Abstract
The recognition of taxonomic ranks in the Linnean classification system is largely arbitrary. Some authors have proposed the use of divergence time as a universally standardized criterion. Agaricus (Agaricaceae, Agaricales) is a mushroom genus that contains many species of high commercial value. Recent studies using ITS sequence data discovered 11 new phylogenetic lineages within the genus, however their taxonomic ranks were uncertain due to the lack of criteria to define them within traditional taxonomy. In this study, we analyzed ITS sequence data from 745 collections (nearly 600 being newly generated) including 86 from type specimens of previously recognized subgenera and sections. Many monophyletic groups were recognized, but most basal relationships were unresolved. One hundred and fourteen representatives of the identified ITS clades were selected in order to produce a multi-gene phylogeny based on combined LSU, tef-1α, and rpb2 sequence data. Divergence times within the multi-gene phylogeny were estimated using BEAST v1.8. Based on phylogenetic relationships and with respect to morphology, we propose a revised taxonomic system for Agaricus that considers divergence time as a standardized criterion for establishing taxonomic ranks. We propose to segregate Agaricus into five subgenera and 20 sections. Subgenus Pseudochitonia is substantially emended; circumscription of the subgenera Agaricus and Flavoagaricus is restricted to taxa of sections Agaricus and Arvenses, respectively; and two new subgenera (Minores and Spissicaules) are introduced. Within Pseudochitonia, sections Bivelares, Brunneopicti, Chitonioides, Nigrobrunnescentes, Sanguinolenti and Xanthodermatei are maintained, but the latter two are reduced because we raise subsection Bohusia to sectional rank and a clade within section Xanthodermatei is formally introduced as section Hondenses; and sections Rubricosi, Crassispori, Flocculenti, and Amoeni are introduced. Section Laeticolores is placed in the subgenus Minores and sections Rarolentes and Subrutilescentes are placed in the subgenus Spissicaules. Twenty-two new species belonging to various sections are described. This work exemplifies that ITS data, while useful at lower taxonomic levels (i.e., detection of species and species groups), are of limited value for inferring deeper phylogenetic relationships. Finally, we suggest that the establishment of a standardized taxonomic system based on divergence times could result in a more objective, and biologically more meaningful, taxonomic ranking of fungi.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Current systematic and taxonomic research focuses on organizing and classifying species, genera, and higher ranks using a combination of characters, including molecular, morphological, ecological, and biogeographical data (e.g., Hyde et al. 2013, Maharachchikumbura et al. 2015). However, as several biologists have pointed out, the Linnean system lacks universal standardized criteria that can comprehensively recognize taxonomic ranks. Fifty years ago, W. Hennig suggested that a taxonomic rank should reflect its geological age, and that time divergence could be used as a universally standardized criterion in the systematics of all known organisms (Hennig 1966). However, it was not until the advent of DNA sequence data analyses and molecular clocks that this idea was further considered.
One of the first studies that aimed at reconciling taxonomic ranks and time divergence estimates is that of Avise and John (1999). These authors studied the molecular evolution of fish (14 species and 9 genera), anthropoid primates (7 species in several families), and fruit flies (14 species of Drosophila). They found a notable disparity in the ages of lineages and their taxonomic rank; for example, all fruit flies, shared common ancestors within a single genus more than 40 million years ago, while some primates formed different families a few million years ago, and cichlid fish were placed in several different genera that formed a few thousand years ago. As a result, they proposed a standardized temporal scheme for biological classification using approximate dates of nodes in an evolutionary tree, as a universal ranking criterion.
Advances in divergence time estimation based on DNA sequence data provide a way to convert molecular change into evolutionary time (Robinson and Robinson 2001; Drummond et al. 2006; Drummond et al. 2012). Fossil isotopic ages aid estimation of molecular divergence time analyses by providing points of calibration (Berbee and Taylor 2010). Two fungal fossil specimens – one within the order Agaricales (Berbee and Taylor 2010) and the other within the order Hymenochaetales (Smith et al. 2004) – provide a means for divergence time calibrations in the basidiomycetes. Although recent reports have successfully established divergence times in fungi (Hibbett and Matheny 2009), these studies did not use these data for the establishment of a taxonomic system.
The saprotrophic genus Agaricus is the type genus of the family Agaricaceae (Agaricales), and has a worldwide distribution. Three hundred and seventy-five species are currently recognized in the genus (Zhao et al. 2011). However, the count should now exceed 400 due to recent discoveries of new species (Chen et al. 2012; 2015; Gui et al. 2015; Lebel 2013; Lebel and Syme 2012; Parra 2013; Wang et al. 2015; Zhao et al. 2012). Agaricus includes many species that have high nutritional and medicinal value, leading to it being a well-studied genus (Largeteau et al. 2011; Wisitrassameewong et al. 2012). Historically, the bulk of morphotaxonomic and systematic foundations in Agaricus has stemmed from mycological work in Europe, North America, tropical Asia, and Africa (Bohus 1995; Cappelli 1984; Heinemann 1978; Kerrigan 1986; Konrad and Maublanc 1952; Kühner and Romagnesi 1953; Möller 1950, 1952; Pilát 1951; Moser 1967–1983; Singer 1986; Wasser 1980). In recent years, Agaricus has been well-studied in Europe in a combination of molecular (ITS) and morphological analyses (Parra 2008, 2013).
The first molecular phylogenetic studies in Agaricus were carried out by Mitchell (1999) from the use of ITS and LSU sequence data from 16 species. Later, Geml et al. (2004) conducted analyses with a larger sample (42 species), revealing six clades. Some sections have been shown to be monophyletic (Arvenses, Bivelares, Xanthodermatei), while others (Sanguinolenti and Spissicaules) were not (Challen et al. 2003; Geml et al. 2004; Kerrigan et al. 2005, 2008). In a more global analysis based on the ITS sequence data from 128 species, an eight-section taxonomic system was proposed (Parra 2008, 2013), including seven new strongly supported and four moderately supported unnamed clades, mainly from tropical areas (Zhao et al. 2011). Recently, four additional sections, Brunneopicti, Nigrobrunnescentes, Rarolentes and Subrutilescentes, have been recognized from tropical and temperate regions (Chen et al. 2015; Parra et al. 2014; Wang et al. 2015; Kerrigan 2016 in press), bringing to twelve the number of currently recognized sections in Agaricus. Historically, the genus Agaricus has been separated into three subgenera Agaricus, Lanagaricus and Conioagaricus using morphological and macrochemical reactions characters (Heinemann 1956a, b; 1978; Singer 1986). The first two subgenera are well accepted by most mycologists (Parra 2013), however the subgenus Coniagaricus is heterogenous; its members have an epithelium pileipellis that is completely different from all Agaricus species from the other subgenera, which lead Heinemann (1978) to suggest that members of this section should be relocated to other genera, even though he erected this subgenus (Heinemann 1978). Unfortunately, DNA data are not available for the subgenus Conioagaricus, which would help clarify its taxonomic position.
This study samples members of each of the twelve sections currently recognized and reports a new classification of subgenera and sections within Agaricus based on extensive morphological examination of specimens in combination with analysis of multi-gene sequence data. We propose a new classification system of the genus that reflects evolutionary divergence time, while concomitantly redefining taxonomic groups based on phylogenetic relationships.
Materials and methods
Sampling
Our dataset is composed of ITS sequences from 745 specimens (including 64 type specimens of known species and 22 herein newly introduced types), mainly from tropical and temperate areas of Asia (China, Thailand, and Malaysia), Europe (France, Spain) and North America (Canada and the U.S.A.), representing each of the 12 sections currently recognized (Parra 2008, 2013; Chen et al. 2015; Parra et al. 2014; Wang et al. 2015; Kerrigan 2016 in press), as well as seven new clades (Zhao et al. 2011). ITS sequence data for nearly 600 collections are new contributions. Nearly 150 other ITS sequences are mainly from our previous studies (Chen et al. 2015, Wang et al. 2015; Zhao et al. 2011). In the latter case, we only used sequence data attached to voucher specimens, or on whose identification we felt we could confidently rely (Callac and Guinberteau 2005; Chen et al. 2012, 2015; Kerrigan 2016 in press; Kerrigan et al. 2005, 2008; Thongklang et al. 2014; Parra 2008, 2013; Wang et al. 2015; Zhao et al. 2011, 2012, 2013).
Morphological examination
The protocol of morphological study, description of morphological characters and macrochemical reactions follow Largent’s methodology (Largent 1986a, b) and our previous studies (Parra 2008; Zhao et al. 2011). Specimens are deposited in the Herbarium Mycologicum Academiae Sinicae (HMAS); CGAB herbarium (INRA); Luis A. Parra private herbarium (LAPAG); Mae Fah Luang University Herbarium (MFLU) and BIOTEC Bangkok Herbarium (BBH). Herbarium acronyms are those of Thiers (http://sweetgum.nybg.org/ih/). Faces of fungi numbers (Facesoffungi) and Fungal Names numbers (FN) are as explained in Jayasiri et al. (2015) and Fungal names (2013). Colour terms are according to Kornerp and Wanscher (1978) or the Online Auction Colour ChartTM (http://OnlineAuctionColorChart.com). Measurements of basidiospores, basidia and cheilocystidia are calculated from at least 20 measurements. Basidiospore measurements include the range of all spores length by width, then \( \overline{x} \), the mean of all spores ± SD (Standard Deviation), Q, the range of the quotient length/width of all basidiospores, and Qm, the mean Q of all spores ± SD.
PCR amplification and sequencing
Genomic DNA was extracted from dried specimens using E.Z.N.A. Forensic DNA Kit (OMEGA Bio-Tek, Norcross, GA, USA) or using 96-well plates (Dentinger et al. 2010). We used primers ITS4 and ITS5 (White et al. 1990) for the ITS1-5.8S-ITS2 region (ITS) of rDNA region, primers LROR and LR5 (Moncalvo et al. 2000, 2002) for the nuclear LSU-rDNA region, primers EF1-983 F and EF1-1567R (Morehouse et al. 2003) for translation elongation factor alpha (tef-1α), and primers b6F and b7.1R (Matheny et al. 2007) for RNA polymerase II subunit II (RPB2). Genes were amplified by polymerase chain reaction (PCR) using the procedures mentioned in Zhao et al. (2011); Moncalvo et al. (2000); Morehouse et al. (2003); Matheny et al. (2007). The PCR products were sent to Shuoyang Biotech Company (Kunming city, China) for sequencing or were sequenced at the Royal Ontario facilities with an Applied Biosystems 3730 DNA Analyzer (Life Technologies, Carlsbad, CA, USA).
Preliminary ITS tree
The 745 ITS sequences used in this study could not be unambiguously aligned because of numerous insertions/deletions. Nevertheless, in order to obtain a better a priori understanding of clade diversity within Agaricus, we conducted ITS analyses of these sequences using neighbor-joining bootstrapping (NJ) in PAUP*4.0 from various alignment methods (not shown). This preliminary NJ tree served as a map for sampling strategies, both for sequencing other genes and for the divergence time analysis. One hundred and fourteen samples representing species from each of the recognized sections, main lineages within each section, and ‘unclassified’ taxa, were subsequently selected for generating LSU, tef-1α, and rpb2 sequence data.
Divergence time estimation of clades (crown vs. stem ages)
The term “clade” refers to a monophyletic group of organisms, which includes the most recent common ancestor of all of its members and their descendants (UCMP Glossary: Phylogenetics http://www.ucmp.berkeley.edu/glossary/gloss1phylo.html). The last common ancestor of a living clade plus all of its descendants is called the crown group. The stem group –which may include extinct lineages– refers to the lineage(s) that originated between the crown age and divergence time between the crown group and the sister clade (Budd 2001; Budd and Jensen 2000). This means that for any given group of taxa the crown age is always younger than the stem age (Stadler et al. 2014). The length of branch between stem ancestor and crown clade depends on factors such the timescale, the net diversification rate, and the species richness of the clade (McPeek and Brown 2007; Stadler et al. 2014). Here we use stem ages to reflect divergence times, as crown ages may change with taxon sampling.
Calibration strategies for estimation of the stem age of Agaricus
We conducted a fossil-calibrated analysis to estimate the time of divergence between Agaricus and its token outgroup Heinemannomyces within the Agaricomycetes. We selected 30 Agaricus species representative of the main sections and ITS clades within the genus for which we had LSU and tef-1α sequence. We integrated these data into the Agaricomycetes matrix of Sánchez-Ramírez et al. (2015) that used two fossils: Archaeomarasmius leggetti Hibbett et al., an agaricoid fruiting body preserved in 90 million years old Dominican amber (Hibbett et al. 1997) as representative of the minimum age of Agaricales; and Quatsinoporites cranhamii S.Y. Sm. et al., a poroid fruiting body from Apple Bay on Vancouver Island from 113 million years ago (Smith et al. 2004) as representative of the minimum age of Hymenochaetales. Members of the Boletales + Atheliales were set as the sister clade of Agaricales. The Agaricus + Heinemannomyces sequence data were aligned using MUSCLE v3.6 Edgar (2004a, b), and then combined with a previously aligned matrix with these outgroups as in Sánchez-Ramírez et al. (2015) using Mesquite v2.75 (Maddison and Maddison 2007). Outgroups in this matrix included 84 species from Agaricomycetes, members from the orders Agaricales, Boletales, and Hymenochaetales, and the agaricoid clades hygrophoroid, tricholomatoid, and marasmioid. Introns in the tef-1α region and a few poorly aligned sites in LSU were removed from the analysis.
Divergence times were estimated using BEAST v1.8 (Drummond et al. 2012). We first constructed an XML file with BEAUTI v1.8. Single gene alignments were imported as separate partitions. Clock and substitution models were set to be unlinked (independently estimated for each gene partition), while the tree prior parameters were set to be linked across partitions (concatenation). As substitution models, we used the GTR + G + I and SYM + G + I, for LSU and tef-1α, respectively, as suggested by jModelTest v2 (Darriba et al. 2012). We used the uncorrelated lognormal relaxed clock model (Drummond et al. 2006; Lepage et al. 2007), specifying a gamma distribution for the ulcd.mean parameter with a shape of 1.0, scale of 1E-3, and offset 0. On the calibrated nodes, we specified a prior gamma distribution with an arbitrarily long tail (scale of 50) and offset ages of 90 and 113 Ma for Agaricales and Hymenochaetales, respectively (Sánchez-Ramírez et al. 2015). We ran four independent Monte Carlo Markov Chains of 50 million generations, logging states every 5000 generations. We compared the log files of each run in Tracer v1.6 (Rambaut et al. 2013; http://tree.bio.ed.ac.uk/software/tracer/) evaluating convergence and mixing, ensuring that effective sample sizes (ESS) were at least 200. An ultrametric maximum-clade-credibility (MCC) tree was summarized using TreeAnnotator 1.8, discarding 10 % of states as burn-in and annotating clades with ≥ 0.8 posterior probability.
Divergence time estimation within Agaricus
We selected 114 taxa from a total of 745 Agaricus samples for dating analyses based on the preliminary ITS NJ tree and per-sample data completeness. We used ITS, LSU, tef-1α, and rpb2 sequence data, and Heinemannomyces as the outgroup taxon (Table 1). Each gene was separately aligned in MUSCLE 3.6 with default settings (Edgar 2004a, b). Ambiguously aligned regions were removed from phylogenetic analyses. Single-gene phylogenies were constructed using Bayesian methods and possible significant conflicts among the single-gene trees were tested (none were detected; data not shown). The dating analysis was performed from the concatenated data set in a manner similar to that described above, using BEAST v1.8; however, we calibrated the nodes by using the highest posterior density (HPD) age values for the Agaricus + Heinemannomyces divergence. In this case, we used normal distribution prior on the treeModel.rootHeight parameter, which had a mean of 66 Ma and a standard deviation of 1 Myr. The substitution models for LSU and tef-1α were the same as above, while for ITS and RPB2 they were TVM + I + G and TrN + I + G, respectively, as indicated by jModelTest v2.
Establishment of subgenera and sections of Agaricus
We used the following criteria to recognize subgenera and sections: (i) they must be monophyletic and statistically well-supported in the multi-gene analyses; (ii) their respective stem ages should be roughly equivalent, and subgenera stem ages must be older than section stem ages; and (iii) they should be identifiable phenotypically, whenever possible. With regard to the second criterion, estimated stem ages for subgenera and sections were estimated to be ca. 30 Ma and ca. 20 Ma, respectively (Table 2). However we kept the divergence times of sections Agaricus and Arvenses, because they are the only sections in subgenera Agaricus and Flavoagaricus, respectively.
Additional ITS analysis within some sections
There were a few taxonomically important specimens (type specimens or type species of known sections) for which we obtained ITS sequences, but not LSU, tef-1α and/or rpb2 sequences. For these we performed independent analyses of ITS sequences in order to identify “proxy” specimens that could represent them in the multigene phylogeny (Table 1). ITS sequence data were initially aligned using MUSCLE 3.6 with default settings (Edgar 2004a, b), then manually adjusted in Mesquite (http://mesquiteproject.org/mesquite/mesquite. html). The alignments have been submitted to TreeBase (TreeBase No. 18680 and 18682). Maximum-likelihood analyses were performed using RAxML v7 (Stamatakis 2006) with a GTR + G substitution model. To assess the statistical support of clades we ran 1000 fast-bootstrap (BS) replications under the GTR-CAT approximation. Bayesian analysis was performed with MrBayes-3.1.2 (Huelsenbeck and Ronquist 2001; Ronquist and Heulsenbeck 2003), with four chains (one cold, three incrementally heated) for10,000,000 generations and Trees were sampled every 100 generations. Those trees sampled prior to searches reaching a split deviation frequency value of 0.01 were discarded as the burn-in, and the remaining trees were used to calculate Bayesian posterior probabilities (PPs).
Results and discussion
Hennig (1966) and Avise and John (1999) suggested that time divergence could serve as a more objective and biologically informative criterion for the delimitation of taxonomic ranks. Here we present the first case study that attempts to use this criterion for the systematics revision of a fungal genus while trying to minimally disrupt its taxonomic ranks as they were recognized in the past. The limitations and uncertainties of molecular divergence time estimation have been discussed in van Tuinen and Torres (2015).
Estimation of the age of Agaricus
The ultrametric MCC tree of the Agaricomycetes (Supplementary Fig. 1) supports the stem ages of the order Hymenochaetales, Boletales + Atheliales, and Agaricales to be (mean values) 158, 141, and 151 Ma, respectively. The mean estimated stem age of Agaricus was 66 Ma.
Estimation of the age of subgenera and sections of Agaricus
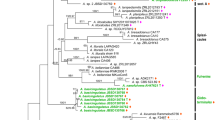
The MCC tree for Agaricus (Fig. 1) shows five monophyletic clades with stem ages of 30–33 Ma. These clades are named A, B, C, D, and E, all of them having PP values ≥ 0.99 (Fig. 1). We propose to rank them as subgenera. Within these five clades, 18 subclades that diverged 18–26 Ma ago were strongly supported statistically. We propose to rank them as sections.
Maximium Clade Credibility tree of Agaricus based on ITS, LSU, tef-1α, and rpb2 genes sequences with the outgroup Heinemannomyces sp.. Posterior probability which are equal and above 80 % are annotated at the internodes. The 95 % highest posterior density of divergence time estimation are marked by horizontal bars
Clade A is proposed as subgenus Minores. It includes section Minores as reported in Zhao et al. (2011); it diverged 30 Ma ago and is separated into two 26 Ma-old clades (A1 represents section Minores, A2 are section unknown and section Laeticolores).
Clade B, which is sister to Clade A, is proposed as subgenus Flavoagaricus. This clade is equivalent to section Arvenses in Geml et al. (2007), Parra (2013) and Zhao et al. (2011). Because this clade diverged 30 Ma ago, we propose to raise it to the subgenus level using the older name “Flavoagaricus”, which now includes solely section Arvenses.
Clade C (33 Ma) includes four clades (C1–4), which range in stem ages from 18 to 23 Ma. Clade C4 is roughly equivalent to the section Spissicaules (Heinemann 1978; Parra 2008). Clade C2 is equivalent to clade TRIII in Zhao et al. (2011) and is described as a new section Amoeni in the present study. Clades C1 and C3 correspond to sections Subrutilescentes and Rarolentes (Kerrigan 2016).
