Abstract
Present research work emphasizes the integrated approach of heavy metal pollution indices coupled with multivariate statistical analysis to evaluate the health risk from groundwater of the Kadava River Basin. In view of this, forty representative groundwater samples were collected from dug and bore wells during pre-monsoon seasons of 2011 from rural habitations which are actively used for drinking and irrigation. The heavy metals viz., Pb, Cd, Zn, Fe, Mn, Cr, Cu, Co and Ni were analyzed through ICP-AES and compared with the BIS standards for drinking appropriateness. The analytical results exhibited that, Pb and Ni exceeded the maximum allowable concentration in all samples; while, Cr in 95% samples and Fe in 92.5% samples found beyond the safe limits. Heavy metal contamination assessment has been performed by using Heavy metal Pollution Index (HPI), Hazard Index (HI), Heavy metal Evaluation Index (HEI) and Degree of Contamination (Cd). The HPI results depicts that, 65 and 10% groundwater samples characterizes the medium and high pollution extent class. The human exposure risk based on HI confirms that groundwater is not suitable for drinking to all age groups as the value of HI ≥ 1. HEI results shows that 82.5% groundwater samples fall in high pollution extent category and only 17.5% samples in medium pollution extent category caused due to excessive presence heavy metals. Cd result illustrates the widespread unsuitability of 97.5% groundwater samples due to high pollution load from heavy metals and 2.5% samples comes under moderate pollution extent. The correlation among the heavy metal indices depicts that HPI with HEI (r = 1) and Cd (0.825); moreover. HEI with Cd (r = 0.825) are strongly associated; hence, it confirms that, all these indices are significant to assess the heavy metal contamination risk in the study area. PCA, CA, CM and pollution indices analysis corroborate that, high contents of heavy metals in groundwater of Kadava River Basin is owed to land use pattern, intense agriculture, leaching of fertilizers and pesticides and domestic waste into the aquifer system. The outcomes of research work may help to local planners and policy makers to prevent the health risk by the implementation of appropriate monitoring and mitigation measures for contaminated aquifers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater is an important natural fresh water resource used for drinking and domestic activities worldwide. As concern India, due to the lack of constrained drinking water supply system, 90% rural and 30% urban population depends on groundwater for drinking and domestic uses (Jaiswal et al. 2003; Wagh et al. 2016a; Katalakute et al. 2016). Generally, groundwater is considered to be safe for drinking than the surface water due to the infiltration of contaminants by the soil media; also it has consistent temperature, natural quality and low vulnerability (Todd 1980; Panaskar et al. 2016). Heavy metals contamination in groundwater is a serious issue worldwide due to growing population and economic development; where metals enter through many natural and anthropogenic activities. The natural processes include weathering of rocks and soils, decomposition of living matter and atmospheric fallout; while, the anthropogenic activities particularly mining and mineral processing, domestic, agricultural and industrial wastes (Reiners et al. 1975; Pawar et al. 2014; Wagh et al. 2016b; Patel et al. 2017; Mukate et al. 2015, 2017; Merrikhpour and Jalali 2015). The heavy metals behavior in aquifer systems is complicated; mainly depend on the source of water, local lithology and the bio-geochemical process in elemental conditions (WHO 1993). Since, industrial revolution the heavy metals contents in groundwater is proliferating globally due to the enhanced use in many public and private sectors. Several studies proved that, heavy metal pollution in water resources is occurring through different ways viz., overuse of fertilizers and pesticides, untreated industrial waste, solid waste dumping sites, acid mine drainage, etc., (Kale et al. 2010; Bhuiyan et al. 2010; Belkhiri et al. 2017; Olagunju et al. 2017; Panaskar et al. 2014). In general, the metal solubility in soil and water are primarily governed by pH, metal concentration, organic carbon, ion exchange capacity and the oxidation state of mineral components and redox potential of the system (Musa et al. 2013).
The chemical elements dissolved less than one milligram per liter of water known as trace elements (U.S.G.S. 1993). The heavy or trace metals in small quantities are essential for the normal functioning and metabolism of human body; but, in excess can be toxic to human health. Nevertheless, heavy metals are not easily degradable by biologically or chemically; therefore, get accumulated for longer periods causing health deterioration to a considerable level. Human could be exposed to heavy metals in three main way viz., direct intake, inhalation and dermal absorption through exposure of contaminated air and water (US EPA 1989). The excess concentration of heavy metals in groundwater is used as the indicator of contamination (Pawar and Pawar 2016). Many researchers accomplished the significance of trace elements to human health, plant growth and understood the mechanisms of metal transport (Pawar and Nikumbh 1999; Gupta et al. 2008; Bhuiyan et al. 2010; Kale et al. 2010). Heavy metal contamination is one of the crucial health problems in the world due to its persistence and accumulation behavior which pose serious threats to living organism and ecosystem (Belkhiri et al. 2017).
