Abstract
The purpose of this study was to establish a simplified operational process for lactic acid (LA) production from inedible starchy biomass by open fermentation using thermotolerant Lactobacillus rhamnosus DUT1908. One step simultaneous liquefaction, saccharification and fermentation (SLSF) was proposed to produce LA using aging paddy rice with hull (APRH) as feedstock. First, a robust microbial strain was obtained by adaptive laboratory evolution under high temperature stress. As a result, L. rhamnosus DUT1908 showed high thermotolerance up to 50 °C and high efficiency of substrate utilization. Then, the performance of this thermotolerant l-lactic acid producing strain was demonstrated. Finally, various fermentation strategies were compared for LA production from APRH, including simultaneous saccharification and fermentation (SSF) and SLSF. In one-step open SLSF process, 107.8 g/L lactic acid was obtained with a productivity of 3.4 g/(L.h) and a yield to theoretical glucose of 0.89 g/g. This is the highest yield and productivity of lactic acid reported on starchy residues, and provides an efficient route for the development of high value-added products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
l-lactic acid (LA) is a versatile block-building chemical that is widely used in the food, cosmetics textiles, pharmaceutical, and chemical industries [1, 2]. Demand for LA has increased dramatically in recent years due to its great potential as the monomer of biodegradable plastic polylactic acid (PLA) for substitution of petroleum-derived plastics, e.g. polyethylene, polypropylene and polystyrene [3]. The production cost of l-lactic acid could be reduced to $1.34–1.46 per kg, but the expectation was around $0.9 per kg for PLA use. The total production cost is mainly from the raw materials cost and separation cost. The raw substrate materials for LA production constitute 40–70% of the total production cost, which is a challenge for cost-effective LA production [1]. Currently, LA is produced from glucose derived from edible starchy materials, which not only contributes to a significant proportion of LA production costs but also competes with food supply. Therefore, lignocellulosic biomass as an alternative inexpensive, non-food substrate has attracted much attention in recent years [1, 3]. However, lignocellulose utilization in LA production remain considerable challenges including lignocellulose pretreatment, the efficient utilization of pentose, inhibitory to LA producing strains by unwanted compounds, and wastewater treatment [4]. The major disadvantages make the production cost of lignocellulose-dervied LA not comparable to starchy-based LA.
Besides lignocellulosic biomass, agro-industrial wastes or byproduct streams, inedible aging paddy rice have been suggested as alternative carbon and/or nitrogen sources for LA production [5,6,7]. Aging paddy rice is rejected for use as foodstuff due to its less tasty flavor and loss of nutrition in comparison to fresh paddy rice. In general, aging paddy rice is generally milled removing the paddy hull and mainly used in feedstuff which brings low value-added profit [5]. Aside from abundant starch, aging paddy rice with hull (APRH) also contains other important nutrients such as fibers, amino acids, vitamins, and minerals than polished rice [8]. The fermentative effect using the different raw materials of unpolished rice and polished from aging paddy was compared according to the report by Lu et al. [5]. The results indicated that 90.80 g/L d-lactic acid with a yield to unpolished rice of 0.73 g/g and a productivity of 1.50 g/(L.h) was reached. The yield was higher about 8.71 and 5.79% than that of polished rice and fresh corn, respectively. Because the unit price of unpolished rice was nearly one half of that of the fresh corn and polished rice. The raw materials cost of LA production decreased by almost 50–60% when unpolished rice was used. Therefore, inedible APRH was selected as a cheap and excellent nutrient source for LA production in this study.
