Abstract
The objective of this work was to collect and summarize relevant literature on the anatomy, histology, and imaging of apical support of the upper vagina and the uterus provided by the cardinal (CL) and uterosacral (USL) ligaments. A literature search in English, French, and German languages was carried out with the keywords apical support, cardinal ligament, transverse cervical ligament, Mackenrodt ligament, parametrium, paracervix, retinaculum uteri, web, uterosacral ligament, and sacrouterine ligament in the PubMed database. Other relevant journal and textbook articles were sought by retrieving references cited in previous PubMed articles. Fifty references were examined in peer-reviewed journals and textbooks. The USL extends from the S2 to the S4 vertebra region to the dorsal margin of the uterine cervix and/or to the upper third of the posterior vaginal wall. It has a superficial and deep component. Autonomous nerve fibers are a major constituent of the deep USL. CL is defined as a perivascular sheath with a proximal insertion around the origin of the internal iliac artery and a distal insertion on the cervix and/or vagina. It is divided into a cranial (vascular) and a caudal (neural) portions. Histologically, it contains mainly vessels, with no distinct band of connective tissue. Both the deep USL and the caudal CL are closely related to the inferior hypogastric plexus. USL and CL are visceral ligaments, with mesentery-like structures containing vessels, nerves, connective tissue, and adipose tissue.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apical support for the uterus and upper vagina is provided by the cardinal (CL) and uterosacral (USL) ligaments [1]. These ligaments are critical in pelvic organ prolapse (POP) as, in addition to apical prolapse, they are strongly related to anterior vaginal wall descent [2, 3]; the most common form of POP being present in 83–87 % cases [4]. Importantly, the anterior vaginal wall is the site of failure in 72 % of recurrences [5]. Despite more than a century of cadaver- and surgery-based research on these ligaments, controversies still exist regarding terminology, definition, composition, and even their existence. Magnetic resonance (MR) imaging has provided new insight due to the possibility of studying these structures in living women without any dissection or tissue-fixing artifact [6, 7]. Furthermore, their contents have been revealed using modern histological techniques such as immunohistochemistry [8]. Given the importance of these structures to pelvic organ support, a summary of what is known about them is appropriate. Although many prior articles are concerned with the ligaments, as they relate to pelvic organ support, there is also a body of literature related to radical pelvic surgery that contains information about their structure and anatomical relationships. We therefore performed a literature review to clarify discrepancies in terminology, anatomy, histology, and imaging of the CL and USL.
Materials and methods
A search of literature in the English, French, and German languages was carried out using the keywords apical support, cardinal ligament, transverse cervical ligament, Mackenrodt ligament, parametrium, paracervix, retinaculum uteri, the web, uterosacral ligament, and sacrouterine ligament in the PubMed database, limiting the search to human studies. Articles that might yield additional original observations but not identified by our search, as well as literature published before 1966, were sought by pulling references cited in the retrieved articles. We also identified articles by searching bibliographies in gynecology and anatomy textbooks related to the CL and USL.
Results
In all, 50 references were examined in peer-reviewed journals and textbooks (Table 1).
Uterosacral ligament (USL)
Gross anatomy
Gross anatomy of the USL is shown in Fig. 1. The USL was first described in the early 1900s in the English literature [9–11]. Initial observations led to the conclusion that it was an important support of the uterus [12]. Blaisdell [9] described uterosacral fibers that attached to the fascia covering the levator ani, coccygeus, and obturator muscles, as well as the presacral fascia. Later, Campbell [13], after dissecting 33 cadavers, noted that whereas the ligaments are useful in surgery, they should not be credited with undue supportive value.
Axial section of a female pelvis: a gross anatomy with the uterus (Ut), ovary (Ov), cervix (Cx), and uterosacral ligament (USL); b magnification of the USL with its superficial (USL s ) and deep (USL d ) parts; c gross anatomy with the uterus (Ut), cervix (Cx), vagina (Vg), coccygeus muscle (Cocc m ) and cardinal ligament (CL); d magnification of the CL region (CL) between the cervix and the pelvic sidewall. The deep USL (USL d ) is visualized
Origin and insertion points
Campbell [13] defined the USL as condensations of fibroelastic and smooth muscle tissue containing autonomic nerves. They represented the lateral boundaries of the posterior cul-de-sac and were positioned lateral to the rectum and medial to the ureters. They attached distally to the posterolateral aspect of the cervix at the level of the internal cervical os and at the lateral vaginal fornix and proximally to the presacral fascia and the S2–S4 sacral vertebrae. Although most investigators reported the USL to originate from S2 to S4 sacral vertebrae, Buller et al. [14] found the origin of the USL to be fanlike at the sacrum (S1–S3 and variably S4), narrowing to its smallest width just proximal to the cervix. Other dense fibrous attachments were found connecting to the sacrospinous ligament. Both Buller et al. and Campbell found fibrous tissue that attached the sacral portion of the ligament to the presacral fascia and the periosteum. Their observations indicated that fibers of the USL and CL were consistently intermingled at the cervical portion, with fibers that extended anteriorly above the internal os and posteriorly onto the proximal third of the vagina. The ligament had a fan shape, with mean widths of 5.2 ± 0.9 cm, 2.7 ± 1.0, and 2.0 ± 0.5 cm at the sacral, intermediate, and cervical portions, respectively. Ercoli et al. [15] defined the USL as emanating from a connective tissue condensation of the visceral pelvic fascia (endopelvic fascia). It originated between S2 and S4 sacral foramina and ischial spines. Fibers forming the USL converge toward the dorsolateral portion of the uterine cervix. On the other hand, Cole et al. [16] described the USL as extending distally to the fusion of the vagina with the levator ani muscle. Fritsch et al. [17], in a plastinated cross-sectional anatomical study, could find no direct attachment of the USL to the bone of the sacrum itself but to adjacent structures. Imaging findings from this study that confirm that fact are described below.