Clade D is composed of twelve well-supported clades (clades D1–12, Fig. 1) which diverged 18 to 26 Ma ago. We propose to recognize these clades at section level. Sections Chitonioides (clade D1), Bivelares (clade D2), Brunneopicti (clade D9) and Nigrobrunnescentes (clade D11) are supported with high PP values, in agreement with previous studies (Parra et al. 2014; Wang et al. 2015; Chen et al. 2015; Didukh et al. 2005; Challen et al. 2003). Section Xanthodermatei was suggested to include three monophyletic groups (represented here by A. xanthodermus Genev., A. bisporiticus Nawaz, Callac, Thongklang & Khalid, and A. hondensis Murrill) based on earlier studies (Geml et al. 2007; Callac and Guinberteau 2005; Kerrigan et al. 2005; Zhao et al. 2011, 2012, Thongklang et al. 2014). However, we found that this section is polyphyletic, and splits into two lineages (clades D3 and D4). Clade D4 contains A. xanthodermus, the type species of section Xanthodermatei, while clade D3 comprises samples ZRL2012611 and ZRL2012014 and is distant from clade D4. Section Sanguinolenti was split into two subsections (Sanguinolenti and Bohusia) by Parra (2008), which were polyphyletic in Zhao et al. (2011). In the present study, these two lineages (represented here by A. sylvaticus Schaeff. and A. bohusii Bon, respectively) are represented as clades D7 and D8. Tropical clades TRb was not well-supported in previous studies (Zhao et al. 2011); however, in this study it splits into well-supported clades D5 and D6 in Fig. 1, and based on their stem ages, they are treated as two sections. Clade D10, composed of A. pallidobrunneus sp. nov. and A. erectosquamosus sp. nov. is also well-supported, and a more detailed ITS analysis (Fig. 2) shows that it may be related to the tropical clade TRa of Zhao et al. (2011). Clade D12 is another well-supported clade in both the multi-gene and ITS trees, and based on the ITS tree it is related to TRc (Zhao et al. 2011). We found two isolated lineages close to clade D9 and D10, LD2012162 and ZRL2010099, but the present data could not clarify their precise location. Clade E is monophyletic with strong support with a stem age of 31 Ma; it represents the section Agaricus.
Phylogeny of Agaricus subgenus Spissicaules generated from Bayesian analysis of ITS sequences, rooted with A. campestris. Bootstrap support (BS) values > 50 % and Bayesian posterior probability (PP) values > 80 % (or >50% in some main clades) are given at the internodes (PP/BS). “T” means the sequence is from type specimen; a clade with thick branch is corresponding a certain section in Fig. 1
Within-clade relationships based on ITS sequence data
In order to better understand evolutionary relationships within clades in Fig. 1 and for which we have large ITS sampling sequences that align with little ambiguities (and in some cases in order to link taxonomically important samples such as type specimens to a proxy representative in our higher level multigene phylogeny), we performed separate Bayesian and Maximum-likelihood analysis of ITS sequence data
In Clade C (subgenus Spissicaules subg. nov.) we use 31 species (Fig. 2). Subclades C1 and C2, which correspond to sections Subrutilescentes and Amoeni are fully supported. Clade C4 contains A. lanipes (F.H. Møller & Jul. Schäff.) Hlaváček, A. litoralis (Wakef. & A. Pearson) Pilát, and A. bresadolanus Bohus, which were shown to belong to section Spissicaules in previous studies (Parra 2008; Kerrigan 1986), and clade C2 represents the TRIII clade of Zhao et al. (2011). Clade C3 is monophyletic (albeit not well supported), but contains 2 well-supported lineages in the ITS tree and comprises five species.
In Clade D we used 105 sequences representing 81 species, with A. campestris L. as the outgroup (Fig. 3). As in the backbone (multigene) tree (Fig. 1), monophyly of sections Bivelares (clade D2), Chitonioides (clade D1), Brunneopicti (clade D9), and Nigrobrunnescentes (clade D11) are fully supported. Section Xanthodermatei splits into two clades: Clade D4 contains the type species A. xanthodermus with 0.99 PP support and clade D3 with 1 PP support is represented by A. hondensis and allied taxa. The section Sanguinolenti (clade D7), which contains the type species A. sylvaticus, is fully supported (Fig. 1) and we consider it as section Sanguinolenti; however, section Bohusia (clade D8) is monophyletic in the multi-gene tree, but not in the ITS tree (Fig. 3). Clades D5, D6, and D10 are well-supported, while clades D11 and D12 have low support in the ITS tree.
Phylogeny of Agaricus subgenus Pseudochitonia generated from Bayesian analysis of ITS sequences, rooted with A. campestris. Bootstrap support (BS) values > 50 % and Bayesian posterior probability (PP) values > 80 % (or >50% in some main clades) are given at the internodes (PP/BS). “T” means the sequence is from type specimen; a clade with thick branch is corresponding a certain section in Fig. 1
Sections Minores (clade A1) and Laeticolores (clade A2) from subgenus Minores, section Arvenses (clade B) from subgenus Flavoagaricus, and section Agaricus (clade E) from subgenus Agaricus contain type specimens or type species of sections and are well or fully supported as monophyletic groups in the backbone tree (Fig. 1); thus, more extensive ITS analyses were not performed for these groups.
Taxonomy
The diagnostic characters in the field of the genus Agaricus are brown to dark brown, free, never deliquescent lamellae, a pileus easily separable from the stipe and the presence of one or several simple or double annuli. Microscopically, the pileipellis is always a cutis, the hymenophoral trama is mostly slightly interwoven, sometimes regular when young, the hyphae are devoid of clamp connections and the basidiospores lack a true apical pore. Basidiome surface discolouration when touched or rubbed and of the context when bruised or cut, biochemical reactions, and odour are also crucial to the taxonomical characterization at species, section and subgenus level.
The developmental process of annulus formation is often very complex in Agaricus species, so that some terms are used specifically in this genus. For example, the terms superous, inferous and intermediate are used when a mechanical test shows if it peels upwards (generally like a skirt), if it peels downwards (generally like a sock), or if it breaks (generally perpendicular or completely appressed to the stipe), respectively, on pulling (Parra 2008). It must be noticed that the annulus can be inferous, but visually pendant (Parra 2008). Also the annulus may be simple (when smooth in its underside or double (two layers), when floccose or squamose on its underside, and if double it is important to know if this ornamentation is arranged radially like a cogwheel. Furthermore, in some species these two layers can be completely separated, such as in A. duplocingulatus Heinem. All characters of the annulus are basic requiement in the genus Agaricus when characterizing certain subgenera, sections and species.
In order to provide an outline of the Agaricus taxonomic system, the main morphological characters of each section are shown in Table 3, and detailed descriptions of all the taxa at any rank are also given.
1. Agaricus subgenus Agaricus
Facesoffungi number: FoF01165; Fig. see Parra 2008, pp198;540–548
Type species Agaricus campestris L., Sp. Pl. 2: 1173 : Fr., Syst. Mycol. 1:281. 1821.
Agaricus subgenus Agaricus only contains A. section Agaricus
Delimitation of Agaricus subgenus Agaricus and A. section Agaricus
The diagnosis of this subgenus is the same as that of A. section Agaricus. KOH and Schäffer’s reactions negative on white areas of the surface of the pileus. Pileus surface unchanging or slightly yellowing, rarely rufescent on touching. Context often turning pink or strongly reddening and then becoming brownish over time near the connection between the stipe and pileus, and sometimes yellow at the base of the stipe on exposure. Odour usually indistinct or mushroomy, but in some species of anise in young basidiomes. Annulus superous or intermediate, simple or double, membranous or fibrillose. Cheilocystidia absent or indistinct, basidia-like, some species abundant, large, globose, piriform or ovoid, such as A. langei (F.H. Møller) F.H. Møller and A. depauperatus (F.H. Møller) Pilát, never catenulate.
Stem age and phylogenetic support
The subgenus Agaricus has long been considered a group containing all sections of this subgenus in temperate areas (Heinemann 1978; Singer 1986; Parra 2008). This study shows that section Agaricus as represented by the type species A. campestris (clade E in Fig. 1) is a monophyletic group, which is supported with 1 PP value, and this result concurs with previous studies (Geml et al. 2007; Zhao et al. 2011). The present circumscription of the subgenus Agaricus is restricted to that of A. section Agaricus with the remaining temperate sections placed in other subgenera. The stem age of this clade is 30.57 Ma and the isolated phylogenetic placement indicates that this clade should represent a higher rank in this genus.
2. Agaricus subgenus Flavoagaricus Wasser, Fl. Fung. RSS Ukrainicae: 138. 1980.
Facesoffungi number: FoF01166; Fig. see Parra 2013, pp835-849
Type species Agaricus arvensis Schaeff., designated by Wasser, Fl. Fung. RSS Ukrainicae: 138.1980.
Agaricus subgenus Flavoagaricus only contains A. section Arvenses (Konrad & Maubl.) Konrad & Maubl., Encycl. Mycol. 14: 104. 1948.
Delimitation of Agaricus subgenus Flavoagaricus and Agaricus section Arvenses
The diagnosis of this subgenus is the same as that of A. section Arvenses. KOH and Schäffer’s reactions positive on all surfaces of basidiome. The surface of pileus often discolours yellow on touching. Context turning yellow, reddish, or not discolouring on exposure. Odour of anise or bitter almonds. Annulus superous, double with lower layer woolly, floccose, breaking in scales often radially arranged as a cogwheel. Cheilocystidia generally catenulate.
Stem age and phylogenetic support
This subgenus/section is reflected in clade B (Fig. 1) which is a monophyletic group with 1 PP support, and the type species of section Arvenses, A. arvensis, nests well in this clade along with other known species from this section, such as A. essettei Bon, A. abruptibulbus Peck, A. augustus Fr., and A. flocculosipes R.L. Zhao, Desjardin, Guinb. & K.D. Hyde. The analysis also indicates its stem age is 30.06 Ma; thus, it is a natural subgenus, and includes only the section Arvenses.
3. Agaricus subgenus Minores (Fr.) R.L. Zhao & Moncalvo. stat. nov.
Fungal Names: FN570194
≡Agaricus [unranked] Minores Fr., Hymenomyc. Eur.: 281. 1874. [basionym]
Type species: Agaricus comtulus Fr. designated by Heinemann, Bull. Jard. Bot. Etat 26: 42. 1956.
Facesoffungi number: FoF01167; Fig. see Parra 2013, pp 968–975
Etymology: the epithet “Minores” is following the name of section Minores.
Delimitation of Agaricus subgenus Minores
KOH reaction positive, Schäffer’s reaction usually positive, seldom negative. Surface of pileus often discolouring yellow on touching. Context often turning yellow on exposure. Odour of anise or bitter almonds. Basidiomes often slender, small-to-medium sized. Annulus superous, if simple, thin, fragile, smooth on both sides; if double upper side smooth and lower surface floccose or squamose. Cheilocystidia simple, clavate, pyriform, sometimes absent, scattered or rare.
Stem age and phylogenetic support
Subgenus Minores (clade A) is fully supported and its stem age is 30.06 Ma. The phylogeny also has a closer position to the subgenus Flavoagaricus than others.
The subgenus includes sections Minores (Fr.) Henn. and Laeticolores Heinem.
3.1 Agaricus [subgenus Minores] section Minores (Fr.) Henn. in Engl. & Prantl, Nat. Pflanzenfam. 1(1**): 238. 1898.
Type species: Agaricus comtulus Fr. designated by Heinemann, Bull. Jard. Bot. État 26: 42. 1956.
Facesoffungi number: FoF01168; Fig. see Parra 2013, pp 968–975
Delimitation of Agaricus section Minores
The characters of this section are those of subgenus Minores, but the Schäffer’s reaction is positive and the lower surface of the annulus is neither floccose nor squamose. The universal veil is poorly developed or absent.
Stem age and phylogenetic support
This section is reflected in clade A1 in Fig. 1 with 0.95 PP support, and its stem age is 26.07 Ma. In this clade, there are 26 species including the type species of this section and three type specimens. In Zhao et al. (2011), three clades (clades TRV, VI, and VII) were strongly supported based on ITS sequence data, which are composed of 10 unnamed species from tropical areas and A. aridicola Geml, Geiser & Royse ex Mateos, J. Morales, J.Muñoz, Rey & Tovar from temperate areas. These three clades are sister to the section Minores (Zhao et al. 2011). In this study, samples CA848 and LAPAG589 (A. aridicola), which were members of TRV in Zhao et al. (2011), are included. The tree (Fig. 1) shows that CA848 clusters with the new samples CA921, ZRL2011156 and ZRLLD013 in clade A1 (section Minores), which is fully supported with a 12 Ma stem age. Sample LAPAG589 (A. aridicola) nested with other new samples (LAPAG797) as a clade also within clade A1. Another sample, CA846, represents the clade TRVI (Zhao et al. 2011) and clusters with sample ZRL2012357, which is fully supported with a 14 Ma stem age, and also nests in clade A1. Because the clades related to TRV and VI are younger than those sections that we accept, and in the topology they are distributed in other well-supported clades within clade A1, we conclude that the members of TRV and VI should belong to section Minores (clade A1).
3.2 Agaricus [subgenus Minores] section Laeticolores Heinem., Kew Bull. 15(2) : 244. 1961.
Type species: Agaricus laeticolor Heinem. & Gooss.-Font., designated by Heinemann, Kew Bull. 15(2): 244. 1961.
Facesoffungi number: FoF01169; Fig. see Heinemann 1956, p99
Delimitation of section Laeticolores
The characters of this section are those of subgenus Minores, but the Schäffer’s reaction is positive, seldom negative and the lower surface of the annulus is smooth, heavily fibrillose, squamose. The universal veil is developed and is often present on the pileus, while the annulus margin and stipe base forms small squamules.
Stem age and phylogenetic support
This section is represented by only one species, A. rufoaurantiacus Heinem. in Fig. 1, and its stem age is 26.07 Ma. Section Laeticolores was established by Heinemann (1961) based on samples from Africa and Central America in Agaricus subgenus Lanagaricus Heinem. Heinemann included seven species in this section in his world key to the genus Agaricus (Heinemann 1978). One of them, A. rufoaurantiacus has been used in our analyses (LAPAM 15) and this section also is studied in molecular phylogeny by first time.
Clade A2 (section unknown or Laeticolores)
Three specimens (LD2012129 introduced as new species A. candidolutescens in this paper, LAPAM14 and ZRLWXH3161) cluster together (Fig. 1), and they are sister to section Laeticolores (represented by A.rufoaurantiacus). Because of the lack of some important morphological information and the limitation of sampling in section Laeticolores, this section is unresolved in this study.
Agaricus candidolutescens L.J. Chen & R.L. Zhao, sp. nov. Figs. 4a, b and 11.
Fungal Names: FN570195
Facesoffungi number: FoF01170
Etymology: “candido” refers to the white surface of basidiome; “lutescens” refers to slightly yellow of context on cutting.
Typus: THAILAND, Chiang Rai Prov., Doi Pui, 25 July 2012, collector Jie Chen, LD2012129 (MFLU12-0962, holotype; HMAS373994, isotype).
Original description: Pileus 27–42 mm diam., 2 mm broad at disc, convex to applanate; surface dry, smooth, silky, with woolly-fibrillose squamules towards the margin, pure white; margin appendiculate. Lamellae free, crowded, ventricose, intercalated with lamellulae, with more than 5 series, 2.5–4 mm broad, at first pink, then pinkish brown, later dark brown and finally almost black. Stipe 40–55 × 3–8 (10–12 at base) mm, cylindrical with a bulbous base, narrowly hollow, white, staining yellow when bruised, smooth above the ring, fibrillose below the ring. Annulus membranous, simple, white, fragile, upper side smooth, lower side fibrillose. Context firm, at first white on cutting, both in pileus and stipe, and quickly turning yellow at the stipe base, finally after 15 min uniformly brown. Odour of almonds.
Macrochemical reactions: KOH reaction positive yellow, Schäffer’s reaction negative.
Basidiospores 6.4–7.3 (−7.5) × 3.9–4.5 μm, [\( \overline{x} \) = 6.8 ± 0.32 × 4.2 ± 0.2, Q = 1.49–1.77, Qm = 1.6 ± 0.05, n = 20], ellipsoid, smooth, dark brown, thick-walled, without germ pore. Basidia11–13 × 6.5–8 μm, clavate, hyaline, smooth, 4-spored. Cheilocystidia 18–38 × 7.5–13 μm, rare, simple, pyriform or sphaeropedunculate, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 5–9 μm in diam., cylindrical, hyaline, smooth, at times slightly constricted at the septa.
Habitat: in groups, in rich soil of tree stump.
Notes: Compared to all species of section Laeticolores, which is the closest section in phylogeny, A. candidolutescens differs in having a pure white, whole basidiome, and a woolly-fibrillose, pileus margin and lower surface of annulus (Heinemann 1978). In the phylogenetic tree (Fig. 1), unnamed sample ZRLWXH3161 from tropical China is sister to A. candidolutescens; however, this Chinese collection has a dark brown, squamose pileus. In morphology, A. candidolutescens is similar to A. haematoscarcus Heinem. et Gooss. (section Lanosi) in its pure white and woolly-fibrillose pileus, however, A. haematoscarcus has a strong red, blood colour on cutting, wider basidiospores (4.8–5.6 μm wide in Heinemann 1956a; 4.5–5.5 μm wide in Parra 2013), and basidiospores with an acute apex.
4. Agaricus subgenus Pseudochitonia Konrad & Maubl., Icon. Select. Fung. 6, fasc. 6: 61. 1927
Type species: Agaricus pequinii (Boud.) Konrad & Maubl., Icon. Select. Fung. 6, fasc. 6: 61. 1927, here designated by L.A. Parra.
Facesoffungi number: FoF01171; Fig. see Parra 2008, pp641-642
Delimitation of Agaricus subgenus Pseudochitonia
This subgenus contains the most sections in the genus Agaricus. KOH and Schäffer’s reactions are negative or positive. Staining yellowish, pink, reddish brown or indistinct on touching the surfaces or on cutting the context. Odour generally mushroomy and pleasant, sometimes indistinct or also unpleasant, like fish or like ink or phenol, and in this latter case often accompanied with an intense yellow discolouration on cutting and bruising, mainly at pileus margin and stipe base. Annulus variable, superous or inferous, simple or double (typically 2 layers and separated far away). Cheilocystidia present or absent, generally simple and not catenulate.
Stem age and phylogenetic support
This subgenus (clade D) is well-supported in the multi-gene and ITS trees. Its stem age is 30.57 Ma. The subgenus Agaricus is the closest group. There are twelve strongly supported clades (D6 is represented by a single sample in Fig. 1) which diverged 18–23 Ma, and these clades are reflected in twelve sections: Agaricus sect. Bivelares (Kauffman) L.A. Parra, A. sect. Bohusia (L.A. Parra) L.A. Parra & R.L. Zhao stat. nov., A. sect. Brunneopicti Heinem., A. sect. Chitonioides Romagn., A. sect. Crassispori R.L. Zhao, A. sect. Flocculenti J. Chen, K.D. Hyde & R.L. Zhao, A. sect. Hondenses R.L. Zhao & L.A. Parra sect. nov., A. sect. Nigrobrunnescentes K. P. Peterson, Desjardin, & Hemmes, A. sect. Rubricosi R.L. Zhao, A. sect. Sanguinolenti Jul. Schäff & F.H. Møller ex L.A. Parra, A. sect. Trisulphurati Heinem., and A. sect. Xanthodermatei Singer.
4.1 Agaricus [subgenus Pseudochitonia] section Bivelares (Kauffman) L.A. Parra, Fungi Europaei 1. Agaricus L. Allopsalliota Nauta & Bas: 265. 2008.
Type species: Agaricus rodmanii (Peck) Lloyd, designated by Singer, The Agaricales in Modern Taxonomy 3rd ed.: 461. 1975.
