Abstract
The objective of this study was to evaluate ethanol production and bioadsorption with four red seaweeds, Gelidium amansii, Gracilaria verrucosa, Kappaphycus alvarezii and Eucheuma denticulatum. To produce ethanol, thermal acid hydrolysis, enzymatic saccharification and fermentation was carried out. After pretreatment, 38.5, 39.9, 31.0 and 27.5 g/L of monosaccharides were obtained from G. amansii, G. verrucosa, K. alvarezii and E. denticulatum, respectively. Ethanol fermentation was performed with Saccharomyces cerevisiae KCCM 1129 adapted to 80 g/L galactose. The ethanol productions by G. amansii, G. verrucosa, K. alvarezii and E. denticulatum were 18.8 g/L with Y EtOH = 0.49, 19.1 g/L with Y EtOH = 0.48, 14.5 g/L with Y EtOH = 0.47 and 13.0 g/L with Y EtOH = 0.47, respectively. The waste seaweed slurries after the ethanol fermentation were reused to adsorb Cd(II), Pb(II) and Cu(II). Using langmuir isotherm model, Cu(II) had the highest affinity for waste seaweeds with the highest q max and electronegativity values among three heavy metals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Currently, world faces global warming and climate change due to the exhaustion of fossil fuels. Biofuels including bioethanol, biodiesel, biohydrogen and biogas represent alternative transportation fuels [1]. Among bioenergy sources, bioethanol is a promising transportation fuel because it can replace fossil fuels and produce sustainable and eco-friendly energy using a closed CO2 circulation system.
Corn was used as the first generation of biomass for the production of bioethanol. Lignocellulosic biomass has been used as a second-generation source to produce bioethanol. However, both of these biomasses have problems. Corn has moral problems related with its use as food and lignocellulosic biomass tends to require complex pretreatment due to lignin, which is difficult to degrade [2]. Seaweed as a third-generation biomass has a high growth rate, low land usage, high CO2 absorption and has no competition with food resources [3].
Seaweeds are classified into three groups by color: red, brown, and green. Carbohydrate is the main source for ethanol production. Among seaweeds, red seaweeds have the highest carbohydrate contents. Thus, four red seaweeds, Gelidium amansii, Gracilaria verrucosa, Kappaphycus alvarezii and Eucheuma denticulatum were used in this study. Red seaweed consists basically of agar, cellulose, xylane, mannan, and carrageenan. Among these, agar, carrageenan and cellulose are polysaccharides that contain galactose and glucose [4].
Almost all kinds of red seaweed can be converted to bioethanol by degrading their polysaccharides into monosaccharides (galactose and glucose), followed by fermentation with suitable yeasts. However, glucose from hydrolysates causes the repression of galactose uptake. The repression decreases the yield of ethanol production. In order to overcome the repression, many studies have developed the yeast by adaptation. Cho et al. [5] developed the Saccharomyces cerevisiae KCCM 1129 by yeast adaptation with high concentration of galactose to produce ethanol from G. amansii. The final ethanol yield (Y EtOH) was increased to a high level of 0.44. Another study used the adaptation of S. cerevisiae to high concentration of galactose to improve the efficiency of the ethanol fermentation using mixed monosaccharides from G. verrucosa [6]. The ethanol concentration was increased from 13.4 g/L with wild S. cerevisiae to 18.3 g/L with adapted S. cerevisiae.
Heavy metals—such as Cd(II), Pb(II), and Cu(II)—are released into the environment from many sources. Characteristics of metal ions include mobility in ecosystems, and due to their toxicity, metal ions are considered priority pollutants [7]. Also heavy metals can accumulate in organisms and cause various diseases. Potential health effects of Cd(II), Pb(II) and Cu(II) include allergic dermatitis, liver and kidney damages and high blood pressure [8]. Thus, it is necessary to remove heavy metals from the environment. The cell wall of seaweed which has negative charge can act as cationic exchangers in the metal adsorption mechanism. Thus, seaweeds could be good adsorbents due to electrostatic attraction and complexation properties of their cell walls [9]. In this study, four red seaweeds, G. amansii, G. verrucosa, K. alvarezii and E. denticulatum, were used as adsorbents after the production of bioethanol.
