Abstract
Lactic acid (LA) fermentation requires a neutralizer for a physiologically acceptable range. However, a neutralizer generates a large amount of gypsum, an environmental pollutant. Furthermore, the downstream processing is complicated and expensive, comprising 50–70% of the total cost. We previously developed a Lactobacillus delbrueckii FM1, which can produce undissociated LA without neutralizer. Here, we improved FM1 by adaptive evolution at pH 4.5, which generated Adp FM1 showing an ~ 1.80-fold increase in LA production compared to FM1. The LA production via fed-batch fermentation yielded 36.2 g/L of LA, with a productivity of 0.500 g/L/h. However, cell viability was reduced due to the acidic pH and/or end-product inhibition. Therefore, an in situ LA recovery process using an extractive solvent was employed to maintain cell viability. Adp FM1 produced 49.2 g/L of LA via in situ LA-extractive fed-batch fermentation, which was ~ 1.4-fold higher than that without LA extraction. Adp FM1 provided a total LA productivity of 0.512 g/L/h in 96 h. Among the tested strains, Adp FM1 exhibited the highest H+-ATPase activity and a 415-fold increase in H+-ATPase gene expression compared to the parent strain. These results suggest that the in situ LA extractive fermentation process will ease downstream processing and prove to be a more economical and environmentally friendly option compared to the present fermentation. To our knowledge, this is the first report on the production of undissociated L-LA by Lactobacillus using an in situ recovery process, with high LA production levels and productivity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lactic acid (LA) has a long history of use for the preservation of human foodstuffs (Davison et al. 1995). In addition, the demand for LA production has increased due to its considerable potential for use in biotechnological applications in various fields such as the food, cosmetic, pharmaceutical, and chemical industries (Wee et al. 2006). LA is also an important platform chemical (Akerberg and Zacchi 2000; Datta and Henry 2006), as it can be converted into other chemicals, such as acrylic acid, propylene glycol, acetaldehyde, and 2,3-pentanedione, and it serves as a main feedstock in the manufacture of biodegradable polymers and green solvents, which has further increased the demand for LA, especially l-lactic acid (LA) (Datta et al. 1995; Sodergard and Stolt 2002; Tsuji 2002). LA can be produced by both chemical and microbial fermentation methods. However, the chemical synthesis of LA always leads to a racemic mixture, which is a major drawback. Therefore, most of the LA is produced via bacterial fermentation, which offers several advantages over chemical synthesis (Benninga 1990). We have improved various strains of lactic acid bacteria (LAB) which produced high amounts of D- or L-LA from hydrolyzed sucrose (Kadam et al. 2006; Joshi et al. 2010) and from sugarcane bagasse-derived cellulose (Adsul et al. 2007; Singhvi et al. 2010) at neutral pH.
For the industrial production of LA, the main objective is to develop a cost-effective fermentation process that would reduce the cost of production. However, during a typical LA fermentation, the low pH of the broth (due to LA production) inhibits the metabolic activities of the microbial cells (Hongo et al. 1986). One method that is commonly used to minimize the negative effects of undissociated LA accumulation on pH is the addition of neutralizers, such as CaCO3, to the fermentation broth to react with the LA to form calcium lactate salt. However, after fermentation, the calcium lactate needs to be hydrolyzed by corrosive sulfuric acid to regenerate undissociated LA, generating calcium sulfate (gypsum), which is currently disposed of as solid waste. The co-production of large amounts of gypsum (1 ton per 1 ton of LA) is a prominent environmental issue related to conventional LA production, as the gypsum has to be discarded (Abdel-Rahman et al. 2013) and could eventually become an unbearable burden to the environment. In addition, the LA extraction process is intricate and costly because of the resulting high calcium lactate content. LA fermentation using acid-tolerant strains would make the process of LA recovery simpler and easier. Hence, there is a need to develop acid-tolerant microbial strains that can produce LA under acidic conditions. Several efforts have been made to improve the acid tolerance of Lactobacillus (Patnaik et al. 2002; Wang et al. 2007) and S. cerevisiae (Suzuki et al. 2013; Kawahata et al. 2006) strains, either by using rational design based on known mechanisms of LA tolerance or by using gene deletion or knock-down libraries. However, the limited knowledge and complexity of acid-tolerance mechanisms have made it difficult to generate LA-tolerant strains by modifying a few target genes. Therefore, adaptive evolution may be an effective strategy for improving the LA tolerance of individual, shuffled/engineered strains of different genetic and metabolic backgrounds (Baek et al. 2016).
There are a number of product recovery techniques, such as solvent extraction, adsorption, chromatographic methods, diffusion dialysis, membrane use, and electrodialysis. Among the possible separation techniques, reactive extraction is a promising method with a high recovery ratio that can be applied in situ (Krzyzaniak et al. 2013). In situ recovery during fermentation could not only recover LA from the broth but also relieve end-product (LA) inhibition (Abdel-Rahman et al. 2013). Reactive extraction using organic acids with amines and ammonium salts has been very well studied, and LA extraction using trioctylamine (TOA) dissolved in decanol and dodecane was previously reported, and LA was shown to have a high distribution coefficient (Yankov et al. 2004). Major obstacles in extractive fermentation are the toxicity of the solvent system to the microorganism and the difference in the pH optima of the fermentation and extraction with commonly used tertiary amines. The use of polar diluents and low pH increases the extractability, as these conditions often stabilize the LA-amine complex through hydrogen bonding or solvation (Yankov et al. 2005). One method to overcome the difference in pH optima is to develop an acid-tolerant organism. Another method is to remove the LA in situ via a chemical or physical method without pH control (Singhvi et al. 2015).
