Abstract
An integrated approach of pollution evaluation indices and statistical techniques was employed to assess the intensity and sources of pollution in Curtin Lake water, Miri City, East Malaysia. Fe, Pb and Se concentrations in most of the water samples exceed the maximum admissible concentration. The heavy metal evaluation index (HEI) shows strong correlations with heavy metal pollution index (HPI) and degree of contamination (C d), and gives a better assessment of pollution levels. Samples from all the 25 locations in the lake were classified as high in C d and low in HPI compared with the respective critical values. The modified schemes of HPI and C d show comparable results with HEI and indicate that about 48 % of the samples with values lower than mean were classed as low contamination and the remaining samples (52 %) with values greater than the mean were classed as medium contamination. Cluster analysis, principal component analysis and pollution indices reveal that the quality of water is mainly controlled by natural/geogenic processes with minor anthropogenic input. US Salinity Laboratory plot and EC classification were also been used to assess the suitability of lake water for agricultural purpose. The current distribution level of heavy metal in the lake water is of environmental and health concerns and needs attention.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
There is worldwide water quality deterioration primarily contributable to growing human populations and economical development, particularly elevating nutrients leading to eutrophication and heavy metal pollution in the aquatic environment (Krishna et al. 2009; Nriagu and Pacyna 1988; Peierls et al. 1998; Holloway et al. 1998; Li et al. 2008; Pekey et al. 2004). The natural sources of the metals include volcanism, bedrock erosion, atmospheric transport and the release from plants (Krishna et al. 2009; Pekey et al. 2004) and anthropogenic activities; particularly, mining and mineral processing have dominant influences on the biogeochemical cycles of trace metals (Krishna et al. 2009; Nriagu and Pacyna 1988; Nriagu 1989, 1996). Heavy metal pollution leads to serious human health hazards through the food chain and the loss of biodiversity and harms the environmental quality. Recent researches into trace elements and heavy metals show highly interesting records (Zhang et al. 2009). Of which, their spatial variability reflects geological parent materials and anthropogenic sources in geographic heterogeneity (Imperato et al. 2003).
A number of significant studies are available in the literature on heavy metal pollution in water sources. Such works include Brown-Adiuku and Ogezi (1991), Edet and Ntekim (1996), Xibao et al. (1996), Yang et al. (1996), Yiping and Min (1996), Zhongyi (1996). All these studies concluded that there was a need to monitor and assess the water quality on a regular basis. This is due to the increase in concentration of heavy metals in potable water, which increase the threat to health and environment. Also, few methods exist in literature on the development and application of index methods for water quality assessment (Venkata Mohan et al. 1996; Prasad and Jaiprakas 1999; Edet and Offiong 2002; Bhuiyan et al. 2010).
The groundwater potential is limited to some pockets of the coastal region in Malaysia and is generally exploited by rural people to supplement their piped water supply. In Miri City, the surface water is readily available throughout the year and mainly utilized for irrigation and domestic uses. Surface water represents 97 % of the total water use, while groundwater represents 3 %. The proposed study area (Miri) is surrounded by the coastal region, industrial and agricultural areas, squatted colonies and commercial areas. The increase in human population and economic activities in this region has grown in scale; the demand for large-scale supplies of freshwater from various competing end users has increased. Declining quality and quantity of water supply of the area can be attributed to the water pollution and improper management of the existing resource.
A comparative assessment of toxic heavy metals is important for determining the degree of pollution in the environment. However, interpretation of data sets comprising analyses of numerous metals is complicated. One approach of simplifying multivariate data is to generate and use a single value, which may subsequently be used for comparative purposes (Miyai et al. 1985; Nimic and Moore 1991). Methods of integrating numerous variables associated with water quality in a specific index are increasingly desired in national and international scenarios. Therefore, several researchers have developed various indices, technically referred to as water quality indices (WQIs) (Lermontov et al. 2009). Usually, water quality index (WQI) is a practical and comparatively simple approach of evaluating the composite influence of overall pollution and hardly provides evidences of the pollution sources. The pollution indices are proposed to provide a useful and comprehensible guiding tool for water quality executives, environmental managers, decision makers and potential users of a given water system. The WQI was initially developed in the USA by Horton (1965) and has been widely used in Africa and Asia (Shoji et al. 1966; Handa 1981; Erondu and Nduka 1993; Palupi et al. 1995; Li et al. 2009). Statistical approaches, particularly multivariate techniques, are competent for resolving this deficit of WQI and are useful for environmental data reduction and interpretation of multiple elements. Principal components analysis (PCA) and cluster analysis (CA) have been considered as a more trustworthy approach for data mining of matrices from environmental quality assessment (Astel et al. 2007, 2008; Simeonova and Simeonov 2007). However, PCA and CA are widely used in water quality assessment.