In this study, bioethanol was produced from four seaweeds using thermal acid hydrolysis, enzymatic saccharification and fermentation. The four waste red seaweeds were then reused to bind Cd(II), Pb(II) and Cu(II) using various slurry contents. Bioadsorption isotherms were constructed with the Langmuir model to compare the Cd(II), Pb(II) and Cu(II) bioadsorption capacities of the bioadsorbents.
Materials and methods
Raw materials and composition analysis
Gelidium amansii and G. verrucosa were obtained from Gijang, Busan and Wando, Jeonnam, Korea, respectively. K. alvarezii and E. denticulatum was obtained from Biolsystems Co., Ltd., Goheung, Jeonnam, Korea. The seaweeds were dried under sunlight and ground using a hammer mill. Ground seaweeds were filtered through a 355 μm (45-mesh) sieve prior to pretreatment. The protein, lipid, ash and carbohydrate contents of the seaweeds were analyzed by the Feed and Foods Nutrition Research Center at Pukyung National University in Busan, Korea, according to the AOAC method [10].
Thermal acid hydrolysis and enzymatic saccharification
Seaweed 8 % (w/v) was subjected to thermal acid hydrolysis. Various acid concentrations and treatment times were used to optimize the thermal acid hydrolysis conditions. The seaweed was hydrolyzed using 30–110 mM H2SO4 and 30–120 min treatment time at 121 °C. Hydrolysates were adjusted to pH 5.0 with NaOH.
Saccharification was conducted by the addition of 16 units/mL of mixed enzymes, using commercial cellulase, Celluclast 1.5 L (8.4 EGU/mL, Novozymes, Bagsvaerd, Denmark), and a mixture of cellulase and β-glucanase, Viscozyme L (1.2 FBG/mL, Novozymes, Bagsvaerd, Denmark). Saccharification was carried out with 100 mL of seaweed slurry at 45 °C and 150 rpm for 48 h. Samples were taken and monosaccharide and hydroxyl methyl furfural (HMF) concentrations were determined by HPLC. The efficiencies (E PS) of pretreatment using thermal acid hydrolysis and saccharification with enzymes were calculated as follows:
where E PS is the efficiency of pretreatment and saccharification (%), ΔS PS is the increase in monosaccharide content (g/L) during pretreatment and saccharification, and TC is the total carbohydrate (g/L) in G. amansii, G. verrucosa, K. alvarezii or E. denticulatum [11].
Adapted yeast and ethanol fermentation
The seaweed hydrolysate pretreated under optimal conditions was fermented using S. cerevisiae KCTC 1126. Wild-type S. cerevisiae was cultured on YPD agar plates (10 g/L yeast extract, 20 g/L peptone, 20 g/L glucose and 5 g/L agar). A yeast colony from the agar plates was inoculated and cultured at 30 °C, 150 rpm for 24 h with 30 mL of YPD medium (containing 10 g/L yeast extract, 20 g/L peptone and 20 g/L glucose). Galactose adaptation was carried out using S. cerevisiae cultured on YPG medium containing 10 g/L yeast extract, 20 g/L peptone and 20 g/L galactose. After 30 h, 3 mL of yeast was inoculated into 30 mL of various YPHG medium composed of 10 g/L yeast extract, 20 g/L peptone and various galactose concentrations (40–120 g/L) to obtain the optimal YPHG medium. Each S. cerevisiae adapted to high concentration of galactose were cultured at 30 °C, 150 rpm for 30 h [6, 12].
Fermentation was carried out in an Erlenmeyer flask containing 100 mL of seaweed hydrolysate with the adapted yeast, at a cell density of 0.35 g dcw/L. The seaweed hydrolysates were fermented at 30 °C and 200 rpm for 5 days. Samples were taken periodically for analysis. The yield of ethanol production (Y EtOH) was calculated as follows:
where Y EtOH is the ethanol production yield (g/g), EtOHmax is the maximum ethanol concentration obtained during fermentation (g/L) and ΔS PS is the total fermentable monosaccharide content increase (g/L) after pretreatment and saccharification [10]. The maximum Y EtOH is 0.51 by the theoretical calculation of 1 mol of glucose conversion to 2 mol of ethanol.
