Abstract
Exposure to heavy metals pollutions in water predisposes consumers to human and environmental health deterioration. In this study, the extent of heavy metals contamination, water quality index, ecological and human health risk assessments of groundwater resources in Ajao industrial area of Lagos, Nigeria were carried out. Results revealed that Cu is the most prevalent heavy metal, contaminating 85.71% of the analyzed water samples. Based on the groundwater quality index, 76.19% of the samples are of excellent water quality and suitable for drinking, domestic and industrial purposes. However, the quality of 23.81% variedly deteriorated. Ecological risk assessment revealed that 85.71% and 14.29% of the samples pose low and moderate ecological risks, respectively. This assessment also showed that Cu was the major heavy metal posing ecological risk in the industrial area. Based on hazard quotients, Cu impacted the potentiality of chronic diseases than other heavy metals. Health hazard index analysis revealed that children are more exposed to non-carcinogenic chronic health risks due to ingestion of contaminated groundwater than the adult population. Probability of cancer risk (PCR) revealed that 19.05% of the samples pose high Cr cancer risk for both adult and children, while 14.29% pose high Cd and Ni cancer risks. Correlation and factor analyses indicated that the origin of the heavy metals in water is majorly attributed to anthropogenic inputs rather than natural, geogenic processes. Awareness programs towards protecting the groundwater in this area should be launched and encouraged. Moreover, contaminated water should be treated before use.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metals are found naturally on earth. However, anthropogenic activities, such as those in industries, commerce and agriculture, expedite their accumulations in ecosystems (Barzegar et al. 2018; Mgbenu and Egbueri 2019). Again, human developmental activities have led to increased population and waste generation. Many waste products in urban areas have been reported to be sources of heavy metals contamination (Egbueri 2018; Barzegar et al. 2019). The higher the heavy metal concentrations are, the higher their contribution to the deterioration of water resources and the environment will be. Once water resources and the environment are contaminated with excess heavy metals, health risks and hazards become inevitable.
There are various food chains and cycles through which toxic heavy metals can get into humans. Usually, heavy metals enter the plant, animal, and human tissues via different mechanisms such as inhalation, diet/food, and manual handling (US-EPA 2011, 2017). In industrial estates, wastewaters are one of the major carriers of heavy metals that contaminate various water systems. Apart from being leached from industrial and consumer wastes, different water sources can be polluted by leaching of heavy metals trapped in soils. Acid rain, peculiar to many industrial areas, facilitates this process (Fergusson 1990). Furthermore, plants (including edible vegetables) are exposed to heavy metals through the uptake of contaminated water. Animals (e.g., fishes, livestock) feed on the contaminated plants and waters, and hence become loaded with high concentrations of heavy metals. In a long run, humans (directly or indirectly) ingest these contaminated waters, plants and animals (Fergusson 1990; Thanomsangad et al. 2019; Barzegar et al. 2019) and thus become exposed to several health risks.
Although some heavy metals, in small quantities, are generally good for human body building (Chowdhury and Chandra 1987), their excessive accumulation in the body is a big threat to human health. Most heavy metals are cancer-inducing agents, leaving their victims with various cancers and diseases (Fergusson 1990; US-EPA 2017; WHO 2017). Because toxic heavy metals have the tendency to bio-accumulate in the human body and are hard to metabolize, their health hazards become imminent (Lim et al. 2008; Adamu et al. 2014; Subba Rao et al. 2019). When in contact with human tissue(s), they can bind to and interfere with the functionalities of vital cellular components in the body (US-EPA 1989, 1999, 2011, 2017; WHO 2017). Despite such serious toxicity, many raw materials rich in heavy metals are still used in various industrial processes—e.g., in the production of commodities like wine, food, children’s toys and jewelries, batteries, paints, and motor vehicle parts (Finch et al. 2015).
Following the daily indiscriminate dislodgment of wastewaters into the environs of Ajao Industrial Estate, Lagos State (Nigeria), there have been unproven arguments whether the quality of groundwater therein is fit for human consumption and industrial purposes. Unfortunately, there is paucity of literature reporting on the quality and health risks of water resources in this well-populated industrial area. However, Ukah et al. (2018) recently examined the impact of industrial wastewater (from this estate) on the physicochemical and microbiological characteristics of drinking water resources. They found out that many drinking water sources were variedly contaminated. Although Ukah et al. (2018) have reported on the quality of drinking water resources in the Ajao Estate, there is still a need for more researches in this area, especially regarding the environmental and health risks.
To efficiently and effectively ensure that groundwater resources are kept safe in industrial areas, regular monitoring, management and sustainability practices are adopted. The adoption of such practices usually includes an imperative desire to assess the heavy metals concentrations in water and the associated ecological and health risks. Ecological and human health risk assessments of water resources measure and quantify the potential health impacts of heavy metals, based on water quality standards and health risk benchmarks (Barzegar et al. 2018; Mgbenu and Egbueri 2019; Taiwo et al. 2019). These assessments often employ methodologies such as pollution indices, multivariate statistical analyses, non-carcinogenic and carcinogenic risk analyses.
