Abstract
Experiments were carried out to investigate the role of nitric oxide (NO) in ameliorating the negative effects of cadmium stress in tomato seedlings. Plants treated with cadmium (CdCl2, 150 μM) showed reduced growth, biomass yield, pigment content, chlorophyll fluorescence, and gas exchange parameters. Exogenous application of NO donor (sodium nitroprusside) with nutrient solution protected chlorophyll pigments, restored chlorophyll fluorescence and gas exchange parameters, and caused significant enhancements in growth and biomass yield. Cadmium triggered the synthesis of proline and glycine betaine; however, application of NO caused further enhancement of their accumulation, reflecting an obvious amelioration of the cadmium-induced decline in relative water content. Activities of the antioxidant enzymes superoxide dismutase, catalase, ascorbate peroxidase, and glutathione reductase, monodehydroascorbate reductase, dehydroascorbate reductase, and other enzymatic activities of ascorbate-glutathione cycle were enhanced following the application of NO, as compared with those in untreated seedlings under control and cadmium stress conditions. NO increased the flavonoid and total phenol content in Cd-stressed tomato plants. Moreover, NO application restricted the uptake of cadmium and enhanced the accumulation of nutrients in different parts of tomato plants. On the basis of the findings of the present study, we propose that NO has a potential role as a growth promoter for tomato under cadmium stress.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Heavy metal pollution is a major global problem that causes marked reductions in plant growth and crop yield (Singh and Prasad 2014). Cadmium (Cd) is a non-essential heavy metal that exhibits high solubility and is easily available and readily absorbed by plants. The high toxicity of cadmium can be ascribed to its high mobility between soil and plant systems (Groppa et al. 2012). Cd pollution can originate from both natural and anthropogenic sources, including weathering of metal-rich rocks, mining, power stations, mineral fertilizers (particularly phosphate fertilizer), and excessive use of wastewater and sewage sludge for agricultural purposes (Zoffoli et al. 2013). Consuming Cd-containing food leads to renal disorders and the development of weak bones (Horiguchi et al. 2010). Once Cd enters the plant system, visible symptoms, including necrosis and reduced root and shoot growth, start to appear and eventually lead to phytotoxicity (Dias et al. 2013). Cd inactivates several enzymes through its strong ability to bind with cysteine sulfhydryl thiol group (Mendoza-Cozatl et al. 2005). Cd impedes key physiological and biochemical processes, including photosynthetic and respiratory electron transport. Moreover, it severely affects mineral nutrition and water uptake (Ahmad et al. 2011; Hameed et al. 2016). In affecting electron transport systems, Cd promotes the generation of toxic reactive oxygen species (ROS) by leaking electrons to molecular oxygen, thereby inducing oxidative stress (Ahmad et al. 2011). Behaving as a non-redox-active metal, Cd generates ROS through interference with redox homeostasis (Cuypers et al. 2011).
Several endogenous tolerance mechanisms are triggered in plants to avert metal-induced oxidative stress: efficient partitioning and compartmentation of toxic metal ions into vacuoles (Wu and Wang 2011); excess synthesis of phytochelatins and formation of phytochelatin-metal complexes for mediating the exclusion into vacuoles; accumulation of osmotic solutes; and increased activities of antioxidants (Ahmad et al. 2011; Ahanger et al. 2014). Partitioning and compartmentation help plants to maintain toxic ions and substances within physiological limits. For rapid neutralizing and scavenging of ROS, plants upregulate their antioxidant defense system, which includes enzymatic as well as non-enzymatic antioxidants (Ahmad et al. 2011; Ahanger et al. 2015). Superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APX), glutathione reductase (GR), monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase (DHAR) are among the important antioxidant enzymes. The enzymes involved in ascorbate-glutathione cycle are effective in mitigating Cd stress in plants (Wu et al. 2015).
Nitric oxide (NO) is now recognized for its well-known role in signaling and has been reported to have growth-promoting properties and ultimately improves plant productivity (Fatma and Khan 2014). Over the past few years, many studies have examined the role of exogenous NO in regulating growth under normal and abiotic stress conditions, including those induced by Cu, Ni, and Cd (Fatma and Khan 2014; Ahmad et al. 2016). Hermes et al. (2013) have observed the altered expression of genes in response to exogenous application of NO for counteracting oxidative stress. However, the actual mechanism underlying NO-induced stress tolerance remains largely unidentified. In the present study, we therefore sought to elucidate this mechanism by examining the alleviation of cadmium stress promoted by exogenous application of NO.
Tomato (Solanum lycopersicum L.) belongs to family Solanaceae and has the highest commercial consumption among vegetables. It is a rich source of important nutrients, such as β-carotene, lycopene, flavonoids, and ascorbic acid, making it an effective antioxidative and anticarcinogenic food. However, on a global scale, cadmium pollution causes a significant reduction in the growth and productivity of tomato. Accordingly, the present study was undertaken to investigate the role of exogenously applied NO in mitigating cadmium-induced toxicity in tomato.
Materials and methods
Seeds of tomato (S. lycopersicum L.) were sterilized using 5% NaOCl for 5 min. Sterilized seeds were sown in pots filled with sand, perlite, and peat with ratio of 1:1:1. After germination, seedlings were thinned to a uniform number of one seedling per pot. From the time of sowing up to 10 days of seedling growth, pots were supplemented with 200 mL full-strength Hoagland solution every alternate day. After 10 days, seedlings were treated with cadmium (150 μM CdCl2) in the form of modified Hoagland solution. Sodium nitroprusside was used as source of NO (100 μM) donor and was applied with Hoagland solution every alternate day to plants after 1 week of Cd treatment up to 40 days. Pots were maintained in a greenhouse under natural climatic conditions with day/night temperatures of 26 ± 2/15 ± 2 °C, a relative humidity of 70–75%, and an average photoperiod of 18 h light and 6 h dark. After 40 days of treatment, the plants were carefully uprooted and analyzed for different parameters. The biochemical and antioxidant activity estimation was performed in uppermost young leaves.
Estimation of shoot/root length and dry biomass
Shoot and root lengths were measured immediately after uprooting the plants using a manual scale. For the estimation of dry weight, leaves were oven-dried at 70 °C for 24 h.
Estimation of photosynthetic pigments
Pigment content was determined by extracting fresh leaf tissues with acetone (80%) and recording the absorbance at 480, 645, and 663 nm using a spectrophotometer (Arnon 1949).
Estimation of chlorophyll fluorescence and gas exchange parameters
A PAM chlorophyll fluorimeter (H. Walz, Effeltrich, Germany) were adopted for the determination of chlorophyll fluorescence parameters in fully expanded leaves using the method of Li et al. (2007).
An infrared gas analyzer (LCA-4 model, Analytical Development Company, Hoddesdon, England) were used to determine the net photosynthetic rate (Pn), CO2 assimilation rate (A), stomatal conductance (gs), and transpiration rate (E) in upper leaves of the seedling.
Determination of RLWC
Relative leaf water content (RLWC) was estimated in accordance with the method described by Yamasaki and Dillenburg (1999). Briefly, 20 leaf disks were punched from the uppermost fresh leaves and their initial fresh weight was measured. Thereafter, the disks were floated on distilled water for 1 h to record the turgid weight and were then oven-dried for 24 h before recording the dry weight. RLWC was calculated using the following formula:
Estimation of proline and glycine betaine
Proline was estimated by following the method of Bates et al. (1973). A 500-mg leaf sample was macerated in sulfosalicylic acid and centrifuged at 10,000×g for 10 min. Two milliliters of the supernatant was reacted with acid ninhydrin and glacial acetic acid at 100 °C for 1 h. Thereafter, samples were placed on ice and proline in the samples was extracted using toluene. Absorbance was recorded at 520 nm. Amounts of proline, expressed as μM proline g−1 FW, were determined from a standard curve.
