Abstract
This study constitutes the first systematic risk assessment in the Lao PDR of the safety of groundwater for consumption. Groundwater and hair samples were collected from seven Lao provinces to determine the quantitative health impact of heavy metals through ingestion exposure. Contamination levels for arsenic (As; 46.0 %) and barium (Ba; 16.2 %) exceeded World Health Organization (WHO) guidelines, especially in Mekong River floodplains. A USEPA assessment model for health risks from daily groundwater ingestion, with adjustments for local water consumption values, was applied to estimate the size of the population at risk for noncarcinogenic and carcinogenic health problems. As was the only element contributing to noncarcinogenic health risks in all contaminated areas. The populations of Bolikhamxai, Savannakhet, Saravane, Champasak, and Attapeu, moreover, were at risks of cancer. In addition to the As groundwater concentration factor, noncarcinogenic and carcinogenic risks were positively correlated with the average daily dose of As, exposure duration, and subject body weight. The level of As in hair correlated with groundwater consumption and average daily dose of As. 25.5 % of the population (n = 228) showed As levels in hair above the toxicity level.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Groundwater has been increasingly turned to as a source of drinking water in order to limit outbreaks of waterborne infectious diseases. This has unfortunately increased incidences of endemic diseases caused by the presence in groundwater of naturally occurring hazardous chemicals, such as fluoride and arsenic (As) (Howard et al. 2006). Investigations of heavy metals, in particular, have recently become a prime focus of environmental scientists (Caylak 2012a; Caylak and Tokar 2012a, b; Howard et al. 2006). Although heavy metals such as boron (B), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), and zinc (Zn) are required for normal body functioning (Adriano 2001; Caylak 2012a; Caylak and Tokar 2012a, b; Gibney et al. 2009), minute amounts of heavy metals such as As, cadmium (Cd), mercury (Hg), and lead (Pb) are considered highly toxic to humans (Caylak 2012b). Taking into account their potential toxicity, persistence in the environment, accumulative characteristics in human tissue, and the typical vector of daily drinking water ingestion over very long terms, there is considerable potential for serious public health consequences. This is especially true in areas where concentrations of heavy metals exceed World Health Organization (WHO) guidelines for drinking water (Marchiset-Ferlay et al. 2012). Previous reports have noted that the adverse health effects of long-term exposure to heavy metals may lead to, including a variety of cancers, mental retardation and neurological, cardiovascular, kidney, and bone diseases (Adriano 2001; Järup 2003; Prüss-Ustün et al. 2011). For these reasons, chronic exposure to a mixture of heavy metals must be seen as a major public health concern (Wang and Fowler 2008).
In the Lao People’s Democratic Republic (Lao PDR), groundwater is the main source of rural and small town water supplies (United Nations Environmental Programme 2001). Even though several hundred tube wells have been drilled throughout the country (Water Environment Partnership in Asia 2012), few results of groundwater monitoring in the Lao PDR have been reported to date. Of note, a preliminary survey of about 50 existing tube wells in 1999 did show As, Ba, Cr, Fe, and Mn contamination in some of the wells (Fengthong et al. 2002). More recently, groundwater drawn from 61 tube wells located along the Mekong River was found to be contaminated with As, B, Ba, Fe, Mn, and U (Chanpiwat et al. 2011). Based on these findings, it seemed necessary to determine in greater detail the levels of heavy metals in the groundwater in order to ensure its safety for public consumption.
A systematic human health risk assessment for individuals exposed to toxic heavy metals through groundwater consumption has yet to be conducted in the Lao PDR. In addition to the use of human blood and urine as biomarkers to measure exposure to toxic elements, human hair has also been used to indicate the levels and length of exposure to toxic pollutants (Bencko 1995; Rodrigues et al. 2008). Indeed, hair analyses appears to be ideally suited for pilot human health risk assessment studies targeting excessive exposure to toxic elements (Bencko 1995) due to the low cost, simple mode of sample collection, and ease of storage and transportation (Bencko 1995; Esteban and Castaño 2009; Rodrigues et al. 2008). In recent years, human hair has been used to monitor workplace exposure to Cd, Hg, and Pb (Esteban and Castaño 2009). In cases of environmental exposure, human hair has been used to indicate the health implications of the consumption of As-contaminated groundwater (Gault et al. 2008; Phan et al. 2010; Sthiannopkao et al. 2010).
Thus, the objectives of this study are to: (i) determine the levels of toxic heavy metals in the groundwater of the Lao PDR, (ii) determine the heterogeneous distribution of As species in the groundwater, (iii) assess noncarcinogenic and carcinogenic health risks to the population from heavy metals in the groundwater through ingestion exposure, (iv) determine the levels of heavy metals in human scalp hair in relation to noncarcinogenic and carcinogenic risks and the individual average daily dose (ADD) of heavy metals, and (v) quantify the health impacts from heavy metals in the groundwater in each area studied.
Materials and methods
Areas studied
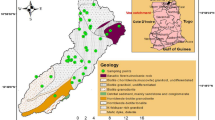
Sampling campaigns were carried out within seven selected provinces in the Lao PDR (Fig. 1). With the exception of Attapeu, located mainly in the Bolavan Plateau in an inactive volcanic area, the six remaining provinces (from north to south) of Vientiane, Bolikhamxai, Khammouan, Savannakhet, Saravane, and Champasak are located along the Mekong River. The Mekong River flows 1,805 km through the Lao PDR (Encyclopedia of the Nations 2012). In general, the Lao PDR has a diverse topography, extending from the floodplain of the Mekong River to the highland areas in the north and east; one quarter of the country is considered lowland, consisting of floodplains and rice paddies (The Wetland Alliance 2012). Khammouan, Savannakhet, Saravane, and Champasak have the main floodplains of the country, and flash floods can occur in Bolikhamxai (Khongsab 2012). Soils in the floodplains are formed from alluvium deposited by the Mekong River, typically comprising either sandy or sandy clay soils, light-colored or sandy with gray or yellow deposits, which are chemically neutral to slightly acidic. The upland soils are more acidic and much less fertile because they are derived from crystalline, granitic, schistose, or sandstone parent rocks (Tuvy Asian 2012). The southern Lao PDR region contains areas of leached and Fe-bearing soils as well as basaltic soils on the Bolavan Plateau.
