Abstract
Recently, high levels of Cr6+ in groundwater have been found and are threatening public health in the Guanzhong Basin of China. For this reason, this study aims to specify the occurrence and spatial distribution of groundwater Cr6+ and to analyze the favorable hydrogeochemical environment elevating its concentration in the Guanzhong Basin. The impacts of Cr6+ on human health were also estimated based on the health risk assessment model recommended by the USEPA. Results show that 45.40% and 37.36% of the groundwater samples contain Cr6+ concentration lower than 10 μg/L and ranging within 11–50 μg/L, respectively. And they are predominantly of HCO3-Ca and HCO3-Ca(Mg) water type. About 17.24% of the total water samples present Cr6+ concentrations exceeding the acceptable limit for drinking purpose of 50 µg/L, and are mainly classified as HCO3-Na water type. Low Cr6+ groundwater is mainly observed in the alluvial aquifer. Cr6+ concentration in the samples from the loess aquifer is higher due to low groundwater velocity caused by the low permeability of loess, as verified by the relationship between Cr6+ and major ions. The relationship between Cr6+ and pH and molar ratio of Na+/(Na+ + Ca2+) suggest that alkaline environment and cation exchange are beneficial to high concentration of Cr6+ in groundwater. Industrial activities are also responsible for the elevation of Cr6+ to some extent. Health risk assessment results show that the adults and children in the study area face higher carcinogenic risks than non-carcinogenic risk induced by Cr6+.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is the primary source of drinking water for approximately 2 billion humans (Gleeson et al. 2010) and supports economic development and ecological environment (Li et al. 2021; Wang et al. 2020; Su et al. 2020). However, heavy metal pollution of groundwater and sediments is seriously affecting the sustainable development of groundwater resources (Kumar et al. 2020; Zhao et al. 2021; Li et al. 2015, 2016; Khan et al. 2020, 2021). Many studies have reported that Cr6+ concentration in groundwater exceeded the acceptable limit of 50 μg/L recommended by the World Health Organization for drinking purpose (Ball and Izbicki 2004; Bourotte et al. 2009; Coyte et al. 2020; He and Li 2020a; Kazakis et al. 2017; Manning et al. 2015; Mills et al. 2011; Vengosh et al. 2016). Chromium in natural groundwater systems is primarily present in two oxidation states, trivalent chromium (Cr3+) and hexavalent chromium (Cr6+). The speciation of chromium depends on pH and redox conditions of groundwater (Rajapaksha et al. 2013; Richard and Bourg 1991). Under alkaline and mild oxidization conditions, Cr6+ will prevail in groundwater, while Cr3+ will predominate under acidic and reduction conditions (Bourotte et al. 2009). Compared to Cr3+, Cr6+ is more water soluble and is comparatively more mobile in groundwater (Coyte et al. 2020; Tseng et al. 2018). In addition, Cr3+ is a micronutrient with a relatively low toxicity, but Cr6+ is a known carcinogen. The intake of high levels of Cr6+ can cause various types of cancer and DNA damage in humans, and this has been reported in a number of studies (Linos et al. 2011; Tseng et al. 2018).
Because of the high toxicity of Cr6+, the occurrence and sources of Cr6+ as well as associated health risks in groundwater have attracted worldwide attention (He and Li 2020a). The occurrence of Cr6+ in groundwater largely depends on the geological background, especially those containing chromium such as ultramafic rocks and serpentinites of ophiolite complexes (Bourotte et al. 2009; Coyte et al. 2020; Equeenuddin and Pattnaik 2020; Kazakis et al. 2015; Lelli et al. 2014; Mills et al. 2011; Oze et al. 2007; Vengosh et al. 2016). The prevailing hydrogeological environment and geochemical processes may influence its concentration, such as pH (Oze et al. 2007), redox environment (Hausladen et al. 2018; Liang et al. 2021), vadose processes (Kazakis et al. 2015; Manning et al. 2015), and cation exchange (Bertolo et al. 2011; Mills et al. 2011). High levels of Cr6+ associated with natural sources are not common in a local region. Therefore, Cr6+ in groundwater can also be associated with various anthropogenic activities, such as coal combustion, fly ash deposits (Kazakis et al. 2017), paint manufacturing (Hausladen et al. 2018), mining activities (Kumari et al. 2017), leather tanning, phosphate fertilizer manufacturing (Molina et al. 2009; Vasileiou et al. 2019), as well as the overexploitation of groundwater (Gu et al. 2015; Testa et al. 2004).
The Guanzhong Basin lies in the middle of Shaanxi Province, being an important part of the Yellow River Basin. It belongs to the semi-humid and semiarid zone. It is also the most densely populated area and the most important agricultural area in northwest China (Deng et al. 2021; Ren et al. 2021). Limited surface water makes groundwater the main source of drinking water or other domestic purposes in this area, especially in rural and isolated urban areas. Groundwater polluted by nitrogen, fluoride and arsenic has been widely reported for a long time in the Guanzhong Basin and drawn a great deal of scholars’ attention (Li et al. 2014; Luo et al. 2014; Wu and Sun 2016; Zhang et al. 2018, 2019; Zhu et al. 2006). However, high concentration of Cr6+ has also been found in the groundwater of Guanzhong Basin in recent years (Dong et al. 2018; Lei et al. 2019; Qiao et al. 2020). There are also many areas near the Guanzhong Basin that are polluted by Cr6+, such as Yuncheng and Hejin in the west of Shanxi Province, Yan’an, Yulin in the northern of Shaanxi (Li 2006; Su et al. 2017; Tian and Wu 2019; Zhang 2012). According to the report, about 4% of the rural drinking water had concentrations of Cr6+ exceeding the acceptable level (50 μg/L) in the Guanzhong Basin, third only after fluoride and nitrogen, and even higher than arsenic (Chang et al. 2019). The rate of Cr6+ exceeding standard limit of groundwater (5.83%) was higher than that of surface water (0.27%) (Lei et al. 2019). Further, the studies on Cr6+ contamination of groundwater in the Guanzhong Basin are limited, and the distribution and occurrence of Cr6+ as well as associated influences on human health are not well understood. Accordingly, the main objectives of this study are (1) to delineate the occurrence and spatial distribution of groundwater Cr6+ in the Guanzhong Basin, (2) to analyze the controlling factors influencing the concentration of groundwater Cr6+, and (3) to estimate the health risks caused by Cr6+. The results of this study may provide a scientific guidance for improving local groundwater quality to reduce the risk of Cr6+ exposure and support ecological protection and high-quality development in the Yellow River basin.