The monitoring of heavy metals concentration is an essential to avoid health implications. Therefore, it becomes immensely important to monitor the concentration of various heavy metals to determine the degree of pollution and impacts on health and ecosystem. However, by interpreting the large dataset to identify the overall impact on water quality is complicated and time consuming. The water quality index is a simpler way to process the multiple dataset to conclude the overall quality in single dimensionless number (Horton 1965). In general, the numerous water quality variables are integrated in a specific index are designed to represent the water quality at regional and global scale. Consequently, for heavy metal contamination assessment the most practiced indices are HPI, HEI, Cd and HI which gives a composite influence of several metals on overall groundwater quality (Singh et al. 2017; Brindha et al. 2016; Omran 2016). The HPI, HEI and Cd are evaluated by using the ratios of measured values of heavy metals and their permissible limits defined by the BIS (Bhuiyan et al. 2010). Therefore, it summarizes the combined effects of several heavy metals considered harmful to conclude the overall contamination in an easier manner. The HI is non-carcinogenic risk caused by consumption of heavy metals containing groundwater, calculated separately for infants, children and adult. Several researchers has used the heavy metal pollution indices in their respective region to assess the source and severity of metal contamination (Prasad and Sangita 2008; Pawar and Pawar 2016; Kumar et al. 2012; Vetrimurugan et al. 2016; Herojeet et al. 2015; Singh et al. 2017). In addition to indices the multivariate statistical analysis also proved its efficiency to execute the multiple data sets to interpret the deteriorated quality in comprehensible way.
Previous studies of Kadava River basin were mainly focused on groundwater quality and its suitability for drinking and irrigation (Wagh et al. 2017a, b, 2018). The study area has intense agriculture, where application of fertilizers and pesticides are usual; these factors may largely influence the concentration of trace element in groundwater. Agricultural soil is more vulnerable to heavy metal contamination compared to other soil types due to the frequent application of agrochemicals to enhance the crop yield (Pakade et al. 2014). Soil salinization is a severe problem in the arid environment due to the high evaporation and quality of water used for irrigation coupled with low rainfall. In addition, other factors viz., soil composition, climatic conditions, land use and land cover and water management practices etc., (Hussain et al. 2010). In case of the Kadava River basin, study on heavy metal content in groundwater has not been carried out so far. Henceforth, the possibility of toxicity to human health from exposure of heavy metals at considerable magnitude over a time period could be estimated using human health risk assessment. Therefore, the present study initiated with the main objectives (1) to analyses the heavy metal content in groundwater samples of Kadava River Basin (2) to assess the health risk to human due to drinking groundwater containing heavy metals with the help of HPI, HEI, Cd and HI. (3) to perform multivariate statistical analysis to identify the causative variables among analyzed heavy metals. The outcomes of the study may be helpful to local habitants to avoid health risk related problems.
Study area
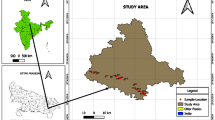
The Kadava River Basin located in Nashik District, Maharashtra, India (longitude 73°55:74°15′E and latitude 19°55′:20°25′N) with total geographical area of 1053 km2 (Fig. 1). Geologically, it is a part of the Deccan Plateau underlain by basalt of upper Cretaceous to lower Eocene age and comprising ‘Pahoehoe’ and ‘Aa’ lava flows (GSI 2001). The Kadava is a tributary of Godavari River, originates in locally known Sahyadri hills of Western Ghats and flows in NW to SE direction and confluences with Godavari River at Nandur-Madmeshwar dam located in Niphad Tehsil. The annual average rainfall is around 700 mm, out of that 80% occurs from South-West monsoonal winds (June–September). The temperature ranges from 5 to 40 °C in winter and summer seasons and characterize as semi-arid climate. The groundwater occurs under unconfined, semi confined to confined conditions below 20–25 m ground level (CGWB 2014).
Materials and methods
A total of forty (40) representative groundwater samples were collected from different dug/bore wells during April 2011. Samples were filtered through the 0.45 µm Millipore filter paper using Millipore glass assembly unit in 100 ml PET bottles. Further, samples were acidified with conc. HNO3 and kept in the ice-box. The samples were analysed on ICP-AES (ICPE-9000, Shimazdu) for Pb, Cd, Zn, Fe, Mn, Cr, Co, Cu and Ni. The Merck’s multi-elements ten (10) standard of 0.01, 0.05, 0.1, 0.25, 0.5, 1.0, 2.0, 5.0, 10.0 and 20 mg/l were used for linear curve preparation. Further, run the samples and record the readings. The blank and standards were run at regular intervals for 10 samples and each sample analysed in triplet to maintain the accuracy. The methodology adopted for health risk assessment of heavy metals is executed as shown in Fig. 2. First step is heavy metals assessment for potential health effects to human through carcinogenic and non-carcinogenic risk assessment based on USEPA and ICMR guidelines. Carcinogenic risk is calculated based on different indices such as HPI, HEI and Cd and non-carcinogenic risk assessment is computed through hazard index. The statistical analysis of heavy metals is carried out through correlation matrix, principle component analysis and cluster analysis. The multivariate statistical analysis performed through SPSS 22 v software and MS Excel.