Bioadsorption
After fermentation, fermented seaweeds were centrifuged (388×g, 10 min) and freeze–dried for 3 days. Dried slurries were ground with a hammer mill and filtered through a 355 μm (45-mesh) sieve prior to bioadsorption. Standard solutions of Cd(II), Pb(II) and Cu(II) were prepared and diluted to 50 ppm. Each solution was adjusted to pH 5 using 5 N NaOH, the optimal pH for bioadsorption [12].
Bioadsorption was carried out using various slurry contents (mg/L) of the four waste seaweeds, added to 100 mL of neutralized metal solutions. The mixtures were agitated at 30 °C and 150 rpm for 2 h. Slurries were centrifuged and filtered for analysis using ICP-MS. Samples were taken periodically for the determination of heavy metal concentrations. The bioadsorption ratio (%) was calculated as follows:
where C f is the final heavy metal concentration in solution (mg/L) and C i is the initial heavy metal concentration in solution (mg/L) [13].
The mechanism of bioadsorption was explained using Langmuir isotherms. The Langmuir model is defined in the following equation:
where q max is the bioadsorption capacity of the adsorbent (mg/g), K L is the Langmuir bioadsorption constant (L/mg), C e is the equilibrium metal ion concentration in solution (mg/L), q e is the equilibrium metal ion concentration on the adsorbent (mg/g) [14].
Analytical method
Enzyme activities were determined by the procedures described by Mandels et al. [14] and Kubicek et al. [16]. The concentrations of monosaccharides including glucose and galactose, 5-hydroxymethylfurfural (5-HMF) and ethanol were determined by HPLC (Agilent 1100 Series, Agilent. Inc., Santa Clara, CA, USA) equipped with a refractive index detector (RID). The Bio-Rad Aminex HPX-87H column (300.0 × 7.8 mm) was maintained at 65 °C and concentrations and components of samples were determined using 5 mM H2SO4 at 0.6 mL/min and a column temperature of 65 °C.
Concentrations of Cd(II), Pb(II) and Cu(II) were determined by ICP-MS (Perkin Elmer, Shelton, USA, Optima 7300DV, Nexion 300D) with an electron multiplier detector. The RF power of ICP MS was 1300 W with 18.3 L/min plasma gas flow rate, 0.97 L/min nebulizer gas flow rate and 1.2 L/min auxiliary gas flow rate [17].
Results and discussion
Composition of the four red seaweeds
The compositions of G. amansii, G. verrucosa, K. alvarezii and E. denticulatum were analyzed by the AOAC method. The total carbohydrate contents were 74.4, 67.0, 72.7 and 64.7 % (w/v), respectively (Table 1). Red seaweed has a 20–30 % greater carbohydrate content than brown and green seaweeds [18]. Thus, red seaweeds were used to produce ethanol in this study.
Thermal acid hydrolysis
The thermal acid hydrolysis of seaweeds with sulfuric acid increases the monosaccharide contents of the degraded from biomass, giving higher sugar yields than that without sulfuric acid treatment [19]. Thus, various sulfuric acid concentrations and pretreatment times were examined using 8 % (w/v) seaweed slurry contents to determine the optimal pretreatment conditions. The effects of sulfuric acid concentrations and pretreatment times are shown as concentrations of monosaccharide after thermal acid hydrolysis in Fig. 1a, b. Optimal sulfuric acid concentrations for of G. amansii, G. verrucosa, K. alvarezii and E. denticulatum were 50, 70, 90 and 90 mM, respectively (Fig. 1a). Pretreatment of biomass with high concentrations of acid has been reported to produce high concentrations of monosaccharide [20]. However, each of the seaweeds had an optimal acid concentration among 50–90 mM to obtain the maximum monosaccharide yield. The optimal thermal acid hydrolysis time (30–120 min) was determined using the optimal sulfuric acid concentration. As shown in Fig. 1b, the four seaweeds had the highest monosaccharide concentrations at 90 min of treatment time. Following thermal acid hydrolysis, the monosaccharide concentration of G. amansii was 25.0 g/L galactose, that of G. verrucosa was 29 g/L, containing 23.8 g/L galactose and 5.2 g/L glucose, that of K. alvarezii was 23 g/L, with 20.5 g/L galactose and 2.5 g/L glucose and that of E. denticulatum was 22.8 g/L monosaccharide, with 19.3 g/L galactose and 3.5 g/L glucose. Most of the monosaccharide was galactose, derived from agar. The structure of agar is softer than that of cellulose; thus agar is hydrolyzed by the acid more readily [16].
