Abstract
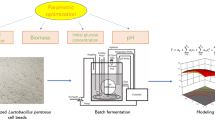
In this study, the immobilization technology was used to improve the LA yield and shorten the fermentation time. The optimum conditions to immobilize Lactobacillus pentosus ATCC 8041 cell were determined by Taguchi design L16 (45). The immobilized L. pentosus ATCC 8041 cells prepared by 2% sodium alginate (SA) and 6% polyvinyl alcohol (PVA) with the immobilization process by 0.10 M calcium chloride (CaCl2) and 2.5% boric acid (H3BO3) had the best performance of LA yield at the temperature of 35 °C, which is significantly higher than that of L. pentosus ATCC 8041 free cells. These cells maintained the stable and efficient performance in 15 repeated batch fermentation, and they also have excellent mechanical strength to keep from breakage caused by cell growth and agitation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Immobilized cell fermentation is an effective way to shorten the fermentation time and improve the product yield based on high dense of cells encapsulated in immobilized cell beads [1]. Gel encapsulation is the most widely used method of cell immobilization [2]. The carrier solution is usually a mixed solution of sodium alginate (SA) and polyvinyl alcohol (PVA), and the calcium chloride (CaCl2) solution and boric acid (H3BO3) are usually used as cross-linking agents [3]. Alginate is a kind of true block copolymers consisting of homopolymeric regions of (1 → 4)-linked β-d-mannuronic acid (M) and α-l-guluronic acid (G) as MM, GG and MG blocks [4]. In CaCl2 solution, the reaction between Ca2+ and GG blocks occurs instantaneously and irreversibly as an ionic cross-link process [5]. PVA is an organic polymer containing diols on the side chain. Those diols are sensitive to borate ions in H3BO3 solution and form cross-link bonds with them [6]. Alginate-PVA gel has many excellent properties compared with other carrier materials, including high mechanical strength, good mass transfer performance, and high activity of immobilized cells. In the hybrid gel, alginate reduces the tendency to agglomeration and improve the surface properties of immobilized cell beads, while PVA promotes mechanical strength and stability [7]. Therefore, alginate-PVA based gel can be considered as a proper and applicable material to prepare immobilized cell beads. In the immobilization process, the concentrations of carrier solutions and cross-linking agent solutions have significant effects on the strength and mass transfer performance of immobilized cell beads [8]. Therefore, appropriate concentrations of carrier solutions and cross-linking agent solutions can ensure that the beads have excellent strength and good mass transfer performance, improving the production yield and shortening the fermentation time [9].
As a traditional industrial product, lactic acid (LA) has been widely used in food, pharmaceutical, cosmetics, and chemical industries [10]. Lactobacillus pentosus (L. pentosus) degrades hexoses via the EmbdenMeyerhoff-Parnas pathway (EMP-P) to produce LA, while it utilizes pentoses via the phosphoketolase pathway (PK-P) [11]. In the absence of glucose, its metabolic pathway changes from homologous fermentation to heterologous fermentation, and acetic acid is also produced as the subproduct during the LA production process [12]. Therefore, L. pentosus can be considered a facultatively heterofermentative organism. The both pathways are always shown as:
Therefore, L. pentosus is a proper bacterium to produce lactic acid in the fermentation process [13] The L. pentosus cell immobilization technology has practical significance in the industrial production of lactic acid [14].
The study presents a search for optimal immobilization conditions using Taguchi design L16 (45). Also, the effect of the application of immobilized cells on fermentation efficiency was investigated.
Materials and methods
Seed culture preparation
Lactobacillus pentosus ATCC 8041 used in this experiment was supplied by the American Type Culture Collection (ATCC). The cells were lyophilized and stored in a refrigerator at − 8 °C. Before immobilization, the cells were activated in de Man, Rogosa and Sharpe broth (MRS broth) at 37 °C and 150 rpm for 8 h on a rotary shaker (GYROMAXTM 747R, Amerex Instruments, Lafayette, CA, USA).
Immobilized cell preparation
Experimental design: Taguchi design L16 (45)
Prior to producing immobilized cells for fermentation experiment, Taguchi design L16 (45) was used to figure out the best combination of concentrations of carrier solutions and cross-linking agent solutions [15]. The concentration levels of sodium alginate (SA), CaCl2, polyvinyl alcohol (PVA), and H3BO3 were designed as Table 1. Factor A, B, C, and D were SA concentration, CaCl2 concentration, PVA concentration, and H3BO3 concentration, respectively.
