Abstract
The uterosacral ligaments (USLs) are important anatomical structures that support the uterus and apical vagina within the pelvis. As these structures are over-stretched, become weak, and exhibit laxity, pelvic floor disorders such as pelvic organ prolapse occur. Although several surgical procedures to treat pelvic floor disorders are directed toward the USLs, there is still a lot that is unknown about their function. This manuscript presents a review of the current knowledge on the mechanical properties of the USLs. The anatomy, microstructure, and clinical significance of the USLs are first reviewed. Then, the results of published experimental studies on the in vivo and ex vivo, uniaxial and biaxial tensile tests are compiled. Based on the existing findings, research gaps are identified and future research directions are discussed. The purpose of this exhaustive review is to help new researchers navigate scientific literature on the mechanical properties of the USLs. The use of these structures remains very popular in reconstructive surgeries that restore and augment the support of pelvic organs, especially as synthetic surgical mesh implants continue to be highly controversial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The uterosacral ligaments (USLs) are the most important supportive structures of the uterus and the apical vagina. Though we call them “ligaments,” they are very different from the strong fibrous bands of connective tissues that comprise articular ligaments. The USLs are composed of collagen, smooth muscle, nerves, blood vessels, and lymphatics.14 This misnomer is representative of a broader lack of understanding of these important yet understudied anatomical structures.
The USLs are membrane-like structures that are typically conceptualized as having three regions: the distal region that attaches at the cervix, the intermediate region, and the proximal region that connects at the sacral spine indirectly via endopelvic fascia.14 They are primarily collagenous, but histologically vary by region. The cervical region of the USLs is mainly comprised of smooth muscle. Smooth muscle content decreases proximally away from the cervix. The sacral region of the USLs is made of looser connective tissue with almost no smooth muscle.14 In addition to smooth muscle and connective tissue, the ligaments are richly innervated. The USLs contain a critical neural nexus called the inferior hypogastric plexus where the hypogastric nerve and the pelvic splanchnic nerves meet and go on to innervate the uterus, apical vagina, bladder, and apical urethra.2,13,80,84
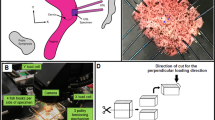
The pelvic organs and their supportive tissues have been analogized as a boat in a dock,52 in which the ropes represent the pelvic ligaments and the water represents the pelvic floor muscles (Fig. 1a). These elements, the water and the ropes, work in tandem to keep the boat, representing the pelvic organs, afloat and in place. However, when the water level goes down as in a dry dock—that is, the pelvic floor muscles are damaged or their supportive mechanism is otherwise diminished—more tension is placed on the ropes (Fig. 1b). Over time, this stretches and damages the ropes, or the apical supportive tissues such as the USLs. The result of this is the eventual failure of the ropes and descent of the boat, or the prolapse of the pelvic organs.
“Boat in the dock” analogy introduced by Norton.52 (a) The pelvic organs represent the boat, the ropes holding the boat to the dock are the ligaments that support the organs from above, and the water represents the pelvic floor muscles. (b) If the water level drops (i.e., loss of support or weakness of the pelvic floor muscles), the boat (organs) hangs on the ropes (ligaments). The ropes stretch out and break, resulting in the boat (organs) falling down (i.e., pelvic organ prolapse (POP)).
Pelvic organ prolapse (POP) is an extremely common disorder estimated to affect up to 50\(\%\) of adult women in the US.5 POP is characterized by the displacement of pelvic organs from their normal anatomical positions. It can result in physiological symptoms of pain, incontinence, and dyspareunia as well as severe psychological symptoms such as decreased body image, anxiety, and depression.38 An estimated 12.6\(\%\) of women in the US undergo surgery to correct POP in their lifetimes.86 However, success of these surgeries is variable, with reports of reoperation rates as high as 30\(\%\).24,53 The USLs are often targeted as supportive anchors for the pelvic organs in POP reconstructive surgeries. The structural integrity of the USLs also plays a critical role in urinary continence and chronic pelvic pain.45,56 Additionally, the USLs are involved in endometriosis with nearly 70\(\%\) of deep infiltrating endometriotic lesions occurring on the USLs.16
The purpose of this review is to present the current state of knowledge on the mechanics of the USLs, including some relevant information on their anatomy, histology, surgical importance, and microstructural changes associated with POP in order to facilitate future research on these critical structures. Ex vivo uniaxial, in vivo uniaxial, and ex vivo biaxial tensile tests have been conducted in order to characterize their elastic and viscoelastic mechanical properties, but much more work needs to be done. This includes analyzing the impact of life events, identifying risk factors for diseases, applying advanced experimental methods, and establishing suitable animal models. Improved characterization of the support function of the USLs will lead to new diagnostic tools, preventive strategies, and treatment methods for pelvic floor disorders (PFDs) and, ultimately, better healthcare for many women affected by these disorders.
Anatomy and Histology
USLs in Humans
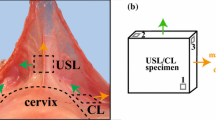
Sometimes referred to as the sacrouterine ligaments or the rectouterine ligaments, the USLs are fan-shaped membrane-like structures that attach to the sacral spine at their proximal ends indirectly via the presacral fascia. They extend distally and anteriorly at an angle slightly greater than 90° from the cephalic-caudal body axis17 around the rectum to connect to the posterolateral region of the cervix and the apical vagina at their distal ends14 (Fig. 2a and 2b). There are conflicting reports regarding the location of the insertion points at the pelvic sidewall. Most investigators agree that the proximal insertion of the USLs occurs at the S2–S4 segments of the sacral spine.59 However, in a cadaveric study by Buller et al.12, the USLs of hemisected pelvises were found to connect consistently to the S1–S3 segments of the sacrum and only occasionally to the S4 segment. An anatomical MRI study conducted by Umek et al.77 corroborated that the USLs’ distal origins span the cervix and apical vagina, but in 82\(\%\) of 61 observed cases in their study, proximal connections occurred at the sacrospinous ligament-coccygeous muscle while only 7\(\%\) showed insertion at the sacrum.
The lengths of USLs are typically measured along the curvature of the ligaments in their main in vivo loading direction. In a cadaveric study of 12 unembalmed pelvises and 5 embalmed hemipelves, the total length of the USLs was reported to range from 12 to 14 cm,84 and in a study by Siddique et al.69 of 6 pelvic blocks with intact uteri, the USLs were reported to have a mean length of 8.7 cm when measured along their superior-lateral edges. When measured caudally from MRI slices containing the most proximal attachments to MRI slices containing the most distal attachments of the ligaments,77 the lengths of the USLs were found to be between 1 and 5 cm, with the mean length being 2.1 ± 0.8 cm, though these measurements do not reflect the anterior angling of the ligaments.
In breadth, or length along direction that is perpendicular to the main in vivo loading direction, the USLs are widest at their proximal sacral ends and taper down to their smallest breadths at the cervix. Buller et al.12 reported mean widths as 5.2 ± 0.9 cm for the sacral portion of the USLs, 2.0 ± 0.5 cm for the cervical portion, and 2.7 ± 1.0 cm for the middle portion. However, the opposite is true of their through-thickness; Vu et al.84 reported that the cervical portion of the USLs was the thickest, ranging from 0.5 to 2 cm. The USLs tapered gradually down to an average of 0.5 cm in the intermediate portion and further to variable thicknesses of 0.5 cm or less in the sacral portion. While the data presented here are inclusive of both USLs (right and left), Umek et al.77 found high variability in the right-to-left symmetry of the USLs. In a random sampling of 20 of their MRI scans, 6 scans showed a longer right USL, 4 scans showed a longer left USL, and 10 scans showed similar lengths for the right and left USLs.77
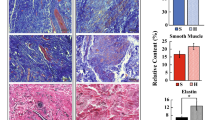
The USLs are commonly conceptualized as having three regions with indistinct boundaries: the proximal or sacral region, the distal or cervical region, and an in-between area referred to as the intermediate region (Fig. 2c). The sizes of these regions in relation to each other are not consistently defined in literature. Most studies of the USLs separate the regions into equal thirds by length12,14,20,37 while a small number of studies do not differentiate by region but rather sample from the entire USLs.19 The cervical third has been described as having a rich blood supply, which diminishes laterally through the USLs, and lymphatic channels, which drain fluid from the cervix and lower uterus towards the sacral lymphatic nodes.14 Histological staining revealed the cervical third as being made predominantly of closely packed smooth muscle bundles that are continuous with the uterine musculature and are interwoven with a network of connective tissue and abundant small nerve bundles. The intermediate third of the USLs was reported as being comprised predominantly of dense collagenous connective tissue with few scattered smooth muscle bundles, a moderate amount of blood vessels, continuation of the lymphatics, fat, and parasympathetic nerves and their ganglia. Finally, the sacral third was reported as almost entirely comprised of loose connective tissue, which is continuous with the presacral fascia and surrounding fat, with minimal blood vessels, lymphatics, and nerves.14 Notably, the lateral USLs contain the inferior hypogastric plexus. This meeting of the hypogastric nerve and the pelvic splanchnic nerves goes on to innvervate the uterus, apical vagina, bladder, and apical urethra.2,13,84 Vu et al.84 posited that it would be more appropriate to distinguish the regions based on their macroscopic contents. Of the 12-14 cm length of the USLs they reported, they defined the cervical region as the most proximal 2–3 cm (roughly 14–25\(\%\) of the total length), the intermediate region as the next 5 cm (roughly 35–42\(\%\)), and the sacral region as the remaining 5.5 cm (roughly 40–46\(\%\)). They described the cervical region as being made up of dense connective tissue but otherwise seemed to agree with Campbell’s macroscopic assessment.14 Histological slides illustrating smooth muscle, collagen, and elastin can be found in Fig. 3.