We previously isolated a mutant strain, L. delbrueckii Uc-3, which produces L-LA with high productivity (Kadam et al. 2006). The acid tolerance of strain Uc-3 was then improved via intergeneric protoplast fusion between Uc-3 and an acid-tolerant Acetobacter pasteurianus strain followed by UV mutagenesis of one of the fusants (Singhvi et al. 2015). In the present study, we further adapted the resulting mutant FM1 by continuous transfer to medium with an acidic pH to improve its acid tolerance. This adaptive evolution method generated an improved strain, designated as Adp FM1, which showed a 1.80-fold increase in LA production compared to that of FM1. We also evaluated Adp FM1 for LA fermentation by batch and fed-batch fermentation without a neutralizing agent. The free LA could be extracted from the broth in a single step by an in situ recovery process using a solvent system consisting of TOA dissolved in decanol and dodecane. In the present study, Adp FM1 produced maximum a total LA concentration of 49.2 g/L, with a productivity of 0.512 g/L/h in 96 h by in situ extractive fermentation. To our knowledge, this is the first report of LA fermentation employing an acid-tolerant Lactobacillus strain producing free LA and its simultaneous recovery from the broth using in a single-step reactive extraction. All strains were then validated for their acid tolerance via enzymatic, physiological, and genetic analyses. Adp FM1 exhibited higher H+-ATPase activity and gene transcription levels compared to other strains. Such acid-tolerant strains will be very useful for developing environmentally and eco-friendly LA production processes, without the generation of gypsum waste.
Materials and methods
Strains, culture media, and composition
L. delbrueckii Uc-3 (NCIM 5219), a high L-LA-producing strain generated by UV mutagenesis, and Acetobacter pasteurianus (NCIM 2314) were obtained from NCIM Resource Centre, NCL (Pune, India). The fusant, F3, was previously generated by protoplast fusion of Uc-3 and A. pasteurianus; F3 was then mutated to obtain FM1 (Singhvi et al. 2015). Stock cultures of all strains were maintained at − 80 °C in 2 mL vials containing a 30% glycerol solution. A. pasteurianus was grown in Acetobacter medium containing (g/L) sorbitol (20.0 g) and yeast extract (5.00 g), pH 4.5. All Lactobacillus strains were cultivated in MRS broth and modified MRS (mMRS) broth containing different glucose concentrations. All experiments were performed in MRS broth at an initial pH of 7.0 without any neutralizing agent for F3, FM1 and Adp FM1.
Improvement of acid tolerance by adaptation
The acid tolerance of strain FM1 was improved by adaptive evolution, which was carried out by growing the strain in MRS medium at an initial pH of 4.5 and continuously transferring after every 48 h for approximately 40 subcultures. During cultivation, the growth rate and metabolite titers were measured to characterize the evolved strains. After the final subculture, acid-tolerant colonies were isolated on solid MRS medium, pH 4.5, and the most efficient strain, Adp FM1, was selected based on its LA production and glucose consumption levels at acidic pH.
l-lactic acid production without a neutralizing agent by batch and fed-batch fermentation at flask level
One milliliter of each glycerol stock culture was inoculated into 10.0 mL of MRS broth (initial pH 7.0) in a screw-cap tube and incubated for 24 h at 42 °C. A portion of this 24-h culture (~ 5.00 mL) was transferred to 100 mL of MRS broth in 250 mL screw-cap conical flasks, and the flasks were incubated at 42 °C with shaking at 150 rpm. Cells from this culture were used as the inoculum (5% v/v) for fermentation medium (mMRS) containing 25.0 g/L (G25), 50.0 g/L (G50), and 100 g/L (G100) of glucose. The fermentation experiments were carried out in screw-cap conical flasks containing 100 mL of fermentation medium at 42 °C with shaking at 150 rpm. Culture samples were harvested and centrifuged at 7000×g for 10 min. Batch and fed-batch fermentation experiments were performed in mMRS medium containing 20.0 g/L glucose. For fed-batch fermentation, 20.0 g/L glucose was fed into the flask every 24 h for 96 h. Then, the sugar, LA, and pH of the supernatant were analyzed.