Pollution evaluation indices
Generally, pollution indices are calculated to determine the appropriateness of water for a specific intended use. In this study the indices namely Heavy metal Pollution Index (HPI), Heavy metal Evaluation Index (HEI), Degree of Contamination (Cd) and Hazard Index (HI) were calculated to define the extent of contamination in groundwater. The HPI and HEI methods used to know the impact of heavy metal concentration on overall quality of groundwater. These indices are evaluated using the ratios of measured values of the parameters and the permissible concentrations of the respective parameters (Bhuiyan et al. 2010). A Hazard Index (HI) is used to calculate the non-carcinogenic risk caused by consumption of heavy metals containing groundwater (Vetrimurugan et al. 2016). The Cd is evaluated by computation of the extent of contamination, calculated individually for each sample of water analyzed as the sum of the contamination factors of each component greater than the permissible limit. Therefore, it summarizes the combined effects of several quality parameters considered harmful in domestic water (Singh et al. 2017).
Heavy metal Pollution Index (HPI)
HPI is rating method that considers the composite influence of individual heavy metal on overall water quality. Hence, many researchers have used HPI as a comprehensive tool to determine the overall quality of water based on heavy metals (Horton 1965; Brown et al. 1970; Herojeet et al. 2015; Vetrimurugan et al. 2016; Vasant et al. 2016). For the calculation of heavy metal indices the BIS (2012) (IS10500: 2012) drinking water quality standards were considered. The weights assigned between 0 and 1 for each metal based on its relative importance in drinking water. As the BIS and other agencies have not set any safe limit for cobalt; hence 08 heavy metals were considered for calculation of HPI by Eq. 1.
where Wi Unit weightage of the ith heavy metal, n Number of heavy metal, Qi Subindex of the ith heavy metal.
The unit weight, Wi, is determined by using Eq. 2
where K Proportionality constant, Si Standard permissible limit for ith heavy metal.
The proportionality constant, K is computed by Eq. 3,
where S1, S2, S3, etc. represent standards for different heavy.
The sub-index, Qi, is calculated by Eq. 4,
where Mi monitored value of heavy metal of the ith heavy metal, Ii ideal value of the ith heavy metal.
The Si, Ii, Wi, and mean HPI values are listed in Table 1. HPI is classified into three classes, viz., Low (0–15), Medium (15–30) and High (> 30) based on its pollution extent (Edet and Offiong 2002; Herojeet et al. 2015).
Heavy metal Evaluation Index (HEI)
HEI gives an overall quality of the water with respect to heavy metals (Edet and Offiong 2002) and is expressed as follows.
where Hc monitored value, HMAC maximum admissible concentration (MAC) of the ith parameter.
Hazard Index
A non-carcinogenic risk due to consumption of groundwater containing heavy metals was assessed by calculating hazard index (HI) based on following equations (US EPA 1989).
where LADD lifetime average daily dose of ingestion of heavy metal through drinking water (mg/kg/day), C concentration of the heavy metal in water (mg/l), IR ingestion rate of water [250 ml/day for infants, i.e., 0–12 months, 1.5 l/day for children (Brindha et al. 2016) and 3 l/day for adults (Planning Commission 2011)], EF exposure frequency (days/year), ED exposure duration [66.4 years (UNDESA 2013)], BW body weight [6.9 kg for infants, 18.7 kg for children and 57.5 kg for adults(ICMR 2009)], AT average time (days).
If individual consumes the same water throughout the year for entire life, then exposure frequency as well as duration will be same as average time. In that case the equation can be modified as
Hazard quotient index (US EPA 1989) is calculated as Eq. 7,
where HQ hazard quotient, RfD Reference dose for a heavy metal that an individual can be exposed to in a day over his/her lifetime without experiencing any deleterious health effect (mg/kg/day).
The RfD value of analyzed heavy metals is represented in Table 2.
The non-carcinogenic risk given by HI is sum of the HQ of all metals.
Degree of Contamination (Cd)
where
Cfi Contamination factor of ith component, CAi Analytical value of ith component, CNi− Maximum permissible concentration of ith component, N Normative values, CNi values were considered as maximum permissible limit.
Results and discussion
In this study, eight heavy metals were analysed and its descriptive statistics and comparison with the BIS standards are represented in Table 3.
Heavy metal contamination
Trace elements distribution and occurrence in the groundwater depends on degree of weathering and mobility of these elements (Handa 1986). The Pb concentration ranges from 0.290 to 1.960 mg/l with an average 0.998 mg/l. As compared with BIS standards, all the groundwater samples exceed the desirable limit (0.01 mg/l). Such, high content may be attributed from the use of lead containing fertilizers and pesticides (Aldrin, Deieldrin, Endosulfan etc.,) applied in the agricultural field. The Cadmium (Cd) is one of the poisonous, non-essentials, bio persistent metal having toxicological properties. The cadmium content deflects from 0.005 to 0.051 mg/l (Avg. 0.27 mg/l). In the study area, 37.5% samples exceed the desirable limit of the BIS (Table 3). The sample number 35 and 39 located in downstream area shows the elevated concentration due to the leaching of fertilizers and pesticides residues.