Histological slides of human USLs using (a) Masson’s trichrome (blue=collagen, red=smooth muscle) and (b) Verhoeff-van Giesson stain (black=elastin, purple=smooth muscle, pink=collagen). Data collected in the study by Baah-Dwomoh et al.3
USLs in Animal Models
The anatomy and histology of the USLs have been studied in some non-human species to establish animal models for PFDs. Non-human primates are generally considered to be the gold standard animal model for such research, but there have been no quantitative comparative studies between their USLs and those of humans. The gross anatomy of the USLs in macaques has been described as very similar to that of humans, with comparable attachments at the sacrum and the cervix, vagina, and cardinal ligaments. The macaque USLs consist of fibrous connective tissue with scattered smooth muscle cells; however the relative contents of the various tissue components have not been quantified.67
Swine have also been evaluated as an animal model for the USLs. Gruber et al.34 identified the USLs in 6 swine as bands of tissue connecting to the lower sacrum and the cervix and upper vagina. Histological staining revealed that the swine USLs were qualitatively similar to those of humans, containing collagen, elastin, smooth muscle, adipose, and nerves. Tan et al.74 performed scanning electron microscopy on 3 USL/cardinal ligament complexes from adult swine. Analysis of the images showed that most collagen fibers were arranged in bundles and oriented in the main in vivo loading direction or at a small angle off of it. The fiber bundles were mostly straight with little crimping. Baah-Dwomoh et al.3 performed histological analysis on adult swine and adult human USLs. The swine and human USLs were similar in collagen and smooth muscle content, with \(83.31\pm 2.13\%\) and \(78.32\pm 1.97\%\) collagen for the swine and human USLs, respectively, and \(16.69\pm 2.13\%\) and \(21.67\pm 1.97\%\) smooth muscle for the swine and human USLs, respectively. However, the swine USLs had a significantly lower relative elastin content at \(5.91\pm 0.69\%\) compared to \(7.01\pm 0.87\%\) for the human USLs.
Mouse and rat USLs have been compared to human USLs by Iwanaga et al.37 In this study, biopsies were collected from the USLs of 5 women with normal pelvic support at their distal insertions. Additionally, the USLs were excised from 4 nulliparous adult mice and 4 rats, and the gross anatomies and histologies of the mouse, rat, and human USLs were compared. The distal human specimens were compared to the entire rodent USLs. The anatomical rodent dissections showed that the mouse and rat USLs had the same insertion points as the human USLs as reported by Campbell14: proximally at the S2-S4 region of the sacral spine and distally at the cervix. The histological images showed that the rodent USLs, similar to the human USLs, were highly heterogenous, composed of smooth muscle, loose connective tissue, blood vessels, and nerves. The rat USLs were more histologically similar to the human USLs than those of the mice. The cross-sectional area of collagen as a fraction of the total area of the ligament in the histological slides was \(0.70\pm 0.11\) for the rats compared to \(0.50\pm 0.04\) for the human USLs. The fraction of smooth muscle for the rats was \(0.27\pm 0.07\) compared to \(0.36\pm 0.03\) for the humans. The mouse USLs contained significantly less collagen, with a fraction of \(0.24\pm 0.03\), and more smooth muscle, with a fraction of \(0.59\pm 0.04\).
USLs in POP Surgeries
The development of POP is rarely tied to a single risk factor, life event, or activity.81 Instead, it is likely caused by a combination of factors, including defects of the supportive structures of the pelvic organs, pelvic nerve or muscle injury, and hormonal deficiencies. While pelvic floor muscle training has shown promise in treating some symptoms of POP, there is no substantial evidence that such training reduces the need for further treatment (e.g., surgery, pessary, drugs, and other physiotherapy).41 Defects of the supportive structures are amenable to surgical repair, and they continue to be a primary focus within the search for the best POP treatment strategy.11 In recent years, the use of synthetic surgical mesh products for POP surgeries has been placed under scrutiny; in 2019 the FDA banned the distribution of mesh products intended for POP repair citing a lack of evidence in safety and efficacy.36 This has led to increased interest and popularity in surgical procedures for POP utilizing native supportive tissues. Most native tissue repair surgeries for POP involve the plication, or folding, of the pelvic ligaments via sutures, which decreases their lengths and increases the tension that they transfer to the attached organs.
According to DeLancey’s classification system,23 the USLs together with the cardinal ligaments provide level I support to the apical vagina and uterus within the healthy pelvic anatomy. Given their important support function, the USLs are often used in POP surgical repairs. In the uterosacral ligament suspension (USLS) procedure, first documented by Miller,50 the USLs are plicated and secured to the vaginal vault after hysterectomy, at approximately the level of the ischial spine, shortening the ligaments and pulling upwards on the vagina. Modifications of this procedure include the McCall culdoplasty, the high USLS, and the USL hysteropexy. In the McCall culdoplasty procedure, the vaginal vault is secured to the proximal USLs and the pouch of Douglas is closed by suturing the two USLs together along the midline, reducing the risk of enterocele development.49 The high USLS operation was described by Shull et al.68 In this procedure, several suspension sutures are placed on each USL higher towards the sacrum, incorporating the coccygeus muscle and sacrospinous ligament for increased support and vaginal length. Lastly, the USL hysteropexy is a uterus-preserving technique in which the USLs are sutured to the uterus rather than the vaginal cuff. Such surgery is preferred in women who may want to have children or are concerned about the potential effect of hysterectomy on their sexual satisfaction.63
The USLS procedures typically do not require the use of synthetic meshes, though mesh products may be used to treat concomitant conditions such as stress urinary incontinence.1 Additional benefits of the USLS procedures are that they restore support by keeping organs and structures in their anatomical positions and allow for vaginal length alteration. However, due to the close proximity of the USLs to ureters, one of the biggest concerns surrounding USLS procedures is risk of ureteral injury, which has been reported to be as high as 11\(\%\).6 USLS surgeries are often performed transvaginally and laparoscopically, resulting in similar outcomes and POP recurrence rates,76 but laparoscopic surgeries reduce the risk of urethral injury.7
According to a meta-analysis of USLS reports, the success rate of USLS procedures, defined as a POP-Q score between 0 and 1,55 varies with type of POP, resulting in 98\(\%\), 87%, and 81\(\%\) rates of successful outcomes for apical, posterior, and anterior prolapse, respectively.47 In such analysis, it was found that additional surgeries were required in 9.4% of the cases to treat recurrent PFDs. In another study by Barber et al.4 where uncontrolled retrospective cases were analyzed, the mean success rate was found to be 85\(\%\) and, on average, approximately 5.8\(\%\) of patients who underwent USLS surgery required additional surgeries to treat recurrent POP. Thus, although procedures utilizing USLs offer high rates of success, the risks of POP recurrence and surgical complications persist. Future studies should investigate methods to quantify the microstructure and mechanical properties of the USLs in vivo so as to tailor surgeries to patient-specific conditions and guide the development of effective mesh implants, ultimately increasing the success rates of USLS procedures.
POP-Associated Microstructural Changes
Pelvic organ prolapse is associated with microstructural changes to the USLs, especially alterations of collagen and other extracellular matrix components (Fig. 4). However, contradictory data exist regarding the exact nature of these changes. Due to the USLs’ high collagen content and its important structural role, comparing the amount and organization of collagen as well as the quantity of factors that promote the synthesis or degradation of collagen in POP and non-POP patients is of significant interest in efforts to elucidate the causes and consequences of POP. These changes commonly involve two primary components of the USLs, collagen type I and collagen type III, which contributes to their strength and elasticity. Several studies have found decreased collagen type I content in the USLs of POP groups compared to healthy groups21,35,43,58,85,88,89,91, though some have found no change in levels of collagen type I.30 There are more data disparities regarding the status of collagen type III, with some groups reporting decreased collagen type III21,35,43,89 while others report an increase30,88 or no change42,91 in collagen type III in the USLs of POP groups compared to those of non-POP groups. Factors which regulate the metabolic activity of collagen are also of special interest, such as matrix metalloproteinases (MMPs) 1, 2, and 9, which are enzymes that degrade collagen. Many studies report upregulation of MMP-1,26,72,78,85 MMP-2,21,31,87,91 and MMP-9,26,91 and their respective genes in patients with POP. Others report no difference in MMP-1,91 MMP-2,57,72 or MMP-9.21,40,57
In addition to collagen, elastin is another structural protein which is thought to be crucial for the reversible extensibility of healthy USLs. Decline in elastin has been linked to POP,33,43 though some studies report no significant differences in the relative amounts of elastin in POP groups versus non-POP groups.39,90 The role of smooth muscle in the supportive function of the USLs is less defined, but reduced smooth muscle content has also been linked to POP.73 Others have reported no differences in the USLs’ smooth muscle content,30,61 but have noted that the smooth muscle in the USLs of POP patients may have structural defects.61
Mechanical Testing Methods
Several experimental studies have been published on the mechanical characterization of the USLs (Fig. 5). Although there is little overlap in regards to specific testing protocols, there are several commonalities in the overall testing approach. The bulk of mechanical studies utilize ex vivo tissues either dissected from animals such as rats, monkeys and swine,3,8,44,51,54,74,75,79 biopsied from human patients,22,60 or removed from cadavers.3,48,62 However, in vivo testing has also been performed on human patients undergoing surgery.46,70 In some cases, the USLs are not isolated from the surrounding tissues and organs but instead they are tested together with the connected pelvic organs and/or other supportive tissues.8,44,46,51,70,74 Some tests involving non-isolated USLs offer findings that describe the function of the USLs in more physiologically relevant loading conditions, but the collected force-displacement data depend highly on the size of the tested specimens. For this reason, such data cannot be easily used to determine the mechanical behavior of USL specimens of different size. When the USLs are tested in isolation, specimens are typically taken from the distal region, adjacent to the cervix (Fig. 2). Specimens can be excised more consistently from the distal ends of the USLs since the proximal regions are arbitrarily defined, and researchers disagree on how exactly the USLs are attached to the sacrum.12,14,27 Moreover, distal portions of the USLs may be removed from a patient during a hysterectomy or similar procedure and, therefore, are more accessible to researchers for mechanical testing.22,60
Uniaxial and biaxial tensile tests have been performed to quantify the mechanical properties of the USLs. Uniaxial tensile tests on rectangular strips of tissue are usually performed in the main in vivo loading direction (MD), or the direction pointing from the distal/cervical end to the proximal/sacral end62,75 (Fig. 2). This direction is the primary loading axis of the USLs as they provide the most support in this direction. However, since the USLs are connected to surrounding tissues in multiple directions, these tests do not simulate the in vivo complex loading conditions. Biaxial tests on square specimens with sides oriented along the MD and the direction perpendicular to it (PD) are used to provide more physiologically-relevant experimental data.