In view of the current state of water quality research in Ajao Estate, this paper aims to examine the extent of heavy metals contamination and ecological and human health risks of contaminated groundwater in this industrial area. The research objectives were to (1) determine the heavy metals contamination levels in the groundwater; (2) identify the possible sources of water contamination using multivariate statistical tools; (3) evaluate the groundwater quality index for human consumption; (4) assess the ecological risk index of the groundwater samples; and (5) assess the non-carcinogenic and carcinogenic health risks associated with the use of the water. This study provides a preliminary, baseline study of the water quality index, ecological and health risks of the heavy metals contaminated water resources in Ajao industrial area. Therefore, it is hoped that the paper will provide insights for groundwater development, monitoring, management and sustainability in the area.
Materials and methods
Description of the study area
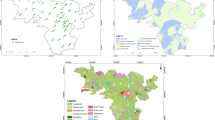
The study area lies within latitudes 6°31′–6°33′N and longitudes 3°18′–3°20′E (Fig. 1) and also within the highly populated area of Oshodi-Isolo Local Government Area, West of Lagos State, Nigeria. The major rivers draining the area are the Ogun, Adiyan, and Osse rivers. The typical drainage system is the lagoons which connect to the Atlantic Ocean. These surface water networks constitute the major recharge sources for the underlying aquifers. Geologically, the Ajao industrial area is within the Dahomey Basin, which stretches from Accra in Ghana, through Republic of Togo and Benin Republic through to Nigeria where it is demarcated from the Niger Delta Basin by the Okitipupa Ridge (Omatsola and Adegoke 1981). Ajao Estate is underlain by two different lithologic units, the coastal plain sand and the alluvial river sand and both are part of the Dahomey Basin (Nwajide 2013). The dominant lithology observed from different hand-dug wells in the study area is characterized by medium to poorly sorted coarse-grained sand and mudrocks (clay and shale) intercalated with sand.
The Ajao area is relatively a lowland within the coastal beaches, extensive inland lagoons and depressions with marsh and mangrove wetland at elevation of 0–2 m and upland areas with moderately drained soils and an elevation of 10–14 m. The coastal plain sands are the main aquifer systems (consisting of three aquiferous layers separated by silts and clays) in the study area (Longe et al. 1987; Kampsax-Kruger and Sshwed Associates 1977). The aquifer systems extend from the outcrop area in the north of the study area to the coast in the south (Longe et al. 1987), and are exploited through hand-dug wells and boreholes. According to Longe et al. (1987), the first aquifer encountered at a depth of 35 m of an average thickness of 6 m stands a higher risk of pollution because of its nearness to the surface. However, the second and third aquifers, which occur between the depths of 40–55 m (about 8 m thick) and 30–90 m (about 32 m thick), respectively, are preferably better aquifers for suitable drinking water supply (Longe et al. 1987).
The population of the area is estimated to be greater than 500,000 residents. The study region is known for several anthropogenic activities ranging from industrial food/wine production, automobile factories and workshops, and numerous fast goods production industries. These industrial activities in and around Ajao estate emit and discharge different kinds of solid and liquid wastes from their production plants on soils and into surface drainage systems. Moreover, part of the liquid industrial effluents could be infiltrating into the groundwater systems, which are the major sources of drinking and industrial water in the Ajao area. The wastes discharged from these industrial activities are characterized by irritating and pungent smell. Collectively, it is believed that the different kinds of industrial wastes generated in this area predispose the inhabitants to water and air pollutions with their associated human health risks.
Sample collection and analysis
To achieve the research objectives, a total of twenty-one groundwater samples were randomly collected (in March–April 2016) with sterilized 1-L plastic bottles, from various boreholes within and around the Ajao Industrial Estate, Lagos, Nigeria. At each sampling point, the source water was used to rinse the sterilized sampling bottle prior to collecting the sample. Coordinate of each sampling point was recorded using a handheld GPS (GARMIN GPSMAP 78S series). The samples were carefully and legibly labeled and placed in an ice-crested cooler to avoid any kind of reactivity or algal growth prior to laboratory analysis (Mgbenu and Egbueri 2019). The physicochemical analysis was done within 48 h of sampling. Water samples were filtered through a cellulose acetate filter (0.45-micron milli-pore filter) before the laboratory analysis.
The groundwater samples were analyzed for the determination of pH, total dissolved solids (TDS), chloride (Cl), and heavy metals (Cu, Fe, Cr, Pb, Zn, Cd, Mn, and Ni). The pH values of the samples were determined in the field using a Testr-2 pH meter after standardizing/calibrating against buffer solution (pH 4.7 and 9.2). The TDS was also measured on site using portable TDS meter (HM Digital COM-100). The heavy metals in water samples were analyzed in the laboratory using specific hollow cathode lamp at a specific wavelength, and then aspirated into the flame of atomic absorption spectrophotometer (AAS) PerkinElmer Analyst 200 (Ukah et al. 2018). All the analytical procedures described in this study followed the recommendations of American Public Health Association (APHA 2005).
