeken A, Vizzini A, Vlasák J, Voigt K, Wanasinghe DN, Wang Y, Weerakoon G, Wen HA, Wen TC, Wijayawardene NN, Wongkanoun S, Wrzosek M, Xiao YP, Xu JC, Yan JY, Yang J, Yang SD, Hu Y, Zhang JF, Zhao J, Zhou LW, Peršoh D, Phillips AJL, Maharachchikumbura SSN (2016) Fungal Divers notes 253–366: taxonomic and phylogenetic contributions to fungal taxa. Fungal Divers 78:1–237
Lindau G (1897) Pyrenomycetineae, Laboulbeniineae. In: Engler A, Prantl K (eds) Die Natürlichen Pflanzenfamilien. Teil. 1, vol 1. Verlag von Wilhelm Engelmann, Leipzig, pp 321–505
Liu B, Du F, Cao JZ (1985) Some new species and new combination of the genus Helvella from China. Acta Mycol Sin 4:208–217
Liu YJ, Whelen S, Hall BD (1999) Phylogenetic relationships among ascomycetes: evidence from an RNA polymerase II subunit. Mol Biol Evol 16:1799–1808
Liu XY, Huang H, Zheng RY (2001) Relationships within Cunninghamella based on sequence analysis of ITS r DNA. Mycotaxon 80:77–95
Liu JK, Phookamsak R, Jones EBG, Zhang Y, Ko-Ko TW, Hu HL, Boonmee S, Doilom M, Chukeatirote E, Bahkali AH, Wang Y, Hyde KD (2011) Astrosphaeriella is polyphyletic, with species in Fissuroma gen. nov., and Neoastrosphaeriella gen. nov. Fungal Divers 51:135–154
Liu JK, Phookamsak R, Doilom M, Wikee S, Li YM, Ariyawansha H, Boonmee S, Chomnunti P, Dai DQ, Bhat J, Romero A, Zhuang WY, Monkai J, Jones EBJ, Chukeatirote E, Ko Ko TW, Zhao YC, Wang Y, Hyde KD (2012) Towards a natural classification of Botryosphaeriales. Fungal Divers 57:149–210
Liu JK, Phookamsak R, Dai DQ, Hyde KH (2014) Roussoellaceae, a new pleosporalean family to accommodate the genera Roussoella and Roussoellopsis. Phytotaxa 181:1–33
Liu JK, Hyde KD, Jones EBG, Ariyawansa HA, Bhat DJ, Boonmee S, Maharachchikumbura SSN, McKenzie EHC, Phookamsak R, Phukhamsakda C, Shenoy BD, Abdel-Wahab MA, Buyck B, Chen J, Chethana KWT, Singtripop C, Dai DQ, Dai YC, Daranagama DA, Dissanayake AJ, Doilom M, Dsouza MJ, Fan XL, Goonasekara ID, Hirayama K, Hongsanan S, Jayasiri SC, Jayawardena RS, Karunarathna SC, Li WJ, Mapook A, Norphanphoun C, Pang KL, Perera RH, Peršoh D, Pinruan U, Senanayake IC, Somrithipol S, Suetrong S, Tanaka K, Thambugala KM, Tian Q, Tibpromma S, Udayanga D, Wijayawardene NN, Wanasinghe DN, Wisitrassameewong K, Zeng XY, Abdel-Aziz FA, Adamčík S, Bahkali AH, Boonyuen N, Bulgakov T, Callac P, Chomnunti P, Greiner K, Hashimoto A, Hofstetter V, Kang JC, Lewis D, Li XH, Liu XZ, Liu ZY, Matsumura M, Mortimer PE, Rambold G, Randrianjohany E, Sato G, Sri-Indrasutdhi V, Tian CM, Verbeken A, von Brackel W, Wang Y, Wen TC, Xu JC, Yan JY, Zhao RL, Camporesi E (2015a) Fungal Divers notes 1–110: taxonomic and phylogenetic contributions to fungal species. Fungal Divers 72:1–197
Liu XY, Udayanga D, Luo ZL, Chen LJ, Zhou DQ, Su HY, Hyde KD (2015b) Backbone tree for Chaetothyriales with four new species of Minimelanolocus from aquatic habitats. Fungal Biol 119:1046–1062
Locquin M (1984) Mycologie Générale et Structurale. Masson, Paris
Lowe DA (1992) Fungal enzymes. In: Arora DK, Elander RP, Murekji KG (eds) Handbook of applied mycology. Fungal biotechnology, vol 4. Marcel Dekker Inc., New York, pp 681–706
Lu BS, Hyde KD (2000) A world monograph of Anthostomella. Fungal Divers Res 4:1–376
Luangsa-ard JJ, Tasanathai K, Mongkolsamrit S, Hywel-Jones NL (2008) Atlas of invertebrate-pathogenic fungi of Thailand, vol 2. BIOTEC, National Science and Tecnology Development Agency, Thailand
Luangsa-ard JJ, Houbraken J, van Doorn T, Hong SB, Borman AM, Hywel-Jones NL, Samson RA (2011) Purpureocillium, a new genus for the medically important Paecilomyces lilacinus. FEMS Microbiol Lett 321:141–149
Lumbsch HT, Huhndorf SM (2010) Myconet volume 14 Part One. Outline of Ascomycota-2009. Fieldiana Life Earth Sci 1:1–922
Luttrell ES (1951) Taxonomy of the pyrenomycetes. U Missouri Stud 24:1–120
Luttrell ES (1989) Morphology of Meliola floridensis. Mycologia 81:192–204
Ma J, Ma LG, Zhang YD, Castañeda-Ruiz RF, Zhang XG (2011a) Pseudospiropes linderae sp. nov. and notes on Minimelanolocus (both anamorphic Strossmayeria) new to China. Nova Hedwig 93:465–473
Ma J, Zhang YD, Ma LG, Zhang XG (2011b) Two new Minimelanolocus species from southern China. Mycotaxon 117:131–135
Maas Geesteranus RA (1967) De fungi van Nederland 2a. Pezizales—deel I (Discina, Helvella, Morchella, Peziza, Rhizina). Wetensch Meded Kon Ned Natuurhist Ver 69:1–72
Maerz A, Paul MR (1950) A dctionary of color. McGraw-Hill book Company, New York
Maharachchikumbura SSN, Guo LD, Cai L, Chukeatirote E, Wu WP, Sun X, Crous PW, Bhat DJ, MaKenzie EHC, Bahkali AH, Hyde KD (2012) A multi-locus backbone tree for Pestalotiopsis, with a polyphasic characterization of 14 new species. Fungal Divers 56:95–129
Maharachchikumbura SSN, Guo LD, Chukeatirote E, McKenzie EHC, Hyde KD (2013) A destructive new disease of Syzygium samarangense in Thailand caused by the new species Pestalotiopsis samarangensis. Trop Plant Pathol 38:227–235
Maharachchikumbura SSN, Guo L-D, Chukeatirote E, Hyde KD (2014a) Improving the backbone tree for the genus Pestalotiopsis; addition of P. steyaertii and P. magna sp. nov. Mycol Prog 3:617–624
Maharachchikumbura SSN, Hyde KD, Groenewald JZ, Xu J, Crous PW (2014b) Pestalotiopsis revisited. Stud Mycol 79:121–186
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang S-K, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu JK, Liu ZY, Norphanphoun C, Shenoy BD, Xiao Y, Bahkali AH, Kang J, Somrothipol S, Suetrong S, Wen T, Xu J (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Bhat DJ, Dayarathne MC, Huang SK, Norphanphoun C, Senanayake IC, Perera RH, Shang QJ, Xiao YP, D’souza MJ, Hongsanan S, Jayawardena RS, Daranagama DA, Konta S, Goonasekara ID, Zhuang WY, Jeewon R, Phillips AJL, Abdel-Wahab MA, Al-Sadi AM, Bahkali AH, Boonmee S, Boonyuen N, Cheewangkoon R, Dissanayake AJ, Kang JC, Li QR, Liu JK, Liu XZ, Liu ZY, Luangsa-ard JJ, Pang KL, Phookamsak R, Promputtha I, Suetrong S, Stadler M, Wen TC, Wijayawardene NN (2016) Families of Sordariomycetes. Fungal Divers 79:1–317
