Abstract
Aims
The effect of different MeJA doses applied prior to or simultaneously with toxic Al on biochemical and physiological properties of Vaccinium corymbosum cultivars with contrasting Al resistance was studied.
Methods
Legacy (Al-resistant) and Bluegold (Al-sensitive) plants were treated with and without toxic Al under controlled conditions: a) without Al and MeJA, b) 100 μM Al, c) 100 μM Al + 5 μM MeJA, d) 100 μM Al + 10 μM MeJA and e) 100 μM Al + 50 μM MeJA. MeJA was applied to leaves 24 h prior to or simultaneously with Al in nutrient solution. After 48 h, Al-concentration, lipid peroxidation (LP), H2O2, antioxidant activity, total phenols, total flavonoids, phenolic compounds and superoxide dismutase activity (SOD) of plant organs were analyzed.
Results
Al-concentrations increased with Al-treatment in both cultivars, being Al, LP and H2O2 concentrations reduced with low simultaneous MeJA application. Higher MeJA doses induced more oxidative damage than the lowest. Legacy increased mainly non-enzymatic compounds, whereas Bluegold increased SOD activity to counteract Al3+.
Conclusions
Low MeJA doses applied simultaneously with Al3+ increased Al-resistance in Legacy by increasing phenolic compounds, while Bluegold reduced oxidative damage through increment of SOD activity, suggesting a diminution of its Al-sensitivity. Higher MeJA doses could be potentially toxic. Studies are needed to determine the molecular mechanisms involved in the protective MeJA effect against Al-toxicity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil acidity (pH < 5.5) solubilizes the aluminum (Al) complex to toxic aluminum (Al3+), which represents the most harmful form for plant crops (Delhaize et al. 2012; Kochian et al. 2015). At lower concentrations, Al3+ negatively affects physiological, biochemical and morphological processes, depending on the plant species, genotypes and degree of tolerance (Barceló and Poschenrieder 2002). The most evident response to Al toxicity in plants is the overproduction of reactive oxygen species (ROS) with the concomitant increase in lipid peroxidation (LP) in the cell membranes. This induces oxidative stress (OS) in cells and organelles, resulting even in cell death (Yamamoto et al. 2002; Guo et al. 2007; Ma et al. 2007). Plants counteract excess ROS by activating antioxidant systems, including enzymatic mechanisms like superoxide dismutase (SOD), which is the first line of defense to scavenge ROS (Wang et al. 2005). In addition, non-enzymatic antioxidant compounds such as total phenols (TP) may also be activated to counteract Al3+-induced OS (Shao et al. 2008). Under stress conditions, the phenol concentrations increase and this causes the Al3+ to have a stronger affinity with phenols than other organic molecules, limiting the Al toxicity (Wang et al. 2015). Another important aspect involved in plant responses to toxic Al is jasmonic acid (JA) (Spollansky et al. 2000). JA and its methyl ester, methyl jasmonate (MeJA), are synthesized from the linoleic and linolenic acids derived from cyclopentanone-based compounds of the jasmonates (Jas) (Creelman and Mullet 1995, 1997; Pauwels et al. 2008; Staswick 2008; Schaller and Stintzi 2009). JA is considered a plant growth regulator and acts as a signal molecule that participates in the regulation of various metabolic pathways. The exposure of plants to toxic metals (TM) stimulates the synthesis and activity of antioxidant metabolites and antioxidant enzymes that can protect plant tissues against stress (Poonam et al. 2013). There is little information about the effects of MeJA application on plants under Al toxicity. Spollansky et al. (2000) and Xue et al. (2008) reported that in Brugmansia candida and Cassia tora plants exposed to Al toxicity and MeJA application, a high lignin accumulation in the cell wall, oxidative stress, peroxidase and NADH activity were observed in the roots of both species. However, reports on fruit species in the presence or absence of MeJA application under other abiotic stresses such as water stress in strawberries (Fragaria x ananassa) (Wang 1999), salinity and radiation in grapevines (Vitisvinifera) (Larronde et al. 2003; Ismail et al. 2012), low temperature in peaches (Prunuspersica) (Menga et al. 2009), and toxic metals (TM) in Arabidopsis thaliana (Maksymiec and Krupa 2002, 2007a;b) are more abundant. These studies involve applying MeJA simultaneously with the stressor. Yet there are no studies that differ in the application time of MeJA and the stress factor and its antioxidant responses in plants. We believe that prior application of MeJA could activate defense mechanisms to counteract stress conditions, preventing its harmful effect. Creelman and Mullet (1995) and Chen et al. (2014) reported that the MeJA effect on plants under toxic metal (TM) stress depends on the intensity of the stress factor as well as the sensitivity of the species or cultivars. In fact, studies performed by Li et al. (2014) indicated that the MeJA treatment significantly enhanced resistance to fungal pathogens in two rice cultivars, but the resistant cultivar maintained a higher level of resistance than the susceptible cultivar under the same treatment. Accordingly, Li et al. (2014) pointed out that studies about defense mechanisms induced by jasmonates are commonly performed on only one cultivar, with the comparison between resistant and susceptible cultivars being important for a better understanding of these mechanisms.
In the aquatic plant Wolffia arrhiza treated with a high JA concentration (100 μM) and increased lead (Pb) toxicity, a decrease was found in chlorophyll and carotenoid pigments. Conversely, at low concentrations of JA (0.1 μM), a decrease in the oxidative damage by Pb in this species was observed, accompanied by an increase in biomass, carbohydrates, proteins, antioxidant concentrations (ascorbic acid and glutathione) and a decrease in LP (Piotrowska et al. 2009). A reduction in LP and increased SOD activity was reported in soybean (Glycine max L.) plants grown under cadmium (Cd) toxicity (500 μM), by adding a low MeJA dose (0.01 μM) (Keramat et al. 2009). Thus, it appears that a low MeJA dose is more effective at reducing the harmful effects of TM in these species.
Although there are few studies related to MeJA and TM, most are in plants of agricultural interest, and in particular fruit crops (Yoon et al. 2010; Ismail et al. 2012). In the last decade the use of natural stimulant compounds such as MeJA has garnered interest due to restrictions in the use of agrochemicals in fruit export. Highbush blueberry (Vaccinium corymbosum L.) is a species native to North America, belonging to the Ericaeae family, which in the last two decades has become an important crop for the nutritional properties of its fruits, which are rich in antioxidant activities and anthocyanin concentrations (Castrejón et al. 2008). Studies performed on highbush blueberry leaves under Al3+ stress have shown different antioxidant capacities and physiological responses depending on the cultivars and degree of resistance to Al toxicity (Reyes-Díaz et al. 2009, 2010). These reports have shown different responses by Brigitta, Bluegold and Legacy to Al toxicity, with Bluegold demonstrating a greater Al sensitivity than Legacy and Brigitta. Therefore, the aim of this work was to evaluate the effect of different MeJA doses applied to leaves at different times (prior to or simultaneously with the application of toxic Al) on the antioxidant performance of roots and leaves of V. corymbosum cultivars.