Immobilization process
Specific amounts of SA and PVA were gradually added to deionized water with continuous agitation at 30 °C and 80 °C, respectively. Two prepared carrier solutions were mixed and sterilized to prepare the SA-PVA transparent hydrogel with certain viscosity as a semi-interpenetrating polymer network [16]. 5 mL centrifuge-concentrated L. pentosus seed culture, corresponding to a cell density of 1.00 × 109 CFU/mL (9.00 log CFU/mL), was injected into the carrier solution with continuous stirring. The fully mixed carrier solution containing L. pentosus cells was injected into the mixed solution of CaCl2 and H3BO3 without agitation to keep the stable shape of beads initially formed, and subsequently stored in a refrigerator to prepare immobilized cell beads with the diameter of 2.5 ± 0.5 mm at 4 °C for 12 h [17]. The prepared immobilized cell beads with a shape of approximate sphere were washed by sterilized deionized water to remove residual CaCl2 and H3BO3, and subsequently transferred into the fresh MRS medium. The immobilized cells were activated at 37 °C and 150 rpm on the shaker for 8 h [18].
Batch fermentation process
Determination of optimum conditions in immobilized cell fermentation
Immobilized L. pentosus ATCC 8041 cell beads prepared under certain conditions were added into a 1.0 L New Brunswick Bioreactor (BIOFLO 110; New Brunswick Scientific Co., Edison, NJ, USA), along with 800 mL fermentation medium consisting of 50 g/L glucose, 5 g/L peptone, 5 g/L yeast extract, 0.5 g/L MgSO4, and 0.5 g/L KH2PO4 [19]. The fermentation pH was maintained at 6.0 by adding 5 mol/L NaOH. Impellers of the bioreactor were disassembled to avoid gel bead damage. Agitation speed was maintained at 150 rpm by a magnetic stirrer. The fermentation was controlled at 35 °C by heat system. The optimum temperature for LA production by immobilized L. pentosus ATCC 8041 cells prepared under optimum conditions was determined by setting the fermentation temperature at 31 °C, 32 °C, 33 °C, 34 °C, 35 °C, 36 °C, 37 °C, 38 °C and 39 °C, respectively. Other fermentation conditions were kept being the same as previous conditions.
Repeated batch fermentation of immobilized cells
The same immobilized cell beads were used for batch fermentation at the optimum temperature found in Sect. 2.3.1 for 720 h and all the other fermentation conditions were kept as mentioned in Sect. 2.3.1. Every 48 h, the fermentation broth was poured out and replaced with the fresh medium. In other words, 15 batches of repeated fermentation were carried out. After each batch was completed, immobilized L. pentosus beads were recovered and washed by sterilized deionized water, and subsequently added to 800 mL fresh fermentation medium for next batch. 50 immobilized cell beads were selected randomly after each batch to detect the number of damaged beads, and the ratio of intact beads was calculated to describe the mechanical strength of beads [8].
Comparison between immobilized cell and free cell batch fermentation
The free cell batch fermentation was conducted as a control experiment at 150 rpm, pH 6.0 and the temperature found from Sect. 2.3.1 in a 1.0 L New Brunswick Bioreactor to compare with the results gathered from immobilized cells. 5 mL centrifuge-concentrated seed culture and 800 mL fermentation medium consisted of 50 g/L glucose, 5 g/L yeast extract, 0.5 g/L MgSO4, and 0.5 g/L KH2PO4 were added.
Determination of substrate and product concentrations
Proton nuclear magnetic resonance spectroscopy (1H NMR) was used to monitor the concentrations of residual glucose and LA [20]. NMR samples consisting of 0.5 mL fermentation sample, 0.4 mL deuterium oxide (Acros organics), and 0.1 mL internal standard were injected into 5-mm-o.d. nuclear magnetic resonance (NMR) tubes (Corning, NY, USA), and then analyzed by 1H NMR spectroscopy [21]. The internal standard was a mixture of 95.5 wt% deuterium oxide, 4.2 wt% glucosamine, 0.2 wt% trimethylamine and 0.1 wt% trimethylsilyl propionate. The signal peak area was integrated using MestReNova software. Glucose concentration and LA concentration were calculated based on the calibration curve developed by the linear relationship between the concentration and peak area.