Zinc (Zn) is an essential element to perform metabolic activities and its deficiency will lead to retarded growth and resistance of body. The Zn value varies from 0.023 to 0.209 mg/l (Avg. 0.065 mg/l) (Table 3). As compared with BIS standards the content of Zn in all groundwater samples is within the desirable limit (5 mg/l) imparting the miniature industrial activities in the study area. The Iron (Fe) is one of the most abundant elements found in earth crust. Iron and Manganese plays an important role in regulation of the biochemical cycle in plants and animals (Ballukarya and Ravi 1999). The Fe concentration ranges from 0.092 to 3.558 mg/l (Avg. 0.837 mg/l). It is observed that 92.5% samples exceed the desirable limit (0.3 mg/l) of the BIS reflecting the impact of agriculture and domestic activities as a possible source (Table 3). Beyond this limit taste/appearance, domestic utility, water supply structures are affected and promote growth of iron bacteria (BIS 2012). The Mn concentration varies from 0.013 to 0.142 mg/l (Avg. 0.043 mg/l) (Table 3). Only sample number 39 surpasses the safe limit of 0.1 mg/l (Table 3). Naturally, chromium occurs in chromite mineral and it replaces Fe3+ and Al3+ (Faust and Aly 1981). Cr content ranges from 0.034 to 3.516 mg/l (Avg. 1.588 mg/l) (Table 3); however, 95% groundwater samples exceeds the desirable limit (0.05 mg/l) set by BIS and such concentration is supposed to be carcinogenic (BIS 10,500:2012). The source of chromium includes intrusive rock weathering, traffic, residential waste, pigments, paints, ceramic, pottery units (Patel et al. 2017). The overexposure of chromium leads to asthma, cough, and shortness of breath, wheezing, anemia, irritation and ulcer (Wilbur et al. 2000). Cobalt (Co) is an essential trace dietary mineral for all animals and micronutrient for bacteria, algae and fungi. The Cobalt content deflects from the 0.001 to 0.267 mg/l (Avg. 0.085 mg/l) (Table 3). The BIS has not set any safe limit for cobalt content in drinking water. Copper (Cu) plays an important role in production of blood hemoglobin, seed production, disease resistance and regulation of water in plants and humans (Davies and Jones 1988). In the groundwater copper ranges from 0.006 to 0.086 mg/l with an average 0.037 mg/l. As per the BIS limit 15% samples exceeds the desirable limit due to waste dumps, domestic waste water, fossil fuels combustion, wood production and phosphate containing fertilizers. The nickel is varying from 0.114 to 0.225 mg/l (Avg. 0.170 mg/l). All the groundwater samples surpass the safe limit of the BIS (Table 3). The elevated concentration is owed to agriculture and domestic activities prevailing in the study area. Excessive nickel exposure may lead to Laryngeal, kidney and prostate cancer (Menzel et al. 1987).
Carcinogenic Risk Assessment Heavy metal Pollution Index (HPI)
The HPI for the Kadava River basin, the HPI values deviates from 476.99 to 1833.65 with mean concentrations 1039.48 for 8 heavy metals in 40 groundwater samples. It is inferred that, all the groundwater samples are above the critical value of 100, hence unfit for human consumption (Fig. 3). The samples located along flow direction are affected mostly due to the accumulation of heavy metals from surface runoff in the lower reaches. In the study area the principle crops are grapes, onion, sugarcane and seasonal crops grown throughout the years. To improve the crop yield, a variety of chemical fertilizers, pesticides, fungicides, weedicides, etc. are applied on large scale by farmers. Since, intense agriculture for prolonged period the accumulation of metals is continued by leaching processes.
Heavy metal Evaluation Index (HEI)
The calculated HEI values swerves from 522.89 to 2010.05 with an average value of 1139.48. There are many HEI classification criterions developed to classify the samples into three categories such as low, medium and high extent of pollution, guided by their respective mean values, and the different levels of contamination (Prasanna et al. 2012). The HEI classification proposed for the present results are as follows: low (HEI < 400), medium (HEI = 400–800) and high (> 800) (Table 4). Table 4 demonstrates that, 82.5% samples represent the high pollution extent caused due to excessive heavy metals and only 17.5% samples represent medium pollution extent.
Degree of Contamination (Cd)
The contamination index (Cd) was used as reference of estimating the degree of contaminants in the form of metals present in particular sample (Al-Ami et al. 1987). The Cd values ranges from 231.56 to 1373.22 with an average value of 711.97. The Cd values further classified as, low (Cd < 40), medium (Cd = 40–80) and high (Cd > 80) (Table 4). All the groundwater samples exceed the value of 80, suggesting that they are highly polluted, hence unfit for drinking due to the high load of heavy metals. Graphical representation (Fig. 3) demonstrated that, values of HPI, HEI and Cd shows similar trends throughout the Kadava River Basin. The HPI, HEI and Cd shows that sample numbers 2, 26, 33, 35 and 39 are worst affected whereas 5, 12, 31 and 40 samples are least affected (Fig. 3). The worst affected samples are located along the flow path in the lower reaches of catchment while least affected samples found in central and upper reaches of the watershed.