Anatomical relationships
USL anatomical relationships are shown in (Fig. 2).
Dissection of an embalmed woman showing the uterus (Ut), cervix (Cx), superficial uterosacral ligament (USL s ) dissected and retracted from the deep USL (USL d ), ureter, cardinal ligament (CL), uterine artery (Uta), internal iliac artery (Iia), umbilical artery (Uma), obturator neurovascular pedicle (Ob), and sacral nervous trunks (Snt) (from [22])
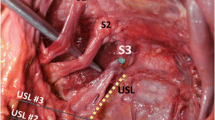
The ureter is lateral to the anterior margin of the USL [14, 18]. Mean [± standard deviation (SD)] distance from the ureter to the USL at the level of the sacrum is 4.1 ± 0.6 cm, at the level of the ischial spine 2.3 ± 0.9 cm, and at the level of the cervical internal os 0.9 ± 0.4 cm. Wieslander et al. [18] noted that sutures placed in the proximal and distal USL measured 1.4 cm on average from the ureter. Discrepancies between these two studies could be related to effects of embalming, pliability of fresh tissue, and the lateral entry point of the needle in the ligament. At the levels of the cervix and ischial spines, the tissues supported a weight >17 kg before failure. Tissue in the sacral region of the ligament was not as strong, failing at 5 kg [14].
Evaluating USL suspension sutures found the ligament to be located close to the first through third sacral foramina, indicating a potential risk of sacral nerve entrapment [18]. Moreover, Siddique et al. demonstrated that the USL crosses the S4 plexus trunk at a mean level of 0.9 cm superior to the ischial spine, the S3 trunk 1.5 cm superior to the ischial spine, the S2 trunk 2.6 cm superior to the ischial spine, and the S1 trunk 3.9 cm superior to the ischial spine [19]. Thus, occasionally, the S1—and more commonly S2–S4 nerve trunks—are vulnerable to injury during USL suspension. These structures pass under the intermediate portion of the USL, in which sutures are commonly placed. These data support the fact that sacral nerves could be ligated if USL suspension sutures are placed lateral to the ligament fibers or too deeply into the pelvic sidewall. From the cervix to its origin at the sacrum, the ligament was 8.7 cm long [95 % confidence interval (CI); 7.5–10.0] [19]. However, in a cadaver study of 12 nonembalmed and five formalin-fixed pelves, Vu et al. [20] measured the USL length as being between 12 and 14 cm.
The ischial spine is consistently found beneath the intermediate portion of the ligament. The superior gluteal vein, which lies medial to the superior gluteal artery, is found directly beneath the sacral portion of the ligament. In the intermediate portion, the middle rectal artery is found near the inferior margin of the USL. The right USL has an apparent greater prominence because of the left-sided deviation of the sigmoid and its mesentery [13].
In studying anatomy and tissue specimens obtained during radical surgery, Butler-Manuel et al. [21] called attention to the fact that the USL is not of similar consistency, thickness, or texture throughout its length or width. It can be divided into superficial and deep sections (Figs. 1 and 2). The superficial component is the structure covered by peritoneum observed on surgery and cadaver dissection when the uterus is pulled upward. The deep portion is obtained after removing the peritoneum and some subperitoneal connective tissue. Histological details concerning these different segments are presented in the next section.
Synthesis
There is general consensus that the USL originates from tissues in the region of S2–S4 sacral vertebrae, with no direct insertion to the bone. Genital tract insertion is at the dorsal margin of the uterine cervix and/or to the upper third of the posterior vaginal wall. The USL is positioned lateral to the rectum and medial to the ureter and has a superficial component covered by peritoneum and a deep retroperitoneal component. It lies nearest to the ureter at the cervix and nearest to the S2–S4 nerve trunks dorsally.
Histology
USL histology is presented in Fig. 3
Campbell [13] studied the USL in 33 cadavers: ten preserved and 23 fresh. Of these, 12 were evaluated histologically. Three distinct histologic ligament regions were identified. At the cervical attachment, the ligament was made up of closely packed bundles of smooth muscle, abundant medium-sized and small blood vessels, and small nerve bundles. The intermediate third of the ligament was composed of predominantly connective tissue and only a few scattered smooth muscle fibers, nerve elements, and blood vessels. The sacral third was almost entirely composed of loose strands of connective tissue and intermingled fat, with few vessels, nerves, and lymphatics. Parasympathetic fibers that supply the pelvic viscera arose from the second through fourth sacral nerve roots and joined sympathetic fibers from the superior hypogastric nerve plexus to form the inferior hypogastric plexus (IHP) or pelvic plexus. Fibers from this plexus followed branches of the internal iliac artery to innervate the pelvic viscera. Many of these fibers coursed through the USL. Tissue fixation and increased time after death before examination were major limitations of this study.
More recently, Cole et al. [16] undertook a histological evaluation of the connective tissue content and organization of the USL in seven fresh cadavers. They found attenuated, poorly organized connective tissue. There were sparse collagen fibers, muscle fibers, and scattered elastin immediately beneath the peritoneum, but they were not clearly organized into a condensed ligamentous structure; few fibroblasts were present. There was also a large amount of adipose tissue in each specimen.
Butler-Manuel et al. [8] collected intraoperative cross-sectional biopsies from the lateral third of the USL and CL of patients undergoing radical versus simple hysterectomy. Quantitative immunohistochemistry was used to demonstrate and quantify nerve content. They found that the ligaments contain autonomic nerves and ganglia, presumed to be extensions of the IHP. Nerve content of the USL and CL differed along their length, with significantly greater nerve content in the middle to lateral thirds toward their origin at the pelvic sidewall compared with the medial third toward the insertion of these ligaments into the uterine body and cervix. The USL had more nerve content than the CL, possibly reflecting differing functions for each ligament. Using traditional histological stains and specific antibodies, other investigators [22] confirmed theses results by showing that the USL contained connective tissue, vessels, nerve fibers, and autonomous ganglia and that no structured ligamentous organization was seen.
