Abstract
Abiotic stresses are the primary sources of crop losses globally. The identification of key mechanisms deployed and established by plants in response to abiotic stresses is necessary for the maintenance of their growth and persistence. Recent discoveries have revealed that phytohormones or plant growth regulators (PGRs), mainly jasmonic acid (JA), have increased our knowledge of hormonal signaling of plants under stressful environments. Jasmonic acid is involved in various physiological and biochemical processes associated with plant growth and development as well as plant defense mechanism against wounding by pathogen and insect attacks. Recent findings suggest that JA can mediate the effect of abiotic stresses and help plants to acclimatize under unfavorable conditions. As a vital PGR, JA contributes in many signal transduction pathways, i.e., gene network, regulatory protein, signaling intermediates and enzymes, proteins, and other molecules that act to defend cells from the harmful effects of various environmental stresses. However, JA does not work as an independent regulator, but acts in a complex signaling pathway along other PGRs. Further, JA can protect and maintain the integrity of plant cells under several stresses by up-regulating the antioxidant defense. In this review, we have documented the biosynthesis and metabolism of JA and its protective role against different abiotic stresses. Further, JA-mediated antioxidant potential and its crosstalk with other PGRs have also been discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Plants grow in atmospheres that execute a diversity of environmental stresses, and the variation of any of these stresses can hinder the normal physiological mechanisms (Raza et al. 2019a). Plants need to replicate and further grow to sustain their existence in harsh environmental conditions, and there are several aids of upholding an equilibrium among plant growth/ development and stress tolerance (Harfouche et al. 2019; Raza et al. 2020a). Being stationary creatures, plants are powerless to evade abiotic stresses merely by moving to an appropriate environment. Therefore, they have advanced mechanisms to pay for the undesirable stressful environment by changing their developmental and physiological mechanisms (Raza et al. 2019a; Harfouche et al. 2019). Although environmental stresses can affect and disrupt their basic functioning mechanism, including amendments in gene expression, biosynthesis of distinct proteins and secondary metabolites, modifications in hormonal signaling and activities of antioxidant enzymes (Nemes et al. 2018; Hasanuzzaman et al. 2013, 2020). Antioxidant enzymes and PGRs play a vital role in determining the plant’s gene expression at the molecular level, which is one of the crucial mechanisms among other physiological developments (Aghdam et al. 2015; Faghih et al. 2017; Farooq et al. 2018; Ghaffari et al. 2020).
In order to respond to the different external as well as internal stimuli, growth, and development of plants must be regulated on their own. In cells, a group of molecules involved in signaling called phytohormone/plant growth regulators (PGRs) is found in a small proportion which is known to regulate the plant responses. Their primary roles in enhancing the plant acclimatization to the fluctuating environment by arbitrating growth, development, nutrients allocation and sink/source transitions have been well recognized by researchers around the world (Wani et al. 2016; Ku et al. 2018; Harfouche et al. 2019; Wang et al. 2020). Even though plant responses against abiotic stresses largely depends upon different factors, PGRs are considered as the essential endogenous substances which modulate the molecular and physiological responses, thus also act as an essential requisite for the survival of these plants as sessile life forms (Wani et al. 2016; Ku et al. 2018; Raza et al. 2019b). PGRs work either at the site of their synthesis or are transported to other parts of the plants where required. These PGRs are jasmonic acid (JA), auxin (IAA), brassinosteroids (BRs), cytokinins (CKs), salicylic acid (SA), abscisic acid (ABA), gibberellins (GAs), ethylene (ET), and strigolactones (SLs) which play a vital role in the growth and the development of the plants (Wani et al. 2016; Ku et al. 2018; Yang et al. 2018; Li et al. 2019; Raza et al. 2019b; Khan et al. 2020).
Notably, JA is the best characterized, well known and most abundant among jasmonates. In plants, JA is not only involved in developmental functions, but also activates the defense responses of plants against the pathogenic attacks and unfavorable environmental conditions such as cold (Mustafa et al. 2018; Habibi et al. 2019), heat (Degu et al. 2016; Balfagón et al. 2019), drought (Parmoon et al. 2019; Ghaffari et al. 2020), salinity (Farhangi-Abriz et al. 2019; Alisofi et al. 2020), heavy metals (Ahmad et al. 2018; Ali et al. 2018), waterlogging (Kamal and Komatsu 2016; Ouli-Jun et al. 2017), elevated ozone (Tuominen et al. 2004; Cui et al. 2016) and UV radiation (Liu et al. 2012). On the other hand, exogenous JA and MeJA have been reported to improve the activities of antioxidant defense enzymes. For instance, under salinity, superoxide dismutase (SOD), and ascorbate peroxidase (APX) in black locust tree (Jiang et al. 2016); SOD, APX, and peroxidase (POD) in strawberry (Faghih et al. 2017); under water deficiet conditions CAT, AsA and POD in Beta vulgaris (Ghaffari et al. 2020); under metal stress, CAT, POD, and SOD activities has been improved and thus enhance the stress tolerance (Aftab et al. 2011).
Jasmonates are derivatives of the fatty acid, including basic compounds like JA, jasmonate iso-leucine conjugate (JA-Ile), and methyl jasmonate (MeJA) (Wasternack and Strnad 2018; Wang et al. 2020). The chemical structure of JA contains a core of 3-oxo-2–20-cis-pentenyl-cyclopentane-1-acetic acid. These endogenous signaling molecules are involved in various developmental processes, and were previously known as stress-related hormones in higher plant species (Wasternack and Xie 2010; Wasternack and Strnad 2018). Since a couple of decades, various transcription factors (TFs) and genes involved in signal transduction process and JA biosynthesis have been recognized, including different activators and inhibitors concerned with environmental signaling (Howe et al. 2018). For instance, JAZ proteins interact with the MYC and MYB TFs and overwhelm the expression of JA-receptive gene (Pauwels and Goossens 2011; Goossens et al. 2017). MYC2, (a JIN1 gene) is a bHLH TF and plays a vital role in the regulation of JA signaling (Dombrecht et al. 2007; Fernández-Calvo et al. 2011) and can interact with several members of the JAZ family repressors (Fernández-Calvo et al. 2011). In addition, ICE1 and ICE2 belong to bHLH TFs, interact with JAZ4 and JAZ9 for the regulation of JA-mediated cold tolerance (Hu et al. 2013). Likewise, MYB, NAC, ERF, and WRKY TFs display noteworthy response to JA signaling, and these TFs control numerous progressions in plants; e.g., the synthesis of tryptophan and glucosinolates is controlled by MYB51 and MYB34 TFs, which also play a vital role in the downstream of MYC2 (Fernández-Calvo et al. 2011). For example, the NAC TFs (ANAC019 and ANAC055) act downstream of MYC2 to modulate cell division, secondary cell wall synthesis, and seed growth (Bu et al. 2008). Two JA-responsive AP2/ERF TFs (AtERF3 and AtERF4) work as repressors to downregulate the expression of their target genes and restrict with the action of other activators (Fujimoto et al. 2000). Furthermore, in Arabidopsis, several WRKY genes, such as WRKY50 (Gao et al. 2011), WRKY57 (Jiang et al. 2014), WRKY22 (Kloth et al. 2016) and WRKY70 (Li et al. 2017), which are regulated by the JA signaling pathway, are mainly connected with plant defense purposes. In another study, WRKY57 TF associate with JAZ4 and JAZ8 proteins to regulate JA-induced leaf senescence in Arabidopsis plants (Jiang et al. 2014).
This review highlighted the physiological and biochemical role of JA under several abiotic stresses. Furthermore, JA-mediated antioxidant defense metabolism, engineered JA biosynthesis, and crosstalk with other hormones have been described to give an overview of how JA helps in improving the abiotic stress tolerance in different plant species.
Biosynthesis and metabolism of jasmonic acid
Jasmonic acid, a distinctive signaling molecule, has now evolved into an established phytohormone that regulates plant reproductive growth, nutrients storage, and assimilates movement (Farhangi-Abriz et al. 2019; Alisofi et al. 2020). Apart from its assertive role in plant growth, JA also enables plants to adapt to different environmental stresses. JA and its formative molecules come from oxygenated octadecanoid fatty acids and have a pentacyclic ring structure (Wasternack and Strnad 2018). It was the cyclopentanone JA that has recently been known to garner the most attention as a plant growth regulator belonging to the Jasmonate family (Wasternack and Xie 2010). Nevertheless, it is clear that the biological activity of plants towards stress stimuli is not limited to JA, but extends to various metabolites and conjugates along with its cyclopentenone precursors, and perhaps vary from them.
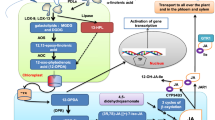
JA biosynthesis typically follows a sequential lipid esterification pathway involving chloroplast and peroxisomes, beginning with the release of α-linolenic acid (α-LeA) from chloroplastic galactolipids (Wang et al. 2019). At first, the emphasis was largely on the mechanical description of enzymatic crystallization involved in JA synthesis. However, findings of Vick and Zimmerman (1983) have made a new proposition, such as a series of lipoxygenase (LOX), a cyclase of hydroperoxide, a reductase and ß-oxidation of the side chain of carboxylic acid. The first hydroperoxide cyclase step was subsequently described as a two-phase allene oxide synthase (AOS) membrane-associated reaction, the highly unstable reaction in which an allene oxide cyclase (AOC) cyclized to 12-oxophytodienoic acid (OPDA). Besides enzymatic reactions, spontaneous hydrolysis stimulates unstable epoxide to α- and ÿ-ketols and nonenzymatic cyclization to racemic OPDA (Brash et al. 1988), which must be considered while assaying enzyme activity, as well as during the quantification of JA and OPDA (Brash et al. 1988). Figure 1 represents the schematic layout of JA biosynthesis and metabolism.
Schematic illustration of JA biosynthesis and metabolism. Hydroperoxy octadecatrienoic acid (HPOT) and oxophytodienoic acid (OPDA) are formed in chloroplast by 13-lipoxygenase (13-LOX), allene oxide synthase (AOS), and allene oxide cyclase (AOC), after the release of α-linolenic acid from galactolipids. After transportation into peroxisomes, OPDA is reduced by OPDA reductase-3 (OPR3) into 3-oxo-2-(2′(Z)-pentenyl)-cyclopentane-1 octanoic acid (OPC-8) and in the presence of acyl-coenzyme A oxidase-1 (ACX1) undertakes ß-oxidation of the side chain of carboxylic acid to (+)-7-iso-JA, which has been initially formed in the right configuration of JA. In the cytosol, conjugation of JA with amino acids, preferentially isoleucine to give JA-Ile which further transformed into JA-Ile methyl ester, JA-Ile glucosyl ester, 12-carboxy-JA-Ile, 12-O-glucosy-JA-Ile, 12-hydroxy-JA-Ile. JA also converted into methyl-JA, 12-O-glycosyl-JA, 12-HSO4-JA, Jasmonyl-amino acid, cis-jasmone, and JA-glycosyl ester
All OPDA producing enzymes are located in the chloroplast and second half of the JA biosynthesis occurs in peroxisomes. At this stage, OPDA is reduced by OPDA reductase preceded by acyl-CoA oxidase (ACX), l-3-ketoacyl-CoA-thiolase (KAT), ß-oxidation enzymes, and multifunctional proteins (MFPs); thus, activated by CoA synthetases and 4-coumaroyl fatty acid co-esters: CoA-ligases. In JA biosynthesis, the stereochemistry of products and intermediates is a key determinant. Naturally occurring enantiomeric forms (7R, 7S) of cis-(+)-7-iso-JA are formed in the AOC catalyzed process. JA-L-Ile has been recognized as the most bioactive JA compound, molecular perception and signaling for JA would always involve an isoleucine conjugate, (+) -7-iso-JA-Ile, having configuration (7R,7S) (Fonseca et al. 2009).