Hence, the present study evaluates the heavy metal concentration in the surface water of Curtin Lake, Miri, East Malaysia. Pollution indices and multivariate approaches (PCA and CA) are used to identify the pollution status and probable sources of pollutants in the lake. The present study has been conducted by comparative evaluations of heavy metal pollution index (HPI), heavy metal evaluation index (HEI) and degree of contamination (C d), which have been successfully used by many researchers (Mohan et al. 1996; Prasad and Jaiprakas 1999; Prasad and Bose 2001; Teng et al. 2004; Prasad and Mondal 2008; Offiong and Edet 1998a, b; Edet and Offiong 2002; Rapant et al. 1999).
Study area
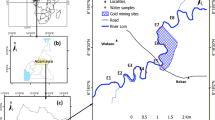
The proposed study area (Fig. 1), Curtin Lake, is located in Curtin University, Sarawak Campus of Miri City, Sarawak State in the east, on the island of Borneo, Malaysia. Sarawak is generally mountainous with the highest range forming the border with Indonesia. The areas of Miri are characterized by a plateau, where young alluvial sediments overlay the folded and monoclinally dipping Late Miocene to Pliocene Lambir and Tukau clastics. The rocks exposed around the Miri City belong to the Middle Miocene Miri formation. Stratigraphically, the rocks belong to the Miri formation; a stack of deltaic cycles forming a layered clay-sand sequence (85 % sand and 15 % clay) with laterally discontinuous sand bodies. The Miri formation [divided as Upper (mostly sand) and Lower part (well-defined beds of shale inter-bedded with sandstones)] is predominantly arenaceous with clay and shale restricted mainly to the lower part. The base of the formation is a gradual transition from the argillaceous Setap shale to the sandy Miri formation (Hutchison 2005). The climate is governed by the regime of the northeast and southwest monsoons. The northeast monsoon blows from October to March, and is responsible for the heavy rains which hit the east coast of the peninsula and frequently cause widespread floods. It also causes the wettest season in the Sarawak State. The southwest monsoon period occurs between May and September and is a drier period. The period between these two monsoons is marked by heavy rainfall. The average temperature throughout the year is very stable (26 °C). In general, Sarawak State experiences more rainfall (3,000–4,000 mm) than the Peninsula. The humidity is high (80 %) due to the high evaporation rate. Out of an annual rainfall volume of 990, 360 km3 is lost to evapotranspiration. The total surface runoff is 566 km3, and about 64 km3 (7 % of the total annual rainfall) contributes to groundwater recharge. However, about 80 % of the groundwater flow returns to the rivers and is therefore not considered an additional resource.
Methodology
Sample collection, and physicochemical and elemental analyses
A total of 25 surface water samples were collected (Fig. 1) in Curtin Lake. The water samples collected below the water surface using 200 ml polyethylene bottles. Prior to sampling, the bottles were rinsed with the water to be sampled and the samples were preserved by acidifying to pH ~ 2 with HNO3 and kept at a temperature of 4 °C until analysis. pH and electrical conductivity measurements were performed in situ with a portable meter. The collected water samples were filtered using a pre-conditioned plastic Millipore filter unit equipped with a 0.45-µm filter membrane for further elemental analysis. The elements (Ca, Mg, Na, K, Al, Ba, Cu, Fe, Ga, In, Li, Mn, Ni, Pb, Rb, Se, Sr, V, U and Zn) were analyzed using inductively coupled plasma-optical emission spectrometer (ICP-OES) Optima 5000 DV Series (Perkin Elmer). It comes with WinLab32 Software which optimizes the work flow and accuracy. Appropriate quality control/quality assurance samples were collected to provide confidence in the data regarding bias and variability. No replicates were analyzed for these samples. Equipment blank was used to test for bias from possible contamination of blank water, which consists of distilled water. This is to verify that decontamination procedures and laboratory protocols are adequate (Koterba et al. 1995).
Pollution evaluation indices
Generally, pollution indices are estimated for a specific use of the water under consideration. The indices used in this study, namely heavy metal pollution index (HPI), heavy metal evaluation index (HEI) and degree of contamination (C d), are determined for the purpose of evaluating drinking and agriculture water quality. The HPI and HEI methods provide an overall quality of the water with regard to heavy metals. These methods are evaluated using the ratios of monitored values of the desired number of parameters and the maximum admissible concentrations of the respective parameters. In the C d method, the quality of water is evaluated by computation of the extent of contamination. The C d is calculated independently for every sample of water analyzed, and is computed as the sum of the contamination factors of each component exceeding the upper permissible limit. Therefore, the C d summarizes the combined effects of a number of quality parameters regarded as unsafe to household water.