Mains EB (1950) Entomogenous species of Akanthomyces, Hymenostilbe and Insecticola in North America. Mycol Soc Am 42:566–589
Mapook A, Boonmee S, Ariyawansa HA, Tibpromma S, Campesori E, Jones EBG, Bahkali AH, Hyde KD (2016) Taxonomic and phylogenetic placement of Nodulosphaeria. Mycol Prog 15:34. doi:10.1007/s11557-016-1176-x
Martin GW (1941) Outline of the fungi. Stud Nat Hist Univ Iowa 18:192–204
Matheny PB (2005) Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales). Mol Phylogenet Evol 35:1–20
Matsushima T (1971) Microfungi of the Solomon Islands and Papua-New Guinea. Published by the author, Kobe, p 78
Mckenzie EHC (1995) Dematiaceous Hyphomycetes on Pandanaceae. V. Sporidesmium sensu lato. Mycotaxon 56:9–29
McNeill J, Barrie FR, Buck WR, Demoulin V, Greuter W, Hawksworth DL, Herendeen PS, Knapp S, Marhold K, Prado J, Prud’homme Van Reine WF, Smith GF, Wiersema JH, Turland NJ (2012) International Code of Nomenclature for algae, fungi, and plants (Melbourne Code). Regnum Veg 154(1):208
Medardi G (2006) Atlante fotografico degli Ascomiceti d’Italia. A.M.B. Fondazione Centro Studi Micologici, Vicenza
Medardi G, Lantieri A, Pfister DH, LoBuglio KF, Cacialli G (2012) Clarification of Peziza fimeti with notes on P. varia collections on dung. Mycotaxon 121:465–476
Micheli PA (1729) Nova plantarvm genera ivxta Tovrnefortii methodvm disposita. Florence
Miller SL, Larsson E, Larsson KH, Verbeken A, Nuytinck J (2006) Perspectives in the new Russulales. Mycologia 98:960–970
Minnis AM, Kennedy AH, Grenier DB, Palm ME, Rossman AY (2012) Phylogeny and taxonomic revision of the Planistromellaceae including its coelomycetous anamorphs: contributions towards a monograph of the genus Kellermania. Persoonia 29:11–28
Minter DW, Holuboá-Jechová V (1981) New or interesting Hyphomycetes on decaying pine litter from Czechoslovakia. Folia Geobot Phytotax Praha 16:195–217
Moesz G (1915) Mykologiai közlemények. II. közlemény. Botanikai Kölzlemények 14:145–158
Möller A (1901) Phycomyceten und Ascomyceten. Untersuchungen aus Brasilien. Botanische Mittheilungen aus den Tropen 9:1–319
Mugambi GK, Huhndorf SM (2009) Molecular phylogenetics of Pleosporales: Melanommataceae and Lophiostomataceae recircumscribed (Pleosporomycetidae, Dothideomycetes, Ascomycota). Stud Mycol 64:103–121
Mugambi GK, Huhndorf SM (2010) Multigene phylogeny of the Coronophorales: morphology and new species in the order. Mycologia 102:185–210
Müller E, Corbaz R (1956) Kulturversuchemitascomyceten III. Sydowia 10:181–188
Müller E, von Arx J (1973) Pyrenomycetes: Meliolales, Coronophorales, Sphaeriales. In: Ainsworth G, Sparrow F, Sussman A (eds) The Fungi IV. Academic Press, New York, pp 87–132
Munk A (1953) The system of Pyrenomycetes. Dansk Bot Arkiv 15:1–163
Munk A (1956) On Metasphaeria coccodes (Karst.) Sacc. (Massarinaceae n. fam.). Friesia 5:303–308
Nag Raj TR (1993) Coelomycetous anamorphs with appendage bearing conidia. Mycologue Publications, Waterloo
Nelsen MP, Lücking R, Grube M, Mbatchou JS, Muggia L, Plata ER, Lumbsch HT (2009) Unravelling the phylogenetic relationships of lichenised fungi in Dothideomyceta. Stud Mycol 64:135–144
Nelsen MP, Lücking R, Aptroot A, Andrew CJ, Cáceres M, Plata ER, Gueidan C, da Silva Canêz L, Knight A, Ludwig LR, Mercado-Díaz JA, Sittiporn P, Lumbsch HT (2014) Elucidating phylogenetic relationships and genus-level classification within the fungal family Trypetheliaceae (Ascomycota: Dothideomycetes). Taxon 63:974–992
Nguyen NH, Landeros F, Garibay-Orijel R, Hansen K, Vellinga EC (2013) The Helvella lacunosa species complex in western North America: cryptic species, misapplied names and parasites. Mycologia 105:1275–1286
Niskanen T, Kytövuori I (2012) Key R: Subgen. Telamonia sects Fulvescentes Melot and Laeti Melot In: Knudsen H, Vesterholt J (eds) Funga Nordica, 2nd revised edition. Agaricoid, boletoid, clavarioid, cyphelloid and gastroid genera. Nordsvamp, Copenhagen, pp 883–885
Nitschke TRJ (1869) Grundlage eines Systems der Pyrenomyceten. Verhandlungen des Naturhistorischen Vereins der Preussischen Rheinlande, Westfalens und des Regierungsbezirks Osnabrück 26:70–77
Nitschke TRJ (1870) Pyrenomycetes Germanici. Eduard Trewendt 2:161–320
Noordeloos ME (1988) Rhodocybe Maire. In: Bas C, Kuyper ThW, Noordeloos ME, Vellinga EC (eds) Flora agaricina neerlandica 1. AA Balkema, Rotterdam, pp 77–82
Noordeloos ME (2004) Entoloma s.l. Fungi Europaei, vol 5a. Edizione Candusso, Alassio, p 617
Núñez M, Ryvarden L (1995) Polyporus (Basidiomycotina) and related genera. Fungiflora, Oslo
Nylander JAA, Wilgenbusch JC, Warren DL, Swofford DL (2008) AWTY (are we there yet?): a system for graphical exploration of MCMC convergence in Bayesian phylogenetics. Bioinformatics 24:581–583
Ogawa J, Sakuradani E, Kishino S, Ando A, Yokozeki K, Shimizu S (2012) Polyunsaturated fatty acids production and transformation by Mortierella alpine and anaerobic bacteria. Eur J Lipid Sci Technol 114:1107–1113
Page RDM (2001) Tree View: tree drawing software for Apple Macintosh and Windows. http://taxonomy.zoology.gla.ac.uk/rod/treeview.html
Park MS, Fong JJ, Lee H, Oh S-Y, Jung PE, Min YJ, Seok SJ, Lim YW (2013) Delimitation of Russula subgenus Amoenula in Korea using three molecular markers. Mycobiology 41:191–201
Pegler DN (1977) A revision of Entolomata (Agaricales) from India and Sri Lanka. Kew Bull 32:189–220
Pegler DN (1986) Agaric flora of Sri Lanka. Kew Bull Addit Ser 12:1–519
Peintner U, Bougher NL, Castellano MA, Moncalvo JM, Moser MM, Trappe JM, Vilgalys R (2001) Multiple origins of sequestrate fungi related to Cortinarius (Cortinaria). Am J Bot 88:2168–2179
Peintner U, Horak E, Moser M, Vilgalys R (2002) Phylogeny of Rozites, Cuphocybe and Rapacea inferred from ITS and LSU rDNA sequences. Mycologia 94:620–629
Peintner U, Moncalvo JM, Vilgalys R (2004) Towards a better understanding of the infrageneric relationships in Cortinarius (Agaricales, Basidiomycota). Mycologia 96:1042–1058
