Abstract
Introduction and hypothesis
The aim was to review the safety and efficacy of pelvic organ prolapse surgery for vaginal apical prolapse.
Methods
Every 4 years and as part of the Fifth International Collaboration on Incontinence we reviewed the English-language scientific literature after searching PubMed, Medline, Cochrane library and Cochrane database of systematic reviews, published up to January 2012. Publications were classified as level 1 evidence (randomised controlled trials (RCT) or systematic reviews), level 2 (poor quality RCT, prospective cohort studies), level 3 (case series or retrospective studies) and level 4 case reports. The highest level of evidence was utilised by the committee to make evidence-based recommendations based upon the Oxford grading system. Grade A recommendation usually depends on consistent level 1 evidence. Grade B recommendation usually depends on consistent level 2 and or 3 studies, or “majority evidence” from RCTs. Grade C recommendation usually depends on level 4 studies or “majority evidence‟ from level 2/3 studies or Delphi processed expert opinion. Grade D “no recommendation possible” would be used where the evidence is inadequate or conflicting and when expert opinion is delivered without a formal analytical process, such as by Delphi.
Results
Abdominal sacral colpopexy (ASC) has a higher success rate than sacrospinous colpopexy with less SUI and postoperative dyspareunia for vault prolapse. ASC had greater morbidity including operating time, inpatient stay, slower return to activities of daily living and higher cost (grade A). ASC has the lowest inpatient costs compared with laparoscopic sacral colpopexy (LSC) and robotic sacral colpopexy (RSC). LSC has lower inpatient costs than RSC (grade B).In single RCTs the RSC had longer operating time than both ASC and LSC (grade B). In small trials objective outcomes appear similar although postoperative pain was greater in RSC. LSC is as effective as ASC with reduced blood loss and admission time (grade C). The data relating to operating time are conflicting. ASC performed with polypropylene mesh has superior outcomes to fascia lata (level I), porcine dermis and small intestine submucosa (level 3; grade B). In a single RCT, LSC had a superior objective and subjective success rate and lower reoperation rate compared with polypropylene transvaginal mesh for vault prolapse (grade B).Level 3 evidence suggests that vaginal uterosacral ligament suspension, McCall culdoplasty, iliococcygeus fixation and colpocleisis are relatively safe and effective interventions (grade C).
Conclusion
Sacral colpopexy is an effective procedure for vault prolapse and further data are required on the route of performance and efficacy of this surgery for uterine prolapse. Polypropylene mesh is the preferred graft at ASC. Vaginal procedures for vault prolapse are well described and are suitable alternatives for those not suitable for sacral colpopexy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
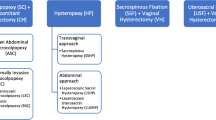
While anterior vaginal prolapse is most common, loss of apical support is usually present in women with prolapse that extends beyond the hymen [1, 2]. There is growing recognition that adequate support for the vaginal apex is an essential component of a durable surgical repair for women with advanced prolapse [3, 4]. Because of the significant contribution of the apex to anterior vaginal support, the best surgical correction of the anterior and posterior walls may fail unless the apex is adequately supported [5, 6]. While recognition of apical defects is one of the biggest challenges in the preoperative evaluation of pelvic support defects, surgical correction of the apex has several good options with relatively high success rates. Apical suspension procedures can be broadly separated into those performed transvaginally and those performed abdominally. Abdominal procedures can be performed via laparotomy or using conventional laparoscopic or robotically assisted-laparoscopic techniques. Although precise estimates are not available, most studies suggest that the vaginal approach is most common with 80–90 % of procedures being performed through this route [7–11]. The individual woman’s surgical history and goals, as well as her individual risks of surgical complications, prolapse recurrence and de novo symptoms affect surgical planning and the choice of procedure for apical POP.
Sacrospinous ligament suspension
One of the most popular and widely reported transvaginal procedures for correcting apical prolapse is sacrospinous ligament suspension (SSLS). First described in 1958 [12], this procedure suspends the vaginal apex to the sacrospinous ligament, either unilaterally or bilaterally, typically using an extraperitoneal approach. Observational series and clinical trials suggest that while apical recurrence after SSLS is uncommon (2.4 to 19 %), recurrence of anterior vaginal prolapse is more problematic (6 to 28.5 %; Table 1). A meta-analysis by Morgan et al. found an overall failure rate at any site of 28.8 % (95 % CI 18.4–36.3 %), with failure of the anterior segment seen in 21.3 % (17.3–25.3 %), apical segment in 7.2 % (95 % CI 4.0–10.4 %) and posterior segment in 6.3 % (95 % CI 4.2–8.4 %). Whether the relatively high rate of anterior vaginal prolapse recurrence seen with SSLS is due to the posterior deflection of the vaginal axis, as many authors suggest [15, 17, 18, 20], or simply represents a general predilection of anterior support to fail after pelvic reconstructive surgery remains unknown [32]. Reoperation rates after SSLS range from 1.3 to 37 %, with all but two series reporting rates of less than 9 % (Table 1).
Information on the functional or QOL outcomes of SSLS is limited. Maher et al. demonstrated significant improvements in condition-specific and generic QOL after SSLS, similar to that after abdominal sacral colpopexy [27]. A meta-analysis of randomised and observational studies found a pooled average for failure to provide relief of prolapse symptoms after SSLS of 10.3 % (95 % CI 4.4–16.2 %) [33]. The pooled average for failure to provide patient satisfaction after SSLS in this analysis was 13 % (95 % CI 7.4–18.6 %) [33]. Although infrequent, serious complications associated with SSLS include buttock pain and sacral/pudendal neurovascular injury. In a review of 22 studies that included 1,229 SSLS procedures, 3 patients (0.2 %) had life-threatening haemorrhage from sacral or pudendal vascular injury and the overall transfusion rate was 2 % [22]. Buttock pain occurred in 3 % of subjects, the vast majority of which resolved by 6 weeks postoperatively [22].
Uterosacral ligament suspension
Uterosacral ligament suspension (USLS) was first described by Miller [34] in 1927 and was later popularised by Shull in the late 1990s [3]. The USLS suspends the vaginal apex to the proximal remnants of the uterosacral ligaments using an intraperitoneal surgical approach. This procedure restores the vagina to its normal axis, avoiding the retroflexion associated with SSLS. The current evidence supporting the use of USLS is limited primarily to uncontrolled retrospective case series and evaluation of these data confirm a mean objective success rate of 85 % (range 48–96 %) and a mean reoperation rate for prolapse of 5.8 % (range 0–12 %; Table 2). A meta-analysis performed by Margulies et al. found pooled rates of anatomical success (POPQ stage 0–1) of 81.2 % (95%CI 67.5–94.5 %) for the anterior segment, 98.3 % (95 % CI 95.7–100 %) for the apical segment and 87.4 % (95 % CI, 67.5–94.5 %) for the posterior segment [47]. Postoperative prolapse symptoms were reported in 5 of the 11 studies in this review and were relieved in 82–100 % of patients. These promising results are balanced by a ureteral kinking/injury rate of 1–11 % with this procedure [47]. A review of 700 consecutive vaginal prolapse surgeries found intraoperative ureteral kinking/injury of 5.9 % directly attributable to USLS. However, 87 % were identified at cystoscopy before the completion of the index surgery and were relieved by removing suspension sutures intraoperatively with no long-term consequences for the patient [48]. Only 3 of the 355 USLS (0.9 %) performed in this series required additional procedures to relieve or correct ureteral obstruction or injury. Margulies et al. identified 10 studies, including a total of 820 women, that reported on the perioperative complications of USLS [47]. The ureteral reimplantation rate in this series was only 0.6 %. Blood transfusions were reported in 1.3 %, cystotomy in 0.1 %, and bowel injury in 0.2 %. To date, no clinical trials comparing the USLS with sacral colpopexy or with SSLS have been published. The Pelvic Floor Disorders Network has an ongoing RCT comparing USLS with SSLS that includes 440 subjects who will be followed for 2 years; results from this trial are expected in 2013 [49].
While the USLS is traditionally performed using an intraperitoneal approach, Dwyer and Fatton have described an extraperitoneal variant of the USLS [50, 51]. In their series of 123 consecutive women undergoing an extraperitoneal USLS, 93 also received anterior and/or posterior synthetic mesh. The overall anatomical success (POPQ stage 0–1) at a mean follow-up of 2 years (range 6 months to 5 years) was 85.5 %, with apical success of 95.4 % [51]. The reoperation rate for recurrent prolapse was 7 %. Ureteral injury occurred in only 1.7 %; however, the blood transfusion rate was 4.9 % and the rate of mesh exposure was 19.3 %.