Materials and methods
Plant material
Two-year-old plants of V. corymbosum cultivars (Legacy and Bluegold), previously classified by Reyes-Díaz et al. (2009, 2010) as Al-resistant and Al-sensitive, respectively were used in this study. Plants from these cultivars were produced in vitro and grown in a substrate of oat shell:sawdust:pine needles at a 1:1:1 proportion. They were provided by Berries San Luis (Quillém, Lautaro, Chile; 38° 29` S, 72° 23` W). Healthy plants of these cultivars with uniform size with a plant height of 35.21 ± 0.16 cm (from crown to apex), and 17.02 ± 0.08 cm (from crown to root tips) in roots were selected.
Growth conditions in nutrient solution
The experiment was carried out in a greenhouse in the Instituto de Agroindustria, Universidad de La Frontera, Temuco, Chile (38°45’S, 72°.40’W). Plants were transferred and grown in a Hoagland nutrient solution [2 mMCa (NO3)2, 3 mM KNO3, 1 mM MgSO4, 0.1 mM KH2PO4 with micronutrients: 25 μM H3BO3, 10 μM MnSO4, 1 mM NH4NO3, 0.07 μM (NH4)6Mo7O24, 2 μM ZnSO4, 0.4 μM CuSO4, 20 μM FeEDTA] under controlled conditions (temperature 25 ± 0.2 °C, 300 μmol m−2 s−1 photosynthetic photon flux and 70 % relative humidity) for seven days for plant conditioning prior to starting the experiment. Solutions were aerated continuously with an aquarium pump and changed twice in the week.
Treatments and experimental design
The experimental design was completely randomized with three replicates per treatment with a total of 60 plants for the two cultivars. After conditioning as described above, plants were treated with or without toxic Al (as AlCl3), with 26.8 % of Al3+as a free metal determined by Geochem speciation (Shaff et al. 2010). The pH was adjusted at 4.5 for 48 h. The MeJA was homogenously applied by spraying on leaves 24 h prior to the application of Al3+ to the nutrient solution or simultaneously with the Al3+ application. The MeJA was dissolved in ultrapure water 1 L (< 1 μS) with 0.05 % (v/v) tween 20 for plants with MeJA, whereas in the controls (without MeJA) 0.05 % v/v tween 20 dissolved in ultrapure water was applied. Dose coverage was 25 ml per plant calculated as total foliar area of plant. Plants were located in screens to avoid drift of MeJA dilution between treatments. The following treatments were applied: a) without Al and MeJA (Control), b) 100 μM Al (Al), c) 100 μM Al + 5 μM MeJA (Al + 5 MeJA), d) 100 μM Al + 10 μM MeJA (Al + 10 MeJA), and e) 100 μM Al + 50 μM MeJA (Al +50 MeJA). Finally, 48 h after adding Al to the nutrient solution, leaves and roots were harvested and stored at −80 °C (Revco Elite Series Ultra-Low Temperature, Thermo Scientific™) for biochemical analyses; subsamples were taken and dried for chemical analysis.
Chemical analysis
Aluminum concentration
Samples were dried in a forced air oven (for 48 h at 70 °C in a Memmert model 410, Schwabach, Germany) until a constant dry weight was reached, and then ground in a mill. Samples were weighed and ashed at 500 °C (JSMF-30 T, electric Muffle Furnace of JSR Research Inc., Korea) for 8 h and then digested with 2 M hydrochloric acid. The Al concentration was determined using a simultaneous multielement atomic absorption spectrophotometer (model 969; UNICAM, Cambridge, UK) as described by Sadzawka et al. (2004).
Biochemical parameters
Lipid peroxidation (LP)
It was used as indicator of damage by oxidative stress. A thiobarbituric acid reacting substance (TBARS) assay according to the modified method of Du and Bramlage (1992) was used. The final malondialdehyde (MDA) products were measured at 532, 600 nm and 440 nm. LP is a good criterion for determining Al resistance in plants (Reyes-Díaz et al. 2010); hence, it was used to establish Al-sensitivity or resistance of the evaluated cultivars.
Hydrogen peroxide concentration (H2O2)
The H2O2 concentration was determined according to Loreto and Velikova (2001). The H2O2 concentration was measured at 390 nm and expressed as μmol g−1 fresh weight.
Total antioxidant activity (AA)
AA was determined in leaves and roots using the DPPH method of Chinnici et al. (2004). The extracts were prepared according to the method used by Reyes-Díaz et al. (2010). Absorbance was measured in a spectrophotometer at 515 nm and expressed in Trolox equivalents (TE).
Total phenols (TP)
TP were determined with the Folin-Ciocalteu reagent using the method of Slinkard and Singleton (1977). Absorbance was measured at 765 nm using a UV/VIS spectrophotometer. Results were expressed as milligrams of chlorogenic acid equivalent per gram of fresh weight (mg CAE g−1 FW).
Flavonoid compound analyses
Total flavonoids were determined using the method of Cheng and Breen (1991) at an absorbance of 510 nm using a UV/VIS spectrophotometer. Results were expressed as micrograms of rutin equivalent per g of fresh weight (μg rutin eq. g−1 FW). The HPLC analysis was performed as described earlier by Ruhland and Day (2000) with minor modifications, at a flow rate of 1.0 ml min−1. The signals were detected at 320 nm and the data were expressed as milli- or micro-grams per g of fresh weight (mg or μg g−1 FW) . The mobile phase was: (A) acidified water (phosphoric acid 10 %) and (B) 100 % acetonitrile, and the eluent gradients were as follows: 0–9 min of 100 % A, 9.1–19.9 min of 81 % A and 19 % B, 20–25 min of 100 % B.
Superoxide dismutase (SOD) activity
The SOD was assayed according to Giannopolitis and Ries (1977) by monitoring the superoxide radical-induced nitro blue tetrazolium (NBT) reduction at 560 nm. The enzymatic activity values were standardized for the protein content according to Bradford’s method (Bradford 1976).
Statistical analysis
The results were based on 3 replicates. All data passed the normality and equal variance tests according to Kolmogorov-Smirnov. Statistical data analyses were carried out by three-way ANOVA (where factors were: treatment, time of MeJA application, and cultivar). Tukey’s test was used to identify means with significant differences (P ≤ 0.05) using the statistical software SAS v. 8.01.
