Abstract
The heavy metal contaminated groundwater results in serious health issues and hence this study attempts to address its sources of contamination using integrated techniques including indexed and statistical methods and its related health hazards. Groundwater pH varied from 5.3 to 8.3 indicating acidic to alkaline in nature. The heavy metal pollution index shows that the groundwater samples vary from low to high pollution class and 21% of the samples exceed the critical limit of 100 implying that they are highly polluted with respect to heavy metals and are unfit for human consumption. The heavy metal evaluation index reveals that all the groundwater samples fall under low pollution. The synthetic pollution index reveals that 2%, 74% and 24% of the samples are suitable, slightly and moderately polluted, respectively, with heavy metals. The water quality index reveals that 19% and 2% of the groundwater samples belong to the poor and very poor water quality category and are spatially situated on the central, northern and southern parts of the study region. Correlation matrix and principal component analysis revealed that weathering of aquifer matrix and anthropogenic activities are accountable for the release of heavy metals into groundwater. Furthermore, R-mode and Q-mode cluster analysis revealed two clusters that are linked to mixed sources including weathering and anthropogenic activities. Based on the hazard quotient, the order of heavy metal impact is Co>Pb>Cd>Zn>As>Mn>Cu>Cr>Fe>Ni for both children and adults. The hazard index values varied from 0.06 to 8.16 for children and from 0.02 to 2.14 for adults. In this study, it is discovered that 43% and 26% of groundwater samples pose a non-carcinogenic health risk in children and adults, respectively. This study highly recommends treatment of contaminated groundwater before consumption in order to protect and maintain public health. The results from this study can be useful for the local municipalities and the policy makers while considering management and mitigation plan to maintain the water quality and to control its adverse effect on human health.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater serves as an important water resource supplying the demand for drinking, domestic, agricultural and industries, especially in regions of arid and semi-arid countries (Wang et al. 2020; Li et al. 2019; Ezugwu et al. 2019; Çiner et al. 2020) and it is usually perceived as safe compared to surface water resources due to its natural filtration of contaminants by the soil as water moves into the groundwater (Sener et al. 2017a, b; Wagh et al. 2018; Edokpayi et al. 2018). However, researchers have reported several cases of groundwater contamination due to various sources (He and Wu 2019). Heavy metal pollution is among the most prominent threat to the quality of groundwater resources owing to its extreme toxicity even at low concentrations (Abou Zakhem and Hafez 2015; Singh and Kamal 2016; Tiwari et al. 2017; Egbueri and Unigwe 2020). Heavy metals enter groundwater through natural processes such as weathering and dissolution of rocks and soils, ion exchange processes, volcanism extruded products, decomposition of living matter and atmospheric matter (Prasanna et al. 2012; Rezaei et al. 2018; Çiner et al. 2020; Wang et al. 2021). Mining, industrial, agricultural, and domestic refuse disposals are some of the common anthropogenic activities that influence the concentration of heavy metals in groundwater resources (Rezaei et al. 2017; Wagh et al. 2018; Giri and Singh 2019; Ahamad et al. 2020). However, the accumulation of heavy metals in ecosystem is exacerbated by anthropogenic activities (Ukah et al. 2019). Accumulated heavy metals are classified as essential (copper, chromium, cobalt, iron, manganese and zinc) and non-essential (arsenic, cadmium and lead) (Çiner et al. 2020). Some heavy metals are required in small amounts for human bodybuilding. However, excess consumption of such heavy metals can be detrimental to health (Haloi and Sarma 2012; Chanpiwat et al. 2014; Boateng et al. 2015; Vetrimurugan et al. 2018; Xiao et al. 2019).
Several numerical and statistical models developed globally are successfully used in water quality assessment. These methods have been shown to provide better insights regarding the quality and health risk statuses of any given drinking water supply (Solangi et al. 2019; Egbueri 2020). For example, the heavy metal pollution index (HPI), heavy metal evaluation index (HEI), synthetic pollution index (SPI), and water quality index (WQI), correlation analysis (CA), principal component analysis (PCA) and hierarchical cluster analysis (HCA) (Mohan et al. 1996; Prasad and Bose 2001; Edet and Offiong 2002; Prasanna et al. 2012; Subba Rao 2012; Solangi et al. 2019; Egbueri 2020; Wu et al. 2020; Li et al. 2015, 2016; Ren et al. 2021). About 40% of the groundwater samples in Nigeria is polluted by heavy metals rendering them inappropriate for human consumption (Egbueri and Unigwe 2020). Different techniques such as PIG, ERI and HCA to assess the drinking water quality of groundwater in Ojoto, Nigeria was utilised. According to the PIG classification, 20% of the groundwater samples were found to be very highly polluted and were found unsuitable for human consumption (Egbueri 2020). The HPI, HEI and Cd results revealed that the majority of groundwater samples in central Bangladesh fall under a low level of pollution and the Cd provided better insight when compared to the other indices (Bodrud-Doza et al. 2016).
Population growth, increase in agricultural activities, rapid urbanization, industrialization, and climate change are among the factors that lead to water quality problems in South Africa (Vhonani et al. 2018). In Southern Africa, about two-thirds of the country’s population depends on groundwater for their domestic purposes (Nel et al. 2009; Vetrimurugan et al. 2017). According to DWAF (2000), 65% of the total water supply in rural areas is derived from groundwater. Direct consumption of groundwater without any form of treatment exposes local communities to various contaminants that may have an adverse impact on human health. About 3.6% of deaths per year are linked to drinking water contamination in South Africa (Nel et al. 2009). Arsenic and lead are the major contaminant sources of groundwater in South Africa (Verlicchi and Grillini 2020).