Abdominal and laparoscopic USLS techniques have also been described. Lowenstein et al. reported a retrospective review of 107 women who underwent prolapse surgery that included an abdominal USLS [52]. In the 75 patients who completed the 1-year follow-up, 12 % reported recurrent or persistent prolapse symptoms and 7 % had an anatomical failure (POPQ stage 2 or greater). Complications were relatively few; however, erosion of the apical sutures (expanded PTFT, Gore-Tex) occurred in 9 % at an average time of 56 months (range 3–75 months) [52]. Rardin et al. reported a retrospective comparison of 96 patients undergoing vaginal USLS with 22 undergoing a laparoscopic USLS procedure and found no significant differences in perioperative morbidity or anatomical or subjective outcomes [53].
Mayo/McCall’s culdoplasty
Like the USLS, the Mayo/McCall’s culdoplasty uses the proximal uterosacral ligaments to suspend the vaginal apex. The major difference is that with the Mayo/McCall procedure the uterosacral ligaments are plicated in the mid-line to obliterate the posterior cul-de-sac. While commonly performed, data describing the outcomes for this procedure are limited (Table 3). Colombo and Milani retrospectively compared the outcomes of a modified McCall’s culdoplasty with those of SSLS (n = 62 in each group) [20]. Recurrence after the McCall’s culdoplasty (Baden–Walker grade ≥2) was 15 % 4 to 9 years after surgery and was not significantly different from the SSLS group. Recurrent anterior vaginal prolapse occurred less frequently in McCall’s group than in the SSLS group (6 vs 21 %, p = 0.04; OR 4.1 [95 % CI 1.3 to 14.2]) [20]. A large retrospective series of 693 women from the Mayo clinic described an 82 % satisfaction rate on subjective follow-up with few complications [54]. The rate of subsequent prolapse repair in this population was 5.2 %. A retrospective case series of 411 women undergoing Mayo culdoplasty found that a more dorsal “deep” placement of sutures through the uterosacral ligaments reduced the incidence of ureteral obstruction compared with other published series [57].
Levator myorrhaphy
In 1961, Francis and Jeffcoate described their retrospective series using levator myorrhaphy in which a wide midline plication of the levator ani muscles is performed to which the vaginal cuff is fixed [58]. A large sponge pack in the rectum is used to avoid overplication and bowel dysfunction. Five of the 35 women responding to the questionnaire had transient ureteral complications, one requiring reoperation. Seventeen women were quite satisfied, while 6 were dissatisfied. Natale et al. compared high levator myorrhaphy with USLS in a randomised clinical trial of 229 women with stage 2–4 prolapse [59]. All women underwent a hysterectomy and all received placement of polypropylene mesh in the anterior vaginal segment. Anatomical success was not significantly different between groups. The mean total vaginal length was significantly shorter after levator myorrhaphy (7.9 vs 8.9 cm, p = 0.04). Urinary, bowel and sexual function did not differ between groups postoperatively. Intraoperative ureteral obstruction was less common in the levator myorrhaphy group (0 vs 7.9 %); however, all cases of ureteral obstruction in the USLS group were corrected intraoperatively with suture removal/replacement with no additional interventions required [59]. Other complications including mesh erosion were similar in the groups.
Iliococcygeus fascia fixation
There are no randomised trials that support the use of this procedure. Several case series have provided some information. Shull reported that apical support was optimal in 39 out of 42 of patients (83 %), but 8 others had apical or other defects [60]. Meeks and colleagues reported a 96 % objective cure in 110 women followed for up to 13 years [61]. In a retrospective case–control study, Maher and colleagues reported similar subjective (91 vs 94 %) and objective (53 vs 67 %) cure rates with iliococcygeus fixation (n = 50) compared with sacrospinous fixation (n = 78) [62].
Transvaginal mesh apical prolapse
Two randomised control trials evaluated transvaginal polypropylene meshes in apical prolapse. Sokol et al. reported a multicentre double-blinded RCT comparing uterosacral colpopexy and native tissue repair (n = 33) with a monofilament polypropylene mesh kit (Prolift, n = 32; Ethicon, Somerville, NJ, USA) for stage 2 or greater uterovaginal prolapse or vaginal prolapse [63].
At 1 year the conventional surgery group had no subsequent surgical interventions compared with 15.6 % in the mesh group (p = 0.017) including 3 for prolapse surgery (2 sacral colpopexy and 1 iliococcygeous fixation) and 2 interventions for mesh exposure. The objective failure rate (any stage 2 or greater prolapse) was 70 % in the conventional surgery group vs 63 % in the mesh group (p > 0.05). The subjective failure rate was also similar in the two groups, 9.1 % in native tissue repairs vs 3.8 % in the mesh group. One patient was transfused and 2 inadvertent cystotomies occurred in the mesh group with no perioperative complications reported in the native tissue group. No differences were seen between the groups utilising a wide variety of validated outcome tools. Unfortunately, owing to the ethics committee imposing a stopping criterion of 15.6 % mesh exposure rate, the study did not recruit the appropriate sample size and is underpowered to detect a significant difference between the groups if it exists.
Maher et al. recently reported results from a randomised trial comparing laparoscopic sacral colpopexy (LSC; n = 53) with a total vaginal mesh kit (Prolift; Ethicon, Somerville, NJ, USA) (n = 55) [64]. LSC was associated with longer operating times (mean difference +52 min [95 % CI 41.5–62.6]), decreased hospital stay (mean difference −0.5 days [95 % CI −0.93 to −0.10]) and quicker return to normal activities (mean difference −5.3 days [95 % CI −8.4 to −2.3]). Two years after surgery, objective success (overall POPQ Stage 0 or 1) was seen in 77 % of the LSC group compared with only 43 % of the TVM group, p < 0.001) [64]. Also, reoperations were significantly higher in the TVM group (22 %) than in the group that received LSC (5 %, p = 0.006).
As seen in Table 4 the success rate of transvaginal meshes for apical prolapse in level 3 evidence is significantly higher and ranges from 87 to 100 % for monofilament polypropylene meshes with mesh erosion rates varying from 0 to 15 %.
Sacral colpopexy
Since its introduction by Lane in 1962 [73], sacral colpopexy has been proven to be an effective and durable technique for correcting apical prolapse. In 2010, approximately 34,000 sacral colpopexies were performed in the USA, representing 11 % of all prolapse surgeries performed during that time period [11]. Traditionally, sacral colpopexy has been performed via a laparotomy (i.e. abdominal sacral colpopexy), but the use of laparoscopic and robotic approaches is increasing.
Abdominal sacral colpopexy
Observational studies and clinical trials suggest that abdominal sacral colpopexy (ASC) is a highly effective procedure for apical prolapse. The success rate of ASC, when defined as lack of apical prolapse, ranges from 78 to 100 % (Table 5). When success is defined as no recurrent prolapse in any segment the published success rates are 56–100 %. A systematic review of ASC performed by Nygaard et al. reported a median reoperation rate for recurrent prolapse of 4.4 % (range 0–18.2 %) and for postoperative stress incontinence of 4.9 % (range 1.2 to 30.9 %) [110]. Clinical trials demonstrate significant improvements in prolapse symptoms, urinary function and quality of life after ASC [27, 106]. There is level 1 evidence that ASC has superior anatomical outcomes compared with SSLS, but this is balanced by longer operating time, longer recovery and higher cost [111]. There are no randomised trials comparing ASC with ULS or with transvaginal mesh procedures. Given the prolonged recovery and unique complications associated with laparotomy, many surgeons reserve sacral colpopexy for patients with apical prolapse thought to be at high risk of failure from a vaginal approach, often considering such factors as age, comorbidities, history of previous prolapse surgery and vaginal length [3, 4, 10, 32, 112]. Unfortunately, there are too few published data to allow an evidence-based decision about which patient with POP will be best served by an ASC relative to other techniques.