Results
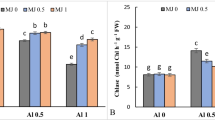
The Al concentration in leaves and roots was generally increased under Al application alone in both cultivars, when compared to the control (P ≤ 0.05; Fig. 1a, b, c and d). Leaves and roots of Bluegold showed 60- and 2.7-fold increase of Al-concentration under Al treatment, respectively than their respective controls (P ≤ 0.05; Fig. 1c, d). Leaves and roots of Legacy showed 4- and 3.4-fold Al increase under Al treatment, respectively in comparison with their respective controls (P ≤ 0.05; Fig. 1a, b). In leaves and roots of Legacy, Al-concentration was lower (54 % and 85 %, respectively) than in leaves and roots of Bluegold (P ≤ 0.05; Fig. 1a, b, c and d), The simultaneous application of MeJA and toxic Al showed that Legacy leaves were able to reduce their Al concentration in all MeJA doses, being reduced by 45 % at the lowest (5 μM) and highest dose of MeJA (P ≤ 0.05; Fig. 1a). By contrast, when MeJA was applied prior to toxic Al3+ only the highest (50 μM) dose of MeJA reduced the Al concentration in leaves (38 %) compared to the Al treatment alone (P ≤ 0.05; Fig. 1a). The Al concentration of Legacy roots showed no statistically significant differences in any of the treatments when MeJA was applied prior to Al3+; however, these concentrations were statistically significantly higher than the control treatment (P ≤ 0.05; Fig. 1b). The situation was different when MeJA was applied simultaneously with Al3+, where a reduction in Al root concentration (13.5 %) at the lower doses of MeJA was found compared to the prior application (Fig. 1b). All combined treatments were able to decrease the Al concentration of both organs in the two cultivars compared to the Al treatment (P ≤ 0.05; Fig. 1c, d). However, in Bluegold leaves, the Al concentration was more reduced when MeJA was applied simultaneously with Al3+compared to previously applied MeJA (P ≤ 0.05; Fig. 1c).
Aluminum concentration (mg kg−1 DW) in leaves (a, c) and roots (b, d) of Legacy and Bluegold cultivars. Values represent the average of 3 replicates ± S.E. and doses in μM of MeJA and Al. Different lowercase letters show statistically significant differences among the treatments at each time of MeJA application (prior or simultaneously) in the same cultivar. Different capital letters show significant differences between MeJA application times for the same treatment and cultivar. Asterisk (*) shows significant differences between cultivars at the same treatment and time of MeJA application (P < 0.05)
Legacy leaves showed significantly higher LP than those of Bluegold (P ≤ 0.05; Fig. 2a, c). The simultaneous application of MeJA and Al3+ reduced leaf LP in all treatments, compared to Al treatment alone (P ≤ 0.05; Fig. 2a). When MeJA was applied prior to Al3+, a reduction in LP was observed at the lowest and highest MeJA doses compared to the simultaneously application of MeJA and Al3+, with the highest LP being obtained with the highest MeJA dose (50 μM) (P ≤ 0.05; Fig. 2a). Legacy roots showed low LP in plants treated with MeJA, regardless of dose and application time, and the LP was lower than in plants treated with Al3+ (P ≤ 0.05; Fig. 2b). The greatest decrease of LP occurred at 10 μM MeJA regardless of application time and with the lowest MeJA dose (5 μM) when applied at the same time as Al3+, these values being lower than the control (P ≤ 0.05; Fig. 2b). Bluegold leaves and roots increased LP (13.7- and 1.8-fold, respectively) under toxic Al compared to the control (P ≤ 0.05; Fig. 2c, d). However, these organs exhibited lower LP at the lowest MeJA treatment when MeJA was applied simultaneously with Al3+ compared to its prior application (P ≤ 0.05; Fig. 2c, d). Similarly, at 10 μM and 50 μM MeJA applied prior to Al3+, a reduction in LP was observed in leaves and roots (7.8- and 1.6-fold, respectively) compared to simultaneous MeJA application (P ≤ 0.05; Fig. 2c, d).
Lipid peroxidation [MDA (nmol g−1 FW)] in leaves (a, c) and roots (b, d) of Legacy and Bluegold cultivars. Values represent the average of 3 replicates ± S.E. and doses in μM of MeJA and Al. Different lowercase letters show statistically significant differences among the treatments at each time of MeJA application (prior or simultaneously) in the same cultivar. Different capital letters show significant differences between MeJA application times for the same treatment and cultivar. Asterisk (*) shows significant differences between cultivars at the same treatment and time of MeJA application (P < 0.05)
No statistically significant differences between Al3+ and control treatments were detected in the H2O2 concentration of Legacy leaves and roots (P ≤ 0.05; Fig. 3a, b). The most noticeable reduction in H2O2 concentration of Legacy leaves (5.7 %) and roots (28.8 %) was with Al3+ and the lowest MeJA dose applied simultaneously as compared to its prior application (P ≤ 0.05; Fig. 3a, b). Instead, in Bluegold the H2O2 concentration increased in leaves (17.8 %) and roots (37.2 %) under Al treatment compared to the control (P ≤ 0.05; Fig. 3c, d). The MeJA application generally decreased the H2O2 concentration compared to the Al treatment (P ≤ 0.05; Fig. 3c, d). Bluegold leaves and roots decreased their H2O2 concentration at lower MeJA treatments applied prior to Al compared to Al treatment alone, reaching similar values to the control with the exception of the highest MeJA treatment in leaves (P ≤ 0.05; Fig. 3c, d)
H2O2 concentration (μmol mg−1 FW) in leaves (a, c) and roots (b, d) of Legacy and Bluegold cultivars. Values represent the average of 3 replicates ± S.E. and doses in μM of MeJA and Al. Different lowercase letters show statistically significant differences among the treatments at each time of MeJA application (prior or simultaneously) in the same cultivar. Different capital letters show significant differences between MeJA application times for the same treatment and cultivar. Asterisk (*) shows significant differences between cultivars at the same treatment and time of MeJA application (P < 0.05)
Generally, AA in Legacy leaves was higher than in the Bluegold cultivar in all treatments compared to the control (P ≤ 0.05; Fig. 4a, c). By contrast, Bluegold roots showed higher AA values than Legacy roots (P ≤ 0.05; Fig. 4b, d). The AA of Legacy leaves was enhanced in all treatments compared to the control (P ≤ 0.05), and the values were very similar between them (Fig. 4a). In Legacy roots, the AA increased by 21.6 % with Al3+ application (P ≤ 0.05; Fig. 4b). The MeJA application in Legacy increased the AA of roots compared to the control, with the exception of the 10 μM MeJA treatment regardless of application time, and 50 μM MeJA applied prior to Al3+ (P ≤ 0.05; Fig. 4b). A slight increase in the AA of Bluegold leaves was frequently observed in all treatments compared to the control (P ≤ 0.05; Fig. 4c). In Bluegold roots, the AA was significantly increased by Al3+ alone and Al + 5 μM MeJA treatment regardless of the time of MeJA application compared to the control (P ≤ 0.05; Fig. 4d).