For estimation of glycine betaine, 500 mg dry plant material was extracted in deionized water (20 mL) after shaking for 24 h at 25 °C. Thereafter, the samples were filtered and then mixed with 2 N H2SO4. A aliquot (0.5-Ml) of the resultant mixture was reacted with cold KI–I2 reagent (0.20 mL) and centrifuged at 10,000×g for 15 min after shaking. The resulting supernatant was carefully removed, and to this, 1,2-dichloroethane was added to dissolve the periodide crystals. After 2 h, the optical density was measured at 365 nm using a spectrophotometer. Amounts of glycine betaine were determined from a standard curve of reference glycine betaine (Grieve and Grattan 1983).
Determination of electrolyte leakage, lipid peroxidation, and hydrogen peroxide
For estimation of hydrogen peroxide (H2O2) production, fresh tissue was extracted in 0.1% TCA and centrifuged at 10,000×g for 10 min. The supernatant (0.5 mL) was mixed with an equal volume of 100 mM potassium phosphate buffer (pH 7.0) and potassium iodide (1 M). Absorbance was recorded at 390 nm, and amounts of H2O2, expressed as nM g−1 FW, were determined from a standard curve (Velikova et al. 2000).
Lipid peroxidation was measured by estimating the formation of malondialdehyde (MDA) content. Fresh leaves were macerated in 0.1% trichloroacetic acid (TCA), and the homogenate was centrifuged at 10,000×g for 5 min. One milliliter of supernatant was reacted with 4 mL thiobarbituric acid (5% TBA prepared in 20% TCA) at 100 °C for 30 min. Thereafter, samples were cooled in an ice bath and were again centrifuged for 10 min at 10,000×g. Optical density was read at 532 and 600 nm (Madhava Rao and Sresty 2000).
We used the method of Dionisio-Sese and Tobita (1998) to determine electrolyte leakage. Twenty fresh leaf disks were transferred to a test tube containing 10 mL distilled water, and electrical conductivity (EC0) was measured. The same samples were boiled at 50 °C (for 20 min) and 100 °C (for 10 min), and electrical conductivities (EC1 and EC2, respectively) were measured at both temperatures. Calculation of electrolyte leakage was performed using the following formula:
Electrolyte leakage = (EC1 − EC0)/(EC2 − EC0) × 100
Determination of antioxidant enzyme activities
Fresh plant tissue was extracted in ice-cold buffer (100 mM potassium phosphate, pH 7.0) containing 1% polyvinyl pyrrolidoneusing a pre-chilled mortar and pestle. The resulting homogenate was centrifuged for 30 min at 12,000×g at 4 °C, and the supernatant was used as an enzyme source for the assay of SOD, CAT, APX, and GR.
Superoxide dismutase (SOD, EC1.15.1.1) activity: The activity of SOD was measured following the method of Dhindsa and Matowe (1981). Activity was measured by monitoring the ability of the enzyme extract to inhibit the photochemical reduction of nitroblue tetrazolium (NBT). The assay mixture contained phosphate buffer (100 mM, pH 7.4), methionine (10 mM), EDTA (1.0 mM), 50 μM riboflavin, 75 μM NBT, and 100 μL enzyme extract. Samples were incubated for 15 min under fluorescent tubes and were read at 560 nm. The amount of protein causing 50% inhibition in the photochemical reduction of NBT was defined as one unit of SOD and was expressed as EU mg−1 protein.
Catalase (CAT, EC1.11.1.6) activity
CAT activity was estimated by following the decomposition of H2O2 at 240 nm for 2 min. Activity was expressed as EU mg−1 protein and an extinction coefficient of 39.4 mM−1 cm−1 was used (Aebi 1984).
Ascorbate peroxidase (APX, EC1.11.1.1) activity
APX activity was assayed in accordance with the method of Nakano and Asada (1981). H2O2-dependent oxidation of ascorbate was monitored at 290 nm for 2 min. An extinction coefficient of 2.8 mM−1 cm−1 was used for calculation of APX activity, which was expressed as EU mg−1 protein.
Glutathione reductase (GR, EC1.6.4.2) activity
The method of Foster and Hess (1980) was used for estimating the activity of GR. The 3-mL reaction mixture contained potassium phosphate buffer (100 mM, pH 7.0), 150 μM NADPH, 1.0 mM EDTA, and 500 μM oxidized glutathione. Changes in absorbance were monitored at 340 nm for 3 min, and activity was expressed as EU mg−1 protein.
Determination of enzymes related to ascorbate-glutathione cycle
Monodehydroascorbate reductase (MDHAR, EC 1.6.5.4) activity
The method developed by Miyake and Asada (1992) was used for the determination of MDHAR. The activity was expressed as μmol NADPH oxidized/(EU mg−1 protein).
Dehydroascorbate reductase (DHAR, EC: 1.8.5.1) activity
The protocol provided by Nakano and Asada (1981) was used to estimate the activity of DHAR. The absorbance was recorded at 265 nm using spectrophotometer (Beckman 640 D, USA) and was expressed as EU mg−1 protein.
Ascorbate and glutathione
The procedure given by Huang et al. (2005) was used for the determination of ascorbic acid. Yu et al. (2003) method was used for the estimation of glutathione pool. GSH content was calculated by subtracting GSSG from total GSH.
Glutathione S-sransferase (GST, EC: 2.5.1.18) activity
The established procedure by Hasanuzzaman and Fujita (2013) was used for the estimation of GST activity. The absorbance was recorded at 340 nm using spectrophotometer (Beckman 640 D, USA) and was expressed as EU mg−1 protein.
Estimation of cadmium and other inorganic elements
Dry plant material (500 mg) was subjected to acid digestion using sulfuric acid and nitric acid (1:5, v/v) at 60 °C for 24 h. The digested material was treated with HNO3/HClO4 mixture (5/1, v/v). Thereafter, the concentration of cadmium and other inorganic elements in shoot and root was estimated using an atomic absorption spectrophotometer (Perkin-Elmer Analyst Model 300) and expressed as μM g−1 DW. For the sulfur estimation turbidimetric method given by Chesnin and Yien (1950) was used.
Statistical analysis
All experiments were repeated three times. Treatment means were statistically analyzed using least significant difference (LSD) analysis of variance for a completely randomized design.
Results
Growth and biomass yield
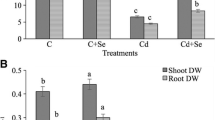
Shoot and root lengths were reduced by 75.94 and 65.52%, respectively, as a consequence of Cd treatment, and the dry weights of shoots and roots were reduced by 75.60 and 74.07%, respectively, compared with those of control plants. Application of NO (100 μM) enhanced the shoot and root length by 51.04 and 48.46%, respectively, relative to cadmium-only-treated plants (Fig. 1a). When applied to Cd-stressed plants, NO (150 Cd + NO) enhanced the dry weight of shoots and roots by 41.17 and 36.36%, respectively, compared with Cd-treated plants alone (Fig. 1b).
Pigment content
Compared to the control plants, chlorophyll a, chlorophyll b, total chlorophyll, and carotenoid contents declined by 60.43, 55.31, 58.69, and 31.08%, respectively, in plants subjected to Cd stress. Exogenous application of NO decreased the negative effect of Cd by enhancing chlorophyll a by 33.33%, chlorophyll b by 36.36%, total chlorophyll by 34.48%, and carotenoids by 13.55%, relative to Cd-stressed plants. In control plants, application of NO (100 μM) increased the contents of chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids by 8.80, 14.54, 10.38, and 15.90%, respectively (Fig. 2).
Chlorophyll fluorescence and gas exchange parameters
Tomato plants exposed to Cd stress (150 μM) reduced the Fv/Fm by 24.70%, ϕPSII by 26.15%, ϕexc by 37.28%, and qp by 30.76% over control plants. A substantial increase of 50.00% in NPQ was also recorded at the same concentration of Cd. Supplementation of NO to Cd-stressed plants showed enhanced levels of Fv/Fm, ϕPSII, ϕexc, and qp by 32.81, 62.50, 32.43, and 23.80%, respectively, over Cd-alone-treated plants. In contrast, the NPQ was observed to decrease by 28.73% through application of NO relative to Cd-treated plants alone (Table 1). The results related to the effect of Cd and NO on gas exchange parameters are depicted in Table 1. Cd (150 μM)-treated plants exhibited decrease in net photosynthetic rate (Pn), CO2 assimilation rate (A), stomatal conductance (gs), and transpiration rate (E) by 32.37, 48.36, 79.62, and 72.98%, respectively, relative to control. When the plants treated with NO in presence of Cd, the gas exchange parameters enhanced over the plants treated with Cd alone. A significant increase in Pn (42.69%), A (12.87%), gs (28.43%), and E (10.34%) was observed when control plants was supplied with NO.