The present study is focused on Maputaland coastal aquifer, South Africa. Rural communities within the area are still without an adequate supply of water resources, as a result, they solely rely on groundwater resources for their domestic water needs. Previous studies conducted in this area revealed that groundwater is highly contaminated with iron, rendering it unsuitable for drinking purpose (Demlie et al. 2014). The ecological impact of metals in beach sediments in marine protected areas shows enrichment of metals which are due to the heavy mineral-rich coastal dunes and past mining activities (Vetrimurugan et al. 2018). The concentrations of cadmium, zinc, lead, manganese, aluminium and iron exceeded the limits of World Health Organization (WHO) standards for drinking water quality of the Maputaland coastal aquifer (Mthembu et al. 2020). Statistical and indexical approaches have not sufficiently explored the extent of metal contamination in the study region. To assess heavy metal levels in drinking water and associated risks to human health, it is imperative to perform a comprehensive study. The main objectives of this study are (1) to evaluate the extent of heavy metal pollution in groundwater using HPI, and HEI (2) assess groundwater quality for drinking purposes by adopting SPI and WQI, and (3) to evaluate potential heavy metal contamination in groundwater using multivariate statistical tools. This study will provide knowledge on major pollution sources and assist water management of South Africa in combating further contamination of groundwater resources.
Methodology
Description of the Study Area
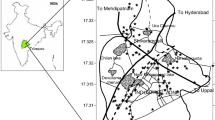
The Maputaland coastal plain of northern KwaZulu Natal extends southerly from Mtunzini to the Mozambique border in the North (Fig. 1). It belongs to the uMkhanyakude district municipality with a geographical area covering approximately 4400 km2. The climate of the study area is humid subtropical with warm summers. The annual rainfall ranges from less than 600 mm per annum inland and rises to approximately 1000 mm per annum along the coast of the study region (Porat and Botha 2008; Ramsay 1996; Watkeys et al. 1993). Land use activities in the area consist of subsistence agriculture, commercial forestry plantations and eco-tourism. The foremost water supply for drinking, domestic and agricultural activities are from groundwater.
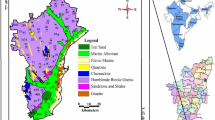
Geologically, the Maputaland coastal plain is underlain by unconsolidated to semi-consolidated sediments of Cretaceous to Quaternary age. Alluvial deposits, arenite, sandstone, and siltstone cover the study area (Fig. 1). Deposits of the Zululand Group consists of Makatini, Mzinene and St. Lucia formations. These sediments are overlain by Miocene aged Uloa formation which is in turn overlain by the cross-bedded calcarenites of the Umkhwelane formation (Meyer et al. 2001). Pleistocene aged Port Durnford and Kosi Bay formations are characterised by loosely consolidated sands, silts, clays and lignite beds (Demlie et al. 2014; Ndlovu 2015). The Holocene aged Sibayi formation is characterised by high coastal dune cordons (Watkeys et al. 1993). The area is covered by sediments that are highly permeable and indorse swift recharge to the aquifers and strongly interact with the wetlands in the region (Mkhwanazi 2010). Groundwater in this area is encountered within the shallow unconfined aquifer that yields approximately 0.5 to 5.0 l/s. (Kelbe et al. 2016; Ndlovu and Demlie 2018). Generally, groundwater flows from the west to the Indian Ocean. The highest groundwater level is approximately 25 m below ground level.
Sampling and Analysis of Groundwater
Groundwater samples were collected from randomly selected 53 bore wells of the Maputaland coastal aquifer during April 2019 (Fig. 1). The wells were purged for approximately 5 min to remove stagnant water prior to sample collection. The groundwater samples were collected and stored using high-density polyethene (HDPE) bottles after filtering with a membrane filter. The 0.5 ml of nitric acid was added to the samples to prevent metal precipitation. The samples were labelled accordingly and stored in the refrigerator at a temperature of 4 °C (Sunkari et al. 2021). Groundwater samples were analysed for pH, As, Fe, Mn, Zn, Pb, Ni, Cr, Cu, Cd and Co.
The pH of the groundwater samples was determined with a multiprobe meter instrument (Aqua Probe A-700). Heavy metals present in water samples were determined by inductively coupled plasma-mass spectrometry (NexION 2000 ICP-MS) instrument using calibration standards (Merck) at the University of Zululand hydrogeochemistry laboratory. Throughout the analytical procedures, the standards of the American Public Health Association (APHA 2005) were followed. Standards and blanks were run to assess the accuracy of the analysis. ArcGIS software v10.5 was employed to plot spatial maps using the IDW (inverse distance weighted) interpolation technique.
Heavy Metal Pollution Assessment
Heavy Metal Pollution Index (HPI)
The heavy metal pollution index (HPI) provides overall quality of water with respect to heavy metals (Mohan et al. 1996). The HPI is computed by first assigning a rating or a weightage (Wi) to each heavy metal. The rating is an arbitrary value between 0 and 1 and its selection depends on the relative importance of each water quality parameter. This weightage (Wi) can be defined as inversely proportional to the recommended standard (Si) for the corresponding parameter (Mohan et al. 1996). In this present study, the standard permissible value (Si) was taken from the SANS standards (SANS 2015). The HPI was computed by the following equation (Mohan et al. 1996);
where Qi is the sub-index of the parameter, Wi is the unit weight of the parameter, and n is the number of parameters considered. The sub-index (Qi) is calculated by the equation
where Mi is the monitored value of the parameter, Ii is the ideal value of the parameter and Si is the standard value of the parameter. In this study, the ideal values for all the metals are given as 0 µg/L since their presence in drinking water is not desired (Vetrimurugan et al. 2017). The negative sign (−) indicates the numerical difference between the two values, ignoring the algebraic sign.