Determination of cell density in immobilized cell beads
The cells were recycled by dissolving immobilized cell beads of 1 g by 0.2 mol/L sodium-citrate solution to prepare the cell solution with the dilution rate of 10–1. The cell solution was subsequently diluted to reach the dilution rate of 10–9 by sterilized water. 0.1 mL diluted cells were inoculated to the MRS agar and cultivated at 37 °C for 2 days, and the cell density can be subsequently calculated [22].
Results and discussions
Result of 1H NMR spectrum
As shown in Fig. 1, 1H NMR was able to measure the concentration of glucose and lactic acid (LA) [23]. The peaks representing the concentration of glucose in the two configurations (α and β) on the spectrum were generated by an anomeric proton region between 4.4 and 5.4 ppm [24]. The signal of LA was formed in the region between 1.30 and 1.35 ppm [25]. Other research reported that the lactic acid peaks were located at 1.42 or 1.44 ppm [26, 27]. The signals of glucose and LA were integrated based on α-glucosamine as the known reference. Bouteille et al. reported that the uncertainty of LA and sugar measurement by 1H NMR was smaller than 5.0% and 3.0%, respectively [27]. Gjersing et al. pointed out that the data collection time of 1H NMR measurement (20 min) was significantly reduced compared with HPLC (1 h) [23].
Optimization of conditions in the immobilization process
The experimental results were analyzed by Design Expert (Version 11) [28]. As shown in Table 2, the maximum and minimum LA yields of immobilized cells prepared by different schemes of conditions were 92.3% and 87.6%, respectively. The maximum yield was 4.7% higher than the minimum yield, and it was 0.9% higher than the scheme reaching second highest LA yield. Therefore, the appropriate scheme of conditions in the immobilization process plays a significant role in improving the fermentation yield of immobilized cells. According to the result, the optimum scheme of conditions in the immobilization process was A2–B1–C2–D3, which meant that the immobilized cell beads prepared by 2% sodium alginate (SA) and 6% polyvinyl alcohol (PVA), and immobilized by 0.10 M CaCl2 and 2.5% H3BO3 had the best fermentation performance. The optimal concentration of SA was 2%, which is consistent with the results of immobilized cell fermentation experiment developed by Goksungur and Guvenc [29]. Immobilized cell beads made from SA solution with a concentration lower than 1.5% are soft and easily broken due to low mechanical strength, and the overgrowth or expansion of the bead diameter might be caused in sugar solutions [30]. The glucose consumption and production rate will decrease if SA concentration is higher than 3%, and SA concentration higher than 6% leads to a decrease of cell activity [31]. The concentration of Ca2+ influences the mass transfer performance of immobilized cell beads [32]. During the immobilization process, the membrane thickness and compactness of the beads continue to increase with the consumption of calcium ions in the cross-link reaction, and this process stops only when the calcium ions are completely consumed [33]. The resistance formed by the dense membrane restricts the diffusion of substrates and nutrients into the beads, resulting in a decrease in yield and production rate [34, 35]. During the immobilization process, CaCl2 concentration is usually between 0.10 and 0.20 M to ensure the progress of the crosslinking reaction and to maintain good mass transfer performance of the beads [19, 34, 36]. When the concentration of sodium alginate is 2%, CaCl2 solution with a concentration of 0.10 M has less Ca2+ reacted with alginate, and less densely packed three-dimensional lattices are formed from the layer to the core of immobilized cell beads. These lattices facilitate the diffusion of substrates and nutrients in beads, thereby promoting the yield and production rate of the target product [34]. The cross-link reaction between PVA and H3BO3 with a suitable concentration can effectively increase the mechanical strength of the immobilized cell beads [37]. The optimum concentration was found as 6%, which agreed with results reported by Bhatnagar et al. and Wang et al. [38, 39]. Bhatnagar et al. observed that the agglomeration of beads occurs if the concentration of PVA is higher than 7%, while the disintegration occurs if the PVA concentration is lower than 5%. The H3BO3 solution with a concentration higher than 3% causes a decrease of the cell viability, so the H3BO3 solution with a concentration of 2–3% is usually chosen [38].