RLWC, proline, and glycine betaine content
RLWC was reduced by 31.57% in cadmium-stressed plants; however, in plants treated with the Cd+NO combination, the percentage reduction was only 21.15% relative to the control (Fig. 3a). In control plants, proline and glycine betaine were observed to increase by 1.02- and 1.09-fold, respectively, following exogenous application of NO. In cadmium-treated plants, the amounts of proline and glycine betaine increased by 4.35- and 8.74-fold, respectively, relative to control, and was further increased by 1.35- and 1.24-fold, respectively, by the application of NO, compared with the Cd-treated plants (Fig. 3b, c).
H2O2, MDA accumulation, and electrolyte leakage
Cadmium treatment caused a considerable increment (185.58%) in the accumulation of H2O2, whereas Cd+NO-treated plants exhibited a 51.38% reduction in H2O2relative to the cadmium-stressed plants. Cadmium stress increased lipid peroxidation and electrolyte leakage by 2.05- and 6.19-fold, respectively. However, in Cd+NO-treated plants, lipid peroxidation declined by 1.59-fold and electrolyte leakage by 1.42-fold, compared with cadmium-treated plants (Fig. 4a–c).
Antioxidant enzyme activity and ascorbate-glutathione cycle
In control plants, exogenous application of NO improved the activity of SOD, CAT, APX, and GR by 6.34, 30.04, 9.04, and 17.29%, respectively. In cadmium-stressed plants, the activity of SOD, CAT, APX, and GR was increased by 82.67, 114.83, 105.18, and 106.22%, respectively, and was further enhanced following NO application by 15.37, 27.70, 32.29, and 38.57%, respectively, compared with the cadmium-treated plants (Fig. 5a, b). Plants treated with Cd exhibited reduced activity of DHAR by 35.62% and MDHAR by 41.18% over control plants. However, supplementation of NO to Cd-stressed plants enhanced the activity of DHAR and MDHAR by 43.39 and 57.56%, respectively, as compared to Cd-alone-treated plants. Cd-treated plants showed increase in GST by 73.43%; however, further increase by 39.35% was also recorded by the application of NO to Cd-stressed plants.
The effect of Cd and NO on the ascorbate-glutathione cycle is presented in Table 2. Cd stress decreased the AsA by 50.00% and GSSG by 30.88% over control; in contrast, GSH enhanced by 43.37% at same concentration of Cd. When these Cd-treated plants were supplemented with NO, enhanced levels of AsA (40.00%), GSH (69.56%), and GSSG (67.60%) were recorded as compared to Cd-alone-treated plants.
Flavonoids and total phenol content
As shown in Table 3, enhanced levels in flavonoid content by 64.38% and total phenol content by 70.57% was observed in seedlings treated with Cd. A further increment by 82.31 and 48.72% in flavonoid and total phenol content, respectively, was recorded in Cd-stressed plants supplemented with NO.
Cd accumulation
NO application resulted in a significant reduction in the uptake and accumulation of cadmium by causing reductions of 67.20, 56.07, and 62.53% in leaf, stem, and root, respectively (Table 4).
Mineral nutrient uptake
Table 5 shows S, Mn, Mg, Ca, and K contents in shoot and root of tomato seedlings under Cd and NO influence. As is evident from the results that under the influence of Cd, the shoot S, Mn, Mg, Ca, and K contents declined by 29.48, 52.40, 50.75, 40.68, and 46.93%, respectively, over control plants. Similar decrease in root S (41.97%), Mn (36.39%), Mg (30.76%), Ca (35.48%), and K (36.87%) was observed with similar concentration of Cd over control. When the CD-treated plants were supplied with NO, enhanced levels in S, Mn, Mg, Ca, and K contents of both shoot and root were recorded.
Discussion
In the present study, exposure of tomato plants to cadmium resulted in a decline in shoot and root lengths and dry weight. Cadmium has been reported to effect growth due to disturbances in normal metabolic activities (Ahmad et al. 2011, 2016). Such a reduction in plant growth under cadmium stress has also been ascribed to impeded nutrient uptake and a decline in water content (Gomes et al. 2013). Growth and developmental events such as cell division and tissue elongation are irreversibly affected by heavy metals through their effect on membrane potential and associated proton pumps (Karcz and Kurtyka 2007). The results of reduced growth and biomass yield due to Cd stress in present study support the findings of Ahmad et al. (2011) in mustard, Li et al. (2013) in soybean, and Khan et al. (2015) in wheat. Application of NO caused a significant improvement in growth parameters, including length and dry weight of shoot and root. Consistent with our results, Basalah et al. (2013) and Mostofa et al. (2014) have also reported amelioration of metal stress by supplementation of NO in wheat and rice, respectively. Recently, Zhao et al. (2016) have similarly demonstrated improved growth and biomass yield in response to exogenous application of NO. The reason might be that NO functions relaxant for cell wall, protects phospholipid bilayer, and helps in cell enlargement and growth of plant (Leshem and Haramaty 1996). Another reason may be NO increases osmotic pressure of a cell and improves the viscosity of cytoplasm (Dong et al. 2014a, b).
Reduction in chlorophyll contents due to cadmium (150 μM) treatment is consistent with the findings of Ahmad et al. (2011) and Khan et al. (2015), who reported a significant decline in the chlorophyll contents of Brassica juncea plants exposed to cadmium. Heavy metal accumulation causes a reduction in the synthesis of chlorophyll pigments by altering the chlorophyll biosynthetic intermediates, and thereby the functioning of the pigment protein complex (Ahanger et al. 2016). However, in the present study, the exogenously sourced NO was observed to help cadmium-treated tomato plants withstand such damage to some extent, and this positive impact of exogenously applied NO may be attributed to its effect on the de novo synthesis of chlorophylls and associated protein components (Ahmad et al. 2016). Studies that support the present findings of NO-mediated protection of chlorophyll against cadmium stress include those of Bai et al. (2015) and Khairy et al. (2016). NO shields the chloroplast membrane from stress-induced destruction, thus protects pigment content (Kausar et al. 2013; Ahmad et al. 2016). Hence, it is evident from the present study that application of NO counters the negative impacts of Cd on photosynthesis by protecting and maintaining the levels of pigments and associated components.
Cd stress decreased the Fv/Fm, ϕPSII, ϕexc, and qp, as has been reported by many biologists with different plants. Li et al. (2015) reported that higher concentrations of Cd (100 μmol L−1) significantly decreased the levels of Fv/Fm, ϕPSII, ϕexc, and qp and in contrast NPQ showed increase. Decrease in Fv/Fm and ϕPSII has also been reported in Lemna minor under combination of CO2 and Cd toxicity by Pietrini et al. (2016). Plants treated with different concentrations of Cd also exhibited decreased chlorophyll fluorescence parameters (Franklin et al. 1992; Gonzalez-Mendoza et al. 2007; Zhang et al. 2007). According to He et al. (2008), Fv/Fm is a strong parameter for stress indicator in plants. Stress-induced decline in Fv/Fm suggests the drop in maximum quantum efficiency PSII centers causing photoinactivation due to increase in non-photochemical quenching (NPG) and dissipates energy in the form of heat, thus damages PSII centers (Franklin et al. 1992). This photoinactivation results in oxidative damage and depletion of PSII reaction center (Baker 2008). Damage to antenna molecules due to heavy metal stress may block transport of electrons from PSII to PSI, thus decreases Fv/Fm and ϕPSII (Mallick and Mohn 2003). They also suggest that Cd replaces manganese (Mn) from water-splitting site that ultimately leads to obstructions in photosynthetic reactions. It has been suggested that stress-induced reduction in chlorophyll fluorescence is attributed to damage to the antenna molecules resulting in partial or complete blockage of electron transport form PSII to PSI (Mallick and Mohn 2003). Another reason of decreasing chlorophyll fluorescence due to Cd stress is by the replacement of Ca2+ by Cd2+ during photoactivation PSII catalytic center (Gonzalez-Mendoza et al. 2007). Thus, it is suggested that Cd acts on the primary site, i.e., water-splitting apparatus of PSII (Gonzalez-Mendoza et al. 2007).
