Abstract
We examined effects of methyl jasmonate (MeJ), with and without N, for the alleviation of the adverse effects of 150 mg kg−1 CdCl2 stress in mentholmint (Mentha arvensis) plants. Exposure of mentholmint plants to Cd stress reduced morphological growth parameters, photosynthetic attributes, chlorophyll content and mineral nutrient assimilation rate. Cd stress significantly increased endogenous leaf and root Cd content by 67.10% and 83.05%, respectively, electrolyte leakage by 67.26%, hydrogen peroxide (H2O2) by 56.66% and malondialdehyde content by 53.97% over that of the control. Cd stress upregulated activities of antioxidant enzymes and increased osmolyte concentration. Application of 1 µM MeJ to Cd-stressed plants partially alleviated the Cd-induced oxidative stress; however, co-application of MeJ with inorganic N reversed the detrimental effects more than did MeJ or N alone. Combined application of MeJ + N further elevated the osmolyte levels and markedly increased mineral nutrient contents and nitrogen use efficiency. MeJ + N significantly reduced the production of reactive oxygen species (ROS) directly or indirectly through higher stimulation of ROS-scavenging enzymes and decreased the root-to-shoot Cd rate of translocation. Cd-induced stomatal inhibition was recovered by MeJ and N. Our study demonstrated the regulatory role of MeJ and N in overcoming Cd stress in mentholmint plants. The study is the first report of regulatory interaction of the exogenous phytohormone (MeJ) with inorganic nutrient (N) for enhancing Cd stress tolerance in mentholmint plants, the same concept can be used for remediation of toxic metal/metalloids in agricultural production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cadmium (Cd) is a common toxic heavy metal (HM) in agricultural soils posing adverse effects on growth, development and yield of crops (Ahmad et al. 2017a, b; Kaur et al. 2017; Yang et al. 2016; Rizwan et al. 2017a, b). Cd is known to hamper vital physiological activities, including photosynthesis, respiration, chlorophyll biosynthesis, assimilation of mineral nutrients and modulation of the signalling cascades of antioxidant systems (Anjum et al. 2014; Ahmad et al. 2015; Kovacik et al. 2017, Su et al. 2017). Cd stress imposes osmotic, ionic, as well as oxidative stress in different plants resulting in the dysfunction of plant metabolism (Kaur et al. 2017; Cuypers et al. 2010; Rizwan et al. 2017a, b). Cd stress is responsible for the overproduction of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2), the hydroxyl radical (OH·), singlet oxygen (1O2) and the superoxide radical (O2•−), which are strong oxidants that cause protein oxidation, lipid peroxidation and DNA and RNA damage (Per et al. 2016; Sewelam et al. 2016).
Plants can cope with the Cd-induced oxidative stress through an efficient signalling network, which involves mineral nutrient homeostasis, production of enzymatic and non-enzymatic antioxidants and accumulation of comparative cytosolutes (Li et al. 2017; Silva et al. 2016; Mnasri et al. 2015). Stress physiologists are involved in developing strategies that underpin Cd-stress tolerance; however, the expanding field is emerging. Of the various adopted practices to reverse HM stress effects, application of plant growth regulators (PGRs) and mineral nutrients to achieve HM stress tolerance remains least explored and discussed.
PGRs crucially mediate plants’ response, metabolism and survival rate under both normal and abiotic environmental pressures. Methyl jasmonate (MeJ) is a methyl ester of jasmonic acid and is a lipid-derived naturally occurring PGR, which has a profound effect on various morphological, physiological, biochemical and antioxidant responses of plants against different stresses (Wasternack 2014; Hanaka et al. 2015; Sharma and Laxmi 2016; Ahmad et al. 2016a, b; Huang et al. 2017). The stimulatory effect of MeJ was found to be dose dependent and concentrations from 10−7 to 10−5 M have enhanced photosynthetic pigments and activity of photosystem II (PSII), which was severely down-regulated by Cd (Kovacik et al. 2011; Maksymiec and Krupa 2002) and Cu stress (Hanaka et al. 2015). It has been observed that MeJ can alleviate the stress imposed by Cd (Ahmad et al. 2017a, b), lead (Pb) (Piotrowska et al. 2009), arsenic (As) (Farooq et al. 2016, 2018), copper (Cu) (Poonam et al. 2013) boron (B) (Aftab et al. 2011) and nickel (Ni) (Sirhindi et al. 2016). On the other hand, studies performed on various plants have established that the addition of N can alleviate a range of toxicities in plants, such as water logging (Roy Chowdhury et al. 2017), UV-B radiation (Correia et al. 2005), freezing (Liu et al. 2017), elevated carbon dioxide (Ruiz-Vera et al. 2017) and metal stress (Giansoldati et al. 2012). Lin et al. (2011) working on rice showed that the deficiency of N under Cd stress significantly reduced chlorophyll, protein and nitrate contents.
Mentha arvensis, also known as mentholmint, belongs to the Lamiaceae family and is a perennial herbaceous plant distributed widely in the temperate regions of Europe, western and central Asia, eastern region of Siberia and North America and east to the Himalayan plane (Lawrence 2007). The plant has been widely cultivated for extraction of useful products which have diaphoretic, antiviral, anti-spasmodic, choleretic, stomachic, carminative, antifungal, antibacterial and vermifuge properties (Naeem et al. 2017), However, the regulatory effect of MeJ and N in reducing toxic effects of Cd stress in mentholmint (M. arvensis) has not been reported.
Nitrogen (N) is an important common limiting mineral nutrient element, which is the main constituent of all amino acids, proteins and a number of N-containing compounds that play a profound role in plant abiotic stress tolerance (Cetner et al. 2017; Mohanty et al. 2018; Siddiqui et al. 2008a, b; Khan et al. 2017). The metabolism of N is one of the fundamental physiological processes in plants in which N-mediated metabolites and related enzymes play a significant role against metal stress (Ren et al. 2017; Ai et al. 2017). The N-assimilation rate has been shown to be up-regulated in plants exposed to elevated metal stress (Khan et al. 2016a, b). N can also act as an important signalling agent, which triggers expression of genes, N-assimilation processes (Thind et al. 2018), metabolism of carbohydrates (Marschner 2011), rate of net photosynthesis (Singh et al. 2016a, b), antioxidant defence and osmoprotectant systems (Siddiqui et al. 2008a, b), PGRs, vitamins and nucleic acids (Beevers and Hageman 1969), chlorophyll biosynthesis (Zhang et al. 2014), and can maintain nutrient homeostasis (Xiao et al. 2017).
Considering the importance, the goals of the present research were to determine the interactive effect of foliar MeJ in the presence or absence of N in countering the detrimental effects of Cd stress in menthol mint.
Materials and Methods
Experimental Methodology
Healthy and uniform suckers of M. arvensis Linn. ‘Kushal’ were sterilized with NaOCl (5%) for 4 min followed by a washing with deionized and double distilled water (DDW) and then transplanted in earthen pots. Each experimental pot (30 × 45 cm) contains 7.5 kg mixture of soil and farmyard manure (6:1). The physicochemical characteristics of the soil were sandy loam texture, pH (1:2) 7.5, EC (1:3) 0.52 m mhos cm−1 and 92.6, 8.5 and 135.4 mg available of critical mineral nutrients, namely N, P and K per kg of soil, respectively. After germination, thinning was manually conducted and 3–4 healthy seedlings were maintained in each pot. The 150 mg kg−1 Cd stress was given basally in the form of cadmium chloride (CdCl2) through the soil at 30 days of transplantation (DAT). Methyl jasmonate (MeJ) and basal nitrogen (N) were given thrice 15 days apart beginning 15 days after Cd stress at 45, 60 and 75 DAT. The concentration of 4 g N per kg soil and MeJ was based on our earlier findings (Mohammad et al. 1998; Khan et al. 2009). The source of N was urea. Each pot was given DDW (200 mL pot−1) on every alternate day to keep the soil moist. The experiment was conducted in net house of Botany Department, Aligarh Muslim University Aligarh, India, with average day/night temperature of 25 ± 3/14 ± 2 °C; relative humidity, 65 ± 5%; photosynthetically active radiation (PAR), 810 ± 20 µmol m−2 s−1; critical photoperiod, 10–12 h. Each experiment was conducted in five replications. The plants were harvested at 90 DAT and biochemical, antioxidant enzyme and osmolyte content were determined in upper young leaves. The Cd, MeJ and N treatments were as follows:
-
0 mg kg−1 Cd + 0 M MeJ + 0 g N; T0 (control);
-
0 mg kg−1 Cd + 1 µM MeJ + 0 g N; T1
-
0 mg kg−1 Cd + 0 M MeJ + 4 g N; T2
-
0 mg kg−1 Cd + 1 µM MeJ + 4 g N; T3
-
150 mg kg−1 Cd + 0 M MeJ + 0 g N; T4
-
150 mg kg−1 Cd + 1 µM MeJ M + 0 g N; T5
-
150 mg kg−1 Cd + 0 M MeJ + 4 g N; T6
-
150 mg kg−1 Cd + 1 µM MeJ + 4 g N; T7
Determination of Growth and Yield Parameters
Shoot and root length were measured using a scale. Samples were kept in an oven and dried and then dry weight (DW) was recorded.
Estimation of Leaf Relative Water Content (LRWC) and Leaf Area
The standard method of Yamasaki and Dillenburg (1999) based on recording fresh, turgid and dry weight of leaf discs was adopted to estimate LRWC. LRWC was calculated using the following formula:
Leaf area (LA) was calculated by using graph sheets.