LA extraction from fermentation broth
A solvent system consisting of TOA (as an extractant), decanol (as modifier), and dodecane (as diluent) was standardized by testing different concentrations to achieve maximum LA extraction from the broth. Batch LA fermentation by Adp FM1 was carried out in mMRS medium containing 25.0 g/L glucose at 42 °C for 48 h. The fermentation broth was centrifuged at 7000×g for 10 min, and the supernatant was used as an aqueous solution for the extraction experiment. Equal volumes (25.0 mL) of aqueous solution containing LA (20.2 g/L) and the standardized solvent system were shaken at 150 rpm for 15–30 min at ambient temperature to attain extraction equilibrium. Then, the phases were allowed to settle in a separatory funnel for 15–30 min at room temperature. After the two phases were separated, the LA concentration in the lower aqueous phase was analyzed, and the concentration in the solvent phase was calculated from the mass balance. After phase separation, the distribution coefficient (Kd) was determined for LA extraction using this solvent system according to the following formula,
where
- Cs:
-
is concentration of LA in the solvent phase at equilibrium
- Caq:
-
is concentration of LA in the aqueous phase at equilibrium
Intermittent LA extractive fermentation by the fed-batch method at flask level
The Adp FM1 strain was evaluated for fed-batch extractive fermentation in mMRS medium containing 100 g/L glucose. A 24-h culture (~ 5.00 mL) was transferred to 100 mL of MRS broth in 250 mL screw-cap conical flasks. The flasks were incubated at 42 °C with shaking at 150 rpm. These cells were then used as the inoculum (5% v/v) for the fermentation medium (mMRS) in screw-cap flasks, and the flasks were incubated anaerobically at 42 °C with shaking at 150 rpm. Every 24 h, up to 96 h, LA was extracted from the fermentation as follows. The fermented broth was centrifuged to separate the cells, and the cell-free broth was mixed with the standardized solvent (20% TOA, 10% decanol, and 70% dodecane) in a 1:1 (solvent/broth) ratio for 15–30 min with shaking. During this incubation, the separated cells were suspended in saline and stored at 4 °C. After shaking, the solvent and broth mixture was transferred to a separatory funnel and allowed to settle for 15–30 min. Then, the phases were separated, and the lower aqueous phase (the medium) was used to continue the fermentation by re-inoculation with the stored cells. Intermittent LA extraction was conducted as described above using fresh solvent.
In situ LA recovery by extractive fermentation at 1 L jar fermenter level
The Adp FM1 strain was cultivated in 10.0 mL of MRS broth for 24 h at 42 °C. A portion of this 24-h grown culture (~ 5.00 mL) was transferred to 100 mL of growth medium in 250 mL screw-cap conical flasks, and the flasks were incubated at 42 °C with shaking at 150 rpm. This pre-culture was then inoculated (at 5%, v/v) into a 1-L jar fermenter (Biott, Tokyo, Japan) containing 250 mL of mMRS medium fed with 20.0 g/L glucose every 24 h until 96 h. LA was extracted after 48 h of fermentation by adding 250 mL of the solvent system (20% TOA, 10% decanol, and 70% dodecane). During fermentation in the fermenter jar, the solvent phase was continuously agitated with the fermented broth. The solvent-saturated broth samples were removed after every 24 h, and the phases were allowed to separate. The lower aqueous phase was analyzed for cell growth, sugar, and LA content.
H+-ATPase activity determination
All the test Lactobacillus strains were grown in MRS medium under anaerobic conditions, and A. pasteurianus NCIM 2314 was aerobically cultivated in Acetobacter medium at an initial pH of 4.5 (except for parent strain, L. delbrueckii Uc-3, which was grown at pH 7.0). After 24 h, samples were centrifuged at 7000×g to separate the cells. The harvested cells were washed thrice with Tris–HCl buffer (50 mM, pH 7.0) and re-suspended in the same buffer (5 mL) containing 20 mM EDTA, 10 mM MgCl2, 100 μg/mL lysozyme (Wako, Osaka, Japan), and 1 mM phenyl methyl sulfonyl fluoride (PMSF). The cell suspension was incubated at 37 °C for 30 min and sonicated. The sonication was performed at 60% amplitude (125 μM) for 5 min with a 2-mm probe under cold conditions. Almost 90% of the cells were disrupted by using this method. The sonicated samples were centrifuged at 7000×g for 10 min, and the supernatant was used as a cell-free extract to analyze H+-ATPase activity.
H+-ATPase activity was determined by measuring the inorganic phosphate (Pi) liberated from ATP according to the method of Fiske and Subbarow (1925)). The enzyme was assayed in a standard reaction mixture consisting of 50 mM Tris–HCl (pH 6.0), 3 mM MgCl2, and 2.5 mM Na-ATP in a final volume of 3 mL. The reaction was initiated by addition of 100 μL of cell-free extract and incubated at 37 °C for 15 min. The reaction was terminated by the addition of 1 mL of 12% (w/v) trichloroacetic acid and centrifuged at 8000×g for 5 min. Then, the supernatant was used to determine inorganic phosphate. The absorbance at 510 nm was measured against a backgrounds sample of the corresponding reaction mixture without cell-free extract. One unit of ATPase activity (IU) was defined as the amount of enzyme required to liberate 1 μmol of Pi from ATP per min. Specific activity was expressed as IU per mg of protein (IU/mg). The total protein concentration in the cell-free extract was determined with the Pierce BCATM Protein Assay kit (Thermo, Rockford, IL, USA).
Analysis of H+-ATPase gene expression by one-step quantitative real-time PCR
All tested Lactobacillus strains, including L delbrueckii Uc-3, fusant F3, mutant FM1, and Adp FM1, were grown in MRS under anaerobic conditions at 42 °C, and A. pasteurianus NCIM 2314 was grown in Acetobacter medium aerobically, at pH 4.5 with shaking at 150 rpm. Then, these strains were inoculated (at 5%) into fermentation medium. After 24 h of fermentation, 2 mL of the broth was collected into an Eppendorf tube and centrifuged at 10,000×g for 10 min. The pellet was suspended in 200 μL of solution A (containing 20 mM sodium acetate, 1 mM EDTA, and 0.5% SDS, pH 5.0) and phenol saturated with 20 mM sodium acetate (pH 5.0) and then mixed well by pipetting. The tubes were then incubated at 60 °C with shaking for 10 min and then centrifuged at 15,000×g for 5 min. After centrifugation, the upper aqueous phase was placed into a new tube. Cold, 100% ethanol (250 μL) was added, mixed well, and then centrifuged at 15,000×g for 5 min. The supernatant was decanted, and 375 μL of cold 70% ethanol was added to rinse the pellet, and then centrifuged at 15,000×g for 30 s. The supernatant was removed, and the RNA pellet was suspended in 50 μL of RNase-free water. Contaminating genomic DNA was digested with DNase I (Takara Bio, Inc., Ohtsu, Japan) for 30 min at 37 °C according to the manufacturer’s instructions as previously described (Singhvi et al. 2017). The samples were stored at − 20 °C. The integrity of the total RNA was determined by electrophoresis on a 2% (w/v) agarose gel, and the RNA concentration and purity (A260/A280 ratio) was determined using a Nanodrop spectrophotometer. This purified RNA was used for quantitative real-time PCR (qRT-PCR) to quantify the expression levels of the H+-ATPase gene.