Ex Vivo Uniaxial Testing: Human USLs
Human USLs have been primarily loaded ex vivo along the MD, that is the direction acting against gravity to support the uterus and apical vagina. To our knowledge, the first mechanical study of the USLs was conducted by Reay Jones et al.60 These authors used uniaxial tensile tests to measure the resilience of USLs from 85 women undergoing hysterectomies, 4 of whom presented with POP. Specimens were taken from their insertion points at the junction of the uterus and cervix, trimmed to \(10\times 10\) mm\(^2\), and loaded at a rate of 10 mm/min. Load-displacement data were collected, and the USL resilience was reported as the area under the load-displacement curve up to the end of the elastic region (Fig. 6a). This metric, which represents the maximum energy absorbed by the tissue when it deforms elastically, quantifies the work done by the tissue in response to the applied external forces that cause it to stretch up to the plastic limit. The median value of the resilience in USLs was reported as 0.022 J. No significant difference was found between the responses of the right and left USLs. The USL resilience in women with POP, 0.004 J, was significantly smaller than that of women without POP, 0.019 J. Resilience decreased with vaginal delivery, patient age, and menopause, but it was not significantly different between primiparous and multiparous women.
After a decade, Martins et al.48 presented data collected via uniaxial tensile testing of human cadaveric USLs. Rectangular specimens with an aspect ratio of 1:2 from the intermediate region of USLs were excised from 15 human cadavers with no history of PFDs. The specimens were pulled at 5 mm/min until failure. The resulting load and displacement data showed nonlinear elastic response with high variability. The mean slope of the linear region of the stress-stretch curves, also referred to as the tangent modulus of the linear region or elastic modulus, was 14.1 ± 1.4 MPa, and the mean ultimate strength was 6.3 ± 0.8 MPa (Fig. 6b). The elastic modulus of the USL was positively correlated with ultimate strength, and the USLs were found to be stronger and stiffer than the round ligaments from the same cadavers. The elastic modulus and ultimate strength of the USL specimens were found to be significantly higher for parous compared with nonparous women.
Concomitantly, Rivaux et al.62 reported the mechanical properties of the USLs excised from 13 female cadavers without POP and compared them to the round and broad ligaments. Ligaments were cut into multiple specimens which were pulled at a rate of 2\(\%\)/s until failure. Nominal stress was recorded, and a video extensometer was used to calculate engineering strain. These stress-strain data showed the nonlinear hyperelastic behavior of the USLs, with high variability. The data were compared to data collected from broad and round ligaments from the same cadavers. The USLs were found to be significantly stiffer than the broad and round ligaments at both low and high levels of deformation when comparison was made using the parameters of Mooney-Rivlin’s constitutive law.
Ex Vivo Uniaxial Testing: Rat, Monkey, and Swine USLs
The pelvic supportive ligaments including the USLs have been mechanically tested together with the vagina in the rodent model by Moalli et al.51 In this study, entire pelvises were dissected from 10 adult virgin rats. The reproductive organs and all of their supportive tissue connections were kept intact, save for the uterine horns and their suspensory ligaments. The specimens retained the spinal column up to the lumbar region, which was embedded in polymethyl methacrylate and clamped in place. They were also clamped at the distal vagina, which was pulled caudally during testing. The specimens were preloaded to 0.015 N and preconditioned from 0 to 2 mm of elongation for 10 cycles at 25 mm/min. They were then pulled to failure at the same elongation rate. The shapes of the load-elongation curves were typical of biological soft tissues, including visible toe and linear regions. The ultimate load at failure ranged from 11.8 to 15.3 N with the average ultimate load at failure being 13.2 ± 1.1 N. The stiffness, defined here as the steepest positive slope of the load-elongation curve over a 1 mm interval of elongation (Fig. 6a), ranged from 2.1 to 4.8 N/mm with a mean value of 2.9 ± 0.9 N/mm. The corresponding elongations at the failure loads ranged from 5.4 to 11.0 mm with an average of 8.9 ± 2.0 mm. The energy absorbed to failure, defined as the area underneath the load-elongation curve up to the point of failure (Fig. 6a), ranged from 0.0259 J to 0.0689 J with an average of 0.0494 ± 0.0127 J. Video recordings of the tests showed that the point of failure coincided with the disruption of the paravaginal connective tissue attachments to the pelvic sidewall and the posterior connective tissue attachments to the levator ani muscles. The USLs failed during continued pulling after this failure point.
Using the experimental methods presented by Moalli et al.51, in a follow-up study by the same group,44 pelvises were dissected from 7 groups of 8 to 13 rats including virgin rats, pregnant rats at two different stages of gestation, rats that had just undergone vaginal delivery or c-section (i.e., abdominal delivery), and rats that were allowed to recover for 4 weeks after vaginal delivery or c-section. Average values of the stiffness were reported as 3.3 ± 0.6 N/mm, 1.6 ± 0.4 N/mm, and 10.1 ± 1.1 N/mm for the virgin, midpregnant, and late pregnant groups, respectively. Average ultimate loads at failure were 14.0 ± 1.4 N, 10.2 ± 1.8 N, and 10.1 ± 1.1 N for the same respective groups. Average stiffnesses immediately post-delivery were 10.8 ± 1.6 N/mm and 9.8 ± 1.4 N/mm, and average ultimate loads at failure were 10.8 ± 1.6 N and 9.8 ± 1.4 N for the vaginal delivery and c-section groups, respectively. Mean stiffnesses 4 weeks post-delivery were 14.0 ± 1.3 N/mm and 17.1 ± 1.0 N/mm, and mean ultimate loads at failure were 14.0 ± 1.3 N and 17.1 ± 1.0 N for the vaginal delivery and c-section groups, respectively. The vagina and supportive tissue complexes of the pregnant and immediate post-delivery rats displayed lower stiffness and ultimate load at failure than those of the virgin rats. The 4 weeks post-delivery groups were not significantly different in stiffness from the virgin rats, and their ultimate loads at failure were not lower.
Nonhuman primates have been used as animal models to test the effects of ovariectomy and subsequent hormone replacement therapy on the mechanics of the USLs by Vardy et al.79 In this study, adult feral cynomolgus macaque monkeys were ovariectomized and placed in three treatment groups for 12 months, including two common hormone replacement therapy regimen groups, conjugated equine estrogens plus continuous medroxyprogesterone acetate and ethinyl estradiol plus norethindrone acetate, and a no treatment group. Dumbbell-shaped sections of USLs were dissected at the uterine insertions. The specimens, which had lengths that ranged between 5 and 15 mm, were subjected to incremental stress relaxation tests along the MD. They were loaded at 0.1 mm/s displacement rate until desired strain levels (ranging from \(5\%\) to \(30\%\)) were reached, they were then allowed to stress relax for about 40 min before being loaded to the next strain level, and they were finally pulled to failure. Strain and stress measured at the end of each stress relaxation, that is the equilibrium stress (Fig. 6d), were collected and fitted to an exponential function. Tensile moduli were then calculated as the slopes of the exponential function at strains of 0\(\%\), 6\(\%\), 12\(\%\), 18\(\%\), 24\(\%\), and 30\(\%\). Tensile moduli at 12\(\%\) strain were reported as 0.08 ± 0.05 MPa for the untreated group, 0.35 ± 0.47 MPa for the conjugated equine estrogens plus continuous medroxyprogesterone acetate group and 0.32 ± 0.28 MPa for the ethinyl estradiol plus norethindrone acetate group. The USLs of both treatment groups were found to have significantly higher tensile moduli than those of the untreated group for 0\(\%\), 6\(\%\), and 12\(\%\) strains, with the trend continuing without statistical significance for the rest of the strain levels. The tensile moduli of the two treatment groups were not found to be significantly different from each other. Mean strength was found to be 0.6 ± 0.4 MPa for a subset of all tested specimens.
The effect of selective estrogen receptor modulators on the mechanical properties of cynomolgus macaque USLs were evaluated by Shahryarinejad et al.66 This study was conducted using experimental methods similar to the ones presented by Vardy et al.79 save for differences in treatment groups and the use of a displacement rate of 1.0 mm/s. Following ovariectomy, 4 groups of macaques were treated for 12 weeks with either a placebo or 1 of 3 selective estrogen receptor modulators-tamoxifen, raloxifene, or ethinyl estradiol-commonly used in postmenopausal and breast cancer prevention therapy. Peak and equilibrium stresses at each strain during incremental relaxation tests were used to generate two sets of stress-strain curves and compute peak and equilibrium tangent moduli via exponential curve fits. While the population sizes were too small to perform statistical analysis, the authors reported that ethinyl estradiol increased the peak tensile modulus of the USLs to about 1.16 MPa from the 0.46 MPa control value. The other two treatments, tamoxifen and raloxifene, decreased the peak tensile moduli of USLs to 0.29 MPa and 0.15 MPa, respectively. All 3 treatments decreased the average equilibrium tensile modulus from 0.34 to 0.11 MPa for the tamoxifen group, 0.05 MPa for the raloxifene group, and 0.13 MPa for the ethinyl estradiol group.