Groundwater quality index (GWQI)
To get a comprehensive summary of quality status and level of pollution, GWQI for human consumption (drinking, domestic and industrial purposes) was examined for the groundwater samples. Three steps were taken in evaluating the GWQI for each of the samples. First, weights (ranging from 1 to 5) were assigned to the physicochemical parameters and heavy metals, according to the parameters’ relative importance in the overall quality of water for drinking and industrial purposes (Rahman et al. 2017; Mgbenu and Egbueri 2019). With the assigned weights, the relative weight of each parameter was calculated using the formula:
where Wi is relative weight, wi is weight of parameter, and n is the total number of parameters. The assigned weights and the calculated relative weights for the eleven analyzed parameters are presented in Table 1.
Second, the quality rating scale for each of the parameters was calculated. This was done by dividing each parameter’s concentration in the water samples by the respective standard value and multiplying the results by 100 (Eq. 2)
where qi is quality rating, Ci is the concentration of each parameter, and Si is the standard value for the parameter.
The last step of the GWQI was the determination of sub-index for each parameter and then the summation of all sub-indices for each sample, as shown in Eqs. 3 and 4, respectively:
where SIi is the sub-index of ith parameter, qi is the quality rating based on concentration of ith parameter and n is the number of parameters. Nigerian Industrial Standard (NIS 2007) and World Health Organization (WHO 2017) standard values were used in this indexing.
Water pollution source identification using multivariate statistical analysis
Different researchers have successfully used multivariate statistics in water quality assessments (Rahman et al. 2017; Wagh et al. 2018; Egbueri 2018, 2019a, b; Barzegar et al. 2016, 2018, 2019; Mgbenu and Egbueri 2019; Egbueri et al. 2019). In this study, two multivariate statistical techniques were employed. The correlation and factor analyses were used to assess the potential sources of heavy metals pollutions in the analyzed groundwater resources. All statistical analyses were performed using SPSS software (v. 22). Input data for the statistical analysis were normalized due to the non-normal distribution of raw data means and differences in units of measurements of the analyzed variables (Barzegar et al. 2016, 2019). For the correlation analysis, correlation coefficients greater than 0.7, 0.5 < r < 0.7 and less than 0.5 were considered as strong, moderate, and weak correlation, respectively (Soltani et al. 2017). In other words, the larger the correlation coefficient is, the stronger the parameters’ association and vice versa. For the factor analysis, the Varimax rotation method, as proposed by Kaiser (1960), was utilized to optimize factor loadings (Barzegar et al. 2017) at eigenvalue ≥ 1. In this study, factor loadings less than 0.5 were considered as low (insignificant), 0.5–0.75 as medium, and above 0.75 as high (Barzegar et al. 2017; Mgbenu and Egbueri 2019).
Ecological risk assessment
The ecological risk index (ERI) for the groundwater was calculated using the functions described in Eqs. 5 and 6 (Taiwo et al. 2019):
where RI is the potential ecological risk factor of each heavy metal; Ti is the toxic-response factor of heavy metal; PI is the pollution index; Cs is the concentration of heavy metals in the sample; and Cb is the corresponding background values. The toxic-response factor of heavy metals is given as: Cd = 30; As = 10; Co, Cu, Ni and Pb = 5; Cr and Zn and Mn = 1 (Taiwo et al. 2019).
Human health risk assessment
Non-carcinogenic health risk assessment
The non-carcinogenic risk assessment involved the calculation of hazard quotients (HQ) and hazard indices (HI) of the individual groundwater samples. Both HQ and HI were calculated after the chronic daily intake (CDI) risk was determined. Employing the guidelines presented by US-EPA (1989), the CDI risks posed by ingesting a single trace element are computed for the children and adult populations as follows:
where CDI is the chronic daily intake otherwise referred to as the exposure dose (mg/kg/day); Cw signifies the contaminant concentration in water (mg/L); IRW represents the water ingestion rate (IRW is equal to 1 L for children and 2 L for adults); EF is used to denote the exposure frequency (EF is equivalent to 365 days per year); ED signifies the exposure duration (adult ED = 70 years while children ED = 6 years); BW is the body weight (equivalent to 70 kg and 15 kg for adult and children, respectively); AT represents the average exposure time (equivalent to 25,550 days and 2190 days for adult and children, respectively) (Mgbenu and Egbueri 2019).
Using Eq. 8, the non-carcinogenic risk of a single element calculated as the HQ is evaluated:
where RfD represents the reference dose of a specific element (mg/kg/day). The RfD equivalent for the heavy metals is 0.7 (Fe), 0.3 (Zn), 0.0035 (Pb), 1.5 (Cr), 0.046 (Mn), 0.04 (Cu), 0.001 (Cd) and 0.02 (Ni) (Duggal et al. 2017; Barzegar et al. 2018; Mgbenu and Egbueri 2019; Thanomsangad et al. 2019).
The final value for the non-carcinogenic risk assessment is the hazard index (HI), which is the summation of the hazard quotient values:
According to US-EPA (1989) and Su et al. (2017), if HI is greater than unity (HI > 1), it implies that the non-carcinogenic health risk of ingesting a particular element is above the acceptable limit, whereas HI < 1 implies that they are within the acceptance limit. Non-carcinogenic risk is, therefore, classified on the basis of HI values into negligible, low risk, medium risk and high risk (Bortey-Sam et al. 2015; Mgbenu and Egbueri 2019).
