Abstract
Methyl jasmonate (MJ) is an important plant growth regulator, involves in various physiological processes of plants. In the present study, role of MJ in tolerance to oilseed rape (Brassica napus L.) roots under arsenic (As) stress was investigated. The treatments were comprised of three MJ doses (0, 0.1, and 1 µM) and two levels of As (0 and 200 µM). Arsenic stress resulted in oxidative damage as evidenced by decreased root growth and enhanced reactive oxygen species and lipid peroxidation. However, plants treated with MJ decreased the H2O2 and O2 ·− contents in roots and have higher antioxidant activities. Importantly, results showed that MJ enhanced the redox states of AsA and GSH, and the related enzymes involved in the AsA–GSH cycle. Moreover, MJ also induced the secondary metabolites related enzymes (PAL and PPO) activities, under As stress. PAL and PPO expression was further increased by MJ application in the roots of B. napus under As stress. MJ also reduced the total As content compared with As alone treated plants. These findings suggest the role of MJ in mitigation of the As-induced oxidative damage by regulating AsA and GSH redox states and by reducing As uptake in both cultivars.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heavy metals are toxic to plants but certain heavy metals are required as an essential element for plant metabolism. However, essential or non-essential heavy metals including some other metalloids in excess amount cause toxicity in plants (Nagajyoti et al. 2010). When plants accumulate the heavy metals at elevated levels, it results in oxidative stress which in turn leads to morpho-physiological changes in plants (Dhankar and Solanki 2011). Arsenic (As) is a ubiquitous non-essential element to plants (Jedynak et al. 2010). Arsenic enters or its presence in the environment is both due to anthropogenic activities, such as the use of As-based pesticides and mining as well as natural including geogenic (Farooq et al. 2016a; Lee et al. 2008; Heikens et al. 2007). Although As is a non-redox active metalloid but its toxicity causes the cellular disruption through induction of oxidative stress via overproduction of reactive oxygen species (ROS) (Farooq et al. 2015). Plants require controlled regulation of intracellular ROS formation, but increased level of ROS results in uncontrolled oxidation which leads to cellular damage and eventual cell death.
Plant hormones such as salicylic acid (SA), abscisic acid (ABA), auxin, brassinosteroid (Br) gibberellic acid (GA), hydrogen sulfide (H2S) and jasmonate (JA) are signaling molecules, involved in plant defense against biotic and abiotic stresses (Ali et al. 2014a; Ryu and Cho 2015; del Amor and Cuadra-Crespo 2011; Bari and Jones 2009). Among these hormones, methyl jasmonate (MJ) an important derivative of JA, involved in several mechanisms related to plant physiological, morphological and biochemical processes. A considerable evidences indicate that JA alleviate the stress conditions in plants tempted due to salinity, drought, wounding, herbivory and plant -pathogens interactions (Dar et al. 2015; Santino et al. 2013; Wu et al. 2012; Yoon et al. 2009; Farmer et al. 2003). Several reports have shown that jasmonate-responsive genes (JRGs) expression is changed under stress conditions such as wounding, insect attack, pathogen infection, ozone stress, and thus play important role in plants stress tolerance (Howe 2004; Halitschke and Baldwin 2004; Dombrowski 2003; Kanna et al. 2003). It has also been observed that exogenous JA application enhanced the antioxidative capability of plants under metal stress condition (Yoon et al. 2009; Farmer 2007). In plants antioxidant system superoxide dismutase (SOD), catalase (CAT) and peroxidases (POD) and ascorbate peroxidase (APX) detoxified the ROS and protect the plant cells from oxidative damage (Islam et al. 2016a, b; Farooq et al. 2016b). In antioxidant metabolism ascorbate and glutathione are also important compounds and play defensive role against oxidative stress. Sasaki-Sekimoto et al. (2005) found that under ozone stress JA application regulate the ascorbate and glutathione metabolism in Arabidopsis. However, few studies have investigated the beneficial roles of MJ on plants under metal stress induced antioxidant responses (Poonam et al. 2013; Yan et al. 2013; Keramat et al. 2010). Nowadays, oilseed rape (Brassica napus L.) is mainly used to complete the edible oil requirements, but it is also considered as a potential candidate for phytoextraction (Ali et al. 2014b). Due to its higher biomass in comparison to natural metal (hyper) accumulators, B. napus contributes to the suitability as a phytoextraction (Grispen et al. 2006) and thus it is of utmost importance to exploit its potential in roots against As stress under the exogenous influence of JA. Most of the work related to the role of MJ has been performed on the leaves and cell culture however the alleviatory effects of MJ especially for root remain insufficient. So, to identify the link between role of MJ and As-induced oxidative stress in roots of plants, we used two cultivars (cv. Zheda 622 and cv. ZS 758) of B. napus plant. The effect of MJ under As stress on plant root length, total As content, ascorbate and glutathione cycle related enzymes and their gene expression in B. napus roots were observed in the present study. Furthermore, the role of MJ in alleviation of plasma membrane damage, antioxidant activities and metabolites changes as well as ultrastructure alterations in B. napus roots were also evaluated.
Materials and methods
Plant material and growth conditions
Seeds of two leading oilseed rape (B. napus L.) cultivars (cvs. Zheda 622 and ZS 758) previously detected as differing in metals tolerance (Farooq et al. 2015) were obtained from the College of Agriculture and Biotechnology, Zhejiang University and used in this study. Mature seeds of both cultivars were germinated in plastic pots (170 mm x 220 mm) filled with peat soil. Three uniform plants per pot were plugged into evenly spaced holes on the pot cover, and placed under ambient conditions (20–24 °C temperature and 55–60% relative humidity) in a green house. After 2 weeks plants were exposed to different concentrations of arsenic (As) (0, 50 and 200 μM) by using the salt NaAsO2 and methyl jasmonate (MJ) (0, 0.1 and 1.0 µM) in different combinations for 14 days. Experiment was performed in four replicates. Analyses were performed at both concentration but due to lower effect at 50 μM and for the sake of conciseness, only the results at the maximum concentration are given. The pH of solution was maintained at 5.7 ± 0.1 with 1 M NaOH or HCl solution. Aeration was given continuously through air pump. Hoagland solution was changed after every 3 days. Fourteen days after treatment, plants roots were thoroughly washed with deionized water to remove surface ions. With respect to laboratory assay, samples were collected either fresh or immediately frozen in liquid N2 and kept frozen until analysis.