Determination of Photosynthetic Attributes
Chlorophyll contents were analysed using a portable SPAD chlorophyll meter (SPAD-502; Konica, Minolta Sensing, Inc., Japan).
Leaf gas exchange measurements viz-net photosynthetic rate (PN), stomatal conductance (gs) and carbon dioxide assimilation rate (Ci) were measured on the uppermost fully expanded leaves in full and bright sunlight between 10:00 and 12:00 h using IRGA (Li-COR 6400, Li-COR, Lincoln, NE, USA).
Chlorophyll fluorescence parameters were recorded with a junior PAM chlorophyll fluorometer (Heinz Mess und Regeltechnik, Heinz Walz Gmbh D-91090, Germany). The fully expanded horizontal Mentha leaves were dark-adapted for 30 min before measuring Fv/Fm (Li et al. 2007).
Determination of Rubisco Content
The Usuda (1985) standard protocol was employed to determine the activity of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco; EC4.1.1.39) by monitoring the oxidation of NADH at 28 °C and 342 nm.
Determination of Osmoprotectants
The content of glycine betaine (GB) was determined according to the method of Grieve and Grattan (1983). The acid ninhydrin based method of Bates et al. (1973) was used to estimate proline content.
Determination of Oxidative Stress Biomarkers
Hydrogen peroxide (H2O2) production, lipid peroxidation in the form of malondialdehyde (MDA) and electrolyte leakage (EL) was estimated using the methods of Velikova et al. (2000), Madhava Rao and Sresty (2000) and Dionisio-Sese and Tobita (1998) respectively. Electrolyte leakage was calculated using the following formula:
The content of superoxide (O2−) was quantified by the estimating the production of nitrite as a result of reaction of hydroxylamine with superoxide anions by employing the method of Wang and Luo (1990). The absorbance of reaction mixture was taken at 530 nm and O2− generation rate was calculated from the standard curve of sodium nitrite and expressed as µg g−1 (FW).
Estimation of Antioxidant Activities
Preparation of Enzyme Extract and Assay
Leaf material was homogenized in a pre-chilled pestle and mortar in 2 mL 100 mM potassium phosphate buffer (pH 7.0) having 1% of polyvinyl pyrrolidone and centrifuged at 12,000×g for 30 min at 4 °C; the supernatant was used to determine different enzyme activities. Superoxide dismutase activity (SOD, EC1.15.1.1) was measured using the nitroblue tetrazolium (NBT) reduction method of Van Rossum et al. (1997). Catalase (CAT: 1.11.1.6) activity was determined by monitoring the decomposition of H2O2 for 2 min at 240 nm (Aebi 1986). Ascorbate peroxidase (APX, 1.11.1.11) activity was assayed according to Nakano and Asada (1981). Glutathione reductase (GR, 1.6.4.2) activity was measured according to the method of Foster and Hess (1980). The activities of these enzymes were expressed in EU mg protein−1. All spectrophotometric analyses used in the present study was conducted using a portable spectrophotometer (ELICO SL 171 MINI SPEC).
Determination of Ascorbate, Carotenoids
Ascorbate contents were measured according to the method of Huang et al. (2005). Carotenoid content in leaves was estimated using the method of Lichtenthaler and Buschmann (2001). The freshly collected leaf samples were homogenized with a mortar and pestle in 100% acetone and then the optical density (OD) was read at 470 nm to determine carotenoid contents.
Determination of Nitrate Reductase (NR) Activity and Nitrogen Use Efficiency
NR activity was determined by adopting the method proposed by Jaworski (1971). A fresh leaf sample (500 mg) was crushed in phosphate buffer (pH 7.5) to which a mixture of 5% isopropanol and 0.2 M potassium nitrate were added, and the mixture was incubated at 30 °C for 120 min. After incubation of the reaction mixture, 0.02% of N-1-naphthyl ethylenediamine dihydrochloride and 1% sulphanilamide was added. The absorption of the coloured solution was recorded at 540 nm. Nitrogen use efficiency (NUE) was calculated asthe ratio of net photosynthesis to N content per unit LA.
Analysis of Root and Leaf Cd
Samples were dried in oven at 85 °C for 24 h and were then digested in a tri-acid (H2SO4, HNO3, HCLO4) mixture at the ratio of 5:1:1v/v at 80 °C on a dry block heater. HNO3 and H2O2 were added to make a transparent solution. The contents were determined by atomic absorption spectrophotometer (GBC, 932 plus; GBC Scientific Instruments, Braeside, Australia) and expressed as µg/g (DW).
Determination of Leaf Mineral Nutrients
Leaf N content was determined by the acid-peroxide digested method of Lindner (1944). The optical density of the reaction medium was read at 525 nm. The method of Fiske and Subbarow (1925) was adopted for P determination. The intensity of the blue colour was recorded at 620 nm.
Determination of Leaf K Content by Flame Emission Spectroscopy
The acid-peroxidase digested aliquot was also used for determining the content of K by flame photometer (Model, ELICO, CL22D, India), which was made to operate at 10 psi pressure (Hald 1947). All the critical nutrient concentrations were expressed in mg g−1 DW.
Scanning Electron Microscopy
Fresh leaf samples were fixed first with 2.5% glutaraldehyde plus 2% paraformaldehyde in 0.1 M phosphate buffer (pH 7.0) for 4 h. The leaves were again fixed with 1% osmium tetroxide in phosphate buffer (pH 7.0) for 50 min and washed with phosphate buffer for 10 min. After fixing, alcohol dehydration (50%, 70%, 80%, 90%, 95% and 100%) was carried out. Finally, leaves were dehydrated in a Carl Zeiss EVO 40 (Germany) scanning electron microscope critical point dryer with liquid CO2. The leaf samples were coated with gold–palladium and observed under a Carl Zeiss EVO 40 (Germany) scanning electron microscope (SEM) at high voltage (15 kV) and magnification of × 1500.
Statistical Analysis
Data were analysed following one-way analysis of variance (ANOVA) using SPSS software version 17. The values represented are means ± SE (n = 5). Different letters indicate significant differences between treatments at P ≤ 0.05. The principal component analysis was performed by Minitab Mtb EXE (2) software.
Results
Effects of MeJ and N on Morphological and Yield Parameters Under Cd Stress
The shoot length decreased significantly (P ≤ 0.05) by 14.53% in T4 (Cd) in comparison to that of the control (T0). Treatment with MeJ to Cd-stressed plants in T5 (Cd + MeJ) showed an increase of 7.70% over Cd-stressed plants. A similar increase of 5.39% was recorded for N application in T6 (Cd + N) over Cd-stressed plants. However, the combined application of MeJ + N in the presence of Cd stress in T7 (Cd + MeJ + N) resulted a more conspicuous and significant increase of 23.24% relative to their individual treatment (Fig. 1a).
a Length of shoot and root, b shoot fresh and dry weight, c leaf relative water content, d leaf area, e maximum PSII efficiency, f photochemical and non-photochemical quenching, g maximum capture efficiency and actual quantum yield, h Rubisco activity of cornmint cultivar ‘Kushal’ grown with/without 150 mg kg−1 Cd in precence of either alone MeJ or N or both. Data are presented as treatments mean ± SE (n = 5). Data followed by same letters are not significantly different by DMRT test at (P ≤ 0.05). 0 mg kg−1 Cd + 0 M MeJ + 0 g N (T0) (control); 0 mg kg−1 Cd + 1 µM MeJ + 0 gN (T1); 0 mg kg−1 Cd + 0 M MeJ + 4 g N (T2); 0 mg kg−1 Cd + 1 µM MeJ + 4 g N (T3); 150 mg kg−1 Cd + 0 M MeJ + 0 g N (T4); 150 mg kg−1 Cd + 1 µM MeJ + 0 g N (T5); 150 mg kg−1 Cd + 0 M MeJ + 4 g N (T6); 150 mg kg−1 Cd + 1 µM MeJ + 4 g N (T7)
The length of root was also negatively influenced by Cd treatment in T4 and it decreased by 26.60% relative to that of the control. Application of MeJ to Cd-stressed plants increased the root length by 36.84% compared to that of the T4 treatment (Fig. 1a). Nitrogen supply in T6 resulted in a significant root length increase of 23.79% over Cd-stressed plants.
A retardation in shoot fresh and dry mass by 22.36% and 46.73%, respectively, was also observed after exposing plants to Cd stress in T4 with respect to that of the control. However, the cumulative treatment of MeJ + N in T7 (Cd + MeJ + N) had a more significant effect and an increase of 35.17% in shoot fresh weight and 52.88% in shoot dry weight with respect to Cd plants in T4 was noticed (Fig. 1b).
MeJ and N Restore LRWC and LA
A significant (P ≤ 0.05) decrease of 30.81% was recorded in LRWC in T4 with respect to that of the T0 control treatment. MeJ in T5 significantly increased LRWC by 26.99% and N in T6 by 24.41%; however, in T7 treatment, LRWC was enhanced by 28.77% with the combined treatment of MeJ and N with respect to Cd-stressed plants (Fig. 1c).
Cd stress decreased LA by 14.22% in T4 relative to that of the control plants. The LA of non-stressed plants in T1 and T2 increased by 13.33% and 10.40%, respectively, in comparison to that of the T0 control plants. The combined effects of MeJ and N in T3 (MeJ + N) was more effective in the non-stressed plants and LA showed a significant increase of 18.18% compared to that of the T0 control plants (Fig. 1d). Regarding Cd stress, MeJ in T5 increased LA by 8.56% and N in T6 increased LA by 5.13%, but MeJ + N in T7 increased LA by 10.11% (Fig. 1d).