Facesoffungi number: FoF01172; Fig. Parra 2008, pp285, 597–602
Delimitation of Agaricus section Bivelares
KOH and Schäffer’s reactions negative. Surface of pileus discolouring reddish or not. Context often turning pink to reddish or unchanging on exposure. Odour indistinct or mushroomy. Basidiomes generally stout, pileus diameter almost equal to stipe length. Annulus intermediate or with one or more inferous annuli, sometimes coexisting with a superous annulus. Cheilocystidia present, mostly clavate, some septate at the base (such as A. subperonatus). No toxic species and easy to reproduce on artificial compost, such as A. bisporus (J.E. Lange) Imbach.
Stem age and phylogenetic support
The section (clade D2) is represented by well-known cultivated species, such as A. bisporus and A. bitorquis (Quél.) Sacc. This section is fully supported as a monophyletic group in analyses of combined multigene sequence data (Fig. 1) and ITS sequence data (Fig. 3). These results are consistent with previous studies (Didukh et al. 2005; Challen et al. 2003; Li et al. 2014). The stem age of this section is 18.16 Ma.
4.2 Agaricus [subgenus Pseudochitonia] section Bohusia (L.A. Parra) L.A. Parra & R.L. Zhao, stat. nov.
Fungal Names: FN570196
≡Agaricus [subgenus Agaricus] subsection Bohusia L.A. Parra, Fungi Europaei 1. Agaricus L. Allopsalliota Nauta & Bas: 159. 2008. [basionym]
Type species: Agaricus bohusii Bon, designated by L.A. Parra, Agaricus L. Allopsalliota Nauta & Bas: 159. 2008.
Facesoffungi number: FoF01173; Fig. see Parra 2008, pp 648–655, 706
Delimitation of Agaricus section Bohusia
KOH reaction positive yellow only at the stipe, Schäffer’s reaction positive red or violaceous purple. Orange-yellow discolouration on stipe surface on touching often present. Context slightly yellow, red or unchanging on exposure. Odour mushroomy. Annulus superous, thick, double. Cheilocystidia present, clavate.
Stem age and phylogenetic support
This section is represented by A. bohusii (LAPAG562) and A. crassisquamosus sp. nov., (ZRL2012607) as a monophyletic clade with strong support in the multi-gene tree (Fig. 1, clade D8). Its stem age is 24.36 Ma. We conclude that subsection Bohusia (Parra 2008) should be raised to sectional level. In the ITS tree (Fig. 3), this new species clusters with specimen WC913 (not included in multi-gene analysis), which is fully supported. Presently, these three species are recognized as members of section Bohusia, and comprise two main lineages; one is represented by A. bohusii (Parra 2008) and the other lineage is A.crassisquamosus sp. nov. and specimen WC913.
Zhao et al. (2011) found that specimens ZRL3012 and ZRL2136 were loosely related to A. bohusii in a previous ITS analysis, but in the present study they did not cluster with A. bohusii and form an isolated clade (Fig. 3). The reliability of their phylogenetic placement needs to be further examined using more gene sequence data.
Agaricus crassisquamosus R.L. Zhao, sp. nov. Figs. 4c, a and 12.
Fungal Names: FN570224
Facesoffungi number: FoF01174
Etymology: epithet “crassisquamosus” refers to the presence of thick squamules on the pileus of this species.
Typus: China, Tibet, Milin, Nanyigou National Forest Park, Alt. 3089 m, E94°21′24″, N29°15′71″, collected by R.L. Zhao, 29 July, 2012, ZRL2012607 (HMAS273991, holotype).
Original description: Pileus 30–60 mm in diam., 5–7 mm thick at the disc, convex, subumbonate; surface dry, completely covered by overed by large, appressed, dark reddish brown (oac523) fibrillose squamules ; margin exceeding and crenate. Lamellae free, crowded, 5–7 mm width, intercalated with lamellulae, brown, dark brown (oac524) when mature. Stipe 60–80 × 6–11 (apex) – 10–20 (base) mm, clavate with a subbulbous base, hollow, surface smooth and white above and below the annulus, becoming yellow on touching. Annulus membranous, superous, pendant, double, the upper side striate, lower side densely fibrillose or floccose, white; Context fleshy, on cutting white both in pileus and stipe, and colour unchanging on exposure. Odour pleasant.
Macrochemical reactions: KOH positive yellow, Schäffer’s reaction positive red.
Basidiospores 6.1–7.3 × 4–4.8 μm [\( \overline{x} \) = 6.4 ± 0.3 × 4.3 ± 0.2, Q = 1.4–1.6, Qm = 1.5 ± 0.1, n = 20], ellipsoid, brown, smooth and thick-walled, without germ pore. Basidia 19–26 × 6–10 μm, clavate, 4-spored. Cheilocystidia 19–48 × 8–18 μm, clavate, pyriform with long stipe, hyaline. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 4–8 μm in diam., long cylindrical, curved, branched, containing brown pigments.
Habitat: scattered in forests dominated by Picea spp.
Notes: This new species is characterized in the field by medium to large basidiomes, and a thick and large, completely squamose-covered pileus. It is similar to A. bohusii in morphology; however, A. bohusii has broadly ellipsoid spores (Qm = 1.24), and clavate to cylindrical cheilocystidia, 6–10 μm wide (Parra 2008), which are different from A. crassisquamosus. The phylogeny also shows that they are different species.
4.3 Agaricus [subgenus Pseudochitonia] section Brunneopicti Heinem., Bull. Jard. Bot. État Bruxelles 26: 71 (1956).
Type species: Agaricus brunneopictus Heinem. & Gooss.-Font., by Heinemann, Bull. Jard. Bot. État Bruxelles 26: 74 (1956).
Facesoffungi number: FoF01175; Fig. see Heinemann 1956, p75
Delimitation of section Brunneopicti
KOH reaction positive or faint, Schäffer’s reaction negative, rarely weakly positive. Context discolouration when bruised faint to strong yellow, orange, rufescent, brownish rufescent or red. Odour from pleasant bitter almond to unpleasant, like phenol or solvent used in marker pens. Pileus roughly covered with punctiform squamules, or brownish, larger squamules. Annulus superous, double or complex with scales or cortinate fibrils on the lower surface. Cheilocystidia usually present, pyriform to broadly clavate. Known only from palaeotropics.
Stem age and phylogenetic support
This section is fully supported as a monophyletic group by multi-gene and ITS analysis (clade D9 in Figs. 1 and 2), and the stem age is 17.75 Ma. This section was introduced by Heinemann (1956a), but a reconstruction has recently been made by Chen et al. (2015) along with several new species (Chen et al. 2015; Karunarathna et al. 2014). Our study is consistent with the previous work (Chen et al. 2015; Zhao et al. 2011).
4.4 Agaricus [subgenus Pseudochitonia] section Chitonioides Romagn., Bull. Soc. Mycol. Fr. 102(1): 118. 1986.
Type species: Agaricus pequinii Boud., designated by Romagnesi, Bull. Soc. Mycol. France 102(1): 118. 1986.
Facesoffungi number: FoF01176; Fig. see Parra 2008, pp. 641–642
Delimitation of section Chitonioides
KOH and Schäffer’s reactions negative. Surface of pileus discolouring indistinct or reddish. Context often turning pink to reddish or unchanging on exposure. Odour indistinct or mushroomy. Basidiomes generally stout, pileus diameter almost equal to stipe length. Annulus inferous, peronate, simple. Cheilocystidia present, clavate, sometimes cylindrical contorted, some septate at the base.
Stem age and phylogenetic support
This section also is fully supported and sister to section Bivelares in multi-gene and ITS analyses (clade D1, Figs. 1 and 2), which concurs with previous studies (Parra 2008; Zhao et al. 2011; Wang et al. 2015). This section was formed 18.16 Ma, which is also in the time range as other sections.
4.5 Agaricus [subgenus Pseudochitonia] section Crassispori R.L. Zhao, sect. nov.
MycoBank: MB814599
Type species: Agaricus lamellidistans R.L. Zhao.
Facesoffungi number: FoF01177; Figs. 5a–d and 13
Etymology: from crassus, crassi- means thick, so “crassispori” referring to the thick walled basidiospores.
Original description and delimitation of section Crassispori
KOH reaction positive, Schäffer’s reaction negative. Not staining on touching and cutting. Smell phenol, ink. Annulus superous, membranous, double and lower side always wooly or cortinate. Cheilocystidia present, shape highly variable, often sinuous, capitate or clavate. Basidiospore with an apical endosporal thickening, shape variable cymbiform or ellipsoid.
Stem age and phylogenetic support
This section is represented by A. variicystis (LD201234) in multi-gene analysis with a stem age of 18.03 Ma. It is fully supported in ITS analysis and comprises A. variicystis sp. nov., A. lamellidistans sp. nov., and A. campestroides Heinem. & Gooss.-Font. This section is sister to section Trisulphurati. Morphologically, the species of this section are distinct in having basidiospores with an apical endospore thickening, which is rare in the genus Agaricus, but common in the genus Micropsalliota. Agaricus campestroides was introduced from Congo. At that time it was reported as a member of A. section Campestres (Heinemann 1956a), which is a homotypic synonym of A. section Agaricus (Cappelli 1984).
Agaricus lamellidistans R.L. Zhao, sp. nov. Figs.5a–d and 13
Fungal Names: FN570197
Facesoffungi number: FoF01178
Etymology: epithet “lamellidistans” refers to the distant lamellae of this species, which is not common in most Agaricus species.
Typus: Thailand, Chiang Mai Prov., Mae Taeng Dist., Tung Joaw village, forest trail, N19°08.07′ E98°38.90′, elev. 1300 m., 5 September 2006, collected by Ruilin Zhao, ZRL3099 (BBH 19615, holotype; HMAS 274007, isotype).
Original description: Pileus 12–36 mm in diam., 2 mm thick at disc, hemisphaerical, extending broad conic, convex with broad umbo and deflexed margin in most cases, some without umbo; surface dry, glabrous to fibrillose, lacking squamules, generally white or cream, disc light orange, brownish orange (6C5), fading to margin, margin exceeding. Lamellae free, less crowded than usual for the genus or slightly distant, intercalated with lamellulae, 4–5 mm broad, ventricose, at first greyish brown (7D2, 7D3), then brownish grey, finally dark brown. Stipe18–80 × 2–4 (apex) –4–5 (base) mm, cylindrical to subclavate, hollow or stuffed with rhizomorphs at the base, white to slightly grey, smooth above the annulus, tomentose below the annulus and almost strigose towards the base, not staining on touching. Annulus membranaceous, superous, pendent, simple, fugacious, white, with radiate striations, up to 4 mm broad. Context firm, white both in pileus and stipe of young basidiomes, and grey to yellowish brown in stipe for mature basidiomes, colour unchanging on exposure. Odour of carbolic acid or ink.
Macrochemical reaction: KOH reaction positive brightly yellow, Schäffer’s reaction negative.
Basidiospores 5.5–6.5 × 3.2–4 μm [\( \overline{x} \) = 6 ± 0.2 × 3.8 ± 0.3, Q = 1.4–2, Qm = 1.61 ± 0.39, n = 20], cymbiform to ellipsoid, smooth, thick-walled, with a slightly apical, endospore thickening, without a germ pore, reddish brown. Basidia13–18 (−22) × 4–7 μm, hyaline, smooth, 4-spored. Cheilocystidia12–32 × 7–16 μm, pyriform, broad-clavate, smooth, hyaline. Pleurocystidia absent. Pileipellis a cutis consisted of hyphae 4–9 μm in diam., light yellow or hyaline, smooth, branched, not constricted at the septa. Annulus consisting of the same hyphae aspileipellis.
Habitat: solitary or scattered in small groups in red soil.
Other material examined: THAILAND, Chiang Mai Prov., Doi Suthep-Pui National Park, Sangasabhasri Lane to Huai Kok Ma village, N18°48.62′ E98°54.60′, elev. 1145 m., 2 July 2005, collected by Thanh Huyen Le, ZRL2081 (BBH 19464; HMAS 274013); same location, 7 June 2006, collected by Thanh Huyen Le, ZRL3034 (BBH 19550); same location, 13 June 2006, collected by Todd Osmundson, ZRL3063 (BBH 19579; HMAS 274004); Chiang Mai Prov., Mae Taeng Dist., Tung Joaw village, forest trail, N19°08.07′ E98°38.90′, elev. 1300 m., 30 June 2006, collected by Ruilin Zhao, ZRL3074 (BBH 19590; HMAS 274012); same location, 5 September 2006, collected by Ruilin Zhao, ZRL3098 (BBH 19614; HMAS 274020).
Notes: This species is often found in the field on the sides of trails in forests of Thailand. It is distinct from most Agaricus species in the distance between lamellae being further than most Agaricus species, which are often crowded. Under the microscope, it is similar to species of section Crassispori in having cymbiform spores with an apical endosporal thickening. The known species A. campestroides belongs to this section, and it can be distinguished from A. lamellidistans as it has a pleasant smell and lacks cheilocystidia, while, A.lamellidistans has an ink smell and abundance of cheilocystidia (Heinemann 1956a).
Agaricus variicystis L.J. Chen, K.D. Hyde & R.L. Zhao, sp. nov. Figs. 5e, f and 14.
Fungal Names: FN570198
Facesoffungi number: FoF01179
Etymology: epithet “variicystis” refers to variable shapes of cheilocystidia in this species.
Typus: THAILAND, Chiang Mai Prov., 3 km down the road from Tharnthong Lodges, 4 June 2012, collector Jie Chen, LD201234 (MFLU12-0878, holotype; HMAS273996, isotype).
Original description: Pileus 53–75 mm in diam., 1–2 mm thick at disc, convex to hemisphaerical, more or less truncate at the disc; surface dry, smooth, occasionally exhibiting very few squamules, on a brownish grey (7C2) background, close to the margin; margin straight. Lamellae free, very crowded, with intercalated lamellulae, 5–7 mm broad, greyish brown to dark brown. Stipe 64–94 × 4–7 (apex) – 8–11 (base) mm, cylindrical to slightly enlarged towards the base, hollow, white, surface smooth, tomentose close to the base (composed of mycelium), staining greyish orange when bruised. Annulus membranous, simple, fragile. Context firm, on cutting pale white both in pileus and stipe, without discolouring on exposure. Odour of phenol.
Macrochemical reactions: KOH reaction positive yellow, Schäffer’s reaction negative.
Basidiospores 5.6–6.4 (−6.8) × 3.4–3.8 (−4.1) μm, [\( \overline{x} \) = 6.1 ± 0.26 × 3.6 ± 0.17, Q = 1.47–1.78, Qm = 1.66 ± 0.04, n = 20], ellipsoid to fusiform, brown, smooth, thick-walled, endospore thickening, without a germ pore. Basidia12–15.5 × 6–6.5 μm, clavate, hyaline, smooth, 4-spored. Cheilocystidia 20–55 × 10–23 μm, abundant, variable in shape, rarely pyriform or broadly clavate, often lageniform to utriform, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 4–10 μm in diam., cylindrical, hyaline, smooth, sometimes slightly constricted at the septa.
Habitat: scattered or in groups, in soil under the bamboo woods or in forest clearings.
Other material examined: THAILAND, Chiang Mai Prov., 3 km down the road from Tharnthong Lodges, 4 June 2012, collector Jie Chen, LD201228 (MFLU 12-0872; HMAS273951).
Notes: This species is distinguished by its uniquely-shaped cheilocystidia, which is very rare in Agaricus. Phylogenetic analyses also support it as a distinct species.
4.6 Agaricus [subgenus Pseudochitonia] section Flocculenti L.J. Chen, K.D. Hyde & R.L. Zhao sect. nov.
MycoBank: MB814600
Facesoffungi number: FoF01180; Figs. 6a, b and 15
Type species: Agaricus erectosquamosus L.J. Chen, K.D. Hyde & R.L. Zhao
Etymology: epithet of “Flocculenti” referring to the pileus and stipe surfaces floccose.
Original description and delimitation of Agaricus section Flocculenti
KOH reaction yellow to orange, Schäffer’s reaction negative. Surface of pileus and stipe not discolouring on bruising. Context lacking discolouration on exposure. Odour indistinct or variable, phenol or pleasant. Universal veil generally developed, such that the surfaces of pileus and stipe are covered in heavily fibrillose squamules. Annulus superous, double, upper layer smooth and lower layer thick, floccose or cogwheel-like. Cheilocystidia present and abundant.
Stem age and phylogenetic support
This section is represented by A. pallidobrunneus and A. erectosquamosus in Fig. 1 as clade D10, which is fully supported and the stem age is 21.89 Ma. In the ITS tree, this clade is also well-supported (1/85 PP/BS) and thus clade D10 is recognized as a new section. The ITS phylogenetic tree indicates that this new section is associated with unnamed sample CA820, which was shown to belong to clade TRa in Zhao et al. (2011) and unnamed samples ZRL2010099 and LD2012162 (Fig. 3) with strong support (0.94/- PP/BS). Because of the lack of other gene sequences from those three unnamed samples, we could not confirm their phylogenetic relationship. Presently we conclude that the strongly supported clade D10 is a new section, and presently contains two new species: A. erectosquamosus and A. pallidobrunneus.
Agaricus erectosquamosus L.J. Chen, K.D. Hyde & R.L. Zhao, sp. nov. Figs. 6a, b and 15
Fungal Names: FN570199
Facesoffungi number: FoF01181
Etymology: the epithet “erectosquamosus” refers to the squamose pileus covered by upturned squames.
Typus: THAILAND, Chiang Mai Prov., Chiang Mai Zoo, 4 August 2012, collector Jie Chen, LD2012165 (MFLU12-0993, holotype; HMAS273992, isotype).
Original description: Pileus 40–50 mm in diam., 5 mm thick at the disc, convex to plane; surface dry, whole capcovered by squamules, erect, dense at the disc, brown against a dirty white background; margin decurved and exceeding. Lamellae free, 5 mm broad, ventricose, crowded, intercalated with lamellulae, reddish brown, brown to dark brown with age. Stipe 45 × 5 mm, cylindrical to slightly clavate upon maturing, hollow, smooth and white above the annulus, and squamose, white to light brown below the annulus, with indistinctive stainingon touching. Annulus membranous, double, large, entire, pendant or subperonate, upper surface smooth, white and lower surface heavy floccose, and often cogwheel-like, white and light brown at the margin. Context fleshy, on cutting pale white both in pileus and stipe, and without discolouration on exposure. Odour indistinct or slightly phenol.
Macrochemical reactions: KOH reaction orange, Schäffer’s reaction negative.
Basidiospores (6.6–) 6.9 –7.6 × 4.1–4.6 (−5) μm, [\( \overline{x} \) = 7.2 ± 0.31 × 4.4 ± 0.2, Q = 1.51–1.73, Qm = 1.63 ± 0.05, n = 20], ellipsoid to oblong, smooth, brown, thick-walled, without a germ pore. Basidia 15–22 × 6.5–7.5 μm, clavate to broadly clavate, hyaline, smooth,4-(2-) spored. Cheilocystidia 18–30 × 9–16.5 μm, abundant, simple, or occasionally in short chains, globose to pyriform or sphaeropedunculate, rarely clavate, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 4–12.5 μm in diam., shortly cylindrical, with brownish membranous pigments, smooth, distinctively constricted at the septa. Annulus composed of hyphae 7–10 μm in diam., which are hyaline and smooth.
Habitat: scattered in soil of grasslands.
Notes: see the notes of A. pallidobrunneus for the details.
Agaricus pallidobrunneus R.L. Zhao, sp. nov. Figs. 6c, d and 16
Fungal Names: FN570225
Facesoffungi number: FoF01182
Etymology: the epithet “pallidobrunneus” refers to light (pallido) brown (brunneus) coloured pileus.
Typus: China, Yunnan Prov., Yongde county, Daxueshan Xiang, Pintian Village, 17 July 2012, collected by R.L. Zhao ZRL2012358 (HMAS273999, holotype).
Original description: Pileus 105 mm in diam., 7 mm thick at disc, convex to plane; surface dry, whole cap covered by fibrils, and broken into small squamules, appressed or upturned, dense at the disc and brown, light brown towards the margin; margin decurved and exceeding. Lamellae free, 11 mm broad, ventricose, crowded, intercalated with lamellulae, reddish brown, brown with age. Stipe110 × 13–20 mm, cylindrical and widening towards base, slightly clavate, hollow, surface white, smooth and white above the annulus, covered by small squamules which are the same as those of the pileus at the lower of annulus, not staining on touching. Annulus membranous, double, large, entire, with crenate edge, pendant, upper surface (upper layer) smooth, white and lower surface (lower layer) floccose, white and light brown towards the margin. Context firm, on cutting greyish white both in pileus and stipe, not discolouring on exposure. Odour pleasant.