The supplementation of NO restored the gas exchange parameters, and chlorophyll fluorescence in the present study has also been reported by Wu et al. (2011) in tomato and Zhang et al. (2006) in barley under light and salt stress, respectively. This beneficial role of NO is due to the uptake of mineral nutrients. According to Wang et al. (2013), enhanced uptake of iron (Fe) and magnesium (Mg) in presence of NO restored chlorophyll synthesis, photosynthesis, and transpiration under salt stress. Dong et al. (2014a, 2014b) also showed enhanced uptake of mineral elements and restoration of stomatal aperture by exogenous NO under Cu stress. This in turn leads to restored gas exchange, chlorophyll fluorescence, photosynthesis, and tolerance to Cd stress in the present study. The role of NO against abiotic stress has been demonstrated by Laspina et al. (2005) in Helianthus annuus under Cd stress, Farooq et al. (2009) in Oryza sativa under drought stress, Dong et al. (2014a, 2014b) in Lolium perenne under Cu stress, and Fatma et al. (2016) in B. juncea under salt stress.
Gas exchange attributes are susceptible to any environmental stress and are reported by many biologists (Ahmad et al. 2011; Asgher et al. 2014; Iqbal et al. 2015). Cd stress decreased the Pn, A, gs, and E in the present study has been also observed in wheat seedlings (Khan et al. 2015). B. juncea plants subjected to different concentrations of Cd also showed decreased behavior in gas exchange parameters and are reported by Per et al. (2016) and Ahmad et al. (2016). Li et al. (2015) reported decrease in gas exchange attributes at high concentrations of Cd in Cd-accumulating plant Elsholtzia argyi. Other heavy metals like arsenic (As) have also been evaluated in decreasing the gas exchange parameters in maize (Anjum et al. 2017). Decline in Pn, A, gs, and E is directly related to the plant biomass yield (Simonova et al. 2007). According to Anjum et al. (2017), Cd stress induced structural deformity in the stomata and due to which all the gas exchange parameters are hampered. Khan et al. (2016) and Per et al. (2016) also reported that reduced functions of stomata leading to decreased conductance under Cd stress are the main cause of reduction in photosynthesis. According to Vassilev et al. (2011), reduced stomatal functions are the main cause of decreased photosynthesis in bean plants under Zn treatment. They also reported reduction in intercellular spaces of leaf mesophyll that leads to hindrance in CO2 flow to the chloroplasts which ultimately effects carboxylase activity of Rubisco. Application of NO enhanced the gas exchange parameters in the present study, and the results corroborate with the findings of Fatma et al. (2016) in B. juncea.
Exogenous application of NO results in greater accumulation of osmotic constituents and mediates stress tolerance through maintenance of cell water content (Ahmad and Sharma 2008; Ahanger et al. 2014). Similar to other osmolytes, proline and glycine betaine play an irreplaceable role in osmoregulation, membrane stability, and stress mitigation in crop plants. It has been reported by several workers that accumulation of supra-optimal levels of proline and glycine betaine does not affect enzyme activity but instead hydrates enzymes, thereby contributing to the restoration of their activity (Kishor et al. 2005). Cadmium-induced accumulation of osmolytes has also been reported by other workers (John et al. 2009; Ahmad et al. 2011; Zhao et al. 2016). The increased accumulation of proline and glycine betaine due to NO application observed in the present investigation clearly indicates the protective role of NO against cadmium. Although results pertaining to the impact of NO on osmolyte accumulation under cadmium stress are very scanty, other studies have reported similar findings, including those of Zhao et al. (2016) in Typha angustifolia, Khan et al. (2012) in B. juncea, and Mostafa et al. (2014) in O. sativa. Exogenous application of NO has been reported to enhance the proline and glycine betaine content in excised leaf tissue of B. juncea (Fatma and Khan 2014). Under stress conditions, accumulation of proline has been attributed to enhanced activity of proline synthesizing enzymes, together with a reduction in proline catabolism (Khan et al. 2015). Moreover, observations in the present study suggest that NO-induced proline accumulation may be due to regulatory control over proline metabolism. Such enhancement in proline and glycine betaine in NO-treated plants resulted in enhancement of the RLWC, which may be due to NO-induced enhancement of hydraulic conductivity. Cd reduces hydraulic conductivity, causing a considerable reduction in cellular turgor, and thereby resulting in a decline in RLWC (Ehlert et al. 2009). The NO-induced increase in water content can contribute to improved wall extensibility, leading to enhancement of cell division and morphological attributes such as leaf area, length, and weight.
In the present study, cadmium stress markedly increased the production of H2O2, thereby inducing lipid peroxidation and electrolyte leakage. Our results showing increased H2O2 production concomitant with lipid peroxidation and electrolyte leakage in cadmium-stressed plants are consistent with those of John et al. (2009) and Ahmad et al. (2011) for mustard. Hossain et al. (2006) have also reported higher electrolyte leakage due to cadmium stress. Cd stress-induced loss of membrane integrity has been attributed to enhanced peroxidation of membrane lipids exhibiting sensitivity to ROS, which results in the leakage of cellular components (Ahmad et al. 2011). Peroxidation of lipids is an important parameter that is employed widely for measuring the magnitude of oxidative stress and is mainly due to enhancement of the activity of lipoxygenase mediating enhanced peroxidation of lipids (Macri et al. 1994). However, NO application was effective in mitigating the negative impact of cadmium to a considerable extent, thereby preventing damage to membrane integrity. Kaya and Ashraf (2015), working with tomato, have also reported the protective role of NO against boron-induced oxidative damage to membranes via the reduction of free radical production. In the present study, the observed decline in lipid peroxidation and membrane leakage in NO-treated tomato plants may have been attributable to the upregulation of the antioxidant system that rapidly eliminates of ROS, including H2O2. In agreement with our findings, Bai et al. (2015) in rye grass and Zhao et al. (2016) in T. angustifolia have also demonstrated that NO mitigates the cadmium-induced oxidative damage to membranes, by reducing the generation of free radicals and hence lipid peroxidation rate. Similarly, Mostofa et al. (2014) have also demonstrated that copper-induced enhancement of H2O2 production and loss of membrane integrity were mitigated by exogenous NO application.
Increases in SOD, CAT, APX, and GR activities as a result of cadmium stress have been reported in Glycine max (Melo et al. 2011), Arachis hypogaea L. (Shan et al. 2012), B. juncea (Ahmad et al. 2011; Irfan et al. 2014), and Solanum melongena L. (Singh and Prasad 2014). Our results showing improved NO-induced antioxidant enzyme activity in cadmium-stressed plants are consistent with the results of Basalah et al. (2013) for wheat and Zhao et al. (2016) for T. angustifolia. Mostofa et al. (2014) have demonstrated improved tolerance in rice, attributable to exogenous application of NO, against copper-induced oxidative damage. NO modulates antioxidant components such as glutathione and ascorbic acid to mediate rapid ROS scavenging and maintain metabolism at an optimal functional level (Mostofa et al. 2014; Zhao et al. (2016). Furthermore, enhancement of the activities of APX and GR predicts the ubiquitous role of NO in H2O2 removal and protection of photosynthetic electron transport by maintaining the concentration of NADH. The NO-induced improvement in the activities of antioxidant enzymes observed in the present study may be attributed to upregulation of antioxidant enzyme coding genes (Ahmad et al. 2016). The antioxidant enzymes APX and GR work in an integrative manner across the ascorbate-glutathione pathway to mediate the maintenance of redox buffer components and protection of cellular structures. In the present study, NO-induced upregulation of GR and APX activities may have protected metabolism by enhancing the GSH/GSSH ratio and associated enzymes of the ascorbate-glutathione pathway (Ahmad et al. 2016). In addition, improvement in the activity of GR restricted the flow of electrons to oxygen, thereby reducing the formation of superoxide radicals, and CAT prevents formation of the more toxic hydroxyl radical (Ahmad et al. 2010). Kaya and Ashraf (2015) observed a significant reduction in oxidative stress in NO-treated tomato plants under boron stress due to improved SOD and peroxidase activity. In B. juncea, Fatma et al. (2016) and Ahmad et al. (2016) demonstrated that exogenous application of NO mitigated the deleterious effect of salinity by causing reduced production of ROS and lipid peroxidation due to upregulated SOD, APX, GR, and CAT activities. Glutathione S-transferase (GST) is also involved in stress tolerance especially under oxidative stress (Conklin and Last 1995). During oxidative stress, the production of endogenous electrophiles is minimized by GST in conjugation with GSH. Roxas et al. (1997) and Cummins et al. (1999) showed that under stress, the GST functions as glutathione peroxidase and protects the plants from the stress injury. GST overexpression provides tolerance to Al toxicity in Arabidopsis (Ezaki et al. 2001).