Assessment of seedlings growth tolerance index (GTI) and As uptake
The length of longest root of each plant was recorded after 14 days of treatment periods. Growth inhibitory rate (%) was calculated according to Wilkins (1978).
For total As concentration analysis, oven dried samples of roots were ash at 550 °C for 20 h in a muffle furnace. After that, by adding 31% (m/v) HNO3 and 17.5% (v/v) H2O2, ash was incubated at 70 °C for about 2 h. The As concentration in the digest was determined using an atomic fluorescence spectroscopy.
Determinations of reactive oxygen species (ROS) and histochemical staining
For hydrogen peroxide (H2O2) measurement we follow the procedure of Velikova et al. (2000) by extracting the root sample in TCA (0.1%, w/v).1 mL KI (1 M) was added in extracted supernatant in presence of 10 mM PBS (pH 7.0) and change in the absorbance was calculated at 390 nm.
For measurement of superoxide radicals (O2 ·−), root samples was homogenized in 65 mM PBS (pH 7.8), and the contents was determined spectrophotometrically as previously described by the method of Jiang and Zhang (2001).
Hydrogen peroxide (H2O2) accumulation was visually detected by using the method of Thordal-Christensen et al. (1997) staining with 3,3-diaminobenzidine (DAB). The O2 ·− accumulation was visualized using the nitrobluetetrazolium (NBT) staining procedure (Romero-Puertas et al. 2004). The NBT and DAB stained roots were seen under a light microscope (model leica MZ 95).
Determinations of lipid peroxidation and cell death content
Lipid peroxidation was determined in terms of malondialdehyde (MDA) content in B. napus plant roots by the method of Zhou and Leul (1999). Root samples (0.5 g) were extracted with 5-mL of 0.1% (w/v) trichloroacetic acid (TCA).1-mL aliquot mixture extract and 4-mL 0.5% thiobarbituric acid in 20% trichloroacetic acid was incubated for 30 min at 95 °C, followed by a quick cooling on ice bath. The absorbance of supernatant was recorded at 532 nm; correction for non-specific absorption was done by subtracting values read at 600 nm.
Evans blue solution (0.25%) was used to stain the roots for cell death contents. After washing two times with distilled water, 50% methanol/1% SDS mixture was used to extraction the dye at 50◦C for 1 h and subsequently measured by spectrophotometrically at 595 nm.
Measurement of endogenous JA concentration
Endogenous JA concentration was determined by using a commercial enzyme-linked immunosorbent ELISA kit (MLBIO tech, China) according to the manufacturer instructions. About 0.1 g of fresh plant tissue was rinsed with 1× saline phosphate-buffer then homogenized in 1 mL PBS and stored overnight at − 20 °C. According to the manufacturer instructions, samples and standards were added and measured spectrophotometrically at a wavelength of 450 nm.
Total RNA extraction and quantitative real-time PCR (RT-qPCR) assay
Total RNA was isolated from root tissues (0.1 g) of different treatments with the reagent Trizol following the method of manufacturer’s procedure. Prime Script TMRT reagent kit (Takara Co. Ltd., Japan) with gDNA (genomic DNA) was used to synthesis the cDNA. By using SYBR® Premix Ex Taq II (Takara Co. Ltd., Japan) cDNA samples were assayed by quantitative real-time PCR (qRT-PCR) in the iCycleriQTM Real-time detection system (Bio-Rad, Hercules, CA, USA). Desire gene primer sequences were made by using the vector NTI, a primer construction tool with the help of sequence databases (http://www.ncbi.nlm.nih.gov). Detail of forward (F) and reverse (R) primer of each gene were presented in the supplementary material Table 1 with PCR conditions.
Biochemical analysis of enzyme activities
Root samples (0.5 g) were homogenized in potassium phosphate buffer (PBS) (50 mM) (pH 7.8) in cold conditions for enzyme activity. Total superoxide dismutase (SOD) was assayed spectrophotometrically at 560 nm by measuring the inhibition of nitro blue tetrazolium (NBT) (Zhang et al. 2008). The 3-mL reaction mixture was comprised of 50 mM PBS, 100 μL of enzyme extract, 13 mM methionine, 75 μM NBT, 2 μM riboflavin and 0.1 mM EDTA. One unit of SOD activity was assayed by the amount of enzyme required to cause 50% inhibition of NBT.
Catalase (CAT) activity was determined in change of absorbance at 240 nm in 3 mL reaction mixture of 50 mM PBS (pH 7.0) with 2 mM EDTA-Na2, 10 mM H2O2 and 100 μL enzyme extract by using the extinction coefficient of H2O2 (39.4 mM cm− 1) for 1 min (Aebi 1984).
The ascorbate peroxide (APX) activity was determined at 290 nm for 30 s after addition of H2O2 according to the method reported by Nakano and Asada (1981), in 3 mL reaction mixture comprised of 100 mM phosphate (pH 7), 0.1 mM EDTA-Na2, 0.3 mM ascorbic acid, 0.06 mM H2O2 and 100 μL enzyme extract. Peroxidase (POD) activity was determined as describe by Zhou and Leul (1999).