Macrochemical reactions: KOH reaction yellow, Schäffer’s reaction negative.
Basidiospores 6.1–7.8 × 3.5–4.5 (−5.2) μm, [\( \overline{x} \) = 6.7 ± 0.4 × 4.3 ± 0.4, Q = 1.4–1.8, Qm = 1.6 ± 0.1, n = 20], ellipsoid to oblong, brown, smooth, thick-walled, without germ pore. Basidia 16–3 × 6–9 μm, clavate to broadly clavate, hyaline, smooth, 4-(2-) spored. Cheilocystidia 17–35 × 11–21 μm, abundant, simple, pyriform or sometimes in short chains, globose to ellipsoid, 11–25 μm in diam., hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 4–18 μm in diam., shortly ellipsoid or cylindrical, with brownish membranous pigments, smooth, distinctively constricted at the septa.
Habitat: solitary in the forest.
Notes: The new species are distinguished by their distinct morphological characteristics: a relatively well-developed universal veil, large basidiospores and lacking discolouration on bruising and context exposure. Although A. erectosquamosus and A. pallidobrunneus phylogenetically nest together, they could easily be separated in the field: A. erectosquamosus has erect, dark brown squamules on the pileus and stipe surface; however, A. pallidobrunneus has light brown fibrillose squamules on the pileus and white fibrillose to fibrillose squamules on the stipe.
4.7 Agaricus [subgenus Agaricus] section Hondenses R.L. Zhao & L.A. Parra sect. nov. Fig. see Kerrigan 1986
Fungal Names: FN570227
Facesoffungi number: FoF01183
Type species: Agaricus hondensis Murrill, Mycologia 4: 296. 1912.
Etymology: epithet “Hondenses” is following the name of type species A. hondensis.
Original description and delimitation of Agaricus section Hondenses
KOH reaction positive yellow, Schäffer’s reaction negative. Surface of pileus and stipe unchanging or becoming reddish on touching. Context turning weakly yellow, then vinaceous pink, reddish brown, seldom green-bluish at base of stipe or unchanging on exposure. Odour generally faint, usually of iodine or phenol, rarely indistinct. Annulus superous, pendant, double, and often cogwheel-like at the lower layer, if large or thick and stiff with short diam. Cheilocystidia present or not, if present often wide clavate, pyriform, sphaeropedunculate, or globose.
Stem age and phylogenetic support
This section is presented as clade D3 with 1 PP support (Figs. 1 and 3), and its stem age is 19.35 Ma. Presently, seven species have been included in this section: A. freirei Blanco-Dios; A. phaeolepidotus (F.H. Møller) F.H. Møller; A. biannulatus A. Mua et al. from Europe (Parra et al. 2011; Parra 2013); A. hondensis from North America (Kerrigan 1986); and three species to be described from China.
All species in section Hondenses had been treated as members of section Xanthodermatei Singer, and they always cluster together in section Xanthodermatei (Callac and Guinberteau 2005; Challen et al. 2003; Geml et al. 2004; Kerrigan et al. 2005; Zhao et al. 2011; Thongklang et al. 2014). However, multi-gene and ITS analysis with extensive samples indicate that section Xanthodermatei is not monophyletic and splits into two parts: clade D3 (ZRL2012611 A. grandiomyces J.L. Zhao & R.L. Zhao nom. prov. and ZRL2012014 A. nigrogracilis R.L. Zhao nom. prov. in Zhou et al. 2016) and clade D4 represented by A. xanthodermus (in Fig. 1). In the extended ITS analyses, because of the increased sampling especially specimens of new section Crassispori (clade D6) and new recognized section Trisulphurati (clade D5), the previous section Xathodermatei also split into those two isolated lineages (D3 and D4). However there is an unexpection in the position of specimen ZRL2012014, where it moves from D3 in the multi-gene tree (Fig. 1) to D4 in the ITS tree (Fig. 3). In order to exclude the contamination error occurred in sequencing, we resequenced this specimen and performed the analyses, and obtained the same results. So presently we could not clarify the placement of specimen ZRL2012014. However another specimen ZRL2012611 (A. grandiomyces) is stable in clade D3 in both trees, and linked with other species of section Hondenses in ITS tree (Fig. 3).
Morphologically, the species of section Hondenses could be separated from section Xanthodermatei by their lack of distinct yellow discolouration on cutting, lack of strong phenol or iodine smell, and annulus often connected as double layers. Based on such phylogenetic topology, stem ages and morphological characteristics, clade D3, named section Hondenses, is separated from clade D4, which represents a more restricted concept of section Xanthodermatei.
4.8 Agaricus subgenus [subgenus Pseudochitonia] section Nigrobrunnescentes K.R. Peterson, Desjardin & Hemmes. Sydowia 52(2): 240. 2000.
Facesoffungi number: FoF01184; Fig. see Peterson et al. 2000, p239
Type species: Agaricus nigrobrunnescens K.R. Peterson, Desjardin & Hemmes, designated by K.R. Peterson, Desjardin & Hemmes, Sydowia 52(2): 238. 2000.
Delimitation of Agaricus section Nigrobrunnescentes
KOH and Schäffer’s reactions negative. Surface of pileus and stipe discolouring reddish brown to dark brown on bruising. Context often turning pink, vinaceous red or reddish brown on exposure. Odour indistinct or mushroomy, never anise, bitter almond, ink, or phenol. Basidiomes generally stout. Annulus superous or intermediate, simple or double, if double upper side white, striated, and lower side floccose and coloured, pendant. Cheilocystidia present, clavate, pyriform, subglobose, catenulate or not, some septate at the base.
Stem age and phylogenetic support
The section is fully supported in the multi-gene tree (Fig. 1, clade D11) and well-supported in the ITS tree (Fig. 3) with extended samples. Its stem age is 23.30 Ma and matches those of sections of Agaricus. This section is re-recognized by the type species A. nigrobrunnescens of section Nigrobrunnescentes and has been successfully sequenced and incorporated into phylogenetic analyses (Parra et al. 2014; Peterson et al. 2000; Wang et al. 2015). Presently, there are nine species: A. biberi Hlaváček; A. boisseletii Heinem.; A. caballeroi L.A. Parra et al.; A. desjardinii Z.R.Wang et al.; A. erythrosarx T. Lebel; A. fuscovelatus Kerrigan; A. lilaceps Zeller; A. padanus Lancon. and A. nigrobrunnescens K.R. Peterson et al., which have been demonstrated to be members of this section worldwide (Parra 2008; Parra et al. 2014; Wang et al. 2015; Kerrigan 1986; István 2009; Lebel 2013).
4.9 Agaricus [subgenus Pseudochitonia] section Rubricosi R.L. Zhao, sect. nov.
MycoBank: MB814601
Facesoffungi number: FoF01185
Type species: Agaricus kunmingensis R.L. Zhao.
Etymology: the epithet “Rubricosi” is referring to the more or less reddish brown discolouring on touching or cutting.
Original description and delimitation of Agaricus section Rubricosi
KOH reaction indistinct or slightly reddish brown, Schäffer’s reaction negative. Surface of pileus and stipe discolouring reddish brown on bruising or not. Context turning red on exposure. Odour indistinct, pleasant, or phenol. Basidiome slender or stout. Annulus superous, membranous, double and often floccose at the lower side. Cheilocystidia present and abundant, pyriform. Distribution tropical or subtropical.
Stem age and phylogenetic support
Agaricus magnivelaris Pegler (collection No. F2389, Pegler, 1983) along with sample F2187 from tropical America nested together (named as clade TRc) in Zhao et al. (2011) with low support (PP = 85; BS = 61). However, in this study there are nine samples from China and Thailand that cluster in this clade, and they are fully supported in the multi-gene tree and well-supported (0.99 PP) in the ITS tree (Figs. 1 and 3, labeled as clade D12). The stem age is 23.30 Ma. We describe it as a new section here. Phylogenetically, this section is close to sections Nigrobrunnescentes, Bohusia, and Sanguinolenti, and all are reddish discolouring species. However, thus far, all species belonging to section Rubricosi are from the tropics and subtropics. Three new species, plus A. magnivelaris Pegler, which was placed in section Sanguinolenti in past studies, are included in section Rubricosi. Samples ZRLWXH3140 and ZRLWXH3078 from southern China belong in this section well; however, because of lack of morphological characterization they are unnamed in this work.
Agaricus variabilicolor R.L. Zhao, sp. nov. Figs. 7a–e and 17.
Fungal Names: FN570200
Facesoffungi number: FoF01186
Etymology: epithet “variabilicolor” refers to the variable colour of the pileus.
Typus: THAILAND, Chiang Mai Prov., Mae Taeng Dist., Ban Pha Deng village, N 19°17.123′ E 98°44. 009′, elev. 900 m, 10 May 2007, collected by Phongeun Sysouphanthong, ZRL4007 (BBH19637, holotype; HMAS, isotype).
Original description: Pileus 35–80 mm in diam, 4–7 mm thick at the disc, pulvinate, hemisphaerical when young, then expanding convex, top applanate or slightly depressed; covered by fibrillose scales or complete scales, colour variable, mostly brown, but also with light brown scales in some cases, appressed or slightly recurved; margin decurved and often appendiculate. Lamellae free, crowded, intercalated with lamellulae, 4–8 mm broad, at first pink, then reddish brown, brown, finally dark brown. Stipe 50–100 × 7–10 mm, equal to slightly tapering towards the base; narrow hollow or stuffed; white; smooth above the annulus, and scabby or scurfy below the annulus, not discolouring on touching. Annulus membranous, 6–12 mm in diam, pendant, simple, fragile and attached at the margin of pileus or entirely at the stipe, upper side smooth, white, and lower side floccose white or with brown colouration. Context firm, on cutting, at first white, both in pileus and stipe, then quickly turning slightly greyish pick in the pileus and slightly pink in stipe, finally after 15 min uniformly light brown. Odour pleasant.
Macrochemical reactions: KOH reddish brown or not on surface of pileus, no colour change on stipe; Schäffer’s reaction negative.
Basidiospores 4.1–5.9 × 2.4–3.9 μm [\( \overline{x} \) = 5 ± 0.3 × 2.9 ± 0.3, Q = 1.3–2, Qm = 1.6 ± 0.2, n = 60], ellipsoid to elongate ellipsoid, light brown to brown, smooth, thick-walled, without germ pore. Basidia16–23 × 5.5–7.2 μm, clavate or cylindrical, hyaline, smooth, 4-spored. Cheilocystidia abundant, pyriform, broad clavate, 9–24 × 7–14 μm, smooth, hyaline. Pleurocystidia absent. Pileipellis a cutis composed of cells 4–23 × 2.5 μm, distinctly constricted at the septa, contains light brown, brown membranous pigments. Annulus composed of rectangular, ellipsoid, or subsphaerical cells, 3–9 × 2.5 μm, hyaline, branched.
Habitat: gregarious or scattered in forest.
Other material examined: THAILAND, Chiang Mai Prov., Mae Taeng Dist., Ban Pha Deng village, N 19°17.123′ E 98°44. 009′, elev. 900 m, 10 May 2007, collected by Phongeun Sysouphanthong, ZRL4012 (BBH19242); same location, 8 May, collected by Phongeun Sysouphanthong, ZRL4002 (BBH19632).
Agaricus dolichopus R.L. Zhao, sp. nov. Figs. 7f, g and 18
Fungal Names: FN570222
Facesoffungi number: FoF01187
Etymology: the epithet “dolichopus” refers to relatively long stipe of this species.
Typus: CHINA, Yunnan Prov., Dali, Cangshan Mountain, 27 July 2014, collected by M.Q. He. ZRL2014120 (HMAS273989, holotype).
Original description: Pileus 40–45 mm in diam., 3–5 mm thick at disc, plane, surface dry, covered by fibrillose squamules, triangular with slightly upturned tip, brown, dense at the disc and spreading towards the margin; margin straight to decurved and sometimes appendiculate. Lamellae free, 3 mm broad, crowded, intercalated with lamellulae, dark brown when mature. Stipe75–80 × 3–5 mm, equal, hollow, surface smooth or slightly fibrillose, white, not discolouring or slightly red on touching. Annulus thick membranous, 5 mm in diam., entire or torn, pendant, white, upper surface smooth and lower surface heavily floccose with brown patches at the margin. Context firm, on cutting, at first white in both pileus and stipe, then quickly turning slightly rubescent at the centre of pileus and stipe, finally after 15 min uniformly light brown. Odour mushroomy, phenol.
Macrochemical reaction: KOH not distinct or yellow, Schäffer’s reaction negative.
Basidiospores 4.5–5.4 × 2.9–3.7 μm [\( \overline{x} \) = 5.1 ± 0.3× 3.4 ± 0.2, Q = 1.4–1.8, Qm = 1.5 ± 0.1, n = 20], ellipsoid, brown, smooth, thick-walled, without germ pore. Basidia14–19 × 5.6–7.4 μm, clavate, hyaline, smooth, 2- or 4-spored. Cheilocystidia abundant, pyriform, broad clavate, (10–) 14–21 × 10–14 μm, smooth, hyaline. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 3.2–13 μm in diam., slightly constricted at the septa, containinglight brown, to brown pigments. Annulus composed of hyphae 3.2–9 μm in diam., hyaline, branched, cylindrical.
Habitat: solitary in forest.
Other material examined: China, Yunnan Prov., Yimeng County, Dalongkou Forest Park, 17 August 2012, collected by Xie Meng, ZRL2012715 (HMAS273950).
Agaricus kunmingensis R.L. Zhao, sp. nov. Figs. 7h–j and 19.
Fungal Names: FN570221
Facesoffungi number: FoF01188
Etymology: the epithet “kunmingensis” refers to the region “Kunming” from where the holotype was collected.
Typus: CHINA, Yunnan Prov., Kunming, Yeya Lake, 30 June 2012, collected by R.L. Zhao, ZRL 2012007 (HMAS273970, holotype).
Original description: Pileus 20–35 mm in diam, 5 mm thick at the disc, campanulate when young, convex to subumbonate when open, with flat top, 45–70 mm in diam.; surface dry, covered by heavy fibrils completely at first, then broken into slightly recurved, more or less concentric, brown (oac700), light brown (oac683) squamules, on a white background, discolouring reddish brown on touching; margin decurved and exceeding. Lamellae free, 3–6 mm broad, some slightly ventricose, crowded, intercalated with lamellulae, reddish brown (oac599), brown, dark brown with age, Stipe 90–150 × 6–7 (apex) – 10–12 (base) mm when mature, ventricose when young, then cylindrical to slightly clavate, hollow, white, surface smooth or slightly fibrillose, white, discolouring reddish brown on touching. Annulus thick membranous, 5–8 mm in diam., entire, pendant or subperonate, upper surface smooth, white and lower surface, heavily floccose, sometimes the floccose layer incompletely white or light brown. Context firm, on cutting at first white to dirty white in both pileus and stipe, then quickly turning reddish brown, finally after 15 min uniformly light brown. Odour pleasant or indistinct.
Macrochemical reaction: KOH not distinct or slightly reddish brown, Schäffer’s reaction negative.
Basidiospores 4.1–5.3 × 2.7–3.3 μm [= 4.6 ± 0.3× 3 ± 0.2, Q = 1.4–1.7, Qm = 1.5 ± 0.1, n = 20], ellipsoid, brown, smooth, thick-walled, without germ pore. Basidia 17–23 × 6–7.7 μm, clavate, hyaline, smooth, 4-spored. Cheilocystidia abundant in young fruiting body and less when mature, pyriform, broad clavate, 14–25 × 8–16 μm, smooth, hyaline. Pleurocystidia absent. Pileipellis a cutis composed of 6–15 μm in diam. hyphae, slightly constricted at the septa, containing light brown to brown pigments. Annulus composed of 3–9 μm diam., hyaline, branched, cylindrical hyphae.
Habitat: scattered in mixed forests of Ericaceae and Fagaceae.
Other material examined: CHINA, Yunnan Prov., Kunming, Yeya Lake, 30 June 2012, collected by R.L. Zhao, ZRL2012015(HMAS273942).
Notes: These three species differ from A. magnivelaris Pegler as the latter has oblong-ellipsoid basidiospores (Qm = 1.9), while the spores of all new species are ellipsoid with a Qm = 1.5–1.6. The basidiomes of the new species are more or less reddish brown on touching or cutting or in KOH reaction, but in A. variabilicolor and A. dolichopus discolouration is very weak or indistinct; while A. kunmingensis has a distinct reddish brown discolouration in all cases. In the field, A. variabilicolor has a light brown cap which is not darker at the disc of pileus, and has a floccose stipe surface, while A. dolichopus has distinct brown triangular scales, which are darker at the disc, and a smooth to slightly fibrillose stipe surface.
4.10 Agaricus [subgenus Pseudochitonia] section Sanguinolenti Jul. Schäff & F.H. Møller ex L .A. Parra, Agaricus L. Allopsalliota Nauta & Bas, 378. 2008.
≡Agaricus [subgenus Agaricus section Sanguinolenti] subsection Sylvatici L.A. Parra, Agaricus L. Allopsalliota Nauta & Bas, 390. 2008.
Facesoffungi number: FoF 01189; Fig. see Parra 2008, pp600, 697–705
Type species: Agaricus sylvaticus Schaeff., designated by L.A. Parra, Agaricus L. Allopsalliota Nauta & Bas, 378. 2008.
Delimitation of Agaricus section Sanguinolenti
KOH and Schäffer’s reactions negative. Surface of pileus and stipe discolouring reddish brown on bruising. Context turning reddish brown to blood red on exposure. Odour indistinct or mushroomy, never anise, bitter almond, ink or phenol. Basidiome slender or stout. Annulus superous, simple, pendant. Cheilocystidia present, and abundant.
Stem age and phylogenetic support
This section is represented by clade D7, which is fully supported (Figs 1 and 3), and the stem age is 23.45 Ma. The concept of section Sanguinolenti was shown to be a polyphyletic group and composed of three monophyletic lineages in Zhao et al. (2011); two were named as subsections Bohusia and Sylvatici respectively (Parra 2008); and the third clade had been recognized as section Nigrobrunnescentes (Parra et al. 2014; Wang et al. 2015). However, in multi-gene analysis these two “subsections” were formed around 24 Ma. Therefore, we elevate them to sectional level: subsection Bohusia to section Bohusia (described above) and subsection Sylvatici is elevated to sectional level and keeps the older name Sanguinolenti. The species belonging to section Sanguinolenti cluster with type species A. sylvaticus, such as A. benesii (Pilát) Pilát and A. dilutibrunneus sp. nov.
Agaricus dilutibrunneus R.L. Zhao, sp. nov. Figs. 5g–I and 20
Fungal Names: FN570223
Facesoffungi number: FoF01190
Etymology: the epithet “dilutibrunneus” refers to light brown scales on the pileus of this species.
Typus: CHINA, Yunnan Prov., Kunming, Yeya Lake, 30 June 2012, collected by Rui-Lin Zhao, ZRL2012010 (HMAS273990, holotype).
Original description: Pileus 50–70 mm in diam., convex, subumbonate; surface dry, covered by squamules, dense at the disc and spreading towards the margin, light brown on a greyish white background; margin decurved. Lamellae free, crowded, intercalated with lamellulae, brown, dark brown when mature. Stipe around 100 × 10 mm, equal to slightly clavate, abruptly bulbous at base; surface fibrillose, and thickening towards the base, white, staining reddish yellow on touching. Annulus membranous, white, fragile. Context firm, on cutting firstly white in both pileus and stipe, then quickly turning slightly rubescent at the centre of pileus and stipe, finally after 15 min uniformly light brown.
Context white to slightly yellowish white, firm, and discolouring reddish yellow on cutting. Odour unknown.
Macrochemical reaction: KOH unknown, Schäffer’s reaction negative.