Non carcinogenic risk assessment
Hazard Index (HI)
The non-carcinogenic risk calculated through Hazard Index (HI) by considering the eight heavy metals. The HI values ≤ 1 is supposed to be safe and HI values ≥ 1 indicates the non-carcinogenic risk. Graphical representation of HI values indicates that, entire study area has non-carcinogenic risk due to consumption of groundwater (Fig. 4). It is observed that, minimum HI was 10.41, 23.05 and 14.99 mg/kg/day for infants, children and adults, respectively (Fig. 4). Maximum cumulative HI was found in children followed by adults and Infants. It corroborates that children are at highest risk than the infants and adult. The lead, cadmium, chromium and nickel metal content contributed to larger percentage in HI ultimately amplify the potential risk. The HI proved to be a very useful tool in evaluating overall pollution of the ground water. It indicates that, groundwater is critically contaminated by leaching of heavy metals from the agricultural and domestic activities.
Multivariate statistical analysis
The multivariate statistical analysis has been performed through the correlation analysis, principle component analysis (PCA) and cluster analysis (CA) to know the affiliation among different heavy metals and also to measure the most influencing variable affecting the groundwater quality.
Principle component analysis
The PCA performed through varimax with Kaiser Normalization method on data (40 samples for 8 heavy metals) to identify the major components involved in contaminating the groundwater quality (Table 5). Generally, eigen values of the factors signify the common variance in the data and resemblance among the different variable is represented as factor scores. Moreover, the components with eigen value above 1 are significant for interpretation. In PCA, the values are represented in positive and negative based on strong, moderate and weak relationships to differentiate the significant heavy metals affecting groundwater quality. The components with eigen values > 1 and loading values > 0.40 affecting significantly on the overall quality (Liu et al. 2003; Shrestha and Kazama 2007). The four principle components (PCs) were extracted with 71.55% of cumulative variance. The factors loadings, Eigen values (> 1), % of Variance and Cumulative % values are listed in Table 5. The positive loadings indicate the significant deterioration caused to groundwater quality while negative loadings intend that water quality remains unaffected by these parameters (Bhuiyan et al. 2010). In the groundwater dataset PC1, PC2, PC3 and PC4, respectively, explained 24.57, 18.95, 14.25 and 13.76% of total variance. It is observed that, PC1 is profoundly loaded on Pb, Cd, Cr and Ni which are mostly derived from anthropogenic sources i.e. application of chemical fertilizers and pesticides. Generally, trace elements with high positive loading in PC1 typically occur in oxidizing water as soluble oxyanions (Chen et al. 2007). PC2 is influenced by Fe and Mn only due to the inputs from domestic waste and agricultural runoff (Wu et al. 2008). The main sources of Mn are leaching from local bedrock and solubility in water depends on anions and oxidation reduction potential mainly at near-neutral pH (ATSDR 2000; Lorite-Herrera et al. 2008). PC3 is highly positively loaded of Cu (0.649) and moderately with Ni (0.412), while highly negatively loaded with Co (− 0.796). The copper in groundwater is mainly released from waste dumps, domestic waste water, fossil fuels combustion and phosphate enriched fertilizers characterized as anthropogenic sources. Lastly, PC4 is dominated by the positive loading of Ni and negative loading of Zn.
Cluster Analysis (CA)
R-mode cluster analysis is used to characterize the different elemental groupings in the data set, which affects the overall groundwater quality. It is performed by using SPSS 22 v. Cluster analysis represents Cd, Cu, Mn, Zn, Co and Ni are grouped in a single cluster; and Pb, Fe and Cr in another cluster (Fig. 5). This clustering of metals indicates that these elements have same source of origin. Pb, Fe and Cr may be owed to the leaching from anthropogenic inputs such as agricultural and domestic waste (Prasanna et al. 2012).
Correlation matrix (CM)
The Pearson’s correlation coefficient matrix is used to know the relationship among various metals (Table 6). The correlation results were compared with the PCA to recognize the inter-parameters relationships. A significant positive correlation of Pb has been observed with Cd (0.439) and Cr (0.52) indicating a similar source of PC1; Cd with Mn (0.424) and Ni (0.562); Fe with Mn only similar to sources reported for PC2. High content Pb may be attributed to the application of lead containing fertilizers and pesticides. Cobalt and copper is not correlated with any other metals. The correlation among the heavy metal indices depicts that HPI with HEI (r = 1) and Cd (0.825) moreover HEI with Cd (r = 0.825) are strongly correlated. It proves that, all these three indices can be significantly used to assess the heavy metal contamination risk in the study area. It is observed that, there is good agreement between PCA, CA and CM techniques for grouping the causative factors in the analyzed the data sets. It is confirmed that, Pb, Cd and Cr are the significant attributers to elevate the health risk from groundwater.