Carcinogenic health risk assessment
To assess the carcinogenic risks associated with the use of the groundwater, heavy metal evaluation index (HEI), heavy metal pollution index (HPI), contamination index (CI), and probability of cancer risk (PCR) were calculated using the formulae given in Eqs. 10, 11, 12, and 14, respectively. HEI is used in assessing associated carcinogenic risks of a given set of water samples. This is because it gives an overall quality of water with respect to their heavy metals content (Wagh et al. 2018). However, the HPI assesses the ratio of heavy metal pollution and also gives the composite effects of individual heavy metal on the overall quality and suitability of water for drinking (Wagh et al. 2018; Egbueri 2018). The NIS (2007) drinking water quality standards were used for the calculation of heavy metal indices of the various samples:
where Hc is the monitored value and HMAC is the maximum admissible concentration (MAC) of the ith parameter.
where HMC is the heavy metal concentration in water sample; AL is the allowable limit; and n is the number of analyzed heavy metals.
To further assess the carcinogenic exposure and impact of the groundwater resources, the CI was evaluated:
where Cfi is the contamination factor for the ith parameter; CAi is the analytical value for the ith component; and CNi is the upper permissible concentration of the ith parameter.
Probability of cancer risk (PCR) of the drinking groundwater resources was estimated as the incremental threat of an individual developing cancer over a lifetime, resulting from the exposure to a potential carcinogen (Rahman et al. 2017). The PCR of each carcinogenic metal is calculated with Eq. 14:
where SF is the slope factor (mg/kg/day).
An acceptable PCR value is ≤ 1 × 10−6, which means on average, the probability is that approximately 1 per 1,000,000 will develop cancer as a consequence of the exposure to a carcinogen (Lim et al. 2008; Adamu et al. 2014). However, risk in the range of 1 × 10−6 to 1 × 10−4 typically has been reported to be acceptable (US-EPA 1999; Rahman et al. 2017). In this study, the SF values for calculating the PCR of the heavy metals (carcinogens) are given as: Pb (0.0085), Cr (0.05), Cd (0.38), and Ni (0.91).
Results and discussion
Extent of heavy metals contaminations in groundwater samples
The water quality data are presented in Table 2. Additionally, the univariate statistical summary of the water quality, the comparison of the water quality with standard limits and the undesirable effects of parameters that exceeded their limits are presented in Table 3. The pH of fifteen groundwater samples (71.43% of the total samples) was off the standard limits of 6.5–8.5 (Table 2), hence classifying them as slightly acidic water. TDS and Cl concentrations in the samples were below the maximum allowable limits (Tables 2 and 3). Based on the TDS and Cl contents, the groundwater samples are classified as excellent drinking water (Egbueri 2019a). However, results from heavy metals analysis indicate that some of the groundwater samples are variedly contaminated (Fig. 2), and thus are not suitable for human consumption. It was observed that 85.71% of the total water samples are contaminated with Cu (Table 3, Fig. 2). Also, Tables 2 and 3 show that 14.29% of the samples have Ni and Cr contaminations. Moreover, 23.81% of the total samples are contaminated with excess Fe, whereas 9.52% and 4.76% samples are contaminated with excess Pb and Cd, respectively. These contaminated borehole samples could be indicating water from shallow aquifers. Studies have shown that, even in very low contents, many heavy metals are toxic to human health (NIS 2007; Lim et al. 2008; US-EPA 1989, 1999, 2011, 2017; Adamu et al. 2014; Bortey-Sam et al. 2015; WHO 2017; Duggal et al. 2017; Barzegar et al. 2018, 2019; Mgbenu and Egbueri 2019; Subba Rao et al. 2019; Thanomsangad et al. 2019). From the results presented, it is indicated that the consumers of the contaminated water are predisposed to several health risks/effects attributed to excess Cu, Fe, Pb, Ni, Cr, and Cd in water. Nevertheless, all of the samples are free from Zn and Mn pollutions, hence the water consumers do not risk such diseases attributed to their excesses in water (Table 3).
Groundwater quality index (GWQI)
The suitability of the groundwater resources was assessed for drinking, domestic and industrial purposes (involving food processing). The computed GWQI values range from 8.285 to 608.728 and therefore are categorized into six water types, from ‘excellent water’ to ‘unfit for drinking” (Tables 4 and 5). The ranges of the GWQI, percentage of samples in each class, the corresponding status of water quality and their possible uses are summarized in Table 5. Results show that 76.19% of the total samples are in ‘excellent’ category and hence are suitable for drinking, domestic and industrial purposes (Tables 4 and 5). However, 9.52% and 14.29% of the samples are in ‘good water’ and ‘unsuitable for drinking’ categories, respectively. Hence, those in ‘good’ class are relatively suitable for domestic and industrial purposes and the unsuitable samples are not acceptable for drinking, domestic and industrial uses (Brown et al. 1972; Mgbenu and Egbueri 2019). The higher values of GWQI found in the five samples (BH06, BH08, BH12, BH15, and BH16) with deteriorated quality (Table 4) were mainly as a result of higher Fe concentration. Therefore, they require proper treatment before use.
