Abstract
We optimized the production of lactic acid by Lactobacillus plantarum BL011, using acid and enzymatic soybean hull hydrolysates as cultivation media. Selection of cultivation conditions was performed in Erlenmeyer flasks, in orbital shaker at 37 °C and 120 rpm, pH 6.0. Plackett–Burman experimental design was performed as tool to identify the best cultivation temperature and whether medium supplementation should be necessary for further experiments. Crude yeast extract and magnesium sulfate supplementation, temperature of 30 °C, 48 h of cultivation, were the ideal conditions defined in batch cultivations (anaerobiosis and controlled pH), allowing for the production of 39.34 g•L−1 of lactic acid, and a productivity of 0.82 g•(L•h)−1 at 48 h of cultivation. Fed-batch bioreactor cultivations were performed, applying 12-h linear ascending feeding strategy, using enzymatic hydrolysate containing either 90 g•L−1 or 130 g•L−1 of total sugars, resulting in a productivity above 1.5 g•(L•h)−1 and acid lactic concentration of 58.6 g•(L•h)−1, after 48 h of cultivation. Our results demonstrate the possibility of using this abundant waste lignocellulosic biomass hydrolysates to cultivate Lactobacillus and to obtain high concentrations of lactic acid.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In view of worldwide efforts regarding implementation of sustainable processes, the impact of studies to develop potential alternative technologies is of foremost importance. Among other factors, the high costs of raw materials involved in bioprocesses are concerns pushing the development of the biorefinery concept as a sustainable key technology for the next decades Biorefineries are facilities where different processes and technologies are integrated in ways to convert several types of biomass [1, 2], such as agro-industrial byproducts and residues, which are low-cost materials widely available, into high added-value bioproducts [3].
Several non-food biomass have already been used in different bioprocess, such as whey, residual glycerol from biodiesel synthesis, reforesting wood residues, and byproducts of agroindustry, such as sugarcane bagasse, cereals or grains straws, and hulls [2, 4, 5]. The use of these raw materials as substrates for microbial cultivations is an important procedure, allowing the reduction of production costs of culture media, besides assisting in reducing environmental impacts caused by their improper disposal [6, 7]
Lactic acid is an important bioproduct, with ever increasing demand [2, 8, 9]. The global lactic acid market required about 1400 kt in 2021, production which is expected to reach 2000 kt by 2025, which should represent a market value of approximately USD 10 billion, globally [9].
Lactic acid (LAC) occurs naturally as two enantiomeric forms: D-(-)-lactic acid (D-LA) and L-( +)-lactic acid (L-LA). LAC finds several industrial applications, such as acidulants, emulsifier, flavoring, and antimicrobial agent in food industry, as a raw material in pharmaceutical applications, and as an essential precursor for polylactic acid (PLA) production [4, 10, 11]. LAC biotechnological production, in contrast with its chemical synthesis, can yield a high optical purity product, which is very important for its applications [11, 12]. The enantiomeric form L-LAC is mainly used in the food and pharmaceutical (cosmetics applications) industries because it can be completely assimilated by the human organism [5, 13]. In the green chemistry field, both LAC isomers can be used to produce different compounds as building blocks. For instance, it can be used to obtain propylene glycol, acrylic acid, 2,3-pentadione, and, perhaps the most important application, as monomers to produce polylactic acid (PLA) [2, 4, 13, 14]. PLA is a biodegradable polymer with high chemical resistance, which is an interesting alternative to traditional fossil plastics. This polymer can also be used to obtain fibers, nonwoven fabrics, and films among many other applications [15,16,17]. As with lactic acid, the market demand for PLA demand should grow, reaching USD 6.5 billion worldwide by 2025 [2, 9].
So far, most of the industrial production of LAC is obtained by fermentative processes, mostly using pure sugar, simple carbohydrates as raw material, which may represent a high cost [4, 18]. Because of this fact, the utilization of alternative substrates and the development of genetically modified microorganisms have gained interest to be applied in this bioprocess [2, 6, 19,20,21,22,23].
The use of lignocellulosic biomass hydrolysates as an alternative in bioprocess still poses some challenges because they present a mixture of hexoses and pentoses, besides containing some amounts of toxic compounds for microbial cells, such as hydroxymethylfurfural and furfural [6, 8], prompting the search for new strategies and microorganisms capable to grow in these [24,25,26,27,28].
Soybean is among the most cultivated crops in the world, with a global production of approximately 337 million tons [29]. Soybean hull hydrolysates are a potential alternative source to be used in bioprocess since they have high sugar concentrations and produce low concentrations of inhibitory compounds [30,31,32,33]. Some recent studies have shown that soybean hull hydrolysates can be successfully used as substrate for 2,3-butanediol and ethanol production [31,32,33,34]. So far, soybean hull hydrolysate has not been reported to produce LAC.