Enzymatic saccharification
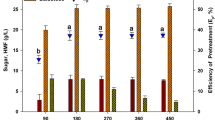
After thermal acid hydrolysis of the four seaweeds, enzymatic saccharification was performed to increase the monosaccharide concentration before fermentation. Enzymatic saccharification was used to hydrolyze the glucans in the red seaweeds [21]. Also, Ahn et al. [22] reported that a mixture of enzymes had a synergistic effect and yielded higher glucose content than a single enzyme. Thus, enzymatic saccharification was carried out using a mixture of Celluclast 1.5 L and Viscozyme L, which consist of with cellulase, β-glucanase and xylanase for 48 h. As shown in Fig. 1c, the effects of enzymatic saccharification were seen as an increase in glucose concentration. Glucose concentration did not increase beyond a 48 h of reaction time. Thus, the optimum enzymatic saccharification time was determined to be 48 h. From enzymatic saccharification, 13.53, 10.93, 8.03 and 4.7 g/L glucose were produced from thermal acid hydrolysates of 8 % (w/v) G. amansii, G. verrucosa, K. alvarezii and E. denticulatum by extracting glucose concentrations from thermal acid hydrolysis, respectively. Thus, total glucose from 8 % (w/v) G. amansii, G. verrucosa, K. alvarezii and E. denticulatum after thermal acid hydrolysis and enzymatic saccharification was 13.57, 16.13, 10.53 and 8.2 g/L, respectively. G. verrucosa obtained the highest glucose concentration among four red seaweeds.
Following thermal acid hydrolysis and enzymatic saccharification using the optimal conditions for the each seaweed, the concentrations of monosaccharides were determined. A summary of the pretreatment conditions is shown in Table 2. After pretreatment with thermal acid hydrolysis and enzymatic saccharification, total monosaccharide concentrations for G. amansii, G. verrucosa, K. alvarezii and E. denticulatum were 38.53, 39.93, 31.01 and 27.49 g/L from total carbohydrates of 59.52, 53.56, 58.12 and 51.76 g/L, respectively. Thus, the E PS values of G. amansii, G. verrucosa, K. alvarezii and E. denticulatum were 64.73, 74.55, 53.35 and 53.11 %, respectively. Among the four seaweeds, G. verrucosa produced the highest monosaccharide level from thermal acid hydrolysis and enzymatic saccharification. Thus, G. verrucosa would be expected to produce the highest concentration of ethanol using this highest concentration of monosaccharides.
Fermentation
Fermentation was carried out with S. cerevisiae adapted to a high concentration of galactose. Generally, wild-type S. cerevisiae can take up both glucose and galactose. However, S. cerevisiae has the preferences to glucose over galactose [23]. Due to the preference to glucose over other monosaccharides, S. cerevisiae decreases ethanol yield. In many previous studies on mixed sugar fermentation, the yeast has been improved by adaptation to high concentrations of monosaccharide to enhance ethanol production [5, 11, 23]. Thus, S. cerevisiae was adapted to increase galactose uptake and in turn enhanced ethanol production. As shown in Fig. 2, YPHG medium with 80 and 120 g/L galactose showed the highest cell growth. Previous study reported that YPHG medium containing 120 g/L galactose to be optimum concentration for the adaptation of S. cerevisiae [5]. However, YPHG medium containing 80 and 120 g/L galactose showed similar yeast growth as 6.40 and 6.41 g dcw/L, respectively. Thus, YPHG medium containing 80 g/L galactose was selected as an optimal adaptation concentration for S. cerevisiae.