Pérez CA, Wingfield MJ, Altier N, Blanchette RA (2013) Species of Mycosphaerellaceae and Teratosphaeriaceae on native Myrtaceae in Uruguay: evidence of fungal host jumps. Fungal biol 117:94–102
Persoon CH (1801) Synopsis methodica fungorum, pp 1–706
Petch T (1931a) Notes on entomogenous fungi. Trans Brit Mycol Soc 16:55–75
Petch T (1931b) New species of Cordyceps, collected during the Whitby foray. Nat Hull 1931:101–103
Petch T (1932) Notes on entomogenous fungi. Trans Brit Mycol Soc 16:209–245
Petersen RH (1968) The genus Clavulinopsis in North America. Mycol Mem 2:1–39
Petersen RH (1978) Notes on clavarioid fungi. XV. Reorganization of Clavaria, Clavulinopsis and Ramariopsis. Mycologia 70:660–671
Petersen RH (1988) The clavarioid fungi of New Zealand. Bull N Z Dept Sci Ind Res 236:1–170
Peterson SW (1995) Phylogenetic analysis of Aspergillus sections Cremei and Wentii, based on ribosomal DNA sequences. Mycol Res 99:1349–1355
Petkovits T, Nagy LG, Hoffmann K, Wagner L, Nyilasi I, Griebel T, Schnabelrauch D, Vogel H, Voigt K, Vágvölgyi C, Papp T (2011) Data partitions, Bayesian analysis and phylogeny of the zygomycetous fungal family Mortierella, inferred from nuclear ribosomal DNA sequences. PLoS One 6:e27507. doi:10.1371/journal.pone.0027507
Petrak F (1925) Mykologische notizen VIII. Ann Mycol 23:1–143
Petrak F (1944) Über die Gattungen Chaetopyrena Pass., Sclerochaeta v. Höhn., Sclerochaetella v. Höhn., Vermiculariella Oud., Chaetosphaeronema Moesz und Pseudophoma v. Höhn. Ann Mycol 42:58–71
Phillips AJL, Alves A, Correia A, Luque J (2005) Two new species of Botryosphaeria with brown, 1-septate ascospores and Dothiorella anamorphs. Mycologia 97:513–529
Phillips AJL, Alves A, Abdollahzadeh J, Slippers B, Wingfield MJ, Groenewald JZ, Crous PW (2013) The Botryosphaeriaceae: genera and species known from culture. Stud Mycol 76:51–167
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa H, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PE, Xu JC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238
Phookamsak R, Monamgoda DS, Li WJ, Dai DQ, Singtripop C, Hyde KD (2015) Poaceascoma helicoides gen et sp. nov., a new genus with scolecospores in Lentitheciaceae. Cryptogam Mycol 36:225–236
Phukhamsakda C, Ariyawansa HA, Phookamsak R, Chomnuti P, Bulgakov TS, Yang JB, Bhat DJ, Bahkali AH, Hyde KD (2015) Muriphaeosphaeria galatellae gen. et sp. nov. in Phaeosphaeriaceae (Pleosporales). Phytotaxa 227:55–65
Pinho DB, Firmino AL, Ferreira-junior WG, Pereira OL (2012) An efficient protocol for DNA extraction from Meliolales and the description of Meliola centellae sp. nov. Mycotaxon 122:333–345
Pinho DB, Firmino AL, Ferreira-Júnior WG, Pereira OL (2013) New Meliolaceae from the Brazilian Atlantic Forest 2: species on host families Annonaceae, Cecropiaceae, Meliaceae, Piperaceae, Rubiaceae, Rutaceae and Tiliaceae. Mycologia 105:697–711
Pinho DB, Junior JH, Firmino AL, Junior BTH, Mizubuti ESG, Pereira OL (2014) Reappraisal of the black mildews (Meliolales) on Hevea brasiliensis. Trop Plant Pathol 39:89–94
Pitt WM, Úrbez-Torres JR, Trouillas FP (2013) Dothiorella vidmadera, a novel species from grapevines in Australia and notes on Spencermartinsia. Fungal Divers 61:209–219
Prado R, Schmitt I, Kautz S, Palice Z, Lücking R, Lumbsch HT (2006) Molecular data place Trypetheliaceae in Dothideomycetes. Mycol Res 110:511–520
Quaedvlieg W, Verkley GJM, Shin H-D, Barretto RW, Alfenas AC, Swart WJ, Groenewald JZ, Crous PW (2013) Sizing up Septoria. Stud Mycol 75:307–390
Quaedvlieg W, Binder M, Groenewald JZ, Summerell BA, Carnegie AJ, Burgess TI, Crous PW (2014) Introducing the consolidated species concept to resolve species in the Teratosphaeriaceae. Persoonia 33:1–40
Quandt CA, Kepler RM, Gams W, Araújo JPM, Ban S, Evans HC, Hughes DP, Humber R, Hywel-Jones N, Li Z, Luangsa-Ard JJ, Rehner SA, Sanjuan T, Sato H, Shrestha B, Sung G-H, Yao Y-J, Zare R, Spatafora JW (2014) Phylogenetic-based nomenclatural proposals for Ophiocordycipitaceae (Hypocreales) with new combinations in Tolypocladium. IMA Fungus 5:121–134
Rabenhorst (1858) Herb myc, ed. 2 no. 725 (in sched)
Rambaut A (2014): FigTree v1.4: Tree figure drawingtool. http://tree.bio.ed.ac.uk/software/figtree/. Accessed 2016 March 10
Rank C, Nielsen KF, Larsen TO, Varga J, Samson RA, Frisvad JC (2011) Distribution of sterigmatocystin in filamentous fungi. Fungal Biol 115:406–420
Rannala B, Yang Z (1996) Probability distribution of molecular evolutionary trees: a new method of phylogenetic inference. J Mol Evol 43:304–311
Rao VG, Subhedar AW (1976) Kamatia—a new genus of hyphomycetes. T Brit Mycol Soc 66:539–541. doi:10.1016/S0007-1536(76)80233-1
Raper KB, Fennell DI (1965) The genus Aspergillus. Williams and Wilkins, Baltimore
Rawla GS (2001) Himalayan species of Russula Pers. ex S.F. Gray. In: Pande PC, Samant SS (eds) Plant diversity of the Himalaya. Gyanodaya Prakashan, Nainital, pp 1–48
Rawla GS (2002) Lactarius D.C. ex S.F. Gray in India—list and critical review. In: Pullaiah P (ed) Biodiversity in India. Regency Publications, New Delhi, pp 221–255
Réblová M (1999) Studies in Chaetosphaeria sensu lato III. Umbrinosphaeria gen. nov. and Miyoshiella with Sporidesmium anamorphs. Mycotaxon 71:13–43
Réblová M, Gams W, Seifert KA (2011) Monilochaetes and allied genera of the Glomerellales, and a reconsideration of families in the Microascales. Stud Mycol 68:163–191
Réblová M, Untereiner WA, Réblová K (2013) Novel evolutionary lineages revealed in the Chaetothyriales (fungi) based on multigene phylogenetic analyses and comparison of its secondary structure. PLoS One 8(5):e63547
Rehner SA (2001) Primers for elongation factor 1-alpha (EF1-alpha). http://www.aftol.org/pdfs/EF1primer.pdf
Richter C, Helaly SE, Thongbai B, Hyde KD, Stadler M (2016) Pyristriatins A and B—pyridino-cyathane antibiotics from the basidiomycete Cyathus cf. striatus. J Nat Prod 79:1684–1688
Rifai MA (1968) The Australasian Pezizales in the herbarium of the Royal Botanic Gardens, Kew. Verhandelingen der Koninklijke Nederlandse Akademie van Wetenschappen, Afd Natuurkunde II 57:1–295