Antioxidant activity (μg TE g−1 FW) in leaves (a, c) and roots (b, d) of Legacy and Bluegold cultivars. Values represent the average of 3 replicates ± S.E. and doses in μM of MeJA and Al. Different lowercase letters show statistically significant differences among the treatments at each time of MeJA application (prior or simultaneously) in the same cultivar. Different capital letters show significant differences between MeJA application times for the same treatment and cultivar. Asterisk (*) shows significant differences between cultivars at the same treatment and time of MeJA application (P < 0.05)
The TP values of leaves and roots were commonly higher in Legacy than in Bluegold (P ≤ 0.05; Fig. 5a, b, c and d). In Legacy leaves significant differences were observed between treatments and control, with the exception of the treatment of Al + 10 μM MeJA (with MeJA applied previous as Al), where no differences were found (P ≤ 0.05; Fig. 5a). The highest TP of Legacy leaves was obtained at 5 μM MeJA, applied prior to Al, and this was higher than the control (33.7 %) and Al (14.9 %) treatments (P ≤ 0.05; Fig. 5a). In Legacy roots, the TP concentrations were enhanced 36 % under toxic Al, and 16.7 % under Al + 5 μM MeJA, and 27 % with Al + 50 μM MeJA compared to the control, independently of the MeJA application time (P ≤ 0.05; Fig. 5b). In Legacy roots, lowest TP concentration (3-fold) were obtained at the treatment of Al + 10 μM MeJA compared with Al treatment at both application times (P ≤ 0.05; Fig. 5b). In Bluegold leaves and roots, TP concentrations increased 1.7- and 1.5-fold, respectively in plants subjected to Al3+ in relation to the control (P ≤ 0.05; Fig. 5c). When MeJA was applied simultaneously with Al3+, leaf TP concentrations significantly increased in all Al + MeJA treatments over the control, with the largest increase (2.2-fold) being at 50 μM Al + MeJA (P ≤ 0.05; Fig. 5c). In Bluegold roots, TP concentration significantly increased with Al treatment alone (35 %), but decreased when MeJA and Al were applied simultaneously reaching values similar to the control (P ≤ 0.05; Fig. 5d).
The total phenols [chlorogenic acid eq (μg g−1 FW)] in leaves (a, c) and roots (b, d) of Legacy and Bluegold cultivars. Values represent the average of 3 replicates ± S.E. and doses in μM of MeJA and Al. Different lowercase letters show statistically significant differences among the treatments at each time of MeJA application (prior or simultaneously) in the same cultivar. Different capital letters show significant differences between MeJA application times for the same treatment and cultivar. Asterisk (*) shows significant differences between cultivars at the same treatment and time of MeJA application (P < 0.05)
Total flavonoids (TF) of Legacy did not show any change among treatments or in their application time (P ≤ 0.05; Table 1). By contrast, Bluegold TF decreased (25 %) in the presence of toxic Al, but the application of the lowest MeJA dose applied simultaneously with Al3+ counteracted this effect (P ≤ 0.05; Table 1). Phenolic compounds of Legacy and Bluegold showed differences in concentrations of chlorogenic acid, rutin, coumaric acid, ferulic acid and myricetin. Caffeic acid was not detected in Bluegold leaves, while quercetin and kaempferol were not detected in either cultivar (P ≤ 0.05; Table 1). In roots of both cultivars, phenolic compounds were not detected as they were below the detection limit of the equipment used.
Chlorogenic acid in Legacy doubled with the application of Al alone compared to the control (P ≤ 0.05; Table 1). The effect of MeJA application was more evident at the lowest MeJA dose when applied prior (3-fold) or simultaneously (1.7-fold) to Al3+ compared to the control (P ≤ 0.05; Table 1). By contrast, a reduction in chlorogenic acid was observed in Bluegold at both MeJA application times and in all treatments compared to the control (P ≤ 0.05; Table 1). An increase in caffeic acid in Legacy with Al and MeJA application was observed at both application times (P ≤ 0.05; Table 1). The high values of caffeic acid were found at Al3+ (2.8-fold) regardless of the MeJA application time, whereas at the lowest MeJA dose a 1.8-fold increase in the previous and 3-fold in the simultaneous MeJA application was detected (P ≤ 0.05; Table 1). Rutin, coumaric and ferulic acids in Legacy decreased with Al treatment (4.7-, 1.8-, and 2.5-fold, respectively), but when the lowest dose of MeJA was applied simultaneously with toxic Al, values similar to the control were achieved (P ≤ 0.05; Table 1). Myricetin concentration in Legacy was augmented (1.8-fold) by adding the lowest MeJA dose at both application times compared to Al3+ treatment (P ≤ 0.05; Table 1). In Bluegold, rutin increased with MeJA application regardless of the application time (P ≤ 0.05; Table 1). Coumaric acid and myricetin practically did not change in Bluegold, whereas ferulic acid decreased in all treatments independently of the MeJA application time (P ≤ 0.05; Table 1).
SOD activity in leaves was higher in Bluegold than in Legacy (P ≤ 0.05; Fig. 6a, c). There was practically no change in its activity among the treatments in Legacy leaves, with the exception of the Al3+ treatment, where a 1.6-fold increase was observed, whereas for the lowest and highest MeJA doses applied simultaneously with Al3+ 1.8- and 1.9-fold increases respectively were found (P ≤ 0.05; Fig. 6a). SOD activity in Legacy roots decreased significantly (1.7-fold) with the Al3+ treatment compared to the control independently of time application, increasing at 5 μM MeJA until reaching control values (P ≤ 0.05; Fig. 6b). A significant 5-fold decrease in SOD activity was also observed at 10 μM MeJA applied prior to Al compared to simultaneous MeJA application (P ≤ 0.05; Fig. 6b). In Bluegold leaves, SOD activity significantly increased (2.9-fold) with Al treatment compared to the control (P ≤ 0.05; Fig. 6c). The highest value of SOD activity was obtained at 10 μM MeJA applied simultaneously with Al3+, representing a 2.2- and 6.4-fold increase compared to the Al3+ treatment alone and to control, respectively (P ≤ 0.05; Fig. 6c). At 5 μM MeJA applied simultaneously with Al3+, SOD activity was somewhat lower than at 10 μM MeJA, showing an increase of 1.4- and 4.2-fold with respect to Al3+ treatment alone and to control, respectively. In Bluegold roots, almost all the SOD values were similar to those of Al treatment and control, with the exception of 10 μM MeJA applied simultaneously with Al3+, where a 2-fold increase of SOD activity was observed (P ≤ 0.05; Fig. 6d).