In order to reduce inter-animal variability, Tan et al.75 performed uniaxial tensile tests on 5 USL specimens and 13 cardinal ligament specimens isolated from a single sow immediately post-partum. Rectangular specimens were preloaded along the MD to 0.25 N and preconditioned from 0.25 to 1.0 N at 0.75 mm/s for 5 cycles. They were then pulled at the same displacement rate until failure occurred while strain was optically measured. The stress-strain curves were found to be nonlinear, exhibiting the typical nonlinear strain stiffening behavior of soft tissue. Mean tangent moduli for the toe and linear regions of the stress-strain curves computed from the USL specimens were reported to be 1.617 ± 1.215 MPa and 29.816 ± 7.378 MPa, respectively and the mean ultimate strength to be 2.767 ± 0.444 MPa. The researchers found the USL specimens to be significantly stronger and stiffer than the cardinal ligament specimens when comparing the ultimate strength and elastic modulus of the linear region of the stress-strain curves.
In Vivo Uniaxial Testing: Human USLs
The force-displacement behavior of the USL/cardinal ligament complex has been investigated in vivo by Smith et al.70 in their pilot study of 17 women in various states of uterine suspensory condition (including healthy non-POP and different stages of POP) while they were undergoing pelvic surgery. In this study, a tenaculum was clamped onto the cervix of each patient while the handle was mounted on a linear actuator. A vaginal speculum was inserted to retract the levator ani muscles in order to reduce the contribution of the pelvic floor to the mechanical response. The tenaculum was pulled at 4 mm/s in the caudal direction until a load of 17.8 N was reached. The load was then held for 60 seconds during which cervical location was measured in relation to the hymen. The stiffness of the suspensory ligaments, measured as the slopes of the measured force-displacement data, was found to increase with increased cervical displacement. It was reported as 4.9 ± 1.4 N/cm with individual specimen stiffness ranging from 3.1 to 8.9 N/cm. The cervical starting positions and range of cervical displacements varied greatly between patients. Due to the limited cohort size, cervix location could not be correlated with ligament stiffness with statistical significance, leaving room for further research on the relationship between POP and apical ligament stiffness.
Further testing of the time-dependent properties of the in vivo USL/cardinal ligament complex of 14 women with POP was conducted by Luo et al.46 using the same testing set up presented by Smith et al.70 While the patients were sedated for pelvic surgery, a linear actuator pulled a tenaculum clamped at the cervix caudally until a 1.1 N preload was applied. Then, the tenaculum was pulled at 4 mm/s until the load reached 17.8 N, at which point the displacement was held constant for 1 min while the decrease in load was recorded. This ramp and hold process was performed three times per patient. The stiffness of the uterine support complex was defined as the difference between the maximum and minimum forces divided by the difference in the cervical displacements at those forces during the ramp phases of the tests. The mean stiffness was 0.49 ± 0.13 N/mm, 0.61 ± 0.22 N/mm, and 0.59 ± 0.2 N/mm for the first, second, and third ramp phases of the tests, respectively. Energy absorbed by the uterine support was defined as the area under the force-displacement curve during the ramp phase of each test and the mean energy was reported to be 0.27 ± 0.07 J, 0.23 ± 0.08 J, and 0.22 ± 0.08 J for the first, second, and third ramp phases of the tests, respectively. The energy absorbed during the first ramp phase was significantly higher than the energy absorbed during the second or third ramp phase, with the decreasing values of absorbed energy indicating increasing stiffness of the tissues after repeated loading. The final relaxation force after the 1 min hold phase was 57\(\%\) of the maximum force reached during the first ramp phase, 64% of the maximum force during the second ramp, and 70\(\%\) of the maximum ramp force during the third ramp. In order to estimate the stiffness of the USLs and cardinal ligaments, the uterine support was modeled as two sets of cables with fixed origins and symmetrical behavior. The model parameters, which included angles and lengths of the ligaments, were obtained from a previous MRI study.17 The mean stiffness of the USLs was estimated to be 0.12 ± 0.04 N/mm. Moreover, the USLs were estimated to have elongated on average 109 ± 32\(\%\) at the maximum force compared to their lengths at rest.
Ex Vivo Biaxial Testing: Human USLs
The elastic and viscoelastic properties of human USLs were first determined by Baah-Dwomoh et al.3 A total of 9 human USL specimens were obtained from the cervical to sacral regions of 4 female cadavers. Square specimens of approximately \(2.3\,\times\,2.3\) cm\(^2\) to 3 \(\times\)3 cm\(^2\) were cut with sides aligned along the main in vivo and perpendicular loading directions as defined in Fig. 7, before being subjected to planar biaxial tensile testing. Following preconditioning in which specimens were loaded and unloaded from 0.1 to 2 N at 0.05 N/s for 10 cycles, the specimens were allowed to recover for 10 min and then pulled equi-biaxially at 0.05 N/s up to a load of 2 N. The 2 N load was then held for 20 min. Throughout testing, digital image correlation was used to calculate strain. During preconditioning/cyclic loading, the mean peak strains as shown in Fig. 6(c) were 4.90\(\%\) and 5.98\(\%\) in the MD and PD, respectively. Stress-strain data were plotted for the pre-creep testing stage, or elastic portion of the tests. Representative data are reported in Fig. 7 for a subset of specimens to show the variability in the elastic response between the two loading directions. Secant moduli were calculated as the slopes of the lines drawn between the first and last points of the curves. The mean secant moduli for the human USLs were 7.76 ± 1.65 MPa and 6.00 ± 1.35 MPa in the MD and PD, respectively. By the end of the creep test, the mean peak strains were 1.09 and 1.15 times the mean initial creep strains in the MD and PD, respectively. The mean creep rates were 0.020 ± 0.003 1/s and 0.028 ± 0.006 1/s in the MD and PD, respectively. No significant differences in mean peak strains, secant moduli, mean creep rates were found between loading directions for the tested human USL specimens.
Biaxial stress-strain data collected from human USL specimens (\(n=6\)) showing variability of the mechanical response in the main in vivo direction (MD, continuous lines) and perpendicular (PD, dashed lines) directions of loading. Data collected in the study by Baah-Dwomoh et al.3
The biaxial mechanical properties of healthy and prolapsed human USLs have been investigated very recently by Danso et al.22 Specimens were obtained from the uterine connection points of the right and left USLs of post-menopausal women who were undergoing hysterectomies. The specimens were biaxially tensile tested along the MD and PD to a target stress of 0.1 MPa. Specimens were preloaded equibiaxially up to the target stress for 10 cycles. Then each specimen was tested equi-biaxially and at four loading ratios: 1:0.5, 1:0.75, 0.75:1, and 0.5:1 at a strain rate of 0.2\(\%\)/s. Each loading regimen was applied for 6 cycles. Load data and strain data were collected during the unloading phase of the 6th unloading cycle. Tangent moduli for the severe POP USLs in the MD fell between 2 and 5 MPa, the values for the 0.5:1 and the 1:0.5 loading regimens, respectively. Tangent moduli ranged from 3.5 to 8.5 MPa for the moderate POP group and from 3.5 to 10 MPa for the non-POP group in the MD for the same respective loading regimens. In the PD, tangent moduli for the severe POP group ranged from 3.5 to 8 MPa for the 1:0.5 and 1:1 loading regimens, respectively. For the moderate POP group, tangent moduli ranged from 4 MPa for the 1:0.5 loading regimen to almost 9 MPa for the 0.5:1 loading regimen. For the non-POP group, tangent moduli in the PD ranged from 4.5 to over 7.5 MPa for the 1:0.5 and 0.75:1 loading regimens, respectively. The USLs of women with severe POP were found to be more extensible and have a lower tangent modulus in the MD than those of women with moderate POP or without POP. This was true for all loading regimens, but extensibility and tangent modulus in the PD were not significantly different among the groups.
Ex Vivo Biaxial Testing: Swine USLs
The first biaxial tests on the USL/cardinal ligament complexes were conducted by Becker and De Vita8 to quantify stress relaxation properties. In their study, USL/cardinal ligament specimens (30\(\times\)30 mm\(^2\)) were isolated from adult swine and subjected to planar biaxial tensile testing along the MD and PD. The specimens were preloaded to 0.04 N and preconditioned from 0.1 to 0.6 N for 10 cycles at a displacement rate of 0.1 mm/s, and then pulled equi-biaxially at 0.1 mm/s until the load on one of the two axes reached selected values between 2 and 12 N, at which point the specimens were held at a constant stretch for 50 min while load data were recorded. The stress-stretch results for the ramping portion of the tests showed nonlinear elastic responses with high variability across tested specimens. The specimens appeared to be qualitatively stiffer in the MD than in the PD, although no statistical comparison was conducted. Stress relaxation results showed rapid relaxation for the first few hundred seconds with the rate of relaxation slowing for the remainder of the tests. Though the elastic behavior of the specimens varied by axial loading direction, the normalized stress relaxation data showed no difference between the two axial loading directions. The stress relaxation and relaxation rate were found to depend on the level of stretch, with stress relaxation rate being higher at lower stretches.