In this study, we evaluated and improved the production of LAC by Lactobacillus plantarum BL011, a wild strain isolated by our group, in soybean hull acid and enzymatic hydrolysates. The work consisted in analyzing several production parameters, such as pH, temperature, and medium supplementation, both in shaker and in bioreactors. Finally, we applied fed-batch strategies to further improve the production of LAC.
2 Materials and methods
2.1 Microorganisms, cell maintenance, and inocula
In this study, we cultivated Lactobacillus plantarum BL011 isolated from Serrano cheese [35]. The strain was kept as a 50% glycerol/MRS suspension at −80 °C. The pre-inocula for the cultivations were carried out in 500 mL Erlenmeyer flasks filled with 100 mL of liquid MRS, for orbital shaker experiments, and with 150 mL for the bioreactor experiments. The flasks were incubated in orbital shakers at 120 rpm, 37 °C for about 12 h until late exponential phase, checked by spectroscopy (1.0 OD at 600 nm). Cells were then collected by centrifugation (3500 g, 4 °C, 15 min), discarding the liquid supernatant. For the orbital shaker experiments, the cell pellets were resuspended directly into the hydrolysate medium to be used as inoculum, while in the bioreactor experiments, cells were resuspended into the supplementation medium (10% working volume fraction). This method was adopted to eliminate possible fermentation products that are produced during pre-inoculum cultivation.
2.2 Soybean hull hydrolysates
Soybean hulls were obtained from local mills (State of Rio Grande do Sul, Brazil, centroid geo-coordinates at 30°51'04"S and 51°48'44"W), as dried material. The solubilization of the hemicellulose fraction of in natura soybean hulls was performed by diluted acid hydrolysis, using a 1% solution of H2SO4 and solid:liquid ratio of 1:10, at 121 °C for 40 min [30]. Solid and liquid fractions were separated by centrifugation (3000 g, 4 °C, 30 min). The remaining solid fraction was washed thoroughly using distilled water to remove the acid residues, whereas the liquid fraction (the acid hydrolysate - AH) was stored for further use. For the enzymatic hydrolysis, the washed solid fraction was incubated in flasks added of CELLUCLAST® 1.5 L (Novozymes, Brazil) at concentration of 15 FPU•g−1 and sodium citrate buffer 1.0 M at solid:liquid ratio of 1/20 for 96 h, 50 °C and incubated in orbital shakers at 120 rpm [31] generating the enzymatic hydrolysate (EH). AH and EH hydrolysates were concentrated under vacuum at 60 °C to reduce water content and to obtain high sugar concentrations to be used as substrates in the cultivations. The hydrolysates were composed of similar concentrations of glucose and xylose (26.16± 0.29 g•L-1 glucose; 22.98± 0.63 g•L-1 xylose), with total sugar concentration of 60 g•L-1 (with arabinose composing the remaining sugars; arabinose was not metabolized by this strain; thus, its concentration was not shown in the graphs). With this sugar composition, glucose concentration in the medium would not be much higher than the concentration of xylose, an approach to avoid the catabolic repression of xylose by glucose.
No other procedures were performed before the fermentations, and the hydrolysates were not detoxified to remove HMF and furfural eventually present.
2.3 Cultivations in orbital shaker
The cultivations were initially performed in orbital shaker flasks, at 37 °C and 120 rpm using a mixture of both hydrolysates [33]. Preliminary experiments tested the technical viability of the process by cultivating the strain in the mixture of soybean acid and enzymatic hydrolysates obtained by vacuum concentration. All experiments in this section were performed in Erlenmeyer flasks of 500 mL, with a 1:5 headspace. The initial pH was set at 6.0, without controlling. Cultivation kinetics were monitored by collecting 2-mL samples every 12 h. All experiments were conducted in duplicates.
2.4 Plackett–Burman experimental design
A Plackett–Burman experimental design with a triplicate at the central point was performed in order to evaluate the influence of the temperature and the need to supplement the hydrolysates in the LAC production. The supplementation tests included crude yeast extract (CYE), which is non-purified, much cheaper material compared to the traditional analytical grade yeast extract; magnesium sulfate (MgSO4); and manganese sulfate (MnSO4). These variables were previously reported to affect the Lactic Acid Bacteria (LAB) metabolism for LAC production [2, 36, 37]. The variable levels are presented in Table 1. The experiments were performed in orbital shaker for 48 h at 120 rpm. After the statistical analysis of the design, validation and control experiments were performed to confirm the results with the significant variables. These experiments were conducted in orbital shaker, at 30 °C and 37 °C, in duplicates.