In studying the superficial and deep part of the USL with the use of nerve-specific antibodies and computer-assisted analysis of immunohistochemical images, Butler-Manuel et al. [21] found a lower percentage of nerve content in the superficial USL than in the deep USL. Sympathetic nerve fibers along with sensory/nociceptive nerves were relatively more abundant than parasympathetic fibers in the deep USL.
These results concerning USL composition were confirmed by Collins et al. [23], who found that the visceral fibers of the IHP were involved in a cadaver dissection study of nerve entrapment at the time of USL fixation. The nerve fibers originated from the S2 and S3 nerve roots. According to the convergence–projection and convergence–facilitation theories of visceral and referred pain in which visceral nerve afferents stimulate painful sensation in somatic spinal nerves, entrapment of these autonomic fibers could cause referred pain in the S2 and S3 dermatomes, leading to symptoms reported in the literature [24].
Gabriel et al. [25] compared the structural components of the USL in women with and without POP. USLs were found to contain approximately 20 % smooth muscle cells. There was no difference in collagen I expression and smooth muscle cell amount between women with and without prolapse. In contrast, collagen III expression was significantly related to prolapse. Later, the same authors [26] reported increased matrix metalloproteinase (MMP-2) expression in USL from women with prolapse.
Synthesis
The USL is a multifaceted, mesentery-like structure containing loose connective tissue, smooth muscle, vessels, and autonomic nerve fibers from the IHP, with contributions from sacral nerves. The USL has more nerve content than the CL. The superficial part consists mainly of smooth muscle peritoneal connective tissue. The deep part consists primarily of nerve fibers (Table 2).
Imaging
USL magnetic resonance (MR) imaging is shown in Fig. 4.
After decades of studying the USL in cadavers and at surgery, authors began documenting it in MR images of living women, as the borders of the ligament are difficult to establish on dissection and ligament removal is somewhat arbitrary. Umek et al. [7] made a quantitative analysis of the USL origin and insertion points using MR imaging. The USL was visible in 87 % of scans. It had a mean craniocaudal distance of 2.1 ± 0.8 cm (range 1–5), calculated from the number of images between the most cranial and the most caudal image, with identifiable origin and insertion points. The difference between this length and the 8- to 14-cm length described above is explained by the fact that the MR craniocaudal measurement does not correspond to the cervix-to-sacrum length measured during dissection, which moreover was made with the ligament under traction, whereas measurements made during imaging represent a length measured at rest. Three regions of origin were found in the MR study: cervix alone (33 %), cervix and vagina (63 %), and vagina alone (4 %) [7]. Proximal insertion points were as follows: sacrospinous ligament–coccygeus muscle complex (82 %), sacrum (7 %), piriformis muscle, and sciatic foramen or ischial spine (11 %). Although USL morphology was similar bilaterally, its craniocaudal extent was greater on the right side. These findings corroborate macroscopic findings by both Campbell [13] and Blaisdell [9], who observed that the sigmoid mesentery caused the left USL to appear less prominent.
Fritsch et al. [17] performed computed tomography (CT) and MR imaging of cadavers that confirmed their anatomic cross-sections by showing an absence of direct USL attachment to the bony sacrum. Similarly, Umek et al. [7] found that the USL does not connect to the bone itself but to fascial structures lying ventral or lateral to the sacrum. In a study of the posterior compartment using MR and three-dimensional reconstruction, Hsu et al. [27] demonstrated that the upper portion of the compartment was bordered by the USL, which was visible in 88 % of the cases and had ventral attachments to both the cervix and vagina. Dorsal attachments were not reported.
Synthesis
The USL is clearly visible in cross-sectional imaging with MR and CT and allow its resting length and attachments to be seen. These studies show that the USL attaches to fascial tissues adjacent to the sacrum and not to the bone of the sacrum itself. Controversial issues such as USL section definitions and length and relationship between USL and CL remain to be resolved by further research.
Cardinal ligament (CL)
Gross anatomy
Gross anatomy of the CL is demonstrated in (Fig. 1c, d). The first mention of a condensation at the base of the broad ligament was by Savage in 1870 [28]. This structure was later named the cardinal ligament by Kocks [29] and the transverse cervical ligament by Mackenrodt [30]. Our review found that eight terms have been invented to describe this structure (Table 3). For simplicity sake, we use the widely applied clinical term cardinal ligament and focus attention on the anatomy and histology of this region rather than on the many names used. In addition, there is continuous disagreement concerning the function of the CL, its structure, contents, and attachments to the uterus and the pelvic sidewall. Gross dissection of this structure was the basis for differing reports and nomenclatures. For instance, Martin [31] used the term retinaculum uteri; Meigs [32] used the term the web to denote fibers connecting the pelvic brim to the uterine cervix.