To achieve an economical and high-efficiency LA fermentative route from starchy residues, different fermentation strategies including simultaneous saccharification and fermentation (SSF), simultaneous liquefaction, saccharification and fermentation (SLSF) were developed and investigated. However, one critical problem in SSF and SLSF strategy was that the optimal temperatures of starch liquefaction and saccharification were higher than that of LA fermentation. In addition, it is quite difficult to sterilized solid materials and the viscosity of starchy biomass increased dramatically after sterilization. Therefore, non-sterilized fermentation with thermotolerant LA producing strains has been attracted much attention in recent years [6, 9, 10]. These thermotolerant LA producing strains include B. coagulans (52 °C) [9], R. microspores (50 °C) [10], E. faecalis (50 °C) [6], L. rhamnosus (42 °C) [11,12,13], L. Delbrueckii (45 °C) [5], etc. Among thermotolerant LA producing strains, Lactobacillus strains have great commercial importance due to high acid tolerance, high yield and productivity, and can be engineered for the selective production of l/d-lactic acid [14]. Among the genus Lactobacillus, L. rhamnosus with a reported thermotolerant temperature up to 42 °C showed low temperature compatibility for one step of liquefaction, saccharification, and fermentation. As a result, the yield and productivity of LA in SSF and SLSF process was not competitive. The yield of LA to theoretical glucose in initial starch ranged from 0.53 to 0.71 g/g and the average productivity ranged from 1.0 to 2.6 g/(L.h) by L. rhamnosus [11,12,13].
The objective of this study was to establish a simplified operational process for LA production efficiently by L. rhamnosus from inedible APRH with open fermentation. First, thermotolerant L. rhamnosus, with optimal growth temperature of 50 °C through adaptive laboratory evolution (ALE) was achieved. Second, the performance of l-lactic acid producing bacterium of L. rhamnosus DUT1908 at high fermentative temperature was investigated. Finally, LA production by thermotolerant L. rhamnosus via SLSF and SSF process from inedible APRH was conducted and compared. This study thus aims to develop an efficient and economical LA production route from inedible starchy biomass.
Materials and methods
Microorganism and strain adaptation
In this study, Lactobacillus rhamnosus DUT1908 derived from Lactobacillus rhamnosus CICC 22825 (China Center of Industrial Culture Collection, Beijing, China) was subjected to adaptive laboratory evolution to enhance its thermotolerant performance. The ALE process was carried out in batch mode and consisted of a subsequent transfer of Lactobacillus rhamnosus CICC 22825 to an adaptive strategy of increasing temperature (40, 42, 45, 47, 50 °C). The culture was propagated with shaking at 40 °C in a 100 mL glucose medium with inoculum concentration of 5% (v/v) and under micro-aerobic conditions. Upon reaching the late exponential growth phase, the culture was transferred to a fresh glucose medium at higher temperature for subsequent cultivation. The culture was grown three times at the same temperature before being exposure to higher temperatures. After this process, the culture was streaked onto an MRS agar and incubated at 50 °C for 48 h. After incubation, the biggest colony designated as L. rhamnosus DUT1908 was selected and used for the fermentation process.
Materials and culture media
Paddy rice was achieved and treated as we described before [6]. The components of APRH contained 61.20% of starch, 8.61% of crude protein, 5.27% of crude fiber, 11.64% of water content, and 13.29% of others. The commercial liquefying enzyme of Liquozyme®COFCO LpH and saccharifying enzyme of SuHong GA COFCO HP used was kindly afforded by COFCO Biochemical CO., Ltd, China. The activities of the liquefying and saccharifying enzymes were determined as 12,300 and 13,060 U/mL, respectively[6]. Corn steep liquor powder (CSLP) with a nitrogen content of 9% (w/w) was purchased from Yuancheng Biotechnology Company, Liaoning, China [15]. It is mainly composed of 54% protein, 8% reducing sugar, 9% ash and 12% lactic acid. All other chemicals were of analytical grade and commercially available.
Seed medium, MRS medium, and CSLP medium were prepared as previously described [6, 16].
