Abstract
A study was conducted to evaluate the heavy metal contamination status of groundwater in Brahmaputra flood plain Barpeta District, Assam, India. The Brahmaputra River flows from the southern part of the district and its many tributaries flow from north to south. Cd, Fe, Mn, Pb, and Zn are estimated by using atomic absorption spectrometer, Perkin Elmer AA 200. The quantity of heavy metals in drinking water should be checked time to time; as heavy metal accumulation will cause numerous problems to living being. Forty groundwater samples were collected mainly from tube wells from the flood plain area. As there is very little information available about the heavy metal contamination status in the heavily populated study area, the present work will help to be acquainted with the suitability of groundwater for drinking applications as well as it will enhance the database. The concentration of iron exceeds the WHO recommended levels of 0.3 mg/L in about 80% of the samples, manganese values exceed 0.4 mg/L in about 22.5% of the samples, and lead values also exceed limit in 22.5% of the samples. Cd is reported in only four sampling locations and three of them exceed the WHO permissible limit (0.003 mg/L). Zinc concentrations were found to be within the prescribed WHO limits. Therefore, pressing awareness is needed for the betterment of water quality; for the sake of safe drinking water. Statistical analysis of the data was carried out using Special Package for Social Sciences (SPSS 16).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The ability of water body to support aquatic life, as well as its suitability for other uses depends on many trace elements. Some metals present in trace quantity are important for life as it helps and regulates many physiological function of the body. The same metal, however, can cause severe toxicological effects on human health and the aquatic ecosystem. For example metals like Cu, iron (Fe), manganese (Mn), Ni, and Zn are essential as micronutrients for life processes in plants and microorganisms while many other metals like Cd, Cr, and Pb have no known physiological activity, they are proved detrimental beyond a certain limit (Marschner 1995; Bruins et al. 2000). Water, the most important natural resource in the world, has the unique property of dissolving and carrying in suspension a huge variety of chemicals and hence water can easily become contaminated (Tiwari and Ali 1988). Water pollution by heavy metals resulting from anthropogenic impact is causing serious ecological problems in many parts of the world. This situation is aggravated by the lack of natural elimination process of metals. The toxic effect of metal depends on its characteristics. They become toxic when complexes are form with organic compound (Akbulut and Tuncer 2011). The chemical composition of groundwater is a measure of its suitability as a source of water for human and animal consumption, irrigation, and for industrial and other purposes. Climate change and urban and industrial development cause severe threat to drinking water integrity as well as sustainability. National and international collaboration is necessary for long-term approaches (Watson and Lawrence 2003). The definition of water quality is therefore not objective, but is socially defined depending on the desired use of water (Babiker et al. 2007). Contamination of groundwater by heavy metals has been given much attention due to their low biodegradability and toxic effects (Numberg 1982; Ramesh et al. 1995). Khalil et al. (2008) reported heavy metal content and their toxicity in soil runoffs and groundwater collected from two mining areas in the region of Marrakech in southern Morocco. High copper and zinc concentrations confirm the acute toxicity shown by MetPLATE™ bioassay in the study. Yayıntas et al. (2007) studied heavy metals and major elements in the wastewater of Kocabas Stream and it is reported that Cr is high in concentration originating from tannery waste. Aktar et al. (2010) in their study found that the surface water of Ganga River around Kolkata in West Bengal is rich in essential micronutrients (Mn, Zn, and Cu) at various locations. The concentration of those metals varied with season, being higher in rainy and lower in winter season. Chakrabarty and Sarma (2011) have reported that a good number of the drinking water sources of Kamrup District of Assam, India were contaminated with cadmium, manganese, and lead. The database of Barpeta District is very poor and very little information is available about the status of heavy metal contamination. The main objectives of this study were to find out the level of selected heavy metals in groundwater from Brahmaputra flood plain in Barpeta District, Assam, India.