Heavy Metal Evaluation Index (HEI)
The HEI yields the overall quality and presence of heavy metal in water (Edet and Offiong 2002). It was computed as follows;
where HC is the monitored value and HMAC is the maximum admissible concentration (MAC) of the parameter taken from SANS (2015).
Assessment of Drinking Water Quality
Synthetic Pollution Index (SPI)
SPI is used to evaluate the degree of pollution of groundwater resources (Solangi et al. 2019), and their drinking suitability (Egbueri and Unigwe 2020). It was computed by the following equation;
where K, Vs, Vo, n and Wi are the constant of proportionality, each parameter’s standard SANS (2015) level, the concentration of each parameter, the total number of observed parameters, and the weight coefficient of each parameter, respectively. Based on the SPI values, water is classified into five categories such as suitable for drinking (SPI < 0.2), slightly polluted water (SPI 0.2–0.5), moderately polluted (SPI 0.5–1.0), highly polluted (SPI 1.0–3.0), and unfit for drinking (SPI > 3.0) (Solangi et al. 2019; Egbueri and Unigwe 2020; Egbueri 2020).
Water Quality Index (WQI)
The WQI was developed by Horton (1965) and it has been widely used by various research scholars to evaluate drinking water quality. Computing the WQI involves five steps; (i) assignment of weights (wi) to each water quality parameter being analysed, (ii) calculation of relative weights (Wi) of parameters using Eq. 8, (iii) quality rating calculation (qi) based on Eq. 8, (iv) determining the sub-index value (SIi) of the analysed parameters based on Eq. 9, (v) calculation of the WQI based on Eq. 10.
where qi represents the parameter quality rating, Ci represents the concentration of the chemical parameter (mg/L), and Si represents the drinking water standard prescribed by SANS/WHO. Weight values (wi) ranging from 1 to 5 were ascribed for each parameter according to their relative significance in the overall quality of drinking water and their indebted effects on human health. A maximum weight of 5 was allocated to the most significant parameters while a weight of 1 was assigned to the least significant parameters. Table 1 shows the weights and relative weights assigned while computing WQI.
Health Risk Assessment
Intake of contaminated groundwater by elevated trace metal content is known to pose threat to human health. Evaluation of non-carcinogenic health risks due to trace metal contaminated groundwater particularly in children is crucial. According to guidelines provided by the United States Environmental Protection Agency (US EPA (1989), the chronic daily intake (CDI) risks caused by ingestion of a single trace element is computed for adults and children using the equation;
where CDI represents chronic daily intake via ingestion pathway (µg/kg/day), C is the concentration of the contaminant in drinking water (µg/L). IR represents the ingestion rate (L/day: 2.2 for adults and 1.8 for children). ED signifies the exposure duration (years: 70 and 6 years for adults and children). EF represents the exposure frequency (days/years: 365 for adults and children). BW is the body weight (kg: 70 for adults while 15 kg is for children). AT signifies the average time (days: 25 550 and 2 190 days for adults and children) (Duggal et al. 2017; Mgbenu and Egbueri 2019; Egbueri 2020; Mthembu et al. 2020). The non-carcinogenic risk of a single trace element is then calculated as the hazard quotient (HQ) using the equation.
where RfD signifies the reference dose of each trace element (µg/kg/day). In this study, the RfD for the different trace metals are given as 0.3 (As), 0.5 (Cd), 40 (Cu), 700 (Fe), 24 (Mn), 1.4 (Pb), 300 (Zn), 0.3 (Co), 3 (Cr), and 20 (Ni) (Duggal et al. 2017; Mgbenu and Egbueri 2019; Egbueri 2020; Mthembu et al. 2020). The hazard index (HI) representing the non-carcinogenic risk of the heavy metal is computed and is obtained by adding the HQs values of each groundwater sample as shown by the following equation;
HI values less than unity indicates that the non-carcinogenic health risk is within the acceptable limit whereas when the HI values are greater than unity it indicates that they are above the acceptable limit (Egbueri and Mgbenu 2020).
Results and Discussion
Heavy Metal Contamination
Table 2 shows the results of physicochemical parameters and their comparison to drinking water standards (WHO 2011; SANS 2015). The groundwater pH varied from 5.3 to 8.3 with an average value of 7.1 resulting the nature of water from acidic to alkaline. The pH of the samples indicated that most of the samples were within the standard limits of SANS (2015) but 30% of the samples were found below 6.5 as prescribed by WHO (2011). The electrical conductivity (EC µS/cm) varied from 115 to 1194 with an average of 330. Concentrations of cadmium are below the detection limit of 4.6 µg/L (mean 0.9 µg/L) and approximately 23% of the samples exceed the SANS (2015) and WHO (2011) standard limits. Zinc ranged from 1.9 to 19 964.5 µg/L (mean 499.9 µg/L). Approximately 2% of the samples exceeded the SANS (2015) and WHO (2011) standard limits for drinking water. Iron concentrations in groundwater ranged from 2.7 to 1 848.6 µg/L with an average range of 140 µg/L. About 11% of the samples have iron concentrations higher than SANS (2015) and WHO (2011) drinking water standard limits. The high iron concentrations may be owed to the leaching process of iron-rich sediments (Demlie et al. 2014). In this study, lead contents varied from below detection limit to 26 µg/L (mean 6.6 µg/L). Approximately, 25% of the samples have lead concentrations exceeding the SANS (2015) and WHO (2011) standard limits. Hence, the samples were classified as unfit for human consumption. Manganese concentrations ranged from 0.4 to 116 µg/L (mean 19.1). About 2% of the samples have manganese concentrations above the SANS (2015) standard limit. It was also observed that arsenic, chromium, copper, cobalt and nickel concentrations are low in groundwater samples and are within the prescribed standard limits of drinking water as prescribed by SANS (2015) and WHO (2011). Furthermore, iron, lead and cadmium were recognised to be dominant heavy metal contaminants in this area. Figure 2a–c shows the spatial distribution of selected heavy metals in the study area. In this study, high iron concentrations are observed in the north-western and south-eastern parts of the area (Fig. 2a). High lead concentrations are recorded in the south-eastern, northern, central, and southwestern parts of the area (Fig. 2b). The spatial distribution of cadmium illustrates that high concentrations are recorded in the central, northern and south-western parts of the area (Fig. 2c). The relationship between groundwater pH and the metal load was computed to classify the water samples (Ficklin et al 1992; Caboi et al 1999). Figure 3 shows that 43% and 57% of the samples are classified as a near-neutral high metal and near-neutral extreme metal. The high acidic pattern plotted against pH could be due to wastewater discharge and the inputs from agrochemicals used during irrigation (Islam et al., 2020; Church, 1998).