Apart from enzymatic antioxidants, non-enzymatic antioxidants like AsA and GSH also played a great role in abiotic stress tolerance in plants (Shao et al. 2008). Cd stress decreased the AsA level as has been reported by Ahmad et al. (2016) in B. juncea and Wang et al. (2017) in Triticum aestivum. Application of NO enhanced the AsA and GSH concentrations in the present study, and the results are in accordance with that of Sun et al. (2015). Wang et al. (2017) also reported that tolerance to Cd stress through AsA was mediated by NO. Xu et al. (2015) reported that the NO induced accumulation of AsA and GSH under Cd stress in two peanut cultivars. The importance of AsA-GSH cycle under Cd stress has been well documented by Anjum et al. (2011) in mung bean. AsA is used as a substrate in detoxification of H2O2 into water by APX, and oxidized form of DHA produces AsA by the help of GSH through AsA-GSH cycle (Anjum et al. 2011). As described in Fig. 6, during the conversion of H2O2 to H2O by APX, MDHA, and DHA is also produced. The production of AsA from MDHA is mediated by MDHAR and from DHA is mediated by DHAR. Overexpression of DHAR, in transgenic tobacco showed enhanced tolerance against aluminum (Al) stress (Yin et al. 2010). Sun et al. (2015) also reported that application of NO enhanced the DHAR activity in wheat roots under Al stress. For the generation of AsA from DHA needs electron donor and the part is played by reduced GSH and it is converted to oxidized glutathione (GSSG) (Fig. 6) (Sun et al. 2015). Conversion of GSH from GSSG is mediated by GR. In the present study, NO application has been found to maintain the GSH pool and also GSH/GSSG ratio. Sun et al. (2015) reported that NO application enhanced the activity and gene expression of DHAR and GR in wheat roots under Al toxicity. They also concluded the high activity and gene expression of GR maintains the high AsA and GSH concentrations. NO increases GSH and GSSG ratios in wheat genotypes, demonstrating outstanding performance against Al stress (Sun et al. 2015). Sun et al. (2015) reported that GSH biosynthesis is not induced by NO, but it activates enzymes of GSH metabolism. NO is having a leading role in protection against heavy metal stress like Cd (Gill et al. 2013; Ahmad et al. 2016). The protective role may be due to its antioxidant nature, and it also induces the activity antioxidant enzymes and genes (Xiong et al. 2010; Gill et al. 2013).
Increase in flavonoids and total phenol content under abiotic stress is regarded as the adaptive strategy against the Cd stress (Marquez-Garcıa et al. 2012; Kapoor et al. 2014; Ahmad et al. 2016). Many other biologists have also reported enhanced levels of flavonoid content and total phenols against Cd stress (Ahmad et al. (2015) in Cannabis sativa; Abd_Allah et al. (2015) in H. annuus). Plants accumulate more flavonoids that provide protection under metal stress (Winkel-Shirley 2001). Phenolic compounds act as antioxidants because they have electron-donating agents (Michalak 2006), and thus quench extra free radicals and decrease production of ROS (Lopes et al. 1999; Jung et al. 2003). Flavonoids have been reported to have protective functions against the abiotic stress (Simontacchi et al. 2015). According to Tossi et al. (2011), NO regulates gene expression involved in flavonoid biosynthesis. So, NO is responsible for the accumulation of flavonoids under stress (Tossi et al. 2011).
In our study, roots accumulated more cadmium in comparison with shoots, which showed less cadmium content, indicating a more efficient sequestration of toxic cadmium ions in the latter. However, application of NO significantly decreased the uptake of cadmium in all plant parts. Basalah et al. (2013) and Zhao et al. (2016) have also reported reduced cadmium uptake due to NO application. NO prevents the uptake and accumulation of toxic heavy metals in the upper parts of plants, resulting in the protection of tomato plants from a cadmium-induced osmotic shift and excess generation of ROS, and hence oxidative stress (Gill and Tuteja 2010). Application of NO to white clover plants also showed decreased accumulation of Cd and may be due to immediate enhancement in internal NO levels (Liu et al. 2015). NO supplementation also promotes membrane transporters that are responsible for Cd removal from root cells and improves uptake of K and Ca (Singh et al. 2009; Xu et al. 2010). NO is also reported to have positive impact on morphology of roots like regulation of later root formation (Correa-Aragunde et al. 2008), growth of primary roots (Fernández-Marcos et al. 2011), formation of adventitious roots (Pagnussat et al. 2004), and development of root hairs (Lombardo et al. 2006). Consistent with the observations in the present study, increased accumulation of Cd in roots relative to shoots has earlier been reported in B. juncea (Ahmad et al. 2011; Irfan et al. 2014).
The decrease in mineral uptake by root and shoot in the present study corroborates with the findings of Gonçalves et al. (2009). Ahmad et al. (2015) also reported restricted uptake of mineral nutrients by B. juncea in presence of Cd. According to Nazar et al. (2012), Cd is in competition with mineral nutrients for the same transporters and elevated levels of Cd results in enhanced uptake of Cd and reduced uptake of mineral nutrients. Another reason is that the activity of H+-ATPase is inhibited under Cd and other heavy metal stress (Burzyński and Kolano 2003). Cd hampers the uptake of mineral nutrients like Ca and K, and Cd enters to the root cells through the transporters/cation channels and is then transported to the shoot (Liu et al. 2015). When Cd is transported through the cations/transporters, it reduces the uptake of other nutrients (Zhang et al. 2014). Supplementation of NO decreased the Cd accumulation and enhanced the uptake of other important nutrients like, Ca, Mg, K, Cu, and Fe (Liu et al. 2015). Ca also acts as secondary messenger in plants, and it quickly responds under metal stresses. The relation between NO and Ca is very close, as NO is reported to activate the Ca2+ influx; it also increases Ca2+ transporters, thus maintains the Ca level in the cell (Courtois et al. 2008; Besson-Bard et al. 2009). In rice plants, application of NO enhanced pectin and hemicellulose which helps in detoxification of Cd (Xiong et al. 2009). This detoxification is due to, NO increased Cd accumulation in cell walls of both root and stem and decreased in cell wall of leaves (Xiong et al. 2009). Uptake of nutrients in ryegrass by NO application under Cd stress has also been reported by Wang et al. (2013). It has been assumed that NO stimulates the PM H+-ATPase and enhanced the uptake of mineral elements (Wang et al. 2013). Thus, NO in plants is having the ability to decrease the Cd translocation from root to shoot (Xiong et al. 2009; Wang et al. 2013). Activities of PM H+-ATPase, V-H+-ATPase, and H+-PPase were enhanced by the external supplementation of NO under metal stress (Cui et al. 2010). Based on these results, it could be concluded that accumulation of Cd in cell wall hampers the transport of Cd to the upper parts of the plants and by the decrease in Cd accumulation and stimulation of PM H+-ATPase, other nutrients might have got chance to be transported.