SLSF and SSF strategies in 5 L bioreactor
SLSF and SSF processes were performed in a 5 L bioreactor (Baoxing Bio, Shanghai, China) with 2 L of working volume under an APRH loading of 20% (w/v) at 50 °C, pH 6.0 and 200 rpm. The liquefying and saccharifying enzymes were both added as 0.5 mL/100 g APRH. The culture pH was controlled by automatic addition of 8 mol/L NaOH. In the SLSF process, the liquefying enzyme and the saccharifying enzyme were added along with the inoculum to the medium. In the SSF process, the liquefying enzyme was first added to the medium for 0.5 h at 75–80 °C, followed by the saccharifying enzyme along with the inoculum at 50 °C. Both the SLSF and SSF processes were not sterilized before incubation and did not require air or nitrogen flushing. The steps involved in the SLSF and SSF strategies were summarized in Fig. 1. The samples were withdrawn at regular intervals and centrifuged at 9720 × g for 10 min to obtain the supernatant for analysis by high-performance liquid chromatography (HPLC).
Analytical methods
The concentrations of biomass, glucose, lactic acid were measured and analyzed as we described before [6]. The optical purity of the l-lactic acid was determined by HPLC equipped with an Astec®CLC-L Chiral column (Sigma Aldrich, Co.). The mobile phase was 5 mmol/L CuSO4 at a flow rate of 1.0 mL/min [6].
Results and discussion
Improving performance of L. rhamnosus DUT1908 by adaptive laboratory evolution
According to the previous studies, during SLSF process, the highest fermentation temperature by L. rhamnosus was up to 42 °C [11, 12]. To ensure the efficient production of LA from APRH by SLSF and SSF processes, robust microbial strains are highly needed under stressful conditions of high temperatures. To improve LA production from aging paddy rice, the ALE experiment under gradually increasing fermentation temperature was performed in this study.
The performance of adaptive L. rhamnosus DUT1908 at high temperatures was investigated for LA production in batch fermentation. As a result, at fermentation temperature of 50 °C, cell growth, glucose consumption and LA production were comparable to what was obtainable at 42 and 47 °C. The final LA titer was 91.68 g/L, with a yield of 0.92 g/g and a productivity of 1.95 g/(L.h) at 47 °C, while the final LA titer was 89.74 g/L, with a yield of 0.91 g/g and a productivity of 1.91 g/(L.h) at 50 °C. The optical purity of l-lactic acid was 95%. In contrast, the original strain of L. rhamnosus grew slowly in medium when the fermentation temperature was over 47 °C and the yield of LA to glucose was only 0.51 g/g. Based on the results of the evolutionary adaptation in this study, the thermotolerant L. rhamnosus DUT1908 was chosen to further improve the efficiency for the SLSF process.
Effect of pH on l-lactic acid production
As the environmental pH will change the charge distribution on the cell membrane and alter its permeability, lower pH thus influenced the in vivo enzyme activities and affected the strain growth [17]. In this study, the influence of pH on LA production by thermotolerant L. rhamnosus DUT1908 was investigated (Fig. 2). From the results, LA titer increased gradually from 57.71 to 91.02 g/L with pH increase from 5.8 to 6.5. The yield of 0.92 g/g with a productivity of 2.46 g/(L.h) was achieved at pH 6.5. The LA titer of 89.74 g/L with a yield of 0.91 g/g and a productivity of 1.91 g/(L.h) was obtained at pH 6.0. Although the highest LA titer and productivity was obtained at pH 6.5, to achieve pH compatibility of liquefaction, saccharification and fermentation process, a lower pH of 6.0 was selected as optimal for LA production by thermotolerant L. rhamnosus DUT1908.