The biaxial creep properties of the USL/cardinal ligament complexes were quantified by Tan et al.74 Square specimens (30\(\times\)30 mm\(^2\)) were excised from the apical vaginal support of sows and subjected to planar biaxial tensile testing along the MD and PD. Following 10 cycles of preconditioning from 0.1 to 0.6 N at 0.1 mm/s, the specimens were pulled equi-biaxially at 0.1 mm/s until either 2 N or 4 N loads were reached in both loading directions. Then, the loads were held constant for two hours while the increases in strains (refer to Fig. 6) were measured using the digital image correlation method. Mean initial strains were larger in the PD, 0.152 at 2 N and 0.263 at 4 N, than in the MD, 0.116 at 2 N and 0.216 at 4 N. The difference was maintained, but without statistical significance, for the mean final strains after 2 hours, with reported values of 1.41 and 1.30 times the initial mean strains in the PD at 2 N and 4 N, respectively, and 1.32 and 1.20 times the initial mean strains in the MD at 2 N and 4 N, respectively. During creep testing, specimens initially strained very quickly, and strain rate decreased over the course of the test. The mean creep rates were 0.054 and 0.050 1/min for the specimens subjected to 2 N in the MD and PD, respectively, and 0.031 and 0.041 1/min for the specimens subjected to 4 N in the MD and PD, respectively. While the observed creep was larger at 2 N than 4 N, the difference was not found to be statistically significant.
The biaxial elastic and viscoelastic properties of the swine USLs have been determined and compared to those of human USLs by Baah-Dwomoh et al.3 Porcine USL specimens were excised from the region between the cervix and the rectum of adult swine. Following the methods previously described for human USLs, peak strains during preconditioning, secant moduli of the pre-creep elastic response, and increased strains during a 20 min long creep were computed. The mean peak strains were 5.70\(\%\) and 8.12\(\%\), the mean secant moduli were 3.51 ± 0.61 MPa and 4.87 ± 1.94 MPa, and the mean creep rates were 0.029 ± 0.008 1/s and 0.031 ± 0.008 1/s in the MD and PD, respectively. The mean strains increased by 1.12 and 1.16 times the mean initial creep strains in the MD and PD, respectively. No significant differences in these quantities were found between either loading directions or human and swine USLs.
The effects of estrogenic mycotoxin consumption on the mechanical properties of swine USLs was investigated in a recent study by Pack et al.54 The toxin used, zearalenone, is produced by fungi and is linked to the onset of POP in swine when consumed. In this study, three groups of pubertal virgin swine were given feed with either the mycotoxin zearalenone dissolved in acetonitrile (2.67 mg of toxin per 1 kg of feed) or just the solvent acetonitrile. The first group was given feed with solvent for 21 days, the second group was given feed with dissolved mycotoxin for 7 days and then feed with solvent but without mycotoxin for 14 days, and the third group was given feed with dissolved mycotoxin for 21 days. Following the 21-day feeding regimens, the swine were sacrificed, and specimens were excised from the mid portion of the USLs between the rectum and the proximal vagina. Square specimens of 30\(\times\)30 mm\(^2\) were loaded into a planar biaxial tensile testing apparatus along MD and PD. The specimens were preconditioned through ten cycles of loading from 0.05 to 0.5 N at a rate of 0.1 mm/s. Following preconditioning, the specimens were preloaded to 0.05 N and stretched at 0.1 mm/s. Digital image correlation was used to calculate strains. No significant difference in average stress between the groups at either 2\(\%\) strain or 4\(\%\) strain was found. The average stresses across the groups at 2\(\%\) strain was reported to be 32.96 ± 4.43 kPa and 40.82 ± 4.22 kPa in the MD and PD, respectively. At 4% strain, the average stresses for the three groups were 63.21 ± 9.69 kPa and 83.38 ± 9.17 kPa in the MD and PD, respectively. There was no significant difference between the two loading directions. For this study, the secant modulus was defined as the slope of a best-fit line between the 2\(\%\) and 4\(\%\) strains on the stress-strain curves. There were no significant differences in secant modulus between the three groups or between the two loading directions. The average secant moduli across the three groups were 1.52 ± 0.27 MPa in the MD and 2.13 ± 0.31 MPa in the PD direction. In all, no significant changes in the swine USLs’ tissue elasticity were found following consumption of zearalenone at the selected moderate dosage.
Discussion
The mechanical properties of the USLs are likely affected by a number of factors, including age, body mass, parity, and menopause, and have been demonstrated to be different between patients who have POP and those who do not. Unfortunately, most published experimental studies do not control for these variables, primarily due to difficulties involved in collecting, processing, and storing human specimens. Some investigators found a significantly negative correlation of USL resilience with vaginal delivery, age, and menopause,60 and others found no significant effects of age, body mass, or menopausal status on USL stiffness.48 The effect of parity on the USLs also remains unclear. Parous women were reported to have stiffer USLs than nulliparous women,48,60 though no differences in the resilience of primiparous versus multiparous USLs were reported.60 The mechanical changes of the USLs during pregnancy are unknown, and MRI analysis revealed that the USLs lengthen throughout the gestational period and remain altered a year after delivery.32 It is not yet clear whether the USLs ever fully recover their mechanical properties from pregnancy and childbirth. In rats, the stiffness of the entire pelvic support complex during pregnancy and immediately post-partum decreased, with the biomechanical properties being restored 4 weeks post-partum.44 Moreover, in women affected by POP, the USLs were found to be less resilient60 and more compliant.22,46
While the relationship between POP and the mechanical properties of the USLs has been investigated to some extent, mechanical alterations of the USLs in connection to other PFDs have been overlooked. For example, there are no studies that characterize changes in the mechanical function of the USLs due to urinary incontinence, despite documented changes in their composition. The content of collagen type III in the USLs of patients with urinary incontinence and POP decreased compared to patients with POP but without urinary incontinence and patients with neither disorder.9,42 Similarly, deep infiltrating endometriosis may modify the contractile properties of the USLs due to lesions that occur on the USLs.16 In some cases, the lesions are implanted within the muscular-connective tissue of the USLs and come into contact with nerves, causing severe chronic pain among other symptoms.10
There exist several points of discrepancy regarding the exact anatomy of the USLs. Anatomical cadaveric dissection studies largely agree that the USLs attach distally at the cervix and proximally at the sacral spine, though there is some disagreement over whether it attached at the S1-S3 or S2-S4 region.59 However, Umek et al.’s77 anatomical MRI study reported the USLs as connecting distally at the cervix and proximally at the sacrospinous ligament and coccygeous muscle. Identification of the exact end points of the USLs through MRI likely depends on the MRI acquisition parameters used, and is complicated by the difficulty of identifying the USLs in images. Most MRI images are taken while the patient is in a supine position and, consequently, they do not accurately portray the anatomy of the USLs in an upright position. Additionally, factors such as fullness of the bladder and rectum, enlarged uterus, fat content, prominent lymphatics, and short distance between cervix and sacrum prohibit or alter the evaluation of the USLs using MRI.77 That said, the USLs may have more anatomical variability than is commonly conceptualized.
Anatomical and mechanical studies seem to skew towards parous subjects, either reporting an average of multiple births per patient,22,46,60,70,77 reporting signs of parity but no medical records,84 or not reporting this information.12,14,62,69 White women also seem to be overrepresented in anatomical studies compared to women of other races with investigators reporting overwhelmingly white study cohorts14,22,77 or not reporting this information.3,12,46,48,60,62,69,70,84 Furthermore, cadaveric studies skew towards older patients and the analysis of their properties can be complicated by other factors; embalming procedures, tissue death and settling in the supine position can make it difficult to translate the findings to living anatomy, microstructure, and mechanics. Ex vivo human studies of the USLs can be performed using fresh tissue specimens. However, due to ethical issues in surgical care, these specimens almost exclusively come from the most distal regions of the USLs. These regions do not reflect the anatomically and structurally different intermediate and sacral regions of the USLs that are often important for USLS surgeries. Overall, inconsistencies in reports about the USLs may indicate that the anatomy and mechanics of these ligaments differ across women. The USLs of large and diverse populations should be investigated as many of the presented studies remain underpowered and skewed.
Though a large portion of the USLs is comprised of smooth muscle and nerve tissues, the contribution of these components to the mechanical function of the USLs remains unknown. Such a contribution, though likely small, could prove consequential to the development of PFDs. It is possible that smooth muscle or nerve tissue in the USLs may become damaged over the course of pregnancy and labor, or simply with aging, eventually contributing to the development of POP. Unfortunately, measuring the contractile properties of human tissue is exceptionally difficult as tissue must be tested either in vivo or immediately following excision to achieve successful contractions ex vivo. Since there are currently no data on the contractile behavior of the USLs, quantifying the contribution of smooth muscle on the mechanics of the USLs using animal models is a clear next step in the research of these important structures.
There is some evidence that nutrition may affect the composition of the USLs and, therefore, the mechanical role of these ligaments. In a study by Findik et al.,29 administration of vitamin C increased collagen I and III in the USLs of pregnant rats. On the other hand, consumption of toxins has been linked to high instances of POP in swine herds.18 How the consumption of toxins influences the USLs in swine has been investigated in a preliminary study by Pack et al.54 Further research in this area is warranted as this knowledge could help in developing new prevention strategies for POP.