Origin and insertion points
Mackenrodt [30] referred to the CL as a stout bundle of fibers emanating from the iliac fossa and inserting into the sidewall of the cervix. Range et al. [33] studied the CL in 18 nonembalmed cadavers and found no structure similar to a skeletal ligament but, rather, areolar connective tissue surrounding blood vessels and the pelvic plexus of nerves, arising near the internal iliac artery and sweeping anteriorly and medially to reach the lateral border of the cervix and vagina (Fig. 2). This condensation was greatest at the lateral margin of the cervix and vagina, extending downward to the level of the pelvic floor. It could not be separated from the thinner, looser endopelvic fascia but did not continue around the vagina and cervix in any bulk. When the uterus was pulled to the opposite side, the CL became more apparent. The vessels appeared to lie in a space between two thick bands extending from the lateral border of the cervix and vagina to the lateral pelvic wall near the origin of the internal iliac artery. In their plastinated anatomical study of the pelvis, Fritsch et al. [17] found no separate band of connective tissue that fastened the cervix and the vault of the vagina to the pelvic sidewall. The paracervical region was mainly filled with adipose tissue, including uterine vessels and nerves, which they thought could be confounded with a ligamentous structure. Kato et al. [34] asserted that the CL was not adequately characterized by anatomists because it could be identified clearly only when the pararectal and paravesical spaces were opened by fingers and instruments. They found a well-defined fascial (ligamentous) structure at the dorsal margin of the CL, dorsal to the cervix. This well-defined fascial structure at the bottom of the CL area consisted of collagenous fibers connecting the cervix to the ischial spine and the endopelvic fascia. The American version of Gray’s anatomy [35] defined the CL as “the fascia over the ventral and dorsal walls of the vagina and cervix that come together at the lateral border of these organs, and the resulting sheets that extend across the pelvic floor as a deeper continuation of the broad ligament.” In 2005,Yabuki et al. [36] undertook a comprehensive cadaver-based dissection study to solve discrepancies regarding CL anatomy. They defined the CL as the bundle that connected the pelvic brim and the uterine cervix. The latter was shown to be a mesentery-like structure covered on its anterior and posterior aspects with visceral endopelvic fascia that was an extension of the perivascular sheath of the internal iliac vessels. In their opinion, the CL was continuous with the hypogastric fascia and did not correspond to any condensation in the base of the broad ligament.
Anatomical relationships
Regarding CL anatomical relationships Range et al. [33] showed that the ureter had a pathway inside the CL at the point at which it crosses under the uterine artery. At this level, vessels became larger and the areolar tissue was less compact and was connected loosely with the superior fascia of the pelvic diaphragm by multiple fine filaments. As the pelvic wall was approached, this tissue fanned out rapidly to become continuous with the general retroperitoneal connective tissue. Peham and Amreich [37], having analyzed the relationship between adjacent organs and connective tissue bands, classified the CL into bladder, vaginal–cervical, and rectal septa. These septa were described as independent bundles that laid parallel to the longitudinal axis of each corresponding organ and did not cross each other. Reiffenstuhl [38] adopted the same concept of horizontal disposition of the septa. However, this theory cannot explain contemporary surgical dissection of the CL, where the rectum is separated from the CL by the USL. Yabuki et al. have studied the CL for several decades [34, 36, 39–43] as it relates to cervical cancer surgery. The CL was observed after opening the paravesical and pararectal spaces. They divided the CL into two parts: the ventral or superficial vascular part, and the dorsal or deep neural part (Table 2). Along with other Japanese authors, they believe that a major part of the IHP is included in the neural part of the CL. However, they also expanded the concept of the CL to assert that it continued to the lateral rectal ligament (one of the neurovascular bundles of the rectum) and made a complete complex [42]. Furthermore, Yabuki et al. classified the pelvic connective tissue into a suspensory and a supporting system [36]. The suspensory system was reported to be a group of true ligaments that had a musculofascial consistency and connected the fascia of the pelvic viscera in a chain-like fashion from the pubis to the sacrum and/or coccyx. It consisted of the pubovesical ligament, superficial layer of the vesicouterine ligament, rectouterine or sacrouterine ligament, and rectococcygeal ligament, which suspended and anchored the pelvic organs to the pubis, sacrum and/or coccyx. On the other hand, the supporting system was a neurovascular fascial complex consisting of the vesicohypogastric fascia, CL, and lateral ligament of the pelvis. The authors’ concept differed from Peham’s in that their definition of the CL was stacked from cranial to caudal and not horizontally.
Given the existence of so many different terms describing pelvic ligaments and fasciae, Ercoli et al. [15] set out to establish a correspondence between the Terminologia Anatomica [44] (official nomenclature) and other commonly used unofficial terms (Table 3). Thus, the CL corresponded to Terminologia Anatomica terms of parametrium and paracervix, as both the parametrium and paracervix consist of connective mesenteries formed mainly by areolar tissue enveloping the visceral branches of the internal iliac vessels during their course toward the uterus and vagina. Conventionally, tissues crossing over the ureter are to be identified with the parametrium, whereas those that cross below the ureter are considered paracervix. In fact, the latter was suggested to be equivalent to the caudal portion of the CL, whereas the parametrium was associated with the cranial portion. In their dissection-based study, these investigators [15] thought that the paracervix could be responsible for the solidity of the CL in pelvic support because it is mainly formed by thick connective mesentery, enveloping the venous root [38], and thinner mesenteries enveloping the inferior vesical and vaginal vessels. The venous root is formed by veins draining the paravisceral venous plexus into the internal iliac vein. However, this concept of paracervix being the caudal portion of the CL or the infraureteral parametrium remains debatable. In a study based upon laparoscopic surgery, cadaver dissection, and MR imaging, Touboul et al. [45] failed to identify the paracervix under the ureter. The only tissue under the ureter corresponded to a connective structure stretching in a ventral-to-dorsal direction on both sides of the rectum, confounding with the USL. Moreover, Hockel et al. [46] analyzed uterovaginal development in serial sections of female human embryos and fetuses and identified no structured CL consisting of dense connective tissue fixing the cervix to the lateral pelvic sidewall. Both investigators explained this discrepancy by dissection artifacts linked to creation of the pararectal and paravesical spaces in other studies [15, 36].
Synthesis
The CL is a mesentery-like structure covered by the visceral pelvic fascia. It is defined as a perivascular sheath with a proximal insertion at approximately the origin of the internal iliac artery and a distal insertion on the cervix and/or vagina. Compared with the USL, CL insertion points are less well identified prior to the creation of pararectal and paravesical spaces.
