Abstract
Groundwater resources can be potentially a target for various sources of contaminations. One major step towards characterization of contamination sources and the associated parameters, is to conduct groundwater quality assessment through different methods. When dealing with heavy metals, calculation of pollution indices is among well-known techniques of contamination characterization. The objectives of this study include primary assessment of physicochemical parameters of the groundwater and heavy metal concentrations and finding distributions using multivariate statistical methods in the study area (i.e. north Isfahan province, Iran). Heavy metal pollution index (HPI) and metal index (MI) were the two indices evaluated for contamination assessment of heavy metals in 35 samples drawn within the study area. Generally, results indicated that the HPI of the groundwater in the study area was less than the critical value (< 100). Based on HPI values, 51% of samples were classified as having low pollution levels, 46% medium pollution levels and 3% high pollution levels. The MI results revealed that two samples in the study area were significantly polluted with heavy metals. Water-type classification according to dominant cations and anions was also conducted and the result identified four types of water: Na–Cl, Na–SO4, Na–HCO3 and Ca–HCO3. Multivariate statistical techniques [namely, correlation analysis and principal component analysis (PCA)] were applied for the evaluation of variations and interpretation of a large complex groundwater quality data set from the study area. The principal component analysis (PCA) extracted four components that control the groundwater chemistry. Findings of our study can be used in devising preventive measures to control pollution in the study area and similar regions where the groundwater resource would be relied upon for drinking purposes in the future.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater resources are among most important sources of fresh water. Similar to surface water resources, water quality is an important environmental issue in groundwater worldwide which largely depends on a number of physicochemical parameters (Kumar Tiwari et al. 2017). Characterization of water quality for groundwater resources should be carried out before making any decisions as how to allocate water for different purposes (drinking, irrigation, industry, etc.). In many regions of the world, groundwater chemistry has been utilized as a measure to determine water quality for drinking and irrigation purposes (Edmunds et al. 2002).
Complexity of geological formations and flow domains are widely observed in groundwater resources. Hydrogeological and geochemical studies are, therefore, warranted for groundwater quality assessment and groundwater resource management, particularly in an event when groundwater quality is potentially degraded by non-natural activities (Colak 2012; Tang et al. 2013; Jabbari et al. 2016). Groundwater sampling and monitoring are also required on an ongoing basis to back up hydrogeological studies and to more accurately assess water quality and propose sustainable management strategies.
Heavy metals are carcinogenic compounds that are among the most common environmental pollutants with their occurrence in water and biota reflecting presence of natural or anthropogenic sources (Adaikpoh et al. 2005; Akoto et al. 2008). Heavy metals exist in water in colloidal, particulate and dissolved phases (Adepoju-Bello et al. 2009) with their occurrence in water bodies being either of natural origin (e.g. eroded minerals within sediments, leaching of ore deposits and volcanism extruded products) or of anthropogenic origin (i.e. solid waste disposal, industrial or domestic effluents) (Marcovecchio et al. 2007). Variations in natural and human activities reflect spatial variations of the aquifer and the hydrochemical parameters of the groundwater. Elevated heavy metal concentrations in water lead to environmental problems which make it necessary to center studies around potential water contamination from metals sources. Industrial, agricultural, and solid waste disposal practices are famous examples of human activities playing role in increasing levels of heavy metals in water, soil, and air (Adekunle et al. 2007; Nagajyoti et al. 2010; Jaishankar et al. 2014).
When studying heavy metals, pollution parameters are generally monitored for assessment of water quality and are then compared with allowable heavy metal concentrations recommended by water standards. Several water quality indices and corresponding applications of index methods have been proposed for estimation of water quality (Edet and Offiong 2002; Bhuiyan et al. 2010). Pollution indices are useful tools for water quality executives, environmental managers, and decision makers as measures which have combined influences of all parameters. In recent years, much attention has been given to evaluation of heavy metal pollution in groundwater using the heavy metal pollution index (HPI) (Rajkumar et al. 2015). The spatial study of heavy metals HPI can be helpful in identifying and quantifying trends in water quality (Mishra et al. 2017). Another index is the general metal index (MI) for drinking water, which takes into account possible additive effects of heavy metals on human health (Enaam Abdullah 2013). MI helps to quickly evaluate the overall quality of drinking water (Enaam Abdullah 2013).
Metals indices can be coupled with statistical methods to obtain more robust results when analyzing water quality data. Multivariate statistical techniques, such as correlation matrix and principal component analysis (PCA), are examples of statistical methods that have been broadly used by researchers around the world for the assessment of water quality (Arora and Mehra 2009; Vieira et al. 2012). Application of multivariate statistical techniques facilitates interpretation of complex data matrices for a better understanding of water quality and a variety of environmental factors.