The human female pelvis has evolved to be unique with no other mammals, including non-human primates, having similar bipedal locomotion and parturition.64,65 This uniqueness constitutes a major obstacle to research on the support function of the USLs as there are no widely accepted animal models for PFDs. In small animal models such as rodents, the USLs are mechanically tested together with other supportive tissues and pelvic organs due to their small sizes44,51 and, from these tests, the mechanical properties of the USLs cannot be isolated. On the other hand, large animal models have been shown to be advantageous since USLs can be separated from other pelvic organs for mechanical testing. However, large animals require specialized housing and surgical facilities with high costs in feed, veterinary care, and surgery. Moreover, these animals have slower growth rates and longer reproductive cycles, further increasing costs and slowing down research. Thus far, monkeys and swine are the only animal models that have been used to mechanically test USLs in isolation from surrounding structures.3,8,54,66,74,75,79 A summary of elasticity metrics reported for these animal models is presented in Fig. 8. However, depending on the scientific research question, other animal models may be better suited to study the role of USLs in relation to PFDs. Comparative studies such as those conducted by Shahryarinejad and Vardy67, Iwanaga et al.37, and Baah-Dwomoh et al.3 are necessary to determine the anatomy, microstructure, and mechanical properties of USLs across species so as to better select animal models and translate research findings to human USLs. Animal models will allow future researchers to bypass the limitations of human tissue collection and to design experiments that control for parity, body mass, age, disease, nutrition, etc.
Mechanical quantities reported by the cited published experimental studies on the elasticity of USLs. The columns denote test type (uniaxial or biaxial tensile tests), and the rows denote animal model (humans, non-human primates, or swine). Note that sec.mod. stands for secant modulus, tan. mod. for tangent modulus, and ten. mod. for tensile modulus.
There is much variability in experimental protocols and methods for testing the mechanical behavior of the USLs. For example, most studies perform cyclic preconditioning before data collection3,8,22,44,51,54,74,75 while others do not.48,60,62,79 Displacement and loading rates also change widely across published studies and, given the viscoelasticity of the USLs, these rates are likely to affect the mechanical data. Existing studies also differ significantly in the data analysis with some investigators presenting load and displacement data44,46,51,60,70 while others present stress, stretch, and strain data.3,8,22,54,66,75,79 While load-displacement data provide valuable information within the contexts of the tested specimens, stress-strain data, being normalized and independent of the size of the specimens, can be better compared across studies. Finally, there is no standardized way of measuring the mechanical properties of the USLs, even from tensile tests. Some studies report the slope of the linear region of the load-displacement, stress-stretch, or stress-strain curves,22,48,75 variably referring to this metric as stiffness, elastic modulus, or tangent modulus. Others present secant modulus,3,46,54,70 as the slope of a line drawn through two points on the stress-strain curve. This metric is useful for stress-strain curves that do not include the linear region. However, there is no consistency between the chosen points for the secant line, making the comparison among such moduli problematic (refer to Fig. 8).
Most of the mechanical experiments on the USLs have been uniaxial tensile tests along the main in vivo loading direction, although the loading direction is sometimes not reported.71 However, in situ, the USLs experience loading in multiple directions, especially at the attachments. Additionally, due to the changing structure of the USLs along the main in vivo loading direction (Fig. 2c), mechanical anisotropy as well as inhomogeneity should be investigated to provide a more complete picture of how these structures function in the body. Biaxial tensile tests have attempted to characterize the USLs in more physiologically relevant loading conditions. Interestingly, no biaxial mechanical studies have found a significant difference in the mechanical properties in the MD and PD of USLs.3,8,22,54,75 On the surface, this may appear to mean that the USLs are elastically isotropic. However, as exemplified in Fig. 7, the stress-strain response is highly variable among USL specimens. Some specimens are stiffer in the MD while others are stiffer in the PD. This nuance is lost when data are averaged across all specimens. Such a disparity in mechanical properties, with some specimens being stiffer in the MD and others in the PD, may relate to the onset of PFDs. Danso et al.22 showed that the USLs of women with severe POP are more compliant in the MD than those of women without POP, while there was no significant difference in the PD between the two groups of women. Directional properties should be further studied in relation to PFDs to better understand how the USL properties are altered. Ideally, diagnostic tools should be developed to quantify the anisotropy, inhomogeneity, and mechanics of the USLs in situ so that individualized treatment plans can be designed.
In vivo mechanical testing of the USLs is invaluable since the USLs are unaltered and their anatomical connections are preserved. In an in vivo study of the USLs,70 some of the inconsistencies that complicate POP description, quantification, and staging were revealed. The POP-Q system is considered to be the standard way to quantify the extent of POP.55 It involves measuring the positions of specific anatomical points in relation to the hymen while the patient is sitting or standing and either coughing or performing the Valsalva maneuver. Smith et al.70 found that, for the majority of their study cohort, the cervical displacement under small applied loads was greater than the maximum extent of POP as measured by the POP-Q system preoperatively. This phenomenon has been observed in several other studies in which little correlation has been found between measurements of POP preoperatively using the standard POP-Q during Valsalva maneuver and the measurements obtained by traction during surgery.15,25,28,82,83 Consequently, the surgical operation that is performed often differs from preoperative plans. Of course, it is also unknown how well the assessments of POP under surgical traction correlate with a patient’s health. Indeed, the fact that these assessments are performed while the patient is supine and under anaesthesia means that the loads that the USLs experience during surgery are very different from those experienced when the patient is in a normal upright position.
Given the escalating safety concerns about the use of surgical mesh implants, the USLs remain the most used endogenous tissues for a wide range of surgeries for PFDs. These surgeries consist primarily of adjusting the length and, consequently, the tension of the USLs in an ad-hoc manner so as to increase the level of apical support that these ligaments provide. However, there are currently no standardized surgical procedures, and the POP recurrence after surgical treatment remains prevalent. New scientific-based guidelines could improve the outcomes of current surgeries. Advanced medical tools and devices that aid in identifying the specific surgery that yields the best outcome must be developed by integrating knowledge about the anatomy, microstructure, and mechanics of the USLs. These tools will be especially valuable as we move toward patient-specific healthcare delivery.
Conclusions
Many great strides have been made in characterizing the mechanical properties of the USLs, but a lot remains to be done. As methodological rigor and technical sophistication increase in mechanics, new efforts become necessary to expand our scientific knowledge of the support function of the USLs. Current research problems in PFDs can only be tackled through concerted interdisciplinary efforts in mechanics, material science, imaging, veterinary medicine, obstetrics, and urogynecology. It is our hope that this synthesized overview on the mechanics of the USLs will facilitate such interdisciplinary efforts toward developing successful methods for POP prevention, diagnosis, and treatment.
References
Amundsen, C. L., B. J. Flynn, and G. D. Webster. Anatomical correction of vaginal vault prolapse by uterosacral ligament fixation in women who also require a pubovaginal sling. J. Urol. 169(5): 1770–1774, 2003.
Azaïs, H., P. Collinet, V. Delmas, and C. Rubod. Rapport anatomique du ligament utérosacré et du nerf hypogastrique pour la chirurgie des lésions d’endométriose profonde. Gynecol. Obstet. Fertil. 41(3): 179–183, 2013.
Baah-Dwomoh, A., M. Alperin, M. Cook, and R. De Vita. Mechanical analysis of the uterosacral ligament: swine vs. human. Ann. Biomed. Eng. 46(12): 2036–2047, 2018.
Barber, M. D., and C. Maher. Apical prolapse. Int. Urogynecol. J. 24(11): 1815–1833, 2013.
Barber, M. D., and C. Maher. Epidemiology and outcome assessment of pelvic organ prolapse. Int. Urogynecol. J. 24(11): 1783–1790, 2013.
Barber, M. D., A. G. Visco, A. C. Weidner, C. L. Amundsen, and R. C. Bump. Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am. J. Obstet. Gynecol. 183(6): 1402–1411, 2000.
Barbier, H. M., M. Z. Smith, C. U. Eto, J. A. Welgoss, W. Von Pechmann, N. Horbach, and D. D. Gruber. Ureteral compromise in laparoscopic versus vaginal uterosacral ligament suspension: a retrospective cohort. Female Pelvic Med. Reconstr. Surg. 21(6): 363–368, 2015.
Becker, W., and R. De Vita. Biaxial mechanical properties of swine uterosacral and cardinal ligaments. Biomech. Model. Mechanobiol. 14(3): 549–560, 2015.
Bergman, A., G. Elia, D. Cheung, N. Perelman, and M. E. Nimni. Biochemical composition of collagen in continent and stress urinary incontinent women. Gynecol. Obstet. Invest. 37(1): 48–51, 1994.
Bonte, H., C. Chapron, M. Vieira, A. Fauconnier, H. Barakat, X. Fritel, M. C. Vacher-Lavenu, and J. B. Dubuisson. Histologic appearance of endometriosis infiltrating uterosacral ligaments in women with painful symptoms. J. Am. Assoc. Gynecol. Laparosc. 9(4): 519–524, 2002.
Brubaker, L., C. Maher, B. Jacquetin, N. Rajamaheswari, P. von Theobald, and P. Norton. Surgery for pelvic organ prolapse. Female Pelvic Med. Reconstr. Surg. 16(1): 9–19, 2010.
Buller, J. L., J. R. Thompson, G. W. Cundiff, L. K. Sullivan, M. A. S. Ybarra, and A. E. Bent. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet. Gynecol. 97(6): 873–879, 2001.
Butler-Manuel, S. A., L. D. Buttery, R. P. A’Hern, J. M. Polak, and D. P. Barton. Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer 89(10): 2144–2144, 2000.
Campbell, R. M. The anatomy and histology of the sacrouterine ligaments. Am. J. Obstet. Gynecol. 59(1): 1–12, 1950.
Chao, F. L., A. Rosamilia, P. L. Dwyer, A. Polyakov, L. Schierlitz, and G. Agnew. Does pre-operative traction on the cervix approximate intra-operative uterine prolapse? a randomised controlled trial. Int. Urogynecol. J. 23(4): 417–422, 2012.