Roger L (1953) Phytopathologie des Pays Chauda. In: Encyclopedia Mycologique, pp 1638–1653
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Rossman AY, Samuels GJ, Rogerson CT, Lowen R (1999) Genera of Bionectriaceae, Hypocreaceae, and Nectriaceae (Hypocreales, Ascomycetes). Stud Mycol 42:1–248
Rossman AY, Farr DF, Castlebury LA (2007) A review of the phylogeny and biology of the Diaporthales. Mycoscience 48:135–144
Rossman AY, Adams GC, Cannon PF, Castlebury LA, Crous PW, Gryzenhout M, Jaklitsch WM, Mejia LC, Stoykov D, Udayanga D, Voglmayr H, Walker DM (2015) Recommendations of generic names in Diaporthales competing for protection or use. IMA Fungus 6:145–154
Saccardo PA (1884) Sylloge Fungorum 3(i–ii):1–860. PA Saccardo, Italy, Padua
Saccardo PA (1889) Sylloge fungorum 8(ix − xvii):1–1143. PA Saccardo, Padua
Saccardo PA (1892) Sylloge fungorum 10(i–xxx):1–964. P.A. Saccardo, Padua
Samson RA, Visagie CM, Houbraken J, Hong SB, Hubka V, Klaassen CH, Perrone G, Seifert KA, Susca A, Tanney JB, Varga J, Kocsubé S, Szigeti G, Yaguchi T, Frisvad JC (2014) Phylogeny, identification and nomenclature of the genus Aspergillus. Stud Mycol 78:141–173
Samuels GJ, McKenzie EHC, Buchanan DE (1981) Ascomycetes of New Zealand. 3. Two new species of Apiospora and their Arthrinium anamorphs on bamboo. N Z J Bot 19:137–149
Sarnari M (1998) Monografia illustrata del Genere Russula in Europa, Italy. Tromo Primo
Schoch CL, Shoemaker RA, Seifert KA, Hambleton S, Spatafora JW, Crous PW (2006) A multigene phylogeny of the Dothideomycetes using four nuclear loci. Mycologia 98:1041–1052
Schoch CL, Crous PW, Groenewald JZ, Boehm EWA, Burgess TI, de Gruyter J, de Hoog GS, Dixon LJ, Grube M, Gueidan C, Harada Y, Hatakeyama S, Hirayama K, Hosoya T, Huhndorf SM, Hyde KD, Jones EBG, Kohlmeyer J, Kruys Å, Li YM, Lücking R, Lumbsch HT, Marvanová L, Mbatchou JS, McVay AH, Miller AN, Mugambi GK, Muggia L, Nelsen MP, Nelson P, Owensby CA, Phillips AJL, Phongpaichit S, Pointing SB, Pujade-Renaud V, Raja HA, Rivas Plata E, Robbertse B, Ruibal C, Sakayaroj J, Sano T, Selbmann L, Shearer CA, Shirouzu T, Slippers B, Suetrong S, Tanaka K, Volkmann-Kohlmeyer B, Wingfield MJ, Wood AR, Woudenberg JHC, Yonezawa H, Zhang Y, Spatafora JW (2009) A class-wide phylogenetic assessment of Dothideomycetes. Stud Mycol 64:1–15
Seaver FJ (1917) Photographs and descriptions of cup-fungi: V. Peziza proteana and Peziza violacea. Mycologia 9:1–3
Seaver FJ (1928) The North American cup-fungi (operculates). Hafner Publishing Company, New-York 377 p
Seifertk K, Morgan-Jones G, Gams W, Kendrick B (2011) The Genera of hyphomycetes. CBS-KNAW Fungal Biodiversity Centre, Utrecht
Senanayake IC, Maharachchikumbura SSN, Hyde KD, Bhat JD, Jones EBG, McKenzie EHC, Dai DQ, Daranagama DA, Dayarathne MC, Goonasekara ID, Konta S, Li WJ, Shang QJ, Stadler M, Wijayawardene NN, Xiao YP, Norphanphoun C, Li QR, Liu XZ, Bahkali AH, Kang JC, Wang Y, Wen TC, Wendt L, Xu JC, Camporesi E (2015) Towards unraveling relationships in Xylariomycetidae (Sordariomycetes). Fungal Divers 73:73–144
Shenoy BD, Jeewon R, Wu W-Y, Bhat DJ, Hyde KD (2006) Ribosomal and RB2 DNA sequence analyses suggest that Sporidesmium and morphologically similar genera are polyphyletic. Mycol Res 110:917–929
Shinmen Y, Shimizu S, Akimoto K, Kawashima H, Yamada H (1989) Production of rachidonic acid by Mortierella fungi: selection of a potent producer and optimization of culture conditions for large-scale production. Appl Microbiol Biotechnol 31:11–16
Shoemaker RA (1976) Canadian and some extralimital Ophiobolus species. Can J Bot 54:2365–2404
Shoemaker RA (1984) Canadian and some extralimital Nodulosphaeria and Entodesmium species. Can J Bot 62:2730–2753
Shoemaker RA, Babcock CE (1989) Phaeosphaeria. Can J Bot 67:1500–1599
Shoemaker RA, Babcock CE (1992) Applanodictyosporous Pleosporales: Clathrospora, Comoclathris, Graphyllium, Macrospora, and Platysporoides. Can J Bot 70:1618
Shoemaker RA, White GP (1985) Lasiosphaeria caesariata with Sporidesmium hormiscioides and L. triseptata with S. adscendens. Sydowia 38:278–283
Silvestro D, Michalak I (2012) RaxmlGUI: a graphical front-end for RAxML. Org Divers Evol 12:335–337
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Königstein 981
Slippers B, Wingfield MJ (2007) Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol Rev 21:75–89
Slippers B, Boissin E, Phillips AJL, Groenewald JZ, Wingfield MJ, Postma A, Burgess T, Crous PW (2013) Phylogenetic lineages in the Botryosphaeriales: a systematic and evolutionary framework. Stud Mycol 76:31–49
Song J, Chen YY, Cui BK, Liu HG, Wang YZ (2014) Morphological and molecular evidence for two new species of Laetiporus (Basidiomycota, Polyporales) from southwestern China. Mycologia 106:1039–1050
Song J, Xing JH, Decock C, He XL, Cui BK (2016) Molecular phylogeny and morphology reveal a new species of Amauroderma (Basidiomycota) from China. Phytotaxa 260:47–56
Sotome K, Hattori T, Ota Y, To-anun C, Salleh B, Kakishima M (2008) Phylogenetic relationships of Polyporus and morphologically allied genera. Mycologia 100:603–615
Sotome K, Hattori T, Ota Y (2011) Taxonomic study on a threatened polypore, Polyporus pseudobetulinus, and a morphologically similar species, P. subvarius. Mycoscience 52:319–326
Spielman LJ (1983) Taxonomy and biology of Valsa Species on hardwoods in North America, with special reference to species on maples. Cornell University, New York
Spielman LJ (1985) A monograph of Valsa on hardwoods in North America. Can J Bot 63:1355–1378
Stadler M (2011) Importance of secondary metabolites in the Xylariaceae as parameters for assessment of their taxonomy, phylogeny, and functional biodiversity. Curr Res Environ Appl Mycol 1:75–133
Stamatakis A (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30:1312–1313
Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the raxml web servers. Syst Biol 57:758–771
Starbäck K (1899) Ascomyceten der ersten Regnellschen Expediton. I. Mit 2 Tafeln. Bihang till Kongl Svenska vetenskaps-akademiens handlingar 25:1–68