In this study, we are focused on assessing the prevailing water quality condition to identify the pollution status and probable sources of pollutants in the study area. This work is primarily aimed to assess physicochemical parameters of groundwater [i.e. pH, electrical conductivity (EC), temperature, and total dissolved solids (TDS)] and calculate HPI and MI indices. The indices are used as quantitative criteria to pinpoint areas with high degree of heavy metal pollution. Spatial frequencies and distributions of heavy metal concentration are then evaluated by applying multivariate statistical methods. Heavy metals of concern include arsenic (As), chromium (Cr), copper (Cu), nickel (Ni), lead (Pb), and zinc (Zn). The result of this research can help identifying segments of groundwater with suitable quality for drinking water purposes.
Study area
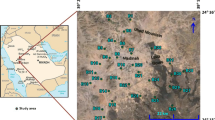
Similar to many other countries in arid and semi-arid regions of the world, groundwater is a major source of water supply for domestic, industrial and agricultural sectors in Iran (Rezaei and Hassani 2017). The area of study of this research is located in north of Isfahan province in Iran which is historically known for low annual precipitations and long droughts. More precisely, this area is located in northern part of the Sanandaj–Sirjan zone and southern part and middle division of central Iran tectonic zone. The study area lies between longitudes 52°15′00″ to 53°15′00″E and latitudes 33°20′05″ to 33°30′05″N, covering an estimated area of approximately 1500 km2 (Fig. 1). Groundwater is an important water resource especially for drinking and agricultural uses in the study area.
Geological features and information of the study area are discussed in this section. The main lithology consists of Eocene volcanic and pyroclastic rocks (Fig. 2). In the Oligocene–Miocene, marl, limestone, sandstone, shale and gypsum units are known as Qom Formation. In the Late Miocene, deposition of sandstone, marl, conglomerate and evaporate rocks known as the upper red formation developed in a molasses-type condition of a sedimentary environment as a result of slow positive movement of the basin (Babaahmadi et al. 2010). Natural sources of heavy metals include volcanism, bedrock erosion, and atmospheric transport. The main sources of potential pollution are deemed to be related to activities in mining, agricultural, municipal and also in part to natural sources.
Geological map of the study area (Amidi and Zahedi 1988)
Materials and method
As the first step in investigation of heavy metals concentrations, samples were drawn from 35 monitoring wells in the area. Specifications and coordinates of sampling stations are listed in Table 1.
Water samples were collected from stations during April and May 2016. Location of sampling stations is demonstrated in Fig. 1 and Table 1. Physicochemical parameters including temperature, pH, electrical conductivity (EC), and total dissolved solids (TDS) were measured in situ, using a multi-parameter portable meter (HATCH, Germany). Groundwater sample collection procedure included drying, cleaning and sterilizing in polyethylene bottles. Samples were, then, labeled, sealed and transported to the laboratory and preserved in the refrigerator at a temperature of about 4 °C until analysis time. Water samples were analyzed for major cations and anions using standard methods within 48 h after sampling. Quality control and analysis procedures for obtaining accurate data were performed, including probe calibration for the in situ parameters. The concentration of heavy metals (i.e. As, Cr, Cu, Ni, Pb, and Zn) was determined using inductively coupled plasma mass spectrometry (ICP-MS) method at the Lab West Laboratories, Australia. The accuracy and precision of the measurements were checked using duplicate samples.
Indexing approach
In this research, two indices, namely, heavy metal pollution index (HPI) and metal index (MI), are applied as quantitative measures of water quality.
Heavy metal pollution index (HPI)
Heavy metal pollution index (HPI) is defined as a quantity reflecting the composite influence of different dissolved heavy metals (Sirajudeen et al. 2014). HPI is a powerful tool for ranking amalgamated effects of individual heavy metals on the overall water quality and determining suitability of groundwater for human consumption with values below 100 safe for consumption (Rizwan et al. 2011). The HPI is a method of assigning a rating or weighting (Wi) for each selected parameter. The rating is a value between zero and one, reflecting the relative importance of individual quality considerations and defined as inversely proposal to the recommended standard for each heavy metal (Mishra et al. 2017). The rating value can also be defined as inversely proportional to the recommended standard (Si) for each parameter (Mohan et al. 1996; Prasad and Kumari 2008). The highest tolerance value for drinking water (Si) refers to the maximum allowable concentration (MAC) in drinking water in the absence of any alternate water source. The desirable maximum value (Ii) indicates the standard limits for the same parameters in drinking water. The HPI index is calculated as follows (Mohan et al. 1996):
The first step involves computing the relative weight (Wi) of each parameter using Eq. 1.
with k being the constant of proportionality.