The Mekong River shapes much of the lifestyle of the Laotian people. The majority of the population (68 %) is concentrated in the fertile Mekong River valley and lowland plains, with the provinces along the Mekong River mostly engaged in agriculture and fisheries (Travel to Laos 2012). Urban life in Laos is limited mainly to the capital city Vientiane (Tuvy Asian 2012). The geographic and demographic information of each area is summarized in Table 1. The total population living in these areas (about three million) accounts for about 60 % of the country’s total population (6.65 million).
Groundwater resources are the main sources of water for both consumption and household use, especially in the small town and rural areas (United Nations Environmental Programme 2001). The water yields of rural supply wells vary from 1 L s−1 to not more than 5 L s−1 (United Nations Environmental Programme 2001). However, well water is not the primary source of drinking water in urban areas; according to a report on the joint monitoring program for water supply and sanitation by WHO and UNICEF (WHO/UNICEF 2012), it was estimated that the status of water supply distribution and availability of bottled water in urban and rural areas in 2008 were 29.6 and 0.3 %, respectively. Moreover, the percentage of protected wells to traditional wells was approximately 20 % in urban areas and 8 % in rural areas.
Groundwater and human hair samples collection
Sampling campaigns for groundwater and human hair samples were conducted in 2008 (May and July) and 2010 (February and March) after the agreement for the ethical use of human subjects was approved by the Ministry of Health, Lao PDR (no. 836/NCHWS). In this study, a total of 148 groundwater and 228 human hair samples were collected. Table 2 summarizes the samples collected in both sampling campaigns.
For groundwater collection, standing water in tube wells was first pumped out for about 10 min. Then, three subsamples for different assessment purposes, including the determination of metal concentration and evaluation of noncarcinogenic and carcinogenic risks, were collected in clean polypropylene bottles that had been previously washed with water drawn from the tube well. Raw water (unfiltered) samples were analyzed for total heavy metal concentrations. The total metal concentrations were then compared with the fourth edition of the WHO guidelines for drinking water quality (WHO 2011) for contamination and safety evaluations of groundwater and to the USEPA reference dose (RfD) (IRIS: Integrated Risk Information System 2011) for both noncarcinogenic and carcinogenic risk assessments. Filtered water samples (using a 0.45-μm pore-sized membrane filter) were analyzed for dissolved As (As(III) and As(V)) concentrations. Here, speciation of inorganic As(III) was determined from samples that were collected using the combination of a 0.45-μm pore-sized membrane filter and an As speciation cartridge. All samples were preserved using concentrated HNO3, kept at 4 °C, and delivered to the laboratory. During the collection of each sample, the pH and reduction–oxidation (redox) potential were also measured using a Horiba D-54 meter; conductivity was measured using an Orion 2-Star meter.
Human hair samples and groundwater collections were conducted at the same time. Hair samples were randomly collected from household members that generally consumed the collected groundwater on a daily basis. About 1 to 2 g of hair from each individual was cut as near as possible to the scalp using stainless steel scissors. The collected hair samples were then placed in labeled and sealed plastic bags before being stored in darkness at ambient temperature until sample analysis.
Sample preparation and analyses
Without pretreatment, both the total metal and dissolved As and As(III) concentrations in the groundwater samples were determined using an inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7500ce). The target elements and their detection limits (μg L−1) were 0.05 As, 0.78 B, 0.03 Ba, 0.05 Cr, 0.07 Cu, 0.04 Mn, 0.04 Ni, and 0.02 Pb.
For analysis, hair samples were first cut into small pieces (0.3 cm) and then washed in order to remove surface hair grease and attached dust particles, following a standardized washing procedure using acetone and deionized water as recommended by the International Atomic Energy Agency (IAEA 1976). In brief, each hair sample was washed in acetone, followed by two washings in deionized water and a final time in acetone. Each washing step took about 10 min. Washed hair samples were then dried in an oven at 60 °C overnight prior to initiating a digestion process. An acid digestion was performed following the method described by Phan et al. (2010). In brief, about 50 to 100 mg of two replicated subsamples of each washed and dried hair sample were weighed into acid-cleaned polyethylene tubes. Then, 1 mL of concentrated HNO3 was added to each hair sample. The digestion was conducted under a closed system at room temperature for 48 h. Next, the acid-digested hair solution was diluted with 9 mL of deionized water and filtered through a 0.45-μm pore-sized membrane filter. Finally, the clear digested solution was analyzed using ICP-MS (Agilent 7500ce) in order to determine the metal concentrations of the same target elements as in groundwater samples. A human hair standard reference material (GBW07601) was treated in the same manner as the sample digestion and analysis to confirm the precision and accuracy of the digestion method.
The accuracy and precision of the ICP-MS measurements were determined using a reagent blank, instrument calibration standard, and standard reference material for trace metals in natural water (SRM 1640).
Human health risk assessment model
Exposure assessment
The magnitudes of the potential chronic health issues of interest (Table 3) as a result of the daily ingestion of specific heavy metals in groundwater were estimated using the following equation (USEPA 1989).
where,
-
ADD is the average daily dose from ingestion of metal in drinking water (mg kg−1 day−1)
-
CW is the concentration of a particular heavy metal in groundwater (mg L−1)
-
IR is the water ingestion rate (L day−1)
-
EF is the exposure frequency (days year−1)
-
ED is the exposure duration (years)
-
BW is the body weight (kg)
-
AT is the average time of life expectancy (days).
Watanabe et al. (2004) and Hossain et al. (2013) reported that the amount of water intake used as the basis for exposure calculation in developing countries, particularly those with warmer climates, generally differs significantly from the quantity of water intake that WHO used to establish the guideline values for drinking water contaminants. Thus, studies that used either the standard amount of water intake (for example, the 2 L day−1 recommended by the WHO) or any secondary data from certain regulatory agencies (such as those recommended in Wang et al. 2011; Batayneh 2012; Giri et al. 2012) might overestimate or underestimate the actual individual exposure.
Therefore, local adjustments to the daily water consumption (IR) value were applied in this present study. Furthermore, to minimize any errors in the assessment of actual individual exposure to heavy metals, the demographic-related information (BW and AT) needed as variables for Eq. (1) were obtained from the individuals using questionnaires.