Steyaert RL (1949) Contributions à létude monographique de Pestalotia de Not. et Monochaetia Sacc. (Truncatella gen. nov. et Pestalotiopsis gen. nov.). Bulletin du Jardin botanique de l’État a Bruxelles 19:285–354
Stolk AC, Malla DS (1971) Penicillium inflatum sp. nov. Persoonia 6:197–200
Stubbe D, Verbeken A (2012) Lactarius subg. Plinthogalus: the European taxa and American varieties of L.lignyotus re-evaluated. Mycologia 104:1490–1501
Stubbe D, Nuytinck J, Verbeken A (2008) Lactarius subgenus Plinthogalus of Malaysia. Fungal Divers 32:125–156
Sturm J (1829) Deutschlands Flora. Abt. III. Die Pilze Deutschlands 2:1–136
Su HY, Hyde KD, Maharachchikumbura SSN, Ariyawansa HA, Luo ZL, Promputtha I, Tian Q, Lin CG, Shang QJ, Zhao YC, Chai HM, Liu XY, Bahkali AH, Bhat JD, McKenzie EHC, Zhou DQ (2016a) The families Distoseptisporaceae fam. nov., Kirschsteiniotheliaceae, Sporormiaceae and Torulaceae, with new species from freshwater in Yunnan Province, China. Fungal Divers. doi:10.1007/s13225-016-0362-0
Su HY, Luo ZL, Liu XY, Su XJ, Hu DM, Zhou DQ, Bahkali AH, Hyde KD (2016b) Lentithecium cangshanense sp. nov. (Lentitheciaceae) from freshwater habitats in Yunnan Province, China. Phytotaxa 267:061–069
Suetrong S, Schoch CL, Spatafora JW, Kohlmeyer J, Volkmann-Kohlmeyer B, Sakayaroj J, Phongpaichit S, Tanaka K, Hirayama K, Jones EBG (2009) Molecular systematics of the marine Dothideomycetes. Stud Mycol 64:155–173
Sung G, Hywel-Jones NL, Sung J-M, Luangsa-Ard JJ, Shrestha B, Spatafora JW (2007) Phylogenetic classification of Cordyceps and the clavicipitaceous fungi. Stud Mycol 57:5–59
Sutton BC (1980) The Coelomycetes. Fungi imperfecti with pycnidia, acervuli and stromata. Commonwealth Mycological Institute, Kew
Sutton BC, Alcorn JL (1974) Neottiosporina. Australasian. J Bot 22:517–530
Svrček M (1970) Über einige arten der Discomycetengattung Peziza [Dill.] L. ex St-Amans. Česká Mykologie 24:57–76
Svrček M (1976) A revision of species of the genus Peziza Dill. ex St-Amans described by. J. Velenovský II. Česká Mykologie 30:35–142
Swofford DL (2002) PAUP: phylogenetic analysis using parsimony, version 4.0b10. Illinois Natural History Survey, Champion, III
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanaka K, Harada Y (2003) Pleosporales in Japan (2): the genus Lophiotrema. Mycoscience 44:115–121
Tanaka K, Hirayama K, Yonezawa H, Sato G, Toriyabe A, Kudo H, Hashimoto A, Matsumura M, Harada Y, Kurihara Y, Shirouzu T, Hosoya T (2015) Revision of the Massarineae (Pleosporales, Dothideomycetes). Stud Mycol 82:75–136
Tangthirasunun N, Silar P, Bhat DJ, Maharachchikumbura SSN, Wijayawardene NN, Bahkali AH, Hyde KD (2015) Morphology and phylogeny of two appendaged genera of coelomycetes: Ciliochorella and Discosia. Sydowia 67:217–226
Tennakoon DS, Hyde KD, Phookamsak R, Wanasinghe DN, Camporesi E, Promputtha I (2016) Taxonomy and Phylogeny of Juncaceicola gen. nov. (Phaeosphaeriaceae, Pleosporinae, Pleosporales). Cryptogam Mycol 37:135–156
Thambugala KM, Ariyawansa HA, Li YM, Boonmee S, Hongsanan S, Tian Q, Singtripop C, Bhat DJ, Camporesi E, Jayawardena R, Liu ZY, Xu JC, Chukeatirote E, Hyde KD (2014) Dothideales. Fungal Divers 68:105–158
Thambugala KM, Chunfang Y, Camporesi E, Bahkali AH, Liu ZY, Hyde KD (2015a) Pseudodidymosphaeria gen. nov. in Massarinaceae. Phytotaxa 231:271–282
Thambugala KM, Hyde KD, Tanaka K, Tian Q, Wanasinghe DN, Ariyawansa HA, Jayasiri SC, Boonmee S, Camporesi E, Hashimoto A, Hirayama K, Schumacher RK, Promputtha I, Liu ZY (2015b) Towards a natural classification and backbone tree for Lophiostomataceae, Floricolaceae, and Amorosiaceae fam. nov. Fungal Divers 74:199–266
Thambugala KM, Daranagama DA, Phillips AJL, Bulgakov TS, Bulgakov DJ, Camporesi E, Bahkali AH, Eungwanichayapant PD, Liu ZY, Hyde KD (2016a) Microfungi on Tamarix. Fungal Divers. doi:10.1007/s13225-016-0371-z
Thambugala KM, Hyde KD, Eungwanichayapant PD, Romero AI, Liu ZY (2016b) Additions to the Genus Rhytidhysteron in Hysteriaceae. Cryptogam Mycol 37:99–116
Tian Q, Liu JK, Hyde KD, Wanasinghe DN, Boonmee S, Jayasiri SC, Luo ZL, Taylor JE, Phillips AJL, Bhat DJ, Li WJ, Ariyawansa H, Thambugala KM, Jones EBG, Chomnunti P, Bahkali AH, Xu JC, Camporesi E (2015) Phylogenetic relationships and morphological reappraisal of Melanommata (Pleosporales). Fungal Divers 74:267–324
Tibpromma S, Promputtha I, Phookamsak R, Boonmee S, Camporesi E, Yang JB, Bhakali AH, McKenzie EH, Hyde KD (2015) Phylogeny and morphology of Premilcurensis gen. nov. (Pleosporales) from stems of Senecio in Italy. Phytotaxa 236(1):40–52
Tsang CC, Chan JFW, Trendell-Smith NJ, Ngan AHY, Ling IWH, Lau SKP, Woo PCY (2014) Subcutaneous phaeohyphomycosis in a patient with IgG4-related sclerosing disease caused by a novel ascomycete, Hongkongmyces pedis gen. et sp. nov.: first report of human infection associated with the family Lindgomyceta. Med Mycol 52:736–747
Tsui CKM, Sivichai S, Rossman AY, Berbee ML (2007) Tubeufia asiana, the teleomorph of Aquaphila albicans in the Tubeufiaceae, Pleosporales, based on cultural and molecular data. Mycologia 99:884–894
Tulasne LR, Tulasne C (1861) Selecta Fungorum Carpologia: Erysiphei
Tzean SS, Chen JL (1990) Cheiromoniliophora elegans gen. et sp. nov. (Hyphomycetes). Mycol Res 94(3):424–427
Udayanga D, Liu X, McKenzie EHC, Chukeatirote E, Bahkali AHA, Hyde KD (2011) The genus Phomopsis: biology, applications, species concepts and names of common phytopathogens. Fungal Divers 50:189–225
Udayanga D, Liu X, McKenzie EHC, Chukeatirote E, Hyde KD (2012) Multi-locus phylogeny reveals three new species of Diaporthe from Thailand. Cryptogam Mycol 33:295–309
Udayanga D, Castlebury LA, Rossman AY, Chukeatirote E, Hyde KD (2014a) Insights into the genus Diaporthe: phylogenetic species delimitation in the D. eres species complex. Fungal Divers 67:203–229
Udayanga D, Castlebury LA, Rossman AY, Hyde KD (2014b) Species limits in Diaporthe: molecular re-assessment of D. citri, D. cytosporella, D. foeniculina and D. rudis. Persoonia 32:83–101