After 48 h of immobilized cell fermentation, the residual glucose concentration was less than 2.53 g/L, which was 5.06% to the initial glucose concentration with the LA yield higher than 0.876 g/g. Under the optimum conditions in the immobilization process, the glucose was consumed more rapidly, with the lowest concentration of 0.09 g/L, 0.18% to the initial glucose concentration. The amount of glucose consumed was slightly higher than the glucose used for LA production, suggesting that a small amount of glucose was utilized for cell growth in each batch during the fermentation process [40]. The density of viable cells in immobilized L. pentosus ATCC 8041 cell beads prepared under optimum conditions was obtained as 4.52 × 109 CFU/g (9.66 log CFU/g) beads, implying a high cell viability of immobilized L. pentosus ATCC 8041 cells prepared under optimum conditions [37].
The results of analysis of variance (ANOVA) were shown in Tables 3 and 4. The factor p-values of A, B, C, and D were less than 0.05. Therefore, the concentrations of SA, CaCl2, PVA, and H3BO3 could be considered to have significant influence on both LA yield and residual glucose concentration in immobilized L. pentosus ATCC 8041 cell fermentation [41].
The temperature effects on immobilized L. pentosus cell fermentation
Lactobacillus pentosus cells can grow and produce LA within a certain temperature range, but the growth and production rate were not the same [42]. As shown in Fig. 2, LA yield increased with increasing temperature in the range of 31–35 °C, and the highest LA yield of immobilized L. pentosus cells was obtained as 0.923 g/g in the batch at 35 °C, with the lowest residual glucose concentration of 0.09 g/L, 0.18% of residual glucose. The LA yield remained at 90.1% in the batch at 39 °C with a small amount of glucose consumption for cell growth, and the LA yield decreased slowly as temperature raised, suggesting that SA-PVA immobilized cell beads prepared under the optimum conditions had good heat resistance to tolerant and adapt to higher temperature [43].
Repeated batch fermentation
The repeated batch fermentation operated with immobilized cells as a cell-recycling system can increase the efficiency of the fermentation process by reducing the time of inoculum preparation [44]. As shown in Table 5, the LA yield in each batch was higher than 0.918 g/g with a productivity of 0.956 g × (L × h)−1 and residual glucose less than 0.26%. The average LA yield in 15 batches was obtained as 0.923 g/g with an average productivity of 0.961 g × (L × h)−1, 0.4% lower than the highest LA yield and productivity, suggesting that the immobilized cells prepared under optimum conditions maintained stable and efficient performance in repeated batch fermentation [45]. All immobilized cell beads remained intact during 15 batches, indicating that the excellent strength of immobilized cell beads prepared under optimum conditions avoided the breakage caused by cell growth and agitation during fermentation process [46].
The comparison of fermentation performance between immobilized L. pentosus cells and free L. pentosus cells
As shown in Fig. 3, after 72 h of free cell fermentation, the LA yield was 0.810 g/g with a residual glucose concentration of 0.62% to initial glucose and a productivity of 0.563 g × (L × h)−1. The fermentation period of immobilized cells was shortened to about 48 h, and the LA yield was 0.923 g/g with the residual glucose concentration of 0.17% to initial glucose and a productivity of 0.961 g × (L × h)−1. When the residual glucose concentration was not significantly different, the LA production of immobilized cell beads prepared under the optimum conditions was 11.3% higher than that of free cell fermentation with 33.3% of fermentation time reduction and 70.7% of productivity improvement [47]. In the fermentation process, the maximum production rate of immobilized cells was higher than free cells and was reached immediately because of a higher cell density after the start of fermentation [48, 49]. The same result was reported by Vatakit and Leenanon, observing that encapsulated L. pentosus cells using fermented purple glutinous rice beverage had the higher cell counts in comparison with free cells [50]. In the initial stage of free cell fermentation, cell growth was dominant, so the LA yield was lower, and the period of LA fermentation was longer [51]. In the immobilized cell fermentation process, cell growth was limited by the space and oxygen/nutrient diffusion, resulting in a higher glucose consumption in lactic acid production to obtain a higher LA yield [52]. Compared to free cell fermentation where the LA yield and productivity were lower because of a higher proportion of glucose utilized for cell growth in a longer period, the LA yield and productivity was improved in immobilized cell fermentation [53].