An EF of 365 days year−1 recommended by the USEPA (1989) was used. The life expectancy at birth of Laotians is 63 years (WHO 2009), for an average life expectancy (AT) of 22,995 days.
The ADD of each metal ingested by each individual was then used for the assessment of both the noncarcinogenic and carcinogenic risks.
Noncarcinogenic risk assessment
The potential noncarcinogenic toxicity of a particular single metal was subsequently estimated through the noncancer hazard quotient (noncancer HQ) shown below (USEPA 1989).
where,
-
ADD is the average daily dose from ingestion of metal in drinking water (mg kg−1 day−1) as a result of Eq. (1)
-
RfD is the USEPA oral reference dose (mg kg−1 day−1) of a particular heavy metal.
As the RfD (Table 3) is the estimated maximum oral daily dose of a toxic substance that is unlikely to cause deleterious noncancerous health effects during a individual’s lifetime, the exposed individual is assumed to be safe when HQ ≤ 1; if HQ > 1, the individual may be vulnerable to the noncancerous effects of that toxic element.
The summation of all noncancer HQs, the noncancer hazard index (noncancer HI), was then calculated in order to express the overall potential noncarcinogenic effects from a mixture of heavy metals through the ingestion of groundwater. If HI > 1, significant noncarcinogenic toxicity through the ingestion pathway could occur (USEPA 1989, 2000). The noncarcinogenic risk (HQ) contribution of each metal to the HI in each area was also calculated.
Carcinogenic risk assessment
As summarized in Table 3, only As could be considered a known human carcinogen. Therefore, only the concentration of As in groundwater was used to assess the probability of cancer developing in individuals over their lifetime, based on the following formula (USEPA 1989).
where,
-
CR is the cancer risk
-
ADD is the average daily dose from ingestion of As (mg kg−1 day−1) as a result of Eq. (1)
-
SF is the oral cancer slope factor of As (1.5 mg kg−1 day−1)
The SF was obtained from the Integrated Risk Information System of the USEPA (IRIS: Integrated Risk Information System 2011).
A schematic summarizing the procedures for assessing the human health risks conducted in this study is shown in Fig. 2.
Statistical analyses
All statistical analyses were performed using the SPSS 15.0 for Windows software package. Prior to statistical analyses, all data sets were tested for normality of distribution using the Kolmogorov–Smirnov test (n > 50), and as the distribution of the data sets were not normally distributed, a nonparametric test (Kruskal–Wallis H) was applied to assess the regional differences in the physical and chemical characteristics of groundwater, the health risk assessment factors (IR, ED, and BW), ADD, HI, HQ, CR, and As concentrations in hair. The significant differences in health risk assessment factors (IR, ED, and BW), ADD, HI, HQ, CR, and As concentrations in hair caused by gender were also tested using the Kruskal–Wallis H test. The relationship between each physical and chemical characteristic of groundwater was then analyzed by Spearman’s rho correlation. The relationship of both noncarcinogenic and carcinogenic risks as well as the levels of As concentrations in hair with ADD, IR, ED, and BW were also examined by Spearman’s rho correlation coefficient. The value of p < 0.01 was used to determine significant differences and significant correlations among the parameters.
Results and discussion
Groundwater chemistry of Lao PDR
The chemical characteristics of groundwater in the Lao PDR are presented in Table 4. In general, a broad range of groundwater sample pH varying from acidic to basic (pH = 3.53 to 8.41) was found. Median pH values show that groundwater from provinces other than Vientiane and Khammouan was relatively neutral. Oxidizing conditions ranging from 18.8 to 222.7 mV (median values) were found in most samples. Some samples displaying reducing groundwater conditions were found in Vientiane (−45.3 mV), Bolikhamxai (−42.0 mV), Saravane (−37.0 mV), Champasak (−22.5 mV), and Attapeu (−1.5 mV).
A significant difference for region was observed in the pH, conductivity, redox potentials, and total concentration of As, B, Ba, Cu, and Ni among the seven study areas (Kruskal–Wallis H, p = 0.000). Compared with the other areas, groundwater from Champasak showed higher concentrations of total As, B, and Ba, with lower redox potentials (Kruskal–Wallis H). This finding indicates that low redox potential values might cause dissolution into the groundwater of heavy metals in soils, particularly in Mekong River sediment. As groundwater in these areas is a drinking water source, the concentrations of As, B, Ba, Cr, Cu, Mn, Ni, and Pb, were compared with WHO guidelines. As Table 5 displays, only As and Ba exceeded the guidelines. About 46 % (68 of 148) and 16.2 % (24 of 148) of the samples contained As and Ba higher than 10 and 700 μg L−1, respectively. In addition, 13.5 % of samples exceeded the national drinking water standard for As of 50 μg L−1. The percent of groundwater with As exceeding the WHO guideline of 10 μg L−1 in these areas is summarized in Table 5. Groundwater with As > 10 μg L−1 was mostly collected from Champasak, where the highest concentration of As (277.75 μg L−1) was also detected (Table 4). However, the levels of As found in this study were lower than the As in groundwater collected from tube wells along the Mekong River in both Cambodia and Vietnam. The levels of As in the Mekong River delta groundwater ranged up to 3,500 μg L−1 in Cambodia and 1,351 μg L−1 in Vietnam (Kim et al. 2011).
Groundwater samples whose As content exceeded WHO guidelines were usually found with Ba concentrations higher than the WHO guideline of 700 μg L−1 (Table 5). Apart from As and Ba, concentrations of other heavy metals were found at levels lower than the WHO guidelines. Therefore, the table confirms that the groundwater samples collected from Vientiane and Khammouan are generally safe and thus can be used by the public, especially as drinking water.