Heavy metal pollution index
HPI index was developed by assigning a rating or weightage (W i ) for each chosen parameter. The rating is an arbitrary value between 0 and 1 and its selection reflects the relative importance of individual quality considerations. It can be defined as inversely proportional to the standard permissible value (S i ) for each parameter (Horton 1965; Mohan et al. 1996; Reddy 1995). In this present study, the concentration limits (i.e., the standard permissible value (S i ) and highest desirable value (I i ) for each parameter) were taken from the WHO standard. The uppermost permissive value for drinking water (S i ) refers to the maximum allowable concentration in drinking water in the absence of any alternate water source. The desirable maximum value (I i ) indicates the standard limits for the same parameters in drinking water (Bhuiyan et al. 2010).
The HPI, assigning a rating or weightage (W i ) for each selected parameter, is determined using the expression below (Mohan et al. 1996):
where Q i and W i are the sub-index and unit weight of the ith parameter, respectively, and n is the number of parameters considered. The sub-index (Q i ) is calculated by
where M i , I i and S i are the monitored heavy metal, ideal and standard values of the ith parameter, respectively. The sign (−) indicates numerical difference of the two values, ignoring the algebraic sign.
Heavy metal evaluation index
HEI gives an overall quality of the water with respect to heavy metals (Edet and Offiong 2002) and is expressed as:
where H c and H mac are the monitored value and maximum admissible concentration (MAC) of the ith parameter, respectively.
Degree of contamination (C d)
The contamination index (C d) summarizes the combined effects of several quality parameters considered harmful to domestic water (Backman et al. 1997) and is calculated as follows:
where
where \( C_{{{\text{f}}_{i} }} , \) \( C_{{{\text{A}}_{i} }} \) and \( C_{{{\text{N}}_{i} }} \) represent contamination factor, analytical value and upper permissible concentration of the ith component, respectively. N denotes the ‘normative value’ and \( C_{{{\text{N}}_{i} }} \) is taken as MAC.
Statistical analysis
The analytical data were subjected to statistical analysis using SPSS software (version 9.0 for Windows). Principal component analysis was used to identify the possible sources of heavy metals. Factor analysis was performed by varimax rotation (Howitt and Cramer 2005), which minimized the number of variables with a high loading on each component, thus facilitating the interpretation of PCA results. Cluster analysis was applied to identify groups of samples with similar heavy metal contents (Panda et al. 2006). CA was formulated according to the Ward-algorithmic method, and the rescaled linkage distance was employed for measuring the distance between clusters of similar metal contents. R-mode CA was used to determine the association of different water quality parameters and pollutant sources. Pearson’s correlation matrix was also used to identify the elements’ relationship.
Results and discussion
Water quality and classification
The physicochemical parameters and total metal concentrations of surface water are shown in Table 1. The electrical conductivity (EC) varied from 330 to 470 µS/cm with a mean of 378.80 µS/cm. The range, mean and standard deviation (SD) of pH are 6.46–8.27, 6.74 and 0.40. The mean metal levels in surface water followed a descending order as: Fe > Al > Ba > Sr > U > Rb > Mn > Se > Li > V > Zn > In > Pb > Cu > Ni > Ga. The descriptive statistics including maximum admissible concentration (MAC) and world standards are given in Table 2. The concentration of Cu, Mn, Ni and Zn are below the MAC values. The concentration of Fe (1607.8–1946.83 µg/l) in all the samples is higher than the MAC of 200 µg/l, 40 % of the samples show Pb in excess of 1.50 µg/l and 28 % of the samples show Se in excess of 10 µg/l. The method of Ficklin et al. (1992), modified by Caboi et al. (1999), was employed for water classification. Figure 2 shows the relationship between total metal contents (mg/l) and pH for the samples. The metal load was computed as Al + Ba + Cu + Fe + Ga + In + Li + Mn + Ni + Pb + Rb + Se + Sr + V + U + Zn (mg/l) and all the samples plot in the field of near-neutral high metal.
Shuhaimi-Othman et al. (2008) reported that the mean metal concentration in surface water of Chini Lake, Peninsular Malaysia was low and within the range of natural background values except for Fe and Al. Aqeel Ashraf et al. (2010) also reported that high nutrient load and concentration of metals, especially mercury in Varsity Lake, West Malaysia. Results from this study indicate that the mean metal concentration of Curtin Lake was high as compared to Chini Lake except for Pb and Zn (Table 3). The concentration of Se is also higher in Curtin Lake as compared to other lakes worldwide (e.g., Masresha Alemayehu et al. 2011; Markert et al. 1997; Singanan et al. 2008) except for Manchar Lake, Pakistan (Kazi et al. 2009).
Pollution evaluation indices
The results of pollution evaluation indices are presented in Table 4. The heavy metal pollution index of all the sampling points have been calculated individually using the international standards (Edet and Offiong 2002) and is represented by HPI, respectively. The range and mean values of HPI were 2.86–7.92 and 4.84. The results of indices showed that the HPI for all the samples were below the critical limit of 100 proposed for drinking water by Prasad and Bose (2001). The heavy metal pollution index calculated with mean concentration values of all metals, including all sampling points is 4.84, which is also well below the critical limit of 100.