In the second step, an individual quality rating (Qi) is computed for each parameter using Eq. 2.
where Mi is the monitored value of heavy metal of ith parameter in the water sample, Ii is the ideal value of the ith parameter, and Si is the standard value of the ith parameter. Third, summing these sub-indices to compute the overall index:
where, Qi is the sub-index of the ith parameter, Wi is the unit weight for the ith parameter, and n is the number of parameters. Generally, the critical value is 100 for drinking water.
Metal index (MI)
The metal index (MI) was preliminarily defined by Tamasi and Cini (2004). This index can be expressed by Eq. 4, where MI is the metal index, C is the concentration of each element in the solution, MAC is the maximum allowed concentration of each element, and the subscript i indicating the ith sample.
The higher the concentration of a metal compared to its respective MAC value, the worse the quality of the water. MI value > 1 is a threshold of warning (Bakan et al. 2010).
Statistical analyses
Multivariate statistical analyses are applied to identify the relationship between groundwater parameters in the study area. Correlation matrix of heavy metal concentrations and calculated indices was constructed to find relations among parameters. The bivariate correlation analysis method was applied to quantify the relation between hydrogeochemical parameters and heavy metals (As, Cr, Cu, Ni, Pb, and Zn). Also, Ca2+, Mg2+, K+, Na+ and HCO3− and SO42− were included in the matrix to capture their potential effect on redox and the metals mobility.
Spearman’s correlation coefficient was utilized for displaying relationships among variables using SPSS 18.0 statistical package (SPSS Inc. 2017). Because the distribution of ions in groundwater samples is non-parametric, the Spearman’s correlation analysis is appropriate to be considered here. A high correlation analysis [correlation coefficient (r) near + 1 or − 1] indicates a good relationship between two variables (either positively or negatively correlated) and a value around zero is reflective of no relationship. It is should be noted that, if the r value is greater than 0.7, two parameters are considered to be strongly correlated; whereas an r value between 0.5 and 0.7, indicates a moderate correlation at 95% significance level (Guey-Shin et al. 2011).
Principal component analysis (PCA) was next performed to reduce the number of variables with meaningful relations. PCA is one of the multivariate statistical methods that can be used to reduce the complexity of the variable analysis and also a better interpretation of large volumes of information and data (Noori et al. 2010). This method allows us to display most of the original variability in a smaller number of dimensions and has been widely used in geochemical and hydrochemical studies (Razo et al. 2004). In this study, PCA with varimax normalized rotation (VNR) was carried out using SPSS 18.0 (Kaiser 1958).
All of the hydrochemical variables measured consisting of T, TDS, EC, pH, anions and cations, heavy metals (As, Cr, Cu, Ni, Pb and Zn), HPI and a MI were used in this statistical studies.
Discussion
Physiochemical parameters of groundwater samples
The physiochemical parameters and statistical summary for all of the 35 water samples are shown in Table 2.
The analytical results of physiochemical parameters were compared with the standard guideline values recommended by the World Health Organization (WHO) for drinking and public health purposes (WHO 2004). In the study area, pH values of groundwater samples vary from 7.05 to 8.76 with a mean of 7.78, indicating a neutral to slightly alkaline water (Fig. 3). TDS values range from 230 to 2200 mg/l, with a mean value of 640 mg/l. Groundwater with high TDS is not suitable for both drinking and irrigation purposes. The maximum allowable limit for the TDS in drinking water is 1000 mg/l (WHO 2004) (Fig. 4). EC and TDS are the most important parameters to demarcate salinity hazard and suitability of water for irrigation purposes. Water EC values vary from 480 to 4300 µS/cm with an average of 1280 µS/cm. It should be noted that maximum allowable limit for EC in drinking water is 1500 µS/cm according to WHO 2004 (Fig. 5). Also, water temperature (T) values vary from 17.2 to 30.1 °C with an average of 21.25 °C (Fig. 6).
Figures 3, 4, 5 and 6 show the spatial distribution of pH, TDS, EC and T values in the study area. The concentration gradient is calculated using the inverse distance weighting interpolation method.