Basidiospores 4.8–5.8 × 3.4–4.2 μm [\( \overline{x} \) = 5.4 ± 0.3× 3.8 ± 0.2, Q = 1.2–1.6, Qm = 1.4 ± 0.1, n = 20], ellipsoid, brown, smooth, dark brown, without germ pore. Basidia 14–19 × 6.7–8.5 (−9) μm, hyaline, smooth, 4-spored. Cheilocystidia composed of pyriform, ellipsoid cells, 17–32 × 13–24 μm; subsphaerical, 10–20 μm in diam, capitate, smooth, hyaline. Pleurocystidia absent. Pileipellis a cutis consistingof 3.5–8 (−13) μm diam., light yellow or hyaline, smooth, branched, hyphae, not constricted at the septa.
Habitat: solitary in forest.
Notes: This species is characterized by its light brown squamules on the pileus and subglobose cheilocystidia in chains. It is most similar to A. benesii (Pilát) Singer, which has a white or light brown cap, and similar shape and size spores. However, the cheilocystidia of A. benesii are clavate and often septate (Parra 2008), which is different from this A. dilutibrunneus. Phylogenetically, A. dilutibrunneus has a distinct position which also indicates that it is a species distinct from A. benesii and A. sylvaticus.
4.11 Agaricus [subgenus Pseudochitonia] section Trisulphurati Heinem., Bull. Jard. Bot. État 26: 91. 1956.
Facesoffungi number: FoF01191; Fig. see Heinemann 1956, PlateXIX, Fig. 9
Type species: Agaricus trisulphuratus Berk., designated by Heinemann Bull. Jard. Bot. État 26: 91. 1956.
Delimitation of section Trisulphurati
KOH reaction positive, Schäffer’s reaction negative. Not staining on touching or cutting. Smell carbolic acid, ink, or mushroomy. Annulus superous, membranous, and lower side always wooly or cortinate. Cheilocystidia present, clavate, or subcapitate. Basidiospore endosporium or not, shape ellipsoid with suprahilar depression (distinct in sample ZRL3014).
Stem age and phylogenetic support
This section (clade D5) is represented by two specimens of A. trisulphuratus, which is fully supported (Fig. 1). It is also fully supported in the ITS analysis (Fig. 3) with extended sampling. The stem age is 18.03 Ma. Heinemann had separated Agaricus into three subgenera: Agaricus, Lanagaricus, and Coniogaricus (Heinemann 1956a, b). The subgenus Lanagaricus is characterized by a woolly universal veil and comprises four sections (Heinemann 1956a). Section Trisulphurati is characterized by a brightly coloured universal veil and thickening of the endospores in the basidiospores. Agaricus trisulphuratus, the type species of section Trisulphurati, has previously been studied, and its phylogenetic position is closely related to sections Bivelares, Chitonioides, and Xanthodermatei (Zhao et al. 2011; Vellinga et al. 2011). In the present study, A. trisulphuratus along with A. ignicolor sp. nov., nest together in the subgenus Pseudochitonia (clade D) with strong support, and also has a similar relationship with those sections. Thus, section Trisulphuratus is now placed in subgenus Pseudochitonia.
Agaricus trisulphuratus Berk. complex, Ann. Mag. Nat. Hist. V, 15: 386, 1885. Figs. 4e, f and 21.
≡Cystoagaricus trisulphuratus (Berk.) Singer, Mycologia 39: 87, 1947.
Facesoffungi number: FoF01192
Pileus mostly 30–60 mm in diam., hemisphaerical, conical, broadly conical, convex, plano-convex, subumbonate when mature, surface dry, covered with acute squamules, floccose, erect at centre and recurved at the margin; golden yellow (5B8), orange, reddish orange (7A8), deep orange (5A8), and fading upon aging or in rain, margin exceeding. Lamellae free, crowded to more crowded, at first white, orange-white (6A2), then greyish-pink, reddish-brown (8E3), greyish-brown (8D3, 6D3), finally brown (7E8), dark brown, edge heteromorphic (colour lighter than those of lamellae). Stipe 20–90 × 3–7 mm, cylindrical; hollow; mostly with rhizomorphs at base; smooth and white above the annulus; flaky and shaggy, same colour as the pileus below the annulus, not discolouring on touching. Annulus pendent, same colour with flaking of stipe surface, in most cases difficult to be recognized from those flakes. Context firm, on cutting white in both pileus and stipe, no discolouring on exposure. Odour none or mushroomy.
Macrochemical reaction: KOH reaction yellow at pileus, Schäffer’s reaction negative.
Basidiospores 5–6 × 3.2–4 μm [\( \overline{x} \) = 5.5 ± 0.6× 3.7 ± 0.3, Q = 1.3–1.8, Qm = 1.48 ± 0.32, n = 20], ellipsoid with suprahilar depression, reddish brown, smooth, thick-walled, without germ pore. Basidia 12–14 × 6–7 (−8) μm, clavate, 4-spored. Cheilocystidia 18–36 × 6–13 (−15) μm, clavate, cylindrical with tapering base, occasionally with 1 septa, surface spiny in some cases, hyaline. Pleurocystidia absent. Pileipellis a cutis composed of 3–5 μm diam., long cylindrical, curved, brunched, fine, spinyhyphae. Annulus and stipitipellis consisting of the same hyphae aspileipellis.
Habitat: mostly solitary, some scattered in small groups in soil.
Other material examined: THAILAND, Chiang Mai, collection data collector unknown, ZRL1003 (SFSU); Chiang Mai Prov., Chiang Dao Cave, 22 July 2004, collected by Ruilin Zhao, ZRL3089 (BBH19605; SFSU); Chiang Mai Prov., Mae Taeng, Ban Mae Sae Village, on Hwy 1095 near 50 km marker, N19°14. 599′ E98°39.456′, elev. 962 m., 26 June 2005, collected byJennifer Kerekes, ZRL2045 (SFSU); same location, 3 July 2005, collected by Ruilin Zhao, ZRL2087 (BBH19470; SFSU); same location, 18 August 2004, collected by Edward Grand, ZRL2128 (BBH19508; SFSU); same location, 3 June 2006, collected by Ruilin Zhao, ZRL3014 (BBH19531; SFSU); same location, 18 June 2006, collected by Ruilin Zhao, ZRL3070 (BBH19586; SFSU); Chiang Mai Prov., Mae Taeng Dist., Ban Pha Deng Village, N 19°17.123′ E 98°44. 009′, elev. 900 m., 11 August 2004, collected by Kevin D Hyde, ZRL2123 (BBH19502; SFSU); same location, 22 May 2006, collected by Ruilin Zhao, ZRL3002 (SFSU); same location, 29 May 2006, collected by Ruilin Zhao, ZRL3009 (BBH19526; SFSU); same location, 24 June 2006, collected by Ruilin Zhao, ZRL3072 (BBH19588; SFSU); Chiang Mai Prov., Mae Taeng Dist., Tung Joaw Village, forest trail, N19°08.07′ E98°38.90′, elev. 1300 m., 3 August 2004, collected by Edward Grand, ZRL2111 (BBH19491; SFSU); Chiang Mai Prov., Mae Taeng Dist., Ban Pha Deng Village, Pathummikaram Temple, forest trail, N 19°06′28.8″ E 98°44′47.3″, elev. 1050 m., 12 June 2004, collected by Ruilin Zhao, ZRL3054 (BBH19570; SFSU); CHINA, Yunnan province, Dali, Cangshan Mountain, Yunnongfeng, 24 July 2014, collected by Sun SY, Bai XM, ZRL2014023 (HMAS273985)., ZRL2014024 (HMAS273935)., ZRL2014025 (HMAS273962), ZRL2014026 (HMAS273927), ZRL2014030 (HMAS273956).
Notes: A. trisulphuratus is type species of section Trisulphurati, and common in forests or grasslands in tropical and subtropical areas. This species also has been reported from Africa (Heinemann 1956a). In the phylogenetic tree (Fig. 2), the samples from Thailand and Malaysia cluster with those from Africa, with strong support. Morphologically, they match the description of Heinemann (1956a).
Agaricus ignicolor R.L. Zhao, sp. nov. Fig. 4g–i and 22
Fungal Names: FN570201
Facesoffungi number: FoF01193
Etymology: “igno” refers to fire, and the species epithet “ignicolor” refers to fire-coloured basidiomes of this species.
Typus: THAILAND, Chiang Mai Prov., Mae Taeng Dist., Ban Pha Deng village, N 19°17.123′ E 98°44. 009′, elev. 900 m, 21 August 2005, collected by Ruilin Zhao, ZRL2132 (BBH 19512, holotype; HMAS 274009, isotype).
Original description: Pileus 55 mm in diam., 2 mm thick at the disc, broadly conic, convex; surface dry, with flaky to sharp warty squamules, erect at the disc then recurved to margin, deep orange, and fading in rain, margin distinctly exceeding. Lamellae free, more crowded, intercalated with lamellulae, 4–5 mm broad, at first white, greyish pink, then dull red (8B3), finally brown, to dark brown. Stipe 22 × 5–7 mm, cylindrical, curved, with rhizomorphs at the base, hollow; smooth and white above the annulus; with thick flaky squamules and deep orange below annulus, not staining on touching. Annulus pendent, superous, and mixed with the stipe squamules, deep orange. Context firm, white to cream in both pileus and stipe, not discolouring on cutting. Odour mushroomy.
Macrochemical reaction: KOH and Schäffer’s reactions negative.
Basidiospores 5–6 × 3–4 μm [\( \overline{x} \) = 5.4 ± 0.4× 3.6 ± 0.4, Q = 1.3–2, Qm = 1.53 ± 0.47, n = 20], ellipsoid, reddish-brown, smooth, thick-walled, without germ pore. Basidia 12–16 × 6–7 μm, clavate, smooth, hyaline. Cheilocystidia 21–30 × 7–13 μm, clavate, broadly clavate, mostly subcapitate with apex 4–7 μm in diam., smooth or with slightly fine spines, hyaline. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 2.5–5 μm in diam., equal, branched, even light yellow, smooth or fine spiny, without constriction at the septa. Annulus and stipitipellis hyphae similar to those of pileipellis.
Habitat: solitary in soils of road banks.
Notes: Agaricus crocopeplus Berk. & Br., A. erythrotrichus Heinem., and A. trisulphuratus are species from section Trisulphurati (Heinemann 1956a). All including this new species, are covered by a developed orange universal veil, which is very distinctive in the field. Agaricus crocopeplus has a white membranous annulus, which differs from the others, which have orange, flaky annuli. Agaricus erythrotrichus has much larger basidiospores (6.2–7.4 × 3.8–4.3 μm) than all others (Heinemann 1956b, 1980). Agaricus ignicolor is most similar to A. trisulphuratus; however, it could be separated by the following characteristics: (i) the edge of annulus of A. ignicolor is orange, while in A. trisulphuratus it is not coloured or slightly white; (ii) the cheilocystidia of A.ignicolor are often capitate, but in A. trisulphuratus they are clavate to cylindrical; and (iii) squamules of A. ignicolor are much more stable, and not easily removed by rain drops. The phylogeny (Fig. 3) also indicates they are different species.
4.12 Agaricus [subgenus Pseudochitonia] section Xanthodermatei Singer, Sydowia 2: 36. 1948.
Facesoffungi number: FoF 01194; Fig. see Parra 2013, pp822-830
Type species: Agaricus xanthodermus Genev. [“xanthoderma”] designated by Singer, Sydowia 2: 36.1948.
Delimitation of Agaricus section Xanthodermatei
KOH reaction positive yellow, Schäffer’s reaction negative. Surface of pileus and stipe often discolouring yellow on bruising. Context turning strongly yellow on exposure, especially at the base of the stipe. Odour mostly iodine or phenol, except some species with a pleasant odour (such as A. murinocephalus R.L. Zhao, Desjardin & K.D. Hyde). Annulus superous, simple, pendent, large. Cheilocystidia present or not. It contains toxic species.
Stem age and phylogenetic support
The emended section Xanthodermatei is represented by Clade D4 which is fully supported (Fig. 1), and well-supported in the ITS tree (Fig. 3). The stem age is 19.58 Ma. Previously, section Xanthodermatei was considered a monophyletic group by most mycologists (Callac and Guinberteau 2005; Challen et al. 2003; Geml et al. 2004; Kerrigan et al. 2005; Parra et al. 2011; Zhao et al. 2011, 2013; Thongklang et al. 2014). Recently, three lineages were revealed in this section based on ITS sequence data after adding some samples from tropical areas (Thongklang et al. 2014). However, in the present multi-gene analysis, the previously conceived section comprises two lineages (reflected in clades D3 and D4) and shows it to be polyphyletic group. The ITS analysis (Fig. 3) using an extended sampling also has the same topology. Based on a combination of stem age and morphological characteristics, those two lineages are ranked as two sections in this study: one is section Hondenses (clade D3 and see above); and the other is emended section Xanthodermatei (clade D4, which contains the type species A. xanthodermus), of which presently A. parvitigrinus Guinb. & Callac, A. xanthosarcus Heinem.& Goos.-Font., A. tollocanensis Callac & G. Mata, A. murinocephalus, and A. microvolvatulus Heinem. A. tibetensis J.L. Zhou and R.L. Zhao nom. prov. and A. tytthocarpus R.L. Zhao nom. prov. have been demonstrated to be members of this section (Zhou et al. 2016). Furthermore, based on a previous phylogenetic analysis (Thongklang et al. 2014), A. bisporiticus, A. californicus Peck, A. endoxanthus Berk. & Broome, A. fuscopunctatus Thongklang, L.J. Chen, Callac & K.D. Hyde, A. iodosmus Heinem., A. laskibarii L.A. Parra & P. Arrill., A. menieri Bon, A. moelleri Wasser, A. moelleroides Guinb. & L.A. Parra, A. placomyces Peck, A. pocillator Murrill, A. pseudopratensis (Bohus) Wasser, and A. xanthodermulus Callac & Guinb. should also belong in this section.
5. Agaricus subgenus Spissicaules (Heinem.) R.L. Zhao & Moncalvo, stat. nov.
Fungal Names: FN570202
Facesoffungi number: FoF 01195
≡Agaricus subsection Spissicaules Heinem., Sydowia 30: 12. 1978. [basionym]
Type species: Agaricus spissicaulis F.H. Møller, designated by Heinemann, Sydowia 30: 12. 1978.
Sections included: sections Amoeni sect. nov.; Rarolentes; Spissicaules; and Subrutilescentes.
Delimitation of Agaricus subgenus Spissicaules
KOH and Schäffer’s reactions negative or weakly positive. Staining yellowish or indistinct, rarely reddish on bruising of the surface of pileus or stipe; context unchanged or turning pink, reddish brown on exposure. Odour strong of almonds with exception of section Subrutilescentes, having an odour similar to Lepiota cristata. Annulus simple, often striate on the upper side. Cheilocystidia present or absent, generally cylindrical to clavate, some basidiole-like, simple and not in chains.
Stem age and phylogenetic support
In the multi-gene analysis (Fig. 1), this subgenus reflects clade C with 0.99 PP support, and its stem age is 33.08 Ma. This phylogeny also shows that the clade composed of subgenera Minores and Flavoagaricus is sister to this subgenus. Subgenus Spissicaules is composed of four lineages, which are all fully supported and their stem ages are from 19–24 Ma. In this study, those four lineages are recognized as four sections: Amoeni sect. nov., Rarolentes, Spissicaules, and Subrutilescentes.
5.1 Agaricus [subgenus Spissicaules] section Spissicaules (Heinem.) Kerrigan, Mycologia 77(1): 141. 1985.
Facesoffungi number: FoF01196
≡Agaricus subsection Spissicaules Heinem., Sydowia 30: 12. 1978.
Type species: Agaricus spissicaulis F.H. Møller, designated by Heinemann, Sydowia 30: 12.1978.
Delimitation of Agaricus section Spissicaulis
KOH and Schäffer’s reactions negative or weakly positive. Surface of pileus and stipe indistinctly discolouring on bruising. Context unchanged or turning pink, reddish on exposure. Odour generally faint, or pleasant. Annulus pendant, simple, superous. Cheilocystidia present, cylindrical to clavate, some septate at base, rarely similar to basidia.
Stem age and phylogenetic support
Clade C4 reflects this section, which is fully supported in multi-gene tree (Fig. 1), and well-supported in ITS tree (Fig. 2). The stem age of section Spissicaules is 21.98 Ma. Presently, the main species of this section are included in this section, such as A. lanipes, A. litoralis, A. bresadolanus, and two new species described in this study.
Agaricus litoraloides R.L. Zhao, sp. nov. Figs. 6e, f and 23
Fungal Names: FN570228
Facesoffungi number: FoF01197
Etymology: the epithet “litoraloides” refers to the morphological similarity of this new species to A. litoralis.
Typus: CHINA,Yunnan Prov., Dali City, Cangshan, 30 July 2011, collected by H.Y. Su SHY2012073026 (HMAS 274021, holotype).
Original description: Pileus 30–80 mm in diam., 7 mm thick at disc, parabolic when young then convex; surface dry, covered by fibrils onthe whole cap, and broken into large squamules (3–5 mm wide), triangular, appressed, dark brown (oac637), reddish brown (oac635); margin exceeding. Lamellae free, 3–5 mm broad, crowded, reddish brown, brown, dark brown with age. Stipe 80–120 × 11–20 mm, cylindrical to slightly clavate; stuffed or hollow; white; surface smooth above the annulus, and erect squamules below the annulus, staining light yellow on touching. Annulus membranous, simple, fragile, pendant, both surfaces smooth and white. Context fleshy, on cutting at first white, both in pileus and stipe, stipe quickly turning slightly rubescent on exposure. Odour strong of almonds.
Macrochemical reactions: KOH reaction unknown, Schäffer’s reaction negative.
Basidiospores 6.7–9 × 4.7-6.1 μm, [\( \overline{x} \) = 8.2 ± 0.5 × 5.4 ± 0.3, Q = 1.3–1.8, Qm = 1.5 ± 0.1, n = 20], ellipsoid, occasionally oblong ellipsoid, brown to dark brown, smooth, thick-walled, without germ pore. Basidia 21–31 × 9.2–11.8 μm, clavate to broadly clavate, hyaline, smooth, 4-spored. Cheilocystidia 12.7–24.3 × 7.8–16 μm, abundant, simple, mostly broadly clavate, pyriform, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 2.5–12.3 μm in diam., cylindrical, with brownish membranous pigments, smooth, not or slightly constricted at the septa. Annulus composed of hyphae 2–10 μm in diam., cylindrical, hyaline, smooth.
Habitat: solitary in the forest.
Other material examined: China, Yunnan Prov., Tengchong county, Datang, Danlonghe Village, 28 July 2011, collected by G.F. Yang ZRL2011249 (HMAS 274019).
Notes: the morphological characters of A. litoraloides are similar to A. litoralis (Nauta 2001) in having large and dark brown scales, large spores, discolouring on touching and cutting, and odour. However the molecular analysis (Fig. 3) clearly supports them as distinct species.
Agaricus planipileus R.L. Zhao, sp. nov. Figs. 6g, h and 24
Fungal Names: FN570226
Facesoffungi number: FoF01198
Etymology: the epithet “planipileus” refers to the applanate pileus of this species.
Typus: CHINA, Yunnan Prov., Tengchong county, Datang, Danlonghe Village, 28 July 2011, collected by R.L. Zhao, ZRL2011250 (HMAS273997, holotype).
Original description: Pileus 70–110 mm in diam., 6–10 mm thick at the disc, applanate, top flat; surface dry, covered by fibrillose squamules, triangular, appressed, light brown (oac646) and easily removed by rain drops, then displaying white background, margin straight and slightly lacerate. Lamellae free, 5–8 mm broad, normal to slightly ventricose, crowded, pink, reddish brown, dark brown (oac610, 636). Stipe 80–200 × 6–20 (apex) – 15–35 (base) mm, cylindrical with a bulbous base, hollow, surface smooth to slightly fibrillose and white above the annulus, floccose squamose and white with light brown tips below the annulus, staining light yellow on touching. Annulus membranous, simple, pendant, upper surfaces smooth and lower surface floccose, 8–12 mm broad, white. Context fleshy, on cutting white, in both pileus and stipe, quickly turning rubescent on exposure. Odour strong of almonds.
Macrochemical reactions: KOH reaction unknown, Schäffer’s reaction negative.