Experimental
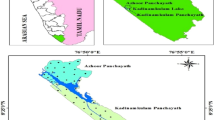
The study area Barpeta (Fig. 1) is situated in the northern bank of River Brahmaputra. Barpeta is renowned in the historical map of Assam as the “Land of the Satras”. The district covers an area of 2,645 km2. The geographical location of the district is between north latitudes of 26′5″ to 26′49″ and east longitude of 90′39″ to 91′17″. Barpeta District falls under Lower Brahmaputra valley zone. The northern part of the district is elevated and covered by forests and tall grass, while the southern part is close to the northern bank of the Brahmaputra low lying flood plain zone. Some hills also break the riverine area just north of the river. The total population of the district is 1,642,420 and ranks fourth in overall ranking among the district population size in Assam. The district has soil cover of younger and older alluvial soil which has undergone diversified pedagogical changes. For the present study, 40 water samples were collected randomly from the floodplain area during winter and pre-monsoon season (November 2008 to April 2009). The samples were collected in 1-L polythene cans after flushing out the tube wells (minimum 10 min) to get the fresh groundwater and compiled together in clean and sterile 1-L polythene cans rinsed with dilute HCl. Analysis of cadmium, iron, lead, manganese, and zinc was done using atomic absorption spectrophotometer (Perkin Elmer AA200) with flow injection analyzing mercury hydride generation system (Model FIAS-100) at 228.80-, 248.33-, 283.31-, 279.48-, and 213.86-nm analytical wavelengths, respectively. The minimum detection limit of AAS is 0.002, 0.005, 0.01, 0.02, and 0.005 mg/L for cadmium, iron, manganese, lead, and zinc, respectively. For the digestion and pre-concentration of the samples, standard methods (APHA 1998) were followed. Nitric acid digestion procedure is used for the preparation of samples. The instrument was used in the limit of precise accuracy and all chemicals and reagents were analytical grade. Double-distilled water was used for all purposes. Different samplings locations of the study are shown in Table 1 and descriptive statistics in the forms of mean, variance, standard deviation, median, range of variation, skewness, and kurtosis are calculated and summarized in tabular forms (Table 2). SPSS® statistical package (Windows 134 version 10.0) was used for data analysis.
Results and discussion
The quality of the groundwater and status of metal pollution were evaluated on the basis of the quality standards as suggested by the guidelines of WHO. Physiographically the major part of the district forms the part of vast alluvial stretch of Brahmaputra River stretching in east–west direction and its northern parts extends up to the foothills of the Bhutan Himalayas. The alluvium deposits are product of interaction of Brahmaputra River and its tributaries. The alluvial plan is made up of boulders, pebbles, cobbles, sand, silt, and clay which are distributed in an unsorted manner. Groundwater in the alluvium formation occurs under semi-confined condition in deeper horizons. The average depth of the water table varies from 2 to 4 m in flood plain areas. The water of the region is mostly alkaline in nature (CGWB 2008). According to the results and findings of the study, the iron concentration is found to be high in most of the samples as they exceed the WHO guideline value of 0.3 mg/L. Singh (2004) found that the amount of iron in Assam was relatively high and above the permissible level in drinking water. The concentration of iron in the study area is not suitable for food processing, dyeing, bleaching, and many activities. Heavier amount of iron can cause serious health problems or premature death. Toxicity of iron may damage the liver, heart, and endocrine glands, leading to debilitating and life-threatening problems such as diabetes, heart failure, and poor growth (Nduka and Orisakwe 2010). Various studies show that the amount of iron in northeastern region is relatively high and almost all states contain iron above the permissible limit. Figure 2 shows range of iron concentration (in milligrams per liter) in the sampling locations. Iron and manganese are commonly found in gravels and rocks principally in an insoluble form. The higher concentration of iron in tube well water may be due to soil origin and used older iron pipes. In a report of Assam Science Technology and Environmental Council, it is also mentioned that the distribution of iron in the shallow groundwater shows high concentration of iron along the northern bank of the Brahmaputra River in Assam. Nine samples of the study area are found to be contaminated by Pb as they exceed WHO permissible limit. Lead is the most significant of all the heavy metals because it is both very toxic and very common. Large difference between mean and median, significant positive skewness and kurtosis value depicts that the distribution of lead in the study area is asymmetric. Groundwater may be contaminated by lead due to enlistment either natural or enriched from anthropogenic activities in the soil and, in some cases, mineral weathering (Buragohain et al. 2010). As Pb-containing minerals are less soluble in water, its concentration is generally low in natural water (Venugopal et al. 2009). Chemical fertilizer used in agricultural field may cause Pb pollution as agriculture is the mainstay of a large majority of the population of Barpeta District but the potential sources of lead in this region are yet to be identified. Manganese in water can promote the growth of iron bacteria, a group of organisms that obtains its energy for growth from the chemical reaction that occurs when manganese mixes with dissolved oxygen. The range of manganese concentration (in milligrams per liter) in the study area is shown in Fig. 3. The studied locations are polluted in terms of manganese concentration as few are crossed and few are approaching WHO guideline value. Manganese values exceed 0.4 mg/L in about 22.5% of the samples. There also exist positive correlation between iron and manganese. Fe and Mn are metals that occur naturally in soils, rocks, and minerals. In the aquifer, groundwater comes in contact with these solid materials dissolving them, releasing their constituents, including Fe and Mn, to the water.