Conclusions
Heavy metals contamination in intensively irrigated area of Kadava River Basin was assessed by using heavy metal pollution indices and multivariate statistical techniques. High levels of Pb, Ni, Cr, Fe and Cd were observed in the groundwater due to natural and agricultural activities. HPI, HEI, Cd and HI indices have been utilized to ascertain the health risk by considering 8 heavy metals. In addition, multivariate statistical tools such as PCA, CA and CM were applied to identify the causative factors which altering the groundwater quality. HPI values indicate that, all the groundwater samples are exceeding the critical limit of 100; hence, unfit for consumption. HEI value suggests that, 17.5% samples have medium pollution extent and 82.5% are representing the high pollution extent. Whereas, Cd illustrates the widespread unsuitability in 97.5% samples due to high pollution load from heavy metals and 2.5% samples represents the moderate pollution extent in the study area. HI values swerves from 10.57 to 46.57; 65% and 10% samples have medium and high pollution extent, respectively which illustrates the elevated risk of heavy metal contamination. The mean value of HI depicts the probability of suffering adverse effects on children (70.64) followed by adults by (45.95) and infants (31.91 mg/kg/day). The increased pollution extent is owed to the elevated content of metals like Pb, Cr and Mn from natural processes and agrochemicals used in farming. The HPI, HEI, Cd and HI values exceeding the threshold values reflect the possibility of health risk hazard; hence, emphasizes the need of monitoring of groundwater to avoid health risk in the Kadava River Basin. To identify the contribution of each metal to the computed indices, PCA, CA and CM has been performed for heavy metals and heavy metal pollution indices (HPI, HEI and Cd). The HEI shows strong correlations with HPI and Cd, depicts the reliability of index to assess the pollution level at its best. The present research highlights the applicability of heavy metal pollution indices coupled with multivariate statistical analysis to ascertain the health risk from heavy metal contamination. The present level of heavy metal concentration in the groundwater of Kadava River Basin is of serious health and environmental concerns and needs immediate attention. A continuous monitoring of groundwater quality is imperative to keep the pollution check and treated drinking water should be supplied to the inhabitants to avoid health implications in future.
References
Al-Ami MY, Al-Nakib SM, Ritha NM, Nouri AM, Al-Assina A (1987) Water quality index applied to the classification and zoning of Al-Jaysh canal, Bagdad, Iraq. J Environ Sci Health A 22:305–319
ATSDR (2000) Toxicological profile for arsenic. US Department of Health and Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, p 428
Ballukarya PN, Ravi R (1999) Characterization of groundwater in the unconfined aquifers of Chennai city, India. Geolog Soc India 54(1):1–11
Belkhiri L, Mouni L, Narany TS, Tiri A (2017) Evaluation of potential health risk of heavy metals in groundwater using the integration of indicator kriging and multivariate statistical methods. Groundw Sustain Dev 4:12–22
Bhuiyan MA, Islam MA, Dampare SB, Parvez L, Suzuki S (2010) Evaluation of hazardous metal pollution in irrigation and drinking water systems in the vicinity of a coal mine area of northwestern Bangladesh. J Hazard Mater 179(1–3):1065–1077
BIS (2012) Indian standards specification for drinking water, BIS: 10500: 2012
Brindha K, Jagadeshan G, Kalpana L, Elango L (2016) Fluoride in weathered rock aquifers of southern India: managed Aquifer recharge for mitigation. Environ Sci Pollut Res 23:8302–8316
Brown RM, McClelland NI, Deininger RA, Tozer RG (1970) A water quality index: do we dare. Water Sewage works 117(10):339–343
CGWB 2014. Central Groundwater Board (CGWB), http://www.cgwb.gov.in/district_profile/maharashtra/nashik.pdf
Chen K, Jiao JJ, Huang J, Huang R (2007) Multivariate statistical evaluation of trace elements in groundwater in a coastal area in Shenzhen. China Environ Pollut 147(3):771–780
Davies BE, Jones LHP (1988) Micronutrients and toxic elements. Russell’s Soil Cond Plant Growth 11:780–814
Edet AE, Offiong OE (2002) Evaluation of water quality pollution indices for heavy metal contamination monitoring. A study case from Akpabuyo-Odukpani area, Lower Cross River Basin (south eastern Nigeria). Geoj 57:295–304. https://doi.org/10.1023/B:GEJO.0000007250.92458.de
Faust SD, Aly OM (1981) Chemistry of natural waters. Butterworths. An Ann Arbor Science Book, Boston p 400
Geological Survey of India (GSI) (2001) District resource Map. GSI, Nashik district
Gupta N, Khan DK, Santra SC (2008) An assessment of heavy metal contamination in vegetables grown in wastewater-irrigated areas of Titagarh, West Bengal, India. Bull Environ Contam Toxicol 80(2):115–118