Heavy Metal Pollution Assessment
Heavy Metal Pollution Index (HPI)
The computed HPI ranged from 0.28 to 128.08 with a mean value of 30.65, respectively (Table 3). The critical value for HPI for drinking water is 100 values < 100 indicates low pollution levels while values of > 100 indicates high water pollution (Mohan et al 1996; Prasad and Bose 2001). In this study, 21% of the samples exceeded the critical value of 100 indicating that these samples are unfit for consumption with respect to heavy metals (Fig. 5). The HPI can be classified into three categories such as low class (< 15), medium class (15–30) and high class (> 30) (Edet and Offiong 2002; Tiwari et al. 2017). Table 4 and Fig. 4a demonstrates that the HPI was categorised into low (71%), medium (4%) and high (25%). The high HPI values were spatially located in the northern, central and south-western part of the area which could be derived from infiltrated pesticides and fertilizers from agricultural land.
Heavy Metal Evaluation Index (HEI)
The values of HEI varied from 0.08 to 10.25 with an average value of 1.83 (Table 3), respectively. The HEI can be classified as low (< 400), medium (400–800) and high pollution (> 800) (Edet and Offiong 2002). In this study, all the groundwater samples were found below the 400 (Table 4; Fig. 4a), respectively. This suggests that the groundwater has low pollution with regards to heavy metals and is safe for human consumption.
Drinking Water Quality Assessment
Synthetic Pollution Index (SPI)
The SPI is employed to classify drinking water into five categories: suitable for drinking (SPI < 0.2), slightly polluted water (SPI = 0.2–0.5), moderately polluted water SPI = 0.5–1.0), highly polluted water (SPI = 1.0–3.0), and unfit for drinking (SPI > 3.0) (Solangi et al. 2019; Egbueri and Unigwe 2020; Egbueri 2020). In this study, the SPI values varied from 0.19 to 1.28 with an average value of 0.47 (Table 3). Based on the SPI classification, 2% of the samples are classified as suitable for drinking, 74% of the samples are classified as slightly polluted with heavy metals while 24% of the samples are classified as moderately polluted (Table 4; Fig. 4b).
Water Quality Index (WQI)
The water quality index results are shown in Table 1. In the present study, the WQI varied from 7.27 to 89.44 with an average of 25.91 (Table 3). The WQI can be classified into five categories such as WQI < 25 indicate excellent water quality; 25–50 indicate good water quality; 50–75 indicate poor water quality; 75–100 indicate very poor water quality, and WQI > 100 indicate unsuitable water quality (Rostami et al 2019). The interpolated spatial distribution map of the study area (Fig. 4 b) depicts that 70% of the groundwater samples fall in the excellent water category, 9% of the samples belong to good water, 19% of the samples belong to poor water quality, and 2% of the samples are unsuitable for drinking purposes. It is shown that groundwater samples that fall in the poor and very poor water category are situated in the central, northern and southern parts of the study region.
Multivariate Statistical Analysis for Identification of Pollution Source
Correlation Matrix (CM)
Correlation matrix identifies the interrelationship among parameters and their possible sources in groundwater (Table 5). Correlation coefficients r > 0.7 is considered as strong, 0.7 > r > 0.5 as moderate and < 0.5 were considered as weak (Egbueri and Mgbenu 2020). The groundwater pH showed a moderate correlation with cadmium (r = 0.57), cobalt (r = 0.55), and HPI (r = 0.55). Iron showed a strong relationship with HEI, this suggests that iron has a key role in determining groundwater quality (Table 5). Lead showed significant correlation with chromium (r = 0.90), cadmium (r = 0.92), cobalt (r = 0.94), HPI (r = 0.96 0 and HEI (r = 0.84). A high concentration of lead and cadmium may be indicative of anthropogenic sources such as agrochemicals (Arslan and Ayyildiz Turan 2015). Chromium has shown strong correlation with cadmium (r = 0.89), cobalt (r = 0.94), HPI (r = 0.99), and HEI (r = 0.71). Furthermore, cadmium show a strong positive correlation with cobalt (r = 0.97), HPI (r = 0.99), and HEI (r = 0.86). Lead, chromium, cadmium and cobalt shows a significantly strong correlation with HPI and HEI. The correlation among heavy metal pollution indices reflected good relation between HPI and HEI (r = 0.87). This indicates that HPI and HEI can be used to evaluate the risk and contamination of heavy metals in the groundwater of this area.