The statistical analysis of the relationship between the physical and chemical characteristics of groundwater displayed significant positive correlations of: (i) total As concentrations with pH (correlation coefficients (r 2) = 0.363), with total concentrations of B (r 2 = 0.659), Ba (r 2 = 0.305), and Mn (r 2 = 0.241) and (ii) total Ba concentrations with total concentrations of B (r 2 = 0.387), Cr (r 2 = 0.269), and Mn (r 2 = 0.409). In addition, significant inverse correlations between the redox potentials and total concentrations of both As and Ba were found, with r 2 of −0.498 and −0.327, respectively. These results clearly suggest that elevated As and Ba concentrations can be found under higher (more basic) pH and reducing environments. This finding agrees with previous reports that As usually displays a sensitive mobilization at pH values of 6.5 to 8.50 under reducing conditions (Phan et al. 2010; Water Sanitation Program 2005). The reducing (anaerobic) conditions that develop from the degradation of organic matter in recently deposited river sediment favors the simultaneous desorption of As- and Ba-bearing minerals in aquifer sediment, such as metal oxides and sulfide minerals, which are the most important mineral sources of As in aquifers (Water Sanitation Program 2005). Ba usually accumulates in the Mn oxides and sulfide minerals of sedimentary deposits (Adriano 2001; Mokrik et al. 2009). This phenomenon is likely the main source of groundwater As in Bolikhamxai, Savannakhet, Saravane, and Champasak—the main floodplains affected by the Mekong River. In Attapeu, deposits from ancient volcanic activity are thought to be the source of a portion of the As and Ba.
The chemistry of As in groundwater
The distribution of As species in groundwater for each area are presented in Fig. 3. A reduced form of As, As(III), was found to be the predominant As species in the reducing groundwater of Saravane and Champasak in which As contamination (>10 μg L−1) was detected. In contrast, As(V) was mainly present in the oxidizing groundwater in Bolikhamxai, Savannakhet, and Attapeu. Overall, As(III) accounted for 40.7 to 50.4 % of the total As concentrations in groundwater collected from Saravane and Champasak, respectively, and under the more oxidizing groundwater environment of Bolikhamxai, Savannakhet, and Attapeu, As(V) accounted for 48.5 to 50.5 % of the total. All As species including As(III), As(V), and particulate As displayed a strong positive significant relationship with the total As concentration, with r 2 of 0.895, 0.802, and 0.702, respectively. Significant positive correlations were also observed between the pH of groundwater and As(III) (r 2 = 0.340) and with As(V) (r 2 = 0.422). Furthermore, As(III) concentrations had significant negative correlations with redox potentials (r 2 = 0.487). Results of these statistical analyses agree well with studies of As distribution in groundwater, which found As(III) more stable and abundant in reducing environments and As(V) predominant in oxidizing environments (Ötleş and Çağindi 2010; Phan et al. 2010).
The dominance of As(III) in groundwater not only provides comprehensive hydrological information about the groundwater environment but also suggests As is of toxicological importance to Laotians, as As(III) is more acutely toxic than As(V) (Adriano 2001; Ötleş and Çağindi 2010).
Noncarcinogenic risk (HQ) assessment of a single heavy metal
To date, there have been few reports on noncarcinogenic effects induced by heavy metals in the groundwater of Laos. A noncarcinogenic risk assessment was therefore conducted in this study based on the results of monitoring heavy metal concentrations in groundwater. The toxicity risks for ingestion of each metal in each area are quantified in Table 6. The noncancer HQs of all eight heavy metals in Vientiane, Khammouan, and Attapeu were below the recommended HQ threshold of 1. The HQs (median values) ranged from 5.90E−07 (Cr) to 8.57E−03 (As) in Vientiane, 4.30E−07 (Cr) to 9.71E−03 (As) in Khammouan, and 6.60E−07 (Cr) to 8.59E−02 (As) in Attapeu. This suggests these heavy metals in the groundwater to pose no adverse noncarcinogenic health threats to these provinces. By contrast, the residents of Bolikhamxai, Savannakhet, Saravane, and Champasak could experience adverse noncarcinogenic health effects (as summarized in Table 3) from groundwater consumption. Notably, As was the only heavy metal of concern that showed the maximum HQ values of 1.20, 1.12, 1.87, and 5.41 in Bolikhamxai, Savannakhet, Saravane, and Champasak, respectively (Table 6). The HQs of chronic As exposure in all areas studied of the Lao PDR were lower than the HQ of As exposure in other As-contaminated areas along the Mekong River in Cambodia, which ranged from 1.29E−03 to 35.67 (Phan et al. 2010). The difference in the HQ from As exposure between this present study and that from groundwater consumption in Cambodia can be ascribed to that country’s higher As groundwater concentrations, which ranged from 0.12 to 1,841.50 μg L−1 (Phan et al. 2010). Though an HQ value above the threshold indicates a greater level of concern, it should also be noted that this probability is not the statistical probability of the effect occurring in a given individual. The noncarcinogenic effects of chronic exposure to As can include damage to the gastrointestinal tract, respiratory tract, skin, liver, cardiovascular system, hematopoietic system, and nervous system (Kapaj et al. 2006; Rahman et al. 2009). Dermal lesions with evidence of hyperpigmentation, hyperkeratosis, desquamation, and hair loss are the most abundant symptoms of chronic As exposure, which usually occur within a period of 5 to 15 years (Duker et al. 2005; Gault et al. 2008). The shortest exposure period reported for the manifestation of As toxicity was about 8 months (Khan et al. 2003).
Overall noncarcinogenic effects (HI) of a mixture of heavy metals through groundwater ingestion
The potential risks of combined noncarcinogenic effects posed by a mixture of all eight heavy metals through daily groundwater ingestion were assessed using the noncancer HI. Table 7 summarizes the potential noncarcinogenic effects in each area. In contrast to previous reports, for example, Phan et al. (2010), Wang et al. (2011), Batayneh (2012), and Giri et al. (2012), in which HI was used to indicate the risk of any single metal of interest, in this present study HI was used as an indicator of the aggregate risk/ risk of mixed trace elements. The noncarcinogenic effects of mixed trace elements occur when HI is higher than 1. As the groundwater from the areas studied was not contaminated by single metals only, and magnified toxic effects from multi-metals might exert synergistic and antagonistic health effects, the noncarninogenic effects of mixed metals were evaluated.