Effect of initial glucose concentration on l-lactic acid production
To develop the fermentation process under a relatively optimal condition, the effect of glucose concentration on LA production by L. rhamnosus DUT1908 at 50 °C was conducted and the results are presented in Fig. 3. The final lactic acid titer increased with the increasing of the initial glucose concentration up to 130 g/L at high fermentation temperature. At an initial glucose concentration of 130 g/L, the l-lactic acid titer reached 106.11 g/L with a yield of 0.93 g/g and a productivity of 1.89 g/(L.h). However, when initial glucose concentration further increased, the residual glucose concentration increased and LA yield decreased obviously. The cell growth was inhibited at the end of fermentation at an initial glucose concentration of 150 g/L. In addition, over 100 g/L lactate added to the medium indicated that cell growth was inhibited significantly compared to previous studies (data not shown). This negative effect was mainly due to the osmotic stress caused by the high level of substrate and the acid stress caused by the high level product [2, 9, 18, 19]. The inhibition mechanisms of products were explained for the following reasons [20]. The cell membrane collapsed due to the change in membrane potential, the cytosol became acidic, and anions inside the cell accumulated. In addition, lactate molarity i.e. Ca2+, NH3+ and Na+ lactates seemed to affect the growth of L. delbrueckii and lactic acid productivity. After the comprehensive comparisons of LA final concentration, yield and productivity, 130 g/L glucose concentration was chosen as the optimal initial glucose concentration for L. rhamnosus DUT1908 at 50 °C.
Effect of nitrogen sources on l-lactic acid production
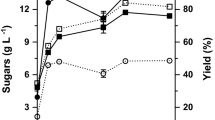
Yeast extract was the most commonly used nitrogen source in fermentation due to abundant B vitamins, which enhanced lactic acid production rates, but the high price hindered its use in large quantities [11]. In an economic analysis, the largest contributor to the production of lactic acid was yeast extract, accounting for about 38% of the overall average cost of production [21]. Various less expensive nitrogen sources, including peptone, corn steep liquor powder, and urea have been reported for their efficiency in the production of lactic acid [11, 15]. In our previous study, LA final concentration using CSLP medium was as high as what was obtained with yeast extract, while LA productivity was slightly higher by microbial consortium [15]. Therefore, the effect of MRS medium and varying CSLP concentration from 16 to 24 g/L on LA production by L. rhamnosus DUT1908 was investigated and the results are presented in Fig. 4. From the results, CSLP medium achieved a similar performance compared to MRS medium and the CSLP concentration had significant effect on productivity of LA, but little effect on final titer and yield of LA. In all the cases, glucose was completely consumed, and the final LA titer was similar as well as LA yield. When initial CSLP concentration was from 16 to 20 g/L, productivity of LA increased from 1.89 to 2.84 g/(L.h) with initial glucose concentration of 130 g/L. However, the concentration of CSLP above 20 g/L did not contribute to a significant increase in LA productivity. From the productivity point of view, 20 g/L was considered as the optimal CSLP concentration for LA production by L. rhamnosus DUT1908. In general, nitrogen sources in MRS medium used for lactic acid production contained 5 g/L yeast extract with a nitrogen content of 9% (w/w), 10 g/L peptone with a nitrogen content of 15% (w/w), 10 g/L beef extract with a nitrogen content of 13% (w/w). In this study, 20 g/L CSLP with a nitrogen content of 9% (w/w), approximately equivalent to half the nitrogen contents in MRS medium, achieved the higher LA concentration and productivity among these two media.
Comparison between SLSF and SSF using inedible APRH
To establish an effective method for the production of l-lactic acid, the SLSF and SSF strategies were contrasted with LA production. Figure 5 presented the fermentation results. LA titer in SSF process reached 91.56 g/L at 32 h while 28.34 g/L of glucose was retained in the medium. According to our previous report [6], about 82 and 78% glucose were obtained at pH 6.0 and 6.5 (50 °C), respectively, using liquefying and saccharifying enzymes dosages at the ratio of 1:1 (0.5 mL enzyme 100 g−1 APRH). At a higher temperature of 90 °C, a hydrolysis yield of about 98% was obtained at pH 6.0. Enzymatic pretreatment for liquefaction separation from saccharification and fermentation in SSF resulted in a release rate of glucose that is higher than the consumption rate of glucose. Thus, the high concentration of reduced sugar inhibited LA production and decreased LA productivity. In one step open SLSF process, 107.83 g/L of LA, with a yield to t theoretical glucose 0.89 g/g and an average productivity of 3.37 g/(L.h) was obtained, with a biomass loading of 20% (w/v). In this study, L. rhamnosus DUT1908 showed perfect high-thermotolerant performance up to 50 °C, which could achieve temperature compatibility of liquefaction, saccharification, and fermentation, and decrease the possibility of contamination by other microorganisms during large-scale fermentation. This enabled its fermentations under open non-sterilized conditions for SLSF process.