Basidiospores 5.7–7.3 × 3.6–4.6 μm, [\( \overline{x} \) = 6.3 ± 0.4 × 4.1 ± 0.3 μm, Q = 1.3–1.8, Qm = 1.5 ± 0.2, n = 20], ellipsoid, brown, smooth, thick-walled, without germ pore. Basidia 14–17 × 7–9 μm, clavate to broadly clavate, hyaline, smooth, 4-(2-) spored. Cheilocystidia 10.7–24 × 7.8–11.8 μm, abundant, simple, pyriform, irregular ellipsoid, wide clavate, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of 3–11.4 μm diam., long cylindrical, smooth, hyphae, with brownish membranous pigments, not constricted at the septa.
Habitat: solitary in the forest.
Other material examined: China, Yunnan Prov., Tengchong county, Datang, Danlonghe Village, 28 July 2011, collected by S.Y. Liu, ZRL2011248 (HMAS253916).
Notes: Several species have light brown to ochre squamules on the pileus, and smaller basidiospores (<7 μm), such as A. bresadolanus and A. litoralis (Nauta 2001; Parra 2008). However, A. litoralis differs from A. planipileus in having a depressed pileus at the disc and a stipe not bulbous at the base. Agaricus bresadolanus has wider ellipsoid spores (Q = 1.24). The phylogenetic analysis also supports them as distinct species.
Agaricus lanipedisimilis P. Callac & R.L. Zhao, sp. nov. Figs. 8a–d and 25.
Fungal Names: FN570220
Facesoffungi number: FoF01199
Etymology: the epithet “lanipedisimilis” refers to the similarity of this species to A. lanipes. From the union of lanipedis, the genitive of lanipes, and similis.
Typus: CHINA, Yunnan Prov., Cangyuan county, Mengjiao, Nangunhe National Natural Reserve, 9 July 2012, collected by R.L. Zhao, ZRL2012193 (HMAS 273998, holotype).
Original description: Pileus 70–90 mm in diam., 3–8 mm thick at disc, parabolic when young and convex; surface dry, completely covered by appressed fibrils and broken into squamulestowards the margin, reddish brown (oac639), margin straight. Lamellae free, 5–8 mm broad, intercalated with lamellulae, crowded, at first white, then pink, reddish brown, finally dark brown. Stipe 100–150 × 8–16 (apex) and 18–30 (base) mm, clavate or cylindrical with wide base or bulbous, hollow, white, surface smooth to slightly fibrillose above the annulus, erected or recurved below the annulus, staining light yellow on touching. Annulus membranous, simple, pendant, upper surfaces smooth and lower surface floccose, up to 20 mm broad, white. Context fleshy, on cutting at first white, both in pileus and stipe, then slowly (after around 5 min) turning slightly pink, both in centre of pileus and stipe. Odour of strong almonds.
Macrochemical reactions: KOH reaction yellow, Schäffer’s reaction negative.
Basidiospores 4.8–6 (−7) × 3.6–4.5 μm, [\( \overline{x} \) = 5.2 ± 0.3 × 4 ± 0.2, Q = 1.1–1.4, Qm = 1.3 ± 0.1, n = 20], broad ellipsoid, brown, smooth, thick-walled, without germ pore. Basidia 14–20 × 6–8.4 μm, clavate to broadly clavate, hyaline, smooth, 4-spored. Cheilocystidia absent. Pleurocystidia absent. Pileipellis a cutis composed of 3–7.6 μm diam., long cylindrical hyphae with brownish membranous pigments, smooth, not constricted at the septa, or occasionally slightly constricted at the terminal cell.
Habitat: solitary in the forest.
Other material examined: China, Yunnan Prov., Cangyuan county, Mengdong, Nangunhe National Natural Reserve, 7 July 2012, collected by Philippe Callac, ZRL2012151 (HMAS253918).
Notes: This new species is characterized by reddish brown fibrillose pilei, floccose stipe from the lower side of annulus to the base, and broadly ellipsoid basidiospores. This species is mostly similar to A. lanipes, which also has these characters. However, A. lanipes has rhizomorphs, brown-coloured fibrillose squamules on the stipe and septate to catenulate cheilocystidia, which are different from the new species (Parra 2008). The phylogenetic analysis also supports them as distinct species. Agaricus planipileus is phylogenetically closest to A. lanipedisimilis, but morphologically different, owing to the light brown pileus and absence of cheilocystidia.
5.2 Agaricus [subgenus Spissicaules] section Rarolentes Kerrigan, nom prov.
Facesoffungi number: FoF01200
Type species: Agaricus butyreburneus designated by Kerrigan, Agaricus of North America, 2016 in press.
Delimitation of Agaricus section Rarolentes
KOH reaction negative on pileus surface, negative to faintly yellow on context, Schäffer’s reaction negative. Pileipellis glabrous or not, pallid. Annulus superous. Surface of pileus and stipe discolouring indistinct on bruising. Context unchanged or turning pink, reddish on exposure. Odour of solvent, rubber, or sometimes of almonds or anise. Annulus sheathed above, pendent. Cheilocystidia present, various, cylindrical to clavate. Basidiopores narrow, long; mean L/W of the type species: 1.51 (Kerrigan 2016 in press).
Stem age and phylogenetic support
Clade C3 reflects this section, which is fully supported in multi-gene analysis (Fig. 1), however it has poor support in the ITS tree (Fig. 2). The stem age of section Rarolentes is 21.98 Ma. This section was recognized by R. Kerrigan from North America (Kerrigan 2016 in press). In the present study there are two pure-white new species, A. albosquamosus and A. leucolepidotus, which have been incorporated and form another lineage within this section. Thus, we propose that the members of this section also contain species with squamules, and not just glabrous, pilei, and their distribution is extended to tropical Asia.
Agaricus albosquamosus L.J. Chen, K.D. Hyde & R.L. Zhao, sp. nov. Figs. 8e–h and 26
Fungal Names: FN570203
Facesoffungi number: FoF01201
Etymology: the epithet “albosquamosus” refers to the white and squamose pileus of this species.
Typus: THAILAND, Chiang Mai Prov., Mae Sa National Park, 16 September 2012, collector Jie Chen, LD2012192 (MFLU12-1017, holotype; HMAS273987, isotype).
Original description: Pileus 36 mm in diam. when young, 65–77 mm in diam., 7–7.5 mm thick at the disc when mature; convex to hemisphaerical, then becoming applanate-convex, finally applanate, occasionally with a slightly depressed centre; surface dry, fibrillose, with fibrillose squamules near the margin, white; in high humidity, the pileus has a reddish tone, margin exceeding to slightly appendiculate. Lamellae free, crowded, intercalated with lamellulae, 5–6 mm broad, at first white, pink, then pinkish brown, finally dark brown. Stipe 98 × 8–12 mm, cylindrical with slightly bulbous, hollow, white, surface, smooth above the annulus and fibrillose below the annulus, not staining on touching. Annulus superous, simple, membranous, thickening towards the edge, upper side surface smooth, lower side surface fibrillose, floccose near the margin. Context firm, on cutting white in both pileus and stipe, not discolouring on exposure. Odour of almonds.
Macrochemical reactions: KOH reaction no discolouration, Schäffer’s reaction negative.
Basidiospores 5.1–6 × 3.4–3.9 μm, [\( \overline{x} \) = 5.5 ± 0.27 × 3.7 ± 0.16, Q = 1.32–1.64, Qm = 1.47 ± 0.05, n = 20], ellipsoid, brown, smooth, thick-walled. Basidia 15–18 × 7–8.5 μm, clavate to broadly clavate, hyaline, smooth, 4-spored or rarely 2-spored, without germ pore. Cheilocystidia 15.5–25 × 9–16.5 μm, abundant, simple, or sometimes in short chains, globose to pyriform or sphaeropedunculate, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 3–9 μm in diam., cylindrical, hyaline, smooth, and at times slightly constricted at the septa.
Habitat: scattered or in groups, in soil under bamboo woods or in a forest clearing.
Other material examined: THAILAND, Chiang Mai Prov., 3 km down the road from Tharnthong Lodge, 4 June 2012, collector Jie Chen, LD201235 (MFLU12-0879).
Notes: this distinct species is characterized by pure white, but squamose pileus, which is different from all other species in this section (Kerrigan 2016 in press).
Agaricus leucolepidotus L.J. Chen & R.L. Zhao, sp. nov. Figs. 8i–j and 27
Fungal Names: FN570204
Facesoffungi number: FoF01202
Etymology: “leuco” refers to white colour, “lepidos” refers to squamous in Greek, so the epithet “leucolepidotus” refers to squamose pileus of this species.
Typus: THAILAND, Chiang Mai Prov., Tharnthong Lodge, 31 May 2012, collector Jie Chen, LD201214 (MFLU12-0858, holotype; HMAS273995, isotype).
Original description: Pileus 43–78 mm in diam., 5 mm thick at the disc, hemisphaerical to convex, then becoming applanate-convex, finally applanate, with a slightly depressed centre; cuticle exceeding the lamellae; surface dry, fibrillose, with fibrillose squamules near the margin, white; margin decurved. Lamellae free, crowded, intercalated with lamellulae, 6 mm broad, at first white, pink, then pinkish brown, finally dark brown. Stipe 94 × 8–10 mm, cylindrical with subbulbous base, hollow, white, surface smooth above the annulus, fibrillose woolly below the annulus, not staining on touching. Annulus superous, simple, at middle of stem, upwards, membranous, thickening towards the edge, upper side surface smooth, lower side surface fibrillose. Context firm, on cutting white in both pileus and stipe, not discolouring on exposure. Odour light of almonds.
Macrochemical reactions: KOH reaction no discolouration, Schäffer’s reaction negative.
Basidiospores (5.8–) 6.5–7.2 (−8.8) × 3.5–4.3 (−5) μm, [\( \overline{x} \) = 6.8 ± 0.29 × 3.8 ± 0.27, Q = 1.60–2.07, Qm = 1.77 ± 0.01, n = 20], ellipsoid to oblong, brown, smooth, thick-walled, without germ pore. Basidia 13.5–16 × 8–10 μm, broadly clavate, hyaline, smooth, 4- or 2-spored. Cheilocystidia 14–21 × 9–12.5 μm, abundant, simple, subglobose to pyriform, hyaline, smooth. Pleurocystidia absent. Pileipellisa cutis composed of hyphae 3–9 μm in diam., cylindrical, hyaline, smooth, and at times slightly constricted at the septa.
Habitat: in groups, in forest clearings.
Notes: This new species is morphologically similar to A. albosquamosus in the field in having a white and squamose pileus (see the above, the new species proposed in this paper); however, it has larger basidiospores. In ITS, they differ by 5 base pairs.
5.3 Agaricus [subgenus Spissicaules] section Amoeni Callac & R.L. Zhao, sect. nov. Figs. 8k–m and 28
MycoBank: MB814602
Facesoffungi number: FoF01203
Type species: Agaricus amoenomyces R.L. Zhao.
Etymology: the epithet “amoeni” refers to the members of this section often has pleasant odour.
Original description and delimitation of Agaricus section Amoeni
KOH and Schäffer’s reactions negative or positive. Surface of stipe discolouring weakly yellow or red on bruising. Context not discolouring or turning slightly orange on exposure. Odour generally pleasant or almonds. Annulus superous, pendant, remains appendiculate, simple, lower side often floccose. Cheilocystidia lacking or similar to basidia, if present generally cylindrical to clavate, some septate at base. Known only from palaeotropics.
Stem age and phylogenetic support
This clade was revealed in Zhao et al. (2011) as clade TRIII with strong support using ITS sequence data. In the present study, it is represented by ZRL3093 and ZRL2010072, which is fully supported in multi-gene and ITS analyses (Figs. 1 and 2, as clade C2), and its stem age is 19.15 Ma. Morphologically, this section is similar to section Brunneopicti, and has variable discolouring on touching and cutting, even in KOH and Schäffer’s reactions. However, the members of this section often have a strong pleasant or almond odour, which is different from section Brunneopicti that smells of phenol. Presently there are four species in this section: one is the known species A. kivuensis which was misplaced in section Brunneopicti by Heinemann (1956a); and three new species described below.
Agaricus amoenomyces R.L. Zhao, sp. nov. Figs. 8k–m and 28
Fungal Names: FN570216
Facesoffungi number: FoF01204
Etymology: the epithet “amoenomyces” refers to pleasant, or beautiful mushrooms.
Typus: CHINA, Xishuangbanna, Mongla, Nangong Mountain. 25 July 2010, collected by R.L. Zhao, ZRL2010072 (HMAS273988, holotype).
Original description: Pileus 44 mm in diam., 6 mm thick at the disc, hemisphaerical, convex, subumbonate with a truncate top; surface dry, with fibrillose scales, more or less concentric, dense at disc, brown against white background, margin incurved. Lamellae free, crowded, intercalated with lamellulae, 7 mm broad, firstly white, then pink, finally reddish brown, dark brown. Stipe 70–100 × 10–12 mm (apex) – 20–28 mm (base), cylindrical and bulbous, narrow hollow, with rhizomorphs at the base, straight, white, surface smooth above the annulus, and fibrillose squamules below the annulus, slightly reddish on touching. Annulus superous, membranous, upper side smooth and white, lower side heavily fibrillose, white with brown fibrils, which is similar to those of the pileus. Context firm, on cutting at first white, both in pileus and stipe, then stipe quickly turning slightly orange on exposure. Odour strong of almonds.
Macrochemical reactions: KOH reaction yellow and Schäffer’s reaction positive or weakly positive.
Basidiospores 4.8–5.5 × 2.9–3 μm [\( \overline{x} \) = 5 ± 0.5 × 2.9 ± 0.1, Q = 1.6–1.8, Qm = 1.7 ± 0.1, n = 20], ellipsoid, light yellowish brown, smooth, thick-walled, without germ pore. Basidia 15–23 × 5–7 μm, clavate, hyaline, 4-spored. Cheilocystidia absent. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 7.5–23 μm in diam., smooth, obviously constricted at the septa, hyaline or light brown, branched.
Habitat: scattered at the banks of roads in forests.
Notes: This new species is distinguished from other species in section Amoeni by its positive KOH and Schäffer’s reactions. The bracelet-like annulus can also distinguish this species from others.
Agaricus suthepensis L.J. Chen, K.D. Hyde & R.L. Zhao, sp. nov. Figs. 9a, b and 29
Fungal Names: FN570206
Facesoffungi number: FoF01205
Etymology: the epithet “suthepensis” refers to the place “Doi Suthep” from where the holotype was collected.
Typus: THAILAND, Chiang Mai Prov., Doi Suthep, 16 July 2012, collector Komsit Wisitrassameewong LD2012100 (MFLU12-0938, holotype; HMAS273993, isotype).
Original description: Pileus 45–65 mm in diam., 5 mm thick at the disc, convex to hemisphaerical when young, applanate when mature, margin reflexed; surface dry, smooth, with dark-brown triangular squamules, dense at the disc, and sparse near the margin, on a white background, margin decurved. Lamellae free, crowded, intercalated with lamellulae, 3 mm broad, at first white, then pink, reddish brown, and finally dark brown. Stipe 54–114 × 6–7 mm (apex) – 9–14 mm (base), cylindrical with bulbous base, hollow, white, smooth above the annulus, fibrillose below the annulus, staining slightly orange on touching. Annulus simple, membranous, with floccose on lower surface, connected with the stipe by cortinate fibrils, white. Context firm, on cutting white, in both pileus and stipe, discolouration on exposure unknown. Odour mushroomy.
Macrochemical reactions: KOH and Schäffer’s reactions negative.
Basidiospores 5–6 × 2.4–3.3 μm [\( \overline{x} \) = 5.5 ± 0.3 × 2.9 ± 0.3, Q = 1.7–2.2, Qm = 1.9 ± 0.2, n = 20], ellipsoid, brown, smooth, thick-walled, without germ pore. Basidia 13–18 × 6–8 μm, clavate, hyaline, 4-spored. Cheilocystidia absent. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 3.3–14 μm in diam., smooth, obviously constricted at the septa, hyaline or light brown, branched.
Habitat: scattered in forests.
Other material examined: Thailand, Chiang Mai Prov., Doi Suthep, 20 June 2010, collector Komsit Wisitrassameewong, NTT042 (MFLU10-1478).
Notes: This new species has negative KOH and Schäffer’s reactions, lacks cheilocystidia, and stains orange on bruising, which are distinctive characters in this section.
Agaricus gratolens Pradeep & R.L. Zhao, sp. nov. Figs. 9c–e and 30
Fungal Names: FN570207
Facesoffungi number: FoF01206
Etymology: the epithet “gratolens” refers to the pleasant odour of the basidiomes
Typus: THAILAND, Chiang Mai Prov., Mae Taeng Dist., Hot Spring, 8 August 2006, collected by Ohnmar Myo Aung, ZRL3093 (BBH19609, holotype; HMAS274011, isotype).
Original description: Pileus 40–45 mm in diam., 2 mm thick at disc, convex with truncate top, margin exceeding; surface dry, with fibrillose scales, light brown, dense at the disc, background cream to light greyish white. Lamellae free, crowded, intercalated with lamellulae, 3 mm broad, firstly white, light brown, then brown, finally dark brown. Stipe 75 × 5 mm, cylindrical, curved, with short rhizomorphs, white, smooth above the annulus, with fibrillose to fibrillose scales below the annulus, staining weakly yellow on touching. Annulus membranous, pendent, simple, superous, fugacious, torn, stretched, white, up to 7 mm broad, low side floccose. Context firm, on cutting white, both in pileus and stipe, then quickly turning slightly ochraceus at the centre of pileus. Odour of almonds.
Macrochemical reaction: KOH and Schäffer’s reactions negative.
Basidiospores 5–6 × 3–4 μm [\( \overline{x} \) = 5.6 ± 0.4× 3.5 ± 0.3, Q = 1.4–1.9, Qm = 1.63 ± 0.27, n = 20], ellipsoid, reddish brown, smooth, thick-walled, without germ pore. Basidia12–14 × 5–7 μm, clavate, smooth, hyaline, 4-spored. Cheilocystidia10–18 × 6–8 μm, broad clavate, ellipsoid, some pyriform, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis consisted of hyphae 5–8 μm in diam., long cylindrical, with dark grey pigment distributed unevenly, smooth. Annulus hyphae of elements 5–10 μm in diam., ellipsoid, cylindrical, hyaline, smooth. Stipitipellis hyphae similar to those of annulus.
Habitat: scattered in rich soil of forests.
Other material examined: INDIA, Kerala State, Trivandrum District, Perayam, 30 Aug. 2006, No. 9679(P) CA956 (=PYP012).
Notes: this new species is similar to A. kivuensis in pileus, basidiospores, and cheilocystidia characteristics. However, the new species has a white stipe surface and stains yellow on touching, while the stipe of A. kivuensis is brown and stains rose. The phylogenetic analysis also supports them as distinct species.
5.4 Agaricus [subgenus Spissicaules] section Subrutilescentes Kerrigan, nom. prov.
Type species: Agaricus subrutilescens, designated by R. Kerrigan, Agaricus of North America, 2016 in press.
Facesoffungi number: FoF01207
Delimitation of Agaricus section
“Stature generally erect, sometimes stocky; pileipellis of radially oriented vinaceous-brown appressed fibrils; flesh unchanging or mildly rufescent; lamellae may bruise rose to red; stipe stuffed-hollow, often with surface fibrils below the annulus, sometimes glabrous; annulus pendant or scant and appressed-pendent; spores relatively small and narrow, size and L/W ranges (for North American species averages): 5.2–5.5 × 3.5–3.7 μm, 1.44–1.51, respectively; Parra (2008), the European A. impudicus (Rea) Pilát has spores ca. 5.9 × 3.6 μm, L/W = 1.64; cheilocystidia various, e.g., clavate, ovoid, subglobose, etc.; KOH reaction on pileus surface teal to olivaceous green; Schäffer’s reaction negative; o-tolidine generally blue everywhere except the lamellae.” Cited from Kerrigan (2016 in press).
Stem age and phylogenetic support
This section is reflected in clade C1 in multi-gene and ITS analyses (Figs 1 and 2), which is fully supported. The stem age is 19.15 Ma. In this clade, A. subrutilescens is the type species of this section and nests well with the three new species: A. brunneopileatus, A. parasubrutilescens, A. inthanonensis, and A. linzhiensis.
