Abstract
Rising concerns over fossil fuel depletion and plastic pollution have driven research into biodegradable alternatives, such as polylactic acid (PLA). Microbial fermentation is preferred for lactic acid production due to its ability to yield enantiomerically pure lactic acid, which is essential for PLA synthesis, unlike the racemic mixture from chemical synthesis. However, commercial lactic acid production using first-generation feedstocks faces challenges related to cost and sustainability. Macroalgae offer a promising alternative with their rapid growth rates and carbon capture capabilities. This review explores recent technological advancements in macroalgae physicochemical characterization, optimization of fermentation conditions, and innovative pretreatment methods to enhance sugar conversion rates for L-LA production. It also covers downstream processes for L-LA recovery, presenting a complete macroalgal biorefinery system. Environmental impacts and economic prospects are assessed through exergy and techno-economic analyses. By valorizing macroalgae detritus, this study underscores its potential to support a sustainable biorefinery industry, addressing economic feasibility and environmental impact.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The escalating global crisis of plastic waste accumulation and the diminishing reserves of fossil fuels are propelling the search for renewable and biodegradable materials to replace conventional plastics. Among the leading alternatives, polylactic acid (PLA) has emerged as a bio-based polymer derived from renewable or recycled resources. Its versatility and biodegradability position PLA as a promising solution to reduce dependence on petrochemical plastics, particularly in packaging applications. The growing production trend of PLA underscores its potential as a valuable material in various applications, including food packaging and medical devices.
Lactic acid (LA) is central to PLA production, traditionally obtained through microbial fermentation using first-generation feedstocks. However, this method has become increasingly controversial due to the “food versus fuel” debate, which may lead to rising food prices and increased pressure on agricultural resources. Macroalgae, as a third-generation feedstock, presents a sustainable alternative that avoids the food-versus-energy conflict associated with first-generation feedstocks. Unlike terrestrial crops, macroalgae do not depend on arable land and exhibit rapid growth rates and high carbohydrate content, positioning it as an ideal candidate for LA production.
Recent investigations have underscored various innovative strategies to improve the effectiveness of converting macroalgae into LA. Scientists have examined particular bacterial strains that are proficient in transforming macroalgal sugars into L-LA, concentrating on refining fermentation conditions to enhance yield while minimizing by-products. The diverse characteristics of macroalgae require customized pretreatment and hydrolysis techniques to optimize sugar extraction, which presents both hurdles and prospects for advancement. For example, the conversion of Eucheuma denticulatum residues into glucose through microwave-assisted autohydrolysis followed by enzymatic hydrolysis has shown promise in terms of energy efficiency [1]. However, challenges such as high energy consumption and enzyme costs remain. Similarly, using Saccharina latissima for acid concentration via forward osmosis has achieved high concentration factors, yet downstream purification remains an issue [2]. Working with multiple algae species has also highlighted downstream purification and reproducibility difficulties, primarily due to varying polysaccharide compositions [3]. These studies underscore the need for more cost-effective and energy-efficient pretreatment methods and standardized downstream processing approaches.
Conventional techniques, such as precipitation and distillation, tend to be demanding in terms of energy and result in considerable waste. It is vital to create alternative separation methods to reduce operational costs and lessen environmental effects, thereby improving the sustainability of LA production. This includes pretreatment strategies and uniform downstream processing methods. Conventional techniques, such as precipitation and distillation, are energy intensive and result in considerable waste. It is vital to create alternative separation methods to reduce operational costs and lessen environmental effects, thereby improving the sustainability of LA production. This includes pretreatment strategies and uniform downstream processing methods.
Exploring a macroalgae-based biorefinery for L-LA production represents a promising path for sustainable biopolymer manufacturing. Despite significant progress, the potential for L-LA production from macroalgae remains relatively untapped, offering opportunities for groundbreaking advancements in biopolymer manufacturing. Future research should optimize pretreatment and hydrolysis methods, develop cost-effective and energy-efficient processes, and fully standardize downstream processing to utilize macroalgae as a sustainable feedstock. This review aims to explore the potential of macroalgae detritus as a viable feedstock for L-LA production. It delves into the characteristics and advantages of using macroalgae in biorefineries, discusses current research, identifies challenges, and suggests future research directions. The structure of the review includes an overview of L-LA and its industrial importance, an introduction to biorefineries and the transition to third-generation feedstocks, and a detailed examination of the potential and challenges of using macroalgae for L-LA production.
Technologies in Lactic Acid Production
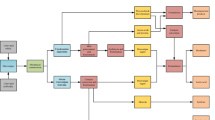
Lactic acid (2-hydroxy propionic acid), commonly known as LA, is an organic compound belonging to the family of carboxylic acids and can normally be found in nature. The physicochemical properties of LA significantly influence its chemical properties. In aqueous solutions, LA exhibits acidic properties and possesses an asymmetric carbon, which results in optical activity. The presence of both carboxyl and hydroxyl groups endows LA with bifunctional reactivity, enhancing its versatility in various chemical reactions. L ( +) lactic acid (L-LA) is one of the stereoisomers of LA. L-LA can be produced through fermentation using lactic acid bacteria (LAB). It is a colorless liquid, odorless, and mild acid taste. LA is a vital chemical building block in PLA production with widespread applications, mainly in the food, chemical, cosmetic, and pharmaceutical industries. The demand for LA has skyrocketed in recent years. The global demand for LA in 2016 was around 1220 kilotons, and it is predicted to grow by 16.2% between 2017 and 2025, reaching a total of 9.8 billion USD by 2025 [4]. There are two ways to produce LA: chemical synthesis and microbial fermentation, as shown in Fig. 1. Chemical synthesis has a racemic mixture (DL-lactic acid), and it is hard to control the physical properties of PLA through a racemic mixture of LA [5]. On the other hand, LA production from microbial fermentation produces optically pure L-LA or D(-) lactic acid (D-LA) with the appropriate microorganism [6].
Synthetic pathway of lactic acid production [6] (Copyright License No: 5841930978822)
Chemical Synthesis of Lactic Acid
LA production involves various chemical processes, including hydrolysis of LA derivatives like esters or nitriles, hydrolysis of alternative substituted propionic acids, decarboxylation of specific 2-methylmalonic acid derivatives, and approaches like reduction, oxidation, rearrangement, and disproportion [5]. Among these, the synthesis of LA from its derivatives is the only commercially viable method. Multiple studies have investigated its chemical synthesis utilizing varying carbon sources. One of these methodologies encompasses the chemical synthesis from petrochemical sources, where ethene is oxidized with palladium (II) chloride to generate acetaldehyde. Through high-pressure liquid phase conditions, assisted with hydrogen cyanide and a base, this acetaldehyde is transformed into lactonitrile. Subsequently, lactonitrile undergoes recovery and purification processes, followed by hydrolysis using sulfuric acid to produce a racemic mix of L- and D-LA [7].
The transformation of glycerol into LA is feasible through hydrothermal treatment, hydrogenolysis, or selective oxidation [8]. Among these, catalytic selective oxidation is a promising strategy because it operates under mild reaction conditions, which aligns with green chemistry principles. An innovative integrated process has been introduced to produce LA and formic acid (FA) simultaneously from glycerol and CO2 [9]. This method addresses the challenges of low productivity and waste production associated with current bio-based LA production technologies. The proposed two-pot/two-step transfer hydrogenation process achieves high yields, catalyst reusability, and efficient product separation. Moreover, the developed esterification method for lactate and formate salts using CO2 significantly reduces sulfuric acid consumption and potassium sulfate waste. Despite the advances in chemical synthesis, microbial fermentation remains a preferred method for LA production due to its lower environmental impact and potential for higher optical purity.
Microbial Fermentation of Lactic Acid
Biotechnology is currently the primary method of producing LA, with approximately 90% of commercial LA produced from the microbial fermentation of bio-based carbohydrates. A triumphant fermentative LA production depends on the temperature, pH, nutrients, substrate concentration, and end-product concentration [10]. Both pH and temperature are closely linked to cellular metabolism, affecting microorganism growth, substrate utilization, and LA production. The control of pH is particularly vital, with most studies indicating an optimal range of 5–6. pH levels outside this range can affect enzyme activity, nutrient transport, and, ultimately, LA production [11]. An optimized pH of around 5.0 was ideal for saccharification but not for high LA production [12]. Stepwise pH control strategies were adopted, with a lower pH in the early stages to mitigate the inhibition from calcium lactate and a higher pH in the stable fermentation phase to reduce low pH inhibition. Different pH control methods significantly impacted LA concentration and yield, demonstrating that the pH environment greatly influences the efficiency of LA production.
Temperature is another crucial element influencing bacterial growth and substrate utilization. The performance of LA production was evaluated at a commercial anaerobic digestion facility using mixed food waste feedstocks [13]. Operating conditions included temperatures ranging from 24 to 35 °C and a low pH of around 3.45. These conditions favored LA as the dominant acid, notably without process optimization. It was observed that a higher temperature and low pH positively influenced the LAB growth and LA production. The results also suggest that grain processing waste and milk paste positively influenced LA concentration, indicating that feedstock composition is crucial in LA fermentation efficiency.
The carbon to nitrogen (C/N) ratio significantly affects LA production. Aligning this ratio with nitrogen sources such as yeast extract, peptone, or meat extract can improve LA production. However, due to the high cost associated with some of these sources, alternative nitrogen sources such as corn steep liquor and corn gluten–wheat bran mixture (CWM) are being investigated [14, 15]. The size of the inoculum is another factor affecting LA production, with optimal sizes varying depending on the microorganism used.
Microbial fermentation has the advantages of low subtract costs, reaction temperature, and energy consumption compared to chemical synthesis. Based on these advantages, most industrial LA production uses microbial fermentation. Using bio-sourced raw materials can increase the market for LA-based polymers [16]. Nutrients, including minerals, vitamins, and nitrogen in inorganic forms, are crucial for the growth and maintenance of LAB. LAB are gram positive, catalase negative, non-sporing, and lack cytochromes. They produce LA predominantly through sugar fermentation. LAB encompasses 20 genera, including Lactobacillus, Lactococcus, Vagococcus, Oenococcus, Aerococcus, Streptococcus, Tetragenococcus, Enterococcus, Pediococcus, Carnobacterium, Weisella, and Leuconostoc [17]. Among these, Lactobacillus is the most abundant, comprising 80 species with varying morphologies from short coccobacilli to long slender rods. The most commonly used species for industrial LA production include Lactobacillus acidophilus, Lactobacillus rhamnosus, and Lactobacillus plantarum [3, 18,19,20]. These strains are commercially available and have been extensively studied for their high LA yield. However, high substrate concentrations and the accumulation of LA as an end-product can inhibit the fermentation process, leading to decreased cell growth, prolonged fermentation periods, and reduced LA productivity.
In summary, the successful production of LA through microbial fermentation hinges on the meticulous control of various process parameters. The optimization of factors such as pH, temperature, C/N ratio, and inoculum size, coupled with cost-effective and environmentally friendly approaches like open fermentation, are crucial to enhancing LA yield and productivity. Exploring innovative solutions to increase bacterial fermentation efficiency and using cheaper, bio-sourced raw materials are crucial for advancing the field of LA fermentation.
Lactic Acid Biorefineries Feedstock and Its Generations
Biorefineries are state-of-the-art facilities that convert renewable biomass into various high-value products such as fuels, chemicals, energy, and materials through integrated and sustainable processes. Biorefineries mimic the operations of an oil refinery, using raw materials such as agricultural waste, forest by-products, algae, and specialty energy crops. The main processes include thermochemical transformation processes such as pyrolysis and gasification and biochemical processes like enzymatic hydrolysis and fermentation and mechanical or chemical extraction techniques. These processes maximize the value extracted from biomass, ensuring the efficient utilization of all components. By converting by-products and residues into additional valuable outputs or energy, biorefineries exemplify resource efficiency and contribute to a circular economy. Despite facing challenges, such as high capital investment and feedstock variability, biorefineries hold significant promise for reducing dependency on fossil fuels, lowering greenhouse gas emissions, and stimulating rural economies. Ongoing research and innovation are focused on enhancing process efficiencies and developing novel biocatalysts to realize the full potential of biorefineries in advancing a sustainable bio-based economy.