Histology
CL histology is depicted in Fig. 3. The microscopic study by Range et al. [33] confirmed that the chief bulk of the CL was blood vessels (mainly veins), nerves arising from the IHP, lymphatic vessels, and their surrounding loose areolar connective tissue. Connections by fine filaments (of collagen) with the superior pelvic diaphragmatic fascia were visible. This areolar tissue was most dense at the site where the fascia was penetrated by blood vessels. Those smooth muscle fibers present were only associated with blood vessel walls and adventitia. Cellular elements, especially fibroblasts, were numerous. There were few isolated elastic fibers outside the vessel walls. The authors clearly concluded that there was no ligament in the sense of a separate band of connective tissue and suggested that the entire mass of retroperitoneal areolar connective tissue supported the pelvic organs. Interestingly, they compared pelvic connective tissue to a piece of chicken wire: “When placed under traction, the chicken wire assumes the appearance of a strong cable, a situation which is duplicated by the paracervical tissues when placed under traction at the time of surgery.”
Later, Kato et al. [34] performed a dissection and histological study of embalmed and fresh cadavers. Although they could clearly visualize the CL between the pararectal and paravesical spaces, no fascial or ligamentous structure was identified on histological analysis, except at the dorsal border of the CL area. Instead, arteries and veins were concentrated in a belt-like area between the uterine cervix and the upper opening of the small pelvic cavity. The fascial structure at the bottom of the CL connected the cervix to the ischial spine and the endopelvic fascia. Nerve distribution in the CL was investigated using myelin staining. The preganglionic pelvic splanchnic nerves were distributed laterally and dorsally to the CL. The IHP was arranged saggitally in a small plate-like manner and was located near the bottom of the CL, close to the fascia described above. Notably, the area of the pelvic splanchnic nerves and plexus was separated from the high vascularity region of the CL area by loose connective tissue.
In 2008, Hoffman et al. [47] analyzed the histopathologic content of the vascular portion of the CL in patients undergoing radical hysterectomy for cervical cancer. Histologic sectioning revealed few nerve twigs in the vascular segment but no large nerve trunks. Ewies et al. [48] studied changes in extracellular matrix proteins in the CL of postmenopausal women with or without POP using a computerized immunohistomorphometric analysis. They found that the CL of prolapsed uteri were characterized by a higher expression of collagen III and tenascin and lower quantities of elastin. Later, they discovered higher levels of estrogen alpha, androgen, and progesterone receptors in the CL of prolapsed uteri [49]. Estrogen-beta receptors were higher in the group with normal pelvic support. In 2010, Salman et al. [50] investigated the CL in women with and without prolapse using light and electron microscopy. They found an altered connective tissue distribution within the CL from women with POP: collagen fibers were fewer and thicker.
Synthesis
The CL consists mainly of vessels, some areolar connective tissue, and some nerve fibers. It can thus be divided into vascular (cranial portion of CL, parametrium) and neural (caudal portion of CL, paracervix) sections. The vascular section is an extension of the perivascular sheath of internal iliac vessel branches going to the genital tract, whereas the neural section is an extension of the IHP (Table 2).
Imaging
In a comparative study between cadaver specimens and MR imaging, Tunn et al. [6] showed that the downward sweep of the CL was visible on standardized MR coronal scans (Fig. 5). However, they acknowledged the fact that this ligament was a complex structure consisting of vessels, nerves, and connective tissue rather than a single band of connective tissue. These findings were confirmed by Touboul et al. [45], who on paracoronal MR scans observed high T2 signals on both sides of the uterus corresponding to the supraureteral lateral parametrium (cranial portion of the CL). Within this structure, lower T2 signals were interpreted as vessels. No structure was observed corresponding to the paracervix. Likewise, Fritsch et al. [17] previously found no CL on CT, MR, or anatomic dissections of fetuses and adults. They found the paracervical region to be filled with mainly adipose tissue, vessels, and nerves.
Synthesis
The CL is best observed on coronal MR scans. It has a characteristic downward sweep from approximately the origin of the internal iliac artery to the genital tract. MR image corresponds mainly to vessels running through the CL. As in dissection studies, imaging could not precisely identify the CL insertion points.
Discussion
Structures that connect the cervix and vagina to the pelvic sidewall, most commonly known as the CL and USL, have been studied using a variety of investigative techniques. These studies were motivated by either a desire to evaluate the role of these ligaments in pelvic organ support or to understand their relationship to radical hysterectomy. This review sought to bring a global approach to the study of these ligaments to allow a synthesis of separate descriptions into a coherent whole (Fig. 6). Considering the number of operative procedures that describe these ligaments for suspension or for oncological operations, an accurate understanding of their structure and nature seems important. This is especially true because the clinical term ligament to some people implies that there is a direct connective tissue attachment between the genital tract and the pelvis—an important misconception.
Anatomical synthesis of cardinal (CL) and uterosacral (USL) ligaments, modified from Kato et al. [34], showing the bladder (B), the uterus (UT), some of the vascular constituents of the CL, which are the uterine artery (Ua) and vein (Uv), the close relationships of the USL with the pelvic plexus (PX), the sacral nerve trunks S2–S4 (Snt), and the hypogastric nerve (Hn), ureter (UR), rectum (Rec), common iliac artery (CIa), external iliac artery and vein (EIav), obturator nerve (On)
Several important conclusions have been obtained from this review. Most importantly, the CL and USL are visceral ligaments with mesentery-like structures containing vessels, nerves, connective tissue, adipose tissue, and lymphatics that connect an organ to the body wall. They vary in the amount of each of these elements from one place to another. It is important to recognize that they are not separate bands of connective tissue similar to skeletal ligaments. The term visceral ligament is used to avoid confusion with skeletal ligaments that connect two bones. This type of flexible mesentery-like support in the pelvis is mechanically logical. The bladder, vagina, and rectum are all distensible organs, and the uterus is highly mobile and must have attachments that allow for normal filling, evacuation, and mobility. Having fixed rigid ligaments would not provide the physiological function required. Abnormal fixation occurs when inadequate rigid meshes for POP repair are used, resulting in impaired bladder and bowel compliance and distension. In addition to support, these organs need appropriate vascularization and innervation provided through the ligaments. The deep USL contains a major conduit of autonomous nerves closely related to the IHP [21, 22]. On the other hand, the CL carry mainly vessels of the internal iliac system that go to the vagina and uterus, and less nerve fibers were found in its content compared with the USL [8].