Table 1 presents a contrast among SLSF process of LA production based on starchy feedstocks in this study and those reported in the literatures. SLSF process was already conducted by LA producing strains of L. rhamnosus [11], L. casei [22, 23], L. delbrueckii [24], B. coagulans [9], and E. faecalis [6]. To the best of our knowledge, the reported optimal temperature of L. rhamnosus was about 42 °C. Therefore, besides SLSF process, the fermentation strategies of two-step fermentation (TSF) and SSF was also focused on using renewable and low-cost starch-based feedstocks by L. rhamnosus with an optimal temperature of 42 °C [11, 13, 25]. TSF strategy is not economical due to the high energy required for the pretreatment of starchy materials. Sterilization is usually employed during TSF process, and the high temperature and pressure results in the Maillard reaction, consequently leading to caramelization and the consumption of some nutrient sources. A relatively high LA titer was obtained from cassava powder according to the study of Wang et al., whereas the yield of LA to initial glucose in cassava powder was not high, with only 0.53–0.68 g/g obtained [11]. The results indicated that LA titer, yield and productivity were highest in SLSF process compared to SSF and TSF. Except for L. rhamnosus, other Lactobacillus strains, e.g. L. casei and L. delbrueckii were also investigated for LA production from starchy materials in SLSF process. A high LA titer of 162.0 g/L, with a yield to initial glucose of 0.78 g/g and a productivity of 3.4 g/(L.h) was achieved using barley starch as feedstock by L. casei NRRL B-441 [23]. The shortcoming in this SLSF process was the low yield of LA and the high dosage of nitrogen source, which included 10 g/L yeast extract and 10 g/L peptone. It should be noted that mixed culture was used to improve LA production in SLSF process from wheat by L. delbrueckii NCIM 2025 and L. casei NCIMB 3254 [24]. Mixed culture of Lactobacilli was superior in the fermentation than a single culture. A relatively high LA titer of 123.0 g/L and yield to initial glucose of 0.86 g/g was achieved while the average productivity of 2.3 g/(L.h) was obtained. In addition, only 1 g/L yeast extract used in mixed culture of Lactobacilli may be due to the symbiotic association to compensate for the nutritional limitations in the media [24]. One step open SLSF with soluble starch, cassava and sorghum flours was investigated using thermophilic B. coagulans IPE22 under 52 °C [9]. Due to the osmotic stress caused by the high level of substrate, glucose conversion rate and LA yield decreased obviously when initial glucose concentration increased over 80 g/L. Finally, LA productions from cassava four and sorghum flour by open SLSF process were investigated, and 61.42 and 55.99 g/L LA were obtained with yields to initial glucose of 0.77 and 0.76 g/g, respectively. Recently, one step open SLSF strategy was also investigated from starchy residues of inedible APRH by Enterococcus faecalis [6]. The final LA titer of 73.75 g/L, with a yield to initial glucose of 0.78 g/g and a productivity of 2.17 g/(L.h) was achieved. A lag phase of over 10 h existed in SLSF process which decreased the yield and productivity of LA. Compared with other reported studies, although LA final concentration was not highest in this study, the productivity and yield of LA was highest which indicated that one step open SLSF process was efficient for inedible APRH bioconversion by thermotolerant L. rhamnosus DUT1908.