2.5 Submerged cultures in bioreactors
The submerged bioreactor cultures were performed in 2-L vessels (Biostat B model, Braum Biotech International, Germany), with 1.5-L working volume [36]. All the bioreactor fermentations were performed at the temperature of 30 °C (selected after the experiments in the section 2.4), using 2 Rushton turbines at agitation speed rate of 300 rpm. In this set of experiments, the strain was cultivated using the mixture of AH and EH supplemented with 7.5 g•L−1 CYE and 0.05 g•L−1 MgSO4 (Table 1). All the experiments were carried out in duplicates. For pH adjusting before starting of fermentations, as well as in the experiments with controlled pH, we used NaOH 10.0 M and H3PO4 5.0 M.
2.5.1 Batch cultivations
In the batch bioreactor fermentations, we investigated the influence of oxygen in the cultivations by testing aerobiosis (300 rpm and aeration rate of 1 vvm air) and anaerobiosis experiments. For the best results, a pH control strategy at 6.0 was carried out.
2.5.2 Fed-batch cultivations
For the fed-batch bioreactor cultivations, a linear feeding strategy was performed. The bioreactor was fed with concentrated enzymatic hydrolysate because it had a higher concentration of glucose. Two feeding strategies were performed: one using a feeding phase of 12 h, making for a total feeding volume of 400 mL of EH, with a sugar concentration of 90 g•L−1 in the feeding medium; another using the same linear feeding strategy, but with a sugar concentration of 130 g•L−1. In both strategies, the feeding media were supplemented with 7.5 g•L-1 CYE and 0.05 g•L−1 MgSO4.
For the fed-batch linear strategy, we calculated the average sugar consumption (giving in g•(L•h)−1) as they were consumed during the exponential LAC production phase, in the best batch bioreactor condition. Based on this, a feeding flow was calculated by Eq. (1):
where F is the feeding flow, in mL•min−1; a and b are feeding constants; and t, the feeding time. For the strategy of 90 g•L−1 sugar, b constant was defined as 0.15 mL•min−1 and the a constant was 1.06•10−2 mL•min−2 for the linear ascending feeding profile of 12 h. For the strategy using 130 g•L−1 sugar, the b constant was defined as 0.10 mL•min−1 and the a constant was 7.59•10−2 mL•min−2.
2.6 Analytical methods
The concentration of sugars (glucose, xylose, and arabinose) and organic acids (lactic, citric, and acetic) was determined by HPLC (Shimadzu, Japan) equipped with a refractive index detector and Aminex HPX-87H column (300•7.8 mm, Bio-Rad, USA). The mobile phase was 5 mM sulfuric acid as eluent, at 45 °C, flow rate of 0.6 mL•min−1, and sample volumes of 20 µL [33]. All the samples were centrifugated (3500 g, 4 °C, 15 min), diluted in distilled water (1:10), and filtered using cellulose acetate membrane, with 0.22-µm pore size (Sartorius, Germany).
The osmotic pressure of the hydrolysates was measured by osmometry, placing 30-µL samples into the chamber of an osmometer (VAPRO 5520, Wescor Inc, USA).
Cell growth could not be measured by either gravimetry or CFU technique, prevented by the characteristics of hydrolysates (fine particles in suspension), which interfered with method precision.
2.7 Kinetic parameters and statistical analysis
The volumetric productivity of lactic acid, QP, (g•(L•h)−1), was determined by the ratio between the amount of LAC produced and the time of cultivation.
Statistical analyses of the experimental design were performed using the software Statistica 12.0 (StatSoft Inc, São Paulo, Brazil) at 5% significance level. Statistical analysis of all experiments was also performed using Statistica 12.0, by submitting the results to analysis of variance (One-Way ANOVA) and Tukey’s test, at 5% significance level.
3 Results and discussion
3.1 Characteristics of acid and enzymatic soybean hull hydrolysates
The acid hydrolysis technique solubilizes lignin and the hemicellulose fraction, liberating C-5 and C-6 sugars, whereas the enzymatic hydrolysis mainly breaks the cellulose chains, producing moieties of glucose. After the acid hydrolysis, the hydrolysate presented a sugar composition of 6.29 ± 1.42 g•L−1 of xylose, 4.56 ± 0.46 g•L−1 arabinose, and 1.22 ± 0.22 g•L−1 of glucose, whereas the enzymatic hydrolysate produced a sugar composition of 12.75 ± 1.18 g•L−1 of glucose and 1.99 ± 0.69 g•L−1 of xylose. After concentration, the hydrolysates were mixed to produce a medium with approximately same concentrations of glucose and xylose. The osmotic pressure of the mixed hydrolysate used in this study for fermentation was 2344.54 ± 167.27 mmol•kg−1. This high osmotic pressure, although similar to other lignocellulosic hydrolysates described in the literature [31,32,33,34], is usually considered too high and would require a robust microorganism capable of growing in such a rash environment. Cortivo et al. [31] evaluated the capacity of recombinant industrial Saccharomyces cerevisiae YRH 396 and YRH 400 strains to ferment sugars from oat and soybean hull hydrolysates into ethanol and xylitol. The osmotic pressure of these hydrolysates was reported to be in between 1385 ± 29 and 3645 ± 57 Osm kg−1 depending on the mixture and method of hydrolysis, but the strains used by the authors were capable of growing in them. However, few microorganisms were described in the literature that are capable of growing in such a high osmotic pressure environment. Bacteria are bounded by semipermeable cytoplasmic membranes, often including aquaporins, with many species also surrounded by a rigid, elastic, and porous cell wall (the murein or peptidoglycan layer) that determines cell shape. Bacterial cells exposed consistently to a very high osmotic pressure must maintain correspondingly high cytoplasmic solute concentrations. Evidence suggests that the regulation of cytoplasmic composition and hydration is a key objective of cellular homeostasis [38].