Study Area
Location and Geography
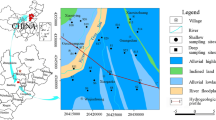
The Guanzhong Basin is located between longitude E107°30′–110°30′ and latitude N34°00′–35°40′. The study area covers 1.9 × 10–4 km2 and constitutes a basin surrounded on three sides by mountain ranges and open to the east. The Wei River flows across the basin from west to east and then empties into the Yellow River (Fig. 1). The occurrence of floods, loess accumulation and the Wei River flowing through this area supplies the geomorphology of the study area. From the north and south piedmont to the center of the basin, the landform types evolve stepwise from a piedmont pluvial plain into a loess tableland and a valley terrace. The general terrain of the study area is high in the west and low in the east, the altitude varies from 325 to 800 m. The climate in the study area is governed by the temperate semiarid and semi-humid monsoon climate characterized with a cold and dry winter and a hot and wet summer. The annual precipitation is 530–700 mm, where about 45% of the precipitation concentrates in July to September. The annual temperature and evaporation are 12–13.6 °C and 1000–1200 mm, respectively.
Geological and Hydrological Settings
Geologically, the Guanzhong Basin is a Cenozoic fault basin formed by Himalayan movement. The basin subsided continuously and accepted deposition during the Late Eocene to Pliocene epochs, thereby accumulating very thick Tertiary fluvial–lacustrine facies in clastic rock. Deposition continued during the Quaternary, a thick layer of unconsolidated sediments overlay the Tertiary rocks. The Quaternary sediments of the basin are mainly lacustrine and alluvial deposits in the central part of the basin, and interbedded with alluvial, diluvial and loess deposits in the marginal areas.
The Guanzhong Basin is a relatively closed basin, which makes it an independent hydrogeological unit. Groundwater is widely distributed in the study area. According to geomorphology and hydrogeological conditions, aquifers in the Guanzhong Basin can be mainly divided into three types of which the first is the alluvial aquifer comprised by sand and sandy gravel. The second is the pluvial aquifer composed of sand, sandy gravel and boulder. The third group is the aeolian loess aquifer formed by loess layers (Fig. 3). The flow direction of groundwater is basically the same as the topography, that is, it flows from the southern and northern margins to the central areas of the basin, from the upper reaches to the lower reaches of the valley, and finally is discharged into rivers and valleys. The groundwater is mainly recharged by rainfall, river leakage, and irrigation infiltration. Groundwater discharge mainly occurs by artificial abstraction, evaporation, and discharging toward the river.
Materials and Methods
Datasets
For this study, the data of groundwater in the Guanzhong Basin were obtained from National Geological Archives of China (Li et al. 2018a). Sample collection and treatment methods can be seen in the paper published by Li et al. (2018a). Conductivity and pH were directly measured in the field using LOVIBOND multiparameter measuring instrument (SD150D) and geographical location names, latitude and longitude were recorded. Simultaneously, two 500 mL water samples were collected and sealed in polyethylene plastic bottles and then sent to the Experimental Testing Center of Xi’an Institute of Geology and Mineral Resource for analysis of the other parameters. All analytical methods followed the national technical regulations (Ministry of Land and Resources of the P. R. China 2006). In addition, the groundwater hydrochemical data were validated by charge balance error percentage (CBE%). Finally, 174 groundwater the samples with CBE% smaller than ± 5% were retained, and 13 hydrochemical parameters were selected for subsequent analysis of this study, including pH, TDS, Na+, K+, Ca2+, Mg2+, Cl−, SO42−, HCO3−, CO32−, NO3−, F− and Cr6+.
Human Health Risk Assessment
Human health risk assessment is an important methodology utilized to assess the probability of deleterious effects on human health to support water quality evaluation and management (Shukla and Saxena 2020). In this study, the non-carcinogenic and carcinogenic health risk of Cr6+ were estimated in two different age groups (adults and children) with the USEPA model (USEPA 1989). But only drinking water ingestion pathway was considered as the most remarkable source of exposure, because all the other exposure pathways such as inhalation and dermal absorption were negligible (Wu et al. 2019, 2020). Because potential health risk is posed only by one contaminant, the non-carcinogenic risk of Cr6+ through drinking water ingestion can be expressed by using the hazard quotient (HQ). Cr6+ is recognized as a Group 1 carcinogen by the WHO (World Health 2017), the carcinogenic risk (CR) of Cr6+ through drinking water ingestion can also be assessed. The computation can be conducted by the following equations (He et al. 2021; Wei et al. 2021; Li et al. 2019a, b).
where RfD indicates the reference dosage for Cr6+ through drinking water ingestion pathway, and the value of RfD for Cr6+ is 0.003 mg/kg/day in this study. SF is the slop factor of Cr6+ (mg/kg/day), and the SF value of Cr6+ is set at 0.5 mg/kg/day. CDI denotes the chronic daily intake (mg/kg/day) and is determined with the following equation (He and Wu 2019; He et al. 2019; Ji et al. 2020).
where C indicates the Cr6+ concentration in groundwater (mg/L); IR is ingestion rate of drinking water (in L/day); EF is the frequency of exposure (days/year); ED is the duration of exposure (years); BW is average body weight of a person (kg), and AT is average time for non-carcinogenic or carcinogenic effects (days). The reference values for computing CDI are determined by USEPA guidelines statistics data and adjusted according to the habits of local residents (Ministry of Environmental Protection of the P. R. China 2013). The values of these parameters are shown in Table 1.