Similar to the noncarcinogenic effects of individual heavy metals (HQ) discussed above, the median values of the HI for Vientiane, Khammouan, and Attapeu were lower than 1, indicating no significant adverse health effects from groundwater consumption in these provinces. Long-term daily consumption of groundwater in Bolikhamxai, Savannakhet, Saravane, and Champasak, however, could cause chronic negative health effects since the maximum HI values for ingestion were 1.34, 1.36, 2.01, and 5.50, respectively. These maximum HI values, in may be noted, were from about 2 to 30 times lower than the HI values for mixed metals recently reported by Phan et al. (2013), who found a range in maximum HI from 2.04 to 35.78 in groundwater collected from As-contaminated areas in Kandal and Kratie in the Mekong Basin in Cambodia.
Overall, the statistical analyses suggest that the significant difference in the average daily dose of As ingestion (ADDAs; Kruskal–Wallis H, p = 0.000) among areas studied was the main factor causing a significant difference in HI values. Indeed, significant correlations between the ADDAs and noncarcinogenic HI (r 2 = 0.987), ADDAs and As concentration in groundwater (r 2 = 0.901), ADDAs and ED (r 2 = 0.249), and ADDAs and BW (r 2 = −0.219) revealed that variation of the HI value can be affected by the As concentration in the groundwater (Asw), ED, and an individual’s BW. These findings agree well with the mathematical models of exposure and noncarcinogenic risk assessment presented in Eqs. (1) and (2), respectively. No significant difference in HI values between genders was found (Kruskal–Wallis H, p = 0.612).
The relative contributions of each heavy metal to HI in each area are depicted in Fig. 4. The percent contribution of each element to the aggregate HI risk in this study was similar to that element’s percent contribution to HI from contaminated groundwater in Cambodia (Phan et al. 2013) and Turkey (Celebi et al. 2014), for which As, Ba, and Mn were the main contributors to the HI index. The groundwater collected along the Mekong River for this present study and that collected in Cambodia by Phan et al. (2013) showed the exact same order of significant elements contributing to HI: As > Mn > Ba. The other elements, B, Cr, Cu, Ni, and Pb, were found to contribute insignificantly to HI.
It is noteworthy that As contributed the highest percentage to HI values, especially in the provinces where the HI values were greater than 1. The percentage that As contributed to the HI values for Bolikhamxai, Savannakhet, Saravane, and Champasak was 85.2, 60.1, 67.8, and 92.0 %, respectively. As was the heavy metal contributing most to the combined potential for noncarcinogenic negative health effects from ingestion of Laos groundwater.
Carcinogenic risk assessment
Only As, among the heavy metals in groundwater, is classified as a known human carcinogen by the International Agency for Research on Cancer and the Integrated Risk Information System of the USEPA (IARC 2011; IRIS: Integrated Risk Information System 2011). Therefore, the carcinogenic risk assessments in this study were based only on As groundwater concentrations. The cancer risks (potential carcinogenic rate) in this study ranged from 1.2E−07 to 2.9E−05 in Vientiane, 1.5E−07 to 5.4E−04 in Bolikhamxai, 2.7E−07 to 1.8E−05 in Khammouan, 1.0E−06 to 5.04E−04 in Savannakhet, 2.1E−06 to 8.4E−04 in Saravane, 2.0E−06 to 2.4E−03 in Champasak, and 1.2E−07 to 1.5E−04 in Attapeu. In practical terms, the USEPA considers cancer risks below 1E-06 to be negligibly small, and risks of 1E−04 to be sufficiently large that remediation is desirable; cancer risks that range between 1E−06 to 1E−04 are generally considered acceptable (USEPA 2011). The cancer risks to the local people of Vientiane and Khammouan could thus be considered “acceptable.” However, at the upper bound of the cancer risks shown in Table 7, about 6, 5, 9, and 30 in 10,000 inhabitants of Bolikhamxai, Savannakhet, Saravane, and Champasak, respectively, could suffer from some type of cancer as a result of daily groundwater consumption.
As the upper bound for cancer risks for these four provinces was higher than the acceptable risk level of 1E−04, it is recommended that a mitigation program for removing As from groundwater be applied to reduce future incidences of cancer. Previous reports also indicate epidemiological evidence for lung, bladder, liver, kidney, colon, prostate, and skin cancers after decades of As exposure (Khan et al. 2003; Duker et al. 2005; Rahman et al. 2009; IRIS: Integrated Risk Information System 2011; Kim et al. 2011). However, no carcinogenic effects from As in the areas studied were observed, as the ED were not long enough for cancers to develop; the median and average ED to As-contaminated groundwater in this study were 7 and 8.2 years, respectively.
The risk of arsenical cancers (CR) in this study was strongly dependent on As levels in groundwater (r 2 = 0.901) and the ADD (r 2 = 1.000). Other factors, individual BW (r 2 = −0.219) and ED (r 2 = 0.249), were also significantly associated with the As cancer risk. Although there was a significant difference in the cancer risk for areas (Kruskal–Wallis H, p = 0.000), no significant differences in cancer risks between genders were observed (Kruskal–Wallis H, p = 0.060). These results agree with Phan et al. (2010), who found the risk of As poisoning for Cambodian residents correlated well with As groundwater concentration, ADD, ED, and IR.
Arsenic contents in human hair
Hair, a keratin-rich tissue, has been recently used as a reliable indicator of the early stages of deleterious health effects from toxicant exposure. As hair is protein rich in thiol groups, which bind well to metal ions, the levels of exposed toxicants in an individual were found to be well-correlated with their levels in each individual’s hair (Gault et al. 2008).
Considering that As was the only heavy metal that could have both noncarcinogenic and carcinogenic effects, for the sake of simplicity, only As concentrations in human hair are discussed in this study.
Analyses of acid-digested hair samples revealed that the As concentrations ranged from 0.01 to 9.85 mg kg−1 (Fig. 5), with median and average values in the whole population (n = 228) being 0.56 and 0.80 mg kg−1, respectively. The percentage recovery of the As concentration in the human hair reference material (GBW07601) was 100.74 ± 6.92. According to Arnold et al. (1990), the normal As concentration in human hair ranges from 0.08 to 0.25 mg kg−1, with a level of >1.00 mg kg−1 being an indication of toxic effects. As shown in Fig. 7, approximately 25.5 % of the population in this study had As levels in hair higher than 1.00 mg kg−1. The remainder had As levels in normal (0.08–0.25 mg kg−1; 18.4 %) or elevated concentrations (>0.25–1.00 mg kg−1; 56.1 %). Savannakhet and Champasak had the highest number of hair samples containing As concentrations exceeding the recommended toxicity levels (Table 8).