Some surgeons have attempted to decrease mesh complications of ASC by using biological materials instead of synthetic mesh. However, the current evidence suggests that biological materials, whether allograft or xenograft, produce inferior anatomical outcomes compared with synthetic mesh, particularly polypropylene, without decreased graft-related complications. Level 1 evidence supports the superiority of polypropylene mesh to fascia lata for objective anatomical support following ASC [109, 113]. A randomised trial of 106 women undergoing ASC compared polypropylene mesh with cadaveric fascia lata and found superior anatomical outcomes in those who received polypropylene at 1 year (success 91 vs 68 %, p = 0.007) and 5 years after surgery (93 vs 62 %, p = 0.02) [109, 113]. There were no differences in graft-related complications overall between the two groups. Several retrospective case series support these data [114, 115, 116). Similarly, level 3 evidence suggests that use of xenografts such as porcine dermis and small intestinal submucosa might also have inferior anatomical success rates compared with polypropylene mesh, with similar rates of graft-related complications [117, 118].
Beyond mesh erosion, reported complications of ASC are generally consistent with those of other major open pelvic surgeries. The systematic review by Nygaard et al. reported that wound complications occurred in 4.6 % (range 0.4 to 19.8 %), haemorrhage or transfusion in 4.4 % (0.2 to 16.9 %), cystotomy in 3.1 % (0.4 to 15.8 %), ureteral injury in 1.0 % (0.8 to 1.9 %) and bowel injury in 1.6 % (0.4 to 2.5 %) [110]. One in 20 women in the CARE trial experienced significant gastrointestinal morbidity after sacral colpopexy. Of 322 women in the study, 19 had symptoms of possible ileus or small bowel obstruction; of these, 4 underwent reoperation for small bowel obstruction, 11 were readmitted for medical management, and 4 had a prolonged initial hospitalisation for gastrointestinal symptoms [119].
Abdominal sacral colpopexy vs sacrospinous ligament suspension
To date, there have been three RCTs that directly compare ASC with SSLS [17, 27, 96]. The Cochrane review on the surgical management of POP by Maher et al. summarises these studies and concludes that these trials provide level 1 evidence that there were no statistically significant differences in objective failure at any site (any pelvic organ prolapse RR 0.77, 95 % CI 0.39 to 1.53), subjective failure (RR 0.53, 95%CI 0.25 to 1.09), reoperation for POP (RR 1.46, 95 % CI 0.19 to 1.11) or patient satisfaction (RR 0.82, 95 % CI 0.32 to 2.06) [111]. However, ASC was superior to SSLS for the following outcomes: prolapse ≤ stage 2 (RR 0.29, 95 % CI 0.09 to 0.97), recurrent vault prolapse (RR 0.23, 95 % CI 0.07 to 0.77), postoperative stress urinary incontinence (RR 0.55, 95 % CI 0.32 to 0.95) and less postoperative dyspareunia (RR 0.39, 95 % CI 0.18 to 0.86). In contrast, ASC was associated with a longer operating time (weighted mean difference [WMD] 21 min, 95 % CI 12 to 30), longer time to recover (WMD 8.3 days, 95 % CI 3.9 to 12.7) and was more expensive (WMD US $1,334, 95 % CI 1,027 to 1,641) than SSLS [111].
Laparoscopic sacral colpopexy
The laparoscopic approach to sacral colpopexy has been adopted by many surgeons over the last decade as an alternative to ASC with the hopes of reproducing the high success rate of the ASC while decreasing the morbidity and delayed recovery associated with laparotomy. Multiple prospective and retrospective case series demonstrate good short to mid-term success rates, with a mean objective success rate of 90.5 % (range 60–100 %, subjective success rates of 79–98 % [120–122] and a mean reoperation rate of 5.9 % (Table 6). To date, no randomised trials have compared LSC with ASC; however, three retrospective comparisons have been published [134, 140, 141]. While results vary somewhat among studies, in general, LSC is associated with a shorter hospital stay, less blood loss, with conflicting data on operating times. Objective outcomes among the groups appear to be similar. Well-designed high-quality clinical trials are necessary to establish independently the effectiveness and safety of the LSC relative to ASC. There is level 1 evidence that LSC provides superior outcomes to total vaginal mesh procedure for women with symptomatic stage 2–4 vaginal vault prolapse, as described above. There are currently no comparative studies, randomised or not, evaluating the relative safety and efficacy of LSC and native tissue (non-mesh) vaginal POP repair.
A recent retrospective study assessed the complication rates in 402 LSC cases [132]. This study compared patients who received concurrent laparoscopically assisted vaginal hysterectomy with those who had had previous hysterectomy. They showed no differences in intra- or perioperative complications and similar rates of mesh erosion between the two groups [132]. Overall, the complication rates for this cohort were 0.75 % for haematoma, 2.2 % for ileus or small bowel obstruction, 1.5 % for bladder injury, 0.75 % for bowel injury and 0.25 % for ureteric injury. At 1 year, the overall mesh erosion rate was 1.2 %. In contrast, Tan-Kim et al. reported on a retrospective series of 188 minimally invasive sacrocolpopexies and found a significantly higher mesh exposure rate in those who received concurrent total vaginal hysterectomy (TVH; 23 %) compared with those who were post-hysterectomy (5 %) or who received a supracervical hysterectomy (5 %) [142]. TVH was found to be an independent risk factor for mesh erosion on multivariate regression analysis in this study (OR 5.67; 95 % CI 2.88–17.10).
Despite the clinical advantages of a laparoscopic approach, the adoption of LSC has been relatively limited, probably because of the steep learning curve associated with attaining laparoscopic suturing and knot tying skills that are required to attach the mesh to the vagina and sacrum. Claerhout et al. evaluated their learning curve in the first 206 cases performed by a single surgeon [130]. Operating times declined rapidly during the first 30 procedures in this series and reached a steady state (175 min) after 90 cases. Using a cumulative sum (CUSUM) approach to evaluate operative time and failures (laparotomy, complications or anatomical failures) they found that adequate learning occurred after 60 cases [130]. Complication rates remained unchanged throughout this series. Akladios et al. found that there was a steady decrease in LSC operative time in a series of the first 48 cases performed, but that a turning point was observed after 18–24 cases [127]. Complication rates were also low throughout this series.
Robotic sacral colpopexy
Because of the relatively long learning curve required for LSC, many surgeons have turned to robotic-assisted surgery in order to offer patients a minimally invasive approach to sacrocolpopexy. Robotic surgical systems have been developed with the goal of facilitating technically difficult procedures by improving the surgeon’s vision, dexterity and ergonomics. No data have been published on the learning curve for robotic sacral colpopexy (RSC); however, expert opinion suggests that the learning curve might be shorter for RSC than with the laparoscopic approach.
The currently available data for RSC are relatively limited and consist primarily of uncontrolled case series, but meta-analysis suggests anatomical outcomes similar to those of ASC and LSC, with objective success rates reported at 60–100 % (mean 93 %), subjective success of 91–94 % and a mean mesh erosion rate of 5 % (Table 7). To date, there have been only two published studies that provide comparative data for the RSC. Geller et al. retrospectively compared 73 patients who received RSC with 105 who received ASC [125]. RSC was associated with less blood loss, longer operative time, shorter length of stay and a higher incidence of fever (4.1 vs 0 %) Anatomical outcomes of the groups 6 weeks after surgery were similar [125].
In the only randomised comparison of RSC to date, Paraiso et al. recently published a clinical trial that provides level 1 evidence that RSC results in longer operating time and increased pain and costs compared with LSC [143]. This single-centre, blinded, randomised trial compared RSC (n = 40) with LSC (n = 38) in women with stage 2–4 post-hysterectomy vaginal prolapse. Total operative time was chosen as the primary outcome for this study serving as a proxy measure for surgical efficiency. Total operative time was significantly longer in the robotic group compared with the laparoscopic group (+67-min difference; 95 % confidence interval [CI] 43–89; P < 0.001) [143]. Anaesthesia time, total time in the operating room, total sacral colpopexy time and total suturing time were all significantly longer in the robotic group. Participants in the robotic group also had significantly higher pain at rest and with activity during weeks 3 through 5 after surgery and required longer use of non-steroidal anti-inflammatory drugs (median, 20 compared with 11 days, P < 0.005). The robotic group incurred greater costs than the laparoscopic group (@mean difference + $1,936; 95 % CI $417–$3,454; P = 0.008) [143]. Both groups demonstrated significant improvement in vaginal support and functional outcomes 1 year after surgery, with no differences between groups. It is worth noting that the surgeons in this study had considerable experience of LSC.