Fermentations using the adapted S. cerevisiae with four 8 % (w/v) hydrolyzed red seaweeds were carried out for 6 days. As shown in Fig. 3a, G. amansii was fermented with 25 g/L galactose and 13.5 g/L glucose by S. cerevisiae adapted to YPHG including 80 g/L galactose. Glucose was totally consumed during the initial 24 h and galactose was used gradually until 72 h the ethanol production level was 18.74 g/L with a Y EtOH of 0.49. According to a previous study, G. amansii with wild-type S. cerevisiae exhibited a Y EtOH value of 0.38 [24]. These results indicate that S. cerevisiae adapted to a high concentration of galactose showed good ethanol production performance. The ethanol fermentation by G. verrucosa and the highest monosaccharide concentrations is shown in Fig. 3b. G. verrucosa hydrolysate contained various concentrations of mixed sugars, 23.8 g/L galactose and 16.1 g/L glucose. Successful utilization of monosaccharides from G. verrucosa as a biomass requires efficient uptake of both sugars for high-yield conversion to ethanol. All of the glucose was taken up within in 24 h and the galactose was totally consumed within in 72 h. The final ethanol concentration was 19.1 g/L with a Y EtOH of 0.48. Kumar et al. [25] reported a Y EtOH of 0.43 for G. verrucosa with wild-type S. cerevisiae. S. cerevisiae adapted to a high concentration of galactose could use both glucose and glactose and exhibited a higher Y EtOH. The adapted S. cerevisiae consumed glucose by 48 h and galactose gradually until 72 h using hydrolysates of K. alvarezii and E. denticulatum as shown in Fig. 3c, d, respectively. The ethanol production with K. alvarezii and E. denticulatum was 14.5 g/L with a Y EtOH of 0.47 and 13.0 g/L with a Y EtOH of 0.47, respectively. Compared with previous reports, the ethanol yields (Y EtOH) of the adapted S. cerevisiae were higher than those of the wild-type S. cerevisiae, with a Y EtOH of 0.41 [26, 27].
During the ethanol fermentation using adapted S. cerevisiae and the four red seaweed hydrolysates, the concentration of HMF as an inhibitor compound decreased to near zero after 48 h, which corresponds well with the time point at which glucose was consumed completely (Fig. 3). Accordingly, this result suggests that S. cerevisiae KCTC 1126 converted HMF into a product, such as 2,5-bis-hydroxymethylfuran [11].
Due to the yeast adaptation, the ethanol production yield of G. amansii, G. verrucosa, K. alvarezii and E. denticulatum were ranging from 0.47 to 0.49. Comparing to previous studies [24–27] using wild-type S. cerevisiae, the yield of ethanol production from G. amansii, G. verrucosa, K. alvarezii and E. denticulatum were ranging from 0.38 to 0.47. These results indicate that adapted S. cerevisiae to a high concentration of galactose showed good ethanol production performance. Cho et al. [6] also reported that S. cerevisiae adapted to YPHG including 120 g/L galactose brings a higher Y EtOH and shorter fermentation time than non-adapted S. cerevisiae. Galactose has been utilized in S. cerevisiae through the Leloir pathway, in which galactose is converted to glucose-6-phosphate by the enzymes [28, 29]. These enzymes are encoded by a GAL gene family. In wild S. cerevisiae, GAL gene family repressed by glucose phosphorylation pathway, thus galactose consumption and metabolic rates were decreased by the presence of glucose [11]. Previous study [6] reported that in the adapted S. cerevisiae, expression of GAL gene family was increased due to decreased transcript levels of repressor gene products. Thus, the adaptation of yeasts to galactose could enhance the expression of enzymes from the GAL gene family genetic network to ensure efficient sugar metabolism for the uptake of galactose and reduce the transcript levels of repressor genes.