Statistical analyses found no significant difference in As hair content between areas studied (Kruskal–Wallis H, p = 0.020), or between genders (Kruskal–Wallis H, p = 0.039), suggesting gender did not influence As accumulation in the hair of the Lao subject population. However, significant positive correlations between As concentration in hair (Ash) and As concentration in groundwater (Asw; r 2 = 0.311), Ash and IR (r 2 = 0.177), and Ash and ADD (r 2 = 0.230) were found (Fig. 6); i.e., As accumulation in hair is likely affected by Asw, IR, and ADD. The reason a significant difference in As content in hair was not observed though there was a significant difference in regional Asw could be due to differences in other socioeconomic status and lifestyle (health-related) behaviors, such as poverty, illiteracy, perception of As and arsenicosis, dietary habits, nutritional status, smoking, and concurrent exposure to other substances (Adriano 2001; Arsenic Policy Support Unit 2006; Hopenhayn 2006; Sampson et al. 2008). Moreover, community and social awareness, including of As mitigation projects and public education, are other factors affecting the uptake of As, and subsequently the prevalence of arsenicosis (Arsenic Policy Support Unit 2006).
In terms of using Ash as an indicator of human health risk, significant positive correlations can be found between both Ash and noncarcinogenic risk from ingestion exposure (HI) and between Ash and carcinogenic risk (CR). Interestingly, these two significant correlations can only be found in the female population (Fig. 7a, b). A sociocultural background in which women generally have lower status and less social value than men was taken to be the reason for higher arsenicosis in women in Bangladesh (Arsenic Policy Support Unit 2006). In this case, exposed females are generally the most likely family members to be malnourished, lower in weight, and less likely to be literate. This finding was in contrast to Chojnacka et al. (2010) and Sthiannopkao et al. (2010), who found no influence of gender on As level in human hair from either occupational or environmental exposures. Further study on the influence of gender, metabolic activity, other possible routes of exposure, and the nutritional status of Laotian people should be conducted to better understand factors affecting the metabolism and accumulation of As in hair.
Nevertheless, significant positive correlations between the ADD and the elemental concentration in hair of Ba (r 2 = 0.661), Cr (r 2 = 0.518), Cu (r 2 = 0.232), and Mn (r 2 = 0.353) were found. This indicates that human hair can be used to determine the exposure and accumulation levels of not only As but also Ba, Cr, Cu, and Mn.
Quantitative summary of human health risks from groundwater ingestion
Kim et al. (2011) indicated the Lao PDR population size at risk from the As in groundwater was still largely unknown; the present study therefore undertook a quantitative assessment of the population at risk. The percent of residents exposed to potential negative health effects through groundwater ingestion are summarized in Table 8. Noncarcinogenic and carcinogenic health risk assessments reveal that only the residents of Vientiane and Khammouan are safe from such effects. People in Bolikhamxai, Savannakhet, Saravane, and Champasak are at risk to both noncarcinogenic and carcinogenic effects. Approximately (respectively) 7.4, 4.2, 11.8, and 25.0 % of the population in these provinces are exposed to noncarcinogenic ill health effects; about 22.2 % of the population in Bolikhamxai, 33.3 % in Savannakhet, 32.4 % in Saravane, 76.8 % in Champasak, and 28.6 % in Attapeu are at risk to cancers, with the cancer risks ranging from 2 to 30 in 10,000 exposures. The population of Champasak was most at risk for both noncarcinogenic and carcinogenic effects, in line with Champasak’s having the highest As groundwater concentration (discussed in “Groundwater chemistry of Lao PDR”).
Conclusions
This study clearly revealed significant human health risks through the ingestion of groundwater in the Lao PDR. As was the only element that posed both noncarcinogenic (HI) and carcinogenic (CR) health risks to the populations of Bolikhamxai, Savannakhet, Saravane, Champasak, and Attapeu. These risks were mainly governed by the Asw, ADD, ED, and an individual’s BW. This present work pointed out the areas with high risks of negative health effects. The computation of human health risks from ingestion exposure suggested that the residents of Bolikhamxai, Savannakhet, Saravane, and Champasak are exposed to both noncarcinogenic and carcinogenic effects in which the prevalence of cancer varied from 2 to 30 in 10,000 exposures. In addition, this study confirmed that elemental concentrations of As, Ba, Cr, Cu, and Mn in human scalp hair can be used to reflect the exposure levels of these elements through groundwater ingestion.
It is highly recommended that mitigation measures be conducted in the areas at risk, with the particular aim of lowering the concentrations of As, Ba, and Mn to meet WHO drinking water standards. Public health awareness should moreover be raised through educational and public health programs for a sustainable national environment.
References
Adriano, D. C. (2001). Trace elements in terrestrial environments: biogeochemistry, bioavailability, and risks of metals (2nd ed.). New York: Springer.
Arnold, H. L., Odam, R. B., & James, W. D. (1990). Diseases of the skin: clinical dermatology (8th ed.). Philadelphia: W.B. Saunders.
Arsenic Policy Support Unit (2006). Selected papers on the social aspects of arsenic and arsenic mitigation in Bangladesh. Available from http://phys4.harvard.edu/~wilson/arsenic/references/selected_social_papers.pdf. Accessed 13 August 2012.
Batayneh, A. T. (2012). Toxic (aluminum, beryllium, boron, chromium and zinc) in groundwater: health risk assessment. International Journal of Environmental Science and Technology, 9, 153–162.
Bencko, V. (1995). Use of human hair as a biomarker in the assessment of exposure to pollutant in occupational and environmental settings. Toxicology, 101, 29–39.
Caylak, E. (2012a). Health risk assessment for trace metals, polycyclic aromatic hydrocarbons and trihalomethanes in drinking water of Cankiri, Turkey. E-Journal of Chemistry, 9(4), 1976–1991.
Caylak, E. (2012b). Health risk assessment for arsenic in water sources of Cankiri Province of Turkey. CLEAN-Soil, Air, Water, 40(7), 728–734.
Caylak, E., & Tokar, M. (2012a). Metallic and microbial contaminants in drinking water of Cankiri, Turkey. E-Journal of Chemistry, 9(2), 608–614.