Conclusion
The optimum conditions for preparing immobilized L. pentosus cells
The immobilized L. pentosus cells prepared by 2% sodium alginate (SA) and 6% polyvinyl alcohol (PVA), and immobilized by 0.10 M CaCl2 and 2.5% H3BO3 have the best performance of lactic acid (LA) yield in the fermentation process, with an excellent strength to avoid the breakage caused by cell growth and agitation, which can also maintain the stable and efficient performance in repeated batch fermentation.
The temperature effects on LA yield in the immobilized L. pentosus cell fermentation
The highest LA yield of immobilized L. pentosus fermentation is obtained at 35 ℃. The immobilized cells prepared under the optimum conditions have good heat resistance to maintain a high LA yield at higher temperature.
The comparison of fermentation performance between immobilized L. pentosus cells and free L. pentosus cells
Compared with the free cell fermentation of L. pentosus, a higher LA yield can be reached in a significantly shortened period during the immobilized L. pentosus cell fermentation process under the same fermentation conditions.
The contents in this paper are the results of independent research. All data and pictures are authentic and reliable. In addition to the references already indicated in the text, the research results of this paper do not include any content of copyright enjoyed by others. Other individuals and groups contributing to the research work involved in this paper have been clearly identified in this paper. This research does not involve human participants or animals. The publication of the paper has been agreed by the authors and does not involve potential conflicts of interest. I am fully aware of the legal liability of this statement.
References
Chibata I, Wingard LB (2014) Immobilized microbial cells: applied biochemistry and bioengineering, vol 4. Elsevier, New York
Tao XQ, Lu GN, Liu JP, Li T, Yang LN (2009) Rapid degradation of phenanthrene by using Sphingomonas sp. GY2B immobilized in calcium alginate gel beads. Int J Environ Res Public Health 6(9):2470–2480
Kong HJ, Smith MK, Mooney DJ (2003) Designing alginate hydrogels to maintain viability of immobilized cells. Biomaterials 24(22):4023–4029
Grasdalen H, Larsen B, Smisrod O (1981) 13C-NMR studies of monomeric composition and sequence in alginate. Carbohyd Res 89(2):179–191
Lee KY, Mooney DJ (2012) Alginate: properties and biomedical applications. Prog Polym Sci 37(1):106–126
Tang Y, Pang L, Wang D (2017) Preparation and characterization of borate bioactive glass cross-linked PVA hydrogel. J Non-Cryst Solids 476:25–29
Zhan J, Jiang S, Pan L (2013) Immobilization of phospholipase A1 using a polyvinyl alcohol-alginate matrix and evaluation of the effects of immobilization. Braz J Chem Eng 30(4):721–728
Xue L, Huang Z, Luo Z, Cao C, Chen Y, Chen R (2009) Optimization of the techniques of S. cerevisiae immobilization by sodium alginate and PVA. Liquor Mak Sci Technol 2:27–30
Wang H, Seki M, Furusaki S (1995) Mathematical model for analysis of mass transfer for immobilized cells in lactic acid fermentation. Biotechnol Prog 11(5):558–564
Vijayakumar J, Aravindan R, Viruthagiri T (2008) Recent trends in the production, purification and application of lactic acid. Chem Biochem Eng Q 22(2):245–264
Bustos G, Moldes AB, Cruz JM, Domínguez JM (2005) Influence of the metabolism pathway on lactic acid production from hemicellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol Prog 21(3):793–798
Garde A, Jonsson G, Schmidt AS, Ahring BK (2002) Lactic acid production from wheat straw hemicellulose hydrolysate by Lactobacillus pentosus and Lactobacillus brevis. Biores Technol 81(3):217–223
Kourkoutas Y, Xolias V, Kallis M, Bezirtzoglou E, Kanellaki M (2005) Lactobacillus casei cell immobilization on fruit pieces for probiotic additive, fermented milk and lactic acid production. Process Biochem 40(1):411–416
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31(6):877–902
Taguchi G, Yokoyama Y (1993) Taguchi methods: design of experiments, vol 4. Amer Supplier Institute, Cairo