Moreover, JA can always be converted into inactive, partially active, and active compounds. To date, JA and its formative molecules are believed to have at least twelve metabolic pathways (Ruan et al. 2019). These pathways may involve in amino acid carboxylation, esterification, conjugation, sulfation, hydroxylation, methylation, decarboxylation, O-glycosylation as well as the development of 12-OH-JA derivatives in lactone. Although most of JA signaling homeostasis provide different JA-Ile derivatives, some reactions in particular during stress feedback and developmental responses such as leaf movement can lead to active compounds (Jimenez-Aleman et al. 2015; Ruan et al. 2019).
The use of many genetic and biochemical methods in recent years has significantly advanced our understanding of nested loops in JA biosynthesis and metabolism. Although there has been substantial progress in understanding these processes, some aspects remain unknown and demand the urgency of further research. Will the initial stages of JA biosynthesis be taking place on esterified and/or free fatty acids? It raises the question about what the substrates are and how they are being fed into the JA biosynthetic pathway.
Jasmonic acid in abiotic stress tolerance
The vital role of JA or MeJA in plant stress tolerance and adaptation has been widely documented. Interestingly, JA can improve the tolerance of plants to a variety of abiotic stresses (Table 1; Fig. 2). Plants have advanced numerous JA-mediated physiological, biochemical, and molecular mechanisms to retort, adjust, and attain tolerance to several abiotic stresses either alone or in combination (Fig. 3). Under low or high concentration, JA is known as a signaling molecule which stimulates the signal transduction pathways in response to many abiotic stresses (Figs. 3, 4). Mainly, Jasmonate-ZIM domain (JAZ) and Jasmonate-associated VQ-Motif GENE1 (JAV1, also known as VQ22) families play a vital role in JA signaling and JA-mediated plant defense (Fig. 4). In contrast, JAZ act as negative regulators of JA signaling (Chini et al. 2007; Thines et al. 2007). The JA-associated degradation of JAZs leads to transcriptional reprogramming of immense array of genes controlled by TFs, such as MYC2, leading to the activation of defense responses and the modulation of several processes of plant growth and development (Qi et al. 2011; Song et al. 2013; Goossens et al. 2017; Wasternack and Strnad, 2018). Likewise, JAV1 family of repressor proteins act a leading regulator in JA-mediated plant defense. JAV1 is a member of a family of plant-specific proteins with the conserved short amino acid sequence motif FxxhVQxhTG, where “x” denotes any amino acid and “h” denotes a hydrophobic residue (Jing and Lin 2015). This family plays several roles in plant defense responses, stress tolerance, and growth and development (Jing and Lin 2015). In the subsequent sections, we summarized the dynamic role of JA-mediated plant tolerance to a variety of abiotic stresses.
Jasmonic acid as a signaling molecule regulates the different plant processes by JA and its conjugate in response to several abiotic stresses, or in developmental progression, and also in basic features of agronomical importance of crop plants. Read text and Fig. 4 for further information about JA signaling
Jasmonic acid signaling pathway under normal and stress conditions. JA in response to defense is regulated by JAZ and JAV1 family of repressor proteins. JAZ proteins bind to the TFs and recruit corepressors, such as NINJA and TPL, to repress gene transcription in the absence of the active form of JA. In response to stressed conditions, induced JA attach with the F-box protein (COI1), recognition component of the SCF ubiquitin E3 ligase complex to promote ubiquitination of JAZ and JAV1. Accordingly, the degradation of both repressor proteins via the 26S proteasome derepress specific TFs and activate developmental and defense responses. Under normal conditions, JA content is low, JAV1, and JAZ proteins bind to various TF and limit their activity. Under stress conditions, JA content increases and degrade JAZ proteins, resulting in active TF that up-regulate genes involved in stress responses. TPL TOPLESS, COI1 coronatine insensitive1, SCF Skp1-Cul1-F-box protein, TFs transcription factors
Salinity stress
Salinity severely hinders the plant growth through ionic toxicity [sodium ion (Na+) and chloride (Cl−)] and osmotic effects or by a combination of both factors (Marriboina and Attipalli 2020); and JA helps to improve the plant growth and stress tolerance (Table 1). Shahzad et al. (2015a) observed that exogenous JA could improve Na+ exclusion in roots by decreasing the Na+ uptake hence facilitating the way forward for salt tolerance in two genotypes of maize. During the first phase of stress, JA level increased, and findings suggest that it might be indirectly involved in the inhibition of leaf growth in salt-sensitive maize genotypes. As the growth examines exposed that JA supply in root medium prevents shoot extension growth and both maize genotypes were sensitive to the inhibitory effects of JA. In tomato, endogenous JA enhanced salt tolerance mainly through maintaining reactive oxygen species (ROS) homeostasis (Abouelsaad and Renault 2018).
In order to lower the water stress in canola, 0.5 mM JA was sprayed under different NaCl levels (40, 70, 100 mM). Salinity lowered the lateral and primary root growth along with weight, density, diameter, and root–water content, while improved the ratio of root length/root weight. Foliar spray of JA increased the overall root growth but decreased the shoot dry weight (SDW) and primary root growth with no alteration in root–water content under altered salt-stress level (Farhangi-Abriz et al. 2019). The effect of MeJA studied on two tomato genotypes showed that salinity stress resulted in lowered biochemical and physiological parameters. The different concentrations (10, 20, 30, 40, 50, and 60 µM) of MeJA sprayed on the control and stressed plants resulted in significant improvement in growth, biochemical, and physiological characteristics of tomato plants (Manan et al. 2016). Bitter melon was treated with 50 µM JA under 50, 150, 300 mM NaCl concentrations. Exogenous JA supplementation alone or together with NaCl improve the growth attributes as well as hyper-accumulation of soluble sugar, amino acids, proline, and proteins; and lowering the activity of hydrogen peroxide (H2O2) decomposing enzymes (Alisofi et al. 2020).
Examination of the effects of MeJA on chemical and volatile components in two contrasting salt-stressed cultivars of basil showed that foliar application of MeJA not only increased the essential oil content but also produced obvious effects on the main oil components. Taken together, it significantly increased antioxidant activity and improved the overall plant defense (Talebi et al. 2018). Transcriptional analysis has shown that an enzyme Arabidopsis lipoxygenase3 (LOX3) is, involved in the synthesis of JA, is induced in plants in response to the high salinity levels. In contrast to wild type, mutant lox3 indicated hypersensitivity to salinity in germination as well as during various developmental phases. LOX3 mutant with salt-sensitive phenotypes was rescued using MeJA, thus proposing that salinity induced impairment in mutant could be mediated by JA (Ding et al. 2016).
Drought stress
Generally, drought stress (DS) or water deficit (WD) condition increases ion toxicity and restricts or/and reduced the plant growth, development, leaf and stem dry weight, canopy, root development and turgor pressure; specifically, reduced the net photosynthesis rate, and stomata conductance (Alam et al. 2014; Ilyas et al. 2017; Yosefi et al. 2020). In various studies, it has been indicated that signaling pathways of JA are linked with DS alleviation (Table 1). After DS, transient as well as the rapid increase of JA content was found in citrus (de Ollas et al. 2013) and Arabidopsis (Balbi and Devoto 2008) plants, but JA content was limited to basal level with stress prolongation. MeJA has also been reported to improve resistance against DS in soybean (Mohamed and Latif 2017), peanut (Todaka et al. 2015), rice (Dhakarey et al. 2017), and Brassica oleracea (Wu et al. 2012) plants by enhancing antioxidant enzyme activity and osmoprotectants.
The effect of JA has been studied on thyme spp comprising Thymus daenensis and T. vulgaris under DS. JA (200 and 400 µL) treatment were given to plants in normal, slight, and mild DS. Irrigation levels had noteworthy effects on plant growth in the form of plant height, leaf area, dry weight, and branches number. Treatment with JA inclined the essential oil harvest and main oil components. p-cymene, γ-terpinene, and carvacrol percentage were higher in extracted oil in stressed situations than nonstressed plants while thymol percentage reduced in DS. Notably, JA significantly enhanced the thymol and carvacrol content, root length, antioxidant activity, and plant height in thyme spp while lowered the yield of essential oil and γ-terpinene amount (Alavi-Samani et al. 2015). Previously, Ilyas et al. (2017) studied the effects of JA on drought-stressed wheat plants. Before applying water stress, seed primed by 100 µM JA and its application mitigated the effects of DS in wheat plants. The results showed that 100 µM JA was found to be more effective as its application increased the germination up to 27% (Ilyas et al. 2017). In sugar beet plants, foliage supplementation of JA (0.5–10 µM) increased the activity of antioxidant enzymes and improved the tolerance against WD condition. JA application also increases the yield by 21 and 24%, respectively, with both concentrations (Ghaffari et al. 2020).
Recent findings have shown that, under DS, JA interact antagonistically with CKs and regulated the process of xylem development from procambial cells in Arabidopsis (Jang and Choi 2018). Scientists have compared the root proteome plus morpho-physiological characteristics of rice wild-type plants with JA mutant cpm2 (coleoptile phytomorphogenesis 2) having disrupted AOC gene for understanding JA role in DS condition. cpm2 mutant has greater stomatal conductance, higher shoot ABA plus improved water use efficacy (WUE) than wild type in DS. AOC was expectedly abundant in the wild type under DS. Moreover, cell growth, cell wall synthesis, and numerous proteins take part in secondary metabolism were abundant in roots of cpm2 mutant plants (Dhakarey et al. 2017). Under DS, strawberry plants were treated with exogenous JA (0.01 and 0.05 mM) treatment. JA treatment lowered the RWC, photosynthesis pigments, total protein contents, and increased the activities of antioxidant enzymes, including malondialdehyde (MDA), H2O2, and proline contents (Yosefi et al. 2020). Similarly, in maize plants, exogenous MeJA (20 μM) reduced the harmful effects of drought-induced oxidative stress by lowering the levels of MDA, LOX activity, and H2O2; it also increased the proline, carbohydrate, and total soluble sugar contents, activities of the antioxidants (CAT, POD, and SOD) were also increased (Tayyab et al. 2020).
Cold stress
Cold stress categorized into chilling (0–15 °C) and freezing (< 0 °C) temperature are considered as serious environmental issues and has been described to limit crop productivity. Mainly, cold stress, can cause changes in cytoplasm viscosity, enzyme activities and inducing chlorosis, necrosis, and membrane damage in tropical and subtropical plants. However, temperature above the optimal for growth can harmfully influence plant processes and cellular machinery by damaging cell homeostasis (Ding et al. 2019). Recent studies provided evidence that JA is involved in senescence of leaf as well as tolerance towards cold stress. Notably, JA levels found higher in senescent leaves than in nonsenescent leaves. Exogenous JA application encourages leaf senescence and expressing genes associated with leaf senescence; thus, increased freezing tolerance in Arabidopsis plants (Hu et al. 2017). Owing to the multifaceted association, JA regulates the C-repeat binding factor (CBF) pathway to upregulate downstream cold-responsive genes and eventually increases cold tolerance (Hu et al. 2017). Likewise, JA has been found to control chilling through induction of ROS-avoidance enzymes (Sharma and Laxmi 2016), and improve the cold stress tolerance in different plants (Table 1).
Scientists have examined the physiological reply of Taraxacum pieninicum towards JA application during cold storage 4 °C plus shoot micro-propagation. Results have revealed that during preculture JA lowered considerably shoots growth deprived of effect on the rate of proliferation. The supplementation of JA (24–72 µM) overcome the cold stress effects that was established through reduced proline accumulation and thiobarbituric acid reactive substance (TBARS). The proliferation of shoots and roots became more active in re-growth later cold storage united to JA treatment while root elongation reserved in this situation (Kamińska et al. 2018). Subject to storing temperature as well as cultivar, blood orange found sensitive towards chilling injury (Cl). Methyl salicylate (MeSA), γ‐aminobutyric acid (GABA), and MeJA treatment after harvesting appeared to alleviate the Cl. 20 and 40 mM aqueous solution of GABA provided through vacuum infiltration at 20 °C for 8 min at 30 kPa, while MeJA and MeSA treatment provided independently at 50 µM and 100 µM by dipping fruit in a 20 L container at 20 °C for 18 h. These all treatment resulted in lowering the Cl by decreasing electrolyte leakage (EL), H2O2 level, MDA, and improved proline content. Effective findings come with 100 µM—MeSA, 50 µM—MeJA and 40 µM—GABA that improved the activity of antioxidant enzymes like SOD, CAT, phenylalanine ammonia-lyase (PAL), and APX while lowered the activity of polyphenol oxidase and POD (Habibi et al. 2019).