Results and Discussion
Cr6+ Concentration in Groundwater
The basic statistical analysis of groundwater Cr6+ concentrations was performed (Table 2). The analytical results indicate that the Cr6+ values range from 1 to 220 μg/L with an average of 29 μg/L and a median of 16 μg/L. According to the groundwater quality standard of China (General Administration of Quality Supervision, Inspection and Quarantine of the P. R. China and Standardization Administration of the P. R. China 2017), the Cr6+ concentrations of groundwater in the study area can be divided into four intervals: ≤ 10, 11–50, 50–100 and > 100 μg/L, respectively. Table 2 summarizes the percentages of groundwater samples classified by the four intervals. As shown in Table 2, the Cr6+ concentrations of the groundwater samples are mainly in the range of ≤ 10 μg/L and 11-50 μg/L, accounting for 45.40% and 37.36% of the groundwater samples, respectively. In addition, about 17.24% of all groundwater samples measured for Cr6+ (30 out of 174) exceeded the allowable limit for drinking purpose (50 µg/L), which indicates a potential cancer risk to human health.
Hydrogeochemical Characteristics in Different Cr6+ Concentration Intervals
The statistics for groundwater chemistry characteristics between different Cr6+ concentration intervals are listed in Table 3. Groundwater samples with Cr6+ concentration < 10 μg/L are characterized by highest mean concentrations of Ca2+, low levels of TDS and lowest mean concentrations of pH, Na+, Mg2+, Cl−, HCO3− and F−. Groundwater with Cr6+ concentrations of 10–50 μg/L have lowest mean levels of TDS and SO42−, and the pH and other major ion (Na+, Mg2+, Cl−, HCO3− and F−.) concentrations are a little higher than groundwater with Cr6+ < 10 μg/L, but concentrations of Ca2+ are lower by comparison. For Groundwater with Cr6+ concentrations of 50–100 μg/L, their mean concentrations of TDS, Na+, Mg2+, Cl−, HCO3− and F− are elevated, and the Ca2+ concentrations become even lower. Further, groundwater samples with Cr6+ concentration > 100 μg/L have the lowest mean concentrations of Ca2+ (mean 42.6 mg/L), and highest mean concentrations of TDS, Na+, Mg2+, Cl− SO42−, F−. These results indicate the occurrence of Cr6+ is greatly related to the interaction between water and rock, because the concentration of major ions increases with the increase of Cr6+ concentration interval except Ca2+.
Figure 2 plots the Piper diagram (He and Li 2020b) for the four Cr6+ concentration intervals of the groundwater samples to study the hydrochemical types of groundwater. This figure indicates that the groundwater samples are predominantly of the HCO3-Ca(Mg) water types, and this agrees with the findings of Duan et al. (2011). It shows that carbonate dissolution is an important process regulating the major anions. The groundwater samples with Cr6+ concentrations < 50 μg/L are clearly distinguished from those > 50 μg/L. Groundwater in Cr6+ concentration intervals ≤ 10 μg/L and 11–50 μg/L are mainly of HCO3-Ca and HCO3-Ca(Mg) type, and a portion of groundwater samples can be classified as HCO3-Na or SO4·Cl-Na types. Groundwater having Cr6+ concentrations > 50 μg/L are classified as HCO3-Na type, and several samples are plotted in SO4·Cl-Na or SO4·Cl-Ca·Mg type zone. The observation of Piper diagram suggests that low concentrations of Cr6+ are associated with high Ca2+ and that high Cr6+ samples are low Ca2+ and high Na+ water.
Spatial Distribution of Cr6+ in Groundwater
The spatial distribution of Cr6+ is delineated in Fig. 3. As a whole, the concentration of groundwater Cr6+ in the north of Weihe River is higher than that in the south of Weihe River. Groundwater Cr6+ concentrations ≤ 10 μg/L are mainly found in the alluvial aquifer and pluvial aquifer occurring at the northern base of the Qinling Mountain. Most of the groundwater samples from the pluvial aquifer located in the north of the Wei River and most of the loess aquifer show that the Cr6+ concentrations range from 11 to 50 μg/L. Zones with groundwater Cr6+ concentrations > 50 μg/L are mainly distributed in the loess aquifer and a portion of alluvial aquifer in the Yanliang district, Xianyang City, Qian, Jingyang, Sanyuan and Pucheng Counties, and these areas are high-risk zones to health issues due to high Cr6+ concentrations exceeding the limit for drinking. Previous studies have also revealed the occurrence of Cr6+ in both groundwater and surface water in the loess areas of China (He and Li 2020a; Xiao et al. 2019). Groundwater samples with Cr6+ concentration > 100 μg/L were concentrated in Xianyang City and Sanyuan County, which may induce a greater risk of disease associated with excessive Cr6+ concentrations. The above results indicate that the geological characteristics of loess may promote the occurrence of Cr6+.
To understand how loess affects the concentration of Cr6+ in groundwater, total Cr in the soil of the Guanzhong Basin was measured in 2019. The spatial distribution of total Cr in the soil is generated. As shown in Fig. 4, the total Cr levels in the soil are higher in the south of the Weihe River Basin than in the north of the basin, and higher in the west than in the east of the basin, which is similar to continental-scale spatial distribution of chromium in catchment sediment/alluvial soil of China published by Yan et al. (2021). However, the total Cr distribution in soil is inconsistent with that of Cr6+ in groundwater in the Guanzhong Basin, because Cr is present in the loess in the form of Cr3+ instead of Cr6+ as revealed by the research pf He and Li (2020a). This indicates that the total Cr in sediments does not have direct effect on the occurrence and distribution of Cr6+ in groundwater of the Guanzhong Plain. However, the loess provides the geogenic source of Cr3+ to form groundwater Cr6+. Thus, it can be inferred that the high concentration of Cr6+ may be attributed to the low groundwater velocity caused by the low permeability of loess. Lower groundwater seepage velocity can be associated with longer residence time of the groundwater, favoring the transform of Cr3+ in the loess into Cr6+ in groundwater. This is consistent with the previous result that the occurrence of Cr6+ is greatly related to water–rock interactions.