The degree of contamination (C d) was used as reference of estimating the extent of metal pollution (Al-Ami et al. 1987). The range and mean values of C d were 2.57–6.11 and 4.01. C d may be classified into three categories (Edet and Offiong 2002; Backman et al. 1997) as follows: low (C d < 1), medium (C d = 1–3) and high (C d > 3). All the samples exceed 3, suggesting that they are highly polluted. On the contrary, the HPI values for all the locations are lower than 100, the critical value (Prasad and Bose 2001) for drinking water.
The heavy metal evaluation index (Edet and Offiong 2002) was used for a better understanding of the pollution indices. The HEI values ranged from 8.57 to 12.11 with a mean value of 10.01. The mean deviations and percentage deviation for all the indices were computed for each sampling point (Table 4); 48 % of the samples (S6, S8–S15, S21, S24 and S25) fell below the respective mean values of HEI. Interestingly, C d, and HPI values of these same samples were below the respective mean value of the indices. These values of C d and HPI which fall below their respective mean values and their corresponding negative percent deviations suggest relatively better quality as observed by Prasad and Bose (2001), Edet and Offiong (2002).
C d, HPI and HEI values show similar trends at various sampling points (Fig. 3) and also significant correlations are observed among the values (Table 5). However, there are some differences between the results of C d and HPI regarding the water quality of the analyzed samples. Therefore, HEI has been used to synchronize the criteria for various pollution indices. By following the approach of Edet and Offiong (2002), the calculated HEI values have been classified in terms of pollution levels as low, medium and high. Different HEI criteria values have been developed for the samples, guided by their respective mean values, and the different levels of contamination are demarcated by a multiple of the mean values. Therefore, the proposed HEI criteria for the samples are as follows: low (HEI < 10), medium (HEI = 10–20) and high (>20). The present level shows that 48 % of samples are within the low zone, while 52 % fall within the medium zone.
The existing water quality schemes for HPI and C d have also been modified following the mean approach of HEI, and the results are presented in Table 6. Thus for the C d, 48 and 52 % of the samples, respectively, are classed as low and medium zones. For the HPI, 56 and 44 % are classified as low and medium contamination.
For examine the contribution of the key metals to the computed indices, correlation was performed between the indices (C d, HPI and HEI) and heavy metal concentrations. From the analysis, Fe, Pb, Li and U show significant correlations with all the indices, suggesting that these metals are the major contributory parameters (Table 5), where Li and U are not considered in the indices calculation due to lack of standard values. Significant correlations are also observed among the values of HPI, HEI and C d.
The HEI and reclassification schemes of HPI and C d show comparable results, and the HEI method may be used as the simple criteria of assessing the quality of water in the lake. Thus, samples S6, S8–S15, S21, S24 and S25 may be considered as less contaminated, whereas S1–S5, S7, S16–S20, S22 and S23 are moderately contaminated by metal pollutants.
Pollution source identification
The principal component analysis was used to further explore the extent of metal pollution and for source identification (Dragovíc et al. 2008; Franco-Uría et al. 2009). Varimax rotation method (Gotelli and Ellison 2004) was used to maximize the sum of the variance of the factor coefficients, which better explained the possible groups/sources that influenced the water system.
Five factors were extracted for surface water data set with eigenvalues >1. The calculated factor loadings, together with cumulative percentage and percentages of variance explained by each factor, are listed in Table 7. The factors in samples led to reductions of the initial dimensions of the data set, which explained about 76 % of the total variance. The calculated factor scores for all the samples are presented in Table 7. Positive scores in PCA indicate that water samples are affected by the presence of the parameters that are significantly loaded on a specific factor, whereas negative scores suggest that water quality is essentially unaffected by those parameters (Prasanna et al. 2010)
PC1, PC2, PC3, PC4 and PC5 explain about 21, 18, 17, 10 and 8 % of the total variance, respectively. PC1 is highly loaded on EC, pH, Ba, Ga, In and Li, which are mostly distributed in S1, S2, S4, S8, S9 and S12. PC1 explains leaching of materials from the soil surfaces or in the sediment of water soils. PC2 is loaded on Al, Fe, Mn, Li and U and could represent a geogenic source component. Fe and Mn could be released by leaching of parent materials from the soil horizon to the water. The solubility of Fe and Mn minerals is strongly redox controlled, particularly at near-neutral pH (Lorite-Herrera et al. 2008). The dissolved Al concentration in surface water is controlled by the solubility of primary silicate weathering products such as kaolinite or illite (Prasanna et al. 2010). These parameters are importantly distributed in S1, S5, S15, S16, S18, S20 and S21, since these samples retain high scores for PC2 (Table 6). PC3 is mostly contributed by Cu, Ni, Se and Zn, representing atmospheric input in the lake water originating from the surrounding industrialized regions (Steinnes and Henriksen 1993; Hanssen et al. 1980), which are significantly distributed in S1, S3, S13 and S14. PC4 is loaded on Pb only, which occurs as an important parameter in S2–S6, S16 and S23. Pb occurs naturally in the environment as geochemical alteration of sulfide minerals. However, most of the Pb concentrations are also found as a result of anthropogenic activities such as automobile exhaust (Bhuiyan et al. 2010). PC5 is loaded on Sr and could be ascribed to geochemical alteration/weathering of sulfate minerals present in the sediment horizon. PC5 shows high scores for S4, S5, S7, S9, S20 and S21, which suggest that Sr is important indicator in these samples.