Biorefineries can be classified as first generation, second generation, or third generation, depending on the sources of the substrate. Biomass is a composite biogenic organic substance that is renewable, non-fossilized, and created through natural or manmade processes. The most critical factor in the fermentation process to produce biofuels and biopolymers is selecting biomass feedstock, which contributes the most significant proportion of the production costs. Figure 2 depicts biofuels and valuable chemicals synthesis routes from different generation feedstocks. The first-generation biomass comprises comestible agricultural crops such as maize, sorghum, sugarcane, and food grains. As shown in Fig. 2, first-generation feedstock as L-LA substrate is the simplest and fastest among all the renewable resources. However, the use of first-generation feedstock as L-LA feedstock suffers from the food versus energy dispute since food prices have risen concurrently with the expansion of the L-LA industry. The drawbacks in first-generation feedstock have subsequently led to the development of second-generation feedstock.
In contrast to readily accessible carbon sources, second-generation feedstocks comprise lignocellulosic biomass such as forest and agricultural waste. Lignocellulose is an abundant carbon–neutral bioenergy source. The common raw material for LA production is lignocellulose biomass, e.g., corncobs, bagasse, and wood-processing waste [21]. Lignocellulose biomass comprises equivalent levels of cellulose (40–50%), hemicellulose (20–35%), and lignin (10–25%) [22]. The chemical constituents of various types of biomasses can differ significantly. L-LA production from lignocellulosic biomass requires additional processing steps such as removing lignin, size reduction, pretreatment, and hydrolysis for monomeric sugar production [23]. Due to its high lignin concentration, lignocellulosic biomass is difficult to hydrolyze into fermentable sugars. Owing to the fact that lignocellulosic biomass is made of cellulose, hemicellulose, and lignin, pretreatment is required to eliminate the crosslinked hemicellulose–lignin barrier surrounding cellulose. Therefore, the production of L-LA from lignocellulosic biomass is currently not cost-viable due to many technological hurdles that must be solved before their full potential can be realized.
Third-generation biomass refers to macroalgae. Macroalgae is a sustainable and renewable resource because of its advantages over conventional biomass sources. Macroalgae cultivation does not require fresh water and arable land and thus does not compete with food crops. Moreover, it does not contain lignin, which is essential for biomass processing. Macroalgae regards third-generation biomass marine as a promising renewable feedstock for biofuel production [24]. This marine biomass consists of protein, lipids, and carbohydrates. Due to their nutritional qualities, macroalgae proteins are a supplementary supply of dietary proteins for humans and animals [25]. Macroalgae lipids mainly comprise triglycerides, phospholipids, free fatty acids, and glycolipids, which can produce high-octane biodiesel and other liquid fuels through extraction and transesterification [26]. These macroalgae carbohydrates, such as furans, ethanol, and acetone, are another potential contributor to value-added biofuels and biomaterials generation [27]. Lignin is a critical constraint in producing terrestrial-based LA from lignocellulosic biomass, resisting degradation. Compared to the complex nature of lignocellulosic biomass, the absence of the lignin fraction in macroalgae makes it an eco-friendlier feedstock for L-LA production, avoiding extra processes such as delignification and detoxification of lignin-originated inhibiting compounds.
Macroalgae-Based Biorefinery Concept
Biorefinery is a term that refers to the process of converting biomass into high-value chemicals and biofuels. Macroalgae-based biorefinery has enormous potential for developing new bio-products and bio-energy generation, owing to macroalgae’s unique chemical structures and composition. Introducing macroalgae biorefinery concepts would minimize reliance on petroleum while having a favorable environmental impact. Macroalgae can be categorized into red, brown, and green based on their color, morphology, and chemical composition. The carbohydrate (25–60%), protein (5–47%), and fat (5%) content varies across macroalgae taxonomically. The biorefinery platform derived from macroalgae feedstock is depicted in Fig. 3.
Macroalgae biorefinery concept to produce high-valued chemicals [28] (Copyright License No: 5841930519225)
Furthermore, macroalgae have a high growth rate and short harvesting time. From Fig. 4, the brown and red macroalgae were cultivated in much larger quantities than the green macroalgae. The trend of cultivated macroalgae has increased in recent decades. The world production of marine macroalgae, or seaweed, has doubled from 18.6 million tons in 2009 to 35.1 million tons in 2020 [29]. The worldwide macroalgae cultivation market is expected to reach USD 16.7 billion in 2020. It has forecasted a USD 30.2 billion market capitalization by 2025 [28]. This substantial growth in macroalgae production is primarily driven by the rising demand for its applications in agriculture and biofuel production. Seaweed farming is practiced in relatively minor numbers of countries, dominated by East and Southeast Asia countries.
World production of farmed macroalgae from 2009 to 2020 [29]
The typical and major green macroalgae (Chlorophyta) genera are Ulva, Codium, and Halimeda species. Carotenoids and chlorophylls a and b are the primary photosynthetic pigments found in green macroalgae. The main constituents of green macroalgae are carbohydrates (53–70% dry weight), which comprise cellulose and hemicellulose but are dominated by the complex sulfated heteropolysaccharide ulvan found in the cell walls of Chlorophyta. Ulvan is a water-soluble sulfated polysaccharide composed of ulvanobiouronic acid 3-sulfate that is made up of repeating units of sulfated rhamnose and glucuronic acid, iduronic acid, or acid xylose [30].
Brown macroalgae (Phaeophyta) are rich in carbohydrates such as mannitol, alginate, cellulose, laminarin, fucoidan, carotenoids, proteins, lipids, and omega-3 fatty acids, as well as secondary metabolites such as polyphenols. Mannitol is an essential component of brown macroalgae, and it is a six-carbon sugar alcohol. Mannitol has a lower calorific value than most other sugars, making it a good sweetener for diabetic diets [31]. Fucoidans are polysaccharides with predominantly fucose and sulfated groups. Numerous pharmaceutical researchers have intensively investigated fucoidans isolated from different brown macroalgae due to their biological properties such as antiviral, antioxidant, anticancer, and anti-inflammatory [32].
Red (Rhodophyta) or red algae may be grown in temperate, subtropical, and tropical climates. Eucheuma denticulatum, Kappaphycus alvarezii, Chondrus crispus, and Sarcothalia crispata are the most industrially significant red algae species due to their main polysaccharides are carrageenan and agar [33]. The red macroalgae Eucheuma cottonii (EC), also known as Kappaphycus alvarezii, can be found in abundance on the inner sides of coral reefs around marine of Southeast Asia like the Philippines, Indonesia, and Malaysia. The main EC cultivators of this species are located on the east coast region of Sabah in the Southeast Asia Region [34]. The extracted EC contains almost pure κ-carrageenan and less than 10% of ι-carrageenan [35]. κ-Carrageenan is used as a gelling, stabilizing, and water-binding agent in the food industry. After extracting κ-carrageenan, the remaining residue is Eucheuma cottonii residues (ECRs). Several kinds of research have used this biomass waste as an alternative feedstock for biofuel and biochemical production [23, 34, 36,37,38]. However, there is still a lack of research on using macroalgae as a feedstock for L-LA production. ECRs can be further saccharified into glucose and fermented to produce L-LA with LAB. Therefore, a suitable pretreatment tailored toward macroalgae biomass is required, especially in biorefinery processes where L-LA generation takes center stage.
Pretreatment and Hydrolysis of Macroalgae Biomass
The biomass pretreatment method must avoid the generation of inhibitory products, reduce the crystallinity of cellulose, and increase the porosity of the cellulosic materials, in which cell wall–bound carbohydrates become accessible for hydrolysis, preventing early degradation or loss of sugars, and be profitable [39]. These pretreatment processes generally influenced the cost of energy conversion. Biomass pretreatment can be categorized into conventional and hybrid pretreatment pathways. Traditional pretreatment methods included physical pretreatment, chemical pretreatment, and biological pretreatment.
Biological Pretreatment
Biological pretreatment is a proponent of ecological sustainability development and maintenance [40]. These techniques use microbes and enzymes or enzymatic systems derived from entire microbes (mainly fungi and bacteria). Biological pretreatment is a more intentional process that requires more time and carefully managed environmental conditions for microbial development. Biological pretreatment of macroalgae isolated from the Mexican Caribbean obtained a higher 20% methane yield using a Bm-2 strain (Trametes hirsuta) [41]. However, most enzymes are relatively unstable, costly, and challenging to recover after the industrial process.
Physical Pretreatment
The fundamental goal of physical pretreatment is to increase the biomass interact surface area and degree of polymerization by decreasing biomass particle size, cellulose crystallinity, and polymerization. Chemical-free physical treatments include various forms such as thermal, freezing and thawing, milling, grinding, chipping, gamma- or microwave irradiation, and ultrasonic therapy. Physical pretreatment reduced algae particle size and then boosted the effectiveness of the subsequent reaction. Physical techniques have a significant, energy-consuming drawback, mainly when applied to scale-up.
Chopping or milling biomass is frequently used to enhance the surface area-to-volume ratio and diminish the particle size and crystallinity of the biomass. Hence, biomass size reduction using milling improves the hydrolysis of complex polysaccharides to sugars for fermentation. The effect of combining two milling modes (vibro-ball and centrifugal milling) toward the enzymatic saccharification of Ulva lactuca (green macroalgae) and Gelidium sesquipedale (red macroalgae) was studied [42]. The results showed that the total sugars released from both species increased by up to 126 and 129% after vibro-ball milling and centrifugal milling pretreatment, respectively.
Microwaves (MW) are low-frequency non-ionizing electromagnetic radiation that generates heat by utilizing the dipole rotation of materials [43]. MW heating can start and stop the process instantly compared to traditional heating. MW is highly energy efficient due to rapid and selective heating, resulting in a fast reaction rate at a low reaction time. Thus, the hydrogen bonds of biomass are interrupted, and dissolved ions are moved to promote their diffusion into the matrix using this technique.
Ultrasonic waves create differential pressure in a solution to boost physical (mechanoacoustic) and chemical (sonochemical) processes [44]. Ultrasonic pretreatment performance is highly dependent on the kind of biomass, the ultrasonic frequency, intensity, and time. Physical factors such as turbulence, micro jets, micro-level mixing, and shock waves are responsible for the effectiveness of ultrasonic pretreatments, which make the biomass more suited for future process stages. Typically, the ultrasonic frequency and time are directly proportional to pretreatment efficiency, where increasing the ultrasonic frequency and time increases the pretreatment efficiency. However, pretreatment efficiency has been observed to plateau at 100 kHz [44]. Ultrasound pretreatment is frequently used in conjunction with chemical treatments.
Chemical Pretreatment
The alkaline pretreatment mechanism is based on dissolution and saponification, which results in the breakdown of the crystallinity of the cell membrane [45]. The common alkali used in alkali pretreatment are Ca(OH)2, NaOH, KOH, CaO, and NH4OH. The three primary parameters affecting alkali pretreatment are alkali concentration, pretreatment temperature, and residence duration.
Ozonolysis employs ozone to predominantly degrade lignin, with minimal impact on hemicellulose and negligible effect on cellulose. A study showed that applying ozone for 90 min at pH 3.0 led to significant delignification of sugarcane bagasse, resulting in production rates of 59% cellulose, 22% hemicellulose, and 6% lignin, with lignin being reduced by up to 217% [46]. Ozonolysis has multiple benefits, including efficient lignin removal, the absence of harmful byproducts, and the capability to implement the reaction under ambient pressure and temperature [7]. Comparisons between ozonolysis and acid pretreatment reveal that while acid pretreatment initially yields more sugar, it also produces inhibitory compounds such as 5-hydroxymethyl furfural (5-HMF) and levulinic acid, which hinder fermentation efficiency [47]. In contrast, ozonolysis avoids generating these inhibitors, leading to higher ethanol production efficiency despite lower sugar yields. The absence of inhibitors and operation at ambient temperatures make ozonolysis a potentially more viable and cost-effective alternative for bioethanol production from marine algae [48]. Nonetheless, the process incurs high costs due to the considerable volume of ozone required.
Acid pretreatment (sulfuric acid) has been successfully developed for the pretreatment of macroalgae biomass. Although sulfuric acid is a powerful agent for cellulose pretreatment, it is toxic, corrosive, and hazardous. Concentrated acid pretreatment with high temperature will also lead to the formation of inhibiting by-productions such as furfural, 5-hydroxymethylfurfural (5-HMF), levulinic acid, and caffeic acid, which can affect the growth of the microorganisms during fermentation [49]. This emphasizes the significance of decreasing the temperature during dilute sulfuric acid pretreatment. For instance, a study achieved a 55.2% glucose yield in Enteromorpha intestinalis by lowering the acid concentration to 0.27MH2SO4 [50]. Similarly, Kappaphycus alvarezii was pretreated with dilute acid followed by enzymatic hydrolysis, resulting in a glucose yield of nearly 66.7% using 0.18 M H2SO4 [51].