Notably, MeJA and hot-air treatment found effective in lowering the CI in peach fruit. Hot air treatment delivered to peach fruit for 3 d at 37 °C and 10 µmol L−1 vapor of MeJA at 24 h prior to storage under 5 °C. These treatments resulted in higher sucrose level as compared to control. Also improved SPS (sucrose phosphate synthase) expression and activity, and lowered invertase (AI) activity have been observed. These findings suggest that improved sucrose related to high SPS and low Al, increases tolerance to chilling witnessed in MeJA and hot-air-treated peach fruits (Yu et al. 2016). Dragon fruit treatment with MeJA resulted in improved postharvest antioxidant enzyme activities and physicochemical characteristics in cold storage (Mustafa et al. 2018).
Heat stress
Heat stress (HS) is one of the major devastating environmental stresses which causes significant crop losses around the globe (Raza et al. 2019a, b). Owing to alteration in global climate, the magnitude and frequency of HS have intensified. It has been reported to result in an excessive yield of ROS, which is known to cause impairment in plant proteins, nucleic acids, and lipids (Siddiqui et al. 2015; Hasanuzzaman et al. 2013, 2020). JA application has been found to play a vital to improve the HS tolerance (Table 1).
Pea plants pretreated with high MeJA concentrations, i.e., 50, 100, and 200 µM were subjected to HS at 40 °C, and cold stress at 4 °C, whereas control/optimum temperature of 20 °C was kept for 72 h. MeJA damaged the morphological and physiological functions of the pea plant under these temperatures, while up-regulated the JA and down-regulated the SA and ABA (Shahzad et al. 2015b). Xu et al. (2016) treated the cell-suspension cultures of Aquilaria sinensis with HS and studied how JA affected the buildup of sesquiterpene. Exogenous application of JA and its methyl ester get accumulated after HS shock. Gene expression involved in JA biosynthesis pathway up-regulated significantly, and sesquiterpene compounds get accumulated. JA inhibitor, i.e. nordihydroguaiaretic acid (NDGA) have shown to block all such effects. Also, MeJA application to A. sinensis has displayed sturdiest effects on sesquiterpene biosynthesis compared to H2O2 and SA. These findings suggest that JA is an important signal transducer in a cascade of intracellular signal resulted from HS, and ultimately JA shows the vital part in sesquiterpene compounds accumulation (Xu et al. 2016).
Plants face high light (HL) along with high temperature, and such conditions are found as a solemn threat towards agriculture yield since photosynthesis is sensitive to elevated temperature and high light intensity. Photosystem II (PSII) is one of the major targets of HS and HL as the degree of photo-inhibition is dependent on the stability of PSII damage rate (induced through LS) and PSII repair rate (impaired in HS) (Balfagón et al. 2019). The Arabidopsis response against HS and HL have been studied, and the results showed that a combination of these stress resulted in enhanced JA-IIe and JA accumulation. Further, JA biosynthesis mutants have shown improved sensitivity to HS and HL when applied simultaneously. These observations indicated that JA has a major role in plants’ acclimation to a combination of HL and HS (Balfagón et al. 2019). The grape berries treated with HL (2500 μmol m−2 s−1), high temperature (40 °C), JA (200 μM), menadione (120 μM), and ABA (3.026 mM) showed high metabolic fluctuations depicting the pre-veraison barriers. At veraison stage, flavonoid accretion boosted the berry flexibility to cue-induced alterations (Degu et al. 2016).
Waterlogging stress
Waterlogging may lead to the formation of hypoxic conditions by affecting the light intensity, formation of toxic substances, and gas diffusion due to rapid depletion of oxygen level in soil (Fukao et al. 2019). It has been reported to increase the endogenous JA concentration in Adzuki beans through the octadecanoid pathway. Moreover, high expression of JA and ABA under high water influx prevented the plants against osmotic stress (Ullah et al. 2017). In waterlogged citrus plants, the stress caused a transient increase in JA levels throughout the experimental phase preceding the JA and ABA accumulation. The increase was observed in different tested genotypes at different periods, whereas significant differences in basal levels of JA in leaves of all genotypes was observed with sensitive species having the lowest levels followed by the hybrid varieties of citrus (Arbona and Gómez-Cadenas 2008). Radhakrishnan and Lee (2013) studied the role of spermine (Spm) under osmotic stress in soybean and concluded that endogenous production of JA helped plants to acclimatize under osmotic stress. This regulation of JA through Spm treatment might reorganize the membrane lipids under osmotic stress in soybean. Moreover, Table 1 illustrated a summary of several experiments showing the positive role of JA under waterlogging stress.
The JA regulated flooding stress response in soybean suggested a significant increase in the asparagine synthase and beta-amylase proteins, which stimulated the nitrogen transport and storage and breakdown of the starch, respectively. In contrast, JA-mediated activation of beta-amylase proteins was further suggested to be involved in controlling the root growth and starch breakdown. The proteomic study indicated an association of glutathione S-transferase (GST) with the peroxide levels leading to less oxidative damage under flooding stress (Kamal and Komatsu 2016). The pathogenesis-related protein TaBWPR1.2 was hypothesized to be involved in waterlogging response in wheat through JA signaling. The JA treated roots did not show any morphological response, but it significantly increased the transcript level of TaBWPR1.2 gene after 3 d of waterlogging treatment. Thus, JA just activated the signaling pathway in seminal roots through gene upregulation and provided tolerance against the stress (Haque et al. 2014).
Heavy metals/metalloids stress
Owing to the documented toxicological effects and high bioaccumulation in food crops, heavy metals have globally appeared as a topic of major concern (Edelstein and Ben-Hur 2018). The range of physiological, biochemical, and metabolic impacts has been reported, which are linked with plants grown at heavy metal polluted sites (Table 1). These include a reduction in plant growth, promotion of leaf senescence, and inhibition of photosynthesis (Edelstein and Ben-Hur 2018; Raza et al. 2020b; Siddiqui et al. 2020).
A study on the role of JA to alleviate the cadmium (Cd) stress in Faba bean revealed that supplementation of JA mitigated the stress on plant growth and biomass yield. It consequently enhanced the chlorophyll (Chl) synthesis by reducing Cd uptake by plants which previously might have resulted due to down-regulation or destruction of enzymes involved in Chl biosynthesis. JA application was further associated with the production of osmolyte and enzymatic antioxidants, which provided protection against the metal stress (Ahmad et al. 2017). Zhao et al. (2016) found that endogenous JA can effectively limit the Cd absorption and translocation to the leaves and other aerial parts of the tomato. They reported a positive role of JA in the regulation of soluble sugar for osmotic adjustment to maintain the RWC under high Cd concentrations. The JA mitigated the Cd-induced oxidative damage given low MDA, EL, and H2O2 content and high activities of antioxidative enzymes, including SOD, POD, and CAT. In another study, Lei et al. (2020) showed that Cd-treated Arabidopsis plants quickly induces the expression of genes helping endogenous JA synthesis, and afterwards upsurges the JA level in Arabidopsis roots. Moreover, exogenous MeJA improves Cd caused chlorosis of new leaves by reducing the Cd level in root cell sap and shoot and reducing the expression of the AtIRT1, AtHMA2 and AtHMA4 genes helping Cd uptake and long-distance translocation, correspondingly. Further, mutation of one of the significant JA synthesis gene (AtAOS) significantly increases the expression of other genes responsible for Cd uptake and translocation, and it also confers improved sensitivity to Cd toxicity. Finally, findings show that Cd-induced JA works by the JA signaling pathway and eases Cd toxicity in Arabidopsis through the modulation of Cd uptake and translocation genes (Lei et al. 2020).
Sirhindi et al. (2015) reported that JA treated Glycine max seedlings managed the antioxidant machinery by the production of SOD, POD, CAT, and APX activity by scavenging the nickel (Ni) induced free radicals. It resulted in a significant increase in soluble protein content by protecting the DNA synthesis of total proteins. Likewise, Azeem (2018) studied the impacts of exogenous application of JA on maize and concluded that plants develop different resistance mechanisms under metal stress. JA improved the plant growth by reducing Ni induced negative impact of oxidative stress on plant growth, biomass, and low protein content. Likewise, JA was found beneficial for soybean plants in attenuating the damage caused by Ni toxicity. JA primed seeds showed better plant growth by improving the photosynthetic efficiency, osmotic imbalance, activity of glyoxalase system, and ROS-detoxification enzymes. It reduced the ROS and LOX activity which positively correlated with the reduction in EL and MDA production, while maintaining high ascorbate and GSH levels, thus regulating the antioxidants enzymatic and nonenzymatic biochemical activities (Mir et al. 2018).
Boron (B) induced toxicity is known to cause an inhibitory effect in Artemisia annua, which is an essential source of artemisinin, an antimalarial drug. Foliar application of MeJA was tested as an attempt to ameliorate the toxic effects of B, thus improving the plant utility. The results showed that MeJA supplementation enhanced the growth, photosynthetic activity, antioxidant enzymes synthesis, and reduced the lipid peroxidation through ROS scavenging mechanism (Aftab et al. 2011). The lead (Pb) treated tomato seedlings primed with JA showed a decline in the Pb uptake and better growth with respect to root and shoot length. JA attenuated the MDA and oxygen-derived free radicals by lowering the expression of respiratory burst oxidase (RBO) and P-type ATPase transporter genes under stress. Moreover, it up-regulated the expression of genes encoding antioxidative enzymes glutathione reductase (GR), GST, PPO, POD, and CAT and increased the nonenzymatic antioxidants (AsA, GSH, and tocopherol) (Bali et al. 2019). The chelation of heavy metals is essential for the regulation of plant growth under metal stress. Along with other protective roles of JA, it has been reported to effectively reduce the expression of heavy metal conveyor proteins and serve as an agent to regulate the synthesis of phytochelatins (Sofy et al. 2020). A study carried out to find the impacts of JA on Lycopersicon esculentum suggested an increase in the metal cheating compounds including nonprotein, protein-bound, and total thiols under the Pb stress through activation of the phytochelatin biosynthetic pathway (Bali et al. 2018). These metal-chelating compounds produced as a result of activation may bind to the Pb ions, thus reducing the toxicity and metal stress (Bali et al. 2019). However, the JA application in Brassica juncea showing Pb-subcellular distribution did not affect the production of phytochelatins. This suggests the presence of other pathways which may get involved in the signal transduction pathway of phytochelatins (Agnihotri and Seth, 2020).
Ultraviolet (UV) radiation stress
Ultraviolet (UV) radiation in the range of 280–320 nm (UV-B) has been documented as one of the critical environmental stress factors affecting plant health globally (Yao et al. 2015). The effects of UV-B on hormonal regulation has been unevenly divided into two groups, i.e., inhibition of growth-promoting hormones; and the improvement of stress-induced defense hormones (Vanhaelewyn et al. 2016). In tobacco, the lack of UV-B-induced antiherbivore protection in the jasmonate deficient variety suggested that jasmonate signaling primarily provided resistance against the insect herbivory. On the contrary, in wild-type plants, it led to high accumulation of phenylpropanoid derivatives to improve the plant defense mechanism and amplified the expression of wound-response and jasmonate-inducible genes like trypsin proteinase inhibitor (TPI) (Demkura et al. 2010). JA was found to improve the tomato plant resistance to thrips (herbivorous arthropods) under UV exposure. UV did not affect JA levels in any genotype, but a significant increase in the JA-Ile concentration was recorded in Moneymaker cultivar treated with high UV radiations. Absence of resistance against thrips in jasmonate-deficient mutant (def-1) under high and low UV proved the defensive role of JA. Where wildtype (Castlemart) cultivar showed comparatively higher resistance against thrips, the silver damage (thrips feeding damage to leaves) induced the JA-responsive genes and increased in JA-Ile levels under high UV exposure (Escobar-Bravo et al. 2019). Table 1 showing the protective role of JA under UV radiation.
