Abstract
Introduction and hypothesis
We studied the geometry of and changes in structures that play an important role in stabilizing the pelvic system during pregnancy using a numerical system at different gestational ages and postpartum.
Methods
We developed a parturient numerical model to assess pelvic structures at different gestational stages (16, 32, and 38 weeks) and postpartum (2 months and 1 year) using magnetic resonance imaging (MRI). Organs, muscles, and ligaments were segmented to generate a 3D model of the pelvis. We studied changes in the length of uterosacral ligaments (USL) and thickness of the puborectal portion of the levator ani muscle (LAM) during and after pregnancy. We used this model to perform finite element (FE) simulation and analyze deformations of these structures under stress from the increase in uterine weight.
Results
Analysis reveals an increase in the length of US ligaments at 16, 32, and 38 weeks. Two months after delivery, it decreases without returning to the length at 16 weeks of pregnancy. Similar changes were observed for the puborectal portion of the LAM. Variations observed in these structures are not equivalent to other anatomical structures of pelvic suspension. FE simulation with increased uterus weight does not lead to those findings.
Conclusion
This analysis brings new elements and a new focus for discussion relating to changes in pelvic balance of parturient women that are not simply linked to the increase in uterine volume.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Female genital prolapse is a major common health problem in which pathophysiology remains unclear. However, some events in women’s obstetrical records, such as pregnancy, multiparity, and vaginal birth, are now identified as risk factors [1]. From the beginning of pregnancy, changes occur in the stability of pelvic statistics shown by changes in Pelvic Organ Prolapse Quantification system (POP-Q) scores, most often without symptoms [2]. The main hypothesis to explain such modifications is related to biomechanical properties of pelvic tissues [3].
Finite element (FE) simulation is commonly used in biomechanics, particularly in gynecology, to analyze system mobility in physiological and pathological conditions [4, 5]. FE simulation is a computer modeling technique for solving problems of engineering and mathematical physics by taking into account the geometry of the studied system, the behavior of material of involved structures, and loading conditions applied. There are other systems allowing the study of the pelvic system during the delivery [6, 7], so the tool can be used to analyze the behavior of anatomical structures undergoing different stresses, such as uterus weight increase during pregnancy.
Our research aimed at studying the geometry of structures that play an important role in balancinc the pelvis, such as levator ani muscle (LAM) and uterosacral ligaments (USL) [8]. We are investigating the use of a magnetic resonance imaging (MRI)-generated numerical model of the pregnant woman at different gestational ages and postpartum to analyze geometrical changes these structures undergo.
Materials and methods
Thanks in part to research and techniques mastered by our team, we developed a numerical model of the parturient at different gestational ages and postpartum [4]. A volunteer parturient (30 years old) who gave free and informed consent (no internal review board approval acquired) underwent MRI at 16, 32, and 38 weeks of gestation (WG) and 2 months and 1 year after delivery (postpartum). This patient had medical/surgical history. She had a cesarean section for labor dystocia at 4 cm cervical dilatation at 41 weeks’ GA. Fetal biometry was standard, with a 3260-g birth weight (20th percentile, Hadlock references). The cephalic measurement was head circumference (HC) 35 cm (90th percentile), and amniotic fluid measurement during the pregnancy was normal. The patient was 52 kg weight before pregnancy, with a normal 14-kg weight gain during pregnancy.
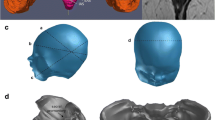
We performed T1, T2, and proton-density MRI sequences without contrast and under medical control. We obtained a cross-sectional view of the pelvis in transverse, coronal, and sagittal planes. A medical doctor trained to read MRI performed manual segmentation of the different anatomic structures. His competences ensured proper identification of each structure and validation of selections. AVIZO software (Standard Edition 7® Visualization Sciences Group VSG, SAS) was used for segmentation. Each slice was analyzed for organs, bones, ligaments, and muscles. By superimposing all 2D slices, we generated a 3D model for each GA analyzed. (Fig. 1). The 3D numerical model was a standard format commonly used for visualizing different structures; however, it has some flaws due to reconstruction algorithms specific to the software. To analyze these structures accurately, we reworked the representations to obtain a viable geometric model using CATIA® (Dassault System) software (Fig. 1). This step was validated by analyzing the deviation between models to ensure accurate representation of the initial anatomical structures. We obtained a final anatomical representative model that allows both geometrical analysis and FE simulation.