Hydrogeochemical facies and classification (water types)
The Piper (1944) diagram was used to identify water types in the study area. The chemical composition of groundwater is dependent on the geology, especially lithology, and solution kinetics, flow patterns of the aquifer and anthropogenic activities which take place within the aquifer system. To find the dominant hydrochemical type and characterization of waters in the study area, groundwater samples are plotted onto trilinear Piper’s diagrams based on major ion concentrations in meq/l (Piper 1944). The Piper plot (Fig. 7) shows that groundwater in the study area consists of four water types: Na–Cl, Na–SO4, Na–HCO3 and Ca–HCO3. This suggests that there might be a considerable number of different processes influencing the chemistry of groundwater in the study area and indicating the variable nature of the groundwater chemistry. Also, Fig. 8 shows the distribution of water types in groundwater of the study area. The results show that the majority of samples (17 samples, 49%) belong to the mixed Na–SO4 water type. Ca–HCO3 water type (one sample, 3%): this type indicates mineral dissolution (probably secondary carbonate and silicate minerals) with sufficient recharge from fresh water (Ako et al. 2012; Srinivasamoorthy et al. 2014). In Na–Cl water type (eight samples, 23%), the final stage of evolution of water types from recharge zone to discharge zone (i.e. bicarbonate → sulfate → chloride) can be observed in water samples of the study area at the discharge zone. In addition, water–evaporitic mineral interactions can be other potential reasons for the presence of chloride type in this zone (Todd and Mays 2005; Merkel and Planer-Friedrich 2008). Na–HCO3 (nine samples, 26%) is another water type which can be a result of ion exchange.
Classification of water
To classify water samples of the study area, the relationship between pH and metal load (defined as As + Cd + Cr + Cu + Fe + Mn + Ni + Pb + Zn in mg/l) was employed (Ficklin et al. 1992; Caboi et al. 1999). Figure 9 shows the relationship between total metal contents (mg/l) and pH of the analyzed samples. The results indicate that the majority of samples (86%, 30 samples) are classified as near neutral–extreme metal (stations 1–20, 22–23, 25–26, 28–31, 34–35), while five samples (14%) show near neutral–high metal (stations 21, 24, 27, 32, 33). The high metal contents of these stations can lead to serious threats for drinking water consumers.
Heavy metals concentrations
Quality of water in the study area, with regard to the concentration of heavy metals (As, Cu, Cr, Ni, Pb, and Zn) is assessed herein. The concentrations of heavy metals in water samples are listed in Table 3 and are compared with heavy metals MACs from WHO (2004). The variation of heavy metals in the study area water at different locations is also presented in Fig. 10. The average heavy metal concentration in the groundwater samples follows this series (in decreasing order): lead > nickel > zinc > copper > arsenic > chrome (Table 3).
Arsenic is a ubiquitous toxicant and a carcinogenic element associated with a wide range of adverse human health effects (WHO 2004; Tseng et al. 2002; Navas-Acien et al. 2006). Weathering and erosion of As-bearing minerals release As into surface water and groundwater resources (Wang and Mulligan 2006a, b; Armienta and Segovia 2008; Naidu and Bhattacharya 2009). The average Arsenic concentration in the study area was 0.023 mg/l, with a range of 0.0017–0.232 (Table 3). Higher concentrations of As at stations 1, 18, 19, 20 and 30 may be attributed to natural processes and anthropogenic sources. Natural processes in the study area consist of evaporation of water due to high temperature and low rate of rain falls in the last decade drought, which enhances a number of salts, trace, and toxic elements. Anthropogenic sources in the study area include the use of arsenical pesticides for protecting crops in the region, mainly. The As concentration at 17 stations is higher than the corresponding WHO values (Fig. 11). Also, transferring of As into the water system during water–acidic volcanic rock interactions are another source of arsenic in groundwater of the study area. The copper content in the water samples ranged from 0.0005 to 0.0709 mg/l with an average value of 0.0301 mg/l (Table 3). The higher level of Cu in some stations is attributed to runoff from farming areas (especially at stations 15 and 17) (Fig. 12). It is noteworthy to state that higher levels of Cu in some stations are due to existence of copper indices in the study area (natural processes). Nickel concentrations ranged from 0.011 to 0.078 mg/l with an average of 0.044 mg/l (Table 3). The Ni concentration at eight stations is higher than the corresponding WHO values (Fig. 13). Zinc concentrations ranged from 0.0005 to 0.035 mg/l with an average of 0.039 mg/l (Table 3). Zinc belongs to a group of trace metals, essential for growth in humans, animals, and plants and is potentially dangerous for the biosphere when present in high concentrations. High Zn concentrations were found at station 4, which can be attributed to the excessive use of zinc sulfate, an important constituent of fertilizers tremendously used in the region. Chromium is present in small quantities in nature. Chromium is extremely irritating and toxic to human body tissue owing to its oxidizing potential and the permeability of biological membranes (Anderson et al. 1996). Chromium levels in the study area ranged from 0.0005 to 0.040 mg/l, with an average of 0.0019 mg/l (Table 3). Lead concentrations ranged from 0.0005 to 0.27 mg/l with an average of 0.056 mg/l (Table 3). It has been observed that sampling stations 23 and 25 have lead levels above the WHO standard of 10 µg/l (Fig. 14). The Pb concentration at two stations is higher than the corresponding WHO values. Lead contamination of the groundwater in the study area can be the result of entry from agricultural run-off containing phosphate fertilizers, and human and animal excreta.