Superoxide dismutase activity (U mg−1 prot) in leaves (a, c) and roots (b, d) of Legacy and Bluegold cultivars. Values represent the average of 3 replicates ± S.E. and doses in μM of MeJA and Al. Different lowercase letters show statistically significant differences among the treatments at each time of MeJA application (prior or simultaneously) in the same cultivar. Different capital letters show significant differences between MeJA application times for the same treatment and cultivar. Asterisk (*) shows significant differences between cultivars at the same treatment and time of MeJA application (P < 0.05)
Discussion
This study focused on the effect of time and dose of MeJA application on the antioxidant performance in blueberry cultivars under toxic Al. Substantial differences in the doses and application times were observed, demonstrating that simultaneous application and a low dose of MeJA (5 μM) was able to reduce the Al toxicity of V. corymbosum by reducing the Al concentration and oxidative damage (LP and H2O2 concentration), whereas the antioxidant performance (phenols and SOD activity) was differentially activated in leaves and roots according the Al resistance of the cultivars (Fig. 1, 2, 3, 5 and 6 and Table 1).
Typical symptoms of Al toxicity in leaves and roots of blueberry plants were observed when plants were subjected to toxic Al (Fig. 1, 2 and 3). This is consistent with the data reported by Reyes-Díaz et al. (2009,2010), Inostroza-Blancheteau et al. (2012), and Manquián et al. (2013). Changes in AA systems due to Al stress were reported by Inostroza-Blancheteau et al. (2011), where high Al concentration in V. corymbosum plants increased LP, showing a high gene expression of glutathione S-transferase (GST) and aldehyde dehydrogenase (ALDH) associated with the enhancement of Al toxicity according to the blueberry cultivars Al resistance or Al sensitivity. Our findings demonstrated that the highest AA and phenolic concentration is organ-dependent, being higher in leaves (Fig. 4a, c) followed by roots (Fig. 4b, d) and fruits (Ehlenfeldt and Prior, 2001; Ribera et al. 2010). In this sense, in Legacy (Al-resistant) leaves, the Al treatment increased chlorogenic and caffeic acids and myricetin, whereas rutin and ferulic acid as well as coumaric acid were reduced in the same treatment (Table 1). By contrast, in Bluegold (Al-sensitive) leaves, caffeic acid was not detected (Table 1). These data are consistent with those of Manquián et al. (2013), who reported an increase in chlorogenic acid and rutin by Al stress in Legacy, but this result is not consistent with the reduced rutin concentration found in our study (Table 1). On the other hand, it has been reported that, as constituents of cell walls, phenolic acids protect against biotic and abiotic stresses (Eraso and Hartley, 1990). In blueberry cultivars the richness and abundance of phenolic compounds depend on the species (Wang et al. 2015). Lowbush blueberry (V. angustifolium) is richer in chlorogenic acid and quercetin glycosides (Harris et al. 2007; Wang et al. 2015), whereas in rabbiteye blueberry (V. ashei) flavan-3-ols, proanthocyanidins, chlorogenic acid and flavonol glycosides were the major phenolic compounds in leaf extracts (Matsuo et al. 2010; Wang et al. 2015). In this sense, Wang et al. (2015) analyzed leaves from 104 blueberry cultivars, identifying 28 phenolic compounds. Based on the results of a hierarchical cluster dendrogram analysis, the 104 blueberry cultivars were clustered into three groups, and Legacy and Bluegold were in different groups. This may explain the differences observed in our study, where the chlorogenic acid in the leaves was higher in Legacy than in Bluegold. In addition, the absence of any phenol compounds in Legacy (quercetin and kaempferol) and in Bluegold (quercetin, kaempferol and caffeic acid) may also explain this difference (Table 1).
Our findings also demonstrated that the reduction in the oxidative damage by the application of the lowest dose of MeJA applied simultaneously with toxic Al, triggered an increase in the antioxidant mechanism responses as: total phenols (Fig. 5), total flavonoids and phenolic compounds (Table 1) as well as SOD activity (Fig. 6) in both cultivars. These results are consistent with those reported by Rudell et al. (2002); Jung (2004); Chen et al. (2006); Keramat et al. (2009); Wang et al. (2009); Ruiz-García et al. (2012); Poonam et al. (2013); Chen et al. (2014).
By contrast, the higher MeJA + Al3+ doses induced oxidative damage similar to those demonstrated by the Al treatment alone (Fig. 2 and 3). Furthermore, with these MeJA doses, antioxidant parameters did not provide evidence of a better response (Fig. 4). Despite the few reports about MeJA application under stress in woody plants, most of them used higher doses of MeJA than those used in our study. In this context, studies performed with MeJA in Gossypium hirsutum (Cotton), Pyrus bretschneideri (pear) and Betula pubescens under various stresses used doses of MeJA from 2.5 to 50 mM (Gao et al. 2004; Mäntylä et al. 2014; Konan et al. 2014). Nonetheless, Konan et al. (2014) found that cotton plants treated with 20 mM of MeJA and biotic stress showed toxicity symptoms and altered total phenolic concentrations. Similar results regarding MeJA toxicity under biotic stress have been reported in other species by Heijari et al. (2008) and Moreira et al. (2009). Lower concentrations of MeJA (0.1–10 μM) applied to the woody species Kandelia obovata subjected to Cd toxicity showed that regardless of the MeJA dose, lipid peroxidation decreased without significant differences among them, but Cd concentration increased compared to the Cd treatment alone. Based on this evidence, we selected 5 μM MeJA as the lowest dose of this phytohormone.
The time application of MeJA was a crucial factor for reducing the Al concentration in leaves, regardless of the Al resistance of the study cultivars (Fig. 1a, c). In Phaseolus coccineus plants the application on leaves of 10 μM MeJA prior (1 h and 24 h) to the addition of toxic Cu (50 and 100 μM) indicated that 1 h prior MeJA application was more efficient at decreasing the Cu concentration at 50 μM Cu, whereas at 100 μM Cu, a MeJA application 24 h prior was more effective than 1 h (Hanaka et al. 2016). Nevertheless, Konan et al. (2014) reported that 5 mM and 10 mM MeJA applied 72 or 48 h prior to pathogen stress increased total phenols compared with pre-treatment at 24 h. Therefore, the time of MeJA application and stress intensity are key factors for response to MeJA application in plants.