Bioadsorption of heavy metals to four waste red seaweed slurries
Following the ethanol production, the wastes of the four red seaweeds were dried and ground for the adsorption of heavy metals. Slurries containing various levels (1–25 g/L) of waste seaweed, were used to adsorb 50 ppm Cd(II), Pb(II) and Cu(II) solutions. Bioadsorption was carried out at pH 5 at 30 °C and 150 rpm for 2 h according to Murphy et al. [12].
As shown in Fig. 4, the bioadsorption ratio depended on the increase in biomass content. As shown in Fig. 4a, the four waste seaweed slurries bound Cd(II). The bioadsorption ratio increased with biomass content up to 20 g/L waste seaweed. Thus, the optimal biomass concentration was 20 g/L for bioadsorption of Cd(II) using the four seaweed slurries in aqueous solution. The bioadsorption ratio of Pb(II) increased as the waste biomass content increased (Fig. 4b). The four waste seaweed slurries showed similar trends in adsorbing Pb(II). The Pb(II) concentration did not increase further at >10 g/L biomass. The adsorption of Cu(II) to waste seaweed by the four waste seaweed slurries is shown in Fig. 4c. Bioadsorption ratios of Cu(II) were >90 % using >5 g/L waste seaweed slurries.
These trends of adsorption of heavy metals to waste seaweeds are expected because the availability of exchangeable sites for the heavy metal ions increases with increasing bioadsorbent content. However, bioadsorption efficiency did not increase over the optimal adsorbent contents [Cd(II): 20 g/L, Pb(II): 10 g/L and Cu(II): 5 g/L]. Ibrahim et al. [30] reported that this is attributable to the partial aggregation of biomass, which reduces the effective surface area for bioadsorption. Thus, the minimum concentrations of waste seaweed slurries for adsorbing 50 ppm of Cd(II), Pb(II) and Cu(II) were 20, 10 and 5 g/L, respectively. Cu(II) has the higher bioadsorption ratio compare to Cd(II) and Pb(II).
As shown in Fig. 5, bioadsorption isotherm was drawn to identify the surface properties and affinity of the waste biomass bioadsorbent. To compare the bioadsorption capacities of bioadsorbents for different heavy metals, the Langmuir isotherm model was used in this study. The Langmuir isotherm assumes that chemisorption occurs between the adsorbent and adsorbate by the formation of an ionic or covalent bond [31].
The equilibrium metal ion concentration in the solution (mg/L) (C e) and the equilibrium metal ion concentration on the adsorbent (mg/g) (q e) exhibited a linear relationship (Fig. 5). All cases showed linear relationships (r 2 = 0.92–0.99). Thus, the equilibrium data fitted the Langmuir model well [31].
Maximum adsorption capacities (q max), Langmuir bioadsorption constants (K L) and coefficients of determination (r 2) are shown in Table 3. The q max ranged from 2.19 mg/g to 11.43 mg/g. q max is a good measure for comparing different adsorbents for the same adsorbate. However, the waste seaweed slurries had similar q max values for the same heavy metal. Of the three heavy metals, copper showed the highest q max values of 10.98–11.43 mg/g and for cadmium and lead were 2.19–3.34 and 4.37–4.57 mg/g, respectively. This indicates that four waste seaweed slurries had higher uptake capacity to copper than cadmium and lead. Many studies of bioadsorbents for removal of cadmium, lead and copper have been reported [32–34]. Yu et al. [32] reported that q max values for a group of nine seaweeds to metal ions were; cadmium: 0.8–1.2 mmol/g, lead: 1.10–1.6 mmol/g and copper: 1.0–1.2 mmol/g. Comparing to those q max of nine seaweeds, q max in this study showed; cadmium: 2.19–3.34 mg/g, lead: 4.37–4.57 mg/g and copper: 10.98–11.43 mg/g. The bioadsorption capacities of the four waste seaweed slurries for the three heavy metals were higher than those of dried biomass that had not been used for ethanol production. Thus, the four red seaweed wastes have considerable potential for the removal of cadmium, lead and copper from industrial waste water.