Principal Component Analysis (PCA)
The principal component analysis identifies sources of heavy metals in groundwater. Varimax rotation was used with Kaiser normalization. Component loadings, Eigen values, percentage of variance and cumulative percentages of the identified principal components (PCs) are shown in Table 6. Figure 6a–c shows the spatial distribution maps of the principal components that were extracted. PCA revealed three principal components that accounted for 70.18% of the total variance. PC1 which explains 37.59% of the total variance has significant positive loadings of lead, chromium, cadmium and cobalt. The presence of these heavy metals in PC1 revealed the contribution of anthropogenic sources such as the application of agricultural fertilizers and pesticides, leaching or infiltration of domestic wastes and garden refuse from the Mbazwana landfill site (Mthembu et al. 2020). The highest values of PC1 are situated in the northern, central and southern parts of the study area, especially in samples BH12, BH15-16, BH26, BH47-53 (Fig. 6a). PC2 accounted for 19.65% of total variance with significant loadings of pH and cobalt, and moderate loading of zinc. This suggests that the groundwater pH is responsible for the release of cobalt and zinc into groundwater. Moderate PC2 values are distributed throughout the study area with the highest values seen at the central part of the study area at sample BH21 (Fig. 6b). The presence of zinc is owed to the use of agricultural fertilizers (Vetrimurugan et al. 2016; Rezaei et al. 2019). PC3 explains 12.9% of the total variance with a negative moderate loading of arsenic, strong positive loading of manganese and moderate loading of nickel. The negative loading of arsenic suggests a different source of origin. The strong and moderate loadings of manganese and nickel may be due to the weathering of manganese and nickel-bearing minerals. Landfill leachate may also contribute to the occurrence of manganese in the groundwater of this area. For PC3, the distributed loadings were high on the eastern and north-western part of the study area for samples BH27-28, BH51-52 (Fig. 6c). For better understanding, three principal components were overlaid into a single map using fuzzy overlay (Fig. 6d). The highest loadings are observed in the northern and central part of the study area with moderate values spread throughout the study area. This indicates that the samples in the northern and central parts of the study area are highly influenced by anthropogenic and weathering processes. This also corresponds with the high values of heavy metals observed in these locations (Fig. 2a–c).
Hierarchical Cluster Analysis (HCA)
The potential sources of pollutants in groundwater of this area were further investigated by carrying out cluster analysis. R-mode cluster analysis was carried out to evaluate the sources of heavy metals in groundwater of this area. R-mode cluster analysis revealed two groups of clusters (Fig. 7a). Cluster 1 consists of pH, chromium, cadmium, cobalt, arsenic, nickel and lead. This cluster indicates the influence of both geogenic (weathering of rock minerals) and anthropogenic sources (domestic wastes and agricultural fertilisers) in the study area. Cluster 2 comprises manganese, copper, iron and zinc and is due to the influences of geogenic and anthropogenic sources. Q-mode cluster analysis was also used to determine the similarities that exist between sampling points. Two groups of clusters were identified by Q-mode cluster analysis (Fig. 7b). Cluster 1 consisted of 46 sampling locations. The samples of this cluster are mainly located throughout the entire study area and its characteristics may be linked to the rocks found in this area such as arenite, sandstone, siltstone and alluvium. Furthermore, these sample locations are characterised by elevated average concentrations of iron, zinc, copper and manganese (Fig. 7b). These high levels are associated with PC2 and PC3, respectively. This cluster is associated with pollution by mixed sources i.e. weathering of soil or rock minerals and anthropogenic sources (agrochemicals). Based on average concentrations, pH, arsenic, nickel and copper were elevated in cluster 1 than in cluster 2. Cluster 2 included 7 sampling locations. Cluster 2 samples are spatially situated in the southern, central and north-western parts of the study area. This cluster is characterised by rocks such as arenite, sandstone and alluvium. These sampling locations have the highest concentrations of zinc, iron, manganese, and lead. These high levels correspond to PC1, PC2, and PC3, respectively. This cluster is also linked to mixed sources i.e. weathering of soil or rock minerals and anthropogenic sources. According to average values, iron, manganese, zinc, lead, chromium, cadmium and cobalt were greater in cluster 2 than those in cluster 1.
Health Risk Assessment
Health risk assessment evaluated the potential health risks of ingestion of trace metal contaminated groundwater in children and adults of the study region. Table 7 outlines the statistical summary for the HQ of the selected heavy metals. The spatial variation of HQ and HI of children and adults are shown in Fig. 8a, b. The mean values of HQ followed the following decreasing trend: Co>Pb>Cd>Zn>As>Mn>Cu>Cr>Fe>Ni for both children and adults (Table 7; Fig. 8a, b). The average HQ values suggest that Co has shown the highest of the total non-carcinogenic health risk in the study region. The HI values for groundwater samples varied from 0.06 to 8.16 for children and from 0.02 to 2.14 for adults, respectively (Fig. 8a, b). HI value for certain elements is greater suggesting non-carcinogenic health risk for ingestion. Likewise, an HI value below one implies that it is within the acceptable limit. In this study, it was discovered that 43% and 26% of groundwater samples pose a non-carcinogenic health risk in children and adults, respectively.
Conclusions
The present study addresses evaluating the extent of heavy metal contamination and their source of origin in groundwater of Maputaland coastal aquifer using indexed and statistical analysis. The following conclusions were made:
-
Groundwater is acidic to alkaline in nature. The dominance of heavy metals is in the order; Zn>Fe>Cu>Mn>Pb>Cr>Ni>Co>Cd>As. Of all the heavy metals, lead, cadmium and iron exceeded the WHO and SANS standard limits for drinking water in 25%, 23% and 11% of the samples.
-
The HPI varied from a minimum of 0.28 to a maximum of 128.08. Based on the HPI classification, the samples varied from low to high pollution class. About 21% of the samples have HPI values greater than the critical limit of 100, suggesting that they are critically contaminated with respect to heavy metals and are unfit for human consumption.
-
The SPI and WQI were used to evaluate the drinking water suitability. Based on SPI results, 2% of the samples were suitable while 74% and 24% were classified as slightly and moderately polluted with heavy metals, respectively.
-
The WQI revealed that 19% and 2% of the groundwater samples belong to the poor and very poor water quality category and are spatially situated on the central, northern and southern parts of the study region.