Water pollution source identification using multivariate statistical analysis
Correlation analysis (CA)
Pearson’s correlation matrix, shown in Table 6, was produced using the IBM SPSS (v. 22). Generally, pH, TDS, and Cl have no significant correlation with the heavy metals. This signifies differences in their origins (Barzegar et al. 2018, 2019; Egbueri 2018; Mgbenu and Egbueri 2019). While the pH, TDS and Cl concentrations in the groundwater are believed to be mainly controlled by geogenic, natural processes, the heavy metals are mainly attributed to anthropogenic sources. The TDS, usually indicating the amount of dissolved minerals in water, is known to be influenced by cations and anions concentrations (Egbueri 2019a). The Cl could be linked to Cl-bearing (e.g., aluminosilicates) silicate mineral weathering (Soltani et al. 2017; Barzegar et al. 2017; Egbueri 2019a), while the pH (which indicated slight acidity) could be linked to acid rain, which is often observed in industrial areas. Additionally, the insignificant correlation between the pH and the heavy metals suggests that the pH is not the main factor affecting the mobility of these metals in the groundwater (Barzegar et al. 2016). It is believed that the mineralogy of geological deposits in an area significantly controls the chemistry and quality of water passing through them (Singh 1987; Utom et al. 2013; Egbueri 2019b). Based on the geology of the study area, the alluviums and the coastal plain sands are not geologic deposits naturally rich in heavy metals (Nwajide 2013; Egbueri 2018; Mgbenu and Egbueri 2019); rather they are thought to be porous and permeable enough to allow the passage of the trace element-laden water into the aquifer system. Nevertheless, a moderate correlation exists between Zn and TDS (Table 6). This suggests that Zn2+ significantly influences the TDS of the samples (Egbueri 2019b).
The correlation matrix (Table 6) revealed that most of the heavy metals have significant associations with one another, signifying similarity of sources (Barzegar et al. 2017; Egbueri 2019a, b). It was observed that Fe has a significant correlation with all the heavy metals. In this study, the presence of excess Fe in the samples can be attributed to both natural and anthropogenic processes. The natural processes that could release Fe in water include redox conditions (Barzegar et al. 2018) of the aquifer system and the rusting effects of water supply pipes. Anthropogenic sources that could release Fe in the water include paints and plastics (Bhutiani et al. 2017), metallic industrial waste leachates and mechanic workshop activities. Zn has a positive correlation with Cu, Cr, Mn and Cd. Their possible sources are attributed to industrial activities such as heavy chemicals production processes, poor waste (paints, e-waste, etc.) disposal in dumpsites, waste combustion and industrial wastewater disposal into drainage systems and soils (Egbueri 2018; Barzegar et al. 2019). Pb has a positive correlation with Cr and Cd, indicating possible sources from sewage sludge, effluents from textile tanning industries, automobile mechanic workshops, batteries, industrial plants and industrial air conditioning coolants (Bhutiani et al. 2017; Egbueri 2018; Garba and Abubakar 2018; Mgbenu and Egbueri 2019). The correlation between Ni and Cd suggests that their origin could also be from Ni/Cd batteries (Garba and Abubakar 2018).
Factor analysis (FA)
The principal component factors were extracted using the Varimax rotation (with Kaiser normalization) and are presented in Table 7. Three factors, which explained 82.324% of the total variance, with ≥ 1 eigenvalue were chosen. The FA results identified the most important factors contributing to the water quality of Ajao industrial area. As a matter of fact, the FA results successfully validated the CA results. The first factor explained 34.371% of the total variance including strong positive loadings on Fe Pb, Cu, Cr, and Cd. Although the origin of Fe is partly attributed to geogenic processes in the correlation analysis, this class is also made up of parameters peculiar to anthropogenic sources rather than mineralogical weathering. The Cr, Cd, Cu, and Pb could be leached from contaminated industrial soils and industrial wastes such as wires, petrochemicals, automobile batteries, tires and electronic wastes. (Bhutiani et al. 2017; Egbueri 2018; Garba and Abubakar 2018). The second factor explained 29.705% of the total variance and included Zn, Ni, Cr, and Mn. This class of parameters is typical of sources linked to anthropogenic inputs from industrial activities such as discharge of heavy chemical wastes, automobile wastes, and paints (Bhutiani et al. 2017; Egbueri 2018; Mgbenu and Egbueri 2019). Based on the geological setting of the Ajao area, there are no reports of rocks or soil deposits rich in heavy metals that could release these potentially toxic elements into the groundwater systems (Singh 1987; Utom et al. 2013; Egbueri 2019b; Mgbenu and Egbueri 2019). Finally, the third factor explained 18.248% of the total variance and included such parameters as pH, TDS, Cl, and Zn which are characteristically attributed to geogenic processes. However, the negative loading of pH in this factor class could suggest that it has no control over the TDS and Cl contents in water. The occurrence of Zn (a readily mobile element) in this factor class could mean that it may have had both geogenic and anthropogenic origins (Bhutiani et al. 2017; Barzegar et al. 2019).