Hydrogeochemical Factors Controlling Cr6+ Concentrations
Hydrogeochemical processes are accountable for the occurrence of the Cr6+ in groundwater. The analysis of the relationship between Cr6+ and the other chemical components in groundwater is helpful for achieving a further understanding of the occurrence of groundwater Cr6+ in a study area (Fig. 5). As shown in Fig. 5a, there is weak linear relationship between groundwater Cr6+ and pH, but the higher concentrations of Cr6+ do tend to occur between about pH 7.7–8.5. This indicates that an alkaline environment is more conducive to Cr6+ occurrence in groundwater, because of stronger sorption and greater tendency toward reduction under neutral and acid environment (Bertolo et al. 2011; Coyte et al. 2020).
As shown in Fig. 5b, c, there are a positive correlation between Cr6+ and Na+, and a negative relationship between Cr6+ and Ca2+ in groundwater, indicating that occurrence mechanism for high Cr6+ concentration is likely related to Ion exchange between Ca2+ and Na+ ions, which removed Ca2+ from groundwater by replacing with Na+ on the surfaces of the clay minerals. The exchange occurs particularly in the aquifer sediment matrixes consisting of fine particles like clay and silty clay. Bourotte et al. (2009) also found cation exchange between Ca2+ and Na+ may result in the desorption of Cr6+ to the aquifer.
In this study, Chloro alkaline indices (CAI-1 and CAI-2) proposed by Schoeller was used to evaluate the process of cation exchange. CAI-1 and CAI-2 can be calculated as follows, where all ions are expressed in meq/L (Li et al. 2018b).
When CAI-1 and CAI-2 both have negative values, indicating that cation exchange processes occur between Ca2+ in groundwater is exchanged for adsorbed Na+ in sediment. However, if the indices were both positive, the exchange occurred in the reverse order. As shown in Fig. 6a, negative CAI-1 and CAI-2 values in most of the groundwater samples indicate that cation exchange of Na+ in sediment against Ca2+ in the groundwater may prevail across the entire basin. Meanwhile, the relationship between Cr6+ concentration and the molar ratio of Na+ to (Na+ + Ca2+) is showed to signify the impact of cation exchange on Cr6+ concentrations in the study area (Fig. 6b). Figure 6b indicates that the intensive cation exchange led to the molar ratio of Na+ to (Na+ + Ca2+) approaching to one and high Cr6+ concentration. This is because cation exchange decreases the concentration of Ca2+ and seems to driving the dissolution of carbonate minerals, increasing the pH and HCO3− concentration. Further, the increasing monovalent Na+ concentrations compared to the divalent Ca2+ concentrations can reduce the repulsive potential between the positive hydrous metal oxide surfaces and negative ions with the help of alkaline conditions, and thus promote the desorption of Cr6+ anions (CrO42−) to the aquifer following the counterion effects (Liu et al. 2018; Zachara et al. 1987).
Impacts of Human Activities on Cr6+
Human activities can affect the occurrence and distribution of Cr6+. Industrial emissions can affect the concentration of Cr6+ in groundwater through leaching. As shown in Fig. 3, groundwater samples with high concentrations of Cr6+ are accompanied by industrial pollution sources and solid waste dump sites. Furthermore, the information of industrial pollution sources listed in Table 4 shows that many industrial pollution sources, such as electroplating, printing and dyeing, fertilizer manufacturing and other enterprises, are widely distributed in the high Cr6+ areas. These industrial pollution sources can discharge a large amount of chromium-containing wastes and/or wastewater to the environment, causing the elevation of Cr6+ concentration in the surrounding groundwater. For example, Xianyang downtown has densely distributed industries, correspondingly groundwater is heavily polluted by Cr6+ (Dong et al. 2018; Wang et al. 2012).
The use of fertilizer in agriculture may also be a potential factor affecting the concentration of Cr6+ in groundwater, because phosphate fertilizer usually contains a certain amount of chromium, particularly in inferior phosphate fertilizer. In the Guanzhong Basin, the NO3− concentration in groundwater ranges from 0.16 to 373.00 mg/L with an average of 49.57 mg/L, indicating that the agricultural activities affect the groundwater quality due to fertilizer as well as animal and human wastes (Duan et al. 2011). However, Cr6+ concentration in groundwater shows a poor correlation with NO3− (Fig. 5d), suggesting that input from agricultural activities do not affect the Cr6+ level in groundwater.
Health Impacts of Groundwater Cr6+
High Cr6+ level in groundwater may pose serious health hazards to local populace through multiple exposure pathways, especially for the drinking intake of untreated water. Therefore, the health risk assessment methodology was used to estimate the non-carcinogenic and carcinogenic health risks to adults and children by Cr6+. Table 5 presents the statistical results of the risk assessment to adults and children when they are exposed through drinking water intake.
For the non-carcinogenic risk, if HQ > 1 is considered that there is a certain health risk. The values of HQ for adults range from 0.010 to 2.229 with an average value of 0.294, with 7.47% samples exceeding the acceptable limit value. However, the HQ values for children range from 0.014 to 3.127 with an average value of 0.413, about 12.07% of the total samples with the HQ values than 1. Above results indicate that non-carcinogenic health risk caused by Cr6+ is under the acceptable level through drinking water ingestion pathway in most of study area. Meanwhile, children are at higher non-carcinogenic risk than adults owing to lower body weight for children.