Qi et al. (2018) explored the role of JA in enhancing the plant resistance to lepidopteran larvae using A. thaliana, Nicotiana attenuate, Oryza sativa, and Zea mays. In A. thaliana, UV-B enhanced the plant defense against Spodoptera litura through an increase in secondary metabolites via JA and/ or JA-Ile pathway and herbivory-induced defense metabolite glucosinolates (GSs) content. Furthermore, the JA impaired N. attenuate, and O. sativa showed JA-dependent pathway against UV-B induced defense response. Mung bean showed induction of JA under elevated UV-B in HUM 1 and HUM 12 cultivars. The values were comparatively high in HUM 12, showing better plant defense response and resistance against UV-B stress along with enzymatic and nonenzymatic antioxidants (Choudhary and Agrawal 2014).
Elevated ozone
Elevated ozone (O3) generates ROS that causes lesions and induce programmed cell death in plants (Castagna et al. 2007). The defensive role of exogenous MeJA against ozone-induced injury was studied in A. thaliana, which showed low ET emissions and ion leakage in response to the applied concentration of 100 µM MeJA. The JA-induced gene expression through RNA gel blot analysis also showed high expression of AtVSP1, a JA inducible gene, in wild type plant under O3 stress (Kanna et al. 2003). A study carried out by Cui et al. (2016) revealed the role of elevated O3 levels in the modulation of the JA signaling pathway in tomato. Encarsia formosa is a globally recognized parasitoid used for the biological control of whiteflies. Results indicated that high emission rate of volatile compounds protected plants from the damaging effect of whiteflies. Furthermore, the presence of E. formosa was promoted by the emission of volatile organic compounds (VOCs), produced in response to the synergistic action of whitefly infestation and elevated levels of O3 production. Some more examples are shown in Table 1.
Pellegrini et al. (2013) found that endogenous JA, an important phyto-regulator of O3 response, showed a successive increase in its accumulation in lemon balm as a response to short-term O3 exposure. Further investigation revealed that the ET and JA production regulated the O3 induced cell death and the protecting function of JA was more prominent due to their antagonistic function after the early hours of fumigation, thus limiting the leaves lesion formation. In addition, an O3 induced increase in the transcript abundance of JA biosynthesis genes allene oxide synthase 2 (AOS2) and AOC was observed in L. esculentum. The active transcription of JA-inducible gene proteinase inhibitor II (PINII) confirmed the activation of the JA pathway in response to the O3 treatment (Castagna et al. 2007). Elevated O3 enhanced the foliar JA levels and up-regulated the LOX and proteinase inhibitors (PIs) activity in wild-type tomato under Helicoverpa armigera infestation. It also regulated the JA defense pathway and protected plants against oxidative stress and H. armigera herbivory under elevated O3 concentration to provide better adaptation environment for the plants to grow (Ren et al. 2015).
Role of JA-mediated antioxidant defense system under stressful environment
Plants develop various physiological and biochemical adaptations using hormone-dependent signaling pathways to deal with abiotic stress. As a multifunctional phytohormone, JA not only promotes plant growth but also upgrades plant defense response under stressful environment (Raza et al. 2019a, b; Wang et al. 2020). It appears that JA, either alone or in conjunction with other plant hormones, can alleviate plant stress and improve plant growth. Besides this, they also contribute to the synthesis of osmolytes, accumulation of metabolites, and up-regulation of antioxidant metabolism (Nafie et al. 2011; Farooq et al. 2018; Ghaffari et al. 2020). Here, Table 2 illustrates the JA-mediated antioxidant defense system to enhance abiotic stress tolerance in different plant species. Whereas, Fig. 5 indicating the vital role of JA as a plant antioxidant defense regulator under multiple abiotic stresses.
A model representing the vital role of JA as a plant antioxidant defense regulator under multiple abiotic stresses. Exogenous JA increases the activities of both enzymatic and nonenzymatic antioxidant defense enzymes and reduces the production of ROS; ultimately help plants to cope with a variety of abiotic stresses including abiotic-induce oxidative stress. On the other hand, it also improves the endogenous JA and the expression level of antioxidant enzyme encoding genes under stress conditions. Further, it also improves several physiological, biochemical, and molecular mechanisms as explained in Fig. 3. However, some directions are still required more attention, such as at what growth stage? At what dose and what is actual tolerance mechanisms concerning the natural crosstalk with other phytohormones?
Although studies have shown JA to be an important plant growth and development regulator, recently its effects on plant defense mechanisms inducing stress tolerance have been of considerable interest. Endogenous JA levels are found to increase in plants that experience specific stress conditions; however, exogenous application of JA increases plant stress resistance by inducing antioxidant activity. In muskmelon, JA-mediated cellular defense signaling was triggered by both primary and secondary metabolism as evident by stress regulating indices, such as ascorbate metabolites, phenolics, and antioxidant enzymes (Nafie et al. 2011). In pepper plants, MeJA treatment mitigated the waterlogging-induced damages by increasing the activities of CAT, POD, and SOD (Ouli-Jun et al. 2017).
In different plants like Arabidopsis, tomato, and potato the endogenous JA level has been reported to increase under salt stress (Pedranzani et al. 2003; Ellouzi et al. 2013; De Domenico et al. 2019). In another study, Jiang et al. (2016) reported that exogenous MeJA application improved the black locust tree's salinity tolerance by stimulating SOD and APX activities. Similarly, MeJA treated strawberry seedlings downgraded the adverse effects of salinity stress, as shown by considerably higher POD, APX, and SOD enzymatic activities (Faghih et al. 2017). According to Farhangi-Abriz and Ghassemi-Golezani (2018), in soybean plants, JA application improved the osmotic and oxidative injuries by increasing the activities of APX and SOD under salinity. Similarly, MeJA also enhanced cauliflower drought resistance by activating both the enzymatic and nonenzymatic antioxidant systems (Wu et al. 2012). Similarly, under drought stress, exogenous JA (0.5 mM) improved GR, and Gly I activities in B. napus; MDHAR activity in B. campestris; and DHAR, GR, GPX, Gly I, and Gly II activities in B. juncea and increase the stress tolerance (Alam et al. 2014). Under water deficit condition, Ghaffari et al. (2020) found the increased activities of CAT, AsA, and POD in sugar beet plants. Likewise, exogenous MeJA reduced the damaging effects of drought-induced oxidative stress by lowering the levels of MDA, LOX activity, and H2O2 contents in maize plants. It also increased the proline, carbohydrate, and total soluble sugar contents, and activities of CAT, POD, and SOD (Tayyab et al. 2020).
Many studies have elaborated the interlinkages between JA signaling network in plants exposed to the toxicity of different heavy metals. Previous research reports have identified that MeJA treatment can mitigate Cd-induced oxidative stress in various plants, including Arabidopsis, chilli pepper, and European black nightshade, through the higher antioxidant activity of APX, CAT, and SOD (Yan et al. 2013, 2015). Thus, the use of MeJA considerably attenuates the damages to plants caused by heavy metals through increasing antioxidant enzyme activity and secondary metabolites production. Exogenous MeJA treatment can counteract plant boron (B) toxicity by stimulating the antioxidant defense enzymes (CAT, POD, and SOD) and impeding lipid peroxidation (Aftab et al. 2011). Resulting in JA may safeguard plants from the negative effects related to B toxicity. Under Ni toxicity, JA-treated soybean plants significantly increased the activities of SOD, CAT, GPX, GST, APX, MDHAR, DHAR, and GR; and it also stimulated the Gly system activity (Mir et al. 2018), and help soybean plants to combat the Ni toxicity. The Pb treated tomato seedlings primed with JA, up-regulated the expression of genes encoding antioxidative enzymes GR, GST, PPO, POD, and CAT and enhanced the activities of AsA, GSH, and tocopherol under Pb toxicity (Bali et al. 2019). Moreover, JA signaling may also contribute to plant adaptation under cold stress by modulating the antioxidant defense. For instance, Habibi et al. (2019) treated blood orange fruit seedlings with MeJA (50 µM) and reported the improved the activities of SOD, CAT, PAL, and APX. MeJA treatment also reduced the activity of polyphenol oxidase and POD under cold stress (Habibi et al. 2019). Under chilling stress, Abidi et al. (2015) found the enhanced activities of POD, CAT, and APX with the foliar treatment of MeJA in peach fruits. Enhanced activities of antioxidant defense help peach fruits to cope with chilling stress. In various plants, such as tomato, mango, peach, loquat, cowpea, guava, and pomegranate MeJA treatment appears to alleviate cold stress by increasing the synthesis of antioxidants, the release of phenolic compounds, and accumulation of heat shock proteins (Meng et al. 2009; Sayyari et al. 2011; Aghdam et al. 2015). Altogether, the above-referred findings reinforce that JA can effectively improve plant stress tolerance by increasing the activity of antioxidant defense system.
Interaction and crosstalk with other phytohormones
The balance between defense mechanisms, growth and development, is a very complicated process in regulatory plant networks, and researchers have found difficulties in understanding the role of different hormones and their crosstalk in those processes. Therefore, studying the hormonal interplay is essential to understand the hormonal reactions in plant stress, growth, and development. Several studies have shown that JA plays a significant role in regulating plant growth and development under stress conditions, and the hormonal interplay between JA and other PGRs associated with different developmental processes. A hormonal crosstalk involves both positive and negative feedback, which can affect the hormonal biosynthesis, transport and signaling (Vos et al. 2015; Ku et al. 2018). Moreover, this synergistic or antagonistic association between JA and other PGRs help plants to develop tolerance against different abiotic stresses (Wasternack and Strnad 2018; Wang et al. 2020). Figure 6 illustrates the crosstalk and interaction with other phytohormones.
An overview of JA-mediated crosstalk with other phytohormones signaling pathways. Notably, MYC2 is the key element involved in communication among JA and GA. DELLAs interact with JAZ repressors, relieving MYC2 from JAZ repression, and enable JA-mediated defense responses by the activation of MYC2. MYC2 is also positively regulated by ABA. On the other hand, MYC2 inhibits SA regulation in response to abiotic stress. The JAZ inhibition of EIN mediates JA and ET signaling synergy in plant tolerance, while the reciprocal counteraction among MYC2 and EIN facilitates JA and ET signaling resentment. Abbreviations are defined in the text
Insight into the mechanism of JA activity in plants under abiotic stress and their crosstalk with other PGRs is critical. Previous studies have reported a new insight into the use of specific molecular mechanisms to regulate plant gene expression through stress-related and growth-related signaling pathways (Pauwels et al. 2010). There is an interaction between the auxin and JA signaling pathways, and auxin can stimulate the expression of JA repressive gene (TIFY10A/JAZ1) in Arabidopsis (Grunewald et al. 2009). There is little evidence regarding the crosstalk between JA and CKs, but it is noticeable that MeJA can maintain the adequate amount of CKs concentration needed for plant development. For instance, the MeJA application modulated the activity and gene expression of CK oxidase under salinity stress. It regulated the concentration of CKs in wheat by regulating the activity of CK dehydrogenase/oxidase (Avalbaev et al. 2016).