Cadmium occurs mostly in association with zinc and gets into water from corrosion of zinc-coated (“galvanized”) pipes and fittings. Cd is reported in only four sampling locations and three of them exceed the WHO permissible limit (0.003 mg/L). In natural surface waters, the concentration of zinc is usually below 10 μg/L and in groundwaters, 10–40 μg/L (Elinder 1986). In tap water, the zinc concentration can be much higher as a result of the leaching of zinc from piping and fittings (Nriagu 1980). All the water samples in the present study fall below the current standard for zinc, which is 4 ppm (WHO 2004). Positive kurtosis and skewness value pointed towards sharp zinc distribution with a long right tail in the study area. The intensity of metal contamination of groundwater in the district follows the trend Fe > Mn > Pb > Zn > Cd while compared with the mean value. Positive skewness of the studied parameter indicates the distribution with an asymmetric tail extending towards more positive value.
The information of relationship of the measured variables are unknown, therefore one-tailed test of significance was carried out. Pearson correlation analysis showed (Table 3) that the parameters of the analyzed samples were weakly and moderately correlated to each other at p < 0.05 level. A significant positive correlation was found to exist between Zn and Cd (0.302) and Pb (0.292) signifying their similar source of geogenic origin and mobility. It is also observed that some of the water quality parameters are negatively correlated. A correlation between Fe and Mn (0.200) was also observed in the collected water samples of the study region.
Conclusion
The assessment of groundwater metal toxicity, sampled from selected locations, shows that some samples are contaminated and this contamination is attributed to its elevated concentration of Fe, Mn, and Pb. Different statistical estimations, viz. standard deviation, variance, skewness, and kurtosis, performed for each parameter indicate that their distribution in the study area is widely off normal with a long asymmetric tail either on the right or left side of the median. All the water samples analyzed in the present investigation are contaminated with iron and partially with manganese, lead, zinc, and cadmium. Therefore, the water should be subjected to suitable chemical or biological treatments especially for keeping Fe and Mn within the prescribed safe levels. The sampling locations which have higher metal concentration, i.e., above the WHO permissible limit, can be used to search for the source of pollution, for planning prevention measures, and to prevent pollution. The present study provides the baseline data for the assessment of metal contamination in the flood plain area of Barpeta District, Assam. Periodical monitoring should be carried out to check the rise in metal concentrations of groundwater.
References
Akbulut, N. E., & Tuncer, A. M. (2011). Accumulation of heavy metals with water quality parameters in Kızılırmak River Basin (Delice River) in Turkey. Environmental Monitoring and Assessment, 173, 387–395. doi:10.1007/s10661-010-1394-7.
Aktar, Md. W., Paramasivam, M., Ganguly, M., Purkait, S., & Sengupta, D. (2010). Assessment and occurrence of various heavy metals in surface water of Ganga River around Kolkata: a study for toxicity and ecological impact. Environmental Monitoring and Assessment, 160, 207–213. doi:10.1007/s10661-008-0688-5.
APHA (American Public Health Association) (1998). Standard method for examination of water and wastewater (20th ed.). Washington, DC: APHA.
Babiker, I. S., Mohamed, M. A. A., & Hiyama, T. (2007). Assessing groundwater quality using GIS. Water Resour Manage, 21, 699–715. doi:10.1007/s11269-006-9059-6.