Heavy metals indices
The descriptive summary statistics of heavy metals and MACs are shown in Table 4. The concentration of Cr, Cu, and Zn were below the MAC in drinking water. The concentration of As (11% samples), Ni (66% samples) and Pb (54% samples) are more than the MAC (Table 4).
For HPI calculation, the concentration limits (i.e. MAC, highest permissive value for drinking water (Si) and the maximum desirable value (Ii) for each heavy metal) were retrieved from the World Health Organization standard (WHO 2004).
The unit weight (Wi) for various water quality parameters is assumed to be inversely proportional to the maximum admissible concentration (MAC) for the corresponding parameter (Table 5).
\(\sum {{W_i}} {Q_i}=3.7808;\quad \sum {{W_i}} =0.1112\)
Mean HPI for all of the samples is calculated as 3.7808/0.1112 = 34 (Table 6).
Similarly, the HPI values for 35 samples in the study area, As, Cu, Cr, Ni, Pb, and Zn, are calculated and the results are given in Table 7 (Fig. 16). Also, bar diagram of HPI values at sampling stations is shown in Fig. 15.
Maximum value of HPI is 129.75, which is associated to station 20 and is potentially due to leaching of heavy metals from industries, such as fertilizers, pigments etc., located within the study area. This sampling site is adjacent to the Ardestan city (7 km distance). The HPI in this station is higher than the threshold index value of 100, indicating critical contamination with heavy metals. The median deviation for the HPI indices was computed for each sampling station (Table 7). The median deviation on the more negative side indicates a slightly better quality with respect to heavy metals. To apply these HPI indices in the present study, the scales were slightly modified using multiples of the median as a criterion. For this purpose, the values are divided into three classes to demarcate the different levels of contamination as low, medium, and high (Table 8). Using scaled HPI values, 51% of samples were classified as having low pollution levels, 46% with medium and 3% with high pollution levels (Fig. 16). Figure 17 shows the spatial distribution of MI values in the study area based on the inverse distance weighting interpolation method.
Computed MI values for groundwater samples are presented in Table 9. The mean MI value is 3.88. Water quality classification using MI has been conducted by Lyulko et al. (2001) and Caerio et al. (2005) (Table 10).
Water quality classification using MI (Table 11) was carried out (Fig. 18).
The MI results show that two samples in the study area are significantly contaminated with heavy metals (sample numbers: 1 and 25). These sampling sites are located on Late Eocene (granodiorite, diorite, and gabbro) rock unit. The contamination may be due to the phenomenon of volcanism and bedrock erosion in the study area. Nineteen samples were observed strongly polluted with heavy metals (sample numbers 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 20, 23 and 30). This can be attributed to the phenomenon of volcanism, bedrock erosion and mining activities (often copper). Six samples were moderately polluted with heavy metals (sample numbers 18, 22, 26, 28, 29 and 31). Bedrock erosion in the study area can be a potential reason. Seven samples were slightly polluted with heavy metals (sample numbers 19, 24, 27, 32, 33, 34 and 35). This can be a result of agricultural activities in the region. One sample was clean with respect to heavy metal (sample number 21). This sampling site is near the Ardestan city and is located on a hillside with andesite–basalt rock unit. Figure 19 shows the spatial distribution of MI values in the study area based on the inverse distance weighting interpolation method.
Statistical analyses
The correlation coefficient matrix for the hydrochemical parameters, anions and cations, and heavy metals are presented in Table 12.
The highest correlation exists between EC and TDS (r = 0.99) because all of the dissolved components cause increased ionic concentration, as well as increased EC concentration. EC and TDS are moderately related to temperature (r = 0.678 and 0.672, respectively). A strong positive correlation was observed between TDS with Mg2+, Na+, and SO42− (r = 0.710, 0.772, and 0.942, respectively) and also between HCO3− with Na+ (r = 0.667). Also, the strong positive correlation observed between K+ with Ca2+ and Mg2+ (r = 0.818 and 0.759, respectively). This implies that these ions were derived from the common source. Ca2+ and Mg2+ presented a strong positive correlation (r = 0.855), indicating a common source. The pH was negatively correlated with all metals with the exception of lead. The positive correlation between pH and Pb in this research is remarkable and consistent with the redox potential of the metal relative to hydrogen. The correlation relations show that there is a significant positive relation between nickel and copper (r = 0.757, p < 0.01), and also between nickel with lead and MI (r = 0.695 and 0.714, respectively). The correlation studies show that the relation between copper and nickel with arsenic is negative (r = − 0.550 and r = − 0.506, respectively) and indicate one vaiable increases as the other decreases, and vice versa (Figs. 11, 12, 13). The correlation between HPI and MI is significant (r = 0.850) and the results show the similar trends at various sampling locations. MI shows high positive correlation with Ni (r = 0.714) and Pb (0.758). HPI has moderate negative relation with Zn (r = -0.521).