In addition, combining mild dilute acid treatment with other treatments may be a beneficial strategy for increasing cellulose degradability and enzymolysis efficiency. The microwave-chemical pretreatment is a hybrid pretreatment in which the treatments are delivered in a particular order to reduce the external cost. Reducing sugar production efficiency from macroalgae with different pretreatment and hydrolysis methods is described in Table 1. A study reported that the maximum total reducing sugar was 74.84% from Eucheuma denticulatum (ED) using dilute acid hydrolysis with microwave-assisted heating [52]. The optimum galactose yield of 50.7% was achieved under microwave-assisted dilute acid hydrolysis with 0.1 M H2SO4 at 120 °C for 25 min [53].
Catalytic Conversion of Pretreated Cellulosic Biomass
Hydrolysis of processed cellulosic biomass is a critical step in the valorization of renewable resources for the sustainable production of biofuels and biochemicals. This process is fundamental to the biorefinery concept, entailing the degradation of the intricate cellulosic structures into fermentable sugars, unlocking the potential for a wide range of value-added applications. The intrinsic recalcitrance of cellulose, characterized by its robust and intricate cellulose matrix, poses a significant challenge to effective hydrolysis. However, recent advancements in pretreatment technologies have substantially enhanced the accessibility of these polysaccharides, thereby improving hydrolysis efficiency.
Innovative techniques incorporating chemical and enzymatic methods have been developed, with each approach contributing distinctively to the breakdown of cellulosic fibers. These strategies effectively raise the quantity of fermentable sugars and reduce the production of inhibitory by-products, thereby advancing the efficacy of downstream processes. Moreover, exploring novel catalysts and engineered enzymes offers an auspicious avenue for optimizing hydrolysis conditions, further augmenting the conversion rates. As the quest for sustainable alternatives to fossil fuels intensifies, the hydrolysis of pretreated cellulosic biomass stands at the forefront of research, promising a future where renewable biomass can effectively supplant non-renewable resources in energy and material production.
Enzymatic Hydrolysis
The enzymatic hydrolysis of cellulose and hemicellulose into sugars is a promising and efficient process catalyzed by cellulase enzymes. This approach is preferred owing to its mild reaction conditions and minimal processing equipment demands. Cellulase typically comprises three enzyme types that work together to transform cellulose into glucose methodically. Initially, the β-1,4-glycosidic bonds found in glucan chains are cleaved by endoglucanase [58]. This results in the production of shorter chains that have reducing and non-reducing ends. Subsequently, exoglucanase acts on these chains, releasing cellobiose from both chain ends. Finally, β-glucosidase splits the cellobiose into individual glucose molecules. Although enzymatic hydrolysis of cellulose can yield between 75 and 95% monosaccharides at 50°C over several days, the costs and challenges of recycling these enzymes pose significant obstacles to their commercial-scale application [59].
Dilute Acid Hydrolysis
The incorporation of dilute acid catalysts markedly enhances the hydrolysis rate of polysaccharides. The complex and macromolecular crystalline structure of cellulose is a pivotal contributor to the chemical resilience of biomass. Consequently, selecting appropriate cellulose types as raw materials and the judicious use of diluted acid plays a vital role in the degree of cellulose breakdown. The method of dilute acid hydrolysis is frequently employed to extract fermentable sugars from carrageenan-rich biomass owing to its straightforward approach, cost-efficiency, and scalability [52, 57]. Nonetheless, applying acid and heat during algae biomass hydrolysis can lead to monosaccharides and sugar degradation by-products. Notably, dehydrated forms of glucose and galactose derived from the cellulose and carrageenan of macroalgae are more susceptible to degradation than the hydrated D-glucose and D-galactose. This leads to the formation of a fermentation inhibitor named 5-hydroxymethylfurfural (5-HMF) [60]. It is essential to recognize that using diluted acid for the random cleavage of polysaccharides often leads to suboptimal fermentation processes.
Consequently, integrating acidolysis with complementary physical or biological methods has been employed to release a tremendous amount of fermentable sugars, thereby enhancing microbial fermentation conversion. Adjusting the sulfuric acid concentration for optimal hydrolysis is critical in this context. Previous research studied on the hydrolysis of Spirogyra peipingensis (a type of green algae) using boiling water (100°C) showed that increasing the acid concentration from 4 to 10% substantially boosted the yield of reducing sugar from 0.4 to 0.55 g/g [61]. Hence, opting for milder acid hydrolysis conditions is recommended for the saccharification of algae biomass, setting the stage for efficient fermentation [53].
Microorganisms and Fermentation Strategies for Lactic Acid Production
LA fermentation involves various microbes such as bacilli, fungi, LAB, and metabolically engineered strains. LAB are micro-aerophilic or anaerobic, thriving in temperatures between 5 and 45°C with an optimal pH range of 5.5–6.5. They require nutrients like vitamins, minerals, fatty acids, carbohydrates, amino acids, peptides, and nucleotide bases. The presence of the L-lactate dehydrogenase (ldhL) gene in specific microbes encodes the L-LDHL enzyme, which is responsible for the exclusive conversion of pyruvate to L-LA. LAB, a group of low guanine-cytosine content gram-positive bacteria within the Lactobacillaceae family, are commonly employed for L-LA production [62]. Renowned for their ability to generate L-LA through carbohydrate metabolism, LAB is particularly efficient when processing macroalgal hydrolysate, which contains mixed rare sugars requiring complete metabolization for optimal L-LA yield.
A critical factor in LAB’s metabolic proficiency is the carbon catabolite repression (CCR) regulatory mechanism [63]. CCR adeptly manages the uptake and metabolism of carbohydrates, ensuring the efficient use of cellular resources. It prioritizes the assimilation and metabolic pathways of preferred carbon sources over less favorable ones, thus facilitating the metabolism of multiple carbohydrates at varying rates [64]. CCR in LAB involves several critical components for transporting and metabolizing rare sugars. These include the sugar phosphotransferase system (PTS), enzyme I (EI), phosphocarrier histidine protein (HPr), and the catabolite control protein A (CcpA) [65]. The PTS is integral to sugar uptake and phosphorylation, utilizing energy from phosphoenolpyruvate (PEP) to phosphorylate sugars and aid in their transport and metabolism. This process involves a phosphoryl transfer from PEP to various proteins, ultimately facilitating the absorption and metabolism of sugars by LAB cells. CcpA, a critical transcriptional regulator in CCR, influences gene expression related to non-preferred carbon sources when preferred sources are available. By binding to catabolite-responsive elements in gene promoter regions, CcpA modulates gene expression, thereby optimizing carbohydrate metabolism [65]. Interestingly, certain strains, such as Bacillus coagulans, can metabolize sugars like xylose alongside glucose for L-LA production [66].
Operational Modes and Fermentation Approaches
LA fermentation can be conducted using various operational modes, including batch, fed-batch, semi-continuous (repeated batch), and continuous modes. Batch fermentation typically results in higher LA concentration, whereas continuous fermentation achieves greater productivity [67]. During batch fermentation, a substrate, inoculum, and neutralizing agent are used to control pH levels [68]. This method is advantageous for producing a concentrated product with minimal contamination risk [69]. However, it faces challenges such as reduced productivity due to end-product inhibition and limited cell concentration from nutrient availability. Excess sugar concentration can cause substrate inhibition, leading to prolonged lag phases and cell lysis, thereby decreasing sugar utilization and fermentation rates [70].
In contrast, fed-batch fermentation is preferred for mitigating substrate inhibition by maintaining low substrate concentrations throughout the process [71]. High substrate levels can inhibit cell growth and LA productivity, causing product inhibition [72]. Optimizing substrate feeding, controlling substrate levels, and selecting appropriate feeding strategies such as constant, exponential, or intermittent feeding techniques are essential to maximize product concentration.
Separate hydrolysis and fermentation (SHF) is a process that carries out biomass hydrolysis and microbial fermentation sequentially. The hydrolysis of polysaccharides from pretreated biomass was first investigated for optimal conditions and followed by microbial fermentation. The first stage of SHF involves the hydrolysis of the substrate. This can be achieved through various pretreatment methods, which aim to break down the rigid and crystalline structures of lignocellulosic waste, such as wheat straw. A combination of physical methods (like milling and steam treatment) and chemical methods (such as NaOH treatment) can be employed to enhance the efficiency of this process [73]. The hydrolysis stage releases fermentable sugars, which are then utilized in the subsequent fermentation process. The second stage, fermentation, typically involves the use of microorganisms like Saccharomyces cerevisiae to convert the hydrolyzed sugars into ethanol. The separation of hydrolysis and fermentation stages in SHF allows for the use of specialized organisms and conditions tailored for each step. This can lead to higher ethanol yields and more efficient substrate processing. High LA productivity (0.85 g/g) was achieved through SHF using enzymatic hydrolysis at 100 rpm, 52°C for 14 h, followed by fermentation using Bacillus coagulans A534 [67]. However, the disadvantages of SHF are that it is time consuming and requires higher enzyme loads [74].
Simultaneous saccharification and fermentation (SSF) is a one-stage process that simultaneously undergoes enzymatic hydrolysis with fermentation to obtain value-added products from biomass. SSF has several advantages over other fermentative methods, including shortening production time and reducing inhibitory compounds. The challenge of SSF is finding equilibrium conditions between enzymatic hydrolysis and microbial fermentation. The optimum pH (pH 4.5–5.5) and temperature (50–55°C) for enzymatic hydrolysis are usually incompatible with fermentation [75].
Bacteria selection for LA microbial fermentation is vital to produce the desired product L-LA. There are four primary producers of lactic acid type–producing bacteria: LAB, Bacillus strains, Escherichia coli, and Corynebacterium glutamicum. LAB is a gram-positive, acid-tolerant group, either rod-shaped (bacilli) or spherical (cocco), which is generally found in plants, meats, and dairy products. There are two types of fermentation for LA production using LAB: homofermentative (only produced LA) and heterofermentative (produced LA and other by-products). Lactobacilli strains are commonly used in industrial LA production because of their long history of industrial-scale production without adverse health effects on consumers or production workers. Bacillus spp., also known as the hay bacillus or grass bacillus, is a gram-positive, catalase-positive bacterium found in soil and the gastrointestinal tract of ruminants and humans.
The selection of appropriate bacteria for LA production is crucial for optimizing yield and efficiency. Lactobacillus and Bacillus sp. are commonly utilized due to their robust fermentation capabilities and high LA yields under various conditions. Lactobacillus rhamnosus has demonstrated significant potential for LA production. For instance, utilizing cassava bagasse as a substrate, this strain yielded 0.88 g/g under SSF at 50°C and a pH range of 5.0 to 6.4 [12]. Similarly, utilizing paddy rice hydrolysate yielded 0.89 g/g under batch SSF conditions at 50°C and pH 6.0, demonstrating its adaptability to various substrates and fermentation conditions [76]. Lactobacillus paracasei, though less commonly highlighted, also shows promise, achieving a yield of 0.96 g/g from an unspecified substrate under batch fermentation at 37°C and pH 6.0, indicating its efficiency in LA production [77]. Bacillus coagulans, renowned for their thermotolerant properties, stand out in high-temperature fermentations. This strain produced a yield of 0.87 g/g from sugarcane bagasse hemicellulosic hydrolysate under batch SHF at 52°C and pH 6.0 [78]. It also demonstrated high efficiency with Eucheuma denticulatum extract and residues, achieving yields of 0.87 and 0.97 g/g, respectively, under various fermentation conditions [1, 53]. Macroalgae, particularly Eucheuma sp., are rich in polysaccharides and have fast growth rates, making them ideal for sustainable biorefinery processes. The use of macroalgae not only supports high LA yields but also contributes to environmental sustainability by utilizing marine resources [1, 18, 53]. Selecting specific bacterial strains and optimizing fermentation conditions, including temperature, pH, and substrate type, are critical for maximizing LA yields and enhancing production efficiency. Table 2 presents a detailed comparison of LA production using different substrates and bacteria, highlighting the superior performance of Bacillus coagulans and Lactobacillus sp. under tailored fermentation processes.
Recovery and Purification of Lactic Acid
The extraction of LA, an essential organic compound in various industrial sectors, has become increasingly important. Its applications range from food preservation and pharmaceutical formulations to biodegradable polymer precursors. The quality and utility of LA are significantly influenced by impurities in the fermentation broth, such as proteins, salts, and other organic acids. Consequently, understanding the impact of these impurities is essential for maintaining the optimal quality and effectiveness of LA. This necessity has spurred demand for more efficient extraction methods. Many techniques for LA recovery from the fermentation broth, including distillation, precipitation, ion exchange, membrane technology, and reactive solvent extraction, have been explored [87]. The current state of LA production and purification combines traditional and innovative approaches, emphasizing the integration of sustainable raw materials, advanced microbial production methods, and enhanced purification techniques. This integration is pivotal for industry growth while adhering to environmental standards. Table 3 presents the advantages and disadvantages of various recent LA recovery techniques.