One-step qRT-PCR was performed by using the One-step SYBR® PrimeScript™ RT-PCR Kit II (Perfect Real Time, TaKaRa Bio Inc.) on an Mx3000P Q-PCR Detection System. With the One-step SYBR® PrimeScript™ RT-PCR Kit II, cDNA was synthesized from the purified RNA using PrimeScript Reverse Transcriptase, and the cDNA was amplified by PCR with TaKaRa Ex Taq HS DNA Polymerase in a single tube. The PCR amplification products were monitored in real time by SYBR Green I detection. Reverse transcription was carried out under the following conditions: 5 min at 42 °C and 10 s at 95 °C. Then, the PCR was performed in 40 cycles of 5 s at 95 °C, 34 s at 57 °C, and 30 s at 72 °C. The primers used for qRT-PCR verification were designed with Primer 3 software based on the available DNA sequences of L. delbrueckii and A. pasteurianus (Table 1). The 2−△△Ct method was used to quantify levels of the target gene, H+-ATPase, and 16S rRNA was used as an internal reference. Since the parent strain, L. delbrueckii Uc-3, could not grow under acidic conditions without a neutralizing agent, it was used as a control, whereas all other strains grown at acidic pH were the test strains. Based on these results, the ratio of the target mRNA in the sample was calculated. The data obtained, i.e., the CT, were analyzed using the comparative critical threshold (∆∆CT) method, according to the following equations.
Relative expression level = 2−∆∆CT
Analytical methods
Cell growth was analyzed spectrophotometrically (UV-1600 visible spectrophotometer; BioSpec, Shimadzu, Tokyo, Japan) at a wavelength of 660 nm. LA and sugar were determined using an HPLC system (US HPLC-1210; Jasco, Tokyo, Japan) equipped with a SUGAR SH-1011 column (Shodex, Tokyo, Japan). The culture samples (1 mL) were centrifuged at 7000×g for 10 min at 4 °C. Then, the supernatant was diluted in ultrapure water, filtered through a membrane filter (Dismic-13HP, 0.45 μM; Advantec, Tokyo, Japan), and finally injected in the HPLC system under the following conditions: column temperature, 50 °C; mobile phase, 3 mM HClO4; flow rate, 1.0 mL min−1; and injection volume, 20 μL. The concentrations of residual sugars and fermentation products were calculated using calibration curves of standard solutions. The optical purity of LA was measured using a BF-5 biosensor (Oji Scientific Instruments, Hyogo, Japan) according to the manufacturer’s instructions as described previously (Abdel-Rahman et al. 2011). LA in the aqueous and solvent phases was analyzed at different time intervals during fermentation. The total LA produced in the fermentation system was calculated by determining the LA concentration in the aqueous and solvent phases according to the following equation:
where
- Σ[LA]:
-
total LA produced (g)
- Caq:
-
concentration of LA in aqueous broth (g/L)
- Vaq:
-
volume of aqueous broth phase (L)
- CS:
-
concentration of LA in solvent (g/L)
- VS:
-
volume of solvent phase (L)
The total LA concentration, productivity, and yield were defined according to the following equation:
where
- CLA:
-
total LA concentration (g/L)
- Σ[LA]:
-
total LA produced (g)
- Vaq:
-
volume of aqueous broth phase (L)
where
- PLA:
-
total LA productivity (g/L/h)
- CLA:
-
total LA concentration (g/L)
- F.T.:
-
fermentation time (h)
where
- YLA:
-
yield of LA at maximum total LA based on glucose consumed (g/g)
- Max CLA:
-
maximum total LA concentration (g/L)
- CGlu:
-
maximum glucose consumed (g/L)
Results
Comparative LA fermentation by Lactobacillus strains
We used L. delbrueckii Uc-3, F3, FM1, and Adp FM1 for LA fermentation and compared LA production and growth in mMRS medium containing G25 at pH 7.0 without any neutralizing agent. Also, the optical purity was checked using biosensor device for all the fermented broth samples which was approximately 99.9% L-LA. Among the tested strains, Adp FM1 produced the highest amount of LA (18.1 g/L) within 24 h of fermentation (Table 2). Adp FM1 also showed LA productivity of 0.750 g/L/h which was ~ 1.80-fold greater than that of the improved mutant FM1. Based on its superior performance in LA fermentation, Adp FM1 was selected for further studies.