Conclusions
Exogenous application of NO allayed the cadmium-induced damage to a range of morphophysiological attributes. NO enhanced the osmolytes and maintained the pigment content, water status, and chlorophyll fluorescence in Cd-stressed seedlings; NO also prevented oxidative damage by upregulating antioxidant enzymes, thereby promoting a significant decline in ROS-induced lipid peroxidation and electrolyte leakage. NO boosts mineral uptake and reduced Cd accumulation, thus minimizes the Cd effect on seedlings. On the basis of the findings of the present study, we propose that NO has a potential role as a growth promoter for tomato under cadmium stress.
References
Abd_Allah EF, Hashem A, Alqarawi AA, Alwathnani Hend A (2015) Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak J Bot 47(2):785–795
Aebi H (1984) Catalase in vitro. Method Enzymol 105:121–126
Ahanger MA, Agarwal RM, Tomar NS, Shrivastava M (2015) Potassium induces positive changes in nitrogen metabolism and antioxidant system of oat (Avena sativa L cultivar Kent). J Plant Inter 10:211–223
Ahanger MA, Moad-Talab N, Abd-Allah EF, Ahmad P, Hajiboland R (2016) Plant growth under drought stress: significance of mineral nutrients. In “Water stress and crop plants: a sustainable approach.” Ed. Ahmad P. Wiley Blackwell. pp. 649–668
Ahanger MA, Tyagi SR, Wani MR, Ahmad P (2014) Drought tolerance: roles of organic osmolytes, growth regulators and mineral nutrients. In: Ahmad P, Wani MR (eds) Physiological mechanisms and adaptation strategies in plants under changing environment, vol I. Springer, New York, pp 25–56
Ahmad P, Abdel Latef AA, Hashem A, Abd Allah EF, Gucel S, Tran L-SP (2016) Nitric oxide mitigates salt stress by regulating levels of osmolytes and antioxidant enzymes in chickpea. Front Plant Sci 7:347
Ahmad P, Jaleel CA, Salem MA, Nabi G, Sharma S (2010) Roles of Enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit Rev Biotechnol 30(3):161–175
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [(Brassica juncea L.) Czern. & Coss.] plants can be alleviated by salicylic acid. South Afri J Bot 77:36–44
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LS (2015) Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS One 10(1):e0114571n.d.
Ahmad P, Sharma S (2008) Salt stress and phyto-biochemical responses of plants – a review. Plant Soil Environ 54(3):89–99
Anjum NA, Umar S, Iqbal M, Khan NA (2011) Cadmium causes oxidative stress in mung bean [Vigna radiata (L.) Wilczek] by affecting antioxidant enzyme systems and ascorbate-glutathione cycle metabolism. Russ J Plant Physiol 58:92–99
Anjum SA, Tanveer M, Hussain S, Ashraf U, Khan I, Wang L (2017) Alteration in growth, leaf gas exchange, and photosynthetic pigments of maize plants under combined cadmium and arsenic stress. Water Air Soil Pollut 228:13
Arnon DI (1949) Copper enzymes in isolated chloroplasts, polyphenol oxidase in Beta vulgaris L. Plant Physiol 24:1–15
Asgher M, Khan NA, Khan MIR, Fatma M, Masood A (2014) Ethylene production is associated with alleviation of cadmium-induced oxidative stress by sulfur in mustard types differing in ethylene sensitivity. Ecotoxicol Environ Safety 106:54–61
Bai XY, Dong YJ, Xu LL, Kong J, Liu S (2015) Effects of exogenous nitric oxide on physiological characteristics of perennial ryegrass under cadmium and copper stress. Russ J Plant Physiol 62(2):237–245
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Basalah MO, Ali HM, Al-Whaibi MH, Siddiqui MH, Sakran AM, Al Sahli AA (2013) Nitric oxide and salicylic acid mitigate cadmium stress in wheat seedlings. J Pure Appl Microbiol 7:139–148
Bates L, Waldren RP, Teare JD (1973) Rapid determination of free proline for water stress studies. Plant Soil 39:205–207
Besson-Bard A, Gravot A, Richaud P, Auroy P, Duc C, Gaymard F, Taconnat L, Renou JP, Pugin A, Wendehenne D (2009) Nitric oxide contributes to cadmium toxicity in Arabidopsis by promoting cadmium accumulation in roots and by up-regulating genes related to iron uptake. Plant Physiol 149:1302–1315
Burzyński M, Kolano E (2003) In vivo and in vitro effects of copper and cadmium on the plasma membrane H+-ATPase from cucumber (Cucumis sativus L.) and maize (Zea mays L.) roots. Acta Physiol Plant 25:39–45
Chesnin L, Yien CH (1950) Turbidimetric determination of available sulfates. Proc Soil Sci Soc Am J 15:149–151
Conklin PL, Last RL (1995) Differential accumulation of antioxidant mRNAs in Arabidopsis thaliana exposed to ozone. Plant Physiol 109:203–212
Correa-Aragunde N, Lombardo C, Lamattina L (2008) Nitric oxide: an active nitrogen molecule that modulates cellulose synthesis in tomato roots. New Phytol 179:386–396
Courtois C, Besson A, Dahan J, Bourque S, Dobrowolska G, Pugin A, Wendehenne D (2008) Nitric oxide signalling in plants: interplays with Ca2+ and protein kinases. J Exp Bot 59:155–163
Cui XM, Zhang YK, Wu XB, Liu CS (2010) The investigation of the alleviated effect of copper toxicity by exogenous nitric oxide in tomato plants. Plant Soil Environ 56:274–281
Cummins I, Cole DJ, Edwards R (1999) A role for glutathione transferases functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J 18:285–292
Cuypers A, Smeets K, Ruytinx J, Opdenakker K, Keunen E, Remans T, Horemans N, Vanhoudt N, Van Sanden S, Van Belleghem F, Guisez Y, Colpaert J, Vangronsveld J (2011) The cellular redox state as a modulator in cadmium and copper responses in Arabidopsis thaliana seedlings. J Plant Physiol 168:309–316
Gonzalez-Mendoza D, Gil FEY, Santamaria JM, Zapata-Perez O (2007) Multiple effects of cadmium on the photosynthetic apparatus of Avicennia germinans L. as probed by OJIP chlorophyll fluorescence measurements. Z Naturforschung C 62:265–272
Dhindsa RS, Matowe W (1981) Drought tolerance in two mosses: correlated with enzymatic defense against lipid peroxidation. J Exp Bot 32:79–91
Dias MC, Monteiro C, Moutinho-Pereira J, Correia C, Goncalves B, Santos C (2013) Cadmium toxicity affects photosynthesis and plant growth at different levels. Acta Physiol Plant 35:1281–1289
Dionisio-Sese ML, Tobita S (1998) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Dong Y, Xu L, Wang Q, Fan Z, Kong J, Bai X (2014a) Effects of exogenous nitric oxide on photosynthesis, antioxidative ability, and mineral element contents of perennial ryegrass under copper stress. J Plant Interact 9:402–411
Dong YJ, Jinc SS, Liu S, Xu LL, Kong J (2014b) Effects of exogenous nitric oxide on growth of cotton seedlings under NaCl stress. J Soil Sci Plant Nutr 14:1–13
Ehlert C, Maurel C, Tardieu F, Simonneau T (2009) Aquaporin mediated reduction in maize root hydraulic conductivity impacts cell turgor and leaf elongation even without changing transpiration. Plant Physiol 150:1093–1104
Ezaki B, Katsuhara M, Kawamura M, Matsumoto H (2001) Different mechanisms of four aluminum (Al)-resistant transgenes for Al toxicity in Arabidopsis. Plant Physiol 127:918–927
Farooq M, Basra SMA, Wahid A, Rehman H (2009) Exogenously applied nitric oxide enhances the drought tolerance in fine grain aromatic rice (Oryza sativa L.) J Agro Crop Sci 195:254–261
Fatma M, Khan NA (2014) Nitric oxide protects photosynthetic capacity inhibition by salinity in Indian mustard. J Funct Environ Bot 4(2):106–116
Fatma M, Masood A, Per TS, Khan NA (2016) Nitric oxide alleviates salt stress inhibited photosynthetic performance by interacting with sulfur assimilation in mustard. Front Plant Sci 7:521