Upreti DK, Pant G (1993) Notes on Arthopyrenia species from India. Bryologist 96(2):226–232
van Beyma Thoe Kingma FH (1939–1940) Beschreibung einiger neuer pilzarten aus dem Centraalbureau voor Schimmelcultures, Baarn (Nederland). Antonie van Leeuwenhoek 6:263–290
van Ryckegem G, Aptroot A (2001) A new Massarina and a new Wettsteinina (Ascomycota) from freshwater and tidal reeds. Nova Hedwig 73:161–166
van Tuinen D, Zhao B, Gianinazzi-Pearson V (1998) PCR in studies of AM fungi: from primers to application. In: Varma AK (ed) Mycorrhizal manual. Springer, Berlin, pp 387–399
Van Vooren N (2003) Étude systématique et nomenclaturale des pézizes blanches III. Peziza proteana et sa forme sparassoides. Bull Mycol. Bot Dauphiné-Savoie 170:47–58
Vilgalys R, Hester M (1990) Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J Bacteriol 172:4238–4246
Voglmayr H, Mayer V, Maschwitz U, Moog J, Djieto-Lordon C, Blatrix R (2010) The diversity of ant-associated black yeasts: insights into a newly discovered world of symbiotic interactions. Fungal Biol 115:1077–1091
von Arx JA (1987) Plant-pathogenic Fungi. J. Cramer, Berlin
von Arx JA, Müller E (1975) A re-evaluation of the bitunicate ascomycetes with keys to families and genera. Stud Mycol 9:1–159
von Höhnel F (1917) Fragmente zur Mykologie (XXIII. Mitteilung, Nr. 1154 bis 1188). Sitzungsberichte der Kaiserlichen Akademie der Wissenschaften Math.-naturw. Klasse Abt I 128:589
Wagner L, Stielow B, Hoffmann K, Petkovits T, Papp T, Vágvölgyi C, de Hoog GS, Verkley G, Voigt K (2013) A comprehensive molecular phylogeny of the Mortierellales (Mortierellomycotina) based on nuclear ribosomal DNA. Persoonia 30:77–93
Wanasinghe DN, Jones EBG, Camporesi E, Boonmee S, Karunarathna SC, Thines M, Mortimer PE, Xu J, Hyde KD (2014) Dematiopleospora mariae gen. sp. nov., from Ononis spinosain Italy. Cryptogam Mycol 35:105–117
Wanasinghe DN, Jones EBG, Camporesi E, Mortimer PE, Xu JC, Bahkali Ah, Hyde KD (2015) The genus Murispora. Cryptogam Mycol 36:419–448
Wanasinghe DN, Jonese EBG, Camporesi E, Dissanayakec AJ, Kamolhan S, Mortimer PE, Xu JC, Abd-Elsalam KA, Hyde KD (2016) Taxonomy and phylogeny of Laburnicola gen. nov. and Paramassariosphaeria gen. nov. (Didymosphaeriaceae, Massarineae, Pleosporales). Fungal Biol (in press)
Wang CL, Lin CC (2004) Five new records of Ascomycetes in Taiwan. Fungal Sci 19:21–29
Wang Z, Johnston PR, Takamatsu S, Spatafora JW, Hibbett DS (2006) Toward a phylogenetic classification of the Leotiomycetes based on rDNA data. Mycologia 98:1065–1075
Wang XL, Kang ZS, Huang LL, Wei J (2011a) Re-evaluation of pathogens causing Valsa canker on apple in China. Mycologia 103:317–324
Wang M, Zhao YC, Zhao Q, Zhou DQ (2016) Helvella sublactea sp. nov. (Helvella) from southwestern China. Phytotaxa 253(2):131–138
Watling R, Lee SS (1998) Ectomycorrhizal fungi associated with members of the Dipterocarpa in Peninsular Malaysia II. J Trop For Sci 10(4):421–430
Watson W (1929) The classification of lichens. II. New Phytol 28:85–116
Weber NS (1972) The genus Helvella in Michigan. Mich Bot 11:147–201
Weber NS (1975) Notes on Western species of Helvella. I. Nova Hedwigia. Beihefte 51:25–38
Wehmeyer LE (1975) The pyrenomycetous fungi. Mycol Mem 6:1–250
White TJ, Bruns T, Lee S, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Wijayawardene DNN, McKenzie EHC, Hyde KD (2012) Towards incorporating anamorphic fungi in a natural classification–checklist and notes for 2012. Mycosphere 3:157–228
Wijayawardene NN, Song Y, Bhat DJ, McKenzie EHC, Chukeatirote E, Wang Y, Hyde KD (2013) Wojnowicia viburni sp. nov. from China and its phylogenetic placement. Sydowia 65:181–190
Wijayawardene NN, Crous PW, Kirk PM, Hawksworth DL, Boonmee S, Braun U, Dai DQ, D’souza MJ, Diederich P, Dissanayake A, Doilom M, Hongsanan S, Jones EBG, Groenewald JZ, Jayawardena R, Lawrey JD, Liu JK, Lücking R, Madrid H, Manamgoda DS, Muggia L, Nelsen MP, Phookamsak R, Suetrong S, Tanaka K, Thambugala KM, Wanasinghe DN, Wikee S, Zhang Y, Aptroot A, Ariyawansa HA, Bahkali AH, Bhat DJ, Gueidan C, Chomnunti P, De Hoog GS, Knudsen K, Li WJ, McKenzie EHC, Miller AN, Phillips AJL, Piątek M, Raja HA, Shivas RS, Slippers B, Taylor JE, Tian Q, Wang Y, Woudenberg JHC, Cai L, Jaklitsch WM, Hyde KD (2014a) Naming and outline of Dothideomycetes—2014 including proposals for the protection or suppression of generic names. Fungal Divers 69:1–55
Wijayawardene NN, Hyde KD, Bhat DJ, Camporesi E, Schumacher RK, Chethana KWT, Wikee S, Bahkali AH, Wang Y (2014b) Camarosporium-like species are polyphyletic in Pleosporales; introducing Paracamarosporium and Pseudocamarosporium gen. nov. in Montagnulaceae. Cryptogamie Mycol 35:177–198
Wijayawardene NN, Hyde KD, Bhat DJ, Goonasekars ID, Nadeeshan D, Camporesi E, Schumacher RK, Wang Y (2015) Additions to brown spored coelomycetous taxa in Massarinae, Pleosporales: introducing Phragmocamarosporium gen. nov. and Suttonomyces gen. nov. Cryptogam Mycol 36:213–224
Wijayawardene NN, Hyde KD, Wanasinghe DN, Papizadeh M, Goonasekara ID, Camporesi E, Bhat DJ, McKenzie EHC, Phillips AJL, Diederich P, Tanaka K, Li WJ, Tangthirasunun N, Phookamsak R, Dai DQ, Dissanayake AJ, Weerakoon G, Maharachchikumbura SSN, Hashimoto A, Matsumura M, Bahkali AH, Wang Y (2016) Taxonomy and phylogeny of dematiaceous coelomycetes. Fungal Divers 77:1–316
Wikee S, Lombard L, Nakashima C, Motohashi K, Chukeatirote E, Cheewangkoon R, McKenzie EHC, Hyde KD, Crous PW (2013) A phylogenetic re-evaluation of Phyllosticta (Botryosphaeriales). Stud Mycol 76:1–29
Wingfield MJ, De Beer ZW, Slippers B, Wingfield BD, Groenewald JZ, Lombard L, Crous PW (2012) One fungus, one name promotes progressive plant pathology. Mol Plant Pathol 13:604–613
Winton LM, Stone JK, Hansen EM, Shoemaker RA (2007) The systematic position of Phaeocryptopus gaeumannii. Mycologia 99:240–252
Wood M, Stevens F (2015) http://www.mykoweb.com/CAF/species/Cyathus_striatus.html