In terms of the carcinogenic risk, there are multiple standards for the acceptable levels of cancer risk, such as 10−4 in Netherlands, 10−5 in Canada and New Zealand, and 10−6 in America and Australia. In this study, value of carcinogenic risk above 10−4 is considered as unacceptable. The values of carcinogenic risk range from 6.51E−06 to 1.43E−03 with a mean value of 1.89E−04 for adults and vary from 1.83E−06 to 4.02E−04 with a mean value of 5.31E−05 for children. 50.57% of the total samples for adults and 16.67% of the sample for children exceed the acceptable limit of the carcinogenic risk (10−4). It is obvious that the carcinogenic risk of Cr6+ has a higher impact than non-carcinogenic risk for adults and children. However, in contrast to the non-carcinogenic risk, the carcinogenic risk of Cr6+ for adults is significantly higher than that for children, because the longer exposure duration leads to the accumulation of Cr6+ in the body. The study results indicate that groundwater treatment is necessary to reduce the Cr6+ concentration for drinking or other domestic purposes, especially in rural areas where water is not treated. Otherwise, the carcinogenic risk of Cr6+ continues to increase as children grow up.
Conclusions
Groundwater polluted by nitrogen, fluorine and arsenic has been well known and attracted much attention in the Guanzhong Basin, but high concentrations of Cr6+ has also been found in recent years. In this study, 13 parameters of 174 groundwater samples were obtained in the Guanzhong Basin. The occurrence and factors influencing the concentration of groundwater Cr6+ were analyzed. The non-carcinogenic and carcinogenic health risks of groundwater Cr6+ through drinking water ingestion were assessed. The main conclusions are summarized:
-
(1)
Groundwater samples from the Guanzhong Basin display Cr6+ concentrations of 1–220 μg/L. 45.40% and 37.36% of the groundwater samples contain Cr6+ concentrations ranged ≤ 10 μg/L and 11–50 μg/L, respectively. And they are mainly of HCO3-Ca and HCO3-Ca(Mg) type. Around 17.24% present Cr6+ concentrations exceeding the allowable value for drinking purpose of 50 µg/L and are predominantly classified as HCO3-Na type. The concentrations of major ions increase with the increase of Cr6+ concentration interval except Ca2+.
-
(2)
Groundwater with low concentration of Cr6+ is mainly found in the alluvial aquifer, whereas high concentration of Cr6+ is associated with the loess aquifer. Low groundwater velocity caused by the low permeability of loess indicates longer residence time of the groundwater, which may favor Cr6+ generation in loess aquifer.
-
(3)
Alkaline environment and cation exchange of Na+ in sediment against Ca2+ in the groundwater are more conducive to Cr6+ formation in groundwater. Industrial activities can discharge a large amount of chromium-containing solid wastes and/or wastewater, and cause the elevation of Cr6+ concentration in the surrounding groundwater, while fertilizer use in agriculture has no apparent effect on Cr6+ occurrence in groundwater.
-
(4)
The non-carcinogenic risk caused by Cr6+ is low for adults and children through drinking water ingestion. However, the carcinogenic risk is very high, and the carcinogenic risk of Cr6+ for adults is significantly higher than that for children. Groundwater treatment is necessary to reduce the Cr6+ concentration for drinking or other domestic purposes, especially in rural areas.
Data Availability
All processed data generated or used during the study appear in the submitted article.
References
Ball JW, Izbicki JA (2004) Occurrence of hexavalent chromium in ground water in the western Mojave Desert, California. Appl Geochem 19:1123–1135. https://doi.org/10.1016/j.apgeochem.2004.01.011
Bertolo R, Bourotte C, Hirata R, Marcolan L, Sracek O (2011) Geochemistry of natural chromium occurrence in a sandstone aquifer in Bauru Basin, São Paulo State, Brazil. Appl Geochem 26:1353–1363. https://doi.org/10.1016/j.apgeochem.2011.05.009
Bourotte C, Bertolo R, Almodovar M, Hirata R (2009) Natural occurrence of hexavalent chromium in a sedimentary aquifer in Urânia, State of São Paulo, Brazil. An Acad Bras Ciênc 81:227–242. https://doi.org/10.1590/S0001-37652009000200009
Chang F, Lei P, Meng Z, Zheng J, Zhang T (2019) Analysis on monitoring results of toxicological indicators of rural drinking water in Shaanxi Province from 2016 to 2018. J Hyg Res 48:739–744 (In Chinese)
Coyte RM, McKinley KL, Jiang S, Karr J, Dwyer GS, Keyworth AJ, Davis CC, Kondash AJ, Vengosh A (2020) Occurrence and distribution of hexavalent chromium in groundwater from North Carolina, USA. Sci Total Environ 711:135135. https://doi.org/10.1016/j.scitotenv.2019.135135
Deng L, Xu B, Yang X, Hu A (2021) Water quality and health risk assessment based on hydrochemical characteristics of tap and large-size bottled water from the main cities and towns in Guanzhong Basin, China. Environ Earth Sci 80:139. https://doi.org/10.1007/s12665-021-09415-x
Dong Y, Yan Z, Zhang X, Yang T, Chen Y, Jiang B (2018) Analysis on distribution and pollution factors of key pollutants in groundwater of Xianyang City. Groundwater 40:80–83 (in Chinese)
Duan L, Wang W, Yang X, Jiang G (2011) Temporal and spatial variation of “three nitrogen” on shallow groundwater in Guanzhong Basin and its control measures. J Arid Land Resour Environ 25:133–137 (In Chinese)
Equeenuddin SM, Pattnaik BK (2020) Hydrogeochemical evolution of hexavalent chromium at the Sukinda ultramafic complex in eastern part of India. Geochemistry 80:125633. https://doi.org/10.1016/j.chemer.2020.125633