Fernández-Marcos M, Sanz L, Lewis DR, Muday GK, Lorenzo O (2011) Nitric oxide causes root apical meristem defects and growth inhibition while reducing PIN-FORMED 1 (PIN1)-dependent acropetal auxin transport. Proc Natl Acad Sci U S A 108:18506–18511
Foster JG, Hess JL (1980) Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Franklin LA, Levavasseur G, Osmond CB, Henley WJ, Ramus J (1992) Two components of onset and recovery during photoinhibition of Ulva rotundata. Planta 186:399–408
Gill SS, Hasanuzzaman M, Nahar K, Tuteja N (2013) Importance of nitric oxide in cadmium stress tolerance in crop plants. Plant Physiol Biochem 63:254–261
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gomes MP, Marques TCLLSM, Soares AM (2013) Cadmium effects on mineral nutrition of the Cd-hyperaccumulator Pfaffia glomerata. Biologia 68(2):223–230
Gonçalves JF, Antes FG, Maldaner J, Pereira LB, Tabaldi LA, Rauber R, Rossato LV, Bisognin DA, Dressler VL, Flores EM, Nicoloso FT (2009) Cadmium and mineral nutrient accumulation in potato plantlets grown under cadmium stress in two different experimental culture conditions. Plant Physiol Biochem 47:814–821
Grieve CM, Grattan SR (1983) Rapid assay for determination of water-soluble quaternary-amino compounds. Plant Soil 70:303–307
Groppa MD, Ianuzzo MP, Rosales EP, Vazquez SC, Benavides MP (2012) Cadmium modulates NADPH oxidase activity and expression in sunflower leaves. Biol Plant 56:167–171
Hameed A, Rasool S, Azooz MM, Hossain MA, Ahanger MA, Ahmad P (2016) Heavy Metal Stress: Plant Responses and Signaling. In: Ahmad P (ed) Plant metal interaction: Emerging remediation techniques, Elsevier Academic Press. pp. 557–584
Hasanuzzaman M, Fujita M (2013) Exogenous sodium nitroprusside alleviates arsenic-induced oxidative stress in wheat (Triticum aestivum L.) seedlings by enhancing antioxidant defense and glyoxalase system. Ecotoxicology 22:584–596
He JY, Ren YF, Zhu C, Yan YP, Jiang DA (2008) Effect of Cd on growth, photosynthetic gas exchange, and chlorophyll fluorescence of wild and Cd-sensitive mutant rice. Photosynthetica 46:466–470
Hermes VS, Dall’asta P, Amaral FP, Anacleto KB, Arisi ACM (2013) The regulation of transcription of genes related to oxidative stress and glutathione synthesis in Zea mays leaves by nitric oxide. Biol Plant 57:620–626
Horiguchi H, Aoshima K, Oguma R, Sasaki S, Miyamoto K, Hosoi Y, Katoh T, Kayam F (2010) Latest status of cadmium accumulation and its effects on kidneys, bone, and erythropoiesis in inhabitants of the formerly cadmium-polluted Jinzu River Basin in Toyama, Japan, after restoration of rice paddies. Int Arch Occup Environ Health 83:953–970
Hossain Z, Mandal AKA, Datta SK, Biswas AK (2006) Isolation of a NaCl tolerant mutant of Chrysanthemum morifolium by gamma radiation: in vitro mutagenesis and selection by salt stress. Funct Plant Biol 33:91–101
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increasedsensitivity to salt stress in ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178:84–91
Irfan M, Ahmad A, Hayat S (2014) Effect of cadmium on the growth and antioxidant enzymes in two varieties of Brassica juncea. Saudi J Biol Sci 21:125–131
John R, Ahmad P, Gadgil K, Sharma S (2009) Cadmium and lead-induced changes in lipid peroxidation, antioxidative enzymes and metal accumulation in Brassica juncea L. at three different growth stages. Arch Agron Soil Sci 55(4):395–405
Jung C, Maeder V, Funk F, Frey B, Sticher H, Frosserd E (2003) Release of phenols from Lupinus albus L. roots exposed to Cu and their possible role in Cu detoxification. Plant Soil 252(2):301–312
Kapoor D, Kaur S, Bhardwaj R (2014) Physiological and biochemical changes in Brassica juncea Plants under Cd-induced stress. Biomed Res Int 2014:726070
Karcz W, Kurtyka R (2007) Effect of cadmium on growth, proton extrusion and membrane potential in maize coleoptile segments. Biol Plant 51:713–719
Kausar F, Shahbaz M, Ashraf M (2013) Protective role of foliar-applied nitric oxide in Triticum aestivum under saline stress. Turk. J Bot 37:1155–1165
Kaya C, Ashraf M (2015) Exogenous application of nitric oxide promotes growth and oxidative defense system in highly boron stressed tomato plants bearing fruit. Sci Hort 185:43–47
Khairy AIH, Oh MJ, Lee SM, Kim DS, Roh KS (2016) Nitric oxide overcomes Cd and Cu toxicity in in vitro-grown tobacco plants through increasing contents and activities of rubisco and rubisco activase. Biochimie Open 2:41–51
Khan MIR, Nazir F, Asgher M, Per TS, Khan NA (2015) Selenium and sulfur influence ethylene formation and alleviate cadmium-induced oxidative stress by improving proline and glutathione production in wheat. J Plant Physiol 173:9–18
Khan MN, Siddiqui MH, Mohammad F, Naeem M (2012) Interactive role of nitric oxide and calcium chloride in enhancing tolerance to salt stress. Nitric Oxide 27:210–218
Khan NA, Asgher M, Per TS, Masood A, Fatma M, Khan MIR (2016) Ethylene potentiates sulfur-mediated reversal of cadmium inhibited photosynthetic responses in mustard. Front Plant Sci 7:1628
Kishor PBK, Sangam S, Amrutha RN, Sri Laxmi P, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88:424–438
Laspina NV, Groppa MD, Tomaro ML, Benavides MP (2005) Nitric oxide protects sunflower leaves against Cd-induced oxidative stress. Plant Sci 169:323–330
Leshem YY, Haramaty E (1996) Plant aging: the emission of NO and ethylene and effect of NO-releasing compounds on growth of pea (Pisum sativum) foliage. J Plant Physiol 148:258–263
Li P, Cai R, Gao H, Peng T, Wang Z (2007) Partitioning of excitation energy in two wheat cultivars with different grain protein contents grown under three nitrogen applications in the field. Physiol Plant 129:822–829
Li Q, Lu Y, Shil Y, Wang T, Ni K, Xu L, Liu S, Wang L, Xiong Q, Giesy JP (2013) Combined effects of cadmium and fluoranthene on germination, growth and photosynthesis of soybean seedlings. J Environ Sci 25(9):1936–1946
Li S, Yang W, Yang T, Chen Y, Ni W (2015) Effects of cadmium stress on leaf chlorophyll fluorescence and photosynthesis of Elsholtzia argyi—a cadmium accumulating plant. Int J Phytoremed 17:85–92
Liu S, Yang R, Pan Y, Ma M, Pan J, Zhao Y, Cheng Q, Wu M, Wang M, Zhang L (2015) Nitric oxide contributes to minerals absorption, proton pumps and hormone equilibrium under cadmium excess in Trifolium repens L. plants. Ecotoxicol Environ Saf 119:35–46
Lombardo MC, Graziano M, Polacco JC, Lamattina L (2006) Nitric oxide functions as a positive regulator of root hair development. Plant Signal Behav 1:28–33
Lopes GKB, Shulman HM, Hermes-Lima M (1999) Polyphenol tannic acid inhibits hydroxyl radical formation from Fenton reaction by complexing ferrous ions. Biochim Biophys Acta/GS 1472:142–152
Macri F, Braidot E, Petrusa E, Vianello A (1994) Lipoxygenase activity associated to isolated soybean plasma membranes. Biochim Biophys Acta 1215:109–114
Madhava Rao KV, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeon pea (Cajanus cajan L. Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Mallick N, Mohn FH (2003) Use of chlorophyll fluorescence in metal-stress research: a case study with the green microalga Scenedesmus. Ecotoxicol Environ Saf 55:64–69