Whereas there was general agreement among authors that a structure called the USL exists [51], some authors [17, 46, 51] deny the existence of the CL. Their studies sought to see whether there is a band of connective tissue, separate from the vessels and nerves, that could satisfy what many students and clinicians think of as a ligament. In other words, they sought to determine whether there is a band of connective tissue attaching the cervix to the pelvis that is distinct from the neurovascular elements. Fritsch [17] and Hockel [46] found no thick band of tissue corresponding to the CL and did not consider the perivascular sheath of the internal iliac vessels going to the genital tract to constitute a ligament. If one accepts the fact that the term cardinal ligament refers to a visceral ligament, which is a mesentery-like structure that connects the uterus to the pelvic sidewall, then there is no disagreement concerning the findings. It is the term, rather than the structure, that is controversial. There are a number of visceral ligaments (e.g., suspensory ligament of the ovary, lateral umbilical ligaments, triangular ligament of the liver, etc.) that refer to visible ridges of tissue that do not contain a dense connective tissue attaching two structures, but mainly consist of vessels.
As we point show in Table 3, many terms have been used for these ligaments. Terminologia Anatomica, a major internationally accepted authority of anatomical terminology, uses the terms parametrium and paracervix. We favor these terms to avoid the implication that there is a separate skeletal-type ligament involved. Although we agree that these are useful words, the common use of the terms CL and USL in current literature justifies their study. The CL can be assumed to be a structure within the parametrium and the paracervix. Instead of relating to a clearly defined ligament structure, it probably corresponds to a region of retroperitoneal areolar tissue associated with vessels supporting the pelvic organs. In the end, the terminology issues boil down to what definition and significance are given to entities. These problems with terminology are, in fact, not new. As the father of the scientific method, Sir Francis Bacon observed more than 300 years ago: “Whereas the meaning ought to govern the term, the term in effect governeth the meaning” [52].
Connective tissue is one component of ligaments that is highly responsible for pelvic support, as it is a living structure that provides the supporting matrix for almost every organ in the body. It consists of cells such as fibroblasts and smooth muscle cells surrounded by fibers and amorphous ground substance. Many loose and irregularly arranged fibers can condense along lines of tension, as Range et al.’s “pulled chicken wire” analogy suggests [33], so the fact that it is not dense connective tissue does not mean it lacks mechanical strength. The resilience of connective tissue is thought to be affected by two factors: an increased ratio of weaker, type III collagen to stronger type I collagen, often seen with wound healing after injury, trauma, or surgery; and an inherent abnormality of tissues histologically characterized by a decrease in tissue cellularity [53]. Kokcu et al. [54] showed higher collagen content and decreased cellularity in connective tissue of patients with POP compared with patients without prolapse. They suggested that decreased fibroblasts and increased collagen type III content could be associated with pelvic floor dysfunction. Elastin fibers did not differ between the two groups, suggesting that elastic fibers most probably do not play a significant role in the etiology of POP. These findings were confirmed by studies on both the USL [25] and the CL [48, 50].
Several authors have called attention to regional differences in these ligaments. The superficial USL described by Butler-Manuel et al. [21] is the structure observed by surgeons during laparoscopy and laparotomy but only if the uterus is pulled anteriorly and placed under tension. On MR imaging, this is the component that is rarely and difficultly visualized, because, as opposed to its deep counterpart, it contain less nerve fibers and vessels [21]. The USL described on MR by Umek et al. [7] and Hsu et al. [27] is, in fact, the deep component. Compared with classical cadaver dissection [13, 14, 18, 19], the authors noted more frequent attachments to the sacrospinous ligament—the coccygeus muscle junction instead to the sacral S2–S4 vertebrae, and found it to be shorter than after cadaver dissection, as MR measurements were in the craniocaudal direction, and ligaments in vivo have tone and are not held under tension. In addition, there are differences in structure depending on how close to the uterus or pelvic sidewall samples are taken [14, 20].
There are somewhat conflicting opinions about the differing compositions of the CL and USL. This arises due to three factors: variable composition of ligaments, differing sites of sampling, and the inherent difficulty in separating them on dissection near the uterus, where they intermingle with one another. The USL and CL are mesentery-like structures with many elements. In the region near the cervix in particular, they intermingle with one another, and any attempt to obtain tissue in this region is based on somewhat arbitrary divisions created during dissection. Most studies reviewed for this article were dissection based using either cadavers or during surgery. Dissection and tissue fixing can produce major artifacts. To visualize the CL, Yabuki et al. [36] opened the paravesical and pararectal spaces. On doing this, they probably took as a single structure CL and USL fibers, as the latter is supposed to be situated medially to the rectum (Fig. 6). Consequently, they divided the CL into a vascular (cranial) and a neural (caudal) portion. This neural portion consists of parasympathetic nerve fibers distributed to the bladder and rectum. On the other hand, investigators [8, 20–22] examining primarily the USL found it consisted of a superficial fibrous and a deep neurovascular section. Similarly to the neural CL, the deep USL was found to contain autonomous nerves and ganglia, closely related to the IHP. In fact, the neural portion of the CL and the deep USL might correspond to the same entity observed from different viewpoints. This structure is most probably the IHP, which is usually a less-well-defined plexus than the superior hypogastric plexus, and lies close to where the ureter passes under the uterine artery [55, 56].