The interaction between toxic metals and Jas is limited, and the mechanisms are mostly unknown (Keramat et al. 2009; Piotrowska et al. 2009). It is known that the increase in metal toxicity and oxidative damage can be decreased by the participation of antioxidant mechanisms induced by Jas (Maksymiec and Krupa, 2002; Maksymiec et al., 2007b; Keramat et al. 2009; Piotrowska et al. 2009; Chen et al. 2014). Based on our results, we suggest that MeJA stimulated the antioxidant mechanisms to counteract the damage induced by toxic Al (Fig. 6 and Table 1). A high affinity for Al ions joining carboxylic groups of phenolic compounds in the cell wall limits the entry of available Al3+ inside the cells, decreasing the effect of toxic Al (McDonald et al. 1996). Therefore, we think that the retention of Al in the cell wall by MeJA application may be one of the first responses to minimize Al damage in the Al-resistant cultivar due to an increase in phenolic compounds with the lowest MeJA doses applied simultaneously with toxic Al (Table 1). However, in the case of the Al-sensitive cultivar subjected to the same treatment as above, the reduction in the Al concentration in tissues showed a relationship with the activation of the enzymatic activity (SOD) (Fig. 6c, d). Comparing the doses of MeJA used in our study with those reported for other fruit species, it appears that our doses are lower, suggesting that doses are highly dependent on the stress condition and plant species (Wang 1999; Larronde et al. 2003; Menga et al. 2009; Ismail et al. 2012). Our results suggest that there is an optimal range of MeJA for each species that can counteract a determined stress; outside this range phytotoxicity occurs (Keramat et al. 2009). Furthermore, at higher doses MeJA could saturate the MeJA receptors in the membrane as has been reported in Arabidopsis subjected to saline and pathogen stress (An et al. 2008; Yoon et al. 2010). Another alternative might be the activation of defense mechanisms stimulated by joint Al action with simultaneous MeJA application, inducing changes in the ROS concentration. It has been reported that Al and MeJA could use H2O2 as a second messenger under stressful conditions (Hu et al. 2009; Liu et al. 2014). In Cassia tora roots grown with 10 μM Al and 10 μM MeJA was observed increases in H2O2 accumulation, lignin production in the root cell wall, AA activation, phenylalanine ammonia-lyase (PAL) and lipoxygenases (LOX) (Xue et al. (2008). It is important to note that MeJA and Al are linked to the early activation of programmed cell death (PCD) (Pan et al. 2001; Zhang and Xing, 2008), suggesting that both Al and MeJA use the apoplastic H2O2 to trigger PCD. In this context, Zhang and Xing (2008) and Huang et al. (2014) determined the start time of ROS production in cells of A. thaliana and peanuts under MeJA and Al stress able to trigger activation of the antioxidant mechanisms. These authors also reported changes related to early ROS production under MeJA application and Al stress, respectively. Further, Sivaguru et al. (2013) described that Al-induced ROS production could be involved in the signaling, regulation and expression of the SbMATE (Sorghum bicolor multidrug and toxic compound extrusion) located in the root plasma membrane and related to citrate efflux, which regulates the entry of Al. Our results suggest that H2O2 could regulate a higher Al uptake in blueberry (Fig. 1 and Fig. 3a, b, c and d) given that the H2O2 concentration had greater values under Al3+in both cultivars (Fig. 3a, b, c and d). This behavior was more evident in the Al-sensitive cultivar (Bluegold) than in the Al-resistant cultivar (Legacy) (Fig. 3a, b, c and d). Interestingly, our findings also showed that Al concentration in tissues decreased concomitantly with a decrease in H2O2 concentration at the lowest MeJA doses applied simultaneously with the toxic Al (Fig. 1a, b, c, d and 3a, b, c, d).
Differential responses in the leaves and roots of blueberry plants under toxic Al and MeJA application were also found in total phenols and phenolic compounds (Fig. 5a, b, c, d and Table 1). Under Al toxicity and MeJA application these compounds were more abundant in leaves than in roots (Fig. 5a, b, c and d and Table 1). In this sense, we suggest that in leaves phenolic compounds are induced as an antioxidant mechanism to counteract Al stress, while in roots this could be related to a high organic acid production to counteract the harmful effect of Al (Fig. 5a, b, c and D). These suggestions agree with those of Hanaka et al. (2016), who reported stimulation of organic acid in Phaseolus coccineus treated with Cu and MeJA during short- and long-term exposure.
Conclusion
Simultaneous Al and MeJA application induced AA in both cultivars compared to prior MeJA application to toxic Al. Additionally, the Al-resistant cultivar increased mainly non-enzymatic compounds to counteract Al toxicity, whereas the Al-sensitive cultivar increased the SOD activity under Al toxicity and MeJA application. Phenols were more abundant in leaves than in roots, suggesting that in leaves these compounds are induced as an antioxidant mechanism to counteract Al stress, while in roots this could be related to a high organic acid production. Low doses of MeJA applied simultaneously with toxic Al increased Al resistance in Legacy and decreased the oxidative damage in Bluegold, suggesting a decrease of Al-sensitivity in the latter cultivar. Therefore, the application of a low dose of MeJA could be a good alternative for reducing the negative effects of Al toxicity in blueberry, decreasing Al concentration in tissues and strengthening the antioxidant mechanisms. Contrarily, higher doses of MeJA could be potentially toxic. Finally, more studies are needed to determine the molecular mechanisms involved in the protective effect of MeJA against Al toxicity.