To analyze US ligaments, we measured length increased according to GA and in post-partum. Calculation of US ligament length was based on curves modeled under CATIA. Each ligament was represented by six curves located in the upper, central and lower part, inside and outside, corresponding to the limits of 3D reconstructions (Fig. 2). For each GA, the average length of left and right ligaments was calculated from a total of 60 measurements.
We analyzed puborectal, pubovisceral, and iliococcygeal LAM, focussing mainly on the puborectalis, by measuring changes in muscle thickness. The muscle is represented by two CATIA-generated surfaces (internal and external). One thousand points of analysis are generated on a regular basis on the internal surface, which enabled us to calculate the gap separating it from the external surface to assess muscle thickness (Fig. 3).
Thereafter, we used this model to perform simulation by FE and analyzed structures by applying a weight increase equivalent to the combined weight of uterus, fetus, placenta, and amniotic fluid during different stages of pregnancy [9]. Implementation of this model is based on previous work by our team [4, 5, 11]. We generate a FE mesh of the whole geometric model derived from the MRI at 16 WG, then applied different uterine weights varying from 660 g at 12 WG to 4850 g at 38 WG. We performed a second simulation with imposed displacement of the cervix based on MRI position at different GAs. For both loading conditions, FE simulation enabled us to obtain a distorted geometry and analyze the impact of modifications on ligaments and muscle structures. We then compared USL size by using the same technique as we increased uterus weight.
Results
USL
In the process of creating our geometric model, we observed that USLs increased in size. This was confirmed with reconstruction of the geometric model (Fig. 2). Indeed, the study of USLs at different GAs shows an increase in length (16, 32, and 38 WG, 40, 46, and 51 mm, respectively). At 2 months and 1 year postpartum, involution occurred, but the structures did not return to normal size; the average of six curves was 45 mm at 1 year postpartum versus 40 mm at 16 WG. Length also vary widely at 38 WG (25—95 mm). At that stage, 50% of values are between 42 and 84 mm. At 16 WG, 32 WG, and postpartum, values were less dispersed and remained <60 mm (Fig. 4a).
Levator ani
For the levator ani, MRI slices do not permit observation of changes in thickness as easily as for USLs. There is an increase in thickness depending on the stage of pregnancy, followed by a decrease postpartum. Here, too, involution is not complete 2 months after delivery, since there is no return to first-trimester pregnancy values (Fig. 4b). At 16 WG, the puborectal muscle is 3.3-mm thick and increases to 4.5 mm at 32 WG, then 5.3 mm at 38 WG, then decreases postpartum (17 weeks after 38 WG evaluation), reaching 4.4 mm 1 year later. Of the 4000 points studied, half were between 1.9 and 4.7 mm at 16 WG vs. 4.2–6.7 mm at 38 WG.
Variations in USLs and puborectal muscle do not seem to be equivalent with other muscle structures. We also assessed the thickness of other levator ani muscles: pubovisceral and iliococcygeal. The pubovisceral muscle increases slightly from 5.0 mm (16 WG) to 5.5 mm (38 WG).
Numerical simulation
FE simulations with increasing loads corresponding to the increase in uterus weight during pregnancy did not lead to the same results. Indeed, even with maximum uterus weight, USLs did not reach the levels observed on MRI sequences (Fig. 5).