Determination of glutathione metabolites and ascorbate content
Reduced glutathione (GSH) was measured by the method of Fadzilla et al. (1997). Ground tissue of roots was suspended in 5 mL of 10% trichloroacetic acid (TCA) and centrifuged at 15,000×g for 15 min. Supernatant of an amount 150 µL was added in to the 100 µL DTNB, (6 mM) and 700 µL NADPH (0.3 mM) in the presence of 50 µL glutathione reductase (10 units mL− 1), monitored at 412 nm. Standard curve was made for analysis of GSH contents. To determine GSSG content, supernatant in amount of 120 µL was mixed with 2-vinylpyridine (10 µL) with triethanolamine (20 µL) and incubated at 25 °C for 25 min. Solution was analyzed at at 412 nm and calibration curve was developed for GSSG contents. Glutathione reductase (GR) activity was determined with the oxidation of NADPH for 1 min at 340 nm (extinction coefficient 6.2 mM cm− 1) (Jiang and Zhang 2002). The concentration of AsA was determined according to Łukasik et al. (2012).
Secondary metabolism-related enzyme assay
Based on reaction product of cinnamic acid, the phenylalanine ammonia-lyase (PAL) activity was determined according to the procedure of Dai et al. (2006). One unit of PAL activity is the change in absorbance at 290 nm of enzyme extract. We followed the Ruiz et al. (1999) to determine the polyphenol peroxidase (PPO) activity by monitoring the absorbance at 370 nm.
Transmission electron microscopy
Small root sections were fixed overnight in 2.5% glutaraldehyde. After 24 h phosphate buffer (PBS) was used to wash the samples and post fixed in 1% osmium oxide (OsO4) for 1 h. Again after washing with PBS, samples were dehydrated with ethanol and embedded in Spurr’s resin overnight. Specimens in ultrathin sections (80 nm) were mounted on copper grids and examined through transmission electron microscope at 60.0 kV.
Statistical analysis
The analysis of variance (ANOVA) was computed to determine the statistically differences while Duncan’s multiple range test applied for the calculation of confidence level at 95% by using the statistical package SPSS, (Chicago, IL, USA).
Results
Effects of MJ and As on root growth rate inhibition (GRI) and total As content
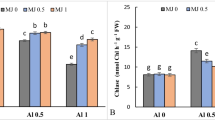
Data regarding root growth inhibition of B. napus seedlings under different treatments of As and MJ has been presented in Fig. 1. After 14 days of As stress, the reduction of root length was 36 and 47% in ZS 758 and Zheda 622 respectively, compared to control plants (Fig. 1). However, exogenous application of MJ improved the decrease in root length under As stress in both cultivars, and the maximum effect was observed at 1 µM MJ (Fig. 1a). The root growth of B. napus plants increased by 26% (ZS 758) and 35% (Zheda 622) after MJ application (1 µM), when compared with As alone stressed plants. Analysis of total As concentration revealed that cultivar Zheda 622 roots had higher As contents than ZS 758. However, As uptake in plant roots was alleviated after the MJ application, especially for the treatment of 1 µM MJ. Application of MJ to As stressed (MJ 1 + 200 µM) plants reduced the As concentration in both cultivars by 27 and 25% in ZS 758 and Zheda 622 respectively (Fig. 1b).
Effects of different treatments of methyl jasmonate (MJ) and arsenic (As) on a growth rate inhibition (GRI) % and b As uptake in roots of two cultivars of B. napus. Different letters showed statistically significant differences (p ≤ 0.05) and vertical bars on graphs represent the mean ± standard error
Exogenous MJ alleviated As-induced ROS accumulation in B. napus roots
In order to evaluate the As-induced oxidative stress, H2O2 and O2 ·− contents representing the major reactive oxygen species (ROS) were estimated (Fig. 2). Application of MJ treatments alone had no significant effect on H2O2 and O2 ·−. MJ treated plants, under As stress showed significantly lower H2O2 and O2 ·−contents in comparison with alone As-treated B. napus root (Fig. 2a, b). As shown in Fig. 2a, As exposure resulted in significant increase in H2O2 content, while application of MJ resulted in decrease in H2O2 content, however more reduced contents was observed in ZS 758. In turn, O2 ·− content significantly decreased by MJ application regardless of cultivars compared with As alone treated plants (Fig. 2b). Histochemical staining results also showed that MJ application modulated the As stress-triggered ROS accumulation in both B. napus cultivars root.
Effects of different treatments of methyl jasmonate (MJ) and arsenic (As) on oxidative stress in terms of ROS such as a hydrogen peroxide (H2O2) and b superoxide (O2 ·−) contents c lipid peroxidation (MDA) and d plasma membrane integrity (cell death content) in roots of two cultivars of B. napus. Different letters showed statistically significant differences (p ≤ 0.05) and vertical bars on graphs represent the mean ± standard error
Exogenous MJ mediated lipid peroxidation and plasma membrane integrity in B. napus
Malondialdehyde (MDA) concentration enhanced in both cultivars of B. napus when exposed to alone As stress compared with the non-treated plants (Fig. 2c). The enhanced concentration of MDA was 101% in ZS 758 and by 178% in Zheda 622, compared with their corresponding controls. Lower MDA concentration was observed in ZS 758 than in Zheda 622. The concentration of MDA, however, significantly decreased after the MJ application as compared with alone As-stressed treatment (Fig. 2c). The MJ application was significant in cultivar ZS 758 than in cultivar Zheda 622 and more effective at higher MJ level (1 µM) than at lower MJ level (0.1 µM). Similarly, both B. napus cultivars exhibited significantly higher level of plasma membrane integrity under As stress (Fig. 2c). Application of MJ to B. napus help in sustaining the membrane integrity in roots, as exhibited by lower cell death content in comparison with stressed plants (Fig. 2d).