R-mode cluster analysis was also performed to understand the physicochemical and elemental groupings in the data set and the results are presented in Fig. 4. Parameters belonging to the same cluster are likely to have originated from a common source. The R-mode CA performed on the samples produced four clusters. Cluster 1 includes pH, EC, Ba, Li, Ga and In; cluster 2 consists of Cu, Zn, Ni and Se; cluster 3 contains Fe, Mn and U; cluster 4 includes Pb, Sr, Al, V and Rb. It reflects the influence of natural hydrogeochemical processes (leaching of materials from the soils) and minor anthropogenic input. Even though there are some differences between the CA and PCA results, a good agreement between the two statistical techniques is evident in all the data sets analyzed.
Correlation matrix (CM)
The Pearson’s correlation coefficient matrices for the analyzed parameters are presented in Table 8. The inter-parameters relationships support the results obtained from PCA, and the CM has also been useful in revealing some new association of metals that have not been properly stated in the PCA analysis. A significant correlation has been observed in the samples. pH significantly correlates with Ba (r = 0.94), Cu (r = 0.70), Ga (r = 0.63), Li (r = 0.81) and Ni (r = 0.60). EC also shows significant correlations with Ba (r = 0.52) and Li (r = 0.66). These results are similar to that of PC1 in the previous section. Metal pairs Ba–Li, Ba–Cu, Ba–Ga and Ba–Ni correlate significantly with respective correlation coefficient (r) values of 0.74, 0.60, 0.54 and 0.50, respectively, indicating a similar source of PC1. Fe correlates with Li (r = 0.62) and Mn (r = 0.69) similar to sources reported for PC2 in the PCA analysis of the samples. A significant correlation exists between Cu and Ni (r = 0.60), Zn (r = 0.76) and Se (0.51), matching with PC3 described in the previous section.
Irrigation water quality
The suitability of water for irrigation is conditional on the effects of mineral constituents of water on both the plant and soil. Excess amount of dissolved ions in irrigation water affects plants and agricultural soil physically and chemically, thus reducing the productivity (Bahar and Reza 2010). Electrical conductivity is a good measure of salinity hazard and it reflects the TDS in water. According to the irrigation-based EC classification by Ragunath (1987), all the samples fall in the range of 250–750 µs/cm, indicating good category and that the water can be used for irrigation purpose. The US Salinity Laboratory (USSL) also suggested a plot for ranking the irrigation water, wherein sodium absorption ratio (SAR) was plotted against specific conductance. SAR was calculated from the relation, SAR = [Na/(Ca++ + Mg++)1/2]. Sodium and salinity are the two important parameters, which can indicate the suitability of water for irrigation purposes. In the USSL plot (Fig. 5), all the samples fall in the C2S1 zone, indicating medium salinity and low sodium hazard. Therefore, these lake waters can be used for irrigation on almost all soils, with little hazards in the development of harmful level of exchangeable sodium (Hem 1985).
Conclusion
Pollution evaluation indices, principal component analysis, cluster analysis and correlation matrix (CM) have been used to assess the intensity and sources of pollution in the Curtin Lake. The concentrations of Fe in all samples are higher than the MAC, while 40 and 28 % of the samples show Pb and Se concentrations above the MAC. The surface water of this lake is characterized as near-neutral high metal, respectively. C d suggests that all samples are highly polluted (C d > 3), whereas HPI indicates that all the samples are within the critical limit (HPI > 100). A better water quality classification of the samples is attained by using heavy metal evaluation index. The HEI criteria assigned 52 % (HEI < 10), 48 % (HEI = 10–20) and 0 % (HEI > 20) of the samples in the categories of low, medium and high pollution, respectively. Comparable results to HEI are obtained when the existing HPI and C d schemes are reclassified using a multiple of the mean, as in the case of HEI.
Principal component analysis with the support of cluster analysis identified that natural/geogenic source (weathering and leaching of parent materials) and anthropogenic impact (from non-point sources) are responsible for controlling the variability of physicochemical parameters and metal contents in the lake water. That the CA and PCA results give a good agreement between the two statistical tools is evident in all the data sets analyzed.