Discussion
We developed 3D numerical model to evaluate geometric evolution of USLs and LAM during pregnancy and postpartum. Many 3D numerical and FE models have been developed to simulate childbirth [12,13,14,15]. Nevertheless, these models have not been used for geometrical analysis of pelvic structures during pregnancy. To our knowledge we report the first results of such analysis. These models are anatomically limited and do not depict the entire pelvic floor [12]. Our goal was to integrate a maximum number pelvic floor structures and thus focused on the three LAM structures and the USLs.
We previously developed a numerical model that simulates childbirth and USLs during the second phase of labor [10]. However, it was based on MRI at 34 WG within the framework of neonatal screening. The sequences used did not ensure good visibility of the muscle and ligaments. USLs and muscles were thus integrated into the model using anatomical reconstruction rather than MRI segmentation, so geometrical analysis was not perfectly representative of reality. Our new model is obtained from MRI sequences performed at various GAs using the proton-density sequence. We were thus able to integrate ligaments and muscles through segmentation and obtained a model anatomically closer to reality.
USLs are essential to ensure a balanced anatomical pelvic system [10, 16]. To our knowledge, there has been no published research focusing on changes in USLs during pregnancy. Studying ligaments on a corpse or during surgery is relatively difficult [10, 11]. Some authors have used MRI to describe these structures anatomically and reported good visibility with the proton-density sequence [16]. Numerical simulation helped highlight the important role they play in pelvic floor stasis [17]. Our geometrical model allows in-depth analysis of these ligaments. We found they increase in length during pregnancy and that even 1 year postpartum, USLs do not return to initial values (16 WG). We might first assume that such an increase is linked to their implantation on the uterus. However, they are implanted at the cervix–isthmus junction, which undergoes few changes due to increased weight during pregnancy.
We also focused on the pelvic muscle to assess changes during pregnancy, studying each part of the LAM separately. Indeed, studies have shown that this muscle undergoes maximum stress during delivery [7, 18, 19]. Delancey et al. showed that the stretch ratio of the LAM, particularly the puborectal portion, can be multiplied by 3.26 at the end of the second stage of labor, during delivery of the fetal head [7]. It is known that maximum stretch ratio for striated muscle tissue is 1.5. So far, the theory put forward to account for the tolerance phenomenon is changes in biomechanical properties of tissue in pregnant women [20]. Our study shows that geometrical changes do occur in this muscle, particularly in thickness. These modifications might allow and enhance its resistance to birth trauma. Analysis of the LAM at 2 months and 1 year postpartum revealed values higher than those observed at 16 WG, leading to the conclusion that complete return to initial pelvic system geometry does not occur after pregnancy and childbirth.
Using the FE simulation, we attempted to simulate the impact of uterus weight gain during pregnancy on USL length. We did not observe the same behavior of the USL using our simulation compared with changes observed in real life based on the four MRI models. It seems to indicate that changes in the USL length are not only the result of uterus weight gain and suggests that other factors influence the change of anatomical structures during pregnancy.
A significant increase in POP has been described during pregnancy. A moderate, usually asymptomatic, change in POP-Q score has been described between the first and third trimesters [2]. Thereafter, spontaneous regression was observed during the year following childbirth and was faster if the patient had a cesarean section [21]. Our findings related to USLs and LAM showed concomitant geometric changes with clinical evolution during pregnancy and after childbirth. The clinical asymptomatic modification might reflect these geometrical changes.
We know that USLs and LAM play an important role in pelvic floor stasis [17]. Different works show that these structures undergo important stress during delivery, even if they seem more resistant than other muscle structures [Changes that take place during pregnancy could take part in this modification of behavior and prepare them for the upcoming stress of childbirth. Moreover, we know that vaginal childbirth has a role in pelvic floor disorders [22]. Even though our patient had a cesarean section, some modifications in USL length and thickness remained after delivery, suggesting that some modification in the pelvic floor begins during pregnancy, not simply during vaginal childbirth. It would have been interesting to analyze LAM and USL size and thickness before pregnancy, but we have no MRI for our patient at that stage. Nevertheless, postpartum values did not return to 16 WG values, so we hypothesize that changes in these structures were not as important before pregnancy and at the GA of 16 weeks.