MJ alleviated As-induced antioxidant enzymes activities in B. napus roots
Results showed that As stress significantly increased SOD and APX activities in both cultivars. SOD activity was greater as 45 and 27% in ZS 758 and Zheda 622 respectively, as compared with their respective controls (Fig. 3a). MJ alone did not show any significant difference but considerably showed significant effect at 1 µM when supplied with As. Under stress condition, application of MJ further enhanced the SOD activity by 36% (ZS 758) and 39% (Zheda 622) (Fig. 3a). APX activity in the plants exposed to As stress (200 µM) was 62% greater in Zheda 622 and 39% in ZS 758 than that of control. MJ application significantly increased APX activity in both cultivars, but considerably no significant difference was noted among the cultivars (Fig. 3b). POD and CAT activities were decreased by 45 and 3% in ZS 758 and 57 and 5% in Zheda 622, respectively (Fig. 3c, d). Application of MJ further enhanced the activities of CAT and POD as compared to As stressed plants (Fig. 3c, d).
Effects of different treatments of methyl jasmonate (MJ) and arsenic (As) on antioxidant enzymes activities of a superoxide dismutase (SOD), b ascorbate peroxidase (APX), c catalase (CAT) and d peroxidase (POD) in roots of two cultivars of B. napus. Different letters showed statistically significant differences (p ≤ 0.05) and vertical bars on graphs represent the mean ± standard error
MJ induced changes in glutathione cycle related enzymes under As stress
Transcriptional analysis of γECS gene, a key enzyme for glutathione biosynthesis was performed in 14-days-old B. napus seedlings after MJ and As treatments (Fig. 4a). γECS genes showed an up-regulation after As treatment by 1.95- and 1.53-fold in ZS 758 and Zheda 622 respectively; however, their expression was further significantly up-regulated after MJ application by 3.02- and 2.74-fold respectively. The GSH contents in As exposed significantly increased in both cultivars. MJ (1 µM) treatment to As exposed seedling further increased the GSH contents as compared with As treated plants (Fig. 4b). Similarly, GR gene in ZS 758 showed an up-regulation of 0.25-fold under As treatment and 0.48-fold after MJ (1 µM) treatment to stress plants (Fig. 4c). In contrast, Zheda 622 showed significant decrease of 0.11-fold in GR gene after As treatment. While, expression of GR was up-regulated by 0.41-fold after MJ (1 µM) treatment to stressed plants (Fig. 4c). Similarly, GR activity significantly increased under As alone and after MJ + As treatment in ZS 758. While in Zheda 622 a significant decrease was observed when treated with As alone (200 µM) as compared with control plants. Application of MJ reduced the As effects and increased the GR activity as compared with As alone treated plants (Fig. 4d).
Effects of different treatments of methyl jasmonate (MJ) and arsenic (As) on expression of a Gamma-glutamylcysteine synthetase (γECS), b reduced glutathione content (GSH), c glutathione reductase activity (GR), and d related gene expression of GR, e oxidized glutathione content (GSSG) and f ratio of GSH/GSSG, GR in roots of two cultivars of B. napus. Different letters showed statistically significant differences (p ≤ 0.05) and vertical bars on graphs represent the mean ± standard error
GSSG contents decreased significantly by 37% in ZS 758 and 41% in Zheda 622 under As alone treatment, compared with control plants (Fig. 4e). Alone MJ treatments showed no significance differences with the control. When MJ was applied under As stress, it increased the GSSG contents significantly by 35 and 45%, in ZS 758 and Zheda 622 respectively. The maximum contents of GSSG contents was observed by the addition of 1 µM MJ to As stressed plants in both cultivars (Fig. 4e). In contrast, the similar ratio of GSH/GSSG was observed under control condition while significantly increased under As alone and MJ + As treatments, as compare to control plants. However, among As alone and MJ + As treated plants no significant differences in GSH/GSSG ratio was observed in both cultivars (Fig. 4f).
AsA content and DHAR gene expression in B. napus roots under MJ and As application
Arsenic stress significantly decreased AsA contents by 14% in ZS 758 and 13% in Zheda 622 as compared to control plants (Fig. 5a). MJ treatments showed a synergetic effect on AsA contents in both cultivars under As stress and it increased the AsA contents in ZS 758 by 10% and in Zheda 622 by 7% as compared with As alone stress plants (Fig. 5a). The transcript level of DHAR was up-regulated after As-exposure by 0.21-fold in cultivar ZS 758 and 0.2-fold in Zheda 622 as compared to control plants (0.16-fold) (Fig. 5b). MJ application at 0.1 and 1 µM, the DHAR showed an up-regulation of 0.23-, 0.21-fold in ZS 758 and 0.24- and 0.22-fold in Zheda 622, respectively (Fig. 5b).
Effects of different treatments of methyl jasmonate (MJ) and arsenic (As) on a ascorbate contents (AsA), b gene expression of dehydroascorbate reductase (DHAR) c jasmonate contents (JA) and d gene expression of lipoxygenase (LOX) in roots of two cultivars of Brassica napus. Different letters showed statistically significant differences (p ≤ 0.05) and vertical bars on graphs represent the mean ± standard error
Effects of As stress and MJ on JA content and LOX gene expression
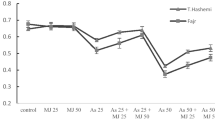
Data regarding JA contents in the roots of two B. napus cultivars are presented in Fig. 4. JA contents were significantly decreased in both cultivars which were more obvious in Zheda 622 when treated with As alone. However, the exogenous application of MJ significantly increased the total JA contents in both B. napus cultivars as compared with control, while the higher contents was observed in Zheda 622 compared with ZS 758 (Fig. 5c). Furthermore, the analysis of JA synthesis pathway gene lipoxygenase 2 (LOX 2) also showed a significant increase under As stress conditions (Fig. 5d). LOX showed significant up-regulation upon exposure in ZS 758 and Zheda 622 (1.78- and 1.54-fold respectively). In both cultivars, better effect was observed at MJ 1 µM + 200 µM As level and the expression of LOX was found to be up-regulated by 2.01- fold in ZS 758 and twofold in Zheda 622.