Influence of MeJ and N on Photosynthetic Attributes
The SPAD value declined by 31.37% in Cd-treated plants relative to that of the control (Table 1). Supplementing Cd-stressed plants with MeJ or N improved SPAD content by 24.04% and 17.34%, respectively, as compared to that of Cd-stressed plants. The combined application of MeJ + N (T7) to Cd-treated plants further increased total Chl content by 29.97% as compared to Cd alone.
The PN decrease was 44.26% in Cd-stressed plants (T4) relative to that of the control (T0) (Table 1). Supplementing Cd-stressed plants with MeJ or N improved PN by 34.91% and 12.85% in the T5 and T6 treatments, respectively. The combined supply of MeJ and N in T7 further increased the rate of PN by 48.79% as compared to that of Cd-stressed plants alone.
The decrease in gs and carbon dioxide assimilation efficiency by Cd was nullified completely by MeJ in plants receiving N input in T7 (Cd + MeJ + N) and the values were 4.92 and 8.51% relative to Cd-stressed plants in T4 (Table 1).
The chlorophyll fluorescence parameters showed a varied trend at the respective treatments. Cd stress in T4 decreased the maximal PSII (Fv/Fm) by 39.13% (Fig. 1e), photochemical quenching (qP) by 16.86% (Fig. 1f), actual quantum yield (f PSII) by − 21% (Fig. 1g) and maximum capture efficiency (f exc) by 36.11% (Fig. 1g), with respect to that of the control T0. Non-photochemical quenching (NPQ) increased by 38.02% (Fig. 1f). Treatment of Cd-stressed plants with MeJ in T5 increased Fv/Fm by 31.14%, qP by 12.65%, f PSII by 23.91% and f exc by 19.29%, but reduced NPQ by 12.67% relative to that of Cd-stressed plants alone. The combined dose of MeJ and N in T7 increased Fv/Fm, qP, f PSII and f exc by 44%, 14.81%, 40.67% and 48.88%, respectively, and decreased NPQ by 22.53% with respect to that of the Cd-stressed plants alone.
The activity of Rubisco showed a significant decrease of 28.66% in the T4 treatment relative to that of the control plants (Fig. 1h). A non-significant increase by N supply in T2 was noted relative to that of the control plants. The combined treatment of MeJ and N in T7 (MeJ + N) enhanced Rubisco activity by 42.21% over that of Cd-stressed plants.
Effects of MeJ and N on Osmoprotectants
Cd at the T4 concentration enhanced the accumulation of proline and GB by 1.52- and 2.90-fold, respectively, in comparison to that of the control (Fig. 2a). Furthermore, the highest proline and GB contents were found in treatment T7 (MeJ + N) with the combined supply of MeJ and N, as compared to N and MeJ alone under Cd stress. Treatment with Cd + MeJ + N in T7 exhibited a 1.69-fold higher GB content than Cd-fed plants in T4 (Fig. 2a).
a Contents of proline and glycine betaine, b superoxide ion (O2−), c electrolyte leakage and d TRABS and H2O2 of cornmint cultivar ‘Kushal’ grown with/without 150 mg kg−1 Cd in precence of either alone MeJ or N or both. Data are presented as treatments mean ± SE (n = 5). Data followed by same small and capital letters are not significantly different by DMRT test at (P ≤ 0.05). 0 mg kg−1 Cd + 0 M MeJ + 0 g N (T0) (control); 0 mg kg−1 Cd + 1 µM MeJ + 0 gN (T1); 0 mg kg−1 Cd + 0 M MeJ + 4 g N (T2); 0 mg kg−1 Cd + 1 µM MeJ + 4 g N (T3); 150 mg kg−1 Cd + 0 M MeJ + 0 g N (T4); 150 mg kg−1 Cd + 1 µM MeJ + 0 g N (T5); 150 mg kg−1 Cd + 0 M MeJ + 4 g N (T6); 150 mg kg−1 Cd + 1 µM MeJ + 4 g N (T7)
Effects of MeJ and N on TRABS Content, EL, H2O2 and O2 − Production
Cd caused a significant increase in TRABS content, EL and H2O2 accumulation by 53.97%, 67.26% and 56.66%, respectively, in T4 as compared to that of the T0 control treatment (Fig. 2c, d). Application of MeJ to plants receiving Cd and N, reduced the TRABS content by 47.12%, EL by 55.68% and H2O2 accumulation by 42.66% in T7 relative to that of Cd-only (T4) treated plants alone.
Cd stress leads to a significant (P ≤ 0.05) increase in O2− content by 52.72% as compared to T0 control plants. Both MeJ and N reduced O2− production in Cd-treated plants with respect to Cd-treated plants, however, decrease was more significant (P ≤ 0.05) when Cd-treated plants received MeJ in presence on N at T7 by 34.62% as compared to only Cd contaminated plants T4 (Fig. 2b).
MeJ and N Enhanced the Activity of Antioxidant Enzymes
Cd stress in T4 enhanced activities of SOD, CAT, APX and GR by 83.72%, 6.02%, 40.49% and 32.11%, respectively, with respect to that of the T0 control plants. Moreover, application of MeJ and N to plants in the T7 treatment increased the SOD by 64.65%, CAT by 8.77%, APX by 34.83% and GR by 56.47% as compared to that of the T4 treatment (Fig. 3a–d). However, no significant increase was noted in SOD, APX or GR activities in the T1and T2 treatments as compared to that of the T0 plants.
Activities of a SOD, b CAT, c APX, d GR, e AsA and f carotenoid content of cornmint cultivar ‘Kushal’ grown with/without 150 mg kg−1 Cd in precence of either alone MeJ or N or both. Data are presented as treatments mean ± SE (n = 5). Data followed by same letters are not significantly different by DMRT test at (P ≤ 0.05). 0 mg kg−1 Cd + 0 M MeJ + 0 g N (T0) (control); 0 mg kg−1 Cd + 1 µM MeJ + 0 gN (T1); 0 mg kg−1 Cd + 0 M MeJ + 4 g N (T2); 0 mg kg−1 Cd + 1 µM MeJ + 4 g N (T3); 150 mg kg−1 Cd + 0 M MeJ + 0 g N (T4); 150 mg kg−1 Cd + 1 µM MeJ + 0 g N (T5); 150 mg kg−1 Cd + 0 M MeJ + 4 g N (T6); 150 mg kg−1 Cd + 1 µM MeJ + 4 g N (T7)
Enhancement of Ascorbate and Carotenoids with Supplementation with MeJ and N
Cd stress caused a significant (P ≤ 0.05) decrease of 42.88% in AsA in the T4 treatment with respect to that of the T0 control. The Cd-induced decrease in AsA was significantly overcomed by supplementing with MeJ and N in combination in T7 rather than with individual treatment of MeJ or N relative to that of the Cd-stressed plants in T4 treatment (Fig. 3e).
The carotenoid content showed a non-significant increase when control plants were given MeJ or N in T1 and T2, respectively. Cd stress increased the carotenoid content by 50.84% with respect to that of the T0 (control) plants. However, treatment T7 enhanced the carotenoid content by 52.30% compared to the Cd-only treatment (Fig. 3f).
Nitrate Reductase Activity and Nitrogen Use Efficiency
Plants treated with Cd in T4 showed a significant decrease in NR activity by 55.00% with respect to that of the T0 control plants (Fig. 4a). Plant supplementation with a combined dose of MeJ + N in T3 increased the activity of NR by 35.48% over that of the control plants. Similarly, the combined dose of MeJ + N to Cd-stressed pants in T7 significantly (P ≤ 0.05) increased the activity of NR by 57.14% as compared to that of Cd-only stressed plants.
Activity of a NR and b nitrogen use efficiency of cornmint cultivar ‘Kushal’ grown with/without 150 mg kg−1 Cd in precence of either alone MeJ or N or both. Data are presented as treatments mean ± SE (n = 5). Data followed by same letters are not significantly different by DMRT test at (P ≤ 0.05). 0 mg kg−1 Cd + 0 M MeJ + 0 g N (T0) (control); 0 mg kg−1 Cd + 1 µM MeJ + 0 gN (T1); 0 mg kg−1 Cd + 0 M MeJ + 4 g N (T2); 0 mg kg−1 Cd + 1 µM MeJ + 4 g N (T3); 150 mg kg−1 Cd + 0 M MeJ + 0 g N (T4); 150 mg kg−1 Cd + 1 µM MeJ + 0 g N (T5); 150 mg kg−1 Cd + 0 M MeJ + 4 g N (T6); 150 mg kg−1 Cd + 1 µM MeJ + 4 g N (T7)
Photosynthetic-NUE exhibited a decreasing trend with the Cd application in T4 and the decrease was 1.64-fold with respect to that of the control plants. However, the combined treatment of MeJ and N in T7 alleviated the decrease in NUE and the increase was 1.47-fold with respect to that of the Cd-stressed plants in T4 (Fig. 4b).
Stomatal Response Studied Under Electron Microscopy
In the T0 control plants, normal stomatal aperture along with characteristic guard cells were observed, but with Cd stress, closed stomata were observed in T4. However, plants receiving MeJ in presence of N decreased the Cd effects on stomata and MeJ along with N prevented the stomatal closure in T7 (Fig. 5a–c).