Our results are based on one patient only. Before widening our sample, we chose to start with a comprehensive study to justify MRI on pregnant women. Even though there was no demonstrable increase in the occurrence of disease or disability for short-term fetal electromagnetic field exposure after the first 3 months [23,24,25], we performed MRI—without obvious medical benefit—on a volunteer patient. Even though we could not predict the evolution of birth when we began studying this patient, we wanted to determine its value before performing MRIs in more pregnant women. Nevertheless, these data seem relevant, since they identify the ideal GA at which to evaluate during pregnancy its effect on pelvic ligaments and muscles. This preliminary result shows the feasibility of conducting studies with a larger series of patients and a minimized number of MRIs in an effort to simulate vaginal delivery [26].
Conclusion
Our research highlights changes occurring in the pelvic geometrical system from the second trimester to 1-year postpartum. This analysis brings some new elements and a new focus for discussion relating to changes in a parturient’s pelvic ligaments and muscles that are not simply linked to the increase in volume and size of the uterus. It could explain some clinical changes in stasus during and after pregnancy. Our findings might also account for the capacity of such structures to support stress related to vaginal delivery.
References
Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109(6):1396–403.
O’Boyle AL, O’Boyle JD, Ricks RE, Patience TH, Calhoun B, Davis G. The natural history of pelvic organ support in pregnancy. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(1):46–9. discussion 49
Rahn DD, Ruff MD, Brown SA, Tibbals HF, Word RA. Biomechanical properties of the vaginal wall: effect of pregnancy, elastic fiber deficiency, and pelvic organ prolapse. Am J Obstet Gynecol. 2008 May;198(5):590.e1–6.
Mayeur O, Witz J-F, Lecomte P, Brieu M, Cosson M, Miller K. Influence of Geometry and Mechanical Properties on the Accuracy of Patient-Specific Simulation of Women Pelvic Floor. Ann Biomed Eng. 2015.
Jeanditgautier E, Mayeur O, Brieu M, Lamblin G, Rubod C, Cosson M. Mobility and stress analysis of different surgical simulations during a sacral colpopexy, using a finite element model of the pelvic system. Int Urogynecol J. 2016.
Parente MPL, Natal Jorge RM, Mascarenhas T, Fernandes AA, Martins J. a. C. The influence of the material properties on the biomechanical behavior of the pelvic floor muscles during vaginal delivery. J Biomech. 2009;42(9):1301–6.
Ashton-Miller JA, Delancey JOL. On the biomechanics of vaginal birth and common sequelae. Annu Rev Biomed Eng. 2009;11:163–76.
Wei JT, De Lancey JOL. Functional anatomy of the pelvic floor and lower urinary tract. Clin Obstet Gynecol. 2004;47(1):3–17.
C. Courpotin, C Polonovski, M Voyer, J-C Chaumeil. Importance de la nutrition prénatale. In: Nutrition et renutrition en pratique pédiatrique. 1992.
Nichols DH. Types of genital prolapse. Postgrad Med. 1969;46(5):183–7.
Buller JL, Thompson JR, Cundiff GW, Krueger Sullivan L, Schön Ybarra MA, Bent AE. Uterosacral ligament: description of anatomic relationships to optimize surgical safety. Obstet Gynecol. 2001;97(6):873–9.
Lepage J, Cosson M, Mayeur O, Brieu M, Rubod C. The role of childbirth research simulators in clinical practice. Int J Gynaecol Obstet Off Organ Int Fed Gynaecol Obstet 2015.
Yan X, Kruger JA, Li X, Nielsen PMF, Nash MP. Modeling the second stage of labor. Wiley Interdiscip Rev Syst Biol Med. 2016;8(6):506–16.