Agaricus parasubrutilescens Callac & R.L. Zhao, sp. nov. Figs. 9f–i and 31
Fungal Names: FN570218
Facesoffungi number: FoF 01208
Etymology: the epithet “parasubrutilescens” refers to its closely related phylogenetic position to A. subrutilescens.
Typus: CHINA, Yunnan Prov., Dali, Cangshan, 24 July 2014 collected by S.Y. Su ZRL2014076 (HMAS274000, holotype).
Original description: Pileus 85–125 mm in diam., 6–7 mm thick at disc convex, plano-convex with broad umbo, top truncate, margin deflexed; surface dry, entirely covered with fibrils, thick and dark at the centre, then broken towards margin into more minute squamules-fibrilles, not concentrically arranged, appressed, brown (6E6, 6E8), with red tinge when wet, margin exceeding. Lamellae free, crowded, intercalated with lamellulae, 6 mm broad, at first white, then light brown (7D4), brown, finally dark brown. Stipe 120–150 × 8–10 mm (apex) – 15–16 mm (base), clavate, cylindrically subbulbous, smooth and white above the annulus, with large squamules, brown or white below the annulus, not staining on touching. Annulus membranous, 10–25 mm broad, large, pendent, simple, superous, entire, lower side heavily fibrillose. Context firm, on cutting at first, white both in pileus and stipe, then turning quickly slightly orange on centre of pileus and stipe on exposure. Odour of molds, mushroomy.
Macrochemical reactions: KOH and Schäffer’s reactions negative.
Basidiospores 4.2–6 × 3–3.5 μm [\( \overline{x} \) = 4.9 ± 0.4× 3.1 ± 0.2, Q = 1.3–2, Qm = 1.58 ± 0.42, n = 20], ellipsoid, brown, smooth, thick-walled, without germ pore. Basidia 13–20 × 5–7 μm, clavate, 4-spored. Cheilocystidia 15–28 × 10–19 μm, pyriform, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis comprising3–7.5 μm diam., brownish grey, brown, smooth, hyphae, constricted at the septa in most cases. Annulus consisting of hyphae 3–10 μm in diam., hyaline, cylindrical, some inflated-clavate, smooth, constricted at the septa. Stipitipellis consisting of hyphae 5–12.5 diam., hyaline, smooth, elongate cylindrical, curved and branched hyphae.
Habitat: solitary in rich soil of forest.
Other material examined: THAILAND, Chiang Mai Prov., Doi Inthanon National Park, junction of Highway 1009 and road to Mae Chem, N19°31.58′ E 98°29.64′, elev. 1700 m., 27 June 2005, collected by Dennis E Desjardin, ZRL2054 (BBH19438, SFSU) and ZRL2055 (BBH19439, SFSU); same location, 5 June 2006, collected by Todd Osmundson, ZRL3020 (BBH19537, SFSU); Chiang Mai Prov., Mae Taeng, Ban Mae Sae Village, on Hwy 1095 near 50 km marker, N19°14. 599′ E98°39.456′, elev. 962 m., 26 June 2005, collected by Ruilin Zhao, ZRL2042 (BBH19427, SFSU). CHINA, Yunnan Prov., Dali, Cangshan, 31 July, 2011, collected by H.Y. Su SHY2011073115 (HMAS 274014); same location, 24 July 2014 collected by H.Y. Su, ZRL2014086 (HMAS273975), ZRL2014090 (HMAS273928), ZRL2014113 (HMAS253913), ZRL2014114 (HMAS273979); Yunnan Prov., Chuxiong city, Zixishan Hill, 21 June 2011, collected by R.L. Zhao, ZRL2011015 (HMAS274016); same location, 22 June 2011, collected by R.L. Zhao, ZRL2011027 (HMAS273926); Guangdong Prov., Lechang, 15 Sept. 2011, collected by X.H. Wang, ZRLWXH3151 (HMAS273959); Yunnan Prov., Nanjian County, Wuliangshan National Natural Reserve, 3 July 2012, collected by Oliver Raspe, ZRL2012025 (HMAS273968); Yunnan Prov., Cangyuan county, Nangunhe National Natural Reserve, 9 July 2012, collected by R.L. Zhao ZRL2012196 (HMAS273971); Yunnan Prov., Xianggelila, Tacheng, 5 August 2014, collected by M.L. Xu and M.Q. He, ZRL2014416 and ZRL2014419; Yunnan Prov., Weixi County, Yongchun 5 August 2014, collected by H.Y. Su, ZRL2014413 and ZRL2014448 (HMAS253914).
Agaricus inthanonensis L.J. Chen, K.D. Hyde & R.L. Zhao, sp. nov.
MycoBank: MB814603
Facesoffungi number: FoF01209
Etymology: the epithet “inthanonensis” refers to the place “Doi Inthanon” from where the holotype was collected.
Typus: THAILAND, Chiang Mai Prov., Doi Inthanon, Sit 1, N 19°06′28.8″ E 98°44′47.3″, elv. 1050 m., 1 July 2010, collector, Jie Chen, LD014 (MFLU10-0747, holotype).
Original description: Pileus 61 mm in diam., 4 mm thick at the disc, convex to hemisphaerical; surface dry, appressed with fibrillose squamules near the margin and thick fibrils at the disc, brown (7E6) against white background, dense at the disc; margin inflexed and eroded. Lamellae free, crowded, intercalated with lamellulae, 4 mm in width, ventricose, reddish grey (8B2) to brown, dark brown with age. Stipe 136 × 6–8 (−12 at the base) mm, cylindrical and subbulbous at the base, with rhizomorphs at the base, hollow, surface smooth and white above the annulus, appressed fibrillose brown squamulose below the annulus, discolouring yellowish brown on touching. Annulus 12 × 15 mm, membranous, white, lower side with brown to margin, upper side smooth, lower side fibrillose floccose, skirt-like. Context firm, discolouring on cutting unknown. Odour woody.
Macrochemical reaction: KOH reaction yellow, Schäffer’s greyish violet or negative.
Basidiospores 5.5–6 × 3.2–4 μm [\( \overline{x} \) = 5.9 ± 0.7 × 3.9 ± 0.7, Q = 1.37–1.81, Qm = 1.51 ± 0.3, n = 20], ellipsoid, brown, smooth, thick-walled, without germ pore. Basidia 16–24 × 7–9 μm, clavate, hyaline, 4-spored. Cheilocystidia 15–19 × 10–14 μm, globose, cystiform, smooth, hyaline. Pleurocystidia absent. Pileipellis a cutis composed of hyphae 5–10 μm in diam., smooth, some constricted at the septa, with dark brown and membranous pigments, rarely branched.
Habitat: solitary on leaf litter in the forest dominated by Castenopsis and Lithocarpus.
Other material examined: CHINA, Yunnan Prov., Xishangbanna, Mengsong, 25 May 2012, collector Samantha C. Karunarathna LDMS22 (MFLU15-2400; HMAS274018).
Agaricus brunneopileatus Callac & R.L. Zhao, sp. nov. Figs. 10a–d and 33
Fungal Names: FN570217
Facesoffungi number: FoF 01210
Etymology: the epithet “brunneopileatus” refers to the brown pileus of this species.
Typus: CHINA, Yunnan Prov., Cangyuan county, Mengjiao, Nuozhang Village, 6 July 2012, collected by O. Raspé and R.L. Zhao, ZRL2012115 (HMAS274001, holotype).
Original description: Pileus 60–80 mm in diam., 5 mm thick at disc, parabolic when young, then convex; surface dry, completely covered by fibrils and broken into appressed squamules, triangular, dense and darker at the disc, dark brown (oac639), reddish brown discolouring on touching, margin decurved. Lamellae free, crowded, intercalated with lamellulae, 5 mm broad, firstly white, then pink, finally brown dark brown. Stipe 110–150 × 7–8 (apex) – 10–15 (base) mm, cylindrical and bulbous at base, hollow, surface smooth and white above the annulus, heavily fibrillose, brown below the annulus, not staining on touching. Annulus membranous, pendant, simple, superous, up to 16 mm broad, upper side smooth and white, lower side heavily fibrillose to smooth when mature, with brown tone. Context fleshy, on cutting white, and no discolouration on exposure. Odour similar to that of Lepiota cristata.
Macrochemical reaction: KOH and Schäffer’s reactions negative.
Basidiospores 4.9–5.8 × 2.5–3.6 μm [\( \overline{x} \) = 5.4 ± 0.3× 3.2 ± 0.2, Q = 1.6–1.9, Qm = 1.7 ± 0.1, n = 20], ellipsoid, reddish brown, smooth, thick-walled, without germ pore. Basidia 12.6–18 × 6.2–8.8 μm, clavate, smooth, hyaline, 4-spored. Cheilocystidia 12–24 × 7–13 μm, pyriform, ellipsoid, occasionally 1–2 septate at the base, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis comprising hyphae 3.2–6.6 μm in diam., long cylindrical, with brown pigments, smooth, slightly constricted at the septa. Annulus hyphae of elements 3–8.6 μm in diam, cylindrical, hyaline, smooth.
Habitat: solitary in forest.
Other material examined: China, Yunnan Prov., Dali, Yangbi County, Sangbuliao Village, 28 July 2014, collected by H.Y Su, ZRL2014144 (HMAS253915).
Agaricus linzhiensis R.L. Zhao, sp. nov. Figs. 10e–g and 34
Fungal Names: FN570230
Facesoffungi number: FOF01211
Etymology: the epithet “linzhiensis” refers to "Linzhi", the place where the holotype was collected.
Typus: CHINA, Tibet, Linzhi, Milin county, Nanyigou National Forest Park, 29 July 2012, collected by R.L. Zhao, ZRL 2012618 (HMAS 274022, holotype).
Original description: Pileus 60–93 mm in diam., 5–7 mm thick at the disc, plano-convex, and margin uplifted, deep split; surface dry, squamules dense at the disc, sparse and smaller towards margin, brown (oac645) against cream to light greyish-white background, margin ncurved, exceeding and slightly appendiculate. Lamellae free, crowded, intercalated with lamellulae, 4 mm broad, at first white, then light brown, brown, finally dark brown. Stipe 50–60 × 10–15 mm, cylindrical, curved, white, smooth above the annulus, squamulose and dense towards the base and somewhat concentric below the annulus, not staining on touching. Annulus membranous, pendant, simple, superous, torn, and remains attached at the edge of pileus, up to 7 mm broad, white to light yellow, upper side smooth and lower side floccose to smooth upon maturing. Context fleshy, at first white on cutting, then rapidly becoming slightly reddish brown on exposure in stipe. Odour similar to those of Lepiota cristata.
Macrochemical reaction: KOH reaction yellow and Schäffer’s reaction weakly positive.
Basidiospores 5–6.4 × 3.7–4.8 μm [\( \overline{x} \) = 5.7 ± 0.4× 4.2 ± 0.3, Q = 1.2–1.5, Qm = 1.3 ± 0.1, n = 20], ovoid to wide ellipsoid, reddish-brown, smooth, thick-walled, without germ pore. Basidia 15–20 × 6.8–8.8 μm, clavate, smooth, hyaline, 2- or 4-spored. Cheilocystidia 11–24 × 7.3–14 μm, long ellipsoid, subsphaerical, pyriform, occasionally 2–3 elements in chains, hyaline, smooth. Pleurocystidia absent. Pileipellis a cutis comprising hyphae 3.5–7 (−10)μm in diam., long cylindrical, dark grey or not, smooth, slightly constricted at the septa. Annulus hyphae of elements 3.8–9 (−14)μm in diam, ellipsoid, cylindrical, hyaline, smooth.
Habitat: solitary in forest.
Notes: the species of section Subrutilescentes are characterized by vinaceous-brown, reddish brown, appressed fibrils on the pileus, context without discolouring or weakly to mildly rufescent on exposure, relatively long spores (ellipsoid, Q = 1.44−1.64 for North American and European specimens) and variable colour changing in KOH (negative, olivaceous green, positive yellow) (Kerrigan 2016 in press; Parra 2008). Those four new species, A. parasubrutilescens, A. inthanonensis, A. brunneopileatus and A. linzhiensis are generally have those typical morphological characters of section Subrutilescentes with the exceptions of A. linzhiensis has wider basidiospores (broadly ellipsoid, Qm = 1.3 ± 0.1) and A. brunneopileatus has elongate ellipsoid (Qm = 1.7 ± 0.1) spores. The cheilocystidia in section Subrutilescentes are generally clavate to pyriform, however, in A. inthanonensis they are globose to subglobose. The type species of this section, A. subrutilescens, has globose cheilocystidia in some cases, but the context becomes greenish on exposure, while in A. inthanonensis it becomes yellow (Kerrigan 1986).
Agaricus parasubrutilescens is similar to A. impudicus in morphology mostly, but differs in its smaller spores and lack of red discolouration of the pileus when bruised, while A. impudicus had the spores with the size of 5.9 × 3.6 μm and the lamellae turns red on bruising. Agaricus parasubrutilescens differs from A. subrutilescens by the new species has a slightly reddish yellow discolouring on cutting and a negative KOH reaction. Agaricus subrutilescens does not change colour on cutting, and turns greenish in KOH. The Chinese specimens ZRL2014289 and SHY20120706064 and the American specimen RWK1940 are phylogenetically most closely related to A. parasubrutilescens (Fig. 2), however, those collections have recurved, brown fibrillose squamules, concentrated at the disc, which are different from those of A.
parasubrutilescens.
Conclusions
Reconstruction of Agaricus taxonomic system. Prior to this study, the genus Agaricus was separated into three subgenera, Agaricus, Lanagaricus, and Coniogaricus (Heinemann 1956, 1978; Singer 1986; Parra 2008; 2013) and eight sections. These well-accepted sections were mainly established from temperate areas, such as Europe and North America (Parra 2008, 2013; Kerrigan 1986; Nauta 2001). Heinemann’s system for subgenera involved Agaricus from tropical Africa and Southwest Asia (Heinemann 1956a, b, 1978). However, the lack of DNA sequence data has hindered its full acceptance by mycologists. A recent phylogenetic study, using ITS sequence data focused on tropical species, but also accommodated all known sections from temperate regions, has revealed seven novel clades with strong support (named TRI–VII) and four less well-supported clades (named TRa–d) from tropical areas (Zhao et al. 2011). Based on this research, clade TRI has been reconstructed as the tropical-specific section Brunneopicti (established by Heinemann) (Chen et al. 2015); and another section Nigrobrunnescentes (established by K. P. Peterson, Desjardin, & Hemmes. in 2000) has been reinstated due to the type specimen of this section having been successfully sequenced in 2012 (Parra et al. 2014; Wang et al. 2015). There are ten well-resolved sections including Spissicaules and Sanguinolenti, which have been demonstrated to be polyphyletic.
In this study, we conducted a combined analysis of ITS and multi-gene sequence data, that included taxa from all previously recognized sections and seven of the eleven recently discovered, but unnamed clades (Zhao et al. 2011). Based on a phylogenetic consensus using combined multi-gene sequence analyses and considering morphology, we propose a revised taxonomic system for Agaricus that also takes into account stem age as a criterion for standardizing taxonomic ranks (Avise and Johns 1999). The genus Agaricus is divided into five subgenera and 20 sections. Two new subgenera are introduced (Minores and Spissicaules), one of which is emended (subgenus Pseudochitonia), and the subgenera Agaricus and Flavoagaricus are emended with a restricted circumscription. The subgenus Lanagaricus (Heinemann 1956a, b) becomes a heterotypic synonym of the subgenus Pseudochitonia. Five new sections are introduced (Rubricosi, Crassispori, Flocculenti, Hondenses, and Amoeni), and two remain with a much more restricted circumscription (Xanthodermatei and Sanguinolenti). Sections Trisulphurati and Laeticolores, which were placed in the subgenus Lanagaricus by Heinemann (1956a), are placed in the subgenera Pseudochitonia and Minores, respectively in this study. Subsection Bohusia (Parra 2008) is elevated to the rank of section. Finally, 22 new species are introduced with illustrated accounts.
The multi-gene analysis presented here largely agrees with the ITS phylogeny depicted in Zhao et al. (2011) in recognizing the monophyly of sections Bivelares, Chitonioides, Agaricus, Minores, Arvenses, Nigrobrunnescentes, and Brunneopicti. Section Xanthodermatei comprised a loose monophyletic group in Zhao et al. (2011) Here, with expanded sampling of both sequence data and taxa, section Xanthodermatei clearly splits into two distinct groups in both multi-gene and ITS analyses. Thus we emend section Xanthodermatei and propose a new section Hondenses. Section Sanguinolenti was shown to be polyphyletic and was segregated into two subsections by Parra (2008); here, subsection Bohusia is raised to sectional rank. Section Spissicaules was also shown to be polyphyletic, and composed of four clades by Zhao et al. (2011). Kerrigan (2016 in press) emended the section Spissicaules and to introduce the sections Subrutilescentes and Rarolentes. Here, we have named the fourth clade as a new section Amoeni. Sections Laeticolores and Trisulphurati were analyzed here for the first time, and are shown here to be monophyletic.
More robust phylogenetic topology using multi-gene sequence data. A comparison of phylogenetic classifications in Agaricus that only used ITS (Zhao et al. 2011), with that of multi-gene analysis (Fig. 1), shows that in general, only the younger clades in the ITS tree that were well-supported, are consistently retrieved in the multi-gene phylogeny, whereas deeper, older nodes were generally not or weakly supported. Clades TRI (section Brunneopicti), TRIII (section Amoeni), TRV, and TRVI from the ITS phylogeny (Zhao et al. 2011) were well-supported in the multi-gene phylogeny: their estimated ages are 18, 19, 13, and 14 Ma, respectively (Fig. 1). In contrast, clades TRb and TRc, with older stem ages (23 and 19 MA, respectively) were weakly supported in the ITS phylogeny, but were strongly supported in the multi-gene analysis. These observations corroborate the fact that ITS sequence data are useful for detecting younger groups, but are of limited value for older, more ancient lineages (Hunag 2012). Zhao et al. (2011) were tempted to recognize TRV and TRVI as new sections, however, this study indicates that they are 13 and 14 Ma old and embedded in a larger clade with many members of section Minores. In this study, we only recognize sections that are approximately the same age clades (ca. 20 Ma), and thus we conclude that TRV and TRVI should remain in section Minores.
Taxonomic ranking by using divergence times. Recognition of taxonomic groups has, to date, mainly been based on phenotype and phylogenetic reconstruction with different ranks being applied in a subjective manner (e.g. Ariyawansa et al. 2014; Phookamsak et al. 2014). Comprehensive studies on species concepts (e.g., Mayden 1997; The Marie Curie SPECIATION Network 2012) are moving towards understanding speciation. However, standard definitions for the higher ranks lack universal, standardized criteria. This results in constant changes to the taxonomic system, especially in complex groups, which is not helpful to the end user. Hennig (1966) stated “If systematics is to be a science, it must bow to the self-evident requirement that objects to which the same label is given must be comparable in some way”. It is therefore crucial to establish universal criteria for defining taxonomic ranks. The may be extremely challenging across all organisms as Avise and John (1999) found notable disparity in the ages of lineages and their taxonomic rank of fish, anthropoid primates and fruit flies. For example, all fruit flies, shared common ancestors within a single genus more than 40 million years ago, while some primates formed different families a few million years ago, and cichlid fish were placed in several different genera that formed a few thousand years ago. Our study is an example towards providing a more objective taxonomic ranking by using divergence times; if such standardized rankings are adopted it could help to stabilize systematics. It has been suggested that divergence times could be used as universal criteria in taxonomic ranking (Hennig 1966; Avise and John 1999). However, this approach has not been adopted in systematic research until recently (for instance see Talavera et al. 2012). We are not aware of any similar studies with fungi.