This review article is an effort to clarify current knowledge about the USL and CL. There are still uncertainties and discrepant viewpoints about the anatomy of the subperitoneal pelvic organ support in women. Studying these structures is not only important for academic insight but also for the clinical application of improving and optimizing surgery both for pelvic floor dysfunction and malignant diseases.
Abbreviations
- CL:
-
Cardinal ligament
- USL:
-
Uterosacral ligament
- POP:
-
Pelvic organ prolapse
- IHP:
-
Inferior hypogastric plexus or pelvic plexus
- MR:
-
Magnetic resonance
- CT:
-
Computed tomography
References
DeLancey JO (1992) Anatomic aspects of vaginal eversion after hysterectomy. Am J Obstet Gynecol 166:1717–1724
Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L (2006) Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol 195:1837–1840
Summers A, Winkel LA, Hussain HK, DeLancey JO (2006) The relationship between anterior and apical compartment support. Am J Obstet Gynecol 194:1438–1443
Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A (2002) Pelvic organ prolapse in Women’s Health Initiative: gravity and gravidity. Am J Obstet Gynecol 186:1160–1166
Fialkow MF, Newton KM, Weiss NS (2008) Incidence of recurrent pelvic organ prolapse 10 years following primary surgical management: a retrospective cohort study. Int Urogynecol J Pelvic Floor Dysfunct 19:1483–1487
Tunn R, DeLancey JO, Quint EE (2001) Visibility of pelvic organ support system structures in magnetic resonance images without an endovaginal coil. Am J Obstet Gynecol 184:1156–1163
Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JO (2004) Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol 103:447–451
Butler-Manuel SA, Buttery LD, A'Hern RP, Polak JM, Barton DP (2000) Pelvic nerve plexus trauma at radical hysterectomy and simple hysterectomy: the nerve content of the uterine supporting ligaments. Cancer 89:834–841
Blaisdell FE (1917) The anatomy of the sacrouterine ligaments. Anat Rec 12:22
Deaver JB (1903) Surgical anatomy. P.Blakiston's Son & Co., Philadelphia
Montgomery EE (1905) Practical gynecology second edition. P.Blakiston's Son & Co., Philadelphia
Fothergill W (1908) The supports of the pelvic viscera: a review of some recent contributions to pelvic anatomy, with a clinical introduction. J Obstet Gynecol Br Emp 13:18–28
Campbell RM (1950) The anatomy and histology of the sacrouterine ligaments. Am J Obstet Gynecol 59:1–12
Buller JL, Thompson JR, Cundiff GW, Krueger Sullivan L, Schon Ybarra MA, Bent AE (2001) Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol 97:873–879
Ercoli A, Delmas V, Fanfani F, Gadonneix P, Ceccaroni M, Fagotti A et al (2005) Terminologia anatomica versus unofficial descriptions and nomenclature of the fasciae and ligaments of the female pelvis: a dissection-based comparative study. Am J Obstet Gynecol 193:1565–1573
Cole EE, Leu PB, Gomelsky A, Revelo P, Shappell H, Scarpero HM et al (2006) Histopathological evaluation of the uterosacral ligament: is this a dependable structure for pelvic reconstruction? BJU Int 97:345–348
Fritsch H, Hotzinger H (1995) Tomographical anatomy of the pelvis, visceral pelvic connective tissue, and its compartments. Clin Anat 8:17–24
Wieslander CK, Roshanravan SM, Wai CY, Schaffer JI, Corton MM (2007) Uterosacral ligament suspension sutures: anatomic relationships in unembalmed female cadavers. Am J Obstet Gynecol 197:672.e1–672.e6
Siddique SA, Gutman RE, Schon Ybarra MA, Rojas F, Handa VL (2006) Relationship of the uterosacral ligament to the sacral plexus and to the pudendal nerve. Int Urogynecol J Pelvic Floor Dysfunct 17:642–645
Vu D, Haylen BT, Tse K, Farnsworth A (2010) Surgical anatomy of the uterosacral ligament. Int Urogynecol J Pelvic Floor Dysfunct 21:1123–1128
Butler-Manuel SA, Buttery LD, Polak JM, A'Hern R, Barton DP (2008) Autonomic nerve trauma at radical hysterectomy: the nerve content and subtypes within the superficial and deep uterosacral ligaments. Reprod Sci 15:91–96
Ramanah R, Parratte B, Arbez-Gindre F, Maillet R, Riethmuller D (2008) The uterosacral complex: ligament or neurovascular pathway? Anatomical and histological study of fetuses and adults. Int Urogynecol J Pelvic Floor Dysfunct 19:1565–1570
Collins SA, Downie SA, Olson TR, Mikhail MS (2009) Nerve injury during uterosacral ligament fixation: a cadaver study. Int Urogynecol J Pelvic Floor Dysfunct 20:505–508
Flynn MK, Weidner AC, Amundsen CL (2006) Sensory nerve injury after uterosacral ligament suspension. Am J Obstet Gynecol 195:1869–1872
Gabriel B, Denschlag D, Göbel H, Fittkow C, Werner M, Gitsch G, Watermann D (2005) Uterosacral ligament in postmenopausal women with or without pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 16:475–479
Gabriel B, Watermann D, Hancke K, Gitsch G, Werner M, Tempfer C, zur Hausen A (2006) Increased expression of matrix metalloproteinase 2 in uterosacral ligaments is associated with pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct 17:478–482
Hsu Y, Lewicky-Gaupp C, DeLancey JO (2008) Posterior compartment anatomy as seen in magnetic resonance imaging and 3-dimensional reconstruction from asymptomatic nulliparas. Am J Obstet Gynecol 198:651.e1–651.e7