Multivariate statistical analysis of CM and PCA confirm the release of heavy metals into groundwater which is controlled by geogenic (weathering of rocks and minerals), anthropogenic sources (domestic wastes and agricultural fertilizers) and mixed sources including weathering of parent material and anthropogenic sources.
-
HQs is in the order: Co>Pb>Cd>Zn>As>Mn>Cu>Cr>Fe>Ni for both children and adult. The HI values varied from 0.06 to 8.16 for children and from 0.02 to 2.14 for adults. In this study, it was discovered that 43% of groundwater samples pose non-carcinogenic health risk in children while 26% of the samples posed health risks in adults.
-
Water in this region is moderately polluted with heavy metals, the residents are at risk of adverse health effects. Accordingly, since children's HQs and HIs are higher than adult’s, they are more likely to be at risk for non-carcinogenic health conditions.
-
This study could be useful to the local municipalities and water management authorities to monitor the water quality of the area before supply and consumption. The study also recommends that a proper mitigation plan should be enforced by the policy makers to ensure public health and to prevent further contamination of the aquifer.
Data Availability
This article includes all the data used in this study. Original data can be obtained on reasonable request.
References
Abou Zakhem B, Hafez R (2015) Heavy metal pollution index for groundwater quality assessment in Damascus Oasis, Syria. Environ Earth Sci 73(10):6591–6600. https://doi.org/10.1007/s12665-014-3882-5
Ahamad A, Raju NJ, Madhav S (2020) Trace elements contamination in groundwater and associated human health risk in the industrial region of southern Sonbhadra, Uttar Pradesh, India. Environ Geochem Health 42(10):3373–3391. https://doi.org/10.1007/s10653-020-00582-7
American Public Health Association (APHA) (2005) Standard methods for examination of water and wastewater, 21st edn. American Public Health Association, Washington
Arslan H, Ayyildiz Turan N (2015) Estimation of spatial distribution of heavy metals in groundwater using interpolation methods and multivariate statistical techniques; its suitability for drinking and irrigation purposes in the Middle Black Sea Region of Turkey. Environ Monit Assess. https://doi.org/10.1007/s10661-015-4725-x
Boateng TK, Opoku F, Acquaah SO, Akoto O (2015) Pollution evaluation, sources and risk assessment of heavy metals in hand—dug wells from Ejisu—Juaben Municipality, Ghana. Environ Syst Res. https://doi.org/10.1186/s40068-015-0045-y
Bodrud-Doza M, Islam ARMT, Ahmed F, Das S, Saha N, Rahman MS (2016) Characterization of groundwater quality using water evaluation indices, multivariate statistics and geostatistics in central Bangladesh. Water Sci 30(1):19–40. https://doi.org/10.1016/j.wsj.2016.05.001
Caboi R, Cidu R, Fanfani L, Lattanzi P, Zuddas P (1999) Environmental mineralogy and geochemistry of the abandoned Pb–Zn Montevecchio-Ingurtosu mining district, Sardinia, Italy. Chron Rech Min 534:21–28
Chanpiwat P, Lee BT, Kim KW, Sthiannopkao S (2014) Human health risk assessment for ingestion exposure to groundwater contaminated by naturally occurring mixtures of toxic heavy metals in the Lao PDR. Environ Monit Assess 186(8):4905–4923. https://doi.org/10.1007/s10661-014-3747-0
Church MR (1998) Acidic deposition: acidification of surface waters. Encyclope- dia of hydrology and lakes. Encyclopedia of earth science. Springer, Dordrecht. https://doi.org/10.1007/1-4020-4497-6_5
Çiner F, Daanoba E, Burak S, Şenbaş A (2020) Geochemical and multivariate statistical evaluation of trace elements in groundwater of Niğde Municipality, South - Central Turkey: implications for arsenic contamination and human health risks assessment. Arch Environ Contam Toxicol. https://doi.org/10.1007/s00244-020-00759-2
Demlie M, Hingston E, Mnisi Z (2014) A study of the sources, human health implications and low cost treatment options of iron rich groundwater in the northeastern coastal areas of KwaZulu-Natal, South Africa. J Geochem Explor 144:504–510. https://doi.org/10.1016/j.gexplo.2014.05.011
Duggal V, Rani A, Mehra R, Balaram V (2017) Risk assessment of metals from groundwater in northeast Rajasthan. J Geol Soc India 90(1):77–84
DWAF (2000) Policy and strategy for groundwater quality management in South Africa. Water quality management series. Department of Water Affairs and Forestry, Pretoria
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57(4):295–304. https://doi.org/10.1023/B:GEJO.0000007250.92458.de
Edokpayi JN, Enitan AM, Mutileni N, Odiyo JO (2018) Evaluation of water quality and human risk assessment due to heavy metals in groundwater around Muledane area of Vhembe District, Limpopo Province, South Africa. Chem Cent J 12(1):1–16. https://doi.org/10.1186/s13065-017-0369-y
Egbueri JC (2020) Groundwater quality assessment using pollution index of groundwater (PIG), ecological risk index (ERI) and hierarchical cluster analysis (HCA): a case study. Groundw Sustain Dev 10(October):100292. https://doi.org/10.1016/j.gsd.2019.100292
Egbueri JC, Mgbenu CN (2020) Chemometric analysis for pollution source identification and human health risk assessment of water resources in Ojoto Province, southeast Nigeria. Appl Water Sci 10:98. https://doi.org/10.1007/s13201-020-01180-9
Egbueri JC, Unigwe CO (2020) Understanding the extent of heavy metal pollution in drinking water supplies from Umunya, Nigeria: an indexical and statistical assessment. Anal Lett. https://doi.org/10.1080/00032719.2020.1731521
Ezugwu CK, Onwuka OS, Egbueri JC, Unigwe CO, Ayejoto DA (2019) Multi-criteria approach to water quality and health risk assessments in a rural agricultural province, southeast Nigeria. HydroResearch 2:40–48. https://doi.org/10.1016/j.hydres.2019.11.005
Ficklin DJWH, Plumee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. In: Kharaka YK, Maest AS (eds) Water–rock interaction, vol 7. Balkema, Rotterdam, pp 381–384
Giri S, Singh AK (2019) Assessment of metal pollution in groundwater using a novel multivariate metal pollution index in the mining areas of the Singhbhum copper belt. Environ Earth Sci 78(6):1–11. https://doi.org/10.1007/s12665-019-8200-9
Haloi N, Sarma HP (2012) Heavy metal contaminations in the groundwater of Brahmaputra flood plain: an assessment of water quality in Barpeta District, Assam (India). Environ Monit Assess 184(10):6229–6237. https://doi.org/10.1007/s10661-011-2415-x
He S, Wu J (2019) Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, northwest China. Expo Health 11(2):125–137. https://doi.org/10.1007/s12403-018-0289-7
Horton R (1965) An index number system for rating water quality. J Water Pollut Control Fed 37:300–306.