General Administration of Quality Supervision, Inspection and Quarantine of the P. R. China, Standardization Administration of the P. R. China (2017) Standard for groundwater quality (GB/T 14848-2017). Beijing: China Standards Press (in Chinese)
Gleeson T, VanderSteen J, Sophocleous MA, Taniguchi M, Alley WM, Allen DM, Zhou Y (2010) Groundwater sustainability strategies. Nat Geosci 3:378–379. https://doi.org/10.1038/ngeo881
Gu X, Dang X, Yang B, Chang L, Li X, You X, Wang H, Wang Q (2015) The distribution regularity and sources of six-valence chromium in groundwater from Wuqi County, Yanan City. Northwest Geol 48:190–203 (In Chinese)
Hausladen DM, Alexander-Ozinskas A, McClain C, Fendorf S (2018) Hexavalent chromium sources and distribution in California groundwater. Environ Sci Technol 52:8242–8251. https://doi.org/10.1021/acs.est.7b06627
He S, Wu J (2019) Hydrogeochemical characteristics, groundwater quality and health risks from hexavalent chromium and nitrate in groundwater of Huanhe Formation in Wuqi County, northwest China. Expo Health 11(2):125–137. https://doi.org/10.1007/s12403-018-0289-7
He X, Li P (2020a) Surface water pollution in the Middle Chinese Loess Plateau with special focus on hexavalent chromium (Cr6+): occurrence, sources and health risks. Expo Health 12:385–401. https://doi.org/10.1007/s12403-020-00344-x
He S, Li P (2020b) A MATLAB based graphical user interface (GUI) for quickly producing widely used hydrogeochemical diagrams. Geochemistry 80(4):125550. https://doi.org/10.1016/j.chemer.2019.125550
He X, Wu J, He S (2019) Hydrochemical characteristics and quality evaluation of groundwater in terms of health risks in Luohe aquifer in Wuqi County of the Chinese Loess Plateau, northwest China. Hum Ecol Risk Assess 25(1–2):32–51. https://doi.org/10.1080/10807039.2018.1531693
He X, Li P, Wu J, Wei M, Ren X, Wang D (2021) Poor groundwater quality and high potential health risks in the Datong Basin, northern China: research from published data. Environ Geochem Health 43(2):791–812. https://doi.org/10.1007/s10653-020-00520-7
Ji Y, Wu J, Wang Y, Elumalai V, Subramani T (2020) Seasonal variation of drinking water quality and human health risk assessment in Hancheng City of Guanzhong Plain, China. Expo Health 12(3):469–485. https://doi.org/10.1007/s12403-020-00357-6
Kazakis N, Kantiranis N, Voudouris KS, Mitrakas M, Kaprara E, Pavlou A (2015) Geogenic Cr oxidation on the surface of mafic minerals and the hydrogeological conditions influencing hexavalent chromium concentrations in groundwater. Sci Total Environ 514:224–238. https://doi.org/10.1016/j.scitotenv.2015.01.080
Kazakis N, Kantiranis N, Kalaitzidou K, Kaprara E, Mitrakas M, Frei R, Vargemezis G, Tsourlos P, Zouboulis A, Filippidis A (2017) Origin of hexavalent chromium in groundwater: the example of Sarigkiol Basin, Northern Greece. Sci Total Environ 593–594:552–566. https://doi.org/10.1016/j.scitotenv.2017.03.128
Khan R, Saxena A, Shukla S (2020) Evaluation of heavy metal pollution for River Gomti, in parts of Ganga Alluvial Plain, India. SN Appl Sci 2:1451. https://doi.org/10.1007/s42452-020-03233-9
Khan R, Saxena A, Shukla S (2021) Assessment of the impact of COVID-19 lockdown on the heavy metal pollution in the River Gomti, Lucknow city, Uttar Pradesh, India. Environ Qual Manag. https://doi.org/10.1002/tqem.21746
Kumar A, Kumar A, Cabral-Pinto MMS, Chaturvedi AK, Shabnam AA, Subrahmanyam G, Mondal R, Gupta DK, Malyan SK, Kumar SS, Khan SA, Yadav KK (2020) Lead toxicity: health hazards, influence on food chain, and sustainable remediation approaches. Int J Environ Res Public Health 17(7):2179. https://doi.org/10.3390/ijerph17072179
Kumari B, Tiwary RK, Srivastava KK (2017) Physico-chemical analysis and correlation study of water resources of the Sukinda chromite mining area, Odisha, India. Mine Water Environ 36:356–362. https://doi.org/10.1007/s10230-016-0409-1
Lei P, Meng Z, Zheng J, Chang F (2019) Analysis on monitoring results of toxicological indicators of rural drinking water in Shaanxi in 2017. Mod Prev Med 46:1112–1115 (in Chinese)
Lelli M, Grassi S, Amadori M, Franceschini F (2014) Natural Cr(VI) contamination of groundwater in the Cecina coastal area and its inner sectors (Tuscany, Italy). Environ Earth Sci 71:3907–3919. https://doi.org/10.1007/s12665-013-2776-2
Li Q (2006) Analysis on distribution and formation causes of high-chrome groundwater in Yuncheng City. Ground Water 28:31–33 (in Chinese)
Li P, Qian H, Wu J, Chen J, Zhang Y, Zhang H (2014) Occurrence and hydrogeochemistry of fluoride in alluvial aquifer of Weihe River, China. Environ Earth Sci 71:3133–3145. https://doi.org/10.1007/s12665-013-2691-6
Li P, Qian H, Howard KWF, Wu J (2015) Heavy metal contamination of Yellow River alluvial sediments, northwest China. Environ Earth Sci 73(7):3403–3415. https://doi.org/10.1007/s12665-014-3628-4
Li P, Wu J, Qian H, Zhou W (2016) Distribution, enrichment and sources of trace metals in the topsoil in the vicinity of a steel wire plant along the Silk Road economic belt, northwest China. Environ Earth Sci 75(10):909. https://doi.org/10.1007/s12665-016-5719-x
Li C, Ma H, Wu Y (2018a) An Inorganic Index dataset of groundwater in the Guanzhong Basin (2015). Geol China 45:31–38. https://doi.org/10.12029/gc2018Z203
Li P, Wu J, Tian R, He S, He X, Xue C, Zhang K (2018b) Geochemistry, hydraulic connectivity and quality appraisal of multilayered groundwater in the Hongdunzi Coal Mine, northwest China. Mine Water Environ 37(2):222–237. https://doi.org/10.1007/s10230-017-0507-8