Chapron, C., A. Fauconnier, M. Vieira, H. Barakat, B. Dousset, V. Pansini, M. Vacher-Lavenu, and J. B. Dubuisson: Anatomical distribution of deeply infiltrating endometriosis: surgical implications and proposition for a classification. Hum. Reprod. 18(1): 157–161, 2003.
Chen, L., R. Ramanah, Y. Hsu, J. A. Ashton-Miller, and J. O. L. DeLancey. Cardinal and deep uterosacral ligament lines of action: MRI based 3D technique development and preliminary findings in normal women. Int. Urogynecol. J. 24(1): 37–45, 2013.
Christensen, C. M., C. J. Mirocha, G. H. Nelson, and J. F. Quast. Effect on young swine of consumption of rations containing corn invaded by Fusarium roseum. Appl. Microbiol. 23(1): 202–202, 1972.
Cole, E. E., P. B. Leu, A. Gomelsky, P. Revelo, H. Shappell, H. M. Scarpero, and R. R. Dmochowski. Histopathological evaluation of the uterosacral ligament: Is this a dependable structure for pelvic reconstruction? BJU Int. 97(2): 345–348, 2006.
Collins, S. A., S. A. Downie, T. R. Olson, and M. S. Mikhail. Nerve injury during uterosacral ligament fixation: acadaver study. Int. Urogynecol. J. 20(5): 505–508, 2009.
Connell, K. A., M. K. Guess, H. Chen, V. Andikyan, R. Bercik, and H. S. Taylor. HOXA11 is critical for development and maintenance of uterosacral ligaments and deficient in pelvic prolapse. J. Clin. Investig. 118(3): 1050–1055, 2008.
Danso, E. K., J. D. Schuster, I. Johnson, E. W. Harville, L. R. Buckner, L. Desrosiers, L. R. Knoepp, and K. S. Miller. Comparison of biaxial biomechanical properties of post-menopausal human prolapsed and non-prolapsed uterosacral ligament. Sci. Rep. 10(1): 1–14, 2020.
DeLancey, J. O. L. Anatomic aspects of vaginal eversion after hysterectomy. Am. J. Obstet. Gynecol. 166(6): 1717–1728, 1992.
Diwadkar, G. B., M. D. Barber, B. Feiner, C. Maher, and J. E. Jelovsek. Complication and reoperation rates after apical vaginal prolapse surgical repair: a systematic review. Obstet. Gynecol. 113(2): 367–373, 2009.
Doumouchtsis, S. K., N. Gauthaman, A. Khunda, M. Basu, K. Dadhwal, Y. V. Gayle, and C. M. Durnea. Comparison between the Valsalva maneuver and intraoperative traction measurements in pelvic organ prolapse assessment. Int. J. Gynecol. Obstet. 139(3): 358–362, 2017.
Dviri, M., E. Leron, J. Dreiher, M. Mazor, and R. Shaco-Levy. Increased matrix metalloproteinases-1,-9 in the uterosacral ligaments and vaginal tissue from women with pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 156(1): 113–117, 2011.
Ercoli, A., V. Delmas, F. Fanfani, P. Gadonneix, M. Ceccaroni, A. Fagotti, S. Mancuso, and G. Scambia. Terminologia anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am. J. Obstet. Gynecol. 193(4): 1565–1573, 2005.
Fayyad, A., S. Hill, V. Gurung, S. Prashar, and A. R. B. Smith. How accurate is symptomatic and clinical evaluation of prolapse prior to surgical repair? Int. Urogynecol. J. 18(10): 1179–1183, 2007.
Findik, R. B., F. Ilkaya, S. Guresci, H. Guzel, S. Karabulut, and J. Karakaya. Effect of vitamin C on collagen structure of cardinal and uterosacral ligaments during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 201: 31–35, 2016.
Gabriel, B., D. Denschlag, H. Göbel, C. Fittkow, M. Werner, G. Gitsch, and D. Watermann. Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int. Urogynecol. J. 16(6): 475–479, 2005.
Gabriel, B., D. Watermann, K. Hancke, G. Gitsch, M. Werner, C. Tempfer, and A. Zur Hausen. Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int. Urogynecol. J. 17(5): 478–482, 2006.
Gautier, E. J. D., O. Mayeur, J. Lepage, M. Brieu, M. Cosson, M., and C. Rubod. Pregnancy impact on uterosacral ligament and pelvic muscles using a 3D numerical and finite element model: preliminary results. Int. Urogynecol. J. 29(3): 425–430, 2018.
Goepel, C. Differential elastin and tenascin immunolabeling in the uterosacral ligaments in postmenopausal women with and without pelvic organ prolapse. Acta Histochem. 110(3): 204–209, 2008.
Gruber, D. D., W. B. Warner, E. D. Lombardini, C. M. Zahn, and J. L. Buller. Anatomical and histological examination of the porcine vagina and supportive structures: in search of an ideal model for pelvic floor disorder evaluation and management. Female Pelvic Med. Reconstr. Surg. 17(3): 110–114, 2011.
Han, L., L. Wang, Q. Wang, H. Li, and H. Zang. Association between pelvic organ prolapse and stress urinary incontinence with collagen. Exp. Ther. Med. 7(5): 1337–1341, 2014.
Holt, E. US FDA rules manufacturers to stop selling mesh devices. Lancet 393(10182), 1686, 2019.
Iwanaga, R., D. J. Orlicky, J. Arnett, M. K. Guess, K. J. Hurt, and K. A. Connell. Comparative histology of mouse, rat, and human pelvic ligaments. Int. Urogynecol. J. 27(11): 1697–1704, 2016.
Jelovsek, J. E., and M. D. Barber. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Am. J. Obstet. Gynecol. 194(5): 1455–1461, 2006.
Kökçü, A., F. Yanik, M. Cetinkaya, T. Alper, B. Kandemir, and E. Malatyalioglu. Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch. Gynecol. Obstet. 266(2): 75–78, 2002.
Leegant, A., L. C. Zuckerwise, K. Downing, J. Brouwer-Visser, C. Zhu, M. J. Cossio, F. Strube, X. Xie, E. Banks, and G. S. Huang. Transforming growth factor-\(\beta\)1 and extracellular matrix protease expression in the uterosacral ligaments of patients with and without pelvic organ prolapse. Female Pelvic Med. Reconstr. Surg. 21(1): 53, 2015.
Li, C., Y. Gong, and B. Wang. The efficacy of pelvic floor muscle training for pelvic organ prolapse: a systematic review and meta-analysis. Int. Urogynecol. J. 27(7): 981–992, 2016.
Liapis, A., P. Bakas, A. Pafiti, M. Frangos-Plemenos, N. Arnoyannaki, and G. Creatsas. Changes of collagen type III in female patients with genuine stress incontinence and pelvic floor prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 97(1): 76–79, 2001.
Liu, C., Y. Wang, B. S. Li, Q. Yang, J. M. Tang, J. Min, S. S. Hong, W. J. Guo, and L. Hong. Role of transforming growth factor \(\beta\)-1 in the pathogenesis of pelvic organ prolapse: a potential therapeutic target. Int. J. Mol. Med. 40(2): 347–356, 2017.
Lowder, J. L., K. M. Debes, D. K. Moon, N. Howden, S. D. and Abramowitch, P. A. Moalli. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet. Gynecol. 109(1): 136–143, 2007.
Ludwig, S., S. Göktepe, P. Mallmann, and W. Jäger. Evaluation of different ‘tensioning’ of apical suspension in women undergoing surgery for prolapse and urinary incontinence. In Vivo 34(3): 1371–1375, 2020.
Luo, J., T. M. Smith, J. A. Ashton-Miller, and J. O. L. DeLancey. In vivo properties of uterine suspensory tissue in pelvic organ prolapse. J. Biomech. Eng. 136: 2, 2014.
Margulies, R. U., M. A. M. Rogers, and D. M. Morgan. Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am. J. Obstet. Gynecol. 202(2): 124–134, 2010.
Martins, P., A. L. Silva-Filho, A. M. R. M. Fonseca, A. Santos, L. Santos, T. Mascarenhas, R. M. N. Jorge, and A. M. Ferreira. Strength of round and uterosacral ligaments: a biomechanical study. Arch. Gynecol. Obstetr. 287(2): 313–318, 2013.
McCall, M. L. Posterior culdeplasty: surgical correction of enterocele during vaginal hysterectomy: a preliminary report. Obstet. Gynecol. 10(6): 595–602, 1957.
Miller, N. F. A new method of correcting complete inversion of the vagina. Surg. Gynecol. Obstet. 44: 550–554, 1927.
Moalli, P. A., N. S. Howden, J. L. Lowder, J. Navarro, K. M. Debes, S. D. Abramowitch, and S. L. Y. Woo. A rat model to study the structural properties of the vagina and its supportive tissues. Am. J. Obstet. Gynecol. 192(1): 80–88, 2005.
Norton, P. A. Pelvic floor disorders: the role of fascia and ligaments. Clin. Obstet. Gynecol. 36(4): 926–938, 1993.
Olsen, A. L., V. J. Smith, J. O. Bergstrom, J. C. Colling, and A. L. Clark. Epidemiology of surgically managed pelvic-organ prolapse and urinary incontinence. Obstet. Gynecol. 89(4): 501–506, 1997.
Pack, E., J. Stewart, M. Rhoads, J. Knight, S. Clark, D. G. Schmale III, and R. De Vita. Effects of short-term moderate ZEN consumption on uterosacral ligament elasticity in pubertal gilts. Res. Vet. Sci. 133: 202–209, 2020.
Persu, C., C. R. Chapple, V. Cauni, S. Gutue, and P. Geavlete. Pelvic organ prolapse quantification system (POP-Q)-a new era in pelvic prolapse staging. J. Med. Life 4(1): 75, 2011.