Human and ecological health risk assessments
Non-carcinogenic health risk assessment
Median values from the HQ assessment revealed that the various impacts of the heavy metals to cause chronic non-carcinogenic risks for adult is Cu > Zn > Fe > Pb > Mn > Ni > Cr > Cd and for children Mn > Cu > Zn > Fe > Pb > Ni > Cr > Cd. Based on the HQ impacts, Cu is the priority pollutant for the adult population, whereas Mn is the priority pollutant for the children population (Mgbenu and Egbueri 2019). The hazard indices for non-carcinogenic health risk assessment (for both adult and children) of the Ajoa industrial area are summarized in Table 8. Results show that 52.38%, 42.86%, and 4.76% of the total groundwater samples pose medium, high and negligible chronic health risks to the adult consumers. However, it was observed that most of the samples (85.72%) predispose the children in this area to high chronic health risks (Table 8). Therefore, this assessment reveals that children are more exposed to non-carcinogenic chronic health risks than the adult population.
Ecological risk index
The calculated ecological risk indices (ERI) for the groundwaters range from 0.400 to 272.250 (Table 4). According to the ERI classification reported by Taiwo et al. (2019), 85.71% of the total samples pose low ecological risks, whereas 14.29% pose moderate ecological risks (Table 9). However, because most of the samples are contaminated with Cu, its impact on the ERI became very obvious and predominant. Therefore, this study indicates that Cu was the major heavy metal posing an ecological risk in the study area.
Carcinogenic health risk assessment
Table 4 shows the carcinogenic HPI, HEI, and CI values for the individual groundwater samples. However, the classification summary of the samples based on HPI, HEI and CI results are also presented in Table 9. On the basis of the HPI and HEI classifications, 100% of the groundwater samples have low heavy metals pollutions and therefore pose low carcinogenic health risks to the water consumers. Nevertheless, the CI classification revealed that 95.24% of the samples pose no carcinogenic health risk, while 4.76% have moderate pollution and would pose a medium carcinogenic health risk (Wagh et al. 2018).
Table 10 presents the results of the probability of cancer risk of four metals (Cr, Cd, Pb and Ni) for both adult and children populations. Based on the acceptable range for cancer risk of ≤ 1 × 10−6 to 1 × 10−4 (US-EPA 1999; Lim et al. 2008; Adamu et al. 2014; Rahman et al. 2017), 19.05% of the total samples have high Cr cancer risk for adult and children and 14.29% have high Cd and Ni cancer risks (Table 10). All of the water samples have negligible cancer risk (for both adult and children populations) due to Pb contamination.
Conclusions
This paper has examined the extent of heavy metals contaminations in water, possible sources of water pollution, groundwater quality index, ecological risk and human health risks (both non-carcinogenic and carcinogenic) associated with ingesting groundwater resources in densely populated Ajao industrial area, Lagos State, Nigeria. Based on the findings of this study, the following conclusions and recommendations are made:
- 1.
TDS and Cl contents were below the standard maximum allowable limits. However, measurements of water pH showed that the majority of the samples were slightly acidic, hence off the set limits of 6.5–8.5.
- 2.
Of all the eight analyzed heavy metals, Cu is the most prevalent, contaminating 85.71% of the total analyzed samples. However, no Zn and Mn pollutions were recorded; their contents in water were below the maximum allowable limits of the NIS (2007) and WHO (2017).
- 3.
Multivariate statistical analysis revealed that both natural (geogenic) and anthropogenic origins may have impacted the chemistry and quality of groundwater resources in the Ajao industrial area. However, anthropogenic origins were more important. The sources of the heavy metals in water are majorly attributed to anthropogenic inputs.
- 4.
Based on the GWQI, majority of the samples (about 76.19% of the total) are of excellent water quality, hence are suitable for drinking, domestic and industrial purposes. However, 23.81% of the samples deteriorated in quality.
- 5.
Based on the ERI, 85.71% of the total samples pose low ecological risks, whereas 14.29% pose moderate ecological risks. Cu was the major heavy metal posing an ecological risk in the study area.
- 6.
Based on the HQ, Cu impacted the potentiality of chronic diseases than other heavy metals. On the basis of the HI, children are more exposed to non-carcinogenic chronic health risks than the adult population. It was learned that about 42.86% of the total groundwater samples pose high chronic health risks to the adult consumers, whereas 85.72% of the samples predispose the children in this area to high chronic health risks.
- 7.
Based on the PCR, 19.05% of the total samples have high Cr cancer risk for both adult and children, while 14.29% have high Cd and Ni cancer risks. Cancer risk due to Pb pollution was negligible.
- 8.
Contaminated water should be adequately treated before use, especially for consumption purposes.
- 9.
The contaminated groundwater samples could be indicating the shallowness of aquifer. Therefore, the residents are advised to avoid shallow aquifers and drill for deeper aquifer sources that are better insulated from surface processes and contaminations.