The separation and purification of LA from fermentation broths have traditionally relied on methods like distillation and precipitation. These techniques have been established over decades and form the backbone of industrial LA production. However, they have their challenges and limitations.
Distillation is a widely used technique for separating components based on differences in their volatilities. In the context of LA separation, distillation is often employed to separate it from aqueous solutions. Reactive distillation, a process intensification technique for reversible liquid-phase chemical reactions, is pivotal in the recovery of LA. It overcomes the limitations of reaction equilibrium, enhancing the conversion of reactants. This process is particularly effective for the esterification of LA, represented by the reversible reaction of LA with ethanol to form ethyl lactate and water. Homogeneous catalysts like sulfuric acid, anhydrous hydrogen chloride, or ion-exchange resins are commonly used. The latter offers advantages like low corrosion, ease of separation, and reusability. Alcohols such as methanol, ethanol, 2-propanol, and butanol can be employed in esterification, with ethanol being a renewable option, though butanol and methanol are economically attractive.
The study concentrates on managing highly interconnected reactive distillation processes for purifying raw lactic acid through esterification followed by hydrolysis [88]. It compares methanol and butanol methods with different recycling stream configurations and control structures. The study reveals that processes with a single recycling stream are easier to control. A temperature control structure with a single composition controller proves adequate for the butanol process, while the methanol process requires additional composition measurements. The research underscores the significance of choosing appropriate control structures to optimize the efficiency and effectiveness of the reactive distillation process in LA purification.
Molecular distillation, or short-path distillation, separates homogeneous liquid mixtures with low volatility, high molecular mass, and high thermosensitivity. It differs from conventional evaporation as it eliminates vapor–liquid equilibrium by placing the hot evaporation surface and the cold condensation surface within the mean free path of evaporated molecules. This setup ensures unobstructed travel of molecules to the condenser. Without a solvent, molecular distillation prevents product contamination, obviating the need for further purification.
In summary, reactive and molecular distillation are critical in the recovery and purification of LA, each offering specific advantages. Reactive distillation facilitates efficient esterification and hydrolysis of LA, while molecular distillation provides a solvent-free, thermally gentle method suitable for high molecular mass and thermosensitive substances. Through their distinct mechanisms, both processes contribute significantly to the sustainable and efficient production of high-purity LA. While distillation is advantageous due to its simplicity and effectiveness for certain mixtures, it can be energy intensive and less effective for azeotropic or close-boiling mixtures. The high energy requirement for vaporization, especially when dealing with large volumes, can be a significant cost and environmental impact drawback.
The calcium precipitation process, involving calcium lactate production and subsequent LA recovery, is known for its cost-effectiveness and simplicity, which is especially beneficial for processing impure or low-concentration LA solutions. However, significant downsides include the generation of substantial gypsum amounts, posing disposal and environmental challenges. The classical calcium lactate process, typically carried out in non-corrosive reactors to prevent fermentation fluid contamination, involves batch fermentation with a mixture of fermentable sugars and complex nitrogen sources, maintaining a pH between 5.5 and 6.0. Active fermentation completes in 2–6 days, after which calcium lactate is filtered, decomposed with sulfuric acid, and further processed through steps like reactive distillation and esterification to produce high-purity LA.
Despite its maturity and widespread use, the calcium lactate crystallization–acidolysis process has limitations, including long filtration steps, labor intensity, and the production of environmentally harmful by-products like gypsum, with roughly 1 ton of gypsum generated per ton of LA produced. This has led to the exploration of alternative methods, such as the magnesium lactate production process [89]. This process uses MgO as a neutralizer to react with LA, forming easily crystallizable magnesium lactate. The subsequent steps involve acidification, extraction, and re-extraction, leading to a more environmentally friendly process with no solid or liquid waste discharge and lower energy consumption.
In industrial settings, the purification of LA mainly relies on precipitation methods. Excess base, like calcium carbonate or hydroxide, is added to the fermentation broth to neutralize LA and maintain a pH conducive to LA producers. Calcium lactates are then recovered by distillation, re-acidification using sulfuric acid, LA release, and gypsum generation. Technical-grade LA undergoes esterification for high-purity products, followed by sequential recovery processes. Although this method is established, it requires large amounts of sulfuric acid and generates gypsum waste, posing environmental issues.
Recent research has focused on alternative recovery methods, such as solvent extraction, membrane separation, and emulsion liquid membrane, to address the environmental and cost concerns associated with traditional calcium precipitation methods. These emerging technologies offer the potential for more sustainable and efficient LA recovery, aligning with the increasing focus on green and cost-effective processes in the industry. Overall, despite the significant role of traditional methods in advancing the LA industry, their limitations in energy efficiency, environmental impact, and scalability have spurred interest in more sustainable and efficient separation techniques.
Ion Exchange
Ion exchange and affinity chromatography have become increasingly prevalent in LA purification. Ion exchange chromatography, leveraging resins that selectively bind LA, is highly effective in achieving purity and specificity. It extracts LA from fermentation broths by selectively capturing and releasing it in a more concentrated form. Affinity chromatography, though less widely used, offers exceptional selectivity, particularly with specific ligands designed to bind LA under designated conditions. These methods excel in precision and handling complex mixtures yet are constrained by resin capacity and the necessity for meticulous control of operating conditions. A significant advancement in this field is demonstrated by using Amberlite IR120 and IRA-67 resins in a stirred tank, effectively purifying LA from sugarcane juice, resulting in high concentration and mass recovery [84]. This method simplifies the purification process and exhibits potential for industrial scalability, underscoring its viability for large-scale applications.
Membrane Filtration
Membrane-based separation technologies, including electrodialysis, ultrafiltration, and nanofiltration, have become crucial techniques in the separation and recovery of LA. Membrane separation has also gained prominence, valued for its low energy requirements and suitability for large-scale operations. Significant advancements have been made in the recovery of L-LA from the organic fraction of municipal solid waste, where a series of filtration processes followed by electrodialysis achieved a recovery rate of 51.5% with 98.7% purity [90]. This method surpassed the efficiency reported in a previous study, significantly reducing impurity content to about 0.3 g/L [91]. In addition, integrating ultrafiltration and nanofiltration for component separation in fermentation broths, combined with ion exchange and vacuum-assisted evaporation, has resulted in LA with a purity exceeding 99.5% [83].
Despite the high purity achieved through membrane technologies, L-LA extraction faces challenges such as high costs, membrane fouling, and polarization issues. Emulsion liquid membrane (ELM) technology has emerged as a promising breakthrough, driven by kinetics rather than solvent affinity, and has achieved an impressive extraction efficiency of 96.59% for L-LA [92]. However, using non-renewable resources as organic solvents in ELM raises sustainability concerns, prompting a shift toward vegetable oils as greener alternatives [92,93,94].
Membrane fouling, caused by particle adsorption or deposition, is a fundamental issue in membrane technology, leading to concentration polarization, reduced membrane flux, and decreased separation efficiency [95]. Addressing this challenge requires strategies like pretreatment and physical or chemical regeneration of membranes. With ongoing development, integrating LA production and purification processes offers a promising route to enhance productivity and quality under more sustainable and practical conditions. Central to recent advancements in LA purification is the selective permeability of novel membrane materials, including composite and mixed-matrix membranes.
Reactive Solvent Extraction
There has been a paradigm shift from conventional partitioning to reactive solvent extraction. This process goes beyond differential solubility and embraces a chemical reaction as its cornerstone. The central aspect of this innovative approach is the formation of a transient complex between the target solute and a reactive agent within the solvent phase. This leads to a notable enhancement in the extractability of solute. This interaction is typically a reversible reaction, which significantly improves both the selectivity and efficiency of the extraction process. The complex decomposes after successfully transferring to the organic phase, releasing the purified solute. The ingenuity of this method lies in its ability to finesse the extraction of solutes that are otherwise resistant to traditional liquid–liquid separation techniques.
The separation of LA through reactive solvent extraction has seen significant advancements, particularly in developing hybrid processes incorporating reactive extraction techniques. A notable example is a two-stage extraction process that combines salting-out and reactive extraction, effectively recovering LA from corn stover hemicellulose-derived liquor [96]. This method achieved an extraction efficiency of 89.4% after five successive cycles with back-extraction, demonstrating an improvement over the 83% efficiency obtained with a one-step extraction under optimized conditions [96]. Notably, this method is energy-efficient and avoids gypsum waste production, as it does not involve calcium salts. However, the requirement for expensive equipment for efficient high mass transfer rate separation and large quantities of toxic extractants add to the operational costs and may limit the scalability of LA production.
Critical factors in extractant selection for LA separation include selectivity, chemical stability, corrosivity, toxicity, and viscosity. High-viscosity extractants often require diluents to enhance extraction capability [97]. Reactive solvent extraction has been effective in purifying LA, which is characterized by low distribution coefficients of impurities and high coefficients for LA. Previous research investigated the extraction efficiency of rice bran oil for LA extraction, using ionic liquid (Aliquat 336) as a stabilizer [92]. They achieved an LA extraction efficiency of about 90% through process parameter adjustment and optimization, indicating strong application potential [92, 98, 99]. Further statistical optimization using response surface methodology (RSM) led to an additional 3% increase in extraction efficiency.
Another study employed RSM, utilizing a mixture of tertiary amines like trioctylamine (TOA) and trindecylamine (TDA) for their high basicity and superior extraction power [100]. After optimization, they achieved an LA extraction efficiency of up to 98.5% with a high rate (9.36 × 10−9 mol/(cm2.s)). These results demonstrated the effectiveness of tertiary amine mixtures as extractants and the exceptional performance of emulsion liquid membrane technology in LA extraction. TOA can form stable complexes with LA, enhancing its efficiency in the separation process. This chelating effect facilitates the selective extraction of LA, reducing interference from other components in the mixture.
Furthermore, the compatibility of TOA with various organic solvents makes it adaptable to different extraction conditions, contributing to its versatility and applicability. This chelating effect allows selective LA extraction with minimal interference from other mixture components. The adaptability of TOA in various organic solvents increases the versatility of the extraction process. Studies have shown that TOA-based extraction has achieved high yields and purity levels in LA recovery with minimal environmental impact, highlighting its sustainable potential [101].
Cell Immobilization
Biomass materials, including microorganisms and enzymes, are increasingly utilized in environmental protection due to their ease of production, eco-friendliness, and high economic efficiency in environmental applications. However, challenges arise with certain biomass materials, such as saccharomycetes, which can be difficult to recycle and lack practicality. Furthermore, many biomass materials demand specific external environmental conditions, limiting their widespread use. Cell immobilization offers a solution for the problems mentioned because it improves cell density and maintains catalytic activity in repeated use by physical or chemical means to position or limit free cells to a specific region of space [102]. The most common immobilization methods are cell adsorption (attachment) on a solid carrier and entrapment in a membrane. Immobilized cell technology has significantly impacted LA production, providing better yields, improved process control, and the capability to use waste substrates effectively. The combination of this technology with advanced bioprocessing tactics offers excellent potential for sustainable and efficient LA production in the future.
Entrapment
Entrapment as a technique for immobilizing LAB involves encasing cells within a gel matrix within a polymeric network. This network, composed of natural or synthetic polymers, is constructed to be both porous and robust, ensuring efficient diffusion of substrates and products while protecting the microorganisms from adverse reaction conditions. Polymers such as alginate, agarose, carrageenan, and polyacrylamide are commonly employed in this process, especially in the biosynthesis of high-value products like pharmaceuticals.
Entrapment techniques use spherical particles containing cells obtained by dripping a polymer-cell suspension into a solution containing precipitate-forming counter ions or thermal polymerization. The common materials used in cell entrapment or encapsulation are calcium alginate, sodium alginate, polyvinyl alcohol, κ-carrageenan, and chitosan [103,104,105]. κ-Carrageenan has been widely used to immobilize cells [106]. Research has shown that the addition of κ-carrageenan to enzyme encapsulation can enhance enzymatic activity by utilizing low molecular weight substrates [107]. To overcome the disadvantages of the common immobilized carriers, immobilized Bacillus coagulans in κ-carrageenan as support, forming a microcapsule with the assistance of glutaraldehyde will provide cell-free products and minimize the mass transfer limitation of the substrate to Bacillus coagulans for LA production. Glutaraldehyde acts as a crosslinking with bi-functional reagents in the entrapment in κ-carrageenan microcapsule and was found to be effective in reducing leakage [108].