Islam F, Zakir HM, Rahman A, Sharmin S (2020) Impact of industrial wastewater irrigation on heavy metal deposition in farm soils of Bhaluka area, Bangladesh. J Geogr Environ Earth Sci Int 24(3):19–31. https://doi.org/10.9734/JGEESI/2020/v24i330207
Kelbe BE, Grundling AT, Price JS (2016) Modelling water-table depth in a primary aquifer to identify potential wetland hydrogeomorphic settings on the northern Maputaland Coastal Plain, KwaZulu-Natal, South Africa. Hydrogeol J 24:249–265
Li P, Qian H, Howard KWF, Wu J (2015) Heavy metal contamination of Yellow River alluvial sediments, northwest China. Environ Earth Sci 73(7):3403–3415. https://doi.org/10.1007/s12665-014-3628-4
Li P, Wu J, Qian H, Zhou W (2016) Distribution, enrichment and sources of trace metals in the topsoil in the vicinity of a steel wire plant along the Silk Road economic belt, northwest China. Environ Earth Sci 75(10):909. https://doi.org/10.1007/s12665-016-5719-x
Li P, He X, Guo W (2019) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an City on the Loess Plateau of northwest China. Hum Ecol Risk Assess 25(1–2):11–31. https://doi.org/10.1080/10807039.2018.1553612
Meyer R, Talma AS, Duvenhage AWA, Eglington BM, Taljaard J, Botha JP, Verwey J, van der Voort I (2001) Geohydrological Investigation and evaluation of the Zululand coastal aquifer. WRC Report No. 221/1/1.Pretoria. WRC
Mgbenu CN, Egbueri JC (2019) The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Appl Water Sci 9:22. https://doi.org/10.1007/s13201-019-0900-5
Mkhwanazi MN (2010) Establishment of the relationship between the sediments mineral composition and groundwater quality of the primary aquifers in the Maputaland coastal plain. Dissertation, University of Zululand
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A31:283–289
Mthembu PP, Elumalai V, Brindha K, Li P (2020) Hydrogeochemical processes and trace metal contamination in groundwater: impact on human health in the Maputaland coastal aquifer, South Africa. Expo Health. https://doi.org/10.1007/s12403-020-00369-2
Ndlovu MS, Demlie M (2018) Statistical analysis of groundwater level variability across KwaZulu-Natal Province. S Afr Environ Earth Sci 77(21):1–15. https://doi.org/10.1007/s12665-018-7929-x
Ndlovu M (2015) Hydrogeological conceptual modelling of the Kosi Bay lake system, North eastern South Africa. Dissertation, University of KwaZuluNatal
Nel J, Xu Y, Batelaan O, Brendonck L (2009) Benefit and implementation of groundwater protection zoning in South Africa. Water Resour Manag 23(14):2895–2911. https://doi.org/10.1007/s11269-009-9415-4
Porat N, Botha G (2008) The luminescence chronology of dune development on the Maputaland coastal plain, southeast Africa. Quatern Sci Rev 27:1024–1046
Prasad B, Bose JM (2001) Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41(1–2):183–188. https://doi.org/10.1007/s002540100380
Prasanna MV, Praveena SM, Chidambaram S, Nagarajan R, Elayaraja A (2012) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci 67(7):1987–2001. https://doi.org/10.1007/s12665-012-1639-6
Ramsay PJ (1996) 9000 Years of sea-level change along the southern African coastline. Quatern Int 31(1989):71–75. https://doi.org/10.1016/1040-6182(95)00040-P
Ren X, Li P, He X, Su F, Elumalai V (2021) Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch Environ Contam Toxicol 80(1):74–91. https://doi.org/10.1007/s00244-020-00772-5
Rezaei A, Hassani H, Jabbari N (2017) Evaluation of groundwater quality and assessment of pollution indices for heavy metals in North of Isfahan Province, Iran. Sustain Water Resour Manag. https://doi.org/10.1007/s40899-017-0209-1
Rezaei A, Hassani H, Hassani S, Jabbari N, Mousavi SBF, Rezaei S (2019) Evaluation of groundwater quality and heavy metal pollution indices in Bazman basin southeastern Iran. Groundwater Sustain Dev 9:100245. https://doi.org/10.1016/j.gsd.2019.100245
Rostami AA, Isazadeh M, Shahabi M, Nozari H (2019) Evaluation of geostatistical techniques and their hybrid in modelling of groundwater quality index in the Marand Plain in Iran. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-019-06591-z
SANS 241-2015. South Africa National Standard 2015. Drinking wate; part 1: Microbiological, physical, aesthetic and chemical determinands; part 2: application of SANS 241-1. Available online: https://www.mwa.co.th/download/prd01/iDW_standard/South_African_Water_Standard_SANS_241-2015.pdf. Accessed 31 Oct 2019.