Li P, He X, Guo W (2019a) Spatial groundwater quality and potential health risks due to nitrate ingestion through drinking water: a case study in Yan’an City on the Loess Plateau of northwest China. Hum Ecol Risk Assess 25(1–2):11–31. https://doi.org/10.1080/10807039.2018.1553612
Li P, He X, Li Y, Xiang G (2019b) Occurrence and health implication of fluoride in groundwater of loess aquifer in the Chinese Loess Plateau: a case study of Tongchuan, northwest China. Expo Health 11(2):95–107. https://doi.org/10.1007/s12403-018-0278-x
Li P, Karunanidhi D, Subramani T, Srinivasamoorthy K (2021) Sources and consequences of groundwater contamination. Arch Environ Contam Toxicol 80(1):1–10. https://doi.org/10.1007/s00244-020-00805-z
Liang J, Huang X, Yan J, Li Y, Zhao Z, Liu Y, Ye J, Wei Y (2021) A review of the formation of Cr(VI) via Cr(III) oxidation in soils and groundwater. Sci Total Environ 774:145762. https://doi.org/10.1016/j.scitotenv.2021.145762
Linos A, Petralias A, Christophi CA, Christoforidou E, Kouroutou P, Stoltidis M, Veloudaki A, Tzala E, Makris KC, Karagas MR (2011) Oral ingestion of hexavalent chromium through drinking water and cancer mortality in an industrial area of Greece—an ecological study. Environ Health 10:50. https://doi.org/10.1186/1476-069X-10-50
Liu Y, Jin M, Ma B, Wang J (2018) Distribution and migration mechanism of fluoride in groundwater in the Manas River Basin, Northwest China. Hydrogeol J 26:1527–1546. https://doi.org/10.1007/s10040-018-1780-8
Luo K, Zhang S, Tian Y, Gao X (2014) Arsenic distribution pattern in different sources of drinking water and their geological background in Guanzhong Basin, Shaanxi, China. Acta Geol Sin Engl Ed 88:984–994. https://doi.org/10.1111/1755-6724.12251
Manning AH, Mills CT, Morrison JM, Ball LB (2015) Insights into controls on hexavalent chromium in groundwater provided by environmental tracers, Sacramento Valley, California, USA. Appl Geochem 62:186–199. https://doi.org/10.1016/j.apgeochem.2015.05.010
Mills CT, Morrison JM, Goldhaber MB, Ellefsen KJ (2011) Chromium(VI) generation in vadose zone soils and alluvial sediments of the southwestern Sacramento Valley, California: a potential source of geogenic Cr(VI) to groundwater. Appl Geochem 26:1488–1501. https://doi.org/10.1016/j.apgeochem.2011.05.023
Ministry of Land and Resources of the P. R. China (2006) The specification of testing quality management for geological laboratories (DZ/T 0130.6-2006). Beijing: China Standards Press (in Chinese)
Ministry of Environmental Protection of the P. R. China (2013) Exposure factors handbook of Chinese population (adults and children). China Environmental Science Press, Beijing (in Chinese)
Molina M, Aburto F, Calderón R, Cazanga M, Escudey M (2009) Trace element composition of selected fertilizers used in Chile: phosphorus fertilizers as a source of long-term soil contamination. Soil Sediment Contam 18:497–511. https://doi.org/10.1080/15320380902962320
Oze C, Bird DK, Fendorf S (2007) Genesis of hexavalent chromium from natural sources in soil and groundwater. Proc Natl Acad Sci 104:6544. https://doi.org/10.1073/pnas.0701085104
Qiao J, Zhu Y, Jia X, Ma S, Niu X, Liu J (2020) Distributions of arsenic and other heavy metals, and health risk assessments for groundwater in the Guanzhong Plain region of China. Environ Res 181:108957. https://doi.org/10.1016/j.envres.2019.108957
Rajapaksha AU, Vithanage M, Ok YS, Oze C (2013) Cr(VI) formation related to Cr(III)-muscovite and birnessite interactions in ultramafic environments. Environ Sci Technol 47:9722–9729. https://doi.org/10.1021/es4015025
Ren X, Li P, He X, Su F, Elumalai V (2021) Hydrogeochemical processes affecting groundwater chemistry in the central part of the Guanzhong Basin, China. Arch Environ Contam Toxicol 80(1):74–91. https://doi.org/10.1007/s00244-020-00772-5
Richard FC, Bourg ACM (1991) Aqueous geochemistry of chromium: a review. Water Res 25:807–816. https://doi.org/10.1016/0043-1354(91)90160-R
Shukla S, Saxena A (2020) Groundwater quality and associated human health risk assessment in parts of Raebareli district, Uttar Pradesh, India. Groundw Sustain Dev 10:100366. https://doi.org/10.1016/j.gsd.2020.100366
Su H, Kang W, Xu Y, Wang J (2017) Assessment of groundwater quality and health risk in the oil and gas field of Dingbian County, Northwest China. Expo Health 9:227–242. https://doi.org/10.1007/s12403-016-0234-6
Su Z, Wu J, He X, Elumalai V (2020) Temporal changes of groundwater quality within the groundwater depression cone and prediction of confined groundwater salinity using Grey Markov model in Yinchuan area of northwest China. Expo Health 12(3):447–468. https://doi.org/10.1007/s12403-020-00355-8
Testa SM, Guertin J, Jacobs J, Avakian C (2004) Sources of chromium contamination in soil and groundwater. CRC Press, Boca Raton, pp 143–164
Tian R, Wu J (2019) Groundwater quality appraisal by improved set pair analysis with game theory weightage and health risk estimation of contaminants for Xuecha drinking water source in a loess area in Northwest China. Hum Ecol Risk Assess 25:132–157. https://doi.org/10.1080/10807039.2019.1573035
Tseng C-H, Lei C, Chen Y-C (2018) Evaluating the health costs of oral hexavalent chromium exposure from water pollution: a case study in Taiwan. J Clean Prod 172:819–826. https://doi.org/10.1016/j.jclepro.2017.10.177
USEPA (1989) Risk assessment guidance for superfund, volume I: human health evaluation manual (Part A). Office of Emergency and Remedial Response, Washington DC