Another notable benefit of immobilization cell technology is its application in valorizing waste substrates. A recent study produced LA from saline cheese whey using isolated lactobacilli, which can endure high salt concentrations, employed immobilization techniques in repeated batch fermentation [109]. This method confirmed the possibility of utilizing waste substrates to produce LA and emphasized immobilized cells’ durability under strenuous circumstances. This approach demonstrated the feasibility of using waste substrates for LA production and highlighted the robustness of immobilized cells in challenging conditions. This method allows for high cell density immobilization and protects the cells against harsh environmental factors. However, it presents specific challenges, including limited diffusion of substrates and products and potential reduction in cell activity due to the constrained mobility of the entrapped cells. The use of these materials, particularly in the encapsulation of LAB, represents a balance between providing a conducive environment for cellular processes and mitigating the limitations inherent in the entrapment technique.
Encapsulation
Encapsulation involves enclosing LAB cells within protective coatings, preventing cell leakage and contamination. Alginate and chitosan-based matrices are frequently used for encapsulation, providing a controlled microenvironment for fermentation. Encapsulation is a sophisticated technique employed in microbial fermentation, allowing for the confinement and protection of LAB within a matrix. This process enhances fermentation efficiency, stability, and overall bioprocess performance. Encapsulation methods involve the creation of protective coatings around individual bacterial cells or cell aggregates, creating a controlled microenvironment that supports optimal growth and LA production. The use of immobilized cell technology in LA production has been shown to improve product yield and productivity. Previous studies demonstrated that optimizing the conditions for immobilized Lactobacillus pentosus cell fermentation resulted in a maximum LA yield of 0.938 g/g glucose with productivity of 2.213 g/(L × h) [110]. This optimization was achieved by applying response surface methodology, highlighting the potential of immobilized cell technology in enhancing the efficiency of LA production processes. Another research recently observed a 97% L-LA yield when encapsulating Lactobacillus rhamnosus in polyvinyl alcohol [111]. However, this technology faces challenges, including higher costs, potential cell damage during immobilization, carrier softening, and gradual cell leakage during prolonged continuous operation.
Alginate and chitosan, naturally derived, are commonly used matrices for encapsulating LAB cells. The encapsulation process entails the suspension of LAB cells in either alginate or chitosan solution, followed by droplet formation utilizing techniques such as extrusion or emulsification. These droplets subsequently solidify into beads or particles via ionic crosslinking or gelation. The encapsulating matrix acts as a barrier, shielding LAB cells from harsh environmental factors, mechanical stresses, and potential contaminants in the fermentation broth. This protection enhances cell viability and overall fermentation efficiency. Besides that, encapsulation provides a controlled microenvironment where nutrient availability, pH, and oxygen levels can be tailored to support optimal microbial growth and LA production. This climate-controlled setting reduces discrepancies within fermentation conditions, resulting in dependable and duplicable outcomes.
Alginate, a polysaccharide derived from brown seaweed, forms a hydrogel matrix by reacting with divalent cations such as calcium ions [112]. The resulting gel structure offers mechanical stability while allowing for nutrient exchange and metabolic activity. Alginate encapsulation protects LAB cells from external stressors, such as shear forces during agitation, pH variations, and the presence of inhibitory compounds [110]. The encapsulated LAB cells remain viable within the gel matrix, maintaining their functionality over extended fermentation periods.
Chitosan, derived from chitin, is another popular matrix for encapsulation due to its biocompatibility and antimicrobial properties [113]. Similar to alginate, chitosan forms gel-like structures in the presence of polycations, providing a protective shield around LAB cells. Chitosan encapsulation safeguards the cells and enhances their survival under adverse conditions. The electrostatic interactions between chitosan and bacterial surfaces promote cell adhesion within the matrix, enhancing cell retention and stability.
Physiochemical Adsorption
Physiochemical adsorption is an immobilization technique that involves the attachment of LAB cells onto solid surfaces through non-covalent interactions, including electrostatic forces and hydrophobic interactions [114]. It involves the physical adhesion of bacterial cells onto a carrier surface. This method is favored for its simplicity and cost-effectiveness. Materials commonly used for adsorption include activated carbon, ceramics, and various polysaccharides like chitosan. The primary advantage of adsorption is its minimal impact on cell viability, allowing the bacteria to retain their metabolic activity. The process of physiochemical adsorption commences with the selection of a suitable matrix, typically composed of materials like alginate, chitosan, or other biopolymers. These matrices possess surface properties that facilitate the adhesion of LAB cells. The non-covalent nature of the adsorption process ensures minimal disturbance to LAB cells, preserving their viability and metabolic activity. The immobilization matrices can be adapted to different scales, allowing for a seamless transition from laboratory-scale experiments to industrial applications. Continuous fermentation setups benefit from the stability of immobilized cells, reducing the need for frequent inoculations. However, one of the limitations of this method is the potential for desorption, where cells may detach from the carrier under certain conditions, such as changes in pH or ionic strength.
Crosslinking
Crosslinking immobilization is a pivotal technique in biotechnology, particularly in enhancing the stability and functionality of probiotic microorganisms. This method involves creating networks of covalent or ionic bonds to stabilize the structure of biomolecules or cells within a matrix. The crosslinked structure protects the encapsulated entities and allows controlled release and targeted delivery, which is crucial in the pharmaceutical and food industries.
The crosslinked cryostructured monoliths were prepared using Lactobacillus reuteri for biocatalysis in the biobased industry, specifically for converting glycerol to valuable chemicals [115]. Polyethyleneimine/modified polyvinyl alcohol (PEI/PVA) was used as a crosslinker, yielding highly stable monoliths with maintained viability and biocatalytic activity. The optimal conditions in a continuous plug flow reactor produced 19.7 g/L of 3-hydroxypropionaldehyde (3HPA), highlighting the efficiency of the immobilization technique.
Another study explored the microencapsulation of Lactobacillus plantarum in alginate-chitosan beads using dual crosslinking by Na-tripolyphosphate (Na-TPP) [116]. The study demonstrated that dual crosslinked beads exhibited higher viability under simulated gastrointestinal conditions than single crosslinked beads. The enhanced stability of these microcapsules indicates their potential in site-specific delivery applications, showcasing the efficacy of the crosslinking immobilization technique in preserving probiotic survivability and facilitating controlled release.
Both studies emphasize the significance of crosslinking immobilization in enhancing the stability and functional efficiency of biocatalysts and probiotics. The choice of crosslinkers and the crosslinking method are critical in determining the final properties of the immobilized cells, such as stability, activity, and release behavior. These advancements in immobilization technology hold promise for diverse applications in biotechnology and healthcare.
Exergy and Techno-Economic Analyses of Third-Generation L-Lactic Acid Biorefinery Systems
Deciphering the Role of Exergy Analysis in Process Refinement
Exergy analysis, deeply rooted in the second law of thermodynamics, emphasizes that energy quality inevitably degrades through each transformation. This analytical method transcends mere energy content evaluation, extending to the potential of energy to perform practical work. It offers a nuanced perspective on the quality and degradation of energy during various transformation processes. By quantifying the maximum useful work possible when an energy or material flow equilibrates with a reference state through reversible processes, exergy analysis emerges as a critical tool for assessing the feasibility and sustainability of bioenergy systems [117].
This approach evaluates energy quality by examining factors like temperature, pressure, and composition. It measures the effectiveness of energy conversion by contrasting actual work output against the maximal work potential of the energy input. A pivotal aspect of exergy analysis is its ability to identify areas where energy is dissipated or wasted within a system, thereby offering pathways to enhance energy efficiency [118]. Exergy analysis is a valuable tool for improving the thermodynamic efficiency of individual components and entire systems. It optimizes processes by reducing energy losses, thereby increasing operational efficiency. In addition, it facilitates the comparison of various energy sources and technologies, aiding in selecting efficient and environmentally sustainable ones.
By integrating energy quantity and quality considerations, exergy analysis provides a comprehensive energy efficiency assessment, contributing to the overall sustainability of processes and systems. This approach is beneficial in pinpointing inefficiencies and areas for improvement, promoting enhanced energy efficiency, and supporting sustainable, eco-friendly industrial practices. Such comprehensive energy analysis is crucial in achieving higher efficiency and sustainability in industrial systems, as highlighted in various studies on biorefineries. These studies demonstrate significant exergy losses in processes, underscoring the need for optimization to improve sustainability and reduce environmental impacts.
Building on the principles of exergy analysis, a study has been conducted on an exergy analysis of a biorefinery process aimed at co-producing third-generation L-LA and electricity from Eucheuma denticulatum residues (EDRs) [119]. The study evaluates the thermodynamic performance of three distinct biorefinery scenarios using process simulation in Aspen Plus V10. The scenarios include (I) L-LA production without a pretreatment unit, (II) L-LA production with microwave-assisted autohydrolysis (MAA) pretreatment, and (III) L-LA production integrated with electricity generation and waste management systems. The exergy analysis revealed that the MAA pretreatment was highly effective, reducing the biomass requirement by 70.2% to produce 1000 kg/h of L-LA. The fermentation unit, wastewater treatment (WWT) unit, and combined heat and power (CHP) unit were identified as the primary contributors to exergy destruction across all scenarios. The L-LA recovery and MAA pretreatment units demonstrated high exergy efficiency, with 99.37 and 99.68%, respectively. Scenario III, which incorporated power generation and waste management, achieved the highest functional exergy efficiency at 22.12% and a normalized exergy destruction of 0.73, highlighting its superior performance in converting waste streams into value-added products and reducing reliance on fossil fuel–derived energy.
Another study expanded upon this analysis by conducting a comprehensive sustainability assessment of converting red macroalgae waste, specifically Eucheuma cottonii (ECR) residue, into PLA through a biorefinery approach [120]. The study examines three distinct scenarios: (1) a biorefinery process for the production of L-LA, (2) L-LA production integrated with fertilizer production and wastewater treatment, and (3) PLA production combined with fertilizer production and wastewater treatment. Utilizing Aspen Plus V12.1 for simulation, the study offers a detailed exergetic and exergoeconomic analysis to evaluate and compare the performance of each scenario. In scenario 1, the biorefinery process solely produces L-LA at an output rate of 5194 kg/h, consuming 12,079 kg/h of ECR. Scenario 2 incorporates additional fertilizer production and wastewater treatment units, yielding 1609 kg/h of organic fertilizer and 1233 kg/h of reusable water alongside L-LA. Scenario 3 extends the process to produce PLA, resulting in an output of 4142 kg/h of PLA and an increased production of reusable water at 1443 kg/h, accompanied by a rise in energy consumption from 28,272.53 kW in scenario 1 to 30,296.15 kW in scenario 3. The exergy analysis indicates that the simultaneous saccharification and fermentation (SSF) unit is the largest contributor to exergy loss across all scenarios, primarily due to heat lost to the surroundings and the irreversible biochemical reactions involved. Scenario 3 demonstrates the highest functional exergy efficiency at 19.54%, reflecting a more effective utilization of input exergy for valuable product formation, in contrast to 10.74 and 14.15% for scenarios 1 and 2, respectively.
These analyses emphasize the importance of selecting efficient drying and pretreatment techniques based on thermodynamic performance, thereby contributing to the evolution of "zero-waste" designs and reducing non-renewable energy consumption. Through methodical exergy analysis, this body of work advances our understanding of bioenergy systems and paves the way for future research to elevate the sustainability of energy production processes.
Techno-economic Analysis of Third-Generation L-LA Biorefinery System
Techno-economic analysis (TEA) evaluates the economic performance of industrial processes to achieve cost-effective and sustainable plant development. This assessment is crucial for understanding the market value of L-LA, which is influenced by factors such as feedstock cost, cultivation techniques, transportation, and technology costs. The TEA of L-LA production from renewable resources has been extensively investigated, mainly focusing on economic feasibility at an industrial scale. However, studies on L-LA production costs from macroalgae are limited in the literature. Comparative TEA studies reveal significant differences in economic feasibility between macroalgae and traditional lignocellulosic biorefineries, with the latter often being less economically viable due to higher pretreatment and delignification costs [121].