Root and Leaf Cd Content
The plants accumulated higher Cd in roots (~ 11.92 µg−1 DW) than in leaves (~ 3.04 µg−1 DW). Application of N or MeJ reduced Cd content both in roots and leaves, but the higher reduction occurred in plants supplied with the combined treatment of N and MeJ in T7, in which significant (P ≤ 0.05) reductions of 73.68% and 61.74% in leaves and roots, respectively, were observed relative to that of Cd-treated plants alone (Fig. 6).
Content of Cd in root and leaf of cornmint cultivar ‘Kushal’ grown with/without 150 mg kg−1 Cd in precence of either alone MeJ or N or both. Data are presented as treatments mean ± SE (n = 5). Data followed by same letters are not significantly different by DMRT test at (P ≤ 0.05). 0 mg kg−1 Cd + 0 M MeJ + 0 g N (T0) (control); 0 mg kg−1 Cd + 1 µM MeJ + 0 gN (T1); 0 mg kg−1 Cd + 0 M MeJ + 4 g N (T2); 0 mg kg−1 Cd + 1 µM MeJ + 4 g N (T3); 150 mg kg−1 Cd + 0 M MeJ + 0 g N (T4); 150 mg kg−1 Cd + 1 µM MeJ + 0 g N (T5); 150 mg kg−1 Cd + 0 M MeJ + 4 g N (T6); 150 mg kg−1 Cd + 1 µM MeJ + 4 g N (T7)
Mineral Nutrient Contents
Under Cd stress, the contents of mineral nutrients (N, P, K) decreased by 49.62%, 29.41% and 14.58%, respectively, in T4 in comparison to that of the T0 (control) plants (Table 2). However, in the control plants supplemented with MeJ + N, N content increased significantly (P ≤ 0.05) by 31.79% relative to that of the control plants. In T7, the dual application of MeJ and N to Cd-stressed plants significantly increased the N content by 41.73% over Cd-stressed plants in T4. The P content increased with both MeJ and N in T5 and T6, respectively, but the increase was non-significant with respect to Cd-stressed plants in T4. K content increased by 36.68% with the combined dose of MeJ and N in T7 with respect that of the Cd-stressed plants in T4 (Table 2).
Principal Component Analysis (PCA)
PCA was used to assess the relation between Cd stress, MeJ and N application on various physiological and biochemical properties in mentholmint plants. According to PCA, growth and photosynthetic characters were in opposite direction with root and leaf Cd content, H2O2, O2•−, EL and TRABS content. In contrast, mineral mutrient contents were in positive direction with growth and photosynthetic parameters. Plant growth and photosynthesis under Cd stress increased with the application of MeJ and N via increase of mineral nurient contents and photosynthesis and by regulation of antioxidants and osmolytes. This was proved by PCA that antioxidants and osmolytes characters lie between plant growth and photosynthesis and oxidative stress biomarkers (Fig. 7).
Principal component analysis (PCA) of various parameters of mentholmint plants under cadmium (Cd) and methyl jasmonate (MeJ) or nitrogen (N). SL shoot length, RL root length, SFM shoot fresh mass, SDM shoot dry mass, LRWC leaf relative water content, LA leaf area, PN net photosynthesis, gs stomatal conductance, Ci intercellular CO2 concentration, N nitrogen, P phosphorous, K potassium, GB glycine betaine, superoxide ion (O2−), EL electrolyte leakage, TRABS thiobarbituric acid reactive substances, H2O2 hydrogen peroxide, SOD superoxide dismutase activity, CAT catalase, APX ascorbate peroxidise, GR glutathione reductase, AsA ascorbate, Fv/Fm maximum PSII efficiency, Qp photochemical, NPq non-photochemical quenching, NR nitrate reductase, NUE nitrogen use efficiency
Discussion
Cd is a non-essential toxic biometal that alters the physiology and metabolism of plants (Kaur et al. 2017; Anetor et al. 2016; Ahmad et al. 2015). In the present study, we observed significant repression of growth parameters because of Cd stress and our results are in agreement with the earlier results of Ahmad et al. (2017a, b) for Vicia faba, Per et al. (2016) for Brassica juncea, Shen et al. (2017) for Brassica campestris and Rizwan et al. (2017a, b) for Oryza sativa. Restricted plant growth because of Cd results from its cumulative effects on the uptake of water and minerals, metabolism of sugars, photosynthetic efficiency, cell division and elongation and enzyme activity (Shen et al. 2017; Ahmad et al. 2015). N supplementation protected M. arvensis from effects of Cd stress because application of N in the present study increased the mineral nutrient content, particularly N, and thus many N-containing metabolites and compounds that benefit enzyme activities associated with plant growth (Shah et al. 2017). N supplementation also promotes cell division and elongation of the mesophyll cells resulting in enhanced growth of leaf blades (Mac Adam et al. 1989), reflecting the significant enhancement in the photosynthetic efficiency and production of assimilates. Application of N and MeJ prevented the toxic effects of Cd on the uptake of essential elements, such as N, P and K, and improved the photosynthetic N-use efficiency, therefore resulting in greater allocation of N to Rubisco proteins. Similar to our results, Per et al. (2016) observed MeJ-mediated alleviation of Cd stress through increased nutrient assimilation. Yan et al. (2013) demonstrated significant alleviation of Cd toxicity in Capsicum after the application of MeJ. In the present study, application of N + MeJ exhibited maximal alleviation of Cd-induced deleterious effects on growth, suggesting the possible existence of crosstalk between N and MeJ.
Cd treatment reduced LRWC in the present study and similar decrease in LRWC has been reported in mustard and faba bean under Cd stress (Ahmad et al. 2011, 2017a, b). It was interesting to observe that application of N and MeJ caused a significant increase in the LRWC under normal and stress conditions probably because of the greater uptake of mineral nutrients, such as K, and the production of proline and GB. Li et al. (2007) have demonstrated that N supplementation increases root development in Cd-stressed plants and hence optimum water absorption capacity is maintained to a considerable extent. Cd stress significantly reduced LA and this might be due to reduced supply and incorporation of compounds to be utilized in plant growth in addition to restricted LRWC and cell division. Supply of N and MeJ restores the metabolic activity of leaf tissues and increases nutrient assimilation dynamics in plants (Fan et al. 2016; Iqbal et al. 2015; Gomez et al. 2010). Aplication of optimum N nutrition has the capacity to alleviate abiotic stress in crops plants by sustaining various metabolic activities even at reduced tissue water potential (Saud et al. 2017). In the present study, N application increase LRWC and this might be due to optimum water tissue balance, similar N-mediated increase in LRWC in wheat plants under N management has been reported (Akram et al. 2014). MeJ also alleviates decrease in LRWC probably by maintaining optimum water balance in mentholmint plants.
Cd significantly decreased the SPAD value and leaf gas exchange parameters in the present study. Cd triggers degradation of chlorophyll proteins by enhancing chlorophyllase activity and affecting the activity of their bio-synthesizing enzymes (Assche and Clijsters 1990; Alyemini et al. 2018). A reduction in chlorophyll content because of Cd treatment has been previously reported by Masood et al. (2012a, b), Khan et al. (2016a, b) in mustard and Abd-Allah et al. (2015) in sunflower plants. In agreement with our results, Singh et al. (2016a, b) and Per et al. (2016) also demonstrated individually the N and MeJ-induced protection of photosynthesis under NaCl- and Cd-stressed in mustard plants. N supply has been observed to alleviate abiotic stress-triggered deleterious effects on photosynthetic potential by increasing N-assimilation, activity of Rubisco and photosynthetic-NUE (Akram et al. 2014; Singh et al. 2016a, b; Iqbal et al. 2015). Reports describing the combined effect of N and MeJ are rare; however, in the present study, application of MeJ along with N improved N-assimilation (N content and NR activity) and resulted in the maintenance of Rubisco functioning and hence reduced the cyclic electron around PSII, thereby restricting the generation of toxic O2− (Makino 2003) which is agreement with our results. In addition, MeJ and N regulated the stomatal functioning under Cd stress (Fig. 5a–c) leading to continuous gas flow exchange and the associated gas exchange parameters. Similar effects of added N on increasing photosynthesis in B. juncea plants was reported by Mohammad et al. (1998) and Siddiqui et al. (2008), Malus prunifolia (Huang et al. 2018). In the present study, both N and MeJ mediated modulation of photosynthesis (increased PN) which may have resulted from the increased diffusion rate of CO2 from the atmosphere (increased A) into the intercellular spaces via the open stomatal aperture under Cd stress (Fig. 5c). Regulation and reversal of Cd-induced inhibition of PSII activity by the exogenous application of MeJ (Per et al. 2016) has been reported; however, reports regarding photosynthetic regulation involving the combined effect of N and MeJ on PSII activity under Cd stress are not available. Moreover, higher proline and GB content in plants because of N and MeJ either individually or jointly protected the carboxylase activity of Rubisco and the activity of PSII in the present study by quickly eliminating ROS and maintaining the tissue water content.