A meta-analysis of observational studies on robotic gynaecological surgery, found that the currently available evidence shows that for most gynaecological procedures studied robotic surgery achieved a shorter hospital stay and less blood loss than open surgery [150]. However, no clinically significant improvements were noted when robotic surgery was compared with conventional laparoscopic surgery in benign gynaecological procedures [150]. The current evidence, while limited, suggests that these conclusions are also applicable for RSC (Table 8). RSC probably has a shorter learning curve than LSC and thus may be more generalisable; however, published evidence for this is currently lacking. In surgeons with advanced laparoscopic skills, RSC offers no clinical benefit compared with LSC and results in longer operating times, greater cost and greater postoperative pain.
Obliterative procedures
Obliterative surgery, such as total colpocleisis (also called colpectomy/colpocleisis) or the LeFort partial colpocleisis, corrects POP by reducing the pelvic viscera back into the pelvis and closing off the vaginal canal either in part or entirely [151]. Obliterative procedures are less commonly performed in Europe, Asia and Australia compared with the USA, and are usually reserved for women who are elderly, medically compromised and no longer sexually active [152]. The purported advantages of obliterative surgery in this population are decreased operative time, decreased perioperative morbidity, and an extremely low prolapse recurrence risk. The obvious disadvantage is the elimination of the potential for vaginal intercourse. Preoperative counselling is essential when choosing between the obliterative and reconstructive options. A systematic review of colpocleisis published in 2006 noted that colpocleisis appears to be nearly 100 % effective in correcting pelvic organ prolapse; however, until recently little was known about postoperative functional or quality of life outcomes [151]. In the last few years a number of reports evaluating symptom improvement and changes in quality of life after colpocleisis have been reported [153–156]. Overall, these series have found high rates of patient satisfaction and significant functional improvement, with low rates of regret for loss of sexual function [153–155]. Barber et al. reported results from a multicentre study of obliterative surgery using a prospective cohort design with a concurrent control group of age-matched women undergoing vaginal reconstructive surgery [153]. Despite permanent alterations in sexual function, significant improvements in bladder, bowel and prolapse symptoms as well as body image were noted after surgery with no differences between those who received colpocleisis and those who underwent reconstructive surgery. Additionally, significant and clinically important improvements were noted in bodily pain, vitality, social functioning, role-emotional, and mental health summary scales of the SF-36 [153]. Similarly, in a study on a retrospective cohort of women over the age of 65 comparing women who underwent colpocleisis (n = 45) and a similar group of women who underwent reconstructive surgery with transvaginal mesh (Prolift, Ethicon Women’s Health and Urology) Murphy et al. found that improvements in condition-specific quality of life and postoperative patient satisfaction were comparable in the two treatment groups [157].
The Pelvic Floor Disorders Network has reported on a large series of women undergoing colpocleisis (n = 153) with 1-year follow-up [154]. All pelvic symptom scores and related bother significantly improved at 3 and 12 months, and 125 patients (95 %) said they were either “very satisfied” or “satisfied” with the outcome of their surgery [154]. Bothersome stress and urge incontinence were present before surgery in 54 % and 41 % of subjects respectively. Forty percent of subjects received a concurrent mid-urethral sling at the time of their colpocleisis and the rates of bothersome stress and urge incontinence 1 year after surgery were 14 % and 15 % respectively. Similarly, bothersome bowel symptoms were present in 77 % of subjects at baseline. One year after surgery, the majority of bothersome bowel symptoms resolved, particularly obstructive and incontinence symptoms, and development of new bowel symptoms was uncommon (0–14 %) [156].
While obliterative procedures are predominantly performed in elderly, frail women who often have multiple co-morbidities, the rate of serious adverse events after this procedure appears to be low. In general, major complications due to performance of surgery on the elderly (e.g. cardiac, pulmonary and cerebrovascular complications) occur at a rate of approximately 2 % [151]. Major complications due to the surgery itself (e.g. pyelonephritis, blood transfusion) occur at a rate of approximately 4 % [151]. A systematic review of published series of colpocleisis from 1966 to 2004 reported a surgical mortality rate of approximately 1 in 400 cases [151]. One complication that appears to be uniquely associated with obliterative surgery is the development of de novo rectal prolapse after surgery [158, 159]. Collins et al. in a retrospective cohort of 916 women undergoing vaginal POP surgery at one institution found that the incidence of postoperative full-thickness rectal prolapse in women who were 65 years old or more who underwent obliterative surgery was 3 out of 74 (4.1 %; 95 % CI, 1.4–11), with an estimated odds ratio of 22 (95 % CI, 2.3–196; P < 0.002) compared with women who were 65 years old or more who underwent reconstructive surgery [158].
Conclusions
-
Level 1 evidence suggests that ASC has a higher success rate than sacrospinous colpopexy, with less SUI and postoperative dyspareunia. ASC had greater morbidity including operating time, inpatient stay, slower return to activities of daily living and higher cost (grade A).
-
ASC has the lowest inpatient cost compared with LSC and RSC. LSC has lower inpatient costs than RSC (grade B).
-
In single RC's the RSC had a longer operating time than both ASC and LSC (grade B). In small trials objective outcomes appear similar although postoperative pain was greater in RSC.
-
LSC is as effective as ASC, with reduced blood loss and admission time (grade C). The data relating to operating times are conflicting
-
ASC performed with polypropylene mesh has superior outcomes to fascia lata (level I) and porcine dermis and small intestine submucosa (level 3; grade B)
-
In a single RCT, LSC had a superior objective and subjective success rate and lower reoperation rate than polypropylene transvaginal mesh for vault prolapse (grade B).
-
Level 3 evidence suggests that vaginal uterosacral ligament suspension, McCall culdoplasty, iliococcygeus fixation and colpocleisis might be relatively safe and effective interventions (grade C)
References
Swift SE (2000) The distribution of pelvic organ support in a population of female subjects seen for routine gynecologic health care. Am J Obstet Gynecol 183(2):277–285
Delancey JO (2002) Fascial and muscular abnormalities in women with urethral hypermobility and anterior vaginal wall prolapse. Am J Obstet Gynecol 187(1):93–98
Shull BL (1999) Pelvic organ prolapse: anterior, superior, and posterior vaginal segment defects. Am J Obstet Gynecol 181(1):6–11
Toozs-Hobson P, Boos K, Cardozo L (1998) Management of vaginal vault prolapse. Br J Obstet Gynaecol 105(1):13–17
Rooney K, Kenton K, Mueller ER, FitzGerald MP, Brubaker L (2006) Advanced anterior vaginal wall prolapse is highly correlated with apical prolapse. Am J Obstet Gynecol 195(6):1837–1840
Hsu Y, Chen L, Summers A, Ashton-Miller JA, DeLancey JO (2008) Anterior vaginal wall length and degree of anterior compartment prolapse seen on dynamic MRI. International urogynecology journal and pelvic floor dysfunction 19(1):137–142
Brown JS, Waetjen LE, Subak LL, Thom DH, Van den Eeden S, Vittinghoff E (2002) Pelvic organ prolapse surgery in the United States, 1997. Am J Obstet Gynecol 186(4):712–716
Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL (1997) Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol 89(4):501–506
Boyles SH, Weber AM, Meyn L (2003) Procedures for pelvic organ prolapse in the United States, 1979–1997. Am J Obstet Gynecol 188(1):108–115
Brubaker L (2005) Burch Colposuspension at the time of sacrocolpopexy in stress continent women reduces bothersome stress urinary symptoms: The CARE randomized trial. J Pelvic Surg 11(1):S5
http://www.fda.gov/downloads/MedicalDevices/Safety/AlertsandNotices/UCM262760.pdf.