The recent study investigates an innovative integrated biorefinery approach for the co-production of third-generation (3G) bioethanol and L-LA from red macroalgae cellulosic residues (MCR) [122]. This study used Aspen Plus V10 to simulate a biorefinery converting 15,883.3 kg/h of red macroalgae cellulosic residues into 3856.8 kg/h of anhydrous bioethanol, 6488.04 kg/h of L-LA, and 4479.48 kg/h of fertilizer while generating 5233.79 kWh of electricity. The study highlighted product yields of 0.24 kg of anhydrous ethanol, 0.41 kg of L-LA, and 0.28 kg of fertilizer per kilogram of MCR, with high purity levels of 99.7 wt.% for bioethanol and 92.8 wt.% for L-LA. The economic analysis revealed a payback period of 6.25 years and an ROI of 30.8%, indicating substantial economic potential. Integrating Industry 5.0 principles, including human–robot interaction, enhanced operational efficiency and productivity.
Another research has conducted a techno-economic analysis to produce LA from Laminaria sp. in a facility with an 11-ton capacity for LA per hour [123]. The carbohydrate composition of Laminaria sp. was determined to be 81.3% by weight, with Lactobacillus casei capable of fermenting 61.6% of it. The optimal production setup included pretreatment with a hot water wash, followed by enzymatic hydrolysis, fermentation, and subsequent purification of LA from the fermentation medium. This method achieved an LA yield of 45.1% from fermentable sugars. Including the potential for alginate fermentation, which accounts for 31.3% by weight, increased the yield to 47.6%. The minimum viable selling price for LA was $3.83/kg, reflecting a 34% difference from the current market price. Using Laminaria latissimi biomass, which has higher levels of laminarin and mannitol, can improve LA yield and reduce the price differential to 28%. With successful alginate fermentation, a payback period of approximately 11 years may be achievable.
The combined exergy and techno-economic analyses of L-LA production from macroalgae biorefineries underscore the importance of optimizing process efficiency and economic viability. Key considerations include minimizing environmental impacts through efficient biomass utilization and integrating advanced waste management and energy recovery systems. The most significant environmental impact of acquiring macroalgal biomass is primarily due to the energy-demanding cultivation methods in tanks and preprocessing techniques like drying. It is vital to optimize this initial phase to improve overall performance. Achieving a high yield of LA per biomass unit is critical for minimizing environmental impacts and facilitating cost-effective production. This can be done by either refining the pretreatment-hydrolysis process to improve reducing sugar recovery or by engineering the fermentation process to effectively utilize most of the extracted sugars. These comprehensive assessments pave the way for future research to enhance the sustainability and efficiency of bioenergy production processes.
Challenge and Future Perspectives
Advancements in third generation L-LA production from macroalgae signify a sustainable leap in biorefinery technologies, yet face hurdles that impede their full-scale industrial application. Among these are refining biorefinery operations for effectively breaking macroalgae into fermentable sugars and enhancing fermentation efficiency. Scalability and economic viability demand rigorous optimization of these steps [121]. Furthermore, the development of microbial strains capable of resisting hydrolysate inhibitors and maintaining high performance in large-scale fermentation processes is crucial. The composition of macroalgae, which can vary significantly due to environmental factors, requires adopting flexible and adaptive processing strategies. Addressing these issues is crucial for fully leveraging macroalgae’s potential in sustainable biomanufacturing.
Exploring integrated biorefinery models that aim to co-produce high-value compounds such as proteins, lipids, and pigments in conjunction with L-LA could significantly enhance the economic appeal of using macroalgae as a feedstock [124]. Achieving this goal necessitates a deepened understanding and enhancement of downstream processes to ensure efficient separation and purification of these valuable co-products.
As a tool to assess the efficiency and sustainability of biorefinery systems, exergy analysis plays a crucial role in identifying areas where energy use can be minimized, thereby enhancing the overall process sustainability [119]. Incorporating exergy analysis in the evaluation of macroalgae-based L-LA production can provide insights into optimizing process conditions, reducing energy consumption, and minimizing waste. Future perspectives entail addressing these technological and economic challenges through advanced research and development. Emphasis should be placed on developing transgenic macroalgae strains with improved biomass conversion traits and resilience to variable cultivation conditions. Furthermore, addressing the significant water demand for macroalgae cultivation necessitates innovative approaches to water management, including the implementation of recycled streams and evaporation control in system designs to curtail utility costs and conserve resources.
Conclusions
The escalating concerns over plastic pollution have spurred a notable shift toward biodegradable plastics, with polylactic acid (PLA) emerging as a key player due to its reliance on lactic acid (LA) as a primary precursor. This trend underscores the pressing need for sustainable LA production methods that pivot from fossil fuels to renewable resources, with fermentation-based approaches gaining commercial traction. However, the viability of these methods often stumbles upon the high costs associated with substrates and downstream processing, alongside the demand for LA of high optical purity. This review positions macroalgae detritus as a promising substrate for LA production, attributing its potential to rapid growth rates and a rich polysaccharide composition. Through examining third-generation LA biorefinery approaches, this study evaluates the viability of macroalgae detritus from exergy, economic, and environmental standpoints, integrating exergy analysis and life cycle assessments to map out the feasibility landscape. Despite the promise, achieving commercial-scale production with high yields, productivity, and optical purity of LA requires further technological advancements in macroalgae processing. The successful optimization of these technologies could herald a new era for macroalgae as a cornerstone feedstock in the biofuel and biochemical industries, marking a significant step toward sustainable biopolymer manufacturing.
Data Availability
The datasets supporting the current study are available from the corresponding author on reasonable request.
References
Chai CY, Tan IS, Foo HCY et al (2021) Sustainable and green pretreatment strategy of Eucheuma denticulatum residues for third-generation L-lactic acid production. Bioresour Technol 330:124930. https://doi.org/10.1016/j.biortech.2021.124930
Garcia-Aguirre J, Alvarado-Morales M, Fotidis IA, Angelidaki I (2020) Up-concentration of succinic acid, lactic acid, and ethanol fermentations broths by forward osmosis. Biochem Eng J 155:107482. https://doi.org/10.1016/J.BEJ.2019.107482
Nagarajan D, Oktarina N, Chen PT et al (2022) Fermentative lactic acid production from seaweed hydrolysate using Lactobacillus sp. And Weissella sp. Bioresour Technol 344:126166. https://doi.org/10.1016/J.BIORTECH.2021.126166
Singhvi M, Zendo T, Sonomoto K (2018) Free lactic acid production under acidic conditions by lactic acid bacteria strains: challenges and future prospects. Appl Microbiol Biotechnol 102:5911–5924. https://doi.org/10.1007/s00253-018-9092-4
Singhvi M, Zendo T, Gokhale D, Sonomoto K (2018) Greener L-lactic acid production through in situ extractive fermentation by an acid-tolerant Lactobacillus strain. Appl Microbiol Biotechnol 102:6425–6435. https://doi.org/10.1007/s00253-018-9084-4
Ahmad A, Banat F, Taher H (2020) A review on the lactic acid fermentation from low-cost renewable materials: recent developments and challenges. EnvironTechnol Innov 20:101138. https://doi.org/10.1016/j.eti.2020.101138
Kassim MA, Meng TK, Kamaludin R, et al (2022) Bioprocessing of sustainable renewable biomass for bioethanol production. Value-Chain of Biofuels 195–234. https://doi.org/10.1016/B978-0-12-824388-6.00004-X
Wang K, Yang Z, Ma Y et al (2022) Recent advances in the utilization of glycerol for the production of lactic acid by catalysis. Biofuels, Bioprod Biorefining 16:1428–1454. https://doi.org/10.1002/bbb.2410
Park J, Valekar AH, Oh KR et al (2023) Merging biomass and CO2 utilization; process design and assessment on simultaneous production of lactic acid and formic acid from glycerol and CO2. Chem Eng J 463:142410. https://doi.org/10.1016/j.cej.2023.142410
Rawoof SAA, Kumar PS, Vo DVN et al (2021) Production of optically pure lactic acid by microbial fermentation: a review. Environ Chem Lett 19:539–556. https://doi.org/10.1007/s10311-020-01083-w
Mostafa YS, Alamri SA, Hashem M et al (2020) Thermostable cellulase biosynthesis from Paenibacillus alvei and its utilization in lactic acid production by simultaneous saccharification and fermentation. Open Life Sci 15(1):798–798. https://doi.org/10.1515/biol-2020-0104
Chen H, Chen B, Su Z et al (2020) Efficient lactic acid production from cassava bagasse by mixed culture of Bacillus coagulans and lactobacillus rhamnosus using stepwise pH controlled simultaneous saccharification and co-fermentation. Ind Crops Prod 146:112175. https://doi.org/10.1016/j.indcrop.2020.112175
Bühlmann CH, Mickan BS, Tait S et al (2021) Lactic acid from mixed food wastes at a commercial biogas facility: effect of feedstock and process conditions. J Clean Prod 284:125243. https://doi.org/10.1016/j.jclepro.2020.125243
Jiang X, Liu X, Xu H et al (2021) Improvement of the nutritional, antioxidant and bioavailability properties of corn gluten-wheat bran mixture fermented with lactic acid bacteria and acid protease. Lwt 144:111161. https://doi.org/10.1016/j.lwt.2021.111161
Unban K, Puangkhankham N, Kanpiengjai A et al (2020) Improvement of polymer grade l-lactic acid production using lactobacillus rhamnosus SCJ9 from low-grade cassava chips by simultaneous saccharification and fermentation. Processes 8:1–17. https://doi.org/10.3390/PR8091143
Nagarajan D, Nandini A, Di DC et al (2020) Lactic acid production from renewable feedstocks using poly(vinyl alcohol)-immobilized Lactobacillus plantarum 23. Ind Eng Chem Res 59:17156–17164. https://doi.org/10.1021/acs.iecr.0c01422
Tang H, Huang W, Yao YF (2023) The metabolites of lactic acid bacteria: classification, biosynthesis and modulation of gut microbiota. Microb Cell 10:49–62. https://doi.org/10.15698/mic2023.03.792
Chong SL, Tan IS, Foo HCY et al (2022) Ultrasonic-assisted molten salt hydrates pretreated Eucheuma cottonii residues as a greener precusor for third-generation L-lactic acid production. Bioresour Technol 364:128136. https://doi.org/10.1016/j.biortech.2022.128136
Machado J, Rossi DM, Ayub MAZ (2024) Batch and fed-batch strategies of lactic acid production by Lactobacillus plantarum BL011 using soybean hull hydrolysates as substrate. Biomass Convers Biorefinery 14:3249–3259. https://doi.org/10.1007/s13399-022-02544-8
Granget C, Manikandan NA, Amulya K et al (2024) Brewer’s spent grain as a self-sufficient feedstock for homofermentative production of optically pure L-lactic acid using Lactobacillus rhamnosus. Environ Technol Innov 34:103582. https://doi.org/10.1016/j.eti.2024.103582
Sindhu R, Binod P, Pandey A, Gnansounou E (2020) Agroresidue-based biorefineries. Refining biomass residues for sustainable energy and products, 642. https://doi.org/10.1016/B978-0-12-818996-2.00011-9