Previous research indicates a complex nexus between JA-ABA. Both signposted pathways appeared to finetune each other's responses and other metabolic pathways against abiotic stress (de Ollas and Dodd 2016). Besides, the role of ABA receptor gene (PYL4) in the regulation of metabolic reprogramming in tobacco and Arabidopsis was also reported during JA signaling (Lackman et al. 2011). The study drew up the relation between JA responses and core–ABA signaling mechanism, which can help track the elicitor-induced reprogramming of plant growth and metabolism. Moreover, the relationship between ABA and JA has been identified following plant response regulation under drought stress (de Ollas et al. 2015). Further, JA signaling is finetuned by the core repressor of GA signaling pathway, DELLA proteins, by competitively binding of MYC2 with JAZ proteins. Highly stable DELLA proteins compete with MYC2 to bind to JAZs without GA and trigger MYC2, which in turn, activates JA-responsive gene expression (Hou et al. 2010). Conversely, JA and SA are two of the basic pathways of biochemical response which can be triggered by environmental stressors. Both of these function as essential signaling molecules that are responsible for plant defense response. In addition, multiple studies have shown the antagonistic activity of JA and SA signaling pathways (Spoel et al. 2003; Van der Does et al. 2013). The interplay between JA and BRs also play a crucial role in plant development and stress responses (Yang et al. 2011). In rice, a mutually antagonistic association was proposed between the JA and the BR pathway (Nahar et al. 2013).Likewise, JAs are being argued to contribute to plant stress responses, and their association with other plant hormones has also been reported (Per et al. 2018). Ethylene response factors (ERFs) is considered to be a key regulatory channel for stress signaling and the number of hormones like JA and ethylene (Muller and Munne-Bosch 2015). Moreover, both ABA-dependent and -independent pathways seem to control JAs potential effects. In rice, drought resistance is associated with the JA signaling pathway. The OsbHLH148 protein which gives drought tolerance in rice interacts with OsJAZ1 to activate the expression of drought response factor (OsDREB1) (Seo et al. 2011). Exogenously applied JA has been observed to increase ABA levels in different plant species under either control or drought conditions (Sánchez‐Romera et al. 2014). Latest experiments using the JA-insensitive mutant, amido-synthetase1-1, exposed to drought, salt, and heat stresses revealed blocked expression of ERF1, indicating that JA and ethylene are necessary for induction of ERF1 under different abiotic stresses. JAs foster stomatal closure, and it was suggested that the water stress impede the conversion of 12-OPDA to JA. OPDA then works either independently or in combination with ABA to facilitate stomatal closure; directing increased drought tolerance (Savchenko et al. 2014). Therefore, JAs have been found to play a vital role in stomatal closure under drought stress. Referring to the effects of JA, auxins are generally positive stomatal opening regulators and assist in the upregulation of JAZ1 genes belonging to a TIFY protein family, which function as JA signalling repressors (Chini et al., 2007; Thines et al., 2007). Another significant phytohormone SA suppresses the JA-inducible RSOsPR10, which encodes a root-specific PR protein in rice under drought and salt treatments (Takeuchi et al., 2011).
Further, JA and ethylene function as antagonists in controlling the responses to heat stress. Zhu et al. (2011) demonstrated an interplay between JA and ethylene signaling pathways through the interaction of JAZ and its targets EIN3/EIL1. A combined application of the MeJA and SA boost in citrus chilling resistance mechanisms. On the other hand, the involvement of cold TF inducer of CBF expression, ROS avoidance mechanism used by both JA and SA signaling pathways highlights the potential crosstalk between JA–SA signal transduction pathways to combat cold stress (Sharma and Laxmi 2016). Conclusively, JAs mitigate the negative impact of abiotic stress. Understanding of physiological and molecular processes in plant resistance to multiple stresses would be critical for the development of new crop varieties that will be better equipped to deal with inevitable climate changes. Also, JA signaling factors and their functions in crosstalk remains to be revealed at organ, tissue or cellular levels. Future research on unrevealing the significant insights into the function and control of JA and their crosstalk with PGRs in combined stress can bring promising results.
Engineered JA biosynthesis to enhance plant abiotic stress tolerance
Classical biotechnological approaches aimed at improving plant abiotic stress tolerance are directed towards strengthening the plant endogenous defense mechanisms; however, it is typically followed by growth setback and yield losses due to the crosstalk between developmental and stress–response pathways. Hormonal crosstalk is expected to influence the defense response, growth pattern, and eventually abiotic stress tolerance in plants. Enhanced understanding of the hormones and their interactions during plant immunity sets the stage for the development of plants with improved tolerance to abiotic stress, preserving overall plant fitness (Nemhauser et al. 2006; Vos et al. 2015; Wani et al. 2016). Genetic engineering has provided new opportunities to build resistance against abiotic stress among several economically valuable crops. The effectiveness of transgenic research, however, is dependent mainly on successful plant transformation procedure to ensure that transgenes are stable within the plant genome with functional expression. A significant number of plant species have been genetically altered by rapid developments in transformation technologies (Kausch et al. 2019).
Negative modulation of known SA signaling regulator, nonexpressor of PR Genes 1 (NPR1) by post-translation mechanisms stimulated the JA signaling (Saleh et al. 2015). The DELLA proteins, considered as GA negative regulators, were also found to positively regulate the JA signaling pathways. In the absence of GA, a JA-regulating transcriptive factor MYC2 may directly interfere with DELLA protein binding to a negative JAZ, enabling the crosstalk between the JA and GA pathways (Hou et al. 2010). The hormonal crosstalk hubs or proteins associated with various hormonal signaling pathways may; therefore, be called NPR1 and DELLA proteins (Hou et al. 2010; Saleh et al. 2015).
To date, much has been reported about JA-responsive gene transcription and regulation that has been driven by the master TF known as MYC2 (Song et al. 2013, 2014). There is relatively little research regarding terminating the JA-mediated transcriptional response and their driving mechanisms. Liu et al. (2019) explicitly demonstrated that MYC2, a bHLH TF encoding JIN gene, binds to the G-box (CACGTG) and G-box-associated hexamers and can interact with many members of the JAZ repressors (Fig. 5) (Dombrecht et al. 2007; Fernández-Calvo et al. 2011). Further, it controls the termination of JA signaling by activating a specific bHLH protein group called MYC2-targeted bHLH (MTB). JA-mediated transcriptional responses are regulated negatively by these MTB proteins due to their confrontational effect on the functioning of the transcriptional activation complex MYC2-MED25 (Breeze 2019). MTB proteins impede the formation of transcriptional activation complex and contend with MYC2 to its target gene promoters for binding sites. Resultantly, MYC2 and MTB proteins develop negative feedback to stop JA signals (Zhai and Li 2019; Wang et al. 2019). Several genes have already been identified, including JA receptors, but their respective roles still need to be explored. Next-generation gene-editing tools, such as CRISPR/Cas9, have opened up new avenues for targeting MTB or related genes for engineering abiotic stress tolerance in crops.
Interestingly, JA is important for plant growth and not only act as a development regulator, but also mediate response to environmental stress. JA metabolism and signaling pathways are suitable targets for genetic engineering to achieve improvement in plants abiotic stress tolerance. The genome-editing systems like CRISPR/Cas9 have recently been one of the revolutionary technologies in modern agricultural biotechnology. CRISPR technology, which was principally adopted as being a non-GM (genetically modified) tool, could expand our efforts into the sustainable tolerance of multiple abiotic stresses in future crops (Zafar et al. 2020; Moradpour and Abdulah 2020). Moreover, yield improvement for higher agricultural production using these genome editing technologies is paramount to combat the detrimental effects of climate change on the contemporary agriculture sector (Raza et al. 2019a; Zafar et al. 2020; Moradpour and Abdulah 2020).
Engineering the JA biosynthesis pathway without creating negative side effects will be a major challenge. Furthermore, the overall genetic cascade of JA could be investigated by comparing JA enriched/deficient plants once they have been subjected to stressed or nonstressed conditions. And by figuring out the key nodes in the JA-biosynthetic pathway, whose modulation could be effective without the associated penalties for abiotic stress tolerance. In the end, it will depend on the degree of success to use those pathways for engineering abiotic stress tolerance in crop plants.
Concluding remarks and future perspectives
During the past few years, JA has gained quite an attention due to its substantial participation as a developmental and defense signaling molecule against various abiotic stresses. Most of the changes introduced by JA are linked with alteration in physiological, biochemical, and molecular mechanisms through up or down-regulation of gene expression. Altogether, most of the scientists who have examined the effects of JA under different abiotic stresses stated that these PGRs decreased the production of ROS and increased the tolerance to several abiotic stresses by enhancing antioxidant activity in plants. Notably, the mode of action of JA is not similar under a variety of environmental stresses due to the divergence of PGR signals and interactions with other hormonal signals. Many genes and TFs (activators/direct regulations, or repressors/indirect regulations) have been elaborated in the core JA signaling pathways, facilitating responses to external stress signals. The JAZ and MYC cis-regulatory elements have particularly been found to play an essential role in the JA signaling pathway via the amalgamation of supervisory TFs (MYC) and connected genes (JAZ, AOS1, AOC, LOX2, and COI1), as briefly explained in Fig. 4. So far, 13 elements have been recognized in Arabidopsis, most of which have two conserved domains, i.e., the central domain called the ZIM domain, and the C-terminal JA-connected domain (Pauwels et al. 2010; Shyu et al. 2012; Thireault et al. 2015). Nevertheless, investigation on plant perception of various stress signals followed by JA production and response to such stresses has not been fully revealed. Studies showed that there is an interaction and crosstalk (synergistic or antagonistic) between JA and other PGRs’ signaling pathways for modulation of the plant responses under normal and stressful environment. Therefore, future investigations on the exploitation of key insights into the role and regulation of JA in a combination of stresses can deliver yield promising results. In addition, JA signaling mechanisms and their functions in signaling crosstalk at organ, tissue, or cell levels are also untouched and yet to be fully understood.
In the recent years, improvements in omics approaches, such as genomics, transcriptomics, proteomics, and metabolomics have facilitated further clues on complex gene–protein interactions and linked networks. These approaches also enable a better examination of the regulatory networks of PGRs and crosstalk under many abiotic stresses. However, PGR signaling mechanisms are complex and changeable, mainly under complex and unpredictable situations. Therefore, there is a need to fully explore the potential of omics approaches for the identification of JA-related genes, proteins, and metabolites for the development of climate–resilient plants. Further, the discovery of JA-related metabolic pathways can provide new insights to fully understand the signaling network under stressful environment, and related researches should be prolonged to various growth conditions, from lab to field. Moreover, the engineered JA-mediated metabolic pathways can open new visions into the current knowledge and help to further explore the JA-mediated stress tolerance mechanisms.