Water quality analysis clearly shows that the elements (e.g., Fe, Pb and Se) released from natural hydrogeochemical processes with minor anthropogenic activities have a high potential for contaminating the lake water. Based on USSL plot and EC classification, the lake water is suitable for irrigation purposes. The contamination of the lake water by some heavy metals poses serious threat to ecological habitat and needs attention. Hence, this work gives background information on toxic metals and their possible sources in the surface water of Curtin Lake. This work has also highlighted the importance of an integrated approach of pollution evaluation indices and multivariate statistical methods in pollution studies of surface water systems.
References
Al-Ami MY, Al-Nakib SM, Ritha NM, Nouri AM, Al-Assina A (1987) Water quality index applied to the classification and zoning of Al-Jaysh canal, Bagdad, Iraq. J Environ Sci Health A(22):305–319
Aqeel Ashraf M, Jamil Maah M, Yuoff I (2010) Water quality characterization of Varsity Lake, University of Malaya, Kuala Lumpur, Malaysia. E J Chem 7(S1):S245–S254
Astel A, Tsakovski S, Barbieri P, Simeonov V (2007) Comparison of self-organizing maps classification approach with cluster and principal components analysis for large environmental data sets. Water Res 41:4566–4578. doi:10.1016/j.watres.2007.06.030
Astel A, Tsakovski S, Simeonov V, Reisenhofer E, Piselli S, Barbieri P (2008) Multivariate classification and modeling in surface water pollution estimation. Anal Bioanal Chem 390:1283–1292. doi:10.1007/s00216-007-1700-6
Backman B, Bodis D, Lahermo P, Rapant S, Tarvainen T (1997) Application of a groundwater contamination index in Finland and Slovakia. Environ Geol 36:55–64. doi:10.1007/s002540050320
Bahar MdM, Reza MdS (2010) Hydrochemical characteristics and quality assessment of shallow groundwater in a coastal area of Southwest Bangladesh. Environ Earth Sci 61:1065–1073
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392. doi:10.1016/j.jhazmat.2009.08.085
Brown-Adiuku ME, Ogezi AE (1991) Heavy metal pollution from mining practices: a case study of Zurak. J Min Geol 27(2):205–211
Caboi R, Cidu R, Fanfani L, Lattanzi P, Zuddas P (1999) Environmental mineralogy and geochemistry of the abandoned Pb–Zn Montevecchio-Ingurtosu mining district, Sardinia, Italy. Chron Rech Miniere 534:21–28
Dragovíc S, Mihailovíc N, Gajíc B (2008) Heavy metals in soils: distribution, relationship with soil characteristics and radionuclides and multivariate assessment of contamination sources. Chemosphere 72(3):491–549. doi:10.1016/j.chemosphere.2008.02.063
Edet AE, Ntekim EEU (1996) Heavy metal distribution in groundwater from Akwa Ibom State, eastern Niger delta-A preliminary pollution assessment. Global J Pure Appl Sci 2(1):67–77
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo–Odukpani area, Lower Cross River Basin (southeastern Nigeria). GeoJournal 57:295–304. doi:10.1023/B:GEJO.0000007250.92458.de
Erondu ES, Nduka EC (1993) A model for determining water quality index (WQI) for the classification of the New Calabar River at Aluer-Port Harcourt, Nigeria. J Environ Stud A44:131–134
Ficklin DJWH, Plumee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. In: Kharaka YK, Maest AS (eds) Water–rock interaction, vol 7. Balkema, Rotterdam, pp 381–384
Franco-Uría A, López-Mateo C, Roca E, Fernández-Marcos ML (2009) Source identification of heavy metals in pastureland by multivariate analysis in NW Spain. J Hazard Mater 165(1–3):1008–1015. doi:10.1016/j.jhazmat.2008.10.118
Gotelli NJ, Ellison AM (2004) A primer of ecological statistics, 1st edn. Sinauer Associates, Sunderland
Handa BK (1981) An integrated water-quality index for irrigation use. Indian J Agric Sci 51:422–426
Hanssen JE, Rambaek JP, Semb A, Steinnes E (1980) Atmospheric deposition of trace elements in Norway. In: Darblos D, Tollan A (eds) Ecological impact of acid precipitation. SNSF-project, Oslo-As, pp 116–117
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. USGS Water Supply Paper 2254:117–120
Holloway JM, Dahlgren RA, Hansen B, Casey WH (1998) Contribution of bedrock nitrogen to high nitrate concentrations in stream water. Nature 395:785–788. doi:10.1038/27410
Horton RK (1965) An index system for rating water quality. J Water Pollut Control Fed 37:300–306