References
An SH, Choi HW, Hwang IS, Hong JK, Hwang BK (2008) A novel pepper membrane-located receptor-like protein gene CaMRP1 is required for disease susceptibility, methyl jasmonate insensitivity and salt tolerance. Plant Mol Biol 67:519–533
Barceló J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75–92
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castrejón A, Eichholz I, Rohn S, Kroh LW, Huyskens-Keil S (2008) Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. J Food Chem 109:564–572
Chen H, Jones AD, Howe GA (2006) Constitutive activation of the jasmonate signaling pathway enhances the production of secondary metabolites in tomato. FEBS Lett 580:2540–2546
Chen J, Yan Z, Li X (2014) Effect of methyl jasmonate on cadmium uptake and antioxidative capacity in Kandelia obovata seedlings under cadmium stress. Ecotoxicol Environ Saf 104:349–356
Cheng GW, Breen PJ (1991) Activity of phenylalanine ammonia-lyase (PAL) and concentrations of anthocyanins and phenolics in developing strawberry fruit. J Amer Soc Hort Scl 116:865–869
Chinnici F, Bendini A, Gaiani A, Riponi C (2004) Radical scavenging activities of peels and pulps from cv. Golden delicious apples as related to their phenolic composition. J Agr Food Chem 52:4684–4689
Creelman RA, Mullet JE (1995) Jasmonic acid distribution and action in plants: Regulation during development and response to biotic and abiotic stress. Proc Natl Acad Sci U S A 92:4114–4119
Creelman RA, Mullet JE (1997) Oligosaccharins, brassinolides, and jasmonates: nontraditional regulators of plant growth, development, and gene expression. Plant Cell 9:1211–1223
Delhaize E, Ma JF, Ryan PR (2012) Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17:341–348
Du Z, Bramlage WJ (1992) Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J Agric Food Chem 40:1556–1570
Ehlenfeldt MK, Prior RL (2001) Oxygen radical absorbance capacity (ORAC) and phenolic and anthocyanin concentrations in fruit and leaf tissues of highbush blueberry. J Agric Food Chem 49:2222–2227
Eraso F, Hartley RD (1990) Monomeric and dimeric constituents of plant cell walls possible factors influencing wall biodegradability. J Sci Food Agric 51:163–170
Gao XP, Wang XF, Lu YF, Zhang LY, Shen YY, Liang Z, Zhang DP (2004) Jasmonic acid is involved in the water-stress-induced betaine accumulation in pear leaves. Plant Cell Environ 27:497–507
Giannopolitis CN, Ries SK (1977) Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol 59:309–314
Guo TR, Zhang GP, Zhang YH (2007) Physiological changes in barley plants under combined toxicity of aluminum, copper and cadmium. Colloids Surf B 57:182–188
Hanaka A, Wójcik M, Dresler S, Mroczek-Zdyrska M, Maksymiec W (2016) Does methyl jasmonate modify the oxidative stress response in Phaseolus coccineus treated with Cu? Ecotoxicol Environ Saf 124:480–488
Harris CS, Burt AJ, Saleem A, Le PM, Martineau LC, Haddad PS, Bennett SAL, Arnason JT (2007) A single HPLC-PAD-APCI/MS method for the quantitative comparison of phenolic compounds found in leaf, stem, root and fruit extracts of Vaccinium angustifolium. Phytochem Anal 18:161–169
Heijari J, Nerg AM, Kainulainen P, Vuorinen M, Holopainen JK (2008) Long-term effects of exogenous methyl jasmonate application on Scots pine (Pinus sylvestris) needle chemical defense and diprionid sawfly performance. Entomol Exp Appl 128:162–171
Hu X, Li W, Chen Q, Yang Y (2009) Early signal transduction linking the synthesis of jasmonic acid in plant. Plant Signal Behav 4:696–697
Huang W, Yang X, Yao S, LwinOo T, He H, Wang A, Li C, He L (2014) Reactive oxygen species burst induced by aluminum stress triggers mitochondria-dependent programmed cell death in peanut root tip cells. Plant Physiol Biochem 82:76–84
Inostroza-Blancheteau C, Reyes-Díaz M, Aquea F, Nunes-Nesi A, Alberdi M, Arce-Johnson P (2011) Biochemical and molecular changes in response to aluminium-stress in highbush blueberry (Vaccinium corymbosum L). Plant Physiol Biochem 49:1005–1012
Inostroza-Blancheteau C, Rengel Z, Alberd M, Mora M, Aquea F, Arce-Johnson P, Reyes-Díaz M (2012) Molecular and physiological strategies to increase aluminum resistance in plants. Mol Biol Rep 39:2069–2079
Ismail A, Riemann M, Nick P (2012) The jasmonate pathway mediates salt tolerance in grapevines. J Exp Bot 63:2127–2139
Jung SJ (2004) Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. Plant Physiol Biochem 42:225–231
Keramat B, Kalantari KM, Arvin MJ (2009) Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant (Glycine max L.). Afr J Microbiol Res 3:240–244
Kochian LV, Pineros MA, Liu J, Magalhaes JV (2015) Plant Adaptation to acid soils: The molecular basis for crop aluminum resistance. Annu Rev Plant Biol 66:571–598
Konan YKF, Kouassi KM, Kouakou KL, Koffi E, Kouassi KN, Sekou D, Kone M, Kouakou TH (2014) Effect of Methyl Jasmonate on Phytoalexins Biosynthesis and Induced Disease Resistance to Fusarium oxysporum f. sp. Vasinfectum in Cotton (Gossypium hirsutum L.). Intl J Agron 2014:1–11
Larronde F, Gaudillière JP, Krisa S, Decendit A, Deffieux G, Mérillon JM (2003) Airborne methyl jasmonate induces stilbene accumulation in leaves and berries of grapevine plants. Am J Enol Vitic 54:60–63
Li Y, Nie Y, Zhang Z, Ye Z, Zou X, Zhang LH, Wang Z (2014) Comparative proteomic analysis of methyl jasmonate-induced defense responses in different rice cultivars. Proteomics 14:1088–1101
Liu J, Li Z, Wang Y, Xing D (2014) Overexpression of ALTERNATIVE OXIDASE1a alleviates mitochondria-dependent programmed cell death induced by aluminium phytotoicity in Arabidopsis. J Exp Bot 65:4465–4478
Loreto F, Velikova V (2001) Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol 127:1781–1787
Ma B, Wan J, Shen ZG (2007) H2O2 production and antioxidant responses in seeds and early seedlings of two different rice varieties exposed to aluminum. Plant Growth Regul 52:91–100
Maksymiec W, Krupa Z (2002) Jasmonic acid and heavy metals in Arabidopsis plants- a similar physiological responses to both stressors? J Plant Physiol 159:509–515
Maksymiec W, Krupa Z (2007a) Effects of methyl jasmonate and excess copper on root and leaf growth. Biol Plant 51:322–326
Maksymiec W, Wójcik M, Krupa Z (2007b) Variation in oxidative stress and photochemical activity in Arabidopsis thaliana leaves subjected to cadmium and excess copper in the presence or absence of jasmonate and ascorbate. Chemosphere 66:421–427
Manquián K, Zúñiga GE, Barrientos H, Escudey M, Molina M (2013) Effect of aluminum on antioxidant activity and phenolic compounds content in in vitro cultured blueberries. Bol Latinoam Caribe Plant Med Aromat 12:603–611