Proline and GB are common compatible organic osmoprotectants having regulatory roles in imparting tolerance to plants against abiotic stresses (Per et al. 2017; Sirhindi et al. 2016; Chen and Murata 2009). There is direct relationship of proline and N as available N in plants is directly stored as proline (Rhodes et al. 1999). Proline biosynthesis relies on an N source, as well as the application method (Neuberg et al. 2010) and in the present study, application of N along with MeJ increased the synthesis of proline under normal conditions and was maintained to highest level in Cd-treated plants with maximal accumulation observed in N + MeJ supplemented Cd seedlings. Accumulation of proline under Cd stress is a prevalent phenomenon in plants. Greater accumulation of proline and GB in N + MeJ treated Cd-stressed conditions could stabilize the process of protein synthesis and protein structure, thus preventing stress-mediated protein denaturation and maintaining enzyme activities (Sharmila and Saradhi 2002), and could lead to rapid detoxification of Cd-triggered ROS. Zakery-Asl et al. (2014) also reported that N application in Phaseolus vulgaris plants results in an increment of proline content in root and foliar organs via increasing the activities proline bio-synthesizing enzymes. Enhanced osmolyte accumulation during metal stress serves (i) to balance the water potential of plants (Sirhindi et al. 2016), (ii) as a metal chelator (Sharma et al. 1998), (iii) as free radical neutralization (Zhao 2011) and (iv) as an energy source for growth revival after stress release (Kavi Kishor and Sreenivasulu 2014). MeJ is also known to induce enzymes related to proline biosynthesis during stress conditions (Chen and Kao 1993) and greater accumulation of osmolytes results from the up-regulation and down-regulation of biosynthetic and catabolic genes of osmolyte generating pathways, respectively (Ahanger et al. 2017). Gao et al. (2004) found that the exogenously applied MeJ induced significant increases in GB biosynthesis in pear plants subjected to water stress conditions.
Cd stress triggered excessive generation of ROS causing increased peroxidation of membranes and leakage of essential cellular components and protein modification (Srivastava et al. 2014; Guo et al. 2016). Previously, increased ROS after Cd treatment has been reported in crops such as mustards, (Masood et al. 2012a, b) chickpea (Ahmad et al. 2016a, b) and tomato (Singh et al. 2018). Reduced ROS accumulation in MeJ and N supplemented seedlings may be attributed to the maintenance of efficient ROS-scavenging. In agreement with this observation, Per et al. (2016) have also demonstrated significant reduction in ROS generation, EL and TBARS under application of MeJ. MeJ has earlier been evidenced to change the fatty acid composition of membranes which makes them less prone to the damages done by ROS (Wang 1999). Moreover, optimizing ROS concentration can maintain the intimate crosstalk of ROS with other stress signalling components, such as transcription factors, leading to better elicitation of stress responses (Ahanger et al. 2017). N application to Cd-treated soybean plants resulted in a decrease of Cd stress, which was correlated with a reduction in Cd accumulation (Konotop et al. 2012) which is in aggrement with the present results. Further strengthening and protection against Cd was mediated by MeJ application, which was observed as significantly reduced endogenous Cd ion concentrations (Fig. 6) and such dilution in endogenous Cd after N and MeJ application may be attributed to greater biomass production. Similar ameliorative effects of exogenous MeJ against metal stress have been reported in rice (Singh and Shah 2014) and oil seed rape (Farooq et al. 2018) seedlings.
N-mediated alleviation of Cd stress via increased antioxidant enzyme activities has been reported in poplar plants by up-regulation and improved gene expression of antioxidant enzymes because of N supply has been reported in Populus plants under Cd stress (Zhang et al. 2014). Similarly, N-mediated alleviation of Cd stress via increased antioxidant enzyme activities has been reported in poplar plants by Zhang et al. (2017). Sirhindi et al. (2016) demonstrated that MeJ application protected Glycine max from the damaging effects of Ni by increasing the gene expression of antioxidant enzymes leading to improved metabolic and photosynthetic efficiency. Similar to these reports, application of MeJ and N in the present study significantly reduced the Cd-mediated excess accumulation of ROS by stimulating the activities of SOD, CAT, APX and GR. The higher activity of antioxidant enzymes in response to MeJ may be attributed to the direct interaction of MeJ with ROS (Chen et al. 2014). Increased CAT, APX and GR activity because of MeJ application in Cd-stressed plants has been reported by others as well (Per et al. 2016; Yan et al. 2015; Keramat et al. 2009). Increased APX and GR activity in N and MeJ supplemented plants resulted in rapid elimination of H2O2 via the AsA-GSH pathway. Application of MeJ + N further elevated the APX and GR activity, and increased GR activity by MeJ treatment in Arabidopsis thaliana plants has been reported to impart metal-stress tolerance at the post-translational level (Xiang 1998). Strengthening of the antioxidant system because of MeJ + N application apparently reduced ROS production and their consequent effects leading to alleviation of Cd-induced oxidative stress in M. arvensis. Greater GR activity and the content of AsA, and carotenoids in MeJ and N treated plants protected the photosynthetic electron transport by maintaining the optimal concentration of NADP and the redox homeostasis for maintaining the activity of key enzymes and levels of ROS. A similar ameliorative role of MeJ via increased antioxidant enzyme activities during Cd stress has been reported by Per et al. (2016) in B. juncea, Yan et al. (2015) in Solanum nigrum, Keramat et al. (2009) in Glycine max and Chen et al. (2014) in Kandelia obovata.
In the present study, contents of carotenoids increased under Cd stress. The contents of AsA decreased with Cd stress, which is in agreement with the finding of Chao et al. (2010) for rice plants who observed a decline in AsA content in response to Cd toxicity. AsA is a potent antioxidant that scavenges excess ROS in plants (Akram et al. 2017). Supplementation of MeJ + N further enhanced the AsA contents in the Cd-stressed plants, which could further protect the metabolism of M. arvensis. Increased AsA contents because of MeJ in Cd-treated K. obovata was also reported by Chen et al. (2014) and MeJ-induced up-regulated expression of genes encoding enzymes for AsA biosynthesis has been reported in A. thaliana (Wolucka et al. 2005). Greater production of AsA and carotenoids after the supplementation of N and MeJ may have improved the potentiality of M. arvensis plants to withstand the Cd stress by modulating the antioxidant defence pathways, such as AsA-GSH.
Supplementation of N and MeJ positively regulated the uptake and assimilation of N via improvement of NR activity. Greater uptake and assimilation of N sources, such as nitrates, mediates increased incorporation of available N into amino acids (Goel and Singh 2015). Similar decreases in N-assimilation by Cd stress have been reported by Gill et al. (2012) in Lepidium sativum. Enhanced N-assimilation after proper mineral application can be beneficial in arbitrating the demand for the key precursor molecules required for the synthesis of stress responsive metabolites, such as proline (Pandey and Agarwal 1998). Increased NUE because of N supplementation has been reported in Chile peppers (Huez Lopez et al. 2011). Hampered uptake capacity of nutrients because of Cd stress can be one of the possible reasons for restricted N-assimilation and external application of N and MeJ may have increased the expression of proteins required for enhanced mineral uptake at the root level, making them available for enzymes, such as NR, and hence increase NUE. Availability of nutrients and plant growth regulators increases N-assimilation under adverse environmental conditions (Iqbal et al. 2011; Singh et al. 2016a). Therefore, it can be suggested that increased N-assimilation because of N and MeJ supplementation might increase the synthesis of metabolites, optimizing the antioxidant defence and osmoprotectant systems in Cd-stressed plants and leading to protection of photosynthesis.
Supplementation of MeJ and N reduced the translocation of Cd from root-to-shoot leading to significant declines in oxidative damage. Similar effects of MeJ in the reduction of Cd toxicity have been reported by Per et al. (2016) in B. juncea and Yan et al. (2013) in Capsicum frutescens. Exogenous phytohormone application has also been reported to modulate metal toxicity by reducing the root-to-shoot metal translocation rate (Singh et al. 2015).
Cd exhibits direct competition with important mineral nutrients (Nazar et al. 2012; Asgher et al. 2014). Our results showed that Cd accumulation considerably reduced the uptake of essential mineral nutrients, which was mitigated by application of N and MeJ (Table 2). N and MeJ restore the mechanism of root-to-shoot translocation of key mineral elements and these observations are in agreement with Kováčik et al. (2011) for Scenedesmus quadricauda and Gomez et al. (2010) for Solanum lycopersicum. Rossato (2002) while working on Brassica napus observed the positive regulatory role of N and MeJ in increasing uptake and assimilation of mineral elements. Positive effects of N and MeJ were obvious when applied jointly and proved to be of considerable importance in the mitigating Cd stress. Increased uptake of mineral nutrients by Bothriochloa ischaemum was observed because of application of N (Ai et al. 2017). Plants showing enhanced uptake and assimilation of key mineral elements exhibited greater accumulation of free amino acids and tolerance to abiotic stresses (Kavi Kishor and Sreenivasulu 2014; Singh and Shah 2014).
Conclusion
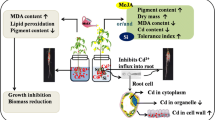
Cadmium stress induced growth and photosynthetic inhibition, and enhanced ROS biosynthesis. Uptake and assimilation of mineral nutrients, stomatal movements and photosynthetic pigments declined in M. arvensis because of Cd; however, exogenous supplementation of MeJ and N optimized ROS metabolism reversing the negative effects of Cd-induced oxidative damage on physio-morphological attributes by protecting chlorophyll, Rubisco and stomatal functioning, therefore leading to growth maintenance. Such Cd stress mitigating roles of N and MeJ can be attributed to their cumulative effects on antioxidant metabolism, nutrient assimilation and osmolyte synthesis. More importantly, the maintenance of redox components, such as AsA, and carotenoids, because of N and MeJ application may have led to significant protection of the photosynthetic system. Decreased root-to-shoot translocation of Cd in N and MeJ treated M. arvensis seedlings could be exploited for its improved growth and productivity on metal and metalloid effected soils. A schematic representation drawn from the present work that how MeJ and N mediates Cd changes to improve growth and photosynthesis in given in Fig. 8.