Effects of As stress and MJ on plant secondary metabolites
Secondary metabolites analysis showed that the activities of PAL and PPO in B. napus roots were increased after 14-days of As exposure, being 70 and 4% in ZS 758, and 25 and 4% in Zheda 622 respectively (Fig. 6a, b). MJ treatment alone had no effect but when applied with As it significantly further increased the PAL and PPO activities by 52 and 6% in ZS 758 and 60 and 5% in Zheda 622, respectively as compared with As alone treatment (Fig. 6a, b). PAL and PPO genes showed an up-regulation of 1.38-, 0.74-fold in ZS 758 and 1.27-, 0.63-fold in Zheda 622 under As alone treatment, as compared with control plants (Fig. 6c, d). Application of MJ under As stress also led a significant increases in PAL and PPO transcripts compared with As alone treated plants. After As treatment, the increase in PAL and PPO transcript was 1.86 and 1.25- fold in ZS 758 and 1.71 and 1.12-fold in Zheda 622 respectively, as compared to control plants (Fig. 6c, d).
Effects of different treatments of methyl jasmonate (MJ) and arsenic (As) on a, b secondary metabolites activities and c, d related gene expression of phenylalanine ammonia-lyase (PAL), and polyphenol peroxidase (PPO) in roots of two cultivars of B. napus. Different letters showed statistically significant differences (p ≤ 0.05) and vertical bars on graphs represent the mean ± standard error
Effects of As stress and MJ on root ultrastructure
Transmission electron microscopic (TEM) micrographs revealed well-defined cytoplasm and organelles, smaller vacuoles, and bigger nuclei and nucleoli in the roots of control plants (Fig. 7). Numerous alterations were noted in the root ultra-structure cells under As stress. Increase in the size of vacuole, disappearance of the nucleolus and disruption of the nucleus and nuclear membrane were some of the features, which could be observed more evidently in both the cultivars (Fig. 7a–f). Moreover, in cultivar Zheda 622 vacuoles were larger in size and the structure of nucleus was more irregular as compared to ZS 758 (Fig. 7b). While the supplementation of MJ alleviated the damage to the nucleus caused by As stress (Fig. 7c, f). In As + MJ (1 µM) treated plants, MJ reduced the effect of As stress and improved the structure of nucleus and nuclear membrane in comparison with the plants exposed to alone As stress.
TEM micrographs of nucleus of root tip cells of two B. napus cultivars. a Control of cultivar ZS 758. b Cultivar ZS 758 exposed to (200 µM) As alone. c Cultivar ZS 758 exposed to As (200 µM) + MJ (1 µM). d Control of Zheda 622. e Cultivar Zheda 622 exposed to (200 µM) As alone. f Cultivar Zheda 622 exposed to As (200 µM) + MJ (1 µM). N nucleus; Nu nucleolus; NM nuclear membrane; and Vac vacuole
Discussion
Heavy metal stress is among the important abiotic stresses that affects various physiological and biochemical aspects (e.g., plant growth, energy, protein synthesis, lipid metabolism) associated with plant growth and development (Hossain and Komatsu 2013; Farooq et al. 2016a). Root bioassay of B. napus seedlings showed that root elongation was significantly reduced under As stress. This could be explained as the negative effects of As on cell elongation. However, the stress produced by As was alleviated when the B. napus roots treated with an exogenous MJ application. Exposure of As resulted in inhibition of root elongation in both cultivars (ZS 758 and Zheda 622) of B. napus, while the effects of MJ, improved As tolerance in B. napus plants observed as increase in root elongation and reduced As uptake (Fig. 1a, b). These results are consistent with the earlier reports of Yan et al. (2013) who suggested that MJ mitigated the inhibitory effect of Cd on root growth.
Metalloid is known to cause oxidative injury in plant tissue by enhancing reactive oxygen species (ROS) levels. The excess H2O2 and O2 ·− contents caused by As stress resulted increase in lipid peroxidation (MDA) and plasma membrane integrity, which were considered markers for membrane lipid peroxidation (Garg and Singla 2011; Farooq et al. 2015). In biological membrane system, lipid peroxidation impairs the membrane functions and causes the inactivation of enzymes and membrane-bound receptors. The present study showed that As treated B. napus roots had high levels of H2O2 and O2 ·− (Fig. 2a, b) linked with the increased MDA content and plasma membrane integrity (Fig. 2c, d). Under As stress condition, roots of MJ-treated B. napus plants accumulated less H2O2, O2 ·−and MDA, and had lower plasma membrane integrity as compared to As treated plants (Fig. 2). These results suggest that MJ may sustain the oxidative stress by maintaining the steady-state ROS intracellular concentrations and play a defensive role in alleviating the As-induced membrane damage. Our results are in the agreement with those found in Phaseolus coccineus where MJ exhibited protective effect in alleviating the lipid peroxidation under metal stress (Hanaka et al. 2016).
Plants have a wide range of defense mechanisms to tolerate the toxic effects of metal including the antioxidant activities that are good indicator of internal cell situation. The roots of cultivar ZS 758 experienced low oxidative damage and had higher activities of antioxidant enzymes as compared to Zheda 622 which showed overwhelming production of ROS under As stress (Fig. 3). MJ application is reported to reduce metal toxicity by preventing the oxidative stress in roots of P. coccineus and Solanum nigrum (Yan et al. 2015; Hanaka et al. 2016). MJ application significantly increased the antioxidant enzyme activities (Fig. 3) and alleviated the oxidative damage induced by As stress.