Mäntylä E, Blande JD, Klemola T (2014) Does application of methyl jasmonate to birch mimic herbivory and attract insectivorous birds in nature? Arthropod Plant Interact 8:143–153
Matsuo Y, Fujita Y, Ohnishi S, Tanaka T, Hirabaru H, Kai T, Sakaida H, Nishizono S, Kouno I (2010) Chemical constituents of the leaves of rabbiteye blueberry (Vaccinium ashei) and characterisation of polymeric proanthocyanidins containing phenylpropanoid units and A-type linkages. Food Chem 121:1073–1079
McDonald M, Mila I, Scalbert A (1996) Precipitation of metal ions by plant polyphenols: optimal conditions and origin of precipitation. J Agric Food Chem 44:599–606
Menga X, Hana J, Wang Q, Tian S (2009) Changes in physiology and quality of peach fruits treated by methyl jasmonate under low temperature stress. Food Chem 114:1028–1035
Moreira X, Sampedro L, Zas R (2009) Defensive responses of Pinus pinaster seedlings to exogenous application of methyl jasmonate: Concentration effect and systemic response. Environ Exp Bot 67:94–100
Pan JW, Zhu MY, Chen H (2001) Aluminum-induced cell death in root-tip cells of barley. Environ Exp Bot 46:71–79
Pauwels L, Morreel K, De Witte E, Lammertyn F, Van Montagu M, Boerjan W, Inzé D, Goossens A (2008) Mapping methyl jasmonate-mediated transcriptional reprogramming of metabolism and cell cycle progression in cultured Arabidopsis cells. Proc Natl Acad Sci U S A 105:1380–1385
Piotrowska A, Bajguz A, Godlewska-żyłkiewicz B, Czerpak R, Kamińska M (2009) Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lemnaceae). J Exp Bot 66:507–513
Poonam S, Kaur H, Geetika S (2013) Effect of jasmonic acid on photosynthetic pigments and stress markers in Cajanus cajan (L.) Mill sp. seedlings under copper stress. Am J Plant Sci 4:817–823
Reyes-Díaz M, Alberdi M, Mora ML (2009) Short-term aluminum stress differentially affects the photochemical efficiency of photosystem II in Highbush Blueberry genotypes. J Amer Soc Hort Sci 134:14–21
Reyes-Díaz M, Inostroza-Blancheteau C, Millaleo R, Cruces E, Wulff-Zottele C, Alberdi M, Mora ML (2010) Long-term aluminum exposure effects on physiological and biochemical features of Highbush Blueberry cultivars. J Amer Soc Hort Sci 135:212–222
Ribera AE, Reyes-Díaz M, Alberdi M, Zuniga GE, Mora ML (2010) Antioxidant compounds in skin and pulp of fruits change among genotypes and maturity stages in Highbush blueberry (Vaccinium corymbosum L.) grown in southern Chile. J Soil Sci Plant Nutr 10:509–536
Rudell DR, Mattheis JP, Fan X, Fellman JK (2002) Methyl jasmonate enhances anthocyanin accumulation and modified production of phenolics and pigments in “Fuji” apples. J Amer Soc Hort Sci 127:435–441
Ruhland CT, Day TA (2000) Effects of ultraviolet-B radiation on leaf elongation, production and phenylpropanoid concentrations of Deschampsia antarctica and Colobanthus quitensis in Antarctica. Physiol Plant 109:244–251
Ruiz-García Y, Romero-Cascales I, Gil-Muñoz R, Fernández-Fernández JI, López-Roca JM, Gómez-Plaza E (2012) Improving grape phenolic content and wine chromatic characteristics through the use of two different elicitors: Methyl jasmonate versus benzothiadiazole. J Agric Food Chem 60:1283–1290
Sadzawka AM, Grez R, Carrasco MA, Mora ML (2004) Métodos de análisis de tejidos vegetales. Comisión de normalización y acreditación, sociedad chilena de la ciencia del suelo, In: Editorial salesianos impresores. Santiago, Chile, p. 105
Schaller A, Stintzi A (2009) Enzymes in jasmonate biosynthesis - Structure, function, regulation. Phytochemistry 70:1532–1538
Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV (2010) GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil 330:207–214
Shao HB, Chu LY, Shao MA, Jaleel CA, Mi HM (2008) Higher plant antioxidants and redox signaling under environmental stresses. C R Biologies 331:433–441
Sivaguru M, Liu J, Kochian LV (2013) Targeted expression of SbMATE in the root distal transition zone is responsible for sorghum aluminum resistance. Plant J 76:297–307
Slinkard K, Singleton VL (1977) Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic 28:29–55
Spollansky TC, Pitta-Alvarez SI, Giulietti AM (2000) Effect of jasmonic acid and aluminum on production of tropane alkaloids in hairy root cultures of Brugmansia candida. Electron J Biotechnol 3:72–75
Staswick PE (2008) JAZing up jasmonate signaling. Trends Plant Sci 13:66–71
Wang SY (1999) Methyl jasmonate reduces water stress in strawberry. J Plant Growth Regul 18:127–134
Wang FZ, Wang QB, Kwon SY, Kwak SS, Su WA (2005) Enhanced drought tolerance of transgenic rice plants expressing a pea manganese superoxide dismutase. J Plant Physiol 162:465–472
Wang SY, Chen C, Wang CY (2009) The influence of light and maturity on fruit quality and flavonoid content red raspberries. Food Chem 112:676–684
Wang LJ, Wu J, Wang HX, Li SS, Zheng XC, Du H, Xu YJ, Wang LS (2015) Composition of phenolic compounds and antioxidant activity in the leaves of blueberry cultivars. J Funct Foods 16:295–304
Xue YJ, Tao L, Yang ZM (2008) Aluminum-induced cell wall peroxidase activity and lignin synthesis are differentially regulated by jasmonate and nitric oxide. J Agric Food Chem 56:9676–9684
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2002) Aluminum toxicity is associated with mitochondrial dysfunction and the production of reactive oxygen species in plant cells. Plant Physiol 128:63–72
Yoon JY, Hamayun M, Lee SK, Lee IJ (2010) Methyl jasmonate alleviated salinity stress in soybean. J Crop Sci Biotechnol 12:63–68
Zhang L, Xing D (2008) Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 49:1092–1111
Acknowledgments
We are very grateful for FONDECYT Project N° 1120917 which supported this work and PhD fellowship N°21110919, both from the Comisión Nacional de Investigación Científica y Tecnológica (CONICYT) of the Government of Chile, as well as the 2013 DI 13-2017 and 2015 DI 15-2015 Projects from the Dirección de Investigación at the Universidad de La Frontera, Temuco, Chile. We would like to thank Graciela Muñoz Pozo for her valuable assistance in the laboratory, and Dr. Helen Lowry for revising the language of the manuscript. Finally, we wish to thank the Doctorado en Ciencias de Recursos Naturales Program and the Laboratorio de Fisiología y Bioquímica Vegetal at the Universidad de La Frontera, Temuco, Chile.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Richard J. Simpson.
Rights and permissions
About this article
Cite this article
Ulloa-Inostroza, E.M., Alberdi, M., Meriño-Gergichevich, C. et al. Low doses of exogenous methyl jasmonate applied simultaneously with toxic aluminum improve the antioxidant performance of Vaccinium corymbosum . Plant Soil 412, 81–96 (2017). https://doi.org/10.1007/s11104-016-2985-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2985-z