Petros, P. P. Severe chronic pelvic pain in women may be caused by ligamentous laxity in the posterior fornix of the vagina. Aust. N. Z. J. Obstet. Gynaecol. 36(3): 351–354, 1996.
Phillips, C. H., F. Anthony, C. Benyon, and A. K. Monga. Urogynaecology: collagen metabolism in the uterosacral ligaments and vaginal skin of women with uterine prolapse. BJOG 113(1): 39–46, 2006.
Putra, I. G. M., I. G. N. Warsita, K. Suwiyoga, I. B. G. F. Manuaba, I. N. G. Budiana, and A. A. G. P. Wiradnyana. Low expression of collagen type I in sacrouterine ligament as risk factor of stage III-IV uterine prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol., 2020.
Ramanah, R., M. B. Berger, B. M. Parratte, and J. O. L. DeLancey. Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int. Urogynecol. J. 23(11): 1483–1494, 2012.
Reay Jones, N. H. J., J. C. Healy, L. J. King, S. Saini, S. Shousha, and T. G. Allen-Mersh. Pelvic connective tissue resilience decreases with vaginal delivery, menopause and uterine prolapse. Br. J. Surg. 90(4): 466–472, 2003.
Reisenauer, C., T. Shiozawa, M. Oppitz, C. Busch, A. Kirschniak, T. Fehm, and U. Drews. The role of smooth muscle in the pathogenesis of pelvic organ prolapse-an immunohistochemical and morphometric analysis of the cervical third of the uterosacral ligament. Int. Urogynecol. J. 19(3): 383–389, 2008.
Rivaux, G., C. Rubod, B. Dedet, M. Brieu, B. Gabriel, and M. Cosson. Comparative analysis of pelvic ligaments: a biomechanics study. Int. Urogynecol. J. 24(1): 135–139, 2013.
Romanzi, L. J., and R. Tyagi. Hysteropexy compared to hysterectomy for uterine prolapse surgery: Does durability differ? Int. Urogynecol. J. 23(5): 625–631, 2012.
Rosenberg, K. and W. Trevathan. Birth, obstetrics and human evolution. BJOG 109(11): 1199–1206, 2002.
Schimpf, M., and P. Tulikangas. Evolution of the female pelvis and relationships to pelvic organ prolapse. Int. Urogynecol. J. 16(4): 315–320, 2005.
Shahryarinejad, A., T. R. Gardner, J. M. Cline, W. N. Levine, H. A. Bunting, M. D. Brodman, C. J. Ascher-Walsh, R. J. Scotti, and M. D. Vardy. Effect of hormone replacement and selective estrogen receptor modulators (SERMs) on the biomechanics and biochemistry of pelvic support ligaments in the cynomolgus monkey (Macaca fascicularis). Am. J. Obstet. Gynecol. 202(5): 485–e1, 2010.
Shahryarinejad, A., and M. D. Vardy. Comparison of human to macaque uterosacral–cardinal ligament complex and its relationship to pelvic organ prolapse. Toxicol. Pathol. 36(7-suppl): 101S–107S, 2008.
Shull, B. L., C. Bachofen, K. W. Coates, and T. J. Kuehl. A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am. J. Obstet. Gynecol. 183(6): 1365–1374, 2000.
Siddique, S. A., R. E. Gutman, M. A. S. Ybarra, F. Rojas, and V. L. Handa. Relationship of the uterosacral ligament to the sacral plexus and to the pudendal nerve. Int. Urogynecol. J. 17(6); 642–645, 2006.
Smith, T. M., J. Luo, Y. Hsu, J. Ashton-Miller, and J. O. L. DeLancey. A novel technique to measure in vivo uterine suspensory ligament stiffness. Am. J. Obstet. Gynecol. 209(5): 484–e1, 2013.
Srivastav, S., R. T. Jamil, and Zeltser. Valsalva maneuver, 2019.
Strinic, T., M. Vulic, S. Tomic, V. Capkun, I. Stipic, and I. Alujevic. Matrix metalloproteinases-1,-2 expression in uterosacral ligaments from women with pelvic organ prolapse. Maturitas 64(2): 132–135, 2009.
Takacs, P., M. Nassiri, M. Gualtieri, K. Candiotti, and C. A. Medina. Uterosacral ligament smooth muscle cell apoptosis is increased in women with uterine prolapse. Reprod. Sci. 16(5): 447–452, 2009.
Tan, T., N. M. Cholewa, S. W. Case, and R. De Vita. Micro-structural and biaxial creep properties of the swine uterosacral-cardinal ligament complex. Ann. Biomed. Eng. 44(11): 3225–3237, 2016.
Tan, T., F. M. Davis, D. D. Gruber, J. C. Massengill, J. L. Robertson, and R. De Vita. Histo-mechanical properties of the swine cardinal and uterosacral ligaments. J. Mech. Behav. Biomed. Mater. 42: 129–137, 2015.
Turner, L. C., E. S. Lavelle, and J. P. Shepherd. Comparison of complications and prolapse recurrence between laparoscopic and vaginal uterosacral ligament suspension for the treatment of vaginal prolapse. Int. Urogynecol. J. 27(5): 797–803, 2016.
Umek, W. H., D. M. Morgan, J. A. Ashton-Miller, and J. O. L. DeLancey. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet. Gynecol. 103(3): 447, 2004.
Usta, A., K. Guzin, M. Kanter, M. Ozgül, and C. S. Usta. Expression of matrix metalloproteinase-1 in round ligament and uterosacral ligament tissue from women with pelvic organ prolapse. J. Mol. Histol. 45(3): 275–281, 2014.
Vardy, M. D., T. R. Gardner, F. Cosman, R. J. Scotti, M. S. Mikhail, A. O. Preiss-Bloom, J. O. Williams, J. M. Cline, and R. Lindsay. The effects of hormone replacement on the biomechanical properties of the uterosacral and round ligaments in the monkey model. Am. J. Obstet. Gynecol. 192(5): 1741–1751, 2005.
Vercellini, P., G. Aimi, M. Busacca, G. Apolone, A. Uglietti, and P. G. Crosignani. Laparoscopic uterosacral ligament resection for dysmenorrhea associated with endometriosis: results of a randomized, controlled trial. Fertil. Steril. 80(2): 310–319, 2003.
Vergeldt, T. F. M., M. Weemhoff, J. IntHout, and K. B. Kluivers. Risk factors for pelvic organ prolapse and its recurrence: a systematic review. Int. Urogynecol. J. 26(11): 1559–1573, 2015.
Vierhout, M. E., J. Stoutjesdijk, and J. Spruijt. A comparison of preoperative and intraoperative evaluation of patients undergoing pelvic reconstructive surgery for pelvic organ prolapse using the pelvic organ prolapse quantification system. Int. Urogynecol. J. 17(1): 46–49, 2006.
Vineyard, D. D., T. J. Kuehl, K. W. Coates, and B. L. Shull. A comparison of preoperative and intraoperative evaluations for patients who undergo site-specific operation for the correction of pelvic organ prolapse. Am. J. Obstet. Gynecol. 186(6): 1155–1159, 2002.
Vu, D., B. T. Haylen, K. Tse, and A. Farnsworth. Surgical anatomy of the uterosacral ligament. Int. Urogynecol. J. 21(9): 1123–1128 (2010).
Vulic, M., T. Strinic, S. Tomic, V. Capkun, I. A. Jakus, and S. Ivica. Difference in expression of collagen type i and matrix metalloproteinase-1 in uterosacral ligaments of women with and without pelvic organ prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 155(2): 225–228, 2011.
Wu, J. M., C. A. Matthews, M. M. Conover, V. Pate, and M. J. Funk. Lifetime risk of stress incontinence or pelvic organ prolapse surgery. Obstet. Gynecol. 123(6): 1201, 2014.
Yılmaz, N., G. Ozaksit, Y. K. Terzi, S. Yılmaz, B. Budak, O. Aksakal, and F.İ. Şahin. HOXA11 and MMP2 gene expression in uterosacral ligaments of women with pelvic organ prolapse. J. Turk. Ger. Gynecol. Assoc. 15(2): 104, 2014.
Yucel, N., A. Usta, K. Guzin, M. Kanter, E. Bilgic, N. O. Ozel, and M. Ozgul. Immunohistochemical analysis of connective tissue in patients with pelvic organ prolapse. J. Mol. Histol. 44(1): 97–102, 2013.
Zeng, C., J. Liu, H. Wang, Y. Zhou, J. Wu, and G. Yan. Correlation between autophagy and collagen deposition in patients with pelvic organ prolapse. Female Pelvic Med. Reconstr. Surg. 24(3): 213–221, 2018.
Zhao, B. H., and J. H. Zhou. Decreased expression of elastin, fibulin-5 and lysyl oxidase-like 1 in the uterosacral ligaments of postmenopausal women with pelvic organ prolapse. J. Obstet Gynaecol. Res. 38(6): 925–931, 2012.
Zhu, Y. P., T. Xie, T. Guo, Z. Sun, L. Zhu, and J. H. Lang. Evaluation of extracellular matrix protein expression and apoptosis in the uterosacral ligaments of patients with or without pelvic organ prolapse. Int. Urogynecol. J., 2020. https://doi.org/10.1007/s00192-020-04446-7.
Acknowledgments
Funding was provided by NSF Grant No. 1804432.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Associate Editor Joel D. Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Donaldson, K., Huntington, A. & De Vita, R. Mechanics of Uterosacral Ligaments: Current Knowledge, Existing Gaps, and Future Directions. Ann Biomed Eng 49, 1788–1804 (2021). https://doi.org/10.1007/s10439-021-02755-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02755-6