A schematic representation showing the potential mechanism of Cd stress alleviation by combined treatment of exogenous MeJ and N. Cd stress causes oxidative stress in plants by orchestrating the generation of ROS and stomatal inhibition. Exogenous application of MeJ and N in the present study enhances the N-assimilation by directly stimulating the activity of NR and N content or by signalling pathways. Additionally, MeJ directly enhanced osmolytes, nutrient contents and activity of various antioxidants. The redox status of antioxidant defence system optimizes the ROS balance, which was altered by Cd stress. Efficient ROS metabolism maintained by MeJ and N improved growth and photosynthesis in Cd persistence in Mentha arvensis plants. Cd cadmium, N nitrogen, MeJ methyl jasmonate, ROS reactive oxygen species. Pointed arrows show promoting and blunt arrows show inhibitory interactions respectively
References
Abd-Allah EF, Hashem A, Alqarawi AA, Alwathnani HA (2015) Alleviation of adverse impact of cadmium stress in sunflower (Helianthus annuus L.) by arbuscular mycorrhizal fungi. Pak J Bot 47:785–795
Aebi H (1986) Catalase in vitro. Method Enzymol 105:121–126
Aftab T, Khan MMA, Idrees M, Naeem M, Hashmi N (2011) Methyl jasmonate counteracts boron toxicity by preventing oxidative stress and regulating antioxidant enzyme activities and artemisinin biosynthesis in Artemisia annua L. Protoplasma 248:601–612
Ahanger MA, Akram NA, Ashraf M, Alyemeni MN, Wijaya L, Ahmad P (2017) Plant responses to environmental stresses—from gene to biotechnology. AoB Plants 9:4. https://doi.org/10.1093/aobpla/plx025
Ahmad P, Nabi G, Ashraf M (2011) Cadmium-induced oxidative damage in mustard [Brassica juncea (L.) Czern. & Coss.] plants can be alleviated by salicylic acid. South Afr J Bot 77:36–44
Ahmad P, Sarwat M, Bhat NA, Wani MR, Kazi AG, Tran LSP (2015) Alleviation of cadmium toxicity in Brassica juncea L. (Czern. & Coss.) by calcium application involves various physiological and biochemical strategies. PLoS ONE. https://doi.org/10.1371/journal.pone.0114571
Ahmad P, Abdel Latef AA, Abd_Allah EF, Hashem A, Sarwat M, Anjum NA, Gucel S (2016a) Calcium and potassium supplementation enhanced growth, osmolyte secondary metabolite production, and enzymatic antioxidant machinery in cadmium-exposed chickpea (Cicer arietinum L.). Front Plant Sci 7:513. https://doi.org/10.3389/fpls.2016.00513
Ahmad P, Rasool S, Gul A, Sheikh SA, Akram NA, Ashraf M, Gucel S (2016b) Jasmonates: multifunctional roles in stress tolerance. Front Plant Sci 7:813. https://doi.org/10.3389/fpls.2016.00813
Ahmad P, Alyemeni MN, Wijaya L, Alam P, Ahanger MA, Alamri SA (2017a) Jasmonic acid alleviates negative impacts of cadmium stress by modifying osmolytes and antioxidants in faba bean (Vicia faba L.). Arch Agron Soil Sci 63:1889–1899
Ahmad P, Ahanger MA, Alyemeni MN, Wijaya L, Alam P (2017b) Exogenous application of nitric oxide modulates osmolyte metabolism, antioxidants, enzymes of ascorbate-glutathione cycle and promotes growth under cadmium stress in tomato. Protoplasma 255:79–93
Ai Z, Wang G, Liang C, Liu H, Zhang J, Xue S, Liu G (2017) The effects of nitrogen addition on the uptake and allocation of macro-and micronutrients in Bothriochloa ischaemum on Loess Plateau in China. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01476
Akram M, Iqbal M, Jamil M (2014) The response of wheat (Triticum aestivum L.) to integrating effects of drought stress and nitrogen management. Bul J Agr Sci 20:275–286
Akram NA, Shafiq F, Ashraf M (2017) Ascorbic acid-A potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00613
Alyemeni MN, Ahanger MA, Wijaya L, Alam P, Bhardwaj R, Ahmad P (2018) Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 255:459–469
Anetor JI, Uche CZ, Ayita EB, Adedapo SK, Adeleye JO, Anetor GO, Akinlade SK (2016) Cadmium level, glycemic control, and indices of renal function in treated type II diabetics: implications for polluted environments. Front Public Health. https://doi.org/10.3389/fpubh.2016.00114
Anjum NA, Umar S, Iqbal M (2014) Assessment of cadmium accumulation, toxicity, and tolerance in Brassicaceae and Fabaceae plants-implications for phytoremediation. Environ Sci Pollut Res 21:10286–10293
Asgher M, Khan MIR, Anjum NA, Khan NA (2014) Minimising toxicity of cadmium in plants—role of plant growth regulators. Protoplasma 252:399–413
Assche F, Clijsters H (1990) Effects of metals on enzyme activity in plants. Plant Cell Environ 13:195–206
Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Beevers L, Hageman RH (1969) Nitrate reduction in higher plants. Ann Rev Plant Physiol 20:495–522
Cetner MD, Kalaji HM, Goltsev V, Aleksandrov V, Kowalczyk K, Borucki W, Jajoo A (2017) Effects of nitrogen-deficiency on efficiency of light-harvesting apparatus in radish. Plant Physiol Biochem 119:81–92
Chao YY, Hong CY, Kao CH (2010) The decline in ascorbic acid content is associated with cadmium toxicity of rice seedlings. Plant Physiol Biochem 48:374–381
Chen CT, Kao CH (1993) Osmotic stress and water stress have opposite effects on putrescine and proline production in excised rice leaves. Plant Growth Regul 13:197–202
Chen TH, Murata N (2009) Glycinebetaine protects plants against abiotic stress: mechanisms and biotechnological applications. Plant Cell Environ 34:1–20
Chen J, Yan Z, Li X (2014) Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol Environ Saf 104:349–356
Correia CM, Pereira JMM, Coutinho JF, Björn LO, Torres-Pereira JMG (2005) Ultraviolet-B radiation and nitrogen affect the photosynthesis of maize: a Mediterranean field study. Eur J Agr 22:337–347
Cuypers A, Plusquin M, Remans T, Jozefczak M, Keunen E, Gielen H, Nawrot T (2010) Cadmium stress: an oxidative challenge. Biometals 23:927–940
Dionisio-Sese ML, Tobita S (1988) Antioxidant responses of rice seedlings to salinity stress. Plant Sci 135:1–9
Fan JW, Du YL, Wang BR, Turner NC, Wang T, Abbott LK, Li FM (2016) Forage yield, soil water depletion, shoot nitrogen and phosphorus uptake and concentration, of young and old stands of alfalfa in response to nitrogen and phosphorus fertilisation in a semiarid environment. Field Crops Res 198:247–257
Farooq MA, Gill RA, Islam F, Ali B, Liu H, Xu J, Zhou W (2016) Methyl jasmonate regulates antioxidant defense and suppresses arsenic uptake in Brassica napus L. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00468
Farooq MA, Islam F, Yang C, Nawaz A, Gill RA, Ali B, Zhou W (2018) Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate–glutathione cycle in oilseed rape roots. Plant Growth Regul 84:135–148
Fiske CH, Subbarow Y (1925) The colorimetric determination of phosphorus. J Biol Chem 66:375–400
Foster JG, Hess JL (1980) Responses of superoxide dismutase and glutathione reductase activities in cotton leaf tissue exposed to an atmosphere enriched in oxygen. Plant Physiol 66:482–487
Gao XP, Wang XF, Lu YF, Zhang LY, Shen YY, Liang Z, Zhang DP (2004) Jasmonic acid is involved in the water-stress-induced betaine accumulation in pear leaves. Plant Cell Environ 27:497–507
Giansoldati V, Tassi E, Morelli E, Gabellieri E, Pedron F, Barbafieri M (2012) Nitrogen fertilizer improves boron phytoextraction by Brassica juncea grown in contaminated sediments and alleviates plant stress. Chemosphere 87:1119–1125
Gill SS, Khan NA, Tuteja N (2012) Cadmium at high dose perturbs growth, photosynthesis and nitrogen metabolism while at low dose it up regulates sulfur assimilation and antioxidant machinery in garden cress (Lepidium sativum L.). Plant Sci 182:112–120
Goel P, Singh AK (2015) Abiotic stresses downregulate key genes involved in nitrogen uptake and assimilation in Brassica juncea L. Plos one 10:e0143645. https://doi.org/10.1371/journal.pone.0143645
Gómez S, Ferrieri RA, Schueller M, Orians CM (2010) Methyl jasmonate elicits rapid changes in carbon and nitrogen dynamics in tomato. New Phytol 188:835–844
Grieve CM, Grattan SR (1983) Rapid assay for determination of water soluble quaternary ammonium compounds. Plant Soil 70:303–307
Guo H, Hong C, Chen X, Xu Y, Liu Y, Jiang D, Zheng B (2016) Different growth and physiological responses to cadmium of the three Miscanthus species. PLoS ONE 11:e0153475. https://doi.org/10.1371/journal.pone.0153475
Hald PM (1947) The flame photometer for the measurement of sodium and potassium in biological materials. J Biol Chem 167:499–510