- 10.
Residents and industrialists are advised to embrace adequate sanitation practices and regular water pollution/quality monitoring. Regular monitoring strategy would help to observe changes in water quality during mid- to long-term periods, thus identifying potential trends that may lead to sustainable water management plans (Barzegar et al. 2019). This paper is only a preliminary study and was based on data from 21 groundwater (borehole) samples. The study could be expanded using more sophisticated investigative approaches in the future by collecting more samples across the area.
References
Adamu, C. I., Nganje, T. N., & Edet, A. (2014). Heavy metal contamination and health risk assessment associated with abandoned barite mines in Cross River State, southeastern Nigeria. Environment Nanotechnology Monitoring and Management. https://doi.org/10.1016/j.enmm.2014.11.001.
APHA. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington DC: American Public Health Association.
Barzegar, R., Moghaddam, A. A., Adamowski, J., et al. (2018). Assessing the potential origins and human health risks of trace elements in groundwater: A case study in the Khoy plain, Iran. Environmental Geochemistry and Health. https://doi.org/10.1007/s10653-018-0194-9.
Barzegar, R., Moghaddam, A. A., Soltani, S., Fijani, E., Tziritis, E., & Kazemian, N. (2017). Heavy metal(loid)s in the groundwater of Shabestar area (NW Iran): source identification and health risk assessment. Exposure and Health. https://doi.org/10.1007/s12403-017-0267-5.
Barzegar, R., Moghaddam, A. A., Soltani, S., et al. (2019). Natural and anthropogenic origins of selected trace elements in the surface waters of Tabriz area, Iran. Environmental Earth Science,78, 254. https://doi.org/10.1007/s12665-019-8250-z.
Barzegar, R., Moghaddam, A. A., & Tziritis, E. (2016). Assessing the hydrogeochemistry and water quality of the Aji-Chay River, northwest of Iran. Environmental Earth Sciences,75, 1486. https://doi.org/10.1007/s12665-016-6302-1.
Bhutiani, R., Kulkarni, D. B., Khanna, D. R., & Gautam, A. (2017). Geochemical distribution and environmental risk assessment of heavy metals in groundwater of an industrial area and its surroundings, Haridwar, India. Energ Ecology and Environment,2(2), 155–167. https://doi.org/10.1007/s40974-016-0019-6.
Bortey-Sam, N., Nakayama, S. M., Ikenaka, Y., et al. (2015). Health risk assessment of heavy metals and metalloid in drinking water from communities near gold mines in Tarkwa, Ghana. Environmental Monitoring and Assessment,187(7), 397.
Brown, R. I. C. E., McClellan, N. I., Deinniger, R. A., et al. (1972). A water quality index crashing physiological barrier. Indicators of Environmental Quality,1, 173–182.
Chowdhury, B. A., & Chandra, R. K. (1987). Biological and health implications of toxic heavy metal and essential trace element interactions. Progress in Food Nutrition Science,11(1), 55–113.
Duggal, V., Rani, A., Mehra, R., et al. (2017). Risk assessment of metals from groundwater in northeast Rajasthan. Journal of the Geological Society of India,90(1), 77–84.
Egbueri, J. C. (2018). Assessment of the quality of groundwaters proximal to dumpsites in Awka and Nnewi metropolises: a comparative approach. International Journal of Energy and Water Resources. https://doi.org/10.1007/s42108-018-0004-1.
Egbueri, J. C. (2019a). Water quality appraisal of selected farm provinces using integrated hydrogeochemical multivariate statistical, and microbiological technique. Modeling Earth Systems and Environment. https://doi.org/10.1007/s40808-019-00585-z.
Egbueri, J. C. (2019b). Evaluation and characterization of the groundwater quality and hydrogeochemistry of Ogbaru farming district in southeastern Nigeria. SN Applied Sciences. https://doi.org/10.1007/s42452-019-0853-1.
Egbueri, J. C., Mgbenu, C. N., & Chukwu, C. N. (2019). Investigating the hydrogeochemical processes and quality of water resources in Ojoto and environs using integrated classical methods. Modeling Earth Systems and Environment. https://doi.org/10.1007/s40808-019-00613-y.
Fergusson, J. E. (1990). The heavy elements: chemistry, environmental impact and health effects. Oxford: Pergamon Press.
Finch, L. E., Hillyer, M. M., & Leopold, M. C. (2015). Quantitative analysis of heavy metals in children’s toys and jewelry: a multi-instrument multitechnique exercise in analytical chemistry and public health. Journal of Chemical Education,92, 849–854.
Garba, S. T., & Abubakar, M. A. (2018). Source and Distribution of the Heavy Metals: Pb, Cd, Cu, Zn, Fe, Cr, and Mn in Soils of Bauchi Metropolis, Nigeria. American Journal of Engineering Research,7(2), 13–23.
Kaiser, H. F. (1960). The application of electronic computers to factor analysis. Educational and Psychological Measurement,20, 141–151.