In this study, we provide a robust phylogenetic framework for the genus Agaricus, and ranks at the subgenus and section levels, using multi-gene sequence data from a worldwide sampling. Taxonomic ranks are determined using divergence times from a fossil-calibrated phylogeny. In order to comply with the present sections, the monophyletic clades are ranked as sectional or subgenera based on divergence times. We use the stem age ca. 20 Ma as one of criteria to set the section ranks, in order to keep the well accepted sections which sections have a stem age ca. 20 Ma; and those of subgenera ca. 30 Ma. Some monophyletic clades cannot be determined due to their much younger ages. The reconstructed taxonomic system for Agaricus aims to standardize systematic decisions, relative to a good taxonomic sampling. This logic follows that lower ranks should be younger than higher ranks; the ranks in same level should have similar divergence time. The number of monophyletic clades in Agaricus that we rank as sections and subgenera in this study are larger than those from traditional schemes (Parra 2008, 2013; Heinemann 1976; Kerrigan 1986). When we compare the sections and subgenera using morphological characters, it is hard to apply distinct morphologies to separate them.
In this paper we explore criteria that could be used as principles in taxonomic classification above the species level. It is feasible that the systematics of all organisms could be arranged using the criteria of divergence times, so that the systematics of all organisms could be represented as a whole, even though they have completely different morphologies and distant phylogenetic relationships.
References
Ariyawansa HA, Tanaka K, Thambugala KM, Phookamsak R, Tian Q, Camporesi E, Hongsanan S, Monkai J, Wanasinghe DN, Chukeatirote E, Kang JC, Xu JC, McKenzie EHC, Jones EBG, Hyde KD (2014) A molecular phylogenetic reappraisal of the Didymosphaeriaceae (= Montagnulaceae). Fungal Divers 68:69–104
Avise JC, John GC (1999) Proposal for a standardized temporal scheme of biological classification for extant species. Proc Natl Acad Sci U S A 96:7358–7363
Berbee ML, Taylor JW (2010) Dating the molecular clock in fungi – how close are we? Fungal Biol Rev 24:1–16
Bohus G (1995) Agaricus studies XIII. Key to the subgenus Agaricus from Europe. Mikol Közlem 34(1):5–35
Budd G, Jensen S (2000) A critical reappraisal of the fossil of the bilaterian phyla. Biol Rev 75:253–295
Budd G (2001) Climbing life’s tree. Nature 412:487
Callac P, Guinberteau J (2005) Morphological and molecular characterization of two novel species of Agaricus section Xanthodermatei. Mycologia 97:416–424
Cappelli A (1984) Agaricus L.: Fr. (Psalliota Fr.). Libreria editrice Biella Giovanna, Saronno, p 558
Challen MP, Kerrigan RW, Callac P (2003) A phylogenetic reconstruction and emendation of Agaricus section Duploannulatae. Mycologia 95(1):61–73
Chen J, Zhao RL, Karunarathna S, Callac P, Raspé O, Bahkali AH, Hyde KD (2012) Agaricus megalosporus: a new species in section Minores. Cryptogamie Mycol 33:145–155
Chen J, Zhao RL, Parra LA, Guelly AK, Kesel AD, Rapior S, Hyde KD, Chukeatirote E, Callac P (2015) Agaricus section Brunneopicti: a phylogenetic reconstruction with descriptions of four new taxa. Phytotaxa 192:145–168
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772
Dentinger BM, Margaritescu S, Moncalvo J-M (2010) Rapid and reliable high-throughput methods of DNA extraction for use in barcoding and molecular systematics of mushrooms. Mol Ecol Resour 10:628–633
Didukh M, Vilgalys R, Wasser SP, Isikhuemhen OS, Nevo E (2005) Notes on Agaricus section Duploannulati using molecular and morphological data. Mycol Res 109:729–740
Drummond AJ, Ho SYW, Phillips MJ, Rambaut A (2006) Relaxed phylogenetics and dating with confidence. PLoS Biol 4(5), e88
Drummond AJ, Suchard MA, Xie D, Rambaut A (2012) Bayesian phylogenetics with BEAUti and BEAST 1.7. Mol Biol 29:1969–1973
Edgar RC (2004a) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797
Edgar RC (2004b) MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinforma 5:113
Fungal Names (2013). http://fungalinfo.im.ac.cn/fungalname/fungalname.html
Geml J, Geiser DM, Royse DJ (2004) Molecular evolution of Agaricus species based on ITS and LSU rDNA sequences. Mycol Prog 3(2):157–176
Geml J, Laursen GA, Nusbaum HC, Taylor DL (2007) Two new species of Agaricus from the Subantarctic. Mycotaxon 100:193–208
Gui Y, Zhu GS, Callac P, Hyde KD, Parra LA, Chen J, Yang TJ, Huang WB, Gong GL, Liu ZY (2015) Agaricus section Arvenses: three new species in highland subtropical Southwest China. Fungal Biol 119(2–3):79–94
Heinemann P (1956a) Champignons récoltés au Congo Belge par Mme M Goossens-Fontana, II Agaricus Fr ss. Bull Jard Bot État 26:1–127
Heinemann P (1956b) Champignons récoltés au Congo Belge par Mme M Goossens-Fontana, II Agaricus, Note complémentaire. Bull Jard Bot État 26:325–333
Heinemann P (1961) Agarici Austroamericani I, Agarics of Trinidad. Kew Bull 15:231–248, pl. 1
Heinemann P (1978) Essai d’une clé de determination des genres Agaricus et Micropsalliota. Sydowia 30:6–37
Heinemann P (1980) Les genres AgaricusetMicropsalliota en Malaisie et en Indonésie. Bull Jard Bot Belg 50:3–68
Hennig W (1966) Phylogenetic systematics. Univ. of Illinois Press, Urbana
Hibbett DS, Matheny PB (2009) The relative ages of ectomycorrhizal mushrooms and their plant hosts estimated using Bayesian relaxed molecular clock analyses. BMC Biol 7:13
Hibbett DS, Grimaldi D, Donoghue MJ (1997) Fossil mushrooms from Miocene and Cretaceous ambers and the evolution of Homobasidiomycetes. Am J Bot 84(8):981–991
Huelsenbeck JP, Ronquist F (2001) MrBayes: Bayesian inferance of phylogeny. Biometrics 17:754–755
Hunag Y (2012) Molecular phylogenetics. Science Press, Beijing, p 533
Hyde KD, Jones EBG, Liu JK, Ariyawansa H, Boehm E, Boonmee S, Braun U, Chomnunti P, Crous PW, Dai DQ, Diederich P, Dissanayake A, Doilom M, Doveri F, Hongsanan S, Jayawardena R, Lawrey JD, Li YM, Liu YX, Lücking R, Monkai J, Muggia L, Nelsen MP, Pang KL, Phookamsak R, Senanayake I, Shearer CA, Suetrong S, Tanaka K, Thambugala KM, Wijayawardene NN, Wikee S, Wu HX, Zhang Y, Aguirre-Hudson B, Alias SA, Aptroot A, Bahkali AH, Bezerra JL, Bhat DJ, Camporesi E, Chukeatirote E, Gueidan C, Hawksworth DL, Hirayama K, Hoog SD, Kang JC, Knudsen K, Li WJ, Li XH, Liu ZY, Mapook A, McKenzie EHC, Miller AN, Mortimer PE, Phillips AJL, Raja HA, Scheuer C, Schumm F, Taylor JE, Tian Q, Tibpromma S, Wanasinghe DN, Wang Y, Xu JC, Yan JY, Yacharoen S, Zhang M (2013) Families of Dothideomycetes. Fungal Divers 63:1–313
István N (2009) Az Agaricus biberi Azonosítása és elöfordulása magyarországon. Mikol Közlem - Clusiana 48(1):37–44
Jayasiri SC, Hyde KD, Ariyawansa HA, Bhat J, Buyck B, Cai L, Dai YC, Abd-Elsalam KA, Ertz D, Hidayat I, Jeewon R, Jones EBG, Bahkali AH, Karunarathna SC, Liu JK, Luangsa-ard JJ, Lumbsch HT, Maharachchikumbura SSN, McKenzie EHC, Moncalvo JM, Ghobad-Nejhad M, Nilsson H, Pang KL, Pereira OL, Phillips AJL, Raspé O, Rollins AW, Romero AI, Etayo J, Selçuk F, Stephenson SL, Suetrong S, Taylor JE, Tsui CKM, Vizzini A, Abdel-Wahab MA, Wen TC, Boonmee S, Dai DQ, Daranagama DA, Dissanayake AJ, Ekanayaka AH, Fryar SC, Hongsanan S, Jayawardena RS, Li WJ, Perera RH, Phookamsak R, de Silva NI, Thambugala KM, Tian Q, Wijayawardene NN, Zhao RL, Zhao Q, Kang JC, Promputtha I (2015) The faces of fungi database: fungal names linked with morphology, molecular and human attributes. Fungal Divers 74:3–18
Kerrigan RW (1986) Agaricales of California. Vol. 6. Agaricaceae. Mad River Press, Eureka, pp 1–62
Kerrigan RW (2016) Agaricus of North America. New York Botanical Garden Press, Bronx, (in press)
Kerrigan RW, Callac P, Challen M, Guinberteau J, Parra LA (2005) Agaricus section Xanthodermatei: a phylogenetic reconstruction with commentary on taxa. Mycologia 97(6):1292–1315
Kerrigan RW, Callac P, Parra LA (2008) New and rare taxa in Agaricus section Bivelares (Duploannulati). Mycologia 100(6):876–892
Karunarathna SC, Guinberteau J, Chen J, Vellinga EC, Zhao RL, Chukeatirote E, Yan JY, Hyde KD, Callac P (2014) Two new species in Agaricus Tropical Clade I. Chiang Mai J Sci 41:771–780
Konrad P, Maublanc A (1952) Les Agaricales. Lechevalier, Paris
Kornerp A, Wanscher JH (1978) Methuen handbook of colour, 3rd edn. Eyre Methuen, London
Kühner P, Romagnesi H (1953) Flore analytique des champignons superieurs (Agarics, Boletes, Chanteralles). Masson et CIE, Paris
Largent DL (1986a) How to identify mushrooms to genus vol. 1. Macroscopic Features. Mad River Press, Eureka, p 166
Largent DL (1986b) How to identify mushrooms to genus vol. 3. Microscopic Features. Mad River Press, Eureka, p 148
Largeteau ML, Llarena-Hernández RC, Regnault-Roger C, Savoie J-M (2011) The medicinal Agaricus mushroom cultivated in Brazil: biology, cultivation and non-medicinal valorization. Appl Microbiol Biotechnol 92:897–907
Lebel T (2013) Two new species of sequestrate Agaricus (section Minores) from Australia. Mycol Prog 12:699–707
Lebel T, Syme A (2012) Sequestrate species of Agaricus and Macrolepiota from Australia: new species and combinations and their position in a calibrated phylogeny. Mycologia 104:496–520
Lepage T, Bryant D, Philippe H, Lartillot NA (2007) General comparison of relaxed molecular clock models. Mol Biol Evol 24(12):2669–2680
Li SF, Xi YL, Qi CX, Liang QQ, Wei SL, Li GJ, Zhao D, Li SJ, Wen HA (2014) Agaricus taeniatus sp. nov., a new member of Agaricus sect. Bivelares from northwest China. Mycotaxon 129:187–196
Maddison W, Maddison D (2007) Mesquite 2. http://mesquiteproject.org
Maharachchikumbura SSN, Hyde KD, Jones EBG, McKenzie EHC, Huang S-K, Abdel-Wahab MA, Daranagama DA, Dayarathne M, D’souza MJ, Goonasekara ID, Hongsanan S, Jayawardena RS, Kirk PM, Konta S, Liu J-K, Liu Z-Y, Norphanphoun C, Shenoy BD, Xiao Y, Bahkali AH, Kang J, Somrothipol S, Suetrong S, Wen T, Xu J (2015) Towards a natural classification and backbone tree for Sordariomycetes. Fungal Divers 72:199–301
Matheny PB, Wang Z, Binder M, Curtis JM, Lim YM, Nilsson RH, Hughes KW, Hofstetter V, Ammirati JF, Schoch CL, Langer E, Langer G, McLaughlin DJ, Wilson AW, FrØslev T, Ge ZW, Kerrigan RW, Slot GC, Yang ZL, Baroni TJ, Fischer M, Hosaka K, Matsuura K, Seidl MT, Vauras J, Hibbett DS (2007) Contributions of rpb2 and tef-1α to the phylogeny of mushrooms and allies (Basidiomycota, Fungi). Mol Phylogenet Evol 43:430–451
Mayden RL (1997) A hierarchy of species concepts: the denouement in the saga of the species problem. In: Claridge MF, Dawah HA, Wilson MP (eds) In species: the units of biodiversity. Chapman & Hall, London, pp 381–424
McPeek MA, Brown JM (2007) Clade age and not diversification rate explains species richness among animal taxa. Am Nat 169, E000
Mitchell AD (1999) Phylogenetic relationships of Agaricus species based on ITS-2 and 28S ribosomal DNA sequences. Mycologia 91(5):811–819
Möller FH (1950) Danish Psalliota species I. Friesia, IV, pp 1–60
Möller FH (1952) Danish Psalliota species II. Friesia, IV, pp 135–220
Moncalvo J-M, Lutzoni FM, Rehner SA, Johnson J, Vilgalys R (2000) Phyligenetic relationships of Agaric fungi based on nuclear large subunit ribosomal DNA sequences. Syst Biol 49:278–305
Moncalvo JM, Vilgalys R, Redhead SA, Johnson JE, James TY, Aime MC, Hofstetter V, Verduin SJW, Larsson E, Baroni TJ, Thorn RG, Jacobsson S, Clémençon H, Miller OK Jr (2002) One Hundred and Seventeen Clades of Euagarics. Mol Phylogenet Evol 23:357–400
Morehouse EA, James TY, Ganley ARD, Vilgalys R, Berger L, Murphy PJ, Longcore JE (2003) Multilocus sequence typing suggests that the chytrid pathogen of amphibians is a recently emerged clone. Mol Ecol 12:395–403
Moser M (1967–1983) Kleine Kryptogamenflora: Die Röhrlinge und Blatterpilze (polyporales, Boletales, Agaricales, Russulales). Gustav Fischer Verlag, Stuttgart
Nauta MM (2001) Agaricus L. and Allopsalliota Nanta et Bas. In: Noordeloos ME, Kuyper TW, Vellinga EC (eds) Flora Agaricina Neerlandica, Critical monographs on families of Agarics and Boleti occurring in the Netherlands. Vol. 5 Family Agaricaceae. A.A. Balkema Publishers, Lisse, pp 23–62
Parra LA (2008) Agaricus L. Allopsalliota, Nauta & Bas, Fungi Europaei, Volume 1. Edizioni Candusso, Alassio, p 824
Parra LA (2013) Agaricus L. Allopsalliota, Nauta & Bas, Fungi Europaei, Volume 1A. Candusso Edizioni s.a.s, Alassio, p 1168
Parra LA, Mua A, Cappelli A, Callac P (2011) Agaricus biannulatus sp. nov., a new species of the section Xanthodermatei collected in Sardinia and Sicily. Micol Veget Medit 26:3–20
Parra LA, Muñoz G, Callac P (2014) Agaricus caballeroi sp. nov., una nueva especie de la sección Nigrobrunnescentes recolectada en España. Micol Veget Medit 29:21–38
Peterson KR, Desjardin ED, Hemmes DE (2000) Agaricales of the Hawaiian Islands. 6. Agaricxaceae I. Agariceae: Agaricus and Melanophyllum. Sydowia 52(2):204–257
Phookamsak R, Liu JK, McKenzie EHC, Manamgoda DS, Ariyawansa HA, Thambugala KM, Dai DQ, Camporesi E, Chukeatirote E, Wijayawardene NN, Bahkali AH, Mortimer PEXJC, Hyde KD (2014) Revision of Phaeosphaeriaceae. Fungal Divers 68:159–238
Pilát A (1951) The Bohemian species of the genus Agaricus. Acta musei Nationalis Pragae, VII B
Rambaut A, Suchard M, Drummond AJ (2013) Tracer 1.6 http://tree.bio.ed.ac.uk/software/tracer/
Robinson NE, Robinson AB (2001) Molecular clocks. Proc Natl Acad Sci U S A 98(3):944–949
Ronquist F, Heulsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sánchez-Ramírez S, Tulloss RE, Amalfi M, Moncalvo JM (2015) Palaeotropical origins, boreotropical distribution and increased rated of diversification in a clade of edible ectomycorrhizal mushrooms (Amanita section Caesareae). J Biogeogr 42(2):351–363
Singer R (1986) The Agaricales in modern taxonomy, 4th edn. Koeltz Scientific Books, Koenigsternm
Smith SY, Currah RS, Stockey RA (2004) Cretaceous and Eocene poroid hymenophores fromVancouver Island, British Columbia. Mycologia 96(1):180–186
Stadler T, Rabosky DL, Ricklefs RE, Bokma F (2014) On age and species richness of higher taxa. Am Nat 184:447–455
Stamatakis A (2006) RaxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 22(21):2688–2690
Talavera G, Lukhtanov LA, Pierce NE, Vila R (2012) Establishing criteria for higher-level classification using molecular data: the systematics of Polyommatus blue butterflies (Lepidoptera, Lycaenidae). Cladistics 29(2):166–192
The Marie Curie SPECIATION Network (2012) What do we need to know about speciation? Trends Ecol Evol 27:27–39
Thongklang N, Nawaz R, Khalid AN, Chen J, Hyde KD, Zhao RL, Parra LA, Hanif M, Moinard M, Callac P (2014) Morphological and molecular characterization of three Agaricus species from tropical Asia (Pakistan, Thailand) reveals a new group in section Xanthodermatei. Mycologia 106:1220–1232
van Tuinen M, Torres CR (2015) Potential for bias and low precision in molecular divergence time estimation of the Canopy of Life: an example from aquatic bird families. Front Genet 6:203. doi:10.3389/fgene.2015.00203
Vellinga EC, Sysouphanthong P, Hyde KD (2011) The family of Agaricaceae: phylogenies and two new white-spored genera. Mycologia 103(3):494–509
Wang ZR, Parra LA, Callac P, Zhou JL, Fu WJ, Dui SH, Hyde KD, Zhao RL (2015) Edible species of Agaricus (Agaricaceae) from Xinjiang Province (Western China). Phytotaxa 202(3):185–197
Wasser SP (1980) Flora Fungorum RSS Ucrainicae: Agaricaceae Cohn. Naukova Dumka, Kiev
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic, New York, pp 315–322
Wisitrassameewong K, Karunarathna SC, Thongklang N, Zhao RL, Callac P, Chukeatirote E, Bahkali AH, Hyde KD (2012) Agaricus subrufescens: new to Thailand. Chiang Mai J Sci 39(2):281–291
Zhao RL, Desjardin DE, Callac P, Parra LA, Guinbereau J, Soytong K, Karunarathna S, Zhang Y, Hyde KD (2013) Two species of Agaricus sect. Xanthodermatei from Thailand. Mycotaxon 122:187–195
Zhao RL, Hyde KD, Desjardin DE, Raspé O, Soytong K, Guinberteau J, Karunarathna SC, Callac P (2012) Agaricus flocculosipes sp. nov., a new potentially cultivatable species from the palaeotropics. Mycoscience 53:300–311
Zhao RL, Karunarathna S, Raspé O, Parra LA, Guinberteau J, Moinard M, Kesel AD, Barroso G, Courtecuisse R, Hyde KD, Guelly AK, Desjardin DE, Callac P (2011) Major clades in tropical Agaricus. Fungal Divers 51:279–296
Zhou JL, Su SY, Su HY, Wang B, Hyde KD, Zhao RL (2016) A description of eleven new species of Agaricus sections Xanthodermatei and Hondenses collected from Tibet and the surrounding areas. Phytotaxa (in press)
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China to RLZ (Project IDs 31000013, 31360014 and 31470152), the Thailand Research Fund to KDH (grant BRG 5580009), and the Natural Sciences and Engineering Research Council of Canada and the ROM Governors to JMM. Dr. Richard Kerrigan made valuable comments and suggestions to improve this paper. Drs. Wang Xiang-Hua, Su Hong-Yang, István Nagy, Samantha C. Karunarathna and Phongeun Sysouphanthong lended specimens.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
(PDF 459 kb)
Rights and permissions
About this article
Cite this article
Zhao, RL., Zhou, JL., Chen, J. et al. Towards standardizing taxonomic ranks using divergence times – a case study for reconstruction of the Agaricus taxonomic system. Fungal Diversity 78, 239–292 (2016). https://doi.org/10.1007/s13225-016-0357-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13225-016-0357-x