Hanaka A, Maksymiec W, Bednarek W (2015) The effect of methyl jasmonate on selected physiological parameters of copper-treated Phaseolus coccineus plants. Plant Growth Regul 77:167–177
Huang C, He W, Guo J, Chang X, Su P, Zhang L (2005) Increased sensitivity to salt stress in an ascorbate-deficient Arabidopsis mutant. J Exp Bot 56:3041–3049
Huang H, Gao H, Liu B, Qi T, Tong J, Xiao L, Song S (2017) Arabidopsis MYB24 regulates jasmonate-mediated stamen development. Front Plant Sci 8:1525
Huang L, Li M, Zhou K, Sun T, Hu L, Li C, Ma F (2018) Uptake and metabolism of ammonium and nitrate in response to drought stress in Malus prunifolia. Plant Physiol Biochem 127:185–193
Huez López MA, Ulery AL, Samani Z, Picchioni G, Flynn R (2011) Response of chile pepper (Capsicum annuum L.) to salt stress and organic and inorganic nitrogen sources: I. growth and yield. Trop Subtrop Agroecol 14(1):757–763
Iqbal N, Nazar R, Syeed S, Masood A, Khan NA (2011) Exogenously-sourced ethylene increases stomatal conductance, photosynthesis, and growth under optimal and deficient nitrogen fertilization in mustard. J Exp Bot 62:4955–4963
Iqbal N, Umar S, Khan NA (2015) Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J Plant Physiol 178:84–91
Jaworski EG (1971) Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun 43:1274–1279
Kaur R, Yadav P, Sharma A, Thukral AK, Kumar V, Kohli SK, Bhardwaj R (2017) Castasterone and citric acid treatment restores photosynthetic attributes in Brassica juncea L. under Cd(II) toxicity. Ecotoxicol Environ Saf 145:466–475
Kavi Kishor PB, Sreenivasulu N (2014) Is proline accumulation per se correlated with stress tolerance or is proline homeostasis a more critical issue? Plant Cell Environ 37:300–311
Keramat B, Kalantari KM, Arvin MJ (2009) Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.) Afr. J Microbiol Res 3:240–244
Khan NM, Siddiqui MH, Mohammad F, Naeem M, Khan MMA (2009) Calcium chloride and gibberellic acid protect linseed (Linum usitatissimum L.) from NaCl stress by inducing antioxidative defence system and osmoprotectant accumulation. Acta Physiol Plant 32:121–132
Khan MIR, Khan NA, Masood A, Per TS, Asgher M (2016a) Hydrogen peroxide alleviates nickel-inhibited photosynthetic responses through increase in use-efficiency of nitrogen and sulfur, and glutathione production in mustard. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00044
Khan NA, Asgher M, Per TS, Masood A, Fatma M, Khan MIR (2016b) Ethylene potentiates sulfur-mediated reversal of cadmium inhibited photosynthetic responses in mustard. Front Plant Sci 7:1628. https://doi.org/10.3389/fpls.2016.01628
Khan A, Tan DKY, Afridi MZ, Luo H, Tung SA, Ajab M, Fahad S (2017) Nitrogen fertility and abiotic stresses management in cotton crop: a review. Environ Sci Pollut Res 24:14551–14566
Konotop Y, Mezsaros P, Matusikova I, Batsmanova L, Taran N (2012) Application of nitrogen nutrition for improving tolerance of soybean seedlings to cadmium. Environ Exp Bot 10:139–144
Kováčik J, Klejdus B, Štork F, Hedbavny J, Bačkor M (2011) Comparison of methyl jasmonate and cadmium effect on selected physiological parameters in Scenedesmus quadricauda (Chlorophyta, Chlorophyceae). J Phycol 47:1044–1049
Kováčik J, Babula P, Hedbavny J (2017) Comparison of vascular and non-vascular aquatic plant as indicators of cadmium toxicity. Chemosphere 180:86–92
Lawrence BM (2007) Mint: the genus Mentha. CRC Press, Boca Raton
Li JG, Jin SL, Chen YQ, Lin GL, Han XR, Li TQ, Zhu E (2007) Effects of nitrogen fertilizer on the root morphology and cadmium accumulation in low cadmium treatment Sedum alfredii Hance. Chin Agric Sci Bull 23:260–265
Li K, Yu H, Li T, Chen G, Huang F (2017) Cadmium accumulation characteristics of low-cadmium rice (Oryza sativa L.) line and F1 hybrids grown in cadmium-contaminated soils. Environ Sci Poll Res 24:17566–17576
Lichtenthaler HK, Buschmann C (2001) Current protocols in food analytical chemistry. Wiley, New York
Lin YL, Chao YY, Huang WD, Kao CH (2011) Effect of nitrogen deficiency on antioxidant status and Cd toxicity in rice seedlings. Plant Growth Regul 64:263–273
Lindner R (1944) Rapid analytical methods for some of the more common inorganic constituents of plant tissues. Plant Physiol 19:76
Liu BY, Lei CY, Liu WQ (2017) Nitrogen addition exacerbates the negative effects of low temperature stress on carbon and nitrogen metabolism in moss. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01328
MacAdam JW, Volenec JJ, Nelson CJ (1989) Effects of nitrogen on mesophyll cell division and epidermal cell elongation in tall fescue leaf blades. Plant Physiol 89:549–556
Madhava Rao KV, Sresty TVS (2000) Antioxidative parameters in the seedlings of pigeonpea (Cajanus cajan (L.) Millspaugh) in response to Zn and Ni stresses. Plant Sci 157:113–128
Makino A (2003) Rubisco and nitrogen relationships in rice: leaf photosynthesis and plant growth. Soil Sci Plant Nutr 49:319–327
Maksymiec W, Krupa Z (2002) The in vivo and in vitro influence of methyl jasmonate on oxidative processes in Arabidopsis thaliana leaves. Acta Physiol Plant 24:351–357
Marschner H (2011) Marschner’s mineral nutrition of higher plants. Academic Press, Cambridge
Masood A, Iqbal N, Khan NA (2012a) Role of ethylene in alleviation of cadmium-induced photosynthetic capacity inhibition by sulphur in mustard. Plant Cell Environ 35:524–533
Masood A, Iqbal N, Khan MIR, Khan NA (2012b) The coordinated role of ethylene and glucose in sulfur-mediated protection of photosynthetic inhibition by cadmium. Plant Signal Behav 7:1420–1422
Mnasri M, Ghabriche R, Fourati E, Zaier H, Sabally K, Barrington S, Ghnaya T (2015) Cd and Ni transport and accumulation in the halophyte Sesuvium portulacastrum: implication of organic acids in these processes. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00156
Mohammad F, Khan T, Afridi R, Fatma A (1998) Effect of nitrogen on carbonic anhydrase activity, stomatal conductance, net photosynthetic rate and yield of mustard. Photosynthetica 34:595–598
Mohanty S, Swain CK, Tripathi R, Sethi SK, Bhattacharyya P, Kumar A, Gautam P (2018) Nitrate leaching, nitrous oxide emission and N use efficiency of aerobic rice under different N application strategy. Arch Agric Soil Sci 64:465–479
Naeem M, Aftab T, Idrees M, Singh M, Ali A, Khan MMA, Varshney L (2017) Modulation of physiological activities, active constituents and essential oil production of Mentha arvensis L. by concomitant application of depolymerised carrageenan, triacontanol and 28-homobrassinolide. J Essen Oil Res 29:179–188
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nazar R, Iqbal N, Masood A, Khan MIR, Syeed S, Khan NA (2012) Cadmium toxicity in plants and role of mineral nutrients in its alleviation. Am J Plant Sci 03:1476–1489
Neuberg M, Pavlíková D, Pavlík M, Balík J (2010) The effect of different nitrogen nutrition on proline and asparagine content in plant. Plant Soil Environ 56:305–311
Pandey R, Agarwal R (1998) Water stress-induced changes in proline contents and nitrate reductase activity in rice under light and dark conditions. Physiol Mol Biol Plants 4:53–57
Per TS, Khan NA, Masood A, Fatma M (2016) Methyl jasmonate alleviates cadmium-induced photosynthetic damages through increased S-assimilation and glutathione production in mustard. Front Plant Sci. https://doi.org/10.3389/fpls.2016.01933
Per TS, Khan NA, Reddy PS, Masood A, Hasanuzzaman M, Khan MIR, Anjum NA (2017) Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol Biochem 115:126–140
Piotrowska A, Bajguz A, Godlewska-Żyłkiewicz B, Czerpak R, Kamińska M (2009) Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). Environ Exp Bot 66:507–513
Poonam S, Kaur H, Geetika S (2013) Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Millsp. Seedlings under copper stress. Am J Plant Sci 04:817–823
Ren B, Dong S, Zhao B, Liu P, Zhang J (2017) Responses of nitrogen metabolism, uptake and translocation of maize to waterlogging at different growth stages. Front Plant Sci. https://doi.org/10.3389/fpls.2017.01216
Rhodes D, Varlues PE, Sharp RE (1999) Role of amino acids in abiotic stress resistance. In: Singh BK (ed) Plant amino acids: biochemistry and biotechnology. Marcel Dekker, New York, pp 319–356
Rizwan M, Ali S, Hussain A, Ali Q, Shakoor MB, Zia-ur-Rehman M, Asma M (2017a) Effect of zinc-lysine on growth, yield and cadmium uptake in wheat (Triticum aestivum L.) and health risk assessment. Chemosphere 187:35–42