References
Abidi W, Cantín CM, Jiménez S, Giménez R, Moreno MÁ, Gogorcena Y (2015) Influence of antioxidant compounds, total sugars and genetic background on the chilling injury susceptibility of a non-melting peach (Prunus persica (L.) Batsch) progeny. J Sci Food Agric 95:351–358
Abouelsaad I, Renault S (2018) Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J Plant Physiol 226:136–144
Aftab T, Khan MMA, Idrees M, Naeem M, Hashmi N (2011) Methyl jasmonate counteracts boron toxicity by preventing oxidative stress and regulating antioxidant enzyme activities and artemisinin biosynthesis in Artemisia annua L. Protoplasma 248:601–612
Aghdam MS, Sevillano L, Flores FB, Bodbodak S (2015) The contribution of biotechnology to improving post-harvest chilling tolerance in fruits and vegetables using heat-shock proteins. J Agric Sci 153:7–24
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P (2018) Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate–glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255:79–93
Ahmad P, Alyemeni MN, Wijaya L, Alam P, Ahanger MA, Alamri SA (2017) Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch Agron Soil Sci 63:1889–1899
Ahmadi F, Karimi K, Struik P (2018) Effect of exogenous application of methyl jasmonate on physiological and biochemical characteristics of Brassica napus L. cv. Talaye under salinity stress. South Afr J Bot 115:5–11
Alam MM, Nahar K, Hasanuzzaman M, Fujita M (2014) Exogenous jasmonic acid modulates the physiology, antioxidant defense and glyoxalase systems in imparting drought stress tolerance in different Brassica species. Plant Biotechnol Rep 8:279–293
Alavi-Samani SM, Kachouei MA, Pirbalouti AG (2015) Growth, yield, chemical composition, and antioxidant activity of essential oils from two thyme species under foliar application of jasmonic acid and water deficit conditions. Hortic Environ Biotechnol 56:411–420
Ali E, Hussain N, Shamsi IH, Jabeen Z, Siddiqui MH, Jiang L-x (2018) Role of jasmonic acid in improving tolerance of rapeseed (Brassica napus L.) to Cd toxicity. J Zhejiang Uni Sci B 19:130–146
Alisofi S, Einali A, Sangtarash MH (2020) Jasmonic acid-induced metabolic responses in bitter melon (Momordica charantia) seedlings under salt stress. J Hortic Sci Biotechnol 95:247–259
Anjum S, Wang L, Farooq M, Khan I, Xue L (2011) Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J Agron Crop Sci 197:296–301
Arbona V, Gómez-Cadenas A (2008) Hormonal modulation of citrus responses to flooding. J Plant Growth Regul 27:241
Asghari M, Hasanlooe AR (2015) Interaction effects of salicylic acid and methyl jasmonate on total antioxidant content, catalase and peroxidase enzymes activity in “Sabrosa” strawberry fruit during storage. Sci Hortic 197:490–495
Avalbaev A, Yuldashev R, Fedorova K, Somov K, Vysotskaya L, Allagulova C, Shakirova F (2016) Exogenous methyl jasmonate regulates cytokinin content by modulating cytokinin oxidase activity in wheat seedlings under salinity. J Plant Physiol 191:101–110
Azeem U (2018) Ameliorating nickel stress by jasmonic acid treatment in Zea mays L. Russ Agric Sci 44:209–215
Agnihotri A, Seth CS (2020) Does jasmonic acid regulate photosynthesis, clastogenecity, and phytochelatins in Brassica juncea L. in response to Pb-subcellular distribution?. Chemosphere 243:125361
Bali S, Kaur P, Kohli SK, Ohri P, Thukral AK, Bhardwaj R, Ahmad P (2018) Jasmonic acid induced changes in physio-biochemical attributes and ascorbate-glutathione pathway in Lycopersicon esculentum under lead stress at different growth stages. Sci Total Environ 645:1344–1360
Balbi V, Devoto A (2008) Jasmonate signalling network in Arabidopsis thaliana: crucial regulatory nodes and new physiological scenarios. New Phytol 177:301–318
Balfagón D, Sengupta S, Gómez-Cadenas A, Fritschi FB, Azad RK, Mittler R, Zandalinas SI (2019) Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol 181:1668–1682
Bali S, Jamwal VL, Kaur P, Kohli SK, Ohri P, Gandhi SG, Bhardwaj R, Al-Huqail AA, Siddiqui MH, Ahmad P (2019) Role of P-type ATPase metal transporters and plant immunity induced by jasmonic acid against Lead (Pb) toxicity in tomato. Ecotoxicol Environ Saf 174:283–294
Brash AR, Baertschi SW, Ingram CD, Harris TM (1988) Isolation and characterization of natural allene oxides: unstable intermediates in the metabolism of lipid hydroperoxides. Proc Natl Acad Sci 85:3382–3386
Breeze E (2019) Master MYCs: MYC2, the jasmonate signaling “master switch”. Am Soc Plant Biol 31:9–10
Bu Q, Jiang H, Li CB, Zhai Q, Zhang J, Wu X, Sun J, Xie Q, Li C (2008) Role of the Arabidopsis thaliana NAC transcription factors ANAC019 and ANAC055 in regulating jasmonic acid-signaled defense responses. Cell Res 18:756–767
Castagna A, Ederli L, Pasqualini S, Mensuali-Sodi A, Baldan B, Donnini S, Ranieri A (2007) The tomato ethylene receptor LE-ETR3 (NR) is not involved in mediating ozone sensitivity: causal relationships among ethylene emission, oxidative burst and tissue damage. New Phytol 174:342–356
Chen H-J, Fu T-Y, Yang S-L, Hsieh H-L (2018) FIN219/JAR1 and cryptochrome1 antagonize each other to modulate photomorphogenesis under blue light in Arabidopsis. PLoS Genet 14:e1007248
Chini A, Fonseca S, Fernández G, Adie B, Chico JM et al (2007) The JAZ family of repressors is the missing link in jasmonate signalling. Nature 448:666–671
Choudhary KK, Agrawal S (2014) Cultivar specificity of tropical mung bean (Vigna radiata L.) to elevated ultraviolet-B: Changes in antioxidative defense system, nitrogen metabolism and accumulation of jasmonic and salicylic acids. Environ Exp Bot 99:122–132
Cui H, Wei J, Su J, Li C, Ge F (2016) Elevated O3 increases volatile organic compounds via jasmonic acid pathway that promote the preference of parasitoid Encarsia formosa for tomato plants. Plant Sci 253:243–250
De Domenico S, Taurino M, Gallo A, Poltronieri P, Pastor V, Flors V, Santino A (2019) Oxylipin dynamics in Medicago truncatula in response to salt and wounding stresses. Physiol Plant 165:198–208
de Ollas C, Arbona V, GóMez-Cadenas A (2015) Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ 38:2157–2170
de Ollas C, Dodd IC (2016) Physiological impacts of ABA–JA interactions under water-limitation. Plant Mol Biol 91:641–650
de Ollas C, Hernando B, Arbona V, Gómez-Cadenas A (2013) Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol Plant 147:296–306
Degu A, Ayenew B, Cramer GR, Fait A (2016) Polyphenolic responses of grapevine berries to light, temperature, oxidative stress, abscisic acid and jasmonic acid show specific developmental-dependent degrees of metabolic resilience to perturbation. Food Chem 212:828–836
Demkura PV, Abdala G, Baldwin IT, Ballaré CL (2010) Jasmonate-dependent and-independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiol 152:1084–1095
Dhakarey R, Raorane ML, Treumann A, Peethambaran PK, Schendel RR, Sahi VP, Hause B, Bunzel M, Henry A, Kohli A (2017) Physiological and proteomic analysis of the rice mutant cpm2 suggests a negative regulatory role of jasmonic acid in drought tolerance. Front Plant Sci 8:1903
Ding H, Lai J, Wu Q, Zhang S, Chen L, Dai Y-S, Wang C, Du J, Xiao S, Yang C (2016) Jasmonate complements the function of Arabidopsis lipoxygenase 3 in salinity stress response. Plant Sci 244:1–7
Ding Y, Shi Y, Yang S (2019) Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol 222:1690–1704
Dombrecht B, Gang PX, Sprague SJ et al (2007) MYC2 differentially modulates diverse jasmonate-dependent functions in Arabidopsis. Plant Cell 19:2225–2245
Edelstein M, Ben-Hur M (2018) Heavy metals and metalloids: sources, risks and strategies to reduce their accumulation in horticultural crops. Sci Hortic 234:431–444
Ellouzi H, Hamed KB, Asensi-Fabado MA, Müller M, Abdelly C, Munné-Bosch S (2013) Drought and cadmium may be as effective as salinity in conferring subsequent salt stress tolerance in Cakile maritima. Planta 237:1311–1323
Escobar-Bravo R, Chen G, Kim HK, Grosser K, van Dam NM, Leiss KA, Klinkhamer PG (2019) Ultraviolet radiation exposure time and intensity modulate tomato resistance to herbivory through activation of jasmonic acid signaling. J Exp Bot 70:315–327
Faghih S, Ghobadi C, Zarei A (2017) Response of strawberry plant cv. ‘Camarosa’to salicylic acid and methyl jasmonate application under salt stress condition. J Plant Growth Regul 36:651–659
Farhangi-Abriz S, Alaee T, Tavasolee A (2019) Salicylic acid but not jasmonic acid improved canola root response to salinity stress. Rhizosphere 9:69–71
Farhangi-Abriz S, Ghassemi-Golezani K (2018) How can salicylic acid and jasmonic acid mitigate salt toxicity in soybean plants? Ecotoxicol Environ Saf 147:1010–1016
Farooq MA, Islam F, Yang C, Nawaz A, Gill RA, Ali B, Song W, Zhou W (2018) Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate–glutathione cycle in oilseed rape roots. Plant Growth Regul 84:135–148
Fedina I, Nedeva D, Georgieva K, Velitchkova M (2009) Methyl jasmonate counteract Uv-B Stress in barley seedlings. J Agron Crop Sci 195:204–212
Fernández-Calvo P, Chini A, Fernández-Barbero G et al (2011) The Arabidopsis bHLH transcription factors MYC3 and MYC4 are targets of JAZ repressors and act additively with MYC2 in the activation of jasmonate responses. Plant Cell 23:701–715
Fonseca S, Chini A, Hamberg M, Adie B, Porzel A, Kramell R, Miersch O, Wasternack C, Solano R (2009) (+)-7-iso-Jasmonoyl-l-isoleucine is the endogenous bioactive jasmonate. Nat Chem Biol 5:344–350
Fukao T, Barrera-Figueroa BE, Juntawong P, Peña-Castro JM (2019) Submergence and waterlogging stress in plants: a review highlighting research opportunities and understudied aspects. Front Plant Sci 10:340
Fujimoto SY, Ohta M, Usui A, Shinshi H, Ohme-Takagi M (2000) Arabidopsis ethylene-responsive element binding factors act as transcriptional activators or repressors of GCC box-mediated gene expression. Plant Cell 12:393–404
Ghaffari H, Tadayon MR, Nadeem M, Razmjoo J, Cheema M (2020) Foliage applications of jasmonic acid modulate the antioxidant defense under water deficit growth in sugar beet. Spanish J Agric Res 17:0805
Ghasemnezhad M, Javaherdashti M (2008) Effect of methyl jasmonate treatment on antioxidant capacity, internal quality and postharvest life of raspberry fruit. Caspian J Environ Sci 6:73–78
Gao QM, Venugopal S, Navarre D, Kachroo A (2011) Low oleic acid-derived repression of jasmonic acid-inducible defense responses requires the WRKY50 and WRKY51 proteins. Plant Physiol 155:464–476
Goossens J, Mertens J, Goossens A (2017) Role and functioning of bHLH transcription factors in jasmonate signalling. J Exp Bot 68:1333–1347
Grunewald W, Vanholme B, Pauwels L, Plovie E, Inze D, Gheysen G, Goossens A (2009) Expression of the Arabidopsis jasmonate signalling repressor JAZ1/TIFY10A is stimulated by auxin. EMBO Rep 10:923–928
Habibi F, Ramezanian A, Rahemi M, Eshghi S, Guillén F, Serrano M, Valero D (2019) Postharvest treatments with γ-aminobutyric acid, methyl jasmonate, or methyl salicylate enhance chilling tolerance of blood orange fruit at prolonged cold storage. J Sci Food Agric 99:6408–6417