Ge X, Chang C, Zhang L et al (2018) Conversion of lignocellulosic biomass into platform chemicals for biobased polyurethane application. Adv Bioenergy 3:161–213. https://doi.org/10.1016/bs.aibe.2018.03.002
Tan IS, Lam MK, Foo HCY et al (2020) Advances of macroalgae biomass for the third generation of bioethanol production. Chinese J Chem Eng 28:502–517. https://doi.org/10.1016/j.cjche.2019.05.012
Behera S, Singh R, Arora R et al (2015) Scope of algae as third generation biofuels. Front Bioeng Biotechnol 2:1–13. https://doi.org/10.3389/fbioe.2014.00090
Morais T, Inácio A, Coutinho T et al (2020) Marine science and engineering seaweed potential in the animal feed: a review. J Mar Sci Energy 8:559. https://doi.org/10.3390/jmse8080559
Kumar M, Sun Y, Rathour R et al (2020) Algae as potential feedstock for the production of biofuels and value-added products: opportunities and challenges. Sci Total Environ 716:137116. https://doi.org/10.1016/J.SCITOTENV.2020.137116
Kostas ET, Adams JMM, Ruiz HA et al (2021) Macroalgal biorefinery concepts for the circular bioeconomy: a review on biotechnological developments and future perspectives. Renew Sustain Energy Rev 151:111553. https://doi.org/10.1016/J.RSER.2021.111553
González-Gloria KD, Rodríguez-Jasso RM, Shiva et al (2021) Macroalgal biomass in terms of third-generation biorefinery concept: current status and techno-economic analysis – a review. Bioresour Technol Reports 16:100863. https://doi.org/10.1016/J.BITEB.2021.100863
The State of World Fisheries and Aquaculture (2022). https://www.fao.org/3/cc0461en/online/cc0461en.html. Accessed 18 Jan 2024
Lahaye M, Brunel M, Bonnin E (1997) Fine chemical structure analysis of oligosaccharides produced by an ulvan-lyase degradation of the water-soluble cell-wall polysaccharides from Ulva sp. (Ulvales, Chlorophyta). Carbohydr Res 304:325–333. https://doi.org/10.1016/S0008-6215(97)00270-X
Morgan H, Kumar S, Orlygsson J et al (2018) Fermentation of mannitol extracts from brown macro algae by thermophilic clostridia. Front Microbiol 9:1–13. https://doi.org/10.3389/fmicb.2018.01931
Flórez-Fernández N, Domínguez H, Torres MD (2019) Advances in the biorefinery of Sargassum muticum: valorisation of the alginate fractions. Ind Crops Prod 138:111483. https://doi.org/10.1016/j.indcrop.2019.111483
Peñuela A, Robledo D, Bourgougnon N et al (2018) Environmentally friendly valorization of Solieria filiformis (Gigartinales, Rhodophyta) from IMTA using a biorefinery concept. Mar Drugs 16:487. https://doi.org/10.3390/md16120487
Tan IS, Lee KT (2016) Comparison of different process strategies for bioethanol production from Eucheuma cottonii: an economic study. Bioresour Technol 199:336–346. https://doi.org/10.1016/j.biortech.2015.08.008
Nurani W, Anwar Y, Batubara I et al (2024) Kappaphycus alvarezii as a renewable source of kappa-carrageenan and other cosmetic ingredients. Int J Biol Macromol 260:129458. https://doi.org/10.1016/j.ijbiomac.2024.129458
Thompson TM, Young BR, Baroutian S (2019) Advances in the pretreatment of brown macroalgae for biogas production. Fuel Process Technol 195:106151. https://doi.org/10.1016/j.fuproc.2019.106151
Fan L, Zhang H, Li J et al (2020) Algal biorefinery to value-added products by using combined processes based on thermochemical conversion: a review. Algal Res 47:101819. https://doi.org/10.1016/j.algal.2020.101819
Nallasivam J, Francis Prashanth P, Harisankar S et al (2022) Valorization of red macroalgae biomass via hydrothermal liquefaction using homogeneous catalysts. Bioresour Technol 346:126515. https://doi.org/10.1016/J.BIORTECH.2021.126515
Yu H, Xiao W, Han L, Huang G (2019) Characterization of mechanical pulverization/phosphoric acid pretreatment of corn stover for enzymatic hydrolysis. Bioresour Technol 282:69–74. https://doi.org/10.1016/j.biortech.2019.02.104
Parvathy Eswari A, Anu Alias Meena R, Yukesh Kannah R, et al (2019) Bioconversion of marine waste biomass for biofuel and value-added products recovery. Elsevier Inc, Amsterdam
Tapia-Tussell R, Avila-Arias J, Maldonado JD et al (2018) Biological pretreatment of Mexican Caribbean macroalgae consortiums using Bm-2 strain (Trametes hirsuta) and its enzymatic broth to improve biomethane potential. Energies 11:494. https://doi.org/10.3390/en11030494
Amamou S, Sambusiti C, Monlau F et al (2018) Mechano-enzymatic deconstruction with a new enzymatic cocktail to enhance enzymatic hydrolysis and bioethanol fermentation of two macroalgae species. Molecules 23:174. https://doi.org/10.3390/molecules23010174
Esquivel-Hernández DA, García-Pérez JS, López-Pacheco IY et al (2022) Resource recovery of lignocellulosic biomass waste into lactic acid - trends to sustain cleaner production. J Environ Manage 301:113925. https://doi.org/10.1016/J.JENVMAN.2021.113925
Bussemaker MJ, Zhang D (2013) Effect of ultrasound on lignocellulosic biomass as a pretreatment for biorefinery and biofuel applications. Ind Eng Chem Res 52:3563–3580. https://doi.org/10.1021/ie3022785
Preethi BJR, Varjani S et al (2022) Breakthrough in hydrolysis of waste biomass by physico-chemical pretreatment processes for efficient anaerobic digestion. Chemosphere 294:133617. https://doi.org/10.1016/J.CHEMOSPHERE.2022.133617
Hermansyah, Cahyadi H, Fatma, et al (2021) Delignification of lignocellulosic biomass sugarcane bagasse by using ozone as initial step to produce bioethanol. Polish J Environ Stud 30:4405–4411.https://doi.org/10.15244/pjoes/132263
Sulfahri MS, Husain DR et al (2020) Fungal pretreatment as a sustainable and low cost option for bioethanol production from marine algae. J Clean Prod 265:121763. https://doi.org/10.1016/j.jclepro.2020.121763
Sulfahri MS, Langford A, Tassakka ACMAR (2020) Ozonolysis as an effective pretreatment strategy for bioethanol production from marine algae. Bioenergy Res 13:1269–1279. https://doi.org/10.1007/s12155-020-10131-w
Ravindran R, Jaiswal AK (2016) A comprehensive review on pre-treatment strategy for lignocellulosic food industry waste: challenges and opportunities. Bioresour Technol 199:92–102. https://doi.org/10.1016/j.biortech.2015.07.106
Nguyen TH, Sunwoo IY, Ra CH et al (2019) Acetone, butanol, and ethanol production from the green seaweed Enteromorpha intestinalis via the separate hydrolysis and fermentation. Bioprocess Biosyst Eng 42:415–424. https://doi.org/10.1007/s00449-018-2045-6
Ra CH, Nguyen TH, Jeong G-T, Kim S-K (2016) Evaluation of hyper thermal acid hydrolysis of Kappaphycus alvarezii for enhanced bioethanol production. Bioresour Technol 209:66–72. https://doi.org/10.1016/j.biortech.2016.02.106
Teh YY, Lee KT, Chen WH et al (2017) Dilute sulfuric acid hydrolysis of red macroalgae Eucheuma denticulatum with microwave-assisted heating for biochar production and sugar recovery. Bioresour Technol 246:20–27. https://doi.org/10.1016/j.biortech.2017.07.101
Tong KTX, Tan IS, Foo HCY et al (2021) Third-generation L-lactic acid production by the microwave-assisted hydrolysis of red macroalgae Eucheuma denticulatum extract. Bioresour Technol 342:125880. https://doi.org/10.1016/j.biortech.2021.125880
Sunwoo IY, Ra CH, Jeong GT, Kim SK (2016) Evaluation of ethanol production and bioadsorption of heavy metals by various red seaweeds. Bioprocess Biosyst Eng 39:915–923. https://doi.org/10.1007/s00449-016-1571-3
Jeong GT, Kim SK, Park DH (2015) Application of solid-acid catalyst and marine macro-algae Gracilaria verrucosa to production of fermentable sugars. Bioresour Technol 181:1–6. https://doi.org/10.1016/J.BIORTECH.2015.01.038
Hessami MJ, Phang SM, Salleh A, Rabiei R (2018) Evaluation of tropical seaweeds as feedstock for bioethanol production. Int J Environ Sci Technol 15:977–992. https://doi.org/10.1007/s13762-017-1455-3
Hessami MJ, Cheng SF, Ambati RR et al (2019) Bioethanol production from agarophyte red seaweed, Gelidium elegans, using a novel sample preparation method for analysing bioethanol content by gas chromatography. 3 Biotech 9:1–8. https://doi.org/10.1007/s13205-018-1549-8
Obeng EM, Adam SNN, Budiman C et al (2017) Lignocellulases: a review of emerging and developing enzymes, systems, and practices. Bioresour Bioprocess 4:16. https://doi.org/10.1186/s40643-017-0146-8
Hassan N, Khairil Anwar NAK, Idris A (2020) Strategy to enhance the sugar production using recyclable inorganic salt for pre-treatment of oil palm empty fruit bunch (OPEFB). BioResources 15:4912–4931. https://doi.org/10.15376/biores.15.3.4912-4931
Pham VT, Guan CY, Han PC et al (2021) Acid-catalyzed hydrothermal treatment of sewage sludge: effects of reaction temperature and acid concentration on the production of hydrolysis by-products. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01495-w
Sulfahri, Husain DR, Wulandari DP et al (2019) Biosugar production from algae Spirogyra peipingensis by acid and enzymatic hydrolysis processes. J Phys Conf Ser: 1341: https://doi.org/10.1088/1742-6596/1341/2/022012
Abedi E, Hashemi SMB (2020) Lactic acid production – producing microorganisms and substrates sources-state of art. Heliyon 6:e04974. https://doi.org/10.1016/j.heliyon.2020.e04974
Chen YY, Dong L, Alam MA, et al (2022) Carbon catabolite repression governs diverse physiological processes and development in Aspergillus nidulans. MBio 13: https://doi.org/10.1128/MBIO.03734-21
Tan J, Abdel-Rahman MA, Numaguchi M et al (2017) Thermophilic Enterococcus faecium QU 50 enabled open repeated batch fermentation for l-lactic acid production from mixed sugars without carbon catabolite repression. RSC Adv 7:24233–24241. https://doi.org/10.1039/c7ra03176a
Lu Y, Song S, Tian H et al (2018) Functional analysis of the role of CcpA in Lactobacillus plantarum grown on fructooligosaccharides or glucose: a transcriptomic perspective. Microb Cell Fact 17:1–11. https://doi.org/10.1186/s12934-018-1050-4
Ahorsu R, Cintorrino G, Medina F, Constantí M (2019) Microwave processes: a viable technology for obtaining xylose from walnut shell to produce lactic acid by Bacillus coagulans. J Clean Prod 231:1171–1181. https://doi.org/10.1016/j.jclepro.2019.05.289
Olszewska-Widdrat A, Alexandri M, López-Gómez JP et al (2020) Batch and continuous lactic acid fermentation based on a multi-substrate approach. Microorganisms 8:1–14. https://doi.org/10.3390/microorganisms8071084
Abdel-Rahman MA, Hassan SED, Azab MS et al (2019) High improvement in lactic acid productivity by new alkaliphilic bacterium using repeated batch fermentation integrated with increased substrate concentration. Biomed Res Int 2019:1. https://doi.org/10.1155/2019/7212870
Kumar A, Udugama IA, Gargalo CL, Gernaey KV (2020) Why is batch processing still dominating the biologics landscape? Towards an integrated continuous bioprocessing alternative. Processes 8:1–19. https://doi.org/10.3390/pr8121641
Nwamba MC, Sun F, Mukasekuru MR et al (2021) Trends and hassles in the microbial production of lactic acid from lignocellulosic biomass. Environ Technol Innov 21:101337. https://doi.org/10.1016/j.eti.2020.101337
Teworte S, Malcı K, Walls LE et al (2022) Recent advances in fed-batch microscale bioreactor design. Biotechnol Adv 55:107888. https://doi.org/10.1016/j.biotechadv.2021.107888
Peetermans A, Foulquié-Moreno MR, Thevelein JM (2021) Mechanisms underlying lactic acid tolerance and its influence on lactic acid production in Saccharomyces cerevisiae. Microb Cell 8:111–130. https://doi.org/10.15698/mic2021.06.751
Mehrotra T, Zaman MN, Prasad BB et al (2020) Rapid immobilization of viable Bacillus pseudomycoides in polyvinyl alcohol/glutaraldehyde hydrogel for biological treatment of municipal wastewater. Environ Sci Pollut Res 27:9167–9180. https://doi.org/10.1007/s11356-019-07296-z
Reis CER, Libardi Junior N, Bento HBS et al (2023) Process strategies to reduce cellulase enzyme loading for renewable sugar production in biorefineries. Chem Eng J 451:138690. https://doi.org/10.1016/j.cej.2022.138690
Aulitto M, Fusco S, Nickel DB et al (2019) Seed culture pre-adaptation of Bacillus coagulans MA-13 improves lactic acid production in simultaneous saccharification and fermentation. Biotechnol Biofuels 12:1–11. https://doi.org/10.1186/s13068-019-1382-2