Savage H (1870) The surgery, surgical pathology and surgical anatomy of the female pelvic organs. J Churchill & Sons, London
Kocks J (1886) Die normale und pathologische lage und gestalt des uterus sowie deren mechanik. Max Cohen & Sohn, Bonn
Mackenrodt A (1895) Ueber die ursachen der normalen und pathologischen lagen des uterus. Arch F Gynak 48:393–421
Martin E (1911) Der haftapparat der weiblichen genitalien. S. Karger, Berlin
Meigs JV (1951) Radical hysterectomy with bilateral pelvic lymph node dissections: a report of 100 patients operated on five or more years ago. Am J Obstet Gynecol 62:854–870
Range RL, Woodburne RT (1964) The gross and microscopic anatomy of the transverse cervical ligament. Am J Obstet Gynecol 90:460–467
Kato T, Murakami G, Yabuki Y (2002) Does the cardinal ligament of the uterus contain a nerve that should be preserved in radical hysterectomy? Anat Sci Int 77:161–168
Clemente CD (ed) (1985) Gray’s anatomy, 30th American edn. Lea & Febiger, Philadelphia, pp 1575–1576
Yabuki Y, Sasaki H, Hatakeyama N, Murakami G (2005) Discrepancies between classic anatomy and modern gynecologic surgery on pelvic connective tissue structure: harmonization of those concepts by collaborative cadaver dissection. Am J Obstet Gynecol 193:7–15
Peham HV, Amreich J (1934) Operative gynecology (translated by Ferguson LK). JB Lippincott, Philadelphia
Reiffenstuhl G (1982) The clinical significance of the connective tissue planes and spaces. Clin Obstet Gynecol 25:812–820
Yabuki Y, Asamoto A, Hoshiba T, Nishimoto H, Kitamura S (1991) Dissection of the cardinal ligament in radical hysterectomy for cervical cancer with emphasis on the lateral ligament. Am J Obstet Gynecol 164:7–14
Yabuki Y, Asamoto A, Hoshiba T, Nishimoto H, Satou N (1996) A new proposal for radical hysterectomy. Gynecol Oncol 62:370–378
Yabuki Y (1997) Cardinal ligament dissection based on a new theory. CEM J Gynecol Oncol 2:278–287
Yabuki Y, Asamoto A, Hoshiba T, Nishimoto H, Nishikawa Y, Nakajima T (2000) Radical hysterectomy: an anatomic evaluation of parametrial dissection. Gynecol Oncol 77:155–163
Kato T, Murakami G, Yabuki Y (2003) A new perspective on nerve-sparing radical hysterectomy: nerve topography and over-preservation of the cardinal ligament. Jpn J Clin Oncol 33:589–591
Terminologia Anatomica (1998) International anatomical terminology/Federative Committee on Anatomical Terminology (FCAT). Thieme, Stuttgart
Touboul C, Fauconnier A, Zareski E, Bouhanna P, Daraï E (2008) The lateral infraureteral parametrium: myth or reality? Am J Obstet Gynecol 199:242.e1–242.e6
Höckel M, Horn LC, Fritsch H (2005) Association between the mesenchymal compartment of uterovaginal organogenesis and local tumour spread in stage IB-IIB cervical carcinoma: a prospective study. Lancet Oncol 6:751–756
Hoffman MS, Williams V, Salihu HM, Gunasekaran S, Sayer RA, Hakam A, Roberts WS (2008) The vascular portion of the cardinal ligament: surgical significance during radical hysterectomy for cervical cancer. Am J Obstet Gynecol 199:191.e1–191.e7
Ewies AA, Al-Azzawi F, Thompson J (2003) Changes in extracellular matrix proteins in the cardinal ligaments of post-menopausal women with or without prolapse: a computerized immunohistomorphometric analysis. Hum Reprod 18:2189–2195
Ewies AA, Thompson J, Al-Azzawi F (2004) Changes in gonadal steroid receptors in the cardinal ligaments of prolapsed uteri: immunohistomorphometric data. Hum Reprod 19:1622–1628
Salman MC, Ozyuncu O, Sargon MF, Kucukali T, Durukan T (2010) Light and electron microscopic evaluation of cardinal ligaments in women with or without uterine prolapse. Int Urogynecol J Pelvic Floor Dysfunct 21:235–239
Fritsch H, Zwierzina M, Riss P (2011) Accuracy of concepts in female pelvic floor anatomy: facts and myths! World J Urol Oct 15 [Epub ahead of print]
Bacon F (1937) Of unity and religion. Essays, civil and moral 1625. P.F Collier and Sons, New York
Norton PA (1993) Pelvic floor disorders: the role of fascia and ligaments. Clin Obstet Gynecol 36:926–938
Kokcu A, Yanik F, Cetinkaya M, Alper T, Kandemir B, Malatyalioglu E (2002) Histopathological evaluation of the connective tissue of the vaginal fascia and the uterine ligaments in women with and without pelvic relaxation. Arch Gynecol Obstet 266:75–78
Mauroy B, Bizet B, Bonnal JL, Crombet T, Duburcq T, Hurt C (2007) Systematization of the vesical and uterovaginal efferences of the female inferior hypogastric plexus (pelvic): applications to pelvic surgery on women patients. Surg Radiol Anat 29:209–217
Mauroy B, Demondion X, Bizet B, Claret A, Mestdagh P, Hurt C (2007) The female inferior hypogastric (= pelvic) plexus: anatomical and radiological description of the plexus and its afferences–applications to pelvic surgery. Surg Radiol Anat 29:55–66
Acknowledgment
We thank Chelsea Noel for editing figures
Conflicts of interest
John DeLancey receives research support from American Medical Systems, Johnson & Johnson, Kimberly Clark and Procter & Gamble.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramanah, R., Berger, M.B., Parratte, B.M. et al. Anatomy and histology of apical support: a literature review concerning cardinal and uterosacral ligaments. Int Urogynecol J 23, 1483–1494 (2012). https://doi.org/10.1007/s00192-012-1819-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-012-1819-7