Hanaka A, Wójcik M, Dresler S, Mroczek-Zdyrska M, Maksymiec W (2016) Does methyl jasmonate modify the oxidative stress response in Phaseolus coccineus treated with Cu? Ecotoxicol Environ Saf 124:480–488
Haque ME, Abe F, Mori M, Oyanagi A, Komatsu S, Kawaguchi K (2014) Characterization of a wheat pathogenesis-related protein, TaBWPR-1.2, in seminal roots in response to waterlogging stress. J Plant Physiol 171:602–609
Harfouche AL, Jacobson DA, Kainer D, Romero JC, Harfouche AH, Mugnozza GS, Moshelion M, Tuskan GA, Keurentjes JJ, Altman A (2019) Accelerating climate resilient plant breeding by applying next-generation artificial intelligence. Trends Biotechnol 37:1217–1235
Hasanuzzaman M, Bhuyan MB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9:681
Hasanuzzaman M, Nahar K, Alam M, Roychowdhury R, Fujita M (2013) Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int J Mol Sci 14:9643–9684
Hou X, Lee LYC, Xia K, Yan Y, Yu H (2010) DELLAs modulate jasmonate signaling via competitive binding to JAZs. Dev Cell 19:884–894
Howe GA, Major IT, Koo AJ (2018) Modularity in jasmonate signaling for multistress resilience. Ann Rev Plant Biol 69:387–415
Hu Y, Jiang Y, Han X, Wang H, Pan J, Yu D (2017) Jasmonate regulates leaf senescence and tolerance to cold stress: crosstalk with other phytohormones. J Exp Bot 68:1361–1369
Hu Y, Jiang L, Wang F, Yu D (2013) Jasmonate regulates the INDUCER OF CBF expression-C-repeat binding factor/dre binding factor1 cascade and freezing tolerance in Arabidopsis. Plant Cell 25:2907–2924
Huang X, Li J, Shang H, Meng X (2015) Effect of methyl jasmonate on the anthocyanin content and antioxidant activity of blueberries during cold storage. J Sci Food Agric 95:337–343
Ilyas N, Gull R, Mazhar R, Saeed M, Kanwal S, Shabir S, Bibi F (2017) Influence of salicylic acid and jasmonic acid on wheat under drought stress. Commun Soil Sci Plant Anal 48:2715–2723
Jang G, Choi YD (2018) Drought stress promotes xylem differentiation by modulating the interaction between cytokinin and jasmonic acid. Plant Signal Behav 13:e1451707
Jiang M, Xu F, Peng M, Huang F, Meng F (2016) Methyl jasmonate regulated diploid and tetraploid black locust (Robinia pseudoacacia L.) tolerance to salt stress. Acta Physiol Plant 38:106
Jiang Y, Liang G, Yang S, Yu D (2014) Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell 26:230–245
Jing Y, Lin R (2015) The VQ motif-containing protein family of plant-specific transcriptional regulators. Plant Physiol 169:371–378
Jimenez-Aleman GH, Machado RA, Görls H, Baldwin IT, Boland W (2015) Synthesis, structural characterization and biological activity of two diastereomeric JA-Ile macrolactones. Org Biomol Chem 13:5885–5893
Kamal AHM, Komatsu S (2016) Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J Proteom 133:33–47
Kamińska M, Tretyn A, Trejgell A (2018) Effect of jasmonic acid on cold-storage of Taraxacum pieninicum encapsulated shoot tips. Plant Cell Tiss Org Cult 135:487–497
Kanna M, Tamaoki M, Kubo A, Nakajima N, Rakwal R, Agrawal GK, Tamogami S, Ioki M, Ogawa D, Saji H (2003) Isolation of an ozone-sensitive and jasmonate-semi-insensitive Arabidopsis mutant (OJI1). Plant Cell Physiol 44:1301–1310
Karaman S, Ozturk B, Genc N, Celik S (2013) Effect of preharvest application of methyl jasmonate on fruit quality of plum (Prunus Salicina L. indell cv. “Fortune”) at harvest and during cold storage. J Food Process Preserv 37:1049–1059
Kausch AP, Nelson-Vasilchik K, Hague J, Mookkan M, Quemada H, Dellaporta S, Fragoso C, Zhang ZJ (2019) Edit at will: Genotype independent plant transformation in the era of advanced genomics and genome editing. Plant Sci 281:186–205
Khan N, Bano A, Ali S, Babar MA (2020) Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul 90:189–203
Ku Y-S, Sintaha M, Cheung M-Y, Lam H-M (2018) Plant hormone signaling crosstalks between biotic and abiotic stress responses. Int J Mol Sci 19:3206
Kloth KJ, Busscher-Lange WGL, J, Van Haarst JC, Kruijer W, Bouwmeester HJ, Dicke M, Jongsma MA, (2016) AtWRKY22 promotes susceptibility to aphids and modulates salicylic acid and jasmonic acid signalling. J Exp Bot 67:3383–3396
Lackman P, González-Guzmán M, Tilleman S, Carqueijeiro I, Pérez AC, Moses T, Seo M, Kanno Y, Häkkinen ST, Van Montagu MC (2011) Jasmonate signaling involves the abscisic acid receptor PYL4 to regulate metabolic reprogramming in Arabidopsis and tobacco. Proc Natl Acad Sci 108:5891–5896
Lei GJ, Sun L, Sun Y, Zhu XF, Li GX, Zheng SJ (2020) Jasmonic acid alleviates cadmium toxicity in Arabidopsis via suppression of cadmium uptake and translocation. J Integr Plant Biol 62:218–227
Li J, Zhong R, Palva ET (2017) WRKY70 and its homolog WRKY54 negatively modulate the cell wall-associated defenses to necrotrophic pathogens in Arabidopsis. PLoS ONE 12:e0183731
Li X, Zhang L, Ahammed GJ, Li Y-T, Wei J-P, Yan P, Zhang L-P, Han X, Han W-Y (2019) Salicylic acid acts upstream of nitric oxide in elevated carbon dioxide-induced flavonoid biosynthesis in tea plant (Camellia sinensis L.). Environ Exp Bot 161:367–374
Liu X, Chi H, Yue M, Zhang X, Li W, Jia E (2012) The regulation of exogenous jasmonic acid on UV-B stress tolerance in wheat. J Plant Growth Regul 31:436–447
Liu X, Tang S, Dou Z, Li G, Liu Z, Wang S, Ding C, Ding Y (2016) Effects of MeJA on the physiological characteristics of japonica rice wuyunjing 24 and ningjing 3 during early grain filling stage under heat stress. Chin J Rice Sci 30:291–303
Liu Y, Du M, Deng L, Shen J, Fang M, Chen Q, Lu Y, Wang Q, Li C, Zhai Q (2019) MYC2 regulates the termination of jasmonate signaling via an autoregulatory negative feedback loop. Plant Cell 31:106–127
Manan A, Ayyub C, Pervez MA, Ahmad R (2016) Methyl jasmonate brings about resistance against salinity stressed tomato plants by altering biochemical and physiological processes. Pak J Agric Sci 53:35–41
Marriboina S, Attipalli RR (2020) Hydrophobic cell-wall barriers and vacuolar sequestration of Na+ ions are among the key mechanisms conferring high salinity tolerance in a biofuel tree species,Pongamia pinnata L. pierre. Environ Exp Bot 171:103949
Meng X, Han J, Wang Q, Tian S (2009) Changes in physiology and quality of peach fruits treated by methyl jasmonate under low temperature stress. Food Chem 114:1028–1035
Mir MA, Sirhindi G, Alyemeni MN, Alam P, Ahmad P (2018) Jasmonic acid improves growth performance of soybean under nickel toxicity by regulating nickel uptake, redox balance, and oxidative stress metabolism. J Plant Growth Regul 37:1195–1209
Mohamed HI, Latif HH (2017) Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol Mol Biol Plant 23:545–556
Moradpour M, Abdulah SNA (2020) CRISPR/dCas9 platforms in plants: strategies and applications beyond genome editing. Plant Biotechnol J 18:32–44
Müller M, Munné-Bosch S (2015) Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169:32–41
Mustafa MA, Ali A, Seymour G, Tucker G (2016) Enhancing the antioxidant content of carambola (Averrhoa carambola) during cold storage and methyl jasmonate treatments. Postharv Biol Technol 118:79–86
Mustafa MA, Ali A, Seymour G, Tucker G (2018) Treatment of dragonfruit (Hylocereus polyrhizus) with salicylic acid and methyl jasmonate improves postharvest physico-chemical properties and antioxidant activity during cold storage. Sci Hortic 231:89–96
Nafie E, Hathout T, Mokadem A, Shyma A (2011) Jasmonic acid elicits oxidative defense and detoxification systems in Cucumis melo L. cells. Braz J Plant Physiol 23:161–174
Nahar K, Kyndt T, Hause B, Höfte M, Gheysen G (2013) Brassinosteroids suppress rice defense against root-knot nematodes through antagonism with the jasmonate pathway. Mol Plant Microb Int 26:106–115
Nemhauser JL, Hong F, Chory J (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126:467–475
Nemes R, Koltai E, Taylor AW, Suzuki K, Gyori F, Radak Z (2018) Reactive oxygen and nitrogen species regulate key metabolic, anabolic, and catabolic pathways in skeletal muscle. Antioxidants 7:85
Ouli-Jun ZCH, Zhou-Bin L, Ge W, Bo-Zhi Y, Xue-Xiao Z (2017) Mitigation of waterlogging-induced damages to pepper by exogenous MeJA. Pak J Bot 49:1127–1135
Parmoon G, Ebadi A, Jahanbakhsh S, Hashemi M (2019) Physiological response of fennel (Foeniculum vulgare Mill.) to drought stress and plant growth regulators. Russ J Plant Physiol 66:795–805
Pedranzani H, Racagni G, Alemano S, Miersch O, Ramírez I, Peña-Cortés H, Taleisnik E, Machado-Domenech E, Abdala G (2003) Salt tolerant tomato plants show increased levels of jasmonic acid. Plant Growth Regul 41:149–158
Pellegrini E, Trivellini A, Campanella A, Francini A, Lorenzini G, Nali C, Vernieri P (2013) Signaling molecules and cell death in Melissa officinalis plants exposed to ozone. Plant Cell Rep 32:1965–1980
Pauwels L, Goossens A (2011) The JAZ proteins: a crucial interface in the jasmonate signaling cascade. Plant Cell 23:3089–3100
Pauwels L, Barbero GF, Geerinck J, Tilleman S, Grunewald W, Pérez A et al (2010) NINJA connects the co-repressor TOPLESS to jasmonate signalling. Nature 464:788–791
Per TS, Khan NA, Masood A, Fatma M (2016) Methyl jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front Plant Sci 7:1933
Per TS, Khan MIR, Anjum NA, Masood A, Hussain SJ, Khan NA (2018) Jasmonates in plants under abiotic stresses: crosstalk with other phytohormones matters. Environ Exp Bot 145:104–120
Qi J, Zhang M, Lu C, Hettenhausen C, Tan Q, Cao G, Zhu X, Wu G, Wu J (2018) Ultraviolet-B enhances the resistance of multiple plant species to lepidopteran insect herbivory through the jasmonic acid pathway. Sci Rep 8:1–9
Qi T, Song S, Ren Q, Wu D, Huang H, Chen Y, Fan M, Peng W, Ren C, Xie D (2011) The Jasmonate-ZIM-domain proteins interact with the WD-repeat/bHLH/MYB complexes to regulate Jasmonate-mediated anthocyanin accumulation and trichome initiation in Arabidopsis thaliana. Plant Cell 23:1795–1814
Radhakrishnan R, Lee I-J (2013) Spermine promotes acclimation to osmotic stress by modifying antioxidant, abscisic acid, and jasmonic acid signals in soybean. J Plant Growth Regul 32:22–30
Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y, Xu J (2019a) Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants 8:34
Raza A, Mehmood SS, Tabassum J, Batool R (2019b) Targeting plant hormones to develop abiotic stress resistance in wheat. In: Wheat production in changing environments. Springer, pp 557–577
Raza A, Ashraf F, Zou X, Zhang X, Tosif H (2020a) Plant Adaptation and Tolerance to Environmental Stresses: Mechanisms and Perspectives. In: Plant Ecophysiology and Adaptation under Climate Change: Mechanisms and Perspectives I. Springer, pp 117–145
Raza A, Habib M, Kakavand SN, Zahid Z, Zahra N, Sharif R, Hasanuzzaman M (2020b) Phytoremediation of cadmium: physiological, biochemical, and molecular mechanisms. Biology 9:177