Biosorption in algae has mainly been attributed to sulphate groups on the seaweed where both electrostatic attraction and complexation can play a role [33]. The tendency towards ionic bonding between atoms increases with increasing difference in the electronegativity of the atoms. Electronegativity is a property which is the measure of atomic power in a molecule to attract electrons [34]. The electronegative values of cadmium, lead and copper are 1.7, 1.7 and 1.90, respectively [35]. Thus, copper which has the highest electronegativity among the three heavy metals had the strongest affinity with the waste seaweed bioadsorbents. Characteristics of the waste seaweed after the ethanol production make the seaweed potentially excellent heavy metals bioadsorbent from industrial waste water.
Conclusion
Gelidium amansii, G. verrucosa, K. alvarezii and E. denticulatum were used as biomasses and adsorbents. Bioethanols of 18.7, 19.1, 14.5 and 13.0 g/L were produced from the monosaccharides obtained from thermal acid hydrolysis and enzymatic saccharification of G. amansii, G. verrucosa, K. alvarezii and E. denticulatum using S. cerevisiae adapted to 80 g/L galactose, respectively. After ethanol fermentation, the seaweeds were reused for the adsorption of heavy metals. Cu(II) had the highest q max values (11.0–11.4 mg/g) with the four waste seaweeds, due to a higher electronegativity than Pb(II) and Cd(II). These results, suggest that red seaweed slurries can be good biomasses for bioethanol production and bioadsorption to remove heavy metals from industrial waste water.
References
Hoekman SK (2009) Biofuels in the US-challenges and opportunities. Renew Energy 34:14–22
Balat M (2010) Production of bioethanol form lignocellulosic materials via the biochemical pathway: a review. Energy Convers Manag 52:858–875
Singh A, Nigam PS, Murphy JD (2011) Renewable fuels from algae: an answer to debatable land based fuels. Bioresour Technol 102:10–16
Fu XT, Kim SM (2010) Agarase: review of major sources, categories, purification method, enzyme characteristics and applications. Mar Drugs 8:200–218
Cho HY, Ra CH, Kim SK (2014) Ethanol production from the seaweed Gelidium amansii, using specific sugar acclimated yeasts. J Microbiol Biotechnol 24:264–269
Ra CH, Kim YJ, Lee SY, Jeong GT, Kim SK (2015) Effects of galactose adaptation in yeast for ethanol fermentation from red seaweed, Gracilaria verrucosa. Bioprocess Biosyst Eng 38:1715–1722
Demirbas A (2008) Heavy metal adsorption onto agro-based waste materials: a review. J Hazard Mater 157:220–229
Ahmaruzzaman M (2011) Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv Collloid Interface Sci 116:36–59
Adel-Ghani NT, El-Chaghaby GA (2014) Biosorption for metal ions removal from aqueous solutions: a review of recent studies. Int J Latest Res Sci Technol 3:24–42
Sanchez-Machado DI, Lopez-Cervantes J, Paserio-Losada P, Lopez-Hernandez J (2004) Fatty acid, total liqid, proten and ashcontents of processed edible seaweed. Food Chem 85:439–444
Ra CH, Jeong GT, Shin MK, Kim SK (2013) Biotransformation of 5-hydroxymethylfurfural (HMF) by Scheffersomyces stipites during ethanol fermentation of hydrolysate of the seaweed Gelidium amansii. Bioresour Technol 140:421–425
Murphy V, Hughes H, McLoughlin P (2007) Cu(II) binding by dried biomass of red, green and brown macroalgae. Water Res 41:731–740
Vinod VTP, Sashidhar RB (2011) Bioremediation of industrial toxic metals with gum kondagogu (Cochlospermum gossypium): a natural carbohydrate biopolymer. Indian J Biotechnol 10:113–120
Langmuir I (1918) The adsorption of gases on plane surfaces of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc 40:1361–1403