Handa BK (1986) Trace elements content of groundwater in the basaltic rocks in some parts of Indian Peninsula. In: Hydrogeology of volcanic terranes. University of Poona, Pune, pp 83–104
Herojeet R, Rishi MS, Kishore N (2015) Integrated approach of heavy metal pollution indices and complexity quantification using chemometric models in the Sirsa Basin, Nalagarh valley, Himachal Pradesh, India. Chin J Geochem 34(4):620–633
Horton RK (1965) An index number system for rating water quality. J Wat Pollut Con Fed 37(3):300–305
Hossein Pour M, Lashkaripour GR, Dehghan P (2014) Assessing the effect of heavy metal concentrations (Fe, Pb, Zn, Ni, Cd, As, Cu, Cr) on the quality of adjacent groundwater resources of Khorasan steel complex. Int J Plant Anim Environ Sci 4:511–518
Hussain G, Alquwaizany A, Al-Zarah A (2010) Guidelines for irrigation water quality and water management in the Kingdom of Saudi Arabia: an overview. J Appl Sci 10(2):79–96
ICMR (Indian Council of Medical Research) (2009) Nutrient requirements and recommended dietary allowances for Indians. A report of the expert group of the ICMR, Hyderabad, India, p 334
Jaiswal RK, Mukherjee S, Krishnamurthy J, Saxena R (2003) Role of remote sensing and GIS techniques for generation of groundwater prospect zones towards rural development–an approach. Int J Remote Sens 24(5):993–1008
Kale SS, Kadam AK, Kumar S, Pawar NJ (2010) Evaluating pollution potential of leachate from landfill site, from the Pune metropolitan city and its impact on shallow basaltic aquifers. Environ Monit Assess 162(1–4):327–346
Katalakute G, Wagh V, Panaskar D, Mukate S (2016) Impact of drought on environmental, agricultural and socio-economic status in Maharashtra State, India. Nat Resour Conserv 4(3):35–41
Kumar PS, Delson PD, Babu PT (2012) Appraisal of heavy metals in groundwater in Chennai city using a HPI model. Bull Environ Contam Toxicol 89(4):793–798
Liu ZY, Chiu KC, Xu L (2003) Strip line detection and thinning by RPCL-based local PCA. Pattern Recogn Lett 24(14):2335–2344
Lorite-Herrera M, Jimenez-Espinosa R, Jimeneze-Millan J, Hiscock KM (2008) Integrated hydrochemical assessment of the Quaternary alluvial aquifer of the Guadalquivir River, Southern Spain. Appl Geochem 23:2040–2054. https://doi.org/10.1016/j.apgeochem.2008.03.013
Menzel DB, Deal DL, Tayyeb MI, Wolpert RL, Boger JR, Shoaf CR, … Francovitch RJ (1987) Pharmacokinetic modeling of the lung burden from repeated inhalation of nickel aerosols. Toxicol Lett 38(1–2):33–43
Merrikhpour H, Jalali M (2015) Geostatistical assessment of solid–liquid distribution coefficients (K d) for Cd, Cu, Pb and Zn in surface soils of Hamedan, Iran. Model Earth Syst Environ 1(4):47
Mukate SV, Panaskar DB, Wagh VM, Pawar RS (2015) Assessment of groundwater quality for drinking and irrigation purpose: A case study of Chincholikati MIDC area, Solapur (MS), India. SRTMUs J Sci 4(1):58–69
Mukate S, Panaskar D, Wagh V, Muley A, Jangam C, Pawar R (2017) Impact of anthropogenic inputs on water quality in Chincholi industrial area of Solapur, Maharashtra, India. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2017.11.001
Musa OK, Shaibu MM, Kudamnya EA (2013) Heavy metal concentration in groundwater around Obajana and its environs, Kogi State, North Central Nigeria. Am Int J Contemp Res 3(8):170–177
Olagunju E, Ariyibi E, Awoyemi M, Adebayo A, Dasho O, Adenika C (2017) Application of geochemical and geophysical approach to environmental impact assessment: a case study of Emirin active open dumpsite, Ado-Ekiti Southwestern Nigeria. Model Earth Syst Environ 3(4):1543–1555
Omran ESE (2016) Environmental modelling of heavy metals using pollution indices and multivariate techniques in the soils of Bahr El Baqar, Egypt. Model Earth Syst Environ 2(3):119
Pakade YB, Chahal V, Chand P, Nagpal A, Katnoria JK (2014) Evaluation of heavy metals contamination and its genotoxicity in agricultural soil of Amritsar, Punjab, India. Int J Res Chem Environ 4(4):20–28
Panaskar DB, Wagh VM, Pawar RS (2014) Assessment of groundwater quality for suitability of domestic and irrigation from Nanded Tehsil, Maharashtra, India. SRTMUs J. Sci, 3(2), 71–83
Panaskar DB, Wagh VM, Muley AA, Mukate SV, Pawar RS, Aamalawar ML (2016) Evaluating groundwater suitability for the domestic, irrigation, and industrial purposes in Nanded Tehsil, Maharashtra, India, using GIS and statistics. Arab J Geosci 9(13):615
Patel P, Raju NJ, Reddy BSR, Suresh U, Sankar DB, Reddy TVK (2017) Heavy metal contamination in river water and sediments of the Swarnamukhi River Basin, India: risk assessment and environmental implications. Environ Geochem Health 40(2):609–623
Pawar NJ, Nikumbh JD (1999) Trace element geochemistry of groundwaters from Behedi basin, Nasik District. Maharashtra J Geol Soc India 54:501–514