Khalid I, Ahmad M, Minhas MU, Barkat K (2018) Preparation and characterization of alginate-PVA-based semi-IPN: controlled release pH-responsive composites. Polym Bull 75(3):1075–1099
Zhu GL, Hu YY, Wang QR (2009) Nitrogen removal performance of anaerobic ammonia oxidation co-culture immobilized in different gel carriers. Water Sci Technol 59(12):2379–2386
García EA, Montoro BP, Benomar N, Castillo-Gutiérrez S, Estudillo-Martínez MD, Knapp CW, Abriouel H (2019) New insights into the molecular effects and probiotic properties of Lactobacillus pentosus pre-adapted to edible oils. LWT 109:153–162
Lee KH, Choi IS, Kim YG, Yang DJ, Bae HJ (2011) Enhanced production of bioethanol and ultrastructural characteristics of reused Saccharomyces cerevisiae immobilized calcium alginate beads. Biores Technol 102(17):8191–8198
Bubb WA (2003) NMR spectroscopy in the study of carbohydrates: characterizing the structural complexity. Concepts Magn Reson Part A Educ J 19(1):1–19
Holzgrabe U (2010) Quantitative NMR spectroscopy in pharmaceutical applications. Prog Nucl Magn Reson Spectrosc 57(2):229–240
Sanders ER (2012) Aseptic laboratory techniques: plating methods. J Vis Exp 63:e3064
Gjersing E, Happs RM, Sykes RW, Doeppke C, Davis MF (2013) Rapid determination of sugar content in biomass hydrolysates using nuclear magnetic resonance spectroscopy. Biotechnol Bioeng 110(3):721–728
Mittal A, Scott GM, Amidon TE, Kiemle DJ, Stipanovic AJ (2009) Quantitative analysis of sugars in wood hydrolyzates with 1H NMR during the autohydrolysis of hardwoods. Biores Technol 100(24):6398–6406
Buyondo JP (2013) Kinetic studies of lactic acid production from wood extract hydrolysate via batch and continuous fermentation processes. State University of New York College of Environmental Science and Forestry
Nord LI, Vaag P, Duus JØ (2004) Quantification of organic and amino acids in beer by 1H NMR spectroscopy. Anal Chem 76(16):4790–4798
Bouteille R, Gaudet M, Lecanu B, This H (2013) Monitoring lactic acid production during milk fermentation by in situ quantitative proton nuclear magnetic resonance spectroscopy. J Dairy Sci 96(4):2071–2080
Arabi M, Ghaedi M, Ostovan A, Tashkhourian J, Asadallahzadeh H (2016) Synthesis and application of molecularly imprinted nanobeads combined ultrasonic assisted for highly selective solid phase extraction trace amount of celecoxib from human plasma samples using design expert (DXB) software. Ultrason Sonochem 33:67–76
Goksungur Y, Guvenc U (1999) Production of lactic acid from beet molasses by calcium alginate immobilized Lactobacillus delbrueckii IFO 3202. J Chem Technol Biotechnol 74(2):131–136
Gilson CD, Thomas A (1995) Ethanol production by alginate immobilised yeast in a fluidised bed bioreactor. J Chem Technol Biotechnol Int Res Process Environ Clean Technol 62(1):38–45
Najafpour G, Younesi H, Ismail KSK (2004) Ethanol fermentation in an immobilized cell reactor using Saccharomyces cerevisiae. Biores Technol 92(3):251–260
Chrastil J (1991) Gelation of calcium alginate. Influence of rice starch or rice flour on the gelation kinetics and on the final gel structure. J Agric Food Chemistry 39(5):874–876
Blandino A, Macias M, Cantero D (1999) Formation of calcium alginate gel capsules: influence of sodium alginate and CaCl2 concentration on gelation kinetics. J Biosci Bioeng 88(6):686–689
Idris A, Suzana W (2006) Effect of sodium alginate concentration, bead diameter, initial pH and temperature on lactic acid production from pineapple waste using immobilized Lactobacillus delbrueckii. Process Biochem 41(5):1117–1123
Bandi S, Kunamneni A, Polur E (2003) Investigation on neomycin production with immobilized cell of Streptomyces marinensis Vuv-5 in calcium alginate matrix. AAP Pharm Sci Tech 20:220
Seifan M, Samani AK, Hewitt S, Berenjian A (2017) The effect of cell immobilization by calcium alginate on bacterially induced calcium carbonate precipitation. Fermentation 3(4):57
Tian X, Liao Q, Liu W, Wang YZ, Zhu X, Li J, Wang H (2009) Photo-hydrogen production rate of a PVA-boric acid gel granule containing immobilized photosynthetic bacteria cells. Int J Hydrogen Energy 34(11):4708–4717