Lactic acid production by batch and fed-batch fermentation
In batch fermentation with G50, Adp FM1 produced 23.1 g/L of LA, with a productivity of 0.320 g/L/h and a yield of 0.780 g/g in 72 h (Fig. 1a). However, in the fermentation with G100, cell growth (as measured by the OD660) was inhibited due to the higher sugar concentration, which resulted in low LA production (Fig. 1b). The maximum LA produced was 10.2 g/L, with a productivity of 0.141 g/L/h in 72 h. In contrast, in fed-batch fermentation, cell growth was not inhibited, and 36.2 g/L of LA was produced, with a productivity of 0.502 g/L/h and a yield of 0.872 g/g in 72 h (Fig. 1c). We determined the cell viability of Adp FM1 during fed-batch fermentation. The percentage of viable cells started to decrease after 48 h of fermentation, which could be due to inhibition by the acidic pH/end product (Fig. 1d).
Lactic acid (LA) production by the Lactobacillus adapted mutant Adp FM1 in mMRS medium by batch fermentation containing 50.0 g/L (a) and 100 g/L (b) glucose and by fed-batch fermentation (c) at pH 7.0 (initial) without neutralizing agent. Cell viability of Adp FM1 in mMRS medium during fed-batch fermentation (d). Symbols: open circles, glucose; closed circles, LA; open triangles, pH; and closed triangles, cell growth (OD660). The data points represent the means and standard deviations of results from three independent experiments. The standard deviation is less than the size of symbols if no error bars are seen
Extractive fermentation of LA by fed-batch method at flask level
Firstly, the solvent system for extracting LA from the broth was standardized, and 20% TOA (as an extractant), 10% decanol (as a modifier), and 70% dodecane (as a diluent) was found to be optimal for LA extraction and was also non-toxic to the cells (data not shown). The Kd for LA was estimated to be 3.55 ± 0.123.
Adp FM1 was evaluated for LA extractive fermentation in mMRS medium without using neutralizing agent by fed-batch method. Initially, intermittently LA extractive fermentation was carried out on a flask-culture scale. LA extraction started after 48-h fermentation, and after every 24-h time interval, the broth was centrifuged and cells were separated out. LA was extracted by adding standardized solvent system at the ratio of 1:1 (v/v). In case of, product removal started after 48 h of fermentation, a maximum total LA concentration of 52.2 g/L, with a total productivity of 0.543 g/L/h and a yield of 0.883 g/g was obtained in 96 h (Fig. 2a). LA production by extractive fermentation was nearly 1.4 times higher than that of the control, without extraction (Fig. 1c). The sugar utilization rate was also improved by extractive fermentation. We determined the viability of Adp FM1 during extractive fermentation, with extraction starting at 48 h of fed-batch fermentation. As shown in Fig. 2b, cell viability decreased with fermentation time but was improved compared to that of the control (Fig. 1d) and was correlated with increased LA production. Similar results were obtained when product removal started after 24 h of fermentation (data not shown).
Intermittent LA extractive fed-batch fermentation in mMRS medium at pH 7.0 (initial) by Adp FM1 without a neutralizing agent at flask level. a LA extraction was initiated after 48 h of fermentation and was conducted every 24 h (vertical arrows). b Cell viability profile of Adp FM1 during fed-batch extractive fermentation with product removal starting at 48 h. Symbols: open circles, glucose; closed circles, total LA concentration; open triangles, pH; closed triangles, cell growth (OD660); open squares, LA concentration in medium after extraction (breaking line); closed squares, LA concentration in medium before extraction (continuous line). The data points represent the means and standard deviations of results from three independent experiments. The standard deviation is less than the size of symbols if no error bars are seen
In situ LA recovery in one step by extractive fermentation
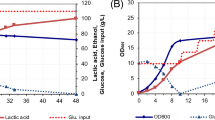
Fed-batch extractive LA fermentation by the Adp FM1 strain was evaluated by in situ LA recovery. The maximum total LA concentration was 49.2 g/L, with a productivity of 0.512 g/L/h and a yield of 0.870 g/g in 96 h (Fig. 3a). LA production (49.2 g/L) was improved nearly 1.4 times as compared to the control, a fed-batch fermentation without LA extraction (36.2 g/L). We determined the cell viability profile of Adp FM1 during in situ LA extractive fermentation. As shown in Fig. 3b, the cell viability decreased with fermentation time but was improved compared to that of the control (Fig. 1d), which is correlated with increased LA production. The pH of the broth had a marked effect on the amount of LA extracted from the broth. Table 3 shows the kinetic parameters of in situ LA recovery as compared to those of other fermentation conditions.
In situ LA extractive fed-batch fermentation in mMRS medium at pH 7.0 (initial) by Adp FM1 without a neutralizing agent in a 1-L jar fermenter. a LA extraction was initiated after 48 h of fermentation (vertical arrows). b Cell viability of Adp FM1 during fed-batch extractive fermentation with product removal starting at 48 h of fermentation. Symbols: open circles, glucose; closed circles, total LA concentration; open triangles, pH; closed triangles, cell growth (OD660); open squares, LA concentration in the medium. The data points represent the means and standard deviations of results from three independent experiments. The standard deviation is less than the size of symbols if no error bars are seen
H+-ATPase activity and transcription levels under acidic condition
At acidic pH, cells exhibited exportation protons and acid anions through the H+-ATPase and efflux pumps, respectively, which leads to high ATP consumption (van Maris et al. 2004). Therefore, we attempted to detect the H+-ATPase activities of all strains grown at acidic pH 4.5 (except for the parent strain L. delbrueckii Uc-3, which was grown at pH 7.0). As shown in Table 4, among the tested Lactobacillus strains, Adp FM1 exhibited higher H+-ATPase-specific activity (0.255 IU/mg-protein). The Adp FM1 strain, which showed higher LA productivity, exhibited correspondingly high H+-ATPase-specific activity when grown in MRS at pH 4.5.