Sederl J (1958) Zur Operation des Prolapses der blind endigenden Sheiden. Geburtshilfe Frauenheilkd 18:824–828
Morley GW, DeLancey JO (1988) Sacrospinous ligament fixation for eversion of the vagina. Am J Obstet Gynecol 158(4):872–881
Imparato E, Aspesi G, Rovetta E, Presti M (1992) Surgical management and prevention of vaginal vault prolapse. Surg Gynecol Obstet 175(3):233–237
Shull BL, Capen CV, Riggs MW, Kuehl TJ (1992) Preoperative and postoperative analysis of site-specific pelvic support defects in 81 women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol 166(6 Pt 1):1764–1768, discussion 1768–1771
Pasley WW (1995) Sacrospinous suspension: a local practitioner’s experience. Am J Obstet Gynecol 173(2):440–445, discussion 445–448
Benson JT, Lucente V, McClellan E (1996) Vaginal versus abdominal reconstructive surgery for the treatment of pelvic support defects: a prospective randomized study with long-term outcome evaluation. Am J Obstet Gynecol 175(6):1418–1421
Paraiso MF, Ballard LA, Walters MD, Lee JC, Mitchinson AR (1996) Pelvic support defects and visceral and sexual function in women treated with sacrospinous ligament suspension and pelvic reconstruction. Am J Obstet Gynecol 175(6):1423–1430
Penalver M, Mekki Y, Lafferty H, Escobar M, Angioli R (1998) Should sacrospinous ligament fixation for the management of pelvic support defects be part of a residency program procedure? The University of Miami experience. Am J Obstet Gynecol 178(2):326–329
Colombo M, Milani R (1998) Sacrospinous ligament fixation and modified McCall culdoplasty during vaginal hysterectomy for advanced uterovaginal prolapse. Am J Obstet Gynecol 179(1):13–20
Meschia M, Bruschi F, Amicarelli F, Pifarotti P, Marchini M, Crosignani PG (1999) The sacrospinous vaginal vault suspension: critical analysis of outcomes. Int Urogynecol J Pelvic Floor Dysfunct 10(3):155–159
Sze EH, Karram MM (1997) Transvaginal repair of vault prolapse: a review. Obstet Gynecol 89(3):466–475
Lantzsch T, Goepel C, Wolters M, Koelbl H, Methfessel HD (2001) Sacrospinous ligament fixation for vaginal vault prolapse. Arch Gynecol Obstet 265(1):21–25
Lovatsis D, Drutz HP (2002) Safety and efficacy of sacrospinous vault suspension. Int Urogynecol J Pelvic Floor Dysfunct 13(5):308–313
Cruikshank SH, Muniz M (2003) Outcomes study: a comparison of cure rates in 695 patients undergoing sacrospinous ligament fixation alone and with other site-specific procedures—a 16-year study. Am J Obstet Gynecol 188(6):1509–1512, discussion 1512–1515
Nieminen K, Huhtala H, Heinonen PK (2003) Anatomic andfunctional assessment and risk factors of recurrent prolapse after vaginal sacrospinous fixation. Acta Obstet Gynecol Scand 82(5):471–478
Maher CF, Qatawneh AM, Dwyer PL, Carey MP, Cornish A, Schluter PJ (2004) Abdominal sacral colpopexy or vaginal sacrospinous colpopexy for vaginal vault prolapse: a prospective randomized study. Am J Obstet Gynecol 190(1):20–26
Hefni MA, El-Toukhy TA (2006) Long-term outcome of vaginal sacrospinous colpopexy for marked uterovaginal and vault prolapse. Eur J Obstet Gynecol Reprod Biol 127(2):257–263
Toglia MR, Fagan MJ (2008) Suture erosion rates and long-term surgical outcomes in patients undergoing sacrospinous ligament suspension with braided polyester suture. Am J Obstet Gynecol 198(5):600e1–600e4
Aigmueller T, Riss P, Dungl A, Bauer H (2008) Long-term follow-up after vaginal sacrospinous fixation: patient satisfaction, anatomical results and quality of life. Int Urogynecol J Pelvic Floor Dysfunct 19(7):965–969
Chou LY, Chang DY, Sheu BC, Huang SC, Chen SY, Chang WC (2010) Clinical outcome of transvaginal sacrospinous fixation with the Veronikis ligature carrier in genital prolapse. Eur J Obstet Gynecol Reprod Biol 152(1):108–110
Weber AM, Richter HE (2005) Pelvic organ prolapse. Obstet Gynecol 106(3):615–634
Morgan DM, Rogers MA, Huebner M, Wei JT, Delancey JO (2007) Heterogeneity in anatomic outcome of sacrospinous ligament fixation for prolapse: a systematic review. Obstet Gynecol 109(6):1424–1433
Miller N (1927) A new method of correcting complete inversion of the vagina. Surg Gynecol Obstet 44:550–554
Jenkins VR 2nd (1997) Uterosacral ligament fixation for vaginal vault suspension in uterine and vaginal vault prolapse. Am J Obstet Gynecol 177(6):1337–1343, discussion 1343–1344
Comiter CV, Vasavada SP, Raz S (1999) Transvaginal culdosuspension: technique and results. Urology 54(5):819–822
Barber MD, Visco AG, Weidner AC, Amundsen CL, Bump RC (2000) Bilateral uterosacral ligament vaginal vault suspension with site-specific endopelvic fascia defect repair for treatment of pelvic organ prolapse. Am J Obstet Gynecol 183(6):1402–1410, discussion 1410–1411
Shull BL, Bachofen C, Coates KW, Kuehl TJ (2000) A transvaginal approach to repair of apical and other associated sites of pelvic organ prolapse with uterosacral ligaments. Am J Obstet Gynecol 183(6):1365–1373
Karram M, Goldwasser S, Kleeman S, Steele A, Vassallo B, Walsh P (2001) High uterosacral vaginal vault suspension with fascial reconstruction for vaginal repair of enterocele and vaginal vault prolapse. Am J Obstet Gynecol 185(6):1339–1342, discussion 42–43
Amundsen CL, Flynn BJ, Webster GD (2003) Anatomical correction of vaginal vault prolapse by uterosacral ligament fixation in women who also require a pubovaginal sling. J Urol 169(5):1770–1774
Silva WA, Pauls RN, Segal JL, Rooney CM, Kleeman SD, Karram MM (2006) Uterosacral ligament vault suspension: five-year outcomes. Obstet Gynecol 108(2):255–263
Antovska SV, Dimitrov DG (2006) Vaginosacral colpopexy (VSC)—a new modification of the Mc Call operation using vaginosacral ligaments as autologous sliding grafts in posthysterectomy vault prolapse. Bratisl Lek Listy 107(3):62–72
Wheeler TL 2nd, Richter HE, Duke AG, Burgio KL, Redden DT, Varner RE (2006) Outcomes with porcine graft placement in the anterior vaginal compartment in patients who undergo high vaginal uterosacral suspension and cystocele repair. Am J Obstet Gynecol 194(5):1486–1491
de Boer TA, Milani AL, Kluivers KB, Withagen MI, Vierhout ME (2009) The effectiveness of surgical correction of uterine prolapse: cervical amputation with uterosacral ligament plication (modified Manchester) versus vaginal hysterectomy with high uterosacral ligament plication. Int Urogynecol J Pelvic Floor Dysfunct 20(11):1313–1319
Doumouchtsis SK, Khunda A, Jeffery ST, Franco AV, Fynes MM (2011) Long-term outcomes of modified high uterosacral ligament vault suspension (HUSLS) at vaginal hysterectomy. Int Urogynecol J 22(5):577–584
Baden WF, Walker TA (1972) Genesis of the vaginal profile: a correlated classification of vaginal relaxation. Clin Obstet Gynecol 15(4):1048–1054
Margulies RU, Rogers MA, Morgan DM (2010) Outcomes of transvaginal uterosacral ligament suspension: systematic review and metaanalysis. Am J Obstet Gynecol 202(2):124–134
Gustilo-Ashby AM, Jelovsek JE, Barber MD, Yoo EH, Paraiso MF, Walters MD (2006) The incidence of ureteral obstruction and the value of intraoperative cystoscopy during vaginal surgery for pelvic organ prolapse. Am J Obstet Gynecol 194(5):1478–1485
Barber MD, Brubaker L, Menefee SA et al (2009) Operations and pelvic muscle training in the management of apical support loss trial: design and methods. Contemp Clin Trials 30(2):178–189