Ren Q, Sun Y, Guo H, Wang C, Li C, Ge F (2015) Elevated ozone induces jasmonic acid defense of tomato plants and reduces midgut proteinase activity in Helicoverpa armigera. Entomol Exp Appl 154:188–198
Ruan J, Zhou Y, Zhou M, Yan J, Khurshid M, Weng W, Cheng J, Zhang K (2019) Jasmonic acid signaling pathway in plants. Int J Mol Sci 20:2479
Saleh A, Withers J, Mohan R, Marqués J, Gu Y, Yan S, Zavaliev R, Nomoto M, Tada Y, Dong X (2015) Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 18:169–182
Sánchez-Romera B, Ruiz-Lozano JM, Li G, Luu DT, Martínez-Ballesta MDC, Carvajal M, Zamarreño AM, García-Mina JM, Maurel C, Aroca R (2014) Enhancement of root hydraulic conductivity by methyl jasmonate and the role of calcium and abscisic acid in this process. Plant Cell Environ 37:995–1008
Savchenko T, Kolla VK, Wang CQ et al (2014) Functional convergence of oxylipin and abscisic acid pathways controls stomatal closure in response to drought. Plant Physiol 164:1151–1160
Siddiqui MH, Alamri S, Khan MN, Corpas FJ, Al-Amri AA, Alsubaie QD, Ali HM, Kalaji HM, Ahmad P (2020) Melatonin and calcium function synergistically to promote the resilience through ROS metabolism under arsenic-induced stress. J Hazard Mat 15:122882
Siddiqui MH, Al-Khaishany MY, Al-Qutami MA, Al-Whaibi MH, Grover A, Ali HM, Al-Wahibi MS (2015) Morphological and physiological characterization of different genotypes of faba bean under heat stress. Saudi J Biol Sci 22:656–663
Sayyari M, Babalar M, Kalantari S, Martínez-Romero D, Guillén F, Serrano M, Valero D (2011) Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem 124:964–970
Seo JS, Joo J, Kim MJ, Kim YK, Nahm BH, Song SI, Cheong JJ, Lee JS, Kim JK, Choi YD (2011) OsbHLH148, a basic helix-loop-helix protein, interacts with OsJAZ proteins in a jasmonate signaling pathway leading to drought tolerance in rice. Plant J 65:907–921
Shahzad A, Pitann B, Ali H, Qayyum M, Fatima A, Bakhat H (2015) Maize genotypes differing in salt resistance vary in jasmonic acid accumulation during the first phase of salt stress. J Agron Crop Sci 201:443–451
Shahzad R, Waqas M, Khan AL, Hamayun M, Kang S-M, Lee I-J (2015) Foliar application of methyl jasmonate induced physio-hormonal changes in Pisum sativum under diverse temperature regimes. Plant Physiol Biochem 96:406–416
Sharma M, Laxmi A (2016) Jasmonates: emerging players in controlling temperature stress tolerance. Front Plant Sci 6:1129
Shyu C, Figueroa P, de Pew CL, Cooke TF, Sheard LB, Moreno JE, Katsir L, Zheng N, Browse J, Howea GA (2012) JAZ8 lacks a canonical degron and has an EAR motif that mediates transcriptional repression of jasmonate responses in Arabidopsis. Plant Cell 24:536–550
Singh I, Shah K (2014) Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 108:57–66
Sirhindi G, Mir MA, Abd-Allah EF, Ahmad P, Gucel S (2016) Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front Plant Sci 7:591
Sirhindi G, Mir MA, Sharma P, Gill SS, Kaur H, Mushtaq R (2015) Modulatory role of jasmonic acid on photosynthetic pigments, antioxidants and stress markers of Glycine max L. under nickel stress. Physiol Mol Biol Plants 21:559–565
Song S, Huang H, Gao H, Wang J, Wu D, Liu X, Yang S, Zhai Q, Li C, Qi T (2014) Interaction between MYC2 and ETHYLENE INSENSITIVE3 modulates antagonism between jasmonate and ethylene signaling in Arabidopsis. Plant Cell 26:263–279
Song S, Qi T, Fan M, Zhang X, Gao H, Huang H, Wu D, Guo H, Xie D (2013) The bHLH subgroup IIId factors negatively regulate jasmonate-mediated plant defense and development. PLoS Genet 9:e1003653
Sofy MR, Seleiman MF, Alhammad BA, Alharbi BM, Mohamed HI (2020) Minimizing adverse effects of pb on maize plants by combined treatment with jasmonic, salicylic acids and proline. Agronomy 10:699
Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Métraux JP, Brown R, Kazan K, Van Loon LC (2003) NPR1 modulates cross-talk between salicylate-and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15:760–770
Talebi M, Moghaddam M, Pirbalouti AG (2018) Methyl jasmonate effects on volatile oil compounds and antioxidant activity of leaf extract of two basil cultivars under salinity stress. Acta Physiol Plant 40:34
Tamaoki M, Nakajima N, Kubo A, Aono M, Matsuyama T, Saji H (2003) Transcriptome analysis of O 3-exposed Arabidopsis reveals that multiple signal pathways act mutually antagonistically to induce gene expression. Plant Mol Biol 53:443–456
Takeuchi K, Gyohda A, Tominaga M, Kawakatsu M, Hatakeyama A, Ishii N, Shimaya K, Nishimura T, Riemann M, Nick P, Hashimoto M (2011) RSOsPR10 expression in response to environmental stresses is regulated antagonistically by jasmonate/ethylene and salicylic acid signaling pathways in rice roots. Plant Cell Physiol 52:1686–1696
Tayyab N, Naz R, Yasmin H, Nosheen A, Keyani R, Sajjad M, Hassan MN, Roberts TH (2020) Combined seed and foliar pre-treatments with exogenous methyl jasmonate and salicylic acid mitigate drought-induced stress in maize. PLoS ONE 15:e0232269
Thireault C, Shyu C, Yoshida Y, St Aubin B, Campos ML, Howe GA (2015) Repression of jasmonate signaling by a non-TIFY JAZ protein in Arabidopsis. Plant J 82:669–679
Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, Liu G, Nomura K, He SY, Howe GA, Browse J (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448:661–665
Todaka D, Shinozaki K, Yamaguchi-Shinozaki K (2015) Recent advances in the dissection of drought-stress regulatory networks and strategies for development of drought-tolerant transgenic rice plants. Front Plant Sci 6:84
Tuominen H, Overmyer K, KeinaÈnen M, Kollist H, Kangasjärvi J (2004) Mutual antagonism of ethylene and jasmonic acid regulates ozone-induced spreading cell death in Arabidopsis. Plant J 39:59–69
Ullah I, Waqas M, Khan MA, Lee I-J, Kim W-C (2017) Exogenous ascorbic acid mitigates flood stress damages of Vigna angularis. Appl Biol Chem 60:603–614
Ulloa-Inostroza EM, Alberdi M, Meriño-Gergichevich C, Reyes-Díaz M (2017) Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum. Plant Soil 412:81–96
Van der Does D, Leon-Reyes A, Koornneef A, Van Verk MC, Rodenburg N, Pauwels L, Goossens A, Körbes AP, Memelink J, Ritsema T, Van Wees SC (2013) Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 25:744–761
Vick BA, Zimmerman DC (1983) The biosynthesis of jasmonic acid: a physiological role for plant lipoxygenase. Biochem Biophys Res Commun 111:470–477
Vos IA, Moritz L, Pieterse CM, Van Wees S (2015) Impact of hormonal crosstalk on plant resistance and fitness under multi-attacker conditions. Front Plant Sci 6:639
Vanhaelewyn L, Prinsen E, Van Der Straeten D, Vandenbussche F (2016) Hormone controlled UV-B responses in plants. J Exp Bot 67:4469–4482
Wang F, Yu G, Liu P (2019) Transporter-mediated subcellular distribution in the metabolism and signaling of jasmonates. Front Plant Sci 10:390
Wang J, Song L, Gong X, Xu J, Li M (2020) Functions of jasmonic acid in plant regulation and response to abiotic stress. Int J Mol Sci 21:1446
Wani SH, Kumar V, Shriram V, Sah SK (2016) Phytohormones and their metabolic engineering for abiotic stress tolerance in crop plants. Crop J 4:162–176
Wasternack C, Strnad M (2018) Jasmonates: News on occurrence, biosynthesis, metabolism and action of an ancient group of signaling compounds. Int J Mol Sci 19:2539
Wasternack C, Xie D (2010) The genuine ligand of a jasmonic acid receptor: improved analysis of jasmonates is now required. Plant Signal Behav 5:337–340
Wu H, Wu X, Li Z, Duan L, Zhang M (2012) Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J Plant Growth Regul 31:113–123
Xu Y-H, Liao Y-C, Zhang Z, Liu J, Sun P-W, Gao Z-H, Sui C, Wei J-H (2016) Jasmonic acid is a crucial signal transducer in heat shock induced sesquiterpene formation in Aquilaria sinensis. Sci Rep 6:21843
Yan Z, Chen J, Li X (2013) Methyl jasmonate as modulator of Cd toxicity in Capsicum frutescens var. fasciculatum seedlings. Ecotoxicol Environ Saf 98:203–209
Yan Z, Zhang W, Chen J, Li X (2015) Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol Plant 59:373–381
Yang CJ, Zhang C, Lu YN, Jin JQ, Wang XL (2011) The mechanisms of brassinosteroids' action: from signal transduction to plant development. Mol Plant 4:588–600
Yang YX, Wu C, Ahammed GJ, Wu C, Yang Z, Wan C, Chen J (2018) Red light-induced systemic resistance against root-knot nematode is mediated by a coordinated regulation of salicylic acid, jasmonic acid and redox signaling in watermelon. Front Plant Sci 9:899. https://doi.org/10.3389/fpls.2018.00899
Yosefi A, Akbar Mozafari A, Javadi T (2020) Jasmonic acid improved in vitro strawberry (Fragaria × ananassa Duch.) resistance to PEG-induced water stress. Plant Cell Tiss Org Cult 142:549–558
Yao Y, You J, Ou Y, Ma J, Wu X, Xu G (2015) Ultraviolet-B protection of ascorbate and tocopherol in plants related with their function on the stability on carotenoid and phenylpropanoid compounds. Plant Physiol Biochem 90:23–31
Yu L, Liu H, Shao X, Yu F, Wei Y, Ni Z, Xu F, Wang H (2016) Effects of hot air and methyl jasmonate treatment on the metabolism of soluble sugars in peach fruit during cold storage. Postharvest Biol Technol 113:8–16
Zafar SA, Zaidi SS, Gaba Y, Singla-Pareek SL, Dhankher OP, Li X, Mansoor S, Pareek A (2020) Engineering abiotic stress tolerance via CRISPR/Cas-mediated genome editing. J Exp Bot 71:470–479
Zaid A, Mohammad F (2018) Methyl jasmonate and nitrogen interact to alleviate cadmium stress in Mentha arvensis by regulating physio-biochemical damages and ROS detoxification. J Plant Growth Regul 37:1331–1348
Zhai Q, Li C (2019) The plant Mediator complex and its role in jasmonate signaling. J Exp Bot 70:3415–3424
Zhao S, Ma Q, Xu X, Li G, Hao L (2016) Tomato jasmonic acid-deficient mutant spr2 seedling response to cadmium stress. J Plant Growth Regul 35:603–610
Zhu Z, An F, Feng Y, Li P, Xue L, Mu A, Jiang Z, Kim J-M, To TK, Li W (2011) Derepression of ethylene-stabilized transcription factors (EIN3/EIL1) mediates jasmonate and ethylene signaling synergy in Arabidopsis. Proc Natl Acad Sci 108:12539–12544
Acknowledgements
The authors are thankful to the scientists whose excellent work has been cited in this study which helped us to gain insight into the presented area and ultimately help us to prepare an up-to-date review.
Funding
There was no external funding for this research.
Author information
Authors and Affiliations
Contributions
AR conceived the idea. All authors equally contributed in writing. AR and MSM prepared the figures. AR, SC, ZZ, and MSM prepared the tables. AR, MHS, and MH, proofread and improved the manuscript. All authors have read and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Additional information
Communicated by Neal Stewart.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Raza, A., Charagh, S., Zahid, Z. et al. Jasmonic acid: a key frontier in conferring abiotic stress tolerance in plants. Plant Cell Rep 40, 1513–1541 (2021). https://doi.org/10.1007/s00299-020-02614-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-020-02614-z