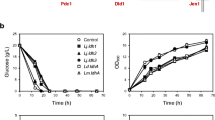
The effect of acidic conditions on the expression of H+-ATPase during LA fermentation, leading to the enhanced production of LA, was examined by qRT-PCR. Among the tested Lactobacillus strains, Adp FM1 exhibited the highest transcriptional levels of the Acetobacter ATP synthase F1 subunit, which was found to be 415-fold higher than that of the parent strain, L. delbrueckii Uc-3. The F3 and FM1 strains showed 138- and 359-fold higher gene expression, respectively, at pH 4.5, which was considerably higher than the expression levels at pH 7.0 (Fig. 4). In all strains, H+-ATPase gene expression was higher at pH 4.5 than at pH 7.0. In addition, the transcriptional levels of Lactobacillus F0-F1 ATP synthase subunit C was examined in all strains; however, the expression levels were lower than that of the Acetobacter ATP synthase F1 subunit gene (data not shown). The parent strain, L. delbrueckii Uc-3, showed very negligible levels of H+-ATPase gene expression, whereas the fusant (F3) exhibited comparatively higher H+-ATPase gene expression, as was observed in Acetobacter. These results suggest that the H+-ATPase gene was transferred during protoplast fusion; hence, F3 acquired the acid tolerance property of the parent Acetobacter strain. Further, as F3 was UV mutagenized and adapted to enhance LA production, generating FM1 and Adp FM1, these strains also showed increased H+-ATPase gene expression under acidic conditions. The enzyme activities of all tested strains were well correlated with the mRNA expression profiles.
Discussion
Currently, LA production by conventional processes requires the addition of a neutralizing agent, such as CaCO3, due to the acid sensitivity of LA-producing Lactobacillus strains, making the downstream processing more complicated and costly. Hence, there is a need to develop robust strains for commercial LA production under acidic conditions. Previously, we improved the acid tolerance of an L-LA-producing Lactobacillus strain by protoplast fusion of A. pasteurianus and L. delbrueckii Uc-3 (Singhvi et al. 2015). In the present study, we further improved the acid tolerance of a mutant strain, FM1, derived from the fusant by adaptation under acidic conditions, which generated an adapted strain, Adp FM1, with enhanced free LA production. LA production from cheese whey using low pH-tolerant LAB strains is currently being studied, which showed that test strains with differing acid tolerances required different neutralizing agent contents (Juodeikiene et al. 2016). Baek et al. (2017) improved the acid tolerance of genetically modified yeast by adaptive laboratory evolution, which yielded strains with higher LA tolerance and D-LA production. In this study, the adapted strain, Adp FM1, produced more LA (18.1 g/L) than the parent L. delbrueckii Uc-3 strain (0.911 g/L), the fusant F3 (7.10 g/L), and the mutant FM1 (10.2 g/L) within 24 h of fermentation (Table 2). Hence, strain Adp FM1 was used for further batch and fed-batch fermentation studies. Although a high glucose concentration caused substrate inhibition in batch fermentation (Fig. 1a, b), fed-batch fermentation enabled a maximum LA production of 36.2 g/L, with a productivity of 0.502 g/L/h and a yield of 0.872 g/g (Fig. 1c). During fed-batch fermentation, nearly 50% of the sugar was not utilized; hence, cell viability was examined (Fig. 1d). After 48 h of LA fermentation, Adp FM1 showed a loss of viability, which might be due to acidic pH/end-product (LA) inhibition.
Marinova and Yankov (2009) studied the toxicity of solvents to Lactobacillus casei, and most of the tested alcohols, including 1-octanol, showed high phase toxicity. It is known that the toxicity of alcohols decreases with increasing alkyl chain length, and n-alkanes are practically non-toxic (Playne and Smith 1983; Martak et al. 1997). Hence, we optimized a solvent system to maximize LA extraction and minimize toxicity to the microbial cells, and the optimal system comprised 20% TOA, 10% decanol, and 70% dodecane (data not shown). We conducted in situ LA recovery by extractive fermentation using the optimized solvent system. Using this system, the cells were largely unaffected, and 70–75% of the LA could be extracted from the fermentation broth in one step. Compared to previously reported strains, our strain, Adp FM1, produced a higher amount of total LA (49.2 g/L), with a productivity of 0.512 g/L/h and a yield of 0.870 g/g in 96 h (Fig. 3a). However, Adp FM1 produced only 10.2 g/L of LA, with a productivity of 0.141 g/L/h by batch fermentation. Thus, LA production was improved almost fivefold using fed-batch fermentation with in situ LA recovery as compared to that with the batch fermentation. We compared the results of the present study to previously reported extractive fermentations. Table 5 shows that, until now, there were no studies reporting the production of undissociated LA with high productivity using an in situ recovery process. It is also known that the acidic pH of the fermentation broth aids in direct LA extraction using solvent systems without the need for acidifying the broth in downstream processing.