Dwyer PL, Fatton B (2008) Bilateral extraperitoneal uterosacral suspension: a new approach to correct posthysterectomy vaginal vault prolapse. Int Urogynecol J Pelvic Floor Dysfunct 19(2):283–292
Fatton B, Dwyer PL, Achtari C, Tan PK (2009) Bilateral extraperitoneal uterosacral vaginal vault suspension: a 2-year follow-up longitudinal case series of 123 patients. Int Urogynecol J Pelvic Floor Dysfunct 20(4):427–434
Lowenstein L, Fitz A, Kenton K, FitzGerald MP, Mueller ER, Brubaker L (2009) Transabdominal uterosacral suspension: outcomes and complications. Am J Obstet Gynecol 200(6):656e1–656e5
Rardin CR, Erekson EA, Sung VW, Ward RM, Myers DL (2009) Uterosacral colpopexy at the time of vaginal hysterectomy: comparison of laparoscopic and vaginal approaches. J Reprod Med 54(5):273–280
Webb MJ, Aronson MP, Ferguson LK, Lee RA (1998) Posthysterectomy vaginal vault prolapse: primary repair in 693 patients. Obstet Gynecol 92(2):281–285
Montella JM, Morrill MY (2005) Effectiveness of the McCall culdeplasty in maintaining support after vaginal hysterectomy. Int Urogynecol J Pelvic Floor Dysfunct 16(3):226–229
Koyama M, Yoshida S, Koyama S et al (2005) Surgical reinforcement of support for the vagina in pelvic organ prolapse: concurrent iliococcygeus fascia colpopexy (Inmon technique). Int Urogynecol J 16(3):197–202
Aronson MP, Aronson PK, Howard AE, Morse AN, Baker SP, Young SB (2005) Low risk of ureteral obstruction with “deep” (dorsal/posterior) uterosacral ligament suture placement for transvaginal apical suspension. Am J Obstet Gynecol 192(5):1530–1536
Francis W, Jeffcoate T (1961) Dyspareunia following vaginal operations. J Obstet Gynaecol Br Commonw 68:1–10
Natale F, La Penna C, Padoa A, Agostini M, Panei M, Cervigni M (2010) High levator myorrhaphy versus uterosacral ligament suspension for vaginal vault fixation: a prospective, randomized study. Int Urogynecol J Pelvic Floor Dysfunct 21(5):515–522
Shull BL, Capen CV, Riggs MW, Kuehl TJ (1993) Bilateral attachment of the vaginal cuff to iliococcygeus fascia: an effective method of cuff suspension. Am J Obstet Gynecol 168(6 Pt 1):1669–174, discussion 1674–1677
Meeks GR, Washburne JF, McGehee RP, Wiser WL (1994) Repair of vaginal vault prolapse by suspension of the vagina to iliococcygeus (prespinous) fascia. Am J Obstet Gynecol 171(6):1444–1452, discussion 1452–1454
Maher CF, Murray CJ, Carey MP, Dwyer PL, Ugoni AM (2001) Iliococcygeus or sacrospinous fixation for vaginal vault prolapse. Obstet Gynecol 98(1):40–44
Sokol AI, Iglesia CB, Kudish BI, Gutman RE, Shveiky DBR, Sokol ER (2012) One-year objective and functional outcomes of a randomized clinical trial of vaginal mesh for prolapse. Am J Obstet Gynecol 206(1):86e1–86e9
Maher CF, Feiner B, DeCuyper EM, Nichlos CJ, Hickey KV, O’Rourke P (2011) Laparoscopic sacral colpopexy versus total vaginal mesh for vaginal vault prolapse: a randomized trial. Am J Obstet Gynecol 204(4):360e1–360e7
Abdel-Fattah M, Ramsay I, West of Scotland Study Group (2008) Retrospective multicentre study of the new minimally invasive mesh repair devices for pelvic organ prolapse. BJOG 115(1):22–30
Gauruder-Burmester A, Koutouzidou P, Rohne J, Gronewold M, Tunn R (2007) Follow-up after polypropylene mesh repair of anterior and posterior compartments in patients with recurrent prolapse. Int Urogynecol J Pelvic Floor Dysfunct 18(9):1059–1064
Moore RD, Mitchell GK, Miklos JR (2012) Single-incision vaginal approach to treat cystocele and vault prolapse with an anterior wall mesh anchored apically to the sacrospinous ligaments. Int Urogynecol J 23(1):85–91
Fatton B, Amblard J, Debodinance P, Cosson M, Jacquetin B (2007) Transvaginal repair of genital prolapse: preliminary results of a new tension-free vaginal mesh (Prolift technique)—a case series multicentric study. Int Urogynecol J Pelvic Floor Dysfunct 18(7):743–752
Belot F, Collinet P, Debodinance P, Ha Duc E, Lucot JP, Cosson M (2005) [Risk factors for prosthesis exposure in treatment of genital prolapse via the vaginal approach].Gynecol. Obstet Fertil 33(12):970–974
van Raalte HM, Lucente VR, Molden SM, Haff R, Murphy M (2009) One-year anatomic and quality-of-life outcomes after the Prolift procedure for treatment of posthysterectomy prolapse. Am J Obstet Gynecol 199(6):694e1–694e6
Milani AL, Withagen MI, Vierhout ME (2009) Trocar-guided total tension-free vaginal mesh repair of post-hysterectomy vaginal vault prolapse. Int Urogynecol J Pelvic Floor Dysfunct 20(10):1203–1211
McDermott CD, Terry CL, Woodman PJ, Hale DS (2011) Surgical outcomes following total Prolift: colpopexy versus hysteropexy. Aust N Z J Obstet Gynaecol 51(1):61–66
Lane F (1962) Repair of posthysterectomy vaginal-vault prolapse. Obstet Gynecol 20:72–77
Biertho I, Dallemagne B, Dewandre JM et al (2004) Intravaginal slingplasty: short term results. Acta Chir Belg 104(6):700–704
Foote AJ, Ralph J (2007) Infracoccygeal sacropexy. Aust N Z J Obstet Gynaecol 47(3):250–251
Mattox TF, Moore S, Stanford EJ, Mills BB (2006) Posterior vaginal sling experience in elderly patients yields poor results. Am J Obstet Gynecol 194(5):1462–1466
Vardy MD, Brodman M, Olivera CK, Zhou HS, Flisser AJ, Bercik RS (2007) Anterior intravaginal slingplasty tunneller device for stress incontinence and posterior intravaginal slingplasty for apical vault prolapse: a 2-year prospective multicenter study. Am J Obstet Gynecol 197(1):104 e1–8
Neuman M, Lavy Y (2008) Posterior intra-vaginal slingplasty for the treatment of vaginal apex prolapse: medium-term results of 140 operations with a novel procedure. Eur J Obstet Gynecol Reprod Biol 140(2):230–233
de Tayrac R, Mathe ML, Bader G, Deffieux X, Fazel A, Fernandez H (2008) Infracoccygeal sacropexy or sacrospinous suspension for uterine or vaginal vault prolapse. Int J Gynaecol Obstet 100(2):154–159
Lee YS, Han DH, Lee JY, Kim JC, Choo MS, Lee KS (2010) Anatomical and functional outcomes of posterior intravaginal slingplasty for the treatment of vaginal vault or uterine prolapse: a prospective, multicenter study. Korean J Urol 51(3):187–192
Amrute KV, Eisenberg ER, Rastinehad AR, Kushner L, Badlani GH (2007) Analysis of outcomes of single polypropylene mesh in total pelvic floor reconstruction. Neurourol Urodyn 26(1):53–58
Addison WA, Livengood CH 3rd, Sutton GP, Parker RT (1985) Abdominal sacral colpopexy with Mersilene mesh in the retroperitoneal position in the management of posthysterectomy vaginal vault prolapse and enterocele. Am J Obstet Gynecol 153(2):140–146
Baker KR, Beresford JM, Campbell C (1990) Colposacropexy with Prolene mesh. Surg Gynecol Obstet 171(1):51–54
Snyder TE, Krantz KE (1991) Abdominal-retroperitoneal sacral colpopexy for the correction of vaginal prolapse. Obstet Gynecol 77(6):944–949
Timmons MC, Addison WA, Addison SB, Cavenar MG (1992) Abdominal sacral colpopexy in 163 women with posthysterectomy vaginal vault prolapse and enterocele. Evolution of operative techniques. The J Reprod Med 37(4):323–327
van Lindert AC, Groenendijk AG, Scholten PC, Heintz AP (1993) Surgical support and suspension of genital prolapse, including preservation of the uterus, using the Gore-Tex soft tissue patch (a preliminary report). Eur J Obstet Gynecol Reprod Biol 50(2):133–139