Kampsax-Kruger and Sshwed Associates (1977) Final Report: Underground water resources of the Metropolitan Lagos. Lagos State Ministry of works, 170 p.
Lim, H. S., Lee, J. S., Chon, H. T., et al. (2008). Heavy metal contamination and health risk assessment in the vicinity of the abandoned Songcheon Au–Ag mine in Korea. Journal of Geochemical Exploration,96, 223–230.
Longe, E. O., Malomo, S., & Olorunniwo, M. A. (1987). Hydrogeology of Lagos metropolis. Journal of African Earth Science,6(3), 163–174.
Mgbenu, C. N., & Egbueri, J. C. (2019). The hydrogeochemical signatures, quality indices and health risk assessment of water resources in Umunya district, southeast Nigeria. Applied Water Science,9, 22. https://doi.org/10.1007/s13201-019-0900-5.
NIS (Nigerian Industrial Standard). (2007). Nigerian standard for drinking water quality. Nigerian Industrial Standard,554, 13–14.
Nwajide, C. S. (2013). Geology of Nigeria’s sedimentary basins (p. 565p). Lagos: CSS Bookshops Ltd.
Omatsola, M. E., & Adegoke, O. S. (1981). Tectonic evolution of Cretaceous stratigraphy of the Dahomey basin. Journal of Mining and Geological Engineering,18(1), 130–137.
Rahman, M. M., Islam, M. A., Bodrud-Doza, M., et al. (2017). Spatio-temporal assessment of groundwater quality and human health risk: A case study in Gopalganj Bangladesh. Exposure and Health. https://doi.org/10.1007/s12403-017-0253-y.
Singh, G. (1987). Mine water quality deterioration due to acid mine drainage. International Journal of Mine Water,6, 49–61.
Soltani, S., Asghari Moghaddam, A., Barzegar, R., Kazemian, N., & Tziritis, E. (2017). Hydrogeochemistry and water quality of the Kordkandi-Duzduzan plain, NW Iran: application of multivariate statistical analysis and PoS index. Environmental Monitoring and Assessment,189, 455.
Su, H., Kang, W., Xu, Y., et al. (2017). Assessing groundwater quality and health risks of nitrogen pollution in the Shenfu mining area of Shaanxi Province. Northwest China: Exposure and Health. https://doi.org/10.1007/s12403-017-0247-9.
Subba Rao, N., Srihari, C., Spandana, B. D., et al. (2019). Comprehensive understanding of groundwater quality and hydrogeochemistry for the sustainable development of suburban area of Visakhapatnam, Andhra Pradesh, India. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2019.1571403.
Taiwo, A. M., Michael, J. O., Gbadebo, A. M., et al. (2019). Pollution and health risk assessment of road dust from Osogbo metropolis, Osun state, Southwestern Nigeria. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2018.1563478.
Thanomsangad, P., Tengjaroenkul, B., Sriuttha, M., et al. (2019). Heavy metal accumulation in frogs surrounding an e-waste dump site and human health risk assessment. Human and Ecological Risk Assessment. https://doi.org/10.1080/10807039.2019.1575181.
Ukah, B. U., Igwe, O., & Ameh, P. (2018). The impact of industrial wastewater on the physicochemical and microbiological characteristics of groundwater in Ajao-Estate Lagos, Nigeria. Environmental Monitoring and Assessment,190, 235.
US-EPA (US Environmental Protection Agency) (1989) Risk assessment guidance for superfund, vol. 1, Human health evaluation manual (Part A) Office of Emergency and Remedial Response, Washington, DC.
US-EPA (US Environmental Protection Agency). (1999). A risk assessment–multi way exposure spread sheet calculation tool. Washington, DC: United States Environmental Protection Agency.
US-EPA (US Environmental Protection Agency) (2011) Exposure Factors Handbook, 2011 Edition (Final Report). EPA/600/R-09/052F. Washington DC.
US-EPA (US Environmental Protection Agency) (2017) National Recommended Water Quality Criteria—Aquatic Life Criteria Table and Human Health Criteria Table.
Utom, A. U., Odoh, B. I., & Egboka, B. C. E. (2013). Assessment of hydrogeochemical characteristics of groundwater quality in the vicinity of Okpara coal and Obwetti fireclay mines, near Enugu town, Nigeria. Applied Water Science,3, 271–283.
Wagh, V. M., Panaskar, D. B., Mukate, S. V., et al. (2018). Health risk assessment of heavy metal contamination in groundwater of Kadava River Basin, Nashik, India. Modeling Earth Systems and Environment. https://doi.org/10.1007/s40808-018-0496-z.
WHO. (2017). Guidelines for drinking water quality (3rd ed.). Geneva: World Health Organization.
Acknowledgements
The authors wish to thank all who assisted in conducting this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest regarding this paper.
Rights and permissions
About this article
Cite this article
Ukah, B.U., Egbueri, J.C., Unigwe, C.O. et al. Extent of heavy metals pollution and health risk assessment of groundwater in a densely populated industrial area, Lagos, Nigeria. Int J Energ Water Res 3, 291–303 (2019). https://doi.org/10.1007/s42108-019-00039-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42108-019-00039-3