Mandels M, Andreoti R, Roche C (1976) Measurement of saccharifying cellulose. Biotechnol Bioeng Symp 6:21–23
Kubicek CP (1982) β-Glucosidase excretion by Trichoderma pseudokoningii correlation with cell wall bound β-1,3-glucanase activities. Arch Microbiol 132:349–354
Sunwoo IY, Ra CH, Kown SJ, Heo JH, Kim YJ, Kim JW, Shin JH, Ahn EJ, Cho YK, Kim SK (2014) Ethanol production from red, brown and green seaweeds and biosorption of heavy metals by waste seaweed slurry from ethanol production. KSBB J 29:414–420
Marinho-Soriano E, Fonseca PC, Carneiro MAA, Moreia WSC (2006) Seasonal variation in the chemical composition of two tropical seaweeds. Bioresour Technol 97:2402–2406
Kahar P, Taku K, Tanaka S (2010) Enzymatic digestion of corncobs retreated with low strength of sulfuric acid for bioethanol production. J Biosci Bioeng 110:453–458
Redding AP, Wang Z, Keshwani DR, Cheng J (2010) High temperature dilute acid pretreatment of coastal Bermuda grass for enzymatic hydrolysis. Bioresour Technol 102:1415–1424
Yanagisawa M, Kawai S, Murata K (2013) Strategies for the production of high concentrations of bioethanol from seaweeds. Bioengineered 4:224–235
Ahn DJ, Kim SK, Yun HS (2012) Optimization of pretreatment and saccharification for the production of bioethanol from water hyacinth by Saccharomyces cerevisiae. Bioprocess Biosyst Eng 35:35–41
Ernandes JR, William JW, Stewart GG (1992) Simultaneous utilization of galactose and glucose by Saccharomyces spp. Biotechnol Adv 6:233–238
Park JH, Hong JY, Jang HC, Oh SG, Kim SH, Yoon JJ, Kim YJ (2012) Use of Gelidium amansii as a promising resource for bioethanol: a practical approach for continuous dilute-acid hydrolysis and fermentation. Bioresour Technol 108:83–88
Kumar S, Gupta R, Kumar G, Sahoo D, Kuhad RC (2013) Bioethanol production from Gracilaria verrucosa, a red alga, in a biorefinery approach. Bioresour Technol 135:150–156
Meinita MDN, Kang JY, Jeong GT, Koo HM, Park SM, Hong YK (2012) Bioethanol production from the acid hydrolysate of the carrageenophyte Kappaphycus alvarezii (cottonii). J Appl Phycol 24:857–862
Kim MJ, Kim JS, Ra CH, Kim SK (2013) Bioethanol production from Eucheuma spinosum using various yeasts. KSBB J 28:315–318
Timson DJ (2007) Galactose metabolism in Saccharomyces cerevisiae. Dyn Biochem Process Biotechnol Mol Biol 1:63–73
Bro C, Knudsen S, Regenberg B, Olsson L, Nielsen J (2005) Improvement of galactose uptake in Saccharomyces cerevisiae through overexpression of phosphoglucomutase: example of transcript analysis as a tool in inverse mentabolic engineering. Appl Environ Microbiol 71:6465–6472
Ibrahim WM (2011) Biosorption of heavy metal ions from aqueous solution by red macroalgae. J Hazrd Mater 192:1827–1835
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: characterization of biosorptive capacity and investigation of mechnisms. J Colloid Interface Sci 275:131–141
Yu Q, Matheickal JT, Yin P, Kaewsarn P (1999) Heavy metal uptake capacities of common marine macro algal biomass. Wat Res 33:1534–1537
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Wat Res. 37:4311–4330
Davidge RW (1979) Mechanical behavior of ceramics. Cambridge University Press, London, pp 3–4
Seo DC, Yu K, DeLaune RD (2008) Comparision of monometal and multimetal adsorption in Mississippi River alluvial wetland sediment: batch and column experiments. Chemosphere 73:1757–1764
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2013R1A1A2059095).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunwoo, I.Y., Ra, C.H., Jeong, GT. et al. Evaluation of ethanol production and bioadsorption of heavy metals by various red seaweeds. Bioprocess Biosyst Eng 39, 915–923 (2016). https://doi.org/10.1007/s00449-016-1571-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-016-1571-3