Caylak, E., & Tokar, M. (2012b). Investigating chemical and microbiological contaminants in drinking water of Cankiri Province, Turkey. Environmental Earth Sciences, 67(7), 2015–2025.
Celebi, A., Sengour, B., & Klove, B. (2014). Human health risk assessment of dissolved metals in groundwater and surface waters in the Melen watershed, Turkey. Journal of Environmental Science and Health, Part A: Toxic/Hazardous Substances and Environmental Engineering, 49, 153–161.
Chanpiwat, P., Sthiannopkao, S., Cho, K. H., Kim, K. W., San, V., Suvannathong, B., & Vongthavady, C. (2011). Contamination by arsenic and other trace elements of tube-well water along the Mekong River in Lao PDR. Environmental Pollution, 159, 567–576.
Chojnacka, K., Michalak, I., Zielińska, A., Górecka, H., & Górecki, H. (2010). Inter-relationship between elements in human hair: the effect of gender. Ecotoxicology and Environmental Safety, 73, 2022–2028.
Duker, A. A., Carranzan, E. J. M., & Hale, M. (2005). Arsenic geochemistry and health. Environment International, 31, 631–641.
Encyclopedia of the Nations (2012). http://www.nationsencyclopedia.com/geography/Indonesia-to-Mongolia/Laos.html. Accessed 25 May 2012.
Esteban, M., & Castaño, A. (2009). Non-invasive matrices in human biomonitoring: a review. Environment International, 35, 438–449.
Fengthong, T., Dethoudom, S., & Keosavanh, O. (2002). Drinking water quality in the Lao People’s Democratic Republic. ESCAP-IWMI seminar on environmental and public health risks due to contamination of soils, crops, surface and groundwater from urban, industrial and natural sources in South East Asia. Hanoi, Vietnam, 10–12 December 2002.
Gault, A. G., Rowland, H. A. L., Charnock, J. M., Wogelius, R. A., Gomez-Morilla, I., Vong, S., Leng, M., Samreth, S., Sampson, M. L., & Polya, D. A. (2008). Arsenic in hair and nails of individuals exposed to arsenic-rich groundwaters in Kandal province, Cambodia. Science of the Total Environment, 393, 168–176.
Gibney, M. J., Labham-New, S. A., Cassidy, A., & Vorster, H. H. (2009). Introduction to human nutrition (2nd ed.). West Sussex: Wiley-Blackwell.
Giri, S., Mahato, M. K., Singh, G., & Jha, V. N. (2012). Risk assessment due to intake of heavy metals through the ingestion of groundwater around two proposed uranium mining areas in Jharkland, India. Environmental Monitoring and Assessment, 184, 1351–1358.
Hopenhayn, C. (2006). Arsenic in drinking water: impact on human health. Elements, 2(2), 103–107.
Hossain, M. A., Rahman, M. M., Murrill, M., Das, B., Roy, B., Dey, S., Maity, D., & Chakraborti, D. (2013). Water consumption patterns and factors contributing to water consumption in arsenic affected population of rural West Bengal, India. Science of the Total Environment, 463–464, 1217–1224.
Howard, G., Bartram, J., Pedley, S., Schmoll, O., Chorus, I., & Berger, P. (2006). Groundwater and public health. In O. Schmoll, G. Howard, J. Chilton, & I. Chorus (Eds.), Protecting groundwater for health. UK: Cornwall.
IAEA. (1976). IAEA/RL/50 Activation analysis of hair as an indicator of contamination of man by environmental trace element pollutants, a cooperative report on the co-ordinated research programme: Nuclear-based methods for analysis of pollutants in human hair, Vienna, October 1976.
IARC: International Agency for Research on Cancer. (2011). IARC monographs on the evaluation of carcinogenic risks to humans, Supplement 7: overall evaluations of carcinogenicity: an updating of IARC monographs volumes 1 to 42. Available from http://monographs.iarc.fr/ENG/Monograohs/suppl17-19.pdf. Accessed 26 June 2011.
IRIS: Integrated Risk Information System (2011). Available from http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showSubstanceList. Accessed 27 July 2011.
Järup, L. (2003). Hazards of heavy metal contamination. British Medical Bulletin, 68, 167–182.
Kapaj, S., Peterson, H., Liber, K., & Bhattacharya, P. (2006). Human health effects from chronic arsenic poisoning—a review. Journal of Environmental Science and Health, Part A, 41, 2399–2428.
Khan, M. M., Sukauchi, F., Sonoda, T., Washio, M., & Mori, M. (2003). Magnitude of arsenic toxicity in tube-well drinking water in Bangladesh and its adverse effects on human health including cancer: evidence from a review of the literature. Asian Pacific Journal of Cancer Prevention, 4, 7–14.
Khongsab, S. (2012). Flood management and mitigation measures. Available from http://www.iwhr.com/zgskyww/rootfiles/2010/06/23/1276836877899907-1276836877901705.pdf. Accessed 27 May 2012.
Kim, K. W., Chanpiwat, P., Hoang, T. H., Phan, K., & Sthiannopkao, S. (2011). Arsenic geochemistry of groundwater in Southeast Asia. Frontiers of Medicine, 5(4), 420–433.
Marchiset-Ferlay, N., Savanovitch, C., & Sauvant-Rochat, M. P. (2012). What is the best biomarker to assess arsenic exposure via drinking water? Environment International, 39, 150–171.
Mokrik, R., Karro, E., Savitskaja, L., & Drevaliene, G. (2009). The origin of barium in the Cambrian-Vendian aquifer system, North Estonia. Estonian Journal of Earth Sciences, 58(3), 193–208.
Ötleş, S., & Çağindi, Ö. (2010). Health importance of arsenic in drinking water and food. Environmental Geochemistry and Health, 32, 367–371.
Phan, K., Sthiannopkao, S., Kim, K. W., Wong, M. H., Sao, V., Hashim, J. H., Yasin, M. S. M., & Aljunid, S. M. (2010). Health risk assessment of inorganic arsenic intake of Cambodia residents through groundwater drinking pathway. Water Research, 44, 5777–5788.