Moreover, AsA and GSH are well-known antioxidants in the AsA–GSH cycle (Noctor and Foyer 1998). GSH biosynthetic pathway in plants can be determined by γECS and GR, both are the important key enzymes for the GSH synthesis and recycling pathway. The regulatory role of MJ in glutathione redox homeostasis can be given by the expression of γECS (Shan and Liang 2010). The expression of γECS in stressed and MJ treated plants supported the enhanced level in GSH content in comparison with As alone treated plants. Similarly, an increase in γECS was observed in ozone stress by Sasaki-Sekimoto et al. (2005). Further, Xiang and Oliver (1998) also found that JA treatment increased the γECS transcript level and GSH content, as well as GR activity and expression (Fig. 4). The present study showed the ability of MJ treated plants to cope with As stress correlated with maintenance of glutathione redox states. This was achieved as increasing GSH content, GR activity and GSH/GSSG ratio in accordance with the findings of Singh and Shah (2014), who also experienced similar induction in GSH metabolites in rice seedlings treated with MJ under Cd stress. Results showed that GSSG content was decreased significantly and this decrease possibly due to induction of GR activity that was suggested to play an important role in the GSH regeneration from GSSG (Gill and Tuteja 2010).
Parallel to GSH, AsA is also an important enzyme, plays a crucial role in cellular defense (Singh and Shah 2014). AsA is recycled by DHAR with the help of GSH as a reductant. Previously, it has been reported that AsA activation through recycling pathway is important for increasing AsA content (Chen et al. 2003). Thus, with AsA content we also reported the expression of DHAR, an AsA recycling pathway gene. We found that MJ induced the DHAR expression, as well also increased the AsA content (Fig. 5). It has been reported that under ozone and water stress jasmonate induces the accumulation of AsA content and increases the transcript level of DHAR (Sasaki-Sekimoto et al. 2005; Shan and Liang 2010) which is consistent with our result under As stress. So, MJ activates the GSH and AsA metabolism and compounds involved in metabolism that provides protection against oxidative damage.
In addition to the above-mentioned enzymes mechanism, secondary metabolites are naturally present in plants and also play diverse roles in physiological processes (Chen et al. 2009). In present study, MJ-treated plants exhibited higher activities of PAL and PPO compared with non-MJ-treated plants (Fig. 6). Further, secondary enzymes such as PAL and PPO as well as their transcripts were induced by MJ and/or As (Fig. 6). In addition, the induction of PAL and PPO genes treated with MJ, may assist the plants to cope with the oxidative stress, in consistence with the finding of Ahammed et al. (2013) who also found that EBr helps in alleviating the oxidative stress in tomato leaves through the induction of these defense compounds.
LOX is an important key gene in JA synthesis. JA plays a key role in regulating a wide range of physiological functions, such as signal transduction, development and senescence, and protection from oxidative stress through enhancing the antioxidant activities and defense gene induction, under heavy metal stress conditions (Devoto and Turner 2003; Wasternack and Hause 2013). Higher level of JA suggests a defensive role in mitigation of excesses ROS during metal stress (Dar et al. 2015). Our results showed that MJ treatment significantly increased the JA content (Fig. 5). This increase in JA content might be possibly due to the enhanced expression of LOX that subsequently increased the synthesis of JA. Arsenic stress significantly lowered the JA contents in B. napus roots (Fig. 5). However, the application of MJ significantly increased the JA contents in both cultivars. In a similar study, Chen et al. (2014) also examined the decrease of JA contents in Kandelia obovata, while the MJ application increased the JA content. Similarly, the expression of LOX 2 in both the cultivars enhanced after 1 µM MJ application under As stress (5). Under abiotic stress condition MJ may be attributed as an inducer of LOX gene expression in different plant such as potato (Geerts et al. 1994), soybean (Park et al. 1994) and Arabidopsis (Melan et al. 1993).
There are few findings of about cellular level changes occur in root tips under the effect of MJ and As. Ultrastructure alteration study provide the insights at cellular level changes in response to oxidative stress (Ali et al. 2013; Caasilit et al. 1997). The present study reported that root tips showed changes in different cell organelles under As stress (200 μM) (Fig. 7). The root tip cells of cultivar Zheda 622 were significantly injured under As stress as compared to ZS 758. Recently, B. napus roots were found to be severely damaged under heavy metal stress (Ali et al. 2014a; Farooq et al. 2015). However, when As-stressed plants treated with MJ, well developed cell structure was observed in root tips. This describes the MJ improving capability in root that was also obvious from antioxidant enzymes study. Exogenously applied MJ clearly recovered the cell damage observed in roots of B. napus cultivars, but these positive effects were more pronounced in ZS 758. Thus, we can conclude that under As stress MJ application, could increase the antioxidant activities and decrease the generation of ROS concentration, leading to reduction of the ultra-structure damage in B. napus root tips.