Some inhibitory compounds commonly formed during the hydrolysis process were also detected in the mixed hydrolysate, including acetic acid and formic acid, but the concentrations of both acids were very low (< 1 g•L−1), as were the concentrations of HMF (~0.25 g•L−1) and furfural (~0.08 g•L−1), which should not present any relevant influence over cell metabolism.
3.2 Orbital shaker cultivations
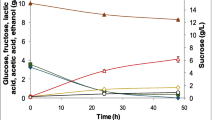
Preliminary experiments in orbital shaker were carried out to verify the viability of the process of growing L. plantarum BL011 in the mixture of hydrolysates since this medium has never been described before for LAB cultures. The initial sugar concentration for these experiments was 60 g•L-1 of fermentable sugars (glucose and xylose, Fig. 1). Because the strain was unable to metabolize arabinose, the concentration of this sugar was not plotted in the figures. The results of this experiment are represented in Fig. 1.
This mixed hydrolysate strategy was used in all subsequent experiments. Glucose and xylose are consumed simultaneously between 6 and 12 h of cultivation. In between 12 and 36 h, xylose is consumed more slowly, while glucose continues to be rapidly metabolized and is depleted. After a likely adapting phase of almost 6 h, the strain starts producing LAC up to 72 h of fermentation (43.27 ± 0.63 g•L−1, corresponding to a productivity of 0.60 ± 0.01 g•(L•h)−1), with a consequent drop in the pH, which stabilized at 4.3. Table 2 presents the main results obtained for LAC production using shaker cultivation. Acetic acid was also produced (2 g•L−1), but no other metabolites, usually produced by heterofermentative LAB, were detected. Some researchers have already reported that there are LAB strains that can produce lactic acid from different carbon sources without necessarily producing secondary metabolites [2, 39].
3.3 Evaluation of temperature and supplementation on LAC production
A Placket-Burman experimental design was run and analyzed using the Statistica 12 software, with confidence level of significant variables > 95% as a tool to evaluate whether should be any influence of the temperature and supplementation of the soybean hull hydrolysates concerning the lactic acid production.
The matrix of the experimental design is presented in Table 3. The quadratic coefficient (R2 = 0.86) suggests a good representation for the impacts of tested variables on the production of lactic acid. Significant variables, as can be seen in the Pareto Diagram (see Supplementary Material), were temperature (x1, −3.5296, negative influence, suggesting the use of temperature at 30 °C) and the positive effects of supplementation with yeast extract (x2, 3.3444) and the addition of magnesium sulfate (x4, 3.37256). The medium supplementation with manganese sulfate (x3) was not significant. The best values for LAC production and productivity (40 g•L−1 and 0.83 g•(L•h)−1 (run 4, Table 3) and 38.38 g•L−1 0.80 g•(L•h)−1 (run 6, Table 3)) were met at temperature 30 °C (negative effect) and MgSO4 supplementation of 0.1 g•L−1 (positive effect), showing that lower temperatures can be used for the LAC production. The Pareto graph of this Plackett-Burman experimental design is presented in the Supplementary Material.
Validation experiments were carried out to confirm this result (Table 4), in which L. plantarum BL011 was cultivated at 30 °C and 37 °C, supplementing the soybean hydrolysate with CYE and MgSO4 at levels 0 and +1 (Table 1). The results showed no statistically significant differences between the temperatures, nor for the supplementation level. Based on this, fermentation conditions at 30 °C and supplementation with MgSO4 and CYE at level 0 (0.05 g•L-1 and 7.5 g•L−1, respectively) were adopted in the next experiments. Relating to the temperature, the value of 30 °C was adopted according to runs 4 and 6 (Table 3), where the highest LAC production was found.