Grunberger W, Grunberger V, Wierrani F (1994) Pelvic promontory fixation of the vaginal vault in sixty-two patients with prolapse after hysterectomy. J Am Coll Surg 178(1):69–72
Lecuru F, Taurelle R, Clouard C, Attal JP (1994) Surgical treatment of genito-urinary prolapses by abdominal approach. Results in a continuous series of 203 operations. Ann Chir 48(11):1013–1019
Brubaker L (1995) Sacrocolpopexy and the anterior compartment: support and function. Am J Obstet Gynecol 173(6):1690–1695, discussion 1695–1696
de Vries MJ, van Dessel TH, Drogedndijk AC, de Haas I, Huikenshoven FJ (1995) Short-term results and long-term patients’ appraisal of abdominal colposacropexy for treatment of genital and vaginal vault prolapse. Eur J Obstet Gynecol Reprod Biol 59:35–38
Hardiman PJ, Drutz HP (1996) Sacrospinous vault suspension and abdominal colposacropexy: success rates and complications. Am J Obstet Gynecol 175(3 Pt 1):612–616
Sullivan ES, Longaker CJ, Lee PY (2001) Total pelvic mesh repair: a ten-year experience. Dis Colon Rectum 44(6):857–863
Occelli B, Narducci F, Cosson M et al (1999) Abdominal colposacroplexy for the treatment of vaginal vault prolapse with or without urinary stress incontinence. Ann Chir 53(5):367–377
Patsner B (1999) Abdominal sacral colpopexy in patients with gynecologic cancer: report of 25 cases with long-term follow-up and literature review. Gynecol Oncol 75(3):504–508
Sze EH, Kohli N, Miklos JR, Roat T, Karram MM (1999) A retrospective comparison of abdominal sacrocolpopexy with Burch colposuspension versus sacrospinous fixation with transvaginal needle suspension for the management of vaginal vault prolapse and coexisting stress incontinence. Int Urogynecol J Pelvic Floor Dysfunct 10(6):390–393
Lo TS, Wang AC (1998) Abdominal colposacropexy and sacrospinous ligament suspension for severe uterovaginal prolapse: a comparison. J Gynecol Surg 14:59–64
Collopy BT, Barham KA (2002) Abdominal colporectopexy with pelvic cul-de-sac closure. Dis Colon Rectum 45(4):522–526, discussion 526–529
Culligan PJ, Murphy M, Blackwell L, Hammons G, Graham C, Heit MH (2002) Long-term success of abdominal sacral colpopexy using synthetic mesh. Am J Obstet Gynecol 187(6):1473–1480, discussion 1481–1482
Lefranc JP, Atallah D, Camatte S, Blondon J (2002) Long term follow up of posthysterectomy vaginal vault prolapse abdominal repair: a report of 85 cases. J Am Coll Surg 195:352–358
Lindeque BG, Nel WS (2002) Sacrocolpopexy-a report on 262 consecutive operations. S Afr Med J 92(12):982–985
Medina CA, Pietro PA, Whitted RW, Penalver M (2002) The use of dura mater allografts for abdominal sacral colpopexy. J Pelvic Surg 8:247–251
Brizzolara S, Pillai-Allen A (2003) Risk of mesh erosion with sacral colpopexy and concurrent hysterectomy. Obstet Gynecol 102(2):306–310
Podratz KC, Ferguson LK, Hoverman VR, Lee RA, Symmonds RE (1995) Abdominal sacral colpopexy for posthysterectomy vaginal vault descensus. J Pelvic Surg 1:18–23
Hilger WS, Poulson M, Norton PA (2003) Long-term results of abdominal sacrocolpopexy. Am J Obstet Gynecol 189(6):1606–1610, discussion 1610–1611
Higgs P, Goh J, Krause H, Sloane K, Carey M (2005) Abdominalsacral colpopexy: an independent prospective long-term follow-up study. Aust N Z J Obstet Gynaecol 45(5):430–434
Brubaker L, Nygaard I, Richter HE et al (2008) Two-year outcomes after sacrocolpopexy with and without burch to prevent stress urinary incontinence. Obstet Gynecol 112(1):49–55
Jeon MJ, Moon YJ, Jung HJ et al (2009) A long-term treatment outcome of abdominal sacrocolpopexy. Yonsei Med J 50(6):807–813
Huebner M, Krzonkalla M, Tunn R (2009) Abdominal sacrocolpopexy—standardized surgical technique, perioperative management and outcome in women with posthysterectomy vaginal vault prolapse. Gynakol Geburtshilfliche Rundsch 49(4):308–314
Tate SB, Blackwell L, Lorenz DJ, Steptoe MM, Culligan PJ (2010) Randomized trial of fascia lata and polypropylene mesh for abdominal sacrocolpopexy: 5-year follow-up. Int Urogynecol J 22(2):137–143
Nygaard IE, McCreery R, Brubaker L et al (2004) Abdominal sacrocolpopexy: a comprehensive review. Obstet Gynecol 104(4):805–823
Maher C, Feiner B, Baessler K, Adams EJ, Hagen S, Glazener CM (2010) Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 4, CD004014
Maher C, Baessler K, Glazener CM, Adams EJ, Hagen S (2004) Surgical management of pelvic organ prolapse in women. Cochrane Database Syst Rev 4, CD004014
Culligan PJ, Blackwell L, Goldsmith LJ, Graham CA, Rogers A, Heit MH (2005) A randomized controlled trial comparing fascia lata and synthetic mesh for sacral colpopexy. Obstet Gynecol 106(1):29–37
FitzGerald MP, Mollenhauer J, Bitterman P, Brubaker L (1999) Functional failure of fascia lata allografts. Am J Obstet Gynecol 181(6):1339–1344
Flynn MK, Webster GD, Amundsen CL (2005) Abdominal sacral colpopexy with allograft fascia lata: one-year outcomes. Am J Obstet Gynecol 192(5):1496–1500
Gregory WT, Otto LN, Bergstrom JO, Clark AL (2005) Surgical outcome of abdominal sacrocolpopexy with synthetic mesh versus abdominal sacrocolpopexy with cadaveric fascia lata. Int Urogynecol J Pelvic Floor Dysfunct 16(5):369–374
Claerhout F, De Ridder D, Van Beckevoort D et al (2010) Sacrocolpopexy using xenogenic acellular collagen in patients at increased risk for graft-related complications. Neurourol Urodyn 29(4):563–567
Quiroz LH, Gutman RE, Shippey S (2008) Abdominal sacrocolpopexy: Anatomic outcomes and complications with Pelvicol, autologous and synthetic graft materials. Am J Obstet Gynecol 198(5):557e1–557e5
Whitehead WE, Bradley CS, Brown MB (2007) Gastrointestinal complications following abdominal sacrocolpopexy for advanced pelvic organ prolapse. Am J Obstet Gynecol 197(1):78e1–78e7
Higgs PJ, Chua HL, Smith AR (2005) Long term review of laparoscopic sacrocolpopexy. BJOG 112(8):1134–1138
Rivoire C, Botchorishvili R, Canis M et al (2007) Complete laparoscopic treatment of genital prolapse with meshes including vaginal promontofixation and anterior repair: a series of 138 patients. J Minim Invasive Gynecol 14(6):712–718
Sarlos D, Brandner S, Kots L, Gygax N, Schaer G (2008) Laparoscopic sacrocolpopexy for uterine and post-hysterectomy prolapse: anatomical results, quality of life and perioperative outcome-a prospective study with 101 cases. Int Urogynecol J Pelvic Floor Dysfunct 19(10):1415–1422
Price N, Slack A, Jackson SR (2010) Laparoscopic hysteropexy: the initial results of a uterine suspension procedure for uterovaginal prolapse. BJOG 117(1):62–68
Sergent F, Resch B, Loisel C, Bisson V, Schaal JP, Marpeau L (2011) Mid-term outcome of laparoscopic sacrocolpopexy with anterior and posterior polyester mesh for treatment of genito-urinary prolapse. Eur J Obstet Gynecol Reprod Biol 156(2):217–222
Geller EJ, Siddiqui NY, Wu JM, Visco AG (2008) Short-term outcomes of robotic sacrocolpopexy compared with abdominal sacrocolpopexy. Obstet Gynecol 112(6):1201–1206
Sabbagh R, Mandron E, Piussan J, Brychaert PE, le Tu M (2010) Long-term anatomical and functional results of laparoscopic promontofixation for pelvic organ prolapse. BJU Int 106(6):861–866
Akladios CY, Dautun D, Saussine C, Baldauf JJ, Mathelin C, Wattiez A (2010) Laparoscopic sacrocolpopexy for female genital organ prolapse: establishment of a learning curve. Eur J Obstet Gynecol Reprod Biol 149(2):218–221