Márquez-García B, Ángeles Fernández-Recamales M, Córdoba F (2012) Effects of cadmium on phenolic composition and antioxidant activities of Erica andevalensis. J Bot 2012:936950
Melo LCA, Alleoni LRF, Carvalho G, Azevedo RA (2011) Cadmium- and barium-toxicity effects on growth and antioxidant capacity of soybean (Glycine max L.) plants, grown in two soil types with different physicochemical properties. J Plant Nutr Soil Sci 1–13
Mendoza-Cozatl D, Loza-Tavera H, Hernandez-Navarro A, Moreno-Sanchez R (2005) Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol Rev 29:653–671
Michalak A (2006) Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Polish J Environ Studies 15:523–530
Miyake C, Asada K (1992) Thylakoid bound ascorbate peroxidase in spinach chloroplast and photoreduction of its primary oxidation product monodehydroascorbate radicals in thylakoids. Plant Cell Physiol 33:541–553
Mostofa MG, Seraj ZI, Fujita M (2014) Exogenous sodium nitroprusside and glutathione alleviate copper toxicity by reducing copper uptake and oxidative damage in rice (Oryza sativa L.) seedlings. Protoplasma 251:1373–1386
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol 22:867–880
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 3:1476–1489
Pagnussat GC, Lanteri ML, Lombardo MC, Lamattina L (2004) Nitric oxide mediates the indole acetic acid induction activation of a mitogen-activated protein kinase cascade involved in adventitious root development. Plant Physiol 135:279–286
Per TS, Khan NA, Masood A, Fatma M (2016) Methyl jasmonate alleviates cadmiuminduced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front Plant Sci 7:1933
Pietrini F, Bianconi D, Massacci A, Iannelli MA (2016) Combined effects of elevated CO2 and Cd-contaminated water on growth, photosynthetic response, Cd accumulation and thiolic components status in Lemna minor L. J Hazard Mater 309:77–86
Roxas VP, Smith RK, Allen ER, Allen RD (1997) Overexpression of glutathione S-transferase/glutathione peroxidase enhances the growth of transgenic tobacco seedlings during stress. Nat Biotechnol 15:988–991
Shan S, Liu F, Li C (2012) Effects of cadmium on growth, oxidative stress and antioxidant enzyme activities in peanut (Arachis hypogaea L.) seedlings. J Agric Sci 4(6):142–151
Shao HB, Chu LY, Zhao HL, Kang C (2008) Primary antioxidant free radical scavenging and redox signalling pathways in higher plant cells. Int J Biol Sci 4:8–14
Simontacchi M, Galatro A, Ramos-Artuso F, Santa-María GE (2015) Plant survival in a changing environment: the role of nitric oxide in plant responses to abiotic stress. Front Plant Sci 6:977
Singh A, Prasad SM (2014) Effect of agro-industrial waste amendment on Cd uptake in Amaranthus caudatus grown under contaminated soil: an oxidative biomarker response. Ecotoxicol Environ Saf 100:105–113
Singh HP, Kaur S, Batish DR, Sharma VP, Sharma N, Kohli RK (2009) Nitric oxide alleviates arsenic toxicity by reducing oxidative damage in the roots of Oryza sativa (rice). Nitric Oxide 20:289–297
Sun C, Liu L, Yu Y, Liu W, Lu L, Jin C, Lin X (2015) Nitric oxide alleviates aluminum-induced oxidative damage through regulating the ascorbate-glutathione cycle in roots of wheat. J Integr Plant Biol 57:550–561
Tossi V, Amenta M, Lamattina L, Cassia R (2011) Nitric oxide enhances plant ultraviolet-B protection up-regulating gene expression of the phenylpropanoid biosynthetic pathway. Plant Cell Environ 34:909–921
Vassilev A, Nikolova A, Koleva L, Lidon F (2011) Effects of excess Zn on growth and photosynthetic performance of young bean plants. J Phytol 3:58–62
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain treated bean plants, protective role of exogenous polyamines. Plant Sci 151:59–66
Wang QH, Liang X, Dong YJ, Xu LL, Zhang XW, Hou J, Fan ZY (2013) Effects of exogenous nitric oxide on cadmium toxicity, element contents and antioxidative system in perennial ryegrass. Plant Growth Regul 69:11–20
Wang Z, Li Q, Wu W, Guo J, Yang Y (2017) Cadmium stress tolerance in wheat seedlings induced by ascorbic acid was mediated by NO signaling pathways. Ecotoxicol Environ Saf 135:75–81
Winkel-Shirley B (2001) Flavonoid biosynthesis. a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126:485–493
Wu X, Zhu W, Zhang H, Ding H, Zhang HJ (2011) Exogenous nitric oxide protects against salt-induced oxidative stress in the leaves from two genotypes of tomato (Lycopersicon esculentum Mill.) Acta Physiol Plant 33:1199–1209
Wu Y, Wang WX (2011) Accumulation subcellular distribution and toxicity of inorganic mercury and methyl mercury in marine phytoplankton. Environ Pollut 159:3097–3105
Wu Z, Zhao X, Sun X, Tan Q, Tang Y, Nie Z, Qu C, Chen Z, Hu C (2015) Antioxidant enzyme systems and the ascorbate–glutathione cycle as contributing factors to cadmium accumulation and tolerance in two oilseed rape cultivars (Brassica napus L.) under moderate cadmium stress. Chemosphere 138:526–536
Xiong J, An LY, Lu H, Zhu C (2009) Exogenous nitric oxide enhances cadmium tolerance of rice by increasing pectin and hemicellulose contents in root cell wall. Planta 230:755–765
Xiong J, Fu G, Tao L, Zhu C (2010) Roles of nitric oxide in alleviating heavy metal toxicity in plants. Arch Biochem Biophys 497:13–20
Xu J, Wang W, Yin H, Liu X, Sun H, Mi Q (2010) Exogenous nitric oxide improves antioxidative capacity and reduces auxin degradation in roots of Medicago truncatula seedlings under cadmium stress. Plant Soil 326:321–330
Xu LL, Fan ZY, Dong YJ, Kong J, Bai XY (2015) Effects of exogenous salicylic acid and nitric oxide on physiological characteristics of two peanut cultivars under cadmium stress. Biol Plant 59:171–182
Yamasaki S, Dillenburg LC (1999) Measurements of leaf relative water content in Araucaria angustifolia. Rev Bras Fisiol Veg 11:69–75
Yin L, Wang S, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K (2010) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231:609–621
Yu CW, Murphy TM, Lin CH (2003) Hydrogen peroxide–induces chilling tolerance in mung beans mediated through ABA-independent glutathione accumulation. Funct Plant Biol 30:955–963
Zhang J, Chen J, Hu Y, Mo Y (2007) Effects of cadmium stress on photosynthetic function of leaves of Lemna minor L. J Agro-Environ Sci 6:2027–2032
Zhang L, Wang Y, Zhao L, Shi S, Zhang L (2006) Involvement of nitric oxide in light-mediated greening of barley seedlings. J Plant Physiol 163:818–826
Zhang X, Gao B, Xia H (2014) Effect of cadmium on growth, photosynthesis, mineral nutrition and metal accumulation of bana grass and vetiver grass. Ecotoxicol Environ Saf 106:102–108
Zhao H, Jin Q, Wang Y, Chu L, Li X, Xu Y (2016) Effects of nitric oxide on alleviating cadmium stress in Typha angustifolia. Plant Growth Regul 78:243–251
Zoffoli HJ, do Amaral-Sobrinho NM, Zonta E, Luisi MV, Marcon G, Tolon Becerra A (2013) Inputs of heavy metals due to agrochemical use in tobacco fields in Brazil’s southern region. Environ Monit Assess 185:2423–2437
Acknowledgements
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group (no. RG-1438-039).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Néstor Carrillo
Rights and permissions
About this article
Cite this article
Ahmad, P., Ahanger, M.A., Alyemeni, M.N. et al. Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255, 79–93 (2018). https://doi.org/10.1007/s00709-017-1132-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00709-017-1132-x