Conclusions
Thermotolerant L. rhamnosus DUT1908 was obtained by adaptive laboratory evolution. This strain could tolerate a rather high temperature up to 50 °C, which was the highest tolerant temperature reported up to now. One-step open SLSF using inedible APRH was developed to get 107.8 g/L of LA titer with a yield to theoretical glucose of 0.89 g/g and productivity of 3.4 g/(L.h). To the best of our knowledge, such high yield and productivity of LA have rarely been reported from starchy feedstock. Due to the advantages of low raw materials cost, simplified operation and energy saving, one-step open SLSF process using inedible APRH showed an efficient way for LA production in large-scale application.
References
Abdel-Rahman MA, Sonomoto K (2016) Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J Biotechnol 236:176–192. https://doi.org/10.1016/j.jbiotec.2016.08.008
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31(6):877–902. https://doi.org/10.1016/j.biotechadv.2013.04.002
Liu G, Sun JE, Zhang J, Tu Y, Bao J (2015) High titer L-lactic acid production from corn stover with minimum wastewater generation and techno-economic evaluation based on Aspen plus modeling. Bioresour Technol 198:803–810. https://doi.org/10.1016/j.biortech.2015.09.098
Zhang Z, Li Y, Zhang J, Peng N, Liang Y, Zhao S (2020) High-titer lactic acid production by Pediococcus acidilactici PA204 from corn stover through fed-batch simultaneous saccharification and fermentation. Microorganisms. https://doi.org/10.3390/microorganisms8101491
Lu ZD, Lu MB, He F, Yu LJ (2009) An economical approach for d-lactic acid production utilizing unpolished rice from aging paddy as major nutrient source. Bioresour Technol 100(6):2026–2031. https://doi.org/10.1016/j.biortech.2008.10.015
Sun YQ, Yang Y, Liu HH, Wei CX, Qi WB, Xiu ZL (2020) Simultaneous liquification, saccharification and fermentation of l-lactic acid using aging paddy rice with hull by an isolated high-thermotolerant Enterococcus faecalis DUT1805. Bioprocess Biosyst Eng 43:1717–1724. https://doi.org/10.1007/s00449-020-02364-y
Wang Y, Cai D, He ML, Wang Z, Qin PY, Tan TW (2015) Open fermentative production of l-lactic acid using white rice bran by simultaneous saccharification and fermentation. Bioresour Technol 198:664–672. https://doi.org/10.1016/j.biortech.2015.09.010
Das M, Banerjee R, Bal S (2008) Evaluation of physicochemical properties of enzyme treated brown rice (Part B). Lwt-Food Sci Technol 41(10):2092–2096. https://doi.org/10.1016/j.lwt.2007.11.018
Wang YJ, Cao WF, Luo JQ, Qi BK, Wan YH (2019) One step open fermentation for lactic acid production from inedible starchy biomass by thermophilic Bacillus coagulans IPE22. Bioresour Technol 272:398–406. https://doi.org/10.1016/j.biortech.2018.10.043
Trakarnpaiboon S, Srisuk N, Piyachomkwan K, Yang ST, Kitpreechavanich V (2017) l-Lactic acid production from liquefied cassava starch by thermotolerant Rhizopus microsporus: characterization and optimization. Process Biochem 63:26–34. https://doi.org/10.1016/j.procbio.2017.08.019
Wang L, Zhao B, Liu B, Yang C, Yu B, Li Q, Ma C, Xu P, Ma Y (2010) Efficient production of L-lactic acid from cassava powder by Lactobacillus rhamnosus. Bioresour Technol 101(20):7895–7901. https://doi.org/10.1016/j.biortech.2010.05.018
Gao MT, Kaneko M, Hirata M, Toorisaka E, Hano T (2008) Utilization of rice bran as nutrient source for fermentative lactic acid production. Bioresour Technol 99(9):3659–3664. https://doi.org/10.1016/j.biortech.2007.07.025