Bhatnagar Y, Singh GB, Mathur A, Srivastava S, Gupta S, Gupta N (2016) Biodegradation of carbazole by Pseudomonas sp. GBS. 5 immobilized in polyvinyl alcohol beads. J Biochem Technol 6(3):1003–1007
Wang P, Chen Z, Li J, Wang L, Gong G, Zhao G, Liu H, Zheng Z (2013) Immobilization of Rhizopus oryzae in a modified polyvinyl alcohol gel for L (+)-lactic acid production. Ann Microbiol 63(3):957–964
Jamali NS, Rashidi NFD, Jahim JM, Sompong O, Jehlee A, Engliman NS (2019) Thermophilic biohydrogen production from palm oil mill effluent: effect of immobilized cells on granular activated carbon in fluidized bed reactor. Food Bioprod Process 117:231–240
Sakoda JM, Cohen BH, Beall G (1954) Test of significance for a series of statistical tests. Psychol Bull 51(2):172
Delgado A, Brito D, Peres C, Noe-Arroyo F, Garrido-Fernández A (2005) Bacteriocin production by Lactobacillus pentosus B96 can be expressed as a function of temperature and NaCl concentration. Food Microbiol 22(6):521–528
Liu J, Pan D, Wu X, Chen H, Cao H, Li QX, Hua R (2018) Enhanced degradation of prometryn and other s-triazine herbicides in pure cultures and wastewater by polyvinyl alcohol-sodium alginate immobilized Leucobacter sp. JW-1. Sci Total Environ 615:78–86
Kurade MB, Waghmode TR, Xiong J-Q, Govindwar SP, Jeon B-H (2019) Decolorization of textile industry effluent using immobilized consortium cells in upflow fixed bed reactor. J Clean Prod 213:884–891
Watanabe I, Miyata N, Ando A, Shiroma R, Tokuyasu K, Nakamura T (2012) Ethanol production by repeated-batch simultaneous saccharification and fermentation (SSF) of alkali-treated rice straw using immobilized Saccharomyces cerevisiae cells. Biores Technol 123:695–698
John RP, Nampoothiri KM, Pandey A (2007) Production of L (+) lactic acid from cassava starch hydrolyzate by immobilized Lactobacillus delbrueckii. J Basic Microbiol 47(1):25–30
Wang Z, Wang Y, Yang ST, Wang R, Ren H (2010) A novel honeycomb matrix for cell immobilization to enhance lactic acid production by Rhizopus oryzae. Biores Technol 101(14):5557–5564
Senthuran A, Senthuran V, Mattiasson B, Kaul R (1997) Lactic acid fermentation in a recycle batch reactor using immobilized Lactobacillus casei. Biotechnol Bioeng 55(6):841–853
Brodelius P (1985) The potential role of immobilization in plant cell biotechnology. Trends Biotechnol 3(11):280–285
Vatakit T, Leenanon B (2017) Effects of prebiotics and cells encapsulation on survivability of Lactobacillus pentosus PR01 in probiotic fermented purple glutinous rice beverage. Asian J Microbiol Biotechnol Environ Exp Sci 19:1–10
Maier RM, Pepper IL (2015) Bacterial growth. Environmental microbiology. Elsevier, Oxford, pp 37–56
Shinmyo A, Kimura H, Okada H (1982) Physiology of α-amylase production by immobilized Bacillus amyloliquefaciens. Eur J Appl Microbiol Biotechnol 14(1):7–12
Chen CC, Lan CC, Pan CL, Huang MY, Chew CH, Hung CC, Chen PH, Lin HTV (2019) Repeated-batch lactic acid fermentation using a novel bacterial immobilization technique based on a microtube array membrane. Process Biochem 20:20
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no potential conflict of interest.
Ethical standards
The contents in this paper are the results of independent research. All data and pictures are authentic and reliable. In addition to the references already indicated in the text, the research results of this paper do not include any content of copyright enjoyed by others. Other individuals and groups contributing to the research work involved in this paper have been clearly identified in this paper. This research does not involve human participants or animals. The publication of the paper has been agreed by the authors and does not involve potential conflicts of interest. I am fully aware of the legal liability of this statement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, J., Huang, J., Guo, H. et al. Optimization of immobilization conditions for Lactobacillus pentosus cells. Bioprocess Biosyst Eng 43, 1071–1079 (2020). https://doi.org/10.1007/s00449-020-02305-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-020-02305-9