Rizwan M, Ali S, Akbar MZ, Shakoor MB, Mahmood A, Ishaque W, Hussain A (2017b) Foliar application of aspartic acid lowers cadmium uptake and Cd-induced oxidative stress in rice under Cd stress. Environ Sci Poll Res 24:21938–21947
Rossato L (2002) Nitrogen storage and remobilization in Brassica napus L. during the growth cycle: effects of methyl jasmonate on nitrate uptake, senescence, growth, and VSP accumulation. J Exp Bot 53:1131–1141
Roy Chowdhury S, Brahmanand PS, Manikandan N, Ambast SK (2017) Effect of N application on its utilization and gaseous exchange in cat tail (Typha elephantina) under waterlogged condition. Ind J Plant Physiol 22:263–266
Ruiz-Vera UM, De Souza AP, Long SP, Ort DR (2017) The role of sink strength and nitrogen availability in the down-regulation of photosynthetic capacity in field-grown Nicotiana tabacum L. at elevated CO2 concentration. Front Plant Sci. https://doi.org/10.3389/fpls.2017.00998
Saud S, Fahad S, Yajun C, Ihsan MZ, Hammad HM, Nasim W, Amanullah H, Arif M, Alharby H (2017) Effects of nitrogen supply on water stress and recovery mechanisms in kentucky bluegrass plants. Front Plant Sci 8:983. https://doi.org/10.3389/fpls.2017.00983
Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: reactive oxygen species at the cross-road. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00187
Shah JM, Bukhari SAH, Zeng JB, Quan XY, Ali E, Muhammad N, Zhang GP (2017) Nitrogen (N) metabolism related enzyme activities, cell ultrastructure and nutrient contents as affected by N level and barley genotype. J Integr Agric 16:190–198
Sharma M, Laxmi A (2016) Jasmonates: emerging players in controlling temperature stress tolerance. Front Plant Sci. https://doi.org/10.3389/fpls.2015.01129
Sharma SS, Schat H, Vooijs R (1998) In vitro alleviation of heavy metal-induced enzyme inhibition by proline. Phytochemistry 49:1531–1535
Sharmila P, Pardha Saradhi P (2002) Physiology and biochemistry of metal toxicity and tolerance in plants. Springer, Netherlands, pp 179–199
Shen G, Niu J, Deng Z (2017) Abscisic acid treatment alleviates cadmium toxicity in purple flowering stalk Brassica campestris L. ssp. chinensis var. purpurea Hort.) seedlings. Plant Physiol Biochem 118:471–478
Siddiqui MH, Khan MN, Mohammad F, Khan MMA (2008a) Role of nitrogen and gibberellin (GA3) in the regulation of enzyme activities and in osmoprotectant accumulation in Brassica juncea L. under salt stress. J Agric Crop Sci 194:14–224
Siddiqui MH, Mohammad F, Khan MN, Khan MMA (2008b) Cumulative effect of soil and foliar application of nitrogen, phosphorus, and sulfur on growth, physico-biochemical parameters, yield attributes, and fatty acid composition in oil of erucic acid-free rapeseed-mustard genotypes. J Plant Nutr 31:1284–1298
Silva AJ, Nascimento CWA, Gouveia-Neto AS (2016) Assessment of cadmium phytotoxicity alleviation by silicon using chlorophyll a fluorescence. Photosynthetica 55:648–654
Singh I, Shah K (2014) Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 108:57–66
Singh AP, Dixit G, Mishra S, Dwivedi S, Tiwari M, Mallick S, Tripathi RD (2015) Salicylic acid modulates arsenic toxicity by reducing its root to shoot translocation in rice (Oryza sativa L.). Front Plant Sci 6:340. https://doi.org/10.3389/fpls.2015.00340
Singh M, Singh VP, Prasad SM (2016a) Responses of photosynthesis, nitrogen and proline metabolism to salinity stress in Solanum lycopersicum under different levels of nitrogen supplementation. Plant Physiol Biochem 109:72–83
Singh M, Singh VP, Prasad SM (2016b) Nitrogen modifies NaCl toxicity in eggplant seedlings: assessment of chlorophyll a fluorescence, antioxidative response and proline metabolism. Biocatal Agric Biotechnol 7:76–86
Singh S, Singh A, Srivastava PK, Prasad SM (2018) Cadmium toxicity and its amelioration by kinetin in tomato seedlings vis-à-vis ascorbate-glutathione cycle. J Photochem Photobiol B 178:76–84
Sirhindi G, Mir MA, Abd-Allah EF, Ahmad P, Gucel S (2016) Jasmonic acid modulates the physio-biochemical attributes, antioxidant enzyme activity, and gene expression in Glycine max under nickel toxicity. Front Plant Sci. https://doi.org/10.3389/fpls.2016.00591
Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey RS (2014) Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251:1047–1065
Su C, Jiang Y, Li F, Yang Y, Lu Q, Zhang T, Xu Q (2017) Investigation of subcellular distribution, physiological, and biochemical changes in Spirodela polyrhiza as a function of cadmium exposure. Environ Exp Bot 142:24–33
Thind HS, Singh Y, Sharma S, Goyal D, Singh V, Singh B (2018) Optimal rate and schedule of nitrogen fertilizer application for enhanced yield and nitrogen use efficiency in dry-seeded rice in north-western India. Arch Agric Soil Sci 64:196–207
Usuda H (1985) The activation state of ribulose 1,5-bisphosphate carboxylase in maize leaves in dark and light. Plant Cell Physiol. https://doi.org/10.1093/oxfordjournals.pcp.a077047
Van Rossum MW, Alberda M, van der Plas LH (1997) Role ofoxidative damage in tulip bulb scale micropropagation. Plant Sci 130:207–216
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci 151:59–66
Wang SY (1999) Methyl jasmonate reduces water stress in strawberry. J Plant Growth Regul 18:127–134
Wang AG, Luo GH (1990) Quantitative relation between the reaction of hydroxylamine and superoxide anion radicals in plants. Plant Physiol Commun 6:55–57
Wasternack C (2014) Action of jasmonates in plant stress responses and development—applied aspects. Biotechnol Adv 32:31–39
Wolucka BA, Goossens A, Inzé D (2005) Methyl jasmonate stimulates the de novo biosynthesis of vitamin C in plant cell suspensions. J Exp Bot 56:2527–2538
Xiang C (1998) Glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Xiao Y, Li Y, Che Y, Deng S, Liu M (2017) Effects of biochar and nitrogen addition on nutrient and Cd uptake of Cichorium intybus grown in acidic soil. Int J Phytorem. https://doi.org/10.1080/15226514.2017.1365342
Yamasaki S, Dillenburg LR (1999) Measurements of leaf relative water content in Araucaria angustifolia. Rev Brasilleira Fisiol Vegetal 11:69–75
Yan Z, Chen J, Li X (2013) Methyl jasmonate as modulator of Cd toxicity in Capsicum frutescens var. fasciculatum seedlings. Ecotoxicol Environ Saf 98:203–209
Yan Z, Zhang W, Chen J, Li X (2015) Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol Plant 59:373–381
Yang L, Ji J, Harris-Shultz KR, Wang H, Wang H, Abd-Allah EF, Hu X (2016) The dynamic changes of the plasma membrane proteins and the protective roles of nitric oxide in rice subjected to heavy metal cadmium stress. Front Plant Sci 7:190
Zakery-Asl MA, Bolandnazara S, Oustanb S (2014) Effect of salinity and nitrogen on growth,sodium, potassium accumulation, and osmotic adjustment of halophyte Suaedaa egyptiaca (Hasselq.) Zoh. Arch Agron Soil Sci 60:785–792
Zhang F, Wan X, Zheng Y, Sun L, Chen Q, Zhu X, Liu M (2014) Effects of nitrogen on the activity of antioxidant enzymes and gene expression in leaves of Populus plants subjected to cadmium stress. J Plant Interact 9:599–609
Zhang F, Li J, Huang J, Lin L, Wan X, Zhao J, Chen Q (2017) Transcriptome profiling reveals the important role of exogenous nitrogen in alleviating cadmium toxicity in poplar plants. J Plant Growth Regul 36:942–956
Zhao Y (2011) Cadmium accumulation and antioxidative defenses in leaves of Triticum aestivum L. and Zea mays L. Afr J Biotechnol 10:2936–2943
Acknowledgements
AZ is thankful to UGC (New Delhi) India and Aligarh Muslim University, Aligarh India for providing the research fellowship No. BTM-2015-04-GH-7403. We are also grateful to University Sophisticated Instrumentation Facility (USIF) A.M.U., Aligarh for SEM analysis. We are also thankful to Prof. Athar Ali Khan and Prof. Aquil Ahmad from Department of Statistics and Operation Research AMU., Aligarh (India) for their help during statistical analysis.
Author information
Authors and Affiliations
Contributions
AZ designed the experiment. AZ carried out experimental analysis. AZ wrote the draft and revised the manuscript, while FM overall supervised the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that No conflict of interest exists.
Rights and permissions
About this article
Cite this article
Zaid, A., Mohammad, F. Methyl Jasmonate and Nitrogen Interact to Alleviate Cadmium Stress in Mentha arvensis by Regulating Physio-Biochemical Damages and ROS Detoxification. J Plant Growth Regul 37, 1331–1348 (2018). https://doi.org/10.1007/s00344-018-9854-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9854-3