3.4 Batch fermentations
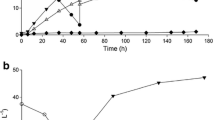
Batch bioreactor cultivations under the conditions set up by the experimental design were conducted in which we could analyze the effects of pH control and aeration over LAC production. We first tested the influence of aeration or anaerobiosis, leaving the pH uncontrolled (Figs. 2 and 3) to determine whether this variable would be important in the process. Although LAB normally produces high amounts of lactic acid under anaerobiosis, these bacteria possess aerotolerant metabolism and can also produce LAC in the presence of oxygen [2, 4]. Results of experiments under oxygen limitation (300 rpm and 1 vvm) are presented in Fig. 2. The highest LAC concentration of 33.09 g•L−1 (productivity of 0.69 g•(L•h)−1) was obtained at 48 h of cultivation. However, under anaerobiosis (Fig. 3), a faster kinetic and higher LAC production were obtained, (36.6 g•L−1 and productivity of 0.76 g•(L•h)−1) at 48 h of cultivation.
The pH profile in both experiments dropped similarly until stabilization at 4.3, slowing the strain metabolism and LAC production. Although lactic acid bacteria have cellular mechanisms of protection against a pH drop, they present inhibition by product [2]. The effects caused by medium acidification were reported in a recent study, in which is shown that L. delbrueckii subsp. bulgaricus directed pyruvate metabolism towards acetyl-CoA instead of lactate [40], directing the production of fatty acids, which changes in the cell membrane fluidity. The same metabolism was described for L. casei and L. rhamnosus [41, 42].
Bioreactor cultivation under anaerobiosis and pH controlled at 6.0 were then performed and the results are presented in Fig. 4. This condition produced the best results concerning LAC production and productivities in batch fermentations. The cultivation time could be reduced to 24 h, increasing the productivity in approximately 52% in this specific time. The profile of sugar consumption also shows an increase in the ability of cells to consume xylose, which was twofold improved, a condition important in the development of this process. In all bioreactor experiments, a small amount of acetic acid was produced, a normal metabolite associated with heterolactic fermentations, where LAB can produce acetic acid from metabolizing pentoses [4].
The incomplete xylose consumption is possibly related to lack of enzymes involved in the metabolic pathway, and some reports address this constrain by genetic engineering of LAB. In a recent study, xylose assimilating genes encoding xylose isomerase and xylulokinase were cloned into L. plantarum NCIMB 8826 [43]. The recombinant strain was capable to consume all the sugars, including the pentoses, in a medium obtained from the hydrolysis of corn stover.
Our results compare very well with reports on the literature in which authors used lignocellulosic biomass hydrolysates in LAB cultivations. In a recent work, the authors cultivated L. casei TISTR 390 in sugarcane bagasse hydrolysate, containing 34 g•L−1 total sugars in batch bioreactors at 37 °C and uncontrolled pH (initial pH 7.0), obtaining 21.3 g•L−1 of lactic acid after 120 h [44]. In comparison, L. rhamnsosus B103 was cultivated in batch bioreactors, at 37 °C and pH controlled at 6.2, producing 57 g•L−1 of lactic acid after 48 h, but the medium used was a dairy industry residue containing 90 g•L−1 of total sugar (60 g•L−1 lactose) and not a lignocellulosic hydrolysate [27].
3.5 Fed-batch fermentations
Fed-batch cultivations were carried out under the best conditions obtained in the previous experiments. This strategy was used to determine whether sugar-controlled feeding would have a positive impact on LAC production. The feeding substrate was the enzymatic hydrolysate with sugar concentrations of 90 and 130 g•L−1 (Figs. 5 and 6, respectively). Feeding starts at 24 h of batch cultivation (vertical dashed line), when cells consumed almost all glucose in the medium (the first fed-batch with feeding for 12 h is presented in Fig. 5).
Lactic acid production kinetic by L. plantarum BL011 in fed-batch using the feeding sugar concentration of 90 g•L−1 with 12 h of feeding, at 30 °C, 300 rpm stirring rate, anaerobiosis, and pH controlled in 6.0. Labels: glucose (∆), xylose (○), lactic acid (▲), and acetic acid (●). Results are the mean of duplicates
Lactic acid production kinetic by L. plantarum BL011 in fed-batch using the feeding sugar concentration of 130 g•L−1 with 12 h of feeding, at 30 °C, 300 rpm stirring rate, anaerobiosis, and pH controlled in 6.0. Labels: glucose (∆), xylose (○), lactic acid (▲), and acetic acid (●). Results are the mean of duplicates
The sugar concentration, especially glucose, was kept close to exhaustion throughout the feeding period in both sets of experiments, indicating that the strategy was successful. In comparison with the batch bioreactor experiments, there was a marked increase in LAC concentrations (51.12 g•L−1 at 48 h), approximately 32% increase in the volumetric productivity, as is shown in Table 5.
Figure 6 shows the kinetics when we increased the sugar concentration of feeding medium to 130 g•L−1 total sugars. This experiment produced the highest LAC concentration and productivity, compared to all bioreactor cultivations (Table 5), reaching 50.26 g•L−1 LAC at the end of feeding (36 h of cultivation) and 58.59 g•L−1 LAC after 48 h of run.