Sun Y, Liu H, Yang Y et al (2021) High-efficient L-lactic acid production from inedible starchy biomass by one-step open fermentation using thermotolerant Lactobacillus rhamnosus DUT1908. Bioprocess Biosyst Eng 44:1935–1941. https://doi.org/10.1007/s00449-021-02573-z
Tian X, Liu X, Zhang Y et al (2021) Metabolic engineering coupled with adaptive evolution strategies for the efficient production of high-quality L-lactic acid by Lactobacillus paracasei. Bioresour Technol 323:124549. https://doi.org/10.1016/j.biortech.2020.124549
Alves de Oliveira R, Schneider R, Vaz Rossell CE et al (2019) Polymer grade L-lactic acid production from sugarcane bagasse hemicellulosic hydrolysate using Bacillus coagulans. Bioresour Technol Reports 6:26–31. https://doi.org/10.1016/j.biteb.2019.02.003
Bahry H, Abdalla R, Pons A et al (2019) Optimization of lactic acid production using immobilized Lactobacillus rhamnosus and carob pod waste from the Lebanese food industry. J Biotechnol 306:81–88. https://doi.org/10.1016/j.jbiotec.2019.09.017
Cubas-Cano E, González-Fernández C, Ballesteros I, Tomás-Pejó E (2020) Efficient utilization of hydrolysates from steam-exploded gardening residues for lactic acid production by optimization of enzyme addition and pH control. Waste Manag 107:235–243. https://doi.org/10.1016/J.WASMAN.2020.04.003
Jiang S, Xu P, Tao F (2019) L-Lactic acid production by Bacillus coagulans through simultaneous saccharification and fermentation of lignocellulosic corncob residue. Bioresour Technol Reports 6:131–137. https://doi.org/10.1016/j.biteb.2019.02.005
Wei Q, Zhang W, Ren X (2019) Recovery and separation of lactic and malic acids from fermentation broth with N,N,N,N-tetrabutylbutanediamide by reactive liquid−liquid extraction and stripping. SDRP J Food Sci Technol 4:934–945. https://doi.org/10.25177/jfst.4.8.ra.584
Lee HD, Lee MY, Hwang YS et al (2017) Separation and purification of lactic acid from fermentation broth using membrane-integrated separation processes. Ind Eng Chem Res 56:8301–8310. https://doi.org/10.1021/acs.iecr.7b02011
de Oliveira PZ, Vandenberghe LP de S, Soccol CR (2023) Lactic acid production using sugarcane juice as an alternative substrate and purification through ion-exchange resins. Fermentation 9(10).https://doi.org/10.3390/fermentation9100879
Matsumoto M, Takemori S, Tahara Y (2020) Lactic acid permeation through deep eutectic solvents-based polymer inclusion membranes. Membranes (Basel) 10:1–8. https://doi.org/10.3390/membranes10090244
Yıldız E, Lalikoglu M, Aşçı YS, Sırma Tarım B (2023) Investigation of reactive extraction of monocarboxylic acids with menthol-based hydrophobic deep eutectic solvent by response surface methodology. Sep Sci Technol 58:1450–1459. https://doi.org/10.1080/01496395.2023.2192382
Tanapawarin R, Thitiprasert S, Wasinee B et al (2016) Improved lactic acid productivity by simultaneous recovery during fermentation using resin exchanger. KKU Res J: 193–199. https://doi.org/10.14456/kkurj.2016.11
Su, CY, Yu, CC, Chien, IL, & Ward, DJ (2015). Control of highly interconnected reactive distillation processes: purification of raw lactic acid by esterification and hydrolysis. Ind Eng Chem Res, 54, 6932–6940. https://doi.org/10.1021/ie5039133
Wang Y, Cai D, Chen C et al (2015) Efficient magnesium lactate production with in situ product removal by crystallization. Bioresour Technol 198:658–663. https://doi.org/10.1016/j.biortech.2015.09.058
López-Gómez JP, Alexandri M, Schneider R et al (2020) Organic fraction of municipal solid waste for the production of L-lactic acid with high optical purity. J Clean Prod 247:119165. https://doi.org/10.1016/j.jclepro.2019.119165
Olszewska-Widdrat A, Alexandri M, López-Gómez JP et al (2019) Production and purification of L-lactic acid in lab and pilot scales using sweet sorghum juice. Fermentation 5:1–10. https://doi.org/10.3390/fermentation5020036
Kumar A, Thakur A, Panesar PS (2018) Statistical optimization of lactic acid extraction using green emulsion ionic liquid membrane (GEILM). J Environ Chem Eng 6:1855–1864. https://doi.org/10.1016/j.jece.2018.01.037
Jusoh N, Noah NFM, Othman N (2019) Extraction and recovery optimization of succinic acid using green emulsion liquid membrane containing palm oil as the diluent. Environ Prog Sustain Energy 38:1–9. https://doi.org/10.1002/ep.13065
Ahmad AL, Shah Buddin MMH, Ooi BS, Kusumastuti A (2017) Utilization of environmentally benign emulsion liquid membrane (ELM) for cadmium extraction from aqueous solution. J Water Process Eng 15:26–30. https://doi.org/10.1016/j.jwpe.2016.05.010
Lech M, Trusek A (2018) Batch electrodialysis of lactic acid obtained from lab fermentation. Polish J Chem Technol 20:81–86. https://doi.org/10.2478/pjct-2018-0042
Lan K, Xu S, Li J, Hu C (2019) Recovery of lactic acid from corn stover hemicellulose-derived liquor. ACS Omega 4:10571–10579. https://doi.org/10.1021/acsomega.9b00794
Komesu A, de Oliveira JAR, Martins LH da S, et al (2017) Lactic acid production to purification: a review. BioResources 12:4364–4383. https://doi.org/10.15376/biores.12.2.4364-4383
Kumar A, Thakur A, Panesar PS (2019) A comparative study on experimental and response surface optimization of lactic acid synergistic extraction using green emulsion liquid membrane. Sep Purif Technol 211:54–62. https://doi.org/10.1016/j.seppur.2018.09.048
Kumar A, Thakur A, Panesar PS (2018) Lactic acid extraction using environmentally benign green emulsion ionic liquid membrane. J Clean Prod 181:574–583. https://doi.org/10.1016/j.jclepro.2018.01.263
Thakur A (2019) Lactic acid extraction from aqueous systems by emulsion liquid membrane separation process using statistical experimental design. Polytechnica 2:62–76. https://doi.org/10.1007/s41050-019-00015-0
Kumar A, Thakur A (2019) Reactive extraction of lactic acid using environmentally benign green solvents and a synergistic mixture of extractants. Sci Iran 26:3456–3467.https://doi.org/10.24200/sci.2019.52233.2610
Bakshi A, Shemansky JM, Chang C, Binder BM (2015) History of research on the plant hormone ethylene. J Plant Growth Regul 34:809–827. https://doi.org/10.1007/s00344-015-9522-9
Hassan ME, Yang Q, Xiao Z (2019) Covalent immobilization of glucoamylase enzyme onto chemically activated surface of κ-carrageenan. Bull Natl Res Cent 43:102. https://doi.org/10.1186/s42269-019-0148-0
Khudhair HA, Ismail ZZ (2019) New application of single and mixed immobilized cells for furfural biodegradation. Bioremediat J 23:32–41. https://doi.org/10.1080/10889868.2019.1569585
Wang J, Huang J, Laffend H et al (2020) Optimization of immobilized Lactobacillus pentosus cell fermentation for lactic acid production. Bioresour Bioprocess 7:15. https://doi.org/10.1186/s40643-020-00305-x
Kavitake D, Kandasamy S, Devi PB, Shetty PH (2018) Recent developments on encapsulation of lactic acid bacteria as potential starter culture in fermented foods – a review. Food Biosci 21:34–44. https://doi.org/10.1016/j.fbio.2017.11.003
Malhotra I, Basir SF (2020) Immobilization of invertase in calcium alginate and calcium alginate-kappa-carrageenan beads and its application in bioethanol production. Prep Biochem Biotechnol 50:494–503. https://doi.org/10.1080/10826068.2019.1709979
Tan IS, Lee KT (2015) Immobilization of β-glucosidase from Aspergillus niger on κ-carrageenan hybrid matrix and its application on the production of reducing sugar from macroalgae cellulosic residue. Bioresour Technol 184:386–394. https://doi.org/10.1016/j.biortech.2014.10.146
Dosuky AS, Elsayed TR, Yousef ET et al (2022) Isolation, identification, and application of lactic acid-producing bacteria using salted cheese whey substrate and immobilized cells technology. J Genet Eng Biotechnol 20:26. https://doi.org/10.1186/s43141-022-00316-5
Wang J, Huang J, Guo H et al (2020) Optimization of immobilization conditions for Lactobacillus pentosus cells. Bioprocess Biosyst Eng 43:1071–1079. https://doi.org/10.1007/s00449-020-02305-9
Radosavljević M, Lević S, Belović M et al (2021) Encapsulation of Lactobacillus rhamnosus in polyvinyl alcohol for the production of L-(+)-lactic acid. Process Biochem 100:149–160. https://doi.org/10.1016/j.procbio.2020.10.006
Jurić S, Tanuwidjaja I, Fuka MM et al (2021) Encapsulation of two fermentation agents, Lactobacillus sakei and calcium ions in microspheres. Colloids Surfaces B Biointerfaces 197:1–11. https://doi.org/10.1016/j.colsurfb.2020.111387
Ahmad SI, Ahmad R, Khan MS et al (2020) Chitin and its derivatives: structural properties and biomedical applications. Int J Biol Macromol 164:526–539. https://doi.org/10.1016/j.ijbiomac.2020.07.098
Schmieg B, Schimek A, Franzreb M (2018) Development and performance of a 3D-printable poly(ethylene glycol) diacrylate hydrogel suitable for enzyme entrapment and long-term biocatalytic applications. Eng Life Sci 18:659–667. https://doi.org/10.1002/elsc.201800030
Zaushitsyna O, Dishisha T, Hatti-Kaul R, Mattiasson B (2017) Crosslinked, cryostructured Lactobacillus reuteri monoliths for production of 3-hydroxypropionaldehyde, 3-hydroxypropionic acid and 1,3-propanediol from glycerol. J Biotechnol 241:22–32. https://doi.org/10.1016/j.jbiotec.2016.11.005
Vaziri AS, Alemzadeh I, Vossoughi M (2018) Improving survivability of Lactobacillus plantarum in alginate-chitosan beads reinforced by Na-tripolyphosphate dual cross-linking. Lwt 97:440–447. https://doi.org/10.1016/j.lwt.2018.07.037
Soltanian S, Aghbashlo M, Almasi F et al (2020) A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers Manag 212:112792. https://doi.org/10.1016/j.enconman.2020.112792
Mahian O, Mirzaie MR, Kasaeian A, Mousavi SH (2020) Exergy analysis in combined heat and power systems: a review. Energy Convers Manag 226:113467. https://doi.org/10.1016/j.enconman.2020.113467
Chung MRWY, Tan IS, Foo HCY, Lam MK (2022) Exergy analysis of a biorefinery process for co-production of third-generation L-lactic acid and electricity from Eucheuma denticulatum residues. Energy 242:122968. https://doi.org/10.1016/j.energy.2021.122968
Tan YL, Tan IS, Foo HCY et al (2024) Biorefinery-based sustainability assessment of macroalgae waste valorization to polylactic acid: exergy and exergoeconomic perspectives. Biochem Eng J 204:109219. https://doi.org/10.1016/j.bej.2024.109219
Tong KTX, Tan IS, Foo HCY et al (2022) Advancement of biorefinery-derived platform chemicals from macroalgae: a perspective for bioethanol and lactic acid. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-022-02561-7
Wong KH, Tan IS, Foo HCY et al (2022) Third-generation bioethanol and L-lactic acid production from red macroalgae cellulosic residue: prospects of Industry 5.0 algae. Energy Convers Manag 253:115155. https://doi.org/10.1016/j.enconman.2021.115155
Grasa ET, Ögmundarson Ó, Gavala HN, Sukumara S (2021) Commodity chemical production from third-generation biomass a techno-economic of lactic acid. Biofuels, Bioprod Biorefining 15:257–281. https://doi.org/10.1002/bbb.2160
Lin HTV, Huang MY, Kao TY et al (2020) Production of lactic acid from seaweed hydrolysates via lactic acid bacteria fermentation. Fermentation 6:12–14. https://doi.org/10.3390/FERMENTATION6010037
Acknowledgements
The authors thank the financial support provided by the Fundamental Research Grant Scheme (FRGS/1/2024/TK05/CURTIN/02/2) from the Ministry of Higher Education (MOHE), Malaysia.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Author information
Authors and Affiliations
Contributions
Soo Ling Chong: writing—original draft and Project administration. Inn Shi Tan: supervision, conceptualization, writing—reviewing, and editing. Henry Foo Chee Yew: validation. Man Kee Lam: investigation. Keat Teong Lee: investigation and validation.
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chong, S.L., Tan, I.S., Foo, H.C.Y. et al. Third-Generation L-Lactic Acid Biorefinery Approaches: Exploring the Viability of Macroalgae Detritus. Bioenerg. Res. (2024). https://doi.org/10.1007/s12155-024-10801-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12155-024-10801-z