Vasileiou E, Papazotos P, Dimitrakopoulos D, Perraki M (2019) Expounding the origin of chromium in groundwater of the Sarigkiol basin, Western Macedonia, Greece: a cohesive statistical approach and hydrochemical study. Environ Monit Assess 191:509. https://doi.org/10.1007/s10661-019-7655-1
Vengosh A, Coyte R, Karr J, Harkness JS, Kondash AJ, Ruhl LS, Merola RB, Dywer GS (2016) Origin of hexavalent chromium in drinking water wells from the piedmont aquifers of North Carolina. Environ Sci Technol Lett 3:409–414. https://doi.org/10.1021/acs.estlett.6b00342
Wang Y, He L, Ma Y, Li B, Li J, Zan B (2012) Study on distribution of chromium (VI) and its migration characters in Shaanxi district in Weihe River. J Northwest A & F Univ Nat Sci Ed 40:129–134, 140 (in Chinese)
Wang D, Wu J, Wang Y, Ji Y (2020) Finding high-quality groundwater resources to reduce the hydatidosis incidence in the Shiqu County of Sichuan Province, China: analysis, assessment, and management. Expo Health 12(2):307–322. https://doi.org/10.1007/s12403-019-00314-y
Wei M, Wu J, Li W, Zhang Q, Su F, Wang Y (2021) Groundwater geochemistry and its impacts on groundwater arsenic enrichment, variation, and health risks in Yongning County, Yinchuan Plain of northwest China. Expo Health. https://doi.org/10.1007/s12403-021-00391-y
World Health Organization (2017) Guidelines for drinking-water quality: fourth edition incorporating first addendum. World Health Organization, Geneva
Wu J, Sun Z (2016) Evaluation of shallow groundwater contamination and associated human health risk in an alluvial plain impacted by agricultural and industrial activities, mid-west China. Expo Health 8:311–329. https://doi.org/10.1007/s12403-015-0170-x
Wu J, Zhou H, He S, Zhang Y (2019) Comprehensive understanding of groundwater quality for domestic and agricultural purposes in terms of health risks in a coal mine area of the Ordos basin, north of the Chinese Loess Plateau. Environ Earth Sci 78(15):446. https://doi.org/10.1007/s12665-019-8471-1
Wu J, Zhang Y, Zhou H (2020) Groundwater chemistry and groundwater quality index incorporating health risk weighting in Dingbian County, Ordos Basin of Northwest China. Geochemistry 80(4):125607. https://doi.org/10.1016/j.chemer.2020.125607
Xiao J, Wang L, Deng L, Jin Z (2019) Characteristics, sources, water quality and health risk assessment of trace elements in river water and well water in the Chinese Loess Plateau. Sci Total Environ 650:2004–2012. https://doi.org/10.1016/j.scitotenv.2018.09.322
Yan T, Wang X, Liu D, Chi Q, Zhou J, Xu S, Zhang B, Nie L, Wang W (2021) Continental-scale spatial distribution of chromium (Cr) in China and its relationship with ultramafic-mafic rocks and ophiolitic chromite deposit. Appl Geochem 126:104896. https://doi.org/10.1016/j.apgeochem.2021.104896
Zachara JM, Girvin DC, Schmidt RL, Resch CT (1987) Chromate adsorption on amorphous iron oxyhydroxide in the presence of major groundwater ions. Environ Sci Technol 21:589–594. https://doi.org/10.1021/es00160a010
Zhao H, Song F, Su F, Shen Y, Li P (2021) Removal of cadmium from contaminated groundwater using a novel silicon/aluminum nanomaterial: an experimental study. Arch Environ Contam Toxicol 80(1):234–247. https://doi.org/10.1007/s00244-020-00784-1
Zhang L (2012) The mechanism research of chromium pollution in groundwater of Hejin City. Taiyuan University of Technology, Master (in Chinese)
Zhang Y, Wu J, Xu B (2018) Human health risk assessment of groundwater nitrogen pollution in Jinghui canal irrigation area of the loess region, northwest China. Environ Earth Sci 77:273. https://doi.org/10.1007/s12665-018-7456-9
Zhang Y, Xu B, Guo Z, Han J, Li H, Jin L, Chen F, Xiong Y (2019) Human health risk assessment of groundwater arsenic contamination in Jinghui irrigation district, China. J Environ Manag 237:163–169. https://doi.org/10.1016/j.jenvman.2019.02.067
Zhu C, Bai G, Liu X, Li Y (2006) Screening high-fluoride and high-arsenic drinking waters and surveying endemic fluorosis and arsenism in Shaanxi province in western China. Water Res 40:3015–3022. https://doi.org/10.1016/j.watres.2006.06.026
Acknowledgements
This research was supported by the National Natural Science Foundation of China (42072286 and 41761144059), the Special Funds for Basic Scientific Research of Central Colleges (300102299301 and 300102291507), the Fok Ying Tong Education Foundation (161098), and the Ten Thousand Talent Program (W03070125). The editor and reviewers are greatly acknowledged for their useful comments and careful edits on the original version of this manuscript. Their comments have helped us a lot in improving the quality of the manuscript.
Author information
Authors and Affiliations
Contributions
L Wang conducted the investigation, data collection, developed the suitable methodology, and was involved with the writing of the manuscript. P Li guided with the conceptualization of the work and provided supervision in the presented work as well as the writing and editing of the manuscript. R Duan and X He helped in visualization, review, and editing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declared that they have no conflict of interest.
Research Involving Human Participants and/or Animals
Not applicable.
Consent to Participate and Publish
All authors agreed to participate and publish.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, L., Li, P., Duan, R. et al. Occurrence, Controlling Factors and Health Risks of Cr6+ in Groundwater in the Guanzhong Basin of China. Expo Health 14, 239–251 (2022). https://doi.org/10.1007/s12403-021-00410-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12403-021-00410-y