So far, few studies use similar strategies of feeding of lignocellulosic biomass hydrolysates to produce LAC. Zhang et al. [43] obtained 61.4 g•L−1 of lactic acid, with a productivity of 0.32 g•(L• h)−1 in cultures of genetically modified L. plantarum NCIMB 8826 ΔldhL1 growing in corn stover hydrolysate (fed-batch SSF process in Erlenmeyers flasks at 37 °C and 150 rpm). In another study, L. rhamnosus B103 was cultivated in dairy industry waste (containing lactose from whey), producing 143.7 g•L−1 LAC, with a productivity of 1.49 g•(L•h)−1 using a pH-stat strategy [27]. Hu et al. [45] cultivated L. pentosus FL0421 in a fed-batch SSF strategy, using a NaOH pretreated and washed corn stover as substrate (at 37 °C and pH 6.0), producing 92.3 g•L−1 LAC in 48 h (productivity 1.92 g•(L•h) −1).
Therefore, the results obtained in the fed-batch experiments in our work are promising approaches to use this cheap medium for the LAC production.
4 Conclusions
For the first time it is shown that soybean hull hydrolysates obtained from acid and enzymatic treatments are potential substrate for the bioproduction of lactic acid by LAB, specifically Lactobacillus plantarum. The hydrolysis of this agro-industrial byproduct results in a sugar-rich medium, low in concentration of inhibitory compounds, but showing a high osmotic pressure, which is usually toxic for microbial cells. The LAB L. plantarum BL011 proved to be a robust strain, capable of converting the sugars in the hydrolysates into lactic acid, even under those harsh medium conditions. Controlling the pH had a positive impact on this process, reducing the necessary fermentation time to a short 24 h, significantly increasing the process productivity. Finally, the fed-batch strategy presented statistically improved lactic acid concentration and productivity in comparison with the batch bioreactors, being a promising tool to cultivate L. plantarum in hydrolysates at an industrial scale.
References
Cherubini F (2010) The biorefinery concept: using biomass instead of oil for producing energy and chemicals. Energy Convers Manag 51:1412–1421. https://doi.org/10.1016/j.enconman.2010.01.015
Alves de Oliveira R, Komesu A, Vaz Rossell CE, Maciel Filho R (2018) Challenges and opportunities in lactic acid bioprocess design—from economic to production aspects. Biochem Eng J 133:219–239. https://doi.org/10.1016/j.bej.2018.03.003
Sillanpää M, Ncibi C (2017) A sustainable bioeconomy. Springer International Publishing, Cham
Cubas-Cano E, González-Fernández C, Ballesteros M, Tomás-Pejó E (2018) Biotechnological advances in lactic acid production by lactic acid bacteria: lignocellulose as novel substrate. Biofuels, Bioprod Biorefining 12:290–303. https://doi.org/10.1002/bbb.1852
Krishna BS, Nikhilesh GSS, Tarun B, et al (2019) Industrial production of lactic acid and its applications. Compr Biotechnol 208–217
Isikgor FH, Becer CR (2015) Lignocellulosic biomass: a sustainable platform for the production of bio-based chemicals and polymers. Polym Chem 6:4497–4559. https://doi.org/10.1039/C5PY00263J
Iqbal HMN, Kyazze G, Keshavarz T (2013) Advances in the valorization of lignocellulosic materials by biotechnology: an overview. BioResources 8:. https://doi.org/10.15376/biores.8.2.3157-3176
Abdel-Rahman MA, Sonomoto K (2016) Opportunities to overcome the current limitations and challenges for efficient microbial production of optically pure lactic acid. J Biotechnol 236:176–192. https://doi.org/10.1016/j.jbiotec.2016.08.008
Grand New Research (2019) Lactic acid market size worth $8.77 billion by 2025. https://www.grandviewresearch.com/ Accessed 20 Aug 2020
Djukić-Vuković A, Mladenović D, Ivanović J et al (2019) Towards sustainability of lactic acid and poly-lactic acid polymers production. Renew Sustain Energy Rev 108.https://doi.org/10.1016/j.rser.2019.03.050
Castillo Martinez FA, Balciunas EM, Salgado JM et al (2013) Lactic acid properties, applications and production: a review. Trends Food Sci Technol 30:70–83. https://doi.org/10.1016/j.tifs.2012.11.007
Das D, Goyal A (2012) Lactic acid bacteria in food industry. Microorganisms in sustainable agriculture and biotechnology. Springer, Netherlands, Dordrecht, pp 757–772