Şener Ş, Şener E, Davraz A (2017a) Assessment of groundwater quality and health risk in drinking water basin using GIS. J Water Health 15(1):112–132. https://doi.org/10.2166/wh.2016.148
Şener Ş, Şener E, Davraz A (2017b) Evaluation of water quality using water quality index (WQI) method and GIS in Aksu River (SW-Turkey). Sci Tot Environ 584–585:131–144. https://doi.org/10.1016/j.scitotenv.2017.01.102
Singh G, Kamal RK (2016) Heavy metal contamination and its indexing approach for groundwater of Goa mining region, India. Appl Water Sci 7(3):1479–1485. https://doi.org/10.1007/s13201-016-0430-3
Solangi GS, Siyal AA, Babar MM, Siyal P (2019) Groundwater quality evaluation using the water quality index (WQI), the synthetic pollution index (SPI), and geospatial tools: a case study of Sujawal district, Pakistan. Hum Ecol Risk Assess. https://doi.org/10.1080/10807039.2019.1588099
Subba Rao N (2012) PIG: a numerical index for dissemination of groundwater contamination zones. Hydrol Process 26(22):3344–3350. https://doi.org/10.1002/hyp.8456
Sunkari ED, Abu M, Zango MS (2021) Geochemical evolution and tracing of groundwater salinization using different ionic ratios, multivariate statistical and geochemical modeling approaches in a typical semi-arid basin. J Contam Hydrol 236:103742. https://doi.org/10.1016/j.jconhyd.2020.103742
Tiwari AK, De Maio M, Amanzio G (2017) Evaluation of metal contamination in the groundwater of the Aosta Valley Region, Italy. Int J Environ Res 11(3):291–300. https://doi.org/10.1007/s41742-017-0027-1
Ukah BU, Egbueri JC, Unigwe CO, Ubido OE (2019) Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. Int J Energy Water Resour 3(4):291–303. https://doi.org/10.1007/s42108-019-00039-3
US-EPA (US Environmental Protection Agency) (1989) Risk assessment guidance for superfund, vol. 1, Human health evaluation manual (Part A) Office of Emergency and Remedial Response, Washington, DC.
Verlicchi P, Grillini V (2020) Surfacewater and groundwater quality in South Africa and Mozambique-analysis of the most critical pollutants for drinking purposes and challenges in water treatment selection. Water (switzerland). https://doi.org/10.3390/w12010305
Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM (2016) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci. https://doi.org/10.1007/s13201-016-0472-6
Vetrimurugan E, Brindha K, Elango L (2017) Human exposure risk assessment due to heavy metals in groundwater by pollution index and multivariate statistical methods: an application from South Africa. Water 9:234
Vetrimurugan E, Shruti VC, Jonathan MP, Roy PD, Rawlins BK, Rivera-Rivera DM (2018) Metals and their ecological impact on beach sediments near the marine protected sites of Sodwana Bay and St. Lucia, South Africa. Mar Pollut Bull 127(December 2017):568–575. https://doi.org/10.1016/j.marpolbul.2017.12.044
Vhonani GN, Vetrimurugan E, Rajmohan N (2018) Irrigation return flow induced mineral weathering and ion exchange reactions in the aquifer, Luvuvhu catchment, South Africa. J Afr Earth Sci 149:517–528. https://doi.org/10.1016/j.jafrearsci.2018.09.001
Wagh VM, Panaskar DB, Mukate SV, Gaikwad SK, Muley AA, Varade AM (2018) Health risk assessment of heavy metal contamination in groundwater of Kadava River Basin, Nashik, India. Model Earth Syst Environ 4:969–980
Wang D, Wu J, Wang Y, Ji Y (2020) Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: analysis, assessment, and management. Expo Health 12(2):307–322. https://doi.org/10.1007/s12403-019-00314-y
Wang L, Li P, Duan R, He X (2021) Occurrence, controlling factors and health risks of Cr6+ in groundwater in the Guanzhong Basin of China. Expo Health. https://doi.org/10.1007/s12403-021-00410-y
Watkeys MK, Mason TR, Goodman PS (1993) The rôle of geology in the development of Maputaland, South Africa. J Afr Earth Sci 16:205–221
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Wu J, Li P, Wang D, Ren X, Wei M (2020) Statistical and multivariate statistical techniques to trace the sources and affecting factors of groundwater pollution in a rapidly growing city on the Chinese Loess Plateau. Hum Ecol Risk Assess 26(6):1603–1621. https://doi.org/10.1080/10807039.2019.1594156
Xiao J, Wang L, Deng L, Jin Z (2019) Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess Plateau. Sci Total Environ 650:2004–2012. https://doi.org/10.1016/j.scitotenv.2018.09.322
Acknowledgements
Authors from the University of Zululand express their gratitude to National Research Foundation (NRF), South Africa (NRF/NSFC Reference: NSFC170331225349 Grant No: 110773) for providing grants and Department of Research and Innovation, the University of Zululand for support in buying Ion Chromatography instrument for this research. Dr. Peiyue Li is grateful for the financial support granted by the National Natural Science Foundation of China (41761144059).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical Approval
All authors agree that they do not have any affiliation with any organization or institute in regard to financial and/or other interests.
Life Science Reporting
This research did not involve any life science threat.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mthembu, P.P., Elumalai, V., Li, P. et al. Integration of Heavy Metal Pollution Indices and Health Risk Assessment of Groundwater in Semi-arid Coastal Aquifers, South Africa. Expo Health 14, 487–502 (2022). https://doi.org/10.1007/s12403-022-00478-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-022-00478-0