Pawar NJ, Pawar JB (2016) Intra-annual variability in the heavy metal geochemistry of ground waters from the Deccan basaltic aquifers of India. Environ Earth Sci 75(8):654
Pawar RS, Panaskar DB, Wagh VM (2014) Characterization of groundwater using water quality index of solapur industrial belt, Maharashtra, India. Int J Res Eng Technol 2(4):31–36
Planning Commission (2011) Faster, sustainable and more inclusive growth: An approach to the 12th five year plan (No. id: 4452)
Prasad B, Sangita K (2008) Heavy metal pollution index of ground water of an abandoned open cast mine filled with fly ash: a case study. Mine Water Environ 27(4):265–267
Prasanna MV, Praveena SM, Chidambaram S, Nagarajan R, Elayaraja A (2012) Evaluation of water quality pollution indices for heavy metal contamination monitoring: a case study from Curtin Lake, Miri City, East Malaysia. Environ Earth Sci 67(7):1987–2001
Reiners WA, Marks RH, Vitousek PM (1975) Heavy metals in subalpine and alpine soils of New Hampshire. Oikos 26(3):264–275
Shrestha S, Kazama F (2007) Assessment of surface water quality using multivariate statistical techniques: a case study of the Fuji river basin, Japan. Environ Model Softw 22(4):464–475
Singh R, Venkatesh AS, Syed TH, Reddy AGS, Kumar M, Kurakalva RM (2017) Assessment of potentially toxic trace elements contamination in groundwater resources of the coal mining area of the Korba Coalfield, Central India. Environ Earth Sci 76(16):566
Todd DK (1980) Groundwater hydrology, 2nd edn. Wiley, New York
U.S.G.S., (1993) National water summary 190–1991 Stream water qualityUSGS water supply paper No. 2400 pp 59
UNDESA (United Nations Department of Economic and Social Affairs) (2013) World Population Prospects. Population Division Database. Detailed indicators 2012 Revision
US EPA (1989) Risk assessment guidance for superfund, volume 1: human health evaluation manual (part A) ((EPA/540/1–89/002: interim final). Office of Emergency and Remedial Response, Washington
Vasant W, Dipak P, Aniket M, Ranjitsinh P, Shrikant M, Nitin D, Manesh A, Abhay V (2016) GIS and statistical approach to assess the groundwater quality of nanded Tehsil, (MS) India. In: Proceedings of first international conference on information and communication technology for intelligent systems, Vol 1. Springer, Cham pp. 409–417
Vetrimurugan E, Brindha K, Elango L, Ndwandwe OM (2016) Human exposure risk to heavy metals through groundwater used for drinking in an intensively irrigated river delta. Appl Water Sci 6:1–14
Wagh VM, Panaskar DB, Varade AM, Mukate SV, Gaikwad SK, Pawar RS, Muley AA, Aamalawar ML (2016a) Major ion chemistry and quality assessment of the groundwater resources of Nanded tehsil, a part of southeast Deccan Volcanic Province, Maharashtra, India. Environ Earth Sci 75(21):1418
Wagh VM, Panaskar DB, Muley AA, Mukate SV, Lolage YP, Aamalawar ML (2016b) Prediction of groundwater suitability for irrigation using artificial neural network model: a case study of Nanded tehsil, Maharashtra, India. Model Earth Syst Environ 2(4):196
Wagh VM, Panaskar DB, Muley AA, Mukate SV (2017a) Groundwater suitability evaluation by CCME WQI model for Kadava River Basin, Nashik, Maharashtra, India. Model Earth Syst Environ 3:1–9
Wagh VM, Panaskar DB, Muley AA (2017b) Estimation of nitrate concentration in groundwater of Kadava river basin-Nashik district, Maharashtra, India by using artificial neural network model. Model Earth Syst Environ 3(1):36
Wagh V, Panaskar D, Muley A, Mukate S, Gaikwad S (2018) Neural Network Modelling for Nitrate Concentration in groundwater of Kadava River basin, Nashik, Maharashtra, India. Groundw Sustain Dev. https://doi.org/10.1016/j.gsd.2017.12.012
WHO (1993) Guidelines for drinking water supply quantity, 2nd edn. I. Recommendations. World Health Organization, Geneva, pp-180–181
Wilbur S, Ingerman L, Citra M, Osier M, Wohlers D (2000) Toxicological profile for chromium. US Department of Health and Human Services. Public Health Service, Agency for Toxic Substances and Disease Registry, 1–419
Wu XH, Qian H, Yu DM, Zhang Q, Yan ZZ, Wang W, Ji YD, Liu HM, Mao ZL, Zhao Q (2008) Investigation and assessment of rational allocation of groundwater resources in the Yinchuan plain. Geological Publishing House, Beijing
Acknowledgements
The authors wish to express their thanks to the Director, School of Earth Sciences, Swami Ramanand Teerth Marathwada University, Nanded, India for providing necessary facilities during present research work. Authors are also thankful to anonymous reviewers for their valuable suggestion to strengthen the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wagh, V.M., Panaskar, D.B., Mukate, S.V. et al. Health risk assessment of heavy metal contamination in groundwater of Kadava River Basin, Nashik, India. Model. Earth Syst. Environ. 4, 969–980 (2018). https://doi.org/10.1007/s40808-018-0496-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40808-018-0496-z