The rotated factor pattern after extraction using varimax rotation is given in Table 13. According to the results of the initial eigen values, five principal components extracted by scree plot explained 85.3% of the total variance (Fig. 20), which is quite good and can be relied upon to identify the main sources of variation in the hydrochemistry. Principal component analysis of different parameters is presented in Table 13.
The first component (PC1), with 41.93% of the total variance, indicates that EC and TDS have high loading and reflecting the physicochemical characteristics of water quality while Temperature and Cr have moderate loading. Also, there are high positive loadings of Ca2+, Mg2+, Na+, SO42− and Cl−, and probably shows the result of mineral water reactions in the study area.
The second component (PC2), with 19.39% of the total variance, contains high loadings for HCO3−, Cu and Ni. PC1 and PC2 are assumed to be indicative of the natural processes and water–rock interaction.
The third component (PC3), with 10.1% of the total variance, indicates that K+ and As have high loading while pH and HPI have moderate loading and can be ascribed to the natural hydrogeochemical evolution of groundwater by groundwater–geological interaction. Also, it reflects that the source of As in the groundwater is the weathering process of minerals and anthropogenic sources (arsenical pesticides for protecting crops).
The fourth component (PC4) which contributes 7.8% of the total variance, indicates that Pb has high loading while MI has moderate loading and can be the result of entry from agricultural run-off.
The five component (PC5) contributes 6.1% of the total variance and is only associated with Zn. It can be attributed to the excessive use of zinc sulfate, which is an important constituent of fertilizers used in the region.
Conclusion
In this research, water quality and hydrogeochemistry characteristics of groundwater in north Isfahan of Iran have been investigated through analyzing samples from 35 stations. The quality of water in the study area, with regard to heavy metals (As, Cu, Cr, Ni, Pb, and Zn) has also been assessed. The Piper diagram showed that the groundwater in the study area has four types: Na–Cl, Na–SO4, Na–HCO3 and Ca– HCO3. This result suggests that there might be a considerable number of different processes influencing the chemistry of groundwater in the study area and indicating the variable nature of the groundwater chemistry. A maximum HPI value of 129.75 was obtained for a sampling site adjacent to Ardestan City. The HPI in this location is higher than the critical index value of 100, indicating critical contamination with heavy metals. Using HPI index metric, 51% of samples were classified as having low pollution levels, 46% medium pollution levels and 3% high pollution levels. Also, the MI results showed that two samples in the study area are significantly contaminated with a heavy metal.
Findings of the correlation analysis and PCA method show a strong impact of anthropogenic and agricultural sources for pollution load in the study area. Results indicate that there are water samples in the study area with the best quality for irrigation and drinking purposes in the future (e.g., stations 21, 22, 24, 26, 27, 31, 32, 33, 34 and 35). Using the methodology proposed in this work, one could perform similar analyses and nominate groundwater wells with suitable quality for different beneficial uses (drinking, industrial, agricultural, etc.).
References
Adaikpoh EO, Nwajei GE, Ogala GE (2005) Heavy metal concentrations in coal and sediments from River Ekulu in Enugu Coal City of Nigeria. J Appl Sci Environ Manag 9(3):5–8
Adekunle I, Adetunji M, Gbadebo A, Banjoko O (2007) Assessment of groundwater quality in a typical rural settlement in southwest Nigeria. Int J Environ Res Public Health 4(4):307–318
Adepoju-Bello A, Ojomolade OO, Ayoola GA, Coker HAB (2009) Quantitative analysis of some toxic metals in domestic water obtained from Lagos metropolis. Niger J Pharm 42(1):57–60
Ako AA, Shimada J, Hosono T, Kagabu M, Ayuk AR, Nkeng GE, Takem GEE, Takounjou ALF (2012) Spring water quality and usability in the Mount Cameroon area revealed by hydrogeochemistry. Environ Geochem Health. doi:https://doi.org/10.1007/s10653-012-9453-3
Akoto O, Bruce TN, Darko G (2008) Heavy metal pollution profiles in streams serving the Owabi reservoir. Afr J Environ Sci Tech 2(11):354–359