Wyka SA, Broders KD (2016) The new family Septorioidea, within the Botryosphaeriales and Septorioides strobi as a new species associated with needle defoliation of Pinus strobus in the United States. Fungal Biol. doi:10.1016/j.funbio.2016.04.005
Xia JW, Ma LG, Castañeda-Ruiz RFC, Zhang XG (2014) Minimelanolocus bicolorata sp. nov., Paradendryphiopsis elegans sp. nov. and Corynesporella bannaense sp. nov. from southern China. Mycoscience 55:299–307
Xu L, Kusakari S, Hosomi A, Toyoda H, Ouchi S (1999) Postharvest diseases of grapes caused by Pestalotiopsis spp. Ann Phytopathol Soc Jpn 65:305–311
Xue HJ, Zhou LW (2012) Polyporus submelanopus sp. nov. (Polyporales, Basidiomycota) from Northwest China. Mycotaxon 122:433–441
Yang Z (1994) Maximum likelihood phylogenetic estimation from DNA sequences with variable rates over sites: approximate methods. J Mol Evol 39:306–314
Yang Q, Fan ZL, Crous PW, Liang YM, Tian CM (2015) Cytospora from Ulmus pumila in Northern China. Mycol Prog 14:74. doi:10.1007/s11557-015-1096-1
Yarwood CE (1973) Pyrenomycetes: Erysiphales. In: Ainsworth GC, Sparrow FK, Sussman AS (eds) The fungi: an advanced treatise, vol IVA. Academic Press, New York, pp 71–86
Ying JZ, Zang M (1994) Economic macrofungi from southwestern China. Sicence Press, Beijing 21
Yu K, Walther G, Van Diepeningen AD, Gerrits Van den Ende AHG, Li R-Y, Moussa TAA, Almaghrabi OA, de Hoog GS (2015) DNA barcoding of clinically relevant Cunninghamella species. Med Mycol 53:99–106
Zhang N, Castlebury LA, Miller AN, Huhndorf SM, Schoch CL, Seifert KA, Rossman AY, Rogers JD, Kohlmeyer J, Volkmann-Kohlmeyer B, Sung GH (2006) An overview of the systematics of the Sordariomycetes based on four-gene phylogeny. Mycologia 98:1077–1088
Zhang Y, Wang HK, Fournier J, Crous PW, Jeewon R, Pointing SB, Hyde KD (2009a) Towards a phylogenetic clarification of Lophiostoma/Massarina and morphologically similar genera in the Pleosporales. Fungal Divers 38:225–251
Zhang Y, Schoch CL, Fournier J, Crous PW, de Gruyter J, Woudenberg JHC, Hirayama K, Taranaka K, Pointing SB, Hyde KD (2009b) Multi-locus phylogeny of the Pleosporales: a taxonomic, ecological and evolutionary re-evaluation. Stud Mycol 64:85–102
Zhang YD, Ma J, Ma LG, Zhang XG (2010) A new species of Minimelanolocus from Fujian, China. Mycotaxon 114:373–376
Zhang H, Hyde KD, Mckenzie EHC, Bahkali AH, Zhou DQ (2012a) Sequence data reveals phylogenetic affinities of Acrocalymma aquatica sp. nov., Aquasubmersa mircensis gen. et sp. nov. and Clohesyomyces aquaticus (freshwater coelomycetes). Cryptogamie Mycol 33:333–346
Zhang Y, Crous PW, Schoch CL, Hyde KD (2012b) Pleosporales. Fungal Divers 53:1–221
Zhang TT, Tong X, Lin YR, Hou CL (2015) A new species and a new combination of Terriera based on morphological and molecular data. Mycol Prog 14:54
Zhao RL, Desjardin DE, Soytong K, Hyde KD (2006) Proposed synonyms in Cyathus. Mycotaxon 97:327–335
Zhao RL, Jeewon R, Desjardin DE, Soytong K, Hyde KD (2007) Ribosomal DNA phylogenies of Cyathus: is the current infrageneric classification appropriate? Mycologia 99:385–395
Zhao RL, Desjardin DE, Soytong K, Hyde KD (2008) A new species of bird’s nest fungi: characterisation of Cyathus subglobisporus sp. nov. based on morphological and molecular data. Persoonia 21:71–76
Zhao CL, Cui BK, Dai YC (2013) New species and phylogeny of Perenniporia based on morphological and molecular characters. Fungal Divers 58:47–60
Zhao CL, Chen H, Song J, Cui BK (2015a) Phylogeny and taxonomy of the genus Abundisporus (Polyporales, Basidiomycota). Mycol Prog 14:38
Zhao Q, Tolgor B, Zhao YC, Yang ZL, Hyde KD (2015b) Species diversity within the Helvella crispa group (Ascomycota: Helvellaceae) in China. Phytotaxa 239:130–142
Zhao Y, Si L, Liu D, Proksch P, Zhou D, Lin W (2015c) Truncateols A-N, new isoprenylated cyclohexanols from the sponge-associated fungus Truncatella angustata with anti-H1N1virus activity. Tetrahedron 71:2708–2718
Zhao Q, Sulayman M, Zhu XT, Zhao YC, Yang ZL, Hyde KD (2016a) Species clarification of the culinary Bachu mushroom in Western China. Mycologia. doi:10.3852/16-002
Zhao Q, Zhang XL, Li SH, Chai HM, Bahkalid AH, Hyde KD (2016b) New species and records of saddle fungi (Helvella, Helvellaceae) from Jiuzhaigou Natural Reserve, China. Mycoscience. doi:10.1016/j.myc.2016.07.005
Zhaxybayeva O, Gogarten JP (2002) Bootstrap, Bayesian probability and maximum likelihood mapping: exploring new tools for comparative genome analyses. BMC Genom 3:4
Zheng RY, Chen GQ (1994) Cunninghamella phaeospora var. multiverticillata var. nov. and its mating with var. phaeospora. Mycosystema 7:1–11
Zheng RY, Chen GQ (1998) Cunninghamella clavata sp. nov., a fungal with an unusual type of branching of sporophore. Mycotaxon 69:187–198
Zheng RY, Chen GQ (2001) A monograph of Cunninghamella. Mycotaxon 80:1–75
Zhou ZW, Huang H (1991) Base composition of DNA and classification of Mucorales. Mycosystema 4:1–14
Zhou JL, Zhu L, Chen H, Cui BK (2016) Taxonomy and phylogeny of Polyporus group Melanopus (Polyporales, Basidiomycota) from China. PLOS One 11(8):e0159495
Zhuang WY (2004) Preliminary survey of the Helvella from Xinjiang, China. Mycotaxon 90:35–42
Zhuang WY, Yang ZL (2008) Some pezizalie an fungi from alpine areas of southwestern China. Mycol Monten 10:235–249
Zogg H (1962) Die Hysteriaceaes. str. und Lophiaceae, unter besonderer Berücksichtigung der mitteleuropäischen Formen. Beitr Kryptogamenflora Schweiz 11:1–190
Acknowledgements