Granese R, Candiani M, Perino A, Romano F, Cucinella G (2009) Laparoscopic sacrocolpopexy in the treatment of vaginal vault prolapse: 8 years experience. Eur J Obstet Gynecol Reprod Biol 146:227–231
Deprest J, De Ridder D, Roovers JP, Werbrouck E, Coremans G, Claerhout F (2009) Medium term outcome of laparoscopic sacrocolpopexy with xenografts compared to synthetic grafts. J Urol 182(5):2362–2368
Claerhout F, Roovers JP, Lewi P, Verguts J, De Ridder D, Deprest J (2009) Implementation of laparoscopic sacrocolpopexy—a single centre’s experience. Int Urogynecol J Pelvic Floor Dysfunct 20(9):1119–1125
North CE, Ali-Ross NS, Smith AR, Reid FM (2009) A prospective study of laparoscopic sacrocolpopexy for the management of pelvic organ prolapse. BJOG 116(9):1251–1257
Stepanian AA, Miklos JR, Moore RD, Mattox TF (2008) Risk of mesh extrusion and other mesh-related complications after laparoscopic sacral colpopexy with or without concurrent laparoscopic-assisted vaginal hysterectomy: experience of 402 patients. J Minim Invasive Gynecol 15(2):188–196
Agarwala N, Hasiak N, Shade M (2007) Laparoscopic sacral colpopexy with Gynemesh as graft material—experience and results. J Minim Invasive Gynecol 14(5):577–583
Paraiso MF, Walters MD, Rackley RR, Melek S, Hugney C (2005) Laparoscopic and abdominal sacral colpopexies: a comparative cohort study. Am J Obstet Gynecol 192(5):1752–1758
Rozet F, Mandron E, Arroyo C, Andrews H, Cathelineau X, Mombet A, Cathala N, Vallancien G et al (2005) Laparoscopic sacral colpopexy approach for genito-urinary prolapse: experience with 363 cases. Eur Urol 47(2):230–236
Ross JW, Preston M (2005) Laparoscopic sacrocolpopexy for severe vaginal vault prolapse: five-year outcome. J Minim Invasive Gynecol 12(3):221–226
Gadonneix P, Ercoli A, Salet-Lizée D et al (2004) Laparoscopic sacrocolpopexy with two separate meshes along the anterior and posterior vaginal walls for multicompartment pelvic organ prolapse. J Am Assoc Gynecol Laparosc 11(1):29–35
Antiphon P, Elard S, Benyoussef A et al (2004) Laparoscopic promontory sacral colpopexy: is the posterior, recto-vaginal, mesh mandatory? Eur Urol 45(5):655–661
Cosson M, Rajabally R, Bogaert E, Querleu D, Crépin G (2002) Laparoscopic sacrocolpopexy, hysterectomy, and burch colposuspension: feasibility and short-term complications of 77 procedures. JSLS 6(2):115–119
Hsiao KC, Latchamsetty K, Govier FE, Kozlowski P, Kobashi KC (2007) Comparison of laparoscopic and abdominal sacrocolpopexy for the treatment of vaginal vault prolapse. J Endourol 21(8):926–930
Klauschie JL, Suozzi BA, O’Brien MM, McBride AW (2009) A comparison of laparoscopic and abdominal sacral colpopexy: Objective outcome and perioperative differences. Int Urogynecol J Pelvic Floor Dysfunct 20(3):273–279
Tan-Kim J, Menefee SA, Luber KM, Nager CW, Lukacz ES (2011) Prevalence and risk factors for mesh erosion after laparoscopic-assisted sacrocolpopexy. Int Urogynecol J 22(2):205–212
Paraiso MF, Jelovsek JE, Frick A, Chen CC, Barber MD (2011) Laparoscopic compared with robotic sacrocolpopexy for vaginal prolapse: a randomized controlled trial. Obstet Gynecol 118(5):1005–1013
Shariati A, Maceda JS, Hale DS (2008) Da Vinci assisted laparo scopic sacral colpopexy: surgical technique on a cohort of 77 patients. J Pelvic Surg 14:163–171
Kramer BA, Whelan CM, Powell TM, Schwartz BF (2009) Robot-assisted laparoscopic sacrocolpopexy as management for pelvic organ prolapse. J Endourol 23(4):655–658
Akl MN, Long JB, Giles DL et al (2009) Robotic-assisted sacrocolpopexy: technique and learning curve. Surg Endosc 23(10):2390–2394
Chan SS, Pang SM, Cheung TH, Cheung RY, Chung TK (2011) Laparoscopic sacrocolpopexy for the treatment of vaginal vault prolapse: with or without robotic assistance. Hong Kong Med J 17(1):54–60
Geller EJ, Parnell BA, Dunivan GC (2011) Pelvic floor function before and after robotic sacrocolpopexy: one-year outcomes. J Minim Invasive Gynecol 18(3):322–327
Moreno SJ, Ortiz OE, Fernandez PC, Galante RI, Corral RJ, Preito NS (2011) Long-term outcomes after robotic sacrocolpopexy in pelvic organ prolapse: prospective analysis. Urol Int 86:414–418
Reza M, Maeso S, Blasco JA, Andradas E (2010) Meta-analysis of observational studies on the safety and effectiveness of robotic gynaecological surgery. Br J Surg 97(12):1772–1783
FitzGerald MP, Richter HE, Siddique S, Thompson P, Zyczynski H (2006) Colpocleisis: a review. Int Urogynecol J Pelvic Floor Dysfunct 17(3):261–271
Jelovsek JE, Maher C, Barber MD (2007) Pelvic organ prolapse. Lancet 369(9566):1027–1038
Barber MD, Amundsen CL, Paraiso MF, Weidner AC, Romero A, Walters MD (2007) Quality of life after surgery for genital prolapse in elderly women: obliterative and reconstructive surgery. Int Urogynecol J Pelvic Floor Dysfunct 18(7):799–806
Fitzgerald MP, Richter HE, Bradley CS et al (2008) Pelvic support, pelvic symptoms, and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct 19(12):1603–1609
Hullfish KL, Bovbjerg VE, Steers WD (2007) Colpocleisis for pelvic organ prolapse: Patient goals, quality of life, and satisfaction. Obstet Gynecol 110(2 Pt 1):341–345
Gutman RE, Bradley CS, Ye W, Markland AD, Whitehead WE, Fitzgerald MP (2009) Effects of colpocleisis on bowel symptoms among women with severe pelvic organ prolapse. Int Urogynecol J 21(4):461–466
Murphy M, Sternschuss G, Haff R, van Raalte H, Saltz S, Lucente V (2008) Quality of life and surgical satisfaction after vaginal reconstructive vs obliterative surgery for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol 198(5):573e1–573e7
Collins SA, Jelovsek JE, Chen CC, Gustilo-Ashby AM, Barber MD (2007) De novo rectal prolapse after obliterative and reconstructive vaginal surgery for urogenital prolapse. Am J Obstet Gynecol 197(1):84e1–84e3
von Pechmann WS, Mutone M, Fyffe J, Hale DS (2003) Total colpocleisis with high levator plication for the treatment of advanced pelvic organ prolapse. Am J Obstet Gynecol 189(1):121–126
Acknowledgements
This publication results from the work of the Committee on Pelvic Organ Prolapse Surgery, part of the 5th International Consultation on Incontinence, held in Paris in February 2012, under the auspices of the International Consultation on Urological Diseases, and enabled by the support of the European Association of Urology.
The authors wish to acknowledge the fine work of previous consultations led by Professor Linda Brubaker.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
On behalf of Committee 15 “Surgical Management of Pelvic Organ Prolapse” from the 5th International Consultation on Incontinence held in Paris, February 2012
This work has been previously published as: Maher C, Baessler K, Barber M, Cheon C, Deitz V, DeTayrac R, Gutman R, Karram M, Sentilhes L (2013) Surgical management of pelvic organ prolapse. In: Abrams, Cardozo, Khoury, Wein (eds) 5th International Consultation on Incontinence. Health Publication Ltd, Paris, Chapter 15 and modified for publication in International Urogynaecology Journal.
Rights and permissions
About this article
Cite this article
Barber, M.D., Maher, C. Apical prolapse. Int Urogynecol J 24, 1815–1833 (2013). https://doi.org/10.1007/s00192-013-2172-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00192-013-2172-1