Naveena BJ, Altaf M, Bhadriah K, Reddy G (2005) Selection of medium components by Plackett-Burman design for production of l(+) lactic acid by Lactobacillus amylophilus GV6 in SSF using wheat bran. Bioresour Technol 96:485–490. https://doi.org/10.1016/j.biortech.2004.05.020
Cui F, Li Y, Wan C (2011) Lactic acid production from corn stover using mixed cultures of Lactobacillus rhamnosus and Lactobacillus brevis. Bioresour Technol 102:1831–1836. https://doi.org/10.1016/j.biortech.2010.09.063
Grewal J, Khare SK (2018) One-pot bioprocess for lactic acid production from lignocellulosic agro-wastes by using ionic liquid stable Lactobacillus brevis. Bioresour Technol 251:268–273. https://doi.org/10.1016/j.biortech.2017.12.056
Zhang Y, Vadlani PV (2013) d-Lactic acid biosynthesis from biomass-derived sugars via Lactobacillus delbrueckii fermentation. Bioprocess Biosyst Eng 36:1897–1904. https://doi.org/10.1007/s00449-013-0965-8
Ulery BD, Nair LS, Laurencin CT (2011) Biomedical applications of biodegradable polymers. J Polym Sci Part B Polym Phys 49:832–864. https://doi.org/10.1002/polb.22259
Wang Y, Tashiro Y, Sonomoto K (2015) Fermentative production of lactic acid from renewable materials: recent achievements, prospects, and limits. J Biosci Bioeng 119:10–18. https://doi.org/10.1016/j.jbiosc.2014.06.003
Zhou C-H, Xia X, Lin C-X et al (2011) Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem Soc Rev 40:5588. https://doi.org/10.1039/c1cs15124j
Chen Y, Wu C, Fan X et al (2020) Engineering of Trichoderma reesei for enhanced degradation of lignocellulosic biomass by truncation of the cellulase activator ACE3. Biotechnol Biofuels 13:62. https://doi.org/10.1186/s13068-020-01701-3
Hasunuma T, Kondo A (2012) Consolidated bioprocessing and simultaneous saccharification and fermentation of lignocellulose to ethanol with thermotolerant yeast strains. Process Biochem 47:1287–1294. https://doi.org/10.1016/j.procbio.2012.05.004
Cunha JT, Romaní A, Inokuma K et al (2020) Consolidated bioprocessing of corn cob-derived hemicellulose: engineered industrial Saccharomyces cerevisiae as efficient whole cell biocatalysts. Biotechnol Biofuels 13:138. https://doi.org/10.1186/s13068-020-01780-2
Tenhaef N, Kappelmann J, Eich A et al (2021) Microaerobic growth-decoupled production of α-ketoglutarate and succinate from xylose in a one-pot process using Corynebacterium glutamicum. Biotechnol J 16:2100043. https://doi.org/10.1002/biot.202100043
Sakdaronnarong C, Srimarut N, Lucknakhul N et al (2014) Two-step acid and alkaline ethanolysis/alkaline peroxide fractionation of sugarcane bagasse and rice straw for production of polylactic acid precursor. Biochem Eng J 85:49–62. https://doi.org/10.1016/j.bej.2014.02.003
Cai W, Chen Q, Xuan H et al (2019) One-pot synthesis of lactic acid from cellulose over a sulfonated Sn-KIT6 catalyst. Korean J Chem Eng 36:513–521. https://doi.org/10.1007/s11814-019-0236-8
Abdel-Rahman MA, Xiao Y, Tashiro Y et al (2015) Fed-batch fermentation for enhanced lactic acid production from glucose/xylose mixture without carbon catabolite repression. J Biosci Bioeng 119:153–158. https://doi.org/10.1016/j.jbiosc.2014.07.007
Bernardo MP, Coelho LF, Sass DC, Contiero J (2016) L-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Brazilian J Microbiol 47:640–646. https://doi.org/10.1016/j.bjm.2015.12.001
Aso Y, Tsubaki M, Dang Long BH et al (2019) Continuous production of d-lactic acid from cellobiose in cell recycle fermentation using β-glucosidase-displaying Escherichia coli. J Biosci Bioeng 127:441–446. https://doi.org/10.1016/j.jbiosc.2018.09.011
USDA (2020) United States Department of Agriculture. Foreign agriculture service. Oilseeds world mark trade https//apps.fas.usda.gov/ Accessed: 20 Aug 2020
Cassales A, de Souza-Cruz PB, Rech R, Záchia Ayub MA (2011) Optimization of soybean hull acid hydrolysis and its characterization as a potential substrate for bioprocessing. Biomass Bioenerg 35:4675–4683. https://doi.org/10.1016/j.biombioe.2011.09.021
Cortivo PRD, Hickert LR, Hector R, Ayub MAZ (2018) Fermentation of oat and soybean hull hydrolysates into ethanol and xylitol by recombinant industrial strains of Saccharomyces cerevisiae under diverse oxygen environments. Ind Crops Prod 113:10–18. https://doi.org/10.1016/j.indcrop.2018.01.010