When the pH of the medium is below the pKa of LA (3.86), extracellular LA can diffuse into the cytosol, passing through the plasma membrane, where it dissociates into lactate and protons (van Maris et al. 2004). Cells counteract this intracellular acidification through proton extrusion by increasing H+-ATPase activity, which results in a significant loss in available energy for growth and other essential metabolic functions. However, over time, it will no longer be possible for the cell to maintain its pH within a physiologically acceptable range, which leads to growth inhibition and ultimately cell death. We investigated the H+-ATPase activities of both parental strains, the fusant F3, and the mutant FM1 and Adp FM1 strains. A. pasteurianus exhibited the highest H+-ATPase-specific activity (0.431 IU/mg-protein), whereas, among the Lactobacillus strains, the Adp FM1 mutant strain exhibited the highest H+-ATPase-specific activity (0.255 IU/mg-protein; Table 4). It was reported that high levels of H+-ATPase correspond to high acid tolerance in batch culture (Shobharani and Halami 2014).
Further, the acid tolerance of the test strains was confirmed using a genetic approach, qRT-PCR analysis of H+-ATPase expression levels at pH 4.5 and 7.0. The parent strain, A. pasteurianus, showed the highest levels of Acetobacter ATP synthase F1 subunit transcription at pH 4.5 and pH 7.0, which were 526- and 302-fold higher than that of L. delbrueckii Uc-3 cells grown in MRS. Among the Lactobacillus strains, F3, FM1, and Adp FM1 also expressed the Acetobacter ATP synthase F1 gene, at levels 138-, 359-, and 415-fold higher than that expressed by L. delbrueckii Uc-3 cells, respectively, at pH 4.5, which was considerably higher than that expressed at pH 7.0 (Fig. 4). However, H+-ATPase expression was higher in the Acetobacter strain than in all tested Lactobacillus strains at pH 4.5 and pH 7.0. These results indicated the presence of the H+-ATPase gene in all the fusant and mutant strains, which is expressed under acidic conditions during LA fermentation. It is known that H+-ATPase expression corresponds to high levels of acid tolerance; hence, we think that the fusant strain acquired the acid tolerance property from Acetobacter through protoplast fusion, and it was maintained in FM1 and Adp FM1.
In conclusion, we employed adaptive evolution at acidic pH to improve the LA tolerance of a mutant strain (FM1) derived from a fusant (F3). The obtained strain, Adp FM1, showed a 1.80-fold increase in LA production under acidic conditions (without the addition of CaCO3) compared to the mutant FM1. Adp FM1 has the advantage of being more acid tolerant, which means that it can grow and ferment sugars under acidic conditions without the need for a neutralizing agent. In contrast to other reported Lactobacillus strains, Adp FM1 produced higher amounts of free LA (~ 49.2 g/L) using extractive fermentation with an in situ recovery process. To the best of our knowledge, this is the first description of free LA production by in situ extractive fermentation, with the highest reported productivity by a Lactobacillus strain under acidic conditions without a neutralizing agent. Production of free LA from glucose by Adp FM1 is feasible and efficient, as the downstream processing is easier and more cost effective. The development and use of such acid-tolerant strains will provide new methods for commercial LA fermentation.
References
Abdel-Rahman MA, Tashiro Y, Zendo T, Shibata K, Sonomoto K (2011) Isolation and characterisation of lactic acid bacterium for effective fermentation of cellobiose into optically pure homo L-(+)-lactic acid. Appl Microbiol Biotechnol 89:1039–1049
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902
Adsul MG, Varma AJ, Gokhale DV (2007) Lactic acid production from waste sugarcane bagasse derived cellulose. Green Chem 9:58–62
Akerberg C, Zacchi G (2000) An economic evaluation of the fermentative production of lactic acid from wheat flour. Bioresour Technol 75:119–126
Baek SH, Kwon EY, Kim SY, Hahn JS (2016) GSF2 deletion increases lactic acid production by alleviating glucose repression in Saccharomyces cerevisiae. Sci Rep 6:34812–34824
Baek SH, Kwon EY, Bae SJ, Cho BR, Kim SY, Hahn JS (2017) Improvement of D-lactic acid production in Saccharomyces cerevisiae under acidic conditions by evolutionary and rational metabolic engineering. Biotechnol J 12:1700015–1700021
Benninga HA (1990) A history of lactic acid making. Kluyver Academic Publishers, Dordrecht
Datta R, Henry M (2006) Lactic acid: recent advances in products, processes and technologies—a review. J Chem Technol Biotechnol 81:1119–1129
Datta R, Tsai S, Bonsignor P, Moon S, Frank J (1995) Technological and economic potential of poly(lactic acid) and lactic acid derivatives. FEMS Microbiol Rev 16:221–231
Davison BE, Llanos RL, Cancilla MR, Redman NC, Hillier AJ (1995) Current research on the genetics of lactic acid production in lactic acid bacteria. Int Dairy J 5:763–784
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorous. J Biol Chem 66:375–389
Gao MT, Shimamura T, Ishida N, Takahashi H (2011) pH-uncontrolled lactic acid fermentation with activated carbon as an adsorbent. Enz Microb Technol 48:526–530