K.D. Hyde would like to thank the Thailand Research Fund grant no RSA5980068 entitled Biodiversity, phylogeny and role of fungal endophytes on above parts of Rhizophora apiculata and Nypa fruticans and the Chinese Academy of Sciences, Project Number 2013T2S0030, for the award of Visiting Professorship for Senior International Scientists at Kunming Institute of Botany. Financial support by the German Academic Exchange Service (DAAD) and the Thai Royal Golden Ph.D. Jubilee-Industry program (RGJ) for a joint TRF-DAAD PPP (2012–2014) academic exchange grant to K.D. Hyde and M. Stadler, and the RGJ for a personal grant to B. Thongbai (No. Ph.D/0138/2553 in 4.S.MF/53/A.3) is gratefully acknowledged. Chayanard Phukhamsakda (PHD/0020/2557) acknowledges the The Royal Golden Jubilee Ph.D. Program under the Thailand Research Fund. Mingkwan Doilom acknowledges the Royal Golden Jubilee Ph.D. Program (PHD./0072/2553 in 4.S.M.F./53/A.2) under the Thailand Research Fund. Ausana Mapook is grateful to Research and Researchers for Industries (RRI) PHD57I0012. Rungtiwa Phookamsak sincerely appreciates The Royal Golden Jubilee Ph. D. Program (PHD/0090/2551 in 4. S. MF/51/A.1) under the Thailand Research Fund for financial support. Qi Zhao thanks the National Natural Science Foundation of China (No. 31360015) and the CAS/SAFEA International Partnership Program for Creative Research Teams, and the Knowledge Innovation Program of the Chinese Academy of Sciences (No. KSCX2-EW-Z-9 and KIB2016002). KNAR acknowledges support from the University Grants Commission (UGC), India, in the form of a Rajiv Gandhi National Fellowship (Grant No. F. 14-2(SC)/2009 (SA-III)) and the permissions given to him for collecting agaric specimens from the forests of Kerala by the Principal Chief Conservator of Forests, Government of Kerala (WL12-4042/2009 dated 05-08-2009). This Project was funded by the National Plan for Science, Technology and Innovation (MAARIFAH), King Abdulaziz City for Science and Technology, Kingdom of Saudi Arabia, Award Number (12–BIO2840–02). B.K. Cui thanked for the finance by the Fundamental Research Funds for the Central Universities (No. 2016ZCQ04) and the National Natural Science Foundation of China (Project No. 31422001). We would like to thank Dr. Marcela E. S. Cáceres for translating the German description of Clavulinopsis, the Conselho Nacional de Desenvolvimento Científico (CNPq) for the master scholarship of LSAN, the Pós-Graduação em Biologia de Fungos (UFPE, Brazil), CNPq (Protax 562106/2010-3, Sisbiota 563342/2010-2, Universal 472792/2011-3) and FACEPE (APQ-0788-2.03/12) for financing this research. H.B. Lee was supported by the Graduate Program for the Undiscovered Taxa of Korea, and by the Project on Survey and Discovery of Indigenous Fungal Species of Korea, funded by NIBR and NNIBR of the Ministry of Environment (MOE), and in part by a fund from National Institute of Animal Science under Rural Development Administration, Republic of Korea. Aniket Ghosh, Priyanka Uniyal and R.P. Bhatt are grateful to the Head, Department of Botany & Microbiology, HNB Garhwal University, Srinagar Garhwal for providing all kinds of facilities during the present study. Kanad Das and Abhishek Baghela are thankful to the Director, Botanical Survey of India, Kolkata and Director, MACS’ Agharkar Research Institute, Pune respectively for providing facilities. UGC provided fellowship to Aniket Ghosh and Priyanka Unial. Field assistance rendered by Mr. Tahir Mehmood and Mr. Upendra Singh (HNBGU) are also duly acknowledged. Tuula Niskanen, Kare Liimatainen, Ilkka Kytövuori, Joe Ammirati, Bálint Dima, and Dimitar Bojantchev would like to acknowledge Heino Vänskä for the help with nomenclature. We are grateful to the curators of H and S. This work was partially supported by the Ministry of Environment, Finland (YM38/5512/2009) and Oskar Öflunds Stiftelse. The authors thanks Dr. Kerstin Voigt for the inestimable help in critical reviewing the lower fungi entries, the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) and Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco (FACEPE) for the postgraduate scholarships to Diogo X. Lima and Carlos A. F. de Souza, respectively. We also thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and FACEPE for financial support through the projects: ‘Mucoromycotina in upland forests from the semi-arid of Pernambuco’ (CNPq—458391/2014-0), and ‘Diversity of Mucoromycotina in different ecosystems of the Pernambuco’s Atlantic Rainforest’ (FACEPE—APQ 0842-2.12/14). Z.L Luo and H.Y Su would like to thank the National Natural Science Foundation of China (Project ID: 31460015) for financial support on Study of the distribution pattern and driving factors of aquatic fungal diversity in the region of Three Parallel Rivers. C. Phukhamsakda would like to thank Dr. Matthew P. Nelsen for his valuable suggestions. Saranyaphat Boonmee thanks to the Thailand Research Fund, project number TRG5880152 and Mae Fah Luang University for a Grant Number 2559A30702006”. C.G. Lin and Y. Wang thank for the finance by the National Natural Science Foundation of China (No. NSFC 31560489) and Fundamental Research on Science and Technology, Ministry of Science and Technology of China (2014FY120100). Haixia Wu would like to thank Dr. Shaun Pennycook for his kindly nomenclatural review and thanked for the finance by the National Natural Science Foundation of China (Project No. 31300019). S.C. Karunarathna, P.E. Mortimer and J.C. Xu would like to thank the World Agroforestry Centre, East and Central Asia Office; Key Laboratory for Plant Diversity and Biogeography of East Asia, Kunming Institute of Botany, Chinese Academy of Science; the Chinese; Ministry of Science and Technology, under the 12th 5-year National Key Technology Support Program (NKTSP)2013BAB07B06 integration and comprehensive demonstration of key technologies on Green Phosphate-mountaion Construction and the CGIAR Research Program 6: Forest, Trees and Agroforestry for partial funding. The National Research Council of Thailand (NRCT), projects—Taxonomy, phylogeny and cultivation of Lentinus species in northern Thailand (NRCT/55201020007) is also thanked. K. Tanaka and A. Hashimoto would like to thank the Japan Society for the Promotion of Science (JSPS; 26291084, 16K07474, 16J07243).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hyde, K.D., Hongsanan, S., Jeewon, R. et al. Fungal diversity notes 367–490: taxonomic and phylogenetic contributions to fungal taxa. Fungal Diversity 80, 1–270 (2016). https://doi.org/10.1007/s13225-016-0373-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-016-0373-x