Bruins, M. R., Kapil, S., & Oehme, F. W. (2000). Microbial resistance to metals in the environment. Ecotoxicology and Environmental Safety, 45, 198–207. doi:10.1006/eesa.1999.1860.
Buragohain, M., Bhuyan, B., & Sarma, H. P. (2010). Seasonal variations of lead, arsenic, cadmium and aluminium contamination of groundwater in Dhemaji district, Assam, India. Environmental Monitoring and Assessment, 170(1–4), 345–351. doi:10.1007/s10661-s10661-009-1237-6.
CGWB. (2008). Ground water information booklet Barpeta district, Assam, CGWB. North Eastern region, Ministry of Water Resource, Govt. of India. Guwahati: Central Ground Water Board
Chakrabarty, S., & Sarma, H. P. (2011). Heavy metal contamination of drinking water in Kamrup district, Assam, India. Environmental Monitoring and Assessment, 179, 479–486. doi:10.1007/s10661-010-1750-7.
El Khalil, H., El Hamiani, O., Bitton, G., Ouazzani, N., & Boularbah. (2008). Heavy metal contamination from mining sites in South Morocco: monitoring metal content and toxicity of soil runoff and groundwater. Environmental Monitoring and Assessment, 136, 147–160. doi:10.1007/s10661-007-9671-9.
Elinder, C. G. (1986). Zinc. In L. Friberg, G. F. Nordberg, & V. B. Vouk (Eds.), Handbook on the toxicology of metals (2nd ed., pp. 664–679). Amsterdam: Elsevier.
Marschner, H. (1995). Mineral nutrition of higher plants. London: Academic Press.
Nduka, J. K., & Orisakwe, O. E. (2010). Water-quality issues in the Niger Delta of Nigeria: a look at heavy metal levels and some physicochemical properties. Environmental Science and Pollution Research. doi:10.1007/s11356-010-0366-3.
Nriagu, J. O. (Ed.). (1980). Zinc in the environment. Part I, ecological cycling. New York: Wiley.
Numberg, H. W. (1982). Yoltametric trace analysis in ecological chemistry of toxic metals. Pure and Applied Chemistry, 54(4), 853–878.
Ramesh, R., Shiv Kumar, K., Eswaramoorthi, S., & Purvaja, G. R. (1995). Migration and contamination of major and trace elements in ground water of Madras city, India. Environmental Geology, 25, 126–136.
Singh, A. K. (2004). Arsenic contamination in groundwater of North Eastern India. Roorkee: Proceedings of national seminar on hydrology held at National Institute of Hydrology.
Tiwari, T. N., & Ali, M. (1988). Water quality index for Indian rivers. In R. K. Trivedy (Ed.), Ecology and pollution of Indian rivers (1st ed., pp. 271–286). New Delhi: Ashish.
Venugopal, T., Giridharan, L., Jayaprakash, M., & Periakali, P. (2009). Environmental impact assessment and seasonal variation study of the groundwater in the vicinity of River Adyar, Chennai, India. Environmental Monitoring and Assessment, 149, 81–97. doi:10.1007/s10661-008-0185-x.
Watson, S. B., & Lawrence, J. (2003). Drinking water quality and sustainability. Water Quality Research Journal of Canada, 38(1), 3–13.
WHO. (2004). Guidelines for drinking water quality (3rd ed.). Geneva: World Health Organization.
Yayıntas, O. T., Yılmaz, S., Turkogluv, M., & Dilgin, Y. (2007). Determination of heavy metal pollution with environmental physicochemical parameters in waste water of Kocabas Stream (Biga, Canakkale, Turkey) by ICP-AES. Environmental Monitoring and Assessment, 127, 389–397. doi:10.1007/s10661-006-9288-4.
Acknowledgments
Nabanita Haloi is thankful to the University Grants Commission, New Delhi for financial assistance in the form of Rajib Gandhi National Fellowship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haloi, N., Sarma, H.P. Heavy metal contaminations in the groundwater of Brahmaputra flood plain: an assessment of water quality in Barpeta District, Assam (India). Environ Monit Assess 184, 6229–6237 (2012). https://doi.org/10.1007/s10661-011-2415-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-011-2415-x