Amidi SM, Zahedi M (1988) Geology map of Kashan 1:250,000 sheets. Geology Survey of Iran, Tehran
Anderson RA, Bryden NA, Polansky MM, Gautschi K (1996) Dietary chromium effects on tissue chromium concentrations and chromium absorption in rats. J Trace Elem Exp Med 9:11–25
Armienta MA, Segovia N (2008) Arsenic and fluoride in the groundwater of Mexico. Environ Geochem Health 30(4):345–353
Arora J, Mehra NK (2009) Seasonal dynamics of zooplankton in a shallow eutrophic, man-made hyposaline lake in Delhi (India): role of environmental factors. Hydrobiologia 626:27–40
Babaahmadi A, Safaei H, Yassaghi A, Vafa H, Naeimi A, Madanipour S, Ahmadi M (2010) A study of quaternary structures in the Qom region, West Central Iran. J Geodyn 50:355–367
Bakan G, Boke Ozkoc H, Tulek S, Cuce H (2010) Integrated environmental quality assessment of Kızılırmak river and its coastal environment. Turk J Fish Aquat Sci 10:453–462
Bhuiyan MAH, Parvez L, Islam MA, Dampare SB, Suzuki S (2010) Heavy metal pollution of coal mine-affected agricultural soils in the northern part of Bangladesh. J Hazard Mater 173:384–392
Caboi R, Cidu R, Fanfani L, Lattanzi P, Zuddas P (1999) Environmental mineralogy and geochemistry of the abandoned Pb–Zn Montevecchio-Ingurtosu mining district, Sardinia, Italy. Chron Rech Min 534:21–28
Caerio S, Costa MH, Ramos TB, Fernandes F, Silveira N, Coimbra A, Painho M (2005) Assessing heavy metal contamination in Sado estuary sediment: an index analysis approach. Ecol Indic 5:155–169
Colak M (2012) Heavy metal concentrations in sultana-cultivation soils and sultana raisins from Manisa(Turkey). Environ Earth Sci 67:695–712. doi:https://doi.org/10.1007/s12665011-1511-0
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring.A study case from Akpabuyo–Odukpani area, lower cross river basin (Southeastern Nigeria). GeoJournal 57:295–304
Edmunds WM, Carrillo-Rivera JJ, Cardona A (2002) Geochemical evolution of groundwater beneath Mexico city. J Hydrol 258:1–24
Enaam Abdullah J (2013) Quality assessment for Shatt Al-Arab River using heavy metal pollution index and metal index. J Environ Earth Sci 3(5):114<background-color:#008B8B;>–</background-color:#008B8B;>120
Ficklin DJWH, Plumee GS, Smith KS, McHugh JB (1992) Geochemical classification of mine drainages and natural drainages in mineralized areas. In: Kharaka YK, Maest AS (eds) Water–rock interaction, vol 7. Balkema, Rotterdam, pp 381–384
Guey-Shin S, Bai-You C, Chi-Ting C, Pei-Hsuan Y, TsunKuo (2011) Applying factor analysis combined with Kriging and information entropy theory for mapping and evaluating the stability of groundwater quality variation in Taiwan. Int J Environ Res Public Health 8C:1084–1109
Jabbari N, Aminzadeh F, Barros PJ (2016) Hydraulic fracturing and the environment: risk assessment for groundwater contamination from well casing failure. J Stoch Environ Res Risk Assess. doi:https://doi.org/10.1007/s00477-016-1280-0
Jaishankar M, Mathew BB, Shah MS, Gowda KRS (2014) Biosorption of few heavy metal ions using agricultural wastes. J Environ Pollut Hum Health 2(1):1–6
Kaiser HF (1958) The varimax criterion for analytic rotation in factor analysis. Psychometrika 23:187–200
Kumar Tiwari A, Singh AK, Kumar M (2017) Assessment of groundwater quality of Pratapgarh district in India for suitability of drinking purpose using water quality index (WQI) and GIS technique. J Sustain Water Resour Manag. doi:https://doi.org/10.1007/s40899-017-0144-1
Lyulko T, Ambalova T, Vasiljeva T (2001) To integrated water quality assessment in Latvia, MTM (monitoring tailor-made) III. Proceedings of international workshop on information for sustainable water management, Netherlands, pp 449–452
Marcovecchio JE, Botte SE, Freije RH (2007) Heavy metals, major metals, trace elements. In: Nollet LM (ed) Handbook of water analysis, 2nd edn. CRC Press, London, pp 275–311
Merkel B, Planer-Friedrich B (2008) Groundwater geochemis try—a practical guide to modeling of natural and contaminated aquatic systems. Springer, Berlin
Mishra S, Jumar A, Yada S, Singhal MK (2017) Assessment of heavy metal contamination in water of Kali river using principle component and cluster analysis, India. J Sustain Water Resour Manag. doi:https://doi.org/10.1007/s40899-017-0141-4