Hongo M, Nomura Y, Iwahara M (1986) Novel method of lactic acid production by electrodialysis fermentation. Appl Environ Microbiol 52:314–319
Joshi DS, Singhvi MS, Khire JM, Gokhale DV (2010) Strain improvement of Lactobacillus lactis for D-lactic acid production. Biotechnol Lett 32:517–520
Juodeikiene G, Zadeike D, Bartkiene E, Klupsaite D (2016) Application of acid tolerant Pedioccocus strains for increasing the sustainability of lactic acid production from cheese whey. LWT Food Sci Technol 72:399–406
Kadam SR, Patil SS, Bastawde KB, Khire JM, Gokhale DV (2006) Strain improvement of Lactobacillus delbrueckii NCIM 2365 for lactic acid production. Process Biochem 41:120–126
Kawahata M, Masaki K, Fujii T, Iefuji H (2006) Yeast genes involved in response to lactic acid and acetic acid: acidic conditions caused by the organic acids in Saccharomyces cerevisiae cultures induce expression of intracellular metal metabolism genes regulated by Aft1p. FEMS Yeast Res 6:924–936
Krzyzaniak A, Leeman M, Vossebeld F, Visser TJ, Schuur S (2013) Novel extractants for the recovery of fermentation derived lactic acid. Purif Technol 111:82–89
Marinova NA, Yankov DS (2009) Toxicity of some solvents and extractants towards Lactobacillus casei cells. Bulg Chem Commun 41:368–373
Martak J, Sabolova E, Schlosser S, Rosenberg M, Kristofikova L (1997) Toxicity of organic solvents used in situ in fermentation of lactic acid by Rhizopus arrhizus. Biotechnol Tech 11:71–75
Matsumoto M, Nishimura M, Kobayashi H, Kondo K (2016) Extractive fermentation of lactic acid with Hiochi bacteria in a two-liquid phase system. Ferment Technol 5:1–6
Patnaik R, Louie S, Gavrilovic V, Perry K, Stemmer W, Ryan M, del Cardayre S (2002) Genome shuffling of Lactobacillus for improved acid tolerance. Nat Biotechnol 20:707–712
Playne MJ, Smith BR (1983) Toxicity of organic extraction reagents to anaerobic bacteria. Biotechnol Bioeng 25:1251–1265
Shobharani P, Halami PM (2014) Cellular fatty acid profile and H+-ATPase activity to assess acid tolerance of Bacillus sp. for potential probiotic functional attributes. Appl Microbiol Biotechnol 98:9045–9058
Singhvi MS, Joshi DS, Adsul MG, Varma AJ, Gokhale DV (2010) D (−) lactic acid production from cellobiose and cellulose by Lactobacillus lactis mutant RM2-24. Green Chem 12:1106–1109
Singhvi MS, Gurjar GS, Gupta VS, Gokhale DV (2015) Biocatalyst development for lactic acid production at acidic pH using inter-generic protoplast fusion. RSC Adv 5:2024–2031
Singhvi MS, Zendo T, Iida H, Gokhale DV, Sonomoto K (2017) Stimulation of D-and L-lactate dehydrogenases transcriptional levels in presence of diammonium hydrogen phosphate resulting to enhanced lactic acid production by Lactobacillus strain. J Biosci Bioeng 124:674–679
Sodergard A, Stolt M (2002) Properties of lactic acid based polymers and their correlation with composition. Prog Polym Sci 27:1123–1163
Suzuki T, Sakamoto T, Sugiyama M, Ishida N, Kambe H, Obata S, Kaneko Y, Takahashi, Harashima S (2013) Disruption of multiple genes whose deletion causes lactic-acid resistance improves lactic-acid resistance and productivity in Saccharomyces cerevisiae. J Biosci Bioeng 115:467–474
Tik N, Bayraktar E, Mehmetoglu U (2001) In situ reactive extraction of lactic acid from fermentation media. J Chemical Technol Biotechnol 76:764–768
Tsuji F (2002) Autocatalytic hydrolysis of amorphous-made polylactides: effects of L-lactide content, tacticity, and enantiomeric polymer blending. Polymer 43:1789–1796
van Maris JA, Winkler AA, Porro D, van Dijken JP, Pronk JT (2004) Directed evolution of pyruvate decarboxylase-negative Saccharomyces cerevisiae, yielding a C2-independent, glucose-tolerant, and pyruvate-hyperproducing yeast. Appl Environ Microbiol 70:159–166
Wang YH, Li Y, Pei XL, Yu L, Feng Y (2007) Genome-shuffling improved acid tolerance and L-lactic acid volumetric productivity in Lactobacillus rhamnosus. J Biotech 129:510–515
Wee YJ, Kim JN, Ryu HW (2006) Biotechnological production of lactic acid and its recent applications. Food Technol Biotechnol 44:163–172
Yankov D, Molinier J, Albet J, Malmary G, Kyuchoukov G (2004) Lactic acid extraction from aqueous solutions with tri-n-octylamine dissolved in decanol and dodecane. Biochem Eng J 21:63–71
Yankov D, Molinier J, Kyuchoukov G, Albet J, Malmary G (2005) Improvement of the lactic acid extraction. Extraction from aqueous solutions and simulated fermentation broth by means of mixed extractant and TOA, partially loaded with HCl. Chem Biochem Eng Q 19:17–24
Acknowledgements
Mamata Singhvi was supported by a fellowship (no. P16100) from the Japan Society for the Promotion of Science (JSPS).
Funding
This work was partially supported by a Research Fellow JSPS grant (no. 16F16100) and a grant from JSPS KAKENHI (grant no. JP16F1610).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Rights and permissions
About this article
Cite this article
Singhvi, M., Zendo, T., Gokhale, D. et al. Greener L-lactic acid production through in situ extractive fermentation by an acid-tolerant Lactobacillus strain. Appl Microbiol Biotechnol 102, 6425–6435 (2018). https://doi.org/10.1007/s00253-018-9084-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-018-9084-4