Howitt D, Cramer D (2005) Introduction to SPSS in psychology: with supplement for releases 10, 11, 12 and 13. Pearson, Harlow
Hutchison CS (2005) Geology of North-West Borneo; Sarawak. Brunei and Sabah. Elsevier, Amsterdam
Imperato M, Adamo P, Naimo D, Arienzo M, Stanzione D, Violante P (2003) Spatial distribution of heavy metals in urban soils of Naples city (Italy). Environ Pollut 124:247–256. doi:10.1016/s0269-7491(02)00478-5
Kazi TG, Arain MB, Jamali MK, Jalbani N, Afridi HI, Sarfraz RA, Baig JA, Shah Abdul Q (2009) Assessment of water quality of polluted lake using multivariate statistical techniques: a case study. Ecotoxicol Environ Saf 72:301–309
Koterba MT, Wilde FD, Lapham WW (1995) Groundwater data collection protocols and procedures for the national water quality assessment program—collection and documentation of water quality samples and related data. US Geological Survey Open-File Report, 95–399, p 113
Krishna AK, Satyanarayanan M, Govil PK (2009) Assessment of heavy metal pollution in water using multivariate statistical techniques in an industrial area: a case study from Patancheru, Medak District, Andhra Pradesh, India. J Hazard Mater 167:366–373. doi:10.1016/j.jhazmat.2008.12.131
Lermontov A, Yokoyama L, Lermontov M, Machado MAS (2009) River quality analysis using fuzzy water quality index: Ribeira do Iguape river watershed, Brazil. Ecol Ind 9:1188–1197. doi:10.1016/j.ecolind.2009.02.006
Li S, Xu Z, Cheng X, Zhang Q (2008) Dissolved trace elements and heavy metals in the Danjiangkou Reservoir, China. Environ Geol 55:977–983. doi:10.1007/s00254-007-1047-5
Li Z, Fang Y, Zeng G, Li J, Zhang Q, Yuan Q, Wang Y, Ye F (2009) Temporal and spatial characteristics of surface water quality by an improved universal pollution index in red soil hilly region of South China: a case study in Liuyanghe River watershed. Environ Geol 58:101–107. doi:10.1007/s00254-008-1497-4
Lorite-Herrera M, Jimenez-Espinosa R, Jimeneze-Millan J, Hiscock KM (2008) Integrated hydrochemical assessment of the Quaternary alluvial aquifer of the Guadalquivir River, Southern Spain. Appl Geochem 23:2040–2054. doi:10.1016/j.apgeochem.2008.03.013
Markert B, Pedrozo F, Geller W, Friese K, Korhammer S, Baffico G, Diaz M, Wolfl S (1997) A contribution to the study of the heavy-metal and nutritional element status of some lakes in the southern Andes of Patagonia (Argentina). Sci Total Environ 206:1–15
Masresha Alemayehu E, Skipperud L, Rosseland BO, Zinabu GM, Meland S, Teien HC, Salbu B (2011) Speciation of selected trace elements in three Ethiopian Rift Valley Lakes (Koka, Ziway, and Awassa) and their major inflows. Sci Total Environ 409:3955–3970
Miyai M, Tada F, Nishida H (1985) Analysis of the composition of heavy metal pollution in Japanese river sediments by principal component analysis, Japan. J Limnol 46:169–173
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A31:283–289
Nimic DA, Moore JN (1991) Prediction of water-soluble metal concentrations in fluvially deposited tailings sediments, Upper Clark Fork Valley, Montana, USA. Appl Geochem 6:635–646. doi:10.1016/0883-2927(91)90074-Y
Nriagu JO (1989) A global assessment of natural sources of atmospheric trace metals. Nature 338:47–49. doi:10.1038/338047a0
Nriagu JO (1996) A history of global metal pollution. Science 272:223–224
Nriagu JO, Pacyna JM (1988) Quantitative assessment of worldwide contamination of air, water and soils by trace-metals. Nature 333:134–139. doi:10.1038/333134a0
Offiong OE, Edet AE (1998a) Water quality assessment in Akpabuyo, Cross River basin, southeastern Nigeria. Environ Geol 34(2–3):167–174. doi:10.1007/s002540050268
Offiong OE, Edet AE (1998b) Surface water quality evaluation in Odukpani, Calabar Flank, southeastern Nigeria. Environ Geol 36(3–4):343–348. doi:10.1007/s002540050350
Palupi K, Sumengen S, Inswiasri S, Agustina L, Nunik SA, Sunarya W, Quraisyn A (1995) River water quality study in the vicinity of Jakarta. Water Sci Technol 39:17–25
Panda UC, Sundaray SK, Rath P, Nayak BB, Bhatta D (2006) Application of factor and cluster analysis for characterization of river and estuarine water systems—a case study: Mahanadi River (India). J Hydrol 331:434–445. doi:10.1016/j.jhydrol.2006.05.029
Peierls BL, Caraco NF, Pace ML, Cole JJ (1998) Human influence on river nitrogen. Nature 350:386–387. doi:10.1038/.350386b0