Ourique LJ, Rocha CC, Gomes RCD et al (2020) Bioreactor production of 2,3-butanediol by Pantoea agglomerans using soybean hull acid hydrolysate as substrate. Bioprocess Biosyst Eng 43:1689–1701. https://doi.org/10.1007/s00449-020-02362-0
Cortivo PRD, Machado J, Hickert LR et al (2019) Production of 2,3-butanediol by Klebsiella pneumoniae BLh-1 and Pantoea agglomerans BL1 cultivated in acid and enzymatic hydrolysates of soybean hull. Biotechnol Prog 35:e2793. https://doi.org/10.1002/btpr.2793
Hickert LR, Cruz MM, Dillon AJP et al (2014) Fermentation kinetics of acid–enzymatic soybean hull hydrolysate in immobilized-cell bioreactors of Saccharomyces cerevisiae, Candida shehatae, Spathaspora arborariae, and their co-cultivations. Biochem Eng J 88:61–67. https://doi.org/10.1016/j.bej.2014.04.004
Souza CFV de, Dalla Rosa T, Ayub MAZ (2003) Changes in the microbiological and physicochemical characteristics of Serrano cheese during manufacture and ripening. Brazilian J Microbiol 34.https://doi.org/10.1590/S1517-83822003000300016
Coghetto CC, Vasconcelos CB, Brinques GB, Ayub MAZ (2016) Lactobacillus plantarum BL011 cultivation in industrial isolated soybean protein acid residue. Brazilian J Microbiol 47.https://doi.org/10.1016/j.bjm.2016.06.003
Sumitha V, Christy Mathelin R, Sivanandham M (2018) Effect of major and minor nutrients on lactic acid production using biodiesel waste-derived crude glycerol as a carbon source by Lactobacillus casei NCIM 2125. Energy Sources, Part A Recover Util Environ Eff 40:. https://doi.org/10.1080/15567036.2018.1475519
Wood JM (2015) Bacterial responses to osmotic challenges. J Gen Physiol 145:381–388. https://doi.org/10.1085/jgp.201411296
Holzapfel WH WB (2014) Lactic acid bacteria. John Wiley & Sons, Ltd, Chichester, UK
Zhai Z, Douillard FP, An H et al (2014) Proteomic characterization of the acid tolerance response in L actobacillus delbrueckii subsp. bulgaricus CAUH1 and functional identification of a novel acid stress-related transcriptional regulator Ldb0677. Environ Microbiol 16:1524–1537. https://doi.org/10.1111/1462-2920.12280
Broadbent JR, Larsen RL, Deibel V, Steele JL (2010) Physiological and transcriptional response of Lactobacillus casei ATCC 334 to Acid Stress. J Bacteriol 192:2445–2458. https://doi.org/10.1128/JB.01618-09
Koponen J, Laakso K, Koskenniemi K et al (2012) Effect of acid stress on protein expression and phosphorylation in Lactobacillus rhamnosus GG. J Proteomics 75:1357–1374. https://doi.org/10.1016/j.jprot.2011.11.009
Zhang Y, Vadlani PV, Kumar A et al (2016) Enhanced D-lactic acid production from renewable resources using engineered Lactobacillus plantarum. Appl Microbiol Biotechnol 100:279–288. https://doi.org/10.1007/s00253-015-7016-0
Oonkhanond B, Jonglertjunya W, Srimarut N et al (2017) Lactic acid production from sugarcane bagasse by an integrated system of lignocellulose fractionation, saccharification, fermentation, and ex-situ nanofiltration. J Environ Chem Eng 5:2533–2541. https://doi.org/10.1016/j.jece.2017.05.004
Hu J, Lin Y, Zhang Z et al (2016) High-titer lactic acid production by Lactobacillus pentosus FL0421 from corn stover using fed-batch simultaneous saccharification and fermentation. Bioresour Technol 214:74–80. https://doi.org/10.1016/j.biortech.2016.04.034
Funding
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnologico (CNPq, Brazil), CAPES (Brazil, Funding Code 001), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS, RS).
Author information
Authors and Affiliations
Contributions
Jonas Machado: experimental procedures; data analyses; and primary writing.
Daniele M Rossi: experimental procedures; data analyses; writing revision; and supervision.
M A Z Ayub: experimental procedures; project concept; data analyses; writing revision; supervision; and funding resources.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Machado, J., Rossi, D.M. & Ayub, M.A.Z. Batch and fed-batch strategies of lactic acid production by Lactobacillus plantarum BL011 using soybean hull hydrolysates as substrate. Biomass Conv. Bioref. 14, 3249–3259 (2024). https://doi.org/10.1007/s13399-022-02544-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02544-8