Conclusion
Present study revealed the important role of MJ in the protection process against As-toxicity in the roots of B. napus by preventing oxidative damage with the enhancement of glutathione metabolism. Interestingly, we found that higher level of 200 μM As on AsA–GSH cycle was potentiated by the exogenous treatment of MJ and helped to maintain membrane integrity and root elongation by reducing lipid peroxidation and cell death content. Moreover, exogenous MJ application also maintained the root function under As stress by reducing the ROS accumulation, probably through modulating the AsA-GSH redox status, enhanced antioxidant enzyme activities and secondary metabolites. The employment of comprehensive systems biological approaches is needed to determine the detailed elucidation of MJ signaling in abiotic stress, which stands as a challenging future perspective.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 10:121–126
Ahammed GJ, Choudhary SP, Chen S, Xia X, Shi K, Zhou Y, Yu J (2013) Role of brassinosterods in alleviation of phenanthrene-cadmium co-contamination-induced photosynthetic inhibition and oxidative stress in tomato. J Exp Bot 64:199–213
Ali B, Tao Q, Zhou Y, Gill RA, Ali S, Rafiq MT, Xu L, Zhou WJ (2013) 5-aminolevolinic acid mitigates the cadmium-induced changes in Brassica napus as revealed by the biochemical and ultra-structural evaluation of roots. Ecotoxicol Environ Saf 92:271–280
Ali B, Qian P, Jin R, Ali S, Khan M, Aziz R, Tian T, Zhou WJ (2014a) Physiological and ultra-structural changes in Brassica napus seedlings induced by cadmium stress. Biol Plant 58:131–138
Ali B, Mwamba TM, Gill RA, Yang C, Ali S, Daud MK, Wu YY, Zhou WJ (2014b) Improvement of element uptake and antioxidative defense in Brassica napus under lead stress by application of hydrogen sulfide. Plant Growth Regul 74:261–273
Bari R, Jones JD (2009). Role of plant hormones in plant defence responses. Plant Mol Biol 69:473–488
Caasilit M, Whitecross MI, Nayudu M, Tanner GJ (1997) UV-B irradiation induce differential leaf damage, ultrastructural changes and accumulation of specific phenolic compounds in rice cultivars. Aust J Plant Physiol 24:261–274
Chen Z, Young TE, Ling J, Chang SC, Gallie DR (2003) Increasing vitamin C content of plants through enhanced ascorbate recycling. Proc Natl Acad Sci USA 100:3525–3530
Chen F, Liu C-J, Tschaplinski TJ, Zhao N (2009) Genomics of secondary metabolism in populus: interactions with biotic and abiotic environments. Crit Rev Plant Sci 28:375–392
Chen J, Yan Z, Li X (2014) Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol Environ Saf 104:349–356
Dai LP, Xiong ZT, Huang Y, Li MJ (2006) Cadmium-induced changes in pigments, total phenolics, and phenylalanine ammonia-lyase activity in fronds of Azolla imbricata. Environ Toxicol 21:505–512
Dar TA, Uddin M, Khan MMA, Hakeem KR, Jaleel H (2015) Jasmonates counter plant stress: a review. Environ Exp Bot 115:49–57
del Amor FM, Cuadra-Crespo P (2011) Alleviation of salinity stress in broccoli using foliar urea or methyl-jasmonate: analysis of growth, gas exchange, and isotope composition. Plant Growth Regul 63:55–62
Devoto A, Turner JG (2003) Regulation of jasmonate-mediated plant responses in Arabidopsis. Ann Bot 92:329–337
Dhankar R, Solanki R (2011) Effect of copper and zinc toxicity on physiological and biochemical parameters in Vigna mungo (L.) Hepper. Int J Pharm Bio Sci 2:553–565
Dombrowski JE (2003) Salt stress activation of wound-related genes in tomato plants. Plant Physiol 132:2098–2107
Fadzilla NM, Finch RP, Burdon RH (1997) Salinity, oxidative stress and antioxidant responses in root cultures of rice. J Exp Bot 48:325–331
Farmer EE (2007) Plant biology: jasmonate perception machines. Nature 448:659–660
Farmer EE, Alme´ras E, Krishnamurthy V (2003) Jasmonates and related oxylipins in plant responses to pathogenesis and herbivory. Curr Opin Plant Biol 6:372–378
Farooq MA, Gill RA, Ali B, Wang J, Islam F, Ali S, Zhou WJ (2015) Subcellular distribution, modulation of antioxidant and stress-related genes response to arsenic in Brassica napus L. Ecotoxicology 25:350–366
Farooq MA, Islam F, Ali B, Najee U, Maod B, Gill RA, Yane G, Siddique KHM, Zhou W (2016a) Arsenic toxicity in plants: cellular and molecular mechanisms of its transport and metabolism. Environ Exp Bot 132:42–52
Farooq MA, Li L, Ali B, Gill RA, Wang J, Ali S, Gill MB, Zhou WJ (2016b) Oxidative injury and antioxidant enzymes regulation in arsenic-exposed seedlings of four Brassica napus L. cultivars. Environ Sci Pollut Res 22:10699–10712
Garg N, Singla P (2011) Arsenic toxicity in crop plants: physiological effects and tolerance mechanisms. Environ Chem Lett 9:303–321
Geerts A, Feltkamp D, Rosahl S (1994) Expression of lipoxygenase in wounded tubers of Soianum tuberosum L. Plant Physiol 105:269–277
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Grispen VMJ, Nelissen HJM, Verkleij JAC (2006) Phytoextraction with Brassica napus L.: a tool for sustainable management of heavy metal contaminated soils. Environ Pollut 144:77–83
Halitschke R, Baldwin IT (2004) Jasmonates and related compounds in plant insect interactions. J Plant Growth Regul 23:238–245
Hanaka A, Wójcik M, Dresler S, Mroczek-Zdyrska M, Maksymiec W (2016) Does methyl jasmonate modify the oxidative stress response in Phaseolus coccineus treated with Cu? Ecotoxicol Environ Saf 124:480–488
Heikens A, Panaullah GM, Meharg AA (2007) Arsenic behaviour from groundwater and soil to crops: impacts on agriculture and food safety. Rev Environ Contam Toxicol 189:43–87
Hossain Z, Komatsu S (2013) Contribution of proteomics studies towards understanding plant heavy metal stress response. Front Plant Sci. doi:10.3389/fpls.2012.00310
Howe GA (2004) Jasmonates as signals in the wound response. J Plant Growth Regul 23:223–237
Islam F, Yasmeen T, Arif MS, Ali S, Ali B, Hameed S, Zhou WJ (2016a) Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul 80:23–36
Islam F, Ali B, Wang J, Farooq MA, Gill RA, Ali S, Wang D, Zhou W (2016b) Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol Biochem 107:82–95