Lepage J, Jayyosi C, Lecomte-Grosbras P, Brieu M, Duriez C, Cosson M, et al. Biomechanical pregnant pelvic system model and numerical simulation of childbirth: impact of delivery on the uterosacral ligaments, preliminary results. Int Urogynecology J. 2014.
Oliveira DA, Parente MPL, Calvo B, Mascarenhas T, Natal Jorge RM. Numerical simulation of the damage evolution in the pelvic floor muscles during childbirth. J Biomech. 2016;49(4):594–601.
Umek WH, Morgan DM, Ashton-Miller JA, DeLancey JOL. Quantitative analysis of uterosacral ligament origin and insertion points by magnetic resonance imaging. Obstet Gynecol. 2004;103(3):447–51.
Cosson M, Rubod C, Vallet A, Witz JF, Dubois P, Brieu M. Simulation of normal pelvic mobilities in building an MRI-validated biomechanical model. Int Urogynecology J. 2013;24(1):105–12.
Li X, Kruger JA, Nash MP, Nielsen PMF. Anisotropic effects of the levator ani muscle during childbirth. Biomech Model Mechanobiol. 2011;10(4):485–94.
Hoyte L, Damaser MS, Warfield SK, Chukkapalli G, Majumdar A, Choi DJ, et al. Quantity and distribution of levator ani stretch during simulated vaginal childbirth. Am J Obstet Gynecol. 2008 Aug;199(2):198.e1–5.
Ulrich D, Edwards SL, Su K, White JF, Ramshaw JAM, Jenkin G, et al. Influence of reproductive status on tissue composition and biomechanical properties of ovine vagina. PLoS One. 2014;9(4):e93172.
Chen Y, Li F-Y, Lin X, Chen J, Chen C, Guess M. The recovery of pelvic organ support during the first year postpartum. BJOG Int J Obstet Gynaecol. 2013;120(11):1430–7.
Volløyhaug I, Mørkved S, Salvesen Ø, Salvesen K. Pelvic organ prolapse and incontinence 15-23 years after first delivery: a cross-sectional study. BJOG Int J Obstet Gynaecol. 2015;122(7):964–71.
Baker PN, Johnson IR, Harvey PR, Gowland PA, Mansfield P. A three-year follow-up of children imaged in utero with echo-planar magnetic resonance. Am J Obstet Gynecol. 1994;170(1 Pt 1):32–3.
Elster AD. Does MR imaging have any known effects on the developing fetus? AJR Am J Roentgenol. 1994;162(6):1493.
Revised guidance on acceptable limits of exposure during nuclear magnetic resonance clinical imaging. Br J Radiol. 1983 Dec;56(672):974–7.
Mayeur O, Jeanditgautier E, Witz J-F, Lecomte-Grosbras P, Cosson M, Rubod C, et al. Evaluation of Strains on Levator Ani Muscle: Damage Induced During Delivery for a Prediction of Patient Risks. In: Computational Biomechanics for Medicine [Internet]. Springer, Cham; 2017 [cited 2017 Jun 21]. p. 135–46. Available from: https://springerlink.bibliotecabuap.elogim.com.doc-distant.univ-lille2.fr/chapter/10.1007/978-3-319-54481-6_12
Acknowledgements
University Lille and Lille medical faculty for the financial support of this project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
E. Jean Dit Gautier, O. Mayeur, J. LePage, and M. Brieu report no conflict of interest. C. Rubod led training courses with Olympus and Nordicpharma. M. Cosson led training courses with Boston, AMS, and Olympus, and is a consultant, accepting honoraria and payment for research from Allergan, Boston, and AMS.
Rights and permissions
About this article
Cite this article
Jean Dit Gautier, E., Mayeur, O., Lepage, J. et al. Pregnancy impact on uterosacral ligament and pelvic muscles using a 3D numerical and finite element model: preliminary results. Int Urogynecol J 29, 425–430 (2018). https://doi.org/10.1007/s00192-017-3520-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-017-3520-3