Li Z, Lu JK, Yang ZX, Han L, Tan TW (2012) Utilization of white rice bran for production of l-lactic acid. Biomass Bioenerg 39:53–58. https://doi.org/10.1016/j.biombioe.2011.12.039
Abedi E, Hashemi SMB (2020) Lactic acid production—producing microorganisms and substrates sources-state of art. Heliyon 6(10):e04974. https://doi.org/10.1016/j.heliyon.2020.e04974
Sun YQ, Xu ZZ, Zheng YF, Zhou JJ, Xiu ZL (2019) Efficient production of lactic acid from sugarcane molasses by a newly microbial consortium CEE-DL15. Process Biochem 81:132–138. https://doi.org/10.1016/j.procbio.2019.03.022
Coelho LF, de Lima CJB, Bernardo MP, Contiero J (2011) D(-)-Lactic acid production by Leuconostoc mesenteroides B512 using different carbon and nitrogen sources. Appl Biochem Biotechnol 164(7):1160–1171. https://doi.org/10.1007/s12010-011-9202-6
Ouyang J, Ma R, Zheng Z, Cai C, Zhang M, Jiang T (2013) Open fermentative production of l-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresour Technol 135:475–480. https://doi.org/10.1016/j.biortech.2012.09.096
Siragusa S, De Angelis M, Calasso M, Campanella D, Minervini F, Di Cagno R, Gobbetti M (2014) Fermentation and proteome profiles of Lactobacillus plantarum strains during growth under food-like conditions. J Proteomics 96:366–380. https://doi.org/10.1016/j.jprot.2013.11.003
Ye LD, Zhao H, Li Z, Wu JC (2013) Improved acid tolerance of Lactobacillus pentosus by error-prone whole genome amplification. Bioresour Technol 135:459–463. https://doi.org/10.1016/j.biortech.2012.10.042
Nakano S, Ugwu CU, Tokiwa Y (2012) Efficient production of d-(-)-lactic acid from broken rice by Lactobacillus delbrueckii using Ca(OH)2 as a neutralizing agent. Bioresour Technol 104:791–794. https://doi.org/10.1016/j.biortech.2011.10.017
Altaf M, Naveena BJ, Reddy G (2007) Use of inexpensive nitrogen sources and starch for l(+) lactic acid production in anaerobic submerged fermentation. Bioresour Technol 98(3):498–503. https://doi.org/10.1016/j.biortech.2006.02.013
John RP, Nampoothiri KM, Pandey A (2006) Simultaneous saccharification and fermentation of cassava bagasse for l-(+)-lactic acid production using Lactobacilli. Appl Biochem Biotechnol 134(3):263–272. https://doi.org/10.1385/Abab:134:3:263
Linko YY, Javanainen P (1996) Simultaneous liquefaction, saccharification, and lactic acid fermentation on barley starch. Enzyme Microb Tech 19(2):118–123. https://doi.org/10.1016/0141-0229(95)00189-1
John RP, Nampoothiri KM, Pandey A (2006) Simultaneous saccharification and l-(+)-lactic acid fermentation of protease-treated wheat bran using mixed culture of lactobacilli. Biotechnol Lett 28(22):1823–1826. https://doi.org/10.1007/s10529-006-9159-7
Gao C, Ma CQ, Xu P (2011) Biotechnological routes based on lactic acid production from biomass. Biotechnol Adv 29(6):930–939. https://doi.org/10.1016/j.biotechadv.2011.07.022
Acknowledgements
This work was supported by the National Natural Science Foundation of China [Grant Numbers 22078043].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sun, Y., Liu, H., Yang, Y. et al. High-efficient l-lactic acid production from inedible starchy biomass by one-step open fermentation using thermotolerant Lactobacillus rhamnosus DUT1908. Bioprocess Biosyst Eng 44, 1935–1941 (2021). https://doi.org/10.1007/s00449-021-02573-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-021-02573-z