Jedynak L, Kowalska J, Kossykowska M, Golimowski M (2010) Studies on the uptake of different arsenic forms and the influence of sample pretreatment on arsenic speciation in White mustard (Sinapis alba). Microchem J 94:125–129
Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize seedlings. Plant Cell Physiol 42:1265–1273
Jiang M, Zhang J (2002) Water stress-induced abscisic acid accumulation triggers the increased generation of reactive oxygen species and up-regulates the activities of antioxidant enzymes in maize leaves. J Exp Bot 53:2401–2410
Kanna M, Tamaoki M, Kubo A (2003). Isolation of an ozone-sensitive and jasmonate semi-insensitive Arabidopsis mutant (oji1). Plant Cell Physiol 44:1301–1310
Keramat B, Kalantari KM, Arvin MJ (2010) Effects of methyl jasmonate treatment on alleviation of cadmium damages in soybean. J Plant Nutr 33:1016–1025
Lee JS, Lee SW, Chon HT, Kim KW (2008) Evaluation of human exposure to arsenic due to rice ingestion in the vicinity of abandoned Myungbong Au–Ag mine site, Korea. J Geochem Explor 96:231–235
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods 25:402–408
Łukasik I, Golawska S, Wojcicka A (2012) Effects of cereal Aphid infestation on ascorbate contents and ascorbate peroxidase activity in Triticale. Pol J Environ Stud 21:1937–1941
Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK (1993) An Arabidopsis thaiiana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate. Plant Physiol 101:441–450
Nagajyoti PC, Lee KD, Sreekanth TVM (2010) Heavy metals, occurrence and toxicity for plants: a review. Environ Chem Lett 8:199–216
Nakano Y, Asada K (1981) Hydrogen-peroxide is scavenged by ascorbate-specific peroxidase in spinach-chloroplasts. Plant Cell Physiol 22:867–880
Noctor G, Foyer CH (1998) Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol 49:249–279
Park TK, Holland MA, Laskey JG, Polacco J (1994) Germination-associated lipoxygenase transcripts persist in maturing soybean plants and are induced by jasmonate. Plant Sci 96:109–117
Poonam S, Kaur H, Geetika S (2013) Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Mill sp. seedlings under copper stress. Am J Plant Sci 4:817–823
Romero-Puertas MC, Rodriguez-Serrano M, Corpas FJ, Gomez M, del Rio LA, Sandalio LM (2004) Cadmium-induced subcellular accumulation of O2 ·– and H2O2 in pea leaves. Plant Cell Environ 27:1122–1134
Ruiz JM, Garcia PC, Rivero RM, Romero L (1999) Response of phenolic metabolism to the application of carbendazim plus boron in tobacco. Physiol Plant 106:151–157
Ryu H, Cho YG (2015) Plant hormones in salt stress tolerance. J Plant Biol 58:147–155
Santino A, Taurino M, De Domenico S, Bonsegna S, Poltronieri P, Pastor V, Flors V (2013) Jasmonate signaling in plant development and defense response to multiple (a)biotic stresses. Plant Cell Rep 32:1085–1098
Sasaki-Sekimoto Y, Taki N, Obayashi T, Aono M, Matsumoto F, Sakurai N, Suzuki H, Hirai MY, Noji M, Saito K, Masuda T, Takamiya K, Shibata D, Ohta H (2005) Coordinated activation of metabolic pathways for antioxidants and defence compounds by jasmonates and their roles in stress tolerance in Arabidopsis. Plant J 44:653–668
Shan C, Liang Z (2010) Jasmonic acid regulates ascorbate and glutathione metabolism in Agropyron cristatum leaves under water stress. Plant Sci 178:130–139
Singh I, Shah K (2014) Exogenous application of methyl jasmonate lowers the effect of cadmium-induced oxidative injury in rice seedlings. Phytochemistry 108:57–66
Thordal-Christensen H, Zhang Z, Wei YD, Collinge DB (1997) Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley-powdery mildew reaction. Plant J 11:1187–1194
Velikova V, Yordanov I, Edreva A (2000) Oxidative stress and some antioxidant system in acid rain treated bean plants: protective role of exogenous polyammines. Plant Sci 151:59–66
Wasternack C, Hause B (2013) Jasmonates: biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the review. Ann Bot 111:1021–1058
Wilkins DA (1978) The measurement of tolerance to endemic factors by means of root growth. New Phytol 80:623–633
Wu H, Wu X, Li Z, Duan L, Zhang M (2012) Physiological evaluation of drought stress tolerance and recovery in cauliflower (Brassica oleracea L.) seedlings treated with methyl jasmonate and coronatine. J Plant Growth Regul 31:113–123
Xiang CB, Oliver DJ (1998) Arabidopsis glutathione metabolic genes coordinately respond to heavy metals and jasmonic acid in Arabidopsis. Plant Cell 10:1539–1550
Yan Z, Chen J, Li X (2013) Methyl jasmonates modulator of Cd toxicity in Capsicum frutescens var. fasciculatum seedlings. Ecotoxicol Environ Saf 98:203–209
Yan Z, Zhang W, Chen J, Li X (2015) Methyl jasmonate alleviates cadmium toxicity in Solanum nigrum by regulating metal uptake and antioxidative capacity. Biol Plant 59:373–381
Yoon JY, Hamayun M, Lee SK, Lee IJ (2009) Methyl jasmonate alleviated salinity stress in soybean. J Crop Sci Biotechnol 12:63–68
Zhang WF, Zhang F, Raziuddin R, Gong HJ, Yang ZM, Lu L, Ye QF, Zhou WJ (2008) Effects of 5-aminolevulinic acid on oilseed rape seedling growth under herbicide toxicity stress. J Plant Growth Regul 27:159–169
Zhou WJ, Leul M (1999) Uniconazole-induced tolerance of rape plants to heat stress in relation to changes in hormonal levels, enzyme activities and lipid peroxidation. Plant Growth Regul 27:99–104
Acknowledgements
This study was supported by the National High Technology Research and Development Program of China (2013AA103007), the Special Fund for Agro-scientific Research in the Public Interest (201303022), the National Natural Science Foundation of China (31570434, 31650110476), Jiangsu Collaborative Innovation Center for Modern Crop Production, and the Science and Technology Department of Zhejiang Province (2016C02050-8).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Farooq, M.A., Islam, F., Yang, C. et al. Methyl jasmonate alleviates arsenic-induced oxidative damage and modulates the ascorbate–glutathione cycle in oilseed rape roots. Plant Growth Regul 84, 135–148 (2018). https://doi.org/10.1007/s10725-017-0327-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-017-0327-7