Phan, K., Phan, S., Huoy, L., Suy, B., Wong, M. H., Hashim, J. H., Yasin, M. S. M., Aljunid, S. M., Sthiannopkao, S., & Kim, K. W. (2013). Assessing mixed trace elements in groundwater and their health risk of residents living in the Mekong River basin of Cambodia. Environmental Pollution, 182, 111–119.
Prüss-Ustün, A., Vickers, C., Haefliger, P., & Bertollini, R. (2011). Knowns and unknowns on burden of disease due to chemicals: a systematic review. Environmental Health, 10, 9.
Public Administration and Service Authority (2009). About Lao PDR. Available from http://www.pacsa.gov.la/index.php?option=com_content&view=article&id=18&Itemid=83&lang=en. Accessed 29 May 2012.
Rahman, M. M., Ng, J. C., & Naidu, R. (2009). Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environmental Geochemistry and Health, 31, 189–200.
RAIS: The Risk Assessment Information System (2011). http://rais.ornl.gov/. Accessed 27 July 2011.
Rodrigues, J. L., Batista, B. L., Nunes, J. A., Passos, C. J. S., & Barbosa, F. (2008). Evaluation of the use of human hair for biomonitoring the deficiency of essential and exposure to toxic elements. Science of the Total Environment, 405, 370–376.
Sampson, M. L., Bostick, B., Chiew, H., Hangan, J. M., & Shantz, A. (2008). Arsenicosis in Cambodia: case studies and policy response. Applied Geochemistry, 23, 2976–2985.
South Laos Tour (2012). Provinces of Laos PDR. http://www.southlaostour.com/Laos-Province/. Accessed 29 May 2012.
Sthiannopkao, S., Kim, K. W., Cho, K. H., Wantala, K., Sotham, S., Sokuntheara, C., & Kim, J. H. (2010). Arsenic levels in human hair, Kandal Province, Cambodia: the influences of groundwater arsenic, consumption period, age and gender. Applied Geochemistry, 25, 81–90.
The Wetland Alliance (2012). http://www.wetlandsalliance.org/about-us/working-areas/laos. Accessed 27 May 2012.
Travel to Laos (2012). People and culture of Laos. Available from http://www.traveltolaos.com/laos-travel-guides/lao-travel-information/people-culture-of-laos/. Accessed 27 May 2012.
Tuvy Asian Books (2012). The land of Laos. http://www.tuvy.com/Countries/Laos/land_of_laos.html. Accessed 27 May 2012.
United Nations Environmental Programme (2001). State of the environment, Lao PDR, 2001. http://www.rrcap.unep.org/reports/soe/laosoe.cfm. Accessed 4 May 2012.
USEPA. (1989). Risk assessment guidance for superfund, volume 1: human health evaluation manual (part A) ((EPA/540/1-89/002: interim final). Washington DC: Office of Emergency and Remedial Response.
USEPA. (2000). Supplementary guidance for conducting health risk assessment of chemical mixtures (EPA/630/R-00/002). Washington DC: Risk Assessment Forum.
USEPA (2011). HH: Risk characterization. http://www.epa.gov/region8/r8risk/hh-risk.html. Accessed 27 July 2011.
Viridor Waste Ltd (2009). Viridor New England energy from waste project: Technical data for HHRA generic assessment criteria (402-0036-00350). http://www.devon.gov.uk/plandoc259_4975.pdf. Accessed 27 July 2011.
Wang, G., & Fowler, B. A. (2008). Roles of biomarkers in evaluating interactions among mixtures of lead, cadmium and arsenic. Toxicology and Applied Pharmacology, 233(1), 92–99.
Wang, Z., Chai, L., Wang, Y., Yang, Z., Wang, H., & Wu, X. (2011). Potential health risk of arsenic and cadmium in groundwater near Xiangjiang River, China: a case study for risk assessment and management of toxic substances. Environmental Monitoring and Assessment, 175, 167–173.
Watanabe, C., Kawata, A., Sudo, N., Sekiyama, M., Inaoka, T., Bae, M., & Ohtsuka, R. (2004). Water intake in an Asian population living in arsenic-contaminated area. Toxicology and Applied Pharmacology, 198, 272–282.
Water Environment Partnership in Asia (2012). State of water environmental issues: Lao PDR. Available from http://www.wepa-db.net/policies/state/laos/overview.htm. Accessed 4 May 2012.
Water Sanitation Program. (2005). Towards a more effective operational response: arsenic contamination of groundwater in South and East Asian countries. New Delhi: The World Bank.
WHO (2009). Life expectancy. http://apps.who.int/gho/data/?theme=country&vid=11900. Accessed 8 May 2012.
WHO. (2011). Guidelines for drinking-water quality (4th ed.). Geneva: WHO Press.
WHO/UNICEF (2012). Joint monitoring programme for water supply and sanitation: estimation for the use of improved drinking-water sources (Lao People’s Dem Republic). http://www.wssinfo.org/. Accessed 29 May 2012.
Acknowledgments
This research was financially supported by the International Environmental Analysis and Education Center (formerly known as the International Environmental Research Center (IERC)), the Gwangju Institute of Science and Technology, and Dong-A University, Republic of Korea. The authors wish to deliver their appreciation to Dr. Kitirote Wantala of the Department of Chemical Engineering, Faculty of Engineering, Khon Kaen University (Thailand), Mr. Piyanat Sawat-aue of the School of Energy, Environment and Materials, King Mongkut’s University of Technology Thonburi (Thailand), and Ms. Pham Thi Diem Phuong of Ho Chi Minh University of Technology (Vietnam) for their laboratory assistance, as well as to Ms. Doungkamon Phihusut of the School of Environmental Science and Engineering, Gwangju Institute of Science and Technology (Republic of Korea) for her assistance in groundwater collection. Dennis McDermott, PhD assisted with the English editing of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Information on funding sources and research ethical agreement in human subjects
The ethical agreement pertaining to use of human subjects was approved by the Ministry of Health, Lao PDR (no. 836/NCHWS).
Rights and permissions
About this article
Cite this article
Chanpiwat, P., Lee, BT., Kim, KW. et al. Human health risk assessment for ingestion exposure to groundwater contaminated by naturally occurring mixtures of toxic heavy metals in the Lao PDR. Environ Monit Assess 186, 4905–4923 (2014). https://doi.org/10.1007/s10661-014-3747-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10661-014-3747-0