Pekey H, Karaka D, Bakoglu M (2004) Source apportionment of trace metals in surface waters of a polluted stream using multivariate statistical analyses. Mar Pollut Bull 49:809–818
Prasad B, Bose JM (2001) Evaluation of the heavy metal pollution index for surface and spring water near a limestone mining area of the lower Himalayas. Environ Geol 41:183–188. doi:10.1007/s002540100380
Prasad B, Jaiprakas KC (1999) Evaluation of heavy metals in ground water near mining area and development of heavy metal pollution index. J Environ Sci Health A34(1):91–102
Prasad B, Mondal KK (2008) The impact of filling an abandoned open cast mine with fly ash on ground water quality: a case study. Mine Water Environ 27(1):40–45. doi:10.1007/s10230-007-0021-5
Prasanna MV, Chidambaram S, Shahul Hameed A, Srinivasamoorthy K (2010) Study of evaluation of groundwater in Gadilam basin using hydrogeochemical and isotope data. Environ Monit Assess 168:63–90. doi:10.1007/s10661-009-1092-5
Ragunath HM (1987) Groundwater. Wiley Eastern Ltd, New Delhi
Rapant S, Raposova M, Bodis D, Marsina K, Slaninka I (1999) Environmental geochemical mapping program in the Slovak Republic. J Geochem Explor 66:151–158. doi:10.1016/S0375-6742(99)00037-0
Reddy SJ (1995) Encyclopaedia of environmental pollution and control, vol 1. Environmental Media, Karlia
Shoji H, Yamamota T, Nakakaga N (1966) Factor analysis of stream pollution of the Yodo River system. Air Water Pollut 10:291–299
Shuhaimi-Othman M, Ahmad A, Mushrifah I, Lim EC (2008) Seasonal influence on water quality and heavy metals concentration in Tasik Chini, Peninsular Malaysia. In: Sengupta M, Dalwani R (eds) Proceedings of Tall2007: the 12th World Lake Conference, pp 300–303
Siegel FR (2002) Environmental geochemistry of potentially toxic metals. Springer, Berlin
Simeonova P, Simeonov V (2007) Chemometrics to evaluate the quality of water sources for human consumption. Microchim Acta 156:315–320. doi:10.1007/s00604-006-0643-5
Singanan M, Wondimu L, Tesso M (2008) Water quality of Wenchi Crater Lake in Ethiopia. Maejo Int J Sci Technol 02(02):361–373
Steinnes E, Henriksen A (1993) Metals in small Norwegian lakes: relation to atmospheric deposition of pollutants. Water Air Soil Pollut 71:167–174
Teng Y, Ni S, Jiao P, Deng J, Zhang C, Wang J (2004) Eco-environmental geochemistry of heavy pollution in Dexing Mining Area. Chin J Geochem 23(4):349–358. doi:10.1007/BF02871307
Venkata Mohan S, Nithila P, Jayarama Reddy S (1996) Estimation of heavy metals in drinking water and development of heavy metal pollution index. J Environ Sci Health A31(2):283–289
World Health Organisation (WHO) (1993) Guidelines for drinking water quality, Recommmendations. Geneva, Switzerland
World Health Organisation (WHO) (2004) Guidelines for drinking water quality (3rd edn.) (ISBN: 9241546387). Retrived from http://www.who.int/water_sanitation_health/dwq/guidelines/en/
Xibao C, Shen Z, Licheng Z (1996) A study of the physicochemical speciation of heavy metals in waters of rivers and lakes in the Changjiang River valley, China. GeoJournal 40(1–2):187–195. doi:10.1007/BF00222544
Yang W, Liensheng Y, Jianxun Z (1996) Effect of metal pollution on the water quality in Taiku Lake. GeoJournal 40(1–2):197–200. doi:10.1007/BF00222545
Yiping H, Min Z (1996) The water quality of Lak Taihu and its protection. GeoJournal 40(1–2):39–44. doi:10.1007/BF00222529
Zhang W, Feng H, Chang J, Qu J, Xie H, Yu L (2009) Heavy metal contamination in surface sediments of Yangtze River intertidal zone: an assessment from different indexes. Environ Pollut 157(5):1533–1543. doi:10.1016/j.envpol.2009.01.1007
Zhongyi W (1996) Surface water chemical changes due to human activities in the Tarim Basin. GeoJournal 40(1–2):25–29. doi:10.1007/BF00222527
Acknowledgments
The authors wish to express their thanks to Curtin Sarawak Research Fund (CSRF) for providing necessary financial support to carry out this study. They are also thankful to the School of Science and Technology, Universiti Malaysia Sabah, Kota Kinabalu, Malaysia for helping in the analysis of heavy metals in the water samples.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasanna, M.V., Praveena, S.M., Chidambaram, S. et al. Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci 67, 1987–2001 (2012). https://doi.org/10.1007/s12665-012-1639-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12665-012-1639-6