Mohan SV, Nithila P, Reddy SJ (1996) Estimation of heavy metal in drinking water and development of heavy metal pollution index. J Environ Sci Health A31:283–289
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8(3):199–216
Naidu R, Bhattacharya P (2009) Arsenic in the environment—risks and management strategies. Environ Geochem Health 1:1–8
Navas-Acien A, Silbergeld EK, Streeter RA, Clark JM, Burke TA, Guallar E (2006) Arsenic exposure and type 2 diabetes: a systematic review of the experimental and epidemiological evidence. Environ Health Perspect 114:641–646 48
Noori R, Sabahi MS, Karbassi AR, Baghvand A, Taati Zadeh H (2010) Multivariate statistical analysis of surface water quality based on correlations and variations in the data set. Desalination 260:129–136
Piper AM (1944) A graphic procedure in the geochemical interpretation of water-analyses. Eos Trans Am Geophys Union 25(6):914–928
Prasad B, Kumari S (2008) Heavy metal pollution index of groundwater of an abandoned open cast mine filled with fly ash: a case study. Mine Water Environ 27(4):265–267
Rajkumar H, Madhuri S, Naval K (2015) Integrated approach of heavy metal pollution indices and complexity quantification using chemometric models in the Sirsa basin, Nalagarh valley, Himachal Pradesh, India. Chin J Geochem 34(4):620–633
Razo I, Carrizales L, Castro J, D´ıaz-Barringa F, Monroy M (2004) Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollut 152:129–152
Rezaei A, Hassani H (2017) Hydrogeochemistry study and groundwater quality assessment in the north of Isfahan,Iran. J Environ Geochem Health. doi:https://doi.org/10.1007/s10653-017-0003-x
Rizwan R, Gurdeep S, Manish Kumar J (2011) Application of heavy metal pollution index for ground water quality assessment in Angul district of Orissa, India. Int J Res Chem Environ 1(2):118–122
Siegel FR (2002) Environmental geochemistry of potentially toxic metals. Springer, Berlin
Sirajudeen J, Arul Manikandan S, Manivel V (2014) Heavy metal pollution index of ground water of Fathima Nagar area near Uyyakondan Channel, Tiruchirapalli District, Tamilnadu, India. World J Pharm Pharmaceut Sci 4(1):967–975
Srinivasamoorthy K, Gopinath M, Chidambaram S, Vasanthavigar M, Sarma VS (2014) Hydrochemical characterization and quality appraisal of groundwater from Pungar sub-basin,Tamilnadu, India. JKing Saud Univ Sci 26:37–52
Statistical Package for the Social Sciences (SPSS) Inc (2017) SPSS 18. http://www.spss.en.softonic.com
Tamasi G, Cini R (2004) Heavy metals in drinking waters from Mount Amiata (Tuscany, Italy). Possible risks from arsenic for public health in the province of Siena. Sci Total Environ 327:41–51
Tang WZ, Ao L, Zhang H, Shan BQ (2013) Accumulation and risk of heavy metals in relation to agricultural intensification in the river sediments of agricultural regions. Environ Earth Sci. doi:https://doi.org/10.1007/s12665-013-2779-z
Todd DK, Mays LW (2005) Ground-water hydrology. Wiley, New York
Tseng CH, Tseng CP, Chiou HY, Hsueh YM, Chong CK, Chen CJ (2002) Epidemiologic evidence of diabetogenic effect of arsenic. Toxicol Lett 133:69–76
Vieira JS, Pires JCM, Martins FG, Vilar VJP, Boaventura RAR, Botelho CMS (2012) Surface water quality assessment of Lis river using multivariate statistical methods. Water Air Soil Pollut 223:5549–5561. doi:https://doi.org/10.1007/s11270-012-1267-5
Wang S, Mulligan CN (2006a) Occurrence of arsenic contamination in Canada: sources, behavior and distribution. Sci Total Environ 366(2–3):701–721
Wang S, Mulligan CN (2006b) Effect of natural organic matter on arsenic release from soils and sediments into groundwater. Environ Geochem Health 28:197–214
World Health Organisation (WHO) (2004) Guidelines for drinking-water quality, recommendations, 3rd edn. Word Health Organization, Geneva
Acknowledgements
The authors would like to thank the Amirkabir University of Technology (Polytechnic Tehran) and Water Resources Management of Isfahan Province for supporting this research. The contribution of Adonis Fard Mousavi, Samira Rezaei and Bashir Shokouh Saljughi are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rezaei, A., Hassani, H. & Jabbari, N. Evaluation of groundwater quality and assessment of pollution indices for heavy metals in North of Isfahan Province, Iran. Sustain. Water Resour. Manag. 5, 491–512 (2019). https://doi.org/10.1007/s40899-017-0209-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40899-017-0209-1